Abstract
Recently, we have described a panel of metastasis-associated antigens in the rat, i.e., of molecules expressed on metastasizing, but not on nonmetastasizing tumor lines. One of these molecules, recognized by the monoclonal antibody D6.1 and named accordingly D6.1A, was found to be abundantly expressed predominantly on mesenchyme-derived cells. The DNA of the antigen has been isolated and cloned. Surprisingly, the gene product proved to interfere strongly with coagulation.
The 1.182-kb cDNA codes for a 235–amino acid long molecule with a 74.2% homology in the nucleotide and a 70% homology in the amino acid sequence to CO-029, a human tumor-associated molecule. According to the distribution of hydrophobic and hydrophilic amino acids, D6.1A belongs to the tetraspanin superfamily. Western blotting of D6.1A-positive metastasizing tumor lines revealed that the D6.1A, like many tetraspanin molecules, is linked to further membrane molecules, one of which could be identified as α6β1 integrin. Transfection of a low-metastasizing tumor cell line with D6.1A cDNA resulted in increased metastatic potential and provided a clue as to the functional role of D6.1A. We noted massive bleeding around the metastases and, possibly as a consequence, local infarctions predominantly in the mesenteric region and all signs of a consumption coagulopathy. By application of the D6.1 antibody the coagulopathy was counterregulated, though not prevented.
It has been known for many years that tumor growth and progression is frequently accompanied by thrombotic disorders. Our data suggest that the phenomenon could well be associated with the expression of tetraspanin molecules.
Tumor progression is a multistep process that requires detachment from the primary tumor, migration through the extracellular matrix and penetration through the basal membrane, only in case of hematogenous metastasis adaptation to the circulation pressure and attachment to the endothelia of the vessel wall and in lymphatic and hematogenous spread settlement and growth in distant organs (19).
According to this array of distinct requirements, metastatic cells as well as their surrounding cells have been shown to display qualitatively or quantitatively altered patterns of gene expression (38). These comprise cell–cell and cell–matrix adhesion molecules (18), production and activation of a variety of matrix-degrading enzymes, their activators and inhibitors (61), and the components of the extracellular matrix (44). It is important to note that so far no single metastogen has been identified; instead, all metastasis-associated alterations are part of physiological programs, like placentation, embryogenesis, organogenesis, stem cell differentiation, and lymphocyte activation (15, 24, 56, 66). One family of metastasis-associated molecules are variant isoforms of the adhesion molecule CD44 (70), which meanwhile are known to be involved particularly in lymphocyte activation (3, 21, 63), but also in organogenesis (20, 26, 33, 67). The metastasizing rat adenocarcinoma line whereof CD44v was originally isolated (22) displays on its surface a panel of additional molecules that are detected neither on the nonmetastasizing subline (39), nor on a variety of nonmetastasizing tumors of different histology, but are expressed on other metastasizing rat tumors (13). This strengthens the contention that they are not individual-specific entities, but indeed metastasis associated. Four of these antigens have been looked at for their expression under physiological conditions and all four were found to be expressed by a variety of tissues in the adult rat, besides being upregulated during the implantation process (13). Here we report on one of these antigens, D6.1A, which has been cloned and sequenced. The molecule belongs to the tetraspanin superfamily and is the homologue of the human carcinoma-associated CO-029 antigen (62). Like most of the tetraspanin molecules (9, 31, 37, 50, 64) it appears to associate with further membrane-integrated structures. Transfection of the molecule in a low-metastasizing tumor line resulted in an increased metastatic capacity. Most surprisingly, animals suffered from peri-tumoral bleeding, local infarctions in distant organs and all signs of a consumption coagulopathy.
Materials and Methods
Animals, Tumors, and Monoclonal Antibodies
BDX rats were obtained from Charles River (Sulzfeld, Germany). They were kept under specific pathogen-free conditions and fed with conventional diet and water at libitum. They were used for experiments at the age of 8–10 wk.
The following lines were used: BSP73ASML (ASML; metastasizing pancreatic adenocarcinoma of the BDX strain)1, BSp73AS (AS; nonmetastasizing pancreatic adenocarcinoma of the BDX strain; references 40, 71), BSp73AS-14 (AS-14; BSp73AS cells transfected with CD44v4-v7 cDNA; reference 22), Regressor and Progressor, two metastasizing colon carcinomas of the BDIX strain, kindly provided by F. Martin (47, 51). BSp73AS-D6.1A (BSp73AS transfected with D6.1A cDNA; AS-D6.1A) is described below.
The mAbs D6.1 (mIgG1) and D5.7 (mIgG1) recognize surface molecules on metastasizing rat tumor lines and have been recently described (13, 39); mAb 3-9 (mIgG1) binds to a gallium chelate complex (72) and was used as control antibody. Ox42 (ECACC, catalog no. 87081803; mIgG2a) binds to the integrin αm chain. Anti-β1 (Ha2/5; hamster IgM), anti-α3 (AB1920; rabbit IgG), anti-α4 (MR-α4-1; mouse IgG2a), anti-α5 (Hmα5-1; hamster IgG), and anti-α6β1 (MB1410; mIgG1), as well as FITC-labeled polyclonal anti-hamster Ig, anti-rabbit IgG, and anti-mouse IgG were obtained commercially.
cDNA Library and Selection of Clones Coding for Surface Molecules
mRNA was prepared from the colon carcinoma cell line Regressor and oligo(dT)/NotI–primed cDNA was prepared using the Librarian Kit (Invitrogen, San Diego). The cDNA was inserted unidirectionally into the mammalian expression vector pcDNA3 after ligation of BstXI adapters and restriction digestion with NotI. The ligated DNA was transformed into Escherichia coli/TOP10F′. The size of the library was estimated to ∼2 × 107 clones.
For the isolation of clones coding for metastasis-associated surface molecules, the cDNA was transfected into COS-7 cells by DEAE dextran. Positive clones were enriched by panning (4, 52). In short, 5 × 106 cells were incubated with 10 μg/ml purified mAb D6.1 (60 min at 4°C). Nonbound antibody was removed by washing and the antibody-coated cells were poured on petri dishes coated with polyclonal goat anti–mouse IgG. After 3 h incubation at room temperature, nonadherent cells were removed by washing. Plasmid DNA of the adherent cells was isolated according to Hirt (27). Genomic DNA and proteins were separated by centrifugation. The plasmid DNA/RNA mixture was digested with RNase A (20 μg/ml) and proteinase K (50 μg/ml), followed by a phenol–chloroform extraction to remove remaining protein. The plasmid DNA was precipitated and used for the transformation of E. coli by electroporation. The procedure was repeated three times and COS cell transfection was performed by spheroplast fusion. After the third panning procedure the plasmid DNA of 12 single colonies was used for DEAE transfection of COS-7 cells. After 3 d, cells were analyzed for antigen expression by FACS®. The cDNA of positive clones was sequenced according to the method of Sanger et al. (49).
Fluorescence In Situ Hybridization
For isolation of chromosomal clones of the CO-029 gene a P1-derived artificial chromosome (PAC; 35) library was screened with the D6.1A cDNA (ICRF library no. 704; Resource Center Primary Data Base, German Human Genome Project, Max Planck Institute for Molecular Genetics, Berlin, Germany). Two positive clones (LLNLP704D12229Q13 and LLNLP704M22126Q13) were isolated and used for fluorescence in situ hybridization (FISH) analysis. PAC-DNA was labeled with DIG-11-dUTP (Boehringer Mannheim GmbH, Mannheim, Germany) by nick translation. Suppression of repetitive sequences, denaturation, hybridization, and fluorescence detection were performed as described (14). Anti–DIG-mouse IgGκ (Boehringer Mannheim GmbH) and Cy3-conjugated sheep anti–mouse IgG (Dianova, Hamburg, Germany) were used to detect digoxigenin-labeled probes. Chromosomes were counterstained with DAPI. Analysis was performed using a Zeiss Axiophot microscope. Images were collected and merged using a cooled CCD camera (KAF 1400; Photometrics, Tuscon, AZ) and IPLab Spectrum software.
Transfection of a D6.1A-negative Tumor Line with D6.1A cDNA
The tumor line AS was transfected by electroporation. Transfected cells were selected by growth in medium containing 600 μg/ml G418. Surviving cells were either enriched by FACS® sorting and/or by single cell cloning. Distinct clones have been designated by subscripted roman numbers, i.e., AS-D6.1AI, AS-D6.1AII, and AS-D6.1AIII.
Western Blot and Immunoprecipitation of Biotinylated Membrane Proteins
For immunoprecipitation, surface membrane proteins were labeled with NHS-Biotin (Boehringer Mannheim GmbH) according to Meier et al. (41). Cells were lysed in immunoprecipitation buffer (10 mM Tris, pH 7.4, 150 mM NaCl, 1 mM MgCl2, 1 mM CaCl2, 2 mM PMSF, 5 mM iodoacetamid, and either 1% CHAPS or 1% NP-40) for 30 min at 4°C and cleared by centrifugation (13,000 rpm, 4°C, 30 min). Lysates were precleared overnight by addition of 1/10 vol protein G–Sepharose including anti-hamster Ig, where required (1:1 in PBS). Afterwards, lysates corresponding to ∼1–5 × 106 cells were incubated with mAb (5 μg/ml or one-fifth the volume of undiluted hybridoma supernatant) for 2 h at 4°C and subsequently, where required, with an anti-hamster Ig (2 h at 4°C). Protein G–Sepharose (1:1 in PBS, 1/10th of the total volume) was added for 60 min. Immune complexes were washed six times with immunoprecipitation buffer and eluted from protein G–Sepharose with Laemmli buffer. SDS-PAGE (34) was performed using gradient gels as indicated in the figure legends. Separated proteins were transferred to polyvinylidene difluoride (PVDF) membranes and bands were visualized after incubation with streptavidin– HRP using the ECL detection system (Amersham Buchler GmbH, Braunschweig, Germany). For the detection of coimmunoprecipitated D6.1A, immune complexes were separated by SDS-PAGE and blotted onto a PVDF membrane. Detection of D6.1A was performed according to standard procedures by successive incubation with D6.1 hybridoma supernatant and sheep anti–mouse IgG-HRP. For visualization the ECL detection system was used.
