Abstract
INCENP is a tightly bound chromosomal protein that transfers to the spindle midzone at the metaphase/anaphase transition. Here, we show that an INCENP truncation mutant (INCENP382–839) associates with microtubules but does not bind to chromosomes, and coats the entire spindle throughout mitosis. Furthermore, an INCENP truncation mutant (INCENP43–839) previously shown not to transfer to the spindle at anaphase (Mackay, A.M., D.M. Eckley, C. Chue, and W.C. Earnshaw. 1993. J. Cell Biol. 123:373–385), is shown here to bind chromosomes, but is unable to target to the centromere. Thus, association with the chromosomes, and specifically with centromeres, appears to be essential for INCENP targeting to the correct spindle subdomain at anaphase. An INCENP truncation mutant (INCENP1–405) that targets to centromeres but lacks the microtubule association region acquires strong dominant-negative characteristics. INCENP1–405 interferes with both prometaphase chromosome alignment and the completion of cytokinesis. INCENP1–405 apparently exerts its effect by displacing the endogenous protein from centromeres. These experiments provide evidence of an unexpected link between this chromosomal protein and cytokinesis, and suggest that one function of INCENP may be to integrate the chromosomal and cytoskeletal events of mitosis.
Although it is now clear that chromosomes are not simply passive objects that are acted upon by the cytoskeleton during mitosis, the extent of their contribution to cytoskeletal events such as cytokinesis remains controversial. At one extreme, it has been suggested that chromosomes are completely dispensable for mitotic events once a bipolar spindle has been assembled (Zhang and Nicklas, 1996). However, other studies suggest that although nuclei are not needed for the initiation of cytokinesis, they are required for cleavage furrows to progress to completion (Sluder et al., 1986; Rappaport, 1991). It now appears that cytokinesis requires a pair of complementary stimuli; one initiating furrow formation, and the other allowing the process to go to completion (Rappaport, 1991). The former is likely to be cytoskeletal in origin, whereas the latter may originate on or near the chromatin. How and where these factors act is not clear, and in particular, the role of the spindle midzone in promoting furrowing is presently controversial. On one hand, certain studies suggest that an ordered spindle midzone with segregating chromosomes has an essential role in the stimulation of furrowing in somatic cells (Cao and Wang, 1996; Wheatley and Wang, 1996). However, other studies suggest that furrows can form midway between asters that are not connected by an ordered midzone (Eckley et al., 1997; Rieder et al., 1997).
One candidate for a nuclear factor involved in stabilizing cleavage furrows is inner centromere protein (INCENP)1, a “chromosomal passenger” protein (Cooke et al., 1987; Earnshaw and Bernat, 1990). Passengers are tightly associated with chromosomes in the early stages of mitosis, and many of these proteins transiently concentrate at centromeres during metaphase (Earnshaw and Mackay, 1994). Passengers abruptly disassociate from the chromosomes at the metaphase/anaphase transition and associate with the stem body material coating the overlapping antiparallel microtubules of the central spindle (Buck and Tisdale, 1962). During anaphase, a portion of INCENP also concentrates in the presumptive cleavage furrow before myosin II or radixin and before any hint of furrowing, making it one of the earliest known markers for furrow assembly (Eckley et al., 1997). A second passenger protein, TD-60, is also an early marker of furrow assembly (Martineau et al., 1995). We have suggested that passenger proteins might be mitotic cytoskeletal proteins whose association with chromosomes during prometaphase and metaphase targets them to the sites where they act during anaphase and telophase (Earnshaw and Bernat, 1990).
cDNA cloning revealed little about the mechanism of INCENP function, as the 96-kD predicted protein was unrelated to other proteins in the database (Mackay et al., 1993). However, transient transfection analysis revealed that distinct regions of the protein are required for microtubule binding and transfer from the chromosomes to the spindle at the metaphase/anaphase transition (Mackay et al., 1993). Chicken INCENP expressed in human and pig cells colocalizes with the endogenous INCENP, suggesting that both the binding partners and cell cycle signals that regulate INCENP movements are conserved between birds and mammals.
The present study involved the use of several INCENP truncation mutants to further analyze the function of this protein in mitosis. We identified a domain required for association with interphase microtubules in the carboxy-terminal portion of INCENP and a region necessary for the protein to target to centromeres in the amino-terminal portion of the molecule. We then showed that association of INCENP with chromosomes and targeting to the centromere appear to be essential for correct transfer of the protein to the spindle midzone at anaphase. A truncated INCENP molecule, INCENP1–405, targets centromeres but lacks the carboxy-terminal microtubule association domain and has a strong dominant-negative effect on mitosis in mammalian cells, disrupting both prometaphase chromosome congression and the completion of cytokinesis. This disruptive phenotype is dependent upon the ability of the truncated polypeptide to target to centromeres. The results presented here provide strong support for the hypothesis that one role of INCENP and other chromosomal passenger proteins may be to integrate the chromosomal and cytoskeletal events of mitosis.
Materials and Methods
Buffers and Reagents
KB buffer is 10 mM Tris-HCl, pH 7.7, 150 mM NaCl, 0.1% BSA. A/2 buffer is 7.5 mM Tris-HCl, pH 7.4, 40 mM KCl, 1.0 mM K-EDTA, pH 7.4, 0.375 mM spermidine, 0.15 mM spermine. TE buffer is 10 mM Tris-HCl, pH 8.0, 1 mM EDTA. Microinjection buffer is 140 mM KCl in 1 mM potassium phosphate buffer, pH 7.1.
Electroporation of Cells
Transfections by electroporation were as previously described (Mackay et al., 1993). For each transfection, 20 μg of DNA was added to 2 × 106 trypsinized, rinsed pig (LLCPK) cells resuspended in 0.4 ml Opti-MEM I (GIBCO BRL, Gaithersburg, MD). Cells were pulsed at 250–300 V, 960 μF in a 0.4-cm cuvette using a Gene Pulser (Bio-Rad Laboratories, Hercules, CA) and were then plated onto glass coverslips in Opti-MEM with 10% fetal bovine serum. Except where noted, transiently transfected cells were analyzed 18 h after electroporation.
Quantitative Proliferation Assay
Microcolonies of 16–32 LLCPK or HeLa cells on gridded coverslips were injected with an expression construct encoding INCENP1–405 in the pECE vector (Ellis et al., 1986; Tomkiel et al., 1994). In control experiments, adjacent colonies were injected with an expression construct encoding INCENP43–839. The growth of the microinjected colonies was monitored by cell counting over the next 36 h. Data (see Fig. 6 A) were obtained by summing the counts for several different colonies for each construct to minimize local growth variations and maximize the accuracy of scoring. All observations of microinjected colonies used an inverted microscope (Nikon Corp., Tokyo, Japan) equipped with an incubator that maintained the stage at 37°C.
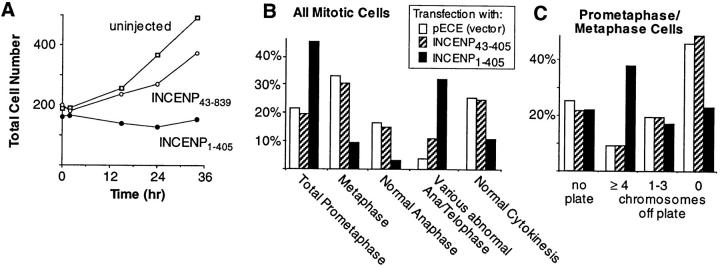
Figure 6.
Phenotypic analysis of dominant-negative INCENP mutant in mammalian cells. (A) INCENP1–405 inhibits colony growth. Colonies of 16–32 HeLa cells were microinjected with plasmid constructs encoding either INCENP1–405 or INCENP43–839 at time t = 0 (abscissa). The aggregate number of cells in all injected colonies is summed on the ordinate. In this experiment, 52% of the cells in colonies injected with INCENP1–405 were binucleate. This contrasts with 8% binucleate cells in colonies injected with INCENP43–839 and 4% binucleate cells in uninjected colonies. (B) Expression of INCENP1–405 causes the accumulation of cells in prometaphase and in ana/ telophase. Slides were prepared from cultures transfected either with the pECE vector alone (158 cells scored), or the vector encoding either INCENP1–405 (129 cells scored) or INCENP43–405 (75 cells scored). For each transfected construct, mitotic cells were scored blind for their morphology in mitosis. For the latter two transfections, only cells expressing the transfected INCENP were included in the scoring. (C) For a more detailed analysis of prometaphase congression, a separate series of transfections was performed. Slides were prepared from cultures transfected either with the pECE vector alone (87 cells scored), or the pECE vector encoding either INCENP1–405 (100 cells scored) or INCENP43–405 (96 cells scored). As in B, transfected cells were scored blind for their morphology in mitosis.
