Abstract
Functional studies on the α6β4 integrin have focused primarily on its role in the organization of hemidesmosomes, stable adhesive structures that associate with the intermediate filament cytoskeleton. In this study, we examined the function of the α6β4 integrin in clone A cells, a colon carcinoma cell line that expresses α6β4 but no α6β1 integrin and exhibits dynamic adhesion and motility on laminin-1. Time-lapse videomicroscopy of clone A cells on laminin-1 revealed that their migration is characterized by filopodial extension and stabilization followed by lamellae that extend in the direction of stabilized filopodia. A function-blocking mAb specific for the α6β4 integrin inhibited clone A migration on laminin-1. This mAb also inhibited filopodial formation and stabilization and lamella formation. Indirect immunofluorescence microscopy revealed that the α6β4 integrin is localized as discrete clusters in filopodia, lamellae, and retraction fibers. Although β1 integrins were also localized in the same structures, a spatial separation of these two integrin populations was evident. In filopodia and lamellae, a striking colocalization of the α6β4 integrin and F-actin was seen. An association between α6β4 and F-actin is supported by the fact that α6β4 integrin and actin were released from clone A cells by treatment with the F-actin– severing protein gelsolin and that α6β4 immunostaining at the marginal edges of clone A cells on laminin-1 was resistant to solubilization with Triton X-100. Cytokeratins were not observed in filopodia and lamellipodia. Moreover, α6β4 was extracted from these marginal edges with a Tween-40/deoxycholate buffer that solubilizes the actin cytoskeleton but not cytokeratins. Three other carcinoma cell lines (MIP-101, CCL-228, and MDA-MB-231) exhibited α6β4 colocalized with actin in filopodia and lamellae. Formation of lamellae in these cells was inhibited with an α6-specific antibody. Together, these results indicate that the α6β4 integrin functions in carcinoma migration on laminin-1 through its ability to promote the formation and stabilization of actin-containing motility structures.
The integrin α6β4, a receptor for the laminins, is essential for the organization and maintenance of epithelial structure (11, 57). In many epithelia, this integrin mediates the formation of stable adhesive structures termed hemidesmosomes that link the intermediate filament cytoskeleton with the extracellular matrix (3, 16). Indeed, the ability of α6β4 to associate with intermediate filaments distinguishes it from other integrins that interact primarily with the actin cytoskeleton (21). The importance of this integrin in epithelial structure has been reinforced by the recent generation of β4-knockout mice that exhibit gross alterations in epithelial morphology and loss of anchorage to the basement membrane (11, 57).
Although the α6β4 integrin is also expressed in many carcinomas, its biological functions in these epithelial- derived tumors have not been well studied (43). We and others have argued that α6β4 may be associated with the process of carcinoma invasion (6, 12, 26, 43, 45). Initially, this argument was based on immunohistochemical data that correlated α6β4 expression and localization with invasive carcinoma (12, 45, 56). More recently, we demonstrated that ectopic expression of α6β4 in β4-deficient colon carcinoma cells significantly increased the rate at which these cells invaded laminin matrices (6). Such data that associate α6β4 with carcinoma invasion, however, are not consistent with the established role for this integrin in the formation of stable and rigid adhesive structures and maintenance of cell polarity in normal epithelial cells because invasive carcinoma cells are characterized by their dynamic interactions with extracellular matrices and their rapid rate of migration, as well as a loss of polarity (24). A priori, these dynamic functions of carcinoma cells would be impeded by the presence of α6β4-containing hemidesmosomes. In fact, hemidesmosomes are not commonly observed in invasive carcinoma cells, although α6β4 expression often persists (for review see reference 43). The hypothesis can be derived from these observations that α6β4 is associated with different functions in invasive carcinoma cells than in normal epithelial cells. Moreover, this functional difference may derive from the interaction of α6β4 in carcinoma cells with cytoskeletal proteins and other molecules that do not interact with this integrin in normal cells.
In this study, we investigated the function and cytoskeletal associations of α6β4 in invasive colon carcinoma cells that migrate on laminin-1 matrices. The data obtained implicate α6β4 in cell migration, and they demonstrate that this integrin is localized in cell structures associated with motility, namely filopodia and lamellae. Importantly, we also show that α6β4 associates with the actin cytoskeleton in filopodia and lamellae and that it participates in their formation and stabilization.
Materials and Methods
Cells and Antibodies
The clone A cell line was originally isolated from a human, poorly differentiated colon adenocarcinoma (10), and its in vitro properties and repertoire of integrin receptors have been described previously (8, 32, 34, 54). The CCL-228 colon carcinoma and the MDA-MB-231 breast carcinoma cell lines were obtained from American Type Culture Collection (Rockville, MD). The MIP-101 colon carcinoma cell line has been described previously (8). Cells were grown in RPMI 1640 medium containing 10 mM Hepes, penicillin (50 U/μl), streptomycin (50 μg/ml), and 10% FBS.
The following mAbs were used in this study: mouse mAb 2B7 (integrin α6) prepared in our laboratory (46); rat mAb GoH3 (integrin α6) from Immunotech (Westbrook, ME); mouse mAb K20 (integrin β1); mouse mAb MC-13 (integrin β1) provided by Steven Akiyama (National Institutes of Health, Bethesda MD) (1); mouse mAb A9 (integrin β4) provided by Thomas Carey (University of Michigan, Ann Arbor, MI); mouse anti–pan-cytokeratin (a mixture of antibodies that recognizes cytokeratins 1, 4, 5, 6, 8, 10, 13, and 19) from Sigma Chemical Co. (St. Louis, MO).
Cell Migration Assays
To assay the migration of clone A cells, bacteriological dishes were coated with 10–100 μg of laminin-1 prepared from the EHS sarcoma as described (27) or collagen type I (Collaborative Research, Waltham, MA) for 2 h at room temperature and then blocked with PBS containing 1% BSA for 1 h. Clone A cells in exponential growth were removed from culture dishes and resuspended in serum-free RPMI 1640 medium containing 10 mM Hepes and 0.1% BSA. The cells were then plated at low density (1 × 104/ cm2) on the matrix-coated dishes and allowed to adhere for 30 min in a humidified atmosphere with 5% CO2 at 37°C. In some experiments, integrin-specific antibodies (2B7 or MC-13, 10 μg/ml) or a nonspecific mouse IgG control (10 μg/ml) were added to the cells either before the cells were plated or after the cells had adhered for the 30 min. The dishes were then sealed with parafilm and placed on a microscope stage heated to 37°C. For image analysis, an inverted microscope (model Diaphot 300; Nikon, Inc., Melville, NY) with phase contrast optics was used. This microscope was connected to a CCD camera (Dage-MTI, Michigan City, IN), a frame-grabber (Scion, Frederick, MD), and a 7600 Power Macintosh computer (Cupertino, CA) to capture the images. Images were collected for 1 h and analyzed with IPlab Spectrum image analysis software.
Migration speed was determined by following cell centroid displacements as a function of time for 1 h at intervals of 15 min. For each individual experiment, 30–40 cells were analyzed. A frame-by-frame analysis of filopodia at intervals of 1 min for 1 h was used to differentiate filopodia from retraction fibers and to monitor the formation and stabilization of filopodia. Lamellar area was determined by tracing their contour and quantifying the area digitally.
Indirect Immunofluorescence Microscopy
Clone A cells were plated on matrix-coated dishes as described above and incubated for 1 h in a humidified atmosphere with 5% CO2 at 37°C. The cells were then fixed for 20 min at room temperature with a buffer containing 4% paraformaldehyde, 100 mM KCl, 300 mM sucrose, 2 mM EGTA , 2 mM MgCl2, and 10 mM Pipes at pH 6.8. Based on a previously described extraction protocol (4, 13), the cells in some experiments were extracted for 1 min at 4°C before fixation with either a “membrane” buffer containing 0.5% Triton X-100, 100 mM KCl, 300 mM sucrose, 10 mM EGTA, 2 mM MgCl2, 1 mM PMSF, and 10 mM Pipes at pH 6.8 or for 5 min at room temperature with a “cytoskeletal” buffer containing 1% Tween-40, 0.5% sodium deoxycholate, 10 mM NaCl, 2 mM MgCl2, 1 mM PMSF, and 20 mM Tris-HCl, pH 7.4, and then fixed for 20 min in the paraformaldehyde buffer. Subsequently, the fixed cells were rinsed with PBS and incubated with a blocking solution that contained 1% albumin and 5% donkey serum in PBS for 30 min. Primary antibodies (GoH3, 1:50; K20, 1:50; pan-cytokeratin, 1:200) and/or FITC phalloidin (20 μg/ml) in blocking solution were immunoreacted separately or in combination with the cells for 30 min. The cells were rinsed three times and either a fluorescein-conjugated donkey anti–mouse or a rhodamine-conjugated donkey anti–rat IgG (Jackson ImmunoResearch Labs, West Grove, PA) in blocking buffer (1:150) were used separately or in combination to stain the cells for 30 min. Cells were rinsed with PBS and mounted in a mixture (9:1) of glycerol and PBS, pH 8.5, containing 0.1% propylgallate. The dishes were cut into slides and examined by confocal microscopy (model LSM; Carl Zeiss, Inc., Thornwood, NY).
