Abstract
Laminins, heterotrimers of α, β, and γ chains, are prominent constituents of basal laminae (BLs) throughout the body. Previous studies have shown that laminins affect both myogenesis and synaptogenesis in skeletal muscle. Here we have studied the distribution of the 10 known laminin chains in muscle and peripheral nerve, and assayed the ability of several heterotrimers to affect the outgrowth of motor axons. We show that cultured muscle cells express four different α chains (α1, α2, α4, and α5), and that developing muscles incorporate all four into BLs. The portion of the muscle's BL that occupies the synaptic cleft contains at least three α chains and two β chains, but each is regulated differently. Initially, the α2, α4, α5, and β1 chains are present both extrasynaptically and synaptically, whereas β2 is restricted to synaptic BL from its first appearance. As development proceeds, α2 remains broadly distributed, whereas α4 and α5 are lost from extrasynaptic BL and β1 from synaptic BL. In adults, α4 is restricted to primary synaptic clefts whereas α5 is present in both primary and secondary clefts. Thus, adult extrasynaptic BL is rich in laminin 2 (α2β1γ1), and synaptic BL contains laminins 4 (α2β2γ1), 9 (α4β2γ1), and 11 (α5β2γ1). Likewise, in cultured muscle cells, α2 and β1 are broadly distributed but α5 and β2 are concentrated at acetylcholine receptor–rich “hot spots,” even in the absence of nerves. The endoneurial and perineurial BLs of peripheral nerve also contain distinct laminin chains: α2, β1, γ1, and α4, α5, β2, γ1, respectively. Mutation of the laminin α2 or β2 genes in mice not only leads to loss of the respective chains in both nerve and muscle, but also to coordinate loss and compensatory upregulation of other chains. Notably, loss of β2 from synaptic BL in β2−/− “knockout” mice is accompanied by loss of α5, and decreased levels of α2 in dystrophic α2dy/dy mice are accompanied by compensatory retention of α4. Finally, we show that motor axons respond in distinct ways to different laminin heterotrimers: they grow freely between laminin 1 (α1β1γ1) and laminin 2, fail to cross from laminin 4 to laminin 1, and stop upon contacting laminin 11. The ability of laminin 11 to serve as a stop signal for growing axons explains, in part, axonal behaviors observed at developing and regenerating synapses in vivo.
In skeletal muscles, a continuous sheath of basal lamina (BL)1 surrounds each muscle fiber and passes through the synaptic cleft at the neuromuscular junction. Thus, most of the BL separates the muscle fiber membrane from interstitial connective tissue, whereas a small fraction (∼0.1%) separates the muscle from the nerve. These extrasynaptic and synaptic portions of the BL play several important roles in the development and function of the muscle and the neuromuscular junction, respectively. Components of extrasynaptic BL regulate myogenesis in embryos, contribute to tensile strength in adults, and serve as a scaffold to orient regenerating myotubes after muscle damage. Components of synaptic BL organize differentiation of the pre- and postsynaptic membranes in embryos, inactivate neurotransmitter in adults, and guide reinnervation after nerve damage (for reviews see Sanes, 1994, 1995).
Key molecules in these processes are the laminins, glycoproteins that are major components of BLs in all tissues. Laminin was initially isolated from tumor-derived matrix as a trimer of α1, β1, and γ1 chains (formerly A, B1, and B2; Chung et al., 1979; Timpl et al., 1979; Burgeson et al., 1994). A homologue of the α1 chain, originally called merosin and now renamed α2, was subsequently isolated from muscle and shown to be a major component of extrasynaptic BL (Lievo and Engvall, 1988; Ehrig et al., 1990). Similarly, β2 (originally s-laminin) was identified as a component of synaptic BL (Chiu and Sanes, 1984; Hunter et al., 1989a ). Laminins containing the α2 chain are adhesive for myoblasts (Schuler and Sorokin, 1995), and recombinant laminin β2 fragments regulate outgrowth of motor axons (Hunter et al., 1989b ; Porter et al., 1995). Thus, based on their distribution in vivo and their effects in vitro, the α2 and β2 chains were hypothesized to be involved in myogenesis and synaptogenesis, respectively. Recent genetic analyses in mice have provided strong support for these hypotheses: targeted mutation of the laminin β2 gene leads to aberrant structural and functional maturation of neuromuscular junctions (Noakes et al., 1995a ), and a naturally occurring hypomorphic allele of laminin α2 (α2dy/dy) gives rise to severe muscular dystrophy (Sunada et al., 1994; Xu et al., 1994b ). Some cases of human familial muscular dystrophy have also been shown to result from mutation of the laminin α2 gene (Hayashi et al., 1993; Helbling-Leclerc et al., 1995; Sunada et al., 1995; Nissinen et al., 1996).
Despite the strong evidence that laminins are crucial for neuromuscular development, it has been difficult to elucidate their precise roles for several reasons. First, additional laminin chains and a total of 11 αβγ heterotrimers (laminins 1–11; Table I) have now been identified in vertebrates. The distribution of the new chains in muscle has not yet been reported, so the identity of the laminin trimers in synaptic and extrasynaptic BL remains unclear. Second, mutation of a single laminin or collagen IV chain gene leads to coordinate loss of some chains and compensatory retention of others in kidney BLs (Kashtan and Kim, 1992; Gubler et al., 1995; Noakes et al., 1995b ; Cosgrove et al., 1996; Miner and Sanes, 1996). Similar interactions might occur in muscle, complicating analyses of the α2dy/dy and laminin β2−/− phenotypes. Third, laminins α2 and β2 are present in the BLs of peripheral nerve as well as muscle (Sanes et al., 1990), so mutant phenotypes might reflect both neurogenic and myogenic defects. Finally, functional studies of laminin β2 have been limited to recombinant fragments (Hunter et al., 1989b ; Porter and Sanes, 1995; Porter et al., 1995) and a single heterotrimeric form (laminin 4; Brandenberger et al., 1996). It may be inappropriate to extrapolate from the activities of these preparations to those of synaptic laminins.
Table I.
Laminin Isoforms in Vertebrates
| Trimer | Composition | |
|---|---|---|
| laminin 1 | α1 β1 γ1 | |
| laminin 2 | α2 β1 γ1 | |
| laminin 3 | α1 β2 γ1 | |
| laminin 4 | α2 β2 γ1 | |
| laminin 5 | α3 β3 γ2 | |
| laminin 6 | α3 β1 γ1 | |
| laminin 7 | α3 β2 γ1 | |
| laminin 8 | α4 β1 γ1 | |
| laminin 9 | α4 β2 γ1 | |
| laminin 10 | α5 β1 γ1 | |
| laminin 11 | α5 β2 γ1 |
For references see Miner et al. (1997).
To address these issues, we have analyzed the expression of all 10 known laminin chains in developing and adult muscles and nerves of wild type, α2dy/dy , and β2−/− mice. We have also documented expression of putative synaptic laminins by cultured muscle, and assayed the effects of several laminin trimers on the outgrowth of motor axons. We show that the laminin isoforms of synaptic, extrasynaptic, and nerve BLs change as development proceeds. In adults, the α4, α5, and β2 chains are all concentrated in synaptic BL, but they are distributed and regulated in different ways, and could form three distinct trimers (laminins 4, 9, and 11). Both the α5 and the β2 chains are lost from synaptic sites in β2 mutants, which display severe synaptic defects, but both are retained in α2 mutants, in which synaptic defects are mild. Moreover, laminin 11 (α5β2γ1) serves as a potent stop signal for motor axons in vitro whereas laminin 4 (α2β2γ1) does not. Together, these results focus attention on laminin 11 as a critical organizer of synaptic development.
Materials and Methods
Animals
Mice deficient in laminin β2 and littermate controls were generated and genotyped as described by Noakes et al. (1995a). When maintained on high-fat rodent chow after weaning, the β2−/− mutants do not gain weight but do live until P28–P35. Mice homozygous for a mutation in the laminin α2 gene (Lama2dy/dy) and littermate controls were purchased from Jackson Laboratories (C57BL/6J-Lama2dy/dy; Bar Harbor, ME). Embryos were taken from timed pregnant ICR or C57BL6 mice, bred in our colony.
Antibodies
Monoclonal antibodies to rat laminin β1 (C21 and C22), β2 (D5, D7, D19, and D27), and γ1 (D18) chains, rabbit antisera to recombinant laminin α4 and α5 chains, and a guinea pig antiserum to laminin β2 were produced in this laboratory and have been described previously (Sanes and Chiu, 1983; Hunter et al., 1989a ; Sanes et al., 1990; Green et al., 1992; Miner et al., 1997). Rat monoclonal antibodies to mouse laminin α1 (clones 198 and 200; Sorokin et al., 1992) were gifts from L. Sorokin (Institute for Experimental Medicine, Erlangen, Germany). Rabbit antiserum to human laminin α2, which cross-reacted with the mouse protein, was provided by P. Yurchenco (Robert Wood Johnson Medical School, Piscataway, NJ; see Miner et al., 1997). Rabbit antiserum to mouse laminin α3 (Aberdam et al., 1994) was a gift from D. Aberdam (Institut National de la Sante et de la Recherche Medicale [INSERM] U385, Nice, France). Rabbit antiserum to laminin-5 (α3β3γ2) was a gift of R. Burgeson (Massachusetts General Hospital, Charlestown, MA). A rat monoclonal antibody to laminin β1 (5A2; Abrahamson et al., 1989; Martin et al., 1995) was a gift from D. Abrahamson (University of Alabama, Birmingham, AL). Rat anti–mouse laminin γ1 was purchased from Chemicon International, Inc. (Temecula, CA). FITC- and HRP-conjugated, goat anti–rabbit antibodies were from Boehringer Mannheim Corp. (Indianapolis, IN); FITC-conjugated, goat anti–rat antibodies were from Cappel/Organon Teknika (Durham, NC); Cy3-goat anti–rabbit antibodies were from Jackson Immunoresearch Laboratories (West Grove, PA); biotinylated, goat anti–guinea pig antibodies were from Sigma Chemical Co. (St. Louis, MO); and HRP-avidin was from Zymed Labs Inc. (South San Francisco, CA).
All results on laminin α1 were confirmed with two monoclonal antibodies, 198 and 200, which react with distinct epitopes (Sorokin et al., 1992). All results on laminin 5 (α3β3γ2) were obtained using an antibody that recognizes all three chains (Marinkovich et al., 1992), and were confirmed using an antibody specific for the α3 chain, which binds an epitope present in both α3A and α3B isoforms (Aberdam et al., 1994; Miner et al., 1997). To date, β3 and γ2 have been found only in association with α3 (Table I), so absence of α3 provides indirect support for absence of β3 and γ2. These antibodies stained a subset of lung BLs intensely in our hands (data not shown). All results on laminins α4 and α5 were confirmed with two separately generated rabbit antisera to each chain; the specificity of these sera has been documented previously (Miner et al., 1997).
Laminin Heterotrimers
Laminin 1 (α1β1γ1) from the mouse EHS tumor matrix, and laminin 2 (α2β1γ1) from human placenta, were purchased from GIBCO BRL/Life Technologies (Gaithersburg, MD). Purified laminin 4 (α2β2γ1) and a second sample of laminin 2, both purified from human placenta, were generous gifts of Y.-S. Cheng and P. Yurchenco (Robert Wood Johnson Medical School; Cheng et al., 1997). Laminin 11 (α5β2γ1) was purified from the conditioned medium of rat Schwannoma D6P2T cells by a modification of the method described by Chiu et al. (1992). By immunoblotting using antibodies described above, the “laminin 11” fraction was found to be rich in α5, β2, and γ1, but to contain little or no α1, α2, α3, α4, or β1.
Neuronal Cultures
To generate patterned substrates, plastic culture dishes were first coated with a thin layer of nitrocellulose (type BA85; Schleicher and Schuell, Keene, NH) (Lagenaur and Lemmon, 1987). A pattern was then formed by applying ∼1-μl drops of test proteins in PBS supplemented with 2 mM EDTA and 1 mg/ml sulforhodamine-101 (Sigma Chemical Co., St. Louis, MO). After incubation in a humidified chamber for 1–5 h at room temperature, dishes were flushed repeatedly to remove unbound material, and then coated for 2 h at room temperature with laminin 1 (20 μg/ml in PBS; GIBCO BRL) to support cell attachment and initiate neurite outgrowth. Dishes were rinsed with PBS or PBS/BSA and used immediately for neurite outgrowth assays.
