Abstract
The interaction of cells with fibronectin generates a series of complex signaling events that serve to regulate several aspects of cell behavior, including growth, differentiation, adhesion, and motility. The formation of a fibronectin matrix is a dynamic, cell-mediated process that involves both ligation of the α5β1 integrin with the Arg-Gly-Asp (RGD) sequence in fibronectin and binding of the amino terminus of fibronectin to cell surface receptors, termed “matrix assembly sites,” which mediate the assembly of soluble fibronectin into insoluble fibrils. Our data demonstrate that the amino-terminal type I repeats of fibronectin bind to the α5β1 integrin and support cell adhesion. Furthermore, the amino terminus of fibronectin modulates actin assembly, focal contact formation, tyrosine kinase activity, and cell migration. Amino-terminal fibronectin fragments and RGD peptides were able to cross-compete for binding to the α5β1 integrin, suggesting that these two domains of fibronectin cannot bind to the α5β1 integrin simultaneously. Cell adhesion to the amino-terminal domain of fibronectin was enhanced by cytochalasin D, suggesting that the ligand specificity of the α5β1 integrin is regulated by the cytoskeleton. These data suggest a new paradigm for integrin-mediated signaling, where distinct regions within one ligand can modulate outside-in signaling through the same integrin.
Fibronectins are a family of high molecular weight, multidomain glycoproteins that circulate in the plasma as soluble, protomeric molecules, and are also found in an insoluble, multimeric form within the extracellular matrix. Fibronectin contains multiple binding sites, including those for sulfated glycosaminoglycans, gelatin, fibrin, and cell surface integrin receptors (43, 47, 72). As a result, fibronectin plays an important role in orchestrating a variety of adhesive and migratory events that occur during embryogenesis, angiogenesis, thrombosis and hemostasis, inflammation, and wound repair (31). Fibronectin expression is widely distributed in embryos (31, 68) and is essential for embryogenesis (18), providing pathways for neural crest and mesodermal migration (18, 31, 68). Cell-mediated fibronectin polymerization also occurs during the repair phase following tissue injury, where it promotes the migration and attachment of fibroblasts, endothelial cells, monocytes, and neutrophils into the wound area (10, 31). In addition, altered deposition of a fibronectin matrix has been correlated with several pathological events. Increased deposition of a fibronectin matrix has been associated with atherosclerosis and fibrosis (7, 38, 67), while restoration of fibronectin matrix assembly in transformed cells has been correlated with a reduction in tumorigenicity (19).
Adherent cells polymerize an insoluble fibronectin matrix by assembling cell- or plasma-derived soluble fibronectin into insoluble fibrils (44). In one of the initial steps of matrix assembly, cell surfaces bind the amino-terminal region of fibronectin in a reversible and saturable manner (39, 54). Subsequent homophilic binding interactions are thought to promote the polymerization of the fibronectin molecule into an insoluble matrix (8, 24, 25, 41, 42) and allow for the regeneration of the cell surface amino-terminal–binding site (44). The binding of the amino terminus of fibronectin to cell surface receptors, termed matrix assembly sites (39), is mediated by the first five type I repeats, which appear to function as a single-binding unit (66). Fibronectin fragments and recombinant constructs missing the amino-terminal region are not assembled into a fibrillar matrix (40, 62, 66). In addition, the presence of excess amino-terminal fibronectin fragment blocks the binding of intact fibronectin to cell surfaces and inhibits matrix assembly (39). The molecule(s) that mediates the initial binding of the amino terminus of fibronectin to cell surfaces has not been identified.
Cellular adhesion to fibronectin is mediated primarily by the α5β1 integrin receptor that interacts with the Arg-Gly-Asp (RGD) sequence within fibronectin's tenth type III module (30, 48). The importance of the α5β1 integrin in fibronectin matrix assembly has been demonstrated in several studies. Overexpression of the α5β1 integrin in CHO cells results in increased fibronectin deposition (19). Antibodies directed against the α5β1 integrin inhibit fibronectin fibril formation (1, 17). In addition, anti-β1 integrin antibodies have been shown to inhibit binding of the amino-terminal fragment to the cell surface, suggesting that the α5β1 integrin can regulate the expression of matrix assembly sites (17). More recently, amino-terminal fibronectin fragments were shown to colocalize with α5β1 integrins at sites of focal adhesions (9, 15).
The interaction of cells with the extracellular matrix via cell surface integrins generates a series of complex signaling events that serve to regulate several aspects of cell behavior, including growth, differentiation, adhesion, and motility (30). Ligation of the α5β1 integrin with the RGD sequence of fibronectin results in the local accumulation of signaling molecules and cytoskeletal components at sites of focal adhesions, and stimulates the tyrosine phosphorylation of specific proteins associated with focal adhesions, including focal adhesion kinase (FAK)1 (21, 35, 59), paxillin (6, 69), tensin (5), and p130cas (46, 71). Focal adhesions, defined morphologically as regions of close cell substratum contact, are dynamic structures that serve to structurally link the extracellular matrix with the cytoskeleton during cell adhesion, and facilitate the transduction of signaling events triggered by extracellular matrix molecules (34). The localization of amino-terminal fibronectin fragments to sites of focal adhesions (15) suggests the possibility that intracellular signals may be generated in response to the interaction of the amino terminus of fibronectin with the matrix assembly site during fibronectin polymerization.
Fibronectin matrix assembly involves both ligation of the α5β1 integrin with the RGD sequence in fibronectin and binding of the amino terminus of fibronectin to cell surface matrix assembly sites. To examine the cellular response initiated upon binding of the amino-terminal domain of fibronectin to cell surfaces, cycloheximide-pretreated cells were allowed to adhere to either recombinant amino-terminal (70 kD) or cell adhesive (III9,10) fragments of fibronectin. In this study, we demonstrate that amino-terminal fragments of fibronectin promote α5β1 integrin-mediated cell adhesion and induce the assembly of novel focal contact structures, which are distinct from those observed after the interaction of the α5β1 integrin with the RGD-containing III9,10 modules of fibronectin. Differential ligation of the α5β1 integrin with either the amino-terminal or RGD domain of fibronectin results in differences in the level of tyrosine phosphorylation of FAK and paxillin. Treatment of suspended cells with cytochalasin D upregulates cell adhesion to amino-terminal fibronectin fragments, suggesting that the ligand specificity of the α5β1 integrin is regulated by the cytoskeleton. In addition, the amino terminus of fibronectin promotes an increase in cell motility in haptotatic assays. Taken together, these data indicate that the interaction of cells with the amino terminus of fibronectin generates specific intracellular signaling events that represent a unique pathway for cell communication with the extracellular matrix. These studies suggest a mechanism, whereby the α5β1 integrin coordinates and modulates cell function by interacting with separate regions of the fibronectin molecule.
Materials and Methods
Reagents and Antibodies
Gel electrophoresis supplies were from Bio-Rad Laboratories (Richmond, VA). Unless otherwise indicated, chemical reagents were obtained from Sigma Chemical Co. (St. Louis, MO). The following monoclonal antibodies were purchased: anti-integrin subunits α2 (P1E6), α3 (P1B5), α5 (mAb16), β1 (mAb13), and β3 (7F12) were purchased from Collaborative Biomedical Products/Becton Dickinson (Bedford, MA); anti-integrin αvβ3 (LM609), talin, and α-actinin were from Chemicon International Inc. (Temecula, CA); anti-fibronectin (3E3), anti-integrin subunits β5 (P1F6), and β1 (P4C10) were purchased from Life Technologies/GIBCO BRL (Gaithersburg, MD); paxillin, phosphotyrosine (PY20), and FAK were purchased from Transduction Laboratories (Lexington, KY); anti-vinculin was purchased from Sigma Chemical Co. Polyclonal antibodies to FAK (BC3) were purchased from Upstate Biotechnology Inc. (Lake Placid, NY). The polyclonal anti-α5 antibody, 4318, was a generous gift from Kenneth Yamada (National Institutes of Health, Bethesda, MD). The murine anti–human fibronectin monoclonal antibody, 9D2, was a gift from Deane Mosher (University of Wisconsin, Madison, WI). The epitope recognized by 9D2 is found within the III1 module (8).
Cell Culture
Human foreskin fibroblasts, A1-F, were a gift from Lynn Allen-Hoffmann (University of Wisconsin, Madison, WI). A1-Fs were cultured in DME (GIBCO BRL) supplemented with 10% FBS (Sterile Systems, Logan, UT).
Purification of Fibronectin and Fibronectin Fragments
Human plasma fibronectin was purified from a fibronectin- and fibrinogen-rich by-product of factor VIII production by ion exchange chromatography on DEAE-cellulose (Pharmacia Biotechnology Inc., Piscataway, NJ), as previously described (40). Fig. 1 shows a schematic of the various fragments and constructs used in this study. The 70-kD amino-terminal fragment of fibronectin was generated by limited digestion of intact fibronectin with cathepsin D, followed by gelatin affinity chromatography, as previously described (40). The 70-kD fragment was further cleaved into a 27-kD amino-terminal fragment and a 40-kD gelatin-binding fragment by limited digestion with trypsin (3). The 27- and 40-kD fragments were then separated by gelatin affinity chromatography. For affinity chromatography, experiments involving isolated α5β1 integrins, the 27- and 70-kD fragments were further purified by immunoabsorbing contaminating fibronectin fragments with the anti-fibronectin antibody, 9D2 (8), covalently coupled to protein A-Sepharose (Pharmacia Biotechnology Inc.). Immunoblot analysis indicated that these preparations contained less than 1 ng of III10-containing fibronectin fragments per 10 μg of 70- or 27-kD fragment (data not shown). A preparation containing the 120-kD cell-binding fragment was generated by limited digestion of fibronectin with chymotrypsin and isolated from the unbound fractions of sequential gelatin– and heparin–Sepharose affinity columns (Pharmacia Biotechnology Inc.), as previously described (50). Purity of all fragments was assessed by SDS-PAGE and proteins were frozen at −80°C until use.
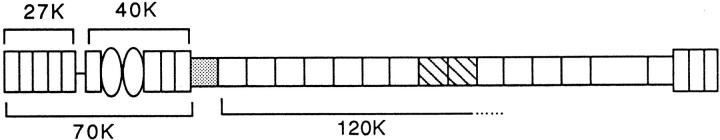
Figure 1.
Fibronectin fragments. Schematic representation of fibronectin molecule. Shown are the 27- (cathepsin-trypsin: amino-terminal), 40- (cathepsin-trypsin: gelatin binding), 70- (cathepsin: amino-terminal), and 120-kD (chymotrypsin: cell binding) fragments. Rectangles, type I modules; ovals, type II modules; squares, type III modules; hatched squares, III9 and III10 modules.
Preparation of Recombinant Fibronectin Modules
Recombinant rat 70 kD (r70 kD) and 40 kD (r40 kD) proteins were produced using a baculovirus expression system, as previously described (65). Recombinant proteins were purified from SF21 insect cells grown in serum-free SF900-II medium (GIBCO BRL) on columns of gelatin-agarose (65). SF21 cells do not produce any detectable fibronectin. Therefore, the r70 and r40 kD proteins produced by these cells do not contain any contaminating fibronectin.
Recombinant human III9,10 (rIII9,10) was expressed in BL21(DE3) bacteria as a fusion protein composed of glutathione-S-transferase (GST) and III9 and III10, as previously described (25). To separate III9,10 from GST, fusion proteins were incubated with TPCK-trypsin-agarose beads (1 U/mg) for 30 min at 20°C. Following incubation, the TPCK-trypsin-agarose beads were removed by centrifugation. rIII9,10 was further purified by passing the digested material over a glutathione agarose column. Purity of recombinant protein preparations was assessed by SDS-PAGE and proteins were frozen at −80°C until use.
Preparation of α5β1 Integrins
The α5β1 integrin was isolated from term placenta by affinity chromatography on 120-kD Sepharose columns, according to the method of Pytela et al. (52, 53). Placenta (∼500 g) was dissected free of chorion, amnion, and umbilical cord, and homogenized briefly with an equal volume of buffer A (50 mM Tris, pH 7.4, 150 mM NaCl, 1 mM CaCl2, 1 mM MgCl2, 1 mM MnCl2, 2 mM PMSF) containing 0.5 mM Pefablock (Boehringer Mannheim Biochemicals, Indianapolis, IN). The homogenate was centrifuged at 16,000 g for 20 min at 4°C and washed three times with buffer A. The pellet was extracted for 1 h on ice with an equal volume of 50 mM octyl-β-d-thioglucopyranoside (OGPS) in buffer A and centrifuged at 27,000 g for 20 min at 4°C. The supernatant was incubated overnight at 4°C with 120 kD-Sepharose (1 mg 120 kD/ml Sepharose). The suspension was packed into a chromatography column and washed with 15 mM OGPS in buffer A. Bound material was eluted with 20 mM EDTA, 25 mM OGPS in buffer A, and then passed over a wheat germ agglutinin column (Vector Labs Inc., Burlingame, CA) pre-equilibrated with buffer B (0.1% NP-40 in buffer A). The wheat germ agglutinin column was washed with buffer B and eluted with 200 mM N-acetyl glucosamine in buffer B. Purity of the α5β1 integrin preparation was ⩾90% as assessed by SDS-PAGE electrophoresis, followed by Coomasie blue staining. Identity of the α5β1 integrin was confirmed by immunoblotting with the anti-β1 antibody, P4C10. Purified α5β1 integrin was also immunoblotted with the anti-fibronectin antibody, 3E3, which recognizes an epitope within the III9-10 modules of fibronectin (49). Fibronectin was not detected upon development of the immunoblot with enhanced chemiluminescence.
