Abstract
Matrix metalloproteinases (MMPs) regulate ductal morphogenesis, apoptosis, and neoplastic progression in mammary epithelial cells. To elucidate the direct effects of MMPs on mammary epithelium, we generated functionally normal cells expressing an inducible autoactivating stromelysin-1 (SL-1) transgene. Induction of SL-1 expression resulted in cleavage of E-cadherin, and triggered progressive phenotypic conversion characterized by disappearance of E-cadherin and catenins from cell–cell contacts, downregulation of cytokeratins, upregulation of vimentin, induction of keratinocyte growth factor expression and activation, and upregulation of endogenous MMPs. Cells expressing SL-1 were unable to undergo lactogenic differentiation and became invasive. Once initiated, this phenotypic conversion was essentially stable, and progressed even in the absence of continued SL-1 expression. These observations demonstrate that inappropriate expression of SL-1 initiates a cascade of events that may represent a coordinated program leading to loss of the differentiated epithelial phenotype and gain of some characteristics of tumor cells. Our data provide novel insights into how MMPs function in development and neoplastic conversion.
The extracellular matrix (ECM)1 microenvironment of cells is critical for determination of cellular behavior and differentiation (for review see Adams and Watt, 1993; Lochter and Bissell, 1995; Ashkenas et al., 1996). Matrix metalloproteinases (MMPs) modify the cellular microenvironment thereby playing important roles during development and tumor progression. Experimental manipulation of net MMP activity can alter signaling through ECM receptors with dramatic effects on cell migration, invasion, apoptosis, neurite extension, epithelial branching morphogenesis, and expression of tissue-specific genes (for review see MacDougall and Matrisian, 1995; Coussens and Werb, 1996). Although most MMPs are secreted extracellularly, cell surface–bound MMPs catalyze focal dissolution of ECM (Monsky et al., 1993; for review see Basbaum and Werb, 1996; Brooks et al., 1996). A wide variety of ECM proteins and certain cell surface molecules are substrates for MMPs (Gearing et al., 1994; Ochieng et al., 1994; Levi et al., 1996; Preece et al., 1996). Whereas MMPs are thought to shape cellular function by remodeling ECM, targeting of cell surface molecules may also play a role in their mode of action, although the latter possibility has not been elucidated.
To understand MMP function in mammary epithelial cells, we have focused on stromelysin-1 (SL-1), because of its diverse molecular targets (Lochter and Bissell, 1997). In the normal mammary gland, SL-1 is synthesized by fibroblasts but not by epithelial cells (Witty et al., 1995). Although SL-1 is produced at all stages of postnatal mammary gland development, its expression is highest during involution (Talhouk et al., 1992; Li et al., 1994; Witty et al., 1995). Intermediate levels of SL-1 are associated with phases of ductal branching and alveolar morphogenesis. The lowest level of SL-1 expression is found in the resting mammary gland. Targeting of an autoactivating mutant of SL-1 to mammary epithelia in vivo increases branching morphogenesis during puberty and impairs lactogenic differentiation during pregnancy (Sympson et al., 1994). Later in life, SL-1 transgenic mice develop preneoplastic lesions and invasive breast tumors (Sympson et al., 1995). In culture, overexpression of SL-1 in a mammary cell strain that contains both epithelial and mesenchymal cell types produces an involutionlike phenotype with induction of apoptosis (Boudreau et al., 1995). Furthermore, SL-1 is used by mammary tumor cells for invasion (Lochter et al., 1997) and has been cloned a number of times as a metastasis-specific gene (for review see MacDougall and Matrisian, 1995; Coussens and Werb, 1996). But how expression of SL-1 could lead to such diverse consequences has remained undetermined. In this study, we have transfected the functionally normal mouse mammary epithelial cell line SCp2 (Desprez et al., 1993) with a cDNA coding for an autoactivating rat SL-1 transgene under the control of a tetracycline (Tet)-regulated promoter. We describe the resulting cascade of molecular and functional alterations in the absence of a stromal component.
Materials and Methods
Generation of Expression Vector for Epitope-tagged SL-1 and Transfection of SCp2 Cells
HA.11, a hemagglutinin epitope tag comprising the amino acid sequence YDVPDYA and the nucleotide sequence TACGACGTACCAGACTACGCA was added to the 3′ end of the cDNA coding for the autoactivating M1 mutant (Val92–Gly92 mutation) (Sanchez-Lopez et al., 1988; Sympson et al., 1994) of the rat SL-1 cDNA (SL-1:M1) by PCR. The PCR product generated with the primers GCAACAGCTGGTTTAATTGT (5′ primer) and CCTTCCATGGATCTTCTTATGCGTAGTCTGGTACGTCGTAACAATTAAACCAGCTG (3′ primer) was ligated into the NcoI sites of SL-1:M1 at positions 1,301 and 1,492 in CA10:SL-1:M1 (Sympson et al., 1994). The tagged SL-1:M1 was excised from CA10:SL-1:M1 and inserted into the EcoRI sites of the pTet-Splice expression vector (Life Technologies, Gaithersburg, MD). The final vector (pTet-Splice-SL-1:M1-tag) was used for transfection of SCp2 cells together with the pTet.tTAk vector (Life Technologies) coding for the tTAK activator protein and a vector containing a neomycin cassette (SV40neo) (Schmidhauser et al., 1992). Transfection of SCp2 cells was then carried out with lipofectin reagent (Life Technologies) according to the manufacturer's instruction. 1.2 × 106 cells were incubated for 24 h in 5 ml OptiMEM (Life Technologies) containing 40 μl lipofectin, 5 μg pTet-Splice-SL-1:M1-tag, 5 μg pTet. tTAK, and 0.5 μg SV40neo. Subsequently, the medium was replaced with serum-containing medium with 4.8 μM Tet (Sigma Chemical Co., St. Louis, MO). 2 d after transfection, cells were selected by addition of 200 μg/ml Geneticin (Life Technologies) to the culture medium. Surviving individual colonies were expanded and screened for induction of the tagged SL-1 transgene after omission of Tet from the culture medium.
Cell Culture
SCp2 cells and the mouse mammary carcinoma cell line SCg6 (Desprez et al., 1993; Lochter et al., 1977) were routinely maintained and passaged in serum-containing medium as described (Desprez et al., 1993). Selected clonal cell lines p2S6, p2S7, and p2S10, which inducibly expressed SL-1, and the cell line p2C, which was established in the same manner as the p2S cell lines but did not express the SL-1 transgene, were routinely grown in serum-containing medium with 100 μg/ml Geneticin and 4.8 μM Tet to maintain selection pressure and to silence transgene expression. The same medium, but without Tet, was also used for long-term induction of SL-1 for longer than 13 d.
All other experiments were performed in chemically defined medium consisting of DME/F12, 5 μg/ml insulin, 5 μg/ml transferrin, 5 ng/ml selenium (added as ITS medium supplement; Sigma Chemical Co.), and 50 μg/ml gentamicin with or without 4.8 μM Tet. In some experiments, a reconstituted basement membrane (Matrigel; Collaborative Biomedical Products, Bedford, MA) was added at a final concentration of 150–200 μg/ml (Streuli et al., 1995). To study milk protein expression, Matrigel and the lactogenic hormones prolactin (3 μg/ml; Sigma Chemical Co.), and hydrocortisone (1 μg/ml; Sigma Chemical Co.) were added for 6 or 7 d. The hydroxamic acid MMP inhibitor 3-(N-hydroxycarbamoyl)-2(R)-isobutylpropionyl-l-tryptophan methylamide (GM6001) and an inactive structural homologue (N-tert-butyloxycarbony)-l-leucine-l-tryptophan (GM1210) (gifts from Dr. R. Galardy, Glycomed Corporation, Alameda, CA) (Grobelny et al., 1992) were dissolved at a concentration of 100 mM in DMSO and added to the culture medium at a final concentration of 100 μM. GM6001 is a general inhibitor of all MMPs with dissociation constant of enzyme–inhibitor complexes (K i) values <100 nM (Grobelny et al., 1992; Gijbels et al., 1994).
Conditioned medium was generated by incubating subconfluent cultures in 10-cm dishes with 5 ml of chemically defined medium for 2 d. Conditioned medium was then mixed at a 1:1 ratio with fresh culture medium before addition to SCp2 cells.
For indirect immunofluorescence, cells were plated at a density of 30,000 cells/cm2 onto poly-l-lysine–treated glass coverslips as described (Lochter et al., 1997).
For coculture of SCp2 and p2S cells, both cell types were mixed at a 1:1 ratio, plated at a density of 30,000 cells/cm2 onto poly-l-lysine–coated glass coverslips and maintained for 6 d in chemically defined medium. Before mixing the two cell types, SCp2 cells were labeled for 50 min at 37°C with 40 μg/ml of the lipophilic, red fluorescent dye, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiI; Molecular Probes, Eugene, OR) in serum-containing medium with 1.6% ethanol as described (Honig and Hume, 1986).
Invasion assays were performed for 2–4 d in modified Boyden chambers (Collaborative Biomedical Products) coated with Matrigel as described (Lochter et al., 1997).
Analysis of Cellular Morphology and Indirect Immunofluorescence
For analysis of cellular morphology, cells were maintained for 12 d on glass coverslips, fixed, and then stained with toluidine blue as described (Lochter et al., 1991).
For indirect immunofluorescence, cells cultured on glass coverslips were fixed with methanol/acetone (1:1) for 15 min at −20°C, washed twice with PBS, and then blocked by incubation with 15% FBS in PBS for 1 h at ambient temperature. Cells were then reacted for 1 h at ambient temperature with mouse monoclonal antibody against E-cadherin (C20820) (1:100 dilution; Transduction Laboratories, Lexington, KY), mouse monoclonal antibody against β-catenin (1:100 dilution; Transduction Laboratories), mouse monoclonal antibody against vimentin (1:200 dilution; Sigma Chemical Co.), or rabbit polyclonal antibody cocktail against cytokeratins (1:20 dilution; Dako Corp., Carpintera, CA) in 15% FBS in PBS. After three washes with PBS, cells were incubated at ambient temperature for 30 min with Texas red–conjugated, goat anti–mouse IgG (Caltag Laboratories, Burlingame, CA) or donkey anti–rabbit IgG (Amersham Corp., Arlington Heights, IL) antibodies, or FITC-conjugated, goat anti–mouse IgG (Caltag Laboratories), or donkey anti–rabbit IgG (Amersham Corp.) antibodies, all at a 1:100 dilution in 15% FBS in PBS. Cells were then washed twice with PBS, incubated with 4′,6-diamidino-2-phenylindole (DAPI) for 1 min to visualize nuclei, and washed two more times with PBS and once with water before mounting with Vectashield (Vector Laboratories, Inc., Burlingame, CA).
For immunofluorescence analysis of cocultured SCp2 and p2S cells, cells were permeabilized for 30 s with digitonin and fixed as described (Crowley and Horwitz, 1995). This procedure insured that cells were permeabilized without removing DiI from cell membranes. Subsequent immunostaining was performed as described above.
