Abstract
Myofibroblasts are unusual cells that share morphological and functional features of muscle and nonmuscle cells. Such cells are thought to control liver blood flow and kidney glomerular filtration rate by having unique contractile properties. To determine how these cells achieve their contractile properties and their resemblance to muscle cells, we have characterized two myofibroblast cell lines. Here, we demonstrate that myofibroblast cell lines from kidney mesangial cells (BHK) and liver stellate cells activate extensive programs of muscle gene expression including a wide variety of muscle structural proteins. In BHK cells, six different striated myosin heavy chain isoforms and many thin filament proteins, including troponin T and tropomyosin are expressed. Liver stellate cells express a limited subset of the muscle thick filament proteins expressed in BHK cells. Although these cells are mitotically active and do not morphologically differentiate into myotubes, we show that MyoD and myogenin are expressed and functional in both cell types. Finally, these cells contract in response to endothelin-1 (ET-1); and we show that ET-1 treatment increases the expression of sarcomeric myosin.
Myofibroblasts are cells of mesenchymal origin that are characterized by a fibroblastic appearance with some ultrastructural features of muscle cells. First described in wound retraction, they are found in almost all organ systems including the external theca of the ovarian follicle, the pulmonary alveolar septa, and the stromal cells of the rat intestinal villi (Kaye et al., 1968; O'Shea, 1970; Gabbiani et al., 1971; Kapanci et al., 1974; Skalli et al., 1986; Skalli and Gabbiani, 1988; Czernobilisky et al., 1989; Sappino et al., 1990). They are also implicated in pathological situations, such as liver cirrhosis and kidney glomerular fibrosis (for review see Skalli and Gabbiani, 1988). It has been hypothesized that myofibroblasts have specialized contractile properties in vivo since they can contract in response to endothelin 1 (ET-1),1 a vasoconstrictive peptide that is activated during injury (Kon et al., 1989; Moore et al., 1992).
The myofibroblasts of liver are called stellate cells and are located in the space of Disse, which is the region between the hepatocytes and the sinusoids. They are involved in retinoid metabolism and are the main producers of extracellular matrix proteins (Wake, 1971; De Leuw et al., 1984). During liver injury, the stellate cells undergo an activation process characterized by a decrease in vitamin A content and an increase in desmin, an intermediate filament protein normally found in muscle. Their close proximity to the endothelial lining of the sinusoids and their contractility in response to ET-1 have led to the hypothesis that they might function as liver pericytes, which are cells that can be found wrapped around capillaries (Diaz-Flores et al., 1991; Ogata et al., 1993; Housset et al., 1995). In the kidney, the mesangial cells located in the glomeruli are myofibroblasts (Johnson et al., 1992). These myofibroblasts are involved in the generation of mediators of inflammation, synthesis of cytokines, production and breakdown of basement membrane, as well as uptake of macromolecules (Elema et al., 1976; McCausland et al., 1977; Martin et al., 1989; Ohyama et al., 1990; Abboud et al., 1991). In addition, they contract and relax in response to vasoactive agents, including ET-1 (Schlondorff et al., 1984; Schlondorff, 1987; Badr et al., 1989). Their contractile properties are thought to control the rate of glomerular filtration by changing capillary surface area and to mediate wound healing after glomerular injury.
The expression of sarcomeric proteins has traditionally been thought to be restricted to striated muscle where their assembly into the sarcomere is highly regulated and ordered. Nonmuscle cells express many homologues of sarcomeric proteins, but there is significant sequence divergence between contractile proteins of muscle and nonmuscle cells. For example, nonmuscle myosin and striated muscle myosin are only 50% identical, and can have up to a 10-fold difference in their ATPase activities. In contrast, all eight striated muscle myosins share >80% amino acid sequence identity (for review see Weiss and Leinwand, 1996). Myofibroblasts have been broadly characterized by their expression of two proteins found in muscle, smooth muscle α-actin and desmin (for review see Sappino et al., 1990). There have been reports of additional muscle proteins expressed in myofibroblasts. An adult skeletal fast IId MyHC gene was found to be expressed in a liver stellate cell line (Ogata et al., 1993). In addition, one study has shown that BHK cells, which we propose are kidney myofibroblasts, express several muscle proteins, including titin (Schaart et al., 1991).
Very little is known about the mechanisms whereby myofibroblasts achieve their contractile properties nor about the components that define their “muscle-like” phenotype. To define and determine how these cells achieve their intermediate phenotype and to define the molecules that confer contractility to these cells, we have characterized two cell lines corresponding to liver and kidney myofibroblasts. The liver stellate cell line was derived from a cirrhotic rat liver, and we present data that BHK cells are a model of glomerular mesangial cells. We found extensive expression of sarcomeric proteins in BHK cells and more limited expression in liver stellate cells. Two myogenic regulators, myogenin and MyoD, appear to be responsible for activating the muscle program of gene expression in these two myofibroblast cell types. We also demonstrate that ET-1 is a potent stimulus of contraction and that this contraction is correlated with a stimulation of striated muscle protein gene expression.
Materials and Methods
Cell Culture
The liver stellate cell line, 2G, a gift from Dr. M. Rojkind (Albert Einstein College of Medicine, New York), was maintained in MEM supplemented with 10% FBS (HyClone, Logan, UT), penicillin G at 50 U/ml, and streptomycin at 50 μg/ml, 1% MEM nonessential amino acids, 2 mM l-glutamine. BHK cells were obtained from Dr. R. Goldman (Northwestern University, Chicago, IL) and were grown in DME with 10% tryptose phosphate broth (Sigma Chemical Co., St. Louis, MO), 10% BCS (HyClone), penicillin G at 50 U/ml, and streptomycin at 50 μg/ml. Cells were grown at 37°C in a 5% humidified CO2 incubator. Cells were treated for 24 h with ET-1 (Sigma Chemical Co.) at a final concentration of 10−10 M and ET-A and ET-B antagonist, PD145065 (Sigma Chemical Co.), at a final concentration of 5 × 10−7 M in serum-free medium. Transient transfections were performed using lipofectamine as described by the manufacturer (GIBCO BRL, Gaithersburg, MD). Myosin light chain reporter constructs were a gift from Dr. N. Rosenthal (Harvard Medical School, Cambridge, MA).
Immunochemistry
Mouse liver was fixed overnight at 4°C in fresh 4% formaldehyde in PBS (171 mM NaCl, 6 mM Na2HPO4, 6 mM KH2PO4, and 3 mM KCl). Tissue was embedded in paraffin following standard procedures (Watkins, 1989). 6-μm sections were deparaffinized, blocked overnight at 4°C in 5% normal goat serum (NGS), 2.5% BSA in PBS. HRP-labeled secondary antibody was visualized with 3-amino-9-ethylcarbazole (Vector Labs, Inc., Burlingame, CA). Antibody incubations were performed overnight at 4°C. Sections were counterstained with Gill's hematoxylin to visualize nuclei (Vector Labs, Inc.). Coverslips were fixed in 3.7% formaldehyde in PBS for 5 min, permeabilized in ice-cold acetone for 2 min, and then blocked in 10% NGS for 1 h at room temperature. Subsequent antibody incubations were performed for 1 h at room temperature.
