Abstract
Cell adhesion molecules (CAMs) are important mediators of cell–cell interactions and regulate cell fate determination by influencing growth, differentiation, and organization within tissues. The human pancarcinoma antigen KSA is a glycoprotein of 40 kD originally identified as a marker of rapidly proliferating tumors of epithelial origin. Interestingly, most normal epithelia also express this antigen, although at lower levels, suggesting that a dynamic regulation of KSA may occur during cell growth and differentiation. Recently, evidence has been provided that this glycoprotein may function as an epithelial cell adhesion molecule (Ep-CAM). Here, we report that Ep-CAM exhibits the features of a morphoregulatory molecule involved in the development of human pancreatic islets. We demonstrate that Ep-CAM expression is targeted to the lateral domain of epithelial cells of the human fetal pancreas, and that it mediates calcium-independent cell–cell adhesion. Quantitative confocal immunofluorescence in fetal pancreata identified the highest levels of Ep-CAM expression in developing islet-like cell clusters budding from the ductal epithelium, a cell compartment thought to comprise endocrine progenitors. A surprisingly reversed pattern was observed in the human adult pancreas, displaying low levels of Ep-CAM in islet cells and high levels in ducts. We further demonstrate that culture conditions promoting epithelial cell growth induce upregulation of Ep-CAM, whereas endocrine differentiation of fetal pancreatic epithelial cells, transplanted in nude mice, is associated with a downregulation of Ep-CAM expression. In addition, a blockade of Ep-CAM function by KS1/4 mAb induced insulin and glucagon gene transcription and translation in fetal pancreatic cell clusters. These results indicate that developmentally regulated expression and function of Ep-CAM play a morphoregulatory role in pancreatic islet ontogeny.
Induction and maintenance of tissue differentiation during development depends on the coordinated spatiotemporal expression of specialized molecules that regulate cell-to-cell and cell-to-matrix interactions (Ekblom et al., 1986; Edelman, 1991, 1992; Takeichi, 1991, 1995; Trelstad, 1984). Since the pioneering work of Holtfreter (1939) and Moscona (1952), who first recognized the existence of cell type–specific adhesive properties in multicellular organisms, the functional portrait of cell adhesion molecules (CAMs)1 has evolved from that of simple “binding molecules” to the modern concept of “morphoregulatory molecules.” In fact, their coordinated action appears to be involved in the regulation of cell growth, differentiation, adhesion, migration, and three-dimensional organization within tissues during morphogenesis (Crossin et al., 1985; Ekblom et al., 1986; Edelman et al., 1991; Edelman, 1992; Takeichi, 1991, 1995).
An exquisite example of timely regulated morphogenesis is provided by the cell growth, differentiation, and organization of pancreatic islets of Langerhans, representing the endocrine compartment of mammalian pancreas (Langerhans, 1869; Orci and Unger, 1975; Orci, 1982). It is currently thought that islet cells originate from undifferentiated progenitors resident within the ductal epithelium of the fetal pancreas (Pictet and Rutter, 1972; Teitelman and Lee, 1987; Alpert et al., 1988; Herrera et al., 1991; Gu and Sarvetnick, 1993). This process involves cell budding, growth, migration into the surrounding mesenchyme, and differentiation into the highly organized islet clusters (Pictet et al., 1972; for review see Slack, 1995). Evidence has been provided for a role of adhesion molecules of the cadherin family in the morphogenesis of the pancreas (Thiery et al., 1982, 1984; Edelman et al., 1983; Hatta and Takeichi, 1986; Nose and Takeichi, 1986; Levi et al., 1991; Sjödin et al., 1995), and in the development of islet clusters (Dahl et al., 1996). Similarly, adhesion molecules of the immunoglobulin superfamily such as neuronal (N)-CAM have been found dynamically expressed in the pancreas and in other organs of endodermal origin during development (Edelman et al., 1983; Rutishauser, 1984; Crossin et al., 1985). In addition, we and others have demonstrated the involvement of cadherins and N-CAM in islet cell–cell adhesion (Langley et al., 1989; Begemann et al., 1990; Rouiller et al., 1990, 1991; Bauer et al., 1992; Moller et al., 1992), and the regulation of islet cell types' organization by calcium-independent adhesion molecules such as N-CAM (Rouiller et al., 1991; Cirulli et al., 1994).
Among the molecules possibly involved in tissue morphogenesis, the pancarcinoma antigen KSA (alias EGP40, 17-1A, ESA, etc.) is particularly interesting (Varki et al., 1984; Edwards et al., 1986; Spurr et al., 1986; Bumol et al., 1988). This antigen, originally identified as an abundantly expressed glycoprotein in tumors of epithelial origin, is found at lower levels in most simple, pseudostratified and transitional normal epithelia (Moldenhauer et al., 1987; Momburg et al., 1987). Fetal epithelia exhibit stronger immunoreactivity for KSA antigen than the adult mature tissues (Varki et al., 1984), suggesting a dynamic regulation of its expression during epithelial ontogeny. Recently, Litvinov and colleagues provided evidence that EGP40 (alias KSA) exhibits the features of a typical cell–cell adhesion molecule when transfected in murine and human tumor cell lines (Litvinov et al., 1994a ,b). The name of Ep-CAM (epithelial cell adhesion molecule) for this antigen was therefore proposed.
To identify novel morphoregulatory molecules possibly involved in pancreatic islet development, we have investigated the expression and function of Ep-CAM in the developing human pancreas. We demonstrate that Ep-CAM expression is targeted to the lateral domain of epithelial cells of the human fetal pancreas, and that it mediates calcium-independent adhesion. In addition, we show that Ep-CAM expression is developmentally regulated during cell growth and endocrine determination of islet cells. Based on properties and roles ascribed to classical cell–cell adhesion molecules in histogenesis and organogenesis (Edelman and Crossin, 1991; Takeichi, 1995), we propose that Ep-CAM represents a novel morphoregulatory molecule delivering specific developmental signals at key stages of pancreatic islet morphogenesis.
Materials and Methods
Tissues
Midgestation human fetal pancreata (HFP) ranging from 18 to 20 wk of gestational age were obtained through nonprofit organ procurement programs (Advanced Bioscience Resources, Oakland, CA; Anatomic Gift Foundation, Laurel, MD), which also obtained consent for tissue donation. Samples of human adult pancreas and pancreatic islets were prepared at The Diabetes Research Institute (University of Florida, Miami, FL) as previously described (Ricordi et al., 1988). Use of human tissues was approved by appropriate Institutional Review Boards.
Immunofluorescence, Confocal Microscopy, and Morphometric Analysis
Triple immunofluorescent labeling for Ep-CAM, insulin, and glucagon, or Ep-CAM, Ki-67, and insulin were performed on 8-μm-thick cryostat sections prepared from snap frozen fetal (18–20 wk of gestation) and adult pancreases. Sections were mounted on glass slides, dried at room temperature for 1 h, fixed in freshly made 4% formaldehyde (from paraformaldehyde) for 20 min at 4°C, permeabilized in 0.1% saponin for 5 min at room temperature, and then incubated in 50 mM glycine in PBS to saturate reactive groups generated by formaldehyde fixation. Nonspecific binding was blocked by incubation of sections in PBS containing 2% donkey serum (DS) (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) and 1% BSA fraction V (Sigma Chemical Co., St. Louis, MO) for 1 h at room temperature. After extensive washes in PBS-DS (0.2% DS, 0.1% BSA, 5 mM glycine), sections were incubated for 1 h at room temperature with a mixture of primary antibodies: anti–Ep-CAM KS1/4 mAb (5 μg/ml) (Varki et al., 1984), IgG fraction of a sheep antiinsulin polyclonal antiserum (5 μg/ml; The Binding Site, Birmingham, United Kingdom), rabbit antiglucagon polyclonal antiserum (1:200 dilution; Chemicon International, Inc., Temecula, CA) or rabbit anti–Ki-67 affinity-purified IgGs (2.5 μg/ml; Dako Corp., Carpinteria, CA) In separate sections, a mixture of normal sheep, rabbit, and mouse IgGs was used as control reference for specificity of primary antibodies. After several washes in PBS-DS, sections were incubated for 1 h at room temperature with a cocktail of F(ab′)2 fractions from secondary antibodies (Jackson ImmunoResearch Laboratories, Inc.): lissamine rhodamine–conjugated affinity-purified donkey anti–sheep IgGs (5 μg/ml) (preadsorbed on chicken, guinea pig, hamster, horse, human, mouse, rabbit, and rat serum proteins); FITC-conjugated affinity-purified donkey anti–rabbit IgGs (5 μg/ml) (preadsorbed on bovine, chicken, goat, guinea pig, hamster, horse, human, mouse, rat, and sheep serum proteins); indodicarbocyanine (Cy5)-conjugated affinity-purified donkey anti–mouse IgGs (5 μg/ml) (preadsorbed on bovine, chicken, goat, guinea pig, hamster, horse, human, rabbit, rat, and sheep serum proteins). After six washes at 5 min each with PBS-DS, sections were mounted in slow fade medium (Molecular Probes, Inc., Eugene, OR), and then viewed on a microscope (model Axiovert 35M; Carl Zeiss, Inc., Thornwood, NY) equipped with a laser scanning confocal attachment (model MRC-1024; Bio-Rad Laboratories, Hercules, CA), using a 40× 1.3 NA objective lens. Fluorescent images relative to each marker were collected by using the 488-, 568-, and 647-nm excitation lines from an argon/ krypton mixed gas laser. Color composite images were generated using Adobe Photoshop 4.0 (Adobe Systems Inc., Mountain View, CA) running on a computer (model SuperMac S910/250; UMAX Computer Corp., Fremont, CA), and then printed with a Fujix Pictrography 3000 color printer (Fuji Photo Film U.S.A., Inc., Elmsford, NY).
Microscopic fields acquired from fetal (n = 50) and adult (n = 72) pancreatic sections immunostained for Ep-CAM, insulin, and glucagon were analyzed for pixel intensity of Ep-CAM–specific immunoreactivity using National Institutes of Health (NIH) Image software (Bethesda, MD). Pixel's intensity units (0–255) were recorded from a total of 250 domains of cell–cell contact for each cell type (ductal, endocrine, epithelial undifferentiated, and exocrine) in both fetal and adult pancreatic specimens.
Immunoelectron Microscopy
HFP cells cultured as monolayers (Beattie et al., 1996) were fixed with 4% formaldehyde in PBS for 30 min, washed in PBS, and then permeabilized with a solution containing 0.1% Triton X-100 and 2% normal goat serum in PBS for 20 min. Cells were incubated with primary antibodies (KS1/4 mAb or anti-EcadEC5 polyclonal antibody [Fannon et al., 1995]) for 1 h, washed in buffer, and then incubated with biotinylated goat anti–mouse or anti–rabbit IgG for 1 h at 4°C. After washes in PBS, the cells were incubated in an avidin–biotin complex for 1 h, washed again in PBS, and then reacted for 4–6 min in 0.05 mg/ml diaminobenzidine tetrahydrochloride (Sigma Chemical Co.) and 0.01% H2O2 in PBS. The cells were then postfixed with 1% OsO4 in PBS for 1 h, rinsed in distilled water, dehydrated in an ethanol series, and then embedded in Durcupan resin (Electron Microscopy Sciences, Fort Washington, PA). Ultrathin (80-nm) sections and semithin (1-μm) sections were cut using a diamond knife and an ultramicrotome (model Ultracut E; Leica USA, Deerfield, IL) and then mounted on uncoated copper grids. Thin sections were imaged at 80 keV using an electron microscope (model 100CX or 2000FX; JEOL Ltd., Akashima, Japan) and then semithick sections were imaged at 300–400 keV using an intermediate voltage electron microscope (model 4000EX; JEOL, Ltd). Stereo pair electron micrographs were obtained by tilting the specimen +5°.
Generation of Islet-like Cell Clusters and Cell Monolayers
HFP at 18–24 wk of gestation were minced in small pieces and then digested in a collagenase solution (6 mg/ml per HBSS; collagenase-P; Boehringher Mannheim Biochemicals, Indianapolis, IN) for 15 min at 37°C in a shaking water bath (150 cycles/min) as described previously (Otonkoski et al., 1993). Cell clusters resulting from this procedure were washed in cold HBSS and then placed in culture in RPMI-1640 (Sigma Chemical Co.) containing 11 mM glucose and supplemented with 10% normal human serum. This culture condition, applied for 3–5 d in nonadherent culture dishes, allows the formation of smooth islet-like cell clusters (ICCs). ICCs contain mostly undifferentiated epithelial cells (Beattie et al., 1994; Otonkoski et al., 1994, 1996) and between 5 and 10% endocrine cells (mainly insulin- and glucagon-producing cells). Cells within ICCs can be induced to proliferate in vitro to threefold when cultured in the presence of 10 ng/ ml of recombinant human hepatocyte growth factor/scatter factor (rhHGF/SF) (Otonkoski et al., 1994; Beattie et al., 1996). Cultures of ICCs in Petri dishes coated with the extracellular matrix 804G, as recently described by Langhofer and co-workers (Langhofer et al., 1993), were supplemented with 10 ng/ml rhHGF/SF, and then used to generate cell monolayers. Under these culture conditions, cells forming the ICCs adhere, migrate, and proliferate to produce large monolayers that result from a 30-fold increase in cell number (Beattie et al., 1996). Cell monolayers prepared in this manner were therefore used as an in vitro model of pancreatic epithelial cell growth.
