Abstract
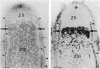
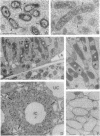
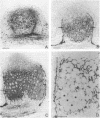
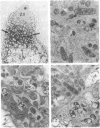
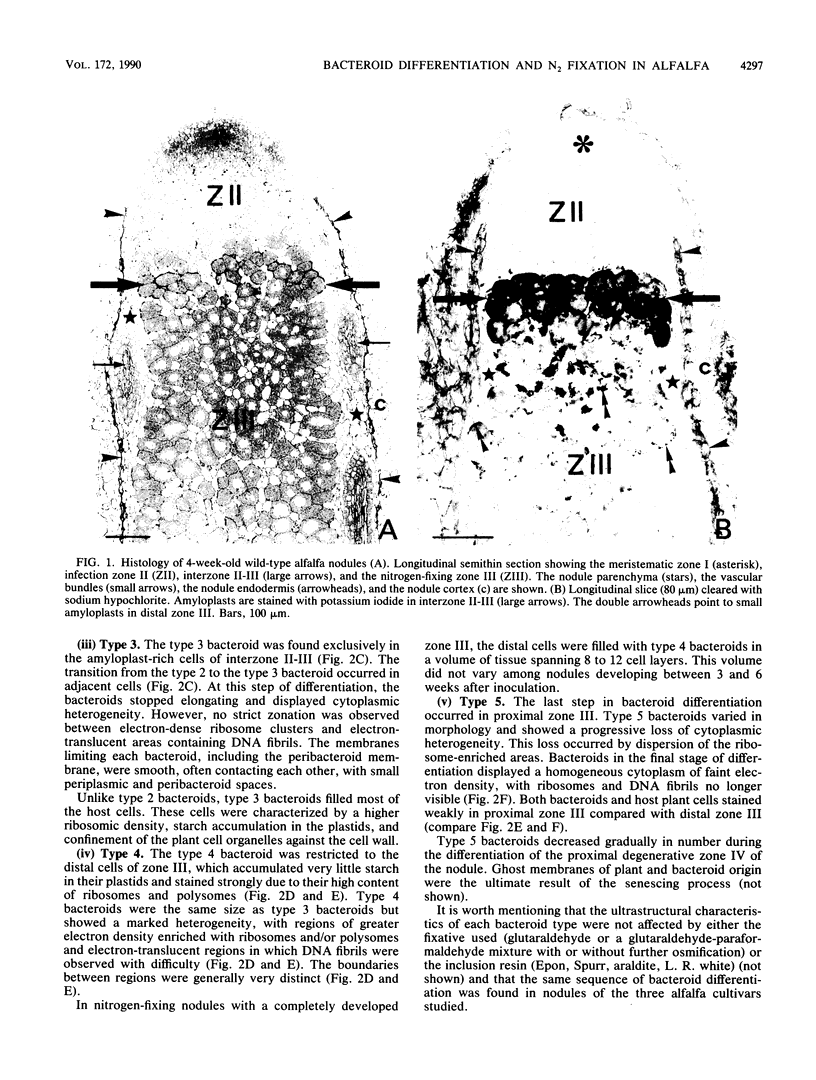
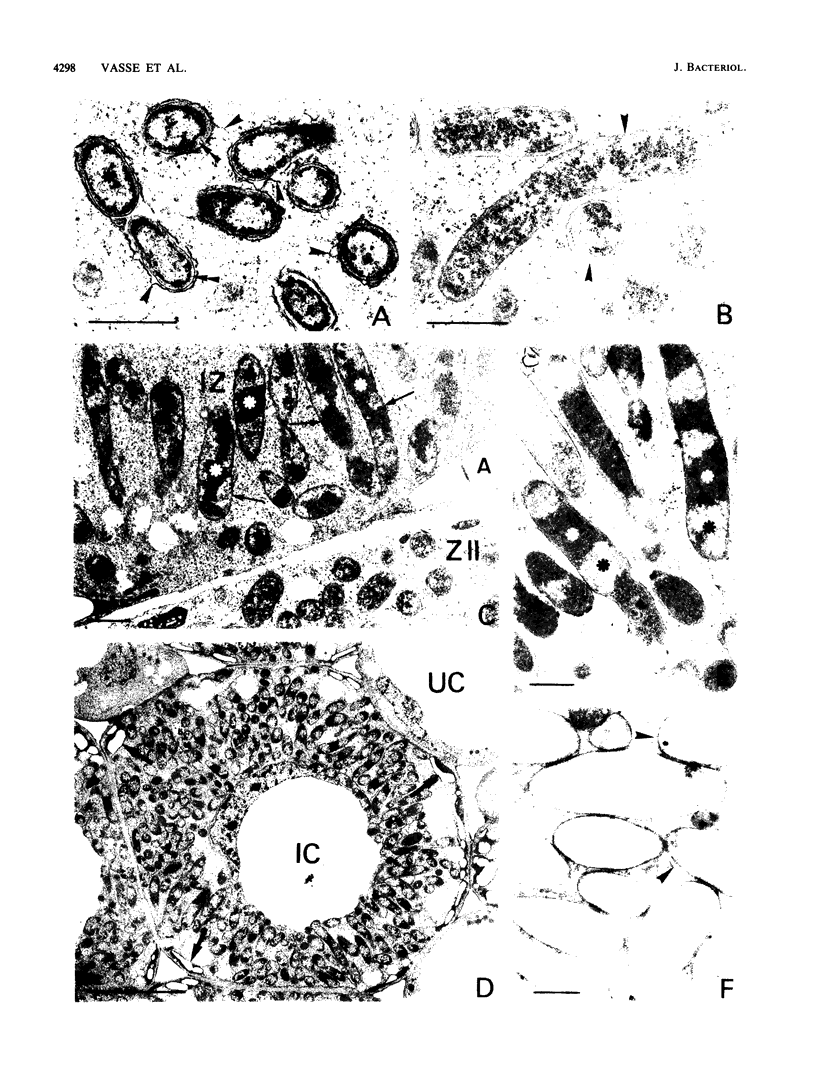
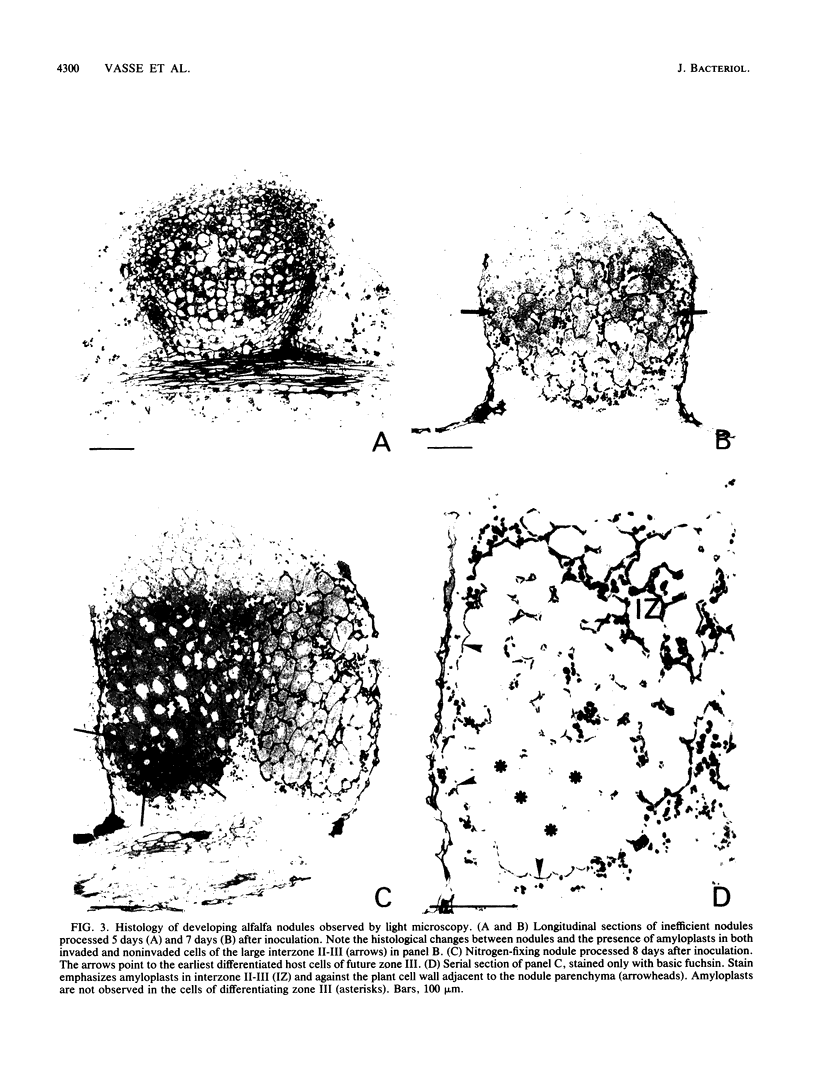
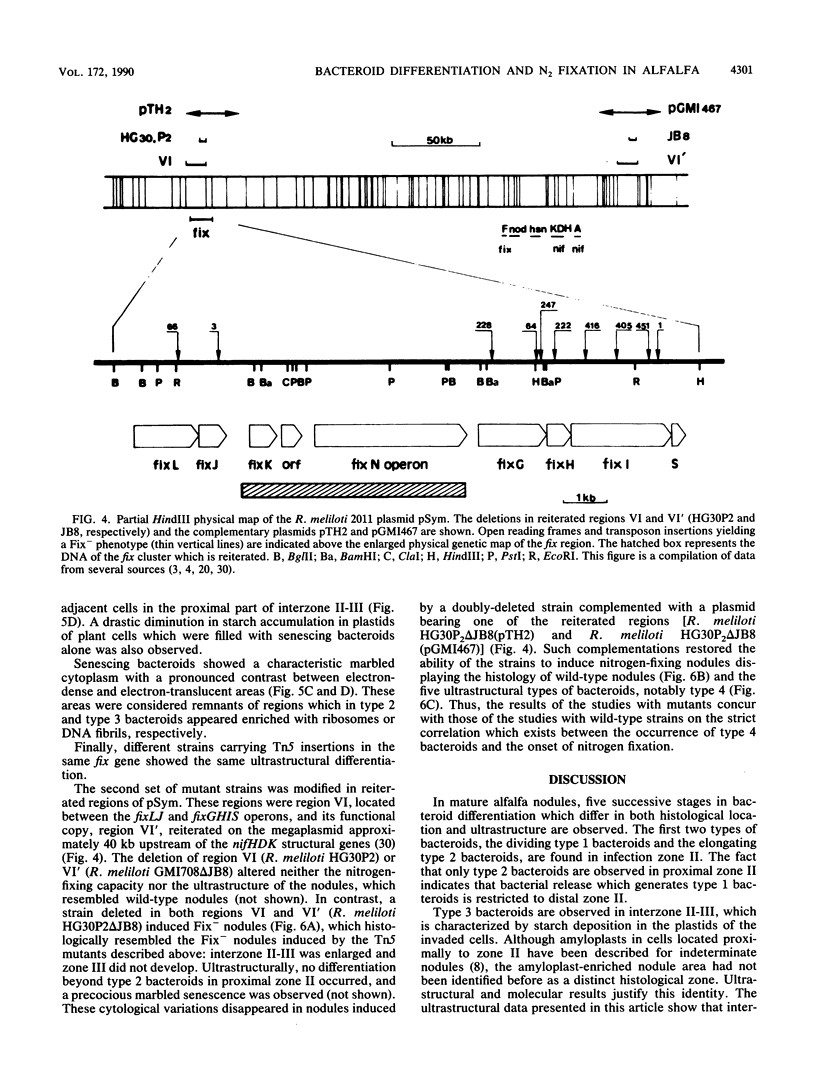
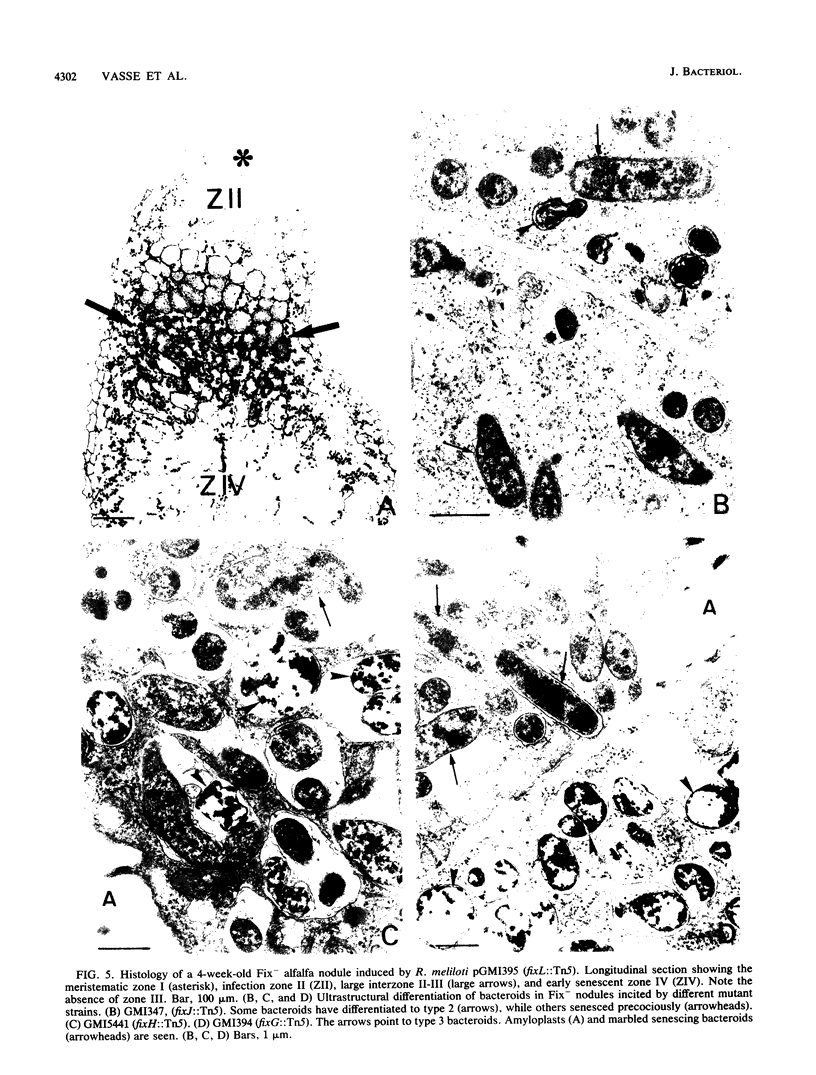
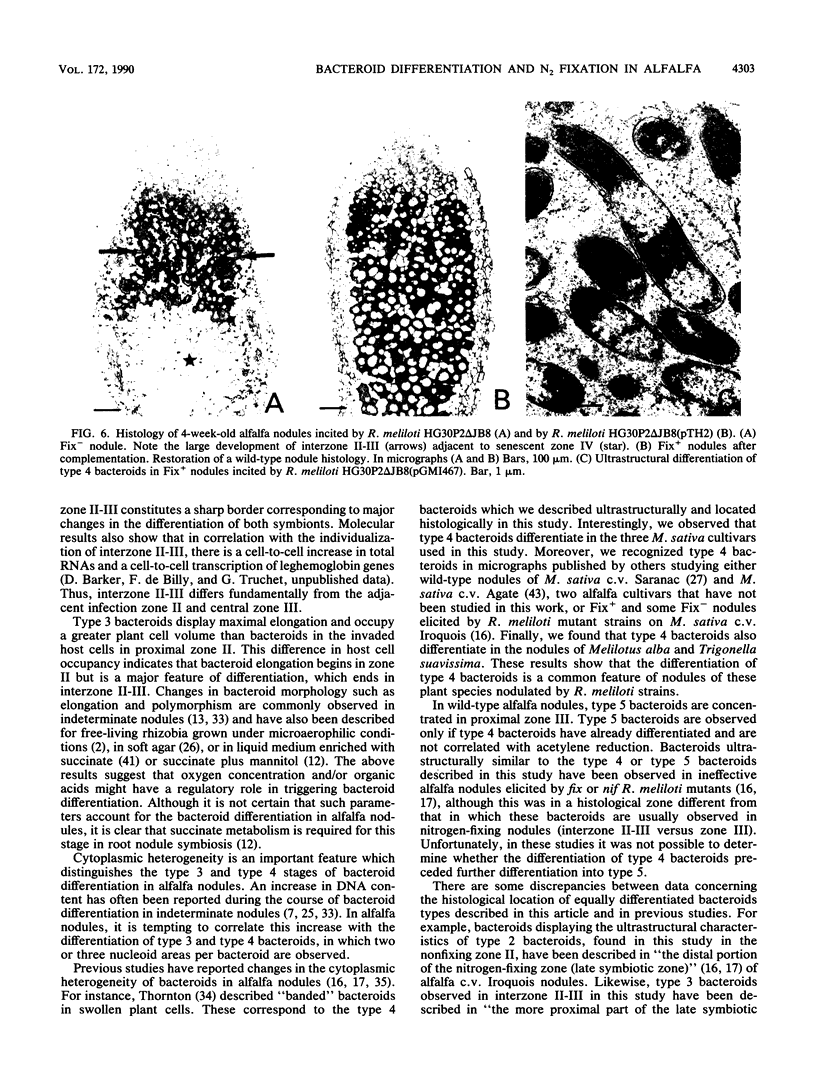
Bacteroid differentiation was examined in developing and mature alfalfa nodules elicited by wild-type or Fix- mutant strains of Rhizobium meliloti. Ultrastructural studies of wild-type nodules distinguished five steps in bacteroid differentiation (types 1 to 5), each being restricted to a well-defined histological region of the nodule. Correlative studies between nodule development, bacteroid differentiation, and acetylene reduction showed that nitrogenase activity was always associated with the differentiation of the distal zone III of the nodule. In this region, the invaded cells were filled with heterogeneous type 4 bacteroids, the cytoplasm of which displayed an alternation of areas enriched with ribosomes or with DNA fibrils. Cytological studies of complementary halves of transversally sectioned mature nodules confirmed that type 4 bacteroids were always observed