Chick ciliary ganglia were dissected from E8-9 embryos, digested in 0.05% trypsin in Ca2+/Mg2+-free HBSS for 15 min at 37°C, and dissociated by trituration. Cells were washed in MEM (No. 11095; GIBCO BRL) containing 10% FCS (Hyclone, Logan, UT), and resuspended in MEM supplemented with 25 mM Hepes, 7% heat inactivated horse serum (Hyclone), 3% FCS, 1 mM glutamine, 1 mM glucose, penicillin, and streptomycin. Aliquots of 30 μl containing 0.2 ganglion equivalent (∼800 neurons) were suspended from culture dish lids in a 37°C, 7% CO2 incubator. Cell clusters formed in 3–6 h within the hanging drops. The clusters were then transferred to culture dishes and positioned near patches of test substrates. Neurons were grown for 30–36 h in culture medium containing 1% chick eye extract (Nishi and Berg, 1981), and then fixed with formaldehyde.
For analysis, cultures were viewed with phase optics to visualize neurites and rhodamine optics to visualize the substrate border. Two categories of neurites were counted. Group A were those that extended on laminin 1 and terminated on or near the substrate border, in a swath extending from 50 μm onto the laminin 1 to 10 μm over onto the test substrate. Group B were neurites that began on laminin 1 and extended greater than 10 μm beyond the border. Neurites initiated on the test substrate, and neurites that did not approach a border were not scored. Inhibition of crossing was calculated as (A ÷ [A + B]).
Because this assay was designed to test the behavior of neurites in response to various laminins, it was crucial to rule out the possibility that test substrates were acting indirectly by reducing the concentration of laminin 1 available to the neurons. To this end, we stained patterned substrates with antisera specific for laminin 1, and found that laminin 1 immunoreactivity was not detectably decreased in regions that had previously been spotted with BSA solutions of ⩽1 mg/ml or laminins 2, 4, or 11 at 40 μg/ml. Biological effects reported below for laminins 4 and 11 were observed at <40 μg/ml.
Muscle Cell Culture
The RMo rat muscle cell line (Merrill, 1989) was cultured in F10 medium containing 15% FCS and 3% chick embryo extract. Nearly confluent cultures were induced to differentiate and form myotubes by medium replacement with DME containing 4% horse serum. After 6 d a soluble 95-kD fragment of rat agrin (Ferns et al., 1993) was added to cultures for 24 h to promote clustering of acetylcholine receptors (AChRs) and associated proteins. C2 mouse muscle cells were cultured as described in Martin et al. (1995).
Immunohistochemistry
Freshly frozen tissues were sectioned in a cryostat at 4–8 μm and fixed with 2% paraformaldehyde in PBS for 10 min. Fixed sections were blocked with 0.1 M glycine in PBS (10 min), and incubated overnight at 4°C with antibodies diluted in a solution of 2% BSA and 0.1% (wt/vol) saponin (Sigma Chemical Co.) in PBS. After washing, bound antibodies were detected with species-specific, fluorochrome-conjugated secondary antibodies, and then washed, mounted in glycerol containing p-phenylenediamine, and observed with epifluorescent illumination. Where appropriate, rhodamine-α-bungarotoxin (50 nM; Molecular Probes, Eugene, OR) was included with the second antibodies, to label AChRs. Rabbit antilaminin α4 and guinea pig anti-β2 recognized only denatured antigen (Miner et al., 1997), so sections to be labeled with these antibodies were pretreated with 0.05% SDS in PBS at 50°C for 20 min. AChR were labeled in denatured sections by incubating sections in rhodamine-α-bungarotoxin both before fixation and after denaturation.
Immunoblotting and Immunoprecipitation
Samples were heated to 95°C for 5 min in SDS-PAGE sample buffer, with or without the reducing agent DTT, then subjected to SDS-PAGE on 7% (reduced) or 3.5% (nonreduced) gels. Proteins were transferred to nitrocellulose membranes (Schleicher and Schuell) in 25 mM Tris, pH 9.5, 130 mM glycine, 0.1% SDS, and 10% (vol/vol) methanol at 320 mA for 24 h at 4°C. Positions of major bands were visualized with Ponceau S and marked. After blocking with a solution of 5% nonfat dry milk (Schnucks, St. Louis, MO) and 0.3% Tween-20 in PBS, filters were cut into strips and incubated with antibodies overnight. Bound mouse and rabbit antibodies were detected with HRP second antibodies and chemiluminescent substrates (Renaissance; DuPont-NEN, Boston, MA). Guinea pig antibodies were detected with biotinylated second antibody and HRP-avidin.
For immunodepletion, aliquots of a protein A–Sepharose CL-4B conjugate (Pharmacia Biotechnology Inc., Piscataway, NJ) were loaded with either antilaminin α5 or preimmune serum (1.5 μl/μl resin), blocked with 2 mg/ml BSA (immunoglobulin-free; Sigma Chemical Co.), and then washed extensively with PBS plus 2 mM EDTA. Samples of laminin 11 were then added (0.25 μg/μl resin) and incubated 6 h or overnight at room temperature. The supernatant was withdrawn and tested for effects on neurite outgrowth as described above.
Results
Diversity of Laminin Chains in Adult Muscle and Nerve
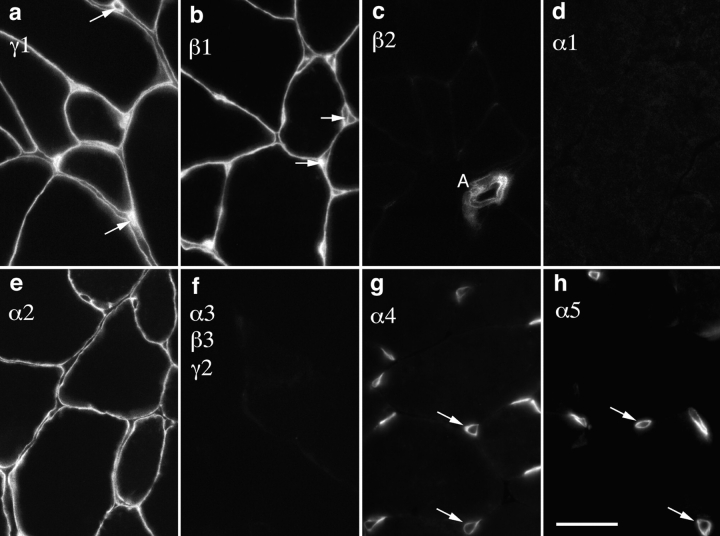
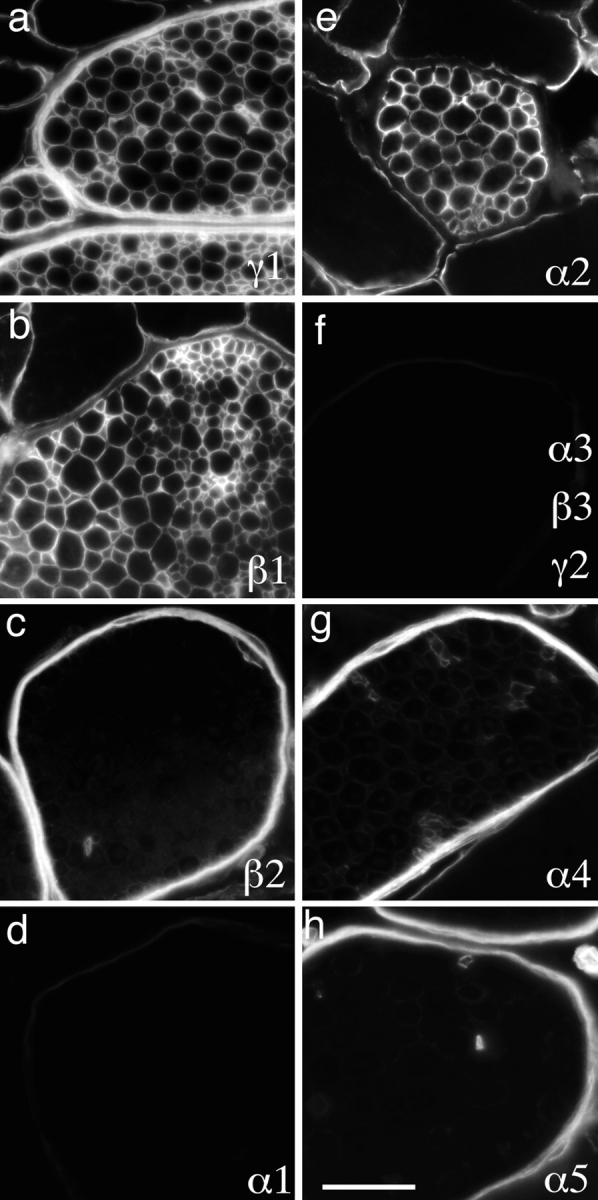
We first asked which of the 10 known laminin chains (α1–5, β1–3, γ1, and γ2) were present in the BL that ensheathed adult mouse muscle fibers. Antibodies to the α2, β1, and γ1 chains intensely stained this BL (Fig. 1, a, b, and e). In contrast, α1, α3, β3, and γ2 were undetectable in muscle (Fig. 1, d and f). The α4, α5, and β2 chains were also undetectable in extrasynaptic BL (Fig. 1, c, g, and h), although they were present at synaptic sites, as detailed below. Thus, consistent with previous reports (Engvall et al., 1990; Sanes et al., 1990; Vachon et al., 1996) and subject to caveats discussed below (see Discussion), the predominant laminin in adult muscle fiber BL appears to be the α2β1γ1 heterotrimer, laminin 2 (Table I).
Figure 1.
Laminins of adult muscle fiber BL. Sections of adult mouse intercostal muscle were stained with antibodies specific for the indicated laminin chains. Muscle fiber BL was rich in the α2, β1, and γ1 chains, but contained little or no α1, α3–5, β2, β3, or γ2. Capillary BL (examples shown by arrows) was α4-, α5-, β1-, and γ1-positive, and arteriolar BL contained β2 (A in c), α5, and γ1 (not shown). Bar, 25 μm.
We also examined intramuscular nerves, which are almost invariably present in sections of skeletal muscle. Such nerves contain two distinct types of BL. One is the multilammelar perineurial BL that coats the fibroblast-derived perineurium, which in turn surrounds fascicles of Schwann cell axon units (Bunge et al., 1989). The second is the endoneurial BL that surrounds individual Schwann cells, which in turn ensheathe one myelinated or several unmyelinated axons. Perineurial BL was rich in laminins α4, α5, β2, and γ1, and was devoid of detectable α1–3, β1, β3, or γ2. Endoneurial BL, in contrast, was rich in α2, β1, and γ1 but contained little or no α1, α3–5, β2, β3, or γ2 (Fig. 2). Thus, the predominant laminin of endoneurial Schwann cells, like that of muscle fibers, is likely to be laminin 2, whereas perineural BL contains laminins 9 and 11.
Figure 2.
Laminins of adult peripheral nerve. Sections of adult mouse intercostal muscles were stained with antibodies specific for the indicated laminin chains, and the internal intercostal nerves examined. Endoneurial BL (surrounding individual axon– Schwann cell units) was rich in the α2, β1, and γ1 chains. Perineurial BL, surrounding fascicles of nerve fibers, was rich in α4, α5, β2, and γ1. Neither contained detectable α1, α3, β2, or γ2. Bar, 50 μm.
Finally, three distinct vascular BLs were readily identifiable in muscle: those of capillaries, arterioles, and venules. Capillary BL contained laminins α4, α5, β1, and γ1 but not α1–3, β3, or γ2; β2 was detected with some but not all antibodies, as previously described in rat muscles (Sanes et al., 1990). Arteriolar BL contained α5, β2, and γ1, but little α4 and no detectable α1–3, β1, β3, or γ2. Venous BL contained β1 instead of β2 but was otherwise similar to arteriolar BL (Fig. 1; and data not shown). Thus, capillary BL is likely to be rich in laminins 8 and 10, arteriolar BL in laminin 11 and venous BL in laminin 10; capillary BL may also contain laminins 9 and 11.
Differential Distribution of Laminin Chains in Synaptic BL
Three BLs with distinct cellular origins are joined at the edge of the neuromuscular junction (Fig. 3 a): extrasynaptic BL (produced by muscle cells and fibroblasts), Schwann cell BL (produced by Schwann cells and fibroblasts), and the BL of the synaptic cleft (produced by nerve and muscle) (Sanes, 1994, 1995). Moreover, ultrastructural studies of membrane and cytoskeletal proteins have defined two distinct domains within the synaptic cleft; AChRs, rapsyn, and utrophin are concentrated in what are called primary clefts, at the crests of junctional folds, whereas neural cell adhesion molecule (N-CAM), sodium channels, and ankyrin are concentrated along the sides of the folds in secondary clefts (Fertuck and Salpeter, 1976; Covault and Sanes, 1986; Flucher and Daniels, 1989; Bewick et al., 1992). It therefore seemed possible, but had not been shown, that BLs of the primary and secondary clefts were molecularly distinct as well.
Figure 3.
