Abstract
The mammalian endopeptidase furin is a type 1 integral membrane protein that is predominantly localized to the TGN and is degraded in lysosomes with a t1/2 = 2–4 h. Whereas the localization of furin to the TGN is largely mediated by sorting signals in the cytosolic tail of the protein, we show here that targeting of furin to lysosomes is a function of the luminal domain of the protein. Inhibition of lysosomal degradation results in the accumulation of high molecular weight aggregates of furin; aggregation is also dependent on the luminal domain of furin. Temperature and pharmacologic manipulations suggest that furin aggregation occurs in the TGN and thus precedes delivery to lysosomes. These findings are consistent with a model in which furin becomes progressively aggregated in the TGN, an event that leads to its transport to lysosomes. Our observations indicate that changes in the aggregation state of luminal domains can be potent determinants of biosynthetic targeting to lysosomes and suggest the possible existence of quality control mechanisms for disposal of aggregated proteins in compartments of the secretory pathway other than the endoplasmic reticulum.
The endopeptidase furin is a type I integral membrane glycoprotein (see scheme in Fig. 1 a), which, at steady state, is predominantly localized to the TGN (Bosshart et al., 1994; Molloy et al., 1994; Schäfer et al., 1995; Shapiro et al., 1997). Furin has also been detected within compartments of the endosomal–lysosomal system (Bosshart et al., 1994; Sariola et al., 1995), which are most likely intermediates in the cycling of the protein between the TGN and the plasma membrane (Chapman and Munro, 1994; Molloy et al., 1994), or in its transport to lysosomes for degradation (Bosshart et al., 1994).
Figure 1.
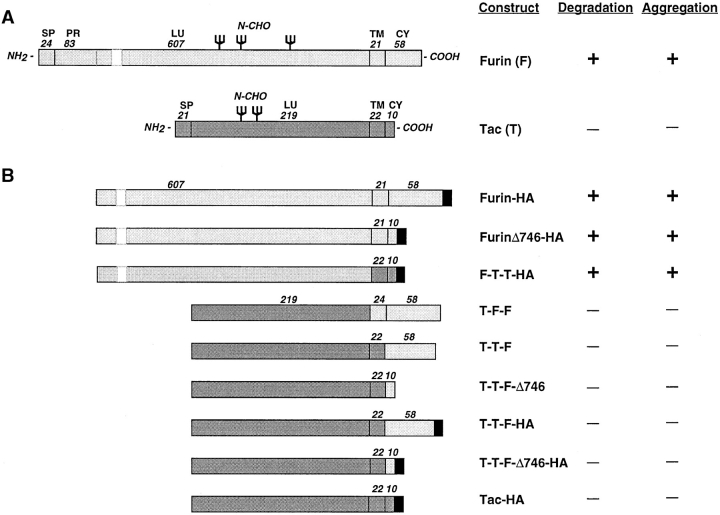
Schematic representation of constructs used in this study and summary of results. (A) The diagrams show the position and number of amino acid residues of the signal peptide (SP), pro-region (PR), luminal (LU), transmembrane (TM), and cytosolic (CY) domains of furin and Tac. The figure also indicates the approximate positions of sites of N-glycan addition (N-CHO) in both molecules. The gap in the furin luminal domain represents a segment that was omitted to draw the schemes to scale. (B) The diagrams represent the structures of different furin-Tac chimeras after cleavage of the signal peptide and pro-region segments. In naming the chimeras, F and T were used to designate domains derived from furin and Tac, respectively. Some of the constructs were tagged by addition of an HA epitope (YPYDVPDYA) at the COOH terminus, as shown in the figure. In some constructs, a FLAG epitope (DYKDDDDK) was used in place of the HA epitope. The number of residues in the transmembrane and cytosolic domains of the chimeras are indicated.
During its transit through the secretory pathway, furin undergoes several posttranslational modifications. In the ER, nascent furin receives three N-linked oligosaccharides chains (see Fig. 1 a, N-CHO) and undergoes sequential cleavage of its NH2-terminal signal peptide (Fig. 1 a, SP) and pro-region (Fig. 1 a, PR) (Leduc et al., 1992; Rehemtulla et al., 1992; Molloy et al., 1994). The pro-region fragment remains noncovalently bound to furin as the protein moves from the ER to the Golgi complex (Anderson et al., 1997). In the TGN, there is an additional cleavage in the pro-region fragment that causes its dissociation from the rest of the molecule (Anderson et al., 1997). Furin then establishes transient residence within the TGN and engages in cycling between the TGN and the plasma membrane (Chapman and Munro, 1994; Molloy et al., 1994). Somewhere along this cycling pathway, a fraction of the furin molecules are cleaved near the luminal–transmembrane boundary, resulting in the release of the soluble luminal domain into the extracellular space (Wise et al., 1990; Bosshart et al., 1994; Molloy et al., 1994; Vey et al., 1994; Creemers et al., 1996). The population of furin that is not cleaved eventually becomes degraded by a lysosomal process with a t1/2 = 2–4 h (Bosshart et al., 1994).
In view of the complexity of furin's trafficking patterns, it is not surprising that the protein contains several post-Golgi sorting determinants. The cytosolic domain of furin has at least two sorting signals that are responsible for its localization to the TGN and its internalization and retrieval from the plasma membrane (Bosshart et al., 1994; Chapman and Munro, 1994; Molloy et al., 1994; Schäfer et al., 1995; Voorhees et al., 1995). One is a tyrosine-based signal (YKGL; residues 758–761) (Schäfer et al., 1995; Voorhees et al., 1995) that is similar to other signals that interact with the medium chains of the clathrin-associated adaptor complexes AP-1 and AP-2 (Ohno et al., 1995, 1996; Boll et al., 1996; for review see Marks et al., 1997). The other signal is a sequence rich in acidic amino acid residues (WQEECPSDSEEDEGRGER; residues 766– 783) (Schäfer et al., 1995; Voorhees et al., 1995) that becomes phosphorylated on serine residues by casein kinase II (Jones et al., 1995; Takahashi et al., 1995). The phosphorylated acidic sequence has recently been shown to interact with the AP-1 adaptor complex (Dittié et al., 1997).
Previous work from our laboratory suggested that the two cytosolic sorting signals are not the only determinants of furin trafficking in post-Golgi compartments. Indeed, deletion of all but 11 amino acids from the cytosolic tail, including the tyrosine-based and acidic signals, resulted in loss of TGN localization; yet, the truncated protein remained mostly intracellular (Bosshart et al., 1994). Like full-length furin, the truncated protein was found to be degraded in lysosomes at a relatively rapid rate (Bosshart et al., 1994). Addition of the same 11 cytosolic amino acids of furin to the luminal and transmembrane domains of the α chain of the interleukin-2 receptor (Tac),1 a plasma membrane protein, did not alter the cell surface localization and turnover of the protein, suggesting that this sequence did not have lysosomal targeting activity per se (Bosshart et al., 1994). These observations pointed to the existence of additional targeting information within the luminal and/ or transmembrane domains of furin. In the present study, we demonstrate that the rapid turnover of furin is a property of the luminal domain of the protein. Furthermore, we show that lysosomal targeting mediated by the furin luminal domain correlates with formation of high molecular weight aggregates, a process that most likely occurs within the TGN. Thus, our results suggest that progressive aggregation of the luminal domain of furin in the TGN results in its delivery to lysosomes for degradation. These observations emphasize the importance of the aggregation state of proteins in post-Golgi trafficking pathways and suggest the possible existence of quality control mechanisms for disposal of aggregated proteins in compartments of the secretory pathway other than the ER (Hammond and Helenius, 1995).
Materials and Methods
Recombinant DNA Procedures
Schematic representations of recombinant constructs used in this study are shown in Fig. 1. Mouse furin (Hatsuzawa et al., 1990) constructs tagged with either the FLAG (Hopp et al., 1988) or hemagglutinin (HA) (Wilson et al., 1984) epitopes and cloned into the pCDL-SRα vector (Takebe et al., 1988) were described previously (Bosshart et al., 1994). The promoter region of pCDL-SRα has SV40 early promoter and human T-cell lymphotrophic virus I long terminal repeat sequences that drive expression of the transgenes in cells from different mammalian species. Tac (Leonard et al., 1984) constructs and Tac-furin chimeras cloned into pCDM8 (Seed, 1987) were also described previously (Bosshart et al., 1994; Voorhees et al., 1995). The pCDM8 plasmid has a human cytomegalovirus promoter that yields good expression levels in primate cells. The construct F-T-T-HA encodes a fusion of residues 1–714 of mouse furin with residues 241–273 of Tac, plus the HA epitope; the construct was made using the double PCR method of Higuchi et al. (1988), and then cloned into pCDL-SRα.
Cells
Rat basophilic leukemia (RBL) cells (clone 2H3) were provided by H. Metzger (National Institutes of Health [NIH], Bethesda, MD). HeLa cells were obtained from the American Type Culture Collection (Rockville, MD). Swei cells (human B-lymphoblastoid) were the gift of M. Marks (University of Pennsylvania, Philadelphia, PA). All cells were cultured in DME (Biofluids, Inc., Rockville, MD) containing 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin (complete medium). Stable transfection of RBL cells and transient transfection of HeLa cells were done as described previously (Humphrey et al., 1993; Bosshart et al., 1994; Marks et al., 1995). Single-cell clones of stably transfected RBL cells were isolated by limiting dilution and screened by immunofluorescence microscopy. Only clones that expressed moderate levels of the transfected constructs were used. RBL lines expressing Tac or a Tac-DKQTLL construct (Letourneur and Klausner, 1992) were the gift of R. Klausner (NIH).
Transient Transfections
HeLa cells were transfected using calcium phosphate precipitation as described (Marks et al., 1996). Typically, 10–40% of the cells were transfected using this procedure. Cells were used 36–40 h after transfection for metabolic labeling or immunofluorescence microscopy.
Antibodies
The antibodies Fur1, Fur2 (Bosshart et al., 1994), and DC16 (a gift from R. Angeletti, Albert Einstein College of Medicine, New York) directed to different luminal epitopes of furin, were described before (Bosshart et al., 1994, Shapiro et al., 1997). The antibody M2 to the FLAG epitope was obtained from International Biotechnologies, Inc. (New Haven, CT), and the antibody HA-11 to the HA epitope was obtained from BAbCO (Richmond, CA). The mouse monoclonal antibody 7G7.B6 (referred to as 7G7; Rubin et al., 1985), directed to a luminal epitope of the human Tac antigen, was produced from a hybridoma clone obtained from the American Type Culture Collection. A polyclonal antibody to lamp-1 was a gift from M. Fukuda (La Jolla Cancer Research Foundation, La Jolla, CA).
