Cell crawling is thought to be the result of three coordinated motility behaviors: (a) protrusion and adhesion of the front end, probably driven by actin assembly; (b) traction force that leads to the advance of the nucleus and bulk cytoplasm; and (c) release and retraction of the tail in most cell types. (In neurons, the last step is highly modified, and an axon elaborates from behind the advancing growth cone.) As Mitchison and Cramer (15) point out, it is the traction step that is least understood and seems most central to productive locomotion. Two papers in JCB now shed new light, both mechanical and molecular, on the traction step of cell crawling. A report in this issue of JCB on growth cone movements from the Forscher group (19) and a paper from the Borisy lab (20) on keratocyte motility provide new insights into the role of myosin in traction. Specifically, the two papers clarify the mechanical connections to the motor that produce the motile output of various animal cells. These papers also provide insight into how a conserved mechanism for animal cell crawling could underlie visually different crawling behavior. Indeed, the cell types used in the new studies are at two extremes of cell crawling behavior. Neuronal growth cones change shape markedly during their inconstant, stop-and-go advance, which is accompanied by a variety of seemingly futile motile behaviors such as waving of, extending, and retracting filopodia, and a persistent retrograde flow of cortical actin. In contrast, fish keratocytes appear to glide smoothly over a culture surface, moving continuously with little or no change in aspect. Retrograde actin flow is not typically seen in these cells; indeed, most actin filaments remain stationary with respect to the substratum during movement (21).
Suter et al. (19) have extended their observations of the retrograde (toward the cell body) flow of cortical actin that occurs on the surface of growth cones of cultured Aplysia bag cell neurons. This behavior has been observed in other cultured cells as well (22, 23). Cortical actin, apparently as a newly assembled, cross-linked network, arises from the most distal margin of the growth cone and then moves smoothly backward across the thin cytoplasmic veil of the lamella only to disappear, presumably by disassembly, when it reaches the thicker, microtubule-rich, central region of cytoplasm. Suitably adherent beads on the cell surface can “go with the flow” on Aplysia neurons and be transported smoothly backward at the same speed as the actin (4, 10), as if the beads were riding an escalator. A key role for myosin contraction in powering this continuous retrograde movement is supported by pharmacological evidence (12). When Aplysia growth cones contact another cell, the actin flow slows at a rate that was inversely proportional to the rate of growth cone advance (10), suggesting a single motor underlying both the cortical flow and growth cone advance.
This older work has now been extended with two innovations. One is the use of beads coated with the cell-surface Aplysia cell adhesion molecule (ApCAM),1 or with antibodies to ApCAM that ride the flow, which revealed two states of coupling to the actin flow. Second, the use of glass needles to restrain these beads allowed for direct application and qualitative assessment of forces independent of the biological phenomena. This simple, reliable, external assay of motive forces in cell locomotion vastly increases the confidence in mechanical interpretations, revealing simple Newtonian behaviors and reducing the dependence on biological assumptions.