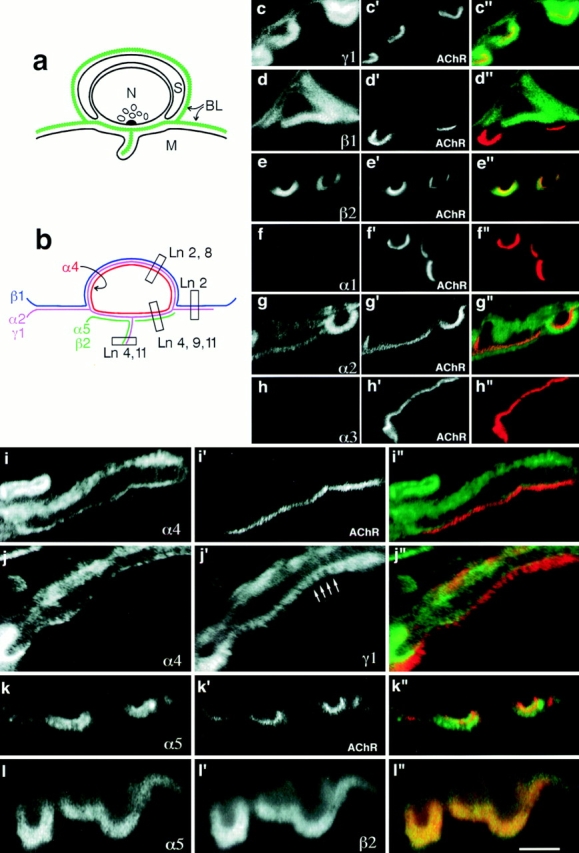
Laminins of adult synaptic BL. (a) Sketch of the neuromuscular junction, showing extrasynaptic, synaptic cleft, and Schwann cell BLs, and the distinct locations of BLs in primary and secondary clefts. (b) Summary of results obtained by confocal microscopy. Constituent heterotrimers deduced from the chain composition are also shown. (c–l) Sections of adult muscle, double labeled with contrasting fluorophores and photographed separately as indicated. The resulting images were combined using Photoshop (Adobe Systems, Mountain View, CA) (c′′– l′′) with the view in c–l shown in green and the view in c′–l′ shown in red. α-Bungarotoxin, which stains AChRs, marks the crests of junctional folds. By reference to this AChR-rich region, the BLs of extrasynaptic regions, Schwann cells, and the troughs of junctional folds (i.e., arrows in j′) can be distinguished. Bar in l is 8 μm for c–h and 5 μm for i–l.
We assessed the distribution of laminins in synaptic regions by using confocal microscopy to examine sections double labeled with chain-specific antibodies to laminins plus rhodamine-α-bungarotoxin, which binds to AChRs at the crests of junctional folds. This single counterstain allowed us to resolve all four domains in synaptic and perisynaptic BL, as is evident in micrographs of sections stained for γ1, which is present throughout the BL (Fig. 3, c and j′; see C24 antigen in Sanes and Chiu, 1983). The BL of the primary synaptic cleft is seen as a fine line adjacent and external to the AChR-rich domain. BL extending into the depths of the folds appears as fine struts that run ∼1 μm from the crests toward the interior of the muscle fiber (Fig. 3 j′). Stretches of BL lateral to the AChR-rich region are extrasynaptic. Finally, Schwann cell BL is a few microns external to the AChR-rich crest.
Three laminin α chains (α2, α4, and α5) were present at synaptic sites, but each had a distinct distribution. The α2 chain was codistributed with γ1, being present in the extrasynaptic, primary cleft, junctional fold, and Schwann cell BLs (Fig. 3, c and g). The α4 chain was present in Schwann cell and primary cleft BLs, but was absent from extrasynaptic BL and from the BL of junctional folds (Fig. 3 i). In contrast, α5 was present in both primary cleft and junctional fold BLs, but was absent from extrasynaptic and Schwann cell BLs (Fig. 3 k). β1 was present in extrasynaptic and Schwann cell but not synaptic BLs (Fig. 3 d; see Sanes et al., 1990), whereas β2, like α5, was present in crest and fold BLs (Fig. 3 e). Laminins α1, α3, β3, and γ2 were undetectable (Fig. 3, f and h; and data not shown).
To confirm these localizations, we used species-specific second antibodies to compare the distributions of pairs of laminin chains. For example, Fig. 3 j shows a section double labeled with antibodies to α4 and γ1. Codistribution of the two chains in primary cleft and Schwann cell BLs is evident, as is the extension of γ1 into the α4-free junctional folds. Double labeling for β2 and α5 confirmed that these two chains are codistributed throughout synaptic BL (Fig. 3 l).
Together, these observations reveal that each of the four BLs that abut synaptic sites bears a distinct complement of laminin chains: α2, β1, and γ1 extrasynaptically (laminin 2); α2, α4, α5, β2, and γ1 in the primary cleft (laminins 4, 9, and 11); α2, α5, β2, and γ1 in the BL of junctional folds (laminins 4 and 11); and α2, α4, β1, and γ1 in the BL that covers Schwann cells (laminins 2 and 8; Fig. 3 b).
Laminin Isoform Transitions in Developing Muscle
In some tissues, it has been shown that the complement of laminin chains in individual BLs changes during development (Jaakkola et al., 1993; Miner and Sanes, 1994; Virtanen et al., 1995, 1996; Miner et al., 1997). We therefore asked whether the laminin chains present when muscles and neuromuscular junctions are forming differed from those present in adult synaptic and extrasynaptic BL. We used intercostal muscles for this study because myogenesis, synaptogenesis, and BL formation have all been intensively studied in these muscles (see Kelly and Zacks, 1969a , b ; Chiu and Sanes, 1984; Rosen et al., 1992).
Intercostal myoblasts begin fusing to form an initial cohort of myotubes, called primary myotubes, on embryonic days (E) 10 and 11, and the myotubes soon begin to assemble a BL. By E 11.5, numerous patches of BL are present on myotube surfaces, but a continuous lamina is not yet present (see Rosen et al., 1992 for references). These patches of BL contained α2, α5, β1, and γ1 chains (Fig. 4, a, c, and f; and data not shown). The α3, α4, β2, β3, and γ2 chains were undetectable (Fig. 4, d and e; and data not shown). Thus, the BL of newly formed myotubes contains laminin 10, as well as the adult trimer, laminin 2.
Figure 4.
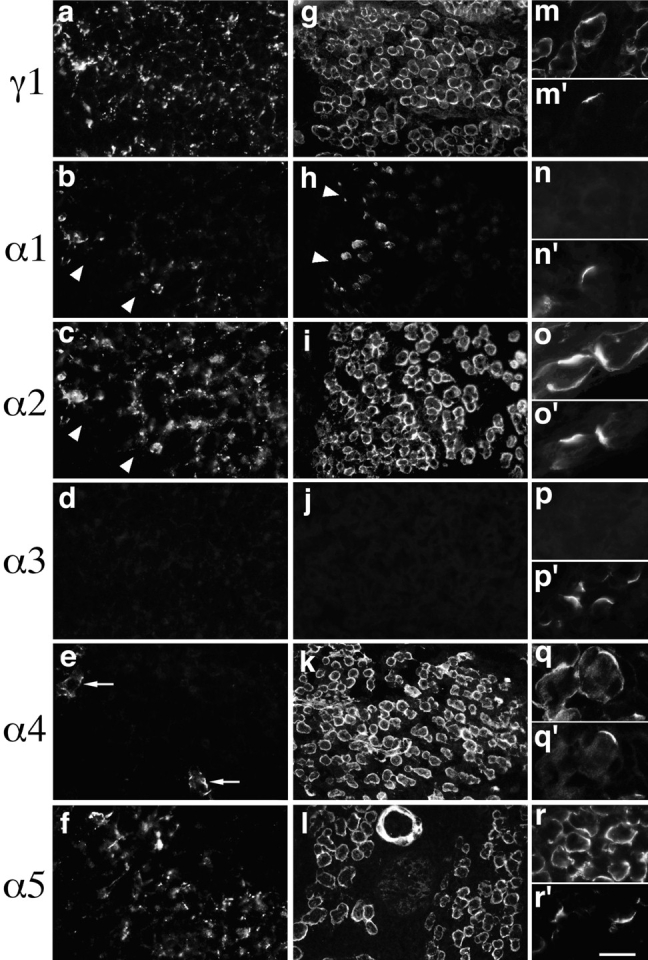
Laminins of embryonic muscle. Sections of intercostal muscle from E 11.5 (a–f) or E 15 (g–r) embryos were stained with antibodies specific for the indicated laminin chains. Some sections were also double labeled with rhodamine-α-bungarotoxin (m′–r′). The patchy BL that partially coats myotubes at E 11.5 contains α2, α5, and γ1 chains and, in areas abutting ribs (arrowheads), α1. Note that the section shown in b and c was double labeled with anti-α1 and anti-α2, showing that α1 is present in a subset of α2-containing BLs. At E11, α4 is detectable only in blood vessels (e, arrows). By E 15, the BL is continuous, and contains α4 in addition to α2, α5, and γ1. The α and γ chain complement of synaptic and extrasynaptic BL is qualitatively similar at this age (m– r), although β2 is already selectively localized to synaptic sites (data not shown). Bar in r represents 20 μm for a–f, 40 μm for g–l, and 16 μm for m–r.
Interestingly, α1 was also present in muscle at E 11.5, but was largely restricted to the ends of myotubes, in regions abutting the ribs (Fig. 4 b). Double labeling with anti-α1 plus anti–N-CAM (a marker of myogenic cells; Sanes et al., 1986; Rosen et al., 1992) confirmed that the α1 was associated with muscle (rather than with tendons or cartilage; data not shown), and double labeling with anti-α1 plus anti-α2 confirmed that α1 was associated with α2-containing BLs (Fig. 4, b and c). Because myogenesis is believed to proceed in large part at the ends of fibers (Zhang and McLennan, 1995), we speculate that laminin α1 may be expressed by myoblasts just as they fuse with myotubes.
BL deposition around primary myotubes continues between E 11 and 13. In addition, a second cohort of myotubes, called secondary myotubes, begins to form on E 14. By E 15, most myotubes bear a continuous BL sheath. As on E 11.5, this BL was rich in laminins α2, α5, β1, and γ1, and α1 remained confined to the ends of myotubes (Fig. 4, g–i, and l; and data not shown). In contrast, levels of α4 increased dramatically after E 11.5, and this chain was present throughout all myotube BLs by E 15 (Fig. 4 k). Thus, laminin 8 appears to join laminins 2 and 10 as primary myotubes mature and secondary myotubes form.
Synaptogenesis begins in intercostal areas at E 13, and rudimentary neuromuscular junctions are readily detectable by E 14 (Kelly and Zacks, 1969b ; Noakes et al., 1993). To assess early stages in the formation of synaptic BL, we double-labeled sections of E 15 intercostal with antilaminins plus rhodamine-α-bungarotoxin. The α2, α4, α5 and γ1 subunits were all present in synaptic as well as extrasynaptic areas. Interestingly, α2, α4, and γ1 appeared to be enriched in synaptic BL, but α5 did not (Fig. 4, m–r). As reported previously for rat intercostals (Chiu and Sanes, 1984), the β2 chain appeared soon after AChR clusters formed, and was restricted to synaptic sites at all stages, whereas β1 was present both synaptically and extrasynaptically at early stages of synaptogenesis (data not shown; note that the C1/C4 and C21/C22 antigens studied by Chiu and Sanes, are now known to be laminins β2 and β1, respectively [Sanes et al., 1990]).
Further changes in the composition of muscle BL occurred perinatally as myotubes matured into myofibers. Extrasynaptic levels of α4 and α5 were markedly lower at birth than at E 15, and neither subunit was detectable by the end of the first postnatal week. At the synapse, the intensity of α5 staining rose postnatally, while levels of α4 remained modest, and the β1 subunit was gradually lost from these regions. The β2 subunit remained confined to synaptic sites, and levels of α2 and γ1 remained high both synaptically and extrasynaptically throughout development (data not shown). We also noted one developmental change in the BL of intramuscular nerves during this period: in embryos and during the first 2 postnatal wk, endoneurial BL contained α4 as well as α2, whereas adult endoneurium contained only α2 (data not shown; compare Figs. 2 g and 8 k).
In summary, the laminin chain composition of both extrasynaptic and synaptic BL changes during development, but in different ways. The α2 and γ1 chains are present in both domains at all stages of development; α4 and α5 are initially present throughout the BL, and then lost from extrasynaptic BL; the β1 chain is initially ubiquitous, and then lost from synaptic BL; and β2 is confined to synaptic sites from its first appearance. From the perspective of deduced trimeric structure, laminin 2 predominates extrasynaptically at all stages, but is joined transiently by laminin 1 near the ends of fibers and by laminins 8 and 10 throughout the fiber length. Synaptically, the embryonic presence of β1 suggests that the β1-containing trimers (laminins 2, 8, and 10) are present initially but then lost, whereas the β2-containing trimers (laminins 4, 9, and 11) appear slightly later and are retained.