in the half of the nodule expressing nitrogenase activity, while the presence of type 5 bacteroids could never be correlated with acetylene reduction. Bacteria with a transposon Tn5 insertion in pSym fix genes elicited the development of Fix- nodules in which bacteroids could not develop into the last two ultrastructural types. The use of mutant strains deleted of DNA fragments bearing functional reiterated pSym fix genes and complemented with recombinant plasmids, each carrying one of these fragments, strengthened the correlation between the occurrence of type 4 bacteroids and acetylene reduction. A new nomenclature is proposed to distinguish the histological areas in alfalfa nodules which account for and are correlated with the multiple stages of bacteroid development.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aguilar O. M., Kapp D., Pühler A. Characterization of a Rhizobium meliloti fixation gene (fixF) located near the common nodulation region. J Bacteriol. 1985 Oct;164(1):245–254. doi: 10.1128/jb.164.1.245-254.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batut J., Daveran-Mingot M. L., David M., Jacobs J., Garnerone A. M., Kahn D. fixK, a gene homologous with fnr and crp from Escherichia coli, regulates nitrogen fixation genes both positively and negatively in Rhizobium meliloti. EMBO J. 1989 Apr;8(4):1279–1286. doi: 10.1002/j.1460-2075.1989.tb03502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhard W. A new staining procedure for electron microscopical cytology. J Ultrastruct Res. 1969 May;27(3):250–265. doi: 10.1016/s0022-5320(69)80016-x. [DOI] [PubMed] [Google Scholar]
- David M., Daveran M. L., Batut J., Dedieu A., Domergue O., Ghai J., Hertig C., Boistard P., Kahn D. Cascade regulation of nif gene expression in Rhizobium meliloti. Cell. 1988 Aug 26;54(5):671–683. doi: 10.1016/s0092-8674(88)80012-6. [DOI] [PubMed] [Google Scholar]
- Faucher C., Maillet F., Vasse J., Rosenberg C., van Brussel A. A., Truchet G., Dénarié J. Rhizobium meliloti host range nodH gene determines production of an alfalfa-specific extracellular signal. J Bacteriol. 1988 Dec;170(12):5489–5499. doi: 10.1128/jb.170.12.5489-5499.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardiol A. E., Truchet G. L., Dazzo F. B. Requirement of succinate dehydrogenase activity for symbiotic bacteroid differentiation of Rhizobium meliloti in alfalfa nodules. Appl Environ Microbiol. 1987 Aug;53(8):1947–1950. doi: 10.1128/aem.53.8.1947-1950.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gourret J. P., Fernandez-Arias H. Etude ultrastructurale et cytochimique de la différenciation des bactéroïdes de Rhizobium trifolii Dangeard dans les nodules de Trifolium repens L. Can J Microbiol. 1974 Aug;20(8):1169–1181. [PubMed] [Google Scholar]
