Abstract
In different systems, cyclic adenosine monophosphate (cAMP) either blocks or promotes cell cycle progression in mid to late G1 phase. Dog thyroid epithelial cells in primary culture constitute a model of positive control of DNA synthesis initiation and G0-S prereplicative phase progression by cAMP as a second messenger for thyrotropin (TSH). The cAMP-dependent mitogenic pathway is unique as it is independent of mitogen-activated protein kinase activation and differs from growth factor–dependent pathways at the level of the expression of several protooncogenes/transcription factors. This study examined the involvement of D-type G1 cyclins and their associated cyclin-dependent kinase (cdk4) in the cAMP-dependent G1 phase progression of dog thyroid cells. Unlike epidermal growth factor (EGF)+serum and other cAMP-independent mitogens, TSH did not induce the accumulation of cyclins D1 and D2 and partially inhibited the basal expression of the most abundant cyclin D3. However, TSH stimulation enhanced the nuclear detection of cyclin D3. This effect correlated with G1 and S phase progression. It was found to reflect both the unmasking of an epitope of cyclin D3 close to its domain of interaction with cdk4, and the nuclear translocation of cyclin D3. TSH and EGF+serum also induced a previously undescribed nuclear translocation of cdk4, the assembly of precipitable cyclin D3–cdk4 complexes, and the Rb kinase activity of these complexes. Previously, cdk4 activity was found to be required in the cAMP-dependent mitogenic pathway of dog thyrocytes, as in growth factor pathways. Here, microinjections of a cyclin D3 antibody showed that cyclin D3 is essential in the TSH/ cAMP-dependent mitogenesis, but not in the pathway of growth factors that induce cyclins D1 and D2. The present study (a) provides the first example in a normal cell of a stimulation of G1 phase progression occurring independently of an enhanced accumulation of cyclins D, (b) identifies the activation of cyclin D3 and cdk4 through their enhanced assembly and/or nuclear translocation, as first convergence steps of the parallel cAMP-dependent and growth factor mitogenic pathways, and (c) strongly suggests that this new mechanism is essential in the cAMP-dependent mitogenesis, which provides the first direct demonstration of the requirement for cyclin D3 in a G1 phase progression.
The D- and E-type cyclins and the cyclin-dependent kinases (cdks)1 they activate, are now recognized to be key regulators of G1 to S phase progression in mammalian cells (Reed, 1991; Sherr, 1993). Overexpression of cyclin D1 or E in fibroblasts shortens their G1 interval, decreases cell size, and renders the cell less dependent on mitogens for S phase entry (Quelle et al., 1993; Resnitzky and Reed, 1995), indicating that both G1 cyclins can be rate-limiting factors for S phase initiation. However, their functions are likely to be quite different (Resnitzki and Reed, 1995). Microinjection of antisense plasmids or neutralizing antibodies to cyclin D1 in serum-stimulated fibroblasts (Baldin et al., 1993; Lukas et al., 1994), or an antibody to cyclin D2 in lymphocytes (Lukas et al., 1995b ), during early to mid G1 phase prevents cellular DNA synthesis, suggesting that cyclin D–dependent kinases execute critical functions during middle to late G1 but are not required thereafter (Sherr, 1994). Until now, no such G1 phase requirement has been demonstrated for cyclin D3, which is more distantly related to the other two cyclins D (Sherr, 1993). Cyclins D bind to and activate cdk4 (Matsushime et al., 1994) or the closely related kinase cdk6 (Meyerson and Harlow, 1994; Tam et al., 1994). The major activity of cyclin D1–cdk4/6 is to phosphorylate and inactivate proteins of the Rb family (Weinberg, 1995; Bartek et al., 1996) that in turn releases active transcription factors, including those of the E2F family (Bejiersbergen et al., 1996; Lukas et al., 1996a ). In cells lacking functional Rb proteins, the microinjections of a cyclin D1–blocking antibody (Lukas et al., 1995a ), or of the cdk4 inhibitor p16ink4A (Medema et al., 1995; Lukas et al., 1995c ), fail to block DNA synthesis. By contrast, the lack of functional Rb protein does not obviate the requirement of cyclin E for DNA synthesis initiation (Ohtsubo et al., 1995). A fundamental role of D-type cyclins is thus to integrate extracellular signals with the cell cycle machinery by neutralizing the Rb inhibitory pathway (Weinberg, 1995; Bartek et al., 1996; Sherr, 1996). By contrast cyclin E is crucial for initiation of DNA synthesis (Ohtsubo et al., 1995; Lukas et al., 1997). Cyclin E transcription is regulated in part by Rb via E2F proteins, which provides a mechanism for cyclin E induction downstream to cdk4 activation (Ohtani et al., 1995; Geng et al., 1996).
The three D-type cyclins (D1, D2, and D3) are combinatorially expressed in a cell lineage–specific manner in proliferating mammalian cells. It is unclear whether they carry out redundant functions in response to different signals or whether their roles are distinct. In addition to activating cdk4 and cdk6 (Sherr, 1994), they might play distinct roles in cell differentiation (Kato et al., 1993; Kiess et al., 1995). Moreover, whereas cyclin D2 and cyclin D3 can also activate cdk2, cyclin D1–cdk2 complexes are inactive (Higashi et al., 1996). D-type cyclins are synthesized as long as growth factor stimulation persists and exhibit only moderate oscillations during the cell cycle, in contrast to other cyclins, with peak levels achieved near G1-S (Baldin et al., 1993; Sewing et al., 1993; Lukas et al., 1995b ). However they are rapidly degraded when mitogens are withdrawn, regardless of the position of the cell in the cycle (Sherr, 1994).
How cAMP, probably through PKA, can impinge on the cell cycle machinery to either inhibit or induce cell proliferation in different systems is still poorly understood (Boynton and Whitfield, 1983; Dumont et al., 1989, 1992; Roger et al., 1995). In some cell types, cAMP has been found to inhibit mitogenic stimulations in mid-G1 or at the restriction point in late G1 as in rat hepatocytes (Vintermyr et al., 1989) and murine macrophages, which involves the inhibition of Rb protein phosphorylation (Christoffersen et al., 1994; Kato et al., 1994). In this case, early mitogenic responses may not be affected by cAMP (Rock et al., 1992), but the G1 block by cAMP was ascribed to result from a suppression of the expression of cyclin D1 (Cocks et al., 1992; Sewing et al., 1993; L'Allemain et al., 1997), cyclin D3 (Ward et al., 1996), and/or an increased expression of the cdk inhibitor p27kip1 (Kato et al., 1994; Ward et al., 1996; L'Allemain et al., 1997). In sharp contrast with these systems, cAMP as the main second messenger for thyrotropin (TSH) and thyroid-stimulating antibodies elicits in thyroid gland in vivo a pathway leading to hyperplasia (goiter) and tumorigenesis (Dumont et al., 1989, 1992; Lyons et al., 1990; Ledent et al., 1992; Parma et al., 1993; Coppée et al., 1996). Dog thyroid epithelial cells in primary culture reproduce these proliferative effects and constitute a model of positive control of DNA synthesis initiation and G0-S prereplicative phase progression by TSH and cAMP (Roger et al., 1983, 1995, 1997; Dumont et al., 1992). In this system, cAMP positively controls a late G1 restriction point (Roger et al., 1987a ; Baptist et al., 1993). In contrast to what is observed in the signaling cascades elicited by growth factors and tumor promoters in this system, the cAMP-dependent mitogenic stimulation does not involve the phosphorylation and nuclear translocation of MAP kinases (Lamy et al., 1993), it poorly induces c-Fos protein accumulation (Baptist et al., 1995), it downregulates c-jun mRNA (Reuse et al., 1991) and, after a short initial induction, c-myc mRNA and protein (Pirson et al., 1996). Despite this early divergence, the cAMP- dependent and -independent mitogenic pathways of dog thyrocytes converge on the phosphorylation of Rb family proteins (Coulonval et al., 1997), late common changes of subcellular localization and phosphorylation of cdk2 and cdc2, and common induction of cyclin A and cdc2 (Baptist et al., 1996).
The passage of normal cells through the restriction point has been suggested to require both the induction of D-type cyclins and a reduction of p27kip1 concentration in response to growth factors (Sherr, 1996). Recently, we have observed in dog thyroid cells that, paradoxically, p27kip1 expression is increased during the cAMP-dependent mitogenic stimulation but not in response to EGF+serum stimulation (Depoortere et al., 1996). Here we compare, using this system, the expression, subcellular localization, activation, and requirement of cyclins D and cdk4 in the cAMP-dependent and cAMP-independent cell cycles, and demonstrate a major involvement of cyclin D3 in the positive regulation by cAMP of the cell cycle at the restriction point.
Materials and Methods
Primary Cultures of Dog Thyroid Follicular Cells
Dog thyrocytes, seeded as follicles (2 × 104 cells/cm2) were cultured in monolayer in the following mixture that constitutes the control medium (Roger et al., 1987b ): DME + Ham's F12 medium + MCDB104 medium (2:1:1, by volume; GIBCO BRL, Paisley, Scotland), supplemented with ascorbic acid (40 μg/ml), insulin (5 μg/ml; Sigma Chemical Co., St. Louis, MO), and antibiotics. The medium was changed every other day. At day 4, the cells were quiescent and were treated with the following stimulants: bovine TSH (Sigma Chemical Co.), murine EGF (Collaborative Research, Waltham, MA), recombinant human hepatocyte growth factor (HGF; a kind gift of T. Nakamura, Osaka University Medical School), forskolin (Calbiochem-Novabiochem Corp., La Jolla, CA), 12-O-tetradecanoylphorbol 13-acetate (TPA; Sigma Chemical Co), and fetal bovine serum (Sera-Lab, Sussex, UK).
