Abstract
Thyroid hormone (T3 or 3,5,3′-triiodothyronine) plays a causative role during amphibian metamorphosis. To investigate how T3 induces some cells to die and others to proliferate and differentiate during this process, we have chosen the model system of intestinal remodeling, which involves apoptotic degeneration of larval epithelial cells and proliferation and differentiation of other cells, such as the fibroblasts and adult epithelial cells, to form the adult intestine. We have established in vitro culture conditions for intestinal epithelial cells and fibroblasts. With this system, we show that T3 can enhance the proliferation of both cell types. However, T3 also concurrently induces larval epithelial apoptosis, which can be inhibited by the extracellular matrix (ECM). Our studies with known inhibitors of mammalian cell death reveal both similarities and differences between amphibian and mammalian cell death. These, together with gene expression analysis, reveal that T3 appears to simultaneously induce different pathways that lead to specific gene regulation, proliferation, and apoptotic degeneration of the epithelial cells. Thus, our data provide an important molecular and cellular basis for the differential responses of different cell types to the endogenous T3 during metamorphosis and support a role of ECM during frog metamorphosis.
Organogenesis and tissue remodeling require not only extensive cell proliferation and differentiation, but also selective elimination of unwanted cells. Such cell removal occurs through well-controled genetic programs, leading to programmed cell death (apoptosis) with a series of distinguished morphological changes (Wyllie et al., 1980; Jacobson et al., 1997). Extensive studies in recent years have identified and characterized many of the genes that participate in cell death during various physiological and pathological processes. However, relatively little is known about how cell death is controlled spatially and temporally during development, and how cell specificity of apoptosis is achieved.
Amphibian metamorphosis is one of the best studied developmental systems where extensive cell removal occurs (Dodd and Dodd, 1976; Gilbert and Frieden, 1981; Gilbert et al., 1996). This process systematically transforms different tadpole organs to adult forms. Some tissues such as the tail are tadpole specific and are completely resorbed during metamorphosis. Others, like the hindlimb, develop de novo from undifferentiated blastema cells. The rest of the organs, such as the intestine, are present in both the premetamorphic tadpoles and post metamorphic frogs, but are drastically remodeled during metamorphosis (Dodd and Dodd, 1976; Dauca and Hourdry, 1985; Yoshizato, 1989; Shi and Ishuzuya-Oka, 1996). Interestingly, cell death appears to take place in all three types of transformations, although most dramatically during organ resorption. Early studies, particularly microscopic examinations, have revealed that cell death during tissue resorption and remodeling occurs through apoptosis (Kerr et al., 1974; Ishizuya-Oka and Shimozawa, 1992a ; Ishizuya-Oka and Ueda, 1996; Izutsu et al., 1996). However, the lack of a proper in vitro system has so far hampered the understanding of the molecular mechanism underlying this apoptotic process.
Thyroid hormone (T3 or 3, 5, 3′-triiodothyronine)1 plays an essential causative role during amphibian metamorphosis (Gilbert and Frieden, 1981; Kikuyama et al., 1993; Gilbert et al., 1996). The hormone is known to directly regulate gene transcription through thyroid hormone receptors (TRs), which are nuclear transcription factors (Tsai and O'Malley, 1994; Yen and Chin, 1994; Mongelsdorf et al., 1995; Shi et al., 1996). Many of the genes that are regulated by T3 during metamorphosis have been identified and characterized (Shi, 1994; Brown et al., 1996; Gilbert et al., 1996). Among the so-called early response genes (those that change their mRNA levels within 1 d of T3 treatment of premetamorphic tadpoles) there are genes encoding transcription factors, extracellular matrix (ECM) modification/digestion enzymes, and ECM components (Shi, 1994; Brown et al., 1996; Gilbert et al., 1996). Noticeably absent among them are genes directly involved in programmed cell death, such as interleukin-1β–converting enzyme (ICE)-like proteases and Bcl-2 familiar members, etc. (White, 1996). Although it is possible that such genes are among the yet to be identified early T3 response genes, it is more likely that such genes are further downstream.
To investigate how T3 controls cell fate during tissue remodeling, we have established conditions for in vitro cultures of tadpole intestinal epithelial and fibroblastic cells. Addition of T3 to the culture medium causes cell death of the larval epithelial cells with typical apoptotic morphology. In contrast, fibroblastic cells are refractory to the hormone-induced cell death; instead, T3 induces the proliferation of those cells. We further show that the epithelial apotosis in vitro can be blocked by some known inhibitor of mammalial cell death and by ECM.
Materials and Methods
Isolation and Culturing of Tadpole Intestinal Epithelial and Fibroblastic Cells
Tadpole intestinal fragments were isolated from the posterior small intestine of premetamorphic tadpoles at stage 57/58 (Nieuwkoop and Faber, 1956) and then digested with collagenase and dispase (Ishizuya-Oka and Shimozawa, 1992b ). The dissociated cells (predominantly epithelial cells, >80%) were cultured overnight at 25°C on plastic dishes in 60% L15 medium supplemented with 10% fetal calf serum (GIBCO BRL, Gaithersburg, MD). The serum was treated with resin (AGI-X8; Bio-Rad Laboratories, Hercules, CA) to remove thyroid hormone (Samuels et al., 1979). After overnight culturing, the epithelial cells were then transferred to a new dish after gentle shaking (tightly attached mesenchymal cells were left behind).
To isolate both epithelial cells and fibroblasts, the anterior small intestinal fragments were digested and cultured as above. The anterior small intestine contains the single intestinal fold where connective tissue is abundant (Marshall and Dixon, 1978; Ishizuya-Oka and Shimozawa, 1987a ). Thus, upon transferring the epithelial cells after overnight culturing on plastic dishes, the vast majority (>80%) of cells remaining attached to the dish were fibroblasts (referred to as fibroblasts throughout this article).
It should be pointed out that the epithelial cells isolated from the anterior and posterior small intestine behaved identically in vitro in the presence or absence of T3 (see Results), and were thus used without distinction in this study.
DNA Fragmentation Assessment by ELISA
The tadpole intestinal epithelial cells or fibroblasts isolated above were labeled overnight in the presence of 10 μM of 5-bromo-2′-deoxyl-uridine (BrdU) at 25°C. The cells were collected by centrifugation at 250 g and 2 × 104 cells/well were cultured in a 96-well plastic culture plate containing different concentrations of T3 for indicated times. The cells were lysed and the supernatant was assayed for DNA fragmentation (cellular DNA fragmentation ELISA Kit; Boehringer Mannheim, Mannheim, Germany) according to the manufacturer's instructions.
DNA Content Analysis
The primary culture of tadpole intestine epithelial cells was treated with or without 100 nM T3. The cells were harvested by trypsinization and fixed in 1% formaldehyde in PBS on ice for 15 min. After centrifugation, the cell pellets (∼5 × 105 cells) were suspended in 2 ml of 0.1% sodium citrate–0.3% NP-40 solution, pH 7.5, and were passed through a 60-μM nylon mesh (Spectramesh; Fisher Scientific Co., Pittsburgh, PA). The cells were stained with 50 μg/ml of propidium iodide and then treated with 50 μg/ml of RNase A at 37°C for 30 min. The fluorescence intensity of individual cells was measured with a flow cytometer (FacScan Immunosystem; Becton Dickinson, Franklin Lakes, NJ).
Agarose Gel Electrophoresis Analysis of DNA Fragmentation during Cell Death
The primary epithelial cells were cultured with or without 100 nM T3 for 1 d. The cells were collected by centrifugation at 500 g for 5 min at 4°C and then lysed in 10 mM Tris-HCl, pH 8, 100 mM NaCl, 25 mM EDTA, 0.5% sodium dodecyl sulfate, and 0.1 μg/ml proteinase K. The lysate was incubated overnight at 50°C. After extraction with an equal volume of phenol/ chloroform/isoamyl alcohol (25:24:1), the DNA in the lysate was precipitated with ethanol, redissolved in H2O, and treated with RNase A (DNase free, 10 μg/ml) at 37°C for 2 h. The sample was again extracted with an equal volume of phenol/chloroform/isoamyl alcohol and precipitated with ethanol. 20 μg of the final purified DNA were fractionated on a 1.2% agarose gel, stained with ethidium bromide, and visualized under ultraviolet light.
Cell Proliferation Assay
Intestinal epithelial cells or fibroblasts were cultured overnight at 25°C in 96-well plastic plates or 6-well plates with or without different matrix coating (5 × 104 cells/well) in the presence of or absence of 100 nM T3 and/or 600 ng/ml CsA. [3H]Thymidine was added at 1 μCi/ml. After another 5 h at 25°C, the cells were then lysed by repeated freezing and thawing. The [3H]thymidine incorporated into genomic DNA was then measured by scintillation counting.
Cell Culturing on Matrix-coated Plastic Dishes
The epithelial cells were cultured on 6-well plastic plates coated with various matrices (Becton Dickinson Labware, Bedford, MA; 1–50 × 104 cells/ well) in the presence or absence of indicated concentrations of T3. For cell death measurement, the cultured cells were isolated by trypsinization and then pelleted and lysed. The lysates were transferred to a 96-well dish for the ELISA assay. For cell proliferation assay, 10 μCi [3H]thymidine was added after overnight treatment with or without T3 and then the cells were incubated in the 1-ml medium for another 5 h. The cells were then isolated by trypsinization and then pelleted and lysed for [3H]thymidine incorporation assay.
RNA Isolation and Analysis
Intestinal epithelial cells from stage 57/58 or stage 64 tadpoles were cultured on plastic dishes with or without ECM coatings in the presence or absence 100 nM T3 and/or 600 ng/ml CsA or 10 ng/ml FK506. After 1 d of culturing, the total RNA was isolated by using RNAzol (Tel-Test, Inc., Friendwood, TX) and quantified by absorption at 260 nM.
Total RNA was electrophoresed on a 1% agarose–formaldehyde gel and transferred onto a GeneScreen membrane (NEN Life Science Products, Boston, MA) after partial hydrolysis with NaOH (Maniatis et al., 1982; Ranjan et al., 1994). Hybridization was done by using the cDNA probes of Xenopus intestinal fatty acid binding protein (IFABP; Shi and Hayes, 1994), Na+/PO4 3− cotransporter (Ishizuya-Oka et al., 1997), and rpL8 (Shi and Liang, 1994). After overnight hybridization at 42°C in 50% formamide, 5 × SSPE, 0.2% SDS, 10% dextran sulfate, 5× Denhardt's solution, and 100 μg/ml denatured salmon sperm DNA, the filters were washed three times for 5–10 min each at room temperature in 2 × SSC and 0.2% SDS. Stringent washes were then done twice for 25 min each in 0.25 × SSC and 0.2% SDS at 65°C.
