Abstract
The asymmetric distribution of stable, posttranslationally modified microtubules (MTs) contributes to the polarization of many cell types, yet the factors controlling the formation of these MTs are not known. We have found that lysophosphatidic acid (LPA) is a major serum factor responsible for rapidly generating stable, detyrosinated (Glu) MTs in serum-starved 3T3 cells. Using C3 toxin and val14 rho we showed that rho was both necessary and sufficient for the induction of Glu MTs by LPA and serum. Unlike previously described factors that induce MT stability, rho induced the stabilization of only a subset of the MTs and, in wound-edge cells, these stable MTs were appropriately oriented toward the leading edge of the cell. LPA had little effect on individual parameters of MT dynamics, but did induce long states of pause in a subset of MTs near the edge of the cell. Rho stimulation of MT stability was independent of actin stress fiber formation. These results identify rho as a novel regulator of the MT cytoskeleton that selectively stabilizes MTs during cell polarization by acting as a switch between dynamic and stable states of MTs rather than as a modulator of MT assembly and disassembly.
In many cells there are at least two populations of microtubules (MTs)1 distinguishable by their rates of turnover. Dynamic MTs have a half-life of minutes and comprise the largest subset of MTs in proliferating and undifferentiated cells (Saxton et al., 1984; Schulze and Kirschner, 1986). In contrast, stable MTs (or more properly, stabilized MTs, since they are derived from dynamic MTs) have a half-life of an hour or more and are minor components of undifferentiated cells (Schulze et al., 1987; Webster et al., 1987). Stabilized MTs are found at elevated levels in polarized and differentiated cells (Gundersen and Bulinski, 1986, 1988; Gundersen et al., 1989; Houliston and Maro, 1989; Baas and Black, 1990; Pepperkok et al., 1990; Warn et al., 1990; Bulinski and Gundersen, 1991; MacRae et al., 1991).
There are reasons to think that the stabilized MTs may perform distinct functions from those performed by the dynamic MTs. In many cases, stabilized MTs have been shown to accumulate posttranslationally modified forms of tubulin (e.g., detyrosinated [Glu] tubulin; Gundersen et al., 1984, 1987; Gundersen and Bulinski, 1986; Webster et al., 1987; Kreis, 1987) and/or acetylated tubulin (Piperno et al., 1987; Schulze et al., 1987). The presence of modified tubulin subunits serves to biochemically distinguish stabilized from dynamic MTs. Nonetheless, available evidence suggests that posttranslational modification is a consequence, not a cause of MT stability (Khawaja et al., 1988; Webster et al., 1990; also see Fig. 3). We have recently found that vimentin intermediate filaments are preferentially coaligned with the subset of stabilized, detyrosinated MTs (Glu MTs) in the lamella of locomoting 3T3 cells and that this interaction is specific for Glu tubulin versus tyrosinated (Tyr) tubulin (Gurland and Gundersen, 1995; Kreitzer, G., and G.G. Gundersen, manuscript submitted for publication). These results suggest that posttranslational modifications of tubulin are not involved in altering the stability of MTs, but rather in regulating the interaction of other organelles with stabilized MTs.
Figure 3.
LPA stimulates formation of nocodazole- and dilution-resistant MTs. For nocodazole stability, SFM-treated cells were refed SFM alone (a) or SFM containing 1 μM LPA (b) or 0.5% CS (c) for 5 min. Nocodazole was added to a final concentration of 2 μM and cells were incubated for 30 min. Cells were then detergent extracted to remove monomeric tubulin, washed, fixed, and stained for β-tubulin. For dilution resistance, SFM-treated cells were refed SFM alone (d) or SFM containing 1 μM LPA (e) for 5 min. The cells were then washed, detergent extracted, washed again, and incubated in PEM at 37°C for 1 h. Coverslips were fixed and stained with antibodies to β-tubulin. Bar, 20 μm.
In a number of cases, the formation of stabilized MTs during cell polarization and differentiation occurs specifically in the region of the cell undergoing polarization. For example, in polarized fibroblasts migrating into an in vitro wound, the stabilized MTs are not found throughout the cell, but are concentrated in the lamella (Gundersen and Bulinski, 1988; Nagasaki et al., 1992). Stabilized MTs are found along the bipolar axis of elongating myoblasts just before myoblast fusion (Gundersen et al., 1989). Stabilized MTs also form selectively in growing neurites during neurite outgrowth (Baas and Black, 1990; Bulinski and Gundersen, 1991). These results suggest that there are signaling pathways that are involved in stimulating MT stabilization both spatially and temporally.
Regulation of MT stabilization is further suggested by observations that the levels of stabilized MTs change rapidly in response to cell-associated and soluble external factors. Stabilized, Glu MTs in locomoting fibroblasts are rapidly lost upon cell–cell collision or after treatment with isolated plasma membranes (Nagasaki et al., 1992, 1994). In serum-starved fibroblasts, MT stabilization can be triggered by serum factors relatively rapidly (30–60 min), or more slowly by TGF-β (Gundersen et al., 1994), suggesting that the MT stabilization is acutely regulated by soluble as well as cell-associated external factors. Protein phosphatase inhibitors can selectively depolymerize stable MTs in the same time frame, suggesting some involvement of protein phosphorylation in the control of stabilized MT formation (Gurland and Gundersen, 1993). To date, however, there are no reports that changes in MT stability occur as rapidly as changes in the actin cytoskeleton, where cytokines and growth factors stimulate changes in actin dynamics and assembly in minutes or even seconds (Condeelis, 1993; Hall, 1994).
How external signals are transmitted to stabilize MTs is completely unknown. Earlier work suggested that mitogenic lipid lysophosphatidic acid (LPA) was an important serum component involved in maintaining MT stability (Nagasaki and Gundersen, 1996). We now show that LPA can rapidly alter MT stability in serum-starved 3T3 fibroblasts. LPA stimulates a number of intracellular signaling pathways and we show that the one involving the small GTP-binding protein, rho, mediates the LPA stimulation of MT stability. Importantly, rho stabilizes only a subset of the MTs, unlike other factors that when introduced into cells stabilize the entire population of MTs. Thus, rho is the first intracellular signaling molecule identified capable of regulating MT stability locally, selectively, and rapidly. Combined with earlier results on the regulation of the actin cytoskeleton by rho (Ridley et al., 1992; Hall, 1994), our results suggest that rho is involved in the coordinate regulation of the MT and actin cytoskeletons.
