Abstract
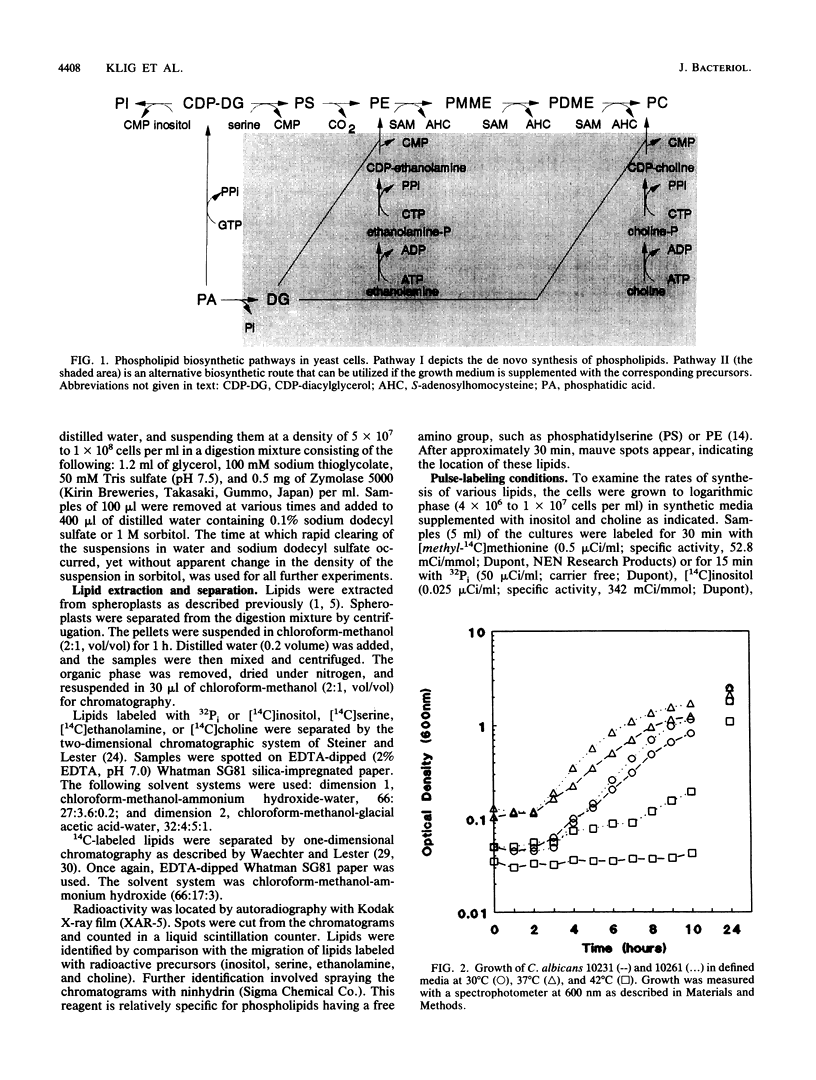
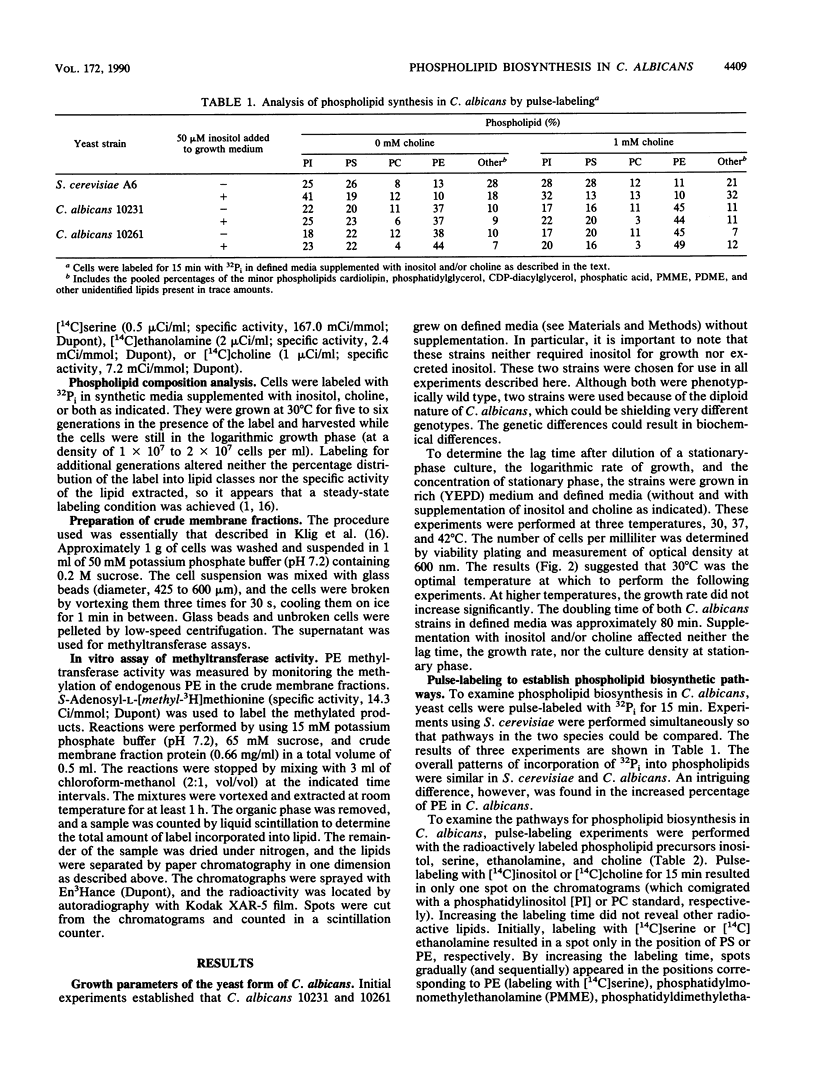
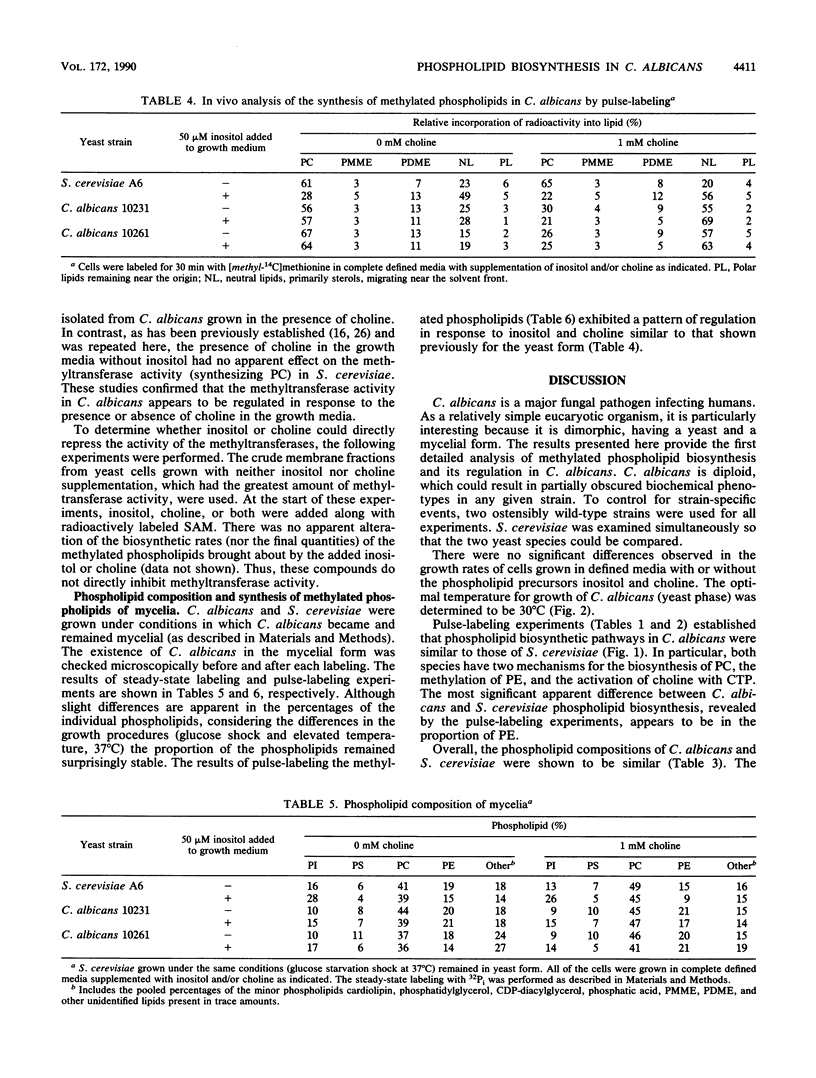
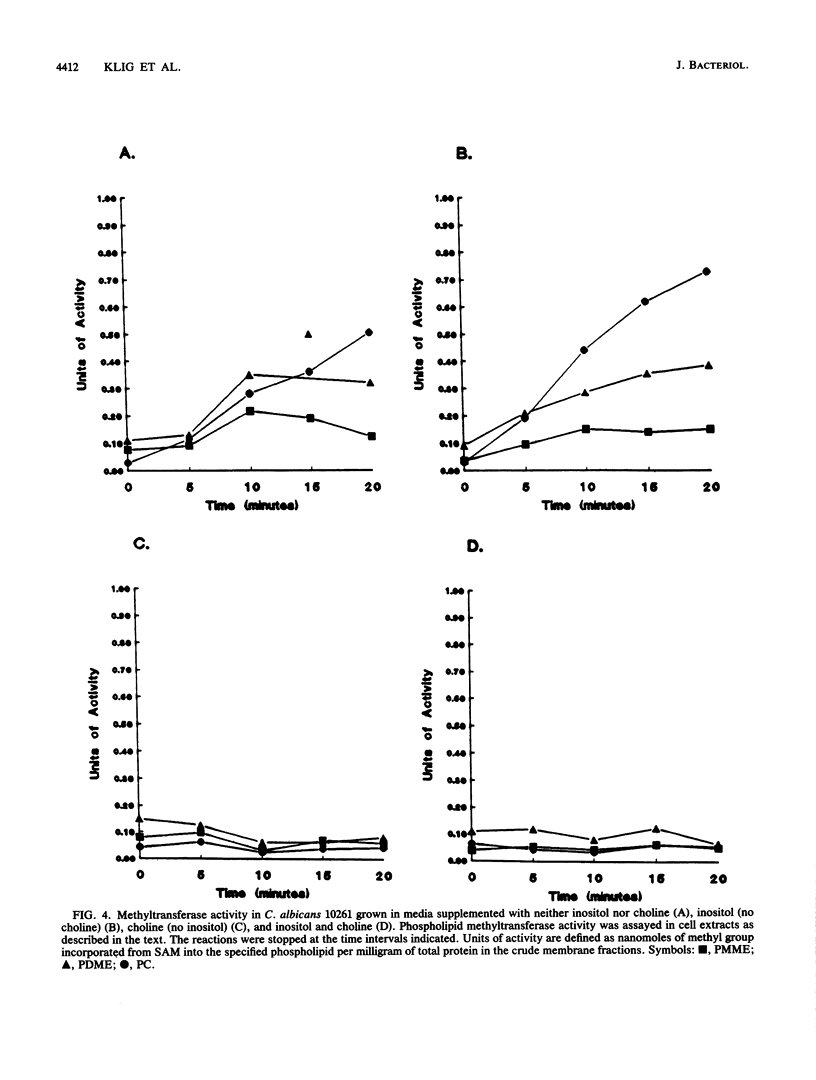
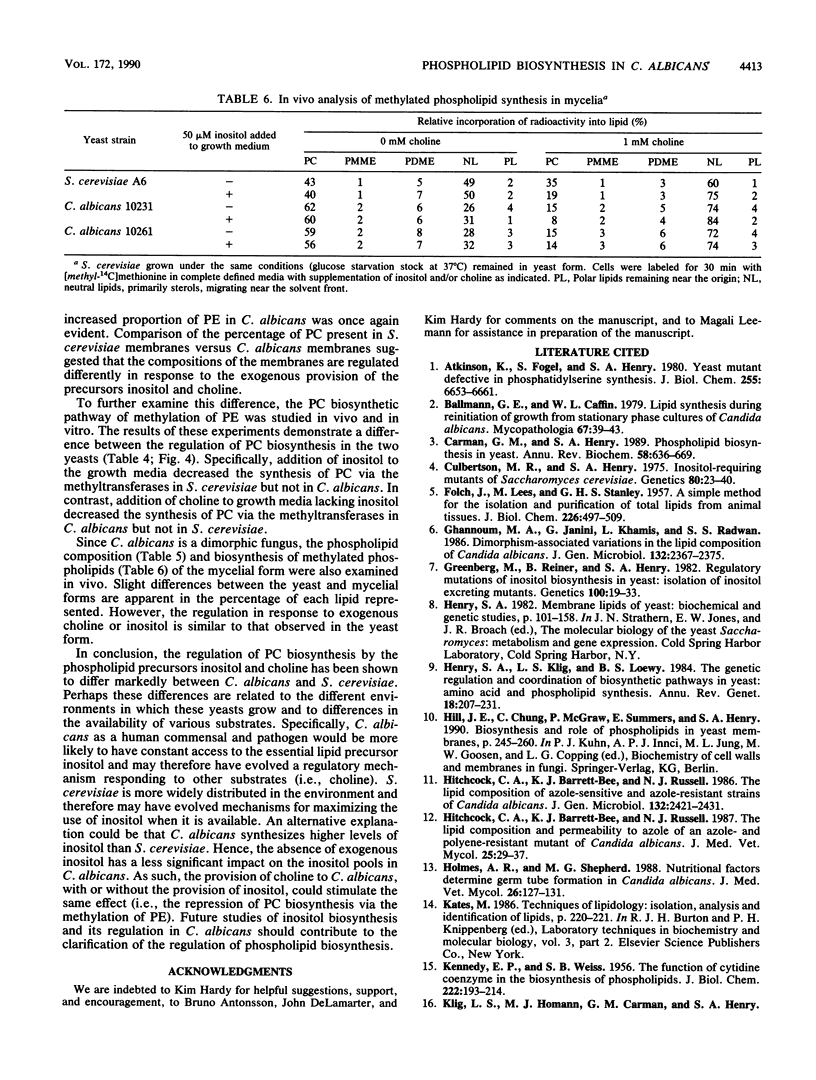
Phospholipid metabolism in the pathogenic fungus Candida albicans was examined. The phospholipid biosynthetic pathways of C. albicans were elucidated and were shown to be similar to those of Saccharomyces cerevisiae. However, marked differences were seen between these two fungi in the regulation of the pathways in response to exogenously provided precursors inositol and choline. In S. cerevisiae, the biosynthesis of phosphatidylcholine via methylation of phosphatidylethanolamine appears to be regulated in response to inositol and choline; provision of choline alone does not repress the activity of this pathway (G. M. Carman and S. A. Henry, Annu. Rev. Biochem. 58:636-669, 1989). The same pathway in C. albicans responds to the exogenous provision of choline. Possible explanations for the observed differences in regulation are discussed.
Full text
PDF
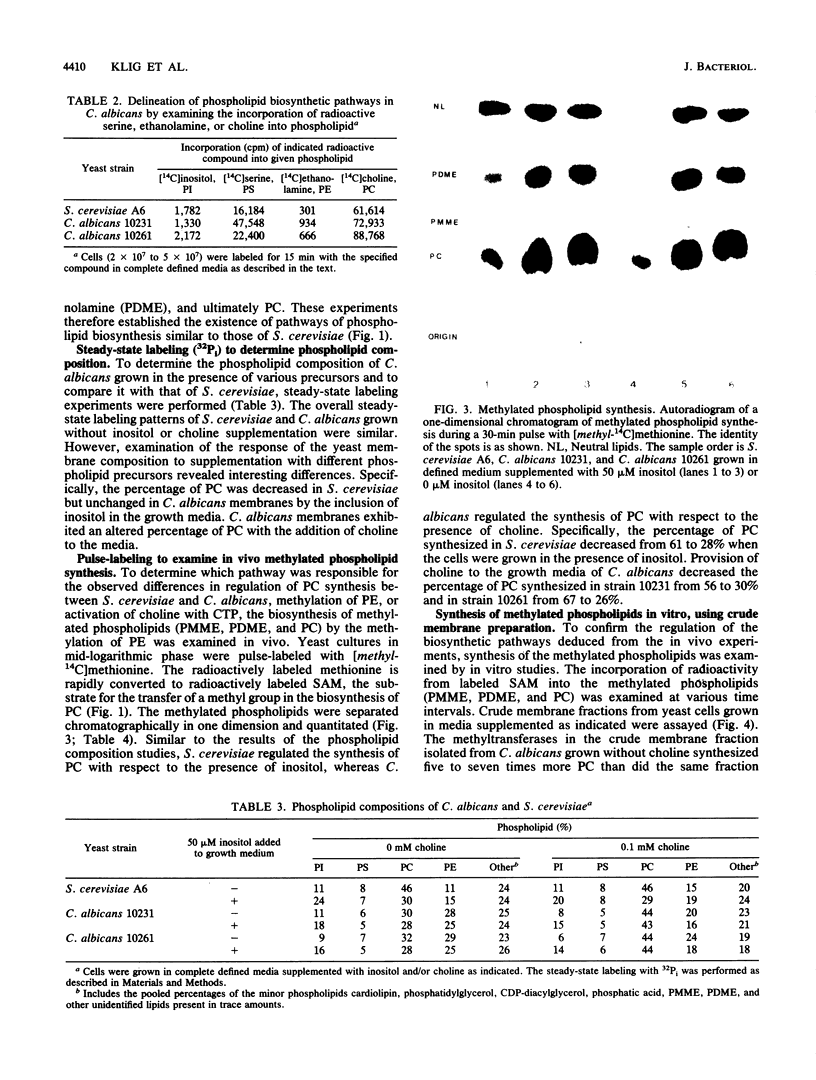

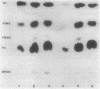