Actin-severing Experiments
Clone A cells (2 × 106) suspended in RPMI-H with 0.1% albumin were plated on laminin-1–coated dishes and incubated for 1 h at 37°C. The following steps were done at 4°C. The medium was removed and a membrane buffer (see above) was added for 30 s and removed by aspiration. A “low calcium” buffer (25 μm CaCl2, 100 mM KCl, 300 mM sucrose, 10 mM EGTA, 2 mM MgCl2 , leupeptin [10 μg/ml], aprotinin [1 μg/ml], pepstatin [5 μg/ml], and 10 mM Pipes, pH 6.8) was used to remove the membrane buffer by washing the cells four times with gentle rocking. Subsequently, the low calcium buffer containing 200 nM gelsolin (kindly provided by Dr. Paul Janmey, Harvard Medical School, Boston, MA) and 50 μg/ml of GC-globulin (Calbiochem, La Jolla, CA) was added to the cells and incubated for 30 min. Control cells were treated with the low calcium buffer alone. An equal volume of membrane buffer was added to the cells for 30 s to terminate the reaction. The buffer was removed and collected in microfuge tubes, centrifuged at 12,000 rpm for 10 min, and immunoprecipitated with the 2B7 antibody. The immune complexes were resolved by SDS-PAGE and immunoblotted with an anti–β4 integrin polyclonal antibody elicited against the last 20 amino acids of the β4 cytoplasmic tail.
Results
The Integrin α6β4 Functions in the Migration of Clone A Cells on Laminin-1 Matrices
Clone A cells were chosen to investigate the possible function of the α6β4 integrin in the migration of carcinoma cells for several reasons. These cells, which were derived from a poorly differentiated colon carcinoma, are invasive both in vitro (8) and in vivo (53). They adhere avidly to laminin-1 (34) and, in fact, can mediate dynamic adhesion on laminin-1 under laminar flow conditions (54). Clone A cells are also advantageous because they express the α6β4 but not the α6β1 integrin laminin receptor (32, 34), enabling the study of the α6β4 integrin in the absence of α6β1 function and the use of α6-specific reagents to target the α6β4 integrin specifically. Clone A cells also employ a β1 integrin (α2β1) as a laminin-1 receptor and for this reason are useful for studying functional differences between β1 and β4 integrin laminin receptors (32, 34).
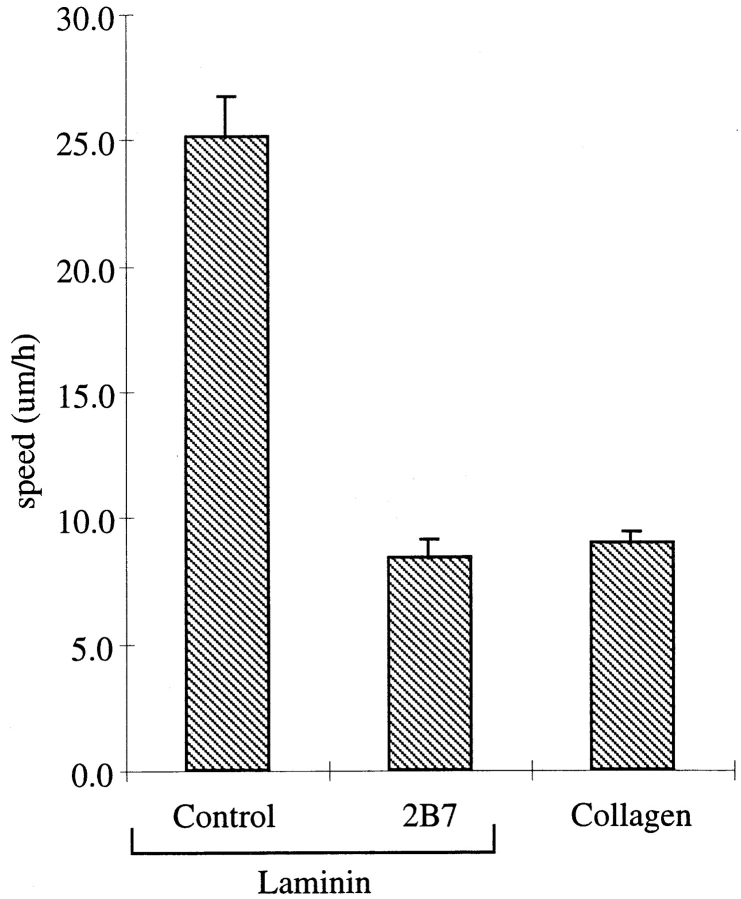
The migration of clone A cells was examined by time-lapse videomicroscopy in serum-free conditions. Clone A cells exhibit random migration (mean rate = 25 μM/h) when plated on laminin-1. As shown in Fig. 1, this rate of migration on laminin-1 is approximately threefold greater than on an equivalent concentration of collagen type I, even though these cells adhere equally well to both matrices (34). Varying the concentration of collagen type I in these assays did not induce migration, suggesting a specific role for laminin in stimulating clone A migration (data not shown).
Figure 1.
Random migration of clone A cells on laminin-1 is dependent on the integrin α6β4. Clone A cells were plated on laminin-1 or collagen I-coated dishes (10 μg/ ml) and incubated at 37°C for 30 min before the addition of either a control mouse IgG (10 μg/ml) or the α6 integrin–specific mAb 2B7 (10 μg/ml). Migration was analyzed by time-lapse videomicroscopy as described in the Materials and Methods section. The mean cell speed (i.e., displacement of the cell centroid as a function of time) obtained from the analysis of 30–40 cells for each experimental condition is reported in this bar graph. Error bars represent SEM.
The α6β4 integrin functions in migration on laminin-1 based on the finding that treatment of clone A cells with an α6-specific mAb (2B7) (46) inhibited this migration significantly (66%) (Fig. 1), but it did not detach the cells from laminin-1 (Fig. 2). In contrast, the cells “rounded up” and detached after exposure to mAb 13, a β1-specific mAb (1) (data not shown). These mAb inhibition data suggest different functions for the α6β4 integrin and β1 integrins in mediating the dynamic interactions of clone A cells with laminin-1.
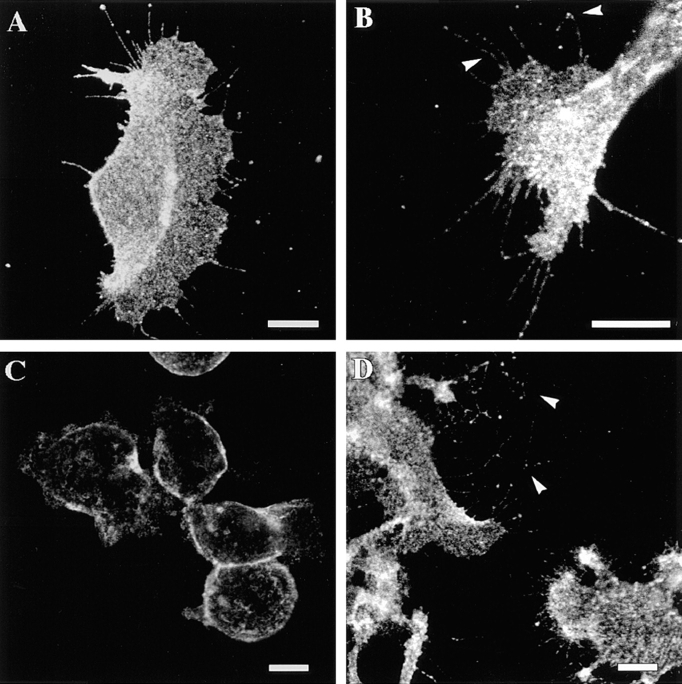
Figure 2.
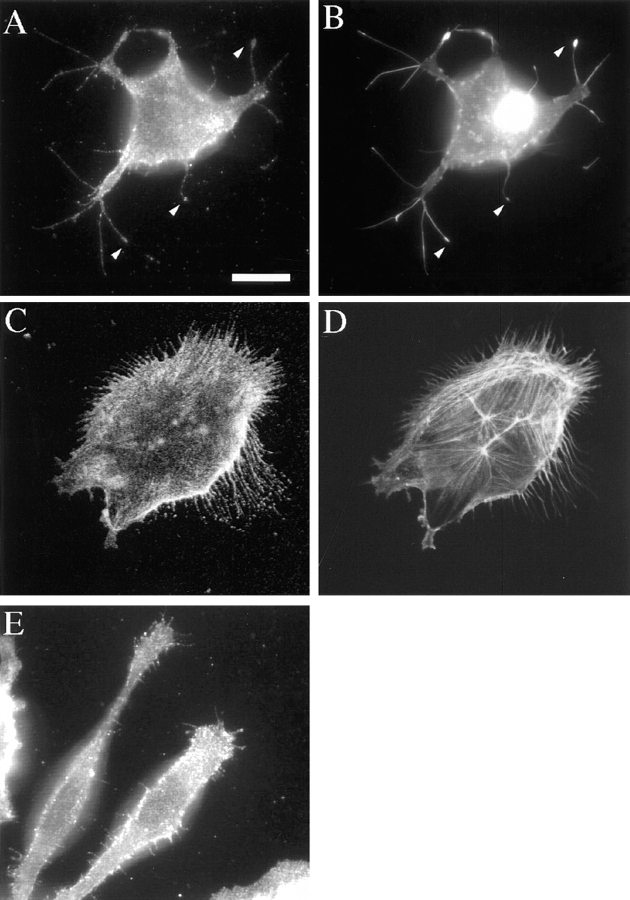
The motile morphology of clone A cells on laminin-1 is dependent on the integrin α6β4. Cells were plated on either laminin- 1–coated (A and C) or collagen I–coated (D) dishes and incubated at 37°C for 30 min before the addition of either (A) control mouse IgG (10 μg/ml) or (C) 2B7 mAb (10 μg/ml). After 30 min, the cells were photographed using phase contrast optics. Note the presence of prominent fan-shaped lamellae in cells on laminin-1 (A) and their digitally traced area in B. The α6-specific mAb 2B7 inhibits formation of these lamellae (C). Prominent lamellae are not seen when clone A cells are plated on collagen I (D). Bar, 20 μm.