Transplantation Experiments and Glucose Tolerance Test
Four athymic nude (nu/nu) BALB/c mice were transplanted under the kidney capsule with 500 ICCs/mouse, as previously described (Sandler et al., 1985; Beattie et al., 1994). At the time of transplant, ICCs contain ∼5– 10% endocrine cells, <10% mesenchymal cells, and ∼80% undifferentiated epithelial cells (Beattie et al., 1994), whereas after 12 wk in vivo, ∼80% of the grafted cells have differentiated into endocrine tissue (Beattie et al., 1997). Therefore, we used this in vivo model to study the expression pattern of Ep-CAM during islet cell development from immature pancreatic epithelial cells. After 12 wk, the mice were fasted for 4 h, anesthetized, and then blood samples were collected from the external jugular vein before and 30 min after an intraperitoneal injection of glucose (3 g/Kg of body weight). Blood samples were then tested for the presence of human C peptide using a human-specific radioimmunoassay, as previously described (Beattie et al., 1994).
Flow Cytomery
HFP were minced and digested in collagenase (3 mg/ml per HBSS; collagenase-P, Boehringher Mannheim Biochemicals) for 15 min at 37°C in a shaking water bath (150 cycles/min). Cell clusters resulting from this procedure were washed in cold PBS containing 0.2 mM EDTA, resuspended in a nonenzymatic cell dissociation medium (Sigma Chemical Co.) and then incubated at 37°C for 10 min applying gentle pipetting every minute. The single cell suspension resulting from this protocol was washed in HBSS containing 1% BSA (HBSS-BSA), and then incubated for 45 min at 4°C with either anti–Ep-CAM KS1/4 mAb (1 μg/ml) or isotype-matched control IgGs. After washing in HBSS-BSA, cells were incubated with FITC-conjugated donkey anti–mouse IgGs (H + L chains) for 45 min at 4°C. After washing in HBSS-BSA, samples were then analyzed on a FACScanTM flow cytometer (Becton Dickinson, Sparks, MD).
Production of Fab′ Fragments
Control or anti-KSA (Ep-CAM) Fab′ fragments were generated by using an immobilized papain digestion protocol (Pierce Chemical Co., Rockford, IL) with either 50 mg of control mouse IgGs or 50 mg of KS1/4 mouse mAb. After separation of Fab′ fragments according to the manufacturer's directions, Fab′s were concentrated by ultrafiltration using Centricon-10 units (Amicon Corp., Beverly, MA) and then resuspended in sterile PBS at a concentration of 1 mg/ml. Purity of Fab′ fraction was checked by analyzing a sample of Fab′ in a 10% agarose gel and followed by Coomassie blue staining. Reactivity of KS1/4 Fab′ to Ep-CAM was confirmed by flow cytometry.
Cell Aggregation and Antibody Perturbation Assay
A single cell suspension was prepared from HFP, as detailed above, and used to test cell–cell aggregation mediated by “calcium-dependent” and “calcium-independent” adhesion systems. These pancreatic cells were divided into two pools and then washed in Krebs-Ringer bicarbonate buffer containing 1% BSA, 10 mM glucose, 10 mM Hepes, 50 μg/ml of DNase I, and either 1 mM CaCl2 or 0.2 mM EDTA. Each of the two cell pools was resuspended at a concentration of 106 cells/ml and then further divided into two aliquots to test the ability of pancreatic cells to reaggregate either in the presence of a control Fab′ fragment or in the presence of an anti– Ep-CAM Fab′ fragment (100 μg/ml). Cell aggregation for each sample was performed in triplicate for 45 min at 37°C in a shaking water bath (100 cycles/min). Cell aggregation was monitored by counting the number of events in a particle counter (ZM-Coulter counter; Coulter Corp., Miami, FL) at the beginning and at the end of the aggregation period, as previously described (Rouiller et al., 1990, 1991; Cirulli et al., 1993).
Effect of Anti–Ep-CAM Fab′ on Fetal Pancreatic Cell Differentiation
Single cell suspensions prepared from HFP, as detailed above, were plated in medium containing either anti–Ep-CAM KS1/4 mAb or control Fab′ fragments (100 μg/ml). Fab′s (50 μg/ml) were added to the cultures daily to ensure constant saturation of Fab′s blocking activity. After 5 d in these culture conditions cells were harvested, and then total RNA was prepared to test insulin and glucagon transcript levels in an RNase protection assay as described (Otonkoski et al., 1993; Mally et al., 1994). In some experiments, samples of both control and anti–Ep-CAM–treated cells were used for DNA and insulin protein determinations (Otonkoski et al., 1993).
Immunoblotting
Samples of cultured ICCs, pancreatic cell monolayers, fetal and adult human islets, and human insulinomas were extracted in 20 mM Tris, 1% Triton X-100, pH 7.2, for 30 min at 4°C. Insoluble material was pelletted by spinning at 10,000 g for 20 min and then supernatants were recovered. Total proteins, 5 μg from each sample, were diluted in electrophoresis sample buffer, boiled for 5 min, and then subjected to 10% SDS–acrylamide gel electrophoresis. Proteins were then electrotransferred onto polyvinylene difluoride (PVDF) membranes (Millipore Corp., Waters Chromatography, Bedford, MA). After blocking in Blotto solution (Tropix, Inc., Bedford, MA), membranes were incubated for 2 h at room temperature with either KS1/4 mAb (1 μg/ml), or an isotype-matched mouse IgG. After extensive washings, PVDF membranes were incubated for 1 h at room temperature with a peroxidase-conjugated goat anti–mouse antibody (1: 5,000 dilution). Antibody binding was revealed by a chemiluminescence detection system (KLM, Gaithersburg, FL) following the manufacturer's instructions.
RNA Isolation and Hormone Transcriptional Analysis
Total cellular RNA was isolated by the acid guanidinium thiocyanate method (Chomczynski and Sacchi, 1987) and then quantitated spectrophotometrically. Transcriptional analyses were performed using a multiprobe RNase protection assay (Otonkoski et al., 1993). All probes used were of human origin and previously described (Otonkoski et al., 1993; Mally et al., 1994). Yeast tRNA (10 μg) was included as a negative control. Quantitation of band intensities was performed by scanning densitometry (model LKB UltroScan XL Laser; Pharmacia Biotech., Inc., Piscataway, NJ) and integrated using GelScan XL software (Pharmacia Biotech, Inc.). Different exposure times were used to ensure that all signals fell within the linear range of the densitometer. The probe-specific mRNA signals were normalized to the cyclophilin signal in each sample to account for differences in sample loading between lanes.
Statistics
Where applicable, data were analyzed using Stat View 4.01 software (Abacus Concepts, Inc., Berkeley, CA) for determination of mean, standard deviation, and parametric statistics (t test).
Results
Ep-CAM Expression Highlights the Epithelial Compartment of the Developing Human Pancreas
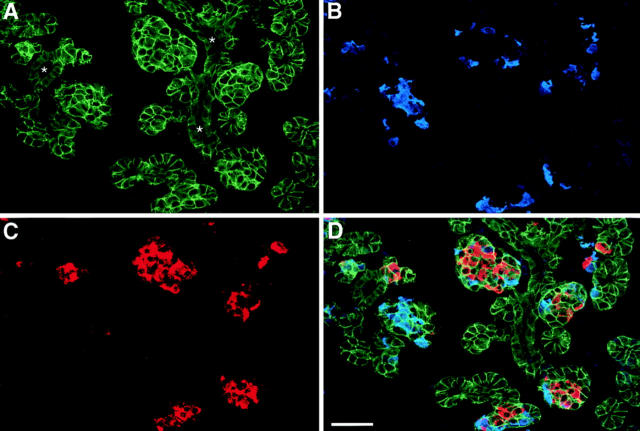
In humans, the development of the endocrine compartment of the pancreas, the islets of Langerhans, occurs during fetal life, whereas differentiation, growth, and expansion of the exocrine acinar tissue is a perinatal event (Fukayama et al., 1986; Jaffe, 1991; Carrere et al., 1992). In all mammals studied, these two functionally distinct compartments are believed to derive from common precursors present in the ductal epithelium engaging separate differentiation programs at distinct developmental stages (Like and Orci, 1972; Pictet and Rutter, 1972; Pictet et al., 1972; Clark and Grant, 1983; Stefan et al., 1983; Teitelman and Lee, 1987; Alpert et al., 1988; Von Dorsche et al., 1989; Gu et al., 1994). At a midgestational age (16–20 wk), the epithelial compartment of the human pancreas consists of a branching ductal tree giving rise to numerous clusters of cells that appear to protrude and invade into the surrounding mesenchyme. To identify the expression pattern of Ep-CAM within the developing human pancreas, 8-μm cryostat sections from a human fetal pancreas of 18 wk were used for the simultaneous identification of Ep-CAM–, insulin- and glucagon-positive cells by triple immunofluorescence. As shown in Fig. 1, Ep-CAM–specific immunoreactivity (A, green) highlights the epithelial compartment of the fetal pancreas, allowing the identification of ducts and clusters of cells budding from the ductal epithelium (asterisks). Many of these budding cell clusters contain insulin- (Fig. 1 C, red) and glucagon-positive cells (Fig. 1 B, blue), a feature consistent with the current notion that endocrine cells arise from precursors present within the ductal epithelium (Pictet et al., 1972; Teitelman and Lee, 1987; Alpert et al., 1988; Gu and Sarvetnick, 1993). Notably, Ep-CAM expression appears to be restricted to sites of cell–cell contacts in a pattern similar to that observed for classical adhesion molecules, suggesting its involvement in the regulation of pancreatic epithelial cell–cell adhesion.
Figure 1.
Ep-CAM expression identifies the epithelial compartment of the human fetal pancreas. Triple immunofluorescence, followed by confocal microscopic analysis, was performed on 8-μm cryostat sections from a human fetal pancreas of 18 wk to simultaneously identify Ep-CAM–, insulin-, and glucagon-positive cells. (A) Ep-CAM–specific immunoreactivity that highlights the cell– cell boundaries of epithelial cells. The strongest immunoreactivity for Ep-CAM is detected in cell clusters budding from the ducts (asterisks). Notably, many cell clusters emerging from the ductal epithelium contain large numbers of glucagon- (B) and insulin-positive cells (C). (D) Combined fluorophore spectra. Yellow color results from the colocalization of green and red fluorescences, specific for Ep-CAM and insulin, respectively. Representative of nine independent experiments using five independent donors (18–20 wk of gestation). Bar, 50 μm.
Ep-CAM Expression Is Targeted to the Lateral Domain of HFP Epithelial Cells
To gain insight on the distribution of Ep-CAM to specific cellular domains, cell monolayers prepared from HFPs were immunolabeled for Ep-CAM by an indirect peroxidase method. Sections were cut either through a horizontal plane parallel to the cell monolayer, or through a vertical plane perpendicular to the monolayer, and then imaged at 300–400 keV using an intermediate voltage electron microscope (JEOL 4000EX). A recently described anti–E-cadherin antiserum (EcadEC5) (Fannon et al., 1995) was used as a positive reference for the identification of cell–cell adhesion complexes. To obtain a three- dimensional view of the regions of cell–cell contacts, stereo pair images were obtained by tilting the specimens +5°. Fig. 2 shows a set of stereo pairs of Ep-CAM–stained cells cut through either the horizontal plane (A) or through a vertical plane (B). As seen in Fig. 2, panels A, electron-dense stain is specifically localized to regions of cell–cell contacts, delineating the rim of three cells in contact with one another. Stereoviewing of these semithick preparations reveals that staining is discontinuous and concentrated in discrete regions of tight cell–cell association.
Figure 2.
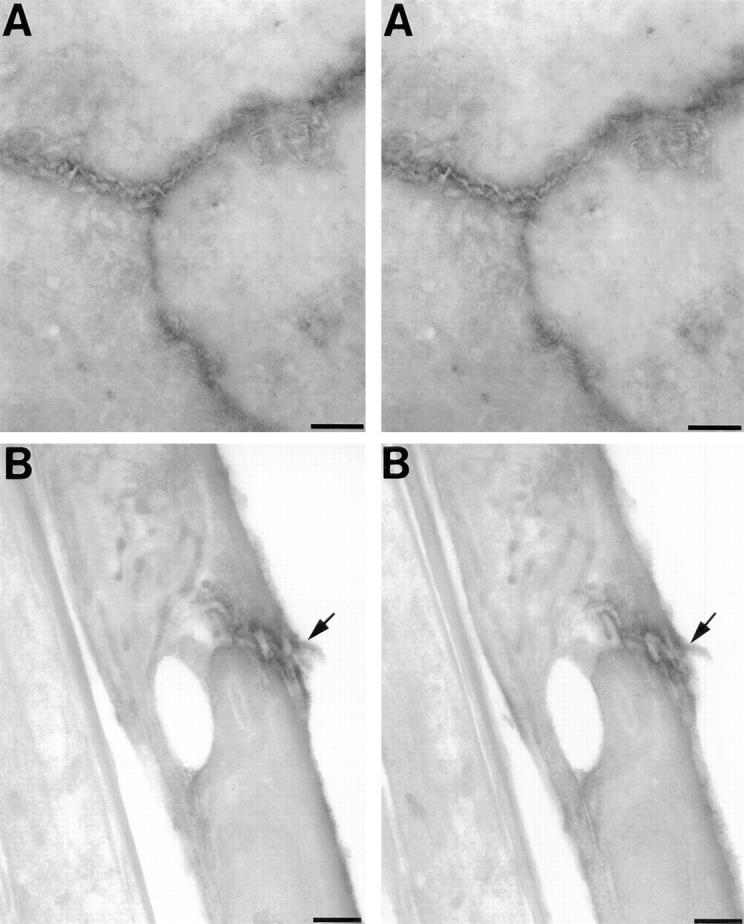
Electron microscopic identification of Ep-CAM at sites of cell–cell contact. Cell monolayers prepared from HFPs were immunolabeled for Ep-CAM by indirect peroxidase method, sectioned either through the horizontal plane (A) or a vertical plane perpendicular to the cell monolayer (B), and then imaged at 300–400 keV using an intermediate voltage electron microscope (JEOL 4000EX). Stereo pair images were obtained by tilting the specimens +5°. As seen in panels A, electron-dense staining specifically localizes to regions of cell–cell contacts, depicting the rim of three cells contacting each other. Panels B reproduce a stereo pair image collected from an Ep-CAM-stained sample sectioned through the z axis. This view identifies basal (left end side), lateral, and apical domains (right end side) of cells in contact. Most of the electron-dense staining appears targeted to the lateral domain of two cells in contact (arrow), and then localizes to interdigitated philopodia-like structures contributed by both cells. Bars, 1 μm.