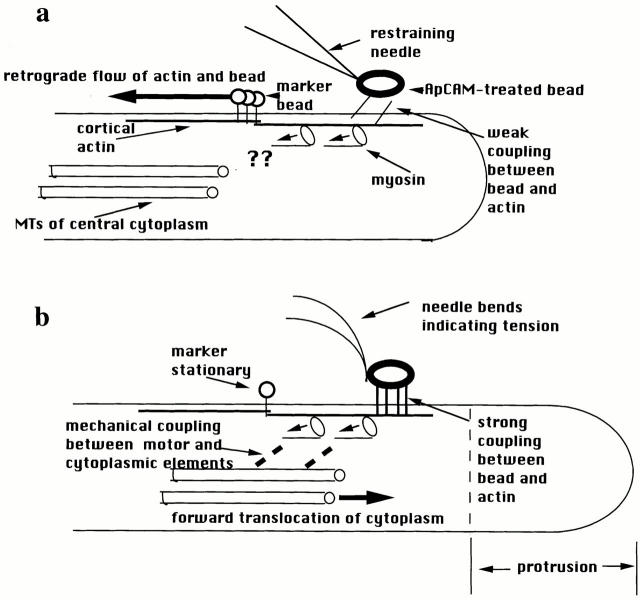
Suter et al. (19) find that ApCAM beads sprinkled on the top surface of the growth cone couple to the retrograde actin flow. For the first 10–20 min, these beads simply stop moving when restrained by the needle, but the retrograde flow continues uninterrupted as shown by the movement of smaller, non-ApCAM beads used as markers. Therefore, ApCAM beads initially couple weakly to the actin flow and can slip easily if restrained (Fig. 1 a). After a variable latency period, however, restraint of the bead produces a set of integrated mechanical responses that the authors call a “restrained bead interaction” (Fig. 1 b). First, the needle bends backward, indicating force generated from within the cell. Second, on-axis beads marking retrograde flow slow down and stop as the tension increases, suggesting that the same mechanism driving actin flow is responsible for needle bending. This result also indicates strong mechanical coupling between the ApCAM bead and the retrograde actin flow that is firm enough to support the force generated by the cell. At the same time, both the protrusion and traction phases of motility occur in the growth cone. Cytoplasm moves forward along the radial axis between the ApCAM bead and the cell margin, i.e., protrusion, which is probably the result of the slowing of retrograde flow. Previous results suggest that actin assembly at the distal margin continues independent of retrograde flow, so that when it slows or stops, the margin of the cell moves forward, driven by actin assembly (10, 12). The new observation is that during a restrained bead interaction the central microtubule-rich cytoplasm also surges forward, which is the key traction movement of growth cone crawling. That is, tension forces are seen to naturally produce the “engorgement” phase of growth cone advance (5). Forward movement of the central cytoplasm after bead restraint indicates a mechanical continuum from the ApCAM bead on the surface through to the central cytoplasm. Since ApCAM is a member of the Ig family of cell adhesion molecules (17), the current evidence is, to the best of our knowledge, the first demonstration of force transduction and connection to the cytoskeleton through an Ig family receptor. This complements the recent finding of robust mechanical links from integrins through to the nucleus (13). Connections from ApCAM to the central cytoplasm were confirmed when release of bead restraint and its accompanying tension were immediately followed by retreat of the central cytoplasm toward its original position and revival of retrograde flow by the marker and ApCAM beads. In our view, this is strong evidence that the same motor that powers retrograde actin flow in static cells does indeed drive the engorgement phase characteristic of growth cone translocation, a somewhat controversial idea. The rearward actin flow and forward flow of central cytoplasm are both the result of the same motor, confirmed by the observation that on-axis beads marking retrograde flow slow down and stop at a rate that is inversely proportional to the rate of central cytoplasmic movement, as previously observed for homophilic growth cone interactions (10).
Figure 1.
Highly schematic diagram of the forces and mechanical connections inferred from the results of Suter et al. (19). (a) During the latency period after attachment of ApCAM-coated beads, restraint of the bead causes it to stop, with no evidence of tension exerted in the needle. Retrograde actin flow continues uninterrupted, suggesting a weak, slipping interaction between the bead and the underlying actin network. The question marks are intended to illustrate that the results provide no information about the presence or absence of connection(s) between the central cytoplasm and the myosin driving retrograde flow. (b) After a latency period, firm connections develop between the ApCAM bead and the actin, associated with clustering of ApCAM beneath the bead. Restraint of the bead now causes development of tension in the needle and retrograde flow to stop. Because the actin is no longer able to slip relative to the surface, the tension generated by the myosin motors is now accomodated by forward flow of cytoplasm at the leading edge and in the central microtubule-containing cytoplasm. This indicates mechanically resistant connections among the surface, actin, myosin motors, and surrounding cytoplasm. See text for further explanation.
An automobile analogy may help clarify how the above conclusions were reached, as the traction observations comprise a simple stick–slip mechanical interaction. Consider a car stuck in the mud applying constant power to its drive wheels (myosin acting to produce force). Because the connection to the environment is fluid, the wheels spin, producing no forward motion. This slip interaction is equivalent to the retrograde actin flow. If the mud now solidifies, like the coupling of the ApCAM bead to the actin after a latency period, and the ground is now immovable (like the needle restraining the actin flow), then the stick interaction occurs and the car moves forward, as does the central cytoplasm in the growth cone. If there is some combination of car movement (central cytoplasmic movement) and wheel spinning (retrograde actin flow), then these rates will be inversely proportional as required by Newton's third law because the same force is driving both.
