Abstract
The cell–cell adhesion molecule N-cadherin, with its associated catenins, is expressed by differentiating skeletal muscle and its precursors. Although N-cadherin's role in later events of skeletal myogenesis such as adhesion during myoblast fusion is well established, less is known about its role in earlier events such as commitment and differentiation. Using an in vitro model system, we have determined that N-cadherin– mediated adhesion enhances skeletal muscle differentiation in three-dimensional cell aggregates. We transfected the cadherin-negative BHK fibroblastlike cell line with N-cadherin. Expression of exogenous N-cadherin upregulated endogenous β-catenin and induced strong cell–cell adhesion. When BHK cells were cultured as three-dimensional aggregates, N-cadherin enhanced withdrawal from the cell cycle and stimulated differentiation into skeletal muscle as measured by increased expression of sarcomeric myosin and the 12/101 antigen. In contrast, N-cadherin did not stimulate differentiation of BHK cells in monolayer cultures. The effect of N-cadherin was not unique since E-cadherin also increased the level of sarcomeric myosin in BHK aggregates. However, a nonfunctional mutant N-cadherin that increased the level of β-catenin failed to promote skeletal muscle differentiation suggesting an adhesion-competent cadherin is required. Our results suggest that cadherin-mediated cell–cell interactions during embryogenesis can dramatically influence skeletal myogenesis.
The association of similarly fated cells is a critical aspect of embryonic development. The cadherin family of proteins mediates cell–cell adhesion through homophilic interactions and thus promotes homogeneity within tissues (Takeichi, 1995). Their spatiotemporal pattern of expression during development suggests cadherins play an important role in the formation and maintenance of tissues (Takeichi, 1988). The best-characterized cadherins are classical cadherins including E-cadherin (uvomorulin), N-cadherin, and P-cadherin. Cadherins are calcium-dependent transmembrane proteins that interact intracellularly with a group of proteins known as catenins (Wheelock and Knudsen, 1991; Kemler, 1993; Gumbiner, 1996). The catenins link the cadherins to the actin cytoskeleton and are required for full adhesive activity of most cadherins (Nagafuchi and Takeichi, 1988, 1989; Ozawa et al., 1990; Knudsen et al., 1995; Rimm et al., 1995). β-catenin, which interacts with cadherins directly, has a role in signal transduction and the specification of cell fate (McCrea and Gumbiner, 1991; Ozawa and Kemler, 1992; Miller and Moon, 1996). Cadherin-mediated junctions serve as signaling centers and disruption of these junctions can lead to growth and developmental defects (Huber et al., 1996; Larue et al., 1996).
The formation of skeletal muscle is a developmental process that requires the commitment of mesodermal precursors, withdrawal from the cell cycle, and differentiation of myoblasts into terminally differentiated multinucleate myofibers (Olson, 1992). This process is controlled through a number of developmental checkpoints that regulate cell cycle arrest and the expression of skeletal muscle–specific genes (Ludolph and Konieczny, 1995). Skeletal muscle– specific transcription factors, including MyoD, control the program of tissue-specific transcription within the developing myoblasts and serve as early markers of skeletal myogenesis (Olson and Klein, 1994).
In one myogenic model system, the pluripotent P19 embryonal carcinoma cells can be induced to form skeletal muscle through the addition of DMSO or exogenous MyoD (Edwards et al., 1983). The induction of differentiation by either of these methods requires aggregation of cells in suspension (Edwards et al., 1983; Skerjanc et al., 1994). This requirement for close contact of similar cells during skeletal muscle myogenesis is known as the community effect. The community effect is important for the differentiation of somites, cell lines, and embryonic stem cells into skeletal muscle (Gurdon et al., 1993; Kato and Gurdon, 1993; Slager et al., 1993; Skerjanc et al., 1994; Cossu et al., 1995). Cadherin-mediated adhesion has been implicated in the community effect and skeletal muscle differentiation (Gurdon et al., 1993; George-Weinstein et al., 1997).
Injection of mRNA encoding a dominant-negative cadherin into early Xenopus laevis embryos blocks the expression of MyoD in the early stages of skeletal muscle myogenesis (Holt et al., 1994). N-cadherin has been identified as a predominant cadherin in developing skeletal muscle and thus most studies have focused on its role in myogenesis. For example, function perturbing antibodies to N-cadherin inhibit skeletal muscle differentiation by primitive streak stage chick epiblast cells in vitro (George-Weinstein et al., 1997). Perturbation studies also demonstrated N-cadherin plays a role in myoblast interaction, the formation of myotubes, and in myofibrillogenesis (Knudsen et al., 1990; Mege et al., 1992; Peralta Soler and Knudsen, 1994). Recently, N-cadherin has been shown to play a role in the migration of skeletal muscle precursors to the limb bud (Brand-Saberi et al., 1996).
Our laboratory is interested in the role of cadherins in myogenesis and the potential role cadherins play in the specification of cell fate. In these studies we used the BHK cell line 2254-62.2. BHK cells exhibit myogenic potential through the expression of MyoD and low levels of sarcomeric myosin (Schaart et al., 1991; this study). However, they do not contain detectable cadherin. We introduced exogenous cadherin into this cell line to investigate the role of cadherins in myogenesis. Our results demonstrate that cadherins dramatically increase the expression of sarcomeric myosin and thus promote the differentiation of BHK cells into skeletal muscle. Differentiation in these cells is dependent on the formation of aggregates, or a community effect, with concomitant withdrawal from the cell cycle.
Materials and Methods
Cells
The BHK 2254-62.2 cell line was obtained from American Type Culture Collection (Rockville, MD) and grown in DME (Fisher Scientific, Pittsburgh, PA) with 7% FBS.
Antibodies
Myosin was detected with the mouse monoclonal anti–chicken pectoralis myosin antibody MF20 (Developmental Studies Hybridoma Bank; Bader et al., 1982). The 12/101 antibody is a skeletal muscle–specific mouse monoclonal antibody to newt skeletal muscle homogenate (Developmental Studies Hybridoma Bank, Johns Hopkins University, Baltimore, MD; Kintner and Brockes, 1984). N-cadherin was detected with 6B3 (George-Weinstein et al., 1997) or 13A9 (Knudsen et al., 1995), β-catenin was detected with 15B8 (Johnson et al., 1993), E-cadherin was detected with the E9 rat monoclonal anti–E-cadherin antibody (Wheelock et al., 1987). Mouse monoclonal antibodies to E- and P-cadherin were purchased from Transduction Laboratories (Lexington, KY). A polyclonal pan–cadherin antibody was purchased from Sigma Chemical Co. (St. Louis, MO). Monoclonal anti–bromo-deoxyuridine (BrdU)1 was purchased from Amersham Corp. (Arlington Heights, IL). Polyclonal anti-MEF2 recognizing all isoforms was purchased from Santa Cruz Biotechnology (Santa Cruz, CA). The polyclonal anti-MyoD was a gift from A.J. Harris (University of Otago Medical School, Otago, New Zealand).
Transfection of BHK Cells
Transcription of the chicken N-cadherin cDNA was controlled by the human β-actin promoter in the expression vector pHβApr-1neo (pHβ-Ncad) (Murphy-Erdosh et al., 1995) (gift of G. Grunwald, Thomas Jefferson University, Philadelphia, PA). A HindIII fragment containing the entire human E-cadherin cDNA was cloned into the HindIII site of pHβApr-1neo to create pHβ-Ecad (Lewis et al., 1997). A mutant cDNA encoding an N-cadherin missing 390 bp from the extracellular domain (Fujimori and Takeichi, 1992) was cloned into pHβApr-1neo to create pHβ-NcadΔ. BHK cells were cotransfected with 1.0 μg of cadherin DNA and 0.1 μg of pLKpac, which served as a more efficient selectable marker than the neomycin resistance gene within pHβApr-1neo. pLKpac was constructed by placing an AvaII/SmaI fragment containing the puromycin resistance gene from pBSpacΔp in pLK-neo-1 (de la Luna et al., 1988; Hirt et al., 1992). Control cells were transfected with pLKpac alone. Transfections were carried out using Lipofectamine (GIBCO BRL, Gaithersburg, MD) according to the manufacturer's instructions.