Cycloheximide Pretreatment
A1-F fibroblasts were seeded onto 100 mm tissue culture dishes (Corning/ Costar, Cambridge, MA) at 106 cells/ml in DME containing 10% FBS, and allowed to reach confluence (3–4 d). To minimize endogenous fibronectin levels during experimental procedures, cells were washed three times with serum-free DME, and pretreated for 3.5 h with cycloheximide (20 μg/ml) in DME containing 0.4% ReduSerII (Upstate Biotechnology Inc.; 39). Cells were detached by incubation in PBS containing 0.5 mM EDTA, followed by 0.08% trypsin (GIBCO BRL). Trypsin activity was inhibited by the addition of 10× excess of soybean trypsin inhibitor and cells were washed three times with DME/0.4% ReduSerII. Cycloheximide (20 μg/ml) was present at all stages of detachment and washing. Cells were resuspended in serum-free DME/0.4% ReduSerII/20 μg/ml cycloheximide, and allowed to recover from trypsinization at 37°C for 60–90 min before plating onto various adhesive substrates.
Cell Attachment Assays
To quantitate cell adhesion to various fibronectin constructs, r40 kD, r70 kD, and rIII9,10 were diluted to 10 μg/ml in PBS, and coated onto 24-well tissue culture plates (Corning/Costar) for 3 h at 37°C. Wells were blocked with BSA at 10 mg/ml for 30 min. Control wells were coated with BSA alone. Cycloheximide-pretreated A-1F fibroblasts were seeded into precoated 24-well tissue culture plates at 105 cell/well. In some wells, anti-integrin antibodies, GRGDS or GRGES peptides (Life Technologies Inc./GIBCO BRL), heparin, or EDTA were added at time of seeding. After a 5-h incubation at 37°C, cells were washed three times in serum-free media, fixed with 1% paraformaldehyde, and stained with 0.5% crystal violet. After staining, cells were washed with water, solubilized with 0.1% SDS, and the absorbance was determined at 540 nM.
70-kD Fragment Affinity Chromatography
r70-, 70-, 27-, 40-, and 120-kD proteolytic fibronectin fragments were coupled to cyanogen bromide-activated Sepharose-4B (Pharmacia Biotechnology Inc.), according to manufacturer's instructions. Each protein was coupled at a molar concentration of 14.3 μM (70 kD = 1 mg/ml). The amount of protein coupled to each resin was determined by monitoring the absorbance at 280 nM of all buffers and washes during the coupling and blocking procedures. Coupling efficiency was ⩾96% for all proteins. Coupled resins were blocked with 0.5% protease-free BSA (Intergen, Purchase, NY) and pre-equilibrated with lysis buffer before use.
Media was removed from cycloheximide-pretreated A-1F fibroblasts, and cells were washed twice with ice-cold PBS. Cells (5 × 106 cells/ml lysis buffer) were lysed on ice using 60 mM OGPS in Tris buffer (50 mM Tris buffer, pH 7.4, containing 150 mM NaCl, 1 mM CaCl2, 1 mM MgCl2, 0.5% BSA, 2 mM PMSF, 1 mM Pefablock, 0.5 mg/ml soybean trypsin inhibitor, 25 μg/ml leupeptin and 25 μg/ml pepstatin; 52). Cell lysates were clarified by centrifugation at 15,000 g for 15 min at 4°C and supernatants were rocked overnight at 4°C with the protein-conjugated resins (1 ml supernatant/ml packed resin). For experiments involving isolated α5β1 integrins, purified α5β1 integrins were diluted in 50 mM OGPS/Tris buffer and rocked overnight at 4°C with the protein-conjugated resins (∼5 μg of α5β1 integrin/0.5 ml packed resin). Resins were washed with 60 mM OGPS in Tris buffer, packed into columns, and washed with an additional 10 column volumes of 15 mM OGPS in Tris buffer. The columns were eluted with 20 mM EDTA and 25 mM OGPS in Tris buffer (52). Fractions (1 ml) were precipitated with trichloroacetic acid, dissolved in nonreducing sample buffer, and analyzed by SDS-PAGE and immunoblotting.
Immunoprecipitation
r70 kD, rIII9,10, and fibronectin were diluted to 10 μg/ml in PBS and coated onto 100 mm tissue culture dishes for 3 h at 37°C. Unbound protein was removed and plates were blocked with BSA at 10 mg/ml for 30 min. Cycloheximide-pretreated A-1F fibroblasts were prepared as described above and seeded at 2 × 106 cell/plate in DME/0.4% ReduSerII/cycloheximide into precoated 100 mm tissue culture dishes. After a 15-h incubation at 37°C, media was removed and cells were washed twice with ice-cold PBS. Cells were incubated on ice for 20 min with 1 ml cold RIPA buffer (50 mM Tris, pH 7.6, containing 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 1 mM EDTA, 1 mM EGTA, 25 μg/ml leupeptin, 25 μg/ml aprotinin, 200 μM sodium orthovanadate, 1 mM H2O2, 0.1% sodium azide, 0.5 mg/ml soybean trypsin inhibitor, and 2 mM PMSF). Cells were scraped from the dish and lysates were clarified by centrifugation at 15,000 g for 30 min at 4°C. Supernatants were assayed for protein concentration using bicinchroninic acid reagents (Pierce, Rockford, IL), according to manufacturer's instructions. 400 μg aliquots were adjusted to equal volume, precleared with mouse IgG and protein A–Sepharose (Pharmacia Biotechnology Inc.) for 1 h at 4°C, and incubated with 4 μg primary antibody for 1 h on ice. Protein A–Sepharose was added to tubes and aliquots were incubated with rocking for an additional 1 h at 4°C. Immunoprecipitates were washed four times with RIPA buffer, solubilized with reducing sample buffer, and analyzed by SDS-PAGE and immunoblotting.
Gel Electrophoresis and Immunoblotting
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed according to Laemmli (36) using a discontinuous buffer system (Bio-Rad Laboratories). Samples were diluted 1:1 in gel buffer containing 4% SDS and 20% glycerin in 0.05 M Tris, pH 6.8. Some samples were reduced with 2% β-mercaptoethanol before electrophoresis. For immunoblotting, electrophoretic transfer of proteins to nitrocellulose (Schleicher & Schuell Inc., Keene, NH) was performed in a transblot apparatus (Bio-Rad Laboratories), according to manufacturer's instructions. Blots were incubated with 3% BSA in Tris-buffered saline, pH 7.6, containing 0.1% Tween-20 (TBS-T), and immunoblotted with primary antibody (anti-phosphotyrosine, PY20, at 1:5,000; anti-β1, P4C10, at 1:1,000; anti-α5, 4318, at 1:1,000; anti-α3, P1B5, at 1:1,000) followed by a 1:6,000 dilution of either goat anti–rabbit or goat anti–mouse peroxidase-linked secondary antibody (Bio-Rad Laboratories). Immunoblots were developed using enhanced chemiluminescence (Amersham Corp., Arlington Heights, IL) according to the manufacturer's protocol. After detection, the immunoblots were stripped in 62.5 mM Tris, pH 6.7, 2% SDS, 100 mM β-mercaptoethanol at 70°C for 60 min. Before reprobing, blots were re-equilibrated in TBS and reblocked with 3% BSA in TBS-T.
Immunofluorescence Microscopy
18-mm glass coverslips were coated for 3 h at 37°C with 10 μg/ml of either fibronectin, rIII9,10, or r70 kD, diluted in PBS. Unbound protein was removed and coverslips were blocked with BSA for an additional 30 min. Cycloheximide-pretreated A1-F fibroblasts were seeded onto coverslips in 12-well cluster dishes (Corning /Costar) at 4 × 104 cells/well in DME/ 0.4% ReduSerII/20 μg/ml cycloheximide. After a 15-h incubation at 37°C, media was removed and cells were fixed with 2% paraformaldehyde in Small's cytoskeletal buffer (63), and permeabilized with ice-cold 0.5% Triton X-100. Cells were incubated for 60 min with primary antibody diluted 1:300 in Small's cytoskeletal buffer followed by incubation with fluorescein-conjugated goat anti–mouse antibody (Cappel Laboratories, Durham, NC) for an additional 60 min. Cells were stained for β1 integrins using the monoclonal anti-β1 antibody, AIIB2 (a gift of C. Damsky, University of California at San Francisco, San Francisco, CA), followed by incubation with a fluorescein-conjugated goat anti–rat antibody (Cappel Laboratories). Cells were washed, mounted, and then examined using an Olympus microscope.
Haptotaxis Assay
Cell migration in the presence of r70 kD, rIII9,10, or fibronectin was assayed using individual modified Boyden chambers. Polycarbonate filters (8 μm pore, 13 mm diam) (Nucleopore Corp., Pleasanton, CA) were precoated on one side with various concentrations of either r70 kD, rIII9,10, or fibronectin diluted in PBS. Control filters were precoated with BSA. Fibroblast-conditioned media containing 0.4% ReduSerII and 20 μg/ml cycloheximide was added to the lower chamber, and filters were placed between the upper and lower chambers so that the protein-coated side faced the lower compartment. Cycloheximide pretreated A-1F fibroblasts were seeded into the upper chamber at 105 cell/well in DME/0.4%/ReduSerII/20 μg/ml cycloheximide and cells were incubated for 12 h at 37°C. Filters were then separated from the chamber and cells on the upper surface were removed. Filters were fixed and stained with Diff-Quik (Baxter Scientific, McGaw Park, IL), mounted on glass slides, and the number of cells that had migrated to the lower surface were quantitated by light microscopy (magnification of 200). Five random microscopic fields were counted per well and experiments were performed in triplicate.
Results
A Recombinant 70-kD Amino-terminal Fibronectin Fragment Supports Cell Adhesion via an α5β1-dependent Mechanism
Cellular adhesion to fibronectin is mediated primarily by the α5β1 integrin, which binds to fibronectin through the RGD sequence contained within the III10 module (49, 50, 53). Adherent cells also express sites on their cell surfaces which bind the amino-terminal portion of fibronectin in a specific and saturable manner (39, 54). To determine whether the amino-terminal region of fibronectin could also support cell adhesion, fibroblasts were incubated in 24-well tissue culture plates precoated with either recombinant 70 kD (r70 kD), recombinant 40 kD (r40 kD), or BSA. The 27-kD amino-terminal fragment contains the first five type I repeats that are required for fibronectin binding to cell surfaces, whereas the 40-kD gelatin-binding fibronectin fragment lacks these five type I repeats and does not bind to cell surfaces (Fig. 1; 39, 54). Recombinant fibronectin fragments were used to eliminate the possibility that contaminating fragments, present in proteolytic preparations, mediated cell adhesion. To inhibit endogenous fibronectin synthesis, newly confluent fibroblast monolayers were pretreated for 3.5 h with 20 μg/ml cycloheximide (40), and washed extensively before trypsinization and reseeding onto fragment-coated wells. Cycloheximide was continuously present during the pretreatment and reseeding steps, and the adhesion assay. As shown in Fig. 2 A, r70 kD supported cell adhesion, whereas r40 kD did not. Similar results were obtained using 70- and 40-kD proteolytic fragments derived from human fibronectin (data not shown). The number of cells which adhered to r70 kD was typically 60–70% of the total number of cells adherent to the rIII9,10 modules of fibronectin (data not shown). Adhesion of cells to r70 kD was inhibited with RGD peptides, the anti-β1 antibody, mAb13 (1), and EDTA (Fig. 2 A). Partial inhibition of cell adhesion to r70 kD was observed with the anti-α5 antibody, mAb16 (1) (Fig. 2 A). In contrast, antibodies to integrin subunits α2, α3, β3, β5, or to the integrin αvβ3 (Fig. 2 B), as well as RGE peptides (Fig. 2 A), had no effect on adhesion of cells to r70 kD. In addition, adhesion to r70 kD was not inhibited by excess heparin (Fig. 2 A), suggesting that heparan sulfate proteoglycans do not mediate adhesion to r70 kD. Cells isolated from fibronectin-null mice and cultured in serum-free media were also able to adhere specifically to r70 kD-coated wells, demonstrating that adhesion to r70 kD can occur in the complete absence of fibronectin (J. Sottile, unpublished observations). Taken together, these results indicate that the amino terminus of fibronectin can support cell adhesion and further, suggest that this adhesion may be mediated by the α5β1 integrin.
Figure 2.
