Abstract
Regulation of the actin cytoskeleton may play a crucial role in cell motility and cancer invasion. We have produced a monoclonal antibody (NCC- Lu-632, IgM, k) reactive with an antigenic protein that is upregulated upon enhanced cell movement. The cDNA for the antigen molecule was found to encode a novel isoform of nonmuscle α-actinin. This isoform (designated actinin-4) was concentrated in the cytoplasm where cells were sharply extended and in cells migrating and located at the edge of cell clusters, but was absent from focal adhesion plaques or adherens junctions, where the classic isoform (actinin-1) was concentrated. Actinin-4 shifted steadily from the cytoplasm to the nucleus upon inhibition of phosphatidylinositol 3 kinase or actin depolymerization. The cytoplasmic localization of actinin-4 was closely associated with an infiltrative histological phenotype and correlated significantly with a poorer prognosis in 61 cases of breast cancer. These findings suggest that cytoplasmic actinin-4 regulates the actin cytoskeleton and increases cellular motility and that its inactivation by transfer to the nucleus abolishes the metastatic potential of human cancers.
Even after the successful removal of a primary cancer by surgery, recurrences often develop at distant sites. Cancer metastasis is a complicated process involving local invasion by cancer cells, infiltration into vascular and lymphatic vessels, survival in the circulation, extravasation, and growth at secondary sites. In this sequence of events, local invasion may play an important role as the initial step in cancer metastasis (Filder et al., 1978; Nicolson, 1988; Zetter, 1990; Aznavoorian et al., 1993). The mechanisms by which cancer invasion occurs are poorly understood, but one of the requirements is enhancement of cell motility. In fact, enhanced movement of cancer cells has been reported to be correlated with metastatic potential in animal models and with a poor prognosis in human cancers (Hosaka et al., 1978; Haemmerli and Strauli, 1981; Partin et al., 1989).
Although cell motility is regulated by several cellular and extracellular factors, the bundling and subcellular distribution of the actin cytoskeleton directly determines cell motility (Stossel, 1993; Lauffenburger and Horwitz, 1996; Cramer et al., 1997). α-Actinin is thought to cross-link actin filaments and connect the actin cytoskeleton to the cell membrane. So far, three isoforms of human α-actinin have been identified: nonmuscle actinin-1, muscle actinin-2, and muscle actinin-3 (Millake et al., 1989; Youssoufian et al., 1990; Beggs et al., 1992). Nonmuscle actinin-1 has been reported to associate with cell adhesion molecules, such as integrin β1 and α-catenin, and is believed to play an important role in stabilizing cell adhesion and regulating cell shape and cell motility (Otey et al., 1990, 1993; Glück et al., 1993; Glück and Ben-Ze'ev, 1994; Knudsen et al., 1995).
In this study, we looked for a new molecular marker that is associated with cell motility and cancer invasion and obtained an mAb. cDNA for the antigen was found to encode a novel isoform of nonmuscle actinin. Notably, this actinin isoform was found to be translocated into the nucleus in a certain population of human cancer cells and in several cell lines. We report here that the subcellular localization of this newly identified actinin may indicate the enhanced motility of cancer cells and predict the metastatic potential of human cancer.
Materials and Methods
Cell Culture and Chemicals
The human giant cell-lung cancer cell line, Lu-65, was established previously in our laboratory (Yamada et al., 1985). Primary cultured human foreskin keratinocytes (HFK)1 were purchased from Seikagaku Co. (Tokyo, Japan) and were cultured as instructed by the supplier. Primary normal human uterine endometrial fibroblasts were isolated and cultured as described previously (Zhang et al., 1995). PC-10, a human squamous lung cancer cell line, was provided by Dr. Y. Hayata (Tokyo Medical College, Tokyo, Japan). A-431 is a human vulvar epidermoid cancer cell line (Giard et al., 1973), and KU7 is a urinary bladder cancer cell line. TE 4, TE 6, TE 7, and TE 10 were derived from esophageal cancers (Nishihira et al., 1993), and MCF7 and R27 were derived from breast cancers (Westley, 1979; Cavailles et al., 1988). The oral floor cancer cell line IMC2, normal embryonic lung fibroblast MRC5, and colon cancer cell line WiDr were obtained from Riken Cell Bank (Tsukuba, Japan). Colon cancer cell line SW480 was obtained from the American Type Culture Collection (Rockville, MD).
HFK were treated with 50 ng/ml wortmannin (Sigma Chemical Co., St. Louis, MO) for 15 min and then washed. After incubation for 24 h, the cells were examined by immunofluorescence microscopy as described below. For actin depolymerization, the cells were treated with 2 μg/ml cytochalasin D (Sigma Chemical Co.) for 2 h.
Production of Antibodies
BALB/c mice were immunized with a lysate of Lu-65 cells, and hybridomas were produced as described previously (Hirohashi et al., 1984). The hybridoma supernatants were then screened immunohistochemically for reactivity with acetone-fixed paraffin-embedded human tissues, as described below.
Rabbit polyclonal anti–actinin-4 antibody was raised against a KLH-conjugated synthetic peptide, MGDYMAQEDDWC (containing amino acids 1–11, Fig. 1), and affinity-purified.
Figure 1.

Deduced amino acid sequence of actinin-4 (upper) in comparison with actinin-1 (lower). The nucleotide sequence of actinin-4 is available from GenBank/EMBL/DDBJ under accession number D89980. Asterisks represent identical amino acids and dots represent conserved amino acids. The sequences conserved among α-actinin isoforms are underlined: actin-binding domain (amino acids 111–125, single underlining), PIP2-binding domain (150–165, double underlining), and EF-hand calcium regulation domains (742–770 and 783–811, lined boxes).
cDNA Cloning and DNA Sequencing
A lambda gt11 cDNA expression library of primary cultured HFK (CLONTECH Laboratories, Inc., Palo Alto, CA) was immunoscreened with mAb NCC-Lu-632, as described previously (Young and Davis, 1983), and a positive 950-bp cDNA clone (ck-20-2) was isolated. For isolation of full-length cDNA of actinin-4 and -1, a cDNA library of mesothelioma MS-1/CDDP cells was screened with synthetic oligonucleotides specific to each actinin. A 3.5-kb actinin-4 cDNA clone, near full-length as determined by Northern blot analysis (Fig. 2 B), was isolated, and both strands were sequenced using an autosequencer (model ABI 377; Perkin Elmer, Foster City, CA).
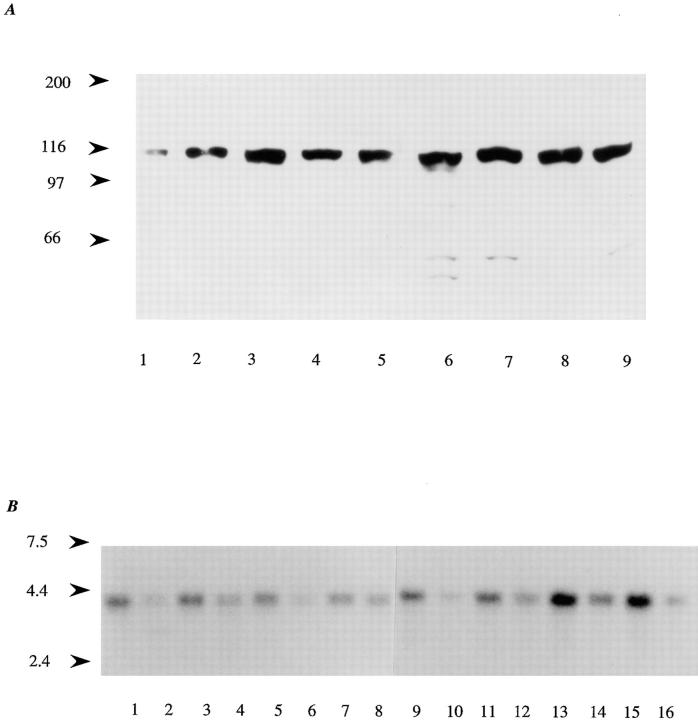
Figure 2.
Detection of actinin-4 protein and mRNA. (A) Immunoblot analysis of actinin-4 in HFK and various human cancer cell lines with mAb NCC-Lu-632. Actinin-4 protein was detected in cell lysates from HFK (lane 1), lung cancer cell lines Lu-65 (lane 2) and PC-10 (lane 3), vulvar cancer cell line A-431 (lane 4), and esophageal cancer cell lines TE 4 (lane 5), TE 6 (lane 6), TE 7 (lane 7), TE 10 (lane 8), and TE 11 (lane 9). Molecular masses (in kD) are shown on the left. (B) Expression of actinin-4 mRNA in normal human tissues. Human multiple tissue Northern blots I and II (CLONTECH Laboratories) were hybridized with an actinin-4–specific oligonucleotide. Each lane contains 2 μg of poly(A)+ RNA of human adult tissues: heart (lane 1), brain (lane 2), placenta (lane 3), lung (lane 4), liver (lane 5), skeletal muscle (lane 6), kidney (lane 7), pancreas (lane 8), spleen (lane 9), thymus (lane 10), prostate (lane 11), testis (lane 12), ovary (lane 13), small intestine (lane 14), colon (lane 15), and peripheral blood leukocytes (lane 16). Molecular masses (in kb) are shown on the left side.