Photomicrographs of clone A cells on laminin-1 demonstrate the morphological changes induced by inhibiting the α6β4 integrin. Clone A cells exhibited a fan-shaped appearance on laminin-1 with prominent lamellae and numerous filopodia (Fig. 2, A and B). Treatment of these cells with 2B7 antibody either before or after plating on laminin-1 had a modest effect on inhibiting cell spreading, but it markedly inhibited the formation of lamellae (Fig. 2 C). In contrast to laminin-1, clone A cells plated on collagen type I were well spread but did not exhibit prominent lamellae (Fig. 2 D).
The Integrin α6β4 Participates in the Dynamic Formation of Actin-based Motility Structures: Filopodia and Lamellae
We analyzed the sequence of events by which filopodia and lamellae lead to cell translation on laminin-1 using time-lapse videomicroscopy to understand how the α6β4 integrin contributes to migration. Subsequent to attachment and spreading on laminin-1, numerous filopodia protruded from clone A cells, as evidenced by the sequence of three cells shown in Fig. 3. Only characteristic threadlike structures that actively protruded from the cells were considered to be filopodia. At later times, filopodia also protruded from the lamellae that had formed in these cells. Video analysis revealed that nascent filopodia exhibited a wide range of motion until they either retracted or were stabilized by anchoring to the laminin-1 substratum. Stabilization was detected when the filopodia anchored to laminin-1 at one or more points along their length, a process that restricted their movement and prevented their retraction. When stabilization occurred at a point proximal to the tip of the filopodium, the filopodium usually continued to extend or move freely distal to the attachment point forming a conspicuous angle, the vertex being the anchoring point (Fig. 3, A–C). The formation of such angles was observed in 80% of the stabilized filopodia examined.
Figure 3.
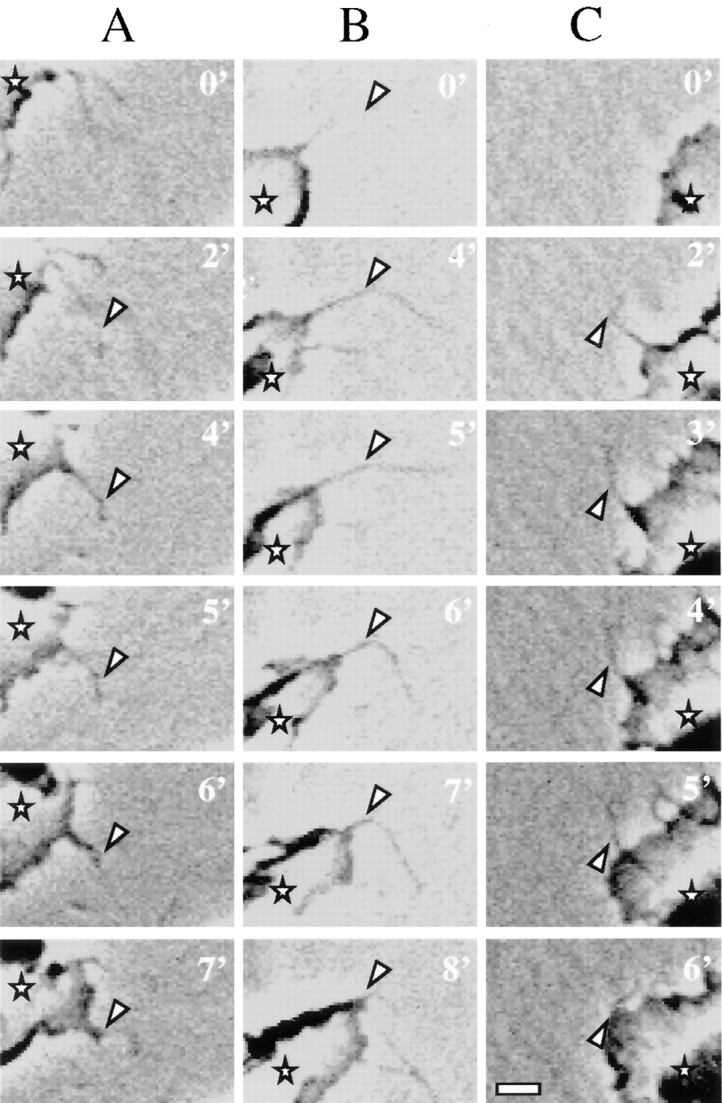
Dynamics of filopodia and lamellae in clone A cells on laminin-1. Cells plated on laminin-1 were analyzed by time-lapse videomicroscopy. Each column shown (A–C) represents a sequence of frames recorded at the specified times from different cells migrating on laminin-1. Arrowheads indicate points at which the filopodia stabilize on the laminin-1 matrix. Asterisks denote the protruding lamella. In A, the filopodium shown stabilized at 2 min and formed an angle at the point indicated by the arrowhead. Note that only the anchoring point is attached because both proximal and distal segments shift positions at later times, during which the lamella extended following the direction of the filopodium. In B and C, the filopodia shown stabilized at 4 and 2 min, respectively. In contrast to the filopodium shown in A, the entire segment of these filopodia proximal to the vertex of the angle became immobilized and the lamellae extended in the direction of these stabilized filopodia. Bar, 5 μm.
The extension of a lamella occurred frequently after the stabilization of a filopodium (Fig. 3, A–C). Such lamellae extruded from the roots of stabilized filopodia and followed the direction of these filopodia (Fig. 3, A–C). Subsequently, the cell bodies translocated in the direction of well-developed lamellae, as described previously (2). Thus, the stabilization of filopodia appeared to be a major determinant of the direction of cell migration. The time that clone A cells moved in a specific direction on laminin-1 was relatively brief (<1 h) because of more active lamellae at other sites on the cell and the collapse of the initial lamellae. This frequent change in direction gave the appearance of a random walk.
We studied the influence of the α6β4 integrin on clone A cells by inhibiting the action of this integrin with the 2B7 mAb and assessing the qualitative and quantitative effects of this inhibition on lamellar dynamics. The formation of lamellae was markedly inhibited (80%) within 60 min of adding the 2B7 mAb to motile cells (Fig. 4). However, the cells inhibited with 2B7 remained well spread (Fig. 2), suggesting a specific function for the α6β4 integrin in the formation of lamellae and not in cell attachment or spreading. Similarly, quantification of higher magnification video images revealed that 2B7 inhibited filopodial formation significantly and that the filopodia that did form in the presence of this inhibitory antibody stabilized much less frequently. Specifically, 48% of filopodia stabilized in control cells compared with 3% in 2B7-treated cells (Fig. 5). In contrast to these effects of 2B7 on clone A cells, the addition of mAb 13, a β1 integrin–specific mAb, caused the cells to round-up and subsequently detach as mentioned above.
Figure 4.
Formation of lamellae in clone A cells on laminin-1 requires the α6β4 integrin. Clone A cells were plated on laminin-1–coated dishes and incubated for 30 min at 37°C before the addition of 2B7 (10 μg/ml) or a control IgG. The cells were photographed after 1 h, and their lamellar area (μm2/cell) was determined by digital image analysis (see example in Fig. 2 B). 50 cells were analyzed for each condition. Error bar represents SEM.
Figure 5.
Inhibition of the integrin α6β4 reduces the formation and stabilization of filopodia in clone A cells on laminin-1. Clone A cells were plated on laminin-1–coated dishes and incubated for 30 min at 37°C before the addition of either 2B7 (10 μg/ml) or a control IgG (10 μg/ml) and analysis by time-lapse videomicroscopy. For each condition, five cells were monitored for 1 h at a frequency of one frame per minute and each frame was analyzed for the active formation of filopodia. A filopodium was considered stabilized if it remained immobile for several frames. The data shown represent the number of filopodia that either formed or stabilized/cell/hour. Error bar represents SEM.
The Integrin α6β4 Is Localized in Filopodia and Lamellae in Areas Distinct from β1 Integrins
To gain insight into how the α6β4 integrin influences the formation of filopodia and lamellae, we analyzed the spatial distribution of this integrin by indirect immunofluorescence microscopy using GoH3, an α6-specific mAb (48). Identical immunostaining results were obtained with A9, a β4-specific mAb (data not shown).
The α6β4 integrin was distributed throughout the cell body, as well as in the lamellae and filopodia of clone A cells plated on laminin-1. In the lamellae, it exhibited a fine grainy pattern of staining (Fig. 6, A and B). In the filopodia themselves, α6β4 staining was localized in discrete clusters that were distributed throughout the shaft (Fig. 6 B). At points of filopodial angling, a concentration of α6β4 staining was usually observed (Figs. 6 B and 7, C and D). In contrast to cells plated on laminin-1, clone A cells plated on collagen type I displayed a diffuse distribution of α6β4 staining with few cluster formations evident on the ventral surface of the cells (Fig. 6 C).
Figure 6.
