Abstract
Osmotic cell swelling activates Cl− channels to achieve anion efflux. In this study, we find that both the tyrosine kinase inhibitor herbimycin A and genetic knockout of p56lck, a src-like tyrosine kinase, block regulatory volume decrease (RVD) in a human T cell line. Activation of a swelling-activated chloride current (ICl−swell) by osmotic swelling in whole-cell patch-clamp experiments is blocked by herbimycin A and lavendustin. Osmotic activation of ICl−swell is defective in p56lck-deficient cells. Retransfection of p56lck restores osmotic current activation. Furthermore, tyrosine kinase activity is sufficient for activation of ICl−swell. Addition of purified p56lck to excised patches activates an outwardly rectifying chloride channel with 31 pS unitary conductance. Purified p56lck washed into the cytoplasm activates ICl−swell in native and p56lck-deficient cells even when hypotonic intracellular solutions lead to cell shrinkage. When whole-cell currents are activated either by swelling or by p56lck, slow single-channel gating events can be observed revealing a unitary conductance of 25–28 pS. In accordance with our patch-clamp data, osmotic swelling increases activity of immunoprecipitated p56lck. We conclude that osmotic swelling activates ICl−swell in lymphocytes via the tyrosine kinase p56lck.
When cells are exposed to hypotonic stress, initial swelling is followed by regulatory volume decrease (RVD).1 In lymphocytes, RVD involves activation of volume-sensitive anion channels, leading to membrane depolarization and thereby opening voltage-activated potassium channels (Deutsch and Lee, 1988). This produces a net loss of KCl, resulting in regulatory decrease of intracellular osmolarity and driving H2O out of the cell. Anion channels activated by osmotic stress are expressed in a wide variety of nonexcitable and excitable tissues (for review see Lang et al., 1997), and are thought to mediate an efflux of osmotically active anions in response to increased cellular volume (Cahalan and Lewis, 1988).
Biophysical and pharmacological differences have been observed between swelling-activated anion channels found in lymphocytes and other tissues (Kunzelmann et al., 1989; Solc and Wine, 1991; Sorota, 1992; Lewis et al., 1993). Most of these channels are outwardly rectifying and possess unitary conductances ranging from 20 to 90 pS. At least three different anion channels have been characterized at the single channel level in membrane patches from lymphocytes (Lewis and Cahalan, 1988; Nishimoto et al., 1991; Garber, 1992). However, a clear relationship between single-channel currents and whole-cell currents is lacking. Although the genes for a number of different Cl− channel proteins have been cloned, the proteins forming swelling activated anion channels in lymphocytes have not yet been identified. Their activation mechanism is even less completely understood. In lymphocytes, neither an increase in cytosolic calcium nor in membrane area is necessary for activation of volume-sensitive anion channels (Ross et al., 1994; Ross and Cahalan, 1995). However, participation of cytoskeletal elements in channel activation has been demonstrated (Levitan et al., 1995). Most interestingly, the presence of intracellular ATP is necessary for maintenance or repeated activation of the swelling-activated anion current (Lewis et al., 1993; Ross et al., 1994). Recent work has shown inhibition of volume-sensitive chloride current in heart cells by tyrosine-kinase inhibitors (Sorota, 1995) and regulation of the cystic fibrosis transmembrane conductance regulator (CFTR) channel by tyrosine phosphorylation (Fischer and Machen, 1996). In this study we have examined the role of a src-like lymphocyte tyrosine kinase, p56lck, in the activation of swelling- activated anion channels in lymphocytes.
Materials and Methods
Cell Culture and Stimulation
Jurkat and p56lck-deficient JCaM1.6 cells were obtained from the American Type Culture Collection (Rockville, MD), p56lck reconstituted JCaM1.6 (JCaM1.6/Lck+) were a gift from Dr. A. Weiss (University of California, San Francisco, CA). The cells were grown in RPMI-1640 medium supplemented with 10% FCS, 10 mM Hepes, pH 7.4, 2 mM l-glutamine, 1 mM sodium pyruvate, 100 μM nonessential amino acids, 100 U/ml penicillin, 100 μg/ml streptomycin (all purchased from Life Technologies, Eggenstein, Germany), and 50 μM β-mercaptoethanol. JCaM1.6/Lck+ were cultured in 250 μg/ml hygromycin, which was removed 48–72 h before beginning of experiments. Stable expression of p56lck was verified (data not shown). The specific src-like tyrosine kinase inhibitor herbimycin A (Calbiochem, Bad Soden, Germany) was added to the cells at a concentration of 10 μM 7–8 h before the experiments.
Solutions
Cells were superfused with modified Ringer's containing 145 mM NaCl, 5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM glucose, and 10 mM Hepes, pH 7.4 (310 mOsmol/kg). The internal pipette solution was used to separate Cl− currents in lymphocytes (Ross et al., 1994) and contained 160 Cs+-glutamate, 0.1 mM CaCl2, 2 mM Mg Cl2, 1.1 mM EGTA, 4 mM Na2ATP, and 10 mM Hepes, pH 7.2 (330 mOsmol/kg). For hypotonic intracellular conditions, this solution was diluted as indicated. For hypertonic conditions, sucrose was added to obtain the indicated osmolality. When varying ATP concentrations, 2 mM Na glutamate replaced 1 mM Na2ATP, respectively. Purified p56lck was purchased from Upstate Biotechnology Inc. (Lake Placid, NY). All other chemicals were obtained from Sigma Chemical Co. (Deisenhofen, FRG). Osmolality was measured using a freezing point osmometer (Knauer, Berlin, FRG).
