Abstract
We have investigated the potential role of contactin and contactin-associated protein (Caspr) in the axonal–glial interactions of myelination. In the nervous system, contactin is expressed by neurons, oligodendrocytes, and their progenitors, but not by Schwann cells. Expression of Caspr, a homologue of Neurexin IV, is restricted to neurons. Both contactin and Caspr are uniformly expressed at high levels on the surface of unensheathed neurites and are downregulated during myelination in vitro and in vivo. Contactin is downregulated along the entire myelinated nerve fiber. In contrast, Caspr expression initially remains elevated along segments of neurites associated with nascent myelin sheaths. With further maturation, Caspr is downregulated in the internode and becomes strikingly concentrated in the paranodal regions of the axon, suggesting that it redistributes from the internode to these sites. Caspr expression is similarly restricted to the paranodes of mature myelinated axons in the peripheral and central nervous systems; it is more diffusely and persistently expressed in gray matter and on unmyelinated axons. Immunoelectron microscopy demonstrated that Caspr is localized to the septate-like junctions that form between axons and the paranodal loops of myelinating cells. Caspr is poorly extracted by nonionic detergents, suggesting that it is associated with the axon cytoskeleton at these junctions. These results indicate that contactin and Caspr function independently during myelination and that their expression is regulated by glial ensheathment. They strongly implicate Caspr as a major transmembrane component of the paranodal junctions, whose molecular composition has previously been unknown, and suggest its role in the reciprocal signaling between axons and glia.
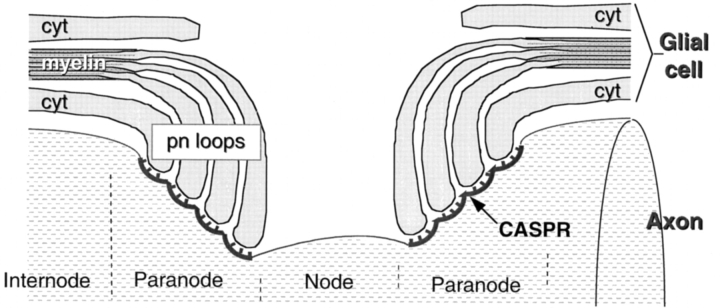
Myelinated nerve fibers play a critical role in the vertebrate nervous system by promoting the efficient and rapid propagation of action potentials via saltatory conduction (Huxley and Stämpfli, 1949). This mode of conduction requires the organization of myelinated fibers into longitudinal domains that are anatomically and functionally distinct. Three domains—the internode, the paranodal region, and the node of Ranvier— form as the result of, and can be distinguished by, specific interactions between axons and myelinating glial cells, i.e., Schwann cells in the peripheral nervous system and oligodendrocytes in the central nervous system (Peters et al., 1991; Salzer, 1997). In the internode, the most stereotypic portion of myelinated fibers, the axon is separated from the inner glial membrane by a regular space of 12 nm or more and is surrounded by a compact myelin sheath. In the paranodal region, the compact myelin lamellae open up into a series of cytoplasmic (paranodal) loops that spiral around and physically invaginate the axon. These glial loops are closely apposed to the axon, being separated by a gap of only 2.5–3 nm, and form a series of septate-like junctions with the axon (shown schematically in Fig. 8). In electron micrographs of longitudinal sections through the paranodal region, these junctions appear as a series of ladder-like densities that arise from the outer leaflet of the axolemma and contact the glial membranes. At the nodes, which represent gaps between the myelin sheaths, the axon is relatively exposed to the extracellular environment. In larger myelinated fibers, Schwann cell microvilli and astrocytic processes are also closely associated with the nodal axolemma in the peripheral and central nervous system (PNS and CNS)1, respectively.
Figure 8.
Schematic structure of the nodal region and the location of Caspr. The longitudinal organization of a myelinated axon at the node of Ranvier is shown. Myelinated axons contain three distinct domains: the internode, where the axon is surrounded by a compact myelin sheath; the paranodal region, where the axon is invaginated by and forms septate-like junctions with paranodal loops of the glial cell; and the node itself. The location of Caspr in the axon membrane is illustrated.
Each of these regions of myelinated fibers are distinct with respect to their function in impulse conduction and organization of voltage-gated channels. Thus, voltage-gated sodium channels are strikingly concentrated (∼1,500/μm2) at the node of Ranvier, enabling regeneration of the action potential. Na+/K+ ATPase and Na+/Ca++ exchangers are also enriched at the node (for a recent review see Waxman and Ritchie, 1993). Delayed rectifier potassium channels are enriched in the juxtaparanodal regions, where they may contribute to repolarization and ionic homeostasis (Wang et al., 1993; Mi et al., 1995). By contrast, the internode, which exhibits a reduced capacitance, has significantly lower concentrations of these voltage-gated channels. The mechanisms that regulate the distinct distributions of voltage-gated channels along the axon are not well understood. Paracrine and juxtacrine signals from glial cells appear to initiate clustering of sodium channels and other nodal components (Kaplan et al., 1997; for review see Salzer, 1997). The paranodal junctions may also contribute by providing a physical barrier that prevents lateral diffusion of ion channels and thereby separates these distinct domains (Rosenbluth, 1976; Gumbiner and Louvard, 1985).
The molecules that mediate the interactions between axons and myelinating glial cells at the paranodal junctions have been elusive. Several proteins of the glial cell are concentrated in the paranodal loops and mediate interactions between the glial processes rather than with the axon. Among these are E cadherin, which is likely to mediate adherens-like junctions between these processes (Fannon et al., 1995), and connexin 32, which is a component of the gap junctions that form between these glial processes (Scherer et al., 1995). The myelin-associated glycoprotein (MAG), which is enriched in the inner glial membrane and appears to promote adhesion to the axon in the internode, is enriched in paranodal loops in the PNS but not the CNS (for review see Quarles et al., 1992). Its function in the paranodal loops of Schwann cells is presently unclear. Several Ig-related cell adhesion molecules (CAMs) on the axon, including specific isoforms of neurofascin and NrCAM, are concentrated at the node of Ranvier, rather than in the paranodes, and may have a role in sodium channel localization (Davis et al., 1996).
Recent studies suggested that contactin/F3/F11 might mediate interactions between axons and glial cells (Peles et al., 1995; Koch et al., 1997). Contactin/F3/F11 is a GPI-anchored neural Ig CAM that promotes nerve fiber outgrowth. It was recently shown to be expressed by Schwann cells in the chick (Willbold et al., 1997) and by rat oligodendrocytes (Koch et al., 1997), although characterization of its expression by these cells in vivo, including its distribution along myelinated fibers, is still incomplete.
Contactin is associated with the transmembrane protein Caspr (contactin-associated protein), which has been suggested to be a coreceptor with contactin (Peles et al., 1997b ). Caspr is a type I integral membrane protein with a molecular mass of 190 kD that is highly expressed in the CNS; it is also present at reduced levels in several extraneuronal tissues (Peles et al., 1997b ). It copurifies with contactin when the carbonic anhydrase domain of the receptor protein tyrosine phosphatase β is used as an affinity ligand. Contactin and Caspr also coimmunoprecipitate, suggesting that they are constitutively complexed. The extracellular domain of Caspr contains a series of laminin G–like and EGF-like domains characteristic of the neurexins; accordingly, Caspr is a member of the neurexin superfamily. The cytoplasmic segment of Caspr contains potential binding sites for SH3 domain–containing proteins and band 4.1 proteins but lacks the carboxy-terminal motif found in other neurexins that binds to PDZ domains. Of interest, the Drosophila homologue of Caspr, Neurexin IV (Baumgartner et al., 1996), which has a similar extracellular domain organization, and a 30% amino acid identity (Peles et al., 1997a ; Littleton et al., 1997), is expressed by glial cells and is a component of their septate junctions that compose the blood–nerve barrier (Baumgartner et al., 1996).
We now report that contactin and Caspr/Neurexin IV are differentially expressed and localized during myelination. Of particular note, Caspr, which is expressed exclusively by neurons, becomes concentrated in the paranodal junctions of mature myelinated fibers in both the CNS and PNS, whereas contactin is substantially downregulated. In unmyelinated fibers and in nascent myelinated fibers, Caspr has a more diffuse localization along the length of the axon, suggesting that it redistributes from the internode to the paranode. These results implicate Caspr as a major component of the septate junctions that form between axons and paranodal loops and suggest that it may mediate reciprocal signaling between axons and myelinating glial cells.
Materials and Methods
Antibodies
Monoclonal antibodies used in these studies included the anti-MAG monoclonal antibody MA513 (gift of M. Schachner, Swiss Federal Institute of Technology, Zürich, Switzerland) and anti-MBP monoclonal antibody SMI 94 (Sternberger Monoclonals, Baltimore, MD). Rabbit polyclonal antisera included an anti-contactin/F3 antiserum (gift of C. Goridis, Institut de Biologie du Développement de Marseille, CNRS/INSERM/Université de la Mediterranée, Marseille, France); anti-Caspr antiserum 60/61 generated against a bacterial fusion protein containing the Caspr cytoplasmic domain and the corresponding preimmune serum (Peles et al., 1997b ).
Tissue Culture Methods
Primary rat Schwann cell and dorsal root ganglion (DRG) neuron cultures and myelinating Schwann cell/neuron cocultures were established as described previously (Einheber et al., 1993) with minor modifications. Neonatal rat Schwann cells were maintained in standard media consisting of DME (Whittaker Bioproducts, Inc., Walkersville, MD), 10% FBS (HyClone Laboratories, Inc., Logan, UT), and 2 mM glutamine (Life Technologies, Gaithersburg, MD) and were amplified in standard media supplemented with 5 ng/ml GGF2 (Cambridge Neurosciences, Cambridge, MA) and 4 μM forskolin (Sigma Chemical Co., St. Louis, MO). To examine Caspr and contactin expression by immunocytochemistry, 50,000 Schwann cells were plated onto poly-l-lysine–coated 12-mm coverslips and kept in standard media for a minimum of 3 d before fixation.
