Abstract
The major glucose transporter of the parasitic protozoan Leishmania enriettii exists in two isoforms, one of which (iso-1) localizes to the flagellar membrane, while the other (iso-2) localizes to the plasma membrane of the cell body, the pellicular membrane. These two isoforms differ only in their cytosolic NH2-terminal domains. Using immunoblots and immunofluorescence microscopy of detergent-extracted cytoskeletons, we have demonstrated that iso-2 associates with the microtubular cytoskeleton that underlies the cell body membrane, whereas the flagellar membrane isoform iso-1 does not associate with the cytoskeleton. Deletion mutants that remove the first 25 or more amino acids from iso-1 are retargeted from the flagellum to the pellicular membrane, suggesting that these deletions remove a signal required for flagellar targeting. Unlike the full-length iso-1 protein, these deletion mutants associate with the cytoskeleton. Our results suggest that cytoskeletal binding serves as an anchor to localize the iso-2 transporter within the pellicular membrane, and that the flagellar targeting signal of iso-1 diverts this transporter into the flagellar membrane and away from the pellicular microtubules.
Differential targeting of integral membrane proteins plays a critical role in defining specialized compartments and structures in eukaryotic cells. Considerable attention has been given to the mechanisms of protein targeting in yeast and higher eukaryotic cells. One well-characterized mechanism for selective retention of integral membrane proteins involves association of the protein with the cytoskeleton. Band 3 (or AE1, anion exchanger 1), a mammalian erythrocyte anion exchanger, associates with ankyrin (4, 8), which binds directly to the cytoskeletal protein β-spectrin. Ankyrin acts as a tether, effectively securing band 3 to the cytoskeleton. The cytoskeletal tethering has been shown to be critical for maintaining erythrocyte cell morphology and for restricting AE1 to the polarized apical or basolateral cell surface of epithelial cells (1, 9). In epithelial cells, the Na+, K+-ATPase associates with ankryin in a similar manner (14, 19, 24, 26). In neurons, the glycine receptor associates with gephyrin, a protein that binds to microtubules (17). Cytoskeletal tethering functions to localize and concentrate the glycine receptor at postsynaptic sites where these receptors function in neurotransmission (16, 18). Thus, cytoskeletal association of specific integral membrane proteins is likely to be widespread among different cell types and organisms, and is a major mechanism for restricting proteins to discrete membrane subdomains.
The plasma membranes of trypanosomatid protozoa such as trypanosomes and Leishmania species can be divided into at least three structurally and functionally distinct subdomains (2): (a) the pellicular membrane that surrounds the cell body, (b) the flagellar membrane that covers the flagellum, and (c) the flagellar pocket membrane, an invagination of the plasma membrane that is located at the base of the flagellum. These membranes are physically contiguous, and together constitute the plasma membrane surface of the parasite. Currently, however, little is known about how these membranes differ in protein composition, or how proteins are targeted to and retained within each subdomain.
We have shown previously (30) that two closely related isoforms of the major glucose transporter from the parasitic protozoan Leishmania enriettii are differentially localized. Isoform-1 (iso-1)1 is located primarily in the flagellar membrane, whereas isoform-2 (iso-2) is localized primarily to the pellicular membrane surrounding the cell body. Hence, although these two membranes are contiguous, the differential localization of these glucose transporter isoforms underscores the fact that they have different protein compositions, and constitute distinct domains of the plasma membrane. Both isoforms are also present in the membrane of the flagellar pocket, the functionally specialized domain of the plasma membrane that receives proteins ultimately destined for the pellicular and flagellar membranes or secreted into the extracellular medium (38). These two glucose transporter isoforms differ only in their cytosolic NH2-terminal domains; iso-1 has a 130–amino acid domain that is completely different in sequence from the 48–amino acid NH2-terminal domain of iso-2 (see Fig. 1). Because the isoforms differ only in their NH2-terminal domains and are identical in sequence throughout the rest of the protein, the NH2-terminal region of iso-1 is likely to contain a flagellar targeting signal. The presence of a flagellar targeting signal is supported by experiments in this paper that demonstrate that chimeric proteins containing the NH2-terminal domain of iso-1 are targeted to the flagellar membrane.
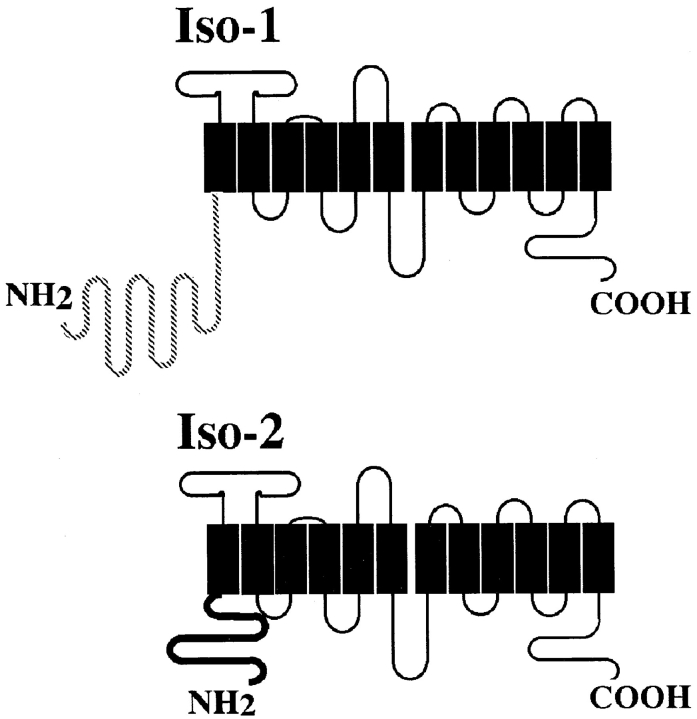
Figure 1.
Schematic diagram showing the putative membrane topology of Pro-1 glucose transporter isoforms iso-1 and iso-2. Black boxes represent putative transmembrane domains. Loops above the boxes represent extracellular domains and the loops below the boxes represent intracellular domains. The hatched line of iso-1 and the bold black line for iso-2 indicate that the only sequence differences between the two isoforms occur within the NH2-terminal domains. Iso-1 has a 130–amino acid NH2-terminal domain, while iso-2 has a 48–amino acid NH2-terminal domain.
Leishmania and other members of the Kinetoplastida have unusual cytoskeletons comprised of subpellicular microtubules that are attached to the inner surface of the pellicular membrane, and that provide the cell with its characteristic shape (6, 35). The linkage between these subpellicular microtubules and the membrane is likely to involve direct or indirect interaction of integral membrane proteins with the microtubules. The other surface membranes (those surrounding the flagellar pocket, the flagellar adhesion zone, and the flagellum itself) are devoid of subpellicular microtubules. The flagellum also contains internal cytoskeletal elements including the flagellar axoneme and the paraflagellar rod (37). One potential explanation for differential targeting of the two glucose transporter isoforms between these specialized membrane compartments is that each isoform interacts differently with various cytoskeletal components. To investigate this possibility, we have fractionated parasites into detergent-insoluble cytoskeletons and detergent-soluble supernatants, and have also monitored the association of each glucose transporter isoform with each fraction. These experiments reveal that the pellicular membrane iso-2 tightly associates with the subpellicular microtubules, whereas the flagellar iso-1 is released into the supernatant by detergent solubilization. These results suggest that iso-2 anchors in the cell body membrane by tethering to the subpellicular microtubules, while iso-1 targets to the flagellar membrane by structural information present in its unique NH2-terminal domain. Similar interactions may direct other parasite pellicular membrane or flagellar membrane proteins to their correct subcellular addresses.
Materials and Methods
Plasmid Constructs
The plasmid pX63Hyg (7) was used for expression of epitope-tagged constructs in L. enriettii. The 13 COOH-terminal amino acids of rat GLUT2 (TVQMEFLGSSETV; 36), which were used as the epitope tag, were introduced into iso-1– and iso-2–containing plasmids as previously described (30). The polylinker 5′ to iso-1 was modified to create a new BglII restriction site by digesting at the 5′ SmaI site and ligating to a BglII linker. NH2-terminal deletion constructs of iso-1 removing the first 10, 20, 25, 30, 50, and 100 amino acids were created. For these constructs, a primer containing a BglII restriction site, a Kozak sequence (AGCAGC), and encoding a methionine and the first six amino acids following the region to be deleted, was used as the forward primer. An antisense primer encoding the six–amino acids upstream of the unique ClaI site within the coding region (5) and the ClaI site was used as the reverse primer to amplify the NH2-terminal region of iso-1. The amplified fragments were gel-purified, digested with BglII and ClaI, and cloned into a pX63Hyg.iso1 vector containing the GLUT2 epitope-tagged iso-1 (30) using the internal ClaI site and the BglII site in the upstream polylinker region. The regions containing the deletions were sequenced as reported previously (5) to confirm that the correct constructs had been generated.
The plasmid pAlt-Neo (20) was used for expression of the chimeras containing the NH2-terminal cytoplasmic domains of either iso-1 or iso-2 fused to the body of the D2 hexose transporter (21). A DNA sequence encoding the first 130 amino acids of iso-1 or the first 48 amino acids of iso-2 (the NH2-terminal domains) followed by amino acids 108 to 112 of D2 (a sequence encoding the beginning of the first predicted transmembrane domain and containing an NruI site used for subcloning) were introduced into a D2-containing plasmid by PCR-based mutagenesis (30). The regions of these chimeras amplified by PCR were sequenced to ensure that no unintended sequence alterations had occurred during the PCR.