Northern Blot Analysis
Multiple tissue Northern blots I and II were purchased from CLONTECH Laboratories and hybridization was performed using a 32P-labeled oligonucleotide probe (5′-CGCTTCTGAGTTAGGGCCCCCA-3′) specific to actinin-4, as described previously (Sambrook et al., 1989). The quality and quantity of electrophoresed mRNA was determined by rehybridization of the same blot with a human β-actin cDNA probe.
Extraction of Cell Lysate and Western Blot Analysis
Cells were extracted with lysis buffer (10 mM Hepes, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1% NP-40, 1 mg/ml NaN3, 0.5 mM Na3Vo4, 10 nM β-glycerophosphate, 1 mM PMSF, 1 μg/ml leupeptin, 1 μg/ml pepstatin A) on ice for 30 min before centrifugation (12,000 g, 15 min). Cell lysates (50 μg protein) were separated by SDS-PAGE (Laemmli, 1970) and transferred to Hybond–polyvinyl difluoride membranes (Amersham International, Buckinghamshire, UK) (Towbin et al., 1979). After incubation with antibodies at 4°C overnight, the blots were detected using a horseradish peroxidase–conjugated anti–mouse IgG+IgM (IBL, Fujioka, Japan) or anti–rabbit Ig antibody and ECL Western blotting detection reagents (Amersham International), as instructed by the suppliers. For blotting of whole cell lysates, cells were lysed directly with Laemmli buffer (62.5 mM Tris-HCl, pH 6.8, 2% SDS, 5% glycerol, 715 mM 2-mercaptoethanol, 0.0025% bromophenol blue) (Harlow and Lane, 1988).
Glutathione S-Transferase Fusion Protein Production and In Vitro Translation
A cDNA fragment of actinin-4 (encoding amino acids 410–664) and the corresponding site of actinin-1 (amino acids 418–672) were amplified by PCR and subcloned into pGEX plasmids (Pharmacia Biotech, Uppsala, Sweden). Glutathione S-transferase (GST) fusion proteins expressed in Escherichia coli were affinity-purified on glutathione–Sepharose 4B (Pharmacia Biotech) as described previously (Shibata et al., 1996).
l-[35S]methionine (Amersham International) labeled polypeptides were synthesized from cDNA clones containing the entire coding regions for actinin-1 and -4, using the TNT reticulocyte lysate system (Promega Corp., Madison, WI) as instructed by the supplier. The synthesized polypeptides were analyzed by SDS-PAGE and autoradiography as described previously (Yamada et al., 1995).
Actin-binding Assay
Purified chicken gizzard actin (Sigma Chemical Co.) was coupled to CNBr-activated Sepharose–4B beads (Pharmacia Biotech) (Prendergast and Ziff, 1991). The beads were incubated with 35S-incorporated in vitro translation products in lysis buffer overnight at 4°C. After extensive washing with lysis buffer, the beads were boiled in Laemmli buffer and analyzed by SDS-PAGE and autoradiography (Yamada et al., 1995).
A cell lysate of the lung cancer cell line PC-10 was also incubated overnight at 4°C with the actin-coupled beads. After thorough washing, the beads were boiled in Laemmli buffer and analyzed by immunoblotting as described above.
Immunoprecipitation
In vitro translation products of actinins were incubated with a monoclonal antibody against chicken α-actinin (BM-75.2, IgM, k; Sigma Chemical Co.), anti–actinin-4 polyclonal antibody, normal mouse IgM, or normal rabbit IgG (Sigma Chemical Co.) overnight at 4°C and precipitated with anti–mouse IgM agarose (for mouse antibodies) (Sigma Chemical Co.) or protein G plus agarose (for rabbit antibodies) (Santa Cruz Biotechnology, Santa Cruz, CA). SDS-PAGE and autoradiography were carried out as described previously (Yamada et al., 1995).
Immunofluorescence Microscopy and “Wound” Assay
Cells were cultured on glass coverslips, fixed with 4% paraformaldehyde and 2% sucrose in PBS, and made permeable with 0.2% Triton X-100. After incubation with antibodies, actinins were detected with biotinylated anti–mouse IgM or biotinylated anti–rabbit IgG and Texas red–avidin D or FITC-conjugated avidin D (Vector Laboratories, Burlingame, CA). Actin filaments were visualized with FITC-phalloidin (Sigma Chemical Co.), and the cells were examined with a Zeiss LSM410 microscope (Thornwood, NY).
Cells were grown to confluency on glass coverslips, and a “wound” was then introduced in the monolayers with a plastic pipette tip (Glück et al., 1993; Glück and Ben-Ze'ev, 1994). After incubation for 24 h, the cells were fixed and examined as described above.
Immunohistochemistry
Acetone-fixed paraffin-embedded human tissues (Sato et al., 1986) were selected from the surgical pathology file of the National Cancer Center Hospital (Tokyo, Japan). 5-μm thin sections were stained with mAb NCC-Lu-632 using an immunoperoxidase avidin-biotin complex (ABC) kit (Vector Laboratories), as described previously (Sato et al., 1986). Histological subtyping of breast cancer was done by two independent certified pathologists, according to the criteria described previously (Rosen and Oberman, 1992).
Statistical Analysis
The postsurgical survival curves for a total of 61 patients with stages I and II breast cancer (Rosen and Oberman, 1992) were expressed as described by Kaplan and Meier (1958). Statistical significance was determined by the generalized Wilcoxon test.
Results
Molecular Cloning of Actinin-4 cDNA
mAb NCC-Lu-632 (IgM, k) was selected on the basis of its intriguing immunohistochemical reactivity, as described below. A cDNA clone encoding the antigen molecule recognized by the mAb was isolated by immunoscreening of the HFK lambda gt11 cDNA library.
The 3.5-kb cDNA clone contained a 2,652-bp open reading frame encoding 884 amino acids (Fig. 1), with a predicted molecular mass of 102 kD. The mAb NCC-Lu-632 reacted consistently with the ∼100-kD antigen molecule in immunoblot analyses (Fig. 2 A), and in vitro translation of the full-length cDNA yielded a major protein of ∼100 kD (Fig. 3 C). This coding region and the deduced amino acid sequence showed a high degree of similarity to human α-actinin-1 (Millake et al., 1989; Youssoufian et al., 1990) (80.0% nucleotide and 86.7% amino acid similarity). It contained structures conserved among α-actinin family members, including the actin-binding domain (Kuhlman et al., 1992) (amino acids 111–125; Fig. 1), pleckstrin-homology (PH) domain containing a phosphatidylinositol 4, 5-biphosphate (PIP2)-binding site (Fukami et al., 1996) (amino acids 150–165), and two EF-hand calcium regulation domains (Witke et al., 1993; Imamura et al., 1994) (amino acids 742–770 and 783–811). Following the nomenclature proposed by Beggs et al. (1992), this gene was termed actinin-4.
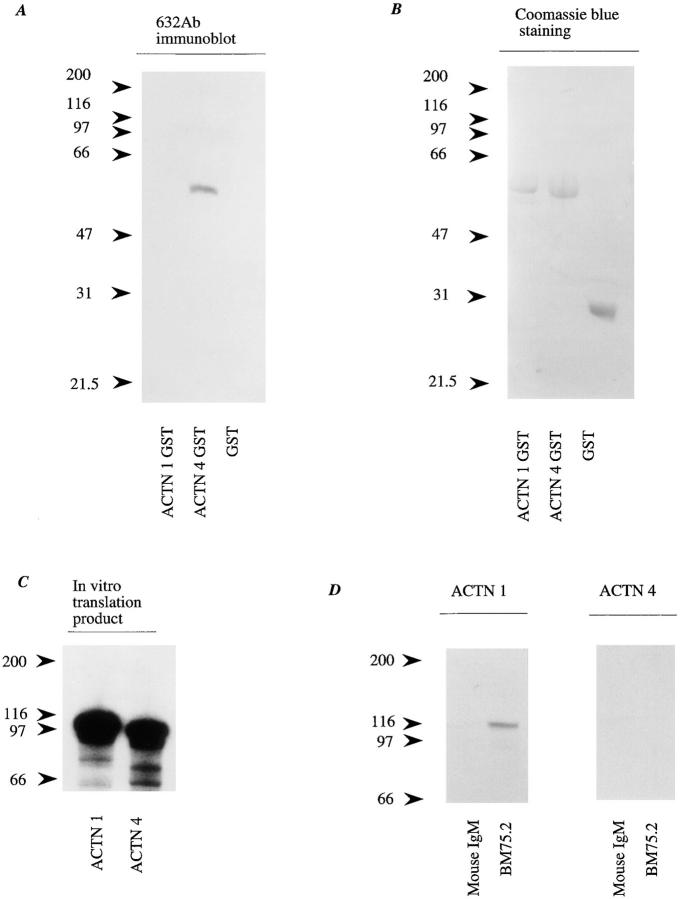
Figure 3.
