Abstract
A checkpoint mechanism operates at the metaphase/anaphase transition to ensure that a bipolar spindle is formed and that all the chromosomes are aligned at the spindle equator before anaphase is initiated. Since mistakes in the segregation of chromosomes during meiosis have particularly disastrous consequences, it seems likely that the meiotic cell division would be characterized by a stringent metaphase/ anaphase checkpoint. To determine if the presence of an unaligned chromosome activates the checkpoint and delays anaphase onset during mammalian female meiosis, we investigated meiotic cell cycle progression in murine oocytes from XO females and control siblings. Despite the fact that the X chromosome failed to align at metaphase in a significant proportion of cells, we were unable to detect a delay in anaphase onset. Based on studies of cell cycle kinetics, the behavior and segregation of the X chromosome, and the aberrant behavior and segregation of autosomal chromosomes in oocytes from XO females, we conclude that mammalian female meiosis lacks chromosome-mediated checkpoint control. The lack of this control mechanism provides a biological explanation for the high incidence of meiotic nondisjunction in the human female. Furthermore, since available evidence suggests that a stringent checkpoint mechanism operates during male meiosis, the lack of a comparable checkpoint in females provides a reason for the difference in the error rate between oogenesis and spermatogenesis.
The metaphase to anaphase transition is governed by a cell cycle checkpoint which monitors chromosome alignment and spindle integrity (for review see references 11, 26, 28, 38). This checkpoint delays anaphase until all chromosomes are properly positioned at the metaphase plate, thereby reducing the likelihood of segregation errors at anaphase. In some organisms, the failure of alignment of even a single chromosome prevents the cell from initiating anaphase (33, 38). The resultant cell cycle delay may be extensive, causing the cell to degenerate without completing the division. However, in many cell types the checkpoint is eventually overridden and division occurs regardless of the chromosome error (4, 23).
The signaling mechanism by which chromosome alignment influences cell cycle progression remains unclear. However, the kinetochore, the proteinaceous structure flanking the centromere, is an essential component. During chromosome alignment, bipolar microtubule attachments are formed through a series of kinetochore–microtubule attachments/detachments and the polymerization/depolymerization of microtubules (for review see reference 30). The poleward forces resulting from the attachment of sister kinetochores to opposite spindle poles at mitotic metaphase are counterbalanced by sister chromatid cohesion forces, allowing the chromosome to stably align at the spindle equator in the characteristic metaphase configuration. The poleward forces also create tension on sister kinetochores, which induces the dephosphorylation of kinetochore proteins (15, 23, 25, 31, 32). Although the way in which tension induces this biochemical change remains unknown, it appears to be a prerequisite for anaphase initiation (5, 12). Disrupting the tension on a single kinetochore by severing its microtuble attachments reverses the biochemical alteration and causes a delay in anaphase onset (32). Since anaphase is initiated if tension is artificially applied to the unattached kinetochore, the signal for delay is thought to emanate from kinetochores which are not under tension (23).
Meiotic cell division is unique because the centromeres of homologous chromosomes rather than those of sister chromatids segregate from each other at anaphase of the first division. In most species, genetic exchange (recombination) between homologues is essential in ensuring their segregation at anaphase I. As chromosomes condense to undergo the first meiotic division, the sites of exchange become visible as chiasmata. At the first meiotic division, chiasmata are thought to function in two ways to ensure the proper segregation of homologous chromosomes; firstly, by maintaining homologues in a paired orientation that promotes the capture of their kinetochores by opposite spindle poles and, secondly, by providing a counterbalance to the forces acting on opposing kinetochores, and thus allowing the homologous pair to congress to the spindle equator (29; for review see references 6, 14).
The central role of recombination in meiotic chromosome segregation suggests that the segregation of an achiasmate chromosome will be impaired. Indeed, chromosomes present as unpaired univalents at the first meiotic division have been reported to undergo premature sister chromatid separation (i.e., equational division), and/or to lag at anaphase, and/or to induce metaphase arrest (4, 7, 24). In certain organisms, however, achiasmate chromosomes are a characteristic of normal meiosis, either because recombination does not occur (e.g., male Drosophila) or because one or more pairs of chromosomes is always achiasmate (e.g., female Drosophila and a few mammalian species where the sex chromosomes are achiasmate). These species have evolved alternate mechanisms for the alignment and segregation of nonexchange chromosomes (for review see references 35, 39).
In the mouse, the effect of an achiasmate chromosome on the meiotic cell division appears to be gender specific. In the male mouse, the presence of a univalent chromosome results in metaphase arrest and subsequent spermatocyte degeneration, rendering the animal sterile (2, 37). In contrast, female mice with an XO sex chromosome constitution are fertile, indicating that the presence of a univalent chromosome does not have the same disruptive effect during the alignment and segregation of chromosomes in female meiosis. This gender-specific difference could have several different explanations: (a) female meiosis may have evolved a specialized mechanism for segregating achiasmate chromosomes at meiosis I; (b) the checkpoint monitoring chromosome alignment at metaphase I may be inefficient in female meiosis, allowing errors to go undetected; or (c) the checkpoint may efficiently detect errors in chromosome alignment and induce a delay in anaphase onset, but oocytes (unlike spermatocytes) do not degenerate, they simply wait out the delay.
In a previous study of chromosome alignment and segregation in oocytes from XO females, we determined that, in a proportion of cells, the single X chromosome behaves like univalent chromosomes in some plant and insect species and segregates equationally at meiosis I (17). However, in a proportion of metaphase cells, the univalent X chromosome was unable to make stable bipolar microtubule attachments and an unaligned X chromosome was observed. Despite this behavior, metaphase arrest was an extremely rare event. However, it was unclear whether this was because of the lack of a checkpoint mechanism or whether the delay induced by the checkpoint had eventually been overridden.
To determine if the presence of an unaligned chromosome activates the checkpoint mechanism and delays anaphase onset during female meiosis, we studied meiotic cell cycle progression in oocytes from XO females and XX littermate controls. We observed a significant delay in the congression of the univalent X chromosome, overt failure of the X chromosome to stably align at metaphase in a significant proportion of cells, and errors in the alignment of other chromosomes. Surprisingly, no delay in anaphase onset was evident. These observations suggest that the control of mammalian female meiosis is fundamentally different from mitotic cells and male meiotic cells. Since the metaphase/anaphase checkpoint provides an important means of correcting errors in alignment that would predispose to errors in chromosome segregation, a gender-specific difference in this cell cycle control mechanism may provide an explanation for the high meiotic chromosome error rate in the human female.