The integrin α6β4 is localized in lamellae and filopodia in clone A cells on laminin-1. Cells plated on laminin-1 (A, B, and D) or collagen I (C) were fixed and processed for immunofluorescence using the rat GoH3 (anti-α6) mAb followed by a rhodamine-conjugated anti–rat antibody as described in the Materials and Methods section. The confocal images shown represent optical sections of the ventral surface. (A) Note the presence of α6β4 staining on the lamellae and in the filopodia at the leading edge (right side of the cell), as well as in retraction fibers at the trailing edge (left side of cell). (B) A higher magnification demonstrates the clustered appearance of α6β4 integrin in the filopodia and its presence at points of filopodial angling. (D) Fibers that are positive for α6β4 staining (arrowheads) are apparently left behind by the advancing cell on the left. (C) Clone A cells plated on collagen I exhibit only a diffuse pattern of α6β4 staining on their ventral surface. Bars, 10 μm.
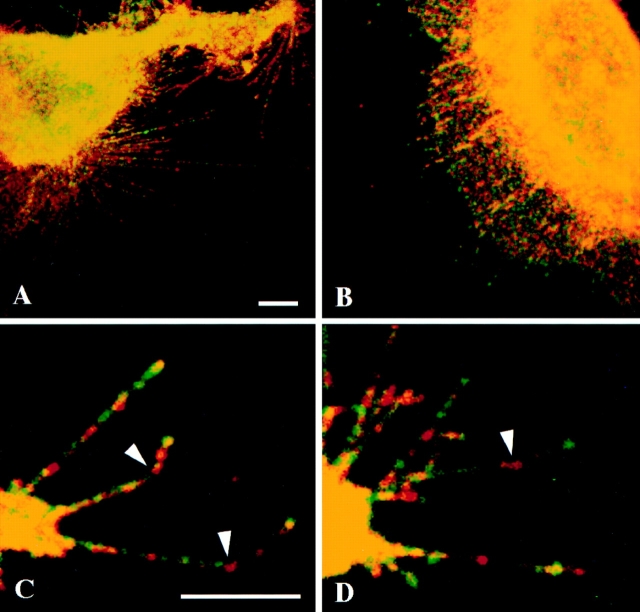
Figure 7.
Distinct localization of α6β4 and β1 integrins in the filopodia and lamellae of clone A cells on laminin-1. Clone A cells were plated on laminin-1 for 1 h at 37°C and processed for double immunofluorescence as described using rat GoH3 mAb and the mouse K-20 mAb followed by a combination of a TRITC-conjugated anti–rat antibody and an FITC-conjugated anti–mouse antibody that do not cross-react. The ventral surface of the cells was analyzed by confocal microscopy. Red, GoH3 mAb; green, K-20 mAb; yellow, colocalization. (A) Several filopodia and retraction fibers show a segregated distribution of α6β4 and β1 staining. (B) In lamellae, α6β4 staining is largely segregated from β1 staining except in the streak-shaped areas where filopodia project into the lamella. (C and D) Higher magnification images of filopodia showing the spatial segregation of α6β4 and β1 staining and the presence of α6β4 in the angles of filopodia (arrowheads). Bar, 5 μm.
The α6β4 integrin was expressed intensely in characteristic retraction fibers of clone A cells on laminin-1 (Figs. 6 A and 8 A). Retraction fibers were identified by their unique appearance at the trailing edges of fan-shaped cells (Fig. 2). In these fan-shaped cells, there was a clear gradient of α6β4 towards the rear that peaked in intensity in retraction fibers (Fig. 6 A). In addition, some retraction fibers that were enriched in α6β4 expression were observed detached from cells, most likely remnants of migrating cells (Fig. 6 D).
Figure 8.
Enhanced localization of the α6β4 integrin in retraction fibers of clone A cells on laminin-1. Cells plated on laminin-1 for 1 h at 37°C were processed for double immunofluorescence as described using the rat GoH3 mAb and mouse K-20 mAb followed by a combination of a TRITC-conjugated anti–rat antibody and an FITC-conjugated anti–mouse antibody that do not cross-react. The ventral surface of the cells was analyzed by confocal microscopy. (A) GoH3 mAb. (B) K-20 mAb. Note the presence of α6β4 staining in retraction fibers (A, arrowheads) at the trailing edge of the cells. Staining of β1 integrin is absent in these fibers (B). Bar, 10 μm.
Because β1 integrin function is also critical in the interaction of clone A cells with laminin-1 (32, 34, 54), we compared the distribution of β1 integrin with that of the α6β4 integrin by double immunostaining using GoH3 and a β1-specific mAb, K20. Although β1 integrin was also localized in filopodia and lamellae, a striking difference in the localization of β4 and β1 integrin staining was evident in these structures (Fig. 7, A–D). In filopodia, discrete clusters or patches of β1 and α6β4 integrin staining were apparent. As noted above, α6β4 staining was observed frequently at points of filopodial angling (Fig. 7, C and D). There were also some regions of overlapping staining, most consistently at the tips of filopodia (Fig. 7, C and D). The staining pattern of the α6β4 and β1 integrins was segregated in the lamellae as well, except at those points where the root of a filopodium projected into the lamella (Fig. 7 B). In the characteristic retraction fibers of clone A cells on laminin-1, the double immunostaining revealed that α6β4 expression predominated over β1 integrin expression (Fig. 8, A and B).
Association of α6β4 with the Actin Cytoskeleton in Motile Structures
Our observations that the α6β4 integrin functions in cell migration and that it is localized in actin-containing structures such as filopodia and lamellae suggest that it does interact with the actin cytoskeleton in clone A cells. However, the majority of studies on the α6β4 integrin and the cytoskeleton have focused on its association with intermediate filaments, especially in hemidesmosomes (5, 11, 15, 22, 49, 51). Very little is known, in fact, about possible interactions of α6β4 with the actin cytoskeleton. For this reason, we assessed the association of the α6β4 integrin with both the actin cytoskeleton and cytokeratins in clone A cells plated on laminin-1 by indirect immunofluorescence microscopy using the GoH3 mAb and either phalloidin or a pan-cytokeratin antibody.
Visualization of clone A cells that had been stained with the GoH3 mAb and phalloidin revealed a striking colocalization of the α6β4 integrin and F-actin in filopodia and at the edges of the lamellae (Fig. 9, A–D). In contrast, cytokeratins were excluded from filopodia and the edges of lamellae in clone A cells plated on laminin-1 based on the staining pattern observed with the pan-cytokeratin antibody (Fig. 10 A). Cytokeratin staining was concentrated largely in the cell body and proximal portions of lamellae. Costaining with GoH3 did not indicate any significant association of the α6β4 integrin and cytokeratins in filopodia or at the edges of lamellae (Fig. 10 A).
Figure 9.

α6β4 integrin colocalizes with F-actin in filopodia of clone A cells on laminin-1. Cells plated on either laminin-1 (A–D and F) or collagen I (E) at 37°C for 1 h were processed for double immunofluorescence as described using the rat GoH3 mAb followed by a rhodamine-conjugated anti–rat antibody and FITC-conjugated phalloidin. The confocal images shown represent optical sections of the ventral surface of the cells. (A and C) GoH3. (B, D, E, and F) Phalloidin. A and B demonstrate colocalization of α6β4 and F-actin in a group of filopodia. D shows the formation of actin cables on the top lamella that project into filopodia. These filopodia are enriched in α6β4 (C). E shows the presence of polygonal actin cables in clone A cells plated on collagen I. In F, the cells were incubated with 2B7 antibody for 30 min before fixation. Note the disappearance of actin cables (remaining protrusions are presumably retraction fibers, see text). Bars, 10 μm.
Figure 10.
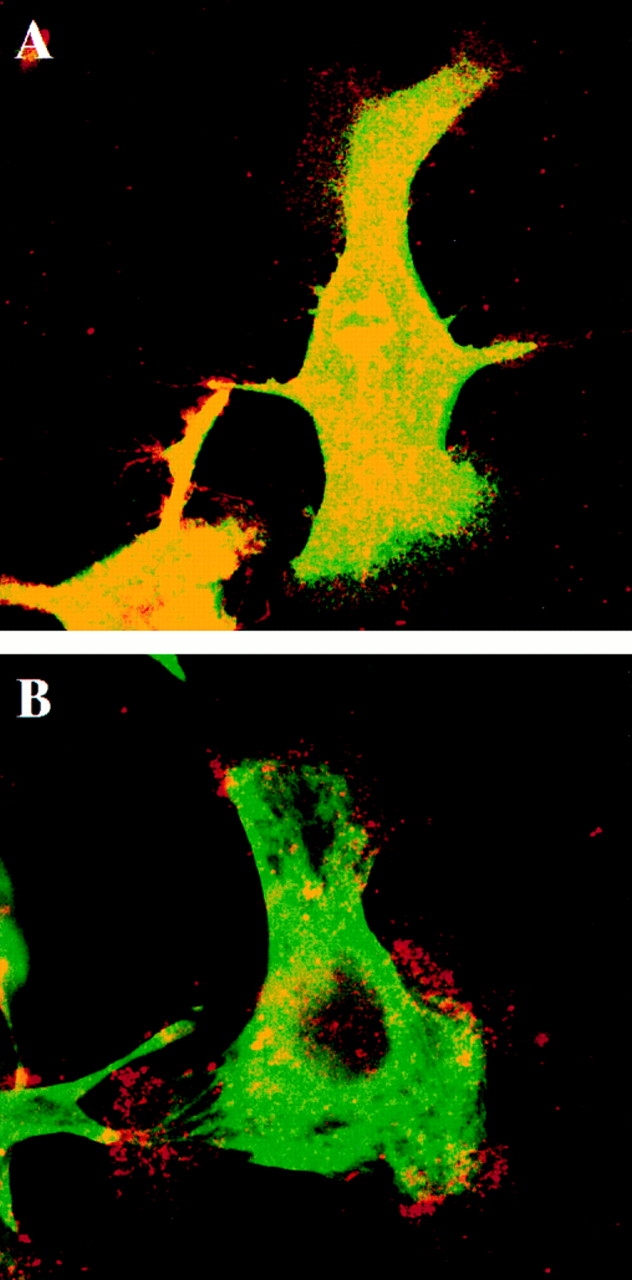
The integrin α6β4 localized at the marginal areas of clone A cells on laminin-1 does not colocalize with cytokeratins. Cells were plated on laminin and incubated for 1 h at 37°C . In A, the cells were fixed immediately after the incubation period in paraformaldehyde and then permeabilized with Triton X-100. In B, cells were extracted first with a Triton X-100 buffer before fixation. In C, the cells were extracted with a Tween-40/deoxycholate buffer before fixation. After fixation, the cells in A–C were stained by a double immunofluorescence protocol using rat GoH3 mAb and a mouse pan-cytokeratin mAb antibody followed by a combination of a TRITC-conjugated anti–rat antibody and a FITC-conjugated anti–mouse antibody that do not cross-react. The ventral surface of the cells was analyzed by confocal microscopy. Red, GoH3; green, cytokeratin; yellow, colocalization. In A and B, the marginal areas (edges of lamellae, filopodia, and retraction fibers) exhibit positive α6β4 staining but no cytokeratin staining. Note in B the persistence of α6β4 staining in marginal clusters arranged in streaks that are likely portions of filopodia. In C, the Tween-40/deoxycholate buffer extracted most of the α6β4 staining in filopodia and lamellae. However, α6β4 staining persisted at the base of lamellae where it colocalized with cytokeratins. The cytokeratin staining was digitally “overexposed” to detect any possible cytokeratin expression. Bar, 10 μm.
