Abstract
During each cell cycle, the yeast vacuole actively partitions between mother and daughter cells. This process requires actin, profilin, an unconventional myosin (Myo2p), and Vac8p. A mutant yeast strain, vac8, is defective in vacuole inheritance, specifically, in early vacuole migration. Vac8p is a 64-kD protein found on the vacuole membrane, a site consistent with its role in vacuole inheritance. Both myristoylation and palmitoylation are required for complete Vac8p localization. Interestingly, whereas myristoylation of Vac8p is not required for vacuole inheritance, palmitoylation is essential. Thus, palmitoylation appears to play a more direct role in vacuole inheritance. Most of the VAC8 sequence encodes 11 armadillo (Arm) repeats. Arm repeats are thought to mediate protein–protein interactions, and many Arm proteins have multiple functions. This is also true for Vac8p. In addition to its role in early vacuole inheritance, Vac8p is required to target aminopeptidase I from the cytoplasm to the vacuole. Mutant analysis demonstrates that Vac8p functions separately in these two processes. Vac8p cosediments with actin filaments. Vac8p is related to β-catenin and plakoglobin, which connect a specific region of the plasma membrane to the actin cytoskeleton. In analogy, Vac8p may link the vacuole to actin during vacuole partitioning.
During each cell cycle, cytoplasmic organelles are partitioned into daughter cells in order to maintain organelle integrity and proper cell division. For organelles that cannot be synthesized de novo, inheritance is essential. However, even for organelles that can be synthesized de novo, inheritance is still advantageous especially in a stressful or competitive environment (Warren and Wickner, 1996).
Whereas high copy number organelles may randomly diffuse into daughter cells, low copy number organelles use specific mechanisms for segregation. The vacuole of Saccharomyces cerevisiae exists in low copy number. Vacuole inheritance is coordinated with the cell cycle and spatially directed. Before bud emergence, the vacuole migrates to the incipient bud site (Hill et al., 1996). The maternal vacuole forms membranous tubules or a series of connected vesicles, termed the “segregation structure,” that extend into the emerging bud (Weisman and Wickner, 1988; Gomes de Mesquita al., 1991; Raymond et al., 1992). These vesicles fuse in the bud to found the daughter cell vacuole. Donation of vacuolar membrane and contents continues via the segregation structure until M phase, when the segregation structure disappears. In principle, vacuole inheritance involves early vacuole migration, formation of the segregation structure and its movement, detachment of the segregation structure, and fusion of the tubules or vesicles.
We have recently discovered that vacuole movement is actin based and that myosin (Myo2p) serves as the motor (Hill et al., 1996). Specific mutations in actin, Myo2p, or profilin abolish normal vacuole inheritance. Moreover, the placement of actin cables and Myo2p in relationship with vacuole membranes is consistent with their having a direct role. These studies also suggested that the vacuole migrates to the presumptive bud site before bud emergence. There is increasing evidence that the movement of several other yeast organelles is coordinated with changes in the actin cytoskeleton. Filamentous actin undergoes significant changes early in the cell cycle. Initially, cortical actin patches are distributed throughout the surface of the cell (reviewed in Lew and Reed, 1995). Approximately 15 min before START (a regulatory point in G1), the cortical actin patches form a ring at the presumptive bud site, and actin cables appear and polarize toward this site. Portions of the Golgi, secretory vesicles and mitochondria also localize there (Preuss et al., 1992; TerBush and Novick, 1995; Simon et al., 1997). How any of these organelles interacts with actin remains unknown.
Molecules required for homotypic vacuole fusion, a late step in vacuole inheritance, have also been identified. Yeast NSF (Sec18p), α-SNAP (Sec17p), a t-SNARE (Vam3p), a v-SNARE (Nyv1p), and a small GTPase (Ypt7p) are required for in vitro vacuole fusion (Haas et al., 1995; Haas and Wickner, 1996; Mayer et al., 1996; Mayer and Wickner, 1997; Nichols et al., 1997). A heterodimer formed by thioredoxin and IB 2 (IB 2 is a cytosolic inhibitor of vacuolar proteinase B) acts synergistically with Sec18p in vacuole fusion (Xu and Wickner, 1996; Xu et al., 1997). Moreover, deletion of the genes encoding thioredoxin or IB 2 results in a vacuole inheritance defect in vivo.
Genetic selections have uncovered a number of vacuole segregation (vac)1 mutants (Weisman et al., 1990; Shaw and Wickner, 1991; Gomes de Mesquita et al., 1996; Wang et al., 1996). Based on the vacuolar morphology, these mutants were grouped into three classes (Wang et al., 1996; Bonangelino et al., 1997). Most of the corresponding wild-type genes have not yet been determined. The class I mutants appear to arrest early in vacuole inheritance. vac8 is a class I vac mutant with multilobed vacuoles. Analysis of vacuole inheritance in zygotes suggests that Vac8p is not freely diffusible in the cytoplasm and, therefore, is probably membrane associated. vac8 is also defective in cytoplasm-to-vacuole protein targeting (the Cvt pathway), whereas endocytosis and protein sorting from the Golgi to vacuole (the Vps pathway) are normal (Wang et al., 1996). Here we show that VAC8, which encodes an armadillo (Arm) repeat-containing protein, has a direct role in vacuole inheritance. Vac8p is both myristoylated and palmitoylated and localized on the vacuolar membrane, a site consistent with its roles. Mutational analysis demonstrates that Vac8p functions separately in both vacuole inheritance and the Cvt pathway. We also find that vac8 is defective in the migration of the vacuole to the presumptive bud site. Moreover, Vac8p cosediments with actin filaments in vitro. Vac8p is most closely related by sequence to the Drosophila armadillo protein (Riggleman et al., 1989) and its vertebrate homologues β-catenin (McCrea et al., 1991) and plakoglobin (Franke et al., 1989), proteins that link actin filaments to the plasma membrane and form the adherens junction. These results suggest that Vac8p may function early in vacuole inheritance by linking the vacuole membrane to the actin cytoskeleton.
Materials and Methods
Strains, Media, and Genetic and Molecular Biological Techniques
The S. cerevisiae strains used in this study were LWY7213 (MATa leu2-112, 2-3 ura3-52 trp1-Δ901 lys2-801 suc2-Δ9 Δade8::HIS3; Wang et al., 1996), RHY6210 (MATα leu2-112, 2-3 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9 pep4-Δ1137; Gomes de Mesquita et al., 1996), LWY2074 (LWY7213 vac8-1; Wang et al., 1996), and LWY2887 (MATα leu2-112, 2-3 ura3-52 trp1-Δ901 lys2-801 suc2-Δ9 Δvac8::HIS3; this study). For studies of vacuole migration to the presumptive bud site, the wild-type strain LWY7235 (MATa leu2,3-112 ura3-52 his3-Δ200 trp1-Δ901 lys2-801 suc2-Δ9) was used. This strain is essentially isogenic to LWY7213. Yeast cells were grown in the following media, yeast extract–peptone dextrose (YPD), synthetic complete (SC), and sporulation medium. Standard genetic manipulations were performed as described (Kaiser et al., 1994).
Construction of plasmids used in this study are described below. DNA manipulations were performed using standard methods (Sambrook et al., 1989). DNA sequencing was performed by the University of Iowa DNA Core Facility. PCR was carried out using Vent polymerase (New England Biolabs, Beverly, MA).
Cloning of VAC8
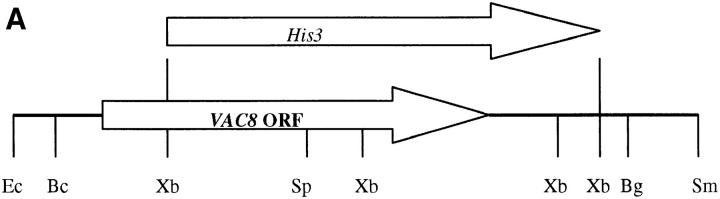
VAC8 was cloned by complementation of a growth defect of vac8-1 on yeast extract–peptone medium containing 1.5 M glycerol at 33°C. vac8-1 cells were transformed with a centromere genomic YCp50 library (Rose et al., 1987). Of the 100,000 transformants screened, 37 transformants exhibited both growth on glycerol plates and normal vacuole inheritance. Plasmids were rescued from several transformants. Partial sequences from one plasmid were used to search the yeast genome database and five open reading frames were identified. Subcloning identified the complementing activity in a 2.43-kb BclI–BglII fragment (see Fig. 1 A). This fragment was subcloned into the BamHI site of pRS416 and pRS426 to generate pYW8 and pYW10, respectively.
Figure 1.

Sequence analysis of the VAC8 gene. (A) Restriction map of the 2,972-bp SmaI–EcoRI fragment containing VAC8. The Δvac8::HIS3 disruption construct is shown. Ec, EcoRI; Bc, BclI; Xb, XbaI; Sp, SphI; Bg, BglII; Sm, SmaI. (B) Alignment of 11 Arm repeats from Vac8p. Shading indicates that at least 3 of the 11 residues are identical or equivalent (K, R, and H; D and E; L and I were considered equivalent). An asterisk indicates a space. The consensus sequence from the 11 repeats are also shown, where plus indicates K, R, or H; minus indicates D or E; X indicates any amino acids. (C) Acylation motifs in Vac8p and other proteins. Myristoylated glycines and palmitoylated cysteines are highlighted (Milligan et al., 1995).
VAC8 Deletion Strain
The 2.97-kb SmaI–EcoRI VAC8 fragment was cloned into pUC19 to generate plasmid pYW13. A 1.8-kb XbaI–XbaI HIS3 fragment was cloned into XbaI digested pYW13 to generate pYW18. A 2.9-kb SmaI–EcoRI fragment from the pYW18 containing VAC8 3′ noncoding sequence, 5′ noncoding sequence, and the first 80 codons was used to transform a wild-type diploid. Colony PCR confirmed that one copy of the VAC8 gene was deleted in the diploid. The Δvac8 strain was obtained from tetrad dissection.
Construction of Mutated VAC8 Clones
The putative myristoylation site, Gly2, was mutated to Ala in pYW13 using the Transformer Mutagenesis Kit (CLONTECH, Palo Alto, CA). The mutagenesis primer was 5′-GCA ACT ATG GCT TCA TGT TGT AG-3′. The selection primer was 5′-GTG CCA CCT GTC GTC TAA GAA ACC-3′. The mutated VAC8 was then subcloned into pRS415.
To mutate all the putative acylation sites in the acylation motif (the Gly residue and the neighboring three Cys residues, see Fig. 1 C), the BclI– SphI fragment in pYW13 was amplified into two fragments by two PCR reactions. For reaction one, the forward primer V15 was 5′-GGA ATT CGG CAG AAT AGT GCT GTC ATT G-3′, and the backward primer V29 was 5′-GGG GT A CCT GAA GCC ATA GTT GCA AAT GTA TAT ATA G-3′. The EcoRI and KpnI sites are underlined, respectively. In reaction two, the forward primer V28 was 5′-GGG GTA CCA GTA GCT TGA AAG ATT CTT CAG ACG AG-3′, and the backward primer V18 was 5′-TCC CCG CGG TCA GCG GCC GCT AGA TAC GGC GTG ACA TTG-3′. The KpnI site is underlined and was introduced to facilitate the subcloning. The mutated nucleotides are in bold face. The two amplified fragments were digested with EcoRI–KpnI and KpnI–SphI, respectively, and subcloned simultaneously into pUC19 digested with EcoRI–SphI. The EcoRI–SphI fragment was then isolated and cloned into pYW8 that had been digested with EcoRI–SphI to generate plasmid pYW34. Mutation of only the three Cys residues was performed as above but primer V31 5′-GGG GT A CCT GAA CCC ATA GTT GCA AAT GTA TAT ATA G-3′ was used instead of V29. The KpnI site is underlined and the mutated nucleotides are in bold face. All the desired mutations and PCR products were verified by sequencing.
