Abstract
Centrosomes repeatedly reproduce in sea urchin zygotes arrested in S phase, whether cyclin-dependent kinase 1–cyclin B (Cdk1-B) activity remains at prefertilization levels or rises to mitotic values. In contrast, when zygotes are arrested in mitosis using cyclin B Δ-90, anaphase occurs at the normal time, yet centrosomes do not reproduce. Together, these results reveal the cell cycle stage specificity for centrosome reproduction and demonstrate that neither the level nor the cycling of Cdk1-B activity coordinate centrosome reproduction with nuclear events. In addition, the proteolytic events of the metaphase–anaphase transition do not control when centrosomes duplicate. When we block protein synthesis at first prophase, the zygotes divide and arrest before second S phase. Both blastomeres contain just two complete centrosomes, which indicates that the cytoplasmic conditions between mitosis and S phase support centrosome reproduction. However, the fact that these daughter centrosomes do not reproduce again under such supportive conditions suggests that they are lacking a component required for reproduction. The repeated reproduction of centrosomes during S phase arrest points to the existence of a necessary “licensing” event that restores this component to daughter centrosomes during S phase, preparing them to reproduce in the next cell cycle.
Centrosome reproduction, or duplication, in animal cells is thought to start when the centrioles lose their orthogonal arrangement near the onset of DNA synthesis, and short daughter centrioles are first seen at the proximal end of each mature centriole (Robbins et al., 1968; Rattner and Phillips, 1973; Kuriyama and Borisy, 1981; Wheatley, 1982). The centrosome as a whole splits at a variable time in G2 with pairs of mother–daughter centrioles going to each daughter centrosome (Aubin et al., 1980; Kochanski and Borisy, 1990). In specifying when the centrosome reproduces, it is important to bear in mind that these morphological events mark the times when the steps of centrosome reproduction are well underway and do not necessarily indicate when they are initiated. The assembly of the essential precursor structures must have occurred at earlier times in the cell cycle.
The events of centrosome reproduction must be tightly coordinated with nuclear events because the division of the cell will inevitably be abnormal if the centrosome fails to reproduce at the proper time or if it reduplicates before mitosis. The mechanisms that ensure the essential coordination between nuclear and centrosomal events during the cell cycle are not well understood. Much of what is known about the controls for centrosomal events has come from studies on cleavage stage zygotes. In these zygotes, the minimal essential controls can be experimentally tested without the complication of maintaining cell growth or centrosomal subunit synthesis, as is the case for somatic cells (see Balczon et al., 1995). In sea urchin zygotes, nuclear activities, such as the timed transcription of RNAs for key centrosomal subunits, the replication of DNA, or nuclear “signals” are not part of the pathway(s) that control centrosome reproduction (Lorch 1952; Sluder et al., 1986). In addition, findings that repeated centrosome reproduction proceeds in the complete absence of protein synthesis (Gard et al., 1990; Sluder et al. 1990) reveals that centrosome reproduction is not limited by the required synthesis of centrosomal subunits at each cell cycle and the zygote can regulate the assembly of centrosomes from preexisting pools of subunits whose sizes are not limiting. Together, these data reveal that strictly cytoplasmic mechanisms control centrosomal events during the cell cycle.
A logical candidate for a cytoplasmic control that could provide the essential coordination between nuclear and centrosomal events is the activity cycle of the cyclin-dependent kinase 1–cyclin B complex (Cdk1-B),1 historically referred to as p34cdc2–cyclin B (Arion et al., 1988; Labbe et al., 1989; Gautier et al., 1990). Cdk1-B is said to constitute the major cell cycle “engine” that drives the cell into and out of mitosis (Murray and Kirschner 1989; Murray et al. 1989; Glotzer et al., 1991; Luca et al., 1991). However, demonstrations of repeated centrosome reproduction in the absence of cyclin B synthesis or a nuclear cycle (Sluder and Lewis, 1987; Gard et al., 1990; Sluder et al., 1990) led to the proposal that centrosomal and nuclear events may be controlled by different metabolic pathways (Sluder et al., 1990). Nevertheless, the normal coordination between nuclear and centrosomal events forces the search for a cytoplasmic activity that links these two key aspects of the cell's preparations for mitosis.
In the present study we tested whether the absolute value of Cdk1-B activity defines periods in the cycle when centrosome reproduction can occur in the same fashion that it serves to coordinate other events during cell cycle progression. In both fission yeast and Xenopus egg extracts, high levels of Cdk1-B activity have been shown to prevent DNA rereplication, which in turn orders the S and M phases (Hayles et al., 1994; Dahnmann et al., 1995; Mahbubani et al., 1997). Also, Cdk1-B activity may function to coordinate cytokinesis with the metaphase–anaphase transition by preventing the assembly of the contractile apparatus until the drop in its activity has occurred at the onset of anaphase (Satterwhite et al., 1992; Wheatley et al., 1997). Centrosomes will repeatedly reproduce under conditions of low Cdk1-B activity (Gard et al., 1990; Sluder et al., 1990) yet do not reproduce when mitosis is moderately prolonged by mercaptoethanol, an admittedly blunt instrument (Mazia et al., 1960; Sluder and Begg, 1985). Perhaps elevated Cdk1-B activity just before and during mitosis prevents centrosome reduplication, while the precipitous drop in its activity at the metaphase–anaphase transition allows the pathways for centrosome reproduction to start, resulting in the appearance of procentrioles (Tournier et al., 1991).
We also tested the possibility that proteolysis at anaphase onset determines when centrosome reproduction begins. Timed proteolysis ensures that certain cell cycle events and transitions are irreversible (for reviews see Murray 1995; King et al., 1996). For example, the coordinate proteolytic degradation of cyclin B and proteins that link daughter chromosomes together coordinates the onset of anaphase chromosome movement with the commitment of the cell to exit mitosis at the metaphase–anaphase transition (Glotzer et al., 1991; Holloway et al., 1993; Wheatley et al., 1997). In a similar vein, a putative requirement for proteolysis of specific centrosomal proteins at the metaphase–anaphase transition could entrain the centrosome cycle with the completion of mitosis (see Biggins et al., 1996; McDonald and Byers, 1997).
Finally, we determined if repeated centrosome reproduction is dependent on specific cell cycle stages. Previous studies have shown that centrosomes duplicate multiple times when the cell cycle is arrested in S phase by agents that block DNA synthesis (Sluder and Lewis, 1987; Raff and Glover, 1988; Balczon et al., 1995). Whether centrosomes repeatedly reproduce during grossly prolonged mitosis and G1 phases of the cell cycle has not been thoroughly examined. Even though cell cycle stage and Cdk1-B activity are normally interrelated, we have been able to test these two parameters independently.
Materials and Methods
Unless otherwise stated, all reagents were purchased from Sigma Chemical Co. (St. Louis, MO).
Living Material and Light Microscopy
Lytechinus pictus and Lytechinus variagatus were purchased from Marinus, Inc. (Long Beach, CA) and Susan Decker (Hollywood, FL) respectively. Eggs and sperm were obtained by intracoelomic injection of 0.5 M KCl as previously described (Fuseler, 1973) and cultured in natural sea water (NSW) at 16–18°C (L. pictus) or 20–21°C (L. variagatus). Individual zygotes were observed and photographed in vivo using a microscope (model ACM; Carl Zeiss, Inc., Thornwood, NY) modified for polarization microscopy. In certain experiments, astral birefringence was augmented by treating the zygotes for 2–5 min with 2% hexylene glycol in NSW (Sluder et al., 1990). Photographs were recorded either on Plus X film developed in Microdol-X developer or on T-Max film developed with T-Max developer (Eastman Kodak, Rochester, NY). Alternatively, real time video and time-lapse video images were captured using a CCD camera controlled by an image processor (Argus 20; Hamamatsu, Bridgewater, NJ) and stored on the hard drive of a PC, using an AV Master video capture card (Fast Multimedia AG, Munich, Germany), Adobe Premier 4.0, and Photoshop 4.0 (Adobe Systems, Inc., Mountain View, CA).
For microinjection experiments, eggs were fertilized, diluted into Ca2+-free sea water (CFSW), and passed twice through a 100-μm Nitex screen (Tetko Inc., Elmsford, NY) to remove the fertilization envelopes. Demembranated eggs were cultured in CFSW, and then before prophase, they were placed in a microinjection chamber as described in Kiehart (1982). Zygotes were injected with ∼0.5% of their volume in prophase and then observed.
