Abstract
The M glycoprotein from the avian coronavirus, infectious bronchitis virus (IBV), contains information for localization to the cis-Golgi network in its first transmembrane domain. We hypothesize that localization to the Golgi complex may depend in part on specific interactions between protein transmembrane domains and membrane lipids. Because the site of sphingolipid synthesis overlaps the localization of IBV M, we asked whether perturbation of sphingolipids affected localization of IBV M. Short-term treatment with two inhibitors of sphingolipid synthesis had no effect on localization of IBV M or other Golgi markers. Thus, ongoing synthesis of these lipids was not required for proper localization. Surprisingly, a third inhibitor, d,l-threo-1-phenyl-2-decanoylamino-3-morpholino- 1-propanol (PDMP), shifted the steady-state distribution of IBV M from the Golgi complex to the ER. This effect was rapid and reversible and was also observed for ERGIC-53 but not for Golgi stack proteins. At the concentration of PDMP used, conversion of ceramide into both glucosylceramide and sphingomyelin was inhibited. Pretreatment with upstream inhibitors partially reversed the effects of PDMP, suggesting that ceramide accumulation mediates the PDMP-induced alterations. Indeed, an increase in cellular ceramide was measured in PDMP-treated cells. We propose that IBV M is at least in part localized by retrieval mechanisms. Further, ceramide accumulation reveals this cycle by upsetting the balance of anterograde and retrograde traffic and/ or disrupting retention by altering bilayer dynamics.
The organelles of the classical secretory pathway must maintain their identity despite a large flux of lipids and proteins. Two models have emerged to explain how proteins can be maintained in specific compartments (Machamer, 1993; Nilsson and Warren, 1994). The retention model proposes that proteins are efficiently anchored in the appropriate compartment. The retrieval model proposes that proteins are continually recycled from later compartments. The two models are not mutually exclusive; indeed most proteins within the secretory pathway probably use both mechanisms for localization, albeit to differing extents. An example of this is the localization of the ER resident protein BiP, which contains a KDEL retrieval signal but is only slowly secreted when this signal is removed (Munro and Pelham, 1987). This suggests that other parts of the molecule may contain retention information.
The M glycoprotein from the avian coronavirus, infectious bronchitis virus (IBV),1 is a model protein for studying localization to the early secretory pathway. Immunoelectron microscopy showed that IBV M expressed in the absence of other IBV proteins was found in the tubulo- vesicular structures at the entry or cis face of the Golgi stack, as well as the first or second cisterna of the Golgi stack (Machamer et al., 1990; Sodeik et al., 1993). We will refer to this region as the cis-Golgi network (CGN; Mellman and Simons, 1992). Defined in this way, we would consider the CGN to at least partially overlap with the intermediate compartment (IC), defined by such markers as ERGIC-53 or p58 (Schweizer et al., 1988; Lahtinen et al., 1996). The first transmembrane domain of IBV M is sufficient to target chimeric proteins to the CGN (Swift and Machamer, 1991; Machamer et al., 1993). Although the transmembrane domains of other Golgi proteins also contain targeting information, no consensus motif for localization has been identified (for review see Colley, 1997). We are intrigued by the possibility that the targeting of Golgi membrane proteins may in part depend on interactions between their transmembrane domains and specific membrane lipids.
Independent studies demonstrate that the lipid compositions of membranes differ at each stage of the secretory pathway (Keenan and Morre, 1970; Cluett et al., 1997; for review see van Meer, 1993). Sphingolipids, including sphingomyelin (SM) and glucosylceramide (GlcCer), the precursor to all gangliosides, are one class of lipids thought to increase in relative concentration through the secretory pathway. Ceramide, the precursor of sphingolipids, is synthesized in the ER. In rat liver, ceramide is converted into the different classes of sphingolipids by enzymes localized to the CGN and the cis- and medial-Golgi cisternae (Futerman et al., 1990; Futerman and Pagano, 1991). When expressed from a recombinant vaccinia virus, IBV M is localized to the CGN in several cell types, and its localization presumably overlaps with that of SM and GlcCer synthase activities. This led us to speculate that there may be a link between sphingolipid synthesis and the localization of IBV M. To address this question we tested the effects of three sphingolipid synthesis inhibitors on the steady-state localization of IBV M. We observed a dramatic redistribution of IBV M induced by one of these inhibitors, the glucosylceramide analogue d,l-threo-1-phenyl-2-decanoylamino- 3-morpholino-1-propanol (PDMP). Use of upstream inhibitors coupled with lipid analysis suggested that the PDMP effects are mediated by the accumulation of the precursor ceramide. Because IBV M can be induced to move to the ER, we propose that IBV M is at least in part localized by retrieval mechanisms.
Materials and Methods
Materials
FCS was from Atlanta Biologicals (Norcross, GA); fumonisin B1 was from Calbiochem (La Jolla, CA); chromatography plates were from E. Merck (Darmstadt, Germany); endoglycosidase H (endo H) from New England Biolabs (Beverly, MA); N-hexanoylsphingosine (C6Cer), lipid standards, and PDMP were from Matreya (Pleasant Gap, PA); [3H]palmitate (50 Ci/mmol) was from DuPont-NEN (Wilmington, DE); Pro-Mix (>1,000 mCi/mmol [35S]methionine) was from Amersham Intl. (Arlington Heights, IL); DME, tissue culture–grade trypsin, and penicillin-streptomycin were from GIBCO BRL (Gaithersburg, MD); all other reagents were from Sigma Chemical Co. (St. Louis, MO).
Antibodies were obtained as follows: monoclonal anti-Bip, Stressgen (Victoria, BC, Canada); monoclonal anti-giantin and monoclonal anti-ERGIC-53, Hans-Peter Hauri (Basel, Switzerland); polyclonal anti–β-COP, Jennifer Lippincott-Schwartz (National Institutes of Health, Bethesda, MD); polyclonal α-mannosidase II, Marilyn Farquhar (University of California at San Diego, La Jolla, CA) and Kelley Moreman (University of Georgia, Athens, GA); polyclonal antibodies to IBV M and vesicular stomatitis virus (VSV) were prepared as described (Machamer and Rose, 1987; Weisz et al., 1993, respectively); Texas red– and FITC-conjugated secondary antibodies, Jackson ImmunoResearch (West Grove, PA).
Methods
Cell Culture.
Tissue culture cells were grown in DME supplemented with 5% (BHK-21) or 10% (Vero) FCS. Cells were grown at 37°C in an atmosphere of 5% CO2.
Pulse–Chase Labeling.
Pulse–chase experiments were performed as previously described (Rosenwald et al., 1992; Weisz et al., 1993). Briefly, cells were plated in 35-mm dishes the night before the experiment to be 90% confluent the next day. VSV (San Juan strain, Indiana serotype) was adsorbed for 30 min in 0.5 ml of serum-free DME. At 4 h after infection, the cells were starved for methionine for 15 min. Cells were then pulsed for 5 min with 50 μCi 35S-Pro-mix and chased for the indicated amount of time in the presence or absence of the indicated drugs. The isopropanol carrier alone had no effect on the rate of transport of VSV G protein. After the chase, cells were washed once with cold PBS and lysed in detergent. VSV G protein was then immunoprecipitated, treated with endo H as described (Rosenwald et al., 1992), separated by SDS-PAGE, and visualized by fluorography. Endo H–sensitive and -resistant forms of the VSV G protein were quantitated by densitometry.
Indirect Immunofluorescence Microscopy.
These experiments were performed as previously described (Swift and Machamer, 1991). Briefly, cells were infected for 30 min with a recombinant vaccinia virus encoding IBV M, and the indicated treatments were begun 4 h after infection. For experiments using exogenous ceramides, the soluble short-chain analogues of ceramide were added to cells as a complex with 0.34 mg/ml defatted BSA as described (Pagano and Martin, 1988). After treatment, cells were fixed, permeabilized, and stained with the appropriate antibody. Images were acquired using a microscope (Axioskop; Zeiss, Inc., Thornwood, NY) equipped with epifluorescence and a CCD camera (Photometrics Sensys, Tucson, AZ) using IP Lab software (Signal Analytics Corp., Vienna, VA). All images shown are the raw data collected at 1×1 binning with a gain of 1.
