Abstract
Expression of muscle-specific β1D integrin with an alternatively spliced cytoplasmic domain in CHO and GD25, β1 integrin-minus cells leads to their phenotypic conversion. β1D-transfected nonmuscle cells display rounded morphology, lack of pseudopodial activity, retarded spreading, reduced migration, and significantly enhanced contractility compared with their β1A-expressing counterparts. The transfected β1D is targeted to focal adhesions and efficiently displaces the endogenous β1A and αvβ3 integrins from the sites of cell–matrix contact. This displacement is observed on several types of extracellular matrix substrata and leads to elevated stability of focal adhesions in β1D transfectants. Whereas a significant part of cellular β1A integrin is extractable in digitonin, the majority of the transfected β1D is digitonin-insoluble and is strongly associated with the detergent-insoluble cytoskeleton. Increased interaction of β1D integrin with the actin cytoskeleton is consistent with and might be mediated by its enhanced binding to talin. In contrast, β1A interacts more strongly with α-actinin, than β1D. Inside-out driven activation of the β1D ectodomain increases ligand binding and fibronectin matrix assembly by β1D transfectants. Phenotypic effects of β1D integrin expression in nonmuscle cells are due to its enhanced interactions with both cytoskeletal and extracellular ligands. They parallel the transitions that muscle cells undergo during differentiation. Modulation of β1 integrin adhesive function by alternative splicing serves as a physiological mechanism reinforcing the cytoskeleton– matrix link in muscle cells. This reflects the major role for β1D integrin in muscle, where extremely stable association is required for contraction.
Integrins are a large family of transmembrane heterodimeric receptors that play a key role in cell adhesion to extracellular matrix (Hynes, 1992). Integrin receptors serve a dual purpose, linking extracellular matrix to the actin cytoskeleton and providing bidirectional transmission of signals between the extracellular matrix and the cytoplasm (Schwartz et al., 1995; Yamada and Miyamoto, 1995; Burridge and Chrzanowska-Wodnicka, 1996). At least two major actin-binding proteins, talin and α-actinin, are thought to interact directly with the cytoplasmic domain of several β subunits, providing a link to the actin cytoskeleton (Horwitz et al., 1986; Otey et al., 1990; Hemler et al., 1994). Among integrins, β1 is typically the most abundant and ubiquitously expressed subunit associated with a number of α subunits to form distinct heterodimers. These interact with a variety of extracellular matrix and cell adhesion molecules (Hynes, 1992). The entire structure of the β1 integrin cytoplasmic domain is critical for integrin– cytoskeleton interaction (Hayashi et al., 1990; LaFlamme et al., 1992; Reszka et al., 1992; Ylanne et al., 1993; Lewis and Schwartz, 1995).
Integrin functions within the cell can be regulated at different levels. These include cell type–specific biosynthesis of certain integrin heterodimers, maturation and processing of the receptors, as well as their transport to the cell surface (Hynes, 1992). Another level of control of integrin function is through regulation of the ligand-binding affinity of integrins on the cell surface. This type of regulation involves conformational changes within integrins. The conformational state of the extracellular domains (activation) of integrins is regulated via their cytoplasmic tails and is referred to as inside-out signaling (Ginsberg et al., 1992; O'Toole et al., 1994; Schwartz et al., 1995). Thus, deletions or mutations of certain residues in the cytoplasmic domains of α and β subunits can either increase or inhibit the ligand-binding activity of integrin receptors (Takada et al., 1992; O'Toole et al., 1994, 1995). The activation state of integrins can also be controlled by some lipid metabolites (Hermanowski-Vosatka et al., 1992; Smyth et al., 1993) and small GTP-binding proteins (Zhang et al., 1996; Hughes et al., 1997). Finally, functional properties of integrin receptors can be modulated by alternative splicing involving their cytoplasmic tails.
So far, four cytoplasmic domain variants of the β1 integrin subunit have been described. Besides the major β1A isoform, characteristic for all known cell types except red blood cells and terminally differentiated striated muscles, two minor cytoplasmic domain isoforms of β1 integrin, β1B and β1C, have been characterized (Altruda et al., 1990; Languino and Ruoslahti, 1992). Although their functions remain uncertain, it has been speculated that β1B can serve as a negative regulator of cell adhesion during development, whereas β1C can strongly inhibit cell growth (Balzac et al., 1993, 1994; Meredith et al., 1995). The alternatively spliced sequences of β1B and β1C have no homology to the major β1A isoform and are unable to localize to cell–matrix adhesion sites apparently because of impaired interaction with the actin cytoskeleton (Balzac et al., 1993; Meredith et al., 1995). Interestingly, β1B and β1C variants have been found only in humans, whereas the fourth β1 isoform, β1D, is highly conserved at least throughout vertebrate evolution, suggesting an important role for this muscle-specific variant (van der Flier et al., 1995; Zhidkova et al., 1995; Baudoin et al., 1996; Belkin et al., 1996).
β1 integrin is localized at junctional structures of striated muscles (Bozyczko et al., 1989). Expression of the β1 integrin subunit as well as the ligand occupation of β1-containing heterodimers is essential for myodifferentiation and the formation of sarcomeric cytoarchitecture (Menko and Boettiger, 1987; Volk et al., 1990). Integrin-mediated cytoskeleton-matrix linkage has to be distinct in muscle cells because of high tensile forces transmitted across the membrane and enhanced stability of muscle adhesive structures. This implies a modified function for β1 integrin in muscles. This function is now attributed primarily to β1D cytoplasmic domain variant, which is a major β1 isoform that completely displaces β1A integrin in differentiated striated muscles (Belkin et al., 1996). Its cytoplasmic domain is highly homologous to that of β1A, including conservation of both NPXY motifs involved in the regulation of ligand-binding affinity (Tamkun et al., 1986; Argraves et al., 1987; Zhidkova et al., 1995; van der Flier et al., 1995). β1D accumulates at all major cell–matrix adhesion sites both in skeletal muscle fibers and cardiomyocytes. α7β1D is a predominant integrin in adult skeletal and heart muscle tissues (Belkin et al., 1996). However, other α subunits, including α5 and α6A, can pair with β1D in developing heart muscle (Brancaccio et al., 1997). The data obtained so far lead to the suggestion that β1D integrin plays a crucial role in linking the subsarcolemmal cytoskeleton to the surrounding extracellular matrix in muscle tissues (Belkin et al., 1996; Fassler et al., 1996).
Upon transfection into nonmuscle cells, β1D is targeted to focal adhesions, proving that the muscle-specific isoform of β1 integrin is able to interact with the nonmuscle cytoskeleton as well (Belkin et al., 1996). To get an insight in the functional properties of this integrin, we have expressed human β1D and β1A cytoplasmic domain isoforms in CHO cells and the mouse GD25 cell line. GD25 cells lack endogenous β1 integrin as a consequence of gene inactivation (Wennerberg et al., 1996). Here we report that the expression of β1D integrin in nonmuscle cells leads to a conversion of cellular phenotype. The observed alterations in cell morphology, inhibition of spreading and motility, as well as an increase in the ligand-binding affinity, fibronectin matrix assembly and contractility, are caused by an enhanced association of β1D integrin with both the actin cytoskeleton and extracellular matrix ligands. β1D-mediated enhancement of actin–membrane attachment is, at least in part, due to a higher affinity interaction of this integrin with the focal adhesion protein, talin. The altered structure of the β1D cytoplasmic domain causes a conformational change of its ectodomain via inside-out signaling mechanisms, leading to activation of ligand binding. Reinforcement of the cytoskeleton–matrix association by β1D reflects a key role for this integrin as a cytoskeleton–matrix linker, strengthening adhesive structures in muscle tissues.
Materials and Methods
Antibodies and Reagents
The following antibodies against β1 integrin were used in this study: TS2/ 16 mAb to human β1 subunit, which activates ligand binding by β1-containing heterodimers was a gift from Dr. M. Hemler (Dana-Farber Cancer Institute, Boston, MA) (Hemler et al., 1984; Arroyo et al., 1992); function-blocking P4C10 mAb against human β1 integrin (Carter et al., 1990) was from GIBCO BRL (Gaithersburg, MD); 102DF5 mAb against human β1 integrin (Ylanne and Virtanen, 1989); 12G10 mAb, which reacts with activated (high affinity conformation for ligand binding) human β1 (Mould et al., 1995); 9EG7 mAb reacting with ligand-, Mg2+-, Mn2+-induced, Ca2+- inhibited epitope on human β1 integrin subunit (Bazzoni et al., 1995); A1A5 mAb against human β1 integrin (Hemler et al., 1984), conjugated with fluorescein and rabbit polyclonal antibody against human β1 integrin (Belkin et al., 1990). 7E2 mAb against hamster β1 integrin and inhibitory PB1 mAb against intact hamster α5β1 heterodimer were generous gifts from Dr. R. Juliano (University of North Carolina, Chapel Hill, NC) (Brown and Juliano, 1985, 1988). Isoform-specific antibodies against β1A and β1D integrins were described earlier (Belkin et al., 1996).
Blocking anti–mouse αv H9.2B8 mAb (Moulder et al., 1991) was obtained from PharMingen (San Diego, CA). Rabbit polyclonal antibodies against αv, α3, and α5 cytoplasmic domains were described earlier (Defilippi et al., 1992; Balzac et al., 1994). mAb 8d4 against talin was obtained from Sigma Chemical Co.(St. Louis, MO) and mAb 1682 against α-actinin was from Chemicon International, Inc. (Temecula, CA). Rabbit polyclonal antibody against platelet myosin II, cross-reacting with nonmuscle myosin, was a gift from Dr. R. Adelstein (National Institutes of Health, Bethesda, MD). Rabbit polyclonal antibody against human plasma fibronectin (Fn) was provided by Dr. L.B. Chen (Dana-Farber Cancer Institute).
Human plasma Fn was from GIBCO BRL. Recombinant, 12-kD cell-binding Fn fragment corresponded to the tenth Arg-Gly-Asp–containing (cell-binding), type III Fn repeat. Cytochalasin D was from Sigma Chemical Co.. Digitonin was purchased from Sigma Chemical Co. and purified by dissolving in water, filtering, and lyophilization before use. 35S-Translabel and methionine- and cysteine-free medium were from ICN Biomedicals Inc. (Costa Mesa, CA). Na125I and [32P]orthophosphate were from Dupont-NEN (Boston, MA).
Expression Constructs, Transfection, and Cell Culture
Human full-length cDNAs encoding β1A integrin or β1D integrin in SV40-based expression vector pECE (Ellis et al., 1986), were transfected into CHO cells or β1-minus GD25 cell line (Wennerberg et al., 1996), and transfectants were selected as described (Belkin et al., 1996). More than 95% of cells in each population expressed human β1 integrin; the expression levels of the transfected β1A and β1D integrins were very similar and comparable to the level of the endogenous hamster β1 integrin subunit in CHO cells and close to the levels of the endogenous αv and β3 integrins in GD25 transfectants. CHO transfectants were cultured in Ham's F12 medium with 10% FBS, and GD25 transfectants were cultured in DME plus 10% FBS.
Morphological Analysis and Spreading
For analysis of cell phenotype, CHO transfectants were plated and cultured for 1 d on Fn-coated dishes. Phase-contrast photographs of live β1A-CHO and β1D-CHO cells were taken on an inverted microscope. The outlines of randomly chosen cells not in contact with other cells were analyzed by the computer Tracer V1.0 software (Dunn and Brown, 1986). The spread area, cell perimeter, and two morphometric parameters of cell shape, cell dispersion and elongation, were calculated as characteristics of cell spreading and polarization. For analysis of the time course of spreading, β1A- and β1D-transfected CHO and GD25 cells were plated in serum-free medium on Fn, laminin, vitronectin, or on immobilized mAb TS2/16 to human β1 integrin (Balzac et al., 1994; Belkin et al., 1996). After specific periods of time, cells were fixed with formaldehyde, stained with Coomassie brilliant blue (Balzac et al., 1994), and then photographed.
Measurements of the Ligand-Binding Affinity
The binding of the 125I-labeled Fn(III)10 fragment to β1A-CHO, β1D-CHO, β1A-GD25, and β1D-GD25 cells in suspension was quantified as described (O'Toole et al., 1990; Wu et al., 1995). Since CHO cells express the endogenous α5β1, and β1-minus GD25 cells express αvβ3 as a major Fn-binding integrin (Wennerberg et al., 1996), inhibitory mAbs PB1 against hamster α5β1 or H9.2B8 against mouse αv were used for CHO and GD25 cells, respectively. In some experiments, blocking P4C10 mAb against human β1 integrin was used in combination with either PB1 mAb (for CHO cells) or H9.2B8 mAb (for GD25 cells). Cells (0.2 ml of 5 × 106 cells/ml) in Tyrode's buffer were incubated with specified concentrations of 125I-labeled Fn(III)10 fragment (sp act 0.12 mCi/nM) for 30 min at 37°C either alone or in the presence of 10 μg/ml of purified TS2/16 mAb, which activates human β1 integrins. Coincubation with an excess of unlabeled Fn(III)10 fragment (0.5 mg/ml) was used to determine and subtract the nonspecific background binding. 50-μl aliquots were layered on 0.3 ml of 20% sucrose in Tyrode's buffer and centrifuged for 3 min at 12,000 rpm. Radioactivity associated with the cell pellet was determined in a gamma counter.
Flow Cytometry
Cell surface expression of the transfected human β1A or β1D integrins in CHO and GD25 transfectants was assessed with 102DF5 and TS2/16 mAbs, whose binding to the β1 subunit is conformation independent. Their expression levels were compared to those of the endogenous hamster β1 integrin (examined with 7E2 mAb). 12G10 mAb reacting with activated human β1 integrin subunit (Mould et al., 1995) and conformation-specific 9EG7 anti–human β1 mAb (Bazzoni et al., 1995) were used either in the absence of Mn2+ ions or in the presence of 1 mM Mn2+. Fluorescein- labeled, affinity-purified donkey anti–mouse IgG (Chemicon International, Inc.) was used as secondary antibody.