Zymograms, Immunoblots, and Immunoprecipitations
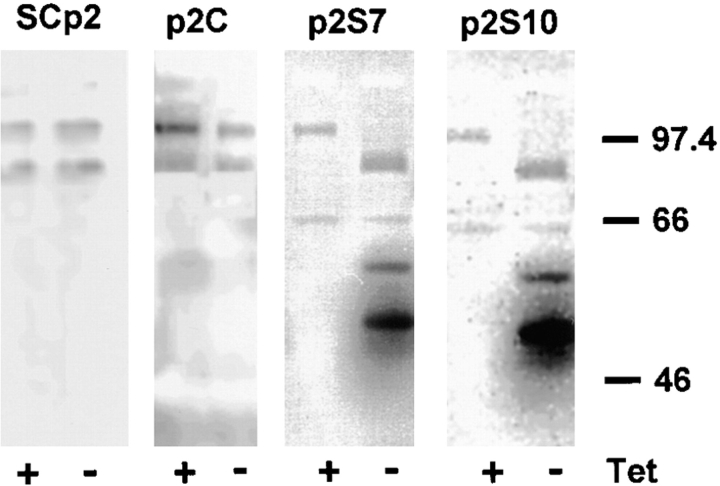
For zymograms on conditioned medium, subconfluent cells maintained in 10-cm dishes were preinduced to express SL-1 for 1 d. 5 ml of chemically defined medium was then added to the cells, collected 2 d later, and concentrated 20-fold with Centricon filter units (Amicon Corp., Danvers, MA) according to the manufacturer's instructions. Casein and gelatin zymography was subsequently performed as described (Fisher and Werb, 1995; Lochter et al., 1997). Identification of all gelatinolytic bands shown in Fig. 7 as MMPs (not shown) was achieved by incubation of gels with 1,10-phenanthroline and GM6001 (Fisher and Werb, 1995).
Figure 7.
Activation and induction of endogenous MMPs by SL-1. Negative images of gelatin zymograms of medium conditioned by SCp2, p2C, p2S7, and p2S10 cells maintained for 3 d in the presence (+) or absence (−) of Tet. Positions of molecular mass markers are indicated.
For immunoblots, cells grown in 10-cm dishes were lysed with radioimmunoprecipitation assay (RIPA) buffer (30 mM NaCl, 50 mM Tris-HCl, pH 7.4, 5 mM ethylenediamine tetraacetic acid, 1% NP-40, 1% deoxycholic acid, 0.1% SDS) containing 20 μg/ml aprotinin (Sigma Chemical Co.) and 1 mM 4-(2-aminoethyl)benzenesulonylfluoride (Calbiochem-Novabiochem Corp., La Jolla, CA). Extraction of cells with Triton X-100 was performed as described (Näthke et al., 1994). Samples containing 10 μg of protein were separated on 8% SDS–polyacrylamide slab gels under reducing conditions (Laemmli, 1970) and transferred onto Immobilon-P membranes (Amersham Corp.). Membranes were then blocked for 1 h with TBST (50 mM Tris, pH 7.5, 100 mM NaCl, 0.1% Tween 20) containing 5% skim milk powder, and then incubated for 1 h at ambient temperature with monoclonal antibody against E-cadherin (C20820; 1:5,000 dilution) or β-catenin (1:5,000 dilution) in the same solution. Blots were then washed with TBST and treated with horseradish peroxidase–conjugated, sheep anti–mouse IgG antibodies (Amersham Corp.) in 5% skim milk powder in TBST. Bands were visualized with an enhanced chemiluminescence detection kit (Amersham Corp.) according to the manufacturer's instructions. For immunoblots for detection of β-casein, RIPA extracts from an equivalent of 105 cells were resolved on 12% SDS–polyacrylamide gels and 4% BSA was used as a blocking reagent. β-casein was detected with a rabbit polyclonal antibody against mouse milk proteins followed by horseradish peroxidase–conjugated, donkey anti–rabbit IgG antibodies (1:1,500 dilution; Amersham Corp.). Immunoblots with mouse monoclonal antibody HA.11 directed against the hemagglutinin epitope tag (1:50 dilution, BAbCO, Richmond, CA) were performed on medium conditioned for 1 or 2 d by an equivalent of 106 cells.
Immunoprecipitations on cell lysates were carried out with cells that had been metabolically labeled for 16 h with 200 μCi [35S]methionine (Amersham Corp.) per ml culture medium. For biotinylation of cell surface proteins, cells were washed three times with PBS and once with Hepes-buffered saline (135 mM NaCl, 5 mM KCl, 0.5 mM Na2HPO4, 10 mM Hepes, pH 7.5) after radiolabeling. Cells were then incubated for 1 h at 4°C with 100 μg/ml Sulfo-NHS-Biotin (Pierce Chemical Co., Rockford, IL) in Hepes-buffered saline. Radiolabeled cells that were or were not cell surface biotinylated were washed three times with chemically defined medium, and then lysed in NP-40 lysis buffer (150 mM NaCl, 50 mM Tris, pH 7.5, 1% NP-40). Antibodies to integrin subunits β1, β4, and α6 (all from PharMingen, San Diego, CA), antibody to the extracellular domain of E-cadherin (ECCD-2) (a gift from Dr. Takeichi, Kyoto University, Kyoto, Japan [Shirayoshi et al., 1986]), and antibody C20820 to the cytoplasmic domain (Transduction Laboratories) were added to cell lysates at a final concentration of 10 μg/ml, and then incubated overnight at 4°C. Subsequently, protein G–agarose (Sigma Chemical Co.) was added and incubation was carried out for 1 h at 4°C. Protein G–agarose beads were washed three times with NP-40 lysis buffer and twice with 50 mM Tris, pH 7.5. Immunoprecipitants were separated on 5% SDS–polyacrylamide gels under nonreducing conditions for integrins, or on 8% SDS–polyacrylamide gels under reducing conditions for E-cadherin. Gels were either dried and processed for autoradiography, or blotted onto Immobilon-P membranes, blocked with 5% skim milk in TBST, and then incubated with peroxidase-conjugated streptavidin (1:100 dilution; Amersham Corp.) before detection of biotinylated proteins with the ECL detection system. For detection of E-cadherin cleavage products released from SCp2 cells by conditioned medium from p2S cells, SCp2 cells were incubated with conditioned medium for 12 h at 37°C. The culture medium was then supplemented with BSA at a final concentration of 1 mg/ml and processed for immunoprecipitation as described above.
ELISA
Cells were plated at a density of 6,000 cells per well into 96-well plates. After designated culture periods, cells were fixed with methanol/acetone (1:1) for 15 min at −20°C and washed twice with PBS. Plates were then incubated for 1 h at ambient temperature with 15% FBS in PBS and subsequently for 1 h at ambient temperature with polyclonal antibody against cytokeratins (1:1,000 dilution), mouse monoclonal antibody against E-cadherin (1:5,000 dilution; C20820), mouse monoclonal antibody against β-catenin (1:5,000 dilution), or mouse monoclonal antibody against γ-catenin (1: 5,000 dilution; Transduction Laboratories). After three washes with PBS, plates were incubated for 1 h at ambient temperature with horseradish peroxidase–conjugated, donkey anti–rabbit IgG antibodies (1:2,500 dilution; Amersham Corp.) or sheep anti–mouse IgG antibodies (1:2,500 dilution; Amersham Corp.) in PBS containing 15% FBS. Plates were then washed six times with PBS and developed with 100 μl/well 1 mg/ml azino-bis-ethylbenzthiazolinesulfonic acid, and 0.1 μl/ml H2O2 in 100 mM sodium acetate, 50 mM sodium phosphate, pH 4.2, for 30 min at 37°C. Absorbance was measured at 405 nm.
Reverse Transcription (RT)-PCR
Extraction of total RNA and RT-PCR were performed exactly as described (Lochter et al., 1997), except that all primers were annealed at 50°C. GCCGAGGCCATGGTGCTACA was used as 5′ primer and TCAGCCCCATCTGGATTGCG as 3′ primer for amplification of hepatocyte growth factor (HGF) mRNA; ATAAGGGTGAGAAGACTGTTCTGTCGCAC was used as 5′ primer and TCCGCTGTGTGTCCATTTAG as 3′ primer for amplification of keratinocyte growth factor (KGF) mRNA; and TGCAATGCAGACGTAGTGATC was used as 5′ primer, and TGACCACGCGCAAGAACCA as 3′ primer for amplification of tissue inhibitor of metalloproteinases-2 (TIMP2) mRNA. 40 cycles of amplification were performed for detection of HGF and KGF and 27 cycles for detection of TIMP2. To verify the identity of amplified sequences, Southern hybridizations were performed as described (Lochter et al., 1997), with oligodeoxynucleotides complementary to the mRNA sequence of the gene examined: AGGGACATCATGCTCATTCACAGCACTGTG for HGF, ATAAGGGTGAGAAGACTGTTCTGTCGCACC for KGF, and TTGCACTCACAGCCCATCTGGTACCTGTGG for TIMP2.