Drug Treatments
1 × 10−4 M emetine and 1 × 10−5 M anisomycin were prepared together in natural sea water immediately before each experiment (Sluder et al., 1990). Eggs were suspended in drug-containing sea water either 30 min before fertilization, or at varying times after fertilization. Aphidicolin was dissolved as a 5 mg/ml stock in DMSO. For each experiment, the drug was diluted into NSW to a final concentration of 10 μg/ml. Eggs were continuously treated with aphidicolin from before fertilization as previously described (Sluder and Lewis, 1987).
Cyclin B Δ-90 mRNA
mRNA, containing a 5′ 7-methyl guanosine cap, was synthesized from a pET3b vector (for T7Δ90) and from FpΔ13TF1 vector (for T7Δ13; see Murray et al., 1989; Glotzer et al., 1991 for cloning details) using the Ambion mMESSAGE mMACHINE™ In Vitro Transcription Kit (Austin, TX). The mRNA yield from each transcription reaction was determined as per the kit instructions, using percent incorporation of a trace nucleotide ([α-32P]GTP) added to the reaction mixture. Transcribed mRNA was separated from unincorporated nucleotides by a G50 spin column, subjected to a phenol/chloroform extraction followed by a chloroform extraction, and then it was twice precipitated with ethanol and stored at −20°C. Before use, the mRNA pellet was suspended in RNAse-free dH2O at a concentration of 1 mg/ml.
Antibromodeoxyuridine Fluorescence Microscopy
To fertilize eggs, 5-bromo-2′-deoxyuridine (BrdU) was added to a final concentration of 300 μg/ml. Aliquots of zygotes were allowed to settle onto coverslips and were fixed in −20°C methanol. Zygotes were postfixed for 4 h in 4 M HCl at room temperature, neutralized in PBS (2.7 mM KH2PO4, 2.7 mM Na2HPO4, 150 mM NaCl, 2.7 mM KCl, pH 7.4), and processed for immunofluorescence microscopy using a mouse monoclonal anti-BrdU antibody (1:40 dilution; Boehringer Mannheim Corp., Indianapolis, IN) followed by a Texas red–conjugated goat anti–mouse secondary antibody (1:500 dilution; Molecular Probes, Inc., Eugene, OR). Immunolabeled zygotes were viewed and photographed with a microscope (Axiophot; Carl Zeiss, Inc.) equipped for epifluorescence.
Refertilization
Eggs were fertilized in NSW, and the fertilization envelopes were removed as described above. These zygotes were maintained in CFSW. At times up to 180 min after the initial fertilization, zygotes were then refertilized by suspending them in 20 ml final volume NSW and then adding 2 ml of fresh diluted sperm (made by diluting 1 ml “dry” sperm in 50 ml NSW). Refertilized zygotes were allowed to settle under 1 g and then suspended in CFSW. To determine the extent of refertilization, as compared with polyspermy at the initial fertilization, fertilized eggs and refertilized eggs from the same culture were treated with Hoescht 33482 and viewed by epifluorescence microscopy. The number of extra pronuclei in each zygote was counted (100 zygotes per count).
Histone H1 Kinase Assays
Unfertilized eggs were dejellied by suspending them for 3 min in artificial sea water (435 mM NaCl, 40 mM MgCl2, 15 mM Mg SO4, 11 mM CaCl2, 10 mM KCl, 5 mM Hepes, pH 5.0). A 1% (vol/vol) suspension of eggs in NSW was then divided into three cultures and kept suspended with stir paddles (60 rpm) in 100-ml beakers. One culture was fertilized and served as control. To the second, emetine and anisomycin were added to a final concentration of 1 × 10−4 M and 1 × 10−5 M, respectively, and then fertilized. The third culture was fertilized and emetine and anisomycin were added at the same concentrations 55 min after fertilization. In separate experiments, eggs were split into two cultures: the first was fertilized and served as a control; the second was fertilized and treated continuously with 10 μg/ml aphidicolin beginning at fertilization. For all experiments, two 3-ml aliquots were taken at each time point, and the average H1 kinase activity for these two aliquots used for each data point.
Samples were prepared and assayed for histone H1 kinase activity by the methods of Suprynowicz et al. (1994) as modified by Sluder et al. (1995), using purified histone H1 (kindly supplied by Dr. James Maller, University of Colorado, Denver, CO; also purchased from Ambion Inc.). Samples were electrophoresed using SDS-PAGE, and quantification of H1 kinase activity was performed with a PhosphorImager SF and Image Quant Software version 3.3 (Molecular Dynamics, Sunnyvale, CA) as previously described (Sluder et al., 1995).
Electron Microscopy
Zygotes were followed in a microinjection chamber. Before fixation, individual zygotes were photographed, removed from the chamber, and fixed for 90 min in 2% osmium tetroxide in 0.4 M sodium acetate buffer (Harris, 1962; Rieder et al., 1985; Sluder and Rieder, 1985). After dehydration and embedment in Epon-Araldite, zygotes were serially sectioned (0.25–0.5 μm/ section) and stained with uranyl acetate and lead citrate, and the relevant sections were viewed and photographed on the Wadsworth Center high-voltage electron microscope operated at 800–1,000 kV.
Results
Centrosome Reproduction and Cdk1-B Kinase Activity during Prolonged S Phase
Sea urchin zygotes were arrested in S phase of the first cell cycle by continuous treatment with 10 μg/ml aphidicolin beginning 5 min after fertilization. Aphidicolin, a specific inhibitor of DNA polymerase-α, prevents the incorporation of [3H]thymidine into nuclear DNA by at least 95% in these zygotes (Ikegami et al., 1979; Sluder and Lewis, 1987). The residual [3H]thymidine incorporation observed may be due to either mitochondrial DNA synthesis or the repair of DNA damage by the β and γ polymerases (Ikegami et al., 1978, 1979). Over a 7-h incubation in the drug (equivalent of five cell cycles), the number of asters progressively increased, with some zygotes containing more than eight asters (Table I) (also see Sluder and Lewis, 1987). To determine if repeated centrosome reproduction during prolonged S phase is peculiar to the first cell cycle, zygotes were treated with 10 μg/ml aphidicolin starting at first prophase. These embryos divided once with normal timing and then arrested before second mitosis. The number of asters in these embryos progressively increased over a period of 7 h, with some containing greater than eight asters per blastomere (Table I).
Table I.
Asters per Zygote
| Number of asters seen 360 min after fertilization | Aphidicolin added at fertilization | Aphidicolin added at first prophase | ||
|---|---|---|---|---|
| 2 | 19 (8%) | 14 (6%) | ||
| 4 | 88 (37%) | 102 (47%) | ||
| 6 | 23 (10%) | 58 (27%) | ||
| 8 | 76 (33%) | 31 (14%) | ||
| >8 | 27 (12%) | 14 (6%) | ||
| Total zygotes | 233 (100%) | 219 (100%) |
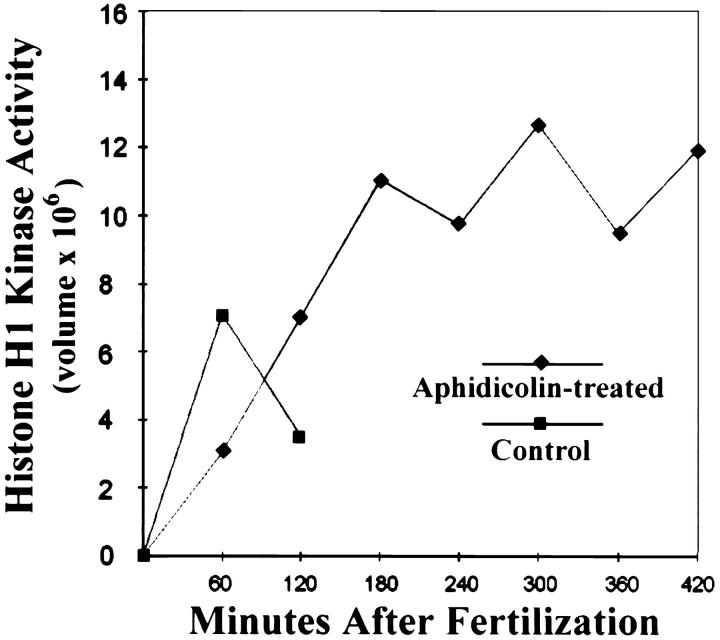
We monitored Cdk1-B activity in zygotes treated with aphidicolin from the time of fertilization, at 60-min intervals for 7 h after fertilization. Histone H1 kinase activity progressively increased, and by 2 h after fertilization it reached the same or higher levels than those at first mitosis in the same female control culture (Fig. 1). We note that H1 kinase activity in aphidicolin-treated zygotes rises slowly compared with control cultures (Fig. 1) (also see Geneviere-Garrigues et al., 1995; Sluder et al., 1995).
