Abstract
The folding and trafficking of tropoelastin is thought to be mediated by intracellular chaperones, although the identity and role of any tropoelastin chaperone remain to be determined. To identify proteins that are associated with tropoelastin intracellularly, bifunctional chemical cross-linkers were used to covalently stabilize interactions between tropoelastin and associated proteins in the secretory pathway in intact fetal bovine auricular chondrocytes. Immunoprecipitation of tropoelastin from cell lysates after cross-linking and analysis by SDS-PAGE showed the presence of two proteins of ∼74 kD (p74) and 78 kD (p78) that coimmunoprecipitated with tropoelastin. Microsequencing of peptide fragments from a cyanogen bromide digest of p78 identified this protein as BiP and sequence analysis identified p74 as the peptidyl-prolyl cis–trans isomerase, FKPB65. The appearance of BiP and FKBP65 in the immunoprecipitations could be enhanced by the addition of brefeldin A (BFA) and N-acetyl-leu-leu-norleucinal (ALLN) to the culture medium for the final 4 h of labeling. Tropoelastin accumulates in the fused ER/Golgi compartment in the presence of BFA if its degradation is inhibited by ALLN (Davis, E.C., and R.P. Mecham. 1996. J. Biol. Chem. 271:3787–3794). The use of BFA and other secretion-disrupting agents suggests that the association of tropoelastin with FKBP65 occurs in the ER. Results from this study provide the first identification of a ligand for an FKBP in the secretory pathway and suggest that the prolyl cis–trans isomerase activity of FKBP65 may be important for the proper folding of the proline-rich tropoelastin molecule before secretion.
Tropoelastin is a soluble 70-kD protein that is cross-linked in the presence of extracellular microfibrils to form insoluble elastic fibers. These fibers are an abundant component of the extracellular matrix where they provide the critical function of elasticity to tissues such as blood vessels, lung, and skin (Mecham and Davis, 1994). Apart from cleavage of a signal sequence as the completed polypeptide chain enters the ER (Karr and Foster, 1981; Saunders and Grant, 1984; Grosso and Mecham, 1988), the tropoelastin monomer remains relatively unchanged as it traverses the secretory pathway en route to the cell surface, with no glycosylation or proteolytic processing.
In a previous study, we reported that tropoelastin undergoes selective degradation in the ER as a consequence of being retained in that compartment by brefeldin A (BFA)1 treatment (Davis and Mecham, 1996). Similar to other proteins that undergo ER-associated degradation (Inoue et al., 1991; Wileman et al., 1991; Thrift et al., 1992), the degradation of tropoelastin can be inhibited by the cysteine protease inhibitor, N-acetyl-leu-leu-norleucinal (ALLN). Because tropoelastin has the property of being able to undergo a phase transition and form a coacervate when at high concentrations under physiological conditions (Cox et al., 1974), it was postulated that the accumulation of tropoelastin within the fused ER/Golgi compartment during BFA treatment may lead to coacervation of the protein and thus its subsequent recognition and degradation as a misfolded protein. Consistent with this hypothesis was the observation of a lag period during which time tropoelastin accumulated for 1 h before rapid degradation of the protein (Davis and Mecham, 1996).
The increased concentration of tropoelastin in the fused ER/Golgi compartment may also exhaust an existing supply of chaperones that would normally fold and traffic tropoelastin to the Golgi, again leading to misfolding and degradation. A protective function for an ER chaperone has been suggested by the work of Jain and colleagues (1994) who demonstrated that the interaction of procollagen I with the ER chaperone, colligin/hsp47, protects the molecule from degradation by a serine protease in the ER. Because the expression of hsp47 predominates in collagen-secreting cells (Saga et al., 1987) and its synthesis closely correlates to that of collagen under many conditions (Clarke et al., 1993), the possibility has been raised that a population of molecular chaperones may exist that are specific for certain substrates (Nagata, 1996).
To investigate the possibility of “substrate-specific” chaperones, primary cultures of fetal bovine chondrocytes were studied for the presence of potential molecular chaperones for tropoelastin. In culture, tropoelastin is a major protein produced by these cells, and is secreted and assembled into an insoluble extracellular elastin matrix. Using the membrane permeable chemical cross-linkers dithiobis- (succinimidyl propionate) (DSP) and disuccinimidyl suberate (DSS), we have identified two proteins that coimmunoprecipitate with tropoelastin under cross-linking conditions in intact cells. Sequence analysis of these proteins has identified them as the ER chaperone BiP and the 65-kD FK506-binding protein FKPB65. FKBP65 is a member of the highly conserved family of intracellular receptors called immunophilins (Schreiber, 1991; Sigal and Dumont, 1992; Galat, 1993). Based on their ability to bind either of the two immunosuppressant drugs, cyclosporin A or FK506, the immunophilins have been divided into two groups, cyclophilins and FKBPs, respectively. To date, six members of the FKBP family have been identified and named according to their respective molecular weights: FKBP12 (Harding et al., 1989; Siekierka et al., 1989), FKBP13 (Jin et al., 1991), FKBP25 (Galat et al., 1992; Jin et al., 1992; Wiederrecht et al., 1992), FKBP52 (Peattie et al., 1992), FKBP54 (Smith et al., 1993) and, most recently, FKBP65 (Coss et al., 1995). Of these, only FKBP13 has been specifically localized to the ER (Nigam et al., 1993). The presence of a putative signal sequence, glycosylation sites and an ER retention sequence (HEEL; Pelham, 1990) at the COOH terminus of FKBP65 (Coss et al., 1995), however, predicts that this protein will also be contained within the ER.
Apart from the property of peptidyl-prolyl cis–trans isomerization, which is common to all immunophilins, no specific function or ligand for FKBPs in the ER has been identified. Results from this study thus provide the first identification of a ligand for an FKBP in the secretory pathway. That this ligand is tropoelastin, a protein with a large percentage of proline residues, suggests that the prolyl isomerase activity of FKBP65 may be important for tropoelastin folding, trafficking, and ultimate assembly into elastic fibers.
Materials and Methods
Cells and Reagents
Bovine ears were obtained from fetuses of 160–180 d of gestation at a local slaughterhouse. Fetal bovine chondrocytes (FBCs) were obtained by collagenase digestion of the auricular cartilage as previously described (Mecham, 1987). All experiments were conducted with first passage cells grown in Dulbecco's modified Eagle's medium supplemented with l-glutamine, nonessential amino acids, antibiotics, and 10% fortified bovine calf serum (Hyclone, Logan, UT).
For metabolic labeling, [4,5-3H]l-leucine (1 mCi/ml) and [35S]l-cysteine, (10 mCi/ml) were purchased from ICN Biomedicals, Inc. (Irvine, CA) and Pro-mix l-[35S] in vivo cell labeling mix (14.3 mCi/ml) was purchased from Amersham Corp. (Arlington Heights, IL). Dialyzed FBS was purchased from Hyclone. Protease inhibitors, ε-amino-n-caproic acid, phenylmethylsulfonyl fluoride, ethylenediamine-tetraacetic acid, and N-ethylmaleimide were purchased from Sigma Chemical Co. (St. Louis, MO) and used in the lysis buffer at final concentrations of 10 mM, 2.5 mM, 5 mM, and 5 mM, respectively. Immune complexes were precipitated using a 1:1 slurry of protein A immobilized on Trisacryl (Pierce, Rockford, IL) in lysis buffer.
Reagents used during metabolic labeling included ALLN, BFA, monensin, and bafilomycin A1 (Sigma Chemical Co.). ALLN and BFA were both used at a final concentration of 10 μg/ml from 10 mg/ml stocks stored at −20°C in ethanol or DMSO, respectively. Monensin was prepared fresh as a 10-mM stock in ethanol and used at a final concentration of 10 μM. Bafilomycin A1 was stored at −20°C as 100 μM stock in DMSO and used at a final concentration of 1 μM. For chemical cross-linking, DSP and DSS were purchased from Pierce and made fresh as 0.1 M stocks in DMSO immediately before use.
