Abstract
The protein 4.1 superfamily is comprised of a diverse group of cytoplasmic proteins, many of which have been shown to associate with the plasma membrane via binding to specific transmembrane proteins. Coracle, a Drosophila protein 4.1 homologue, is required during embryogenesis and is localized to the cytoplasmic face of the septate junction in epithelial cells. Using in vitro mutagenesis, we demonstrate that the amino-terminal 383 amino acids of Coracle define a functional domain that is both necessary and sufficient for proper septate junction localization in transgenic embryos. Genetic mutations within this domain disrupt the subcellular localization of Coracle and severely affect its genetic function, indicating that correct subcellular localization is essential for Coracle function. Furthermore, the localization of Coracle and the transmembrane protein Neurexin to the septate junction display an interdependent relationship, suggesting that Coracle and Neurexin interact with one another at the cytoplasmic face of the septate junction. Consistent with this notion, immunoprecipitation and in vitro binding studies demonstrate that the amino-terminal 383 amino acids of Coracle and cytoplasmic domain of Neurexin interact directly. Together these results indicate that Coracle provides essential membrane-organizing functions at the septate junction, and that these functions are carried out by an amino-terminal domain that is conserved in all protein 4.1 superfamily members.
Cell–cell communication within an epithelium plays vital roles in the maintenance of epithelial character and in correct specification of cell fate. Epithelial tissues exhibit an apicobasal polarity with the apical domain separated from the basolateral domain by a junctional complex. In Drosophila and other invertebrate epithelia, the apicolateral junctional complex consists of an apical adherens junction and a more basal septate junction (Poodry and Schneiderman, 1970; Tepass and Hartenstein, 1994). The septate junction, characterized by ladder-like septa in electron micrographs, is an invertebrate specific junction which has been proposed, based upon similar molecular components, to be functionally analogous to the vertebrate tight junction (Willott et al., 1993). The molecular characterization of numerous proteins that function within an array of signaling pathways has revealed that the junctional complex is a primary site for cellular interactions. Studies in Drosophila demonstrate that the receptor tyrosine kinases Sevenless and the EGF receptor homologue are localized to the apical junctional complex and enriched at the adherens junction (Tomlinson et al., 1987; Zak and Shilo, 1992). In addition, the Notch receptor and its transmembrane ligands, Delta and Serrate, have also been localized to the adherens junction (Fehon et al., 1991; Thomas et al., 1991; Kooh et al., 1993). Furthermore, Armadillo, the Drosophila homologue of β-catenin, is a component of the wingless signaling pathway and makes up part of the molecular architecture of the adherens junction through interactions with junctionally localized cadherins (Peifer and Wieschaus, 1990; Peifer et al., 1991).
The protein 4.1 superfamily comprises a large group of cytoplasmic proteins, many of which have been shown to associate with the plasma membrane (for review see Sato et al., 1992; Arpin et al., 1994; Takeuchi et al., 1994; McCartney and Fehon, 1997; Tsukita et al., 1997; Vaheri et al., 1997). Members of this superfamily include protein 4.1, talin, the ezrin/radixin/moesin (ERM)1 subfamily, the Neurofibromatosis 2 tumor suppressor Merlin, Drosophila Expanded, several protein tyrosine phosphatases (Hendriks et al., 1995; Higashitsuji et al., 1995), and at least two nonmuscle myosins (Chen et al., 1996; Weil et al., 1996). The unifying structural characteristic of this family is a region of ∼300 amino acids, usually situated near the amino terminus of the protein. Studies of several family members have shown that this region binds to the cytoplasmic tail of specific transmembrane proteins, thereby facilitating their localization to the cytoplasmic face of the plasma membrane (Rees et al., 1990). Interest in the protein 4.1 superfamily has increased because of recent evidence, which suggests that members of this family participate in important cell signaling events. For example, Merlin, the product of the Neurofibromatosis 2 tumor-suppressor gene, is involved in growth regulation (Rouleau et al., 1993; Trofatter et al., 1993). Additionally, members of the ERM subfamily have been implicated in Rho-dependent signaling (Hirao et al., 1996; Mackay et al., 1997) and in a signaling pathway involving hepatocyte growth factor (Crepaldi et al., 1997).
As the prototypic member of this superfamily, protein 4.1 has been extensively studied. These studies have revealed that the erythrocyte isoform of protein 4.1 cross-links the plasma membrane to the underlying cytoskeleton. This function is carried out through protein–protein interactions with glycophorin C at the membrane using sequences within the conserved amino-terminal domain of protein 4.1, and with spectrin and actin using a more carboxyl-terminal domain (Marchesi, 1985). At the erythrocyte membrane, protein 4.1 exists in a ternary complex with glycophorin C and a palmitoylated glycoprotein, p55 (Marfatia et al., 1994, 1995), a member of a growing family of membrane-associated guanylate kinase (MAGUK) proteins. A defining structural motif of MAGUK proteins is the presence of one to several PDZ domains (PSD-95, DLG, ZO-1), which are known to be involved in protein– protein interactions (Kim et al., 1995). Many signaling molecules found at the plasma membrane contain PDZ domains, leading to the speculation that they function in the clustering of signaling complexes at particular plasma membrane domains (Fanning and Anderson, 1996).
Four members of the protein 4.1 superfamily, coracle (cor), Merlin, Moesin, and expanded have been identified in Drosophila (Boedigheimer and Laughon, 1993; Fehon et al., 1994; McCartney and Fehon, 1996), thereby providing powerful genetic tools to aid in the functional analysis of this protein superfamily. We have previously described a Drosophila homologue of protein 4.1, Coracle, that is found at the septate junction in ectodermally derived epithelial cells (Fehon et al., 1994). Mutations in coracle are embryonic lethal with defects manifested during the process of dorsal closure. In addition, coracle mutations dominantly suppress the rough eye phenotype of Ellipse, a hypermorphic allele of the EGF-receptor homologue. Two other proteins that localize to the septate junction in epithelial cells bear striking resemblance to vertebrate proteins known to interact with protein 4.1. The first is Drosophila Neurexin (NRX), a member of a growing family of proteins initially identified as synapse-specific receptors (Petrenko et al., 1991; Baumgartner et al., 1996). The cytoplasmic domain of NRX shares 68% similarity to the cytoplasmic domain of glycophorin C, and Coracle protein is mislocalized in a strong hypomorphic allele of Nrx, suggesting the possibility of a direct interaction between Coracle and NRX similar to that of protein 4.1 with glycophorin C (Baumgartner et al., 1996). The other septate junction resident protein that is similar to a known protein 4.1 interactor is Discs Large (DLG), one of the prototypic members of the MAGUK protein family. Mutations in dlg lead to partial to complete loss of septate junctions, loss of apicobasal polarity, and neoplastic overgrowth of imaginal tissue, suggesting that DLG is a critical component of the septate junction (Woods and Bryant, 1991; Woods et al., 1996). Furthermore, a human homologue of DLG, hDLG, has been shown to bind to protein 4.1 (Lue et al., 1994), raising the possibility that Coracle and DLG interact.
To explore the role of Coracle in organizing proteins at the septate junction, we have initiated molecular-genetic and biochemical studies to identify regions that are functionally significant. We show that the amino-terminal 383 amino acids of Coracle define a functional domain that is necessary and sufficient for septate junction localization. In addition, we demonstrate that Coracle binds to the cytoplasmic domain of NRX in vitro and that in vivo these two proteins display an interdependent relationship that is essential for maintenance of their proper localization at the septate junction.
Materials and Methods
Drosophila Stocks
All fly stocks were maintained on standard corn meal, yeast, molasses, agar medium. Canton S was used as the wild-type stock. w 1118 flies were used for the transformation of coracle transgenes using the mini-w + vector pCaSpeR-hs (Thummel et al., 1988). The coracle alleles used in this study are described elsewhere. Nrx mutant fly stocks (Nrx 46, Nrx 4025, Nrx 4304, and Nrx 14) were described previously (Baumgartner et al., 1996). Df(3L) vin 7 was obtained from the Bloomington Drosophila stock center at Indiana University (Bloomington, IN).
Sequencing of Mutant coracle Alleles
Genomic DNA was obtained using standard methods from flies homozygous for either cor 4 or cor 6, which had been rescued to adulthood by ubiquitous expression of a coracle cDNA transgene (Fehon et al., 1994). coracle genomic DNA was amplified using intron-specific coracle primers. The resulting PCR products were sequenced using the AmpliCycle sequence kit (Perkin Elmer Corp., Branchburg, NJ). The sequence was verified on both strands.
coracle Transgenes
The transformation vector pCaSpeR-hs (Thummel et al., 1988) was modified to include an amino-terminal myc epitope by annealing the two primers consmyc S (sense) 5′-AAT TCA CCA TGG AGC AAA AGC TCA TTT CTG AAG AGG ACT TGA GGC CTA A and consmyc A (antisense) 5′-GAT CTT AGG CCT CAA GTC CTC TTC AGA AAT GAG CTT TTG CTC CAT GGT G, which produce a duplex with overhanging EcoRI and BglII ends. This fragment was subsequently cloned into an EcoRI/BamHI cut pCaSpeR-hs vector (all restriction enzymes were obtained from New England Biolabs Inc., Beverly, MA). Digestion of the modified vector with StuI allowed PCR generated coracle transgenes to be cloned in-frame immediately downstream of the myc epitope.
