Abstract
We have investigated the interactions of two nuclear-encoded preproteins with the chloroplast protein import machinery at three stages in import using a label-transfer crosslinking approach. During energy-independent binding at the outer envelope membrane, preproteins interact with three known components of the outer membrane translocon complex, Toc34, Toc75, and Toc86. Although Toc75 and Toc86 are known to associate with preproteins during import, a role for Toc34 in preprotein binding previously had not been observed. The interaction of Toc34 with preproteins is regulated by the binding, but not hydrolysis of GTP. These data provide the first evidence for a direct role for Toc34 in import, and provide insights into the function of GTP as a regulator of preprotein recognition. Toc75 and Toc86 are the major targets of cross-linking upon insertion of preproteins across the outer envelope membrane, supporting the proposal that both proteins function in translocation at the outer membrane as well as preprotein recognition. The inner membrane proteins, Tic(21) and Tic22, and a previously unidentified protein of 14 kD are the major targets of crosslinking during the late stages in import. These data provide additional support for the roles of these components during protein translocation across the inner membrane. Our results suggest a defined sequence of molecular interactions that result in the transport of nuclear-encoded preproteins from the cytoplasm into the stroma of chloroplasts.
As a consequence of its dual genetic origin, the chloroplast must import the vast majority of its polypeptide components from their site of synthesis in the cytoplasm. The import of nuclear-encoded proteins into the chloroplast is mediated by interactions between the intrinsic NH2-terminal targeting signal (transit sequence) of the preprotein and a common recognition and translocation machinery at the chloroplast envelope (for review see Cline and Henry, 1996; Fuks and Schnell, 1997; Lübeck et al., 1997). The general import machinery is composed of independent translocon complexes in the inner and outer envelope membranes (Schnell et al., 1997). The translocon at the outer envelope membrane of chloroplasts (Toc)1 complex mediates the initial binding of cytoplasmic preproteins at the chloroplast surface and their subsequent ATP/GTP-dependent translocation across the outer membrane. Translocation across the inner membrane is mediated by the translocon at the inner envelope membrane of chloroplasts (Tic) complex and is driven by ATP hydrolysis in the stroma. The Toc and Tic complexes associate at contact sites between the outer and inner envelope membranes to facilitate direct translocation of preproteins from the cytoplasm to the stroma (Schnell and Blobel, 1993; Nielsen et al., 1997). A fraction of Toc and Tic complexes can be coimmunoprecipitated (Nielsen et al., 1997), suggesting that a portion of the outer and inner membrane import machineries may be stably linked under steady state conditions.
Initial binding of the preprotein to the Toc complex is mediated by a trimeric complex composed of Toc34, Toc75, and Toc86 (Waegemann and Soll, 1991; Hirsch et al., 1994; Kessler et al., 1994; Schnell et al., 1994; Seedorf et al., 1995; Tranel et al., 1995). Antibody inhibition (Hirsch et al., 1994) and covalent crosslinking studies (Perry and Keegstra, 1994; Ma et al., 1996) indicate a role for Toc86 in preprotein binding. Toc75 appears to form at least part of the translocation channel at the outer membrane translocon because it is efficiently crosslinked to preproteins in the presence of low concentrations of ATP and GTP (0.1 mM) that promote insertion of the preprotein across the outer membrane (Perry and Keegstra, 1994; Ma et al., 1996). Experimental evidence for the function of Toc34 in import is lacking, but it has been proposed that it acts in concert with Toc86 to regulate preprotein recognition. This proposal is consistent with the observations that both Toc34 and Toc86 are GTP-binding proteins (Hirsch et al., 1994; Kessler et al., 1994; Seedorf et al., 1995) and that GTP is required at the early stages of import (Olsen and Keegstra, 1992; Kessler et al., 1994).
Several components of the inner membrane translocon have been identified. An integral inner membrane component, Tic110 (Schnell et al., 1994; Wu et al., 1994; Lübeck et al., 1996), has been detected in complexes containing proteins at late stages in import. Tic110 coimmunoprecipitates with two stromal chaperones, the hsp60 homologue, cpn60 (Kessler and Blobel, 1996), and the chloroplast ClpC homologue (Nielsen et al., 1997). These observations suggest that Tic110 may function as a docking site for molecular chaperones that participate in driving transport across the inner membrane or in folding of newly imported proteins in the stroma. Two additional components of the chloroplast envelope, IAP21 and IAP25, have been covalently crosslinked to modified versions of the precursor to the small subunit of ribulose-1,5-bisphosphate carboxylase (pS) at an intermediate stage in import (Ma et al., 1996). On the basis of the localization at the inner membrane (Kouranov, A., and D.J. Schnell, unpublished observation) and in accordance with the newly adopted uniform nomenclature for chloroplast import components (Schnell et al., 1997), we now refer to IAP21 and IAP25 as Tic(21) and Tic22, respectively. The ability to directly crosslink Tic(21) and Tic22 to preproteins suggests that they form part of the recognition or translocation channel at the inner membrane.
Although several components of the Toc and Tic complexes have been identified based on their association with preproteins, the sequence of molecular interactions that result in the import of preproteins from the cytoplasm to the stroma has not been mapped. In this report, we have investigated the interactions of two preproteins with the Toc and Tic complexes using site-specific crosslinking to intermediates at three known stages in import: preprotein recognition, outer membrane translocation, and inner membrane translocation. Our results indicate that Toc34, Toc75, and Toc86 directly interact with preproteins during recognition at the outer membrane. The interaction of preproteins with Toc34 is regulated by GTP binding. Toc86 and Toc75 are associated with preproteins during outer membrane translocation, suggesting that both proteins may participate in protein conductance at this membrane. Tic(21), Tic22, and a previously unidentified protein, designated Tic(14), are the major crosslinking targets during inner membrane translocation.
Materials and Methods
Preparation of Crosslinking Substrates
Plasmid pET21b-pS-protA encoding the pS-protA preprotein was constructed as previously described (Schnell and Blobel, 1993). Plasmid pET21b-pFd-protA-1 encoding pFd-protA-1 was generated by PCR-directed mutagenesis of pET-pFd-protA (Ma et al., 1996) with primers that introduced the following changes in the pFd coding region: a methionine to cysteine conversion at position −1 and a glutamate–phenylalanine insertion at position +4. The remainder of the pFd-protA-1 sequence was identical to pFd-protA (Ma et al., 1996). Plasmid pET21b-pS-protA-1 encoding pS-protA-1 was generated by PCR-directed mutagenesis of pET21b-pS-protA to change the cysteine at position −1 to serine. The remainder of the pS-protA-1 sequence was identical to pS-protA. Plasmid pET21b-pS-protA-2 encoding pS-protA-2 was generated by PCR-directed mutagenesis of pET21b-pS-protA-1 with primers that introduced the following changes: a valine to glutamate conversion at position +39 and the elimination of the cysteine at position +41. The remainder of the pS-protA-2 sequence was identical to pS-protA-1.
The pS-protA, pS-protA-1, pS-protA-2, and pFd-protA-1 polypeptides were expressed in Escherichia coli BL21 (DE3) containing COOH-terminal extensions of six histidine residues. The preproteins were purified from extracts by affinity chromatography on a nickel–chelate matrix according to the supplier's recommendations (Novagen, Inc., Madison, WI).
The iodination of the heterobifunctional crosslinking reagent, N-[4[(p-azidosalicylamido)butyl]-3′(2-pyridyldithio)propionamide (APDP; Pierce, Inc., Rockford, IL) and the coupling of [125I]APDP to preproteins were performed as previously described (Ma et al., 1996). The molar ratio of [125I]APDP to cysteine residues for each preprotein was 1.5:1 in all coupling reactions. The crosslinking substrates were stored at −80°C in 0.1 M NaHCO3, pH 9.0, containing 8 M urea.
