Abstract
Muscle cells are frequently subjected to severe conditions caused by heat, oxidative, and mechanical stresses. The small heat shock proteins (sHSPs) such as αB-crystallin and HSP27, which are highly expressed in muscle cells, have been suggested to play roles in maintaining myofibrillar integrity against such stresses. Here, we identified a novel member of the sHSP family that associates specifically with myotonic dystrophy protein kinase (DMPK). This DMPK-binding protein, MKBP, shows a unique nature compared with other known sHSPs: (a) In muscle cytosol, MKBP exists as an oligomeric complex separate from the complex formed by αB-crystallin and HSP27. (b) The expression of MKBP is not induced by heat shock, although it shows the characteristic early response of redistribution to the insoluble fraction like other sHSPs. Immunohistochemical analysis of skeletal muscle cells shows that MKBP localizes to the cross sections of individual myofibrils at the Z-membrane as well as the neuromuscular junction, where DMPK has been suggested to be concentrated. In vitro, MKBP enhances the kinase activity of DMPK and protects it from heat-induced inactivation. These results suggest that MKBP constitutes a novel stress-responsive system independent of other known sHSPs in muscle cells and that DMPK may be involved in this system by being activated by MKBP. Importantly, since the amount of MKBP protein, but not that of other sHSP family member proteins, is selectively upregulated in skeletal muscle from DM patients, an interaction between DMPK and MKBP may be involved in the pathogenesis of DM.
Myotonic dystrophy (DM)1 is an autosomal dominantly inherited disease characterized primarily by myotonia and progressive muscle weakness, although it is also associated with a range of other abnormalities, including cardiac conduction defects, cataracts, testicular and ovarian atrophy, diabetes, and mental dysfunction (Harper, 1989). In 1992, positional cloning studies identified the responsible mutation as CTG repeat expansions in a gene encoding a putative serine/threonine kinase (DMPK) (Brook et al., 1992; Fu et al., 1992; Mahadevan et al., 1992). However, unlike the case of Duchennne muscular dystrophy, this finding did not lead directly to accelerated progress in the study of the underlying molecular mechanism of the disease because the mutation is located in the 3′-untranslated region of the gene, and thus DMPK, if expressed, should work normally in DM patients. If one assumes that a change in the function of DMPK is involved in the pathogenesis of DM, then a possible mechanism is that the CTG repeat expansions affect the expression level of DMPK by impairing a certain step in gene expression such as transcription, mRNA processing, or translation (Taneja et al., 1995). In fact, numerous studies to quantify the levels of DMPK mRNA or protein in the muscles of affected patients have been undertaken to validate this possibility. Although there are some conflicting results (Sabouri et al., 1993; Bhagwati et al., 1996), most studies have reported decreases in the level of DMPK mRNA and protein, suggesting the involvement of DMPK levels in the pathogenesis of DM (Fu et al., 1993; Maeda et al., 1995; Wang et al., 1995). Very recently, two papers clearly demonstrated that CUG expansion results in the nuclear retention of DMPK transcripts, which probably leads to a reduction in DMPK protein (Davis et al., 1997; Hamshere et al., 1997).
The demonstration that neither DMPK knockout nor transgenic mice show the profound phenotypes seen in DM patients suggests that the underlying molecular mechanism of DM is not simply a lack of or excess amounts of the DMPK protein (Jansen et al., 1996; Reddy et al., 1996). However, it is important that mice lacking DMPK demonstrate late onset myopathy with weakness and some abnormalities in the skeletal muscle fibers (Jansen et al., 1996; Reddy et al., 1996), whereas mice that overexpress DMPK develop cardiomyopathy (Jansen et al., 1996). These results suggest that the DMPK protein plays an essential role in maintaining muscle structure and function and support the possibility that changes in DMPK levels contribute to the pathology of DM. These results raise the question of how a lack or excess of DMPK protein causes muscle disorder.
Heat shock proteins (HSPs), whose synthesis is induced by heat or other stresses, are divided into several groups on the basis of their molecular masses (Linquist and Craig, 1988). HSPs with low molecular masses of 15–30 kD, observed in most species, have been shown to form large aggregates in the cytosol and are called small heat shock proteins (sHSP) (Caspers and Bhat, 1995). So far, four mammalian sHSPs, HSP27,2 αA- and αB-crystallin, and p20, have been identified (Hickey and Weber, 1982; Ingolia and Craig, 1982; Klemenz et al., 1991; Head et al., 1994; Kato et al., 1994), and some have been shown to work as molecular chaperones (Jakob et al., 1993 ; Rao et al., 1994) and confer thermotolerance when overexpressed in mammalian cells (Lavoie et al., 1993a ,b, 1995; Iwaki et al., 1994; Mehlen et al., 1995). Recent results on HSP27 and αB-crystallin suggest that sHSPs confer stress resistance by stabilizing the actin cytoskeleton (Lavoie et al., 1993b , 1995; Iwaki et al., 1994). Interestingly, three of these proteins, HSP27, αB-crystallin, and p20, are highly expressed in muscle cells and together form large oligomeric complexes (Kato et al., 1994). These results suggest that sHSPs may play important roles in the stress resistance of muscle cells, which are often subjected to severe conditions.
In this study, we searched for DMPK-binding proteins in a human skeletal muscle cDNA library with the expectation that such binding proteins would provide clues to the physiological function of DMPK and thus suggest a molecular basis for the pathogenesis of DM. Here, we report that a novel member of the small heat shock protein family, designated MKBP (myotonic dystrophy protein kinase binding protein), specifically associates with DMPK. It activates DMPK kinase activity in vitro and protects it from heat-induced inactivation. Importantly, the expression of MKBP in skeletal muscles from DM patients is selectively upregulated, suggesting that a protein interaction between DMPK and MKBP is involved in the pathogenesis of DM. Based on the results presented here, we propose that DMPK is involved in the stress-responsive system in muscle cells.
Materials and Methods
cDNA Cloning
A 2.0-kb EcoRI-BamHI fragment derived from DMPK/SRD (Sasagawa et al., 1994) was cloned in-frame with the GAL4-DNA–binding domain into pGBT9 (CLONTECH Laboratories, Inc., Palo Alto, CA). The resultant plasmid (DMPK [1–541]/pGBT) was sequentially transformed with 1 × 106 cDNAs from a human skeletal muscle library (CLONTECH Laboratories, Inc.) into yeast HF7C. The transformants were plated onto SD medium lacking histidine, tryptophan, leucine, and uracil, and containing 20 mM 3-aminotriazole (Sigma Chemical Co., St. Louis, MO). The plates were incubated at 30°C for 6 d and His+ colonies were assayed for β-galactosidase activity by a filter assay to identify true positive colonies. The cDNAs coding human αB-crystallin and HSP27 were cloned by PCR from the same library described above using appropriate synthetic oligonucleotide primers. The validity of the PCR products was established by sequencing.
Antibodies
Anti-MKBP polyclonal antisera, c-2 and MKC148, were generated against the glutathione-S-transferase (GST)–MKBP fusion protein and a synthetic polypeptide encoding amino acid residues 148–167 (RGGRHLDTEVNEVYISLLPA) of MKBP, respectively. Before use, anti-GST IgG in the c-2 antiserum was depleted by incubation with purified GST immobilized on glutathione beads (Pharmacia Biotech, Piscataway, NJ). Anti– human αB-crystallin antiserum, anti–human HSP27 monoclonal antibody, and anti–mouse HSP25 antiserum were purchased from StressGen Biotechnologies Corp. (Victoria, British Columbia, Canada).
Electrophoresis and Western Blot Analysis
Tissue samples were homogenized and sonicated in 2% SDS, 1 mM EDTA, 70 mM Tris-HCl, pH 6.7, and 10% glycerol and heated at 100°C for 5 min. Protein concentration was determined with DC Protein Assay kit (Bio-Rad Laboratories, Inc., Hercules, CA) using BSA as a protein standard. 2-Mercaptoethanol and Bromo Phenol blue were added to a final concentration of 125 mM and 0.02%, respectively, and the samples were denatured again at 100°C for 5 min. Protein samples were electrophoresed on 12% polyacrylamide gels in the presence of SDS. For two- dimensional PAGE, tissues were solubilized in 8 M urea, 0.5% NP-40, and 5% 2-mercaptoethanol, and electrophoresed as described (Suzuki et al., 1995). Protein transfer for immunostaining was performed as described (Suzuki et al., 1995). All immunoblots were visualized by chemiluminescence ECL (Amersham Corp., Arlington Heights, IL).