The following coracle transgenes were generated by PCR amplification from a full length coracle cDNA clone (Fehon et al., 1994): cor 1–383 (bp 370–1,516), cor 1–323 (bp 370–1,337), cor 1–167 (bp 370–868), cor 106–167 (bp 685–868), cor 106–383 (bp 685–1,516), cor 207–383 (bp 988–1,516), cor 378–1698 (bp 1,501–5,544), cor 378–1591 (bp 1,501–5,141), cor 1585–1698 (bp 5,122–5,544). The sequence of all PCR amplified regions was confirmed by sequencing using standard methods (Fehon et al., 1991).
To specifically immunoprecipitate cor 4 mutant protein from a large collection of embryos, a transformation construct providing genetic rescue of coracle mutations that lacked the epitope recognized by the guinea pig anti-Coracle antibody was constructed by fusing the Drosophila ubiquitin promoter (Lee et al., 1988) to the cDNA2 isoform of coracle (Fehon et al., 1994) and placing this expression cassette within pCaSperR-4 (Thummel et al., 1988). For this construct, a 1.3-kb EcoRI/PvuII fragment representing a portion of the 3′ end of cDNA2 was ligated into an EcoRI/PvuII (partial) cut pRmHa-3 construct containing the 3′ end of coracle. The resulting plasmid was digested with EcoRI and XbaI to excise a 2.8-kb fragment which contained the 3′ half of cDNA2 and the ADH polyadenylation signal. This fragment was ligated along with an 885-bp SacI/EcoRI fragment containing the 5′ half of cDNA2 into a SacI/XbaI digested RHXpHSS7-pUp2 vector. A 6.1-kb NotI fragment containing the ubiquitin promoter, the coracle cDNA2 coding sequence and the ADH polyadenylation signal was then excised and subcloned into a NotI digested pCaSpeR-4 vector. Transformation of all these constructs was performed as described (Rebay et al., 1993). For each construct from 4–10 independent transformant lines were established.
Transient Transfection of Schneider 2 Cells
The maintenance, transfection, induction, and immunofluorescent analysis of Schneider 2 (S2) cells were performed as described (Fehon et al., 1990). The heat shock regimen consisted of a 30-min heat shock at 38°C followed by 2 h recovery at 25°C.
Antibody Preparation, Immunolocalization, and Cuticle Preparations
A DLG fusion protein was generated by cloning a PCR-generated fragment of nucleotides 2,653–3,360 comprising amino acids 757–993 (Woods and Bryant, 1991) into SmaI cut pGEX 2 (Smith and Johnson, 1988; Pharmacia Diagnostics AB, Uppsala, Sweden). The fusion protein was purified as described (Frorath et al., 1991). Polyclonal antiserum was raised against the DLG fusion protein in mice. Mouse monoclonal antiserum against the myc epitope was obtained as monoclonal supernatant from cell line 1-9E10.2 (Evan et al., 1985) using standard procedures (Harlow and Lane, 1988). Guinea pig polyclonal antiserum against Coracle has been previously described (Fehon et al., 1994). Rabbit polyclonal antiserum against NRX was provided by H. Bellen (Baylor College of Medicine, Baylor, TX) and has been previously described (Baumgartner et al., 1996). All secondary antibodies were obtained from Jackson ImmunoResearch Laboratories (West Grove, PA).
Mutant embryos were unambiguously identified either using marked balancers that express β-gal driven by the ftz promoter or by counterstaining the embryos with antibodies against Coracle or NRX. Immunostaining of embryos was performed as described (Fehon et al., 1991). For the genetic rescue of NRX localization, wild-type embryos or embryos of the genotypes cor 5; hscor 1–383 or cor 5; hscor 378–1698 were collected for 2 h at 25°C, aged for 4 h at 25°C, heat shocked for 1 h at 38°C, and then allowed to recover for 7 h at 25°C. The embryos were subsequently fixed and stained as described above. A Zeiss LSM410 laser scanning confocal microscope with a krypton/argon laser (Carl Zeiss Inc., Thornwood, NY) was used for the confocal microscopy. Antibodies were used at the following dilutions: anti-Coracle 1:10,000; anti-DLG 1:250; anti-myc 1:100; and anti-NRX 1:5,000. Cuticle preparations were mounted in Hoyer's medium (Ashburner, 1989).
Immunoprecipitation and Western Blot Analysis
Wild-type and cor 4 mutant embryos ubiquitously expressing the cDNA2 isoform of Coracle (Fehon et al., 1994) were collected for 3 h at 25°C, and then aged for ∼12–14 h at room temperature. Transgenic embryos expressing Cor 1–383 or Cor 378–1698 were collected for 3 h at 25°C, aged for ∼10–12 h at room temperature, and were then heat shocked for 1 h at 38°C followed by a 2 h recovery at 25°C. Embryos were then dechorionated in 50% commercial bleach (12% sodium hypoclorite) for 3 min and rinsed several times in ice-cold lysis buffer (20 mM Hepes, pH 7.0, 50 mM NaCl, 1 mM EDTA, 0.5 mM EGTA, 10 mM DTT, 1% Triton X-100). Embryos were homogenized in a 10× vol of ice-cold lysis buffer that included PMSF and leupeptin at a 1:1,000 dilution in a tight-fitting dounce homogenizer. The homogenate was drawn through a 27 gauge tuberculin needle 5–10 times, and then centrifuged at 16,000 g for 15 min at 4°C. After removing the upper lipid layer, the soluble lysate was collected and incubated for 30–60 min with primary antibody. Primary antibodies used were guinea pig anti-Coracle at 1:200 and anti-myc 1-9E10.2 at 1:1. Immunoreactive complexes were precipitated by centrifugation (8,000 g for 1 min at 4°C) following incubation with zysorbin (Zymed Labs Inc., South San Francisco, CA) for 30–60 min at 4°C. The complexes were washed 8–10 times in lysis buffer, and then boiled in 1× SDS sample buffer (Laemmli, 1970). Protein samples separated on 3–15% SDS-PAGE were transferred to nitrocellulose for 1 h at 100 V at 4°C. Blots were blocked in 5% nonfat milk in TBS plus 0.1% Tween-20 for 1 h at room temperature, and then incubated overnight at 4°C in primary antibody (anti-NRX at 1:10,000, anti-DLG at 1:5,000). After washing in TBS Tween-20 and incubation in blocking reagent with horseradish peroxidase–coupled secondary antibodies for 3–4 h at room temperature, the immunoreactive proteins were visualized using chemiluminescent autoradiography (Pierce, Rockford, IL).
In Vitro Binding Studies
A Coracle fusion protein was constructed by cloning the entire coracle cDNA3 sequence (Fehon et al., 1994) into the SmaI site of pGEX 2TK (Pharmacia Diagnostics AB). Purified Coracle fusion protein was prepared as described (Frangioni and Neel, 1993) with the exception that 1% Triton X-100 was used in place of sarkosyl. The glutathione-S-transferase (GST) moeity was removed by thrombin cleavage followed by adsorption to glutathione agarose (Sigma Chemical Co., St. Louis, MO). Purified Coracle was labeled with 32P using γ-32P dATP and the catalytic subunit of protein kinase (from bovine heart, Sigma Chemical Co.) according to manufacturers instructions. Labeled protein was purified from unincorporated nucleotides through a G25 column (Pharmacia Diagnostics AB) and fractions containing the highest concentration of TCA precipitable counts were used for the binding reactions. A Coracle fusion protein of amino acids 608–1,285 has been previously described (Fehon et al., 1994), and was purified as just described. A NRX fusion protein was constructed by cloning the cytoplasmic domain of NRX (bp 3,993–4,127) into the SmaI site of pGEX 3. NRX fusion protein was purified as just described. GST-NRX was covalently linked to CNBr-activated Sepharose (Pharmacia Diagnostics AB) at a concentration of ∼1 mg protein per ml beads and blocked in binding buffer (20 mM Hepes, pH 7.0, 50 mM NaCl, 1 mM EDTA, 0.5 mM EGTA, 5 mM DTT) plus 1% BSA for at least 2 h at 4°C. As a control, purified GST was covalently linked to CNBr-activated Sepharose (Pharmacia Diagnostics AB) at a concentration of ∼1 mg protein per ml beads. 100 μl of a 50% slurry of NRX or GST-linked beads (∼50 μg protein) was incubated with 5–10 μg of 32P-labeled Coracle in 250–400 μl of binding buffer ± a 10-fold molar excess of unlabeled proteins for 30 min at room temperature with gentle shaking. After washing three times with binding buffer, the beads were added to 3 ml of scintillation fluid (Safety-Solve, Research Products International Corp., Mount Prospect, IL) and counted in a scintillation counter (LS6000SC; Beckman, Fullerton, CA). Three replicates were performed for each treatment and means plus standard errors were determined.
Results
Coracle Protein Produced in cor4 and cor6 Mutant Embryos Is Mislocalized to the Cytoplasm
Coracle protein is normally localized to the septate junction in ectodermally derived epithelial cells (Fehon et al., 1994). Using optical microscopy, this localization is most obvious in the polarized epithelia of the salivary glands and hindgut (Fig. 1, B and C, respectively). To identify regions of Coracle responsible for this localization, we examined its subcellular localization in a collection of coracle point mutations with moderate to severe phenotypes. Two severe alleles, cor 4 and cor 6, produce Coracle protein which is mislocalized to the cytoplasm (Fig. 1, D–G). The Coracle protein in cor 4 mutant embryos is expressed at nearly wild-type levels (Fig. 1), but with a molecular mass roughly 10 kD smaller than the wild-type protein (data not shown). Sequence analysis revealed that the molecular lesion responsible for this allele is an in-frame deletion of 78 amino acids (amino acids 68–146) in the conserved amino-terminal region (CNTR; Fig. 1 A). Analysis of the cor 6 mutation showed that it is a missense mutation of Leu39 to Gln in the CNTR (Fig. 1 A). Coracle protein in embryos homozygous for cor 6 is expressed at much lower levels based upon immunofluorescence (Fig. 1) and by immunoblot (data not shown). These results, together with the observation that both cor 4 and cor 6 behave genetically as strong hypomorphs (Lamb, R.S., R.E. Ward, and R.G. Fehon, manuscript in preparation), suggest that correct subcellular localization is essential for Coracle function.