Materials and Methods
Cell Culture, Serum-free Treatment, and Wounding
NIH-3T3 cells (passage no. 127–137) were cultured in DME and calf serum as previously described (Gundersen and Bulinski, 1988). Cells were seeded onto acid-washed glass coverslips, grown to confluency (2–3 d), and starved in serum-free medium (SFM) as previously described (Gundersen et al., 1994). For some experiments, monolayers were wounded by scraping off a narrow strip of cells with a jeweler's screwdriver (Gundersen and Bulinski, 1988) and then used immediately or after 2 h.
Lipid Treatments
Serum-starved cells were treated with lipids by adding lipids directly from stock solutions to SFM. The extent of Glu MT induction (see below) was compared with that obtained in 0.5% calf serum (CS), which gave a maximal response (Gundersen et al., 1994). In some experiments, LPA or CS was pretreated with phospholipase B (PLB; 0.5 U/nmol LPA; 1 U/10 μl CS) at 37°C for 2 h, and then boiled for 4 min to destroy PLB activity. PLB was from Sigma Chemical Co. (no. P8914; St. Louis, MO) and was dissolved in calcium and magnesium free Hanks' balanced salt solution containing 20 mM Hepes, pH 8.0 (CMFH). The following lipids were obtained from Sigma Chemical Co. except where noted: LPA, l-α-lysophosphatidic acid (1-oleoyl; Sigma Chemical Co. or Avanti Polar Lipids, Alabaster, AL); LPG, l-α-lysophosphatidylglycerol (1-oleoyl; Avanti Polar Lipids); PA, l-α-phosphatidic acid (dioleoyl); LPC, l-α-lysophosphatidylcholine (1-oleoyl); LPE, l-α-lyosphosphatidylethanolamine (1-oleoyl); LPS, l-α-lysophosphatidyl- l-serine; LPI, l-α-lysophosphatidylinositol; OAG, 1-oleoyl-2-acetyl-sn-glycerol; PAF, 1-O-hexadecyl-2-acetyl-sn-glycero-3-phosphocholine (platelet- activating factor); and LPAF, 1-O-hexadecyl-sn-glycero-3-phosphocholine (lysoplatelet-activating factor). Stock solutions were prepared as follows: PAF and LPAF were prepared in water at 5 mg/ml. LPA and LPG were at 1 mM in CMFH containing 1 mM fatty acid–free BSA; PA, LPC, LPE, LPS, LPI, and OAG were dissolved in 50% ethanol at 5 mg/ml. All lipids were stored at −80°C and mixed into SFM just before adding to the cells.
Assessment of the Induction of Glu MTs
The extent of the induction of Glu MTs was assessed microscopically, essentially as previously described (Gundersen and Bulinski, 1988; Gundersen et al., 1994). In brief, cell monolayers that had been fixed and immunofluorescently labeled for Glu and Tyr MTs (see below) were examined with a Nikon 60× Plan Apochromat oil objective (NA 1.4) on a Nikon Optiphot fluorescence microscope. The extent of the induction of Glu MTs was determined on a cell-to-cell basis by scoring cells for the presence of a significant number of MTs (⩾10) that were brightly labeled with antibody to Glu tubulin. As has been found in numerous other studies (Gundersen et al., 1984, 1989, 1994; Webster et al., 1987; Gundersen and Bulinski, 1988), these brightly labeled Glu MTs are clearly distinguishable from the bulk of the MTs that contain very low levels of Glu tubulin.
Assessment of MT Stability
To determine the resistance of MTs to nocodazole, SFM-treated cells that had been refed fresh SFM alone or SFM containing 1 μM LPA or 0.5% CS for 5 min were treated with 2 μM nocodazole for 30 min (still in the presence of LPA or CS). Previously, we had determined that 2 μM nocodazole was optimal for breaking down most of the MTs in SFM-treated NIH-3T3 cells (Gundersen et al., 1994). After nocodazole treatment, cells were detergent extracted under conditions that remove monomeric tubulin, but preserve MTs (Gundersen et al., 1994), and then fixed in −20°C methanol for 5 min. The nocodazole resistance of MTs in cells that had been microinjected with 1.2 mg/ml val14 rho or 1 mg/ml Hu IgG (as a control), was determined in the same way, except that the nonextractable marker, 0.5 mg/ml IFA mouse antibody to intermediate filaments (Pruss et al., 1981), was comicroinjected and cells were incubated for 30 min before treating with 2 μM nocodazole for 60 min, extracting, and fixing.
To assess the resistance of MTs to dilution, SFM-treated cells were refed SFM or SFM containing 1 μM LPA for 5 min, washed quickly with warm EBSS, then PEM (0.1 M PIPES, pH 6.9, 1 mM EGTA, and 1 mM MgCl2), and then extracted for 1 min in 0.2% Triton X-100 in PEM. After extraction, cells were washed with warm PEM and then incubated in PEM for 1 h at 37°C. The cells were then fixed in −20°C methanol.
