Abstract
Exocytic organelles undergo profound reorganization during myoblast differentiation and fusion. Here, we analyzed whether glycoprotein processing and targeting changed during this process by using vesicular stomatitis virus (VSV) G protein and influenza virus hemagglutinin (HA) as models. After the induction of differentiation, the maturation and transport of the VSV G protein changed dramatically. Thus, only half of the G protein was processed and traveled through the Golgi, whereas the other half remained unprocessed. Experiments with the VSV tsO45 mutant indicated that the unprocessed form folded and trimerized normally and then exited the ER. It did not, however, travel through the Golgi since brefeldin A recalled it back to the ER. Influenza virus HA glycoprotein, on the contrary, acquired resistance to endoglycosidase H and insolubility in Triton X-100, indicating passage through the Golgi. Biochemical and morphological assays indicated that the HA appeared at the myotube surface. A major fraction of the Golgi-processed VSV G protein, however, did not appear at the myotube surface, but was found in intracellular vesicles that partially colocalized with the regulatable glucose transporter. Taken together, the results suggest that, during early myogenic differentiation, the VSV G protein was rerouted into developing, muscle-specific membrane compartments. Influenza virus HA, on the contrary, was targeted to the myotube surface.
When myoblasts differentiate into myotubes, organelles of the exocytic pathway undergo a profound reorganization. Accordingly, the Golgi apparatus disperses into Golgi elements, parts of which assume a perinuclear localization, and the rest relocate independently of the nuclear positions (Tassin et al., 1985; Metsikkö et al., 1992). This organization of the Golgi complex persists in adult myofibers (Ralston, 1993; Rahkila et al., 1996). Microtubules are arranged longitudinally and lose their organization centers during early myogenesis (Tassin et al., 1985). In adult myofibers, the Golgi elements are associated with perinuclear and longitudinal microtubule bundles (Rahkila et al., 1997). The ER also assumes a peripheric organization along with the appearance of the multinucleated stage (Gu et al., 1989). The whole exocytic transport machinery appears to be organized differently in the multinucleated muscle cells as compared to those in the mononucleated stage.
Simultaneously with, or soon after the reorganization of the export machinery, muscle-specific membrane systems, including the sarcoplasmic reticulum (SR)1 and transverse (T) tubules, begin to develop. It is generally thought that the SR arises as a specialized ER structure, but it remains obscure how the SR membrane system receives its specific components. The T tubules arise simultaneously with the SR and form an anastamosing network inside the multinucleated cells (Franzini-Armstrong, 1991; Flucher et al., 1993). In adult muscle fibers, T tubules are continuous with the plasma membrane, but they exhibit their own specific protein and lipid compositions (Rosemblatt et al., 1981; Haimovich et al., 1987; Jorgensen et al., 1990). Immunofluorescence studies with T tubule-specific antibodies suggest that T tubule precursors appear very early during development as an intracellular compartment (Flucher et al., 1991). A vesicular compartment containing the regulatable glucose transporter is closely associated with the T tubules and appears during early myogenesis (Mitsumoto and Klip, 1992). Accordingly, the regulatable glucose transporter in muscle cells is externalized into the T tubules after insulin or exercise stimulation (Douen et al., 1990; Hirshman et al., 1990; Marette et al., 1992).
It is not exactly known whether the organelle reorganization and development of the muscle-specific membrane systems during myotube formation affect membrane protein processing in the Golgi, nor whether nor how membrane trafficking pathways are rearranged. Enveloped virus glycoproteins have been widely used as tools for studying the maturation and transport processes in mononucleated cells. The spike proteins of those viruses budding at the cell surface have been found to be targeted in a polarized fashion in epithelial cells, neurons, and osteoclasts (Rodriguez-Boulan and Pendergast, 1980; Dotti and Simons, 1990; Salo et al., 1996). Here, we have analyzed the exocytic trafficking of multinucleated myotubes using the viral glycoprotein model. The results show that changes of transport pathways and Golgi processing occur after early myoblast differentiation. Thus, only about half of the G protein travels through the Golgi and, instead of being externalized, the Golgi-processed form is destined into an intracellular vesicular compartment. The other half seems to fold, trimerize, and exit the ER, but deviates from the transport pathway leading to the Golgi. Influenza virus hemmagglutinin (HA), on the contrary, is efficiently transported through the Golgi to the cell surface. Therefore, differential processing and membrane transport for the influenza virus HA and VSV G protein arise during myotube formation.
Materials and Methods
Cell Culture
Rat L6 myoblasts (Yaffe, 1968) were obtained from American Type Culture Collection (Rockville, MD). They were grown in DME (Gibco Laboratories, Grand Island, NY) containing 5% fetal calf serum (Gibco Laboratories), in an atmosphere of 5% CO2. Fusion was induced by replacing the medium with DME containing 1% horse serum (Gibco Laboratories) and 0.4 U/ml of insulin (differentiation medium; Metsikkö and Väänänen, 1993). Myotubes appeared within 2 or 3 d. Thereafter, the differentiation medium was replaced with the growth medium consisting of DME, 5% fetal calf serum, and 5 μM cytosine arabinoside. Myotubes were used for experiments after a 3-d growth in the differentiation medium, unless otherwise stated.
Primary myotubes were obtained by cultivating satellite cells isolated from rat flexor digitorum brevis muscle on Petri dishes. Briefly, the muscle was excised and treated with collagenase (1 mg/ml; Worthington Biochemical Corp., Lakewood, NJ) for 1 h at 37°C, followed by trituration with a pipette. The satellite cells were plated together with myofibers on Matrigel (Becton-Dickinson, Oxford, UK), and then cultured in DME containing 5% horse serum and 20% controlled process serum replacement (Sigma Chemical Co., St. Louis, MO). Fusion of the satellite cells into myotubes occurred within 2 wk and the myotubes were then used for experiments. Myofibers were isolated from rat footpads as described (Bekoff and Betz, 1977; Rahkila et al., 1996). Satellite cells were removed and then myofibers were plated and cultivated on Matrigel.
Infections
Vesicular stomatitus virus (VSV) was of the Indiana serotype and the temperature-sensitive mutant tsO45-6 (Griffiths et al., 1985) was used. Viral multiplicities were obtained by plaque titrations on BHK cells as described (Matlin et al., 1983). Myoblasts and myotubes were infected at a multiplicity of 10 for 1 h at 37° (VSV) or 32°C (tsO45). Influenza virus was of the WSN strain, generously provided by A. Helenius (Yale University, New Haven, CT). WSN stocks were grown in MDCK cells and then plaque titrations were performed on MDCK cells as described (Matlin et al., 1981). Myoblasts and myotubes were infected for 1 h at a multiplicity of five per nucleus, whereas myofibers were infected at a multiplicity of 20. The plasmid pSemliki Forest virus (SFV)1–VSV G (Rolls et al., 1994) was generously provided by D.-J. Opstelten (Karolinska Institute, Stockholm, Sweden). The recSFV–VSV G particles were prepared as described (Olkkonen et al., 1994). When L6 myotubes were infected with the recSFV–VSV G particles, G protein expression was detected by metabolic pulse-chase labeling and subsequent SDS-PAGE analysis.
Metabolic Labeling and SDS-PAGE
Pulse-chase labeling with [35S]methionine (Amersham Corp., Bucks, UK) was performed at 6 h after infection for VSV-infected cells and at 8 h after infection for WSN virus-infected cells. A 10-min pulse labeling (50 μCi/ml) was performed, followed by various chase times in the presence of a 10-fold excess of the normal methionine concentration. After the chase, cells were solubilized in PBS, pH 7.3, containing 1% Triton X-100, 1% deoxycholate, and 1 mM phenylmethylsulfonyl fluoride. Immunoprecipitation of viral glycoproteins was performed using specific antisera and protein A–Sepharose (Sigma Chemical Co.). Endoglycosidase H (endo H; Boehringer Mannheim, Mannheim, Germany) digestion was performed as described by Pesonen et al. (1984) or Bennett et al. (1988). SDS-PAGE was performed as described by Laemmli (1970). Dried gels were exposed to Fuji RX film (Tokyo, Japan). Quantitation of the viral glycoprotein bands was performed with a PhosphorImager SI (Molecular Dynamics, Sunnyvale, CA).
Surface Biotinylation
Surface biotinylation of L6 myoblasts and myotubes was performed using sulfo-NHS-biotin (Pierce Chemical Co., Rockford, IL). Biotinylation was carried out after [35S]methionine pulse-chase labeling by the method of Lisanti et al. (1988), as described before (Kellokumpu et al., 1995). Biotinylated proteins were adsorbed onto immobilized streptavidin (Boehringer Mannheim) according to the manufacturer's instructions, released by boiling in SDS sample buffer, and then separated using SDS-PAGE.
Antibodies and Immunofluorescence Studies
Cells were fixed with 3% paraformaldehyde in PBS. 1% Triton X-100 solution in PBS was used for permeabilization. The antibodies against VSV G protein have been described (Metsikkö et al., 1992). Antibodies against the gel-purified HA band of the WSN strain (Martin and Helenius, 1991) were provided by A. Helenius. Alternatively, antibodies raised against HA virosomes were used. The virosomes were prepared by octylglucoside dialysis as described (Stegmann et al., 1987) and then injected intradermally into rabbits, three times at 2-wk intervals. The antiserum was adsorbed with fixed, nonpermeabilized, WSN-infected L6 myoblasts for 30 min at room temperature, released with 0.5 M glycine buffer, pH 2.5, and then neutralized immediately. The resulting antibodies revealed only the HA band in infected L6 myoblasts by Western blotting. Monoclonal antibody against mannosidase II (Baron and Garoff, 1990) was purchased from BAbCO (Richmond, CA). Anti-p58 antibodies have been described (Saraste and Svensson, 1991). Monoclonal antibody against the VSV G protein tail (clone P5D4; Kreis, 1986) was purchased from Sigma Chemical Co., and monoclonal antidihydropyridine receptor α-subunit antibody and anticalsequestrin antibody were from Affinity BioReagents Inc. (Golden, CO). Polyclonal antibodies against regulatable glucose transporter were from Calbiochem-Novabiochem Corp. (La Jolla, CA). Primary antibodies were applied to the cells at appropriate dilutions and then incubated for 1 h at 37°C. TRITC- or FITC-conjugated swine anti–rabbit IgG and Texas red or FITC-conjugated goat anti–mouse IgG, were used as secondary antibodies at 1:200 dilutions, and then incubated for 1 h at 37°C. Hoechst dye 32258 (Sigma Chemical Co.) was used to detect nuclear positions. Samples were mounted using Mowiol Hoechst, Frankfurt, Germany and then viewed with a laser scanning confocal microscope (Leica Lasertechnik, Heidelberg, Germany). When indicated, Matrox Inspector software (Matrox Electronic System LTD, Quebec, Canada) was used to increase the contrast of the images and to generate the fluorescence intensity data by the line profile function. The graphs of line profile data and the correlation coefficients were produced using SigmaPlot software (SPSS, Inc., Chicago, IL).