Antibodies
For immunoprecipitation experiments and Western blot analysis, a monoclonal tropoelastin antibody, BA-4, raised to bovine α-elastin (Wrenn et al., 1986) and a polyclonal FKBP65 antipeptide antibody were used. For production of the FKBP65 antibody, a synthetic peptide to the COOH terminus of mouse FKBP65 (Coss et al., 1995) was prepared using FastMoc chemistry on an ABI 431A peptide synthesizer. The sequence of the peptide was confirmed using an ABI 473 protein sequencer. The peptide made was C-LKSDEDQERVHEEL and thus targets the same antigenic epitope as the Pep 4 antibody prepared by Coss and colleagues (Coss et al., 1995). Antibody production from the synthetic peptide was performed as previously described (Mariencheck et al., 1995). The antibody was used as an IgG fraction without further purification.
Metabolic Labeling and Chemical Cross-linking
Confluent monolayers of FBCs, grown in P-100 tissue culture plates, were washed with leucine-free or cysteine/methionine-free medium containing 5% dialyzed FBS and incubated in this medium for 1 h. Each dish of cells was metabolically labeled with either 50 μCi of [3H]leucine in 3 ml of leucine-free medium containing 5% dialyzed FBS for tropoelastin labeling, or 60 μCi of [35S]cysteine plus 60 μCi of [35S]Pro-mix in cysteine/methionine-free medium for labeling of cross-linked proteins. Any treatment of cells during the pulse with BFA, ALLN, monensin, or bafilomycin A1 is as indicated in the text and figure legends. After metabolic labeling, the medium was removed and cell layers were washed with room temperature PBS. To each dish, 1 ml of PBS was added and the cell layers scraped up, transferred to microfuge tubes, and pelleted at 1,000 g. The cell pellets were then resuspended in 100 μl of PBS and 2 μl of either DSP stock, DSS stock, or DMSO (carrier), was added. Cross-linking was carried out at room temperature with periodic gentle vortexing. After 45 min, 900 μl of PBS was added to each tube and the cross-linking reaction was quenched by the addition of 20 μl/ml of a 1-M glycine stock (pH 9.2). The samples were vortexed and left at room temperature for 10 min before the addition of 10 μl/ml of a 1-M glycine stock (pH 7.2) to lower the pH. After a wash in PBS, 1 ml of cold lysis buffer (50 mM Tris-HCl [pH 8.0], 150 mM NaCl, 1% NP-40) with protease inhibitors was added to each tube and the tubes were rotated at 4°C for 30 min. Cellular debris was pelleted by centrifugation and the cell lysates transferred to clean microfuge tubes for immunoprecipitation.
Sucrose Density Gradient Analysis
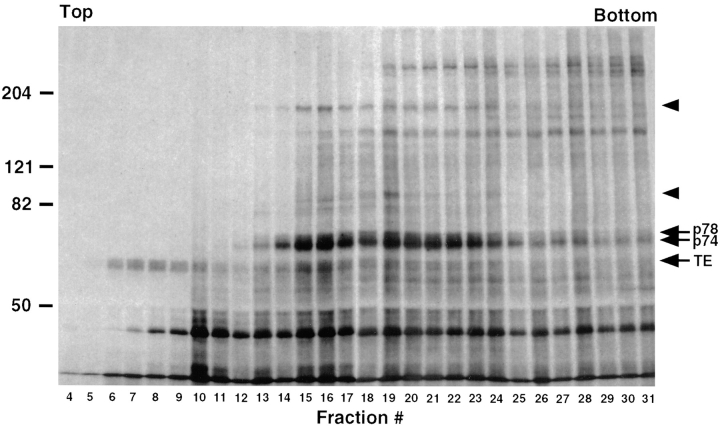
For analysis of DSP cross-linked complexes, two P-100 culture dishes of postconfluent FBCs were metabolically labeled with [35S]cysteine and [35S]Pro-mix for 18 h, chemically cross-linked with DSP, and then lysed as described above. The lysate (1 ml) was precleared by incubation with 10 μg/ml normal mouse IgG for 2 h followed by an additional hour with 25 μl of protein A immobilized on Trisacryl. The protein A–Trisacryl was pelleted by centrifugation and the lysate was layered over a 10-ml gradient of 5–25% sucrose prepared with a buffer of 50 mM Tris-HCl (pH 8.0) containing 150 mM NaCl and 0.1% Triton X-100. The gradient was spun in a Beckman SW-41 rotor at 288,000 g for 34 h at 4°C. After centrifugation, 32 fractions of 350 μl each were collected from the top of the gradient and immunoprecipitated for tropoelastin as described below.
Immunoprecipitation
Lysates were precleared by incubation with 10 μg/ml normal mouse IgG for 2 h followed by an additional hour with 25 μl of protein A immobilized on Trisacryl. The protein A–Trisacryl was pelleted by centrifugation and the supernatants transferred to clean tubes containing 100 μl of 50 mg/ml BSA in lysis buffer and 10 μg of BA-4 antibody or 50 μg of FKBP65 antibody. Lysates were incubated overnight at 4°C with gentle agitation. The following day, 40 μl of protein A–Trisacryl was added to each tube and incubated for 1 h at 4°C with gentle agitation. The immune complexes were pelleted and the pellets washed three times with 50 mM Tris-HCl, and 400 mM NaCl (pH 8.0), and one time with 10 mM Tris-HCl (pH 6.8). After the final wash, each pellet was resuspended in 35 μl of Laemmli sample buffer containing DTT and incubated at 100°C for 6 min. The samples were electrophoresed on SDS-polyacrylamide gels and then fixed and treated with EN3HANCE (NEN Research Products, Boston, MA) for 1 h for 3H-containing gels and 1 M salicylic acid in 15% methanol for 30 min for 35S-containing gels. Gels were then dried and exposed to Kodak XAR-5 X-ray film. Exposure times were ∼24 h for all gels except the sucrose density gradient (Fig. 3) that was exposed for 2 wk. All immunoprecipitation experiments were conducted a minimum of three times to ensure reproducibility of results. For densitometric quantitation, autoradiographic bands were scanned using NIH image. Each band for each of the three proteins was scanned four times with different sized areas to ensure accurate and consistent measurements. The same four areas were used for each experimental condition. The average of the four measurements was plotted.
Figure 3.
Sedimentation analysis of DSP cross-linked protein complexes. Cells were labeled with [35S]cysteine/ methionine for 18 h, cross-linked with DSP, lysed, and the lysate fractionated over a 5–25% sucrose density gradient. Fractions were immunoprecipitated for tropoelastin and analyzed by SDS-PAGE under reducing conditions and fluorography (2 wk exposure). Two pools of tropoelastin are evident; a population not cross-linked by DSP (fractions 6–10) and a second population cross-linked to p74 and p78 (fractions 14–24). Note that two additional bands are seen to codistribute with the tropoelastin-p74–p78 in the center of the gradient (arrowheads).
Western Blot Analysis
After electrophoresis, cross-linked samples were transferred to nitrocellulose and nonspecific-binding sites were blocked with 5% dry milk in TBS (20 mM Tris base, 137 mM NaCl [pH 7.6]) containing 0.1% Tween-20 overnight at 4°C. All remaining rinses, washes, and antibody dilutions were in 2% dry milk, 0.05% Tween-20 in TBS. The next day, the blot was rinsed and incubated in primary antibody for 1 h. After two quick rinses and 1 × 15 min and 2 × 5 min washes, the blot was then incubated for 1 h with HRP-linked sheep anti–mouse F(ab′)2 (Amersham Corp.) for detection of tropoelastin, or HRP-linked donkey anti–rabbit F(ab′)2 for detection of FKBP65. After a wash, as described above, and final rinse in TBS, the blot was incubated with enhanced chemiluminescence (ECL) detection reagents (Amersham Corp.) according to the manufacturer's directions.
Cyanogen Bromide Treatment and Microsequencing
To obtain sufficient protein for microsequencing, tropoelastin was immunoprecipitated from FBCs in six P-100 culture dishes after cross-linking with DSP, as described above. The sample was loaded in a single lane, electrophoresed, and transferred to ProBlott membrane (Applied Biosystems Inc., Foster City, CA) according to the manufacturer's directions. After the transfer, the membrane was rinsed in several changes of distilled water, stained with 0.1% Coomassie blue R-250 in 50% methanol for 2 min, and destained with several changes of 50% methanol containing 10% acetic acid. The membrane was then rinsed with several changes of distilled water and air dried. Each band to be sequenced was excised from the dry membrane, cut into 2–3 mm2 pieces and placed in a microfuge tube. To each tube, 150 μl of 150 mM cyanogen bromide (CNBr) in 70% (vol/vol) formic acid was added and the tubes were incubated overnight in the dark at room temperature. Excess reagent was removed by vacuum centrifugation, and when dry, the slices were washed with 50 μl deionized water by vortexing and redried. The dried pieces of the blot were then loaded into the reaction chamber of an ABI 473 protein sequencer for sequence analysis. Sequence obtained from the CNBr digest was confirmed with the o-phthalaldehyde (OPA) blocking technique (Brown-Augsburger et al., 1995), a way of sequencing a single peptide in a peptide mixture.