Production of Laminins α4 and α5 by Muscle Cells
To understand how laminins act, it is important to know which cells make them. This issue is of particular importance for synaptic BL, which is known to contain contributions from both muscle and nerve (Sanes, 1995), and might also contain products of Schwann cells. Myogenic cells are known to synthesize the laminin α1, α2, β1, β2, and γ1 chains (Green et al., 1992; Kroll et al., 1994; Schuler and Sorokin, 1995; Vachon et al., 1996), but synthesis of α4 or α5 by muscle has not yet been reported. To address this issue, we used the rat muscle cell line, RMo (Merrill, 1989). We have previously shown that RMo cells express β1, β2, and γ1 chains, and hitherto unidentified α-like chains (Green et al., 1992). Here, we asked whether the α-like chains corresponded to α4 or α5.
Proteins of RMo myotubes were separated by SDS-PAGE, and then immunoblotted. Antisera to laminin α4 recognized a protein of ∼200 kD, and antisera to α5 recognized a protein of ∼400 kD (Fig. 5 a, lanes 3 and 4). These apparent molecular weights were similar to those obtained previously from rat lung and kidney tissue extracts immunoblotted with these same antisera (Miner et al., 1997). Antiserum to laminin 1 recognized bands of ∼400, ∼220, and ∼200 kD (Fig. 5 a, lane 2), presumably representing the α1, β1, and γ1 chains, respectively. As expected, anti-β2 bound to a protein of ∼190 kD (Fig. 5 a, lane 5). Non-immune serum showed no reaction with any of the laminin chains (Fig. 5 a, lane 1). Thus, muscle cells synthesize not only the laminin α1, α2 (see below), β1, β2, and γ1 chains, but also α4 and α5.
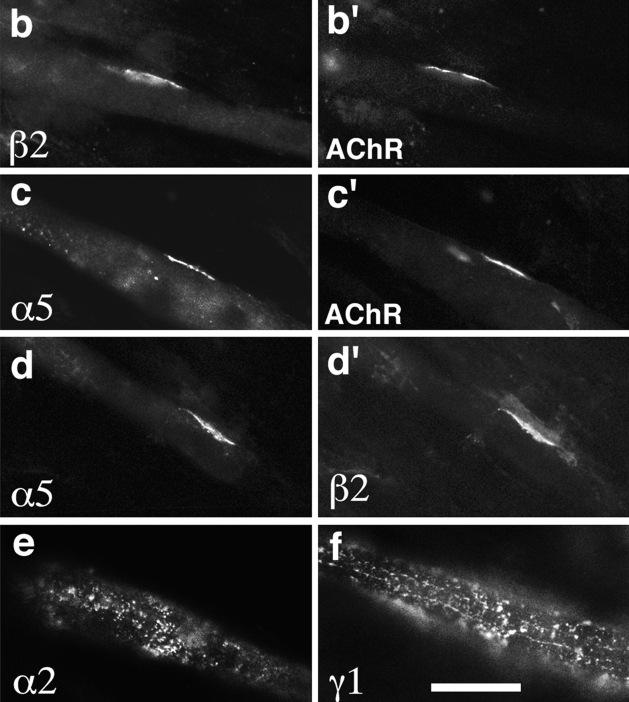
Figure 5.
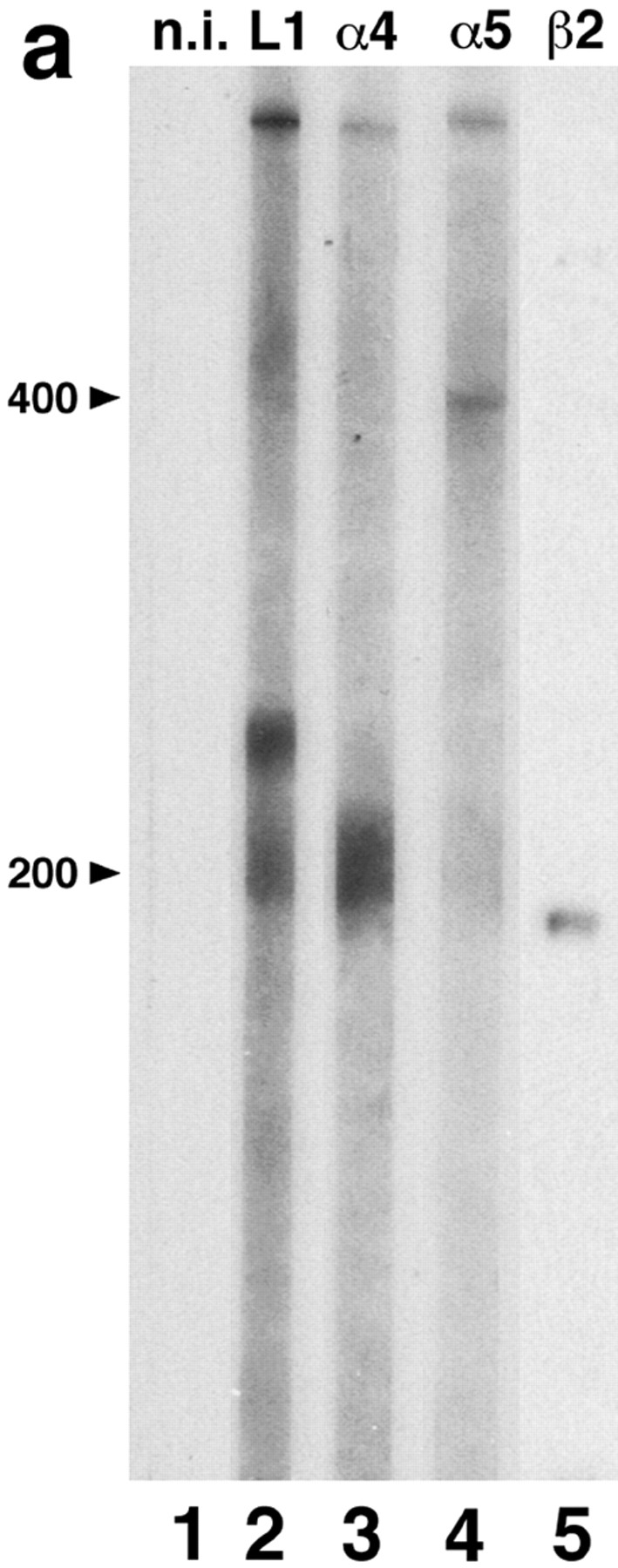
Synthesis and distribution of laminin chains in RMo myotubes. (a) Immunoblot of cell lysate with nonimmune serum (lane 1), antilaminin 1 (lane 2), antilaminin α4 (lane 3), antilaminin α5 (lane 4), or antilaminin β2 (lane 5). (b–d) Micrographs of cultures labeled with antibodies specific for the indicated laminin chains. The cultures shown in b and c were counterstained with rhodamine-α-bungarotoxin (b′, c′), and the culture shown in d was counterstained with antilaminin β2 (d′). The α2 (e), β1 (not shown), and γ1 (f) chains are broadly distributed on the myotube surface, whereas α5 and β2 are colocalized in small patches that abut AChR-rich domains on the myotube membrane. Bar in f is 20 μm for b, c, e, and f, and 30 μm for d.
We and others have previously shown that myotubes can differentially localize laminin β chains in the absence of nerves: β1 is broadly distributed throughout the myotube BL, whereas β2 is largely restricted to small patches of BL that cover AChR-rich domains (“hot spots”) of the plasma membrane (Silberstein et al., 1982; Sanes and Lawrence, 1983; Martin et al., 1995). Here, we used RMo cells to ask whether the ability to differentially distribute α chains is also cell autonomous, or whether it requires nerves and/or Schwann cells. Unfortunately, available anti-α4 sera require antigen denaturation, which turned out to be incompatible with determination of surface localization. However, extracellular deposits of laminins α5 and β2 were both clearly concentrated in AChR-rich regions (Fig. 5, b and c). Double labeling with anti-α5 and anti-β2 showed that the two chains were generally codistributed (Fig. 5 d). In contrast, the α2, β1, and γ1 chains were broadly distributed on the myotube surface (Fig. 5, e and f; and data not shown). Thus, myotubes can not only synthesize multiple laminin α chains, but also differentially distribute them. Moreover, the nerve is not necessary for the selective association of laminin 11 with regions that resemble postsynaptic membrane.
We also immunostained C2 and primary mouse myotubes with antibodies to laminin α5. In contrast to results obtained with RMo cells, α5 was associated with both AChR-rich and AChR-poor regions of myotubes in these preparations (data not shown). Since α5 becomes restricted to synaptic sites in vivo as development proceeds, it may be that postsynaptic differentiation progresses to a later stage in RMo than in C2 or primary myotubes.
Compensation and Coregulation in Laminin α2 and β2 Mutants
Mice with mutations in two laminin chain genes are available: naturally occurring α2dy/dy mice, in which levels of α2 are markedly reduced (Arahata et al., 1993; Sunada et al., 1994, 1995; Xu et al., 1994a ,b), and β2−/− mice, in which a null mutation was introduced by homologous recombination (Noakes et al., 1995a ). The α2dy/dy mice exhibit severe muscular dystrophy with only minor perturbation of neuromuscular junctions (Carbonetto, 1977; Banker et al., 1979; Law et al., 1983; Desaki et al., 1995) whereas synaptic maturation is markedly aberrant but muscles are nearly normal in β2−/− mice (Noakes et al., 1995a ). These results implicate the laminin α2 and β2 chains in myogenesis and synaptogenesis, respectively. However, interpretation of the mutant phenotypes requires understanding which laminin trimers are present in the BLs of mutant muscle and peripheral nerve. We therefore assessed the distribution of other laminin chains in α2dy/dy and β2−/− muscle.
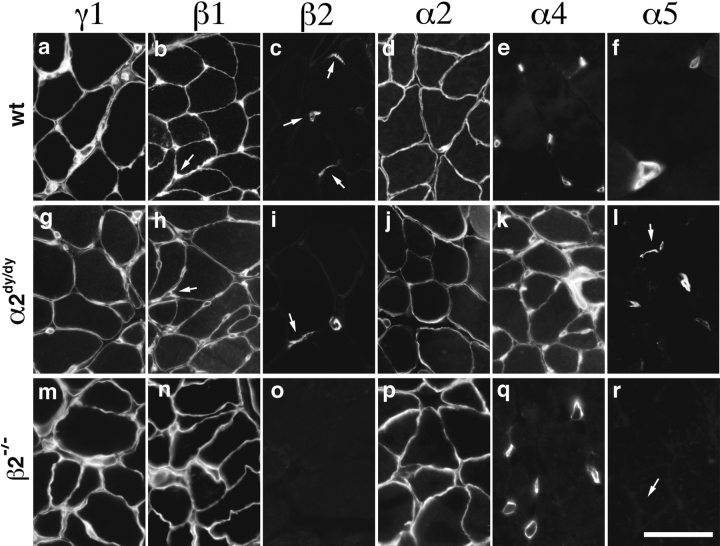
In extrasynaptic BL of α2dy/dy muscles, laminin α2 immunoreactivity was markedly reduced in intensity and was patchy rather than continuous in distribution (Fig. 6 j). This incomplete loss has been noted previously, and is consistent with dy/dy being an allele that decreases α2 levels but does not affect the size of the α2 polypeptide (Sunada et al., 1994; Xu et al., 1994a ). In contrast, levels of β1 and γ1 immunoreactivity were only slightly lower in α2dy/dy muscles than littermate control muscles (Fig. 6, a, b, g, and h), consistent with previous reports in α2dy/dy mice (Sunada et al., 1994; Xu, et al., 1994a) and in human merosin-deficient dystrophy (Hayashi et al., 1993; Sewry et al., 1995). Assuming that native laminins are all heterotrimers (Burgeson et al., 1994), this pattern implies a compensatory increase in the level of another α chain. Indeed, immunoreactivity for laminin α4 was undetectable in controls but intense in α2dy/dy extrasynaptic BL (Fig. 6, e and k). This compensation was specific, in that the laminin α1, α3, α5, and β2 chains remained undetectable extrasynaptically (Fig. 6, c, f, i, and l; and data not shown). Thus, laminin 8 may compensate for the loss of laminin 2 in α2dy/dy muscle.
Figure 6.
Laminins of α2dy/dy and β2−/− muscle. Sections of intercostal muscle from α2dy/dy (g–l) or β2−/− mutants (m–r) or from controls (a–f) were stained with antibodies specific for the indicated laminin chains. In α2dy/dy muscle, partial loss of α2 leads to appearance or retention of α4 but not α5. Extrasynaptic BL of β2−/− mutants does not differ from that of controls. Arrows mark synaptic sites (localized with rhodamine- α-bungarotoxin; not shown) in c, h, i, l, and r. Bar in r is 40 μm for a–l and 20 μm for m–r.