Results
SL-1 Expression Leads to Loss of Epithelial Function and Establishment of Scattered Morphology and Invasive Behavior
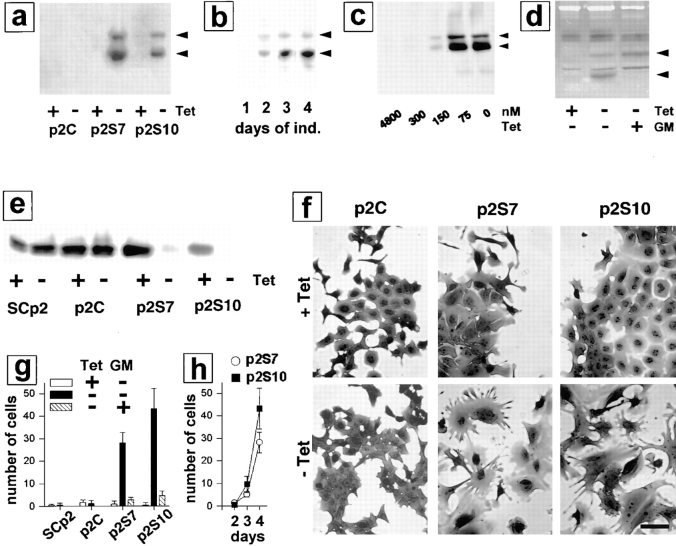
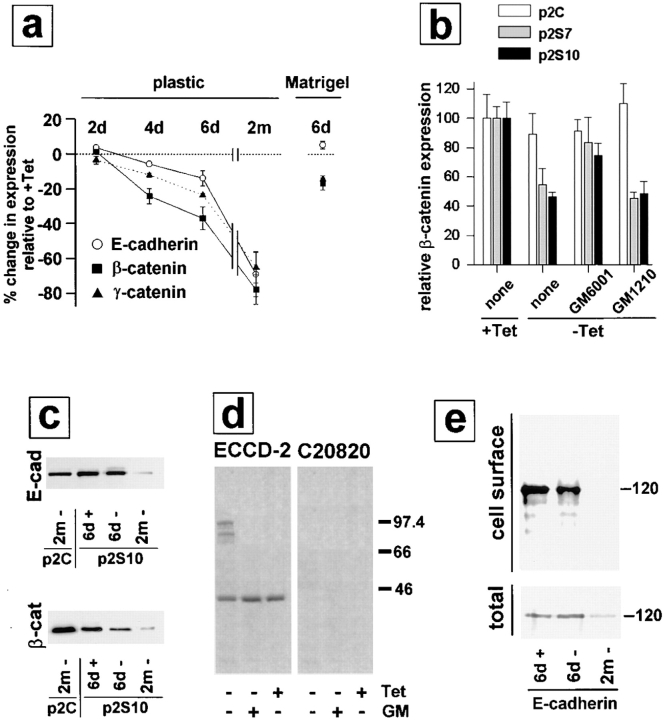
To determine the effects of MMPs on mammary epithelial cells, we used a rat SL-1 cDNA with a Val92–Gly92 mutation that produces autoactivation of the SL-1 proenzyme (Sanchez-Lopez et al., 1988). In this way, SL-1 activity is not dependent on any endogenous cellular activation of the protease. To aid in detection of the transgene, a 7–amino acid epitope tag from the influenza virus hemagglutinin protein was added at the 3′ end of the SL-1 cDNA, which was subcloned into an eukaryotic expression vector under the control of a Tet-regulated promoter (Shockett et al., 1995). The SL-1 construct was transfected into the functionally normal, untransformed mouse mammary epithelial cell line SCp2 (Desprez et al., 1993; Lochter et al., 1997), and maintained in the presence of 4.8 μM Tet, which silences expression of the transgene (Shockett et al., 1995). Three cell clones, p2S6, p2S7, and p2S10 (referred to collectively as p2S cells) that inducibly expressed SL-1 upon omission of Tet from the culture medium were selected (Fig. 1 a). Results presented in this study were similar for the three cell clones. To be concise, only data on p2S7 and p2S10 cells are shown. A fourth cell line, p2C, which was derived from SCp2 cells by the same transfection and selection procedure as p2S cells but did not express detectable amounts of SL-1 after omission of Tet from the culture medium (Fig. 1 a), and the parental SCp2 cells were used as negative controls. Whereas little SL-1 was secreted by p2S cells 2 d after SL-1 induction (Fig. 1 b), significant secretion of SL-1 into the culture medium was detectable by 3 d after removal of Tet. The overall pattern of secreted proteins did not change appreciably when SL-1 was induced for 6 d (not shown), indicating that omission of Tet from the culture medium and expression of the SL-1 transgene did not interfere with overall protein production, and did not lead to random proteolytic cleavage of secreted proteins. As determined by casein zymography, the amount of SL-1 secreted by p2S cells 3 d after induction was similar to the amount of SL-1 secreted by mouse mammary carcinoma cell lines (Lochter et al., 1997), and by fibroblasts prepared from mouse mammary glands of 10-wk-old virgin mice (data not shown), suggesting that the expression level of the SL-1 transgene in p2S cells is physiologically relevant. As little as 300 nM Tet completely repressed expression of SL-1 (Fig. 1 c). Once Tet was removed, activation, but not expression of SL-1 proenzyme in p2S cells was prevented by addition of the peptide hydroxamic acid MMP inhibitor GM6001 to the culture medium (Fig. 1 d). All experiments were done in chemically defined medium. Under these conditions, induction of SL-1 transgene expression did not affect cell number or survival, as determined in three independent experiments by cell counts, bromodeoxyuridine incorporation and terminal deoxynucleotidyl transferase–mediated dUTP nick end labeling (TUNEL) analysis (not shown).
Figure 1.
Effects of SL-1 on cell scattering, lactogenic differentiation, and invasion of mammary epithelial cells. (a) P2C, p2S7, and p2S10 cells were maintained for 3 d in the presence (+) or absence (−) of Tet. Medium conditioned by cells for 2 d was analyzed for expression of the SL-1 transgene by immunoblotting with a monoclonal antibody against the HA.11 epitope tag. Both latent SL-1 proenzyme (61 kD, top arrowhead; also in b–d) and active SL-1 (49 kD, bottom arrowhead; also in b–d) could be detected in conditioned medium from p2S7 and p2S10 cells, but not p2C cells. (b) Medium conditioned by p2S10 cells for 1 d was analyzed for the presence of SL-1 protein 1, 2, 3, and 4 d after omission of Tet from the culture medium (days of ind.) by immunoblotting with antibodies against the HA.11 epitope tag. (c) p2S10 cells were maintained for 3 d in the presence of increasing concentrations of Tet. Medium conditioned by cells for 2 d was analyzed for expression of the SL-1 transgene by immunoblotting with antibodies against the HA.11 epitope tag: 300 nM Tet was sufficient to completely inhibit SL-1 induction in p2S10 cells. (d) p2S10 cells were maintained for 3 d in the presence (+) or absence (−) of Tet and in the presence (+) or absence (−) of GM6001 (GM). Medium conditioned by cells for 2 d was analyzed for expression and activation of the SL-1 transgene by casein substrate gel zymography. Zymograms are shown as negative images. Note that activation, but not production of SL-1 is inhibited by GM6001. (e) Immunoblot detection of β-casein in SCp2, p2C, p2S7, and p2S10 cells cultured for 6 d with Matrigel and lactogenic hormones and in the presence (+) or absence (−) of Tet. (f) P2C, p2S7, and p2S10 cells were maintained for 12 d in the presence (+ Tet) or absence (− Tet) of Tet, fixed and stained with toluidine blue. (g) Invasion of Matrigel by SCp2, p2C, p2S7, and p2S10 cells in the presence (white bars) or absence (black bars) of Tet, or in the absence of Tet and in the presence of GM6001 (hatched bars; GM). Means and standard deviations from three independent experiments are shown. (h) Invasion of p2S7 (○) and p2S10 (▪) cells maintained in the absence of Tet for 2, 3, and 4 d after plating onto Matrigel. Means and standard deviations from three independent experiments are shown. Bar, 20 μM.
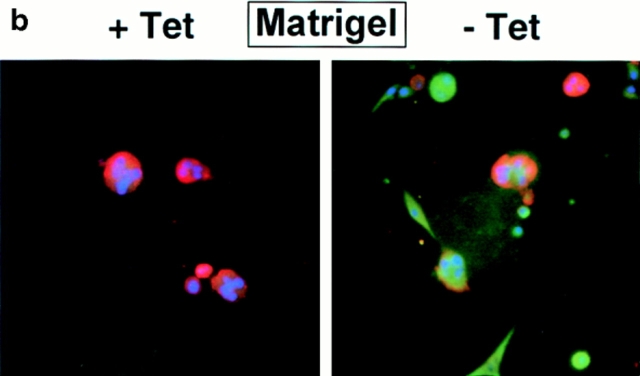
The functional phenotype of mouse mammary epithelial cells in culture and in vivo is defined by their ability to produce milk proteins (Emerman et al., 1977). The presence of both a basement membrane and lactogenic hormones are required for milk protein production of primary mouse mammary epithelial cells and mouse mammary epithelial cell lines (Barcellos-Hoff et al., 1989; Desprez et al., 1993). In vivo, ectopic expression of SL-1 in mammary epithelium of transgenic mice leads to inhibition of milk protein expression (Sympson et al., 1994). We found that expression of SL-1 by p2S cells cultured in the presence of Matrigel and lactogenic hormones strongly impaired production of the major milk protein β-casein (Fig. 1 e). Control cells were unaffected by the presence or absence of Tet in the culture medium. On a tissue culture plastic substratum, p2S cells maintained in the presence of Tet and SCp2 (not shown) and p2C cells maintained either in the presence or absence of Tet formed typical epithelial monolayers with most cells in close contact with neighboring cells (Fig. 1 f). In contrast, when SL-1 was induced in p2S cells by omission of Tet from the culture medium the cells lost well- delineated cell–cell contacts and adopted a fusiform morphology (Fig. 1 f). These changes in cellular morphology were barely detectable 6 d after SL-1 induction (not shown), but became obvious 12 d after SL-1 induction (Fig. 1 f). A “scattered” mesenchymal morphology is typical of the phenotype that epithelial cells assume during migration and invasion (for review see Birchmeier et al., 1996; Gilles and Thompson, 1996). As early as 4 d after SL-1 induction, p2S cells expressing SL-1 were able to migrate through Matrigel in a modified Boyden chamber assay, whereas noninduced cells were not invasive (Fig. 1, g and h). This invasive activity was abolished when a MMP inhibitor, GM6001, was added to the assay (Fig. 1 g). Thus, SL-1, which is used by mouse mammary tumor cells to invade basement membranes (Lochter et al., 1997), can also be used for migration and invasion by untransformed mammary epithelial cells.
SL-1 Triggers Cleavage of E-cadherin and Regulates β-Catenin Expression
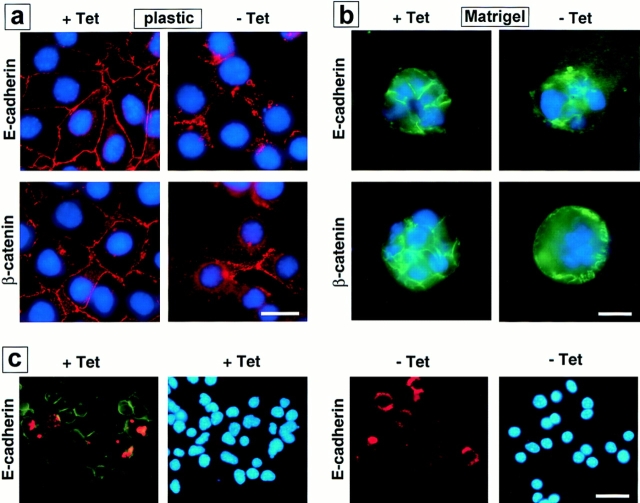
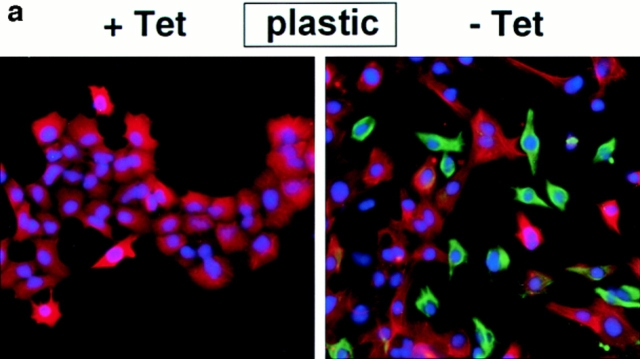
Because cell scattering and invasion requires dissolution of stable cell–cell contacts (for review see Birchmeier et al., 1996; Gilles and Thompson, 1996), we examined whether SL-1 affected cell–cell adhesion systems maintained by the major epithelial adhesion molecule E-cadherin. Both E-cadherin and its intracellular binding partner β-catenin were present at cell–cell contacts in p2S cells maintained in the presence of Tet (Fig. 2, a and b). However, by 6 d after induction of SL-1 there was a marked decrease in both of these molecules at cell–cell junctions (Fig. 2, a and b). SL-1 disrupted the integrity of cell–cell junctions in cells maintained both in the absence and presence of Matrigel (Fig. 2, a and b). E-cadherin and β-catenin localization at sites of cell–cell contact was unaffected by Tet in control SCp2 and p2C cells (not shown).
Figure 2.