The dissociated neuronal cultures consisted of 4,000 rat embryonic day 16 or 17 DRG neurons plated onto 12-mm glass coverslips coated with ammoniated rat tail collagen (Biomedical Technologies, Inc., Stoughton, MA). These cultures were maintained in standard neuronal media, which consists of MEM (Life Technologies) supplemented with 10% FBS, 2 mM glutamine, 0.4% glucose (Sigma Chemical Co.), and 50 ng/ml 2.5S NGF (Bioproducts for Science, Inc., Indianapolis, IN). These cultures were treated for 2.5 wk with 5-fluorodeoxyuridine and uridine (both at 10−5 M) (Sigma Chemical Co.), which were added to the standard neuronal media in alternate feedings to eliminate nonneuronal cells.
Myelinating Schwann cell/neuron cocultures were prepared by seeding purified DRG neuron cultures with 200,000 Schwann cells/coverslip in standard neuronal media. The media was replaced the next day with N2 media, which consists of 5 mg/ml insulin (Sigma Chemical Co.), 10 mg/ml transferrin (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA), 20 nM progesterone (Sigma Chemical Co.), 100 mM putrescine (Sigma Chemical Co.), 30 nM selenium (Sigma Chemical Co.), and 2 mM glutamine in a 1:1 mixture of DME and Ham's F-12 (Life Technologies) supplemented with 2.5S NGF. The cultures were maintained in N2 media for 3 d to allow the Schwann cells to populate the neurites. To initiate basal lamina formation and myelination, the cultures were fed the standard neuronal media supplemented with 50 μg/ml ascorbic acid (Sigma Chemical Co.).
O-2A progenitors and oligodendrocytes were prepared as described previously (Canoll et al., 1996a ). To examine Caspr and contactin expression on oligodendrocytes by immunocytochemistry, 50,000 O-2A cells were plated onto poly-l-lysine–coated Lab-Tek chamber slide wells (Fisher Scientific, Pittsburgh, PA) and kept in DM+ media for 3 d before fixation.
Immunoblotting
Cultures of DRG neurons and Schwann cells were lysed in a solution containing 95 mM NaCl, 25 mM Tris-Cl, pH 7.4, 10 mM EDTA, 2% SDS, 1 mM PMSF, 10 μg/ml aprotinin, and 20 μM leupeptin. O-2A and oligodendrocyte lysates were prepared as described (Canoll et al., 1996a ). Sciatic nerve lysates were prepared from nerves immersed in liquid nitrogen after their dissection (Einheber et al., 1993). Protein concentrations of the lysates were determined by the Micro BCA method (Pierce Chemical Co., Rockford, IL). Lysates were subjected to SDS gel electrophoresis and blotted onto nitrocellulose. Blots were first probed with primary antibody against Caspr followed by 125I-labeled protein A (Amersham Corp., Arlington Heights, IL). Appropriate regions of the blots were cut out and reincubated with antibodies to contactin/F3 and 125I–protein A. The blots were then scanned and quantitated on a Molecular Dynamics (Sunnyvale, CA) phosphorimager.
For detergent extraction experiments, cultures grown in 35-mm dishes were rinsed with Dulbecco's PBS (dPBS; Life Technologies) and extracted with 1% Triton X-100 in dPBS in the presence of protease inhibitors at 4°C for 30 min. The supernatants containing the Triton X-100–solubilized proteins were then collected, and the remaining insoluble material was further extracted in gel sample buffer containing 2.5% SDS for 10 min at room temperature. All samples were spun at 12,000 g for 30 min at room temperature, and equal volumes of the cleared supernatants were fractionated on a 7.5% acrylamide SDS gel and blotted to nitrocellulose. The blot was probed with Caspr polyclonal antiserum and 125I–protein A and scanned on a phosphorimager to quantitate the immunolabeled bands.
Light Microscopy and Immunofluorescence
Immunofluorescence microscopy was done as described previously (Einheber et al., 1993) with minor modifications. Cultures grown on coverslips were washed with dPBS (Life Technologies), fixed with 4% paraformaldehyde in dPBS for 15 min, washed in dPBS, and then permeabilized with 100% methanol at −20°C for 15 min. The coverslips were then washed with dPBS and blocked for 30 min with Leibovitz's L-15 media (Life Technologies) supplemented with 10% heat-inactivated FBS. The coverslips were incubated for 1 h at room temperature with primary antibodies diluted in the blocking solution, washed three times with blocking solution, and incubated for 1 h at room temperature with species-specific affinity-purified rhodamine-conjugated donkey anti–rabbit IgG or fluorescein-conjugated donkey anti–mouse IgG (Chemicon International, Inc., Temecula, CA) diluted 1:100 in the blocking solution. The coverslips were washed five times, mounted in Citifluor (Ted Pella, Inc., Redding, CA) on glass slides, and examined by epifluorescence microscopy.
For tissue immunocytochemistry, vibratome sections of brain (60 μm thick) and teased sciatic nerve fibers were prepared from acrolein perfused adult Sprague Dawley rats and incubated with primary antibodies as described previously (Einheber et al., 1996). Primary antibodies bound to the tissue were detected using the avidin–biotin complex (ABC) peroxidase method and the chromagen 3,3′diaminobenzidine (Aldrich Chemical Co., Milwaukee, WI) (Einheber et al., 1996).
Immunoelectron Microscopy
Tissue sections immunolabeled with Caspr polyclonal antibody were processed for silver-enhanced immunogold labeling as described (Chan et al., 1990). Briefly, immunolabeled tissue was incubated for 2 h in 1:50 dilution of 1-nm colloidal gold–conjugated goat anti–rabbit IgG (Amersham Corp.), postfixed in 2% glutaraldehyde in PBS for 10 min, and reacted with silver solution using the intenSEM kit (Amersham Corp.).
For electron microscopy, the immunolabeled sections were then fixed for 1 h in 2% osmium tetroxide, dehydrated through a graded series of ethanols and propylene oxide, and embedded in Epon 812 between two sheets of Aclar plastic. Ultrathin sections (50 nm) were cut from the Epon– tissue interface, collected on copper grids, counterstained with 5% uranyl acetate and Reynold's lead citrate, and examined with an electron microscope (model 201; Philips Electron Optics, Mahwah, NJ).
Northern Analysis
Total RNA was prepared from Schwann cells, O-2A progenitors, and oligodendroglia (consisting of a mixed population of O4+/O1− and O1+ cells), rat brain, and postnatal sciatic nerves by CsCl2 gradient centrifugation (Chirgwin et al., 1979). Equal samples (10 μg) of total RNA were electrophoresed in 1% agarose, 2.2 M formaldehyde gels, transferred to nylon membranes (Duralon; Stratagene, La Jolla, CA) in 6× SSC, and UV cross-linked. Blots were prehybridized, hybridized, and washed using standard techniques; the final stringency of the wash was 0.2× SSC at 65°C for 30 min. cDNAs used as probes included an ∼500-bp fragment to rat Caspr and an ∼1.5-kb fragment to human contactin as described (Peles et al., 1997b ). 32P-labeled cDNA probes with specific activities of 2–5 × 109 cpm/mg were prepared by primer extension with random hexamers using the Prim-a-gene kit (Promega Corp., Madison, WI) according to the manufacturer's instructions.
Results
Caspr and Contactin Are Distinctly Expressed by Neurons and Myelinating Glia
We first determined the expression patterns of contactin/ F3/F11 (contactin) and Caspr using well-characterized antibodies that recognize the extracellular and the cytoplasmic domains of these proteins, respectively. Purified cultures of sensory neurons, Schwann cells, oligodendrocyte progenitors, and differentiated progenitors (consisting of both O4+/O1− prooligodendrocytes and O1+ oligodendrocytes) were prepared, and expression was analyzed by immunofluorescence (Fig. 1). Contactin is expressed robustly by neurons (Fig. 1 A), by oligodendrocytes (Fig. 1 E), and by their progenitors but not by Schwann cells (Fig. 1 C). By contrast, Caspr expression is restricted to neurons and their processes (Fig. 1 B); no staining of Schwann cells (Fig. 1 D) or of cells in the oligodendrocyte lineage (Fig. 1 F) was observed. Both Caspr and contactin are diffusely distributed along the entire surface of neurons and their processes.
Figure 1.
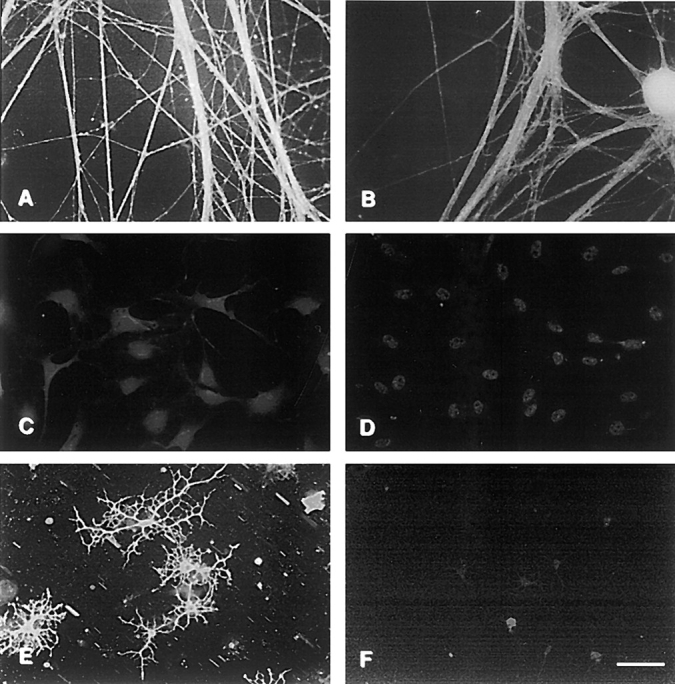
Expression of contactin and Caspr by neurons, Schwann cells and oligodendrocytes. Primary cultures of sensory neurons (A and B), Schwann cells (C and D), and oligodendrocytes (E and F) were stained with antibodies to contactin (A, C, and E) and Caspr (B, D, and F). Bar, 50 μm.