Northern Blot Analysis
Multiple-tissue Northern blot membranes (CLONTECH Laboratories, Inc.) were used to analyze the tissue distribution of MKBP. Total RNA preparations from C2C12 cells were obtained using a Quick Prep Total RNA Extraction kit (Pharmacia Biotech). RNAs were separated in 1% agarose gels and blotted onto Hybond-N nylon membranes. Membranes were probed with a cDNA fragment containing the full-length coding region of each sHSP. Membrane-washing conditions were as follows: For multiple-tissue Northern blot, four times in 2× SSC, 0.05% SDS at room temperature for 10 min followed by two times in 0.1× SSC, 0.1% SDS at 50°C for 40 min; for the analysis of the total RNA from C2C12 cells, three times in 2× SSC, 0.1% SDS at room temperature for 10 min followed by two times in 0.2× SSC, 0.1% SDS at 47°C for 30 min. Autoradiography was carried out at −70°C using Fuji x-ray films (Tokyo, Japan) with an intensifying screen.
Assays for Protein–Protein Interaction Analysis
For two-hybrid analysis, vectors were constructed by subcloning the corresponding regions of the cDNA into pGBT9 or pGAD10 (CLONTECH Laboratories, Inc.), except for constructs for DMPK (KD) and protein kinase C (PKC)-ε (KD), which were prepared in pAS2 (CLONTECH Laboratories, Inc.). Interactions were confirmed by observing growth on histidine-lacking plates and by the acquisition of β-galactosidase activity. For the immunoprecipitation assay, COS1 cells transiently transfected with the appropriate expression vectors were suspended in lysis buffer containing 20 mM Tris-HCl, pH 7.5, 100 mM NaCl, 5 mM EGTA, 2 mM EGTA, 10 mM MgCl2, 1 mg/ml BSA, 10% glycerol, 2 mM Na3VO4, 20 mM NaF, 2 mM pyrophosphate, 1 mM DTT, 10 μg/ml leupeptin, and 2 mM PMSF and homogenized mildly in a Dounce homogenizer. The resultant extract was clarified by centrifugation (15,000 rpm, 30 min) and then mixed with anti-T7 tag monoclonal antibody (Novagen, Inc., Madison, WI) immobilized on protein G–Sepharose 4B (Pharmacia Biotech). After incubation for 2 h at 4°C, the resin-bound complexes were washed with the same lysis buffer, and the bound proteins were analyzed by 10% SDS-PAGE followed by immunoblotting. Blot overlay assays were performed essentially as described previously (Suzuki et al., 1995). Briefly, purified recombinant DMPK expressed in Escherichia coli using the pET-15b expression vector (Novagen, Inc.) (0.5 μg) was blotted onto polyvinylidene difluoride membranes. The membrane was blocked with 5% (wt/vol) skimmed milk powder in PBS and incubated with GST or GST–MKBP (30 μg/ml) in buffer A (20 mM Tris-HCl, pH7.5, 100 mM NaCl, 10 mM MgCl2, 1 mg/ml BSA, 0.5% Triton X-100, 0.5 mM DTT, and 5 μg/ml leupeptin) at room temperature for 2 h. The bound proteins were detected with anti-GST antibody.
Protein Kinase Assay
The kinase reaction was carried out in 20 μl of kinase assay buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 10 mM MgCl2,, and 0.01% leupeptin) containing 10 μM [γ-32P]ATP (185 GBq/mmol) with or without exogenous substrates or additives. When using PKCα, which was expressed in and purified from SF21 insect cells, 0.5 mM CaCl2, 25 μg/ml phosphatidyl serine, and 50 ng/ml TPA were added, and the amount of [γ-32P]ATP was lowered to 1.85 GBq/mmol. After incubation for 30 min at 30°C (10 min in the case of PKCα), the reaction mixture was boiled in SDS sample buffer and analyzed in 12% SDS-PAGE and autoradiography. To monitor the chaperone-like activity of MKBP, reaction mixtures without ATP were incubated for 15 min (11 min for PKCα) at 43°C. After brief cooling, the kinase reaction was started by the addition of [γ-32P]ATP as described above. In this assay, recombinant sHSPs free from the carrier protein GST were used. These were prepared by subcloning the cDNA fragment covering the complete open reading frame of each sHSP into pGEX-6P (Pharmacia Biotech). The GST fusion proteins obtained were purified on glutathione Sepharose 4B, and the sHSPs were specifically eluted by digesting the linker site with PreScission Protease (Pharmacia Biotech) according to the manufacturer's directions. Because this protease is a recombinant protein fused to GST, it is trapped by the glutathione resin and does not contaminate the sHSP preparations.
Cell Cultures
Primary cultures of cardiac myocytes were prepared from ventricles of 1-d-old ICR mice as described previously (Nyui et al., 1997). To prepare the soluble fraction, cells from a 60-mm culture dish were suspended in 300 μl of PBS containing 1 mM EGTA, 1 mM DTT, 10 μg/ml leupeptin, 5 μg/ml aprotinin, and 1 mM PMSF, sonicated at 0°C for 30 s, and centrifuged at 130,000 g for 40 min. The C2C12 mouse myoblast cell line was kindly provided by Shosei Yoshida (National Institute of Neuroscience, NCNP, Kodaira, Tokyo, Japan). C2C12 cells were maintained in DME supplemented with 10% FCS. To induce differentiation, the cells were cultured on collagen-coated tissue culture plates (Corning, Tokyo, Japan) and then switched to a serum-free ITS medium consisting of DME supplemented with insulin (10 μg/ml), transferrin (5 μg/ml), and sodium selenite (10 nmol). For heat treatment, differentiated C2C12 cells (3 d in ITS medium) were exposed to transitory heatshock by floating on a water bath at 44°C for 15 min. The cells were recovered after appropriate times at 37°C. After washing with cold PBS, the cells were scraped and total RNA was extracted.
Treatment of Rat Hindlimb Muscle and Preparation of Tissue Extracts
SD rats (11-wk-old) were killed under ether anesthesia, and the hindlimb muscles (gastrocnemius) were immediately removed and subjected to heat treatment for 20 min as described previously (Kato et al., 1994). The tissues were frozen in liquid nitrogen and stored at −80°C. The tissues were ground into powder in liquid nitrogen and homogenized in 10 vol (vol/wt) of extraction buffer (20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 10 mM MgCl2, 2 mM EGTA, 1 mM DTT, 10 μg/ml leupeptin, 5 μg/ml aprotinin, and 1 mM PMSF). The homogenates were centrifuged at 130,000 g for 40 min at 4°C, and the supernatants were used for gel filtration analysis or protein analysis. Pellets were solubilized in the same volume of SDS solubilizing buffer as the extraction buffer used above.
Gel Filtration
50 μl of mouse cardiomyocyte or rat hindlimb muscle extract was loaded onto a prepacked column of Superose 12 (PC3.2/30) set on the SMART System (Pharmacia Biotech) and equilibrated with the appropriate extraction buffer for each sample at 4°C. Blue dextran (>2,000 kD), bovine γ-globulin (154 kD), BSA (67 kD), chicken ovalbumin (45 kD), and lysozyme (14 kD) were used as molecular mass standards.
Immunohistochemistry
Human limb muscle specimens (mostly from biceps bracii, and, in some cases, rectus femoris) were obtained for diagnostic purposes with informed consent. The procedures for immunohistochemical observation were described previously (Hayashi et al., 1993).