Immunoelectron Microscopy
All incubations were performed at 4°C unless otherwise indicated. Cells were grown on coverslips that were washed with cold PBS three times for 5 min each, fixed with 4% paraformaldehyde in PBS for 30 min, and then washed with PBS as above. Cells were permeabilized with 1% Triton X-100 for 1 min, rinsed with PBS, and then free aldehydes were quenched with 0.05% sodium borohydride in PBS for 1 h at 4°C. Cells were again rinsed in PBS, and then blocked overnight at 4°C in 2% NGS in PBS. Cells were washed three times for 5 min each in 1% BSA, 0.05% Tween-20 in PBS (BSA/Tween/PBS). Primary antibody was diluted in 1% BSA, 0.05% Tween-20, and then incubated overnight. Cells were washed three times for 5 min each with BSA/Tween/PBS and incubated for 12–16 h with secondary antibody, 5 nm gold-conjugated, goat anti–mouse IgG (Ted Pella, Redding, CA). Cells were then washed with 0.05% Tween-20 in PBS, five times for 10 min at room temperature. Cells were then washed in 0.1 M sodium cacodylate, pH 7.4, and postfixed with 2.5% glutaraldehyde in 0.1 M sodium cacodylate. Coverslips were removed from the culture dishes for embedding, staining, and sectioning as previously described (Luft, 1961; Sabatini et al., 1963). Serial sections were cut parallel to the plane of the substratum, and examined in an electron microscope (100C; JEOL USA Inc., Peabody, MA) at an accelerating voltage of 80 kV.
Immunoblot Analysis
Protein expression of liver stellate cells and BHK cells was examined by immunoblotting. 100-mm dishes were washed twice in PBS, scraped in cytoskeletal buffer (50 mM KCl, 10 mM KPO4, 2 mM MgCl, 0.5 mM EDTA, 2 mM DTT), and then lysed by freeze thawing. Lysate was diluted in Laemmli sample buffer (Laemmli, 1970). Protein concentration was determined by Bradford assay, and 10 μg of each protein sample were electrophoresed on a 7.5% SDS-PAGE, transferred to 0.2 μm nitrocellulose membrane (Schleicher and Schuell, Keene, NH), and then membrane blocked with 5% nonfat milk in PBS for 2 h at room temperature. Electrophoresis and electroblotting followed standard protocols (Laemmli, 1970; Towbin et al., 1979). Primary antibody incubations were performed at 4°C overnight. Secondary antibody incubation was performed at room temperature for 2 h. Immunoreactive proteins were visualized using chemiluminescence (Amersham Corp., Arlington Heights, IL).
Proliferation Assay
Cells were plated at density of 7.44 × 105 in 100-mm dishes and maintained in serum-free medium for 24 h and treated with 10−10 M ET-1 for 24 h. Cells were trypsinized with 1 ml of trypsin/EDTA and counted with an hemocytometer 24 h after addition of ET-1.
Collagen Contraction Assay
Collagen solution, vitrogen 100 (Celltrix Laboratories, Palo Alto, CA), containing liver stellate cells (2 × 105 cells/ml) or BHK (7.5 × 105 cells/ml) were prepared as previously described (Moon and Tranquillo, 1993), with the exception that 0.5 ml of partially gelled collagen was pipetted into wells containing warm silicon oil instead of 0.1 ml of collagen.
CAT Assays on Cultured Cell Lysates
Transient transfections were performed using lipofectamine as described by the manufacturer (GIBCO BRL). 48 h after transfections, dishes were washed three times in PBS. Cell lysate was prepared by freeze thawing. The amount of protein in the supernatant was measured by the Bradford assay using BSA as the standard (Bio-Rad Laboratories, Hercules, CA). chloramphenicol acetyl transferase (CAT) assays were performed on 10 μg of total protein. CAT assays were done by TLC with an incubation for 2 h at 37°C (Gorman et al., 1982).
Antibodies
Primary monoclonal antibodies were F59 (Miller et al., 1989), antiembryonic myosin heavy chain, F.1652 (a gift from Dr. H. Blau, Stanford University School of Medicine, Palo Alto, CA), antiperinatal myosin heavy chain, N3.36 (a gift from Dr. H. Blau), anti-MyoD and antimyogenin (Novocastra, Burlingame, CA), antisarcomeric actin, HUC1-1 (a gift from Dr. J. Lessard, University of Cincinnati, Cincinnati, OH), anti-myosin light chain (MLC) 1,3 (a gift from Dr. F. Stockdale, Stanford University School of Medicine). Monoclonal antibodies against tropomyosin, troponin T (TnT), desmin, and titin were purchased from Sigma Chemical Co. Secondary antibodies conjugated to fluorescein and HRP were obtained from Jackson Immunoresearch Laboratories (West Grove, PA). Actin was visualized with rhodamine phalloidin (Molecular Probes, Eugene, OR).
Results
Expression of Sarcomeric Proteins in BHK and Liver Stellate Cells
Previous reports of the expression of muscle proteins in myofibroblasts (Table I) led us to determine the extent of sarcomeric protein gene expression in both BHK and liver stellate cells by immunocytochemistry and Western blotting using isoform-specific antibodies. In addition to expressing smooth muscle α-actin and desmin, they also express many skeletal muscle structural proteins (Table I). Both the number and category are different between the two myofibroblast cell types. As shown in Table I, the liver stellate cells express three skeletal muscle myosin heavy chain isoforms (MyHCs) including one developmental isoform and two adult isoforms. To confirm that sarcomeric proteins are expressed in vivo in liver myofibroblasts, serial sections from a mouse liver were stained with a sarcomeric MyHC antibody. As shown in Fig. 1, the staining is localized to the liver stellate cells.
Table I.
Muscle Protein Expression in Myofibroblasts*
| In Vivo‡ | Liver stellate cells | BHK cells | L cells | |||||
|---|---|---|---|---|---|---|---|---|
| Sarcomeric MyHC | + | + | − | |||||
| Embryonic | − | + | − | |||||
| Perinatal | + | + | − | |||||
| Adult skeletal fast IIa | + | + | − | |||||
| Adult skeletal fast IIb | − | + | − | |||||
| Adult skeletal fast IId | + | + | + | − | ||||
| α-Cardiac MyHC | − | − | − | |||||
| β MyHC | − | + | − | |||||
| Sarcomeric MLC | − | + | − | |||||
| Sarcomeric actin | − | + | − | |||||
| Sarcomeric tropomyosin (α,β) | − | + | − | |||||
| Sarcomeric troponin T | − | + | − | |||||
| Smooth-muscle MyHC | + | + | + | − | ||||
| Smooth-muscle actin | + | + | + | − | ||||
| Titin | − | − | − | |||||
| Desmin | + | + | + | − |
Cells were grown on coverslips under normal growth conditions. Coverslips were fixed and stained as described in Materials and Methods. 100-mm dishes were scraped in Laemmli sample buffer. Lysate was used for immunoblotting.
Previous in vivo reports in myofibroblasts (Benzonana et al., 1988; Sappino et al., 1990; Ogata et al., 1993).
Figure 1.