Formation of Cell Aggregates
Aggregates were formed by harvesting cells with trypsin/EDTA and resuspending the cells at 1.5 × 105 cells per ml in DME with FBS. 20-μl drops of media containing 3,000 cells per drop were pipetted onto the inner surface of the lid of a Petri plate. The lid was then placed on the Petri plate so that the drops were hanging from the lid with the cells suspended within them. To eliminate evaporation within the hanging drops, 5 ml of PBS was placed in the bottom of the Petri plate. For analysis, aggregates were harvested by pipette and dispersed for counting, extracted for Western blot analysis, or replated on slides for immunofluorescence microscopy.
Cell Growth Assay
A total of 1.5 × 105 cells were plated in 20-μl drops containing 3,000 cells per drop and either suspended from Petri plate lids or placed as individual drops on tissue culture dishes. Cells were grown for 72 h and harvested with trypsin for cells on culture dishes or dispersed into single cells with trypsin for cells in hanging aggregates. Cells were counted microscopically using a hemocytometer and viability determined using Trypan blue exclusion.
BrdU Labeling of Cells
BHK cells were labeled with a 1:1,000 dilution of cell proliferation labeling reagent (Amersham Corp.) containing BrdU and fluoro-deoxyuridine for 2.5 h and fixed using paraformaldehyde. BrdU was detected with a monoclonal anti-BrdU antibody.
Western Blot Analysis
Cell monolayers were harvested by scraping, or suspended cell aggregates were collected by pipet. The cells were washed in PBS, and extracted in a 10-fold excess of 1× SDS sample buffer (50 mM Tris, pH 6.8, 20 mM DTT, 2% SDS, 0.1% bromphenol blue, 10% glycerol). Insoluble material was removed by centrifugation. An equal amount of protein from each sample was separated on SDS-polyacrylamide gels and electrophoretically transferred to nitrocellulose as described (Knudsen et al., 1995). Blots were blocked with 3% bovine serum albumin in Tris-buffered saline containing 0.05% Tween 20. Proteins of interest were detected with various primary monoclonal and polyclonal antibodies and the appropriate species-specific, alkaline phosphatase–conjugated secondary antibodies (Fisher Scientific). Blots were developed with nitro blue tetrazolium and 5-bromo-4-chloro-3-indolyl phosphate. Prestained molecular weight markers were purchased from Bio Rad Laboratories (Richmond, CA). High range molecular weight markers include myosin (205 kD), β-galactosidase (116.5 kD), bovine serum albumin (77 kD), and ovalbumin (46.5 kD). Low range molecular weight markers include phosphorylase B (110 kD), bovine serum albumin (84 kD), ovalbumin (47 kD), carbonic anhydrase (33 kD), soybean trypsin inhibitor (24 kD), and lysozyme (16 kD). The presence of dye in the prestained standards causes some proteins to migrate at the indicated molecular weights rather than their true molecular weights.
Immunofluorescence
Cells were grown on glass slides for 2 d and fixed in methanol for 10 min at −20°C. Staining was performed as described (Knudsen et al., 1995). For double labeling, cells were labeled sequentially for BrdU incorporation and myosin expression using anti-BrdU, indocarbocyanate conjugated to anti–mouse IgG (Jackson Immuno Research, West Grove, PA), MF20, and FITC conjugated to anti–mouse IgG2b (ICN Biomedicals, Irvine, CA).
Results
Exogenous N-cadherin Promotes Adhesion and Increases β-catenin Levels in BHK Cells
The BHK 2254-62.2 cells used in this study were derived from BHK-21/C13 cells isolated from BHK. The BHK-21/ C13 cells exhibit skeletal muscle characteristics including the presence of desmin, titin, and muscle-specific tropomyosin, and the ability to fuse into multinucleate myotubes (Frank et al., 1982; Quinlan and Franke, 1982; Schaart et al., 1991; Van der Loop et al., 1996). The subline BHK 2254-62.2 used in this study expresses MyoD and low levels of sarcomeric myosin but does not fuse. These cells exhibit fibroblastlike morphology with poor cell–cell adhesion. Immunofluorescence light microscopy revealed that the BHK cells lack detectable N-, E-, or P-cadherin (not shown). Moreover, β-catenin was found at low levels in the cytoplasm but was absent at the plasma membrane, suggesting the cells lack significant levels of transmembrane cadherin. In aggregation assays, the BHK cells exhibit a small degree of calcium-dependent cell–cell adhesion, which may result from a low level of an unidentified cadherin or from some other adhesion mechanism (Urushihara et al., 1977; data not shown). The BHK cells do express N-CAM and thus exhibit calcium-independent aggregation (not shown).
Given the significant role of cadherins in many aspects of skeletal muscle development, and the myogenic potential of the BHK cells that lack detectable cadherins, these cells presented a unique model system to examine the role of cadherins in differentiation. Thus, we transfected the BHK cells with exogenous cadherin and examined the effect of its expression on a biochemical marker of differentiation, i.e., sarcomeric myosin. We initiated our studies with N-cadherin due to its established role in myogenesis.
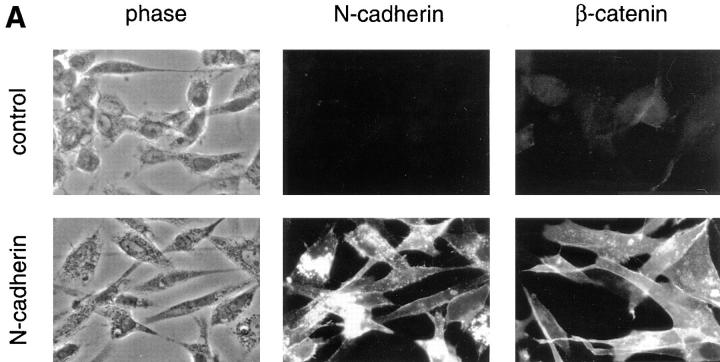
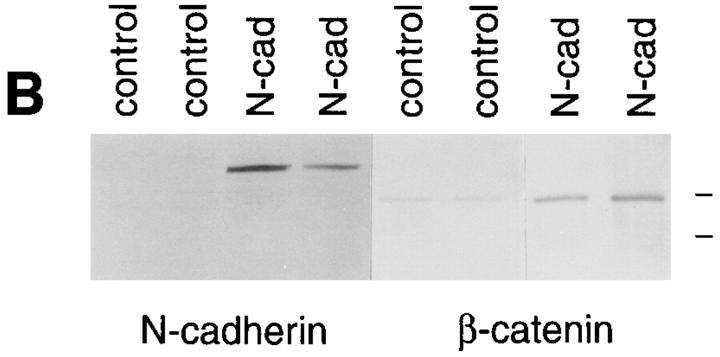
We transfected chicken N-cadherin into BHK cells and established several cell lines that express stable levels of N-cadherin (Fig. 1). The exogenous N-cadherin localized to sites of cell–cell adhesion but had little effect on the morphology of cells in monolayer cultures (Fig. 1 A), although it increased their ability to aggregate in suspension in the presence of calcium (not shown). Introduction of the exogenous cadherin resulted in a dramatic increase in the levels of β-catenin detected at cell–cell junctions by immunofluorescence light microscopy (Fig. 1 A) and in cell extracts by Western blot analysis (Fig. 1 B). This increase in catenin levels upon introduction of exogenous cadherin in cells lacking endogenous cadherin has been shown previously (Nagafuchi et al., 1991; Tanihara et al., 1994).
Figure 1.
Immunofluorescent light microscopic analysis of N-cadherin and β-catenin in control and N-cadherin– transfected BHK cells. (A) BHK cells containing either pHβ-Ncad plus pLKpac (N-cadherin) or pLKpac alone (control) were grown on slides, fixed, and stained for the presence of either N-cadherin with 6B3 or β-catenin with 15B8. Both N-cadherin and β-catenin localized to sites of cell–cell contact in the transfected cells but not in control cells. (B) Extracts from two independent clones stably expressing exogenous N-cadherin and from two control cell lines were resolved by SDS-PAGE and immunoblotted with antibodies to N-cadherin (6B3) or β-catenin (15B8). Two separate gels were probed for either N-cadherin or β-catenin. Low range molecular weight markers were used and the positions of the 110- and 84-kD bands are shown at the right side of the figure.