Adhesion and Proliferation Assays
In adhesion studies D6.1A-transfected clones as well as control clones were labeled with 51Cr and were seeded either on a monolayer of adherent cells or on plates coated with components of the extracellular matrix, i.e., hyaluronic acid (HA), collagen type I (CO-I), type III (CO-III), and type IV (CO-IV), laminin (LA), fibronectin (FN), and vitronectin (VN), or on plates coated with the D6.1 antibody. The antibody as well as the components of the extracellular matrix, except for HA were coated at 10 μg/ml; HA was coated at a concentration of 100 μg/ml. Where indicated, anti-D6.1 (10 μg/ml) was added to the culture medium. Cells were incubated for 20 min to 4 h and were washed repeatedly. The adherent cells were lysed by 2% SDS, transferred into counting vials, and counted in a γ-counter. The percentage of adherent cells is shown.
Proliferation of D6.1A-transfected cells was tested by [3H]thymidine incorporation. Cells were seeded either on substrate-coated or on D6.1-coated plates, where the supernatant contained either a control antibody or D6.1. [3H]thymidine was added immediately or after 12–72 h. After 8 h of incubation with [3H]thymidine, cells were trypsinized and transferred to a β-counter to evaluate thymidine incorporation.
Coagulation Assays
The following coagulation parameters were evaluated in the sera of tumor-bearing rats by automatic sample processing (Elektra 1600; Bade, Munich, Germany): Quick, partial prothrombin time (PTT) and the fibrinogen content of the serum. The Quick assay represents a group test for exogenous coagulation factors. After addition of thromboplastin reagent (thromboplastin, Ca++, phospholipids) the time for coagulation (fibrinogen → fibrin) will be determined. PPT is a group test for endogenous coagulation factors. Plasma will be incubated with contact activators that initiate activation of factor XII, XI, and high molecular mass kininogen. Phospholipids are added to replace platelets. CaCl2 is added after preactivation and the coagulation time is determined. For the determination of fibrinogen, the plasma is diluted in Veronal buffer and prewarmed before the addition of thrombin reagent to determine the coagulation time. The D-dimer assay determines exclusively fragments of fibrin (not of fibrinogen). The fragments are created by cross-linking the D part of two fibrin molecules, which are separated from the remaining part of the molecule by plasmin. D-dimers are elevated in the state of overactivity of the clotting system. They are determined by an ELISA using a D-dimer–specific antibody.
Metastasis Assay
BDX rats received 5 × 105 tumor cells, subcutaneously (s.c.), intravenously (i.v.), or intrafootpad (i.f.p.). In the latter case the tumor and the draining lymph node were excised by amputation at the knee as soon as the primary tumor reached a diameter of 0.5 cm. Animals were observed for local tumor growth, weight loss, anemia, and shortage of breath and were killed before reaching a moribund stage. Metastasis formation was controlled macroscopically and by histology.
Results
Recently, we have reported on the physiological expression of a molecule detected only on metastatic sublines of a pancreatic and a colon adenocarcinoma, but not on nonmetastatic lines (13). On one of the metastasizing tumor lines, ASML, the molecule, recognized by the mAb D6.1, has a molecular mass of 24–40 kD. The epitope recognized by D6.1 was sensitive to reducing agents, i.e., it could only be detected under nonreducing conditions in Western blots. By tunicamycin treatment, the 24-kD band became by far the most prominent, suggesting that the D6.1-antigen (D6.1A) is a glycoprotein. Evaluation of whether the molecule may be expressed not only on metastasizing tumor cells, but also on nontransformed cells revealed that the D6.1A is expressed in many different tissues including epithelia, endothelia, smooth and striated musculature, as well as nerve tissues. During ontogeny its expression was strongly upregulated in stromal elements at the site of implantation, in the decidua and the myometrium towards the placenta. Besides, a population of bone marrow cells and the majority of monocytes stained with D6.1, while the molecule was not detected on resting B or T cells. By this abundant pattern of expression on the one side and the absence of the D6.1A on nonmetastasizing rat tumor cells on the other, we became interested in exploring its molecular nature to possibly unravel its function.
Cloning of D6.1A cDNA
After three rounds of transfection of COS7 cells with plasmid DNA of a cDNA library derived from a metastatic rat colon carcinoma line and transfection of COS7 cells with plasmid DNA of 12 bacterial colonies, two clones were isolated that gave positive FACS® staining with the D6.1 mAb. Western blot analysis confirmed that the cells expressed a D6.1-reactive molecule of an estimated molecular mass of 24 kD. Sequencing of the D6.1A cDNA revealed a 1,182-bp sequence with an open reading frame for 235 amino acids (AA) starting with the 5′ ATG codon, which is flanked by a consensus sequence for the initiation of translation. The 5′ untranslated region spans 229 nucleotides. Within the 227 bases of the 3′ untranslated region there is a consensus sequence for poly(A)+ addition (Fig. 1). The nucleotide sequence of the D6.1A revealed a 74.2% homology to a human tetraspanin molecule, CO-029, which has been found on lung, colon, and pancreatic tumors, but has not been detected on nontransformed cells (62).
Figure 1.

Nucleotide sequence of D6.1A in comparison to CO-029. The nucleotide sequences of D6.1 (bold) and of CO-029 are shown. Congruent nucleotides are marked by asterisks. The consensus sequence for the initiation of translation is underlined, the consensus sequence for addition of the poly(A)+ tail is underlined with dots, and the stop codon is double underlined. These data are available from GenBank/EMBL/DDBJ under accession number Y13275.
Genes of several members of the tetraspanin superfamily have been found in clusters (54), providing evidence for gene duplication. Therefore, we wanted to know whether D6.1A/CO-029 may also be located close to additional tetraspanin genes. This was tested in the human system by in situ hybridization. As shown in Fig. 2, the CO-029 gene was found on q12 of chromosome 12, where, indeed, two additional members of the tetraspanin family, SAS and CD63, have been located (29).
Figure 2.
Chromosomal localization of CO-029. The FISH analysis of the CO-029 gene was done with PAC clone LLNLP704D12229Q13. The hybridization was performed with a metaphase preparation of immortalized lymphocytes. A G-banded chromosome 12 is shown on the top of the figure and the same metaphase spread is shown in the FISH on the bottom. CO-029 maps to chromosomal band 12q12 (arrow).
The D6.1A Molecule
The AA sequence of D6.1A and CO-029 show up to 70% identity and up to 80% similarity. According to the AA sequence, the D6.1A molecule has an isoelectric point of pH 7.63 and a theoretical molecular mass of 25.58 kD, which corresponds to the smallest molecule detected on the metastatic Regressor/Progressor and ASML tumor lines. The distribution of hydrophobic and hydrophilic AA classifies the molecule, like CO-029, as an integral transmembrane protein of type III (69; Fig. 3). Four hydrophobic sequences of ∼25 AA are separated by hydrophilic AA. Between the transmembrane domains 1 and 2 there is a small and between the transmembrane domains 3 and 4 there is a larger extracellular domain. The NH2 and the COOH terminus, as well as a short sequence of 4 AA between the transmembrane domains 2 and 3 are located in the cytoplasm. The molecule contains the 11 conserved cystine residues of the tetraspanin family. There are two potential N-glycosylation sites (one on CO-029). Near the COOH terminus there is a consensus sequence for interaction with tyrosine phosphatases. The AA YCQI are a consensus sequence for association with SH2 domain containing proteins (59).
Figure 3.
Amino acid sequence and proposed organization of D6.1A. (A) Amino acid sequence of D6.1A and CO-029: the 11 conserved cystine residues of the tetraspanin superfamily molecules are printed in bold, the two potential N-glycosylation sites are underlined by dots and the four hydrophobic regions are underlined. The consensus sequence for binding of tyrosine phosphatases are printed in italics. The AA sequence YCQI (double underlined) represents a recognition sequence for the association with SH2 domain containing proteins. The identity between D6.1A and CO-029 is 70.2%, the similarity is 81.7%. (B) Hydrophobicity plot according to Kyte-Doolittle (33a) the peptide structure (according to the HUSAR program) of the AA sequence shown above reveals the four hydrophobic regions and the intermitted hydrophilic AA. The structure allows to group the molecule into the transmembrane proteins type III.