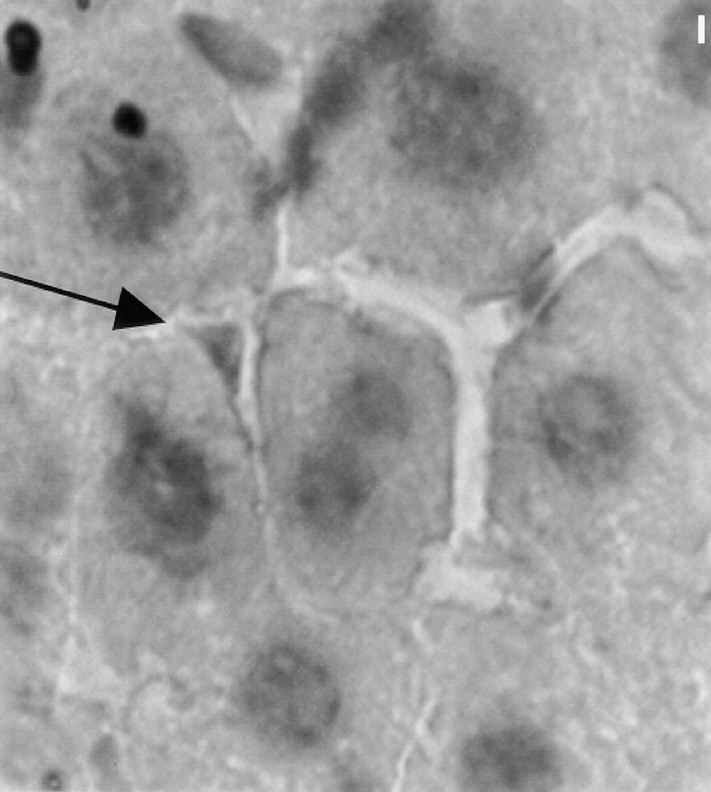
Sarcomeric MyHC is expressed in vivo in liver stellate cells. Immunoperoxidase labeling of sections of liver tissue was performed to visualize liver cell types expressing sarcomeric MyHC by using F59. Only perisinusoidal cells are positive for sarcomeric MyHC (arrow). Bar, 10 μm.
BHK cells express the entire program of developmental and adult skeletal MyHC isoforms tested thus far. Neither cell line expresses α-cardiac MyHC, which suggests that only the skeletal muscle program is activated in these two myofibroblast cell types. Unlike the liver stellate cell line, the BHK cell line also expresses sarcomeric thin filament proteins, including α and β tropomyosin and TnT. To determine whether the expression of sarcomeric proteins was homogeneous across the cell population, cells were stained with an antibody that recognizes all sarcomeric MyHCs, F59 (Miller et al., 1989). Approximately 10% of liver stellate cells expressed sarcomeric MyHC in culture, whereas 30% of BHK cells expressed sarcomeric MyHC under standard growth conditions (Fig. 2). In those cells expressing sarcomeric MyHC, the level of expression corresponds to 0.01% of total protein in liver stellate cells and 0.1% of total protein in BHK cells. This compares to 0.2–0.5% of total protein for nonmuscle MyHC (data not shown).
Figure 2.
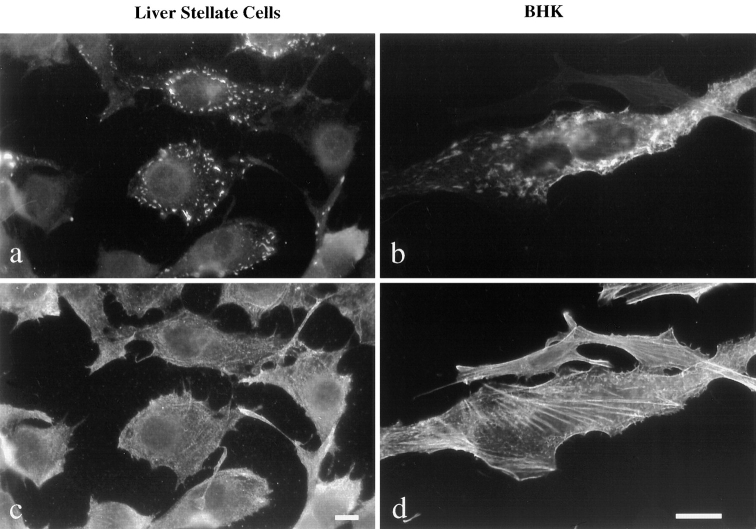
Localization of nonmuscle and sarcomeric MyHC expressed in liver stellate cells and BHK cells. Indirect immunofluorescence was performed to visualize MyHC in liver stellate cells (a and c) and BHK cells (b and d). Sarcomeric MyHC (a and b) forms punctate structures around the plasma membrane of liver stellate cells whereas it is more uniformly distributed in the cytoplasm of BHK cells. Sarcomeric (a and b) and nonmuscle MyHC (c and d) show partial colocalization in BHK cells, but not in liver stellate cells. Bars, (a and c) 10 μm, and (b and d) 20 μm.
To determine whether the sarcomeric MyHC expressed in BHK and liver stellate cells interacts with the nonmuscle cytoskeleton, sarcomeric and nonmuscle MyHC were visualized by double-label immunofluorescence. There was no colocalization of sarcomeric and nonsarcomeric MyHC in liver stellate cells (Fig. 2, a and c), whereas there was some colocalization of the two MyHCs in BHK cells but no appearance of the sarcomeric MyHC in the stress fibers (Fig. 2, b and d). In skeletal muscle, tropomyosin and TnT interact with the actin filaments. To determine the localization of sarcomeric tropomyosin and TnT in BHK cells, double-label immunofluorescence was performed. As shown in Fig. 3, there is colocalization of tropomyosin and TnT with the actin filament network of the cell.
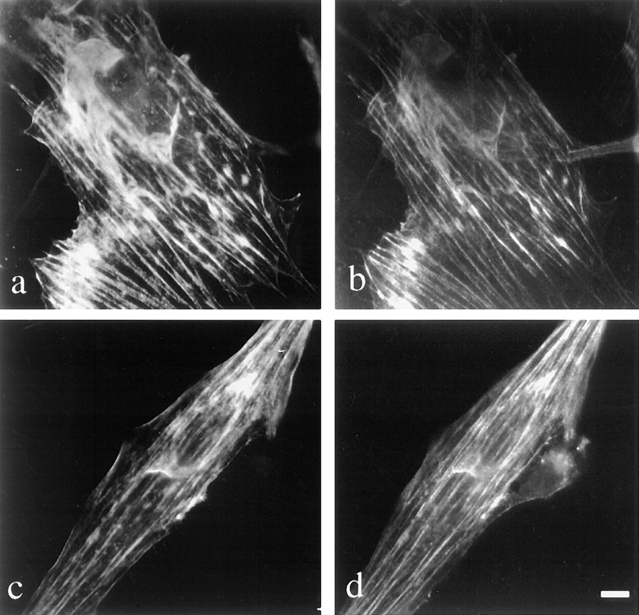
Figure 3.
Localization of sarcomeric thin filament proteins in BHK cells. Indirect immunofluorescence was performed to localize sarcomeric α and β tropomyosin (a), sarcomeric TnT (c), and the actin filaments (b and d) using anti-TnT, antisarcomeric tropomyosin, and rhodamine phalloidin. Sarcomeric TnT and sarcomeric α and β tropomyosin colocalize with the actin filaments. Bar, 10 μm.
Organization of Sarcomeric MyHC in BHK and Liver Stellate Cells
If the expressed sarcomeric MyHC is to play a unique contractile function in these two myofibroblast cell types, it might be expected to be organized into filamentous structures that are distinct from the nonmuscle MyHC. It is well established that muscle and nonmuscle MyHC filaments are very different in size and organization (Citi et al., 1987; Moncman et al., 1993; Verkhovsky et al., 1995). Sarcomeric thick filaments are 1.5 μm in length and composed of 100–200 MyHC molecules, whereas nonmuscle MyHC is organized into 0.25–0.35-μm minifilaments composed of 16–28 MyHC molecules (for review see Korn and Hammer, 1988). To examine the organization of sarcomeric MyHC in BHK and liver stellate cells, immunogold electron microscopy was carried out. Sarcomeric MyHC expressed in BHK and liver stellate cells formed bundles of filaments as determined by immunogold labeling (Fig. 4, a and b) whereas nonmuscle MyHC did not (Fig. 4, c and d). From this analysis, it is clear that there are no sarcomeres in either of these cell lines and there is no apparent periodicity to the structures formed by the muscle MyHC.