Results
The DMPK Kinase Domain Binds Specifically to the α-Crystallin Domain of MKBP, a Novel Member of the sHSP Family
To obtain clues as to the physiological function of myotonic dystrophy protein kinase (DMPK), we searched for DMPK-binding proteins in a human skeletal muscle cDNA library using a yeast two-hybrid system. As shown in Fig. 1 a, we used the first 541 amino acids of DMPK, DMPK (1–541), which contains the NH2-terminal kinase domain and the α-helical coiled-coil domain (Sasagawa et al., 1994), as a probe to identify a cDNA clone (D2-1) (Fig. 1 b). The clone also interacted with the first 358 residues of DMPK, DMPK (KD), suggesting that the protein encoded by D2-1 interacts with the kinase domain of DMPK (Fig. 1 b). This clone with a 0.9-kb insert encodes a novel protein that shows striking similarity to members of the sHSP family (Fig. 2; the partial sequence of D2-1 has already been reported as one of the eye muscle autoantigens in thyroid-associated ophthalmopathy) (Elisei et al., 1993). Based on the size of the mRNA (see below) and a comparison with other sHSPs, we identified a methionine codon preceded by a Kozak's consensus sequence located 23 amino acid residues downstream of the cloning junction. Thus this protein, which we named MKBP, contains 182 amino acid residues and has an estimated molecular mass of 20,295 kD. As shown in Fig. 2, MKBP represents the most diverged member of this family, showing more than 30% overall sequence identity to each known human sHSP (Caspers and Bhat, 1995). The greatest similarity (42% or greater identity) occurs in the α-crystallin domain, which is shared by all sHSPs and is thought to be important for their molecular chaperone activity (Jakob et al., 1993; Rao et al., 1994). Further analysis using an MKBP mutant, MKBP (64–182), showed that the interaction between DMPK and MKBP is mediated by this α-crystallin domain (Fig. 1 b). In addition, the other members of the sHSP family abundant in skeletal muscle, αB-crystallin and HSP27, do not interact with DMPK (Fig. 1 b). Therefore, the interaction between DMPK and MKBP is not due to some nonspecific binding activity of MKBP as a molecular chaperone. Moreover, MKBP does not interact with the kinase domain (1352–2420) of PKC-ε, PKC-ε (KD), confirming that the observed binding of MKBP is specific for DMPK (Fig. 1 b).
Figure 1.
MKBP specifically interacts with the kinase domain of DMPK. (a) Shown are molecular structures of the two major DMPK isoforms, tentatively designated as DMPK-1 (Jansen et al., 1992) and DMPK-2 (Sasagawa et al., 1994), and the corresponding regions used in two-hybrid screening. (b) Specificity of the interaction of MKBP with DMPK detected in the yeast two-hybrid assay. The growth on −ULWH plates, which lack uracil, leucine, tryptophan, and histidine, indicates the interaction (lower panels). The cotransfected plasmid vectors are indicated (DNA-binding domain fusion/Activation domain fusion). D2-1 represents a plasmid clone obtained during screening. MKBP (full) represents a plasmid in which the extra sequence preceding the initiation codon in D2-1 was deleted. 3-aminotriazole was added to suppress the background growth of yeast. The same results were also confirmed by β-galactosidase filter assay (data not shown). (c) In vivo interaction between DMPK and MKBP detected in COS1 cells. Immune complexes precipitated with anti-T7 epitope antibody (top, middle), or total cell extracts (bottom) were assayed for DMPK and MKBP content by immunoblotting. MKBP was detected with the anti-MKBP antiserum c-2 (see Fig. 3). (d) Blot overlay assay showing the direct interaction between recombinant DMPK (1–541) and MKBP. Histidine-tagged DMPK (1–541) (0.5 μg) purified from E. coli (lanes 2–6) or molecular mass markers (total protein amount = ∼5 μg) (lane 1) were blotted onto a membrane after SDS-PAGE separation and assayed for MKBP binding by overlaying purified GST–MKBP or GST. The binding of the overlaid protein was detected by immunostaining with anti-GST antibody. Note that the molecular mass markers did not bind GST-MKBP (lane 1).
Figure 2.

Deduced amino acid sequence of human MKBP and alignment with four human sHSPs. Black boxes indicate amino acid identity. The α-crystallin domain is indicated by the double-headed arrow. The amino acid sequence used to generate the anti-MKBP antibody, MKC148, is underlined. The table shows the percent amino acid identity among sHSPs in the alignment of the α-crystallin domain as calculated by the DNASIS program (Hitachi Software Engineering Co., Ltd., Tokyo, Japan). The amino acid residues of the sHSPs used for comparison are as follows; 66–148 for MKBP, 87–169 for HSP27, 64–144 for αA-crstallin, 67–149 for αB-crystallin, and 66–148 for p20. The sequence data for MKBP are available from GenBank/EMBL/DDBJ under accession number D8961.
The physical association between DMPK and MKBP was independently confirmed by an immunoprecipitation assay on extracts of COS1 cells transiently transfected with expression vectors encoding T7 epitope-tagged DMPK and wild-type MKBP (Fig. 1 c). MKBP immunoprecipitated with the anti-T7 antibody only when T7-DMPK (the full-length isoform corresponding to DMPK-2 in Fig. 1 a) as well as T7-DMPK (1–541) were coexpressed in the cell. Furthermore, an in vitro solid-phase binding assay (overlay) using purified recombinant DMPK (1–541) and MKBP proteins clearly demonstrated a direct interaction between DMPK and MKBP. As shown in Fig. 1 d, GST–MKBP, but not GST, binds to histidine-tagged DMPK (1–541) blotted onto a membrane. The binding affinity did not depend very much on the presence of ATP.
MKBP Is the Fourth Member of the sHSP Family That Is Highly Expressed in Muscle Cells
Northern blot analysis of mRNA from human tissues showed a single transcript of ∼1.0 kb expressed highly in skeletal and cardiac muscle as well as ubiquitously at lower levels (Fig. 3 a).
Figure 3.
MKBP is highly expressed in skeletal muscle and heart. (a) Northern blot of MKBP mRNA in adult human tissues. Each lane contained 2 μg of poly (A)+ RNA isolated from the indicated tissues. An x-ray film was exposed at −70°C for 14 d with an intensifying screen. (b) Western blot analysis showing the expression of three members of the sHSP family in muscle cells. Identical blots on which the extracts of human skeletal muscle (lane 1), mouse hindlimb skeletal muscle (lane 2), and mouse cardiac muscle (lane 3) were transferred after SDS-PAGE separation were probed with antibodies against three kinds of sHSPs. c-2 depleted indicates serum from which anti-MKBP IgG was specifically depleted using the antigen. (c) Characterization of another anti-MKBP antibody, MKC148, raised against a COOH-terminal sequence of MKBP. The extract of human skeletal muscle was analyzed using the indicated antisera. MKC148 was used for immunohistochemical analysis in Fig. 6. (d) Two-dimensional PAGE analysis of human skeletal muscle. The blot membrane was probed with the indicated mixture of antisera. Arrowheads indicate the positions of HSP27 (pI = 5.95) used as a PI marker. The positions of αB-crystallin and MKBP are indicated by open triangles. The arrow indicates a nonspecific spot stained by the anti–αB-crystallin antibody (see b).
Antiserum (c-2) against recombinant MKBP detected a protein of 22 kD in skeletal and cardiac muscle (Fig. 3 b). Another anti-MKBP antibody (MKC148) raised against the COOH-terminal sequence specific for MKBP (amino acid residues 148–167) also detected the same band (Fig. 3 c). Because MKBP and αB-crystallin show similar mobilities on one-dimensional SDS-PAGE, we also performed two-dimensional PAGE analysis and confirmed the expression of the MKBP protein in human skeletal muscle as well as the specificity of c-2 (Fig. 3 d). c-2 detected spots consistent with the predicted pI of 4.98 for MKBP (open triangle in lower panel), whereas anti-αB-crystalline antiserum reacted only with spots corresponding to the pI of 6.92 for αB-crystalline (Fig. 3 d, open triangle in upper panel). Using these specific antibodies and each purified recombinant protein as a standard, we estimated the protein concentration of MKBP and αB-crystallin in skeletal muscle cell. MKBP was found to present at high levels (0.65 μg/ mg protein) in rat hindlimb muscle (gastrocnemius) extracts, comparable to the concentration of αB-crystallin (0.72 μg/mg protein).