FACS™ Analysis of Transfected Cells
HeLa cells were cotransfected with 18 μg of the indicated INCENP-containing plasmid, and 2 μg of E1P green fluorescent protein (GFP)–pSX, the gift of N.B. Cole and J. Lippincott-Schwartz (both from National Institutes of Health, Bethesda, MD). This plasmid encodes a chimera of hsELP1 and GFP (S65T) (Cole et al., 1996). 18 h after electroporation, cells were trypsinized and then sorted on a FACS™ Star Plus (Beckton and Dickinson Co., Mountain View, CA) using an excitation wavelength of 490 nm and detection of GFP fluorescence at 511 nm. 500,000 cells were collected in each case for cells cotransfected with E1P-GFP–pSX and constructs encoding INCENP1–405, INCENP43–405, or vector alone. Transfected and endogenous INCENP levels were evaluated by SDS-PAGE and immunoblotting. INCENP was detected with rabbit serum 1186, with detection by enhanced chemiluminescence (Amersham Corp., Arlington Heights, IL).
Strategy for Cloning INCENP1–405–pECE
Clone INCENPII–pECE (Mackay et al., 1993) was digested with BglII and AflII (Life Technologies Inc., Gaithersburg, MD). The fragment containing the vector and encoding residues 1–405 of the INCENP open reading frame (ORF) was blunted with Klenow fragment (New England Biolabs Inc., Beverly, MA) (Sambrook et al., 1989). A single copy of the NheI linker containing an amber stop codon in all three frames (CTAGCTAGCTAG; New England Biolabs Inc.) was ligated to the fragment (Lathe et al., 1984).
Production of Anti-INCENP Antibodies
Rabbit antisera ra1- and ra2-INCENP to the carboxy terminus of chicken INCENP and rabbit sera 1005 and 1186, made by immunization with the full-length INCENP polypeptide produced in baculovirus-infected Sf9 cells have been described (Mackay et al., 1993; Eckley et al., 1997). A guinea pig antiserum (JH2045) specific for the carboxy-terminal portion of HeLa and LLCPK INCENP was generated by immunization with INCENP398–839, cloned in vector pT7-7, and then expressed in Escherichia coli BL21 (DE3). JH 2045 serum was affinity purified from nitrocellulose strips containing the antigen and then was used at a dilution of 1:3 in immunofluorescence.
Immunofluorescence
Cytological spreads were prepared by centrifugation using methods developed in the Earnshaw lab (Earnshaw and Migeon, 1985). Spreads were fixed with 4% paraformaldehyde and postfixed with cold (−20°C) methanol before being processed for immunofluorescence. Cells expressing transfected INCENP were fixed with absolute methanol (−20°C) or 4% paraformaldehyde followed by a postfix with cold (−20°C) methanol. After fixation, coverslips were processed as previously described (Mackay et al., 1993), using 2% goat serum to minimize nonspecific staining.
Microtubules were labeled by mouse monoclonal antibody Tu27B (Caceres et al., 1983), the gift of C. Rieder (Wadsworth Labs, Albany, NY). DNA was visualized by staining with 4,6-diamidino-2-phenylindole (DAPI, 0.5 μg/ml; Calbiochem-Novabiochem Corp., La Jolla, CA). Coverslips were mounted in Mowiol plus 5 mg/ml n-propyl gallate (Sigma Chemical Co., St. Louis, MO) as an antifade agent. Images were recorded using a silicon-intensified tube (SIT) camera (DAGE-MTI, Inc., Michigan City, IN) as previously described (Mackay et al., 1993).
Results
Identification of a Domain Required for INCENP Targeting to Centromeres
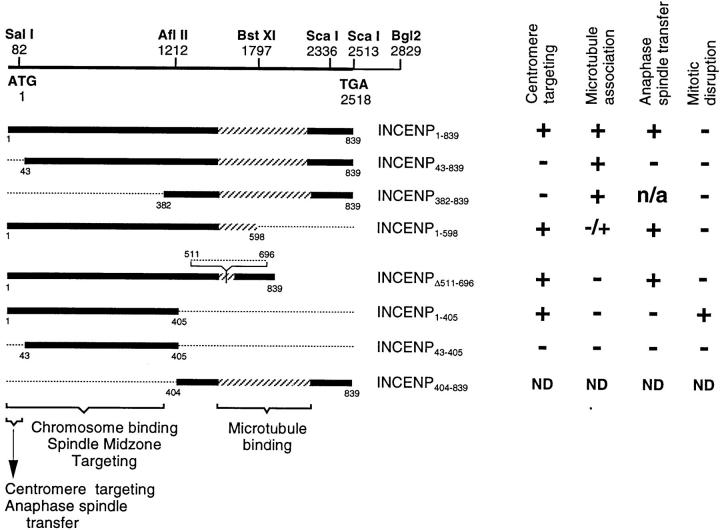
We used transient transfection assays with a number of truncation mutants to map INCENP regions that are essential for centromere targeting and transfer to the spindle during anaphase. The constructs and results are summarized in Fig. 1. In transfected cells expressing INCENP at extremely high levels (“jackpot” cells), INCENP accumulates in the cytoplasm, where it colocalizes with microtubules (Mackay et al., 1993) and causes a dramatic change in the organization of the microtubule network (Fig. 2 A). Based on this assay for microtubule association, we have found that the transfer of INCENP from the chromosomes to the mitotic spindle is unexpectedly complex: an INCENP truncation mutant (INCENPΔ511–696) that fails to associate with microtubules in the interphase cytoplasm (Mackay et al., 1993) does transfer to the spindle, whereas another mutant (INCENP43–839) that can associate with microtubules (Fig. 2 B) is defective in spindle transfer (Mackay et al., 1993).
Figure 1.
INCENP open reading frame and deletion mutants used in this study. (Top) Map of the INCENP cDNA showing the position of important restriction sites. Below this are the various INCENP constructs used in this study. The region of putative coiled-coil (Berger et al., 1995) is stippled. (Bottom) Known functional regions required for chromosome binding and centromere targeting (this study) and for transfer to the spindle and microtubule binding (Mackay et al., 1993). The results of expression of each species in cultured cells are summarized on the right. Because our molecular cloning analysis (Mackay et al., 1993) revealed that the two INCENP polypeptides are likely to be the alternatively spliced products of a single gene, we now refer to this gene and both of its products as INCENP.
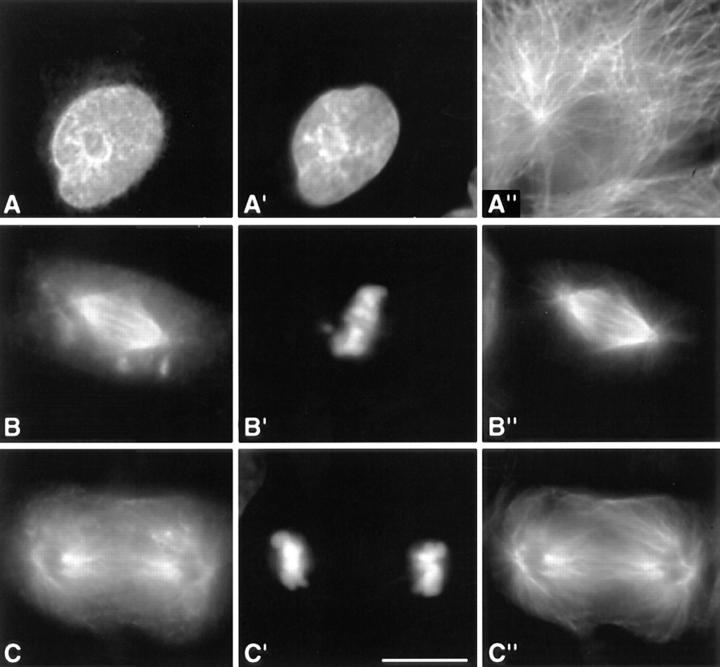
Figure 2.