Fn Matrix Assembly Assays
β1A-CHO, β1D-CHO, as well as β1A-GD25 and β1D-GD25 cells did not assemble Fn matrix well when confluent cell monolayers were cultured in growth medium containing 1% FBS for 2 d. To boost the formation of Fn matrix, exogenous human plasma Fn was added at 200 nM concentration for 2 d to the confluent cell monolayers grown on glass coverslips. Inhibitory mAbs PB1 against hamster α5β1 and H9.2B8 against mouse αv were used to block Fn matrix assembly by the endogenous Fn-binding integrins in CHO and GD25 cells, respectively. Activating TS2/16 and inhibitory P4C10 mAbs were used for the transfected human β1A and β1D integrins. After 2 d, cell monolayers were fixed and stained with anti-Fn antibody. Stained cells were observed using a Zeiss epifluorescence microscope (Carl Zeiss Inc., Thornwood, NY) and representative fields were photographed using equal exposure lengths on Kodak T-Max 400 film (Eastman Kodak, Rochester, NY).
To quantitate Fn incorporation into deoxycholate-insoluble matrix, confluent β1A-CHO, β1D-CHO, β1A-GD25, and β1D-GD25 cultures were incubated for 2 d with 100, 200, or 300 nM of 125I-labeled Fn (sp act 0.08 mCi/nM) in growth medium containing 1% FBS and blocking and activating mAbs as specified above. Deoxycholate-insoluble fraction was obtained from cell monolayers as described (McKeown-Longo and Mosher, 1985; Wu et al., 1993, 1995).125I-labeled Fn incorporated into the deoxycholate-insoluble extracellular matrix was analyzed by reducing SDS-PAGE (6% running gel) and autoradiography. Iodinated Fn bands were cut out and counted in a gamma counter.
Migration Assays
Migratory properties of β1A-CHO, β1D-CHO, β1A-GD25, and β1D-GD25 cells were examined by a wound closure assay and time lapse videomicroscopy. For the wound closure assay, confluent cell monolayers grown on Fn-coated coverslips were wounded by dragging a sterile 1-mm pipette tip across the monolayer to create cell-free fields (Romer et al., 1994). 2 d later, glass coverslips were fixed with formaldehyde, stained with Coomassie blue, and then photographed.
For time lapse videomicroscopy, β1A- and β1D-transfected CHO and GD25 cells were plated on plastic dishes coated with 10 μg/ml of human plasma Fn. Five to six cells were scanned per field in eight different fields, every 20 min for 4 h. The displacement of the cell center as a function of time was calculated for each cell using nonoverlapping time intervals. To block the endogenous Fn receptors, PB1 mAb was used for CHO transfectants and H9.2B8 mAb for GD25 transfectants. TS2/16 was used as the activating mAb and P4C10 as the blocking mAb for the transfected human β1A and β1D integrins.
Analysis of the Association of β1A and β1D Integrins with α Subunits
β1D-CHO cells as well as β1A- and β1D-GD25 transfectants were lysed in buffer containing 1% Triton X-100 in 50 mM TrisCl, 150 mM NaCl, pH 7.5, and protease inhibitors. Each lysate was clarified by centrifugation, divided into four equal parts, and the transfected human β1 integrins were immunoprecipitated using TS2/16 mAb, whereas α3, α5, and αv subunits were immunoprecipitated with antibodies against cytoplasmic domains of these integrins. The resulting immunoprecipitates were run on 10% gel and blots were probed with the isoform-specific antibodies against β1A or β1D integrins.
Localization of the Transfected β1A and β1D Integrins and the Endogenous β1A and αv Subunits and Analysis of their Association with the Actin Cytoskeleton
To localize the transfected and the endogenous β1 integrins, as well as the endogenous αv integrins in the transfectants, cells cultured on Fn-coated coverslips were fixed with formaldehyde and permeabilized with 0.5% Triton X-100 in PBS. CHO transfectants were costained with fluorescein-labeled A1A5 mAb to human β1 and rhodamine-labeled 7E2 mAb to hamster β1 integrin. GD25 cells were double stained with mouse fluorescein–labeled A1A5 mAb and rabbit anti-αv antibody followed by rhodamine-labeled donkey anti–rabbit antibody (Chemicon International Inc.). Stained cells were observed using epifluorescence with a Zeiss Axiophot microscope and photographed using Kodak T-Max 400 film.
To study whether the solubility of integrins in digitonin correlates with their cytoskeletal association, 35S-labeled β1A-CHO and β1D-CHO cells, either untreated or treated for 1 h with 10 μM of cytochalasin D, were fractionated into soluble and cytoskeleton-associated fractions by sequential extraction at 4°C with 0.1% digitonin in 50 mM Pipes, 1 mM MgCl2, 1 mM EGTA, 1 mM EDTA, pH 6.9, and then with radioimmunoprecipitation assay (RIPA) buffer (50 mM TrisCl, 150 mM NaCl, 1% Triton X-100, 0.5% Na-deoxycholate, and 0.1% SDS, pH 7.5). Both buffers contained 10 μg/ml leupeptin, 10 μg/ml pepstatin, and 0.5 mM PMSF as protease inhibitors. The transfected β1A and β1D integrins were immunoprecipitated from digitonin- and RIPA-soluble fractions using TS2/16 mAb. 35S-labeled β1 immunoprecipitates were run on 10% gels and analyzed by autoradiography.
To assess the association of the transfected and the endogenous β1 integrin subunits and the endogenous αv integrins with the actin cytoskeleton, 35S-labeled CHO and GD25 transfectants were sequentially extracted with digitonin and RIPA buffers as described above. Immunoprecipitation of the transfected human β1A and β1D integrins from both cellular fractions was performed with TS2/16 mAb. 7E2 mAb antibody was used for the endogenous hamster β1A integrin. Rabbit anti-αv antibody was used to immunoprecipitate the endogenous αvβ3/αvβ5 integrins from GD25 transfectants. 35S-labeled β1 and αv immunoprecipitates were analyzed by SDS-PAGE on 10% gels and subsequent autoradiography.
Analysis of the Association of β1A and β1D Integrins with Talin and α-Actinin by Coimmunoprecipitation
To compare the association of β1A and β1D integrins with talin and α-actinin, 5 × 106 transfected cells were incubated in suspension with 10 μg of either purified TS2/16 mAb, 12G10 mAb, or 7E2 mAb at 4°C for 30 min on a rotator. In the case of 12G10 mAb, cells were either preincubated for 5 min with 1 mM Mn2+ or used in the absence of Mn2+. Cells were centrifuged (1,000 rpm, 3 min) and the pellets were extracted for 3 min on ice with buffer containing 0.5% digitonin in 50 mM Pipes, 1 mM MgCl2, 1 mM EGTA, 1 mM EDTA, pH 6.9, with 10 μg/ml leupeptin, 10 μg/ml pepstatin, and 0.5 mM PMSF. Under these conditions ∼80–90% of cellular β1 integrins was extracted. Cell extracts were centrifuged (12,000 rpm, 30 min, 4°C) and the resulting supernatants incubated at 4°C for 45 min with donkey anti–mouse IgG immobilized on protein A–Sepharose beads. Immunoprecipitates were washed with the same buffer, boiled in SDS sample buffer, and then run on 10% gels. Proteins were transferred onto Immobilon membranes (Millipore Corp., Bedford, MA) and blotted with either rabbit polyclonal antibody to human β1 integrin, 8d4 mAb against talin, or 1682 mAb against α-actinin. To verify the equal amount of β1A and β1D isoforms in the immunoprecipitates, the blots were stripped and reprobed with the isoform-specific antibodies against β1A and β1D (Belkin et al., 1996).
Interaction of Talin and α-Actinin with β1A and β1D Cytoplasmic Domain Peptides
Talin was purified from human platelets as described earlier (Collier and Wang, 1982). α-Actinin purification from chicken gizzards was performed as described (Otey et al., 1990). Talin and α-actinin were iodinated using 125I and Iodobeads (Pierce Chemical Co., Rockford, IL). The proteins were labeled to a specific activity of 1.2 × 106 cpm/μg for talin and 7.5 × 106 cpm/μg for α-actinin.
Full-length cytoplasmic domain peptides of β1A and β1D integrins (Belkin et al., 1996) were iodinated using Iodogen method. Both peptides were initially tested for their binding to the microtiter wells in the range of 1–150 μM concentrations in buffer containing 50 mM TrisCl, 150 mM NaCl, pH 7.5. Since they bound similarly to the wells, saturating 50 μM concentration of β1A and β1D peptides was used in subsequent experiments to immobilize them on plastic 96-well microtiter plates for 1 h at 37°C. After blocking with 2% BSA in 50 mM TrisCl, 150 mM NaCl, wells with the bound peptides were incubated with 1 nM of 125I-talin or 125I– α-actinin and 1 nM to 1 μM concentrations of unlabeled talin or α-actinin in the same buffer with 0.1% BSA for 4 h at 37°C. After the incubations, wells were washed three times with the same buffer and bound radioactivity was measured in a gamma counter. Nonspecific background was determined and subtracted for talin and α-actinin binding to BSA-coated wells.
Measurements of Cellular Contractility and Myosin Light Chain Phosphorylation
Silicone rubber substrata for assessing cellular contractility were made as described previously (Harris et al., 1980; Danowski, 1989). The UV glow discharge polymerization was used in combination with gold-palladium coating (Chrzanowska-Wodnicka and Burridge, 1996). 5- and 12-s polymerization was used for CHO and GD25 transfectants, respectively. Cells were plated on the cross-linked rubber substrata in growth medium with 10% FBS and photographed on the next day.
Possible changes in myosin light chain phosphorylation were examined as described (Chrzanowska-Wodnicka and Burridge, 1996). Briefly, subconfluent β1A-CHO and β1D-CHO cells cultured on Fn-coated, 35-mm dishes, were labeled for 4 h with 20 μCi/ml of 35S-Translabel and 100 μCi/ml of [32P]orthophosphate in phosphate-free medium. Cells were washed with PBS and equal amounts of material, as judged by 35S-incorporated radioactivity, were taken for immunoprecipitation with antimyosin antibody, followed by protein A–Sepharose beads. Immunoprecipitates were washed, boiled in SDS sample buffer, and then run on 15% polyacrylamide gel. Phosphorylated myosin light chain bands were visualized by autoradiography using three sheets of aluminum foil to block traces of 35S radiation.
Results
β1D Integrin Alters Cell Morphology and Inhibits Spreading
The levels of surface expression of the transfected human β1A and β1D, measured with mAbs 102DF5 and TS2/16, were very close to each other in both CHO and GD25 transfectants (Table I). They were also similar to the levels of the endogenous β1A in CHO transfectants and αv integrins in GD25 cells (see Fig. 9, I and J). No difference in association of the transfected β1A and β1D with endogenous α subunits was found in the two types of transfectants (Fig. 7).
Table I.
Expression Levels and Activation States of Transfected Human β1A and β1D Integrins in CHO and GD25 Transfectants
| mAb 12G10 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Cell type | mAb 102DF5* | mAb 12G10* | mAb 12G10 + 1mM Mn2+ * | mAb 12G10 + 1 mM Mn 2+‡ | ||||
| β1A-CHO | 43.1 | 9.2 | 33.6 | 27.4 | ||||
| β1D-CHO | 34.4 | 21.8 | 28.2 | 77.3 | ||||
| β1A-GD25 | 42.2 | 12.2 | 32.4 | 37.7 | ||||
| β1D-GD25 | 38.9 | 29.1 | 36.7 | 79.3 | ||||
| mAb 9EG7 | ||||||||
| mAb TS2/16* | mAb 9EG7* | mAb 9EG7 + 1mM Mn2+ * | mAb 9EG7 + 1 mM Mn 2+‡ | |||||
| β1A-GD25 | 105 | 39 | 89 | 43.8 | ||||
| β1D-GD25 | 95 | 69 | 78 | 88.4 |
Values are mean fluorescence intensities from a representative experiment.
Values are percentages.
Figure 9.
