Abstract
The cadherins are a family of homophilic adhesion molecules that play a vital role in the formation of cellular junctions and in tissue morphogenesis. Members of the integrin family are also involved in cell to cell adhesion, but bind heterophilically to immunoglobulin superfamily molecules such as intracellular adhesion molecule (ICAM)–1, vascular cell adhesion molecule (VCAM)–1, or mucosal addressin cell adhesion molecule (MadCAM)–1. Recently, an interaction between epithelial (E-) cadherin and the mucosal lymphocyte integrin, αEβ7, has been proposed. Here, we demonstrate that a human E-cadherin–Fc fusion protein binds directly to soluble recombinant αEβ7, and to αEβ7 solubilized from intraepithelial T lymphocytes. Furthermore, intraepithelial lymphocytes or transfected JY′ cells expressing the αEβ7 integrin adhere strongly to purified E-cadherin–Fc coated on plastic, and the adhesion can be inhibited by antibodies to αEβ7 or E-cadherin.
The binding of αEβ7 integrin to cadherins is selective since cell adhesion to P-cadherin–Fc through αEβ7 requires >100-fold more fusion protein than to E-cadherin–Fc. Although the structure of the αE-chain is unique among integrins, the avidity of αEβ7 for E-cadherin can be regulated by divalent cations or phorbol myristate acetate. Cross-linking of the T cell receptor complex on intraepithelial lymphocytes increases the avidity of αEβ7 for E-cadherin, and may provide a mechanism for the adherence and activation of lymphocytes within the epithelium in the presence of specific foreign antigen. Thus, despite its dissimilarity to known integrin ligands, the specific molecular interaction demonstrated here indicates that E-cadherin is a direct counter receptor for the αEβ7 integrin.
The cadherins constitute a family of cell surface adhesion molecules that are involved in calcium- dependent homophilic cell to cell adhesion (Takeichi, 1990). The best studied human cadherins, E-, P-, N-, and VE-cadherin, have a restricted tissue distribution: E- and P-cadherin are expressed in epithelial tissues (Nose and Takeichi, 1986; Shimoyama et al., 1989a), N-cadherin is found mainly on neural cells (Hatta et al., 1987), and VE-cadherin is found on vascular endothelium (Lampugnani et al., 1992). Homophilic binding between cadherins on adjacent cells is vital for the maintenance of strong cell to cell adhesion in these tissues. For example, E-cadherin is required for the formation of adherens junctions between mature epithelial cells (Boller et al., 1985; Gumbiner et al., 1988) and is involved in Langerhans cell adhesion to keratinocytes (Tang et al., 1993), and VE-cadherin is needed for the maintenance of lateral association between endothelial cells (Lampugnani et al., 1992). During development cadherins are critically involved in the cell sorting required for tissue morphogenesis (Takeichi, 1995), and loss of E-cadherin function contributes to the metastasis of a variety of carcinomas (Ben-Ze'ev, 1997). The extracellular regions of mature mammalian cadherins are comprised five “CAD” modules of ∼110 amino acids. Crystallographic and biochemical studies indicate that cadherins probably form dimers on the cell surface (Shapiro et al., 1995; Nagar et al., 1996), and that interaction with dimeric cadherins on opposing cell surfaces can lead to the formation of “zipper-like” cell junctions (Shapiro et al., 1995; Tomschy et al., 1996).
The integrins are a second family of transmembrane adhesion molecules that are involved in both cell to cell and cell to matrix interactions. At least 15 α chains associate with 8 β chains to form a large number of heterodimeric integrins that can be classified into several major subfamilies based on their shared use of a particular β chain (Hynes, 1992). Members of three such subfamilies, the β1, β2, and β7 integrins, are commonly found on leukocytes. The expression of β1 integrins is widespread (for example, α5β1, CD49e/CD29, is found on T cells, granulocytes, platelets, fibroblasts, endothelium, and epithelium), whereas the β2 and β7 integrins have a restricted pattern of expression. For example, αLβ2 (CD11a/CD18) is expressed on most lymphocytes and many myeloid cells but not on other cell types, and αEβ7 (CD103/unclustered) is found on >95% of intestinal intraepithelial lymphocytes (iIEL)1 and on other mucosal T cells, macrophages, and mast cells, but on only 2% of peripheral blood lymphocytes (Cerf-Bensussan et al., 1987; Smith et al., 1994; Tiisala et al., 1995). The major ligands of the integrins fall into two categories: cell surface molecules that are members of the immunoglobulin superfamily (such as vascular cell adhesion molecule [VCAM]–1, intracellular adhesion molecule [ICAM]–1, 2, 3, and mucosal addressin cell adhesion molecule [MadCAM]–1) and a variety of large extracellular proteins (such as fibronectin, vitronectin, fibrinogen, and complement component iC3b; Hynes, 1992). A common feature of both groups is the presence of exposed acidic amino acids crucial for integrin binding (Bergelson and Hemler, 1995). Many integrins exist in states of low or high avidity for their ligands, and these states can be regulated from within the cell (Hynes, 1992). For example, the avidity of αLβ2 on resting T cells can be increased by stimulation through the T cell receptor (TCR) with anti–CD3 mAbs (Dustin and Springer, 1989; van Kooyk et al., 1989).
Recently, we reported that E-cadherin on human epithelial cells may be a ligand for the mucosal lymphocyte integrin, αEβ7, and a similar interaction has been suggested in the mouse (Cepek et al., 1994; Karecla et al., 1995). mAbs to E-cadherin or to αEβ7 block IEL adherence to epithelial cells, and transfection of cells with αEβ7 confers upon them the ability to adhere to cells transfected with E-cadherin (Cepek et al., 1994). E-cadherin has been defined extensively as a homophilic adhesion molecule, and its sequence is not related to known cell surface or extracellular integrin ligands. Thus, the concept that αEβ7 and E-cadherin are counter receptors is at variance with current knowledge of both integrin and cadherin interactions. Moreover, as we and others have pointed out, the indirect methods used in the studies to date are insufficient to conclusively demonstrate a direct physical interaction between these two adhesion receptors (Cepek et al., 1994; Erle, 1995; Karecla et al., 1995; Takeichi, 1995). For example, it is clear that antibodies to one integrin (e.g., αVβ3) can cause specific transdominant inhibition of the function of another integrin (e.g., α5β1; Blystone et al., 1995; Díaz-González et al., 1996) or could result in steric hindrance of an adjacent receptor. Furthermore, expression of an exogenous gene in a transfected cell can have profound effects on the surface expression of a variety of other proteins (Marks et al., 1996), and the transfection of constructs containing integrin β1 tails into fibroblasts alters the function of endogenous integrins (LaFlamme et al., 1994). Such effects can lead to erroneous conclusions about the interactions that are directly involved in adhesion.
Given that the interaction of αEβ7 on intraepithelial lymphocytes with its receptor on epithelial cells is likely to be crucial for the normal development, function, and/or retention of lymphocytes in the epithelium (Cepek et al., 1993; Erle, 1995), we sought to determine if αEβ7 and E-cadherin are directly interacting counter receptors. In addition, we investigated whether this interaction is specific among cadherins and whether it can be regulated through alterations in αEβ7 avidity.
Materials and Methods
Materials
DNA manipulating enzymes were purchased from New England Biolabs Inc. (Beverly, MA). Oligonucleotides were obtained from Oligotech (Boston, MA). Other chemicals were purchased from Sigma Chemical Co. (St. Louis, MO). ICAM-1–Fc (the entire extracellular region of human ICAM-1 fused to the hinge and Fc portion of human IgG1) was a generous gift of Dr. Lloyd Klickstein (Brigham and Women's Hospital, Boston, MA). Purified human IgG1 was obtained from Calbiochem-Novabiochem Corp. (La Jolla, CA).
mAbs
The mAb used (all mouse IgG against human antigens) were as follows: E4.6 (anti–E-cadherin, IgG1; Cepek et al., 1994), HECD-1 (anti–E-cadherin, IgG1; Shimoyama et al., 1989b), NCC-CAD299 (anti–P-cadherin, IgG1; Shimoyama et al., 1989b), HML-1 (anti-αEβ7, IgG2a; Cerf-Bensussan et al., 1987), BerACT-8 (anti-αEβ7, IgG1; Kruschwitz et al., 1991), αE7-1 (anti-αEβ7, IgG2a; Russell et al., 1994), αE7-2 (anti-αEβ7, IgG1; Russell et al., 1994), αE7-3 (anti-αEβ7, IgG1; Russell et al., 1994), D6.21 (anti-αLβ2, IgG1; Cepek et al., 1993), TS1/22 (anti-αLβ2, IgG1; Sanchez-Madrid et al., 1982), TS1/18 (anti-β2, IgG1; Sanchez-Madrid et al., 1983), ACT-1 (anti-α4β7, IgG1; Lazarovits et al., 1984), B5G10 (anti-α4, IgG1; Hemler et al., 1987), 4B4 (anti-β1, IgG1; Morimoto et al., 1985), RR1/1 (anti–ICAM-1, IgG1; Rothlein et al., 1986), SPVT3b (anti-CD3ε, IgG2a; Spits et al., 1983), OKT4 (anti-CD4, IgG2b; obtained from American Type Culture Collection [ATCC], Rockville, MD), OKT8 (anti-CD8α, IgG2a; ATCC), W6/32 (anti-MHCI, IgG2a; Barnstable et al., 1978), TCR-δ1 (anti–TCR-δ, IgG1; Band et al., 1987), P3 (control IgG1; Kohler and Milstein, 1975), RPC5.4 (control IgG2a; Mohit and Fan, 1971).
Cells
Human iIEL were isolated as previously described (Roberts et al., 1993; Russell et al., 1996). The iIEL were stimulated with PHA-P (Difco Laboratories Inc., Detroit, MI) and irradiated feeder cells (80% PBMC and 20% JY lymphoblastoid cells) in 2 nM IL-2, 4% (vol/vol) heat-inactivated FBS (Hyclone Laboratories Inc., Logan, UT), 50 μM 2-mercaptoethanol, and Yssel's medium at 10% CO2 (Russell et al., 1996). The iIEL lines 496 and 194 were maintained by periodic restimulation as described and were used in adhesion assays after 2–8 wk. At the time of assay, IEL496 was 100% CD3+, 90% CD8+, 10% CD4+, 95% αLβ2 + (MFI ∼400), 97% αEβ7 + (MFI ∼700), and IEL194 was 100% CD3+, 60% CD8+, 40% CD4+, 95% αLβ2 + (MFI ∼500), 80% αEβ7 + (MFI ∼600) by FACS® analysis. Both lines maintained expression of αEβ7 without addition of exogenous TGF-β1.
A subline of the human B lymphoblastoid cell line, JY, that expresses the α4β7 integrin (JY′), was kindly provided by Dr. Martin Hemler (Dana-Farber Cancer Institute, Boston, MA; Chan et al., 1992) and was maintained in 10% (vol/vol) heat-inactivated FBS (Hyclone Laboratories Inc.), RPMI-1640 (GIBCO BRL, Gaithersburg, MD) at 37°C, and 5% CO2. Human embryonic kidney HEK293 cells (obtained from ATCC) were maintained in 10% (vol/vol) heat-inactivated FBS (Hyclone Laboratories Inc.) and DME Medium (GIBCO BRL) at 37°C in 10% CO2. COS-7 cells (obtained from ATCC) were grown in 10% (vol/vol) NuSerum (Collaborative Research, Inc., Waltham, MA), 10 mM Hepes, 2 mM l-glutamine, and DMEM (GIBCO BRL) at 10% CO2. Human breast epithelial 16E6.A5 cells were maintained as described (Cepek et al., 1993).
Construction of E- and P-Cadherin–Fc Expression Vectors
E-cadherin–Fc.
A double-stranded DNA adapter containing a 5′ Ngo MI cohesive end, the final five codons of the human E-cadherin extracellular region, and a 3′ XhoI cohesive end was produced by annealing the complimentary oligonucleotides JON1 (5′-CCGGCGTCTGTAGGAAGC-3′) and JON2 (5′-TCGAGCTTCCTACAGACG-3′). This adapter was then ligated to the 3′-end of an EcoRV–NgoMI fragment encoding the rest of the extracellular region of human E-cadherin derived from the plasmid pERF-1 (kindly provided by Dr. David Rimm, Yale University, New Haven, CT; Rimm and Morrow, 1994). After removal of excess adapters by centrifugation through a Centricon-100 filter (Amicon Corp., Danvers, MA), the resulting EcoRV–XhoI fragment was introduced inframe, upstream of coding for the hinge and Fc region of human IgG1 in a derivative of pCDM8 (pCDM8/Fc; Chen and Nelson, 1996) also cleaved with EcoRV and XhoI. The sequence of the junctional region is shown in Fig. 1 a. To confirm the integrity of the construct, the nucleotide sequence of the junctional region of the E-cadherin–Fc construct was determined by double-stranded sequencing using the Sequenase kit (United States Biochemical Corp., Cleveland, OH) according to the manufacturer's protocol. Finally, the E-cadherin–Fc cDNA was excised from pCDM8 using EcoRV and NotI and ligated into the expression vector pCEP4 (Invitrogen Corp., Carlsbad, CA) cleaved with PvuII and NotI.
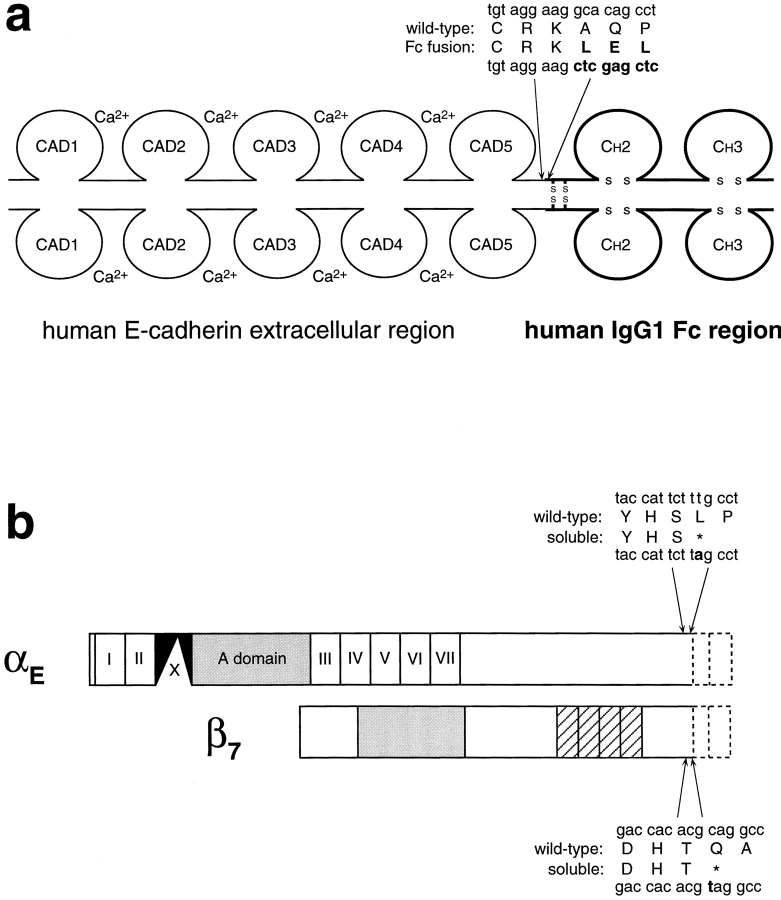
Figure 1.
