Abstract
The mechanisms through which the small GTPases Rac1 and Cdc42 regulate the formation of membrane ruffles, lamellipodia, and filopodia are currently unknown. The p21-activated kinases (PAKs) are direct targets of active Rac and Cdc42 which can induce the assembly of polarized cytoskeletal structures when expressed in fibroblasts, suggesting that they may play a role in mediating the effects of these GTPases on cytoskeletal dynamics.
We have examined the subcellular localization of endogenous PAK1 in fibroblast cell lines using specific PAK1 antibodies. PAK1 is detected in submembranous vesicles in both unstimulated and stimulated fibroblasts that colocalize with a marker for fluid-phase uptake. In cells stimulated with PDGF, in v-Src–transformed fibroblasts, and in wounded cells, PAK1 redistributed into dorsal and membrane ruffles and into the edges of lamellipodia, where it colocalizes with polymerized actin. PAK1 was also colocalized with F-actin in membrane ruffles extended as a response to constitutive activation of Rac1. PAK1 appears to precede F-actin in translocating to cytoskeletal structures formed at the cell periphery. The association of PAK1 with the actin cytoskeleton is prevented by the actin filament-disrupting agent cytochalasin D and by the phosphatidylinositol 3-kinase inhibitor wortmannin. Co-immunoprecipitation experiments demonstrate an in vivo interaction of PAK1 with filamentous (F)-actin in stimulated cells. Microinjection of a constitutively active PAK1 mutant into Rat-1 fibroblasts overexpressing the insulin receptor (HIRcB cells) induced the formation of F-actin- and PAK1-containing structures reminiscent of dorsal ruffles. These data indicate a close correlation between the subcellular distribution of endogenous PAK1 and the formation of Rac/Cdc42-dependent cytoskeletal structures and support an active role for PAK1 in regulating cortical actin rearrangements.
A variety of growth factors, oncogenes, chemokines, and extracellular matrix components induce dramatic morphological and cytoskeletal changes in cells. The polymerization of cortical actin and the associated production of membrane ruffles and lamellipodia are important components of cellular motile responses and may regulate other aspects of cellular signaling as well (Stossel, 1993; Mitchison and Cramer, 1996). Recent work has implicated members of the Rho family of GTPases as mediators of cytoskeletal changes (Ridley et al., 1992; Hall, 1994; Kozma et al., 1995; Nobes and Hall, 1995). Rac1 mediates the effects of many hormones and oncogenes on formation of cortical actin structures (Hall, 1994). Thus, introduction of dominant negative forms of Rac into cells inhibits, while active Rac mutants effectively induce, membrane ruffling, lamellipod formation, and pinocytosis (Ridley et al., 1992). Similarly, the related GTPase Cdc42 regulates the extension of actin filament bundles into filopodia (Kozma et al., 1995; Nobes and Hall, 1995). Both Rac and Cdc42 also induce the formation of multimolecular focal complexes distinct from the focal adhesions induced by Rho (Nobes and Hall, 1995).
The mechanisms by which Rac and Cdc42 initiate and regulate the formation of cytoskeletal structures are not currently understood. Evidence has been obtained that in some systems Rac and related GTPases can regulate actin polymerization through their ability to modulate cellular levels of phosphatidylinositol 4-monophosphate via phosphatidylinositol (PI)1 5-kinase (Chong et al., 1994; Hartwig et al., 1995) and/or arachidonic acid release via regulation of PLA2 (Peppelenbosch et al., 1995). Recently, a direct target for active Rac has been identified as a family of serine/thrionine kinases known as p21-activated kinases or PAKs (Manser et al., 1994, 1995; Bagrodia et al., 1995b ; Knaus et al., 1995; Martin et al., 1995). The activity of PAKs is stimulated by the binding of GTP-bound Rac or Cdc42. Reports show that both G protein-coupled receptors and cytokine receptors regulate PAK activity (Knaus et al., 1995; Zhang et al., 1995). PAKs have been implicated in phosphorylation of the p47phox component of the Rac-regulated NADPH oxidase (Knaus et al., 1995) and in the activation of a Rac/Cdc42-controlled kinase cascade leading to stimulation of the stress-activated MAP kinases, p38 and JNK (Bagrodia et al., 1995a ; Zhang et al., 1995).
The ability of PAKs to regulate MAP kinase activities is analogous to the role of the PAK homolog Ste20 in the pheromone response pathway of Saccharomyces cerevisiae (Herskowitz, 1995), where it regulates a MAP kinase signaling cascade. Ste20 also plays important roles in regulating polarized cell growth, presumably through effects on the actin cytoskeleton (Chant and Stowers, 1995; Cvrckova et al., 1995; Leeuw et al., 1995; Zarzov et al., 1996), as does pak1 + in fission yeast (Ottilie et al., 1995). Recently, we have demonstrated that mammalian PAK1 can initiate cytoskeletal rearrangements reminiscent of those produced by Rac and/or Cdc42 (Sells et al., 1997). Introduction of activated forms of PAK1 into Swiss 3T3 fibroblasts causes the formation of membrane ruffles, lamellipodia, and filopodia. In this paper, we provide further evidence in support of a role for PAK1 in regulating actin assembly by demonstrating that endogenous PAK1 becomes co-localized with filamentous (F)-actin at the edge of lamellipodia and in dorsal and membrane ruffles induced by distinct upstream stimuli. PAK1 physically associates with polymerized actin in PDGF-stimulated cells and may play a role in initiating and/or integrating the formation of F-actin–containing cytoskeletal structures.
Materials and Methods
Cell Culture and Preparation for Analysis
Cell culture and maintenance techniques were identical to those described in Ridley (1995). Swiss 3T3 cells were grown in DME containing 10% fetal bovine serum. v-src–transformed NIH3T3 cells and their control cells were grown in DME containing 10 μg/ml G418 (Luttrell et al., 1988). 5–7 d after seeding, the confluent cells were incubated overnight (16 h) in DME to obtain serum-free conditions. Immediately before fixation, cells were treated with 3–5 ng/ml PDGF BB (Upstate Biotechnology, Inc., Lake Placid, NY). In some experiments, cells were pretreated with 10 μM cytochalasin D or 100 nM wortmannin for 10 min before stimulation with PDGF.
Cells were prepared for adhesion assays as described in Hotchin and Hall (1995). Briefly, confluent Swiss 3T3 cells were serum starved overnight and then trypsinized and washed in serum-free DME. The cells were resuspended in the same buffer and stimulated with 5 ng/ml PDGF and plated onto coverslips coated with 50 μg/ml fibronectin. Cells were fixed at the indicated times after plating.
Transfection of Swiss 3T3 Cells with Semliki Forest Virus
The cDNA fragment encoding Rac1 wild-type and mutations Q61L and T17N were amplified by PCR using primers that contain a BamHI restriction enzyme site and a myc tag at the 5′ end. The myc-tagged Rac constructs were subcloned into the BamHI site of pSFV3 (Life Technologies, Gaithersburg, MD) and recombinant virus generated. Swiss 3T3 cells were infected with Rac or Lac Z containing virus in serum-free media and allowed to grow 13–15 h before experiment. Gene expression was confirmed by immunofluorescence using anti-myc (9E10).
Affinity Purification of Antibodies
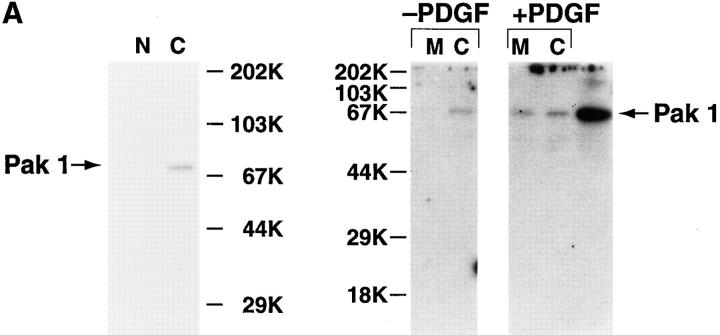
PAK1 was purified as a glutathione-S-transferase fusion protein from Escherichia coli as described in Knaus et al. (1995) and was coupled to cyanogen bromide-activated Sepharose 4B (Pharmacia Fine Chemicals, Piscataway, NJ). Anti-PAK1 antibody R626, prepared as detailed in Knaus et al. (1995), and 2124/3, directed against PAK1 residues 174–306, were affinity-purified using this resin as described in Schneider et al. (1982). The monospecificity of the affinity-purified polyclonal anti-PAK1 antibodies was confirmed by Western blotting against Swiss 3T3 subcellular fractions (see Fig. 9).