N-cadherin Promotes Myogenesis Only in Aggregated BHK Cells
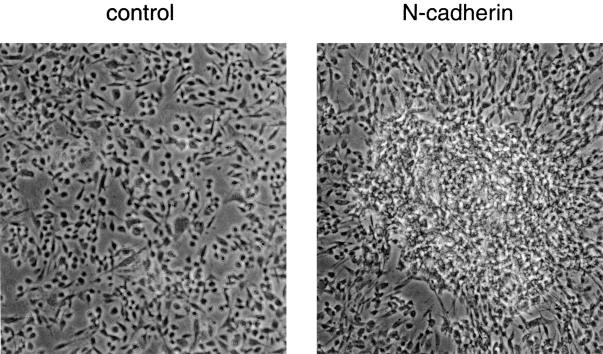
Introduction of N-cadherin had no detectable effect on the overall level of sarcomeric myosin in BHK cells growing as a monolayer (Fig. 2 B), which at first glance suggested that N-cadherin does not promote differentiation. Differentiation of skeletal muscle cell lines can be triggered by removing FBS or other growth factors. A similar approach with monolayer cultures of BHK cells failed to induce differentiation, even when the cells expressed exogenous N-cadherin. However, other cells with myogenic potential, such as P19 teratocarcinoma cells or embryonic stem cells, are induced to differentiate into skeletal muscle through aggregation in suspension (Edwards et al., 1983; Slager et al., 1993). Aggregation likely mimics conditions in which tight cell–cell contacts form in vivo, such as within somites. To replicate these conditions, we brought the BHK cells into close contact by culturing them in hanging drops of DME containing 7% FBS, and examined the effect of this aggregation on their differentiation. Aggregation of cells containing exogenous N-cadherin resulted in the formation of large aggregates that did not disperse after replating the cells for 48 h (Fig. 3). In contrast, the suspended control cells lacking N-cadherin failed to form stable aggregates and readily dispersed upon replating (Fig. 3).
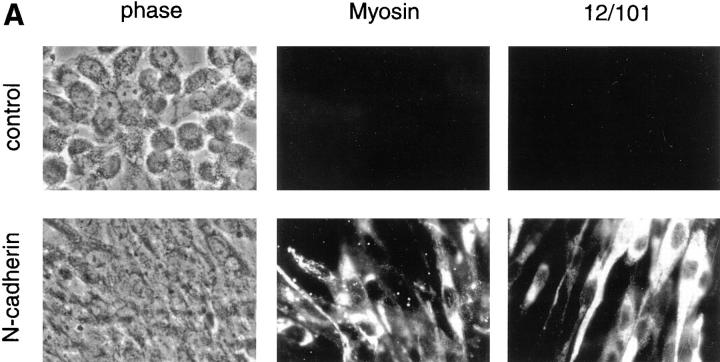
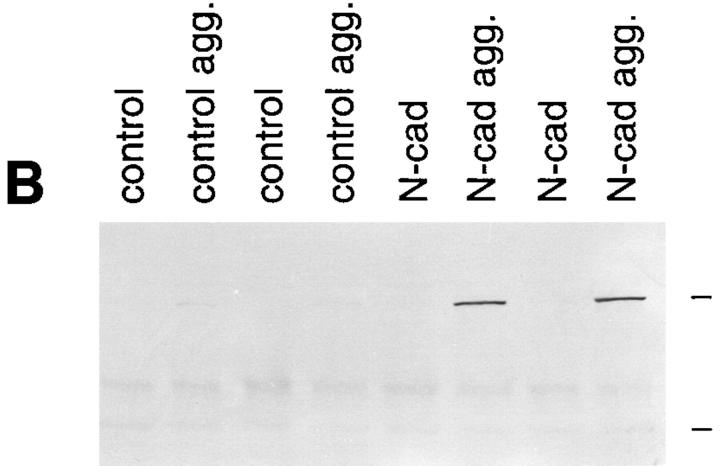
Figure 2.
N-cadherin increases sarcomeric myosin and the 12/101 antigen in aggregated BHK cells. Control cells and cells expressing exogenous N-cadherin were aggregated and examined for the expression of two markers of skeletal muscle differentiation. (A) Cells were aggregated for 24 h, then collected, and placed on slides for 48 h. Sarcomeric myosin was detected with MF20 and the 12/101 antigen was detected with a monoclonal antibody. The apparent differences in the number and morphology between control cells and cells expressing N-cadherin are due to the different cell–cell adhesion properties of the cells (Fig. 3). (B) Two independent clones expressing N-cadherin (N-cad) and two control clones were grown as a monolayer and as aggregates in hanging drops (agg.) for 72 h. The cells were collected and extracted in SDS sample buffer. Extracts were resolved by SDS-PAGE, transferred to nitrocellulose, and immunoblotted with MF20 to sarcomeric myosin. High range molecular weight markers were used and the positions of the 205- and 116.5-kD bands are indicated at the right side of the figure. No increase in myosin or 12/101 was seen in N-cadherin–expressing cells grown in a monolayer.
Figure 3.
N-cadherin promotes aggregation in BHK cells. BHK cells expressing exogenous N-cadherin (N-cadherin) and control cells (control) were placed in hanging drops for 24 h to promote aggregation. The cells were then collected, placed on slides, and grown for 48 h to permit strong attachment to the slides. During this time N-cadherin– expressing cells remained as aggregates whereas the control cells spread out as a monolayer. Cells were fixed and observed by phase contrast microscopy.
Aggregation of the N-cadherin–containing transfectants by suspension culture resulted in a dramatic increase in sarcomeric myosin heavy chain, a marker of muscle differentiation, whereas control cell aggregates showed no detectable change in myosin expression (Fig. 2). The increase in myosin expression in the N-cadherin transfectants was paralleled by an increase in the 12/101 antigen, a marker of skeletal muscle differentiation (Kintner and Brockes, 1984) (Fig. 2 A). To test the specificity of the N-cadherin–mediated induction of differentiation, we also aggregated the BHK cells with the lectins wheat germ agglutinin and concanavalin A. Aggregation in the presence of either of these lectins did increase cell–cell adhesion but did not increase sarcomeric myosin levels, suggesting N-cadherin has a role in promoting differentiation beyond simple agglutination of the cells (not shown). The differentiation process observed in the N-cadherin–containing BHKs proceeded over a 72-h-period in suspension culture with a gradual increase in myosin expression (not shown). For each of the following experiments we examined differentiation in suspension cultures at the 72-h-time point.
N-cadherin Alters the Growth of BHK Cells in Aggregates
Differentiation into skeletal muscle requires coordinated withdrawal from the cell cycle and activation of myogenic transcription factors (Ludolph and Konieczny, 1995). Since N-cadherin was capable of promoting differentiation of BHKs in aggregates but not the monolayer cultures, we were interested in its potential to affect the growth rate of these cells in monolayer and suspension cultures.
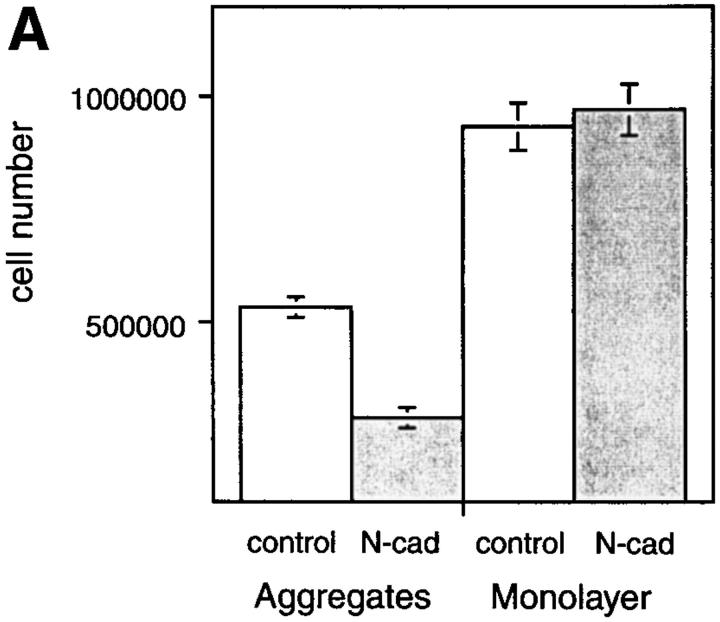
Exogenous N-cadherin had no effect on the proliferation of BHK cells grown as monolayer cultures in the presence of serum (Fig. 4 A). However, when BHK cells were cultured as aggregates in the presence of serum, their growth decreased significantly in comparison to the attached cells. Moreover, this inhibition was enhanced in cells expressing N-cadherin (Fig. 4 A). There was no difference in the percentage of nonviable cells in any of the cultures. These results suggest that suspension culture induces withdrawal from the cell cycle and that this is further enhanced by N-cadherin. The inhibition of cell proliferation in control aggregates did not stimulate differentiation. However, the enhanced inhibition of growth induced by N-cadherin in the aggregates may play a role in promoting their differentiation into skeletal muscle.