Structure of soluble recombinant E-cadherin–Fc and truncated αEβ7. (a) Structure of the human E-cadherin–Fc fusion protein. The sequence of the extracellular juxtamembrane region of wild-type E-cadherin and the alterations resulting from fusion with the human Fc region are shown. Regions corresponding to the Fc portion are shown in bold. The corresponding sequences in P-cadherin, tgc cct gga ccc tgg aaa (encoding CPGPWK), become tgc cct gga ctc gag ctc (encoding CPGLEL) in the P-cadherin–Fc fusion protein. (b) Structure of soluble truncated αEβ7. In the αE chain, the EF hand–like repeats are labeled I to VII, the extra “X” domain is shown in black, and the A domain in grey. In the β7 chain, the β integrin conserved region that may resemble an A domain (Lee et al., 1995) is shown in grey, and the cysteine-rich repeats are hatched. The transmembrane and cytoplasmic regions that were removed from each chain are shown as dotted lines. The change made in the cDNA sequence of each chain to introduce a stop codon immediately before the transmembrane region is shown in bold.
P-Cadherin–Fc.
An adapter fragment encoding the final 93 codons of the human P-cadherin extracellular region was generated by PCR from a human P-cadherin cDNA in pBR322 (kindly provided by Dr. S. Hirohashi, National Cancer Center Research Institute, Tokyo, Japan; Shimoyama et al., 1989b) using the primers JON4 (5′-GGCGTGCCACCTACCTTATCAT-3′) and JON5 (5′-TTTTTTCTCGAGTCCAGGGCAGGTTTCGAC-3′) and cloned plaque-forming unit (PFU) polymerase (Stratagene, La Jolla, CA) according to the manufacturer's recommendations, with 25 cycles of 94°C 1 min/55°C 1 min/72°C 1 min. After digestion with BsaBI and XhoI, this adapter was ligated to the 3′-end of a HindIII–BsaBI fragment encoding the rest of the extracellular region of human P-cadherin. After digestion of the ligation products with HindIII and XhoI and purification of the required fragment by Agarose gel electrophoresis and USBioclean (United States Biochemical Corp.), the resulting HindIII–XhoI fragment was introduced into pCDM8/Fc cleaved with HindIII and XhoI. The sequence of the junctional region is indicated in the legend of Fig. 1 a. After sequencing as described above, the P-cadherin–Fc cDNA was excised with HindIII and NotI, and inserted into pCEP4 cleaved with the same enzymes.
DNA for transfections was prepared using a Maxi-prep kit (QIAGEN Inc., Chatsworth, CA).
Construction of αE and β7 Expression Vectors
Full-Length αE.
A full-length open reading frame encoding human αE was generated from cDNA clones described previously (Shaw et al., 1994). The final construct included the HindIII–NsiI fragment of clone 38, the NsiI–BsaBI fragment of clone 2-54, the BsaBI–AccI fragment of clone 1-39A, the AccI–BglII fragment of clone 2-54, and the BglII–XhoI fragment of clone 3-15. The full-length cDNA was introduced into the XhoI site of the expression vector pSRα-neo (Takebe et al., 1988) to produce pSRα-neo/αE.
Truncated αE.
PCR was used to introduce a stop codon and HindIII site immediately upstream of the transmembrane region of human αE cDNA (see Fig. 1 b). PCR with the primers 5′-AAGAATGGCATTCAGTGAGC-3′ and 5′-GGGAAGGTTGATGATAGGCTAAGAATGGTAC-3′ amplified a product that was subsequently cleaved with BglII and HindIII to generate a 180-bp fragment from the end of the αE extracellular region. This fragment was then introduced into the BglII site of the full-length αE cDNA to generate an open reading frame for the entire extracellular portion of human αE. After sequencing as described above, the truncated αE cDNA was introduced in either the sense or antisense direction into the HindIII site of the expression vector pAPRM8 (Wong and Farrell, 1991) to produce pAPRM8/tαEs and pAPRM8/tαEas.
Truncated β7.
A full-length human β7 cDNA was derived by ligation of the XhoI–EcoRI fragment from a partial β7 cDNA (kindly provided by Dr. G.W. Krissansen, University of Auckland, New Zealand; Yuan et al., 1990) and the NotI–XhoI fragment of another partial β7 cDNA cloned from an IEL cDNA library (Shaw et al., 1994). To introduce a stop codon and EcoRI site immediately upstream of the transmembrane region of human β7 cDNA, PCR with the primers 5′-TCGCTGCCAATGTGGAGTATG-3′ and 5′-GGGGAATTCACAATGGCCTACGTGTGGTCTGC-3′ was carried out. The product obtained was subsequently cleaved with BsmI and EcoRI to generate a 400-bp fragment from the end of the β7 extracellular region. This fragment was then introduced into the BsmI and EcoRI sites of pBluescript containing the β7 cDNA to generate an open reading frame for the entire extracellular portion of human β7 (see Fig. 1 b). After sequencing as described above, the truncated β7 cDNA was excised from pBluescript with EcoRI and introduced in either the sense or antisense direction into the EcoRI site of the expression vector pAPRM8 to produce pAPRM8/tβ7s and pAPRM8/tβ7as.
Production of Cadherin–Fc Proteins
HEK293 cells (106 cells per 75-cm2 flask) were stably transfected with 25 μg plasmid DNA using the Mammalian transfection kit (Stratagene Inc.). After growth for 24 h in nonselective medium the cells were transferred to 96-well tissue culture plates and incubated in selective medium containing 300 μg/ml hygromycin B. After 15 d, supernatants from wells containing resistant colonies were assayed for fusion proteins by ELISA.
To produce the cadherin–Fc proteins, transfected cells were grown in triple-layer 500-cm2 flasks (Nunc, Roskilde, Denmark) in 10% (vol/vol) Ultralow Ig FBS (GIBCO BRL), 300 μg/ml hygromycin B, and DMEM. After 5–10 d of culture, the medium was harvested and filtered through a 0.2-μm membrane. The E- and P-cadherin–Fc fusion proteins were then purified on separate, previously unused GammaBind G–Sepharose columns (Pharmacia Biotech Sverige, Uppsala, Sweden). The columns were washed with TBS and 1 mM CaCl2, pH 7.4, and then eluted with 0.2 M glycine and 1 mM CaCl2, pH 2.3. Fractions containing purified fusion proteins were dialyzed into TBS and 1 mM CaCl2, pH 7.4 and then stored at −20°C. The purity of fusion protein was assessed by SDS-PAGE and Coomassie blue staining, and the concentration was determined by Bradford assay using BSA as a standard (Bio-Rad Labs., Hercules, CA).
Production of Soluble 35S-labeled Recombinant αEβ7 Integrin
Soluble recombinant αEβ7 was produced by COS-7 cells after transient transfection using DEAE-dextran (Coligan et al., 1994, Unit 10-14) with the plasmids pAPRM8/tαEs and pAPRM8/tβ7s. Control transfections were carried out with the antisense constructs pAPRM8/tαEas and pAPRM8/tβ7as. After incubation for 48 h in complete medium, the cells were washed once with PBS and then 1 mCi 35S-Express (Dupont-NEN, Boston, MA) in 6 ml 10% (vol/vol), dialyzed FBS, 5% DMEM, 85% methionine- and cysteine-free DMEM (GIBCO BRL), 10 mM Hepes, and 2 mM l-glutamine was added. After incubation at 37°C for 24 h, the medium, containing labeled secreted proteins, was filtered through a 0.2-μm membrane.
Generation of JY′-αE and JY′-Vector Cell Lines
To produce cells expressing cell surface αEβ7, JY′ cells that express endogenous β7 integrin chain were transfected by electroporation with 4 μg pSRα-neo/αE or with the pSRα-neo vector alone as a control. Then transfected cells were selected by culture in 0.5 mg/ml G418. To generate the JY′-αE line, αEβ7-expressing transfectants were isolated by positive selection with the anti-αEβ7 mAb BerACT-8 on magnetic goat anti–mouse immunoglobulin dynabeads according to the manufacturer's recommendations (Dynal A.S., Oslo, Norway) and by flow cytometric sorting. FACS® was carried out as previously described (Parker et al., 1990). The clone J6.7 was finally isolated by limiting dilution. The αEβ7 expressed on JY′-αE cells was indistinguishable from that on IEL when immunoprecipitated from 125I surface-labeled cells with the anti–αEβ7 mAb, HML-1 (not shown).
Adhesion Assays
Unless otherwise stated, the wells of Linbro 96-well microtiter plates (ICN Flow Laboratories, Horsham, MA) were coated with human IgG1 Fc-containing proteins in 50 μl/well TBS and 1 mM CaCl2, pH 7.4, for 18 h at 4°C. The wells were subsequently washed twice with 20 mM Hepes, 137 mM NaCl, and 3 mM KCl, pH 7.4 (HBS), with 1 mM CaCl2, and was then blocked with 1% BSA (Calbiochem-Novabiochem Corp.), HBS, and 1 mM CaCl2 for 2 h at room temperature. In assays in which the effects of divalent cations were assessed, coating and blocking was carried out in HBS and 10 mM EDTA. In assays in which adhesion to P- and E-cadherin–Fc was compared, the wells were coated with 1 μg/well goat anti–human IgG polyclonal antibody (Zymed, San Francisco, CA) in 100 μl TBS, pH 7.4, and blocked as described above before addition of Fc fusion proteins.
IEL or transfected JY′ cells were labeled with BCECF-AM (Molecular Probes, Eugene, OR) as previously described (Cepek et al., 1993). During labeling of JY′ cells, 10% (vol/vol) heat-inactivated normal human serum was included to block Fc receptors. Adhesion assays were carried out in 0.1% BSA and HBS with combinations of MnCl2, MgCl2, CaCl2, or 1 mM EGTA, as indicated (see text). In antibody blocking experiments, cells or wells were preincubated with mAbs for 10 min at 4°C as described in the text. For cell activation experiments, cells were preincubated with antibodies or 50 ng/ml PMA at 4°C for 15 min. Adhesion assays were carried out as described previously (Cepek et al., 1993) with the following modifications. Labeled cells were brought into contact with the microtiter plate wells by centrifugation at 60 g for 2 min (IEL) or 1 min (JY′). After incubation at 37°C for 10 min, nonadherent cells were removed by washing with 1 mM MnCl2, 1 mM MgCl2, 1 mM CaCl2, and HBS at 37°C unless the effect of divalent cations was being assessed, in which case HBS alone was used. Since in these assays the fluorescence of input cells was quenched to some degree by the presence of adhesion buffer, but the percent bound was determined after removing the buffer, some apparent readings of >100% are obtained.
Homophilic adhesion assays were carried out as described above with the following modifications. 16E6.A5 cells were released from culture dishes using 0.02% (wt/vol) trypsin, 2 mM CaCl2, and HBS to minimize proteolysis of cadherins. After adding 2 vol of 0.04% (wt/vol) soy bean trypsin inhibitor, HBS, and washing twice with HBS, the cells were resuspended in 0.1% BSA, HBS, and 1 mM CaCl2 and allowed to settle onto the microtiter plate wells for 10 min at 4°C. After incubation at 37°C for 30 min, and washing twice with HBS and 1 mM CaCl2, the percentage of bound cells was determined using a fluorogenic assay of endogenous cellular phosphatase activity (Tolosa and Shaw, 1996).
Surface Labeling of iIEL with 125I
Cultured iIEL (4 × 107) were isolated by centrifugation on Ficoll-Paque (Pharmacia Biotech Sverige) and subjected to cell surface labeling with 2 mCi Na125I (Dupont-NEN) using the lactoperoxidase method (Coligan et al., 1994, Unit 8-11). The labeled cells were then lysed in 0.5% Triton X-100, TBS, and 4 mM iodoacetamide, 1 mM phenylmethylsulfonyl fluoride for 4 h at 4°C. Insoluble material was removed by centrifugation at 12,000 g for 20 min.
Immunoadsorption
Batches of 125I-labeled IEL lysate or 35S-labeled transfected COS-7 medium were supplemented divalent cations (see text) and precleared twice with 0.4% (vol/vol) normal rabbit serum, 1.5% (vol/vol) protein A–Sepharose (Pharmacia), and 1.5% (wt/vol) Pansorbin (Calbiochem-Novabiochem Corp.) for a total of 24 h at 4°C. Subsequently, aliquots were incubated with mouse mAbs or human IgG1 Fc-containing proteins for 3 h at 4°C. Then, 10 μl protein A–Sepharose resin was added to each tube, and the incubation at 4°C was continued for a further 3 h. For immunoprecipitations using mouse IgG1 mAbs, protein A–Sepharose precoated with rabbit anti–mouse immunoglobulin polyclonal antibody (Cappel, West Chester, PA) was used. Then the immobilized complexes were washed six times with TBS containing the same concentration of divalent cations used during the adsorption steps. For immunoadsorption from IEL lysates, 0.1% Triton X-100 was also present during washing. Proteins bound to the resin were eluted by boiling in 3% (wt/vol) SDS, 10% (vol/vol) glycerol, 50% (wt/vol) urea, and 60 mM Tris, pH 7, before analysis by SDS-PAGE.
SDS-PAGE
SDS-PAGE on 7.5% (wt/vol) polyacrylamide gels (Protogel; National Diagnostics, Atlanta, GA) was carried out as described (Coligan et al., 1994, Unit 8-4). Samples were reduced by the inclusion of 25 mM dithiothreitol. Radiolabeled proteins were visualized by autoradiography using Biomax MR and MS film (Kodak, Rochester, NY) and quantitated using phosphorimaging and the ImageQuant package (Molecular Dynamics Inc., Sunnyvale, CA).
Statistical Analysis
P values testing the hypothesis that two populations had equal means were calculated using a two-tailed Welch t test (which assumes the populations follow a normal distribution, but not that they have equal variance). Also, a nonparametric two-tailed Mann-Whitney test (which does not assume normal distributions) was used to test whether the population medians were equal. Calculations were carried out using the Instat package (Graphpad Software, San Diego, CA).