Myelination Regulates the Expression and Distribution of Caspr
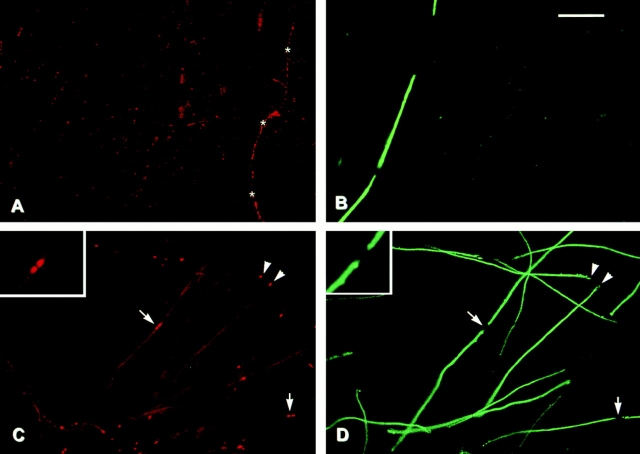
We next examined contactin and Caspr expression in established cocultures of Schwann cells and DRG neurons (Fig. 2). These cultures contained significant numbers of myelinated fibers as visualized by staining for myelin basic protein (MBP), which is a component of compact myelin (Fig. 2, B and D). Contactin (Fig. 2 A) is significantly downregulated in the cocultures in both ensheathed and myelinated fibers compared with isolated primary neurons. Contactin continues to be expressed at relatively high levels on the occasional unensheathed neurite that persists in such cultures (Fig. 2 A, asterisks). These results indicate that direct Schwann cell contact is responsible for the downregulation of contactin expression.
Figure 2.
Contactin and Caspr expression in myelinating cocultures. Schwann cells were added to cultures of dissociated sensory neurons and allowed to repopulate the neurites. Ascorbic acid and serum were added to promote myelination, and cultures were fixed after an additional 3 wk and stained for contactin (A) and Caspr (C). Corresponding fields (B and D) were stained for MBP. Asterisks in A indicate an unensheathed fiber that expresses contactin at high levels. Arrows in C and D indicate nodes of Ranvier; arrowheads indicate isolated paranodes. The insets in C and D show the node in the center of the field at higher power. Bar, 50 μm.
Caspr expression is also significantly reduced in the cocultures (Fig. 2 C). Of particular note, there is a dramatic change in its distribution, with very high levels present at the ends of the myelin sheaths. This concentration of Caspr is located just beyond the compact myelin sheath (as visualized by MBP staining) in the region of the paranodes. In the case of isolated myelin segments, Caspr is present at either end of the myelin segment (Fig. 2, C and D, arrowheads). Where two myelin segments approach each other to form a node, Caspr staining appears as a doublet. Two representative nodes are indicated by the arrows in Fig. 2, C and D, and a higher power view of the node in the center of the field is shown in the inset. Of note, the Caspr staining is found within the gap of MBP staining but does not appear to extend into the node itself.
Caspr Is Downregulated with Myelination and Is Associated with a Detergent Insoluble Fraction
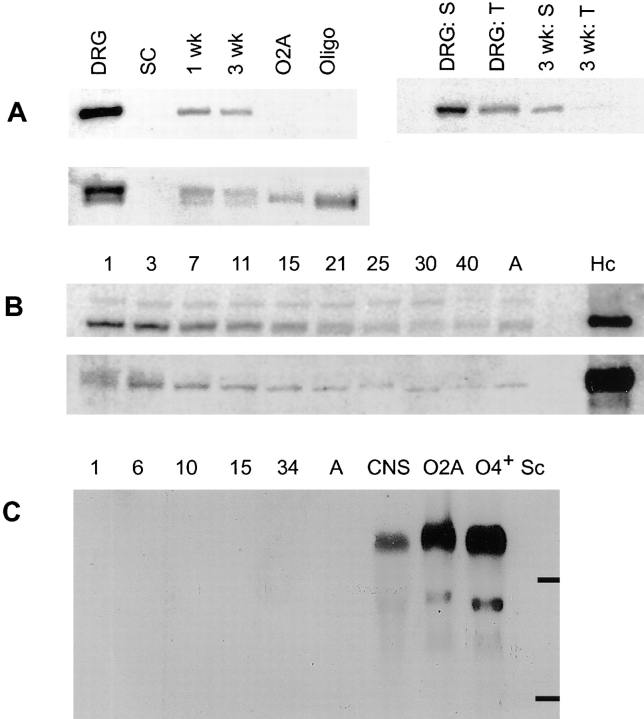
To assess more accurately the relative levels of expression of these proteins in the cultures, we performed Western blotting using 125I-labeled protein A as a reporter (Fig. 3). We compared culture lysates (50 μg) of neurons, Schwann cells, and Schwann cell/neuron cocultures after 1 or 3 wk in myelinating conditions; lysates (25 μg) of oligodendrocyte progenitors and differentiated O4+ oligodendroglia were also analyzed (Fig. 3 A). Caspr migrated with an expected molecular mass of ∼190 kD (Fig. 3 A, upper panels); in several experiments we also observed a minor band of ∼50 kD that may be a proteolytic fragment (data not shown). Consistent with immunostaining results, DRG neurons expressed Caspr at robust levels, whereas it was undetectable in Schwann cell and oligodendrocyte lysates. Caspr expression was greatly reduced in the cocultures compared with the neurons (more than 10-fold in several experiments). A progressive reduction in Caspr levels was also noticeable between 1 and 3 wk of coculture.
Figure 3.
Immunoblot and Northern analysis of contactin and Caspr expression. (A) Caspr and contactin expression in cultured cells and association of Caspr with the detergent insoluble complex. 50 μg of protein lysate prepared from neuron (DRG), Schwann cell (SC), 1-wk-old, and 3-wk-old myelinating cocultures and 25 μg of lysate from oligodendrocyte progenitors (O-2A) and O4+ oligodendroglia (Oligo) were fractionated by SDS-PAGE, blotted onto nitrocellulose, and probed with antiserum specific for Caspr (top left) or contactin (bottom). The top right of A is an immunoblot of samples prepared from DRGs and 3-wk-old myelinating cultures extracted with Triton X-100 (T) and SDS (S) probed with the Caspr antiserum. (B) Changes in Caspr and contactin expression during postnatal sciatic nerve development. 100 μg of protein lysate, prepared from sciatic nerves of rats at postnatal days 1, 3, 7, 11, 15, 21, 25, 30, 40, and adult (A) were separated by SDS-PAGE, blotted onto nitrocellulose, and probed with either Caspr (B, top) or contactin (B, bottom) antisera. Note that the amounts of Caspr and contactin detected in sciatic nerve were considerably smaller than that in 100 μg of hippocampal lysate (Hc). (C) Northern blot analysis of contactin expression. A Northern blot with 10 μg per lane of total RNA isolated from postnatal day 1, 6, 10, 15, 34, and adult sciatic nerves, total rat brain (CNS) and cultured O-2A progenitors, O4+ oligodendroglia, and Schwann cells (Sc) was probed with a 32P-labeled human contactin cDNA probe. Bars indicate the position of 28S and 18S rRNA.
In parallel, we examined whether Caspr might be part of a cytoskeleton-enriched, detergent-insoluble complex. We extracted neuron cultures and myelinating cocultures (at 4 wk) with 1% Triton X-100 (Fig. 3 A, lanes T), solubilized the remaining material with SDS (Fig. 3 A, lanes S), and analyzed both fractions by Western blotting (Fig. 3 A, top right). Only ∼25% of the total Caspr in the neuron cultures was extracted by Triton (Fig. 3 A, compare lane DRG:T to DRG:S). In the myelinating cocultures, less than 10% was extracted by Triton ( Fig. 3 A, compare lane 3 wk:T to 3 wk:S), suggesting that even more of the Caspr is part of a detergent-insoluble complex. In related studies, we have found that Caspr staining persists in the cocultures after Triton extraction, whereas MBP and MAG are completely removed (data not shown). Likewise, extraction of brain membrane fractions with a variety of nonionic detergents (e.g., Triton, Brij, digitonin, octylglucoside) released less than half of the total Caspr in each case (data not shown). These results indicate that Caspr is associated with a detergent-insoluble, cytoskeleton fraction in vitro and in vivo.
We also examined contactin expression by Western blotting. Contactin is expressed by neurons and oligodendrocytes, but not by Schwann cells, and is similarly progressively downregulated in the cocultures (Fig. 3 A, bottom). Of note, contactin is detected as a doublet on neurons and as a single band on oligodendrocytes. We had previously found that the upper band of this doublet is removed by phosphatidylinositol phospholipase C, whereas the lower band is phosphatidylinositol phospholipase C–resistant and is likely a preform of contactin (Rosen et al., 1992). As shown in Fig. 3 B, Caspr and contactin expression also significantly decrease during postnatal sciatic nerve development (day 1 through adulthood). At all times, they are present at substantially reduced levels in sciatic nerves when compared with hippocampus (Fig. 3 B, lane Hc) used as a CNS control.