Materials and Methods
Production of XO Female Mice
Oocytes were obtained from XO female mice and XX sibling controls. XO females were produced on two different inbred strain backgrounds using the following breeding strategy: (a) chromosomally normal C57BL/6 females were mated to C57BL/6 males carrying the Y* mutation (9), and (b) chromosomally normal C3H females were mated to C3H males carrying the patchy fur (Paf) mutation (22). Approximately 20% of the female offspring produced from each mating had an XO sex chromosome constitution. Bone marrow specimens were collected at the time of autopsy (10) for subsequent karyotyping of donor females. However, to ensure that the meiotic analyses were unbiased, all meiotic experiments were completed without knowledge of the karyotype.
Oocyte Collection, Culture, and Fixation
Oocytes were liberated from the ovaries of 3.5-week-old females by piercing antral follicles with 26-g needles (Becton and Dickinson, Co., Franklin Lakes, NJ). Meiotically arrested oocytes at the germinal vesicle stage were collected and placed in 10 μl drops of Waymouth's MB752/1 medium (GIBCO BRL, Gaithersburg, MD) supplemented with 10% fetal bovine serum and 0.23 mM sodium pyruvate, overlaid with mineral oil (E.R. Squibb and Sons, Inc., Princeton, NJ), and incubated at 37°C in an atmosphere of 5% CO2 in air.
To obtain a synchronous cell population, oocytes were scored after 2 h in culture for nuclear envelope breakdown (indicating resumption of meiosis I). Only oocytes resuming meiosis within the first 2 h of culture were included in the study, and these oocytes were incubated for a total of 6, 8, 10, 12, 14, or 16 h. At the end of each culture period oocytes were embedded in a fibrin clot (bovine fibrinogen type IV; Calbiochem-Novabiochem Corp., La Jolla, CA; bovine thrombin; Sigma Chemical Co., St Louis, MO) attached to a microscope slide as previously described (17). Embedded oocytes were immediately fixed in 2% formaldehyde, 1% Triton X-100, 0.1mM Pipes, 5 mM MgCl2, and 2.5 mM EGTA for 30 min at 37°C. After fixation, oocytes were washed for 10 min in 0.1% normal goat serum (NGS;1 GIBCO BRL)/PBS and blocked for ⩾1 h at 37°C in PBS wash solution containing 10% NGS, 0.02% sodium azide, and 0.1% Triton X-100. Oocytes were stored in this wash solution at 4°C until immunofluorescence staining was performed.
Immunofluorescence and Fluorescence In Situ Hybridization (FISH)
Oocytes were incubated with a 1:2,000 dilution in PBS of a primary mouse monoclonal antibody to acetylated tubulin (Sigma Chemical Co.) for 1 h at 37°C, and then washed in 10% NGS/PBS for 1 h at 37°C. The primary antibody was detected with a 1:100 dilution in PBS of an FITC-conjugated goat anti–mouse IgG (Accurate Chemical and Scientific Corp., Westbury, NY), and then washed for 1 h at 37°C. Oocytes were stained with 100 ng/ ml propidium iodide and a coverslip was applied with 50% glycerol/4× SSC and 0.1 μg/ml p-phenylenediamine mounting medium and sealed with rubber cement. Oocytes were analyzed on an epifluorescent microscope (model Axioplan, Carl Zeiss, Inc., Thornwood, NY) and the meiotic stage of each oocyte was classified based on the chromosome configuration and spindle morphology as detailed below.
Oocytes from XO and control sibling females produced on the C57BL/6 background were hybridized as described previously (17) with the DNA probe, DXWas 70 (American Type Culture Collection, Rockville, MD), which recognizes repetitive sequences near the centromere of the mouse X chromosome (8). The probe was labeled with digoxigenin (Boehringer Mannheim Biochemicals, Indianapolis, IN) and detected with FITC-conjugated anti-digoxigenin (Boehringer Mannheim Biochemicals). After hybridization and detection, oocytes were analyzed on a confocal microscope (model 600: Bio-Rad Laboratories, Hercules, CA) to characterize the meiotic behavior of the univalent X chromosome. Three-dimensional optical sectioning was utilized to determine the position of the fluorescently labeled X chromosome in individual oocytes.
Meiotic Classification and Stastical Analysis
Oocytes were classified into four different meiotic stages on the basis of chromosome configuration and spindle morphology. Oocytes at the prometaphase stage had condensed bivalent chromosomes either arranged in a rosette pattern with some evident microtubule staining but no distinct spindle pole formation, or loosely arranged chromosomes on a short round spindle with discreet pole formation. Oocytes scored as metaphase I had tightly aligned chromosomes equidistantly positioned between the poles of a barrel-shaped bipolar spindle. Anaphase/telophase I oocytes had two distinct groups of chromosomes positioned on an elongated bipolar spindle with a spindle midzone often discernible, and in metaphase II– arrested oocytes, one-half of the chromosome complement was tightly aligned and positioned equidistant between the poles of a bipolar spindle within the oocyte, and the other half was present in the first polar body.
To determine if the overall rate of meiotic progression differed between oocytes from XO and XX females, a contingency χ2 analysis was performed on the distribution of meiotic stages represented in each culture group. That is, for each time point, the number of oocytes at prometaphase, metaphase, anaphase, and metaphase II was compared between the XO and XX groups.
Results
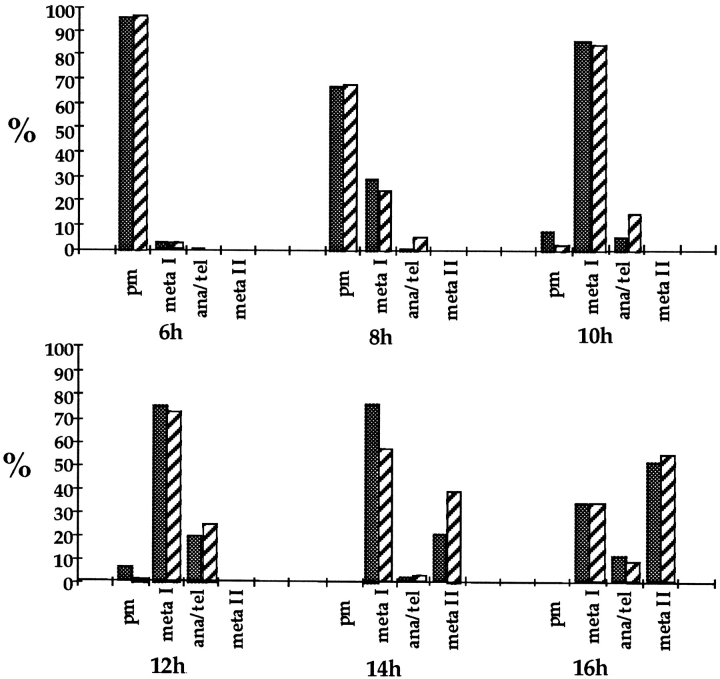
The rate of meiotic cell cycle progression was analyzed by studying groups of oocytes from XO and XX female siblings produced on the inbred C57BL/6 background at successive time points during the first meiotic division. The distribution of meiotic stages for groups of oocytes cultured for 6, 8, 10, 12, 14, and 16 h is presented in Table I and shown graphically in Fig. 1. Each time point represents ⩾250 oocytes, with ⩾100 oocytes each from XO females and controls. As can be seen from Fig. 1, this time course provided access to all stages of the first meiotic division; prometaphase stage oocytes predominated among oocytes analyzed after 6 and 8 h in culture, metaphase I was the predominant stage at the 10-, 12-, and 14-h time points, and the majority of oocytes had reached metaphase II arrest after 16 h in culture. Although anaphase/telophase figures were observed among the oocytes scored at all time points, they never predominated, reflecting the brief duration of this stage.