To study the interaction of the α6β4 integrin with the cytoskeleton in more detail, we used an in situ extraction scheme that solubilizes proteins to an extent that correlates with their cytoskeletal associations (4, 13). Clone A cells adherent to laminin-1 were extracted with either a 0.5% Triton X-100 buffer that removes most of the soluble protein and phospholipid but not the actin and intermediate filament cytoskeletons, or a two-detergent buffer (1.0% Tween-40/0.5% deoxycholate) that removes the bulk of the actin cytoskeleton but not intermediate filaments and associated proteins (4, 13). Subsequent to extraction, the cells were fixed and costained with integrin-specific mAbs (GoH3 or K20) and cytoskeletal-specific reagents (phalloidin or pan-cytokeratin mAbs). Extraction of clone A cells with the Triton X-100 buffer revealed that the α6β4 and F-actin colocalization observed in unextracted cells is preserved in clusters at proximal sites in filopodia, as well as at the roots of filopodia that project into the lamellae (Fig. 11, A and B). Several of these colocalization sites were also the origins of actin filament bundles (Fig. 11, A and B). In contrast, α6β4 did not colocalize with cytokeratins in filopodia and distal sites of many of the lamellae either in unextracted cells (Fig. 10 A) or after the Triton X-100 buffer extraction (Fig. 10 B). These results suggest that α6β4 is retained at the cell edges because of its association with actin and not with cytokeratins. In agreement with this possibility, these marginal areas of actin-associated α6β4 integrin were removed by the Tween/ deoxycholate buffer. As shown in Fig. 10 C, the only GoH3-positive staining that remained after this buffer was the staining that colocalized with cytokeratins at the base of lamellae, the lamellae themselves having been largely removed by the Tween/deoxycholate buffer. F-actin was extracted by the Tween/deoxycholate buffer, as indicated by a lack of phalloidin staining (Fig. 11 D). Interestingly, most of the β1 integrin staining was removed from cells on laminin-1 by the Triton X-100 buffer, suggesting a weaker interaction of β1 integrins with the cytoskeleton than with the α6β4 integrin in these cells (Fig. 11 C). Also, most of the α6β4 staining was extracted by the Triton X-100 buffer in cells plated on collagen I, indicating that the association of α6β4 with actin is dependent on laminin-1 (data not shown). Together, these results reinforce the hypothesis that the retention of α6β4 in filopodia and edges of lamellae of clone A cells on laminin-1 is mediated by the actin cytoskeleton. In these motile structures, the only apparent association of α6β4 with cytokeratins occurs at the base of the lamellae.
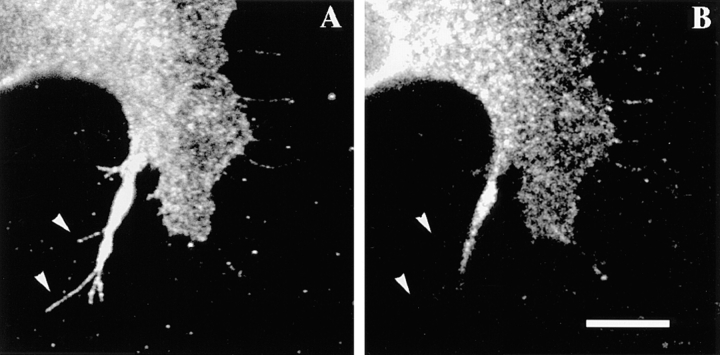
Figure 11.
The integrin α6β4 is associated with F-actin. Cells were plated on laminin and incubated for 1 h at 37°C . In A–C, the cells were extracted first with a Triton X-100 buffer before fixation with paraformaldehyde. In D, the cells were extracted with a Tween-40/deoxycholate buffer before fixation. After fixation, the cells in A and B were double immunostained using rat GoH3 mAb followed by a rhodamine-conjugated anti–rat antibody (A) and FITC-conjugated phalloidin (B). In C, the cells were stained with mouse K-20 mAb antibody followed by an FITC-conjugated anti–mouse antibody. In D, the cells were stained with phalloidin. The ventral area of the cells was analyzed by confocal microscopy. Note in A and B the colocalization of α6β4 (A) and F-actin (B) at the roots of filopodia (arrowheads). Several of these colocalization areas are in continuity with actin cables (B). Bar, 10 μm.
The presence of α6β4 at the origin sites of actin filament bundles described above prompted us to examine the possibility of a functional relationship. The actin bundles were usually organized as multiple cables that ran parallel to the margins of lamellae and were most commonly observed in fan-shaped cells (Fig. 9 D). The α6β4 integrin colocalized with F-actin at the termini of several of these parallel actin bundles, which were in continuity with filopodia, but it was not localized along the bundles themselves (Fig. 9, C and D; Fig. 11). These actin bundles were not organized into polygonal arrays traversing the nuclear area, such as those that are characteristic of fibroblasts. Interestingly, however, clone A cells on collagen type I did exhibit such a polygonal array of filament bundles (Fig. 9 E). The parallel actin bundles were no longer evident when cells plated on laminin-1 were treated with the 2B7 antibody, suggesting that the α6β4 integrin is critical for their formation (Fig. 9 F).
Biochemical Evidence for an Association of the α6β4 Integrin with Actin Filaments
The data presented above strongly support an association of the α6β4 integrin with actin filaments in clone A cells. To obtain biochemical evidence for this association, we used the actin-severing protein gelsolin to assess whether severing actin filaments would liberate α6β4. Clone A cells adherent to laminin-1 were extracted with the 0.5% Triton X-100 buffer to remove soluble proteins and then treated with gelsolin. The proteins liberated from extracted cells treated with gelsolin, as well as from extracted cells treated with the buffer alone, were analyzed for the presence of α6β4 by immunoprecipitation with 2B7 and subsequent immunoblotting with a polyclonal antibody specific for the β4 cytoplasmic domain. As shown in Fig. 12, the 200-kD β4 subunit was liberated from gelsolin-treated cells but not from cells treated with buffer alone. Moreover, the material obtained from the gelsolin-treated cells but not the control cells was enriched in actin (Fig. 12).
Figure 12.
The actin-severing protein gelsolin releases α6β4 integrin from permeabilized clone A cells. Cells were plated on laminin-1 and incubated for 1 h at 37°C. After permeablization with a Triton X-100 buffer, the cells were incubated with either gelsolin (G) or control buffer (C) for 30 min. The gelsolin-liberated fraction was immunoprecipitated with an α6-specific antibody (2B7), subjected to SDS-PAGE, and immunoblotted with a β4-specific polyclonal antibody. An aliquot of the gelsolin-liberated fraction was subjected to SDS-PAGE and stained with Coomassie blue to detect the 43-kD actin band that was evident in the gelsolin-treated but not the control cells.
Functional Association of the α6β4 Integrin with Filopodia and Lamellae in Other Carcinoma Cells
We explored the possibility that the functional and topographical properties of the α6β4 integrin observed in clone A cells could be extended to other carcinoma cells that express α6β4. The CCL-228 and MIP-101 colon carcinoma cells have been shown previously to express the α6 integrin subunit exclusively associated with β4 (19), while the MDA-MB-231 breast carcinoma cells express primarily the α6β4 heterodimer (data not shown). These carcinoma cells were analyzed by indirect immunofluorescence using an anti-β4 antibody (A9) and FITC-phalloidin. Although these carcinoma cells differed markedly in their morphology on laminin-1, all of them exhibited a fine grainy pattern of β4 staining on their ventral surfaces (Fig. 13, A–E). More specifically, β4 was localized in discrete clusters in filopodia, retraction fibers, and lamellae. A striking colocalization of β4 and actin was seen in these structures similar to the results obtained with clone A cells. Interestingly, MIP-101 cells exhibited long filopodia with distinct “actin nodes” that were enriched in β4 staining. (Fig. 13, A and B).