Since several members of the tetraspanin superfamily have been described to associate with other membrane proteins, particularly with β1 integrins (reviewed in references 23, 37), it became of interest whether D6.1A would also associate with integrins. When the D6.1A was precipitated by using a mild detergent (CHAPS), it mainly coprecipitated from ASML and Progressor with one molecule in the molecular range of 120–130 kD (Fig. 4 A). By the described frequent association of tetraspanin molecules with integrins we first evaluated by FACS® analysis the expression of several integrins on ASML, Progressor, and AS (Fig. 4 B). All lines expressed the β1 chain, but the expression level was higher on ASML and Progressor as compared with AS cells. Interestingly, when AS cells were transfected with D6.1A cDNA, expression of the β1 integrin chain was found to be augmented. A similar phenomenon was seen with the β2 chain. The β2 chain was not expressed by ASML and Progressor, but on ∼20% of AS cells. Again, expression of the β2 chain was upregulated on D6.1A cDNA–transfected AS cells. None of the lines expressed the β3, the α4 and the α5 chain. All lines reacted with an α6β1-specific antibody. The αm chain was not expressed on ASML and Progressor, but on AS and D6.1A cDNA–transfected AS cells.
Figure 4.
Coimmunoprecipitation of D6.1A. (a) ASML, AS, and Progressor (2 × 106 cells/lane) were biotinylated and subsequently lysed using CHAPS (C) and NP-40 (N) containing buffers, respectively. The immunoprecipitation was performed with D6.1, D5.7, and 3-9 (control IgG1). SDS-PAGE (4–20% gradient gel) was run under reducing conditions. Using the mild detergent CHAPS a high molecular mass protein of 120–130 kD has been precipitated with D6.1 from ASML and Progressor, but not from AS. The protein is not seen with the control antibody 3-9, nor with D5.7, which recognizes a molecule expressed at comparable intensity to D6.1A on ASML and Progressor. Furthermore, the molecule could not be detected using the strong detergent NP-40. (b) Expression of integrins on ASML, Progressor, AS, and D6.1A cDNA–transfected AS cells was evaluated by FACS® analysis. The lines were stained with anti-β1, anti-β2, anti-β3, anti-α3, anti-α4, anti-α5, anti-α6β1, anti-αm, and, for comparison with the mAb D6.1 and D5.7. The negative control (second, FITC-labeled antibody only) and the stained cells are shown. All four lines express β1, α3, and α6, but are negative for β3, α4, and α5 integrin. The β2 and the αm chain are only expressed on AS and AS-D6.1A cells. The metastasis-associated molecules, D6.1A and D5.7A, were expressed on ASML and Progressor, but not on AS cells. It should be noted that D6.1A, D5.7A, and β1 integrin are expressed at comparable intensity on ASML and Progressor cells. (c) ASML cells were lysed using CHAPS containing buffer. The immunoprecipitation was performed with D6.1, D5.7, and anti-β1, anti-α4, and anti-α5 integrins. Precipitates, corresponding to 2 × 105 cells were run under nonreducing conditions on a 12% SDS gel. After blotting the membrane was incubated with D6.1. Using the mild detergent CHAPS, D6.1A was precipitated with D6.1 and with the anti-β1 integrin. A very weak band was seen with D5.7. There was not precipitate with the anti-α4 and the anti-α5 integrins, α4 and α5 being not expressed on ASML cells. (d and e) Progressor cells were lysed using CHAPS containing buffer and the immunoprecipitation was performed as described in c. For immunoprecipitation D6.1, D5.7, anti-β1, anti-α4, and anti-α5 (d) and anti-β1, anti-α3, anti-α4, anti-α5, and anti-α6β1 (e) integrins were used. Precipitates, corresponding to 2 × 105 cells were run under nonreducing conditions on a 12% SDS gel. After blotting the membrane was incubated with D6.1. In lysates of Progressor cells, D6.1A was precipitated with D6.1, with the anti-β1 integrin, with the α3 and with the α6β1 integrin.
By immunoprecipitates of lysates from ASML (Fig. 4 C) and Progressor (Fig. 4 D), it could be demonstrated that D6.1A was, indeed, associated with β1 integrin. No precipitate was detected with anti-α4 and anti-α5, which are not expressed on ASML and Progressor (Fig. 4 C). Furthermore, as shown for Progressor (Fig. 4 E), D6.1A coprecipitated with α3 as well as with α6β1.
Tetraspanin molecules have also been described to colocalize with CD44 (32). Since expression of CD44v4-v7, found at high level on the ASML as well as on the Regressor and Progressor lines, is associated with tumor progression, it was tempting to speculate that D6.1A might coprecipitate with CD44 variant isoforms. But a CD44-containing coprecipitate could be detected with none of the lines (data not shown). Finally we were interested whether D6.1A may coprecipitate with additional metastasis-associated molecules detected on ASML and Progressor (13, 39). Using precipitates of ASML, we noted a very weak staining with D6.1 of a molecule (D5.7A) precipitated by D5.7 (Fig. 4 C), which could be identified as the rat homologue of EGP314 (Würfel et al., manuscript in preparation). According to FACS® analysis (see Fig. 4 B), D6.1A, D5.7A, and the β1 integrin are expressed at comparable levels at the ASML and the Progressor lines. Thus, if at all, the association between D6.1A and D5.7A is much weaker than the association between D6.1A and the integrins (Fig. 4, C–E). No coprecipitate could be detected using the strong detergent NP-40 (data not shown).
D6.1A in Cell–Cell and Cell–Substrate Adhesion
Tetraspanin molecules, mainly by their nature as molecular facilitators (37), have been described to influence cell adhesion. To evaluate whether D6.1A may be involved in cell substrate adhesion the D6.1A-positive Progressor line, as well as D6.1A-transfected AS, clones were tested for adhesion to a panel of extracellular matrix molecules. As shown in Fig. 5, transfection of AS with D6.1 cDNA led to a slightly increased adhesion to plastic and to HA-coated plates. Adhesion to other components of the extracellular matrix was not altered by D6.1A transfection. By addition of D6.1 to the culture medium, adhesion to plastic and HA was reduced, which confirmed some involvement of D6.1A in adhesion to plastic and HA. Besides, adhesion to collagen type I was diminished. Different from the transfected clones, adhesion of the metastasizing tumor lines Regressor (not shown) and Progressor to plastic and extracellular matrix molecules was not influenced by the D6.1 mAb. Thus, D6.1A does not appear to be a cell–substrate adhesion molecule, but can probably, in concert with additional surface molecule(s), alter adhesiveness in general (plastic) and towards HA in particular.
Figure 5.

Influence of D6.1A on substrate adhesion. (A) Mock- and D6.1A-transfected AS cells were labeled with 51Cr and 5 × 104 cells were seeded on 96-well plates, uncoated or coated with a variety of substrates. The percentage of cells (mean of triplicates + SD) adhering after 60 min of incubation are shown. Similar values were observed after 30 and 120 min, while at later time points differences were vanishing (data not shown). Adhesion of the two D6.1A-transfected clones to plastic and HA was increased. (B) A constitutively D6.1A-positive line (Progressor) and 2 D6.1A-transfected AS clones (AS-D6.1AI and AS-D6.1AII) were labeled with 51Cr and 5 × 104 cells were seeded on 96-well plates, uncoated or coated with a variety of substrates. The culture medium contained titrated amounts of an IgG1 mAb of irrelevant specificity or of the D6.1 mAb. The percentage of cells (mean of triplicates + SD) adhering after 60 min of incubation in the presence of 10 μg/ml mAb are shown. The D6.1 mAb interferes with the binding of the transfected lines towards plastic, HA and CO-I. The antibody had no influence on the binding of the Progressor line.
Cell–cell adhesion was not influenced by D6.1A. This accounted for the adhesiveness of D6.1A-positive cells to D6.1A-negative as well as D6.1A-positive cells (data not shown).
D6.1A and Cell Proliferation
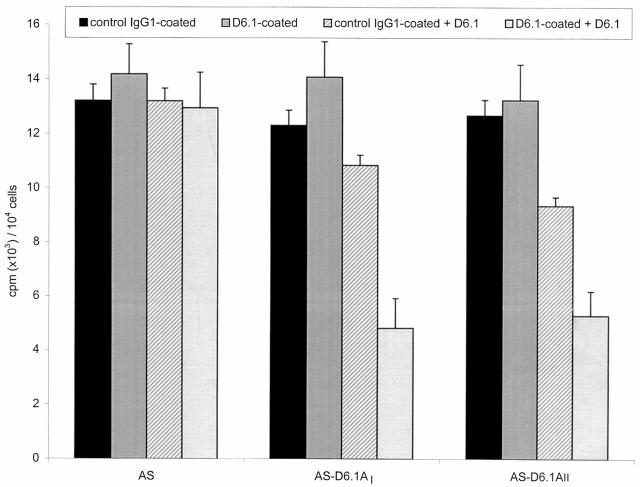
Tetraspanin molecules have also been described to be involved in cell proliferation, mainly via associated molecules (reviewed in reference 29). A possible involvement of D6.1A in proliferation was tested by two approaches. First we evaluated proliferative activity of the D6.1A-positive tumor lines Regressor and Progressor seeded on substrate-coated plates in dependence on the presence of the D6.1 mAb. Only one of the two lines showed enhanced proliferation in response to fibronectin, which could be inhibited by D6.1 (data not shown). Because the transfected clones provide a more stringent system, next we compared the influence of plastic-bound D6.1 on the proliferative activity of D6.1A-transfected vs mock-transfected lines. As could have been expected, proliferation of D6.1A-negative clones was unaltered and addition of D6.1 to the culture medium had no effect. Instead, proliferation of the D6.1A-transfected clones was inhibited in the presence of D6.1 in the culture medium, although it was unaltered on D6.1-coated plates (Fig. 6). Because coated D6.1 did not influence proliferative activity, this finding, too, argues against a direct involvement of D6.1A in cell proliferation, but supports a role as molecular facilitator (37).
