Abstract
Here, we report on the analysis of keratin 18 null mice. Unlike the ablation of K8, which together with K18 is expressed in embryonic and simple adult epithelia, K18 null mice are viable, fertile, and show a normal lifespan. In young K18 null mice, hepatocytes were completely devoid of keratin filaments. Nevertheless, typical desmosomes were formed and maintained. Old K18 null mice, however, developed a distinctive liver pathology with abnormal hepatocytes containing K8-positive aggregates. These stained positively for ubiquitin and MM120-1 and were identified as Mallory bodies, one hallmark of human alcoholic hepatitis. This is the first demonstration that the ablation of one keratin leads to the accumulation of its single partner. Another striking finding was the absence or drastic down regulation of K7 in several tissues despite its ongoing transcription. Moreover, K18 null mice revealed new insights in the filament-forming capacity of the tail-less K19 in vivo. Due to the unexpected secondary loss of K7, only K8/19 are expressed in the uterine epithelium of K18 null mice. Immunoelectron microscopy of this tissue demonstrated the presence of typical K8/19 IF, thus highlighting in vivo that K19 is a fully competent partner for K8.
Among known intermediate filament (IF)1 proteins, keratins are the most diverse group, represented in mammals by approximately 15 type I and II genes (Fuchs and Weber, 1994). They are expressed as sets of one or several pairs during embryonic development and tissue differentiation. Unlike most other IFs, keratin IF assemble from coiled-coil heterodimers that first form tetramers, and then IF (Coulombe and Fuchs, 1990; Hatzfeld and Weber, 1990; Steinert, 1990). Single keratins are unable to form IF in vitro (Steinert et al., 1976; Hatzfeld and Franke, 1985) or in cultured cells where they become quickly proteolysed (Domenjoud et al., 1988; Kulesh et al., 1989; Magin et al., 1990; Bader et al., 1991). If combined in vitro, any type I and II keratin subunits have the intrinsic property of forming heterotypic IF, leading to the hypothesis of keratin promiscuity (Hatzfeld and Franke, 1985). One of the few measurable properties of individual keratin complexes in vitro is their different stability upon dissociation/association in the presence of increasing concentrations of urea (Franke et al., 1983). These data suggested that keratin 8/18 (K8/18) form less stable IF than the epidermal pair K5/14. Experiments using plasmon surface resonance and viscosimetry have also provided evidence that keratin complexes and IF formed from different subunits were of different stability (Hofmann and Franke, 1997).
Recently, the discovery of point mutations in epidermal keratin genes (Bonifas et al., 1991; Coulombe et al., 1991; Lane et al., 1992), preceded by transgenic mice expressing mutant keratin subunits (Vassar et al., 1991), were shown to cause a number of dominantly inherited human skin disorders like epidermolysis bullosa simplex and epidermolytic hyperkeratosis (Corden and McLean, 1996). Such point mutations disrupt the integrity of keratin filaments followed by cytolysis and skin blistering or hyperkeratosis (Fuchs, 1994; McLean and Lane, 1995; Corden and McLean, 1996), thus demonstrating the importance of keratins as cytoskeletal proteins in epidermis.
The functional role of nonepidermal keratins is less clear. Cultured cells of simple epithelial origin grow normally in the absence of cytoplasmic IF (Klymkowsky, 1981; Venetianer et al., 1983), arguing that IF might be required to establish or maintain the differentiated state in vivo. Antibody-mediated disruption of K8/18 filaments in the early mouse embryo failed to block early development (Emerson, 1988). This surprising result was confirmed by K8 knockout mice, which can reach adulthood (Baribault et al., 1993, 1994), depending on the genetic background. In one strain, these mice died around day 12 from yet unknown tissue damage. In a different strain, they survived to adulthood suffering from colorectal hyperplasia and inflammation. The overall architecture of K8-expressing mouse epithelia was established and maintained in all strains tested in the absence of keratin IF (Baribault et al., 1994).
In vivo, keratin IF are built from distinct pairs, like K8/18 typical of hepatocytes, or K5/14 of basal cells in all stratified epithelia (Moll et al., 1982; Lane, 1993). Whether the organization and functional properties of IF in tissues like colon, which express K7, 8, 18, 19, and 20 (Moll et al., 1982), is different from those in hepatocytes, is presently unknown. A few experiments were carried out to disrupt the balance of simple epithelial keratins. Whereas the overexpression of K8 or 18 in transgenic mice seemed to produce no phenotype (Abe and Oshima, 1990; Casanova et al., 1995), overexpression of a human K18 gene carrying a point mutation similar to that in epidermal keratin genes led to filament disruption in hepatocytes, causing hepatocyte fragility and chronic hepatitis (Ku et al., 1995, 1996). A similar albeit less dramatic phenotype was observed in mice expressing human K14 in liver (Albers et al., 1995).
A remarkable alteration of keratin IF organization in hepatocytes can be seen after long-term alcohol abuse in humans as well as after chronic intoxication with drugs like griseofulvin in mice, namely, the cytoplasmic accumulation of keratin-containing aggregates, termed Mallory bodies (MBs; Mallory, 1911; Denk et al., 1975, 1979; Franke et al., 1979; Jensen and Gluud, 1994 a,b). MBs consist predominantly of K8 and variable amounts of K18 assembled in a nonfilamentous manner (Hazan et al., 1986; Zatloukal et al., 1991). In addition to keratin, nonkeratin components, such as the MB-specific MM120-1 antigen, a protein with an apparent molecular mass of 62–65 kD that is recognized by the antibody SMI 31, and ubiquitin are constituents of MBs (Lowe et al., 1988; Zatloukal et al., 1990; Preisegger et al., 1992).
To further analyze the role of simple epithelial keratins, we inactivated the murine K18 gene by homologous recombination in ES cells. Furthermore, K18 null mice can address the issue of K19's function in vivo, because certain epithelia of K18 null mice, like kidney and uterus, express only K8 and 19. Among all keratins, K19 is a unique polypeptide due to the lack of a tail domain (Bader et al., 1986) and its expression pattern (Moll et al., 1982). Due to its frequent coexpression with K8 and 18, K19 could be considered a simple epithelial keratin. However, its presence in cells of the hair follicle and in the basal epidermis (Bartek et al., 1985; Markey et al., 1992; Michel et al., 1996) also justified its classification as an epidermal keratin (Lane, 1993). Possibly, the lack of a tail domain is a prerequisite for K19 to fulfill specific functions in both settings.
The analysis of K18 null mice further allows the comparison of tissue-specific effects of a type I keratin- to those of a type II keratin knockout. This is especially interesting in certain epithelia, like hepatocytes, where only K8 and 18 are expressed and where they are known to be involved in pathological alterations like the formation of Mallory bodies (Denk et al., 1979; Jensen and Gluud, 1994 a,b). As we show here, K18 null mice are viable and fertile. They displayed no fetal lethality due to liver hemorrhage, indicating that subunit proteins of the same polymer may have complementary but not identical functions. Most importantly, old K18 null mice developed a specific liver pathology, defined by the accumulation of K8-positive aggregates.
Materials and Methods
DNA Constructs
The targeting vector was constructed from a genomic keratin 18 λ phage kindly provided by T. Morita (Osaka University, Osaka, Japan; Ichinose et al., 1988). The targeting vector contains two arms of mouse keratin 18 genomic DNA; a 5′–1.2-kb StuI-EcoRV (positions 1,202–2,485 in the K18 gene sequence, Genbank/EMBL/DDBJ Accession No. M22832) and a 3′– 4.7-kb EcoRI (positions 3,542)-BamHI (positions 8,200) fragment, separated by the mouse phosphoglycerate-kinase (PGK) promoter–driven HPRT minigene (PGK/pDWM1; Magin et al., 1992). The HPRT minigene replaces most of exon 1 and part of exon 2. We prepared two vectors, one with the HPRT minigene in sense and one in the antisense orientation, with respect to the K18 transcription unit. This construct was inserted into a Bluescript II SK+ vector containing a Herpes simplex virus thymidine kinase gene also driven by the mouse PGK promoter. The targeting vector was linearized with ClaI, so that vector sequences were downstream of the thymidine kinase gene (Fig. 1). For electroporation, DNA was purified by Qiagen columns (Düsseldorf, Germany), followed by phenol/chloroform extraction and ethanol precipitation.
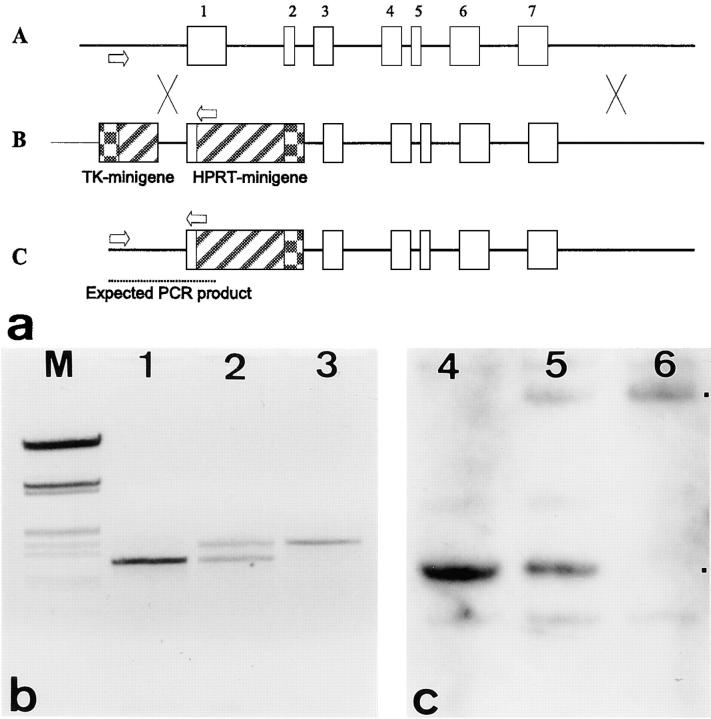
Figure 1.
(a and A) Structure of the murine keratin 18 locus. Arrows indicate the position of PCR primers used to identify the wild-type and targeted alleles in A–C. Exons are indicated by numbered open boxes. (B) The targeting vector consists of an HPRT minigene replacing most of exon 1, intron 1, and exon 2. The 5′-homology is ∼1.1 kb and the 3′- homology, 4.7 kb. A thymidine kinase minigene (TK-minigene) is included for selection against random integration. The vector was linearized such that the thymidine kinase minigene was protected by vector sequences. (C) The targeted K18 locus. X between A and B indicates regions of homologous recombination. (b) PCR products representing the genotypes of wild-type (lane 1), hetero- (2), and homozygous (3) K18 mice. M size marker (λ EcoRI/HindIII). Products were separated on 1.2% agarose gels. (c) Southern blot analysis of the three genotypes of mice. Here, DNA restricted with EcoRI and labeled with a K18- 5′ probe is shown. The presence of a 5.5-kb EcoRI–fragment is indicative of the targeted and that of a 2.1-kb fragment of the wild-type allele. The position of fragments representing wild-type and targeted alleles is indicated by dots.