Indirect Immunofluorescence
Fixation and staining were performed as previously described (Gundersen et al., 1984, 1994). Indirect immunofluorescence for most experiments was performed with a rabbit peptide antibody specific for Glu tubulin (SG; Gundersen et al., 1984) and a mouse monoclonal antibody specific for actin (C4D6; Lessard, 1988). In some experiments, cells were also stained with a rat monoclonal antibody to Tyr tubulin (YL1/2; Kilmartin, 1982). YL1/2 was the generous gift of Dr. J. Kilmartin (Medical Research Council, Cambridge, UK). For the MT stability experiments, the SG antibody and a mouse monoclonal antibody (3F3) reactive with all β-tubulin isoforms, were used as previously described (Khawaja et al., 1988). C4D6 and 3F3 were generously provided by Dr. J. Lessard (University of Cincinnati, OH). Secondary antibodies were 1:400 dilution of high F/P fluorescein-conjugated goat anti–rabbit IgG (Cappel, Durham, NC), 1:200 dilutions of rhodamine-conjugated donkey anti–mouse or donkey anti–rat IgG (Jackson ImmunoResearch Laboratories, West Grove, PA), 1:100 dilutions of coumarin-conjugated donkey anti–rat or Cy5-conjugated donkey anti–rat (Jackson ImmunoResearch Laboratories). Injected human IgG was detected with the secondary antibody coumarin-conjugated donkey anti– human IgG at a 1:50 dilution. Injected IFA antibody was detected with the rhodamine-conjugated donkey anti–mouse antibody at a 1:200 dilution.
Images were captured with a SIT camera (MTI model 65; Dage-MTI, Michigan City, IN) or a cooled CCD camera with a Kodak KAF 1400 chip (1,317 × 1,035 pixels; MicroMAX; Princeton Instruments, Trenton, NJ), processed using Image 1 or Metamorph imaging software (both from Universal Imaging Corp., West Chester, PA).
Microinjection
Cells were pressure microinjected using back-loaded glass capillaries and a Narshige micromanipulator (Narshige, Greenvale, NY; Gurland and Gundersen, 1995). We estimate that ∼10% of the cell volume was routinely injected.
Wild-type rhoA and the constitutively active mutant, val14 rhoA, were expressed from the pGEX-2T vector as glutathione-S-transferase fusion proteins (Smith and Johnson, 1988) and cleaved and purified according to the method of Ridley et al. (1992). By SDS–gel electrophoresis, these preparations were 30–50% pure. Proteins were concentrated using a collodion bag apparatus and dialyzed against H-KCl (10 mM Hepes, pH 7.4, 140 mM KCl) plus 1 mM MgCl2. Rho proteins at 2 mg/ml were comicroinjected with 1 mg/ml human IgG to identify injected cells. Rho vectors were the generous gift of Dr. A. Hall (University College, London, UK).
RhoA GTP-γS and GDP-βS treatments were performed by incubating 2 mg/ml rhoA with 2 mM EDTA and 1 mM GTP-γS or GDP-βS at 30°C for 30 min. Samples were then put on ice, MgCl2 added to a concentration of 15 mM, and dialyzed overnight against H-KCl plus 5 mM MgCl2. The concentration of the injected samples was 1.2 mg/ml. Protein concentration was determined with the BCA protein assay (Sigma Chemical Co.) using BSA as the standard.
Purified botulinum C3 toxin was a kind gift from Dr. K. Aktories (Universitat Freiburg, Germany). C3 was stored at −80°C and thawed and diluted to 1 or 10 μg/ml in H-KCl just before use. C3 was microinjected into serum-starved cells before treating cells with LPA or CS.
In Vivo MT Measurements
Rhodamine-labeled tubulin (R-tubulin) was prepared as described previously (Mikhailov and Gundersen, 1995). Monolayers of serum-starved cells were wounded, allowed to recover for 2 h, and microinjected with 5–6 mg/ml R-tubulin. Cells were rinsed with fresh SFM and the R-tubulin was allowed to incorporate overnight. Fluorescence video microscopy was performed as described previously (Mikhailov and Gundersen, 1995), with the following modifications: cells were perfused with warm 10 mM Hepes-buffered, pH 7.4, phenol red-free, bicarbonate-free DME (Sigma Chemical Co.) containing 5 mg/ml fatty acid–free BSA, 10 mM lactic acid, and Oxyrase (Oxyrase Inc., Ashland, OH; 3 units per 5 ml of medium) to reduce photodamage (Mikhailov and Gundersen, 1995). Images (32 frame average) were captured every 10 s using an ISIT camera (DAGE-MTI), digitized with Image 1 software and stored on an OMDR.
Measurements and dynamics calculations were performed using Image-1 (Universal Imaging Corp.) and Lotus 1,2,3 software, as described (Dhamodharan and Wadsworth, 1995; Mikhailov and Gundersen, 1995). Microtubules in sequential images were traced using a mouse-driven cursor, and the computer calculated the change in position of the ends. The direction was manually recorded. Differences of position of <0.5 μm in sequential images were considered within measurement error and defined as pauses (Sammak and Borisy, 1988; Dhamodharan and Wadsworth, 1995). Microtubule dynamic instability parameters were calculated from image sequences taken at 10-s intervals. Rescue frequency, catastrophe frequency, and dynamicity were determined as described by others (Dhamodharan and Wadsworth, 1995). Values for percent time growing, shortening, or pausing were calculated for individual MTs by computing the time spent in each phase. Statistical comparisons were made using Student's t test.
Cytochalasin D Treatment
Monolayers of serum-starved 3T3 cells were wounded, fed SFM with or without 0.5 μM cytochalasin D (Sigma Chemical Co.), and incubated for 15 min. SFM containing 1 μM LPA was then diluted 1:10 into the medium, mixed well, and incubated for an additional 90 min. Coverslips were fixed and stained as above.
Results
LPA Induces the Formation of Glu MTs in Serum-starved NIH-3T3 Cells
In a previous study (Nagasaki and Gundersen, 1996), we found that removal of LPA from serum caused a contact inhibition–like response (diminished lamellepodia, reduced motility, and loss of stable Glu MTs) in locomoting fibroblasts. The loss of stable, Glu MTs from the lamella of the locomoting fibroblasts upon LPA removal suggested that LPA might be an important factor in regulating the stability of MTs. To determine whether LPA is capable of inducing the formation of stable MTs, we examined the effects of LPA and other lipids on serum-starved NIH-3T3 fibroblasts, which are dependent on added factors to generate stable MTs (Gundersen et al., 1994).