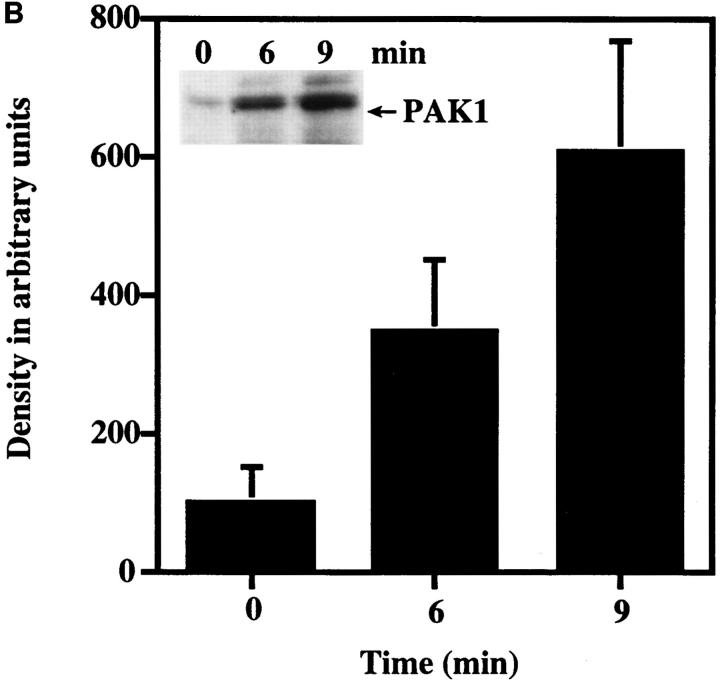
Figure 9.
(A) Detection of PAK1 in Swiss 3T3 subcellular fractions. Serum-starved cells with either no addition (−PDGF) or with addition (+PDGF) of 5 ng/ml PDGF for 10 min were separated into membrane (M) and cytosolic (C) fractions and immunoblotted for PAK1 using affinity-purified anti-PAK1 antibody, as described in Materials and Methods. Alternatively, highly purified nuclei (N) were prepared as described in Materials and Methods for immunoblotting. PAK1 was detected as a single band at 68-kD that comigrated with authentic human PAK1 expressed in Cos cells (last lane). (B) Detection of PAK1 in the membrane fraction during PDGF stimulation. Membrane fractions of quiescent Swiss 3T3 cells with either no addition or at 6 and 9 min after the addition of 5 ng/ml of PDGF were immunoblotted for PAK1 using affinity-purified anti-PAK1 antibody. Quantitation of the 68-kD band was by phosphoimager analysis. The mean density of the 68-kD band from unstimulated membrane fractions was set at 100%. The data shown represent the mean ± SEM of three separate experiments. The presence of 20– 40% of total PAK1 immunoreactivity in the isolated membrane fraction from PDGF-stimulated but not unstimulated cells was consistently observed. (Inset) Representative Western blot of membrane fractions blotted for PAK1. Arrow indicates 68-kD band.
Immunofluorescence Microscopy
Cells were prepared and fixed according to Ridley (1995). Briefly, quiescent Swiss 3T3 cells on coverslips were stimulated with 3 to 5 ng/ml PDGF for 10 min and fixed in either 3% paraformaldehyde or 3.7% formaldehyde (Sigma Chemical Co., St. Louis, MO) for 15 min, or in 100% MeOH at −20°C for 10 min. After fixation with formaldehyde, the cells were permeabilized for 5 min in 0.2% Triton X-100. Coverslips were then incubated with 0.5 μg/ml rhodamine phalloidin (Molecular Probes, Inc., Eugene, OR) and 20 μg/ml affinity-purified polyclonal anti-PAK1 R626; 20 μg/ml affinity-purified polyclonal anti-PAK1 2124/3; 1:50 polyclonal anti-Rac1 R785 (Quinn et al., 1993); 1:100 anti-BiP (StressGen, Biotechnologies, Victoria, BC, Canada); 1:50 anti-cytochrome oxidase (Molecular Probes, Inc.); or 1:10 anti–LAMP-2 (Granger et al., 1990) for 1 h. After extensive washing in 10 mM Hepes/0.5 M NaCl buffer, pH 7.4, containing 0.1% saponin, the coverslips were stained with 1:200 fluorescein-conjugated anti–rabbit IgG (Cappel Laboratories, Cochranville, PA) and 1:50 rhodamine-conjugated anti–mouse IgG, or 1:200 fluorescein-conjugated anti–mouse IgG and 1:50 rhodamine-conjugated anti–rabbit IgG for 1 h. For localization of focal complexes, cells were incubated for 1 h with rabbit polyclonal anti-PAK1 and mouse monoclonal anti-phosphotyrosine (4G10; Upstate Biotechnology, Inc.), mouse monoclonal anti-vinculin (Sigma Chemical Co.), or mouse monoclonal anti-actin (Sigma Chemical Co.). After washing, the coverslips were stained with fluorescein-conjugated goat anti–rabbit IgG and rhodamine-conjugated goat anti–mouse IgG (Cappel). Washed coverslips were mounted in Slow Fade (Molecular Probes, Inc.) and examined by light and confocal microscopy.
Cells were examined using a confocal scanning microscope (MRC600; Bio Rad, Hercules, CA) equipped with an argon–krypton mixed gas laser. The confocal unit was attached to an inverted microscope (IM35M; Zeiss, Inc., Thornwood, NY) and the data collected using a 63× oil immersion lens. Side by side images were collected using the K1/K2 block combination, merged together, and processed with the COMOS software (Bio Rad). Images were photographed on a slave monitor, the Focus Imagecorder Plus (Focus Graphics, Inc.) with a 35-mm camera back and Kodak print film (Royal Gold ASA25).
Cellular controls treated with anti-PAK1 alone or fluorescein-labeled goat anti–rabbit antibody alone did not show significant fluorescence in either the fluorescein or rhodamine channels. Cells treated with either rhodamine phalloidin or primary antibody, followed by either fluorescein- or rhodamine-conjugated secondary antibody, did not exhibit any crossover fluorescence between the fluorescein and rhodamine channels.
Subcellular Fractionation
Quiescent, serum-starved Swiss 3T3 cells were incubated with ±5 ng/ml PDGF for 6, 9, or 10 min before fractionation by the method of Krek et al. (1992). Cells were then harvested in ice-cold trypsin–EDTA and washed in 1 mM Hepes/NaCl buffer, pH 7.5. Approximately 107 cells were resuspended in 300 μl ice-cold hypotonic buffer containing 20 mM Hepes-KOH, pH 7.5, 5 mM KCl, 1.5 mM MgCl2, 1 mM dithiothreitol, and protease cocktail (1 μg/ml each of chymostatin, leupeptin, and pepstatin, 1 mM PMSF, 2 μg/ml aprotinin, 0.2 mM sodium vanadate). After incubation on ice for 10 min, cells were homogenized using 15 strokes in a Dounce homogenizer and centrifuged at 2,000 rpm for 10 min. The resulting supernatant was spun at 10,000 g for 1 h in a centrifuge (TL-100; Beckman Instruments, Fullerton, CA) according to Thom et al. (1977). The membrane pellet obtained was dissolved in 250 μl 1× Laemmli sample buffer. 50 μl of 4× sample buffer was added to the supernatant (cytosolic) fraction to bring the total volume to 250 μl. Highly purified Swiss 3T3 nuclei, prepared according to the procedure of Schreiber et al. (1989), were generously provided by P. Maher, (The Scripps Research Institute, La Jolla, CA); these nuclear preparations have been characterized and shown to be free of contaminating organelles (Maher, 1996). The samples were analyzed on 10% SDS-polyacrylamide gels and either stained with Coomassie brilliant blue to analyze total protein or Western blotted using a 1:1,000 dilution of affinity-purified PAK1 antibody or a 1:500 dilution of rabbit polyclonal anti-actin (Sigma Chemical Co.). Proteins were then detected either by autoradiography with I125 protein A or alkaline phosphatase-conjugated anti–rabbit IgG (Bio Rad). Bands of interest were quantified by scanning densitometry using the phosphoimager system from Molecular Dynamics (Sunnyvale, CA).
Wounding Technique
The wound healing model (Todaro et al., 1967) was used to obtain polarized Swiss 3T3 cells. Confluent cells were wounded by a 1–2-mm-wide slash through the cell monolayer using a sharp razor blade. Polarized cells were fixed and stained for PAK1 and F-actin, as described, at 3 and 6 h after wounding.
Coimmunoprecipitation Assay
Quiescent, serum-starved Swiss 3T3 cells were stimulated with 5 ng/ml PDGF for 10 min and harvested from plates by addition of 500 μl ice-cold lysis buffer containing 1% NP40/100-mm plate. The clarified cell lysates were incubated with 25 μl of either anti-PAK1 or preimmune serum. Both batches of antibody were preadsorbed against an actin/CNBr/Sepharose resin, as described in Dharmawardhane et al. (1991), to eliminate IgG molecules that may bind to actin. After 2 h at 4°C, 80 μl of 50% protein A-Sepharose was added to the mixture and incubated for 1 h at 4°C. The immunoprecipitates were recovered and washed twice in wash buffer with 1% NP40 containing 200 mM KCl and four times in the same buffer without NP40. The use of the high ionic strength buffer inhibits nonspecific binding of actin to IgG (Fechheimer et al., 1979). The washed immunoprecipitates were resuspended in 100 μl 1× Laemmli sample buffer and analyzed by SDS-PAGE and Western blotting using anti-actin, as described above. The 42-kD actin band was quantified by scanning densitometry (Molecular Dynamics).
Analysis of Triton-Insoluble Cytoskeletons
Triton-insoluble cytoskeletons were isolated from quiescent- or PDGF-stimulated Swiss 3T3 cells using procedures modified from Dharmawardhane et al. (1991) and Burgess et al. (1989). Confluent cells on 100-mm Petri dishes were lysed in 1 ml of pH 7.5 buffer containing 75 mM KCl, 2 mM MgCl2, 5 mM EDTA, 5 mM DTT, 20 mM PIPES, 1 mM ATP, protease inhibitors, and 0.5% Triton X-100. After extraction on ice for 5 min, the lysates were centrifuged at 14,000 rpm for 3 min at 4°C. The Triton-insoluble cytoskeletons recovered in the pellets were dissolved in 100 μl of 1× Laemmli sample buffer and analyzed by SDS-PAGE and Western blotting for PAK1.