Lipid Synthesis Assays.
Cells were seeded onto 6-cm plastic dishes 2 d before the experiment so that they were 90% confluent the day of the experiment. Cells were incubated in DME supplemented with 50 μg/ml cycloheximide, and half of the dishes were also incubated with 5 mM βCA. 1 h later, fresh medium containing cycloheximide and [3H]palmitate (100 μCi/ dish) was added, along with either 1% isopropanol (control and βCA) or 100 μM PDMP and 5 mM βCA if indicated. 1 h later, cells were trypsinized and washed off the plate in 0.8 ml cold PBS. To normalize lipid levels, 50 μl were removed for a protein assay by the method of Bradford (1976). Lipids were then extracted by the method of Bligh and Dyer (1959). Labeled samples were doped with 10 μg cold ceramide to allow for visualization. Samples were run on 10 × 10 cm high performance thin-layer chromatography plates with a mobile phase of chloroform/glacial acetic acid (9:1; Abe et al., 1992). Plates were sprayed with water to visualize the ceramide bands that were scraped and counted after the addition of scintillation fluid. Alternatively, plates were dipped in 10% 4,5-diphenyloxazole in chloroform for visualization by fluorography (Henderson and Tocher, 1992).
Results
Inhibition of Sphingolipid Synthesis by PDMP but Not βCA or FB1 Causes a Mislocalization of IBV M Protein to the ER
To test whether ongoing sphingolipid synthesis was necessary for the correct localization of the IBV M protein, we used three inhibitors of sphingolipid synthesis: β-chloroalanine (βCA; Medlock and Merrill, 1988), fumonisin B1 (FB1; Wang et al., 1991), and PDMP (Vunnam and Radin, 1980; Inokuchi and Radin, 1987). The biosynthetic pathway for sphingolipids with the sites of inhibition of these drugs is shown in Fig. 1. Both βCA (5 mM) and FB1 (100 μM) inhibited the incorporation of radiolabeled precursors into sphingolipids in BHK-21 cells by >90% (data not shown). Consistent with previous results (Rosenwald et al., 1992), we found that PDMP (100 μM) inhibited GlcCer synthesis by ∼90% and SM synthesis by ∼50% in BHK-21 cells (data not shown).
Figure 1.
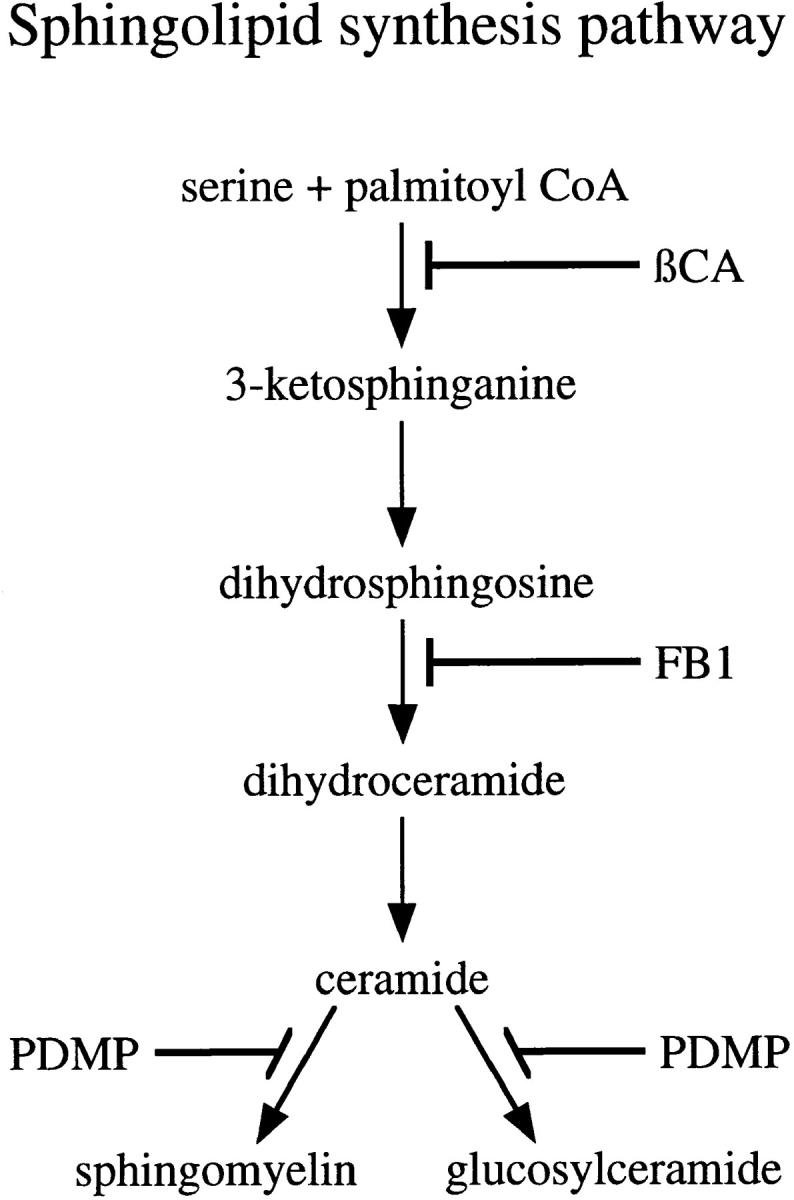
Sphingolipid synthesis pathway. The pathway for sphingolipid biosynthesis in mammalian cells and the steps inhibited by the drugs used in this study are indicated.
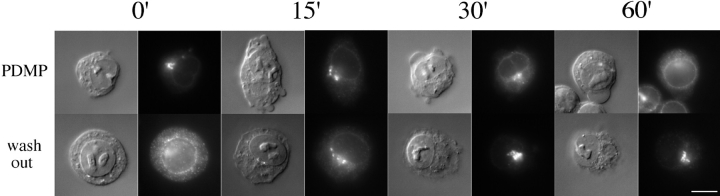
To express the IBV M protein, BHK-21 cells were infected with a recombinant vaccinia virus encoding IBV M (Machamer and Rose, 1987). 4 h after infection the cells were treated with cycloheximide for 1 h to chase newly synthesized M protein out of the ER. Sphingolipid synthesis inhibitors were then added and the cells incubated for another 1 h before processing for immunofluorescence (Fig. 2). In control cells, IBV M exhibited a tight, juxtanuclear staining pattern that colocalized with Golgi markers. The Golgi localization pattern of IBV M was unchanged by treatment with either βCA or FB1, suggesting that ongoing sphingolipid synthesis is not required for proper localization of IBV M. In contrast, PDMP-treated cells showed a marked change in the staining pattern of the IBV M protein after 1 h. The localization of IBV M in the presence of PDMP changed from Golgi to ER based on the absence of strong juxtanuclear staining and the presence of nuclear rim staining and a tubulo-reticular staining pattern that colocalized with ER markers. Treatment of infected cells with lower concentrations of PDMP had no effect on IBV M localization and very little effect on SM synthesis (data not shown). We next tested the kinetics of PDMP-induced mislocalization of IBV M. Cells were infected as before and treated for 1 h with cycloheximide. Then cells were treated with PDMP for varying lengths of time up to 1 h (Fig. 3). Redistribution was first observed at 15 min and became maximal at 60 min. This redistribution was rapidly reversible, such that 30 min after washing out PDMP, IBV M had moved back to the Golgi region.
Figure 2.