5′- and 3′-specific probes for murine K7, 8, 18, 19, and 20 were subcloned in Bluescript II SK+ (Stratagene, Heidelberg, Germany). Gel-purified inserts were random prime–labeled using a kit from Boehringer Mannheim (Mannheim, Germany) and 50 μCi of 32P-dCTP (Amersham Buchler GmbH, Braunschweig, Germany).
Cell Culture and Electroporation
Culture conditions for the embryonic stem cell line HM-1 and selection of targeted colonies were described previously (Stacey et al., 1994). Electroporation was done with 200 μg of linearized vector DNA at 800 V and 3 μF. Enrichment after double selection with HAT and ganciclovir was ∼15-fold.
Preparation and Analysis of Genomic DNA
Colonies surviving the HAT/ganciclovir selection regime were transferred into 24-well plates (Becton Dickinson, Heidelberg, Germany) and approximately half of each was processed for PCR analysis (Selfridge et al., 1992). Genomic DNA was purified with a kit (Qiagen Inc.). The primers used were specific for the sequence upstream of the StuI site of cytokeratin 18: GTCCTCAGCACCCTGTAACCTG (Genbank/EMBL/DDBJ Accession No. M22832, nucleotides 1,316–1,337) and for the HPRT minigene: AGCCTACCCTCTGGTAGATTGTCG (HPRT–cDNA sequence, positions 960–983, see (Selfridge et al., 1992). Reaction conditions were as described before (Selfridge et al., 1992) with the following time/temperature parameters: 25 s/95°C, 25 s/65°C and 1 min/72°C for 35 cycles. An Omnigene thermocycler (MWG, Ebersberg, Germany) was used. Taq polymerase was purchased from GIBCO BRL (Karlsruhe, Germany). Genomic DNA from colonies, which produced a PCR product, was isolated from confluent embryonic stem cell cultures by proteinase K and RNAase A (200 μg/ml of both) digestion at 37°C overnight, followed by phenol/chloroform extraction and ethanol precipitation. Genomic Southern blotting was carried out under stringent conditions (hybridization at 42°C in 50% formamide, 5× SSPE, 5× Denhardt, 100 μg/ml herring sperm DNA, 1% SDS, 10% dextran sulfate; four washes for 15 min each at 68°C in 0.1× SSPE, 0.1 SDS) to analyze the structure of the targeted allele by hybridization with three probes: (a) a 3′-EcoRI fragment outside the targeting vector; (b) the HPRT minigene; and (c) a 5′-EcoRI fragment located upstream of the targeting vector.
Generation and Identification of Knockout Mice
Generation of knockout mice was done as described before (Porter et al., 1996) using BALB/c and C57BL/6 blastocysts as recipients. Animals were bought from Harlan Winkelmann (Borken, Germany) with SPF status. Mice were genotyped using a PCR assay of mouse tail DNA, which included the two primers described for identifying ES cell colonies and an additional primer downstream of the EcoRV site: CGGTCTGGATTCCACCCATTC (Genbank/EMBL/DDBJ Accession No. M22832, positions 2,535–2,555). This PCR reaction gave two products; one specific for the wild type (1.3 kb) and one for the altered allele (1.5 kb).
RNA Analysis of Litters
Animals were killed by cervical dislocation and tissues were snap-frozen in liquid nitrogen immediately after dissection. Total RNA was extracted as described by Chomczynski and Sacchi (1987) with minor modifications. 5 mM vanadyl-ribonucleotide (GIBCO-BRL, Karlsruhe, Germany) was included in all purification steps. Aliquots of various total RNA preparations (30 μg) were loaded on 1.4% agarose gels in the presence of formamide/formaldehyde as denaturant and RNA was transferred to Genescreen membranes (New England Nuclear, Dreieich, Germany) by capillary transfer using 10× SSPE as buffer (Sambrook, 1989). Hybridization and washing conditions were as described above for Southern blotting.
Preparation of Protein Extracts and Western Blotting
Protein extracts enriched in cytoskeletal proteins were prepared from frozen tissue by homogenization as described previously (Magin et al., 1983). Total protein extracts were prepared as before (Schröder et al., 1997). The following proteinase inhibitors were included in all buffers: Aprotinin at 0.1 μg/ml, E-64 at 0.5 μg/ml and Pefabloc SC at 100 μg/ml (all from Boehringer Mannheim). One- and two-dimensional gel electrophoresis were carried out as described (Magin et al., 1983; Bader et al., 1991). Proteins were transferred electrophoretically onto nitrocellulose membranes (0.2 μm pore size; Schleicher & Schuell, Dassel, Germany) using 25 mM sodium borate, 1 mM EDTA, pH 9.2, as transfer buffer in a wet blot apparatus (BioRad Laboratories, München, Germany). Immunodetection was performed with peroxidase-coupled secondary antibodies (Dianova, Hamburg, Germany) and enhanced chemiluminescence with reagents from Pierce (St. Augustin, Germany) as described (Schröder et al., 1997). Primary antibody dilutions were as follows: Troma 1 and 3 (K8 and 19, respectively), 1:5,000; Ks 18.04 (K18; Progen, Heidelberg, Germany) diluted 1:8,000; Ks 20.10 (K20, Progen; and from R. Moll, Halle, Germany) diluted 1:1,000; RCK 105 and OVTL 12–30 (K7; Eurodiagnostics, Maastricht, The Netherlands; and from F. Ramaekers, University of Maastricht, Maastricht, The Netherlands) diluted 1:1,000. Antibodies to desmosomal proteins were kindly provided by W.W. Franke (German Cancer Research Center, Heidelberg, Germany) and D. Garrod (University of Manchester, Manchester, UK). Secondary antibodies were diluted 1:30,000.
Immunofluorescence
Frozen sections (5 μm thick) were fixed in acetone at −20°C for 10 min and dried for a few hours before further processing. All antibodies were diluted in PBS containing 1% BSA. The primary monoclonal antibody dilutions were as follows: Troma 1 and 3 (K8 and 19; a kind gift of H. Baribault, Cancer Center, La Jolla, CA) 1:50, Ks 18.04 (K18; Progen) 1:30, RCK 105 and OVTL 12–30 (K7, kindly provided by F. Ramaekers and from Eurodiagnostics) 1:20, Ks 20.10 (K20) 1:10, II- 5F (antidesmoplakin, kindly provided by D. Garrod, University of Manchester, Manchester, UK) 1:10, mAB 121 (antiplectin, kindly provided by R. Owaribe, University of Nagoya, Nagoya, Japan) 1:200, MM 120-1 (directed against MBs, provided by H. Denk, University of Graz, Austria) undiluted, Z 0458 (ubiquitin), diluted 1:100 (Dako Corp., Hamburg, Germany). FITC-, Texas red-, Cy2- or Cy3 labeled secondary antibodies were diluted as recommended by the manufacturers (Dianova, Medac, both Hamburg, Germany). Primary mouse monoclonal antibodies were detected with subclass-specific secondary antibodies (Medac) to minimize background. Slides were mounted in Mowiol (Calbiochem, Bad Soden, Germany) containing 0.1% Dabco (Sigma Chemical GmbH, Munich, Germany).
Conventional and Immunogold Electron Microscopy
Fixation of tissue specimen and staining procedures were performed as described (Mertens et al., 1996; Ruiz et al., 1996).
Results
Targeting the Mouse Keratin 18 Gene
The K18 targeting vector was constructed from a λ phage harboring K18 genomic sequences and contained 5.9 kb of overall homology (Fig. 1 A). We noted that the targeting vector containing the HPRT minigene in the same orientation as the K18 transcription unit yielded at least 10-fold less colonies than the one in the opposite orientation. ES cells, transfected with the former construct, also produced two- to threefold less HPRT- mRNA than those with the latter. Only colonies derived from the latter were analyzed further. Among 72 colonies analyzed after HAT/ganciclovir selection, nine proved to be positive by PCR and Southern blotting (Fig. 1, B and C). The three clones of HM-1 cells carrying the correctly targeted K18 allele gave 100% germline transmission when injected into BALB/c or C57Bl/6 blastocyts. Chimaeras were bred to BALB/c, Bl/6, 129/Sv, or MF-1 animals. Most results were obtained with intercrosses between 129/Sv and MF-1, or from animals on a 129/Sv background.
Homozygous K18 Null Mice Are Viable and Fertile and Have a Normal Lifespan
Heterozygous K18 males were mated to heterozygous and wild-type females. In all strains tested, the expected frequency of homozygotes was observed at birth. K18 null mice have a normal lifespan compared to wild-type animals. Up to the age of 4 mo, K18 mice of both genotypes were normal as judged by morphological or histological inspection. A consistent feature in 18-mo-old mice was a marked hepatomegaly. There were no other organ abnormalities. This is in contrast to K8 null mice, which suffered from an increased embryonic lethality, possibly due to liver hemorrhage on a C57Bl/6 background. With respect to the findings in K8 null fetuses, it can be concluded that the liver phenotype of the corresponding knockout mice is different. Whereas K8 null mice developed colorectal hyperplasia as adults in the strain FVB/N (Baribault et al., 1993, 1994), no anorectal prolapse was observed in K18 null animals. This highlights that type I and II keratins provide complementary but not identical functions.
K18 mRNA and Protein Are Reduced in Heterozygotes and Absent in All Tissues from Homozygotes
In hepatocytes of adult liver, K18 and its type II partner K8 are the only two keratins expressed. Given that 50% of K8 knockout mice suffered embryonic lethality at day 12 and displayed liver hemorrhage, one might have suspected a similar phenotype in K18 null mice. To analyze whether a null allele had been generated, we tested total protein or cytoskeletal extracts from normal and K18 null mice for keratin expression by SDS-PAGE and Western blotting with specific keratin antibodies. No K18 was found in any of the protein extracts examined, indicating that the knockout for K18 was complete. Also, K8 was absent in hepatocytes of K18 null mice (Fig. 2) in agreement with the rapid degradation of single keratins in the absence of a partner keratin (Domenjoud et al., 1988; Kulesh et al., 1989; Magin et al., 1990). The absence of functional K18 was also confirmed by Western blotting with the broad range antibody IFA (not shown; Pruss et al., 1981), which binds to the COOH-terminal end of keratin rod domains (Magin et al., 1987). The amounts of K18 and its partner K8 as judged by SDS-PAGE analysis were slightly reduced but present in equimolar amounts in heterozygotes, indicating that in vivo the steady-state amount of keratins is determined by that of its corresponding partner (Fig. 2).