In normal adult muscle, levels of laminin α2 were significantly higher in synaptic than in extrasynaptic regions of the muscle fiber surface (see above). Paradoxically, levels of α2 were more strikingly reduced in synaptic than in extrasynaptic regions of α2dy/dy muscle, leaving synaptic and Schwann cell BLs nearly devoid of α2 immunoreactive material (Fig. 7 a). As if in compensation, levels of α4 were more markedly increased synaptically than extrasynaptically in α2dy/dy muscle (Fig. 7 b). Levels of α5 and β2 were similar in synaptic BL of wild-type and α2dy/dy mice, and α1, α3, and β1 were absent from control and mutant synapses alike (Figs. 6, c and i; and 7, c and d; and data not shown). Thus, the apparent ratios of synaptic laminins are altered in α2dy/dy. Levels of laminin 11 are similar at control and mutant synapses, but levels of laminin 4 are dramatically reduced and levels of laminin 9 are increased in the mutant. Therefore, laminin 4 is not required for qualitatively normal synaptic structure and function.
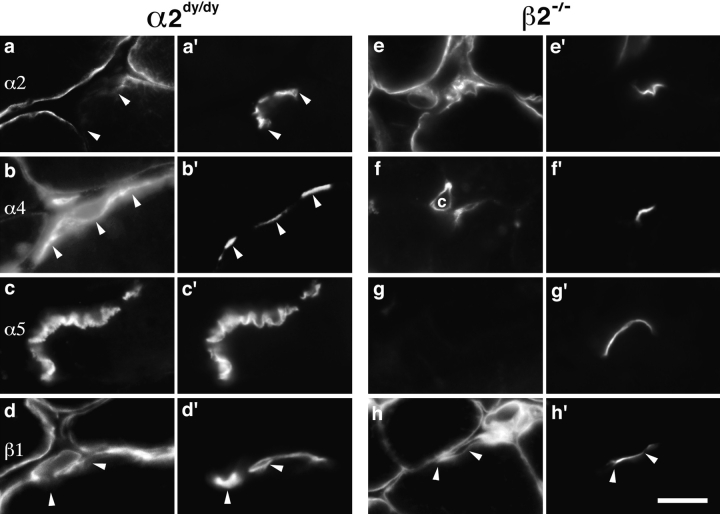
Figure 7.
Laminins of synaptic BL in α2dy/dy and β2−/− mutants. Sections of intercostal muscle from adult α2dy/dy (a–d) or β2−/− mutants aged 14 d (e–h) were double stained with antibodies specific for the indicated laminin chains (a–h) plus rhodamine-α-bungarotoxin (a′–h′). Specific loss of α2 from synaptic sites in α2dy/dy leads to increased levels of α4, but no detectable changes in the distribution of α5 or β1. Loss of β2 from synaptic sites in β2−/− leads to coordinate loss of α5 and increased levels of β1 but not marked changes in the distribution of α2 or α4. Bar, 15 μm.
A different pattern of compensation was observed in the BL of laminin β2−/− mutants. No alterations were detectable in extrasynaptic laminins, consistent with the restriction of β2 to synaptic sites at all stages of development (Fig. 6, m–r). However, the laminin composition of mutant synaptic BL was altered in three ways. First, as expected, laminin β2 was absent (Fig. 6 o). Second, and unexpectedly, laminin α5 was undetectable at synapses in β2−/− mutants at all stages examined, from P10 though P35 (Fig. 7 g; and data not shown). Third, laminin β1, which is undetectable at control and α2dy/dy synapses, was clearly present in β2−/− synaptic BL (Fig. 7 h). These changes in synaptic BL were specific, since levels of α2 and γ1 were not greatly reduced at β2−/− synapses (Fig. 7 e; and data not shown). Laminin α4 also remained concentrated at synaptic sites in β2−/− muscle (Fig. 7 f); however, we were unable to determine whether this α4 was present in synaptic BL per se as well as in the closely apposed BL of Schwann cells, which protrude into synaptic clefts in β2−/− mutants (Noakes et al., 1995a ). Together, these alterations suggest that the normal synaptic laminins (laminins 4, 9, and 11) are replaced by laminin 2 and possibly laminin 8, but not laminin 10 in β2−/− mutants. In that laminin 4 is apparently dispensable for synaptogenesis (as shown by the α2dy/dy mice; see above), these results focus attention on laminins 9 and 11 as regulators of neuromuscular development.
To extend our analysis of compensation and coregulation, we assessed the distribution of laminin chains in α2dy/dy and β2−/− intramuscular nerves. As detailed above, α2 is normally found in endoneurial BL and β2 in perineurial BL. In endoneurial BL of the α2dy/dy mutant, levels of α2 were markedly reduced and levels of α4 were increased (Fig. 8, d and e). Thus, α4 appears to compensate for α2 in nerve as it does in muscle. In perineurial BL of the β2−/− mutant, β2 and α5 were both absent and α4 was considerably reduced (Fig. 8, i–l). Thus, α5 and β2 appear to be coregulated in nerve as in muscle. The laminin compositions of perineurial BL in the α2dy/dy mutant and of endoneurial BL in the β2−/− mutant were qualitatively normal (compare Figs. 2 and 8).
Figure 8.
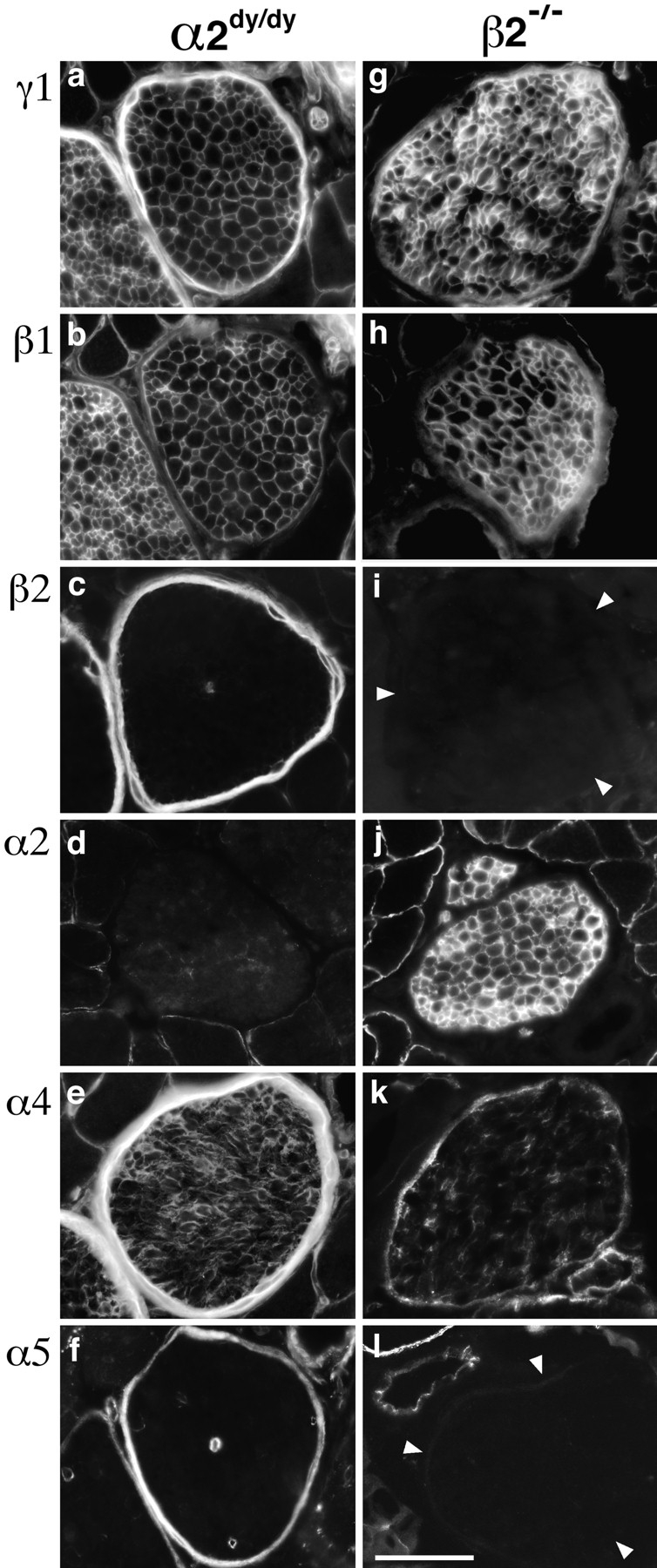
Laminins of peripheral nerve in α2dy/dy and β2−/− mutants. Intramuscular nerves from intercostal muscles were stained with antibodies specific for the indicated laminin chains. Wild-type nerves are shown in Fig. 2. Loss of α2 in α2dy/dy leads to a compensatory increase in α4 immunoreactivity in the endoneurium. Loss of β2 in β2−/− leads to a coordinate loss of α5, and reduction in α4 from the perineurium. Bar, 50 μm.
Distinct Response of Motor Axons to Laminins 1, 2, 4, and 11
As neuromuscular junctions mature in embryos or regenerate after nerve injury in adults, motor axons encounter synaptic BL. Analysis of the laminin β2−/− mutant mice suggests that laminin β2 is one of the components that arrests the growth of motor axons and promotes their differentiation into nerve terminals (Patton, B.L., and J.R. Sanes, in preparation). Likewise, we have shown that recombinant β2 fragments have outgrowth-stopping and differentiation-promoting activities in vitro (Hunter et al., 1991; Porter et al., 1995; Patton, B.L., and J.R. Sanes. 1995. Soc. Neurosci. Abstr. 13:799). Here, we have presented evidence that synaptic β2 may be associated with three distinct heterotrimers: laminins 4, 9, and 11. It was therefore important to assess the effects of native β2 in heterotrimeric form, and to ask whether the β2-containing heterotrimers have distinct bioactivities. Accordingly, we assessed outgrowth from embryonic chick ciliary neurons on substrates coated with one of four different heterotrimers: laminins 1, 2, 4, or 11. (Purified laminin 9 was not available to us.) Ciliary neurons, like spinal motor neurons, innervate striated muscle in vivo. Moreover, they are easily isolated, recognize original synaptic sites on skeletal muscle fibers (Covault et al., 1987), and stop growing in response to recombinant laminin β2 in vitro (Porter et al., 1995).
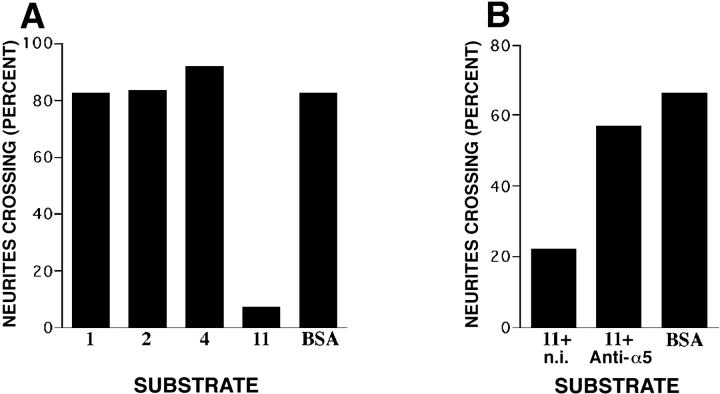
Initially, we plated dissociated ciliary neurons on substrates coated with purified laminin 1, 2, 4, or 11, and then fixed and viewed them 30 h later. Most neurons extended neurites on laminins 1, 2, or 4, as reported previously (Weaver et al., 1995; Brandenberger et al., 1996) (Fig. 9, a and b). In contrast, neurons adhered to but did not extend neurites on laminin 11 (Fig. 9 c). Likewise, little outgrowth was observed when neurons were plated on mixtures of laminins 1 and 11, indicating that laminin 11 inhibited neurite outgrowth and did not merely lack outgrowth-promoting activity (data not shown). In this respect, laminin 11 behaved like recombinant β2 fragments, which we have previously shown to support adhesion of, but inhibit outgrowth from ciliary motoneurons (Porter et al., 1995).
Figure 9.