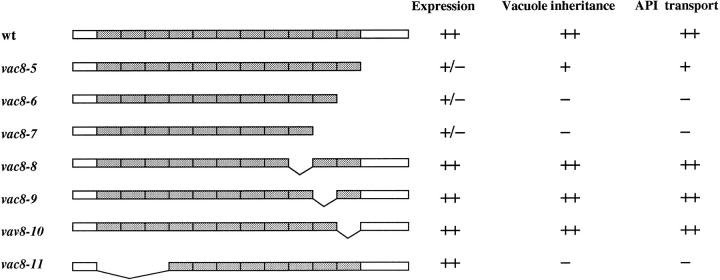
For the constructs used in Fig. 8, sequential deletions from the COOH terminus were carried out by PCR; internal deletions were carried out with the Transformer Mutagenesis Kit (CLONTECH, Palo Alto, CA).
Figure 8.
Deletion analysis of Vac8p. Δvac8 strains harboring CEN plasmids with different VAC8 constructs were analyzed for expression, vacuole inheritance and API transport. ++, normal; +, slightly defective; +/−, greatly reduced expression; −, defective.
Vac8p Antiserum and Purification
To make a GST-VAC8 fusion, the VAC8 coding region was amplified by PCR and cloned into the EcoRI–NcoI sites of pGEX-KG (Guan and Dixon, 1991). The fusion protein was induced in E. coli and purified using a glutathione column following the manufacturer's instructions (Pharmacia Biotech Inc., Piscataway, NJ). Vac8p antiserum was generated in rabbits by the Pocono Rabbit Farm & Laboratory, Inc. (Canadensis, PA).
To affinity purify Vac8p antibodies, 2.7 mg GST-VAC8 fusion protein was conjugated to a 1-ml slurry of Affi-Gel 10 beads following the manufacturer's instructions (Bio-Rad Laboratories, Hercules, CA). Vac8p antiserum was first passed through a GST protein column (kindly provided by Dr. David Price, University of Iowa). The flowthrough was then loaded onto the GST-VAC8 fusion protein column. The column was washed with PBS buffer and the antibodies eluted with 50 mM glycine-HCl, pH 2.5.
Western Blotting
Western analysis of aminopeptidase I (API) was performed as described (Harding et al., 1995). To detect Vac8p from total cell extracts, exponentially growing cells were lysed by vigorous agitation with 0.5-mm glass beads in lysis buffer (0.3 M sorbitol, 10 mM Tris, pH 7.5, 0.1 M NaCl, 1 mM MgCl2, 1.0 mM EDTA, and 1 mM PMSF) containing protease inhibitor cocktail (Xu and Wickner, 1996). Extracts were precleared by centrifugation at 500 g for 10 min and added to an equal volume of 2× Laemmli sample buffer (Laemmli, 1970). Extracts were boiled for 5 min before loading on a 10% SDS–polyacrylamide gel. Proteins were transferred to nitrocellulose membrane and subjected to Western analysis. Vac8p antiserum was used at 1:4,000 dilution and HRP-coupled goat anti–rabbit IgG at 1:10,000 dilution. Blots were developed using the ECL kit (Amersham Corp.).
Cell Fractionation and Extraction
Precleared cell extracts were generated as above and separated into pellet (P13) and supernatant (S13) fractions by centrifugation at 13,000 g for 10 min. The S13 fraction was further separated into pellet (P100) and supernatant (S100) fractions by centrifugation at 100,000 g for 30 min. For extraction experiments, the P13 fraction was resuspended in lysis buffer. Aliquots of the P13 fraction were adjusted to one of the following conditions, 1% Triton X-100, 1.6 M urea, 1 M NaCl, 0.1 M Na2CO3, 1 M hydroxylamine (pH 7.0), or left untreated and incubated for 30 min on ice. The samples were then respun to generate the second round of P13 and S13 fractions. Equal portions of cell extracts from each fraction were applied to an SDS–polyacrylamide gel. Proteins were transferred to nitrocellulose and Vac8p detected with anti-Vac8p serum.
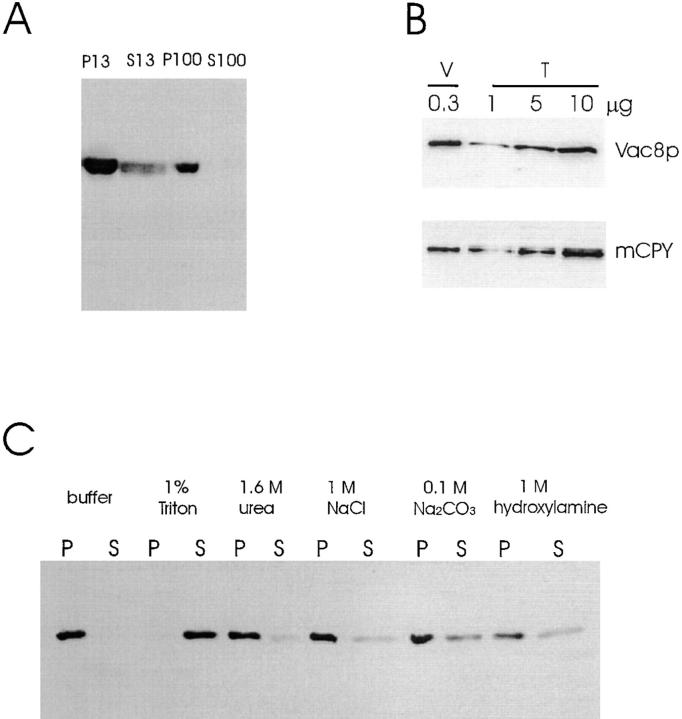
Vacuoles were isolated as described (Haas, 1996). In brief, cells were grown in YPD medium to a density of OD600 0.6–1.0. 1,000 OD600 units cells were pelleted, resuspended in 50 ml DTT/Pipes buffer (100 mM Pipes/KOH, pH 9.4, 10 mM DTT), and incubated at 30°C for 10 min. Cells were then collected and spheroplasted for 25 min at 30°C in 15 ml buffer (0.6 M d-sorbitol, 0.16 × YPD, and 50 mM KPi, pH 7.5) containing 1 mg oxalyticase (Enzogenetics, Corvallis, OR). Spheroplasts were lysed in 2.5 ml 15% Ficoll solution (in 10 mM Pipes/KOH, pH 6.8, and 200 mM d-sorbitol) containing 70 μg DEAE-dextran and transferred to a centrifuge tube. 3 ml of 8%, 3 ml of 4%, and 2 ml of 0% of Ficoll solution were added sequentially to create a step gradient. Vacuoles were recovered from the 4%/0% Ficoll interface after centrifugation at 110,000 g for 90 min at 4°C. Vacuolar protein concentration was determined with the Bradford reagent (Bio-Rad Laboratories). 0.3 μg vacuolar protein was used for Western analysis (see Fig. 4 B).
Figure 4.
Vac8p is associated with vacuolar membranes. (A) Precleared extracts from wild-type cells (LWY7213) were subjected to sequential differential centrifugation as described in Materials and Methods. Equal portions of cell extracts (0.75 OD600 unit equivalents) were separated by SDS-PAGE, transferred to nitrocellulose and probed with Vac8p antiserum. (B) Vacuoles were isolated as described (Haas, 1995). Vacuoles (V, 0.3 μg protein) and precleared total cell extracts (T, 1 μg, 5 μg and 10 μg) were dissolved in SDS-PAGE sample buffer (Laemmli, 1970) and analyzed by immunoblot with Vac8p antiserum and CPY antiserum. (C) The P13 fraction in A was resuspended in lysis buffer. Aliquots of the P13 fraction was treated for 30 min on ice with various reagents indicated. The samples were then respun to generate the second round of P13 and S13 fractions. The presence of Vac8p in the P13 and S13 fractions was determined by Western blot.
Lipid Labeling and Immunoprecipitation
Labeling with [3H]myristic acid was performed as described (Herman et al., 1991). Yeast cells were grown in SC media. Cells (25 OD600 units) were collected and resuspended in 5 ml SC media. Cells were preincubated with 20 μg/ml cerulein (Sigma Chemical Co.) for 20 min at 30°C. 1.0 mCi [3H]myristic acid (specific activity: 60 Ci/mmol; American Radiolabeled Chemicals Inc., St. Louis, MO) was added and the cells were labeled for 60 min at 30°C.
Labeling with [3H]palmitic acid was performed as described (Deschenes and Broach, 1987). Cells (65 OD600 units) from SC media were collected and resuspended in 25 ml SC media, and then incubated with 3 μg/ml cerulein and 500 μCi [3H]palmitic acid (specific activity: 60 Ci/ mmol; American Radiolabeled Chemicals Inc.) for 4 h at 30°C.
After labeling, the cells were washed once and lysed in 300 μl lysis buffer. For detection of myristoylation, 300 μl IP buffer (50 mM Tris-HCl, pH 7.0, 200 mM NaCl, 1% Triton X-100, 0.5% deoxycholate, and 0.1% SDS) was added and then cell extracts were agitated for 1 min. For detection of palmitoylation, cell extracts were subjected to 13,000 g centrifugation for 10 min. The membrane fraction was solubilized with 300 μl IP buffer. Both preparations were centrifuged at 13,000 g for 10 min. 60–80 μl Vac8p antiserum was added to the supernatant and the resulting solution was incubated overnight. Subsequently, protein A beads were added and the suspension was incubated for 4 h. The beads were washed three times with IP buffer. Antigen was eluted with sample buffer (30 mM Tris-HCl, pH 6.7, 12% [wt/vol] SDS, 6% [vol/vol] glycerol, and 0.1 μg/ml bromphenol blue; Mitchell et al., 1994) at 65°C for 10 min and subjected to SDS-PAGE and fluorography.
Cosedimentation of Vac8p with F-Actin
High speed total cell extracts (for wild-type cells) or cytosol (for Δvac8 and the myristoylation-minus mutant vac8-2) were generated as described (Haas, 1996) using the lysis buffer (20 mM Pipes/KOH, pH 6.8, 250 mM sorbitol, 100 mM K(OAc), 50 mM KCl, 5 mM MgCl2, 1 mM Mg-ATP, 1 mM PMSF, and 2× protease inhibitors cocktail [Xu and Wickner, 1996]). For total cell extracts, the lysis buffer also contained 0.5% (wt/vol) Triton X-100. Protein concentration was adjusted to 10 mg/ml. Aliquots of them were kept at −80°C for <1 wk.
Before use, the total cell extracts and cytosol were thawed and spun in a Beckman TL-100 tabletop centrifuge using a TLA-100 fixed angel rotor for 1 h at 65,000 rpm. Freshly purified yeast G-actin was in G-buffer (5 mM Tris-HCl, pH 7.5, 0.1 mM CaCl2, 0.1 mM ATP, 2 mM PMSF, and 1× protease inhibitors) at a concentration of 2 mg/ml. A 100-μl mixture contained 20 μl total cell extracts or cytosol, 20 μl fresh G-actin, and G-buffer. No G-actin was added in controls. For the experiments using Δvac8 cytosol, 10 μl purified GST-Vac8 protein (2.6 μg) was added. The mixtures were incubated for 1 h on ice and polymerized actin was pelleted using the same rotor for 30 min at 60,000 rpm. The supernatant was carefully taken and mixed with 100 μl 2× Laemmli sample buffer (Laemmli, 1970). 100 μl F-buffer (5 mM Tris-HCl, pH 7.5, 2 mM MgCl2, 50 mM KCl, 5 μM DTT, 0.1 mM CaCl2, 0.1 mM ATP, 2 mM PMSF, and 1× protease inhibitors) was added to the pellet and centrifuged as before. This pellet was resuspended in 40 μl 1× Laemmli sample buffer. 5 μl of the pellet fraction and the first supernatant fraction were separated by SDS-PAGE and the proteins transferred to a nitrocellulose membrane. The membrane was cut into two halves. The top half was probed with Vac8p antiserum and the bottom half was probed with the serum to the yeast ubiquitin–protein hydrolase Yuh1p (provided by Dr. Robert Cohen, University of Iowa). In addition, 0.1 μl of the pellet fraction was separated by SDS-PAGE. The proteins were transferred to a nitrocellulose membrane and probed for the presence of actin.