Antibodies
Mouse monoclonal antibodies to cyclin D1 (DCS-6; Lukas et al., 1994), cyclin D2 (DCS-3 and -5; Lukas et al., 1995b ), and cyclin D3 (DCS-22 and -29; Bartkova et al., 1996) were characterized previously. DCS-28, -20, -24, and -27 are new mouse monoclonal antibodies generated upon immunization of BALB/c mice with bacterially produced human cyclin D3, using techniques described in Lukas et al. (1994). The mouse monoclonal antibodies DCS-31, -32, and -35 against recombinant cdk4 were generated using similar techniques. Epitope mapping of monoclonal antibodies against cyclin D3 was performed using an ELISA technique based on a library of biotinylated 20-mer peptides (with 5–amino acid overlap) covering the complete sequence of cyclin D3 (Bartkova, J., unpublished data). Rabbit polyclonal antibodies against cdk4 (C22) and Rb (C15) were from Santa Cruz Biotechnology (Santa Cruz, CA).
Gel Electrophoresis and Immunodetection of Proteins
Cell proteins were separated by PAGE and immunodetected after Western blotting as previously described (Baptist et al., 1995). 125I-Labeled anti–mouse antibody from sheep (ICN Pharmaceuticals Inc., Irvine, CA) and 125I-protein A (Amersham International, Little Chalfont, UK) were used as secondary reagents to detect monoclonal and polyclonal antibodies, respectively. Autoradiographs were quantitated by densitometry (Ultroscan; Bio-Rad Laboratories, Hercules, CA).
Indirect Immunofluorescence
Cells in petri dishes (2 × 104 cells/cm2) were fixed with 2% paraformaldehyde for 90 s at 4°C and then with methanol for 10 min at −20°C, permeabilized with 0.1% Triton X-100 in PBS, pH 7.5, at room temperature and blocked for 30 min with normal sheep serum (5% in PBS containing 0.3% bovine serum albumin [PBS/BSA]; Baptist et al., 1993, 1996). For staining of cyclin D3, D2, D1, or cdk4 cells were then incubated overnight at 4°C with the respective mouse monoclonal antibodies (culture supernatant: 1/3; ascite liquid: 1/200). After washing, cells were successively incubated for 2 h at room temperature with biotinylated sheep anti–mouse immunoglobulins (1/50, RPN1001; Amersham International) and for 1 h with fluorescein-streptavidin (1/30, RPN1232; Amersham International).
For double staining of proliferating cell nuclear antigen PCNA used as a cell cycle marker (Baptist et al., 1993, 1996) and cyclin D3 using two mouse monoclonal antibodies, fixed cells were incubated overnight at 4°C with DCS-22 and then for 2 h at room temperature with biotinylated anti-mouse immunoglobulins and for 1 h with Texas red streptavidin. Washed cells were then successively incubated for 30 min with normal mouse serum (1/100), for 2 h with unconjugated anti-mouse immunoglobulin G Fab fragment (50 g/ml; Jackson Immuno-Research Laboratories, West Grove, PA), for 1 h with PC10 (1/400; DAKOPATTS, Copenhagen, Denmark), and for 1 h with fluorescein-conjugated anti-mouse immunoglobulin (1/50; Amersham International). This “sandwich” procedure is designed to avoid binding of PC10 (the PCNA monoclonal antibody) to the first secondary antibody and binding of the second secondary antibody to the cyclin D antibody.
To unmask the DCS-22 epitope, cells were fixed and permeabilized as above, and then incubated for 10 min at room temperature with a solution of 0.01% trypsin (ICN Pharmaceuticals Inc.) in MCDB104 Hepes-buffered culture medium. This dilution of trypsin was established from a range of different dilutions in preliminary experiments. The trypsin digestion was stopped by rapidly rinsing cells with PBS/BSA and incubating them with normal sheep serum as above. Cells were then normally processed for immunofluorescent detection of cyclin D3 using DCS-22.
Photometry
The nuclear immunofluorescence of cyclin D3 was quantitated using a photometric head attached to the Zeiss Axiovert 135 microscope (Carl Zeiss Inc., Thornwood, NY) and a 100× oil immersion lens exactly as described previously (Baptist et al., 1995). Fluorescence was measured from ∼100 nuclei selected at random in each dish. All the conditions were assayed in duplicate with an excellent reproducibility.
DNA Synthesis
Cells in 3-cm Petri dishes were incubated either for 1 or 24 h before fixation in the presence of 10−4 M 8-bromo-deoxyuridine (BrdU) and 2 10−6 M fluorodeoxycytidine. Cells were fixed and the incorporation of BrdU into nuclei was revealed by immunofluorescence as described (Roger et al., 1992). The percentage of BrdU-labeled nuclei was evaluated by counting 1,000 nuclei per dish.
Immunoprecipitation
Subconfluent cultures of thyrocytes that contain the same number of cells 20 h after stimulation by TSH, EGF+serum, or none were washed with calcium/magnesium-free PBS and lysed in 1 ml lysis buffer (Xiong et al., 1992) containing 150 mM NaCl, 50 mM Tris-HCl, pH 7.5, 0.5% NP-40, 50 mM NaF, 1 mM sodium orthovanadate, DTT, and protease inhibitors (pefablock, leupeptin). 1 ml of precleared cellular lysate was incubated with 2 g of antibody at 4°C for 2 h (monoclonal antibody against cyclin D3 [DCS-28] or against cdk4 [DCS-35]). A rabbit anti–mouse immunoglobulin antibody was added in the last 30 min. The immune complexes were recovered by incubation with protein A/G Agarose (Santa Cruz Biotechnology) for 1 h. The protein A/G Agarose immune complexes were suspended in SDS lysis buffer, boiled for 4 min, and analyzed on 10% SDS– polyacrylamide gels. The proteins were immunodetected as described above using either the DCS-22 cyclin D3 antibody or the DCS-35 cdk4 antibody. Cyclin D3 and cdk4 were not precipitated by a control mouse immunoglobulin G or irrelevant mouse monoclonal antibodies in the present conditions.
Rb Kinase Assay
Cells were suspended in IP buffer (50 mM N-2-hydroxyethylpiperazine- N′-2-ethanesulfonic acid [Hepes, pH 7.5] 150 mM NaCl, 1 mM EDTA, 2.5 mM EGTA, 1 mM DTT, and 0.1% Tween 20) containing 10% glycerol, 0.1 mM phenylmethylsulfonyl fluoride, 10 μg of leupeptin per ml, 20 U of aprotinin per ml, 10 mM β-glycerophosphate, 1 mM NaF, and 0.1 mM sodium orthovanadate, and sonicated at 4°C (Virsonic 475, full microtip power two times for 10 s each time; Matsushime et al., 1994). Lysates were clarified by centrifugation at 10,000 g for 5 min, and the supernatants were precipitated for 2–6 h at 4°C with protein A/G–Agarose beads precoated with saturating amounts of the indicating antibodies. Protein A/G–Agarose was pretreated with rabbit anti–mouse to provide a suitable affinity matrix. Immunoprecipitated proteins on beads were washed four times with 1 ml of IP buffer and twice with the kinase buffer (50 mM Hepes, pH 7.5, 10 mM MgCl2, 1 mM dithiothreitol). The beads were suspended in 30 μl of kinase buffer containing substrate (1 μg of soluble glutathione S-transferase-Rb COOH terminus fusion protein [GST-Rbc] prepared from Escherichia coli transformed with pGEX-Rb [773-928] as previously described [Meyerson and Harlow, 1994]) and 2.5 mM EGTA, 10 mM β-glycerophosphate, 0.1 mM sodium orthovanadate, 1 mM NaF, 50 μM ATP, and 5 μCi of γ-[32P]ATP. After incubation for 30 min at 30°C with occasional mixing, the samples were boiled in polyacrylamide gel sample buffer containing SDS and separated by electrophoresis. Phosphorylated proteins were visualized by autoradiography or phosphorimaging (PhosphorImager; Molecular Dynamics, Inc., Sunnyvale, CA) of the dried slab gels.
Microinjection of Dog Thyroid Cells
Thyrocytes were microinjected at day 4 of the culture as described (Dremier et al., 1997) with affinity-purified monoclonal antibodies against cyclin D3 (DCS-29) or cyclin D1 (DCS-6). At this time, cells were quiescent and well spread. As the capacity to proliferate can differ for each follicle-derived cell cluster, but is generally homogeneous within each, one half of each cell cluster was microinjected, the other half being used as a control. The microinjected and nonmicroinjected cells were stimulated by various mitogenic factors just after the microinjection. BrdU (10-4M) and fluorodeoxycytidine (2 × 10−6 M) were added 16 h later and cells were fixed 40 h after the stimulation with methanol for 10 min at −20°C. Injected cells were identified by biotinylated anti-mouse antibody followed by Texas red–coupled streptavidin (incubations with the antibodies for 1 h). BrdU incorporation was then codetected as described (Roger et al., 1992), using a FITC-coupled anti-BrdU antibody (overnight incubation; Becton Dickinson, Mountain View, CA) and all the nuclei were counterstained with Hoechst 33342 dye. The fraction of cells entering into DNA synthesis was estimated by the percentage of BrdU-labeled nuclei. All the countings were realized independently by two persons.
All the experiments were performed three to five times with similar results.
Results
After 4 d without mitogenic agents but in the presence of insulin, dog thyrocytes were spread and quiescent. Cells were then stimulated to proliferate using either TSH (maximum cAMP-dependent mitogenic stimulation [Roger et al., 1983]), or EGF+serum (maximum cAMP-independent mitogenic stimulation), or these factors in combination. As previously shown (Roger et al., 1987b ; Baptist et al., 1993) the first S-phase cells appeared 18–20 h after stimulation by TSH or EGF+serum (Fig. 1). S-phase began earlier (14 h) in response to the combination of EGF+serum+TSH. This illustrates the fact that TSH (cAMP) cooperates with cAMP-independent mitogens by stimulating steps that are rate limiting for DNA synthesis initiation (Roger et al., 1987a ; Baptist et al., 1993, 1996). The combination of TSH and EGF+serum also resulted in an increase of the fraction of cells that enter S phase (Roger et al., 1987b ; Fig. 1).
Figure 1.