Subcellular localization of E-cadherin and β-catenin after SL-1 induction. (a–b) P2S10 cells were maintained for 6 d in the absence (a) or presence (b) of Matrigel and in the presence (+ Tet) or absence (− Tet) of Tet. Cells were analyzed by indirect immunofluorescence for distribution of E-cadherin and β-catenin (red in a, green in b). Cells were counterstained with DAPI to visualize nuclei (blue). (c) SCp2 cells were labeled with DiI (red) and cocultured with p2S10 cells for 6 d in the presence (+ Tet) or absence (− Tet) of Tet. Cells were then permeabilzed with digitonin, fixed, and then immunostained for E-cadherin (green). Cells were counterstained with DAPI (blue). Bars: (a and b) 10 μM; (c) 25 μm.
To exclude the possibility that the effects observed with SL-1 were observable only in the p2S cell clones selected, SCp2 cells were labeled with the lipophilic red fluorescent dye DiI and cocultured with p2S cells in the absence of Tet on tissue culture plastic. E-cadherin (Fig. 2 c) and β-catenin (not shown) immunofluorescence was dramatically reduced after brief detergent extraction in both the SCp2 cells that did not express the SL-1 transgene and p2S cells that expressed SL-1. Thus, the phenotypic alteration induced by SL-1 was not cell autonomous and could also be induced in trans. Furthermore, these data show that the effects of SL-1 are because of its extracellular activity.
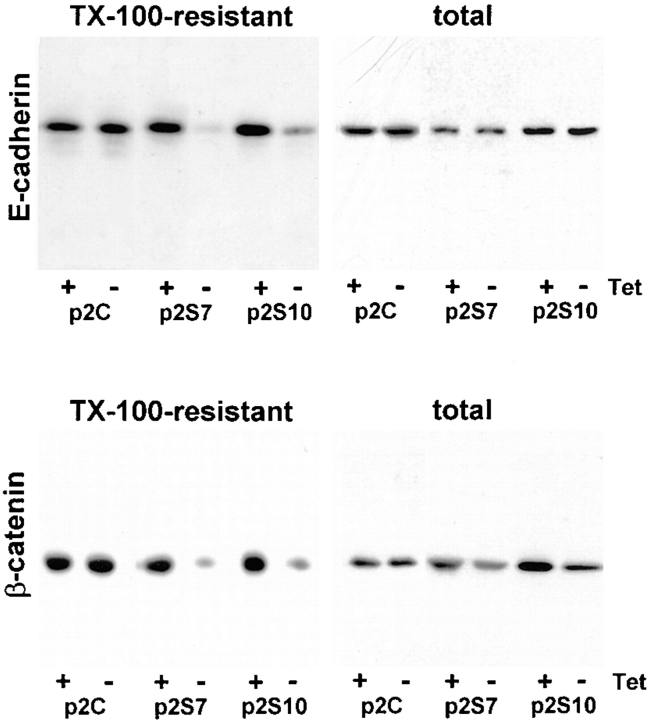
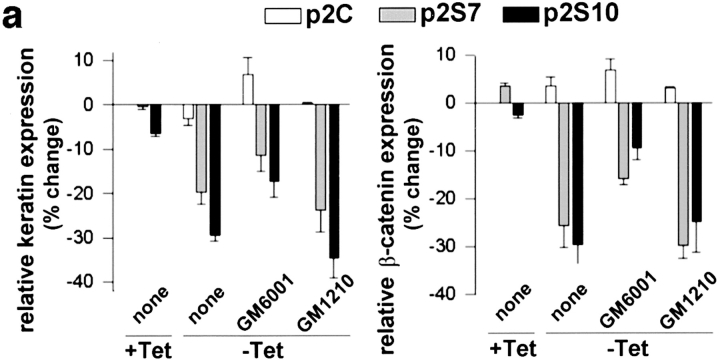
We observed an increased detergent solubility of E-cadherin and β-catenin in cells exposed to SL-1, which suggests a reduced association with the cytoskeleton (Näthke et al., 1994). Total E-cadherin protein, as determined by immunoblotting, was not altered, but the Triton X-100– resistant, E-cadherin pool was greatly diminished upon SL-1 induction in p2S cells for 6 d (Fig. 3). For β-catenin, both total and Triton X-100–resistant pools were reduced 6 d after induction of SL-1 (Figs. 3 and 4 a). This reduction in β-catenin levels required the proteolytic activity of SL-1, because it was prevented by GM6001, but not by its inactive structural homologue GM1210 (Fig. 4 b). A significant reduction of β-catenin levels occurred first 4 d after SL-1 induction (Fig. 4 a and not shown). When p2S cells were maintained in the presence of Matrigel, β-catenin expression also decreased as a result of SL-1 induction, albeit to a lesser extent than in the absence of Matrigel (Fig. 4 a). Results similar to β-catenin were obtained also for γ-catenin (Fig. 4 a; and not shown). With prolonged induction of SL-1 in p2S cells by culture for 2 mo on tissue culture plastic without Tet, β-catenin levels declined further and E-cadherin expression was repressed (Fig. 4, a and c).
Figure 3.
Total and cytoskeleton-associated E-cadherin and β-catenin after SL-1 induction. P2C, p2S7 and p2S10 cells were cultured for 6 d on tissue culture plastic in the presence (+) or absence (−) of Tet. They were then either directly lysed in RIPA buffer (total) or briefly extracted with Triton X-100 before cell lysis in RIPA buffer (TX-100-resistant) before analysis of expression of E-cadherin and β-catenin by immunoblotting.
Figure 4.
Expression of E-cadherin and β-catenin in p2S cells cultured in the absence and presence of Matrigel. (a) Quantification of E-cadherin (○), β-catenin (▪), and γ-catenin (▴) expression by ELISA on p2S10 cells maintained on plastic and induced to express SL-1 for 2, 4, and 6 d (2d, 4d, and 6d) or 2 mo (2m), or on p2S10 cells induced to express SL-1 for 6 d in the presence of Matrigel. Results are expressed as percent increase or decrease in expression of cells maintained in the absence of Tet as compared to cells maintained in the presence of Tet. Means and standard deviations from three independent experiments are shown. (b) Quantification of β-catenin expression by ELISA on p2C (white bars), p2S7 (gray bars), and p2S10 (black bars) cells maintained on tissue culture plastic for 6 d in the presence (+ Tet) or in the absence (− Tet) of Tet with no further additives (none) or with GM6001 or its inactive homologue GM1210. Results are normalized with expression levels obtained in the presence of Tet set to 100. Means and standard deviations from three independent experiments are shown. (c) Immunoblots for total E-cadherin (E-cad) and β-catenin (β-cat) expressed by p2C and p2S10 cells maintained for 6 d (6d) or 2 mo (2m) in the presence (+) or absence (−) of Tet. (d) [35S]methionine-labeled SCp2 cells were treated for 12 h with conditioned medium from p2S10 cells induced (− Tet) or not induced (+ Tet) to express SL-1 for 6 d. The conditioned medium from p2S10 cells maintained without Tet was either supplemented (+ GM) or not supplemented (− GM) with GM6001. E-cadherin was subsequently immunoprecipitated from the cell culture medium with a monoclonal antibody against the extracellular (ECCD-2) or intracellular (C20820) domain of E-cadherin. The amount of cleaved E-cadherin detected in the medium, however, constituted only a minor part of the total E-cadherin produced by p2S cells (not shown). (e) Cell surface proteins of [35S]methionine-labeled p2S10 cells maintained for 6 d in the presence of Tet (6d +), or for 6 d (6d −), or 2 mo (2m −) in the absence of Tet were biotinylated, immunoprecipitated with antibodies against E-cadherin, and then visualized with peroxidase-conjugated streptavidin (cell surface). Autoradiograms of total immunoprecipitated E-cadherin protein (total), on the same membranes as used for immunoblotting, are shown for comparison.
Because MMPs have cell surface substrates in addition to ECM substrates, we asked whether proteolytic removal of the extracellular domain of E-cadherin by SL-1 occurred. Indeed, after exposure of SCp2 cells to medium conditioned by p2S cells maintained in the absence of Tet, soluble 95- and 80-kD fragments reacting with ECCD-2 were recovered from the medium (Fig. 4 d). Consistent with this finding, cell surface E-cadherin was slightly reduced in p2S cells induced to produce SL-1 for 6 d (Fig. 4 e). E-cadherin cleavage did not occur in the presence of GM6001, indicating that E-cadherin is either a direct substrate of SL-1 or that SL-1 activates or induces other MMPs that could degrade E-cadherin. A 40-kD form of E-cadherin was precipitated from the medium regardless of whether SL-1 was induced or not (Fig. 4 d).
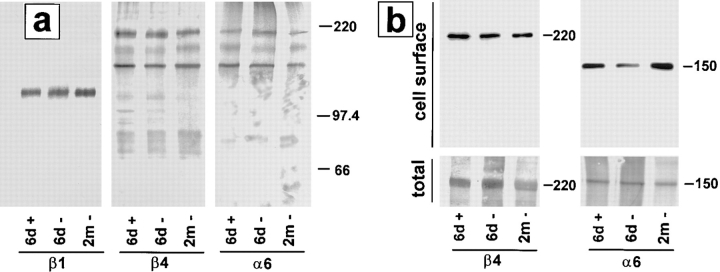
Cell–ECM contacts are also altered during epithelial cell scattering (for review see Adams and Watt, 1993; Gilles and Thompson, 1996). We therefore examined the impact of SL-1 on expression of integrin subunits β1, β4, and α6, which have been implicated in functional differentiation of mammary epithelial cells (Streuli et al., 1991; Boudreau et al., 1995; Weaver et al., 1997). The α6 integrin subunit was selectively coimmunoprecipitated with β4, but not with β1, indicating that p2S cells expressed α6β4, but not α6β1 integrin (Fig. 5 a), whether SL-1 was induced or not. Expression of total β1, β4, and α6 was not altered by SL-1, even after 2 mo of induction (Fig. 5 a). However, there was a reproducible, transient modulation in cell surface expression of the α6 integrin subunit that appeared to be decreased at day 6 after SL-1 induction, but not 2 mo after SL-1 induction (Fig. 5 b). Thus, integrin alterations appeared to be minor, whereas E-cadherin biochemistry changed dramatically after SL-1 induction.
Figure 5.
Effect of SL-1 induction on expression of integrins. (a) Cell lysates of [35S]methionine-labeled p2S10 cells maintained for 6 d in the presence of Tet (6d +), or for 6 d (6d −), or 2 mo (2m −) in the absence of Tet were immunoprecipitated with antibodies against integrin subunits β1, β4, and α6. Positions of molecular mass markers of 220, 97.4, and 66 kD are indicated. (b) Cell surface proteins of [35S]methionine-labeled p2S10 cells maintained for 6 d in the presence of Tet (6d +), or for 6 d (6d −), or 2 mo (2m −) in the absence of Tet were biotinylated, immunoprecipitated with antibodies against integrin subunits β4 and α6, and visualized with peroxidase-conjugated streptavidin (cell surface). Autoradiograms of total immunoprecipitated β4 and α6 protein (total), on the same membranes as used for immunoblotting, are shown for comparison.