Vacuole Staining with FM4-64 and Localization by Immunofluorescence
Observation of vacuole inheritance with FM4-64 in vegetative cells and zygotes was performed as described (Wang et al., 1996).
Immunolocalization of Vac8p was performed as described (Berkower et al., 1994) except duration of the primary antibody incubation was 3–4 h. Immunofluorescence for detection of vacuole polarization was performed as described (Hill et al., 1996). Actin was visualized using goat anti–yeast actin serum (a generous gift from Drs. T. Karpova and J. Cooper, Washington University, St. Louis, MO) affinity purified against yeast actin. The affinity-purified Vac8p antibody and the 60-kD vacuolar ATPase mouse monoclonal antibody (Molecular Probes, Inc., Eugene, Oregon) were used at a 1:50 dilution. The affinity-purified actin antibody and all secondary antibodies were used at a 1:200 dilution. Oregon Green 488–conjugated goat anti–rabbit IgG (Molecular Probes, Inc.) was used to detect Vac8p. Lissamine rhodamine–conjugated donkey anti–mouse IgG (Jackson ImmunoResearch Laboratories Inc.) was used to detect the 60-kD subunit of the v-ATPase. Cy2 conjugated donkey anti–goat IgG (Jackson ImmunoResearch Laboratories Inc.) was used to detect actin.
Confocal Imaging
Images were obtained with a laser scanning confocal system (MRC-1024; Bio-Rad Laboratories) mounted on a Nikon Optiphot microscope. Dual detection was performed with separate photomultiplier tubes and the resultant images were merged. For each field, a z-series of 5–10 steps was scanned and a single step was selected (for Vac8p localization) or all steps were projected for a single view (for vacuole migration studies) using the MRC-1024 LaserSharp software. Adobe Photoshop was used for any further processing of images.
Results
Isolation and Sequence Analysis of VAC8
The vac8-1 mutant was isolated by fluorescence-activated cell sorting enrichment (Wang et al., 1996). We observed that vac8-1 failed to grow on yeast extract–peptone medium containing 1.5 M glycerol at 33°C. To identify the VAC8 gene, vac8-1 was transformed with a yeast genomic centromere YCp50 library (Rose et al., 1987). Of 100,000 transformants, 37 grew on this selection medium and displayed normal vacuole inheritance. The plasmids from these strains were rescued. One open reading frame (ORF), YEL013w (accession number P30998), was identified that complemented both the vacuole inheritance and API transport defects of vac8-1. A VAC8 deletion strain was constructed and crossed to vac8-1. The diploid was sporulated and dissected. All spores from 11 tetrads showed vacuole inheritance defects, demonstrating that YEL013w is the authentic VAC8 gene. All 37 candidate plasmids contained the VAC8 gene as assessed by Western or PCR analysis.
VAC8 encodes a protein containing 578 amino acids with no apparent signal sequence or transmembrane domain. Sequence analysis using the BLAST algorithm revealed Vac8p as a member of the Arm repeat-containing protein family. Among all the Arm repeat proteins, Vac8p is most closely related to Drosophila armadillo and its vertebrate homologues, β-catenin and plakoglobin. When the sequences of armadillo and its homologues were compared with all the putative ORFs of the S. cerevisiae genome, only two relatives were identified. The first is VAC8. The second, SRP1, encodes the yeast homologue of importin α and is involved in nuclear import (Yano et al., 1994). The homology of Vac8p with armadillo/β-catenin/plakoglobin is more significant than that of Srp1p. Vac8p has 11 contiguous 40–42-amino acid Arm repeats, a 28–amino acid NH2 terminus, and a 98–amino acid COOH terminus. The alignment of the 11 Arm repeats and their consensus sequence is shown in Fig. 1 B. A prominent feature of the COOH terminus is an unusual stretch of nine Asn residues.
Examination of the Vac8p NH2 terminus revealed potential acylation sites (see Fig. 1 C). Similar acylation motifs are present in subfamilies of heterotrimeric G protein α subunits and the Src kinase family (see Milligan et al., 1995). In these motifs, the Gly residue in position 2 is modified cotranslationally by myristic acid and the Cys residues are modified posttranslationally by palmitic acid. These types of lipid modifications have been shown to be important for protein localization and protein–protein interactions (reviewed in Casey, 1995; Milligan et al., 1995).
Vac8p Is Required for Vacuole Inheritance
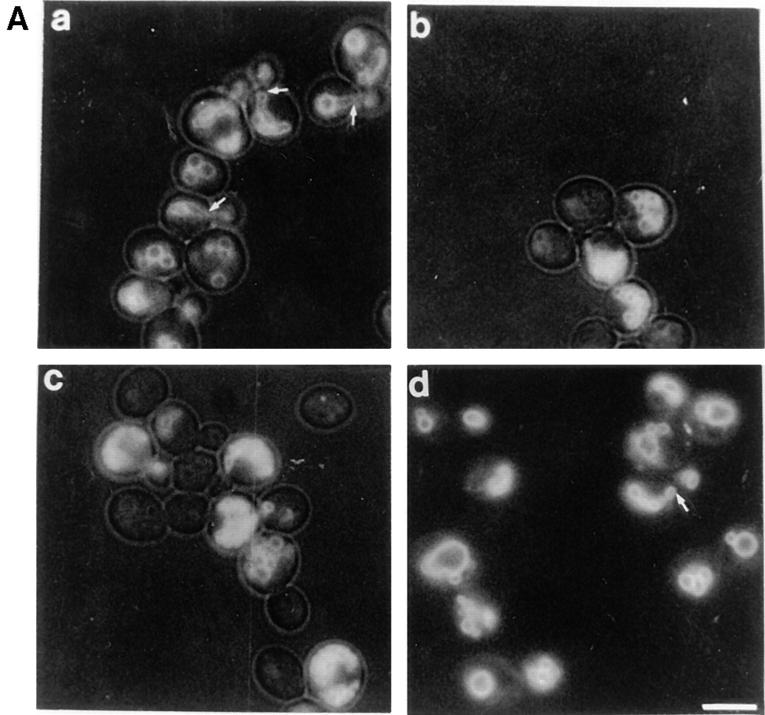
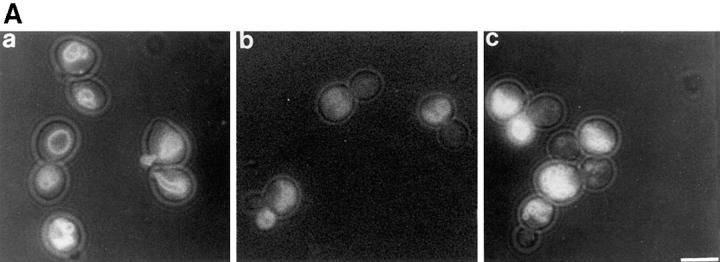
Yeast cells that lack VAC8 (Δvac8) are viable but grow more slowly than wild-type cells. To investigate the requirement for Vac8p in vacuole inheritance, wild-type and Δvac8 cells were labeled with FM4-64 for 1 h and chased for 3 h. The newly formed buds will contain labeled vacuoles only if they inherit a portion of the mother cell's vacuole. As shown in Fig. 2 A, in contrast to the wild-type cells, the buds in Δvac8 either lack vacuoles or contain small faintly labeled vacuoles. Moreover, note the absence of segregation structures, membranous connections between the mother and bud vacuoles. Segregation structures, a hallmark of normal vacuole inheritance, are prominent in the wild-type strain (Fig. 2 A, arrows). Loss of Vac8p function also perturbs vacuolar morphology; there are often more than six lobes clustered in Δvac8 vacuoles, compared with wild-type vacuoles where usually one to six lobes are present. For additional examples of wild-type vacuolar morphology versus a fragmented vacuolar morphology see Fig. 7 A. High copy VAC8 did not suppress any of the vac mutants listed below. Likewise, when double mutants were constructed between vac8 and vac9, vac11, vac12, act1-101, act1-135, or myo2-66, no synthetic phenotypes were observed. act1-101, act1-135, and myo2-66 all display severe vacuole inheritance defects (Hill et al., 1996).
Figure 2.
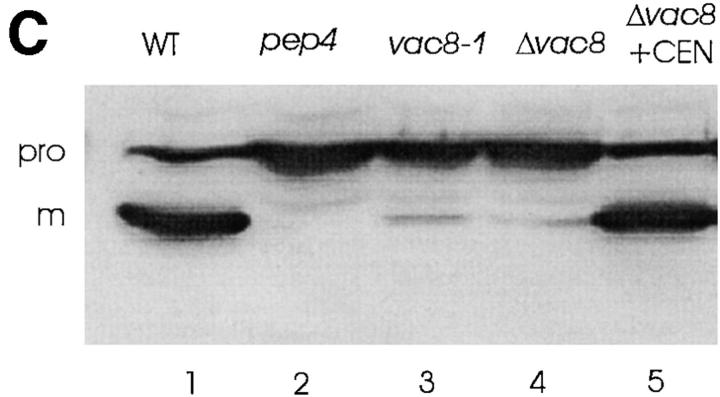
Vac8p is required for both vacuole inheritance and API transport. (A) Cells were labeled with FM4-64 for 1 h. Free dye was removed and cells were resuspended in fresh medium and allowed to grow for 3 h at 23°C. The vacuoles were viewed by fluorescence microscopy. Segregation structures are indicated with arrows. a, Wild-type; b, vac8-1; c, Δvac8; d, Δvac8 with CEN VAC8 plasmid. (B) Vac8p is required for polarization of the vacuole to the presumptive bud site. Fixed spheroplasts were double labeled with actin antibody (green) and 60-kD v-ATPase antibody (red), detected with Cy2 conjugated donkey anti–goat IgG and Lissamine rhodamine–conjugated donkey anti–mouse IgG, respectively. (a and b) Wild-type, (c and d) Δvac8. (C) Cell extracts were prepared as described in Materials and Methods. Equal amounts of cell extract (0.3 OD600 equivalents) were subjected to Western analysis with anti-API serum. The positions of precursor (pro) and mature (m) API are shown. Lane 1, wild-type; lane 2, pep4; lane 3, vac8-1; lane 4, Δvac8; lane 5, Δvac8 with CEN VAC8 plasmid. Bars, 5 μm.
Figure 7.
Phenotypic analysis of the acylation mutants. (A) Δvac8 strains harboring CEN plasmids with different VAC8 alleles were examined for vacuole inheritance as done in Fig. 2 A. (a) vac8-2; (b) vac8-3; (c) vac8-4. (B) API transport was examined as done in Fig. 2 B. Bar, 5 μm.
In recent studies we found that vacuole partitioning initiates before bud emergence (Hill et al., 1996). With the limited number of unbudded cells included in that study, we detected a portion of the vacuole juxtaposed with the cortical actin ring at the presumptive bud site, and the rest of the vacuole aligned with the actin cables. These observations suggest that this early vacuole migration is dependent on the vacuole interacting with the actin cytoskeleton. To further characterize Vac8p's role in vacuole inheritance, vacuole placement was examined in cells from a population of asynchronously growing yeast. Actin and vacuole membranes in fixed cells were detected with antiactin antibody and anti–60-kD vacuolar ATPase subunit, respectively. All unbudded cells with a polarized actin cytoskeleton were scored. In 79% of the wild-type yeast, the vacuole was polarized along the actin cytoskeleton, with a portion of the vacuole juxtaposed with the presumptive bud site (Fig. 2 B and Table I). We assume that the polarization of the actin cytoskeleton occurs before vacuole polarization. Thus, cells that have not yet polarized their vacuoles may be further away from START. In Δvac8 only 26% of the polarized cells had vacuoles coincident with the presumptive bud site. At least 57% of the Δvac8 population is clearly unpolarized, compared with 21% of the wild-type population. Thus, Vac8p facilitates this early step in vacuole inheritance.