Antibody specificity to actinin-1 and -4. (A) A polypeptide of actinin-4 (amino acids 410–664) and a corresponding site of actinin-1 (amino acids 418–672) were expressed as GST fusion proteins in E. coli. Immunoblot analysis revealed that NCC- Lu-632 mAb reacts only with the actinin-4 GST fusion protein (ACTN4 GST), and not with the actinin-1 GST fusion protein (ACTN1 GST) or GST alone (GST). Molecular masses (in kD) are shown on the left. (B) Coomassie blue staining of the blot corresponding to that in A, demonstrating proper protein loading in each lane. (C) In vitro translation products of actinin-1 and -4. SDS-PAGE and autoradiography reveal 35S-labeled actinin-1 and -4 proteins. Molecular masses (in kD) are shown on the left. (D) In vitro translation products of actinin-1 (ACTN 1) and actinin-4 (ACTN 4) were immunoprecipitated by anti–chicken actinin mAb BM-75.2 or normal mouse IgM. Actinin-1 but not actinin-4 was precipitated with BM-75.2. Molecular masses (in kD) are shown on the left.
Expression of Actinin-4 mRNA in Normal and Neoplastic Cells
Northern hybridization was carried out with an actinin- 4–specific 22-bp oligonucleotide probe to avoid cross- hybridization with actinin-1. As shown in Fig. 2 B, intense expression of 3.5-kb actinin-4 mRNA was detected in the ovary (Fig. 2 B, lane 13) and colon (lane 15), and faint expression was detected in all tissues examined. No gross structural alterations of actinin-4 mRNA were detected in any of the human carcinoma cell lines examined (data not shown).
Specificity of Antibodies
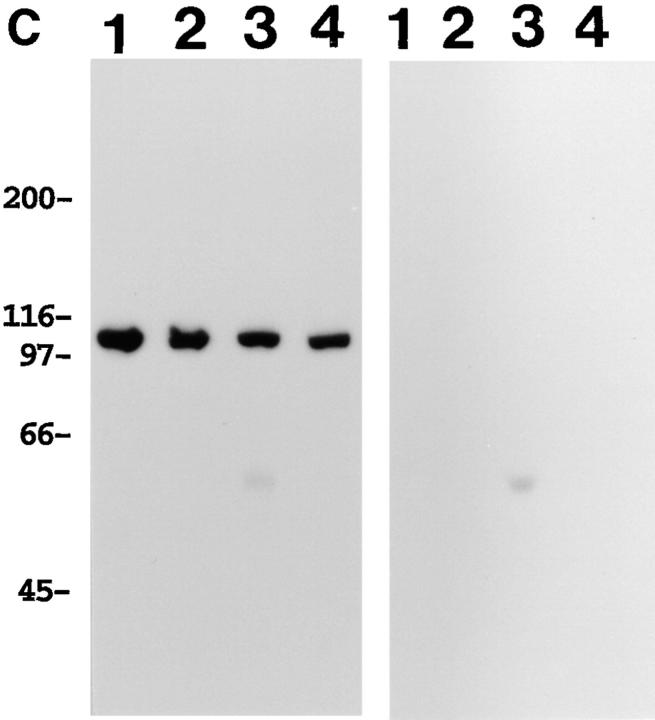
To determine the specific reactivity of mAb NCC-Lu-632 with actinin-4, amino acids 410–664 of actinin-4 and a corresponding site of actinin-1 (Millake et al., 1989) (amino acids 418–672) were expressed as a GST fusion protein in E. coli. Immunoblot analysis revealed that the mAb reacted only with actinin-4 GST fusion protein, and not with actinin-1 GST fusion protein or GST alone (Fig. 3 A), confirming the specificity of mAb NCC-Lu-632 for actinin-4.
Immunoprecipitation analyses revealed that an mAb against chicken actinin, BM-75.2, was reactive with the in vitro translation product of cDNA of actinin-1, but not with that of actinin-4 (Fig. 3 D).
Immunoblot analyses revealed that the polyclonal antibody raised against the NH2-terminal amino acids of actinin-4 reacted only with a single band of the same molecular weight as NCC-Lu-632 mAb (Fig. 4 A). Immunoprecipitation analyses revealed that this polyclonal antibody was reactive with the in vitro translation product of the cDNA of actinin-4, but not with that of actinin-1 (Fig. 4 B), thus confirming its specificity.
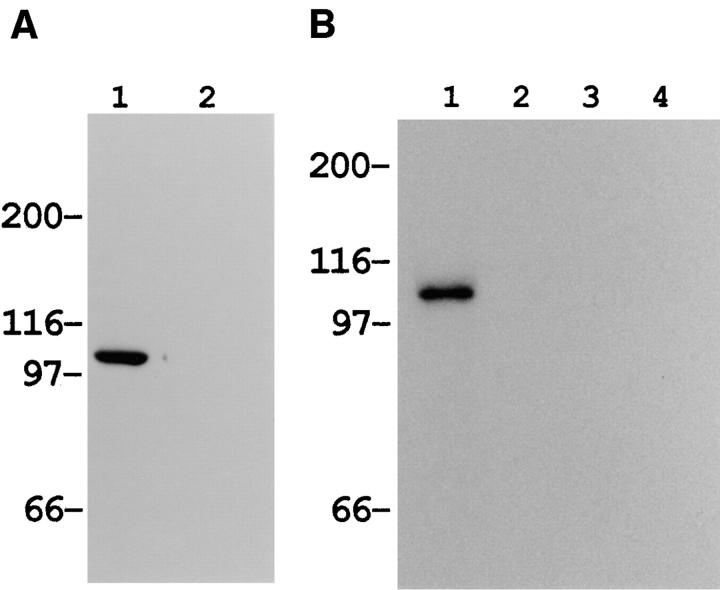
Figure 4.
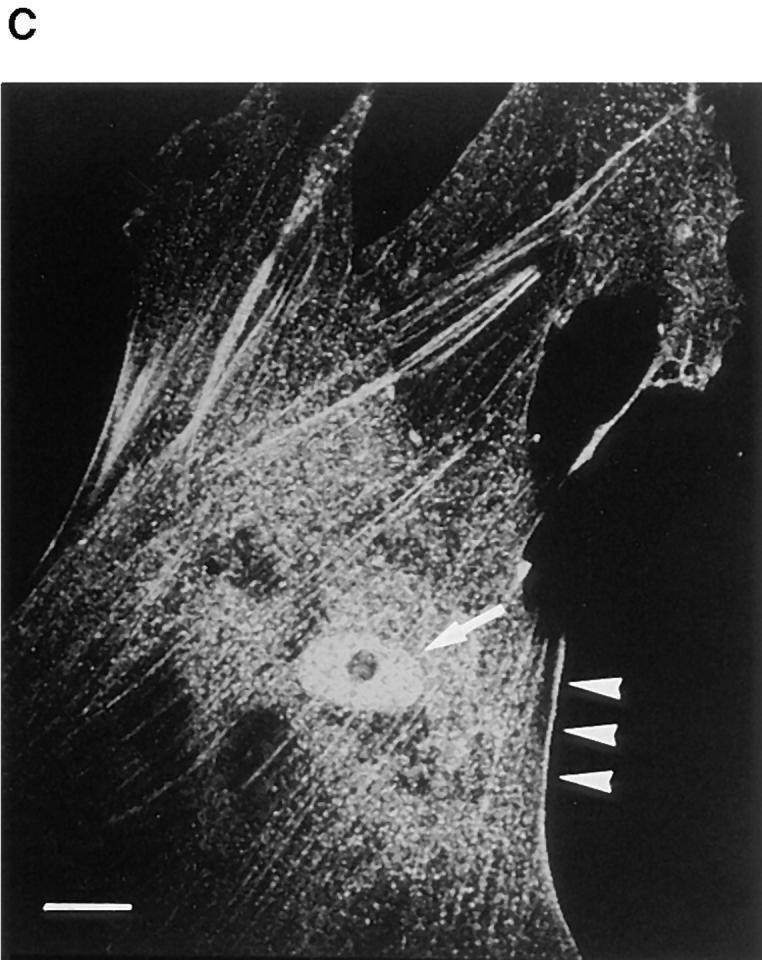
Reactivity of polyclonal antibody against actinin-4 peptide. (A) Immunoblot analysis reveals that the polyclonal antibody raised against actinin-4 peptide (lane 1) reacts with a single protein of ∼100 kD in the cell lysate of WiDr cells. A blot with normal rabbit IgG (lane 2) is included as a negative control. Molecular masses (in kD) are shown on the left. (B) In vitro translation products of actinin-4 (lanes 1 and 3) and actinin-1 (lanes 2 and 4) were immunoprecipitated by the anti–actinin-4 polyclonal antibody (lanes 1 and 2) or normal rabbit IgG (lanes 3 and 4). SDS-PAGE and autoradiography reveal that this polyclonal antibody is reactive with actinin-4 protein, but not with actinin-1. (C) Confocal immunofluorescence microscopy showing the subcellular localization of actinin-4 in lung fibroblasts MRC-5. Arrow indicates the nuclear staining, and arrowheads indicate linear staining of actinin-4 along actin stress fibers. Bar, 5 μm.