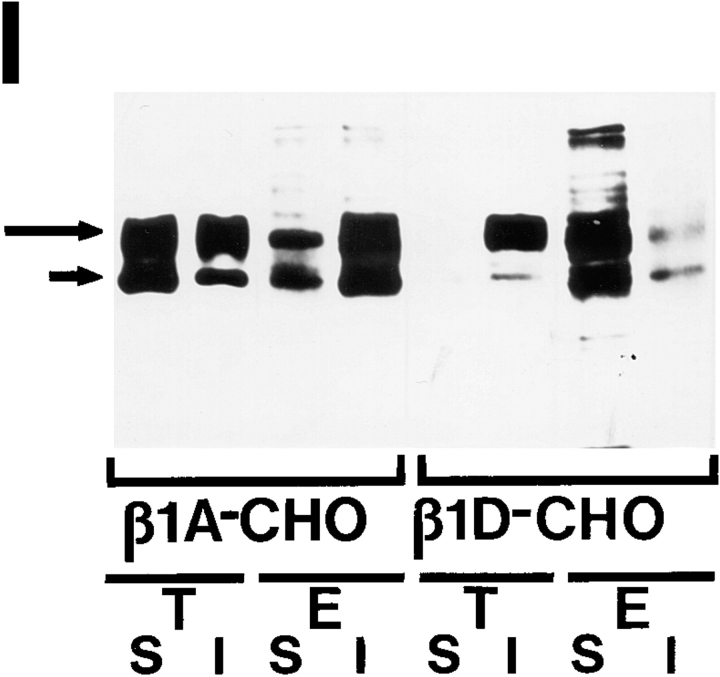
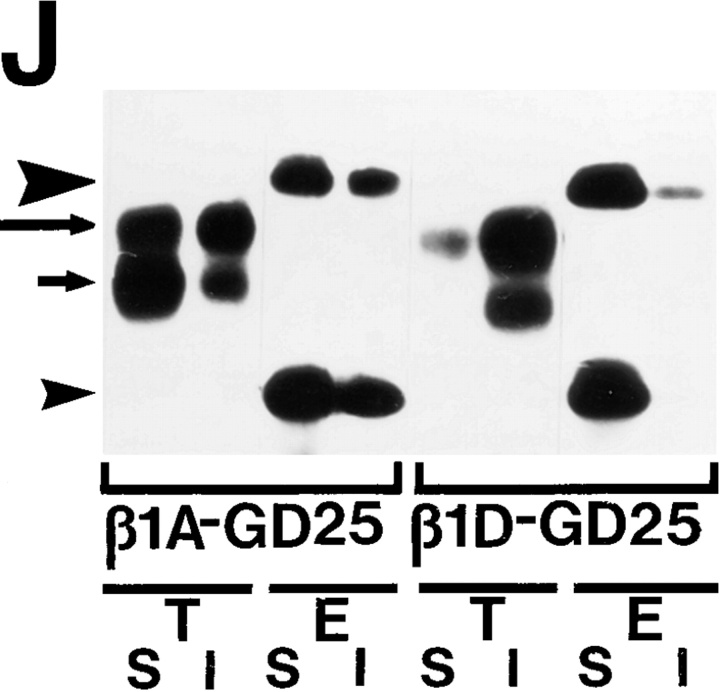
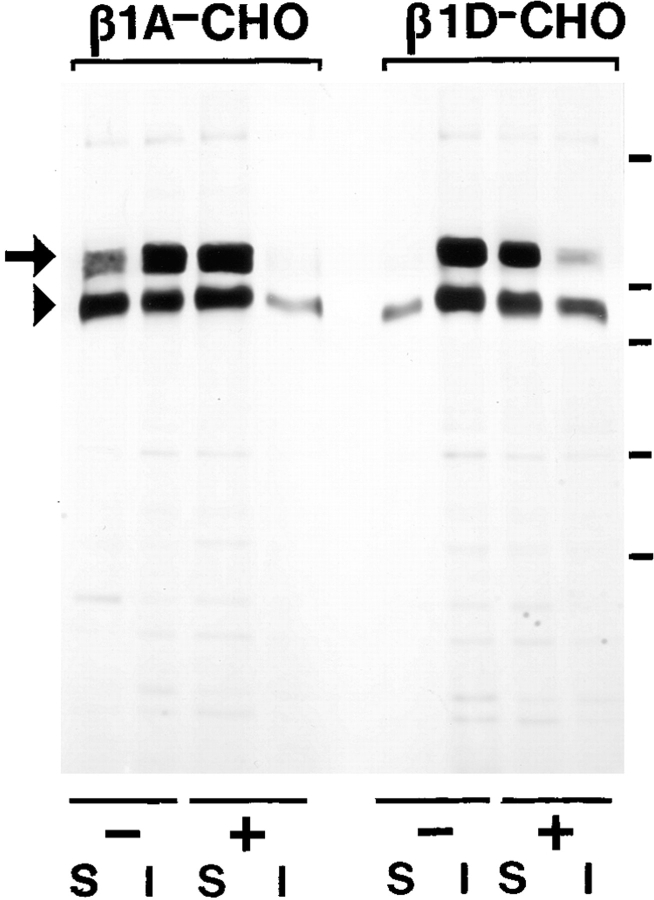
β1D interacts more strongly than β1A with the actin cytoskeleton and displaces the endogenous β1A and αv integrins from focal adhesions. (A–D) Localization of the transfected β1A and β1D and the endogenous β1A integrins in CHO transfectants. β1A-CHO (A and C) and β1D-CHO (B and D) cells were double stained for human β1A (A) and hamster β1A (C) integrins, or for human β1D (B) and hamster β1A (D) integrins. (E–H) Localization of the transfected β1A and β1D and the endogenous αv integrins in GD25 transfectants. β1A-GD25 (E and G) and β1D-GD25 (F and H) cells were double stained for human β1A (E) and mouse αv (G) integrins, or for human β1D (F) and mouse αv (H) integrins. Note colocalization of the transfected β1A with the endogenous β1A and αv integrins, whereas transfected β1D integrin displaces the endogenous β1A from focal adhesions in CHO transfectants and the endogenous αv integrins from focal adhesions in GD25 transfectants. (I and J) Association of the transfected β1A and β1D and the endogenous β1A and αv integrins with the actin cytoskeleton in CHO and GD25 transfectants. 35S-Labeled integrins were immunoprecipitated from digitonin-soluble (S) and digitonin-insoluble (I) fractions of β1A- and β1D-transfected CHO (I) and GD25 (J) cells. (T), transfected human β1A or β1D integrin; (E), endogenous hamster β1A (I) or mouse αvβ3/αvβ5 (J) integrins. Long arrows point to the mature β1 integrin subunit, short arrows mark the precursor of the β1 subunit. The large arrowhead marks the αv subunit and the small arrowhead marks the associated β3 and β5 subunits, not resolved under conditions of this electrophoresis. Unlike the transfected β1A, β1D is present predominantly in the digitonin-insoluble (cytoskeletal) fraction, whereas the endogenous hamster β1A in β1D-CHO and mouse αvβ3/αvβ5 integrins in β1D-GD25 cells are mostly digitonin-soluble. Bars, 50 μm.
Figure 7.
Association of β1A and β1D integrins with α subunits in CHO and GD25 transfectants. Immunoprecipitates containing the transfected human β1 integrin subunit (1), α3 subunit (2), α5 subunit (3), or αv subunit (4) from β1D-CHO (A and B), β1A-GD25 (C and D), and β1D-GD25 (E and F) cells were run on 10% gel and subjected to immunoblotting with the isoform-specific antibodies against β1A (A, C, and E) and β1D (B, D, and F) integrins.
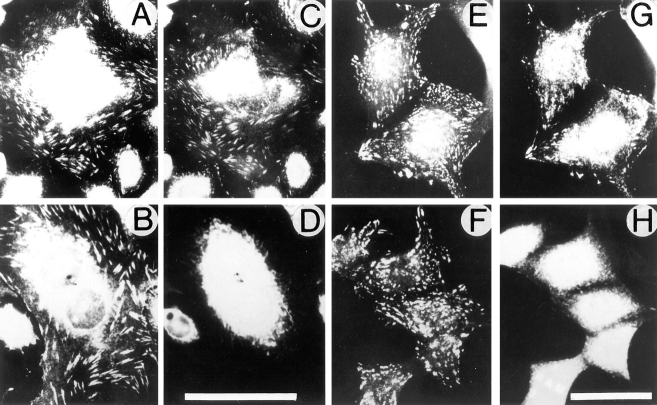
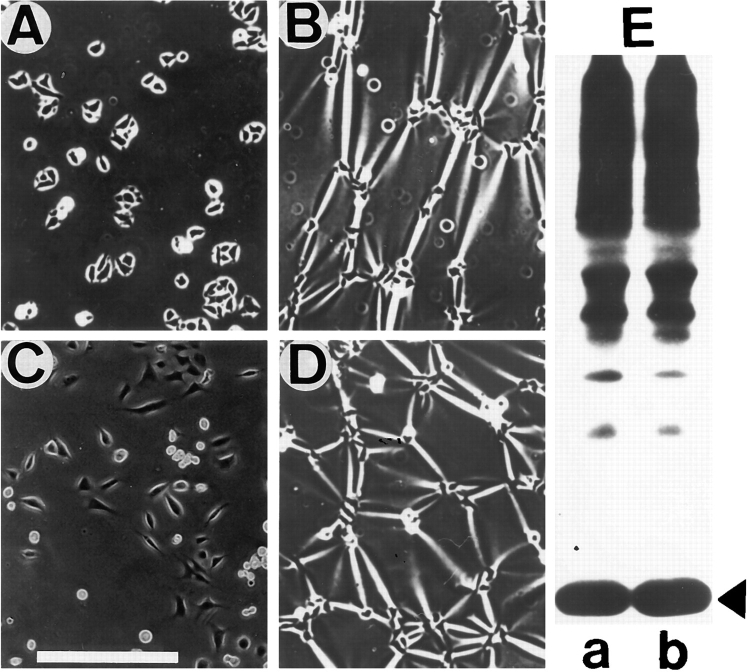
To determine possible effects of β1A and β1D integrin expression on cell morphology, CHO transfectants were grown on Fn for 1 d (Fig. 1, A and B). β1D-CHO cells appear more rounded with fewer cytoplasmic extensions at their periphery than their β1A-transfected counterparts. Statistical analysis of the cell shape was performed for randomly chosen β1A-CHO and β1D-CHO cells (Dunn and Brown, 1986). This demonstrated that spread areas were similar for β1A and β1D transfectants, but the average cell perimeter was ∼20% lower for β1D-CHO cells (Table II). Two cell shape parameters, dispersion and elongation, representing measures of cell multipolarity and bipolarity, respectively, were significantly lower for β1D transfectants. These data indicated that pseudopodial activity and cell polarization were reduced in CHO cells expressing β1D integrin.
Figure 1.
Altered morphology and inhibited spreading of CHO and GD25 cells expressing β1D integrin. β1A-CHO (A) and β1D-CHO (B) cells were plated on Fn and cultured for 1 d. β1A-CHO (C, E, and G) and β1D-CHO (D, F, and H) cells were plated in serum-free medium on Fn for 30 min (C and D), or 1 h (E and F); or on TS2/16 mAb against human β1 integrin for 2 h (G and H). β1A-GD25 (I and K) and β1D-GD25 (J and L) cells were plated in serum-free medium on Fn (I and J) or vitronectin (K and L) for 1 h. Cells were fixed with formaldehyde and stained with Coomassie blue. Bar, 50 μm.
Table II.
Cell Shape Parameters of β1A-CHO and β1D-CHO Cells
| Cell type | Area, μm2‡ | Perimeter, μm‡ | Dispersion‡ | Elongation‡ | ||||
|---|---|---|---|---|---|---|---|---|
| β1A-CHO* | 814 ± 37 | 147 ± 4 | 0.237 ± 0.016 | 1.059 ± 0.065 | ||||
| β1D-CHO* | 742 ± 34 | 118 ± 3 | 0.112 ± 0.009 | 0.769 ± 0.045 |
107 cells were analyzed for β1A-CHO population and 109 cells for β1D-CHO population.
Given are means and SE.
To analyze the time course of spreading, β1A and β1D transfectants were plated on purified Fn (Fig. 1, C–F). Spreading of CHO cells expressing β1D integrin on Fn was significantly delayed compared with β1A transfectants. When both β1A and β1D transfectants were plated on TS2/16 mAb specific for human β1 integrin, β1D-CHO were less spread than β1A-CHO cells (Fig. 1, G and H). Notably, β1D transfectants also spread more slowly on laminin, vitronectin, and 7E2 mAb against hamster β1 integrin (data not shown), indicating that inhibition of spreading was not limited to a certain type of substrate. Likewise, spreading of β1D-GD25 cells on both Fn and vitronectin was significantly delayed compared with the spreading of β1A-GD25 cells (Fig. 1, I–L). These observations suggested that retardation of spreading was a general phenomenon of β1D expression.
Constitutive Activation of the β1D Integrin Ectodomain Increases Ligand Binding
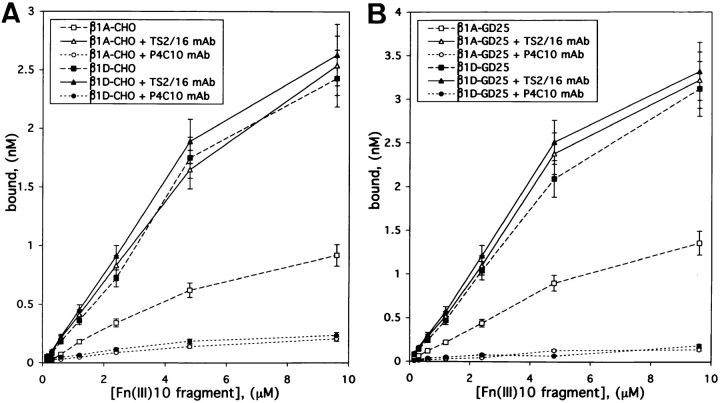
To characterize the affinity states of β1A and β1D integrins, the binding of the Fn(III)10 fragment to β1A- and β1D-transfected CHO and GD25 cells in suspension was examined. In the presence of function-blocking mAbs PB1 and H9.2B8, which inhibited endogenous Fn-binding hamster α5β1 and mouse αvβ3 integrins, respectively, β1D transfectants exhibited a significant increase in binding the soluble ligand compared with β1A-expressing cells (Fig. 2, A and B). TS2/16 mAb, which activates human β1 integrin, markedly increased Fn(III)10 fragment binding to both β1A-CHO and β1A-GD25 cells, whereas the ligand binding by β1D transfectants in the presence of TS2/16 mAb remained unaffected (Fig. 2, A and B). Blocking P4C10 mAb against human β1 integrin completely abolished the binding of Fn(III)10 fragment to all types of transfectants (Fig. 2, A and B), therefore proving its specificity.
Figure 2.
Increased ligand binding by β1D integrin. Ligand-binding properties of β1A- and β1D-transfected CHO (A) and GD25 (B) cells. Binding of 125I-labeled Fn(III)10 fragment to β1A (open marks) and β1D (filled marks) transfectants either in the absence (squares) or presence of the activating anti–human β1 integrin TS2/16 mAb (triangles), or the function-blocking anti– human β1 integrin P4C10 mAb (circles) was determined as described in the Materials and Methods. The endogenous Fn-binding hamster α5β1 and mouse αvβ3 integrins were blocked by preincubation of the CHO and GD25 cells with the inhibitory PB1 and H9.2B8 mAbs, respectively. Note that β1A-transfected, but not β1D-transfected CHO and GD25 cells, display a significant increase in Fn(III)10 fragment binding in the presence of activating TS2/16 mAb. Depicted are the means from triplicate measurements.