Kinetics of cell cycle progression after mitogenic stimulation of quiescent dog thyrocytes. 4-d-old dog thyrocytes were stimulated at time 0 by TSH (1 mU/ml), EGF (25 ng/ml)+serum (10%), or a combination of TSH, EGF, and serum (10%). 1 h before fixation at the times indicated, cells were incubated with BrdU. BrdU incorporation was detected by immunofluorescent staining and the percentage of cells in S phase was determined.
Differential Expression of D-type Cyclins during Distinct Mitogenic Stimulations
The various mouse cyclin D1, D2, and D3 monoclonal antibodies used throughout this study were monospecific in Western immunoblotting analyses. In immunoblotting experiments, as illustrated in Fig. 2, very different exposure times were necessary to obtain comparable autoradiography signals for the three cyclins D: 1–3 d for the detection of cyclin D3, ∼10 d for cyclin D1, and ∼3 wk for cyclin D2 (Fig. 2 A). In some experiments cyclin D2 was even undetectable. Similar data, i.e., strongest signals for the detection of cyclin D3 as compared with cyclin D1 and even more to cyclin D2, were obtained using several different monoclonal antibodies used at similar concentrations (compare the detections with similar exposure times of cyclin D1 [Fig. 2 A] and cyclin D3 [Fig. 2 B]; and data not shown). This indicates that cyclin D3 is the most abundant one in dog thyrocytes, unlike in most cell types used until now for proliferation studies.
Figure 2.
Western blotting analyses of the accumulation of the three cyclins D in dog thyrocytes after stimulation by different mitogenic treatments. Cells remained quiescent in control conditions (C) or were stimulated by TSH (1 mU/ml), EGF (25 ng/ ml)+serum (10%; ES), or the combination of these factors (ES+TSH) for 8 to 32 h. (A) Immunoblot autoradiograph. 30 μg of cellular proteins were loaded per lane. The exposure durations were different for the autoradiographical detection of the different cyclins D: cyclin D1 (DCS-6), 8 d; cyclin D2 (DCS-3), 16 d; cyclin D3 (DCS-22), 2 d. ND, not done or lost. (B) Detection of cyclin D3 with DCS-29 from control cells (C) and cells stimulated by TSH (T) or ES for 20 h. This autoradiograph was exposed for 8 d like the cyclin D1 immunoblot shown in A, in order to illustrate the relative abundances of cyclin D3 and cyclin D1. It also shows the high specificity of the DCS-29 antibody used in the microinjection experiments of Figs. 10 and 11. (C) Densitometry quantitation of cyclin D1 from the autoradiograph details in A. (D) Densitometry quantitation of cyclin D3 (shown with a more precise kinetics from another dog thyrocyte primary culture in order to illustrate the qualitative reproducibility compared with the results in A). In C and D, the relative amount of cyclin D1 or cyclin D3 after cell stimulation is expressed compared with the average (fixed as 100) of values obtained at different times from unstimulated (control) cells in the same experiments.
In response to EGF+serum, cyclins D1 and D2 were markedly induced. This effect was detectable 12 h after stimulation, and maximum at 16–20 h, but at 26 h the expression of cyclins D1 and D2 decreased (Fig. 2, A and C), consistently with a maximum accumulation in mid to late G1 as in other systems (Baldin et al., 1993; Lukas et al., 1994, 1995b ). At all the time points, TSH did not stimulate cyclin D1 and D2 expression and even slightly inhibited it in the presence of EGF+serum (Fig. 2, A and C). At variance with cyclins D1 and D2, cyclin D3 was already abundant in quiescent control cells, its expression was less affected by mitogens, and it did not disappear at late cell cycle stages (Fig. 2, A, B, and D). EGF+serum and EGF+serum+TSH stimulated cyclin D3 accumulation by ∼50%, as first detected 12 h after stimulation. On the contrary, TSH inhibited by ∼50% the basal expression of cyclin D3 during the whole prereplicative phase (until 24 h). At late time points of TSH stimulation (26–32 h), after many cells had already reached S phase, cyclin D3 levels increased (Fig. 2, A and D). This effect could result from the delayed induction by TSH of insulin receptors (Burikhanov et al., 1996) and the moderate stimulation of cyclin D3 accumulation by insulin (unpublished observations).
HGF and TPA qualitatively mimicked the EGF+serum action on the expression of cyclins D (data not shown). Thus, TSH markedly differs from all the cAMP-independent mitogens studied so far, as it triggers DNA synthesis and cell cycle progression without stimulating the accumulation of cyclins D during G1 phase.
TSH Enhances the Immunofluorescence Detection of Cyclin D3
Using indirect immunofluorescence, the presence and subcellular location of D-type cyclins were studied at various time points (8, 12, 16, 20, 26, 32 h) after stimulation. Using DCS-6 (cyclin D1), DCS-3 or -5 (cyclin D2), and DCS-22 (cyclin D3), the three cyclins D were detected as mostly nuclear proteins (Fig. 3). EGF+serum treatment increased their nuclear staining in 30–50% of cells, starting at 12 h of stimulation (Fig. 3), in agreement with immunoblotting data on the accumulation of these proteins (Fig. 2). Also consistent with the immunoblotting analyses, TSH did not induce cyclin D1 and D2 nuclear stainings at any time points during the prereplicative phase (Fig. 3 illustrating the 20 h time point and data not shown). However, in sharp contrast with the inhibition of cyclin D3 expression detected on immunoblots of TSH-treated cells (Fig. 2), we observed with the same antibody (DCS-22) that TSH like EGF+serum markedly enhanced the immunofluorescent labeling of cyclin D3 in the nuclei of a majority of cells (first seen 16 h after TSH stimulation; gradually increased afterwards; Fig. 3). This effect of TSH was mimicked by the adenylyl cyclase activator forskolin (not shown).
Figure 3.
Immunofluorescence labeling of cyclin D1 (DCS-6), cyclin D2 (DCS-3), and cyclin D3 (DCS-22) in dog thyrocytes stimulated by different mitogenic treatments. Quiescent 4-d-old cells were stimulated for 20 h by TSH (1 mU/ml), EGF (25 ng/ ml)+serum (10%; ES), or remained in control condition (C). Exposure times of the photographs were the same for the different cell treatments, but were shorter for cyclin D3 than cyclin D1 and much longer for cyclin D2.
The nuclear DCS-22 immunofluorescent staining of cyclin D3 was measured by photometry from 200 individual cells per condition 20 h after the addition of various mitogens. The individual responses of cells were heterogeneous. Compared with the average fluorescence measured from the nuclei of control cells, TSH in the presence of insulin enhanced the DCS-22 detection of cyclin D3 by a factor of 2–8 in 51% of the cell population. When comparing the different cAMP-dependent and cAMP-independent mitogenic treatments, a good correlation (r = 0.929) was found between the average intensity of the nuclear labeling of cyclin D3 and the subsequent accumulation of BrdU-labeled cells during a further 28 h incubation (Fig. 4). Thus, the increase of the nuclear immunoreactivity of cyclin D3, in response to various treatments was a better predictive factor of their mitogenic potency than the accumulation of this protein. The enhanced nuclear immunoreactivity of cyclin D3 might therefore reflect an important event for cell cycle progression. The correlation between the enhanced immunoreactivity of cyclin D3 and cell cycle progression was further assessed by the analysis of the double immunofluorescence labeling of cyclin D3 (DCS-22) and PCNA, which has been previously demonstrated in dog thyroid cells as a marker of the different phases of the cell cycle (Baptist et al., 1993, 1996). In response to either TSH or EGF+serum, the nuclear immunoreactivity of cyclin D3 was strongly increased in all the cells in late G1 and S (Fig. 5). There were also some cells with a low PCNA immunoreactivity but an intense DCS-22 staining (Fig. 5). Whether such cells were at a G1 stage before PCNA appearance and would eventually enter S phase is not known.
Figure 4.
Correlation between the effects of various mitogenic treatments on DNA synthesis and on the increase of cyclin D3 reactivity to DCS-22. 4-d-old quiescent dog thyrocytes were stimulated with either TSH (1 mU/ml), EGF (25 ng/ml; E), TPA (10 ng/ml; TPA), HGF (40 ng/ml; H), EGF+serum (10%; ES) or remained in the usual control conditions in the presence of insulin (5 μg/ml; C). Some cells were maintained since the beginning of the culture in the absence of insulin (α). 20 h after stimulation (i.e., when a maximum of cells are in mid to late G1), cells were fixed and processed for cyclin D3 immunofluorescent labeling using DCS-22. The nuclear fluorescence was measured over 200 cells per conditions. For each condition, the proliferative activity was evaluated 48 h after mitogenic stimulation by the incorporation of BrdU during the last 24 h of incubation, and plotted as a function of the average intensity of cyclin D3 immunofluorescence.
Figure 5.
Double immunofluorescence labeling of cyclin D3 (detected using DCS-22), and PCNA used as a cell cycle marker in the same cells. Before fixation, 4-d-old dog thyrocytes were stimulated for 26 h with TSH (1 mU/ml), EGF (25 ng/ml)+serum (10%; ES), or remained quiescent in control medium (C) for this time. Arrowheads and large arrows show cells identified, respectively, in late G1 and S phase as described previously (Baptist et al., 1993, 1996). Small arrows show G0/G1 cells with a low PCNA staining.