Actin-binding Activity In Vitro
From the deduced amino acid sequence (Fig. 1), actinin-4 was predicted to contain an actin-binding domain (Hemmings and Critchley, 1992; Kuhlman et al., 1992). To confirm this, in vitro binding experiments were performed using actin-coupled agarose beads (Prendergast and Ziff, 1991). 35S-labeled actinin-4 recombinant protein, produced by in vitro translation, was incubated overnight with agarose beads coupled to actin. After extensive washing, SDS-PAGE and autoradiography revealed labeled polypeptides whose size was consistent with the predicted size of actinin-4 (Fig. 5 A).
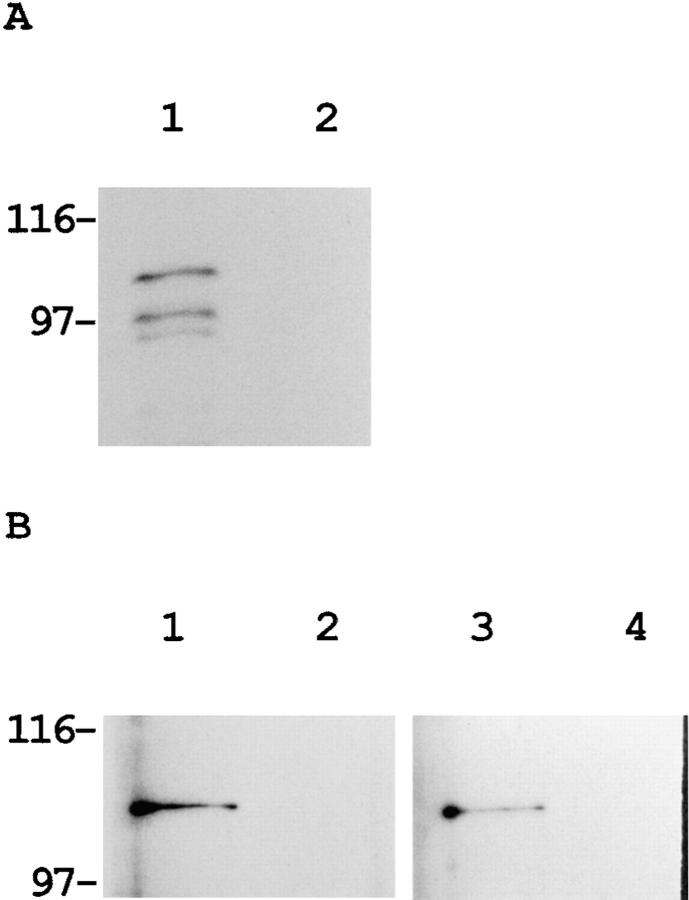
Figure 5.
Actin-binding activity of actinin-4. (A) Direct association of actinin-4 with actin. In vitro translation product of actinin-4 was incubated with chicken gizzard actin-conjugated Sepharose 4B beads (lane 1) or Sepharose 4B beads alone (lane 2). After extensive washing, SDS-PAGE and autoradiography revealed that 35S-labeled actinin-4 protein was retained only in actin-coupled beads (lane 1). Molecular masses (in kD) are shown on the left. (B) Cell lysate of human squamous cell carcinoma cell line (PC-10) was incubated with actin-conjugated (lanes 1 and 3) or control (lanes 2 and 4) Sepharose 4B beads. Bound proteins were analyzed by immunoblotting. Approximately 100-kD proteins of actinin-1 (lane 1) and actinin-4 (lane 3) were detected with monoclonal antibodies BM-75.2 and NCC-Lu-632, respectively. Molecular masses (in kD) are shown on the left.
Similarly, a cell lysate of the lung cancer cell line PC-10 was incubated overnight with actin-agarose beads and washed thoroughly. Immunoblot analysis with mAb NCC-Lu-632 revealed the retention of actinin-4 on actin-agarose beads (Fig. 5 B), confirming the actin-binding activity of actinin-4.
Distinct Subcellular Localization of Two Nonmuscle Actinins
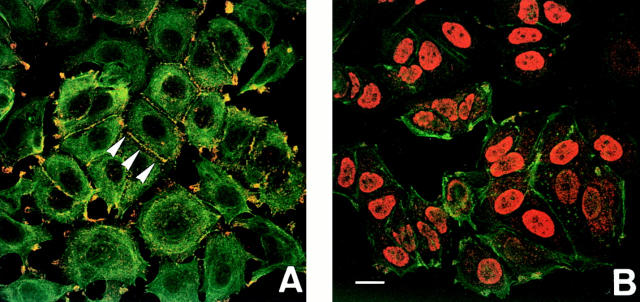
Confocal immunofluorescence microscopy revealed that actinin-1 was localized specifically at the ends of actin stress fibers (Fig. 6, A, C, and E) and adherens junctions (Fig. 7 A), as described previously (Lazarides, 1975; Wehland et al., 1979; Knudsen et al., 1995). In contrast to this cell membrane–associated localization of actinin-1, actinin-4 protein was colocalized with actin stress fibers and was dispersed in the cytoplasm and nucleus (Fig. 6, B, D, and F). The specific immunocytochemical reactivity of monoclonal antibody NCC-Lu-632 was further confirmed by the polyclonal antibody raised against the NH2-terminal amino acids of actinin-4 (Fig. 4 C). In most cancer cell lines including PC10, A431, SW480, TE4, TE6, TE7, TE10, and R27, actin stress fibers were poorly developed and actinin-4 was diffusely dispersed in the cytoplasm. Characteristically, actinin-4 was highly concentrated in the cytoplasm where the cells were sharply extended (Fig. 8, B, E, and H). Actinin-4 was stained intensely at the edges of cell clusters (Fig. 8, C, F, and I). The difference in subcellular localization between these two nonmuscle isoforms suggested that they were associated with different functions.
Figure 6.
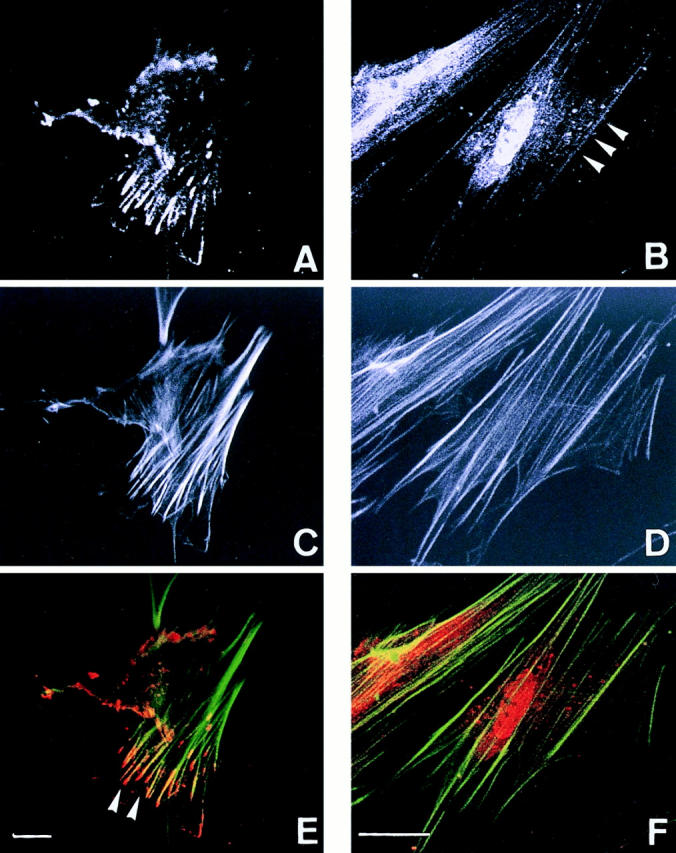
Confocal fluorescence microscopy showing actinins and the actin cytoskeleton in uterine endometrial fibroblasts. (A, C, and E) Double fluorescence of actinin-1 detected by BM-75.2 mAb (A and red in E) and actin by phalloidin-conjugated FITC (C and green in E) is shown. Actinin-1 is localized at the ends of actin stress fibers (E, arrowheads). (B, D, and F) Double fluorescence of actinin-4 detected by NCC-Lu-632 mAb (B and red in F) and actin by phalloidin-conjugated FITC (D and green in F) is shown. Actinin-4 is colocalized specifically with actin stress fibers (B, arrowheads). Bars, 5 μm.