Chloroplast Isolation and Subfractionation
Intact chloroplasts were isolated from 10- to 14-d-old pea seedlings (Pisum sativum variant Green Arrow) by homogenization and Percoll silica gel gradient centrifugation as previously described (Pain and Blobel, 1987). Isolated chloroplasts were resuspended in 50 mM Hepes-KOH, pH 7.7, 0.33 M sorbitol (HS buffer) to a concentration equivalent to 2 to 3 mg chlorophyll/ml.
To prepare chloroplast envelope membranes, intact chloroplasts were lysed under hypertonic conditions and separated into soluble and membrane fractions by differential centrifugation as described by Keegstra and Yousif (1986). The total membrane fraction was separated into envelope and thylakoid membrane fractions by flotation into linear sucrose gradients as previously described (Schnell et al., 1994).
Precursor Binding and Crosslinking Reactions
Isolated intact chloroplasts were incubated in HS buffer containing 50 mM KOAc, 4 mM MgOAc (import buffer) in the presence of 400 nM nigericin for 15 min at 26°C in the dark to deplete them of endogenous ATP. All binding reactions were performed in the dark using chloroplasts equivalent to 2 mg chlorophyll in a reaction volume of 1 ml. For the binding of precursor in the absence of ATP (energy-independent binding), 20 U of apyrase (Sigma Chemical Co., St. Louis, MO) was added to the chloroplast suspension during the ATP depletion reaction. For the binding of precursor in the presence of ATP (early import intermediate), ATP-depleted chloroplasts were incubated in the presence of 0.1 mM MgATP and 0.1 mM MgGTP for 5 min at 26°C. For the import reactions, ATP-depleted chloroplasts were incubated in the presence of 2 mM MgATP for 5 min at 26°C. After the pretreatments, urea-denatured preprotein was added to a final concentration of 200 nM, and the incubation was continued for 10 min (energy-independent binding and early import intermediate) or 20 min (late import stage) at 26°C. The chloroplasts were transferred to the bottom half of a glass petri dish on ice and irradiated from above with a chromato-vu transilluminator (UVP, Inc., Upland, CA) at 312 nm at a distance of 5 cm for 20 min with constant shaking. Thermolysin treatment of intact chloroplasts was performed as described previously (Cline et al., 1984) using 0.1 mg thermolysin/ml for 30 min on ice. The chloroplasts were collected by centrifugation for 30 s at 6,000 g in a microcentrifuge, subfractionated as described above, and the envelope fractions were analyzed by SDS-PAGE.
Sample Analysis and Quantitation
All crosslinked samples were resolved by SDS-PAGE on 12% polyacrylamide gels. The radioactive signals in dried gels were captured and quantitated using a Phosphorimager SI (Molecular Dynamics, Inc., Sunnyvale, CA) with the IPLab gel scientific image processing version 1.5c program (Signal Analytics, Vienna, VA). All images of radioactive gels were captured using the IP Labgel software. Image labels were added using Canvas version 2.1 software (Deneba Systems, Inc., Miami, FL), and images were printed on an XLS 8600 PS printer (Kodak, Rochester, NY).
Results
Similar Envelope Import Components Are Crosslinked to Two Chimeric Preproteins
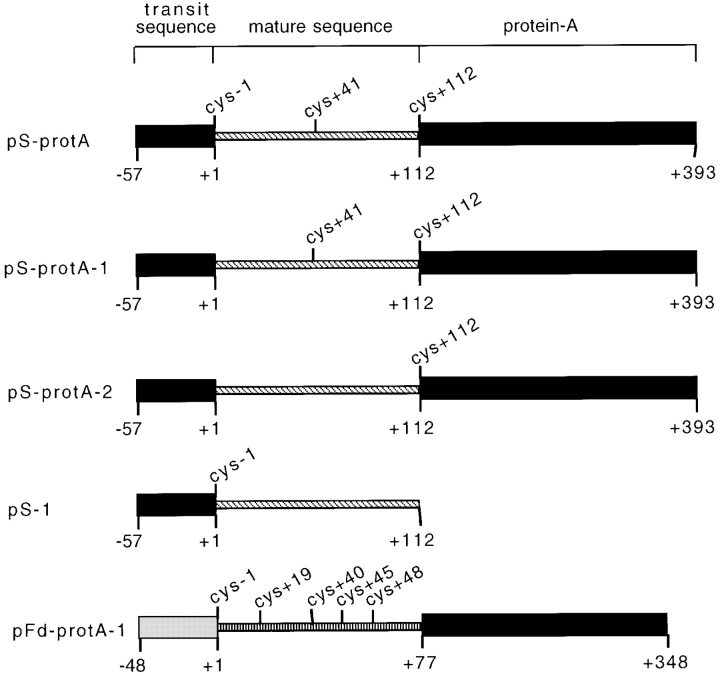
We performed label transfer crosslinking experiments with preproteins at each of the known stages in import to map the specific molecular interactions between preproteins and components of the outer and inner membrane translocons. Two chimeric preproteins, pS-protA and pFd-protA-1, were used as crosslinking substrates for these studies (Fig. 1). The pS-protA and pFd-protA-1 preproteins consisted of pS or preferredoxin (pFd) fused at their COOH termini to the IgG binding domains of staphylococcal protein A, respectively. Both pS-protA and pFd-protA-1 were modified with the photoactivatible, cleavable crosslinking reagent, [125I]APDP, at cysteine residues by a disulfide exchange reaction to yield the crosslinking substrates (Fig. 1). The modifications of pS-protA and pFd-protA-1 with [125I]APDP had a negligible effect on the binding and import characteristics of the preproteins in in vitro assays using intact chloroplasts (data not shown). The pS-protA and pFd-protA-1 proteins provided crosslinking substrates derived from two preproteins of similar size with crosslinking groups at comparable positions within their transit sequences and at additional sites within their mature sequences.
Figure 1.
Schematic diagram of the crosslinking substrates used in this study. The names of the preproteins used as crosslinking substrates are indicated to the left of each figure. The positions of the transit sequences, mature sequences, and IgG-binding domain of protein A are indicated by boxed regions. The positions of [125I]APDP-reactive cysteines are indicated above each figure. The scale in amino acid residues is indicated at the bottom of each figure. The +1 residue refers to the amino-terminal residue of the mature polypeptides.
To investigate the interactions of preproteins with translocon components, pS-protA and pFd-protA-1 were crosslinked to isolated chloroplasts at three known stages in import. These stages are defined as energy-independent binding observed in the presence of the NTP hydrolyzing enzyme, apyrase (Ma et al., 1996); an early import intermediate observed in the presence of 0.1 mM ATP and GTP (Cline et al., 1985; Schnell et al., 1993); or a late stage in import observed in the presence of 2 mM ATP and 0.1 mM GTP (Schnell et al., 1993). The late stage of import contains both proteolytically processed and unprocessed import substrates. The processed substrate previously was shown to represent a late import intermediate that is simultaneously inserted across the outer and inner membranes. The presence of the late import intermediate at this stage provided the potential to identify components of the inner membrane translocon.
Isolated chloroplasts were depleted of endogenous ATP by incubation in the dark at 26°C in the presence of the proton ionophore, nigericin. Energy-depleted chloroplasts were incubated with apyrase or NTP and either pS-protA or pFd-protA-1 and irradiated to induce photocrosslinking. After crosslinking, the chloroplasts were reisolated and fractionated to yield a total envelope membrane fraction. The envelope proteins were treated under reducing conditions to cleave the crosslinker and resolved by SDS-PAGE. Cleavage of the crosslinker with reducing agents releases the 125I-ADP moiety from the preprotein, but it remains covalently attached to the target protein, allowing the crosslinked products to be visualized directly by fluorography.