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkinson K., Fogel S., Henry S. A. Yeast mutant defective in phosphatidylserine synthesis. J Biol Chem. 1980 Jul 25;255(14):6653–6661. [PubMed] [Google Scholar]
- Ballmann G. E., Caffin W. L. Lipid synthesis during reinitiation of growth from stationary phase cultures of Candida albicans. Mycopathologia. 1979 Mar 30;67(1):39–43. doi: 10.1007/BF00436239. [DOI] [PubMed] [Google Scholar]
- Carman G. M., Henry S. A. Phospholipid biosynthesis in yeast. Annu Rev Biochem. 1989;58:635–669. doi: 10.1146/annurev.bi.58.070189.003223. [DOI] [PubMed] [Google Scholar]
- Culbertson M. R., Henry S. A. Inositol-requiring mutants of Saccharomyces cerevisiae. Genetics. 1975 May;80(1):23–40. doi: 10.1093/genetics/80.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- Ghannoum M. A., Janini G., Khamis L., Radwan S. S. Dimorphism-associated variations in the lipid composition of Candida albicans. J Gen Microbiol. 1986 Aug;132(8):2367–2375. doi: 10.1099/00221287-132-8-2367. [DOI] [PubMed] [Google Scholar]
- Greenberg M. L., Reiner B., Henry S. A. Regulatory mutations of inositol biosynthesis in yeast: isolation of inositol-excreting mutants. Genetics. 1982 Jan;100(1):19–33. doi: 10.1093/genetics/100.