Abstract
We have previously shown that p115, a vesicle docking protein, binds to two proteins (p130 and p400) in detergent extracts of Golgi membranes. p130 was identified as GM130, a Golgi matrix protein, and was shown to act as a membrane receptor for p115. p400 has now been identified as giantin, a Golgi membrane protein with most of its mass projecting into the cytoplasm. Giantin is found on COPI vesicles and pretreatment with antibodies inhibits both the binding of p115 and the docking of these vesicles with Golgi membranes. In contrast, GM130 is depleted from COPI vesicles and inhibition of the GM130 on Golgi membranes, using either antibodies or an NH2-terminal GM130 peptide, inhibits p115 binding and vesicle docking. Together these results suggest that COPI vesicles are docked by giantin on the COPI vesicles and GM130 on Golgi membranes with p115 providing a bridge.
Transport through the exocytic pathway is a discontinuous process, vesicles pinching off from one membrane compartment before fusing with the next (Rothman and Wieland, 1996). Coat protein (COP)1II vesicles carry selected cargo from the ER to Golgi apparatus (Rexach et al., 1994; Barlowe, 1995; Campbell and Schekman, 1997) whereas COPI vesicles have been implicated in cargo transport through the Golgi stack (Malhotra et al., 1989; Orci et al., 1997). COPI vesicles are also involved in retrograde transport back to the ER of those components that need to be recycled or salvaged (Cosson and Letourneur, 1994; Letourneur et al., 1994). They may also be involved in ER to Golgi transport of components other than cargo molecules (Bednarek et al., 1995).
Targeting of COPI and COPII vesicles to specific membrane compartments is thought to be mediated by soluble N-ethylmaleimide–sensitive fusion protein (NSF) attachment protein (SNAP) receptors (SNAREs) (Söllner et al., 1993; Rothman and Wieland, 1996). Each transport vesicle carries one or more vesicle (v)-SNAREs, which interact with the cognate target (t)-SNARE on the recipient membrane. Assembly of the SNARE pair is strictly regulated by several different types of protein including members of the Ypt/rab family of small GTPases (Lian et al., 1994; Sogaard et al., 1994), and the sec1 family of proteins (Dascher and Balch, 1996; Lupashin and Waters, 1997). Together these control the fidelity of SNARE pairing, which eventually leads to vesicle fusion. This is catalyzed by NSF and accessory SNAPs, though the precise mechanism and order of events is still unclear (Mayer et al., 1996).
Pairing of the v- and t-SNAREs is preceded by a step in which the vesicle docks with a potential target membrane. This type of docking can occur before the removal of the COPI coat and has also been referred to as a tethering process catalyzed by what have been termed “Velcro” factors (Pfeffer, 1996). One such factor is p115, which was originally identified as a protein needed for intra-Golgi transport (Waters et al., 1992) and has also been implicated in fusion of transcytotic vesicles with the plasma membrane (Barroso et al., 1995). The yeast homologue, Uso1p, is required for ER to Golgi transport (Nakajima et al., 1991), and acts upstream of the formation of SNARE pairs (Lupashin et al., 1996; Sapperstein et al., 1996). Yeast strains deleted for Uso1p can survive if they overexpress certain ER to Golgi v-SNAREs (Sapperstein et al., 1996). This is most simply explained as a mass action effect, the decreased efficiency with which the vesicle docks to the membrane being compensated by the increased efficiency with which the paired SNARE state can form. Rotary shadowing has shown that p115 and Uso1p are elongated homodimers with two globular heads and a predicted coiled-coil tail, reminiscent of myosin II (Sapperstein et al., 1995; Yamakawa et al., 1996). The tail is long (p115 = 45 nm; Uso1p = 150 nm) compared to the width of a transport vesicle (∼50 nm). This again is consistent with a vesicle docking function.
The docking function of p115 is perhaps most vividly demonstrated during mitosis in animal cells when the Golgi apparatus undergoes extensive fragmentation (Warren, 1993; Levine et al., 1995). Up to two-thirds of Golgi membrane is converted into COPI vesicles in vitro through continued budding in the absence of fusion (Misteli and Warren, 1994; Sönnichsen et al., 1996). Fusion appears to be inhibited because p115 can no longer bind to its membrane receptor, GM130 (Levine et al., 1996; Nakamura et al., 1997). GM130 was originally identified as a component of the Golgi matrix or scaffold (Nakamura et al., 1995). It is a tightly bound peripheral membrane protein mostly located on the cis side of the Golgi apparatus. The binding site for p115 is located within the NH2-terminal 75 amino acids, and mitotic phosphorylation in this region inhibits p115 binding (Nakamura et al., 1997). This is thought to prevent the docking of COPI vesicles leading to their accumulation and consequent fragmentation of the Golgi apparatus.
GM130 provides a target membrane docking site for p115 but this does not explain how p115 is bound to the docked COPI vesicles. When used as an affinity ligand to probe detergent extracts of Golgi membranes, p115 was shown to bind to an ∼400-kD protein in addition to GM130 (Nakamura et al., 1997). We have now identified this protein as giantin, a Golgi membrane protein with most of its mass projecting into the cytoplasm (Linstedt and Hauri, 1993; Seelig et al., 1994). We provide evidence that COPI vesicles are docked by giantin on the vesicles and GM130 on the Golgi membranes, bridged by p115.
Materials and Methods
Materials
The following antibodies were used for Western blotting and/or immunogold labeling: polyclonal antibodies NN5 against GM130 (Nakamura et al., 1995); polyclonal antibodies against a mixture of recombinant giantin polypeptides P1-P5 (Seelig et al., 1994); a mixture of affinity-purified antibodies against these peptides (for cryolabeling); mAb 8A6 against p115 (Waters et al., 1992); and mAbs M3A5 and mAD against β-COP (Allan and Kreis, 1986; Duden et al., 1991). In competition studies membranes or cytosol were pretreated with the peptide N73pep, comprising the first 73 NH2-terminal amino acids of GM130 (Nakamura et al., 1997), and an antiserum against this peptide (NN15).
Interphase Incubation and Fractionation of Golgi Membranes
1.0-ml incubations of purified rat liver Golgi stacks with interphase cytosol in the presence of 20 μM GTPγS, followed by fractionation of the membranes on 30–50% sucrose equilibrium gradients were performed as described (Sönnichsen et al., 1996).
Western Blotting and Quantitation
Membrane and protein pellets were dissolved in SDS-PAGE sample buffer, and proteins separated on 6%, in some cases 4–10% polyacrylamide gradient gels. After transfer to nitrocellulose (Hybond C; Amersham Life Science, Little Chalfont, UK) blots were blocked using PBS containing 10% (wt/vol) milk, and antibodies were diluted in the same mixture. HRP-conjugated, goat anti–rabbit or anti–mouse antibodies (Tago, Buckingham, UK) were used to detect primary antibodies. Bands were visualized by enhanced chemiluminescence (ECL; Amersham Life Science).
Films were scanned by a high resolution scanner at 300 dpi. Pixel densities were determined using NIH Image 1.51 (National Institutes of Health, Bethesda, MD). Standard curves were constructed using serially diluted total incubations or purified p115.
Purification of COPI-coated Vesicles and Golgi Remnants
Incubations of rat liver Golgi stacks and interphase or mitotic cytosol in the presence of 20 μM GTPγS were performed as described (Sönnichsen et al., 1996). Typically, for 10 binding assays, four 1.0-ml incubations were carried out for 30 min (interphase) or 20 min (mitotic) at 37°C, the salt concentration was raised to 250 mM KCl by addition of a 3 M stock solution to release COPI vesicles, and the larger Golgi remnants were sedimented in 150-μl aliquots at 10,000 g av in a horizontal centrifuge (Eppendorf Inc.) for 15 min at 4°C. Supernatants were pooled in two aliquots of 2.0 ml, and COPI vesicles were purified by sedimentation through a layer of 500 μl 35% sucrose (wt/wt) in gradient buffer (25 mM Tris-HCl, pH 7.3, 2.5 mM MgCl2, 250 mM KCl) onto a 50-μl cushion of 50% sucrose (wt/wt) in KHM (20 mM Hepes/KOH, pH 7.2, 2.5 mM Mg-acetate, 90 mM K-acetate) for 2 h at 55,000 rpm in a TLS55 rotor (Beckman Instruments, Inc., Fullerton, CA). Vesicles were resuspended in KHM, supplemented with 0.2 M sucrose. Quantitative analysis of electron micrographs showed that >85% of the membranes isolated by this procedure were COPI-coated vesicle membranes.