Fig. 2, panels B represent a stereo pair image collected from an Ep-CAM–stained sample sectioned perpendicularly to the monolayer plan. Viewing from this perspective facilitates the identification of basal, lateral, and apical domains of cells in contact. Notably, most of the electron-dense staining appears targeted to the lateral domain of two cells in contact (arrow) and localizes to interdigitated filopodia-like structures contributed to this junction by both cells. Occasionally, some staining localizes to small plasma membrane ruffles at the apical pole of the cells. In contrast, no detectable staining was found on the basal pole of HFP cells, excluding the contribution of Ep-CAM to cell interactions with basal membranes.
The high concentration of electron-dense staining observed for Ep-CAM is reminiscent of more typical cell adhesion complexes such as those observed in samples immunostained for E-cadherin (Fig. 3 A), a classical epithelial cell adhesion molecule. Fig. 3, panels B is a stereo pair of a sample incubated with mouse IgG2a used as negative control for KS1/4 mAb. These data strongly support the involvement of Ep-CAM in the establishment and/or maintenance of epithelial cell–cell interactions.
Figure 3.
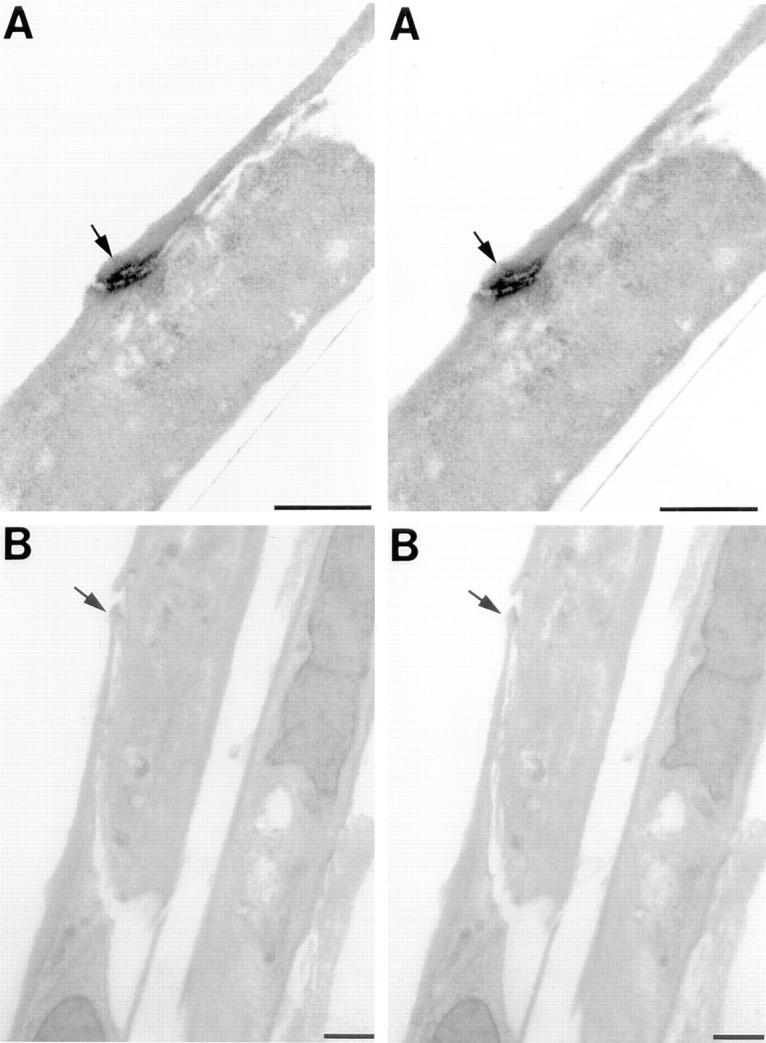
Identification of E-cadherin in junctional complexes of fetal pancreatic epithelial cells. Samples sectioned through a vertical plane and prepared as in Fig. 2 were immunolabeled for E-cadherin with an anti–E-cadherin antiserum (EcadEC5) (Fannon et al., 1995) to identify functional adhesion complexes in HFP cell monolayers. Panels A represent a stereo pair image of two cells in contact with each other, displaying strong E-cadherin–specific electron-dense staining at the site of contact (arrow). The apical pole of these cells is on the left. Panels B reproduce a stereo pair view of a sample incubated with an isotype-matched antibody used as negative control for KS1/4 mAb. The arrow shows a site of cell–cell contact between two cells. Bars, 1 μm.
Ep-CAM Mediates Cell–Cell Adhesion of HFP Epithelial Cells
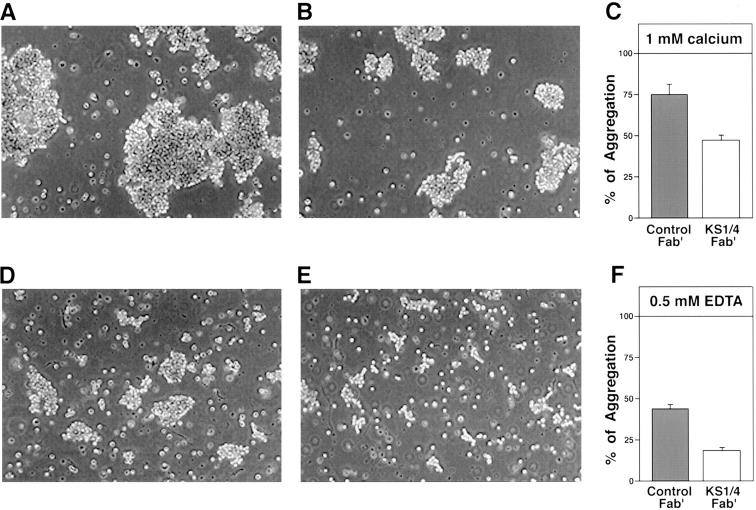
To investigate the involvement of Ep-CAM in pancreatic epithelial cell–cell adhesion, we performed reaggregation experiments following classical protocols previously used for the functional characterization of other cell adhesion molecules, either in transfected or embryonic cells (Takeichi, 1977; Hoffman, 1992), or in primary pancreatic islet cells (Rouiller et al., 1990, 1991; Cirulli et al., 1993). After collagenase digestion of HFPs, small clusters of cells were further dissociated using a nonenzymatic dissociation medium (Sigma Chemical Co.) to obtain a free cell suspension. Immunostaining for Ep-CAM followed by flow cytometric analysis demonstrated that >95% of the cells obtained with this procedure are epithelial. This cell suspension was then used to perform short-term (45 min) reaggregation experiments at 37°C in the presence or absence of calcium, to discriminate between calcium-dependent and calcium-independent adhesion mechanisms (Sperry, 1963). Each of these experimental conditions was also carried out in the presence or absence of anti–Ep-CAM antibodies used either as whole immunoglobulins (mAb 323A3 [Edwards et al., 1986]) or as Fab′ fractions (mAb KS1/4). Fig. 4 shows a representative example of an aggregation assay using HFP cells. As seen in Fig. 4 A, incubation of HFP cells for 45 min at 37°C in the presence of calcium and control Fab′ fragments yielded large aggregates that accounted for 74.6% of total aggregation (Fig. 4 C, gray bar), whereas aggregation performed in the absence of calcium (0.5 mM EDTA) allowed the formation of much smaller cell clusters (Fig. 4 D), accounting for only 47% of total aggregation (Fig. 4 F, gray bar). The qualitative and quantitative difference observed in ion dependence of HFP cell aggregation properties suggest that these cells use both classical calcium-dependent and calcium-independent mechanisms of adhesion to associate to each other. When HFP cells were reaggregated under the same conditions but in the presence of anti–Ep-CAM Fab′ fragments (from KS1/4 mAb), reaggregation dynamics were affected both in the presence and absence of calcium. Fig. 4 B shows the qualitative appearance of cell clusters obtained in the presence of calcium and anti–Ep-CAM Fab′ fragments. Under these conditions, cell aggregation was partially inhibited, yielding much smaller clusters and accounting for only ∼44% of total aggregation (Fig. 4 C, open bar). This residual aggregation is likely to be mediated by cell adhesion molecules of the cadherin superfamily. In contrast, aggregation of HFP cells in the absence of calcium appeared to be dramatically affected by the blockade of Ep-CAM (Fig. 4 E). In this condition, very small cell clusters were observed, accounting for only 18.9% of total aggregation (Fig. 4 F, open bar). Similar inhibitory effects were observed in separate experiments by using mAb 323A3, previously characterized by Litvinov and colleagues as function blocking for Ep-CAM (Litvinov et al., 1984a). Our results demonstrate that KS1/4 mAb is also a function-blocking antibody for Ep-CAM, and that Ep-CAM is a major adhesion molecule mediating epithelial cell–cell association in the developing human pancreas.
Figure 4.
Ep-CAM mediates calcium- independent aggregation of fetal pancreatic epithelial cells. Freshly dissociated pancreatic epithelial cells were reaggregated in the presence (A–C) or absence (D–F) of calcium, to discriminate between calcium-dependent and -independent adhesion mechanisms. A shows the qualitative appearance of cell aggregates obtained in the presence of control Fab′ fragments, whereas B depicts clusters formed in the presence of anti–Ep-CAM Fab′ fragments (from KS1/4 mAb). This mAb reduced the size of cell aggregates, causing an inhibition of cell aggregation from 74.6 to 44% (C) (P < 0.001). In the absence of calcium, the aggregation observed in the presence of a control Fab′ (D and F) is decreased from 47 to 18.9% (E and F) (P < 0.001). Values in C and F are expressed as mean ± SEM of four independent experiments, using four independent donors (18–20 wk of gestation).
Expression of Ep-CAM Is Upregulated in Developing Islet Cells Budding from the Ductal Epithelium
Our immunofluorescence studies on HFP at 18 wk of gestation demonstrated a unique expression pattern of Ep-CAM. Fig. 5 shows a representative field collected from a section of HFP stained by two-color immunofluorescence for Ep-CAM (A, green) and insulin (B, red). The two fluorophore spectra are merged in Fig. 5 C. Although Ep-CAM–specific fluorescence weakly marked ductal epithelial cells (Fig. 5 C, arrow), it strongly highlighted regions of cell–cell contact within large clusters of epithelial cells budding from the ducts (Fig. 5, A and C). Some of these cell clusters comprise insulin-producing cells in their core, suggesting that they may represent developing islets of Langerhans. Other epithelial cell clusters, also showing high levels of Ep-CAM, lacked detectable expression of islet hormones. The possibility that these clusters of undifferentiated epithelial cells comprise exocrine precursors remains speculative because no exocrine markers are expressed in the human pancreas at this developmental stage (Fukayama et al., 1986; Jaffe, 1991; Carrere et al., 1992; Mally, 1994, and data not shown). Thus, a unique expression pattern of Ep-CAM characterizes morphologically distinct cell populations in situ, i.e., ductal cells displaying low levels of the antigen, and ICCs and clusters of undifferentiated epithelial cells showing high levels of Ep-CAM. The existence of at least two distinct cell populations identified by Ep-CAM was further confirmed by flow cytometric analysis of cell suspensions. As shown in Fig. 5 D, two cell populations are identified by immunostaining with the KS1/4 mAb. One population exhibits low levels of Ep-CAM and accounts for ∼30% of total cells (Fig. 5 D, arrow), whereas the other displays high levels of Ep-CAM expression and accounts for ∼65% of total cells (Fig. 5 D, arrowhead). We suggest that Ep-CAMlow cells identified by flow cytometry are likely to correspond in situ to ductal cells (Fig. 5 C, arrow), whereas the Ep-CAMhigh cell population (Fig. 5 D, arrowhead) comprises developing ICCs (Fig. 5 C, arrowheads) and undifferentiated epithelial cells. Overall, the data show that modulation of Ep-CAM expression accompanies the budding of islet cells from the ductal epithelium.
Figure 5.