MKBP Exists in an Oligomeric Complex Separate from Other sHSPs in Muscle Cells
Since sHSPs expressed in muscle cells have been shown to associate with one another to form a large oligomeric complex (Kato et al., 1992, 1994), we tested the possibility that MKBP is also a component of this complex. Soluble proteins extracted from primary cultures of mouse cardiomyocytes or rat hindlimb skeletal muscle were subjected to gel filtration and analyzed by immunoblotting. In both cell types, MKBP was found to exist in an oligomeric complex (∼150 kD) separate from the complex containing αB-crystallin and HSP27 (>500 kD) (Fig. 4 a). This result was also supported by yeast two-hybrid assays monitoring the interactions between the three members of the sHSP family (Fig. 4 b); strong homo- and heterooligomeric αB-crystallin and HSP27 activities were observed in this assay, consistent with the previous biochemical analysis (Kato et al., 1992). On the other hand, MKBP interacted with neither αB-crystallin nor HSP27, whereas it showed homophilic binding activity. Therefore, we conclude that MKBP works independently of the known sHSPs in muscle cells by forming a separate oligomeric complex. Interestingly, the apparent molecular masses of MKBP in skeletal muscle and cardiomyocytes differ slightly. This suggests the possibility of different MKBP complex compositions in these cells. Although Kato et al. have demonstrated that p20 in soluble rat diaphragm extracts exists mainly in two forms of oligomeric complexes with apparent molecular masses larger than 500 kD and lower than 67 kD but none of ∼150 kD (Kato et al., 1994), there remains the possibility that some fraction of p20 associates with MKBP.
Figure 4.
MKBP exists in an oligomeric complex separate from the complex composed of αB-crystallin and HSP27 in muscle cells. (a) Size fractionation of oligomeric complexes formed by each member of the sHSP family in mouse cardiac muscle (top) and rat skeletal muscle (bottom). The soluble fraction from each muscle cell type was gel-filtered through Superose 12 and analyzed by immunoblotting. c-2 was used to detect MKBP. (b) Interactions between three members of the sHSP family as monitored by the yeast two-hybrid assays. The region of the cDNA corresponding to the complete open reading frame of each sHSP was subcloned into pGBT9 (for fusion with the DNA-binding domain of GAL4) or pGAD424 (for fusion with the activation domain of GAL4) and subjected to assays. The same results were also confirmed by β-galactosidase filter assay (data not shown).
Heat Shock Does Not Induce the Accumulation of MKBP mRNA, but Causes an Early Redistribution into the Insoluble Fraction
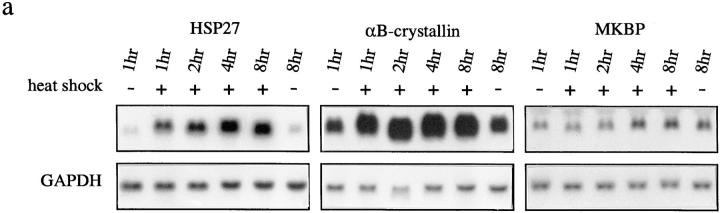
Cells once exposed to environmental stress have been suggested to acquire an increased capacity to survive subsequent stress by accumulating HSPs (Linquist and Craig, 1988). In many cell lines, the expression of sHSPs has also been shown to be induced by heat and other stresses, suggesting that they also contribute to the acquisition of stress resistance in cells (Klementz et al., 1991; Head et al., 1994). In a mouse myoblast cell line, C2C12, which expresses at least three members of the mammalian sHSP family, HSP27, αB-crystallin, and MKBP, we also observed the dramatic accumulation of the mRNA for HSP27 and αB-crystallin within 1 h after heat treatment (44°C 15 min). However, the expression of MKBP was not induced at all, even after 8 h, and the levels did not change even when more severe heat treatments were applied (44°C 1 h or 46°C 15 min) (Fig. 5 a; data not shown).
Figure 5.
MKBP is a unique stress-responsive protein. (a) Northern blot analysis of total RNA from differentiated C2C12 cells extracted at the indicated times after heat treatment (44°C 15 min). Three identical blots were hybridized with each sHSP probe as indicated at the top. The same membranes were rehybridized with a probe for glyceraldehyde- 3 -phosphate dehydrogenase (GAPDH) to confirm that the amount of RNA in each lane were the same. (b) Heat-inducible redistribution of MKBP from the cytoplasm to the insoluble fraction in rat skeletal muscle. After incubation at the indicated temperature for 20 min, the freshly excised hindlimb muscle from 11-wk-old SD rats was homogenized and fractionated into soluble (S) and insoluble (P) fractions by centrifugation (130,000 g, 40 min). T indicates the total homogenate before centrifugation. Each sample was separated in 12% SDS-PAGE and analyzed by immunoblotting (top). Each membrane was stained with Coomassie brilliant blue (CBB) after immunoblot analysis (bottom).
In addition to transcriptional upregulation, heat shock has been suggested to induce an immediate redistribution of preexisting sHSPs from the cytosolic fraction to the insoluble fraction. As shown in Fig. 5 b, MKBP also showed a similar response to heat shock. At physiological temperature, almost 50% of the MKBP in the skeletal muscle of the rat hindlimb was extracted in the soluble fraction, whereas after heat shock treatment (44 or 46°C 20 min), increased amounts of MKBP were found in the insoluble fraction (Fig. 5 b). These results suggest that MKBP not only shows sequence homology with other known sHSPs but also, like the others, behaves as a stress responsive protein.
MKBP Localizes to Z-Membranes and the Neuromuscular Junction in Human Skeletal Muscle
Fig. 6 shows the subcellular immunolocalization of MKBP in human skeletal muscle. In cross section, anti-MKBP antiserum, MKC148, shows an antigen-specific, dotlike staining pattern that may correspond to individual cross sections of myofibril fibers (Fig. 6, a–c). On the other hand, in longitudinal section, the staining pattern shows an ordered striation identified as the Z-line from the overlapping pattern produced by desmin staining (Fig. 6, d–f). In addition to the predominant staining in the cytoplasm, MKBP also localizes to the neuromuscular junction identified by rhodamine-labeled α-bungarotoxin (Fig. 6, g–i). Based on the recent demonstration that DMPK localizes predominantly to the neuromuscular junction (Maeda et al., 1995; Whiting et al., 1995), these results support the notion that DMPK and a fraction of MKBP colocalize endogenously at the neuromuscular junction in skeletal muscle cells.
Figure 6.

MKBP localizes to the Z-membrane and neuromuscular junction in human skeletal muscle. Shown are cross sections (a–c and g–i) and longitudinal sections (d–f) of wild-type (wt; a, c, and d–f) and DM (b and g–i) skeletal muscle. Sections were stained with MKC148 (mk; a, b, d, and g), anti-MKBP IgG-depleted MKC148 (co; c), antidesmin antibody (des; e), or α-bungarotoxin (BT; h). f and i represent the superimposed images of d and e, and g and h, respectively. Bar: (a–c and g–i) 25 μm; (d–f) 5 μm.