Figure 1.
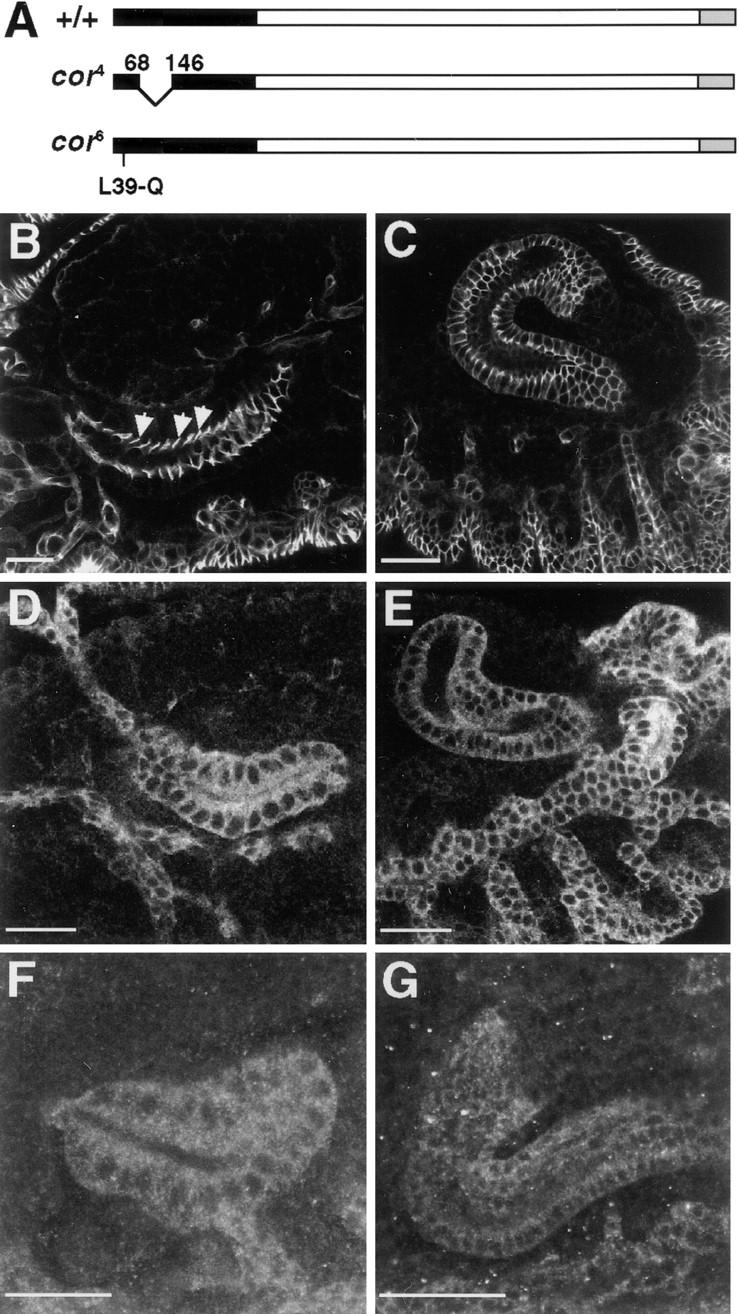
Coracle protein is mislocalized in embryos homozygous for the cor 4 and cor 6 mutations. (A) Diagramatic representation of Coracle protein showing the molecular lesions responsible for the cor 4 and cor 6 alleles. The CNTR is depicted in black. (B–G) Confocal optical sections of salivary glands (B, D, and F) and hindguts (C, E, and G) from wild-type (B and C), cor 4 (D and E) and cor 6 (F and G) mutant embryos stained with guinea pig polyclonal antibodies against Coracle. In this and all subsequent figures, anterior is to the left. In wild-type epithelia, Coracle is localized to the apicolateral membrane in the region of the septate junction (arrows in B), whereas in cor 4 and cor 6 tissues, Coracle is found throughout the cytoplasm. Bars, 25 μm.
The Amino-terminal 383 Amino Acids of Coracle Are Necessary and Sufficient for Localization to the Septate Junction
To address more definitively the role of the CNTR in subcellular localization, we initiated a structure/function analysis of Coracle by generating the coracle deletion mutations shown in Fig. 2. These constructs were transfected into S2 Drosophila cultured cells, heat shocked to induce expression, and analyzed by indirect immunofluorescence to determine the subcellular localization of the expressed proteins. Although S2 cells do not form an epithelium or display junctions, transiently transfected full-length Coracle is found predominately associated with the plasma membrane (Fig. 2 B), consistent with its membrane association in embryonic epithelia and in imaginal discs. Similarly, Cor 1–383, encoding the entire CNTR (Fehon et al., 1994), is also predominately associated with the plasma membrane, indicating that this region is sufficient for membrane localization in S2 cells (Fig. 2 C). To delimit the region responsible for this subcellular localization, we generated several smaller fragments of the amino-terminal region and expressed them in S2 cells. In every case, the resulting recombinant protein was found in the cytoplasm and showed no appreciable association with the plasma membrane (Fig. 2, D–G). Even Cor 1–323, which only removes 60 amino acids from the carboxyl terminus of the CNTR, was cytoplasmically localized (Fig. 2 D), indicating that an intact CNTR is necessary for correct subcellular localization. Consistent with this notion, Cor 378–1698, which contains all of Coracle except the CNTR, is also found throughout the cytoplasm as are all smaller fragments derived from this region (Fig. 2, H–J).
Figure 2.
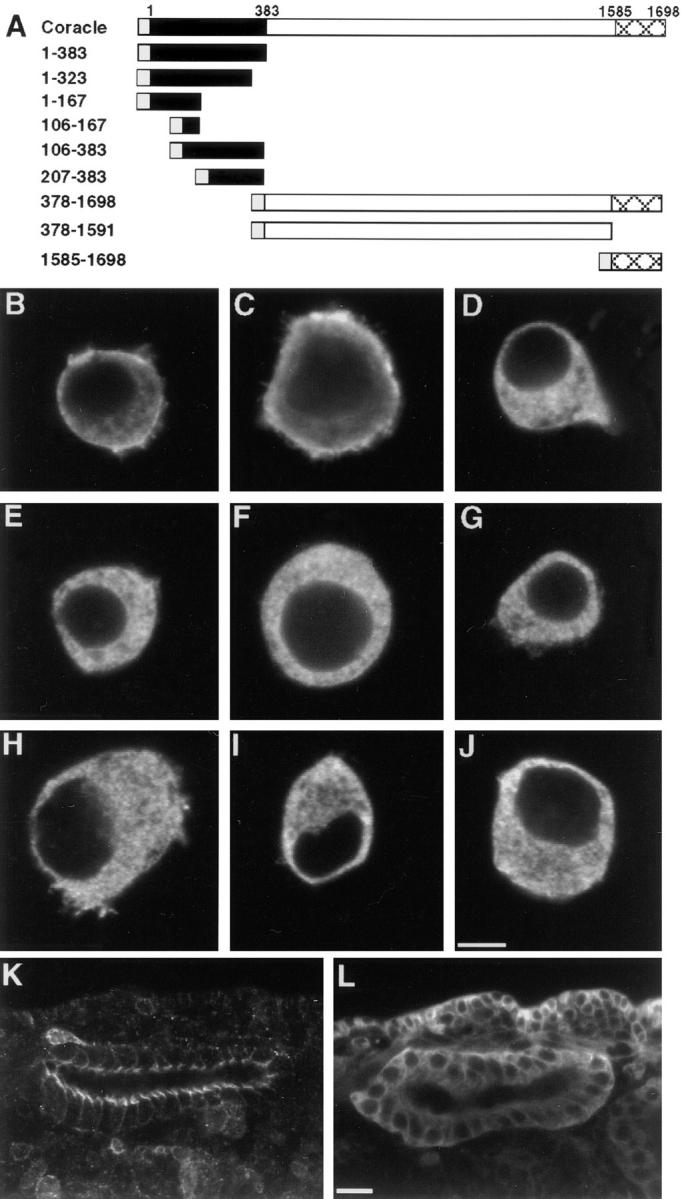
The CNTR of Coracle is necessary and sufficient for membrane localization in S2 cultured cells and septate junction localization in transgenic embryos. (A) Diagramatic representation of Coracle deletion mutants. The CNTR is depicted in black, the myc epitope tag in grey, and the carboxy-terminal–conserved region in broad crosshatch. (B–J) Confocal optical sections of S2 cells transfected with full length coracle (B) or coracle deletion constructs (C–J) stained with antibodies against Coracle (B) or the myc epitope (C–J). (B) full length Coracle. (C) Cor 1–383. (D) Cor 1–323. (E) Cor 1–167. (F) Cor 106–167. (G) Cor 106– 383. (H) Cor 378–1698. (I) Cor 378–1591. (J) Cor 1585–1698. Wild-type Coracle and Cor 1–383 show distinct membrane localization whereas the other deletion constructs are primarily cytoplasmic. (K and L) Confocal optical sections of salivary glands in transgenic embryos expressing Cor 1–383 (K) or Cor 378–1698 (L) stained with antibodies against the myc epitope. Note that the apicolateral localization of Cor 1–383 in transgenic embryos is similar to that of Coracle in wild-type embryos (Fig. 1 B), whereas Cor 378–1698 is distributed throughout the cytoplasm. Bars, (B–J) 5 μm; (K and L) 10 μm.