Figure 4.
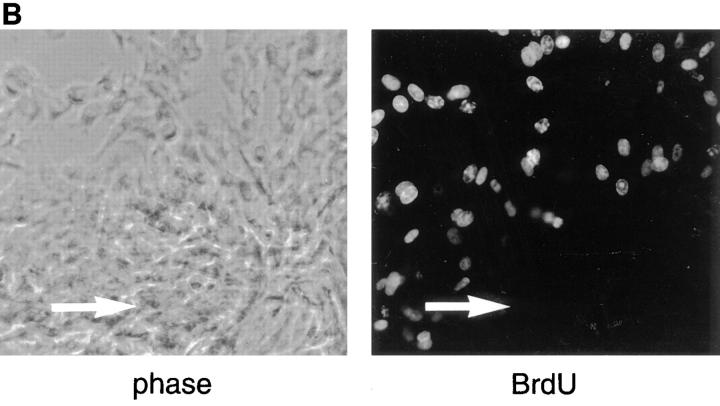
Culturing BHK cells in aggregates inhibits their growth, and this inhibition is enhanced by N-cadherin. (A) An equal number of cells (1.5 × 105) expressing N-cadherin (N-cad) or of control cells (control) was grown for 72 h as hanging drops (aggregates) or as individual drops on tissue culture dishes (monolayer). Cells were harvested and dispersed into single cells with trypsin and counted microscopically using a hemocytometer. Viability was determined by Trypan blue exclusion. Viability was >95% in all of the cultures. The mean of four individual experiments is shown and the standard deviation is indicated. Student's test was used to determine there was no significant difference between the growth of N-cadherin–expressing cells and control cells cultured as monolayers (P = 0.3736). There were significant differences between the growth of control cells in aggregates vs monolayers (P < 0.001), the growth of N-cadherin– expressing cells in aggregates vs monolayers (P < 0.001), and the growth of N-cadherin–expressing cells vs control cells cultured as aggregates (P < 0.001). (B) BHK cells expressing N-cadherin were placed in hanging drops for 24 h to promote aggregation. The cells were collected, placed on slides, and grown for 24 h to permit strong attachment to the slides. The cells were then labeled with BrdU and probed for BrdU using a monoclonal anti-BrdU. The position of the aggregate is indicated by an arrow in each panel.
Given the inhibition of growth induced by N-cadherin– mediated aggregation and the role of N-cadherin in the promotion of myogenesis, we wanted to determine whether the cells expressing myosin were postmitotic. Our experiments demonstrated that cells within the N-cadherin– expressing aggregates express myosin (Fig. 2), whereas the majority of those in a monolayer around the aggregate did not. We examined the mitotic state of the cells within these aggregates using BrdU labeling and found that BrdU was incorporated into many cells on the periphery of the aggregates, whereas the vast majority of cells within the aggregates failed to incorporate BrdU (Fig. 4 B). Double labeling using anti-myosin and anti-BrdU monoclonal antibodies and isotype-specific secondary antibodies revealed that myosin positive cells on the periphery of the aggregate did not incorporate BrdU. A total of 517 cells within 10 independent fields on the periphery of an aggregate were analyzed and 45% of the cells were positive for BrdU, 16% were positive for myosin, but none were positive for both myosin and BrdU. The lack of BrdU incorporation within the myosin positive aggregates provides further evidence that N-cadherin promotes differentiation and withdrawal from the cell cycle.
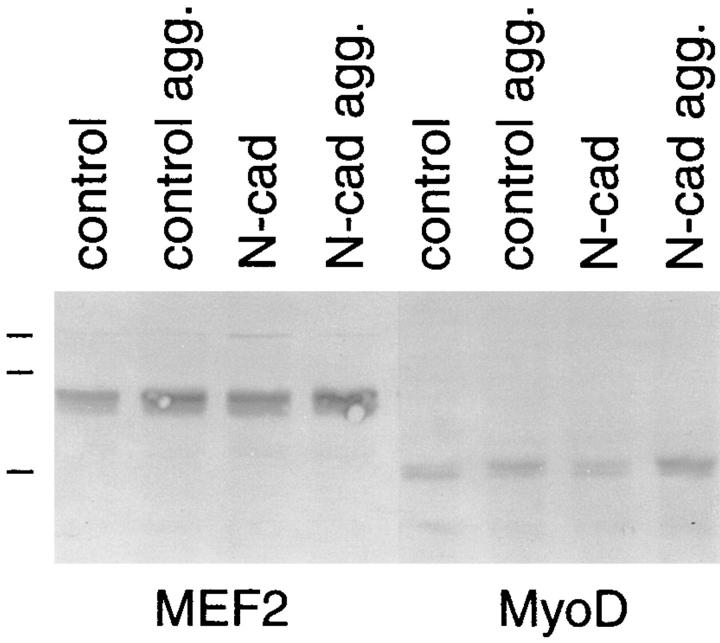
Two of the transcription factors that cooperate to activate skeletal muscle myogenesis, MyoD and MEF2, both respond to growth control signals during differentiation (Olson et al., 1991; Ludolph and Konieczny, 1995; Molkentin et al., 1996). Given our finding that N-cadherin inhibits proliferation and enhances sarcomeric myosin levels in aggregated BHK cells, we were interested in the possible effect N-cadherin–mediated aggregation may have on levels of MyoD and MEF2. Therefore, we examined the levels of MEF2 and MyoD in suspended aggregates using Western blot analysis. We found that suspension culture with concomitant withdrawal from the cell cycle slightly increased the levels of MEF2 and MyoD above those found in cells grown as a monolayer (Fig. 5). However, the presence of N-cadherin did not have any detectable effect on the levels of MyoD or MEF2 in either monolayer or suspension cultures (Fig. 5).
Figure 5.
N-cadherin does not alter levels of myogenic transcription factors in BHK cells. BHK cells expressing exogenous N-cadherin (N-cad) and control cells (control) were grown as a monolayer and as hanging drops (agg.) for 72 h and prepared for Western blot analysis as described in Fig. 2. Blots were probed with polyclonal anti-MEF2 and MyoD. Low range molecular weight markers were used and the positions of the 110-, 84-, and 47-kD bands are indicated on the figure.
E-cadherin Also Promotes the Differentiation of BHK Cells
N-cadherin is expressed by skeletal muscle precursors and has been implicated in the differentiation of skeletal muscle (George-Weinstein et al., 1997). Other cadherins, such as E-cadherin, can induce strong cell–cell adhesion but are not found in skeletal muscle. Therefore, we sought to determine whether stimulation of myogenesis by N-cadherin in BHK cells was specific to N-cadherin or if another cadherin, even one not normally expressed by skeletal muscle, also could stimulate differentiation. Therefore, we transfected the BHK cells with human E-cadherin. Similar to those with N-cadherin, cells expressing E-cadherin showed increased cell–cell adhesion in an aggregation assay (not shown) and also had increased levels of β-catenin relative to control cells (Fig. 6). Moreover, cells containing E-cadherin showed differentiation properties similar to those with N-cadherin (i.e., upon aggregation in suspension culture, the cells expressed increased levels of sarcomeric myosin) (Fig. 6). These results demonstrate that cadherin-mediated stimulation of skeletal muscle differentiation is not N-cadherin specific, and likely can be accomplished by any cadherin expressed by skeletal muscle cells or their precursors.
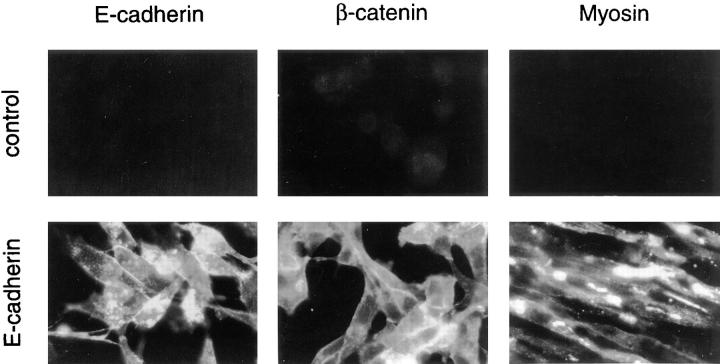
Figure 6.