To further assess the difference in the conformation of the ectodomains of the transfected β1A and β1D, a flow cytometry analysis was used with mAb 12G10. This antibody recognizes preferentially a Mn2+- and ligand-induced conformation of the human β1 integrin subunit (Mould et al., 1995; Mould, 1996). The amount of 12G10 mAb bound to the cell surface was significantly higher for β1D-expressing CHO and GD25 cells, than for β1A-expressing cells. Moreover, the binding of 12G10 mAb was almost unchanged for β1D-CHO and β1D-GD25 cells in the presence of Mn2+, whereas the 12G10 mAb binding to both types of β1A transfectants was dramatically increased by Mn2+ (Table I). Another mAb, 9EG7, whose binding to human β1 integrin is stimulated by ligands and Mn2+, but inhibited by Ca2+ (Bazzoni et al., 1995), also reacted more strongly with β1D-GD25 compared with β1A-GD25 cells (Table I). Together, these results showed that in β1D- expressing cells in suspension the majority (∼77–88%) of β1D integrins were constitutively activated on the cell surface, whereas only ∼27–44% of the transfected β1A integrins were present in the activated state.
β1D Enhances Fn Matrix Assembly
Fn biosynthesis and secretion levels in CHO and GD25 cells were not altered by β1A or β1D expression and appeared to be identical for each pair of transfectants (data not shown). Fn matrix assembly by β1A- and β1D-transfected cells was analyzed with the exogenous Fn by both immunofluorescence and measurements of 125I-Fn incorporation into deoxycholate-insoluble matrix (McKeown-Longo and Mosher, 1985; Wu et al., 1993, 1995). Using different concentrations of the exogenous Fn, we consistently observed an enhanced Fn matrix assembly by β1D transfectants compared to their β1A-expressing counterparts (Fig. 3 A). In our subsequent experiments we used 200 nM of exogenous Fn for matrix assembly studies with the transfectants. There was no difference in Fn matrix assembly between β1A and β1D transfectants in the presence of blocking P4C10 mAb against human β1 integrin, showing that the observed effects can be ascribed specifically to the expressed β1D (Fig. 3 B).
Figure 3.
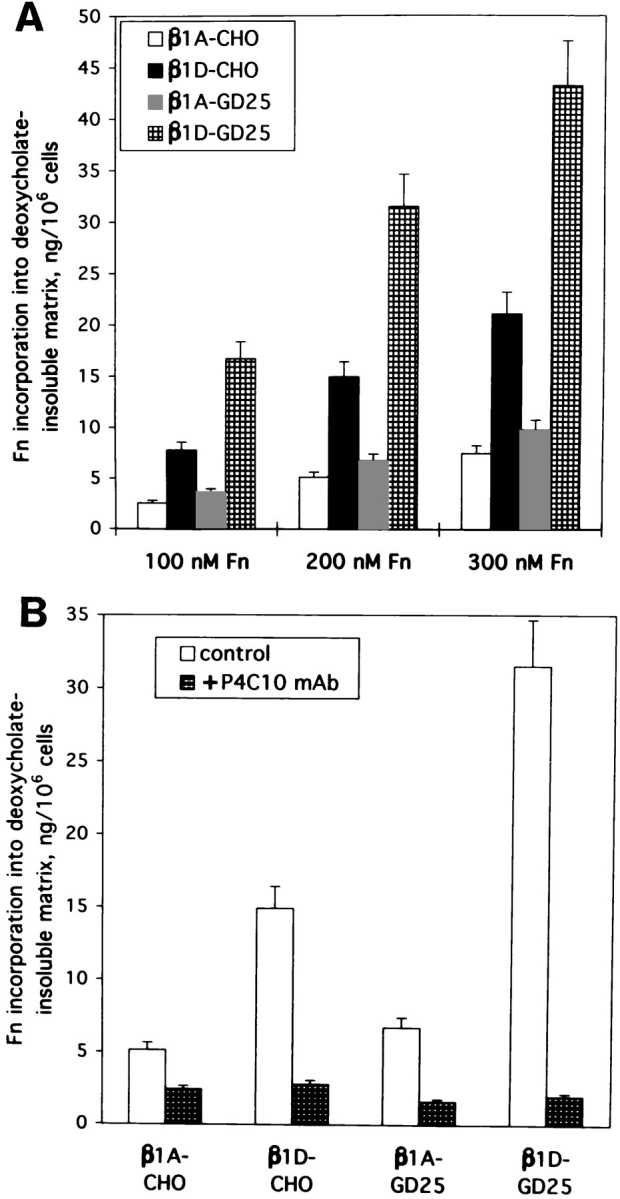
β1D integrin enhances Fn matrix assembly. (A and B) Incorporation of exogenous Fn into deoxycholate-insoluble matrix by β1A- and β1D-transfected CHO and GD25 cells. 125I-Labeled deoxycholate-insoluble Fn was visualized by SDS electrophoresis on 6% gels under reducing conditions, after autoradiography. 125I-Fn bands were cut out and radioactivity was counted in a gamma counter. Bars represent the means of triplicate determinations. (A) Cells were cultured for 2 d with 100, 200, or 300 nM of exogenous Fn. (B) Cells were cultured for 2 d with 200 nM of exogenous Fn in the absence or in the presence of function-blocking P4C10 mAb against the transfected human β1 integrins.
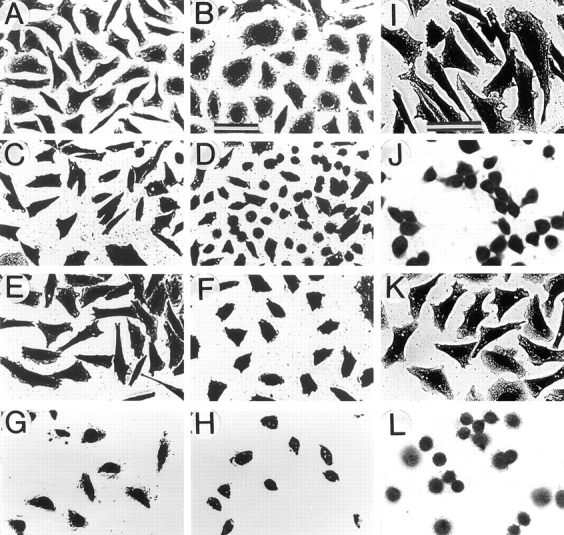
In the presence of blocking anti–hamster α5β1 integrin mAb PB1 (for CHO cells) or inhibitory anti–mouse αv integrin mAb H9.2B8 (for GD25 cells), β1D transfectants assembled more abundant meshwork of Fn fibrils, than β1A-expressing counterparts (Fig. 4, A, B, E, F, and I, a, b, e, and f). Activating mAb TS2/16 significantly increased Fn matrix assembly by β1A-CHO and β1A-GD25 cells, but did not change the levels of assembly for β1D-transfected CHO and GD25 cells (Fig. 4, C, D, G, H, and I, c, d, g, and h). Quantitation of 125I-Fn incorporated into the extracellular matrix showed a five- to sixfold increase in Fn assembly by β1D compared with β1A integrin in CHO and GD25 cells (Fig. 4, I and J). Interestingly, whereas mAb TS2/16 caused two- to threefold increase in Fn matrix assembly by β1A integrin for both types of transfectants, these levels appeared still much lower than those exhibited by β1D integrin (Fig. 4, I and J).
Figure 4.
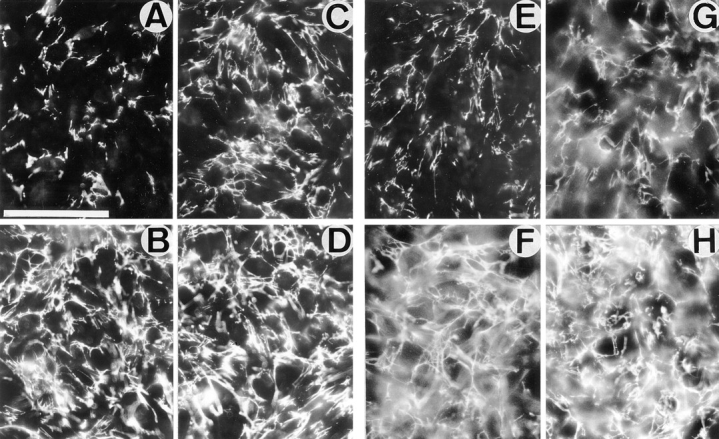
Activation of β1D integrin extracellular domain contributes to the increased β1D-mediated assembly of Fn matrix. (A–H) Immunofluorescent detection of Fn matrix deposition. Confluent monolayers of β1A-CHO (A and C), β1D-CHO (B and D), β1A-GD25 (E and G), and β1D-GD25 (F and H) cells were cultured for 2 d with 200 nM of exogenous human plasma Fn either in the absence (A, B, E, and F) or presence (C, D, G, and H) of activating anti–human β1 TS2/16 mAb. Inhibitory PB1 (A–D) and H9.2B8 (E–H) mAbs were used in the growth media to block the endogenous Fn-binding α5β1 and αvβ3 integrins, respectively. Note that more abundant Fn matrix was assembled by β1D-transfected cells (B, D, F, and H). A significant increase in Fn matrix assembly occurred when β1A-transfected cells (C and G), but not β1D-transfected cells (D and H) were incubated in the presence of TS2/16 mAb. Bar, 200 μm. (I and J) Biochemical evaluation of Fn matrix assembly. Cells were cultured for 2 d with 200 nM of exogenous Fn. (I) 125I-Labeled Fn was visualized by SDS-PAGE and autoradiography after it had been incorporated into deoxycholate-insoluble matrix of β1A-CHO (a and c), β1D-CHO (b and d), β1A-GD25 (e and g) and β1D-GD25 (f and h) cells either in the absence (I: a, b, e, and f; J, dark bars) or in the presence (I: c, d, g, and h; J, hatched bars) of activating TS2/16 mAb. mAbs PB1 (a–d) and H9.2B8 (e–h) were used as blocking antibodies for the endogenous Fn-binding integrins. 125I-Fn was used as a marker for SDS-PAGE (i). Molecular weight markers (from top to bottom) are 200, 116, 97, and 68 kD. They are indicated to the left of the gel. (J) 125I-Fn bands for β1A and β1D transfectants from the experiments shown in I were cut out and quantitated in a gamma counter. Bars in J represent the means of triplicate measurements for two independent experiments.
β1D Integrin Inhibits Cell Migration
Initially, migratory properties of β1A- and β1D-transfected CHO and GD25 cells were analyzed using a monolayer wounding assay. In 2-d wound closure experiments, β1D transfectants exhibited significantly slower migration rates than β1A-expressing cells (Fig. 5, A–D). When migratory behavior of β1A and β1D transfectants was analyzed on Fn substrate by time lapse videomicroscopy, β1D-expressing cells migrated three- to fourfold slower than β1A-transfected cells. Again, blocking P4C10 mAb against human β1 integrin completely abolished the effects of β1D on cell migration (Fig. 5 E).
Figure 5.
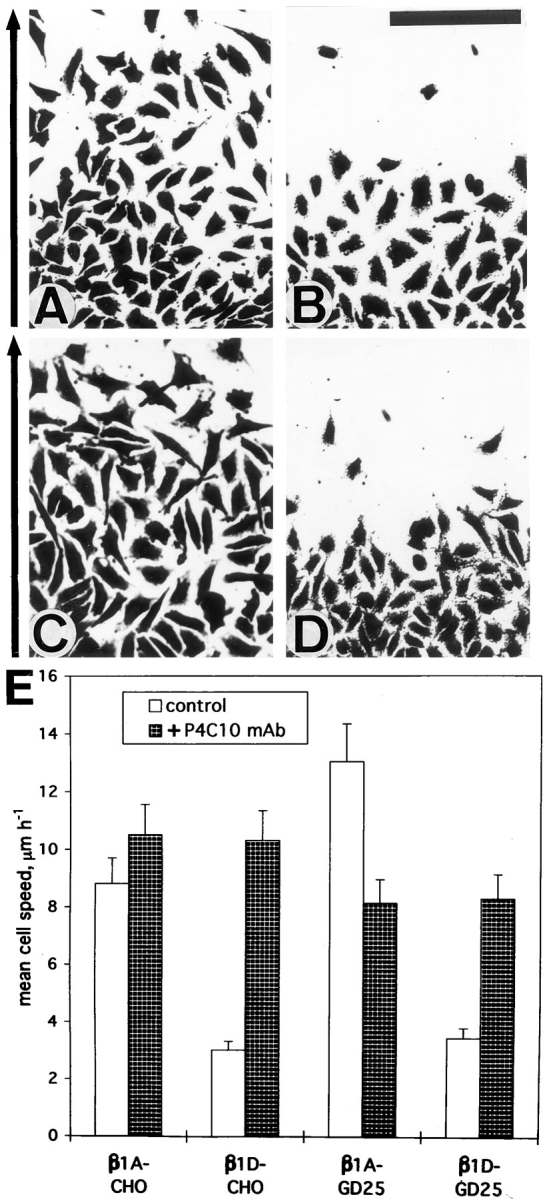
Expression of β1D integrin reduces migration. (A–D) Wounding assays. Confluent monolayers of β1A-CHO (A), β1D-CHO (B), β1A-GD25 (C), and β1D-GD25 (D) cells were scraped to generate 1-mm-wide wounds. After 2 d, cells were fixed, stained and then photographed. The direction of cell migration is shown to the left of the micrographs. (E) Time lapse videomicroscopy analysis of migratory behavior of β1A- and β1D-transfected CHO and GD25 cells. Either untreated cells (light bars), or cells in the presence of blocking P4C10 mAb against human β1 integrin (dark bars) were observed. Bar, 200 μm.