To assess the in vivo function of these recombinant proteins, we generated transgenic flies using P-element– mediated germline transformation and determined the subcellular localization of the induced proteins during embryogenesis by indirect immunofluorescence. In all cases, the subcellular localization of these recombinant proteins was consistent with our observations in the S2 cell assay. Specifically, the CNTR fragment, Cor 1–383, is associated with the membrane in the region of the apical junctional complex in a pattern that is very similar to the localization of Coracle expressed in wild-type embryos (compare Figs. 2 K to 1 B). Cor 1–383 displays the same pattern of localization in embryos homozygous for cor 5 (data not shown), a null genetic background that has no detectable Coracle protein (Lamb., R.S., manuscript in preparation), indicating that sequences within the CNTR alone are responsible for this localization. Also consistent with the S2 cell assay, Cor 378–1698 in transgenic embryos is found throughout the cytoplasm and is excluded from the nucleus (Fig. 2 L). The expression levels of the smaller fragments derived from the CNTR were usually substantially reduced, although in all cases where the recombinant proteins were detected, they were found throughout the cytoplasm and not associated with the membrane (data not shown). Taken together, these experiments demonstrate that the amino-terminal 383 amino acids are both necessary and sufficient for correct subcellular localization in cultured S2 and embryonic epithelial cells.
Similarities between coracle and Neurexin Embryonic Phenotypes
Given the evidence that protein 4.1 localizes to the erythrocyte membrane through an interaction with the cytoplasmic tail of glycophorin C (Marchesi, 1985), it seems likely that Coracle localizes to the plasma membrane via an interaction with a septate junction resident transmembrane protein. Two recent results implicate NRX in this possible interaction with Coracle at the septate junction. First, Coracle localization is altered in Nrx mutant embryos (Baumgartner et al., 1996), and second, 8 of the 12 amino acid residues shown to be required for the binding of protein 4.1 to glycophorin C are conserved in NRX (Littleton et al., 1997). We therefore sought to examine further the interaction between Coracle and NRX through comparison of their genetic phenotypes, by more extensive investigation of the localization requirements for each protein, and through biochemical assays for direct physical interactions.
A previous study (Baumgartner et al., 1996) has reported defects in dorsal closure in severe Nrx alleles similar to those reported for coracle alleles (Fehon et al., 1994). To understand better the relationship between these genes, we compared the embryonic phenotypes of coracle and Nrx mutant embryos. Cuticle preparations of coracle and Nrx mutant embryos display similar dorsal closure defects (Fig. 3, A and B), although the penetrance of this phenotype is greater in coracle than in Nrx mutant embryos. The most penetrant phenotype shared between coracle and Nrx is a “weakening” of the cuticle in which the ventral denticle belts are faint, with finer, less opaque, and less abundant denticles. Additionally, both coracle and Nrx mutants display a very penetrant necrosis of the salivary glands (Fig. 3, C and D). Two other phenotypes reported for Nrx mutants (Baumgartner et al., 1996) are apparent in strong coracle alleles: the tracheae fail to inflate, and muscular contractions are severely diminished late in embryogenesis (data not shown).
Figure 3.
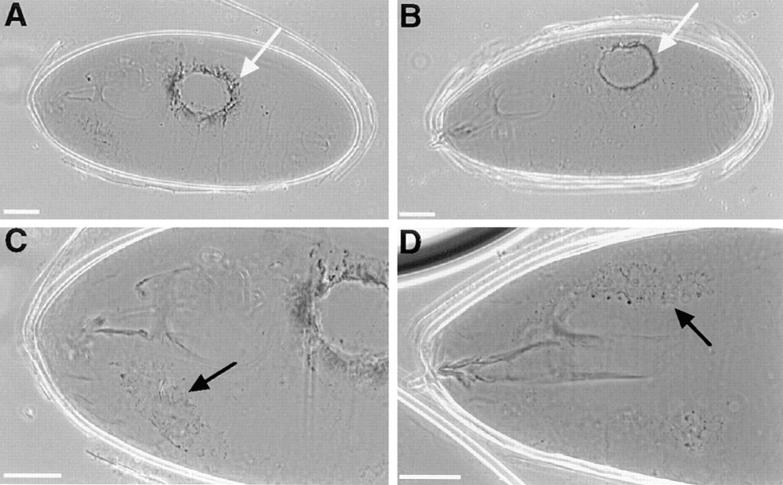
coracle and Nrx mutant embryos display similar defects in dorsal closure and in the salivary glands. Cuticle preparations of cor 5 (A and C), Nrx 46 (B) and Nrx 4304 (D) mutant embryos. The dorsal holes in coracle and Nrx mutant embryos are very similar (white arrows in A and B), as is the necrosis of the salivary glands (black arrows in C and D). Bars, 50 μm.
Interdependence of Coracle and NRX for Proper Localization
Baumgartner and colleagues (1996) have recently reported that in embryos mutant for a strong Nrx allele, Coracle protein was no longer tightly localized to the septate junction. To confirm and extend this observation, we have examined the subcellular localization of Coracle in embryos homozygous for null (Df[3L]vin 7) and strongly hypomorphic (Nrx 46, Nrx 4025, and Nrx 4304) Nrx alleles by indirect immunofluorescence. In all these cases, Coracle protein is mislocalized in the mutant embryos (Fig. 4). Rather than being tightly associated with the septate junction, the majority of the Coracle protein, although still membrane associated, has a more basolateral distribution (Fig. 4, compare D with G). In addition, some of the Coracle protein is distributed throughout the cytoplasm. Furthermore, the overall expression level of Coracle in the internal epithelia of the salivary gland and hindgut is considerably reduced relative to the expression in the epidermis. This mislocalization of Coracle is specific and does not reflect a gross alteration in the integrity of the septate junction, as judged by the nearly wild-type appearance of DLG in these embryos (compare Fig. 4 E with 4 H). This result suggests that the targeting of Coracle to the septate junction requires either a direct interaction with NRX or a NRX- dependent complex.
Figure 4.
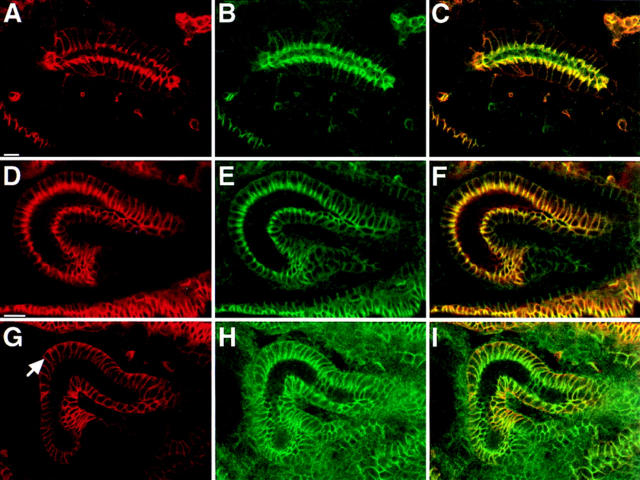
Coracle protein is mislocalized in Nrx mutant embryos. Confocal optical sections of salivary glands (A–C) and hindguts (D–I) from wild-type (A–F) and Nrx 46 mutant embryos (G–I) doubly stained with antibodies against Coracle (red) and either NRX (green in B and C) or DLG (green in E, F, H, and I). Coracle and NRX colocalize along the apicolateral membrane in the region of the septate junction in wild-type embryos (A–C). Similarly, DLG colocalizes with Coracle at the septate junction in wildtype embryos (D– F). However, in Nrx 46 mutant embryos Coracle protein spreads along the entire lateral membrane (arrow in G) and is found in the cytoplasm whereas DLG protein remains concentrated in the region of the septate junction. Bars, 10 μm.
A previous study (Baumgartner et al., 1996) has indicated that in contrast to the dependence of Coracle localization on NRX expression, NRX does not depend on Coracle expression for its localization. However, the coracle allele used for this study, cor 2, expresses a protein with an intact CNTR that localizes to the cell membrane (Fehon et al., 1994) and retains partial function (Lamb, R.S., manuscript in preparation). It is, therefore, still possible that NRX is dependent on Coracle for proper subcellular localization. To address this question, we took advantage of recently isolated coracle alleles (Lamb, R.S., manuscript in preparation). In weaker coracle alleles, NRX protein targets correctly to the septate junction in embryonic salivary glands (Fig. 5 D). However, in null and strongly hypomorphic coracle alleles (cor 5 and cor 6, respectively), NRX extends basolaterally so that it is expressed along the entire lateral membrane (Figs. 5 F and 6 B). In addition, we have observed punctate cytoplasmic NRX staining in the salivary glands and in the epidermis of both weakly and strongly hypomorphic coracle embryos (Fig. 5, C and E). This effect on the localization of NRX is specific, as we find no detectable difference in the localization of DLG in coracle mutant embryos (data not shown).
Figure 5.