Results
Production of Human E- and P-cadherin–Fc Fusion Proteins
Constructs encoding the extracellular portion of either human E-cadherin or P-cadherin were linked in frame to a construct encoding the Fc region of human IgG1 (including the hinge, CH2, and CH3 domains). Transfection of HEK293 cells and selection with hygromycin B led to the generation of stable lines expressing soluble E-cadherin– Fc or P-cadherin–Fc fusion proteins. The cadherin–Fc fusion proteins were expected to be dimeric due to the presence of disulfide bonds in the Fc region (Fig. 1 a), possibly similar to cadherin dimers on the cell surface (Shapiro et al., 1995; Nagar et al., 1996).
After purification on protein G–Sepharose, SDS-PAGE in reducing conditions revealed the presence of proteins of the expected size for both E- and P-cadherin–Fc monomers (∼120 kD, Fig. 2). Minor species of 135 or 130 kD were also present in preparations of the E- and P-cadherin fusion proteins, respectively. Since cadherins are synthesized as proproteins and the sizes of these bands match those predicted for the immature forms, it is likely that a small proportion of partially processed procadherin is present in each case. In nonreducing conditions both fusion proteins migrate at ∼240 kD (Fig. 2) as expected for dimeric fusion proteins linked through disulfide bonds in the hinge of the Fc region. A small proportion of monomeric cadherin fusion protein is also present in each case. In an ELISA, E-cadherin–Fc but not P-cadherin–Fc is recognized by the antihuman E-cadherin mAb E4.6, and P-cadherin–Fc but not E-cadherin–Fc is recognized by the antihuman P-cadherin mAb NCC-CAD299, further confirming the identity of the two recombinant products (not shown).
Figure 2.
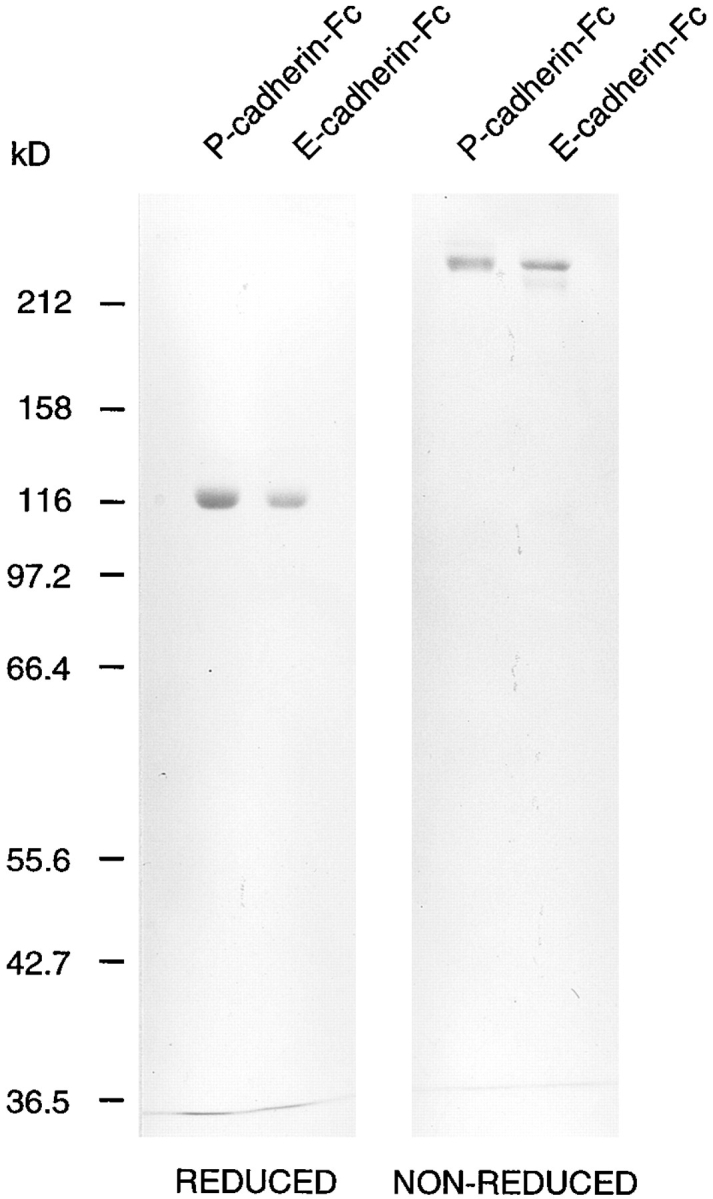
SDS-PAGE of purified recombinant P- and E-cadherin–Fc fusion proteins. Approximately 2 μg of P-cadherin–Fc and 1.5 μg of E-cadherin–Fc protein were subjected to 7.5% SDS-PAGE in reducing and nonreducing conditions and Coomassie blue staining.
E-Cadherin–Fc Supports Adhesion of IEL
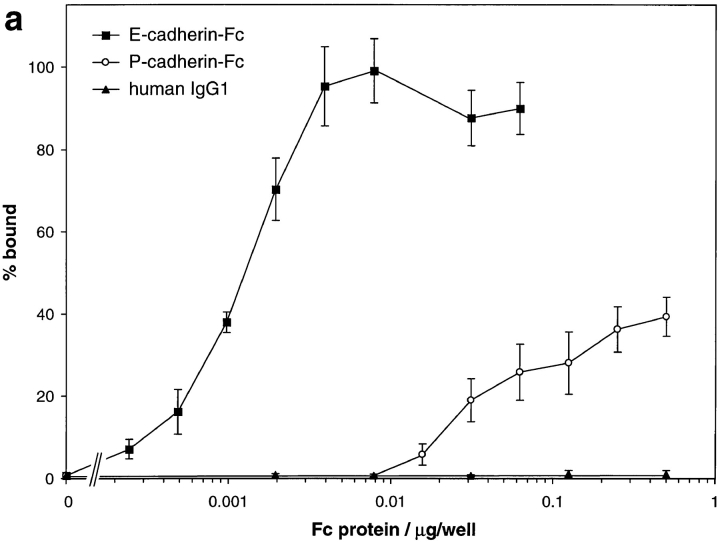
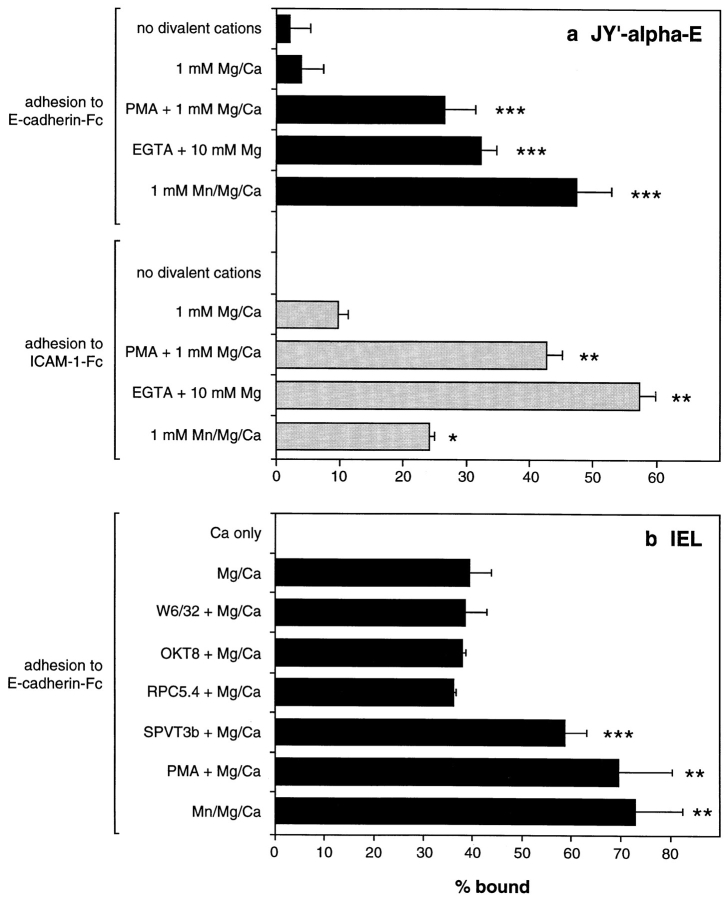
Cell surface E-cadherin has been proposed to be a ligand for the IEL integrin αEβ7 (Cepek et al., 1994; Karecla et al., 1995). To test the capacity of the cadherin–Fc fusions to support adhesion of IEL, we immobilized the proteins via antihuman IgG antibody on polystyrene microtiter plate wells. Binding of IEL to wells coated with subnanogram quantities of E-cadherin–Fc could be detected in the presence of 1 mM MnCl2, 1 mM MgCl2, and 1 mM CaCl2 (Fig. 3 a). At 1 ng/well, ∼40% of the IEL adhered, and maximal adhesion occurred at 10 ng/well of E-cadherin– Fc. Thus, a dose-dependent adhesion of IEL to the E-cadherin fusion protein was clear. Indeed, for IEL expressing similar levels of αEβ7 and αLβ2, adhesion to human E-cadherin–Fc was similar or greater than that seen to human ICAM-1–Fc (data not shown and see Fig. 3 b). In contrast, adhesion to P-cadherin–Fc required >100-fold more fusion protein and did not reach 100% at any coating concentration. 40% adhesion was seen to P-cadherin–Fc at 500 ng/well. No adhesion could be detected to wells coated with 500 ng/well human IgG1 (Fig. 3 a).
Figure 3.
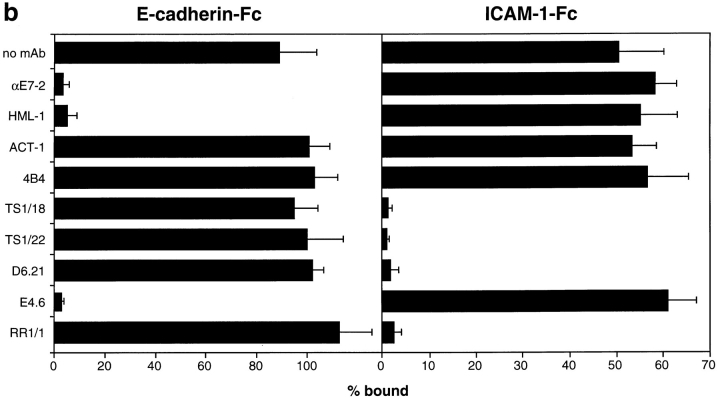
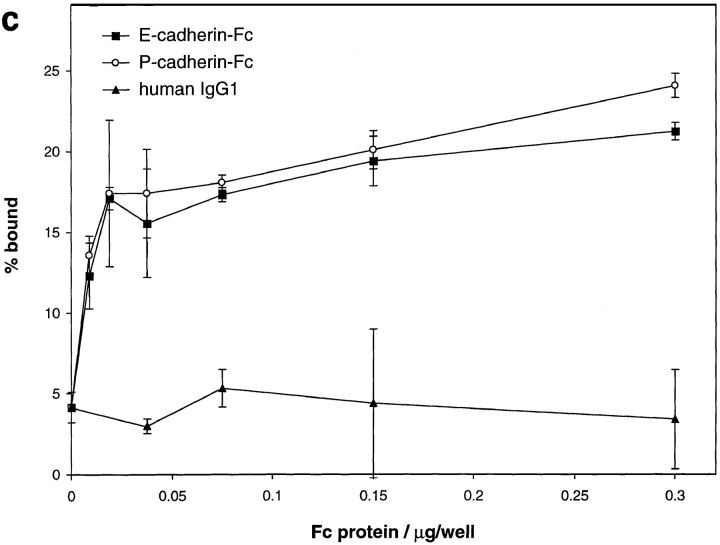
Adhesion of cultured intestinal IEL and epithelial cells to E- and P-cadherin–Fc. (a) Dose response of IEL adhesion to E- and P-cadherin–Fc. Serial dilutions of purified E-cadherin– Fc, P-cadherin–Fc, and human IgG1 were immobilized on microtiter plate wells coated with polyclonal goat anti– human IgG antibody and blocked with BSA. The adhesion of cultured IEL was determined in the presence of 1 mM MnCl2, 1 mM MgCl2, 1 mM CaCl2, 0.1% BSA, HBS, pH 7.4, as described in Materials and Methods. Adhesion was determined in two independent experiments, each in triplicate, and the results are expressed as the mean percent bound ± 1 SD (n = 6). (b) Inhibition of adhesion of IEL to E-cadherin–Fc and ICAM-1–Fc by mAbs. Microtiter plate wells were coated directly with 0.06 μg/well of human E-cadherin–Fc or 0.6 μg/well ICAM-1–Fc protein, and blocked with BSA. The adhesion of cultured IEL was determined in the presence of 1 mM MnCl2, 1 mM MgCl2, 1 mM CaCl2, 0.1% BSA, HBS, pH 7.4, and various mAbs. The purified mAbs αE7-2 (anti-αEβ7), D6.21 (anti-αLβ2), 4B4 (anti-β1), ACT-1 (anti-α4β7), E4.6 (anti–E-cadherin), and RR1/1 (anti–ICAM-1) were used at 10 μg/ml. Ascites fluid containing HML-1 (anti-αEβ7), TS1/22 (anti-αLβ2), and TS1/18 (anti-β2) were used at a dilution of 1:50. All antibodies were preincubated with IEL for 10 min on ice before addition to the plate except for RR1/1 and E4.6, which were preincubated in the microtiter plate. All mAbs were mouse IgG1 except HML-1 (mouse IgG2a). The results are expressed as the mean percent bound + 1 SD (n = 5). (c) Dose response of 16E6.A5 cell adhesion to E- and P-cadherin–Fc. Microtiter plates were coated as described for a. Adhesion was determined as described in Materials and Methods and the results are expressed as the mean percent bound ± 1 SD (n = 3). The adhesion of 16E6.A5 cells to E-cadherin–Fc is inhibited by >95% with the anti–E-cadherin mAb HECD-1, but not with the anti–P-cadherin mAb NCC-CAD299. In contrast, the adhesion of 16E6.A5 cells to P-cadherin–Fc is inhibited by 80% with the anti–P-cadherin mAb NCC-CAD299, but not with the anti– E-cadherin mAb HECD-1 (data not shown).
Adhesion of PHA-activated peripheral blood lymphocytes (PBL) to E-cadherin–Fc could not be detected (data not shown). This is consistent with the fact that only 2– 5% of PBL express αEβ7 (Cerf-Bensussan et al., 1987). The E- and P-cadherin–Fc proteins support similar levels of adhesion of 16E6.A5 epithelial cells (Cepek et al., 1993) that express similar levels of E- and P-cadherin, suggesting that both fusion proteins are equally able to support cadherin-mediated homophilic adhesion (Fig. 3 c).