Cell Culture and Transfection
Promastigotes of L. enriettii were cultured at 26°C in DME-L medium (12) containing 5% FCS and 5% bovine embryonic fluid. Parasites were transfected by electroporation (15) of plasmid DNA using a Gene Pulser (Bio-Rad Laboratories, Richmond, CA) apparatus. 1 d after transfection, hygromycin (Calbiochem Corp., La Jolla, CA) was added to a final concentration of 50 μg/ml to the culture, and the parasites were maintained under these conditions until drug-resistant parasites grew out (usually ∼4 wk). The hygromycin concentration was then increased to 200 μg/ml for maintenance of transfected parasites. Parasites transfected with pALT-Neo–based plasmids were selected with 10 μg/ml of G418 (GIBCO BRL, Gaithersburg, MD) 1 d after transfection, and the drug concentration was increased to 200 μg/ml after parasites grew out.
Antibody Preparation
Polyclonal antiserum against the glutathione S-transferase fusion protein containing the 25 COOH-terminal amino acids of a rat GLUT2 was purchased from East Acres Biologicals (Southbridge, MA), and was used for immunofluorescence at a dilution of 1:500. For Western blots, polyclonal antiserum against a maltose-binding protein fusion protein (New England Biolabs, Beverly, MA; 11, 23) containing the 13 COOH-terminal amino acids of a rat GLUT2 was used. The antiserum was prepared by Cocalico Biologicals, Inc. (Reamstown, PA), and was used at a dilution of 1:500.
The anti-P1C (Pro-1 COOH terminus) antibody, directed against the 32 COOH-terminal amino acids of the Pro-1 glucose transporters, has been described previously (30). The anti-P1L (Pro-1 loop) antiserum was raised against a glutathione S-transferase fusion protein containing amino acids 160–227 of iso-2 (5), which are contained within the first extracellular loop of Pro-1. This antiserum was affinity-purified (30) over a Sepharose column (Bio-Rad Laboratories) containing this fusion protein, concentrated by passing the affinity-purified serum over a protein A–Sepharose column (Sigma Chemical Co., St. Louis, MO), and used at a dilution of 1:500 for protein blots. The specificity of each antiserum was confirmed using immunoblots by competition with the fusion protein used to raise the antiserum as described previously (30); the same concentration of native glutathione S-transferase did not compete the signal from either antiserum.
The mouse anti–α-tubulin monoclonal antibody raised against native chick brain microtubules was obtained from Amersham Corp. (Arlington Heights, IL). The E7 mouse anti–human β-tubulin monoclonal antibody was prepared by Dr. Michael Klymkowsky, and was obtained from the Developmental Studies Hybridoma Bank maintained by the Department of Pharmacology and Molecular Sciences, Johns Hopkins University School of Medicine (Baltimore, MD), and the Department of Biological Sciences, University of Iowa (Iowa City, IA), under contract N01-HD- 2-3144 from the National Institute of Child Health and Human Development. The anti-D2 and anti-MIT affinity-purified antibodies, which were directed against the COOH-terminal hydrophilic domains of each transporter, have been described previously (10, 22).
Cell Lysates and Immunoblots
For preparation of total cell lysates, parasites at a density of 1–2 × 107/ml were pelleted, washed with PBS, resuspended in Laemmli sample buffer (32) to a density of 0.2–1 × 109 cells/ml, and immediately heated to 65°C for 5 min. Samples containing 10 μl of lysate were reheated to 65°C for 3 min, loaded onto 10% SDS-polyacrylamide gels, separated by standard methods (32), and electroblotted onto a nitrocellulose membrane using a Mini Trans-Blot apparatus (Bio-Rad Laboratories) according to the manufacturer's instructions. Blots were developed using the ECL chemiluminescence system (Amersham Corp.) and goat anti–rabbit IgG coupled to horseradish peroxidase (Boehringer Mannheim Biochemicals, Indianapolis, IN) as detailed in the manufacturer's instructions, and the developed blots were exposed to XAR-5 film (Eastman Kodak Co., Rochester, NY).
Cytoskeleton Preparation
Leishmania cytoskeletons were prepared using the method of Schneider et al. (33). In brief, parasites were grown to 1–2 × 107 cells/ml and washed 3× in ice-cold PBS. The pellet of cells was resuspended in MME (10 mM MOPS, pH 6.9, 0.1 mM EGTA, 1 mM MgSO4, and 0.1% Triton X-100) plus protease inhibitors (1 μM each of leupeptin, aprotinin, and chymostatin) at a concentration of 4 × 107 cells/ml. The cells were incubated on ice for 10 min, and were centrifuged at 3,000 g in an Eppendorf microcentrifuge (Brinkmann Instruments Inc., Westbury, NY) for 5 min. The pellet was washed once with PBS and resuspended to 1/20 of the MME volume in PBS/Laemmli Sample Buffer at a 1:1 mix. The supernatant was concentrated to 1/10 of the original volume with a Microcon 10 microconcentrator (Amicon, Beverly, MA) to approximate the pellet concentration, and 0.25 volume of 4× Laemmli sample buffer was added to the supernatant. Both supernatant and pellet were heated at 60°C for 5 min. For protein gels and immunoblots, 10 μl of pellet and 20 μl of supernatant were run per lane. For immunofluorescence, the pellets were prepared as described above, except that they were resuspended in 1/2 the original volume of PBS after centrifugation and washing.
Immunofluorescence Microscopy
For immunofluorescence imaging, parasites were pelleted, washed twice in PBS, resuspended at a density of ∼107 cells/ml, and attached to poly- l-lysine–coated coverslips. For cytoskeleton preps, pellets were prepared as described above, resuspended at a density of ∼107 cytoskeletons/ml, and attached to poly-l-lysine–coated coverslips. The adherent parasites/ cytoskeletons were fixed with 100% methanol at −20°C for 15 min. After fixation, coverslips were rinsed in PBS and incubated in PBS plus 2% goat serum for 15 min. Antiserum was added at the appropriate dilution in PBS plus 2% goat serum, and incubated for 1 h at room temperature. Coverslips were rinsed three times in PBS, and then incubated for 1 h with goat anti–rabbit IgG coupled to FITC (fluorescein isothiocynate; 1:200 dilution) (Molecular Probes, Inc., Eugene, OR) or to rhodamine B (1:200; Biosource International, Camarillo, CA) or with goat anti–mouse IgG coupled to Bodipy Oregon green (1:200) or Texas red (1:800; both from Molecular Probes, Inc.) in PBS plus 2% goat serum. Coverslips were rinsed five times with PBS and then mounted on slides in 50 mM Tris, pH 8.0, 90% glycerol, and 20 mg/ml n-propyl-gallate (Sigma Chemical Co.). For confocal microscopy, samples were examined with a confocal laser scanning microscope as described previously (30).
Results
Fractionation of Glucose Transporter Isoforms into Detergent Soluble and Insoluble Phases
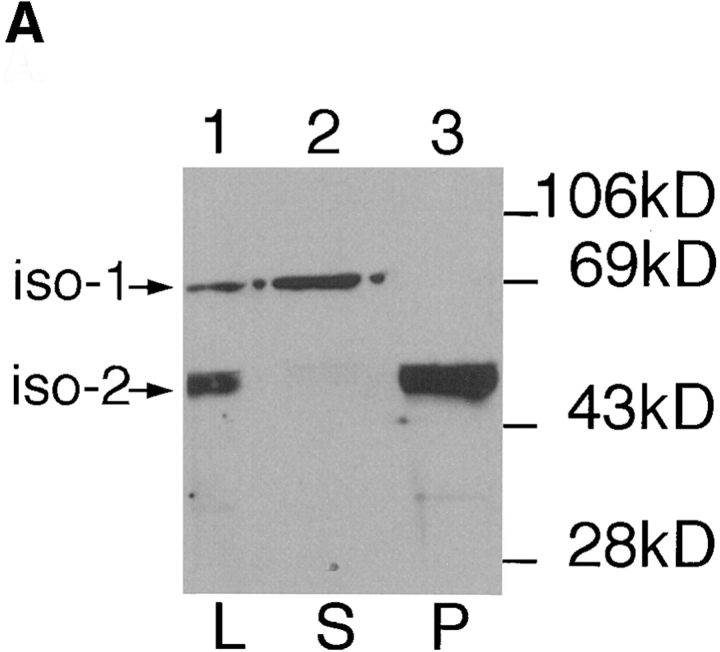
One possible explanation for the differential localization of the glucose transporter isoforms iso-1 and iso-2 is that each isoform interacts with the parasite cytoskeleton in a different way. The cytoskeleton of Kinetoplastid parasites, containing the subpellicular microtubules and the flagellar axoneme, can be separated from the cytosolic and membrane components of the cell by solubilization with nonionic detergents such as Triton X-100 followed by centrifugation to quantitatively pellet the cytoskeletons (31). To test the possible association of each glucose transporter isoform with the detergent-insoluble cytoskeleton, we prepared soluble and insoluble fractions from L. enriettii promastigotes and probed immunoblots of each fraction with the anti-P1C antibody directed against the COOH-terminal hydrophilic domain that is conserved in both isoforms (Fig. 1). Whereas immunoreactive proteins of ∼65 and 50 kD are present in the total cell lysates (Fig. 2 A, lane 1), the ∼65-kD protein is quantitatively associated with the supernatant (lane 2), whereas the ∼50-kD protein is quantitatively associated with the detergent-insoluble fraction (lane 3).