Golgi remnants in the 10,000 g pellets were washed in gradient buffer with 10% sucrose (wt/wt), and recovered through a layer of 15% sucrose (wt/wt) in gradient buffer onto a 50-μl cushion of 50% sucrose (wt/wt) in KHM for 1 h at 55,000 rpm and 4°C in a TLS55 rotor. Membranes were resuspended at a concentration of 2.0 mg/ml in KHM, supplemented with 0.2 M sucrose.
Cryoelectron Microscopy and Immunogold Labeling
COPI-coated vesicles were purified and processed for cryoelectron microscopy as described in Sönnichsen et al. (1996). Blocks were cut on an Ultracut UCT microtome (Leica, AG, Heerbrugg, Switzerland) with an EMFCS cryoattachment at −114°C and sections collected on Formvar/ carbon-coated copper grids.
Grids were incubated at room temperature on a drop of 50 mM NH4Cl, and nonspecific binding was blocked with 1% (wt/vol) fish skin gelatine in PBS for 30 min. Sections were labeled for 40 min with affinity-purified anti-giantin antibody, washed on five drops of PBS with fish skin gelatine over 10 min, and then incubated with protein A coupled to 10-nm gold (Dept. of Cell Biology, Utrecht School of Medicine, Utrecht, Netherlands) for 40 min. The grids were washed for 20 min on sequential drops of PBS, and for 30 min with water before staining with 2% uranyl acetate for 6 min at room temperature followed by incubation on 2% methylcellulose containing 0.2% uranyl acetate for 10 min on ice. Excess methyl cellulose was removed using filter paper and the grids air dried. Sections were observed in an electron microscope (CM10; Philips Electron Optics, Mahwah, NJ).
Purification of Rat Liver p115, Biotinylation and Coupling to Streptavidin Beads
Rat liver p115, purified as described in Levine et al. (1996) was biotinylated and coupled to streptavidin beads (Nakamura et al., 1997). Beads were resuspended in HKT buffer (10 mM Hepes/KOH, pH 7.4, 100 mM KCl, 0.5% Triton X-100, 1 mM EDTA, 1 mM DTT) and blocked with 5 mg/ml soybean trypsin inhibitor.
Solubilization of Golgi Membranes or COPI Vesicles and Binding to Bio-p115 Beads
Rat liver Golgi stacks (100 μg) or purified COPI vesicles from 4.0 ml of interphase incubation (80–100 μg; see above) were incubated in 2× HKT buffer plus protease inhibitors (Boehringer Mannheim GmbH, Mannheim, Germany) for 30 min on ice, diluted 1:1 with water and clarified for 10 min at 14,000 rpm in a microfuge. Extracts were incubated with 10 μl of p115 beads for 1 h at 4°C on a wheel. Beads were washed four times with 500 μl HKT, bound proteins eluted with 500 μl HKT containing 0.5 M KCl and precipitated with 10% TCA. Samples were analyzed by SDS-PAGE, followed by Coomassie staining or Western blotting, probing with antibodies against giantin, GM130, and p115. Pretreatment of Golgi membranes with interphase or mitotic cytosol was performed as described in Nakamura et al. (1997). For antibody competition experiments Golgi extracts were preincubated with 5 μl of giantin antibodies or preimmune serum for 2 h at 4°C before adding p115 beads.
Cross-linking of Bio-p115 to Giantin on COPI Vesicles
Purified COPI vesicles (100 μg) were incubated with 1.2 μg of biotinylated p115 in KHM, 0.2 M sucrose for 15 min on ice. Samples were adjusted to 50% sucrose (wt/wt) with a 60% sucrose solution in KHM, overlaid with 1 ml of 45% sucrose (wt/wt) in KHM, and vesicles were recovered in the top 300 μl after flotation at 55,000 rpm for 2 h at 4°C in a TLS55 rotor. Cross-linker 3,3′-dithiobis(sulfosuccinimidyl-propionate) (DTSSP) (Pierce Chemical Co., Piscataway, NJ) was added to a final concentration of 70 μM in 500 μl KHM, incubated for 30 min on ice and quenched with 1 mM Tris/HCl, pH 7.2. After the incubation, COPI vesicles were extracted with 2× HKT containing 0.5 M KCl and protease inhibitors for 30 min on ice. Extracts were clarified for 10 min at 14,000 rpm in a microfuge and incubated with 15 μl of streptavidin beads for 1 h at 4°C. Beads were washed three times in incubation buffer, and twice with KHM before addition of 40 μl SDS sample buffer containing 50 mM DTT to cleave the cross-linker. Unbound proteins were precipitated from the supernatant with 10% TCA. Proteins were fractionated by SDS-PAGE, transferred to nitrocellulose and probed with antibodies against giantin, β-COP, and p115.
Binding of p115 to COPI Vesicles and Golgi Remnants
COPI-coated vesicles or light Golgi remnants (8–10 μg) were incubated in 100 μl of binding buffer (KHM, supplemented with 0.2 M sucrose, 1 mM DTT, 2 mM ATP) with purified p115 (Levine et al., 1996) for 15 min on ice. For competition experiments, membranes were pretreated with 1 μl of anti-giantin or anti-GM130 NN15 serum for 15 min on ice before addition of 100 ng p115, or the same amount of p115 was incubated with a 70-fold molar excess of peptide N73pep for 15 min on ice before addition of the membranes. COPI vesicles were recovered by a high speed centrifugation at 100,000 rpm for 30 min in a TL100 rotor (Beckman Instruments, Inc.) through a 100-μl layer of 35% sucrose (wt/wt) in KHM. Golgi remnants were recovered through a 100-μl layer of 15% sucrose (wt/wt) in KHM in the same rotor. The recovery of COPI vesicles was 80–90%, assessed by quantitation of the Western blot signal for β-COP. The recovery of Golgi remnants was 70–80%, assessed using GM130. These recoveries were not significantly affected by the different treatments of the membranes.
COPI Vesicle Release Assay
Stacked Golgi membranes were incubated for 15 min on ice with 0.5 M KCl in KHM, 0.2 M sucrose, recovered on a 5-μl cushion of 2 M sucrose by sedimentation at 10,000 g av for 10 min in a horizontal centrifuge (Eppendorf Inc.), and resuspended in KHM, 0.2 M sucrose. 10–15 μg of salt-washed membranes were incubated with interphase cytosol (8–10 mg/ml) in 100 μl KHM, and 0.2 M sucrose, in the presence of an ATP-regenerating system and 20 μM GTPγS for 30 min at 37°C. To release COPI vesicles from Golgi remnants the salt concentration was raised to 250 mM KCl. In some experiments the cytosol was pretreated with a 10-, 30-, or 100-fold molar excess of GM130 peptide over cytosolic p115 for 15 min on ice, and no salt was added after the incubation at 37°C. Golgi remnants were sedimented at 15,000 g for 15 min at 4°C in a microfuge. Released COPI vesicles were recovered from the supernatant by sedimentation through a layer of 35% sucrose (wt/wt) in KHM at 100,000 rpm for 30 min in a TL100.2 rotor (Beckman Instruments, Inc.). Pellets were analyzed by SDS-PAGE and Western blotting, probing for β-COP, GM130, and p115.
Docking Assay
In a standard assay, 8 μg of salt-washed Golgi membranes were incubated with 8–10 μg of purified COPI vesicles and 75 ng of purified p115 in 100 μl of KHM, 0.2 M sucrose, 2 mM ATP, 0.5 mM GTP, 1 mM DTT for 10 min at 30°C. GTPγS (0.1 mM) was also used instead of GTP and gave similar results. Generally, the incubation was started by adding the Golgi membranes. Golgi membranes and docked vesicles were sedimented at 10,000 g av in a horizontal Eppendorf centrifuge for 10 min. COPI vesicles in the supernatant were recovered by sedimentation at 100,000 rpm for 30 min in a TL100 rotor (Beckman Instruments, Inc.). In some experiments, Golgi membranes (1–2 mg/ml) were pretreated with antibodies against giantin or GM130 in a dilution of 1:100 for 15 min on ice, reisolated by sedimentation at 10,000 g onto a 5-μl cushion of 2 M sucrose, and then resuspended in incubation buffer. COPI vesicles were pretreated with antibodies against giantin or GM130 during the purification procedure. After pooling the released vesicles from the salt supernatants, antibodies (dilution 1:100) and vesicles were left on ice for 15 min, before reisolation through 35% sucrose by high speed centrifugation. In all experiments the recovery of Golgi membranes in the medium speed pellet was >95%, assessed by quantitation of the GM130 signal. The combined recovery of COPI vesicles from both the medium and high speed pellets was 80–90%, assessed using the β-COP signal.