Results
To identify molecular chaperones that assist in the secretion and assembly of tropoelastin, FBCs were chosen because these cells continue to synthesize and secrete tropoelastin monomers when established in primary culture. Unlike many elastogenic cell types, FBCs in culture also assemble the monomers into an insoluble extracellular elastic fiber (Lee et al., 1994). It is hypothesized, therefore, that within these cells, the protein must associate with an appropriate series of folding and trafficking chaperones to allow for ultimate assembly outside the cell.
Tropoelastin Is Cross-linked into Higher Molecular Weight Complexes by DSS
Because tropoelastin is rapidly secreted (Davis and Mecham, 1996), initial experiments were conducted with BFA to accumulate the protein within the cell and allow for maximal interaction with potential molecular chaperones. In all instances, the cysteine protease inhibitor ALLN was included with BFA to inhibit the selective degradation of tropoelastin that occurs as a result of its retention in the fused ER/Golgi compartment (Davis and Mecham, 1996). With an abundance of leucine residues, and only two cysteines and no methionines, [3H]leucine was used to metabolically label tropoelastin in FBCs for 4 h in the presence of BFA and ALLN. After cross-linking with DSP, immunoprecipitation of tropoelastin from the cell lysate revealed one wide band at ∼70 kD upon separation by SDS-PAGE under reducing conditions (Fig. 1 A). Two isoforms of tropoelastin are expressed by these cells, thus the wide band actually represents a doublet (see Figs. 4 A and 6). When the nonreducible cross-linker DSS was used, the tropoelastin band was reduced in density and four additional high molecular weight bands were evident (Fig. 1 A). To determine if these bands represented tropoelastin monomers cross-linked by DSS into higher molecular weight complexes, the experiment was repeated under nonradioactive conditions and the proteins were transferred and probed for tropoelastin by Western analysis. As seen in Fig. 1 B, the identical pattern of four bands was revealed in the DSS sample, whereas no immunoreactive bands for tropoelastin were observed in the DSP sample.
Figure 1.
Cross-linking of tropoelastin into higher molecular weight complexes by DSS treatment. (A) Intact FBCs labeled for 4 h with [3H]leucine in the presence of BFA and ALLN were cross-linked with either DSP or DSS and then lysed and immunoprecipitated for tropoelastin. Analysis by SDS-PAGE under reducing conditions showed higher molecular weight complexes in the DSS-treated cells (arrows) and a reduced intensity of tropoelastin monomer at 70 kD (TE). (B) FBCs treated for 4 h with BFA and ALLN were cross-linked, lysed, and immunoprecipitated for tropoelastin. After SDS-PAGE, the proteins were transferred to nitrocellulose and blotted for tropoelastin. Immunoreactive bands in the DSS lane (arrows) indicate nonreducible cross-linked complexes of tropoelastin with other proteins. (PC) pre-clear.
Figure 4.
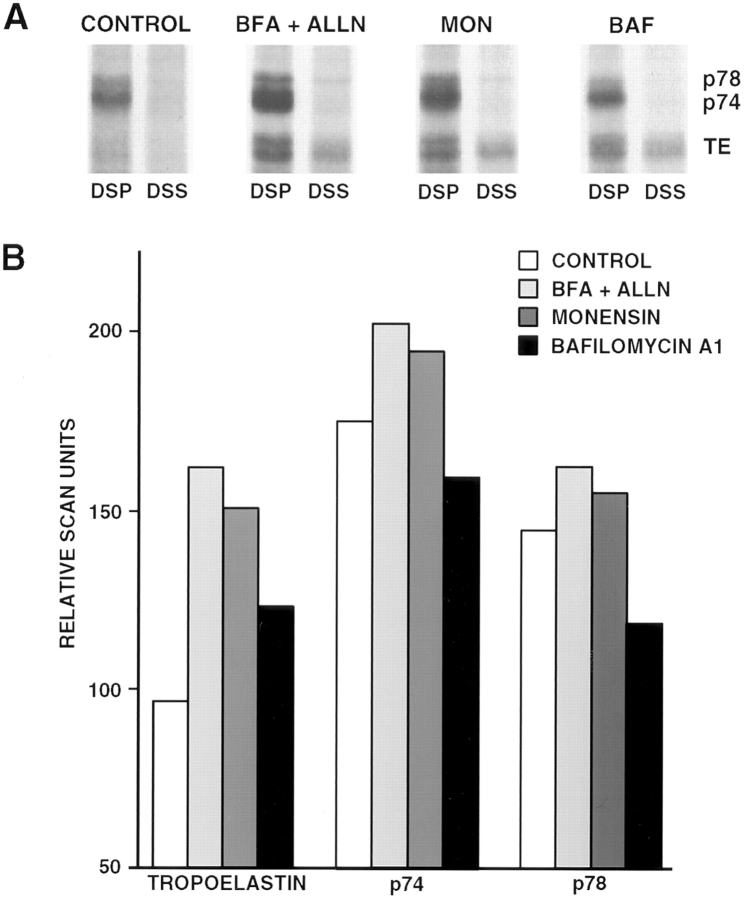
Effect of secretion-disrupting agents on the association of p74 and p78 with tropoelastin. (A) FBCs were metabolically labeled with [35S]cysteine/methionine for 16 h with either no treatment (control) or treatment with BFA + ALLN, monensin, or bafilomycin A1 for the final 4 h of the labeling period. After cross-linking with either DSP or DSS, the cells were lysed and immunoprecipitated for tropoelastin. Samples were analyzed by SDS-PAGE under reducing conditions. A concurrent increase in p74 and p78 was observed along with tropoelastin in cells treated with either BFA + ALLN or monensin. No increase in p74 and p78 was observed in bafilomycin A1-treated cells as compared to control cells despite an increase in tropoelastin. (B) Densitometric scanning of the bands in A confirm the lack of increased p74 and p78 coimmunoprecipitation in bafilomycin A1-treated cells. Note that the amount of p74 and p78 decreases as the secretion block moves progressively farther along the secretory pathway.
Figure 6.
Bovine FKBP65 is cross-linked to tropoelastin in the secretory pathway of FBCs. FBCs were treated with BFA + ALLN for 4 h before cross-linking with either DSP or DSS. Cell lysates were immunoprecipitated for either tropoelastin or FKBP65, separated by SDS-PAGE under reducing conditions, transferred, and blotted for either the same protein or the opposite protein. Lane 2 shows that FKBP65 can be detected in the cross-linked cell lysate after immunoprecipitation with tropoelastin antibody and lane 5 shows that tropoelastin can be detected in the FKBP65 immunoprecipitation after cross-linking. Note that when the nonreducible cross-linker is used (lane 3), FKBP65 is detected in a higher molecular weight complex that must also contain tropoelastin (arrowhead). This experiment was designed to maximize the ability to detect the cross-linked proteins and should not be interpreted in a quantitative manner because the amount of cell lysate used was increased threefold for conditions where the blotting antibody was different than that used for immunoprecipitation. The ECL exposure times were also adjusted as follows: lanes 1–3, ∼30 s; lane 4, ∼30 s after waiting 2 h for the signal to decay; lane 5, ∼2 min.