Antibodies to αEβ7 and E-Cadherin Block IEL Adhesion to E-Cadherin–Fc
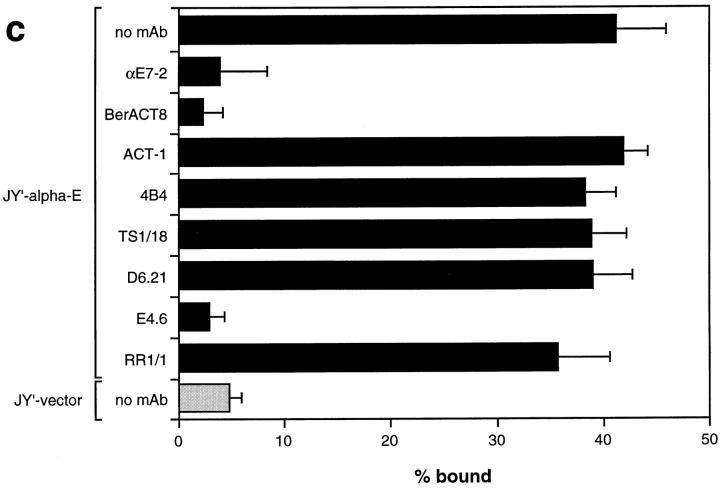
To determine if lymphocyte αEβ7 was responsible for adhesion of IEL to E-cadherin–Fc, we attempted to block the interaction with mAbs to human lymphocyte surface integrins. The binding of IEL to E-cadherin–Fc coated directly on plastic was completely blocked by anti–αEβ7 mAbs HML-1, BerACT8, αE7-1, and αE7-2 and by the anti–E-cadherin mAb E4.6, while adhesion to ICAM-1–Fc was not affected (Fig. 3 b and data not shown). In contrast, the anti-β2 integrin mAb, TS1/18, the anti–αLβ2 mAbs, TS1/22 and D6.21, and the anti–ICAM-1 mAb RR1/1 all prevented IEL adhesion to ICAM-1–Fc, but not to E-cadherin–Fc. The anti-β1 integrin mAb, 4B4, blocked adhesion of IEL to human fibronectin (not shown) but not to E-cadherin–Fc or ICAM-1–Fc. A blocking mAb to α4β7 (ACT-1) also did not inhibit IEL adhesion to E-cadherin– Fc or ICAM-1–Fc (Fig. 3 b). Adhesion of IEL to P-cadherin–Fc was also completely blocked by the anti–αEβ7 mAb, αE7-2, but was unaffected by the anti–αLβ2 mAb, D6.21 (data not shown). Thus, adhesion of IEL to E- or P-cadherin–Fc involves αEβ7, and adhesion to ICAM-1–Fc involves αLβ2.
JY Cells Transfected with αE Adhere to E-Cadherin–Fc
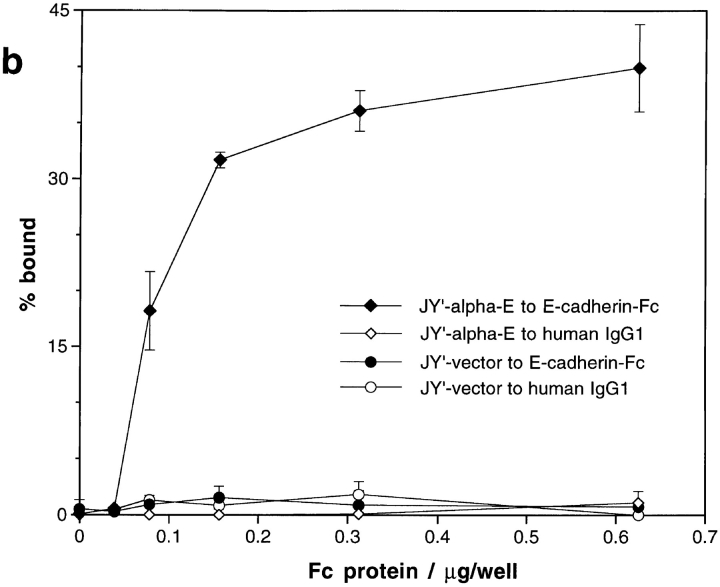
To confirm that expression of exogenous αEβ7 would confer upon a cell the ability to adhere to E-cadherin–Fc, adhesion assays were performed with JY′ cells transfected with a full-length human αE-encoding cDNA construct. JY′ cells, or JY′ cells transfected with vector only (JY′-vector), expressed the α4β7 integrin, very little β1 integrin, but no αEβ7 by FACS®. JY′ transfected with αE (JY′-αE) had levels of α4 and β1 integrins similar to untransfected or JY′-vector cells, but now also expressed αEβ7 (99% αEβ7 +, MFI ∼80). Both JY′-vector and JY′-αE transfectants also expressed similar levels of αLβ2 (Fig. 4 a).
Figure 4.
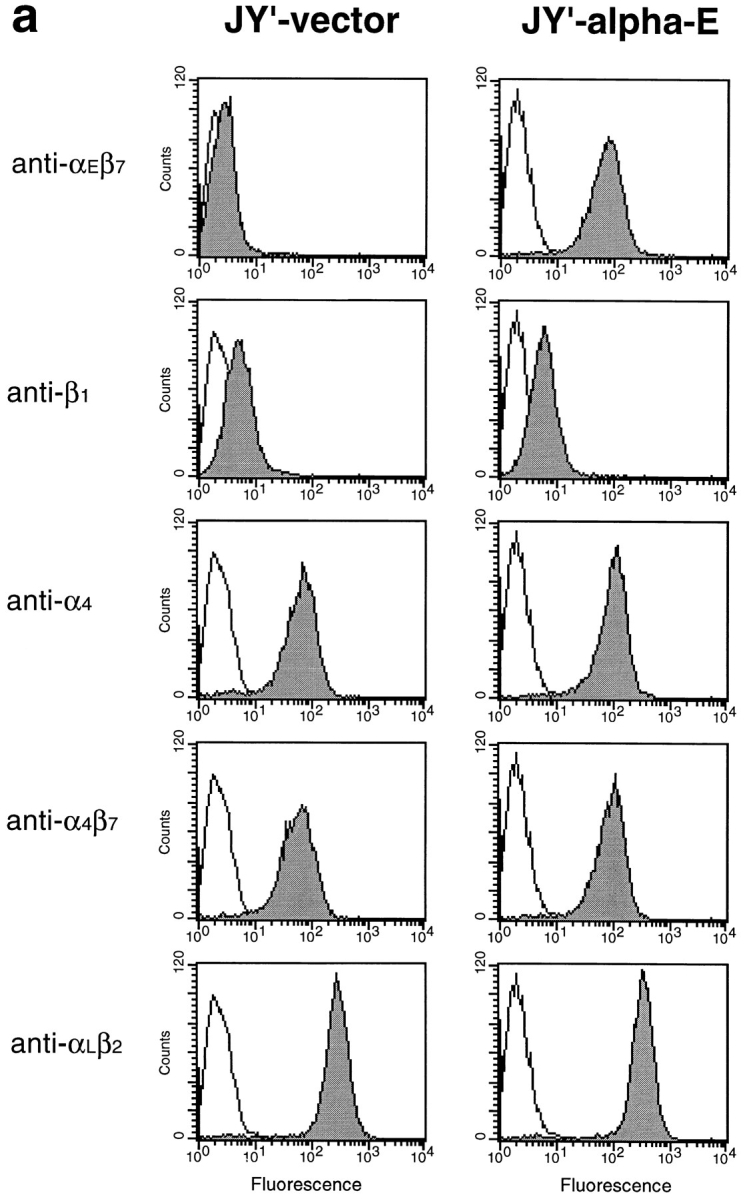
Analysis of JY′ cells transfected with αE cDNA. (a) Flow cytometric analysis of the cell surface expression of integrins on JY′ cells transfected with pSRα-neo vector alone (JY′-vector) or with pSRα-neo/αE (JY′-αE). The staining with control mAb P3 is shown unshaded. Staining with αE7-2 (anti-αEβ7), 4B4 (anti-β1), B5G10 (anti-α4), ACT-1 (anti-α4β7), and TS1/22 (anti-αLβ2) is shown shaded. All mAbs were mouse IgG1. (b) Adhesion of transfected JY′ cells to E-cadherin–Fc. Microtiter plate wells were coated directly with serial dilutions of E-cadherin–Fc or human IgG1, and blocked with BSA. The adhesion of JY′-vector and JY′-αE cells was determined in the presence of 1 mM MnCl2, 1 mM MgCl2, 1 mM CaCl2, 0.1% BSA, HBS, pH 7.4, as described in Materials and Methods. The results are expressed as the mean percent bound ± 1 SD (n = 3). (c) Inhibition of adhesion of JY′-αE cells to E-cadherin–Fc by mAbs. Microtiter plate wells were coated directly with 0.63 μg/well of E-cadherin–Fc or human IgG1, and blocked with BSA. The adhesion of transfected JY′ cells in the presence of various mAbs was determined as described for IEL in Fig. 3 b. The purified mAbs αE7-2, and BerACT8 (anti-αEβ7), ACT-1 (anti-α4β7), 4B4 (anti-β1), D6.21 (anti-αLβ2), E4.6 (anti–E-cadherin), and RR1/1 (anti–ICAM-1) were used at 10 μg/ml. Ascites fluid containing TS1/18 (anti-β2) was used at a dilution of 1:100. All mAbs were mouse IgG1. The results are expressed as the mean percent bound + 1 SD (n = 4). Adhesion of JY′-vector and JY′-αE cells to human IgG1 in this experiment was <3%.
In the presence of manganese, JY′ cells transfected with vector alone did not adhere to E-cadherin–Fc coated directly on microtiter plate wells at any concentration tested. However, under the washing conditions used, 40% of JY′-αE cells adhered to 600 ng/well E-cadherin–Fc (Fig. 4 b). In contrast, both cell lines adhered to ICAM-1–Fc (not shown) while neither adhered to the control Fc protein human IgG1 (Fig. 4 b). The higher percent binding observed for IEL to E-cadherin–Fc when compared with transfected JY′ cells probably reflects the higher surface expression of αEβ7 on IEL (on IEL, mean fluorescence intensity [MFI] ∼600; on JY′-αE, MFI ∼80).
The adhesion of JY′-αE cells to E-cadherin–Fc was blocked from 40% cells bound to <5% (the level seen for JY′-vector cells) by mAbs to αEβ7 (αE7-2 and BerACT8) and E-cadherin–Fc (E4.6), but not by blocking mAbs to αLβ2, α4β7, β1, or β2 integrins or to ICAM-1 (Fig. 4 c). In contrast, the adhesion of JY′-αE cells to ICAM-1–Fc was blocked by antibodies to αLβ2 (TS1/22 and D6.21), β2 integrins (TS1/18), and ICAM-1 (RR1/1), but not by antibodies to αEβ7, α4β7, or β1 integrins (data not shown). These mAb-blocking experiments further confirm that the cell adhesion measured is mediated by αEβ7 binding to E-cadherin–Fc, or by αLβ2 binding to ICAM-1–Fc.
E-Cadherin–Fc Binds Directly to 125I-labeled αEβ7 from a Lysate of IEL
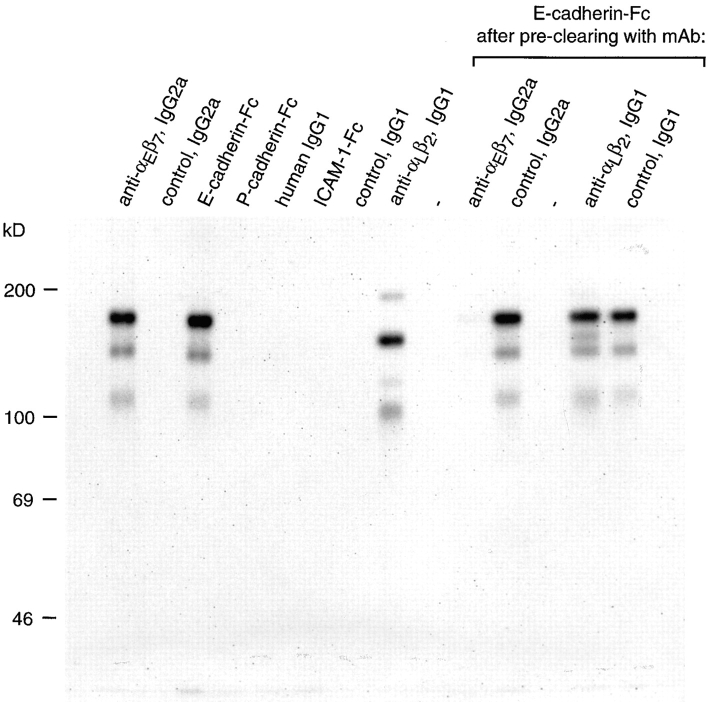
To demonstrate that E-cadherin molecules interact directly with αEβ7 molecules, the proteins that bound to E-cadherin–Fc from a 125I-labeled IEL lysate in the presence of 1 mM MnCl2, 1 mM MgCl2, and 1 mM CaCl2 were analyzed. Since the fusion protein contains a human IgG1 Fc region that binds to protein A, a standard immunoadsorption procedure was used. Proteins of the expected size for αEβ7 (175, 135, and 110 kD) and αLβ2 (160 and 100 kD) were visible on SDS-PAGE in nonreducing conditions after immunoprecipitation with anti–αEβ7 and anti–αLβ2 mAbs, respectively (Fig. 5). Remarkably, E-cadherin–Fc bound to radiolabeled species identical in size and relative intensity to those seen with the anti–αEβ7 mAb (Fig. 5, compare lanes 1 and 3), but distinct from those seen with the anti–αLβ2 mAb (Fig. 5, compare lanes 3 and 8). In contrast, no radiolabeled species were detected binding to the P-cadherin–Fc or IgG1 proteins. After immunoadsorption with the ICAM-1–Fc, proteins of the expected size for αLβ2 could be visualized only on overexposed phosphorimages (not shown). While the same number of cell equivalents were used in each of the human IgG1 containing protein-binding experiments, ∼40-fold fewer cell equivalents were required to immunoadsorb an equal amount of radiolabeled αEβ7 with the anti–αEβ7 mAb compared with E-cadherin–Fc (see legend to Fig. 5). This is consistent with the expected lower affinity of a cell adhesion receptor interaction compared with that of an antibody–antigen interaction.
Figure 5.