Volume Measurements
Volume changes were measured on the stage of an inverted microscope (Axiovert 135; Carl Zeiss, Oberkochen, Germany) by video imaging. Cells were loaded with 2 μM calcein-AM (Molecular Probes, Eugene, OR) for 15 min. Excitation was performed at 497 nm using a monochromator (Uhl, Munich, Germany). Video images were recorded at 521 nm and digitized (IMG8; Lindemann and Meiser, Homburg, Germany). The fluorescent cell area was analyzed using the PC version of the public domain NIH Image program (ImagePC; Scion Corp., Frederick, MD; available on the Internet via http://rsb.info.nih.gov/nih-image). Relative volume (V) was calculated from the image area (A) with the following relation:
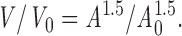 |
Patch-Clamp Recordings
Whole-cell currents were recorded at 31°C using an EPC-9 patch-clamp amplifier and Pulse software (Heka, Lambrecht, Germany). Experiments were performed on an Axiovert 135 microscope and cells were observed using a video system to note volume changes. Pipettes were pulled to a resistance of 2–5 MΩ from borosilicate glass. For high resolution recordings, pipettes were coated with sylgard (Dow Corning, Midland, MI). Capacitive transients were cancelled using the Cslow-compensation of the amplifier. Series resistance was not compensated. Cells were held at 0 mV and voltage ramps or pulses of the indicated size were applied every 5 or 20 s. The current signal was filtered at 1 kHz and digitized at a 5-kHz sampling rate. By convention, anionic inward fluxes are shown as positive (outward) currents.
Immunoprecipitation and p56lck Assay
Cell stimulation was terminated by lysis in 25 mM Hepes, pH 7.4, 0.1% SDS, 0.5% sodium deoxycholate, 1% Triton X-100, 125 mM NaCl, 10 mM of each sodium fluoride, Na3VO4, and sodium pyrophosphate and 10 μg/ml of each aprotinin and leupeptin (radioimmunoprecipitation assay buffer) for immunoprecipitation of p56lck. After lysis, DNA and cell debris were pelleted by centrifugation at 20,000 g for 15 min, and the supernatants were subjected to immunoprecipitation of p56lck using an anti-p56lck polyclonal antibody (Upstate Biotechnology Inc.). Control immunoprecipitates were performed with irrelevant affinity-purified polyclonal rabbit immunoglobulins. Immunoprecipitates were incubated overnight at 4°C. After addition of anti–rabbit, IgG-coupled agarose, incubation was continued for at least 60 min. Immunocomplexes were washed four times in lysis buffer, twice in kinase buffer (25 mM Hepes, pH 7.0, 150 mM NaCl, 10 mM MnCl2, 1 mM Na3VO4, 5 mM DTT, and 0.5% NP-40), and then resuspended in the same buffer. The kinase reaction was initiated by addition of 10 μCi [32P]γATP (3,000 Ci/mmol; Du Pont-NEN, Boston, MA) and ATP (10 μM) in kinase buffer. The samples were incubated at 30°C for 20 min, the reaction was stopped with reducing 5× SDS sample buffer and 10% SDS-PAGE was performed, followed by autoradiography. An aliquot of the immunoprecipitates was analyzed by immunoblotting for detection of equal amounts of p56lck. Immunoblots were developed by incubation with HRP-conjugated protein G (BIO RAD Laboratories, München, Germany) and a chemiluminescence kit (Amersham, Braunschweig, Germany).
Results
RVD Depends on Tyrosine Kinase Activity
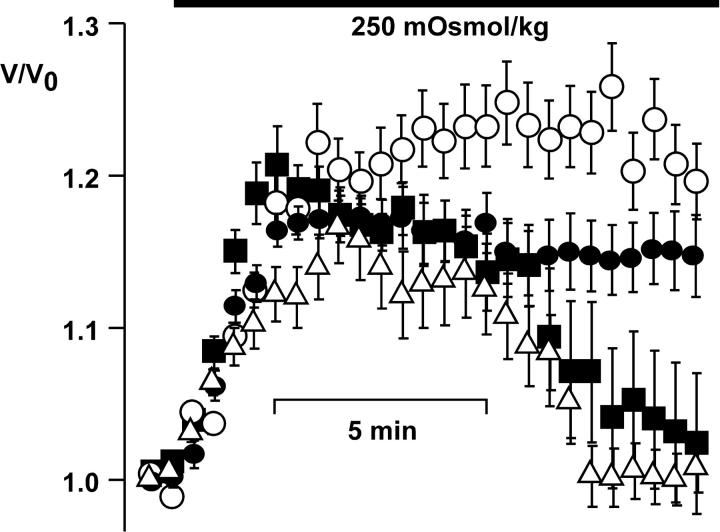
When Jurkat T lymphocytes were superfused with 80% Ringer's (250 mOsmol/kg), RVD restored the initial volume after 13 min in control cells (Fig. 1). RVD was completely blocked in cells preincubated with 10 μM herbimycin A and was defective in JCaM1.6 cells, a Jurkat subclone deficient for the src-like tyrosine kinase p56lck. Retransfection of p56lck restored RVD in JCaM1.6 cells. To elucidate the mechanism of RVD block by inhibition of src-like tyrosine kinases, we studied the volume-sensitive anion current with the patch-clamp method.
Figure 1.
RVD is blocked by herbimycin A. Cells were superfused with hypotonic solution as indicated. After the initial swelling, control cells restored their volume within 13 min (▪; n = 24). No RVD was seen in cells pretreated with herbimycin A (○; n = 13) or in p56lck-deficient JCaM1.6 cells (•; n = 12). RVD was restored in JCaM1.6 cells retransfected with p56lck (▵; n = 30). Error bars show SEM.