Results
Cell Type-specific Responses to Thyroid Hormone in Primary Intestinal Cell Cultures
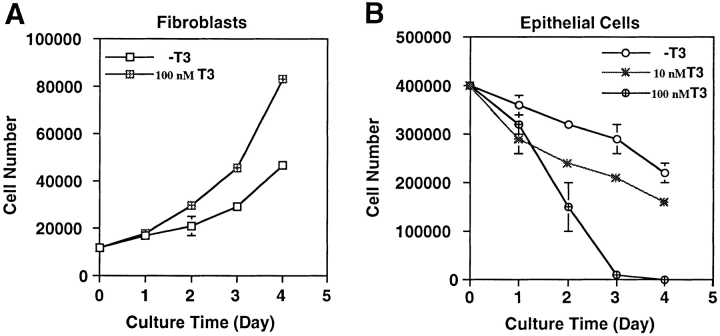
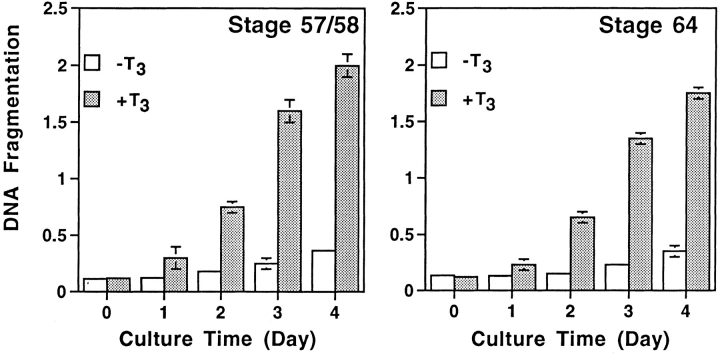
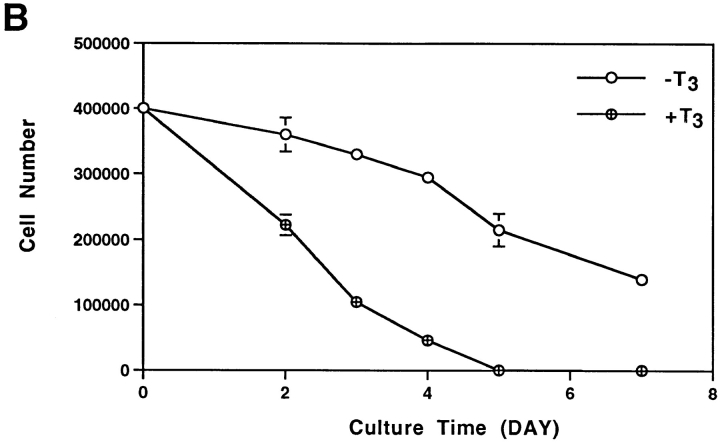
To investigate how T3 induces the degeneration of larval epithelium and proliferation and differentiation of adult cell types in the intestine, we dissociated the anterior small intestine of stage 57/58 Xenopus laevis tadpoles and isolated both the epithelial cells and the rest of the intestinal cells, which were predominantly mature and immature fibroblasts (McAvoy and Dixon, 1977; Ishizuya-Oka and Shimozawa, 1987a , b ). Upon culturing in vitro in the presence of 10% T3-depleted calf serum, the fibroblastic cells slowly proliferated, with a doubling time of ∼2.5 d (Fig. 1 A). In contrast, the viable epithelial cells gradually decreased in number with ∼60% of live cells remaining after 4 d of culturing (Fig. 1 B). Interestingly, addition of 100 nM T3 had contrasting effects on the cultured primary cells. The T3 treatment doubled the proliferation rate of the fibroblasts (Fig. 1 A) while drastically stimulating the degeneration of the epithelial cells (Fig. 1 B). Even at 10 nM, close to the endogenous plasma concentration at the climax of metamorphosis (Leloup and Buscaglia, 1977), T3 caused considerable reductions in epithelial cell survival (Fig. 1 B).
Figure 1.
Contrasting effects of thyroid hormone on tadpole intestinal epithelial and fibroblastic cells. The fibroblasts (A) and epithelial cells (B) were isolated from stage 57/58 of tadpole small intestine and then cultured on a six-well plastic dish in 60% L-15 medium containing 10% T3- depleted fetal bovine serum at 25°C in the presence or absence of 10 or 100 nM T3. The live cells were counted daily by trypan blue staining. Note that the epithelial cell number decreased even when no exogenous T3 was present. This could be because of the residual T3 in the treated serum. DNA fragmentation and flow cytometry analyses (Figs. 2 and 3) indicated that at least part of this decrease was due to apoptosis.
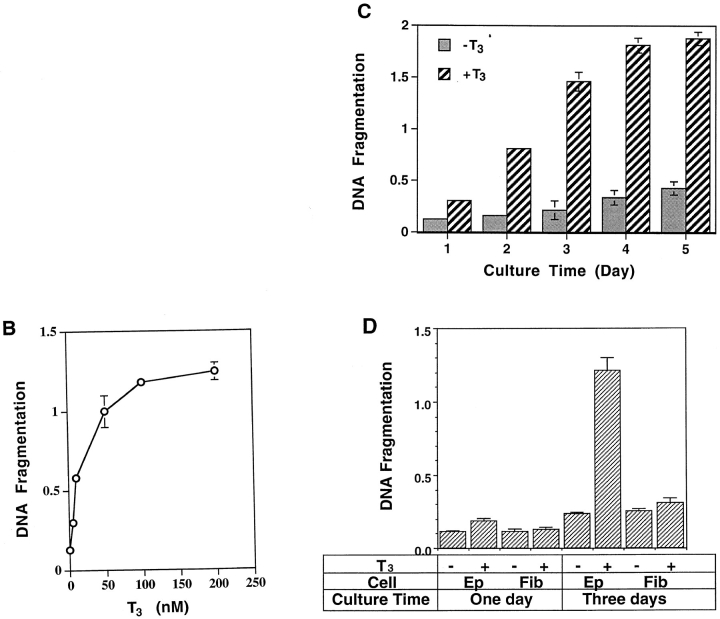
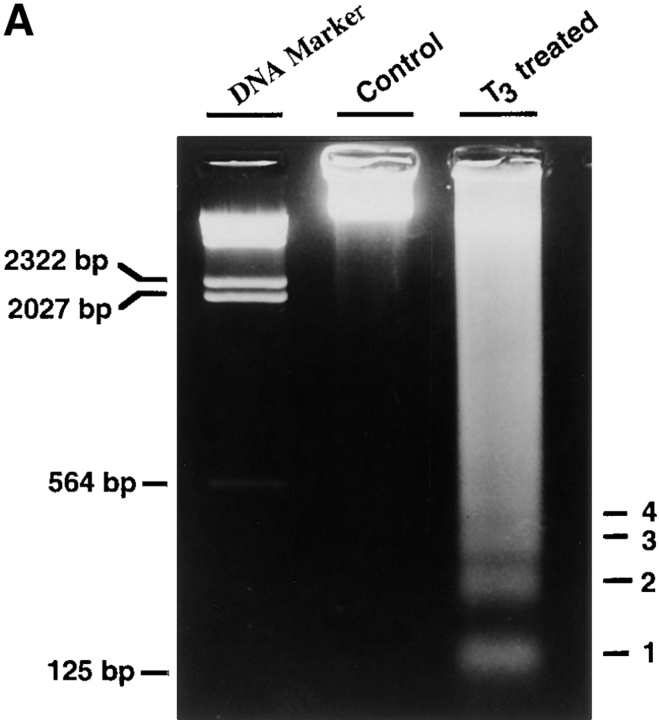
A major property of programmed cell death in mammals is the formation of a ladder of multinucleosomal-sized genomic DNA fragments. To determine whether T3-induced larval epithelial cell degeneration in vitro also possesses such changes, genomic DNA was isolated from epithelial cells cultured for 1 d in the presence or absence of T3 and analyzed on an agarose gel. The results clearly showed that T3 induced a nucleosomal DNA fragmentation ladder (Fig. 2 A).
Figure 2.
The tadpole intestinal epithelial cells but not the fibroblasts respond to T3 by undergoing programmed cell death. (A) T3-treatment of epithelial cells resulted in the formation of a nucleosome-sized DNA ladder. The epithelial cells were cultured on plastic dishes in the absence (Control) or presence (T3 treated) of 100 nM T3 for 1 d. The genomic DNA was isolated, electrophoresed on an agarose gel, stained with ethidium bromide, and visualized under ultraviolet light. The DNA bands equivalent to the lengths of the DNA in 1–4 nucleosomes were labeled on the right. (B) T3 induces dose-dependent DNA fragmentation in the tadpole intestinal epithelial cells. The epithelial cells were cultured on plastic dishes in the presence of different concentrations of T3 for 3 d and then DNA fragmentation was measured using the ELISA methods. Note that DNA fragmentation was detectible with as low as 5–10 nM of T3, similar to that in the plasma during metamorphosis (Leloup and Buscaglia, 1977), and plateaued at 100 nM T3. (C) Kinetics of T3-induced epithelial cell DNA fragmentation. The intestinal epithelial cells were cultured in the presence of 100 nM T3 for 1–5 d and then DNA fragmentation was analyzed with the ELISA method. Note that DNA fragmentation reached the maximum after 3 or 4 d of treatment. (D) T3 induces DNA fragmentation in the epithelial cells but not the fibroblasts. Intestinal epithelial cells and fibroblasts were isolated and cultured on 96-well plastic dishes (2 × 104 cells/well) for 1 or 3 d in the presence or absence of 100 nM T3. DNA fragmentation was then determined by using the ELISA method.
Using an ELISA assay designed to measure the extent of nuclear DNA fragmentation, we found that T3 caused epithelial cell death in a dose-dependent manner, with extensive cell death occurring at physiological concentrations (5–10 nM; Leloup and Buscaglia, 1977) of T3 and the maximal cell death at 100 nM of T3 (Fig. 2 B), in agreement with the cell survival data above (Fig. 1 B). Kinetically, the extent of DNA fragmentation was detectable by the ELISA assay after 1 d of T3 treatment (Fig. 2 C), consistent with the DNA fragmentation detected by the agarose gel assay (Fig. 2 A). It continued to increase ⩽4 or 5 d of T3 treatment (Fig. 2, C and D). In contrast to the epithelial cells, the fibroblasts showed no detectable DNA fragmentation above backgrounds at even 100 nM T3 (Fig. 2 D). Thus, T3 induces cell death specifically in the larval epithelium.