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry S. A., Klig L. S., Loewy B. S. The genetic regulation and coordination of biosynthetic pathways in yeast: amino acid and phospholipid synthesis. Annu Rev Genet. 1984;18:207–231. doi: 10.1146/annurev.ge.18.120184.001231. [DOI] [PubMed] [Google Scholar]
- Hitchcock C. A., Barrett-Bee K. J., Russell N. J. The lipid composition and permeability to azole of an azole- and polyene-resistant mutant of Candida albicans. J Med Vet Mycol. 1987 Feb;25(1):29–37. doi: 10.1080/02681218780000041. [DOI] [PubMed] [Google Scholar]
- Hitchcock C. A., Barrett-Bee K. J., Russell N. J. The lipid composition of azole-sensitive and azole-resistant strains of Candida albicans. J Gen Microbiol. 1986 Sep;132(9):2421–2431. doi: 10.1099/00221287-132-9-2421. [DOI] [PubMed] [Google Scholar]
- Holmes A. R., Shepherd M. G. Nutritional factors determine germ tube formation in Candida albicans. J Med Vet Mycol. 1988 Apr;26(2):127–131. [PubMed] [Google Scholar]
- KENNEDY E. P., WEISS S. B. The function of cytidine coenzymes in the biosynthesis of phospholipides. J Biol Chem. 1956 Sep;222(1):193–214. [PubMed] [Google Scholar]