Kinase Assays
PAK1 activity was assayed as described in Knaus et al. (1995) using both autophosphorylation and phosphorylation of the exogenous substrate myelin basic protein (MBP) to assess activity. Unstimulated cells or cells stimulated with 3 ng/ml PDGF for the indicated times were lysed and immunoprecipitated with PAK1 antibody as described above for actin analysis. The immunoprecipitates were washed twice with 1 ml of lysis buffer with 1% NP40, twice in the same buffer without NP40, and twice with kinase buffer consisting of 50 mM Hepes, pH 7.5, 10 mM MgCl2, 2 mM MnCl2, 0.2 mM DTT. Washed pellets were then subjected to in vitro kinase assays (Knaus et al., 1995).
Microinjection of PAK1 Mutants into HIRcB Cells
Microinjections were performed as described in Martin et al. (1996). Briefly, HIRcB cells grown on glass coverslips were rendered quiescent by starvation for 36–48 h in serum-free DME. Expression vectors (Sells et al., 1997) for PAK1 wild-type and PAK1 (H83L,H86L) suspended in microinjection buffer (5 mM NaPO4 and 100 mM KCl, pH 7.4) at a concentration of 0.1 mg/ml, were injected directly into the nucleus using glass capillary needles. 6–8 h after injection, cells were fixed with 3.7% formaldehyde in PBS and fluorescently stained for protein expression and actin localization. Primary incubation with anti-myc (9E10) ascites fluid (1:500 in PBS for 1 h at room temperature) was followed by incubation with a fluorescein-conjugated goat anti–mouse antibody (Jackson ImmunoResearch, West Grove, PA) diluted 1:100 in PBS for 1 h at room temperature. Rhodamine-phalloidin (Sigma Chemical Co.) was added to the secondary incubation at a concentration of 0.5 μg/ml.
Results
PAK1 Is Activated by PDGF in Swiss 3T3 Cells
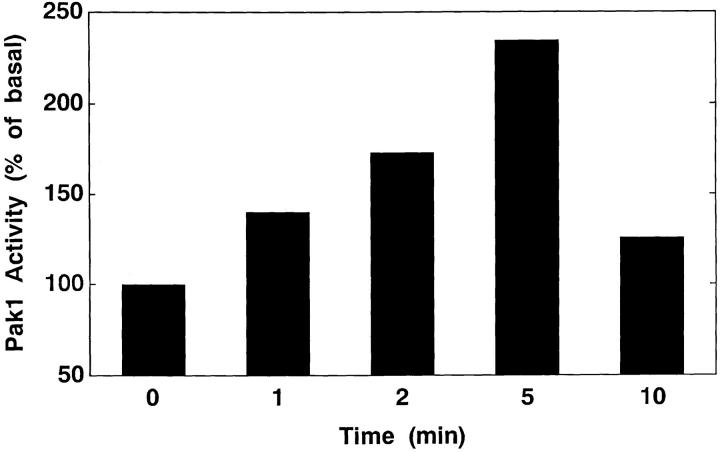
We examined the effect of PDGF on the kinase activity of endogenous PAK1 in Swiss 3T3 cells. PAK1 autocatalytic activity was rapidly stimulated by PDGF (Fig. 1), with enhanced activity detected within 1 min and maximum stimulation seen between 2 and 5 min in various experiments. PDGF also stimulated phosphorylation of the exogenous substrate, MBP, in the PAK1 immunoprecipitates (not shown).
Figure 1.
Stimulation of PAK1 activity by PDGF in Swiss 3T3 cells. Swiss 3T3 cells were stimulated with 3 ng/ml PDGF for the indicated times and PAK1 activity determined in immunoprecipitates by in vitro kinase assays as described in Materials and Methods. Activity at t = 0 was set as 100%. Results shown are representative of four similar experiments.
PAK1 Localizes to Pinocytic Vesicles in Unstimulated Swiss 3T3 Cells
Since stimulation of cells by PDGF induces the formation of Rac-dependent cytoskeletal structures (Ridley et al., 1992; Hall, 1994) and increases PAK1 activity, we examined the subcellular localization of endogenous PAK1 in unstimulated and PDGF-stimulated Swiss 3T3 cells using affinity-purified polyclonal antibody against full length PAK1. In unstimulated cells, PAK1 was sparsely distributed throughout the cytoplasm but was particularly evident in structures that had the appearance of elongated vesicles (Fig. 2, A and B). These structures were dispersed throughout the cell, with increased abundance apparent around the nucleus. An identical vesicular distribution was observed with the PAK1 peptide antibody. Rac1 was present in similar structures as determined using polyclonal anti-Rac1, R785 (Quinn et al., 1993), and a commercial anti-Rac1 (Santa Cruz Biotechnology, Santa Cruz, CA).
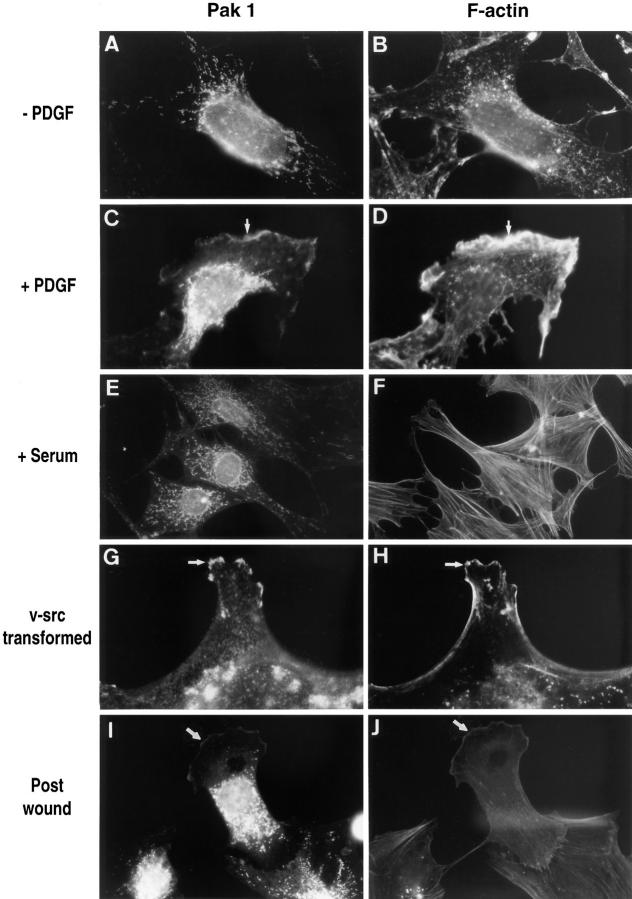
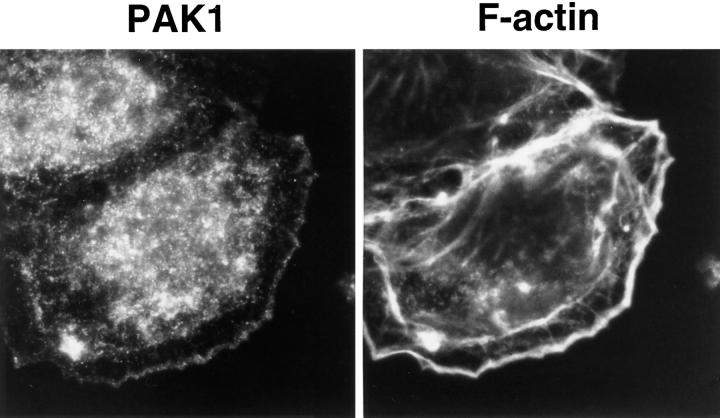
Figure 2.
PAK1 and F-actin localization in Swiss 3T3 fibroblasts. (Left column) Cells stained with affinity-purified anti-PAK1 antibody; (right column) cells stained with rhodamine phalloidin. (A and B) Serum-starved cells with no addition or (C and D) stimulated for 10 min with 3 ng/ml PDGF. (E and F) Cells in fetal bovine serum. (G and H) v-Src–transformed 10 t1/2 cells; untransformed 10 t1/2 controls were similar in appearance to the serum-starved Swiss 3T3 controls in A and B and are thus not shown here. I and J show a polarized Swiss 3T3 cell migrating into the area of a wound. All procedures were as described in Materials and Methods. Arrows indicate areas where PAK1 and F-actin colocalize in membrane ruffles. Micrographs shown are at 5,000× (A–F, I, and J) and 7,000× (G and H).