Figure 1.
Histone H1 kinase activities in control zygotes (squares) and zygotes treated with aphidicolin (diamonds) to prevent DNA synthesis. Both cultures came from a common pool of zygotes. H1 kinase activity in control zygotes (squares) rises at first mitosis and then drops at the metaphase–anaphase transition. Ordinate: H1 kinase activity expressed as “volume,” which is the sum of the pixel values of the H1 band minus background as determined in the PhosphorImager. Abscissa: minutes after fertilization.
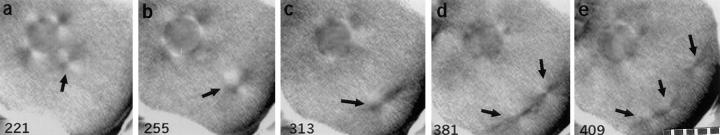
In addition, using time-lapse video recordings we followed individual living zygotes with intact nuclei, starting 3 h after application of the drug at fertilization, a time when Cdk1-B activity should be at mitotic or supramitotic levels. We found that asters repeatedly doubled during S phase with high Cdk1-B activity (Fig. 2). We also found that the period of aster doubling in zygotes treated with aphidicolin (average 148 min, range 40–257 min, n = 20) is longer and more variable than that of controls (average 47 min, range 35–55 min, n = 17). Since the asters we followed doubled at least three times, we conclude that we observed centrosome reproduction, not centrosome splitting.
Figure 2.
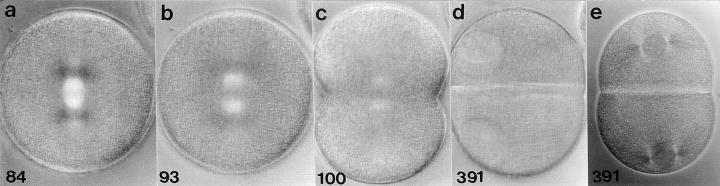
Repeated centrosome reproduction with high Cdk1-B activity in a zygote arrested in S phase with aphidicolin. Here we have focused on one aster that has separated from the nucleus and doubles twice starting 221 min after fertilization. (a) Four asters at the nucleus; one aster is detaching (arrow). (b) The detached aster migrates away from the nucleus. (c) The detached aster has begun to double. (d) This aster has split into two asters (arrows). (e) These asters split again (arrows); the fourth aster is out of the plane of focus. Minutes after fertilization are shown in the lower corner of each frame. Polarization optics. 10 μm per scale division.
Centrosome Reproduction during Prolonged Mitosis
To determine if mitosis is a cell cycle phase that supports repeated centrosome reproduction, we microinjected mRNA coding for nondegradable sea urchin cyclin B (cyclin B Δ-90) (Glotzer et al., 1991) into zygotes at prophase of first mitosis. Of the 149 zygotes injected, all underwent nuclear envelope breakdown (NEB), assembled a bipolar spindle of normal appearance (Fig. 3 a), and initiated anaphase with normal timing (Fig. 3 b). All but two of these zygotes failed to exit mitosis, as judged by the persistence of a birefringent spindle and lack of nuclear envelope reformation. The division of the other two zygotes we attribute to inadequate doses of injected mRNA. Cyclin B Δ-90–injected zygotes remained arrested in mitosis for an average of 216 min after NEB (range: 153–586 minutes). In comparison, control zygotes spend on average 19 min between NEB and anaphase onset (Sluder et al., 1994). Injected zygotes eventually died with a behavior suggestive of apoptosis. Fading of spindle birefringence was followed by the cytoplasm turning granular; then the cortex began a series of irregular dynamic distortions that terminated with the lysis of the zygote.
Figure 3.
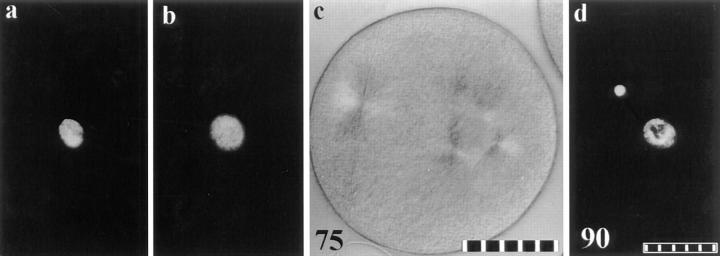
Microinjection of cyclin B Δ-90 mRNA at first prophase arrests zygotes in mitosis. (a) The zygote enters mitosis and forms a normal bipolar spindle. The refractile sphere in the lower left portion of the zygote is a drop of oil used to cap the micropipet. (b) Anaphase onset occurs at the normal time. (c) Both of the spindle poles have split and a tetrapolar spindle forms. (d) This zygote shortly before fixation for serial section ultrastructural analysis. (e) Each spindle pole of this zygote contains only a single centriole. All four centrioles in this zygote are shown in this frame. (a–d) Polarization optics. Minutes after fertilization are shown in the lower left corner of each frame. 10 μm per scale division. Bar: (e) 0.25 μm.
For all cells arrested in mitosis, both spindle poles split at or before anaphase onset, separated, and formed a tetrapolar spindle (Fig. 3, c and d). With the exception of one zygote, the number of asters never increased past four. To distinguish if this doubling of asters during mitosis reflected a splitting of the two centrosomes into half centrosomes, or a full centrosome reproduction, we followed a zygote after injection with mRNA coding for cyclin B Δ-90 (Fig. 3). After anaphase onset, both spindle poles split (Fig. 3 c), and at 237 min after NEB (Fig. 3 d), we fixed this cell for serial semithick section ultrastructural analysis. We located and completely reconstructed all four centrosomes and found only one centriole in each (Fig. 3 e). Our previous work has shown that this is characteristic of spindle pole splitting without reproduction during prolonged mitosis (Sluder and Begg, 1985; Sluder and Rieder, 1985).
To asses chromosome behavior after cyclin B Δ-90 injection, we treated the zygotes with Hoescht 33442 and followed seven individuals with both polarization and fluorescence optics. The chromosomes condensed and congressed to the metaphase plate in a normal fashion upon assembly of a bipolar spindle (Fig 4, a and d). In anaphase, the daughter chromosomes disjoined and moved towards the opposite spindle poles (Fig. 4, b and e). However, later the condensed chromosomes were distributed throughout the tetrapolar spindle (Fig. 4, c and f). Thus, cyclin B Δ-90 prevents exit from mitosis but does not inhibit the proteolysis of proteins required for chromosome disjunction (see Holloway et al., 1993; Wheatley et al., 1997). Together, these observations reveal that centrosomes split but do not reproduce during prolonged mitosis even though the proteolytic events of the metaphase–anaphase transition occur.
Figure 4.
Anaphase chromosome disjunction in a zygote injected with cyclin B Δ-90 mRNA. (a–c) Polarization micrographs. (d–f) Fluorescence micrographs of the chromosomes of the same cell at the same times. (a and d) A normal prometaphase spindle has formed, with the chromosomes aligned at the metaphase plate. The oil droplet used to cap the injection needle can be seen below the spindle. (b and e) Mid-anaphase. The sister chromatids have disjoined and are moving towards the spindle poles. (c and f) Both spindle poles have split, and a tetrapolar spindle has formed. The disjoined chromatids are dispersed throughout the central region of the tetrapolar spindle. Minutes after fertilization are shown in the lower left corner of the lower frames. 10 μm per scale division.
To be certain that the mitotic arrest was a direct consequence of cyclin B Δ-90 expression, we microinjected the same concentration of mRNA encoding either Xenopus triglobin (n = 27) or a degradable form of sea urchin cyclin B (cyclin B Δ-13; n = 8) (Murray et al., 1989). Zygotes injected with these mRNAs progressed through mitosis with normal timing and morphology (data not shown).