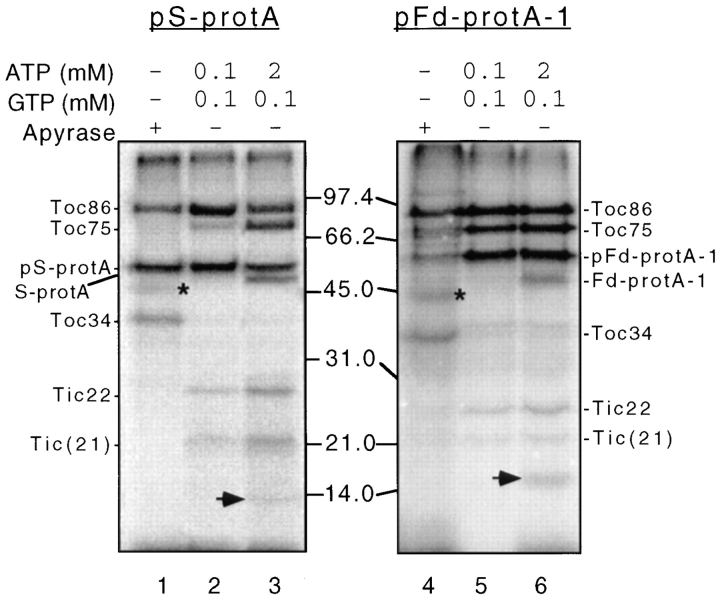
The results of crosslinking are shown in Fig. 2. The pS-protA and pFd-protA-1 substrates migrate at 52 and 55 kD, respectively, and are detected in all lanes of Fig. 2. Both preproteins retain a significant amount of radiolabel even after treatment with reducing agents, indicating that irradiation induces significant intra- or intermolecular crosslinking of the substrates themselves. The overall pattern of crosslinking to envelope components with pS-protA and pFd-protA-1 is remarkably similar at all stages of import (Fig. 2). This observation is consistent with previous proposals that preproteins use a common import machinery at the envelope.
Figure 2.
Crosslinking of pS-protA and pFd-protA-1 to envelope polypeptides at three stages in import. Energy-depleted chloroplasts (2 mg chlorophyll) were incubated with 200 nM urea denatured pS-protA and pFd-protA-1 in a standard import assay-containing apyrase (+ Apyrase), or the indicated concentrations of ATP (ATP) and GTP (GTP) at 26°C in the dark. The chloroplasts were irradiated at 312 nM for 20 min on ice with a transilluminator at a distance of 5 cm. Reisolated chloroplasts were fractionated to yield a total envelope membrane fraction. Envelope membranes (50 μg of protein) were analyzed by SDS-PAGE under reducing conditions and fluorography. The molecular masses of standard proteins are indicated at the center of the figures. The positions of pS-protA, S-protA, pFd-protA-1, Fd-protA-1, Toc86, Toc75, Toc34, Tic22, and Tic(21) are shown to the left and right of the figures. Asterisks indicate the position of the 45-kD crosslinked protein, and arrowheads indicate the position of the 14-kD crosslinked protein.
As previously observed (Ma et al., 1996), Toc86 and Toc75 are crosslinked at the energy-independent binding of both pS-protA (Fig. 2, lane 1) and pFd-protA-1 (Fig. 2, lane 4). In addition, a 34-kD crosslinked protein coincident with Toc34 is present at the binding stage (Fig. 2, lanes 1 and 4). Fig. 3 shows that anti-Toc34 serum immunoprecipitates the 34-kD crosslinked product from pS-protA crosslinking reactions, confirming that this polypeptide is Toc34. Similar results were obtained with pFd-protA-1 crosslinked membranes (data not shown). The results in Fig. 2 are consistent with the hypothesis that Toc34 participates in preprotein recognition at the outer membrane.
Figure 3.
Immunoprecipitation of the crosslinked 34-kD protein with anti-Toc34. Envelope membranes (50 μg protein) from a pS-protA crosslinking reaction (lane 1, Env) were reduced with 100 mM DTT, dissolved under denaturing conditions, and immunoprecipitated with anti-Toc34 serum (anti-34) or anti-Toc34 preimmune serum (PI). The immunoprecipitate of anti-Toc34 reactions (B) and the unbound supernatant (U) were analyzed by SDS-PAGE and fluorography.
Crosslinking of preproteins to Toc34 was not detected in previous studies using similar methods (Perry and Keegstra, 1994; Ma et al., 1996). As is demonstrated below (see Fig. 8), Toc34 crosslinking is very sensitive to the presence of NTPs. Therefore, we believe that the short apyrase pretreatment used in the previous studies was insufficient to completely deplete chloroplasts of exogenous NTPs (Ma et al., 1996). In the present study, we found it necessary to extend the apyrase preincubation to obtain consistent Toc34 crosslinking.
Figure 8.
Energy requirements for pS-protA-1 crosslinking to Toc34. Energy-depleted chloroplasts (2 mg chlorophyll) were incubated with 200 nM urea-denatured pS-protA-1 in the presence of apyrase (+ Apyrase), or 0.1 mM ATP (+ATP), GTP (+GTP), GDP (+GDP), or GMP (+GMP) for 10 min at 26°C in the dark and crosslinked by UV irradiation. The envelope fraction (50 μg protein) from crosslinked chloroplasts was analyzed by SDS-PAGE and fluorography. The positions of pS-protA-1, Toc86, and Toc34 are shown to the left of the figure.
A minor crosslinked band of 45 kD (Fig. 2, asterisk) is apparent with both substrates (Fig. 2, lanes 1 and 4) at energy-independent binding. The identity of this protein is unknown. Additional faint crosslinked bands are observed at 70 and 60 kD with pFd-protA-1 (Fig. 2, lane 4). The identity of these two proteins was not investigated because crosslinking was only observed with pFd-protA-1. We focused our studies on proteins found in crosslinking reactions with both proteins because they are likely to represent components of the general import apparatus.
Toc86 and Toc75 are crosslinked to the early import intermediates of both pS-protA and pFd-protA-1 (Fig. 2, lanes 2 and 5), with the levels of Toc75 crosslinking significantly increasing over those observed at the binding stage of import. Crosslinking to Toc34 decreases to undetectable levels in the presence of exogenous ATP and GTP (Fig. 2, lanes 2 and 5). In addition to the outer membrane components, two components of the inner membrane translocon, Tic(21) and Tic22, are crosslinked to the early import intermediates (Fig. 2, lanes 2 and 5).
Toc75 and Toc86 are major crosslinked products in the outer membrane under import conditions (i.e., 2 mM ATP, 0.1 mM GTP) with pS-protA (Fig. 2, lane 3) and pFd-protA-1 (Fig. 2, lane 6). These results are consistent with the observation that both processed and precursor forms of the substrates are present at this stage (Fig. 2, lanes 3 and 6) and that late import intermediates span both the outer and inner membrane translocons (Schnell et al., 1994). The inner membrane import components, Tic(21) and Tic22, also are major crosslinked products at the late stage in import (Fig. 2, lanes 3 and 6). In addition to these known components, a previously unidentified crosslinked product is apparent at ∼14 kD (Fig. 2, lanes 3 and 6, arrowhead). The identity of this protein has not been determined, although immunoblot analysis indicates that it is not the mature small subunit of rubisco (data not shown). This polypeptide is a new candidate for a component of the inner membrane translocon.
The crosslinking data in Fig. 2 suggest a sequence of events in which the preprotein successively inserts more deeply into the outer and inner membrane translocons en route to the stroma. The label transfer crosslinking technique provides a method to assess the degree of preprotein insertion into the translocon complexes by performing crosslinking with preproteins that have been engineered with crosslinking sites at various positions within their primary structures. To take advantage of this method, two derivatives of pS-protA were generated by site-directed mutagenesis to eliminate the cysteine at position −1 in the transit sequence (pS-protA-1) or the cysteines at both position −1 and +41 (pS-protA-2; Fig. 1). These crosslinking substrates allowed us to examine the extent of envelope translocation of pS-protA at each stage in import by determining the extent of crosslinking to outer and inner membrane translocon components.