Osmotic Activation of a Chloride Current (ICl) Requires a Tyrosine Kinase
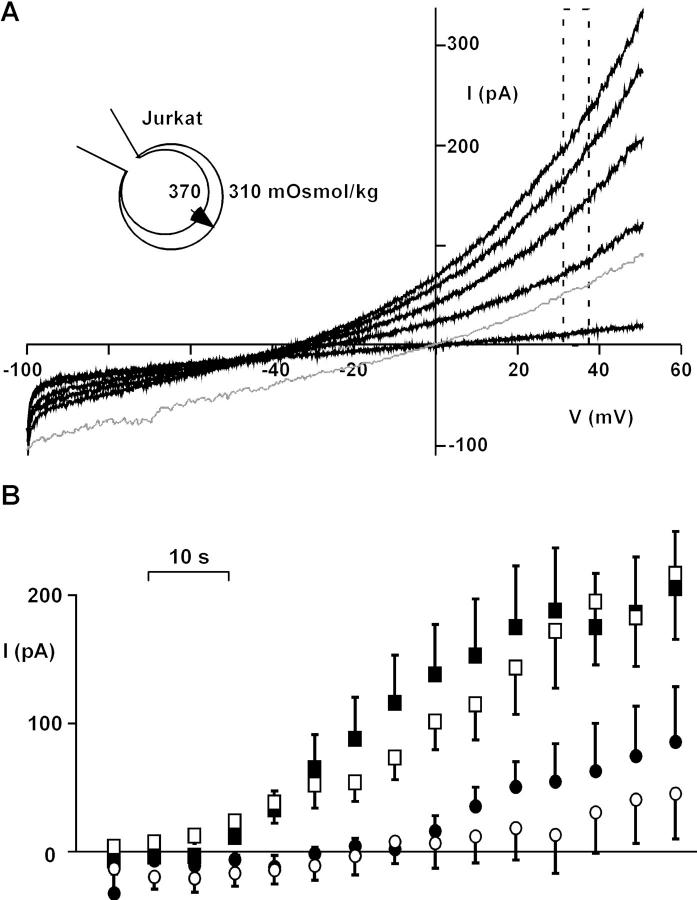
Osmotic swelling of Jurkat T cells induced a Cl− current 10–20 s after washing a hypertonic pipette solution into the cell. The current was characterized by strong outward rectification and a poor permeability to intracellular glutamate (Fig. 2 A). The swelling-activated Cl− current (ICl−swell) did not undergo visible inactivation using 200-ms pulses up to 80 mV, and was blocked by 500 μM diisothiocyanato-2-2-stilbenesulfonic acid (DIDS), as previously described (Lewis et al., 1993).
Figure 2.
Osmotic activation of ICl−swell is defective in p56lck-deficient JCaM1.6 cells. (A) Osmotic swelling activates an outwardly rectifying anion conductance. The reversal potential <−40 mV and the small inward current indicate poor permeability for intracellular glutamate. Traces are shown 1, 28, 42, 56, and 77 s after break-in. The lower trace (thin line) was recorded from a cell with symmetrical Cl− concentrations 35 s after break-in, demonstrating intrinsic outward rectification. No leak correction was performed. Instantaneous IV-plots were obtained using 200-ms voltage ramps. (B) Outward currents at 35 mV in Jurkat cells (▪), p56lck-deficient JCaM1.6 cells (•), p56lck-retransfected JCaM1.6/ lck+ cells (□), and Jurkat cells pretreated for 8 h with 10 μM herbimycin A (○; n = 13, 10, 10, and 8, respectively). Error bars show SEM. Leak currents determined at −55 mV were subtracted.
In contrast, the development of the outwardly rectifying current was attenuated and delayed in JCaM1.6 cells. Osmotic activation of the current was rescued in JCaM1.6/ lck+ cells retransfected with p56lck. Furthermore, ICl−swell was blocked in Jurkat cells by the inhibitors of src-like tyrosine kinases herbimycin A and lavendustin. The time course of outward currents is summarized in Fig. 2 B. Table I shows dose-dependent block by lavendustin (IC50 ≈ 10 nM) and compares outward currents using different genetic and pharmacological manipulations.
Table I.
Tyrosine Kinase Inhibitors Block ICl–swell
| Osmolality | p56lck | I | SEM | n | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mOsmol/kg | U/ml | pA | pA | |||||||||
| Jurkat + herbimycin A | 370 | 0 | 45.7 | 36.7 | 8 | a | ||||||
| Jurkat + lavendustin 10 μM | 370 | 0 | 7.8 | 6.2 | 4 | b | ||||||
| Jurkat + lavendustin 100 nM | 370 | 0 | 20.3 | 33.6 | 3 | c | ||||||
| Jurkat + lavendustin 10 nM | 370 | 0 | 72.5 | 11.1 | 5 | d | ||||||
| Jurkat | 370 | 0 | 210.5 | 35.1 | 14 | a–e | ||||||
| Jcam1.6 | 370 | 0 | 100 | 47.9 | 12 | e, f | ||||||
| Jcam1.6, p56lck retransfected | 370 | 0 | 247.3 | 41 | 12 | f | ||||||
| Jcam1.6 | 300 | 10 | 38.1 | 8.3 | 9 | g, h | ||||||
| Jcam1.6 | 300 | 0 | 8.9 | 2 | 5 | g | ||||||
| Jurkat | 260 | 10 | 107.1 | 44 | 5 | i, h | ||||||
| Jurkat | 260 | 0 | 6.9 | 2.4 | 6 | i | ||||||
Whole-cell Cl− currents 2 min after break-in. Osmolality of the pipette solution was varied to swell or shrink the respective cell types. Lavendustin added 5 min before recordings blocked the swelling-induced Cl− current in a dose-dependent manner: a–i, significantly different data pairs (t test, P < 0.05). Current activated by purified p56lck in the pipette was significantly larger in Jurkat cells when compared with Jcam1.6 cells. Extracellular osmolality was always 310 mOsmol/kg.