Results
Binding of Giantin to p115
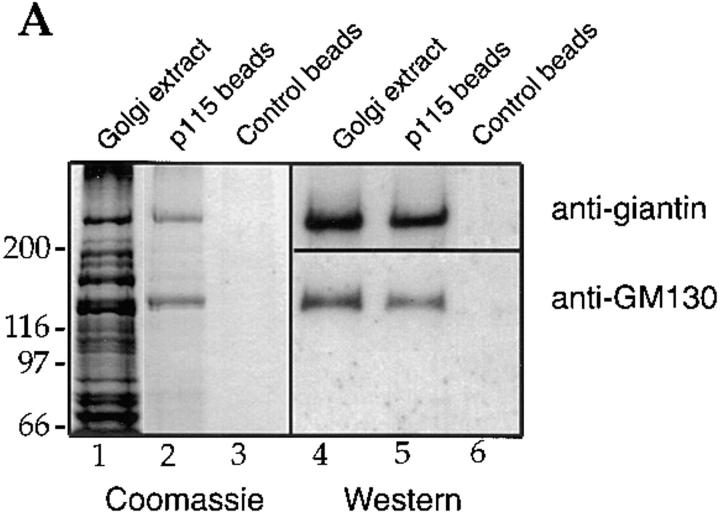
We showed previously that p115 binds to just two proteins in extracts of Golgi membranes (Nakamura et al., 1997). Purified rat liver Golgi membranes were extracted with 0.5% Triton X-100 and 200 mM KCl, diluted twofold, and the clarified supernatant (Fig. 1 A, lane 1) incubated with biotinylated p115 that had been immobilized on streptavidin beads. Proteins of 130 kD and ∼400 kD bound to p115 but not control beads (Fig. 1 A, cf. lanes 2 and 3), and the protein of 130 kD was identified as GM130 (Fig. 1 A, lane 5), a component of the Golgi matrix (Nakamura et al., 1995). We have now identified the ∼400 kD protein as giantin, a type II Golgi membrane protein with most of its mass projecting into the cytoplasm (Linstedt and Hauri, 1993; Seelig et al., 1994). p400 reacted on Western blots with polyclonal antibodies specific for giantin (Fig. 1 A lane 5). Proteolytic fragments of p400 extracted from the gel were analyzed by mass spectrometry and were found to be a perfect match for rat giantin fragments generated from the sequence in the database (Pappin et al., 1993). Pretreatment of the Golgi extract with anti-giantin antibodies inhibited subsequent binding of p400 to p115 beads by ∼80% (Fig. 1 B, lane 2). GM130 was unaffected by this treatment (Fig. 1 B, lane 2).
Figure 1.
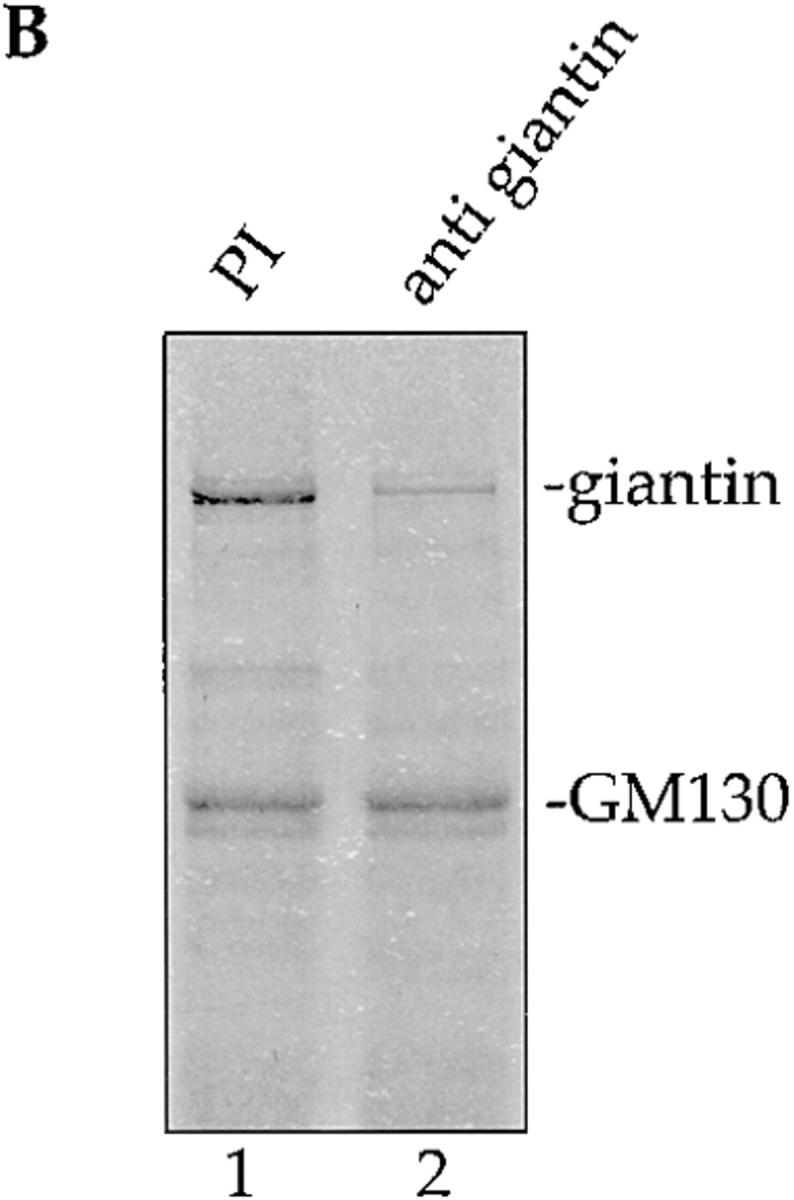
p115 binds to giantin. (A) Rat liver Golgi membranes were solubilized with buffer containing 0.5% Triton X-100, the extract cleared by centrifugation, and then incubated with biotin-p115 immobilized on streptavidin beads, or control beads. The clarified Golgi extract (lanes 1 and 4; 20% of total) and proteins bound to p115 beads (lanes 2 and 5; 50% of total) or control beads (lanes 3 and 6; 50% of total) were fractionated by SDS-PAGE and either stained with Coomassie blue or transferred to nitrocellulose and probed for GM130 and giantin, using specific antibodies. Molecular weight markers are shown in kD. (B) The clarified Triton X-100 extract of Golgi membranes was either preincubated with preimmune serum (PI, lane 1) or with anti- giantin antibodies (lane 2) before incubation with p115 beads. Bound proteins were fractionated by SDS-PAGE and visualized by Coomassie staining. (C) Golgi membranes were incubated for 10 min at 37°C with interphase HeLa cytosol (lane 1), mitotic HeLa cytosol (lane 2), or mitotic HeLa cytosol inactivated by pretreatment with staurosporine (lane 3). After solubilization of the reisolated membranes with Triton X-100, the cleared supernatants were incubated with p115 beads. Bound proteins were fractionated by SDS-PAGE, transferred to nitrocellulose, and then probed for GM130 and giantin.
Giantin does not bind to p115 via GM130. This was shown by exploiting the fact that mitotic GM130 does not bind to p115 (Nakamura et al., 1997). Golgi membranes were incubated with HeLa cytosol from interphase or mitotic cells. In one experiment, the mitotic cytosol was inactivated by prior incubation with the general kinase inhibitor, staurosporine. After incubation, the membranes were extracted with Triton X-100 and salt, and the clarified extract incubated with p115 beads. Bound proteins were fractionated by SDS-PAGE followed by transfer to nitrocellulose filters and probing for GM130 and giantin. As shown in Fig. 1 C, GM130 bound to p115 after treatment with interphase cytosol or inactivated mitotic cytosol (Fig. 1 C, lanes 1 and 3) but binding was abolished after incubation with mitotic cytosol (Fig. 1 C, lane 2). In contrast, treatment with mitotic cytosol had little effect on the binding of giantin to p115. The level was very similar to that observed after treatment with interphase or inactivated mitotic cytosol (Fig. 1 C, cf. lane 2 with lanes 1 and 3). These data not only show that giantin binds to p115 in the absence of GM130 but also that this binding is not mitotically regulated.