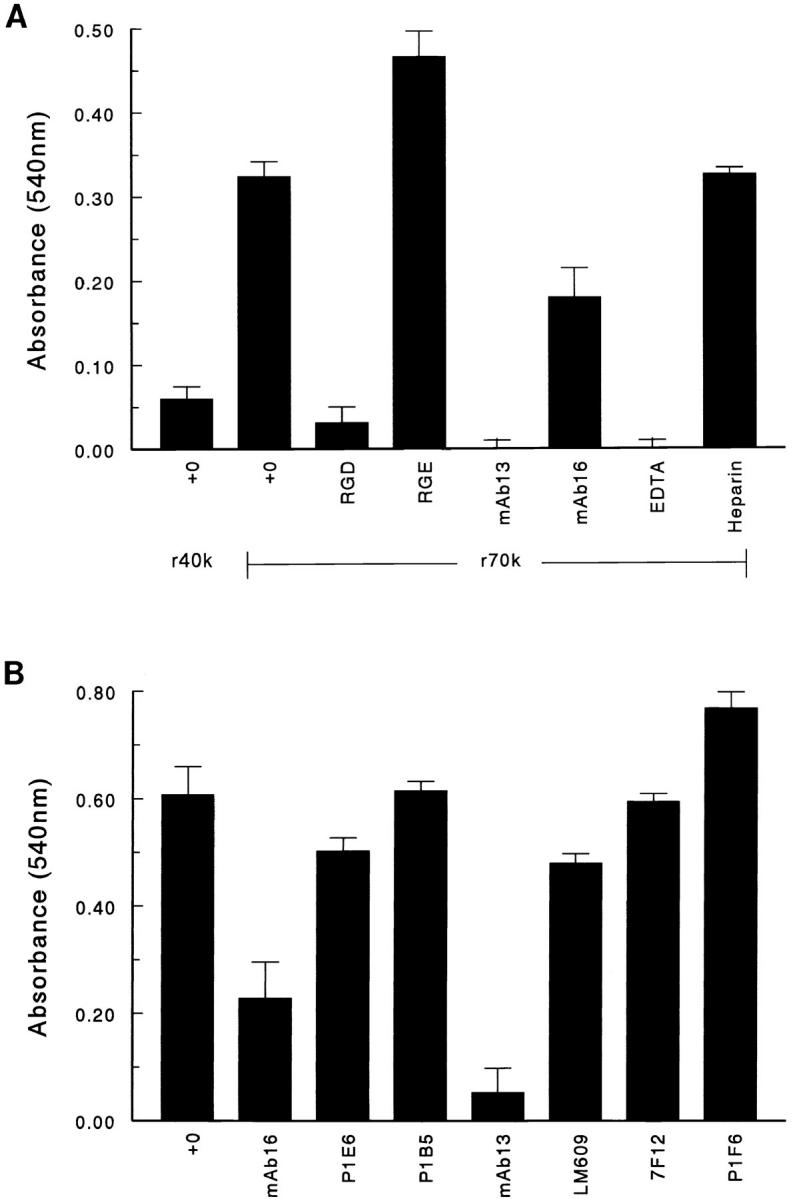
Adhesion of fibroblasts to r70 kD. Fibroblasts were pretreated with 20 μg/ml cycloheximide for 3.5 h as indicated under Materials and Methods. Cells were resuspended in DME/ 0.4% Redu-SerII containing 20 μg/ml cycloheximide, and plated at 105 cell/well in 24-well tissue culture plates precoated with 10 μg/ml r70-kD, r40 kD, or BSA. Cells were allowed to attach for 5 h in the absence (+0) or presence of (A) RGD or RGE peptides (0.5 mg/ml), the anti-β1 antibody, mAb 13 (20 μg/ml), the anti-α5 antibody, mAb 16 (20 μg/ml), EDTA (5 mM), or heparin (100 μg/ml) or (B) antibodies to the following integrin subunits (20 μg/ml): β1 (mAb13), αvβ3 (LM609), β3 (7F12), α2 (P1E6), α3 (P1B5), or α5 (mAb16). Cells were then washed, fixed with paraformaldehyde, and stained with 0.5% crystal violet. After staining, cells were washed, solubilized with 0.1% SDS, and the absorbance was determined at 540 nM. The absorbance obtained on BSA-coated wells was subtracted from all wells. Data are expressed as the mean absorbance at 540 nM ± the standard error and represent one of three experiments done in triplicate.
Binding of the α5β1 Integrin to the Amino Terminus of Fibronectin
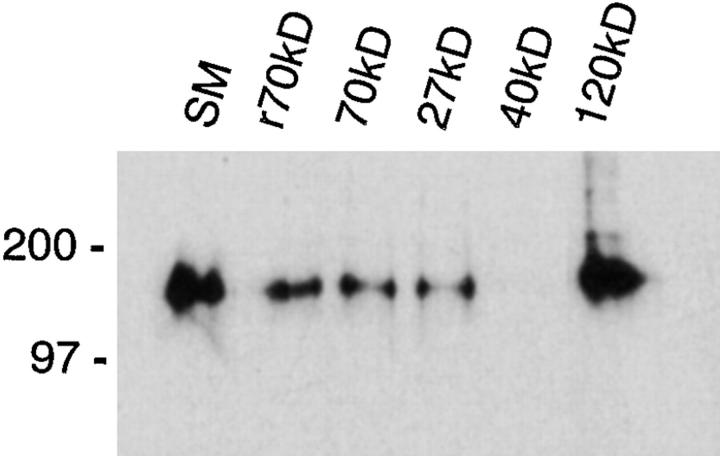
Our studies indicate that the adhesion of fibroblasts to r70 kD occurs through an α5β1 integrin–dependent mechanism. To determine whether the amino terminus of fibronectin is capable of directly interacting with the α5β1 integrin, affinity chromatography experiments were conducted. Purified α5β1 integrins were incubated with r70-, 70-, 27-, 40-, and 120-kD affinity columns. Resins were eluted with 20 mM EDTA and fractions were analyzed by SDS-PAGE and immunoblotting. As shown in Fig. 3, purified α5β1 integrins bound to the r70-, 70-, 27-, and 120-kD affinity columns. In contrast, α5β1 integrins were not detected in fractions eluted from the 40-kD affinity column (Fig. 3). To verify that the 40-kD fragment had been coupled to the affinity resin, the ability of gelatin to bind to the 40-kD affinity resin was determined (2). In experiments not shown, the 40-kD affinity column supported gelatin binding, whereas the 120-kD affinity column did not.
Figure 3.
70-kD affinity chromatography of purified α5β1 integrins. Purified α5β1 integrins were diluted in Tris buffer, pH 7.4, containing 50 mM n-OGPS (final concentration ∼10 μg/ml), and incubated for 15 h at 4°C with resins coupled to either recombinant 70-kD (r70kD), 70-kD (70kD), 27-kD (27kD), 40-kD (40kD), or 120-kD (120kD) fibronectin fragments (∼5 μg α5β1/ resin). Resins were eluted with 20 mM EDTA. Eluted fractions were precipitated with trichloroacetic acid and resuspended in nonreducing sample buffer before electrophoresis into 10% SDS-PAGE gels. Gels were transferred to nitrocellulose and immunoblotted with the anti-β1 antibody, P4C10. Approximately 1 μg of the starting material (SM) is shown. Molecular mass standards are myosin (200 kD) and phosphorylase B (97.4 kD).
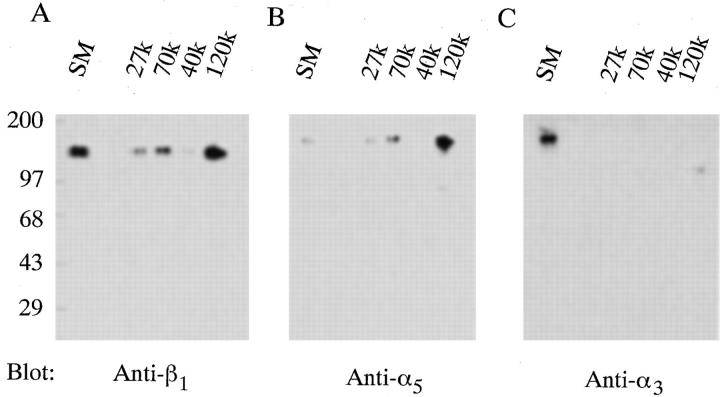
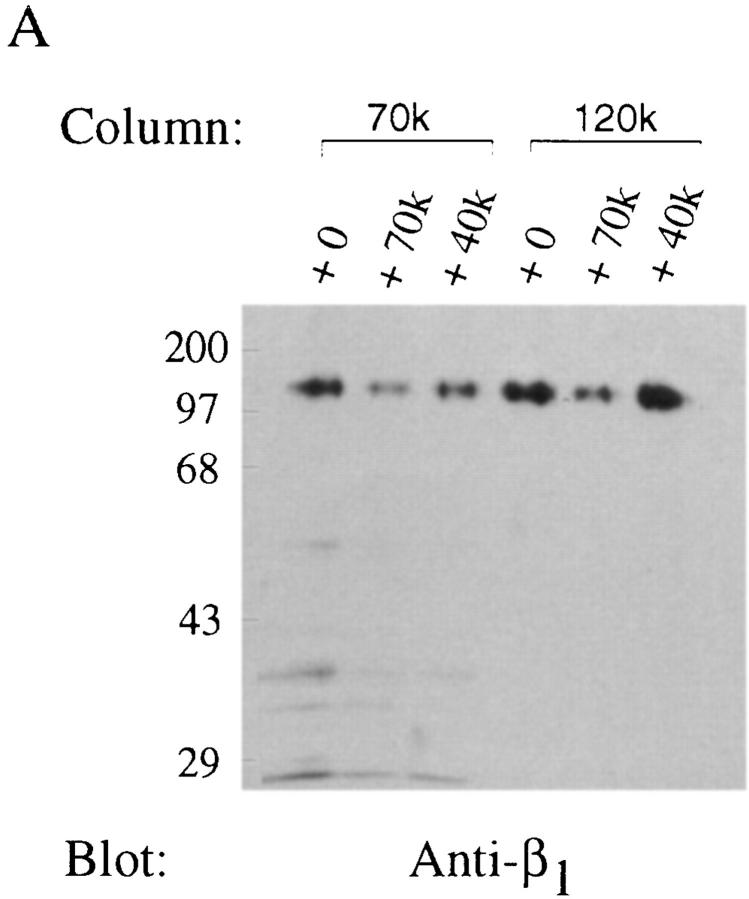
To determine whether other fibronectin-binding integrins could interact with the amino terminus of fibronectin, affinity chromatography experiments were performed using whole cell lysates. Cycloheximide-pretreated fibroblasts were lysed using n -OGPS. Clarified cell lysates were split into fractions of equal volume and applied to either a 27-, 40-, 70-, or 120-kD affinity column. Resins were eluted with 20 mM EDTA and fractions were analyzed by SDS-PAGE and immunoblotting. As shown in Fig. 4 A, β1 integrin subunits bound to the 27- and 70-kD affinity columns and were eluted with EDTA. In contrast, β1 integrin subunits were not detected in fractions eluted from the 40-kD affinity column (Fig. 4 A). As previously demonstrated (52), β1 integrin subunits were detected in fractions eluted from the 120-kD affinity column with EDTA (Fig. 4 A).
Figure 4.
70-kD affinity chromatography of cycloheximide-pretreated cell lysates. Fibroblasts were pretreated with cycloheximide as indicated under Materials and Methods. Cells were lysed using 60 mM n-OGPS, and clarified lysates were incubated for 15 h at 4°C with resins coupled to 27 (27k), 70 (70k), 40 (40k), or 120-kD (120k) fibronectin fragments. The starting material (SM) is shown. Resins were eluted with 20 mM EDTA. Eluted fractions were precipitated with trichloroacetic acid and resuspended in nonreducing sample buffer before electrophoresis into 10% SDS-PAGE gels. Gels were transferred to nitrocellulose and immunoblotted (Blot) with anti-β1 (A) antibodies. Blots were then stripped and reprobed using antibodies against α5 (B) or α3 (C) subunits. Molecular mass standards are myosin (200 kD), phosphorylase B (97.4 kD), BSA (68 kD), and ovalbumin (43 kD).
To determine the specificity of the interaction of the amino terminus of fibronectin with the β1 integrin subunit, immunoblots were stripped and sequentially reprobed with antibodies directed against either the α5 or the α3 (Fig. 4 C) integrin subunits. As shown in Fig. 4 B, α5 integrin subunits were detected in fractions eluted from the 27-, 70-, and 120-kD columns, but not from the 40-kD column. In contrast, α3 integrin subunits were not detected in fractions eluted from either the 27-, 40-, 70-, or 120-kD column, although the α3 integrin subunit was present in the unfractionated lysate (Fig. 4 C). Furthermore, no staining was detected when blots were stripped and reprobed using antibodies directed against either the β3 or β5 integrin subunits, even though these integrins were present in the original lysate (data not shown). Taken together, these studies demonstrate that the α5β1 integrin can bind specifically to the amino-terminal type I repeats of fibronectin.