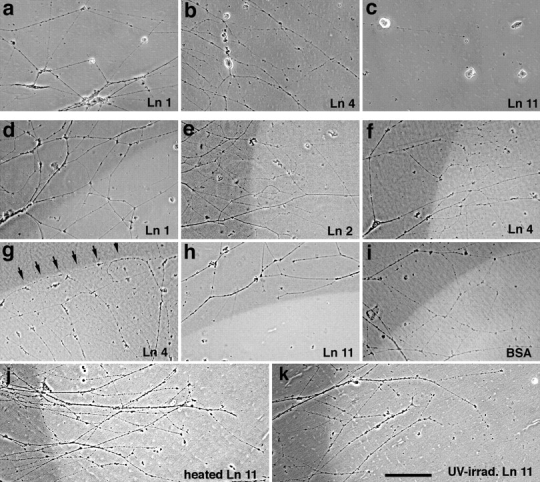
Growth of ciliary neurites on laminins (a–c). Neurons from embryonic chick ciliary ganglia were plated on substrates coated with laminin 1 (20 μg/ml; a), laminin 4 (20 μg/ml; b) or laminin 11 (30 μg/ml; c). Neurites extend on laminins 1 and 4 but only seldom on laminin 11. (d–l) Clusters of ciliary neurons were plated on patterned substrates, consisting of fields of laminin 1 and spots in which the laminin was coated atop test substrates: laminin 1 (100 μg/ml; d), laminin 2 (50 μg/ml; e), laminin 4 (50 μg/ml; f and g), laminin 11 (50 μg/ml; h, j and k), or BSA (100 μg/ml; i). In j and k, the laminin 11 was denatured by UV irradiation (j), or heating (k) before laminin 1 was applied. To mark borders, the test substances were mixed with the fluorescent dye sulforhodamine. Fields were photographed with a combination of phase and rhodamine optics, so that areas bearing test substrate appear bright. Neurons were plated on laminin 1 in d–f and h–k, and on the test substrate in g. Neurites cross freely over borders of additional laminin 1, laminin 2, BSA, or denatured laminin 11. Neurites growing on laminin 1 also cross freely onto a laminins 1 and 4 mixture, but neurites growing on the mixture seldom cross onto laminin 1. Neurites growing on laminin 1 seldom cross onto the laminins 1 and 11. Bar, 150 μm.
Based on these results, we plated clusters of ciliary neurons on a uniform field of laminin 1, and then observed neurites that encountered a patch containing a mixture of laminin 1 plus laminins 2, 4, or 11. Neurites crossed freely onto either laminin 2 or laminin 4 (Fig. 9, e and f). Likewise, mixtures of laminin 1 plus either BSA or additional laminin 1 had no discernible effect on outgrowth (Fig. 9, d and i). In contrast, neurites seldom grew from laminin 1 onto a mixture of laminins 1 and 11 (Figs. 9 h and 10 a). The effect of laminin 11 was abolished by thermal denaturation or UV irradiation (as in Porter et al., 1995) (Fig. 9, j and k), further indicating that the native preparation was actively inhibitory rather than merely inactive. The inhibitory activity was depleted by precipitation with antisera to α5 (Fig. 10 b), confirming that the bioactivity was attributable to laminin 11 itself rather than to a contaminant in the preparation. From these results, we conclude that laminin 11 can serve as a stop signal for motor neurites.
Figure 10.
Ciliary neurites distinguish laminin 11 from laminins 1, 2, and 4. (a) The frequency with which neurites growing on laminin 1 crossed onto a mixture of the indicated composition was determined as illustrated in Fig. 9 and detailed in Materials and Methods. Concentrations of proteins were as given in Fig. 9 legend. (b) Absorption with anti-α5 coupled to protein A–agarose depleted the inhibitory activity in laminin 11. Treatment of the laminin 11 with preimmune serum (from the same rabbits) plus protein A–agarose had little or no effect. Bars show values from 21 to 80 neurites (mean = 53) in A, and 67–80 neurites (mean = 75) in B.
In the assays scored in Fig. 10, ciliary neurons were plated onto laminin 1 and their growth onto another laminin was monitored. In some cases, however, clusters of ciliary neurons were plated onto the mixed substrate, so that neurites encountered the border from the opposite direction. For mixtures of laminins 1 and 2, neurites readily traversed the border in both directions. As noted above, outgrowth was sparse in mixtures of laminins 1 and 11, so crossing onto laminin 1 was difficult to evaluate. Interestingly, however, neurites seldom crossed from laminins 1 and 4 onto laminin 1 (Fig. 9 g). In that laminin 1 clearly promotes neurite outgrowth (i.e., is not inhibitory) on its own, this result indicates that ciliary neurons prefer laminin 4 to laminin 1 as a substrate. Thus, two different synaptic laminins have distinct effects on neurite outgrowth.
Discussion
In previous studies, we have analyzed the distribution, regulation, and function of the laminin β chains in muscle (Chiu and Sanes, 1984; Hunter et al., 1989a ,b; Sanes et al., 1990; Noakes et al., 1995a ; Porter et al., 1995). Here, we have extended these analyses to the α chains, with special emphasis on the newly discovered α4 and α5. Our main results are as follows. (a) Cultured muscle cells express four different α chains (α1, α2, α4, and α5), and developing muscles incorporate all four into BLs, each in a distinct pattern. (b) Synaptic and extrasynaptic BL acquire distinct complements of laminin chains as development proceeds: α2, α4, α5, and β1 are initially present both synaptically and extrasynaptically, whereas β2 is restricted to synaptic BL from its first appearance; α2 remains broadly distributed; α4 and α5 become restricted to synaptic BL; and β1 becomes restricted to extrasynaptic BL. (c) Likewise, cultured muscles cells restrict α5 and β2 to AChR-rich hot spots but broadly distribute α2 and β1, even in the absence of nerves. (d) Laminin isoforms mark two distinct domains within adult synaptic BL: α2, α5, β2, and γ1 are present in both the primary cleft and junctional folds, whereas α4 is restricted to the primary cleft. (e) The endoneurial and perineurial BLs of peripheral nerve each contain distinct laminin chains (α2, β2, γ1, and α4, α5, β1, γ1, respectively). (f) Mutation of the laminin α2 and β2 genes leads to coordinate loss and compensatory upregulation of other chains. (g) Motor axons respond in distinct ways to different laminin heterotrimers: they grow freely between laminin 1 and laminin 2, fail to cross from laminin 4 to laminin 1, and stop upon contacting laminin 11. The ability of laminin 11 to serve as a stop signal for growing axons explains, at least in part, axonal behaviors observed at developing and regenerating synapses in vivo.
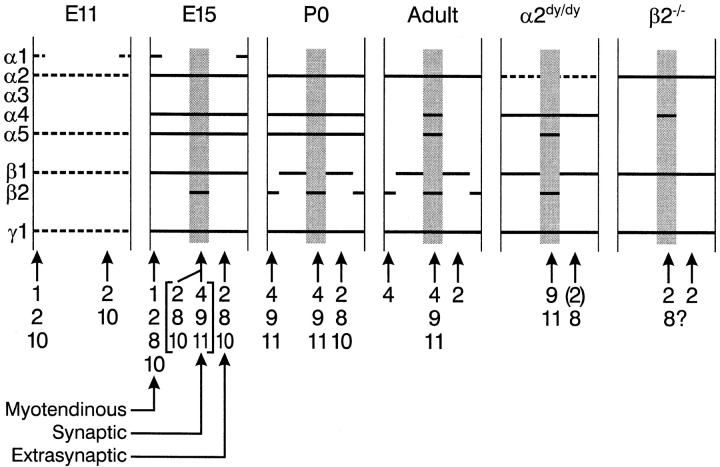
Fig. 11 summarizes the main patterns of laminin chain expression that we have documented in muscle. The figure also indicates the heterotrimers (as named in Table I) that we deduce to be present at various stages and sites. This interpretation depends, however, on two assumptions. The first is that all possible αβγ heterotrimers are formed from α1–5, β1, β2, and γ1, whenever the constituent chains are present. We know of no evidence against this idea, but it has not been critically tested. The second is that no other laminin chains are present in muscle besides those for which we have probes. In fact, there are some indications that other chains exist. For example, perineurial BL of β2−/− muscle contains α4 and γ1 but little or no β1–3. If all laminins are heterotrimers, there may be another β chain to be discovered in perineurium. Thus, the assignments of trimers made in Fig. 11 and throughout the text must be regarded as provisional.
Figure 11.
Distribution of laminin chains in synaptic and extrasynaptic BL of developing, adult, and mutant muscle. The diagram summarizes data illustrated in Figs. 1, 3, 4, 6, and 7, and is discussed in the text. Laminins of myotendinous regions were determined for wild type but not mutant muscles. Heterotrimers that could be assembled from the component chains are indicated below each part.
Previously, we showed that synaptic BL contains two α chains: α2, which is present throughout muscle fiber BL, and a synapse-specific α chain that reacted with the 4C7 monoclonal antibody (Sanes et al., 1990). At the time, we believed that 4C7 reacted solely with the α1 chain, but this now seems unlikely (Miner et al., 1997). Indeed, we show here that α1 is not found in synaptic BL, but that α4 and α5 are. Thus, subject to the caveats above, we conclude that there are three synaptic laminins: 4, 9, and 11. Any or all may play important roles in the development and maintenance of synapses, but several results focus our attention on laminin 11. First, isolated muscle cells can localize both the α5 and the β2 subunits even in the absence of nerves, thus suiting this laminin to a role as a synaptic retrograde signal. Second, both subunits are lost from synaptic sites in β2−/− mutants, which show severe synaptic defects (Noakes et al., 1995), whereas both are retained in α2dy/dy mice, which show mild synaptic defects (Carbonetto, 1977; Banker et al., 1979; Law et al., 1983; Desaki et al., 1995). In contrast, laminin 4 is lost from α2dy/dy synapses. Third, laminin 11 serves as a stop signal for elongating motor neurites, a property previously ascribed to synaptic BL (Sanes et al., 1978).
The bioactivity of laminin 11 is consistent with the synaptic defects observed during reinnervation of β2−/− mutant muscle, in which regenerating axons often extend beyond original synaptic sites (Patton, B.L., and J.R. Sanes, in preparation). However, the coordinate loss of the α5 and β2 chains from mutant synaptic BL raises the question of which chain is responsible for the stopping activity. In support of α5 is the observation that laminin 4 (α2/β2/γ1) does not inhibit neurite outgrowth. In support of β2 is the observation that recombinant β2 fragments exert a potent stopping activity very much like that shown here for native laminin 11 (Porter et al., 1995). One intriguing possibility is that the inhibitory activity resides in the β2 chain, but that it is context dependent, being favored by combination with α5 but opposed by combination with α2.
Recently, Brandenberger et al. (1996) also showed that β2-containing laminin 4 promotes outgrowth of neurites from ciliary neurons and argued against the idea that synaptic laminins serve as stop signals for motor axons. Our results are consistent with theirs, but our new data lead us to question several of their conclusions. First, contrary to their assertion, laminin 4 is not the sole synaptic laminin. Previous findings suggested the existence of at least one additional synaptic laminin (Sanes et al., 1990), and our new results suggest that laminins 4, 9, and 11 are all β2-containing synaptic laminins. Moreover, the phenotypes of the α2dy/dy and β2−/− mutants discussed above suggest that laminins 9 and/or 11 are more critical for synaptic function than is laminin 4. Second, based on their finding that laminin 4 does not inhibit neurite outgrowth, Brandenberger et al. (1996) concluded that laminin β2 is unlikely to be involved in the process that leads motor axons to stop growing at synaptic sites. In contrast, our studies of laminin-coated substrates demonstrate that β2-containing laminin 11 is a potent inhibitor of neurite outgrowth. Third, they confirmed our previous reports (Hunter et al., 1989b ; Porter et al., 1995) that the tripeptide sequence LRE affects the adhesiveness of a recombinant β2 fragment, and that mutation of this sequence decreases the ability of the fragment to inhibit neurite outgrowth. However, they showed that LRE is inactive when inserted into a recombinant fragment of a chicken cartilage matrix protein, which is predicted to form a coiled-coil structure similar to that of the LRE-containing domain of β2. They therefore argue that the LRE site is unlikely to be active in the native protein. Our results with laminin 11 raise the alternative possibility, that the activity of the LRE site may be context dependent. For example, the juxtaposition of β2 with α5 might disrupt the coiled-coil conformation of the former. Finally, Brandenberger et al. (1996) noted that ciliary neurons extended neurites of the same mean length on substrates of laminins 2 and 4, and therefore concluded that neurite outgrowth on laminin 2 was indistinguishable from that on laminin 4. However, when we observed neurites growing from either laminins 1 and 2 or 1 and 4 onto laminin 1, we found that neurites were blind to laminin 2 but sensitive to laminin 4 borders. Thus, these two trimers do have distinguishable effects on neurites.