Table I.
Polarization of the Vacuole in Unbudded Cells
| Polarized | Unpolarized | Ambiguous | ||||
|---|---|---|---|---|---|---|
| WT (n = 89) | 79% | 21% | 0% | |||
| Δvac8 (n = 99) | 26% | 57% | 17% |
Asynchronous cultures of both wild-type and Δvac8 cells were fixed and stained for actin and the vacuole membrane. Confocal images of the cells were obtained and individual unbudded cells evaluated for polarization of the vacuole to the presumptive bud site.
Vac8p Is Required for Protein Targeting from the Cytoplasm to Vacuole
The vac8 mutant has no detectable defect in endocytosis or targeting protein to the vacuole via the Vps pathway (Wang et al., 1996). However, vac8 is defective in the Cvt pathway. Cell extracts were separated by SDS-PAGE and probed with serum generated against aminopeptidase I (API), a protein marker for the Cvt pathway (Klionsky et al., 1992). In wild-type cells, a significant amount of API precursor was transported into the vacuole and processed to the mature form (Fig. 2 C). The maturation of API is dependent on the presence of active proteinase A (encoded by PEP4; Klionsky et al., 1992). However, in Δvac8 cells almost all the API was in the precursor form. The vacuole inheritance and API transport defects in Δvac8 were complemented by a plasmid containing the VAC8 gene (Fig. 2, A and C). These results demonstrate that Vac8p is required for both vacuole inheritance and protein transport from the cytoplasm to vacuole.
Identification of the VAC8 Gene Product
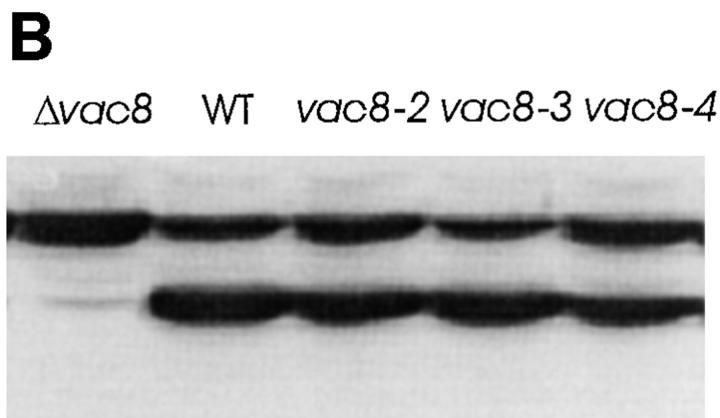
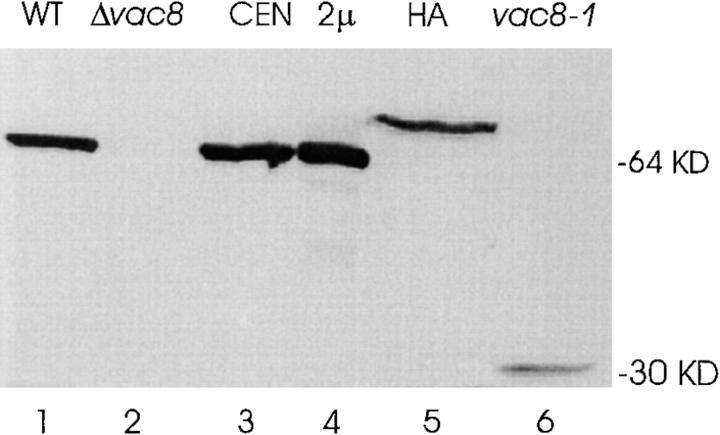
To detect Vac8p, antibodies were raised to a recombinant GST–Vac8p fusion protein. As seen in Fig. 3, in agreement with the predicted molecular weight, the Vac8p antiserum specifically recognized a protein of ∼64 kD in wild-type cells and Δvac8 cells transformed with a VAC8-containing plasmid, whereas the 64-kD band was absent in Δvac8 cells. Moreover, the level of Vac8p produced from the 2μ plasmid is greater than the level expressed from a centromere plasmid. Interestingly, vac8-1 produced a 30-kD truncated VAC8 gene product. As expected, the VAC8 gene product from a 6× HA COOH-terminal–tagged construct (VAC8-HA) had a slightly lower mobility. All these results confirmed the identity of Vac8p. However, vac8-1 and VAC8-HA each produced a significantly lower level of protein. This is consistent with other data that suggest that the 98–amino acid residue COOH-terminal tail is involved in stabilization of the protein (see below).
Figure 3.
Identification of the VAC8 gene product. Cells were grown in SC medium and cell extracts generated by breakage with glass beads. Precleared cell extracts were separated by SDS-PAGE, transferred to nitrocellulose and probed with Vac8p antiserum. Lane 1, 10 μg protein, wild-type; lane 2, 10 μg protein, Δvac8; lane 3, 10 μg protein, Δvac8 with CEN VAC8; lane 4, 5 μg protein, Δvac8 with 2μ VAC8; lane 5, 50 μg protein, Δvac8 with CEN VAC8-HA; lane 6, 50 μg protein, vac8-1.
Vac8p Is Associated with the Vacuolar Membrane
To study the function of Vac8p, its subcellular localization was determined. Precleared wild-type cell extracts were separated into pellet (P13) and supernatant (S13) fractions by 13,000 g centrifugation. The S13 fraction was further separated into pellet (P100) and supernatant (S100) fractions by 100,000 g centrifugation. Equal portions of cell extracts from each fraction were analyzed. Most Vac8p (>80%) was in the P13 fraction with the remainder in the P100 fraction (Fig. 4 A). No Vac8p was found in the S100 soluble fraction.
The P13 fraction is enriched in cellular membranes, including those from vacuoles. We used two methods, vacuole fractionation and double label immunofluorescence, to determine whether Vac8p associates specifically with the vacuolar membrane. Vacuoles were isolated by discontinuous Ficoll gradient centrifugation (Haas, 1996). Vac8p was enriched 45 times in vacuole fractions, similar to vacuolar marker protein carboxypeptidase Y (CPY) that was enriched 30 times (Fig. 4 B). In contrast, when the same amount of proteins in Fig. 4 B was probed with antibodies to a ER marker, dolichol phosphate mannose synthase (Dpm1p), and Dpm1p was only found in the total cell extracts not in the vacuoles (data not shown). The slightly lower enrichment for CPY compared with Vac8p is probably due to vacuoles that lysed, releasing CPY, during the isolation procedure. In immunolocalization experiments (see Fig. 6 A), affinity-purified anti-Vac8p polyclonal antibodies specifically stained the vacuolar membrane in wild-type cells. No immunostaining was observed in Δvac8. The vacuolar membrane localization was confirmed by double label immunofluorescence with antibodies to the 60-kD vacuolar ATPase subunit. The anti-HA monoclonal antibodies also localized HA-tagged Vac8p on the vacuolar membrane (not shown). Vac8p was present on the vacuolar membrane at all stages of the cell cycle. Examination of living cells with GFP-tagged Vac8p also revealed that Vac8p constitutively resides on the vacuolar membrane (data not shown). The location of Vac8p is consistent with its functioning in both vacuole inheritance and API transport.
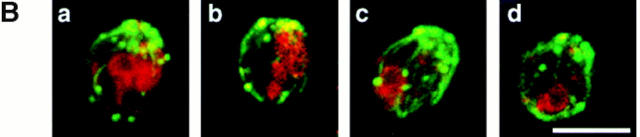
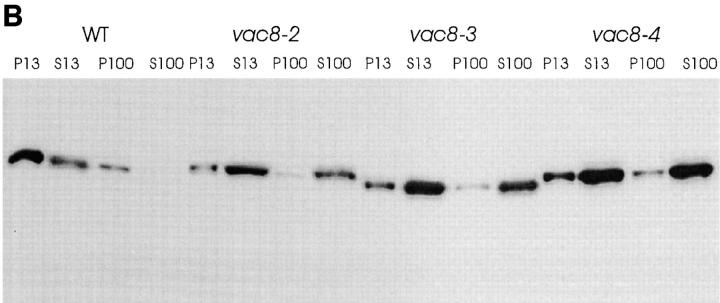
Figure 6.
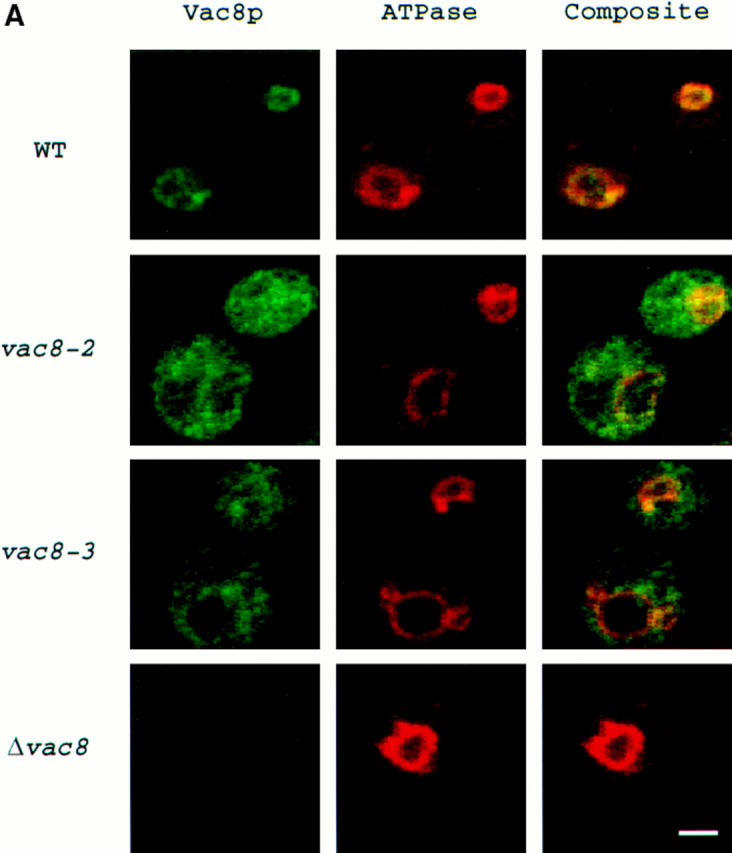
Mislocalization of Vac8p in the acylation mutants. (A) Cells were processed and incubated with both affinity-purified Vac8p antibody (green) and 60-kD v-ATPase monoclonal antibody (red), detected by Oregon Green 488–conjugated goat anti–rabbit IgG and Lissamine rhodamine–conjugated donkey anti– mouse IgG, respectively. Δvac8 harboring CEN plasmids with different VAC8 alleles was used. (B) Fractionation and determination of Vac8p was performed as in Fig. 4 A. Δvac8 harboring CEN plasmids with different VAC8 alleles was used. Lanes 1–4, WT; lanes 5–8, vac8-2; lanes 9–12, vac8-3; lanes 12–16, vac8-4. Bar, 2 μm.
To determine the nature of Vac8p association with the vacuolar membrane, the P13 fraction was resuspended and treated for 30 min on ice with various reagents. The treated P13 fractions were subjected to 13,000 g centrifugation to generate new P13 and S13 fractions. An aliquot of each fraction was separated by SDS-PAGE and probed with Vac8p antiserum (Fig. 4 C). Treatment of the P13 fraction with 1.6 M urea or 1 M NaCl extracted little Vac8p from the membrane, suggesting that Vac8p association with the membrane is not simply via protein–protein interactions. Since Vac8p lacks transmembrane domains but contains acylation motifs at the NH2 terminus, the membrane association of Vac8p most likely relies on lipid–membrane interactions. This is consistent with the result that Vac8p could be quantitatively released from the membrane when incubated with a nonionic detergent (1% Triton X-100). Treatment with 0.1 M Na2CO3, pH 11, or 1 M hydroxylamine, pH 7.0, extracted a portion of Vac8p (30%) from the membrane. These two reagents can cleave the thioester bonds that often link palmitoyl moieties to proteins (Omary and Trowbridge, 1981). This result further implicates lipid–membrane interactions in the anchoring of Vac8p.