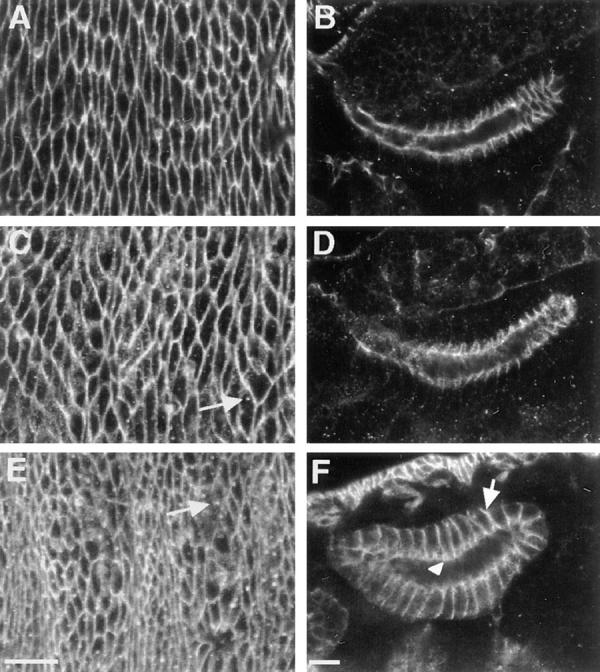
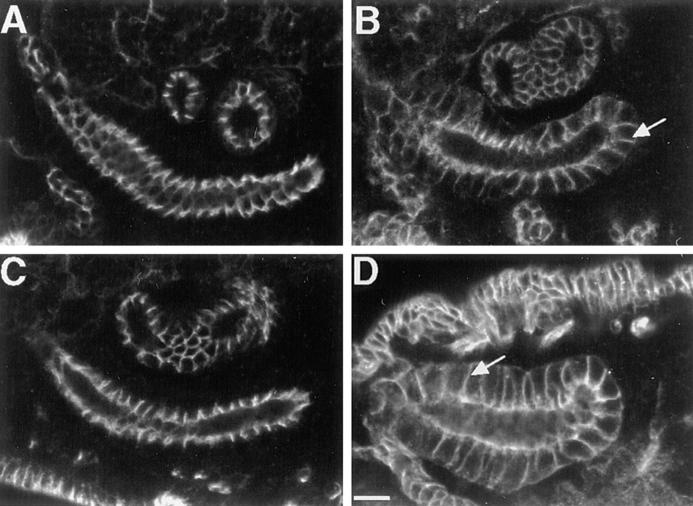
NRX protein is mislocalized in coracle mutant embryos. Confocal optical sections of epidermis (A, C, and E) and salivary glands (B, D, and F) in wild-type (A and B), cor 10 (C and D), and cor 6 (E and F) mutant embryos stained with antibodies against NRX. In cor 6 mutant embryos NRX spreads basally along the lateral membrane (arrow in F), although some enhanced NRX staining is still apparent in the region of the septate junction (arrowhead in F). Note also the punctate cytoplasmic staining in the epidermis of both cor 10 and cor 6 mutant embryos (arrows in C and E). Bars, 10 μm.
Figure 6.
The CNTR of Coracle can rescue the mislocalization of NRX in coracle mutant embryos. NRX staining in salivary glands from wild-type embryos (A), cor 5 mutant embryos carrying the hscor 1–383 transgene uninduced (B), cor 5; hscor 1–383 induced to express the recombinant protein (C), or cor 5; hscor 378–1698 induced (D). NRX localizes along the entire lateral membrane in cor 5 embryos that do not express the CNTR (arrows in B and D) whereas cor 5 mutant embryos induced to express the CNTR display properly localized NRX (compare C with A). Bar, 10 μm.
Given the observations that Coracle function is required for maintenance of NRX localization at the septate junction and that the CNTR of Coracle is sufficient to correctly target Coracle to the septate junction, it is possible that sequences within the CNTR are sufficient for the maintenance of NRX localization. To test this idea, we induced expression of the CNTR in the cor 5 null genetic background and examined the subcellular localization of NRX by indirect immunofluorescence. NRX protein is mislocalized along the entire basolateral membrane in cor 5 mutant embryos (Fig. 6 B). In contrast, cor 5 embryos that have been induced to express Cor 1–383 display NRX localization identical to that of wild-type embryos (Fig. 6, compare C with A). However, cor 5 mutant embryos that express the remainder of Coracle (Cor 378–1698) fail to “rescue” the NRX localization defect and display a NRX distribution identical to cor 5 mutant embryos (Fig. 6, compare D with B). Taken together, these results indicate that there is an interdependence of Coracle and NRX for proper localization to the septate junction, and that sequences within the CNTR of Coracle are essential for this function.
Interactions between Coracle and Neurexin
The observed codependence of Coracle and NRX for normal localization to the septate junction suggests that these proteins interact at the cytoplasmic face of the septate junction. We have addressed this idea in two ways, by asking if Coracle and NRX coprecipitate in extracts of embryonic tissues, and by examining the binding of Coracle and NRX in vitro. Consistent with the notion that they do interact, immunoprecipitation of Coracle from wild-type embryonic lysates results in a coprecipitation of NRX (Fig. 7 A). This coprecipitation is specific to Coracle and NRX, because DLG, another septate junction associated protein, is not detected in anti-Coracle immunoprecipitates (Fig. 7 B).
Figure 7.
NRX coimmunoprecipitates with Coracle from embryonic lysates. (A) Embryonic lysates from wild-type embryos (lanes 2 and 3) or cor 4 embryos (lanes 4 and 5, see Materials and Methods) were immunoprecipitated with guinea pig polyclonal anti-Coracle antibody (lanes 3 and 5) or preimmune serum (lanes 2 and 4). After 3–15% SDS-PAGE and transfer to nitrocellulose, they were immunoblotted with anti-NRX antibodies. Lane 1 is a whole cell lysate. Molecular weight standards in kD are indicated on the left. Sample loading was adjusted to ensure equal quantities of wild-type or cor 4-derived Coracle protein in the immunoprecipitates. Note the presence of NRX in anti-Coracle immunoprecipitates from wild-type but not cor 4 mutant embryonic lysates. (B) The same blot as in A was stripped (Kaufmann et al., 1987) and immunoblotted with anti-DLG antibodies. DLG does not coprecipitate with Coracle in wild-type embryonic lysates. (C) Embryonic lysates from wild-type embryos expressing hsCor 1–383 (lanes 2 and 3) or hsCor 378–1698 (lanes 4 and 5) were immunoprecipitated with mouse monoclonal anti-myc antibodies to specifically immunoprecipitate the induced recombinant protein (lanes 3 and 5) or with preimmune serum (lanes 2 and 4). The immunoprecipitates were subjected to the same treatment as in A. Induction and immunoprecipitation of recombinant proteins was verified by immunoblot (data not shown). Note the coprecipitation of NRX with Cor 1–383 but not with Cor 378–1698.
Based upon the observation that the CNTR of Coracle is sufficient for the maintenance of NRX localization, it seemed likely that the coprecipitation of NRX with Coracle was mediated through the CNTR. To examine this possibility, we prepared lysates from embryos homozygous for cor 4 (a deletion of 78 amino acids within the CNTR) carrying a cor + transgene that lacks the epitope recognized by the guinea pig antibody, but provides full genetic function (see Materials and Methods; Schweizer, L.Z., and R.G. Fehon, unpublished observations). Immunoprecipitation of Coracle from this lysate precipitates only the cor 4-derived Coracle protein. NRX protein does not coprecipitate with the cor 4 protein (Fig. 7 A), indicating that the CNTR of Coracle is necessary for the observed interaction with NRX. Furthermore, immunoprecipitates of just the CNTR (Cor 1–383) from transgenic embryos did coprecipitate NRX, whereas immunoprecipitates from embryos expressing the rest of Coracle (Cor 378–1698) did not (Fig. 7 C), indicating that the CNTR is not only necessary but also sufficient for interaction with NRX. Thus the essential sequences for binding NRX or a NRX-containing complex are contained within the amino-terminal 383 amino acids of Coracle.
To determine whether the coprecipitation of Coracle and NRX in embryonic lysates was mediated through a direct interaction between these two proteins, we examined binding in vitro between bacterially expressed recombinant proteins. A Coracle fusion protein representing the cDNA3 isoform of coracle (Fehon et al., 1994) specifically bound to the cytoplasmic domain of NRX immobilized onto a column. This interaction was efficiently competed with a 10-fold molar excess of unlabeled Coracle (Table I). However, this interaction was not significantly affected with a 10-fold molar excess of unlabeled GST nor with a Coracle fusion protein containing the central divergent region of Coracle (amino acids 608–1,285). Similar binding was observed between the NRX cytoplasmic tail and just the CNTR of Coracle (data not shown). These results demonstrate that Coracle and NRX interact directly via the CNTR of Coracle and the cytoplasmic tail of NRX.
Table I.
In Vitro Binding between Coracle and NRX
| Bound protein | Labeled protein | Competition | % relative binding* | |||
|---|---|---|---|---|---|---|
| NRX | 32P-COR | none | 100 | |||
| GST | 32P-COR | none | 33.9 ± 8.3 | |||
| NRX | 32P-COR | 10 × COR | 36.6 ± 3.4 | |||
| NRX | 32P-COR | 10 × GST | 90.9 ± 3.1 | |||
| NRX | 32P-COR | 10 × COR608–1285 | 118.0 ± 8.8 |
Relative to COR and NRX, no competition ± standard error
Discussion
We have dissected the Coracle protein structurally to identify functionally important regions of this member of the protein 4.1 superfamily. Using in vitro mutagenesis, we have shown that the amino-terminal 383 amino acids of Coracle are sufficient and necessary for membrane association in S2 Drosophila cultured cells, and for correct subcellular localization to the septate junction in transgenic embryos. Genetic mutations that affect this domain disrupt the subcellular localization of Coracle and severely affect its genetic functions. Furthermore, the localization of Coracle and the transmembrane protein Neurexin to the septate junction display an interdependent relationship, suggesting that they interact with one another. Consistent with this notion, immunoprecipitation and in vitro binding assays demonstrate that the amino-terminal 383 amino acids of Coracle and the cytoplasmic domain of Neurexin interact directly.