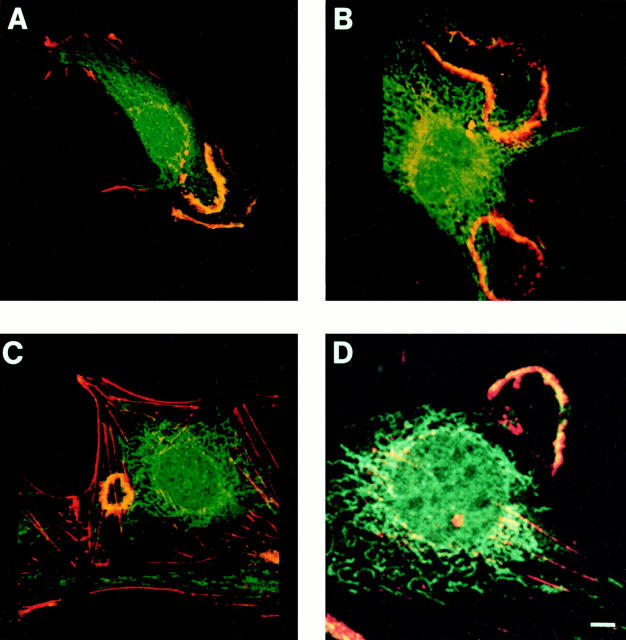
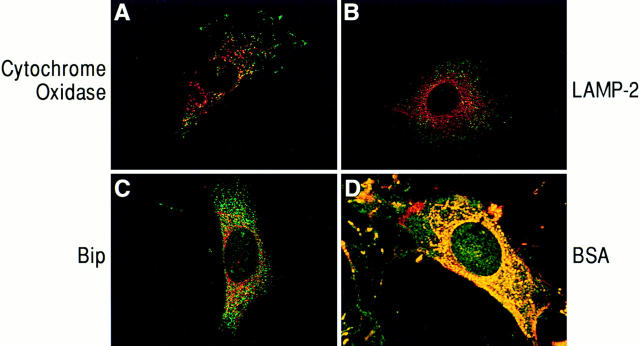
Confocal imaging (see Fig. 4) indicated that these assemblies did not colocalize with F-actin and were found closely apposed to the plasma membrane. The vesicular structures did not appear to be artifacts of the method of fixation, as they were detected using either formaldehyde, glutaraldehyde, or methanol as fixative. They were not disrupted by treatment with cytochalasin D, an inhibitor of actin polymerization, but pretreatment of cells with Triton X-100 before fixation disrupted the vesicles to which PAK1 localized, suggesting they are indeed membranous in nature. These vesicles did not represent mitochondria, as shown by costaining with PAK1 antibody and an antibody to cytochrome oxidase, a mitochondrial membrane component (Fig. 3 A). Using various organelle markers, we were able to establish that PAK1 was not colocalized with endoplasmic reticulum (anti-Bip-2, Fig. 3 C) or with lysosomes and late endosomes (anti-Lamp-2, Fig. 3 B). The distribution of PAK1 was also distinct from Golgi (anti-α-mannosidase) or microtubules (anti-tubulin); data not shown. However, when cells were allowed to take up BSA-rhodamine as a marker for fluid phase uptake, the BSA colocalized with the PAK1-containing vesicles as determined by confocal microscopy (Fig. 3 D). In support of an association of PAK1 with pinocytic vesicles, we observed that PAK1 became diffusely distributed throughout the cytoplasm when the cells were treated with amiloride, a potent inhibitor of Na+/H+ exchange, which is known to block pinocytosis (West et al., 1989; data not shown).
Figure 4.
PAK1 and F-actin distribution after PDGF addition. Confocal microscopy was performed on Swiss 3T3 cells after PDGF stimulation for 10 min, as described in Materials and Methods. Red, rhodamine-phalloidin staining of F-actin; green, fluorescein staining of PAK1; yellow, merged images indicating the areas of colocalization. PAK1 and F-actin are colocalized in dorsal ruffles (A and C), membrane ruffles (A and B), and lamellipodia (D). Bar, 15 μm.
Figure 3.
Confocal micrographs of cells immunostained for PAK1 and cytoplasmic organelle markers. Swiss 3T3 cells in serum were fixed in 100% methanol and costained with anti-PAK1, and antibodies to organelle markers as described in Materials and Methods. (A, B, and D) Red, rhodamine staining of cytochrome oxidase (mitochondrial marker), LAMP-2 (lysosomal marker), and BSA, respectively; green, fluorescein staining of PAK1. (C) Green, fluorescein staining of BiP (endoplasmic reticulum marker); red, rhodamine staining of PAK1; yellow, areas of colocalization.
PAK1 Localizes to Sites of Actin Assembly in PDGF-stimulated Swiss 3T3 Cells
Very little PAK1 was detected in direct association with the plasma membrane in the unstimulated cells, which also contained a minimal cytoskeleton (Fig. 2, A and B). When cells were treated with 10% fetal calf serum, we observed extensive formation of actin stress fibers (Fig. 2 F). However, PAK1 did not localize to the stress fibers (Fig. 2, E and F), nor did it accumulate at the plasma membrane under these conditions. In contrast to the distribution of PAK1 in resting cells, when cells were stimulated with PDGF we observed that PAK1 now became evident in areas of active cytoskeletal rearrangement. At 10 min after stimulation, PAK1 was detected in membrane ruffles and at the edges of lamellipodia (Figs. 2, C and D, and 4). We observed that endogenous Rac1 was also localized to areas of active membrane ruffling (not shown). Such changes in cortical actin induced by PDGF are known to be Rac dependent and are inhibited by the PI 3-kinase inhibitor wortmannin (Wennström et al., 1994; Nobes et al., 1995). Treatment of the PDGF-stimulated Swiss 3T3 cells with either the F-actin–disrupting agent cytochalasin D (10 μM) or the PI 3-kinase inhibitor wortmannin (100 nM) caused the complete loss of membrane ruffles, and in the presence of these inhibitors we no longer observed membrane-associated PAK1.
The apparent colocalization of PAK1 with F-actin in membrane ruffles was verified by confocal microscopy (Fig. 4). PAK1 was detected in large membrane ruffles that often appeared at one end of the cell (Fig. 4, A and D). In some cells we observed ruffles that contained PAK1 primarily at the very edge of the active ruffle, while smaller ruffles deficient in PAK1 could be seen on the same cell (e.g., see cell in Fig. 4 A). In cells expressing dorsal ruffles the overlap of PAK1 with F-actin was particularly evident (Fig. 4, B and C). In data obtained from three representative experiments (481 total cells counted), we detected PAK1 associated with 117 of 166 membrane ruffles (70%), in 86 of 88 lamellipodia (97%), and in 199 of 216 dorsal ruffles (92%). While few in number in Swiss 3T3 cells, in areas where actin microspikes or filopodial extensions were evident, PAK1 was found to colocalize with F-actin in 18 of 20 (90%) of these structures as well.
Because the localization of PAK1 in the polarized fibroblasts appeared to be primarily at the leading edge, we examined the localization of PAK1 in Swiss 3T3 cells during a wound healing response. About 3 h after the initial wounding, fibroblasts display a polarization response at the edge of the wound and begin to migrate into the wound itself (Conrad et al., 1993). As shown in Fig. 2 (I and J), PAK1 is found within the leading lamellae of the polarized cells migrating towards the wound.
PAK1 Localizes to Cortical Actin Structures in v-src–transformed 10 T1/2 Fibroblasts
To determine whether PAK1 was associated with similar cytoskeletal structures in cells in which actin rearrangements were induced by means other than PDGF, we examined v-src–transformed 10 T1/2 cells. As compared to a control cell line, the v-src–transformed cells were characterized by loss of actin stress fibers, extension of numerous lamellipodia, and areas of active membrane ruffling (Fig. 2, G and H), as previously described (Luttrell et al., 1988). PAK1 was clearly localized in membrane ruffles and particularly at the ruffling tips of lamellipodial extensions. Taken together, these data show that induction of cortical actin structures by distinct stimuli in different cell types is associated with the translocation of PAK1 into these structures.
Rac Induces Relocalization of PAK1 into Membrane Ruffles
We examined whether induction of membrane ruffling by expression of a constitutively active Rac1 was sufficient to induce the redistribution of PAK1 into the ruffles. Quiescent Swiss 3T3 cells were infected using Semliki Forest virus containing the cDNAs for wild-type and mutant Rac1. Expression of lac Z gene control, wild-type Rac1, or inactive Rac1 T17N had no effect on cell morphology or PAK1 distribution (not shown). Cells expressing constitutively active Rac1 Q61L formed extensive peripheral membrane ruffles. Fig. 5 shows that PAK1 becomes relocalized into the area of membrane ruffling induced by the activated Rac1. Thus, Rac-mediated cytoskeletal rearrangement is associated with the movement of PAK1 into the induced structures.
Figure 5.
PAK1 and F-actin localization in Swiss 3T3 cells expressing dominant active Rac1. Swiss 3T3 cells infected with Rac1 (Q61L) virus were fixed after 14 h and immunostained for endogenous PAK1 as described in Materials and Methods. (Left) PAK1; (right) staining for F-actin using rhodamine-phalloidin.
PAK1 Localization Precedes F-Actin Assembly in PDGF-induced Ruffles
To examine the redistribution of PAK1 in response to PDGF in more detail, we analyzed cells at various times after stimulation with PDGF (Fig. 6). At early times up to 3 min after stimulation there was little F-actin staining, and PAK1 was found in the pinocytic vesicles only. By 6 min after PDGF addition, we observed PAK1 staining in what appeared to be early dorsal ruffles at the cell surface. Surprisingly, these structures did not contain F-actin. However, at longer times (9 min) polymerized actin appeared in dorsal ruffles, where they colocalized with PAK1 by confocal microscopy, as in Fig. 4. These data suggest that translocation of PAK1 either precedes recruitment of F-actin into the forming ruffles, or that PAK1 may be involved in the polymerization of F-actin at these sites.
Figure 6.