Immunofluorescence Microscopy
Cells were grown to 70–80% confluence on glass coverslips and fixed for 15 min at room temperature with 2% formaldehyde in PBS. After washing in PBS, the cells were incubated for 1 h with primary antibodies in 0.1% saponin/PBS. After rinsing in PBS, cells were further incubated for 30 min with a mixture of tetramethyl rhodamine– and fluorescein-conjugated secondary antibodies (Jackson Immunoresearch, West Grove, PA) in 0.1% saponin/PBS. The coverslips were washed once more in PBS and mounted onto glass slides with Fluoromount G (Southern Biotechnology Associates Inc., Birmingham, AL). Samples were examined with a confocal microscope (Zeiss LSM 410, Carl Zeiss Inc., Thornwood, NY).
Metabolic Labeling, Immunoprecipitation, and Electrophoresis
Cells were pulse labeled for 30 min with [35S]methionine (EXPRE35S35S; DuPont-NEN, Boston, MA) in methionine-free medium, chased for various times in complete medium, and proteins were immunoprecipitated with antifurin or anti-Tac antibodies, as described in previous publications (Bosshart et al., 1994; Voorhees et al., 1995). Furin constructs were immunoprecipitated with mixtures of antibodies, including Fur1, Fur2, DC16, and, whenever appropriate, anti-FLAG (M2) or anti-HA (HA-11) antibodies. Immunoprecipitates were separated by SDS-PAGE on either 8% acrylamide or 4–20% acrylamide gradient gels and labeled bands revealed by fluorography.
Sedimentation Velocity Analyses
Metabolically labeled cells were solubilized for 15 min at 4°C in 1% (wt/ vol) Triton X-100, 0.5% sodium deoxycholate, 0.3 M NaCl, 50 mM Tris-HCl, pH 7.4 (lysis buffer). Lysates (0.5 ml) were cleared by centrifugation for 15 min at 15,000 g at 4°C, applied to the top of 12-ml, linear 5–20% (wt/vol) sucrose gradients, centrifuged for 16 h at 4°C in a SW41 rotor at 39,000 RPM (188,000 g), and fractions collected as described (Bonnerot et al., 1994). Each of 15 fractions collected from the gradients was subjected to immunoprecipitation with antibodies to furin, Tac, or epitope tags. Peak fractions for migration of a human major histocompatibility complex (MHC)-class I molecule (∼57 kD) and the human transferrin receptor (∼190 kD), used as size markers for integral membrane proteins, were identified by immunoprecipitation of gradient fractions from metabolically labeled Swei cells, as described (Bonnerot et al., 1994).
Chemical Cross-linking
Metabolically labeled cells were solubilized as described above, except that Hepes-NaOH, pH 7.4, was used in place of Tris-HCl in the lysis buffer, because the amino groups in Tris react with the cross-linking reagent. The cleared cell lysate (0.5 ml) was incubated at room temperature with different concentrations (0.1–3 mM) of dithio bis-sulfosuccinimidylpropionate (DTSSP). After 20 min, the reactions were quenched with an equal volume (0.5 ml) of Tris-containing lysis buffer, and then the cross-linked samples were subjected to immunoprecipitation. DTSSP has an intermolecular disulfide bond between its reactive groups that allows the cross-link between the proteins to be broken by treatment with reducing agents. Thus, all immunoprecipitates were run on SDS-PAGE under nonreducing conditions to evaluate the extent of cross-linking, and under reducing conditions to demonstrate that the cross-linked protein corresponded to furin.
Pharmacologic Manipulations
Lysosomal protein degradation was inhibited by incubation with either 50 mM NH4Cl, 200 μM chloroquine or LPEM mixture (100 μg/ml leupeptin, 100 μg/ml pepstatin, 100 μg/ml E64, and 20 mM methionine methyl ester) (Bosshart et al., 1994) in complete medium containing 20 mM Hepes-NaOH buffer, pH 7.1. Aluminum fluoride (AlF4 −) was generated by the addition of 1 μl/ml 30 mM to AlCl3 to complete medium containing 20 mM Hepes-NaOH buffer, pH 7.1. After vortexing, 10 μl/ml of 1 M NaF was added to the medium, vortexed, and then immediately added to cells.
Results
Lysosomal Degradation of Furin
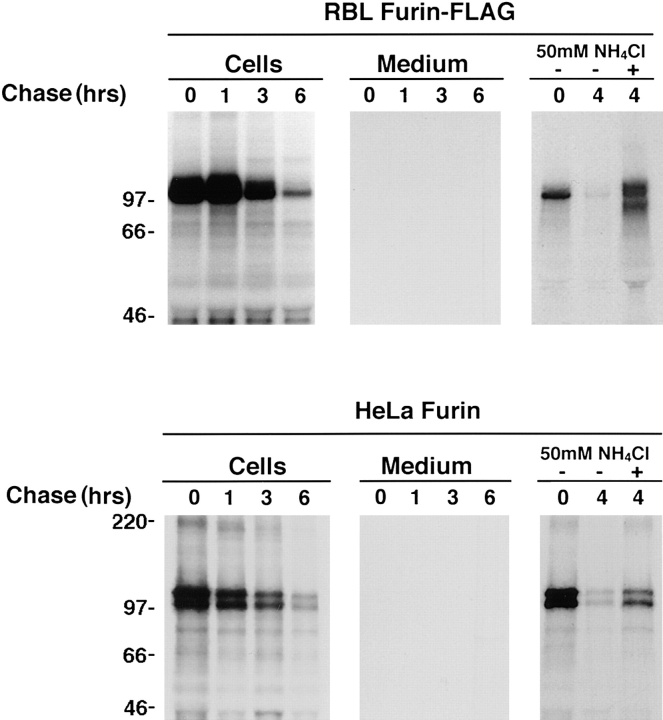
As previously demonstrated (Bosshart et al., 1994), a furin-FLAG construct expressed stably in RBL cells was degraded with a t1/2 = 2–4 h (Figs. 2 and 3). A small amount of an ∼80-kD cleavage product of furin, observed after long exposure of autoradiograms, was released into the medium; however, this species did not account for the rapid loss of furin from the cells. The degradation of furin was not caused by the presence of the FLAG epitope, as furin-HA in RBL cells (Fig. 4) and untagged furin in HeLa cells (Fig. 2) were degraded with similarly rapid rates. Degradation occurred after transport through the Golgi complex (Bosshart et al., 1994), and was inhibited by NH4Cl (Figs. 2 and 4) and other inhibitors of lysosomal proteolysis (Bosshart et al., 1994), suggesting that it took place within lysosomes. Thus, the relatively rapid turnover of furin appears to be an intrinsic property of the protein, independent of the cells in which it is expressed and of the presence or absence of epitope tags.
Figure 2.
Lysosomal turnover of furin in transfected cells. Stably transfected RBL cells expressing furin-FLAG and transiently transfected HeLa cells expressing untagged furin were analyzed by pulse chase metabolic labeling as described in Materials and Methods. Cells were pulse labeled for 30 min with [35S]methionine and chased for various times in the absence (−) or presence (+) of 50 mM NH4Cl as indicated in the figure. Furin-FLAG was isolated from cells and media by immunoprecipitation with a mixture of antibodies, including Fur1, Fur2, DC16, and M2; untagged furin was isolated using the same antibody mixture, except for M2. The presence of an ∼80-kD soluble furin species in the media was detected upon prolonged exposure of the autoradiograms.
Figure 3.
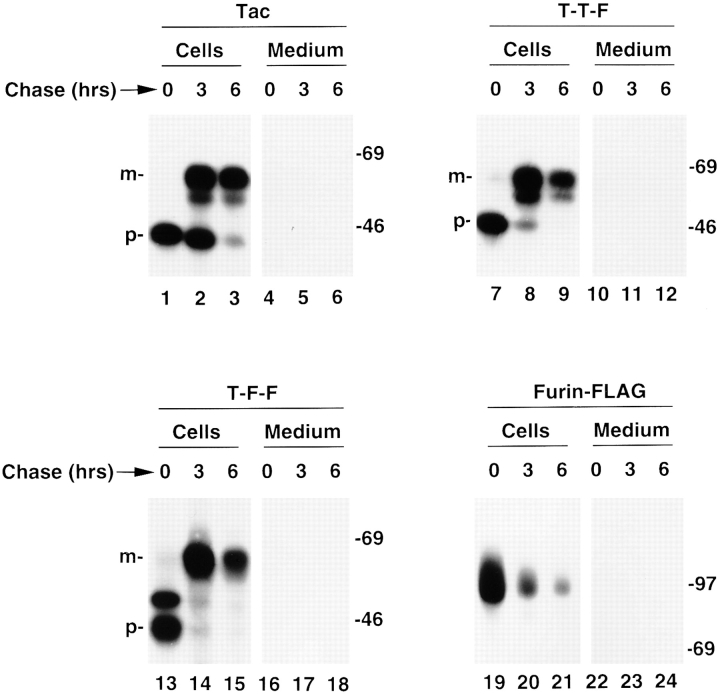
Rapid turnover of furin is not mediated by the transmembrane and cytosolic domains. Stably transfected RBL cells expressing Tac, T-T-F, T-F-F, or furin-FLAG (see Fig. 1 for structures) were pulse labeled with [35S]methionine for 30 min and chased for 0, 3, or 6 h. Tac species were isolated by immunoprecipitation with the monoclonal antibody 7G7 and furin species with a mixture of Fur1, Fur2, and DC16. The symbols p and m point to the ER precursor and Golgi-processed, mature forms of the Tac proteins, respectively. The positions of molecular weight markers (expressed as 10−3 × M r) are shown at right.
Figure 4.