Table I.
The Distribution of Meiotic Stages in Oocytes from XO and XX Females Produced on the C57BL/6 Background
| 6 h | 8 h | 10 h | 12 h | 14 h | 16 h | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| XO | XX | XO | XX | XO | XX | XO | XX | XO | XX | XO | XX | |||||||||||||
| Prometaphase | 133 | 122 | 108 | 94 | 9 | 36 | 3 | 5 | — | — | — | — | ||||||||||||
| 97.1% | 96.1% | 68.4% | 67.1% | 2.4% | 8.6% | 1.1% | 4.8% | — | — | — | — | |||||||||||||
| Metaphase I | 4 | 4 | 40 | 43 | 316 | 360 | 206 | 79 | 171 | 286 | 85 | 50 | ||||||||||||
| 2.9% | 3.1% | 25.3% | 30.7% | 83.2% | 85.7% | 73.3% | 76% | 58.4% | 76.7% | 35.9% | 36.5% | |||||||||||||
| Ana/Telophase | — | 1 | 10 | 3 | 55 | 24 | 72 | 20 | 8 | 9 | 22 | 16 | ||||||||||||
| — | 0.8% | 6.3% | 2.1% | 14.5% | 5.7% | 25.6% | 19.2% | 2.7% | 2.4% | 9.3% | 11.7% | |||||||||||||
| Metaphase II | — | — | — | — | — | — | — | — | 114 | 78 | 130 | 71 | ||||||||||||
| — | — | — | — | — | — | — | — | 38.9% | 20.9% | 54.9% | 51.8% | |||||||||||||
| Totals | 137 | 127 | 158 | 140 | 380 | 420 | 281 | 104 | 293 | 373 | 237 | 137 | ||||||||||||
| χ2 2 = 1.1 | χ2 2 = 3.8 | χ2 2 = 29.3 | χ2 2 = 6.5 | χ2 2 = 26.5 | χ2 2 = 0.6 | |||||||||||||||||||
| P < 0.9 | P < 0.5 | P < 0.005 | P < 0.05 | P < 0.005 | P < 0.9 | |||||||||||||||||||
Figure 1.
The distribution of meiotic stages in oocytes from XO and XX females produced on the C57BL/6 background. The proportion of oocytes from XO (striped bars) and XX (solid bars) females at each meiotic stage (prometaphase [pm], metaphase I [meta I], anaphase/telophase I [ana/tel], and metaphase II [meta II]) after 6, 8, 10, 12, 14, and 16 h in culture.
Resumption of Meiosis I and Congression
The rate of meiotic resumption, as determined by the proportion of cells undergoing nuclear envelope breakdown within the first 2 h of culture, was not different for oocytes from XO and XX females. The overall frequency of nuclear envelope breakdown was 66.1% (1,778 out of 2,689) for oocytes from XO females and 63.9% (1,645 out of 2,573) for oocytes from XX females. Only oocytes undergoing nuclear envelope breakdown within the first 2 h of culture were included in subsequent analyses.
During prometaphase in the first few hours after nuclear envelope breakdown, chromosome condensation occurs, the first meiotic spindle is organized, and the chromosomes begin to congress to the spindle equator. Virtually all oocytes (97.1% of oocytes from XO females and 96.1% from XX females) analyzed after 6 h in culture were at prometaphase. Furthermore, no obvious difference in the rate of chromosome congression (i.e., progression from prometaphase to metaphase I) was observed between oocytes from the two types of females (Fig. 2). After 6 h in culture, a small proportion of oocytes, 2.9% from XO females and 3.1% from controls, had reached metaphase I. The proportion of metaphase I oocytes increased to 25.3 and 30.7% for XO and XX controls, respectively, after 8 h and by 10 h, the majority of oocytes (83.2% from XO females and 85.7 from controls) were at metaphase I and a proportion (14.5% from XO females and 5.7 from controls) had initiated anaphase.
Figure 2.
Rate of congression to metaphase I. The proportion of oocytes from XO (open squares) and XX (closed squares) females that had completed congression (aligned at metaphase or initiated anaphase) after 6, 8, and 10 h in culture (note that ⩽20% of metaphase oocytes from XO females contained an X chromosome that failed to congress, although all other chromosomes were properly aligned at the spindle equator).
Anaphase Onset
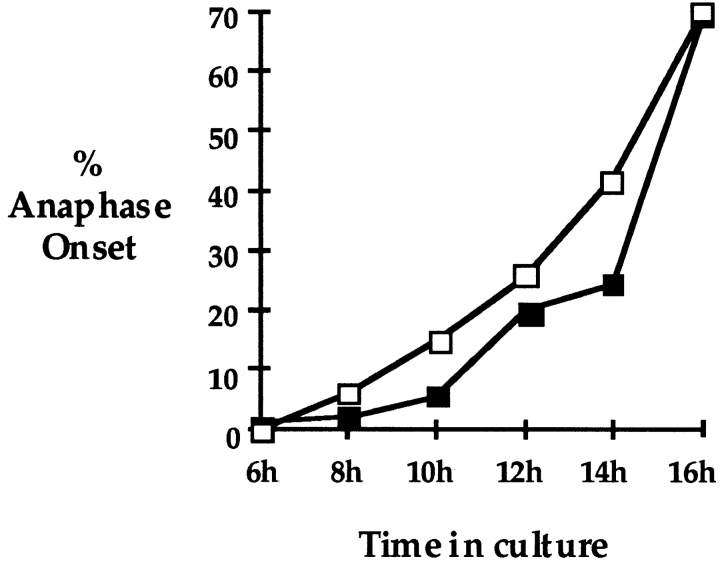
As cells began to progress beyond metaphase I, the distribution of meiotic stages became different for oocytes from XO females and controls (Table I and Fig. 1). The first appreciable group of anaphase/telophase cells was observed at 8 h and, at this time point, a greater proportion of oocytes from XO females had initiated anaphase. By 10 h in culture, the proportion of anaphase oocytes from XO females was more than twice that of the control females (14.5 and 5.7%, respectively), and the distribution of meiotic stages for the two types of females was significantly different (χ2 2 = 29.25; P <0.005). Moreover, this trend was apparent in oocytes cultured for 12 and 14 h in culture; 25.6% of oocytes from XO females and 19.2% from XX females had initiated anaphase after 12 h and by 14 h, the proportion of oocytes that had exited metaphase I (i.e., anaphase/telophase oocytes and oocytes arrested at second meiotic metaphase arrest) increased to 41.6 and 23.3% for oocytes from XO and XX females, respectively. As an increasing proportion of oocytes accumulated at metaphase II arrest, the difference in the distribution of meiotic stages of oocytes from the two types of females was no longer apparent and, after 16 h in culture, the majority of the oocytes from both types of females (64.1% from XO females and 63.5% from controls) had exited metaphase and were at either anaphase/telophase or had completed the first meiotic division, extruded a polar body, and arrested at metaphase II. The data on anaphase onset are summarized in Fig. 3 where the proportion of oocytes that had progressed beyond metaphase I is plotted against time in culture for XO females and control siblings.