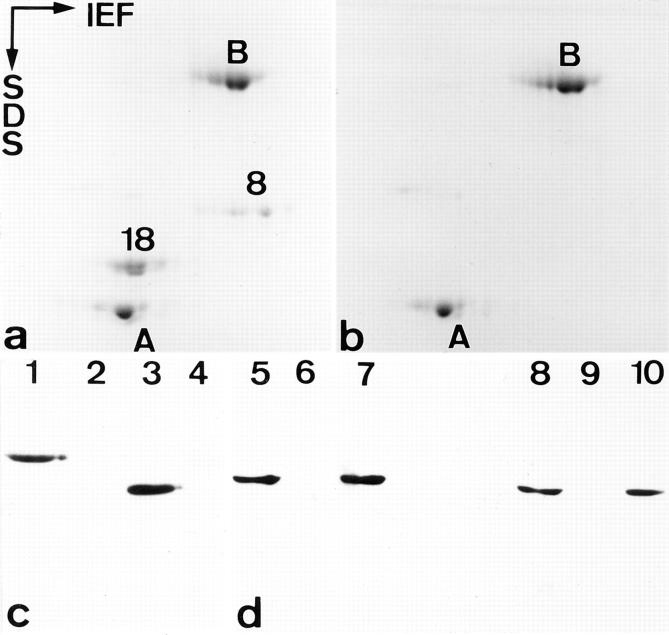
Figure 2.
Detection of liver keratins. (a and b) Analysis of cytoskeletal proteins from livers of normal (a) and homozygous (b) K18 mice by two-dimensional gel electrophoresis. Proteins were separated by isoelectric focusing (IEF) in the first and by SDS-PAGE in the second dimension. 8 and 18 indicate the position of K8 and 18; B and A indicate bovine serum albumin and actin added as reference markers. Note isoelectric keratin variants in wild type and the loss of both keratins in homozygous mice. (c and d) Western blot analysis of total proteins (one dimensional gel electrophoresis) from livers of normal (lanes 1, 3, 5, and 8), hetero- (7 and 10) and homozygous (2, 4, 6, and 9) K18 knockout mice. mAB Troma 1 was used to detetct K8 in lanes 1, 2, and 5–7. mAB Ks 18.04 was used to detect K18 in lanes 3, 4, and 8–10. Bound antibodies were visualized by enhanced chemiluminescence. The amounts of K8 and 18 appear similar in normal and heterozygous mice (d). Compare lane 5 to 7 and 8 to 10 in d.
When total RNA prepared from normal and K18 null mouse livers was probed for the expression of K8 and 18, the mRNA for K18 was absent (Fig. 3), confirming the Western blot results. The mRNA level for K8 was unaltered. In agreement with findings from K8 null mice (Baribault et al., 1994), where the converse was found for K18 mRNA, we concluded that K8 and 18 are regulated independently at the transcriptional level, despite their close chromosomal localization (Waseem et al., 1990).
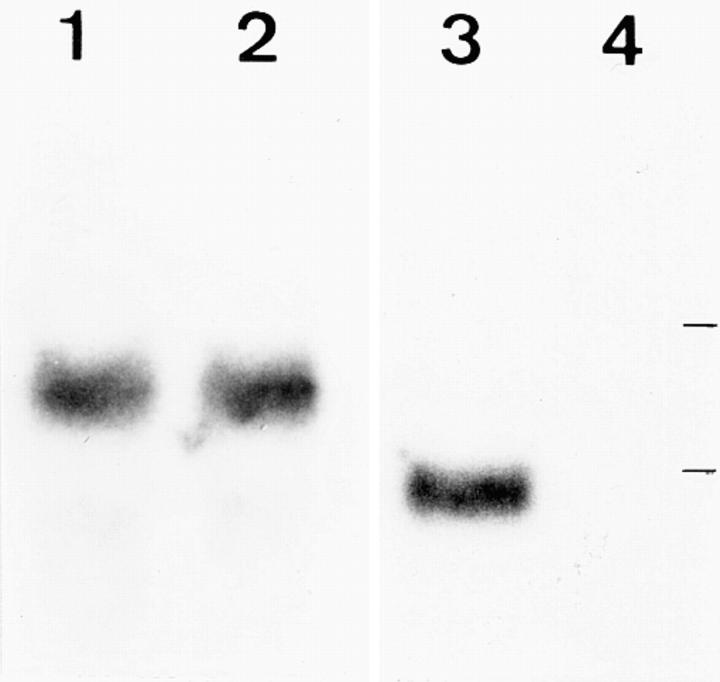
Figure 3.
Northern blot analysis of total liver RNA using random prime–labeled specific probes. The band for K8 mRNA was of equal intensity in normal (lane 1) and homozygous (lane 2) K18 mice. The mRNA for K18 was present in normal (lane 3) and absent in homozygous (lane 4) mice. Lines indicate positions of Escherichia coli 16- and 23 S- rRNAs.
To examine other potential consequences of K18 ablation, we analyzed mRNA and protein expression in tissues with a more complex keratin expression pattern. The Northern blot analysis (Fig. 4) showed that, apart from the loss of K18, the level of K7, 8, 19, and 20 mRNAs remained largely unaltered. We noted a particularly strong expression of mouse K20, which was by far the most prominent keratin mRNA among those investigated. Interestingly, the high level of mRNA did not correlate with the low and rather restricted expression of the corresponding protein (not shown).
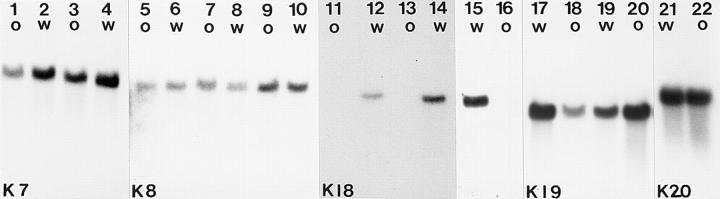
Figure 4.
Northern blot analysis of total RNA using random prime–labeled specific probes. (K7) RNAs hybridized to a K7 probe (1–4), (K8) to a K8 probe (5–10), (K18) to a K18 probe (11–16), (K19) to a K19 (17–20), and (K20) to a K20 probe (21–22). RNA from the following tissues were analyzed: uterus (1, 2, 5, 6, 13, 14, 19, and 20), colon (3, 4, 7, 8, 11, 12, 17, 18, 21, and 22), kidney (9, 10, 15, and 16). w and o indicate tissues from wild-type and K18 null tissues, respectively. Except for the absence of K18 mRNA in uterus (13), colon (11), and kidney (16) of homozygotes, the level of other keratin mRNAs remained largely unchanged in the two genotypes.
The Absence of Keratin Filaments Does Not Interfere with Desmosome Formation in Hepatocytes of Young K18 Null Mice
In cryosections of K18 null mouse liver, up to the age of 4 mo, we noted the complete absence of keratins by indirect immunofluorescence, in line with results from Northern and Western blotting. Histological analysis revealed the presence of small necrotic foci in liver sections from K18 null mice (Magin, T.M., unpublished observations). Our analysis included a panel of monoclonal antibodies and two polyclonal antisera directed against simple epithelial keratins, all of which failed to stain hepatocyte IF (Fig. 5). In control sections, antibodies to K8 and 19 strongly labeled bile duct epithelia known to express K7, 8, 18, and 19 (not shown). Here, the K8/19 staining was predominantly luminal, in agreement with a recent report on the participation of K19 in the formation of the apical cytoskeleton in cultured epithelial cells (Salas et al., 1997).
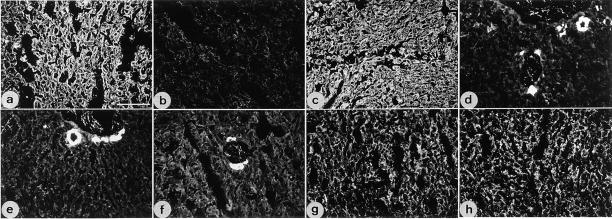
Figure 5.
Immunofluorescence analysis of liver from wild-type (a, c, e, and g) and young K18 null mice (b, d, f, and h). Fixed cryosections were stained with monoclonal antibodies to K18 (a and b), K8 (c and d), K19 (e and f), and desmoplakin I and II (g and h). Note the presence of K8 and 18 in hepatocytes of wild-type (a and c) and the lack of staining in homozygous mice (b and d). In the null mice, K8 staining is restricted to bile duct epithelial cells (d). K19 immunoreactivity was restricted to bile duct epithelia in both genotypes (e and f). Staining with an antibody against desmoplakins I and II indicates the presence of desmosomes with no recognizable differences between both genotypes (g and h). Bar, 95 μm.
In epithelia, keratins are closely associated with desmosomes and hemidesmosomes (Koch and Franke, 1990; Schwarz et al., 1990). Whereas the loss of plakoglobin (Ruiz et al., 1996), BPAG-1 (Guo et al., 1995) and of plectin (McLean et al., 1996) is known to abolish the connection with keratin IF, the consequences of a keratin IF loss for the formation and/or distribution of desmosomes has not been studied before in vivo. We, therefore, stained frozen sections with antibodies to desmosomal proteins. As judged by conventional immunofluorescence microscopy, no difference in the number and distribution of desmosomes could be observed (Fig. 5). The additional analysis of ultrathin liver sections by electron microscopy confirmed the typical ultrastructure of desmosomes without attachment of keratin IF (Fig. 6). It is evident from these and other observations (Denk et al., 1985) that desmosomes form and are maintained in the absence of keratin filaments.
Figure 6.
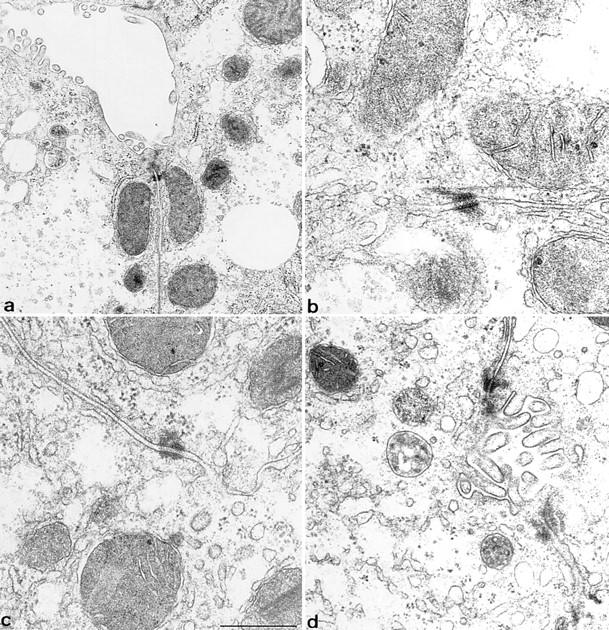
Ultrastructural analysis of hepatocytes from normal (a and b) and young K18 null mice (c and d). A typical desmosome with keratin IF attached in wild-type mice is seen in b. Note the absence of IF in the cytoplasm and at sites of desmosomes in K18 knockout mice (c and d). The nature of the nonfilamentous material attached to a desmosome in c is unknown. Desmosomes appear normal in the absence of IF. Bar, 0.5 μm.