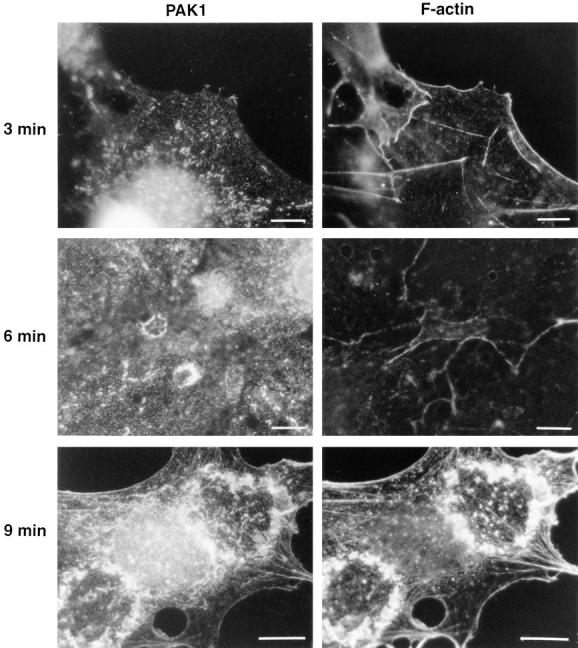
Time course of PAK1 and F-actin localization after PDGF stimulation. (Left column) Cells stained with affinity-purified anti-Pak1 antibody; (right column) cells stained with rhodamine-phalloidin. Cells were fixed at 3, 6, and 9 min after stimulation with PDGF, as described in Materials and Methods. Bars, 15 μm.
Constitutively Active PAK1 Mutant Induces Formation of Dorsal Ruffles
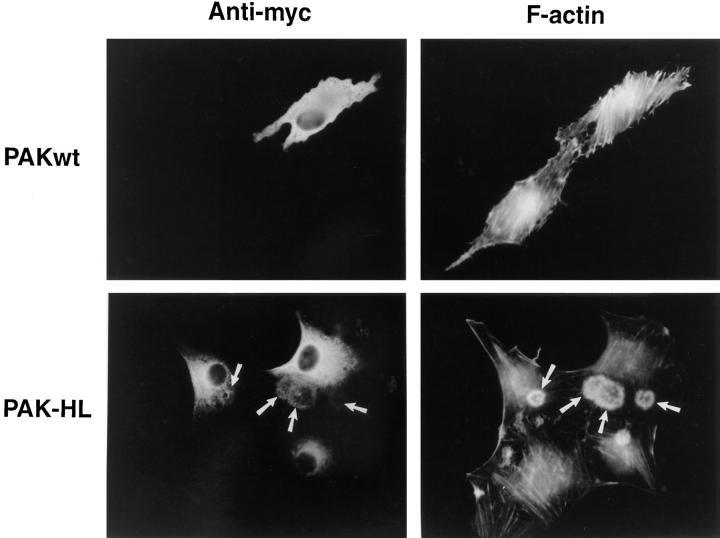
We have demonstrated that a dominant active PAK1 (H83L,H86L) mutant is capable of inducing the formation of polarized lamellipodia and ruffles in Swiss 3T3 cells (Sells et al., 1997). We examined whether this activated form of PAK1 could induce the formation of dorsal ruffles in HIRcB cells, as these cells normally exhibit a dramatic dorsal ruffling response when stimulated with insulin (Martin et al., 1996). As shown in Fig. 7, PAK1(H83L,H86L) induced the formation of circular ruffles reminiscent of the dorsal ruffles seen at early times in Swiss 3T3 cells (compare Figs. 4 C and 6). These dorsal ruffles contained both F-actin and PAK1(H83L,H86L). Interestingly, insulin has been shown to stimulate PAK activity (Tsakiridis et al., 1996), and we observed that endogenous PAK1 was localized in the insulin-induced dorsal ruffles of nontransfected HIRcB cells (data not shown).
Figure 7.
Actin localization in HIRcB cells injected with PAK1. Quiescent HIRcB cells were microinjected with myc-tagged expression vectors carrying wild-type (top row) or mutant (H83L, H86L) PAK1 cDNA (bottom row). (Left column) Cells stained with anti-myc to localize injected PAK1 and to identify expressing cells. (Right column) Same cells stained with rhodamine-phalloidin to detect F-actin.
PAK1 and Focal Complexes
Swiss 3T3 cells trypsinized from quiescent cultures were plated on fibronectin immediately after PDGF addition. In contrast to the cells shown in Figs. 2–6, these cells are just forming cell–substrate attachments and exhibit a spreading phenotype at early times. We observed that PAK1 is found at the cell periphery at 15 min after addition of PDGF and plating on fibronectin, while there is little rhodamine-phalloidin staining evident at the edges of the cells (Fig. 8). Staining of cells at this stage with an anti-actin antibody to detect both F- and G-actin confirmed this observation, as there was very little actin detected at the cell edge (data not shown). By 20 min after PDGF and plating on fibronectin, a wave of F-actin begins to move toward the cell periphery. Similar actin “rings” or peripheral “arcs” have been described previously in human erythroleukemia cells spreading on fibronectin (Niu and Nachmias, 1994), as well as in fibroblasts after stimulation with epidermal growth factor (Chang et al., 1995) and during epithelial cell migration after wounding (Nusrat et al., 1992). At 25–30 min after plating, this spreading F-actin colocalizes with the PAK1 in membrane ruffles at the edge of the cell. Membrane ruffles containing both PAK1 and F-actin are fully formed by 45 min.
Figure 8.
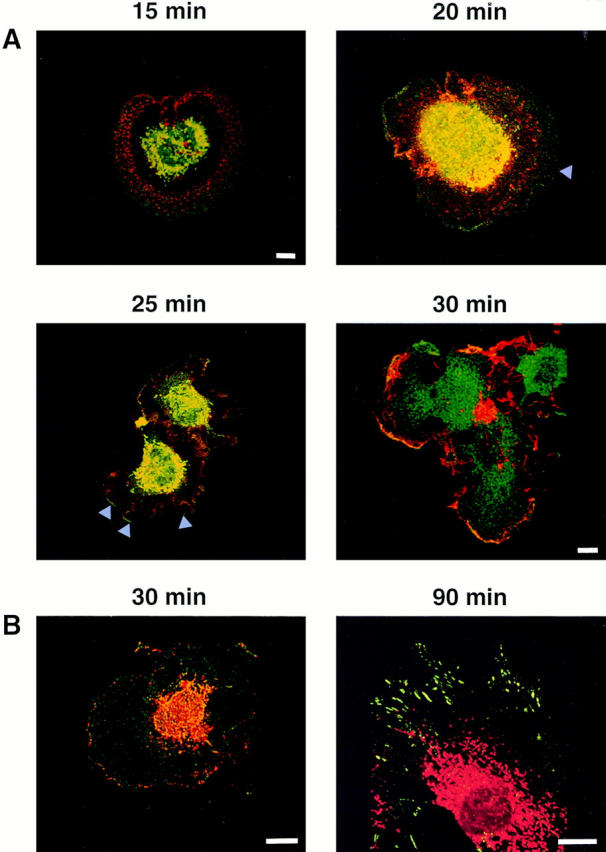
Distribution of PAK1 and F-actin in Swiss 3T3 cells plated on fibronectin. (A) PAK1 and F-actin distribution after stimulation with PDGF and plating on a fibronectin matrix. Confocal micrographs of quiescent Swiss 3T3 cells at 15, 20, 25, and 30 min after stimulation are shown. Red, Rhodamine-phalloidin staining of F-actin; green, fluorescein staining of PAK1; yellow, merged images indicating the areas of colocalization. (B) PAK1 and phosphotyrosine localization after PDGF stimulation when plated on fibronectin. Confocal micrographs of Swiss 3T3 cells at 30 and 90 min after stimulation are shown. (30 min) Red, Rhodamine staining of phosphotyrosine; green, fluorescein staining of PAK1; yellow, merged images indicating the areas of colocalization. (90 min) Red, rhodamine staining of PAK1: green, fluorescein staining of phosphotyrosine. Bars, 15 μm.
Focal complexes have been shown to be triggered by the combined effect of extracellular matrix, via activation of integrin receptors, and activity of Rac and Cdc42 (Hotchin and Hall, 1995). PAK1(H83L,H86L) can induce the formation of focal contacts when introduced into Swiss 3T3 fibroblasts attached to cell surfaces (Sells et al., 1997), and the ability of kinase-active PAK1 mutants to cause actin stress fiber and focal complex dissolution has been reported (Manser et al., 1997; Sells et al., 1997). The PAK-induced focal complexes appear identical to focal complexes induced by Rac and Cdc42 (Nobes and Hall, 1995) in that they contain both vinculin and phosphotyrosine-containing proteins. We observed that PAK1 was detected in the cell periphery of fibronectin-plated cells as a pattern of discrete point contacts reminiscent of focal complexes (Fig. 8 B, left). Staining with antibodies against phosphotyrosine (and vinculin; not shown) revealed only intermittent colocalization of PAK1 with the phosphotyrosine-containing focal complexes. There were clearly distinct foci of phosphotyrosine staining that were devoid of PAK1 and vice versa. While overlap was noted in some complexes, these were only a fraction of the total. The relationship of the PAK1-containing foci to the phosphotyrosine- and vinculin-containing complexes is thus unclear. It is possible that the points of staining observed represent distinct stages in focal complex assembly/disassembly with which PAK1 is transiently associated. PAK1 clearly was not detected in classical Rho-dependent focal adhesions (Fig. 8 B, right).