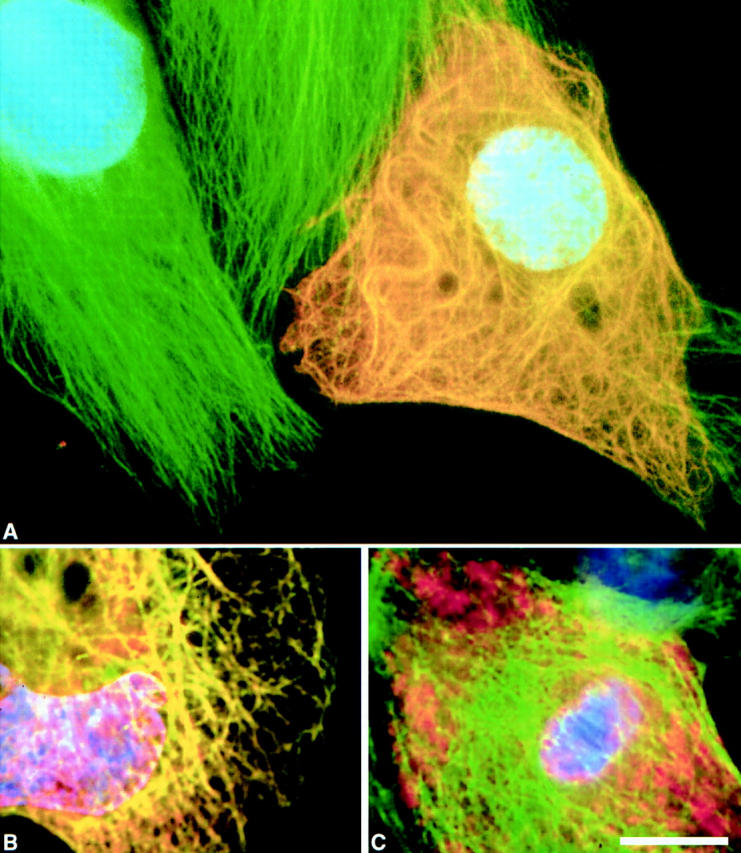
Deletion of the carboxy-terminal region of INCENP abolishes the ability of the protein to bind interphase microtubules. In these images, INCENP is red, microtubules are green, and DNA is blue. (A) Bundling of cytoplasmic microtubules in a jackpot cell expressing wild-type INCENP1–839. Neighboring nonexpressing cells have normal microtubule arrays. (B) INCENP43–839 associates with microtubules in a jackpot cell. (C) Failure of INCENP1–405 to associate with and bundle microtubules in a jackpot cell. Bar, 10 μm.
If microtubule association is not the key to spindle association, then what is? Most chromosome passenger proteins become highly concentrated at centromeres before anaphase onset (Earnshaw and Mackay, 1994). Therefore, it is possible that centromere targeting in metaphase is required for spindle transfer in anaphase. To test this possibility, we have examined the distribution of INCENP in mitotic chromosomes obtained by centrifuging colcemid-arrested cells onto coverslips. This experiment enabled us to visualize the localization of both endogenous porcine and transfected wild-type chicken INCENP in the inner centromere, flanked by a marker for the kinetochore domain (Fig. 3, A and B). In striking contrast, INCENP43–839 was found to be defective in centromere targeting, instead painting the entire chromosome (Fig. 3 D). Two other INCENP truncation mutants (Fig. 1, INCENPΔ511–696 and INCENP1–598) that are defective in microtubule binding but positive for spindle transfer, both showed strong centromere targeting (Fig. 3 C; data not shown). Thus, the amino-terminal 42 amino acids of INCENP are essential for centromere targeting, and this targeting correlates with the ability to transfer to the spindle at anaphase.
Figure 3.
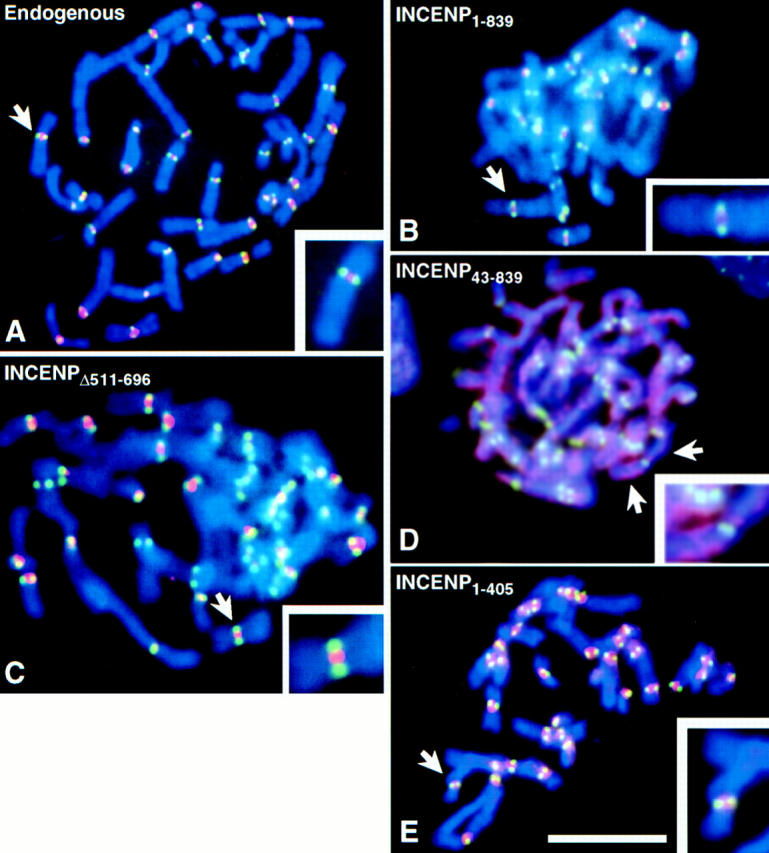
Deletion of residues 1–42 abolishes the ability of INCENP to concentrate in the inner centromere. LLCPK chromosomes were stained for INCENP (red), kinetochores (green), and DNA (blue). (A) Endogenous (porcine) INCENP is concentrated in the inner centromere. (B, C and E) Transfected full-length INCENP1–839, INCENPΔ511–696, and INCENP1–405 are all concentrated in the inner centromere. (D) Transfected INCENP43–839 fails to concentrate at centromeres, but instead paints the entire chromosome. Bar, 10 μm.
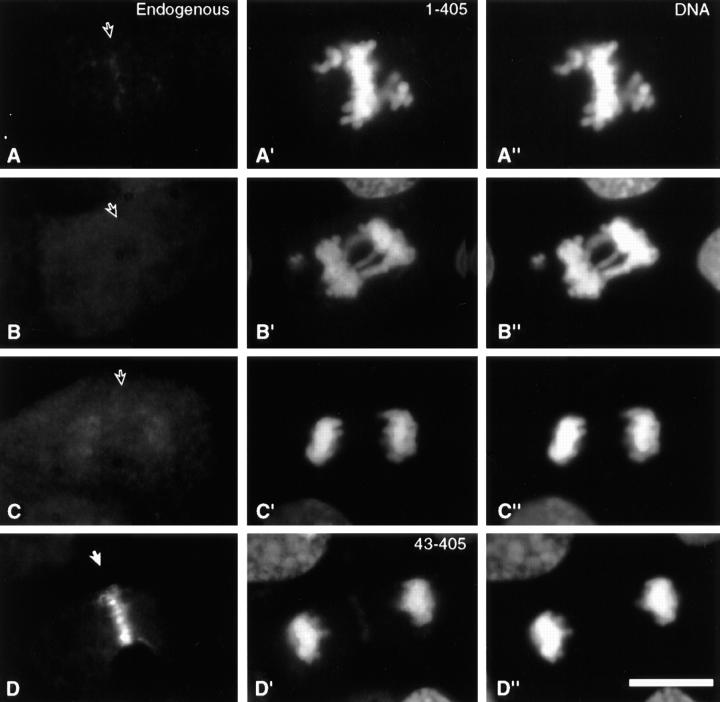
To test the hypothesis that centromere targeting alone is necessary and sufficient for INCENP to transfer to the spindle at anaphase onset, we constructed an INCENP truncation mutant (Fig. 1, INCENP1–405) that showed strong targeting to centromeres (Fig. 3 E), and no detectable association with microtubules in the cytoplasm of jackpot cells (Fig. 2 C). In selected cells where the INCENP1–405 was expressed at nearly endogenous levels and no obvious perturbation of mitotic events was observed, INCENP1–405 localization resembled that of wild-type INCENP in early mitosis through metaphase (Fig. 4, A–B′). However, after anaphase onset, when the endogenous INCENP transferred to the spindle (Fig. 4 C), INCENP1–405 remained on the separating sister chromatids (Fig. 4 C′). Low levels of INCENP1–405 could be detected in the intercellular bridge of cells undergoing cytokinesis, although most of the transfected protein remained in the daughter nuclei (Fig. 4, compare D and D′). In control experiments, a further truncation mutant, INCENP43–405 (Fig. 1), behaved like INCENP1–405 with two important exceptions. First, INCENP43–405 failed to target to centromeres at metaphase (Fig. 4 B′′). Second, at telophase, INCENP43–405 was essentially undetectable in the intercellular bridge (Fig. 4 D′′). As described below, INCENP1–405 is a strong dominant mutant that disrupts mitotic events, whereas INCENP43–405 expression produces no deleterious effects on mitotic progression.