Accumulation of K8-Positive Aggregates in Hepatocytes of Old K18 Null Mice
When frozen sections of 18-mo-old K18 null mice were stained, ∼20–30% of hepatocytes showed irregular K8-positive aggregates of variable size scattered throughout their cytoplasm. K8-aggregates were distributed in the cytoplasm and/or in the cell periphery of single or grouped hepatocytes (Fig. 7). K8-staining cells were often enlarged when compared to cells without K8 accumulation. Immunostaining for K7, 19, and 20, desmin and vimentin failed to detect any of these IF proteins in K8-positive or -negative hepatocytes (not shown). Double immunofluorescence analysis with antibodies to ubiquitin and the Mallory body antigen, MM120-1, in combination with K8 antibodies revealed that the aggregates contained K8, ubiquitin, and the MM120-1 protein, thus resembling MBs in human livers with alcoholic hepatitis (Fig. 7). In contrast to old K18 null mice, neither abnormal keratin structures nor ubiquitin- or MM120-1–positive cytoplasmic depositions were found in control sections from age-matched wild-type mice.
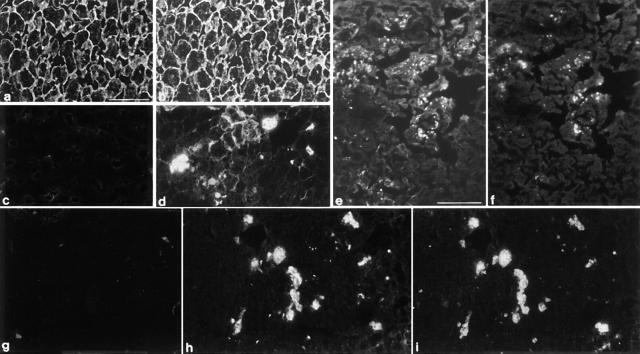
Figure 7.
Immunofluorescence analysis of liver from 18-mo-old K18 null (c–f, h and i) and wild-type mice (a, b, and g). Fixed cryosections were stained with monoclonal antibodies to K18 (a and c), K8 (b, d, and e), M 120 (g and h), and an antiserum specific for ubiquitin (f and i). Double immunofluorescence analysis for K8 and 18 (a–d). Typical K18 and K8 staining in liver sections from wild-type mice (a and b). Note the absence of K18 in old knockout mice (c) and the formation of K8 immunoreactive aggregates in liver sections from old wild-type mice (d). These K8-positive aggregates are present in the cell periphery or as large, cytoplasmic inclusions. Double immunofluorescence for K8 and ubiquitin of sections from K18 null mice (e and f). Note that most but not all K8-positive aggregates (e) also stain positively for ubiquitin (f). Lack of Mallory body-specific staining in wild-type liver sections (g). Double immunofluorescence (MM 120-1 and ubiquitin) of sections from K18 null mice (h and i). Note the double staining of Mallory bodies with MM 120-1 (h) and ubiquitin (i). Bars: (a–d and g and h) 45 μm; (e) 65 μm.
The Lack of K7 Uncovers Strict In Vivo Pairing Rules
Previous in vitro studies have shown that any type I and II keratins can assemble to heterotypic complexes or IF (Hatzfeld and Franke, 1985; Eichner et al., 1986; Magin et al., 1987; Paladini et al., 1996), whereas in vivo, they form specific pairs (Moll et al., 1982; Sun et al., 1985; Cooper and Sun, 1986). As many simple epithelia coexpress the type II K7 and 8 along with type I K18, 19, and 20 (Moll et al., 1982; Lane, 1993), our K18 null mice offered a unique opportunity to analyze possible consequences on keratin pairing.
When we compared frozen sections of uterus from normal and homozygous K18 mice, we noted not only the lack of K18, but also that of K7 (Fig. 8), which was in agreement with our Western blot analysis of cytoskeletal extracts (Fig. 9). The lack of K7 or its drastic reduction was not restricted to uterus but observed also in bile duct and kidney epithelia (not shown). In contrast, the expression and distribution of K8 and K19 seemed unaltered in the tissues examined (Figs. 8 and 10).
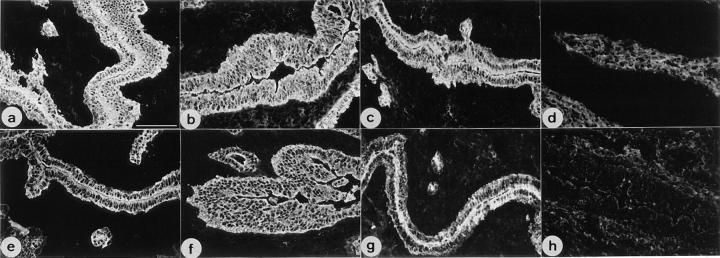
Figure 8.
Immunofluorescence analysis of uterus from wild-type (a, c, e, and g) and K18 null mice (b, d, f, and h). Fixed cryosections were stained with monoclonal antibodies to K8 (a and b), K18 (c and d), K19 (e and f) and K7 (g and h). Note the absence of K7 staining (h) in addition to that of K18 (d) in K18 null mice. An increased staining of the connective tissue due to the reaction of secondary antibodies in sections from knockout mice was observed in d and h. Bar, 95 μm.
Figure 9.
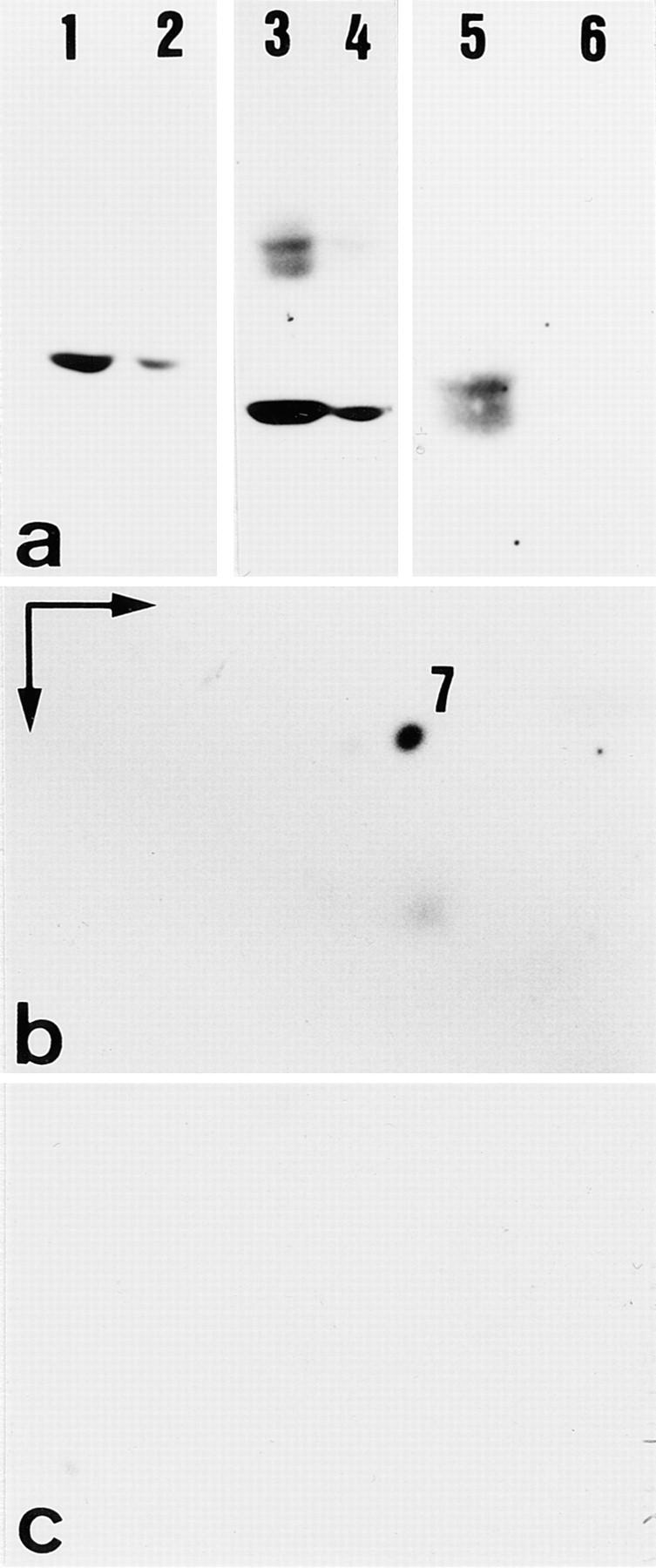
Altered keratin expression in uterus of K18 null mice. (a–c) Western blot analysis of total proteins separated by SDS-PAGE. (a) Proteins separated by SDS-PAGE. Note the slight reduction of K8 (lanes 1 and 2, reacted with mAB Troma 1) and 19 (lanes 3 and 4, reacted with mAB Troma 3) in extracts from K18 null mice (lanes 2 and 4) compared to those from wild-type mice (lanes 1 and 3). The nature of the upper bands in lane 3 is unknown. (lanes 5 and 6) Detection of K18 with mAB Ks 18.04 in wild-type (lane 5), but not in K18 null animals (lane 6). The doublet in lane 5 probably indicates some degradation. (b and c) Analysis of cytoskeletal proteins from uterus of wild-type (b) and homozygous K18 mice (c) by two-dimensional gel electrophoresis, followed by Western blot analysis. Proteins were separated by isoelectric focusing (IEF) in the first and by SDS-PAGE in the second dimension. Note the presence of K7 in wild-type (b) and its absence in K18 null mice (c) as judged by Western blotting with mAB OVTL 12–30 and enhanced chemiluminescence.
Figure 10.