Centrosome Reproduction and Levels of Cdk1-B Kinase Activity: Inhibition of Protein Synthesis
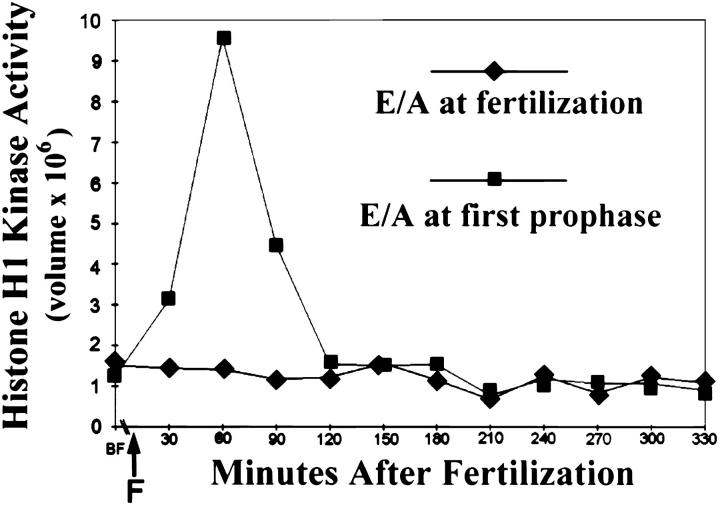
When protein synthesis in sea urchin zygotes is completely blocked from before fertilization, centrosomes repeatedly reproduce in a slow and asynchronous fashion (Table II; see also Sluder et al., 1990). In addition, we quantified Cdk1-B activity in such zygotes at 30-min intervals for 3 h. As shown in Fig. 5 (diamonds), H1 kinase activity remained at prefertilization or basal levels for the duration of the experiment.
Table II.
Asters per Zygote
| Number of asters per cell 360 min after fertilization | Emetine/anisomycin added at fertilization | Emetine/anisomycin added at prophase | ||||
|---|---|---|---|---|---|---|
| Single cell (no division) | Two-cell embryo (asters per blastomere) | |||||
| 2 | 282 (42%) | 45 (58%) | 570 (100%) | |||
| 4 | 81 (12%) | 19 (24%) | − | |||
| 6 | 47 (7%) | 13 (18%) | − | |||
| 8 | 121 (18%) | − | − | |||
| >8 | 141 (21%) | − | − | |||
| Total zygotes | 672 (100%) | 78 (100%) | 570 (100%) | |||
Figure 5.
Histone H1 kinase activity in zygotes treated with protein synthesis inhibitors (E/A) beginning before fertilization (diamonds) and at prophase of first mitosis (squares). Both cultures came from a common pool of zygotes. The arrow (F) indicates time of fertilization. Note that the first time point for each experiment was taken 15 min before fertilization and represents the basal level of H1 kinase activity in unfertilized eggs. Each data point represents the average histone H1 kinase activity for two samples taken at each time. The two curves represent actual PhosphorImager values and were not normalized to each other. Ordinate: H1 kinase activity expressed as “volume,” which is the sum of the pixel values of the H1 band minus background as determined in the PhosphorImager. Abscissa: minutes after fertilization.
However, we observed a remarkably different pattern of centrosome reproduction when we blocked protein synthesis during the second cell cycle. Zygotes were treated continuously with translation inhibitors beginning at prophase of first mitosis; the time course of Cdk1 activity in these cultures is shown in Fig. 5 (squares). We found that H1 kinase activity rose to control levels during mitosis and returned to the basal level after the metaphase–anaphase transition where it remained thereafter. These zygotes divided once with normal timing (Fig. 6, a–c), presumably because they had completed all preparations for first division before protein synthesis was inhibited. The two blastomeres reformed nuclei and arrested in interphase before second NEB (Fig. 6 d). In all cases, each blastomere contained two asters at opposite sides of the nucleus for up to 8 h or the equivalent of seven division cycles (Table II). Although astral birefringence was typically low, the number of asters could be readily determined by careful through focusing of the microscope or by treating the zygotes with 2% hexylene glycol to augment astral birefringence. For display purposes, we show a zygote treated with 2% hexylene glycol (Fig. 6 e).
Figure 6.
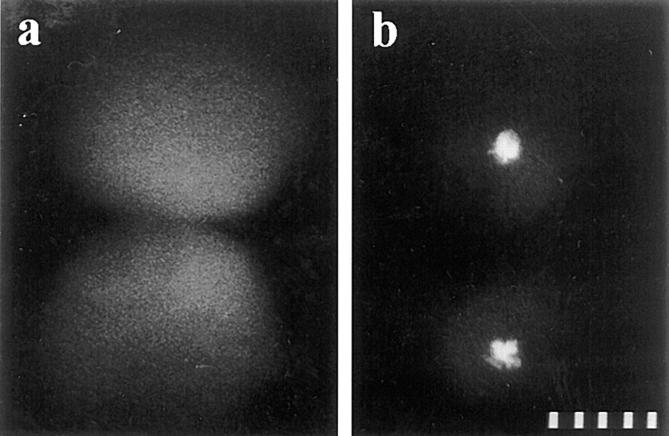
The development of a zygote treated with protein synthesis inhibitors beginning at first prophase. (a) Prometaphase. (b) Anaphase. (c) Initiation of cytokinesis at telophase. (d) The blastomeres arrest in interphase of the second cell cycle, and the nuclei become enlarged over time. The two asters in each blastomere are weakly birefringent and thus not visible in this micrograph. (e) Another zygote from the same culture, also arrested in second interphase. This zygote has been treated with 2% hexylene glycol to augment astral birefringence. Minutes after fertilization are shown in the lower corner of each panel. Polarization optics. 10 μm per scale division.
The slight asynchrony in the development of zygotes in our cultures meant that some individuals had not reached prophase by the time we applied the translation inhibitors. As a consequence, these cells were arrested before first mitosis and did not undergo first NEB, and the centrosomes reproduced asynchronously, yielding as many as six asters (Table II). Thus, in the same culture, we found examples that clearly show different patterns of centrosome reproduction for cell cycle arrest before and after first mitosis.
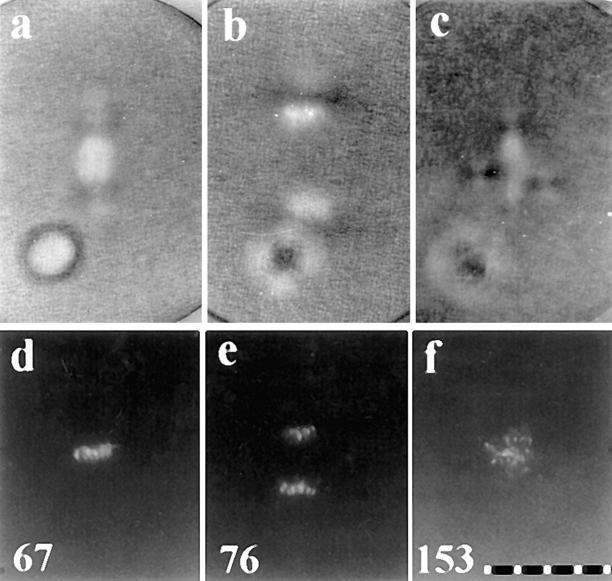
To determine if the two asters formed in each blastomere reflected the splitting, or complete reproduction, of the centrosome inherited from first mitosis, we followed individual zygotes and fixed them for serial semithick section ultrastructural analyses. Fig. 7 a shows a living zygote just before fixation, 164 min after fertilization. We serially reconstructed three of the four asters in this zygote and found that each centrosome contained two centrioles of normal appearance (Fig. 7, a–c). The zygote shown in Fig. 7 d was fixed 345 min after fertilization. Serial reconstruction of all four asters revealed that each centrosome contained two centrioles (Fig. 7, d–g). These observations reveal that the centrosome inherited by each blastomere at the end of mitosis undergoes only one round of complete reproduction when the cell cycle is arrested under these conditions.
Figure 7.