Binding of αEβ7 from an IEL lysate to E-cadherin–Fc. Cultured intestinal IEL were surface labeled with 125I and solubilized as described in the text. Batches of lysate were subjected to immunoadsorption with mAbs or human IgG1 Fc containing proteins in the presence of 1 mM MnCl2, 1 mM MgCl2, 1 mM CaCl2. Samples were resolved by 7.5% SDS-PAGE in nonreducing conditions, and visualized by autoradiography. The precipitations with αE7-1 and RPC5.4 mAbs (both mouse IgG2a) represent the material obtained using 0.1 μl ascites from 7 × 104 cell equivalents, for TS1/22 and P3 mAbs (both mouse IgG1, with rabbit anti–mouse IgG) using 0.5 μl ascites from 7 × 105 cell equivalents, and for E-cadherin–Fc, P-cadherin–Fc, ICAM-1–Fc and human IgG1 using 5 μg fusion protein from 3 × 106 cell equivalents. For the preclearing experiments, batches of 1.5 × 106 cell equivalents were preabsorbed with the stated antibodies and protein A–Sepharose, before immunoadsorption with 2.5 μg E-cadherin–Fc.
To further confirm that the proteins bound by anti-αEβ7 and the E-cadherin fusion protein were the same, batches of lysate were precleared with mAbs to αEβ7 (αE7-1), αLβ2 (TS1/22), or the control mAbs RPC5.4 and P3, before exposure to E-cadherin–Fc (Fig. 5). Prior immunodepletion with the anti–αEβ7 mAb prevented the subsequent binding of material from the IEL lysate to E-cadherin–Fc. Preclearing with the other mAbs had no such effect. Thus, the predominant 125I-labeled protein from the IEL cell surface that interacts with E-cadherin–Fc in these conditions is αEβ7.
E-Cadherin–Fc Binds to Soluble Recombinant αEβ7
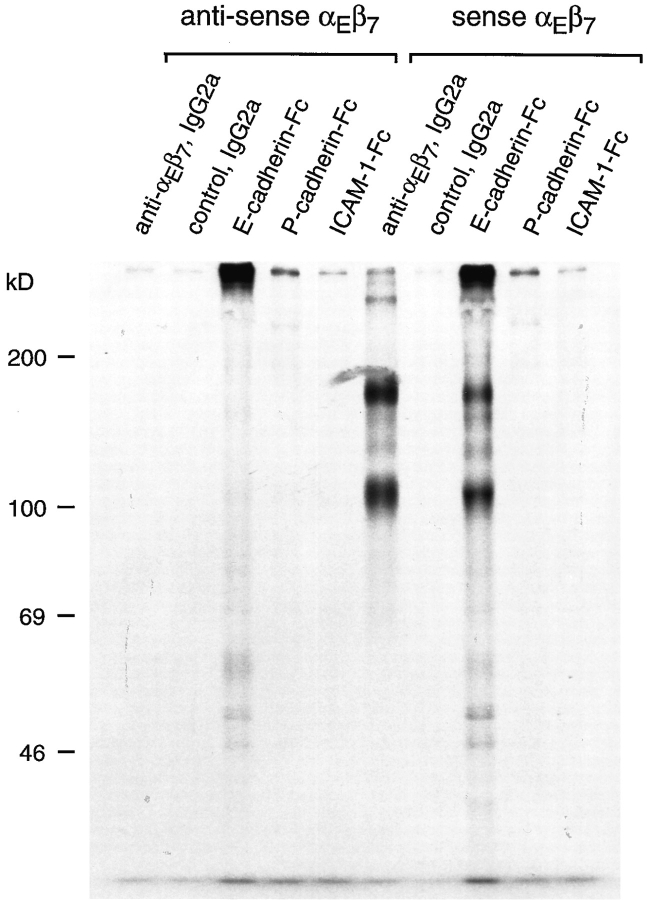
To produce a soluble form of αEβ7, stop codons were introduced immediately upstream of the transmembrane coding regions in the cDNAs encoding the human αE and β7 proteins (see Fig. 1 b, and Materials and Methods). Supernatant containing 35S-labeled soluble αEβ7 was produced by transient transfection of COS-7 cells followed by metabolic labeling. Proteins in the supernatant were then subjected to immunoprecipitation with a panel of anti– αEβ7 antibodies that recognize at least three distinct αE epitopes (Russell et al., 1994). In the medium from cells transfected with plasmids encoding truncated αE and β7 in the sense orientation, all the anti–αEβ7 mAbs tested (αE7-1, αE7-2, αE7-3, HML-1, and BerACT8), but not a control mAb (TCR-δ1), precipitated two major bands of 140 and 105 kD on reducing SDS-PAGE. On nonreducing SDS-PAGE, two bands of 170 and 100 kD were observed (Fig. 6 and data not shown). These sizes correspond to those expected for the truncated αE and β7 chains, respectively. No such proteins were precipitated from the medium of COS-7 cells transfected with constructs containing truncated αE and β7 cDNAs in the antisense orientation. These results confirm the secretion of a soluble form of αEβ7 that retains all of the epitopes of cell surface αEβ7 that were tested.
Figure 6.
Direct binding of soluble recombinant αEβ7 to E-cadherin–Fc. COS-7 cells transiently transfected with either αE and β7 constructs in the sense or in the antisense orientation were metabolically labeled with 35S amino acids as described in the text. The medium was then made 1 mM with respect to MnCl2 and subjected to immunoadsorption with antibodies or Fc-fusion proteins. Samples were resolved on 7.5% SDS-PAGE in nonreducing conditions, and visualized by autoradiography. The precipitations with αE7-1 and RPC5.4 mAbs (both mouse IgG2a) represent the material obtained using 0.25 ml medium and 0.5 μl ascites, and for E-cadherin–Fc, P-cadherin–Fc, and ICAM-1–Fc, using 1.0 ml medium and 5 μg fusion protein. Aggregated material is present in the both control and test E-cadherin–Fc adsorptions.
We then sought to demonstrate binding of recombinant soluble αEβ7 integrin to the human E-cadherin–Fc fusion. Both the anti–αEβ7 mAb αE7-1 and the E-cadherin–Fc protein were able to bind proteins of the expected size for soluble αEβ7 (Fig. 6). No detectable soluble αEβ7 bound to the control mAb RPC5.4, or the fusion proteins P-cadherin–Fc and ICAM-1–Fc. Furthermore, these proteins were not bound by E-cadherin–Fc in medium from COS-7 cells transfected with the antisense αE and β7 constructs (Fig. 6). Thus, E-cadherin–Fc interacts with soluble αEβ7 in the absence of other cellular proteins that are present in the IEL lysate used previously.
The Avidity of Cell Surface αEβ7 Is Regulated
Although the avidity of many integrins is regulated from within the cell, similar regulation of αEβ7 has not previously been reported. Furthermore, the structure of the αE chain is unique among integrins due to the presence of an extra domain in the extracellular region, membrane distal of the A domain, that is cleaved in the mature protein (the “X” domain, see Fig. 1 b; Shaw et al., 1994). This raises the possibility that regulation of the avidity of αEβ7 due to conformational changes could be different from other integrins. Therefore, studies were performed to investigate the regulation of αEβ7 avidity on JY′-αE cells and IEL, and to compare it to the well studied αLβ2 integrin.
JY′ cells transfected with αE adhered poorly to E-cadherin–Fc in the presence of 1 mM MgCl2 and 1 mM CaCl2 in the absence of manganese. The addition of 1 mM manganese caused a 12-fold or greater rise in the adhesion of JY′-αE cells to E-cadherin–Fc. PMA stimulation of JY′-αE also caused a sevenfold increase in binding to E-cadherin–Fc (Fig. 7 a). Adhesion of JY′-αE cells to ICAM-1–Fc was also enhanced by manganese or PMA, suggesting that both αEβ7 and αLβ2 can be activated by similar means. Relative to the effect of manganese, PMA was better able to stimulate adhesion of JY′-αE to ICAM-1–Fc than to E-cadherin–Fc. The reason for this difference is unclear, but valid comparisons are difficult since αLβ2 is expressed at a higher level than αEβ7 on JY′-αE cells (Fig. 3 a).
Figure 7.
Regulation of JY′-αE cell and IEL adhesion to E-cadherin–Fc and ICAM-1–Fc. (a) The adhesion of JY′-αE cells to 0.6 μg/well directly coated E-cadherin–Fc and ICAM-1–Fc under various conditions. EDTA (10 mM) was present during coating and blocking of the microtiter plates, and was washed out with HBS before adding cells. The adhesion buffer was 0.1% BSA/ HBS, pH 7.4, containing divalent cations and 50 ng/ml PMA or 1 mM EGTA where appropriate. The results are expressed as mean percent adhesion + 1 SD (n = 6 for adhesion to E-cadherin–Fc, n = 3 for adhesion to ICAM-1–Fc). The percent adhesion to human IgG1 under the each condition was subtracted from the results (<5% in each case). (b) The adhesion of IEL to 0.1 μg/well E-cadherin–Fc under various conditions. The adhesion buffer was 0.05 mM MgCl2, 1 mM CaCl2, 0.1% BSA, HBS, pH 7.4, unless otherwise stated. Ascites containing antibodies (all mouse IgG2a) to MHC I (W6/32), CD8α (OKT8), nonbinding control (RPC5.4), and CD3 (SPVT3b) were used at a dilution of 1:100 and cross-linked with 10 μg/ml rabbit anti–mouse IgG polyclonal antibody. Where appropriate, 1 mM MnCl2 or 50 ng/ml PMA was also included. The results are expressed as mean percent adhesion + 1 SD (n = 6, except for experiments with OKT8 and RPC5.4 where n = 3). In both a and b, P values calculated by Welch's alternate t test compared with the Mg/Ca alone are as follows: ***P < 0.0001, **P < 0.001, *P < 0.01. In addition, for adhesion to E-cadherin–Fc, P values by the Mann-Whitney test compared with Mg/Ca alone are: ***P = 0.002, **P < 0.005 (see Materials and Methods).
In contrast to JY′ transfectants, >90% of IEL adhered to E-cadherin–Fc in 1 mM MgCl2 and 1 mM CaCl2 and the addition of manganese or PMA had little enhancing effect on adhesion (not shown). Thus IEL, which are maintained in culture in the presence of IL-2 with periodic PHA-stimulation, have constitutively active αEβ7 in these conditions. To study regulation of αEβ7 on IEL, we carried out assays in 0.05 mM MgCl2 and 1 mM CaCl2. In these conditions of limiting MgCl2, just under 40% of IEL adhere to E-cadherin–Fc (Fig. 7 b). The addition of manganese or PMA causes an almost twofold increase in the percentage of IEL adhering to E-cadherin–Fc. Moreover, cross-linking of the TCR complex with a mouse anti–CD3 mAb, followed by anti–mouse immunoglobulin polyclonal antibody, also causes a significant increase in IEL adhesion to E-cadherin–Fc (P < 0.0001, by Welch's alternate t test, see Fig. 7 b). Similar treatment of IEL with antibodies to CD8α or MHC class I had no such effect. Cross-linking of the TCR on IEL also increases the avidity of αLβ2 for ICAM-1, and of β1-integrins for fibronectin (data not shown), as has been reported for other T cells (Dustin and Springer, 1989; Shimizu et al., 1990; van Kooyk et al., 1989).
It is known that calcium is required for the rigidification of the structure of E-cadherin (Nagar et al., 1996; Pokutta et al., 1994) and for E-cadherin–mediated homotypic cell to cell adhesion (Hyafil et al., 1981; Yoshida and Takeichi, 1982). However, in the presence of magnesium, even when calcium has been depleted with EGTA, both JY′-αE cells (Fig. 7 a) and IEL (not shown) adhere to E-cadherin–Fc. Thus, the heterophilic interaction of E-cadherin appears to be independent of calcium, at least in this system. It is unlikely that this is due to restriction of conformational changes within plate-bound E-cadherin–Fc, since similar results were found whether the fusion protein was immobilized directly on plastic or through its Fc region on antiimmunoglobulin antibodies (not shown). In contrast, the adhesion of E-cadherin–expressing 16E6.A5 epithelial cells to E-cadherin–Fc is dependent on calcium (not shown). Also, since mouse E-cadherin does not have appreciable affinity for magnesium (Hyafil et al., 1981), and magnesium cannot support homophilic adhesion to E-cadherin–Fc (not shown), it is unlikely that magnesium substitutes for calcium in the cadherin structure. The results also suggest that calcium is not required for the function of αEβ7 integrin. This is also the case for αLβ2 binding to ICAM-1 (Fig. 7 a; Shimizu and Mobley, 1993; Stewart et al., 1996).
In summary, αEβ7, like αLβ2, can exist in both high and low avidity states on the cell surface. In common with αLβ2–mediated adhesion to ICAM-1, treatment of cells expressing αEβ7 in a low avidity state with manganese, with PMA, with anti–CD3 antibodies, or by removing calcium and elevating magnesium, leads to increased adhesion to E-cadherin–Fc.
The Direct Binding of E-Cadherin–Fc to Solubilized αEβ7 Is Modulated by Divalent Cations
We wished to determine if the effects of divalent cations on cell adhesion to E-cadherin–Fc were due to a direct influence on the binding of αEβ7 molecules to E-cadherin– Fc molecules. The binding of E-cadherin–Fc to 125I-labeled αEβ7 in an IEL lysate was analyzed by immunoadsorption as described in Fig. 5, but in the presence of various concentrations of divalent cations (Fig. 8). No binding of E-cadherin–Fc to αEβ7 was detected in the presence of 5 mM EDTA, confirming the cation dependency of the interaction (Fig. 8 b). Binding of E-cadherin–Fc to αEβ7 was clear in the presence of 1 mM MgCl2 and 1 mM CaCl2, but was increased eightfold by the addition of 1 mM MnCl2 (Fig. 8 b). Approximately equal quantities of αEβ7 were immunoadsorbed by the anti–αEβ7 mAb αE7-1 in all conditions, confirming that the differences seen in binding to the E-cadherin–Fc fusion protein were not due to dissociation or degradation of the αEβ7 chains (Fig. 8 a). Furthermore, since cadherins are known to be protease sensitive in the absence of calcium, we confirmed that an equal quantity of E-cadherin–Fc protein was present in each condition by Coomassie Blue staining of the immunoadsorbed material (Fig. 8 b, ii).
Figure 8.