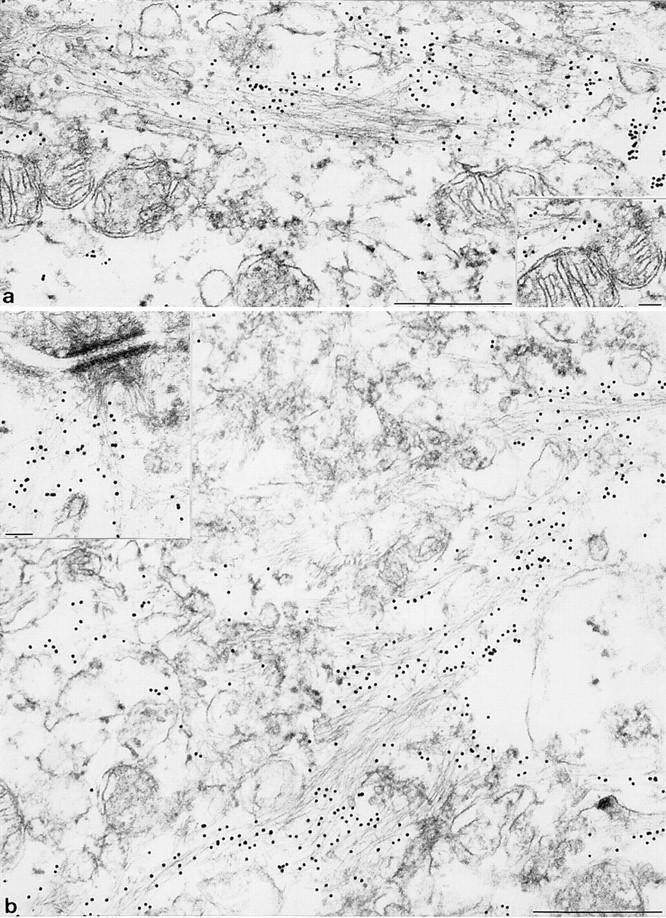
Immune electron microscopy of keratin IF in uterine epithelia from wild-type (a) and K18 null mice (b). Wild-type mice express K7, 8, 18, and 19 in uterus epithelium. (a) IF arranged in loose bundles were identified as keratins after labeling with specific primary antibodies followed by secondary antibodies coupled to 10 nm gold particles. The inset in a shows the interaction of keratin IF with a mitochondrion. (b) The appearance of keratin IF in corresponding epithelium of K18 knockout mice is highly similar if not identical to that in a. This shows that the tail-less K19 forms bona fide IF with K8. Inset in b: typical interaction of K8/19 IF with a desmosome. Keratin IF were detected either with mAB lu-5 (recognizing all keratins) or monospecific K19 antisera. Bars: (a and b) 5 μm; (inset) 0.1 μm.
To see whether K7 might be soluble and was, therefore, missing in cytoskeletal extracts of K18 null mice, we also performed immunoblotting of total proteins with K7 antibodies. Here we detected trace amounts of K7 (not shown). At the same time, the level of K7 mRNA remained unchanged in all tissues examined (Fig. 4). The relative amount of K8 and 19 remained equal in K18 null mice, but was slightly reduced in homozygous compared to normal mice. The presence of K8 and K19 in the cytoskeletal fraction suggested that these two keratins were able to form insoluble structures in vivo. Therefore, we went on to study their distribution by light and electron microscopy.
The Tail-less K19 Forms Bona Fide Intermediate Filaments with K8 and Seems To Replace K18 in Functional Terms
It is well established that keratin head domains are essential for filament formation (Albers and Fuchs, 1987, 1989; Bader et al., 1991; Wilson et al., 1992; Hatzfeld and Burba, 1994). The role of the tail domain is more controversial. Whereas some experiments have documented typical IF formed from K8 and 19 in vitro and in cultured cells (Hatzfeld and Weber, 1990; Bader et al., 1991), others have suggested that the lack of its tail domain renders K19 unable to form IF (Lu and Lane, 1990).
In a number of tissues like kidney and uterine epithelium, the forced absence of K18 left K8 and the tail-less K19 as the only keratins expressed, giving us the unique opportunity to analyze K8/19 structures in greater detail. As judged by immune electron microscopy, they formed bona fide IF. Filaments appeared smooth and in typical loose arrays in wild type (Fig. 10 A), in K18 null mice (Fig. 10 B), and when attached to desmosomes in a manner indistinguishable from those observed with other keratin combinations. We also noted the insertion of keratin IF to the surface of mitochondria (inset in Fig. 10 A). The overall number of IF appeared similar in K18 null mice and in wild-type animals, in agreement with immunofluorescence data.
Immunofluorescence analysis of several additional tissues, including colon, small intestine, kidney, vagina, lung, and pancreas demonstrated that the overall appearance of keratin IF in those tissues of K18 null mice appeared similar to that of wild-type mice, despite the absence of K18. We concluded that in those tissues K19 and K20 seem to be functional equivalents of K18. This could explain why we have not observed a hyperplasia of the colon epithelium that was seen in K8 null mice. Our observations clearly show that in vivo K19 is a fully competent partner for K8, at least in simple epithelia of the mouse.
Discussion
During the last few years, considerable progress has been made in understanding the cytoskeletal functions of keratins in skin. These were revealed by the discovery of point mutations in epidermal keratin genes (Fuchs, 1994; McLean and Lane, 1995) and by the establishment of conventional transgenic (Vassar et al., 1991) or knockout mice (Lloyd et al., 1995; Porter et al., 1996; for a review, see Magin, 1997). The function of simple epithelial keratins is less clear due to the small number of mouse models and to the lack of genetic disorders caused by mutations in these keratin genes (for a recently discovered K18 mutation see Ku et al., 1997). The overall phenotype of K8 (Baribault et al., 1993, 1994) and 18 knockout mice is less dramatic than that of K10 or 14 knockout animals (Lloyd et al., 1995; Porter et al., 1996; Reichelt et al., 1997).
Given that K8 and 18 are coexpressed and represent subunits of the same 10-nm filament throughout embryonic development and many adult epithelia (Jackson et al., 1980; for a review see Lane, 1993), one might have assumed a similar phenotype for K8 and K18 knockout mice. Surprisingly, K18 null mice are viable, fertile, and have a normal life expectancy. However, they develop a specific liver pathology defined by the accumulation of K8-positive Mallory bodies in old animals. Mid-gestational death accompanied by liver hemorrhage or colorectal hyperplasia in adults, which were the hallmarks of K8 null mice (Baribault et al., 1993, 1994), were not associated with the K18 knockout.
Desmosomes Form and Are Maintained in the Absence of Keratin IF
In the mouse, desmosomes form first in trophectodermal cells of the 32-cell stage embryo (Ducibella et al., 1975; Jackson et al., 1980), preceded by the presence of K8 and 18 (Chisholm and Houliston, 1987; Emerson, 1988; Lane, 1993), which at that stage are not well organized as a typical cytoskeleton. It has been proposed that the interaction of desmosomes with IF allows the transduction of forces among epithelial cells (Arnn and Staehelin, 1981). Regarding the formation of desmosomes, several authors have provided evidence that plaque components are assembled in the cytoplasm, attached to or in close association with keratin IF (Jones and Goldman, 1985; Green et al., 1987; Pasdar and Nelson, 1988; Pasdar et al., 1991), invoking a requirement of keratin IF for desmosome formation. In that respect K18 knockout mice have allowed us to analyze desmosomes in the absence of keratin IF ab origine. Clearly, hepatocyte desmosomes have a typical appearance and size distribution in the absence of IF as judged by electron microscopy. By light microscopy we have found no evidence for a reduced amount of desmosomes or an increased cytoplasmic staining of desmoplakin which would be indicative of an increased turnover rate or instability of desmosomes. With respect to cell–cell interaction, we have not detected areas of cell separation, which would indicate a reduced desmosome-mediated adhesion. Taken together one can conclude that at least in liver of K18 null mice the formation and maintenance of desmosomes can occur in the absence of keratin IF.
Accumulation of K8 Aggregates in Hepatocytes of Old K18 Null Mice
The most surprising finding in hepatocytes of old K18 null mice was the accumulation of K8-positive aggregates, which developed in the absence of other keratins, vimentin, or desmin. These aggregates were identified as Mallory bodies by double immunofluoresecence with antibodies directed against ubiquitin and the Mallory body-specific antigen MM120-1 (Zatloukal et al., 1990). Based on in vitro assays (Steinert et al., 1976; Hatzfeld and Franke, 1985), as well as cell microinjection or transfection studies (Domenjoud et al., 1988; Kulesh et al., 1989; Magin et al., 1990; Bader et al., 1991), it has been demonstrated that single keratins do not form IF, are unstable, and become rapidly degraded in vivo. This was confirmed in vivo in K8 (Baribault et al., 1993) and young K18 null mice. At present, neither the triggers nor the point of time of K8 accumulation in hepatocytes are known. With regard to the observed alignment of K8 aggregates at the cell periphery or close to desmosomes, it is tempting to speculate that they could be initiated by the formation of homopolymeric K8 assemblies, which are initially protected against proteolysis. In due course, these initial K8 seeds would increase in size and redistribute also within the cytoplasm. These K8-containing aggregates might acquire anomalous tertiary structures, and become recognized by ubiquitin-targeting mechanisms (Hershko and Ciechanover, 1994; Haas, 1997). Due to their large size, however, they might be relatively protease resistant.
The slow process of keratin aggregation and the absence of a functional IF cytoskeleton in K18 null mice has also been found in a variety of diseases, like human alcoholic hepatitis (Denk et al., 1979), blistering skin disorders (Corden and McLean, 1996), or insulin-dependent diabetes in mice (Blessing et al., 1995). The development of K8 aggregates in old K18 null mice is the first example of the formation Mallory bodies, resulting simply from a keratin gene knockout.
The Lack of K7 in K18 Null Mice: Pairing Specificity of Keratins
Corroborative data on the regulation of keratin expression indicate that most genes are regulated at the transcriptional level (Jorcano et al., 1984; Oshima, 1992; Fuchs and Weber, 1994), with the possible exception of K6 in epidermis (Tyner and Fuchs, 1986). The continued transcription of K8 in our K18 knockout mice, as well as other studies (Knapp et al., 1989; Baribault et al., 1993, 1994; Lloyd et al., 1995; Porter et al., 1996; Reichelt et al., 1997), have not provided evidence for an interdependent control of types I and II keratin genes. Based on these studies, it rather seems that fine tuning of keratin expression involves the formation of heterotypic protein polymers. The rapid turnover or accumulation of single, unpaired keratins indicate a lack of translational feedback mechanisms in this gene family like those operating in the tubulin gene family (Yen et al., 1988).