T3 Stimulates the Proliferation of Both Larval Epithelial Cells and Fibroblasts
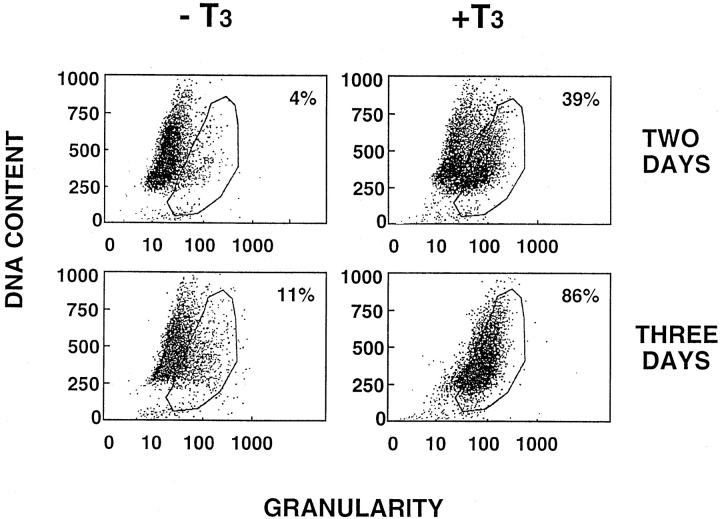
Although differentiated and fully functional, larval intestinal epithelial cells are capable of proliferation (McAvoy and Dixon, 1977; Ishizuya-Oka and Shimozawa, 1987a ). To investigate whether T3 causes apoptosis of the proliferating epithelial cells, we analyzed T3-treated primary cell cultures using flow cytometry. The larval epithelial cells cultured in the absence of T3 for 2 or 3 d had ⩽10% of the cells in the region of high granularity, i.e., apoptotic region (Fig. 3, encircled area). In contrast, ∼40 and 90% of the cells were in the apoptotic region when treated with T3 for 2 and 3 d, respectively (Fig. 3), in agreement with the cell survival analysis in Fig. 1 (B). Interestingly, the DNA content of the apoptotic cells ranged from subdiploid to nearly tetraploid (Fig. 3), suggesting that epithelial cells at different stages of the cell cycle, including the S- and G2-phases, were susceptible to T3-induced cell death. The results further indicated that larval epithelial cells could proliferate under the in vitro culture conditions, and that T3 did not block this proliferation. Instead, T3 may induce both apoptosis and cell proliferation.
Figure 3.
Flow cytometry analysis indicates that epithelial cells undergo apoptosis in response to T3 at different stages of the cell cycle. The epithelial cells were cultured in the presence or absence of 100 nM T3 for 2 or 3 d. The cells were then analyzed by flow cytometry. Although the exact boundary between the live cells and apoptotic cells (encircled area) was difficult to determine with precision, the results clearly showed that cells with different DNA contents or at different cell cycle stages (G2 at the top and G1 at the bottom) were present in the apoptotic region (reflected by the increased cellular granularity). Note that after 3 d of treatment, essentially all cells were in the apoptotic region, and were shown to be dead by trypan blue staining and DNA fragmentation (Figs. 1 and 2).
To directly investigate the possible effect of T3 on intestinal cell proliferation, the [3H]thymidine incorporation assay was performed. Both the epithelial cells and fibroblasts had similar levels of [3H]thymidine incorporated in the absence of T3 (Fig. 4). T3 treatment stimulated [3H]thymidine incorporation in both the epithelial cells and fibroblasts to a similar extent. Thus, both epithelial cells and fibroblasts can proliferate in vitro with similar rates, and T3 causes nearly a twofold increase in this proliferation for these two cell types of the tadpole intestine.
Figure 4.
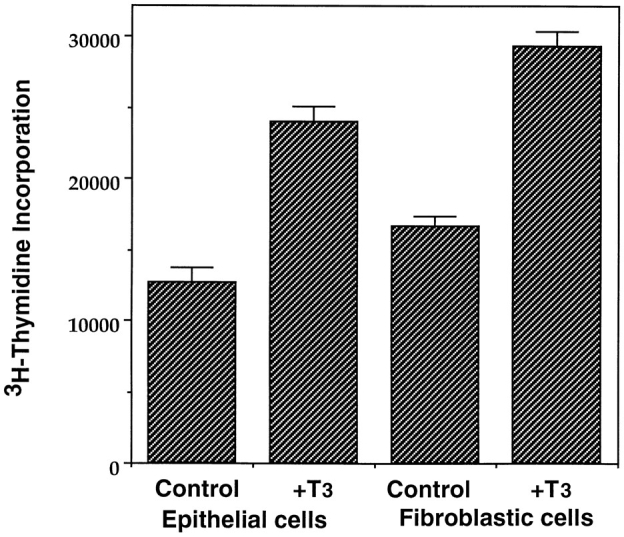
T3 stimulates the proliferation of both the intestinal epithelial cells and fibroblasts. Intestinal epithelial cells and fibroblasts were cultured overnight on 96-well plastic dishes (5 × 104 cells/well) in the presence or absence of 100 nM T3. 0.1 μCi of [3H]thymidine was added to the 0.1-ml culture medium/well and incubated for another 5 h. The amount of [3H]thymidine incorporated into genomic DNA was then measured.
Gene Regulation by T3 in In Vitro Epithelial Cell Culture
T3 is known to regulate gene expression during amphibian metamorphosis (Shi, 1994; Brown et al., 1996; Gilbert et al., 1996). In Xenopus laevis intestine, >20 genes have been shown to be regulated either directly or indirectly by T3 (Shi and Ishizuya-Oka, 1996). Among them, IFABP (Ishizuya-Oka et al., 1994; Shi and Hayes, 1994) and a Na+/ PO4 3− cotransporter gene (Ishizuya-Oka et al., 1997) have been shown to be expressed in the intestinal epithelium. To determine whether the T3-induced epithelial apoptosis in vitro has a similar gene regulation profile as in tadpoles, RNA was isolated from epithelial cells cultured for 1 d in the presence or absence of 100 nM T3 and then analyzed by Northern blot analysis.
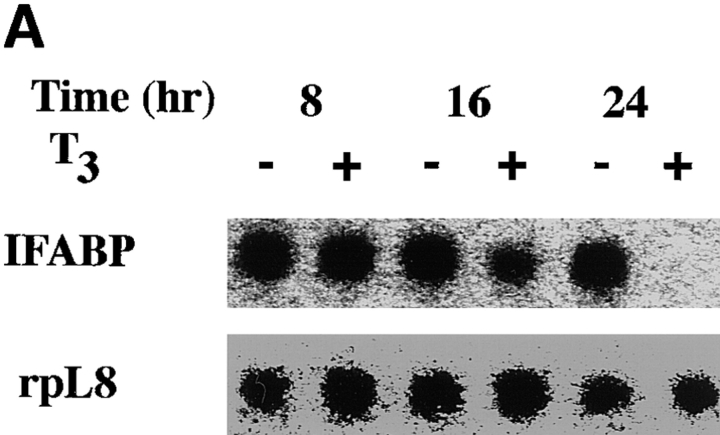
When the intestinal epithelial cells from stage 57/58 tadpoles were cultured in the presence of 100 nM T3, the levels of IFABP mRNA were not significantly affected within 16 h, but downregulated after 24 h (Fig. 5 A), in agreement with the suggestion that IFABP gene is not a direct T3- response gene (Shi and Hayes, 1994). Similarly, the expression of Na+/PO4 3− cotransporter gene was drastically downregulated after 1 d of T3 treatment (Fig. 5 B). The IFABP gene is known to be downregulated by T3 in tadpoles (Shi and Hayes, 1994). The Na+/PO4 3− cotransporter gene, on the other hand, is known to be first upregulated and then downregulated when premetamorphic tadpoles of stage 56 or younger are treated with 5 nM T3 (Ishizuya-Oka et al., 1997). Its mRNA level peaks around stages 58–60 in the intestine during normal development (Ishizuya-Oka et al., 1997). Thus, it is not surprising that T3 treatment of intestinal epithelial cells from stage 57/58 tadpoles resulted in the downregulation of this gene (Fig. 5).
Figure 5.
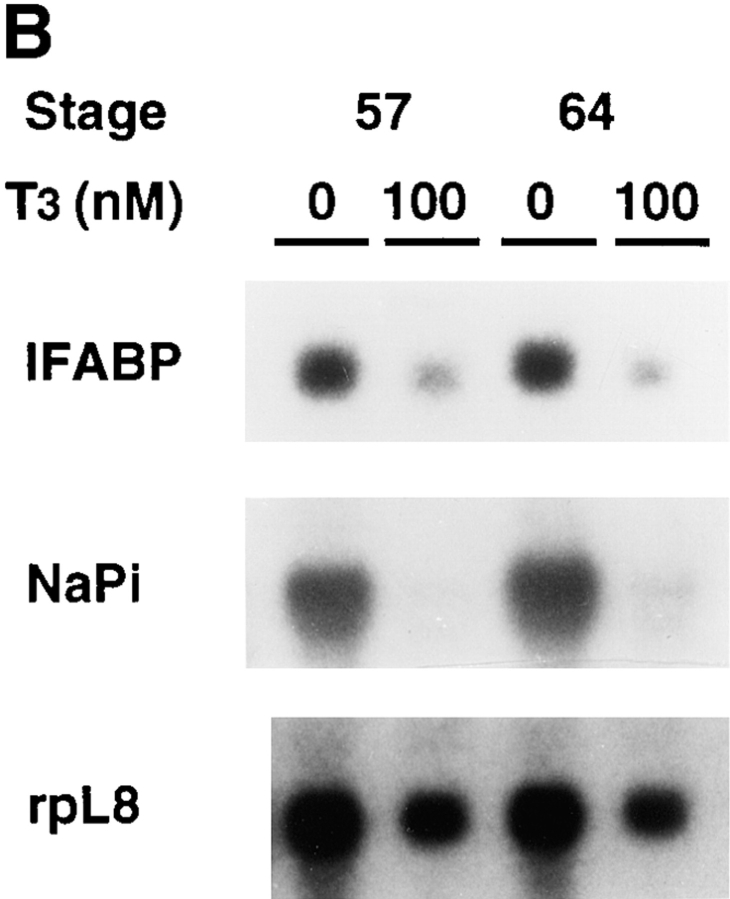
T3 treatment of intestinal epithelial cells leads to the downregulation of two known epithelial specific genes. (A) Kinetics of the downregulation of IFABP gene by T3 in vitro. Epithelial cells from stage 57/58 tadpole intestine were cultured on plastic dishes in the presence of 100 nM T3 for indicated numbers of hours and then RNA was isolated for Northern blot analysis of IFABP mRNA. (B) Regulation of IFABP and Na+/ PO4 3− cotransporter genes by T3 in vitro. Epithelial cells from stage 57/58 or stage 64 tadpoles were isolated and cultured on plastic dishes in the presence or absence of 100 nM T3 for 1 d. The RNA was isolated and analyzed by Northern blot hybridization with the cDNA probes for IFABP and intestinal Na+/PO4 3− cotransporter (NaPi). Note that both genes were downregulated in the stage 57/58 epithelial cells as expected from their expression during normal development (Shi and Hayes, 1994; Ishizuya-Oka et al., 1994, 1997). However, their downregulation in stage 64 epithelial cells appeared to contradict with expectation (see text for discussion). The hybridization with rpL8 served as a loading control.