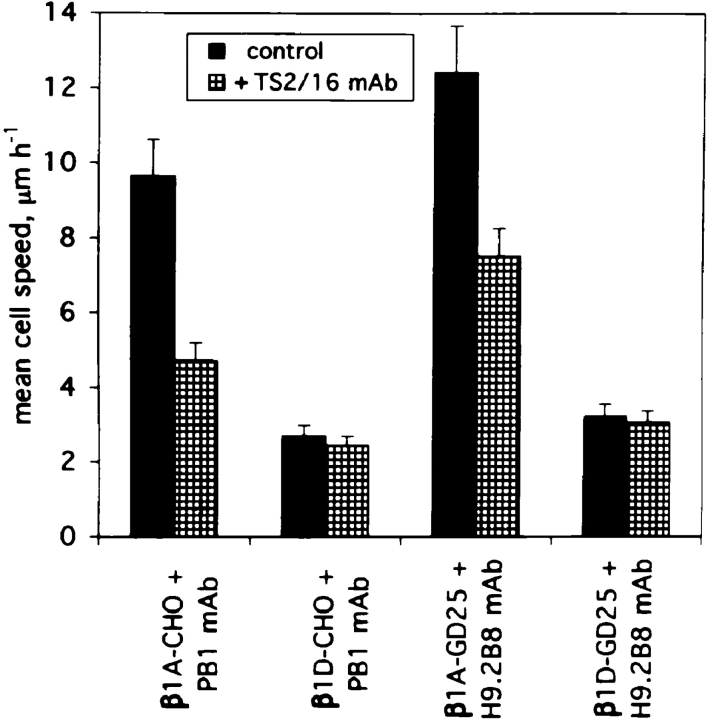
Migration of β1A- and β1D-expressing cells was also examined on Fn by time lapse videomicroscopy in the presence of blocking mAbs PB1 for CHO and H9.2B8 for GD25 transfectants (Fig. 6). In both cases when the endogenous Fn-binding integrins were inhibited, the mean cell speed of β1D transfectants appeared to be drastically reduced compared to that of β1A-expressing cells. Activating TS2/16 mAb significantly decreased migration mediated by β1A integrin but did not alter the migratory behavior of β1D-expressing cells. The migration rates of β1A-transfected CHO and GD25 cells treated with TS2/16 mAb still exceeded substantially the migration rates of β1D-transfectants.
Figure 6.
The role of activation of the β1D ectodomain in the decreased migration of β1D transfectants. Cell migration of β1A and β1D transfectants on Fn was analyzed by time lapse videomicroscopy. Inhibitory mAbs PB1 and H9.2B8 were used to block the endogenous Fn-binding α5β1 integrin in CHO and αvβ3 integrin in GD25 transfectants. The experiments were performed in the absence (dark bars) or presence (hatched bars) of activating TS2/16 mAb.
Interaction of β1A and β1D Integrins with α Subunits in CHO and GD25 Transfectants
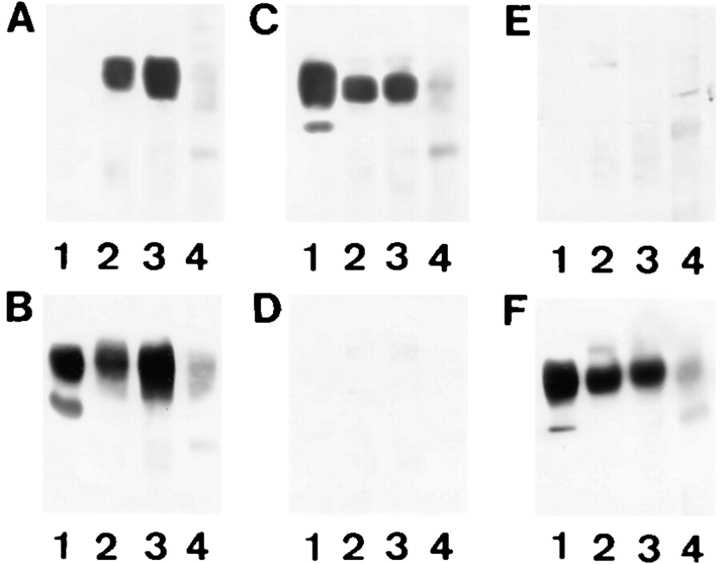
Many of the observed properties of β1D including enhanced ligand binding, elevated Fn matrix assembly, and decreased cell motility could be explained by different mode of association of β1A and β1D integrins with α subunits in the transfected cells. Therefore, we examined α subunit association for β1A and β1D in CHO and GD25 transfectants. Among various β1-associated α subunits, α3, α5, and very small amount of αv were detected in both types of transfectants by immunoprecipitation (Fig. 7). Immunoblotting of the corresponding immunoprecipitates from β1D-CHO (Fig. 7, A and B), β1A-GD25 (Fig. 7, C and D), and β1D-GD25 (Fig. 7, E and F) cells showed that equal amounts of β1D and β1A integrins were associated with α3 and α5 subunits in CHO and GD25 transfectants.
Cytoskeletal Association of β1A and β1D Integrins Correlates with Their Insolubility in Digitonin
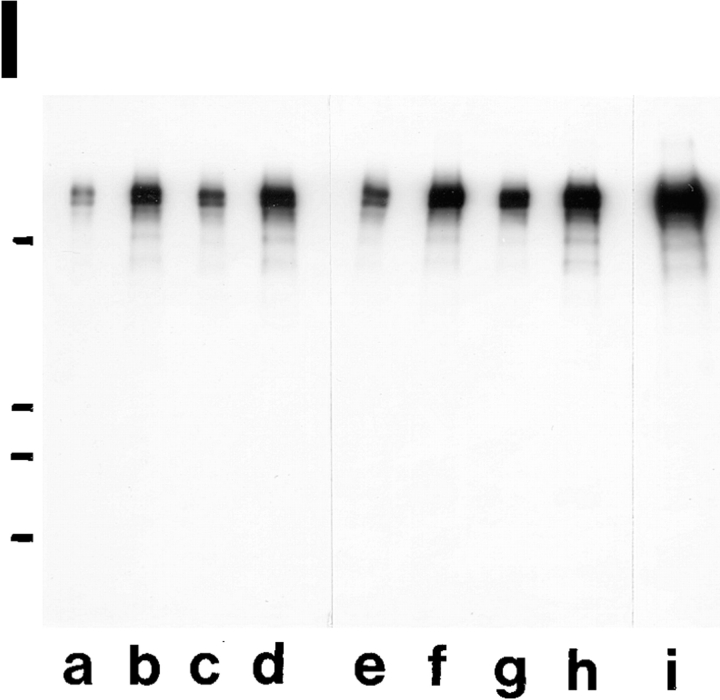
To determine whether there is a difference in cytoskeletal association between β1A and β1D integrins, we designed a method of sequential extraction using digitonin and RIPA buffers for 35S-labeled cell cultures, followed by integrin immunoprecipitation from both cellular fractions. To test whether integrin insolubility in digitonin is determined by the mode of integrin–cytoskeleton association, we compared β1 integrin immunoprecipitates from digitonin and RIPA fractions of untreated and cytochalasin D–treated β1A- and β1D-transfected CHO cells (Fig. 8). Treatment of cultured cells with cytochalasin D shifted almost all β1A and the majority of β1D to the digitonin-soluble fraction. These experiments demonstrated that insolubility of β1 integrins in digitonin depends on their association with the actin cytoskeleton.
Figure 8.
Digitonin insolubility of β1A and β1D integrins is ascribable to cytoskeletal association. 35S-labeled, transfected β1 integrins were immunoprecipitated from digitonin-soluble (S) and digitonin-insoluble (I) fractions of either untreated (−) or cytochalasin D–treated (+) β1A-CHO and β1D-CHO cells. Arrow, the mature β1 subunit; and arrowhead, the precursor form. Note a significant increase in solubility of β1A and β1D integrins in 0.1% digitonin after cytochalasin D treatment. Molecular weight markers (from top to bottom) are 200, 116, 97, 68, and 43 kD. They are indicated to the right of the gel.
β1D Integrin Displaces the Endogenous β1A and αv Subunits from Focal Adhesions and Associates Strongly with the Digitonin-insoluble Cytoskeleton
Since β1D and β1A integrins have structurally different cytoplasmic domains, and the two types of transfectants displayed dissimilar phenotypes, we next attempted to compare cytoskeletal interactions of the β1A and β1D isoforms. To localize the transfected and the endogenous β1 integrins in CHO transfectants, cells grown on Fn were double stained with anti–human β1 and anti–hamster β1 mAbs. In β1A-CHO cells, both the transfected and the endogenous β1A subunits colocalized at focal adhesions (Fig. 9, A and C). β1D integrin was prominently localized at focal adhesions of β1D-CHO cells. Surprisingly, no endogenous β1A integrin was detected in focal adhesions of β1D transfectants grown on Fn or other extracellular matrix proteins (Fig. 9, B and D; and data not shown). Similarly, both the transfected β1A and β1D integrins were targeted to focal adhesions of GD25 transfectants on Fn (Fig. 9, E and F). The endogenous αv subunit of Fn-binding αvβ3 integrin in β1A-GD25 cells was at least partially colocalized with β1A at sites of cell–matrix contact (αv does not pair with β1 integrins in GD25 cells; Wennerberg et al., 1996). In contrast, β1D displaced the endogenous αv integrins from focal adhesions (Fig. 9, G and H). Therefore, the displacement of β1A and αv integrins from focal adhesions by expressed β1D was a general phenomenon, suggesting a considerably stronger association of β1D integrin with the actin cytoskeleton.
To define biochemically the modes of β1A and β1D interaction with the cytoskeleton, both the transfected β1A and β1D, as well as the endogenous β1A and αv integrins were immunoprecipitated from soluble and cytoskeleton-associated fractions of 35S-labeled transfectants grown on Fn (Fig. 9, I and J). In β1A-CHO cells, the transfected β1A was equally distributed between the soluble and the cytoskeletal fractions, wheras the majority of the endogenous hamster β1A subunit was associated with the cytoskeleton. In contrast, β1D was found exclusively in the cytoskeletal fraction, whereas almost all the endogenous β1A integrin was present in the soluble fraction of β1D-CHO cells, displaced from the cytoskeleton (Fig. 9 I). Cytoskeletal associations of the transfected β1A and β1D integrins in GD25 transfectants were similar to those observed in CHO transfectants. Again, much of the transfected β1A integrin was digitonin soluble. However, the majority of β1D integrin was digitonin insoluble, whereas most of the endogenous αvβ3/αvβ5 integrins were displaced from their association with the cytoskeleton by the transfected β1D (Fig. 9 J).
Differential Interaction of β1A and β1D Integrins with Talin and α-Actinin
At least two cytoskeletal proteins, talin and α-actinin, are known to interact in vitro with the cytoplasmic domain of β1A integrin (Horwitz et al., 1986; Otey et al., 1990). To identify cytoskeletal proteins, associated preferentially with the β1D integrin subunit in vivo, antibody clustering of the transfected β1A and β1D integrins on the surface of CHO and GD25 transfectants was used in combination with subsequent immunoprecipitation and analysis of the immunoprecipitates (Miyamoto et al., 1995). Several cytoskeletal proteins, including actin, talin, α-actinin, and vinculin coprecipitated with the transfected β1 integrins (data not shown). When β1A and β1D immunoprecipitates, obtained with activating TS2/16 anti–human β1 mAb, were compared by immunoblotting with mAb 8d4 against talin, a significantly stronger talin band was detected in association with β1D integrin in both CHO and GD25 transfectants (Fig. 10, A, a, a′, b, and b′; and D, a, a′, b, and b′). This preferential association of talin with β1D compared with β1A integrin did not depend on the nature of anti-β1 mAb used for clustering. 12G10 mAb, which recognizes a Mn2+- and ligand-induced conformation of human β1 integrin, precipitated more talin from β1D-CHO than from β1A-CHO cells both in the absence or in the presence of 1 mM Mn2+ (Fig. 10, A, c, c′, d, and d′). In control immunoprecipitations with the 7E2 mAb against the endogenous hamster β1 integrin, equal amounts of talin were detected in association with the endogenous β1A in CHO transfectants (Fig. 10, A, e, and e′). Interestingly, when anti–human β1 integrin immunoprecipitates from both types of transfectants were probed for α-actinin, more α-actinin was detected in association with β1A than with β1D integrin (Fig. 10, A, f, and f′; and D, c, and c′). To ensure equal amounts of these isoforms in the immunoprecipitates, all the samples used in these experiments were also examined with the isoform-specific antibodies against β1A (Fig. 10, B and E) and β1D (Fig. 10, C and F) integrins (Belkin et al., 1996). Besides the immunoprecipitates from β1A-CHO and β1D-CHO cells with the conformation-specific mAb 12G10 in the absence of Mn2+ (Fig. 10, B, c; and C, c′), all other samples contained equal amounts of β1 integrins (Fig. 10, B, C, E, and F).
Figure 10.