The above results provide strong support for the hypothesis that growth cone advance depends on engagement and disengagement of cell surface receptors with underlying actin networks within the cytoplasm (11, 15). Essentially, a two-state clutch (strong and weak coupling, or stick and slip) exists between the membrane proteins and the cytoskeleton (Fig. 1). Variable engagement of surface receptors to the underlying cytoskeleton had previously been demonstrated (2). However, the forces applied were small and there was no clear relationship between behaviors of cell crawling and the observed differences in engagement. Suter et al. (19) provide persuasive evidence that traction force requires different, firmer connections between surface receptors and the cytoskeleton than the connections required simply for retrograde flow of surface markers, which are weak and slip easily. A “slipping clutch” may also explain the finding that, in some cells, surface-marking beads move at a fraction of the rate of the underlying actin flow (22). Suter et al. (19) also provide an initial clue to the molecular basis of the two clutch states: a clustering of ApCAM occurs along with the strongly coupled state. Thus, the two states may simply reflect differences in the local concentration of coupling interactions. Also confirmed is the notion that growth cone advance involves central cytoplasm pulled forward by tension (6), which requires the now demonstrated firm links between the source of tension and the cytoplasm. Myosin appears to be the source of this tension, based on the current evidence linking cytoplasmic movement with retrograde flow and the earlier results that retrograde flow depends on myosin ATPase activity (12). A major question is what cytoplasmic architecture might underlie the connections between the cell surface, the motor, and the deep cytoplasm. The diagram in Fig. 1 illustrates the mechanical aspects suggested by the new results but is clearly unrealistic in terms of cytoskeletal arrangements. For example, although some actin filaments in lamellar regions of growth cones are oriented parallel to the axis of movement, most filaments form a crisscross network (9). Further insight into the nature and functioning of some of the connections is provided by the paper on fish keratocytes (20).
Fish keratocytes are wing-shaped cells whose long axis is perpendicular to the direction of advance. The forward arc of the cell consists of a broad, thin lamella and the rear part of the cell contains the nucleus and cell body. Keratocytes' peculiar ghostly locomotion, in which the cell moves smoothly and continuously at high speed (10 μm/min) with essentially no change in shape, is thought to be due to nearly perfect coordination among the protrusion, traction, and retraction phases (15). It is known from watching keratocytes move on and deform rubber sheets that the cell exerts most of its traction force near the two lammellar sides (8), rather than along the front–back axis as one might expect. Curiously, the cytoplasm and organelles of the cell body rotate like the drum of a steamroller as the cell moves forward (1). Svitkina et al. (20) address these unusual phenomena in a study that confirms previous observations of a marked gradient in the actin filament network (18): dense at the lamellar margin, less dense toward the cell body, but then very dense in the form of actin bundles in the transition region between the lamella and cell body. The new data indicate that myosin is also arrayed in a gradient. Fluorescent myosin II, as bipolar filaments, formed small clusters near the lamellar margin that increased in size toward the cell body, aggregating into a network in the transition region. In moving cells, the myosin clusters in the lamella are initially stationary with respect to the substratum, so that as the cell moves, the cell body gets closer to the clusters and the lamellar margin moves further away. In static cells, myosin clusters move slowly backward toward the cell body.
Fig. 2 illustrates a model “dynamic network contraction” proposed by Svitkina et al. (20) to explain their results. Actin assembly is nucleated at the lamellar margin to form a network that is disassembled nearer the nucleus, as observed for several cell types (2). Myosin filaments and clusters also arise spontaneously within the network, as previously observed in fibroblasts (14). The actomyosin-based contraction of this network pulls the cell body forward and also causes the network to collapse into bundles at the transition zone (Fig. 2 b). This concentration of cytoskeletal filaments near the bottom of the cell body creates a drag force that, combined with the forward translocation force, causes the cell body to rotate (just as the nondrive wheels of a car spin from being dragged along the ground) (Fig. 2 d). The authors postulate a stick-slip clutch connecting the cell body to the cytoskeletal network to explain how the cell body can be pulled forward by the contracting network and also rotate. Typically, contractile mechanisms have been assumed to involve contraction parallel to the direction of advance (15, 16), but a network postulated to contract uniformly throughout would exert a significant fraction of the force perpendicular to the direction of advance. This new model could then explain the substantial traction forces exerted laterally (8). The uniform contraction produces leftward and rightward forces at the sides of the lamella that cannot be accommodated by net leftward or rightward locomotion of the cell because of cell integrity, but would stretch the cell out perpendicular to the direction of advance. Nevertheless, the forces on a rubber substratum would be seen to be greatest at these lamellar sides, and not along the front-to-back axis where the forces are accommodated by net locomotion.