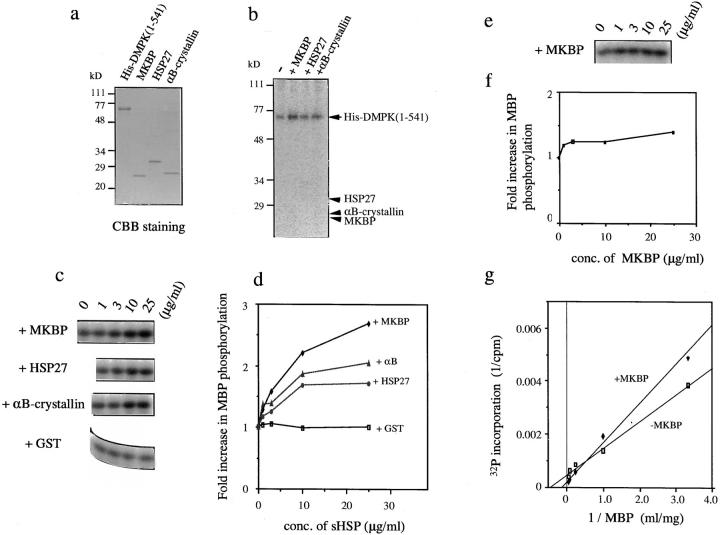
MKBP Activates the Kinase Activity of DMPK and Confers Thermoresistance
To assess the physiological significance of the interaction between DMPK and MKBP, we performed an in vitro kinase assay using recombinant of DMPK (1–541) and sHSPs (Fig. 1 d). For these assays, we excised the carrier protein, GST, from each GST fusion protein of sHSP to eliminate possible steric hindrance (Fig. 7 a). Interestingly, gel filtration analysis revealed that, in contrast with the situation in vivo (Fig. 4), these purified sHSPs all exist in oligomeric complexes with apparent molecular sizes ranging from 150 to 200 kD under the assay conditions used (MKBP and HSP27, ∼150 kD; αB-crystalline, ∼200 kD, data not shown). As shown in Fig. 7 b, recombinant DMPK (1–541) shows autophosphorylation activity as reported previously (Dunne et al., 1994); however, it does not phosphorylate MKBP, suggesting that MKBP is not a downstream target of DMPK. On the other hand, the data in Fig. 7 b also suggest that when sHSPs coexist in the reaction mixture, DMPK autophosphorylation increases, and that this effect is greatest when MKBP is in the solution (3.4-fold for MKBP, 1.8-fold for HSP27, and 2.4-fold for αB-crystallin). This enhancement of DMPK kinase activity was further confirmed using myelin basic protein (MBP) as a substrate (James et al., 1996). In contrast with GST or BSA (data not shown), the existence of very low concentrations of sHSPs (25 μg/ml) in the solution increases the phosphorylation of MBP by DMPK two- to threefold, and among the sHSPs examined, MKBP was again found to be the most effective (Fig. 7, c and d). Since MKBP does not affect MBP phosphorylation by PKCα (Fig. 7, e and f), the observed effect of MKBP is suggested to be specific for DMPK. Fig. 7 g shows a Lineweaver-Burk plot derived from the substrate dose dependence data for MBP phosphorylation by DMPK. It shows that MKBP increases not only the maximal rate, V max, of phosphorylation, but also the Michaelis constant, K m, excluding the possibility that MKBP increases MBP phosphorylation by mediating the interaction between DMPK and MBP (if so, K m should be lowered). Rather, MKBP lowers the affinity of DMPK for MBP, suggesting that it alters the recognition of DMPK by affecting the tertiary structure of the kinase domain.
Figure 7.
MKBP enhances the kinase activity of DMPK. (a) Coomassie blue staining of the recombinant proteins used (0.8 μg of His-DMPK [1–541] and 0.4 μg of each sHSP) after separation on 12% SDS-PAGE. (b) In vitro kinase assay using sHSPs as substrates. 50 ng (40 nM) of His-DMPK (1–541) was incubated in kinase assay buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 10 mM MgCl2,, and 0.01% leupeptin) containing 10 μM [γ-32P]ATP (185 GBq/mmol) for 30 min at 30°C in the presence (second through fourth lanes) or absence (first lane) of sHSPs (1 μg) as indicated at the top. Reactions were stopped by boiling in sample buffer and analyzed by 12% SDS-PAGE and autoradiography. The position of each recombinant protein is indicated. While DMPK autophosphorylation was observed, no sHSPs were phosphorylated. (c) The effect of MKBP on the phosphorylation of MBP by DMPK. 20 μg of MBP were phosphorylated by His-DMPK (1–541) under the same conditions as b in the presence of sHSP or GST. Shown are the results of autoradiography demonstrating the incorporation of 32P into MBP. The additives and their concentrations are indicated. The data are quantitatively analyzed in d. (e) The effect of MKBP on the phosphorylation of MBP by PKCα. Two micrograms of MBP were phosphorylated by PKCα (10 ng) in kinase buffer containing 0.5 mM CaCl2, 25 μg/ml phosphatidyl serine, 50 ng/ml TPA, and 10 μM [γ-32P]ATP (1.85 GBq/mmol) for 10 min at 30°C with various concentrations of MKBP. The data are quantitatively analyzed in f. (g) A Lineweaver-Burk plot derived from the result on MBP dose dependence of 32P incorporation. The intercept on the vertical axis gives 1/V max, whereas that on the horizontal axis gives −1/K m. The estimated K m values for MBP by DMPK (1–541) are 2.4 and 6.4 mg/ml in the absence and presence of 25 μg/ml MKBP, respectively.
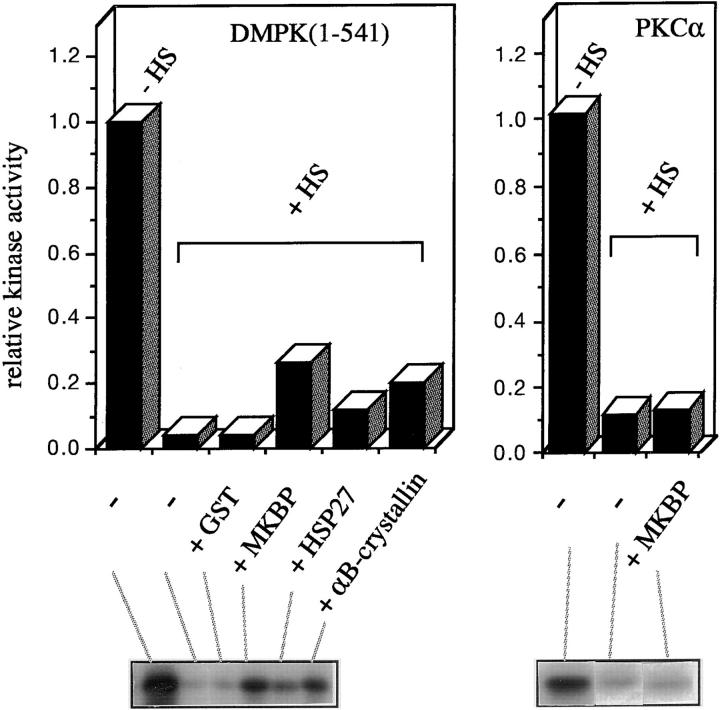
Because HSP27, as well as αB-crystallin, has been shown to have chaperone activity, we next examined the possibility that MKBP also has a chaperone-like activity to suppress the heat-induced inactivation of DMPK (Fig. 8). While the kinase activity of His-DMPK (1–541) decreases to less than 4.3% after incubation at 43°C for 15 min, 26.4% of the activity remains when 50 μg/ml MKBP is present with the kinase during the heat treatment. Since this difference (larger than sixfold) cannot be explained by the enhancement of the remaining DMPK activity (Fig. 7, c and d), these data suggest that MKBP suppresses the heat-induced inactivation of DMPK by binding to its kinase domain. Similar but smaller effects were also observed in the case of HSP27 and αB-crystallin. However, MKBP hardly inhibits the inactivation of PKCα, suggesting again that the effect is specific for DMPK.
Figure 8.
MKBP protects DMPK from heat-induced inactivation. His–DMPK (1–541) or PKCα was incubated at 43°C with or without the indicated additives (50 μg/ml) for 15 min for DMPK and for 11 min for PKCα. After heat treatment, the activity of each kinase was examined by monitoring 32P incorporation into MBP.