Metabolic Labeling with [35S]Cysteine/Methionine Reveals Two Proteins Cross-linked to Tropoelastin in the Secretory Pathway
Although tropoelastin was detected in high molecular weight complexes after cross-linking with DSS, no additional protein(s) were evident on the autoradiograph in the DSP cross-linked sample. It was assumed, therefore, that the protein(s) to which tropoelastin was cross-linked was not efficiently labeled. For this reason, the experiment was repeated using [35S]cysteine/methionine as the radiolabel. To ensure that the cross-linking of tropoelastin to another protein in the secretory pathway was not a brefeldin A–induced event, the experiment was first conducted in the absence of any secretion-disrupting drug. After cross-linking with DSP, cell lysates immunoprecipitated for tropoelastin demonstrated the presence of a thin, faint band at 78 kD (p78) and a more prominent band at 74 kD (p74) (Fig. 2 A). The band of tropoelastin at 70 kD was barely discernible because tropoelastin is not well labeled by [35S]cysteine/methionine. In the cell lysate from the DSS cross-linked sample, no bands at these molecular weights were observed, indicating that p74 and p78 were cross-linked to tropoelastin in higher molecular weight complexes.
Figure 2.
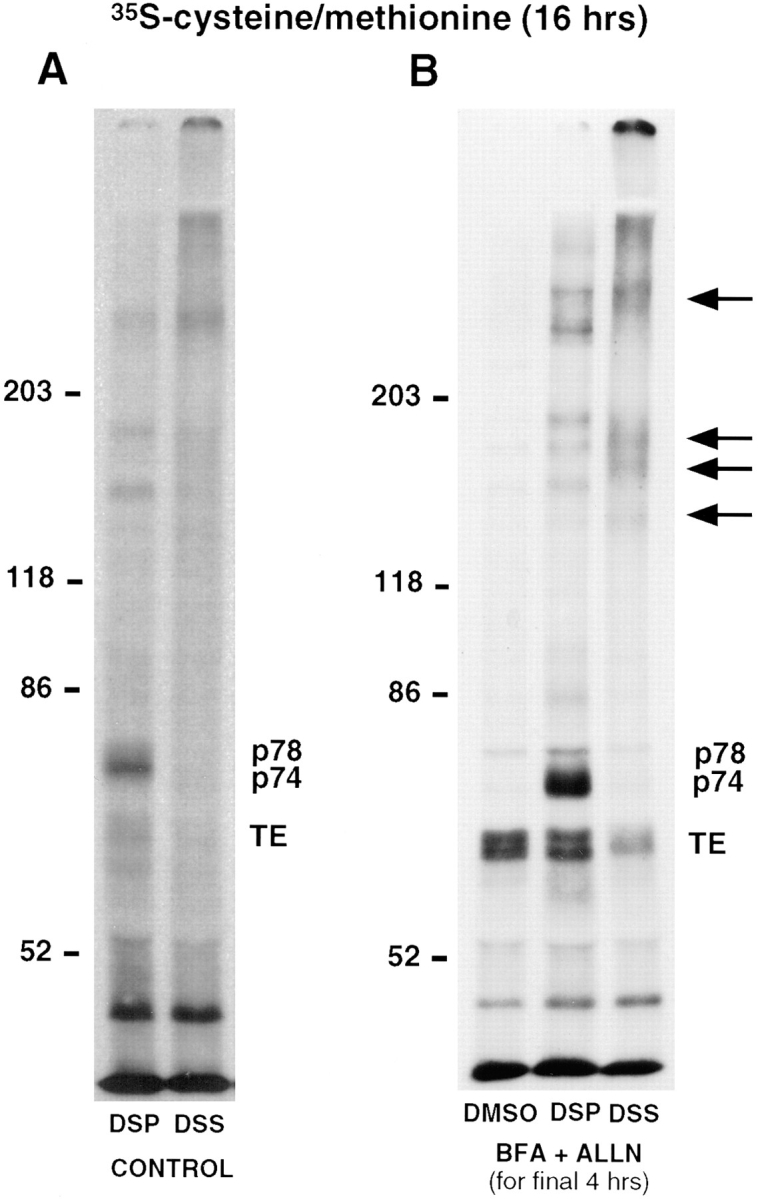
35S-labeling allows the identification of two proteins that are cross-linked to tropoelastin. FBCs were labeled with [35S]cysteine/methionine for 16 h and (A) either left untreated (CONTROL) or (B) treated with BFA and ALLN (BFA + ALLN) for the final 4 h of the labeling period. After metabolic labeling, intracellular proteins were cross-linked with either DSP or DSS, lysed, and immunoprecipitated for tropoelastin. One of the BFA + ALLN samples was treated with an equivalent concentration of DMSO alone (vehicle) rather than cross-linker. Analysis by SDS-PAGE under reducing conditions showed the presence of two additional bands in the DSP-treated cells (p74 and p78). These bands were greatly enhanced after BFA-treatment. In the DSS cross-linked lysates, high molecular weight bands in the same position as were seen in Fig. 1 were observed (arrows), and as expected, the immunoprecipitated bands of tropoelastin (TE), p74 and p78, were all reduced in density. Note that p78 coimmunoprecipitates with tropoelastin in the absence of cross-linker.
In these experiments, tropoelastin was continually being secreted during the 16-h labeling period, so the amount of radiolabeled tropoelastin within the cell would be constant and its association with any chaperone protein would be relatively short-lived in control (untreated) cells. To retain more tropoelastin inside the cell and potentially enhance the association of tropoelastin with p74 and p78, FBCs were labeled for 16 h with [35S]cysteine/methionine and treated with BFA and ALLN during the final 4 h of the labeling period. Under these conditions, a prominent band of tropoelastin was observed in the DSP cross-linked cell lysates together with increased amounts of p74 and p78 (Fig. 2 B). This result suggests that the p74 and p78 proteins are present with tropoelastin in the fused ER/Golgi compartment. As expected with the nonreducible cross-linker DSS, the tropoelastin band was reduced in amount and higher molecular weight bands of cross-linked proteins similar to those seen in Fig. 1 were observed. In addition, no p74 was observed in the DSS cross-linked lysate, indicating that all of this protein was immunoprecipitated by being cross-linked to tropoelastin. A small amount of p78, however, was evident suggesting that some p78 could be coimmunoprecipitated with tropoelastin without being directly cross-linked to it. This is supported by the observation that a similar amount of p78 is seen in cell lysates incubated with DMSO alone (Fig. 2 B).
Sedimentation Analysis of the DSP Cross-linked Proteins
To investigate the nature of the tropoelastin complex and associated proteins, a DSP cross-linked lysate was sedimented over a 5–25% sucrose density gradient. Fractions were collected, immunoprecipitated for tropoelastin, and separated on a reducing gel. Fig. 3 shows that a considerable proportion of tropoelastin was present in the light fractions. This indicates that a population of intracellular tropoelastin was either not associated with any other protein in the cell or unaffected by this particular type of cross-linker. This result is consistent with the presence of a band of tropoelastin in immunoprecipitations of DSS cross-linked lysates (Fig. 2 B). Although only weakly labeled by the [35S]cysteine/methionine, tropoelastin was also observed in the center of the gradient together with p74 and p78. In addition, the sedimentation analysis showed several other bands that distributed in the same fractions. Increasing evidence suggests that BiP may be part of a set of resident ER chaperones that complex together with secretory proteins as they fold and assemble in the ER (Lin et al., 1993; Nigam et al., 1994; Kuznetsov et al., 1997). This “folding complex” is reported to include BiP, ERp72, grp94, and grp170. The molecular weights of two of the bands that cosediment with the tropoelastin-p74–p78 complex are similar to those expected for the two ER chaperones, grp94 and grp170. The identity of these proteins, however, remains to be determined. Note that the cells for this experiment were not treated with BFA and ALLN at the end of the labeling period. This provides evidence that the association of p74 and p78 with tropoelastin occurs under normal conditions of tropoelastin trafficking and is not a consequence of BFA-induced accumulation of tropoelastin in the fused ER/Golgi compartment.