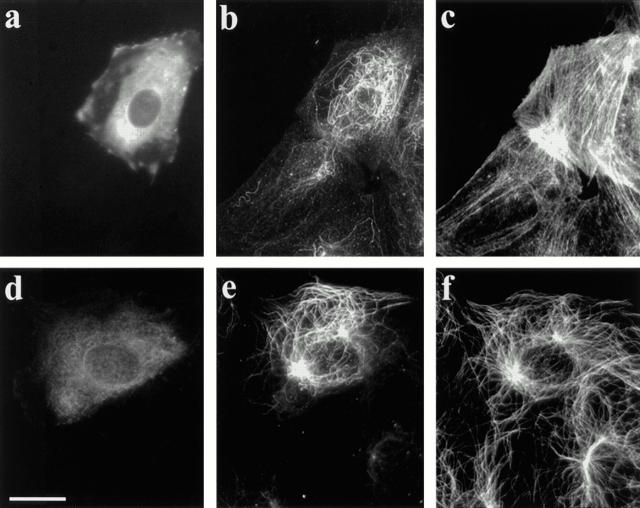
Serum-starved NIH 3T3 cells contained virtually no Glu MTs (Fig. 1 a) but numerous Tyr MTs (Fig. 1 b) by immunofluorescence with Glu and Tyr tubulin specific antibodies as previously reported (Gundersen et al., 1994). When these cells were incubated for 2 h with 1 μM LPA there was a substantial increase in the number of Glu MTs in virtually every cell (Fig. 1 c). LPA did not induce a dramatic increase in the total number of MTs, as represented by the Tyr MTs (Fig. 1 d). The increase in Glu MTs was comparable to that seen with 0.5% CS (Fig. 1 e) and demonstrated that LPA was sufficient to stimulate the formation of Glu MTs. We also detected an increase in Glu tubulin levels, but not total tubulin levels, in LPA- or CS-treated cells by western blot analysis (data not shown; see Gundersen et al., 1994). This confirmed that the increased Glu MT staining was not due to the unmasking of Glu tubulin epitopes, but rather reflected a selective increase in Glu MTs.
Figure 1.
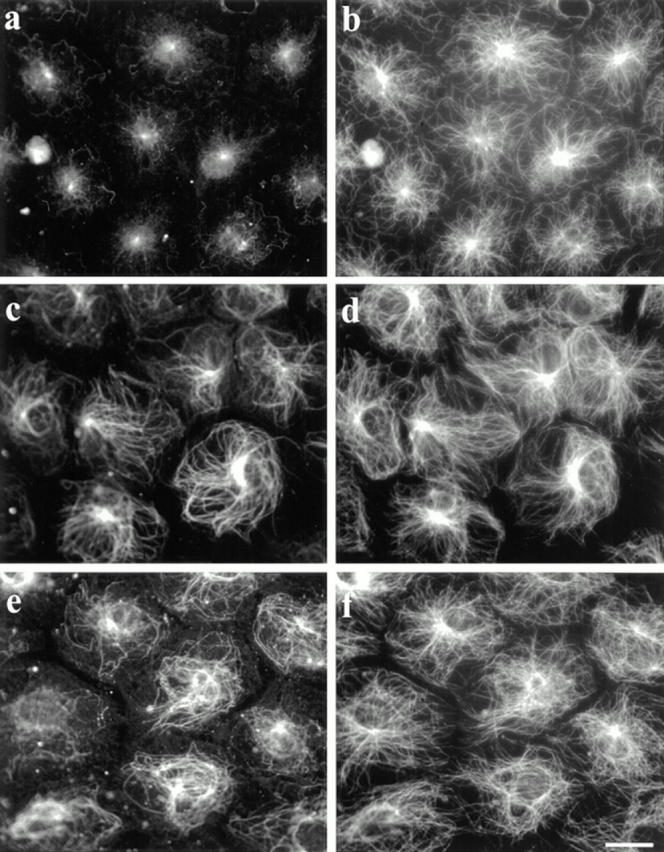
LPA induces Glu MT formation in serum-starved 3T3 fibroblasts. SFM-treated cells were refed SFM (a and b), SFM containing 1 μM LPA (c and d), or SFM containing 0.5% CS (e and f) for 2 h. Cells were fixed and stained with antibodies specific for Glu (a, c, and e) or Tyr (b, d, and f) tubulin. Bar, 20 μm.
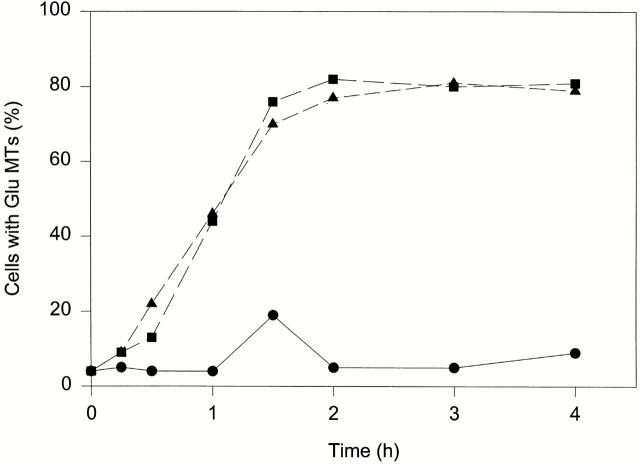
The induction of Glu MTs by LPA was rapid and was nearly identical to that seen with CS. Treatment of serum-starved NIH-3T3 cells with 1 μM LPA for 1 h resulted in a 10-fold increase in the number of cells containing brightly stained Glu MTs (Fig. 2). A significant increase in the number of cells with Glu MTs was detected within 30 min of LPA addition. This rapid induction of Glu MTs distinguishes LPA from other factors that initiate a much slower increase in Glu MTs (Gundersen et al., 1994).
Figure 2.
LPA and CS induce Glu MTs with a similar time course. SFM-treated cells were refed SFM alone (•) or SFM containing 1 μM LPA (▪) or 0.5% CS (▴) and then incubated for the indicated time before fixation and staining for Glu MTs. Scoring was as described in Table I. Results shown are averages of three separate experiments.