PDMP but not βCA or FB1 mislocalize IBV M to the ER. BHK-21 cells infected with a recombinant vaccinia virus encoding IBV M were treated 4 h after infection with 50 μg/ml cycloheximide for 1 h. Either 5 mM βCA, 100 μM FB1, or 100 μM PDMP was then added to the indicated samples for 1 h, at which time the cells were fixed and prepared for indirect immunofluorescence with antibodies directed against IBV M and Texas red secondary antibodies. For each experimental group, the Nomarski image is shown on the left and the fluorescence image on the right. Bar, 10 μm.
Figure 3.
The redistribution of IBV M induced by PDMP was rapid and reversible. BHK-21 cells infected with a recombinant vaccinia virus encoding IBV M were treated 4 h after infection with 50 μg/ml cycloheximide for 1 h. PDMP (100 μM) was added, and dishes were fixed at the indicated times and prepared for indirect immunofluorescence. After 1 h in PDMP, the remaining dishes were washed three times with 1 ml DME with serum and incubated with cycloheximide-containing medium for the indicated time before being prepared for indirect immunofluorescence. For each experimental series, the Nomarski image is shown on the left and the fluorescence image on the right. Bar, 10 μm.
PDMP Treatment Does Not Mislocalize Golgi Markers, but Does Mislocalize ERGIC-53
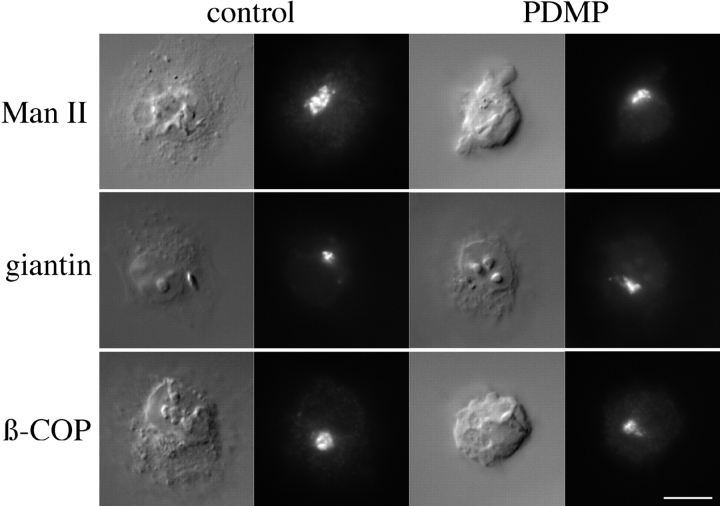
We next asked if PDMP mislocalized other Golgi markers. Cells were infected and treated as described above and then stained with antibodies to two integral membrane proteins of the Golgi stack, giantin (Linstedt and Hauri, 1993) and mannosidase II (Man II; Moremen and Robbins, 1991; Velasco et al., 1993). Treatment of cells with 100 μM PDMP for 1 h had no effect on the localization of either protein (Fig. 4), suggesting that the morphology of the Golgi was not greatly altered. We also examined the localization of β-COP, a peripheral membrane protein of the stack and CGN involved in vesicular traffic (Oprins et al., 1993). As seen in Fig. 4, the distribution of β-COP appeared unaffected in treated cells.
Figure 4.
PDMP did not affect localization of endogenous Golgi markers. BHK-21 cells were incubated for 1 h with cycloheximide and then incubated for 1 h in medium with 1% isopropanol (control) or 100 μM PDMP. Cells were then fixed and prepared for indirect immunofluorescence with antibodies to Man II, giantin, or β-COP and Texas red secondary antibodies. For each experimental series, the Nomarski image is shown on the left and the fluorescence image on the right. Bar, 10 μm.
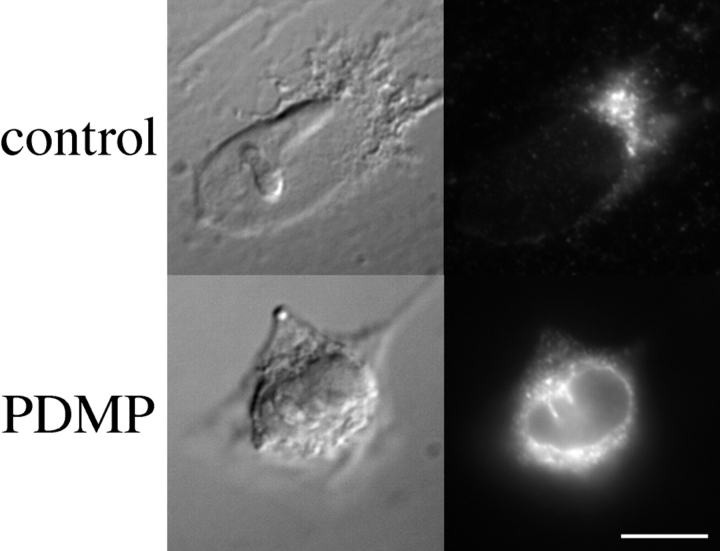
We then looked at a protein which cycles through the cis-Golgi. As we did not have access to antibodies that crossreact with known markers in BHK-21 cells, we examined ERGIC-53 in Vero cells. IBV M localizes to the CGN in these cells and is also redistributed to the ER by PDMP (data not shown). ERGIC-53 is an IC protein that cycles between the ER, IC, and Golgi stack (Lippincott-Schwartz et al., 1990; Schindler et al., 1993). Vero cells were treated with cycloheximide for 1 h and then with or without 100 μM PDMP for an additional hour before being fixed and prepared for immunofluorescence (Fig. 5). Similar to the effects on IBV M, PDMP treatment shifted the steady-state distribution of ERGIC-53 to the ER. In addition, neither βCA nor FB1 had any effect on the localization of ERGIC-53 (data not shown).
Figure 5.
PDMP induced the redistribution of the endogenous IC protein, ERGIC-53, to the ER. Vero cells were incubated with cycloheximide for 1 h and then incubated for 1 h in the presence either 1% isopropanol (control) or 100 μM PDMP. Cells were then fixed and prepared for indirect immunofluorescence with antibodies to ERGIC-53. For each experimental group, the Nomarski image is shown on the left and the fluorescence image on the right. Bar, 10 μm.
PDMP but Not βCA or FB1 Slows the Anterograde Traffic of VSV G
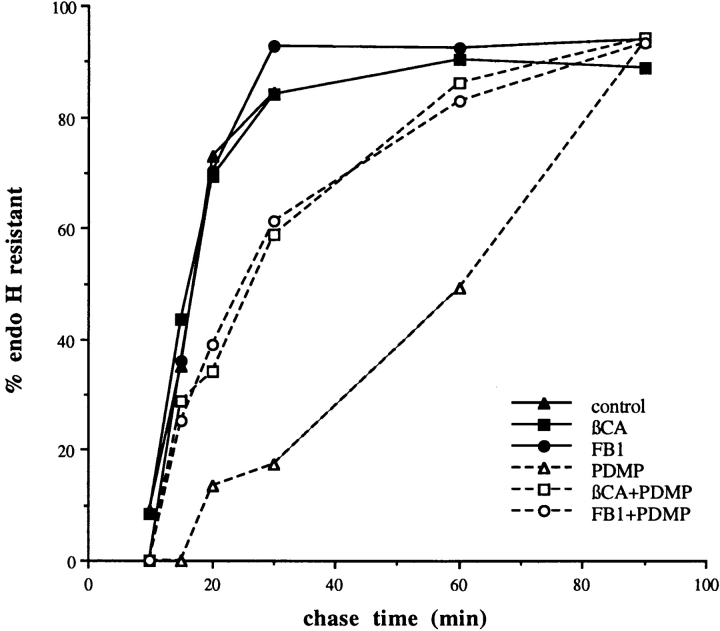
The above immunofluorescence results suggested that PDMP might generally alter the distribution of proteins that have a dynamic localization mechanism. Interestingly, it has been shown that PDMP slows the rate of both anterograde vesicular traffic (Rosenwald et al., 1992) and endocytosis (Chen et al., 1995) in CHO cells. We tested whether PDMP also slowed anterograde traffic in BHK-21 cells. We used a plasma membrane protein, the well-characterized G protein from VSV. A pulse–chase experiment was performed either in the presence or absence of PDMP, βCA, or FB1. The rate of accumulation of endo H–resistant VSV G was used as an assay for the rate of arrival at the medial-Golgi. Neither βCA nor FB1 had any effect on the rate of anterograde traffic. However, PDMP increased the half-time for transport by ∼2.5-fold from 18 to 44 min (Fig. 6).