The Association of Tropoelastin with p74 and p78 Occurs within Early Compartments of the Secretory Pathway
The association of p74 and p78 with tropoelastin as it travels through the secretory pathway was investigated by using several different secretion disrupting agents to block the path of tropoelastin in various compartments within the cell during the final 4 h of the labeling period. In control cells, a strong band of p74 and a diffuse band of p78 were observed in the DSP cross-linked sample despite the tropoelastin band being barely evident (Fig. 4 A). This result is consistent with Fig. 2 A and confirms that the association of tropoelastin with p74 and p78 occurs under normal conditions when the trafficking of tropoelastin is unimpeded. When BFA was used together with ALLN, there was a concurrent increase in both the amount of tropoelastin and associated proteins, p74 and p78, in the DSP cross-linked cells as compared to untreated cells. Monensin, which blocks transport of proteins from the cis to trans side of the Golgi (Tartakoff, 1983), also resulted in a proportional increase in both tropoelastin and the associated proteins as determined by coimmunoprecipitation. Unlike brefeldin A, monensin does not cause retrograde fusion of the Golgi cisternae with the ER and thus the results obtained indicate that the association of tropoelastin with p74 and p78 is not induced by the brefeldin A alteration of cell organelle architecture. In contrast to brefeldin A and monesin, treatment of cells with bafilomycin A1 for the final 4 h of the pulse did not enhance the amount of p74 or p78 that was cross-linked to tropoelastin over that obtained in control cells despite more tropoelastin being immunoprecipitated under these conditions. This observation was confirmed by densitometric scanning of the radiolabeled bands (Fig. 4 B). Because bafilomycin A1 results in the accumulation of secreted proteins at the level of the trans-Golgi network (Henomatsu et al., 1993), these results suggest that both p74 and p78 are localized in early compartments of the secretory pathway. Note that the use of ALLN in the monensin and bafilomycin A1 incubations was not necessary, as tropoelastin readily accumulates in the Golgi without any apparent degradation by the ER- associated, ALLN-sensitive mechanism (Davis, E.C., and R.P. Mecham, manuscript submitted for publication).
Microsequence Analysis Identifies p78 as BiP and p74 as Bovine FKBP65
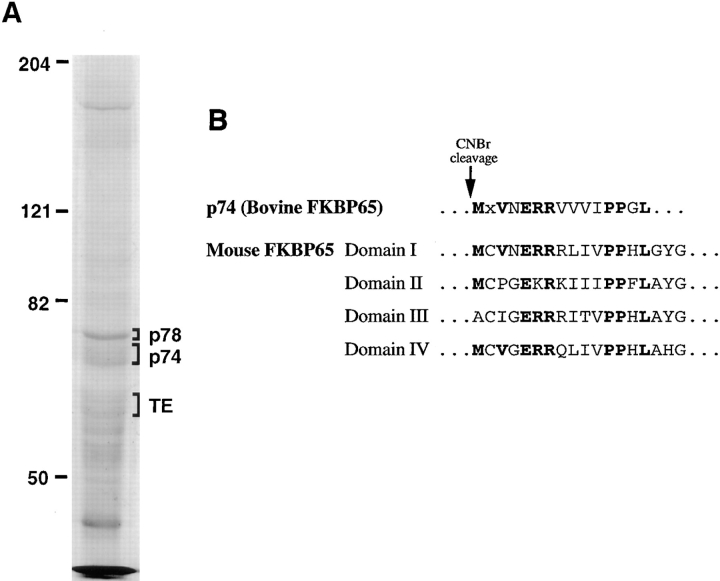
The DSP cross-linked lysate was scaled up approximately sixfold to obtain sufficient protein for microsequence analysis. After immunoprecipitation with the tropoelastin antibody, proteins were separated by SDS-PAGE and transferred to PVDF membrane. After staining with Coomassie blue R-250, the p78 and p74 bands were easily recognized. The p74 band, however, was diffuse and often appeared as two bands (Fig. 5 A). Initial attempts to sequence protein from these bands failed due to a blocked amino terminus, most likely the consequence of the cross-linking reagent interacting with the α amino group. To obtain internal sequence, the p74 and p78 bands were cut from the blot and the proteins were cleaved in situ with CNBr. The resulting peptide mixture was then sequenced and, as expected, multiple sequences were obtained for both proteins. For p78, the definitive location of proline residues in the peptide mixture was determined. Using additional bands of CNBr-cleaved p78, the mixture was then sequenced again until a proline residue was exposed at the amino terminus. OPA was then used to selectively block the primary amines at the ends of each peptide in the mixture. Because OPA does not react with secondary amines, the peptide with the NH2-terminal proline remained unblocked and could be independently sequenced (Brown-Augsburger et al., 1995). The resulting sequences from several proline-containing peptides sequenced in this manner allowed the identification of p78 as the ER chaperone, BiP.
Figure 5.
Microsequencing identifies p78 as BiP and p74 as bovine FKBP65. (A) FBCs were treated with BFA + ALLN for 4 h, cross-linked with DSP, lysed and immunoprecipitated using an antibody to tropoelastin. Samples were separated by SDS-PAGE, transferred to PVDF membrane, and stained with Coomassie blue. Areas indicated on the right were cut from the blot and treated with CNBr before sequencing. Unique sequences from p78 obtain following OPA treatment identified p78 as BiP. Separate sequencing of the two p74 bands following CNBr digestion showed that both bands were the same protein. (B) Alignment of the amino acid sequence from a portion of each of the four PPIase domains in mouse FKBP65 as reported by Coss and colleagues (1995) with the sequence determined for p74 after CNBr treatment. Note the high degree of homology between the PPIase domains (bold) that allows a definitive sequence for p74 to be obtained from the peptide mixture.
Independent microsequencing after CNBr cleavage of each of the two bands that form the p74 doublet showed that both bands were the same protein. Remarkably, when the CNBr digest of p74 was sequenced, a clear single sequence was distinguished against a minor background of residues, even though the digest contained a peptide mixture. The derived sequence was used to search GenBank/ EMBL/DDBJ and a high degree of homology was obtained with the peptidyl-prolyl cis–trans isomerase (PPIase) domains of the mouse FK506-binding protein, FKBP65 under accession number L07063 (Coss et al., 1995). From the sequence of mouse FKBP65, it was evident that the strong sequence that was obtained for p74 was a result of the high degree of homology between the four PPIase domains in FKBP65 (Fig. 5 B).
Tropoelastin Is Cross-linked to FKBP65 in the Secretory Pathway of FBCs
To detect FKBP65 in FBCs and investigate its association with tropoelastin, a peptide antibody was made to the COOH terminus of the protein using the published sequence of mouse FKBP65 (Coss et al., 1995). The association of the two proteins was investigated by Western analysis after cross-linking and immunoprecipitation. Immunoblotting of DSP cross-linked lysates with the FKBP65 antibody after immunoprecipitation with the same antibody showed the doublet of FKBP65 at 74 kD and an additional band at 100 kD (Fig. 6). The appearance of FKBP65 as a doublet is consistent with the studies of Coss and colleagues (1995). The identity of the 100-kD protein that cross-reacts with the antibody is unknown. When immunoprecipitation of DSP cross-linked lysates was carried out with the tropoelastin antibody and subsequently blotted for FKBP65, a prominent wide band was observed at a molecular weight identical to that seen by blotting with the FKBP65 antibody. In contrast, if the nonreducible cross-linker DSS was used, the 74-kD band was absent and a weak band of immunoreactivity was observed at a higher molecular weight (Fig. 6). This result provides direct evidence that tropoelastin is associated with FKBP65 in the secretory pathway. The ability of the FKBP65 antibody to coimmunoprecipitate tropoelastin with FKBP65 after cross-linking was verified by the detection of a tropoelastin doublet in DSP cross-linked lysates following FKBP65 immunoprecipitation (Fig. 6). Because only that amount of tropoelastin in association with FKBP65 would be immunoprecipitated by the FKBP65 antibody following cross-linking, a relatively weak signal was expected as compared to when tropoelastin was blotted from a tropoelastin immunoprecipitation. To get a strong enough signal, therefore, the amount of cells used was increased and the ECL times extended for blots probed with antibodies that differ from those used for immunoprecipitation (Fig. 6, lanes 2, 3, and 5). When the nonreducible cross-linker DSS was used instead of DSP and the cell lysate immunoprecipitated with the FKBP65 antibody, no immunoreactive tropoelastin could be detected on the blot (not shown). This was expected as the already weak tropoelastin signal would likely be distributed in several areas of high molecular weight due to the various complexes formed among tropoelastin, FKBP65, and other proteins.
Discussion
The present study has identified BiP and FKBP65 as molecular chaperones for tropoelastin in the secretory pathway of FBCs using chemical cross-linking in intact cells. Although in some experiments brefeldin A was used to retain the tropoelastin within the cell and enhance the tropoelastin–chaperone interactions, the association of BiP and FKBP65 with tropoelastin was also shown to occur in untreated cells. This observation demonstrates that the tropoelastin–chaperone interactions are physiologically significant and not a result of distorting the secretory pathway or disrupting the transport of secreted proteins.