SL-1 Induction Leads to Alterations in Expression of Intermediate Filament Cytoskeleton Proteins
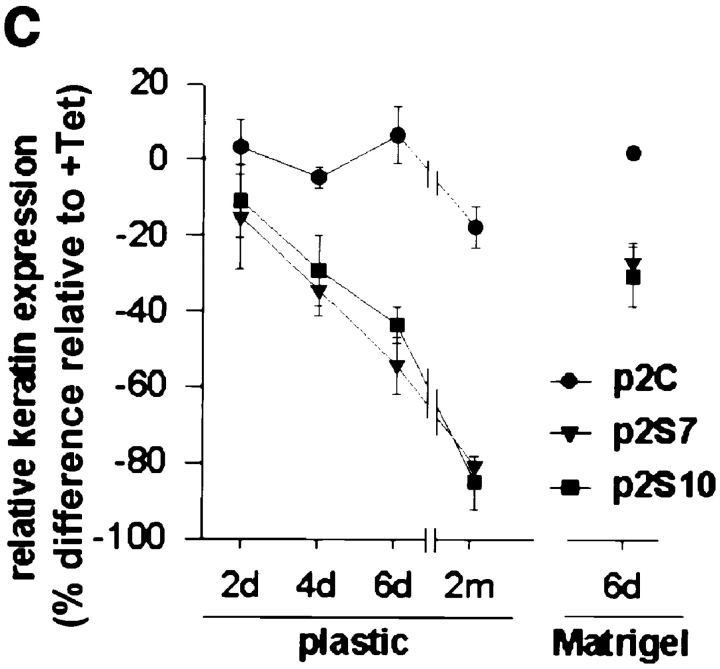
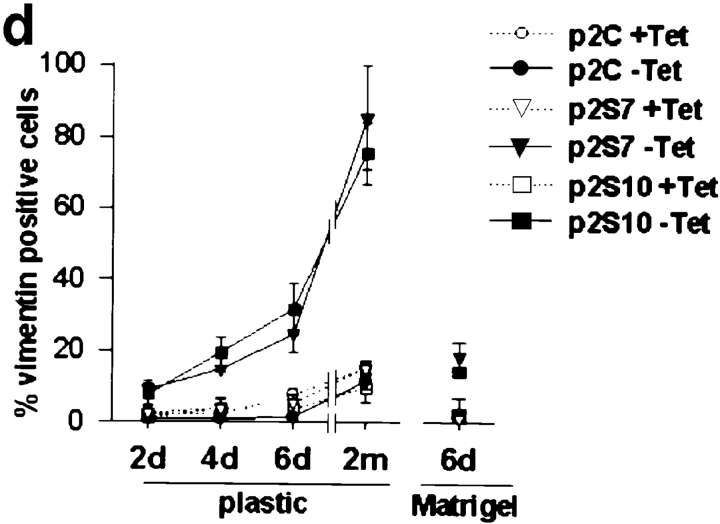
Epithelial cells that are migratory and invasive often downregulate their expression of cytokeratins and upregulate vimentin (for review see Birchmeier et al., 1996; Gilles and Thompson, 1996). The p2S cells contained cytokeratin intermediate filaments in the presence of Tet, and did not express vimentin (Fig. 6, a and b). Upon induction of SL-1, p2S cells began to express vimentin, even when cultured with Matrigel (Fig. 6, a and b). Some cells expressed vimentin alone, whereas others coexpressed vimentin with various levels of cytokeratins (Fig. 6, a and b), suggesting dual expression of vimentin and cytokeratins during transition to the complete loss of cytokeratins. In p2S cells that were maintained on tissue culture plastic and induced to express SL-1 for 6 d, cytokeratin expression decreased to ∼50% of control, as measured by ELISA assay (Fig. 6 c). Furthermore, ∼30% of the cells became vimentin positive, as determined by counting the number of cells reacting with antibodies against vimentin (Fig. 6 d). Addition of GM6001 at the time of Tet removal inhibited this phenotypic transition (not shown). After 2 mo of SL-1 induction, ∼80% of the cells were vimentin positive, whereas total cytokeratin expression decreased to 15% of control levels (Fig. 6, c and d). The transition from a cytokeratin cytoskeleton to a vimentin cytoskeleton in p2S cells occurred even in the presence of a basement membrane, albeit more slowly. When Tet was removed for 6 d in cultures containing Matrigel, ∼15% of the cells became vimentin positive, and total cytokeratin expression decreased to ∼70% of control levels (Fig. 6, c and d). Taken together these results show that, both in the presence and absence of a basement membrane, SL-1 induction leads to inhibition of expression of epithelial cytokeratins concomitant with expression of the mesenchymal intermediate filament protein vimentin.
Figure 6.
Effect of SL-1 induction on cytokeratin and vimentin expression. (a–b) P2S10 cells were maintained for 6 d in the absence (a) or presence (b) of Matrigel and in the presence (+ Tet) or absence (− Tet) of Tet. Cells were analyzed by indirect immunofluorescence for expression of cytokeratins (red) and vimentin (green). Cells were counterstained with DAPI (blue) to visualize nuclei. Note that in the presence of Matrigel most cells are round and form multicellular aggregates. (c) Quantification of cytokeratin expression by ELISA on p2C (•), p2S7 (▾), and p2S10 (▪) cells maintained on plastic and induced to express SL-1 for 2, 4, and 6 d (2d, 4d, and 6d), or 2 mo (2m), or on cells maintained in the presence of Matrigel for 6 d. Results are expressed as the percent increase or decrease in cytokeratin expression of cells maintained in the absence of Tet as compared to cells maintained in the presence of Tet. Means and standard deviations from three independent experiments are shown. (d) Quantification of the number of vimentin expressing p2C (circles), p2S7 (triangles) and p2S10 (squares) cells maintained on plastic or in the presence of Matrigel, and induced (black symbols) or not induced (white symbols) to express SL-1 for the times indicated above. Results are expressed as the percentage of cells displaying vimentin immunoreactivity. Means and standard deviations from three independent experiments are shown.
SL-1 Alters Endogenous MMPs and Induces KGF Expression in p2S Cells
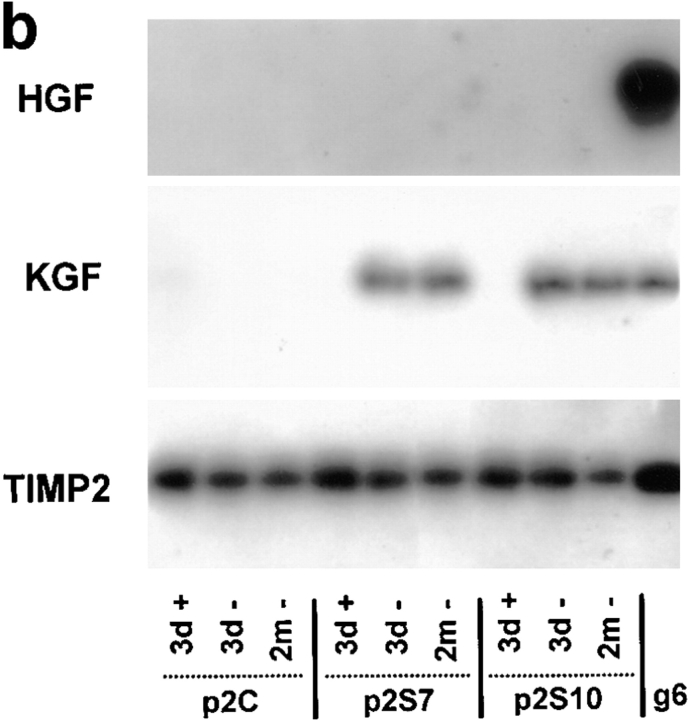
The observation that SL-1 induced a cascade of apparent epigenetic changes in p2S cells, characterized by induction of vimentin and downregulation of cytokeratins and β-catenin, raises the question of whether the regulation of other genes important for mammary epithelial function also changed in response to SL-1 action. Because a SL-1 transgene induces endogenous MMP expression in mice (Alexander et al., 1996), and inhibition of SL-1 expression in mammary carcinoma cells also reduces the synthesis of other MMPs (Lochter et al., 1997), we first determined whether other MMP activities were altered after SL-1 induction. We observed that endogenous gelatinase B was activated as early as 3 d after removal of Tet from the culture medium (Fig. 7). Moreover, additional gelatinolytic bands of 58 and 51 kD were upregulated, the latter corresponding to the molecular weight of collagenase-3 (Fig. 7). Interestingly, endogenous SL-1 was a late gene induced by the SL-1 transgene, not present at day 6, but appearing between 2 and 8 wk after SL-1 induction (not shown).
Cell scattering and loss of E-cadherin–mediated cell adhesion can be induced in other types of epithelial cells by growth factors such as KGF, HGF, and transforming growth factor-β (TGF-β) (Weidner et al., 1990; Miettinen et al., 1994; Savagner et al., 1994; Kitagawa et al., 1996). We therefore asked whether the SL-1–induced scattering and dedifferentiation are secondary to an induction of these scattering and/or dedifferentiation factors. If this were the case, the conditioned medium from p2S cells induced to express SL-1 should contain factors that could lead to a loss of differentiation in SCp2 cells even when MMP function is inhibited by GM6001. Consistent with the coculture experiment (Fig. 2 c), treatment of SCp2 cells for 6 d with medium conditioned by p2S cells generated in the absence of Tet for 6 d, resulted in reduction of β-catenin and cytokeratin levels (Fig. 8 a). Significantly, these alterations were also observed, albeit to a lesser extent, when the experiment was performed in the presence of GM6001 (Fig. 8 a), suggesting that SL-1 induces secreted factors that are not themselves MMPs. We thus determined whether KGF, HGF, or TGF-β were produced by p2S cells. Neither KGF nor HGF mRNA was expressed by p2S cells cultured in the presence of Tet (Fig. 8 b). In contrast, expression of SL-1 for 3 d led to induction of KGF, but not HGF mRNA (Fig. 8 b). TIMP2 mRNA, which was used as a positive control in this assay, was unchanged. KGF mRNA expression was maintained in cells induced to express SL-1 for 2 mo (Fig. 8 b). However, recombinant KGF, alone, was unable to alter β-catenin or cytokeratin expression in p2S or SCp2 cells (not shown), suggesting that additional factors were needed for the effects observed with conditioned medium. TGF-β, as measured by the mink lung cell assay, was secreted in a latent form by p2S cells (not shown). Total amounts of secreted TGF-β or its activation were not significantly altered by SL-1 expression (not shown). Taken together, these data indicate that the regulation of at least one growth factor gene (KGF) is downstream from SL-1 action, but that KGF alone is not sufficient for the phenotypic conversion observed.
Figure 8.
Induction of KGF by induction of SL-1. (a) Quantification of cytokeratin (keratin) and β-catenin expression by ELISA on SCp2 cells that had been incubated for 6 d with medium conditioned by p2C (white bars), p2S7 (gray bars), or p2S10 (black bars) cells maintained in the presence (+ Tet) or absence (− Tet) of Tet. GM6001 or GM1210 were added or not added (none) to conditioned medium from cells maintained in the absence of Tet. Results are expressed as the percent increase or decrease in cytokeratin or β-catenin expression compared to SCp2 cells maintained with conditioned medium from p2C cells cultured in the presence of Tet. Means and standard deviations from three independent experiments are shown. (b) Southern hybridization of RT-PCR products obtained with primers specific for HGF, KGF, or TIMP2 on total RNA prepared from p2C, p2S7, and p2S10 cells maintained for 3 days in the presence of Tet (3d +), or 3 d (3d −) or 2 months (2m −) in the absence of Tet, or from the mouse mammary carcinoma cell line SCg6 (g6).