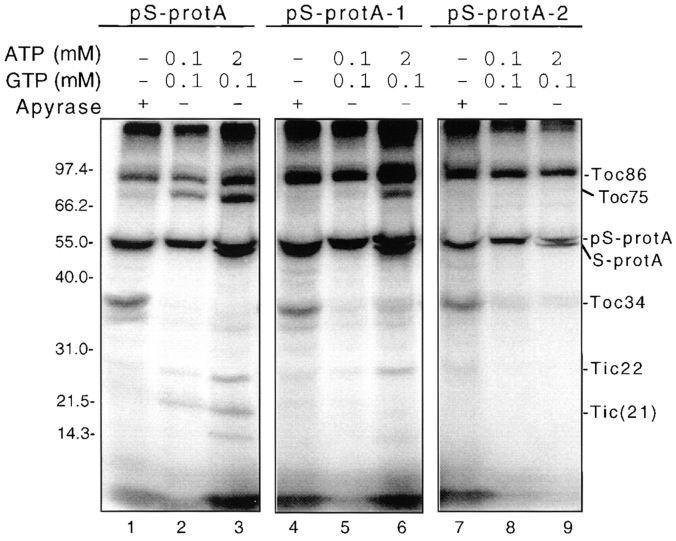
The results of crosslinking to the pS-protA derivatives is shown in Fig. 4. The patterns of crosslinked proteins with pS-protA, pS-protA-1, and pS-protA-2 are very similar in the absence of energy (Fig. 4, compare lanes 1, 4, and 7). As previously observed, Toc34 and Toc86 are the major crosslinked products with pS-protA at this stage. Toc86 and Toc34 also are efficiently labeled with pS-protA-1 and pS-protA-2 (Fig. 4, lanes 4 and 7). These results indicate that residues as far as 112 amino acids from the transit sequence of the preprotein are associated with these two outer membrane components during initial binding of the preprotein.
Figure 4.
Crosslinking of pS-protA, pS-protA-1, or pS-protA-2 to envelope polypeptides at three stages in import. Energy- depleted chloroplasts (2 mg chlorophyll) were incubated with 200 nM urea-denatured pS-protA (pS-protA), pS-protA-1 (pS-protA-1), or pS-protA-2 (pS-protA-2) in the presence of apyrase (+ Apyrase), or the indicated concentrations of ATP (ATP) and GTP (GTP) at 26°C in the dark. The chloroplasts were irradiated at 312 nM for 20 min on ice with a transilluminator at a distance of 5 cm. Reisolated chloroplasts were fractionated to yield a total envelope membrane fraction. Envelope membranes (50 μg of protein) were analyzed by SDS-PAGE under reducing conditions and fluorography. The molecular masses of standard proteins are indicated at the center of the figures. The positions of pS-protA, S-protA, Toc86, Toc75, Toc34, Tic22, and Tic(21) are shown to the left and right of the figures.
In the presence of 0.1 mM ATP and GTP, Toc86 is a major crosslinking target in the outer membrane with all three substrates (Fig. 4, lanes 2, 5, and 8). Crosslinking of all three substrates to Toc34 is dramatically reduced, indicating that the interaction of Toc34 with the preprotein is sensitive to the presence of NTPs (Fig. 4, lanes 2, 5, and 8). In contrast, crosslinking to Toc75 and the inner membrane components, Tic(21) and Tic22, is observed only with pS-protA (Fig. 4, lanes 2, 5, and 8). These data suggest that only the NH2-terminal residues of pS-protA have inserted into or across the outer membrane translocon at this early import intermediate stage.
In the presence of concentrations of ATP that promote insertion of the preproteins across the inner membrane, Toc86 is still crosslinked to all three substrates (Fig. 4, lanes 3, 6, and 9). Toc75 and Tic22 crosslink to both pS-protA and pS-protA-1 (Fig. 4, lanes 3, and 6). Therefore, the transit sequence and at least 41 amino acids of the preprotein have translocated across the outer membrane and are exposed to the outer face of the inner membrane at the late stage of import. The integral inner membrane protein, Tic(21), and the 14-kD protein of unknown identity are crosslinked only to pS-protA (Fig. 4, lane 3), suggesting that only the NH2-terminal regions of the preprotein have engaged these components. The pS-protA-2 substrate does not label Toc75 or inner membrane components at this stage (Fig. 4, lane 8) indicating that the COOH-terminal region of the pS domain and the entire protein A domain of the preprotein remain exposed to the cytoplasmic side of the outer membrane.
Toc86 Functions in Preprotein Recognition and Translocation at the Outer Membrane Translocon
Toc86 is consistently and efficiently crosslinked to all substrates at each stage in import (Fig. 4), indicating a key role for this protein in the outer membrane translocon. However, the relationship of crosslinking to the function of Toc86 is unclear. Toc86 has been proposed to represent a receptor for preproteins at the outer envelope and has previously been shown to crosslink to the transit sequence of pS in the absence of energy or in the presence of 0.1 mM ATP and 0.1 mM GTP (Perry and Keegstra, 1994; Ma et al., 1996). Therefore, one possibility to explain crosslinking to Toc86 at each stage in import is that preproteins accumulate at the Toc86 receptor site en route to the translocation channel even in the presence of ATP concentrations that promote import. An alternative explanation is that Toc86 serves additional functions in import, such as participating in membrane translocation, and that the association of preproteins with Toc86 may undergo significant changes upon energy-dependent insertion across the outer membrane. To further investigate the differences in preprotein binding at the two early stages of import, we mapped the sites of preprotein crosslinking to Toc86 using the outer membrane impermeable protease, thermolysin (Cline et al., 1984). Thermolysin digests the 34-kD cytoplasmic GTP-binding domain of Toc86 leaving a 52-kD membrane- protected domain (Fig. 5 B; Hirsch et al., 1994; Schnell et al., 1994). As a result, thermolysin treatment of crosslinked Toc86 allowed us to determine whether crosslinking of the preprotein occurred via the cytoplasmic domain or the membrane-protected domain of the protein. Crosslinking to the membrane-protected domain of Toc86 would be consistent with a role for this protein in translocation.
Figure 5.
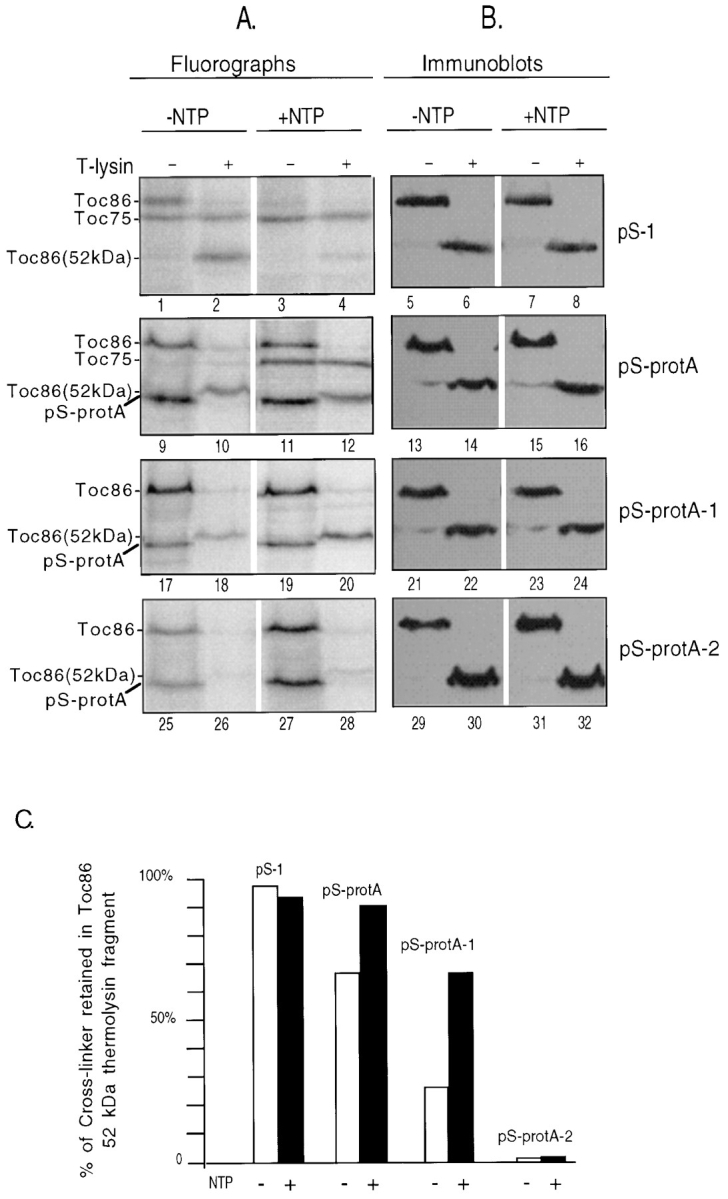
Crosslinking of pS-protA substrates to the membrane-protected domain of Toc86. Energy-depleted chloroplasts (2 mg chlorophyll) were crosslinked to pS-1 (pS-1), pS-protA (pS-protA), pS-protA-1 (pS-protA-1), or pS-protA-2 (pS-protA-2) in the presence of apyrase (−NTP) or 0.1 mM ATP and GTP (+NTP) for 10 min at 26°C in the dark as described in Fig. 4. After crosslinking, chloroplasts were treated in the absence (−T-lysin) or presence (+T-lysin) of 0.2 mg/ml thermolysin on ice for 30 min. The envelope membrane fractions from these chloroplasts were resolved by SDS-PAGE and analyzed by (A) fluorography or (B) immunoblotting with anti-Toc86 sera. The positions of Toc75, Toc86, the 52-kD proteolytic fragment of Toc86 (Toc86 (52 kD)), pS-protA, pS-protA-1, and pS-protA-2 are indicated at the left of the figures. (C) Quantitation of the percent of radiolabeled crosslinker that is retained in the 52-kD membrane-protected fragment of Toc86 after thermolysin digestion from A.