The LPA induction of Glu MTs was concentration dependent. A significant increase in the number of cells with Glu MTs was detected with 10 nM LPA, and the maximal response occurred with 100 nM (Table I). The effects of LPA were also specific, as even closely related lipids were ineffective even at 1-10 μM (Table I). Among the lipids tested were PA, which has an additional fatty acid chain; LPI, LPE, LPC, LPG, and LPS, which have head groups; and PAF and LPAF, which contain ether-linked fatty acids but are otherwise structurally similar. Pretreatment of LPA with PLB, which cleaves the acyl side chain of lysophospholipids, abrogated the ability of LPA to induce Glu MTs (Table I). PLB pretreatment of CS also reduced the ability of the serum to induce Glu MTs (Table I). We conclude that LPA can specifically increase Glu MTs in cells and is one of the major serum factors involved in regulating MT stability.
Table I.
Induction of Glu MTs in Serum-starved NIH-3T3 Fibroblasts by LPA and Related Lipids
| Treatment | Concentration | Glu MTs | ||
|---|---|---|---|---|
| μM | ||||
| CS | 0.5% | +++ | ||
| LPA | 1 | +++ | ||
| 0.1 | +++ | |||
| 0.01 | ++ | |||
| 0.001 | + | |||
| PA | 10 | − | ||
| LPC | 10 | − | ||
| LPG | 10 | + | ||
| LPE | 1 | − | ||
| LPI | 1 | − | ||
| LPS | 1 | − | ||
| OAG | 10 | − | ||
| PAF | 10 | − | ||
| LPAF | 10 | + | ||
| CS + PLB* | 0.5% | ± | ||
| LPA + PLB* | 0.1 | − |
SFM-treated cells were refed SFM alone as a control or SFM containing the indicated components at the indicated concentration for 2 h, then stained with antibodies to Glu tubulin. 100 cells were examined and scored for the presence of Glu MTs according to the criteria in Materials and Methods. The response was then ranked according to the following scheme: − = no change from control (<10% of cells with Glu MTs); + = just detectable increase over controls (10–30% with Glu MTs); ++ = half-maximal response (30–70% of cells with Glu MTs); +++ = maximal response (>70% of cells containing Glu MTs).
CS and LPA were treated with 1 U PLB/10 μL CS, and 0.5 U PLB/nmole LPA at 37°C for 2 h (see Materials and Methods).
LPA Induces a Rapid Increase in Stable MTs
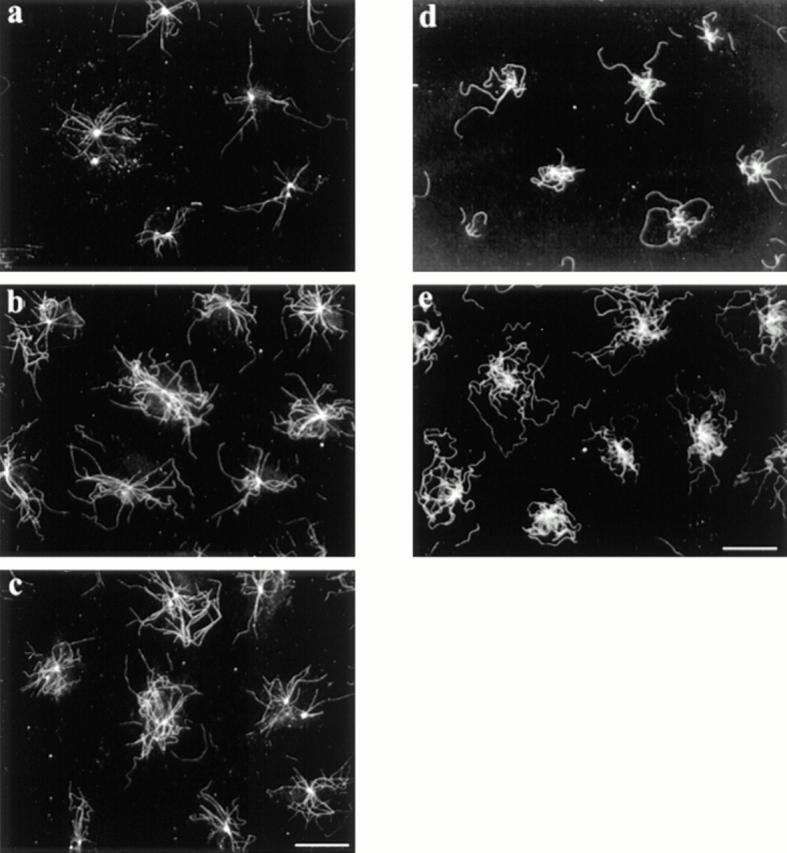
To confirm that the LPA-induced Glu MTs reflected an increase in the number of stabilized MTs rather than solely an increase in detyrosination, we tested the resistance of MTs to nocodazole, which selectively depolymerizes dynamic MTs at concentrations of 1–10 μM (Khawaja et al., 1988). Serum-starved cells were treated with 0.1 μM LPA or 0.5% CS for 5 min, followed by 2 μM nocodazole (still in the presence of LPA or CS) for 30 min to depolymerize dynamic MTs (Fig. 3). Whereas cells in SFM had few MTs after nocodazole treatment (Fig. 3 a), cells incubated with LPA or CS contained numerous nocodazole-resistant MTs (Fig. 3, b and c). These stable MTs also reacted with antibodies to Glu tubulin (not shown). This confirms that LPA induces MT stabilization and suggests that the stabilization induced by LPA is rapid.