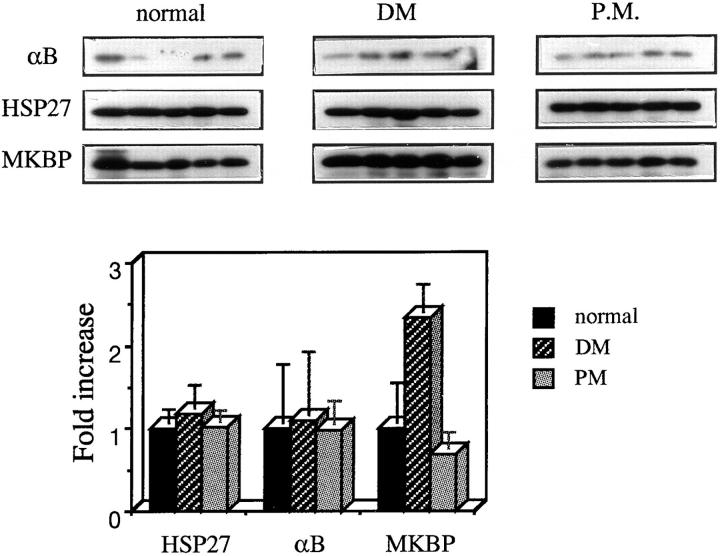
MKBP Is Selectively Upregulated in DM Patients
In a first attempt to address the question of whether the interaction between DMPK and MKBP is involved in the pathogenesis of DM, we analyzed the amount of MKBP protein in skeletal muscle from DM patients. As demonstrated clearly in Fig. 9, the amount of MKBP is commonly upregulated in these muscles compared with skeletal muscle from normal subjects, whereas no changes in the amounts of αB-crystallin and HSP27 are observed. In addition, no change in the amount of MKBP was detected in the case of polymyositis (PM), indicating that the change in the MKBP level does not result from muscle degeneration itself. Consistent with these results, immunostaining of DM muscle with MKC148 sometimes shows increased cytosolic background staining in addition to dotlike staining (Fig. 6, b and g). In some cases, the dotlike staining is enlarged (Fig. 6 b).
Figure 9.
MKBP is specifically upregulated in skeletal muscle from DM patients. Skeletal muscle biopsy samples from normal, DM, and PM adults (6.75 μg/lane) were analyzed by immunoblotting after 12% SDS-PAGE separation. The antibodies used are indicated on the left. Protein amounts in each lane were confirmed to be equal by Coomassie brilliant blue staining of the same blot membrane after immunostaining (data not shown). The results as quantified densitometrically are shown in the lower histogram. Similar results were obtained with additional samples from five DM patients (data not shown).
Discussion
Since muscle cells are frequently subjected to severe conditions caused by heat, oxidative, and mechanical stresses, especially during exercise (Brooks et al., 1971a ,b; Davis et al., 1982), there must be some mechanisms to protect cells from damage. Several lines of evidence suggest that sHSPs, which are constitutively highly expressed in muscle cells, play roles in maintaining myofibrillar integrity against such stresses. Atomi et al. (1991) suggested that αB-crystallin localizes to Z-bands, and its dramatic decrease is a characteristic feature of disuse atrophy of the slow muscle fibers. In addition, HSP27 and αB-crystallin have been suggested to bind to actin filaments (Miron et al., 1991; Bennardini et al., 1992) and confer thermoresistance by affecting their structure and stability (Lavoie et al., 1993a ,b; 1995; Iwaki et al., 1994).
In this work, we identified a novel member of the sHSP family, MKBP, which is also constitutively expressed in skeletal and cardiac muscles. We demonstrated that MKBP not only shows strong homology with other sHSPs but also has features characteristic of sHSPs (Figs. 4 and 5). MKBP shows homooligomeric activity and forms aggregates in muscle cytosol. Furthermore, it redistributes to the insoluble fraction in response to heat shock, suggesting that it is one of the stress responsive proteins found in muscle cells. However, the present results also indicate that MKBP is a unique member of the sHSP family since it forms an independent complex distinct from the complex formed by other sHSPs such as HSP27 and αB-crystallin. In addition, its expression is not induced by heat treatment. Based on these results, we conclude that MKBP is involved in a novel stress responsive system distinct from the known system composed of HSP27, αB-crystallin, and p20 (Kato et al., 1994), and that both systems may work independently to confer stress resistance to muscle cells. The intracellular localization of MKBP at the Z-membranes and neuromuscular junction suggests a role for the protein in protecting the components of these muscle-specific structures.
Various stresses other than heat, such as heavy metal exposure, hypertonicity, and the stimulation with tumor necrosis factor α, have been shown to induce the expression of HSP27 and αB-crystallin (Head et al., 1994). Therefore, there may be some stress stimulations that induce the expression of MKBP. In fact, we observed that the protein levels of MKBP, but not αB-crystallin, increase in response to stretch stimulation in primary cultures of neonatal mouse cardiomyocytes (Suzuki, A., Y. Sugiyama, N. Nyu-i, and S. Ohno, unpublished results). MKBP might play a role in a system by which muscle cells respond to mechanical stress.
We identified MKBP by searching for DMPK binding proteins. Three independent experiments established the specific and direct interaction between DMPK and MKBP. The localization of MKBP at the neuromuscular junction where DMPK is also suggested to be concentrated (Fig. 6, h–j) (Maeda et al., 1995; Whiting et al., 1995), as well as the selective change in the amount of MKBP in skeletal muscle from DM patients (Fig. 9), further suggest an intrinsic interaction between these proteins. We also found that MKBP is not significantly phosphorylated by DMPK but rather activates the kinase activity of DMPK two- to threefold and protects it from heat-induced inactivation. An analysis of the kinetic parameters of DMPK also suggests the possibility that MKBP binding changes the tertiary structure of the kinase domain of DMPK (Fig. 7 g). These results, although indirect, suggest that MKBP can work as a kind of molecular chaperone for DMPK at least in vitro. Recently, a growing number of intracellular signaling molecules, including various kinases, receptors, and transcription factors, have been found to be constitutively associated with chaperone molecules such as HSP90 and immunophilins (Rutherford and Zuker, 1994; Pennisi, 1996). Based on these observations, the regulated folding or assembly of signaling molecules by chaperone molecules is beginning to be recognized as a general mechanism by which signal transduction pathways are controlled. With regard to sHSP, HSP27 was very recently shown to bind and activate PKB/Akt/RAC-protein kinase in response to heat or oxidative stress (Konishi et al., 1997). Taken together, it seems reasonable to speculate that one of the physiological functions of MKBP may be to act as a molecular chaperone specific for DMPK that stabilizes and protects its kinase activity. Furthermore, if there are some stress stimulations that regulate the MKBP expression level, MKBP might act as a signal transducer activating DMPK in response to such stimulations (for example, stretch stimulation as stated above). The data in Figs. 7 and 8 show that HSP27 and αB-crystallin also have a similar, although smaller, effect on DMPK. However, because several observations in vivo suggest the specificity of the interaction between DMPK and MKBP (Figs. 1 and 9), we speculate that there are unknown factors that make MKBP the main sHSP affecting the activity of DMPK in vivo. In this sense, it should be noted that the size of the oligomeric structure of recombinant MKBP is similar to that observed in vivo (Fig. 4), while those of HSP27 and αB-crystallin are different (data not shown).
Although CTG repeat expansions in the 3′-untranslated region of the DMPK gene are responsible for DM, the molecular basis of the pathogenesis of the disease, including even the involvement of the DMPK protein, remains controversial. However, recent studies on DMPK knockout mice have demonstrated that DMPK plays an essential role in maintaining muscle structure and function, and thus its absence does contribute to some aspects of the disease (Reddy et al., 1996). Therefore, the following hypothesis based on the present results may provide a partial explanation for the molecular basis of the disease: DMPK is involved in the stress-responsive system by being activated by MKBP, and its absence or a reduction in its level impairs its activity to protect cells against stresses. This would result in the accumulation of cellular damage leading to the gradual muscle degeneration observed in the late stages in knockout mice (Reddy et al., 1996) and in DM patients. In this context, the selective upregulation of MKBP observed in patients (Fig. 9) might be explained as a sign of a feedback mechanism trying to compensate for the reduction in DMPK.
To our knowledge, this is the first report suggesting a correlation between a specific muscular dystrophy and the stress-responsive system of muscle cells. Although characterized by skeletal muscle wasting and myotonia, DM is a multisystem disorder with diverged phenotypes (Harper, 1989). The tissue distributions of DMPK and MKBP transcripts correlate well (Fig. 3; Jansen et al., 1992), and although their levels are very low, they are expressed ubiquitously. Therefore, the pathological mechanism we have hypothesized might also work in tissues other than muscle. Of course, some of the diverged phenotypes may arise via other unknown mechanisms not related to the function of DMPK. To understand the molecular basis of this complex disease, much further study is needed. Also, with respect to our hypothetical picture, a great many gaps, including the identity of the specific substrate of DMPK, remain to be filled. However, the finding of a stress-responsive protein that activates DMPK provides a new opportunity to clarify the currently obscure DMPK signaling pathway. In addition, it provides novel and important insight into the molecular basis underlying some of the essential phenotypes of this disease.