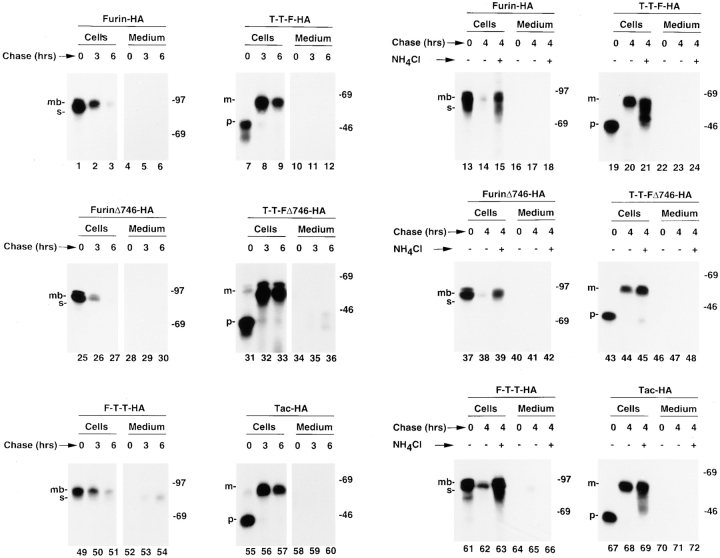
The luminal domain of furin is necessary and sufficient for its rapid lysosomal degradation. Stably transfected RBL cells expressing various furin and Tac chimeras (see Fig. 1 for structures) were pulse labeled with [35S]methionine for 30 min and chased for different times in the absence (−) or presence (+) of 50 mM NH4Cl. Tac and furin species were isolated with the same antibodies mentioned in the legend to Fig. 3. The positions of relative molecular mass markers (expressed as 10−3 × M r) are shown at right. The symbols p and m point to the ER precursor and Golgi-processed, mature forms of the Tac proteins, respectively. The symbols mb and s mark the positions of membrane-bound (∼100 kD) and soluble (∼80 kD) forms of furin, respectively, as previously described (Bosshart et al., 1994).
Rapid Degradation of Furin Is Not Mediated by Its Cytosolic or Transmembrane Domains
To map the topologic domain(s) of furin that determine its rapid lysosomal turnover, we examined the fate of chimeric proteins made by exchanging domains between furin and the long-lived plasma membrane protein, Tac (Fig. 1 a). One of the chimeras, T-T-F, consisted of the luminal and transmembrane domains of Tac and the cytosolic domain of furin; another chimera, T-F-F, had the luminal domain of Tac and the transmembrane and cytosolic domains of furin (Fig. 1 b). Pulse-chase analyses showed that both chimeras were much more stable than furin-FLAG (Fig. 3). Therefore, the cytosolic and transmembrane domains of furin could not target a reporter protein to lysosomes and were thus not likely responsible for the rapid turnover of furin.
The Luminal Domain Is Largely Responsible for the Rapid Lysosomal Degradation of Furin
Further investigation of the domain(s) of furin that mediate its rapid turnover were performed with chimeric proteins containing the furin luminal domain (the structures are shown in Fig. 1 b), expressed in stably transfected RBL cells. As previously shown (Bosshart et al., 1994), furin-HA was rapidly degraded (Fig. 4). Deletion of residues 747–793 from the cytosolic domain of furin, which include both the tyrosine-based and acidic signals (Schäfer et al., 1995; Voorhees et al., 1995), did not prevent degradation (Fig. 4, furinΔ746-HA). Moreover, a chimera consisting of the luminal domain of furin, the transmembrane and cytoplasmic domains of Tac and the HA epitope (F-T-T-HA) was also rapidly degraded (Fig. 4). The degradation of these proteins was not because of the presence of the HA epitope, since placement of this epitope at the COOH terminus of analogous constructs having the luminal domain of Tac produced proteins that were stable over the time span of the experiments (Fig. 4, T-T-F-HA, T-T-FΔ746-HA, and Tac-HA). Thus, the only structural difference between F-T-T-HA (which is rapidly degraded) and Tac-HA (which is stable) is the in-luminal domain of the proteins. The ability of NH4Cl (Fig. 4, lanes 13–72) and other inhibitors of lysosomal proteolysis (not shown) to inhibit degradation of proteins having the furin luminal domain, confirmed that the degradation occurred by a lysosomal pathway. Taken together, the experiments shown in Figs. 3 and 4 indicate that the luminal domain of furin is both necessary and sufficient for targeting degradation.
Morphologic Evidence That the Luminal Domain of Furin Targets It to Lysosomes
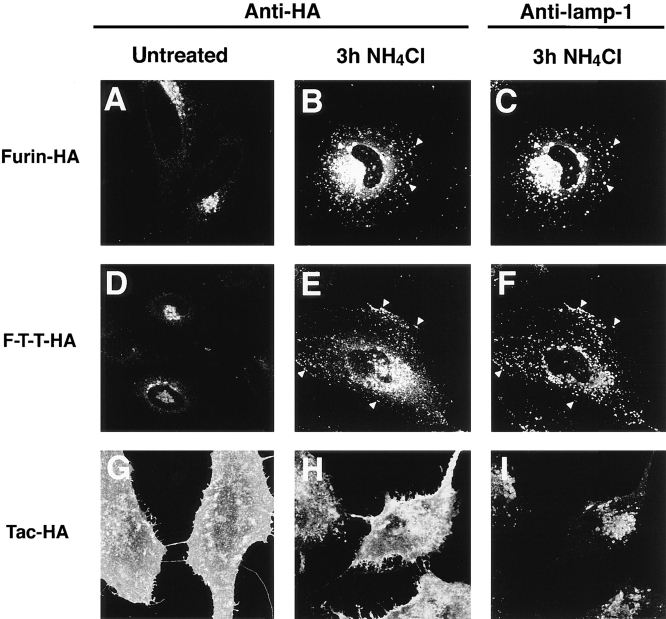
The transport of furin to lysosomes and the role of the luminal domain in this process were also examined by immunofluorescence microscopy. As previously reported (Bosshart et al., 1994), furin-HA was localized to a juxtanuclear structure characteristic of the TGN (Fig. 5 A). After a 3-h incubation with NH4Cl however, a large fraction of furin-HA was localized to lysosomes (Fig. 5 B), as demonstrated by costaining with an antibody to the lysosomal membrane protein lamp-1 (Fig. 5 C). F-T-T-HA displayed weak staining of the ER and the Golgi complex in untreated cells (Fig. 5 D). Treatment with NH4Cl resulted in an increase of the fluorescent signal and the appearance of the protein in lamp-1–positive vesicles (Fig. 5, E and F). As a control for the influence of the HA epitope on protein localization, we examined the distribution of Tac-HA. This protein was found at the plasma membrane in both untreated (Fig. 5 G) and NH4Cl-treated cells (Fig. 5 H), confirming that the HA epitope does not mediate lysosomal targeting. Since F-T-T-HA and Tac-HA differ only in the luminal domains, we concluded that the accumulation of F-T-T-HA in lysosomes is a function of the luminal domain of furin. These morphological observations are consistent with the biochemical data showing that a significant fraction of newly synthesized furin is transported to lysosomes and that the lysosomal targeting is largely mediated by the luminal domain of the protein.
Figure 5.
The luminal domain of furin mediates targeting to vesicles containing the lysosomal membrane protein lamp-1. Transiently transfected HeLa cells expressing furin-HA, Tac-HA, or F-T-T-HA were incubated for 3 h in the absence or presence of 50 mM NH4Cl. Cells were fixed, permeabilized, and then stained with anti-HA and anti–lamp-1 antibodies, and a mixture of tetramethyl rhodamine anti–mouse IgG and fluorescein anti–rabbit IgG. Cells were examined by confocal microscopy as described in Materials and Methods. Notice the localization of furin-HA and F-T-T-HA to vesicles containing lamp-1 in NH4Cl-treated cells (arrowheads in B, C, E, and F).
Inhibition of Lysosomal Degradation Reveals the Formation of High Molecular Weight Aggregates of Furin
The finding that the determinant of rapid turnover of furin is contained within the luminal domain was unexpected because most lysosomal targeting determinants identified to date map to cytosolic domains (Mellman, 1996; Marks et al., 1997). A possible explanation for our observations was that the luminal domain of furin harbored a specific sorting signal for targeting to lysosomes. Alternatively, delivery of furin to lysosomes could be mediated by a change in the physical-chemical properties of the protein at the TGN or at some post-TGN compartment. We decided to examine the aggregation state of furin, because formation of high molecular weight aggregates has been proposed to contribute to protein retention within the ER lumen (for review see Rose and Doms, 1988; Hurtley and Helenius, 1989) and to precede the nonlysosomal degradation of various proteins (Delahunty et al., 1993; Bonnerot et al., 1994). Furthermore, aggregation has been implicated in diverting proteins from the TGN to the regulated secretory pathway (for review see Bauerfeind and Huttner, 1993).