Figure 3.
Anaphase onset. The proportion of oocytes from XO (open squares) and XX (closed squares) females that had initiated anaphase after 6, 8, 10, 12, 14, and 16 h in culture. Oocytes classified as having initiated anaphase include anaphase/telophase I and metaphase II–arrested oocytes.
Anaphase Onset in Oocytes from XO Females on the C3H Inbred Strain Background
To determine if the difference in the rate of anaphase onset observed among oocytes from XO and XX females was unique to the C57BL/6 inbred strain background, oocytes from XO females and XX sibling controls produced on the C3H inbred strain background were analyzed. The distribution of meiotic stages among oocytes cultured for 8 and 10 h is shown in Fig. 4. At both time points a greater proportion of oocytes from XO females had progressed beyond metaphase I. After 8 h, 30.6% of oocytes from XO females and 11.1% from XX females had initiated anaphase and, after 10 h, 93.8% of the oocytes from XO females and 79.1% from controls had progressed beyond metaphase I. The distribution of meiotic stages for the two types of females was significantly different at the 8-h time point (χ2 2= 7.38; P < 0.025), and approached significance at the 10-h time point (χ2 2= 5.991; P < 0.05). Hence, on both the C3H and C57BL/6 inbred strain backgrounds, we observed a more rapid exit from metaphase I in oocytes from XO females.
Figure 4.
The distribution of meiotic stages in oocytes from XO and XX females produced on the C3H background. The proportion of oocytes from XO (striped bars) and XX (solid bars) females at each meiotic stage (prometaphase [pm], metaphase I [meta I], anaphase/telophase I [ana/tel], and metaphase II [meta II]) after 8 and 10 h in culture. The 8-h time point represents 36 oocytes from XX females and 62 from XO females, and the 10-h time point represents 91 oocytes from XX females and 65 from XO females.
Behavior and Segregation of the Univalent X Chromosome
We previously reported an apparent delay in the congression of the univalent X chromosome in prometaphase stage oocytes (17). Likewise, in the present study, FISH with an X chromosome-specific probe demonstrated that the univalent X chromosome was separated from the congressing bivalents (Fig. 5, a and b) in 13.5% (18 out of 133) of prometaphase oocytes after 6 h in culture and in 28.8% (34 out of 108) of prometaphase oocytes after 8 h in culture. Furthermore, the X chromosome failed to align at the spindle equator in 20% of metaphase I oocytes after 8 h (Fig. 5, c and d). The frequency of metaphase I oocytes with a misaligned X chromosome decreased to 13.6% at 10 h, and did not exceed 5% of metaphase I oocytes at the 12-, 14-, and 16-h time points (Table II). In addition, misalignment of chromosomes other than the X chromosome was observed at both metaphase I and II (Fig. 5, e and f). At metaphase I this behavior appeared to be related to the failure of alignment of the X chromosome, since 16 out of 63 (25%) metaphase I oocytes with a misaligned X chromosome also had one or more misaligned autosomal chromosomes. Furthermore, misalignment of an autosomal chromosome was observed in 5% (9 out of 144) of metaphase II–arrested oocytes from XO females, but was not observed in 159 oocytes from control females.
Figure 5.
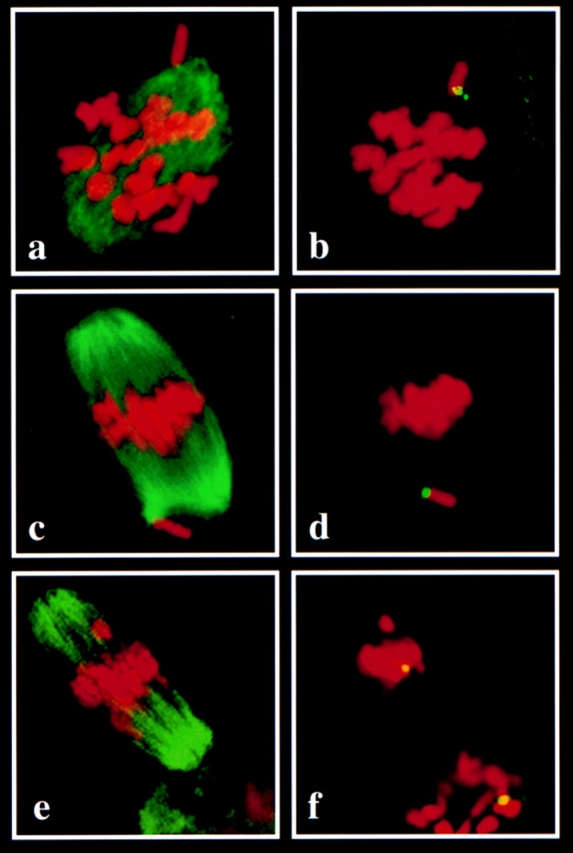
Confocal images of individual oocytes from XO females. The images on the left (a, c, and e) show combined immunofluorescence staining with an antibody to acetylated tubulin to visualize the spindle microtubules (green) and DNA staining with propidium iodide to visualize the chromosomes. The images on the right (b, d, and f) show the same oocyte as the left image, but the immunofluorescence staining has been quenched to allow visualization of the signal resulting from fluorescence in situ hybridization with an X chromosome-specific probe (green/yellow). (a) Prometaphase stage oocyte showing evidence of spindle pole formation and organization of microtubules into a barrel-shaped structure. Condensed chromosomes have begun to congress to the spindle equator, but one chromosome appears clearly separated from the group. (b) FISH with an X-specific probe confirms that the outlying chromosome is the univalent X chromosome. (c) Metaphase I stage oocyte showing all chromosomes except one aligned at the spindle equator. (d) FISH demonstrates that the unaligned chromosome located near one spindle pole is the univalent X chromosome. (e) Metaphase II–arrested oocyte showing all chromosomes but one aligned at the spindle equator. Note that the diffuse microtubule staining below the spindle is the first polar body. (f) FISH demonstrates that the misaligned chromosome is not the X chromosome. Note the presence of two distinct X chromosome signals; one in the group of chromosomes aligned at the spindle equator, and one in the polar body, indicating equational segregation of the univalent X chromosome at the first meiotic division.