Vac8p Is Both Myristoylated and Palmitoylated
The presence of the potential myristoylation and palmitoylation sites at the NH2 terminus and the results from the membrane extraction experiments suggested that Vac8p is acylated. To analyze this directly, we performed in vivo radiolabeling experiments. Cells were labeled with [3H]myristic acid. Total cell extracts were generated in the presence of detergents and Vac8p immunoprecipitated (Fig. 5 A). Vac8p from cells with wild-type VAC8 plasmid incorporated the myristic acid. When the putative myristoylation site glycine residue was mutated (G2S, vac8-2), no Vac8p was labeled. To detect palmitoylation, cells were labeled with [3H]palmitic acid. The 13,000-g membrane fraction (P13) was solubilized and Vac8p immunoprecipitated (Fig. 5 B). Again, incorporation of the palmitic acid occurred in the wild-type protein, but not in the mutant Vac8p protein, where the putative palmitoylation sites were mutated (C4G, C5T, C7S; vac8-3). The lack of labeling in the acylation-minus mutants was not due to problems with the expression of these mutant proteins (Fig. 5 C). Only a minor population of the myristoylation-minus mutant (vac8-2) could be palmitoylated (Fig. 5 B). Although only 20% of the Vac8p in the myristoylation-minus mutant was on the vacuolar membrane (see below), when Vac8p was immunoprecipitated from total cell extracts rather than the P13 membrane fraction, similar levels of palmitoylation were observed (data not shown). This suggests that the cytosolic Vac8p is not palmitoylated and that all the palmitoylated Vac8p associates with vacuolar membrane. Note that acylation affected the electrophoretic mobility of Vac8p (Fig. 5 C). In summary, Vac8p is both myristoylated and palmitoylated in vivo and these modifications most likely occur at the NH2 terminus acylation motif.
Figure 5.
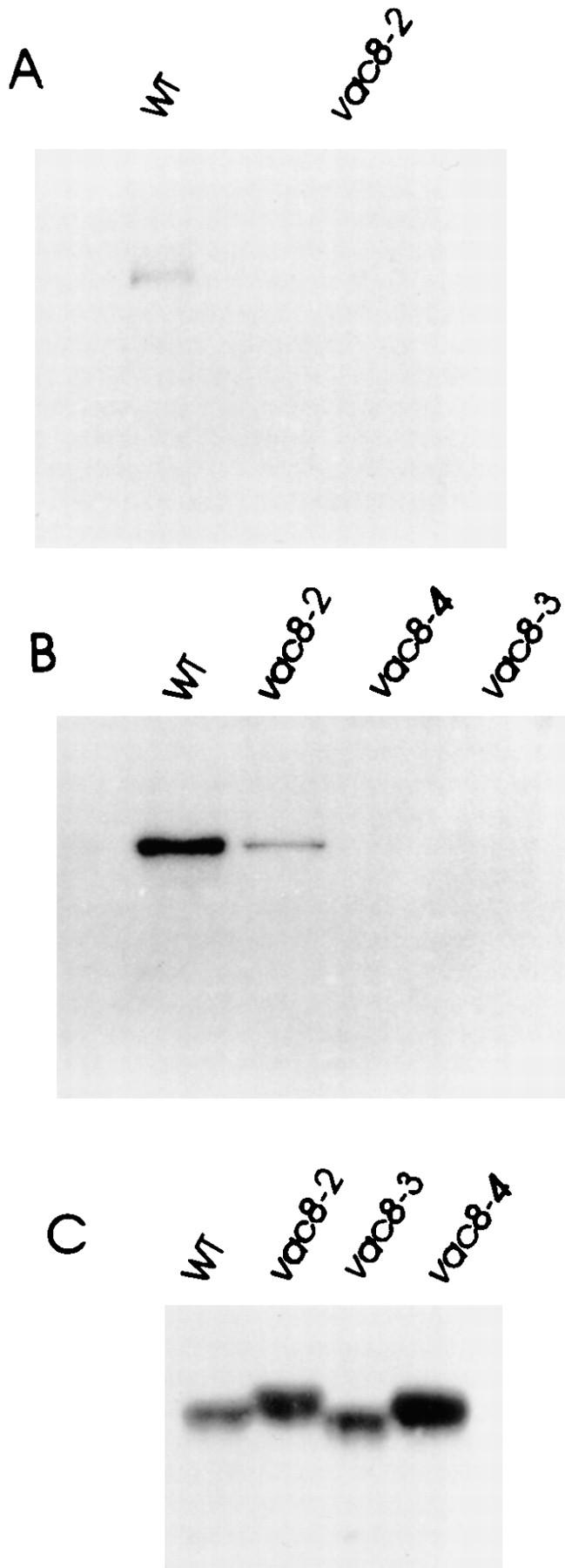
Vac8p is acylated in vivo. Δvac8 yeast harboring CEN plasmids with different VAC8 alleles were labeled in vivo with [3H]myristic acid (A) or [3H]palmitic acid (B). Vac8p was immunoprecipitated with antiserum as described in Materials and Methods. Antigen was eluted and subjected to SDS-PAGE and fluorography. vac8-2, G2A; vac8-3, C4G, C5T, and C7S; vac8-4, G2A, C4G, C5T, and C7S. (C) Cell extracts prepared from Δvac8 harboring CEN plasmids with different VAC8 alleles were analyzed by immunoblot with anti-Vac8p serum. Note the mobility differences in the VAC8 alleles.
Acylation Is Important for Vacuolar Membrane Association of Vac8p
Having established that Vac8p was acylated, we next determined whether the acylation is responsible for Vac8p association with the vacuolar membrane. When double label immunofluorescence experiments were performed, only a small amount of Vac8p from the myristoylation-minus mutant (vac8-2), the palmitoylation-minus mutant (vac8-3) or the myristoylation and palmitoylation double mutant (vac8-4) was associated with the vacuolar membrane (Fig. 6 A, not shown for vac8-4). Fractionation studies showed that, consistent with the immunofluorescence results, Vac8p from the acylation-minus mutants was mostly cytosolic with a small fraction (20%) associated with the P13 membrane fraction (Fig. 6 B). Thus, efficient vacuolar membrane localization requires the presence of both the myristoylation and palmitoylation motifs. Note that a small amount of Vac8p remained in the P13 fraction even in the absence of both myristoylation and palmitoylation (Fig. 6 B, lanes 12–16), indicating that nonacylated Vac8p is still capable of associating with the vacuolar membrane. Most of the membrane-bound, nonacylated Vac8p is released by treatment with urea, NaCl, or Na2CO3 (data not shown).
Palmitoylation Is Essential for Vacuole Inheritance but Not API Transport
To determine whether acylation is important for Vac8p function, the phenotypic consequences of the acylation-minus mutants were examined. Strikingly, whereas vac8-2 showed normal vacuole inheritance and morphology (Fig. 7 A, a), the vacuole inheritance and morphology in vac8-3 were identical to those in Δvac8 cells (b). Thus, palmitoylation is required for Vac8p function in vacuole inheritance, whereas myristoylation is dispensable. When vac8-2 or vac8-3 were labeled with FM4-64 and mated with Δvac8, only 9% of the zygotic buds inherited vacuoles from vac8-3 parental vacuoles, compared with 89% from vac8-2 parental vacuoles (Table II). This further confirms the importance of palmitoylation but not myristoylation for Vac8p function in vacuole inheritance. Because almost equal amounts of Vac8p are present in the membrane fraction of the two mutants, the vacuole inheritance defect in the palmitoylation-minus mutant is unlikely to be due to the mislocalization of Vac8p from the vacuolar membrane; rather, the palmitate group may somehow participate more directly in the vacuole segregation process.
Table II.
Effects of Acylation Site Mutations on Vacuole Inheritance in Zygotes
The strains listed on the left were labeled with FM4-64. After removing FM4-64 from the medium, the labeled cells were mixed with an equal number of cells of the opposite mating type. Cultures were incubated with shaking at 23°C for 4–4.5 h. Cells were examined by fluorescence microscopy and scored for the presence of labeled vacuoles. At least 50 zygotes were scored in each cross.
Based on fractionation studies, 80% of Vac8p in vac8-2 is mislocalized to the cytosol (Fig. 6 B). Since vac8-2 is functional, we examined vacuole inheritance in zygotes to ask whether the cytosolic vac8-2 protein is able to support vacuole inheritance. Δvac8 were labeled and mated with the acylation mutants. If cytosolic Vac8p could support vacuole inheritance, then the labeled Δvac8 parent would be rapidly rescued by cytosolic vac8-2 gene product and would transfer its vacuole material to the bud in a Δvac8 × vac8-2 cross. However, only 10% of zygotes had labeled vacuoles in the buds, similar to that seen in the Δvac8 × VAC8, Δvac8 × vac8-3, and Δvac8 × vac8-4 crosses (Table II). These results suggest that in vac8-2 the vacuolar membrane-bound Vac8p is responsible for vacuole inheritance and the cytosolic Vac8p is not functional.
In contrast with vacuole inheritance, cytoplasm to vacuole targeting of API was normal in all of the acylation-minus mutants (Fig. 7 B). Thus, API targeting requires neither type of acylation. That the palmitoylation-minus mutant is normal for API targeting but not for vacuole inheritance strongly supports the hypothesis that Vac8p has at least two distinct functions, vacuole segregation and the Cvt pathway (Wang et al., 1996).
Deletion Analysis of Vac8p
To identify other portions of Vac8p required for its function, deletions of VAC8 were constructed and the biological consequences assessed. A summary of the results is shown in Fig. 8. Deletion of the COOH-terminal tail 98 residues (vac8-5) significantly reduced the steady state level of its gene products; however, >90% of the cells showed normal vacuole inheritance and API transport was only slightly affected. Thus, low levels of Vac8p suffice and the truncated protein is still functional. Among the Arm repeats, those proximal to the COOH terminus are relatively unimportant, as deletions of a single Arm repeat alone (vac8-8, vac8-9 and vac8-10) had no apparent effect on protein stability or function. When the COOH-terminal tail and either the last Arm repeat or multiple Arm repeats were deleted (vac8-6 and vac8-7), much less protein was produced and normal vacuole inheritance and API transport were completely abolished. A vac8 allele in which the first three Arm repeats was deleted (vac8-11) was expressed at levels similar to wild-type and localized on the vacuolar membrane (Fig. 8 and data not shown), however, vacuole inheritance and API transport were defective. Interestingly, unlike other vac8 alleles, a significant number of cells (∼50%) in vac8-11 showed wild-type vacuolar morphology where the vacuoles contained one to six lobes (data not shown). Thus the vac8-11 protein appears to retain some function. Together, the deletion analyses indicate that the COOH terminus and the proximal Arm repeats play a role in maintaining Vac8p structural integrity, and that Vac8p function at least partially resides in the NH2-terminal repeats.