Figure 2.
Immunoblots of Triton X-100 extracted L. enriettii fractions. L designates total lysate, S designates the supernatant fraction, and P designates the cytoskeletal pellet. iso-1 and iso-2 indicate the bands on the blot that correspond to each of these two glucose transporter isoforms. Samples were separated on a 10% SDS-polyacrylamide gel, blotted onto nitrocellulose, and probed with the indicated antibodies. The blots in A were probed with affinity-purified anti-P1C antibody (at a 1:100 dilution), and the blots in B were probed with anti-P1L (lane 1, 1:500), anti–α-tubulin (lane 2, 1:800), or anti–β-tubulin (lane 3, 1:1,000). Numbers at the left indicate the mobilities of molecular mass markers in kilodaltons.
These results suggest that the major glucose transporter isoform, iso-2, fractionates quantitatively with the detergent-insoluble pellet, and is likely to be associated with the parasite cytoskeleton. This isoform has a predicted molecular mass of 61.4 kD, but migrates more rapidly on SDS-PAGE (30), similar to the anomolously rapid mobility of mammalian glucose transporters (25). Conversely, the less intense slower mobility band present in the supernatant fraction is likely to represent the larger (∼70 kD predicted) and less abundant iso-1 that is located in the flagellar membrane (30). These suggestions are confirmed by epitope-tagging results discussed below. Similar fractionation results have also been obtained (data not shown) using the anti-P1L antibody directed against the first extracellular loop that is conserved in iso-1 and iso-2.
One potential artifact of the preceding fractionation experiments could arise from possible cross-reactivity of the anti-P1C and anti-P1L antibodies with the abundant α-and β-tubulin proteins present in the cytoskeletal pellet. To demonstrate that the ∼50-kD band detected in the pellet is not a tubulin subunit, we probed immunoblots (Fig. 2 B) of the pellet fraction with the anti-P1L antibody (lane 1), and with monoclonal antibodies directed against either chicken α-tubulin (lane 2) or human β-tubulin (lane 3). The distinctly slower mobility of the tubulin subunits compared to the band that reacts with the anti-P1L antibody demonstrates that this latter band does not represent tubulin.
To confirm the results of the immunoblots, we have also examined the detergent-extracted cytoskeletons by immunofluorescence using both the anti-P1C and anti–α-tubulin antibodies. The anti-P1C antibody reacts with the body of the parasite in these cytoskeletal preps, but not with the flagella (Fig. 3, P1C), consistent with the notion that the pellicular membrane iso-2 is associated with the cytoskeleton, but the flagellar iso-1 is not. In contrast, the control anti–α-tubulin antibody reacts with microtubules in both the cell body and the flagellum (Fig. 3, α-tubulin).
Figure 3.
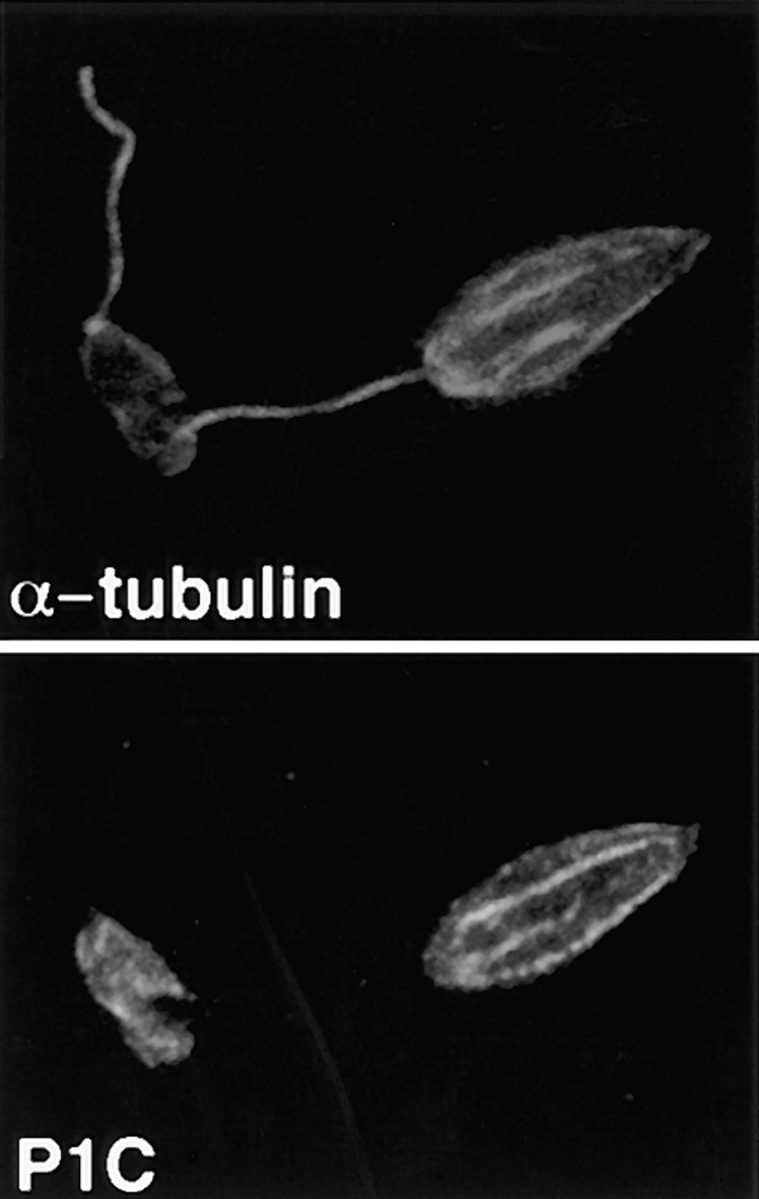
Double-label confocal laser scanning micrographs of Triton X-100–extracted L. enriettii promastigotes stained with anti-P1C and anti– α-tubulin. Cytoskeletons were fixed with methanol, stained with a 1:100 dilution of the anti-P1C antibody and a 1:500 dilution of the anti–α-tubulin antibody, and then with an FITC-conjugated anti–rabbit IgG (P1C) and a rhodamine-conjugated anti–mouse IgG (α-tubulin) secondary antibody. Cytoskeletons were examined by confocal microscopy using illumination at 488 nm to visualize the Pro-1 glucose transporter–complexed FITC antibody (P1C) or at 546 nm to visualize α-tubulin complexed with the rhodamine antibody (α-tubulin). Each micrograph represents a single 0.5-μm section through each field.
To support further the conclusion that the interaction between the parasite glucose transporter and components of the pellet is not the result of nonspecific binding, we have also performed fractionations using lysis buffer containing high concentrations of Triton X-100 (1%), n-octyl-glucoside (0.5%), CHAPS (0.5%), Zwittergent (0.5%), Nonidet-P40 (0.5%), NaCl (1 M), or β-mercaptoethanol (0.5 M). In each case, the ∼50-kD glucose transporter band fractionated quantitatively with the pellet (data not shown). We have also attempted to specifically solubilize the parasite cytoskeleton to demonstrate that disruption of the microtubules releases the glucose transporter from the pellet. The subpellicular microtubules of Kinetoplastid parasites are impervious to classical microtubule-disrupting treatments, including alkaloids such as colchicine, cold, and vinblastine (35 and data not shown). The microtubules of the closely related parasite Trypanosoma brucei can be disrupted by 1 mM CaCl2 or 1 M NaCl (31). We have, however, found that the cytoskeletons of L. enriettii and other Leishmania species are resistant to 1 M NaCl and are disorganized by 1–10 mM CaCl2, but form amorphous aggregates that pellet upon centrifugation.