- HUXLEY H. E., ZUBAY G. Preferential staining of nucleic acid-containing structures for electron microscopy. J Biophys Biochem Cytol. 1961 Nov;11:273–296. doi: 10.1083/jcb.11.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halverson L. J., Stacey G. Signal exchange in plant-microbe interactions. Microbiol Rev. 1986 Jun;50(2):193–225. doi: 10.1128/mr.50.2.193-225.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch A. M., Bang M., Ausubel F. M. Ultrastructural analysis of ineffective alfalfa nodules formed by nif::Tn5 mutants of Rhizobium meliloti. J Bacteriol. 1983 Jul;155(1):367–380. doi: 10.1128/jb.155.1.367-380.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch A. M., Smith C. A. Effects of Rhizobium meliloti nif and fix mutants on alfalfa root nodule development. J Bacteriol. 1987 Mar;169(3):1137–1146. doi: 10.1128/jb.169.3.1137-1146.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber J. D., Parker F., Odland G. F. A basic fuchsin and alkalinized methylene blue rapid stain for epoxy-embedded tissue. Stain Technol. 1968 Mar;43(2):83–87. doi: 10.3109/10520296809115048. [DOI] [PubMed] [Google Scholar]
- Kahn D., David M., Domergue O., Daveran M. L., Ghai J., Hirsch P. R., Batut J. Rhizobium meliloti fixGHI sequence predicts involvement of a specific cation pump in symbiotic nitrogen fixation. J Bacteriol. 1989 Feb;171(2):929–939. doi: 10.1128/jb.171.2.929-939.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerouge P., Roche P., Faucher C., Maillet F., Truchet G., Promé J. C., Dénarié J. Symbiotic host-specificity of Rhizobium meliloti is determined by a sulphated and acylated glucosamine oligosaccharide signal. Nature. 1990 Apr 19;344(6268):781–784. doi: 10.1038/344781a0. [DOI] [PubMed] [Google Scholar]
- Paau A. S., Bloch C. B., Brill W. J. Developmental fate of Rhizobium meliloti bacteroids in alfalfa nodules. J Bacteriol. 1980 Sep;143(3):1480–1490. doi: 10.1128/jb.143.3.1480-1490.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paau A. S., Cowles J. R. Development of Bacteroids in Alfalfa (Medicago sativa) Nodules. Plant Physiol. 1978 Oct;62(4):526–530. doi: 10.1104/pp.62.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paau A. S., Lee D., Cowles J. R. Comparison of nucleic acid content in populations of free-living and symbiotic Rhizobium meliloti by flow microfluorometry. J Bacteriol. 1977 Feb;129(2):1156–1158. doi: 10.1128/jb.129.2.1156-1158.