The finding that the amino-terminal 383 amino acids (the CNTR) of Coracle is responsible for septate junction localization correlates well with similar studies of other protein 4.1 family members. The corresponding region of erythrocyte protein 4.1, the 30-kD domain, has been shown to be required for membrane association through binding to the cytoplasmic domain of glycophorin C and the anion exchanger (Anderson and Lovrien, 1984; Pasternack et al., 1985). Likewise, in ERM proteins, the amino-terminal conserved region has been shown to be responsible for localization to the cytoplasmic face of the cell membrane in cultured cells (Algrain et al., 1993), possibly through an interaction with CD44 (Tsukita et al., 1994). Taken together, these results suggest that sequences conserved in all protein 4.1 superfamily members serve as a subcellular targeting domain.
Although the CNTR is well conserved between different members of this superfamily, it is likely that the differences in sequence between them may affect interactions with different transmembrane partners and thereby provide subcellular specificity. For example, in Drosophila, Coracle is localized to the septate junction whereas the other family members, Moesin, Merlin, and Expanded, are found primarily in the region of the adherens junction (Boedigheimer and Laughon, 1993; McCartney and Fehon, 1996). Once correctly targeted to a specific subdomain of the membrane, each protein may then serve unique functions. Erythrocyte protein 4.1 and the ERM proteins appear to function as membrane-cytoskeletal crosslinkers, whereas other family members, such as the protein tyrosine phosphatases, have divergent functions that are not directly related to cytoskeletal binding (McCartney and Fehon, 1997).
The identification and characterization of the cor 4 mutation, which results from an in-frame deletion of 78 amino acids within the CNTR, provides insight into the functions of Coracle and its localization to the septate junction. Although cor 4 protein is expressed at essentially normal levels, it does not localize correctly to the septate junction (Fig. 1, D and E). Similarly, cor 6, which results from an amino acid substitution at Leu39 within the CNTR (but outside of the region deleted in cor 4), produces a protein that is also mislocalized to the cytoplasm. Attempts to subdivide this region into smaller pieces using in vitro mutagenesis result in proteins that cannot localize to either the cell membrane or to the septate junction (Fig. 2). Taken together, these mutagenesis studies indicate that the CNTR operates as a single functional unit. Furthermore, the observation that both cor 4 and cor 6 mutant embryos display severe embryonic phenotypes (Lamb, R.S., R.E. Ward, and R.G. Fehon, manuscript in preparation) suggests that an intact CNTR is essential not only for subcellular localization, but also for function in the context of the developing embryo. However, the CNTR does not appear to provide full genetic function as determined by the inability of hscor 1–383 to rescue a null coracle allele by repeated daily heat shocks (data not shown). Further studies are underway to more fully examine the properties of this functional domain.
Previous work has indicated that whereas Coracle is dependent on NRX for its localization, NRX is not dependent on Coracle (Baumgartner et al., 1996). We have confirmed that Coracle localization is dependent on NRX, but find in addition that in strong coracle alleles, NRX protein is no longer tightly confined to the septate junction and instead spreads basolaterally (Fig. 5). However, the mislocalization of NRX in coracle mutants is qualitatively different from that of Coracle in Nrx mutants. Whereas Coracle shows no apparent localization to the septate junction in Nrx mutants, in coracle mutants there is an enrichment of NRX at the septate junction. One interpretation of this result is that targeting of NRX to the septate junction occurs properly in cells lacking Coracle, but that subsequent maintenance of NRX localization is disrupted. Alternatively, it is possible that there is residual Coracle function in even the strongest alleles, although phenotypic and biochemical evidence indicates that this is not the case (Lamb, R.S., R.E. Ward, and R.G. Fehon, manuscript in preparation).
The evidence presented here indicates that Coracle functions to organize the transmembrane protein NRX to the septate junction. Similarly, previous studies have shown that transfection of ezrin into mouse thymoma cells leads to the redistribution of ICAM-2 in cellular protrusions known as uropods (Helander et al., 1996). In addition, the observed correlation between the rapid redistribution of ERM proteins and dynamic cell surface projections upon growth factor stimulation (Bretscher, 1989) suggests that this membrane-organizing function is a general property of the protein 4.1 superfamily. Our observation that this activity is contained entirely within the CNTR indicates that this conserved domain has similar functions in all family members.
The interdependence between Coracle and NRX for proper localization suggests that at least one other protein in the presumptive septate junction serves as the initial target for both proteins to be properly localized. Based upon the protein 4.1 paradigm of a ternary complex consisting of protein 4.1, glycophorin C, and p55 (Marfatia et al., 1995), we would predict that a PDZ repeat-containing protein is a part of the complex containing Coracle and NRX. The most likely existing candidate for this additional protein is DLG based upon its extensive sequence similarity to p55. DLG is expressed maternally, and initially is uniformly distributed along the lateral membrane and to a lesser extent throughout the cytoplasm. Coincident with the expression of Coracle and NRX, this subcellular localization is refined to the presumptive septate junction (Woods and Bryant, 1991). This expression pattern might be expected of a protein that serves to “prepattern” the septate junction. However, we have failed to detect any interaction between Coracle and DLG by immunoprecipitation (Fig. 7), and we have observed no genetic interaction between coracle and dlg mutant alleles (data not shown). In addition, the embryonic defects associated with dlg mutants (Perrimon, 1988) are different from those of coracle and Nrx. These results suggest that DLG is not involved in a ternary complex together with Coracle and NRX, despite its structural similarity with p55. The question of whether there is another PDZ repeat–containing protein that functions to stabilize Coracle-NRX interactions remains to be answered, although the structural similarities between the respective Drosophila and human proteins strongly suggest that one exists. Additionally, the recent identification of EBP50 as a PDZ repeat–containing protein that associates with ERM proteins (Reczek et al., 1997) suggests that an interaction with a PDZ repeat-containing protein may be a ubiquitous feature of protein 4.1 members. Regardless, the results described here strongly suggest that at least one other component is involved in Coracle/NRX localization and function. Further studies are currently underway to identify this other possible component using a combination of genetic and biochemical approaches.
The structural components and signaling molecules that function together at the septate junction implicate this region in essential signaling mechanisms required for proper cellular communication and epithelial integrity. The phenotype resulting from zygotic loss of dlg function suggests a requirement for septate junctions in the control of cell proliferation and apicobasal polarity (Stewart et al., 1972; Woods and Bryant, 1989; Woods et al., 1997). Nrx mutant embryos display neuromuscular defects apparently due to a breakdown in the blood-brain barrier, and subtle morphological defects in the ultrastructure of the septate junction, suggesting that the septate junction acts as a barrier to paracellular flow (Baumgartner et al., 1996). In addition, the extracellular domain of NRX has EGF and laminin G repeats (Baumgartner et al., 1996), raising the possibility that it serves as a cell surface receptor. In support of this hypothesis, Neurexins were first identified as synaptic receptors (Petrenko et al., 1991), and have subsequently been proposed to function as receptors in synaptic targeting of neurons (Ullrich et al., 1995). The interdependence between Coracle and NRX for proper localization and their similar embryonic phenotype suggests that they function together in signaling events. The molecular details of these signaling processes are currently unclear, as are their functions. However, the range of observed coracle phenotypes, including embryonic dorsal closure and proliferation defects (Lamb, R.S., R.E. Ward, and R.G. Fehon, manuscript in preparation), suggests that either more than one signaling mechanism is involved, or that this signal has multiple functions during development. Given the membrane-organizing activity of the CNTR of Coracle that we describe here, it is likely that Coracle and the other protein 4.1 family members function to target receptors and their intracellular effectors to specific membrane domains. Precedence for this type of signal-mediating molecular scaffold has been found in the multiple PDZ repeat–containing protein InaD, which functions to bring components of the phototransduction pathway together into a “transducisome” (Tsunoda et al., 1997). Given the increasing evidence that protein 4.1 family members function in concert with PDZ repeat–containing proteins (Marfatia et al., 1994, 1995; Reczek et al., 1997), the hypothesis that Coracle and other protein 4.1 family members link together the molecular components of a signal cascade is particularly intriguing. The identification and characterization of other components of this protein complex at the cytoplasmic face of the septate junction will add to our understanding of the functions of Coracle and the other members of this highly conserved protein superfamily in the context of signal integration during development.
Acknowledgments
We would like to thank H. Bellen (Baylor College of Medicine, Baylor, TX) for providing NRX antibodies and fly stocks, D. Woods (University of California, Irvine, CA) for providing dlg clones, and the Bloomington stock center for fly stocks (Bloomington, IN). We thank L. Schweizer (University of Zurich, Zurich, Switzerland) and D. Mootz (Harvard University, Cambridge, MA) for technical help; and I. Rebay (Whitehead Institute, Massachusetts Institute of Technology, Cambridge, MA), the anonymous reviewers, and our colleagues in the Fehon lab for valuable suggestions and discussions.