Figure 6.
Effect of sphingolipid synthesis inhibitors on transit of VSV G to the medial-Golgi. BHK-21 cells were infected with VSV. At 3.25 h after infection, cells were incubated in the presence or absence of 5 mM βCA or 100 μM FB1. At 4 h after infection, cells were pulse labeled and chased in the presence or absence of 100 μM PDMP for the indicated times. Cells were lysed with detergent and VSV G was immunoprecipitated. After treatment of immunoprecipitates with endo H, samples were electrophoresed and autoradiographed. Scanning densitometry was used to quantitate the amount of endo H–resistant VSV G at each time point. The data shown are from one representative experiment. PDMP, but not βCA or FB1, slowed arrival of VSV G at the medial-Golgi by 2.5-fold. Pretreatment with either βCA or FB1 partially corrected the PDMP effect.
The PDMP Effect on Anterograde Traffic Is Ameliorated by Pretreatment with Either βCA or FB1
Fig. 6 shows that neither βCA nor FB1 had any effect on the transit rate of VSV G, suggesting that the decreased rate with PDMP was not due to a block in the ongoing synthesis of sphingolipids. PDMP inhibits the conversion of ceramide into glycosphingolipids and sphingomyelin. One possible explanation for the effects observed with PDMP was an accumulation of the precursor ceramide. βCA and FB1 act upstream of PDMP (Fig. 1), and the intermediates in sphingolipid synthesis between these steps and ceramide production are thought to be short lived (Merrill and Wang, 1986; Medlock and Merril, 1988). To ask if ceramide accumulation might mediate the PDMP-induced slowing of anterograde traffic, cells were treated with βCA or FB1 before PDMP. We performed a pulse–chase labeling experiment with cells pretreated with either βCA or FB1 for 1 h before being chased in the presence or absence of βCA or FB1 and PDMP (Fig. 6). Pretreatment with the earlier inhibitors reduced the PDMP-induced slowing of anterograde traffic by about half, suggesting that accumulation of newly synthesized ceramide at least partially mediates this effect.
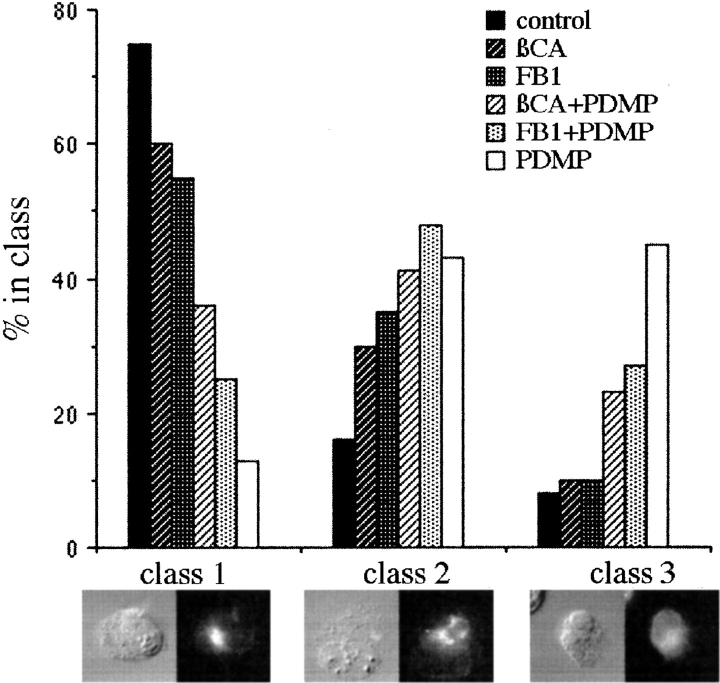
Pretreatment with βCA or FB1 Reduces the PDMP-induced Mislocalization of IBV M Protein
To test whether ceramide was likely to mediate the PDMP-induced mislocalization of IBV M, indirect immunofluorescence was performed as above with cells pretreated with either βCA or FB1 for 1 h before the addition of PDMP. To simplify the quantification, the localization of IBV M was classified into one of three staining patterns. Class 1 was the most commonly seen pattern in untreated cells, i.e., tight juxta-nuclear staining that colocalized with Golgi markers. Class 3 was the most commonly seen pattern in PDMP-treated cells, i.e., diffuse staining with prominent nuclear envelope staining that colocalized with ER markers. Class 2 was intermediate between the class 1 and class 3. Whether this pattern represents an overlap of ER and Golgi staining patterns or an increase in IC staining is not clear. For these experiments, coded samples were quantified by counting at least 100 cells for each sample. The experiment was performed five times, with one representative experiment shown (Fig. 7). Interestingly, when cells were quantified in this way, we observed that both βCA and FB1 caused a slight shift from class 1 to class 2. The significance of this observation is not clear. PDMP dramatically shifted the distribution of IBV M from mainly class 1 in the control cells to mainly class 3. Pretreatment with either βCA or FB1 significantly reduced this shift in the staining pattern of IBV M, suggesting that accumulation of newly synthesized ceramide mediates the effects of PDMP on localization of IBV M.
Figure 7.
Effect of pretreatment with βCA and FB1 on PDMP-induced redistribution of IBV M. Cells were prepared for immunofluorescence as described in Fig. 2 with the exception that indicated samples were treated with either 5 mM βCA or 100 μM FB1 beginning with the cycloheximide chase 4 h after infection. At 5 h after infection, cells were treated with 1% isopropanol (control) or 100 μM PDMP. 1 h later the cells were fixed and prepared for indirect immunofluorescence. Random fields from coded samples were scored for the number of IBV M–expressing cells that fell into the three classes of staining pattern shown. Treating cells with FB1 or βCA before PDMP shifted the IBV M staining pattern towards the control pattern.
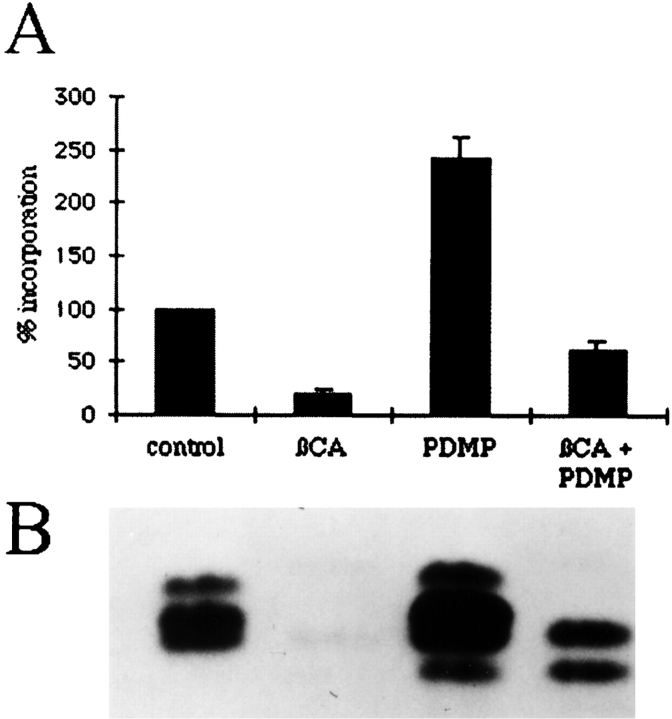
PDMP Increases Cellular Ceramide Levels
The pretreatment experiments suggested that increased levels of ceramide mediate the PDMP-induced effects on IBV M localization. Ceramide can act as a second messenger (for review see Hannun, 1994) and has been suggested to be the mediator of some of the effects of PDMP in CHO cells (Rosenwald and Pagano, 1993). Furthermore, in several systems, cellular levels of ceramide increased upon treatment with PDMP or one of its more soluble analogues (for example see Rani et al., 1995; Posse de Chaves et al., 1997). We measured the levels of ceramide after various treatments by pulse–labeling with [3H]palmitate. After labeling and treatment, cellular lipids were extracted and run on chromatography plates. The appropriate spots corresponding to ceramide were scraped and counted. Consistent with our hypothesis, Fig. 8 A shows that PDMP treatment increased the levels of ceramide 2.5-fold over control levels. Pretreatment with βCA reduced PDMP- induced ceramide accumulation to roughly half of control levels, but this level was still threefold higher than cells treated with βCA alone. Fig. 8 B is a representative fluorograph showing the ceramide region that was quantified in Fig. 8 A. Interestingly, we noticed the appearance of a lower migrating ceramide band in PDMP-treated cells.