PAK1 Translocates to a Membrane Fraction and Is Associated with F-actin in PDGF-stimulated Cells
The PDGF-stimulated redistribution of PAK1 to the plasma membrane in areas of membrane ruffling was verified by preparing subcellular fractions from cells in the presence or absence of PDGF. As shown in Fig. 9 A (right), in unstimulated Swiss 3T3 cells, PAK1 was located primarily in the cytosolic fraction, with very little PAK1 in the membrane fraction. Isolation of a highly enriched nuclear fraction demonstrated the absence of significant immunodetectable PAK1 associated with nuclei, in either the presence or absence of PDGF (Fig. 9 A, left). Treatment of cells with PDGF caused a substantial time-dependent increase in the amount of PAK1 that became associated with the membrane fraction, peaking at 9–10 min (Fig. 9 B). The increase of PAK1 in the membrane fraction at 6 min corresponds to the appearance of PAK1 as discrete circular rings on the cell surface, which precede the formation of dorsal ruffles at 9 min after PDGF addition (Fig. 6). This membrane fraction represents primarily plasma membrane (Thom et al., 1977; Krek et al., 1992), probably containing membrane-associated cytoskeletal elements. It is unlikely that there is significant contamination with the pinocytic vesicles with which PAK1 is associated, as we detected very little PAK1 in this fraction from unstimulated cells. In other experiments, we detected reproducible PDGF-induced increases in the amount of PAK1 found in the Triton X-100–insoluble cytoskeletal fraction (not shown). These findings are consistent with the immunolocalization studies we have described herein.
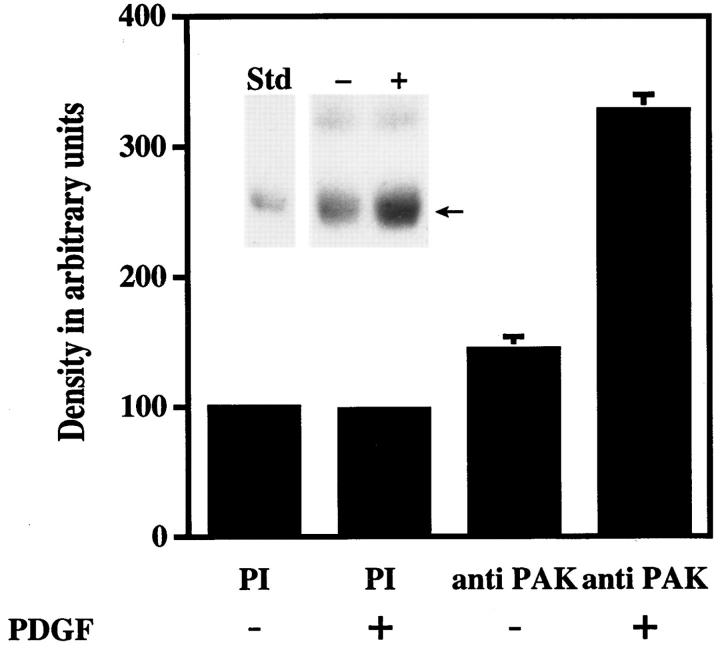
The physical association of PAK1 with F-actin was probed in PAK1 immunoprecipitates (Fig. 10). We observed a band reactive with a specific actin antibody in the PAK1 immunoprecipitates from PDGF-stimulated cells (Fig. 10, inset). Scanning densitometry of the actin band detected in PAK1 immunoprecipitates shows that a statistically significant amount of actin is associated with PAK1 in cell lysates treated with PDGF when compared to the association of actin in the absence of PDGF. The amount of actin associated with anti-PAK1 immunoprecipitates from control cells was similar to the amount of actin associated nonspecifically with immunoprecipitates using pre-immune serum. It cannot be determined at this point whether the association of F-actin with PAK1 immunoprecipitates represents a direct interaction or whether it involves indirect association mediated by other protein components.
Figure 10.
Coprecipitation of PAK1 with F-actin in cellular immunoprecipitates. PAK1 was immunoprecipitated from quiescent Swiss 3T3 cell lysates before and after PDGF addition (5 ng/ ml) and immunoblotted with an anti-actin antibody, as described. Quantitation of the 42-kD actin band (see inset below) was by phosphoimager analysis (Molecular Probes, Inc.). The mean density of the 42-kD band from unstimulated pre-immune serum immunoprecipitates was set at 100%. The data shown represent the mean ± SEM of duplicate determinations from three separate experiments. (Inset) Representative Western blot of PAK1 immunoprecipitates immunostained for actin. Arrow indicates 42-kD actin band that comigrated with the purified rabbit actin (Sigma Chemical Co.) standard (Std). The upper band in the immunoprecipitates is IgG heavy chain.
Discussion
Although PAK1 has been identified as a direct target for the small GTPases Rac and Cdc42, its role in mediating the biochemical responses of these GTPases remains unclear. The present data indicate that PAK1 is closely physically associated with the formation of Rac- and Cdc42-dependent actin structures. Stimuli that induce membrane ruffling and the formation of lamellipodia and filopodia, including PDGF, transformation by v-src and constitutive activation of Rac1, cause PAK1 to associate with these dynamic cytoskeletal structures, where it is closely localized with F-actin.
We observed that PAK1 kinase activity is stimulated within 1 min after addition of PDGF (Fig. 1), and changes in the intracellular distribution of PAK1 occur within 5 min of PDGF stimulation. PAK1 localizes in ring-like structures at the cell surface at early times (6–9 min after stimulation) which ultimately evolve into F-actin–containing dorsal ruffles (Fig. 6). Introduction of a cytoskeletally active PAK1(H83L,H86L) into the insulin-responsive Rat-1 cell line, HIRcB, induced the formation of very similar ring-like structures containing F-actin (Fig. 7). PAK1 colocalizes with F-actin in these dorsal ruffles and in membrane ruffles subsequently formed at later times. When Swiss 3T3 cells were plated on fibronectin-coated surfaces and stimulated with PDGF, we observed that PAK1 clearly preceded the appearance of F-actin in the cell cortex (Fig. 8). Taken together with the results of Sells et al. (1997), these data suggest a role for PAK in initiating the assembly of specific cytoskeletal elements. It is interesting to speculate that PAK may play a role in regulating actin assembly through effects on membrane flux. Bretscher (1996) has described a model by which regulated membrane flow into the leading edge of motile cells drives cell locomotion. While envisioned as a polarized endocytic cycle involving coated pits, regulation of membrane flux via a pinocytic mechanism could also account for the provisions of this model. The formation of dorsal ruffles has been associated with the process of macropinocytosis (Dowrick et al., 1993), and it is of particular interest that we observe PAK1 to colocalize with pinocytic vesicles (Fig. 3). Formation of pinocytic vesicles has been shown to be regulated by Rac (Ridley et al., 1992) and we will direct future studies at determining whether PAK1 is a mediator of the effects of Rac on fluid-phase pinocytosis and, potentially, cell movement.
At later times after cell activation by PDGF, some of the cells assumed a polarized phenotype in which PAK1 became colocalized with F-actin in lamellipodia and associated membrane ruffles at the leading edge or “front” of the cell. PAK1 was clearly absent in the smaller ruffles near the “rear” of the cell (see Fig. 4 A), indicating that it is not a required component for all types of ruffling. A similar distribution of PAK1 at the leading edge was observed in polarized cells during the wound healing response (Fig. 2, I and J). This data suggests that PAK1 may play a role in directed cellular movement by regulating actin dynamics at the leading edge of cells. Alternatively, association of PAK1 with F-actin may be transient, with PAK1 dissociating from the older ruffles at the back of the cell. However, in support of the former, we have observed that the cytoskeletal assemblies induced by PAK1 in fibroblasts appear polarized in nature and are very reminiscent of motile cells (Sells et al., 1997).
The ability of exogenously introduced PAK1 to regulate actin assembly is dependent upon an amino-terminal, proline-rich domain in PAK1 which has a predicted PXXP SH3-binding motif (Sells et al., 1997). We have shown that this domain does serve as a functional SH3-binding site, as it effectively interacts with the second SH3 domain of the Nck adapter protein (Bokoch et al., 1996; Galisteo et al., 1996). It is possible that PAK binds an SH3 domain-containing protein that physically interacts with F-actin, as an analogous situation has been described in S. cerevisiae (Leeuw et al., 1995). In this species, a protein known as Bem1 binds to the yeast PAK homolog, Ste20 kinase, through an SH3 domain present on Bem1. This interaction with Bem1 mediates a physical association of Ste20 with F-actin. In the present study, we have demonstrated that increased amounts of F-actin are also associated with mammalian PAK1 in immunoprecipitates from PDGF-stimulated cells. This interaction is likely to be mediated by additional protein components as well, as we have been unable to detect direct binding of F-actin to PAK1 in preliminary experiments. These data suggest a scenario in which the formation of Rac-GTP induced by PDGF leads to direct PAK1 activation. Binding of Rac-GTP to PAK1 will change the conformation of the amino terminus such that the SH3-binding domain is exposed and/or has a higher affinity for SH3-containing target proteins. These putative target proteins, serving as the functional equivalent of Bem1 in yeast, would enable PAK1 to associate with F-actin. In support of this model, we observed that expression of the constitutively active Rac1(Q61L) mutant in Swiss 3T3 cells induced the relocalization of PAK1 into cortical actin structures (Fig. 5).