Oligomerization Assay
VSV-infected myotubes were pulse labeled with [35S]methionine and then followed by a 90-min chase. The cells were lysed with 1% Triton X-100 in a buffer containing 20 mM MES, 30 mM Tris, and 100 mM NaCl, pH 5.8, and then the lysates were centrifuged in a Beckman SW 50.1 rotor (Sparks, MD) for 16 h on 5–20% sucrose gradients made in 0.1% Triton X-100 in the above buffer, pH 6.3, as described in detail earlier (Doms et al., 1987). Gradient fractions were subjected to SDS-PAGE. The position of the monomeric G protein was determined by centrifuging a lysate of tsO45-infected myotubes that were labeled for 10 min were [35S]methionine at 39°C.
Results
Folding and Export of Proteins Occurs Normally in the Myotube ER
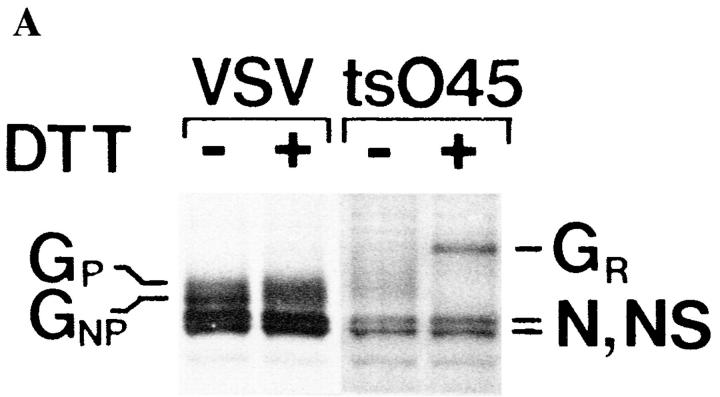
Previous studies have shown that the VSV G protein is rapidly processed by the Golgi of myoblasts, whereas in myotubes and myofibers only half of the G protein acquire endo H resistance (Kellokumpu et al., 1995; Rahkila et al., 1996), indicating arrival in the medial Golgi (Balch and Keller, 1986). Here, we analyzed whether the G protein that remains unprocessed and sensitive to endo H in myotubes was correctly folded and oligomerized by using the fact that DTT, when added to the culture medium, does not reduce the disulfide bridges of correctly folded proteins. The disulfide bridges of misfolded VSV G protein instead are reduced, resulting in a mobility shift into a more slowly migrating species during nonreducing SDS-PAGE (Braakman et al., 1992; Hammond and Helenius, 1994). Thus, we labeled VSV-infected myotubes with [35S]methionine for 10 min and then chased for 90 min. After the chase, the myotubes were subjected to DTT for 15 min. Subsequent SDS-PAGE analysis under nonreducing conditions showed that both the processed and the unprocessed G protein forms were totally resistant to treatment with DTT (Fig. 1 A), suggesting correct folding. Fig. 1 A further shows that the mutant VSV tsO45 G protein, which at 39°C is blocked in the ER (Zilberstein et al., 1980) and remains in a misfolded state, was shifted into a more slowly migrating form upon treatment with DTT.
Figure 1.
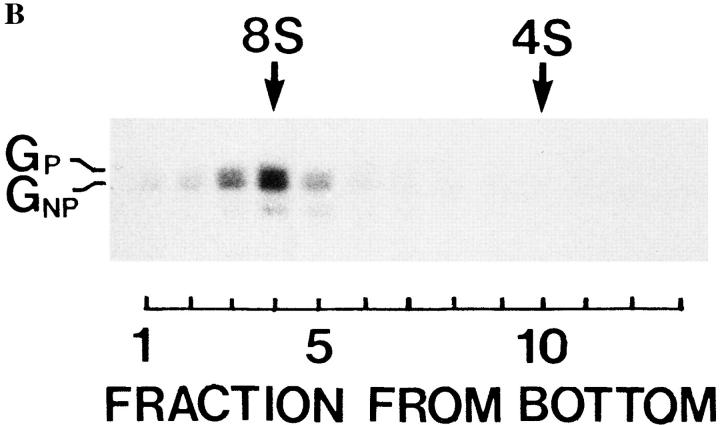
The processed and the nonprocessed G protein forms in myotubes fold and trimerize normally. (A) VSV-infected myotubes were labeled for 10 min with [35S]methionine, followed by a 60-min chase at 37°C. After the chase, cells were incubated for 15 min with or without 5 mM DTT at 37°C. Myotubes were then solubilized and followed by analysis by SDS-PAGE under nonreducing conditions. Neither the processed (GP) nor the nonprocessed (GN P) VSV G protein was affected by the reducing agent. When myotubes infected with the tsO45 mutant virus were labeled with [35S]methionine at 39°C and then treated for 15 min with DTT, the G protein quantitatively shifted into a more slowly migrating form (GR). N and NS are nucleocapsid proteins. (B) Myotubes were labeled with [35S]methionine for 10 min, followed by a 90-min chase, and then solubilized under acidic conditions as described (Doms et al., 1987). Sucrose density–gradient centrifugation was then performed in a Beckman SW 50 rotor for 15 h at 45,000 rpm at 4°C. Gradient fractions were subjected to SDS-PAGE under reducing conditions. Both G protein forms (GP and GNP) cosediment at ∼8S, indicating a trimeric oligomerization state for both the processed and nonprocessed G proteins.
Since previous studies have shown that the VSV G protein in mononucleated cells trimerizes after the folding process (Doms et al., 1987), we determined the oligomerization state of the two G protein forms in myotubes. Sedimentation analysis by sucrose density–gradient centrifugation indicated that both the processed and unprocessed forms had a sedimentation coefficient of 8 S, indicating that both assembled into trimers (Fig. 1 B).
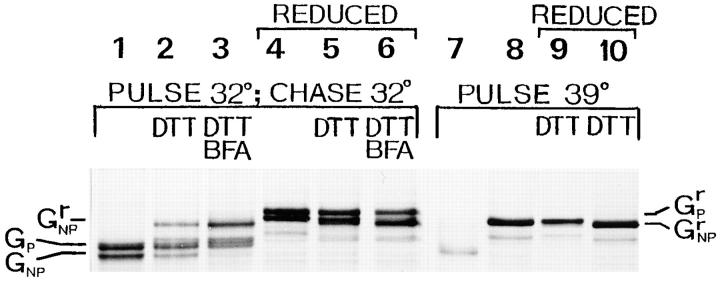
Since trimerization does not guarantee that the G protein is competent to be exported from the ER (Doms et al., 1988), we analyzed whether the nonprocessed trimeric G protein really exited the ER. For this purpose, we exploited the finding of de Silva et al. (1990) that discovered correctly folded tsO45 G protein misfolds when subjected to the ER environment at the nonpermissive temperature of 39°C. We monitored the misfolding process by using the fact that DTT opens the disulfide bridges of incorrectly folded G protein, resulting in a mobility shift into a more slowly migrating species during nonreducing SDS-PAGE. Hence, L6 myotubes infected with the tsO45 mutant were labeled at 32°C and then chased for 90 min at 32°C. Fig. 2 shows the myotubes exhibit the processed and the nonprocessed G protein bands both on nonreducing (lane 1) and reducing SDS-PAGE (lane 4). After the chase period at 32°C, a further 10-min incubation at 39°C in the presence of DTT was performed, during which the G protein in the ER is expected to unfold. We found that only half of the nonprocessed G protein shifted into a less mobile form and was unfolded (Fig. 2, lane 2). The tsO45 G protein, synthesized at 39°C and treated with DTT, quantitatively shifted into the less mobile form (Fig. 2, lane 8). We conclude that at 32°C, half of the nonprocessed G protein had left the ER. When brefeldin A was present for the last 20 min of the chase at 32°C and during the 39°C treatment with DTT, practically all of the nonprocessed G protein was unfolded, but the processed G protein remained in its folded state (Fig. 2, lane 3). The DTT treatment at 39°C appears to slightly increase the mobility of the G protein upon SDS-PAGE under reducing conditions (Fig. 2, lanes 5 and 6). Since brefeldin A is known to recall the G protein from the medial and cis-Golgi to the ER, but not from the trans-Golgi network (Doms et al., 1989), we conclude that the nonprocessed G protein did not pass through the Golgi apparatus.
Figure 2.
Thermosensitivity of the mutant tsO45 G protein in myotubes. Myotubes were infected with the VSV tsO45 mutant and then maintained at 32°C. They were pulse labeled with [35S]methionine for 10 min either at 32°C, followed by a 90-min chase period at 32°C (lanes 1–6), or pulse labeled at 39°C without the chase period (lanes 7–10). Indicated samples were next treated with DTT (5 mM) for 10 min at 39°C. Cells were lysed and then the lysates were subjected to SDS-PAGE without reduction (lanes 1–3 and 7 and 8) or after reduction (marked REDUCED, lanes 4–6 and 9 and 10). After a chase at 32°C, both the processed (GP) and nonprocessed (GNP) G protein forms run faster on nonreducing SDS-PAGE (lane 1) as compared to analysis after reduction (lane 4; GrP, GrNP). Shifting the temperature to 39°C for 10 min in the presence of DTT results in the unfolding of some G protein, as indicated by SDS-PAGE, of a nonreduced sample (lane 2). Lane 5 represents an identical sample analyzed under reducing conditions. When brefeldin A (BFA; 5 μg/ml) was present during the last 20 min of the chase and during the 10-min period at 39°C, practically all the nonprocessed G protein unfolded (lane 3). Lane 6 shows the brefeldin A–treated sample analyzed under reducing conditions. Lane 7 shows a control where a 10-min pulse was performed at 39°C, whereas lane 8 shows a control that was pulse labeled for 10 min at 39°C, and then followed by a 10-min chase at 39°C in the presence of 5 mM DTT. Lanes 9 and 10 show the corresponding samples analyzed under reducing conditions.