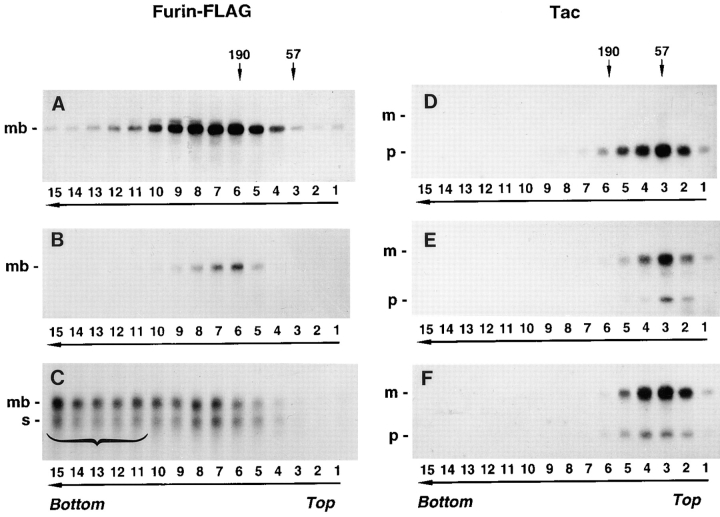
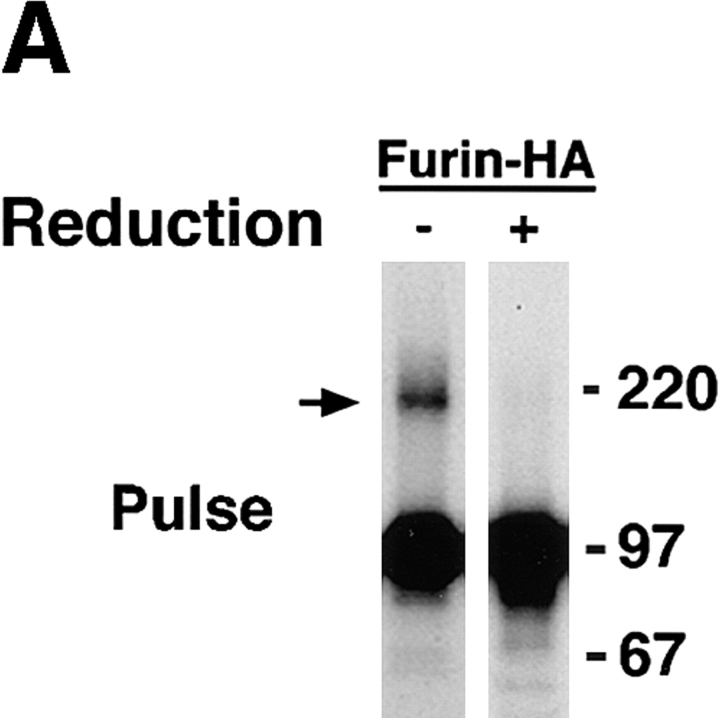
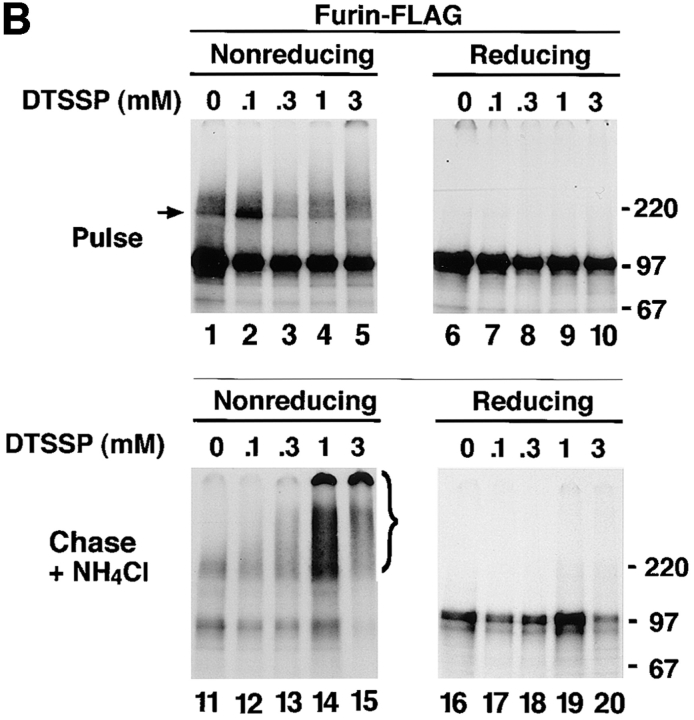
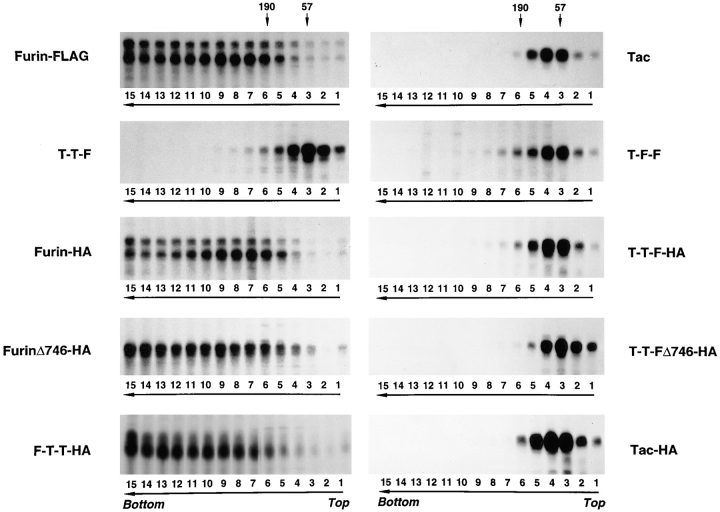
The aggregation state of various furin and Tac constructs was analyzed by sedimentation velocity on sucrose gradients (Martin and Ames, 1961). Newly synthesized furin-FLAG sedimented with a size compatible with it being a small oligomer (i.e., a dimer or a trimer [The type of gradient used in this study was optimized for detecting aggregates and not for obtaining accurate measurements of the size of small oligomers.]) (Fig. 6 A). A more accurate estimation of the size of newly synthesized furin was obtained by chemical cross-linking with DTSSP, an agent having a cleavable disulfide bridge (Fig. 7). Substantial fractions of pulse-labeled furin-HA (Fig. 7 A, arrow) and furin-FLAG (Fig. 7 B, arrow) were cross-linked into an ∼200-kD species, suggesting that newly synthesized ∼100-kD furin polypeptide assembles into a homodimer. Only a fraction of furin-FLAG survived after a 4-h chase (Fig. 6 B); its size, as estimated by sedimentation velocity analysis, was similar to that observed after the pulse (Fig. 6 A). Strikingly, chasing in the presence of 50 mM NH4Cl, resulted in accumulation of high molecular weight aggregates migrating all the way to the bottom of the gradient (Fig. 6 C, bracket). Both the ∼100-kD membrane-bound form of furin and an ∼80-kD soluble, luminal form of furin that accumulates in the presence of 50 mM NH4Cl (Bosshart et al., 1994) were similarly aggregated. The accumulation of high molecular weight aggregates of furin-FLAG after a 4-h chase in the presence of 50 mM NH4Cl could also be detected by cross-linking with DTSSP (Fig. 7 B, bracket). Some of the cross-linked aggregates were so large that they remained at the top of the stacking gel under nonreducing conditions (Fig. 7 B).
Figure 6.
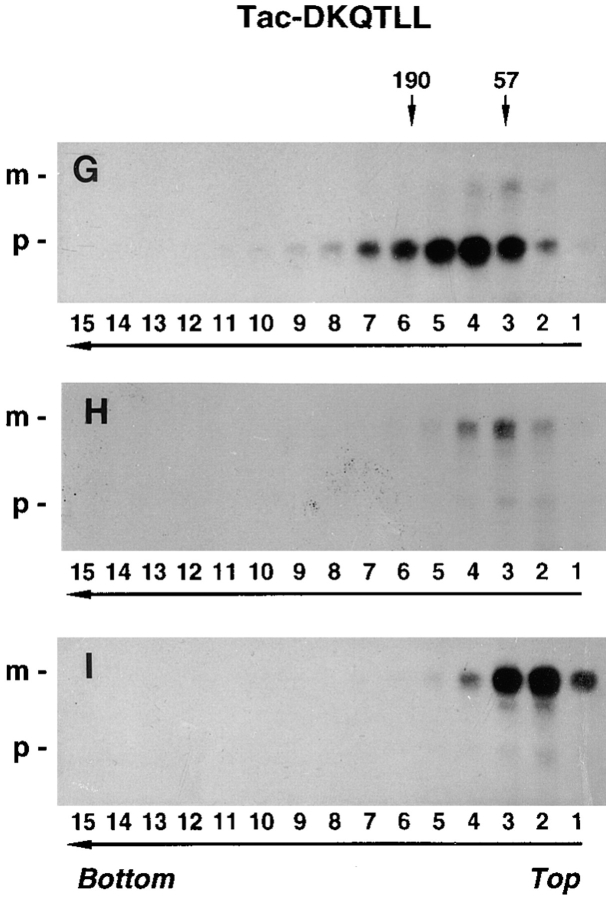
Aggregation of furin-FLAG in NH4Cl-treated cells. Stably transfected RBL cells expressing furin-FLAG, Tac, or Tac-DKQTLL were pulse labeled for 30 min with [35S]methionine and chased for 0 h (A, D, and G), 4 h (B), or 6 h (C, E, F, H, and I) in the absence (A, B, D, E, G, and H) or presence (C, F, and I) of 50 mM NH4Cl. Detergent lysates of the cells were fractionated by sedimentation on 5–20% sucrose gradients for 16 h (see Materials and Methods). Individual gradient fractions (1– 15) were immunoprecipitated with either an antifurin antibody (Fur2) (A–C) or an antibody to Tac (7G7) (D–I), and then analyzed by SDS-PAGE and fluorography. The MHC class I H-2Kb/β2-microglobulin complex (∼57 kD, fraction 3) and the transferrin receptor (∼190 kD, fractions 5 and 6) were used as internal size standards for integral membrane proteins. Gradient fraction numbers, from top to bottom, are indicated. The bracket in C indicates high molecular weight aggregates of furin-FLAG.
Figure 7.
Analysis of the aggregation state of furin by chemical cross-linking. RBL cells expressing either furin-HA (A) or furin-FLAG (B) were pulse labeled with [35S]methionine for 30 min at 37°C. Cells were either lysed immediately or chased for 4 h at 37°C in the presence of 50 mM NH4Cl. Lysates were cross-linked with either 0.1 mM (A) or varying concentrations (B) of DTSSP. Furin-HA (A) was isolated by immunoprecipitation with an antibody mixture containing HA-11, Fur1, and Fur2. Furin-FLAG (B) was immunoprecipitated with M2, Fur1, and Fur2. Samples were resolved by SDS-PAGE under nonreducing or reducing conditions, as indicated in the figure. Arrows point to the position of the furin dimer. The positions of molecular mass markers (expressed as 10−3 × M r) are shown at right.
Both furin-FLAG and untagged furin expressed in HeLa cells were similarly aggregated after a 6-h chase in the presence of NH4Cl (Fig. 8), suggesting that aggregation was not restricted to RBL cells, nor was it caused by the epitope tags. In addition, we observed that chasing in the presence of a mixture of leupepin, E64, pepstatin A and methionine methyl ester (LPEM), each of which inhibits lysosomal degradation by a mechanism distinct from that of NH4Cl (Chen et al., 1988; Bosshart et al., 1994), also resulted in accumulation of furin-FLAG and furin-HA aggregates (Fig. 9). Thus, furin aggregation is not caused by NH4Cl but rather revealed by inhibition of lysosomal degradation, regardless of the inhibitor used.
Figure 8.