p56lck Activates Chloride Conductance without Swelling
If tyrosine phosphorylation is a crucial step in the activation of ICl−swell, addition of constitutively active p56lck to the cytosol should activate ICl−swell in the native as well as the lck-deficient cell line without cell swelling. When using a hypotonic pipette solution (260 mOsmol/kg), Jurkat cells started to shrink shortly after break-in. However, a large ICl could be observed when purified p56lck was added to the intracellular solution (Table I). This current shared with the swelling-activated conductance strong outward rectification, a poor permeability to glutamate and lack of inactivation (Fig. 3, A and B). Because Jurkat cells possess intrinsic p56lck activity, we attempted to activate ICl in the p56lck-deficient subclone JCaM1.6 using hypotonic pipette solutions. In five whole-cell recordings from JCaM1.6 cells patched with a slightly hypotonic pipette solution (300 mOsmol/kg) ICl did not activate and cell volume decreased slowly during the experiment. However, when p56lck was added to the pipette solution, a small outwardly rectifying ICl developed (Fig. 3 C; Table I). The current was completely and reversibly blocked by 500 μM DIDS (Fig. 3 C) and no fast inactivation could be observed (data not shown). Thus, purified lck activates ICl with properties indistinguishable from ICl−swell.
Figure 3.
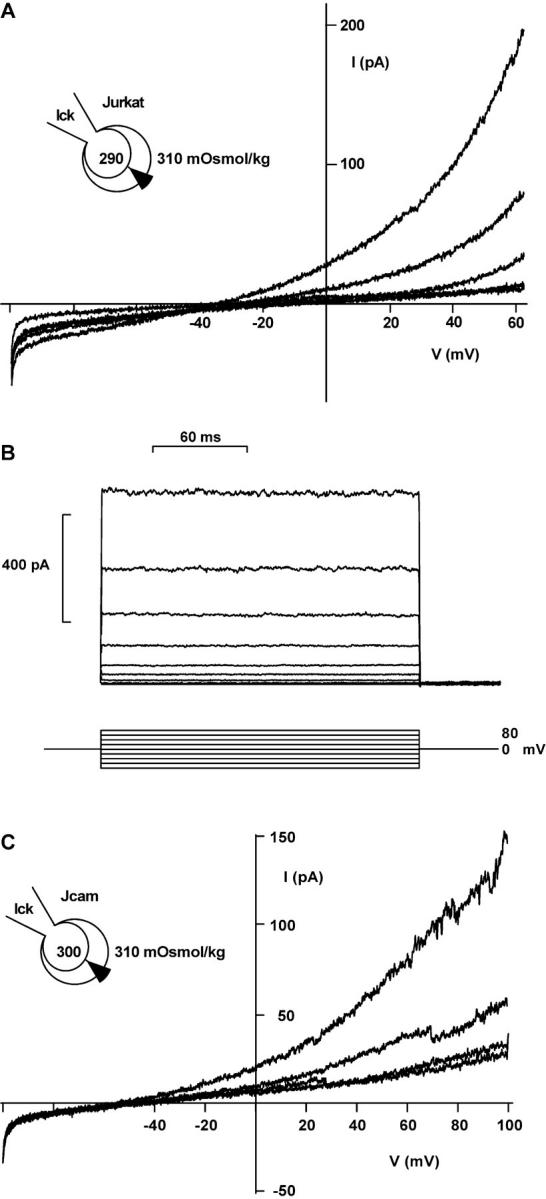
ICl activated by p56lck. (A) ICl in a shrinking Jurkat cell with 10 U/ml purified p56lck in the pipette. Representative traces are shown 1, 121, 218, 284, and 371 s after break-in (no leak subtraction). (B) Lack of inactivation of outward currents induced by purified p56lck. 200-ms pulses beginning at −80 mV, with 20-mV increments, were applied every 5 s. Leak and capacitive currents were subtracted. (C) ICl in a shrinking JCaM1.6 cell with 10 U/ml purified p56lck in the pipette. Traces are shown 307, 373, and 482 s after break-in. The lowest trace shows block of the current with 500 μM DIDS. Note the current steps indicating single-channel transitions. 250-ms voltage ramps, no leak subtraction.
p56lck Activates Cl− Channels in Excised Patches
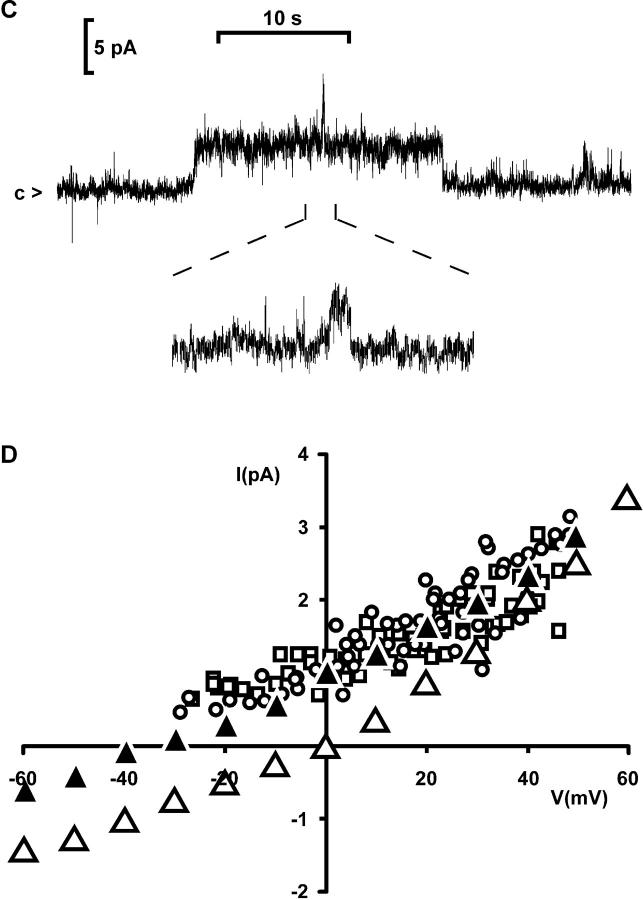
Addition of purified p56lck to the cytosolic surface of excised patches from Jurkat T cells activated an outwardly rectifying Cl− channel (Fig. 4, A and B). No spontaneous activation was observed in 55 excised patches held at 0 mV holding potential without enzyme added. The channel showed outward rectification, even with symmetrical Cl− concentrations, and was selective for Cl− over glutamate (Fig. 4 D).