Epitope Unmasking and Nuclear Translocation of Cyclin D3
The TSH-induced increase of cyclin D3 immunoreactivity was reproduced using several cyclin D3 monoclonal antibodies (DCS-20, 22, 24, 27, and 29, which recognize epitopes located between amino acids 241 and 260 of cyclin D3 sequence close to the “cyclin box”; data not shown). The comparison of immunoblotting and immunofluorescence data thus suggested that these epitopes were inaccessible to these related cyclin D3 antibodies in fixed quiescent cells and unmasked in the nuclei of cells stimulated by TSH (and by EGF+serum). A different situation was revealed using another cyclin D3 monoclonal antibody (DCS-28). This antibody recognizes an epitope localized within the COOH-terminal 20–amino acid residues of cyclin D3; it binds cyclin D3 in Western blots and is the most efficient for precipitating the cyclin D3-associated kinase activity (our unpublished data). Using DCS-28, control quiescent cells were more heavily stained with an overall cytoplasmic and nuclear distribution of the labeling (Fig. 6). In response to TSH, this labeling was not further enhanced, but in many cells it moved to a more nuclear localization that suggests a nuclear translocation (Fig. 6). Thus, the comparison of DCS-22 and DCS-28 immunofluorescent patterns also suggests that DCS-22 epitopes of cyclin D3 were masked in quiescent cells. To substantiate this inaccessibility of the DCS-22 epitope, we gently digested fixed cells with a diluted trypsin solution, a classical method for retrieval of masked antigens. The mild trypsin digestion enhanced the detection of cyclin D3 by DCS-22 in quiescent cells but not in TSH-treated cells, confirming the overall cytoplasmic and nuclear localization of cyclin D3 before stimulation and its nuclear translocation in response to TSH, as revealed using DCS-28 (Fig. 6).
Figure 6.
Immunofluorescence labeling of cyclin D3 in dog thyrocytes. Quiescent 4-d-old cells were stimulated for 20 h by TSH (1 mU/ml) or remained in control condition (C). Cyclin D3 was detected in the same experiment using DCS-22 or DCS-28 as in Fig. 3, or using DCS-22 after a retrieval treatment of masked antigen by trypsin. All the DCS-22 labelings were photographed with the same exposure time. Note the more intense staining of control cells with DCS-28 or with DCS-22 after the trypsin preincubation, and in these two last conditions, the increase of the nuclear staining of cyclin D3 at the expense of its cytoplasmic staining in many TSH-stimulated cells, which suggests a nuclear translocation.
Nuclear Translocation of cdk4
Unlike some other normal cell types (Ewen et al., 1993; Matsushime et al., 1994), quiescent dog thyrocytes expressed cdk4 before mitogenic stimulation, as shown by Western blotting analysis (Fig. 7). In response to TSH or TSH+EGF+serum, but not in all the experiments in response to EGF+serum, the abundance of cdk4 moderately increased (Fig. 7). In various experiments this effect was mostly observed at 20–40 h after stimulation, i.e., when most responding cells were already progressing into S and G2 phases.
Figure 7.
Time course of the accumulation of cdk4 in dog thyrocytes after stimulation by different mitogenic treatments. Cells were stimulated by TSH (1 mU/ ml), EGF (25 ng/ml)+serum (10%; ES), or the combination of these factors (ES+TSH) for 14–32 h. Some cells remained quiescent in control conditions (C) and were analyzed at 14 and 26 h. Autoradiography of cdk4 detection by Western blotting. ND, not done.
The immunofluorescent detection of cdk4 using the DCS-31 and -32 monoclonal antibodies, or the polyclonal antibody from Santa Cruz Biotechnology, showed that cdk4 was distributed in both the cytoplasm and nucleus of quiescent control cells. As first observed 12 h after EGF+serum or 16–20 h after TSH addition, many cells displayed a strong increase of the nuclear staining of cdk4 at the expense of the cytoplasmic labeling (Fig. 8). Since cdk4 contents, as measured by Western blottings, varied little at these times (Fig. 7), this suggests a nuclear translocation of cdk4. The double immunofluorescent labeling of cdk4 and PCNA showed that the nuclear staining of cdk4 remained elevated until mitosis onset in TSH-treated cells. By contrast, in EGF+serum–treated cells, the cdk4 nuclear staining was increased in many cells well before the appearance of PCNA and it decreased at 20–26 h in S-phase cells. In response to the combination of both stimulations (TSH+EGF+serum), cdk4 nuclear staining increased early, at 8–12 h, but remained elevated at later time points like in TSH treatment (data not shown).
Figure 8.
Immunofluorescence labeling of cdk4 in dog thyrocytes. Before fixation, 4-d-old dog thyrocytes were stimulated for 20 h with TSH (1 mU/ml), EGF (25 ng/ml)+serum (10%; ES), or remained quiescent in control condition (C). Note the increase of nuclear staining of cdk4 at the expense of its cytoplasmic staining in many stimulated cells, which suggests a nuclear translocation.
Assembly and Activity of Cyclin D3–cdk4 Complexes
The above immunofluorescence results have suggested that TSH and EGF+serum could induce the formation of nuclear cyclin D3–cdk4 complexes. To test this hypothesis, we precipitated cdk4 using the DCS-35 monoclonal antibody and analyzed by Western blotting the coimmunoprecipitation of cyclin D3. Conversely, cyclin D3 was precipitated using DCS-28 and precipitates were examined for the presence of cdk4 (Fig. 9 A). Cells were used 20 h after stimulation, i.e., when a maximum of cells are in mid to late G1 phase.
Figure 9.
Convergence of cAMP-dependent and -independent mitogenic treatments on cyclin D3–cdk4 complex formation and Rb phosphorylation. 4-d-old dog thyrocytes were stimulated for 20 h with either TSH (1 mU/ml; T), EGF (25 ng/ml)+serum (10%; ES), or remained quiescent in control medium (C) for this time. (A) Assembly of cyclin D3–cdk4 complexes. The complexes were immunoprecipitated as indicated from equal amounts of cell extract using either DCS-28 (cyclin D3) or DCS-35 (cdk4) monoclonal antibodies, and the presence of cyclin D3 and cdk4 was detected by Western blot in these immunoprecipitates. A same autoradiograph exposure is provided throughout in order to allow the direct comparison of amounts of total versus complexed cdk4 and cyclin D3. (B) Cyclin D3–associated Rb kinase activity using GST-Rb COOH-terminal protein (GST-Rbc) and γ-[32P]ATP as substrates. (C) Phosphorylation of Rb detected by its electrophoretic shift in Western blot of whole cell lysates.
In the experiment shown, the amount of total DCS-35–precipitable cdk4 slightly increased in response to TSH (+37% as evaluated by densitometry) and EGF+serum (+33%). The amount of total DCS-28–precipitable cyclin D3 also moderately increased in response to EGF+serum (+60%) and weakly in response to TSH (+20%), which in this last case contrasted with the moderate reduction of cyclin D3 content consistently observed in whole lysates of TSH-stimulated cells (Fig. 2). Thus, TSH treatment might somewhat affect the efficiency of cyclin D3 precipitation by DCS-28 in the present nondenaturing conditions. In contrast to these modest variations of cdk4 and cyclin D3 concentrations and/or immunoreactivity, the amount of cyclin D3 complexed with cdk4 strongly increased in TSH- or EGF+serum–stimulated cells (Fig. 9 A). Both TSH and EGF+serum provoked a 10-fold elevation of the amount of cdk4 coprecipitated with cyclin D3. As grossly evaluated by the comparison of the amounts of cdk4 precipitated by cyclin D3 or cdk4 antibodies, ∼50% of total cdk4 was associated with cyclin D3 in TSH- or EGF+serum–stimulated cells, compared with 5% in control cells (Fig. 9 A).
The Rb-kinase activity in cyclin D3 immunoprecipitates was analyzed using a recombinant COOH-terminal fragment of Rb as substrate (Matsushime et al., 1994). In these precipitates, the phosphorylation of GST-Rb was stimulated by both TSH and EGF+serum and paralleled the presence of cdk4 (Fig. 9 B). TSH and EGF+serum also induced the phosphorylation of Rb demonstrated by its electrophoretic shift in Western blots of total cell lysates (Fig. 9 C; Coulonval et al., 1997). This demonstrates that both TSH and EGF+serum mitogenic stimulations converge on the assembly of active cyclin D3–cdk4 complexes and Rb phosphorylation.
Requirement for Cyclin D3 in cAMP-dependent G1 Phase Progression
To assess whether cyclin D3 is required for cell cycle progression, we microinjected a cyclin D3–specific antibody (DCS-29) into quiescent dog thyrocytes and examined its effect upon S-phase entry induced by different mitogenic factors. DCS-29 (Bartkova et al., 1996) like DCS-22, very efficiently and quite specifically recognized dog cyclin D3 in immunoblotting and immunofluorescence experiments (Fig. 2 B; data not shown). DCS-29 like DCS-22 recognizes an epitope very close to the “cyclin box” domain required for cdk4 activation, and it does not precipitate cyclin D3–associated kinase activity (Bartkova, J., unpublished data).
As shown in Fig. 10 and summarized in Fig. 11, cells microinjected with DCS-29 and stimulated by TSH or the direct adenylyl cyclase activator forskolin were prevented from entering DNA synthesis (70–80% of inhibition), whereas microinjection of a control immunoglobulin did not result in any significant inhibition. By contrast the DCS-6 cyclin D1 antibody did not prevent DNA synthesis induced by TSH (Fig. 10), whereas in parallel microinjection experiments performed under identical conditions the same batch of purified DCS-6 blocked G1 phase progression in the human MCF7 cell line (Fig. 2 in Lukas et al., 1996b ). It is worth mentioning that the DCS-6 epitope is conserved among species and lies within the “cyclin box” domain, which explains that this antibody prevents both the association of cyclin D1 with cdks and DNA synthesis in a variety of human and murine cells that predominantly express cyclin D1 (Lukas et al., 1994, 1995a,b; Ohtsubo et al., 1995). Since DCS-6 did recognize dog cyclin D1 (Figs. 2 A and 3), it had very likely the capacity to neutralize dog cyclin D1 in the present microinjection experiments. The results (Figs. 10 and 11) thus suggest that cyclin D3, but not cyclin D1, is required for the cAMP-dependent G1 phase progression of dog thyrocytes.
Figure 10.