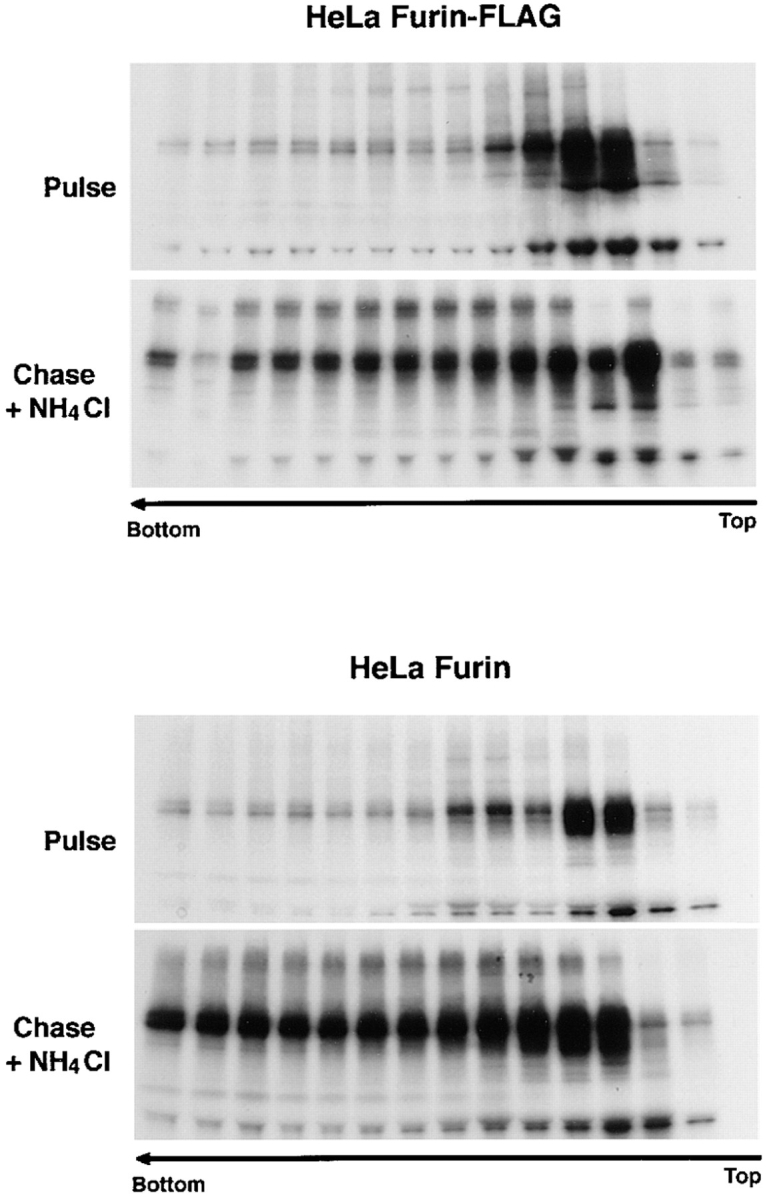
Both furin-FLAG and untagged furin aggregate in HeLa cells. HeLa cells were transiently transfected with plasmids encoding either furin-FLAG or untagged furin. Cells were labeled for 30 min with [35S]methionine and chased for 6 h in the presence or absence of 50 mM NH4Cl. The aggregation state of furin was assessed by sedimentation velocity analysis as described in the legend to Fig. 6.
Figure 9.
The luminal domain mediates aggregation of furin. Stably transfected RBL cells expressing the furin or Tac chimeras indicated in the figure were pulse labeled for 30 min with [35S]methionine and chased for 4 h in the presence of a mixture of nonacidotropic lysosomal inhibitors (LPEM). Detergent-solubilized cells were fractionated by sedimentation on sucrose gradients and Tac and furin species were immunoprecipitated from the fractions as described in the legend to Fig. 6 and in Materials and Methods. Notice that only proteins having the furin luminal domain underwent aggregation during chase in the presence of lysosomal inhibitors.
In contrast to furin-FLAG, Tac, which is transported to the plasma membrane and is relatively stable, did not change its size during chase either in the absence or the presence of NH4Cl (Fig. 6, D–F). Similarly, Tac-DKQTLL, which is targeted to lysosomes by virtue of a di-leucine signal in the cytosolic domain (Letourneur and Klausner, 1992), did not undergo aggregation either in the absence or the presence of NH4Cl in the chase medium (Fig. 6, G–I). From these results, we concluded that inhibition of lysosomal degradation results in the accumulation specifically of furin aggregates and not of proteins like Tac and Tac-DKQTLL, which are sorted by mechanisms unrelated to aggregation.
Aggregation of Furin Is Mediated by Its Luminal Domain
To elucidate the domain(s) of furin required for aggregation, we analyzed the aggregation state of various furin and Tac chimeric proteins when lysosomal degradation was inhibited with the LPEM mixture (Fig. 9). We observed that all the proteins that contained the furin luminal domain became aggregated (furin-FLAG, furin-HA, furinΔ746-HA, and F-T-T-HA), whereas those that had the Tac luminal domain (Tac, T-T-F, T-F-F, T-T-F-HA, T-T-FΔ746-HA, and Tac-HA; see Fig. 1 for structures) did not (Fig. 9). From these experiments, we concluded that, like degradation, the aggregation of furin observed upon inhibition of lysosomal proteolysis was a property of the luminal domain of the protein.
Evidence That Aggregation of Furin Occurs within the TGN
To determine whether the aggregation of furin occurred within the TGN—thus preceding delivery of the protein to lysosomes—or after exit from the TGN, we performed temperature and pharmacologic manipulations that have been previously used to examine TGN-mediated processes. Incubation of cells at 19–20°C has been shown to arrest the trafficking of newly synthesized proteins at the level of the TGN (Griffiths et al., 1985; Sariola et al., 1995). Pulse-chase analyses showed that furin-FLAG was not detectably degraded over a 6-h chase at 19°C (Fig. 10 A, compare with chase at 37°C in Fig. 2), which is consistent with blockage of transport to lysosomes. Interestingly, cross-linking with DTSSP of extracts from cells chased at 19°C demonstrated absence of any high molecular weight aggregates of furin (Fig. 10 B, lane 1) suggesting that aggregation itself was temperature dependent.
Figure 10.
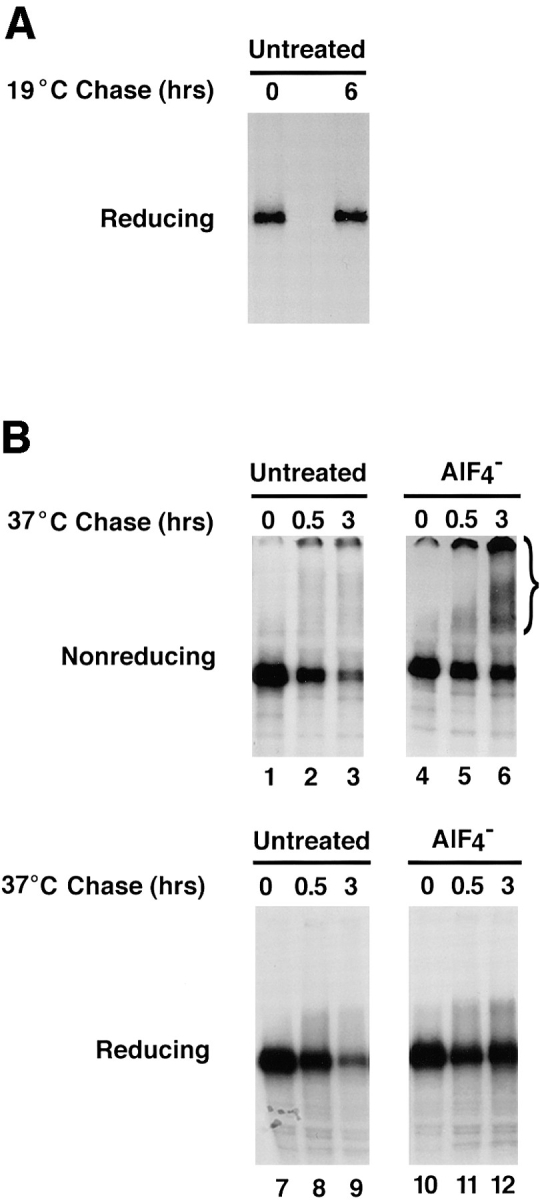
Effects of AlF4 − on aggregation and degradation of furin accumulated in the TGN. (A) RBL cells expressing furin-FLAG were pulse labeled with [35S]methionine for 30 min and chased for either 0 or 6 h at 19°C. Furin-FLAG was isolated by immunoprecipitation with a mixture of the antibodies M2, Fur1, and Fur2. Immunoprecipitates were resolved by SDS-PAGE. (B) RBL cells expressing furin-FLAG were pulse labeled with [35S]methionine for 30 min and chased for 6 h at 19°C. Cells were then further chased for 30 min at 19°C and 6 h at 37°C in the absence (Untreated) or presence of aluminum fluoride (AlF4−). Cell lysates were cross-linked with 1 mM DTSSP and furin-FLAG was immunoprecipitated and analyzed as described in A.
Temperature manipulations were then combined with the use of AlF4 −. AlF4 − activates GTP-binding proteins involved in the recruitment of organellar coats to membranes and inhibits various trafficking events by preventing the normal cycling of coat proteins between membrane and cytosolic pools (Donaldson et al., 1992; Carter et al., 1993; Finazzi et al., 1994). One of the trafficking steps inhibited by AlF4 − is exit from the TGN (Kantanen et al., 1995; Sariola et al., 1995). In an immunofluorescence microscopy experiment similar to that shown in Fig. 5, we verified that AlF4 − blocked transport of furin-HA from the TGN to a lysosomal compartment in chloroquine-treated cells at 37°C (data not shown).
We then analyzed the effects of AlF4 − on degradation and aggregation of furin. We observed that raising the temperature from 19°C to 37°C in the absence of AlF4 − resulted in time-dependent degradation of furin-FLAG that had accumulated in the TGN (Fig. 10 B, lanes 7–9). This was likely because of resumption of transport to lysosomes at 37°C. In contrast, when the temperature shift was performed in the presence of AlF4 −, furin was not degraded over a 3-h period (Fig. 10 B, lanes 10–12), as would be expected from the AlF4 −-induced blockage of exit from the TGN. Analysis by DTSSP cross-linking showed that the furin that was protected from degradation by treatment with AlF4 − existed as high molecular weight aggregates (Fig. 10 B, lanes 4–6, bracket). On the basis of these observations, we concluded that the aggregation of furin occurs most likely in the TGN and thus precedes delivery of the protein to lysosomes.