Figure 4.
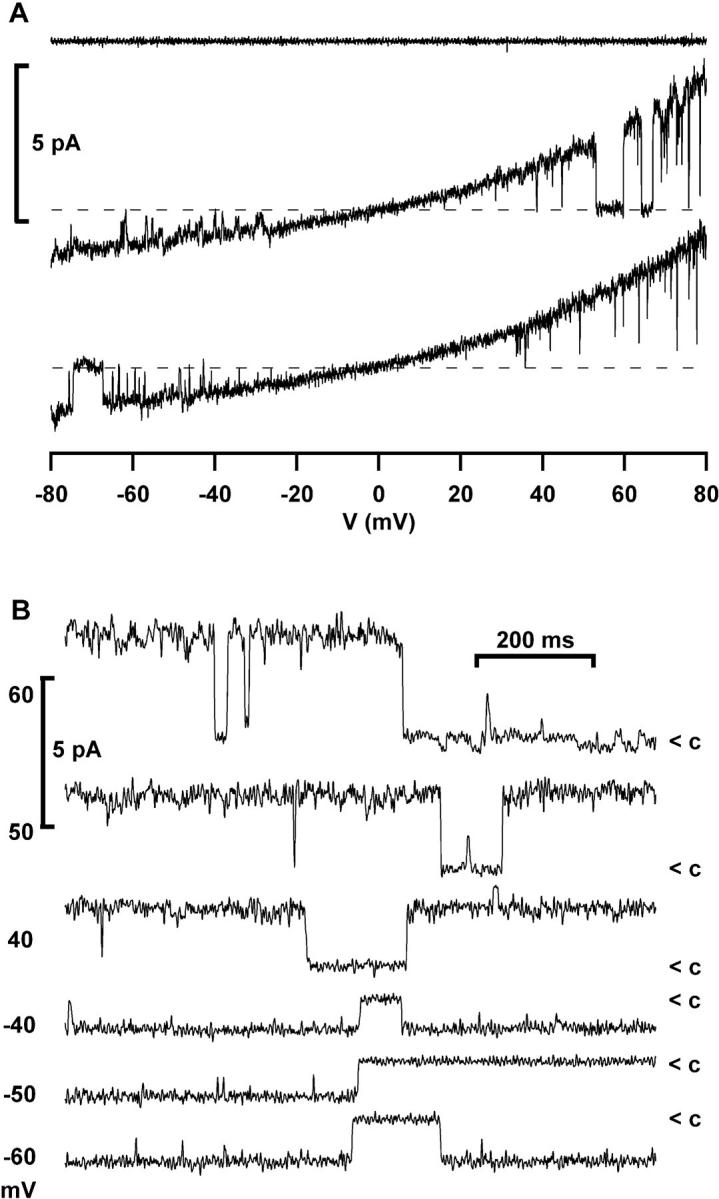
Single channels in excised patches and whole- cell recordings. (A) Activation of an outwardly rectifying Cl− channel by purified p56lck (2 U/ml + 10 μM ATP) added to the internal surface of an excised patch (Jurkat T cells; n = 6). Instantaneous IV-plots were obtained using voltage ramps from −80 to 80 mV. The upper trace was taken before, the lower traces 3 min after addition of the enzyme (symmetrical NaCl solution, leak subtracted). (B) Continuous recordings from an excised inside-out patch activated by purified p56lck, showing closure events (<c) of the outwardly rectifying channel at the indicated holding potentials (filter 500 Hz). (C) Single-channel transitions in a continuous whole- cell recording from a JCaM1.6 cell with 10 U/ml p56lck in the pipette (same conditions as in Fig. 3 C). Upward deflection indicates channel opening. Two channels are visible in this trace. The inset shows a channel opening at a 10-fold magnified time scale. Note the slow gating. The holding potential was 80 mV, filter 500 Hz. (D) Current transitions in whole-cell currents resemble outwardly rectifying chloride channels in excised patches. □, Whole-cell current transitions activated by slow swelling of a Jurkat cell with hypertonic intracellular solution (330 mOsmol/kg, Cs+-glutamate); ○, whole-cell current transitions activated by p56lck in a JCaM1.6 cell with slightly hypotonic intracellular solution (300 mOsmol/kg, Cs+-glutamate); ▴, excised inside-out patch activated by purified p56lck with Cs+-glutamate in the bath; ▵, excised patch activated by purified p56lck with NaCl in the bath. The extracellular solution always contained NaCl (310 mOsmol/kg).
Single-Channel Transitions in Whole-Cell Recordings
Single-channel opening and closing transitions between discrete whole-cell current levels were frequently seen, when ICl was slowly activated by p56lck in JCaM1.6 cells using slightly hypotonic intracellular solution (300 mOsmol/kg; Fig. 3 C). Single-channel openings lasting for several seconds were observed in continuous recordings at constant voltage (Fig. 4 C). The transitions in current traces obtained with voltage ramps frequently spanned more than one channel level. A similar behavior has been described for swelling-activated chloride channels (Meyer and Korbmacher, 1996). With voltage ramps, the current often jumped several times between an open and closed state.