Inhibition of α5β1 Binding to 70 kD by RGD Peptides
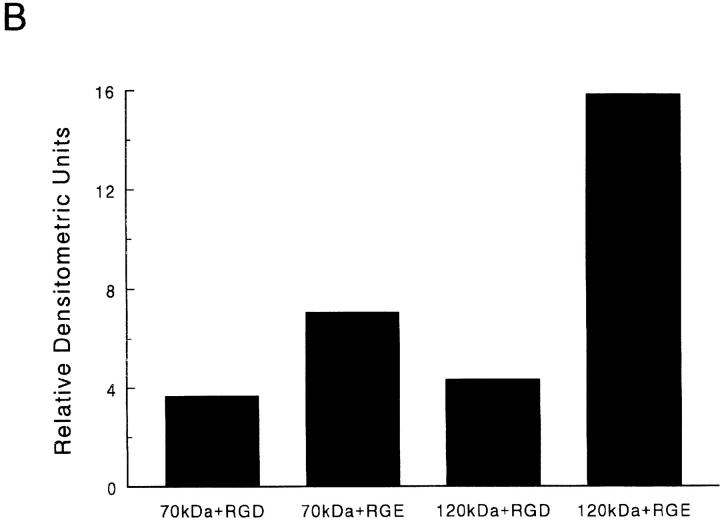
Data obtained from our cell adhesion experiments indicate that addition of excess RGD peptides inhibits cell adhesion to r70 kD (Fig. 2 A). To determine whether RGD peptides could inhibit α5β1 integrin binding to immobilized 70 kD, cell lysates were incubated with either the 70- or 120-kD affinity column in the presence of either excess RGD peptides or the inactive peptide, RGE (52). As shown in Fig. 5, addition of 1 mg/ml RGD peptides to cell lysates resulted in a ∼50% decrease in the binding of α5β1 integrins to 70-kD affinity columns, as compared to the level bound in the presence of RGE peptides (Fig. 5). As expected (52), addition of RGD peptides to cell lysates incubated with the 120-kD affinity column resulted in an ∼75% reduction in the binding of α5β1 integrins to 120 kD compared to that observed in the presence of the RGE peptides (Fig. 5). These data suggest that occupancy of the α5β1 integrin with the RGD sequence of fibronectin inhibits the ability of the α5β1 integrin to interact with fibronectin's amino terminus.
Figure 5.
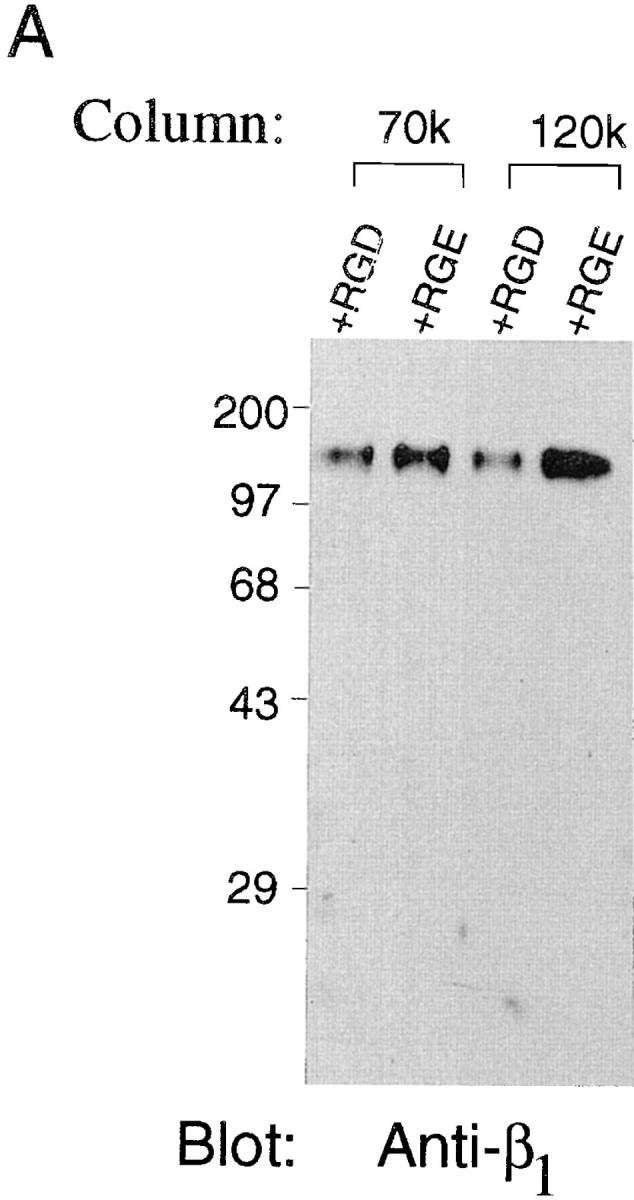
Effect of RGD peptides on α5β1 integrin–binding to 70-kD affinity columns. Fibroblast lysates were generated as indicated in the legend to Fig. 4. Lysates were then incubated with either 70 (70k) or 120-kD (120k) affinity columns in the presence of either GRGDSP (+RGD) or GRGESP (+RGE) at 1 mg/ml for 15 h at 4°C. Resins were eluted with 20 mM EDTA. Eluted fractions were precipitated with trichloroacetic acid and resuspended in nonreducing sample buffer before electrophoresis into 10% SDS-PAGE gels. Gels were transferred to nitrocellulose and immunoblotted (Blot) with an anti-β1 antibody. Molecular mass standards, shown by dashes, are the same as those indicated in the legend to Fig. 4. (B) Densitometric scan of the immunoblot shown in A.
Inhibition of α5β1 Binding to RGD by 70 kD
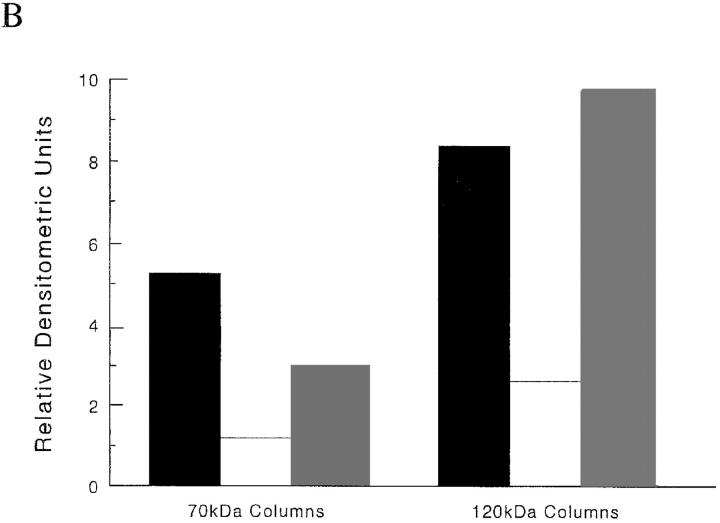
Our data indicate that the α5β1 integrin contains a binding site for the amino terminus of fibronectin, and that the interaction of the α5β1 integrin with r70 kD is inhibited by RGD peptides. To determine whether the amino terminus of fibronectin could competitively inhibit the binding of the α5β1 integrin to the RGD region of fibronectin, cell lysates were incubated with 120-kD affinity columns in the absence or presence of either 70- or 40-kD fibronectin fragments. As shown in Fig. 6, addition of 200 μg/ml of the 70-kD fragment to cell lysates incubated with the 120-kD affinity resin resulted in a ∼70% decrease in the binding of the α5β1 integrin to the 120-kD fragment; addition of an equal molar concentration of the gelatin-binding 40-kD fibronectin fragment had no effect on the binding of α5β1 integrins to 120 kD (Fig. 6). Addition of excess 70-kD fibronectin fragments to cell lysates incubated with 70-kD affinity columns resulted in an ∼80% inhibition of binding of α5β1 integrins to 70 kD (Fig. 6). These data indicate that binding of the amino terminus of fibronectin to the α5β1 integrin attenuates the ability of the integrin to interact with the RGD sequence of fibronectin and suggest that the α5β1 integrin cannot bind to the RGD and amino-terminal domains of fibronectin simultaneously.
Figure 6.
Effect of 70 kD on α5β1 integrin–binding to 120-kD affinity columns. Cell lysates were generated as indicated in the legend to Fig. 4, and incubated with either 70- (70k) or 120-kD (120k) affinity columns in the absence (+0) or presence of 30 μM 70- (+70k) or 40-kD (+40k) for 15 h at 4°C. Eluted fractions were precipitated with trichloroacetic acid and resuspended in nonreducing sample buffer before electrophoresis into 10% SDS-PAGE gels. Gels were transferred to nitrocellulose and immunoblotted (Blot) with an anti-β1 antibody. Molecular mass standards are the same as those indicated in the legend to Fig. 4. (B) Densitometric scan of the immunoblot shown in A; +0 (solid bars); +70 kD (open bars); +40 kD (shaded bars).
Upregulation of Cell Adhesion to r70 kD by Cytochalasin D
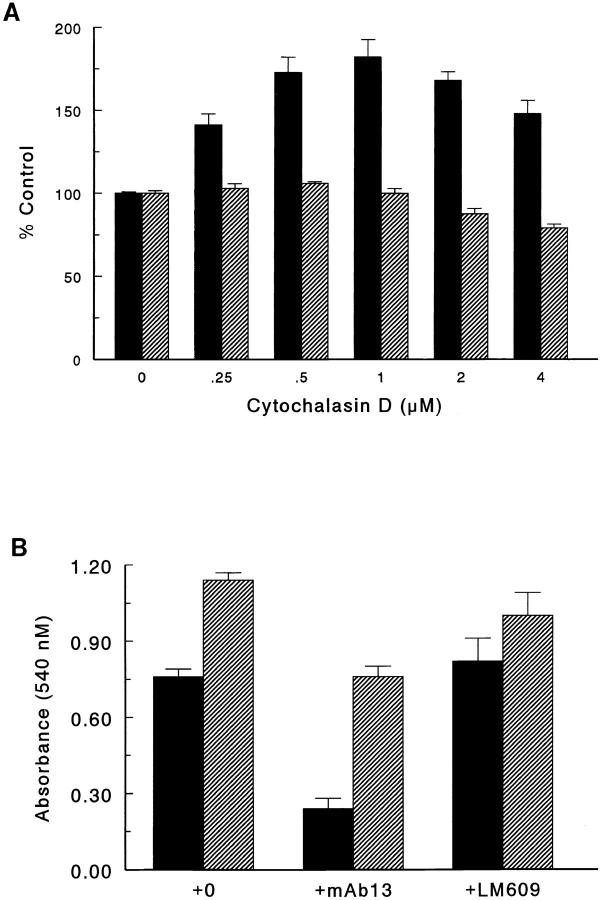
Earlier studies have shown that cytochalasins, which bind to the barbed end of actin and inhibit the growth of actin filaments (11), induce the functional activation of the αmβ2 integrin to promote binding of factor X to monocytes (16). To investigate whether actin redistribution plays a role in the ligand-binding specificity of the α5β1 integrin, the effect of cytochalasin D on cell adhesion to r70 kD was determined. Cycloheximide-treated fibroblasts were incubated with various concentrations of cytochalasin D for 1 h before seeding onto tissue culture wells precoated with r70 kD. After a 4-h incubation at 37°C, unattached cells were removed by washing and adherent cells were quantitated by staining with crystal violet. As shown in Fig. 7 A, pretreatment of fibroblasts with cytochalasin D resulted in a dose-dependent increase in the number of cells adherent to r70 kD. Moreover, cell adhesion to r70 kD was increased 60–80% over control levels by pretreatment of cells with 0.5–1 μM cytochalasin D (Fig. 7 A). To determine whether cytochalasin D promotes a similar increase in cell adhesion to the RGD domain of fibronectin, the effect of cytochalasin D pretreatment on cell adhesion to rIII9,10 was measured in a 30-min assay, at which time the number of adherent cells was ∼70% of the total number of cells in the assay (data not shown). In contrast to the results obtained with cells adherent to r70 kD, cytochalasin D pretreatment resulted in a small, but significant decrease in cell adhesion to rIII9,10 (Fig. 7 A). Pretreatment of cells with cytochalasin D did not promote cell attachment to BSA-coated wells (data not shown). Addition of the anti-β1 integrin antibody, mAb13, to cytochalasin D–pretreated cells inhibited adhesion to r70 kD, whereas addition of the anti-αvβ3 integrin antibody, LM609, had no effect on cell adhesion, indicating that the cytochalasin D–induced increase in cell adhesion to r70 kD was mediated by the β1 integrin (Fig. 7 B). Taken together, these data indicate that treatment of suspended cells with cytochalasin D upregulates β1-integrin–mediated cell adhesion to the amino terminus of fibronectin.
Figure 7.
Upregulation of cell adhesion to r70 kD by cytochalasin D. (A) Cycloheximide-pretreated fibroblasts were incubated with increasing concentrations of cytochalasin D in DME/0.4% Redu-SerII/cycloheximide for 1 h before seeding onto 24-well tissue culture plates precoated with 10 μg/ml of either r70 kD (solid bars) or rIII9,10 (hatched bars). After either a 4-h (r70 kD) or 30-min (rIII9,10) incubation at 37°C, adherent cells were quantitated as indicated in the legend to Fig. 2. Cytochalasin D was present throughout the adhesion assay. Data is presented as percent of cell adhesion measured in the absence of cytochalasin D. (B) Cycloheximide-pretreated fibroblasts were incubated for 1 h with 0.5 μM cytochalasin D before seeding into tissue culture wells precoated with r70 kD (solid bars) in the absence (+0) or presence of either the anti-β1 antibody, mAb 13, or the anti-αvβ3 antibody, LM609. Control cells were pretreated with DMSO alone for 1 h before seeding onto wells precoated with rIII9,10 (hatched bars). After a 4-h incubation at 37°C, adherent cells were quantitated as indicated in the legend to Fig. 2. Data is presented as mean absorbance measured at 540 nM ± standard error and represents one of three experiments performed in triplicate.