Discussion
Furin localizes to the TGN by virtue of specific sorting information contained mainly within its cytosolic domain (Bosshart et al., 1994; Chapman and Munro, 1994; Molloy et al., 1994; Jones et al., 1995; Schäfer et al., 1995; Takahashi et al., 1995; Voorhees et al., 1995). In this study, we present evidence for a role of the luminal domain of furin in targeting to lysosomes for degradation. In addition, we show that inhibition of lysosomal degradation by various pharmacologic agents results in accumulation of aggregated furin in lysosomes. Like the degradation of furin, aggregation is also mediated by the luminal domain of the protein. These observations establish a strong correlation between aggregation and lysosomal targeting (see Fig. 1) and suggest that the two phenomena may be linked. Temperature and pharmacologic manipulations of transport from the TGN suggest that aggregation of furin occurs within the TGN, and precedes its transport to lysosomes. Thus, furin may become progressively aggregated during its residence in the TGN; aggregated furin would then be transported to lysosomes where the protein would be rapidly degraded.
Possible Significance of the Rapid Turnover of Furin
Most endogenous integral membrane proteins in the late secretory and endocytic pathways are degraded by the lysosomal system, although their rates of degradation vary widely. At one end of the range are lysosomal membrane proteins that, despite their localization to lysosomes, exhibit half-lives of several days (Lewis et al., 1985; Barriocanal et al., 1986; Green et al., 1987). At the other end are escort proteins such as the class II MHC-associated invariant chain (Blum and Cresswell, 1988) and the low-density lipoprotein receptor family–associated protein (RAP) (Czekay et al., 1997), which are degraded in late endosomal/ lysosomal compartments soon after passage through the TGN. Rapid lysosomal turnover is not limited to escort proteins but is also a characteristic of cytokine receptors such as the erythropoietin receptor, which is constitutively removed from the plasma and degraded in lysosomes with a t1/2 of <1 h (Neumann et al., 1993). The half-life of furin is therefore longer than that of cytokine receptors but shorter than that of other Golgi and TGN proteins, including TGN38 (originally known as GIMPt), which has a t1/2 of ∼8 h (Yuan et al., 1987).
A previous study had shown that epitope-tagged forms of furin expressed in RBL cells had a t1/2 = 2–4 h (Bosshart et al., 1994). Here, we demonstrate that untagged furin expressed in HeLa cells has a similarly short half-life, thus ruling out that the rapid turnover of furin is caused by addition of epitope tags or by its expression in RBL cells. Since endogenous furin is expressed at very low levels in most cells (van Duijnhoven et al., 1992; Shapiro et al., 1997), it is possible that the aggregation and degradation of the protein observed in this study are because of overexpression. However, the stably transfected RBL cells used in our experiments were selected for moderate expression levels. Furthermore, other proteins expressed at similar levels in RBL cells (e.g., all the Tac constructs) do not undergo aggregation after transport through the Golgi complex, and are degraded only if they have a specific lysosomal targeting signal (e.g., Tac-DKQTLL). Moreover, aggregation and degradation of furin-FLAG were observed in a stably transfected MDCK cell clone that expresses barely detectable levels of the protein (data not shown). These observations suggest that the aggregation and rapid turnover of furin are intrinsic properties of the protein.
The physiological significance of the rapid turnover of furin is unclear. Rapidly turning over proteins often fulfill regulatory functions. In addition to its role as a pro-protein convertase, furin has recently been implicated in the control of cell growth and differentiation (Konda et al., 1997), processes that may require rapid and precise attenuation of furin function. Targeting furin for lysosomal degradation would provide a mechanism for rapid lowering of protein levels under certain growth conditions. Another possibility is that the rapid turnover of furin is a consequence of its targeting to late endosomal compartments, where it might participate in endoproteolytic processing reactions. For example, furin could be involved in processing antigens for presentation by class II MHC molecules or other proteins that are targeted to late endosomal compartments before they are released into the medium. If lysosomal degradation results from a functional requirement to target furin to late endosomal compartments, it is not clear why the protein would not have a “lysosomal avoidance” signal such as that described for the cation- dependent, mannose 6-phosphate receptor (Rohrer et al., 1995), a protein with which furin shares some trafficking pathways. Finally, another possibility that has to be considered is that the aggregation and lysosomal targeting of furin in transfected cells is because of the absence of a specific “chaperone-like” molecule that stabilizes furin in the TGN.
Post-Golgi Aggregation As a Determinant of Lysosomal Targeting
The localization of most integral membrane proteins to organelles of the secretory and endocytic pathways can be explained on the basis of two fundamental mechanisms. The first mechanism relies on the recognition of discrete sorting signals by specific receptorlike molecules, as is the case for tyrosine-based sorting signals that target proteins to compartments of the endosomal/lysosomal system (Mellman, 1996; Marks et al., 1997). The second mechanism depends on a general physical-chemical property of the proteins that leads to retention in certain cellular environments. An example of this type of mechanism is the retention of some proteins in the ER and cis-Golgi cisternae, which may be mediated by formation of large aggregates (Rose and Doms, 1988; Hurtley and Helenius, 1989; Weisz et al., 1993). Both types of mechanism appear to contribute to the trafficking of furin within cells. Whereas the existence of specific sorting signals in the cytosolic domain of furin is now well established (Bosshart et al., 1994; Molloy et al., 1994; Jones et al., 1995; Schäfer et al., 1995; Takahashi et al., 1995; Voorhees et al., 1995), the results of the present study suggest that changes in the aggregation state of the furin luminal domain also contribute to the overall distribution and fate of the protein within cells. Thus furin is an example of a protein for which the concerted action of cytosolic and luminal determinants defines a complex pattern of protein trafficking.
Indirect evidence linking protein aggregation in the biosynthetic pathway with lysosomal targeting has been obtained for other proteins. For example, acidification in vitro was shown to cause aggregation of class II MHC molecules in the absence of bound antigenic peptides; this phenomenon was proposed to be the basis for the lysosomal degradation of unoccupied class II MHC molecules (Germain and Rinker, 1993). Another study demonstrated that treatment with leupeptin caused accumulation of aggregated class II MHC molecules in a lysosomal compartment (Amigorena et al., 1995). A more common observation is enhanced internalization and lysosomal targeting induced by cross-linking of cell surface molecules with antibodies or multivalent ligands (Ukkonen et al., 1986; Weissman et al., 1986). The lysosomal targeting of furin may thus be a manifestation of a general mechanism that diverts aggregated proteins from both biosynthetic and endocytic pathways to lysosomes. An attractive possibility is that this pathway provides a final level of quality control for proteins that become aggregated or otherwise damaged in post-Golgi compartments, in a manner analogous to ER quality control mechanisms (Bonifacino and Klausner, 1994; Hammond and Helenius, 1995).
Possible Mechanisms of Furin Aggregation
We have shown that furin appears to be a homodimer soon after synthesis in the ER (Figs. 6 A and Fig. 7). The fraction of the initial furin that remains after 4–6 h also behaves as a homodimer (Fig. 6 B). Aggregated furin, on the other hand, belongs to a population of molecules that are targeted for lysosomal degradation and can only be revealed by treatment with lysosomal inhibitors. We think that furin aggregates are most likely homo-aggregates; this idea is based on the absence of any other major proteins in furin immunoprecipitates, even after long-term labeling (data not shown). Unlike some aggregates of misfolded proteins formed in the ER (de Silva et al., 1993), furin aggregates are not disulfide bonded since they can be readily dissociated by SDS under nonreducing conditions (Fig. 7).
We speculate that aggregation could be triggered by a conformational change induced by exposure of furin to the environment of the TGN. Indeed, we have observed that recognition of furin by certain antibodies to luminal domain epitopes (e.g., Fur1 and Fur2) increases upon chase in the presence of lysosomal inhibitors (unpublished observations). This is why we used a mixture of antibodies to luminal and cytosolic epitopes in all of our immunoprecipitation experiments. An important event that takes place in the TGN is the dissociation of the furin pro-region fragment (Anderson et al., 1997); we speculate that this dissociation could render the protein more prone to aggregation. The ionic environment of the TGN could also contribute to the aggregation of furin. Several studies have shown that various proteins targeted to secretory granules aggregate at the high Ca+2 concentration and mildly acidic pH of the TGN (Freedman and Scheele, 1993; Shennan et al., 1994; Song and Fricker, 1995b ; Colomer et al., 1996). This aggregation has been proposed to be an integral part of the mechanism by which some proteins are targeted from the TGN to the regulated secretory pathway (Bauerfeind and Huttner, 1993). It is thus conceivable that aggregation of furin may occur by a similar mechanism, and that its degradation may reflect transport to secretory granules that contain lysosomal proteins, as is the case for RBL cells (Bonifacino et al., 1989), or alternative transport to lysosomes in cells that lack regulated secretion (e.g., HeLa cells). The presence of furin in both mature and immature secretory granules has been reported (Song and Fricker, 1995a ; Dittié et al., 1997), and it is possible that aggregation may play a role in sorting furin to these organelles. Finally, it has been speculated that a luminal cysteine-rich domain adjacent to the transmembrane domain of furin and other members of the furin family may play a role in sorting (Nakagawa et al., 1993). It would therefore be of interest to investigate the role of this domain in aggregation and degradation.
Concluding Remarks
Our studies of the intracellular trafficking of furin have uncovered an unusual phenomenon, namely, that the protein becomes aggregated upon transport into the TGN. Aggregation appears to promote targeting of the protein for lysosomal degradation, since the presence of the aggregated protein can only be evidenced by treatment with lysosomal inhibitors. Whereas the aggregation of furin is extensive and causes a profound alteration in the fate of the protein, we expect that more subtle changes in the aggregation state of other integral membrane proteins will also play a role in regulating their transport through secretory and endocytic routes.
Acknowledgments
We thank R. Angeletti, M. Fukuda, R. Klausner, M. Marks, and H. Metzger for generous gifts of reagents and N. Cole and C.E. Ooi for critical review of the manuscript.