The surprising result was, however, that the expression of both the IFABP and Na+/PO4 3− cotransporter genes was downregulated even when the intestinal epithelial cells from stage 64 tadpoles were cultured in vitro in the presence of 100 nM T3 (Fig. 5 B). This contrasts with their upregulation during stages 62–64 when adult epithelial cells differentiate in vivo (Shi and Hayes, 1994; Ishizuya-Oka et al., 1994, 1997). As the epithelial cells at stage 64 are adult type, they are capable of proliferating and differentiating even in the presence of T3 in intact tadpoles. The results here suggest that the epithelial cells dissociated from the mesenchyme, and the basal lamina between the epithelium and the mesenchyme respond to T3 differently. Indeed, the epithelial cells from stage 64 tadpoles underwent cell death just like those from stage 57/58 tadpoles in the presence of T3 as assayed by DNA fragmentation (Fig. 6). It is also interesting to note that cell death was induced by T3 in spite of the copresence of epithelial and mesenchymal cells in vitro (Fig. 6), suggesting that the mere presence of nonepithelial components is not sufficient to prevent epithelial cell death.
Figure 6.
Both larval and adult intestinal cells undergo apoptosis upon T3 treatment in vitro. Cells were dissociated from intestine of stage 57/58 or 64 tadpoles and all of the cells were cultured together in vitro in the presence or absence of 100 nM T3. Cell death was analyzed by using the DNA fragmentation ELISA assay. The cells were predominantly epithelial but a higher portion of mesenchymal cells were present in stage 64 tadpole intestine (McAvoy and Dixon, 1977; Ishizuya-Oka and Shimozawa, 1987a ). Note that cell death were detected for both the larval and adult intestinal cells. Slightly lower levels of T3-induced DNA fragmentation were observed at stage 64, probably reflecting the presence of a slightly higher percentage of nonepithelial cells.
ECM Inhibits Epithelial Cell Death In Vitro
The intestinal epithelium is in close contact with the basal lamina, a special ECM that separates the epithelium from the underlying mesenchyme. It is known that the basal lamina undergoes extensive remodeling during metamorphosis (Ishizuya-Oka and Shimozawa, 1987b ; Murata and Merker, 1991; Shi and Ishizuya-Oka, 1996). Furthermore, the gene expression studies above suggest that dissociated adult intestinal epithelial cells have a distinct response to T3 than when they are in intact animals. Thus, it is likely that ECM plays a role during intestinal remodeling. To investigate such a possibility, we cultured the larval epithelial cells on plastic dishes coated with various components of the basal lamina (laminin, collagen IV, and fibronectin). For a comparison, we also used dishes coated with collagen I, a major component of the connective tissue. Although the cells did not attach to plastic dishes, they attached well to all matrix-coated dishes (Fig. 7 and data not shown). These coatings not only facilitated cell attachment but also produced a more extended or spread-out cell shape, whereas cells on the plastic dish were round. In general, all coatings were found to enhance the epithelial cell survival in vitro, increasing the survival time (when ⩾95% of cells were dead) from ∼1 wk on plastic dishes to ∼2 wk on matrix-coated dishes.
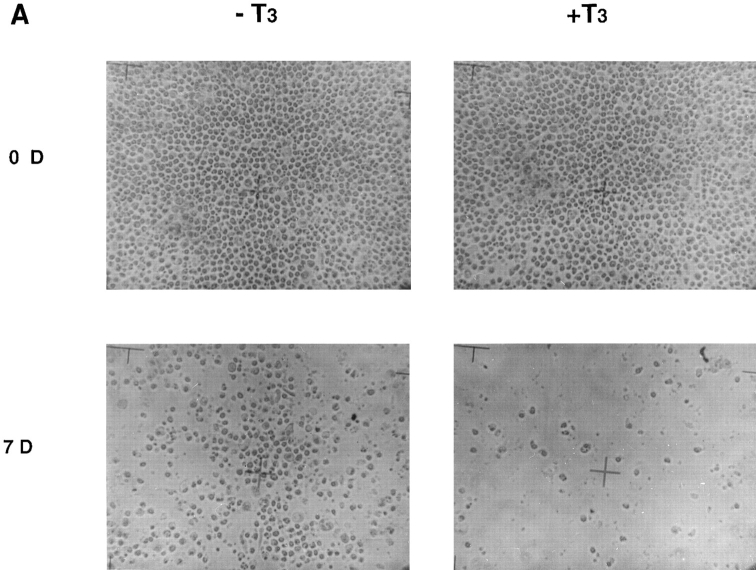
Figure 7.
Intestinal epithelial cells cultured on fibronectin-coated dishes survive longer in the presence and absence of T3 than those on plastic dishes. (A) The cells were cultured on the coated dishes in the presence or absence of 100 nM T3 for 0 or 7 d and then photographed. Note that the cells attached nicely to the coated dishes in contrast to their behavior on plastic dishes, and that some cells were still present after 7 d of T3 treatment, in contrast to those on plastic dishes (Fig. 1). (B) The cells were cultured on fibronectin-coated dishes in the presence or absence of 100 nM T3 and then the live cells were counted at different days after trypsinization and trypan blue staining.
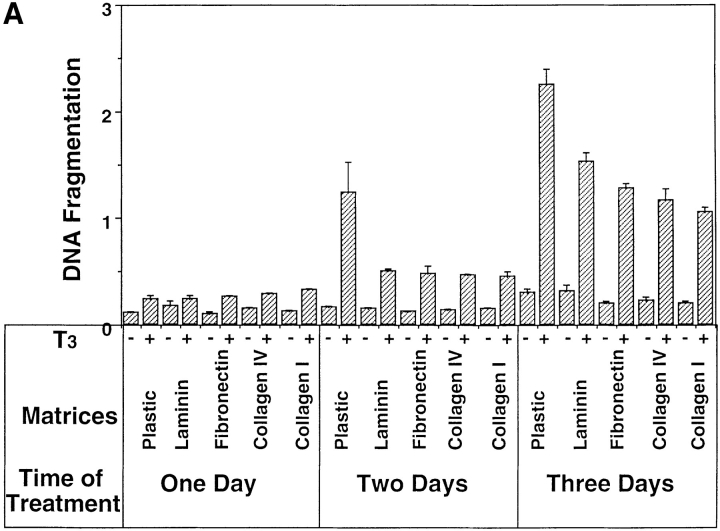
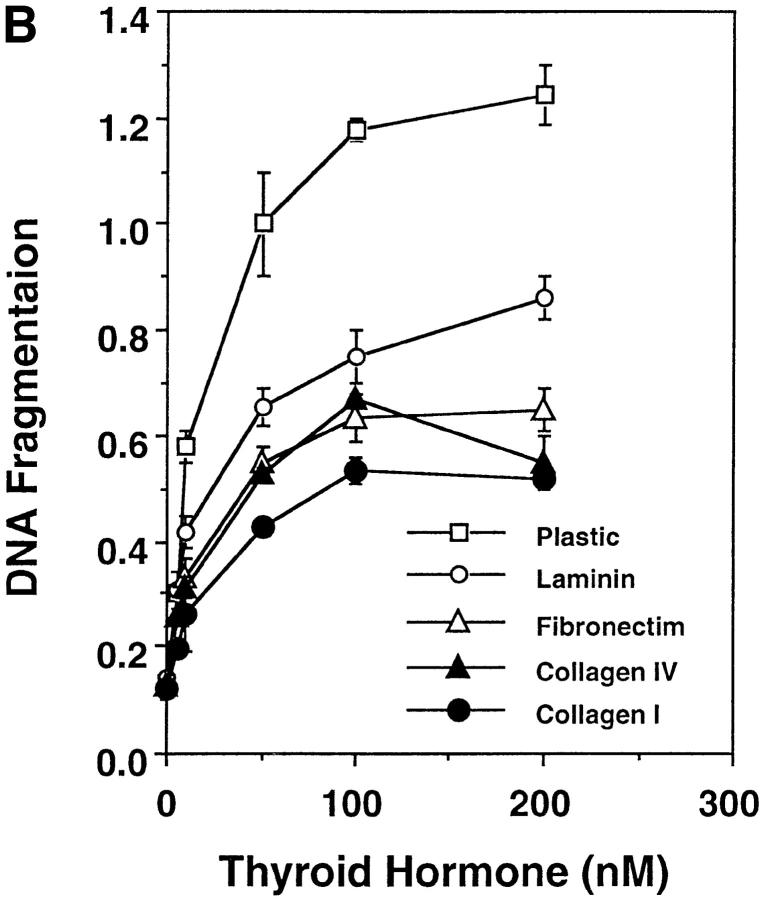
Similar to many other types of cells, the fate of the tadpole intestinal epithelial cells is likely to be controled by a balance of survival and death factors. The enhanced survival of the intestinal epithelial cells on matrix-coated dishes implies that these matrices may inhibit T3-induced epithelial apoptosis. To test this possibility, tadpole intestinal epithelial cells were cultured on fibronectin- (Fig. 7 A) or type I collagen- (data not shown) coated dishes and treated with T3. Although T3 treatment still led to epithelial cell death, >50 and 10% of the cells remained viable after 2 and 4 d of treatment, respectively (Fig. 7 B). Under the same conditions, all of the cells cultured on plastic dishes died after 3 d of treatment (Fig. 1 B). Quantitative analysis of the T3-induced apoptosis by ELISA assay revealed that all matrices inhibited ∼50% of the T3-induced DNA fragmentation after 2 or 3 d of T3 treatment (Fig. 8 A). On the other hand, the T3-induced epithelial apoptosis on different coatings had similar T3-dose responses (Fig. 8 B), indicating the effects of these matrices were not simply due to any possible effects of the matrices on the availability of T3. Instead, the results suggest that the matrices were influencing cellular responses to T3 through yet unknown signal transduction pathways.
Figure 8.