A Northern blot for contactin (Fig. 3 C) confirms its high level expression in oligodendroglia; a major band of ∼6.4 kb and minor bands between 3–4 kb were present, consistent with previous reports (Gennarini et al., 1989). No expression of contactin was detected in Schwann cells or at varying times of sciatic nerve development. By contrast, Caspr mRNA of the expected size (6.2 kb) was detected in the CNS control but not in glia or sciatic nerve samples (data not shown). These results are consistent with the protein studies described above and indicate that Caspr and contactin in sciatic nerve are exclusively of axonal origin.
Redistribution of Caspr during Myelination
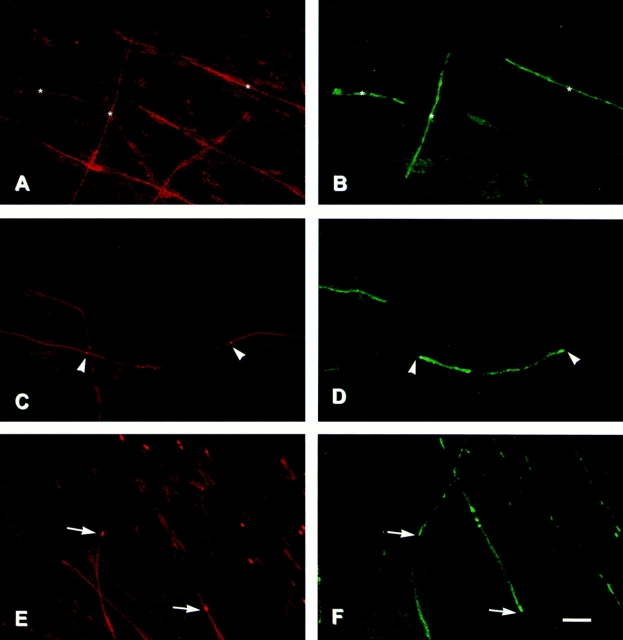
To investigate further the mechanisms by which Caspr becomes concentrated at the paranodes, we have analyzed its distribution at different times of myelination in the coculture system (Fig. 4). Schwann cells were seeded onto preestablished networks of DRG neurites and maintained for several days in a defined media in which Schwann cells proliferate but do not ensheathe or myelinate nerve fibers. Ascorbic acid was then added (day 0) to initiate ensheathment and myelination. Cultures were fixed at various times thereafter and double stained for Caspr and MAG, a myelin-specific protein that is expressed at the onset of myelination (Owens and Bunge, 1989).
Figure 4.
Redistribution of Caspr during myelination in vitro. Schwann cells were added to cultures of dissociated sensory neurons and allowed to repopulate the neurites. Ascorbic acid and serum were added to promote myelination, and cultures were fixed after an additional 4 d (A and B), 6 d (C and D), or 11 d (E and F). Cultures were immunostained for Caspr (A, C, and E) and MAG (B, D, and F). At 4 d, a few MAG-positive, nascent myelin segments were present (three are indicated with asterisks in B). Caspr expression continues to be expressed in the underlying axolemma (A, asterisks). 6 d after adding ascorbate, Caspr has begun to concentrate in some of the forming paranodes (C, arrowheads) associated with maturing myelin sheaths and, at the same time, is becoming attenuated in the corresponding internode. By 11 d, numerous myelin segments are present, and MAG staining in the Schmidt-Lanterman incisures is apparent (F). Caspr is concentrated in multiple paranodes and is substantially reduced in the internodes of mature myelin segments (located above the arrows in E), whereas it continues to be abundant in the internodes of nascent myelin segments (located below the arrows in E). Bar, 25 μm.
New myelin sheaths begin to form on days 3 to 4 in the cocultures and can be detected by their expression of MAG (Fig. 4 B, asterisks). Caspr is initially diffusely expressed along the entire neurite at this time, although at somewhat reduced levels compared with pure populations of neurons (Fig. 4 A). Interestingly, in some instances there appeared to be a slight increase in Caspr expression under some of the forming segments of myelin (data not shown). By 6–7 d, myelin sheaths have begun to compact, as indicated by the redistribution of MAG into the periaxonal glial membrane, Schmidt-Lanterman incisures and the paranodal loops. At the same time, Caspr began to accumulate into the paranodes of a few myelinated segments. (Examples of nascent paranodes are indicated by the arrowheads in Fig. 4 C.) Of additional note, Caspr expression frequently appeared attenuated at the center of corresponding internodes.
By 11 d, there is a striking accumulation of Caspr into multiple paranodes (Fig. 4, E and F). Concentrations of Caspr were invariably associated with well-myelinated segments, particularly those associated with MAG-positive Schmidt-Lanterman incisures, and accumulated into both paranodes of a myelinated segment at approximately the same time. Expression in the internode was reduced in those segments containing concentrations of Caspr at the paranodes compared with other segments that were still in the process of myelination. Two examples are indicated by arrows in Fig. 4, E and F, which mark paranodal accumulations. It may be seen that the mature myelin internodes located above the arrows express less Caspr than the two nascent myelin internodes located below the arrows. Taken together, these results indicate that Caspr accumulation in the paranodal region is a late event that occurs with myelin compaction and maturation and is likely to reflect a redistribution from the internode.
Caspr Is Concentrated in Paranodes in the CNS and PNS
To determine whether Caspr is localized at the paranodes of myelinated fibers in the PNS and CNS in vivo, whole mounts of teased rat sciatic nerve fibers and sections through various regions of the rat brain were immunolabeled with anti-Caspr antibody and examined by light microscopy. Intense paranodal staining for Caspr was observed in sciatic nerve fibers (Fig. 5 A), and virtually all of the fiber tracts in the brain, including the large myelinated axons of the facial nerve (Fig. 5 B) and the small, thinly myelinated axons of the corpus callosum (Fig. 6 A). Minimal staining, if any at all, was evident in the internodes. Paranodal staining was not observed with the corresponding control preimmune serum (data not shown). Although the protein appeared to be absent from the outer surfaces of the paranodal loops, it could not be determined at the light microscopic level whether Caspr immunoreactivity was associated with the inner surfaces of the paranodal loops adjacent to the axon, the axonal membrane, or with both. More precise localization of the protein was resolved by immunoelectron microscopy (see below).
Figure 5.

Caspr is concentrated in the paranodal regions of myelinated fibers in the PNS and CNS. A teased fiber preparation of adult sciatic nerve (A) and a coronal section through the facial nerve in the pons (B) were stained with an antiserum against Caspr using the immunoperoxidase technique and visualized by Nomarski (A) or brightfield microscopy (B). Low-magnification photomicrographs show that Caspr immunoreactivity is essentially restricted to the paranodes in both PNS and CNS fibers. Higher magnifications of individual nodes are shown in the insets. Bar, 20 μm.
Figure 6.
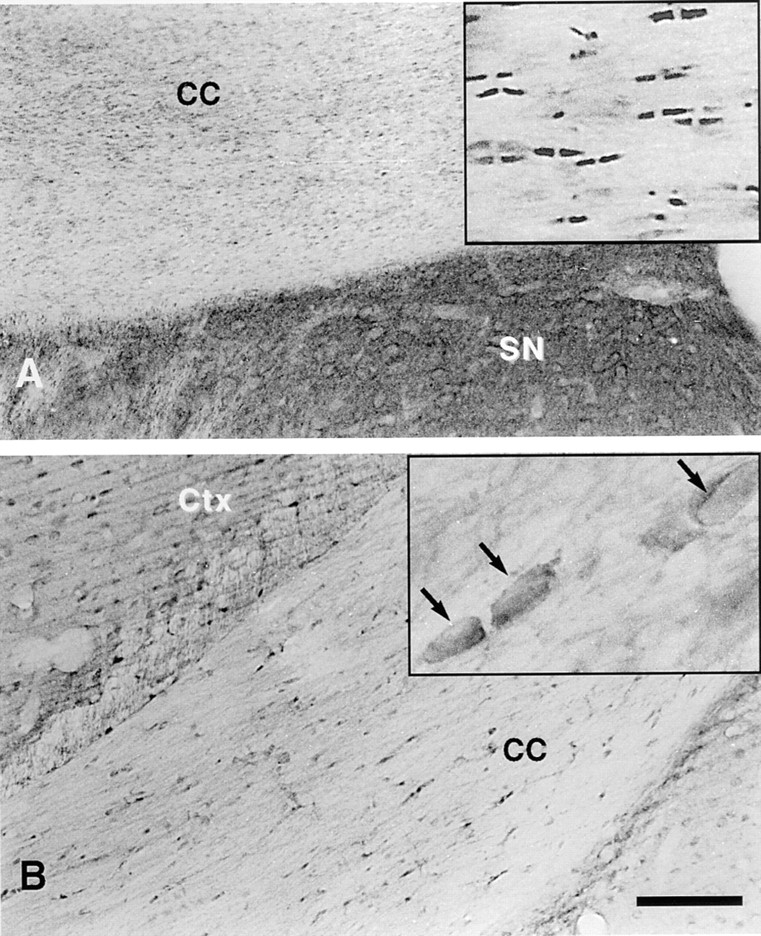
Contactin/F3 is not concentrated in the paranodal region. Staining of the corpus callosum (cc) with antisera against Caspr and contactin/F3 is shown. Caspr staining (A) is concentrated in the paranodal regions of the small myelinated fibers of the corpus callosum (shown at higher magnification in the inset). Contactin/F3 immunoreactivity is concentrated in cells that may be interfascicular oligodendrocytes based on their morphology (arrows in the higher-magnification inset) and location. Minimal contactin/F3 immunoreactivity was apparent in the paranodal regions. Moderately intense but diffuse Caspr immunoreactivity in the lateral septal nucleus (SN in A) and light contactin/F3 staining of cells in the cortex (Ctx in B), some of which appear to be neurons, were also apparent. Bar, 100 μm.