To investigate further the effect of cytochalasin D on cell adhesion to either the amino-terminal or RGD region of fibronectin, cell adhesion to varying substrate coating concentrations was assayed. As shown in Fig. 8 A, pretreatment of cells with 1 μM cytochalasin D resulted in a marked enhancement of cell adhesion over a range of r70-kD coating concentrations as compared to control cells, indicating that cytochalasin D pretreatment increases the efficiency of α5β1 integrin-mediated adhesion to the amino terminus of fibronectin. In contrast, pretreatment of cells with 1 μM cytochalasin D resulted in a decrease in the efficiency of cell adhesion to rIII9,10 (Fig. 8 B). These data indicate that inhibition of actin filament assembly in suspended cells differentially affects α5β1 integrin-mediated cell adhesion to the amino terminus and the RGD region of fibronectin and further, suggest that redistribution of actin filaments may modulate the ligand-binding specificity of the α5β1 integrin by increasing the affinity of the α5β1 integrin for the amino terminus of fibronectin.
Figure 8.
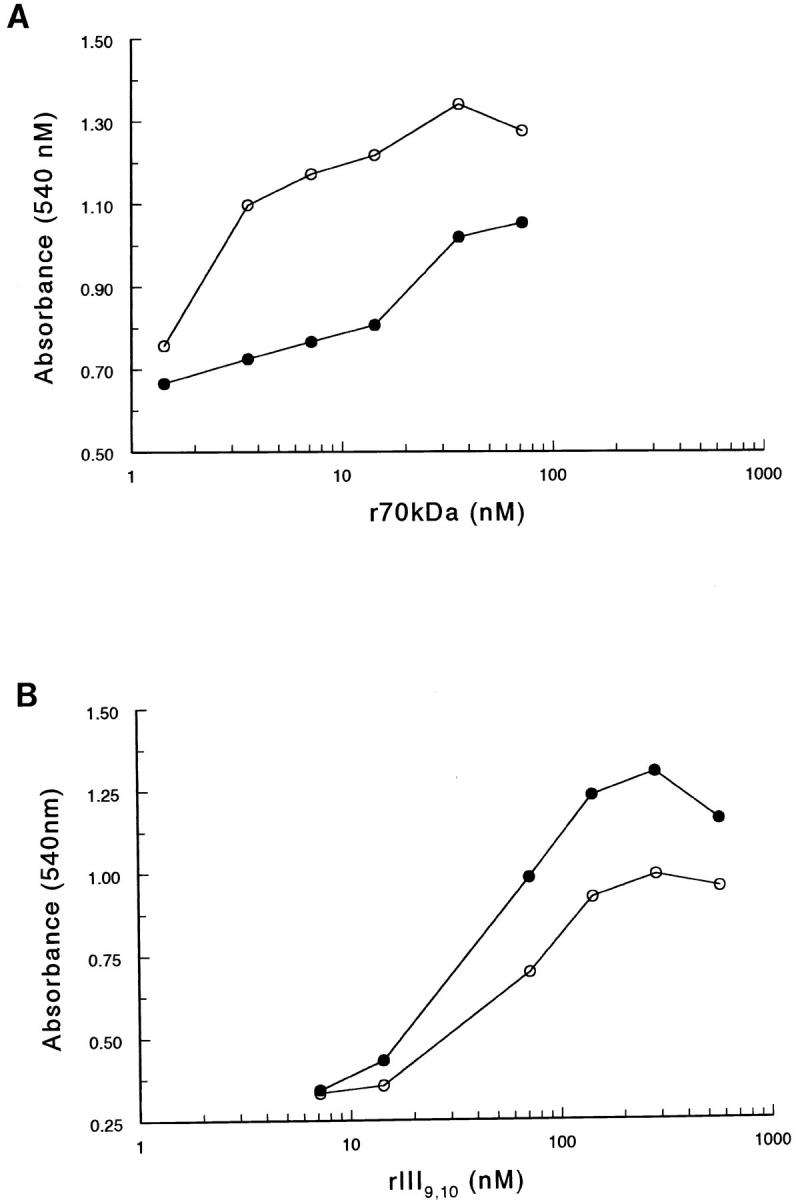
Efficiency of cell adhesion to r70-kD- or rIII9,10-coated substrates after cytochalasin D treatment. Cycloheximide-pretreated fibroblasts were resuspended in DME/0.4% Redu-SerII/ cycloheximide and treated with either 1 μM of cytochalasin D (○) or DMSO (•) for 1 h before seeding onto 24-well tissue culture plates precoated with increasing concentrations of r70 kD (A) or rIII9,10 (B). Adherent cells were quantitated as indicated in the legend to Fig. 2. Data is presented as absorbance measured at 540 nM and represents one of two experiments performed in quadruplicate.
Cellular Adhesion to r70 kD Induces Clustering of β1 Integrins, but Not FAK, at Sites of Focal Adhesions
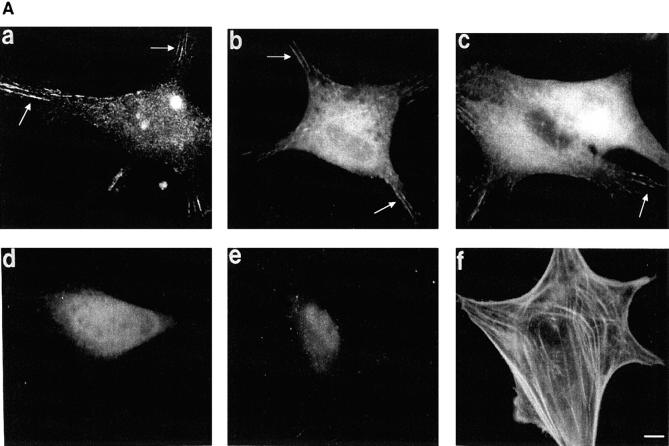
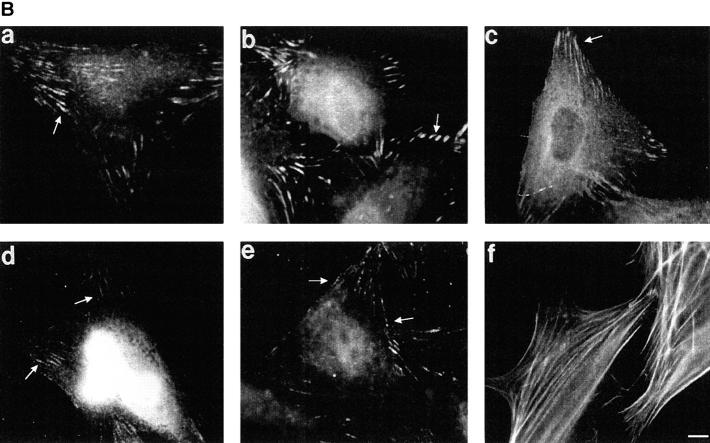
The cytoplasmic region of integrin receptors interacts with cytoskeletal proteins and provides a link between the extracellular matrix and the cytoskeleton at sites of focal adhesions (30). To determine whether adhesion of cells to r70 kD could promote the formation of focal adhesion structures, cycloheximide-pretreated fibroblasts were seeded onto glass coverslips precoated with either r70 kD or rIII9,10, and allowed to adhere and spread for 15 h. After this incubation, cells were fixed, permeabilized, and stained for the presence of various proteins previously shown to be associated with focal contact structures. Cells adherent to r70 kD developed long, thin, focal adhesions, as observed by interference reflection microscopy (data not shown). These focal adhesions showed positive staining for β1 integrins, paxillin, α-actinin (Fig. 9 A, a–c), vinculin, and talin (data not shown). FAK and phosphotyrosine staining were not detected (Fig. 9 A, d and e). In addition, actin was not organized into stress fibers commonly observed in fibronectin-adherent cells (Fig. 9 B, f), but instead was seen primarily in a circular pattern surrounding the nucleus with fibers terminating in cell extensions (Fig. 9 A, f). Cells seeded onto rIII9,10 developed focal adhesion structures which appeared shorter and denser by interference reflection microscopy than those observed on r70-kD cells (data not shown). Focal adhesions in rIII9,10-adherent cells stained positively for β1 integrins, paxillin, α-actinin, (Fig. 9 B, a–c), vinculin, and talin (not shown). In addition, cells seeded onto rIII9,10 showed positive staining for both FAK and phosphotyrosine (Fig. 9 B, d and e), and actin was organized as elongated stress fibers (Fig. 9 B, f). Focal adhesions in fibronectin-adherent cells also stained positively for β1 integrins, vinculin, talin, α-actinin, paxillin, FAK, and phosphotyrosine (data not shown). These data indicate that adhesion of cells to r70 kD recruits a number of cytoskeletal proteins to focal adhesions, but does not recruit FAK to these structures and does not promote actin stress fiber formation. In addition, these focal adhesions do not stain for phosphotyrosine. Thus, these studies suggest that ligation of the α5β1 integrin by the amino terminus of fibronectin stimulates the formation of focal adhesion structures which are distinct from those formed upon ligation of the α5β1 integrin by the RGD sequence of fibronectin.
Figure 9.
Characterization of r70-kD–induced focal adhesions. Cycloheximide-pretreated fibroblasts were resuspended in DME/0.4% ReduSerII containing 20 μg/ ml cycloheximide and seeded at 105 cell/ml onto 18-mm coverslips precoated with either r70 kD (A) or rIII9,10 (B). After a 15-h incubation, cells were fixed, permeabilized, and stained for β1 integrins (a), paxillin (b), α-actinin (c), FAK (d), or phosphotyrosine (e), using a 1:300 dilution of primary antibody followed by a 1:300 dilution of FITC-labeled goat anti–mouse antibody. Cells were stained for actin (f) using fluorescein-conjugated phalloidin. Cells were washed, mounted, and examined on a microscope equipped for epifluorescence. Bar, 10 μm.
Protein Tyrosine Phosphorylation Patterns from Fibroblasts Adherent to r70 kD
Previous studies have demonstrated that cellular adhesion to fibronectin stimulates the tyrosine phosphorylation of a number of focal adhesion-associated proteins, including FAK and paxillin (6). In the present study, cells adherent to r70 kD did not stain for phosphotyrosine, suggesting that a nontyrosine phosphorylated form of paxillin is recruited to focal adhesion structures following adhesion to r70 kD. To further investigate the pattern of tyrosine phosphorylation of cellular proteins in response to adhesion to r70 kD and to compare this pattern to that obtained from cells adherent to either intact fibronectin or rIII9,10, lysates were prepared from cells seeded onto r70 kD, rIII9,10, or fibronectin and analyzed by SDS-PAGE and immunoblotting. Fig. 10 shows an immunoblot of cell lysates probed with an anti-phosphotyrosine antibody. In cells seeded onto either rIII9,10 or intact fibronectin, elevated levels of phosphotyrosine staining could be detected in proteins migrating at ∼125 and 70 kD (Fig. 10, arrowheads). These protein bands most likely correspond to the proteins FAK and paxillin, respectively (6). In contrast, the levels of phosphotyrosine staining of the 125 and 70-kD bands were significantly reduced in lysates obtained from cells adherent to r70 kD (Fig. 10, arrowheads). In addition, adhesion of cells to r70 kD stimulated the tyrosine phosphorylation of a protein migrating at ∼110 kD that was not tyrosine phosphorylated in lysates isolated from cells adherent to either fibronectin or rIII9,10 (Fig. 10, arrow). These data suggest that the interaction of cells with the amino terminus of fibronectin stimulates intracellular tyrosine kinase signaling pathways which are distinct from those stimulated in response to ligation of the α5β1 integrin by the RGD sequence of fibronectin.
Figure 10.