Figure 7.
Nuclear localization of actinin-4. (A) Confocal fluorescence microscopy showing subcellular localization of actinin-1 and the actin cytoskeleton in breast cancer cell line MCF7. Double fluorescence of actinin-1 detected by BM-75.2 mAb (red) and actin by phalloidin-conjugated FITC (green) is shown. Actinin-1 is concentrated specifically at focal adhesions and adherens junctions (arrowheads) (yellow), but not in the nucleus. (B) Confocal fluorescence microscopy showing nuclear localization of actinin-4 and the actin cytoskeleton in breast cancer cell line MCF7. Double fluorescence of actinin-4 detected by NCC-Lu-632 mAb (red) and actin by phalloidin-conjugated FITC (green) is shown. Actinin-4 is separated from the cytoplasmic actin cytoskeleton and localized specifically in the nucleus. (C) Immunoblot analysis of whole cell lysates from oral floor cancer cell line IMC2 (lane 1), urinary bladder cancer cell line KU7 (lane 2), and breast cancer cell lines MCF7 (lane 3) and R27 (lane 4) by mAb NCC-Lu-632 (left) and normal mouse IgM (negative control, right). IMC2, KU7, and MCF7 are cell lines showing the nuclear localization of actinin-4, and R27 is a cell line showing cytoplasmic localization. Molecular masses (in kD) are shown on the left. Bar, 5 μm.
Figure 8.
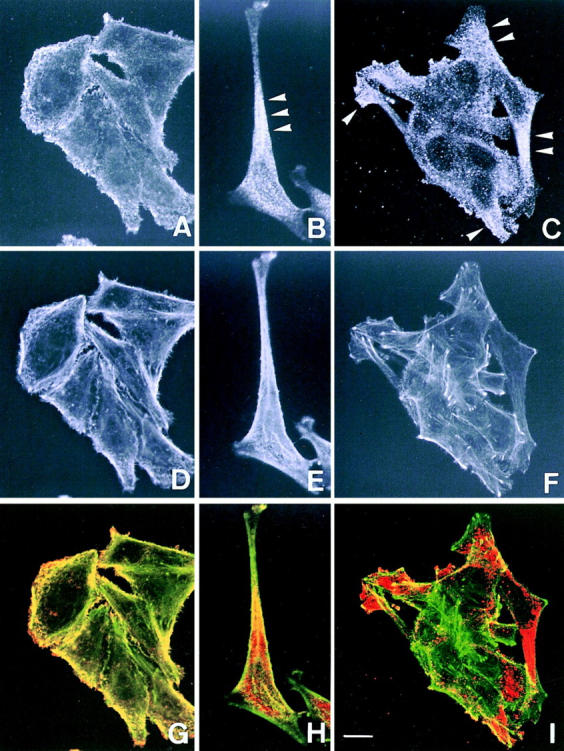
Confocal fluorescence microscopy showing the localization of actinins and the actin cytoskeleton in a colon cancer cell line SW480. (A, D, and G) Double fluorescence of actinin-1 detected by BM-75.2 mAb (A and red in G) and actin by phalloidin-conjugated FITC (D and green in G) is shown. Actinin-1 is colocalized with the cell membrane–associated cortical actin cytoskeleton. (B, E, and H) Double fluorescence of actinin-4 detected by NCC-Lu-632 mAb (B and red in H) and actin by phalloidin-conjugated FITC (E and green in H) is shown. Actinin-4 is concentrated in the sharply extended cytoplasm (B, arrowhead). (C, F, and I) Double fluorescence of actinin-4 detected by NCC-Lu-632 mAb (C and red in I) and actin by phalloidin-conjugated FITC (F and green in I) is shown. Cells at the edge of a cluster overexpress actinin-4 (C, arrowheads). Bar, 5 μm.
Upregulation of Actinin-4 upon Enhanced Cell Movement
From the above immunofluorescence observations, we speculated that the expression of actinin-4 was regulated dynamically by cell movement. An artificial linear defect was introduced in the confluent monolayers of A-431 and R27 cells, and we examined the cells forced to be motile by immunofluorescence microscopy. As shown in Fig. 9, actinin-4 was markedly induced in cells along the edges of the wound (Fig. 9 A) and in cells migrating into the wound (Fig. 9, B–D).
Figure 9.
Wound assay for the detection of actinin-4 in cells forced to be motile. An artificial linear defect was introduced in confluent monolayers of A-431 cells. The expression of actinin-4 is shown by immunofluorescence microscopy. The cells along the edges of the wound (A) and the cells migrating into the wound (B–D) overexpress actinin-4. D is a higher-power view of B. Bars, 10 μm.
Nuclear Translocation of Actinin-4
In a limited number of cell lines, including the breast cancer cell line MCF7, oral floor cancer IMC2, and bladder cancer KU7, it was noticed that actinin-4 was localized exclusively in the nucleus (Fig. 7 B), in contrast to the association of actinin-1 with the cell membrane in these cells (Fig. 7 A). The nuclear staining was not due to cross-reactivity of the mAb with other nuclear proteins because NCC-Lu-632 mAb reacted only with a protein of ∼100 kD in whole-cell lysates of these cell lines (Fig. 7 C).
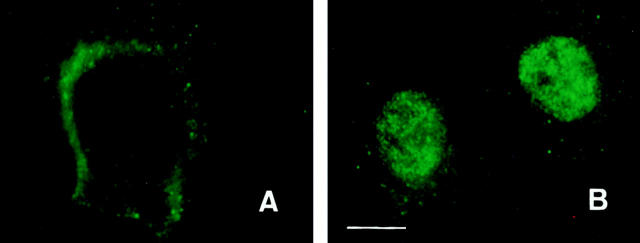
Actinin-4 possesses a conserved PIP2-binding site. PIP2 binds α-actinin through the pleckstrin homology domain and regulates its actin-binding activity (Fukami, 1992; Fukami et al., 1996). PIP2 is one of the substrates of phosphatidylinositol 3 kinase (PI3 kinase). By treating HFK with a PI3 kinase inhibitor, wortmannin (Vemuri, 1994), actinin-4 was steadily translocated into the nucleus (Fig. 10). A bladder cancer cell line, KU7, which is deficient in PI3 kinase expression, also showed nuclear localization of actinin-4 without treatment (data not shown). These findings suggest that inhibition of the PI3 kinase–mediated signaling pathway is involved in the nuclear translocation of actinin-4.
Figure 10.
Immunofluorescence microscopy showing translocation of actinin-4 protein from the cytoplasm to the nucleus. (A) In control untreated HFK, actinin-4 was found to exist in the cytoplasm. (B) After treatment of HFK with wortmannin, actinin-4 protein was translocated into the nucleus. Bar, 5 μm.
Cytochalasin D inhibits actin polymerization (Casella et al., 1981). Treatment with cytochalasin D also induced nuclear translocation (data not shown), suggesting that the nuclear translocation of actinin-4 is also caused by loss of its association with the cytoplasmic actin cytoskeleton.
Subcellular Distribution of Actinin-4 Predicts Poor Prognosis of Breast Cancer
The distribution of actinin-4 was determined immunohistochemically in human tissue using the mAb, NCC-Lu-632. Actinin-4 was expressed in a limited population of normal cells, including erythrocytes, endothelial cells, and epithelial cells in various tissues at their border with stromal connective tissue.