Figure 13.
The α6β4 integrin is localized in actin-containing motility structures in other carcinoma cells. MIP-101 (A and B), MDA-MB-231 (C and D), and CCL-228 (E) carcinoma cells were analyzed by double immunostaining with the β4-specific A9 antibody (A, C, and E) and FITC-phalloidin (B and D). Note the concentration of α6β4 in the actin nodes present in the filopodia of MIP-101 cells (A and B, arrowheads) and the distribution of α6β4 in filopodia, retraction fibers, and lamellae of MDA-MB-231 and CCL-228 cells. (F) An α6-specific antibody inhibits formation of lamellae in CCL-228 cells. Cells were plated on laminin-1 in the presence or absence of 2B7 for 1 h. The cells were photographed, and their lamellar area (μm2/cell) was determined by digital image analysis. 50 cells were analyzed for each condition. Error bar represents SEM. Bar, 20 μm.
We also explored the function of the α6β4 integrin in the dynamic behavior of CCL-228 cells on laminin-1 using the function-blocking 2B7 antibody. As shown in Fig. 13 F, 2B7 markedly inhibited the formation of lamellae, but it did not affect cell attachment. Similar results were obtained with MIP-101 and MDA-MB-231 cells (data not shown). These data indicate that the interaction of α6β4 with actin-containing motility structures is a frequent phenomenon in carcinoma cells.
Discussion
Functional studies on the α6β4 integrin have focused primarily on its role in the organization of hemidesmosomes, stable adhesive structures that associate with the intermediate filament cytoskeleton (5, 15, 22, 49, 51). We report here that the α6β4 integrin can also function in the dynamic process of cell migration on laminin-1 based on data obtained from the analysis of clone A colon carcinoma and other carcinoma cells. Interestingly, our results demonstrate that this integrin participates in a specific aspect of the migration process, the formation and stabilization of filopodia and lamellae. The functional involvement of the α6β4 integrin in the dynamics of these actin-containing motility structures is supported by our findings that α6β4 is localized in filopodia and edges of the lamellae and that it associates with actin and not with cytokeratins in these structures. These specific functions of α6β4 in the migration process of clone A cells are in contrast to a more general function for β1 integrins, which are required for the adhesion and spreading of clone A cells on laminin-1.
The premise for this study was our interest in understanding how the α6β4 integrin contributes to carcinoma progression. Although several studies have linked this integrin to the invasive behavior of various carcinomas, mechanistic data to support a function for α6β4 in a dynamic process such as invasion have been lacking (43 and references therein). Our finding that α6β4 can participate in the formation and function of actin-containing motility structures in colon carcinoma cells clearly suggests an important role for this integrin in cell migration, a process that is critical for tumor invasion and metastasis. Indeed, it will be extremely informative to study the localization and cytoskeletal dynamics of α6β4 in other carcinoma cells in which it has been linked to invasion, such as thyroid and gastric carcinomas (45, 52). In this direction, it is worth mentioning that many aspects of epithelial wound healing, including a loss of cell polarity and an induction of cell migration, resemble the behavior of invasive carcinoma cells exemplified by the fibroblast-like morphology and active motility seen in clone A cells (37). There is some evidence in fact that α6β4 may participate in epithelial wound healing (9, 23, 29, 30, 35, 37), although the nature of this involvement has not been explored. In addition, EGF has been shown to increase the migration of 804G bladder cells by disrupting hemidesmosomes and possibly enabling α6β4 to participate in migration (36). Collectively, these findings raise the possibility of a general function for the α6β4 integrin in epithelial cell migration.
The videomicroscopy data presented indicate that filopodial formation and stabilization play a key role in the migration of clone A cells on laminin-1, reminiscent of earlier studies on cell locomotion by Albrecht-Buehler that ascribed exploratory and sensory capabilities to filopodia (2). Using fibroblasts plated on surfaces coated with gold, he observed that lamellipodia form when filopodia find and contact a gold-coated area and that these lamellipodia follow the direction of the stabilized filopodia. The importance of filopodial contacts in directing cell migration is underscored by the pioneer growth cone model in grasshoppers where a single filopodial contact can re-orient an entire growth cone (41). This sequence of events is consistent with the more recent transport-track model of migration (39). This model implies that stabilized filopodia or any other polarized, actin filament bundle fixed onto a substrate provides the cell with a structure that can be used as a one-way transport track by myosin II motors. Such motors pull other cytoplasmic components bound to substratum-free actin filaments forward (39). This model has also been supported by the respreading of cells after mitosis. Such cells use retraction fibers to guide the spreading edge, and these retraction fibers contain polarized bundles of actin filaments anchored to the substrate at their tips. If the retraction fibers are mechanically detached from the substrate, a spreading edge is not extended (7, 38). These observations are mentioned because we observed a related sequence of events during the migration of clone A cells on laminin-1. The nascent lamellae formed in the direction of filopodia that had stabilized on laminin-1. Moreover, this stabilization is mediated by the α6β4 integrin because function-blocking antibodies substantially reduced the number of stabilized filopodia. Stabilization is probably a direct interaction of α6β4 with laminin-1 at the anchorage point, based on the observation that α6β4 is enriched at the angles of filopodia, sites that are clearly the anchoring points detected by videomicroscopy. This scenario implies that the stabilizing function of the α6β4 integrin is an early event in clone A motility on laminin-1.
One question that arises from our data is whether the role of the α6β4 integrin in filopodial formation and stabilization is unique. Clearly, many cell types that do not express this integrin can form filopodia and migrate on laminin-1. We propose that the α6β4 integrin enhances the process of filopodial extension and stabilization because of its distinctive adhesive properties on laminin-1. Previously, we demonstrated that the α6β4 integrin expressed by clone A cells has an extremely high adhesive strength for laminin-1 (50). Specifically, laminin-1 adhesion mediated by this integrin was able to resist shear forces up to 100 dynes/cm2. The high adhesive strength of this integrin for laminin-1 supports its involvement in filopodial stabilization. Moreover, the fact that we observed α6β4 preferentially expressed in retraction fibers at the trailing edge of migrating cells, as well as in retraction fibers detached from migrating cells, supports the notion that α6β4 interacts avidly with laminin-1 and that this interaction, once formed, is not disrupted easily. A mechanism for releasing the adhesive strength of α6β4 at the rear of the cell must exist, however, because the bulk of this integrin remains with the migrating cell. Indeed, the dynamics of integrins at the rear of migrating cells have been linked to the regulation of cell motility (31, 42).
The data provided here suggest an association of the α6β4 integrin with actin filaments and that this association is dependent on the adhesion of these cells to laminin-1. Specifically, the actin-severing protein gelsolin was able to liberate α6β4 from clone A cells along with actin. Also, the immunostaining studies revealed that both filopodia and the edges of lamellae contain α6β4 distributed in the form of discrete clusters that colocalized with F-actin. Filopodia and many of the margins of lamellae did not contain any cytokeratins detectable by immunofluorescence microscopy using a pan-cytokeratin antibody. An association between α6β4 and F-actin is also supported by the fact that α6β4 immunostaining at the marginal edges of the cell was resistant to solubilization with Triton X-100. Moreover, α6β4 was extracted from these marginal edges with a Tween-40/deoxycholate buffer that has been shown to solubilize most of the actin cytoskeleton but not intermediate filaments (4, 13), providing additional evidence that α6β4 does not associate with cytokeratins in filopodia and the distal areas of lamellae. However, α6β4 appears to associate with cytokeratins at the base of the lamellae based on the colocalization data and the fact that the α6β4 immunostaining in these regions was resistant to the Tween-40/deoxycholate buffer. These observations suggest that a more stable α6β4-mediated adhesion may occur at the base of the lamellae than in filopodia and the margins of the lamellae.
The association of the α6β4 integrin and actin filament bundles is intriguing and it could relate to the mechanism of clone A migration. A parallel arrangement of actin filament bundles was consistently found in fan-shaped lamellae of cells that moved at a higher rate and that persisted in one direction for longer times than cells that did not express such bundles. These parallel actin bundles are probably related to those described earlier as actin arcs that are preferentially seen in very motile cells, in contrast to stress fibers that organize orthogonally and are frequently seen in stationary cells (18, 25, 50). These arcs originate on the ventral margins of the cell and then traverse in parallel across the dorsal surface of the lamella. The integrin α6β4 appears to have a critical role in the formation of these actin filament bundles because they were disrupted by α6-blocking antibodies. Moreover, α6β4 was associated with actin at the origins of these actin filament bundles based on the results obtained with the detergent extraction protocol. Additional studies on the involvement of these actin bundles in cell migration and the role of α6β4 in their function should be insightful.
The α6β4 integrin can participate in filopodial formation on laminin-1 based on the findings that function-blocking antibodies inhibited their formation. Although the mechanism by which α6β4 contributes to filopodial growth remains to be elucidated, an area that should be explored is the involvement of α6β4 in actin dynamics. One model of filopodial growth suggests that substrate- anchored proteins coupled with molecular motors at the base of filopodia regulate the rearward flow of actin, while those at the tip may regulate the polymerization of actin (47). Based on this model and our finding that α6β4 was found in clusters at the roots of filopodia in association with actin, one possibility is that α6β4 functions as a substrate-anchoring molecule that is involved in reducing the rearward flow of actin at the roots of filopodia. Interestingly, reduction of the rearward flow of actin has been shown to induce cell protrusions (33). Another likely possibility is that a signaling cascade initiated by ligation of α6β4 is linked to the mechanism of actin polymerization and filopodial growth. For example, the GTPase cdc42 (28, 40) has been implicated in filopodial formation in fibroblasts, and the possibility that α6β4 regulates the activity of such molecules is attractive.