Figure 8.
Treatment with PDMP increased the level of newly synthesized ceramide by 2.5-fold. BHK-21 cells were incubated with 50 μg/ml cycloheximide and, if indicated, 5 mM βCA. After 1 h, media was replaced with medium containing cycloheximide, 100 μCi [3H]palmitate (50 Ci/mmol), βCA if indicated, and either 1% isopropanol (control and βCA) or 100 μM PDMP. After 1 h, cells were trypsinized to remove them from the dish and the lipids were extracted as described. (A) The spots corresponding to ceramides on the chromatography plates were scraped, extracted, and counted. Four experiments were performed, with duplicate plates for each. Shown are the mean values ± SEM. (B) A representative fluorogram showing the ceramide region of the chromatography plate.
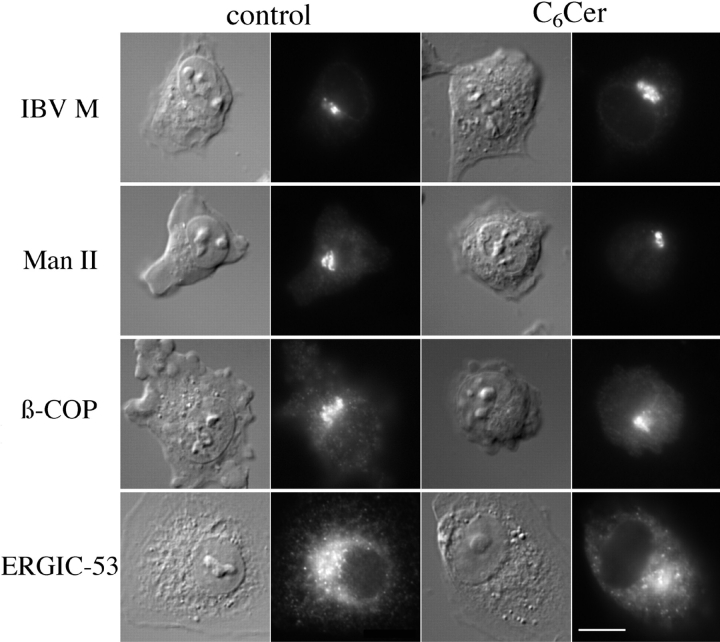
A Soluble Analogue of Ceramide Does Not Affect the Localization of IBV M or ERGIC-53
The pretreatment experiments as well as lipid analysis implicated ceramide as the mediator of PDMP effects. If this were true, addition of a soluble, short-chain analogue of ceramide might mimic the effects of PDMP. Indeed, one such cell-permeable analogue of ceramide, N-hexanoylsphingosine (C6Cer), reproduces the effects of PDMP on anterograde traffic of VSV G in CHO cells (Rosenwald and Pagano, 1993). We first asked whether C6Cer slowed anterograde traffic in BHK-21 cells. A pulse–chase labeling experiment with VSV-infected cells was performed, with cells chased in the absence or presence of 25 μM C6Cer. C6Cer slowed the rate of anterograde traffic in BHK-21 cells ∼2.5-fold (data not shown). We went on to use C6Cer in our indirect immunofluorescence assay. As expected, C6Cer had no effect on the localization of either Man II or β-COP (Fig. 9). However, contrary to our expectation, C6Cer did not alter the localization of either IBV M or ERGIC-53. This suggests that exogenously added C6Cer and ceramide generated by treatment with PDMP do not have the same effects. Two other soluble analogues of ceramide, C2Cer and C8Cer, were also tested and found to have no effect on the localization of IBV M (data not shown).
Figure 9.
Exogenous C6Cer did not redistribute IBV M or ERGIC-53. BHK cells (IBV M, Man II, and β-COP) or Vero cells (ERGIC-53) were infected with a recombinant vaccinia virus expressing IBV M. 4 h after infection the cells were treated with 50 μg/ml cycloheximide. At 5 h after infection cells were incubated for 1 h in serum-free DME with 0.34 mg/ml defatted BSA with or without 25 μM C6Cer. Cells were then prepared for indirect immunofluorescence and stained with the indicated antibodies. For each experimental series, the Nomarski image is shown on the left and the fluorescence image on the right. Bar, 10 μm.
Discussion
Steady-State Localization of IBV M Involves Cycling through the ER
There are two possible mechanisms to maintain the steady-state localization of proteins to specific sites along the secretory pathway: (a) retention in the particular compartment, and (b) retrieval from other compartments (Machamer, 1993; Nilsson and Warren, 1994). The redistribution of IBV M induced by PDMP was surprising, as we expected that if we disrupted its localization, it would move with “bulk flow” to the plasma membrane. That IBV M was redistributed to the ER suggests that it has specific targeting information for retrieval to the ER. Coupled with earlier work in our laboratory on the role of oligomerization of IBV M chimeras in CGN targeting (Weisz et al., 1993), our findings suggest that IBV M maintains its steady-state distribution by both retention and retrieval (Fig. 10 A). We propose two models to explain the redistribution of IBV M protein induced by PDMP. The simplest model (Fig. 10 B, left arrow) is that the M protein normally cycles between the ER and the Golgi at a significant rate, as has been shown for ERGIC-53 (Lippincott-Schwartz et al., 1990; Schindler et al., 1993). The slowing of anterograde traffic would cause the protein to shift its steady-state distribution to the ER, assuming that retrograde traffic is unaffected or perhaps increased by PDMP. A second possibility is that PDMP disrupts retention of the M protein, which then cycles back to the ER by normal retrieval mechanisms (Fig. 10 B, right arrow). These two models are not mutually exclusive, and both effects of PDMP may be necessary to redistribute IBV M to the extent observed.
Figure 10.
