Abstract
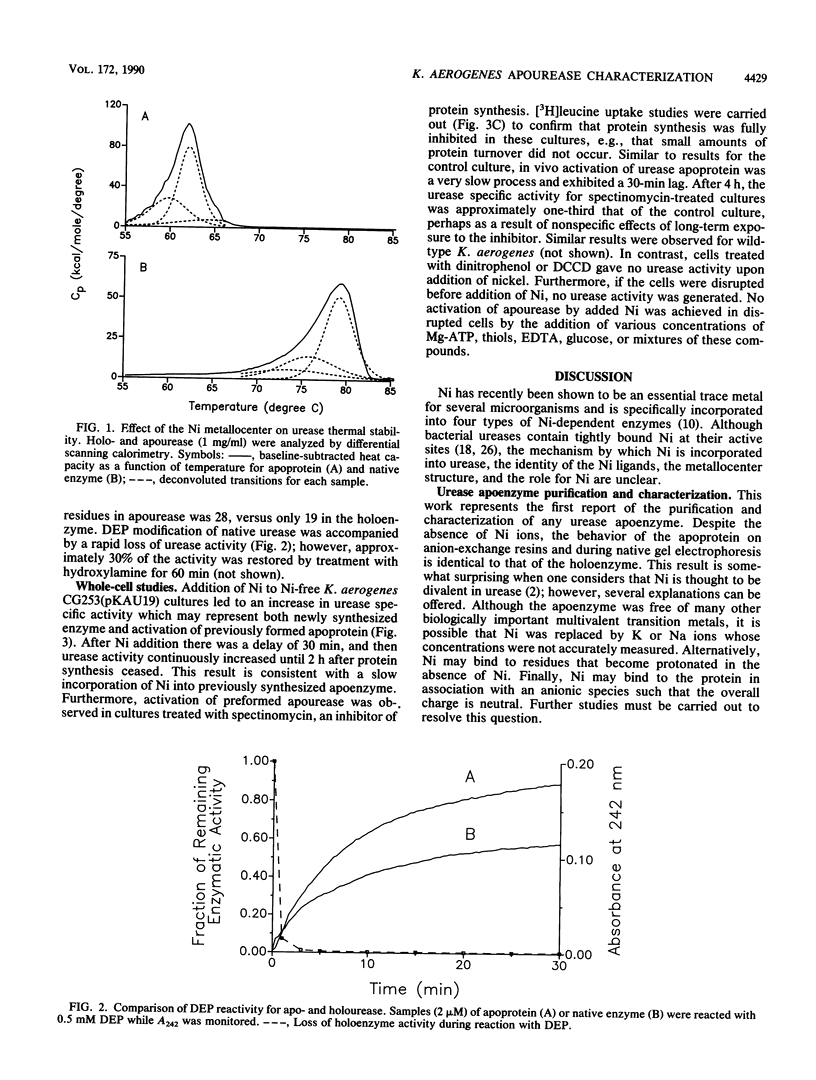
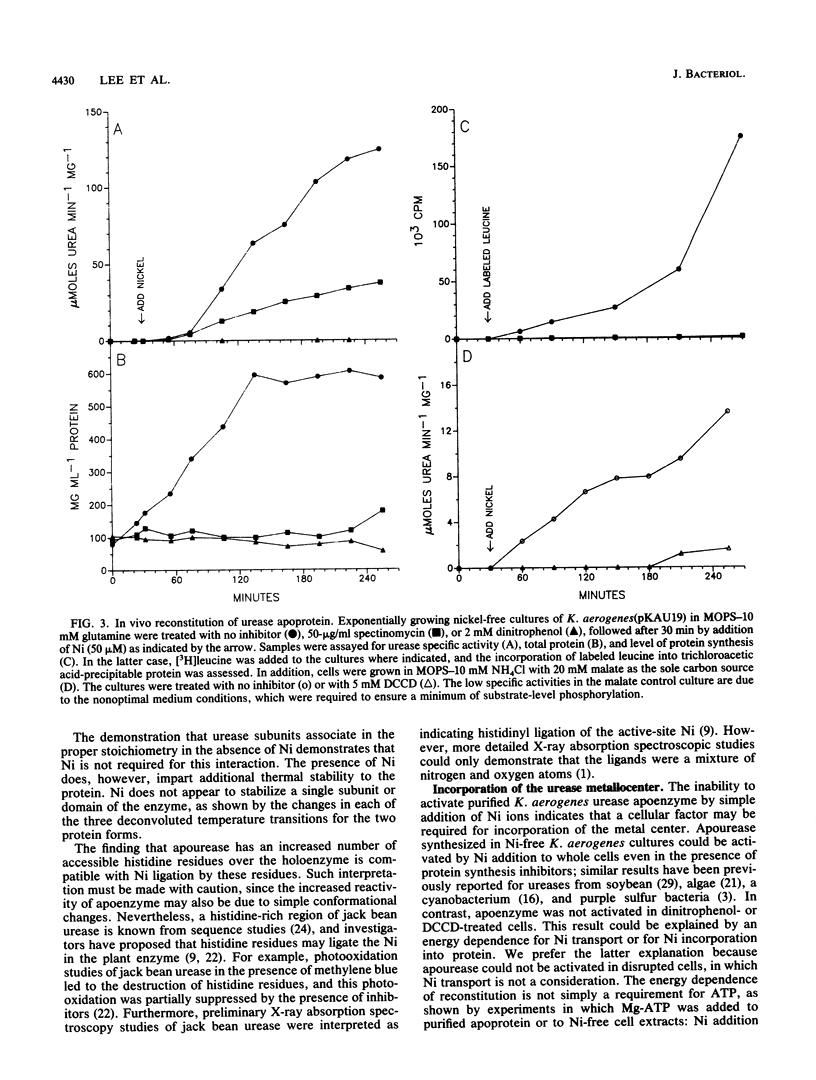
Urease was purified from recombinant Klebsiella aerogenes which was grown in the absence of nickel. The protein was inactive and contained no transition metals, yet it possessed the same heteropolymeric structure as native enzyme, demonstrating that Ni is not required for intersubunit association. Ni did, however, substantially increase the stability of the intact metalloprotein (Tm = 79 degrees C) compared with apoenzyme (Tm = 62 degrees C), as revealed by differential scanning calorimetric analysis. An increased number of histidine residues were accessible to diethyl pyrocarbonate in apourease compared with holoenzyme, consistent with possible Ni ligation by histidinyl residues. Addition of Ni to purified apourease did not yield active enzyme; however, urease apoenzyme was very slowly activated in vivo by addition of Ni ions to Ni-free cell cultures, even after treatment of the cells with spectinomycin to inhibit protein synthesis. In contrast, sonicated cells and cells treated with dinitrophenol or dicyclohexylcarbodiimide were incapable of activating apourease. These results indicate that apourease activation is an energy-dependent process that is destroyed by cell disruption.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alagna L., Hasnain S. S., Piggott B., Williams D. J. The nickel ion environment in jack bean urease. Biochem J. 1984 Jun 1;220(2):591–595. doi: 10.1042/bj2200591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson E. W., Howe H. B., Jr Reversion and interallelic complementation at four urease loci in Neurospora crassa. Mol Gen Genet. 1978 Oct 24;165(3):277–282. doi: 10.1007/BF00332527. [DOI] [PubMed] [Google Scholar]
- Dixon N. E., Gazzola C., Asher C. J., Lee D. S., Blakeley R. L., Zerner B. Jack been urease (EC 3.5.1.5). II. The relationship between nickel, enzymatic activity, and the "abnormal" ultraviolet spectrum. The nickel content of jack beans. Can J Biochem. 1980 Jun;58(6):474–480. doi: 10.1139/o80-063. [DOI] [PubMed] [Google Scholar]