Distribution of GM130 and Giantin
COPI-coated vesicles were generated by incubating Golgi membranes with interphase cytosol and GTPγS for 60 min at 37°C. After the incubation, KCl was added to 250 mM to release the COPI vesicles that were then separated from Golgi remnants using equilibrium sucrose gradients.
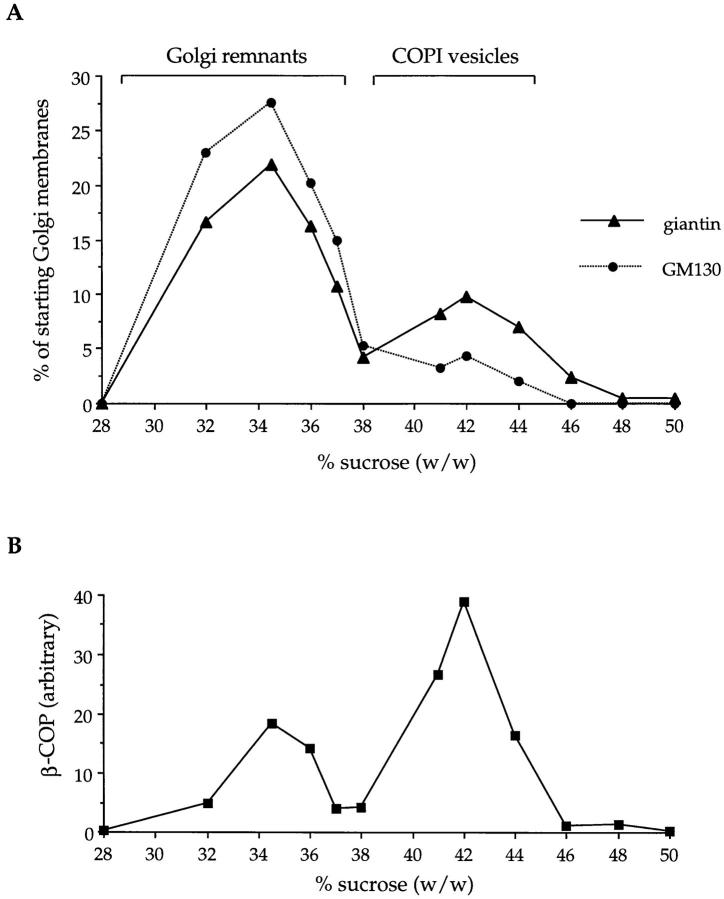
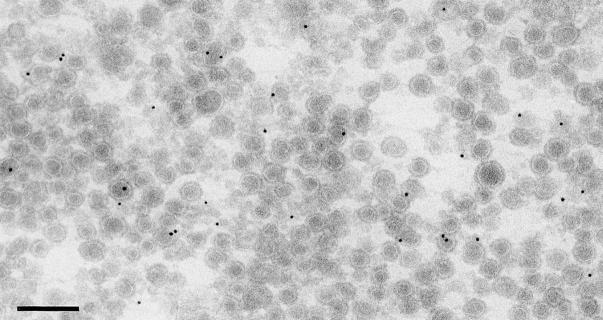
As shown in Fig. 2 A, giantin cofractionated with both the Golgi remnants and the COPI vesicles, marked by the distribution of β-COP (Fig. 2 B). Immunoelectron microscopy was used to show that giantin was in COPI vesicles and not in cofractionating, contaminating membranes. Cryosections were labeled with affinity-purified anti-giantin antibodies that were visualized using 10 nm protein A–gold. As shown in Fig. 3, most if not all of the gold labeling was over or near to COPI vesicle profiles.
Figure 2.
GM130, but not giantin, is depleted from COPI vesicles. Golgi membranes were incubated for 60 min at 37°C with interphase HeLa cytosol in the presence of GTPγS to accumulate COPI vesicles. After addition of salt to release the vesicles from Golgi remnants, samples were centrifuged to equilibrium on a 30–50% sucrose gradient. Membranes were reisolated by centrifugation, fractionated by SDS-PAGE, Western blotted, and then quantitated. (A) GM130 and giantin. (B) β-COP. The fractions containing COPI vesicles and Golgi remnants are indicated.
Figure 3.
Purified COPI vesicles contain giantin by immunoelectron microscopy. COPI vesicles generated as described in the legend to Fig. 2 were fixed, cryosectioned, and labeled with antibodies to giantin followed by protein A coupled to 10-nm gold. Bar, 200 nm.
Calculation showed that 26% of the giantin in starting Golgi membranes was present in the COPI vesicle fractions (Table I). Phospholipid analysis has shown that 24% of membrane bilayer is incorporated into COPI vesicles under these conditions (Sönnichsen et al., 1996). This means that the average density of giantin in the COPI vesicles (Table I, 108%) was about the same or slightly higher compared to the starting membranes.
Table I.
Giantin and GM130 in COPI Vesicles
| Content of COPI Vesicles | ||||
|---|---|---|---|---|
| Amount (Percent of starting RLG) | Average density (Percent of starting RLG) | |||
| Giantin | 26 ± 1.4 | 108 ± 5.8 | ||
| GM130 | 8.5 ± 1.8 | 35 ± 7.5 | ||
COPI vesicles isolated as described in the legend to Fig. 2 were analyzed by Western blotting for their content of giantin and GM130 compared with starting Golgi membranes. Phospholipid analysis showed that 24% of starting Golgi membrane was present in COPI vesicles (Sönnichsen et al., 1996), which permitted calculation of the average density of GM130 and giantin in the vesicles compared with starting membranes (set to 100%).
In contrast, GM130 was largely depleted in the COPI vesicles. GM130, like giantin, cofractionated with COPI vesicles in sucrose gradients (Fig. 2 A) but there was clearly much less GM130 in the vesicles when compared with giantin. This was confirmed by quantitative analysis. The calculated average density of GM130 in COPI vesicles was only about one third of that in the starting membranes (Table I, 35%). Together these data suggest that, during vesicle budding, giantin is incorporated at the prevailing concentration in Golgi membranes whereas GM130 is largely excluded.
Binding of p115 to COPI Vesicles
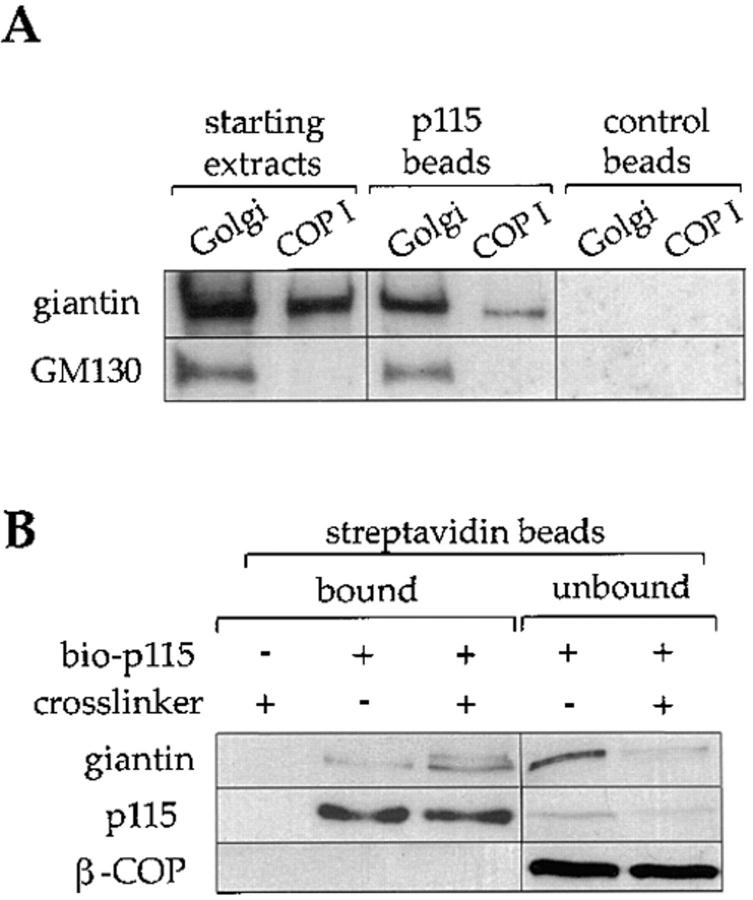
Giantin in COPI vesicles was able to bind p115 under the same conditions previously used for Golgi membranes (Fig. 1 A). Vesicles were extracted with Triton X-100 and salt, and incubated with p115 beads. As shown in Fig. 4 A, giantin extracted from starting Golgi membranes or COPI vesicles, bound to p115 but not control beads. There was little if any binding of GM130 from COPI vesicles, consistent with the low levels that were present in these membranes.