- Loewy B. S., Henry S. A. The INO2 and INO4 loci of Saccharomyces cerevisiae are pleiotropic regulatory genes. Mol Cell Biol. 1984 Nov;4(11):2479–2485. doi: 10.1128/mcb.4.11.2479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lott T. J., Boiron P., Reiss E. An electrophoretic karyotype for Candida albicans reveals large chromosomes in multiples. Mol Gen Genet. 1987 Aug;209(1):170–174. doi: 10.1007/BF00329854. [DOI] [PubMed] [Google Scholar]
- Magee B. B., Koltin Y., Gorman J. A., Magee P. T. Assignment of cloned genes to the seven electrophoretically separated Candida albicans chromosomes. Mol Cell Biol. 1988 Nov;8(11):4721–4726. doi: 10.1128/mcb.8.11.4721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marriott M. S. Isolation and chemical characterization of plasma membranes from the yeast and mycelial forms of Candida albicans. J Gen Microbiol. 1975 Jan;86(1):115–132. doi: 10.1099/00221287-86-1-115. [DOI] [PubMed] [Google Scholar]
- McGraw P., Henry S. A. Mutations in the Saccharomyces cerevisiae opi3 gene: effects on phospholipid methylation, growth and cross-pathway regulation of inositol synthesis. Genetics. 1989 Jun;122(2):317–330. doi: 10.1093/genetics/122.