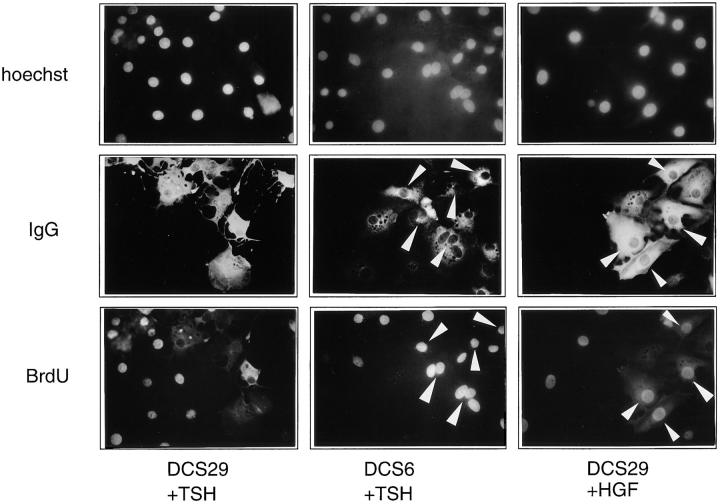
Requirement of cyclin D3 for G1 phase progression stimulated by TSH, but not by the cAMP-independent mitogen HGF. Quiescent dog thyrocytes were injected with the DCS-29 cyclin D3 monoclonal antibody (6 mg/ml), or with the DCS-6 cyclin D1 blocking antibody, and stimulated for 40 h with TSH (1 mU/ml) or HGF (50 ng/ml). BrdU was added 24 h before fixation. Nuclei were identified by Hoechst 33342 staining of DNA. Microinjected cells were identified by the immunodetection of the injected antibody (IgG). BrdU incorporation was coimmunodetected (BrdU). Arrowheads indicate injected cells that have incorporated BrdU. Note that none of the TSH-stimulated cells injected with the DCS-29 cyclin D3 antibody incorporated BrdU, in contrast to neighboring noninjected cells, TSH-stimulated cells injected with the DCS-6 cyclin D1 antibody, and HGF-stimulated cells injected with the DCS-29 cyclin D3 antibody.
Figure 11.
Summary of cyclin D3 antibody microinjection experiments. Quiescent dog thyrocytes were injected by 2 or 6 mg/ml DCS-29 cyclin D3 antibody or a control IgG and processed for immunofluorescent detection of injected immunoglobulin and BrdU as in Fig. 10, after stimulation for 40 h as indicated, with TSH (1 mU/ml), forskolin (Fo; 10−5M), HGF (50 ng/ml), EGF (25 ng/ml)+serum (S), or EGF alone. Results are expressed (mean+SD) relative to the percentages of BrdU-labeled nuclei in neighboring noninjected cells. Average percentage of BrdU-labeled nuclei in noninjected cells were 37% (TSH), 50% (Fo), 46% (HGF), 69% (EGF+S), 35% (EGF), and 3% in control unstimulated cells. The number of separate experiments and the total number of injected cells analyzed are indicated for each condition. In each separate experiment, the stimulation by TSH (or Fo) was compared with one of the cAMP-independent stimulations (HGF, EGF+S, or EGF).
On the other hand, in the same experiments the microinjection of DCS-29 had no or a weak effect on the capacity of dog thyrocytes to enter DNA synthesis after stimulations that induce the accumulation of cyclins D1 and D2, including HGF, EGF+serum, and EGF (Figs. 10 and 11). Whereas the microinjection of 2 mg/ml DCS-29 was sufficient to block DNA synthesis in TSH-treated cells, the microinjection of a higher DCS-29 concentration (6 mg/ml) did not inhibit the entry of cells after the stimulation by HGF and only slightly affected the weaker stimulation by EGF. These data demonstrate that the cell cycle inhibitory effect of DCS-29 in TSH-treated cells is specific, rather than attributable to some nonspecific toxic component in the antibody preparations. The absence of DCS-29 effect in growth factor–stimulated dog thyrocytes did not suggest a lack of cdk4 requirement, since the microinjection of the p16INK4A cdk4 inhibitor completely inhibited the stimulations of DNA synthesis by both TSH and EGF (Lukas et al., 1996b ). Rather, the present observation is consistent with the view that different cyclins D might play overlapping roles in cdk4 activation and G1 phase progression in thyrocytes stimulated by cAMP-independent growth factors.
Discussion
The dog thyrocyte culture system constitutes an interesting model of the diversity of the mechanisms of cell cycle control by distinct intracellular signaling cascades. In these cells, we have previously proposed that the potent MAP kinase–independent mitogenic pathway elicited by TSH via cAMP and the rapidly converging cAMP-independent ones of growth factors and tumor promoters could remain partly separated during most of the G1 phase, and in parallel lead to the commitment for DNA replication (Roger et al., 1987b ; Dumont et al., 1989, 1992; Lamy et al., 1993; Roger et al., 1995, 1997).
This study strongly supports this proposal. In dog thyrocytes as in a variety of cell types, cAMP-independent mitogens including EGF+serum, HGF, and TPA induced the accumulation of cyclins D1 and D2 and slightly enhanced the high basal expression of cyclin D3. In sharp contrast, TSH via cAMP did not induce the accumulation of cyclin D1 and D2 and even partially inhibited the basal expression of cyclin D3 at least during the G0-S prereplicative phase. Moreover, the synergy between TSH and EGF+serum on DNA synthesis initiation was not associated with a similar synergy at the level of cyclin D expression, and TSH even slightly reduced the cyclin D1 accumulation induced by EGF+serum. To our knowledge, there is no precedent for such an observation in the case of a mitogenic stimulation of a normal cell. Mitogenic pathways stimulated by agents as diverse as growth factors through tyrosine kinase receptors (Sewing et al., 1993), activators of protein kinases C (Ajchenbaum et al., 1993), transforming growth factor β (Rao and Kohtz., 1995), or steroid hormones (Musgrove et al., 1993), have all been found to involve the induction of at least one of the three cyclins D, as a prerequisite for the activation of cdk4 and the inactivation of Rb-like cell cycle suppressors. Our results also contrast with the induction of cyclin D2 mRNA by FSH through cAMP recently demonstrated in rat ovarian granulosa cells (Sicinski et al., 1996), and the reported increase of cyclin D1 accumulation in TSH-stimulated rat FRTL5 thyroid cell line (Yamamoto et al., 1996). In different cell systems, cAMP may thus use different strategies, not only to inhibit cell cycle progression (Roger et al., 1995), but also to directly stimulate it.
Strikingly, in dog thyrocytes the effects of cAMP on the accumulation of cyclins D and p27kip1 (Depoortere et al., 1996; a cdk inhibitor whose cellular concentration, as generally considered [Kato et al., 1994; Coats et al., 1996], should decrease as a prerequisite for the passage through the restriction point in response to growth factors) are thus similar to those observed in cells blocked in G1 by cAMP (Cocks et al., 1992; Sewing et al., 1993; Kato et al., 1994; Ward et al., 1996; L'Allemain et al., 1997). Paradoxically, in the dog thyrocyte system they are associated with a late G1 cAMP-dependent commitment to DNA synthesis (Roger et al., 1987a ) and mitosis (Baptist et al., 1993), which involves, as after a cAMP-independent mitogenic stimulation, the phosphorylation of Rb and related p107 and p130Rb2 (Coulonval et al., 1997), the nuclear translocation and phosphorylation of cdk2 and the accumulation of cyclin A and cdc2 (Baptist et al., 1996). This has raised the question of the mechanism of Rb phosphorylation in this cAMP-dependent pathway. Here we demonstrate that the TSH and EGF+serum mitogenic pathways do converge on the assembly of precipitable complexes of cdk4 and cyclin D3, and the stimulation of the Rb kinase activity associated with cyclin D3. The activation of cdk4 is indeed an essential step both in the cAMP-dependent and growth factor mitogenic pathways, since the microinjection of the p16INK4A inhibitor of cdk4 blocked the stimulation of DNA synthesis by TSH and EGF in dog thyrocytes (Lukas et al., 1996b ), exactly as in growth factor–stimulated fibroblasts with a normal Rb function (Lukas et al., 1995c ). These results support the concept that the inactivation of Rb family proteins through phosphorylation by cyclin D–cdk4 is indeed the ultimate integrator of quite diverse mitogenic stimulations at the restriction point (Weinberg, 1995; Bartek et al., 1996; Sherr, 1996; Coulonval et al., 1997).
In TSH-stimulated dog thyrocytes cyclin D3 appears to be the main D-type cyclin involved in cdk4 activation and DNA synthesis induction: (a) cyclins D1 and D2 were not induced (cyclin D2 was often undetectable); (b) cyclin D3 appears to be the most abundant cyclin D in quiescent cultured dog thyrocytes and in the thyroid gland of mice in vivo (Coppée, F., F. Depoortere, J. Bartek, C. Ledent, M. Parmentier, J.E. Dumont, manuscript submitted for publication), like in some T lymphocytes (Tam et al., 1994) but at variance with most cell types used for cell cycle studies. Even after a 50% reduction of its concentration, cyclin D3 remains significantly expressed since it was evaluated to recruit ∼50% of cdk4 molecules in TSH-stimulated dog thyrocytes; (c) the microinjection of the DCS-6–neutralizing antibody to cyclin D1 failed to inhibit TSH-stimulated DNA synthesis, at variance with a wide variety of cell types, but like other cells that weakly express cyclin D1 (Lukas et al., 1994); (d) the microinjection of a cyclin D3 antibody (DCS-29) directly showed that cyclin D3 is required for DNA synthesis in cells stimulated by TSH or forskolin, but very poorly or not at all in cells stimulated by growth factors (HGF, EGF, and the combination of EGF+serum) that induce cyclin D1 and D2. This specific requirement of cyclin D3 is a novel observation. Cyclin D1 or D2 knockouts by gene targeting have only impaired the development of tissues that predominantly express these cyclins, and have suggested partly overlapping functions for the three cyclins D in G1 progression (Sicinsky et al., 1995, 1996; Lahti et al., 1997). In dog thyrocytes, cyclins D1 and/or D2 induced by growth factors but not by TSH might thus compensate for the neutralization of cyclin D3. It remains to be seen whether cyclin D3 knockout in mice could specifically impair the development and/or TSH-dependent hyperplasia of the thyroid gland, but we surmise that in other cell types the requirement for cyclin D3 also might be cryptic in the presence of other cyclins D. Other mechanisms of the differential requirement of cyclins D have not been excluded, such as a critical influence of different cdk inhibitor levels in the parallel mitogenic pathways. Whatever the mechanism(s), the TSH (cAMP)-dependent cell cycle of dog thyrocytes is unique as it mainly depends on cyclin D3 for cdk4 activation and DNA synthesis, which allows this first direct demonstration of a G1 phase requirement for cyclin D3.