Figure 4.
Distribution of endogenous INCENP, INCENP1–405 and INCENP43–405 in LLCPK cells undergoing normal mitosis. Control cells (A–D) or cells transfected with either INCENP1–405 (A′–D′) or INCENP43–405 (B′′–D′′) were stained for INCENP (red), microtubules (green) and DNA (blue). (A and A′) Interphase through prophase: endogenous INCENPs are nuclear. (B, B′, and B′′) Prometaphase through metaphase: both endogenous INCENPs and INCENP1–405 become highly concentrated at centromeres (B′ arrow). INCENP43–405 is distributed all along the chromosomes with no enrichment at centromeres (arrow). (C, C′, and C′′) Anaphase: endogenous INCENP transfers to the central spindle and cell cortex in the cleavage furrow. INCENP1–405 and INCENP43–405 remain on the separated sister chromatids. (D, D′, and D′′) Cytokinesis: endogenous INCENP labels the intercellular bridge intensely; nuclei are unlabeled. INCENP1–405 is found in both daughter nuclei and the intercellular bridge. INCENP43–405 is found in daughter nuclei and is absent from the intercellular bridge. Although quantitation of expression levels in transient transfections is problematic, it is our impression that cells expressing low levels of INCENP1–405 traverse mitosis normally, whereas cells expressing higher levels of the mutant protein frequently exhibit disruptions of mitosis. Bar, 10 μm.
Identification of a Domain Required for INCENP Targeting to the Spindle Midzone
If INCENP is capable of associating with microtubules along their entire lengths in jackpot cells, how is it that the protein targets selectively to the spindle midzone at anaphase, rather than binding throughout the mitotic spindle? The preceding experiments, together with those of an earlier study (Mackay et al., 1993), confirmed that the carboxy-terminal half of INCENP is required for the protein to associate with microtubules in the cytoplasm of jackpot cells. To determine whether the microtubule association region of INCENP contains a spindle midzone–targeting motif, we created a truncated INCENP molecule, INCENP382–839, that contains the microtubule association region, but lacks the amino-terminal half of the protein (Figs. 1 and 5).
Figure 5.
Deletion of residues 1–381 abolishes the ability of INCENP to target to the spindle midzone during mitosis. LLCPK cells expressing INCENP382–839 (A–C) were stained for DNA (A′–C′) and tubulin (A′′–C′′). (A–A′′) INCENP382–839 is concentrated in nuclei during interphase. (B–B′′) INCENP382–839 fails to bind chromosomes during metaphase, instead uniformly coating the spindle microtubules. Several INCENP aggregates are also seen scattered in the cytoplasm. (C–C′′) INCENP382–839 coats the spindle microtubules at anaphase and does not target to the spindle midzone. Bar, 10 μm.
This molecule accumulated in nuclei during interphase (Fig. 5 A). However, as cells entered mitosis, the protein showed no association with the condensing chromosomes. After nuclear envelope breakdown, INCENP382–839 appeared to coat the entire spindle, so that by metaphase (Fig. 5 B), the INCENP staining essentially duplicated that seen with an antitubulin antibody. After anaphase onset, INCENP382–839 showed no preferential association with the central spindle. Instead it continued to coat the spindle microtubules nondiscriminately (Fig. 5 C). These experiments indicate that the microtubule association region of INCENP lacks a specific spindle midzone–targeting signal. Thus, it appears that some activity directed by the amino-terminal portion of INCENP, possibly association with chromosomes or targeting to centromeres, is essential for the correct localization of INCENP to the midzone at anaphase.
INCENP1–405 Is a Dominant Mutant That Interferes with Cell Proliferation
Use of a quantitative colony counting assay (Bernat et al., 1990) revealed that the expression of INCENP1–405 inhibited the proliferation of human (HeLa) and pig (LLCPK) cells. In the experiment shown in Fig. 6 A, HeLa cells were plated sparsely and then allowed to grow into microcolonies. At time t = 0, all cells in selected colonies were microinjected with an expression construct encoding INCENP1–405. By 36 h later the number of cells per colony remained slightly below that at the start of the experiment. Under these conditions, neighboring colonies of uninjected cells grew with a doubling time of ∼25 h. Cells injected with a construct expressing INCENP43–839 showed an initial growth lag, but had nearly doubled by 36 h. Similar results were obtained after injection of full-length wild-type INCENP, INCENP382–839, or INCENP43–405 (data not shown).
INCENP1–405 Interferes with Prometaphase Chromosome Alignment
Transfected cultures expressing INCENP1–405 exhibited an increased percentage of cells in prometaphase and ana/ telophase relative to control transfectants, while showing a concomitant decrease in the number of cells in metaphase and undergoing normal cytokinesis (Fig. 6 B). Control cultures transfected with the pECE vector alone or with INCENP43–405 showed essentially identical levels of cells throughout the various mitotic stages (Fig. 6 B). Thus, INCENP1–405 appears to interfere with both chromosome congression in prometaphase and with events during ana/ telophase.
To examine events during prometaphase in more detail, cultures of HeLa and LLCPK cells were transfected with vector alone or with constructs encoding INCENP1–405 or, as a control, INCENP43–405. Transfected cultures were then scored for the percentages of cells in the various mitotic stages. Relative to the control transfectants, expression of INCENP1–405 had no effect on the frequency of cells with no organized plate (Figs. 6 C and 7 A), nor with up to three monooriented chromosomes. Since the percentage of cells in a particular mitotic phase is proportional to the length of that phase, we conclude that expression of INCENP1–405 does not result in a significant delay in the initiation of congression.
Figure 7.