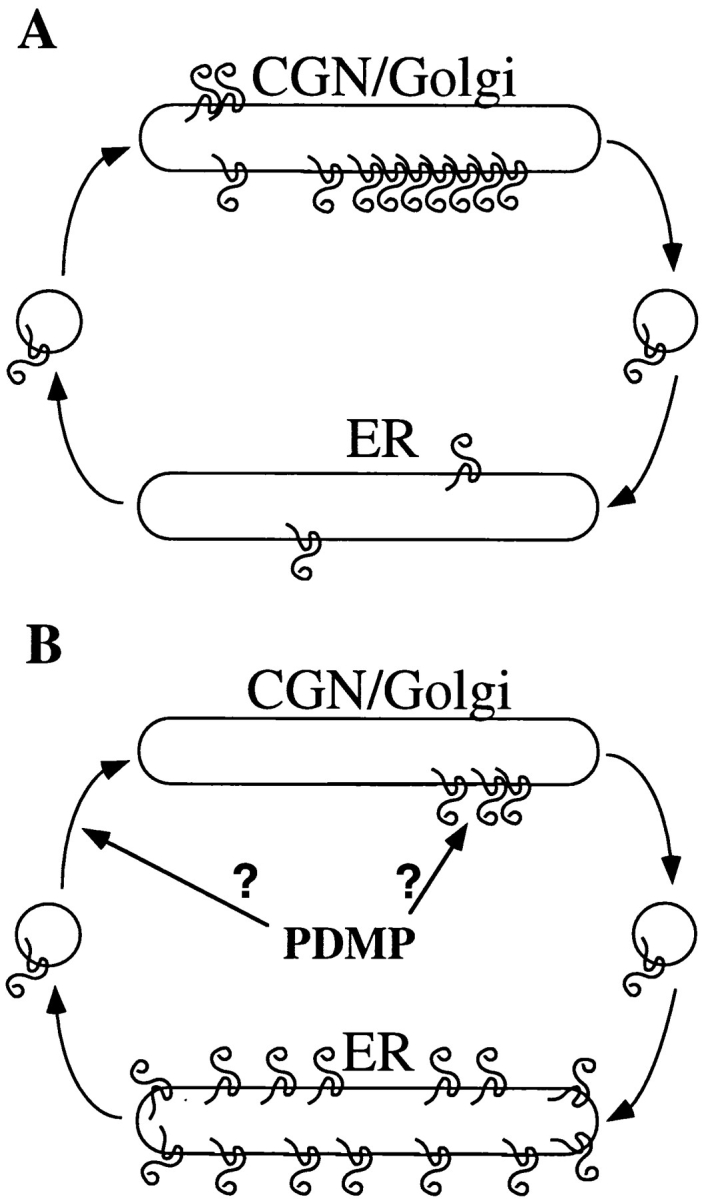
Current models for how IBV M maintains its steady-state distribution and how PDMP might alter this distribution. (A) Evidence presented here shows that IBV M can be induced to redistribute to the ER, indicating that IBV M has information necessary for traffic to the ER. We propose that IBV M normally maintains its steady-state distribution by cycling through the ER at some basal rate. (B) If IBV M is cycling through the ER normally, then its localization would depend on the balance of anterograde and retrograde traffic rates. As PDMP slows anterograde traffic, IBV M could accumulate in the ER when the balance of membrane traffic is disrupted (left arrow). Alternatively, PDMP may act by disrupting the retention of IBV M, causing it to move out of the Golgi (right arrow). It is also possible that redistribution of IBV M requires both effects of PDMP.
Implicit in both models is the idea that IBV M contains retrieval information. PDMP-induced ER redistribution was also observed for ERGIC-53, a dilysine-containing IC marker known to cycle through the ER (Lippincott-Schwartz et al., 1990; Schindler et al., 1993). Double-label immunoelectron microscopy in HeLa cells showed that the distribution of ERGIC-53 and IBV M overlapped significantly, though not completely (Sodeik et al., 1993). This suggests that IBV M may in part use the same, as yet unknown, cellular machinery used by IC proteins to maintain its steady- state localization. Using deletion mutants and chimeric molecules, dissection of IBV M retention and retrieval information should be possible using PDMP as a tool. The pathway followed by IBV M during retrieval will also be important to decipher. Experiments in nontreated cells suggest that the protein may move through later Golgi compartments even though its steady-state distribution is the CGN, because its oligosaccharides are slowly processed (Machamer et al., 1990).
Interestingly, we did not observe redistribution of two Golgi stack markers (Man II and giantin) to the ER with PDMP treatment. Golgi membrane proteins were recently suggested to be highly mobile (Cole et al., 1996b ) and to cycle through the ER (Cole et al., 1996a ). Our results with PDMP suggest that cycling of these proteins does not occur during the time scale of our experiments, and/or that its predominant effect on IBV M is a loss of retention.
Ceramide Mediates the Effects of PDMP
Interestingly, it has been shown that myriocin, an inhibitor of sphingolipid synthesis thought to inhibit the same step as βCA, reduces the rate of transport of a glycosylphosphatidylinositol-linked protein but not other proteins out of the ER in yeast (Horvath et al., 1994). It would be interesting to test whether inhibition of ceramide synthesis in mammalian cells also slows the rate of transport of glycosylphosphatidylinositol-linked proteins in mammalian cells. However, neither βCA nor FB1 had an effect on the rate of anterograde traffic of VSV G or the localization of IC, CGN, or Golgi stack proteins. When we quantified the effects of these two inhibitors on the immunofluorescence staining pattern of IBV M, we did detect a slight shift toward the intermediate staining pattern (Fig. 7). Whether this represents an altered distribution pattern or subtle effects on Golgi structure caused by these inhibitors is unclear. From these results we conclude that ongoing sphingolipid synthesis is not required for either normal rates of anterograde traffic or proper localization of Golgi proteins. However, the glucosylceramide analogue PDMP, at a concentration that inhibits both GlcCer and SM synthases, causes a redistribution of IBV M to the ER and a slowing of anterograde traffic in BHK-21 cells. Why does PDMP have these effects while βCA and FB1 do not? It is likely that PDMP exerts its effects by causing the accumulation of ceramide. Other groups have found that the various effects of PDMP and its active analogues were concomitant with (Uemura et al., 1990; Shayman et al.; 1991; Rani et al., 1995) or actually caused by increases in ceramide concentrations (Abe et al., 1996; Posse de Chaves et al., 1997). In BHK-21 cells treated with 100 μM PDMP for 1 h we measured an increase in newly synthesized ceramide (Fig. 8 A), showing at least a correlation between levels of newly synthesized ceramide and the effects of PDMP on protein traffic and localization. The fact that pretreatment with either βCA or FB1, both of which block the synthesis of ceramide, can ameliorate the effects of PDMP demonstrates that ceramide is at least in part the mediator of these effects. However, the effects of PDMP are not completely abolished by pretreatment, even though βCA pretreatment reduced the level of newly synthesized ceramide by 50% compared to control. One explanation is that ceramide accumulation is only one of the ways that PDMP exerts its effects. Another possibility is that ceramide is being generated by the degradation of preexisting sphingolipids, e.g., at the cell surface or in lysosomes. This ceramide cannot be made back into sphingolipids because the enzymes SM and GlcCer synthases are inhibited in the presence of PDMP. Interestingly, the fluorograph of the ceramide bands generated after the various treatments (Fig. 8 B) shows different relative amounts of ceramide species. We suspect that these ceramides differ in acyl chain lengths (Abe et al., 1992) and that this may be an indication of different sources of ceramide, i.e., de novo synthesis or sphingolipid degradation. We are currently investigating this possibility.
Accumulation of Endogenous Ceramide by PDMP Treatment and Exogenous C6Cer Do Not Have the Same Effects
Based on the pretreatment results, we expected that soluble analogues of ceramide would mimic the effects of PDMP. Rosenwald and Pagano (1993) showed that like 100 μM PDMP, 25 μM C6Cer decreased the rate of transit of an itinerant protein to the medial-Golgi in CHO cells. Consistent with these results, 25 μM C6Cer also slowed anterograde traffic in BHK-21 cells. However, 25 μM C6Cer had no effect on the localization of either the M protein or ERGIC-53. Other short-chain ceramide analogues (C2- and C8-ceramide) also had no effect on IBV M localization. How does this fit with our model that ceramide mediates PDMP effects? One explanation is that the effect on protein localization is independent of the effect on anterograde traffic rates. In this case, it may be that C6Cer and endogenous ceramide have similar ability to bind and affect proteins that regulate anterograde traffic, but C6Cer is unable to bind or affect proteins that regulate protein localization. It has been shown that some short chain analogues of ceramide do not have the same effects as endogenous ceramide (e.g., Wolff et al., 1994). A second possibility is that effects of ceramide are limited to the bilayers in which its concentration increases, and the difference seen between endogenous ceramide and exogenous C6Cer is due to the different intracellular distribution of these molecules. C6Cer would be unlikely to accumulate in compartments where IBV M and ERGIC 53 reside, because it is rapidly converted to sphingolipids there (Rosenwald et al., 1992). Effects on membrane traffic might be more dramatic in compartments where C6Cer accumulates, possibly the trans-Golgi and TGN (Pagano et al., 1989). Consistent with this idea, the rate of movement of VSV G through the late Golgi (measured by sialylation) was slowed to a much greater extent by C6Cer than by PDMP (Rosenwald and Pagano, 1993).