In addition to staining of the fiber tracts, low to moderate levels of diffuse Caspr immunoreactivity were present throughout the neuropil of the brain. An example of such diffuse staining in the lateral septal nucleus is shown in Fig. 6 A (SN). Considerable punctate staining, most likely representing paranodal regions of small myelinated fibers but possibly representing other neuronal structures, was found throughout the neuropil. In general, neuronal cell bodies in most regions of the brain examined were either unlabeled or only lightly labeled by the Caspr antibody.
The distribution of contactin immunoreactivity in brain sections was also examined by light microscopy (Fig. 6 B). In contrast to Caspr, and consistent with the in vitro studies described above, contactin was not concentrated at paranodal regions of myelinated fibers in these sections. However, relatively strong contactin staining of cells, possibly interfascicular oligodendrocytes, was observed in fiber tracts such as the corpus callosum (Fig. 6 B inset, arrows). Light to moderate contactin immunostaining was also prominent in many neurons distributed throughout the brain, such as those of the cerebral cortex (Fig. 6 B).
Caspr Is Localized to the Septate-like Junctions of the Paranodes
To determine more precisely the localization of Caspr within the paranode and to distinguish whether it was associated with neuronal and/or glial cell membranes, the distribution of the protein in the corpus callosum and the facial nerve (within the pons) was examined by immunoelectron microscopy. The results of these studies confirmed the light microscopic observations that Caspr is primarily concentrated at the paranodal regions of myelinated fibers. Furthermore, they showed that the protein is a component of the axonal membrane and not that of the paranodal loops of the glial cell. Representative nodal regions of myelinated fibers in the corpus callosum showing the silver-enhanced immunogold particles denoting the distribution of Caspr are shown in Fig. 7, A–C. Similar patterns of paranodal labeling were observed in sections of the facial nerve (data not shown). Most of the immunolabeling at nodal regions was restricted to the inner surface of the axonal membrane containing the septate-like junctions. An example of the characteristic septae of these junctions are shown at high magnification in the inset of Fig. 7 A. This staining of the inner membrane surface is expected given the reactivity of the antibody with cytoplasmic determinants on Caspr. In favorable planes of section, such as that shown in Fig. 7 B, strikingly intense immunolabeling was observed along the presumptive inner surface of the paranodal axonal membrane. Importantly, virtually no labeling was observed in the nodal gap itself and only occasional particles were detected in the axonal membrane or cytoplasm in the internodes of myelinated fibers. Taken together, these results indicate that Caspr is likely to be a component of the septate-like junctions at the paranodal region.
Figure 7.
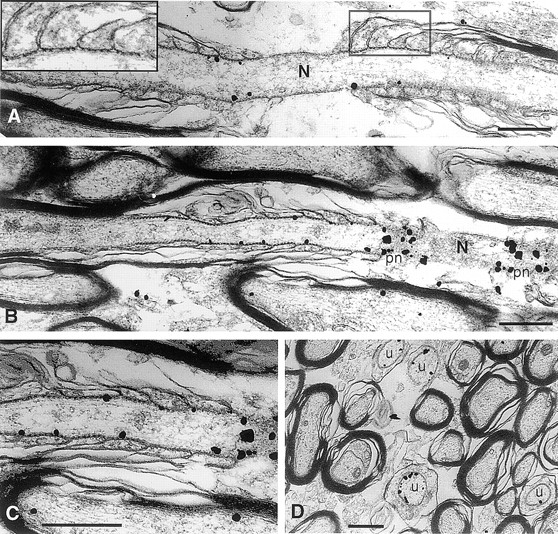
Immunoelectron microscopic localization of Caspr in myelinated and unmyelinated nerve fibers of the corpus callosum. Representative longitudinal sections of nodal regions of myelinated fibers with silver enhanced immunogold particles denoting the distribution of Caspr are shown in A–C. Gold particles were concentrated along the inner surface of the axonal membrane beneath the septate-like junctions located in the paranodal region (A–C). The inset in A shows the septate-like junctions that form between the axon and paranodal glial loops at higher magnification. Extremely dense labeling was observed where the plane of section approached the inner surface of the axonal membrane at the paranodes (pn; B and at higher magnification of the same field in C). Gold particles were occasionally found associated with the axonal membrane or cytoplasm in internodal portions of the myelinated fibers (B and C) but were rarely seen in the node (N). (D) A cross section demonstrating labeling of small caliber axons that are unmyelinated (u). Bars, 0.5 μm.
In addition to its concentration at the paranodal region of myelinated fibers, rather intense Caspr immunogold labeling was also observed around the inner surfaces of axonal membranes of some small-diameter nerve fibers that appeared to be unmyelinated. Examples of such fibers from the corpus callosum cut in cross section are shown in Fig. 7 D (u). These labeled profiles are unlikely to represent cross sections through the paranodal regions of myelinated fibers because they appear to lack the increased number of microtubules in the axon or surrounding myelin lamellae characteristic of this region (Peters et al., 1991). Furthermore, these profiles were often present in clusters within the callosum and much more frequent than would be expected for paranodes, which are quite short in comparison to the length of nerve fibers. These findings suggest that unmyelinated or ensheathed axons in the CNS may continue to express considerable amounts of Caspr and are consistent with the in vitro studies above, which showed that neurons grown in the absence of Schwann cells or those during the early stages of myelination express significant levels of this protein.
Discussion
We have shown that contactin and Caspr, which are both diffusely expressed on unensheathed nerve fibers, have very different fates during myelination. Contactin is nearly undetectable on myelinated fibers, whereas Caspr becomes highly concentrated in the paranodal region. These studies indicate that, although contactin and Caspr interact laterally (i.e., exhibit cis interactions) when they are in the same membrane in other settings (Peles et al., 1997b ), they have distinct roles during myelination and are not obligately associated. These findings also implicate Caspr as a major component of the septate-like paranodal junctions. The expression of contactin and the localization of Caspr are considered further below.
Contactin Expression during Myelination
In characterizing the expression of contactin, we have confirmed a recent report that oligodendrocytes express this protein in culture (Koch et al., 1997). This expression by oligodendrocytes and their progenitors could promote the initial interactions of axons and oligodendrocytes, possibly via a homophilic mechanism. Contactin also interacts with other ligands, notably receptor protein tyrosine phosphatase β (Peles et al., 1995), which is expressed at high levels by radial glial cells and oligodendrocyte progenitors (Canoll et al., 1996b ). Expression of contactin may therefore promote homotypic interactions between progenitors or heterotypic interactions with radial glia on which they appear to migrate (Zerlin et al., 1995). Interestingly, although contactin is robustly expressed by oligodendrocytes and their progenitors in vitro and by what appear to be interfascicular oligodendrocytes in vivo (Fig. 6 B, inset), there is little expression in white matter tracts or compact myelin. Consistent with these findings, other studies have reported contactin expression by neurons and their processes, including occasional axons in white matter, but not in myelin itself (Gennarini et al., 1989; Faivre-Sarrailh et al., 1992). Contactin was recently detected in myelin fractions by biochemical methods (Koch et al., 1997), potentially reflecting the presence of oligodendrocyte membranes in these myelin fractions or, alternatively, a greater sensitivity of the blotting techniques used.
In contrast, Schwann cells do not express contactin based on immunofluorescence, Western, and Northern analyses. These results differ from those of a recent report demonstrating expression of F11, the avian homologue of contactin/F3, in chick Schwann cells in vitro and in sciatic nerve (Willbold et al., 1997). This may reflect differences in contactin expression between avian and mammalian species. We have also found that contactin expression on nerve fibers dramatically declines during postnatal sciatic nerve development, a period of rapid myelination, and during myelination in vitro. This expression pattern resembles that of other adhesion molecules on axons, including NCAM and L1, that are also downregulated during the transition from ensheathment to myelination (Martini and Schachner, 1986; for review see Salzer, 1995). Contactin appears to be persistently expressed at low levels on unensheathed nerve fibers in the cocultures (see Fig. 2), indicating that downregulation depends on direct Schwann cell contact.
Caspr Is the First Marker Specific for Paranodal Junctions
The structure of the paranodal junctions that form between axons and the paranodal loops of oligodendrocytes and myelinating Schwann cells were described over thirty years ago (Bargmann and Lindner, 1964; Andres, 1965), but their molecular composition has remained unknown. A major finding of this study is the identification of Caspr as the first known marker of these junctions (shown diagrammatically in Fig. 8). Caspr seems likely to be a major transmembrane component of these junctions as well. Previous ultrastructural studies demonstrated that septate junction components arise from the axon, extending ∼3–5 nm to contact the glial membranes of the paranodal loops (Peters et al., 1991). Freeze fracture studies have revealed circumferential rows of intramembranous particles within the axolemma at these junctions (Wiley and Ellisman, 1980), potentially corresponding to Caspr. The time course of the focal accumulation of Caspr at these sites, i.e., at later stages of myelination, agrees well with earlier studies demonstrating that septate junctions develop with maturation of the myelinated fiber (Tao-Cheng and Rosenbluth, 1982). Of particular note, Drosophila Neurexin IV, a homologue of Caspr, was recently shown to be an essential component of the septate junctions that form between specialized glial cells (Baumgartner et al., 1996); Neurexin IV mutants lack the transverse septae characteristic of these junctions. Taken together, these results strongly support the notion that Caspr is an integral membrane component of the septate-like junctions at the paranodes.