This work was supported by grants from the National Science Foundation, No. IBN-92066555, and the American Cancer Society, No. DB-84846, to R.G. Fehon. R.E. Ward, and R.S. Lamb were supported by National Institutes of Health Training Grants, No. 5T32 GM07754 and No. GM07184, respectively.
Abbreviations used in this paper
- CNTR
conserved amino-terminal region
- DLG
discs large
- ERM
ezrin/radixin/moesin
- GST
glutathione- S-transferase
- MAGUK
membrane-associated guanylate kinase
- NRX
neurexin
- PDZ
PSD-95, DLG, ZO-1
Footnotes
Address all correspondence to Richard G. Fehon, Duke University, B361 LSRC, Research Drive, Durham, NC 27708-1000. Tel.: (919) 613-8192. Fax: (919) 613-8177. E-mail: rfehon@acpub.duke.edu
References
- Algrain M, Turunen O, Vaheri A, Louvard D, Arpin M. Ezrin contains cytoskeleton and membrane binding domains accounting for its proposed role as a membrane-cytoskeletal linker. J Cell Biol. 1993;120:129–139. doi: 10.1083/jcb.120.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RA, Lovrien RE. Glycophorin is linked by band 4.1 protein to the human erythrocyte membrane skeleton. Nature. 1984;307:655–658. doi: 10.1038/307655a0. [DOI] [PubMed] [Google Scholar]
- Arpin M, Algrain M, Louvard D. Membrane-actin microfilament connections: an increasing diversity of players related to band 4.1. Curr Opin Cell Biol. 1994;6:136–141. doi: 10.1016/0955-0674(94)90127-9. [DOI] [PubMed] [Google Scholar]
- Ashburner, M. 1989. Drosophila: A Laboratory Manual. Vol. 2. Cold Spring Laboratory Press, Cold Spring Harbor, NY. 434 pp.
- Baumgartner S, Littleton JT, Broadie K, Bhat MA, Harbecke R, Lengyel JA, Chiquet-Ehrismann R, Prokop A, Bellen HJ. A Drosophila neurexin is required for septate junction and blood-nerve barrier formation and function. Cell. 1996;87:1059–1068. doi: 10.1016/s0092-8674(00)81800-0. [DOI] [PubMed] [Google Scholar]
- Boedigheimer M, Laughon A. expanded: a gene involved in the control of cell proliferation in imaginal discs. Development. 1993;118:1291–1301. doi: 10.1242/dev.118.4.1291. [DOI] [PubMed] [Google Scholar]
- Bretscher A. Rapid phosphorylation and reorganization of ezrin and spectrin accompany morphological changes induced in A-431 cells by epidermal growth factor. J Cell Biol. 1989;108:921–930. doi: 10.1083/jcb.108.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZY, Hasson T, Kelley PM, Schwender BJ, Schwartz MF, Ramakrishnan WJ, Mooseker MS, Corey DP. Molecular cloning and domain structure of human myosin-VIIa, the gene product defective in Usher syndrome 1B. Genomics. 1996;36:440–448. doi: 10.1006/geno.1996.0489. [DOI] [PubMed] [Google Scholar]
- Crepaldi T, Gautreau A, Comoglio PM, Louvard D, Arpin M. Ezrin is an effector of hepatocyte growth factor-mediated migration and morphogenesis in epithelial cells. J Cell Biol. 1997;138:423–434. doi: 10.1083/jcb.138.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan GI, Lewis GK, Ramsay G, Bishop JM. Isolation of monoclonal antibodies specific for human c-myc proto-oncogene product. Mol and Cell Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning AS, Anderson JM. Protein-protein interactions: PDZ domain networks. Curr Biol. 1996;6:1385–1388. doi: 10.1016/s0960-9822(96)00737-3. [DOI] [PubMed] [Google Scholar]
- Fehon RG, Kooh PJ, Rebay I, Regan CL, Xu T, Muskavitch MAT, Artavanis-Tsakonas S. Molecular interactions between the protein products of the neurogenic loci Notch and Delta, two EGF-homologous genes in Drosophila. . Cell. 1990;61:523–534. doi: 10.1016/0092-8674(90)90534-l. [DOI] [PubMed] [Google Scholar]
- Fehon RG, Johansen K, Rebay I, Artavanis-Tsakonas S. Complex cellular and subcellular regulation of Notch expression during embryonic and imaginal development of Drosophila: implications for notch function. J Cell Biol. 1991;113:657–669. doi: 10.1083/jcb.113.3.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehon RG, Dawson IA, Artavanis-Tsakonas S. A Drosophila homologue of membrane-skeleton protein 4.1 is associated with septate junctions and is encoded by the coraclegene. Development. 1994;120:545–557. doi: 10.1242/dev.120.3.545. [DOI] [PubMed] [Google Scholar]
- Frangioni JV, Neel BG. Solubilization and purification of enzymatically active glutathione S-transferase (pGEX) fusion proteins. Anal Biochem. 1993;210:179–187. doi: 10.1006/abio.1993.1170. [DOI] [PubMed] [Google Scholar]
- Frorath B, Scanarini M, Netter HJ, Abney CC, Liedvogel B, Lakomek HJ, Northemann W. Cloning and expression of antigenic epitopes of the human 68-kDa (U1) ribonucleoprotein antigen in Escherichia coli. . Biotechniques. 1991;11:364–371. [PubMed] [Google Scholar]
- Harlow, E., and D. Lane. 1988. In Antibodies: A Laboratory Manual. Cold Spring Laboratory Press, Cold Spring Harbor, NY. 245–281.
- Helander TS, Carpen O, Turunen O, Kovanen PE, Vaheri A, Timonen T. ICAM-2 redistributed by ezrin as a target for killer cells. Nature. 1996;382:265–268. doi: 10.1038/382265a0. [DOI] [PubMed] [Google Scholar]
- Hendriks W, Schepens J, Bachner D, Rijss J, Zeeuwen P, Zechner U, Hameister H, Wieringa B. Molecular cloning of a mouse epithelial protein-tyrosine phosphatase with similarity to submembranous proteins. J Cell Biochem. 1995;59:418–430. doi: 10.1002/jcb.240590403. [DOI] [PubMed] [Google Scholar]
- Higashitsuji H, Arii S, Furutani M, Imamura M, Kaneko Y, Takenawa J, Nakayama H, Fujita J. Enhanced expression of multiple protein tyrosine phosphatases in the regenerating mouse liver: isolation of PTP-RL10, a novel cytoplasmic-type phosphatase with sequence homology to cytoskeletal protein 4.1. Oncogene. 1995;10:407–414. [PubMed] [Google Scholar]
- Hirao M, Sato N, Kondo T, Yonemura S, Monden M, Sasaki T, Takai Y, Tsukita S, Tsukita S. Regulation mechanism of ERM (ezrin/radixin/ moesin) protein/plasma membrane association: possible involvement of phosphatidylinositol turnover and Rho-dependent signaling pathway. J Cell Biol. 1996;135:37–51. doi: 10.1083/jcb.135.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann SH, Ewing CM, Shaper JH. The erasable Western blot. Anal Biochem. 1987;161:89–95. doi: 10.1016/0003-2697(87)90656-7. [DOI] [PubMed] [Google Scholar]
- Kim E, Niethammer M, Rothschild A, Jan YN, Shen M. Clustering of Shaker-type K+channels by interaction with a family of membrane-associated guanylate kinases. Nature. 1995;378:85–88. doi: 10.1038/378085a0. [DOI] [PubMed] [Google Scholar]
- Kooh PJ, Fehon RG, Muskavitch MA. Implications of dynamic patterns of Delta and Notch expression for cellular interactions during Drosophiladevelopment. Development. 1993;117:493–507. doi: 10.1242/dev.117.2.493. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee H, Simon JA, Lis JT. Structure and expression of ubiquitin genes of Drosophila melanogaster. . Mol Cell Biol. 1988;8:4727–4735. doi: 10.1128/mcb.8.11.4727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littleton JT, Bhat MA, Bellen HJ. Deciphering the function of neurexins at cellular junctions. J Cell Biol. 1997;137:793–796. doi: 10.1083/jcb.137.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lue RA, Marfatia SM, Branton D, Chishti AH. Cloning and characterization of hdlg: the human homologue of the Drosophila discs large tumor suppressor binds to protein 4.1. Proc Natl Acad Sci USA. 1994;91:9818–9822. doi: 10.1073/pnas.91.21.9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay DJG, Esch F, Furthmayr H, Hall A. Rho- and Rac- dependent assembly of focal adhesion complexes and actin filaments in permeabilized fibroblasts: an essential role for ezrin/radixin/moesin proteins. J Cell Biol. 1997;138:927–938. doi: 10.1083/jcb.138.4.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi VT. Stabilizing infrastructure of cell membranes. Ann Rev Cell Biol. 1985;1:531–561. doi: 10.1146/annurev.cb.01.110185.002531. [DOI] [PubMed] [Google Scholar]
- Marfatia SM, Leu RA, Branton D, Chishti AH. Identification of the protein 4.1 binding interface on glycophorin C and p55, a homologue of the Drosophila discs-large tumor suppressor protein. J Biol Chem. 1995;270:715–719. doi: 10.1074/jbc.270.2.715. [DOI] [PubMed] [Google Scholar]
- Marfatia SM, Lue RA, Branton D, Chishti AH. In vitro binding studies suggest a membrane-associated complex between erythroid p55, protein 4.1, and glycophorin C. J Biol Chem. 1994;269:8631–8634. [PubMed] [Google Scholar]
- McCartney BM, Fehon RG. Distinct cellular and subcellular patterns of expression imply distinct functions for the Drosophilahomologues of moesin and the neurofibromatosis 2 tumor suppressor, merlin. J Cell Biol. 1996;133:843–852. doi: 10.1083/jcb.133.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCartney, B.M., and R.G. Fehon. 1997. The ERM family of proteins and their roles in cell-cell interactions. In Cytoskeletal-Membrane Interactions and Signal Transduction. P. Cowin and M.W. Klymkowsky, editors. R.G. Landes Bioscience, Austin, TX. 200–210.