Very recently, during the course of analyzing the promoter region of the human αB-crystallin gene located on chromosome 11q22, Iwaki et al. (1997) identified a gene encoding a novel member of the sHSP family (HSPB2) in a head-to-head orientation with the αB-crystallin gene with an intermediate region of ∼1 kb. Interestingly, this HSPB2 is identical to MKBP. They also demonstrated that, in contrast to αB-crystallin, the HSPB2 mRNA is not detectable in rat lens by Northern blot. Together with the present results, this finding raises intriguing questions as to how the expression of both genes is regulated and how they have evolved.
Acknowledgments
We thank S. Yoshida and Y. Nabeshima for providing us with the C2C12 cell line; H. Sugita, E. Ozawa, and R. Matsuda for stimulating discussion; and M. Okamoto for technical assistance. We are grateful to A. Iwaki and Y. Fukumaki for communication of their results before publication.
This work was supported by a grant [8A-1] from the National Center of Neurology and Psychiatry of the Ministry of Health and Welfare, Japan.
Abbreviations used in this paper
- DM
myotonic dystrophy
- DMPK
myotonic dystrophy protein kinase
- GST
glutathione-S-transferase
- MBP
myelin basic protein
- MKBP
myotonic dystrophy protein kinase binding protein
- PKC
protein kinase C
- sHSP
small heat shock protein
- TPA
12-O-tetradecanoyl phorbol-13-acetate
Footnotes
Please address all correspondence to Dr. Atsushi Suzuki or Dr. Shigeo Ohno, Department of Molecular Biology, Yokohama City University School of Medicine, Kanazawa-ku, Yokohama 236, Japan. Tel.: 81-045-787-2596. Fax: 81-045-785-4140. E-mail: abell@med.yokohama-cu.ac.jp (Dr. Suzuki) or ohnos@med.yokohama-cu.ac.jp (Dr. Ohno).
2. Although the mouse homologue of human HSP27 has been called HSP25, in this paper we standardize the protein to HSP27 irrespective of species to avoid confusion.
References
- Atomi Y, Yamada S, Strohman R, Nonomura Y. αB-crystallin in skeletal muscle: purification and localization. J Biochem. 1991;110:812–822. doi: 10.1093/oxfordjournals.jbchem.a123665. [DOI] [PubMed] [Google Scholar]
- Bennardini F, Wrzosek A, Chiesi M. αB-crystallin cardiac tissue. Association with actin and desmin filaments. Circ Res. 1992;71:288–294. doi: 10.1161/01.res.71.2.288. [DOI] [PubMed] [Google Scholar]
- Bhagwati S, Ghatpande A, Leung B. Normal levels of DM RNA and myotonin protein kinase in skeletal muscle from adult myotonic dystrophy (DM) patients. Biochim Biophys Acta. 1996;1317:155–157. doi: 10.1016/s0925-4439(96)00057-9. [DOI] [PubMed] [Google Scholar]
- Brook JD, McCurrach ME, Harley HG, Buckler AJ, Church D, Aburatani H, Hunter K, Stanton VP, Thirlon J-P, Hudson T, et al. Molecular basis of myotonic dystrophy: expansion of a trinucleotide (CTG) repeat at the 3′ end of a transcript encoding a protein kinase family member. Cell. 1992;68:799–808. doi: 10.1016/0092-8674(92)90154-5. [DOI] [PubMed] [Google Scholar]
- Brooks GA, Hittelman KJ, Faulkner JA, Beyer RE. Tissue temperatures and whole-animal oxygen consumption after exercise. Am J Physiol. 1971a;221:427–431. doi: 10.1152/ajplegacy.1971.221.2.427. [DOI] [PubMed] [Google Scholar]
- Brooks GA, Hittelman KJ, Faulkner JA, Beyer RE. Temperature, skeletal muscle mitochondrial functions, and oxygen debt. Am J Physiol. 1971b;220:1053–1059. doi: 10.1152/ajplegacy.1971.220.4.1053. [DOI] [PubMed] [Google Scholar]
- Caspers G-J, Bhat SP. The expanding small heat-shock protein family, and structure predictions of the conserved “α-crystallin domain”. J Mol Evol. 1995;40:238–248. doi: 10.1007/BF00163229. [DOI] [PubMed] [Google Scholar]
- Davis BM, McCurrach ME, Taneja KL, Singer RH, Housman DE. Expansion of a CUG trinucleotide repeat in the 3′ untranslated region of myotonic dystrophy protein kinase transcripts results in nuclear retention of transcripts. Proc Natl Acad Sci USA. 1997;94:7388–7393. doi: 10.1073/pnas.94.14.7388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KJA, Quintaniha AT, Brooks GA, Packer L. Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun. 1982;107:1198–1205. doi: 10.1016/s0006-291x(82)80124-1. [DOI] [PubMed] [Google Scholar]
- Dunne PW, Walch ET, Epstein HF. Phosphorylation reactions of recombinant human myotonic dystrophy protein kinase and their inhibition. Biochemistry. 1994;33:10809–10814. doi: 10.1021/bi00201a031. [DOI] [PubMed] [Google Scholar]
- Elisei R, Weightman D, Kendall-Taylor P, Vassart G, Ludgate M. Muscle autoantigens in thyroid associated ophthalmopathy: the limits of molecular genetics. J Endocrinol Invest. 1993;16:533–540. doi: 10.1007/BF03348900. [DOI] [PubMed] [Google Scholar]
- Fu Y-H, Pizzuti A, Fenwick RG, Jr, King J, Rainarayan S, Dunne PW, Dubel J, Nasser GA, Ashizawa T, de Jong P, et al. An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science. 1992;255:1256–1258. doi: 10.1126/science.1546326. [DOI] [PubMed] [Google Scholar]
- Fu Y-H DL, Friedman, Richards S, Pearlman JA, Gibbs RA, Pizzuti A, Ashizawa T, Perryman MB, Scarlato G, Fenwick RG, Jr, Caskey CT. Decreased expression of myotonin-protein kinase messenger RNA and protein in adult form of myotonic dystrophy. Science. 1993;260:235–237. doi: 10.1126/science.8469976. [DOI] [PubMed] [Google Scholar]
- Hamshere MG, Newman EE, Alwazzan M, Athwal BS, Brook JD. Transcriptional abnormality in myotonic dystrophy affects DMPK but not neighboring genes. Proc Natl Acad Sci USA. 1997;94:7394–7399. doi: 10.1073/pnas.94.14.7394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper, P.S. 1989. Myotonic Dystrophy, 2nd Ed. W.B. Saunders, London. 325 pp.