Figure 6.
Influence of D6.1A on proliferative activity. Mock- and D6.1A-transfected AS cells were seeded (1 × 104 cells/well) on control IgG1- or D6.1-coated plates. Where indicated the medium contained 10 μg/ml of D6.1. After 24 h of incubation, [3H]thymidine was added for an additional 8 h, cells were harvested and incorporation of [3H]thymidine was determined in a β-counter. The mean of triplicates + SD is shown. Proliferation of AS-D6.1AI and AS-D6.1AII cells was inhibited in the presence of D6.1 in the culture medium.
D6.1A in Metastasis Formation
The D6.1A was originally described as a metastasis-associated molecule and was isolated from a metastasizing tumor line. However, by the circumstantial evidences of D6.1A functioning in concert with additional cell surface molecules, it appeared questionable whether transfection of a weakly metastasizing AS clone with only the D6.1A cDNA would result in tumor progression. In view of these considerations, the outcome of the in vivo metastasis assay was surprising.
Cells were either injected s.c., i.f.p., or i.v. After i.f.p. application, the local tumor was excised together with the popliteal lymph node when the primary tumor reached a mean diameter of 0.5 cm. In the first experiments, two D6.1A-transfected AS clones, AS-D6.1AI and AS-D6.1AII and a mock transfectant (empty vector), AS-mock, were tested. D6.1A expression, indeed, influenced in a subtle way metastasis formation. First, the D6.1A-transfected clones grew slower than the parental line (Table I and Fig. 7). This was seen particularly after the s.c. application, but became also evident after i.f.p. as well as after i.v. application by the prolonged survival time. The phenomenon was most pronounced for the AS-D6.1AI clone, which after i.f.p. application did not at all grow locally. Second, expression of D6.1A facilitated metastasis formation (Table II). After s.c. application of AS-D6.1A, 4 out of 12, after i.f.p. application 7 out of 8, and after i.v. application 9 out of 10 rats showed lung metastasis. The corresponding numbers in animals receiving AS-mock were 0 out of 5 (s.c.), 1 out of 5 (i.f.p.), and 2 out of 5 (i.v.). Third, AS-D6.1AI frequently metastasized to the lung without settlement in the lymph nodes (Table II). This was most apparent after i.f.p. application, where in all three animals that developed lung metastases no growth in the draining lymph nodes was observed. The phenomenon was also noted in some animals developing lung metastases after i.v. application. In the remaining rats, lymph nodes were macroscopically unaltered and the presence of tumor cells was only verified after in vitro culture of the excised nodes. After s.c. application of tumor cells, the axillary and the inguinal lymph nodes were infiltrated in most of the rats. Forth, and most impressively, expression of D6.1A caused massive bleeding in 27 out of 30 rats in the surrounding of the primary tumor and the metastases, even when the metastatic nodules appeared rather small. Frequently (in 15 out of 30 rats), bleeding around the metastases was accompanied by infarctions predominantly in the abdominal vessels, which led to local necrosis of the gut (either the ileum or the colon) or organs of the urogenital tract, like the uterine horns, the ovary, the kidney and the adrenal gland (Table II and Fig. 8).
Table I.
In Vivo Growth of D6.1A cDNA–transfected AS Clones
| Tumor line | Application* | Local tumor | Survival rate | Mean survival time | Range | P value‡ | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AS-mock | s.c. | 6/6 | 0/6 | 36 ± 3.8 | 32–44 | |||||||
| AS-D6.1AI | s.c. | 12/12 | 0/12 | 53 ± 5.7 | 39–58 | <0.001 | ||||||
| AS-mock | i.f.p./excision | 5/5 | 0/5 | 42 ± 14.3 | 30–64 | |||||||
| AS-D6.1AI | i.f.p./no excision | 0/5 | 2/5 | 103 ± 4.2 | 99–110 | <0.001 | ||||||
| AS-D6.1AII | i.f.p./excision | 5/5 | 0/5 | 49 ± 9.3 | 32–59 | 0.304 | ||||||
| AS-mock | i.v. | 0/5 | 29 ± 5.6 | 23–39 | ||||||||
| AS-D6.1AI | i.v. | 0/5 | 61 ± 22.8 | 30–100 | 0.021 | |||||||
| AS-D6.1AII | i.v. | 0/5 | 41 ± 10.1 | 30–58 | 0.041 |
Rats received 5 × 105 tumor cells either s.c., i.f.p. or i.v. After i.f.p. application the tumor and the draining lymph node was excised when the primary tumor reached a mean diameter of 0.5 cm, i.e., after 18 d for AS-mock and after 22 d for AS-D6.1AII. Animals which had received AS-D6.1AI did not develop a primary tumor.
Statistical significance of differences in the survival time have been calculated by the Student's t test.
Figure 7.

In vivo growth behavior of D6.1A-transfected lines. (A–C) A weakly metastasizing mock-transfected and two D6.1A-transfected AS clones (AS-D6.1AI and AS-D6.1AII; 5 × 105 cells) were injected s.c. (A), i.f.p. (B), or i.v. (C). Where indicated the local tumor and the draining lymph node were excised at a time point where the primary tumor reached 0.5 cm in diameter. The growth rate of the tumors (A), the number of surviving rats and the survival time (B and C) are shown. As can be seen, D6.1A-transfected cells grew more slowly (A). Tumorigenicity was not enhanced, but instead appeared to be slightly reduced, with some rats not developing a primary tumor or metastases after i.f.p. application (B). Data are derived from the same animals as analyzed in Table I and II.
Table II.
Growth Characteristics of D6.1A cDNA–transfected AS Clones
| Tumor line | Application* | Lymph node metastasis | Lung | Others | Special features | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Inguinal | Paraaortic/retroperit. | Axillary | ||||||||||||
| AS-mock | s.c. | 1/5 | 0/5 | 3/5 | 0/5 | 0/5 | None | |||||||
| AS-D6.1AI | s.c. | 6/12 | 2/12 | 8/12 | 4/12 | 0/12 | 7/12 Hemorrhaging | |||||||
| AS-mock | i.f.p./excision | 5/5 | 4/5 | 1/5 | 1/5 | 0/5 | None | |||||||
| AS-D6.1AI | i.f.p./no excision | 0/3 | 0/3 | 0/3 | 3/3 | 0/3 | 3/3 Hemorrhaging | |||||||
| AS-D6.1AII | i.f.p./excision | 5/5 | 5/5 | 4/5 | 4/5 | 0/5 | None | |||||||
| AS-mock | i.v. | 2/5 | 2/5 | 1/5 | 2/5 | 0/5 | None | |||||||
| AS-D6.1AI | i.v. | 2/5 | 1/5 | 2/5 | 4/5 | 0/5 | 3/5 Hemorrhaging | |||||||
| AS-D6.1AII | i.v. | 5/5 | 4/5 | 4/5 | 5/5 | 0/5 | 2/5 Hemorrhaging | |||||||
Data are derived from the same animals as in Table I.
Figure 8.
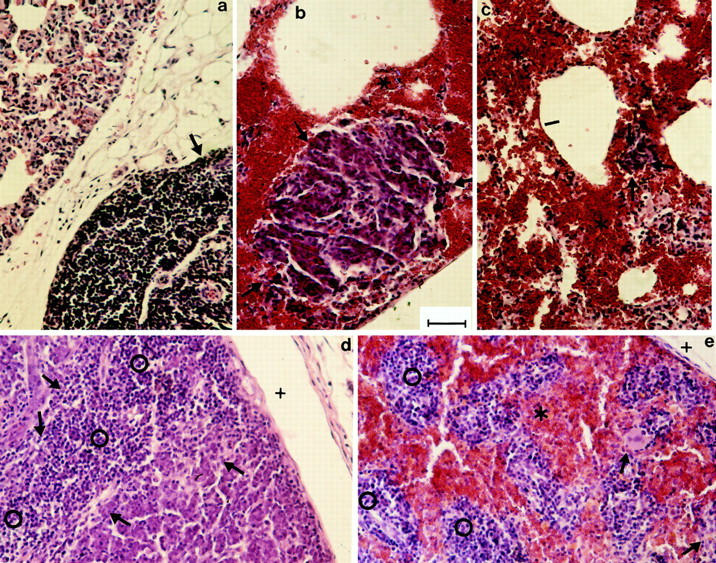

Metastasis formation of a D6.1A-transfected BSp73AS clone. Tissue section of AS-D6.1A and of AS-bearing rats were fixed in formalin and embedded in paraffin. Sections (5 μm) were stained with hematoxylin/eosin. Lung metastasis of AS-bearing (a) and AS-D6.1A–bearing (b and c) rats: the lung section of the AS-bearing rat shows part of a solid metastatic nodule (arrow) adjacent to unaltered lung tissue (alveolus marked by |). In the lung section of AS-D6.1A–bearing rats (b and c) bronchi and alveoli (|) are emphysematous or filled with blood like the interstitium (*). Metastatic nodules are rather small (encircled by arrows). In the lymph node of AS-bearing rats (d), the tumor (arrows) has displaced most of the lymphatic tissue (open circle). The subcapsular sinus is marked by +. In AS-D6.1A–bearing rats (e), few tumor cells (arrow) were detected in the lymph nodes, lymphoid cells (open circle) were dispersed between coagulated blood (*). The lymph node metastasis and lung metastasis was received from rats after s.c. and i.v., respectively, tumor cell application. Tumor-free organs were only altered (as a consequence of tumor-distant infarctions) in AS-D6.1A– bearing rats. As examples the gut, the adrenal gland, and the kidney of AS– (f, h, and j) and AS-D6.1A– (g, i, and k) bearing rats are shown. In the gut of AS-D6.1A–bearing rats, the epithelial layer (open square) is largely destroyed and replaced by infiltrating cells (open circle), the muscularis mucosae (arrow) has disappeared and the muscularis (arrowhead) has been replaced by fibrous tissue. In h and i the medullary region of the adrenal gland is shown. In AS-D6.1A–bearing rats the structure was completely destroyed. Only in some areas (arrowhead) pycnotic cells were still visible, but mostly the cell structure had been completely dissolved and replaced by coagulated blood (mostly erythrocyte ghosts, open square, are seen in this section). In the kidney of AS-D6.1A–bearing rats, glomeruli (arrowhead) were still visible, but tubuli (arrow) could not be identified. The interstitial tissue was filled with coagulated blood (*) and fibrin clots (open square). Bar, 6.1 μm.