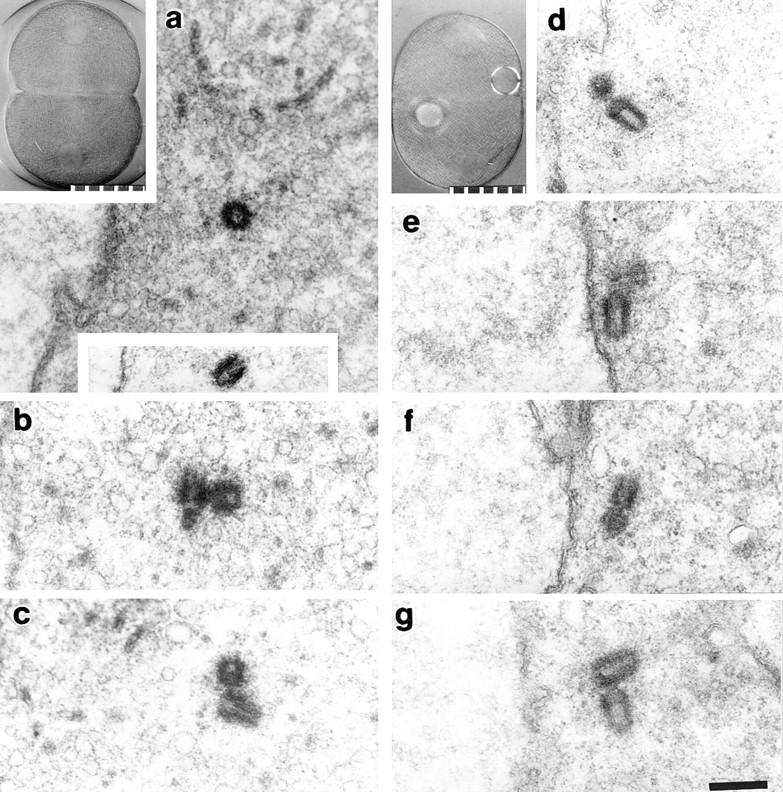
Serial semithick section ultrastructural analysis of centrosomes in two zygotes treated with protein synthesis inhibitors at first prophase. (a–c) Zygote fixed 164 min after fertilization. (a, upper inset) Zygote before fixation. Three centrosomes were completely reconstructed, and each contained two centrioles (a–c). The lower inset in a shows the second centriole in this centrosome, which was located in a different section. The profile of the nuclear envelope can be seen to the left of the lower inset. (d–g) Zygote fixed 345 min after fertilization. (d, inset) Zygote just before fixation; the refractile spheres are oil drops used to mark the zygote for recovery and fixation. All four centrosomes in this zygote were reconstructed, and each contained two centrioles (d–g). All four centrosomes are close to the nuclear envelopes, which are seen running vertically through the center of each panel. (a and d, insets) Polarization optics. 10 μm per scale division. Bar, 2 μm.
Cell Cycle Phase of Zygotes Treated with Translation Inhibitors
Previous work has shown that DNA synthesis proceeds to completion at the normal time in sea urchin zygotes treated with translation inhibitors beginning at fertilization (Wagenaar and Mazia, 1978; Wagenaar, 1983; Strausfeld et al., 1996). Since we found that completely inhibiting protein synthesis at fertilization and first prophase yielded strikingly different patterns of centrosome reproduction, we asked if zygotes in either case had entered and/or completed S phase. Protein synthesis was inhibited beginning at either fertilization or first prophase in the presence of BrdU, an immunoreactive reporter molecule that only incorporates into nuclear DNA during replication (Cawood and Savage, 1983; Gunduz, 1985). The incorporation of BrdU into the nuclei was then assessed by indirect immunofluorescence with an antibody to BrdU.
When protein synthesis was completely inhibited starting before fertilization, BrdU was incorporated into the zygote nucleus (Fig. 8 b) at levels comparable to control cells (Fig. 8 a), consistent with previous reports (Wagenaar and Mazia, 1978; Wagenaar, 1983). To determine if such zygotes remained indefinitely in S phase or had entered G2, we applied translation inhibitors and BrdU at fertilization. Then at times ranging 60–180 min after the initial fertilization, we refertilized the zygotes and fixed them 60 min later to asses BrdU incorporation into the supernumerary sperm nuclei. Our rationale was that the presence or absence of BrdU incorporation into the extra sperm nuclei would indicate if the cytoplasm was or was not in S phase. We note that even at 60 min after the initial fertilization, DNA synthesis in the zygote nucleus should have been finished by at least 15 min (see Hinegardner et al., 1964; Wagenaar, 1983).
Figure 8.
Indirect immunofluorescence using anti-BrdU antibody to assay for DNA synthesis. (a) Nuclear region of a control zygote fixed 50 min after fertilization. (b) Nuclear region of a zygote treated with protein synthesis inhibitors at fertilization and fixed 50 min later. (c) Polarization micrograph of a zygote treated with protein synthesis inhibitors at fertilization and refertilized 180 min after fertilization. Note that the asters associated with the nucleus have reproduced. The late entering sperm pronucleus with its sperm aster can be seen on the left. Minutes after refertilization are indicated in the lower corner of this frame. (d) Anti-BrdU immunofluorescence of a refertilized zygote from the same culture as c. Note that both the zygote nucleus and the late-entering sperm pronucleus have incorporated BrdU. Minutes after refertilization are indicated in the lower corner of this frame. (a, b, and d) Fluorescence optics. (c) Polarization optics. 10 μm per scale division.
We found that 13–26% of the zygotes in the refertilized cultures contained one or more sperm pronuclei, each with an associated sperm aster (Fig. 8 c). Importantly, in all cases the extra sperm pronuclei had incorporated BrdU into their DNA (Fig. 8 d). To ensure that the extra pronuclei were not due to polyspermy at the initial fertilization, the number of supernumerary pronuclei were counted in the refertilized cultures and compared with the same experiment control cultures. The incidence of polyspermy in the control cultures was only 1–3%. Thus, the complete inhibition of protein synthesis from fertilization arrests the zygotes in S phase.
We next asked if the blastomeres from the zygotes treated with translation inhibitors at first prophase enter second S phase. We applied translation inhibitors and BrdU to zygotes starting at prophase of first mitosis and then fixed them 120 min later to assay for BrdU incorporation. In all cases we observed no incorporation of BrdU into nuclear DNA (Fig. 9 a). To control for the possibility that fertilized eggs have a reduced capacity to take up BrdU, as has been suggested (Epel, 1972), we applied translation inhibitors 90 min after fertilization, when the bulk of the culture was in early telophase and starting to synthesize DNA (Hinegardner et al., 1964). 120 min later, we processed these zygotes for immunofluorescence and found that the nuclei had incorporated BrdU (Fig. 9 b). Thus, mitosis does not inhibit the uptake of BrdU, and we conclude that the addition of translation inhibitors at first prophase arrests zygotes before entry into second S phase (G1 equivalent).
Figure 9.
Indirect immunofluorescence using an anti-BrdU antibody to assay for DNA synthesis. (a) This zygote was treated with translation inhibitors and BrdU at first prophase (55 min after fertilization) and fixed 120 min later when it was arrested in second interphase. There is no incorporation of BrdU into nuclear DNA. Background cytoplasmic fluorescence has become evident due to the long camera exposure used. (b) Control for BrdU permeability in prophase zygotes. BrdU was applied in first prophase (55 min after fertilization) and translation inhibitors were applied at telophase (90 min after fertilization) when second DNA synthesis had begun. The zygotes were fixed 120 min later. Incorporation of BrdU into nuclear DNA, as shown here, indicates that BrdU enters prophase/mitotic zygotes. Fluorescence optics. 10 μm per scale division.
Discussion
Cdk1-B Activity and Centrosome Reproduction
We found no correlation between Cdk1-B activity and the ability of centrosomes to repeatedly reproduce. Centrosomes duplicate multiple times when Cdk1-B activity is at mitotic levels, (e.g., in zygotes arrested in S phase by aphidicolin) or at a prefertilization basal level (e.g., in zygotes treated with translation inhibitors from the time of fertilization). Conversely, centrosomes do not reproduce when zygotes are arrested in mitosis with permanently high Cdk1-B activity or are arrested by translation inhibitors before second S phase with basal levels of kinase activity. Thus, we conclude that the coordination between centrosome reproduction and nuclear events is not mediated by either the cycling or the absolute value of Cdk1-B activity.