The association of BiP with a secretory protein in the ER is not surprising. This observation does, however, provide validity for the cross-linking technique because BiP and tropoelastin would be in the same compartment during the synthesis of tropoelastin and, based on the properties of BiP as a molecular chaperone (Gething and Sambrook, 1992) and the hydrophobic nature of tropoelastin, it would be highly probable that the two proteins would interact. The association of tropoelastin with FKBP65 is a novel finding and represents the first ligand to be identified for an FK506-binding protein in the secretory pathway. FKBP65 was initially cloned and sequenced from a JB6 murine epidermal cell cDNA expression library (Coss et al., 1995) and, to date, represents the sixth FKBP to be identified. Sequence analysis predicts the protein to contain a potential membrane-spanning region and four PPIase signature domains. FKBP65 was also shown to be a glycoprotein and to contain a putative signal sequence at its amino terminus. A potential ER retention sequence at the COOH terminus (Coss et al., 1995) predicts that FKBP65 is located in the ER. The association of FKBP65 with tropoelastin, a soluble secreted protein, provides indirect evidence to support this localization. The extent to which FKBP65 may move from the ER to other compartments along the secretory pathway remains to be determined.
Although the predicted molecular mass of FKBP65 is 64,683 D based on the open reading frame of the clone and 60,576 after cleavage of the predicted NH2-terminal signal sequence (Coss et al., 1995), immunoprecipitation and Western blotting of bovine FKBP65 revealed a doublet that migrated at ∼74 kD by SDS-PAGE. This result is consistent with the findings of Coss and colleagues (1995) who suggest that the reason for the decrease in mobility after SDS-PAGE, results from glycosylation of the protein and unique protein structure resulting from nine proline– proline sequences. The reason for migration of the protein as two bands is more uncertain. The upper band was identified to be phosphorylated, however, and thus could account for at least part of the difference (Coss et al., 1995).
The biological role in the ER of the two FKBP PPIases, FKBP13 (Nigam et al., 1993) and FKBP65, is largely unknown. Because PPIases are known to catalyze the otherwise slow conversion between cis- and trans-isomers of peptidyl–prolyl bonds in peptides and proteins, they have been assigned the general function of molecular chaperones in that they likely assist in protein folding. Until our identification of an association between FKBP65 and tropoelastin, however, no known ligand for these proteins had been identified. Although PPIase activity of an FKBP on a ligand in the ER has not been demonstrated, isomerization of cis- to trans-peptide bonds by a member of the cyclophilin family of PPIases, cyclophilin B (Smith et al., 1995), has been shown to be critical for the formation of the triple helical structure of procollagen. In nascent type I collagen, it has been estimated that 16% of the X-proline and 8% of the X-hydroxyproline bonds are in the cis-conformation (Sarkar et al., 1984). In order for collagen to assemble, all of these bonds must be converted to the trans-conformation. The necessity of this conformational change was demonstrated by the ability of cyclosporin A, which competes with procollagen for cyclophilin B, to disrupt the stability, folding, and assembly of collagen (Steinmann et al., 1991; Bächinger et al., 1993). Interestingly, FKBP65 shows a restricted tissue expression (Coss et al., 1995) that is in contrast to the ubiquitous expression of all other FKBPs. This observation suggests that FKBP65 may have a specific role in certain cell types rather than generalized function as a PPIase.
A role for a protein isomerase in the folding of tropoelastin is attractive, based on the fact that ∼12% of the residues in tropoelastin are proline. In contrast to procollagen, little is known concerning the conformational state of these X-proline bonds. The proline residues in tropoelastin are, however, in domains within the primary sequence that are important for secondary and tertiary structure of the molecule. In many of these sequences, proline residues are found at the corner of a β turn that is stabilized by a hydrogen bond between the C-O of residue 1 and the N-H of residue 4 (Urry, 1983). In order for this hydrogen bond to form, the proline must be in the trans-configuration. One important proline-containing sequence is a pentapeptide, GVGVP, that repeats 11 times near the center of bovine tropoelastin (Indik et al., 1990). The repeating β turns formed by the pentapeptide sequences stack into a β spiral that itself has interesting mechanical properties (Urry et al., 1995). By disrupting the β turn substructure, a single cis-proline could alter the formation of the β spiral and hence prevent the proper folding of tropoelastin or alter its elastomeric properties.
Other regions of tropoelastin where PPIase activity may be important are cross-linking domains that consist of lysine pairs separated by one or more proline residues and flanked by additional prolines. Because the presence of proline residues between and around the lysine residues destined for cross-linking would place steric restrictions on the conformation of the cross-linking domains, the activity of a PPIase on the bonds associated with these prolines may be of considerable importance for the formation of the cross-links. On a larger scale, the cis–trans configuration of the X-proline bonds may ultimately play a role in the overall folding of each monomer, such that cross-linking domains between monomers are aligned.
Whereas the importance of a PPIase to act as a proline isomerase is obvious, increasing evidence suggests that these enzymes may play additional, or alternative, roles in the cell. In Drosophila, the ninaA gene codes for an ER localized cyclophilin homologue that is specific for photoreceptor cells. Despite the presence of a potential ER retention signal, ninaA colocalizes with its substrate, Rh1 rhodopsin, in both the ER and in postGolgi secretory vesicles (Colley et al., 1991). Because mutations in the ninaA gene block the transport of Rh1 rhodopsin to the cell surface and lead to an accumulation of the protein in the ER, an intracellular trafficking function for ninaA has been suggested. FKBP52 (also called Hsp56) has also been implicated in a trafficking or targeting event. This PPIase is present in the cytosol where it binds to Hsp90 and forms a heterocomplex with untransformed glucocorticoid receptors (Czar et al., 1994; Owens-Grillo et al., 1995). The role played by FKBP52 in steroid receptor action is uncertain, particularly because PPIase activity is not required for heterocomplex formation nor for proper folding of the hormone-binding domain (Owens-Grillo et al., 1995). Several lines of evidence, however, including nuclear localization of FKBP52, have suggested that FKBP52 functions to target steroid receptor movement to the nucleus rather than acting as an enzyme (Pratt et al., 1993; Owens-Grillo et al., 1996). In light of these studies, it will now be important to determine the extent to which FKBP65 plays a role in the trafficking of tropoelastin through post-ER compartments and in the ultimate assembly of the elastic fiber in the extracellular matrix.
An interesting question is how FKBP65 relates to the 67-kD elastin-binding protein (EBP67) described in earlier studies. EBP67 was originally characterized as a component of a protein complex thought to play a role in elastic fiber assembly on the cell surface (Wrenn et al., 1988). To date, the identity of this protein remains unclear. Candidate proteins include the 67-kD high affinity laminin receptor, galactosyltransferase, 5′ nucleotidase, and several others (for review see Mecham and Hinek, 1996). More recently, Hinek et al. (1993, 1994, 1995) have suggested that a catalytically inactive alternatively spliced form of β-galactosidase is the 67-kD elastin-binding protein and that this protein functions as an intracellular chaperone. They propose that this galactosidase variant transports tropoelastin through the secretory pathway, mediates elastic fiber assembly at the plasma membrane, and then recycles back to the intracellular endosomal compartment where it reassociates with newly synthesized tropoelastin and shuttles it to the cell surface (Hinek et al., 1995). There are fundamental differences between FKBP65 and the 67-kD protein described by Hinek et al. (1995), however, that support separate roles for these proteins within the cell. FKBP65 appears to be localized to early compartments of the secretory pathway consistent with the presence of a putative ER retention signal. It also does not contain a lectin-like domain that could interact with galactose-containing glycoconjugates. Furthermore, there is no evidence that FKBP65 acts to shuttle any protein to the cell surface and may function solely as a folding chaperone in the ER.
The experiments in the present study were originally designed to investigate whether a “substrate-specific” chaperone for tropoelastin is present in cells committed to the production of elastic fiber components. It seems unlikely that FKBP65 is specific for tropoelastin, as recent data has shown several high molecular weight bands that coimmunoprecipitate with FKBP65 even in the absence of cross-linker (Davis, E.C., unpublished observation). Whether these bands represent additional substrates for FKBP65 or other molecular chaperones has yet to be determined. Of particular interest will be to determine if FKBP65 is part of a “chaperone complex” or whether it acts independently to assist in the folding and/or trafficking of tropoelastin.