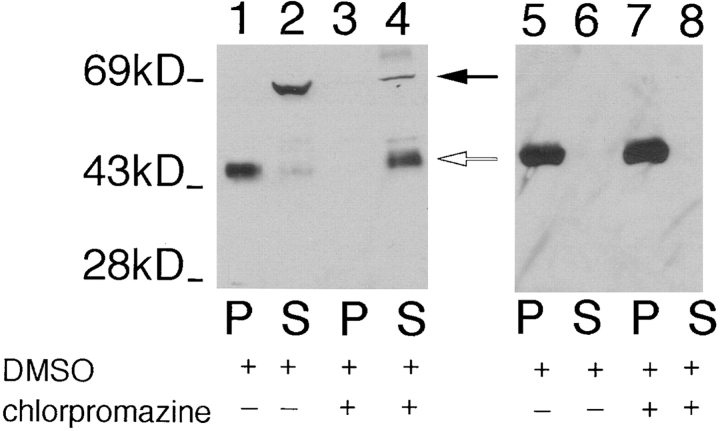
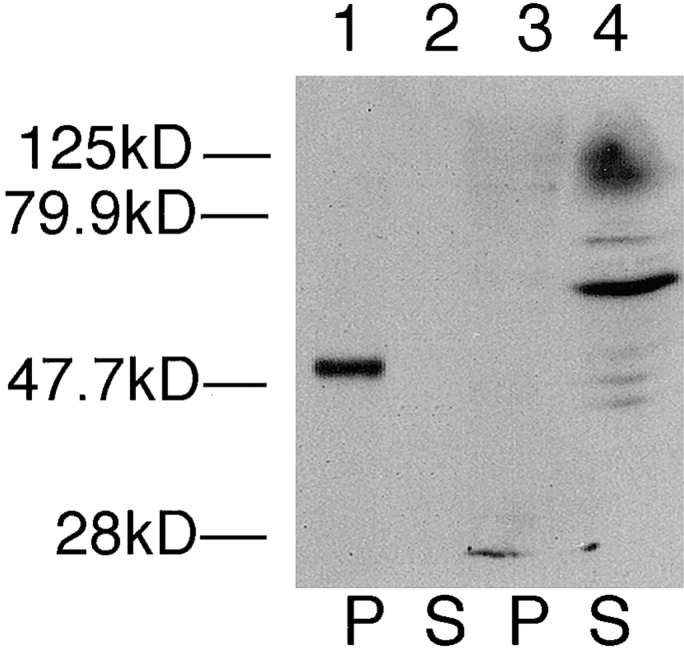
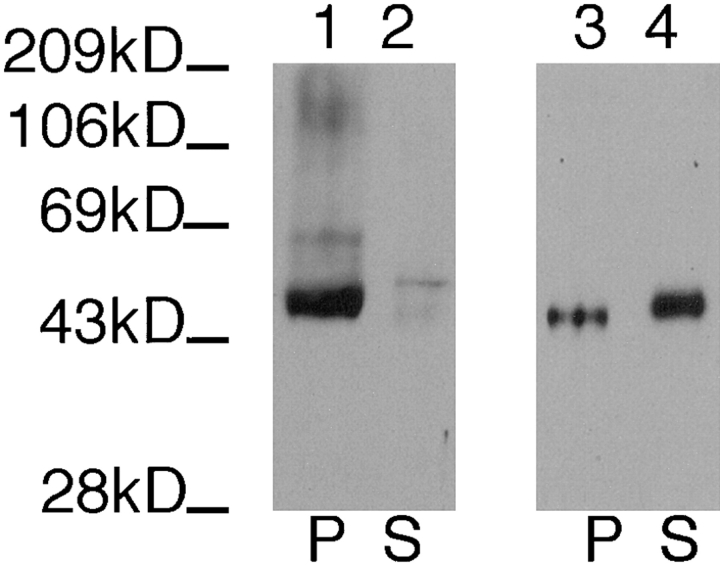
The tricyclic drug chlorpromazine disrupts cytoskeletons of trypanosomatid protozoa (34) including Leishmania (29) when live cells are incubated with drug. To determine whether this cytoskeletal-disrupting drug releases iso-2 from the pellet of a detergent extract, we incubated L. enriettii cells with 1 mM chlopromazine for 30 min, and then prepared fractionated detergent extracts. As shown in Fig. 4 (lanes 3 and 4), this cytoskeletal-disrupting agent releases iso-2 into the supernatant, whereas treatment of control cells with DMSO alone (Fig. 4, lanes 1 and 2) or with a nonspecific cell poison, 0.5% sodium azide (not shown), does not release iso-2 from the detergent-insoluble pellet. One complication with the preceding experiment, however, is that the mechanism of action of chlorpromazine is not known. Although this drug removes pellicular microtubules from the plasma membrane (34), it clearly does not completely depolymerize these microtubules, as they still fractionate with the pellet of the detergent extract (Fig. 4, lanes 7 and 8). The ability of this cytoskeletal-disrupting drug to release iso-2 from the pellet is consistent with the interpretation that iso-2 interacts with the parasite cytoskeleton, and not with some other component of the detergent-insoluble pellet. The absence of a drug that efficiently depolymerizes subpellicular microtubules in this parasite, however, precludes a definitive interpretation of these pharmacological results.
Figure 4.
Immunoblot of pellet (P) and the supernatant (S) fractions from L. enriettii incubated with 10% DMSO (lanes 1, 2, 5, and 6) or 1 mM chlorpromazine in 10% DMSO (lanes 3, 4, 7, and 8), followed by Triton X-100 extraction. The samples were run on an 8% acrylamide gel. The blot containing lanes 1–4 was probed with the anti-P1C antibody (1:100), and the blot containing lanes 5–8 was probed with anti–α-tubulin antibody (1:1,000). The solid arrow indicates the position of iso-1, and the open arrow indicates the position of iso-2. The numbers at the left indicate the migration of molecular mass markers, with molecular masses given in kD.
Fractionation of Individual Epitope Tagged Glucose Transporter Isoforms
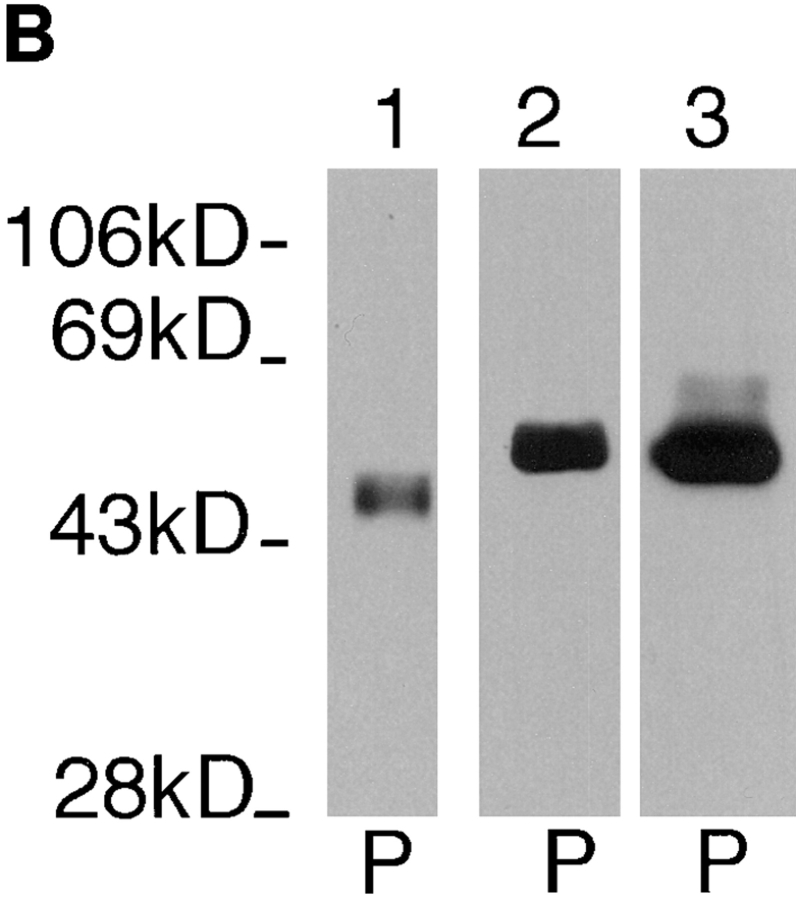
To examine cytoskeletal association of each glucose transporter isoform, we used previously established (30) parasite lines that express either iso-1 (line pX63Hyg.iso1) or iso-2 (line pX63Hyg.iso2) containing a COOH-terminal 13–amino acid epitope tag derived from the COOH terminus of the rat glucose transporter GLUT2 (36). Immunoblots of pellets and supernatants from each cell line probed with the anti-GLUT2 antiserum (Fig. 5) reveal that the ∼50-kD iso-2 fractionates quantitatively with the pellet (Fig. 5, lanes 1 and 2), whereas the ∼65-kD iso-1 fractionates quantitatively with the supernatant (Fig. 5, lanes 3 and 4).
Figure 5.
Immunoblot of pellet (P) and the supernatant (S) fractions from Triton X-100–extracted L. enriettii transfected with plasmids encoding epitope tagged iso-2 (lanes 1 and 2) or epitope-tagged iso-1 (lanes 3 and 4). The blot was probed with an anti-GLUT2 antibody (1: 500). Other symbols are as indicated in Fig. 2.