VSV G and Influenza Virus HA Are Differentially Sorted in the Myotube Intermediate Compartment
The brefeldin A experiment argues that the processed G protein reaches the trans-Golgi network whereas the nonprocessed does not. The fact that the nonprocessed form remains endo H sensitive (Kellokumpu et al., 1995) suggests that it does not reach the medial Golgi either. We next analyzed whether the G protein arrived at the intermediate compartment. Being blocked in the ER at 39°C (Zilberstein et al., 1980) due to a folding defect (Hammond and Helenius, 1994), the G protein of the tsO45 mutant is transported to the intermediate compartment when incubated at 15°C (Lotti et al., 1992). Therefore, myotubes infected for 5 h with the mutant at 39°C were transferred for 2 h to 15°C. Immunofluorescence staining showed that during this incubation period, the G protein accumulated into dots around the nuclei and dispersed in the cytoplasm. Colocalization of the G protein with the intermediate compartment marker, p58 (Saraste and Svensson, 1991), was prominent at 15°C (Fig. 3). Since G protein was seen exclusively in these structures, we conclude that most of it moved from the ER to the intermediate compartment. This localization pattern of the “15°C compartment” is very similar to that observed by immunofluorescence staining for the “20°C compartment” in myotubes (Metsikkö et al., 1992; Kellokumpu et al., 1995).
Figure 3.
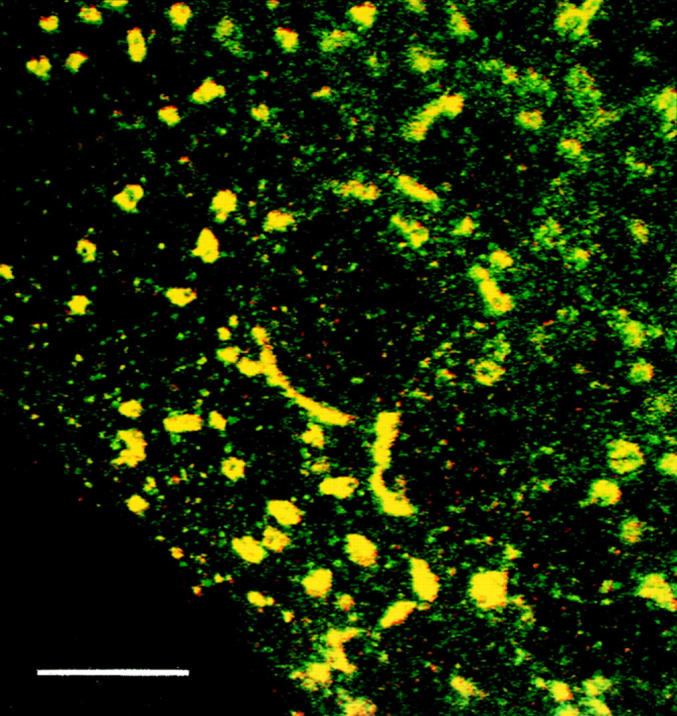
Localization of the VSV tsO45 G protein during a 15°C block. Infected myotubes were maintained for 5 h at 39°C and then followed by a 2-h incubation at 15°C. Double immunofluorescence staining for tsO45 G protein (red) and p58, a marker of the intermediate compartment (green), shows colocalization, indicated by yellow. Monoclonal antibodies against the VSV G protein cytoplasmic tail were used and then visualized with anti–mouse IgG conjugated to Texas red, whereas polyclonal antibodies and anti–rabbit IgG conjugated to FITC were used to detect the p58 protein. Bar, 10 μm.
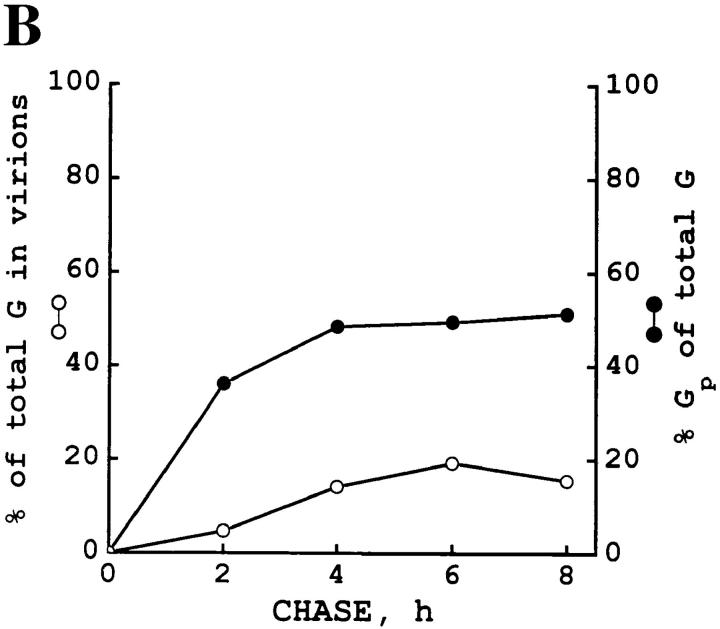
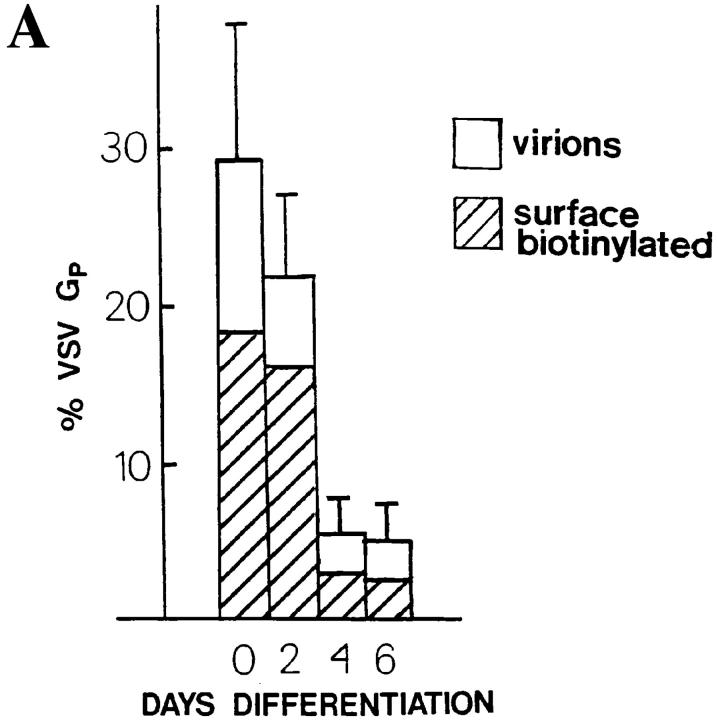
It remains possible that the unprocessed G protein is transported slowly through the Golgi and matures with time. Therefore, we infected L6 myotubes with wild-type VSV, pulse labeled the cells, and then extended the chase time to 8 h. Myotubes remained attached to the dish after this long chase period. We found that the processing of the G protein gradually declined so that further processing practically ceased after a 4-h chase period. After the 8-h chase, the unprocessed form was still present. To exclude the possibility that the unprocessed form was somehow externalized, we analyzed the virions that were released into the medium. We found that only the processed form of the G protein was incorporated into the viral particles. The amount of the G protein chased into the viral particles remained <20% of the total G protein in the system. The results of these experiments are shown in Fig. 4. We conclude that the nonprocessed G protein did not appear at the cell surface.
Figure 4.
A fraction of the VSV G protein remains nonprocessed during a prolonged chase and this form does not appear in viral particles. Infected myofibers were labeled for 10 min with [35S]methionine and then followed by chase periods of 0, 2, 4, 6, and 8 h. After each chase period, cells were solubilized with a buffer containing 1% Triton X-100, 1% deoxycholate, and 1 mM PMSF. The media were collected and clarified by a 5-min centrifugation at 5,000 g, and then centrifugation for 60 min at 75,000 g and 4°C to pellet the virions. (A) SDS-PAGE shows the nonprocessed and processed G protein bands in the cell lysates although only the processed form is present in the virions. 10% of the cell lysates were applied on the gel whereas virions were loaded quantitatively. (B) The fraction of the G protein in the virions is shown at each chase timepoint, together with the fraction of the processed G protein (GP) of the total G protein. Total G protein was counted as a sum of the G protein present in the virions and in the cells. For GP, the processed G protein in the cells and in the virions was counted.
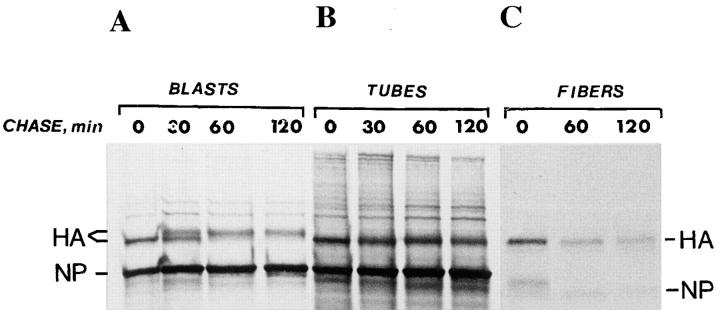
We next analyzed the ER-to-Golgi transport of the influenza virus HA during differentiation, and thus determined the Golgi processing of the HA before and after fusion of L6 myoblasts. When the parent myoblasts were infected and pulse-chase labeled with [35S]methionine, the HA was found to mature, within 2 h, into a species that exhibited a slower mobility during SDS-PAGE than the core-glycosylated ER form (Fig. 5 A). This behavior is typical of several types of mononucleated cells. In the multinucleated myotubes, however, such a mobility shift was not observed (Fig. 5 B). The change in the maturation of HA appeared during the differentiation of myoblasts into myotubes and persisted for at least 10 d. No mobility shift of the HA during pulse-chase labeling of isolated adult myofibers was observed (Fig. 5 C), indicating that the processing changes were permanent. Since a proton channel protein, M2, is expressed during influenza virus infection, it is possible that Golgi processing in the myotubes changed due to intra-Golgi pH variations (Sugrue et al., 1990). However, infection and pulse-chase labeling in the presence of amantadine, which eliminates the proton channel activity of the M2 protein, produced no effect on the results.
Figure 5.