Single-channel transitions could also be observed when Jurkat cells were patched with slightly hypertonic intracellular solution (330 mOsmol/kg). Cell volume increased and ICl−swell activated slowly under these conditions. Transitions in whole-cell currents either activated by p56lck or by slow swelling are compared with single-channel transitions from excised, p56lck-activated patches in Fig. 4 D. We obtained unitary conductances of 25, 28, and 31 pS at 40 mV when constructing IV plots from single-channel transitions activated by swelling or purified p56lck in whole-cell recordings, or activated by p56lck in excised patches, respectively.
Osmotic Cell Swelling Activates p56lck
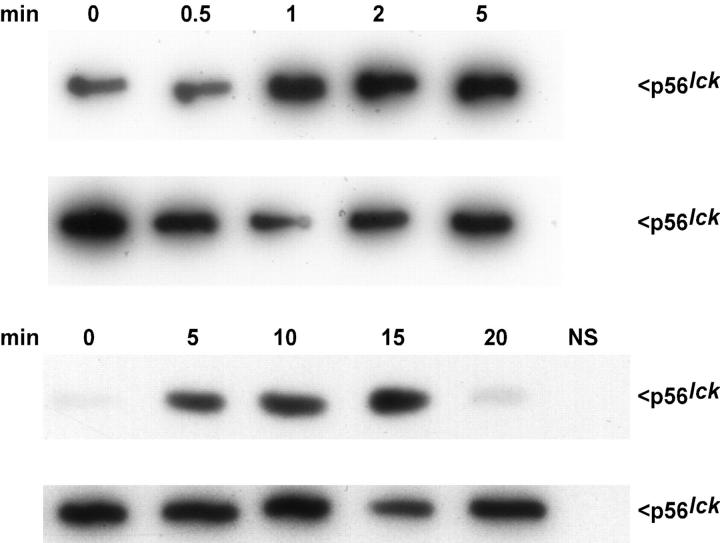
To measure p56lck kinase activity, Jurkat T cells were exposed to hypotonic extracellular solution (250 mOsmol/kg) and in vitro assays were performed on immunoprecipitated p56lck. A transient increase in p56lck activity was observed resembling the time course of RVD. p56lck activation was detected 1 min after exposure to osmotic stress, peaked at 15 min, and then declined rapidly thereafter (Fig. 5). No activation of p56lck was seen when cells were kept in isotonic solution (data not shown).
Figure 5.
Cell swelling activates p56lck. The activity of p56lck induced by hypoosmotic cell swelling is shown at two different time scales. Jurkat cells (4 × 106 per sample) were incubated with 250 mOsmol/kg solution for the indicated time, lysed, and then p56lck was immunoprecipitated from postcentrifugation supernatants. Activity of p56lck was determined by autophosphorylation in the presence of [32P]γATP. Samples were separated by 10% SDS-PAGE and analyzed by autoradiography. Nonspecific control immunoprecipitate performed with irrelevant affinity-purified polyclonal rabbit immunoglobulins is lacking kinase activity (lower panel right lane, NS). The second blot in each panel shows an aliquot of immunoprecipitated p56lck immunoblotted with anti-p56lck antibody demonstrating comparable amounts of protein in each lane. The results are representative of four independent experiments.
Discussion
In this study, we show for the first time that the tyrosine kinase, p56lck, mediates RVD by activating ICl−swell. T cells deficient for p56lck are defective in RVD and activation of ICl−swell, and retransfection of this kinase results in restoration of osmotic current activation.
Several observations suggesting involvement of a phosphorylation event in osmotic current activation have been reported. ATP in the pipette is required for maintenance and repeated activation of ICl−swell in lymphocytes (Lewis et al., 1993; Ross and Cahalan, 1995). A similar ATP dependence has been demonstrated in many different endothelial and epithelial cell types (Gill et al., 1992; Jackson et al., 1994, 1996; Oiki et al., 1994; Meyer and Korbmacher, 1996), although some discrepancies exist between different cells, i.e., the ability of nonhydrolyzable ATP analogues to replace ATP. Furthermore, a delay between the volume change and current activation in the range of 1 min is typically observed, making a direct link between membrane stretch and channel gating unlikely (Lewis et al., 1993; Ross et al., 1994). Direct evidence for involvement of tyrosine phosphorylation in ICl− activation came from pharmacological studies. Inhibitors of tyrosine phosphorylation have been recently reported to inhibit swelling-induced 125I efflux in intestinal epithelium (Tilly et al., 1993) and activation of ICl in cardiac and endothelial cells (Sorota, 1995; Voets et al., 1998). To our knowledge, no specific tyrosine kinase has been linked to the activation of ICl−swell so far.
When compared with the native Jurkat cell line, activation of ICl−swell in JCaM1.6 cells was not completely abolished, but reduced in size and delayed. This could conceivably indicate a permissive role for p56lck-mediated tyrosine phosphorylation. However, the p56lck-deficient cell line JCaM1.6 expresses other src-like kinases like fyn (August and Dupont, 1995). Herbimycin A and lavendustin, which inhibit all src-like kinases, blocked RVD and the osmotic current response. Therefore, we suppose that other src-like kinases can partially substitute for lck in lck-deficient cells. Strong evidence for a crucial role of p56lck comes from the experiments using purified p56lck. When added to the cytosol, p56lck activates a whole-cell chloride current with properties indistinguishable from ICl−swell without cell swelling and opens a Cl− channel in excised patches. Both currents share lack of inactivation, block by 500 μM DIDS, selectivity for Cl−, and outward rectification. The smaller amplitude of the lck-activated current typically observed in JCaM1.6 cells could be attributed to amplification of kinase activity by phosphorylation of the native p56lck present in Jurkat cells.