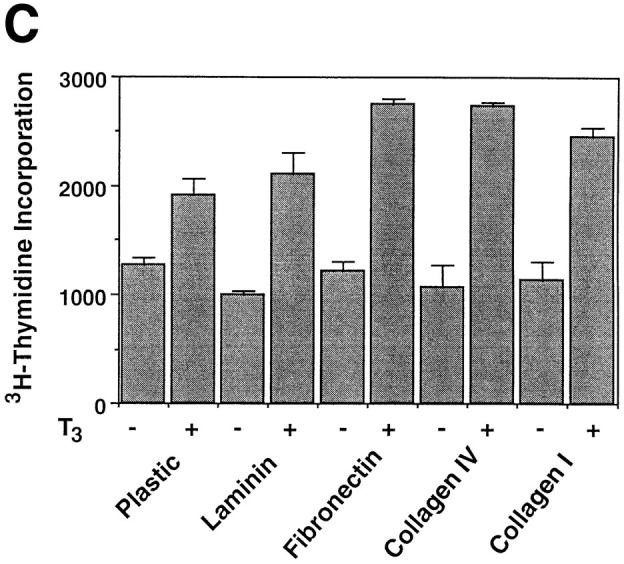
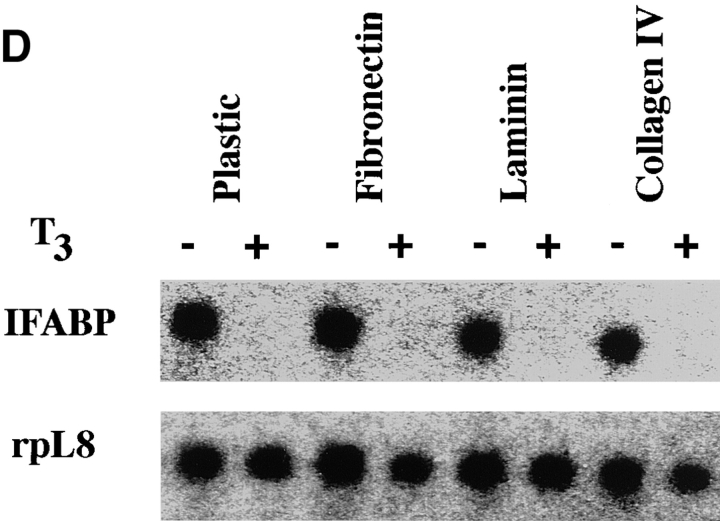
Epithelial cells from stage 57/58 intestine cultured on matrix-coated dishes are more resistant to T3-induced apoptosis, but are similarly stimulated by T3 to proliferate or downregulate IFABP expression. (A) Intestinal epithelial cells were cultured on plastic dishes with or without indicated coatings for 1–3 d in the presence or absence of 100 nM T3. DNA fragmentation was then measured by the ELISA method. (B) Epithelial cells cultured on various matrix-coated dishes have a similar dose response to T3 treatment in spite of their increased resistance to T3-induced cell death. The epithelial cells were cultured on various dishes in the presence of different concentrations of T3 for 3 d and then DNA fragmentation was then determined by the ELISA method. (C) Epithelial cells were cultured overnight on various matrix-coated dishes in the presence or absence of 100 nM T3 (5 × 104 cells/well). 0.1 μCi of [3H]thymidine were added into the 1-ml culture medium/well and then incubated for another 5 h. [3H]thymidine incorporated into genomic DNA was then measured. (D) Epithelial cells were cultured on matrix-coated dishes in the presence or absence of 100 nM T3 for 1 d and then total RNA was isolated for Northern blot analysis of IFABP mRNA. The hybridization with rpL8 served as a control.
In contrast to the effects of ECM on T3-induced cell death, 3H-thymidine incorporation showed that the various matrices had little effect on T3-induced cell proliferation (Fig. 8 C). Similarly, they had no effect on the downregulation of IFABP gene expression by T3 (Fig. 8 D). These results suggest that T3 induces multiple cellular events, each of which is in turn affected by different signal transduction pathways.
Cell Death Inhibition Studies Confirm the Existence of Multiple Signal Transduction Pathways Induced by T3
The results above suggest that the differential responses to T3 of different intestinal cells during amphibian metamorphosis lie mainly with the ability of T3 to induce epithelial apoptosis. The exact mechanism underlying apoptosis remains unknown despite extensive investigations. However, earlier studies in mammals have demonstrated the involvement of ICE-like proteases and nucleases during programmed cell death (Martin and Green, 1995; White, 1996). In addition, the participation of signal transduction pathways involving phosphatases/kinases has also been suggested by the ability of immunosuppressants FK506 and cyclosporin A (CsA) to inhibit activation-induced T cell death (Shi et al., 1989; Birer et al., 1990). To investigate the possible involvement of similar pathways during amphibian metamorphosis, we tested the ability of four of these inhibitors to block T3-induced intestinal epithelial cell death. These include aurintricarboxylic acid (ATA; Shi et al., 1994) which is a nuclease inhibitor, Z-Val-Ala-Asp-floromethalketone (Z-VAD; Muzio et al., 1996; Pronk et al., 1996), which is an ICE-like protease inhibitor, CsA, and Fk506.
Both Z-VAD and ATA inhibited the T3-induced intestinal epithelial cell death (Figs. 9, A and B), suggesting the participation of ICE-like proteases and nucleases, respectively. Of the two immunosuppressants, only CsA inhibited the T3-induced epithelial apoptosis (Figs. 9, A and B). Identical results were obtained with different concentrations of these drugs (data not shown). Thus, CsA has similar effects on T3-induced apoptosis as on activation-induced T cell death, whereas FK506 has different effects on these two apoptotic processes.
Figure 9.
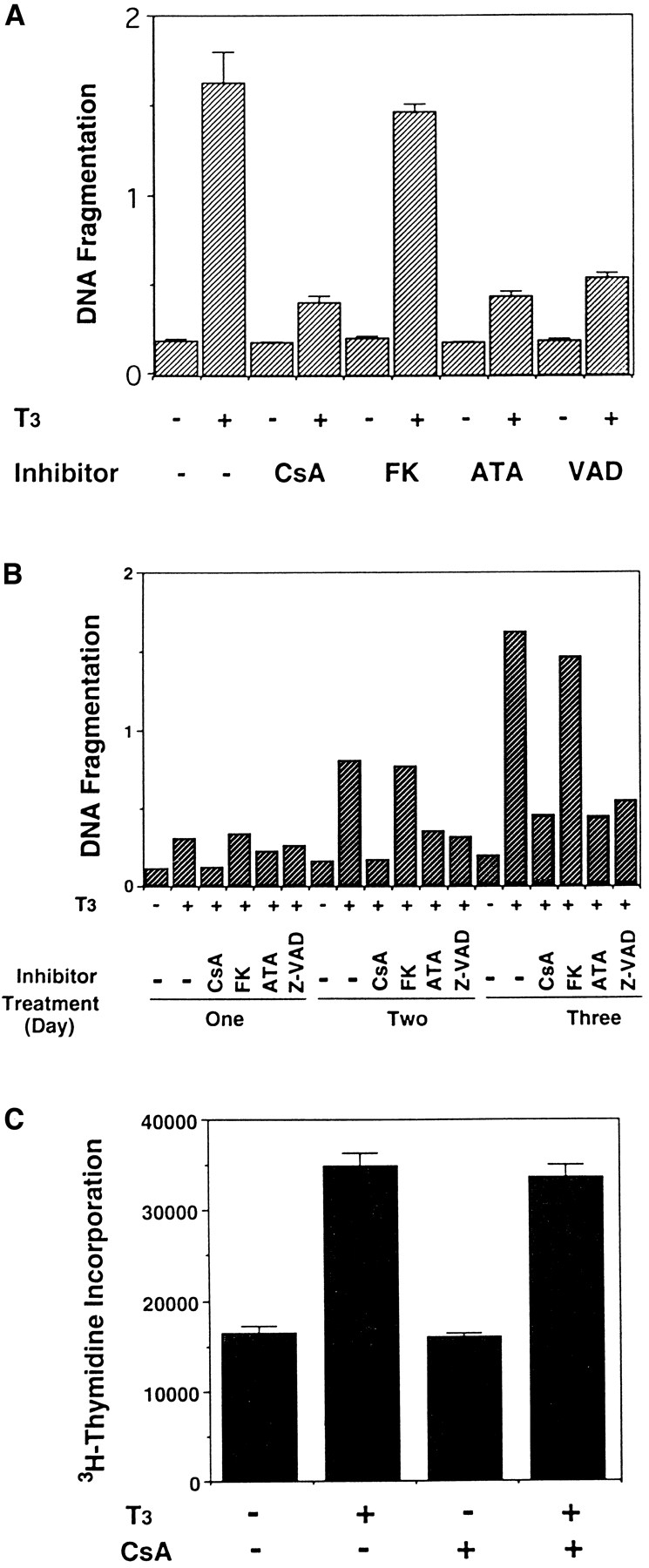
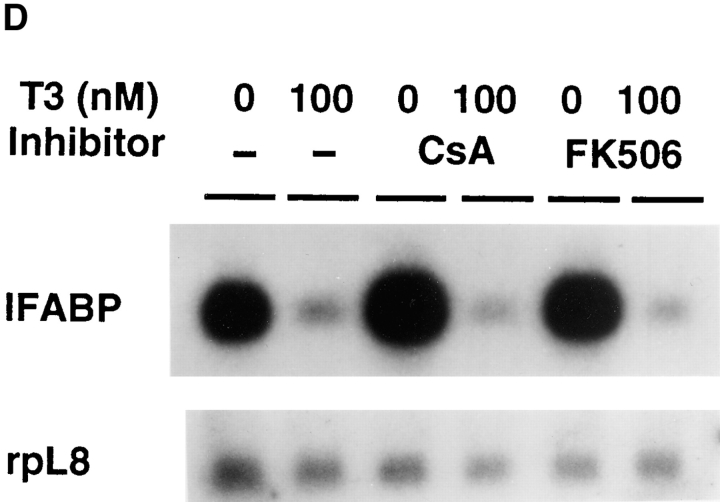
T3-induced intestinal epithelial cell death but not cell proliferation can be inhibited by some but not all known inhibitors of mammalian apoptosis. (A) The epithelial cells were cultured on plastic dishes for 3 d in the presence or absence of 100 nM T3 and/or 300 ng/ml CsA, 10 ng/ml FK506 (FK), 100 μM ATA, and 50 μM Z-VAD (VAD). DNA fragmentation was then measured by the ELISA method. Note that with the exception of FK506, all inhibitors blocked T3-induced epithelial cell DNA fragmentation. None of the drugs had any effect on DNA fragmentation by itself. (B) Time course of the drug inhibition of T3-induced epithelial cell death. Note that again with the exception of FK506, all drugs inhibited cell death throughout the treatment. The concentrations of the drugs used were 600 ng/ml CsA, 10 ng/ml FK506 (FK), 100 μM ATA, and 50 μM Z-VAD (VAD), respectively. (C) CsA does not block T3-induced epithelial cell proliferation. The epithelial cells were cultured in the presence or absence of 100 nM T3 and/or 600 ng/ml CsA for 1 d. Cell proliferation was determined as in Fig. 4. (D) The downregulation of IFABP gene in vitro by T3 is resistant to CsA and FK506. Epithelial cells from stage 57/58 tadpoles were cultured on plastic dishes in the presence or absence of 100 nM T3 and/or 600 ng/ml CsA or 10 ng FK506 (FK) for 1 d. The RNA was then isolated and analyzed as above. Note that FK506 had no effect on either cell death (A and B) or IFABP downregulation. Although CsA could inhibit cell death (A and B), it failed to block T3-induced IFABP gene regulation. The hybridization with rpL8 served as a loading control.
Interestingly, using flow cytometry we observed that CsA blocked the apoptosis of cells at all different stages of the cell cycle, resulting in a profile of cell distribution similar to that of the control cells in the absence of T3 (Fig. 3 A and data not shown). In contrast, CsA had no effect on DNA synthesis both in the presence or absence of T3 (Fig. 9 C). These results suggest that T3 simultaneously induces the cell death and proliferation in the epithelial cells, and only the death pathway is sensitive to CsA. Such a conclusion is also consistent with the fact that T3 stimulates fibroblastic cell proliferation even though it does not cause fibroblastic cell apoptosis.