Once Activated, the Altered Epithelial Phenotype Induced by SL-1 Is Stable in the Absence of Continued SL-1 Activity
The observation that SL-1 induces expression of factors refractory to MMP inhibition suggests that, once SL-1 is induced, it initiates a cascade of alterations that sustain phenotypic conversion, even after a relatively brief pulse of SL-1 expression. To address this possibility, p2S cells were induced to express SL-1 for 6 d, and then cultured in the presence of Tet and GM6001 to inhibit its further transcription and to inactivate the existing SL-1. Over the next 7 d, the altered expression of β-catenin, cytokeratins, and vimentin produced by SL-1 during the initial 6 d in the absence of Tet was sustained, and actually progressed (Fig. 9 a). When cells that had been induced to express SL-1 for 6 d were grown subsequently in the presence of Tet and GM6001 for an additional month, they continued to dedifferentiate towards a mesenchymal phenotype (Fig. 9 a). These results indicate that the effect of SL-1 was stable and irreversible, which is consistent with the hypothesis that p2S cells, once induced to express SL-1, become independent of continued SL-1 action to sustain a dedifferentiated phenotype.
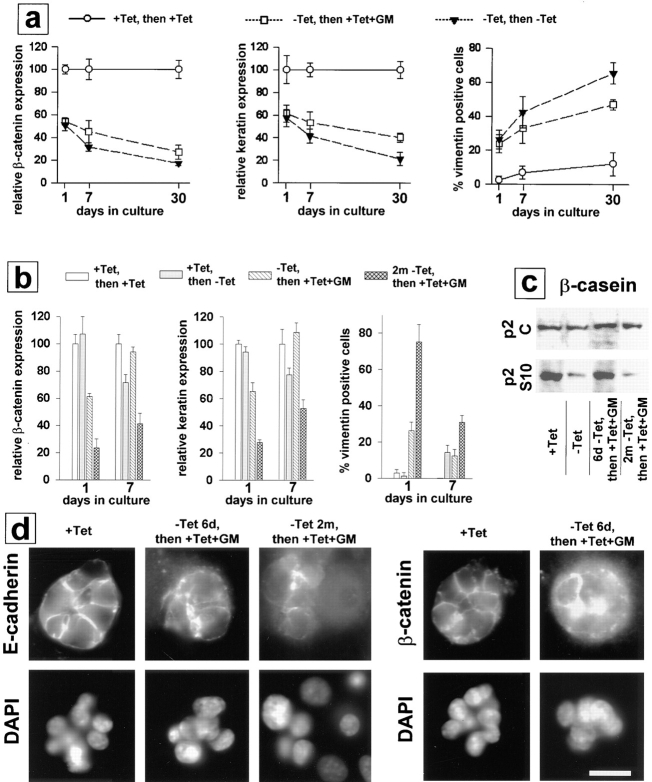
Figure 9.
Irreversibility of effects of SL-1 on phenotypic conversion of p2S cells. (a) P2S10 cells were preinduced to express SL-1 for 6 d. Cells were then maintained on tissue culture plastic and in the presence or absence of Tet, or Tet and GM6001. Expression of β-catenin and cytokeratins (keratin) was determined by ELISA and the number of vimentin-positive cells was counted 1, 7, and 30 d later. ○, cells maintained in the presence of Tet all along; □, cells preinduced to express SL-1 for 6 d and subsequently maintained in the presence of Tet and GM6001; and ▾, cells preinduced to express SL-1 for 6 d and subsequently maintained in the absence of Tet. ELISA results were normalized with values obtained for cells maintained in the presence of Tet set to 100 at each time point. Means and standard deviations from three independent experiments are shown. (b) P2S10 cells were preinduced to express SL-1 for 6 d or 2 mo in the absence of Matrigel. Cells were then maintained with Matrigel and in the presence or absence of Tet or Tet and GM6001. Expression of β-catenin and cytokeratins (keratin) was determined by ELISA and the number of vimentin-positive cells was counted 1 and 7 d later. White bars, cells maintained in the presence of Tet all along; gray bars, cells not preinduced to express SL-1 and subsequently maintained in the absence of Tet; hatched bars, cells preinduced to express SL-1 for 6 d and subsequently maintained in the presence of Tet and GM6001; gray crosshatched bars, cells preinduced to express SL-1 for 2 mo and subsequently maintained in the presence of Tet and GM6001. ELISA results are normalized with values obtained for cells maintained in the presence of Tet set to 100 at each time point. Means and standard deviations from three independent experiments are shown. (c) Immunoblot analysis of β-casein expression by p2C and p2S10 cells maintained with Matrigel and lactogenic hormones for 7 d. Cells were either not preinduced to express SL-1, and then maintained with Matrigel in the presence (+ Tet) or absence (− Tet) of Tet, or were preinduced in the absence of Matrigel to express SL-1 for 6 d (6d − Tet, then + Tet + GM) or 2 mo (2m − Tet, then + Tet + GM), and then maintained in the presence of Tet and GM6001 with Matrigel. (d) Cells were either not preinduced to express SL-1 (+ Tet), or preinduced to express SL-1 for 6 d (− Tet 6d, then + Tet + GM) or 2 mo (− Tet 2m, then + Tet + GM) in the absence of Matrigel before addition of Tet and GM6001 together with Matrigel for 7 d. Cells were then analyzed by indirect immunofluorescence for distribution of E-cadherin and β-catenin. Bar, 10 μm.
The requirement of an intact basement membrane for epithelial differentiation suggests that ECM may stabilize the epithelial phenotype (Barcellos-Hoff et al., 1989; Streuli et al., 1991). However, as described above, SL-1 was able to produce phenotypic conversion also in the presence of Matrigel, but to a lesser degree than on plastic. We therefore investigated whether the phenotype of p2S cells that expressed SL-1 in the absence of Matrigel could be reverted by culturing the cells with Matrigel under conditions where SL-1 activity was suppressed, i.e., in the presence of Tet and GM6001. When cells that were cultured for 6 d without Tet and Matrigel were then cultured for 7 d with Matrigel (with SL-1 activity suppressed), the number of vimentin-positive cells decreased from 30% in preinduced cells to 10%, β-catenin and cytokeratin were restored to normal levels, and the cells reacquired the ability to express milk proteins (Fig. 9, b and c). Nevertheless, E-cadherin and β-catenin were still diffusely distributed inside the cell (Fig. 9 d). After prolonged expression of SL-1 in p2S cells for 2 mo, the addition of Matrigel did not result in restoration of β-casein expression (Fig. 9 c). Although Matrigel was still able to increase cytokeratins and β-catenin, and to reduce vimentin expression in these cells, this reversion was relatively minor, and total expression of these molecules remained significantly different from cultures that had never been exposed to SL-1 (Fig. 9 b). These data indicate that the phenotype induced by SL-1 becomes progressively more irreversible, as cells are maintained in the presence of SL-1 and in the absence of basement membrane components.
Discussion
Proteases in general, and MMPs in particular, are implicated in a staggering number of biological pathways and diseases (Werb, 1997). The goal of the present study was to determine in a well-defined system, and without the complications of a stroma, whether such diverse processes could be brought about by a single MMP such as SL-1. For this purpose, we stably transfected a cDNA coding for an autoactivating rat SL-1 cDNA under the control of an inducible promoter into the functionally normal, nontumorigenic mouse mammary cell line SCp2, which does not express detectable amounts of endogenous SL-1 protein (Desprez et al., 1993; Lochter et al., 1997). In contrast to functionally normal mammary epithelial cells, mouse mammary carcinoma cell lines and fibroblasts in culture express high levels of endogenous SL-1 that are similar to the amount of the SL-1 transgene produced after its induction (Lochter et al., 1997; data not shown). This suggests that the quantities of the SL-1 transgene produced by cells after induction, as well as the effects of the SL-1 transgene on functionally normal mammary epithelial cells described in this study, are physiologically relevant.
We have shown here that expression of SL-1 in SCp2 cells triggers a cascade of events that is progressive in nature and results in a marked loss of the epithelial phenotype. Within 3 d of culture, a time period that was required for maximal induction of the SL-1 transgene, expression of SL-1 led to activation and upregulation of endogenous MMPs and induction of KGF. KGF is a member of the fibroblast growth factor family and normally is expressed only by mesenchymal cells (for review see Rubin et al., 1995). Four days after SL-1 induction, levels of β-catenin and cytokeratins were slightly reduced and a few cells began to express vimentin. 6 d after SL-1 induction, β-catenin and cytokeratin levels dropped by ∼50%, and ∼30% of cells expressed vimentin. At this time, the remaining β-catenin was essentially lost from cell–cell contacts and accumulated in the cytoplasm. E-cadherin also disappeared from the cell surface, but total E-cadherin levels were largely unaltered. Reduction of cytokeratin levels, induction of vimentin expression and loss of E-cadherin and β-catenin from cell–cell contacts were also observed when a recombinant SL-1 was added to culture medium of SCp2 cells (data not shown). When SL-1 transgene expression was sustained for 2 mo, β-catenin and cytokeratin levels declined to <20% of the level of noninduced cells, and ∼90% of cells expressed vimentin. During this long-term induction of SL-1, E-cadherin expression was downregulated. In contrast, expression of integrin subunits β1, β4 and α6 was largely unaffected by SL-1.
In the presence of basement membrane, mammary epithelial cells form three-dimensional cell aggregates that bear striking resemblance to mammary acini in vivo (Barcellos-Hoff et al., 1989; Desprez et al., 1993). However, the loss of the epithelial phenotype occurred regardless of whether SL-1–producing cells were maintained on nonspecific adhesive substrata or in the presence of a reconstituted basement membrane. Upon SL-1 induction on adhesive tissue culture substrata, the molecular alterations were followed by profound cell scattering and adoption of a mesenchymal morphology. In three-dimensional cultures using Matrigel, SL-1 induction inhibited lactogenic differentiation and cells acquired an invasive behavior. These changes indicate that mammary epithelial cells converted from an epithelial to a predominantly mesenchymal phenotype.
The process of conversion of epithelial cells to a mesenchymal phenotype has been variously referred to as epithelial–mesenchymal transition (EMT), epithelial transdifferentiation, epithelio–mesenchymal transformation, or epithelioid-to-mesenchymal transition (for review see Hay, 1995; Birchmeier et al., 1996; Gilles and Thompson, 1996). EMT, at both morphological and molecular levels, is a necessary step for a number of normal developmental processes that require cell migration and ECM invasion (for review see Hay, 1995). Although EMT has not been reported to occur during normal postnatal development of the mammary gland, it is a hallmark of tumor progression associated with the acquisition of invasive/metastatic features of breast carcinomas (for review see Birchmeier et al., 1996; Gilles and Thompson, 1996). The cascade of epigenetic changes that we observed with SL-1 acting on mammary epithelial cells may therefore mimic a program that otherwise occurs mainly during tumor progression.