We and others have previously documented complex patterns of regulation for the laminins and collagens IV of renal BLs (Kashtan and Kim, 1992; Miner and Sanes, 1994, 1996; Gubler et al., 1995; Noakes et al., 1995b ; Cosgrove et al., 1996; Miner et al., 1997). Results presented here extend these phenomena to muscle. First, as in kidney (Miner et al., 1997), individual adult intramuscular BLs can express one (extrasynaptic: α2), two (perineurial: α4+5), or three (synaptic: α2+4+5) α chains, along with a single β and γ chain. Second, the α and β chain complements of individual BLs can change as development proceeds. For example, extrasynaptic muscle BL may progress through at least three compositions with regard to its α chains (α1+2 to α2+5, to α2+4+5, and then to α2). Likewise, in renal glomerular BL, developmental transitions occur in collagen IV chains (α1+2 to α1–5, and then to α3–5), laminin α chains (α1+4 to α1+4+5 to α4+5, and then to α5), and laminin β chains (β1 to β1+2, and then to β2) (Ekblom et al., 1990; Miner and Sanes, 1994; Virtanen et al., 1995; Miner et al., 1997). Third, loss of a single laminin chain from muscle leads to an apparently compensatory appearance of others. For example, decreased expression of α2 in muscle and endoneurial BLs in α2dy/dy mice leads to increased levels of α4, and loss of β2 from synaptic BL leads to increased levels of β1. These changes are reminiscent of those seen in kidney, where deletion of laminin β2 or collagen IV α3–5 from glomerular BL results in increased levels of laminin β1 or collagen IV α1+2, respectively (Kashtan and Kim, 1992; Noakes et al., 1995b ; Cosgrove et al., 1996; Miner and Sanes, 1996). Interestingly, in all of these cases, the compensating chain is one that was normally expressed in embryos and then lost in adults; whether the compensation is best viewed as reexpression or retention of the embryonic phenotype will require further studies of mechanism. Finally, loss of laminin β2 from synaptic and vascular BLs leads to coordinate loss of α5. Likewise, in kidney, mutations in genes encoding any of three collagen IV chains, α3–5, leads to loss of all three chains from glomerular BL (Gubler et al., 1995; Cosgrove et al., 1996; Miner and Sanes, 1996). Together, these results suggest that colocalization of multiple isoforms, isoform transitions during development, and compensation and coregulation in specific deficiency states represent general features of BL assembly and maintenance.
These complexities in composition, development, and regulation are important in considering the roles that laminins play in formation and maintenance of nerve, muscle, and synapse. Potential roles of synaptic laminins are discussed above. As regards muscle, the upregulation or retention of α4 in α2dy/dy mice may be of particular interest. The α2dy/dy mouse exhibits severe muscular dystrophy, and has long been used as an animal model of human dystrophies. The recent findings that the α2 gene is mutated both in α2dy/dy mice and in some humans with congenital dystrophies (Hayashi et al., 1993; Sunada et al., 1994, 1995; Xu et al., 1994b ; Helbling-Leclerc et al., 1995; Nissinen et al., 1996) demonstrates that it is a genotypically as well as phenotypically valid model of human disease. In humans with α2 deficiency, levels of another α-like chain are increased (Mundegen et al., 1995; Sewry et al., 1995; Connolly et al., 1996); the identity of this chain remains uncertain, but our results suggest that it might be α4. If so, this pattern would resemble that seen in dystrophin-deficient dystrophies (mdx in mice, and Duchenne and Becker in humans), in which utrophin, the autosomal homologue of dystrophin, is expressed transiently in developing normal muscles, but retained or upregulated in adult mutant muscle. Recent studies have shown that utrophin attenuates the severity of dystrophy in mdx mice, and raised the possibility that further upregulation of utrophin could be therapeutically beneficial in humans with Duchenne dystrophy (Tinsley et al., 1996; Deconinck et al., 1997; Grady et al., 1997). Likewise, it will be important to ask whether α4 partially compensates for α2 functionally as well as structurally, and whether it may provide an avenue for intervention in the human disease.
Acknowledgments
We are grateful to Dr. J. Lichtman for advice on confocal imaging, and J. Cunningham, and J. Ko for technical assistance.
This work was supported by grants from the National Institutes of Health. J.H. Miner was supported by a Damon Runyon-Walter Winchell Cancer Research Fund fellowship.
Abbreviations used in this paper
- AChR
acetylcholine receptors
- BL
basal lamina
- E
embryonic day
Footnotes
Address all correspondence to Joshua R. Sanes, Department of Anatomy and Neurobiology, Washington University School of Medicine, 660 South Euclid Avenue, St. Louis, MO 63110. Fax: (314) 747-1150. E-mail: sanesj@thalamus.wustl.edu
References
- Aberdam D, Aguzzi A, Baudoin C, Galliano MF, Ortonne JP, Meneguzzi G. Developmental expression of nicein adhesion protein (laminin-5) subunits suggests multiple morphogenic roles. Cell Adhes Commun. 1994;2:115–129. doi: 10.3109/15419069409004431. [DOI] [PubMed] [Google Scholar]
- Abrahamson DR, Irwin MH, St. John PL, Perry EW, Accavitti MA, Heck LW, Couchman JR. Selective immunoreactivities of kidney basement membranes to monoclonal antibodies against laminin: Localization of the end of the long arm and the short arms to discrete microdomains. J Cell Biol. 1989;109:3477–3491. doi: 10.1083/jcb.109.6.3477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arahata K, Hayashi YK, Koga R, Goto K, Lee JH, Miyague Y, Ishii H, Tsukahara T, Takeda S, Woo M, et al. Laminin in animal models for muscular dystrophy: defect of laminin-M in skeletal and cardiac muscles and peripheral nerve of the homozygous dystrophic dy/dy mice. Proc Jpn Acad B. 1993;69:259–264. [Google Scholar]
- Banker BQ, Hirst NS, Chester CS, Fok RY. Histometric and electron cytochemical study of muscle in the dystrophic mouse. Ann NY Acad Sci. 1979;317:115–131. [PubMed] [Google Scholar]
- Bewick GS, Nicholson LVB, Young C, O'Donnell E, Slater CR. Different distributions of dystrophin and related proteins at nerve-muscle junctions. Neuroreport. 1992;3:857–860. doi: 10.1097/00001756-199210000-00009. [DOI] [PubMed] [Google Scholar]
- Brandenberger R, Kammerer RA, Engel J, Chiquet M. Native chick laminin-4 containing the β2 chain (s-laminin) promotes motor axon growth. J Cell Biol. 1996;135:1583–1592. doi: 10.1083/jcb.135.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunge MB, Wood PM, Tynan LB, Bates ML, Sanes JR. Perineurium originates from fibroblasts: demonstration in vitro with a retroviral marker. Science. 1989;243:229–231. doi: 10.1126/science.2492115. [DOI] [PubMed] [Google Scholar]
- Burgeson RE, Chiquet M, Deutzmann R, Ekblom P, Engel J, Kleinman H, Martin GR, Meneguzzi G, Paulsson M, Sanes J, et al. A new nomenclature for the laminins. Matrix Biol. 1994;14:209–211. doi: 10.1016/0945-053x(94)90184-8. [DOI] [PubMed] [Google Scholar]
- Carbonetto S. Neuromuscular transmission in dystrophic mice. J Neurophysiol. 1977;40:836–843. doi: 10.1152/jn.1977.40.4.836. [DOI] [PubMed] [Google Scholar]
- Chen, Y.-S., M.F. Champliaud, R.E. Burgeson, M.P. Marenkovich, and P.D. Yurchenco. 1997. Self-assembly of laminin isoforms. J. Biol. Chem. In Press. [DOI] [PubMed]
- Chiu AY, Sanes JR. Development of basal lamina in synaptic and extrasynaptic portions of embryonic rat muscle. Dev Biol. 1984;103:456–467. doi: 10.1016/0012-1606(84)90333-6. [DOI] [PubMed] [Google Scholar]
- Chiu AY, Ugozolli M, Meiri K, Ko J. Purification and lectin-binding properties of s-laminin, a synaptic isoform of the laminin B1 chain. J Neurochem. 1992;59:10–17. doi: 10.1111/j.1471-4159.1992.tb08869.x. [DOI] [PubMed] [Google Scholar]
- Chung AE, Jaffe R, Freeman IL, Vergnes JP, Braginski JE, Carlin B. Properties of a basement membrane-related glycoprotein synthesized in culture by a mouse embryonal carcinoma-derived cell line. Cell. 1979;16:277–287. doi: 10.1016/0092-8674(79)90005-9. [DOI] [PubMed] [Google Scholar]
- Connolly AM, Pestronk A, Planer GJ, Yue J, Mehta S, Choksi R. Congenital muscular dystrophy syndromes distinguished by alkaline and acid phosphatase, merosin, and dystrophin staining. Neurology. 1996;46:810–814. doi: 10.1212/wnl.46.3.810. [DOI] [PubMed] [Google Scholar]
- Cosgrove D, Meehan DT, Grunkemeyer JA, Kornak JM, Sayers R, Hunter WJ, Samuelson GC. Collagen COL4α3 knockout: a mouse model for autosomal Alport syndrome. Genes Dev. 1996;10:2981–2992. doi: 10.1101/gad.10.23.2981. [DOI] [PubMed] [Google Scholar]
- Covault J, Sanes JR. Distribution of N-CAM in synaptic and extrasynaptic portions of developing and adult skeletal muscle. J Cell Biol. 1986;102:716–730. doi: 10.1083/jcb.102.3.716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covault J, Cunningham JM, Sanes JR. Neurite outgrowth on cryostat sections of innervated and denervated skeletal muscle. J Cell Biol. 1987;105:2479–2488. doi: 10.1083/jcb.105.6.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desaki J, Matsuda S, Sakanaka M. Morphological changes of neuromuscular junctions in the dystrophic (dy) mouse: a scanning and transmission electron microscopic study. J Electron Microsc. 1995;44:59–65. [PubMed] [Google Scholar]
- Deconinck, A.E., J.A. Rafael, J.A. Skinner, S.C. Brown, A.C. Potter, L. Metzinger, D.J. Watt, J.G. Dickson, J.M. Tinsley, and K.E. Davies. 1997. Utrophin-dystrophin deficient mice as a model for Duchenne muscular dystrophy. Cell. In press. [DOI] [PubMed]
- Ehrig K, Leivo I, Argraves WS, Ruoslahti E, Engvall E. Merosin, a tissue-specific basement membrane protein, is a laminin-like protein. Proc Natl Acad Sci USA. 1990;87:3264–3268. doi: 10.1073/pnas.87.9.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekblom M, Klein G, Mugrauer G, Fecker L, Deutzmann R, Timpl R, Ekblom P. Transient and locally restricted expression of laminin A chain mRNA by developing epithelial cells during kidney organogenesis. Cell. 1990;60:337–346. doi: 10.1016/0092-8674(90)90748-4. [DOI] [PubMed] [Google Scholar]
- Engvall E, Earwicker D, Haaparanta T, Ruoslahti E, Sanes JR. Distribution and isolation of four laminin variants; tissue restricted distribution of heterotrimers assembled from five different subunits. Cell Regul. 1990;1:731–740. doi: 10.1091/mbc.1.10.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferns MJ, Campanelli JT, Hoch W, Scheller RH, Hall Z. The ability of agrin to cluster AChRs depends on alternative splicing and on cell surface proteoglycans. Neuron. 1993;11:491–502. doi: 10.1016/0896-6273(93)90153-i. [DOI] [PubMed] [Google Scholar]
- Fertuck HC, Salpeter MM. Quantitation of junctional and extrajunctional acetylcholine receptors by electron microscope autoradiography after 125I-α-bungarotoxin binding at mouse neuromuscular junctions. J Cell Biol. 1976;69:144–158. doi: 10.1083/jcb.69.1.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flucher BE, Daniels MP. Distribution of Na+channels and ankyrin in neuromuscular junctions is complementary to that of acetylcholine receptors and the 43 kd protein. Neuron. 1989;3:163–175. doi: 10.1016/0896-6273(89)90029-9. [DOI] [PubMed] [Google Scholar]
- Grady RM, Teng H, Nichol MC, Cunningham JC, Wilkinson RS, Sanes JR. Skeletal and cardiac myopathies in mice lacking utrophin and dystrophin: a model for Duchenne muscular dystrophy. Cell. 1997;90:729–738. doi: 10.1016/s0092-8674(00)80533-4. [DOI] [PubMed] [Google Scholar]
- Green TL, Hunter DD, Chan W, Merlie JP, Sanes JR. Synthesis and assembly of the synaptic cleft protein S-laminin by cultured cells. J Biol Chem. 1992;267:2014–2022. [PubMed] [Google Scholar]