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel J. J., Yang A. F. Light and electron microscopic studies of nodule structure of alfalfa. Can J Microbiol. 1981 Jan;27(1):36–43. doi: 10.1139/m81-006. [DOI] [PubMed] [Google Scholar]
- Peters N. K., Frost J. W., Long S. R. A plant flavone, luteolin, induces expression of Rhizobium meliloti nodulation genes. Science. 1986 Aug 29;233(4767):977–980. doi: 10.1126/science.3738520. [DOI] [PubMed] [Google Scholar]
- Putnoky P., Grosskopf E., Ha D. T., Kiss G. B., Kondorosi A. Rhizobium fix genes mediate at least two communication steps in symbiotic nodule development. J Cell Biol. 1988 Mar;106(3):597–607. doi: 10.1083/jcb.106.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renalier M. H., Batut J., Ghai J., Terzaghi B., Gherardi M., David M., Garnerone A. M., Vasse J., Truchet G., Huguet T. A new symbiotic cluster on the pSym megaplasmid of Rhizobium meliloti 2011 carries a functional fix gene repeat and a nod locus. J Bacteriol. 1987 May;169(5):2231–2238. doi: 10.1128/jb.169.5.2231-2238.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg C., Boistard P., Dénarié J., Casse-Delbart F. Genes controlling early and late functions in symbiosis are located on a megaplasmid in Rhizobium meliloti. Mol Gen Genet. 1981;184(2):326–333. doi: 10.1007/BF00272926. [DOI] [PubMed] [Google Scholar]