Vac8p Cosediments with Actin Filaments
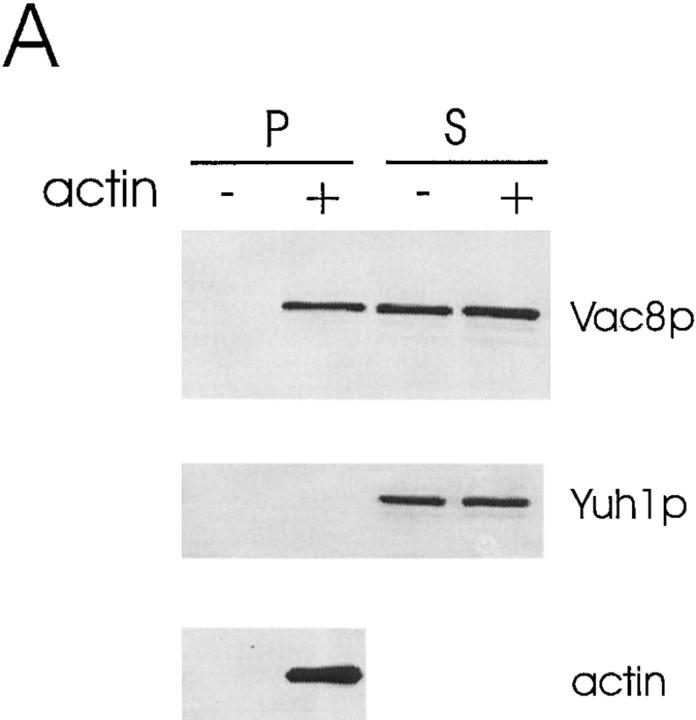
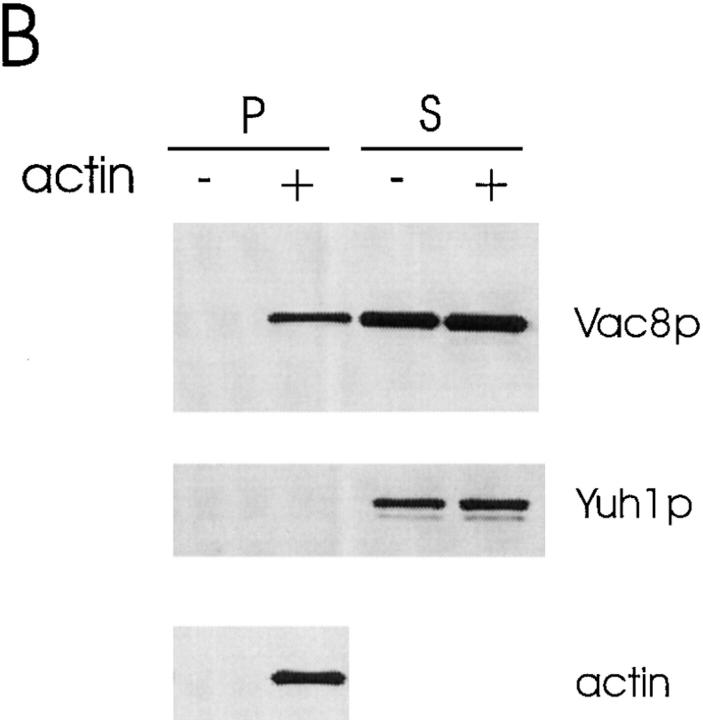
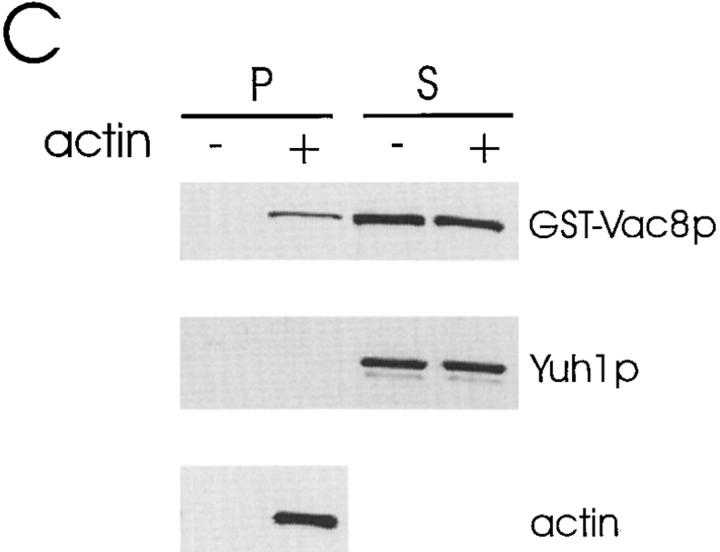
Actin is required for vacuole segregation (Hill et al., 1996). Moreover, Vac8p shares significant homology with β-catenin/plakoglobin and vac8 is defective in early vacuole migration. Therefore, we tested whether Vac8p interacts with actin in vitro. When purified yeast monomeric actin (G-actin) was added to the Triton X-100 solubilized, high speed total cell extracts from wild-type cells under conditions favorable to actin filament (F-actin) formation, G-actin polymerized to form F-actin. In a control experiment we found that when G-actin is added to the same reaction buffer, within 4 min 90% of the G-actin is incorporated into actin filaments. Strikingly, a portion of Vac8p cosedimented with this F-actin (Fig. 9 A). No Vac8p sedimented when exogenous actin was absent. Furthermore, as a control, the cytoplasmic protein yeast ubiquitin–protein hydrolase (Yuh1p; Miller et al., 1989) did not sediment in the presence or absence of actin, suggesting that the Vac8p was specifically associating with the actin filaments and not just nonspecifically being trapped. When the experiments were performed using cytosol from the myristoylation-minus mutant or Δvac8, the soluble, nonacylated Vac8p or recombinant GST–Vac8p also cosedimented with actin (Fig. 9, B and C). These results establish an interaction between Vac8p and actin in vitro. We were unable to test whether Vac8p directly interacts with actin since GST–Vac8p was rapidly degraded under conditions where no cytosol was added. It was also not possible to perform these experiments using only the endogenous actin because after cell breakage, the concentration of actin was too low and the F-actin rapidly depolymerized.
Figure 9.
Vac8p cosediments with actin filaments. 20 μl (2 mg/ml final concentration) of a high speed, Triton X-100 solubilized total cell extract (A) or cytosol from the myristoylation-minus mutant vac8-2 (B), or cytosol from Δvac8 (C) was mixed with 20 μl fresh G-actin (0.4 mg/ml final concentration) and G-buffer in a 100 μl reaction. G-buffer without actin was added to adjust the volume in the controls. For the experiments using Δvac8 cytosol, 10 μl purified GST-Vac8 protein (26 μg/ml final concentration) was added. The mixtures were incubated for 1 h on ice and then the polymerized actin was pelleted. 5 μl of the supernatant fraction (200 μl final) and the washed pellet fraction (50 μl final) were separated by SDS-PAGE and transferred to nitrocellulose membranes. The same membrane was cut into two halves. The top half was probed with Vac8p antiserum and the bottom half was probed with the antiserum to Yuh1p. In addition, 0.1 μl of the pellet fraction was run separately and used to detect actin.
Discussion
Vac8p May Mediate Vacuole Membrane–Actin Interactions
Analyses of vac8 mutants suggest that Vac8p functions in an early step of vacuole partitioning, i.e., vacuole migration or segregation structure formation/transport. Identification and characterization of VAC8 revealed that Vac8p is a 64-kD protein with 11 contiguous Arm repeats flanked by a conserved acylation motif at the NH2 terminus and a 98-residue Asn-rich COOH-terminal tail. Arm repeats have been found in a diverse set of proteins and are thought to mediate protein–protein interactions (Peifer et al., 1994). Identification of Vac8p as an Arm repeat protein suggests at least two possible models for Vac8p function in early vacuole inheritance. First, Vac8p could function in recruiting components to the vacuolar membrane necessary for formation of the segregation structure. In this regard, it is noteworthy that the β-adaptin subunit of the clathrin-associated coat protein complex, a protein involved in vesicle formation, is an Arm repeat protein (Küssel and Frasch, 1995). However, a more intriguing possibility is that Vac8p might interact with the actin cytoskeleton. Our observations that actin, actin-binding proteins, and Myo2p are required for vacuole inheritance suggest that specific components must link the vacuole to the actin cytoskeleton (Hill et al., 1996). As reported here, vac8 is defective in early vacuole migration, a phenotype consistent with a defect in linking the vacuole to the actin cytoskeleton. In fact, myo2-2, which has a severe vac defect, has a similar defect in early vacuole migration (Catlett, N.L., and L.S. Weisman, manuscript in preparation). Most importantly, we find that Vac8p is able to interact with actin filaments in vitro. Further support for this role is that, among all the Arm repeat proteins, Vac8p is most closely related to β-catenin/plakoglobin. β-catenin and plakoglobin link the plasma membrane to the actin filaments at the adherens junction (reviewed in Cowin and Burke, 1996). In analogy, Vac8p may link the vacuole to the actin cytoskeleton, leading first to vacuole migration to the incipient bud site and subsequently to movement of the segregation structure. It remains unclear whether the interaction of Vac8p with actin is direct. The interaction of β-catenin/ plakoglobin with actin can be mediated by α-catenin and α-actinin (reviewed in Cowin and Burke, 1996), or mediated by fascin (Tao et al., 1996). Thus, it is likely that the interaction of Vac8p with actin is mediated through other protein(s). There are no obvious homologues of α-catenin, α-actinin, or fascin in yeast. But, based on the considerable sequence divergence between Vac8p and β-catenin/plakoglobin, it is not surprising that distinct proteins are involved. Experiments designed to test whether Vac8p interacts with actin directly or via other proteins are currently in progress.
Localization, Acylation, and Function of Vac8p
Experiments using immunofluorescence or purified vacuoles demonstrated that Vac8p is bound to the vacuolar membrane. Previously, we had proposed that for any recessive vac mutant, one could predict whether the corresponding wild-type gene product is cytoplasmic or bound in a large complex by assaying vacuole inheritance in a heterozygous zygote (Weisman et al., 1990, Wang et al., 1996) The failure of vac8-1 to be corrected in a heterozygous zygote (Wang et al., 1996) is consistent with its location on the vacuolar membrane.
Vac8p is the second protein in yeast that has been shown to be both myristoylated and palmitoylated, the first was the G protein α-subunit Gpa1p (Song and Dohlman, 1997). Our mutagenesis studies are consistent with the acylation of Vac8p occurring in the conserved NH2-terminal acylation motif. Moreover, in the myristoylation-minus vac8 mutant, Vac8p is still palmitoylated although the level of palmitoylation is greatly reduced. Perhaps the unmyristoylated Vac8p is less accessible to the membrane-bound palmitoyltransferase. Alternatively, since palmitoylation is potentially a reversible process (Milligan et al., 1995), the unmyristoylated Vac8p might have a high rate of depalmitoylation, as was seen in the mammalian G protein αi subunit (Degtyarev et al., 1994). There is controversy over whether palmitoylation is a myristoylation- dependent event (Hallak et al., 1994; Mumby et al., 1994; Resh, 1994). Our results provide new evidence that palmitoylation can occur independent of myristoylation (Degtyarev et al., 1994; Veit et al., 1994; Wilson and Bourne, 1995; Schroeder et al., 1996).
Both myristoylation and palmitoylation are required for efficient vacuolar membrane association of Vac8p. In the palmitoylation-minus vac8 mutant, most of the Vac8p is cytosolic. This agrees with in vitro biophysical studies (Peitzsch and McLaughlin, 1993) that demonstrate that N-myristoyl moieties are not sufficient to foster a stable membrane association. Although palmitoylation confers substantial membrane affinity, Vac8p is also mislocalized in the myristoylation-minus mutant. This probably occurs due to the greatly reduced level of palmitoylation in the myristoylation-minus mutant.
We explored the functional significance of Vac8p acylation. Unexpectedly, vacuole inheritance appears to be normal in the myristoylation-minus mutant. Studies with zygotes indicate that vacuole inheritance in the myristoylation-minus mutant is directed by the small amount of vacuolar membrane-bound Vac8p, not the cytosolic population of Vac8p. We suspect that this unmyristoylated, active Vac8p pool is palmitoylated since palmitoylation is essential. There are at least two ways to explain the activity of nonmyristoylated Vac8p. First, the level of palmitoylated Vac8p may be higher than what is measured if this modification is relatively transient in the absence of myristoylation. Second, vacuole inheritance is unaffected when levels of Vac8p are reduced 5–10-fold. Thus, the small amount of palmitoylated Vac8p may be sufficient for full activity. Both VAC8 with an HA-tag at the COOH terminus and a COOH-terminal tail deleted VAC8 function normally in vacuole inheritance. In these constructs, the level of Vac8p is reduced 5–10-fold from wild-type. Because the myristoylation-minus mutant is active in vacuole inheritance, the significance of Vac8p myristoylation is obscure.