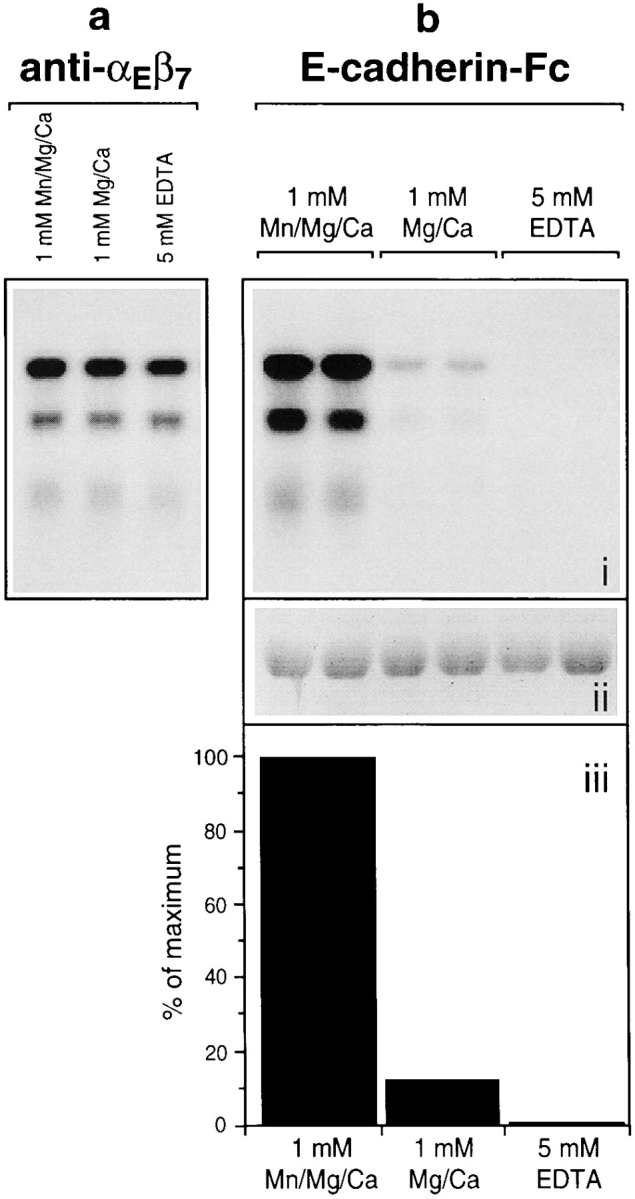
Direct binding of αEβ7 to E-cadherin–Fc is modulated by divalent cations. (a) Immunoadsorption of αEβ7 by an anti– αEβ7 mAb is unaffected by divalent cations. Batches of 125I-labeled IEL lysate were subjected to immunoadsorption with the αE7-1 mAb in the presence of divalent cations or EDTA as shown. Samples were resolved by 7.5% SDS-PAGE in nonreducing conditions, and αEβ7 was visualized by autoradiography. (b) Immunoadsorption of αEβ7 by E-cadherin–Fc is modulated by divalent cations. (i) In duplicate, batches of 125I-labeled IEL lysate were subjected to immunoadsorption with E-cadherin–Fc in the conditions shown in a. (ii) The presence of an equal quantity of E-cadherin–Fc in the immunoadsorbed material was confirmed by 7.5% SDS-PAGE in reducing conditions and Coomassie blue staining. (iii) Quantitation of αEβ7 bound to E-cadherin–Fc in each condition. Phosphorimage quantitation of αEβ7 was based upon the 175- and 135-kD bands corresponding to the αE-chain (Shaw et al., 1994). The averages of duplicates for each band were combined. The precipitations with αE7-1 represent the material obtained using 0.05-μl ascites from 6 × 104 cell equivalents and for E-cadherin–Fc using 5 μg fusion protein from 2 × 106 cell equivalents.
Thus, the cation dependency of αEβ7-mediated cell adhesion to E-cadherin–Fc is paralleled by the cation dependency of αEβ7 binding directly to E-cadherin–Fc. Also, the association of αE with β7 chains is not dependent on calcium or magnesium, as found for mouse αEβ7 (Kilshaw and Murant, 1991), but in contrast to mouse α4β7, an integrin that requires calcium for heterodimer formation in similar experiments (Holzmann et al., 1989).
Discussion
Here, we demonstrate that an E-cadherin fusion protein binds directly both to αEβ7 from solubilized intraepithelial lymphocytes, and to a soluble recombinant form of αEβ7. Furthermore, purified E-cadherin fusion protein can serve as a potent substrate for cell adhesion through the αEβ7 integrin. Thus, although E-cadherin is classically thought to be a homophilic adhesion molecule and is unlike known integrin ligands, it is a direct counter receptor for the αEβ7 integrin.
We found that αEβ7 exhibits selectivity in binding to cadherins. The adhesion of IEL cells to P-cadherin–Fc requires >100-fold more fusion protein than adhesion to E-cadherin–Fc. In both cases the adhesion is dependent on αEβ7, since it is abolished by antibodies to αEβ7. Thus, although human epithelial cells express both E- and P-cadherin, it is likely that E-cadherin is the primary epithelial receptor for αEβ7 in vivo. The idea that αEβ7 is selective in binding cadherins is consistent with the proposed role of αEβ7 in tissue-specific leukocyte adhesion, and with the finding that IEL do not adhere to all cadherin-expressing cells. For example, IEL do not adhere to endothelial cells that possess VE-cadherin (Cepek et al., 1994).
Regulation of αEβ7 avidity for its counter receptor has not previously been reported. An increase in IEL adhesion to epithelial cells in the presence of manganese has been observed (Cepek et al., 1993; Karecla et al., 1995). However, it is not clear that this change is due to regulation of αEβ7 binding to E-cadherin since other receptors, including αLβ2 and ICAM-1, are involved in this complex cell to cell interaction (Cepek et al., 1993; Roberts et al., 1993). Here, the adhesion of cells expressing both αEβ7 and αLβ2 to purified E-cadherin–Fc or ICAM-1–Fc allowed us to compare the regulation of αEβ7 avidity with that of αLβ2 in a system in which only a single integrin counter receptor was available in each case. The αLβ2 integrin on freshly isolated PBL has low avidity for immobilized ICAM-1 in the presence of 1 mM MgCl2 and 1 mM CaCl2. However, in the presence of manganese ions PBL adhere strongly to purified ICAM-1 (Dransfield et al., 1992). Changes in the concentrations and presence of divalent cations are thought to have a direct effect on the conformation of the integrin at the cell surface, leading to increased ligand binding through an increase in the affinity of the integrin and/or changes in clustering of the integrin in the membrane. In addition, signaling from inside the cell can lead to increased integrin avidity. For example, PMA stimulation of resting PBL leads to increased adhesion to ICAM-1 through αLβ2. This type of regulation may involve changes in integrin clustering on the cell surface due to alterations in integrin association with the cytoskeleton (Lub et al., 1995, 1997; Stewart et al., 1996). In contrast to resting PBL, αLβ2 on a proportion of IL-2/PHA-activated PBL has high avidity for ICAM-1 (Lub et al., 1997). Furthermore, the avidity state of integrins transfected into different cell types can vary. For example, αLβ2 expressed on K562 cells is in a constitutively inactive state, while αLβ2 expressed on L cells is constitutively active (Lub et al., 1995).
We find that in 1 mM MgCl2 and 1 mM CaCl2, αE-transfected JY′ cells exhibit poor adhesion to both E-cadherin–Fc and ICAM-1–Fc. Thus, on these cells, both αEβ7 and αLβ2 exist in a low avidity state. However, these cells can be induced to adhere to both integrin ligands by the addition of 1 mM MnCl2. Since parallel changes in the direct binding of solubilized αEβ7 to E-cadherin–Fc were also observed, it is likely that this difference in cell adhesiveness is due to a direct effect of manganese on the conformation of the αEβ7 integrin. Thus, changes in extracellular cations can regulate the affinity of αEβ7 for its ligand. In contrast, cultured IEL, which are maintained in IL-2 with periodic stimulation with PHA and feeder cells, adhere avidly to both E-cadherin–Fc and ICAM-1–Fc in 1 mM MgCl2 and 1 mM CaCl2 even in the absence of manganese. Both αEβ7 and αLβ2 on IEL appear to be maintained in a constitutively active state, like αLβ2 on a proportion of IL-2/PHA-stimulated peripheral blood T cells.
We also show that signaling from inside the cell is able to increase the avidity of αEβ7. The addition of the phorbol ester, PMA, which acts intracellularly to upregulate protein kinase C, leads to increased adhesion of αE-transfected JY′ cells to E-cadherin–Fc or ICAM-1–Fc. In the presence of a suboptimal concentration of magnesium, PMA also enhanced IEL adhesion to E-cadherin–Fc. The physiological triggers for such changes in lymphocyte integrin avidity in vivo remain unclear. In vitro, chemokines such as MCP-1, MIP-1β, and RANTES can activate lymphocyte integrins (Campbell et al., 1996; Carr et al., 1996; Lloyd et al., 1996), and cross-linking of components of the T cell receptor can boost the adhesion of resting PBL to ICAM-1 through αLβ2 (Dustin and Springer, 1989; van Kooyk et al., 1989) or to fibronectin and laminin through β1-integrins (Shimizu et al., 1990). Here, we report that antibody cross-linking of cell surface CD3 is similarly able to increase IEL adhesion to E-cadherin–Fc, while cross-linking of MHC class I or CD8 receptors had no such effect. Thus, recognition by an IEL of an antigen presented by an epithelial cell or a professional antigen-presenting cell expressing E-cadherin (such as a Langerhans cell; Tang et al., 1993) could trigger increased adhesion to that cell through an upregulation of αEβ7 avidity. This interaction may also provide costimulation to the T cell, since anti–αEβ7 antibodies, in common with anti–αLβ2 antibodies, are known to increase T cell proliferation in the presence of suboptimal anti-CD3 (Russell et al., 1994; Sarnacki et al., 1992; Wacholtz et al., 1989), and ICAM-1 on an antigen-presenting cell can costimulate through αLβ2 (Dubey et al., 1995). Such events may be important in arresting lymphocytes within the epithelium when a specific antigen is recognized. In addition, since IEL contact more than one cell when resident within the epithelium, localized upregulation of αEβ7 avidity within an IEL could aid in polarizing lymphocyte interactions toward the relevant antigen-presenting cell.
X-ray crystallography and NMR studies have recently revealed that cadherin modules adopt a tertiary structure rather like immunoglobulin domains (Overduin et al., 1995; Shapiro et al., 1995; Nagar et al., 1996). Thus E-cadherin has a structure resembling that of the known cellular integrin ligands and can now be placed within the family of immunoglobulin-like, integrin-binding proteins. E-cadherin may share another feature of well-defined integrin ligands; the presence of a solvent-exposed acidic residue vital for integrin binding (Bergelson and Hemler, 1995). Mutation of an aspartate or glutamate residue in the CD-loop region in domain 1 of the immunoglobulin superfamily integrin ligands VCAM-1 (Osborn et al., 1994; Renz et al., 1994; Vonderheide et al., 1994; Jones et al., 1995; Wang et al., 1995), ICAM-1, (Staunton et al., 1990; Holness et al., 1995), and MadCAM-1 (Briskin et al., 1996; Viney et al., 1996) abolishes integrin binding. The tenth immunoglobulin-like FN III repeat of fibronectin also has an acidic residue within the RGD sequence on the FG-loop that is involved in integrin binding (Main et al., 1992). In mouse E-cadherin, the BC loop of domain 1 contains a glutamate residue required for adhesion of αEβ7-expressing lymphocytes to E-cadherin–transfected L cells (Karecla et al., 1996). Since this residue is conserved in human E-cadherin and we demonstrate that E-cadherin is a direct counter receptor for αEβ7, it is likely that this amino acid contributes directly to the αEβ7-binding site on E-cadherin, in a manner similar to that proposed for other integrin ligands. It is interesting to note that while three different families of immunoglobulin-like structures with exposed acidic amino acids serve as integrin ligands, the face of the module involved appears to be different in each case. It is also intriguing that both E-cadherin and ICAM-1 are likely to be dimeric on the cell surface (Miller et al., 1995; Reilly et al., 1995; Nagar et al., 1996), and that both are ligands of integrins that contain an A domain in the α chain (αEβ7 and αLβ2, respectively). Since it has been proposed that integrin β-chains also contain a ligand-binding A domain (Lee et al., 1995; Puzon-McLaughlin and Takada, 1996), it is possible that αEβ7 and αLβ2 actually possess two A domains each. As suggested for αLβ2 binding to ICAM-1 (Miller et al., 1995), it is tempting to speculate that the binding of dimeric E-cadherin by αEβ7 could involve both these domains.
Although E-cadherin is a calcium-binding molecule, we find that E-cadherin–Fc binding to αEβ7 is independent of calcium. It seems that calcium is not directly involved in maintenance of the αEβ7 binding site on E-cadherin. Studies with recombinant soluble forms of mouse E-cadherin and Xenopus C-cadherin suggest that homophilic cadherin interactions do require the presence of calcium (Brieher et al., 1996; Tomschy et al., 1996). Interestingly, in their NMR studies of the first module of mouse E-cadherin, Overduin et al. (1995) find that the addition of calcium leads to a large shift in the orientation of histidine-79 within the HAV motif implicated in homophilic E-cadherin interaction (Blaschuk et al., 1990; Shapiro et al., 1995). In contrast, no such shift is found in any of the residues in or around the BC-loop implicated in αEβ7 interaction (Overduin et al., 1995). Thus it is possible that changes in extracellular calcium levels (for review see Maurer et al., 1996) would differentially regulate E-cadherin binding to αEβ7 versus other E-cadherin molecules. However, since calcium is required to rigidify and extend E-cadherin (Pokutta et al., 1994; Nagar et al., 1996) and to protect it from proteolysis (Hyafil et al., 1981; Yoshida and Takeichi, 1982), the lack of calcium dependence for αEβ7-mediated adhesion to the E-cadherin–Fc fusion protein may not hold true on the cell surface.
Although the role of αEβ7 binding to E-cadherin in vivo has yet to be established, the direct, specific, and regulated binding of αEβ7-expressing cells and of αEβ7 itself to the E-cadherin fusion protein very strongly suggests a physiological function for this interaction. Although there is an increase in the number of β7-integrin positive iIEL in chimeric mice with a defect in intestinal E-cadherin expression (Hermiston and Gordon, 1995), this is perhaps not surprising since the observed disruption of the epithelial layer and accompanying infectious and inflammatory response is likely to result in the attraction of many leukocytes into the intestine. In contrast, recently developed αE knockout mice have reduced numbers of intraepithelial IEL (Parker, C.M., unpublished results).
The role in vivo of the low but detectable αEβ7-mediated adhesion of IEL to human P-cadherin is more difficult to assess. The relative abundance of E- and P-cadherin on epithelial cells depends upon the state of cell differentiation (Hirai et al., 1989a ,b), but this may not be the sole determinant of αEβ7-mediated interactions. On a single cell type, cadherins can display differential association with the cytoskeleton and thus exist in distinct pools on the cell surface (Salomon et al., 1992), and E- and P-cadherin are found in separate complexes on A431 cells (Johnson et al., 1993). Cell adhesion through homophilic cadherin–cadherin interactions requires their association with the cytoskeleton through intracellular catenins (Nagafuchi and Takeichi, 1988). However, while E-cadherin lacking its cytoplasmic tail is unable to associate with the catenins and cannot support strong homophilic adhesion, it is able to support adhesion of lymphocytes expressing αEβ7 (Karecla et al., 1996). So, since different pools of cadherins may differ in their availability for interaction with different counter receptors, it is difficult to predict whether or not a high local concentration of a distinct population of P-cadherin on the cell surface could contribute to αEβ7-mediated adhesion.