Table II.
X Chromosome Behavior
| 8 h | 10 h | 12 h | 14 h | 16 h | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Metaphase I oocytes with a misaligned X chromosome* | ||||||||||
| Oocytes from XO females | 8/40 (20%) | 43/316 (13.6%) | 3/206 (1.5%) | 5/171 (2.9%) | 4/85 (4.7%) | |||||
| Oocytes from XX controls | 0/43 (0%) | 0/360 (0%) | 0/79 (0%) | 0/286 (0%) | 0/50 (0%) | |||||
| X chromosome segregation pattern in oocytes from XO females‡ | ||||||||||
| Intact segregation to one spindle pole | 26 (61.9%) | 11 (68.8%) | 49 (79%) | 48 (61.5%) | ||||||
| Equational segregation of sister chromatids | 16 (38.1%) | 5 (31.3%) | 13 (21%) | 30 (38.5%) | ||||||
| Total | 42 | 16 | 62 | 78 | ||||||
The X chromosome was scored as misaligned only when it was clearly separated from the mass of chromosomes at the spindle equator. 16 of the 63 cells with a misaligned X chromosome also had a misaligned autosomal bivalent or univalent chromosome; however, if cells with a misaligned X chromosome are excluded, the frequency of autosomal misalignment (∼1%) was no different in metaphase oocytes from XO females and controls.
For 10- and 12-h time points, segregation was analyzed in anaphase/telophase I stage oocytes and the equational segregation category includes a small number of anaphase cells exhibiting anaphase lagging of the X chromosome. For 14- and 16-h time points only metaphase II–arrested oocytes were analyzed.
The above frequencies of X chromosome misalignment at metaphase almost certainly underestimate the number of metaphase cells in which the X chromosome made a monopolar attachment and was unable to align at the spindle equator. The large number of chromosomes in the murine complement made it difficult to accurately determine the attachment orientation (i.e., monopolar or bipolar) of the univalent X chromosome even with the aid of confocal microscopy. Hence, we adopted a conservative scoring approach in which the X chromosome was scored as misaligned only when it was clearly separated from the mass of chromosomes at the spindle equator. An X chromosome that appeared to have made a monopolar attachment but was located at the periphery of the chromosome mass was not scored as misaligned using this criteria; at the 8-h time point, ∼15% of the metaphase cells fell into this category. Thus, the actual frequency of monopolar attachment at metaphase I probably significantly exceeds the figures given in Table II.
Segregation of the X chromosome at the first meiotic division was evaluated in a subset of anaphase/telophase I and metaphase II oocytes from XO females (Table II). The univalent X chromosome segregated intact to one spindle pole in 63.7% (37 out of 58) of anaphase/telophase I oocytes and 69.3% (97 out of 140) of metaphase II oocytes. In approximately one-third of remaining oocytes at both stages, the univalent X chromosome divided equationally, segregating one sister chromatid to each spindle pole.
Discussion
Aberrant Chromosome Behavior in Oocytes from XO Females Provides Evidence for Lack of Metaphase/Anaphase Checkpoint Control
The fidelity of cell division is ensured by checkpoint mechanisms that assure that strategic events occurring during one phase of the cell cycle are completed before the next phase of the cycle is initiated. One such mechanism operates at the metaphase/anaphase transition to delay the onset of anaphase in cells with defective spindle formation or chromosome alignment. The imposed delay provides an opportunity for the correction of defects that would predispose to errors in chromosome segregation at anaphase.
Meiotic segregation errors have particularly disastrous consequences, since they result in the production of abnormal gametes. Thus, the metaphase/anaphase checkpoint would be expected to be of particular importance during meiotic cell division. Our studies of oocytes from XO females, however, indicate that chromosome misalignment at metaphase does not induce a delay in anaphase onset. Despite an apparent delay in the congression of the X chromosome during prometaphase and a significant proportion of metaphase cells with a misaligned X chromosome, we found no evidence of an accumulation of cells at metaphase I among oocytes from XO females. In addition, the frequency of metaphase cells with a misaligned X chromosome—the category expected to induce a delay at metaphase—decreased rather than increased over time.
The lack of accumulation of cells at metaphase I has several plausible explanations: (a) murine female meiosis may have evolved a specialized mechanism for the segregation of achiasmate chromosomes at meiosis I; (b) female meiosis may have very stringent checkpoint control which is activated by the univalent X chromosome, but rapid error correction makes the resultant delay in anaphase onset undetectable by the method of analysis used in the present study; (c) female meiosis may have a stringent checkpoint control mechanism that is activated by the univalent X chromosome, but error correction may be inefficient and the checkpoint may be overridden after a finite amount of time; (d) the X chromosome may fail to activate the checkpoint by virtue of its univalent status; or (e) the presence of an unaligned chromosome at metaphase may fail to activate the checkpoint because the mechanism is not functional. As detailed below, we interpret our data as support for the last explanation; i.e., that the checkpoint control mechanism is nonfunctional, and we further hypothesize that the lack of chromosome-mediated checkpoint control is a general feature of mammalian female meiosis.
In most species, recombination ensures the segregation of homologues at the first meiotic division; however, a variety of backup mechanisms have evolved for the segregation of achiasmate chromosomes in species where all or several chromosomes in the genome consistently fail to recombine (39). Most of these mechanisms direct the segregation of achiasmate homologues and are based on some physical attachment. Efficient mechanisms for the segregation of unpaired univalent sex chromosomes have, however, been described in several insect species. These backup mechanisms ensure the intact segregation of the univalent to one spindle pole at the first meiotic division in some species but, in others, direct equational segregation of univalent sex chromosomes at the first meiotic division (for review see reference 39). Our FISH studies using an X chromosome-specific probe suggest that both intact and equational segregation of the univalent X chromosome occur in appreciable frequency in oocytes from XO females, thus arguing against the existence of an efficient backup mechanism for the segregation of the univalent X chromosome.