Several reports show that CFTR can be involved in the regulation of outwardly rectifying chloride channels (Gabriel et al., 1993; Schwiebert et al., 1995). Single-channel recordings from fibroblasts transfected with CFTR have revealed the activation of a fast gate by the tyrosine kinase p60c-src, increasing this chloride channel's open probability (Fischer and Machen, 1996). Whereas ICl−swell is easily distinguishable from CFTR channels by its outward rectification and sensitivity to DIDS, the CFTR protein could represent a target for tyrosine phosphorylation, regulating the ICl−swell studied here. Identification, cloning, and purification of the channel protein underlying ICl−swell in lymphocytes will be necessary to determine the phosphorylation target. Therefore, we attempted to characterize the single channel underlying ICl−swell in whole-cell recordings.
The single channel responsible for ICl−swell has not been identified so far. An outwardly rectifying, DIDS-sensitive 40–50 pS chloride channel can be activated by membrane excision from lymphocytes and prolonged depolarization (Garber, 1992). However, ∼1 pS unitary conductance was estimated by stationary noise analysis of ICl−swell in lymphocytes (Doroshenko and Neher, 1992; Lewis et al., 1993). A solution to this discrepancy could come from the observation, that ICl−swell channels in epithelial cells show prolonged open states with minimal channel gating (Jackson and Strange, 1995; Meyer and Korbmacher, 1996). When current variance is analyzed to obtain information about unitary events, gating events could be missed because of their low frequency. Recording at room temperature further slows down gating kinetics and the lack of voltage-dependent inactivation hinders the use of nonstationary noise analysis. In the present study we observed single-channel events in high resolution whole-cell recordings. The channels show extremely slow gating even at 31°C and prolonged open states lasting for several seconds. Similar behavior of swelling-activated chloride channels has been recently described in glioma and kidney epithelial cells (Jackson and Strange, 1995; Meyer and Korbmacher, 1996). We obtain unitary conductances of 25 and 28 pS when constructing IV plots from single channel transitions in whole-cell recordings activated by swelling and by purified p56lck, respectively. Furthermore, we were able to activate a 31-pS, outwardly rectifying Cl− channel in excised membrane patches by adding purified p56lck to the cytosolic surface. Thus, cell swelling and p56lck seem to activate the same chloride channel. The 31-pS outwardly rectifying single channels observed in excised patches may well be responsible for ICl−swell in lymphocytes.
Cell swelling has been previously described to induce tyrosine phosphorylation in an intestinal epithelial cell line (Tilly et al., 1993). Hypoosmotic stimulation of mitogen-activated protein kinase was blocked by tyrosine kinase inhibitors in astrocytes (Schliess et al., 1996). Tyrosine kinase activation is upstream of MAP kinase (Schliess et al., 1996). In Jurkat T lymphocytes we show for the first time activation by hypoosmotic cell swelling of a specific tyrosine kinase that is directly involved in the activation of ICl−swell. Interestingly, the enhanced p56lck activity is transient, following a time course similar to the RVD in intact lymphocytes (Figs. 1 and 5). Thus, a tight feedback control seems to regulate volume and p56lck activity. ICl−swell channels are activated by p56lck and mediate volume decrease by anion efflux, representing an important link in this novel control loop.
Abbreviations used in this paper
- RVD
regulatory volume decrease
- ICl
chloride current
- ICl−swell
swelling-activated chloride current
- CFTR
cystic fibrosis transmembrane conductance regulator
- DIDS
diisothiocyanato-2-2-stilbenesulfonic acid
Footnotes
This work was in part funded by Deutsche Forschungsgemeinschaft Le 792/3-1, La 315/4-3, and Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie/Interdisziplinäres Klinisches Forschungszentrum 01KS9605. I. Szabò is grateful for a European Molecular Biology Organization fellowship.
E. Gulbins and F. Lang contributed equally to this work.
References
- August A, Dupont B. Activation of extracellular signal-regulated protein kinase (ERK/MAP kinase) following CD28 cross-linking: activation in cells lacking p56lck. Tissue Antigens. 1995;46:155–162. doi: 10.1111/j.1399-0039.1995.tb03114.x. [DOI] [PubMed] [Google Scholar]
- Cahalan M.D., and R.S. Lewis. 1988. Role of potassium and chloride channels in volume regulation by T lymphocytes. In Cell Physiology of Blood. R.B. Gunn, and J.C. Parker, editors. Rockefeller University Press, New York. 281–301. [PubMed]
- Deutsch C, Lee SC. Cell volume regulation in lymphocytes. Renal Physiol Biochem. 1988;3:260–276. doi: 10.1159/000173166. [DOI] [PubMed] [Google Scholar]
- Doroshenko P, Neher E. Volume-sensitive chloride conductance in bovine chromaffin cell membrane. J Physiol (Lond) 1992;449:197–218. doi: 10.1113/jphysiol.1992.sp019082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer H, Machen TE. The tyrosine kinase p60c-src regulates the fast gate of the cystic fibrosis transmembrane conductance regulator chloride channel. Biophys J. 1996;71:3073–3082. doi: 10.1016/S0006-3495(96)79501-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel SE, Clarke LL, Boucher RC, Stutts MJ. CFTR and outward rectifying chloride channels are distinct proteins with a regulatory relationship. Nature. 1993;363:263–268. doi: 10.1038/363263a0. [DOI] [PubMed] [Google Scholar]