Differentiation of myoblasts into multinucleated muscle cells induces altered HA processing. L6 myoblasts and myotubes were infected with influenza virus and pulse labeled with [35S]methionine for 10 min and then followed by chase periods as indicated. Myoblasts (A) and myotubes (B) were solubilized in SDS sample buffer and then subjected to SDS-PAGE. In myoblasts, a shift to a less mobile form occurred during the chase, wheras such a shift was not seen in myotubes. (C) Isolated rat myofibers were infected for 15 h and then followed by a 10-min pulse and chase times as indicated. Fibers were extracted with PBS containing 1% Triton X-100 and 1% deoxycholate, followed by immunoprecipitation using antibodies against gel-purified HA and immobilized protein A. No mobility shift during the chase occurred with the myofibers. NP indicates nucleocapsid protein.
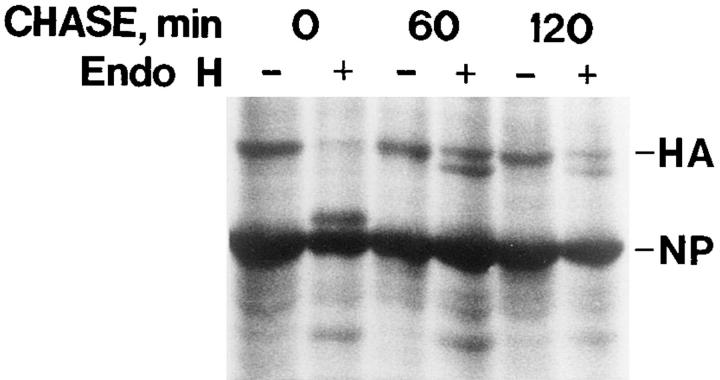
To resolve whether the apparent lack of HA maturation was due to defective transport from the ER to the Golgi, endo H digestion was performed. Fig. 6 shows that endo H resistance was acquired in the myotubes after a 60-min chase period. A totally resistant form, and another form that was partially affected, albeit not to the same extent as the core glycosylated ER form, were present. Similar results were obtained in the presence and absence of amantadine in the chase medium. Since endo H resistance is due to mannosidase II activity in the medial Golgi, these results indicate that the HA reached the medial Golgi.
Figure 6.
HA is processed in the myotube Golgi. Myotubes infected with influenza virus were pulse-chase labeled using [35S]methionine. Amantadine (10 μM) was present in the pulse and chase media. Cells were then solubilized with 200 μl of 0.5% SDS in 50 mM Tris buffer, pH 6.8. After heating for 1 min at 96°C, 800 μl of 0.2 M citrate buffer, pH 5.5, was added. Aliquots (100 μl) were then incubated for 16 h at 37°C, with or without endo H (10 mU). The core-glycosylated HA, seen at zero chase time, is fully sensitive to the enzyme, whereas a totally and a partially resistant form are seen at 60- and 120-min chase times. NP indicates nucleocapsid protein.
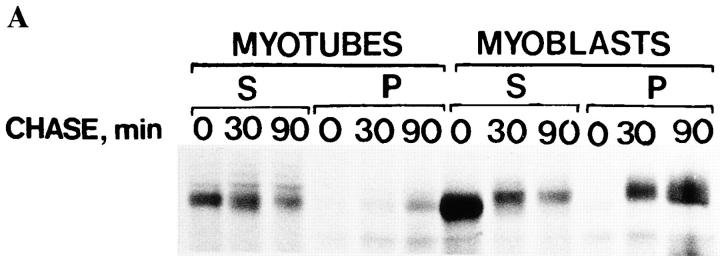
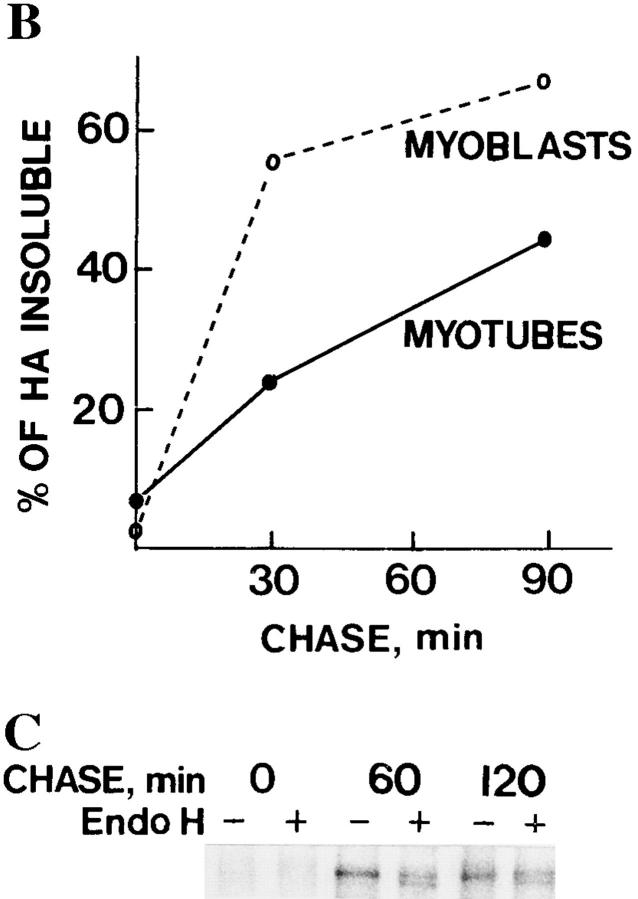
It has been shown that ∼50% of HA becomes insoluble in Triton X-100 upon arrival in the trans-Golgi network (Skibbens et al., 1989; Brown and Rose, 1992). Therefore, we determined the fraction of HA in myoblasts and myotubes that became insoluble in Triton X-100. During a 90-min chase, 67% of the HA became insoluble in myoblasts, whereas ∼45% became insoluble in myotubes (Fig. 7, A and B). Thus, insolubility in Triton X-100 was acquired both in myoblasts and in myotubes, although this occurred more rapidly in the myoblasts. When endo H digestion was performed on the Triton-insoluble fraction of HA in myotubes, it was found to contain both the fully and partially resistant HA forms, approximately in the same ratio as in the total lysate (Fig. 7 C). Since insolubility in Triton X-100 appears when HA arrives in the trans-Golgi network, we conclude that both HA forms, differentiated by endo H digestion, traveled through the Golgi apparatus in myotubes. Thus, VSV G protein and influenza HA were differentially sorted in the ER–Golgi pathway.
Figure 7.
HA acquires Triton X-100 insolubility in myoblasts and myotubes. Influenza virus-infected myotubes and myoblasts were pulse labeled for 10 min with [35S]methionine and then followed by chase times as indicated. Cells were then extracted with 1% Triton X-100, pH 6.5, and then the soluble fractions (S), as well as the remaining pellets (P), were analyzed by SDS-PAGE. Autoradiograms of the HA bands are shown in A, whereas B shows the relative amounts of the HA remaining insoluble at the indicated chase times. The Triton-insoluble pellets of myotubes were subjected to digestion with endo H and then analyzed by SDS-PAGE, shown in C. Totally and partially endo H–resistant HA forms are seen after a 60- and 120-min chase.
VSV G Protein Is Transported into Intracellular Compartments in Myotubes
Our results indicate that the nonprocessed G protein did not pass through the Golgi apparatus and did not appear at the cell surface. The processed G protein arrived in the trans-Golgi network since it could not be recalled to the ER by brefeldin A treatment. Furthermore, the fact that the processed G protein travels more slowly than the nonprocessed form on SDS gels is due to sialic acid addition in the trans-Golgi network (Balch and Keller, 1986). To quantitate the amount of the Golgi-processed G protein that arrived at the cell surface in myotubes, VSV-infected and pulse-labeled cells were surface-biotinylated after a 90-min chase period. Fig. 8 A shows that a decreasing fraction of G protein appears at the cell surface while the differentiation of the L6 myoblasts into myotubes proceeds. The fraction of the processed G protein subject to surface biotinylation dropped from ∼18% in myoblasts to ∼2% in myotubes during the differentiation period when cell–cell fusion and the related organelle reorganization occurred. When the amount of the G protein of the virions, formed at the cell surface (Metsikkö et al., 1992) during the chase period was also determined, 29% of the processed G protein was found to be externalized in myoblasts, whereas 4% was externalized in 6-d-old myotubes. Immunofluorescence staining for the G protein in permeabilized 4-d-old myotubes also showed that a major fraction of the G protein was located intracellularly (Fig. 8 B). Furthermore, our electron microscopy studies have indicated that L6 myotubes do not support intracellular viral budding (data not shown). These data indicate that in the myotubes, a small fraction of the processed G protein was externalized, whereas most remained intracellular.
Figure 8.
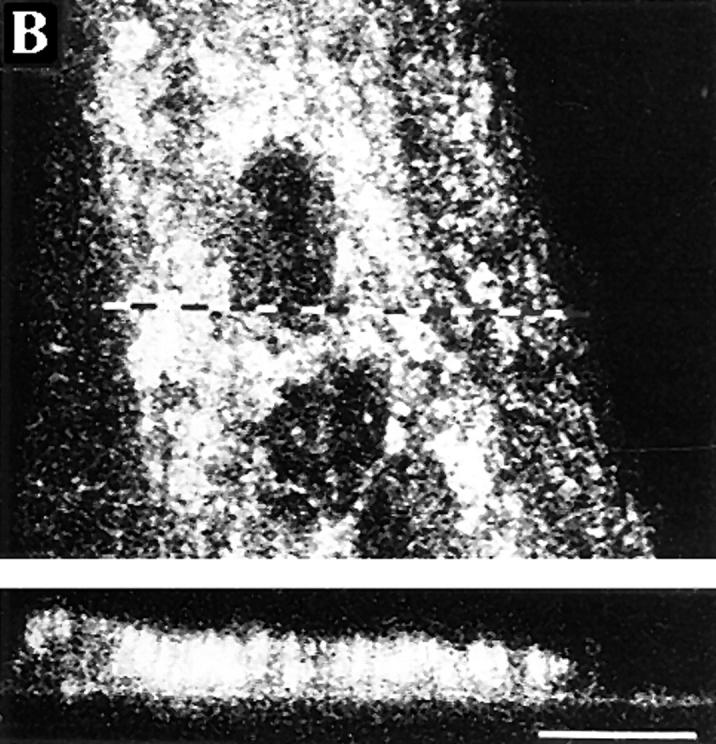
Surface appearance of the VSV G protein decreases during the differentiation of myoblasts into myotubes. (A) Myoblast/myotube cultures were infected with VSV after 0, 2, 4, and 6 d of culture in the differentiation medium. The cells were pulse labeled for 10 min with [35S]methionine and then followed by a 90-min chase and surface biotinylation. Cells were then solubilized and the biotinylated G protein from the lysates adsorbed onto immobilized streptavidin. Conditioning media were also collected and the virions were pelleted. The biotinylated material, cell lysates, and virions were subjected to SDS-PAGE, and the processed G protein bands (GP) quantified using a PhosphorImager. The bars show the percentages of the processed G protein that has externalized (biotinylated G protein and G protein in the virions), in relation to the total GP (cell lysates, biotinylated fractions, and virions). Mean and range of two determinations are shown. Hatched portions show the fraction of GP that was biotinylated and open portions show the fraction of GP in the virions. (B) A confocal section of immunofluorescence staining for the G protein in a permeabilized, 4-d-old VSV-infected myotube. After a 6-h infection period, the cells were incubated with 0.4 mM cycloheximide for 1 h before fixaton. The corresponding xz-section is also shown to indicate that most of the G protein is intracellular. The dotted line shows the position of the xz-section. Polyclonal anti–G protein antibodies were used and TRITC-conjugated anti–rabbit IgG were used for visualization. Bar, 10 μm.