Acknowledgments
The authors thank David Schettler and Clarina Tisdale (Washington University, St. Louis, MO) for cell culture assistance. E.C. Davis was an American Lung Association Research Fellow during initial phases of this work.
This work was supported by National Institutes of Health grants (HL 41926, HL 53325, and AR 41474) to R.P. Mecham and by a Beginning Grant-in-Aid from the American Heart Association - Missouri Affiliate to E.C. Davis.
Abbreviations used in this paper
- ALLN
N-acetyl-leu-leu-norleucinal
- BFA
brefeldin A
- CNBr
cyanogen bromide
- DSP
dithiobis-(succinimidyl propionate)
- DSS
disuccinimidyl suberate
- ECL
enhanced chemiluminescence
- FBCs
fetal bovine chondrocytes
- OPA
o-phthalaldehyde
- PPIase
peptidyl-prolyl cis–trans isomerase
Footnotes
Address all correspondence to Elaine C. Davis, Department of Cell Biology and Neuroscience, The University of Texas Southwestern Medical Center, 5323 Harry Hines Boulevard, Dallas, TX 75235-9039. Tel.: (214) 648-2319. Fax: (214) 648-8694. E-mail: davis16@utsw.swmed.edu
References
- Bächinger HP, Morris NP, Davis JM. Thermal stability and folding of the collagen triple helix and the effects of mutations in osteogenesis imperfecta on the triple helix of type I collagen. Am J Med Genet. 1993;45:152–162. doi: 10.1002/ajmg.1320450204. [DOI] [PubMed] [Google Scholar]
- Brown-Augsburger P, Tisdale C, Broekelmann T, Sloan C, Mecham RP. Identification of an elastin cross-linking domain that joins three peptide chains. J Biol Chem. 1995;270:17778–17783. doi: 10.1074/jbc.270.30.17778. [DOI] [PubMed] [Google Scholar]
- Clarke EP, Jain N, Brickenden A, Lorimer IA, Sanwal BD. Parallel regulation of procollagen I and colligin, a collagen-binding protein and a member of the serine protease inhibitor family. J Cell Biol. 1993;121:193–199. doi: 10.1083/jcb.121.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colley NJ, Baker EK, Stamnes MA, Zuker CS. The cyclophilin homolog ninaA is required in the secretory pathway. Cell. 1991;67:255–263. doi: 10.1016/0092-8674(91)90177-z. [DOI] [PubMed] [Google Scholar]
- Coss MC, Winterstein D, Sowder RC, Simek SL. Molecular cloning, DNA sequence analysis, and biochemical characterization of a novel 65-kDa FK506-binding protein (FKBP65) J Biol Chem. 1995;270:29336–29341. doi: 10.1074/jbc.270.49.29336. [DOI] [PubMed] [Google Scholar]
- Cox BA, Starcher BC, Urry DW. Coacervation of tropoelastin results in fiber formation. J Biol Chem. 1974;249:997–998. [PubMed] [Google Scholar]
- Czar MJ, Owens-Grillo JK, Dittmar KD, Hutchison KA, Zacharek AM, Leach KL, Deibel MR, Pratt WB. Characterization of the protein-protein interactions determining the heat shock protein (hsp90-hsp70-hsp56) heterocomplex. J Biol Chem. 1994;269:11155–11161. [PubMed] [Google Scholar]
- Davis EC, Mecham RP. Selective degradation of accumulated secretory proteins in the endoplasmic reticulum: A possible clearance pathway for abnormal tropoelastin. J Biol Chem. 1996;271:3787–3794. [PubMed] [Google Scholar]
- Galat A. Peptidylproline cis-trans-isomerases: immunophilins. Eur J Biochem. 1993;216:689–707. doi: 10.1111/j.1432-1033.1993.tb18189.x. [DOI] [PubMed] [Google Scholar]
- Galat A, Lane WS, Standaert RF, Schreiber SL. A rapamycin-selective 25-kDa immunophilin. Biochemistry. 1992;31:2427–2434. doi: 10.1021/bi00123a031. [DOI] [PubMed] [Google Scholar]
- Gething M-J, Sambrook J. Protein folding in the cell. Nature. 1992;355:33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- Grosso L, Mecham RP. In vitro processing of tropoelastin: investigation of a possible transport function associated with the carboxy-terminal domain. Biochem Biophys Res Commun. 1988;151:822–826. doi: 10.1016/s0006-291x(88)81129-x. [DOI] [PubMed] [Google Scholar]
- Harding MW, Galat A, Uehling DE, Schreiber SL. A receptor for the immunosuppressant FK506 is a cis-transpeptidyl-prolyl isomerase. Nature. 1989;341:758–760. doi: 10.1038/341758a0. [DOI] [PubMed] [Google Scholar]
- Henomatsu N, Yoshimori T, Yamamoto A, Moriyama Y, Tashiro Y. Inhibition of intracellular transport of newly synthesized prolactin by bafilomycin A1 in a pituitary tumor cell line, GH3cells. Eur J Cell Biol. 1993;62:127–139. [PubMed] [Google Scholar]
- Hinek A, Rabinovitch M. 67-kD elastin-binding protein is a protective “companion” of extracellular insoluble elastin and intracellular tropoelastin. J Cell Biol. 1994;126:563–574. doi: 10.1083/jcb.126.2.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinek A, Keeley FW, Callahan J. Recycling of the 67-kDa elastin binding protein in arterial myocytes is imperative for secretion of tropoelastin. Exp Cell Res. 1995;220:312–324. doi: 10.1006/excr.1995.1321. [DOI] [PubMed] [Google Scholar]
- Hinek A, Rabinovitch M, Keeley F, Okamura-Oho Y, Callahan J. The 67-kD elastin/laminin-binding protein is related to an enzymatically inactive, alternatively spliced form of beta-galactosidase. J Clin Invest. 1993;91:1198–1205. doi: 10.1172/JCI116280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indik, Z., H. Yeh, N. Ornstein-Goldstein, and J. Rosenbloom. 1990. Structure of the elastin gene and alternative splicing of elastin mRNA. In Genes for Extracellular Matrix Proteins. L. Sandell and C. Boyd, editors. Academic Press, New York. 221-250.
- Inoue S, Bar-Nun S, Roitelman J, Simoni RD. Inhibition of degradation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase in vivoby cysteine protease inhibitors. J Biol Chem. 1991;266:13311–13317. [PubMed] [Google Scholar]
- Jain N, Brickenden A, Ball EH, Sanwal BD. Inhibition of procollagen I degradation by colligin: a collagen-binding serpin. Arch Biochem Biophys. 1994;314:23–30. doi: 10.1006/abbi.1994.1407. [DOI] [PubMed] [Google Scholar]
- Jin Y-J, Burakoff SJ, Bierer BE. Molecular cloning of a 25-kDa high affinity rapamycin binding protein, FKBP25. J Biol Chem. 1992;267:10942–10945. [PubMed] [Google Scholar]
- Jin Y-J, Albers MW, Lane WS, Bierer BE, Schreiber SL, Burakoff SJ. Molecular cloning of a membrane-associated human FK506- and rapamycin-binding protein, FKBP-13. Proc Natl Acad Sci USA. 1991;88:6677–6681. doi: 10.1073/pnas.88.15.6677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karr SR, Foster JA. Primary structure of the signal peptide of tropoelastin b. J Biol Chem. 1981;265:5946–5949. [PubMed] [Google Scholar]
- Kuznetsov G, Chen LB, Nigam SK. Multiple molecular chaperones complex with misfolded large oligomeric glycoproteins in the endoplasmic reticulum. J Biol Chem. 1997;272:3057–3063. doi: 10.1074/jbc.272.5.3057. [DOI] [PubMed] [Google Scholar]
- Lee KA, Pierce RA, Davis EC, Mecham RP, Parks WC. Conversion to an elastogenic phenotype by fetal hyaline chondrocytes is accompanied by altered expression of elastin-related macromolecules. Dev Biol. 1994;163:241–252. doi: 10.1006/dbio.1994.1140. [DOI] [PubMed] [Google Scholar]
- Lin H, Masso-Welch P, Di Y-P, Cai J, Shen J, Subjeck JR. The 170-kDa glucose-regulated stress protein is an endoplasmic reticulum protein that binds immunoglobulin. Mol Biol Cell. 1993;4:1109–1119. doi: 10.1091/mbc.4.11.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariencheck MC, Davis EC, Zhang H, Ramirez F, Rosenbloom J, Gibson MA, Parks WC, Mecham RP. Fibrillin-1 and fibrillin-2 show temporal and tissue-specific regulation of expression in developing elastic tissues. Connect Tissue Res. 1995;31:87–97. doi: 10.3109/03008209509028396. [DOI] [PubMed] [Google Scholar]
- Mecham, R.P. 1987. Modulation of elastin synthesis: in vitro models. Methods Enzymol. 144(D):232–246. [DOI] [PubMed]
- Mecham, R.P., and E.C. Davis. 1994. Elastic fiber structure and sssembly. In Extracellular Matrix Assembly and Structure. P.D. Yurchenko, D.E. Birk, and R.P. Mecham, editors. Academic Press, San Diego. 281–314.