SL-1 may have a two-pronged action in inducing phenotypic conversion of epithelial cells because it proteolyzes both the ECM and cell surface molecules such as tumor necrosis factor-α precursor and L-selectin (Gearing et al., 1994; Preece et al., 1996). Here we present evidence that SL-1 also cleaves the cell surface protein E-cadherin either directly, or indirectly by activation or induction of other proteases. Proteolytic removal of extracellular portions of E-cadherin may release soluble β-catenin, which could then associate with the Tcf/Lef family of transcription factors (Behrens et al., 1996; Korinek et al., 1997; Rubinfeld et al., 1997), and subsequently, regulate genes that are associated with the SL-1–induced phenotypic conversion of epithelial dedifferentiation. Alternatively, disruption of homophilic, calcium-dependent cell–cell adhesion may trigger second messenger cascades through increased tyrosine phosphorylation or cAMP production (Ghersi et al., 1996; Trolice et al., 1997). These effects may be enhanced by proteolytic cleavage of basement membrane/ECM. Laminins, which are produced by mammary epithelial cells in culture (Reichman et al., 1989), are good candidates for mediating SL-1 effects, because laminin-1–derived peptides enhance tumor cell invasion (Kanemoto et al., 1990; Bresalier et al., 1995), and cleavage of laminin-5 by gelatinase A promotes locomotion of otherwise nonmotile mammary epithelial cells (Giannelli et al., 1997).
We also discovered that KGF is upregulated after SL-1 induction. KGF belongs to a growing group of growth factors such as acidic fibroblast growth factor, HGF and TGF-β that can trigger EMT (Valles et al., 1990; Weidner et al., 1990; Miettinen et al., 1994; Savagner et al., 1994; Kitagawa et al., 1996). However, KGF alone could not substitute for the effects of SL-1 on mammary epithelial cells. It is possible that other growth factors in addition to KGF are induced by SL-1 in p2S cells, which could facilitate phenotypic conversion of these cells. Interestingly, we have observed that SL-1 augments expression of insulinlike growth factor 2 and basic fibroblast growth factor in other mammary epithelial cell lines, albeit not in p2S cells (data not shown). This indicates that SL-1 can induce different growth factors in different cell types.
The reversibility of the mesenchymal phenotype after removal of the EMT-inducing stimulus is an important feature of many cell culture systems where this phenomenon has been studied (Tucker et al., 1990; Valles et al., 1990; Miettinen et al., 1994; Savagner et al., 1994; Kitagawa et al., 1996). SL-1–induced dedifferentiation and mesenchymal conversion, in contrast, was irreversible, i.e., the loss of epithelial features observed 6 d after SL-1 induction was maintained, and even progressed further when cells were subsequently grown in the presence of Tet and the MMP inhibitor GM6001. The phenotypic conversion was inhibited only when GM6001 was added at the time of SL-1 induction.
When cells induced to express SL-1 for 6 d were confronted with a reconstituted basement membrane in the presence of Tet and GM6001, some but not all of the differentiated phenotypes could be restored; synthesis of β-casein, β-catenin, and cytokeratins were normalized to control levels and the number of vimentin expressing cells dropped. However, E-cadherin and β-catenin did not return to cell–cell contacts. This indicates that, even in the presence of a proper ECM microenvironment, altered E-cadherin and β-catenin signaling systems may still maintain a converted phenotype. When cells were induced to express SL-1 for 2 mo on a tissue culture plastic substratum, very few of epithelial properties were restored by addition of a basement membrane, and milk protein expression could not be recovered. These data show that the phenotypic conversion triggered by SL-1 comprises a hierarchy of events leading to the progressively more stable and irreversible acquisition of mesenchymal features.
Whereas it is clear from our data that SL-1 constitutes an important switch that can determine whether cells progress towards a dedifferentiated and invasive phenotype, the precise molecular mechanism(s) by which the loss of epithelial features or EMT occur(s) remains elusive. It has been proposed that a “master gene” that triggers expression of a set of mesenchyme-specific genes can be induced in epithelial cells (Hay, 1995). A master gene, usually thought of as a transcription factor, would regulate a variety of key genes that define and support the mesenchymal phenotype. As an alternative to this hypothesis, extracellular enzymes such as SL-1 may initiate a multistage/multifactor program that involves the cooperative or synergistic interaction of several proteolytic SL-1 targets, a process that may be relevant for tumor progression in vivo (Newgreen and Minichiello, 1996).
The finding that SL-1 induces transition of epithelium to an invasive, dedifferentiated phenotype is novel and provides a new paradigm for multifunctionality of a major MMP. That SL-1 can act in trans in a “hit-and-run” manner, after a relatively brief period of expression, suggests that in vivo MMP actions and effects could be separated temporally and spatially. The effect of SL-1 on phenotypic conversion also provides a culture model for the multistage tumor initiation and progression observed in WAP-SL-1 transgenic mice (Sympson et al., 1995). Based on our results we propose that SL-1 is an overriding pleiotropic regulator functioning as a master gene capable of triggering a complex program in mammary cells that leads to the progressive conversion of functionally normal epithelial cells to an invasive mesenchymal phenotype, which may eventually become malignant.
Acknowledgments
We are grateful to D. Lam for excellent technical assistance, and to F. Wang, R. Schwarz, and D. Callaghan for helpful technical advice.
This work was supported by funds from the U.S. Department of Energy, Office of Health and Environmental Research (contracts DE-AC03-76-SF00098 and DE-AC03 76-SF01012), and the National Cancer Institute (grant CA 57621), by the Institutional National Research Service Award (5T32 ES07106) (to S. Galosy), and by postdoctoral fellowships from the European Molecular Biology Organization and the California Breast Cancer Research Program (to A. Lochter), and the National Institutes of Health (to J. Muschler).
Abbreviations used in this paper
- DAPI
4′,6-diamidino-2-phenylindole
- ECCD
antibody to the extracellular domain of E-cadherin
- ECM
extracellular matrix
- EMT
epithelial–mesenchymal transition
- HGF
hepatocyte growth factor
- KGF
keratinocyte growth factor
- MMP
matrix metalloproteinase
- RT
reverse transcription
- SL-1
stromelysin-1
- Tet
tetracycline
- TGF-β
transforming growth factor–β
- TIMP2
tissue inhibitor of metalloproteinases–2
Footnotes
Address all correspondence to M.J. Bissell, Life Sciences Division, Building 83, Lawrence Berkeley National Laboratory, University of California, 1 Cyclotron Road, Berkeley, CA 94720. Tel.: (510) 486-4365. Fax: (510) 486-5586. E-mail: mjbissell@lbl.gov
A. Lochter's present address is Center for Clinical and Basic Research, 222 Ballerup Byvej, DK-2750 Ballerup, Denmark.
References
- Adams JC, Watt FM. Regulation of development and differentiation by the extracellular matrix. Development (Camb) 1993;117:1187–1198. doi: 10.1242/dev.117.4.1183. [DOI] [PubMed] [Google Scholar]
- Alexander CM, Howard EW, Bissell MJ, Werb Z. Rescue of mammary epithelial cell apoptosis and entactin degradation by a tissue inhibitor of metalloproteinases-1 transgene. J Cell Biol. 1996;135:1669–1677. doi: 10.1083/jcb.135.6.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashkenas J, Muschler J, Bissell MJ. The extracellular matrix in epithelial biology: shared molecules and common themes in distant phyla. Dev Biol. 1996;180:433–444. doi: 10.1006/dbio.1996.0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcellos-Hoff MH, Aggeler J, Ram TG, Bissell MJ. Functional differentiation and alveolar morphogenesis of primary mammary cultures on reconstituted basement membrane. Development (Camb) 1989;105:223–235. doi: 10.1242/dev.105.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum CB, Werb Z. Focalized proteolysis: spatial and temporal regulation of extracellular matrix degradation at the cell surface. Curr Opin Cell Biol. 1996;8:731–738. doi: 10.1016/s0955-0674(96)80116-5. [DOI] [PubMed] [Google Scholar]
- Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, Birchmeier W. Functional interaction of β-catenin with the transcription factor LEF-1. Nature. 1996;382:638–642. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- Birchmeier C, Birchmeier W, Brand-Saberi B. Epithelial-mesenchymal transitions in cancer progression. Acta Anat (Basel) 1996;156:216–227. doi: 10.1159/000147848. [DOI] [PubMed] [Google Scholar]
- Boudreau N, Sympson CJ, Werb Z, Bissell MJ. Suppression of ICE and apoptosis in mammary epithelial cells by extracellular matrix. Science. 1995;267:891–893. doi: 10.1126/science.7531366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresalier RS, Schwartz B, Kim YS, Duh QY, Kleinman HK, Sullam PM. The laminin α 1 chain Ile-Lys-Val-Ala-Val (IKVAV)-containing peptide promotes liver colonization by human colon cancer cells. Cancer Res. 1995;55:2476–2480. [PubMed] [Google Scholar]
- Brooks PC, Stromblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, Quigley JP, Cheresh DA. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin αvβ3. Cell. 1996;85:683–693. doi: 10.1016/s0092-8674(00)81235-0. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Werb Z. Matrix metalloproteinases and the development of cancer. Chem Biol. 1996;3:895–904. doi: 10.1016/s1074-5521(96)90178-7. [DOI] [PubMed] [Google Scholar]
- Crowley E, Horwitz AF. Tyrosine phosphorylation and cytoskeletal tension regulate the release of fibroblast adhesions. J Cell Biol. 1995;131:525–537. doi: 10.1083/jcb.131.2.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desprez P-Y, Roskelley C, Campisi J, Bissell MJ. Isolation of functional cell lines from a mouse mammary epithelial cell strain: the importance of basement membrane and cell-cell interactions. Mol Cell Differ. 1993;1:99–110. [Google Scholar]
- Emerman JT, Enami J, Pitelka DR, Nandi S. Hormonal effects on intracellular and secreted casein in cultures of mouse mammary epithelial cells on floating collagen membranes. Proc Natl Acad Sci USA. 1977;74:4466–4470. doi: 10.1073/pnas.74.10.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher, S.J., and Z. Werb. 1995. Techniques for studying the catabolism of extracellular matrix components. In Extracellular Matrix: A Practical Approach. M.A. Haralson, and J.R. Hassell, editors. IRL Press Ltd. Oxford. 261–281.