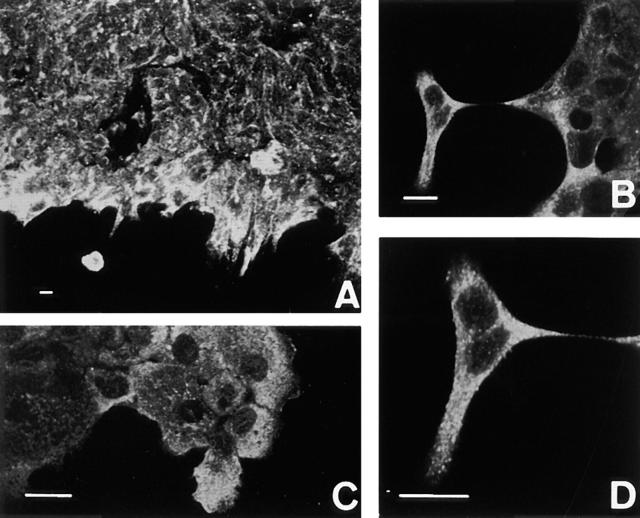
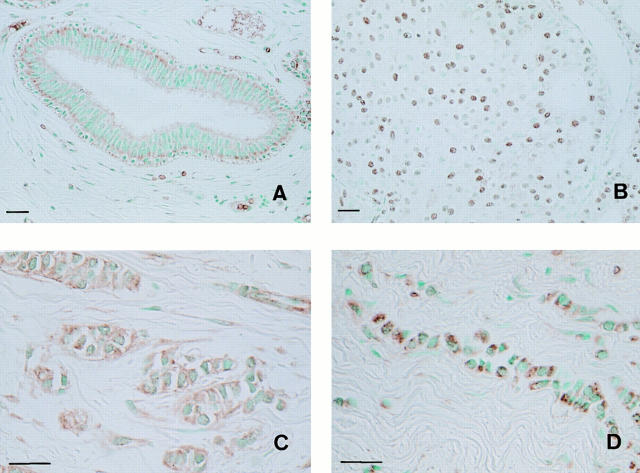
In the ducts of normal mammary glands, the epithelio– stromal and epithelio–myoepithelial borders were stained, but the nucleus of normal mammary gland cells did not stain (Fig. 11 A). Scirrhous carcinoma and invasive lobular carcinoma of the breast manifest highly infiltrative growth into the fibrous stroma, in contrast to low-infiltrative histological types including papillotubular and solid-tubular carcinomas (Rosen and Oberman, 1992). To determine the relationship between the growth pattern of breast cancer and the subcellular localization of actinin-4, 83 cases of invasive breast cancer were examined immunohistochemically. The reactivity of mAb NCC-Lu-632 was classified into three types (Table I): nuclear (type A, Fig. 11 B), combined (type B), and cytoplasmic (type C, Fig. 10, C and D). Histologically, all type A cases were papillotubular or solid-tubular carcinomas that showed low-infiltrative extension (20/20). In type B, the majority of cases were papillotubular and solid tubular carcinomas (25/33) and fewer were scirrhous carcinomas (8/33). In contrast to these two types, most type C cases were scirrhous (15/30) (Fig. 11 C) and invasive lobular carcinomas (6/30) (Fig. 11 D), both of which extended locally in a highly infiltrative manner.
Figure 11.
Immunohistochemical detection of actinin-4 in human tissues. Immunoperoxidase staining (ABC method) was performed on acetone-fixed paraffin-embedded (AMeX) (Sato et al., 1986) human tissues using NCC-Lu-632 mAb. (A) Normal mammary glands. Duct cells of normal mammary glands are stained at the epithelio–stromal and epithelio– myoepithelial borders, but the nuclei of normal mammary gland cells are not stained. (B) In papillotubular carcinoma of the breast, actinin-4 is localized in the nuclei (type A). (C) In scirrhous carcinoma of the breast, the cytoplasm of tumor cells is stained, but the nuclei of tumor cells are not (type C). (D) In invasive lobular carcinoma of the breast, the cytoplasm of the tumor cells is stained (type C). Bars, 30 μm.
Table I.
Correlation between the Subcellular Localization of Actinin-4 and Histological Subtypes of Breast Cancer
| Invasive ductal carcinoma | Invasive lobular carcinoma | |||||
|---|---|---|---|---|---|---|
| Papillotubular and solid tubular carcinoma | Scirrhous carcinoma | |||||
| A. Nuclear | 20 | 0 | 0 | |||
| B. Combined | 25 | 8 | 0 | |||
| C. Cytoplasmic | 9 | 15 | 6 | |||
| Total | 54 | 23 | 6 | |||
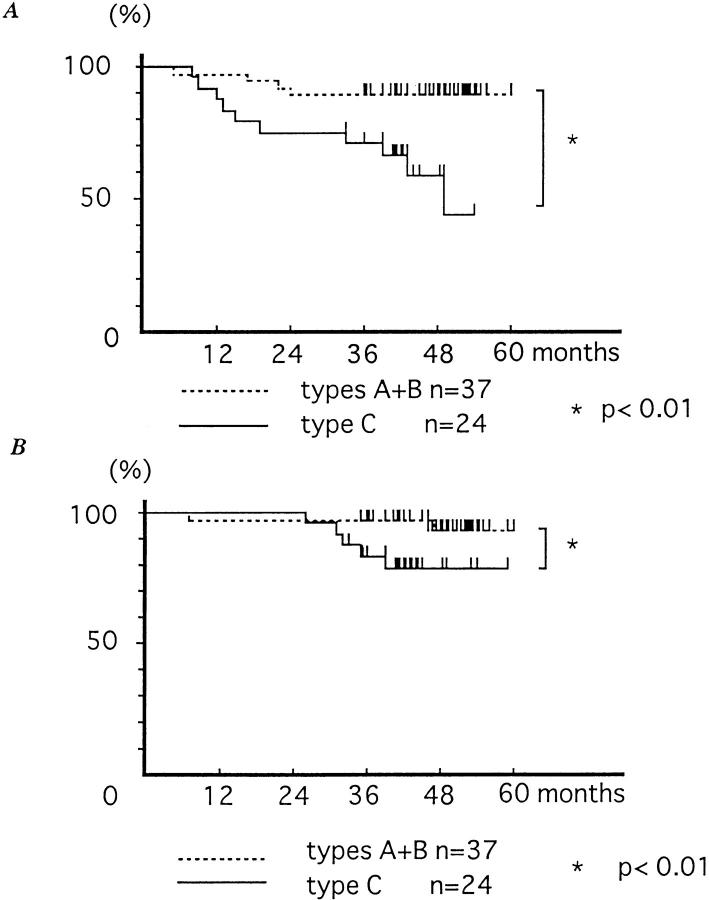
We then determined whether these staining patterns were correlated with prognosis in patients with breast cancer. The relationship between the actinin-4 staining pattern and disease-free and overall survival of 61 patients with relatively early breast cancer (clinical stages I and II [Rosen and Oberman, 1992]) was examined. A significant difference in disease-free survival was observed between types A+B and type C (P < 0.01) (Fig. 12 A). In addition, a significant difference in overall survival was observed between types A + B and type C (P < 0.01) (Fig. 12 B). These findings suggest that the subcellular distribution of actinin-4 may indicate the biological behavior of breast cancer and may be considered a new risk factor for relapse.
Figure 12.
(A) Disease-free survival curves of 61 patients with clinical stages I and II breast cancer, according to the subcellular localization of actinin-4. Disease-free survival curves are drawn using the Kaplan-Meiler method. Significant difference in disease-free survival is observed between types A + B (nuclear) and type C (nonnuclear) (P < 0.01). (B) Overall survival curves of 61 patients with clinical stages I and II breast cancer, according to the subcellular localization of actinin-4. Significant difference in overall survival is observed between types A + B (nuclear) and type C (nonnuclear) (P < 0.01).
Discussion
α-Actinin is a member of the superfamily of actin-binding proteins that includes spectrin, filamin, dystrophin, and fimbrin, by virtue of the similarity of their actin-binding domains (Matsudaira, 1991). α-Actinin binds to the integrin β1 cytoplasmic domain (Otey et al., 1990, 1993) and is believed to link the actin cytoskeleton to focal adhesions in cooperation with other cytoplasmic proteins, including tensin, talin, and vinculin (Burridge et al., 1990; Miyamoto et al., 1995). Actinin is also localized at adherens junctions in connection with α-catenin (Knudsen et al., 1995). Assembly of these structural proteins is believed to play an important role in stabilizing cell adhesion (Burridge et al., 1990; Miyamoto et al., 1995) and suppressing cell motility (Glück et al., 1993; Glück and Ben-Ze'ev, 1994).
In this study, using a newly established mAb, we isolated a cDNA clone encoding a novel isoform of α-actinin, designated actinin-4. Immunofluorescence microscopy demonstrated that the classical isoform, actinin-1, was concentrated in actin stress fiber ends and adherens junctions. In contrast, this novel nonmuscle α-actinin, actinin-4, binds actin filaments at a different subcellular location, thereby exerting functions distinct from those of actinin-1. Actinin-4 is highly concentrated in sharply stretched cells. In the wound assay, the cells forced to be motile along wound edges and cells migrating into the wound overexpressed actinin-4. These findings suggest the involvement of actinin-4 in cell movement. Glück et al. demonstrated that the expression of actinin-1 was reduced in SV-40–transformed 3T3 cells (Glück et al., 1993; Glück and Ben-Ze'ev, 1994). Transfection with actinin-1 cDNA suppressed tumorigenicity. Transfection with actinin-1 cDNA in an antisense orientation increased cell motility, supporting the contradictory roles of the two nonmuscle isoforms with respect to cell movement.