The tsO45 mutant G protein was used to analyze the nature of the intracellular compartment(s) to which the G protein is destined. Accordingly, myotubes were infected with the tsO45 virus for 5 h at 39°C. After this growth period, the G protein located to the myotube ER (Fig. 9 A; Metsikkö et al., 1992). Shifting the temperature from 39° to 15°C resulted in the accumulation of the G protein in perinuclear and dispersed structures (Fig. 9 B). When the temperature was shifted from 39° to 20°C for 2 h, the G protein showed a very similar staining pattern as that seen at 15°C. Furthermore, colocalization with both the intermediate compartment marker, p58 (Fig. 9, C and D), and with the Golgi marker, mannosidase II (Fig. 9, E and F), was seen. This finding is compatible with the idea that, during a 20°C block, the G protein is in the intermediate and the Golgi compartments. When we incubated the myotubes for 2 h at 32°C after the 20°C incubation, the G protein moved into spot-like structures (Fig. 9 G) that no longer colocalized with mannosidase II (Fig. 9 H), indicating that the G protein had left the Golgi apparatus. Similar results were obtained by shifting the temperature from 39°C directly to 32°C, omitting the incubation at 20°C (data not shown).
Figure 9.
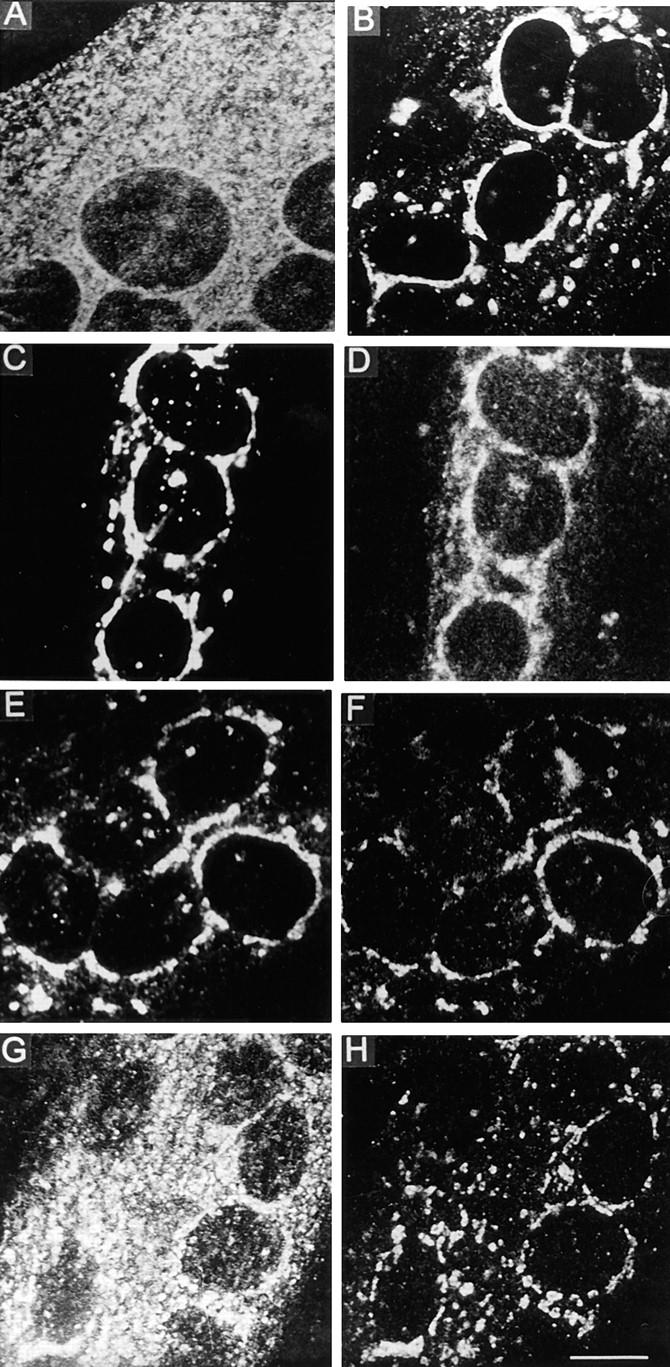
The destination of the VSV G protein in myotubes appears to be an intracellular compartment. VSV tsO45-infected myotubes were maintained for 5 h at 39°C and then stained for the G protein using polyclonal antibodies (A). The temperature was then shifted to 15°C for 2 h, resulting in a staining pattern composed of a perinuclear component and dispersed dots (B). When the temperature was shifted from 39° to 20°C for 2 h, double staining with a monoclonal P5D4 anti–G protein antibody (C) and polyclonal anti-p58 antibodies (D) showed essential colocalization. Double staining for the G protein, using polyclonal antibodies (E), and monoclonal anti–mannosidase II antibody (F), also showed overlapping staining patterns. After the 20°C block, the temperature was raised for 2 h to 32°C (G and H). Double staining for the G protein (G) and mannosidase II (H) now shows that the G protein has left the Golgi compartment. Confocal sections are shown. TRITC-conjugated anti–rabbit IgG and FITC-conjugated anti–mouse IgG were used as secondary antibodies. Bar, 10 μm.
In myofibers, the VSV G protein is localized to the T tubules (Rahkila et al., 1996). To determine whether a subfraction of the intracellular, G protein-containing vesicles in the L6 myotubes represented developing T tubules, double immunofluorescence staining for the dihydropyridine receptor α-subunit and the tsO45 virus G protein was performed. TsO45 virus-infected myotubes were incubated for 5 h at 39°C and then for 2 h at the permissive temperature of 32°C, but colocalization of the two antigens was weak (data not shown). During myogenesis, a vesicular compartment containing the regulatable glucose transporter develops, and we next analyzed the possibility that the G protein was transported into this specific compartment. Double-staining for the glucose transporter and the tsO45 G protein, after a 5-h growth period at 39°C and a 2-h incubation at 32°C, clearly showed a partial colocalization (Fig. 10, A–C). This colocalization was further analyzed by a line profile analysis, shown in the inset on Fig. 10 C. It can be seen that the fluorescence signal for the glucose transporter is accompanied with high signal for the VSV G protein. However, there is a population of VSV G–positive structures without glucose transporter signal (Fig. 10 C, arrowheads in inset). Correlation coefficient between these two fluorescence signals is 0.56 (n = 177, P < 0.05), supporting the idea of partial colocalization. We found partial colocalization of the glucose transporter and VSV G protein also in isolated myofibers, shown in Fig. 10, D–F.
Figure 10.
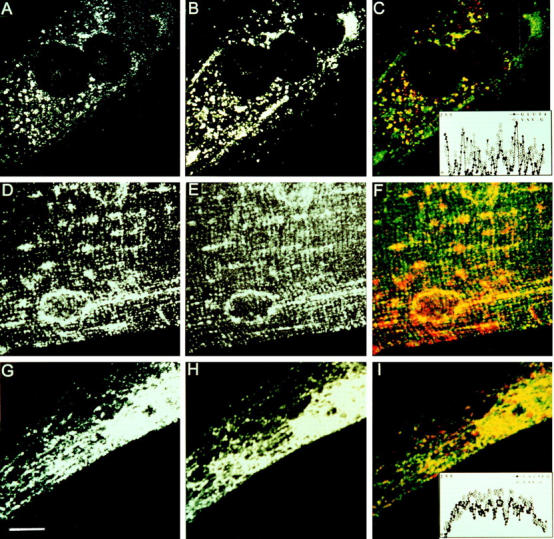
The G protein shows partial colocalization with the regulatable glucose transporter and calsequestrin in muscle cells. (A–C) L6 myotubes were infected with the VSV tsO45 mutant, incubated for 5 h at 39°C, and then followed by a 2-h incubation at 32°C in the presence of 0.4 mM cycloheximide. After permeabilization, double immunofluorescence staining for the regulatable glucose transporter and VSV G protein was performed using polyclonal antibodies for the glucose transporter and P5D4 monoclonal anti–G protein antibody. The glucose transporter was visualized by TRITC-conjugated anti–rabbit IgG (A) and the G protein with FITC-conjugated anti– mouse IgG (B). The color print (C) shows the glucose transporter (red), G protein (green), and colocalization (yellow). The inset is a graphical presentation of a line profile analysis of the fluorescence intensities (y-axis) of the corresponding pixels of glucose transporter (GLUT4) and VSV G protein staining on the x-axis. Arrowheads indicate points of VSV G signal without GLUT4 signal. (D–F) Double labeling for glucose transporter (D) and VSV G protein (E) in an isolated myofiber. Myofibers were infected for 10 h with wtVSV and then followed by a 1-h treatment with cycloheximide (0.4 mM) before fixation. In the color print (F), glucose transporter appears red whereas VSV G protein appears green; yellow indicates colocalization of the two markers. (G–I) Primary myotube culture was infected with recSFV–VSV G particles for 16 h. After 1 h of cycloheximide treatment, cells were fixed, permeabilized, and then subjected to double immunofluorescence staining for calsequestrin and G protein. Calsequestrin was visualized with Texas red–conjugated anti–mouse IgG (G), and then the G protein was visualized using FITC-conjugated anti–rabbit IgG (H). In the color print (I), calsequestrin appears red and G protein appears green; yellow indicates colocalization. The inset in I shows a line profile analysis for calsequestrin and VSV G protein. Matrox Inspector software was used to increase the contrast of the images shown in A–C and G–I. Confocal planes are shown. Bar, 10 μm.