In the context of these data, the absence or drastic reduction of K7 staining in uterine, kidney, and bile duct epithelia of K18 null mice was completely unexpected. We excluded epitope masking of K7 by using several K7 antibodies, all of which failed to react on frozen sections of K18 null mice. At least two possibilities can be envisaged to explain the lack or drastic reduction of K7 in several epithelia of K18 null mice: (a) K7 could be incapable of forming IF with K19 in vivo. This is unlikely given that these two keratins formed IF de novo upon gene transfer into 3T3 fibroblasts (Lu and Lane, 1990). It is nevertheless conceivable, that in natural epithelia, individual keratins have a distinct subcellular distribution making them unavailable for other than their “natural” partner. (b) K7 might be unable to bind to K19 because it is competed out by K8. Recent measurements using viscosimetry and plasmon surface resonance have indeed shown significant differences of keratin affinities in vitro (Hofmann and Franke, 1997), extending previous measurements based on “melting” keratin complexes in the presence of urea (Franke et al., 1983; Hatzfeld and Franke, 1985). Obviously heterotypic complexes between K8/19 seem to assemble faster and have a higher affinity than those between K7 and K19.
The Case of K19: A Normal Keratin without a Tail Domain?
K19 has a unique structure with a short α-helical extension of nine amino acids instead of a typical tail domain (Bader et al., 1986). It is coexpressed with simple epithelial as well as epidermal keratins and has been proposed to be a marker of epithelial stem cells (Moll et al., 1982; Bartek et al., 1985; Michel et al., 1996). Based on cell transfection experiments, it has been proposed that the lack of a proper tail domain rendered K19 unable to form regular IF (Lu and Lane, 1990), supporting the idea that the IF cytoskeleton of K19 expressing cells might be more flexible than that built of other keratins. This view has been challenged by other in vitro and cell transfection studies, showing that K19 would form typical IF (Hatzfeld and Weber, 1990; Bader et al., 1991).
The ablation of K18 had given us a unique opportunity to analyze the properties of K19 in vivo. A striking feature of tissues like kidney, bile duct, and uterus, which in K18 knockout mice transcribed only K8 and 19 genes, was the positive K19 immunoreactivity. Immune electron microscopy of uterine epithelium revealed beyond doubt that K8 and 19 formed IF of normal appearance with an intracellular distribution similar to that of IF in normal mice. K8/19 IF interacted with desmosomes in a classical manner (Schwarz et al., 1990) and were attached to the surface of mitochondria (for an early observation see Mose-Larsen et al., 1982).
This strongly argues in favor of a normal filament-forming capacity of the murine K19 in vivo. Furthermore, these findings are in line with a previous report showing K8/19 filaments in Wilms tumor (Ishii et al., 1989) as well as in vitro studies (Herrmann et al., 1996), demonstrating that desmin and vimentin (type III IF) form IF without tail domains. In functional terms, it still has to be proven that K8/ 19 IF are equivalent to their tail-containing counterparts.
In summary, the generation of K18 null mice has revealed the following major findings: (a) In contrast to K8 null mice, our animals are viable, fertile, and have a normal life expectancy. (b) Desmosomes form, are maintained, and retain full adhesive properties in the absence of keratin IF. (c) Old mice displayed enlarged hepatocytes with K8 aggregates that were identified as Mallory bodies. The development of K8 aggregates in old K18 null mice is the first example of the formation of Mallory bodies resulting simply from a keratin gene knockout. (d) K7 was absent or drastically reduced in several tissues despite continued transcription of its mRNA, suggesting that K8 preferentially assembles with K19 in the absence of K18. (e) The tail-less keratin 19 forms bona fide IF with K8 in vivo and seems able to compensate for the regular K8/18/ 19 IF in functional terms.
Acknowledgments
We thank E.B. Lane (Dundee, UK), D. Garrod, W.W. Franke, K. Owaribe, and H. Baribault (La Jolla, CA) for gifts of antibodies and R. Leube (University of Mainz, Mainz, Germany) for the K20 probe. T. Morita is thanked for the murine K18 gene. T.M. Magin is most grateful to Conny Stumptner (University of Austria, Graz, Austria) for initiating the analysis of old K18 null mice during a stay in his lab.
Abbreviations used in this paper
- IF
intermediate filaments
- MBs
Mallory bodies
- PGK
phosphoglycerate kinase
Footnotes
This work was initially supported by a Wellcome Trust Fellowship (T.M. Magin) and receives funding by the Deutsche Forschungsgemeinschaft (SFB 284) and the Bonner Forum Biomedizin (T.M. Magin).
Address all correspondence to Thomas M. Magin, Universitaet Bonn, Institut fuer Genetik, Abt. Molekulargenetik, Roemerstrasse 164, 53117 Bonn, Germany. Tel: 49 228 73 4444. Fax: 49 228 73 4558. E-mail: t.magin@uni-bonn.de
References
- Abe M, Oshima RG. A single human keratin 18 gene is expressed in diverse epithelial cells of transgenic mice. J Cell Biol. 1990;111:1197–1206. doi: 10.1083/jcb.111.3.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers K, Fuchs E. The expression of mutant epidermal keratin cDNAs transfected in simple epithelial and squamous cell carcinoma lines. J Cell Biol. 1987;105:791–806. doi: 10.1083/jcb.105.2.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albers K, Fuchs E. Expression of mutant keratin cDNAs in epithelial cells reveals possible mechanisms for initiation and assembly of intermediate filaments. J Cell Biol. 1989;108:1477–1493. doi: 10.1083/jcb.108.4.1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnn J, Staehelin LA. The structure and function of spot desmosomes. Int J Dermatol. 1981;20:330–339. doi: 10.1111/j.1365-4362.1981.tb00815.x. [DOI] [PubMed] [Google Scholar]
- Bader BL, Magin TM, Freudenmann M, Stumpp S, Franke WW. Intermediate filaments formed de novo from tail-less cytokeratins in the cytoplasm and in the nucleus. J Cell Biol. 1991;115:1293–1307. doi: 10.1083/jcb.115.5.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader BL, Magin TM, Hatzfeld M, Franke WW. Amino acid sequence and gene organization of cytokeratin no. 19, an exceptional tail-less intemediate filament protein. EMBO (Eur Mol Biol Organ) J. 1986;5:1865–1875. doi: 10.1002/j.1460-2075.1986.tb04438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baribault H, Penner J, Iozzo RV, Wilson-Heiner M. Colorectal hyperplasia and inflammation in keratin 8-deficient FVB/N mice. Genes Dev. 1994;8:2964–2973. doi: 10.1101/gad.8.24.2964. [DOI] [PubMed] [Google Scholar]
- Baribault H, Price J, Miyai K, Oshima RG. Mid-gestational lethality in mice lacking keratin 8. Genes Dev. 1993;7:1191–1202. doi: 10.1101/gad.7.7a.1191. [DOI] [PubMed] [Google Scholar]
- Bartek J, Taylor J, Papadimitriou, Miller N, Millis R. Patterns of expression of keratin 19 as detected with monoclonal antibodies in human breast tissues and tumours. Int J Cancer. 1985;36:299–306. [PubMed] [Google Scholar]
- Blessing M, Rüther U, Franke WW. Ectopic synthesis of epidermal cytokeratins in pancreatic island cells of transgenic mice interferes with cytoskeletal order and insulin production. J Cell Biol. 1993;120:743–755. doi: 10.1083/jcb.120.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifas JM, Rothman AL, Epstein EH. Epidermolysis bullosa simplex: evidence in two families for keratin gene abnormalities. Science. 1991;2554:1202–1205. doi: 10.1126/science.1720261. [DOI] [PubMed] [Google Scholar]
- Casanova L, Bravo A, Were F, Ramirez A, Jorcano JJ, Vidal M. Tissue-specific and efficient expression of the human simple epithelial keratin 8 gene in transgenic mice. J Cell Sci. 1995;108:811–820. doi: 10.1242/jcs.108.2.811. [DOI] [PubMed] [Google Scholar]
- Chisholm JC, Houliston E. Cytokeratin filament assembly in the preimplantation mouse embryo. Development. 1987;101:565–582. doi: 10.1242/dev.101.3.565. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cooper D, Sun TT. Monoclonal antibody analysis of bovine epithelial keratins. Specific pairs as defined by coexpression. J Biol Chem. 1986;261:4646–4654. [PubMed] [Google Scholar]
- Corden LD, McLean WHI. Human keratin diseases: hereditary fragility of specific epithelial tissues. Exp Dermatol. 1996;5:297–307. doi: 10.1111/j.1600-0625.1996.tb00133.x. [DOI] [PubMed] [Google Scholar]
- Coulombe PA, Fuchs E. Elucidating the early stages of keratin intermediate filament assembly. J Cell Biol. 1990;111:153–169. doi: 10.1083/jcb.111.1.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulombe PA, Hutton ME, Letai A, Hebert A, Paller AS, Fuchs E. Point mutations in human keratin 14 genes of epidermolysis bullosa simplex patients: genetic and functional analyses. Cell. 1991;66:1301–1311. doi: 10.1016/0092-8674(91)90051-y. [DOI] [PubMed] [Google Scholar]
- Denk H, Gschnait F, Wolff K. Hepatocellular hyalin (Mallory bodies) in long term griseofulvin-treated mice: a new experimental model for the study of hyalin formation. Lab Invest. 1975;32:773–776. [PubMed] [Google Scholar]
- Denk H, Franke WW, Kerjaschki D, Eckersdorfer R. Mallory bodies in experimental animals and in man. Int Rev Exp Pathol. 1979;20:77–121. [PubMed] [Google Scholar]
- Denk H, Lackinger E, Cowin P, Franke WW. Maintenance of desmosomes in mouse hepatocytes after drug-induced rearrangement of cytokeratin filament material. Demonstration of independence of desmosomes and intermediate-sized filaments. Exp Cell Res. 1985;161:161–171. doi: 10.1016/0014-4827(85)90500-2. [DOI] [PubMed] [Google Scholar]