Figure 4.
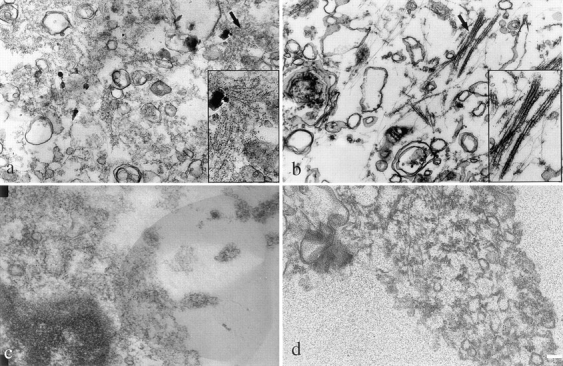
Ultrastructural analysis of sarcomeric MyHC in liver stellate cells and BHK cells. Immunoelectron microscopy was performed to determine the organization of sarcomeric MyHC in liver stellate cells and BHK cells. Monoclonal antibody, F59, was used to detect sarcomeric MyHC in liver stellate (a) and BHK cells (b); anti–α-platelet myosin antibody was used to detect nonmuscle MyHC in liver stellate cells (c) and BHK cells (d). Secondary antibodies were 5 nm gold-conjugated IgG. Arrows denote areas in inset for a and b. Bar, 1 μm.
Myogenic Regulators Control Sarcomeric Gene Expression in Liver Stellate Cells and BHK Cells in an E-Box–Dependent Manner
Myogenic regulatory factors (MRFs) are responsible for controlling the expression of muscle-specific genes during development (for review see Molkentin and Olson, 1996). Since a wide array of “muscle” genes are expressed in myofibroblasts, we hypothesized that myogenic regulators are expressed in BHK and liver stellate cells and are responsible for activation of muscle structural proteins. As determined by immunocytochemistry, MyoD (Fig. 5) and myogenin (data not shown) were detected in 100% of BHK and liver stellate cells. Myf-5 was not detected in either cell line (data not shown).
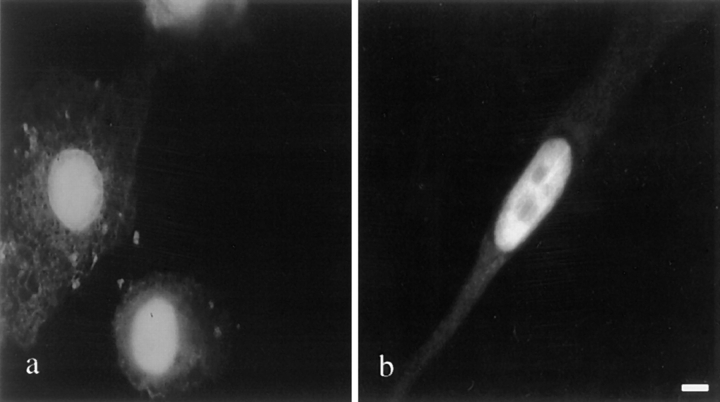
Figure 5.
Localization of MyoD in liver stellate cells and BHK cells. Indirect immunofluorescence was performed to localize MyoD in liver stellate cells (a) and BHK cells (b) using a monoclonal anti-MyoD antibody. MyoD expressed in liver stellate cells and BHK cells has a normal nuclear localization. Bar, 10 μm.
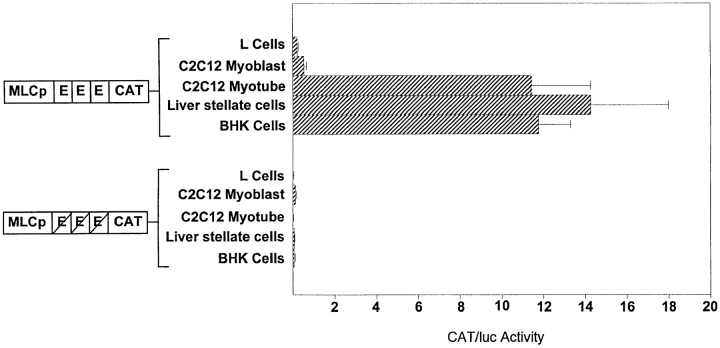
MRFs control muscle gene expression by binding to DNA consensus sequences, called E-boxes, that are found in the control regions of many muscle genes (Buskin and Hauschka, 1989). To determine whether the same programs that regulate gene expression in muscle cells also activate the muscle genes in BHK and liver stellate cells, skeletal myosin light chain (MLC) 1,3 promoter elements linked to the CAT reporter gene (MLC-CAT) were transfected into BHK and liver stellate cells. The wild-type construct, which has three E-boxes, is sufficient to confer muscle-specific gene expression in cultured cells and in transgenic mice, and its activity is dependent on the presence of MRFs (Rosenthal et al., 1989; Wentworth et al., 1991). The second construct is mutated in all three E-boxes and is not expressed in muscle cells (Wentworth et al., 1991). Included as a positive cell control were C2C12 myotubes and as negative controls, C2C12 myoblasts, and L cells. As shown in Fig. 6, the wild-type MLC construct is as active in BHK and stellate cells as in C2C12 differentiated muscle cells. In addition, the activity of the MLC construct in BHK and liver stellate cells is abolished when the E-boxes are mutated, indicating that the expression of this sarcomeric gene in BHK and liver stellate cells is dependent on the presence of the MRFs.
Figure 6.
E-box dependent expression of muscle reporter constructs in myofibroblasts. Previously described MLC reporter constructs were transfected into cell types as indicated (Wentworth et al., 1991). CAT activity is corrected for transfection efficiency by using a luciferase construct as a cotransfection reference plasmid. Transfections were carried out in triplicate. CAT activity is presented as mean value ± standard error.
ET-1–induced Contraction of BHK and Liver Stellate Cells Is Accompanied by an Increase in Sarcomeric MyHC Expression
Tissue repair includes the activation of resident mesenchymal cells to become myofibroblasts and the induction of ET-1 (Mak et al., 1984; Sappino et al., 1990). The activation process involves the development of a contractile phenotype with increased expression of smooth muscle α-actin in vivo (Sappino et al., 1990; Gabbiani, 1994). ET-1 has been shown to induce contractility of cultured stellate cells and mesangial cells (Badr et al., 1989; Rockey et al., 1993). To determine if ET-1 can induce the contraction of BHK and the liver stellate cell line characterized here, we performed a collagen contraction assay in the presence and absence of ET-1, using mouse L cells as a negative control. In the absence of exogenously added ET-1, myofibroblasts exhibited limited contractility, while L cells did not. As shown in Fig. 7, there was a dramatic contractile response to ET-1 that was blocked by an endothelin antagonist in both liver stellate cells and BHK cells.
Figure 7.