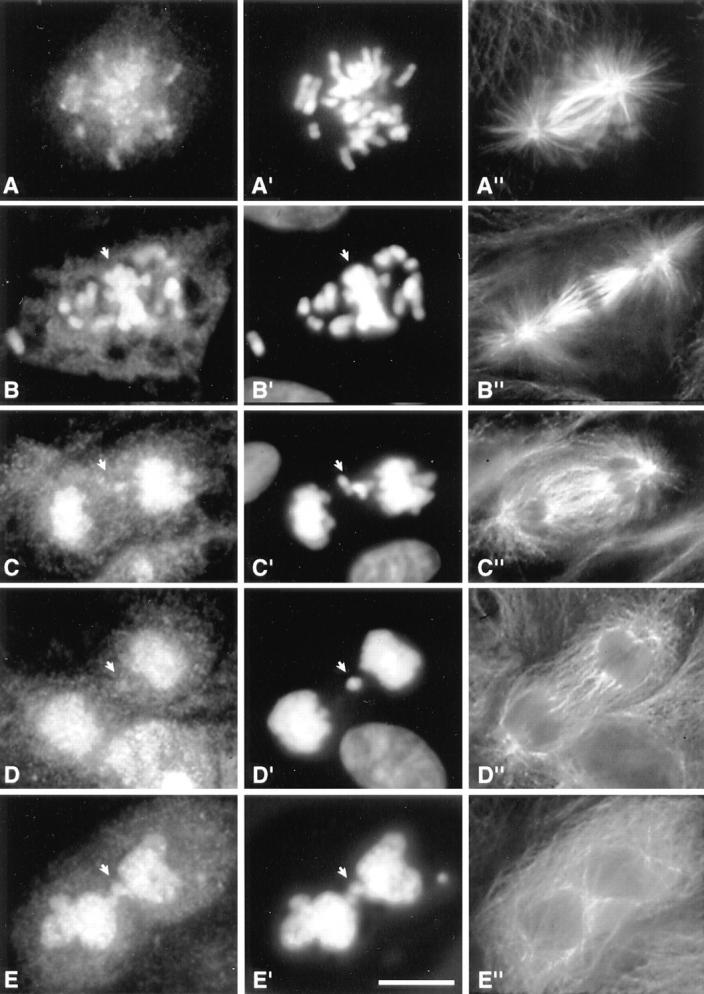
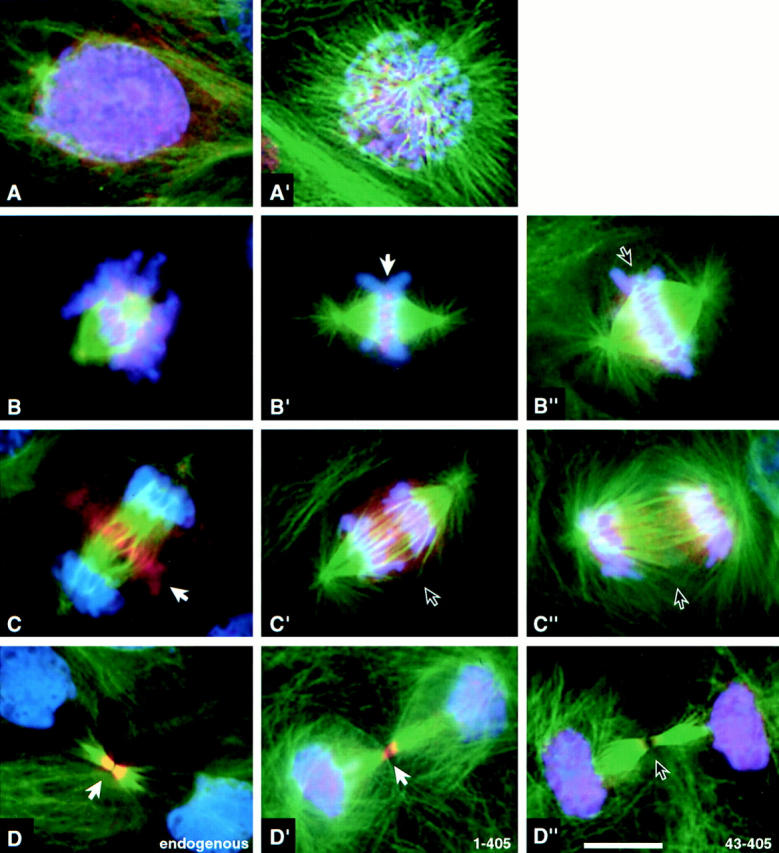
Phenotypes of cells undergoing aberrant mitosis induced by expression of INCENP1–405. (A–E) Transfected INCENP. (A′–E′) DNA stained with DAPI. (A′′–E′′) Microtubules. (A, A′, and A′′) Normal early prometaphase. (B, B′, and B′′) Prometaphase with many chromosomes that have failed to localize to the metaphase plate (arrow). (C, C′, and C′′) Anaphase with lagging chromosomes but a relatively normal central spindle. (D, D′, and D′′) Postmitotic cell with an unusual microtubule array that has characteristics of both anaphase and interphase. Prominent microtubule bundles are absent and the array appears to spread across the entire width of the cell. (E, E′, and E′′) Failed cytokinesis with an interphase microtubule array.
In contrast, a substantial increase (∼25%) was noted in the number of cells expressing INCENP1–405 that had a metaphase plate but with at least four maloriented chromosomes (Figs. 6 C and 7 B). The simplest interpretation of this result is that some cells expressing INCENP1–405 arrest or delay in midprometaphase (the hypercondensation of the chromosomes in Fig. 7 B is consistent with a mitotic delay). Observation of the INCENP signal in indirect immunofluorescence suggests that the more severely affected cells express elevated levels of INCENP relative to the endogenous protein.
The accumulation of cells expressing INCENP1–405 with at least four chromosomes not aligned at the metaphase plate could arise either from a failure of a subset of chromosomes to congress to the spindle midzone or from instability of the plate once congression had occurred. As an initial approach to distinguish between these two alternatives, we fused INCENP1–405 to the Aequorea victoria GFP (Chalfie et al., 1994). Among cells expressing the INCENP1–405–GFP chimera, we could observe cells in which congression appeared to be delayed (data not shown). Thus, these preliminary results provide support for the notion that the expression of INCENP1–405 can interfere with the completion of prometaphase congression in living cells.
The apparent interference with prometaphase congression by INCENP1–405 does not appear to result in a complete block of mitotic progression. As described below, many cells expressing INCENP1–405 were observed in various stages of ana/telophase. As these cells often showed the presence of lagging chromosomes or micronuclei, we deduce that, as described previously in other cell types (see Molè-Bajer, 1958; Sluder, 1979; Rieder and Alexander, 1989; Sluder et al., 1994 for selected examples), mitotic checkpoint control in these cells was not sufficient to completely block mitotic progression in the presence of the mitotic abnormalities caused by expression of INCENP1–405.
INCENP1–405 Disrupts Cytokinesis
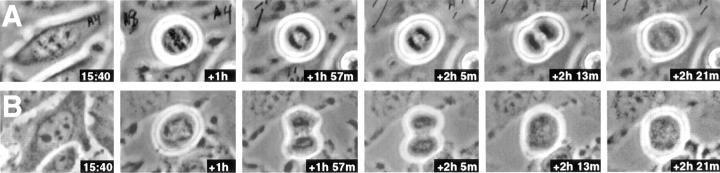
Colonies of microinjected HeLa cells expressing INCENP1–405 contained, on average, 46% binucleate cells by 36 h after injection, strongly suggesting that this protein can interfere with cytokinesis. This prediction was verified by direct examination of living cells (Fig. 8). Microinjected HeLa cells expressing INCENP1–405 traversed mitosis and furrowing was apparently initiated normally. At that point, a number of cells appeared to stall in their progression through cytokinesis. For example, the cell in Fig. 8 B appeared to pause with the furrow constricted about half-way for 12 min before abruptly returning to a rounded configuration. No such disruptions of cytokinesis were observed in cells expressing INCENP43–405 or INCENP43–839 (data not shown). We note that this disruption of cytokinesis was observed in both human (HeLa) and pig (LLCPK) cells expressing INCENP1–405.
Figure 8.
Examples of failures in cytokinesis in cells expressing INCENP1–405. (A and B) Examples of two HeLa cells injected with a plasmid encoding INCENP1–405 at time t = 0. Observation began 15 h, 40 min after microinjection. Both cells show indications of furrowing that ultimately reverse.
Examination of fixed samples revealed the presence of two classes of abnormal ana/telophase cells expressing INCENP1–405. The first consisted of anaphases with lagging chromosomes or chromosome bridges but relatively normal mitotic spindles (Fig. 7 C). The second class, which is unique in our experience, consisted of postmitotic cells in which early G1 nuclei (Fig. 7 D′) were embedded in microtubule arrays with characteristics of both anaphase and interphase (compare Fig. 7, D′′ with the normal microtubule array in C′′ and with the interphase microtubule array in the binucleate cell of E′′). These abnormal arrays showed no evidence of the formation of the prominent microtubule bundles that typically accompany the formation of stem body matrix (refer to Fig. 4 C). In one experiment 129 transfected cells expressing INCENP1–405 were scored for their mitotic morphology and 11 of the cells (8.5%) were found to have this novel spindle morphology. Overall, the appearance of these cells suggests that anaphase was initiated, but that cytokinesis then failed. Thus, it is likely that these cells correspond to the failures in cytokinesis shown in Fig. 8.
INCENP1–405 Disrupts the Localization of the Endogenous INCENPs
The endogenous INCENP was virtually undetectable at all mitotic stages in cells expressing INCENP1–405 when those cells were stained with an antibody directed against the carboxy-terminal half of INCENP (Fig. 9, A–C). In contrast, the endogenous INCENP was readily detected on the same coverslips in adjacent cells that were not expressing the transgene (data not shown) and also in control cells transfected with INCENP43–405 (Fig. 9 D).
Figure 9.