Figure 4.
Biotinylated p115 binds to giantin in COPI vesicles. (A) Triton X-100 extracts of rat liver Golgi membranes or purified COPI vesicles from interphase incubations were incubated with biotinylated p115 immobilized on streptavidin beads, or control beads. Starting extracts (30% of total), and proteins bound to p115 beads or control beads (100% of total) were fractionated by SDS-PAGE, transferred to nitrocellulose, and then probed with antibodies against giantin and GM130. (B) Purified COPI vesicles were incubated with or without biotinylated p115 (bio-p115) for 15 min on ice, the membranes reisolated on a sucrose flotation gradient, and then incubated with or without the cross-linker, DTSSP. After quenching the cross-linker, proteins were extracted with Triton X-100, 0.5 M KCl, and then the extracts were incubated with streptavidin beads. The cross-linker was cleaved by boiling the samples in sample buffer with 50 mM DTT. Bound and unbound fractions were subjected to SDS-PAGE and analyzed by Western blotting, probing with antibodies against giantin, p115, and β-COP. Note that the streptavidin beads quantitatively depleted biotinylated p115 from the extracts.
Cross-linking studies confirmed the binding of biotinylated p115 to giantin in intact COPI vesicles. These were incubated in the presence or absence of biotinylated p115 in the presence or absence of the cleavable cross-linker DTSSP. At the end of the incubation the samples were solubilized with Triton X-100 and 0.5 M KCl, proteins bound to p115 were isolated using streptavidin beads and, after cleavage of the cross-linker using 50 mM DTT, were identified by Western blotting. As shown in Fig. 4 B, most of the giantin did not bind to biotinylated p115 in the absence of cross-linker under these high salt conditions. In the presence of DTSSP two forms of giantin were detected, the larger likely representing giantin dimers. Binding was specific since neither β-COP (Fig. 4 B) nor α-COP (data not shown) were cross-linked to p115. Binding was also quantitative in that >80% of the giantin in the incubation was cross-linked to p115. This rules out the possibility that p115 is binding to giantin on the small percentage of contaminating membranes in the COPI vesicle preparations. Quantitation showed that >85% of the membrane in these preparations was 50–100 nm vesicle profiles with COPI coats.
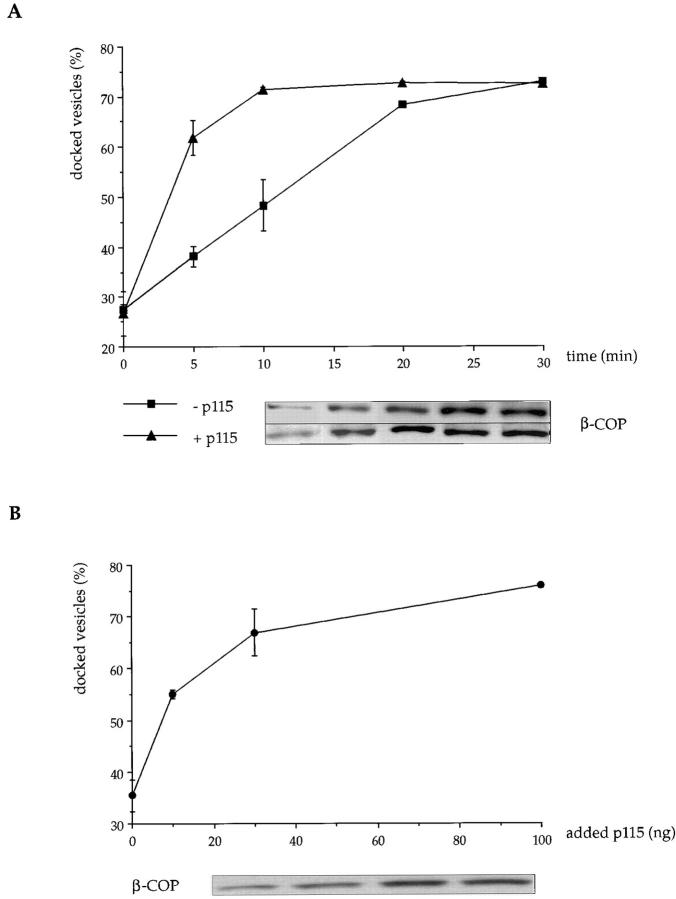
Experiments were also carried out using purified p115, unmodified by biotinylation. As shown in Fig. 5, COPI vesicles containing residual p115 bound exogenous p115 in a saturable manner. At saturation, 1 ng of p115 bound to 80–100 ng COPI vesicle proteins, suggesting that p115 binds to a major protein in COPI vesicles.
Figure 5.
Binding of p115 to COPI vesicles is saturable. Purified COPI vesicles (8–10 μg) were incubated with increasing amounts of p115 for 15 min on ice. Membranes were reisolated by centrifugation through a 35% sucrose cushion, the pellets fractionated by SDS-PAGE and the p115 quantitated after Western blotting (inset shows representative experiment). Vesicle recovery was reproducibly 80–90% as assessed by probing with β-COP antibodies. Results are expressed as the mean of three experiments ± SEM.
The binding of p115 to interphase COPI vesicles was inhibited by ∼60% after preincubation in the presence of anti-giantin antibodies (Fig. 6 A). It was essentially unaffected by preincubation with antibodies to the GM130 NH2 terminus, or in the presence of a peptide derived from the NH2-terminal 73 amino acids of GM130. This peptide has previously been shown to inhibit the binding of p115 to GM130 (Nakamura et al., 1997). Binding was also unaffected when COPI vesicles were prepared using mitotic instead of interphase cytosol (Fig. 6 A). Mitotic phosphorylation of GM130 prevents binding to p115 (Nakamura et al., 1997), whereas mitotic treatment of giantin has little effect (Fig. 1 C). Together these data provide strong evidence that p115 binds to giantin, not GM130, in COPI vesicles.
Figure 6.

p115 binds to giantin on COPI vesicles and GM130 on Golgi remnants. Golgi membranes were incubated at 37°C with interphase (30 min) or mitotic cytosol (20 min) in the presence of GTPγS, salt was added, and samples were fractionated to separate COPI vesicles from Golgi remnants (see Materials and Methods). Samples (8–10 μg) were incubated for 15 min on ice with 100 ng p115. Antibodies to GM130 and giantin were used to pretreat the membranes for 15 min on ice before addition of p115. The GM130 peptide was added together with the p115 at a 70-fold molar excess. Membranes were reisolated, fractionated by SDS-PAGE, the amount of bound p115 quantitated by Western blotting and normalized for β-COP (COPI vesicles) or GM130 (Golgi remnants). Membrane recovery was 80–90% for COPI vesicles and 70–80% for Golgi remnants. Results are expressed as a percentage of the interphase vesicles or remnants ± SEM (n = 3–5, depending on the experiment). The 100% values were 88 ± 5.8 ng p115/10 μg interphase vesicles and 112 ± 18.3 ng/10 μg interphase remnants. (A) Binding to COPI vesicles. (B) Binding to Golgi remnants.
Binding of p115 to Golgi Remnants
The opposite results were obtained when p115 was bound to Golgi remnants recovered from the preparation of COPI vesicles (see Fig. 2 and Materials and Methods). These remnants can be thought of as the membranes that would have acted as the acceptors for the COPI vesicles had the transport cycle not been arrested by the addition of GTPγS.