- Dixon N. E., Gazzola T. C., blakeley R. L., Zermer B. Letter: Jack bean urease (EC 3.5.1.5). A metalloenzyme. A simple biological role for nickel? J Am Chem Soc. 1975 Jul 9;97(14):4131–4133. doi: 10.1021/ja00847a045. [DOI] [PubMed] [Google Scholar]
- Eskew D. L., Welch R. M., Cary E. E. A simple plant nutrient solution purification method for effective removal of trace metals using controlled pore glass-8-hydroxyquinoline chelation column chromatography. Plant Physiol. 1984 Sep;76(1):103–105. doi: 10.1104/pp.76.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillingame R. H. Identification of the dicyclohexylcarbodiimide-reactive protein component of the adenosine 5'-triphosphate energy-transducing system of Escherichia coli. J Bacteriol. 1975 Nov;124(2):870–883. doi: 10.1128/jb.124.2.870-883.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasnain S. S., Piggott B. An EXAFS study of jack bean urease, a nickel metalloenzyme. Biochem Biophys Res Commun. 1983 Apr 15;112(1):279–283. doi: 10.1016/0006-291x(83)91827-2. [DOI] [PubMed] [Google Scholar]
- Hausinger R. P. Nickel utilization by microorganisms. Microbiol Rev. 1987 Mar;51(1):22–42. doi: 10.1128/mr.51.1.22-42.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mackay E. M., Pateman J. A. Nickel requirement of a urease-deficient mutant in Aspergillus nidulans. J Gen Microbiol. 1980 Jan;116(1):249–251. doi: 10.1099/00221287-116-1-249. [DOI] [PubMed] [Google Scholar]
- Meyer-Bothling L E, Polacco J C, Cianzio S R. Pleiotropic soybean mutants defective in both urease isozymes. Mol Gen Genet. 1987 Oct;209(3):432–438. doi: 10.1007/BF00331146. [DOI] [PubMed] [Google Scholar]