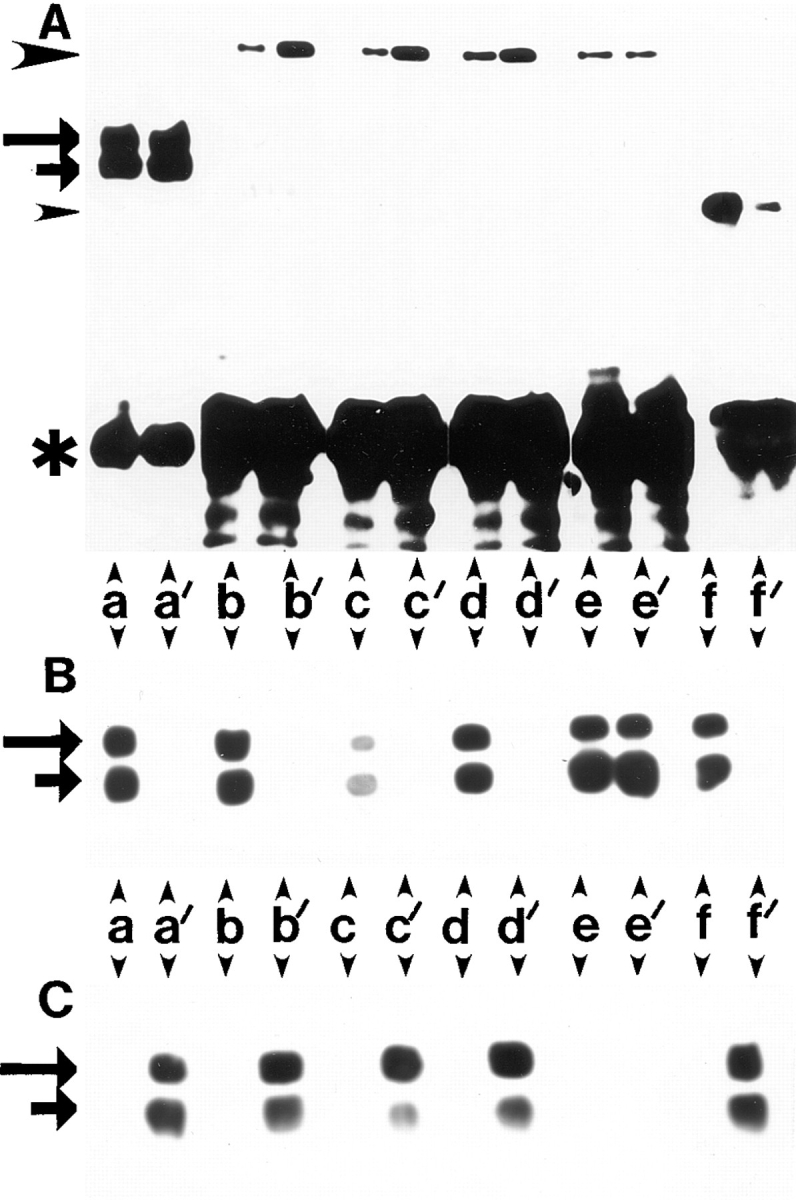
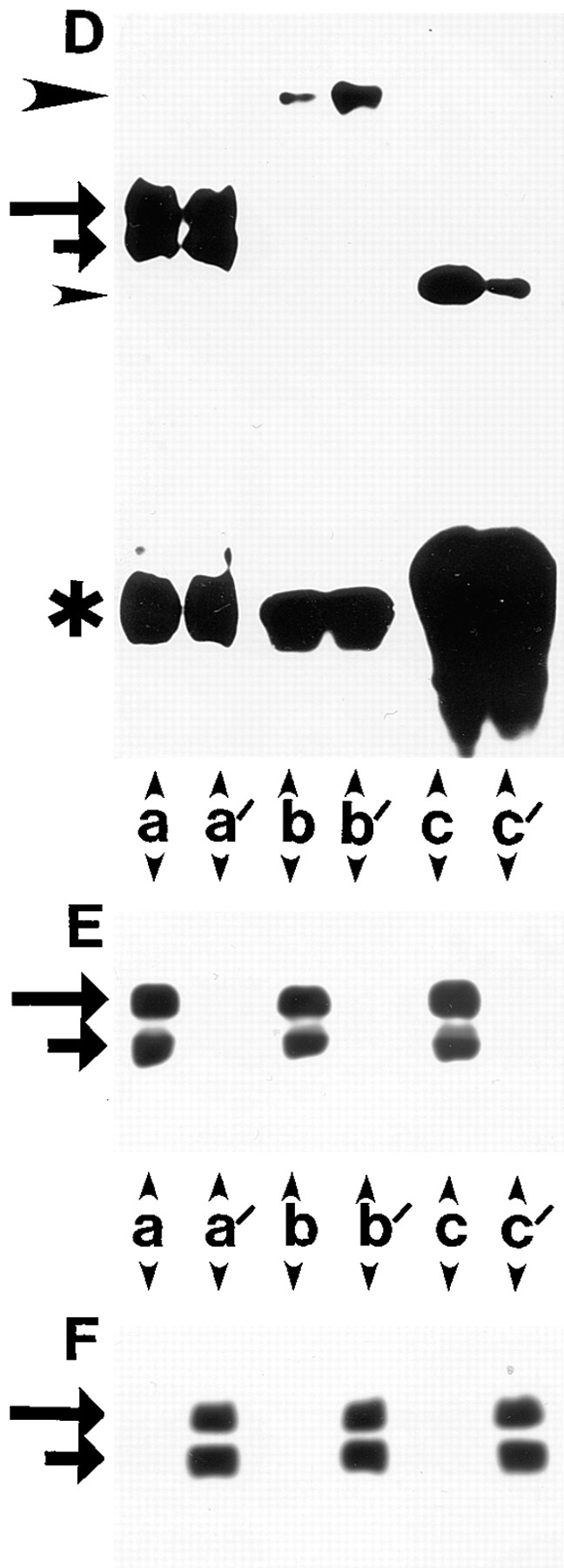
Differential association of β1A and β1D integrins with talin and α-actinin. (A and D) Coimmunoprecipitation of talin and α-actinin with β1D and β1A integrins. (A) CHO transfectants. Human β1A (a–d, and f) or β1D (a′–d′, and f′ ) integrins were immunoprecipitated with TS2/16 mAb (a, a′, b, b′, f, and f ′) or activation-dependent 12G10 mAb either in the absence of Mn2+ (c and c′) or in the presence of 1 mM Mn2+ (d and d′ ). Endogenous hamster β1A was immunoprecipitated from β1A-CHO (e) or β1D-CHO (e′ ) cells with 7E2 mAb. Immunoprecipitates were probed for β1 integrin (a and a′ ), talin (b, b′, c, c′, d, d′, e, and e′ ), or α-actinin (f and f ′ ). (D) GD25 transfectants. Human β1A (a–c) or β1D (a′–c′ ) integrins were immunoprecipitated with TS2/16 mAb and immunoprecipitates were probed for β1 integrin (a and a′ ), talin (b and b′ ), or α-actinin (c and c′ ). Long and short arrows mark the β1 integrin doublet (mature form and precursor, respectively). Large arrowheads point to talin and small arrowheads mark α-actinin. Asterisks indicate IgG heavy chains. (B, C, E, and F) The same immunoprecipitates as in A were probed for β1A (B) or β1D (C) integrins with the isoform-specific antibodies. The same immunoprecipitates as in D were blotted for β1A (E) or β1D (F).
To compare further the interactions of β1A and β1D integrins with talin and α-actinin, in vitro solid phase binding assays were performed with 125I-talin, 125I–α-actinin, and immobilized full-length synthetic peptides, corresponding to the β1A and β1D cytoplasmic domains (Fig. 11). First, we tested the binding of 125I-labeled β1A and β1D cytoplasmic domain peptides to the microtiter wells (Fig. 11 A). The amounts of the β1A and β1D peptides adsorbed to the wells were almost indistinguishable within the wide range of concentrations examined.
Figure 11.
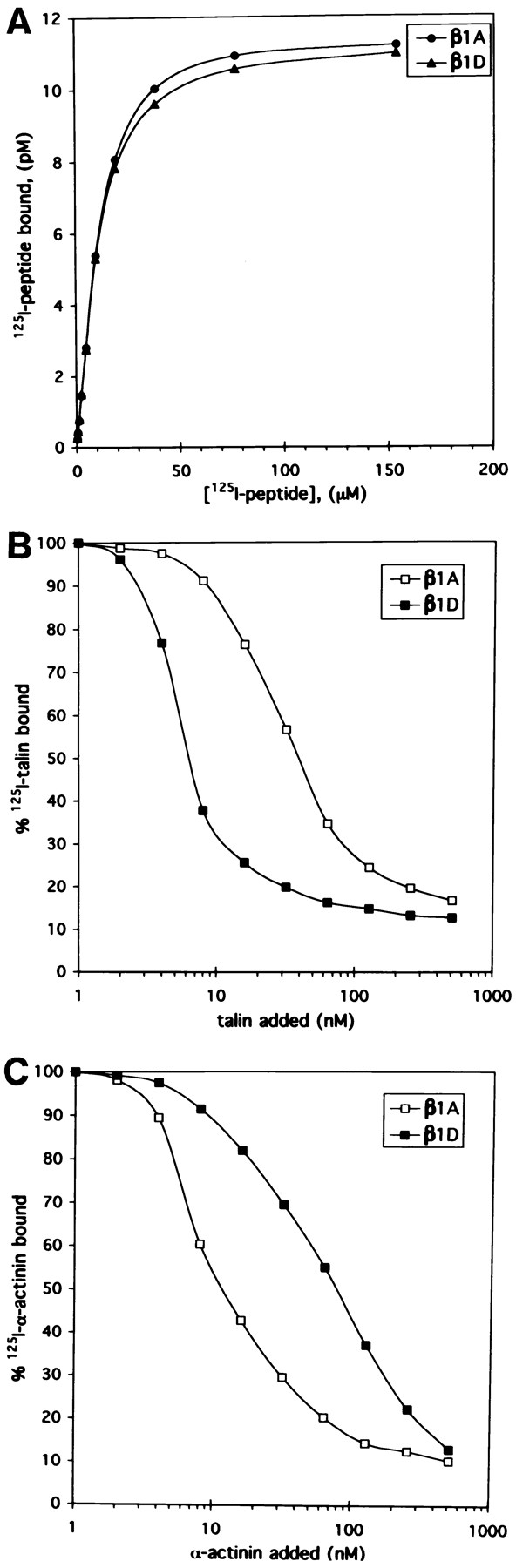
Interaction of talin and α-actinin with β1A and β1D cytoplasmic domain peptides. (A) Binding of full-length 125I-β1A (•) and 125I-β1D (▴) cytoplasmic domain peptides to the microtiter wells. Shown are the average of triplicate determinations. (B and C) Binding of 125I-talin (B) and 125I–α-actinin (C) to the microtiter wells coated with β1A (□) or β1D (▪) cytoplasmic domain peptides in the presence of the excess of unlabeled talin (B) or α-actinin (C). All points are the average of quadruplicate determinations.
Then, using the solid phase binding assay we more fully characterized the interactions of 125I-talin and 125I–α-actinin with the β1A and β1D cytoplasmic domain peptides (Fig. 11, B and C). We found that the binding of 125I-talin to the β1D peptide was severalfold higher than the binding to the β1A peptide. The apparent dissociation constants of 4.2 × 10−8 and 5.9 × 10−9 M were calculated from the competition binding curves for talin interactions with the β1A and β1D cytoplasmic peptides, respectively (Fig. 11 B). Conversely, 125I–α-actinin bound more strongly to the β1A than to the β1D cytoplasmic domain peptide (Fig. 11 C). In this case the dissociation constants were 1.2 × 10−8 M for α-actinin–β1A binding and 8.8 × 10−8 M for α-actinin–β1D binding. Together, the coimmunoprecipitation analyses and in vitro binding data demonstrated that talin interacts more strongly with the cytoplasmic domain of β1D than β1A integrin, whereas α-actinin interacts preferentially with the β1A integrin isoform.
β1D Integrin Increases Cellular Contractility
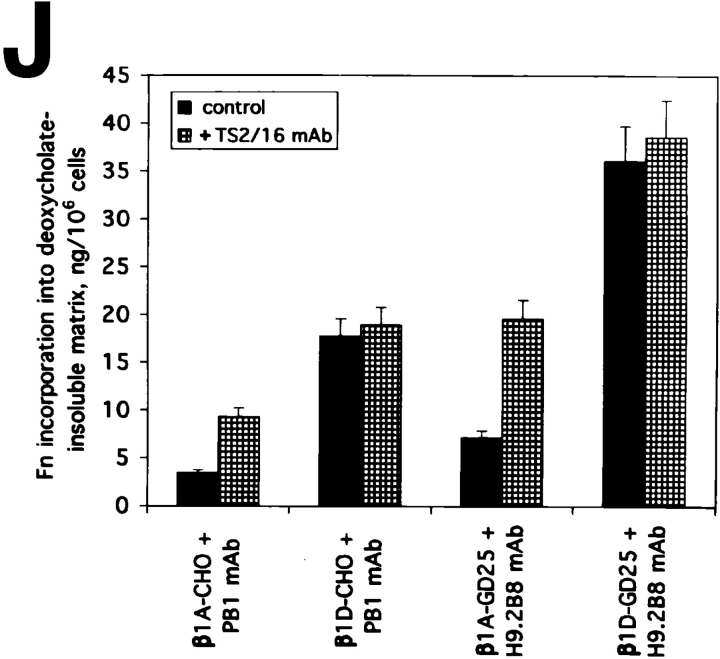
The enhanced association of β1D integrin with the actin cytoskeleton prompted us to examine whether the β1D integrin–cytoskeleton interaction increases contractility. When β1A and β1D transfectants were plated on flexible silicone rubber substrata for 1 d, β1D-transfected cells generated prominent wrinkles within the substrata. However, β1A transfectants were essentially unable to wrinkle these substrata (Fig. 12, A–D). Interestingly, the enhancement of contractility was not accompanied by substantially elevated phosphorylation of myosin regulatory light chains in β1D-CHO cells (Fig. 12 E). Therefore, the observed increase in cellular contractility of β1D transfectants appears to be mostly because of enhancement of actin–membrane attachment, and did not depend on significant changes of myosin ATPase activity.
Figure 12.
β1D integrin elevates cellular contractility without affecting phosphorylation of myosin light chains. (A–D) Rubber substrate contractility assay for β1A and β1D transfectants. β1A-CHO (A), β1D-CHO (B), β1A-GD25 (C), and β1D-GD25 (D) cells were plated for 1 d on silicone rubber substrata and photographed. (E) Myosin light chain phosphorylation in CHO transfectants. Myosin was immunoprecipitated from 32P-labeled β1A-CHO cells (a) and β1D-CHO cells (b). Immunoprecipitates were analyzed by SDS-PAGE and autoradiography. Arrowhead points to myosin light chains. Bar, 200 μm.
Discussion
In this work we have analyzed muscle β1D integrin, comparing its properties with those of the common β1A isoform. We found that the unique cytoplasmic sequence of β1D endows this molecule with the distinctive functional properties. β1D integrin displays an increased affinity for both the extracellular matrix ligands and the actin cytoskeleton. Expression of β1D causes a marked phenotypic conversion of both CHO and β1-deficient GD25 cells. The β1D phenotype includes altered morphology, retarded spreading, enhanced ligand binding, and extracellular matrix assembly, as well as reduced migration and significantly increased contractility.