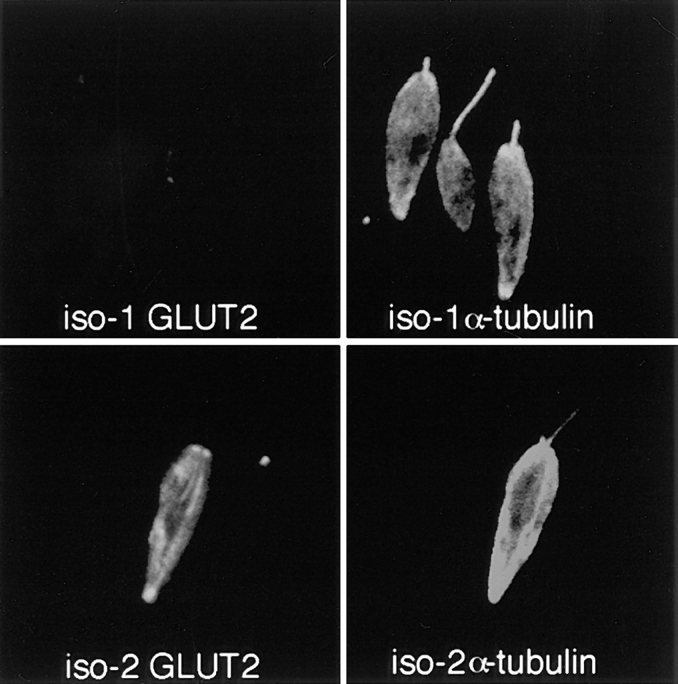
Examination of cytoskeletal pellets derived from each cell line using double-label immunofluorescence (Fig. 6) confirms cytoskeletal association of iso-2, but not iso-1. Thus, cytoskeletons from the iso-1–expressing cell line incubated with the anti-GLUT2 antibody directed against the epitope tag show only background levels of staining (iso-1 GLUT2), confirming the absence of iso-1 in the cytoskeletons. The same cells incubated with the control anti–α-tubulin antibody, however, stain on both the cell bodies and the flagella (iso-1 α-tubulin). In contrast, cytoskeletons from the iso-2–expressing cell line incubated with the anti-GLUT2 antibody stain intensely over the cell body, but not the flagellum (iso-2 GLUT2), indicating the presence of iso-2 in this cytoskeletal fraction associated with the subpellicular microtubules. The control anti–α-tubulin antibody stains both the cell body and the flagellum (iso-2 α-tubulin).
Figure 6.
Double-label confocal laser scanning micrographs of Triton X-100–extracted L. enriettii promastigotes transfected with plasmid encoding epitope-tagged iso-1 (top) or epitope-tagged iso-2 (bottom) and stained with the rabbit anti-GLUT2 antibody directed against the epitope tag (GLUT2) and the murine anti–α-tubulin antibody (α-tubulin). Cytoskeletons were fixed with methanol, stained with 1:500 dilutions of the anti-GLUT2 antibody and anti-α-tubulin antibodies, and then with an FITC-conjugated anti–rabbit IgG (GLUT2) and a rhodamine-conjugated anti–mouse IgG (α-tubulin) secondary antibody. Cytoskeletons were examined by confocal microscopy. Each micrograph represents a single 0.5-μm section through each field.
Partial Characterization of a Flagellar Targeting Signal of Iso-1
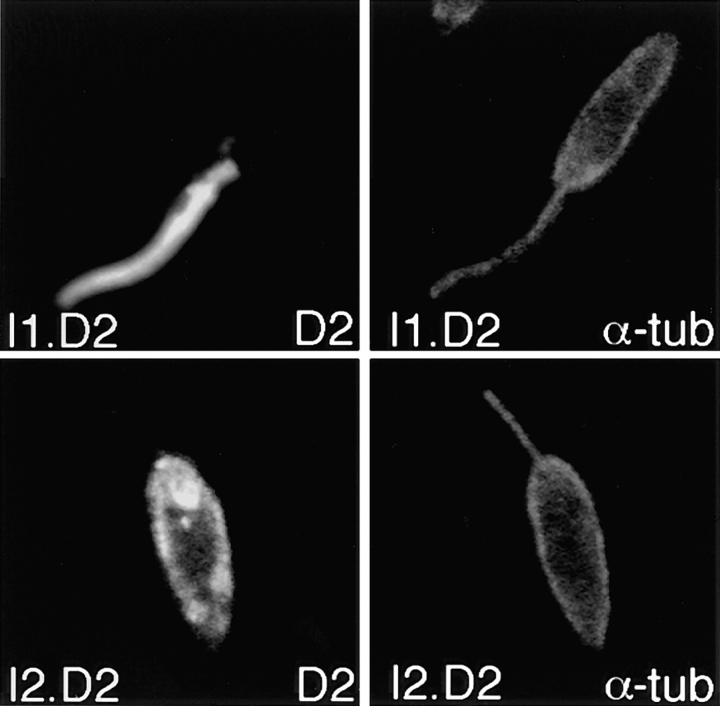
Because iso-1 and iso-2 differ only in their NH2-terminal domains, these regions must contain the information required for differential targeting. One possibility is that the NH2-terminal domain of iso-1 contains a signal that causes trafficking of this isoform to the flagellum. To determine whether the NH2-terminal domain of iso-1 was sufficient to target an integral membrane protein to the flagellar membrane, we created a chimeric construct that replaced the NH2-terminal cytoplasmic domain of a different pellicular membrane transporter, the D2 hexose transporter from the related parasite L. donovani (22), with the NH2-terminal domain of iso-1. This I1.D2 chimeric protein was expressed in L. enriettii cells, and the location of the chimera was determined by immunofluorescence microscopy using an antibody directed against the L. donovani D2 transporter. In contrast to the wild-type D2 transporter, the I1.D2 chimera is targeted to the flagellar membrane (Fig. 7, I1.D2). Thus, the iso-1 NH2-terminal domain contains a dominant flagellar targeting signal that is sufficient to redirect a pellicular membrane protein to the flagellum. As a complementary control, we also prepared a chimera containing the NH2-terminal cytoplasmic domain of iso-2 fused to the body of the D2 hexose transporter. Immunofluorescence images of parasites expressing this I2.D2 chimera reveal that it is targeted to the pellicular membrane, and does not appear in the flagellar membrane (Fig. 7, I2.D2), similar to the previously determined localization of iso-2 (30) and similar to the localization of the L. donovani D2 transporter when it is expressed in either L. donovani (22) or in L. enriettii (data not shown).
Figure 7.
Confocal laser microscopy of L. enriettii parasites overexpressing chimeras of the D2 hexose transporter containing the NH2-terminal domain of iso-1 (I1.D2, top) or the NH2-terminal domain of iso-2 (I2.D2, bottom). Cells were double-stained with the anti–α-tubulin antibody (α-tub, 1:400) and the anti-D2 antibody (D2, 1:200), followed by goat anti–mouse Texas red and goat anti–rabbit Bodipy Oregon green. Each micrograph represents a single 0.5-μm section through each field.
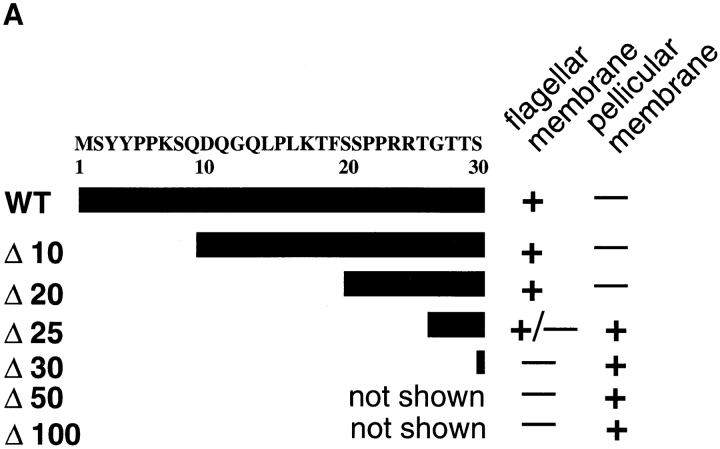
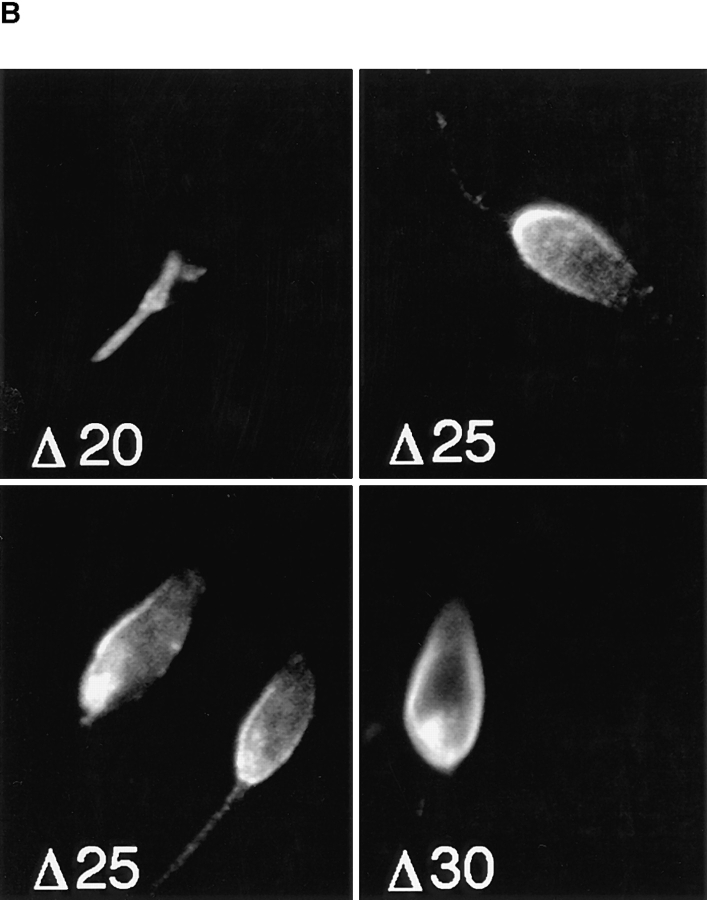
To begin to characterize the flagellar targeting signal, we created a series of NH2-terminal deletions of iso-1 (Fig. 8 A) as detailed in Materials and Methods. Each epitope-tagged construct was transfected into L. enriettii, and the stably transfected cells were assayed by confocal immunofluorescence microscopy for localization of the iso-1 deletion mutants (Fig. 8 B). NH2-terminal deletions of 10 (not shown) and 20 (Fig. 8 B, Δ20) amino acids result in iso-1 mutants that exhibit wild-type localization in the flagellar membrane. Deleting five more amino acids creates a protein that localizes primarily to the pellicular membrane, but with some staining present on the flagella of some parasites (Fig. 8 B, Δ25). Deletion of five additional amino acids results in a protein that localizes exclusively to the pellicular membrane (Fig. 8 B, Δ30), identical to iso-2 targeting. Deletions of 50 and 100 amino acids also exhibit the same pellicular membrane localization as Δ30 (data not shown). Thus, essential iso-1 flagellar targeting information begins at approximately amino acid 25. It is not yet clear whether this targeting information is contained in a discrete contiguous sequence of amino acids as is the case for the internalization signal of the transferin receptor (13), or whether it represents a three-dimensional recognition domain, as is the case for the signals for lysosomal enzyme targeting (3). Nonetheless, these results confirm that the NH2 terminus of iso-1 contains a signal necessary for flagellar targeting.