It is widely thought that the rapid increase in Cdk1-B activity correlated with the onset of mitosis is sufficient to cause nuclear envelope breakdown in zygotes and somatic cells. The fact that the nuclei in aphidicolin-treated zygotes do not necessarily break down when H1 kinase activity reaches mitotic levels, therefore, raises the question of whether the H1 activity we measured was truly due to Cdk1-B, or rather to the activity of Cdk1–cyclin A and/or Cdk2–cyclins A or E. Two groups of studies indicate that the histone H1 kinase activity we measured is predominantly due to Cdk1-B. First, mitotic levels of Cdk1-B activity are not sufficient to drive nuclear envelope breakdown in sea urchin zygotes when the checkpoint for the completion of DNA synthesis is activated (Geneviere-Garrigues et al., 1995; Sluder et al., 1995). These zygotes are not peculiar in this respect, because Lamb et al. (1990) found that microinjection of purified Cdk1–cyclin B into cultured mammalian cells causes them to adopt a prophase morphology, but they do not undergo nuclear envelope breakdown. Also, Heald et al. (1993) found that high levels of cytoplasmic Cdk1-B activity during interphase will not induce premature nuclear envelope breakdown if wee1, a nuclear protein, is overexpressed. In Saccharomyces cerevisiae, the constitutive activation of the cdc28 kinase (budding yeast homologue of cdc2) does not lead to precocious entry into mitosis or bypass the checkpoint for the completion of DNA synthesis (Amon et al., 1992; Sorger and Murray, 1992). Second, previous work with whole sea urchin egg homogenates revealed that greater than 90% of the H1 kinase activity associated with Cdk1 was specifically due to the kinase bound to cyclin B (Geneviere-Garrigues et al., 1995). Similar work with Xenopus egg extracts reported that Cdk2–cyclin A and –cyclin E contributed less than 10% of the total H1 kinase activity (Rempel et al., 1995).
While we conclude that Cdk1-B activity does not coordinate nuclear and centrosomal events in the cell cycle, our results do not rule out the possibility that other Cdk-cyclin combinations play a role in controlling centrosome duplication. Given that centrosomes repeatedly reproduce during prolonged S phase, it will be important to determine the roles played by those cyclin-dependent kinases that have been implicated in the transition from G1 to S, and in maintaining S phase progression, i.e., Cdk1–cyclin A and Cdk2 complexed with cyclin E and/or cyclin A (Strausfeld et al., 1996; also Reynolds, K.D., P.K. Jackson, and T. Stearns, 1996. Mol. Biol Cell. 7:562a).
Cell Cycle Stage Dependence of Centrosome Reproduction
The ability of centrosomes to reproduce is dependent upon the stage of the cell cycle. S phase, when sufficiently prolonged by aphidicolin, permits multiple rounds of complete centrosome reproduction, albeit with a periodicity that is significantly longer than the entire normal cell cycle. In this respect, sea urchin zygotes show the same functional properties as somatic cells. Recently, Balczon et al. (1995) demonstrated repeated centrosome reproduction in CHO cells arrested in S phase with hydroxyurea. Also in the course of our study we were interested to discover the reason why centrosomes repeatedly reproduce when protein synthesis is completely inhibited from the time of fertilization. By using refertilizing sperm as indicators of the state of the cytoplasm, we found that such zygotes were arrested in S phase.
In contrast, M phase does not support centrosome reproduction. When mitosis is prolonged by up to 8 h, the two spindle poles doubled to four at the normal time of telophase but do not increase in number thereafter. The fact that each of the four poles contains only a single centriole demonstrates that the centrosomes have split rather than truly reproduced (see Mazia et al., 1960; Sluder and Begg, 1985; Sluder and Rieder, 1985). Our finding that repeated centrosome reproduction occurs under conditions of high Cdk1-B activity during S phase arrest indicates that the lack of centrosome reproduction during mitosis is not the consequence of high Cdk1-B activity per se.
Even though cyclin B Δ-90 maintains high Cdk1-B activity in the cell, it does not prevent the proteolysis of specific substrates at the metaphase–anaphase transition, such as endogenous cyclin B and the proteins that link chromatids (this report; Glotzer et al., 1991; Holloway et al., 1993; Wheatley et al., 1997). Our finding that centrosomes do not reproduce after this transition, given that the cell remains in mitosis, suggests that proteolysis at the metaphase–anaphase transition is not the limiting event that determines when centrosome reproduction begins. However, our data do not rule out the possibility that proteolysis at some other point in the cell cycle is necessary for centrosome reproduction (see Biggins et al., 1996; McDonald and Byers, 1997).
Centrosome Reproduction “Begins” in S Phase
Zygotes treated with translation inhibitors in first prophase complete first mitosis, and, as expected, arrest before second nuclear envelope breakdown. Importantly, we demonstrated that the two blastomeres are always arrested at a point before the onset of S phase (perhaps a G1 equivalent). For blastomeres arrested at this point in the cell cycle, we found that the aster inherited by each cell at the end of mitosis doubles only once even in zygotes followed for 8 h. Same cell correlative light and serial section ultrastructural analysis of these blastomeres revealed that each daughter aster contained the normal complement of two centrioles. Together, these results indicate that the phase of the cell cycle before entry into S (G1 equivalent) will support the morphological aspects of centrosome reproduction, such as the assembly of daughter centrioles and the splitting/separation of daughter centrosomes. However, the fact that the daughter centrosomes do not reproduce again under such supportive conditions indicates that they are lacking a component required for reproduction. This coupled with our observation that centrosomes will repeatedly reproduce in second division blastomeres arrested in S phase suggests the existence of a critical event in S phase that restores this component to the daughter centrosomes, thereby preparing them to reproduce in the next cell cycle. Although we have no information on the nature of this event, possibilities include a “licensing” of the centrosome for reproduction through the posttranslational modifications of key centrosome components, or the assembly of essential precursor structures needed for the next round of centrosome duplication. This licensing event does not appear to correlate with the acquisition of the protein cennexin, which is first detected on the daughter centrioles at the boundary between G2 and M (Lange and Gull, 1995). Regardless of the mechanism, our results suggest that centrosome reproduction actually begins before the prior round of centrosome maturation is complete.
The expression of the morphological events of centrosome reproduction during G1 is not peculiar to zygotes. Rattner and Phillips (1973) reported procentriole formation as early as 4 h after mitosis in L929 cells, a point well before the onset of S phase at 6–7 h after mitosis. Although the appearance of procentrioles is said to occur at the G1/S boundary, we submit that it is not certain that entry into S phase is essential for the formation of daughter centrioles in cultured cells. It is understandably difficult to precisely determine the timing of the G1/S boundary for cells prepared for ultrastructural analysis from synchronized cultures. In addition, by the time procentrioles have elongated to the point that they are visible in the electron microscope, the assembly process must be well underway.
Control of Centrosome Reproduction and the Cell Cycle
How is centrosome reproduction coordinated with nuclear events during the cell cycle? We propose that the centrosome inherited by a cell in telophase is competent to reproduce (i.e., “licensed”) and that the coordination of centrosome reproduction with the nuclear cycle is achieved by a G1 event that triggers the expression of centrosome reproduction, seen as diplosome disorientation and procentriole assembly. In addition, the centrosome cycle is keyed to the nuclear cycle by an S phase event that is necessary for the daughter centrosomes to acquire reproductive competence and be able to duplicate during the following G1. Thus, the perceived independence of centrosome reproduction from the nuclear events of the cell cycle suggested by a number of previous studies (Sluder and Lewis, 1987; Raff and Glover, 1988; Gard et al., 1990; Sluder et al., 1990; Balczon et al., 1995) is simply due to greatly prolonging the cytoplasmic conditions of S phase, which allows both the expression of centrosome reproduction and the reacquisition of reproductive capacity.
Given that S phase supports repeated centrosome reproduction, why does the centrosome normally reproduce only once during each cell cycle? A possible answer comes from observations that additional rounds of centrosome reproduction are slow during prolonged S phase. The time between rounds of centrosome reproduction during aphidicolin-prolonged S phase is on average three times longer than the normal duration of the entire zygotic cell cycle. Thus, the zygote normally completes S phase well before the centrosomes would reproduce a second time. For CHO cells arrested with hydroxyurea, the period of centrosome doubling appears to be longer than the entire normal cell cycle (Balczon et al., 1995). Therefore, when the cell cycle proceeds at a normal rate, progress into late G1 and entry into S are rate limiting for centrosome reproduction, and the essential coordination between centrosomal and nuclear events is assured.