- Domenjoud L, Jorcano JL, Breuer B, Alonso A. Synthesis and fate of keratins 8 and 18 in nonepithelial cells transfected with cDNA. Exp Cell Res. 1988;179:352–361. doi: 10.1016/0014-4827(88)90274-1. [DOI] [PubMed] [Google Scholar]
- Ducibella T, Albertini DF, Anderson E, Biggers JD. The preimplantation mammalian embryo: characterization of intercellular junctions and their appearance during development. Dev Biol. 1975;45:231–250. doi: 10.1016/0012-1606(75)90063-9. [DOI] [PubMed] [Google Scholar]
- Eichner R, Sun TT, Aebi U. The role of keratin subfamilies and keratin pairs in the formation of human epidermal intermediate filaments. J Cell Biol. 1986;102:1767–1777. doi: 10.1083/jcb.102.5.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson JA. Disruption of the cytokeratin filament network in the preimplantation mouse embryo. Development. 1988;104:219–234. doi: 10.1242/dev.104.2.219. [DOI] [PubMed] [Google Scholar]
- Franke WW, Denk H, Schmid E, Osborn M, Weber K. Ultrastructural, biochemical and immunologic characterization of Mallory bodies in livers of griseofulvin-treated mice. Fimbriated rods of filaments containing prekeratin-like polypeptides. Lab Invest. 1979;40:207–220. [PubMed] [Google Scholar]
- Franke WW, Schiller DL, Hatzfeld M, Winter S. Protein complexes of intermediate-sized filaments: melting of cytokeratin complexes in urea reveals different polypeptide separation characteristics. Proc Natl Acad Sci USA. 1983;80:7113–7117. doi: 10.1073/pnas.80.23.7113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E. Intermediate filaments and disease: mutations that cripple cell strength. J Cell Biol. 1994;125:511–516. doi: 10.1083/jcb.125.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E, Weber K. Intermediate filaments: structure, dynamics, function and disease. Annu Rev Biochem. 1994;63:345–382. doi: 10.1146/annurev.bi.63.070194.002021. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Weber K. Intermediate filaments: structure, dynamics, function, and disease. Annu Rev Biochem. 1994;63:345–382. doi: 10.1146/annurev.bi.63.070194.002021. [DOI] [PubMed] [Google Scholar]
- Green KJ, Geiger B, Jones JCR, Talian JC, Goldman RD. The relationship between intermediate filaments and microfilaments before and during the formation of desmosomes and adhaerens-type junctions in mouse epidermal keratinocytes. J Cell Biol. 1987;104:1389–1402. doi: 10.1083/jcb.104.5.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L, Degenstein L, Dowling J, Yu Q-C, Wollman R, Fuchs E. Gene targeting of BPAG1: abnormalities in mechanical strength and cell migration in stratified epithelia and neurologic degeneration. Cell. 1995;81:233–243. doi: 10.1016/0092-8674(95)90333-x. [DOI] [PubMed] [Google Scholar]
- Haas AL. Evolving roles for ubiquitin in cellular regulation. FASEB (Fed Am Soc Exp Biol) J. 1997;11:1053–1054. doi: 10.1096/fasebj.11.13.9367340. [DOI] [PubMed] [Google Scholar]
- Hatzfeld M, Burba M. Function of type I and type II keratin head domains: their role in dimer, tetramer and filament formation. J Cell Sci. 1994;107:1959–1972. doi: 10.1242/jcs.107.7.1959. [DOI] [PubMed] [Google Scholar]
- Hatzfeld M, Franke WW. Pair formation and promiscuity of cytokeratins: formation in vitro of heterotypic complexes and intermediate-sized filaments by homologous and heterologous recombinations of purified polypeptides. J Cell Biol. 1985;101:1826–1841. doi: 10.1083/jcb.101.5.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzfeld M, Weber K. The coiled coil of in vitro assembled keratin filaments is a heterodimer of type I and II keratins: use of site-specific mutagenesis and recombinant protein expression. J Cell Biol. 1990;110:1199–1210. doi: 10.1083/jcb.110.4.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzfeld M, Weber K. Tailless keratins assemble into regular intermediate filaments in vitro. J Cell Sci. 1990;97:317–324. doi: 10.1242/jcs.97.2.317. [DOI] [PubMed] [Google Scholar]
- Hazan R, Denk H, Franke WW, Lackinger E, Schiller DL. Change of cytokeratin organization during development of Mallory bodies as revealed by a monoclonal antibody. Lab Invest. 1986;54:543–553. [PubMed] [Google Scholar]
- Herrmann H, Haener M, Brettel M, Mueller SA, Goldie KN, Fedtke B, Lustig A, Franke WW, Aebi U. Structure and assembly properties of the intermediate filament protein vimentin: the role of its head, rod and tail domains. J Mol Biol. 1996;264:933–953. doi: 10.1006/jmbi.1996.0688. [DOI] [PubMed] [Google Scholar]
- Hershko A, Ciechanover A. The ubiquitin system for protein degradation. Annu Rev Biochem. 1992;61:761–807. doi: 10.1146/annurev.bi.61.070192.003553. [DOI] [PubMed] [Google Scholar]
- Ichinose Y, Morita T, Zhang FY, Srimahasongcram S, Tondella ML, Matsumoto M, Nozaki M, Matsushiro A. Nucleotide sequence and structure of the mouse cytokeratin endoB gene. Gene. 1988;70:85–95. doi: 10.1016/0378-1119(88)90107-2. [DOI] [PubMed] [Google Scholar]
- Hofmann I, Franke WW. Heterotypic interactions and filament assembly of type I and type II cytokeratins in vitro: viscosimetry and determinations of relative affinities. Eur J Cell Biol. 1997;72:122–132. [PubMed] [Google Scholar]
- Hu B, French SW. 2′,3′-Dideoxyinosine-induced Mallory bodies in patients with HIV. Am J Clin Pathol. 1997;108:280–283. doi: 10.1093/ajcp/108.3.280. [DOI] [PubMed] [Google Scholar]
- Ishii E, Fujimoto J, Hara S, Tanaka S, Hata J. Human sarcomatous Wilms' tumor lines: evidence for epithelial differentiation in clear cell sarcoma of the kidney. Cancer Res. 1989;49:5392–5399. [PubMed] [Google Scholar]
- Jackson BW, Grund C, Schmid E, Buerki K, Franke WW, Illmensee K. Formation of cytoskeletal elements during mouse embryogenesis. Differentiation. 1980;17:161–179. doi: 10.1111/j.1432-0436.1980.tb01093.x. [DOI] [PubMed] [Google Scholar]
- Jensen K, Gluud C. The Mallory body: morphological, clinical and experimental studies (Part 1 of a literature survey) Hepatology. 1994;20:1061–1077. doi: 10.1002/hep.1840200440. [DOI] [PubMed] [Google Scholar]
- Jensen K, Gluud C. The Mallory body: theories on development and pathological significance (Part 2 of a literature survey) Hepatology. 1994;20:1330–1342. [PubMed] [Google Scholar]
- Jones JCR, Goldman RD. Intermediate filaments and the initiation of desmosome assembly. J Cell Biol. 1985;101:506–517. doi: 10.1083/jcb.101.2.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorcano JL, Magin TM, Franke WW. Cell type-specific expression of bovine keratin genes as demonstrated by the use of complementary DNA clones. J Mol Biol. 1984;176:21–37. doi: 10.1016/0022-2836(84)90380-2. [DOI] [PubMed] [Google Scholar]
- Klymkowsky MW. Intermediate filaments in 3T3 cells collapse after intracellular injection of a monoclonal anti-intermediate filament antibody. Nature. 1981;291:249–251. doi: 10.1038/291249a0. [DOI] [PubMed] [Google Scholar]
- Knapp AC, Franke WW. Spontaneous losses of control of cytokeratin gene expression in transformed, non-epithelial human cells occurring at different levels of regulation. Cell. 1989;59:67–79. doi: 10.1016/0092-8674(89)90870-2. [DOI] [PubMed] [Google Scholar]
- Knapp AC, Bosch FX, Hergt M, Kuhn C, Winter S, Simanowski, Schmid E, Regauer S, Bartek J, Franke WW. Cytokeratins and cytokeratin filaments in subpopulations of cultured human and rodent cells of nonepithelial origin: modes and patterns of formation. Differentiation. 1989;42:81–102. doi: 10.1111/j.1432-0436.1989.tb00610.x. [DOI] [PubMed] [Google Scholar]
- Koch PJ, Franke WW. Desmosomal cadherins: another growing multigene family of adhesion molecules. Curr Opin Cell Biol. 1994;6:682–687. doi: 10.1016/0955-0674(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Ku NO, Michie SA, Oshima RG, Omary MB. Chronic hepatitis, hepatocyte fragility, and increased soluble phosphoglycokeratins in transgenic mice expressing a keratin 18 conserved arginine mutant. J Cell Biol. 1995;131:1303–1314. doi: 10.1083/jcb.131.5.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku NO, Wright TL, Terrault NA, Gish R, Omary MB. Mutation of human keratin 18 in association with cryptogenic cirrhosis. J Clin Invest. 1997;99:19–23. doi: 10.1172/JCI119127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulesh DA, Cecena G, Darmon YM, Vassear M, Oshima RG. Posttranslational regulation of keratins: degradation of mouse and human keratins 18 and 8. Mol Cell Biol. 1989;9:1553–1565. doi: 10.1128/mcb.9.4.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane, E.B. 1993. Keratins. In Connective Tissue and Its Heritable Disorders. Wiley-Liss, New York. 237–247.
- Lane EB, Rugg EL, Navsaria H, Leigh IM, Heagerty AHM, Ishida-Yamamoto A, Eady RAJ. A mutation in the conserved helix termination peptide of keratin 5 in hereditary skin blistering. Nature. 1992;356:244–246. doi: 10.1038/356244a0. [DOI] [PubMed] [Google Scholar]
- Liang P, MacRae TH. Molecular chaperones and the cytoskeleton. J Cell Sci. 1997;110:1431–1440. doi: 10.1242/jcs.110.13.1431. [DOI] [PubMed] [Google Scholar]
- Lloyd C, Yu Q-C, Cheng J, Turksen K, Degenstein L, Hutton E, Fuchs E. The basal keratin network of stratified squamous epithelia: defining K15 function in the absence of K14. J Cell Biol. 1995;129:1329–1344. doi: 10.1083/jcb.129.5.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Lane EB. Retrovirus-mediated transgenic keratin expression in cultured fibroblasts: specific domain functions in keratin stabilization and filament formation. Cell. 1990;62:681–696. doi: 10.1016/0092-8674(90)90114-t. [DOI] [PubMed] [Google Scholar]
- Magin, T.M. 1997. Lessons from keratin transgenic and knockout mice. In Subcellular Biochemistry. H. Herrmann and J.R. Harris, editors. Plenum Publishing Corp., London.