The assembly and activation of cyclin D3–cdk4 complexes in TSH-treated cells that were paradoxically associated with a partial inhibition of cyclin D3 accumulation should thus involve a new mechanism. This mechanism might also play a nonessential role (as argued above) in growth factor–dependent G1 phase progression, since the formation of cyclin D3–cdk4 complexes in EGF+serum– stimulated dog thyrocytes is also poorly explained by only a 50% increase of cyclin D3 levels. At variance with some other systems (Ewen et al., 1993; Matsushime et al., 1994), in dog thyrocytes the stimulation of the activity of cdk4 is not associated with important variations of its concentration. In quiescent thyrocytes, the simultaneous expression of cyclin D3 and cdk4 does not suffice for cdk4 activation. This is consistent with observations from rodent fibroblasts that despite constitutive engineered overexpression of cyclin D3 together with cdk4, remain dependent on mitogenic stimulation for the assembly of active cyclin D3–cdk4 complexes (Matsushime et al., 1994). This study provides some new insights about the poorly understood assembly of cyclin D–cdk4 complexes:
(a) As detailed in Results, some but not all the epitopes of cyclin D3 were inaccessible to antibodies in fixed quiescent thyrocytes, but specifically unmasked in cycling cells in response to both cAMP-dependent and cAMP-independent mitogenic stimulations. An attractive speculation would be that DCS-22 and related antibodies are preferentially recognizing the active cyclin D3 molecules in the present cell fixation conditions. Interestingly, the epitope made accessible to these antibodies in stimulated cells is very close to the “cyclin box” domain of interaction with cdk4. The cAMP-dependent activation step in G1 (through modifications of protein–protein interactions or conformational changes) could thus allow both the access of DCS-22 to its antigen and the assembly of cyclin D3–cdk4 complexes.
(b) Both in TSH- and in EGF+serum–treated dog thyrocytes, the formation of cyclin D3–cdk4 complexes was associated with nuclear translocations of cyclin D3 and cdk4 that initially were equally distributed in the cytoplasm and nucleus of quiescent cells. To our knowledge, such nuclear translocations of cyclin D3 and cdk4 have not been previously reported in other systems. They could be crucial for cdk4 activation, as recently suggested by studies of cells engineered to overexpress a cyclin D and cdk4 with various cdk inhibitors (LaBaer et al., 1997; Reynisdottir and Massagué, 1997). The cdk4 translocation occurred later during the prereplicative phase of TSH-treated thyrocytes than in EGF+serum–treated cells, and thus might well correspond to the “X” event we have postulated previously as being especially rate limiting for the initiation of DNA synthesis in TSH-stimulated cells (Baptist et al., 1993). Previously we have evidenced the nuclear translocation of cdk2 and cdc2 at G1/S and G2/M transitions, respectively (Baptist et al., 1996). Subcellular localization might thus be a general determinant of the activation of cyclin-dependent kinases.
(c) Cyclin D3 and cdk4 are not known to contain a nuclear localization signal. Their nuclear accumulation might thus depend on their interaction with other nuclear proteins. After completion of the present experiments, Reynisdottir and Massagué (1997) and La Baer et al. (1997) have demonstrated using cotransfected cell lines that p27kip1 can target cdk4 and D-type cyclins to the nucleus. p27kip1 and other cdk inhibitors of the p21 family might also promote the association of cdk4 with cyclins D (LaBaer et al., 1997). Unlike initial claims (Kato et al., 1994), the presence of p27kip1 in cyclin D-cdk4 complexes does not necessarily prevent their activity (Soos et al., 1996; Blain et al., 1997). The TSH (cAMP)-induced accumulation of p27kip1 we have observed including in the nuclei of G1 and S phase thyrocytes (Depoortere et al., 1996) might thus contribute to the cAMP-dependent nuclear translocation and/or assembly of cyclin D3 and cdk4.
The unique characteristics of the TSH-controlled cAMP-mediated growth pathway might explain how it can be compatible with the cAMP-dependent induction of differentiation expression that is inhibited by cAMP-independent mitogens (Pohl et al., 1990). In addition to their role as cdk4 activators, the different D-type cyclins have distinct influences on differentiation in a variety of cell types (Kato and Sherr, 1993; Ravnik et al., 1995; Wang et al., 1995). Cyclin D1 expression inhibits the terminal differentiation of myoblasts that is associated with a strong induction of cyclin D3 (Kiess et al., 1995; Rao and Kohtz, 1995). Thyroid growth accompanies chronic stimulation of function by TSH. The limited number of cAMP-dependent mitotic cycles of fully differentiated thyrocytes that this process involves (Dumont et al., 1992), must obey specific constrains (Roger et al., 1995). The cAMP-dependent mitogenic pathway could thus be viewed, in thyrocytes and possibly other cells, as a differentiation characteristic, adjunctive to the more general mechanisms operated by growth factors (Roger et al., 1992, 1995). This could help to understand the various odd characteristics of this pathway, such as the lack of cyclins D induction and the specific cyclin D3 requirement.
In summary, this study has precisely identified the earliest observed convergence point of the cAMP-dependent and cAMP-independent mitogenic pathways of dog thyrocytes on cyclin D3 “activation,” cdk4 nuclear translocation, and assembly of active cyclin D3–cdk4 complexes. However, these pathways lately differ in essential steps of the cdk4 activation mechanism: the expression of the various cyclins D and p27kip1 and the requirement for cyclin D3.
Acknowledgments
This study was supported by the Belgium Program on University Poles of Attraction initiated by the Belgian State, Prime Minister's Office, Science Policy Programming, and by grants from the National Fund for Scientific Research (FNRS, Belgium), the Caisse Générale d'Epargne et de Retraite, the Danish Cancer Society, and the Nordic Cancer Union. F. Depoortere and A. Van Keymeulen are fellows of the Fonds pour la Formation a la Recherche dans l'Industrie et l'Agriculture (FRIA), S. Dremier is a fellow of the “Televie”, and P.P. Roger is a Research Associate of the FNRS.
Abbreviations used in this paper
- BrdU
8-bromo-deoxyuridine
- cdk
cyclin-dependent kinase
- HGF
hepatocyte growth factor
- TPA
12-O-tetradecanoylphorbol 13-acetate
- TSH
thyrotropin
Footnotes
Address all correspondence to Pierre Roger, IRIBHN, Campus Erasme, 808 Route de Lennik, B-1070 Brussels, Belgium. Tel.: 322 555 41 53. Fax: 322 555 46 55.
The first two authors made equal contributions to this work.