E-cadherin promotes skeletal muscle myogenesis. Stable cell lines transfected with either pHβ-Ecad plus pLKpac (E-cadherin) or pLKpac alone (control) were aggregated and analyzed by immunofluorescence light microscopy as described in Fig. 2. Both E-cadherin and β-catenin localized to sites of cell– cell contact. E-cadherin was detected with E9, β-catenin was detected with 15B8, and myosin was detected with MF20. As with N-cadherin, increased myosin was observed only when the cells were aggregated in suspension.
Nonfunctional N-cadherin Increases β-catenin but Does Not Promote Differentiation
Enhanced differentiation of BHK cells into skeletal muscle does not appear to be N-cadherin specific. At this point, we wanted to distinguish between cadherin-mediated adhesion and the cadherin-mediated increase in β-catenin. Expression of any exogenous classical cadherin by these cells would be expected to increase both cell–cell adhesion and the level of endogenous β-catenin. Since β-catenin has been implicated in cell fate determination and intracellular signaling (Miller and Moon, 1996), it is possible that the stimulation of differentiation by exogenous cadherin is due to increased levels of β-catenin. To test this possibility we transfected the BHK cells with a cDNA encoding mutant N-cadherin defective in cell–cell adhesion. The intracellular domain of this cadherin is intact and therefore β-catenin levels increase in its presence (Fig. 7). However, introduction of this mutant N-cadherin into BHK cells failed to promote differentiation in either monolayer cultures or aggregates, suggesting that cadherin-mediated adhesion and not simply elevated levels of β-catenin stimulates the differentiation of BHKs into skeletal muscle (Fig. 7).
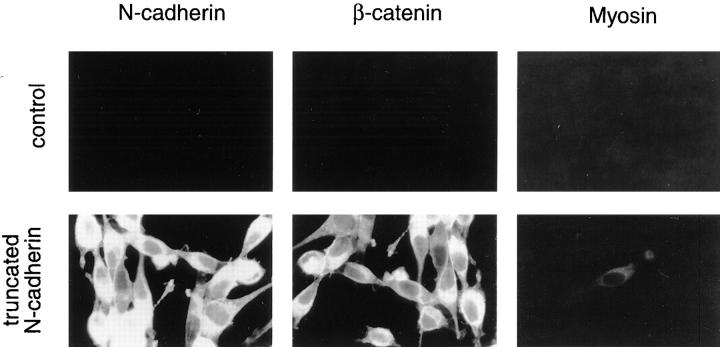
Figure 7.
Nonfunctional N-cadherin increases β-catenin but does not promote skeletal muscle differentiation. Stable cell lines transfected with either pHβ-NcadΔ plus pLKpac (truncated N-cadherin) or pLKpac alone (control) were aggregated and analyzed by immunofluorescence light microscopy as described in Fig. 2. Truncated N-cadherin was detected with 6B3, β-catenin with 15B8, and sarcomeric myosin with MF20. Cells expressing truncated N-cadherin and control cells spread as a monolayer after replating due to lack of strong cell–cell adhesion in either of these cultures.
Discussion
Skeletal muscle precursors contain multiple cadherins that together have an important role in establishing tissue homogeneity. M-cadherin is involved in myotube formation and the adhesion of satellite cells to mature muscle (Donalies et al., 1991; Zeschnigk et al., 1995). N-cadherin is involved in myoblast interaction and the formation of myotubes (Knudsen et al., 1990; Mege et al., 1992). Skeletal muscle precursors also contain cadherin-11 (Kimura et al., 1995), R-cadherin (Inuzuka et al., 1991), and T-cadherin (Ranscht, 1994). Although roles for N-cadherin and M-cadherin in later stages of myogenesis have been firmly established, less is known about the requirement of cadherins in the early stages of myogenesis. Holt et al. (1994) demonstrated that a dominant-negative cadherin mRNA, which perturbs the function of all cadherins, can inhibit early stages of myogenesis in Xenopus embryos. In addition, George-Weinstein et al. (1997) recently showed that function-perturbing antibodies to N-cadherin inhibit the differentiation of cultured primitive streak stage chick epiblast cells into skeletal muscle. Here, we show that N-cadherin– mediated adhesion promotes skeletal myogenesis in a myogenic cell line by increasing the level of sarcomeric myosin but that this effect is not specific for N-cadherin. The lack of N-cadherin specificity is consistent with previous studies in the N-cadherin null mouse, which demonstrated that N-cadherin is not essential for skeletal muscle differentiation (Radice et al., 1997). Since skeletal muscle expresses multiple cadherins, it is likely that upon chronic loss of N-cadherin another cadherin(s) substitutes functionally for it.
The increase in sarcomeric myosin in cadherin-expressing BHK cells required their aggregation in suspension, which disrupts the extracellular matrix interactions found in cells grown as monolayers. In this regard, the BHK cells are similar to P19 teratocarcinoma cells that differentiate to skeletal muscle only after being aggregated in suspension (Edwards et al., 1983). In other systems, perturbation of cell–matrix adhesion inhibits myogenesis. Inhibition of matrix synthesis and perturbation of integrin function with peptides or antibodies inhibit the differentiation of cultured embryonic chick pectoral muscle myoblasts (Menko and Boettiger, 1987; Nandan et al., 1990; Saitoh et al., 1992). Moreover, myogenic cells grown in suspension as single cells growth arrest but fail to differentiate without substrate adhesion (Milasincic et al., 1996). These apparently conflicting results are likely due to differences in the model systems and how the experiments were conducted. It is possible that cadherin-mediated adhesion alters the production of matrix or the interaction of cells with extracellular matrix in a way that favors differentiation. N-cadherin–mediated adhesion also could bypass the signals required for the integrin-mediated promotion of myogenesis.
Many growth factors inhibit myogenesis by negatively regulating myogenic transcription factors, including MyoD and MEF2, that are upregulated during skeletal muscle myogenesis (Vaidya et al., 1989; Olson et al., 1991; Yu et al., 1992; Breitbart et al., 1993; Buchberger et al., 1994). In addition, MyoD positively autoregulates its own expression and in some systems can induce cells to withdraw from the cell cycle through induction of the cell cycle inhibitor p21 (Thayer et al., 1989; Halevy et al., 1995). Therefore, it is not surprising that the aggregated BHK cells that withdrew from the cell cycle showed increased levels of these myogenic transcription factors. However, only the cadherin-expressing BHK cells expressed increased sarcomeric myosin. This may be due to the enhanced growth suppression in the cadherin-expressing cells. Alternatively, cadherin-mediated adhesion may alter the activity of the myogenic transcription factors. The activities of both MyoD and MEF2 are regulated through changes in phosphorylation state and interaction with binding partners (Benezra et al., 1990; Jen et al., 1992; Li et al., 1992; Ornatasky and McDermott, 1996). However, we did not observe a shift in molecular weight of MyoD or MEF2, nor did we see a change in serine phosphorylation of MEF2, suggesting the cadherin did not alter phosphorylation of these transcription factors (not shown).
Cadherins are responsible for the close association of groups of cells with a similar developmental fate. The associated cells then initiate and respond to further developmental signals. One possible result of this cadherin-mediated association is intracellular signaling via the cadherin–catenin complex. Several lines of evidence suggest β-catenin has an important role in development and signal transduction. β-Catenin shares homology with the Drosophila segment polarity protein Armadillo, which also is found in cell junctions (Gumbiner, 1995; Peifer, 1995). Changes in β-catenin levels result in alterations of mesoderm formation in Xenopus and mouse (Heasman et al., 1994; Funayama et al., 1995; Haegel et al., 1995). In addition, signaling by Wnt leads to the accumulation of β-catenin in the cytoplasm and β-catenin can be translocated to the nucleus (Orsulic and Peifer, 1996; Papkoff et al., 1996; Schneider et al., 1996; Yost et al., 1996). Although the role of β-catenin in signal transduction makes it an attractive candidate for an enhancer of myogenesis, our studies suggest that the primary stimulatory effect of exogenous cadherins on myogenesis comes from the cadherin–catenin complex itself and cadherin-mediated adhesion. It is possible, however, that β-catenin stimulates myogenesis through a pathway that is dependent on functional cadherins.