We next analyzed whether the nonprocessed G protein was possibly destined into a compartment representing developing SR. The L6 myotubes, however, were negative for our SR markers. Therefore, we used primary myotubes that show calsequestrin expression and, like L6 myotubes, exhibit dual VSV G protein processing (data not shown). Since we found that during viral infection, VSV buds into intracellular tubular structures in the primary myotubes, we expressed the G protein using the SFV expression system, instead of VSV infection. Double labeling for the expressed G protein and calsequestrin in the primary myotubes showed partial colocalization (Fig. 10, G–I). Line profile analysis (Fig. 10 I, inset) indicated a highly significant correlation coefficient of 0.85 (n = 230).
Influenza Virus HA Appears at the Myotube Surface
Our data show that the HA arrived at the trans-Golgi network (Fig. 7). We next analyzed by confocal microscopy whether the HA appeared at the myotube surface. Fig. 11 A indicates that HA was unevenly distributed at the cell surface, showing punctate labeling. The confocal xz section demonstrates that the interior portions of the myotube were only slightly stained. Electron microscopic analysis of influenza virus-infected myotubes revealed viral budding at the cell surface, but budding into intracellular tubulovesicular structures was also seen (data not shown).
Figure 11.
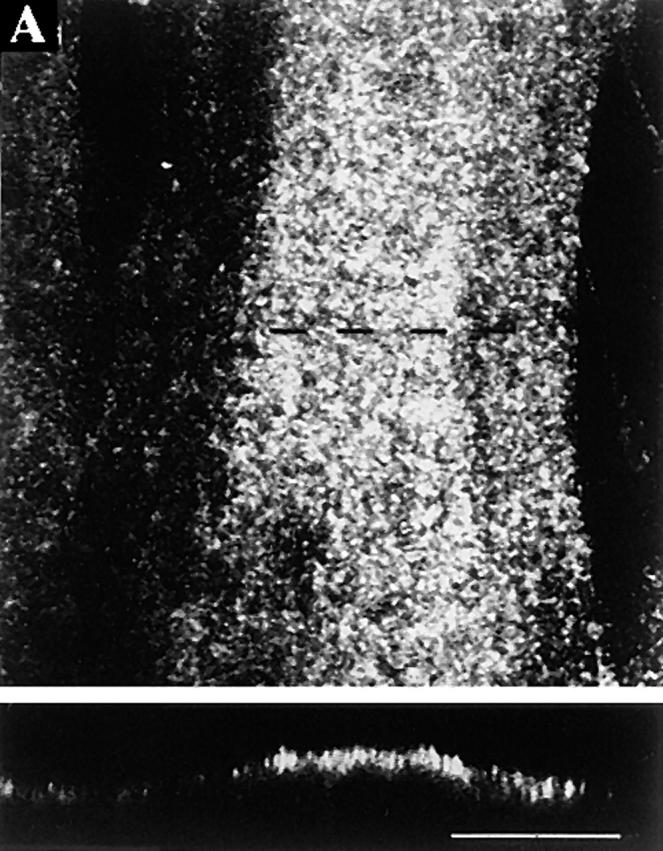
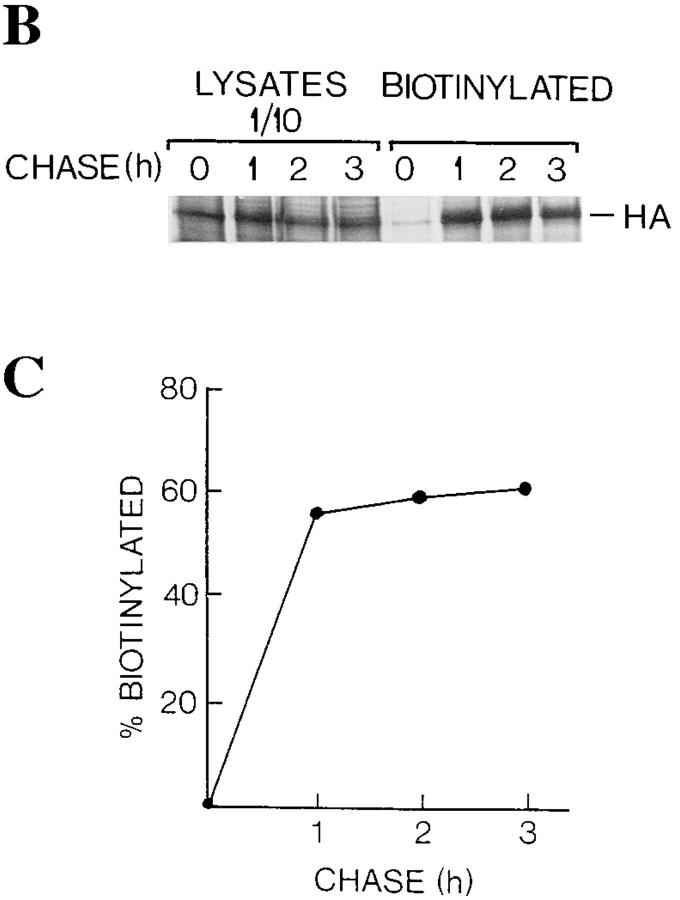
Influenza virus HA is localized at the cell surface in L6 myotubes. Myotubes were maintained in differentiation medium for 3 d and then infected with influenza virus. (A) At 6 h after infection, permeabilized cells were immunostained using antibodies raised against influenza virosomes. A summary projection of 12 confocal sections, scanned at 0.3-μm steps, shows granular staining (top), whereas a xz-section scanned along the dotted line indicates that the granular staining is localized at the cell surface (bottom). (B and C) Influenza virus-infected myotubes were labeled with [35S]methionine for 10 min and then followed by chase periods as indicated. After each chase, cells were subjected to surface biotinylation at 0°C, and then followed by solubilization and adsorbtion of the biotinylated material with immobilized streptavidin. SDS-PAGE profiles of the HA in the cell lysates (10% loaded on the gel) and those bound to streptavidin (100% loaded on the gel) are shown (B). Band intensities were quantified, and then the relative amounts of the HA bound to streptavidin from the total lysates are shown (C). The HA band bound to streptavidin at zero chase time was counted as background. Bar, 10 μm.
To quantify the fraction of the HA at the plasma membrane, surface biotinylation was performed after a 10-min [35S]methionine pulse and various chase periods. Cells were then lysed, lysates were adsorbed onto immobilized streptavidin, and SDS-PAGE was performed. The intensities of the biotinylated and total HA bands were measured with a PhosphorImager. Fig. 11 (B and C) show that >50% of the HA was subject to biotinylation after a 1-h chase period. Taken together, these results indicate that majority of the HA was chased to the cell surface.
Discussion
Our results show that the VSV G protein and influenza HA undergo very different processing and targeting in multinucleated myotubes. This contrasts with the situation in the parent myoblasts where both glycoproteins are targeted to the plasma membrane. Although influenza HA was transported to the cell surface of the multinucleated myotubes, VSV G protein remained mainly intracellular. Furthermore, only half of the VSV G protein was processed in L6 myotubes, as has been reported previously (Kellokumpu et al., 1995). This dual behavior of the VSV G protein is typical of the multinucleated muscle phenotype, since it was found to appear during the differentiation of L6 myoblasts, and persists in adult myofibers (Rahkila et al., 1996). Our unpublished results indicate that the SFV p62 protein underwent efficient post-Golgi cleavage in myoblasts whereas only about half of the p62 was cleaved in myotubes. Furthermore, an increasing fraction of the E1 subunit of the p62–E1 complex remained endo H sensitive when myoblasts differentiated into myotubes, suggesting deviation of the p62–E1 complex from the Golgi pathway. SFV glycoprotein is basolaterally targeted like the VSV G protein, but it remains to be seen whether this behavior is typical of basolateral proteins.
Generally, misfolded proteins are retarded in the ER and, therefore, do not undergo Golgi processing. It seems unlikely, however, that the nonprocessed G protein in myotubes was retarded in the ER due to defective folding, since it was insensitive to DTT and was assembled into trimers. When the mutant tsO45 G protein, synthesized at 32°C, was subjected to the nonpermissive temperature, 39°C, in the presence of a reducing agent, only half of the nonprocessed G protein was reduced. This finding indicates that at least half of the nonprocessed form left the ER. Supporting this idea, Nycodenz-gradient fractionation experiments have shown that after [35S]methionine pulse labeling and a chase period, both G protein forms shifted from the heavy ER fraction into a lighter fraction (Kellokumpu et al., 1995).
When tsO45 virus-infected myotubes were incubated at 15°C, the G protein was seen to colocalize with the intermediate/cis-Golgi marker p58. Previously, vesicular G protein-containing structures were found after a 20°C block, and these structures were interpreted to be deformed Golgi elements (Kellokumpu et al., 1995). Based on the results presented here, a more likely explanation is that the endo H–sensitive G protein fraction, or part of it, was still in the intermediate/cis-Golgi compartment after the 2-h incubation at 20°C. Here we found that brefeldin A recalled the nonprocessed G protein back to the ER, indicating that it did not pass through the Golgi. Furthermore, myotube Golgi was functionally intact, since it processed all of the HA. Since the localization patterns of the tsO45 G protein at 15° and 32°C looked very different, it seems unlikely that the nonprocessed form was blocked in the intermediate compartment. Based on the partial colocalization of the VSV G protein and calsequestrin, we suggest that the nonprocessed G protein was transported from the intermediate/cis-Golgi compartment into a novel muscle-specific compartment that developed during myogenesis. In primary myotubes, evidence has been shown that calsequestrin, a resident SR protein, exits the ER, but does not pass through the Golgi apparatus and is not secreted (Thomas et al., 1989). In nonmuscle cells, on the other hand, calsequestrin was transported through the Golgi into lysosome-resembling structures (Raichman et al., 1995). Therefore, it is tempting to speculate that the nonprocessed fraction of the G protein illustrates a pathway to the SR. Although it remains obscure whether the SR and the ER are the same organelle that have a common lumen, the SR has been reported to contain ER components (Volpe et al., 1992). This could explain why the nonprocessed tsO45 G protein partially unfolded when subjected to 39°C.