Figure 8.
(A) Deletions of the NH2-terminal domain of iso-1. WT represents the wild-type sequence. The Δ numbers represent the number of NH2-terminal amino acids deleted in each construct. The amino acid sequence (single letter code) of the relevant portion of the NH2-terminal domain is indicated at the top with numbers designating the amino acid position starting with the initiating methionine at position 1. (B) Confocal laser scanning microscopy of L. enriettii promastigotes expressing epitope-tagged deletion constructs Δ20, Δ25, and Δ30. Stable cell lines transfected with pX63Hyg.Δ20, pX63Hyg.Δ25, or pX63Hyg.Δ30 were stained with a 1:500 dilution of the anti-GLUT2 antibody and a 1:200 dilution of FITC-conjugated secondary antibody and examined by confocal microscopy. Each micrograph represents a single 0.5-μm section through each field.
Fractionation of Iso-1 NH2 Domain Mutants in Detergent Soluble and Insoluble Phases
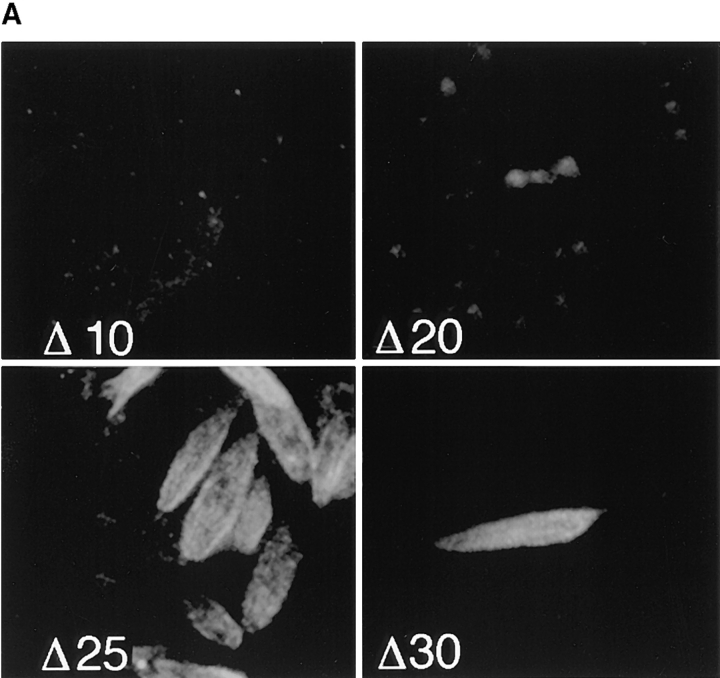
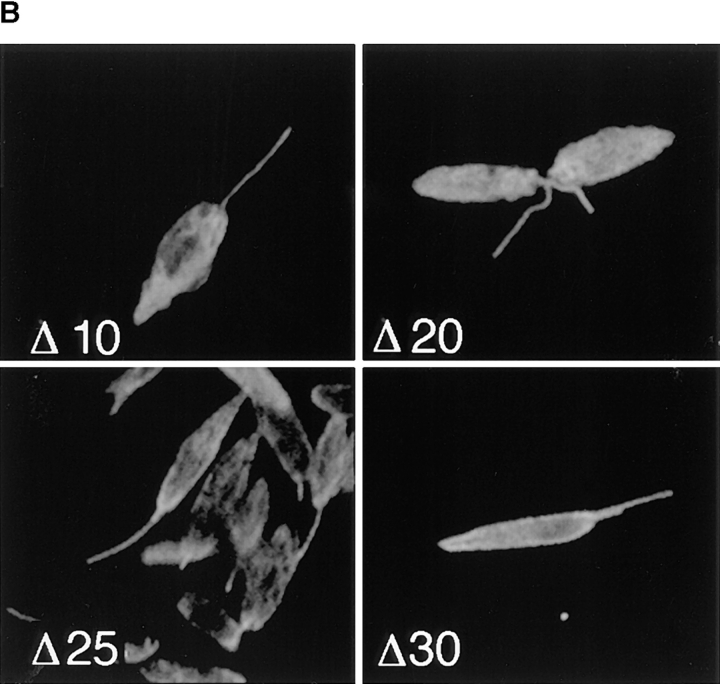
To examine whether the retargeted iso-1 deletion mutants associate with the cytoskeleton, we performed immunofluorescence of detergent-extracted cytoskeletons. Examination of cytoskeletal pellets derived from each cell line using double-label immunofluorescence (Fig. 9) confirms the cytoskeletal association of Δ25 and Δ30. Cytoskeletons from the Δ10- and Δ20-expressing cell line show only background levels of anti-GLUT2 staining (Fig. 9 A, Δ10 and Δ20), consistent with the absence of these truncated transporters in the cytoskeletons. The same cells labeled with the control anti–α-tubulin antibody have staining on flagella and cell bodies (Fig. 9 B), demonstrating that the cytoskeletons remain intact. In contrast, cytoskeletons of Δ25- and Δ30-expressing cells labeled with the anti-GLUT2 antibody stain intensely over the cell body, but not the flagellum (Fig. 9 A, Δ25 and Δ30), indicating that these deletion mutants associate with the subpellicular microtubules. The control anti–α-tubulin antibody stains both the cell body and flagellum (Fig. 9 B, Δ25 and Δ30). Thus, retargeting of iso-1 from the flagellar membrane to the pellicular membrane results in association of the mutant proteins with the cytoskeleton.
Figure 9.
Confocal laser scanning microscopy of Triton X-100–extracted L. enriettii promastigotes expressing epitope-tagged deletion constructs Δ10, Δ20, Δ25, and Δ30. Cytoskeletons from stable cell lines transfected with pX63Hyg.Δ10, pX63Hyg.Δ20, pX63Hyg.Δ25, or pX63Hyg.Δ30 were stained with a 1:500 dilutions of the anti-GLUT2 antibody (A) and the anti–α-tubulin antibody (B) and an FITC- or a rhodamine-conjugated secondary antibody, respectively, and examined by confocal microscopy as described in Fig. 3. Each micrograph represents a single 0.5-μm section through each field.
Cytoskeletal Tethering in Other Leishmania Integral Membrane Proteins
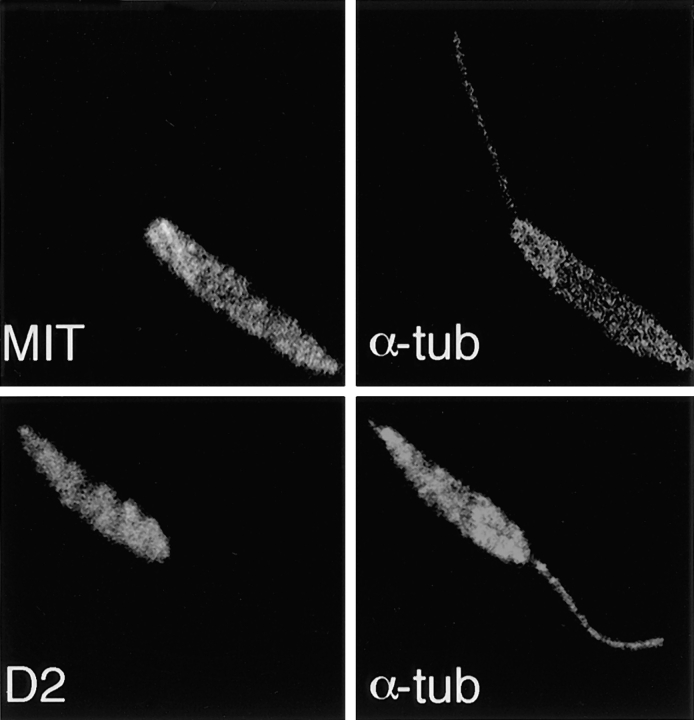
To determine if the phenomenon of cytoskeletal association could be generalized to other integral membrane proteins and to other species of Leishmania, we prepared immunoblots of pellets and supernatants of L. donovani overexpressing the D2 hexose transporter (22) or the MIT myo-inositol transporter (10). Previous studies have shown that D2 localizes to the pellicular membrane (22), whereas MIT localizes to both the pellicular membrane and the flagellar membrane (10). Immunoblots of fractionated D2 hexose transporter and MIT-overexpressing cell lines probed with anti-D2 and anti-MIT antibodies, respectively (Fig. 10), reveal that the ∼50-kD D2 band fractionates exclusively with the pellet (lanes 1 and 2), while the ∼50-kD MIT band fractionates in both the pellet and supernatant (lanes 3 and 4). These immunoblot results were also confirmed with confocal immunofluorescence microscopy, which reveals that both the D2 and MIT transporters are retained in the detergent-extracted cytoskeletons (Fig. 11). Thus, another pellicular membrane transporter (D2) associates with the cytoskeleton, whereas a transporter (MIT) that is in both the pellicular membrane and the flagellar membrane fractionates with both the cytoskeletal pellet and the supernatant.