The requirement of a close community of cells for differentiation has been demonstrated in several developmental systems, and cell–cell adhesion plays an important role in many of these systems (Gurdon et al., 1993). The formation of aggregates by primary thyroid cells alters cell–cell contacts and promotes their differentiation (Yap and Manley, 1993). The differentiation of trophoblast cells in culture also requires their aggregation and is associated with an increase in E-cadherin expression (Rebut-Bonneton et al., 1993). The importance of tissue morphology and the balance between cell–cell and cell–matrix adhesion in development was demonstrated recently in a tumorigenic breast cell line (Weaver et al., 1997). In this model system, nontumorigenic breast cancer cells cultured as three-dimensional aggregates differentiated and formed a basement membrane. In contrast, a spontaneous tumorigenic subline with known oncogenic mutations failed to undergo morphogenesis when grown as aggregates, and instead produced invasive colonies with poor cell–cell adhesion. The addition of integrin function perturbing antibodies to these tumorigenic aggregates promoted the formation of cell–cell junctions, withdrawal from the cell cycle, and differentiation. However, the same antibodies caused decreased cell–cell adhesion in the nontumorigenic cells and inhibited their differentiation. This study, like ours, illustrates the impact that tissue morphology and cellular interactions play in the regulation of cell growth and differentiation. Cellular condensation involving changes in cell shape and cell adhesion is a frequent occurrence in embryogenesis, i.e. somitogenesis. These changes are likely to alter many aspects of cell behavior, including proliferation and expression of transcription factors, all of which contribute to the control of cell and tissue differentiation both in vivo and in vitro.
In summary, our studies show that cadherin-mediated cell–cell adhesion, along with the presence of myogenic transcription factors (i.e., MyoD and MEF2) and withdrawal from the cell cycle, is necessary for skeletal muscle differentiation. Muscle differentiation is not stimulated by increased MyoD/MEF2 and decreased cell proliferation in the absence of cadherin-mediated cell–cell adhesion, even if the cells are brought into close contact. The signal(s) generated by the cadherin-mediated adhesion and the mechanism by which sarcomeric myosin levels are increased are subjects for future studies.
Acknowledgments
We thank L. Myers for expert technical assistance, M. Wheelock and A. Peralta Soler for critical reading of the manuscript, and M. George-Weinstein and K. Linask for helpful discussions. The MF20 and 12/101 antibodies were obtained from the Developmental Studies Hybridoma Bank maintained in the department of Pharmacology and Molecular Sciences, Johns Hopkins University School of Medicine (Baltimore, MD), and the Department of Biological Sciences, University of Iowa (Iowa City, IA) under contract N01-HD-2-3144 from the National Institute of Child Health and Human Development.
Footnotes
Address all correspondence to Karen A. Knudsen, The Lankenau Medical Research Center, 100 Lancaster Avenue, Wynnewood, PA 19096. Tel.: (610) 645-3581. Fax: (610) 645-2205.
1. Abbreviation used in this paper: BrdU, bromo-deoxyuridine.
References
- Bader D, Masaki T, Fischman DA. Immunochemical analysis of myosin heavy chain during avian myogenesis in vivo and in vitro. J Cell Biol. 1982;95:763–770. doi: 10.1083/jcb.95.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benezra R, Davis RL, Lockshon D, Turner DL, Weintraub H. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell. 1990;61:49–59. doi: 10.1016/0092-8674(90)90214-y. [DOI] [PubMed] [Google Scholar]
- Brand-Saberi B, Gamel AJ, Krenn V, Muller TS, Wilting J, Christ B. N-cadherin is involved in myoblast migration and muscle differentiation in the avian limb bud. Dev Biol. 1996;178:160–173. doi: 10.1006/dbio.1996.0206. [DOI] [PubMed] [Google Scholar]
- Breitbart RE, Liang CS, Smoot LB, Laheru DA, Mahdavi V, Nadal-Ginard B. A fourth human MEF2 transcription factor, hMEF2D, is an early marker of the myogenic lineage. Development (Camb) 1993;118:1095–1106. doi: 10.1242/dev.118.4.1095. [DOI] [PubMed] [Google Scholar]
- Buchberger A, Ragge K, Arnold HH. The myogenin gene is activated during myocyte differentiation by pre-existing, not newly synthesized transcription factor MEF-2. J Biol Chem. 1994;269:17289–17296. [PubMed] [Google Scholar]
- Cossu G, Kelly R, Di Donna S, Vivarelli E, Buckingham M. Myoblast differentiation during mammalian somitogenesis is dependent upon a community effect. Proc Natl Acad Sci USA. 1995;92:2254–2258. doi: 10.1073/pnas.92.6.2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Luna S, Soria I, Pulido D, Ortin J, Jimenez A. Efficient transformation of mammalian cells with constructs containing a puromycin-resistance marker. Gene (Amst) 1988;62:121–126. doi: 10.1016/0378-1119(88)90585-9. [DOI] [PubMed] [Google Scholar]
- Donalies MM, Cramer M, Ringwald M, Starzinski-Powitz A. Expression of M-cadherin, a member of the cadherin multigene family, correlates with differentiation of skeletal muscle cells. Proc Natl Acad Sci USA. 1991;88:8024–8028. doi: 10.1073/pnas.88.18.8024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards MK, Harris JF, McBurney MW. Induced muscle differentiation in an embryonal carcinoma cell line. Mol Cell Biol. 1983;3:2280–2286. doi: 10.1128/mcb.3.12.2280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank ED, Tuszynski GP, Warren L. Localization of vimentin and desmin in BHK21/C13 cells and in baby hamster kidney. Exp Cell Res. 1982;139:235–247. doi: 10.1016/0014-4827(82)90248-8. [DOI] [PubMed] [Google Scholar]
- Fujimori T, Takeichi M. Disruption of epithelial cell–cell adhesion by exogenous expression of a mutated nonfunctional N-cadherin. Mol Biol Cell. 1992;4:37–47. doi: 10.1091/mbc.4.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funayama N, Fagotto F, McCrea P, Gumbiner BM. Embryonic axis induction by the armadillo repeat domain of beta-catenin: evidence for intracellular signaling. J Cell Biol. 1995;128:959–968. doi: 10.1083/jcb.128.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George-Weinstein M, Gerhart J, Blitz J, Simak E, Knudsen KA. N-cadherin promotes the commitment and differentiation of skeletal muscle precursor cells. Dev Biol. 1997;185:14–24. doi: 10.1006/dbio.1997.8542. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Signal transduction of beta-catenin. Curr Opin Cell Biol. 1995;7:634–640. doi: 10.1016/0955-0674(95)80104-9. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- Gurdon JB, Lemaire P, Kato K. Community effects and related phenomena in development. Cell. 1993;75:831–834. doi: 10.1016/0092-8674(93)90526-v. [DOI] [PubMed] [Google Scholar]
- Haegel H, Larue L, Ohsugi M, Fedorov L, Herrenknecht K, Kemler R. Lack of beta-catenin affects mouse development at gastrulation. Development (Camb) 1995;121:3529–3537. doi: 10.1242/dev.121.11.3529. [DOI] [PubMed] [Google Scholar]
- Halevy O, Novitch BG, Spicer DB, Skapek SX, Rhee J, Hannon GJ, Beach D, Lassar AB. Correlation of terminal cell cycle arrest of skeletal muscle with induction of p21 by MyoD. Science (Wash DC) 1995;267:1018–1021. doi: 10.1126/science.7863327. [DOI] [PubMed] [Google Scholar]