After crosslinking reactions with pS-protA, pS-protA-1, and pS-protA-2, intact chloroplasts were treated with thermolysin under conditions that previously have been shown to selectively digest cytoplasmically exposed proteins (Cline et al., 1984). The experiment was also performed with a fourth crosslinking substrate, pS-1. This derivative of pS contains a single crosslinking site at position −1 of the transit sequence (Fig. 1) and has previously been shown to crosslink to Toc86 in the absence of energy and in the presence of 0.1 mM ATP and 0.1 mM GTP (Ma et al., 1996). The pS-1 substrate allowed us to selectively investigate the environment of the transit sequence at the two early stages of import. Interestingly, the radiolabeled crosslinker is quantitatively retained in the 52-kD thermolysin fragment of Toc86 after crosslinking to pS-1 in either the absence or presence of added energy (Fig. 5 A, compare lanes 1 and 2, and lanes 3 and 4, and C). These data suggest that the transit sequence is in the vicinity of the membrane-protected domain of Toc86 and may be partially inserted into the outer membrane even in the absence of energy.
Crosslinking of pS-protA occurs to the cytoplasmic and membrane-protected domains of Toc86 in the absence of energy (Fig. 5, lanes 9 and 10, and C) , consistent with the distribution of crosslinker in both the transit sequence and mature sequence of the preprotein. Crosslinking to the membrane-protected domain of Toc86 is dramatically decreased in pS-protA-1 at this stage (Fig. 5 A, lanes 17 and 18, and C), reflecting the absence of a crosslinker within the transit sequence of this preprotein. In the presence of 0.1 mM ATP and 0.1 mM GTP, the relative amount of crosslink label retained in the 52-kD domain increases 1.5-fold for pS-protA (Fig. 5 A, lanes 11 and 12, and C), and 2.5-fold for pS-protA-1 (Fig. 5 A, lanes 19 and 20, and C). Very low levels of crosslinking between pS-protA-2 and the 52-kD fragment are detected in the absence or presence of energy (Fig. 5 A, lanes 25–28, and C), indicating that the distant crosslinking site on this preprotein has not inserted into the outer membrane at either stage in import. We interpret these data to indicate that the transition from energy-independent binding to the early import intermediate results in the insertion of a significant portion of the mature sequence of the preproteins into the outer membrane and that this insertion is, at least, partially mediated by the membrane-protected domain of Toc86. On the basis of these results, we suggest that Toc86 participates both in preprotein recognition and translocation at the outer membrane.
Association of Toc34 with Preproteins Is Regulated by GTP Binding
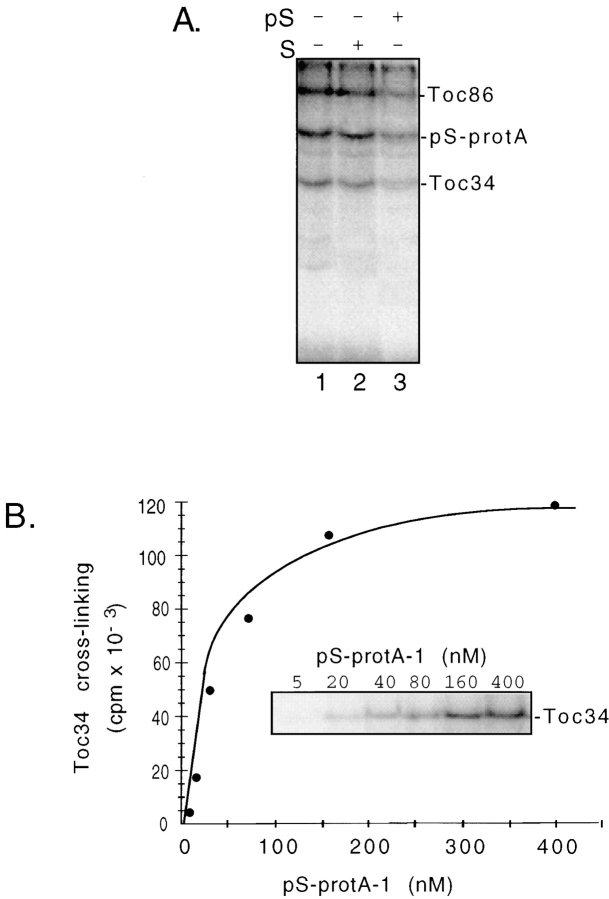
A direct interaction of Toc34 with preproteins has not previously been observed. The crosslinking of Toc34 to preproteins during energy-independent binding supports the proposal that Toc34 participates with Toc86 in the initial interaction of preproteins with the outer envelope translocon. We wished to investigate the nature of the interaction of preproteins with Toc34 to gain insight into its roles at the outer membrane. As a first step, we investigated the specificity of preprotein binding to Toc34. Both Toc34 and Toc86 are major proteins of the outer membrane and both possess significant cytoplasmic domains. Therefore, it is possible that the crosslinking observed with Toc34 and Toc86 in the presence of apyrase represents a nonspecific association of the urea-denatured preprotein with these two components. To eliminate this possibility, we tested the ability of authentic pS or the mature small subunit of ribulose-1,5-bisphosphate carboxylase (S) to compete for the labeling of Toc34 and Toc86 with pS-protA-1 in the crosslinking assay. The pS-protA-1 substrate was used for these experiments because it consistently gave the highest levels of Toc34 labeling in our assays and therefore provided the highest sensitivity for quantitative analysis of crosslinking. Fig. 6 A shows that authentic pS (Fig. 6 A, compare lanes 1 and 3) but not mature small subunit lacking a transit sequence (Fig. 6 A, compare lanes 1 and 2), is able to competitively inhibit the crosslinking of both Toc86 and Toc34 to pS-protA-1. Crosslinking to Toc86 and Toc34 decrease to 15% of the control levels in the presence of a 10-fold excess of authentic pS. As a second test of the selectivity of the Toc34–preprotein association, we tested whether the interaction was saturable. Chloroplasts were crosslinked to increasing concentrations of pS-protA-1 in the absence of energy. Fig. 6 B shows that crosslinking of pS-protA-1 to Toc34 is saturated at ∼400 nM pS-protA-1. Crosslinking to Toc86 was saturated at the same concentration of pS-protA-1 (data not shown). Therefore, the interaction of the preprotein with Toc34 and Toc86 in the absence of energy is dependent upon the presence of a functional targeting sequence and represents a selective interaction with preprotein.