- Hayashi YK, Engvall E, Arikawa-Hirasawa E, Goto K, Koga R, Nonaka I, Sugita H, Arahata K. Abnormal localization of laminin subunits in muscular dystrophies. J Neurol Sci. 1993;119:53–64. doi: 10.1016/0022-510x(93)90191-z. [DOI] [PubMed] [Google Scholar]
- Head MW, Corbin E, Goldman JE. Coordinate and independent regulation of αB-crystallin and HSP27 expression in response to physiological stress. J Cell Physiol. 1994;159:41–50. doi: 10.1002/jcp.1041590107. [DOI] [PubMed] [Google Scholar]
- Hickey ED, Weber LA. Modulation of heat-shock polypeptide synthesis in HeLa cells during hyperthermia and recovery. Biochemistry. 1982;21:1513–1521. doi: 10.1021/bi00536a008. [DOI] [PubMed] [Google Scholar]
- Ingolia TD, Craig EA. Four small Drosophila heat shock proteins are related to each other and to mammalian α-crystallin. Proc Natl Acad Sci USA. 1982;79:2360–2364. doi: 10.1073/pnas.79.7.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaki T, Iwaki A, Tateishi J, Goldman JE. Sense and antisense modification of glial αB-crystallin production results in alterations of stress fiber formation and thermoresistance. J Cell Biol. 1994;125:1385–1393. doi: 10.1083/jcb.125.6.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwaki A, Nagano T, Nakagawa M, Iwaki T, Fukumaki Y. Identification and characterization of the gene encoding a new member of the α-crystallin/small hsp family, closely linked to the αB-crystallin gene in a head-to-head manner. Genomics. 1997;45:386–394. doi: 10.1006/geno.1997.4956. [DOI] [PubMed] [Google Scholar]
- Jakob U, Gaestel M, Engel K, Buchner J. Small heat shock proteins are molecular chaperones. J Biol Chem. 1993;268:1517–1520. [PubMed] [Google Scholar]
- James DW, Haq R, Tamai K, Sabourin LA, Ikeda J-E, Korneluk RG. Investigation of myotonic dystrophy kinase isoform translocation and membrane association. J Biol Chem. 1996;271:15187–15193. doi: 10.1074/jbc.271.25.15187. [DOI] [PubMed] [Google Scholar]
- Jansen G, Mahadevan M, Amemiya C, Wormskamp N, Segers B, Hendriks W, O'Hoy K, Bairk S, Sabourin L, Lennon G, et al. Characterization of the myotonic dystrophy region predicts multiple protein isoform- encoding mRNAs. Nat Genet. 1992;1:261–266. doi: 10.1038/ng0792-261. [DOI] [PubMed] [Google Scholar]
- Jansen G, Groenen PJTA, Bachner D, Jap PHK, Coerwinkel M, Oerlemans F, van den Broek W, Gohlsch B, Pette D, Plomp JJ, et al. Abnormal myotonic dystrophy protein kinase levels produce only mild myopathy in mice. Nat Genet. 1996;13:316–324. doi: 10.1038/ng0796-316. [DOI] [PubMed] [Google Scholar]
- Kato K, Shinohara H, Goto S, Inaguma Y, Morishita R, Asano T. Copurification of small heat shock protein with αB-crystallin from human skeletal muscle. J Biol Chem. 1992;267:7718–7725. [PubMed] [Google Scholar]
- Kato K, Goto S, Inaguma Y, Hasegawa K, Morishita R, Asano T. Purification and characterization of a 20-kD protein that is highly homologous to αB-crystallin. J Biol Chem. 1994;269:15302–15309. [PubMed] [Google Scholar]
- Klemenz R, Froehli E, Steiger RH, Schafer R, Aoyama A. αB-crystallin is a small heat shock protein. Proc Natl Acad Sci USA. 1991;88:3652–3656. doi: 10.1073/pnas.88.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konishi H, Matsuzaki H, Tanaka M, Takemura Y, Kuroda S, Ono Y, Kikkawa U. Activation of protein kinase B (Akt/RAC-protein kinase) by cellular stress and its association with heat shock protein Hsp27. FEBS Lett. 1997;410:493–498. doi: 10.1016/s0014-5793(97)00541-3. [DOI] [PubMed] [Google Scholar]
- Lavoie JN, Gingras-Breton G, Tanguay RM, Landry J. Induction of Chinese hamster HSP27 gene expression in mouse cells confers resistance to heat shock. J Biol Chem. 1993a;268:3420–3429. [PubMed] [Google Scholar]
- Lavoie JN, Hickey E, Weber LA, Landry J. Modulation of actin microfilament dynamics and fluid phase pinocytosis by phosphorylation of heat shock protein 27. J Biol Chem. 1993b;268:24210–24214. [PubMed] [Google Scholar]
- Lavoie JN, Lambert H, Hickey E, Weber LA, Landry J. Modulation of cellular thermoresistance and actin filament stability accompanies phosphorylation-induced changes in the oligomeric structure of heat shock protein 27. Mol Cell Biol. 1995;15:505–516. doi: 10.1128/mcb.15.1.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linquist S, Craig EA. The heat shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- Maeda M CS, Taft, Bush EW, Holder E, Bailey WM, Neville H, Perryman MB, Bies RD. Identification, tissue-specific expression, and subcellular localization of the 80 and 71kD forms of myotonic dystrophy kinase protein. J Biol Chem. 1995;270:20246–20249. doi: 10.1074/jbc.270.35.20246. [DOI] [PubMed] [Google Scholar]
- Mahadevan M, Tsilfidis C, Sabourin L, Shutler G, Ameniya C, Jansen G, Neville C, Narang M, Barcelo J, O'Hoy K, et al. Myotonic dystrophy mutation: an unstable CTG repeat in the 3′ untranslated region of the gene. Science. 1992;255:1253–1255. doi: 10.1126/science.1546325. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Preville S, Chareyron P, Briolay J, Klemenz R, Arrigo A-P. Constitutive expression of human hsp27, Drosophila hsp27, or human αB-crystallin confers resistance to TNF- and oxidative stress-induced cytotoxicity in stably transfected murine L929 fibroblasts. J Immunol. 1995;154:363–374. [PubMed] [Google Scholar]
- Miron T, Vancompernolle K, Vandekerckhove J, Wilchek M, Geiger B. A 25-kD inhibitor of actin polymerization is a low molecular mass heat shock protein. J Cell Biol. 1991;114:255–261. doi: 10.1083/jcb.114.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyui N, Tamura K, Mizuno K, Ishigami T, Yabana M, Kihara M, Fukamizu A, Ochiai H, Umemura S, Murakami K, et al. Angiotensin II is not necessary for stretch-induced MAP kinase activation in cardiomyocytes. Biochem Biophys Res Commun. 1997;235:36–41. doi: 10.1006/bbrc.1997.6706. [DOI] [PubMed] [Google Scholar]
- Pennisi E. Expanding the eukaryote's cast of chaperones. Science. 1996;274:1613–1614. doi: 10.1126/science.274.5293.1613. [DOI] [PubMed] [Google Scholar]
- Rao PV, Horwitz J, Zigler JS., Jr Chaperone-like activity of α-crystallin. J Biol Chem. 1994;269:13266–13272. [PubMed] [Google Scholar]
- Reddy S, Smith DBJ, Rich MM, Leferovich JM, Reilly P, Davis BM, Taran K, Rayburn H, Bronson R, Cros D, et al. Mice lacking the myotonic dystrophy protein kinase develop a late onset progressive myopathy. Nat Genet. 1996;13:325–335. doi: 10.1038/ng0796-325. [DOI] [PubMed] [Google Scholar]
- Rutherford SL, Zuker CS. Protein folding and the regulation of signaling pathways. Cell. 1994;79:1129–1132. doi: 10.1016/0092-8674(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Sabouri LA, Mahadevan MS, Narang M, Lee DSC, Surh LC, Korneluk RG. Effect of the myotonic dystrophy (DM) mutation on mRNA levels of the DM gene. Nat Genet. 1993;4:233–238. doi: 10.1038/ng0793-233. [DOI] [PubMed] [Google Scholar]
- Sasagawa N, Sorimachi H, Maruyama K, Arahata K, Ishiura S, Suzuki K. Expression of a novel human myotonin protein kinase (MtPK) cDNA clone which encodes a protein with a thymopoietin-like domain in COS cells. FEBS Lett. 1994;351:22–26. doi: 10.1016/0014-5793(94)00808-6. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Yoshida M, Ozawa E. Mammalian α1- and β1-syntrophin bind to the alternative splice-prone region of the dystrophin COOH terminus. J Cell Biol. 1995;128:373–381. doi: 10.1083/jcb.128.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneja KL, McCurrach M, Schalling M, Housman D, Singer RH. Foci of trinucleotide repeat transcripts in nuclei of myotonic dystrophy cells and tissues. J Cell Biol. 1995;128:995–1002. doi: 10.1083/jcb.128.6.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Pegoraro E, Menegazzo E, Gennarelli M, Hoop RC, Angelini C, Hoffman EP. Myotonic dystrophy: evidence for a possible dominant-negative RNA mutation. Hum Mol Genet. 1995;4:599–606. doi: 10.1093/hmg/4.4.599. [DOI] [PubMed] [Google Scholar]
- Whiting EJ, Waring JD, Tamai K, Somerville M J, Hincke M, Staines WA, Ikeda J-E, Korneluk RG. Characterization of myotonin dystrophy kinase (DMK) protein in human and rodent muscle and central nervous tissue. Hum Mol Genet. 1995;4:1063–1072. doi: 10.1093/hmg/4.6.1063. [DOI] [PubMed] [Google Scholar]