Figure 2.
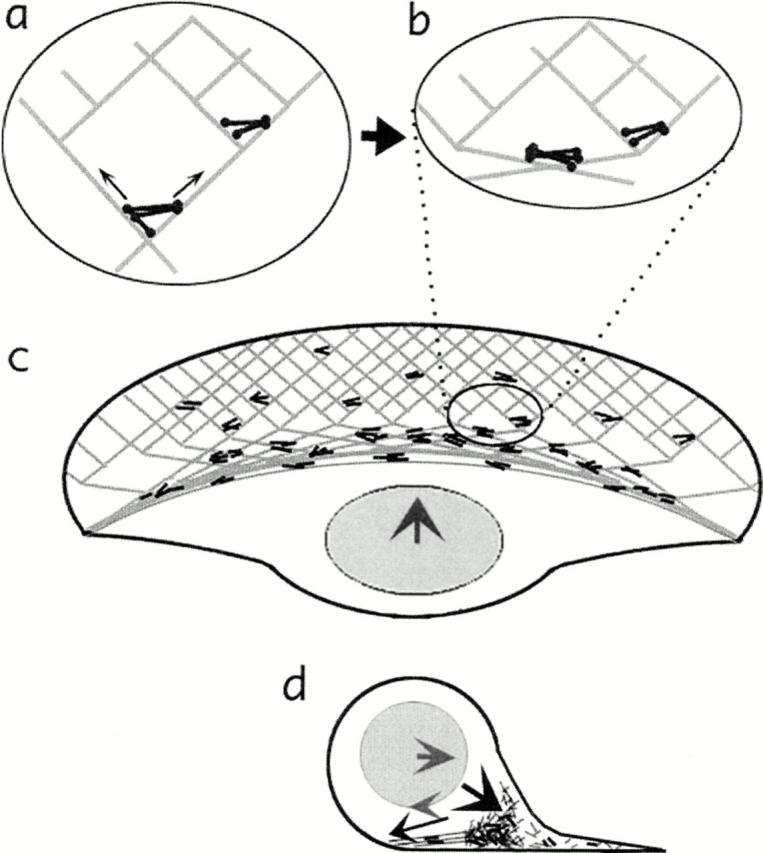
Diagram illustrating the dynamic network model of Svitkina et al. (20) in a locomoting fish keratocyte. (a and b) A network of actin (light grey lines) and myosin (dark bipolar filaments) contracts causing reorganization of the network. Myosin is clustered and actin is brought into alignment as parallel bundles. (c) Seen at the level of the entire cell, this network contraction causes forward translocation of the cell body. (d) In this cross-section, the forward rolling of the cell body is seen as a combination of the forward-directed force and a drag force at the bottom of the cell body/nucleus created by the accumulation of contracted network. Similar to nondrive wheels of a car, the combination of forward force and dragging along the bottom surface causes rotation of a rounded object.