Figure 10.
Immunoblots of Triton X-100 extracts from stably transfected L. donovani promastigotes overexpressing the D2 hexose transporter (lanes 1 and 2) or the MIT myo-inositol transporter (lanes 3 and 4). P designates the pellet (lanes 1 and 3) and S designates the supernatants (lanes 2 and 4) of the detergent extracts. Lanes 1 and 2 were stained with the anti-D2 antibody (1:200), and lanes 3 and 4 were stained with the anti-MIT antibody (1:3,000). Numbers to the left indicate the mobilities of protein molecular mass markers in kilodaltons.
Figure 11.
Confocal laser scanning microscopy of Triton X-100 extracted promastigotes of L. donovani overexpressing the MIT myo-inositol transporter (top) or the D2 hexose transporter (bottom). Each cytoskeleton was double-stained with either the anti-MIT antibody (MIT, 1:1,000) or the anti-D2 antibody (D2, 1:200), followed by the control α-tubulin antibody (α-tub, 1:500). Secondary FITC-conjugated or rhodamine-conjugated antibodies were used to reveal staining of the antitransporter or anti–α-tubulin antibodies, respectively. Each micrograph represents a single 0.5-μm section through each field.
Discussion
Association with cytoskeletal components is often a way of targeting membrane proteins to specific locations in the cell. We have demonstrated that the pellicular membrane glucose transporter isoform of the parasitic protozoan L. enriettii is specifically bound to the parasite cytoskeleton, and that this interaction may serve to constrain this isoform to the cell body domain of the plasma membrane. Conversely, the flagellar glucose transporter isoform, which differs from the pellicular membrane isoform exclusively in the NH2-terminal hydrophilic domain, does not associate with the cytoskeleton. The distinct fractionation properties of these two closely related isoforms underscores the specificity of the interaction between iso-2 and the cytoskeleton, and argues strongly that this association is not simply an artifactual binding of a hydrophobic protein to a cytoskeletal component. This conclusion is reinforced by the fact that iso-2 fractionates quantitatively with the cytoskeleton, and iso-1 fractionates quantitatively with the detergent-soluble supernatant, a result that would be unlikely for a nonspecific interaction. Deletion of the first 25–30 amino acids from the flagellar isoform, however, results in retargeting of this transporter to the pellicular membrane and concomitant association with the cytoskeleton. These results suggest that both isoforms contain the structural information required for cytoskeletal association, but that the flagellar isoform is prevented from establishing this interaction by physical sequestration into a compartment that does not contain the subpellicular microtubules. We do not yet know the molecular details of this cytoskeletal tethering. By analogy to other mammalian membrane proteins that are tethered to the cytoskeleton (AE1, Na+, K+-ATPase, and the glycine receptor), however, it is likely that some linker protein binds the transporter to the microtubules. Additional studies will be required to determine which parts of the iso-2 protein are required for cytoskeletal tethering, and to determine whether linker proteins mediate the association of these glucose transporters with the microtubules.
It is noteworthy that tethering to the subpellicular microtubules occurs for other integral membrane proteins that show complete (D2 hexose transporter) or partial (MIT myo-inositol transporter) localization to the cell body membrane. Although we do not know how many integral membrane proteins are associated with the cytoskeleton in these organisms, the linkage to microtubules of the three transporters studied here suggests that there may be a large family of such proteins. It is also worth noting that biochemical purification of many integral membrane proteins from Kinetoplastid parasites may require that these proteins be released from the cytoskeleton either before or after detergent extraction.
We have demonstrated, using deletion mutagenesis and chimeric constructs, that the unique NH2-terminal domain of iso-1 is both necessary and sufficient for trafficking to the flagellar membrane. Further deletion and point mutagenesis studies are currently in progress to determine whether the flagellar targeting signal is a discrete stretch of amino acids, beginning around amino acid 25, or whether it is structurally more complex. It is likely that this flagellar targeting signal interacts with another protein or proteins required for flagellar targeting, as has been shown for the interaction of the tyrosine-based sorting signal from the mammalian integral membrane protein TGN38 with two clathrin-associated proteins (27). This issue, however, remains to be investigated.
The results reported here suggest a tentative model for differential targeting of the two glucose transporter isoforms. During biosynthesis, both proteins are presumably added to the external membrane of the flagellar pocket, the organelle to which integral membrane and secreted proteins are targeted (38), as well as a membrane that contains both iso-1 and iso-2 (30). Proteins such as iso-1 that contain a flagellar targeting signal may be sorted to the contiguous flagellar membrane, presumably by interaction of a flagellar targeting signal with other proteins that are part of the sorting apparatus. Proteins such as iso-2 that do not contain a flagellar targeting signal may traffic to the pellicular membrane, possibly by default, where they can then interact with the subpellicular microtubules. The cytoskeletal tethering would then constrain these proteins to remain in the cell body membrane. This model underscores the notion that proteins that are differentially sorted into subdomains of a continuous membrane system require not only signals for differential targeting during their trafficking to the membrane, but also a mechanism for retention of each protein at its correct subcellular address. Proteins such as MIT that are present in both membranes might have a weak flagellar targeting signal that would sort only a fraction of the proteins to the flagellar membrane. Conversely, MIT might have a weak cytoskeletal tethering signal that would allow diffusion of some of these transporters into the flagellar membrane.
A major objective of future studies will be to identify structural components of each isoform that are involved in cytoskeletal tethering or flagellar targeting, and to search for other proteins that may interact with these targeting signals to direct each isoform to its correct subcellular address. Since various proteins in different Kinetoplastid protozoa are restricted largely to the pellicular membrane (e.g., the iso-2 and D2 glucose transporters in Leishmania species) or to the flagellar membrane (e.g., iso-1 in L. enriettii and the receptor adenylate cyclases in Trypanosoma brucei [28]), these targeting pathways are likely to be of broad significance in these protozoa.
Acknowledgments
The authors wish to thank Jodi Engstrom and Ken Fish for performing the confocal microscopy presented in this paper. Xiaowen Xu prepared the anti-P1L and anti-P1C antibodies. Marco Sanchez and Andreas Seyfang helped with cell transfections. We also wish to thank Caroline Enns, Eric Barklis, and Buddy Ullman for helpful discussions.
This work was supported by National Institutes of Health Grants AI25920 and AI01162 to S.M. Landfear as well as by a Tartar Fellowship and a National Research Service Award Training Grant to E.L. Snapp. S.M. Landfear is a recipient of the Molecular Parasitology Scholar Award from the Burroughs Wellcome Fund.
Abbreviations used in this paper
- iso-1
isoform 1
- iso-2
isoform 2
Footnotes
Address all correspondence to Scott M. Landfear, Department of Molecular Microbiology and Immunology, Oregon Health Sciences University, 3181 S.W. Sam Jackson Park Road, Portland, OR 97201. Tel.: (503) 494-2426. Fax: (503) 494-6862.
References
- 1.Alper SL, Stuart-Tilley A, Simmons CF, Brown D, Drenckhahn D. The fodrin-ankyrin cytoskeleton of choroid plexus preferentially colocalizes with apical Na+K+-ATPase rather than with basolateral anion exchanger AE2. J Clin Invest. 1994;93:1430–1438. doi: 10.1172/JCI117120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balber AE. The pellicle and the membrane of the flagellum, flagellar adhesion zone, and flagellar pocket: functionally discrete surface domains of the bloodstream form of African trypanosomes. Crit Rev Immunol. 1990;10:177–201. [PubMed] [Google Scholar]
- 3.Baranski TJ, Koelsch G, Hartsuck JA, Kornfeld S. Mapping and molecular modeling of a recognition domain for lysosomal enzyme targeting. J Biol Chem. 1991;266:23365–23372. [PubMed] [Google Scholar]
- 4.Bennett V, Stenbuck PJ. The membrane attachment protein for spectrin is associated with band 3 in human erythrocyte membranes. Nature. 1979;280:468–473. doi: 10.1038/280468a0. [DOI] [PubMed] [Google Scholar]
- 5.Cairns BR, Collard MW, Landfear SM. Developmentally regulated gene from Leishmaniaencodes a putative membrane transport protein. Proc Natl Acad Sci USA. 1989;85:2130–2134. doi: 10.1073/pnas.86.20.7682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang, K.-P. 1990. Cell biology of Leishmania. In Modern Parasite Biology. D.J. Wyler, editor. W.H. Freeman and Co., New York. 79–90.