An interesting aspect of this study is the apparent division of labor between the α6β4 and β1 integrins in mediating clone A interactions with laminin-1. Previously, we demonstrated that clone A cells use the α6β4 and α2β1 integrins to interact with laminin-1 (32, 34). The mAb inhibition data presented here extend these observations by demonstrating that a β1 integrin, presumably α2β1, is essential for the adhesion and spreading of clone A cells on laminin-1 but that α6β4 is not required for these processes. As shown, the α6β4 integrin appears to be involved in a much more specific function in clone A cells, the formation and stabilization of filopodia and lamellae. Although it is difficult to assess the role of β1 integrins in these events directly because function-blocking mAbs cause the cells to detach from laminin-1, it is likely that they are essential. This possibility is supported by our finding that both α6β4 and β1 integrins are localized in filopodia and lamellae. A reasonable hypothesis based on these observations is that the α6β4 and α2β1 integrins mediate distinct signaling pathways in response to their ligation by laminin-1 and that both signaling pathways are required for the migration of clone A cells on laminin-1.
The cytoskeletal associations of the α6β4 integrin that we have characterized in clone A cells should be compared with recent studies on the molecular interactions between α6β4 and cytokeratins (16). There is now evidence that the hemidesmosomal proteins BPAG-2 (bullous pemphigoid antigen) and HD-1 interact with α6β4 (20, 44), and that HD-1 can provide the link between α6β4 and cytokeratins (55). Another hemidesmosomal protein, BPAG-1, has been shown to be essential in the attachment of cytokeratins to the hemidesmosome, although an interaction with α6β4 has not been demonstrated (17). Clone A cells express HD-1 but they do not express either of the BPAG proteins (data not shown), and they do not contain classical hemidesmosomes (32). In this regard, however, another type of hemidesmosome (type II), has been described in a mammary epithelial cell line that contains only α6β4 in association with HD-1 (55). Type II hemidesmosomes have also been observed in HT29-Fu cells, a colon carcinoma cell line that was reverted to a differentiated phenotype by fluorouracil treatment (14). An important difference, however, between these cells and clone A cells is that the distribution pattern of α6β4 in the mammary epithelial and HT29-Fu cells did not suggest an association with actin (14, 55). Although the molecular basis for these differences in the cytoskeletal associations of α6β4 are not known, an invasive carcinoma cell such as clone A may be an extremely useful cell type to define the molecules that link α6β4 to the actin cytoskeleton and the factors that promote this association. In clone A cells, HD-1 is probably not involved in α6β4 linkage with actin because preliminary studies indicate that HD-1 is not present in the filopodia and edges of the lamellae of these cells (Rabinovitz, I., unpublished results). Regardless of the mechanism, however, a shift from a stable interaction of α6β4 with cytokeratins to a more dynamic interaction with the actin cytoskeleton could have important implications for epithelial cell migration and tumor progression.
Acknowledgments
We thank Paul Janmey and Phil Allen for helpful discussions and for providing gelsolin. Margaret Lotz, Leslie Shaw, and Julie Theriot are acknowledged for helpful discussions and comments on the manuscript, and Helen Wang for technical assistance. We also thank Steve Akiyama and Tom Carey for providing antibodies.
This work was supported by National Institutes of Health grants CA44704 and AI39264 and a US Army Breast Cancer Grant.
Note Added in Proof
Since submission of this manuscript, we have shown that the mechanism by which the α6β4 integrin mediates the formation of lamellae involves activation of phosphoinositide 3-OH kinase (Shaw, L.M., I. Rabinovitz, H. Wang, A. Toker, and A.M. Mercurio. 1997. Cell. In press).
Footnotes
Address all correspondence to Arthur M. Mercurio, Beth Israel Deaconess Medical Center-Dana 601, 330 Brookline Ave., Boston, MA 02215. Tel.: (617) 667-7714. Fax: (617) 975-5071. E-mail: amercuri@bih.harvard.edu
References
- 1.Akiyama SK, Yamada SS, Yamada KM. Characterization of a 140-kD avian cell surface antigen as a fibronectin-binding molecule. J Cell Biol. 1986;102:442–448. doi: 10.1083/jcb.102.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albrecht-Buehler G. Filopodia of spreading 3T3 cells. Do they have a substrate-exploring function? . J Cell Biol. 1976;69:275–286. doi: 10.1083/jcb.69.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Borradori L, Sonnenberg A. Hemidesmosomes—roles in adhesion, signaling and human diseases. Curr Opin Cell Biol. 1996;8:647–656. doi: 10.1016/s0955-0674(96)80106-2. [DOI] [PubMed] [Google Scholar]
- 4.Capco DG, Wan KM, Penman S. The nuclear matrix: three-dimensional architecture and protein composition. Cell. 1982;29:847–858. doi: 10.1016/0092-8674(82)90446-9. [DOI] [PubMed] [Google Scholar]
- 5.Carter WG, Kaur P, Gil SG, Gahr PJ, Wayner EA. Distinct functions for integrins α3β1 in focal adhesions and α6β4/bullous pemphigoid antigen in a new stable anchoring contact (SAC) of keratinocytes: relation to hemidesmosomes. J Cell Biol. 1990;111:3141–3154. doi: 10.1083/jcb.111.6.3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chao C, Lotz MM, Clarke AC, Mercurio AM. A function for the integrin α6β4 in the invasive properties of colorectal carcinoma cells. Cancer Res. 1996;56:4811–4819. [PubMed] [Google Scholar]
- 7.Cramer L, Mitchison TJ. Moving and stationary actin filaments are involved in spreading of postmitotic PtK2 cells. J Cell Biol. 1993;122:833–843. doi: 10.1083/jcb.122.4.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daneker GW, Jr, Piazza AJ, Steele GD, Jr, Mercurio AM. Relationship between extracellular matrix interactions and degree of differentiation in human colon carcinoma cell lines. Cancer Res. 1989;49:681–686. [PubMed] [Google Scholar]
- 9.De Luca M, Pellegrini G, Zambruno G, Marchisio PC. Role of integrins in cell adhesion and polarity in normal keratinocytes and human skin pathologies. J Dermatol. 1994;21:821–828. doi: 10.1111/j.1346-8138.1994.tb03296.x. [DOI] [PubMed] [Google Scholar]
- 10.Dexter DL, Barbosa JA, Calabresi P. N,N-dimethylformamide-induced alteration of cell culture characteristics and loss of tumorigenicity in cultured human colon carcinoma cells. Cancer Res. 1979;39:1020–1025. [PubMed] [Google Scholar]
- 11.Dowling J, Yu QC, Fuchs E. β4 integrin is required for hemidesmosome formation, cell adhesion and cell survival. J Cell Biol. 1996;134:559–572. doi: 10.1083/jcb.134.2.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Falcioni R, Kennel SJ, Giacomini P, Zupi G, Sacchi A. Expression of tumor antigen correlated with metastatic potential of Lewis lung carcinoma and B16 melanoma clones in mice. Cancer Res. 1986;46:5772–5778. [PubMed] [Google Scholar]
- 13.Fey EG, Wan KM, Penman S. Epithelial cytoskeletal framework and nuclear matrix-intermediate filament scaffold: three-dimensional organization and protein composition. J Cell Biol. 1983;98:1973–1984. doi: 10.1083/jcb.98.6.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fontao L, Dirrig S, Owaribe K, Kedinger M, Launay JF. Expression of HD1—relationship with the cytoskeleton in cultured human colonic carcinoma cells. Exp Cell Res. 1997;231:319–327. doi: 10.1006/excr.1996.3465. [DOI] [PubMed] [Google Scholar]
- 15.Gomez M, Navarro P, Quintanilla M, Cano A. Expression of α6β4 integrin increases during malignant conversion of mouse epidermal keratinocytes: association of β4 subunit to the cytokeratin fraction. Exp Cell Res. 1992;201:250–261. doi: 10.1016/0014-4827(92)90272-a. [DOI] [PubMed] [Google Scholar]
- 16.Green KJ, Jones JCR. Desmosomes and hemidesmosomes— structure and function of molecular components. FASEB (Fed Am Soc Exp Biol) J. 1996;10:871–881. doi: 10.1096/fasebj.10.8.8666164. [DOI] [PubMed] [Google Scholar]
- 17.Guo L, Degenstein L, Dowling J, Yu QC, Wollmann R, Perman B, Fuchs E. Gene targeting of BPAG1: abnormalities in mechanical strength and cell migration in stratified epithelia and neurologic degeneration. Cell. 1995;81:233–243. doi: 10.1016/0092-8674(95)90333-x. [DOI] [PubMed] [Google Scholar]
- 18.Heath JP. Arcs: curved microfilament bundles beneath the dorsal surface of the leading lamellae of moving chick embryo fibroblasts. Cell Biol Int Rep. 1981;5:975–980. doi: 10.1016/0309-1651(81)90214-9. [DOI] [PubMed] [Google Scholar]