Whether PAK1 amino-terminal binding proteins actually regulate localized actin assembly at appropriate sites or whether this interaction mediates the recruitment of PAK1-target protein complexes to membrane-associated sites of actin assembly is still undetermined. It is of particular interest that we observed PAK1 to precede the appearance of F-actin in the cell cortex of adherent PDGF-stimulated Swiss 3T3 cells (Fig. 6 A). A transient association with focal complexes is suggested by the appearance of PAK1 in focal complex-like structures in stimulated cells, although confocal imaging reveals that there is only partial overlap with the majority of phosphotyrosine-containing focal complexes (Fig. 8 B). As observed with formation of dorsal and membrane ruffles, where PAK1 precedes the apparent recruitment of F-actin, PAK1 may be transiently required during an early stage of focal complex formation and/or during their dissolution as well (Manser et al., 1997; Sells et al., 1997).
Recently, POR1 (partner of Rac) was identified as a potential regulator of Rac-mediated membrane ruffling (Van Aelst et al., 1996). Truncated versions of POR1 block both Ras- and Rac-induced ruffling responses. Addition of POR1 to cells microinjected with dominant active Ras enhanced subsequent ruffling, although POR1 itself could not induce cytoskeletal changes. Therefore, other effectors may also be involved in regulation of the actin cytoskeleton by Rac, and it is possible that POR1 acts in concert with PAK1 to regulate membrane cytoskeletal dynamics. Similarly, Wiscott-Aldrich syndrome protein (WASP) is a target for Cdc42 and has been shown to induce the formation of poorly characterized actin clusters when overexpressed in cells (Symons et al., 1996). Both WASP (Rivero-Lezcan et al., 1995) and PAK (Bokoch et al., 1996; Galisteo et al., 1996) have been shown to interact with distinct SH3 domains on the adapter protein Nck. A complex involving these two proteins could be important for regulation of filopodia by Cdc42.
We have demonstrated in the current study that PAK1 translocates into Rac- and Cdc42-dependent actin structures in Swiss 3T3, 10 t1/2, and Rat-1 (HIRcB) cells, where PAK1 is closely associated with F-actin. This conclusion is supported by immunofluorescence and confocal microscopy, as well as biochemical data. In light of recent evidence that introduction of constitutively active PAK1 proteins into cells is able to modulate the actin cytoskeleton, the current results establish that endogenous PAK1 does translocate to areas of actin polymerization in response to physiological Rac-dependent signals. Moreover, these studies provide additional insights into the role of PAK1 in modulating various aspects of cytoskeletal dynamics, suggesting it may participate in an early stage of actin recruitment and/or polymerization. The molecular mechanisms that regulate the association of PAK1 with F-actin and the protein targets through which PAK1 regulates the cytoskeleton in motile cells remain to be established.
Acknowledgments
This work was supported by National Institutes of Health grants GM39434 and GM44428 (to G.M. Bokoch). S.S. Martin was supported by grant DK33651 to Dr. Jerrold M. Olefsky. This is publication No. 10378-IMM from The Scripps Research Institute.
Abbreviations used in this paper
- F-actin
filamentous actin
- MBP
myelin basic protein
- PI
phosphatidylinositol
Footnotes
Please address all correspondence to Dr. Gary M. Bokoch, Department of Immunology-IMM14, The Scripps Research Institute, 10550 N. Torrey Pines Road, La Jolla, CA 92037. Tel.: (619) 784-8217; Fax: (619) 784-8218.
The authors gratefully acknowledge the technical assistance of Yan Wang, M.D., and Benjamin P. Bohl. We also thank Dr. Malcolm Wood and George Klier for their assistance with confocal microscopy. Dr. Pamela Maher (TSRI) graciously provided highly purified Swiss 3T3 nuclei for these studies, and anti–LAMP-2 antibody was a kind gift of Dr. Bruce Granger (Montana State University, Bozeman, MT). Secretarial services provided by Ms. Antonette Lestelle and helpful discussions with Drs. Sandra Schmid and Klaus Hahn (both of TSRI) are greatly appreciated.
References
- Bagrodia S, Derijard B, Davis RJ, Cerione RA. Cdc42 and PAKmediated signaling leads to JUN kinase and p38MAP kinase activation. J Biol Chem. 1995a;270:27995–27998. doi: 10.1074/jbc.270.47.27995. [DOI] [PubMed] [Google Scholar]
- Bagrodia S, Taylor SJ, Creasy CL, Chernoff J, Cerione RA. Identification of a mouse p21Cdc42/Rac-activated kinase. J Biol Chem. 1995b;270:22731–22737. doi: 10.1074/jbc.270.39.22731. [DOI] [PubMed] [Google Scholar]
- Bokoch GM, Wang Y, Bohl BP, Sells MA, Quilliam LA, Knaus UG. Interaction of the Nck adapter protein with p21-activated kinase (PAK1) J Biol Chem. 1996;271:25746–25749. doi: 10.1074/jbc.271.42.25746. [DOI] [PubMed] [Google Scholar]
- Bretscher MS. Getting membrane flow and the cytoskeleton to cooperate in moving cells. Cell. 1996;87:601–606. doi: 10.1016/s0092-8674(00)81380-x. [DOI] [PubMed] [Google Scholar]
- Burgess DR, Jiang W, Mamajiwalla S, Kinsey W. Intestinal crypt stem cells possess high levels of cytoskeletal-associated phosphotyrosine-containing proteins and tyrosine kinase activity relative to differentiated enterocytes. J Cell Biol. 1989;109:2139–2144. doi: 10.1083/jcb.109.5.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang J, Gill S, Settleman J, Parsons SJ. c-Src regulates the simultaneous rearrangement of the actin cytoskeleton, p190 RhoGAP and p120 RasGAP following epidermal growth factor stimulation. J Cell Biol. 1995;130:355–368. doi: 10.1083/jcb.130.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chant J, Stowers L. GTPase cascades choreographing cellular behavior: movement, morphogenesis, and more. Cell. 1995;81:1–4. doi: 10.1016/0092-8674(95)90363-1. [DOI] [PubMed] [Google Scholar]
- Chong L, Traynor-Kaplan A, Bokoch GM, Schwartz MA. The small GTP-binding protein Rho regulates a phosphatidylinositol 4-phosphate 5-kinase in mammalian cells. Cell. 1994;79:507–513. doi: 10.1016/0092-8674(94)90259-3. [DOI] [PubMed] [Google Scholar]
- Conrad PA, Giuliano KA, Fisher G, Collins K, Matsudaira PT, Taylor DL. Relative distribution of actin, myosin I and myosin II during the wound healing response of fibroblasts. J Cell Biol. 1993;120:1381–1391. doi: 10.1083/jcb.120.6.1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cvrckova F, De Virgilio C, Manser E, Pringle JR, Nasmyth K. Ste20-like protein kinases are required for normal localization of cell growth and for cytokinesis in budding yeast. Genes Dev. 1995;9:1817–1830. doi: 10.1101/gad.9.15.1817. [DOI] [PubMed] [Google Scholar]
- Dharmawardhane S, Demma M, Yang F, Condeelis J. Compartmentalization and actin binding properties of ABP-50: the elongation factor-1-α of Dictyostelium. . Cell Motil Cytoskel. 1991;20:279–288. doi: 10.1002/cm.970200404. [DOI] [PubMed] [Google Scholar]
- Dowrick P, Kenworthy P, McCann B, Warn R. Circular ruffle formation and closure lead to macropinocytosis in hepatocyte growth factor/ scatter factor treated cells. Eur J Cell Biol. 1993;61:44–53. [PubMed] [Google Scholar]
- Fechheimer M, Daiss JI, Cebra JJ. Interaction of immunoglobulin with actin. Mol Immunol. 1979;16:881–888. doi: 10.1016/0161-5890(79)90086-5. [DOI] [PubMed] [Google Scholar]
- Galisteo M, Chernoff J, Su Y, Skolnik EY, Schlessinger J. The adaptor protein nck links receptor tyrosine kinases with the serine-threonine kinase Pak1. J Biol Chem. 1996;271:20997–21000. doi: 10.1074/jbc.271.35.20997. [DOI] [PubMed] [Google Scholar]
- Granger BL, Green SA, Gabel CA, Howe CL, Mellman I, Heleniu A. Characterization and cloning of Igp 100, a lysosomal membrane glycoprotein from mouse and rat cells. J Biol Chem. 1990;265:12036–12043. [PubMed] [Google Scholar]
- Hall A. Small GTP-binding proteins and the regulation of the actin cytoskeleton. Annu Rev Cell Biol. 1994;10:31–54. doi: 10.1146/annurev.cb.10.110194.000335. [DOI] [PubMed] [Google Scholar]
- Hartwig JH, Bokoch GM, Carpenter C, Janmey P, Taylor L, Stossel TP. Thrombin receptor ligation and activated rac uncap actin filament barbed ends through phosphoinositide synthesis in permeabilized human platelets. Cell. 1995;82:643–653. doi: 10.1016/0092-8674(95)90036-5. [DOI] [PubMed] [Google Scholar]