Like human, mouse, canine, and Xenopus E-cadherin, human P-cadherin possesses an acidic residue at the tip of the BC loop in the first cadherin module (Shimoyama et al., 1989b). Thus human E- and P-cadherin may share a similar mode of binding to αEβ7. In contrast, mouse P-cadherin does not possess an acidic residue at this position (Nose et al., 1987), and it has been reported that murine lymphocytes expressing αEβ7 do not adhere to L cells transfected with mouse P-cadherin (Karecla et al., 1996). Although this cell to cell adhesion assay is likely to be less sensitive than the cell to fusion protein assay used here, these findings further complicate the question of the potential importance of αEβ7 binding to P-cadherin. It is possible that in some respects the function of P-cadherin may differ in the two species, and this may be reflected in the different expression pattern of P-cadherin in human and mouse (Shimoyama et al., 1989a,b).
Recently, a second direct heterophilic noncadherin ligand of a cadherin has been identified. The Listeria surface protein internalin binds to E-cadherin and invasion of cells expressing E-cadherin by Listeria in vitro is inhibited by anti–E-cadherin antibodies (Mengaud et al., 1996). Together with studies suggesting that different cadherins can bind to each other (for example N- and R-cadherin) (Takeichi, 1995), it is now clear that the biology of cadherin function is not limited to homophilic interactions, and is more complex than previously imagined.
Acknowledgments
This work was supported by a Wellcome Trust International Prize Travelling Research Fellowship (J.M.G. Higgins), and grants from the National Institutes of Health (NIH; M.B. Brenner), NIH grant AI01212 (D.A. Mandlebrot), the Crohn's and Colitis Foundation of America and NIH grant DK43351 (G.J. Russell), an NIH R29 First Award GM49342 and a Cancer Research Institute Investigator Award (C.M. Parker), NIH grant GM35527 (W.J. Nelson), and a postdoctoral fellowship from the National Kidney Foundation (Y.-T. Chen).
Abbreviations used in this paper
- ICAM
intracellular adhesion molecule
- iIEL
intestinal intraepithelial lymphocytes
- MadCAM
mucosal addressin cell adhesion molecule
- MFI
mean fluorescence intensity
- PBL
peripheral blood lymphocytes
- TCR
T cell receptor
- VCAM
vascular cell adhesion molecule
References
- Band H, Hochstenbach F, McLean J, Hata S, Krangel MS, Brenner MB. Immunochemical proof that a novel rearranging gene encodes the T cell receptor δ subunit. Science. 1987;238:682–684. doi: 10.1126/science.3672118. [DOI] [PubMed] [Google Scholar]
- Barnstable CJ, Bodmer WF, Brown G, Galfre G, Milstein C, Williams AF, Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978;14:9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Ben-Ze'ev A. Cytoskeletal and adhesion proteins as tumor suppressors. Curr Opin Cell Biol. 1997;9:99–108. doi: 10.1016/s0955-0674(97)80158-5. [DOI] [PubMed] [Google Scholar]
- Bergelson JM, Hemler ME. Integrin-ligand binding. Do integrins use a ‘MIDAS touch' to grasp an Asp? . Curr Biol. 1995;5:615–617. doi: 10.1016/s0960-9822(95)00124-2. [DOI] [PubMed] [Google Scholar]
- Blaschuk OW, Sullivan R, David S, Pouliot Y. Identification of a cadherin cell adhesion recognition sequence. Dev Biol. 1990;139:227–229. doi: 10.1016/0012-1606(90)90290-y. [DOI] [PubMed] [Google Scholar]
- Blystone SD, Lindberg FP, LaFlamme SE, Brown EJ. Integrin β3 cytoplasmic tail is necessary and sufficient for regulation of α5β1 phagocytosis by αvβ3and integrin-associated protein. J Cell Biol. 1995;130:745–754. doi: 10.1083/jcb.130.3.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller K, Vestweber D, Kemler R. Cell-adhesion molecule uvomorulin is localized in the intermediate junctions of adult intestinal epithelial cells. J Cell Biol. 1985;100:327–332. doi: 10.1083/jcb.100.1.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brieher WM, Yap AS, Gumbiner BM. Lateral dimerization is required for the homophilic binding activity of C-cadherin. J Cell Biol. 1996;135:487–496. doi: 10.1083/jcb.135.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briskin MJ, Rott L, Butcher EC. Structural requirements for mucosal vascular addressin binding to its lymphocyte receptor α4β7. Common themes among integrin-Ig family interactions. J Immunol. 1996;156:719–726. [PubMed] [Google Scholar]
- Campbell JJ, Qin S, Bacon KB, Mackay CR, Butcher EC. Biology of chemokine and classical chemoattractant receptors: differential requirements for adhesion-triggering versus chemotactic responses in lymphoid cells. J Cell Biol. 1996;134:255–266. doi: 10.1083/jcb.134.1.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr MW, Alon R, Springer TA. The C-C chemokine MCP-1 differentially modulates the avidity of β1 and β2 integrins on T lymphocytes. Immunity. 1996;4:179–187. doi: 10.1016/s1074-7613(00)80682-2. [DOI] [PubMed] [Google Scholar]
- Cepek KL, Parker CM, Madara JL, Brenner MB. Integrin αEβ7mediates adhesion of T lymphocytes to epithelial cells. J Immunol. 1993;150:3459–3470. [PubMed] [Google Scholar]
- Cepek KL, Shaw SK, Parker CM, Russell GJ, Morrow JS, Rimm DL, Brenner MB. Adhesion between epithelial cells and T lymphocytes mediated by E-cadherin and the αEβ7integrin. Nature. 1994;372:190–193. doi: 10.1038/372190a0. [DOI] [PubMed] [Google Scholar]
- Cerf-Bensussan N, Jarry A, Brousse N, Lisowska-Grospierre B, Guy-Grand D, Griscelli C. A monoclonal antibody (HML-1) defining a novel membrane molecule present on human intestinal lymphocytes. Eur J Immunol. 1987;17:1279–1285. doi: 10.1002/eji.1830170910. [DOI] [PubMed] [Google Scholar]
- Chan BM, Elices MJ, Murphy E, Hemler ME. Adhesion to vascular cell adhesion molecule 1 and fibronectin. Comparison of α4β1 (VLA-4) and α4β7on the human B cell line JY. J Biol Chem. 1992;267:8366–8370. [PubMed] [Google Scholar]
- Chen YT, Nelson WJ. Continuous production of soluble extracellular domain of a type-I transmembrane protein in mammalian cells using an Epstein-Barr virus ori-P based expression vector. Anal Biochem. 1996;242:276–278. doi: 10.1006/abio.1996.0466. [DOI] [PubMed] [Google Scholar]
- Coligan, J.E., A.M. Kruisbeek, D.H. Margulies, E.M. Shevach, and W. Strober. 1994. Current Protocols in Immunology. R. Coico, editor. Current Protocols. John Wiley & Sons, Inc., New York.
- Díaz-González F, Forsyth J, Steiner B, Ginsberg MH. Trans-dominant inhibition of integrin function. Mol Biol Cell. 1996;7:1939–1951. doi: 10.1091/mbc.7.12.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dransfield I, Cabañas C, Craig A, Hogg N. Divalent cation regulation of the function of the leukocyte integrin LFA-1. J Cell Biol. 1992;116:219–226. doi: 10.1083/jcb.116.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubey C, Croft M, Swain SL. Costimulatory requirements of naive CD4+T cells. ICAM-1 or B7-1 can costimulate naive CD4 T cell activation but both are required for optimum response. J Immunol. 1995;155:45–57. [PubMed] [Google Scholar]
- Dustin ML, Springer TA. T-cell receptor cross-linking transiently stimulates adhesiveness through LFA-1. Nature. 1989;341:619–624. doi: 10.1038/341619a0. [DOI] [PubMed] [Google Scholar]
- Erle DJ. Intraepithelial lymphocytes. Scratching the surface. Curr Biol. 1995;5:252–254. doi: 10.1016/s0960-9822(95)00052-2. [DOI] [PubMed] [Google Scholar]
- Gumbiner B, Stevenson B, Grimaldi A. The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J Cell Biol. 1988;107:1575–1587. doi: 10.1083/jcb.107.4.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatta K, Takagi S, Fujisawa H, Takeichi M. Spatial and temporal expression pattern of N-cadherin cell adhesion molecules correlated with morphogenetic processes of chicken embryos. Dev Biol. 1987;120:215–227. doi: 10.1016/0012-1606(87)90119-9. [DOI] [PubMed] [Google Scholar]
- Hemler ME, Huang C, Takada Y, Schwarz L, Strominger JL, Clabby ML. Characterization of the cell surface heterodimer VLA-4 and related peptides. J Biol Chem. 1987;262:11478–11485. [PubMed] [Google Scholar]
- Hermiston ML, Gordon JI. Inflammatory bowel disease and adenomas in mice expressing a dominant negative N-cadherin. Science. 1995;270:1203–1207. doi: 10.1126/science.270.5239.1203. [DOI] [PubMed] [Google Scholar]
- Hirai Y, Nose A, Kobayashi S, Takeichi M. Expression and role of E- and P-cadherin adhesion molecules in embryonic histogenesis. I. Lung epithelial morphogenesis. Development. 1989a;105:263–270. doi: 10.1242/dev.105.2.263. [DOI] [PubMed] [Google Scholar]
- Hirai Y, Nose A, Kobayashi S, Takeichi M. Expression and role of E- and P-cadherin adhesion molecules in embryonic histogenesis. II. Skin morphogenesis. Development. 1989b;105:271–277. doi: 10.1242/dev.105.2.271. [DOI] [PubMed] [Google Scholar]
- Holness CL, Bates PA, Little AJ, Buckley CD, McDowall A, Bossy D, Hogg N, Simmons DL. Analysis of the binding site on intercellular adhesion molecule 3 for the leukocyte integrin lymphocyte function-associated antigen 1. J Biol Chem. 1995;270:877–884. doi: 10.1074/jbc.270.2.877. [DOI] [PubMed] [Google Scholar]
- Holzmann B, McIntyre BW, Weissman IL. Identification of a murine Peyer's patch-specific lymphocyte homing receptor as an integrin molecule with an α chain homologous to human VLA4α. Cell. 1989;56:37–46. doi: 10.1016/0092-8674(89)90981-1. [DOI] [PubMed] [Google Scholar]
- Hyafil F, Babinet C, Jacob F. Cell-cell interactions in early embryogenesis: a molecular approach to the role of calcium. Cell. 1981;26:447–454. doi: 10.1016/0092-8674(81)90214-2. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Lewis JE, Li D, Wahl J, Soler AP, Knudsen KA, Wheelock MJ. P- and E-cadherin are in separate complexes in cells expressing both cadherins. Exp Cell Res. 1993;207:252–260. doi: 10.1006/excr.1993.1191. [DOI] [PubMed] [Google Scholar]
- Jones EY, Harlos K, Bottomley MJ, Robinson RC, Driscoll PC, Edwards RM, Clements JM, Dudgeon TJ, Stuart DI. Crystal structure of an integrin-binding fragment of vascular cell adhesion molecule-1 at 1.8 Å resolution. Nature. 1995;373:539–544. doi: 10.1038/373539a0. [DOI] [PubMed] [Google Scholar]
- Karecla PI, Bowden SJ, Green SJ, Kilshaw PJ. Recognition of E-cadherin on epithelial cells by the mucosal T cell integrin αM290β7 (αEβ7) Eur J Immunol. 1995;25:852–856. doi: 10.1002/eji.1830250333. [DOI] [PubMed] [Google Scholar]
- Karecla PI, Green SJ, Bowden SJ, Coadwell J, Kilshaw PJ. Identification of a binding site for integrin αEβ7in the N-terminal domain of E-cadherin. J Biol Chem. 1996;271:30909–30915. doi: 10.1074/jbc.271.48.30909. [DOI] [PubMed] [Google Scholar]
- Kilshaw PJ, Murant SJ. Expression and regulation of β7 (βp) integrins on mouse lymphocytes: relevance to the mucosal immune system. Eur J Immunol. 1991;21:2591–2597. doi: 10.1002/eji.1830211041. [DOI] [PubMed] [Google Scholar]
- Kohler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Kruschwitz M, Fritzsche G, Schwarting R, Micklem K, Mason DY, Falini B, Stein H. Ber-ACT8: new monoclonal antibody to the mucosa lymphocyte antigen. J Clin Path. 1991;44:636–645. doi: 10.1136/jcp.44.8.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFlamme SE, Thomas LA, Yamada SS, Yamada KM. Single subunit chimeric integrins as mimics and inhibitors of endogenous integrin functions in receptor localization, cell spreading and migration, and matrix assembly. J Cell Biol. 1994;126:1287–1298. doi: 10.1083/jcb.126.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampugnani MG, Resnati M, Raiteri M, Pigott R, Pisacane A, Houen G, Ruco LP, Dejana E. A novel endothelial-specific membrane protein is a marker of cell-cell contacts. J Cell Biol. 1992;118:1511–1522. doi: 10.1083/jcb.118.6.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarovits AI, Moscicki RA, Kurnick JT, Camerini D, Bhan AK, Baird LG, Erikson M, Colvin RB. Lymphocyte activation antigens. I. A monoclonal antibody, anti-Act I, defines a new late lymphocyte activation antigen. J Immunol. 1984;133:1857–1862. [PubMed] [Google Scholar]
- Lee JO, Rieu P, Arnaout MA, Liddington R. Crystal structure of the A domain from the α subunit of integrin CR3 (CD11b/CD18) Cell. 1995;80:631–638. doi: 10.1016/0092-8674(95)90517-0. [DOI] [PubMed] [Google Scholar]
- Lloyd AR, Oppenheim JJ, Kelvin DJ, Taub DD. Chemokines regulate T cell adherence to recombinant adhesion molecules and extracellular matrix proteins. J Immunol. 1996;156:932–938. [PubMed] [Google Scholar]
- Lub M, van Kooyk Y, Figdor CG. Ins and outs of LFA-1. Immunol Today. 1995;16:479–483. doi: 10.1016/0167-5699(95)80031-x. [DOI] [PubMed] [Google Scholar]
- Lub M, van Kooyk Y, van Vliet SJ, Figdor CG. Dual role of the actin cytoskeleton in regulating cell adhesion mediated by the integrin lymphocyte function-associated molecule-1. Mol Biol Cell. 1997;8:341–351. doi: 10.1091/mbc.8.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Main AL, Harvey TS, Baron M, Boyd J, Campbell ID. The three-dimensional structure of the tenth type III module of fibronectin: an insight into RGD-mediated interactions. Cell. 1992;71:671–678. doi: 10.1016/0092-8674(92)90600-h. [DOI] [PubMed] [Google Scholar]