In the L6 myotubes, biochemical and morphological analyses showed that a major fraction of both the non- and Golgi-processed G protein accumulated intracellularly. Immunofluorescence studies showed that a fraction of the VSV G protein was targeted to intracellular vesicular structures that were reactive for the regulatable glucose transporter. Although our morphological analyses do not discriminate between the processed and nonprocessed G protein, it is reasonable to believe that the processed form located to glucose transporter vesicles since their nature is endosomal (Martin et al., 1996). The glucose transporter in myotubes is mainly contained in the storage vesicles but it constitutively recycles to the plasma membrane (Ramlal et al., 1988). Interestingly, the processed G protein accumulated into the intracellular pool and was slowly chased to the myotube surface and into virions, mimicking the behavior of the glucose transporter. One possible explanation for the intracellular targeting of the G protein is that the tyrosine-based basolateral targeting signal in the cytoplasmic tail (Thomas et al., 1993) is recognized as an endosomal tyrosine-based sorting signal (for review see Marks et al., 1997) in muscle. Interestingly, influenza virus HA, being an apically targeted glycoprotein, was not transported into intracellular structures. It has been shown that vesicles containing HA use totally different transport mechanisms from the trans-Golgi network as compared to those containing a basolateral protein, such as the VSV G protein (Ikonen et al., 1995). Furthermore, the cytoplasmic tail of HA is not recognized by clathrin adaptors at the trans-Golgi network or the plasma membrane.
The maturation of the influenza HA glycoprotein in multinucleated L6 myotubes differed from that in the prefusion myoblasts. Thus, in the mononucleated myoblasts, a mobility shift in SDS-PAGE gels occurred, as is also observed in fibroblasts or polarized epithelial cells, where HA is expressed apically (Rodriguez-Boulan et al., 1984). In the multinucleated myotubes, however, the mobility shift of HA characterizing its maturation was not seen. It was previously reported that the glycosylation pattern of type 1 glucose transporter changed upon differentiation (Mitsumoto and Klip, 1992). It seems that sugar-modifying enzymes in the Golgi underwent changes during myoblast differentiation and fusion. The finding that both the HA fully resistant to endo H and the partially resistant form became similarly insoluble in Triton X-100 during chasing argues that they both arrived in a specific sphingolipid environment existing in the trans-Golgi network (Brown and Rose, 1992).
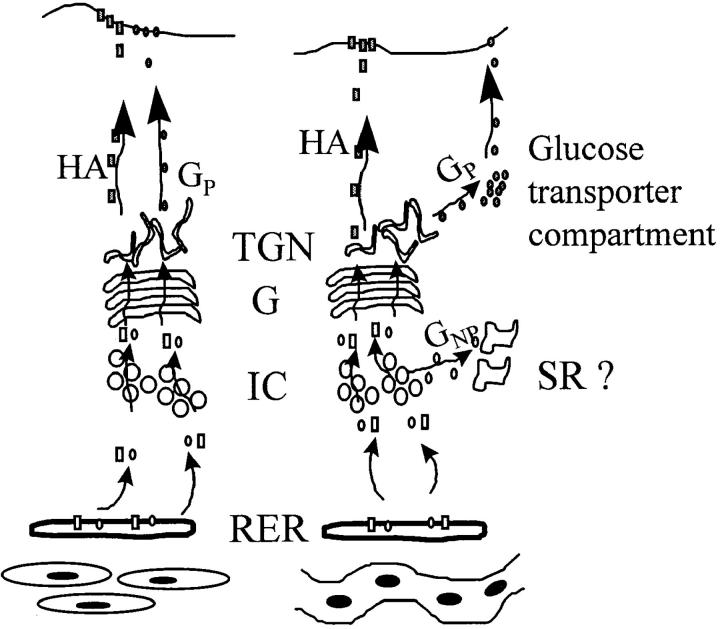
In conclusion, viral glycoproteins exhibit different types of transport routes in muscle cells. Our results suggest that, in the muscle cell pre-Golgi compartment, VSV G protein and influenza HA are differentially sorted. Furthermore, the HA appears at the myotube surface, whereas the VSV G protein seems to be targeted into pre- and post-Golgi–type intracellular vesicles. Our previous report that the VSV G protein appears in myofiber T tubules (Rahkila et al., 1996) fits with the present idea that the Golgi-processed G protein resides in glucose transporter vesicles, since the glucose transporter recycles between the intracellular vesicles and T tubules. It remains to be seen whether the G protein recycles similarly. Fig. 12 summarizes our hypothesis of VSV G protein and influenza HA trafficking in L6 myoblasts and myotubes.
Figure 12.
Postulated transport routes for VSV G protein and influenza HA in L6 myoblasts (left) and in multinucleated myotubes (right). HA travels to the cell surface both in myoblasts and myotubes. In myoblasts, VSV G protein is transported to the cell surface, but in myotubes, it is transported to a pre-Golgi compartment, suggested to be SR, and to a post-Golgi compartment postulated to represent the recruitable glucose transporter compartment. RER indicates rough endoplasmic reticulum; IC indicates intermediate compartment; G indicates Golgi apparatus; TGN indicates trans-Golgi network; GP indicates processed G protein; GNP indicates nonprocessed G protein.
Acknowledgments
This study was supported by a grant from the Academy of Finland to K. Metsikkö (grant number 34510).
Abbreviations used in this paper
- endo H
endoglycosidase H
- HA
hemagglutinin
- SFV
Semliki Forest virus
- SR
sarcoplasmic reticulum
- T
transverse
- VSV
vesicular stomatitis virus
Footnotes
Address all correspondence to Kalervo Metsikkö, Department of Anatomy, University of Oulu, Kajaanintie 52A, FIN-90220 Oulu, Finland. Tel.: (358) 853-75-183. Fax: (358) 853-75-172. E-mail: metsikko@sun3.oulu.fi
References
- Balch WE, Keller DS. ATP-coupled transport of vesicular stomatitis virus G protein. Functional boundaries of secretory compartments. J Biol Chem. 1986;261:14690–14696. [PubMed] [Google Scholar]
- Baron MD, Garoff H. Mannosidase II and the 135 kDa Golgi-specific antigen recognized monoclonal antibody 53FC3 are the same dimeric protein. J Biol Chem. 1990;265:19928–19931. [PubMed] [Google Scholar]
- Bekoff A, Betz W. Properties of isolated adult rat muscle fibers maintained in tissue culture. J Physiol. 1977;271:537–547. doi: 10.1113/jphysiol.1977.sp012013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett MK, Wandinger-Ness A, Simons K. Release of putative exocytic transport vesicles from perforated MDCK cells. EMBO (Eur Mol Biol Organ) J. 1988;7:4075–4085. doi: 10.1002/j.1460-2075.1988.tb03301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braakman I, Helenius J, Helenius A. Manipulating disulfide bond formation and protein folding in the endoplasmic reticulum. EMBO (Eur Mol Biol Organ) J. 1992;11:1717–1722. doi: 10.1002/j.1460-2075.1992.tb05223.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- de Silva AM, Balch WE, Helenius A. Quality control in the endoplasmic reticulum: folding and misfolding of vesicular stomatitis virus G protein in cells and in vitro. J Cell Biol. 1990;111:857–866. doi: 10.1083/jcb.111.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doms RW, Keller DS, Helenius A, Balch WE. Role for adenosine triphosphate in regulating the assembly and transport of vesicular stomatitis virus G protein trimers. J Cell Biol. 1987;105:1957–1969. doi: 10.1083/jcb.105.5.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doms RW, Ruusula A, Machamer C, Helenius J, Helenius A, Rose JK. Differential effects of mutations in three domains on folding, quaternary structure, and intracellular transport of vesicular stomatitis virus G protein. J Cell Biol. 1988;107:89–99. doi: 10.1083/jcb.107.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doms RW, Russ G, Yewdell JW. Brefeldin A redistributes resident and itinerant Golgi proteins to the endoplasmic reticulum. J Cell Biol. 1989;109:61–72. doi: 10.1083/jcb.109.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotti CG, Simons K. Polarized sorting of viral glycoproteins to the axon and dendrites of hippocampal neurons in culture. Cell. 1990;62:63–72. doi: 10.1016/0092-8674(90)90240-f. [DOI] [PubMed] [Google Scholar]
- Douen AG, Ramlal D, Rastogi S, Bilan PJ, Cartee GD, Vranic M, Holloszy JO, Klip A. Exercise induces recruitment of the “insulin responsive glucose transporter.” Evidence for distinct intracellular insulin- and exercise recruitable transporter pools in skeletal muscle. J Biol Chem. 1990;265:13427–13430. [PubMed] [Google Scholar]
- Flucher BE, Terasaki M, Chin H, Beeler TJ, Daniels MP. Biogenesis of transverse tubules in skeletal muscle in vitro. Dev Biol. 1991;145:77–90. doi: 10.1016/0012-1606(91)90214-n. [DOI] [PubMed] [Google Scholar]
- Flucher BE, Takekura H, Franzini-Armstrong C. Development of the excitation-contraction coupling apparatus in skeletal muscle: association of sarcoplasmic reticulum and transverse tubules with myofibrils. Dev Biol. 1993;160:135–147. doi: 10.1006/dbio.1993.1292. [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C. Simultaneous maturation of transverse tubules and sarcoplasmic reticulum during muscle differentiation in the mouse. Dev Biol. 1991;146:353–363. doi: 10.1016/0012-1606(91)90237-w. [DOI] [PubMed] [Google Scholar]
- Griffiths G, Pfeiffer S, Simons K, Matlin K. Exit of newly synthesized membrane proteins from the transcisterna of the Golgi complex to the plasma membrane. J Cell Biol. 1985;101:949–964. doi: 10.1083/jcb.101.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y, Ralston E, Murphy-Erdosh C, Black RA, Hall ZW. Acetylcholine receptor in a C2 muscle cell variant is retained in the endoplasmic reticulum. J Cell Biol. 1989;109:729–738. doi: 10.1083/jcb.109.2.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haimovich B, Schotland DL, Fieles DL, Barchi RL. Localization of sodium channel subtypes in adult rat skeletal muscle using channel-specific monoclonal antibodies. J Neurosci. 1987;7:2957–2966. doi: 10.1523/JNEUROSCI.07-09-02957.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond C, Helenius A. Quality control in the secretory pathway: retention of a misfolded viral membrane glycoprotein involves cycling between the ER, the intermediate compartment, and Golgi apparatus. J Cell Biol. 1994;126:41–52. doi: 10.1083/jcb.126.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirshman MF, Goodyear LJ, Wardzala LJ, Horton ED, Horton ES. Identification of an intracellular pool of glucose transporters from basal and insulin-stimulated rat skeletal muscle. J Biol Chem. 1990;265:987–991. [PubMed] [Google Scholar]