- Garber S. Outwardly rectifying chloride channels in lymphocytes. J Membr Biol. 1992;127:49–56. doi: 10.1007/BF00232757. [DOI] [PubMed] [Google Scholar]
- Gill DR, Hyde SC, Higgins CF, Valverde MA, Mintenig GM, Sepulveda FV. Separation of drug transport and chloride channel functions of the human multidrug resistance P-glycoprotein. Cell. 1992;71:23–32. doi: 10.1016/0092-8674(92)90263-c. [DOI] [PubMed] [Google Scholar]
- Jackson PS, Strange K. Single-channel properties of a volume-sensitive anion conductance. Current activation occurs by abrupt switching of closed channels to an open state. J Gen Physiol. 1995;105:643–660. doi: 10.1085/jgp.105.5.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson PS, Morrison R, Strange K. The volume-sensitive organic osmolyte-anion channel VSOAC is regulated by nonhydrolytic ATP binding. Am J Physiol. 1994;267:1203–1209. doi: 10.1152/ajpcell.1994.267.5.C1203. [DOI] [PubMed] [Google Scholar]
- Jackson PS, Churchwell K, Ballatori N, Boyer JL, Strange K. Swelling-activated anion conductance in skate hepatocytes: regulation by cell Cl−and ATP. Am J Physiol. 1996;270:57–66. doi: 10.1152/ajpcell.1996.270.1.C57. [DOI] [PubMed] [Google Scholar]
- Kunzelman K, Pavenstädt H, Greger R. Properties and regulations of chloride channels in cystic fibrosis and normal airway cells. Pflügers Arch. 1989;415:172–182. doi: 10.1007/BF00370589. [DOI] [PubMed] [Google Scholar]
- Lang F, Busch G, Ritter M, Völkl H, Waldegger S, Gulbins E, Häusinger D. The functional significance of cell volume regulation mechanisms. Physiol Rev. 1998;78:247–306. doi: 10.1152/physrev.1998.78.1.247. [DOI] [PubMed] [Google Scholar]
- Levitan I, Almonte C, Mollard P, Garber SS. Modulation of a volume-regulated Cl−current by f-actin. J Membr Biol. 1995;147:283–294. doi: 10.1007/BF00234526. [DOI] [PubMed] [Google Scholar]
- Lewis RS, Cahalan MD. Subset-specific expression of potassium channels in developing murine T lymphocytes. Science. 1988;239:771–775. doi: 10.1126/science.2448877. [DOI] [PubMed] [Google Scholar]
- Lewis RS, Ross PE, Cahalan MD. Chloride channels activated by osmotic stress in T lymphocytes. J Gen Physiol. 1993;101:801–826. doi: 10.1085/jgp.101.6.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K, Korbmacher C. Cell swelling activates ATP-dependent voltage-gated chloride channels in M-1 mouse cortical collecting duct cells. J Gen Physiol. 1996;108:177–193. doi: 10.1085/jgp.108.3.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimoto I, Wagner JA, Schulman H, Gardner P. Regulation of Cl−channels by multifunctional CaM kinase. Neuron. 1991;6:547–555. doi: 10.1016/0896-6273(91)90057-7. [DOI] [PubMed] [Google Scholar]
- Oiki S, Kubo M, Okada Y. Mg2+ and ATP-dependence of volume-sensitive Cl−channels in human epithelial cells. Jpn J Physiol. 1994;44(Suppl.):77–79. [PubMed] [Google Scholar]
- Ross PE, Cahalan MD. Ca2+influx pathways mediated by swelling or stores depletion in mouse thymocytes. J Gen Physiol. 1995;106:415–444. doi: 10.1085/jgp.106.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross PR, Garber SS, Cahalan MD. Membrane chloride conductance and capacitance in Jurkat T lymphocytes during osmotic swelling. Biophys J. 1994;66:169–178. doi: 10.1016/S0006-3495(94)80754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schliess F, Sinning R, Fischer R, Schmalenbach C, Haussinger D. Calcium-dependent activation of Erk-1 and Erk-2 after hypo-osmotic astrocyte swelling. Biochem J. 1996;320:167–171. doi: 10.1042/bj3200167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwiebert EM, Egan ME, Hwang TH, Fulmer SB, Allen SS, Cutting GR, Guggino WB. CFTR regulates outwardly rectifying chloride channels through an autocrine mechanism involving ATP. Cell. 1995;81:1063–1073. doi: 10.1016/s0092-8674(05)80011-x. [DOI] [PubMed] [Google Scholar]
- Solc CK, Wine JJ. Swelling-induced and depolarization-induced Cl−channels in normal and cystic fibrosis epithelial cells. Am J Physiol. 1991;261:658–674. doi: 10.1152/ajpcell.1991.261.4.C658. [DOI] [PubMed] [Google Scholar]
- Sorota S. Swelling-induced chloride-sensitive current in canine atrial cells revealed by whole-cell patch-clamp method. Circ Res. 1992;70:679–687. doi: 10.1161/01.res.70.4.679. [DOI] [PubMed] [Google Scholar]
- Sorota S. Tyrosine protein kinase inhibitors prevent activation of cardiac swelling-induced chloride current. Pflugers Arch. 1995;431:178–185. doi: 10.1007/BF00410189. [DOI] [PubMed] [Google Scholar]
- Tilly BC, van den Berghe N, Tertoolen LG, Edixhoven MJ, de Jonge HR. Protein tyrosine phosphorylation is involved in osmoregulation of ionic conductances. J Biol Chem. 1993;268:19919–19922. [PubMed] [Google Scholar]
- Voets T, Manolopoulos V, Eggermont J, Ellory C, Droogmans G, Nilius B. Regulation of a swelling-activated chloride current in bovine endothelium by protein tyrosine phosphorylation and G proteins. J Physiol. 1998;506:341–352. doi: 10.1111/j.1469-7793.1998.341bw.x. [DOI] [PMC free article] [PubMed] [Google Scholar]