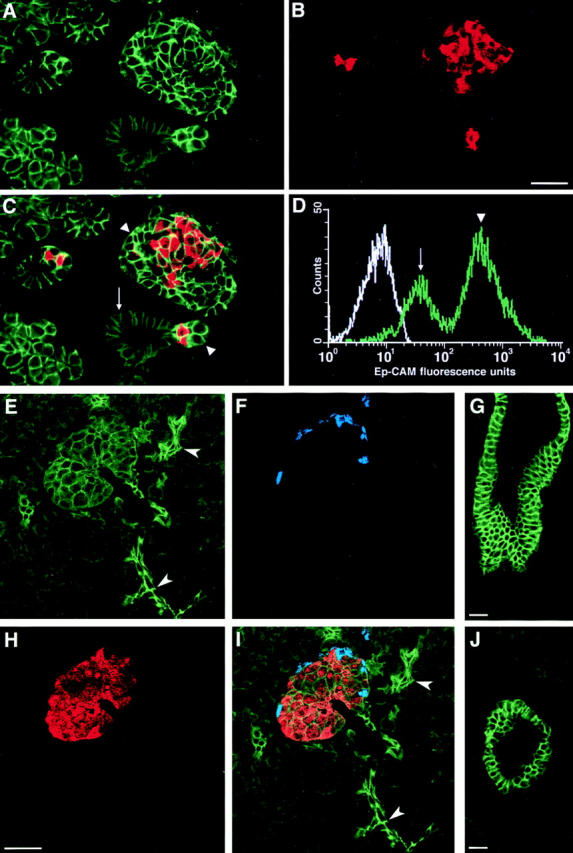
Developmentally regulated expression of Ep-CAM in the human pancreas. Confocal analysis performed on cryostat sections of a HFP, double immunostained for Ep-CAM (A) and insulin (B), reveals the upregulation of Ep-CAM expression in cell clusters budding from the ductal epithelium (A). The two fluorophore spectra are merged in C, where insulin-positive cells (red) are identified within clusters displaying the brightest immunoreactivity for Ep-CAM (green). D represents a typical histogram obtained by flow cytometric analysis after immunolabeling for Ep-CAM of freshly isolated HFP cells. It shows that two distinct populations of Ep-CAM– positive cells are present in the HFP, an Ep-CAMlow (arrow) and an Ep-CAMhigh (arrowhead) population. This pattern, when compared to the in situ detection of Ep-CAM (A and C), suggests that Ep-CAMlow cells comprise ductal cells (C and D, arrows), whereas Ep-CAMhigh cells (C and D, arrowheads) correspond to developing islet cells, and clusters of undifferentiated epithelial cells. The white tracing in D represents the autofluorescence of cells incubated with a mouse IgG2a used as a control reference. The data are representative of nine independent immunostainings using five independent donors (18–20 wk of gestation). Triple immunofluorescent localization was also performed on sections of human adult pancreas to simultaneously identify Ep-CAM, insulin, and glucagon. In this set of experiments, the brightest Ep-CAM– specific immunofluorescence was recorded at the cell–cell boundaries of intercalar ductal cells (E, arrowheads), in interlobular ducts (J), and in main ducts (G). Islets of Langerhans, identified by the insulin- (H) and glucagon-specific fluorescence (F), exhibit a significantly less intense Ep-CAM–specific fluorescence (E). Combined fluorophore spectra (I). The data are representative of four independent experiments, using three independent adult donors (20–56 yr old). Bars: (B) 30 μm; (H and G) 50 μm; (J) 40 μm.
Expression Pattern of Ep-CAM in the Human Adult Pancreas
To investigate whether the Ep-CAM expression pattern observed in fetal pancreata is retained in the adult pancreas, we performed triple immunofluorescence experiments for the simultaneous identification of Ep-CAM, insulin, and glucagon in cryostat sections from adult human pancreas. In contrast to fetal pancreas, the highest levels of expression of Ep-CAM were not identified in endocrine cells, but rather in small intercalar ducts, interlobular, and main ducts. Indeed, as shown in Fig. 5, E and I, adult islets, identified by the insulin (Fig. 5 H) and glucagon staining (Fig. 5 F), displayed low to intermediate levels of Ep-CAM as compared to the bright fluorescent signal recorded at the cell–cell boundaries of intercalar ductal cells (Fig. 5, E and I, arrowheads), and in interlobular and main ducts (Fig. 5, G and J). Often localized intracellularly, exocrine tissue demonstrated weak staining for Ep-CAM.
Quantitative Analysis of Ep-CAM Expression in Fetal and Adult Human Pancreas
To quantitatively evaluate the levels of Ep-CAM expression in specific cell types, confocal images from human fetal and adult pancreata were studied by computer-aided morphometric analysis. Table I shows the pixel intensities of Ep-CAM–specific fluorescence recorded in ductal, endocrine, and epithelial undifferentiated cells of the fetal pancreas, as well as in ductal, islet, and acinar cells of the adult pancreas. Whereas fetal ductal cells displayed a mean pixel intensity of 73.2 ± 12.6, islet cells within the same sections showed a mean pixel intensity of 180.3 ± 21.5 (P < 0.001). Similarly, high levels of Ep-CAM expression (i.e., 197.9 ± 22.5) were measured in clusters of epithelial undifferentiated cells that lack expression of all islet hormones (insulin, glucagon, pancreatic polypeptide, and somatostatin), or exocrine markers such as amylase (data not shown). Conversely, this analysis performed on adult pancreas revealed mean pixel intensities of 179.4 ± 18.3 in ductal cells and 110.8 ± 11.2 in islet cells (P < 0.05). Cells of the exocrine compartment showed significantly lower pixel intensity (56.8 ± 7.1). Thus, ductal and islet cells demonstrate a reverse pattern of Ep-CAM expression in the adult pancreas compared to the fetal pancreas. These results suggest that mechanisms regulating high levels of Ep-CAM expression in fetal islets and undifferentiated cell clusters may remain constitutively operative in adult ductal cells, perhaps marking the ability of this cell compartment to act as a reservoir of islet cell progenitors in adult life (Dudek et al., 1991; Gu et al., 1993).
Table I.
Pixel Intensity of Ep-CAM–specific Immunofluorescence at Regions of Cell–Cell Contact
| Cell types | Fetal pancreas | Adult pancreas | ICCs | Grafts | ||||
|---|---|---|---|---|---|---|---|---|
| Ductal | 73.2 ± 12.6* | 179.4 ± 18.3‡ | 71.1 ± 11.7§ | 177.9 ± 21.3 ¶ | ||||
| Endocrine | 180.3 ± 21.5 | 110.8 ± 11.2 | 185.4 ± 13.5 | 104.8 ± 17.6 | ||||
| Epithelial undifferentiated | 197.9 ± 22.5 | – | 194.1 ± 17.2 | – | ||||
| Exocrine | – | 56.8 ± 7.1 | – | – |
The pixel intensity of Ep-CAM–specific fluorescence was recorded in 250 domains of cell–cell contact for each cell type. Numbers of fields scored in fetal pancreata = 50, adult pancreata = 72, ICCs = 54, grafts = 69. Number of donors = 3 (fetal pancreata at gestational age 18–20 wk). Number of donors of adult pancreata = 3 (age: 20, 32, and 56 yr).
The pixel intensity recorded in ductal cells is significantly different from values detected in endocrine cells and epithelial undifferentiated cells (P < 0.001).
Significantly different from values detected in endocrine cells (P < 0.05) and in exocrine cells (P < 0.001).
Significantly different from values detected in endocrine and epithelial undifferentiated cells (P < 0.001).
Values recorded in grafts' ductal cells differ significantly from those recorded in endocrine cells (P < 0.05).
High Levels of Ep-CAM Expression Characterize Proliferating Epithelial Cells In Situ
The high levels of Ep-CAM expressed by developing islet cell clusters in the fetal pancreas suggest that upregulation of Ep-CAM may be associated with cell proliferation. To test this hypothesis, fetal and adult human pancreata were stained for Ep-CAM, insulin, and the Ki-67 antigen, a nuclear marker of cycling cells (Gerdes et al., 1983). The presence of proliferating events among ductal, endocrine, and exocrine cells was evaluated both qualitatively (Fig. 6) and quantitatively by morphometric analysis of serial tissue sections (Table II). Fig. 6 A represents a reconstruction of four adjacent fields from a fetal pancreas showing a large duct (asterisks) and several adjacent cell clusters. In no instances were Ki-67-positive nuclei (Fig. 6 A, blue) identified among ductal cells weakly stained for Ep-CAM. Conversely, proliferating ductal cells consistently exhibited strong staining for Ep-CAM (Fig. 6 A, arrowheads). Among cells adjacent to the ducts, numerous Ki-67–positive Ep-CAMhigh cells could also be observed. Nevertheless, many Ep-CAMhigh cells appeared to be not cycling. Within cell clusters budding from the ductal epithelium, several insulin-producing cells (Fig. 6, arrows) also positive for the Ki-67 antigen were observed. This latter observation suggests that newly differentiated β cells may contribute to the expansion of the islet cell mass. The fact that developing islet cells exhibited high levels of Ep-CAM is consistent with an immature phenotype, as supported by their inability to secrete hormones in response to secretagogues.
Figure 6.
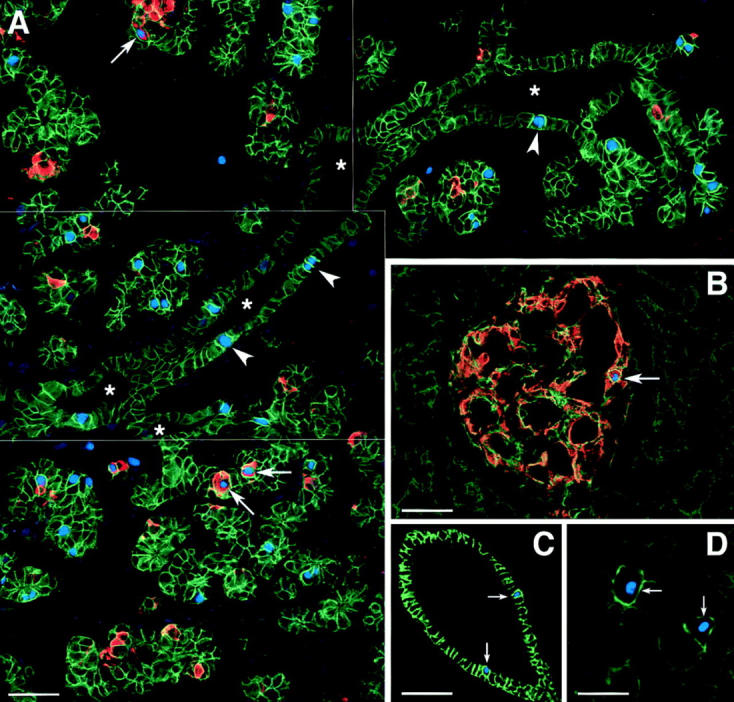
High levels of Ep-CAM expression mark proliferating pancreatic epithelial cells in situ. A shows a reconstruction of four adjacent microscopic fields from a fetal pancreas (18 wk), immunostained for Ep-CAM (green), insulin (red), and Ki-67 (blue). Numerous proliferating cells identified by the nuclear staining for Ki-67 can be observed in cell clusters budding from pancreatic ducts (asterisks), and within the ductal epithelium (arrowheads). Notably, all Ki-67–positive cells exhibited high levels of Ep-CAM–specific immunoreactivity, including those identified within the monolayered ductal epithelium that normally exhibit low levels of Ep-CAM. Many cycling insulin-positive cells were also observed (arrows). Similar analysis performed on sections of human adult pancreas (B–D) also revealed that expression of Ki-67 is always associated with high levels of Ep-CAM. Although rare cycling β cells were observed (B, arrow; refer to Table II), most Ki-67–positive cells were identified in ducts (C, arrows) and in the acinar tissue (D, arrows). Note that even within the exocrine compartment, which overall expresses low levels of Ep-CAM, cycling cells identified by the Ki-67 staining always showed brighter Ep-CAM–specific fluorescence compared to the surrounding noncycling acinar cells. The data are representative of five independent experiments, using three independent donors of both fetal (18–20 wk of gestation) and adult pancreata (20–56 yr old). Bars: (A) 50 μm; (B and C) 80 μm; (D) 40 μm.
Table II.
Distribution of Ki-67–positive Cells in the Human Pancreas
| Cell types | Ki-67–positive cells | Proliferation index | ||
|---|---|---|---|---|
| Fetal pancreas | ||||
| Ductal | 11.70 [122]* | 0.48‡ | ||
| Endocrine | 14.87 [155] | 0.69 | ||
| Epithelial undifferentiated | 52.01 [542] | 1.50 | ||
| Adult pancreas | ||||
| Ductal | 39.12 [367]§ | 2.79 ¶ | ||
| Endocrine | 0.95 [9] | 0.47 | ||
| Acinar | 44.80 [421] | 0.60 |
Expressed as percentage of total number of cells counted. [number of cells counted].
Calculated by correcting the percentage of Ki-67–positive cells for the relative proportion of pancreatic cell types (i.e., ductal cells = 24%, endocrine cells = 21.5%, epithelial undifferentiated = 34.5%, stroma = 20%) (Cirulli, V., unpublished data). Number of fields scored = 96. Total number of cells counted = 819. Number of donors = 3 (fetal pancreata at gestational age 18–20 wk).
Expressed as percentage of total number of cells counted. [Number of cells counted].
Calculated by correcting the percentage of Ki-67–positive cells for the relative proportion of the different pancreatic cell types (i.e., ductal cells = 14%, endocrine cells = 2%, epithelial undifferentiated = 74%, stroma = 10%) (for review see Githens, 1988). Number of fields scored = 202. Total number of cells counted = 797. Number of donors = 3 (age: 20, 32, and 56 yr).
Similar analysis performed on cryostat sections from human adult pancreata revealed fewer cycling events (Fig. 6, B–D). Fig. 6 B shows an islet of Langerhans identified by the insulin-specific staining (red). Relatively higher levels of Ep-CAM expression (Fig. 6, green) were detected in islet cells as compared to the surrounding acinar tissue. In this field, a cycling insulin-positive cell can be identified by the expression of Ki-67 (Fig. 6, blue) in its nucleus (Fig. 6, arrow). Fig. 6 C shows Ki-67–positive nuclei within the ductal epithelium (arrows), a cell compartment that expresses the highest levels of Ep-CAM in the adult pancreas. Interestingly, even within the exocrine compartment, which overall expresses low levels of Ep-CAM, cycling cells identified by the Ki-67 staining always showed brighter Ep-CAM–specific fluorescence, as compared to the surrounding noncycling acinar cells (Fig. 6 D, arrows). Table II summarizes the frequency of Ki-67–positive nuclei in the different cell types of both the fetal and adult human pancreas. To take into account the different representation of the various pancreatic cell types, the percentage of Ki-67–positive cells was corrected for the relative cell mass of the ductal, endocrine, and exocrine pancreatic compartments. This allowed the calculation of a “proliferation index” that reflected the actual frequency of proliferating cells in each compartment. This analysis demonstrates that the cells bearing high levels of Ep-CAM expression (i.e., undifferentiated epithelium in the fetal pancreas and ductal cells in the adult pancreas) exhibit a higher proliferation index (Table II), consistent with the hypothesis that upregulation of Ep-CAM is linked to the high proliferative potential of those cell compartments.