- 7.Cruz A, Coburn CM, Beverley SM. Double targeted gene replacement for creating null mutants. Proc Natl Acad Sci USA. 1991;88:7170–7174. doi: 10.1073/pnas.88.16.7170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding Y, Kobayashi S, Kopito R. Mapping of ankyrin binding determinants on the erythroid anion exchanger, AE1. J Biol Chem. 1996;271:22494–22498. doi: 10.1074/jbc.271.37.22494. [DOI] [PubMed] [Google Scholar]
- 9.Drenckhahn D, Schülter K, Allen DP, Bennett V. Colocalization of band 3 with ankyrin and spectrin at the basal membrane of intercalated cells in the rat kidney. Science. 1985;230:1287–1289. doi: 10.1126/science.2933809. [DOI] [PubMed] [Google Scholar]
- 10.Drew ME, Langford CK, Klamo EM, Russell DG, Kavanaugh MP, Landfear SM. Functional expression of a myo-inositol/H+ symporter from Leishmania donovani. . Mol Cell Biol. 1995;15:5508–5515. doi: 10.1128/mcb.15.10.5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guan C, Li P, Riggs PD, Inouye H. Vectors that facilitate the expression and purification of foreign peptides in Escherichia coliby fusion to maltose-binding protein. Gene. 1987;67:21–30. doi: 10.1016/0378-1119(88)90004-2. [DOI] [PubMed] [Google Scholar]
- 12.Iovannisci DM, Ullman B. High efficiency plating method for Leishmaniapromastigotes in semidefined or completely-defined medium. J Parasitol. 1983;69:633–636. [PubMed] [Google Scholar]
- 13.Jing S, Spencer T, Miller K, Hopkins C, Trowbridge I S. Role of the human transferrin receptor cytoplasmic domain in endocytosis: localization of a specific signal sequence for internalization. J Cell Biol. 1990;110:283–294. doi: 10.1083/jcb.110.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jordan C, Püschel, Koob R, Drenckhahn D. Identification of a binding motif for ankyrin on the α-subunit of Na+,K+-ATPase. J Biol Chem. 1995;270:29971–29975. doi: 10.1074/jbc.270.50.29971. [DOI] [PubMed] [Google Scholar]
- 15.Kapler GM, Coburn CM, Beverley SM. Stable transfection of the human parasite Leishmania majordelineates a 30-kilobase region sufficient for extrachromosomal replication and expression. Mol Cell Biol. 1990;10:1084–1094. doi: 10.1128/mcb.10.3.1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirsch J, Betz H. The postsynaptic localization of the glycine receptor-associated protein gephyrin is regulated by the cytoskeleton. J Neurosci. 1995;15:4148–4156. doi: 10.1523/JNEUROSCI.15-06-04148.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kirsch J, Langosch D, Prior P, Littauer UZ, Schmitt B, Betz H. The 93-kDa glycine receptor-associated protein binds to tubulin. J Biol Chem. 1991;266:22242–22245. [PubMed] [Google Scholar]
- 18.Kirsch J, Wolters I, Triller A, Betz H. Gephyrin antisense oligonucleotides prevent glycine receptor clustering in spinal neurons. Nature. 1993;366:745–748. doi: 10.1038/366745a0. [DOI] [PubMed] [Google Scholar]
- 19.Koob R, Zimmerman M, Schoner W, Drenckhahn D. Colocalization and coprecipitation of ankyrin and Na+, K+-ATPase in kidney epithelial cells. Eur J Cell Biol. 1988;45:230–237. [PubMed] [Google Scholar]
- 20.Laban A, Tobin JF, de Lafaille MAC, Wirth DF. Stable expression of the bacterial neor gene in Leishmania enriettii. . Nature. 1990;343:572–574. doi: 10.1038/343572a0. [DOI] [PubMed] [Google Scholar]
- 21.Langford CK, Ewbank SA, Hanson SA, Ullman B, Landfear SM. Molecular characterization of two genes encoding members of the glucose transporter superfamily in the parasitic protozoan Leishmania donovani. . Mol Biochem Parasitol. 1992;55:51–64. doi: 10.1016/0166-6851(92)90126-5. [DOI] [PubMed] [Google Scholar]
- 22.Langford CK, Kavanaugh MP, Stenberg PE, Zhang W, Landfear SM. Functional expression and subcellular localization of a high K m hexose transporter from Leishmania donovani. . Biochemistry. 1995;34:11814–11821. doi: 10.1021/bi00037a020. [DOI] [PubMed] [Google Scholar]
- 23.Maina CV, Riggs PD, Grandea AG, Slatko BE, Moran LS, Tagliamonte JA, McReynolds LA, Guan C. A vector to express and purify foreign proteins in Escherichia coliby fusion to and separation from maltose binding protein. Gene. 1988;74:365–373. doi: 10.1016/0378-1119(88)90170-9. [DOI] [PubMed] [Google Scholar]
- 24.Morrow JS, Cianci CD, Ardito T, Mann AS, Cashgarian M. Ankyrin links fodrin to the alpha subunit of Na,K-ATPase in Madin-Darby canine kidney cells and in intact renal tubule cells. J Cell Biol. 1989;108:455–465. doi: 10.1083/jcb.108.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mueckler M, Caruso C, Baldwin SA, Panico M, Blench I, Morris HR, Allard WJ, Lienhard GE, Lodish HF. Sequence and structure of a human glucose transporter. Science. 1985;229:941–945. doi: 10.1126/science.3839598. [DOI] [PubMed] [Google Scholar]
- 26.Nelson WJ, Hammerton RW. A membrane-cytoskeletal complex containing Na+, K+-ATPase, ankyrin, and fodrin in Madin-Darby canine kidney (MDCK) cells: implications for the biogenesis of epithelial cell polarity. J Cell Biol. 1989;108:893–902. doi: 10.1083/jcb.108.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohno H, Stewart J, Fournier M-C, Bosshart H, Rhee I, Miyatake S, Saito T, Gallussner A, Kirchhausen T, Bonifacino JS. Interaction of tyrosine-based signals with clathrin-associated proteins. Science. 1995;269:1872–1875. doi: 10.1126/science.7569928. [DOI] [PubMed] [Google Scholar]
- 28.Paindavoine P, Rolin S, Van Assel S, Geuskens M, Jauniaux J, Dinsart C, Huet G, Pays E. A gene from the variant surface glycoprotein expression site encodes one of several transmembrane adenylate cyclases located on the flagellum of Trypanosoma brucei. . Mol Cell Biol. 1992;12:1218–1225. doi: 10.1128/mcb.12.3.1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parson RD, Manian AA, Harcus JL, Hall D, Hewlett EL. Lethal effect of phenothiazine neuroleptics on the pathogenic protozoan Leishmania donovani. . Science. 1982;217:369–371. doi: 10.1126/science.6124040. [DOI] [PubMed] [Google Scholar]
- 30.Piper RC, Xu X, Russell DG, Little BM, Landfear SM. Differential targeting of two glucose transporters from Leishmania enriettii is mediated by an NH2-terminal domain. J Cell Biol. 1995;128:499–508. doi: 10.1083/jcb.128.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robinson D, Beattie P, Sherwin T, Gull K. Microtubules, tubulin, and microtubule-associated proteins of trypanosomes. Methods Enzymol. 1991;196:285–299. doi: 10.1016/0076-6879(91)96027-o. [DOI] [PubMed] [Google Scholar]
- 32.Sambrook, J., E.F. Fritsch, and T. Maniatis. 1989. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 33.Schneider, A., T. Sherwin, S.R., D.G. Russell, K. Gull, and T. Seebeck. 1987. Subpellicular and flagellar microtubules of Trypanosoma brucei brucei contain the same alpha tubulin isoforms. J. Cell Biol. 104:431–438. [DOI] [PMC free article] [PubMed]
- 34.Seebeck T, Gehr P. Trypanocidal action of neuroleptic phenothiazines in Trypanosoma brucei. . Mol Biochem Parasitol. 1983;9:197–208. doi: 10.1016/0166-6851(83)90097-x. [DOI] [PubMed] [Google Scholar]
- 35.Seebeck T, Hemphill A, Lawson D. The cytoskeleton of trypanosomes. Parasitol Today. 1990;9:201–206. doi: 10.1016/0169-4758(90)90069-g. [DOI] [PubMed] [Google Scholar]
- 36.Thorens B, Sarkar HK, Kaback HR, Lodish HF. Cloning and functional expression in bacteria of a novel glucose transporter present in liver, intestine, kidney, and β-pancreatic islet cells. Cell. 1988;55:281–290. doi: 10.1016/0092-8674(88)90051-7. [DOI] [PubMed] [Google Scholar]
- 37.Vickerman K. The ultrastructure of pathogenic flagellates. Ciba Found Symp. 1974;20:171–190. [Google Scholar]
- 38.Webster P, Russell DG. The flagellar pocket of trypanosomatids. Parasitol Today. 1993;9:201–206. doi: 10.1016/0169-4758(93)90008-4. [DOI] [PubMed] [Google Scholar]