References
- Ajchenbaum F, Ando K, De Caprio JA, Griffin JD. Independent regulation of human D-type cyclin gene expression during G1 phase in primary human T lymphocytes. J. Biol Chem. 1993;268:4113–4119. [PubMed] [Google Scholar]
- Baldin V, Lukas J, Marcotte MJ, Pagano M, Draetta G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993;7:812–821. doi: 10.1101/gad.7.5.812. [DOI] [PubMed] [Google Scholar]
- Baptist M, Dumont JE, Roger PP. Demonstration of cell cycle kinetics in thyroid primary culture by immunostaining of proliferating cell nuclear antigen: differences in cyclic AMP-dependent and -independent mitogenic stimulations. J Cell Sci. 1993;105:69–80. doi: 10.1242/jcs.105.1.69. [DOI] [PubMed] [Google Scholar]
- Baptist M, Dumont JE, Roger PP. Intercellular heterogeneity of early mitogenic events: cAMP generalizes the EGF effect on c-Fos protein appearance but not on MAP kinase phosphorylation and nuclear translocation in dog thyroid epithelial cells. Exp Cell Res. 1995;221:160–171. doi: 10.1006/excr.1995.1363. [DOI] [PubMed] [Google Scholar]
- Baptist M, Lamy F, Gannon J, Hunt T, Dumont JE, Roger PP. Expression and subcellular localization of CDK2 and cdc2 kinases and their common partner cyclin A in thyroid epithelial cells: comparison of cyclic AMP-dependent and -independent cell cycles. J Cell Physiol. 1996;166:256–273. doi: 10.1002/(SICI)1097-4652(199602)166:2<256::AID-JCP3>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Bartek J, Bartkova J, Lukas J. The retinoblastoma protein pathway and the restriction point. Curr Opin Cell Biol. 1996;8:805–814. doi: 10.1016/s0955-0674(96)80081-0. [DOI] [PubMed] [Google Scholar]
- Bartkova J, Zemanova M, Bartek J. Abundance and subcellular localisation of cyclin D3 in human tumors. Int J Cancer. 1996;65:323–327. doi: 10.1002/(SICI)1097-0215(19960126)65:3<323::AID-IJC8>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Beijersbergen RL, Bernards R. Cell cycle regulation by the retinoblastoma family of growth inhibitory proteins. Biochim Biophys Acta. 1996;1287:103–120. doi: 10.1016/0304-419x(96)00002-9. [DOI] [PubMed] [Google Scholar]
- Blain SW, Montalvo E, Massagué J. Differential interaction of the cyclin-dependent kinase (cdk) inhibitor p27kip1with cyclin A-cdk2 and cyclin D2-cdk4. J Biol Chem. 1997;272:25863–25872. doi: 10.1074/jbc.272.41.25863. [DOI] [PubMed] [Google Scholar]
- Boynton AL, Whitfield JF. The role of cyclic AMP in cell proliferation: a critical assessment of the evidence. Adv Cyclic Nucleotide Res. 1983;15:193–294. [Google Scholar]
- Burikhanov R, Coulonval K, Pirson I, Lamy F, Dumont JE, Roger PP. Thyrotropin via cyclic AMP induces insulin receptor expression and insulin co-stimulation of growth and amplifies insulin and insulin-like growth factor signaling pathways in dog thyroid epithelial cells. J Biol Chem. 1996;271:29400–29406. doi: 10.1074/jbc.271.46.29400. [DOI] [PubMed] [Google Scholar]
- Christoffersen J, Smeland EB, Stokke T, Tasken K, Anderson KB, Blomhoff HK. Retinoblastoma protein is rapidly dephosphorylated by elevated cyclic adenosine monophosphate levels in human B-lymphoid cells. Cancer Res. 1994;54:2245–2250. [PubMed] [Google Scholar]
- Coats S, Flanagan WM, Nourse J, Roberts JM. Requirement of p27KIP1for restriction point control of the fibroblast cell cycle. Science. 1996;272:877–880. doi: 10.1126/science.272.5263.877. [DOI] [PubMed] [Google Scholar]
- Cocks BG, Vairo G, Bodrug SE, Hamilton JA. Suppression of growth factor-induced CYL1 cyclin gene expression by antiproliferative agents. J Biol Chem. 1992;267:12307–12310. [PubMed] [Google Scholar]
- Coppée F, Gérard AC, Denef JF, Ledent C, Vassart G, Dumont JE, Parmentier M. Early occurrence of metastatic differentiated thyroid carcinomas in transgenic mice expressing the A2a adenosine receptor gene and the human papillomavirus type 16 E7 oncogene. Oncogene. 1996;13:1471–1482. [PubMed] [Google Scholar]
- Coulonval K, Maenhaut C, Dumont JE, Lamy F. Phosphorylation of the three Rb protein family members is a common step of the cAMP-, the growth factors- and the phorbol esters-mitogenic cascades but is not necessary for the hypertrophy induced by insulin. Exp Cell Res. 1997;233:395–398. doi: 10.1006/excr.1997.3582. [DOI] [PubMed] [Google Scholar]
- Depoortere F, Dumont JE, Roger PP. Paradoxical accumulation of the cyclin-dependent kinase inhibitor p27KIPduring the cAMP-dependent mitogenic stimulation of thyroid epithelial cells. J Cell Sci. 1996;109:1759–1764. doi: 10.1242/jcs.109.7.1759. [DOI] [PubMed] [Google Scholar]
- Dremier S, Pohl V, Poteet-Smith C, Roger PP, Corbin J, Doskeland SO, Dumont JE, Maenhaut C. Activation of cyclic AMP-dependent kinase is required but may not be sufficient to mimic cyclic AMP-dependent DNA synthesis and thyroglobulin expression in dog thyroid cells. Mol Cell Biol. 1997;17:6717–6726. doi: 10.1128/mcb.17.11.6717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont JE, Jauniaux JC, Roger PP. The cyclic AMP-mediated stimulation of cell proliferation. Trends Biochem Sci. 1989;14:67–71. doi: 10.1016/0968-0004(89)90046-7. [DOI] [PubMed] [Google Scholar]
- Dumont JE, Lamy F, Roger P, Maenhaut C. Physiological and pathological regulation of thyroid cell proliferation and differentiation by thyrotropin and other factors. Physiol Rev. 1992;72:667–697. doi: 10.1152/physrev.1992.72.3.667. [DOI] [PubMed] [Google Scholar]
- Ewen ME, Sluss HK, Whitehouse LL, Livingston DM. TGFβ inhibition of cdk4 synthesis is linked to cell cycle arrest. Cell. 1993;74:1009–1020. doi: 10.1016/0092-8674(93)90723-4. [DOI] [PubMed] [Google Scholar]
- Geng Y, NgEaton E, Picon M, Roberts JM, Lundberg AS, Gifford A, Sardet C, Weinberg RA. Regulation of cyclin E transcription by E2Fs and retinoblastoma protein. Oncogene. 1996;12:1173–1180. [PubMed] [Google Scholar]
- Higashi H, Suzuki-Takahashi I, Saitoh S, Segawa K, Taya Y, Okuyama A, Nishimura S, Kitagawa M. Cyclin-dependent kinase-2 (cdk2) forms an inactive complex with cyclin D1 since cdk2 associated with cyclin D1 is not phosphorylated by cdk7-cyclin H. Eur J Biochem. 1996;237:460–467. doi: 10.1111/j.1432-1033.1996.0460k.x. [DOI] [PubMed] [Google Scholar]
- Kato JY, Sherr CJ. Inhibition of granulocyte differentiation by G1 cyclin D2 and D3 but not D1. Proc Natl Acad Sci USA. 1993;90:11513–11517. doi: 10.1073/pnas.90.24.11513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato JY, Matsuoka M, Polyak K, Massagué J, Sherr CJ. Cyclic AMP-induced G1 phase arrest mediated by an inhibitor (p27KIP1) of cyclin-dependent kinase 4 activation. Cell. 1994;79:487–496. doi: 10.1016/0092-8674(94)90257-7. [DOI] [PubMed] [Google Scholar]
- Kiess M, Gill RM, Hamel PA. Expression of the positive regulator of cell cycle progression, cyclin D3, is induced during differentiation by myoblasts into quiescent myotubes. Oncogene. 1995;10:159–166. [PubMed] [Google Scholar]
- LaBaer J, Garrett MD, Stevenson LF, Slingerland JM, Standhu C, Chou HS, Fattaey A, Harlow E. New functional activities for the p21 family of cdk inhibitors. Genes Dev. 1997;11:847–862. doi: 10.1101/gad.11.7.847. [DOI] [PubMed] [Google Scholar]
- Lamy F, Wilkin F, Baptist M, Posada J, Roger PP, Dumont JE. Phosphorylation of mitogen-activated protein kinases is involved in the epidermal growth factor and phorbol ester, but not in the thyrotropin/cAMP thyroid mitogenic pathway. J Biol Chem. 1993;268:8398–8401. [PubMed] [Google Scholar]
- Lahti JM, Li H, Kidd VJ. Elimination of cyclin D1 in vertebrate cells leads to an altered cell cycle phenotype, which is rescued by overexpression of murine cyclins D1, D2, D3 but not by a mutant cyclin D1. J Biol Chem. 1997;272:10859–10869. doi: 10.1074/jbc.272.16.10859. [DOI] [PubMed] [Google Scholar]
- L'Allemain G, Lavoie JN, Rivard N, Baldin V, Pouysségur J. Cyclin D1 expression is a major target of the cAMP-induced inhibition of cell cycle entry in fibroblasts. Oncogene. 1997;14:1981–1990. doi: 10.1038/sj.onc.1201038. [DOI] [PubMed] [Google Scholar]
- Ledent C, Dumont JE, Vassart G, Parmentier M. Thyroid expression of an A2 adenosine receptor transgene induces hyperplasia and hyperthyroidism. EMBO (Eur Mol Biol Organ) J. 1992;11:537–542. doi: 10.1002/j.1460-2075.1992.tb05084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas J, Pagano M, Staskova Z, Draetta G, Bartek J. Cyclin D1 protein oscillates and is essential for cell cycle progression in human tumour cell lines. Oncogene. 1994;9:707–718. [PubMed] [Google Scholar]
- Lukas J, Bartkova J, Rohde M, Strauss M, Bartek J. Cyclin D1 is dispensable for G1 control in retinoblastoma gene-deficient cells independently of cdk4 activity. Mol Cell Biol. 1995a;15:2600–2611. doi: 10.1128/mcb.15.5.2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas J, Bartkova J, Welcker M, Petersen OW, Peters G, Strauss M, Bartek J. Cyclin D2 is a moderately oscillating nucleoprotein required for G1 phase progression in specific cell types. Oncogene. 1995b;10:2125–2134. [PubMed] [Google Scholar]
- Lukas J, Parry D, Aagaard L, Mann DJ, Bartkova J, Strauss M, Peters G, Bartek J. Retinoblastoma-protein-dependent cell-cycle inhibition by the tumour suppressor p16. Nature. 1995c;375:503–506. doi: 10.1038/375503a0. [DOI] [PubMed] [Google Scholar]
- Lukas J, Petersen BO, Holm K, Bartek J, Helin K. Deregulated expression of E2F family members induces S-phase entry and overcomes p16INK4A-mediated growth suppression. Mol Cell Biol. 1996a;16:1047–1057. doi: 10.1128/mcb.16.3.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas J, Bartkova J, Bartek J. Convergence of mitogenic cascades from diverse classes of receptors at the cyclin D-cyclin-dependent kinase-pRb-controlled G1 checkpoint. Mol Cell Biol. 1996b;16:6917–6925. doi: 10.1128/mcb.16.12.6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukas J, Herzinger T, Hansen K, Moroni MC, Resnitzki D, Helin K, Reed SI, Bartek J. Cyclin E–induced S phase without activation of the pRb/E2F pathway. Genes Dev. 1997;11:1479–1492. doi: 10.1101/gad.11.11.1479. [DOI] [PubMed] [Google Scholar]