As shown in Fig. 6 B, preincubation of the remnants with anti-giantin antibodies only inhibited the binding of p115 by ∼30%. In contrast, binding was inhibited by ∼70% after pretreatment with anti-GM130 antibodies, or when the incubation was carried out in the presence of the GM130 peptide. The same inhibition was also obtained when the remnants from a mitotic incubation were used in the binding assay (Fig. 6 B). These data suggest that p115 binds to GM130 on Golgi remnants and much less readily to giantin.
Docking of COPI Vesicles
The data so far strongly suggest that p115 binds to giantin in COPI vesicles and GM130 on Golgi membranes but provides no direct evidence that p115 acts as the bridge linking these two so as to dock the vesicles to the membrane. Two approaches were taken to address this question.
The first was to exploit the bound state of COPI vesicles at the end of an interphase incubation in the presence of GTPγS. As part of the purification procedure, COPI vesicles are released by raising the salt concentration to levels comparable to those needed for the release of p115 from Golgi membranes (Levine et al., 1996; Malhotra et al., 1989). If the action of salt is through the release of p115 then it should be possible to mimic this process by adding the GM130 peptide to break the link between p115 and GM130 on Golgi membranes. As shown in Fig. 7 A, the addition of salt leads to a 2.3-fold increase of COPI vesicles in the supernatant (from 43 to 100%). Increasing concentrations of the GM130 peptide mimicked the effect of salt such that a 100-fold excess over cytosolic p115 released >75% of the COPI vesicles compared with the salt treatment. The release of COPI vesicles was inversely correlated with the amounts of p115 left on Golgi remnants. Treatment with salt lowered the p115 level about fourfold (from 100 to 28%), very similar to that obtained using the 100-fold excess of GM130 peptide (30%). These data strongly suggest that p115 mediates the docking of COPI vesicles through binding to GM130.
Figure 7.
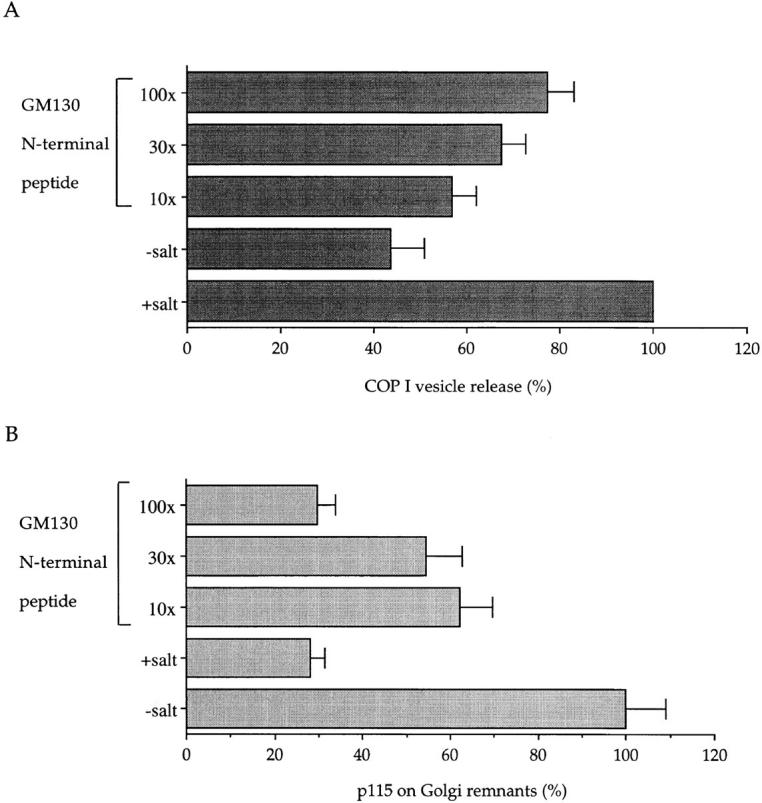
The GM130 peptide releases COPI vesicles from Golgi membranes. Salt-washed Golgi membranes were incubated with interphase cytosol for 30 min at 37°C in the presence of GTPγS. In some experiments, the cytosol was preincubated for 15 min on ice with the GM130 peptide at the indicated molar ratios over cytosolic p115. At the end of the incubation, those samples using untreated cytosol were either left untreated (−salt; GM130 peptide) or treated with KCl at a final concentration of 250 mM to release COPI vesicles (+salt). Golgi remnants were sedimented in a microfuge and released COPI vesicles in the supernatant were recovered by high speed centrifugation through a 35% sucrose cushion. Remnants and vesicles were fractionated by SDS-PAGE and quantitated by Western blotting using antibodies to β-COP and p115. (A) Release of COPI vesicles is expressed as a percentage of the +salt control ± SEM (n = 3). (B) p115 bound to Golgi remnants is expressed as a percentage of the −salt control ± SEM (n = 3).
The second approach was to set up a docking assay using purified COPI vesicles and stacked Golgi membranes. Vesicles with COPI coats locked on by GTPγS are known to bind to Golgi membranes (Malhotra et al., 1989). To show a dependency of docking on p115 it was necessary to remove this protein from Golgi membranes and COPI vesicles. This was difficult to do efficiently so the amounts of membrane used had to be kept to a minimum. Pilot experiments were carried out to determine the least amount of Golgi membranes (containing p115) needed to bind most of the COPI vesicles in the assay. Since these vesicles were in limited supply, a fixed amount of 8–10 μg was chosen, and 8 μg of Golgi membranes was found to be sufficient to bind ∼80% of these added vesicles (data not shown).
Salt washing then rendered these Golgi membranes sensitive to added p115. As shown in Fig. 8, the rate of COPI vesicle docking was dependent both on the amount of p115 added and on the time of the incubation. Interestingly, the extent of docking was the same even in the absence of added p115. This could reflect the presence of residual p115 on the membranes (∼10%) and vesicles (∼15%) that cannot be removed even using high salt. Efforts to remove this residual p115 on Golgi membranes using the GM130 peptide have been partially successful and this does increase the time needed to reach maximum docking. Our inability to completely inhibit docking might reflect a catalytic role for p115 rather than as a stable component of the docked vesicle complex (see Discussion).
Figure 8.
Docking of COPI vesicles to Golgi membranes is facilitated by p115. Purified COPI vesicles (8–10 μg) were incubated with salt-washed Golgi membranes (8 μg) at 30°C for (A) increasing times ± 75 ng p115 or (B) increasing amounts of p115 for 10 min. Docked vesicles were isolated by medium-speed centrifugation, and free vesicles were recovered from the supernatant by high speed centrifugation. Results are expressed as the amount of β-COP in the medium speed pellet as a percentage of that in the combined medium and high speed pellets ± SEM (n = 3). Insets show the β-COP signal in the medium speed pellets of representative binding experiments. Recovery of COPI vesicles was reproducibly 80–90% of the input vesicles.
The role played by giantin and GM130 was assessed at early time points when the docking of COPI vesicles to Golgi membranes was dependent on the amount of added p115 (Fig. 8 B). A 10-min incubation using 75 ng p115 was chosen and the results are presented in Fig. 9. Docking of COPI vesicles was dependent on the presence of Golgi membranes showing that the centrifugation conditions could distinguish between docked and free vesicles. Pretreatment of COPI vesicles with anti-giantin antibodies inhibited docking whereas pretreatment of Golgi membranes had no effect. Conversely, pretreatment of Golgi membranes with anti-GM130 antibodies inhibited docking, whereas pretreatment of COPI vesicles did not. These results agree with those obtained by binding p115 to the individual membrane components and strongly support the idea that giantin is used to dock COPI vesicles to GM130 in Golgi membranes using p115 as the bridge.
Figure 9.
Docking requires giantin in the COPI vesicles and GM130 on Golgi membranes. Purified COPI vesicles (8–10 μg) were incubated with p115 (75 ng) in the absence or presence of salt-washed Golgi membranes (8 μg) for 10 min at 30°C. Where indicated the vesicles or Golgi membranes were pretreated with antibodies to giantin or GM130. Samples were processed as in Fig. 8 and vesicle docking is expressed as a percentage of the control without pretreatment ± SEM (n = 3).
Discussion
We have identified another component involved in docking COPI vesicles to Golgi membranes. Giantin was originally identified using a monoclonal antibody raised against Golgi membranes (Linstedt and Hauri, 1993); and biochemical studies (Linstedt and Hauri, 1993), followed by cloning and sequencing the protein (Seelig et al., 1994), showed that it was a membrane protein anchored at the COOH terminus with most of its mass projecting into the cytoplasm. Sedimentation studies predicted a long, rod-like protein (Linstedt and Hauri, 1993), and this was corroborated by the sequence that predicted extensive coiled-coil regions throughout the protein (Seelig et al., 1994).