Acknowledgments
We are grateful to Drs. Laura Hake and Joel Richter (Worcester Foundation) for their assistance in teaching us how to make mRNA and to Ms. Elizabeth Thompson (University of Massachusetts Medical School) for her invaluable help with the histone H1 kinase assays. The cyclin B constructs and purified histone H1 protein were generously provided by Dr. Michael Glotzer (EMBL, Heidelberg, Germany), and Dr. James Maller (University of Colorado, Denver, CO), respectively. We acknowledge the contributions of Mr. Richard Cole (Wadsworth Center) and Mr. Frederick Miller (University of Massachusetts Medical Center) in working through the trials and tribulations of the electron microscopy and Dr. Sally Wheatley (Worcester Foundation and University of Edinburgh) for critically reading this manuscript. Finally, we would like to thank Dr. Don Cleveland (University of California at San Diego, La Jolla, CA) for providing constructive comments on our study.
This work was supported by National Institutes of Health (NIH) GM 30758 to G. Sluder, NIH GM 40198 to C.L. Rieder, and NIH NCRR-01219, awarded by the Department of Health and Human Services/Public Heath Service, which supports the Wadsworth Center Biological Microscopy and Image Reconstruction Facility as a National Biotechnology Resource. E.H. Hinchcliffe is supported by a Cell Biology of Development Training Grant (NIH HD07312).
Abbreviations used in this paper
- BrdU
5-bromo-2′-deoxyuridine
- Cdk1-B
cyclin-dependent kinase 1–cyclin B
- CFSW
Ca2+-free sea water
- NEB
nuclear envelope breakdown
- NSW
natural sea water
Footnotes
Address all correspondence to Dr. Greenfield Sluder, Department of Cell Biology, University of Massachusetts Medical Center, Shrewsbury Campus, 222 Maple Avenue, Shrewsbury, MA 01545. Tel.: (508) 842-8921, ext. 341. Fax: (508) 842-3915. E-mail: sluder@sci.wfbr.edu
References
- Amon A, Surana U, Muroff I, Nasmyth K. Regulation of p34cdc2 tyrosine phosphorylation is not required for entry into mitosis in S. cerevisiae. . Nature. 1992;355:368–371. doi: 10.1038/355368a0. [DOI] [PubMed] [Google Scholar]
- Arion D, Meijer L, Brizuela L, Beach D. Cdc2 is a component of the M phase-specific H1 kinase: evidence for identity with MPF. Cell. 1988;55:371–378. doi: 10.1016/0092-8674(88)90060-8. [DOI] [PubMed] [Google Scholar]
- Aubin J E, Osborn M, Weber K. Variations in the distribution and migration of centriole duplexes in mitotic PtK2cells studied by immunofluorescence microscopy. J Cell Sci. 1980;43:177–194. doi: 10.1242/jcs.43.1.177. [DOI] [PubMed] [Google Scholar]
- Balczon R, Bao L, Zimmer WE, Brown K, Zinkowski RP, Brinkley BR. Dissociation of centrosome replication events from cycles of DNA synthesis and mitotic division in hydroxyurea-arrested Chinese hamster ovary cells. J Cell Biol. 1995;130:105–115. doi: 10.1083/jcb.130.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggins S, Ivanovska I, Rose MD. Yeast ubiquitin-like genes are involved in duplication of the microtubule organizing center. J Cell Biol. 1996;133:1331–1346. doi: 10.1083/jcb.133.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawood AH, Savage JR. A comparison of the use of bromodeoxyuridine and [3H] thymidine in studies of the cell cycle. Cell Tissue Kinet. 1983;16:51–57. [PubMed] [Google Scholar]
- Dahnmann C, Diffley JFX, Nasmyth KA. S-phase-promoting cyclin-dependent kinases prevent re-replication by inhibiting the transition of replication origins to a pre-replicative state. Curr Biol. 1995;5:1257–1269. doi: 10.1016/s0960-9822(95)00252-1. [DOI] [PubMed] [Google Scholar]
- Epel D. Activation of Na2+-dependent amino acid transport system upon fertilization of sea urchin eggs. Exp Cell Res. 1972;72:74–89. doi: 10.1016/0014-4827(72)90569-1. [DOI] [PubMed] [Google Scholar]
- Fuseler JF. Repetitive procurement of mature gametes from individual sea stars and sea urchins. J Cell Biol. 1973;57:879–881. doi: 10.1083/jcb.57.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gard DL, Hafezi S, Zhang T, Doxsey SJ. Centrosome duplication continues in cycloheximide-treated Xenopusblastulae in the absence of a detectable cell cycle. J Cell Biol. 1990;110:2033–2042. doi: 10.1083/jcb.110.6.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier J, Minshull J, Lohka M, Glotzer M, Hunt T, Maller JL. Cyclin is a component of maturation-promoting factor from Xenopus. Cell. 1990;60:487–494. doi: 10.1016/0092-8674(90)90599-a. [DOI] [PubMed] [Google Scholar]
- Geneviere-Garrigues AM, Barakat A, Doree M, Moreau JL, Picard A. Active cyclin B-cdc2 kinase does not inhibit DNA replication and cannot drive prematurely fertilized sea urchin eggs into mitosis. J Cell Sci. 1995;108:2693–2703. doi: 10.1242/jcs.108.7.2693. [DOI] [PubMed] [Google Scholar]
- Glotzer M, Murray AW, Kirschner MW. Cyclin in degraded by the ubiquitin pathway. Nature. 1991;349:132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- Gunduz N. The use of FITC-conjugated monoclonal antibodies for the determination of S-phase cells with fluorescence microscopy. Cytometry. 1985;6:597–601. doi: 10.1002/cyto.990060615. [DOI] [PubMed] [Google Scholar]
- Harris P. Some structural and functional aspects of the mitotic apparatus in sea urchin embryos. J Cell Biol. 1962;14:475–487. doi: 10.1083/jcb.14.3.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayles J, Fisher D, Woollard A, Nurse P. Temporal order of S phase and mitosis in fission yeast is determined by the state of the p34cdc2-mitotic cyclin complex. Cell. 1994;78:813–822. doi: 10.1016/s0092-8674(94)90542-8. [DOI] [PubMed] [Google Scholar]
- Heald R, McLoughlin M, McKeon F. Human wee1 maintains mitotic timing by protecting the nucleus from cytoplasmically activated Cdc2 kinase. Cell. 1993;74:463–474. doi: 10.1016/0092-8674(93)80048-j. [DOI] [PubMed] [Google Scholar]
- Hinegardner RT, Rao B, Feldman DE. The DNA synthetic period during early development of the sea urchin egg. Exp Cell Res. 1964;36:53–61. doi: 10.1016/0014-4827(64)90159-4. [DOI] [PubMed] [Google Scholar]
- Holloway S, Glotzer M, King RW, Murray AW. Anaphase is initiated by proteolysis rather than by the inactivation of maturation-promoting factor. Cell. 1993;73:1393–1402. doi: 10.1016/0092-8674(93)90364-v. [DOI] [PubMed] [Google Scholar]
- Ikegami S, Amemiya S, Oguro M, Nagano H, Mano Y. Aphidicolin prevents mitotic cell division by interfering with the activity of DNA polymerase-α. Nature. 1978;275:458–460. doi: 10.1038/275458a0. [DOI] [PubMed] [Google Scholar]
- Ikegami S, Amemiya S, Oguro M, Nagano H, Mano Y. Inhibition by aphidicolin of cell cycle progression and DNA replication in sea urchin embryos. J Cell Physiol. 1979;100:439–444. doi: 10.1002/jcp.1041000307. [DOI] [PubMed] [Google Scholar]
- Kiehart DP. Microinjection of echinoderm eggs: apparatus and procedures. Methods Cell Biol. 1982;25:13–31. doi: 10.1016/s0091-679x(08)61418-1. [DOI] [PubMed] [Google Scholar]
- King RW, Deshaies RJ, Peters J-M, Kirschner MW. How proteolysis drives the cell cycle. Science. 1996;274:1652–1659. doi: 10.1126/science.274.5293.1652. [DOI] [PubMed] [Google Scholar]