It is not yet clear how ceramide induces the changes in anterograde traffic and protein localization. Recent studies have shown that ceramide (Hannun, 1994) and its metabolites (Hakomori, 1990) play a major role as second messengers. Preliminary experiments with both the ceramidase inhibitor N-oleoylethanolamine and exogenously added sphingosine suggest that the ceramide breakdown product sphingosine does not play a role in the localization of IBV M (Maceyka, M., and C. Machamer, unpublished observations). Ceramide activates a cytosolic protein phosphatase (Dobrowsky and Hannun, 1992; Wolff et al., 1994) that can be inhibited by okadaic acid. Preliminary experiments suggest that okadaic acid does not block the PDMP-induced changes (Maceyka, M., and C. Machamer, unpublished observations). Ceramide could be activating a protein kinase (Mathias et al., 1991), but this kinase has not been fully characterized. Another possibility is that increased ceramide within the lipid bilayer directly affects the localization of IBV M. Earlier work from our laboratory showed that the first transmembrane domain of IBV M can target chimeras to the CGN (Swift and Machamer, 1991; Machamer et al., 1993). It is possible that CGN bilayers have distinct lipid domains, and that these domains contain different sets of proteins, analogous to glycosphingolipid rafts. Segregation of membrane proteins involved in vesicular traffic, such as SNAREs, would lead to mobile and immobile domains. We hypothesize that under normal conditions, IBV M would be targeted to an immobile domain and escaped molecules would be cycled back through the ER. Elevated levels of ceramide could disrupt these immobile lipid domains, inducing IBV M to cycle. Alternatively, perhaps ceramide binds to IBV M transmembrane domains, preventing an interaction with immobile lipid domains.
Acknowledgments
We thank Drs. E. Cluett, D. Raben, and A. Hubbard for useful discussions and the members of the Machamer lab for critical reading of the manuscript. We also thank H.-P. Hauri, M. Farquhar, K. Moremen, and J. Lippincott-Schwartz for antibodies.
This work was supported by grant GM42522 from the National Institutes of Health.
Abbreviations used in this paper
- CGN
cis-Golgi network
- GlcCer
glucosylceramide
- IBV
infectious bronchitis virus
- IC
intermediate compartment
- PDMP
d,l-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol
- SM
sphingomyelin
- VSV
vesicular stomatitis virus
Footnotes
Address all correspondence to Carolyn E. Machamer, Department of Cell Biology and Anatomy, Johns Hopkins University School of Medicine, 725 N. Wolfe St., Baltimore, MD 21205. Tel.: (410) 955-1809. Fax: (410) 955-4129. E-mail: carolyn_machamer@qmail.bs.jhu.edu
References
- Abe A, Wu D, Shayman JA, Radin NS. Metabolic effects of short-chain ceramide and glucosylceramide on sphingolipids and protein kinase C. Eur J Biochem. 1992;210:765–773. doi: 10.1111/j.1432-1033.1992.tb17479.x. [DOI] [PubMed] [Google Scholar]
- Abe A, Radin NS, Shayman JA. Inhibition of glucosylceramide synthase by synthase inhibitors and ceramide. Biochim Biophys Acta. 1996;1299:333–341. doi: 10.1016/0005-2760(95)00217-0. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bretscher MS, Munro S. Cholesterol and the Golgi apparatus. Science. 1993;261:1280–1281. doi: 10.1126/science.8362242. [DOI] [PubMed] [Google Scholar]
- Chen C-S, Rosenwald AG, Pagano RE. Ceramide as a modulator of endocytosis. J Biol Chem. 1995;270:13291–13297. doi: 10.1074/jbc.270.22.13291. [DOI] [PubMed] [Google Scholar]
- Cluett EB, Kuismanen E, Machamer CE. Heterogeneous distribution of an unusual phospholipid in the Golgi complex. Mol Biol Cell. 1997;8:2233–2240. doi: 10.1091/mbc.8.11.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole NB, Sciaky N, Marotta A, Song J, Lippincott-Schwartz J. Golgi dispersal during microtubule disruption: regeneration of Golgi stacks at peripheral endoplasmic reticulum exit sites. Mol Biol Cell. 1996a;7:631–650. doi: 10.1091/mbc.7.4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole NB, Smith CL, Sciaky N, Terasaki M, Edidin M, Lippincott-Schwartz J. Diffusional mobility of Golgi proteins in membranes of living cells. Science. 1996b;273:797–801. doi: 10.1126/science.273.5276.797. [DOI] [PubMed] [Google Scholar]
- Colley KJ. Golgi localization of glycosyltransferases: more questions than answers. Glycobiology. 1997;7:1–13. doi: 10.1093/glycob/7.1.1-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrowsky RT, Hannun Y. Ceramide stimulates a cytosolic protein phosphatase. J Biol Chem. 1992;267:5048–5051. [PubMed] [Google Scholar]
- Futerman AH, Pagano RE. Determination of the intracellular sites and topology of glucosylceramide synthesis in rat liver. Biochem J. 1991;280:295–302. doi: 10.1042/bj2800295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Futerman AH, Stieger B, Hubbard AL, Pagano RE. Sphingomyelin synthesis in rat liver occurs predominantly at the cis and medial cisternae of the Golgi apparatus. J Biol Chem. 1990;265:8650–8675. [PubMed] [Google Scholar]
- Hakomori S-I. Bifunctional role of glycosphingolipids. Modulators for transmembrane signaling and mediators for cellular interactions. J Biol Chem. 1990;265:18713–18716. [PubMed] [Google Scholar]
- Hannun YA. The sphingomyelin cycle and the second messenger function of ceramide. J Biol Chem. 1994;269:3125–3128. [PubMed] [Google Scholar]
- Henderson, R.J., and D.R. Tocher. 1992. Thin-layer chromotography. In Lipid Analysis: A Practical Approach. R.J. Hamilton and S. Hamilton, editors. Oxford University Press, Oxford. 65–111.