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riggsby W. S., Torres-Bauza L. J., Wills J. W., Townes T. M. DNA content, kinetic complexity, and the ploidy question in Candida albicans. Mol Cell Biol. 1982 Jul;2(7):853–862. doi: 10.1128/mcb.2.7.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd M. G., Poulter R. T., Sullivan P. A. Candida albicans: biology, genetics, and pathogenicity. Annu Rev Microbiol. 1985;39:579–614. doi: 10.1146/annurev.mi.39.100185.003051. [DOI] [PubMed] [Google Scholar]
- Steiner M. R., Lester R. L. In vitro studies of phospholipid biosynthesis in Saccharomyces cerevisiae. Biochim Biophys Acta. 1972 Feb 21;260(2):222–243. doi: 10.1016/0005-2760(72)90035-5. [DOI] [PubMed] [Google Scholar]
- Summers E. F., Letts V. A., McGraw P., Henry S. A. Saccharomyces cerevisiae cho2 mutants are deficient in phospholipid methylation and cross-pathway regulation of inositol synthesis. Genetics. 1988 Dec;120(4):909–922. doi: 10.1093/genetics/120.4.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi A., Dudani A. K., Prasad R. Why choline supplementation did not enhance phosphatidylcholine level in Candida albicans. Biochem Int. 1983 Jan;6(1):119–128. [PubMed] [Google Scholar]