Figure 6.
Selectivity of pS-protA-1 crosslinking to Toc34 and Toc86. (A) Isolated chloroplasts equivalent to 2 mg of chlorophyll were crosslinked to 50 nM pS-protA-1 in the presence of apyrase and 500 nM of unlabeled pS (+pS) or mature S (+S) as described in Fig. 4. Fractions corresponding to envelope membranes (50 μg protein) were analyzed by SDS/PAGE and fluorography. (B) Isolated chloroplasts equivalent to 2 mg of chlorophyll were crosslinked in the presence of apyrase and the indicated concentrations of urea-denatured pS-protA-1 as described in Fig. 4. The envelope membrane fractions from these chloroplasts were resolved by SDS-PAGE and analyzed by fluorography. The inset shows the fluorograph of crosslinking to Toc34. The graph shows quantitative analysis of the crosslinking to Toc34 shown in the inset.
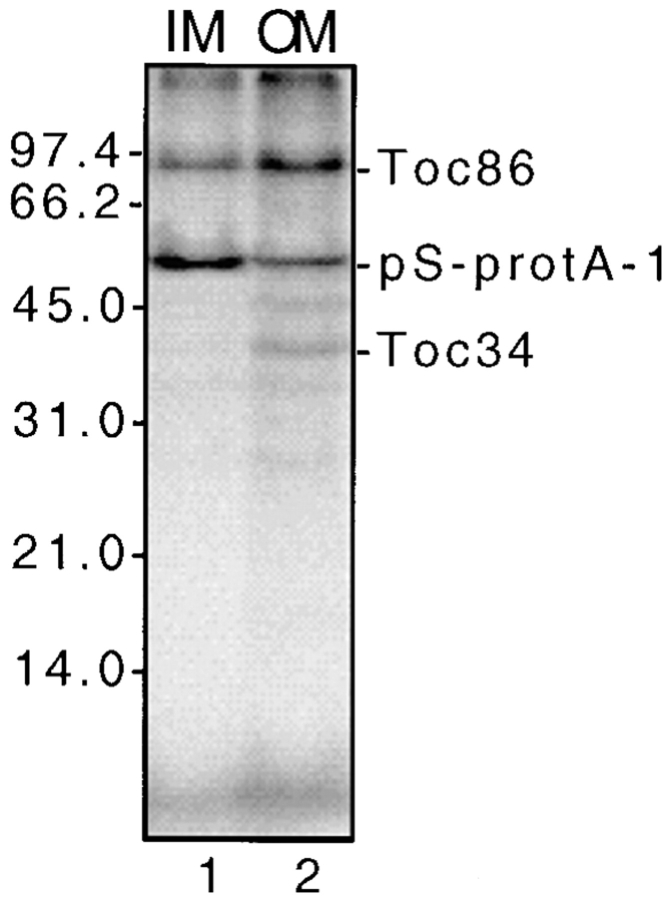
We previously showed that the interaction of Toc86 with preproteins in the absence of NTPs occurred primarily in Toc complexes that were not associated with Tic components at contact sites (Ma et al., 1996). To determine whether Toc34 crosslinking was dependent upon the formation of contact sites, we separated envelope membranes from crosslinking reactions into fractions enriched in outer membrane vesicles and mixed outer–inner membrane vesicles that contain contact sites. Fig. 7 shows that crosslinked Toc34 is enriched in outer membrane vesicles (compare lanes 1 and 2). Therefore, the interaction is not dependent on the formation of contact sites but can occur in “free” Toc complexes.
Figure 7.
Location of crosslinked Toc34 in outer and inner envelope membranes. Envelope membranes from chloroplasts crosslinked to pS-protA-1 in the presence of apyrase were fractionated into outer membrane and inner membrane vesicles by flotation in linear sucrose gradients (see Materials and Methods). The proteins (50 μg) from outer membrane (OM) and inner membrane vesicles (IM) were resolved by SDS-PAGE and analyzed by fluorography. The molecular masses of standard proteins are indicated at the left of the figure. The positions of pS-protA-1, Toc86, and Toc34 are shown to the right of the figure.
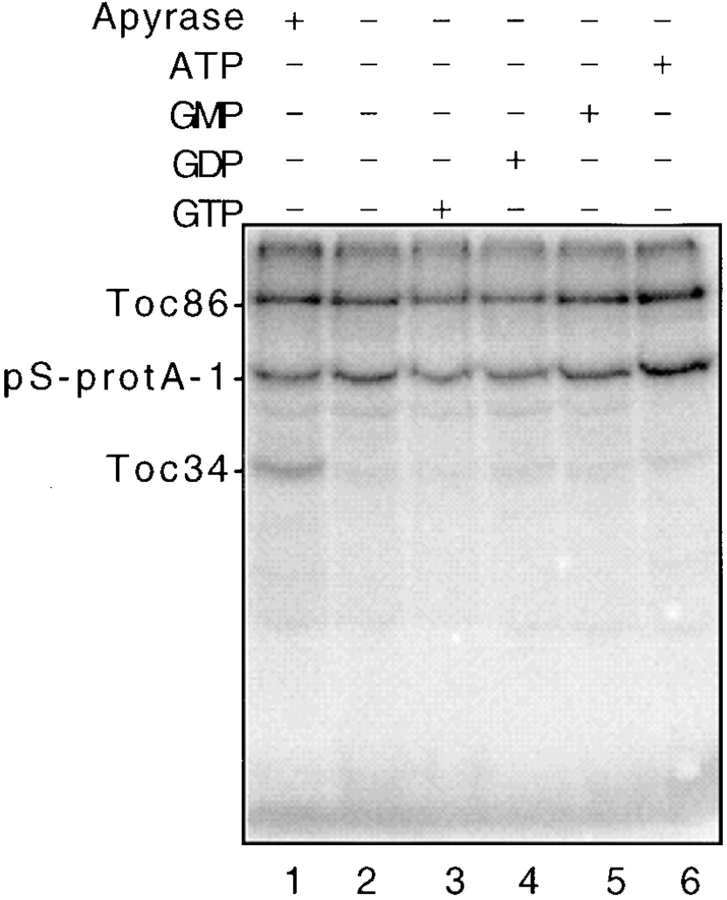
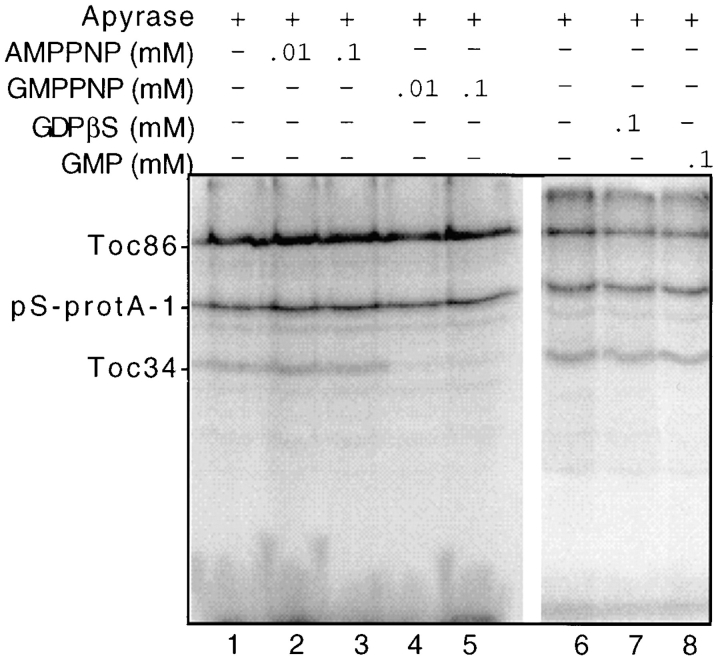
Crosslinking of Toc34 to preproteins was observed only in the absence of added ATP or GTP. This observation suggests that the Toc34–preprotein interaction may be regulated by the intrinsic GTP-binding activity of Toc34 or Toc86. To explore this possibility, we tested the effects of various nucleotides on the crosslinking of pS-protA-1 to Toc34. Energy-depleted chloroplasts were crosslinked to pS-protA-1 in the presence or absence of apyrase, 0.1 mM ATP, 0.1 mM GTP, 0.1 mM GDP, or 0.1 mM GMP. Crosslinking to Toc34 was stimulated only in samples containing apyrase (Fig. 8, lane 1). Even samples without added nucleotide exhibited little crosslinking to Toc34 (Fig. 8, lane 2). These results suggest that sufficient NTP is present in the binding reaction to promote the disruption of the Toc34–preprotein interaction. We attempted to reinvestigate the energy requirements for Toc34 crosslinking by using chloroplasts that had been pretreated with apyrase and reisolated before the addition of nucleotide. However, the pattern of crosslinking was nearly identical to that presented in Fig. 8 (data not shown). Apparently, the physical manipulations of chloroplast reisolation releases sufficient nucleotide to promote dissociation of the Toc34–preprotein interaction.