Various growth factors provide stimuli for cell motility in addition to mitogenic activity (Klagsbrun, 1996). Recently, Hsu et al. (1996) identified a new murine nonmuscle α-actinin isoform, FR-17, as a fibroblast growth factor-1–inducible gene in NIH-3T3 cells. Although it has not been fully sequenced, the deduced COOH-terminal amino acids (1–109; sequence data available from GenBank/ EMBL/DDBJ under accession number U41415/6) available for FR-17 showed 97.2% identity with amino acids 776–884 of human actinin-4, suggesting that this isoform may be a murine homologue of human actinin-4. In addition to FR-17, a computer search revealed that several partial sequences identical to actinin-4 were registered in dbEST databases. These ESTs were isolated from human cDNAs of placenta (accession number AA368907), fetal brain (M85377), Jurkat T-cells (AA312012), and pancreatic islets (C06273, D83843).
The level of another actin-binding protein, thymosin β15, was reported to be elevated in human metastatic prostate cancer and correlated positively with the Gleason score (Bao et al., 1996). In contrast to actinins, thymosins inhibit actin polymerization. Cell motility is regulated by both disassembly and reformation of the actin cytoskeleton. Thymosin β15 has been considered to degrade the static anchorage of the actin cytoskeleton and liberate actin monomers. Actinin-4 may then cross-link actin filaments and reorganize the cytoskeleton, which is essential for cell movement. Further studies are necessary to elucidate how these molecules regulate the actin cytoskeleton and participate in cancer invasion and metastasis.
In this study, we found that actinin-4 existed in the nucleus of a certain population of breast cancers and in several cell lines. From the deduced amino acid sequence, it seems that actinin-4 does not possess any apparent nuclear localization signals (Boulikas, 1993). Although the precise mechanisms of the nuclear transition are still under investigation, we were able to reproduce the nuclear transition of actinin-4 in tissue culture. Actinin-4 was translocated from the cytoplasm to the nucleus by treatment with the PI3 kinase inhibitor, wortmannin (Vemuri, 1994), or by actin depolymerization using cytochalasin D (Casella et al., 1981). PI3 kinase is thought to be one of the key molecules involved in the signaling pathways of activated receptor tyrosine kinases and regulates cell growth, motility, and morphogenesis (Raffioni and Bradshaw, 1992; Royal and Park, 1995). The nuclear localization of actinin-4 was observed mainly in low-infiltrative histological subtypes of breast carcinoma. The abnormal activation of this enzyme may determine the biological behavior of cancer cells and confer metastatic potential to them.
Although histological subtyping is a common procedure in the pathological diagnosis of breast cancer, the correlation between histological subtypes and the outcome of patients in this study did not reach statistical significance (data not shown). However, we demonstrated a significantly poorer disease-free survival and overall survival in breast cancer patients with a nonnuclear localization of actinin-4 (Fig. 12). This difference was probably due to cases of papillotubular and solid-tubular carcinomas, in which the location of actinin-4 was exceptionally cytoplasmic. These cases may have had a high degree of metastatic potential that could not be detected by conventional histological examination.
From the above experimental and clinical observations, we conclude that a cytoplasmic localization may indicate active actin-bundling by actinin-4 and enhanced cell motility. Because no definite marker is currently available for assessment of the metastatic potential of cancer, the subcellular localization of the actinin-4 molecule may represent a potential new biological marker for cancer metastasis and may predict early relapse of breast cancer. Large-scale prospective analyses are necessary to examine this issue, not only for breast cancer but also for other cancers.
Abbreviations used in this paper
- GST
glutathione S-transferase
- HFK
human foreskin keratinocyte(s)
- PH
pleckstrin homology
- PI3
phosphatidylinositol 3
- PIP2
phosphatidylinositol 4,5-biphosphate
Footnotes
K. Honda is a recipient of a Research Resident Fellowship from the Foundation for Promotion of Cancer Research. This research was supported in part by a Grant-in-Aid for the Second Term Comprehensive 10-Year Strategy for Cancer Control from the Ministry of Health and Welfare, Japan.
The nucleotide sequence data reported in this paper will appear in the GenBank/EMBL/DDBJ nucleotide sequence databases under accession number D89980.
Address all correspondence to Setsuo Hirohashi, Pathology Division, National Cancer Center Research Institute, 1-1 Tsukiji 5-chome, Chuo-ku, Tokyo 104, Japan. Tel.: +81-3-3542-2511, ext. 4102. Fax: +81-3-3248-2737. E-mail: shirohas@gan2.ncc.go.jp
References
- Aznavoorian S, Murphy AN, Stetler-Stevenson WG, Liotta LA. Molecular aspects of tumor cell invasion and metastasis. Cancer. 1993;71:1368–1383. doi: 10.1002/1097-0142(19930215)71:4<1368::aid-cncr2820710432>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- Bao L, Loda M, Janmey AP, Stewart R, Anand-Apte B, Zetter BR. Tymosin β15: a novel regulator of tumor cell motility upregulated in metastatic prostate cancer. Nat Med. 1996;2:1322–1328. doi: 10.1038/nm1296-1322. [DOI] [PubMed] [Google Scholar]
- Beggs AH, Byers TJ, Knoll JHM, Boyce FM, Bruns GAP, Kunkel LM. Cloning and characterization of two human skeletal muscle α-actinin genes located on chromosomes 1 and 11. J Biol Chem. 1992;267:9281–9288. [PubMed] [Google Scholar]
- Boulikas T. Nuclear localization signals (NLS) Crit Rev Eukaryotic Gene Expr. 1993;3:193–227. [PubMed] [Google Scholar]
- Burridge K, Nuckolls G, Otey C, Pavalko F, Simon K, Turner C. Actin-membrane interaction in focal adhesions. Cell Differ Dev. 1990;32:337–342. doi: 10.1016/0922-3371(90)90048-2. [DOI] [PubMed] [Google Scholar]
- Casella JF, Flanagan MD, Lin S. Cytochalasin D inhibits actin polymerization and induces depolymerization of actin filaments formed during platelet shape change. Nature. 1981;293:302–305. doi: 10.1038/293302a0. [DOI] [PubMed] [Google Scholar]
- Cavailles V, Augereau P, Garcia M, Rochefort H. Estrogens and growth factors induce the mRNA of the 52K-pro-cathepsin-D secreted by breast cancer cells. Nucleic Acids Res. 1988;16:1903–1919. doi: 10.1093/nar/16.5.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer LP, Siebert M, Mitchison TJ. Identification of novel graded polarity actin filament bundles in locomoting heart fibroblasts. J Cell Biol. 1997;136:1287–1305. doi: 10.1083/jcb.136.6.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filder IJ, Gersten DM, Hart IR. The biology of cancer invasion and metastasis. Adv Cancer Res. 1978;28:149–250. doi: 10.1016/s0065-230x(08)60648-x. [DOI] [PubMed] [Google Scholar]
- Fukami K, Furuhashi K, Inagaki M, Endo T, Hatano S, Takenawa T. Requirement of phosphatidylinositol 4,5-bisphosphate for α-actinin function. Nature. 1992;359:150–152. doi: 10.1038/359150a0. [DOI] [PubMed] [Google Scholar]
- Fukami K, Sawada N, Endo T, Takenawa T. Identification of a phosphatidylinositol 4,5-bisphosphate-binding site in chicken skeletal muscle α-actinin. J Biol Chem. 1996;271:2646–2650. doi: 10.1074/jbc.271.5.2646. [DOI] [PubMed] [Google Scholar]
- Giard DJ, Aaronson SA, Todaro GJ, Arnstein P, Kersey JH, Dosik H, Parks WP. In vitro cultivation of human tumors: establishment of cell lines derived from a series of solid tumors. J Natl Cancer Inst. 1973;51:1417–1423. doi: 10.1093/jnci/51.5.1417. [DOI] [PubMed] [Google Scholar]
- Glück U, Ben-Ze'ev A. Modulation of α-actinin levels affects cell motility and confers tumorigenisity on 3T3 cells. J Cell Sci. 1994;107:1773–1782. doi: 10.1242/jcs.107.7.1773. [DOI] [PubMed] [Google Scholar]
- Glück U, Kwiatkowski DJ, Ben-Ze'ev A. Suppression of tumorigenicity in simian virus 40-transformed 3T3 cells transfected with α-actinin cDNA. Proc Natl Acad Sci USA. 1993;90:383–387. doi: 10.1073/pnas.90.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haemmerli G, Strauli P. In vitro motility of cells from human epidermoid carcinomas. A study by phase-contrast and reflection-contrast cinematography. Int J Cancer. 1981;27:603–610. doi: 10.1002/ijc.2910270506. [DOI] [PubMed] [Google Scholar]
- Harlow, E., and D. Lane. 1988. Preparing total cell lysates for immunoblotting. In Antibodies. A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 481 pp.