Abbreviations used in this paper
- DTSSP
dithio bis-sulfosuccinimidylpropionate
- HA
hemagglutinin epitope
- MHC
major histocompatibility complex
- RBL
rat basophilic leukemia
- Tac
α chain of the interleukin-2 receptor
Footnotes
Address all correspondence to J.S. Bonifacino, CBMB, NICHD, National Institutes of Health, Building 18T Room 101, 18 Library Drive, MSC 5430, Bethesda, MD 20892-5430. Tel.: (301) 496-6368. Fax: (301) 402-0078. E-mail: juan@helix.nih.gov
H. Bosshart's present address is LRF Virus Centre, University of Glasgow, Bearsden Road, Glasgow G61 1QH, United Kingdom.
H. Küster's present address is Ludwig-Maximilians-Universität, Pettenkoferstrasse 8a, 80336 München, Germany.
References
- Amigorena S, Webster P, Drake J, Newcomb J, Cresswell P, Mellman I. Invariant chain cleavage and peptide loading in major histocompatibility complex class II vesicles. J Exp Med. 1995;181:1729–1741. doi: 10.1084/jem.181.5.1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson ED, VanSlyke JK, Thulin CD, Jean F, Thomas G. Activation of the furin endoprotease is a multiple-step process: requirements for acidification and internal propeptide cleavage. EMBO (Eur Mol Biol Organ) J. 1997;16:1508–1518. doi: 10.1093/emboj/16.7.1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barriocanal JG, Bonifacino JS, Yuan L, Sandoval IV. Biosynthesis, glycosylation, movement through the Golgi system, and transport to lysosomes by an N-linked carbohydrate-independent mechanism of three lysosomal integral membrane proteins. J Biol Chem. 1986;261:16755–16763. [PubMed] [Google Scholar]
- Bauerfeind R, Huttner WB. Biogenesis of constitutive secretory vesicles, secretory granules and synaptic vesicles. Curr Opin Cell Biol. 1993;5:628–635. doi: 10.1016/0955-0674(93)90132-a. [DOI] [PubMed] [Google Scholar]
- Blum JS, Cresswell P. Role for intracellular proteases in the processing and transport of class II HLA antigens. Proc Natl Acad Sci USA. 1988;85:3975–3979. doi: 10.1073/pnas.85.11.3975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boll W, Ohno H, Songyang Z, Rapoport I, Cantley LC, Bonifacino JS, Kirchhausen T. Sequence requirements for the recognition of tyrosine-based endocytic signals by clathrin AP-2 complexes. EMBO (Eur Mol Biol Organ) J. 1996;15:5789–5795. [PMC free article] [PubMed] [Google Scholar]
- Bonifacino, J.S., and R.D. Klausner. 1994. Degradation of proteins retained in the endoplasmic reticulum. In Cellular Proteolytic Systems. A. Ciechanover, and A.L. Schwartz, editors. Wiley-Liss, Inc., New York. 137–160.
- Bonifacino JS, Yuan L, Sandoval IV. Internalization and recycling to serotonin-containing granules of the 80K integral membrane protein exposed on the surface of secreting rat basophilic leukaemia cells. J Cell Sci. 1989;92:701–712. doi: 10.1242/jcs.92.4.701. [DOI] [PubMed] [Google Scholar]
- Bonnerot C, Marks MS, Cosson P, Robertson EJ, Bikoff EK, Germain RN, Bonifacino JS. Association with BiP and aggregation of class II MHC molecules synthesized in the absence of invariant chain. EMBO (Eur Mol Biol Organ) J. 1994;13:934–944. doi: 10.1002/j.1460-2075.1994.tb06338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosshart H, Humphrey J, Deignan E, Davidson J, Drazba J, Yuan L, Oorschot V, Peters PJ, Bonifacino JS. The cytoplasmic domain mediates localization of furin to the trans-Golgi network en route to the endosomal/lysosomal system. J Cell Biol. 1994;126:1157–1172. doi: 10.1083/jcb.126.5.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter LL, Redelmeier TE, Woollenweber LA, Schmid SL. Multiple GTP-binding proteins participate in clathrin-coated vesicle-mediated endocytosis. J Cell Biol. 1993;120:37–45. doi: 10.1083/jcb.120.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman RE, Munro S. Retrieval of TGN proteins from the cell surface requires endosomal acidification. EMBO (Eur Mol Biol Organ) J. 1994;13:2305–2312. doi: 10.1002/j.1460-2075.1994.tb06514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Bonifacino JS, Yuan LC, Klausner RD. Selective degradation of T cell antigen receptor chains retained in a pre-Golgi compartment. J Cell Biol. 1988;107:2149–2161. doi: 10.1083/jcb.107.6.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colomer V, Kicska GA, Rindler MJ. Secretory granule content proteins and the luminal domains of granule membrane proteins aggregate in vitro at mildly acidic pH. J Biol Chem. 1996;271:48–55. doi: 10.1074/jbc.271.1.48. [DOI] [PubMed] [Google Scholar]
- Creemers JW, Usac EF, Bright NA, Van de Loo JW, Jansen E, Van de Ven WJM, Hutton JC. Identification of a transferable sorting domain for the regulated pathway in the prohormone convertase PC2. J Biol Chem. 1996;271:25284–25291. doi: 10.1074/jbc.271.41.25284. [DOI] [PubMed] [Google Scholar]
- Czekay RP, Orlando RA, Woodward L, Lundstrom M, Farquhar MG. Endocytic trafficking of megalin/RAP complexes: dissociation of the complexes in late endosomes. Mol Biol Cell. 1997;8:517–532. doi: 10.1091/mbc.8.3.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Silva A, Braakman I, Helenius A. Posttranslational folding of vesicular stomatitis virus G protein in the ER: Involvement of noncovalent and covalent complexes. J Cell Biol. 1993;120:647–655. doi: 10.1083/jcb.120.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delahunty MD, Stafford FJ, Yuan LC, Shaz D, Bonifacino JS. Uncleaved signals for glycosylphosphatidylinositol anchoring cause retention of precursor proteins in the endoplasmic reticulum. J Biol Chem. 1993;268:12017–12027. [PubMed] [Google Scholar]
- Dittié AS, Thomas L, Thomas G, Tooze SA. Interaction of furin in immature secretory granules from neuroendocrine cells with the AP-1 adaptor complex is modulated by casein kinase II phosphorylation. EMBO (Eur Mol Biol Organ) J. 1997;16:4859–4870. doi: 10.1093/emboj/16.16.4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson JG, Cassel D, Kahn RA, Klausner RD. ADP-ribosylation factor, a small GTP-binding protein, is required for binding of the coatomer protein β-COP to Golgi membranes. Proc Natl Acad Sci USA. 1992;89:6408–6412. doi: 10.1073/pnas.89.14.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finazzi D, Cassel D, Donaldson JG, Klausner RD. Aluminum fluoride acts on the reversibility of ARF1-dependent coat protein binding to Golgi membranes. J Biol Chem. 1994;269:13325–13330. [PubMed] [Google Scholar]
- Freedman S, Scheele GA. Regulated secretory proteins in the exocrine pancreas aggregate under conditions that mimic the trans-Golgi network. Biochem Biophys Res Commun. 1993;197:992–999. doi: 10.1006/bbrc.1993.2577. [DOI] [PubMed] [Google Scholar]
- Germain RN, Rinker AG., Jr Peptide binding inhibits protein aggregation of invariant-chain free class II dimers and promotes expression of occupied molecules. Nature. 1993;363:725–728. doi: 10.1038/363725a0. [DOI] [PubMed] [Google Scholar]
- Green SA, Zimmer KP, Griffiths G, Mellman I. Kinetics of intracellular transport and sorting of lysosomal membrane and plasma membrane proteins. J Cell Biol. 1987;105:1227–1240. doi: 10.1083/jcb.105.3.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G, Pfeiffer S, Simons K, Matlin K. Exit of newly synthesized membrane proteins from the trans cisternae of the Golgi complex to the plasma membrane. J Cell Biol. 1985;101:949–964. doi: 10.1083/jcb.101.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond C, Helenius A. Quality control in the secretory pathway. Curr Opin Cell Biol. 1995;7:523–529. doi: 10.1016/0955-0674(95)80009-3. [DOI] [PubMed] [Google Scholar]
- Hatsuzawa K, Hosaka M, Nakagawa T, Nagase M, Shoda A, Murakami K, Nakayama K. Structure and expression of mouse furin, a yeast Kex2-related protease: lack of processing of coexpressed prorenin in GH4C1 cells. J Biol Chem. 1990;265:22075–22078. [PubMed] [Google Scholar]
- Higuchi R, Krummel B, Saiki RK. A general method of in vitro preparation and specific mutagenesis of DNA fragments: study of protein and DNA interactions. Nucleic Acids Res. 1988;16:7351–7367. doi: 10.1093/nar/16.15.7351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp TP, Prickett KS, Price V, Libby R T, March CJ, Cerretti P, Urdal DL, Conlon PJ. A short polypeptide marker sequence useful for recombinant protein identification and purification. Biotechnology. 1988;6:1205–1210. [Google Scholar]
- Humphrey JS, Peters PJ, Yuan LC, Bonifacino JS. Localization of TGN38 to the trans-Golgi network: Involvement of a cytoplasmic tyrosine-containing sequence. J Cell Biol. 1993;120:1123–1135. doi: 10.1083/jcb.120.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurtley SM, Helenius A. Protein oligomerization in the endoplasmic reticulum. Annu Rev Cell Biol. 1989;5:277–307. doi: 10.1146/annurev.cb.05.110189.001425. [DOI] [PubMed] [Google Scholar]
- Jones BJ, Thomas L, Molloy SS, Thulin CD, Fry MD, Walsh KA, Thomas G. Intracellular trafficking of furin is modulated by the phosphorylation state of a casein kinase II site in its cytoplasmic tail. EMBO (Eur Mol Biol Organ) J. 1995;14:5869–5883. doi: 10.1002/j.1460-2075.1995.tb00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantanen ML, Leinikki P, Kuismanen E. Endoproteolytic cleavage of HIV-1 gp160 envelope precursor occurs after exit from the trans-Golgi network (TGN) Arch Virol. 1995;140:1441–1449. doi: 10.1007/BF01322670. [DOI] [PubMed] [Google Scholar]