- Gearing AJ, Beckett P, Christodoulou M, Churchill M, Clements J, Davidson AH, Drummond AH, Galloway WA, Gilbert R, Gordon JL, et al. Processing of tumour necrosis factor-α precursor by metalloproteinases. Nature. 1994;370:555–557. doi: 10.1038/370555a0. [DOI] [PubMed] [Google Scholar]
- Ghersi G, Salamone M, Levi G, Vittorelli ML. Cell adhesion-dependent regulation of cell growth during sea urchin development. Eur J Cell Biol. 1996;69:259–266. [PubMed] [Google Scholar]
- Giannelli G, Falk-Marzillier J, Schiraldi O, Stetler-Stevenson WG, Quaranta V. Induction of migration by matrix metalloproteinase-2 cleavage of laminin-5. Science. 1997;277:225–228. doi: 10.1126/science.277.5323.225. [DOI] [PubMed] [Google Scholar]
- Gijbels K, Galardy RE, Steinman L. Reversal of experimental autoimmune encephalomyelitis with a hydroxamate inhibitor of matrix metalloproteases. J Clin Invest. 1994;94:2177–2182. doi: 10.1172/JCI117578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilles C, Thompson E. The epithelial to mesenchymal transition and metastatic progression in carcinoma. Breast J. 1996;2:83–96. [Google Scholar]
- Grobelny D, Poncz L, Galardy RE. Inhibition of human skin fibroblast collagenase, thermolysin, and Pseudomonas aeruginosaelastase by peptide hydroxamic acids. Biochemistry. 1992;31:7152–7154. doi: 10.1021/bi00146a017. [DOI] [PubMed] [Google Scholar]
- Hay ED. An overview of epithelio-mesenchymal transformation. Acta Anat (Basel) 1995;154:8–20. doi: 10.1159/000147748. [DOI] [PubMed] [Google Scholar]
- Honig MG, Hume RI. Fluorescent carbocyanine dyes allow living neurons of identified origin to be studied in long-term culture. J Cell Biol. 1986;103:171–187. doi: 10.1083/jcb.103.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanemoto T, Reich R, Royce L, Greatorex D, Adler SH, Shiraishi N, Martin GR, Yamada Y, Kleinman HK. Identification of an amino acid sequence from the laminin A chain that stimulates metastasis and collagenase IV production. Proc Natl Acad Sci USA. 1990;87:2279–2283. doi: 10.1073/pnas.87.6.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa K, Murata A, Matsuura N, Tohya K, Takaichi S, Monden M, Inoue M. Epithelial-mesenchymal transformation of a newly established cell line from ovarian adenosarcoma by transforming growth factor-β1. Int J Cancer. 1996;66:91–97. doi: 10.1002/(SICI)1097-0215(19960328)66:1<91::AID-IJC16>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Korinek V, Barker N, Morin PJ, van Wichen D, de Wegner R, Kinzler KW, Vogelstein B, Clevers H. Constitutive transcriptional activation by a β-catenin-Tcf complex in APC−/− colon carcinoma. Science. 1997;275:1784–1787. doi: 10.1126/science.275.5307.1784. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levi E, Fridman R, Miao HQ, Ma YS, Yayon A, Vlodavsky I. Matrix metalloproteinase 2 releases active soluble ectodomain of fibroblast growth factor receptor 1. Proc Natl Acad Sci USA. 1996;93:7069–7074. doi: 10.1073/pnas.93.14.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Strange R, Friis RR, Djonov V, Altermatt HJ, Saurer S, Niemann H, Andres AC. Expression of stromelysin-1 and TIMP-1 in the involuting mammary gland and in early invasive tumors of the mouse. Int J Cancer. 1994;59:560–568. doi: 10.1002/ijc.2910590421. [DOI] [PubMed] [Google Scholar]
- Lochter A, Bissell MJ. Involvement of extracellular matrix in breast cancer. Semin Cancer Biol. 1995;6:165–173. doi: 10.1006/scbi.1995.0017. [DOI] [PubMed] [Google Scholar]
- Lochter, A., and M. Bissell. 1997. Mammary gland biology and the wisdom of extracellular matrix. In Biological Signaling in the Mammary Gland. C.J. Wilde, M. Peaker, and E. Taylor, editors. 77–92.
- Lochter A, Vaughan L, Kaplony A, Prochiantz A, Schachner M, Faissner A. J1/tenascin in substrate-bound and soluble form displays contrary effects on neurite outgrowth. J Cell Biol. 1991;113:1159–1171. doi: 10.1083/jcb.113.5.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochter A, Srebrow A, Sympson CJ, Terracio N, Werb Z, Bissell M J. Misregulation of stromelysin-1 expression in mouse mammary tumor cells accompanies acquisition of stromelysin-1-dependent invasive properties. J Biol Chem. 1997;272:5007–5015. doi: 10.1074/jbc.272.8.5007. [DOI] [PubMed] [Google Scholar]
- MacDougall JR, Matrisian LM. Contributions of tumor and stromal matrix metalloproteinases in tumor progression, invasion and metastasis. Cancer Metastasis Rev. 1995;14:351–362. doi: 10.1007/BF00690603. [DOI] [PubMed] [Google Scholar]
- Miettinen PJ, Ebner R, Lopez AR, Derynck R. TGF-β induced transdifferentiation of mammary epithelial cells to mesenchymal cells: Involvement of type I receptors. J Cell Biol. 1994;127:2021–2036. doi: 10.1083/jcb.127.6.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsky WL, Kelly T, Lin CY, Yeh Y, Stetler SW, Mueller SC, Chen WT. Binding and localization of M(r) 72,000 matrix metalloproteinase at cell surface invadopodia. Cancer Res. 1993;53:3159–3164. [PubMed] [Google Scholar]
- Näthke IS, Hinck L, Swedlow JR, Papkoff J, Nelson WJ. Defining interactions and distributions of cadherin and catenin complexes in polarized epithelial cells. J Cell Biol. 1994;125:1341–1352. doi: 10.1083/jcb.125.6.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newgreen DF, Minichiello J. Control of epitheliomesenchymal transformation. II. Cross-modulation of cell adhesion and cytoskeletal systems in embryonic neural cells. Dev Biol. 1996;176:300–312. doi: 10.1006/dbio.1996.0135. [DOI] [PubMed] [Google Scholar]
- Ochieng J, Fridman R, Nangia-Makker P, Kleiner DE, Liotta LA, Stetler-Stevenson WG, Raz A. Galectin-3 is a novel substrate for human matrix metalloproteinases-2 and -9. Biochemistry. 1994;33:14109–14114. doi: 10.1021/bi00251a020. [DOI] [PubMed] [Google Scholar]
- Preece G, Murphy G, Ager A. Metalloproteinase-mediated regulation of L-selectin levels on leukocytes. J Biol Chem. 1996;271:11634–11640. doi: 10.1074/jbc.271.20.11634. [DOI] [PubMed] [Google Scholar]
- Reichman E, Ball R, Groner B, Friis RR. New mammary epithelial and fibroblastic cell clones in coculture form structures competent to differentiate functionally. J Cell Biol. 1989;108:1127–1138. doi: 10.1083/jcb.108.3.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin JS, Bottaro DP, Chedid M, Miki T, Ron D, Cunha GR, Finch PW. Keratinocyte growth factor as a cytokine that mediates mesenchymal-epithelial interaction. EXS. 1995;74:191–214. doi: 10.1007/978-3-0348-9070-0_10. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B, Robbins P, El-Gamil M, Albert I, Porfiri E, Polakis P. Stabilization of β-catenin by genetic defects in melanoma cell lines. Science. 1997;275:1790–1792. doi: 10.1126/science.275.5307.1790. [DOI] [PubMed] [Google Scholar]
- Sanchez-Lopez R, Nicholson R, Gesnel MC, Matrisian LM, Breathnach R. Structure-function relationships in the collagenase family member transin. J Biol Chem. 1988;263:11892–11899. [PubMed] [Google Scholar]
- Savagner P, Valles AM, Jouanneau J, Yamada KM, Thiery JP. Alternative splicing in fibroblast growth factor receptor 2 is associated with induced epithelial-mesenchymal transition in rat bladder carcinoma cells. Mol Biol Cell. 1994;5:851–862. doi: 10.1091/mbc.5.8.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidhauser C, Casperson GF, Myers CA, Sanzo KT, Bolten S, Bissell MJ. A novel transcriptional enhancer is involved in the prolactin- and extracellular matrix-dependent regulation of β-casein gene expression. Mol Biol Cell. 1992;3:699–709. doi: 10.1091/mbc.3.6.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayoshi Y, Nose A, Iwasaki K, Takeichi M. N-linked oligosaccharides are not involved in the function of a cell-cell binding glycoprotein E-cadherin. Cell Struct Funct. 1986;11:245–252. doi: 10.1247/csf.11.245. [DOI] [PubMed] [Google Scholar]
- Shockett P, Difilippantonio M, Hellman N, Schatz DG. A modified tetracycline-regluated system provides autoregulatory, inducible gene expression in cultured cells and transgenic mice. Proc Natl Acad Sci USA. 1995;92:6522–6526. doi: 10.1073/pnas.92.14.6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli CH, Bissell MJ. Expression of extracellular matrix components is regulated by substratum. J Cell Biol. 1990;110:1405–1415. doi: 10.1083/jcb.110.4.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli CH, Bailey N, Bissell MJ. Control of mammary epithelial differentiation: Basement membrane induces tissue-specific gene expression in the absence of cell-cell interaction and morphological polarity. J Cell Biol. 1991;115:1383–1395. doi: 10.1083/jcb.115.5.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli CH, Schmidhauser C, Bailey N, Yurchenco P, Skubitz AP, Roskelley C, Bissell MJ. Laminin mediates tissue-specific gene expression in mammary epithelia. J Cell Biol. 1995;129:591–603. doi: 10.1083/jcb.129.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sympson CJ, Talhouk RS, Alexander CM, Chin JR, Clift SM, Bissell MJ, Werb Z. Targeted expression of stromelysin-1 in mammary gland provides evidence for a role of proteinases in branching morphogenesis and the requirement for an intact basement membrane for tissue-specific gene expression. J Cell Biol. 1994;125:681–693. doi: 10.1083/jcb.125.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sympson CJ, Bissell MJ, Werb Z. Mammary gland tumor formation in transgenic mice overexpressing stromelysin-1. Semin Cancer Biol. 1995;6:159–163. doi: 10.1006/scbi.1995.0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talhouk RS, Bissell MJ, Werb Z. Coordinated expression of extracellular matrix–degrading proteinases and their inhibitors regulates mammary epithelial function during involution. J Cell Biol. 1992;118:1271–1282. doi: 10.1083/jcb.118.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trolice MP, Pappalardo A, Peluso JJ. Basic fibroblast growth factor and N-cadherin maintain rat granulosa cell and ovarian surface epithelial cell viability by stimulating the tyrosine phosphorylation of the fibroblast growth factor receptors. Endocrinology. 1997;138:107–113. doi: 10.1210/endo.138.1.4836. [DOI] [PubMed] [Google Scholar]
- Tucker GC, Boyer B, Gavrilovic J, Emonard H, Thiery J-P. Collagen-mediated dispersion of NBT-II rat bladder carcinoma cells. Cancer Res. 1990;50:129–137. [PubMed] [Google Scholar]
- Valles AM, Boyer B, Badet J, Tucker GC, Barritault D, Thiery JP. Acidic fibroblast growth factor is a modulator of epithelial plasticity in a rat bladder carcinoma cell line. Proc Natl Acad Sci USA. 1990;87:1124–1128. doi: 10.1073/pnas.87.3.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver VM, Petersen OW, Wang F, Larabell CA, Briand P, Damsky C, Bissell MJ. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo using integrin blocking antibodies. J Cell Biol. 1997;137:231–246. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner KM, Behrens J, Vandekerckhove J, Birchmeier W. Scatter factor: Molecular characteristics and effect on the invasiveness of epithelial cells. J Cell Biol. 1990;111:2097–2108. doi: 10.1083/jcb.111.5.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werb, Z. 1997. Extracellular matrix and cell surface proteolysis: regulating celllular ecology. Cell. In press. [DOI] [PubMed]
- Witty JP, Wright JH, Matrisian LM. Matrix metalloproteinases are expressed during ductal and alveolar mammary morphogenesis, and misregulation of stromelysin-1 in transgenic mice induces unscheduled alveolar development. Mol Biol Cell. 1995;6:1287–1303. doi: 10.1091/mbc.6.10.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]