In addition to contraction, cell crawling requires making and breaking attachments to the substratum (7). Combining ideas about surface-to-cytoplasm connections from Suter et al. (19) with the contractile network ideas from Svitkina et al. (20) produces a speculative model that can explain a variety of the motile phenomena associated with cell crawling. We assume, as in other models, that the motile phenomena observed on the top, free surface of cells reflects mechanisms also functioning on the bottom, attached cell surface. During traction, the actin network is stably and tightly coupled to surface receptors, as shown by Suter et al. (19) that, in turn, are coupled to the substratum. Myosin-generated tension causes network contraction, and the inner cytoplasmic mass or cell body moves forward (Fig. 1 b), but the lamellar actin remains attached to and stationary with the substratum (21), as does myosin suspended within the network (20). Persistent movement is possible because the network is essentially regenerated continuously for each change of location by network assembly at the forward edge and disassembly at the rear. Retrograde movement of actin and/or myosin in stationary cells is the result of network contraction in the presence of weak interactions between the membrane and the network (Fig. 1 a). These slip, allowing the entire network to slip backward relative to the membrane, as shown by Suter et al. (19) for growth cones. (In stationary keratocytes, we imagine that tension rips loose some of the moorings and, combined with network assembly/disassembly, creates a slow retrograde movement of the network.) Presumably, the stop-and-go advance of growth cones then reflects oscillating periods of slip or stick between the cytoskeleton and substratum. And the differences between myosin playing a contractile or transport function in cell traction (15) would seem to narrow considerably, reduced (arguably) to different clutch engagements. Localized contraction of the network combined with protrusive events could underlie membrane ruffling and filopodial waving often seen on the dorsal surface of cells. Both keratocytes and Aplysia growth cones are highly lamellipodial with few of the filopodia that garnish the front ends of many crawling cells. These finger-like projections contain a bundle of uniformly oriented actin (9). Thus, forces and cytoskeletal arrangements would correspond even more closely to that shown in Fig. 1, suggesting little fundamental difference between filopodial and lamellipodial crawling.
Speculations aside, the current work raises many important questions for future study. Some in the field remain skeptical of the simple role for myosin in retrograde flow and cell crawling shown in Fig. 1. In any case, how does the myosin function in motile cells? For example, at least one observation of Suter et al. (19) argues against myosin in Aplysia growth cones being organized as in the lamella of keratocytes. Suter et al. (19) show that traction force can be exerted in a local region of the growth cone while retrograde flow continues elsewhere. That is difficult to explain with a uniformly contracting network. Does Aplysia myosin also move retrogradely during actin flow as predicted by dynamic network contraction? Or put another way, just how general are the findings from fish keratocytes? The evidence for variable coupling between surface receptors and the underlying cytoskeleton also raises a host of questions about the molecular and mechanical bases for this complex-regulated connection. And what underlies the connections between the motor and deep cytoplasm? Is a clutch at this position, postulated by Svitkina et al. (20), a special feature of keratocytes? In cells without rolling motion, there may always be a connection. Whether the motor produces traction could then depend on whether there was a simultaneous connection to a system that permitted the force to be dissipated without moving the cell (“sling mud”). Because of these and many other issues, the pages of JCB are likely to be graced by important advances in our understanding of cell crawling for some time to come.
Footnotes
1. Abbreviation used in this paper: ApCAM, Aplysia cell adhesion molecule.
Address all correspondence to Steven R. Heidemann, Department of Physiology, Giltner Hall, Michigan State University, East Lansing, MI 48824-1101. Tel.: (517) 355-6475. Fax: (517) 355-5125. E-mail: heidemann @psl.msu.edu
References