Induction of Epithelial Cell Growth Is Associated with the Upregulation of Ep-CAM Expression
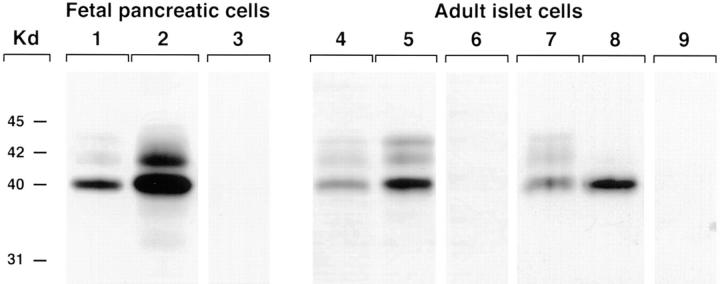
To determine whether upregulation of Ep-CAM is associated with the growth of pancreatic epithelial cells in vitro, we next assessed the expression levels of this antigen by protein immunoblotting. We compared free-floating ICCs, comprising only a limited number of cycling cells (Otonkoski et al., 1993), to cell monolayers generated by culturing fetal pancreatic cells on the 804G extracellular matrix supplemented with rhHGF/SF. This culture condition greatly enhances the proliferation of both fetal (Beattie et al., 1996) and adult human islet cells (Hayek et al., 1995). As shown in Fig. 7, lane 1, a major band with an apparent molecular mass of 40 kD could be identified by the KS1/4 mAb in cell extracts from fetal ICCs cultured as floating clusters. Notably, when cells were plated on the 804G extracellular matrix to promote epithelial cell growth, a remarkable upregulation of Ep-CAM expression was observed (Fig. 7, lane 2). In this latter condition, an additional 42-kD band was also detected. The 40- and 42-kD proteins recognized by the KS1/4 mAb were previously characterized in UCLA-P3 cells as differently glycosylated isoforms of the same antigen (Perez and Walker, 1989). Induction of cell growth in adult islets cultured as monolayers was also associated with a significant upregulation of Ep-CAM (Fig. 7, lane 5) as compared to the levels detected in floating islet cultures (Fig. 7, lane 4). Interestingly, lysates from a human islet β cell tumor (insulinoma) exhibited higher levels of Ep-CAM expression than normal islets (Fig. 7, lanes 8 and 7, respectively). These results indicate that islet cell growth induced by mitogenic stimula in vitro or tumoral transformation in vivo is accompanied by increased expression of Ep-CAM.
Figure 7.
Ep-CAM expression is upregulated by growth stimuli. Western blot analysis of cell extracts from fetal ICCs (lane 1) revealed a band with an apparent molecular mass of 40 kD using the KS1/4 mAb. When cells were plated on the 804G extracellular matrix to promote epithelial cell growth, a remarkable upregulation of Ep-CAM expression was observed (lane 2). In this condition, an additional band of 42 kD was detected. When purified adult islets were cultured either in the absence of growth stimuli (lane 4), or as monolayers on the 804G matrix supplemented with rhHGF/SF (lane 5), a significant upregulation of Ep-CAM expression was observed. Lysates from human insulinomas also showed higher levels of Ep-CAM (lane 8) compared to normal islets (lane 7). Lanes 3, 6, and 9 were loaded with cell lysates from fetal cell monolayers, adult islet monolayers, and human insulinoma, respectively, and then probed with a control mouse IgG2a. The data are representative of seven independent experiments using fetal and adult cells (seven and five independent donors, respectively), and of three independent experiments using human insulinomas (from two independent donors).
Developmentally Regulated Expression of Ep-CAM during Pancreatic Islet Maturation
To follow the expression of Ep-CAM during development of islet clusters, we took advantage of an established in vivo model of endocrine differentiation of HFP cells. In this model, undifferentiated fetal pancreatic ICCs transplanted under the kidney capsule of immune-deficient nude mice develop a significant endocrine cell mass after a 12-wk period (Beattie et al., 1995, 1996; Hayek et al., 1996). Therefore, in the next series of experiments we transplanted four nude mice with 500 human ICCs each. After 12 wk, the mice were tested for the presence of human insulin in the peripheral blood using a human C peptide– specific radioimmunoassay. We detected 81 ± 26 pmol/liter of human C peptide in basal conditions and 424.5 ± 27.8 pmol/liter after intraperitoneal injection of glucose, indicating that the transplanted mice harbored functionally mature human islet β cells. Hematoxylin/eosin staining of the grafts showed numerous large islet-like epithelial clusters (Fig. 8 B, arrowheads) and ductal structures (Fig. 8 B, asterisks and inset). Consistent with our previous reports, such islet cell clusters were immersed in a well-vascularized stromal tissue of mouse origin (Beattie et al., 1994, 1997). None of the grafts developed exocrine tissue, as detected by morphological analysis and immunostaining for amylase (data not shown). This last observation is in agreement with a recent report by Gittes and colleagues, showing that transplantation of enriched epithelial cell preparations from the mouse fetal pancreas develops into islet cells and ductal elements without exocrine tissue (Gittes et al., 1997).
Figure 8.
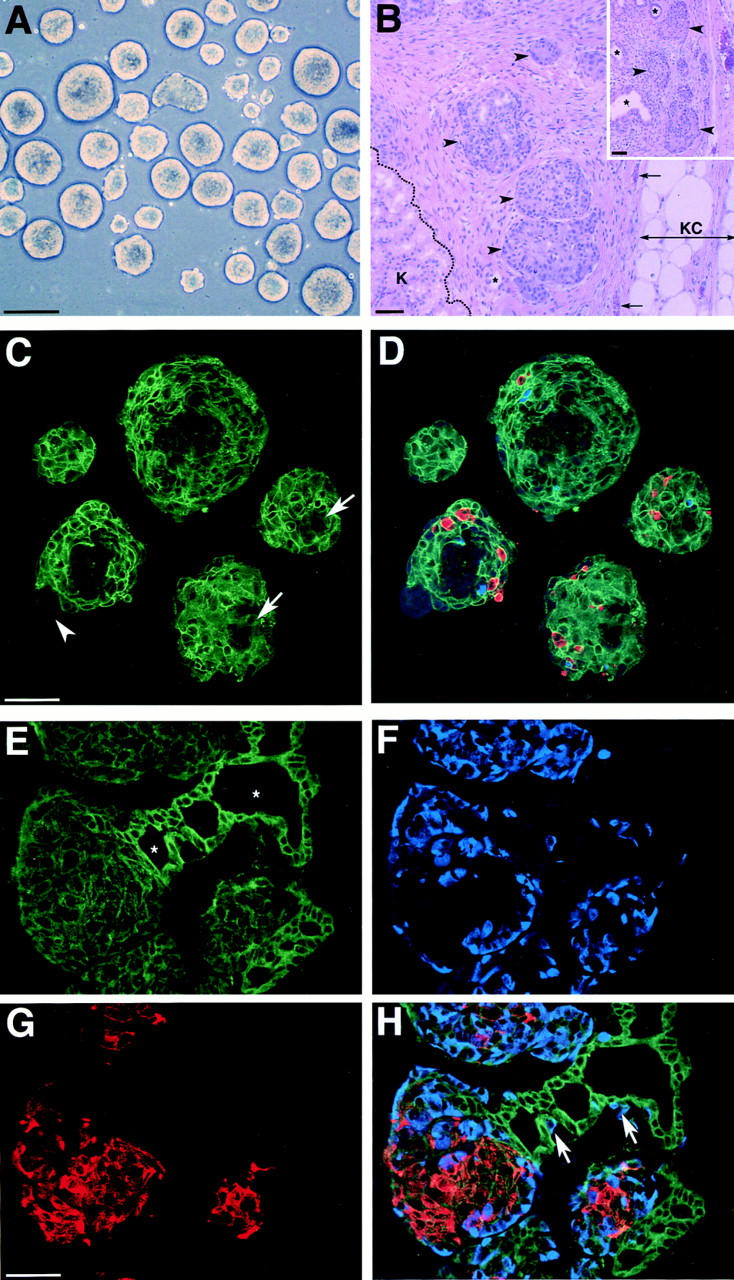
Developmentally regulated expression of Ep-CAM during pancreatic islet maturation. Fetal pancreatic cells cultured as floating ICCs (A) were transplanted under the kidney capsule of nude mice, and grafts were analyzed after 12 wk for the presence of islet tissue (B). Hematossilin/ eosin staining of graft sections revealed the presence of numerous ICCs (arrowheads) and some ductal elements (asterisks) immersed in a well-vascularized stroma (arrows indicate blood vessels). K, mouse kidney; KC, kidney capsule. Three-color immunofluorescence for the simultaneous identification of Ep-CAM (green), insulin (red), and glucagon (blue), was performed in sections from ICCs. C shows the Ep-CAM–specific immunofluorescence that highlights cell–cell boundaries of epithelial cells. Few ductal structures can be identified within these cell clusters (arrows), displaying a dimmer Ep-CAM–specific signal, as in fetal pancreas in situ. A small fraction of mesenchymal cells (<10%) lacking Ep-CAM expression can be identified in some ICCs (arrowheads). Merging of the three fluorophore spectra (D), specific for Ep-CAM (green), insulin (red), and glucagon (blue), shows that only a small fraction of the cells forming ICCs express islet hormones (5–10%), whereas most of the other epithelial cells are undifferentiated. Immunostaining of sections from the grafts after 12 wk in vivo shows that high levels of Ep-CAM (E) are found in ductal elements (asterisks), whereas endocrine cells (glucagon, F; insulin, G) exhibit lower levels, as in the adult pancreas in situ. H shows the merging of the three fluorophore spectra specific for Ep-CAM (green), insulin (red), and glucagon (blue). Bars: (A) 100 μm; (B, C, and G) 50 μm.
We next compared the expression of Ep-CAM in the grafts and in samples of ICCs preserved for histological analysis from the very same pool used to transplant the nude mice (Fig. 8 A). For this purpose, sections from the ICC preparations and grafts were stained by three-color immunofluorescence for Ep-CAM (green), insulin (red), and glucagon (blue). The ICC preparations (Fig. 8, C and D) consisted mostly of epithelial cells strongly stained for Ep-CAM (pixel intensities were 194.1 ± 17.2 in epithelial undifferentiated cells and 185.4 ± 13.5 in endocrine cells, respectively; refer to Table I). Few ductal structures (Fig. 8 C, arrows) exhibiting low levels of Ep-CAM–specific staining (pixel intensity of 71.1 ± 11.7; refer to Table I), and discrete regions occupied by mesenchymal cells lacking Ep-CAM expression (Fig. 8 C, arrowhead) were also observed. Merging of the three fluorophore spectra in Fig. 8 D showed that only a minor fraction of the epithelial cells express insulin and/or glucagon, consistent with the fact that ICCs comprise mostly undifferentiated epithelial cells (Beattie et al., 1997). In contrast, the analysis of the grafts revealed the presence of large clusters of endocrine cells resembling adult islets of Langerhans (Fig. 8, E–H), with insulin-producing cells located in the center and most glucagon cells arranged at the periphery. In addition, well-formed ducts containing numerous glucagon-producing cells were observed (Fig. 8 H, arrows), suggesting an ongoing and/or residual emergence of endocrine cells from the ductal epithelium. Pancreatic polypeptide- and somatostatin-positive cells were also found in the grafts (data not shown). Interestingly, the expression pattern of Ep-CAM in these grafts is reminiscent of that observed in the intact adult human pancreas, with high levels in the ductal epithelium and relatively lower levels in islet cells (Fig. 8 E; pixel intensity of 104.8 ± 17.6 in islet cells versus 177.9 ± 21.3 in ductal cells, respectively; refer to Table I). These results indicate that differentiation of fetal cells into functional mature endocrine cells is associated with downregulation of Ep-CAM.