- Waechter C. J., Lester R. L. Differential regulation of the N-methyl transferases responsible for phosphatidylcholine synthesis in Saccharomyces cerevisiae. Arch Biochem Biophys. 1973 Sep;158(1):401–410. doi: 10.1016/0003-9861(73)90637-1. [DOI] [PubMed] [Google Scholar]
- Waechter C. J., Lester R. L. Regulation of phosphatidylcholine biosynthesis in Saccharomyces cerevisiae. J Bacteriol. 1971 Mar;105(3):837–843. doi: 10.1128/jb.105.3.837-843.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan W. L., Magee P. T. Natural heterozygosity in Candida albicans. J Bacteriol. 1981 Feb;145(2):896–903. doi: 10.1128/jb.145.2.896-903.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan W. L., Partridge R. M., Magee P. T. Heterozygosity and segregation in Candida albicans. Mol Gen Genet. 1980;180(1):107–113. doi: 10.1007/BF00267358. [DOI] [PubMed] [Google Scholar]
- Whelan W. L., Soll D. R. Mitotic recombination in Candida albicans: recessive lethal alleles linked to a gene required for methionine biosynthesis. Mol Gen Genet. 1982;187(3):477–485. doi: 10.1007/BF00332632. [DOI] [PubMed] [Google Scholar]
- Whelan W. L. The genetics of medically important fungi. Crit Rev Microbiol. 1987;14(2):99–170. doi: 10.3109/10408418709104437. [DOI] [PubMed] [Google Scholar]