- Hemmings LK, Kuhlman PA, Critchley DR. Analysis of the actin-binding domain of α-actinin by mutagenesis and demonstration that dystrophin contains a functionally homologous domain. J Cell Biol. 1992;116:1369–1380. doi: 10.1083/jcb.116.6.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirohashi S, Watanabe M, Shimosato Y, Sekine T. Monoclonal antibody reactive with the sialyl-sugar residue of a high molecular weight glycoprotein in sera of cancer patients. Gann. 1984;75:485–488. [PubMed] [Google Scholar]
- Hosaka S, Suzuki M, Goto M, Sato H. Motility of rat ascites hepatoma cells, with reference to malignant characteristics in cancer metastasis. Gann. 1978;69:273–276. [PubMed] [Google Scholar]
- Hsu DKW, Gou Y, Alberts GF, Peifley KA, Winkles JA. Fibroblast growth factor-1-inducible gene FR-17 encodes a nonmuscle α-actinin isoform. J Cell Physiol. 1996;167:261–268. doi: 10.1002/(SICI)1097-4652(199605)167:2<261::AID-JCP9>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Imamura M, Sakurai T, Ogawa Y, Ishikawa T, Goto K, Masaki T. Molecular cloning of low-Ca2+-sensitive-type nonmuscle α-actinin. Eur J Biochem. 1994;223:395–401. doi: 10.1111/j.1432-1033.1994.tb19006.x. [DOI] [PubMed] [Google Scholar]
- Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Statis Assoc. 1958;53:457–481. [Google Scholar]
- Klagsbrun M. The fibroblast growth factor family: structural and biological properties. Prog Growth Factor Res. 1996;167:261–268. doi: 10.1016/0955-2235(89)90012-4. [DOI] [PubMed] [Google Scholar]
- Knudsen KA, Soler AP, Johnson KR, Wheelock MJ. Interaction of α-actinin with the cadherin/catenin cell–cell adhesion complex via α-catenin. J Cell Biol. 1995;130:67–77. doi: 10.1083/jcb.130.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhlman PA, Hemmings L, Critchley DR. The identification and characterisation of an actin-binding site in α-actinin by mutagenesis. FEBS Lett. 1992;304:201–206. doi: 10.1016/0014-5793(92)80619-r. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- Lazarides EB, Burridge K. α-actinin: immunofluorescent localization of a muscle structural protein in nonmuscle cells. Cell. 1975;6:289–298. doi: 10.1016/0092-8674(75)90180-4. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Modular organization of actin crosslinking proteins. Trends Biochem Sci. 1991;16:87–92. doi: 10.1016/0968-0004(91)90039-x. [DOI] [PubMed] [Google Scholar]
- Millake DB, Blanchard AD, Patel B, Critchley DR. The cDNA sequence of a human placental α-actinin. Nucleic Acids Res. 1989;17:6725. doi: 10.1093/nar/17.16.6725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto S, Teramoto H, Coso OA, Gutkind JS, Burbelo PD, Akiyama SK, Yamada KM. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J Cell Biol. 1995;131:791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolson GL. Cancer metastasis: tumor cell and host organ properties important in metastasis to specific secondary sites. Biochim Biophys Acta. 1988;948:175–224. doi: 10.1016/0304-419x(88)90010-8. [DOI] [PubMed] [Google Scholar]
- Nishihira T, Hashimoto Y, Katayama M, Mori S, Kuroki T. Molecular and cellular features of esophageal cancer cells. J Cancer Res Clin Oncol. 1993;119:441–449. doi: 10.1007/BF01215923. [DOI] [PubMed] [Google Scholar]
- Otey CA, Pavalko FM, Burridge K. An interaction between α-actinin and the β1 integrin subunit in vitro. J Cell Biol. 1990;111:721–729. doi: 10.1083/jcb.111.2.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otey CA, Vasquez GB, Burridge K, Erickson BW. Mapping of the α-actinin binding site within the β1 integrin cytoplasmic domain. J Biol Chem. 1993;268:21193–21197. [PubMed] [Google Scholar]
- Partin AW, Shoeniger JS, Mohler LL, Coffey DS. Fourier analysis of cell motility: correlation of motility with metastatic potentials. Proc Natl Acad Sci USA. 1989;86:1254–1258. doi: 10.1073/pnas.86.4.1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast GC, Ziff EB. Mbh1: a novel gelsolin/severin-related protein which binds actin in vitro and exhibits nuclear localization in vivo. EMBO (Eur Mol Biol Organ) J. 1991;10:757–766. doi: 10.1002/j.1460-2075.1991.tb08007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffioni S, Bradshaw RA. Activation of phosphatidylinositol 3-kinase by epidermal growth factor, basic fibroblast growth factor, and nerve growth factor in PC12 pheochromocytoma cells. Proc Natl Acad Sci USA. 1992;89:9121–9125. doi: 10.1073/pnas.89.19.9121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen, P.P., and H.A. Oberman. 1992. Tumors of the mammary gland. Armed Forces Institute of Pathology, Washington, D.C. 115–175.
- Royal I, Park M. Hepatocyte growth factor-induced scatter of Madin-Darby canine kidney cells requires phosphatidylinositol 3-kinase. J Biol Chem. 1995;270:27780–27787. doi: 10.1074/jbc.270.46.27780. [DOI] [PubMed] [Google Scholar]
- Sambrook, J., E.F. Fritsch, and T. Maniatis. 1989. Molecular Cloning. A Laboratory Manual, 2nd Ed. Cold Springs Harbor Laboratory Press, Cold Spring Harbor, NY.
- Sato Y, Mukai K, Watanabe S, Goto M, Shimosato Y. The AMeX method: a simplified technique of tissue processing and paraffin embedding with improved preservation of antigens for immunostaining. Am J Pathol. 1986;125:431–435. [PMC free article] [PubMed] [Google Scholar]
- Shibata T, Ochiai A, Kanai Y, Akimoto S, Gotoh M, Yasui N, Machinami R, Hirohashi S. Dominant negative inhibition of the association between β-catenin and c-erbB-2 by N-terminally deleted β-catenin suppresses the invasion and metastasis of cancer cells. Oncogene. 1996;13:883–889. [PubMed] [Google Scholar]
- Stossel TP. On the crawling of animal cells. Science. 1993;260:1086–1094. doi: 10.1126/science.8493552. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vemuri GSR SE. Wortmannin inhibits serum-induced activation of phosphoinositide 3-kinase and proliferation of CHRF-288 cells. Biochem Biophys Res Commun. 1994;202:1619–1623. doi: 10.1006/bbrc.1994.2118. [DOI] [PubMed] [Google Scholar]
- Wehland J, Osborn M, Weber K. Cell to substratum contacts in living cells. A direct correlation between interference reflection and indirect immunofluorescence microscopy using antibodies against actin and α-actinin. J Cell Sci. 1979;37:257–273. doi: 10.1242/jcs.37.1.257. [DOI] [PubMed] [Google Scholar]
- Westley BR H. Estradiol induced proteins in the MCF7 human breast cancer cell line. Biochem Biophys Res Commun. 1979;90:410–416. doi: 10.1016/0006-291x(79)91250-6. [DOI] [PubMed] [Google Scholar]
- Witke W, Hofmann A, Koppel B, Schleicher M, Noegel AA. The Ca(2+)-binding domains in nonmuscle type α-actinin: biochemical and genetic analysis. J Cell Biol. 1993;121:599–606. doi: 10.1083/jcb.121.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada T, Hirohashi S, Shimosato Y, Kodama T, Hayashi S, Ogura T, Gamou S, Shimizu N. Giant cell carcinomas of the lung producing colony-stimulating factor in vitro and in vivo. Gann. 1985;76:967–976. [PubMed] [Google Scholar]
- Yamada T, Jiping J, Endo R, Gotoh M, Shimosato Y, Hirohashi S. Molecular cloning of a cell-surface glycoprotein that can potentially discriminate mesothelium from epithelium: its identification as vascular cell adhesion molecule 1. Br J Cancer. 1995;71:562–570. doi: 10.1038/bjc.1995.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RA, Davis RW. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci USA. 1983;80:1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youssoufian H, McAfee M, Kwiatkowski DJ. Cloning and chromosomal localization of the human cytoskeletal α-actinin gene reveals linkage to the β-spectrin gene. Am J Hum Genet. 1990;47:62–72. [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Rees MCP, Bicknell R. The isolation and long-term culture of normal human endometrial epithelium and stroma. J Cell Sci. 1995;108:323–331. doi: 10.1242/jcs.108.1.323. [DOI] [PubMed] [Google Scholar]
- Zetter BR. The cellular basis of site-specific tumor metastasis. N Engl J Med. 1990;322:605–612. doi: 10.1056/NEJM199003013220907. [DOI] [PubMed] [Google Scholar]