- Horvath A, Sutterlin C, Manning-Krieg U, Movva NR, Riezman H. Ceramide synthesis enhances transport of GPI-anchored proteins to the Golgi apparatus in yeast. EMBO (Eur Mol Biol Organ) J. 1994;13:3687–3695. doi: 10.1002/j.1460-2075.1994.tb06678.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inokuchi J-I, Radin NS. Preparation of the active isomer of 1-phenyl-2-decanoylamino-3-morpholino-1-propanol, inhibitor of murine glucocerebroside synthetase. J Lipid Res. 1987;28:565–571. [PubMed] [Google Scholar]
- Keenan TW, Morre DJ. Phospholipid class and fatty acid composition of Golgi apparatus isolated from rat liver and comparison with other cell fractions. Biochemistry. 1970;9:19–25. doi: 10.1021/bi00803a003. [DOI] [PubMed] [Google Scholar]
- Lahtinen U, Hellman U, Wernstedt C, Saraste J, Pettersson RF. Molecular cloning and expression of a 58-kDa cis-Golgi and intermediate compartment protein. J Biol Chem. 1996;271:4031–4037. doi: 10.1074/jbc.271.8.4031. [DOI] [PubMed] [Google Scholar]
- Linstedt AD, Hauri H-P. Giantin, a novel conserved Golgi membrane protein containing a cytoplasmic domain of at least 350kDa. Mol Biol Cell. 1993;4:679–693. doi: 10.1091/mbc.4.7.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippincott-Schwartz J, Donaldson JG, Schweizer A, Berger EG, Hauri H-P, Yuan LC, Klausner RD. Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. Cell. 1990;60:821–836. doi: 10.1016/0092-8674(90)90096-w. [DOI] [PubMed] [Google Scholar]
- Machamer CE. Golgi retention signals: do membranes hold the key? . Curr Opin Cell Biol. 1993;5:606–612. doi: 10.1016/0962-8924(91)90001-P. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machamer CE, Rose JK. A specific membrane-spanning domain of a coronavirus E1 glycoprotein is required for its retention in the Golgi region. J Cell Biol. 1987;105:1205–1214. doi: 10.1083/jcb.105.3.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machamer CE, Mentone SA, Rose JK, Farquhar MG. The avian coronavirus E1 protein is targeted to the cis Golgi. Proc Natl Acad Sci USA. 1990;87:6944–6948. doi: 10.1073/pnas.87.18.6944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machamer CE, Grim MM, Esquela A, Chung SW, Rolls M, Ryan K, Swift AM. Retention of a cis Golgi protein requires polar residues on one face of a predicted α-helix in the transmembrane domain. Mol Biol Cell. 1993;4:695–704. doi: 10.1091/mbc.4.7.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias S, Dressler KA, Kolesnick RN. Characterization of a ceramide-activated protein kinase: stimulation by tumor necrosis factor α. Proc Natl Acad Sci USA. 1991;88:10009–10013. doi: 10.1073/pnas.88.22.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medlock KA, Merrill AH. Inhibition of serine palmitoyltransferase in vitro and long-chain base biosynthesis in intact Chinese hamster ovary cells by β-chloroalanine. Biochemistry. 1988;27:7079–7084. doi: 10.1021/bi00418a061. [DOI] [PubMed] [Google Scholar]
- Mellman I, Simons K. The Golgi complex: in vitro veritas? . Cell. 1992;68:829–840. doi: 10.1016/0092-8674(92)90027-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill AH, Wang E. Biosynthesis of long-chain (sphingoid) bases from serine by LM cells. Evidence for introduction of the 4-trans-double bond after de novo biosynthesis of N-acylsphinganines. J Biol Chem. 1986;261:3764–3769. [PubMed] [Google Scholar]
- Moremen KW, Robbins PW. Isolation, characterization and expression of cDNAs encoding murine α-mannosidase II, a Golgi enzyme that controls conversion of high mannose to complex N-glycans. J Cell Biol. 1991;115:1521–1534. doi: 10.1083/jcb.115.6.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munro S, Pelham HRB. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987;48:899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- Nilsson T, Warren G. Retention and retrieval in the endoplasmic reticulum and Golgi apparatus. Curr Opin Cell Biol. 1994;6:517–521. doi: 10.1016/0955-0674(94)90070-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oprins A, Duden R, Kreis TE, Geuze HJ, Slot JW. β-COP localizes mainly to the cis-Golgi side in exocrine pancrease. J Cell Biol. 1993;121:49–59. doi: 10.1083/jcb.121.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano, R.E., and O.C. Martin. 1988. Use of fluorescent analogs of ceramide to study the Golgi apparatus of animal cells. In Cell Biology: A Laboratory Handbook. J.E. Celis, editor. Academic Press, Inc., San Diego. 387–393.
- Pagano RE, Sepanski MA, Martin OC. Molecular trapping of a fluorescent ceramide analogue at the Golgi apparatus of fixed cells: interaction with endogenous lipids provides a trans-Golgi marker for both light and electron microscopy. J Cell Biol. 1989;109:2067–2079. doi: 10.1083/jcb.109.5.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posse de Chaves EI, Bussiere M, Vance DE, Campenot RB, Vance JE. Elevation of ceramide within distal neurites inhibits neurite growth in cultured rat sympathetic neurons. J Biol Chem. 1997;272:3028–3035. doi: 10.1074/jbc.272.5.3028. [DOI] [PubMed] [Google Scholar]
- Rani CSS, Abe A, Chang Y, Rosenzweig N, Saltiel AR, Radin NS, Shayman JA. Cell cycle arrest induced by an inhibitor of glucosylceramide synthase. Correlation with cyclin-independent kinases. J Biol Chem. 1995;270:2859–2867. doi: 10.1074/jbc.270.6.2859. [DOI] [PubMed] [Google Scholar]
- Rosenwald AG, Pagano RE. Inhibition of glycoprotein traffic through the secretory pathway by ceramide. J Biol Chem. 1993;268:4577–4579. [PubMed] [Google Scholar]
- Rosenwald AG, Machamer CE, Pagano RE. Effects of a sphingolipid synthesis inhibitor on membrane transport through the secretory pathway. Biochemistry. 1992;31:3581–3590. doi: 10.1021/bi00129a005. [DOI] [PubMed] [Google Scholar]
- Schindler R, Itin C, Zerial M, Lottspeich F, Hauri H-P. ERGIC-53, a membrane protein of the ER-Golgi intermediate compartment, carries an ER retention motif. Eur J Cell Biol. 1993;61:1–9. [PubMed] [Google Scholar]
- Schweizer A, Fransen JAM, Baechi T, Ginsel L, Hauri H-P. Identification, by a monoclonal antibody, of a 53-kD protein associated with a tubulovesicular compartment at the cis-side of the Golgi apparatus. J Cell Biol. 1988;107:1643–1653. doi: 10.1083/jcb.107.5.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shayman JA, Deshmukh GD, Mahdiyoun S, Thomas TP, Wu D, Barcelon FS, Radin NS. Modulation of renal epithelial cell growth by glucosylceramide. Association with protein kinase C, sphingosine, and diacylglycerol. J Biol Chem. 1991;266:22968–22974. [PubMed] [Google Scholar]
- Sodeik B, Doms RW, Ericsson M, Hiller G, Machamer CE, van't Hof W, van Meer G, Moss B, Griffiths G. Assembly of vaccinia virus: role of the intermediate compartment between the endoplasmic reticulum and the Golgi stacks. J Cell Biol. 1993;121:521–541. doi: 10.1083/jcb.121.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swift AM, Machamer CE. A Golgi retention signal in a membrane-spanning domain of coronavirus E1 protein. J Cell Biol. 1991;115:19–30. doi: 10.1083/jcb.115.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura K-I, Sugiyama E, Tamai C, Hara A, Taketomi T, Radin NS. Effect of an inhibitor of glucosylceramide synthesis on cultured rabbit skin fibroblasts. J Biochem (Tokyo) 1990;108:525–530. doi: 10.1093/oxfordjournals.jbchem.a123236. [DOI] [PubMed] [Google Scholar]
- van Meer G. Transport and sorting of membrane lipids. Curr Opin Cell Biol. 1993;5:661–673. doi: 10.1016/0955-0674(93)90137-f. [DOI] [PubMed] [Google Scholar]
- Velasco A, Hendricks L, Moremen KW, Tulsiani DRP, Touster O, Farquhar MG. Cell type-dependent variations in the subcellular distribution of α-mannosidase I and II. J Cell Biol. 1993;122:39–51. doi: 10.1083/jcb.122.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vunnam RR, Radin NS. Analogs of ceramide that inhibit glucocerebroside synthetase in mouse brain. Chem Phys Lipids. 1980;26:265–278. doi: 10.1016/0009-3084(80)90057-2. [DOI] [PubMed] [Google Scholar]
- Wang E, Norred WP, Bacon CW, Riley RT, Merril AH., Jr Inhibition of sphingolipid biosynthesis by fumonisins: implications for diseases associated with Fusarium moniliforme. . J Biol Chem. 1991;266:14486–14490. [PubMed] [Google Scholar]
- Weisz OA, Swift AM, Machamer CE. Oligomerization of a membrane protein correlates with its retention in the Golgi complex. J Cell Biol. 1993;122:1185–1196. doi: 10.1083/jcb.122.6.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff RA, Dobrowsky RT, Bielawska A, Obeid LM, Hannun Y. Role of ceramide-activated protein phosphatase in ceramide-mediated signal transduction. J Biol Chem. 1994;269:19605–19609. [PubMed] [Google Scholar]