Thus, D6.1A influenced metastasis formation, but in an unexpected way. Besides metastasis formation in the absence of local tumor growth, which is characteristic for the ASML line, AS-D6.1A clones differed from ASML, which metastasizes explicitly via the draining lymph nodes, by the rather weak involvement of the lymphatic system as well as by either the local destruction of capillaries and/or interference with the clotting system.
To support the latter assumption, we screened rats bearing D6.1A-transfected AS cells for clotting activity (Table III). Animals received a s.c. injection of AS-mock or AS-D6.1AI. Five animals in each group were treated i.v. twice per week with 200 μg D6.1, and five with an isotype-matched control antibody. Animals were killed after 5 wk. All five rats receiving AS-D6.1A and a control antibody showed bleeding in the tumor tissue and three out of five had infarctions in the gastrointestinal tract. In AS-D6.1A– bearing rats that received a control antibody, Quick values were strongly reduced and partial prothrombin times were significantly prolonged. Furthermore, the fibrinogen content in the serum was significantly decreased and ranged below 10% of standard values in three out of five rats. Complexes of fibrin fragments (D-dimers) were significantly increased. These findings point towards a severe consumption coagulopathy. Interestingly, in four out of five animals that received D6.1 (200 μg, twice per week, i.v.) throughout the period of tumor growth, Quick, partial prothrombin time, and the fibrinogen content were normalized, whereas complexes of fibrin fragments were still augmented, i.e., D6.1 did not prevent the coagulopathy, but counterregulated it. In line with this laboratory finding was the observation that, with one exception, neither bleeding in the peri-tumoral region nor infarctions were seen. AS-mock–bearing rats showed no bleeding, irrespective of whether they received control IgG or D6.1 and coagulation parameters were unaltered (data not shown). Thus, metastasis promotion by D6.1A likely functions via interference with the clotting system.
Table III.
Evidence of Consumption Coagulopathy in AS-D6.1I Tumor-bearing Rats
| AS-D6.1AI * tumor-bearing rats | Antibody treatment‡ | Quick | PPT | Fibrinogen | D-Dimers | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| standard values | >70% | 25–40 | 150–450 | <0.5 | ||||||||
| % | s | mg/dl | mg/l | |||||||||
| 1 | Control IgG1 | <3 | >140 | <10 | 3 | |||||||
| 2 | Control IgG1 | <3 | >140 | <10 | 2 | |||||||
| 3 | Control IgG1 | <3 | >140 | <10 | 8 | |||||||
| 4 | Control IgG1 | 100 | >140 | 57 | >8 | |||||||
| 5 | Control IgG1 | 42 | 15 | 93 | >8 | |||||||
| 1 | D6.1 | 100 | 25 | 308 | >8 | |||||||
| 2 | D6.1 | 85 | 17 | 229 | nt§ | |||||||
| 3 | D6.1 | 100 | 19 | 261 | >8 | |||||||
| 4 | D6.1 | 100 | 16 | 280 | 6 | |||||||
| 5 | D6.1 | nt | nt | nt | nt |
Tumor cells were injected s.c. (5 × 105) and animals were sacrificed when the mean tumor diameter reached 2 cm.
Rats received 200 μg, i.v., twice per week.
nt, not testable, this accounted particularly for rat 5 in the D6.1 treated group, which apparently displayed features of a hypercoagulopathy.
Discussion
Recently, we have described the physiological expression of a panel of seemingly metastasis-associated molecules, i.e., of structures detected in a rat tumor line metastasizing via the lymphatic system, but not in nonmetastasizing lines (13). The association with metastasis formation was particularly surprising for one molecule recognized by the mAb D6.1, because this metastasis-associated marker was most abundantly expressed in ectoderm-, mesoderm-, and endoderm-derived tissues of the adult rat. The apparent discrepancy between physiological expression and association with tumor progression prompted us to identify its molecular structure.
The molecule recognized by the D6.1 mAb and accordingly named D6.1A belongs to the tetraspanin superfamily, which is characterized by the NH2 and the COOH termini being located within the cytoplasm (69). The D6.1A has two potential extracellular regions, both with potential N-glycosylation sites, which fits the observation that we noted in Western blots of tunicamycin-treated cells of a clear reduction of the molecular mass. Besides which, there is a recognition sequence for the association with SH2 domain–containing proteins (59) and a consensus sequence for binding of tyrosine phosphatases, an association with tyrosine phosphatases being already described for two other members, CD53 and CD63, of the tetraspanin superfamily (12).
The D6.1A shows a high degree of homology to the human CO-029 antigen (62), which originally has been isolated from a colon cancer line, but is also found on cancer cells of the mammary gland, the pancreas and the stomach (53). Distinct to the D6.1A, CO-029 has not been described to be abundantly expressed under physiological conditions (53, 62). However, although expression of some tetraspanin molecules, e.g., CD37 and CD53, is rather restricted, others like CD9, CD63, CD81, and CD82 have been found on a large variety of tissues and cells (6, 31, 42, 55, 65). Despite their abundance, several of them, e.g., CD9, CD63, and CD82, are supposed to be associated with the metastatic phenotype (1, 17, 25, 30, 46).
In terms of function, tetraspanins have been described to be involved in adhesion and motility, proliferation, activation, and differentiation, as well as in metastasis formation or suppression (reviewed in reference 37). It has been suggested that this divergent array of functions may relate to their capacity to group-specific cell surface proteins, mostly integrins (37, 69). Thus, first we asked whether D6.1A may associated with integrins. When testing the currently available antibodies reacting with rat integrins, it was found that ASML, Progressor, Regressor (data not shown), AS cells, and D6.1A cDNA–transfected AS cells expressed the α3, α6, and β1 integrin chains. Coprecipitation studies revealed that D6.1A, indeed, associated with β1, α3 as well as α6β1. None of the cells expressed α4, α5, and β3. AS cells, distinct to the metastasizing lines ASML and Progressor, also stained partly with am and β2. Interestingly, expression of β2, but also of β1 was upregulated on AS-D6.1A as compared with AS. However, with neither ASML nor Progressor were we able to detect an association with CD44s, which also has been reported to associate with tetraspanin molecules (32). Considering other metastasis-associated molecules on ASML and Progressor/Regressor, no association with C4.4A, a molecule with partial homology to the urokinase type plasminogen receptor (Rösel, M., C. Claas, S. Seiter, and M. Zöller, manuscript submitted for publication; data not shown) could be found. With D5.7A, the rat homologue of the panepithelial marker EGP314 (Würfel, J., M. Rösel, C. Claas, S. Seiter, and M. Zöller, manuscript in preparation) D6.1A associated very weakly if at all.
Having shown that D6.1A, like other tetraspanin molecules, coprecipitated with certain integrins, we proceeded with in vitro studies, asking whether our observations fitted into known features of tetraspanin molecules and whether particular functions could explain an involvement of D6.1A in metastatic settlement. Special emphasis was given to uncover differences between ASML vs AS-D6.1A, because, as discussed below, D6.1A exerted distinct functional activities on the metastasizing tumor line ASML as compared with the D6.1A-transfected tumor line AS, which gains the capacity to metastasize, but progresses distinctly from ASML.