Our evidence shows that giantin is one of the two proteins in detergent extracts of Golgi membranes that bind to the vesicle docking protein, p115. The other is GM130 and several lines of evidence strongly suggest that GM130 binding occurs on Golgi membranes and not on COPI vesicles. Antibodies to GM130 inhibited the binding of p115 to Golgi membranes but not COPI vesicles. The same was true using the GM130 NH2-terminal peptide that disrupts the interaction between p115 and GM130. The binding of p115 was also inhibited when Golgi membranes were prepared using mitotic instead of interphase cytosol, whereas there was no difference when COPI vesicles were prepared under the two conditions. We had previously shown that the binding of p115 to GM130 is inhibited under mitotic conditions (Nakamura et al., 1997), so these results are consistent with inactivation of GM130 on Golgi membranes especially since we have now shown that the binding of giantin to p115 is not mitotically regulated. Lastly, GM130 was depleted from COPI vesicles. The average concentration was only about a third of that in starting membranes. Together these data argue that GM130 is the Golgi membrane docking site for p115.
In marked contrast, the binding of p115 to giantin appears to occur largely to the giantin population in COPI vesicles. Anti-giantin antibodies inhibited the binding of p115 to COPI vesicles by ∼60%, compared with 30% for Golgi membranes. The average concentration of giantin in COPI vesicles was about the same as that in starting Golgi membranes, suggesting that it is sampled during vesicle budding at the prevailing concentration. Lastly, p115 bound to giantin extracted from COPI vesicles and could be cross-linked to it in intact vesicles.
These results were obtained by binding p115 either to COPI vesicles or Golgi membranes and the conclusions were strengthened considerably by two approaches addressing the docking of vesicles. The first showed that docked vesicles could be released using the GM130 peptide and this correlated with decreased amounts of p115 on the Golgi membranes. The second showed that the binding of purified COPI vesicles to Golgi membranes required p115, GM130, and giantin. Pretreatment of COPI vesicles with antibodies to giantin, but not GM130, inhibited docking and the converse was true for Golgi membranes.
Taken together, these data support the view that p115 docks vesicles by linking the giantin on the vesicles to GM130 on the Golgi membrane. We cannot yet exclude the possibility that the residual GM130 on the vesicles or the giantin on the membrane plays a role though the available evidence is against this. We also cannot yet determine precisely the role played by p115 in the docking process. As shown in Fig. 8, exogenous p115 stimulates docking, but there seems to be sufficient p115 left on the membranes and vesicles used for the docking assay to permit maximal docking if given enough time. This p115 could either act catalytically bringing giantin and GM130 together, and then moving on to the next pairing, or it could participate stoichiometrically, forming an integral part of the final complex. Until we can analyze the composition of the docked protein complexes we will not be able to decide which of these two mechanisms is operative.
Giantin must be exposed on the surface of COPI vesicles if it is to interact with p115. The predicted length of giantin from sedimentation studies is ∼250 nm (Linstedt et al., 1995), more than sufficient to protrude through the 10–15-nm-thick COPI coat (Malhotra et al., 1989; Oprins et al., 1993). In fact this predicted length is as much as four times the width of a COPI vesicle, which gives rise to the image of transport vesicles endowed with long, filamentous protrusions. Such protrusions would certainly aid in keeping vesicles near to Golgi membranes. They might even help ensure that a COPI vesicle budding from one cisterna becomes attached to the next before budding is complete. This could increase the efficiency of intra-Golgi transport by preventing vesicles leaving the immediate vicinity of the stack.
We do not yet know how many giantin molecules are present on each vesicle. The levels of cryolabeling were low but the antibody inhibition experiments suggest that the majority of vesicles have at least one copy. Calculations indicate up to eight copies per vesicle. These might enter the vesicles passively since they are present at the same average density as in starting membranes. Alternatively, there might be a signal to ensure that each vesicle receives at least one copy. Work by Hauri and colleagues has suggested that information in the COOH-terminal part of the protein is needed for newly synthesised giantin to leave the ER (Linstedt et al., 1995), presumably in COPII vesicles, and this same signal might be used in COPI vesicles.
Other roles for GM130, p115, and giantin are also not excluded by these results. As an example, immunofluorescence microscopy shows giantin surrounding the Golgi apparatus (Linstedt and Hauri, 1993; Seelig et al., 1994) whereas GM130 and p115 are mostly located on the cis side (Shima et al., 1997). This either means that proteins other than p115 and GM130 are involved in the docking of COPI vesicles or that giantin is playing other, perhaps more structural roles, in the organization of the stack as suggested by others (Linstedt and Hauri, 1993; Seelig et al., 1994). In either case, the next step will be to use giantin as an affinity ligand to probe for other Golgi proteins with which it might interact.
Acknowledgments
We thank Drs. T. Kreis, G. Waters, and M. Renz for kindly providing us with antibodies; D. Pappin for analysis of giantin fragments by mass spectrometry; and F. Barr for critical reading of the manuscript.
Abbreviations used in this paper
- COP
coat protein
- DTSSP
3,3′-dithiobis(sulfosuccinimidyl-propionate)
- NSF
N-ethylmaleimide–sensitive fusion protein
- SNAP
soluble NSF attachment protein
- SNARE
SNAP receptor
Footnotes
B. Sönnichsen, E. Jämsä, and B. Dirac-Svejstrup were supported by research fellowships from the Deutsche Forschungsgemeinschaft, Academy of Finland, and Danish Natural Science Research Council, respectively.
Address all correspondence to Graham Warren, Cell Biology Laboratory, Imperial Cancer Research Fund, 44 Lincoln's Inn Fields, London WC2A, 3PX, UK. Tel.: 0171-269-3561. Fax: 0171-269-3417. E-mail: g.warren@europa.lif.icnet.uk
B. Sönnichsen's present address is Cell Biology Program, European Molecular Biology Laboratories, Meyerhofstrasse 1, 69117 Heidelberg, Germany.