The induction of epithelial apoptosis by T3 is presumably through the activation and/or repression of certain genes in the intestine. Currently, it is not known which, if any, of the known T3-regulated genes are involved in epithelial cell death. The ability of T3 to regulate epithelial gene expression in vitro prompted us to investigate whether the cell death inhibitors used above can affect T3-dependent gene regulation. Of particular interest is the immunosuppressants FK506 and CsA. Both drugs are known to inhibit activation-induced T cell death (Shi et al., 1989; Birer et al., 1990). Furthermore, both have been shown to exert their immunosuppressive effect by inhibiting calmodulin-dependent tyrosine phosphatase calcineurin (McKeon, 1991; Schreiber, 1992). This suggests that they may act at an early step of the signal transduction pathway leading to T cell death, in contrast to the inhibitors of ICE-like enzymes or nucleases. Thus, we treated epithelial cells from stage 57/58 tadpole intestine with or without T3 in the presence or absence of CsA and FK506, and then analyzed the effects of such treatment on IFABP expression. Again, T3 treatment alone led to the downregulation of the gene (Fig. 9 D). In agreement with its inability to block the T3-induced apoptosis, FK506 had no effect on the expression of the IFABP gene (Fig. 9 D). Interestingly, under the conditions used, CsA could completely block the T3-induced cell death, but it could not prevent the downregulation of the IFABP gene (Fig. 9 D). Thus, whereas the IFABP gene downregulation does not appear to be a direct effect of T3 (Shi and Hayes, 1994), it may still precede the step of the cell death pathway that is sensitive to CsA inhibition. Alternately and more likely, T3-induced downregulation of the IFABP gene is in a parallel pathway independent of the cell death pathway. Thus, CsA can block T3-induced apoptotic pathways but not the T3-induced cell proliferation or regulation of some T3 response genes.
Discussion
We have successfully cultured cells of the tadpole intestine in vitro and investigated the effects of thyroid hormone on these primary cell cultures. We have demonstrated here that both larval epithelial and fibroblastic cells respond to T3 by increasing their DNA synthesis, and that only the epithelial cells undergo T3-dependent programmed cell death with typical apoptotic properties as observed in mammals. This T3-induced epithelial apoptosis can be inhibited by ECMs. More importantly, our study reveals that T3 induces multiple pathways in the larval epithelial cells.
Primary Cell Cultures of Tadpole Intestine Mimic the Cell-Specific Responses to T3 in Intact Tadpoles
Organ culture experiments have shown that the regulation of amphibian metamorphosis by T3 is organ autonomous (Dodd and Dodd, 1977; Ishizuya-Oka and Shimozawa, 1991; Tata et al., 1991). In the frog intestine, two major cell types exist, epithelial and fibroblasts (Ishizuya-Oka and Shimozawa, 1987a ). While the fibroblasts rapidly proliferate and differentiate during metamorphosis (Ishizuya-Oka and Shimozawa, 1987a ), the larval epithelial cells undergo degeneration through an apoptotic process (McAvoy and Dixon, 1977; Ishizuya-Oka and Shimozawa, 1992a ). Our results indicate that at least part of the intestinal remodeling, i.e., the epithelial cell death, can be reproduced in primary cultures of separated cells in vitro in the presence of T3, suggesting that the apoptotic event is cell autonomous. Furthermore, this T3-dependent apoptosis has the same cell type specificity as in vivo (McAvoy and Dixon, 1977; Ishizuya-Oka and Shimozawa, 1992a , b ).
The fibroblasts, on the other hand, are refractory to T3-induced apoptosis in our in vitro system. This agrees well with their ability to proliferate and differentiate but not undergo apoptosis during natural metamorphosis (Ishizuya-Oka and Shimozawa, 1987a , 1992a , b ). Interestingly, T3 treatment of the fibroblast in vitro leads to an increase in cell proliferation, suggesting that the development of the fibroblasts during metamorphosis is through the action of T3 on those cells directly, i.e., cell autonomous.
A surprising finding is that T3 also stimulates the proliferation of the epithelial cells. However, larval intestinal epithelia cells are known to be capable of dividing in spite of their differentiated phenotype (McAvoy and Dixon, 1977, 1978; Ishizuya-Oka and Shimozawa, 1987a ). Thus, T3 may control a common set of genes present in both the epithelial cells and fibroblasts of the intestine that can facilitate the cell proliferation. What separates the larval epithelial cells from the other major intestinal cells, the fibroblasts, is their apoptotic response to T3. This latter response occurs in epithelial cells at all stages of the cell cycles. The final outcome of the T3 treatment is the total degeneration of the larval epithelial cells both in vivo and in primary cell cultures. This differential effect of T3 on epithelial cells and immature fibroblasts suggests that T3 has the ability to stimulate cell cycle progression, which leads to cell proliferation in non- or less-differentiated cells such as the fibroblasts, or to apoptosis in differentiated cells, such as the epithelial cells.
Concurrent Induction of Multiple Pathways by T3 in the Larval Intestinal Epithelial Cells
Amphibian metamorphosis is perhaps one of the processes where cell death takes place at its extreme. All the tadpole-specific organs, such as the tail and gill, degenerate completely whereas the rest of the organs undergo extensive remodeling or de novo development. Most, if not all, of the organ transformations require the removal of some or all of the existing cells. Early microscopic examinations have shown that the tail resorption and intestinal remodeling involve cell death with typical apoptotic morphologies as observed in mammals (Kerr et al., 1974; Ishizuya-Oka and Shimozawa, 1992a ). Our studies have provided biochemical and cell biological evidence for the programmed cell death via apoptosis in the tadpole intestine.
The induction of intestinal apoptosis by T3 is believed to be through the activation of the cell death pathway and/or the deactivation of cell survival signals. Although many thyroid hormone response genes have been identified in the intestine (Shi and Ishizuya-Oka, 1996), none of them correspond to known cell death or survival genes. However, our inhibition studies clearly indicate the involvement of ICE-like proteases and nucleases, just as in mammalian apoptotic processes (Martin and Green, 1995; White, 1996). Furthermore, the T3-induced epithelial cell death has a typical nucleosomal ladder of DNA fragmentation. Thus, the cell death during the T3-dependent amphibian developmental process possesses many of the characteristics of mammalian apoptotic model systems.
Our studies with immunosuppressants CsA and FK506 show that CsA inhibits T3-induced cell death, similar to that observed for activation-induced T cell death in mammals (Shi et al., 1989; Birer et al., 1990). On the other hand, FK506, which inhibits activation-induced T cell death (Birer et al., 1990), has no effect on T3-induced intestinal epithelial apoptosis. The exact mechanisms by which CsA and FK506 inhibit T cell death are unknown. However, both CsA and FK506 have been shown to be capable of inhibiting calmodulin-dependent phosphatase, and this inhibition has been suggested to be responsible for their effects in T cell death (Shi et al., 1989). Our studies suggest that such a mechanism may not be responsible for the inhibition of T3-induced intestinal cell death by CsA.
An intriguing possibility has been suggested by the recent finding that CsA inhibits the DNA binding activity of the transcription factor Nur77 in T cells (Yazdanbakhsh et al., 1995). Nur77 is required for T cell death, and belongs to the superfamily of nuclear hormone receptors that also include TRs (Liu et al., 1994; Woronicz et al., 1994; Mangelsdorf et al., 1995). It is, therefore, suggested that the inhibition of Nur77 activity by CsA may block the ability of Nur77 to regulate its target genes, thus preventing activation-induced T cell death. As TRs and Nur77 belong to the same receptor family and share many functional features, it is possible that CsA may inhibit TR function, thus blocking the intestinal epithelial cell death. However, our results on the expression of the IFABP gene clearly rule out such a mechanism, as the IFABP gene was downregulated by T3 both in the presence or absence of CsA. Thus, CsA functions either in a parallel pathway independent of the pathway leading to IFABP gene regulation or downstream of the IFABP gene regulation.
Independent of the exact mechanism of CsA action, the fact that CsA can block T3-induced cell death while having no effect on T3-induced downregulation of the IFABP gene, and an increase in cell proliferation supports the idea that T3 induces multiple, indepencent cellular events in the intestinal epithelial cells. These include apoptosis, cell proliferation, and specific regulation of genes that are involved in neither cell death nor cell proliferation. Such a conclusion is also supported by the ability of ECM to inhibit intestinal epithelial cell death but not cell proliferation.
Role of ECM in Epithelial Development during Intestinal Remodeling
The intestinal epithelium is separated from the mesenchyme by a special ECM, the basal lamina, whose major components include laminin, entactin, type IV collagen, and fibronectin, etc. (Hay, 1991; Timpl and Brown, 1996). The ECM serves as a structural support for the cells it surrounds and is essential for the integrity and morphology of an organ. Equally as important, ECM can modulate a number of cellular functions, such as cell migration, morphology, proliferation, differentiation, and death (Hay, 1991; Schmidt et al., 1993; Ruoslahti and Reed, 1994).
Studies in both mammals and amphibians have implicated a role of basal lamina during intestinal development (for review see Louvard et al., 1992; Simon-Assmann and Kedinger, 1993; Shi and Ishizuya-Oka, 1996). During amphibian metamorphosis, extensive remodeling of the intestinal basal lamina (Ishizuya-Oka and Shimozawa, 1987b ; Murata and Merker, 1991) has been observed to coincide with frequent migration of macrophages across the lamina into degenerating larval epithelium and extensive direct contacts between the developing adult epithelial cells and mesenchyme (Ishizuya-Oka and Shimozawa, 1987b , 1992b ).
Our results indicate that basal lamina components laminin, fibronectin, and type IV collagen can directly inhibit T3-induced larval epithelial cell death. Although similar effects observed with type I collagen were essentially absent in the basal lamina, this may reflect the fact that our primary epithelial cell cultures represent an extreme case where all cell–ECM interactions had been removed upon dissociating the epithelial cells. It is very likely that disrupting the interactions between the epithelial cells and different ECM components may have different effects on epithelial behavior in vivo. In this regard, it is interesting to note that a number of matrix metalloproteinase genes are upregulated during intestinal remodeling and tail resorption (Patterton et al., 1995; Brown et al., 1996; Stolow et al., 1996). This family of Zn-dependent extracellular enzymes are capable of digesting various components of the ECM (Alexander and Werb, 1991; Matrisian, 1992; Birkedal-Hansen et al., 1993; Sang and Douglas, 1996). Of particular interest is stromelysin-3, whose substrates in the ECM remain to be identified. This gene has been found to be activated in different organs immediately before and during cell death (Patterton et al., 1995; Brown et al., 1996). More importantly, its spatial and temporal expression correlates precisely with the basal lamina modification in the intestine as summarized above (Ishizuya-Oka et al., 1996). In contrast, the collagenase-3, collagenase-4, and gelatinase A are either minimally regulated or activated only during or toward the end of intestinal epithelial degeneration (Patterton et al., 1995; Stolow et al., 1996). These results argue for a role of specific modification of the basal lamina by metalloproteinases during T3-induced epithelial apoptosis. In support of this, a number of metalloproteinase genes are also activated during apoptotic degeneration of the postlactation mammary gland (Talhouk et al., 1992; Lund et al., 1996); and overexpression of stromelysin-1 in the mammary gland leads to matrix modification and apoptosis (Witty et al., 1995; Alexander et al., 1996).