As an alternative, we tested whether AMPPNP, GMPPNP, GDPβS, or GMP could affect Toc34 crosslinking in the presence of apyrase. AMPPNP and GMPPNP are nonhydrolyzable analogues of ATP and GTP, respectively, that should be unaffected by apyrase. GDPβS, an analogue of GDP, presumably can be hydrolyzed but at a very low rate, and GMP is unaffected by apyrase. The results shown in Fig. 9 demonstrate that 10 μM or greater concentrations of GMPPNP are able to disrupt the Toc34–preprotein interaction (lanes 4 and 5), whereas comparable concentrations of AMPPNP (lanes 2 and 3), GDPβS (lane 7), or GMP (lane 8) did not affect crosslinking. Although GTP is known to be required for protein import into chloroplasts, the specific function(s) of GTP has not been defined. The results presented in Fig. 9 suggest that the binding of GTP regulates the interaction of preproteins with the components of the Toc complex at the early stages of preprotein recognition.
Figure 9.
Effect of nonhydrolizable GTP analogues on the crosslinking of pS-protA-1 to Toc34. Energy-depleted chloroplasts (2 mg chlorophyll) were incubated with 200 nM urea-denatured pS-protA-1 in the presence of apyrase (+ Apyrase), and the indicated concentrations of AMPPNP (AMPPNP), GMPPNP (+GMPPNP), GDPβS (+GDPβS), or GMP (GMP) for 10 min at 26°C in the dark and crosslinked by UV irradiation. The envelope fraction (50 μg protein) from crosslinked chloroplasts was analyzed by SDS-PAGE and fluorography. The positions of pS-protA-1, Toc86, and Toc34 are shown to the left of the figure.
Discussion
Our results suggest a sequence of events during the import of cytoplasmic preproteins across the chloroplast envelope. In this model, cytoplasmic preproteins initially bind to the Toc complex at the outer envelope membrane in a readily reversible reaction that does not require NTP hydrolysis. We refer to this stage as energy-independent binding. This stage in import was not previously recognized as a productive step because of the low levels of preprotein binding detected in standard in vitro binding assays with isolated chloroplasts (Cline et al., 1985; Olsen et al., 1989; Olsen et al., 1992). The binding of preprotein to Toc complexes in the absence of NTPs may be of relatively low affinity, and preprotein could dissociate during the reisolation of chloroplasts. However, covalent crosslinking allowed us to capture a steady state picture of the binding reaction. Our data indicate that the energy-independent binding requires the transit sequence of the preprotein and is saturable (Fig. 6). Both observations are consistent with a specific binding reaction. The concentration of pS-protA that saturates energy-independent binding (400 nM) is nearly identical to the concentrations of pS-protA that we previously showed are necessary to saturate the sites for binding of the early import intermediate (Schnell and Blobel, 1993). This suggests that the number of energy- independent preprotein-binding sites limit the number of preproteins that associate with the Toc complex.
We previously showed that Toc86 and Toc75 are crosslinked to the transit sequence of pS during energy- independent binding (Ma et al., 1996). Based on these results we proposed that the transit sequence receptor site may be composed of domains from both Toc components. In Fig. 5 we show that the transit sequence crosslinks to the membrane-protected domains of Toc75 and Toc86, suggesting that the transit sequence may be partially inserted in the outer membrane translocon at this early recognition step in import.
In this report, we also demonstrate that the preprotein interacts with Toc34 during energy-independent binding (Fig. 2). Although Toc34 forms a stable complex with Toc75 and Toc86, a role for this component in import previously had not been observed. We propose that Toc 34 may regulate the transition from energy-independent binding of the preprotein to a later stage in import through a cycle of GTP binding. This hypothesis is supported by our observation that GMPPNP binding results in the dissociation of the contact between Toc34 and the preprotein (Fig. 9).
There are several possibilities to explain the observed effect of GMPPNP on the Toc34–preprotein interaction. One possibility is that GTP binding may regulate the association/dissociation of Toc34 with other Toc components. Toc34 is detected in association with Toc complexes in the absence of bound preprotein and at all stages in import examined (Schnell et al., 1994; Ma et al., 1996; Nielsen et al., 1997), but the dynamics of this interaction have not been examined. An alternative explanation is that GTP binding induces a conformational change in the Toc complex that is necessary for subsequent insertion of the preprotein across the outer membrane through the protein-conducting channel. In this scenario, GTP binding may serve as a switch that is necessary to commit the preprotein to translocation. Future studies should clarify whether Toc34 crosslinking represents a true binding of preprotein to this component or is a consequence of a conformation of bound preprotein in the Toc complex that brings the crosslinker in close vicinity to Toc34. Furthermore, reconstitution of these interactions should allow us to define whether the observed change in preprotein binding is a consequence of GTP binding at Toc34, Toc86, or both proteins.
Although we have shown GMPPNP regulates the Toc34–preprotein interaction, it is unlikely that this represents the only role for GTP in preprotein binding. Previous data have shown that GMPPNP or slowly hydrolyzable analogues of GTP inhibit the formation of early import intermediates (Olsen et al., 1992; Schnell 1994). Likewise, pS does not crosslink to the inner membrane import components, Tic(21) or Tic22, in the presence of GMPPNP (Fig. 9). These data indicate that GTP hydrolysis is necessary for steps subsequent to energy-independent binding in the import reaction, such as stable insertion across the outer membrane. Therefore, the GMPPNP-driven dissociation of Toc34 and preprotein may represent only the first in a set of GTP-dependent reactions that regulates the targeting reaction.
The second stage of import corresponds to the early import intermediate that has inserted across the outer membrane and is in contact with components of the inner membrane. This step in import requires the hydrolysis of ATP and GTP (Cline et al., 1985; Olsen et al., 1989; Olsen et al., 1992; Schnell et al., 1994). Toc75 is the major target of crosslinking to the transit sequence of pS at this stage in import (Ma et al., 1996). Furthermore, anti-Toc75 antibodies block protein import (Tranel et al., 1995). These observations led to the proposal that Toc75 is a component of the protein-conducting channel in the outer membrane. Here, we show that Toc86 also is in contact with the preproteins that are inserted across the outer membrane. The protease-sensitivity studies in Fig. 5 indicate that this contact occurs through the regions of Toc86 that are embedded in the outer membrane or located within the intermembrane space of the envelope. The regions of contact between the preprotein and Toc86 are significantly different at the energy-independent binding stage and the early import intermediate. Although Toc86 contacts the transit sequence of preproteins during recognition, it remains in contact with mature sequences of the preprotein during outer membrane translocation. On the basis of these results, we propose that Toc86 participates in preprotein translocation at the Toc complex in addition to its role in preprotein recognition. It is possible that the membrane domains of Toc86 and Toc75 interact to form the transport channel in the outer membrane translocon, or that the preprotein associates with a domain of Toc86 in the intermembrane space after insertion across the outer membrane.