Sequential Reorganization of the Axolemma during Myelination
These studies emphasize that the organization of the axon into three distinct longitudinal domains, i.e., the node, the paranodal region, and the internode, occurs sequentially during myelination. Previous studies have demonstrated that initial events in the formation of the node, characterized by clustering of intramembranous particles and sodium channels, occurs at the onset of myelination before compact myelin formation (for review see Black et al., 1995; Salzer, 1997) and can even occur in its absence (Deerinck et al., 1997). In contrast, paranodal junctions form during later stages of myelination, after the node has begun to mature, and require the formation of appropriately compacted myelin (Rosenbluth, 1983). Consistent with the sequential development of these domains, we have found that ankyrin G, a marker of the nodal cytoskeleton (Kordeli and Bennett, 1991), accumulates at the node several days before the accumulation of Caspr in the paranodes (Zanazzi, G., V. Bennett, and J. Salzer, unpublished observations).
The dramatic changes in the distribution of Caspr also indicate that myelination is critical to this sequential reorganization. Caspr is uniformly expressed on the surface of unensheathed DRG neurites. In the cocultures, Caspr remains diffusely expressed along the axon until later stages of myelination (see Fig. 4); it is persistently expressed on unmyelinated axons in the CNS in the adult (see Fig. 7 D). As the myelin sheath matures, Caspr levels dramatically increase in the paranodal region, accumulating in the paranodes of the most mature myelin sheaths first (see Figs. 2 and 4), consistent with morphologic studies of paranodal junction development (Tao-Cheng and Rosenbluth, 1982). Caspr accumulation in the paranodes is accompanied by a simultaneous decrease in the internode (see Fig. 4, C and E). This decrease may reflect, in part, more rapid turnover of Caspr in the internode during myelination (see Fig. 3 A), as well as its redistribution to the paranodes. Interactions with glial ligands and/or elements of the axon cytoskeleton may target Caspr to the paranodal region. The association of Caspr with a detergent insoluble fraction in neurons, myelinating cocultures, and brain membrane fractions as well as specific sequence motifs of its cytoplasmic domain (discussed below) are consistent with the latter possibility.
Potential Role of Paranodal Junctions in Channel Distribution and Signaling
It has been proposed that invertebrate septate junctions serve several functions, including a role analogous to that of tight junctions in providing a transcellular barrier to the passage of solute molecules and in promoting the generation of apical and basal lateral compartments (Lane, 1991). In strong support of this notion, the blood–nerve barrier that forms between the specialized glial cells is disrupted in a Drosophila Neurexin IV mutant (Baumgartner et al., 1996). The paranodal junctions of myelinated fibers probably have a similar role in forming a partial physical barrier. Studies in which electron-dense tracers were infused in peripheral nerves, for example, have demonstrated that these junctions provide a partial barrier to the diffusion of small ions and an absolute barrier for the passage of large molecular weight compounds into the internode (Hirano and Llena, 1995). By retarding ionic movements, these junctions are likely to promote more efficient saltatory conduction. Paranodal junctions may also regulate the distribution of voltage-gated channels. Indirect evidence, including freeze fracture studies, suggests they physically demarcate the boundaries of the node by limiting the lateral diffusion of Na+ channels, thereby confining them to the node (Rosenbluth, 1983). Thus, while the paranodal junctions may not be required for the initial clustering of Na+ channels, they may restrict the subsequent distribution of these channels. Less is known about factors that regulate the distribution of specific voltage-gated K+ channels (Kv1.1 and Kv1.2), which are concentrated in the juxtaparanodal region (Wang et al., 1993; Mi et al., 1995). The paranodal junctions are appropriately positioned to have a similar role in limiting the lateral diffusion of K+ channels but have not yet been directly implicated.
With this report, we have shown that paranodal junctions not only resemble invertebrate septate junctions in their morphology but also share homologous proteins. The molecular characterization of septate junctions has progressed rapidly in recent years, largely from analysis of Drosophila mutants. Invertebrate septate junctions have been found to be associated with actin filaments and associated proteins (Lane and Flores, 1988; Colombo et al., 1993). The localization of a second protein to Drosophila septate junctions, band 4.1–Coracle (Fehon et al., 1994), provides a mechanism for this linkage. Band 4.1 is a prototype of a large family of cytoskeletal proteins that includes the ERM (ezrin/radixin/moesin) family of cytoskeletal proteins and several phosphatases (for review see Tsukita et al., 1997). It functions as a link between transmembrane proteins and the underlying spectrin/actin cytoskeleton (Bennett and Gilligan, 1993). It is of interest that Drosophila Neurexin IV and Caspr both contain cytoplasmic sequences homologous to those in glycophorin, a red blood cell protein, that binds to the erythrocyte band 4.1 protein. Of additional note, in Drosophila Neurexin IV mutants, band 4.1–Coracle is no longer restricted to the septate junctions (Baumgartner et al., 1996). These results provide strong support for a direct interaction of Neurexin IV with band 4.1–Coracle.
While it is not yet known whether Caspr is similarly linked to any band 4.1 superfamily members, current evidence supports its association with the axon cytoskeleton. The spacing between the septae at the paranodes is highly regular (25–30 nm and see Fig. 7 A, inset) (Peters et al., 1991), suggesting an organized structure that might be imposed by cytoskeletal associations. Indeed, previous ultrastructural studies have demonstrated a linkage between membrane proteins of the axon and the underlying cytoskeleton at the paranodes (Ichimura and Ellisman, 1991), although the nature of the proteins has not yet been determined. Finally, we have shown (Fig. 3 A) that Caspr is poorly extracted by nonionic detergents from neurons alone and from myelinating cocultures and brain membrane preparations. Taken together, these results indicate that Caspr is likely to be constitutively associated with the axon cytoskeleton, possibly through a band 4.1 superfamily member. Such a linkage could regulate its targeting to the paranodal junctions.
The structure of the cytoplasmic region of Caspr suggests it may also regulate intracellular signaling pathways in axons. Notably, this segment contains a proline-rich sequence with at least one canonical SH3 domain–binding site (Peles et al., 1997b ). Consistent with this suggestion, fusion proteins containing the SH3 domains of the signaling proteins PLC γ, src, fyn, and the p85 subunit of PI 3-kinase bound to the cytoplasmic domain of Caspr (Peles et al., 1997b ). It is not yet known whether these proteins are associated with paranodal junctions in vivo. However, this would be an attractive possibility, as the paranodal region appears to be a site of local axon–glia signaling. For example, the diameter of the axon in the nodal region is dramatically reduced (up to 80% in large fibers). This reduced diameter reflects alterations in the level of phosphorylation and the packing density of axonal cytoskeletal proteins (Waegh et al., 1992). The rate of axonal transport is also significantly diminished in the paranodal region (Waegh et al., 1992; Fabricius et al., 1993), which is consistent with local signaling. Whether proteins that are linked to Caspr mediate these effects or not will be an important area for future investigation. An additional signaling mechanism in invertebrate septate junctions may be mediated by the PDZ binding domain of Neurexin IV, which is also shared by other neurexins (for review see Littleton et al., 1997). Notably, Neurexin IV may interact with the discs-large tumor suppressor protein, a MAGUK protein that is found in septate junctions and has a role in cell signaling and differentiation (Woods and Bryant, 1991). This interaction may be unique to Drosophila Neurexin IV, as Caspr lacks a PDZ binding domain and several mammalian homologues of discs-large have not been localized to the paranodes (Cho et al., 1992; Müller et al., 1995; Hunt et al., 1996).
An important question is the identity of the glial ligand(s) for Caspr and whether Caspr regulates glial function. Freeze fracture studies have demonstrated that there are glial particles within the paranodal loops that are juxtaposed over axolemmal particles in the paranodal junctions (Wiley and Ellisman, 1980). These glial proteins are candidate ligands for Caspr. To date, they have not been identified nor are any glial proteins known that have a complementary distribution to Caspr. Like all neurexins, the extracellular segment of Caspr contains domains homologous to laminin G domains and EGF repeats (Littleton et al., 1997; Peles et al., 1997). In addition, it contains a number of unique motifs including a discoidin-related (lectin-like) domain, a fibrinogen-related sequence, and repeats of proline, glycine, and tyrosine residues that may reflect multiple ligands. A calcium-dependent ligand for specific neurexin isoforms has been identified, termed neuroligin-1, but it is expressed by only a subset of neurons in the CNS (Ichtchenko et al., 1995). However, the adhesive integrity of the paranodal junctions is also calcium dependent (Blank et al., 1974), perhaps indicative of calcium-dependent adhesion mediated by Caspr.
Caspr is likely to have other functions beyond its role in paranodal junctions. We have observed diffuse, high level expression in gray matter (see Fig. 7) not restricted to paranodes. This may reflect its putative role as a signaling coreceptor with contactin (Peles et al., 1997b ). In addition, septate-like junctions have been noted in the vertebrate central nervous system in other sites, including the pituitary (Kurono et al., 1994) and cerebellum (Sotelo and Llinás, 1972; Laube et al., 1996). It is not yet known whether these junctions are enriched in Caspr. Finally, Caspr is expressed at low levels in several other tissues, including the ovaries and a number of enteric viscera (Peles et al., 1997b ), perhaps reflecting a wider distribution of septate-like junctions or additional roles for this protein.
Caspr and Other Neurexins Are Novel Candidates to Mediate Axon–Glia Interactions
The neurexins comprise a large family of neuronal surface proteins that are candidate mediators of synaptic interactions and, potentially, target recognition (Ushkaryov et al., 1992). A recent study suggests that other neurexins may mediate axon–glia interactions. In particular, a pan-neurexin antibody, raised to a sequence present in neurexins I–III but not Caspr, stained the electromotor nerve of the electric fish at the axon interface with myelinating Schwann cells (Russell and Carlson, 1997). Whether or not other neurexins, in addition to Caspr, also mediate axon–glia interactions in mammals will require further study. This pan-neurexin antibody also stained the perineurial cells of the electromotor nerve, raising the possibility that neurexins may play a more general role in the tight association of cells in this layer. By Northern analysis, we have not detected Caspr mRNA in the sciatic nerve, suggesting it is not expressed in the vertebrate perineurium.