Tetraspanin molecules are known to influence the activation state of adhesion molecules (11), and tumor progression is frequently associated with adhesive phenomena, e.g., loss of homotypic adhesion molecules and/or high expression of cell–matrix adhesion molecules (2). Therefore, next we evaluated a possible influence of D6.1A on cell–matrix as well as cell–cell adhesion. First, we did not obtain any evidence of D6.1A being directly involved in cell–cell adhesion. Considering cell–matrix interactions, D6.1A-transfected as compared with mock-transfected lines displayed improved adhesion towards plastic and hyaluronic acid, which was inhibited by the D6.1 mAb. Since adhesion of constitutively D6.1A-positive cells to plastic and different substrates was not influenced by D6.1, it is tempting to speculate that D6.1A may facilitate binding only via associated surface molecules. One possible candidate could be β1 integrins, the expression of which has been found to be upregulated on AS cells transfected with D6.1A cDNA. Other candidates could be αm or β2, expression of both being restricted to AS cells and expression of the latter also becoming stronger by transfection with D6.1A cDNA. Interestingly, β2 integrin has been described to be required for the firm attachment and transmigration of neutrophils to surface-adherent platelets, thus facilitating extravasation of neutrophils at sites of vascular injury (16). The hematopoietic isoform of CD44, known as hyaluronate receptor (5), also is much more abundantly expressed on AS than on ASML cells (68). Thus, the increased adhesion of the D6.1A cDNA–transfected AS clones could possibly have been due to either a direct engagement of CD44s in a multicomponent complex or an association between the D6.1A-associated β1 integrin and CD44s, evidences for both phenomena being already described (9, 36). Although β2 and CD44s are possible candidates responsible for the differences in adhesion between AS-D6.1A–transfected cells and ASML, it should be remembered that ASML and AS appear to differ by the expression of over 100 gene products (60), whose potential association with D6.1A has not yet been explored. Therefore, additional experiments will be required for any decisive answer.
The same argument also accounts for the influence of D6.1 on the proliferation of D6.1A-transfected AS cells. Although seeding of D6.1A-transfected AS cells on D6.1-coated plates hardly influenced proliferative activity, D6.1 in the culture medium was inhibitory. One possibility could be that depending on the conformation of D6.1A, D6.1 triggers or blocks associated signaling molecules. Interestingly, Lundell et al. (36) described a release from inhibition of proliferation by blocking an integrin ligand interaction, wherein the integrin was associated with CD44. Whether by masking of D6.1A with D6.1 a similar proliferation inhibitory effect can be achieved, or whether this involves associated integrins, remains to be explored. Irrespective of the underlying mechanism, the adhesion and proliferation studies provided further evidence for D6.1A functioning in association with additional surface molecules.
The in vitro studies on the influence of D6.1A on cell– matrix adhesion and cell proliferation, although not opposing an influence of D6.1A, had not provided a clear-cut hint as to an involvement in metastasis formation. Convincing evidence for the latter and a first clue as to the function of D6.1A was derived from the in vivo growth pattern of D6.1A-transfected AS cells. Four features associated with the in vivo growth of D6.1A-transfected cells should be discussed: (a) D6.1A retarded the tumor growth; (b) after i.f.p., but to a lesser extent also after i.v. injection, the incidence of lung metastasis was increased; (c) metastasis formation within the lymphatic tissue was less pronounced; and (d) hemorrhaging in the tumor surrounding tissues was noted in nearly all rats and in distant organs in 15 out of 30 animals injected with AS-D6.1A. No such differences, particularly no hemorrhaging was seen with mock-transfected cells.
The D6.1A-transfected lines shared with the parental metastasizing line, ASML, the retardation of local growth as compared with AS. However, ASML cells metastasize exclusively via the lymphatic system. We have speculated that the lymphatic spread of ASML may be facilitated by CD44v4-v7, which can mediate an interaction between tumor cells and antigen-presenting cells, whereby the supply of cytokines becomes triggered (24, 73). Since the D6.1A molecule is not expressed during lymphocyte activation (13), there is no reason to assume that D6.1A expression on tumor cells facilitates metastasis formation in lymphatic tissue. In fact, some animals whose primary tumor was excised after i.f.p. application did not develop metastasis. This could have been due to a decrease in tumorigenicity. We favor the interpretation that a lack of growth support in the lymphatic tissue hampered metastasis formation.
There remains the question of how expression of D6.1A on AS cells facilitates extravasation of tumor cells. The bleeding within and surrounding the AS-D6.1A tumor has been most impressive, particularly taking into account that in hundreds of ASML-bearing rats, no hemorrhaging nor infarctions in distant organs were ever seen. Other tetraspanin molecules have been described to be involved in activation (CD63) or in aggregation and activation (CD9) of platelets and these phenomena have been associated with conformational changes of platelet-associated integrins (43, 57, 58). The observations that AS-D6.1A–bearing rats suffer from a severe consumption coagulopathy, which was counterregulated by D6.1, strongly support the assumption that D6.1A on the transfected tumor cells initiates in a similar, but as yet undefined way the clotting cascade. It is known that an interaction of platelets with tumor cells leads to an increase in malignancy (28), and the β2 integrin expressed by AS and AS-D6.1A cells, but not on ASML cells, could well account for this association (16). Furthermore, a relationship between malignancies and thrombotic disorders has been known for many years and has been described repeatedly (reviewed in references 10, 48, 74). It is supposed that tumor cells can induce platelet aggregation, which may be accompanied by platelet activation, release of matrix degrading enzymes, and thrombus formation (7, 8, 45). So far, however, the underlying mechanisms have not been unraveled. Considering D6.1A, it is tempting to speculate that binding of D6.1A or of a D6.1A complex initiates directly or indirectly (via activation of endothelial cells) aggregation and activation of platelets, which finally results in a consumption coagulopathy. Furthermore, since in vivo growth of ASML is not accompanied by thrombotic disorder, the comparison of this line with AS-D6.1A will provide an ideal model to unravel under which circumstances and in combination with which associated molecules D6.1A interferes with the clotting system. The findings that AS and ASML express only partly overlapping integrin profiles could well be of interest in unraveling the question.
Taken together, we have isolated a tetraspanin molecule that is abundantly expressed on many cells in the absence of malignant transformation, yet, is associated with the metastatic phenotype. Thus, its metastasis promoting features probably are linked to associated molecules. So far, we have shown that D6.1A associated strongly with β1, α3, and α6β1 integrins. The importance of molecules associated with D6.1A became apparent considering the functional activity of this tetraspanin molecule, particularly the different in vivo growth characteristics of endogenous and by transfection D6.1A-positive tumor cells. The most conspicuous observation has been the consumption coagulopathy observed only in the D6.1A cDNA–transfected AS line. These findings clearly point towards tetraspanin molecules being actively involved in thrombotic disorders that frequently accompany tumor progression (10, 48, 74). By exploring the associations of D6.1A with different integrins in constitutively and by transfection D6.1A-positive lines for example, it should be possible to clarify which molecular complexes hamper or induce activation of the clotting system.
Acknowledgments
We thank the German Resource Center, Max-Planck Institute for Molecular Genetics, Berlin and Department of Molecular Genome Analysis, German Cancer Research Center, Heidelberg for the provision of human PAC and for the PAC screening and K. Hexel, Department of Immune Genetics, German Cancer Research Center, Heidelberg, for fluorescence activated cell sorting.
Abbreviations used in this paper
- AA
amino acid
- AS
BSp73AS tumor line
- AS-14
AS-14 tumor line
- AS-D6.1A
AS-D6.1A tumor line
- ASML
ASML tumor line
- CO-I/III/IV
collagen type I/III/IV
- FISH
fluorescence in situ hybridization
- FN
fibronectin
- HA
hyaluronic acid
- i.f.p.
intrafootpad
- LA
laminin
- PAC
P1-derived artificial chromosome
- PTT
partial prothrombin time
- s.c.
subcutaneously
- PVDF
polyvinylidene difluoride
- VN
vitronectin
Footnotes
This investigation was supported by the Mildred Scheelstiftung für Krebshilfe (M. Zöller) and the Tumorzentrum Heidelberg Mannheim (M. Zöller).
References
- 1.Adachi M, Toshihiko T, Yoshiaki I, Huang CI, Higashiyama M, Miyake M. Correlation of KAI1/CD82 gene expression with good prognosis in patients with non-small cell lung cancer. Cancer Res. 1996;56:1751–1755. [PubMed] [Google Scholar]
- 2.Albelda SM. Role of integrins and other cell adhesion molecules in tumor progression and metastasis. Lab Invest. 1993;68:4–17. [PubMed] [Google Scholar]
- 3.Arch R, Wirth K, Hofmann M, Ponta H, Matzku S, Herrlich P, Zöller M. Participation in normal immune responses of a metastasis-inducing splice variant of CD44. Science. 1992;257:682–685. doi: 10.1126/science.1496383. [DOI] [PubMed] [Google Scholar]
- 4.Aruffo A, Seed B. Molecular cloning of a CD28 cDNA by a high-efficiency COS cell expression system. Proc Natl Acad Sci USA. 1987;84:8573–8577. doi: 10.1073/pnas.84.23.8573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principle cell surface receptor for hyaluronate. Cell. 1990;61:1303–1313. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- 6.Barclay, A.N., M.H. Brown, S.K.A. Law, A.J. Knight, M.G. Tomlinson, and P.A. van der Merwe. 1997. The Leucocyte Antigen Factsbook. Academic Press, New York. 613 pp.