- Heasman J, Crawford A, Goldstone K, Garner-Hamrick P, Gumbiner B, McCrea P, Kintner C, Noro CY, Wylie C. Overexpression of cadherins and underexpression of β-catenin inhibit dorsal mesoderm induction in early Xenopusembryos. Cell. 1994;79:791–803. doi: 10.1016/0092-8674(94)90069-8. [DOI] [PubMed] [Google Scholar]
- Hirt RP, Poulain-Godefroy O, Billotte J, Kraehenbuhl JP, Fasel N. Highly inducible synthesis of heterologous proteins in epithelial cells carrying a glucocorticoid-responsive vector. Gene (Amst) 1992;111:199–206. doi: 10.1016/0378-1119(92)90687-k. [DOI] [PubMed] [Google Scholar]
- Holt CE, Lemaire P, Gurdon JB. Cadherin-mediated cell interactions are necessary for the activation of MyoD in Xenopusmesoderm. Proc Natl Acad Sci USA. 1994;91:10844–10848. doi: 10.1073/pnas.91.23.10844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber O, Bierkamp C, Kemler R. Cadherins and catenins in development. Curr Opin Cell Biol. 1996;8:685–691. doi: 10.1016/s0955-0674(96)80110-4. [DOI] [PubMed] [Google Scholar]
- Inuzuka H, Redies C, Takeichi M. Differential expression of R- and N-cadherin in neural and mesodermal tissues during early chicken development. Development (Camb) 1991;113:959–967. doi: 10.1242/dev.113.3.959. [DOI] [PubMed] [Google Scholar]
- Jen Y, Weintraub H, Benezra R. Overexpression of Id protein inhibits the muscle differentiation program: in vivo association of Id with E2A proteins. Genes Dev. 1992;6:1466–1479. doi: 10.1101/gad.6.8.1466. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Lewis JE, Li D, Wahl J, Peralta A, Soler, Knudsen KA, Wheelock MJ. P- and E-cadherin are in separate complexes in cells expressing both cadherins. Exp Cell Res. 1993;207:252–260. doi: 10.1006/excr.1993.1191. [DOI] [PubMed] [Google Scholar]
- Kato K, Gurdon JB. Single-cell transplantation determines the time when Xenopusmuscle precursor cells acquire a capacity for autonomous differentiation. Proc Natl Acad Sci USA. 1993;90:1310–1314. doi: 10.1073/pnas.90.4.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemler R. From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends Genet. 1993;9:317–321. doi: 10.1016/0168-9525(93)90250-l. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Matsunami H, Inoue T, Shimamura K, Uchida N, Ueno T, Miyazaki T, Takeichi M. Cadherin-11 expressed in association with mesenchymal morphogenesis in the head, somite, and limb bud of early mouse embryos. Dev Biol. 1995;169:347–358. doi: 10.1006/dbio.1995.1149. [DOI] [PubMed] [Google Scholar]
- Kintner CR, Brockes JP. Monoclonal antibodies identify blastemal cells derived from dedifferentiating muscle in newt limb regeneration. Nature (Lond) 1984;308:67–69. doi: 10.1038/308067a0. [DOI] [PubMed] [Google Scholar]
- Knudsen KA, Myers L, McElwee SA. A role for the Ca2+-dependent adhesion molecule, N-cadherin, in myoblast interaction during myogenesis. Exp Cell Res. 1990;188:175–184. doi: 10.1016/0014-4827(90)90157-6. [DOI] [PubMed] [Google Scholar]
- Knudsen KA, Peralta A, Soler, Johnson KR, Wheelock MJ. Interaction of α-actinin with the cadherin/catenin cell–cell adhesion complex via α-catenin. J Cell Biol. 1995;130:67–77. doi: 10.1083/jcb.130.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larue L, Antos C, Butz S, Huber O, Delmas V, Dominis M, Kemler R. A role for cadherins in tissue formation. Development (Camb) 1996;122:3185–3194. doi: 10.1242/dev.122.10.3185. [DOI] [PubMed] [Google Scholar]
- Lewis JE, Wahl JK, Sass KM, Jensen PJ, Johnson KR, Wheelock MJ. Cross-talk between adherens junctions and desmosomes depends on plakoglobin. J Cell Biol. 1997;136:919–934. doi: 10.1083/jcb.136.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Zhou J, James G, Heller-Harrison R, Czech MP, Olson EN. FGF inactivates myogenic helix-loop-helix proteins through phosphorylation of a conserved protein kinase C site in their DNA-binding domains. Cell. 1992;71:1181–1194. doi: 10.1016/s0092-8674(05)80066-2. [DOI] [PubMed] [Google Scholar]
- Ludolph DC, Konieczny SF. Transcription factor families: muscling in on the myogenic program. FASEB (Fed Am Soc Exp Biol) J. 1995;9:1595–1604. doi: 10.1096/fasebj.9.15.8529839. [DOI] [PubMed] [Google Scholar]
- McCrea PD, Gumbiner BM. Purification of a 92-kD cytoplasmic protein tightly associated with the cell–cell adhesion molecule E-cadherin (uvomorulin). Characterization and extractability of the protein complex from the cell cytostructure. J Biol Chem. 1991;266:4514–4520. [PubMed] [Google Scholar]
- Mege RM, Goudou D, Diaz C, Nicolet M, Garcia L, Geraud G, Rieger F. N-cadherin and N-CAM in myoblast fusion: compared localisation and effect of blockade by peptides and antibodies. J Cell Sci. 1992;103:897–906. doi: 10.1242/jcs.103.4.897. [DOI] [PubMed] [Google Scholar]
- Menko AS, Boettiger D. Occupation of the extracellular matrix receptor, integrin, is a control point for myogenic differentiation. Cell. 1987;51:51–57. doi: 10.1016/0092-8674(87)90009-2. [DOI] [PubMed] [Google Scholar]
- Milasincic DJ, Dhawan J, Farmer SR. Anchorage-dependent control of muscle-specific gene expression in C2C12 mouse myoblasts. In Vitro Cell Dev Biol Anim. 1996;32:90–99. doi: 10.1007/BF02723040. [DOI] [PubMed] [Google Scholar]
- Miller JR, Moon RT. Signal transduction through β-catenin and specification of cell fate during embryogenesis. Genes Dev. 1996;10:2527–2539. doi: 10.1101/gad.10.20.2527. [DOI] [PubMed] [Google Scholar]
- Molkentin JD, Li L, Olson EN. Phosphorylation of the MADS-Box transcription factor MEF2C enhances its DNA binding activity. J Biol Chem. 1996;271:17199–17204. doi: 10.1074/jbc.271.29.17199. [DOI] [PubMed] [Google Scholar]
- Murphy-Erdosh C, Yoshida CK, Paradies N, Reichardt LF. The cadherin-binding specificities of B-cadherin and LCAM. J Cell Biol. 1995;129:1379–1390. doi: 10.1083/jcb.129.5.1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuchi A, Takeichi M. Cell binding function of E-cadherin is regulated by the cytoplasmic domain. EMBO (Eur Mol Biol Organ) J. 1988;7:3679–3684. doi: 10.1002/j.1460-2075.1988.tb03249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuchi A, Takeichi M. Transmembrane control of cadherin-mediated cell adhesion: a 94 kD protein functionally associated with a specific region of the cytoplasmic domain of E-cadherin. Cell Regul. 1989;1:37–44. doi: 10.1091/mbc.1.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuchi A, Takeichi M, Tsukita S. The 102 kd cadherin-associated protein: similarity to vinculin and posttranscriptional regulation of expression. Cell. 1991;65:849–857. doi: 10.1016/0092-8674(91)90392-c. [DOI] [PubMed] [Google Scholar]
- Nandan D, Clarke EP, Ball EH, Sanwal BD. Ethyl-3,4-dihydroxybenzoate inhibits myoblast differentiation: evidence for an essential role of collagen. J Cell Biol. 1990;110:1673–1679. doi: 10.1083/jcb.110.5.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EN. Interplay between proliferation and differentiation within the myogenic lineage. Dev Biol. 1992;154:261–272. doi: 10.1016/0012-1606(92)90066-p. [DOI] [PubMed] [Google Scholar]
- Olson EN, Klein WH. bHLH factors in muscle development: dead lines and commitments, what to leave in and what to leave out. Genes Dev. 1994;8:1–8. doi: 10.1101/gad.8.1.1. [DOI] [PubMed] [Google Scholar]
- Olson EN, Brennan TJ, Chakraborty T, Cheng TC, Cserjesi P, Edmondson D, James G, Li L. Molecular control of myogenesis: antagonism between growth and differentiation. Mol Cell Biochem. 1991;104:7–13. doi: 10.1007/BF00229797. [DOI] [PubMed] [Google Scholar]