T. Levine's present address is Medical Research Council LMB, Hills Road, Cambridge CB2 2QH, UK.
References
- Allan VJ, Kreis TE. A microtubule-binding protein associated with membranes of the Golgi apparatus. J Cell Biol. 1986;103:2229–2239. doi: 10.1083/jcb.103.6.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlowe C. COP II - a membrane coat that forms endoplasmic reticulum-derived vesicles. FEBS (Fed Eur Biochem Soc) Lett. 1995;369:93–96. doi: 10.1016/0014-5793(95)00618-j. [DOI] [PubMed] [Google Scholar]
- Barroso M, Nelson DS, Sztul E. Transcytosis-associated protein (TAP)/p115 is a general fusion factor required for binding of vesicles to acceptor membranes. Proc Natl Acad Sci USA. 1995;92:527–531. doi: 10.1073/pnas.92.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarek SY, Ravazzola M, Hosobuchi M, Amherdt M, Perrelet A, Schekman R, Orci L. COP I-coated and COP II-coated vesicles bud directly from the endoplasmic-reticulum in yeast. Cell. 1995;83:1183–1196. doi: 10.1016/0092-8674(95)90144-2. [DOI] [PubMed] [Google Scholar]
- Campbell JL, Schekman R. Selective packaging of cargo molecules into endoplasmic reticulum-derived COPII vesicles. Proc Natl Acad Sci USA. 1997;94:837–842. doi: 10.1073/pnas.94.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosson P, Letourneur F. Coatomer interaction with di-lysine endoplasmic reticulum retention motifs. Science. 1994;263:1629–1631. doi: 10.1126/science.8128252. [DOI] [PubMed] [Google Scholar]
- Dascher C, Balch WE. Mammalian Sly1 regulates syntaxin 5 function in endoplasmic reticulum to Golgi transport. J Biol Chem. 1996;271:15866–15869. doi: 10.1074/jbc.271.27.15866. [DOI] [PubMed] [Google Scholar]
- Duden R, Griffiths G, Frank R, Argos P, Kreis TE. β-COP, a 110 kD protein associated with non-clathrin-coated vesicles and the Golgi complex, shows homology to β-adaptin. Cell. 1991;64:649–665. doi: 10.1016/0092-8674(91)90248-w. [DOI] [PubMed] [Google Scholar]
- Letourneur F, Gaynor EC, Hennecke S, Demolliere C, Duden R, Emr SD, Riezman H, Cosson P. Coatomer is essential for retrieval of dilysine-tagged proteins to the endoplasmic-reticulum. Cell. 1994;79:1199–1207. doi: 10.1016/0092-8674(94)90011-6. [DOI] [PubMed] [Google Scholar]
- Levine TP, Misteli T, Rabouille C, Warren G. Mitotic disassembly and reassembly of the Golgi-apparatus. Cold Spring Harbor Symp Quant Biol. 1995;60:549–557. doi: 10.1101/sqb.1995.060.01.058. [DOI] [PubMed] [Google Scholar]
- Levine TP, Rabouille C, Kieckbusch RH, Warren G. Binding of the vesicle docking protein p115 to Golgi membranes is inhibited under mitotic conditions. J Biol Chem. 1996;271:17304–17311. doi: 10.1074/jbc.271.29.17304. [DOI] [PubMed] [Google Scholar]
- Lian JP, Stone S, Jiang Y, Lyons P, Ferro-Novick S. Ypt1p implicated in v-SNARE activation. Nature. 1994;372:698–701. doi: 10.1038/372698a0. [DOI] [PubMed] [Google Scholar]
- Linstedt AD, Hauri HP. Giantin, a novel conserved Golgi membrane-protein containing a cytoplasmic domain of at least 350-kda. Mol Biol Cell. 1993;4:679–693. doi: 10.1091/mbc.4.7.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linstedt AD, Foguet M, Renz M, Seelig HP, Glick BS, Hauri HP. A C-terminally-anchored Golgi protein is inserted into the endoplasmic-reticulum and then transported to the Golgi-apparatus. Proc Natl Acad Sci USA. 1995;92:5102–5105. doi: 10.1073/pnas.92.11.5102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupashin VV, Waters MG. t-SNARE activation through transient interaction with a rab-like guanosine triphosphatase. Science. 1997;276:1255–1258. doi: 10.1126/science.276.5316.1255. [DOI] [PubMed] [Google Scholar]
- Lupashin VV, Hamamoto S, Schekman RW. Biochemical requirements for the targeting and fusion of ER-derived transport vesicles with purified yeast Golgi membranes. J Cell Biol. 1996;132:277–289. doi: 10.1083/jcb.132.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra V, Serafini T, Orci L, Shepherd JC, Rothman JE. Purification of a novel class of coated vesicles mediating biosynthetic protein transport through the Golgi stack. Cell. 1989;58:329–336. doi: 10.1016/0092-8674(89)90847-7. [DOI] [PubMed] [Google Scholar]
- Mayer A, Wickner W, Haas A. Sec18p (NSF)-driven release of Sec17p (α-SNAP) can precede docking and fusion of yeast vacuoles. Cell. 1996;85:83–94. doi: 10.1016/s0092-8674(00)81084-3. [DOI] [PubMed] [Google Scholar]
- Misteli T, Warren G. COP-coated vesicles are involved in the mitotic fragmentation of Golgi stacks in a cell-free system. J Cell Biol. 1994;125:269–282. doi: 10.1083/jcb.125.2.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima H, Hirata A, Ogawa Y, Yonehara T, Yoda K, Yamasaki M. A cytoskeleton-related gene, USO1, is required for intracellular protein transport in Saccharomyces cerevisiae. J Cell Biol. 1991;113:245–260. doi: 10.1083/jcb.113.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura N, Rabouille C, Watson R, Nilsson T, Hui N, Slusarewicz P, Kreis TE, Warren G. Characterization of a cis-Golgi matrix protein, GM130. J Cell Biol. 1995;131:1715–1726. doi: 10.1083/jcb.131.6.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura N, Lowe M, Levine TP, Rabouille C, Warren G. The vesicle docking protein p115 binds GM130, a cis-Golgi matrix protein, in a mitotically regulated manner. Cell. 1997;89:445–455. doi: 10.1016/s0092-8674(00)80225-1. [DOI] [PubMed] [Google Scholar]
- Oprins A, Duden R, Kreis TE, Geuze HJ, Slot JW. β-COP localizes mainly to the cis-Golgi side in exocrine pancreas. J Cell Biol. 1993;121:49–59. doi: 10.1083/jcb.121.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L, Stamnes M, Ravazzola M, Amherdt M, Perrelet A, Söllner TH, Rothman JE. Bidirectional transport by distinct populations of COPI-coated vesicles. Cell. 1997;90:335–349. doi: 10.1016/s0092-8674(00)80341-4. [DOI] [PubMed] [Google Scholar]
- Pappin DJC, Hojrup P, Bleasby AJ. Rapid identification of proteins by peptide-mass fingerprinting. Curr Biol. 1993;3:327–332. doi: 10.1016/0960-9822(93)90195-t. [DOI] [PubMed] [Google Scholar]
- Pfeffer SR. Transport vesicle docking: SNAREs and associates. Annu Rev Cell Dev Biol. 1996;12:441–461. doi: 10.1146/annurev.cellbio.12.1.441. [DOI] [PubMed] [Google Scholar]
- Rexach MF, Latterich M, Schekman RW. Characteristics of endoplasmic reticulum–derived transport vesicles. J Cell Biol. 1994;126:1133–1148. doi: 10.1083/jcb.126.5.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman JE, Wieland FT. Protein sorting by transport vesicles. Science. 1996;272:227–234. doi: 10.1126/science.272.5259.227. [DOI] [PubMed] [Google Scholar]
- Sapperstein SK, Walter DM, Grosvenor AR, Heuser JE, Waters MG. p115 is a general vesicular transport factor related to the yeast endoplasmic reticulum to Golgi transport factor Uso1p. Proc Natl Acad Sci USA. 1995;92:522–526. doi: 10.1073/pnas.92.2.522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapperstein SK, Lupashin VV, Schmitt HD, Waters MG. Assembly of the ER to Golgi SNARE complex requires Uso1p. J Cell Biol. 1996;132:755–767. doi: 10.1083/jcb.132.5.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelig HP, Schranz P, Schroter H, Wiemann C, Griffiths G, Renz M. Molecular genetic analyses of a 376-kilodalton Golgi complex membrane protein (giantin) Mol Cell Biol. 1994;14:2564–2576. doi: 10.1128/mcb.14.4.2564. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Shima DT, Haldar K, Pepperkok R, Watson R, Warren G. Partitioning of the Golgi apparatus during mitosis in living HeLa cells. J Cell Biol. 1997;137:1211–1228. doi: 10.1083/jcb.137.6.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sogaard M, Tani K, Ye RR, Geromanos S, Tempst P, Kirchhausen T, Rothman JE, Söllner T. A rab protein is required for the assembly of SNARE complexes in the docking of transport vesicles. Cell. 1994;78:937–948. doi: 10.1016/0092-8674(94)90270-4. [DOI] [PubMed] [Google Scholar]
- Söllner T, Whitehart SW, Brunner M, Erdjumentbromage H, Geromanos S, Tempst P, Rothman JE. SNAP receptors implicated in vesicle targeting and fusion. Nature. 1993;362:318–324. doi: 10.1038/362318a0. [DOI] [PubMed] [Google Scholar]
- Sönnichsen B, Watson R, Clausen H, Misteli T, Warren G. Sorting by COP I–coated vesicles under interphase and mitotic conditions. J Cell Biol. 1996;134:1411–1425. doi: 10.1083/jcb.134.6.1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G. Membrane partitioning during cell division. Annu Rev Biochem. 1993;62:323–348. doi: 10.1146/annurev.bi.62.070193.001543. [DOI] [PubMed] [Google Scholar]
- Waters MG, Clary DO, Rothman JE. A novel 115-kD peripheral membrane protein is required for intercisternal transport in the Golgi stack. J Cell Biol. 1992;118:1015–1026. doi: 10.1083/jcb.118.5.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakawa H, Seog DH, Yoda K, Yamasaki M, Wakabayashi T. Uso1 protein is a dimer with 2 globular heads and a long coiled-coil tail. J Struct Biol. 1996;116:356–365. doi: 10.1006/jsbi.1996.0053. [DOI] [PubMed] [Google Scholar]