- Truchet G., Debellé F., Vasse J., Terzaghi B., Garnerone A. M., Rosenberg C., Batut J., Maillet F., Dénarié J. Identification of a Rhizobium meliloti pSym2011 region controlling the host specificity of root hair curling and nodulation. J Bacteriol. 1985 Dec;164(3):1200–1210. doi: 10.1128/jb.164.3.1200-1210.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban J. E., Dazzo F. B. Succinate-Induced Morphology of Rhizobium trifolii 0403 Resembles That of Bacteroids in Clover Nodules. Appl Environ Microbiol. 1982 Jul;44(1):219–226. doi: 10.1128/aem.44.1.219-226.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virts E. L., Stanfield S. W., Helinski D. R., Ditta G. S. Common regulatory elements control symbiotic and microaerobic induction of nifA in Rhizobium meliloti. Proc Natl Acad Sci U S A. 1988 May;85(9):3062–3065. doi: 10.1073/pnas.85.9.3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong C. H., Pankhurst C. E., Kondorosi A., Broughton W. J. Morphology of root nodules and nodule-like structures formed by Rhizobium and Agrobacterium strains containing a Rhizobium meliloti megaplasmid. J Cell Biol. 1983 Sep;97(3):787–794. doi: 10.1083/jcb.97.3.787. [DOI] [PMC free article] [PubMed] [Google Scholar]