- Ikonen E, Tagaya M, Ullrich O, Montecucco C, Simons K. Different requirements for NSF, SNAP, and Rab proteins in apical and basolateral transport in MDCK cells. Cell. 1995;81:571–580. doi: 10.1016/0092-8674(95)90078-0. [DOI] [PubMed] [Google Scholar]
- Jorgensen AO, Arnold W, Shen AC-Y, Yuan S, Gaver M, Campbell KP. Identification of novel proteins unique to either transverse tubules (TS28) or the sarcolemma (SL50) in rabbit skeletal muscle. J Cell Biol. 1990;110:1173–1185. doi: 10.1083/jcb.110.4.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellokumpu S, Sormunen R, Väänänen HK, Metsikkö K. Defective maturation of a viral glycoprotein and partial loss of the Golgi stack structure during in vitro myogenesis. Exp Cell Res. 1995;220:101–111. doi: 10.1006/excr.1995.1296. [DOI] [PubMed] [Google Scholar]
- Kreis TE. Microinjected antibodies against the cytoplasmic domain of vesicular stomatitis virus glycoprotein block its transport to the cell surface. EMBO (Eur Mol Biol Organ) J. 1986;5:931–941. doi: 10.1002/j.1460-2075.1986.tb04306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lisanti MP, Sargiacomo A, Greave L, Saltiel A, Rodriguez-Boulan E. Polarized apical distribution of glycosyl-phosphatidylinositol-anchored proteins in a renal epithelial cell line. Proc Natl Acad Sci USA. 1988;85:9557–9561. doi: 10.1073/pnas.85.24.9557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotti LV, Torrisi M-R, Pascale MC, Bonatti S. Immunocytochemical analysis of the transfer of vesicular stomatitis virus G glycoprotein from the intermediate compartment to the Golgi complex. J Cell Biol. 1992;118:43–50. doi: 10.1083/jcb.118.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marette A, Burdett E, Douen A, Vranic M, Klip A. Insulin induces the translocation of GLUT4 from a unique intracellular organelle to transverse tubules in rat skeletal muscle. Diabetes. 1992;41:1562–1569. doi: 10.2337/diab.41.12.1562. [DOI] [PubMed] [Google Scholar]
- Marks MS, Ohno H, Kirckhausen T, Bonifacio JS. Protein sorting by tyrosine-based signals: adapting to the Ys and wherefores. Trends Cell Biol. 1997;7:124–128. doi: 10.1016/S0962-8924(96)10057-X. [DOI] [PubMed] [Google Scholar]
- Martin K, Helenius A. Nuclear transport of influenza virus ribonucleoproteins: the viral matrix protein (M1) promotes export and inhibits import. Cell. 1991;67:117–130. doi: 10.1016/0092-8674(91)90576-k. [DOI] [PubMed] [Google Scholar]
- Martin S, Tellam J, Livingstone C, Slot JW, Gould GW, James DE. The glucose transporter (GLUT4) and vesicle-associated membrane protein-2 (VAMP-2) are segregated from recycling endosomes in insulin-sensitive cells. J Cell Biol. 1996;134:625–635. doi: 10.1083/jcb.134.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlin KS, Reggio H, Helenius A, Simons K. Infectious entry pathway of influenza virus in a canine kidney cell line. J Cell Biol. 1981;91:601–613. doi: 10.1083/jcb.91.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlin KS, Bainton DF, Pesonen M, Louvard D, Genty N, Simons K. Transepithelial transport of a viral membrane glycoprotein implanted into the apical membrane of Madin-Darby canine kidney cells. I. Morphological evidence. J Cell Biol. 1983;97:627–637. doi: 10.1083/jcb.97.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metsikkö K, Väänänen K. Synthesis and secretion of a 38-kDa glycopolypeptide coincides with L6 myoblast fusion. Int J Dev Biol. 1993;37:305–310. [PubMed] [Google Scholar]
- Metsikkö K, Hentunen T, Väänänen K. Local expression and exocytosis of viral glycoproteins in multinucleated muscle cells. J Cell Biol. 1992;117:987–995. doi: 10.1083/jcb.117.5.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsumoto Y, Klip A. Development regulation of the subcellular distribution and glycosylation of GLUT1 and GLUT4 glucose transporters during myogenesis of L6 muscle cells. J Biol Chem. 1992;267:4957–4962. [PubMed] [Google Scholar]
- Olkkonen VM, Dupree P, Simons K, Liljeström P, Garoff H. Expression of exogenous proteins in mammalian cells with the Semliki Forest virus vector. Methods Cell Biol. 1994;43:43–53. doi: 10.1016/s0091-679x(08)60597-x. [DOI] [PubMed] [Google Scholar]
- Pesonen M, Bravo R, Simons K. Transcytosis of the G protein of vesicular stomatitis virus after implantation into the apical membrane of Madin-Darby canine kidney cells. II. Involvement of the Golgi complex. J Cell Biol. 1984;99:803–809. doi: 10.1083/jcb.99.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahkila P, Alakangas A, Väänänen K, Metsikkö K. Transport pathway, maturation, and targetting of the vesicular stomatitis virus glycoprotein in skeletal muscle fibers. J Cell Sci. 1996;109:1585–1596. doi: 10.1242/jcs.109.6.1585. [DOI] [PubMed] [Google Scholar]
- Rahkila P, Väänänen K, Saraste J, Metsikkö K. Endoplasmic reticulum to Golgi trafficking in multinucleated skeletal muscle fibers. Exp Cell Res. 1997;234:452–464. doi: 10.1006/excr.1997.3633. [DOI] [PubMed] [Google Scholar]
- Raichman M, Panzeri MC, Clementi E, Papazafiri P, Eckley M, Clegg DO, Villa A, Meldolesi J. Differential localization and functional role of calsequestrin in growing and differentiated myoblasts. J Cell Biol. 1995;128:341–354. doi: 10.1083/jcb.128.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston E. Changes in architecture of the Golgi complex and other subcellular organelles during myogenesis. J Cell Biol. 1993;120:399–409. doi: 10.1083/jcb.120.2.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramlal T, Sarabia V, Bilan PJ, Klip A. Insulin-mediated translocation of glucose transporters from intracellular membranes to plasma membrane: sole mechanism of stimulation of glucose transporter in L6 musclecells. Biochem Biophys Res Commun. 1988;157:1329–1335. doi: 10.1016/s0006-291x(88)81020-9. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Pendergast M. Polarized distribution of viral envelope proteins in the plasma membrane of infected epithelial cells. Cell. 1980;20:45–54. doi: 10.1016/0092-8674(80)90233-0. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Paskiet KT, Salas PJ, Bard E. Intracellular transport of influenza virus hemagglutinin to the apical surface of Madin-Darby canine kidney cells. J Cell Biol. 1984;98:308–319. doi: 10.1083/jcb.98.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls MM, Webster P, Balba NH, Rose JK. Novel infectious particles by expression of the vesicular stomatitis virus glycoprotein from a self-replicating RNA. Cell. 1994;79:497–506. doi: 10.1016/0092-8674(94)90258-5. [DOI] [PubMed] [Google Scholar]
- Rosemblatt M, Hidalgo C, Vergara C, Ikemoto N. Immunological and biochemical properties of transverse tubule membranes isolated from rabbit skeletal muscle. J Biol Chem. 1981;256:8140–8148. [PubMed] [Google Scholar]
- Salo J, Metsikkö K, Palokangas H, Lehenkari P, Väänänen HK. Bone-resorbing osteoclasts reveal a dynamic division of basal plasma membrane into two different domains. J Cell Sci. 1996;109:301–307. doi: 10.1242/jcs.109.2.301. [DOI] [PubMed] [Google Scholar]
- Saraste J, Svensson K. Distribution of the intermediate elements operating in ER to Golgi transport. J Cell Sci. 1991;100:415–430. doi: 10.1242/jcs.100.3.415. [DOI] [PubMed] [Google Scholar]
- Skibbens JE, Roth MG, Matlin KS. Differential extractability of influenza virus hemagglutinin during intracellular transport in polarized epithelial cells and nonpolar fibroblasts. J Cell Biol. 1989;108:821–832. doi: 10.1083/jcb.108.3.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmann T, Morselt HWM, Booy FP, van Breemen JFL, Scherphof G, Wilschut J. Functional reconstitution of influenza virus envelopes. EMBO (Eur Mol Biol Organ) J. 1987;6:2651–2659. doi: 10.1002/j.1460-2075.1987.tb02556.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugrue RJ, Badahur G, Zambon MC, Hall-Smith M, Douglas AR, Hay AJ. Specific structural alteration of the influenza haemagglutinin by amantadine. EMBO (Eur Mol Biol Organ) J. 1990;11:3469–3476. doi: 10.1002/j.1460-2075.1990.tb07555.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassin AM, Paintrand M, Berger EG, Bornens M. The Golgi apparatus remains associated with microtubule organizing centers during myogenesis. J Cell Biol. 1985;101:630–638. doi: 10.1083/jcb.101.2.630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas DC, Brewer CB, Roth MG. Vesicular stomatitis virus glycoprotein contains a dominant cytoplasmic basolateral sorting signal critically dependent upon tyrosine. J Biol Chem. 1993;268:3313–3320. [PubMed] [Google Scholar]
- Thomas K, Navarro J, Benson RJJ, Campbell KP, Rotundo RL, Fine RE. Newly synthesized calsequestrin, destined for the sarcoplasmic reticulum, is contained in early/intermediate Golgi-derived clathrin-coated vesicles. J Biol Chem. 1989;264:3140–3145. [PubMed] [Google Scholar]
- Volpe P, Villa A, Podini P, Martini A, Nori A, Panzeri MC, Meldolesi J. The endoplasmic reticulum-sarcoplasmic reticulum connection: distribution of endoplasmic reticulum markers in the sarcoplasmic reticulum of skeletal muscle fibers. Proc Natl Acad Sci USA. 1992;89:6142–6146. doi: 10.1073/pnas.89.13.6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaffe D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc Natl Acad Sci USA. 1968;61:477–483. doi: 10.1073/pnas.61.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberstein A, Snider MD, Porter M, Lodish HF. Mutants of vesicular stomatitis virus blocked at different stages in maturation of the viral glycoprotein. Cell. 1980;21:417–427. doi: 10.1016/0092-8674(80)90478-x. [DOI] [PubMed] [Google Scholar]