In the above experiment, we could not pinpoint the time at which the MTs became stabilized (resistant to nocodazole), since it took 30 min of nocodazole treatment to deplete the cells of the dynamic MTs. To determine when MT stability was achieved, we took advantage of the resistance of stabilized MTs to dilution upon cell permeabilization with detergent (Khawaja et al., 1988). This allowed us to treat cells with LPA briefly and then extract the cells and assess the effects on MTs. Cells were incubated for 5 min with SFM alone or SFM containing 1 μM LPA, then extracted with 0.2% Triton-X 100, and incubated in PEM for 1 h. As shown in Fig. 3 d, this treatment led to depolymerization of virtually all of the MTs in unstimulated cells. In contrast, LPA-treated cells contained a significant number of dilution-resistant MTs (Fig. 3 e). These results confirm that MT stability was rapidly induced by external agents and that LPA was not simply affecting the detyrosination enzyme, tubulin carboxypeptidase. Also, since little Glu staining is detected in cells fixed after 5 min in LPA (not shown), presumably because it takes the carboxypeptidase ∼30 min to generate significant levels of Glu tubulin (see Gundersen et al., 1987), this further confirms that detyrosination is not involved in MT stabilization.
Rho Is Necessary for the Induction of Glu MTs by LPA or Serum
LPA stimulates numerous intracellular signaling cascades through a putative transmembrane receptor (see Moolenar, 1997). Previous work in our laboratory has suggested that many of these pathways were not involved in inducing stable MTs. For example, pertussis toxin did not block CS or LPA stimulation of Glu MTs in serum-starved cells, suggesting that the Gi pathway is not involved (not shown). Protein kinase C activators (octyl-acetyl-glycerol [OAG] or phorbol esters) did not stimulate Glu MT formation (Table I and not shown), and pretreatment of cells with PMA to downregulate protein kinase C did not block LPA induction of Glu MT formation (not shown). Elevating internal calcium levels by microinjecting inositol triphosphate was also ineffective (not shown). In the assay for maintenance of the oriented array of Glu MTs, arachidonic acid had no effect on Glu MTs (Nagasaki and Gundersen, 1996). Thus, a number of the intracellular signaling pathways stimulated by LPA did not seem to be involved in the stabilization of MTs.
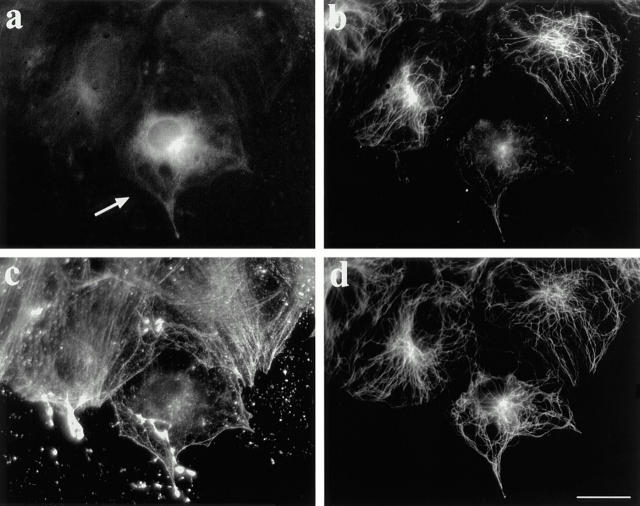
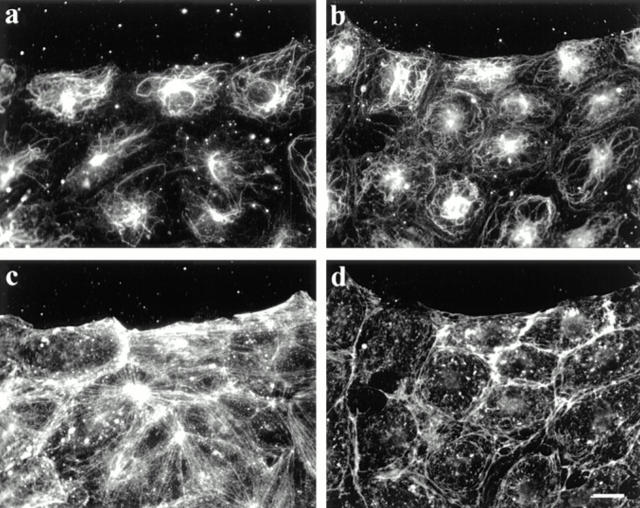
LPA also activates the small GTP-binding protein rho, and in so doing induces the rapid formation of actin stress fibers and focal adhesions (Ridley and Hall, 1992). Several characteristics (rapidity, extent, and dose) of the LPA induction of actin stress fibers were similar to those we found for the induction of MT stability, suggesting that rho may be involved in generating stable MTs. To examine this possibility, we microinjected serum-starved cells with botulinum C3 toxin that ADP-ribosylates and inactivates rhoA (Rubin et al., 1988) and then treated the cells with LPA or serum. Injection of C3 toxin dramatically reduced the capability of the cells to generate Glu MTs after LPA treatment. Most C3-injected cells (65%, n = 189) contained virtually no Glu MTs, as shown in Fig. 4 a. Other C3-injected cells had fewer Glu MTs than uninjected cells. As reported previously (Ridley and Hall, 1992), the C3 toxin also blocked the formation of stress fibers (Fig. 4 c). Importantly, Tyr MTs appeared largely unaffected by C3 toxin (Fig. 4 d). Microinjection of the human IgG marker alone did not block the effect of LPA (not shown). C3 was also sufficient to block induction of Glu MTs in response to 1% CS (not shown). These results show that rho is necessary for induction of Glu MTs by LPA or CS.
Figure 4.
C3 toxin inhibits LPA-induced formation of Glu MTs. Botulinum C3 toxin (1 μg/ml) was microinjected into serum-starved cells along with human IgG as a marker. The medium was then replaced with fresh SFM containing 1 μM LPA and cells were incubated for 90 min. After fixation in −20°C methanol, coverslips were quadrupally stained with antibodies to human IgG (a), Glu tubulin (b), actin (c), and Tyr tubulin (d). Bar, 20 μm.