- Pasternack GR, Anderson RA, Leto TL, Marchesi VT. Interactions between protein 4.1 and band 3. J Biol Chem. 1985;260:3676–3683. [PubMed] [Google Scholar]
- Peifer M, Rauskolb C, Williams M, Riggleman B, Wieschaus E. The segment polarity gene armadillo interacts with the wingless signaling pathway in both embryonic and adult pattern formation. Development. 1991;111:1029–43. doi: 10.1242/dev.111.4.1029. [DOI] [PubMed] [Google Scholar]
- Peifer M, Wieschaus E. The segment polarity gene armadilloencodes a functionally modular protein that is the Drosophila homologue of human plakoglobin. Cell. 1990;63:1167–1178. doi: 10.1016/0092-8674(90)90413-9. [DOI] [PubMed] [Google Scholar]
- Perrimon N. The maternal effect of lethal(1)discs-large-1: a recessive oncogene of Drosophila melanogaster. . Dev Biol. 1988;127:392–407. doi: 10.1016/0012-1606(88)90326-0. [DOI] [PubMed] [Google Scholar]
- Petrenko AG, Perin MS, Bazbek A, Davletov BA, Ushkaryov YA, Geppert M, Sudhof TC. Binding of synaptotagmin to the α-latrotoxin receptor implicates both in synaptic vesicle exocytosis. Nature. 1991;353:65–68. doi: 10.1038/353065a0. [DOI] [PubMed] [Google Scholar]
- Poodry CA, Schneiderman HA. The ultrastructure of the developing leg of Drosophila melanogaster. W Roux's Arch. 1970;166:1–44. doi: 10.1007/BF00576805. [DOI] [PubMed] [Google Scholar]
- Rebay I, Fehon RG, Artavanis-Tsakonas S. Specific truncations of Drosophila Notch define dominant activated and dominant negative forms of the receptor. Cell. 1993;74:319–329. doi: 10.1016/0092-8674(93)90423-n. [DOI] [PubMed] [Google Scholar]
- Reczek D, Berryman M, Bretscher A. Identification of EBP50: a PDZ-containing phosphoprotein that associates with members of the ezrin-radixin-moesin family. J Cell Biol. 1997;139:169–179. doi: 10.1083/jcb.139.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees DJG, Ades SE, Singer SJ, Hynes RO. Sequence and domain structure of talin. Nature. 1990;347:685–689. doi: 10.1038/347685a0. [DOI] [PubMed] [Google Scholar]
- Rouleau GA, Merel P, Lutchman M, Sanson M, Zucman J, Marineau C, Hoang-Suan K, Demczuk S, Desmaze C, Plougastel B, et al. Alteration in a new gene encoding a putative membrane-organizing protein causes neurofibromatosis type 2. Nature. 1993;363:515–521. doi: 10.1038/363515a0. [DOI] [PubMed] [Google Scholar]
- Sato N, Funayama N, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. A gene family consisting of ezrin, radixin and moesin. Its specific localization at actin filament/plasma membrane association sites. J Cell Sci. 1992;103:131–143. doi: 10.1242/jcs.103.1.131. [DOI] [PubMed] [Google Scholar]
- Smith DB, Johnson KS. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Stewart M, Murphy C, Fristrom J. The recovery and preliminary characterization of X chromosome mutants affecting imaginal discs of Drosophila melanogaster. . Dev Biol. 1972;27:71–83. doi: 10.1016/0012-1606(72)90113-3. [DOI] [PubMed] [Google Scholar]
- Takeuchi K, Kawashima A, Nagafuchi A, Tsukita S. Structural diversity of band 4.1 superfamily members. J Cell Sci. 1994;107:1921–1928. doi: 10.1242/jcs.107.7.1921. [DOI] [PubMed] [Google Scholar]
- Tepass U, Hartenstein V. The development of cellular junctions in the Drosophilaembryo. Dev Biol. 1994;161:563–596. doi: 10.1006/dbio.1994.1054. [DOI] [PubMed] [Google Scholar]
- Thomas U, Speicher SA, Knust E. The Drosophila gene Serrateencodes an EGF-like transmembrane protein with a complex expression pattern in embryos and wing discs. Development. 1991;111:749–761. doi: 10.1242/dev.111.3.749. [DOI] [PubMed] [Google Scholar]
- Thummel CS, Boulet AM, Lipshitz HD. Vectors for DrosophilaP element-mediated transformation and tissue culture transfection. Gene. 1988;74:445–456. doi: 10.1016/0378-1119(88)90177-1. [DOI] [PubMed] [Google Scholar]
- Tomlinson A, Bowtell DL, Hafen E, Rubin GM. Localization of the sevenless protein, a putative receptor for positional information in the eye imaginal disc of Drosophila. Cell. 1987;51:143–150. doi: 10.1016/0092-8674(87)90019-5. [DOI] [PubMed] [Google Scholar]
- Trofatter JA, MacCollin MM, Rutter JL, Eldridge R, Kley N, Menon AG, Pulaski K, Haase H, Ambrose CM, Munroe D, et al. A novel moesin-, ezrin-, radixin-like gene is a candidate for the neurofibromatosis 2 tumor suppressor. Cell. 1993;72:791–800. doi: 10.1016/0092-8674(93)90406-g. [DOI] [PubMed] [Google Scholar]
- Tsukita S, Oishi K, Sato N, Sagara J, Kawai A, Tsukita S. ERM family members as molecular linkers between the cell surface glycoprotein CD44 and actin-based cytoskeletons. J Cell Biol. 1994;126:391–401. doi: 10.1083/jcb.126.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukita S, Yonemura S, Tsukita S. ERM (ezrin/radixin/moesin) family: from cytoskeleton to signal transduction. Curr Opin Cell Biol. 1997;9:70–75. doi: 10.1016/s0955-0674(97)80154-8. [DOI] [PubMed] [Google Scholar]
- Tsunoda S, Sierralta J, Sun Y, Bodner R, Suzuki E, Becker A, Socolich M, Zuker CS. A multivalent PDZ-domain protein assembles signalling complexes in a G- protein-coupled cascade. Nature. 1997;388:243–249. doi: 10.1038/40805. [DOI] [PubMed] [Google Scholar]
- Ullrich B, Ushkaryov YA, Sudhof TC. Cartography of neurexins: more than 1000 isoforms generated by alternative splicing and expressed in distinct subsets of neurons. Neuron. 1995;14:497–507. doi: 10.1016/0896-6273(95)90306-2. [DOI] [PubMed] [Google Scholar]
- Vaheri A, Carpen O, Heiska L, Helander T S, Jaaskelainen J, Majander-Nordenswan P, Sainio M, Timonen T, Turunen O. The ezrin protein family: membrane-cytoskeleton interactions and disease associations. Curr Opin Cell Biol. 1997;9:659–666. doi: 10.1016/s0955-0674(97)80119-6. [DOI] [PubMed] [Google Scholar]
- Weil D, Levy G, Sahly I, Levi-Acobas F, Blanchard S, El-Amraoui A, Crozet F, Philippe H, Abitbol M, Petit C. Human myosin VIIA responsible for the Usher 1B syndrome: a predicted membrane-associated motor protein expressed in developing sensory epithelia. Proc Natl Acad Sci USA. 1996;93:3232–3237. doi: 10.1073/pnas.93.8.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willott E, Balda MS, Fanning AS, Jameson B, Van Itallie C, Anderson JM. The tight junction protein ZO-1 is homologous to the Drosophila discs-largetumor suppressor protein of septate junctions. Proc Natl Acad Sci USA. 1993;90:7834–7838. doi: 10.1073/pnas.90.16.7834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods DF, Bryant PJ. Molecular cloning of the lethal (1) discs large-1 oncogene of Drosophila. Dev Biol. 1989;134:222–235. doi: 10.1016/0012-1606(89)90092-4. [DOI] [PubMed] [Google Scholar]
- Woods DF, Bryant PJ. The discs-largetumor suppressor gene of Drosophila encodes a guanylate kinase homologue localized at septate junctions. Cell. 1991;66:451–464. doi: 10.1016/0092-8674(81)90009-x. [DOI] [PubMed] [Google Scholar]
- Woods DF, Hough C, Peel D, Callaini G, Bryant PJ. Dlg protein is required for junctional structure, cell polarity, and proliferation control in Drosophilaepithelia. J Cell Biol. 1996;134:1469–1482. doi: 10.1083/jcb.134.6.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods DF, Wu JW, Bryant PJ. Localization of proteins to the apico-lateral junctions of Drosophila epithelia. Dev Genet. 1997;20:111–118. doi: 10.1002/(SICI)1520-6408(1997)20:2<111::AID-DVG4>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Zak NB, Shilo BZ. Localization of DER and the pattern of cell divisions in wild-type and Ellipseeye imaginal discs. Dev Biol. 1992;149:448–456. doi: 10.1016/0012-1606(92)90299-v. [DOI] [PubMed] [Google Scholar]