Expression of INCENP1–405, but not INCENP43–405, displaces the endogenous INCENP from its normal location. Endogenous INCENP detected using a polyclonal antibody specific for INCENP residues 404–839 is diffusely distributed in transfected cells expressing INCENP1–405 either during prometaphase (A, A′, and A′′), anaphase (B, B′, and B′′) or telophase (C, C′, and C′′). In contrast, the distribution of endogenous INCENP is normal in cells expressing INCENP43–405 (D, D′, and D′′). Staining for the endogenous protein is seen in A–D, with the expected location of INCENP indicated by an arrow in each panel. The expressed truncated INCENPs are seen in A′–D′. A′′–D′′ show the DAPI staining for DNA. Similar results were obtained with cells at all stages of the cell cycle. Bar, 10 μm.
To rule out the possibility that expression of INCENP1–405 somehow causes the endogenous INCENP to be degraded, HeLa cells were electroporated with constructs expressing INCENP1–405 or INCENP43–405 together with a construct expressing a Golgi marker protein (human ELP1) conjugated to GFP (Cole et al., 1996) and sorted by FACS™ 18 h later, yielding a population of transfected cells that was ∼98% pure, as judged by GFP fluorescence. SDS-PAGE and immunoblotting analysis revealed that the levels of endogenous INCENP in cells expressing either INCENP transgene were indistinguishable from wild-type levels (data not shown). In conclusion, INCENP1–405 is likely to exert its dominant-negative effect at least in part by displacing the endogenous protein from its binding sites at centromeres.
Discussion
Chromosome Passengers Are Cytoskeletal Proteins That Function Throughout Mitosis
The major result of this study is the finding that targeting of the chromosomal passenger protein INCENP and its various deletion constructs to chromosomes, and specifically to centromeres, is essential for both the localization and function of these proteins during mitosis. Furthermore, a truncated INCENP molecule (INCENP1–405) that targets to centromeres and remains trapped on the chromosomes behaves as a dominant-negative mutant that interferes with both metaphase chromosome alignment and cytokinesis in cultured cells. Thus, although chromosome associated for the majority of the cell cycle, INCENP can be considered a cytoskeletal protein whose centromeric localization appears to be required for the successful execution of prometaphase congression and cytokinesis.
INCENP1–405 Is a Dominant Mutant That Disrupts Prometaphase Congression
INCENP1–405 binds to chromosomes and targets to centromeres but lacks the motifs required for association with microtubules in jackpot cells. In mitosis, this protein localizes normally from prophase through metaphase, but from anaphase onward it fails to transfer to the spindle, instead remaining bound to the chromosomes. If expressed at elevated levels, INCENP1–405 is toxic to dividing cells, causing defects both early and late in mitosis.
The earliest mitotic defect seen in cells expressing INCENP1–405 is a failure to complete prometaphase chromosome alignment. Approximately 40% of cells expressing INCENP1–405 appear to arrest or delay in midprometaphase with at least four monooriented chromosomes. In theory, this phenotype could arise either by interference with the movement of the chromosomes to the spindle midzone (congression defect), or by destabilization of the metaphase configuration itself: chromosomes might congress, but then subsequently detach and become randomized in the cell. We have previously observed the latter phenotype in cells injected with anticentromere antibodies that interfere with kinetochore structure and stability (Bernat et al., 1990, 1991). In the case of INCENP1–405, we believe that the most likely defect is in congression itself, as we have directly observed a significant delay in chromosome congression in living cells expressing an INCENP1–405–GFP fusion protein (data not shown).
INCENP1–405 might disrupt congression by interfering with the assembly of a chromosomal structure such as the kinetochore. To investigate this possibility, cells microinjected with plasmid encoding INCENP1–405 were monitored by video microscopy. Selected cells undergoing difficulties in mitosis were fixed, embedded, and then subjected to serial sectioning analysis in the electron microscope. No significant changes in kinetochore ultrastructure were observed (Cooke, C.A., A. Mackay, and W.C. Earnshaw, unpublished observations; data not shown).
Alternatively, INCENP1–405 might interfere with the function of plus end–directed motors on the chromosomes such as chromokinesin–Xklp-1 (Vernos et al., 1995; Wang and Adler, 1995), mitotic centromere-associated kinesin (Wordeman and Mitchison, 1995), or centromere protein E (CENP-E) (Yen et al., 1991, 1992; Wood et al., 1997). Interference with CENP-E function either in vitro (Wood et al., 1997) or in vivo (Schaar et al., 1997) has been shown to disrupt prometaphase chromosome alignment. Interestingly, microinjection of antibodies to CENP-E into cultured cells produced an effect very similar to that seen after expression of INCENP1–405: prometaphase cells accumulated in which a limited subset of the chromosomes failed to congress to the metaphase plate (Schaar et al., 1997). Those authors suggested that chromosomes near poles encounter fewer microtubules and therefore, might require more efficient capture mechanisms (presumably involving CENP-E) to congress properly. It will be interesting in future experiments to determine whether expression of INCENP1–405 alters the distribution of CENP-E in mitotic cells.
Finally, it is possible that expression of INCENP1–405 interferes with some other aspect of spindle assembly or function that is required for cells to attain a stable metaphase configuration. In particular, INCENP1–405 might interfere with the function of the stem body matrix, a poorly characterized electron-dense material that coats the antiparallel microtubules of the central spindle (Buck and Tisdale, 1962). Although the stem body matrix is generally thought to function in anaphase, it is possible that this structure has a role earlier in mitosis, e.g., in stabilization of the spindle during formation of the metaphase plate. Immunoelectron microscopy reveals that INCENP can transfer from the chromosomes to the stem body matrix during metaphase (Earnshaw and Cooke, 1991). It is worth noting that three proteins required for prometaphase congression, chromokinesin–Xklp-1 (Vernos et al., 1995), CENP-E (Schaar et al., 1997; Wood et al., 1997), and INCENP (this study) all associate with the stem body matrix later in mitosis.
Disruption of Cytokinesis by INCENP1–405
The second phenotype commonly seen in cells expressing elevated levels of INCENP1–405 was a failure to complete cytokinesis: cleavage furrows constricted to a variable extent, usually approximately halfway, but then abruptly regressed, causing the cell to return to a rounded state. One explanation for such a failure in cytokinesis could be that the cleavage furrow impinges upon lagging chromosomes and is physically prevented from going to completion. In a careful light and electron microscopy study of the terminal phase of cytokinesis (Mullins and Biesele, 1977), it was noted that the presence of chromatin in the intercellular bridge caused cytokinesis to fail, followed by furrow regression and the production of binucleate cells. We have produced an identical phenotype in previous studies from our lab by injecting cells with anticentromere antibodies from patients (Bernat et al., 1990, 1991). We do not believe this effect can explain the present results for two reasons. First, in all cases where chromatin was trapped in the midbody, cytokinesis appeared to proceed normally until it encountered the lagging chromosome. This resulted in the appearance of a highly constricted intercellular bridge with a well-formed midbody containing trapped chromatin (Mullins and Biesele, 1977). This midbody persisted after regression of the furrow. In the present study, cytokinesis apparently failed at a much earlier stage; no fully constricted intercellular bridges were noted, and no midbody remnants were found in binucleate cells produced as a consequence of INCENP1–405 expression. In the earlier study (Mullins and Biesele, 1977), observation of living cells revealed that the stalled intercellular bridge did not regress for 1–9 h. In the present study, furrow regression appeared to occur much more rapidly (in as little as 7 min with an average of 21 ± 16 min in 9 cells followed by video microscopy). Finally, as described below, we have previously shown that a chimeric CENP-B–INCENP45–839 fusion protein also blocks the completion of cytokinesis in human cells (Eckley et al., 1997). Cells expressing that protein showed no obvious disruption of prometaphase events nor evidence of lagging chromosomes.