Inhibition of Ep-CAM–mediated Cell–Cell Interactions Causes Endocrine Differentiation of HFP Cells
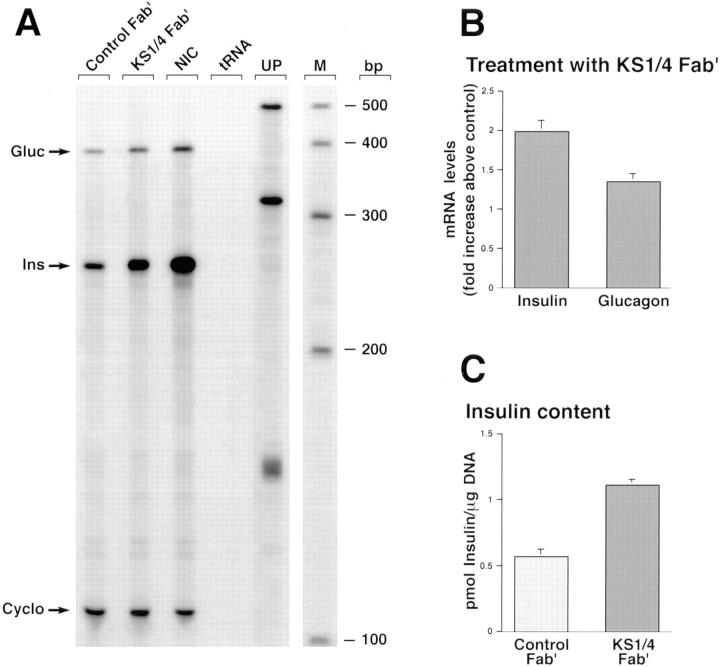
The expression pattern of Ep-CAM in the developing human pancreas suggests that a dynamic modulation of this CAM may be involved in the cell's decision to either grow or differentiate. To assess whether the blockade of Ep-CAM–mediated cell–cell interactions may mimic a state of functional downregulation of Ep-CAM usage/expression and promote endocrine differentiation, HFP cells were cultured as floating ICCs for 5 d in the presence of either Fab′ fragments of KS1/4 mAb or control Fab′ generated from isotype-matched mouse IgGs. At the end of the culture period, control and KS1/4-treated cells were harvested for total RNA isolation and DNA and insulin content determination. Because human HFP express a number of other adhesion molecules, including N-CAM and E-cadherin (Cirulli et al., 1995), we predicted that treatment with KS1/4 Fab′, although exerting a potent inhibitory effect on short-term reaggregation experiments (refer to Fig. 4), should have not affected their ability to reaggregate in long-term cultures into ICCs. In fact, after 5 d of culture both control and KS1/4-treated samples yielded macroscopically similar ICCs (data not shown). As shown in Fig. 9 A, analysis of insulin and glucagon transcripts revealed that treatment with anti–Ep-CAM KS1/4 Fab′ causes a significant increase of both insulin and glucagon gene transcription. Nicotinamide-treated ICCs (Fig. 9, lane NIC) were used in this assay as an internal positive control for induction of endocrine differentiation. In fact, we have previously shown that nicotinamide increases glucagon and insulin gene transcription, as well as the overall number of glucagon- and glucose-responsive insulin-producing cells (Otonkoski et al., 1993). Quantitation of band intensities performed by scanning densitometry showed that the blockade of Ep-CAM (Fig. 9, lane KS1/4 Fab′) produced a 1.9- and 1.4-fold increase, respectively, of insulin and glucagon mRNAs above the levels measured in control ICCs (Fig. 9 B). These findings were further validated by the demonstration of increased insulin protein content in ICCs treated with anti–Ep-CAM KS1/4 Fab′ (Fig. 9 C).
Figure 9.
Blockade of Ep-CAM–mediated cell–cell interactions causes endocrine differentiation of HFP cells. HFP cells cultured as floating ICCs for 5 d in the presence of either Fab′ fragments of KS1/4 mAb or control Fab′ were used for determination of insulin and glucagon transcript levels in a multiprobe RNase protection assay. A shows that treatment with anti–Ep-CAM KS1/4 Fab′ causes a significant increase of both insulin and glucagon gene transcription. Nicotinamide-treated ICCs (lane NIC) were used in the assay as an internal positive control for induction of endocrine differentiation. Yeast tRNA (lane tRNA) was included as a negative control. Lane UP, undigested probes; M, RNA sizing ladder. Quantitation of band intensities performed by scanning densitometry (B) shows that blockade of Ep-CAM produces a 1.9- and 1.4-fold increase, respectively, of insulin and glucagon mRNAs above the levels measured in control ICCs. Note that the insulin protein content is also increased in ICCs treated with anti–Ep-CAM KS1/4 Fab′ (C). A is representative of four independent assays. Data in B and C are expressed as mean ± SEM of four independent experiments. P values for significant differences were: P < 0.002 for both insulin and glucagon transcript levels compared to control in B; P < 0.005 for samples treated with KS1/4 Fab′ compared to control in C.
These results strongly suggest that disengagement of Ep-CAM–mediated cell–cell interactions, by either downregulation of the receptor from the cell surface and/or functional inactivation, may be a signaling event involved in the differentiation of pancreatic islet cells.
Discussion
Adhesion molecules mediating cell–cell interactions play crucial morphoregulatory roles in specifying cell fate during development (Trelstad, 1984; Ekblom et al., 1986; Edelman and Crossin, 1991; Edelman, 1992; Takeichi, 1991, 1995). Here we demonstrate that the pancarcinoma antigen KSA (Varki et al., 1984; Edwards et al., 1986; Spurr et al., 1986; Bumol et al., 1988), alias Ep-CAM (Litvinov et al., 1994a ), is expressed in the human fetal pancreas at sites of epithelial cell–cell contacts and that the antigen mediates a calcium-independent mechanism of cell aggregation. The data also show that expression of Ep-CAM is developmentally regulated during pancreatic islet ontogeny. Thus, high levels of Ep-CAM expression were detected in fetal pancreatic islet cells in situ and after induction of cell growth in vitro. In contrast, endocrine differentiation of immature fetal pancreatic epithelial cells in an in vivo transplantation model was associated with a significant downregulation of Ep-CAM expression. Moreover, blockade of Ep-CAM function in vitro caused endocrine differentiation of fetal pancreatic cells. Taken together, these observations suggest that Ep-CAM exhibits the features of a morphoregulatory molecule.
Our data provide evidence that Ep-CAM upregulation and function is associated with the outgrowth of early endocrine cells from the pancreatic ductal epithelium. We show that Ep-CAM expression is restricted to the epithelial compartment of the fetal pancreas in a pattern reminiscent of the distribution of classical cell–cell adhesion molecules. Furthermore, immunoelectron microscopic analysis of Ep-CAM showed that the predominant subcellular localization of this glycoprotein is targeted to domains of cell–cell contact, suggesting its involvement in cell aggregation. Indeed, our short-term reaggregation experiments clearly demonstrate that Ep-CAM is involved in pancreatic epithelial cell–cell aggregation. Hence, using two different Ep-CAM–specific mAbs, KS1/4 and 323A3, both recognizing extracellular sequences of the molecule, a significant inhibitory effect of pancreatic epithelial cell reaggregation was observed. These functional data provide the first evidence that Ep-CAM mediates cell–cell aggregation of primary (nontumoral) epithelial cells. Based on some sequence homology that Ep-CAM shares with the extracellular matrix protein nidogen, it was previously suggested that this epithelial marker could participate in either intercellular or cell–matrix adhesion of epithelial cells (Thampoe et al., 1988; Mann et al., 1989; Simon et al., 1990). Although a direct involvement of Ep-CAM in cell– cell interactions is supported by our morphological studies and demonstrated by our antibody perturbation experiments, the possibility of Ep-CAM involvement in cell– matrix interactions is unlikely for the following reasons. First, Ep-CAM–specific immunoreactivity was never detected at the basal pole of pancreatic epithelial cells, as shown by our electron microscopic analysis, and secondly, we found that both KS1/4 and 323A3 mAbs are unable to inhibit pancreatic cell adhesion to the 804G matrix (data not shown).
We provide evidence that upregulation of Ep-CAM is linked to cell proliferative events. Thus, cycling cells, identified in situ by the expression of the Ki-67 antigen, consistently displayed the highest levels of Ep-CAM expression. In the fetal pancreas, the highest frequency of proliferating cells was found among Ep-CAMhigh ICCs budding from the ducts. Conversely, in the adult pancreas, the ductal compartment strongly stained for Ep-CAM exhibited the highest proliferation index. Interestingly, in no instances were Ep-CAMlow cells found to proliferate. In fact, we observed that cell compartments such as the fetal ductal epithelium and the adult acinar tissue that overall exhibited low levels of Ep-CAM comprised scattered proliferating cells that expressed increased levels of Ep-CAM. Thus, our studies establish a direct correlation between frequency of proliferating cells and high expression of Ep-CAM in each cell compartment. However, we found that not all Ep-CAMhigh cells within fetal islet cell clusters and adult ducts are actually cycling. This latter observation suggests that upregulation of Ep-CAM may not necessarily coincide with cell division, but rather marks the capability of epithelial cells to respond to an array of mitogenic/developmental signals.
In line with this hypothesis is the observation that remarkable upregulation of Ep-CAM occurs not only in fetal epithelia but also in rapidly growing carcinomas (Varki et al., 1984). Thus, it is possible that cell interactions mediated by this glycoprotein, although ensuring cell–cell association, may be required for the transduction of specific signals necessary for the development of various epithelial organs and for the growth of epithelial tumors. Hence, the association of Ep-CAM upregulation with cell growth in tumors of epithelial origin (Edwards et al., 1986; Moldenhauer et al., 1987; Litvinov et al., 1994a ,b; Velders et al., 1994), and its de novo expression during tumoral transformation in tissues that usually lack this molecule have been extensively documented (Quak et al., 1990; Tsubura et al., 1992; Tellechea et al., 1993). Tumoral transformation of epithelial tissues has been also associated with a decreased expression/function of E-cadherin, a CAM required for the development and maintenance of cellular differentiation (Marrs and Nelson, 1996), and regarded as a tumor suppressor gene (Takeichi, 1993). Interestingly, a recent report by Litvinov and colleagues shows that Ep-CAM can decrease cadherin-mediated cell–cell association, thereby providing a possible mechanism by which Ep-CAM may promote the development of a proliferative phenotype in epithelial cells (Litvinov et al., 1997).
Based on the evidence that pancreatic endocrine cells derive from progenitors resident in the ductal epithelium (Pictet and Rutter, 1972; Pictet et al., 1972; Teitelman and Lee, 1987; Alpert et al., 1988; Gu and Sarvetnick, 1993), our data suggests that upregulation of Ep-CAM occurs at an early developmental stage of islet cell ontogeny. In support of this hypothesis we show that Ep-CAM is upregulated in pancreatic epithelial and islet cells induced to proliferate in vitro, and in spontaneous pancreatic β cell tumors in vivo. We propose that the biological significance of Ep-CAM upregulation in vivo is to transduce specific morphogenetic signals during the emigration and expansion of early endocrine precursors and newly differentiated endocrine cells into the surrounding mesenchyme.
The expression pattern of Ep-CAM that we describe in the fetal and adult pancreas, as well as in our in vivo model of islet cell development from undifferentiated fetal epithelial cells, provides an interesting example of the developmentally regulated expression of this antigen. Thus, whereas in the fetal pancreas the highest levels of Ep-CAM are detected in islet cell clusters growing out of the ductal tree and in clusters of undifferentiated epithelial cells, in adult life the ductal epithelium, rather than islet cells, displays the strongest Ep-CAM–specific immunoreactivity. These observations suggest that human adult pancreatic ducts may retain a higher responsiveness to growth stimuli, a characteristic supported by our data on the relatively higher frequency of proliferative events in this cell compartment. This possibility is also consistent with the current belief that most human adult pancreatic tumors are of ductal origin.
The in vivo and in vitro experimental models of endocrine differentiation described here provide additional evidence for Ep-CAM playing important morphoregulatory roles in pancreatic islet development. Thus, a significant downregulation of Ep-CAM was observed when undifferentiated fetal pancreatic epithelial cells were transplanted and allowed to differentiate in vivo. Hence, upon endocrine differentiation, islet cell clusters developed within the grafts and expressed levels of Ep-CAM similar to those detected in situ in the adult islets. Likewise, when the adhesive function of Ep-CAM was inhibited by specific KS1/4 Fab′ fragments, fetal pancreatic cells underwent endocrine differentiation, demonstrated by the upregulation of insulin and glucagon transcripts, as well as by the increased insulin protein content. These data strongly suggest that manipulations designed to inhibit or reduce Ep-CAM–mediated interactions favor endocrine differentiation. Conversely, upregulation of Ep-CAM negatively regulates endocrine differentiation. In line with this view, the low levels of Ep-CAM expressed in human adult islets may be ineffective in inhibiting hormone gene expression, and only a substantial upregulation of this CAM above threshold levels can endow epithelial cells with a higher responsiveness to growth stimuli or directly regulate cell multiplication. This interpretation is consistent with the most recent view of CAM's function, which encompasses not only cell–cell aggregation within tissues, but also regulation of cell multiplication (Edelman and Crossin, 1991; Sporn et al., 1995), cell differentiation (Kemler et al., 1977; Edelman and Crossin, 1991; Takeichi, 1995), migration and three-dimensional organization within tissues during morphogenesis (Crossin et al., 1985; Friedlander et al., 1989; Edelman and Crossin, 1991; Takeichi, 1991, 1995; Larue et al., 1996), as well as tissue remodeling during wound healing (Watt and Jones, 1993; Krushel et al., 1995), and tumoral transformation in adult organisms (Parish et al., 1987; Hynes, 1989). Thus, CAMs should be regarded as transducers of diverse morphoregulatory messages within cell collectives.
Interestingly, despite its functional involvement in cell adhesion, Ep-CAM does not share significant homology with any of the well-characterized families of adhesion molecules such as CAMs of the immunoglobulin superfamily, cadherins, integrins, and selectins. However, the presence of two EGF-like domains comprised between a cysteine-rich and cysteine-poor domain in the extracellular sequence of Ep-CAM confers some structural homology with members of the Drosophila Notch and the Caenorhabditis elegans Lin family of proteins. Notably, both Notch and Lin gene products are involved in cell–cell signaling pathways controlling cell fate decisions during development (Artavanis-Tsakonas et al., 1995; Katz et al., 1995). With respect to the common structural folding of EGF-like domains shared by many proteins involved in pleiotropic and developmental effects, Ep-CAM also shares some homology with tissue plasminogen activator, regulating proteolysis of extracellular matrix during cell growth and migration (Blasi et al., 1987; Appella et al., 1987). Thus we propose that Ep-CAM belongs to a novel family of epithelial-specific cell surface receptors mediating adhesion and delivering growth/developmental signals. Our findings provide strong evidence for a morphoregulatory role of Ep-CAM in pancreatic islet development.