Further evidence on the role of ECM in epithelial development comes from our analysis on T3-dependent gene regulation. During normal development, the intestinal IFABP and Na+/PO4 3− genes are reactivated in the adult epithelial cells as they differentiate in the presence of T3 (stages 62–66) (Shi and Hayes, 1994; Ishizuya-Oka et al., 1994, 1997). However, when epithelial cells isolated from stage 64 tadpoles were cultured in vitro, T3 treatment led to the downregulation of these genes, just like the cells isolated from premetamorphic tadpoles. Thus, the removal of the ECM and the underlying mesenchyme rendered the adult epithelial cells at stage 64 to undergo apoptosis in response to T3. Although individual ECM components fail to prevent the downregulation of IFABP gene by T3, multiple interactions between epithelial cells and ECM in vivo may be required for proper gene expression in epithelial cells. Alternatively, epithelial–mesenchymal interactions may also play an important role in adult epithelial development as first suggested by the organ culture experiments (Ishizuya-Oka and Shimozawa, 1992b ). However, coculturing mesenchymal and epithelial cells fails to prevent cell death (Fig. 6). Thus, both cell–cell and cell–ECM interactions are likely to be important for adult epithelial development. Our results further suggest that the differentiated intestinal epithelial cells are intrinsically vulnerable to T3-induced death. What prevents the adult epithelial cells from T3- induced apoptosis is partially because of the new ECM– epithelial and/or mesenchymal–epithelial interactions established during metamorphosis. Such a conclusion is also consistent with the self-renewal of adult intestinal epithelium during which epithelial cells gradually migrate as they differentiate toward the crest of the fold, equivalent to mammalian intestinal villus (Shi and Ishizuya-Oka, 1996). After a finite period of time, the cells at the crest but not elsewhere undergo apoptosis, partially because of altered cell–cell and cell–ECM interactions, and are replaced by the newly arrived epithelial cells (McAvoy and Dixon, 1977; Ishizuya-Oka and Ueda, 1996).
It is unclear how ECM influences intestinal epithelial development during metamorphosis. Studies in various model systems have provided evidence for the involvement of cell surface ECM receptors, especially integrins, in transducing the ECM signals (Werb et al., 1989; Damsky and Werb, 1992; Montgomery et al., 1994; Ruoslahti and Reed, 1994; Boudreau et al., 1995; Brown and Yamada, 1995). In one of the best studied model systems, i.e., the development of the mammary gland, it has been proposed that the interaction of ECM with its integrin receptors leads to the activation of focal adhesion tyrosine kinase, which in turn transduces the signal through the mitogen-activated kinase pathway to the nucleus (Roskelley et al., 1995). This or similar mechanisms may be responsible for ECM-mediated transcriptional regulation of gene expression (Roskelley et al., 1994). Such ECM-mediated gene expression may also play a role in regulating the fate of intestinal epithelial cells during T3-dependent metamorphosis.
Acknowledgments
We would like to thank P. Henkart, H. Kleinman, and K. Yamada for helpful discussions and suggestions, and T. Vo (all from National Institutes of Health) for preparation of the manuscript.
Abbreviations used in this paper
- CsA
cyclosporin A
- ECM
extracellular matrix
- ICE
interleukin-1β-converting enzyme
- IFABP
intestinal fatty acid binding protein
- T3
thyroid hormone or 3,5,3′-triiodothryonine
- TR
thyroid hormone receptor
- Z-VAD
Z-Val-Ala-Asp-floromethalketone
Footnotes
M.A. Stolow's present address is OriGene Technologies, Inc., 13 Taft Court, Suite 111, Rockville, MD 20855.
Address all correspondence to Yun-Bo Shi, Laboratory of Molecular Embryology, National Institute of Child Health and Human Development, Building 18T, Room 106, Bethesda, MD 20892-5431. Tel.: (301) 402-1004. Fax: (301) 402-1323. E-mail: shi@helix.nih.gov
References
- Alexander, C.M., and Z. Werb. 1991. Extracellular Matrix Degradation. In Cell Biology of Extracellular Matrix. 2nd ed. E.D. Hay, editor. Plenum Press, New York. pp. 255–302.
- Alexander CM, Howard EW, Bissell MJ, Werb Z. Rescue of mammary epithelial cell apoptosis and entactin degradation by a tissue inhibitor of metalloproteinases-1 transgene. J Cell Biol. 1996;135:1669–1677. doi: 10.1083/jcb.135.6.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birer BE, Mattila PS, Standaert RF, Herzenberg LA, Burakoff SJ, Crabtree G, Schreiber SL. Two distinct signal transmission pathways in T lymphocytes are inhibited by complexes formed between an immunophilin and either FK506 or rapamycin. Proc Natl Acad Sci USA. 1990;87:9231–9235. doi: 10.1073/pnas.87.23.9231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkedal-Hansen H, Moore WGI, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- Boudreau N, Sympson CJ, Werb Z, Bissell MJ. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science. 1995;267:891–893. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DD, Wang Z, Furlow JD, Kanamori A, Schwartzman RA, Rmo BF, Pinder A. The thyroid hormone-induced tail resorption program during Xenopus laevismetamorphosis. Proc Natl Acad Sci USA. 1996;93:1924–1929. doi: 10.1073/pnas.93.5.1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown KE, Yamada KM. The role of integrins during vertebrate development. Dev Biol. 1995;6:69–77. [Google Scholar]
- Damsky CH, Werb Z. Signal transduction by integrin receptors for extracellular matrix: cooperative processing of extracellular information. Curr Biol. 1992;4:772–781. doi: 10.1016/0955-0674(92)90100-q. [DOI] [PubMed] [Google Scholar]
- Dauca, M., and J. Hourdry. 1985. Transformations in the intestinal epithelium during anuran metamorphosis. In Metamorphosis. M. Balls and M. Bownes, editors. The Clarendon Press, Oxford, U.K. pp. 36–58.
- Dodd, M.H.I., and J.M. Dodd. 1976. The Biology of Metamorphosis. In Physiology of the Amphibia. B. Lofts, editor. Acadmic Press, New York. pp. 467–599.
- Gilbert, L.I., and E. Frieden. 1981. Metamorphosis: A Problem in Developmental Biology. 2nd ed. Plenum Press, New York. 563 pp.
- Gilbert, L.I., J.R. Tata, and B.G. Atkinson. 1996. Metamorphosis: Post-embryonic Reprogramming of Gene Expression in Amphibian and Insect Cells. Academic Press, New York. 671 pp.
- Hay, E.D. 1991. Cell Biology of Extracellular Matrix. 2nd ed. Plenum Press, New York. 468 pp.
- Ishizuya-Oka A, Shimozawa A. Development of the connective tissue in the digestive tract of the larval and metamorphosing Xenopus laevis. . Anat Anz. 1987a;164:81–93. [PubMed] [Google Scholar]
- Ishizuya-Oka A, Shimozawa A. Ultrastructural changes in the intestinal connective tissue of Xenopus laevisduring metamorphosis. J Morphol. 1987b;193:13–22. doi: 10.1002/jmor.1051930103. [DOI] [PubMed] [Google Scholar]
- Ishizuya-Oka A, Shimozawa A. Induction of metamorphosis by thyroid hormone in anuran small intestine cultured organotypically in vitro. In Vitro Cell Dev Biol. 1991;27A:853–857. doi: 10.1007/BF02630987. [DOI] [PubMed] [Google Scholar]
- Ishizuya-Oka A, Shimozawa A. Programmed cell death and heterolysis of larval epithelial cells by macrophage-like cells in the anuran small intestine in vivo and in vitro. J Morphol. 1992a;213:185–195. doi: 10.1002/jmor.1052130205. [DOI] [PubMed] [Google Scholar]
- Ishizuya-Oka A, Shimozawa A. Connective tissue is involved in adult epithelial development of the small intestine during anuran metamorphosis in vitro. Roux's Arch Dev Biol. 1992b;201:322–329. doi: 10.1007/BF00592113. [DOI] [PubMed] [Google Scholar]
- Ishizuya-Oka A, Ueda S. Apoptosis and cell proliferation in the Xenopus small intestine during metamorphosis. Cell Tissue Res. 1996;286:467–476. doi: 10.1007/s004410050716. [DOI] [PubMed] [Google Scholar]
- Ishizuya-Oka A, Shimozawa A, Takeda H, Shi Y-B. Cell-specific and spatio-temporal expression of intestinal fatty acid-binding protein gene during amphibian metamorphosis. Roux's Arch Dev Biol. 1994;204:150–155. doi: 10.1007/BF00361110. [DOI] [PubMed] [Google Scholar]
- Ishizuya-Oka A, Ueda S, Shi Y-B. Transient expression of stromelysin-3 mRNA in the amphibian small intestine during metamorphosis. Cell Tissue Res. 1996;283:325–329. doi: 10.1007/s004410050542. [DOI] [PubMed] [Google Scholar]
- Ishizuya-Oka A, Stolow MA, Ueda S, Shi Y-B. Temporal and spatial expression of an intestinal Na+/PO4 3−cotransporter correlates with epithelial transformation during thyroid hormone-dependent frog metamorphosis. Dev Genet. 1997;20:53–66. doi: 10.1002/(SICI)1520-6408(1997)20:1<53::AID-DVG7>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Izutsu Y, Yoshizato K, Tochinai S. Adult-type splenocytes of Xenopus induce apoptosis of histocompatible larval tail cells in vitro. Differentiation. 1996;60:277–286. doi: 10.1046/j.1432-0436.1996.6050277.x. [DOI] [PubMed] [Google Scholar]
- Jacobson MD, Weil M, Raff MC. Programmed cell death in animal development. Cell. 1997;88:347–354. doi: 10.1016/s0092-8674(00)81873-5. [DOI] [PubMed] [Google Scholar]
- Kerr JFR, Harmon B, Searle J. An electron-microscope study of cell eletion in the anuran tadpole tail during spontaneous metamorphosis with special reference to apoptosis of striated muscle fibres. J Cell Sci. 1974;14:571–585. doi: 10.1242/jcs.14.3.571. [DOI] [PubMed] [Google Scholar]
- Kikuyama S, Kawamura K, Tanaka S, Yamamoto K. Aspects of amphibian metamorphosis: hormonal control. Int Rev Cytol. 1993;145:105–148. doi: 10.1016/s0074-7696(08)60426-x. [DOI] [PubMed] [Google Scholar]
- Leloup J, Buscaglia M. La triiodothyronine: hormone de la métamorphose des amphibiens. CR Acad Sci. 1977;284:2261–2263. [Google Scholar]
- Liu Z-G, Smith SW, McLaughlin KA, Schwartz LM, Osborne BA. Apoptotic signals delivered through the T-cell receptor of a T-cell hybrid require the immediate-early gene nur77. Nature. 1994;367:281–284. doi: 10.1038/367281a0. [DOI] [PubMed] [Google Scholar]
- Louvard D, Kedinger M, Hauri HP. The differenitating intestinal epithelial cell: establishment and maintenance of functions through interactions between cellular structures. Annu Rev Cell Biol. 1992;8:157–195. doi: 10.1146/annurev.cb.08.110192.001105. [DOI] [PubMed] [Google Scholar]
- Lund LR, Romer J, Thomasset N, Solberg H, Pyke C, Bissell MJ, Dono K, Werb Z. Two distinct phases of apoptosis in mammary gland involution: proteinase-independent and -dependent pathways. Development. 1996;122:181–193. doi: 10.1242/dev.122.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangelsdorf DJ, Thummel C, Beato M, Herrlich P, Schutz G, Umesono K, Blumberg B, Kastner P, Mark M, Chambon P, Evans RM. The nuclear receptor superfamily: the second decade. Cell. 1995;83:835–839. doi: 10.1016/0092-8674(95)90199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis, T., E.F. Fritsch, and J. Sambrook. 1982. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, New York. 545 pp.