A number of earlier observations support the suggestion that INCENP might have a role in cytokinesis. (a) INCENP is one of the earliest known polypeptides to concentrate in the presumptive cleavage furrow; the protein is detected in this region during midanaphase before any evidence of furrowing (Cooke et al., 1987), and before the accumulation of myosin II and radixin (Eckley et al., 1997). (b) Immunoelectron microscopy reveals that in addition to its association with the stem body matrix, a portion of the INCENP antigen is intimately associated with the cytoplasmic face of the plasma membrane within the cleavage furrow (Earnshaw and Cooke, 1991). (c) In cells undergoing aberrant multipolar cytokinesis, INCENP is always associated with sites of both normal and ectopic furrowing (Eckley et al., 1997). (d) Expression of an artificial protein in which INCENP45–839 was fused to the centromere-targeting motif from CENP-B, a DNA-binding protein that is localized to the same region of the centromeric heterochromatin as INCENP (Cooke et al., 1987; Earnshaw and Cooke, 1991), caused a dominant failure late in cytokinesis (Eckley et al., 1997). Importantly, the terminal phenotype produced by the artificial chimera was distinct from that produced by INCENP1–405. Cleavage failed at a later stage, the furrow did not regress, and cells proceeded into the next cycle joined by an intercellular bridge with a prominent midbody (Eckley et al., 1997). Thus, two different dominant INCENP mutants, which target to centromeres by distinct mechanisms, both interfere with the completion of cytokinesis but produce distinct terminal phenotypes. The fact that the disruption of cytokinesis is not an allele-specific phenotype lends strong support to the notion that INCENP or an interacting protein has an important role in cytokinesis.
Centromere Targeting Is Essential for INCENP Function
We have identified an INCENP region that is essential for centromere targeting and shown that this targeting is crucial for the function of both the wild-type INCENP and its dominant mutant alleles. In fact, the best explanation for the dominant-negative effect of INCENP1–405 is that this protein displaces the wild-type endogenous protein from its centromeric binding sites. This implies that the centromeric targeting of INCENP is not only important for prometaphase congression, which would be expected to involve centromere activity, but also for successful cytokinesis, which has no direct functional link with centromeres.
In metaphase cells, centromeres occupy the region of the spindle with the highest concentration of antiparallel microtubules. This is the region where the stem body assembles, and the centromeres may present INCENP to the spindle in a location or conformation that is required for stem body assembly. The failure of INCENP383–839 to be specifically transported to this domain of the spindle by chromosomes may explain why this protein fails to target to the midzone and instead coats the entire spindle during mitosis (Fig. 5).
Are Chromosomes Necessary for Cytokinesis?
When microsurgery was used to remove all chromosomes from insect spermatocytes in meiosis I after the establishment of a bipolar spindle, the cells appeared to execute anaphase and cytokinesis normally (Zhang and Nicklas, 1996). Although this experiment appeared to argue against a role for chromosomes in the later stages of mitosis, the results are subject to two caveats. First, cytokinesis in spermatocytes does not normally go to completion, at least in mammals. Developing spermatocytes and spermatids remain connected by intercellular bridges 2 or 3 μm in diameter until the time the sperm are shed (Dym and Fawcett, 1971; Dym, 1988). Thus, the process may differ in important respects from that in somatic cells with complete cytokinesis. Second, the experiments did not include an immunochemical analysis for either INCENP or TD-60. If these proteins are, in fact, present in meiotic cells, one or both may detach from the chromosomes either during microsurgery or in vivo at the time of bipolar spindle assembly, thus permitting them to perform their cytoskeletal functions normally even after the removal of the chromosomes. Third, it is possible that even if present, INCENP and TD-60 act preferentially at meiosis II, the division at which sister centromeres disjoin (and which was not examined in the Zhang and Nicklas [1996] study). It will be important in future experiments to determine the fate of INCENP in grasshopper spermatocytes after removal of the chromosomes.
Two other recent studies have also indicated that furrowing in mitotic cells may not require the local presence of a spindle midzone with segregating chromosomes. In one study (Eckley et al., 1997), when adjacent mitotic human osteosarcoma cells were fused manually by needle puncture, they formed V-shaped spindles upon which chromosomes segregated apparently normally. In 4 out of 19 of those cells (21%) an ectopic furrow formed above the open end of the V, where the two poles were connected by a microtubule array that lacked segregating chromosomes. In the second study, PtK1 cells were electrofused, and it was noted that if the spindles were 20 μm apart in the heterokaryons, they stayed separate throughout mitosis (Rieder et al., 1997). In this case furrows formed between the adjacent spindles in 8 out of 30 cases (26%), even though this region of the cell lacked segregating chromosomes. In both cases, immunostaining of these cells for INCENP has revealed the presence of the protein in both the normal and ectopic furrows (Eckley et al., 1997) (Savoian, M., and C. Rieder, unpublished data), thereby revealing that even though the protein is localized to the chromosomes through metaphase, it can relocate to distant parts of the cell cortex as cells establish the cleavage furrow in ana/telophase. The mechanism for this INCENP relocalization is not presently known.
The reversal of cytokinesis after the initiation of furrowing caused by expression of INCENP1–405 in mammalian cells resembles the phenotype seen in micromanipulation experiments using sand dollar eggs where the nucleus was separated from the asters (Rappaport, 1991). Those experiments led Rappaport to conclude that although asters alone are able to trigger the formation of a cleavage furrow, a nuclear factor was necessary to enable the furrowing to proceed to completion. It is tempting to speculate that this nuclear factor might be either INCENP or an INCENP-interacting protein.
The present studies suggest that INCENP is involved in both early and late mitosis, with a dominant-negative INCENP mutant affecting both chromosome congression and cytokinesis. Although INCENP is a nuclear/chromosomal protein for most of the cell cycle, it is required for at least one cytoskeletal function in mitosis. Interestingly, the ability of INCENP to execute this cytoskeletal function requires previous localization of the protein to centromeres. These results are consistent with the hypothesis that one function of INCENP and other chromosomal passenger proteins may be to integrate the chromosomal and cytoskeletal events of mitosis.
Acknowledgments
We thank J. Lippincott-Schwartz for the gift of plasmid encoding hsELP1–GFP, and C. Rieder for the gift of Tu27B monoclonal antibody, and L. Binder. We thank our colleagues from the Institute of Cell and Molecular Biology, Edinburgh, Scotland: J. Brown, I. Davis, K. Hardwick, M. Heck, J. Lewis, S. Wheatley, and S. Winder for their helpful discussions and comments on the manuscript.
This work was supported by a grant from the National Institutes of Health (GM3521) and by a Principal Research Fellowship of the Wellcome Trust to W.C. Earnshaw.
Abbreviations used in this paper
- TD-60
telophase disk protein of 60 kD
- DAPI
4,6-diamidino-Z-phenylindole
- GFP
green fluorescent protein
- INCENP
inner centromere protein
- CENP-E
centromere protein E
Footnotes
A.M. Mackay's present address is Osiris Therapeutics, 2001 Aliceanna St., Baltimore, MD 21231.
Address all correspondence to William C. Earnshaw, Institute of Cell and Molecular Biology, University of Edinburgh, Swann Building, The King's Buildings, Mayfield Road, Edinburgh EH9 3JR Scotland, United Kingdom. Tel.: (44) 131-650-7101. Fax: (44) 131-650-7100. E-mail: bill. earnshaw@ed.ac.uk
References
- Berger B, Wilson DB, Wolf E, Tonchev T, Milla M, Kim PS. Predicting coiled coils by use of pairwise residue correlations. Proc Natl Acad Sci USA. 1995;92:8259–8263. doi: 10.1073/pnas.92.18.8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernat RL, Borisy GG, Rothfield NF, Earnshaw WC. Injection of anticentromere antibodies in interphase disrupts events required for chromosome movement at mitosis. J Cell Biol. 1990;111:1519–1533. doi: 10.1083/jcb.111.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernat RL, Delannoy MR, Rothfield NF, Earnshaw WC. Disruption of centromere assembly during interphase inhibits kinetochore morphogenesis and function in mitosis. Cell. 1991;66:1229–1238. doi: 10.1016/0092-8674(91)90045-z. [DOI] [PubMed] [Google Scholar]
- Buck RC, Tisdale JM. The fine structure of the mid-body of the rat erythroblast. J Cell Biol. 1962;13:109–115. doi: 10.1083/jcb.13.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caceres A, Binder LI, Payne MR, Bender P, Rebhun LI, Steward O. Differential subcellular localization of tubulin and the microtubule- associated protein MAP-2 in brain tissue as revealed by immunocytochemistry with monoclonal hybridoma antibodies. J Neurosci. 1983;4:394–410. doi: 10.1523/JNEUROSCI.04-02-00394.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L-g, Wang Y-I. Signals from the spindle midzone are required for the stimulation of cytokinesis in cultured epithelial cells. Mol Biol Cell. 1996;7:225–232. doi: 10.1091/mbc.7.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC. Green fluorescent protein as a marker for gene expression. Science. 1994;263:802–805. doi: 10.1126/science.8303295. [DOI] [PubMed] [Google Scholar]
- Cole NB, Smith CL, Sciaky N, Terasaki M, Edidin M, Lippincott-Schwartz J. Diffusional mobility of Golgi proteins in membranes of living cells. Science. 1996;273:797–801. doi: 10.1126/science.273.5276.797. [DOI] [PubMed] [Google Scholar]
- Cooke CA, Heck MMS, Earnshaw WC. The INCENP antigens: movement from the inner centromere to the midbody during mitosis. J Cell Biol. 1987;105:2053–2067. doi: 10.1083/jcb.105.5.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dym, M. 1988. The Male Reproductive System. In Cell and Tissue Biology: A Textbook of Histology. L. Weiss, editor. Urban & Schwarzenberg, Baltimore, MD. 930–972.