- Mecham, R.P., and A. Hinek. 1996. Non-integrin laminin receptors. In The Laminins. P. Ekblom and R. Timpl, editors. Harwood Academic Publishers, New York. 159–183.
- Nagata K. Hsp47: a collagen-specific molecular chaperone. TIBS (Trends Biochem Sci) 1996;21:23–26. doi: 10.1016/0968-0004(96)80881-4. [DOI] [PubMed] [Google Scholar]
- Nigam SK, Jin Y-J, Jin M-J, Bush KT, Bierer BE, Burakoff SJ. Localization of the FK506-binding protein, FKBP 13, to the lumen of the endoplasmic reticulum. Biochem J. 1993;294:511–515. doi: 10.1042/bj2940511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigam SK, Goldberg AL, Ho S, Rohde MF, Bush KT, Sherman MY. A set of endoplasmic reticulum proteins possessing properties of molecular chaperones includes Ca2+-binding proteins and members of the thioredoxin superfamily. J Biol Chem. 1994;269:1744–1749. [PubMed] [Google Scholar]
- Owens-Grillo JK, Hoffmann K, Hutchison KA, Yem AW, Deibel MR, Handschumacher RE, Pratt WB. The cyclosporin A-binding immunophilin CyP-40 and the FK506-binding immunophilin hsp56 bind to a common site on hsp90 and exist in independent cytosolic heterocomplexes with untransformed glucocorticoid receptor. J Biol Chem. 1995;270:20479–20484. doi: 10.1074/jbc.270.35.20479. [DOI] [PubMed] [Google Scholar]
- Owens-Grillo JK, Czar MJ, Hutchison KA, Hoffmann K, Perdew GH, Pratt WB. A model of protein targeting mediated by immunophilins and other proteins that bind to hsp90 via tetratricopeptide repeat domains. J Biol Chem. 1996;271:13468–13475. doi: 10.1074/jbc.271.23.13468. [DOI] [PubMed] [Google Scholar]
- Peattie DA, Harding MW, Fleming MA, DeCenzo MT, Lippke JA, Livingston DJ, Benasutti M. Expression and characterization of human FKBP52, an immunophilin that associates with the 90-kDa heat shock protein and is a component of steroid receptor complexes. Proc Natl Acad Sci USA. 1992;89:10974–10978. doi: 10.1073/pnas.89.22.10974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham HRB. The retention signal for soluble proteins of the endoplasmic reticulum. TIBS (Trends Biochem Sci) 1990;15:483–486. doi: 10.1016/0968-0004(90)90303-s. [DOI] [PubMed] [Google Scholar]
- Pratt WB, Czar MJ, Stancato LF, Owens JK. The hsp56 immunophilin component of steroid receptor heterocomplexes: could this be the elusive nuclear localization signal-binding protein? . J Steroid Biochem Mol Biol. 1993;46:269–279. doi: 10.1016/0960-0760(93)90216-j. [DOI] [PubMed] [Google Scholar]
- Saga S, Nagata K, Chen W-T, Yamada KM. pH-dependent function, purification, and intracellular location of a major collagen-binding glycoprotein. J Cell Biol. 1987;105:517–527. doi: 10.1083/jcb.105.1.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar SK, Young PE, Sullivan CE, Torchia DA. Detection of cis and trans X-Pro peptide bonds in proteins by 13C NMR: application to collagen. Proc Natl Acad Sci USA. 1984;81:4800–4803. doi: 10.1073/pnas.81.15.4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders NA, Grant ME. Elastin biosynthesis in chick-embryo arteries. Studies on the intracellular site of synthesis of tropoelastin. Biochem J. 1984;221:393–400. doi: 10.1042/bj2210393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber SL. Chemistry and biology of the immunophilins and their immunosuppressive ligands. Science. 1991;251:283–287. doi: 10.1126/science.1702904. [DOI] [PubMed] [Google Scholar]
- Siekierka JJ, Hung SHY, Poe M, Lin CS, Sigal NH. A cytosolic binding protein for the immunosuppressant FK506 has peptidyl-prolyl isomerase activity but is distinct from cyclophilin. Nature. 1989;341:755–757. doi: 10.1038/341755a0. [DOI] [PubMed] [Google Scholar]
- Sigal NH, Dumont FJ. Cyclosporin A, FK-506, and rapamycin: pharmacologic probes of lymphocyte signal tranduction. Annu Rev Immunol. 1992;10:519–560. doi: 10.1146/annurev.iy.10.040192.002511. [DOI] [PubMed] [Google Scholar]
- Smith DF, Albers MW, Schreiber SL, Leach KL, Deibel MR. FKBP54, a novel FK506-binding protein in avian progesterone receptor complexes and HeLa extracts. J Biol Chem. 1993;268:24270–24273. [PubMed] [Google Scholar]
- Smith T, Ferreira LR, Hebert C, Norris K, Sauk JJ. Hsp47 and cyclophilin B traverse the endoplasmic reticulum with procollagen into pre-Golgi intermediate vesicles. J Biol Chem. 1995;270:18323–18328. doi: 10.1074/jbc.270.31.18323. [DOI] [PubMed] [Google Scholar]
- Steinmann B, Bruckner P, Superti-Furga A. Cyclosporin A slows collagen triple-helix formation in vivo: indirect evidence for a physiologic role of peptidyl-prolyl cis-trans-isomerase. J Biol Chem. 1991;266:1299–1303. [PubMed] [Google Scholar]
- Tartakoff AM. Perturbation of vesicular traffic with the carboxylic ionophore monensin. Cell. 1983;32:1026–1028. doi: 10.1016/0092-8674(83)90286-6. [DOI] [PubMed] [Google Scholar]
- Thrift RN, Drisko J, Dueland S, Trawick JD, Davis RA. Translocation of apolipoprotein B across the endoplasmic reticulum is blocked in a nonhepatic cell line. Proc Natl Acad Sci USA. 1992;89:9161–9165. doi: 10.1073/pnas.89.19.9161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry DW. What is elastin; what is not. Ultrastruct Pathol. 1983;4:227–251. doi: 10.3109/01913128309140793. [DOI] [PubMed] [Google Scholar]
- Urry, D.W., C.-H. Luan, and S.Q. Peng. 1995. Molecular biophysics of elastin structure, function and pathology. In The Molecular Biology and Pathology of Elastic Tissues. Vol. Ciba Foundation Symposium 192. L. Robert, editor. John Wiley & sons, New York. 4–22. [DOI] [PubMed]
- Wiederrecht G, Martin MM, Sigal NH, Siekierka JJ. Isolation of a human cDNA encoding a 25 kDa FK-506 and rapamycin binding protein. Biochem Biophys Res Commun. 1992;185:298–303. doi: 10.1016/s0006-291x(05)80990-8. [DOI] [PubMed] [Google Scholar]
- Wileman T, Kane LP, Terhorst C. Degradation of T-cell receptor chains in the endoplasmic reticulum is inhibited by inhibitors of cysteine proteases. Cell Regul. 1991;2:753–765. doi: 10.1091/mbc.2.9.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrenn DS, Griffin GL, Senior RM, Mecham RP. Characterization of biologically active domains on elastin: identification of a monoclonal antibody to a cell recognition site. Biochemistry. 1986;25:5172–5176. doi: 10.1021/bi00366a028. [DOI] [PubMed] [Google Scholar]