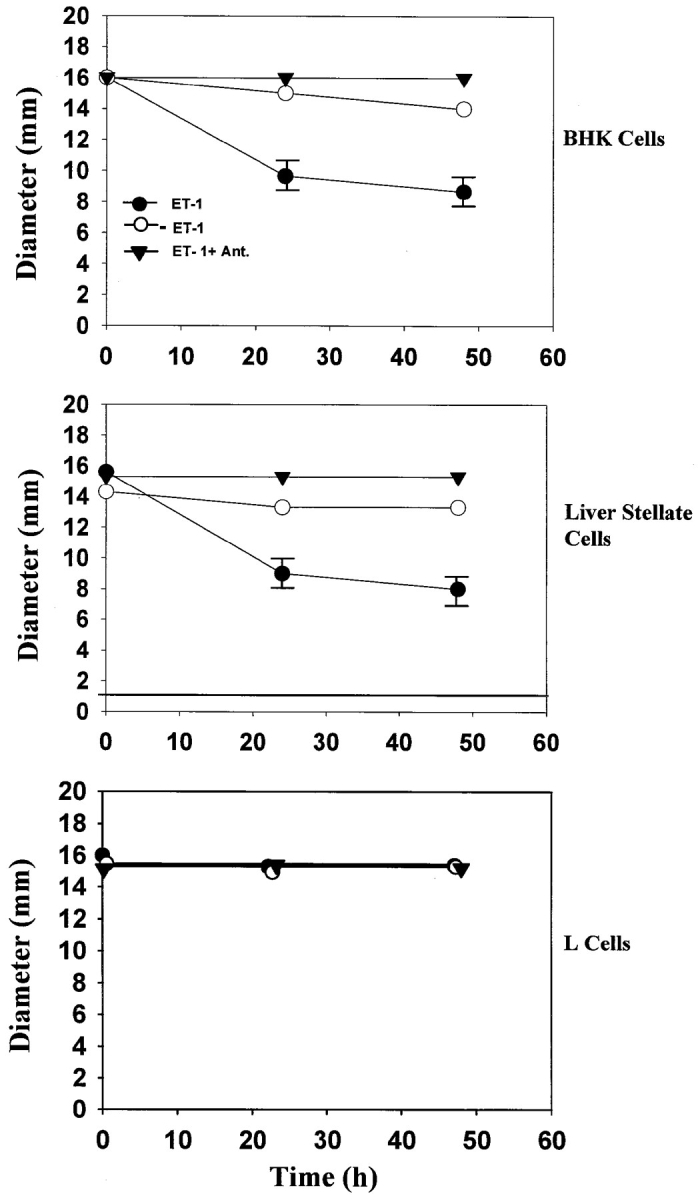
ET-1 dependent contractility of myofibroblasts. Contraction assays were performed as described in Materials and Methods. The change in diameter of the collagen gel was measured after 24 and 48 h in the absence of ET-1 (○) after addition of 10−10 M ET-1 (•) and 5 × 10−7 M ET-1 antagonist (▾). Each contraction assay was performed in triplicate. Data are presented as mean values ± standard error.
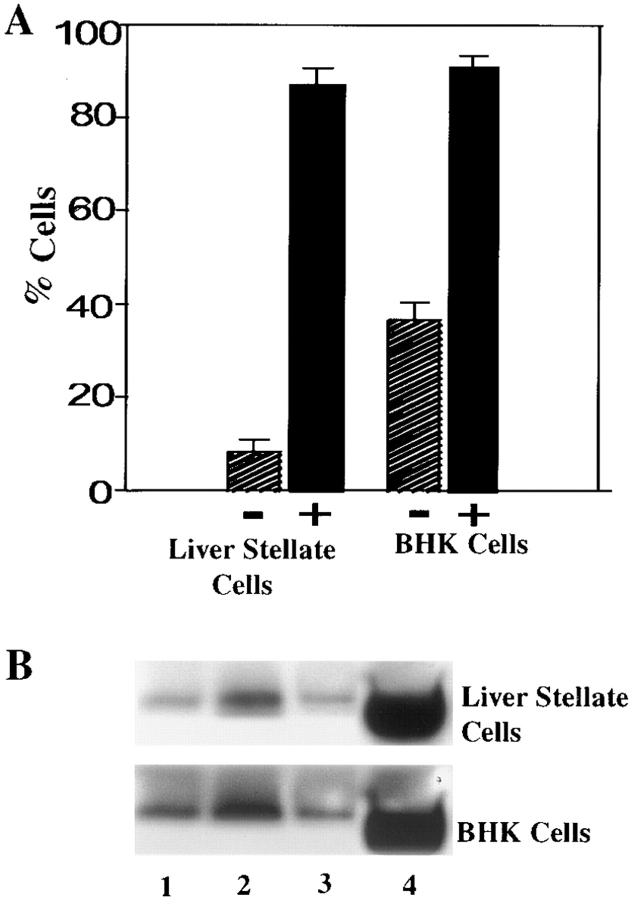
To determine if there was a change in sarcomeric MyHC expression in response to ET-1, cells were grown for 24 h in the presence of ET-1 and scored for the proportion of cells expressing MyHC and the amount of MyHC expressed. To confirm that the proportional increase in MyHC, which was visible by immunofluorescence, was accompanied by an increase in the amount of MyHC protein, Western analysis was also performed on the cells in the absence and presence of ET-1. As shown in Fig. 8, there was a ninefold increase in the proportion of MyHC-positive stellate cells and a threefold increase in the proportion of sarcomeric, MyHC-positive BHK cells. These increases were accompanied by a significant increase in the amount of sarcomeric MyHC (Fig. 8).
Figure 8.
ET-1 increases expression of sarcomeric MyHC in myofibroblasts. (A) Indirect immunofluorescence was performed to determine the number of sarcomeric MyHC-positive liver stellate cells and BHK cells before (−) and after (+) treatment with 10−10 M ET-1 for 24 h. (B) Immunoblotting was performed to determine the increase of sarcomeric MyHC in liver stellate cells and BHK cells in the absence of serum (lane 1), in the presence of 10% serum (lane 2), in the presence of 5 × 10−7 M ET-1 antagonist (lane 3), and in the presence of 10−10 M ET-1 for 24 h (lane 4).
Discussion
We believe that the liver stellate cell line and BHK cells are good culture models for the myofibroblasts of the liver and the kidney, respectively. They satisfy the established criteria for myofibroblasts. They actively produce extracellular matrix proteins and express desmin and smooth muscle α-actin (Tsutsumi et al., 1987; Ramadori et al., 1990; Rockey et al., 1992). In addition, the cells contract in response to treatment with ET-1. The discovery of extensive sarcomeric protein gene expression is consistent with the idea that these cells may be uniquely equipped for the contractile activities required for wound healing.
A key characteristic of these cells is their expression of sarcomeric myosins. Myosins are the molecular motors responsible for movement in all eukaryotic cells. It seems logical to attribute the acquisition of unique contractile capacity of myofibroblasts to the expression of sarcomeric proteins. Sarcomeric myosin was previously thought to perform a single function, that of muscle contraction in the context of the sarcomere, whereas the myosin expressed in nonmuscle cells has been shown to be involved in diverse cellular functions, ranging from cytokinesis to morphogenesis (DeLozanne and Spudich, 1987; Fukui et al., 1990). Given that many nonmuscle cells actively divide, one requirement for the nonmuscle myosin filaments is that they be able to undergo rounds of assembly and disassembly. The muscle sarcomere is a much more stable structure and most changes in its composition are thought to occur by exchange, in and out, of existing structures (Wenderoth and Eisenberg, 1987). Clearly, the sarcomeric proteins expressed in myofibroblasts are able to function in an actively dividing cell. Sarcomeric MyHC in liver stellate cells and BHK cells is organized differently than the nonmuscle MyHC, but despite the presence of most of the components of the sarcomere in BHK cells, sarcomeres do not form. BHK cells are quite remarkable in that they express every skeletal muscle structural protein we have tested (except titin), including ones that are normally developmentally regulated. As mentioned earlier, there is a report that under certain growth conditions, BHK cells can even express titin (Schaart et al., 1991). It seems logical that the sarcomeric motor molecules provide unique contractile properties to myofibroblasts during processes such as wound healing.