- Konda Y, Yokota H, Kayo T, Horiuchi T, Sugiyama N, Tanaka S, Takata K, Takeuchi T. Proprotein-processing endoprotease furin controls the growth and differentiation of gastric mucous cells. J Clin Invest. 1997;99:1842–1851. doi: 10.1172/JCI119351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leduc R, Molloy SS, Thorne BA, Thomas G. Activation of human furin precursor processing endoprotease occurs by an intramolecular autoproteolytic cleavage. J Biol Chem. 1992;267:14304–14308. [PubMed] [Google Scholar]
- Leonard WJ, Depper JM, Crabtree GR, Rudikoff S, Pumphrey J, Robb RJ, Krönke M, Svetlik PB, Peffer NJ, Waldmann TA, et al. Molecular cloning and expression of cDNAs for the human interleukin-2 receptor. Nature. 1984;311:626–631. doi: 10.1038/311626a0. [DOI] [PubMed] [Google Scholar]
- Letourneur F, Klausner RD. A novel di-leucine motif and a tyrosine-based motif independently mediate lysosomal targeting and endocytosis of CD3 chains. Cell. 1992;69:1143–1157. doi: 10.1016/0092-8674(92)90636-q. [DOI] [PubMed] [Google Scholar]
- Lewis V, Green SA, Marsh M, Vihko P, Helenius A, Mellman I. Glycoproteins of the lysosomal membrane. J Cell Biol. 1985;100:1839–1847. doi: 10.1083/jcb.100.6.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MS, Roche PA, van Donselaar E, Woodruff L, Peters PJ, Bonifacino JS. A lysosomal targeting signal in the cytoplasmic tail of the β chain directs HLA-DM to MHC class II compartments. J Cell Biol. 1995;131:351–369. doi: 10.1083/jcb.131.2.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MS, Woodruff L, Ohno H, Bonifacino JS. Protein targeting by tyrosine- and di-leucine-based signals: Evidence for distinct saturable components. J Cell Biol. 1996;135:341–354. doi: 10.1083/jcb.135.2.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks MS, Ohno H, Kirchhausen T, Bonifacino JS. Protein sorting by tyrosine-based signals: adapting to the Ys and wherefores. Trends Cell Biol. 1997;7:124–128. doi: 10.1016/S0962-8924(96)10057-X. [DOI] [PubMed] [Google Scholar]
- Martin RG, Ames BN. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961;236:1372–1379. [PubMed] [Google Scholar]
- Mellman I. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- Molloy SS, Thomas L, VanSlyke JK, Stenberg PE, Thomas G. Intracellular trafficking and activation of the furin proprotein convertase: localization to the TGN and recycling from the cell surface. EMBO (Eur Mol Biol Organ) J. 1994;13:18–33. doi: 10.1002/j.1460-2075.1994.tb06231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Murakami K, Nakayama K. Identification of an isoform with an extremely large Cys-rich region of PC6, a Kex2-like processing endoprotease. FEBS (Fed Eur Biochem Soc) Lett. 1993;327:165–171. doi: 10.1016/0014-5793(93)80163-o. [DOI] [PubMed] [Google Scholar]
- Neumann D, Wikström L, Watowich SS, Lodish HF. Intermediates in degradation of the erythropoietin receptor accumulate and are degraded in lysosomes. J Biol Chem. 1993;268:13639–13649. [PubMed] [Google Scholar]
- Ohno H, Stewart J, Fournier MC, Bosshart H, Rhee I, Miyatake S, Saito T, Gallusser A, Kirchhausen T, Bonifacino J S. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science. 1995;269:1872–1875. doi: 10.1126/science.7569928. [DOI] [PubMed] [Google Scholar]
- Ohno H, Fournier MC, Poy G, Bonifacino JS. Structural determinants of interaction of tyrosine-based sorting signals with the adaptor medium chains. J Biol Chem. 1996;271:29009–29015. doi: 10.1074/jbc.271.46.29009. [DOI] [PubMed] [Google Scholar]
- Rehemtulla A, Dorner AJ, Kaufman RJ. Regulation of PACE propeptide-processing activity: requirement for a post-endoplasmic reticulum compartment and autoproteolytic activation. Proc Natl Acad Sci USA. 1992;89:8235–8239. doi: 10.1073/pnas.89.17.8235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer J, Shweizer A, Johnson KF, Kornfeld S. A determinant in the cytoplasmic tail of the cation-dependent mannose 6-phosphate receptor prevents trafficking to lysosomes. J Cell Biol. 1995;130:1297–1306. doi: 10.1083/jcb.130.6.1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose JK, Doms RW. Regulation of protein export from the endoplasmic reticulum. Annu Rev Cell Biol. 1988;4:257–288. doi: 10.1146/annurev.cb.04.110188.001353. [DOI] [PubMed] [Google Scholar]
- Rubin LA, Kurman CC, Biddison WE, Goldman ND, Nelson DL. A monoclonal antibody 7G7/B6, binds to an epitope on the human interleukin-2 (IL-2) receptor that is distinct from that recognized by IL-2 or anti-Tac. Hybridoma. 1985;4:91–102. doi: 10.1089/hyb.1985.4.91. [DOI] [PubMed] [Google Scholar]
- Sariola M, Saraste J, Kuismanen E. Communication of post-Golgi elements with early endocytic pathway: regulation of endoproteolytic cleavage of Semliki Forest virus p62 precursor. J Cell Sci. 1995;108:2465–2475. doi: 10.1242/jcs.108.6.2465. [DOI] [PubMed] [Google Scholar]
- Schäfer W, Stroh A, Berghöfer S, Seiler J, Vey M, Kruse ML, Kern HF, Klenk HD, Garten W. Two independent targeting signals in the cytoplasmic domain determine trans-Golgi network localization and endosomal trafficking of the proprotein convertase furin. EMBO (Eur Mol Biol Organ) J. 1995;14:2424–2435. doi: 10.1002/j.1460-2075.1995.tb07240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seed B. An LFA-3 cDNA encodes a phospholipid-linked membrane protein homologous to its receptor CD2. Nature. 1987;329:840–842. doi: 10.1038/329840a0. [DOI] [PubMed] [Google Scholar]
- Shapiro J, Sciaky N, Lee J, Bosshart H, Angeletti RH, Bonifacino JS. Localization of endogenous furin in cultured cell lines. J Histochem Cytochem. 1997;45:3–12. doi: 10.1177/002215549704500102. [DOI] [PubMed] [Google Scholar]
- Shennan KI, Taylor NA, Docherty K. Calcium- and pH-dependent aggregation and membrane association of the precursor of the prohormone convertase PC2. J Biol Chem. 1994;269:18646–18650. [PubMed] [Google Scholar]
- Song L, Fricker L. Processing of procarboxypeptidase E into carboxypeptidase E occurs in secretory vesicles. J Neurochem. 1995a;65:444–453. doi: 10.1046/j.1471-4159.1995.65010444.x. [DOI] [PubMed] [Google Scholar]
- Song L, Fricker LD. Calcium- and pH -dependent aggregation of carboxypeptidase E. J Biol Chem. 1995b;270:7963–7967. doi: 10.1074/jbc.270.14.7963. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Nakagawa T, Banno T, Watanabe T, Murakami K, Nakayama K. Localization of furin to the trans-Golgi network and recycling from the cell surface involves Ser and Tyr residues within the cytoplasmic domain. J Biol Chem. 1995;270:28397–28401. doi: 10.1074/jbc.270.47.28397. [DOI] [PubMed] [Google Scholar]
- Takebe Y, Seiki M, Fujisawa J, Hoy P, Yokota K, Arai K, Yoshida M, Arai N. SR alpha promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and the R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol Cell Biol. 1988;8:466–472. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ukkonen P, Lewis V, Marsh M, Helenius A, Mellman I. Transport of macrophage Fc receptors and Fc receptor-bound ligands to lysosomes. J Exp Med. 1986;163:952–971. doi: 10.1084/jem.163.4.952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Duijnhoven HL P, Creemers JWM, Kranenborg MGC, Timmer EDJ, Groeneveld A, van den Ouweland AMW, Roebroek AJM, van de Ven WJM. Development and characterization of a panel of monoclonal antibodies against the novel subtilisin-like proprotein processing enzyme furin. Hybridoma. 1992;11:71–86. doi: 10.1089/hyb.1992.11.71. [DOI] [PubMed] [Google Scholar]
- Vey M, Schäfer W, Berghofer S, Klenk HD, Garten W. Maturation of the trans-Golgi network protease furin: Compartmentalization of propeptide removal, substrate cleavage, and COOH-terminal truncation. J Cell Biol. 1994;127:1829–1842. doi: 10.1083/jcb.127.6.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorhees P, Deignan E, van Donselaar E, Humphrey J, Marks MS, Peters PJ, Bonifacino JS. An acidic sequence within the cytoplasmic domain of furin functions as a determinant of trans-Golgi network localization and internalization from the cell surface. EMBO (Eur Mol Biol Organ) J. 1995;14:4961–4975. doi: 10.1002/j.1460-2075.1995.tb00179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman AM, Klausner RD, Rao K, Harford J B. Exposure of K562 cells to anti-receptor monoclonal antibody OKT9 results in rapid redistribution and enhanced degradation of the transferrin receptor. J Cell Biol. 1986;102:951–958. doi: 10.1083/jcb.102.3.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz OA, Swift AM, Machamer CE. Oligomerization of a membrane protein correlates with its retention in the Golgi complex. J Cell Biol. 1993;122:1185–1196. doi: 10.1083/jcb.122.6.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson IA, Niman HL, Houghten RA, Cherenson AR, Connolly ML, Lerner RA. The structure of an antigenic determinant in a protein. Cell. 1984;37:767–778. doi: 10.1016/0092-8674(84)90412-4. [DOI] [PubMed] [Google Scholar]
- Wise RJ, Barr PJ, Wong PA, Kiefer MC, Brake AJ, Kaufman RJ. Expression of a human proprotein processing enzyme: correct cleavage of the von Willebrand factor precursor at a paired basic amino acid site. Proc Natl Acad Sci USA. 1990;87:9378–9382. doi: 10.1073/pnas.87.23.9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Barriocanal JG, Bonifacino JS, Sandoval IV. Two integral membrane proteins located in the cis-middle and trans-part of the Golgi system acquire sialylated N-linked carbohydrates and display different turnovers and sensitivity to cAMP-dependent phosphorylation. J Cell Biol. 1987;105:215–227. doi: 10.1083/jcb.105.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]