Together, the increased integrin–ligand and integrin– cytoskeleton associations mediated by the β1D isoform, cause a significant reinforcement of the entire cytoskeleton–matrix link. Unlike two other β1 integrin variants β1B and β1C, which are distributed uniformly at the cell surface and are unable to interact with the actin cytoskeleton, β1D is readily targeted to sites of cell–matrix adhesion upon expression in nonmuscle cells. Furthermore, significantly stronger association of β1D with the actin cytoskeleton can be mediated by its enhanced interaction with talin and might change the organization of focal adhesions in β1D transfectants compared with these structures in β1A-expressing cells. However, by immunofluorescence with a large panel of antibodies against cytoskeletal and focal adhesion proteins, we did not observe prominent differences in the organization of stress fibers and focal adhesions between β1D and β1A transfectants. The intensities of immunostaining for talin, vinculin, α-actinin, paxillin, focal adhesion kinase, and phosphotyrosine at focal adhesions were similar in both types of transfectants (data not shown). Apparently, the differences in the affinities between β1A/β1D and talin/α-actinin might be insufficient to detect preferential accumulation of these cytoskeletal proteins at focal adhesions of β1A- and β1D-transfected cells by immunofluorescence. Nevertheless, β1D transfectants appeared to be more contractile without changes in myosin light chain phosphorylation and displayed increased resistance of focal adhesions against disassembly by contractility inhibitors BDM and H7 (data not shown). These observations pointed to the increased stability of focal adhesions in cells expressing β1D integrin that occurs primarily because of enhancement of actin–membrane attachment.
Simultaneously, displacement of the endogenous β1A subunit from sites of cell–matrix adhesion by β1D generates a unique “dominant-positive phenotype” of the transfected nonmuscle cells. In this situation, the β1 integrin-mediated, cytoskeleton–matrix link is built exclusively by the β1D integrin and lacks the β1A isoform. Displacement of the major β1A integrin isoform from cell–matrix adhesion sites appears to be a general phenomenon of β1D expression, caused by its enhanced association with the actin cytoskeleton. This displacement is observed on various extracellular matrix substrata in a number of nonmuscle cells (Belkin, A.M., unpublished data). Similarly, in GD25 transfectants the major endogenous αvβ3 integrin appeared to be displaced from the sites of cell–matrix contact. The altered structure of the β1D integrin cytoplasmic domain also leads to a conformational change in its ectodomain that increases the ligand-binding affinity of β1D-containing heterodimers. The constitutive activation of β1D on the cell surface, combined with its stronger interaction with the actin cytoskeleton, enhance Fn matrix assembly by β1D transfectants (Wu et al., 1995).
Recently, Kucik et al. (1996) demonstrated that nonadhesive inactive state of LFA-1 integrin in lymphocytes is maintained by the cytoskeleton. Both PMA and cytochalasin D were shown to stimulate lymphocyte adhesion, which was accompanied by activation of their major β2 integrin and its release of cytoskeletal constraints. This points to the opposite mechanisms of cytoskeletal control of integrin activation between β2 and β1 integrin subfamilies and might reflect profoundly dissimilar physiology of nonadherent lymphocytes and adhesion-dependent cells, including striated muscles.
Previously, modulation of the ligand-binding affinity of integrin β subunits was demonstrated using either activating antibodies (Arroyo et al., 1992; Faull et al., 1993) or point mutations in the β1 and β3 subunit cytoplasmic domains (Ginsberg et al., 1992; Takada et al., 1992; O'Toole et al., 1994, 1995; Schwartz et al., 1995). Here we present the results showing an existence of physiological mechanism reinforcing the cytoskeleton–matrix link in muscle cells based on modulation of integrin adhesive function. This mechanism involves a novel type of inside-out integrin signaling where activation of the extracellular domain of the integrin β subunit is controlled by alternative splicing of its cytodomain. Since the expression of β1D and β1A isoforms is developmentally regulated in muscle (Belkin et al., 1996), differentiating muscle cells can control the overall strength of cytoskeleton–matrix attachment via alternative splicing of the β1 integrin subunit.
The organization of focal adhesions in β1D-transfected nonmuscle cells appears to be very similar to that found in differentiated muscle cells. In both situations, β1D is the only β1 isoform localized at cell–matrix adhesive structures (Belkin et al., 1996). Therefore, the enhanced cytoskeleton–matrix association and stabilization of focal adhesions, mediated by β1D in the transfectants, might reflect the major role for this integrin in muscle. Since talin, a major structural component of focal adhesions, accumulates at muscle adhesions (Belkin et al., 1986; Tidball et al., 1986), it can also serve as a key cytoskeletal element linking β1D integrin to actin filaments in muscle. In contrast, we found that α-actinin, a focal adhesion component that interacts with β1A in vitro (Otey et al., 1990), binds β1D less strongly than β1A. This correlates with the absence of α-actinin at myotendinous junctions, the major sites of force transmission in muscle (Tidball, 1987). Additionally, certain muscle-specific cytoskeletal proteins, including dystrophin, may contribute to linking β1D integrin to the subsarcolemmal cytoskeleton.
Many existing models of focal adhesion assembly imply that their formation proceeds from outside the cell inward, starting from integrin clustering by their ligands on the cell surface (Yamada and Miyamoto, 1995; Craig and Johnson, 1996). However, rho-stimulated contractility has been shown to drive the formation of integrin-containing focal adhesion complexes from inside the cell (Hotchin and Hall, 1995; Burridge and Chrzanowska-Wodnicka, 1996; Chrzanowska-Wodnicka and Burridge, 1996). Also, recent data on muscle integrins in Drosophila melanogaster point to certain ligand-independent intracellular mechanisms directing localization of βps integrins (analogous to β1 integrins in vertebrates) to sites of their function in embryonic muscles (Martin-Bermudo and Brown, 1996). The altered structure of the β1D cytoplasmic domain and the enhanced interaction of this integrin with the cytoskeleton might determine its ligand-independent targeting to muscle adhesive structures.
Increased Fn matrix assembly by CHO cells expressing β1D can be driven by a combination of higher ligand-binding affinity of this integrin, reinforced actin-membrane association and enhanced contractility of β1D transfectants. Recently, it was shown that the appearance of new Fn matrix assembly sites on the cell surface is stimulated by lysophosphatidic acid, an agent that promotes contractility (Jalink and Moolenaar, 1992; Zhang et al., 1994; Chrzanowska-Wodnicka and Burridge, 1996). Wu and coworkers demonstrated that both integrin activation on the cell surface and integrin–cytoskeleton interactions (postoccupancy events) are essential for the assembly of a Fn matrix (Wu et al., 1995). Our data are in agreement with these findings. The increased Fn matrix assembly mediated by β1D correlates well with the larger proportion of activated integrins on the cell surface, the more stable integrin– cytoskeleton linkage and enhanced contractility in these transfectants. Interestingly, the observed enhancement of Fn matrix assembly by β1D appeared to be greater than the increase in ligand-binding affinity for β1D transfectants. Even though an activation of β1A integrin with TS2/ 16 mAb significantly increased Fn matrix assembly by β1A transfectants, this still did not convert them entirely to the β1D phenotype. These differences might reflect the enhanced integrin–cytoskeleton association and contractility in β1D transfectants.
Finally, the increased ligand-binding, contractility, and Fn matrix deposition contribute to the reduced cell migration of β1D transfectants. In accordance with recent findings of Palecek et al. (1997), activation of the β1A ectodomain reduced the migration of the transfectants at substrate concentrations and integrin expression levels used in our experiments. Notably, integrin activation in the case of β1A transfectants did not decrease their migration rates to the levels characteristic for β1D-expressing cells, again suggesting an important role for integrin–cytoskeleton interactions in the generation of the β1D phenotype. Together, our data demonstrate that the whole set of alterations displayed by cells expressing β1D, is determined by the distinctive structure of the β1D cytoplasmic domain. The alternatively spliced sequence of this β1 integrin isoform both enhances its association with the actin cytoskeleton and increases receptor–ligand interaction due to constitutive activation of the β1D ectodomain. Both these factors contribute to increased Fn matrix assembly and decreased migration of β1D transfectants.
The changes of nonmuscle cells triggered by expression of the β1D isoform are analogous to the transitions that muscle cells undergo during differentiation, accompanied by a gradual increase in the expression of this integrin (Belkin et al., 1996). Thus, growing myotubes possess large, extremely stable adhesions, their spreading is greatly inhibited and even early immature myotubes cease to migrate. Although Fn matrix assembly is strongly decreased in muscle cells, upregulation of synthesis and enhanced deposition of certain laminin isoforms is typical for myodifferentiation both in culture and in vivo. During myodifferentiation, a dramatic increase in cellular contractility has to be counterbalanced by the reinforcement of the cytoskeleton–matrix link. All these phenotypic transitions characteristic for differentiating muscle cells generate a requirement for the novel integrin, strengthening the association between the actin cytoskeleton and the surrounding extracellular matrix. This requirement determines the distinctive properties of β1D and defines a critical role for this β1 integrin cytoplasmic domain in the organization and function of adhesive structures in muscle tissues.
Acknowledgments
We are grateful to Dr. L. Arnold (University of North Carolina at Chapel Hill) for his advice and help with fluocytometry.
This work was supported by National Institutes of Health grants R29 CA72961 to A.M. Belkin, and GM29860 and HL45100 to K. Burridge, and grants from the Italian Association for Research on Cancer, Biomed and Telethon to G. Tarone.
Footnotes
Address all correspondence to A.M. Belkin, Department of Biochemistry, American Red Cross, 15601 Crabbs Branch Way, Rockville, MD 20855. Tel.: (301) 738-0725. Fax: (301) 738-0794. E-mail: Belkina@usa.redcross.org
References
- Altruda F, Cervella P, Tarone G, Botta C, Balzac F, Stefanuto G, Silengo L. A human integrin β1 subunit with a unique cytoplasmic domain generated by alternative mRNA processing. Gene. 1990;95:261–266. doi: 10.1016/0378-1119(90)90369-3. [DOI] [PubMed] [Google Scholar]
- Argraves WS, Suzuki S, Arai H, Thompson K, Piersbacher MD, Ruoslahti E. Amino acid sequence of the human fibronectin receptor. J Cell Biol. 1987;105:1183–1190. doi: 10.1083/jcb.105.3.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo AG, Sanchez-Mateos P, Campanero MR, Martin-Padura I, Dejana E, Sanchez-Madrid F. Regulation of the VLA integrin-ligand interactions through the β1 subunit. J Cell Biol. 1992;117:659–670. doi: 10.1083/jcb.117.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzac F, Belkin AM, Koteliansky VE, Balabanov YV, Altruda F, Silengo L, Tarone G. Expression and functional analysis of a cytoplasmic domain variant of the β1 integrin subunit. J Cell Biol. 1993;121:171–178. doi: 10.1083/jcb.121.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balzac F, Retta SF, Albini A, Melchiorri A, Koteliansky VE, Geuna M, Silengo L, Tarone G. Expression of β1B integrin isoform in CHO cells results in a dominant negative effect on cell adhesion and motility. J Cell Biol. 1994;127:557–565. doi: 10.1083/jcb.127.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudoin C, van der Flier A, Borradori L, Sonnenberg A. Genomic organization of the mouse β1 gene: conservation of the β1D but not of the β1B and β1C integrin splice variants. Cell Adhes Comm. 1996;4:1–11. doi: 10.3109/15419069609010759. [DOI] [PubMed] [Google Scholar]
- Bazzoni G, Shih DT, Buck CA, Hemler ME. Monoclonal antibody 9EG7 defines a novel β1 integrin epitope induced by soluble ligand and manganese, but inhibited by calcium. J Biol Chem. 1995;270:25570–25577. doi: 10.1074/jbc.270.43.25570. [DOI] [PubMed] [Google Scholar]
- Belkin AM, Zhidkova NI, Koteliansky VE. Localization of talin in skeletal and cardiac muscles. FEBS (Fed Eur Biochem Soc) Lett. 1986;20:32–36. doi: 10.1016/0014-5793(86)80505-1. [DOI] [PubMed] [Google Scholar]
- Belkin VM, Belkin AM, Koteliansky VE. Human smooth muscle VLA-1 integrin: Purification, substrate specificity, localization in aorta and expression during development. J Cell Biol. 1990;111:2159–2170. doi: 10.1083/jcb.111.5.2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belkin AM, Zhidkova NI, Balzac F, Altruda F, Tomatis D, Maier A, Tarone G, Koteliansky VE, Burridge K. β1D integrin displaces the β1A isoform in striated muscles: Localization at junctional structures and signaling potential in nonmuscle cells. J Cell Biol. 1996;132:211–226. doi: 10.1083/jcb.132.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozyczko D, Decker C, Muschler J, Horwitz AF. Integrin on developing and adult skeletal muscle. Exp Cell Res. 1989;183:72–91. doi: 10.1016/0014-4827(89)90419-9. [DOI] [PubMed] [Google Scholar]
- Brancaccio, M., S. Cabodi, A.M. Belkin, G. Collo, V.E. Koteliansky, D. Tomatis, F. Altruda, L. Silengo, and G. Tarone. 1997. Differential onset of expression of α7 and β1D integrin during mouse heart and skeletal muscle development. Cell Adhes. Commun. In press. [DOI] [PubMed]
- Brown PJ, Juliano RL. Selective inhibition of fibronectin-mediated cell adhesion by monoclonal antibodies to a cell-surface glycoprotein. Science. 1985;228:1448–1451. doi: 10.1126/science.4012302. [DOI] [PubMed] [Google Scholar]
- Brown PJ, Juliano RL. Monoclonal antibodies to distinctive epitopes on the α and β subunits of the fibronectin receptor. Exp Cell Res. 1988;177:303–318. doi: 10.1016/0014-4827(88)90464-8. [DOI] [PubMed] [Google Scholar]
- Burridge K, Chrzanowska-Wodnicka M. Focal adhesions, contractility and signaling. Annu Rev Cell Dev Biol. 1996;12:463–518. doi: 10.1146/annurev.cellbio.12.1.463. [DOI] [PubMed] [Google Scholar]
- Carter WE, Wayner EA, Bouchard TS, Kaur P. The role of integrins α2β1 and α3β1 in cell–cell and cell–substrate adhesion of human epidermal cells. J Cell Biol. 1990;110:1387–1404. doi: 10.1083/jcb.110.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. J Cell Biol. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier N, Wang K. Human platelet P235: a high Mrprotein which restricts the length of actin filaments. FEBS (Fed Eur Biochem Soc) Lett. 1982;143:205–210. doi: 10.1016/0014-5793(82)80099-9. [DOI] [PubMed] [Google Scholar]
- Craig SW, Johnson RP. Assembly of focal adhesions: progress, paradigms, and portents. Curr Opin Cell Biol. 1996;8:74–85. doi: 10.1016/s0955-0674(96)80051-2. [DOI] [PubMed] [Google Scholar]
- Danowski BA. Fibroblast contractility and actin organization are stimulated by microtubule inhibitors. J Cell Sci. 1989;93:255–266. doi: 10.1242/jcs.93.2.255. [DOI] [PubMed] [Google Scholar]
- Defilippi P, Silengo L, Tarone G. α6β1 integrin (laminin receptor) is downregulated by tumor necrosis factor alpha and interleukin-1 beta in human endothelial cells. J Biol Chem. 1992;267:18303–18307. [PubMed] [Google Scholar]
- Dunn GA, Brown AF. Alignment of fibroblasts on grooved surfaces described by a simple geometric transformation. J Cell Sci. 1986;83:313–340. doi: 10.1242/jcs.83.1.313. [DOI] [PubMed] [Google Scholar]
- Ellis L, Clauser E, Morgan DO, Edery M, Roth RA, Rutter WJ. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromise insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986;45:721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- Fassler R, Rohmedel J, Maltsev V, Bloch W, Lentini S, Guan K, Gullberg D, Hescheler J, Addicks K, Wodus AM. Differentiation and integrity of cardiac muscle cells are impaired in the presence of β1 integrin. J Cell Sci. 1996;109:2989–2999. doi: 10.1242/jcs.109.13.2989. [DOI] [PubMed] [Google Scholar]
- Faull RG, Kovach NL, Harlan JM, Ginsberg MH. Affinity modulation of integrin α5β1: Regulation of the functional response by soluble fibronectin. J Cell Biol. 1993;121:155–162. doi: 10.1083/jcb.121.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg MH, Du X, Plow EF. Inside-out integrin signaling. Curr Opin Cell Biol. 1992;4:766–771. doi: 10.1016/0955-0674(92)90099-x. [DOI] [PubMed] [Google Scholar]
- Harris AK, Wild P, Stopak D. Silicone rubber substrata: a new wrinkle in the study of cell locomotion. Science. 1980;208:177–179. doi: 10.1126/science.6987736. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Haimovich B, Reszka A, Boettiger D, Horwitz AF. Expression and function of chicken integrin β1 subunit and its cytoplasmic domain mutants in mouse NIH 3T3 cells. J Cell Biol. 1990;110:175–182. doi: 10.1083/jcb.110.1.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemler ME, Sanchez-Madrid F, Flotte TJ, Krensky AM, Burakoff SJ, Bhan AK, Springer TA, Strominger JL. Glycoproteins of 210,000 and 130,000 m.w. on activated T cells: cell distribution and antigenic relation to components on resting cells and T cell lines. J Immunol. 1984;132:3011–3018. [PubMed] [Google Scholar]
- Hemler, M.E., J.B. Weitzman, R. Pasqualini, S. Kawaguchi, P.D. Kassner, and F.B. Berdichevsky. 1994. Structure, biochemical properties and biological functions of integrin cytoplasmic domains. In Integrin: The Biological Problem. Y. Takada, editor. CRC Press, Ann Arbor, MI. 1–35.