In summary, we have shown that the expression and distribution of Caspr and contactin are differentially regulated during myelination. Of particular interest is the localization of Caspr to the septate-like junctions of the paranode. This is the first component of these vertebrate junctions to be identified and this, together with its homology to Drosophila Neurexin IV, suggests that it is a major transmembrane component required for their integrity. These findings have important implications for the identity of other proteins that are associated with Caspr in these junctions and for the potential role of Caspr in reciprocal axon–glia signaling.
Acknowledgments
We thank Dr. Josie Schlessinger (New York University Medical Center, New York) for his encouragement and support, Peter Canoll (New York University Medical Center, New York) and Dr. Randy McKinnon (Robert Wood Johnson Medical Center, Piscataway, NJ) for assistance with oligodendrocyte progenitor cultures and lysate preparation, Sabrina Prince (Cornell University Medical College, New York) for assistance with immunocytochemistry, Jody Culkin (New York University Medical Center, New York) for photographic assistance, and John Weider (New York University Medical Center, New York) for digital imaging assistance.
This study was supported by National Institutes of Health (NIH) grants NS26001 and NS33165 (J.L. Salzer) and HL18974, MH42834, and DA08259 (T.A. Milner). W. Ching is a Medical Scientist Trainee supported by NIH training grant 5T32 GM07308 from the National Institute of General Medical Sciences.
Abbreviations used in this paper
- CAM
cell adhesion molecule
- Caspr
contactin-associated protein
- CNS and PNS
central and peripheral nervous system
- dPBS
Dulbecco's PBS
- DRG
dorsal root ganglia
- MAG
myelin-associated glycoprotein
- MBP
myelin basic protein
Note Added in Proof
After this paper was submitted, the cloning and localization of paranodin, a protein identical in sequence to Caspr, was reported (Menegoz, M., P. Gaspar, M. Le Bert, T. Galvez, F. Burgaya, C. Palfrey, P. Ezan, F. Aruos, and J.-A. Girault. 1997. Neuron. 19:319–331).
Footnotes
Address all correspondence to Dr. James L. Salzer, Department of Cell Biology, New York University Medical School, 550 First Avenue, New York, NY 10016. Tel.: (212) 263-5358. Fax: (212) 263-8139.
Elior Peles' current address is Department of Molecular Cell Biology, The Weizmann Institute of Science, Rehovot, Israel.
References
- Andres KH. über die feinstruktur besonderer einrichtungen in markhaltigen nervenfasern des kleinhirns der ratte. Z Zellforsch. 1965;65:701–712. [PubMed] [Google Scholar]
- Bargmann W, Lindner E. über den Feinbau des Nebennierenmarkes des Igels (Erinaceus europaeus L) . Z Zellforsch. 1964;64:868–912. [PubMed] [Google Scholar]
- Baumgartner S, Littleton JT, Broadie K, Bhat MA, Harbecke R, Lengyel JA, Chiquet-Ehrismann R, Prokop A, Bellen HJ. A Drosophila neurexin is required for septate junction and blood-nerve barrier formation and function. Cell. 1996;87:1059–1068. doi: 10.1016/s0092-8674(00)81800-0. [DOI] [PubMed] [Google Scholar]
- Bennett V, Gilligan DM. The spectrin-based membrane skeleton and micron-scale organization of the plasma membrane. Annu Rev Cell Biol. 1993;9:27–66. doi: 10.1146/annurev.cb.09.110193.000331. [DOI] [PubMed] [Google Scholar]
- Black, J.A., H. Sontheimer, Y. Oh, and S.G. Waxman. 1995. The oligodendrocyte, the perinodal astrocyte, and the central node of Ranvier. In The Axon. S. Waxman, J. Kocsis, and P. Stys, editors. Oxford University Press, New York. 116–143.
- Blank WFJ, Bunge MB, Bunge RP. The sensitivity of the myelin sheath, particularly the Schwann cell-axolemmal junction, to lowered calcium levels in cultured sensory ganglia. Brain Res. 1974;67:503–518. doi: 10.1016/0006-8993(74)90498-3. [DOI] [PubMed] [Google Scholar]
- Canoll PD, Musacchio JM, Hardy R, Reynolds R, Marchionni MA, Salzer JL. GGF/neuregulin is a neuronal signal that promotes the proliferation and survival and inhibits the differentiation of oligodendrocyte progenitors. Neuron. 1996a;17:229–243. doi: 10.1016/s0896-6273(00)80155-5. [DOI] [PubMed] [Google Scholar]
- Canoll PD, Petanceska S, Schlessinger J, Musacchio JM. Three forms of RPTP-β are differentially expressed during gliogenesis in the developing rat brain and during glial cell differentiation in culture. J Neurosci Res. 1996b;44:199–215. doi: 10.1002/(SICI)1097-4547(19960501)44:3<199::AID-JNR1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Chan J, Aoki C, Pickel VM. Optimization of differential immunogold-silver and peroxidase labeling with maintenance of ultrastructure in brain section before plastic embedding. J Neurosci Methods. 1990;33:113–127. doi: 10.1016/0165-0270(90)90015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin JM, Przbyla AE, MacDonald RJ, Rutter RJ. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Cho KO, Hunt CA, Kennedy MB. The rat brain postsynaptic density fraction contains a homolog of the Drosophila discs-large tumor suppressor protein. Neuron. 1992;9:929–942. doi: 10.1016/0896-6273(92)90245-9. [DOI] [PubMed] [Google Scholar]
- Colombo A, Bonfanti P, Camatini M. Actin, α-actinin, and vinculin are associated with septate junctions in Insecta. Cell Motil Cytoskel. 1993;26:205–213. doi: 10.1002/cm.970260304. [DOI] [PubMed] [Google Scholar]
- Davis JQ, Lambert S, Bennett V. Molecular composition of the node of Ranvier: identification of ankyrin-binding cell adhesion molecules neurofascin (mucin+/third FNIII domain−) and NrCAM at nodal axon segments. J Cell Biol. 1996;135:1355–1367. doi: 10.1083/jcb.135.5.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deerinck TJ, Levinson SR, Bennett GV, Ellisman MH. Clustering of voltage sensitive sodium channels on axons is independent of direct Schwann cell contact in the dystrophic mouse. J Neurosci. 1997;17:5080–5088. doi: 10.1523/JNEUROSCI.17-13-05080.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einheber S, Milner T, Giancotti F, Salzer J. Axonal regulation of Schwann cell integrin expression suggests a role for α6β4 in myelination. J Cell Biol. 1993;123:1223–1236. doi: 10.1083/jcb.123.5.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einheber S, Schnapp LM, Salzer JL, Cappiello ZB, Milner TA. The regional and ultrastructural distribution of the α8 integrin subunit in the developing and adult rat brain suggests a role in synaptic function. J Comp Neurol. 1996;370:105–134. doi: 10.1002/(SICI)1096-9861(19960617)370:1<105::AID-CNE10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Fabricius C, Berthold CH, Rydmark M. Axoplasmic organelles at nodes of Ranvier. II. Occurrence and distribution in large myelinated spinal cord axons of the adult cat. J Neurocytol. 1993;22:941–954. doi: 10.1007/BF01218352. [DOI] [PubMed] [Google Scholar]
- Faivre-Sarrailh C, Gennarini G, Goridis C, Rougon G. F3/F11 cell surface molecule expression in the developing mouse cerebellum is polarized at synaptic sites and within granule cells. J Neurosci. 1992;12:257–267. doi: 10.1523/JNEUROSCI.12-01-00257.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fannon AM, Sherman DL, Ilyina-Gragerova G, Brophy PJ, Friedrich VL, Jr, Colman DR. Novel E-cadherin mediated adhesion in peripheral nerve: Schwann cell architecture is stabilized by autotypic adherens junctions. J Cell Biol. 1995;129:189–202. doi: 10.1083/jcb.129.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehon RG, Dawson IA, Artavanis-Tsakonas S. A Drosophila homologue of membrane-skeleton protein 4.1 is associated with septate junctions and is encoded by the coracle gene. Development (Camb) 1994;120:545–557. doi: 10.1242/dev.120.3.545. [DOI] [PubMed] [Google Scholar]
- Gennarini G, Cibelli G, Rougon G, Mattei M-G, Goridis C. The mouse neuronal cell surface protein F3: a phosphatidylinositol-anchored member of the immunoglobulin superfamily related to chicken contactin. J Cell Biol. 1989;109:775–778. doi: 10.1083/jcb.109.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner B, Louvard D. Localized barriers in the plasma membrane: a common way to form domains. Trends Biochem Sci. 1985;10:435–438. [Google Scholar]
- Hirano, A., and J.F. Llena. 1995. Morphology of central nervous system axons. In The Axon. S. Waxman, J. Kocsis, and P. Stys, editors. Oxford University Press, New York. 49–67.