Acknowledgments
We are grateful to D.R. Colman (Brookdale Center for Molecular Biology, The Mount Sinai School of Medicine, New York) for critical reading of the manuscript. We would like to thank V. Quaranta and D.R. Salomon (The Scripps Research Institute, La Jolla, CA) for helpful discussions, G. Chamness (Health Science Center, University of Texas, San Antonio, TX) for providing us with the 323A3 mAb, and J.S. Rubin (Laboratory of Cellular and Molecular Biology, National Institutes of Health [NIH], National Cancer Institute, Bethesda, MD) for the hrHGF/SF. We are also grateful to G.F. Billman (The Children's Hospital, San Diego, CA) and U. Di Mario (The University of Rome “La Sapienza”, Rome, Italy) for providing samples of human insulinomas.
This work was supported by an NIH grant (grant number DK-39087), and the Herbert and Perry Fund to A. Hayek. The National Center for Microscopy and Imaging Research is supported by an NIH grant (grant number RR-04050) to M.H. Ellisman. L. Inverardi, C. Ricordi, M.I. Mally, and A. Ptasznik were supported by research grants from the Juvenile Diabetes Foundation International (JDFI). V. Cirulli was supported by a Pilot and Feasibility grant from The Whittier Institute Diabetes Program and by a Career Development Award from the JDFI. L. Crisa is the recipient of a Career Development Award from the JDFI.
Abbreviations used in this paper
- CAM
cell adhesion molecule
- DS
donkey serum
- Ep-CAM
epithelial cell adhesion molecule
- HFP
human fetal pancreas
- ICC
islet-like cell cluster
- N-CAM
neuronal cell adhesion molecule
- rhGHF/SF
recombinant human hepatocyte growth factor/scatter factor
Footnotes
Address all correspondence to Vincenzo Cirulli, The Islet Research Laboratories at the Whittier Institute for Diabetes, Department of Pediatrics, University of California at San Diego, 9894 Genesee Avenue, La Jolla, CA 92037. Tel.: (619) 622-8423. Fax: (619) 558-3495. E-mail: vincenzo@alex.ucsd.edu
References
- Alpert S, Hanahan D, Tietelman G. Hybrid insulin genes reveal a developmental lineage for pancreatic endocrine cell and imply a relationship with neurons. Cell. 1988;53:295–308. doi: 10.1016/0092-8674(88)90391-1. [DOI] [PubMed] [Google Scholar]
- Appella E, Robinson EA, Ullrich SJ, Stoppelli MP, Corti A, Cassani G, Blasi F. The receptor-binding sequence of urokinase. A biological function for the growth-factor module of proteases. J Biol Chem. 1987;262:4437–4440. [PubMed] [Google Scholar]
- Artavanis-Tsakonas S, Matsuno K, Fortini M. Notch signaling. Science. 1995;268:225–232. doi: 10.1126/science.7716513. [DOI] [PubMed] [Google Scholar]
- Bauer GE, Balsamo J, Lilien J. Cadherin-mediated adhesion in pancreatic islet cells is modulated by a cell surface N-acetylgalactosaminylphosphotransferase. J Cell Sci. 1992;103:1235–1241. doi: 10.1242/jcs.103.4.1235. [DOI] [PubMed] [Google Scholar]
- Beattie GM, Levine F, Mally MI, Otonkoski T, O'Brien JS, Salomon DR, Hayek A. Acid β-galactosidase: a developmentally regulated marker of endocrine cell precursors in the human fetal pancreas. J Clin Endocrinol Metab. 1994;78:1232–1240. doi: 10.1210/jcem.78.5.8175983. [DOI] [PubMed] [Google Scholar]
- Beattie GM, Rubin JS, Mally MI, Otonkoski T, Hayek A. Regulation of proliferation and differentiation of human fetal pancreatic islet cells by extracellular matrix, hepatocyte growth factor and cell–cell contact. Diabetes. 1996;45:1223–1228. doi: 10.2337/diab.45.9.1223. [DOI] [PubMed] [Google Scholar]
- Beattie GM, Otonkoski T, Lopez AD, Hayek A. Functional β-cell mass after transplantation of human fetal pancreatic cells. Differentiation or proliferation? Diabetes. 1997;46:244–248. doi: 10.2337/diab.46.2.244. [DOI] [PubMed] [Google Scholar]
- Begemann M, Tan SS, Cunningham BA, Edelman GM. Expression of chicken liver cell adhesion molecule fusion genes in transgenic mice. Proc Natl Acad Sci USA. 1990;87:9042–9046. doi: 10.1073/pnas.87.22.9042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blasi F, Vassalli J-D, Dano J. Urokinase-type plasminogen activator: proenzyme, receptor, and inhibitors. J Cell Biol. 1987;104:801–804. doi: 10.1083/jcb.104.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumol TF, Marder P, De Herdt SJ, Borowitz MJ, Apelgren LD. Characterization of the human tumor and normal tissue reactivity of the KS1/4 monoclonal antibody. Hybridoma. 1988;7:407–415. doi: 10.1089/hyb.1988.7.407. [DOI] [PubMed] [Google Scholar]
- Carrere J, Figarella-Branger D, Senegas-Balas F, Figarella C, Guy-Crotte O. Immunohistochemical study of secretory proteins in the developing human pancreas. Differentiation. 1992;51:55–60. doi: 10.1111/j.1432-0436.1992.tb00680.x. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cirulli V, Halban PA, Rouiller DG. TNF-α modifies adhesion properties of rat islet B-cells. J Clin Invest. 1993;91:1868–1876. doi: 10.1172/JCI116403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirulli V, Ricordi C, Hayek A. E-cadherin, NCAM, and Ep-CAM expression in human fetal pancreata. Transpl Proc. 1995;27:3335. [PubMed] [Google Scholar]
- Cirulli V, Baetens D, Rutishauser U, Halban PA, Orci L, Rouiller DG. Expression of neural cell adhesion molecule (NCAM) in rat islets and its role in islet cell type segregation. J Cell Sci. 1994;107:1429–1436. doi: 10.1242/jcs.107.6.1429. [DOI] [PubMed] [Google Scholar]
- Clark A, Grant AM. Quantitative morphology of endocrine cells in human fetal pancreas. Diabetologia. 1983;25:31–35. doi: 10.1007/BF00251893. [DOI] [PubMed] [Google Scholar]
- Crossin KL, Chuong CM, Edelman GM. Expression sequences of cell adhesion molecules. Proc Natl Acad Sci USA. 1985;82:6942–6946. doi: 10.1073/pnas.82.20.6942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl U, Sjödin A, Semb H. Cadherins regulate aggregation of pancreatic β-cells in vivo. . Development (Camb) 1996;122:2895–2902. doi: 10.1242/dev.122.9.2895. [DOI] [PubMed] [Google Scholar]
- Dudek RW, Lawrence IE, Hill RS, Johnson RC. Induction of islet cytodifferentiation by fetal mesenchyme in adult pancreatic ductal epithelium. Diabetes. 1991;40:1041–1048. doi: 10.2337/diab.40.8.1041. [DOI] [PubMed] [Google Scholar]
- Edelman GM. Morphoregulation. Dev Dyn. 1992;193:2–10. doi: 10.1002/aja.1001930103. [DOI] [PubMed] [Google Scholar]
- Edelman GM, Crossin K. Cell adhesion molecules: implication for a molecular histology. Annu Rev Biochem. 1991;60:155–190. doi: 10.1146/annurev.bi.60.070191.001103. [DOI] [PubMed] [Google Scholar]
- Edelman GM, Gallin WJ, Delouvée A, Cunningham BA, Thiery J-P. Early epochal maps of two different cell adhesion molecules. Proc Natl Acad Sci USA. 1983;80:4384–4388. doi: 10.1073/pnas.80.14.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DP, Grzyb KT, Dressler LG, Mansel RE, Zava DT, Sledge GW, McGuire WL. Monoclonal antibody identification and characterization of a Mr 43,000 membrane glycoprotein associated with human breast cancer. Cancer Res. 1986;46:1306–1317. [PubMed] [Google Scholar]
- Ekblom P, Vestweber D, Kemler R. Cell-matrix interactions and cell adhesion during development. Annu Rev Cell Biol. 1986;2:27–47. doi: 10.1146/annurev.cb.02.110186.000331. [DOI] [PubMed] [Google Scholar]
- Fannon AM, Sherman DL, Ilyina-Gragerova G, Brophy PJ, Friedrich VL, Jr, Colman DR. Novel E-cadherin-mediated adhesion in peripheral nerve: schwann cell architecture is stabilized by autotypic adherens junctions. J Cell Biol. 1995;129:189–202. doi: 10.1083/jcb.129.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander DR, Mege RM, Cuningham BA, Edelman GM. Cell sorting-out is modulated by both the specificity and amount of different cell adhesion molecules (CAMs) expressed on cell surface. Proc Natl Acad Sci USA. 1989;86:7043–7047. doi: 10.1073/pnas.86.18.7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukayama M, Ogawa M, Hayashi Y, Koike M. Development of human pancreas. Differentiation. 1986;31:127–133. doi: 10.1111/j.1432-0436.1986.tb00393.x. [DOI] [PubMed] [Google Scholar]
- Gerdes J, Schwab U, Lemke H, Stein H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer. 1983;31:13–20. doi: 10.1002/ijc.2910310104. [DOI] [PubMed] [Google Scholar]
- Githens S. The pancreatic duct cell: proliferative capabilities, specific characteristics, metaplasia, isolation, and culture. J Pediatr Gastroenterol Nutr. 1988;7:486–506. [PubMed] [Google Scholar]
- Gittes GK, Galante PE, Hanahan D, Rutter WJ, Debas HT. Lineage-specific morphogenesis in the developing pancreas: role of mesenchymal factors. Development (Camb) 1996;122:439–447. doi: 10.1242/dev.122.2.439. [DOI] [PubMed] [Google Scholar]
- Gu D, Sarvetnick N. Epithelial cell proliferation and islet neogenesis in IFN-γ transgenic mice. Development (Camb) 1993;118:33–46. doi: 10.1242/dev.118.1.33. [DOI] [PubMed] [Google Scholar]
- Gu D, Lee MS, Krahl T, Sarvetnick N. Transitional cells in the regenerating pancreas. Development (Camb) 1994;120:1873–1881. doi: 10.1242/dev.120.7.1873. [DOI] [PubMed] [Google Scholar]
- Hatta K, Takeichi M. Expression of N-cadherin adhesion molecules associated with early morphogenetic events in chick development. Nature. 1986;320:447–449. doi: 10.1038/320447a0. [DOI] [PubMed] [Google Scholar]
- Hayek A, Beattie GM, Cirulli V, Lopez AD, Ricordi C, Rubin JS. Growth factor/matrix-induced proliferation of human adult β-cells. Diabetes. 1995;44:1458–1460. doi: 10.2337/diab.44.12.1458. [DOI] [PubMed] [Google Scholar]
- Herrera PL, Huarte J, Sanvito F, Meda P, Orci L, Vassalli J-D. Embryogenesis of the murine endocrine pancreas: early expression of pancreatic polypeptide gene. Development (Camb) 1991;113:1257–1265. doi: 10.1242/dev.113.4.1257. [DOI] [PubMed] [Google Scholar]
- Hoffman, S. 1992. Assays of cell adhesion. In Cell–Cell Interactions. A Practical Approach. B.R. Stevenson, W.J. Gallin, and D.L. Paul, editors. Oxford University Press Inc., New York. 1–30.
- Holtfreter, J. 1939. Tissue Affinity, A Means of Embryonic Morphogenesis. In Foundation of Experimental Embryology. B.H. Willier and J.M. Oppenheimer, editors. Prentice-Hall, Inc., Englewood Cliffs, NJ. 186–225.
- Hynes, R.O. 1989. Oncogenic transformation. In Fibronectins. A. Rich, editor. Springer-Verlag, New York. 301–334.
- Jaffe, R. 1991. The Pancreas. In Textbook of Fetal and Perinatal Pathology. J.S. Wigglesworth and D.B. Singer, editors. Blackwell Scientific Publications, Boston, MA. 1021–1054.
- Katz W, Hill R, Clandinin T, Sternberg P. Different levels of the C. elegansgrowth factor LIN-3 promote distinct vulval precursor fates. Cell. 1995;82:297–307. doi: 10.1016/0092-8674(95)90317-8. [DOI] [PubMed] [Google Scholar]
- Kemler R, Babinet C, Jacob F. Surface antigen in early differentiation. Proc Natl Acad Sci USA. 1977;74:4449–4452. doi: 10.1073/pnas.74.10.4449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krushel LA, Sporn O, Cunningham BA, Crossin KL, Edelman GM. Neural cell adhesion molecule (N-CAM) inhibits astrocyte proliferation after injury to different regions of the adult rat brain. Proc Natl Acad Sci USA. 1995;92:4323–4327. doi: 10.1073/pnas.92.10.4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langerhans, P. 1869. Contributions to the microscopic anatomy of the pancreas. Doctoral dissertation. Friedrich-Wilhelms Universität, Berlin, Germany. 1–39.