- Ornatsky OI, McDermott JC. MEF2 protein expression, DNA binding specificity and complex composition, and transcriptional activity in muscle and nonmuscle cells. J Biol Chem. 1996;271:24927–24933. doi: 10.1074/jbc.271.40.24927. [DOI] [PubMed] [Google Scholar]
- Orsulic S, Peifer M. An in vivo structure-function study of armadillo, the β-catenin homologue, reveals both separate and overlapping regions of the protein required for cell adhesion and for wingless signaling. J Cell Biol. 1996;134:1283–1300. doi: 10.1083/jcb.134.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa M, Kemler R. Molecular organization of the uvomorulin– catenin complex. J Cell Biol. 1992;116:989–996. doi: 10.1083/jcb.116.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa M, Ringwald M, Kemler R. Uvomorulin-catenin complex formation is regulated by a specific domain in the cytoplasmic region of the cell adhesion molecule. Proc Natl Acad Sci USA. 1990;87:4246–4250. doi: 10.1073/pnas.87.11.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papkoff J, Rubinfeld B, Schryver B, Polakis P. Wnt-1 regulates free pools of catenins and stabilizes APC-catenin complexes. Mol Cell Biol. 1996;16:2128–2134. doi: 10.1128/mcb.16.5.2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peifer M. Cell adhesion and signal transduction: the Armadillo connection. Trends Cell Biol. 1995;5:224–229. doi: 10.1016/s0962-8924(00)89015-7. [DOI] [PubMed] [Google Scholar]
- Peralta Soler, A., and K.A. Knudsen. N-cadherin involvement in cardiac myocyte interaction and myofibrillogenesis. Dev Biol. 1994;162:9–17. doi: 10.1006/dbio.1994.1062. [DOI] [PubMed] [Google Scholar]
- Quinlan RA, Franke WW. Heteropolymer filaments of vimentin and desmin in vascular smooth muscle tissue and cultured baby hamster kidney cells demonstrated by chemical crosslinking. Proc Natl Acad Sci USA. 1982;79:3452–3456. doi: 10.1073/pnas.79.11.3452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radice GL, Rayburn H, Matsunami H, Knudsen KA, Takeichi M, Hynes RO. Developmental defects in mouse embryos lacking N-cadherin. Dev Biol. 1997;181:64–78. doi: 10.1006/dbio.1996.8443. [DOI] [PubMed] [Google Scholar]
- Ranscht B. Cadherins and catenins: interactions and functions in embryonic development. Curr Opin Cell Biol. 1994;6:740–746. doi: 10.1016/0955-0674(94)90102-3. [DOI] [PubMed] [Google Scholar]
- Rebut-Bonneton C, Boutemy-Roulier S, Evain-Brion D. Modulation of pp60c-src activity and cellular localization during differentiation of human trophoblast cells in culture. J Cell Sci. 1993;105:629–636. doi: 10.1242/jcs.105.3.629. [DOI] [PubMed] [Google Scholar]
- Rimm DL, Koslov ER, Kebriaei P, Cianci CD, Morrow JS. Alpha 1(E)-catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc Natl Acad Sci USA. 1995;92:8813–8817. doi: 10.1073/pnas.92.19.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh O, Periasamy M, Kan M, Matsuda R. cis-4-Hydroxy-L-proline and ethyl-3,4-dihydroxybenzoate prevent myogenesis of C2C12 muscle cells and block MyoD1 and myogenin expression. Exp Cell Res. 1992;200:70–76. doi: 10.1016/s0014-4827(05)80072-2. [DOI] [PubMed] [Google Scholar]
- Schaart G, Pieper FR, Kuijpers HJ, Bloemendal H, Ramaekers FC. Baby hamster kidney (BHK-21/C13) cells can express striated muscle type proteins. Differentiation. 1991;46:105–115. doi: 10.1111/j.1432-0436.1991.tb00871.x. [DOI] [PubMed] [Google Scholar]
- Schneider S, Steinbeisser H, Warga RM, Hausen P. β-catenin translocation into nuclei demarcates the dorsalizing centers in frog and fish embryos. Mech Dev. 1996;57:191–198. doi: 10.1016/0925-4773(96)00546-1. [DOI] [PubMed] [Google Scholar]
- Skerjanc IS, Slack RS, McBurney MW. Cellular aggregation enhances MyoD-directed skeletal myogenesis in embryonal carcinoma cells. Mol Cell Biol. 1994;14:8451–8459. doi: 10.1128/mcb.14.12.8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slager HG, Van Inzen W, Freund E, Van den Eijnden-Van AJ, Raaij, Mummery CL. Transforming growth factor-β in the early mouse embryo: implications for the regulation of muscle formation and implantation. Dev Genet. 1993;14:212–224. doi: 10.1002/dvg.1020140308. [DOI] [PubMed] [Google Scholar]
- Takeichi M. The cadherins: cell-cell adhesion molecules controlling animal morphogenesis. Development (Camb) 1988;102:639–655. doi: 10.1242/dev.102.4.639. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Morphogenetic roles of classic cadherins. Curr Opin Cell Biol. 1995;7:619–627. doi: 10.1016/0955-0674(95)80102-2. [DOI] [PubMed] [Google Scholar]
- Tanihara H, Kido M, Obata S, Heimark RL, Davidson M, St John T, Suzuki S. Characterization of cadherin-4 and cadherin-5 reveals new aspects of cadherins. J Cell Sci. 1994;107:1697–1704. doi: 10.1242/jcs.107.6.1697. [DOI] [PubMed] [Google Scholar]
- Thayer MJ, Tapscott SJ, Davis RL, Wright WE, Lassar AB, Weintraub H. Positive autoregulation of the myogenic determination gene MyoD1. Cell. 1989;58:241–248. doi: 10.1016/0092-8674(89)90838-6. [DOI] [PubMed] [Google Scholar]
- Urushihara H, Ueda MJ, Okada TS, Takeichi M. Calcium-dependent and -independent adhesion of normal and transformed BHK cells. Cell Struct Funct. 1977;2:289–296. [Google Scholar]
- Vaidya TB, Rhodes SJ, Taparowsky EJ, Konieczny SF. Fibroblast growth factor and transforming growth factor beta repress transcription of the myogenic regulatory gene MyoD1. Mol Cell Biol. 1989;9:3576–3579. doi: 10.1128/mcb.9.8.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Loop FT, Van Eys GJ, Schaart G, Ramaekers FC. Titin expression as an early indication of heart and skeletal muscle differentiation in vitro. Developmental re-organisation in relation to cytoskeletal constituents. J Muscle Res Cell Motil. 1996;17:23–36. doi: 10.1007/BF00140321. [DOI] [PubMed] [Google Scholar]
- Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ. Reversion of the malignant phenotype of human breast cancer cells in three-dimensional culture and in vivo by Integrin blocking antibodies. J Cell Biol. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheelock MJ, Knudsen KA. Cadherins and associated proteins. In Vivo (Athens) 1991;5:505–513. [PubMed] [Google Scholar]
- Wheelock MJ, Buck CA, Bechtol KB, Damsky CH. Soluble 80-kd fragment of cell-CAM 120/80 disrupts cell-cell adhesion. J Cell Biochem. 1987;34:187–202. doi: 10.1002/jcb.240340305. [DOI] [PubMed] [Google Scholar]
- Yap AS, Manley SW. Contact inhibition of cell spreading: a mechanism for the maintenance of thyroid cell aggregation in vitro. Exp Cell Res. 1993;208:121–127. doi: 10.1006/excr.1993.1229. [DOI] [PubMed] [Google Scholar]
- Yost C, Torres M, Miller JR, Huang E, Kimelman D, Moon RT. The axis-inducing activity, stability, and subcellular distribution of β-catenin is regulated in Xenopusembryos by glycogen synthase kinase 3. Genes Dev. 1996;10:1443–1454. doi: 10.1101/gad.10.12.1443. [DOI] [PubMed] [Google Scholar]
- Yu YT, Breitbart RE, Smoot LB, Lee Y, Mahdavi V, Nadal-Ginard B. Human myocyte-specific enhancer factor 2 comprises a group of tissue-restricted MADS box transcription factors. Genes Dev. 1992;6:1783–1798. doi: 10.1101/gad.6.9.1783. [DOI] [PubMed] [Google Scholar]
- Zeschnigk M, Kozian D, Kuch C, Schmoll M, Starzinski-Powitz A. Involvement of M-cadherin in terminal differentiation of skeletal muscle cells. J Cell Sci. 1995;108:2973–2981. doi: 10.1242/jcs.108.9.2973. [DOI] [PubMed] [Google Scholar]