- Hermanowski-Vosatka A, van Strijp JAG, Swiggard WJ, Wright SD. Integrin-modulating factor 1: a lipid that alters the function of leukocyte integrins. Cell. 1992;68:341–352. doi: 10.1016/0092-8674(92)90475-r. [DOI] [PubMed] [Google Scholar]
- Horwitz AF, Duggan E, Buck C, Beckerle M, Burridge K. Interaction of plasma membrane fibronectin receptor with talin: a transmembrane linkage. Nature. 1986;320:531–533. doi: 10.1038/320531a0. [DOI] [PubMed] [Google Scholar]
- Hotchin NA, Hall A. The assembly of integrin adhesion complexes requires both extracellular matrix and intracellular rho/rac GTPases. J Cell Biol. 1995;131:1857–1865. doi: 10.1083/jcb.131.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes PE, Renshaw MW, Pfaff M, Forsyth J, Keivens VM, Shwartz MA, Ginsberg MH. Suppression of integrin activation: a novel function of Ras/Raf initiated MAP kinase pathway. Cell. 1997;88:521–530. doi: 10.1016/s0092-8674(00)81892-9. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: versatility, modulation and signalling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Jalink K, Moolenaar WH. Thrombin receptor activation causes rapid neural cell rounding and neurite retraction independent of classic second messengers. J Cell Biol. 1992;118:411–419. doi: 10.1083/jcb.118.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucik DF, Dustin ML, Miller JM, Brown EJ. Adhesion-activating phorbol ester increases the mobility of leukocyte integrin LFA-1 in cultured lymphocytes. J Clin Invest. 1996;97:2139–2144. doi: 10.1172/JCI118651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFlamme SE, Akiyama SK, Yamada KM. Regulation of fibronectin receptor distribution. J Cell Biol. 1992;117:437–447. doi: 10.1083/jcb.117.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Languino LR, Ruoslahti E. An alternative form of the integrin β1 subunit with variant cytoplasmic domain. J Biol Chem. 1992;267:7116–7120. [PubMed] [Google Scholar]
- Lewis JM, Schwartz MA. Mapping in vivo associations of cytoplasmic proteins with integrin β1 cytoplasmic domain mutants. Mol Biol Cell. 1995;6:151–160. doi: 10.1091/mbc.6.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Bermudo MD, Brown NH. Intracellular signals direct integrin localization to sites of function in embryonic muscles. J Cell Biol. 1996;134:217–226. doi: 10.1083/jcb.134.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeown-Longo PJ, Mosher DF. Interaction of 70,000-mol-wt amino-terminal fragment of fibronectin with the matrix assembly receptor of fibroblasts. J Cell Biol. 1985;100:364–374. doi: 10.1083/jcb.100.2.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menko AS, Boettiger D. Occupation of the extracellular matrix receptor, integrin, is a central point of myogenic differentiation. Cell. 1987;51:51–57. doi: 10.1016/0092-8674(87)90009-2. [DOI] [PubMed] [Google Scholar]
- Meredith J, Jr, Takada Y, Fornaro M, Languino LR, Schwartz MA. Inhibition of cell cycle progression by the alternatively spliced integrin β1C. Science. 1995;269:1570–1572. doi: 10.1126/science.7545312. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Akiyama SK, Yamada KM. Synergistic roles for receptor occupancy and aggregation in integrin transmembrane function. Science. 1995;267:883–885. doi: 10.1126/science.7846531. [DOI] [PubMed] [Google Scholar]
- Mould AP, Garratt AN, Askari JA, Akiyama SK, Humphries MJ. Identification of a novel anti-integrin monoclonal antibody that recognizes a ligand-induced binding site epitope on the β1 subunit. FEBS (Fed Eur Biochem Soc) Lett. 1995;363:118–122. doi: 10.1016/0014-5793(95)00301-o. [DOI] [PubMed] [Google Scholar]
- Mould AP. Getting integrins into shape: recent insights into how integrin activity is regulated by conformational changes. J Cell Sci. 1996;109:2613–2618. doi: 10.1242/jcs.109.11.2613. [DOI] [PubMed] [Google Scholar]
- Moulder K, Roberts K, Shevach EM, Coligan JE. The mouse vitronectin receptor is a T cell activation antigen. J Exp Med. 1991;173:343–347. doi: 10.1084/jem.173.2.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otey CA, Pavalko FM, Burridge K. An interaction between α-actinin and the β1 integrin subunit in vitro. J Cell Biol. 1990;111:721–729. doi: 10.1083/jcb.111.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole TE, Loftus JC, Du X, Glass AA, Ruggeri ZM, Shatill SJ, Plow EF, Ginsberg MH. Affinity modulation of the αIIbβ3 integrin (platelet GPIIb-IIIa) is an intrinsic property of the receptor. Cell Regul. 1990;1:883–893. doi: 10.1091/mbc.1.12.883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole TE, Katagiri Y, Faull RJ, Peter K, Tamura R, Quaranta V, Loftus JC, Shatill SJ, Ginsberg MH. Integrin cytoplasmic domains mediate inside-out signal transduction. J Cell Biol. 1994;124:1047–1059. doi: 10.1083/jcb.124.6.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Toole TE, Ylanne J, Culley BM. Regulation of integrin affinity states through an NPXY motif in the β subunit cytoplasmic domain. J Biol Chem. 1995;270:8553–8558. doi: 10.1074/jbc.270.15.8553. [DOI] [PubMed] [Google Scholar]
- Palecek SP, Loftus JC, Ginsberg MH, Lauffenburger DA, Horwitz AF. Integrin-ligand binding properties govern cell migration speed through cell-substratum adhesiveness. Nature. 1997;385:537–540. doi: 10.1038/385537a0. [DOI] [PubMed] [Google Scholar]
- Reszka AA, Hayashi Y, Horwitz AF. Identification of amino acid sequences in the integrin β1 cytoplasmic domain implicated in cytoskeletal association. J Cell Biol. 1992;117:1321–1330. doi: 10.1083/jcb.117.6.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer LH, McLean N, Turner CE, Burridge K. Tyrosine kinase activity, cytoskeletal organization and motility in human vascular endothelial cells. Mol Biol Cell. 1994;5:349–361. doi: 10.1091/mbc.5.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MA, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- Smyth SS, Joneckis CC, Parise LV. Regulation of vascular integrins. Blood. 1993;81:2827–2843. [PubMed] [Google Scholar]
- Tamkun JW, DeSimone DW, Fonda D, Patel RS, Buck C, Horwitz AF, Hynes RO. Structure of integrin, a glycoprotein involved in transmembrane linkage between fibronectin and actin. Cell. 1986;46:271–282. doi: 10.1016/0092-8674(86)90744-0. [DOI] [PubMed] [Google Scholar]
- Takada Y, Ylanne J, Mandelman D, Puzon F, Ginsberg MH. A point mutation of integrin β1 subunit blocks binding of α5β1 to fibronectin and invasin but not recruitment to adhesion plaques. J Cell Biol. 1992;119:913–921. doi: 10.1083/jcb.119.4.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidball JG. α-actinin is absent from the terminal segments of myofibrils and from subsarcolemmal densities in frog skeletal muscles. Exp Cell Res. 1987;170:469–482. doi: 10.1016/0014-4827(87)90321-1. [DOI] [PubMed] [Google Scholar]
- Tidball JG, O'Halloran T, Burridge K. Talin at myotendinous junctions. J Cell Biol. 1986;103:1465–1472. doi: 10.1083/jcb.103.4.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Flier A, Kuikman I, Baudoin C, van der Neut R, Sonnenberg A. A novel β1 integrin isoform produced by alternative splicing: unique expression in cardiac and skeletal muscle. FEBS (Fed Eur Biochem Soc) Lett. 1995;369:340–344. doi: 10.1016/0014-5793(95)00814-p. [DOI] [PubMed] [Google Scholar]
- Volk T, Fessler LI, Fessler JH. A role for integrin in the formation of sarcomeric cytoarchitecture. Cell. 1990;63:525–536. doi: 10.1016/0092-8674(90)90449-o. [DOI] [PubMed] [Google Scholar]
- Wennerberg K, Lohikangas L, Gullberg D, Pfaff M, Johansson S, Fassler R. β1 integrin-dependent and -independent polymerization of fibronectin. J Cell Biol. 1996;132:227–238. doi: 10.1083/jcb.132.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C, Bauer JS, Juliano RL, McDonald JA. The α5β1 integrin fibronectin receptor, but not the α5 cytoplasmic domain, functions in an early and essential step in fibronectin matrix assembly. J Biol Chem. 1993;268:21883–21888. [PubMed] [Google Scholar]
- Wu C, Keivens VM, O'Toole TE, McDonald JA, Ginsberg MH. Integrin activation and cytoskeletal interaction are essential for the assembly of a fibronectin matrix. Cell. 1995;83:715–724. doi: 10.1016/0092-8674(95)90184-1. [DOI] [PubMed] [Google Scholar]
- Yamada KM, Miyamoto S. Integrin transmembrane signaling and cytoskeletal control. Curr Opin Cell Biol. 1995;7:681–689. doi: 10.1016/0955-0674(95)80110-3. [DOI] [PubMed] [Google Scholar]
- Ylanne J, Virtanen I. The Mr 140,000 fibronectin receptor complex in normal and virus-transformed human fibroblasts and in fibrosarcoma cells: identical localization and function. Int J Cancer. 1989;43:1126–1136. doi: 10.1002/ijc.2910430628. [DOI] [PubMed] [Google Scholar]
- Ylanne J, Chen Y, O'Toole TE, Loftus JC, Takada Y, Ginsberg MH. Distinct roles of integrin α and β subunit cytoplasmic domains in cell spreading and the formation of focal adhesions. J Cell Biol. 1993;122:223–233. doi: 10.1083/jcb.122.1.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Checovich WJ, Peters DM, Albrecht RM, Mosher DF. Modulation of cell surface fibronectin assembly sites by lysophosphatidic acid. J Cell Biol. 1994;127:1447–1459. doi: 10.1083/jcb.127.5.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Vuori K, Wang H-G, Reed JC, Ruoslahti E. Integrin activation by R-ras. Cell. 1996;85:61–69. doi: 10.1016/s0092-8674(00)81082-x. [DOI] [PubMed] [Google Scholar]
- Zhidkova NI, Belkin AM, Mayne R. Novel isoform of β1 integrin expressed in skeletal and cardiac muscle. Biochem Biophys Res Comm. 1995;214:279–285. doi: 10.1006/bbrc.1995.2285. [DOI] [PubMed] [Google Scholar]