In contrast to the myristoylation-minus mutant, the palmitoylation-minus mutant is completely defective in vacuole inheritance. The inheritance defect is not due to the low level of Vac8p on the vacuolar membrane because an almost identical amount of Vac8p is also present on the vacuolar membrane in the myristoylation-minus mutant. Three cysteines were mutated to create the palmitoylation-minus form of Vac8p. It is possible that one or two of these cysteine residues have other functions, for example, involvement in the formation of a protein complex necessary for vacuole inheritance. However, a direct role for palmitoylation in vacuole inheritance is appealing. First, in vitro studies showed that acyl-CoA stimulates vesicle budding and fusion from Golgi cisternae (Pfanner et al., 1989, 1990). It has been suggested that acyl-CoA stimulates Golgi trafficking via protein palmitoylation. Second, yeast vacuole fusion in vitro is stimulated by acyl-CoA (Haas and Wickner, 1996). Moreover, several proteins involved in vesicle fusion are palmitoylated in vivo (Hess et al., 1992; Couve et al., 1995). The palmitoyl group may allow Vac8p to associate with the vacuolar membrane in a defined, productive configuration. Furthermore, the palmitoyl group may also promote interactions of Vac8p with other proteins. It has been shown that palmitoylation of a cysteine pair in the cytoplasmic domain of transforming growth factor-α, a transmembrane protein, mediates an interaction with the cytoplasmic protein p86 (Shum et al., 1996). Finally, the palmitoyl group of Vac8p may modify the vacuolar membrane lipid composition in order to make it suitable for segregation structure formation.
Distinct Roles for Vac8p in Vacuole Inheritance and Protein Targeting
In addition to its defect in vacuole inheritance, vac8 is defective in the Cvt pathway. Analysis of the phenotype of the palmitoylation-minus mutant, coupled with the normal vacuole inheritance in other cvt mutants, strongly suggests that the vacuole inheritance defect is not a secondary defect caused by blockage of the Cvt pathway. Likewise, the vacuole inheritance defects and the Cvt pathway defects are unlikely due to the fragmented vacuole morphology of vac8. 50% of cells of a vac8 allele with the first three Arm repeats deleted have normal vacuolar morphology but are completely defective in vacuole inheritance and in the Cvt pathway. The palmitoylation-minus mutant has a vacuolar morphology identical to Δvac8 but a normal Cvt pathway. These results suggest that Vac8p directly participates in both vacuole inheritance and the Cvt pathway. API is known to transit from the cytoplasm to the vacuole by first forming an oligomer (Kim et al., 1997), and then becoming incorporated into double-membraned vesicles. The vesicles then move to the vacuole and cross the vacuole membrane, resulting in single membrane vesicles within the vacuole that still contain API (Scott et al., 1997). There is significant genetic overlap between the Cvt pathway and autophagy (Harding et al., 1996; Scott et al., 1996). However, Δvac8 is not defective in autophagy. This suggests at least two possibilities. Perhaps API-containing vesicles are not identical with autophagic vesicles, and Vac8p is required for vacuolar uptake of only the API vesicles. Or perhaps Vac8p functions early in the packaging of API into vesicles. Thus, the location of Vac8p active in API transport may be in the cytosol or on the small vesicles. Although we detect most of the Vac8p on the vacuole membrane, we cannot rule out this second activity taking place elsewhere in the cell. Interestingly, lack of palmitoylation had no affect on Vac8p activity in API import.
Like Vac8p, proteins with two functions have been observed in other protein/membrane trafficking systems. First, Sec63p is involved in both nuclear fusion and protein translocation into the ER; Sec63p plays direct roles in each process (Brodsky and Schekman, 1993; Scidmore et al., 1993; Ng and Walter, 1996). Second, yeast Srp1p, an Arm repeat protein, is required for both protein import into the nucleus and nuclear membrane segregation (Yano et al., 1994). Third, the class D VPS gene products are required for both protein sorting from the Golgi to vacuole and vacuole inheritance (Raymond et al., 1992; Weisman and Wickner, 1992). It is likely that some of the VPS gene products play direct roles in vacuole inheritance.
One way that Vac8p may function in both vacuole inheritance and the Cvt pathway is by engaging in two different protein complexes. Glycerol gradient centrifugation experiments reveal Vac8p in a large protein complex on the vacuole membrane (Wang, Y.-X., and L.S. Weisman, unpublished data). The complex may form through protein–protein interactions with the Vac8p Arm repeats. The Arm repeats of β-catenin interact with numerous proteins to perform dual roles in cell–cell adhesion and in a developmental patterning pathway. Each partner binds to a specific subset of these repeats, yet a number of these repeats are required for both pathways (see reviews Adams, 1997; Nusse, 1997). By deletion analysis, we have found that the first three Arm repeats of Vac8p are important for both vacuole inheritance and the Cvt pathway. Possibly, the first three Arm repeats are part of the region where Vac8p interacts with different proteins. In addition, palmitoylation–depalmitoylation may regulate or determine the different functions of Vac8p.
Acknowledgments
We thank Drs. Daniel Klionsky and Robert Cohen for providing API serum and Yuh1 serum, respectively. We thank Kuo-Kuang Wen, Dongmei Cheng, and Vicci Korman for providing purified actin and helpful discussions for the cosedimentation experiments. We thank Tom Monninger and the University of Iowa Central Microscopy Research Facility for guidance in use of the confocal microscope. We gratefully acknowledge the excellent technical assistance of Emily J. Bristow and Michelle Muller. We thank Cecilia Bonangelino and Drs. Robert Cohen, Robert Deschenes, and Peter Rubenstein for valuable discussions and comments on the manuscript.
Abbreviations used in this paper
- API
aminopeptidase I
- Arm
armadillo
- CPY
carboxypeptidase Y
- Cvt
cytoplasm to vacuole targeting
- Vac
vacuole segregation
- WT
wild-type
Footnotes
L.S. Weisman is a Carver Research Scientist. This work was supported by a gift to L.S. Weisman from the Roy J. Carver Charitable Trust, and National Institutes of Health grant GM50403 to L.S. Weisman.
References
- Adams J. Cell adhesion-spreading frontiers, intricate insights. Trends Cell Biol. 1997;7:107–110. doi: 10.1016/S0962-8924(97)01001-5. [DOI] [PubMed] [Google Scholar]
- Berkower C, Loayza D, Michaelis S. Metabolic instability and constitutive endocytosis of STE6, the a-factor transporter of Saccharomyces cerevisiae. . Mol Biol Cell. 1994;5:1185–1198. doi: 10.1091/mbc.5.11.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonangelino CJ, Catlett NL, Weisman LS. Vac7p, a novel vacuolar protein, is required for normal vacuole inheritance and morphology. Mol Cell Biol. 1997;17:6847–6858. doi: 10.1128/mcb.17.12.6847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky JL, Schekman R. A Sec63p-Bip complex from yeast is required for protein translocation in a reconstituted proteoliposome. J Cell Biol. 1993;123:1355–1363. doi: 10.1083/jcb.123.6.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey P. Protein lipidation in cell signaling. Science. 1995;268:221–225. doi: 10.1126/science.7716512. [DOI] [PubMed] [Google Scholar]
- Couve A, Protopopov V, Gerst JE. Yeast synaptobrevin homologs are modified posttranslationally by the addition of palmitate. Proc Natl Acad Sci USA. 1995;92:5987–5991. doi: 10.1073/pnas.92.13.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowin P, Burke B. Cytoskeleton-membrane interactions. Curr Opin Cell Biol. 1996;8:56–65. doi: 10.1016/s0955-0674(96)80049-4. [DOI] [PubMed] [Google Scholar]
- Degtyarev MY, Spiegel AM, Jones TLZ. Palmitoylation of a G protein αisubunit requires membrane localization not myristoylation. J Biol Chem. 1994;269:30898–30903. [PubMed] [Google Scholar]
- Deschenes R, Broach J. Fatty acylation is important but not essential for Saccharomyces cerevisiaeRAS function. Mol Cell Biol. 1987;7:2344–2351. doi: 10.1128/mcb.7.7.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke WW, Goldschmidt MD, Zimblemann R, Mueller HM, Schiller DL, Cowin P. Molecular cloning and amino acid sequence of human plakoglobin, the common junctional plaque protein. Proc Natl Acad Sci USA. 1989;86:4027–4031. doi: 10.1073/pnas.86.11.4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes de Mesquita DS, ten Hoopen R, Woldringh CL. Vacuolar segregation to the bud of S. cerevisiae: An analysis of the morphology and timing in the cell cycle. J Gen Micro. 1991;137:2447–2454. doi: 10.1099/00221287-137-10-2447. [DOI] [PubMed] [Google Scholar]
- Gomes de Mesquita DS, van den Haazel B, Bouwman J, Woldringh CL. Characterization of new vacuolar segregation mutants, isolated by screening for loss of proteinase B self-activation. Eur J Cell Biol. 1996;71:237–247. [PubMed] [Google Scholar]
- Guan KL, Dixon JE. Eukaryotic proteins expressed in Escherichia coli:An improved thrombin cleavage and purification procedure of fusion proteins with glutathione S-transferase. Anal Biochem. 1991;192:262–267. doi: 10.1016/0003-2697(91)90534-z. [DOI] [PubMed] [Google Scholar]
- Haas A. A quantitative assay to measure homotypic vacuole fusion in vitro. Methods Cell Sci. 1996;17:238–294. [Google Scholar]
- Haas A, Wickner W. Homotypic vacuole fusion requires Sec17p (yeast α-SNAP) and Sec18p (yeast NSF) EMBO (Eur Mol Biol Organ) J. 1996;15:3296–3305. [PMC free article] [PubMed] [Google Scholar]
- Haas A, Scheglmann D, Lazar T, Gallwitz D, Wickner W. The GTPase of Saccharomyces cerevisiaeis required on both partner vacuoles for late-stage homotypic membrane-fusion during vacuole inheritance. EMBO (Eur Mol Biol Organ) J. 1995;14:5258–5270. doi: 10.1002/j.1460-2075.1995.tb00210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallak H, Brass LF, Manning DR. Failure to myristoylate the α subunit of Gz is correlated with an inhibition of palmitoylation and membrane attachment, but has no affect on phosphorylation by protein kinase C. J Biol Chem. 1994;269:4571–4576. [PubMed] [Google Scholar]
- Harding TM, Morano KA, Scott SV, Klionsky DJ. Isolation and characterization of yeast mutants in the cytoplasm to vacuole targeting pathway. J Cell Biol. 1995;131:591–602. doi: 10.1083/jcb.131.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding TM, Hefner-Gravink A, Thumm M, Klionsky DJ. Genetic and phenotypic overlap between autophagy and the cytoplasm to vacuole protein targeting pathway. J Biol Chem. 1996;271:17621–17624. doi: 10.1074/jbc.271.30.17621. [DOI] [PubMed] [Google Scholar]
- Herman PK, Stack JH, DeModena JA, Emr SD. A genetic and structural analysis of the yeast Vps15 protein kinase: evidence for a role of Vps15 in vacuolar protein delivery. Cell. 1991;64:425–437. doi: 10.1002/j.1460-2075.1991.tb04981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess DT, Slater TM, Wilson MC, Skeene JHP. The 25kDa synaptosomal-associated protein SNAP-25 is the major methionine-rich polypeptide in rapid axonal transport and a major substrate for palmitoylation in adult CNS. J Neurosci. 1992;12:4634–4641. doi: 10.1523/JNEUROSCI.12-12-04634.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill KL, Catlett NL, Weisman LS. Actin and myosin function in directed vacuole movement during yeast cell division in Saccharomyces cerevisiae. . J Cell Biol. 1996;135:1535–1549. doi: 10.1083/jcb.135.6.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser, C., S. Michaelis, and A. Mitchell. 1994. Methods in Yeast Genetics. Cold Spring Harbor Laboratory Press.