- Gubler M-C, Knebelmann B, Beziau A, Broyer M, Pirson Y, Haddoum F, Kleppel MM, Antignac C. Autosomal recessive Alport syndrome: immunohistochemical study of type IV collagen chain distribution. Kidney Int. 1995;47:1142–1147. doi: 10.1038/ki.1995.163. [DOI] [PubMed] [Google Scholar]
- Hayashi YK, Engvall E, Arikawa HE, Goto K, Koga R, Nonaka I, Sugita H, Arahata K. Abnormal localization of laminin subunits in muscular dystrophies. J Neurol Sci. 1993;119:53–64. doi: 10.1016/0022-510x(93)90191-z. [DOI] [PubMed] [Google Scholar]
- Helbling-Leclerc A, Zhang X, Topaloglu H, Cruaud C, Tesson F, Weissenbach J, Tome FM, Schwartz K, Fardeau M, Tryggvason K, et al. Mutations in the laminin α2-chain gene (LAMA2) cause merosin-deficient congenital muscular dystrophy. Nat Genet. 1995;11:216–218. doi: 10.1038/ng1095-216. [DOI] [PubMed] [Google Scholar]
- Hunter DD, Shah V, Merlie JP, Sanes JR. A laminin-like adhesive protein concentrated in the synaptic cleft of the neuromuscular junction. Nature. 1989a;338:229–234. doi: 10.1038/338229a0. [DOI] [PubMed] [Google Scholar]
- Hunter DD, Porter BE, Bulock JW, Adams SP, Merlie JP, Sanes JR. Primary sequence of a motor neuron-selective adhesive site in the synaptic basal lamina protein S-laminin. Cell. 1989b;59:905–913. doi: 10.1016/0092-8674(89)90613-2. [DOI] [PubMed] [Google Scholar]
- Jaakkola S, Savunen O, Halme T, Uitto J, Peltonen J. Basement membranes during development of human nerve: Schwann cells and perineurial cells display marked changes in their expression profiles for laminin subunits and β1 and β4 integrins. J Neurocytol. 1993;22:215–230. doi: 10.1007/BF01246360. [DOI] [PubMed] [Google Scholar]
- Kashtan CE, Kim Y. Distribution of the α1 and α2-chains of collagen IV and of collagens V and VI in Alport syndrome. Kidney Int. 1992;42:115–126. doi: 10.1038/ki.1992.269. [DOI] [PubMed] [Google Scholar]
- Kelly AM, Zacks SI. The histogenesis of rat intercostal muscle. J Cell Biol. 1969a;42:135–153. doi: 10.1083/jcb.42.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AM, Zacks SI. The fine structure of motor endplate morphogenesis. J Cell Biol. 1969b;42:154–169. doi: 10.1083/jcb.42.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll TG, Peters BP, Hustad CM, Jones PA, Killen PD, Ruddon RW. Expression of laminin chains during myogenic differentiation. J Biol Chem. 1994;269:9270–9277. [PubMed] [Google Scholar]
- Lagenaur C, Lemmon V. An L1-like molecule, the 8D9 antigen, is a potent substrate for neurite extension. Proc Natl Acad Sci USA. 1987;84:7753–7757. doi: 10.1073/pnas.84.21.7753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law PK, Saito A, Fleischer S. Ultrastructural changes in muscle and motor end-plates of the dystrophic mouse. Exp Neurol. 1983;80:361–382. doi: 10.1016/0014-4886(83)90289-3. [DOI] [PubMed] [Google Scholar]
- Leivo I, Engvall E. Merosin, a protein specific for basement membranes of Schwann cells, striated muscle, and trophoblast, is expressed late in nerve and muscle development. Proc Natl Acad Sci USA. 1988;85:1544–1548. doi: 10.1073/pnas.85.5.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinkovich MP, Lunstrum GP, Burgeson RE. The anchoring filament protein kalinin is synthesized and secreted as a high molecular weight precursor. J Biol Chem. 1992;267:17900–17906. [PubMed] [Google Scholar]
- Martin PT, Ettinger AJ, Sanes JR. A synaptic localization domain in the synaptic cleft protein laminin β2 (s-laminin) Science. 1995;269:413–416. doi: 10.1126/science.7618109. [DOI] [PubMed] [Google Scholar]
- Merrill GF. Clonal derivation of a rat muscle cell strain that forms contraction-competent myotubes. In Vitro Cell Dev Biol. 1989;25:471–476. doi: 10.1007/BF02624635. [DOI] [PubMed] [Google Scholar]
- Miner JH, Sanes JR. Collagen IV α3, α4, and α5 chains in rodent basal laminae: Sequence, distribution, association with laminins, and developmental switches. J Cell Biol. 1994;127:879–891. doi: 10.1083/jcb.127.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JH, Sanes JR. Molecular and functional defects in kidneys of mice lacking collagen (α3)IV: Implications for alport syndrome. J Cell Biol. 1996;135:1403–1413. doi: 10.1083/jcb.135.5.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miner JH, Patton BL, Lentz SI, Gilbert DJ, Snider WD, Jenkins NA, Copeland NG, Sanes JR. The laminin α chains: Expression, developmental transitions, and chromosomal locations of α1-5, identification of heterotrimeric laminins 8-11, and cloning of a novel α3 isoform. J Cell Biol. 1997;137:685–701. doi: 10.1083/jcb.137.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundegar RR, von Oertzen J, Zierz S. Increased laminin A expression in regenerating myofibers in neuromuscular disorders. Muscle & Nerve. 1995;18:992–999. doi: 10.1002/mus.880180911. [DOI] [PubMed] [Google Scholar]
- Nishi R, Berg DK. Two components from eye tissue that differentially stimulate the growth and development of ciliary ganglion neurons in cell culture. J Neurosci. 1981;1:505–513. doi: 10.1523/JNEUROSCI.01-05-00505.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissinen M, Helbling LA, Zhang X, Evangelista T, Topaloglu H, Cruaud C, Weissenbach J, Fardeau M, Tome FM, Schwartz K, et al. Substitution of a conserved cysteine-996 in a cysteine-rich motif of the laminin α2-chain in congenital muscular dystrophy with partial deficiency of the protein. Am J Hum Genet. 1996;58:1177–1184. [PMC free article] [PubMed] [Google Scholar]
- Noakes PG, Phillips WD, Hanley TA, Sanes JR, Merlie JP. 43K protein and acetylcholine receptors colocalize during the initial stages of neuromuscular synapse formation in vivo. Dev Biol. 1993;155:275–280. doi: 10.1006/dbio.1993.1025. [DOI] [PubMed] [Google Scholar]
- Noakes PG, Gautam M, Mudd J, Sanes JR, Merlie JP. Aberrant differentiation of neuromuscular junctions in mice lacking s-laminin/ laminin β2. Nature. 1995a;374:258–262. doi: 10.1038/374258a0. [DOI] [PubMed] [Google Scholar]
- Noakes PG, Miner JH, Gautam M, Cunningham JM, Sanes JR, Merlie JP. The renal glomerulus of mice lacking s-laminin/laminin β2: nephrosis despite molecular compensation by laminin β1. Nat Genet. 1995b;10:400–406. doi: 10.1038/ng0895-400. [DOI] [PubMed] [Google Scholar]
- Porter BE, Sanes JR. Gated migration: neurons migrate on but not onto substrates containing S-laminin. Dev Biol. 1995;167:609–616. doi: 10.1006/dbio.1995.1052. [DOI] [PubMed] [Google Scholar]
- Porter BE, Weis J, Sanes JR. A motoneuron-selective stop signal in the synaptic cleft protein S-laminin. Neuron. 1995;14:549–559. doi: 10.1016/0896-6273(95)90311-9. [DOI] [PubMed] [Google Scholar]
- Rosen GD, Sanes JR, LaChance R, Cunningham JM, Roman J, Dean DC. Roles for the integrin VLA-4 and its counter receptor VCAM-1 in myogenesis. Cell. 1992;69:1107–1119. doi: 10.1016/0092-8674(92)90633-n. [DOI] [PubMed] [Google Scholar]
- Sanes, J.R. 1994. The extracellular matrix. In Myology. 2nd edition, A.G. Engel, and C. Franzini-Armstrong, editors. McGraw-Hill Inc., New York. 242– 260.
- Sanes JR. The synaptic cleft of the neuromuscular junction. Semin Dev Biol. 1995;6:163–173. [Google Scholar]
- Sanes JR, Chiu AY. The basal lamina of the neuromuscular junction. Cold Spring Harbor Symp Quant Biol. 1983;48:667–678. doi: 10.1101/sqb.1983.048.01.070. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Lawrence JJ. Activity-dependent accumulation of basal lamina by cultured rat myotubes. Dev Biol. 1983;97:123–136. doi: 10.1016/0012-1606(83)90070-2. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Marshall LM, McMahan UJ. Reinnervation of muscle fiber basal lamina after removal of myofibers. Differentiation of regenerating axons at original synaptic sites. J Cell Biol. 1978;78:176–198. doi: 10.1083/jcb.78.1.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR, Schachner M, Covault J. Expression of several adhesive macromolecules (N-CAM, L1, J1, NILE, uvomorulin, laminin, fibronectin, and a heparan sulfate proteoglycan) in embryonic, adult, and denervated adult skeletal muscle. J Cell Biol. 1986;102:420–431. doi: 10.1083/jcb.102.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR, Engvall E, Butkowski R, Hunter DD. Molecular heterogeneity of basal laminae: Isoforms of laminin and collagen IV at the neuromuscular junction and elsewhere. J Cell Biol. 1990;111:1685–1699. doi: 10.1083/jcb.111.4.1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler F, Sorokin LM. Expression of laminin isoforms in mouse myogenic cells in vitro and in vivo. J Cell Sci. 1995;108:3795–3805. doi: 10.1242/jcs.108.12.3795. [DOI] [PubMed] [Google Scholar]
- Sewry CA, Philpot J, Mahony D, Wilson LA, Muntoni F, Dubowitz V. Expression of laminin subunits in congenital muscular dystrophy. Neuromuscul Disord. 1995;5:307–316. doi: 10.1016/0960-8966(94)00072-h. [DOI] [PubMed] [Google Scholar]
- Silberstein L, Inestrosa NC, Hall ZW. Aneural muscle cell cultures make synaptic basal lamina components. Nature. 1982;295:143–145. doi: 10.1038/295143a0. [DOI] [PubMed] [Google Scholar]
- Sorokin LM, Conzelmann S, Ekblom P, Battaglia C, Aumailley M, Timpl R. Monoclonal antibodies against laminin A chain fragment E3 and their effects on binding to cells and proteoglycan and on kidney development. Exp Cell Res. 1992;201:137–144. doi: 10.1016/0014-4827(92)90357-e. [DOI] [PubMed] [Google Scholar]
- Sunada Y, Bernier SM, Kozak CA, Yamada Y, Campbell KP. Deficiency of merosin in dystrophic dy mice and genetic linkage of laminin M chain gene to dy locus. J Biol Chem. 1994;269:13729–13732. [PubMed] [Google Scholar]
- Sunada Y, Bernier SM, Utani A, Yamada Y, Campbell KP. Identification of a novel mutant transcript of laminin α2 chain gene responsible for muscular dystrophy and dysmyelination in dy2J mice. Hum Mol Genet. 1995;4:1055–1061. doi: 10.1093/hmg/4.6.1055. [DOI] [PubMed] [Google Scholar]
- Timpl R, Rohde H, Robey PG, Rennard SI, Foidart JM, Martin GR. Laminin—a glycoprotein from basement membranes. J Biol Chem. 1979;254:9933–9937. [PubMed] [Google Scholar]
- Tinsley JM, Potter AC, Phelps SR, Fisher R, Trickett JI, Davies KE. Amelioration of the dystrophic phenotype of mdxmice using a truncated utrophin transgene. Nature. 1996;384:349–353. doi: 10.1038/384349a0. [DOI] [PubMed] [Google Scholar]
- Vachon PH, Loechel F, Xu H, Wewer UM, Engvall E. Merosin and laminin in myogenesis; specific requirement for merosin in myotube stability and survival. J Cell Biol. 1996;134:1483–1497. doi: 10.1083/jcb.134.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen I, Laitinen L, Korhonen M. Differential expression of laminin polypeptides in developing and adult human kidney. J Histochem Cytochem. 1995;43:621–628. doi: 10.1177/43.6.7769233. [DOI] [PubMed] [Google Scholar]
- Virtanen I, Laitinen A, Tani T, Paakko P, Laitinen LA, Burgeson RE, Lehto VP. Differential expression of laminins and their integrin receptors in developing and adult human lung. Am J Respir Cell Mol Biol. 1996;15:184–196. doi: 10.1165/ajrcmb.15.2.8703474. [DOI] [PubMed] [Google Scholar]
- Weaver CD, Yoshida CK, de Curtis I, Reichardt LF. Expression and in vitro function of β1-integrin laminin receptors in the developing avian ciliary ganglion. J Neurosci. 1995;15:5275–5285. doi: 10.1523/JNEUROSCI.15-07-05275.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Christmas P, Wu XR, Wewer UM, Engvall E. Defective muscle basement membrane and lack of M-laminin in the dystrophic dy/dy mouse. Proc Natl Acad Sci USA. 1994a;91:5572–5576. doi: 10.1073/pnas.91.12.5572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Wu XR, Wewer UM, Engvall E. Murine muscular dystrophy caused by a mutation in the laminin α2 (Lama2) gene. Nat Genet. 1994b;8:297–302. doi: 10.1038/ng1194-297. [DOI] [PubMed] [Google Scholar]
- Zhang M, McLennan IS. During secondary myotube formation, primary myotubes preferentially absorb new nuclei at their ends. Dev Dyn. 1995;204:168–177. doi: 10.1002/aja.1002040207. [DOI] [PubMed] [Google Scholar]