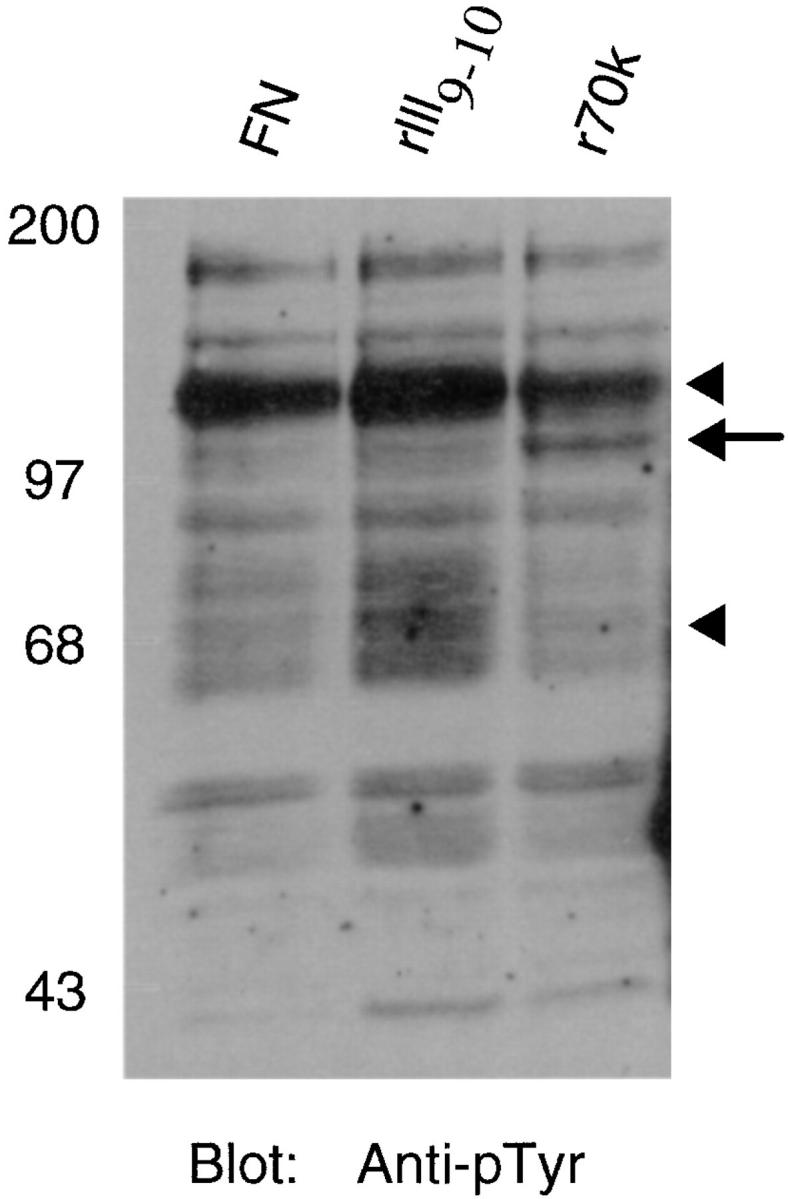
Tyrosine phosphorylation of proteins isolated from cells adherent to r70 kD, rIII9,10, or fibronectin. Cycloheximide-pretreated fibroblasts were resuspended in DME/0.4% ReduSerII containing 20 μg/ml cycloheximide and seeded at 106 cell/ml onto 100-mm tissue culture dishes precoated with fibronectin (FN), rIII9,10, (rIII9 ,10), or r70 kD (r70k). After a 15-h incubation at 37°C, cells were lysed with RIPA buffer, and equal aliquots of clarified lysates were electrophoresed into 8% SDS-PAGE gels. Gels were transferred to nitrocellulose and immunoblotted (Blot) using an anti-phosphotyrosine (Anti-pTyr) antibody. The positions of p125 and p70 are indicated by the arrowheads. The position of p110 is indicated by the arrow to the right of the blot. Molecular mass standards are the same as those indicated in the legend to Fig. 4.
Cellular Adhesion to r70 kD Results in Reduced Levels of Tyrosine Phosphorylation of FAK and Paxillin
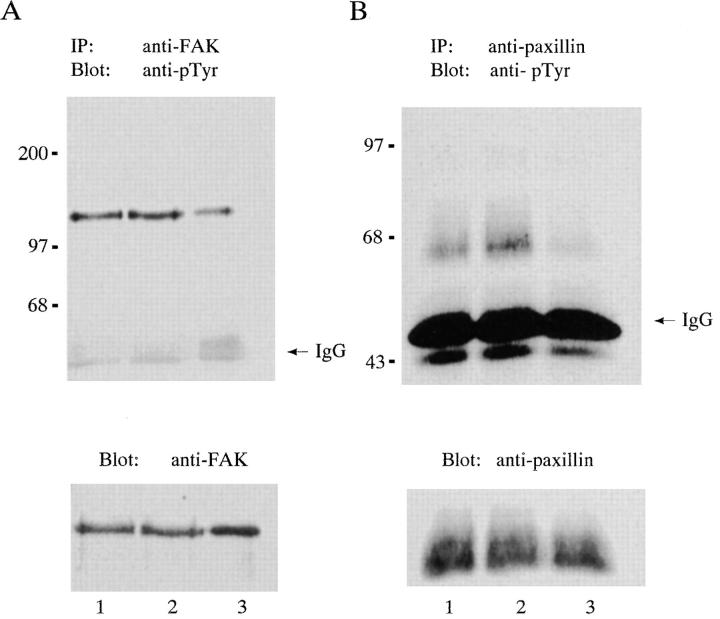
To quantitate the levels of tyrosine phosphorylation of FAK and paxillin generated in response to adhesion to r70 kD, lysates were extracted from cells seeded onto either fibronectin, rIII9,10, or r70 kD and immunoprecipitated with antibodies directed against either FAK (Fig. 11 A) or paxillin (Fig. 11 B). Immunoprecipitates were analyzed by SDS-PAGE and immunoblotting using an anti-phosphotyrosine antibody. To ensure equal loading conditions, immunoblots were stripped and reprobed using antibodies against either FAK (Fig. 11 A) or paxillin (Fig. 11 B). Levels of tyrosine phosphorylation were quantitated by scanning densitometry. The level of tyrosine phosphorylation of FAK in cells adherent to r70 kD was ∼70% less than levels of FAK tyrosine phosphorylation observed in cells adherent to either rIII9,10 or fibronectin (Fig. 11 A). Furthermore, in contrast to rIII9,10- or fibronectin-adherent cells, paxillin was not tyrosine phosphorylated in cells adherent to r70 kD (Fig. 11 B). These data indicate that α5β1 integrin–mediated adhesion to the amino terminus of fibronectin results in significantly reduced levels of FAK tyrosine phosphorylation and does not stimulate tyrosine phosphorylation of paxillin to detectable levels. Taken together, these data suggest that the level of tyrosine phosphorylation of FAK and paxillin may be modulated by the ligand specificity of the α5β1 integrin.
Figure 11.
Phosphotyrosine content of FAK and paxillin isolated from cells adherent to r70 kD, rIII9,10, and fibronectin. Cycloheximide-pretreated fibroblasts were seeded onto 100-mm tissue culture dishes precoated with either fibronectin (lane 1), rIII9,10, (lane 2), or r70 kD (lane 3). After a 15-h incubation at 37°C, cells were lysed with RIPA buffer, and equal aliquots of clarified lysates were immunoprecipitated (IP) with anti-FAK (A) or anti-paxillin (B) antibodies. Immunoprecipitates were separated by SDS-PAGE and analyzed by immunoblotting (Blot) using the anti-phosphotyrosine antibody, PY20 (pTyr). To ensure equal loading, blots were stripped and reprobed with the anti-FAK (A) or anti-paxillin (B) antibodies.
Adhesion to r70 kD Stimulates Migration of Fibroblasts
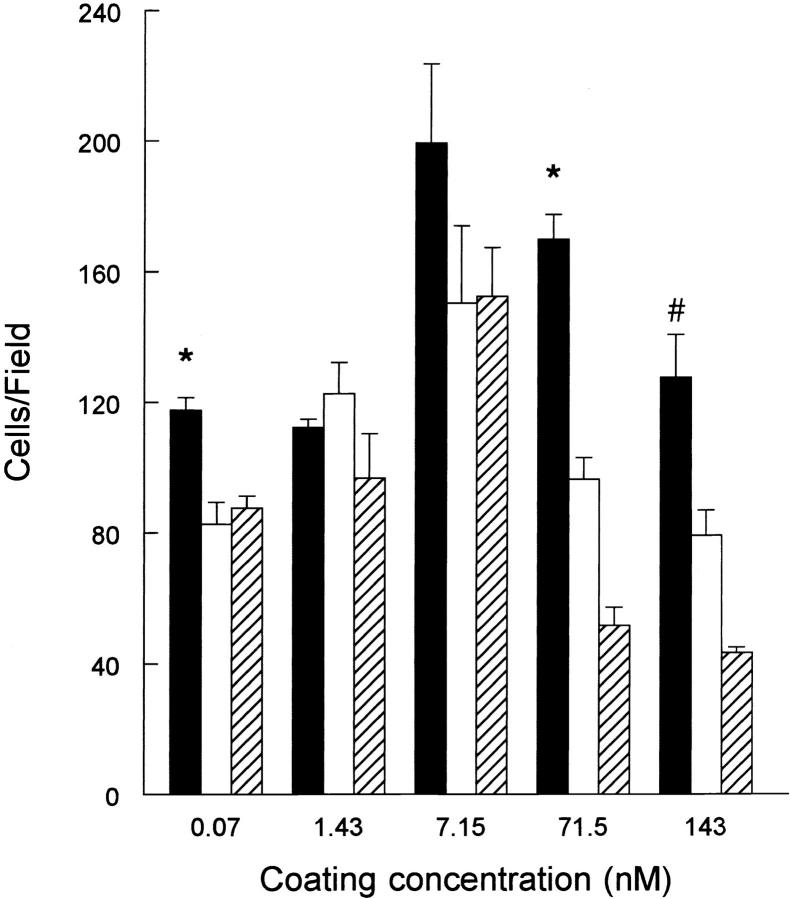
The α5β1 integrin has previously been shown to mediate cell migration on fibronectin-coated substrates (32). Additional studies have demonstrated an inverse relationship between the number and extent of focal adhesions and cell motility on fibronectin (14). Our data indicate that α5β1 integrin–mediated adhesion of cells to r70 kD results in the formation of focal adhesion structures that are distinct in terms of protein composition and phosphotyrosine content from those formed in response to adhesion to rIII9,10. Therefore, to examine cell motility on a r70-kD–coated substrate, cycloheximide-pretreated fibroblasts were seeded into modified Boyden chambers and allowed to migrate through filters which had been precoated on the underside with various concentrations of either r70 kD, rIII9,10, or intact fibronectin. Fibroblast-conditioned media was added to the lower chamber to act as a chemoattractant. As shown in Fig. 12, cells seeded onto filters coated with increasing concentrations of either r70 kD, rIII9,10, or fibronectin displayed a biphasic response with the peak rate of cell migration occurring at a coating concentration of 7.1 nM. This biphasic response is similar to results obtained in previous studies demonstrating that maximal cell migration occurs at intermediate substrate-coating concentrations (13). At both lower (0.07 nM) and higher (71.5 and 143 nM) coating concentrations, the number of cells that migrated through r70-kD–coated filters was significantly greater than the number of cells which migrated through either rIII9,10- or fibronectin-coated filters (Fig. 12). The largest difference in cell migration was observed at a coating concentration of 71 nM (r70-kD–coating concentration of 5 μg/ml); at this concentration, 75% more cells migrated through r70-kD–coated filters than through rIII9,10-coated filters (Fig. 12). These data indicate that the amino terminus of fibronectin is capable of supporting directed cell migration, and that the extent of migration of stimulated fibroblasts on a r70-kD–coated substrate at both low and high coating concentrations is greater than that observed with cells adherent to rIII9,10. Taken together, these data suggest that the amino-terminal and cell-adhesion domains of fibronectin can differentially affect cell motility.
Figure 12.
Haptotaxis of fibroblasts on r70-kD-, rIII9,10- or fibronectin-coated substrates. Cycloheximide-pretreated fibroblasts were resuspended in DME/0.4% ReduSerII containing 20 μg/ml cycloheximide and seeded into individual Boyden chambers at 105 cell/ml onto 8 μm polycarbonate filters precoated on the underside with either r70 kD (solid bars), rIII9,10 (open bars), or fibronectin (hatched bars). After a 12-h incubation at 37°C, filters were removed and the number of cells that had migrated onto the substrate-coated side of the filter were quantitated as indicated under Materials and Methods. Migration results are expressed as cells/high power field (HPF). The average number of cells that migrated on BSA-coated filters was ⩽2/HPF. Data is presented as mean HPF ± standard error and represents one of two experiments performed in triplicate. *Significantly different from rIII9,10 group (P < 0.05). #Significantly different from rIII9,10 group (P < 0.01).
Discussion
At various times during development and wound repair, fibronectin matrix assembly is upregulated to support the functions of cell adhesion, migration, and differentiation. In one of the initial steps of fibronectin matrix assembly, adherent cells bind the amino-terminal region of fibronectin (39, 54). Whereas several earlier studies have characterized the response of cells to RGD-containing fragments of fibronectin (61), in the present study, we have developed a model that allows us to examine the cellular response initiated by the binding of the amino terminus of fibronectin to cell surfaces. The results presented in this study provide evidence that the amino terminus of fibronectin can trigger α5β1 integrin–mediated intracellular signals that are distinct from those generated in response to ligation of the α5β1 integrin with fibronectin's RGD sequence. These findings are the first to demonstrate that the amino-terminal matrix assembly domain of fibronectin directly modulates integrin-mediated tyrosine kinase activity and focal contact assembly.
Previous studies have demonstrated that inhibition of tyrosine kinase activity prevents the tyrosine phosphorylation of FAK and the formation of focal adhesions, suggesting that FAK tyrosine kinase activity is necessary for focal contact formation (6, 56). Other studies, however, suggest that FAK activation plays a role in the modulation, rather than the formation of focal adhesion structures. Overexpression of the carboxyl-terminal noncatalytic domain of FAK, pp41/43FRNK (60), was shown to inhibit tyrosine phosphorylation of endogenous FAK and delay, but not prevent focal adhesion formation (55). Similarly, microinjection of cells with a construct containing the FAK focal adhesion targeting sequence, but not the kinase domain, decreased both the association of endogenous FAK with focal adhesions and focal adhesion tyrosine phosphorylation content, but did not reduce focal adhesion formation (20). Moreover, fibroblasts isolated from FAK-deficient mouse embryos formed phosphotyrosine-containing focal adhesions (33). Our data provide evidence that clustering of occupied β1 integrins and formation of focal adhesions can occur in the absence of FAK clustering and in the presence of reduced tyrosine phosphorylation. Furthermore, whereas clustering of β1 integrins was induced by adhesion to either the amino-terminal or RGD domain of fibronectin, only RGD-mediated ligation induced FAK clustering, suggesting that specific ligand occupancy of the α5β1 integrin, and not β1 clustering, recruits FAK to focal adhesions and modulates tyrosine kinase activity.