- 1.Anderson KI, Wang Y-L, Small JV. Coordination of protrusion and translocation of the keratocyte involves rolling of the cell body. J Cell Biol. 1996;134:1209–1218. doi: 10.1083/jcb.134.5.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choquet D, Felsenfeld DP, Sheetz MP. Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell. 1997;88:39–48. doi: 10.1016/s0092-8674(00)81856-5. [DOI] [PubMed] [Google Scholar]
- 3.Condeelis J. Life at the leading edge: the formation of cell protrusions. Annu Rev Cell Biol. 1993;9:411–444. doi: 10.1146/annurev.cb.09.110193.002211. [DOI] [PubMed] [Google Scholar]
- 4.Forscher P, Smith SJ. Actions of cytochalasins on the organizaton of actin filaments and microtubules in a neuronal growth cone. J Cell Biol. 1988;107:1505–1516. doi: 10.1083/jcb.107.4.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldberg DJ, Burmeister DW. Stages in axon formation: Observation of growth of Aplysiaaxons in culture using video-enhanced contrast-differential interference contrast microscopy. J Cell Biol. 1986;103:1921–1931. doi: 10.1083/jcb.103.5.1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Heidemann SR, Lamoureux P, Buxbaum RE. On the cytomechanics and fluid dynamics of growth cone motility. J Cell Sci. 1991;15(Suppl.):35–44. doi: 10.1242/jcs.1991.supplement_15.6. [DOI] [PubMed] [Google Scholar]
- 7.Lauffenburger DA, Horwitz AF. Cell migration: A physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 8.Lee J, Leonard M, Oliver T, Ishihara A, Jacobson K. Traction forces generated by locomoting keratocytes. J Cell Biol. 1994;127:1957–1964. doi: 10.1083/jcb.127.6.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lewis AK, Bridgman PC. Nerve growth cone lamellipodia contain two populations of actin filaments that differ in organization and polarity. J Cell Biol. 1992;119:1219–1243. doi: 10.1083/jcb.119.5.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin CH, Forscher P. Growth cone advance is inversely proportional to retrograde F-actin flow. Neuron. 1995;14:763–771. doi: 10.1016/0896-6273(95)90220-1. [DOI] [PubMed] [Google Scholar]
- 11.Lin CH, Thompson CA, Forscher P. Cytoskeletal reorganization underlying growth cone motility. Curr Opin Neurobiol. 1994;4:640–647. doi: 10.1016/0959-4388(94)90004-3. [DOI] [PubMed] [Google Scholar]
- 12.Lin CH, Espreafico EM, Mooseker MS, Forscher P. Myosin drives retrograde F-actin flow in neuronal growth cones. Neuron. 1996;16:769–782. doi: 10.1016/s0896-6273(00)80097-5. [DOI] [PubMed] [Google Scholar]
- 13.Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci USA. 1997;94:849–854. doi: 10.1073/pnas.94.3.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McKenna NM, Wang YL, Konkel ME. Formation and movement of myosin-containing structures in living fibroblasts. J Cell Biol. 1989;109:1163–1172. doi: 10.1083/jcb.109.3.1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchison T, Cramer LP. Actin-based cell motility and cell locomotion. Cell. 1996;84:371–379. doi: 10.1016/s0092-8674(00)81281-7. [DOI] [PubMed] [Google Scholar]
- 16.Mitchison T, Kirschner MW. Cytoskeletal dynamics and nerve growth. Neuron. 1988;1:761–772. doi: 10.1016/0896-6273(88)90124-9. [DOI] [PubMed] [Google Scholar]
- 17.Rutishauser U. Adhesion molecules of the nervous system. Curr Opin Neurobiol. 1993;3:709–715. doi: 10.1016/0959-4388(93)90142-l. [DOI] [PubMed] [Google Scholar]
- 18.Small JV, Herzog M, Anderson K. Actin filament organization in fish keratocyte lamellipodium. J Cell Biol. 1995;129:1275–1286. doi: 10.1083/jcb.129.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Suter, D.M., L.M. Errante, V. Belotserkovsky, and P. Forscher. 1998. The Ig superfamily cell adhesion molecule, ApCAM, mediates growth cone steering by substrate-cytoskeletal coupling. J. Cell Biol. In press. [DOI] [PMC free article] [PubMed]
- 20.Svitkina TM, Verkhovsky AB, McQuade KM, Borisy GG. Analysis of the actin-myosin II system in fish epidermal keratocytes: Mechanism of cell body translocation. J Cell Biol. 1997;139:397–415. doi: 10.1083/jcb.139.2.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Theriot JA, Mitchison TJ. Actin filament dynamics in locomoting cells. Nature. 1991;352:126–131. doi: 10.1038/352126a0. [DOI] [PubMed] [Google Scholar]
- 22.Theriot JA, Mitchison TJ. Comparison of actin and cell surface dynamics in motile fibroblasts. J Cell Biol. 1992;119:367–377. doi: 10.1083/jcb.119.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Waterman-Storer CM, Salmon ED. Actomyosin-based retrograde flow of microtubules in the lamella of migrating epithelial cells influences microtubule dynamic instability and turnover and is associated with microtubule breakage and treadmilling. J Cell Biol. 1997;139:417–434. doi: 10.1083/jcb.139.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]