- Kim J, Scott SV, Oda MN, Klionsky DJ. Transport of a large oligomeric protein by the cytoplasm to vacuole protein targeting pathway. J Cell Biol. 1997;137:609–618. doi: 10.1083/jcb.137.3.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klionsky DJ, Cueva R, Yaver DS. Aminopeptidase I of Saccharomyces cerevisiaeis localized to the vacuole independent of the secretory pathway. J Cell Biol. 1992;119:287–299. doi: 10.1083/jcb.119.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Küssel P, Frasch M. Pendulin, a Drosophilaprotein with cell cycle-dependent nuclear localization, is required for normal cell proliferation. J Cell Biol. 1995;129:1491–1507. doi: 10.1083/jcb.129.6.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lew DJ, Reed SI. Cell cycle control of morphogenesis in budding yeast. Curr Opin Genet Dev. 1995;5:17–23. doi: 10.1016/s0959-437x(95)90048-9. [DOI] [PubMed] [Google Scholar]
- Mayer A, Wickner W. Docking of yeast vacuoles is catalyzed by the Ras-like GTPase Ypt7p after symmetric priming by Sec18p (NSF) J Cell Biol. 1997;136:307–317. doi: 10.1083/jcb.136.2.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer A, Wickner W, Haas A. Sec18p (NSF)-driven release of Sec17p (α−SNAP) can precede docking and fusion of yeast vacuoles. Cell. 1996;85:83–94. doi: 10.1016/s0092-8674(00)81084-3. [DOI] [PubMed] [Google Scholar]
- McCrea P, Turck C, Gumbiner B. A homologue of the armadillo protein in Drosophila(plakoglobin) associated with E-cadherin. Science. 1991;254:1359–1361. doi: 10.1126/science.1962194. [DOI] [PubMed] [Google Scholar]
- Miller HI, Henzel WJ, Ridgway JB, Kuang W-J, Chisholm V, Liu C-C. Cloning and expression of a yeast ubiquitin-protein cleaving activity in Escherichia Coli. . Biotechnology. 1989;7:698–704. [Google Scholar]
- Milligan G, Parenti M, Magee AI. The dynamic role of palmitoylation in signal transduction. Trends Biochem Sci. 1995;20:181–186. doi: 10.1016/s0968-0004(00)89004-0. [DOI] [PubMed] [Google Scholar]
- Mitchell DA, Farh L, Marshall T, Deschenes RJ. A polybasic domain allows nonprenylated Ras proteins to function in Saccharomyces cerevisiae. . J Biol Chem. 1994;269:21540–21546. [PubMed] [Google Scholar]
- Mumby SM, Kleuss C, Gilman AG. Receptor regulation of G-protein palmitoylation. Proc Natl Acad of Sci USA. 1994;91:2800–2804. doi: 10.1073/pnas.91.7.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng DT W, Walter P. ER membrane protein complex required for nuclear fusion. J Cell Biol. 1996;132:499–509. doi: 10.1083/jcb.132.4.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols B, Ungermann C, Pelham HRB, Wickner W, Haas A. Homotypic vacuolar fusion mediated by t- and v-SNAREs. Nature. 1997;387:199–202. doi: 10.1038/387199a0. [DOI] [PubMed] [Google Scholar]
- Nusse R. A versatile transcriptional effector of wingless signaling. Cell. 1997;89:321–323. doi: 10.1016/s0092-8674(00)80210-x. [DOI] [PubMed] [Google Scholar]
- Omary MB, Trowbridge IS. Covalent binding of fatty acid to the transferrin receptor in cultured human cells. J Biol Chem. 1981;256:4715–4718. [PubMed] [Google Scholar]
- Peifer M, Berg S, Reynolds AB. A repeating amino acid motif shared by proteins with diverse cellular roles. Cell. 1994;76:789–791. doi: 10.1016/0092-8674(94)90353-0. [DOI] [PubMed] [Google Scholar]
- Peitzsch RM, McLaughlin S. Binding of acylated peptides and fatty acids to phospholipids: pertinence to myristoylated proteins. Biochemistry. 1993;32:10436–10443. doi: 10.1021/bi00090a020. [DOI] [PubMed] [Google Scholar]
- Pfanner N, Oric L, Glick BS, Amherdt M, Arden SR, Malhotra V, Rothman JE. Fatty acyl-coenzyme A is required for budding of transport vesicles from Golgi cisternae. Cell. 1989;59:95–102. doi: 10.1016/0092-8674(89)90872-6. [DOI] [PubMed] [Google Scholar]
- Pfanner N, Glick BS, Amherdt M, Arden SR, Rothman JE. Fatty acylation promotes fusion of transport vesicles with Golgi cisternae. J Cell Biol. 1990;110:955–965. doi: 10.1083/jcb.110.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss D, Mulholland J, Franzusoff A, Segev N, Botstein D. Characterization of the SaccharomycesGolgi complex through the cell cycle by immunoelectron microscopy. Mol Biol Cell. 1992;3:789–803. doi: 10.1091/mbc.3.7.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond CK, Howald-Stevenson I, Vater CA, Stevens TH. Morphological classification of the yeast vacuolar protein sorting mutants: evidence for a prevacuolar compartment in class E vpsmutants. Mol Biol Cell. 1992;3:1389–1402. doi: 10.1091/mbc.3.12.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resh MD. Myristoylation and palmitoylation of Src family members: the fats of the matter. Cell. 1994;76:411–413. doi: 10.1016/0092-8674(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Riggleman B, Wieschaus E, Schedl P. Molecular analysis of the Armadillo locus: uniformly distributed transcripts and a protein with novel internal repeats are associated with the Drosophila segment polarity gene. Genes Dev. 1989;3:96–113. doi: 10.1101/gad.3.1.96. [DOI] [PubMed] [Google Scholar]
- Rose MD, Novick P, Thomas JH, Botstein D, Fink GR. A Saccharomyces cerevisiaegenomic plasmid bank based on a centromere-containing shuttle vector. Gene. 1987;60:237–243. doi: 10.1016/0378-1119(87)90232-0. [DOI] [PubMed] [Google Scholar]
- Sambrook, J., E.F. Fritsch, and T. Maniatis. 1989. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- Schroeder H, Leventis R, Shahinian S, Walton PA, Silvius JR. Lipid-modified, cysteinyl-containing peptides of diverse structures are efficiently S-acylated at the plasma membrane of mammalian cells. J Cell Biol. 1996;134:647–660. doi: 10.1083/jcb.134.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scidmore MA, Okamura HH, Rose MD. Genetic interactions between KAR2 and SEC63, encoding eukaryotic homologues of DnaK and DnaJ in the endoplamic reticulum. Mol Biol Cell. 1993;4:1145–1159. doi: 10.1091/mbc.4.11.1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SV, Hefner-Gravink A, Morano KA, Noda T, Ohsumi Y, Klionsky DJ. Cytoplasm-to-vacuole targeting and autophagy employ the same machinery to deliver proteins to the yeast vacuole. Proc Natl Acad Sci USA. 1996;93:12304–12308. doi: 10.1073/pnas.93.22.12304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott SV, Baba M, Ohsumi Y, Klionsky DJ. Aminopeptidase I is targeted to the vacuole by a nonclassical vesicular mechanism. J Cell Biol. 1997;138:37–44. doi: 10.1083/jcb.138.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw JM, Wickner W. vac2: a yeast mutant which distinguishes vacuole segregation from Golgi-to-vacuole protein targeting. EMBO (Eur Mol Biol Organ) J. 1991;10:1741–1748. doi: 10.1002/j.1460-2075.1991.tb07698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shum L, Turck CW, Derynck R. Cysteines 153 and 154 of transmembrane transforming growth factor-α are palmitoylated and mediate cytoplasmic protein association. J Biol Chem. 1996;271:28502–28508. doi: 10.1074/jbc.271.45.28502. [DOI] [PubMed] [Google Scholar]
- Simon VR, Karmon SL, Pon LA. Mitochondrial inheritance: cell cycle and actin cable dependence of polarized mitochondrial movements in Saccharomyces cerevisiae. . Cell Motil Cytoskel. 1997;37:199–210. doi: 10.1002/(SICI)1097-0169(1997)37:3<199::AID-CM2>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Song J, Dohlman HG. Partial constitutive activation of pheromone receptors by a palmitoylation-site mutant of a G protein alpha subunit in yeast. Biochemistry. 1996;35:14806–14817. doi: 10.1021/bi961846b. [DOI] [PubMed] [Google Scholar]
- Tao YS, Edwards RA, Tubb B, Wang S, Bryan J, McCrea PD. β-catenin associates with the actin-bundling protein fascin in a noncadherin complex. J Cell Biol. 1996;134:1271–1281. doi: 10.1083/jcb.134.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TerBush DR, Novick P. Sec6, Sec8, and Sec15 are components of a multisubunit complex which localizes to small bud tips in Saccharomyces cerevisiae. . J Cell Biol. 1995;130:299–312. doi: 10.1083/jcb.130.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veit M, Nurnberg B, Spicher K, Harteneck C, Ponimaskin E, Schultz G, Schmidt MFG. The α-subunits of G protein G12 and G13are palmitoylated, but not amidically myristoylated. FEBS Lett. 1994;339:160–164. doi: 10.1016/0014-5793(94)80406-0. [DOI] [PubMed] [Google Scholar]
- Wang Y-X, Zhao H, Harding T, Gomes de Mesquita D, Woldringh CL, Klionsky D, Munn AL, Weisman LS. Multiple classes of yeast mutants are defective in vacuole partitioning yet target vacuole proteins correctly. Mol Biol Cell. 1996;7:1375–1389. doi: 10.1091/mbc.7.9.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G, Wickner W. Organelle Inheritance. Cell. 1996;84:395–400. doi: 10.1016/s0092-8674(00)81284-2. [DOI] [PubMed] [Google Scholar]
- Weisman LS, Wickner W. Intervacuole exchange in the yeast zygote: a new pathway in organelle communication. Science. 1988;241:589–591. doi: 10.1126/science.3041591. [DOI] [PubMed] [Google Scholar]
- Weisman LS, Wickner W. Molecular characterization of VAC1, a gene required for vacuole inheritance and vacuole protein sorting. J Biol Chem. 1992;267:618–623. [PubMed] [Google Scholar]
- Weisman LS, Emr SD, Wickner W. Mutants of S. cerevisiaethat block intervacuole vesicular traffic and vacuole division and segregation. Proc Natl Acad of Sci USA. 1990;87:1076–1080. doi: 10.1073/pnas.87.3.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson PT, Bourne HR. Fatty acylation of αz. Effects of palmitoylation and myristoylation on αzsignaling. J Biol Chem. 1995;270:9667–9675. doi: 10.1074/jbc.270.16.9667. [DOI] [PubMed] [Google Scholar]
- Xu Z, Wickner W. Thioredoxin is required for vacuole inheritance in Saccharomyces cerevisiae. . J Cell Biol. 1996;132:787–794. doi: 10.1083/jcb.132.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Z, Mayer A, Muller E, Wickner W. A heterodimer of thioredoxin and IB 2 cooperates with Sec18p (NSF) to promote yeast vacuole inheritance. J Cell Biol. 1997;136:299–306. doi: 10.1083/jcb.136.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano R, Oakes ML, Tabb MM, Nomura M. Yeast Srp1p has homology to armadillo/plakoglobin/β-catenin and participates in apparently multiple nuclear functions including the maintenance of the nucleolar structure. Proc Natl Acad Sci USA. 1994;91:6880–6884. doi: 10.1073/pnas.91.15.6880. [DOI] [PMC free article] [PubMed] [Google Scholar]