After cell attachment to fibronectin, both FAK and paxillin accumulate at sites of focal adhesions and are rapidly phosphorylated on tyrosine residues (6). Overexpression of FAK in chicken embryo cells results in the induction of tyrosine phosphorylation of paxillin (58). In addition, in vitro and in vivo evidence suggests that paxillin may be a substrate for tyrosine phosphorylation by FAK (4, 23, 70). The association of paxillin with FAK has led to the suggestion that FAK activity may be required for targeting of paxillin to focal adhesions (57). More recent evidence, however, indicates that tyrosine phosphorylation of paxillin by FAK is not required for the localization of paxillin to established focal contacts (4). Our data extend these observations by providing evidence that paxillin tyrosine phosphorylation is not required for localization into newly formed r70-kD–induced focal adhesions. In addition, paxillin accumulates in 70-kD–induced focal adhesions in the absence of focal adhesion–associated FAK.
Adhesion of cells to r70 kD stimulated the tyrosine phosphorylation of a protein with a molecular mass of ∼110 kD (p110). Tyrosine phosphorylation of p110 was unique to r70-kD–adherent cells as this protein was not tyrosine phosphorylated in lysates extracted from cells which were adherent to rIII9,10. To date, we have been unable to determine the identity of this protein. Nevertheless, the tyrosine phosphorylation of p110 after cell adhesion to r70 kD provides further evidence that the interaction of cells with the amino terminus of fibronectin stimulates a unique signaling pathway not associated with RGD-mediated cell adhesion.
The ability of the α5β1 integrin to transduce distinct signals upon interacting with either the amino-terminal or RGD region of fibronectin suggests that information specified by different regions of fibronectin may serve to modulate intracellular signals stimulated during processes involving adhesion to fibronectin. The ability of r70 kD to promote an increased haptotactic response versus rIII9,10 suggests the possibility that altering the ligand specificity of the α5β1 integrin may be a mechanism by which cells regulate the rate and/or extent of migration. Cellular migration is a dynamic process, which requires adhesive events that coordinate interactions between the integrin receptor and the actin cytoskeleton to regulate the formation and release of focal adhesions (28). Previous studies have correlated a decrease in actin stress fiber formation (14) and a disruption of focal adhesions (37) with growth factor–stimulated increases in cell motility. In addition, CHO cells expressing αIIbβ3 integrin mutants that increase the organization of actin filaments, displayed a decrease in the rate of cellular migration on a fibrinogen-coated substrate (29). In the present study, adhesion of cells to r70 kD was associated with a decrease in focal contact density, as well as a decrease in actin stress fiber formation as compared to rIII9,10-adherent cells. The ability of the α5β1 integrin to transduce separate signals that give rise to both distinct patterns of actin organization and differences in the extent of cell motility, suggests that the preferential ligation of the α5β1 integrin with either the amino-terminal or RGD region of fibronectin may serve to modulate cell migration on a fibronectin matrix.
Pretreatment of fibroblasts with cytochalasin D differentially affected α5β1 integrin-mediated cell adhesion to the amino-terminal or RGD domains of fibronectin, suggesting that the ligand specificity of the α5β1 integrin is regulated in part by the actin cytoskeleton. The cytochalasin D–induced increase in efficiency of cell adhesion to amino-terminal fibronectin fragments suggests that actin filament redistribution promotes an increase in the affinity of the α5β1 integrin for the amino terminus of fibronectin. Previous studies have demonstrated that the integrin cytoplasmic domain plays a role in modulating the conformation and ligand-binding properties of the extracellular domain of several integrin subclasses, including α5β1 (12, 22, 26, 51). Therefore, as hypothesized by Elemer et al. (16), it is possible that transient alterations in actin filament assembly may function to modulate a cytoskeletal constraint on the cytoplasmic region of the integrin to allow for changes in ligand recognition. Our data are consistent with a model, in which actin filament redistribution releases a conformational constraint on the cytoplasmic domain of the α5β1 integrin, which serves to promote the transition of the α5β1 integrin to a conformation recognized by the amino terminus of fibronectin.
The present study, together with several recent studies (24, 64, 73), suggest the existence of three distinct sites on cell surfaces which interact with fibronectin's amino terminus. We have previously identified a conformation-dependent binding site within the III1 module of fibronectin which binds to the amino terminus of fibronectin (24). More recently, cross-linking studies have identified large molecular mass molecules that interact with the 70-kD fibronectin fragment on cell surfaces (73). Whereas our data clearly demonstrate that the matrix assembly domain of fibronectin binds to the α5β1 integrin and modulates α5β1- dependent signaling pathways, the possible contribution of the large molecular mass molecule to the observed phenotype of cells adherent to the 70-kD fibronectin fragment cannot be ruled out. Similarly, although our experiments were conducted in the presence of cycloheximide, we cannot exclude the possibility that small amounts of fibronectin may contribute to or modify the r70-kD–induced phenotype in cycloheximide-pretreated fibroblasts. Nevertheless, the 70-kD–induced phosphorylation of p110, as well as the novel actin structures observed in cells adherent to 70 kD, provide compelling evidence that the amino-terminal matrix assembly domain of fibronectin modulates α5β1- dependent signaling pathways involved in the regulation of focal adhesion formation and intracellular tyrosine phosphorylation events.
Our studies suggest a model of α5β1 integrin occupancy, in which binding of either the amino-terminal or RGD domain of fibronectin to the α5β1 integrin negatively regulates occupancy by the other fibronectin ligand. Recently, Mould et al. (45) demonstrated that occupancy of the α5β1 integrin with RGD-containing peptides allosterically inhibits the binding of the anti-β1 antibody, mAb13, to the α5β1 integrin, suggesting that occupancy of the α5β1 integrin with RGD induces a conformational change within the integrin, which attenuates the expression of the epitope-recognized mAb 13. In the present study, RGD peptides were shown to inhibit cell adhesion to the amino terminus of fibronectin, whereas amino-terminal fibronectin fragments and RGD peptides were able to cross-compete for binding to the α5β1 integrin, suggesting that these two domains of fibronectin cannot bind to the α5β1 integrin simultaneously. The reciprocal inhibition of α5β1 integrin binding, coupled with the ability of these two regions of fibronectin to generate distinct intracellular signaling events, suggests that these two conformations of the α5β1 integrin may reflect an actin-dependent intramolecular switch that serves to control integrin function. Previous studies have shown that divalent cations, as well as monoclonal antibodies, can affect ligand binding to integrins, suggesting that conformational changes within the integrin can affect ligand specificity (27, 61). Our studies suggest the possibility that the amino terminus of fibronectin and the “classic” cell-binding domain of fibronectin may represent examples of physiologically relevant ligands that can modulate integrin conformation and, furthermore, provide direct evidence that these conformational changes lead to the activation of distinct intracellular signaling pathways. These studies also provide the groundwork for future studies which may elucidate the specific pathways responsible for control of distinct cellular phenotypes previously attributed to β1 integrin function, including cell migration, matrix assembly, and secretion of metalloproteases (27).
Acknowledgments
We thank Susan LaFlamme (Albany Medical College, Albany, NY) for helpful advice and critically reviewing this manuscript.
This work was supported by grants CA69612 (P.J. McKeown-Longo) and HL50549 (J. Sottile) from the National Institutes of Health and grants 950210 (D.C. Hocking) and 950318 (J. Sottile) from the American Heart Association, New York State Affiliate.
Abbreviations used in this paper
- FAK
focal adhesion kinase
- OGPS
octyl-β-d-thioglucopranoside
Footnotes
Address all correspondence to Paula J. McKeown-Longo, Department of Physiology and Cell Biology, Neil Hellman Medical Research Building, Albany Medical College, 47 New Scotland Avenue, Albany, NY 12208. Tel.: (518) 262-5666. Fax: (518) 262-5669. E-mail: paula_mckeown-longo@ccgateway.amc.edu
References
- 1.Akiyama SK, Yamada SS, Chen WT, Yamada KM. Analysis of fibronectin receptor function with monoclonal antibodies: roles in cell adhesion, migration, matrix assembly, and cytoskeletal organization. J Cell Biol. 1989;109:863–875. doi: 10.1083/jcb.109.2.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balian G, Click EM, Crouch E, Davidson JM, Bornstein P. Isolation of a collagen-binding fragment from fibronectin and cold-insoluble globulin. J Biol Chem. 1979;254:1429–1432. [PubMed] [Google Scholar]
- 3.Balian G, Click EM, Bornstein P. Localization of a collagen-binding domain in fibronectin. J Biol Chem. 1980;255:3234–3236. [PubMed] [Google Scholar]
- 4.Bellis SL, Miller JT, Turner CE. Characterization of tyrosine phosphorylation of paxillin in vitroby focal adhesion kinase. J Biol Chem. 1995;270:17437–17441. doi: 10.1074/jbc.270.29.17437. [DOI] [PubMed] [Google Scholar]
- 5.Bockholt SM, Burridge K. Cell spreading on extracellular matrix proteins induces tyrosine phosphorylation of tensin. J Biol Chem. 1993;268:14565–14567. [PubMed] [Google Scholar]
- 6.Burridge K, Turner CE, Romer LH. Tyrosine phosphorylation of paxillin and pp125FAKaccompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J Cell Biol. 1992;119:893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carsons, S., B.B. Lavietes, and H.S. Diamond. 1989. Role of fibronectin in rheumatic diseases. In Fibronectin. D.F. Mosher, editor. Academic Press, San Diego, CA. 327–361.
- 8.Chernousov MA, Fogerty FJ, Koteliansky VE, Mosher DF. Role of the I-9 and III-1 modules of fibronectin in formation of an extracellular matrix. J Biol Chem. 1991;266:10851–10858. [PubMed] [Google Scholar]
- 9.Christopher RA, Kowalczyk AP, McKeown-Longo PJ. Localization of fibronectin matrix assembly sites on fibroblasts and endothelial cells. J Cell Sci. 1997;110:569–581. doi: 10.1242/jcs.110.5.569. [DOI] [PubMed] [Google Scholar]
- 10.Colvin, R.B. 1989. Fibronectin in wound healing. In Fibronectin. D.F. Mosher, editor. Academic Press, New York. 213–254.