- Kochanski RS, Borisy GG. Mode of centriole duplication and distribution. J Cell Biol. 1990;110:1599–1605. doi: 10.1083/jcb.110.5.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama R, Borisy GG. Centriole cycle in Chinese hamster ovary cells as determined by whole-mount electron microscopy. J Cell Biol. 1981;91:814–821. doi: 10.1083/jcb.91.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labbe J-C, Capony J-P, Caput D, Cavadore J-C, Derancourt M, Kaghdad M, Lelias J-M, Picard A, Doree M. MPF from starfish oocytes at first meiotic metaphase is a heterodimer containing one molecule of cdc2 and one molecule of cyclin B. EMBO (Eur Mol Biol Organ) J. 1989;8:3053–3058. doi: 10.1002/j.1460-2075.1989.tb08456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb NJC, Fernandez A, Watrin A, Labbe J-C, Cavadore J-C. Microinjection of p34cdc2kinase induces marked changes in cell shape, cytoskeletal organization, and chromatin structure in mammalian fibroblasts. Cell. 1990;60:151–165. doi: 10.1016/0092-8674(90)90725-t. [DOI] [PubMed] [Google Scholar]
- Lange BMH, Gull K. A molecular marker for centriole maturation in the mammalian cell cycle. J Cell Biol. 1995;130:919–927. doi: 10.1083/jcb.130.4.919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorch IJ. Enucleation of sea urchin blastomeres with or without removal of asters. Q J Microsc Sci. 1952;93:475–486. [Google Scholar]
- Luca FC, Shibuya EK, Dohrmann CE, Ruderman JV. Both cyclin AΔ60 and BΔ97 are stable and arrest cells in M-phase, but only cyclin BΔ97 turns on cyclin destruction. EMBO (Eur Mol Biol Organ) J. 1991;10:4311–4320. doi: 10.1002/j.1460-2075.1991.tb05009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazia D, Harris P, Bibring T. The multiplicity of the mitotic centers and the time-course of their duplication and separation. Biophys Biochem Cytol. 1960;7:1–20. doi: 10.1083/jcb.7.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahbubani HM, Chong JPJ, Chevalier S, Thommes P, Blow JJ. Cell cycle regulation of the replication licensing system: involvement of a Cdk-dependent inhibitor. J Cell Biol. 1997;136:125–135. doi: 10.1083/jcb.136.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell HB, Byers B. A proteosome cap subunit required for spindle pole body duplication in yeast. J Cell Biol. 1997;137:539–553. doi: 10.1083/jcb.137.3.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray AW. Cyclin ubiquitination: the destructive end of mitosis. Cell. 1995;81:149–152. doi: 10.1016/0092-8674(95)90322-4. [DOI] [PubMed] [Google Scholar]
- Murray AW, Kirschner MW. Cyclin synthesis drives the early embryonic cell cycle. Nature. 1989;339:275–280. doi: 10.1038/339275a0. [DOI] [PubMed] [Google Scholar]
- Murray AW, Solomon MJ, Kirschner MW. The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature. 1989;339:280–286. doi: 10.1038/339280a0. [DOI] [PubMed] [Google Scholar]
- Raff JW, Glover DM. Nuclear and cytoplasmic mitotic cycles continue in Drosophila embryos in which DNA synthesis is inhibited by aphidicolin. J Cell Biol. 1988;107:2009–2019. doi: 10.1083/jcb.107.6.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rattner JB, Phillips SG. Independence of centriole formation and DNA synthesis. J Cell Biol. 1973;57:358–372. doi: 10.1083/jcb.57.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rempel RE, Sleight SB, Maller JL. Maternal Xenopus Cdk2- cyclin E complexs function during meiotic and early embryonic cell cycles that lack a G1phase. J Biol Chem. 1995;270:6843–6855. doi: 10.1074/jbc.270.12.6843. [DOI] [PubMed] [Google Scholar]
- Rieder CL, Rupp G, Bowser S. Electron microscopy of semi-thick sections: advantages for biomedical research. J Electron Microsc. 1985;2:11–28. [Google Scholar]
- Robbins EL, Jentzsch G, Micali A. The centriole cycle in synchronized HeLa cells. J Cell Biol. 1968;36:329–339. doi: 10.1083/jcb.36.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterwhite LL, Lohka MJ, Wilson KL, Cisek TY, Corden LJ, Pollard TD. Phosphorylation of myosin-II regulatory light chain by cyclin-p34cdc2. A mechanism for the timing of cytokinesis. J Cell Biol. 1992;118:595–605. doi: 10.1083/jcb.118.3.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluder G, Begg DA. Experimental analysis of the reproduction of spindle poles. J Cell Sci. 1985;76:35–51. doi: 10.1242/jcs.76.1.35. [DOI] [PubMed] [Google Scholar]
- Sluder G, Lewis K. Relationship between nuclear DNA synthesis and centrosome reproduction in sea urchin eggs. J Exp Zool. 1987;244:89–100. doi: 10.1002/jez.1402440111. [DOI] [PubMed] [Google Scholar]
- Sluder G, Rieder CL. Centriole number and the reproductive capacity of spindle poles. J Cell Biol. 1985;100:887–896. doi: 10.1083/jcb.100.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluder G, Miller FJ, Rieder CL. The reproduction of centrosomes: nuclear versus cytoplasmic controls. J Cell Biol. 1986;103:1873–1881. doi: 10.1083/jcb.103.5.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluder G, Miller FJ, Cole R, Rieder CL. Protein synthesis and the cell cycle: centrosome reproduction in sea urchin eggs is not under translational control. J Cell Biol. 1990;110:2025–2032. doi: 10.1083/jcb.110.6.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluder G, Miller FJ, Thompson EA, Wolf DE. Feedback control of the metaphase–anaphase transition in sea urchin zygotes: role of maloriented chromosomes. J Cell Biol. 1994;126:189–198. doi: 10.1083/jcb.126.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluder G, Thompson EA, Rieder CL, Miller FJ. Nuclear envelope breakdown is under nuclear not cytoplasmic control in sea urchin zygotes. J Cell Biol. 1995;129:1447–1458. doi: 10.1083/jcb.129.6.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorger PK, Murray AW. S-phase feedback control in budding yeast independent of tyrosine phosphorylation of p34cdc2 . Nature. 1992;35:365–368. doi: 10.1038/355365a0. [DOI] [PubMed] [Google Scholar]
- Strausfeld UP, Howell M, Descombes P, Chevalier S, Rempel R, Adamczewski J, Maller JL, Hunt T, Blow JJ. Both cyclin A and cyclin E have S-phase promoting (SPF) activity in Xenopusegg extracts. J Cell Sci. 1996;109:1555–1563. doi: 10.1242/jcs.109.6.1555. [DOI] [PubMed] [Google Scholar]
- Suprynowicz FA, Prusmack C, Whalley T. Ca2+triggers premature inactivation of the cdc2 protein kinase in permeabilized sea urchin embryos. Proc Natl Acad Sci USA. 1994;91:6176–6180. doi: 10.1073/pnas.91.13.6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier F, Cyrklaff M, Karsenti E, Bornens M. Centrosomes competent for parthenogenesis in Xenopuseggs support procentriole budding in cell-free extracts. Proc Natl Acad Sci USA. 1991;88:9929–9933. doi: 10.1073/pnas.88.22.9929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenaar EB. The timing of synthesis of proteins required for mitosis in the cell cycle of the sea urchin embryo. Exp Cell Res. 1983;144:393–403. doi: 10.1016/0014-4827(83)90419-6. [DOI] [PubMed] [Google Scholar]
- Wagenaar, E.B., and D. Mazia. 1978. The effects of emetine on first cleavage division in the sea urchin Stronglyocentrotus purpuratus. In Cell Reproduction. E.R. Dirkson, D.M. Prescott, and C.F. Fox, editors. Academic Press, New York. 539–545.
- Wheatley, D.N. 1982. The Centriole: A Central Enigma of Cell Biology. Elsevier Biomedical Press, Amsterdam. 232 pp.
- Wheatley SP, Hinchcliffe EH, Glotzer M, Hyman AA, Sluder G, Wang Y-L. CDK 1 inactivation regulates anaphase spindle dynamics and cytokinesis in vivo. J Cell Biol. 1997;138:385–393. doi: 10.1083/jcb.138.2.385. [DOI] [PMC free article] [PubMed] [Google Scholar]