- Marks MS, Woodruff L, Ohno H, Bonifacino JS. Protein targeting by tyrosine- and dileucine-based signals: evidence for distinct saturable components. J Cell Biol. 1996;135:341–354. doi: 10.1083/jcb.135.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer P, Hohenester E, Engel J. Extracellular calcium-binding proteins. Curr Opin Cell Biol. 1996;8:609–617. doi: 10.1016/s0955-0674(96)80101-3. [DOI] [PubMed] [Google Scholar]
- Mengaud J, Ohayon H, Gounon P, Mege RM, Cossart P. E-cadherin is the receptor for internalin, a surface protein required for entry of L. monocytogenesinto epithelial cells. Cell. 1996;84:923–932. doi: 10.1016/s0092-8674(00)81070-3. [DOI] [PubMed] [Google Scholar]
- Miller J, Knorr R, Ferrone M, Houdei R, Carron CP, Dustin ML. Intercellular adhesion molecule-1 dimerization and its consequences for adhesion mediated by lymphocyte function associated-1. J Exp Med. 1995;182:1231–1241. doi: 10.1084/jem.182.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohit B, Fan K. Hybrid cell line from a cloned immunoglobulin-producing mouse myeloma and a nonproducing mouse lymphoma. Science. 1971;171:75–77. doi: 10.1126/science.171.3966.75. [DOI] [PubMed] [Google Scholar]
- Morimoto C, Letvin NL, Boyd AW, Hagan M, Brown HM, Kornacki MM, Schlossman SF. The isolation and characterization of the human helper inducer T cell subset. J Immunol. 1985;134:3762–3769. [PubMed] [Google Scholar]
- Nagafuchi A, Takeichi M. Cell binding function of E-cadherin is regulated by the cytoplasmic domain. EMBO (Eur Mol Biol Organ) J. 1988;7:3679–3684. doi: 10.1002/j.1460-2075.1988.tb03249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagar B, Overduin M, Ikura M, Rini JM. Structural basis of calcium-induced E-cadherin rigidification and dimerization. Nature. 1996;380:360–364. doi: 10.1038/380360a0. [DOI] [PubMed] [Google Scholar]
- Nose A, Nagafuchi A, Takeichi M. Isolation of placental cadherin cDNA: identification of a novel gene family of cell-cell adhesion molecules. EMBO (Eur Mol Biol Organ) J. 1987;6:3655–3661. doi: 10.1002/j.1460-2075.1987.tb02698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nose A, Takeichi M. A novel cadherin cell adhesion molecule: its expression patterns associated with implantation and organogenesis of mouse embryos. J Cell Biol. 1986;103:2649–2658. doi: 10.1083/jcb.103.6.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn L, Vassallo C, Browning BG, Tizard R, Haskard DO, Benjamin CD, Dougas I, Kirchhausen T. Arrangement of domains, and amino acid residues required for binding of vascular cell adhesion molecule-1 to its counter-receptor VLA-4 (α4β1) J Cell Biol. 1994;124:601–608. doi: 10.1083/jcb.124.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overduin M, Harvey TS, Bagby S, Tong KI, Yau P, Takeichi M, Ikura M. Solution structure of the epithelial cadherin domain responsible for selective cell adhesion. Science. 1995;267:386–389. doi: 10.1126/science.7824937. [DOI] [PubMed] [Google Scholar]
- Parker CM, Groh V, Band H, Porcelli SA, Morita C, Fabbi M, Glass D, Strominger JL, Brenner MB. Evidence for extrathymic changes in the T cell receptor γ/δ repertoire. J Exp Med. 1990;171:1597–1612. doi: 10.1084/jem.171.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokutta S, Herrenknecht K, Kemler R, Engel J. Conformational changes of the recombinant extracellular domain of E-cadherin upon calcium binding. Eur J Biochem. 1994;223:1019–1026. doi: 10.1111/j.1432-1033.1994.tb19080.x. [DOI] [PubMed] [Google Scholar]
- Puzon-McLaughlin W, Takada Y. Critical residues for ligand binding in an I domain-like structure of the integrin beta1 subunit. J Biol Chem. 1996;271:20438–20443. doi: 10.1074/jbc.271.34.20438. [DOI] [PubMed] [Google Scholar]
- Reilly PL, Woska JRJ, Jeanfavre DD, McNally E, Rothlein R, Bormann BJ. The native structure of intercellular adhesion molecule-1 (ICAM-1) is a dimer. Correlation with binding to LFA-1. J Immunol. 1995;155:529–532. [PubMed] [Google Scholar]
- Renz ME, Chiu HH, Jones S, Fox J, Kim KJ, Presta LG, Fong S. Structural requirements for adhesion of soluble recombinant murine vascular cell adhesion molecule-1 to α4β1 . J Cell Biol. 1994;125:1395–1406. doi: 10.1083/jcb.125.6.1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimm DL, Morrow JS. Molecular cloning of human E-cadherin suggests a novel subdivision of the cadherin superfamily. Biochem Biophys Res Commun. 1994;200:1754–1761. doi: 10.1006/bbrc.1994.1656. [DOI] [PubMed] [Google Scholar]
- Roberts AI, O'Connell SM, Ebert EC. Intestinal intraepithelial lymphocytes bind to colon cancer cells by HML-1 and CD11a. Cancer Res. 1993;53:1608–1611. [PubMed] [Google Scholar]
- Rothlein R, Dustin ML, Marlin SD, Springer TA. A human intercellular adhesion molecule (ICAM-1) distinct from LFA-1. J Immunol. 1986;137:1270–1274. [PubMed] [Google Scholar]
- Russell GJ, Parker CM, Cepek KL, Mandelbrot DA, Sood A, Mizoguchi E, Ebert EC, Brenner MB, Bhan AK. Distinct structural and functional epitopes of the αEβ7integrin. Eur J Immunol. 1994;24:2832–2841. doi: 10.1002/eji.1830241138. [DOI] [PubMed] [Google Scholar]
- Russell GJ, Parker CM, Sood A, Mizoguchi E, Ebert EC, Bhan AK, Brenner MB. p126 (CDw101), a costimulatory molecule preferentially expressed on mucosal T lymphocytes. J Immunol. 1996;157:3366–3374. [PubMed] [Google Scholar]
- Salomon D, Ayalon O, Patel-King R, Hynes RO, Geiger B. Extrajunctional distribution of N-cadherin in cultured human endothelial cells. J Cell Sci. 1992;102:7–17. doi: 10.1242/jcs.102.1.7. [DOI] [PubMed] [Google Scholar]
- Sanchez-Madrid F, Krensky AM, Ware CF, Robbins E, Strominger JL, Burakoff SJ, Springer TA. Three distinct antigens associated with human T-lymphocyte-mediated cytolysis: LFA-1, LFA-2, and LFA-3. Proc Natl Acad Sci USA. 1982;79:7489–7493. doi: 10.1073/pnas.79.23.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Madrid F, Nagy JA, Robbins E, Simon P, Springer TA. A human leukocyte differentiation antigen family with distinct alpha-subunits and a common beta-subunit: the lymphocyte function-associated antigen (LFA-1), the C3bi complement receptor (OKM1/Mac-1), and the p150,95 molecule. J Exp Med. 1983;158:1785–1803. doi: 10.1084/jem.158.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarnacki S, Begue B, Buc H, Le Deist F, Cerf-Bensussan N. Enhancement of CD3-induced activation of human intestinal intraepithelial lymphocytes by stimulation of the β7-containing integrin defined by HML-1 monoclonal antibody. Eur J Immunol. 1992;22:2887–2892. doi: 10.1002/eji.1830221120. [DOI] [PubMed] [Google Scholar]
- Shapiro L, Fannon AM, Kwong PD, Thompson A, Lehmann MS, Grübel G, Legrand JF, Als-Nielsen J, Colman DR, Hendrickson WA. Structural basis of cell-cell adhesion by cadherins. Nature. 1995;374:327–337. doi: 10.1038/374327a0. [DOI] [PubMed] [Google Scholar]
- Shaw SK, Cepek KL, Murphy EA, Russell GJ, Brenner MB, Parker CM. Molecular cloning of the human mucosal lymphocyte integrin αEsubunit. Unusual structure and restricted RNA distribution. J Biol Chem. 1994;269:6016–6025. [PubMed] [Google Scholar]
- Shimizu Y, Mobley JL. Distinct divalent cation requirements for integrin-mediated CD4+T lymphocyte adhesion to ICAM-1, fibronectin, VCAM-1, and invasin. J Immunol. 1993;151:4106–4115. [PubMed] [Google Scholar]
- Shimizu Y, Van Seventer GA, Horgan KJ, Shaw S. Regulated expression and binding of three VLA (β1) integrin receptors on T cells. Nature. 1990;345:250–253. doi: 10.1038/345250a0. [DOI] [PubMed] [Google Scholar]
- Shimoyama Y, Hirohashi S, Hirano S, Noguchi M, Shimosato Y, Takeichi M, Abe O. Cadherin cell-adhesion molecules in human epithelial tissues and carcinomas. Cancer Res. 1989a;49:2128–2133. [PubMed] [Google Scholar]
- Shimoyama Y, Yoshida T, Terada M, Shimosato Y, Abe O, Hirohashi S. Molecular cloning of a human Ca2+-dependent cell-cell adhesion molecule homologous to mouse placental cadherin: its low expression in human placental tissues. J Cell Biol. 1989b;109:1787–1794. doi: 10.1083/jcb.109.4.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith TJ, Ducharme LA, Shaw SK, Parker CM, Brenner MB, Kilshaw PJ, Weis JH. Murine M290 integrin expression modulated by mast cell activation. Immunity. 1994;1:393–403. doi: 10.1016/1074-7613(94)90070-1. [DOI] [PubMed] [Google Scholar]
- Spits H, Keizer G, Borst J, Terhorst C, Hekman A, de Vries JE. Characterization of monoclonal antibodies against cell surface molecules associated with cytotoxic activity of natural and activated killer cells and cloned CTL lines. Hybridoma. 1983;2:423–437. doi: 10.1089/hyb.1983.2.423. [DOI] [PubMed] [Google Scholar]
- Staunton DE, Dustin ML, Erickson HP, Springer TA. The arrangement of the immunoglobulin-like domains of ICAM-1 and the binding sites for LFA-1 and rhinovirus. Cell. 1990;61:243–254. doi: 10.1016/0092-8674(90)90805-o. [DOI] [PubMed] [Google Scholar]
- Stewart MP, Cabañas C, Hogg N. T cell adhesion to intercellular adhesion molecule-1 (ICAM-1) is controlled by cell spreading and the activation of integrin LFA-1. J Immunol. 1996;156:1810–1817. [PubMed] [Google Scholar]
- Takebe Y, Seiki M, Fujisawa J, Hoy P, Yokota K, Arai K, Yoshida M, Arai N. SRα promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol Cell Biol. 1988;8:466–472. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. Cadherins: a molecular family important in selective cell-cell adhesion. Annu Rev Biochem. 1990;59:237–252. doi: 10.1146/annurev.bi.59.070190.001321. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Morphogenetic roles of classic cadherins. Curr Opin Cell Biol. 1995;7:619–627. doi: 10.1016/0955-0674(95)80102-2. [DOI] [PubMed] [Google Scholar]
- Tang A, Amagai M, Granger LG, Stanley JR, Udey MC. Adhesion of epidermal Langerhans cells to keratinocytes mediated by E-cadherin. Nature. 1993;361:82–85. doi: 10.1038/361082a0. [DOI] [PubMed] [Google Scholar]
- Tiisala S, Paavonen T, Renkonen R. αEβ7 and α4β7integrins associated with intraepithelial and mucosal homing, are expressed on macrophages. Eur J Immunol. 1995;25:411–417. doi: 10.1002/eji.1830250216. [DOI] [PubMed] [Google Scholar]
- Tolosa E, Shaw S. A fluorogenic assay of endogenous phosphatase for assessment of cell adhesion. J Immunol Methods. 1996;192:165–172. doi: 10.1016/0022-1759(96)00042-7. [DOI] [PubMed] [Google Scholar]
- Tomschy A, Fauser C, Landwehr R, Engel J. Homophilic adhesion of E-cadherin occurs by a co-operative two-step interaction of N-terminal domains. EMBO (Eur Mol Biol Organ) J. 1996;15:3507–3514. [PMC free article] [PubMed] [Google Scholar]
- van Kooyk Y, van de Wiel-van Kemenade P, Weder P, Kuijpers TW, Figdor CG. Enhancement of LFA-1-mediated cell adhesion by triggering through CD2 or CD3 on T lymphocytes. Nature. 1989;342:811–813. doi: 10.1038/342811a0. [DOI] [PubMed] [Google Scholar]
- Viney JL, Jones S, Chiu HH, Lagrimas B, Renz ME, Presta LG, Jackson D, Hillan KJ, Lew S, Fong S. Mucosal addressin cell adhesion molecule-1: a structural and functional analysis demarcates the integrin binding motif. J Immunol. 1996;157:2488–2497. [PubMed] [Google Scholar]
- Vonderheide RH, Tedder TF, Springer TA, Staunton DE. Residues within a conserved amino acid motif of domains 1 and 4 of VCAM-1 are required for binding to VLA-4. J Cell Biol. 1994;125:215–222. doi: 10.1083/jcb.125.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacholtz MC, Patel SS, Lipsky PE. Leukocyte function-associated antigen 1 is an activation molecule for human T cells. J Exp Med. 1989;170:431–448. doi: 10.1084/jem.170.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JH, Pepinsky RB, Stehle T, Liu JH, Karpusas M, Browning B, Osborn L. The crystal structure of an N-terminal two-domain fragment of vascular cell adhesion molecule 1 (VCAM-1): a cyclic peptide based on the domain 1 C-D loop can inhibit VCAM-1-α4 integrin interaction. Proc Natl Acad Sci USA. 1995;92:5714–5718. doi: 10.1073/pnas.92.12.5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong WW, Farrell SA. Proposed structure of the F′ allotype of human CR1. Loss of a C3b binding site may be associated with altered function. J Immunol. 1991;146:656–662. [PubMed] [Google Scholar]
- Yoshida C, Takeichi M. Teratocarcinoma cell adhesion: identification of a cell-surface protein involved in calcium-dependent cell aggregation. Cell. 1982;28:217–224. doi: 10.1016/0092-8674(82)90339-7. [DOI] [PubMed] [Google Scholar]
- Yuan Q, Jiang W, Krissansen GW, Watson JD. Cloning and sequence analysis of a novel β2-related integrin transcript from T lymphocytes: homology of integrin cysteine-rich repeats to domain III of laminin B chains. Int Immunol. 1990;2:1097–1108. doi: 10.1093/intimm/2.11.1097. [DOI] [PubMed] [Google Scholar]