The activation of the myogenic program in muscle precursor cells involves a number of regulatory factors that regulate the expression of sarcomeric proteins (Olson, 1990; Tapscott and Weintraub, 1991; Buckingham, 1992). MyoD, Myf-5, MRF4, and myogenin act as transcriptional activators of many skeletal muscle genes (Davis et al., 1987; Braun et al., 1989; Rhodes et al., 1989; Wright et al., 1989; Braun et al., 1990; Miner and Wold, 1990; Edmonson and Olson, 1989). The expression of MyoD and myogenin in cultured liver stellate cells and BHK cells suggests that the muscle program in these specialized nonmuscle cells is activated by some of the same factors that are used during myogenesis. This is further supported by the transient transfection assays, which indicate that MRF-binding sites in the MLC enhancer are targets for the action of these factors in myofibroblasts. It is very intriguing that these cells express both myogenic regulators and muscle structural proteins, and yet they are not terminally differentiated, nor are they morphologically differentiated. It is also interesting that these two cell types express distinct programs of muscle gene expression, with the liver stellate cells expressing a more limited program. What limits muscle gene expression in liver stellate cells to the thick filament?
The wound healing response is a complex process that involves interplay of extracellular matrix components, cytokines, and biologically active peptides, such as members of the endothelin family (Slavin, 1996; Mutsaers et al., 1997). ET-1 is a powerful vasoconstrictive agent synthesized by vascular endothelial cells (Yanigasawa et al., 1988). Significant amounts of ET-1 are produced by nonendothelial cells such as mesangial cells and liver stellate cells. In the liver, the stellate cells have abundant ET-1 receptors (Housset et al., 1993; Rockey et al., 1995). In the kidney, a number of glomerular cells, including the mesangial cells synthesize ET-1 and also express ET receptors (Kohzuki et al., 1989; Kohan et al., 1992). There is an increased level of ET-1 during liver and kidney injury (Ohta et al., 1991; Moore et al., 1992; Asbert et al., 1993). Cultured liver stellate cells and mesangial cells display increased contractility in response to ET-1 (Badr et al., 1989; Kon et al., 1989; Katoh et al., 1990; Rockey et al., 1993). Furthermore, intravenous injection of ET-1 directly into rat kidneys led to a decrease in glomerular filtration rate and renal blood flow with a concomitant contraction of mesangial cells cultured from the injected rats. These effects were reversed by injection of an anti–ET-1 antibody (Kon et al., 1989). Likewise, liver stellate cells treated with ET antagonists showed a decrease in smooth muscle α-actin expression (Rockey and Chung, 1996). In this study, we confirm that an established liver stellate cell line and BHK cells can contract in response to ET-1. In addition, we demonstrate that there is a significant increase in the number of cells expressing sarcomeric MyHC. This is additional evidence supporting a functional role for the sarcomeric proteins expressed in these two myofibroblast cell types. This contractile response is not accompanied by an increase in cell proliferation (Mayer, D.H.G., and L.A. Leinwand, unpublished observation). We also demonstrate that the contractility of liver stellate cells and mesangial cells is blocked by the presence of an ET-1 antagonist.
There are numerous questions about these intriguing cells that will form the basis for future investigation. Future experiments will be directed towards identifying pathways that lead to myofibroblast cell types in vivo as well as determining the effects of disrupting sarcomeric protein expression on liver stellate cell and BHK cell contractility. Additionally, it will be interesting to determine the block to terminal differentiation that prevents the full differentiation potential of the myogenic regulators.
Abbreviations used in this paper
- ET-1
endothelin 1
- MLC
myosin light chain
- MRF
myogenic regulatory factors
- MyHC
myosin heavy chain isoforms
- NGS
normal goat serum
- TnT
troponin T
Footnotes
Address all correspondence to Leslie A. Leinwand, Department of Molecular, Cellular and Developmental Biology, University of Colorado, Campus Box 347, Boulder, CO 80309-0347. Tel.: (303) 492-7606. Fax: (303) 492-8907. E-mail: leinwand@stripe.colorado.edu
The authors acknowledge C. Saez for initiating this project. We thank M. Buvoli, B. Tompkins, B. Lu, and K. Haubold for assistance with figures. We thank K.L. Vikstrom and O. Roopnarine for critical reading of the manuscript. The authors would like to thank T. Giddings for assistance with electron microscopy.
D.C.G. Mayer was supported by National Institutes of Health grant No. GM29090. The research was supported by NIH grant No. GM29090 to L.A. Leinwand. This work is submitted in partial fulfillment of the requirements for the degree of Doctor of Philosophy in the Sue Golding Graduate Division of Medical Sciences, Albert Einstein College of Medicine, Yeshiva University (New York).
References
- Abboud HE. Resident glomerular cells in glomerular injury: mesangial cells. Semin Nephrol. 1991;11:304–310. [PubMed] [Google Scholar]
- Asbert M, Ginhs A, Ginhs P. Circulating level of endothelin in cirrhosis. Gastroenterology. 1993;104:1485–1491. doi: 10.1016/0016-5085(93)90360-o. [DOI] [PubMed] [Google Scholar]
- Badr KF, Murray JJ, Breyer MD, Takahashi K, Inagami T, Harris RC. Mesangial cell, glomerular and renal vascular responses to endothelin in the rat kidney. J Clin Invest. 1989;83:336–342. doi: 10.1172/JCI113880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benzonana G, Skalli O, Gabbiani G. Correlation between the distribution of smooth muscle and non muscle myosins and α-smooth muscle actin in normal and pathological soft tissues. Cell Motil Cytoskeleton. 1988;11:260–274. doi: 10.1002/cm.970110405. [DOI] [PubMed] [Google Scholar]
- Braun T, Buschhausen-Denker G, Bober E, Tannich E, Arnold HH. A novel human muscle factor related but distinct from MyoD1 induces myogenic conversion in 10T1/2 fibroblasts. EMBO (Eur Mol Biol Organ) J. 1989;8:701–709. doi: 10.1002/j.1460-2075.1989.tb03429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun T, Bober E, Winter B, Rosenthal N, Arnold HH. Myf-6, a new member of the human gene family of myogenic determination factors: evidence for a gene cluster on chromosome 12. EMBO (Eur Mol Biol Organ) J. 1990;9:821–831. doi: 10.1002/j.1460-2075.1990.tb08179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckingham M. Making muscle in mammals. Trends Genet. 1992;8:144–148. doi: 10.1016/0168-9525(92)90373-C. [DOI] [PubMed] [Google Scholar]
- Buskin JN, Hauschka SD. Identification of a myocyte-specific nuclear factor which binds to the muscle-specific enhancer of the mouse muscle creatine kinase gene. Mol Cell Biol. 1989;9:2627–2640. doi: 10.1128/mcb.9.6.2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citi S, Smith RC, Kendrick-Jones J. Effects of light chain phosphorylation and skeletal myosin on the stability of nonmuscle myosin filaments. J Mol Biol. 1987;198:253–262. doi: 10.1016/0022-2836(87)90311-1. [DOI] [PubMed] [Google Scholar]