- Lyons J, Landis CA, Harsh L, Vallar K, Grünewald H, Feichtinger H, Duh QY, Clark OH, Kawasaki E, Bourne HR, McCormick F. Two G protein oncogenes in human endocrine tumors. Science. 1990;249:655–659. doi: 10.1126/science.2116665. [DOI] [PubMed] [Google Scholar]
- Matsushime H, Quelle DE, Shurtleff SA, Shibuya M, Sherr CJ, Kato JY. D-type cyclin-dependent kinase activity in mammalian cells. Mol Cell Biol. 1994;14:2066–2076. doi: 10.1128/mcb.14.3.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema RH, Herrera RE, Lam F, Weinberg RA. Growth suppression by p16ink4requires functional retinoblastoma protein. Proc Natl Acad Sci USA. 1995;92:6289–6293. doi: 10.1073/pnas.92.14.6289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerson M, Harlow E. Identification of G1 kinase activity for cdk6 a novel cyclin D partner. Mol Cell Biol. 1994;14:2077–2086. doi: 10.1128/mcb.14.3.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musgrove FA, Hamilton JA, Lee CSL, Sweeney KJF, Watts CKW, Sutherland RL. Growth factor, steroid, and steroid antagonist regulation of cyclin gene expression associated with changes in T-47D human breast cancer cell cycle progression. Mol Cell Biol. 1993;13:3577–3587. doi: 10.1128/mcb.13.6.3577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtani, K.J., J. Degregori, and J.R. Nevins. 1995. Regulation of the cyclin E gene by transcription factor E2F1. Proc. Natl. Acad. Sci. USA. 92:12146– 12150. [DOI] [PMC free article] [PubMed]
- Ohtsubo M, Theodoras AM, Schumacher J, Roberts JM, Pagano M. Human cyclin E, a nuclear protein essential for the G1-to-S phase transition. Mol Cell Biol. 1995;15:2612–2624. doi: 10.1128/mcb.15.5.2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parma J, Duprez L, Van Sande J, Cochaux P, Gervy C, Mockel J, Dumont JE, Vassart G. Somatic mutations in the thyrotropin receptor gene cause hyperfunctioning thyroid adenomas. Nature. 1993;365:649–651. doi: 10.1038/365649a0. [DOI] [PubMed] [Google Scholar]
- Pirson I, Coulonval K, Lamy F, Dumont JE. c-myc expression is controlled by the mitogenic cAMP-cascade in thyrocytes. J Cell Physiol. 1996;168:59–70. doi: 10.1002/(SICI)1097-4652(199607)168:1<59::AID-JCP8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Pohl V, Roger PP, Christophe D, Pattyn G, Vassart G, Dumont JE. Differentiation expression during proliferative activity induced through different pathways: in situ hybridization study of thyroglobulin gene in thyroid epithelial cells. J Cell Biol. 1990;111:663–672. doi: 10.1083/jcb.111.2.663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quelle DE, Ashmun RA, Shurtleff SA, Kato JY, Bar-Sagi MF, Roussel MF, Sherr CJ. Overexpression of mouse D-type cyclins accelerates G1 phase in rodent fibroblasts. Genes Dev. 1993;7:1559–1571. doi: 10.1101/gad.7.8.1559. [DOI] [PubMed] [Google Scholar]
- Rao SS, Kohtz DS. Positive and negative regulation of D-type cyclin expression in skeletal myoblasts by basic fibroblast growth factor and transforming growth factor beta. A role for cyclin D1 in control of myoblast differentiation. J Biol Chem. 1995;270:4093–4100. doi: 10.1074/jbc.270.8.4093. [DOI] [PubMed] [Google Scholar]
- Ravnik SE, Rhee K, Wolgemuth DJ. Distinct patterns of expression of D-type cyclins during testicular development in the mouse. Dev Genet. 1995;16:171–178. doi: 10.1002/dvg.1020160209. [DOI] [PubMed] [Google Scholar]
- Reed SI. G1-specific cyclins: in search of an S-phase-promoting factor. Trends Genet. 1991;7:95–99. doi: 10.1016/0168-9525(91)90279-Y. [DOI] [PubMed] [Google Scholar]
- Resnitzky D, Reed SI. Different roles for cyclins D1 and E in regulation of the G1-to-S transition. Mol Cell Biol. 1995;15:3463–3469. doi: 10.1128/mcb.15.7.3463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuse S, Pirson I, Dumont JE. Differential regulation of protooncogenes c-jun and jun D expressions by protein tyrosine kinase, protein kinase C, and cyclic AMP mitogenic pathways in dog primary thyrocytes: TSH and cyclic AMP induce proliferation but downregulate c-jun expression. Exp Cell Res. 1991;196:210–215. doi: 10.1016/0014-4827(91)90253-q. [DOI] [PubMed] [Google Scholar]
- Reynisdottir I, Massagué J. The subcellular locations of p15ink4b and p27kip1coordinate their inhibitory interactions with cdk4 and cdk2. Genes Dev. 1997;11:492–503. doi: 10.1101/gad.11.4.492. [DOI] [PubMed] [Google Scholar]
- Rock CO, Cleveland JL, Jackowski S. Macrophage growth arrest by cyclic AMP defines a distinct checkpoint in the mid G1 stage of the cell cycle and overrides constitutive c-myc expression. Mol Cell Biol. 1992;12:2351–2358. doi: 10.1128/mcb.12.5.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger PP, Servais P, Dumont JE. Stimulation by thyrotropin and cyclic AMP of the proliferation of quiescent canine thyroid cells cultured in a defined medium containing insulin. FEBS Lett. 1983;157:323–327. doi: 10.1016/0014-5793(83)80569-9. [DOI] [PubMed] [Google Scholar]
- Roger PP, Servais P, Dumont JE. Regulation of dog thyroid epithelial cell cycle by forskolin, an adenylate cyclase activator. Exp Cell Res. 1987a;172:282–292. doi: 10.1016/0014-4827(87)90387-9. [DOI] [PubMed] [Google Scholar]
- Roger PP, Servais P, Dumont JE. Induction of DNA synthesis in dog thyrocytes in primary culture: synergistic effects of thyrotropin and cyclic AMP with epidermal growth factor and insulin. J Cell Physiol. 1987b;130:58–67. doi: 10.1002/jcp.1041300110. [DOI] [PubMed] [Google Scholar]
- Roger PP, Baptist M, Dumont JE. A mechanism generating heterogeneity in thyroid epithelial cells: suppression of the thyrotropin/cAMP-dependent mitogenic pathway after cell division induced by cAMP-independent factors. J Cell Biol. 1992;117:383–393. doi: 10.1083/jcb.117.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roger PP, Reuse S, Maenhaut C, Dumont JE. Multiple facets of the modulation of growth by cAMP. Vitam Horm. 1995;51:59–191. doi: 10.1016/s0083-6729(08)61038-9. [DOI] [PubMed] [Google Scholar]
- Roger PP, Christophe D, Dumont JE, Pirson I. The dog thyroid culture system: a model of regulation of function, growth and differentiation expression by cAMP and other well defined signaling cascades. Eur J Endocrinol. 1997;137:579–598. doi: 10.1530/eje.0.1370579. [DOI] [PubMed] [Google Scholar]
- Sewing ASB, Burger C, Shalk C, Lucibello FC, Müller R. Human cyclin D1 encodes a labile nuclear protein whose synthesis is directly induced by growth factors and suppressed by cyclic AMP. J Cell Sci. 1993;104:545–555. doi: 10.1242/jcs.104.2.545. [DOI] [PubMed] [Google Scholar]
- Sherr CJ. Mammalian G1 cyclins. Cell. 1993;73:1059–1065. doi: 10.1016/0092-8674(93)90636-5. [DOI] [PubMed] [Google Scholar]
- Sherr CJ. G1 phase progression: cycling on cue. Cell. 1994;79:551–555. doi: 10.1016/0092-8674(94)90540-1. [DOI] [PubMed] [Google Scholar]
- Sherr CJ. Cancer cell cycles. Science. 1996;274:1672–1677. doi: 10.1126/science.274.5293.1672. [DOI] [PubMed] [Google Scholar]
- Sicinski P, Donaher JL, Parker SB, Li T, Fazelli A, Gartner H, Haslam SZ, Bronson RT, Elledge SJ, Weinberg RA. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell. 1995;82:621–630. doi: 10.1016/0092-8674(95)90034-9. [DOI] [PubMed] [Google Scholar]
- Sicinski P, Donaher JL, Geng Y, Parker SB, Gardner H, Park MY, Robker RL, Richards JS, McGimis LK, Biggers JD, et al. Cyclin D2 is an FSH-responsive gene involved in gonadal cell proliferation and oncogenesis. Nature. 1996;384:470–474. doi: 10.1038/384470a0. [DOI] [PubMed] [Google Scholar]
- Soos TJ, Kiyokawa H, Yan JS, Rutin MS, Giordano A, De Blasio A, Bottega S, Wong B, Mendelson J, Koff A. Formation of p27-CDK complexes during the human mitotic cell cycle. Cell Growth Differ. 1996;7:135–146. [PubMed] [Google Scholar]
- Tam SW, Theodoras AM, Shay JW, Draetta GF, Pagano M. Differential expression and regulation of cyclin D1 protein in normal and tumor human cells: association with cdk4 is required for cyclin D1 function in G1 progression. Oncogene. 1994;9:2663–2674. [PubMed] [Google Scholar]
- Vintermyr OK, Mellgren GM, Boe R, Doskeland SO. Cyclic adenosine monophosphate acts synergistically with dexamethasone to inhibit entrance of culture rat hepatocytes into S phase. J Cell Physiol. 1989;141:371–382. doi: 10.1002/jcp.1041410219. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhang Y, Kamen D, Lees E, Ravid K. Cyclin D3 is essential for megakaryocytopoiesis. Blood. 1995;86:3783–3788. [PubMed] [Google Scholar]
- Ward AC, Csar XF, Hoffmann BW, Hamilton JA. Cyclic AMP inhibits expression of D-type cyclins and cdk4 and induces p27KIP1in G-CSF-treated NFS-60 cells. Biochem Biophys Res Commun. 1996;224:10–16. doi: 10.1006/bbrc.1996.0976. [DOI] [PubMed] [Google Scholar]
- Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- Xiong Y, Zhang M, Beach D. D-type cyclins associate with multiple protein kinases and the DNA replication and repair factor PCNA. Cell. 1992;71:505–514. doi: 10.1016/0092-8674(92)90518-h. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Hisai A, Ban T, Saito J, Tahara K, Terano T, Tamura Y, Saito Y, Kitagawa M. Thyrotropin induces G1 cyclin expression and accelerates G1 phase after insulin-like growth factor I stimulation in FRTL-5 cells. Endocrinology. 1996;137:2036–2042. doi: 10.1210/endo.137.5.8612545. [DOI] [PubMed] [Google Scholar]