Active Rho GTPase Stimulates the Formation of Glu MTs in Serum-starved Cells
To determine whether rho was sufficient to stimulate MT stability, we microinjected the constitutively active mutant of rho, val14 rho, into serum-starved 3T3 cells and assayed the cells for Glu MTs. We found that 83% of val14 rho- injected cells (n = 88) showed a dramatic increase in the number of Glu MTs over that in uninjected neighbors (Fig. 5, b and e). As expected, val14 rho also stimulated formation of actin stress fibers (Fig. 5 c). A comparison of the Glu MTs with the Tyr MTs in the injected cells showed that val14 rho induced the stabilization of only a subset of the MTs, since there were many MTs that stained only for Tyr tubulin (Fig. 5, d–f). Consistent with this selective stabilization of a subset of MTs, we did not detect a significant difference in the level of Tyr MTs in injected vs. noninjected cells (Fig. 5 f). The coinjected marker protein, human IgG, had no effect on Glu MTs when microinjected alone (not shown).
Figure 5.
Microinjected val14 rho induces formation of Glu MTs. A wounded monolayer of serum-starved cells was microinjected with val14 rho along with human IgG as a marker and then incubated an additional 90–120 min before fixation and triple immunofluorescence staining (a–c are from one field, d–f are from another). Injected cells were detected with coumarin-conjugated antibodies to human IgG (a and d). Injected cells contain many Glu MTs, whereas uninjected neighboring cells contain few or none (b and e). Val14 rho also induced stress fiber formation, as shown in c with a monoclonal antibody to actin. Tyr MTs were largely unaffected by val14 rho injection (f). Bar, 20 μm.
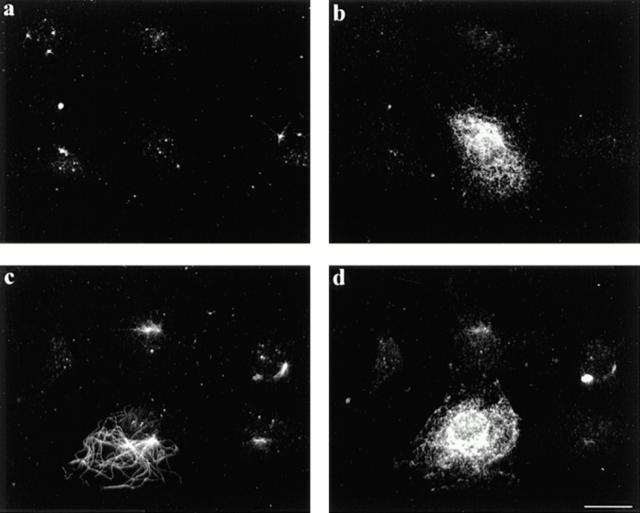
We confirmed the stability of these Glu MTs by testing the resistance of MTs in the val14 rho injected cells to nocodazole (1 h with 2 μM nocodazole). Because it was necessary to extract cells after nocodazole treatment (see Materials and Methods), we comicroinjected a nonextractable marker, IFA antibodies to intermediate filaments, to identify the injected cells (Fig. 6, b and d). Whereas 72% of the val14 rho–injected cells (n = 196) contained nocodazole-resistant MTs (Fig. 6 c), only 20% of control injected cells (n = 92) had nocodazole-resistant MTs (Fig. 6 a). A similar percentage of uninjected cells (18%) had nocodazole resistant MTs, showing that the microinjection alone did not increase the number of cells with stable MTs.
Figure 6.
Microinjected val14 rho induces formation of nocodazole resistant MTs. Serum-starved cells were microinjected with either IFA antibodies (a and b) or IFA antibodies and val14 rho (c and d). Cells were then incubated for 30 min, treated with 2 μM nocodazole for 1 h, and then extracted and fixed. Injected cells were detected with rhodamine-conjugated antibodies to mouse IgG (b and d) and nocodazole-resistant MTs were detected with Glu tubulin antibodies (a and c). Only val14 rho–injected cells contain nocodazole resistant MTs (c). Bar, 20 μm.
The selective stabilization of a subset of MTs in val14 rho–injected cells supports the idea that rho is a bona fide regulator of MT stability in cells. Further support for this notion came from the distribution of Glu MTs in wound edge cells microinjected with val14 rho. Previous studies have shown that the Glu MTs are oriented toward the leading edge of monolayer cells adjacent to an in vitro wound (Gundersen et al., 1989, 1994; Nagasaki et al., 1992). In many wound-edge cells microinjected with val14 rho, we found that the Glu MTs that were formed were localized to the leading edge of the cell (compare Fig. 5, e and f). In fact, at 1 h after treatment, when ∼45% of cells contain Glu MTs with either LPA or rho (see Fig. 2), the proportions of cells with oriented Glu MTs were very similar (76% for val14 rho versus 82% for LPA). Thus, active rho, in the absence of external factors, is sufficient to induce the selective stabilization of MTs with the proper orientation in wound edge cells. These features distinguish rho from many other factors (e.g., taxol and MAPs, see Discussion) that stabilize MTs indiscriminately in cells.
To confirm that active, GTP-bound rho was responsible for the induction of Glu MTs, wild-type recombinant rho was incubated with either GTP-γS or GDP-βS. These nonhydrolyzable guanine nucleotides activate and inhibit rho, respectively. Microinjection of rho–GTP-γS stimulated an increase in Glu MTs and actin stress fibers, while microinjection of the same concentration of rho–GDP-βS had no effect (not shown). These results confirm that the active, GTP-bound form of rho is sufficient to induce formation of Glu MTs in serum-starved cells.
Rho Induces Long Episodes of Pausing in a Subset of MTs
To understand how rho affected MTs, we examined the dynamics of individual MTs in living cells after microinjection of R-tubulin (e.g., see Fig. 7). We compared the dynamics of MTs in serum-starved cells to those treated with LPA, since we showed above that the LPA-induced stabilization of MTs required rho. There was a significant decrease in the dynamicity and a significant increase in percent time paused (from 28 to 42%) in cells treated with LPA (Table II). The growth rate was modestly reduced in LPA, but this difference was less significant (P < 0.05) than those in dynamicity and per cent time paused (P < 0.01). Shrinkage rate and catastrophe and rescue frequencies were all within experimental error. The increase in the mean percent time paused was due mostly to a decrease in the percentage of time spent shortening. These data suggest that LPA may not significantly affect the dynamics of tubulin subunit addition or loss from MTs, but rather may change whether a MT is in an active state (of growing or shrinking) versus an inactive state (see Discussion).