These segregation data also argue against the hypothesis that the checkpoint is activated but the error rapidly corrected, precluding the detection of the resultant delay in anaphase onset. That is, failure of alignment at metaphase I presumably reflects attachment of the univalent X chromosome to only a single spindle pole. With the onset of anaphase, this configuration should result in intact segregation of the univalent X chromosome to the pole to which it is attached. Thus, a rapid correction mechanism would be expected to virtually abolish the intact (i.e., reductional) segregation pattern. In fact, in our studies, nearly two-thirds of all segregants were of this type, providing no support for the hypothesis that an error correction mechanism exists.
These data also argue against the third explanation; i.e., that the checkpoint is activated, but that after a period of delay the cell escapes the checkpoint and enters anaphase. If this explanation were correct, we might expect a time-dependent change in the pattern of X chromosome segregation. That is, the reductional, but not the equational, segregants would be expected to activate the checkpoint and cause a delay in anaphase onset. Thus, with increasing time in culture, we would expect to observe an increase in the proportion of reductional segregants among anaphase preparations. In fact, the proportion of reductional segregants was no different in anaphase cells from 10- and 16-h cultures, suggesting that no such delay had occurred.
It is possible that the misaligned X chromosome escapes detection by the cell cycle surveillance mechanism by virtue of its univalent status. That is, the proper segregation of homologues at the first meiotic division requires that the kinetochores on sister chromatids behave as a functional unit, and there appears to be some physical constraint on sister kinetochores before anaphase I that facilitates this behavior (for review see reference 30). This constraint is not absolute, as evidenced by the fact that univalent chromosomes can segregate equationally at the first meiotic division. However, the ability of sister kinetochores to act in a coordinate fashion during the first meiotic division suggests that attachment of the univalent X chromosome to a single spindle pole at metaphase I may result in the occupation of all kinetochore domains. Although this configuration would not place the X chromosome kinetochore under tension, it may be sufficient to bypass the checkpoint mechanism. Indeed, in at least one meiotic system, the male grasshopper, the X chromosome appears to be exempt from the normal rules of tension-mediated silencing of the kinetochore signal (30, 32).
Although it is possible that the mammalian X chromosome is similarly excluded from checkpoint surveillance, we think this is unlikely. In mammalian male meiosis, there is indirect but, nevertheless, compelling evidence that a tension-mediated checkpoint mechanism is activated by the presence of a univalent X chromosome. In the XO, sex-reversed (XO, Sxr) male mouse, resulting from the transfer of the testis determining gene, Sry, to the distal tip of the X chromosome, spermatogenesis arrests at meiotic metaphase (21, 37). This phenotype is reminiscent of the male mantid, where the presence of a univalent X chromosome triggers extensive metaphase delay, with resultant spermatocyte degeneration (4, 23). Interestingly, meiotic arrest of the XO, Sxr spermatocytes can be overcome by the addition of a small marker chromosome consisting of a centromere and the pseudoautosomal region (3). This small region of homologous noncoding DNA acts as the normal site of pairing between the X and Y chromosomes during male meiosis, and its presence on a marker chromosome presumably allows the X chromosome and the marker to make a stable bipolar attachment and align at metaphase, thus satisfying the requirements for anaphase onset imposed by the cell cycle checkpoint.
Thus, we conclude that the most likely explanation for our observations is that a chromosome-mediated checkpoint mechanism is nonfunctional in the XO oocyte. The aberrant behavior and segregation of autosomal chromosomes in oocytes from XO females provides further support for this hypothesis: Misaligned chromosomes other than the X chromosome were observed at both the first and second meiotic metaphase. Lack of cell cycle control to detect these errors and delay anaphase onset until they are corrected would be expected to result in a corresponding increase in autosomal aneuploidy and, indeed, previous studies suggest a high frequency of autosomal aneuploidy among preimplantation embryos from XO females (16). The errors in metaphase alignment detected in our meiotic studies provide a mechanism for this high error rate.
A Hypothesis: Lack of Chromosome-mediated Checkpoint Control Is a General Feature of Mammalian Female Meiosis
The lack of chromosome-mediated checkpoint control in oocytes from XO females could: (a) be a meiotic defect unique to the XO female, or (b) reflect a general feature of mammalian female meiosis, detectable in our studies because of the presence of the univalent X chromosome.
Although the rate of cell cycle progression from nuclear envelope breakdown to metaphase I was similar in oocytes from XO females and control siblings, anaphase onset appeared to be slightly accelerated in oocytes from XO females. This effect, which was evident on two different genetic backgrounds, could be interpreted to mean that oocytes from XO females prematurely exit metaphase, while oocytes from XX females experience the normal checkpoint control delay. However, we think that this is unlikely to be the case and, indeed, we favor the hypothesis that the lack of checkpoint control is a characteristic of mammalian female meiosis for several reasons. First, it seems likely that a fundamental difference in the meiotic control process which delays anaphase onset in a proportion of oocytes from one type of female but not the other would alter the shape of the anaphase onset curve. However, although anaphase onset for oocytes from XO females was temporally shifted relative to that for control oocytes, the slopes of the two curves were not different (Fig. 3). The inflection in the control curve at 12 h (Fig. 3), which makes it appear as though a proportion of cells delay at metaphase, may reflect the somewhat smaller sample size at this time point. In any event, a similar deviation has not been observed in previous or subsequent meiotic studies of oocytes from control females (data not shown). Secondly, our immunofluorescence studies provide no evidence that lack of checkpoint control is a unique feature of oocytes from XO females. That is, we analyzed the MI spindle and the chromosome alignment of every metaphase cell represented in Table I. Although a low level of chromosome misalignment (∼1%) was observed among metaphase oocytes from control females, the frequency of such cells decreased over time rather than increased, as would be expected if cells with a misaligned chromosome were delayed at metaphase because of the action of a checkpoint mechanism.
Finally, genetic considerations favor the explanation that lack of checkpoint control is a general feature of mammalian female meiosis. In our studies, XO and control siblings were produced on inbred genetic backgrounds. Thus, if the lack of checkpoint control were unique to the XO female, the only genetic explanation for the difference would be altered X chromosome dosage. However, normal males, like XO females, have only a single X chromosome and, as discussed above, the available evidence suggests that a stringent checkpoint mechanism operates during mammalian male meiosis to prevent anaphase onset in cells with a univalent chromosome. Thus, if the checkpoint control defect is unique to the XO female, it is necessary to postulate that the checkpoint control mechanism is controlled either by different genes in males and females—which seems unlikely—or by an X-linked gene that is required in double dose during female meiosis and that has a Y-linked homologue that functions during male meiosis. However, meiotic studies of oocytes from XY females with structurally normal sex chromosomes indicate that the meiotic process in these females, like XO females, lacks chromosome mediated checkpoint control (Hunt, P. unpublished data).