- 19.Hemler ME, Crouse C, Sonnenberg A. Association of the VLA α6 subunit with a novel protein. A possible alternative to the common VLA β1 subunit on certain cell lines. J Biol Chem. 1989;264:6529–6535. [PubMed] [Google Scholar]
- 20.Hopkinson SB, Baker SE, Jones JC. Molecular genetic studies of a human epidermal autoantigen (the 180-kD bullous pemphigoid antigen/BP180): identification of functionally important sequences within the BP180 molecule and evidence for an interaction between BP180 and α6 integrin. J Cell Biol. 1995;130:117–125. doi: 10.1083/jcb.130.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 22.Jones JC, Kurpakus MA, Cooper HM, Quaranta V. A function for the integrin α6β4 in the hemidesmosome. Cell Regul. 1991;2:427–438. doi: 10.1091/mbc.2.6.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Juhasz I, Murphy GF, Yan HC, Herlyn M, Albelda SM. Regulation of extracellular matrix proteins and integrin cell substratum adhesion receptors on epithelium during cutaneous human wound healing in vivo. Am J Pathol. 1993;143:1458–1469. [PMC free article] [PubMed] [Google Scholar]
- 24.Juliano RL, Varner JA. Adhesion molecules in cancer: the role of integrins. Curr Opin Cell Biol. 1993;5:812–818. doi: 10.1016/0955-0674(93)90030-t. [DOI] [PubMed] [Google Scholar]
- 25.Kaiho M, Sato A. Circular distribution of microfilaments in cells spreading in vitro. Exp Cell Res. 1978;113:222–227. doi: 10.1016/0014-4827(78)90106-4. [DOI] [PubMed] [Google Scholar]
- 26.Kimmel KA, Carey TE. Altered expression in squamous carcinoma cells of an orientation restricted epithelial antigen detected by monoclonal antibody A9. Cancer Res. 1986;46:3614–3623. [PubMed] [Google Scholar]
- 27.Kleinman HK, McGarvey ML, Hassell JR, Star VL, Cannon FB, Laurie GW, Martin GR. Basement membrane complexes with biological activity. Biochemistry. 1986;25:312–318. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- 28.Kozma R, Ahmed S, Best A, Lim L. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol Cell Biol. 1995;15:1942–1952. doi: 10.1128/mcb.15.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kurpakus MA, Quaranta V, Jones JC. Surface relocation of α6β4 integrins and assembly of hemidesmosomes in an in vitro model of wound healing. J Cell Biol. 1991;115:1737–1750. doi: 10.1083/jcb.115.6.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Larjava H, Salo T, Haapasalmi K, Kramer RH, Heino J. Expression of integrins and basement membrane components by wound keratinocytes. J Clin Invest. 1993;92:1425–1435. doi: 10.1172/JCI116719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lauffenburger DA, Horwitz AF. Cell migration—a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 32.Lee EC, Lotz MM, Steele GD, Jr, Mercurio AM. The integrin α6β4 is a laminin receptor. J Cell Biol. 1992;117:671–678. doi: 10.1083/jcb.117.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lin CH, Espreafico EM, Mooseker MS, Forscher P. Myosin drives retrograde F-actin flow in neuronal growth cones. Neuron. 1996;16:769–782. doi: 10.1016/s0896-6273(00)80097-5. [DOI] [PubMed] [Google Scholar]
- 34.Lotz MM, Korzelius CA, Mercurio AM. Human colon carcinoma cells use multiple receptors to adhere to laminin: involvement of α6β4 and α2β1 integrins. Cell Regul. 1990;1:249–257. doi: 10.1091/mbc.1.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lotz MM, Nusrat A, Madara JL, Ezzell R, Wewer UM, Mercurio AM. Intestinal epithelial restitution. Involvement of specific laminin isoforms and integrin laminin receptors in wound closure of a transformed model epithelium. Am J Pathol. 1997;150:747–760. [PMC free article] [PubMed] [Google Scholar]
- 36.Mainiero F, Pepe A, Yeon M, Ren Y, Giancotti F. The intracellular functions of α6β4 integrin are regulated by EGF. J Cell Biol. 1996;134:241–253. doi: 10.1083/jcb.134.1.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martin P. Wound healing—aiming for perfect skin regeneration. Science. 1997;276:75–81. doi: 10.1126/science.276.5309.75. [DOI] [PubMed] [Google Scholar]
- 38.Mitchison TJ. Actin based motility on retraction fibers in mitotic PtK2 cells. Cell Motil Cytoskel. 1992;22:135–151. doi: 10.1002/cm.970220207. [DOI] [PubMed] [Google Scholar]
- 39.Mitchison TJ, Cramer LP. Actin-based cell motility and cell locomotion. Cell. 1996;84:371–379. doi: 10.1016/s0092-8674(00)81281-7. [DOI] [PubMed] [Google Scholar]
- 40.Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- 41.O'Connor TP, Duerr JS, Bentley D. Pioneer growth cone steering decisions mediated by single filopodial contacts in situ. J Neurosci. 1990;10:3935–3946. doi: 10.1523/JNEUROSCI.10-12-03935.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Palecek SP, Schmidt CE, Lauffenburger DA, Horwitz AF. Integrin dynamics on the tail region of migrating fibroblasts. J Cell Sci. 1996;109:941–952. doi: 10.1242/jcs.109.5.941. [DOI] [PubMed] [Google Scholar]
- 43.Rabinovitz I, Mercurio AM. The integrin α6β4 and the biology of carcinoma. Biochem Cell Biol. 1996;74:811–821. doi: 10.1139/o96-087. [DOI] [PubMed] [Google Scholar]
- 44.Sanchezaparicio P, Develasco AMM, Niessen CM, Borradori L, Kuikman I, Hulsman EHM, Fassler R, Owaribe K, Sonnenberg A. The subcellular distribution of the high molecular mass protein, HD1, is determined by the cytoplasmic domain of the integrin β4 subunit. J Cell Sci. 1997;110:169–178. doi: 10.1242/jcs.110.2.169. [DOI] [PubMed] [Google Scholar]
- 45.Serini G, Trusolino L, Saggiorato E, Cremona O, Derossi M, Angeli A, Orlandi F, Marchisio P C. Changes in integrin and E-cadherin expression in neoplastic versus normal thyroid tissue. J Natl Cancer Inst. 1996;88:442–449. doi: 10.1093/jnci/88.7.442. [DOI] [PubMed] [Google Scholar]
- 46.Shaw LM, Lotz MM, Mercurio AM. Inside-out integrin signaling in macrophages. Analysis of the role of the α6A β1 and α6B β1 integrin variants in laminin adhesion by cDNA expression in an α6 integrin-deficient macrophage cell line. J Biol Chem. 1993;268:11401–11408. [PubMed] [Google Scholar]
- 47.Sheetz MP, Wayne DB, Pearlman AL. Extension of filopodia by motor-dependent actin assembly. Cell Motil Cytoskel. 1992;22:160–169. doi: 10.1002/cm.970220303. [DOI] [PubMed] [Google Scholar]
- 48.Sonnenberg A, Janssen H, Hogervorst F, Calafat J, Hilgers J. A complex of platelet glycoproteins Ic and IIa identified by a rat monoclonal antibody. J Biol Chem. 1987;262:10376–10383. [PubMed] [Google Scholar]
- 49.Sonnenberg A, Calafat J, Janssen H, Daams H, van der Raaij-Helmer LM, Falcioni R, Kennel SJ, Aplin JD, Baker J, Loizidou M, et al. Integrin α6/β4 complex is located in hemidesmosomes, suggesting a major role in epidermal cell-basement membrane adhesion. J Cell Biol. 1991;113:907–917. doi: 10.1083/jcb.113.4.907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soranno T, Bell E. Cytostructural dynamics of spreading and translocating cells. J Cell Biol. 1982;95:127–136. doi: 10.1083/jcb.95.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stepp MA, Spurr-Michaud S, Tisdale A, Elwell J, Gipson IK. α6β4 integrin heterodimer is a component of hemidesmosomes. Proc Natl Acad Sci USA. 1990;87:8970–8974. doi: 10.1073/pnas.87.22.8970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tani T, Karttunen T, Kiviluoto T, Kivilaakso E, Burgeson RE, Sipponen P, Virtanen I. α6β4 integrin and newly deposited laminin-1 and laminin-5 form the adhesion mechanism of gastric carcinoma. Continuous expression of laminins but not that of collagen VII is preserved in invasive parts of the carcinomas: implications for acquisition of the invading phenotype. Am J Pathol. 1996;149:781–793. [PMC free article] [PubMed] [Google Scholar]
- 53.Thomas, P. 1993. Metastatic potential of human colorectal cancer cell lines. R.G. Landes Co., Austin, TX. 94 pp.
- 54.Tozeren A, Kleinman HK, Wu S, Mercurio AM, Byers SW. Integrin α6β4 mediates dynamic interactions with laminin. J Cell Sci. 1994;107:3153–3163. doi: 10.1242/jcs.107.11.3153. [DOI] [PubMed] [Google Scholar]
- 55.Uematsu J, Nishizawa Y, Sonnenberg A, Owaribe K. Demonstration of type II hemidesmosomes in a mammary gland epithelial cell line, BMGE-H. J Biochem. 1994;115:469–476. doi: 10.1093/oxfordjournals.jbchem.a124361. [DOI] [PubMed] [Google Scholar]
- 56.Van Waes C, Kozarsky KF, Warren AB, Kidd L, Paugh D, Liebert M, Carey TE. The A9 antigen associated with aggressive human squamous carcinoma is structurally and functionally similar to the newly defined integrin α6β4. Cancer Res. 1991;51:2395–2402. [PubMed] [Google Scholar]
- 57.Vanderneut R, Krimpenfort P, Calafat J, Niessen CM, Sonnenberg A. Epithelial detachment due to absence of hemidesmosomes in integrin β4 null mice. Nat Genet. 1996;13:366–369. doi: 10.1038/ng0796-366. [DOI] [PubMed] [Google Scholar]