- 11.Cooper JA. Effects of cytochalasin and phalloidin on actin. J Cell Biol. 1987;105:1473–1478. doi: 10.1083/jcb.105.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crowe DT, Chiu H, Fong S, Weissman IL. Regulation of the avidity of integrin α4β7 by the β7cytoplasmic domain. J Biol Chem. 1994;269:14411–14418. [PubMed] [Google Scholar]
- 13.DiMilla PA, Stone JA, Quinn JA, Albelda SM, Lauffenburger DA. Maximal migration of human smooth muscle cells on fibronectin and type IV collagen occurs at an intermediate attachment strength. J Cell Biol. 1993;122:729–737. doi: 10.1083/jcb.122.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dunlevy JR, Couchman JR. Controlled induction of focal adhesion disassembly and migration in primary fibroblasts. J Cell Sci. 1993;105:489–500. doi: 10.1242/jcs.105.2.489. [DOI] [PubMed] [Google Scholar]
- 15.Dzamba BJ, Bultmann H, Akiyama SK, Peters DM. Substrate-specific binding of the amino terminus of fibronectin to an integrin complex in focal adhesions. J Biol Chem. 1994;269:19646–19652. [PubMed] [Google Scholar]
- 16.Elemer GS, Edgington TS. Microfilament reorganization is associated with functional activation of αmβ2 on monocytic cells. J Biol Chem. 1994;269:3159–3166. [PubMed] [Google Scholar]
- 17.Fogerty FJ, Akiyama SK, Yamada KM, Mosher DF. Inhibition of binding of fibronectin to matrix assembly sites by anti-integrin (α5β1) antibodies. J Cell Biol. 1990;111:699–708. doi: 10.1083/jcb.111.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.George EL, Georges-Labouesse EN, Patel-King RS, Rayburn H, Hynes RO. Defects in mesoderm, neural tube and vascular development in mouse embryos lacking fibronectin. Development. 1993;119:1079–1091. doi: 10.1242/dev.119.4.1079. [DOI] [PubMed] [Google Scholar]
- 19.Giancotti FG, Ruoslahti E. Elevated levels of the α5β1fibronectin receptor suppress the transformed phenotype of Chinese hamster ovary cells. Cell. 1990;60:849–859. doi: 10.1016/0092-8674(90)90098-y. [DOI] [PubMed] [Google Scholar]
- 20.Gilmore AP, Romer LH. Inhibition of focal adhesion kinase (FAK) signaling in focal adhesions decreases cell motility and proliferation. Mol Biol Cell. 1996;7:1209–1224. doi: 10.1091/mbc.7.8.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guan J, Trevithick JE, Hynes RO. Fibronectin/integrin interaction induces tyrosine phosphorylation of a 120kDa protein. Cell Regul. 1991;2:951–964. doi: 10.1091/mbc.2.11.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hibbs ML, Hong X, Stacker SA, Springer TA. Regulation of adhesion to ICAM-1 by the cytoplasmic domain of LFA-1 integrin β subunit. Science. 1991;251:1611–1613. doi: 10.1126/science.1672776. [DOI] [PubMed] [Google Scholar]
- 23.Hildebrand JD, Schaller MD, Parsons JT. Paxillin, a tyrosine phosphorylated focal adhesion-associated protein binds to the carboxyl terminal domain of focal adhesion kinase. Mol Biol Cell. 1996;6:637–647. doi: 10.1091/mbc.6.6.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hocking DC, Sottile J, McKeown-Longo PJ. Fibronectin's III-1 module contains a conformation-dependent binding site for the amino-terminal region of fibronectin. J Biol Chem. 1994;269:19183–19191. [PubMed] [Google Scholar]
- 25.Hocking DC, Smith RK, McKeown-Longo PJ. A novel role for the integrin-binding III-10 module in fibronectin matrix assembly. J Cell Biol. 1996;133:431–444. doi: 10.1083/jcb.133.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hughes PE, O'Toole TE, Ylanne J, Shattil SJ, Ginsberg MH. The conserved membrane-proximal region of an integrin cytoplasmic domain specifies ligand binding affinity. J Biol Chem. 1995;270:12411–12417. doi: 10.1074/jbc.270.21.12411. [DOI] [PubMed] [Google Scholar]
- 27.Humphries MJ. Integrin activation: the link between ligand binding and signal transduction. Curr Opin Cell Biol. 1996;8:632–640. doi: 10.1016/s0955-0674(96)80104-9. [DOI] [PubMed] [Google Scholar]
- 28.Huttenlocher A, Sandborg RR, Horwitz AF. Adhesion in cell migration. Curr Opin Cell Biol. 1995;7:697–706. doi: 10.1016/0955-0674(95)80112-x. [DOI] [PubMed] [Google Scholar]
- 29.Huttenlocher A, Ginsberg MH, Horwitz AF. Modulation of cell migration by integrin-mediated cytoskeletal linkages and ligand-binding affinity. J Cell Biol. 1996;134:1551–1562. doi: 10.1083/jcb.134.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hynes RO. Integrins: a family of cell surface receptors. Cell. 1987;48:549–554. doi: 10.1016/0092-8674(87)90233-9. [DOI] [PubMed] [Google Scholar]
- 31.Hynes, R.O. 1990. Fibronectins. Springer-Verlag, New York.
- 32.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 33.Ilic D, Futura Y, Kanazawa S, Takeda N, Sobue K, Nakatsuji N, Nomura S, Fujimoto J, Okada M, Yamamoto T, Aizawa S. Reduced cell motility and enhanced focal adhesion contact formation in cells from FAK-deficient mice. Nature. 1995;377:539–544. doi: 10.1038/377539a0. [DOI] [PubMed] [Google Scholar]
- 34.Jockusch BM, Bubeck P, Giehl K, Kroemker M, Moscher J, Rothkegel M, Rudiger M, Schluter K, Stanke G, Winkler J. The molecular architecture of focal adhesions. Ann Rev Cell Biol. 1995;11:379–416. doi: 10.1146/annurev.cb.11.110195.002115. [DOI] [PubMed] [Google Scholar]
- 35.Kornberg L, Earp HS, Parsons JT, Schaller M, Juliano RL. Cell adhesion or integrin clustering increases phosphorylation of a focal adhesion-associated tyrosine kinase. J Biol Chem. 1992;267:23439–23442. [PubMed] [Google Scholar]
- 36.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 37.Matsumoto K, Nakamura T, Kramer RH. Hepatocyte growth factor/scatter factor induces tyrosine phosphorylation of focal adhesion kinase (p125FAK) and promotes migration and invasion by oral squamous cell carcinoma cells. J Biol Chem. 1994;269:31807–31813. [PubMed] [Google Scholar]
- 38.McDonald, J.A. 1989. Fibronectin in the lung. In Fibronectin. D.F. Mosher, editor. Academic Press, San Diego, CA. 363–393.
- 39.McKeown-Longo PJ, Mosher DF. Interaction of the 70,000-mol-wt amino-terminal fragment of fibronectin with the matrix-assembly receptor of fibroblasts. J Cell Biol. 1985;100:364–374. doi: 10.1083/jcb.100.2.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McKeown-Longo PJ, Etzler CA. Induction of fibronectin matrix assembly in human fibrosarcoma cells by dexamethasone. J Cell Biol. 1987;104:601–610. doi: 10.1083/jcb.104.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morla A, Ruoslahti E. A fibronectin self-assembly site involved in fibronectin matrix assembly: reconstruction in a synthetic peptide. J Cell Biol. 1992;118:421. doi: 10.1083/jcb.118.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morla A, Zhang Z, Ruoslahti E. Superfibronectin is a functionally distinct form of fibronectin. Nature. 1994;367:193–196. doi: 10.1038/367193a0. [DOI] [PubMed] [Google Scholar]
- 43.Mosher DF. Physiology of fibronectin. Ann Rev Med. 1984;35:561–575. doi: 10.1146/annurev.me.35.020184.003021. [DOI] [PubMed] [Google Scholar]
- 44.Mosher DF, Sottile J, Wu C, McDonald JA. Assembly of extracellular matrix. Curr Opin Cell Biol. 1992;4:810–818. doi: 10.1016/0955-0674(92)90104-k. [DOI] [PubMed] [Google Scholar]
- 45.Mould AP, Akiyama SK, Humphries MJ. The inhibitory anti-β1 integrin monoclonal antibody 13 recognizes an epitope that is attenuated by ligand occupancy. J Biol Chem. 1996;271:20365–20374. doi: 10.1074/jbc.271.34.20365. [DOI] [PubMed] [Google Scholar]
- 46.Nojima Y, Morino N, Mimura T, Hamasaki K, Furuya H, Sakai R, Sato T, Tachibana K, Morimoto C, Yazaki Y, Hirai H. Integrin-mediated cell adhesion promotes tyrosine phosphorylation of p130Cas, a Src homology 3-containing molecule having multiple Src homology 2-binding motifs. J Biol Chem. 1995;270:15398–15402. doi: 10.1074/jbc.270.25.15398. [DOI] [PubMed] [Google Scholar]
- 47.Petersen TE, Thogersen HC, Skorstengaard K, Vibe-Pedersen K, Sahl P, Sottrup-Jensen L, Magnusson S. Partial primary structure of bovine plasma fibronectin: three types of internal homology. Proc Natl Acad Sci USA. 1983;80:137–141. doi: 10.1073/pnas.80.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pierschbacher M, Ruoslahti E. Variants of the cell recognition site of fibronectin that retain attachment-promoting activity. Proc Natl Acad Sci USA. 1984;81:5985–5988. doi: 10.1073/pnas.81.19.5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pierschbacher MD, Ruoslahti E. The cell attachment activity of fibronectin can be duplicated by small synthetic fragments of the molecule. Nature. 1984;309:30–33. doi: 10.1038/309030a0. [DOI] [PubMed] [Google Scholar]
- 50.Pierschbacher MD, Hayman EG, Ruoslahti E. Location of the cell-attachment site in fibronectin with monoclonal antibodies and proteolytic fragments of the molecule. Cell. 1981;26:259–267. doi: 10.1016/0092-8674(81)90308-1. [DOI] [PubMed] [Google Scholar]
- 51.Puzon-McLaughlin W, Yednock TA, Takada Y. Regulation of conformation and ligand binding function of integrin α5β1 by the β1cytoplasmic domain. J Biol Chem. 1996;271:16580–16585. doi: 10.1074/jbc.271.28.16580. [DOI] [PubMed] [Google Scholar]
- 52.Pytela R, Pierschbacher MD, Ruoslahti E. Identification and isolation of a 140 kd cell surface glycoprotein with properties of a fibronectin receptor. Cell. 1985;40:191–198. doi: 10.1016/0092-8674(85)90322-8. [DOI] [PubMed] [Google Scholar]
- 53.Pytela R, Pierschbacher MD, Argraves S, Suzuki S, Ruoslahti E. Arginine-glycine-aspartic acid adhesion receptors. Methods Enzymol. 1987;144:475–489. doi: 10.1016/0076-6879(87)44196-7. [DOI] [PubMed] [Google Scholar]
- 54.Quade BJ, McDonald JA. Fibronectin's amino-terminal matrix assembly site is located within the 29-kDa amino terminal domain containing five type I repeats. J Biol Chem. 1988;263:19602–19609. [PubMed] [Google Scholar]
- 55.Richarson A, Parsons JT. Mechanism for regulation of the adhesion-associated protein tyrosine kinase pp125FAK . Nature. 1996;380:538–540. doi: 10.1038/380538a0. [DOI] [PubMed] [Google Scholar]
- 56.Romer LH, Burridge K, Turner CE. Signaling between the extracellular matrix and the cytoskeleton: tyrosine phosphorylation and focal adhesion assembly. Cold Spring Harbor Symp Quant Biol. 1992;57:193–202. doi: 10.1101/sqb.1992.057.01.024. [DOI] [PubMed] [Google Scholar]
- 57.Schaller MD, Parsons JT. Focal adhesion kinase and associated proteins. Curr Opin Cell Biol. 1994;6:705–710. doi: 10.1016/0955-0674(94)90097-3. [DOI] [PubMed] [Google Scholar]
- 58.Schaller MD, Parsons JT. pp125FAK-dependent tyrosine phosphorylation of paxillin creates a high affinity binding site for crk. Mol Cell Biol. 1995;15:2635–2645. doi: 10.1128/mcb.15.5.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schaller MD, Borgman CA, Cobb BS, Vines RR, Reynolds AB, Parsons JT. pp125FAK, a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci USA. 1992;89:5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schaller MD, Borgman CA, Parsons JT. Autonomous expression of a non-catalytic domain of the focal adhesion associated protein tyrosine kinase. Mol Cell Biol. 1993;13:785–791. doi: 10.1128/mcb.13.2.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schwartz M, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Ann Rev Cell Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- 62.Schwarzbauer JE. Identification of the fibronectin sequences required for assembly of a fibrillar matrix. J Cell Biol. 1991;113:1463–1473. doi: 10.1083/jcb.113.6.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Small JV, Celis JE. Filament arrangements in negatively stained cultured cells: the organization of actin. Eur J Cell Biol. 1978;16:308–325. [PubMed] [Google Scholar]
- 64.Sottile J, Mosher DF. N-terminal type I modules are required for fibronectin binding to fibroblasts and to fibronectin's III-1 module. Biochem J. 1997;323:51–60. doi: 10.1042/bj3230051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sottile J, Selegue J, Mosher DF. Recombinant 70-kDa protein from the amino terminal region of rat fibroblasts inhibits binding of fibronectin to cells and bacteria. Protein Expr Purif. 1990;1:104–110. doi: 10.1016/1046-5928(90)90002-g. [DOI] [PubMed] [Google Scholar]
- 66.Sottile J, Schwarzbauer J, Selegue J, Mosher DF. Five type I modules of fibronectin form a functional unit that binds to fibroblasts and Staphylococcus aureus. . J Biol Chem. 1991;266:12840–12843. [PubMed] [Google Scholar]
- 67.Stenman S, von Smitten K, Vaheri A. Fibronectin and atherosclerosis. Acta Med Scand Suppl. 1980;642:165–170. doi: 10.1111/j.0954-6820.1980.tb10949.x. [DOI] [PubMed] [Google Scholar]
- 68.Thiery, J.P., J.L. Duband, S. Dufour, P. Savagner, and B.A. Imhof. 1989. Role of fibronectins in embryogenesis. In Fibronectin. D.F. Mosher, editor. Academic Press, New York. 181–212.
- 69.Turner CE. Paxillin is a major phosphotyrosine-containing protein during embryonic development. J Cell Biol. 1991;115:201–207. doi: 10.1083/jcb.115.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Turner CE, Miller JT. Primary sequence of paxillin contains putatitive SH2 and SH3 domain binding motifs and multiple LIM domains: identification of a vinculin and pp125FAK binding region. J Cell Sci. 1994;107:1583–1591. doi: 10.1242/jcs.107.6.1583. [DOI] [PubMed] [Google Scholar]
- 71.Vuori K, Ruoslahti E. Tyrosine phosphorylation of p130Casand cortactin accompanies integrin-mediated cell adhesion to the extracellular matrix. J Biol Chem. 1995;270:22259–22262. doi: 10.1074/jbc.270.38.22259. [DOI] [PubMed] [Google Scholar]
- 72.Yamada, K. 1989. Fibronectin domains and receptors. In Fibronectin. D.F. Mosher, editor. Academic Press, New York. 47–121.
- 73.Zhang Q, Mosher DF. Cross-linking of the NH2-terminal region of fibronectin to molecules of large apparent molecular mass. J Biol Chem. 1996;271:33284–33292. doi: 10.1074/jbc.271.52.33284. [DOI] [PubMed] [Google Scholar]