- Marshall JA, Dixon KE. Cell specialization in the epithelium of the small intestine of feeding Xenopus laevis. J Anat. 1978;126:133–144. [PMC free article] [PubMed] [Google Scholar]
- Martin SJ, Green DR. Protease activation during apoptosis: death by a thousand cuts? . Cell. 1995;82:349–352. doi: 10.1016/0092-8674(95)90422-0. [DOI] [PubMed] [Google Scholar]
- Matrisian LM. The matrix-degrading metalloproteinases. Bioessays. 1992;14:455–463. doi: 10.1002/bies.950140705. [DOI] [PubMed] [Google Scholar]
- McAvoy JW, Dixon KE. Cell proliferation and renewal in the small intestinal epithelium of metamorphosing and adult Xenopus laevis. J Exp Zool. 1977;202:129–138. [Google Scholar]
- McAvoy JW, Dixon KE. Cell specialization in the small intestinal epithelium of adult Xenopus laevis: structural aspects. J Anat. 1978;125:155–169. [PMC free article] [PubMed] [Google Scholar]
- McKeon F. When worlds collide: immunosuppressants meet protein phosphatases. Cell. 1991;66:823–826. doi: 10.1016/0092-8674(91)90426-y. [DOI] [PubMed] [Google Scholar]
- Montgomery AMP, Reisfeld RA, Cheresh DA. Integrin αvβ3 rescues melanoma cells from apoptosis in three dimensional dermal collagen. Proc Natl Acad Sci USA. 1994;91:8856–8860. doi: 10.1073/pnas.91.19.8856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata E, Merker HJ. Morphologic changes of the basal lamina in the small intestine of Xenopus laevis during metamorphosis. Acta Anat. 1991;140:60–69. doi: 10.1159/000147038. [DOI] [PubMed] [Google Scholar]
- Muzio M, Chinnaiyan AM, Kischkel FC, O'Rourke K, Shevchenko A, Ni J, Scaffidi C, Bretz JD, Zhang M, Gentz R, et al. FLICE, a novel FADD-homologous ICE/CED-3-like protease, is recruited to the CD95 (Fas/APO-1) death-inducing signaling complex. Cell. 1996;85:817–827. doi: 10.1016/s0092-8674(00)81266-0. [DOI] [PubMed] [Google Scholar]
- Nieuwkoop, P.D., and J. Faber. 1956. Normal Table of Xenopus laevis. North Holland Publishing, Amsterdam. The Netherlands. 243 pp.
- Patterton D, Hayes WP, Shi Y-B. Transcriptional activation of the matrix metalloproteinase gene stromelysin-3 coincides with thyroid hormone-induced cell death during frog metamorphosis. Dev Biol. 1995;167:252–262. doi: 10.1006/dbio.1995.1021. [DOI] [PubMed] [Google Scholar]
- Pronk GJ, Ramer K, Amiri P, Williams LT. Requirement of an ICE-like protease for induction of apoptosis and ceramide generation by REAPER. Science. 1996;271:808–810. doi: 10.1126/science.271.5250.808. [DOI] [PubMed] [Google Scholar]
- Ranjan M, Wong J, Shi Y-B. Transcriptional repression of Xenopus TRβ gene is mediated by a thyroid hormone response element located near the start site. J Biol Chem. 1994;269:24699–24705. [PubMed] [Google Scholar]
- Roskelley CD, Desprez PY, Bissell MJ. Extracellular matrix- dependent tissue-specific gene expression in mammary epithelial cells requires both physical and biochemical signal transduction. Proc Natl Acad Sci USA. 1994;91:12378–12382. doi: 10.1073/pnas.91.26.12378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roskelley CD, Srebrow A, Bissell MJ. A hierarchy of ECM-mediated signalling regulates tissue-specific gene expression. Curr Biol. 1995;7:736–747. doi: 10.1016/0955-0674(95)80117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E, Reed JC. Anchorage dependence, integrins, and apoptosis. Cell. 1994;77:477–478. doi: 10.1016/0092-8674(94)90209-7. [DOI] [PubMed] [Google Scholar]
- Samuels HH, Stanley F, Casanova J. Depletion of L-3, 5, 3′-triiodothyronine and L-thyroxine in euthyroid calf serum for use in cell culture studies of the action of thyroid hormone. Endocrinology. 1979;105:80–85. doi: 10.1210/endo-105-1-80. [DOI] [PubMed] [Google Scholar]
- Sang QA, Douglas DA. Computational sequence analysis of matrix metalloproteinases. J Protein Chem. 1996;15:137–160. doi: 10.1007/BF01887395. [DOI] [PubMed] [Google Scholar]
- Schmidt JW, Piepenhagen PA, Nelson WJ. Modulation of epithelial morphogenesis and cell fate by cell-to-cell signals and regulated cell adhesion. Semin Cell Biol. 1993;4:161–173. doi: 10.1006/scel.1993.1020. [DOI] [PubMed] [Google Scholar]
- Schreiber SL. Immunophilin-sensitive protein phosphatase action in cell signaling pathways. Cell. 1992;70:365–368. doi: 10.1016/0092-8674(92)90158-9. [DOI] [PubMed] [Google Scholar]
- Shi Y-B, Hayes WP. Thyroid hormone-dependent regulation of the intestinal fatty acid-binding protein gene during amphibian metamorphosis. Dev Biol. 1994;161:48–58. doi: 10.1006/dbio.1994.1006. [DOI] [PubMed] [Google Scholar]
- Shi Y-B. Molecular biology of amphibian metamorphosis: A new approach to an old problem. Trends Endocrinol Metab. 1994;5:14–20. doi: 10.1016/1043-2760(94)90116-3. [DOI] [PubMed] [Google Scholar]
- Shi Y-B, Liang VC-T. Cloning and characterization of the ribosomal protein L8 gene from Xenopus laevis. . Biochim Biophys Acta. 1994;1217:227–228. doi: 10.1016/0167-4781(94)90042-6. [DOI] [PubMed] [Google Scholar]
- Shi Y-B, Ishizuya-Oka A. Biphasic intestinal development in amphibians: embryogensis and remodeling during metamorphosis. Curr Top Dev Biol. 1996;32:205–235. doi: 10.1016/s0070-2153(08)60429-9. [DOI] [PubMed] [Google Scholar]
- Shi Y-B, Wong J, Puzianowska-Kuznicka M. Thyroid hormone receptors: mechanisms of transcriptional regulation and roles during frog development. J Biomed Sci. 1996;3:307–318. doi: 10.1007/BF02257960. [DOI] [PubMed] [Google Scholar]
- Shi YF, Sahai BM, Green DR. Cyclosporin A inhibits activation-induced cell death in T-cell hybridomas and thymocytes. Nature. 1989;339:625–626. doi: 10.1038/339625a0. [DOI] [PubMed] [Google Scholar]
- Shi YF, Mogil RJ, Bissonnette RP, Bromley P, Yamaguchi I, Green DR. The role of DNA fragmentation in T cell activation-induced apoptosis in vitro and in vivo. J Immunol. 1994;152:1674–1683. [PubMed] [Google Scholar]
- Simon-Assmann P, Kedinger M. Heterotypic cellular cooperation in gut morphogenesis and differentiation. Semin Cell Biol. 1993;4:221–230. doi: 10.1006/scel.1993.1026. [DOI] [PubMed] [Google Scholar]
- Stolow MA, Bauzon DD, Li J, Segwick T, Liang VC-T, Sang QA, Shi Y-B. Identification and characterization of a novel ollagenase in Xenopus laevis: possible roles during frog development. Mol Biol Cell. 1996;7:1471–1483. doi: 10.1091/mbc.7.10.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talhouk RS, Bissell MJ, Werb Z. Coordinated expression of extracellular matrix-degrading proteinases and their inhibitors regulates mammary epithelial function during involution. J Cell Biol. 1992;118:1271–1282. doi: 10.1083/jcb.118.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tata JR, Kawahara A, Baker BS. Prolactin inhibits both thyroid hormone-induced morphogenesis and cell death in cultured amphibian larval tissues. Dev Biol. 1991;146:72–80. doi: 10.1016/0012-1606(91)90447-b. [DOI] [PubMed] [Google Scholar]
- Timpl R, Brown JC. Supramolecular assembly of basement membranes. Bioessays. 1996;18:123–132. doi: 10.1002/bies.950180208. [DOI] [PubMed] [Google Scholar]
- Tsai M-J, O'Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- Werb Z, Tremble PM, Behrendtsen O, Crowley E, Damsky C. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol. 1989;109:877–889. doi: 10.1083/jcb.109.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E. Life, death, and the pursuit of apoptosis. Genes Dev. 1996;10:1–15. doi: 10.1101/gad.10.1.1. [DOI] [PubMed] [Google Scholar]
- Witty JP, Lempka T, Coffey RJ, Jr, Matrisian LM. Decreased tumor formation in 7,12-dimethylbenzanthracene-treated stromelysin-1 transgenic mice is associated with alterations in mammary epithelial cell apoptosis. Cancer Res. 1995;55:1401–1406. [PubMed] [Google Scholar]
- Woronicz JD, Calnan B, Ngo V, Winoto A. Requirement for the orphan steroid receptor Nur77 in apoptosis of T-cell hybridomas. Nature. 1994;367:277–281. doi: 10.1038/367277a0. [DOI] [PubMed] [Google Scholar]
- Wyllie AH, Kerr JFR, Curie AR. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]
- Yazdanbakhsh K, Choi J-W, Li Y, Lau LF, Choi Y. Cyclosporin A blocks apoptosis by inhibiting the DNA binding activity of the transcription factor Nur77. Proc Natl Acad Sci USA. 1995;92:437–441. doi: 10.1073/pnas.92.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen PM, Chin WW. New advances in understanding the molecular mechanisms of thyroid hormone action. Trends Endocrinol Metab. 1994;5:65–72. doi: 10.1016/1043-2760(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Yoshizato K. Biochemistry and cell biology of amphibian metamorphosis with a special emphasis on the mechanism of removal of larval organs. Int Rev Cytol. 1989;119:97–149. doi: 10.1016/s0074-7696(08)60650-6. [DOI] [PubMed] [Google Scholar]