- Magin TM, Jorcano JL, Franke WW. Translational products of mRNAs coding for non-epidermal cytokeratins. EMBO (Eur Mol Biol Organ) J. 1983;2:1387–1392. doi: 10.1002/j.1460-2075.1983.tb01596.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magin TM, Hatzfeld M, Franke WW. Analysis of cytokeratin domains by cloning and expression of intact and deleted polypeptides in Escherichia coli. EMBO (Eur Mol Biol Organ) J. 1987;6:2607–2615. doi: 10.1002/j.1460-2075.1987.tb02551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magin TM, Bader BL, Freudenmann M, Franke WW. De novo formation of cytokeratin filaments in calf lens cells and cytoplasts after transfection with cDNAs or microinjection with mRNAs encoding human cytokeratins. Eur J Cell Biol. 1990;53:333–348. [PubMed] [Google Scholar]
- Magin TM, McEwan C, Milne M, Pow AM, Selfridge J, Melton DW. A position- and orientation-dependent element in the first intron is required for expression of the mouse HPRT gene in embryonic stem cells. Gene. 1992;122:289–296. doi: 10.1016/0378-1119(92)90217-d. [DOI] [PubMed] [Google Scholar]
- Mallory FB. Cirrhosis of the liver: five different types of lesions from which it may arise. Bull Johns Hopkins Hosp. 1911;22:69–75. [Google Scholar]
- Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci USA. 1997;94:849–854. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markey AC, Lane EB, Macdonald DM, Leigh IM. Keratin expression in basal cell carcinomas. Br J Dermatol. 1992;126:154–160. doi: 10.1111/j.1365-2133.1992.tb07813.x. [DOI] [PubMed] [Google Scholar]
- McLean WH, Lane EB. Intermediate filaments in disease. Curr Opin Cell Biol. 1995;7:118–125. doi: 10.1016/0955-0674(95)80053-0. [DOI] [PubMed] [Google Scholar]
- McLean WHI, Pulkkinen L, Smith FJD, Rugg EL, Lane EB, Bullrich F, Burgeson RE, Amano S, Hudson DL, Owaribe K, et al. Loss of plectin causes epidermolysis bullosa with muscular dystrophy: cDNA cloning and genomic organization. Genes Dev. 1996;10:1724–1735. doi: 10.1101/gad.10.14.1724. [DOI] [PubMed] [Google Scholar]
- Mertens C, Kuhn C, Franke WW. Plakophilins 2a and 2b: constitutive proteins of dual location in the karyoplasm and the desmosomal plaque. J Cell Biol. 1996;135:1009–1025. doi: 10.1083/jcb.135.4.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel M, Torik N, Godbout MJ, Lussier M, Gaudreau P, Royal A, Germain L. Keratin 19 as a biochemical marker of skin stem cells in vivo and in vitro: keratin 19 expressing cells are differentially localized in function of anatomic sites, and their number varies with donor age and culture stage. J Cell Sci. 1996;109:1017–1028. doi: 10.1242/jcs.109.5.1017. [DOI] [PubMed] [Google Scholar]
- Moll R, Franke WW, Schiller DL, Geiger B, Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982;31:11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Moll R, Schiller DL, Franke WW. Identification of protein IT of the intestinal cytoskeleton as a novel type I cytokeratin with unusual properties and expression patterns. J Cell Biol. 1990;111:567–580. doi: 10.1083/jcb.111.2.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mose-Larsen P, Bravo R, Fey SJ, Small JV, Celis JE. Putative association of mitochondria with a subpopulation of intermediate-sized filaments in cultured human skin fibroblasts. Cell. 1982;31:681–692. doi: 10.1016/0092-8674(82)90323-3. [DOI] [PubMed] [Google Scholar]
- Oshima RG. Intermediate filament molecular biology. Curr Opin Cell Biol. 1992;4:110–116. doi: 10.1016/0955-0674(92)90067-m. [DOI] [PubMed] [Google Scholar]
- Paladini RD, Takahashi K, Bravo NS, Coulombe PA. Onset of re-epithelialization after skin injury correlates with a reorganization of keratin filaments in wound edge keratinocytes: defining a potential role for keratin 16. J Cell Biol. 1996;132:381–397. doi: 10.1083/jcb.132.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasdar M, Nelson WJ. Kinetics of desmosome assembly in Madin-Darby canine kidney epithelial cells: temporal and spatial regulation of desmoplakin organization and stabilization upon cell–cell contact. II. Morphological analysis. J Cell Biol. 1988;106:687–695. doi: 10.1083/jcb.106.3.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasdar M, Krzeminiski KA, Nelson WJ. Regulation of desmosome assembly in MDCK epithelial cells: coordination of membrane core and cytoplasmic plaque domain assembly at the plasma membrane. J Cell Biol. 1991;113:645–655. doi: 10.1083/jcb.113.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter RM, Leitgeb S, Melton DW, Swensson O, Eady RAJ, Magin TM. Gene targeting at the mouse cytokeratin 10 locus: severe skin fragility and changes of cytokeratin expression in the epidermis. J Cell Biol. 1996;132:925–936. doi: 10.1083/jcb.132.5.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preisegger K-H, Zatloukal K, Spurej G, Riegelnegg D, Denk H. Common epitopes of human and murine Mallory bodies and Lewy bodies as revealed by a neurofilament antibody. Lab Invest. 1992;66:193–199. [PubMed] [Google Scholar]
- Pruss RM, Mirsky R, Raff RM, Thorpe R, Dowding AJ, Anderton BH. All classes of intermediate filaments share a common antigenic determinant defined by a monoclonal antibody. Cell. 1981;27:419–428. doi: 10.1016/0092-8674(81)90383-4. [DOI] [PubMed] [Google Scholar]
- Reichelt J, Bauer C, Porter RM, Lane EB, Herzog V, Magin TM. Out of balance: consequences of a partial keratin 10 knockout. J Cell Sci. 1997;110:2175–2186. doi: 10.1242/jcs.110.18.2175. [DOI] [PubMed] [Google Scholar]
- Ruiz P, Brinkmann V, Ledermann B, Behrend M, Grund C, Thalhammer C, Vogel F, Birchmeier C, Günthert U, Franke WW, et al. Targeted mutation of plakoglobin in mice reveals essential functions of desmosomes in the embryonic heart. J Cell Biol. 1996;135:215–225. doi: 10.1083/jcb.135.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas PJ, Rodriguez ML, Viciana AL, Vega DE, Salas, Hauri HP. The apical submembrane cytoskeleton participates in the organization of the apical pole in epithelial cells. J Cell Biol. 1997;137:359–375. doi: 10.1083/jcb.137.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook, J., E.F. Fritsch, and T. Maniatis. 1989. Molecular Cloning, Volumes 1–3. Cold Spring Laboratory Press, Cold Spring Harbor, New York.
- Schmidt A, Heid HW, Schäfer S, Nuber UA, Zimbelman R, Franke WW. Desmosomes and cytoskeletal architecture in epithelial differentiation: cell-type-specific plaque components and intermediate filament anchorage. Eur J Cell Biol. 1994;65:229–245. [PubMed] [Google Scholar]
- Schröder R, Mundegar RR, Treusch M, Schlegel U, Blümcke I, Owaribe K, Magin TM. Altered distribution of plectin/HD1 in dystrophinopathies. Eur J Cell Biol. 1997;74:165–171. [PubMed] [Google Scholar]
- Schwarz MA, Owaribe K, Kartenbeck J, Franke WW. Desmosomes and hemidesmosomes: constitutive molecular components. Annu Rev Cell Biol. 1990;6:461–491. doi: 10.1146/annurev.cb.06.110190.002333. [DOI] [PubMed] [Google Scholar]
- Selfridge J, Pow AM, McWhir J, Magin TM, Melton DW. Gene targeting using a HPRT minigene/HPRT-deficient embryonic stem cell system: inactivation of the mouse ERCC-1 gene. Somatic Cell Mol Genet. 1992;18:325–336. doi: 10.1007/BF01235756. [DOI] [PubMed] [Google Scholar]
- Stacey A, Schnieke A, McWhir J, Colman A, Melton DW. Use of double-replacement gene targeting to replace the murine alpha-lactalbumin gene with its human counterpart in embryonic stem cells and mice. Mol Cell Biol. 1994;14:1009–1016. doi: 10.1128/mcb.14.2.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert PM. The two-chain coiled-coil molecule of native epidermal keratin intermediate filaments is a type I-type II heterodimer. J Biol Chem. 1990;265:8766–8774. [PubMed] [Google Scholar]
- Steinert PM, Idler WW, Zimmerman SB. Self-assembly of bovine epidermal keratin filaments in vitro. J Mol Biol. 1976;108:547–567. doi: 10.1016/s0022-2836(76)80136-2. [DOI] [PubMed] [Google Scholar]
- Sun TT, Tseng SC, Huang AJ, Cooper D, Schermer A, Lynch MH, Weiss R, Eichner R. Monoclonal antibody studies of mammalian epithelial keratins: a review. Annu New York Acad Sci. 1985;455:307–329. doi: 10.1111/j.1749-6632.1985.tb50419.x. [DOI] [PubMed] [Google Scholar]
- Trevor K, Linney E, Oshima RG. Suppression of endo B cytokeratin by its antisense RNA inhibits the normal coexpression of endo A cytokeratin. Proc Natl Acad Sci USA. 1987;84:1040–1044. doi: 10.1073/pnas.84.4.1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyner AL, Fuchs E. Evidence for posttranscriptional regulation of the keratins expressed during hyperproliferation and malignant transformation in human epidermis. J Cell Biol. 1986;103:1945–1955. doi: 10.1083/jcb.103.5.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassar R, Coulombe PA, Degenstein L, Albers K, Fuchs E. Mutant keratin expression in transgenic mice causes marked abnormalities resembling a human genetic skin disease. Cell. 1991;64:265–380. doi: 10.1016/0092-8674(91)90645-f. [DOI] [PubMed] [Google Scholar]
- Venetianer A, Schiller DL, Magin T, Franke WW. Cessation of cytokeratin expression in a rat hepatoma cell line lacking differentiated functions. Nature. 1983;305:730–733. doi: 10.1038/305730a0. [DOI] [PubMed] [Google Scholar]
- Waseem A, Gough AC, Spurr NK, Lane EB. Localization of the gene for human simple epithelial keratin 18 to chromosome 12 using polymerase chain reaction. Genomics. 1990;7:188–194. doi: 10.1016/0888-7543(90)90540-b. [DOI] [PubMed] [Google Scholar]
- Wilson AK, Coulombe PA, Fuchs E. The roles of K5 and K14 head, tail, and R/K L L E G E domains in keratin filament assembly in vitro. J Cell Biol. 1992;119:401–414. doi: 10.1083/jcb.119.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen TJ, Machlin PS, Cleveland DW. Autoregulated instability of beta-tubulin mRNAs by recognition of the nascent amino terminus of beta-tubulin. Nature. 1988;334:580–585. doi: 10.1038/334580a0. [DOI] [PubMed] [Google Scholar]
- Zatloukal K, Denk H, Spurej G, Lackinger E, Preisegger K-H, Franke WW. High molecular weight component of Mallory bodies detected by a monoclonal antibody. Lab Invest. 1990;62:427–434. [PubMed] [Google Scholar]
- Zatloukal K, Böck G, Rainer I, Denk H, Weber K. High molecular weight components are main constituents of Mallory bodies isolated with a fluorescence activated cell sorter. Lab Invest. 1991;64:200–206. [PubMed] [Google Scholar]