- Langhofer M, Hopkinson SB, Jones JCR. The matrix secreted by 804G cells contains laminin-related components that participate in hemidesmosome assembly in vitro. . J Cell Sci. 1993;105:753–764. doi: 10.1242/jcs.105.3.753. [DOI] [PubMed] [Google Scholar]
- Langley OK, Aletsee-Ufrecht MC, Grant NJ, Gratzl M. Expression of the neural cell adhesion molecule NCAM in endocrine cells. J Histochem Cytochem. 1989;37:781–791. doi: 10.1177/37.6.2723399. [DOI] [PubMed] [Google Scholar]
- Larue L, Antos C, Butz S, Huber O, Delmas V, Dominis M, Kemler R. A role for cadherins in tissue formation. Development (Camb) 1996;122:3185–3194. doi: 10.1242/dev.122.10.3185. [DOI] [PubMed] [Google Scholar]
- Levi G, Gumbiner B, Thiery J-P. The distribution of E-cadherin during Xenopus laevisdevelopment. Development (Camb) 1991;111:159–169. doi: 10.1242/dev.111.1.159. [DOI] [PubMed] [Google Scholar]
- Like A, Orci L. Embryogenesis of the human pancreatic islets: a light and electron microscopic study. Diabetes. 1972;21(Suppl.):511–534. doi: 10.2337/diab.21.2.s511. [DOI] [PubMed] [Google Scholar]
- Litvinov SV, Velders MP, Bakker HAM, Fleuren GJ, Warnaar SO. Ep-CAM: a human epithelial antigen is a homophilic cell–cell adhesion molecule. J Cell Biol. 1994a;125:437–446. doi: 10.1083/jcb.125.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litvinov SV, Bakker HAM, Gourevitch MM, Velders MP, Warnaar SO. Evidence for a role of the epithelial glycoprotein 40 (Ep-CAM) in epithelial cell–cell adhesion. Cell Adh Comm. 1994b;2:417–428. doi: 10.3109/15419069409004452. [DOI] [PubMed] [Google Scholar]
- Litvinov SV, Balzar M, Winter MJ, Bakker HAM, Briaire-de Bruijin IH, Prins F, Fleuren GJ, Warnaar SO. Epithelial cell adhesion molecule (Ep-CAM) modulates cell–cell interactions mediated by classical cadherins. J Cell Biol. 1997;139:1337–1348. doi: 10.1083/jcb.139.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mally MI, Otonkoski T, Lopez AD, Hayek A. Developmental gene expression in the human fetal pancreas. Pediatric Res. 1994;36:537–544. doi: 10.1203/00006450-199410000-00022. [DOI] [PubMed] [Google Scholar]
- Mann K, Deutzmann R, Aumailley M, Timple R, Raimondi L, Yamada Y, Pan T, Conway D, Chui ML. Amino acid sequence of mouse nidogen, a multidomain basement membrane protein with binding activity for laminin, collagen IV and cells. EMBO (Eur Mol Biol Organ) J. 1989;8:65–72. doi: 10.1002/j.1460-2075.1989.tb03349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs JA, Nelson JW. Cadherin cell adhesion molecules in differentiation and embryogenesis. Int Rev Cytol. 1996;165:159–205. doi: 10.1016/s0074-7696(08)62222-6. [DOI] [PubMed] [Google Scholar]
- Moldenhauer G, Momburg F, Möller P, Schwartz R, Hammerling G. Epithelial-specific surface glycoprotein of Mr 43,000 is a widely distributed human carcinoma marker. Br J Cancer. 1987;56:714–721. doi: 10.1038/bjc.1987.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moller CJ, Christgau S, Williamson MR, Madsen OD, Zhan-Po N, Bock E, Baekkeskov S. Differential expression of neural cell adhesion molecule and cadherins in pancreatic islets, glucagonomas, and insulinomas. Mol Endocrinol. 1992;6:1332–1342. doi: 10.1210/mend.6.8.1406710. [DOI] [PubMed] [Google Scholar]
- Momburg P, Moldenhauer G, Hammerling GJ, Möller P. Immunohistochemical study of the expression of a Mr 34,000 human epithelial-specific surface glycoprotein in normal and malignant tissues. Cancer Res. 1987;47:2883–2891. [PubMed] [Google Scholar]
- Moscona A, Moscona H. Dissociation and aggregation of cells from organ rudiments of the early chick embryos. J Anat. 1952;86:287–301. [PMC free article] [PubMed] [Google Scholar]
- Nose A, Takeichi M. A novel cadherin cell adhesion molecule: its expression patterns associated with implantation and organogenesis of mouse embryos. J Cell Biol. 1986;103:2649–2658. doi: 10.1083/jcb.103.6.2649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L. Macro- and micro-domains in the endocrine pancreas. Diabetes. 1982;31:538–565. doi: 10.2337/diab.31.6.538. [DOI] [PubMed] [Google Scholar]
- Orci L, Unger RH. Functional subdivision of islets of Langerhans and possible role of D-cells. Lancet. 1975;20:1243–1244. doi: 10.1016/s0140-6736(75)92078-4. [DOI] [PubMed] [Google Scholar]
- Otonkoski T, Beattie GM, Mally MI, Ricordi C, Hayek A. Nicotinamide is a potent inducer of endocrine differentiation in cultured human fetal pancreatic cells. J Clin Invest. 1993;92:1459–1466. doi: 10.1172/JCI116723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otonkoski T, Beattie GM, Rubin JS, Lopez AD, Baird A, Hayek A. Hepatocyte growth factor/scatter factor has insulinotropic activity in human fetal pancreatic cells. Diabetes. 1994;43:947–953. doi: 10.2337/diab.43.7.947. [DOI] [PubMed] [Google Scholar]
- Otonkoski T, Cirulli V, Beattie GM, Mally M, Soto G, Rubin JS, Hayek A. A role for hepatocyte growth factor/scatter factor in fetal mesenchyme-induced pancreatic β-cell growth. Endocrinology. 1996;137:3131–3139. doi: 10.1210/endo.137.7.8770939. [DOI] [PubMed] [Google Scholar]
- Parish RW, Schmidhauser C, Schmidt T, Dudler R. Mechanisms of tumor cell metastasis. J Cell Sci Suppl. 1987;8:181–197. doi: 10.1242/jcs.1987.supplement_8.10. [DOI] [PubMed] [Google Scholar]
- Perez MS, Walker LE. Isolation and characterization of a cDNA encoding the KS1/4 epithelial carcinoma marker. J Immunol. 1989;142:3662–3667. [PubMed] [Google Scholar]
- Pictet, R.L., and W.J. Rutter. 1972. Development of the embryonic endocrine pancreas. In Handbook of Physiology. Vol. 1. The Endocrine Pancreas. D. Steiner and N. Freinkel, editors. Williams and Wilkins Company, Baltimore, MD. 25–66.
- Pictet RL, Clark WR, Williams RH, Rutter WJ. An ultrastructural analysis of the developing embryonic pancreas. Dev Biol. 1972;29:436–467. doi: 10.1016/0012-1606(72)90083-8. [DOI] [PubMed] [Google Scholar]
- Quak JJ, Van Dongen G, Brakkee JG, Hayashidda DJ, Balm AJ, Snow GB, Meijer CJ. Production of a monoclonal antibody (K931) to squamous cell carcinoma associated antigen identified as the 17-1A antigen. Hybridoma. 1990;9:377–387. doi: 10.1089/hyb.1990.9.377. [DOI] [PubMed] [Google Scholar]
- Ricordi C, Lacy PE, Finke EH, Olack BJ, Sharp DW. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37:413–420. doi: 10.2337/diab.37.4.413. [DOI] [PubMed] [Google Scholar]
- Rouiller GD, Cirulli V, Halban PA. Differences in aggregation properties and levels of the neural cell adhesion molecule (NCAM) between islet cell types. Exp Cell Res. 1990;191:305–312. doi: 10.1016/0014-4827(90)90019-7. [DOI] [PubMed] [Google Scholar]
- Rouiller GD, Cirulli V, Halban PA. Uvomorulin mediates calcium-dependent aggregation of islet cells, whereas calcium-independent cell adhesion molecules distinguish between islet cell types. Dev Biol. 1991;148:233–242. doi: 10.1016/0012-1606(91)90332-w. [DOI] [PubMed] [Google Scholar]
- Rutishauser U. Developmental biology of a neural cell adhesion molecule. Nature. 1984;310:549–554. doi: 10.1038/310549a0. [DOI] [PubMed] [Google Scholar]
- Sandler S, Andersson A, Schnell A, Mellgren A, Tollemar J, Borg H, Petersson B, Groth CG, Hellerstrom C. Tissue culture of human fetal pancreas. Development and function of B cells in vitroand transplantation of explants to nude mice. Diabetes. 1985;34:1113–1119. doi: 10.2337/diab.34.11.1113. [DOI] [PubMed] [Google Scholar]
- Simon B, Podolsky K, Moldenhauer G, Isselbacher KJ, Gattoni-Cellini S, Brand SJ. Epithelial glycoprotein is a member of a family of epithelial cell surface antigens homologous to nidogen, a matrix adhesion protein. Proc Natl Acad Sci USA. 1990;87:2755–2759. doi: 10.1073/pnas.87.7.2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sjödin A, Dahl U, Semb H. Mouse R-cadherin: expression during the organogenesis of the pancreas and gastrointestinal tract. Exp Cell Res. 1995;221:413–425. doi: 10.1006/excr.1995.1392. [DOI] [PubMed] [Google Scholar]
- Slack JMW. Developmental biology of the pancreas. Development (Camb) 1995;121:1569–1580. doi: 10.1242/dev.121.6.1569. [DOI] [PubMed] [Google Scholar]
- Sperry RW. Chemoaffinity in the orderly growth of nerve fiber patterns and connections. Proc Natl Acad Sci USA. 1963;50:703–710. doi: 10.1073/pnas.50.4.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn O, Edelman GM, Crossin KL. The neural cell adhesion molecule (N-CAM) inhibits proliferation in primary cultures of rat astrocytes. Proc Natl Acad Sci USA. 1995;92:542–546. doi: 10.1073/pnas.92.2.542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr NK, Durbin H, Sheer D, Parkar M, Bobrow L, Bodmer WF. Characterization and chromosomal assignment of a human cell surface antigen defined by the monoclonal antibody AUAI. Int J Cancer. 1986;38:631–636. doi: 10.1002/ijc.2910380503. [DOI] [PubMed] [Google Scholar]
- Stefan Y, Grasso S, Perrelet A, Orci L. A quantitative immunofluorescent study of the endocrine cell populations in the developing human pancreas. Diabetes. 1983;32:293–301. doi: 10.2337/diab.32.4.293. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Functional correlation between cell adhesive properties and some cell surface proteins. J Cell Biol. 1977;75:464–474. doi: 10.1083/jcb.75.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251:1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Cadherins in cancer: implications for invasion and metastasis. Curr Opin Cell Biol. 1993;5:806–811. doi: 10.1016/0955-0674(93)90029-p. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Morphogenetic roles of classic cadherins. Curr Opin Cell Biol. 1995;7:619–627. doi: 10.1016/0955-0674(95)80102-2. [DOI] [PubMed] [Google Scholar]
- Teitelman G, Lee JK. Cell lineage analysis of pancreatic islet cell development: glucagon and insulin cells arise from catecholaminergic precursors present in the pancreatic duct. Dev Biol. 1987;121:454–466. doi: 10.1016/0012-1606(87)90182-5. [DOI] [PubMed] [Google Scholar]
- Tellechea O, Reis JP, Domingues JC, Baptista AP. Monoclonal antibody Ber EP4 distinguishes basal-cell carcinoma from squamous-cell carcinoma of the skin. Am J Dermatol. 1993;15:452–455. doi: 10.1097/00000372-199310000-00007. [DOI] [PubMed] [Google Scholar]
- Thampoe IJ, Ng JSC, Lloyd KO. Biochemical analysis of a human epithelial antigen: differential cell expression and processing. Arch Biochem Biophys. 1988;267:342–352. doi: 10.1016/0003-9861(88)90040-9. [DOI] [PubMed] [Google Scholar]
- Thiery J-P, Duband J-L, Rutishauser U, Edelman GM. Cell adhesion molecules in early chick embryogenesis. Proc Natl Acad Sci USA. 1982;79:6737–6741. doi: 10.1073/pnas.79.21.6737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiery J-P, Delouvée A, Gallin WJ, Cunningham BA, Edelman GM. Ontogenetic expression of cell adhesion molecule L-CAM is found in epithelia derived from the three primary germ layers. Dev Biol. 1984;102:61–78. doi: 10.1016/0012-1606(84)90175-1. [DOI] [PubMed] [Google Scholar]
- Trelstad, R. 1984. Role of Extracellular Matrix in Development. Alan R. Liss, New York. 643 pp.
- Tsubura A, Senzaki H, Sasaki M, Hilgers J, Morii S. Immunohistochemical demonstration of breast-derived and/or carcinoma-associated glycoproteins in normal skin appendages and their tumors. J Cut Pathol. 1992;19:73–79. doi: 10.1111/j.1600-0560.1992.tb01562.x. [DOI] [PubMed] [Google Scholar]
- Varki NM, Reisfeld RA, Walker LE. Antigens associated with a human lung adenocarcinoma defined by monoclonal antibodies. Cancer Res. 1984;44:681–687. [PubMed] [Google Scholar]
- Velders MP, Litvinov SV, Warnaar SO, Gorter A, Fleuren GJ, Zurawski VA, Coney LA. New chimeric anti-pancarcinoma monoclonal antibody with superior cytotoxicity-mediating potency. Cancer Res. 1994;54:1753–1759. [PubMed] [Google Scholar]
- Von Dorsche HH, Falt K, Titlbach M, Reiher H, Hahn H-J, Falkmer S. Immunohistochemical, morphometric and ultrastructural investigations of the early development of insulin, somatostatin, glucagon and PP cells in foetal human pancreas. Diabetes Res. 1989;12:551–556. [PubMed] [Google Scholar]
- Watt FM, Jones PH. Expression and function of keratinocyte integrins. Development. 1993;106(Suppl.):185–192. [PubMed] [Google Scholar]