Thus, we think the simplest explanation for our data is that female meiosis lacks chromosome-mediated checkpoint control. Although the reason for the slight acceleration in anaphase onset among oocytes from XO females remains unclear, it is important to note that X chromosome dosage is altered in the ovarian soma as well as the oocyte in the XO female, hence a somatic effect cannot be excluded. Experiments are currently in progress to determine if the altered cell cycle kinetics that we have observed are somatically mediated or oocyte intrinsic.
Lack of Chromosome-mediated Checkpoint Control Provides an Explanation for the High Meiotic Error Rate in Our Own Species
Given the role of the metaphase/anaphase checkpoint in ensuring proper chromosome segregation, the lack of this important control mechanism during female meiosis is surprising. Indeed, the most plausible explanation is that the cell cycle control machinery does indeed exist, but is operationally impaired because of the excessive volume of the mammalian oocyte. Studies using the Xenopus oocyte extract system developed by Murray (34) have demonstrated that activation of the metaphase/anaphase checkpoint in response to microtubule inhibiting drugs is dependent upon the ratio of extract volume to chromatin content (27). Similarly, in sea urchin embryos, during the first few embryonic cleavage divisions when blastomere volume is still large, errors in chromosome alignment do not induce metaphase delay (36).
Indeed, just as chiasmata ensure homologue segregation in a broad range of species, the lack of chromosome-mediated checkpoint control may be a general feature of oogenesis. In Drosophila, tension-mediated meiotic control works backwards in oogenesis: tension prevents the progression into anaphase. In the absence of functional chiasmata, the metaphase I arrest which characterizes Drosophila oogenesis is abolished and the cell proceeds directly into anaphase (19). Despite the fact that tension is utilized in a fundamentally different way, Drosophila oogenesis also apparently lacks chromosome-mediated checkpoint control, since meiosis proceeds without arrest despite the presence of monooriented or misaligned chromosomes (19, 26).
The lack of chromosome-mediated checkpoint control imposed by the metaphase/anaphase checkpoint would be predicted to result in an elevated meiotic error rate in female meiosis by comparison with male meiosis. In our own species this is indeed the case. An estimated 20% of human conceptions are chromosomally abnormal and virtually all errors are maternal in origin (13, 18). Similarly, in the mouse, the available data suggest that the error rate is substantially higher in oogenesis than spermatogenesis (1).
Although our results provide an important explanation for the high meiotic error rate in our own species, a number of important questions remain. For example, in human female meiosis, the error rate is strongly influenced by maternal age. The basis of the maternal age effect remains to be elucidated. Our results, however, suggest that any age-related changes that even slightly disrupt spindle formation and chromosome alignment could dramatically increase chromosome segregation errors. Similarly, the possibility that metaphase/anaphase checkpoint control may be nonfunctional during early embryonic cleavage divisions deserves attention since it suggests that the early cleavage divisions of the human embryo are also vulnerable to error. This has important clinical implications. It could provide an explanation for the phenomenon of confined placental mosaicism and associated intrauterine growth retardation in human conceptions (20). Moreover, with the advent of human assisted reproductive technology, early cleavage development for a proportion of human conceptions takes place in vitro. Thus, the possibility that the spindle integrity and/or chromosome alignment could be compromised by minor alterations in the culture environment must be carefully evaluated.
Acknowledgments
We wish to thank T. Hassold, S. Hawley, and B. Nicklas for helpful discussions and comments on the manuscript, and E. Baart for technical assistance.
Abbreviations used in this paper
- FISH
fluorescence in situ hybridization
- NGS
normal goat serum
Footnotes
The work was supported by grants R01 HD31866 from the National Institutes of Health and FY96-0621 from the March of Dimes to P.A. Hunt.
Address all correspondence to Patricia A. Hunt, Department of Genetics, School of Medicine, Case Western Reserve University, 10900 Euclid Ave., Cleveland, OH 44106-4955. Tel.: (216) 368-3458. Fax: (216) 368-3432. E-mail: pah13@po.cwru.edu
References
- 1.Bond, D.J., and A.C. Chandley. 1983. Aneuploidy. In Oxford Monographs on Medical Genetics. Vol. 11. Oxford University Press, New York.
- 2.Burgoyne, P.S., and S.K. Mahadevaiah. 1993. Unpaired sex chromosomes and gametogenic failure. In Chromosomes Today. Vol. 11. A.T. Sumner and A.C. Chandley, editors. Chapman and Hall, London. 243–263.
- 3.Burgoyne PS, Mahadevaiah SK, Sutcliffe MJ, Palmer SJ. Fertility in mice requires X-Y pairing and a Y-chromosomal “spermiogenesis” gene mapping to the long arm. Cell. 1992;71:391–398. doi: 10.1016/0092-8674(92)90509-b. [DOI] [PubMed] [Google Scholar]
- 4.Callan HG, Jacobs PA. The meiotic process in Mantis reglidiosaL. males. J Genet. 1957;55:200–217. [Google Scholar]
- 5.Campbell MS, Gorbsky GJ. Microinjection of mitotic cells with the 3F3/2 anti-phosphoepitope antibody delays the onset of anaphase. J Cell Biol. 1995;129:1195–1204. doi: 10.1083/jcb.129.5.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carpenter A TC. Chiasma function. Cell. 1994;77:959–962. doi: 10.1016/0092-8674(94)90434-0. [DOI] [PubMed] [Google Scholar]
- 7.Darlington CD. Misdivision and the genetics of the centromere. J Genet. 1939;37:341–363. [Google Scholar]
- 8.Disteche CM, Candy SL, Aldre DA. Translocation and amplification of an X-chromosome DNA repeat in inbred strains of mice. Nuc Acids Res. 1987;15:4393–4401. doi: 10.1093/nar/15.11.4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eicher EM, Hale DW, Hunt PA, Lee BK, Tucker PK, King TR, Eppig JT, Washburn LL. The mouse Y* chromosome involves a complex rearrangement including interstitial positioning of the Y-pseudoautosomal region. Cytogenet Cell Genet. 1991;57:221–230. doi: 10.1159/000133152. [DOI] [PubMed] [Google Scholar]
- 10.Eicher EM, Washburn LL. Assignment of genes to regions of mouse chromosomes. Proc Natl Acad Sci USA. 1978;75:946–950. doi: 10.1073/pnas.75.2.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gorbsky GJ. Kinetochores, microtubules and the metaphase checkpoint. Trends Cell Biol. 1995;5:143–148. doi: 10.1016/s0962-8924(00)88968-0. [DOI] [PubMed] [Google Scholar]
- 12.Gorbsky GJ, Ricketts WA. Differential expression of a phosphoepitope at the kinetochores of moving chromosomes. J Cell Biol. 1993;122:1311–1321. doi: 10.1083/jcb.122.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hassold T, Hunt PA, Sherman S. Trisomy in humans: incidence, origin and etiology. Curr Opin Genet Dev. 1993;3:398–403. doi: 10.1016/0959-437x(93)90111-2. [DOI] [PubMed] [Google Scholar]
- 14.Hawley, R.S. 1988. Exchange and chromosome segregation in eukaryotes. In Genetic Recombination. R. Kucherlapati and G. Smith, editors. American Society of Microbiology, Washington, D.C. pp. 497–525.