- Hunt CA, Schenker LJ, Kennedy MB. PSD-95 is associated with the postsynaptic density and not with the presynaptic membrane at forebrain synapses. J Neurosci. 1996;16:1380–1388. doi: 10.1523/JNEUROSCI.16-04-01380.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley AF, Stämpfli R. Evidence for saltatory conduction in peripheral myelinated nerve fibers. J Physiol (Lond) 1949;108:315–339. [PubMed] [Google Scholar]
- Ichimura T, Ellisman MH. Three-dimensional fine structure of cytoskeletal-membrane interactions at nodes of Ranvier. J Neurocytol. 1991;20:667–681. doi: 10.1007/BF01187068. [DOI] [PubMed] [Google Scholar]
- Ichtchenko K, Hata Y, Nguyen T, Ullrich B, Missler M, Moomaw C, Sudhof TC. Neuroligin 1: a splice site-specific ligand for β-neurexins. Cell. 1995;81:435–443. doi: 10.1016/0092-8674(95)90396-8. [DOI] [PubMed] [Google Scholar]
- Kaplan MR, Meyer-Franke A, Lambert S, Bennett V, Duncan ID, Levinson SR, Barres BA. Induction of sodium channel clustering by oligodendrocytes. Nature. 1997;386:724–728. doi: 10.1038/386724a0. [DOI] [PubMed] [Google Scholar]
- Koch T, Brugger T, Bach A, Gennarini G, Trotter J. Expression of the immunoglobulin superfamily cell adhesion molecule F3 by oligodendrocyte-lineage cells. Glia. 1997;19:199–212. doi: 10.1002/(sici)1098-1136(199703)19:3<199::aid-glia3>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Kordeli E, Bennett V. Distinct ankyrin isoforms at neuron cell bodies and nodes of Ranvier in myelinated axons of central and peripheral nerves. J Cell Biol. 1991;114:1243–1259. doi: 10.1083/jcb.114.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurono C, Nozaki M, Ohguchi H, Soji T, Herbert DC. Septate-like junctions in the normal male rat pituitary gland. Tissue Cell. 1994;26:913–916. doi: 10.1016/0040-8166(94)90040-x. [DOI] [PubMed] [Google Scholar]
- Lane NJ. Morphology of glial blood-brain barriers. Ann NY Acad Sci. 1991;633:348–362. doi: 10.1111/j.1749-6632.1991.tb15626.x. [DOI] [PubMed] [Google Scholar]
- Lane NJ, Flores V. Actin filaments are associated with the septate junctions of invertebrates. Tissue Cell. 1988;20:211–217. doi: 10.1016/0040-8166(88)90042-0. [DOI] [PubMed] [Google Scholar]
- Laube G, Roper J, Pitt JC, Sewing S, Kistner U, Garner CC, Pongs O, Veh RW. Ultrastructural localization of Shaker-related potassium channel subunits and synapse-associated protein 90 to septate-like junctions in rat cerebellar Pinceaux. Brain Res Mol Brain Res. 1996;42:51–61. doi: 10.1016/s0169-328x(96)00120-9. [DOI] [PubMed] [Google Scholar]
- Littleton JT, Bhat MA, Bellen HJ. Deciphering the function of neurexins at cellular junctions. J Cell Biol. 1997;137:793–796. doi: 10.1083/jcb.137.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martini R, Schachner M. Immunoelectron microscopic localization of neural cell adhesion molecules (L1, N-CAM, and MAG) and their shared carbohydrate epitope and myelin basic protein in developing sciatic nerve. J Cell Biol. 1986;103:2439–2448. doi: 10.1083/jcb.103.6.2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi H, Deerinck TJ, Ellisman MH, Schwarz TL. Differential distribution of closely related potassium channels in rat Schwann cells. J Neurosci. 1995;15:3761–3774. doi: 10.1523/JNEUROSCI.15-05-03761.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller BM, Kistner U, Veh RW, Cases-Langhoff C, Becker B, Gundelfinger ED, Garner CC. Molecular characterization and spatial distribution of SAP97, a novel presynaptic protein homologous to SAP90 and the Drosophiladiscs-large tumor suppressor protein. J Neurosci. 1995;15:2354–2366. doi: 10.1523/JNEUROSCI.15-03-02354.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens GC, Bunge RP. Evidence for an early role for myelin-associated glycoprotein in the process of myelination. Glia. 1989;2:119–128. doi: 10.1002/glia.440020208. [DOI] [PubMed] [Google Scholar]
- Peles E, Nativ M, Campbell PL, Sakurai T, Martinez R, Lev S, Clary DO, Schilling J, Barnea G, Plowman GD, Grumet M, Schlessinger J. The carbonic anhydrase domain of receptor tyrosine phosphatase β is a functional ligand for the axonal cell recognition molecule contactin. Cell. 1995;82:251–260. doi: 10.1016/0092-8674(95)90312-7. [DOI] [PubMed] [Google Scholar]
- Peles E, Joho K, Plowman GD, Schlessinger J. Close similarity between Drosophila neurexin IV and mammalian Caspr protein suggests a conserved mechanism for cellular interactions [letter] Cell. 1997a;88:745–746. doi: 10.1016/s0092-8674(00)81920-0. [DOI] [PubMed] [Google Scholar]
- Peles E, Nativ M, Lustig M, Grumet M, Schilling J, Martinez R, Plowman GD, Schlessinger J. Identification of a novel contactin-associated transmembrane receptor with multiple domains implicated in protein-protein interactions. EMBO (Eur Mol Biol Organ) J. 1997b;16:978–988. doi: 10.1093/emboj/16.5.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters, A., S.L. Palay, and H. deF. Webster. 1991. The Fine Structure of the Nervous System. Oxford University Press, New York. 494 pp.
- Quarles, R.H., D.R. Colman, J.L. Salzer, and B.D. Trapp. 1992. Myelin-associated glycoprotein: structure-function relationships and involvement in neurological diseases. In Myelin: Biology and Chemistry. R. Martensen, editor. CRC Press, Boca Raton, FL. 413–448.
- Rosen CL, Lisanti MP, Salzer JL. Expression of unique sets of GPI-linked proteins by different primary neurons in vitro. J Cell Biol. 1992;117:617–627. doi: 10.1083/jcb.117.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbluth J. Intramembranous particle distribution at the node of Ranvier and adjacent axolemma in myelinated axons of the frog brain. J Neurocytol. 1976;5:731–745. doi: 10.1007/BF01181584. [DOI] [PubMed] [Google Scholar]
- Rosenbluth, J. 1983. Structure of the node of Ranvier. In Structure and Function in Excitable Cells. D.C. Chang, I. Tasaki, J.W.J. Adelman, and H.R. Leuchtag, editors. Plenum Press, New York. 25–52.
- Russell AB, Carlson SS. Neurexin is expressed on nerves, but not at nerve terminals, in the electric organ. J Neurosci. 1997;17:4734–4743. doi: 10.1523/JNEUROSCI.17-12-04734.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzer, J.L. 1995. Mechanisms of adhesion between axons and glial cells. In The Axon. S. Waxman, J. Kocsis, and P. Stys, editors. Oxford University Press, New York. 164–184.
- Salzer JL. Clustering sodium channels at the node of Ranvier: close encounters of the axon-glia kind. Neuron. 1997;18:843–846. doi: 10.1016/s0896-6273(00)80323-2. [DOI] [PubMed] [Google Scholar]
- Scherer SS, Deschênes SM, Xu Y, Grinspan JB, Fischbeck KH, Paul DL. Connexin32 is a myelin-related protein in the PNS and CNS. J Neurosci. 1995;15:8281–8294. doi: 10.1523/JNEUROSCI.15-12-08281.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotelo C, Llinás R. Specialized membrane junctions between neurons in the vertebrate cerebellar cortex. J Cell Biol. 1972;53:271–289. doi: 10.1083/jcb.53.2.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao-Cheng JH, Rosenbluth J. Development of nodal and paranodal membrane specializations in amphibian peripheral nerves. Brain Res. 1982;255:577–594. doi: 10.1016/0165-3806(82)90055-4. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Yonemura S, Tsukita S. ERM (ezrin/radixin/moesin) family: from cytoskeleton to signal transduction. Curr Opin Cell Biol. 1997;9:70–75. doi: 10.1016/s0955-0674(97)80154-8. [DOI] [PubMed] [Google Scholar]
- Ushkaryov YA, Petrenko AG, Geppert M, Sudhof TC. Neurexins: synaptic cell surface proteins related to the α-latrotoxin receptor and laminin. Science. 1992;257:50–56. doi: 10.1126/science.1621094. [DOI] [PubMed] [Google Scholar]
- Waegh SM de, V.M.-Y. Lee, and S.T. Brady. Local modulation of neurofilament phosphorylation, axonal caliber, and slow axonal transport by myelinating Schwann cells. Cell. 1992;68:451–463. doi: 10.1016/0092-8674(92)90183-d. [DOI] [PubMed] [Google Scholar]
- Wang H, Kunkel DD, Martin TM, Schwartzkroin PA, Tempel BL. Heteromultimeric K+ channels in terminal and juxtaparanodal regions of neurons. Nature. 1993;365:75–79. doi: 10.1038/365075a0. [DOI] [PubMed] [Google Scholar]
- Waxman SG, Ritchie JM. Molecular dissection of the myelinated axon. Ann Neurol. 1993;33:121–136. doi: 10.1002/ana.410330202. [DOI] [PubMed] [Google Scholar]
- Wiley CA, Ellisman MH. Rows of dimeric-particles within the axolemma and juxtaposed particles within glia, incorporated into a new model for the paranodal glial-axonal junction at the node of Ranvier. J Cell Biol. 1980;84:261–280. doi: 10.1083/jcb.84.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willbold E, Brümmendorf T, Rathjen FG, Schwartz H, Weiss B. The neural cell recognition molecule F11 is expressed on Müller cells and Schwann cells in vitro. . J Brain Res. 1997;38:71–80. [PubMed] [Google Scholar]
- Woods DF, Bryant PJ. The discs-large tumor suppressor gene of Drosophila encodes a guanylate kinase homolog localized at septate junctions. Cell. 1991;66:451–464. doi: 10.1016/0092-8674(81)90009-x. [DOI] [PubMed] [Google Scholar]
- Zerlin M, Levison SW, Goldman JE. Early patterns of migration, morphogenesis, and intermediate filament expression of subventricular zone cells in the postnatal rat forebrain. J Neurosci. 1995;15:7238–7249. doi: 10.1523/JNEUROSCI.15-11-07238.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]