- Czernobilisky BE, Shezen E, Lifschitz-Mercer B, Fogel M, Luzon A, Jacob N, Skalli O, Gabbiani G. Alpha-smooth muscle actin (α-SM actin) in normal human ovaries, in ovarian stromal hyperplasia and in ovarian neoplasms. Virchows Arch. 1989;57:55–65. doi: 10.1007/BF02899065. [DOI] [PubMed] [Google Scholar]
- Davis RL, Weintraub H, Lassar AB. Expression of a single transfected cDNA converts fibroblasts to myoblasts. Cell. 1987;51:987–1000. doi: 10.1016/0092-8674(87)90585-x. [DOI] [PubMed] [Google Scholar]
- De Leuw AM, McCarthy SB, Geerts A, Knook DL. Purified rat liver fat-storing cells in culture divide and contain collagen. Hepatology. 1984;4:392–403. doi: 10.1002/hep.1840040307. [DOI] [PubMed] [Google Scholar]
- DeLozanne A, Spudich JA. Disruption of the Dictyostelium discoideummyosin heavy chain gene by homologous recombination. Science. 1987;236:1086–1091. doi: 10.1126/science.3576222. [DOI] [PubMed] [Google Scholar]
- Diaz-Flores L, Gutierrez R, Verela H, Rancel N, Valladares F. Microvascular pericytes: a review of their morphological and functional characteristics. Histol Histopathol. 1991;6:269–286. [PubMed] [Google Scholar]
- Edmonson DG, Olson EN. A gene with homology to the mycsimilarity region of MyoD1 is expressed during myogenesis and is sufficient to activate the muscle differentiation program. Genes Dev. 1989;3:628–640. doi: 10.1101/gad.3.5.628. [DOI] [PubMed] [Google Scholar]
- Elema, J.D., Hoyer J.R., and R.L. Vernier. 1976. The glomerular mesangium: uptake and transport of intravenously injected colloidal carbon in rats. Kidney Int. 9:395–406. [DOI] [PubMed]
- Fukui Y, DeLozanne A, Spudich JA. Structure and function of the cytoskeleton of a Dictyostelium discoideummyosin-defective mutant. J Cell Biol. 1990;110:367–378. doi: 10.1083/jcb.110.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbiani G. Modulation of fibroblastic cytoskeletal features during wound healing and fibrosis. Pathol Res Pract. 1994;190:851–853. doi: 10.1016/S0344-0338(11)80988-X. [DOI] [PubMed] [Google Scholar]
- Gabbiani G, Ryan GB, Majno G. Presence of modified fibroblasts in granulation tissue and their possible role in wound contraction. Experientia (Basel) 1971;27:549–550. doi: 10.1007/BF02147594. [DOI] [PubMed] [Google Scholar]
- Gorman CA, Moffat LF, Howard BH. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982;2:1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housset CN, Rockey DC, Bissel DM. Endothelin receptors in rat liver: lipocytes as a contractile target for endothelin 1. Proc Natl Acad Sci USA. 1993;90:9266–9270. doi: 10.1073/pnas.90.20.9266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housset CN, Rockey DC, Friedman SL, Bissel DM. Hepatic lipocytes: a major target for endothelin-1. J Hepatol. 1995;22:55–60. [PubMed] [Google Scholar]
- Johnson RJ, Floege J, Yoshimura A, Lida H, Couser WG, Alpers CE. The activated mesangial cell: a glomerular “myofibroblast”? . J Am Soc Nephrol. 1992;2:190–197. doi: 10.1681/ASN.V210s190. [DOI] [PubMed] [Google Scholar]
- Kapanci Y, Assimacopoulos A, Irle C, Zwahlen A, Gabbiani G. “Contractile interstitial cells” in pulmonary alveolar septa: A possible regulator of ventilation/perfusion ratio? . J Cell Biol. 1974;60:375–392. doi: 10.1083/jcb.60.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh T, Chang H, Uchida S, Okuda T, Kurokawa K. Direct effects of endothelin in the rat kidney. Am J Physiol. 1990;258:397–402. doi: 10.1152/ajprenal.1990.258.2.F397. [DOI] [PubMed] [Google Scholar]
- Kaye GI, Lane N, Pascal PR. Colonic pericryptal fibroblast sheath: replication, migration, and cytodifferentiation of a mesenchymal cell-system in adult tissue. Fine structural aspects of rabbit and human colon. Gastroenterology. 1968;60:515–520. [PubMed] [Google Scholar]
- Kohan DE, Hughes AK, Perkins SL. Characterization of endothelin receptors in the inner medullary collecting duct of the rat. J Biol Chem. 1992;267:12336–12340. [PubMed] [Google Scholar]
- Kohzuki M, Johnson CI, Chai SY, Casley DJ, Mendelsohn FAO. Localization of endothelin receptors in rat kidney. Eur J Pharmacol. 1989;160:193–194. doi: 10.1016/0014-2999(89)90673-0. [DOI] [PubMed] [Google Scholar]
- Kon V, Yoshioka T, Fogo A, Ichikawa I. Glomerular actions of endothelin in vivo. . J Clin Invest. 1989;83:1762–1767. doi: 10.1172/JCI114079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn ED, Hammer JA., III Myosins of nonmuscle cells. Annu Rev Biophys Biophys Chem. 1988;17:23–45. doi: 10.1146/annurev.bb.17.060188.000323. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;237:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Luft JH. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak KM, Leo MA, Lieber CS. Alcoholic liver injury in baboons: transformation of lipocytes to transitional cells. Gastroenterology. 1984;87:188–200. [PubMed] [Google Scholar]
- Martin J, Davies M, Thomas G, Lovett DH. Human mesangial cells secrete a GBM-degrading neutral proteinase and a specific inhibitor. Kidney Int. 1989;36:790–801. doi: 10.1038/ki.1989.264. [DOI] [PubMed] [Google Scholar]
- McCausland IP, Gavin JB, Herdson PB. The distribution of dextran macromolecules in the glomeruli of normal and nephrotoxic rats. Pathology. 1977;9:123–128. doi: 10.3109/00313027709085250. [DOI] [PubMed] [Google Scholar]
- Miller JB, Teal SB, Stockdale FE. Evolutionarily conserved sequences of striated muscle myosin heavy chain isoforms. Epitope mapping by cDNA expression. J Biol Chem. 1989;264:13122–13130. [PubMed] [Google Scholar]
- Miner JH, Wold B. Herculin, a fourth member of the MyoD family of myogenic regulatory genes. Proc Natl Acad Sci USA. 1990;87:1089–1093. doi: 10.1073/pnas.87.3.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molkentin JD, Olson EN. Defining the regulatory networks for muscle development. Curr Opin Gen Dev. 1996;6:445–453. doi: 10.1016/s0959-437x(96)80066-9. [DOI] [PubMed] [Google Scholar]
- Moncman CL, Rindt H, Robbins J, Winkelman DA. Segregated assembly of muscle myosin expressed in nonmuscle cells. Mol Biol Cell. 1993;4:1051–1067. doi: 10.1091/mbc.4.10.1051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon AG, Tranquillo R. Fibroblast-populated collagen microsphere assay of cell traction force: part 1. Continuum model. AIChE (American Institute of Chemical Engineers) J. 1993;39:163–177. [Google Scholar]
- Moore K, Wendon J, Frazer M, Karani J, Williams R, Badr K. Plasma endothelin immunoreactivity in liver disease and the hepatorenal syndrome. N Engl J Med. 1992;327:1774–1778. doi: 10.1056/NEJM199212173272502. [DOI] [PubMed] [Google Scholar]
- Mutsaers SE, Bishop JE, McGrouther G, Laurent GJ. Mechanisms of tissue repair: from wound healing to fibrosis. Int J Biochem Cell Biol. 1997;29:5–17. doi: 10.1016/s1357-2725(96)00115-x. [DOI] [PubMed] [Google Scholar]