- 15.Henderson SA, Koch CA. Co-orientation stability by physical tension: a demonstration with experimentally interlocked bivalents. Chromosoma (Berl) 1970;29:207–216. doi: 10.1007/BF00326079. [DOI] [PubMed] [Google Scholar]
- 16.Hunt PA. Survival of XO mouse fetuses: effect of parental origin of the X chromosome or uterine environment. Development (Camb) 1991;111:1137–1141. doi: 10.1242/dev.111.4.1137. [DOI] [PubMed] [Google Scholar]
- 17.Hunt PA, LeMaire R, Embury P, Mroz K, Sheean L. Analysis of chromosome behavior in intact mammalian oocytes: monitoring the segregation of a univalent chromosome during mammalian female meiosis. Hum Mol Genet. 1995;4:2007–2012. doi: 10.1093/hmg/4.11.2007. [DOI] [PubMed] [Google Scholar]
- 18.Jacobs PA. The chromosome complement of human gametes. Oxford Rev Reprod Biol. 1992;14:48–72. [PubMed] [Google Scholar]
- 19.Jang JK, Messina L, Erdman MB, Arbel T, Hawley RS. Induction of metaphase arrest in Drosophilaoocytes by chiasma-based kinetochore tension. Science. 1995;268:1917–1919. doi: 10.1126/science.7604267. [DOI] [PubMed] [Google Scholar]
- 20.Kalousek DK. Current topic: Confined placental mosaicism and intrauterine fetal development. Placenta. 1994;15:219–230. doi: 10.1016/0143-4004(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 21.Kot MC, Handel MA. Spermatogenesis in XO, Sxr mice: role of the Y chromosome. J Exp Zool. 1991;256:92–105. doi: 10.1002/jez.1402560112. [DOI] [PubMed] [Google Scholar]
- 22.Lane PW, Davisson MT. Patchy Fur (Paf), a semidominant X-linked gene associated with high level of X-Y nondisjunction in male mice. J Hered. 1990;81:43–50. doi: 10.1093/oxfordjournals.jhered.a110923. [DOI] [PubMed] [Google Scholar]
- 23.Li X, Nicklas RB. Mitotic forces control a cell-cycle checkpoint. Nature. 1995;373:630–632. doi: 10.1038/373630a0. [DOI] [PubMed] [Google Scholar]
- 24.Maguire MP. Meiotic behavior of a tiny fragment chromosome that carries a transposed centromere. Genome. 1987;29:744–746. doi: 10.1139/g87-126. [DOI] [PubMed] [Google Scholar]
- 25.McIntosh JR. Structural and mechanical control of mitotic progression. Cold Spring Harbor Symp Quant Biol. 1991;56:613–619. doi: 10.1101/sqb.1991.056.01.070. [DOI] [PubMed] [Google Scholar]
- 26.McKim KS, Hawley RS. Chromosomal control of meiotic cell division. Science. 1995;270:1595–1601. doi: 10.1126/science.270.5242.1595. [DOI] [PubMed] [Google Scholar]
- 27.Minshull J, Sun H, Tonks NK, Murray AW. A MAP kinase-dependent spindle assembly checkpoint in Xenopusegg extracts. Cell. 1994;79:475–486. doi: 10.1016/0092-8674(94)90256-9. [DOI] [PubMed] [Google Scholar]
- 28.Murray AW. Tense spindles can relax. Nature. 1995;373:560–561. doi: 10.1038/373560a0. [DOI] [PubMed] [Google Scholar]
- 29.Nicklas RB. Chromosome segregation mechanisms. Genet. 1974;78:205–213. doi: 10.1093/genetics/78.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicklas RB. How cells get the right chromosomes. Science. 1997;275:632–636. doi: 10.1126/science.275.5300.632. [DOI] [PubMed] [Google Scholar]
- 31.Nicklas RB, Kock CA. Chromosome micromanipulation. III. Spindle fiber tension and the reorientation of mal-orientated chromosomes. J Cell Biol. 1969;43:40–50. doi: 10.1083/jcb.43.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nicklas RB, Ward SC, Gorbsky GJ. Kinetochore chemistry is sensitive to tension and may link mitotic forces to a cell cycle checkpoint. J Cell Biol. 1995;130:929–939. doi: 10.1083/jcb.130.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rieder CL, Schultz A, Cole R, Sluder G. Anaphase onset in vertebrate somatic cells is controlled by a checkpoint that monitors sister kinetochore attachment to the spindle. J Cell Biol. 1994;127:1301–1310. doi: 10.1083/jcb.127.5.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shamu CE, Murray AW. Sister chromatid separation in frog egg extracts requires DNA topoisomerase II activity during sister chromatid separation. J Cell Biol. 1992;117:921–934. doi: 10.1083/jcb.117.5.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Simchen G, Hugerat Y. What determines whether chromosomes segregate reductionally or equationally in meiosis? . Bioessays. 1993;15:1–8. doi: 10.1002/bies.950150102. [DOI] [PubMed] [Google Scholar]
- 36.Sluder G, Miller FJ, Thompson EA, Wolf DE. Feedback control of the metaphase–anaphase transition in sea urchin zygotes: role of maloriented chromosomes. J Cell Biol. 1994;126:189–198. doi: 10.1083/jcb.126.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sutcliffe MJ, Darling SM, Burgoyne PS. Spermatogenesis in XY, Sxra, and XOSxramice: a quantitative analysis of spermatogenesis throughout puberty. Mol Reprod Dev. 1991;30:81–89. doi: 10.1002/mrd.1080300202. [DOI] [PubMed] [Google Scholar]
- 38.Wells WAE, Murray AW. Aberrantly segregating centromeres activate the spindle assembly checkpoint in budding yeast. J Cell Biol. 1996;133:75–84. doi: 10.1083/jcb.133.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolf KW. How meiotic cells deal with non-exchange chromosomes. Bioessays. 1993;16:107–114. doi: 10.1002/bies.950160207. [DOI] [PubMed] [Google Scholar]