- Dym M, Fawcett DW. Further observations on the numbers of spermatogonia, spermatocytes, and spermatids connected by intercellular bridges in the mammalian testis. Biol Reprod. 1971;4:195–215. doi: 10.1093/biolreprod/4.2.195. [DOI] [PubMed] [Google Scholar]
- Earnshaw WC, Migeon B. A family of centromere proteins is absent from the latent centromere of a stable isodicentric chromosome. Chromosoma (Berl) 1985;92:290–296. doi: 10.1007/BF00329812. [DOI] [PubMed] [Google Scholar]
- Earnshaw WC, Bernat RL. Chromosomal passengers: towards an integrated view of mitosis. Chromosoma (Berl) 1990;100:139–146. doi: 10.1007/BF00337241. [DOI] [PubMed] [Google Scholar]
- Earnshaw WC, Cooke CA. Analysis of the distribution of the INCENPs throughout mitosis reveals the existence of three distinct substages of metaphase and early events in cleavage furrow formation. J Cell Sci. 1991;98:443–461. doi: 10.1242/jcs.98.4.443. [DOI] [PubMed] [Google Scholar]
- Earnshaw WC, Mackay AM. The role of non-histone proteins in the chromosomal events of mitosis. FASEB (Fed Am Soc Exp Biol) J. 1994;8:947–956. doi: 10.1096/fasebj.8.12.8088460. [DOI] [PubMed] [Google Scholar]
- Eckley DM, Ainsztein AM, Mackay A, Goldberg IG, Earnshaw WC. Chromosomal proteins and cytokinesis: patterns of cleavage furrow formation and inner-centromere protein positioning in mitotic heterokaryons and midanaphase cells. J Cell Biol. 1997;136:1169–1183. doi: 10.1083/jcb.136.6.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis L, Clauser E, Morgan DO, Edery M, Roth RA, Rutter WJ. Replacement of insulin receptor tyrosine residues 1162 and 1163 compromises insulin-stimulated kinase activity and uptake of 2-deoxyglucose. Cell. 1986;45:721–732. doi: 10.1016/0092-8674(86)90786-5. [DOI] [PubMed] [Google Scholar]
- Lathe R, Kieny MP, Skory S, Lecocq JP. Linker tailing: unphosphorylated linker oligonucleotides for joining DNA termini. DNA. 1984;3:173–182. doi: 10.1089/dna.1984.3.173. [DOI] [PubMed] [Google Scholar]
- Mackay AM, Eckley DM, Chue C, Earnshaw WC. Molecular analysis of the INCENPs (Inner Centromere Proteins): separate domains are required for association with microtubules during interphase and with the central spindle during anaphase. J Cell Biol. 1993;123:373–385. doi: 10.1083/jcb.123.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martineau SN, Andreassen PR, Margolis RL. Delay of HeLa cell cleavage into interphase using dihydrocytochalasin B: retention of a postmitotic spindle and telophase disc correlates with synchronous cleavage recovery. J Cell Biol. 1995;131:191–205. doi: 10.1083/jcb.131.1.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molè-Bajer J. Cine-micrographic analysis of C-mitosis in endosperm. Chromosoma (Berl) 1958;9:332–358. doi: 10.1007/BF02568085. [DOI] [PubMed] [Google Scholar]
- Mullins JM, Biesele JJ. Terminal phase of cytokinesis in D-98S cells. J Cell Biol. 1977;73:672–684. doi: 10.1083/jcb.73.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappaport R. Enhancement of aster-induced furrowing activity by a factor associated with the nucleus. J Exp Zool. 1991;257:87–95. [Google Scholar]
- Rieder, C.L., and S.P. Alexander. 1989. The attachment of chromosomes to the mitotic spindle and the production of aneuploidy in newt lung cells. In Mechanisms of Chromosome Distribution and Aneuploidy. M.A. Resnick and B.K. Vig, editors. Alan R. Liss, New York. 185–194. [PubMed]
- Rieder CL, Khodjakov A, Paliulis LV, Fortier TM, Cole RW, Sluder G. Mitosis in vertebrate somatic cells with two spindles: implications for the metaphase/anaphase transition checkpoint and cleavage. Proc Natl Acad Sci USA. 1997;94:5107–5112. doi: 10.1073/pnas.94.10.5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook, J., F. Fritsch, and T. Maniatis. 1989. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. 545 pp.
- Schaar BT, Chan GKT, Maddox P, Salmon ED, Yen TJ. CENP-E function at kinetochores is esential for chromosome alignment. J Cell Biol. 1997;139:1373–1382. doi: 10.1083/jcb.139.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluder G. Role of spindle microtubules in the control of cell cycle timing. J Cell Biol. 1979;80:674–691. doi: 10.1083/jcb.80.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluder G, Miller FJ, Rieder CL. The reproduction of centrosomes: nuclear versus cytoplasmic controls. J Cell Biol. 1986;103:1873–1881. doi: 10.1083/jcb.103.5.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sluder G, Miller FJ, Tomson EA, Wolf DE. Feedback control of the metaphase/anaphase transition in sea urchin zygotes: role of maloriented chromosomes. J Cell Biol. 1994;126:189–198. doi: 10.1083/jcb.126.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomkiel JE, Cooke CA, Saitoh H, Bernat RL, Earnshaw WC. CENP-C is required for maintaining proper kinetochore size and for a timely transition to anaphase. J Cell Biol. 1994;125:531–545. doi: 10.1083/jcb.125.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernos I, Raats J, Hirano T, Heasman J, Karsenti E, Wylie C. Xklp1, a chromosomal Xenopuskinesin-like protein essential for spindle organization and chromosome positioning. Cell. 1995;81:117–127. doi: 10.1016/0092-8674(95)90376-3. [DOI] [PubMed] [Google Scholar]
- Wang SZ, Adler R. Chromokinesin: a DNA-binding, kinesin-like nuclear protein. J Cell Biol. 1995;128:761–768. doi: 10.1083/jcb.128.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheatley SP, Wang Y. Midzone microtubule bundles are continuously required for cytokinesis in cultured epithelial cells. J Cell Biol. 1996;135:981–989. doi: 10.1083/jcb.135.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood KW, Sakowicz R, Goldstein LSB, Cleveland DW. CENP-E is a plus end-directed kinetochore motor required for metaphase chromosome alignment. Cell. 1997;91:357–366. doi: 10.1016/s0092-8674(00)80419-5. [DOI] [PubMed] [Google Scholar]
- Wordeman L, Mitchison TJ. Identification and partial characterization of mitotic centromere-associated kinesin, a kinesin-related protein that associates with centromeres during mitosis. J Cell Biol. 1995;128:95–105. doi: 10.1083/jcb.128.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen TJ, Compton DA, Earnshaw WC, Cleveland DW. CENP-E, a human centromere associated protein released from chromosomes at the onset of anaphase. EMBO (Eur Mol Biol Organ) J. 1991;10:1245–1254. doi: 10.1002/j.1460-2075.1991.tb08066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen TJ, Li G, Schaar B, Szilak I, Cleveland DW. CENP-E is a putative kinetochore motor that accumulates just prior to mitosis. Nature. 1992;359:536–539. doi: 10.1038/359536a0. [DOI] [PubMed] [Google Scholar]
- Zhang D, Nicklas RB. “Anaphase” and cytokinesis in the absence of chromosomes. Nature. 1996;382:466–468. doi: 10.1038/382466a0. [DOI] [PubMed] [Google Scholar]