The final step of import is the insertion of the preprotein across the inner membrane. Our previous results showed that two inner membrane associated proteins, Tic(21) and Tic22, crosslink to a derivative of pS at an early intermediate stage in import (Ma et al., 1996). We have shown here that both proteins crosslink to a pS-protA and pFd-protA at early and late stages in import providing further support for these proteins as components of the general import machinery of the inner membrane. Site specific cross-linking suggests that Tic22 and Tic(21) interact with preproteins sequentially (Fig. 4). We propose that Tic22 may contain the binding site for the precursor at the inner membrane as it emerges from the Toc complex into the intermembrane space. Tic(21) is likely to form part of the membrane translocation machinery. Detectable crosslinking to an additional inner membrane import component, Tic110, is not observed with either pS-protA or pFd-protA at any stage in import although considerable evidence supports a role for Tic110 in the import process (Schnell et al., 1994; Wu, et al., 1994; Kessler and Blobel, 1996; Lübeck, et al., 1996). Based on its association with the chloroplast hsp60 homolog, cpn60, Kessler and Blobel (1996) have proposed that Tic110 acts as a docking site for the molecular chaperone at the inner membrane. This function would serve to localize the chaperone at the sites of translocation to assist in folding of newly imported preproteins. Such a function for Tic110 would not require direct contact with preproteins.
The pS-protA and pFd-protA substrates crosslinked to two previously unidentified proteins in the envelope. One of these crosslinked products is a 45 kDa polypeptide that is crosslinked to pS-protA and pFd-protA at the energy-independent binding stage of import. The second is 14-kD polypeptide that crosslinks to pS-protA and pFd-protA only at the late stages of import. These proteins represent new candidates for import components that participate in the early and late stages of envelope translocation, respectively.
Acknowledgments
The authors would like to thank Samuel LaSala and Yongkang Ma for their technical assistance and helpful discussions during the preparation of this manuscript.
This work was supported by National Science Foundation grants MCB-9404611 and MCB-9722914 to D.J. Schnell.
Abbreviations used in this paper
- APDP
N-[4-(p-azidosalicylamido)butyl]-3′(2-pyridyldithio)propionamide
- Tic
translocon at the inner envelope membrane of chloroplasts
- Toc
translocon at the outer envelope membrane of chloroplasts
Footnotes
Address all correspondence to Danny J. Schnell, Department of Biological Sciences, Rutgers University, 101 Warren Street, Newark, NJ 07102. Tel.: (973) 353-1082. Fax: (973) 353-1007. E-mail: schnell@andromeda.rutgers.edu
References
- Cline K, Henry R. Import and routing of nucleus-encoded chloroplast proteins. Annu Rev Cell Dev Biol. 1996;12:1–26. doi: 10.1146/annurev.cellbio.12.1.1. [DOI] [PubMed] [Google Scholar]
- Cline K, Werner-Washburne M, Andrews J, Keegstra K. Thermolysin is a suitable protease for probing the surface of intact pea chloroplasts. Plant Physiol. 1984;74:675–678. doi: 10.1104/pp.75.3.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline K, Werner-Washburne M, Lubben TH, Keegstra K. Precursors to two nuclear-encoded chloroplast proteins bind to the outer envelope membrane before being imported into chloroplasts. J Biol Chem. 1985;260:3691–3696. [PubMed] [Google Scholar]
- Fuks B, Schnell DJ. Mechanism of protein transport across the chloroplast envelope. Plant Physiol. 1997;114:405–410. doi: 10.1104/pp.114.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch S, Muckel E, Heemeyer F, von Heijne G, Soll J. A receptor component of the chloroplast protein translocation machinery. Science. 1994;266:1989–1992. doi: 10.1126/science.7801125. [DOI] [PubMed] [Google Scholar]
- Keegstra K, Yousif AE. Isolation and characterization of chloroplast envelope membranes. Methods Enzymol. 1986;118:316–325. [Google Scholar]
- Kessler F, Blobel G. Interaction of the protein import and folding machineries in the chloroplast. Proc Natl Acad Sci USA. 1996;93:7684–7689. doi: 10.1073/pnas.93.15.7684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler F, Blobel G, Patel HA, Schnell DJ. Identification of two GTP-binding proteins in the chloroplast protein import machinery. Science. 1994;266:1035–1039. doi: 10.1126/science.7973656. [DOI] [PubMed] [Google Scholar]
- Lübeck J, Soll J, Akita M, Nielsen E, Keegstra K. Topology of IEP110, a component of the chloroplastic protein import machinery present in the inner envelope membrane. EMBO (Eur Mol Biol Organ) J. 1996;15:4230–4238. [PMC free article] [PubMed] [Google Scholar]
- Lübeck J, Heins L, Soll J. Protein import into chloroplasts. Physiologia Plantarum. 1997;100:53–64. [Google Scholar]
- Ma Y, Kouranov A, LaSala S, Schnell DJ. Two components of the chloroplast protein import apparatus, IAP86 and IAP75, interact with the transit sequence during the recognition and translocation of precursor proteins at the outer envelope. J Cell Biol. 1996;134:315–327. doi: 10.1083/jcb.134.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen E, Akita M, Davila-Aponte J, Keegstra K. Stable association of chloroplastic precursors with protein-translocation complexes that contain proteins from both envelope membranes and a stromal Hsp100 molecular chaperone. EMBO (Eur Mol Biol Organ) J. 1997;16:935–946. doi: 10.1093/emboj/16.5.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen LJ, Keegstra K. The binding of precursor proteins to chloroplasts requires nucleoside triphosphates in the intermembrane space. J Biol Chem. 1992;267:433–439. [PubMed] [Google Scholar]
- Olsen LJ, Theg SM, Selman BR, Keegstra K. ATP is required for the binding of precursor proteins to chloroplasts. J Biol Chem. 1989;264:6724–6729. [PubMed] [Google Scholar]
- Pain D, Blobel G. Protein import in chloroplasts requires a chloroplast ATPase. Proc Natl Acad Sci USA. 1987;84:3288–3292. doi: 10.1073/pnas.84.10.3288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry SE, Keegstra K. Envelope membrane proteins that interact with chloroplastic precursor proteins. Plant Cell. 1994;6:93–105. doi: 10.1105/tpc.6.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell DJ, Blobel G. Identification of intermediates in the pathway of protein import into chloroplasts and their localization to envelope contact sites. J Cell Biol. 1993;120:103–115. doi: 10.1083/jcb.120.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell DJ, Kessler F, Blobel G. Isolation of components of the chloroplast protein import machinery. Science. 1994;266:1007–1012. doi: 10.1126/science.7973649. [DOI] [PubMed] [Google Scholar]
- Schnell DJ, Blobel G, Keegstra K, Kessler F, Ko K, Soll J. A nomenclature for the protein import components of the chloroplast envelope. Trends Cell Biol. 1997;7:303–304. doi: 10.1016/S0962-8924(97)01111-2. [DOI] [PubMed] [Google Scholar]
- Seedorf M, Waegemann K, Soll J. A constituent of the chloroplast import complex represents a new type of GTP-binding protein. Plant J. 1995;7:401–411. doi: 10.1046/j.1365-313x.1995.7030401.x. [DOI] [PubMed] [Google Scholar]
- Tranel PJ, Froehlich J, Goyal A, Keegstra K. A component of the chloroplastic protein import apparatus is targeted to the outer envelope membrane via a novel pathway. EMBO (Eur Mol Biol Organ) J. 1995;14:2436–2446. doi: 10.1002/j.1460-2075.1995.tb07241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waegemann K, Soll J. Characterization of the protein import apparatus in isolated outer envelopes of chloroplasts. Plant J. 1991;1:149–158. [Google Scholar]
- Wu C, Seibert FS, Ko K. Identification of chloroplast envelope proteins in close proximity to a partially translocated chimeric precursor protein. J Biol Chem. 1994;269:32264–32271. [PubMed] [Google Scholar]