- 7.Bastida E, Ordinas A, Giardina SL, Jamieson GA. Differentiation of platelet aggregating effects of human tumor cell lines based on inhibition studies with apyrase, hirudin and phospholipase. Cancer Res. 1982;42:4348–4352. [PubMed] [Google Scholar]
- 8.Belloc C, Lu H, Soria C, Fridman R, Legrand Y, Menashi S. The effect of platelets on invasiveness and protease production of human mammary tumor cells. Int J Cancer. 1995;60:413–417. doi: 10.1002/ijc.2910600324. [DOI] [PubMed] [Google Scholar]
- 9.Berditchevski F, Zutter MM, Hemler ME. Characterization of novel complexes on the cell surface between integrins and proteins with 4 transmembrane domains (TM4 proteins) Mol Biol Cell. 1996;7:193–207. doi: 10.1091/mbc.7.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bick RL. Coagulation abnormalities in malignancy: a review. Semin Thromb Hemostasis. 1992;18:353–372. doi: 10.1055/s-2007-1002575. [DOI] [PubMed] [Google Scholar]
- 11.Boucheix C, Soria C, Mirshahi M, Soria J, Perrot JV, Fournier N, Billard M, Rosenfeld C. Characteristics of platelet aggregation induced by the monoclonal antibody ALB6 (acute lymphoblastic leukemia antigen P24). Inhibition of aggregation by ALB6Fab. FEBS Lett. 1983;161:289–295. doi: 10.1016/0014-5793(83)81027-8. [DOI] [PubMed] [Google Scholar]
- 12.Carmo AM, Wright MD. Association of the transmembrane 4 superfamily molecule CD53 with a tyrosine phosphatase activity. Eur J Immunol. 1995;25:2090–2095. doi: 10.1002/eji.1830250743. [DOI] [PubMed] [Google Scholar]
- 13.Claas C, Herrmann K, Matzku S, Möller P, Zöller M. Developmentally regulated expression of metastasis-associated antigens in the rat. Cell Growth Differ. 1996;7:663–678. [PubMed] [Google Scholar]
- 14.Corvi R, Amler LC, Savelyeva L, Gehring M, Schwab M. MYCN is retained in single copy at chromosome 2 band p23-24 during amplification in human neuroblastoma cells. Proc Natl Acad Sci USA. 1994;91:5523–5527. doi: 10.1073/pnas.91.12.5523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cross JC, Werb Z, Fisher SJ. Implantation and the placenta: key pieces of the development puzzle. Science. 1994;266:1508–1518. doi: 10.1126/science.7985020. [DOI] [PubMed] [Google Scholar]
- 16.Diavoco TG, Roth SJ, Buccola JM, Bainton DF, Springer TA. Neutrophil rolling, arrest, and transmigration across activated, surface-adherent platelets via sequential action of P-selectin and the beta 2-integrin CD11b/CD18. Blood. 1996;88:146–157. [PubMed] [Google Scholar]
- 17.Dong JT, Lamb PW, Rinker-Schaeffer CW, Vukanovic J, Ichikawa T, Isaacs JT, Barrett JC. KAI1, a metastasis suppressor gene for prostate cancer on human chromosome 11p11.2. Science. 1995;268:884–886. doi: 10.1126/science.7754374. [DOI] [PubMed] [Google Scholar]
- 18.Dorudi S, Hart IR. Mechanisms underlying invasion and metastasis. Curr Opin Oncol. 1993;5:130–135. [PubMed] [Google Scholar]
- 19.Fidler IJ, Hart IR. Biological diversity in metastatic neoplasms: origins and implications. Science. 1982;217:998–1003. doi: 10.1126/science.7112116. [DOI] [PubMed] [Google Scholar]
- 20.Fox SB, Fawcett J, Jackson DG, Collins I, Gatter KC, Harris AL, Gearing A, Simmons DL. Normal human tissues, in addition to some tumors, express multiple different CD44 isoforms. Cancer Res. 1994;54:4539–4546. [PubMed] [Google Scholar]
- 21.Galluzzo E, Albi N, Fiorucci S, Merigiola C, Ruggeri L, Tosti A, Grossi CE, Velardi A. Involvement of CD44 variant isoforms in hyaluronate adhesion of human activated T cells. Eur J Immunol. 1995;25:2932–2939. doi: 10.1002/eji.1830251033. [DOI] [PubMed] [Google Scholar]
- 22.Günthert U, Hofmann M, Rudy W, Reber S, Zöller M, Haussmann I, Matzku S, Wenzel A, Ponta H, Herrlich P. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell. 1991;65:13–24. doi: 10.1016/0092-8674(91)90403-l. [DOI] [PubMed] [Google Scholar]
- 23.Hemler ME, Mannion BA, Berditchevski F. Association of TM4SF proteins with integrins: relevance to cancer. Biochim Biophys Acta. 1996;1287:67–71. doi: 10.1016/0304-419x(96)00007-8. [DOI] [PubMed] [Google Scholar]
- 24.Herrlich P, Zöller M, Pals ST, Ponta H. CD44 splice variants: metastases meet lymphocytes. Immunol Today. 1993;14:395–399. doi: 10.1016/0167-5699(93)90141-7. [DOI] [PubMed] [Google Scholar]
- 25.Higachiyama M, Toshihiko T, Yoshiaki I, Adachi M, Huang CI, Koh T, Kodama K, Doi O, Miyake M. Reduced motility related protein-1 (MRP1/CD9) gene expression as a factor poor prognosis in non-small cell lung cancer. Cancer Res. 1995;55:6040–6044. [PubMed] [Google Scholar]
- 26.Hirano H, Screaton GR, Bell MV, Jackson DG, Bell JI, Hodes RJ. CD44 isoform expression mediated by alternative splicing: tissue-specific regulation in mice. Int Immunol. 1994;6:49–59. doi: 10.1093/intimm/6.1.49. [DOI] [PubMed] [Google Scholar]
- 27.Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967;26:365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- 28.Honn KV, Tang DG, Crissman JD. Platelets and cancer metastasis: a causal relationship? . Cancer Metastasis Rev. 1992;11:325–351. doi: 10.1007/BF01307186. [DOI] [PubMed] [Google Scholar]
- 29.Hotta H, Ross AH, Huebner K, Isobe M, Wendeborn S, Chao MV, Ricciardi RP, Tsujimoto Y, Croce CM, Koprowski H. Molecular cloning and characterization of an antigen associated with early stages of melanoma tumor progression. Cancer Res. 1988;48:2955–2962. [PubMed] [Google Scholar]
- 30.Ikeyama S, Koyama M, Yamaoko M, Sasada R, Miyake M. Suppression of cell motility and metastasis by transfection with human motility-related protein (MRP-1/CD9) DNA. J Exp Med. 1993;177:1231–1237. doi: 10.1084/jem.177.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Imai T, Yoshie O. C33 antigen and M38 antigen recognized by monoclonal antibodies inhibitory to syncytium formation by human T cell leukemia virus type 1 are both members of the transmembrane 4 superfamily and associate with each other and with CD4 or CD8 in T cells. J Immunol. 1993;151:6470–6481. [PubMed] [Google Scholar]
- 32.Jones PH, Bishop LA, Watt FM. Functional significance of CD9 association with beta 1 integrins in human epidermal keratinocytes. Cell Adhes Commun. 1996;4:297–305. doi: 10.3109/15419069609010773. [DOI] [PubMed] [Google Scholar]
- 33.Kennel SJ, Lankford TK, Foote LJ, Shinpock SG, Stringer C. CD44 expression on murine tissues. J Cell Sci. 1993;104:373–382. doi: 10.1242/jcs.104.2.373. [DOI] [PubMed] [Google Scholar]
- 33a.Kyte J, Doolittle RF. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 34.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 35.Lehrach, H. 1990. Genetic and physical mapping. In Genome Analysis Volume 1. K.E. Davies and S.M. Tilgham, editors. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 39–81.