- Ogata I, Saez CG, Greenwel P, Ponce ML, Geerts A, Leinwand L, Rojkind M. Rat liver fat-storing cell lines express sarcomeric myosin heavy chain mRNA and protein. Cell Motil Cytoskeleton. 1993;26:125–132. doi: 10.1002/cm.970260204. [DOI] [PubMed] [Google Scholar]
- Ohta K, Hirata Y, Shichiri M, Kanno K, Emori T, Tomika K, Marumo F. Urinary excretion of endothelin 1 in normal subjects and patients with renal diseases. Kidney Int. 1991;39:307–311. doi: 10.1038/ki.1991.38. [DOI] [PubMed] [Google Scholar]
- Ohyama K, Seyer JM, Raghow R, Kang AH. Extracellular matrix phenotype of rat mesangial cells in culture. Biosynthesis of collagen types I, III, IV, and a low molecular weight collagenous component and their regulation by dexamethasone. J Lab Clin Med. 1990;116:219–227. [PubMed] [Google Scholar]
- Olson EN. MyoD family: a paradigm for development? . Genes Dev. 1990;4:1454–1461. doi: 10.1101/gad.4.9.1454. [DOI] [PubMed] [Google Scholar]
- O'Shea JD. An ultrastructural study of smooth-muscle like cells in the theca of the ovarian follicle of the rat. Anat Record. 1970;167:137–140. doi: 10.1002/ar.1091670202. [DOI] [PubMed] [Google Scholar]
- Ramadori G, Veit T, Schwogler S, Dienes HP, Knittel T, Reider H, Meyer zum Buschenfelde K-H. Expression of the gene of the α-smooth muscle-actin isoform in rat liver and in rat fat-storing (ITO) cells. Virchows Archiv B Cell Pathol. 1990;59:349–357. doi: 10.1007/BF02899424. [DOI] [PubMed] [Google Scholar]
- Rhodes SJ, Konienczny SF. Identification of MRF4: a new member of the muscle regulatory factor gene family. Genes Dev. 1989;3:2050–2061. doi: 10.1101/gad.3.12b.2050. [DOI] [PubMed] [Google Scholar]
- Rockey DC. Characterization of endothelin receptors mediating rat hepatic stellate cell contraction. Biochem Biophys Res Commun. 1995;207:725–731. doi: 10.1006/bbrc.1995.1247. [DOI] [PubMed] [Google Scholar]
- Rockey DC, Chung JJ. Endothelin antagonism in experimental hepatic fibrosis. J Clin Invest. 1996;98:1381–1388. doi: 10.1172/JCI118925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockey DC, Boyles JK, Gabbiani G, Friedman SL. Hepatic lipocytes express smooth-muscle actin upon activation in vivo and in culture. J Submicrosc Cytol Pathol. 1992;24:193–203. [PubMed] [Google Scholar]
- Rockey DC, Housset CN, Friedman SL. Activation-dependent contractility of rat hepatic lipocytes in culture and in vivo. J Clin Invest. 1993;92:1795–1804. doi: 10.1172/JCI116769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal N, Kornhauser JM, Donoghue M, Rosen KM, Merlie JP. Myosin light chain enhancer activates muscle-specific, developmentally regulated expression in transgenic mice. Proc Natl Acad Sci USA. 1989;86:7780–7784. doi: 10.1073/pnas.86.20.7780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini DD, Bensch K, Barnett RJ. Cytochemistry and electron microscopy: The preservation of cellular ultrastructure and enzymatic activity by aldehyde fixation. J Cell Biol. 1963;17:19–58. doi: 10.1083/jcb.17.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sappino AP, Schurch W, Gabbiani G. Biology of disease: differentiation repertoire of fibroblastic cells: expression of cytoskeletal proteins as marker of phenotypic regulation. Lab Invest. 1990;63:144–161. [PubMed] [Google Scholar]
- Schaart G, Pieper FR, Kuijpers HJH, Bloemendal H, Ramaekers FC S. Baby hamster kidney (BHK-21/C13) cells can express striated muscle type proteins. Differentiation. 1991;46:105–115. doi: 10.1111/j.1432-0436.1991.tb00871.x. [DOI] [PubMed] [Google Scholar]
- Schlondorff D. The glomerular mesangial cell: an expanding role for a specialized pericyte. FASEB (Fed Am Soc Exp Biol) J. 1987;1:272–281. doi: 10.1096/fasebj.1.4.3308611. [DOI] [PubMed] [Google Scholar]
- Schlondorff D, Satriano JA, Hagege J, Perez J, Baud L. Effect of platelet activating factor and serum treated zymosan on PGE2 synthesis, arachidonic acid release and contraction of cultured rat mesangial cells. J Clin Invest. 1984;73:1227–1231. doi: 10.1172/JCI111309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skalli, O., and G. Gabbiani. 1988. The biology of myofibroblasts: relationships to wound contraction and fibrocontractive diseases. In The Molecular and Cellular Biology of Wound Repair. R.M.F. Clarck, and P.M. Henson, editors. Plenum Publishing Corp., New York. 373–402.
- Skalli O, Ropraz P, Trzeciak A, Benzonana G, Gilessen D, Gabbiani G. A monoclonal antibody for α-smooth muscle actin, a new probe for smooth muscle differentiation. J Cell Biol. 1986;103:2787–2797. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slavin J. The role of cytokines in wound healing. J Pathol. 1996;178:5–10. doi: 10.1002/(SICI)1096-9896(199601)178:1<5::AID-PATH443>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Tapscott SJ, Weintraub H. MyoD and the regulation of myogenesis by basic helix-loop-helix proteins. J Clin Invest. 1991;87:1133–1138. doi: 10.1172/JCI115109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi M, Takada A, Takase S. Characterization of desmin-positive rat liver sinusoidal cells. Hepatology. 1987;7:277–284. doi: 10.1002/hep.1840070212. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrilamide gels to nitrocellulose sheets: procedures and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhovsky AB, Svitkina TM, Borisy GG. Myosin II filament assembly in the active lamella of fibroblasts: Their morphogenesis and role in the formation of actin filament bundles. J Cell Biol. 1995;131:989–1002. doi: 10.1083/jcb.131.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake K. “Sternzellen”: perisinusoidal cells with reference to storage of vitamin A. Am J Anat. 1971;132:429–462. doi: 10.1002/aja.1001320404. [DOI] [PubMed] [Google Scholar]
- Watkins, S. 1989. In situ hybridization and immunohistochemistry. In Current Protocols in Molecular Biology. S.M. Ausubel, R. Brent, R.E. Kingston, D.D. Moore, J.G. Seidman, J.A. Smith, and K. Struhl, editors. John Wiley and Sons. New York.
- Weiss A, Leinwand LA. The mammalian myosin heavy chain gene family. Annu Rev Cell Dev Biol. 1996;12:417–439. doi: 10.1146/annurev.cellbio.12.1.417. [DOI] [PubMed] [Google Scholar]
- Wenderoth MP, Eisenberg BR. Incorporation of nascent myosin heavy chains into thick filaments of cardiac myocytes in thyroid-treated rabbits. J Cell Biol. 1987;105:2771–2780. doi: 10.1083/jcb.105.6.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentworth BM, Donoghue M, Engert JC, Berglund EB, Rosenthal N. Paired MyoD binding sites regulate myosin light chain gene expression. Proc Natl Acad Sci USA. 1991;88:1242–1246. doi: 10.1073/pnas.88.4.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright WE, Binder M, Lin VK. Myogenin, a factor regulating myogenesis, has a domain homologous to MyoD1. Cell. 1989;56:607–617. doi: 10.1016/0092-8674(89)90583-7. [DOI] [PubMed] [Google Scholar]
- Yanigasawa M, Kurihara H, Kimura S, Tomobe Y, Koboyashi M, Mitsui Y, Goto K, Masaki T. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]