- Herskowitz I. MAP kinase pathways in yeast: for mating and more. Cell. 1995;80:187–197. doi: 10.1016/0092-8674(95)90402-6. [DOI] [PubMed] [Google Scholar]
- Hotchin NA, Hall A. The assembly of integrin adhesion complexes requires both extracellular matrix and intracellular rho/rac GTPases. J Cell Biol. 1995;131:1857–1865. doi: 10.1083/jcb.131.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus UG, Morris S, Dong H, Chernoff J, Bokoch GM. Regulation of human leukocyte p21-activated kinases through G protein-coupled receptors. Science (Wash DC) 1995;269:221–223. doi: 10.1126/science.7618083. [DOI] [PubMed] [Google Scholar]
- Kozma R, Ahmed S, Best A, Lim L. The Ras-related protein CDC42Hs and bradykinin promote the formation of peripheral action microspikes and filopodia in Swiss 3T3 fibroblasts. Mol Cell Biol. 1995;15:1942–1952. doi: 10.1128/mcb.15.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krek W, Maridor G, Nigg EA. Casein kinase II is a predominantly nuclear enzyme. J Cell Biol. 1992;116:43–55. doi: 10.1083/jcb.116.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeuw T, Forrest-Lieuvin A, Wu C, Chenevert J, Clark K, Whiteway M, Thomas DY, Leberer E. Pheromone response in yeast: association of Bemlp with proteins of the MAP kinase cascade and actin. Science (Wash DC) 1995;270:1210–1213. doi: 10.1126/science.270.5239.1210. [DOI] [PubMed] [Google Scholar]
- Luttrell DK, Luttrell LM, Parsons SJ. Augumented mitogenic responsiveness to epidermal growth factor in murine fibroblasts that overexpress pp60-src. Mol Cell Biol. 1988;8:497–501. doi: 10.1128/mcb.8.1.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher PA. Nuclear translocation of fibroblast growth factor receptor in response to FGF2. J Cell Biol. 1996;134:529–536. doi: 10.1083/jcb.134.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manser E, Leung T, Salihuddin H, Zhao Z, Lim L. A brain serine/ threonine protein kinase activated by Cdc42 and Rac1. Nature (Lond) 1994;367:40–46. doi: 10.1038/367040a0. [DOI] [PubMed] [Google Scholar]
- Manser E, Chong C, Zhao Z, Leung T, Michael G, Hall C, Lim L. Molecular cloning of a new member of the p21Cdc42/Rac-activated kinase (PAK) family. J Biol Chem. 1995;270:25070–25078. doi: 10.1074/jbc.270.42.25070. [DOI] [PubMed] [Google Scholar]
- Manser E, Huang H-Y, Loo T-H, Chen X-Q, Dong J-M, Leung T, Lim L. Expression of constititively active α-PAK reveal effects of the kinase on actin and focal complexes. Mol Cell Biol. 1997;17:1129–1143. doi: 10.1128/mcb.17.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin GA, Bollag G, McCormick F, Abo A. A novel serine kinase activated by rac1/CDC42Hs-dependent autophosphorylation is related to PAK65 and STE20. EMBO (Eur Mol Biol Organ) J. 1995;14:1970–1978. doi: 10.1002/j.1460-2075.1995.tb07189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S, Rose DW, Saltiel AR, Klippel A, Williams LT, Olefsky JM. Phosphatidylinositol 3-kinase is necessary and sufficient for insulin-stimulated stress fiber breakdown. Endocrinology. 1996;137:5045–5053. doi: 10.1210/endo.137.11.8895379. [DOI] [PubMed] [Google Scholar]
- Mitchison TJ, Cramer LP. Actin-based cell motility and cell locomotion. Cell. 1996;84:371–379. doi: 10.1016/s0092-8674(00)81281-7. [DOI] [PubMed] [Google Scholar]
- Niu MY, Nachmias VT. Two-step mechanism for actin polymerization in human erythroleukemia cells induced by phorbol ester. Cell Motil Cytoskeleton. 1994;27:327–336. doi: 10.1002/cm.970270405. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, Rac, and CDC42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Nobes CD, Hawkins P, Stephens L, Hall A. Activation of the small GTP-binding proteins rho and rac by growth factor receptors. J Cell Sci. 1995;108:225–233. doi: 10.1242/jcs.108.1.225. [DOI] [PubMed] [Google Scholar]
- Nusrat A, Delp C, Madara JL. Intestinal epithelial restitution. Characterization of a cell culture model and mapping of cytoskeletal elements in migrating cells. J Clin Invest. 1992;89:1501–1511. doi: 10.1172/JCI115741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottilie S, Miller PJ, Johnson DI, Creasy CL, Sells MA, Bagrodia S, Frosburg SL, Chernoff J. Fission yeast pak1+encodes a protein kinase that interacts with Cdc42p and is involved in the control of cell polarity and mating. EMBO (Eur Mol Biol Organ) J. 1995;14:5908–5919. doi: 10.1002/j.1460-2075.1995.tb00278.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppelenbosch MP, Qiu R, de Vries-Smits AMM, Tertoolen LGJ, de Laat SW, McCormick F, Hall A, Symons MH, Bos JL. Rac mediates growth factor-induced arachidonic acid release. Cell. 1995;81:1–20. doi: 10.1016/0092-8674(95)90005-5. [DOI] [PubMed] [Google Scholar]
- Quinn MT, Evans T, Loetterle LR, Jesaitis AJ, Bokoch GM. Translocation of Rac correlates with NADPH oxidase activation: evidence for equimolar translocation of oxidase components. J Biol Chem. 1993;268:20983–20987. [PubMed] [Google Scholar]
- Ridley, A.J. 1995. Growth factor-induced actin reorganization in Swiss 3T3 cells. In Methods In Enzymology. W.E. Balch, C.J. Der, and A. Hall, editors. Academic Press, San Diego, CA. 306–313. [DOI] [PubMed]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Rivero-Lezcan OM, Marcella A, Sameshima JH, Robbins KC. Wiskott-Aldrich syndrome protein physically associates with Nck through Src homology 3 domains. Mol Cell Biol. 1995;15:5725–5731. doi: 10.1128/mcb.15.10.5725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider C, Newman RA, Sutherland DR, Asser U, Greaves MF. A one-step purification of membrane proteins using a high efficiency immunomatrix. J Biol Chem. 1982;257:10766–10769. [PubMed] [Google Scholar]
- Schreiber E, Matthias P, Muller MM, Schaffner W. Rapid detection of octamer binding proteins with “mini-extracts”, prepared from a small number of cells. Nucleic Acid Res. 1989;17:6419. doi: 10.1093/nar/17.15.6419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sells MA, Knaus UG, Ambrose D, Bagrodia S, Bokoch GM, Chernoff J. Human p21 activated kinases regulate actin reorganization in mammalian cells. Curr Biol. 1997;7:202–210. doi: 10.1016/s0960-9822(97)70091-5. [DOI] [PubMed] [Google Scholar]
- Stossel TP. On the crawling of animal cells. Science (Wash DC) 1993;260:1086–1096. doi: 10.1126/science.8493552. [DOI] [PubMed] [Google Scholar]
- Symons M, Derry JMJ, Karlak B, Jiang S, Lemahieu V, McCormick F, Francke U, Abo A. Wiskett-Aldrich syndrome protein, a novel effector for the GTPase Cdc42Hs, is implicated in actin polymerization. Cell. 1996;84:723–734. doi: 10.1016/s0092-8674(00)81050-8. [DOI] [PubMed] [Google Scholar]
- Thom D, Powell AJ, Lloyd CW, Rees DA. Rapid isolation of plasma membranes in high yield from cultured fibroblasts. Biochem J. 1977;168:187–194. doi: 10.1042/bj1680187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todaro, G., Y. Matsuya, S. Bloom, A. Robbins, and H. Green. 1967. Growth regulating substances for animal cells in culture. V. Defendras and M. Stoker, editors. Wistar Institute Press, Philadelphia, PA.
- Tsakiridis T, Taha C, Grinstein S, Klip A. Insulin activates a p21-activated kinase in muscle cells via phosphatidylinositol 3-kinase. J Biol Chem. 1996;271:19664–19667. doi: 10.1074/jbc.271.33.19664. [DOI] [PubMed] [Google Scholar]
- Van Aelst L, Joneson T, Bar-Sagi D. Identification of a novel Rac1-interacting protein involved in membrane ruffling. EMBO (Eur Mol Biol Organ) J. 1996;15:3778–3786. [PMC free article] [PubMed] [Google Scholar]
- Wennström S, Hawkins P, Cooke F, Hara K, Yonezawa K, Kasuga M, Jackson T, Claesson-Welsh L, Stephens L. Activation of PI-3 kinase is required for PDGF-stimulated membrane ruffling. Curr Biol. 1994;4:385–393. doi: 10.1016/s0960-9822(00)00087-7. [DOI] [PubMed] [Google Scholar]
- West MA, Bretschez MS, Watts C. Distinct endocytotic pathways in epidermal growth factor-stimulated human carcinoma A-437 cells. J Cell Biol. 1989;109:2731–2739. doi: 10.1083/jcb.109.6.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarzov P, Mazzoni C, Mann C. The SLT2(MPK1) MAP kinase is activated during periods of polarized cell growth in yeast. EMBO (Eur Mol Biol Organ) J. 1996;15:83–91. [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Han J, Sells MA, Chernoff J, Knaus UG, Ulevitch RJ, Bokoch GM. Rho family GTPases regulate p38 MAP kinase through the downstream mediator Pak1. J Biol Chem. 1995;270:23934–23936. doi: 10.1074/jbc.270.41.23934. [DOI] [PubMed] [Google Scholar]