- Mobley H. L., Hausinger R. P. Microbial ureases: significance, regulation, and molecular characterization. Microbiol Rev. 1989 Mar;53(1):85–108. doi: 10.1128/mr.53.1.85-108.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulrooney S. B., Lynch M. J., Mobley H. L., Hausinger R. P. Purification, characterization, and genetic organization of recombinant Providencia stuartii urease expressed by Escherichia coli. J Bacteriol. 1988 May;170(5):2202–2207. doi: 10.1128/jb.170.5.2202-2207.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulrooney S. B., Pankratz H. S., Hausinger R. P. Regulation of gene expression and cellular localization of cloned Klebsiella aerogenes (K. pneumoniae) urease. J Gen Microbiol. 1989 Jun;135(6):1769–1776. doi: 10.1099/00221287-135-6-1769. [DOI] [PubMed] [Google Scholar]
- Sakaguchi K., Mitsui K., Kobashi K., Hase J. Photo-oxidation of Jack bean urease in the presence of methylene blue. J Biochem. 1983 Mar;93(3):681–686. doi: 10.1093/jb/93.3.681. [DOI] [PubMed] [Google Scholar]
- Takishima K., Suga T., Mamiya G. The structure of jack bean urease. The complete amino acid sequence, limited proteolysis and reactive cysteine residues. Eur J Biochem. 1988 Jul 15;175(1):151–165. doi: 10.1111/j.1432-1033.1988.tb14177.x. [DOI] [PubMed] [Google Scholar]
- Todd M. J., Hausinger R. P. Competitive inhibitors of Klebsiella aerogenes urease. Mechanisms of interaction with the nickel active site. J Biol Chem. 1989 Sep 25;264(27):15835–15842. [PubMed] [Google Scholar]
- Todd M. J., Hausinger R. P. Purification and characterization of the nickel-containing multicomponent urease from Klebsiella aerogenes. J Biol Chem. 1987 May 5;262(13):5963–5967. [PubMed] [Google Scholar]
- Winkler R. G., Blevins D. G., Polacco J. C., Randall D. D. Ureide catabolism in nitrogen-fixing legumes. Trends Biochem Sci. 1988 Mar;13(3):97–100. doi: 10.1016/0968-0004(88)90049-7. [DOI] [PubMed] [Google Scholar]
- Winkler R. G., Polacco J. C., Eskew D. L., Welch R. M. Nickel is not required for apourease synthesis in soybean seeds. Plant Physiol. 1983 May;72(1):262–263. doi: 10.1104/pp.72.1.262. [DOI] [PMC free article] [PubMed] [Google Scholar]