- 36.Lundell BI, McCarthy JB, Kovach NL, Verfaillie CM. Activation of β1 integrins on CML progenitors reveals cooperation between β1 integrins and CD44 in the regulation of adhesion and proliferation. Leukemia. 1997;11:822–829. doi: 10.1038/sj.leu.2400653. [DOI] [PubMed] [Google Scholar]
- 37.Maecker HT, Todd SC, Levy S. The tetraspanin superfamily: molecular facilitators. FASEB J. 1997;11:428–442. [PubMed] [Google Scholar]
- 38.Mareel MM, Van Roy FM, Bracke ME. How and when do tumor cells metastasize? . Crit Rev Oncog. 1993;4:559–594. [PubMed] [Google Scholar]
- 39.Matzku S, Wenzel A, Liu S, Zöller M. Antigenic differences between metastatic and nonmetastatic BSp73 rat tumor variants characterized by monoclonal antibodies. Cancer Res. 1989;49:1294–1299. [PubMed] [Google Scholar]
- 40.Matzku S, Komitowski D, Mildenberger M, Zöller M. Characterization of BSp73, a spontaneous rat tumor and its in vivo selected variants showing different metastasizing capacities. Invasion Metastasis. 1983;3:109–123. [PubMed] [Google Scholar]
- 41.Meier T, Arni S, Malarkannan S, Poincelet M, Hoessli D. Immunodetection of biotinylated lymphocyte-surface proteins by enhanced chemiluminescence: a nonradioactive method for cell-surface protein analysis. Anal Biochem. 1992;204:220–226. doi: 10.1016/0003-2697(92)90165-4. [DOI] [PubMed] [Google Scholar]
- 42.Nagira M, Imai T, Ishikawa I, Uwabe KI, Yoshie O. Mouse homologue of C33 antigen (CD82), a member of the transmembrane 4 superfamily: complementary DNA, genomic structure, and expression. Cell Immunol. 1994;157:144–157. doi: 10.1006/cimm.1994.1212. [DOI] [PubMed] [Google Scholar]
- 43.Nieuwenhuis HK, van Oosterhout JJG, Rozenmuller E, van Iwaarden F, Sixma JJ. Studies with a monoclonal antibody against activated platelets: evidence that a secreted 53000 molecular weight lysosome-like granule protein is exposed on the surface of activated platelets in the circulation. Blood. 1987;70:838–845. [PubMed] [Google Scholar]
- 44.Pauli UB, Schwartz DE, Thonar EJ-M, Kuettner KE. Tumor invasion and host extracellular matrix. Metast Rev. 1983;2:129–152. doi: 10.1007/BF00048966. [DOI] [PubMed] [Google Scholar]
- 45.Pearlstein E, Salk PL, Yogeeswaran S, Karpatkin S. Correlation between spontaneous metastatic potential, platelet aggregating activity of cell surface extracts, and cell surface sialylation in 10 metastatic variant derivatives of a rat renal sarcoma line. Proc Natl Acad Sci USA. 1980;77:4336–4339. doi: 10.1073/pnas.77.7.4336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Radford KJ, Mallesch J, Hersey P. Suppression of human melanoma cell growth and metastasis by the melanoma-associated antigen CD63 (ME491) Int J Cancer. 1995;62:631–635. doi: 10.1002/ijc.2910620523. [DOI] [PubMed] [Google Scholar]
- 47.Reisser D, Olsson NO, Martin F. In vivo and in vitro reactivity of rat spleen cells against regressor and progressor colon-cancer cell variants. Int J Cancer. 1993;53:651–656. doi: 10.1002/ijc.2910530421. [DOI] [PubMed] [Google Scholar]
- 48.Rickles FR, Levine M, Edwards RL. Hemostatic alterations in cancer patients. Cancer Metastasis Rev. 1992;11:237–248. doi: 10.1007/BF01307180. [DOI] [PubMed] [Google Scholar]
- 49.Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schick MR, Levy S. The TAPA-1 molecule is associated on the surface of B cells with HLA-DR molecules. J Immunol. 1993;151:4090–4097. [PubMed] [Google Scholar]
- 51.Shimizu T, Pelletier H, Hammann A, Olsson NO, Martin MS, Martin F. Effects of a single injection of anti-asialo GM1 serum on natural cytotoxicity and the growth of a regressive clonic tumor in syngeneic rats. Int J Cancer. 1987;40:676–680. doi: 10.1002/ijc.2910400518. [DOI] [PubMed] [Google Scholar]
- 52.Seed B, Aruffo A. Molecular cloning of the CD2 antigen, the T-cell erythrocyte receptor, by a rapid immunoselection procedure. Proc Natl Acad Sci USA. 1987;84:3365–3369. doi: 10.1073/pnas.84.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sela BA, Steplewski Z, Koprowski H. Colon carcinoma-associated glycoproteins recognized by monoclonal antibodies CO-029 and GA22-2. Hybridoma. 1989;8:481–491. doi: 10.1089/hyb.1989.8.481. [DOI] [PubMed] [Google Scholar]
- 54.Sedlin MF, Rochelle JM, Tomlinson MG, Wright MD. Mapping of the genes for four members of the transmembrane 4 superfamily: mouse CD9, CD63, CD81 and CD82. Immunogenetics. 1995;42:422–425. doi: 10.1007/BF00179406. [DOI] [PubMed] [Google Scholar]
- 55.Shaw AR, Domanska A, Mak A, Gilchrist A, Dobler K, Visser L, Poppema S, Fliegel L, Letarte M, Willett BJ. Ectopic expression of human and feline CD9 in a human B cell line confers β1 integrin-dependent motility on fibronectin and laminin substrates and enhanced tyrosine phosphorylation. J Biol Chem. 1995;270:24092–24099. doi: 10.1074/jbc.270.41.24092. [DOI] [PubMed] [Google Scholar]
- 56.Sher BT, Bargatze R, Holzmann B, Gallatin WM, Matthews D, Wu N, Picker L, Butcher EC, Weissman IL. Homing receptors and metastasis. Adv Cancer Res. 1988;51:361–390. doi: 10.1016/s0065-230x(08)60226-2. [DOI] [PubMed] [Google Scholar]
- 57.Slupsky JR, Seehafer JG, Tang SC, Masellis-Smith A, Shaw ARE. Evidence that monoclonal antibodies against CD9 antigen induce specific association between CD9 and the platelet gpIIβ/IIIα complex. J Biol Chem. 1989;264:12289–12293. [PubMed] [Google Scholar]
- 58.Slupsky JR, Cawley JC, Kaplan C, Zuzel M. Analysis of CD9, CD32 and p67 signaling: use of degranulated platelets indicates direct involvement of CD9 and p67 in integrin activation. Br J Haematol. 1997;96:275–286. doi: 10.1046/j.1365-2141.1997.d01-2011.x. [DOI] [PubMed] [Google Scholar]
- 59.Songyang Z, Shoelson SE, Chaudhuri M, Gish G, Pawson T, Haser WG, King F, Roberts T, Ratnofsky S, Lechleider RJ, et al. SH2 domains recognize specific phosphopeptide sequences. Cell. 1993;72:767–778. doi: 10.1016/0092-8674(93)90404-e. [DOI] [PubMed] [Google Scholar]
- 60.von Stein OD, Thies WG, Hofman M. A high throughput screening for rarely transcribed differentially expressed genes. Nucleic Acids Res. 1997;25:2598–2602. doi: 10.1093/nar/25.13.2598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Stetler-Stevenson WG, Aznavoorian S, Liotta LA. Tumor cell interactions with the extracellular matrix during invasion and metastasis. Annu Rev Cell Biol. 1993;9:541–573. doi: 10.1146/annurev.cb.09.110193.002545. [DOI] [PubMed] [Google Scholar]
- 62.Szala S, Kasai Y, Steplewski Z, Rodeck U, Koprowski H, Linnenbach AJ. Molecular cloning of cDNA for the human tumor-associated antigen CO-029 and identification of related transmembrane antigens. Proc Natl Acad Sci USA. 1990;87:6833–6837. doi: 10.1073/pnas.87.17.6833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Taher TE, Smit L, Griffioen AW, Schilder-Tol EJ, Borst J, Pals ST. Signaling through CD44 is mediated by tyrosine kinases. Association with p56lck in T lymphocytes. J Biol Chem. 1996;271:2863–2867. doi: 10.1074/jbc.271.5.2863. [DOI] [PubMed] [Google Scholar]
- 64.Takahashi S, Doss C, Levy S, Levy R. TAPA-1, the target of an antiproliferative antibody, is associated on the cell surface with the Leu-13 antigen. J Immunol. 1990;145:2207–2213. [PubMed] [Google Scholar]
- 65.Takagi S, Fujikawa K, Imai T, Fukuhara N, Fukudome K, Minegishi M, Tsuchiya S, Konno T, Hinuma Y, Yoshie O. Identification of a highly specific surface marker of T-cell acute lymphoblastic leukemia and neuroblastoma as a new member of the transmembrane 4 superfamily. Int J Cancer. 1995;61:706–715. doi: 10.1002/ijc.2910610519. [DOI] [PubMed] [Google Scholar]
- 66.Thiery JP, Boyer B, Tucker G, Gavrilovic J, Valles AM. Adhesion mechanisms in embryogenesis and in cancer invasion and metastasis. Metastasis (Ciba Found Symp) 1988;141:48–74. doi: 10.1002/9780470513736.ch4. [DOI] [PubMed] [Google Scholar]
- 67.Weber B, Rösel M, Arch R, Möller P, Zöller M. Expression of variant isoforms of CD44 during ontogeny of the rat: evidence for divergent functions of distinct exon combinations. Differentiation. 1996;60:17–29. doi: 10.1046/j.1432-0436.1996.6010017.x. [DOI] [PubMed] [Google Scholar]
- 68.Wirth, K., R. Arch, C. Somasandaram, M. Hofmann, B. Weber, P. Herrlich, S. Matzku, and M. Zöller. 1993. Expression of CD44 isoforms carrying metastasis-associated sequences in newborn and adult rats. Eur. J. Cancer. 29A:1172–1177. [DOI] [PubMed]
- 69.Wright MD, Tomlinson MG. The ins and outs of the transmembrane 4 superfamily. Immunol Today. 1994;15:588–594. doi: 10.1016/0167-5699(94)90222-4. [DOI] [PubMed] [Google Scholar]
- 70.Zöller M. CD44: physiological expression of distinct isoforms as evidence for organ-specific metastasis formation. J Mol Med. 1995;73:425–438. doi: 10.1007/BF00202261. [DOI] [PubMed] [Google Scholar]
- 71.Zöller M, Matzku S, Goerttler K. High incidence of spontaneous transplantable tumours in BDX rats. Br J Cancer. 1978;37:61–66. doi: 10.1038/bjc.1978.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zöller M, Schuhmacher J, Reed J, Maier-Borst W, Matzku S. Establishment and characterization of monoclonal antibodies against an octahedral Gallium chelate. J Nucl Med. 1992;33:1366–1372. [PubMed] [Google Scholar]
- 73.Zöller M. Joint features of metastasis formation and lymphocyte maturation and activation. Curr Top Microbiol Immunol. 1996;213:215–247. doi: 10.1007/978-3-642-61107-0_14. [DOI] [PubMed] [Google Scholar]
- 74.Zuffa M, Kubancok J, Horvath A. The thrombo-embolic disease as a paraneoplastic syndrome. Neoplasma. 1982;28:241–244. [PubMed] [Google Scholar]