Figure 7.
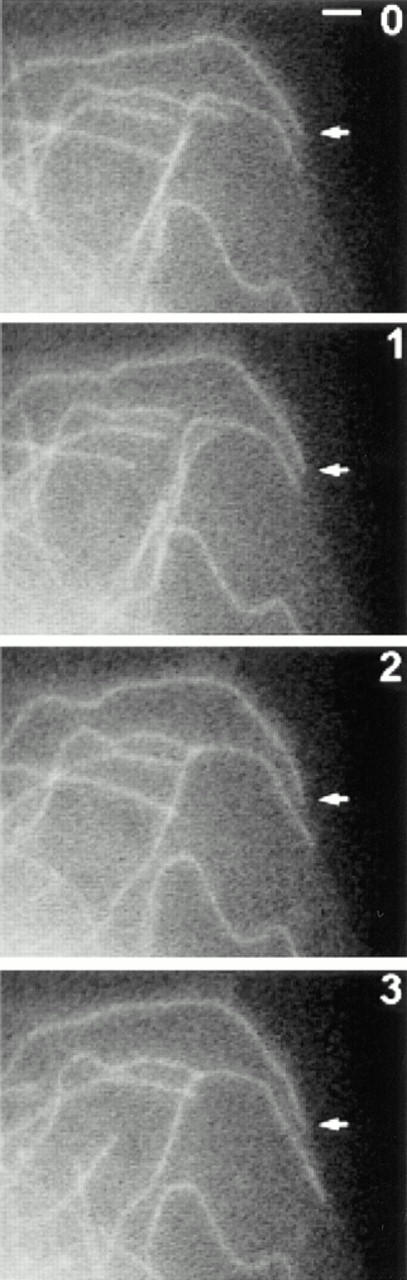
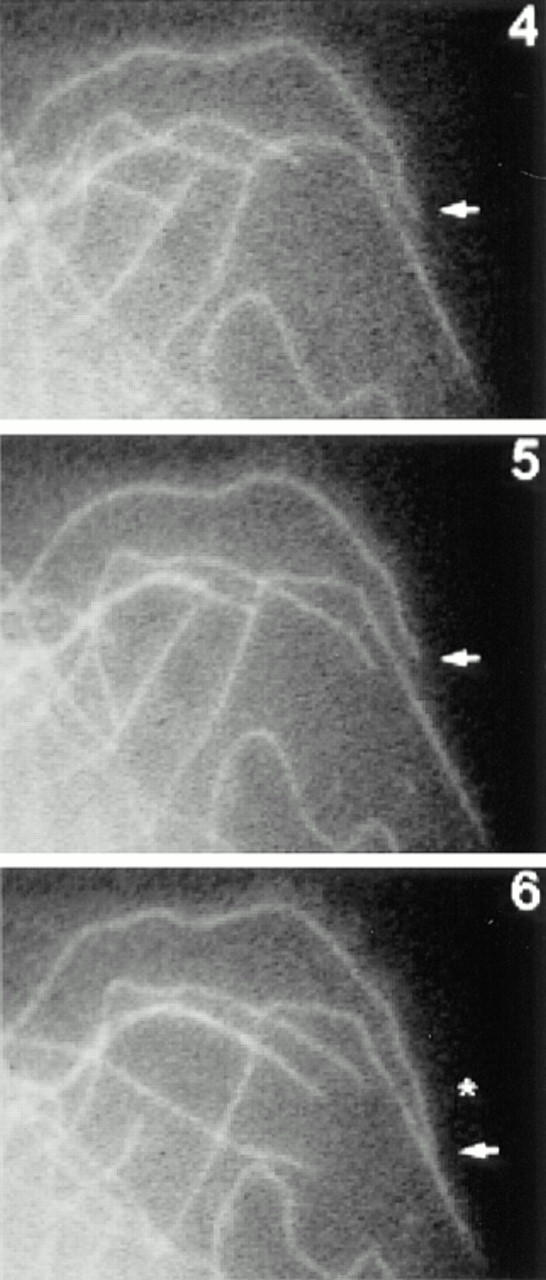
Dynamics of MTs in LPA-treated cells. Serum-starved cells were injected with R-tubulin. The next day cells were treated with 1 μM LPA for 1–2 h and then fluorescent images were acquired at 10-s intervals. Shown are R-tubulin–labeled MTs at the edge of a live cell at 1-min intervals. Many MT ends can be seen to undergo changes in length during the recording. Arrow designates a long-paused MT (see text). An asterisk indicates a change in position of the long-paused MT at 6 min. Bar, 2 μm.
Table II.
MT Dynamics in Serum-free and LPA-treated Cells
| Parameter | SFM | LPA | ||
|---|---|---|---|---|
| Growth rate (μm/min) | 8.3 ± 4.1 | 6.8 ± 2.4* | ||
| Shortening rate (μm/min) | 11.7 ± 5.3 | 10.7 ± 2.8 | ||
| Catastrophe (events/min) | 0.02 ± 0.02 | 0.01 ± 0.01 | ||
| Rescue (events/min) | 0.03 ± 0.03 | 0.03 ± 0.03 | ||
| Dynamicity (events/min) | 0.13 ± 0.07 | 0.09 ± 0.04‡ | ||
| Time growing (%) | 37.5 ± 15.9 | 32.1 ± 15.2 | ||
| Time shortening (%) | 34.5 ± 22.3 | 25.6 ± 12.6 | ||
| Time paused (%) | 28.0 ± 19.5 | 42.3 ± 19.7‡ | ||
| Long-paused MTs (%)‖ | 0 | 13 | ||
| Number of MTs | 53 | 59 |
Data were obtained from 8 cells (SFM) and 6 cells (LPA).
Significant difference at P < 0.05.
Significant difference at P < 0.01.
Long-paused MTs were those paused for greater than 3 min.
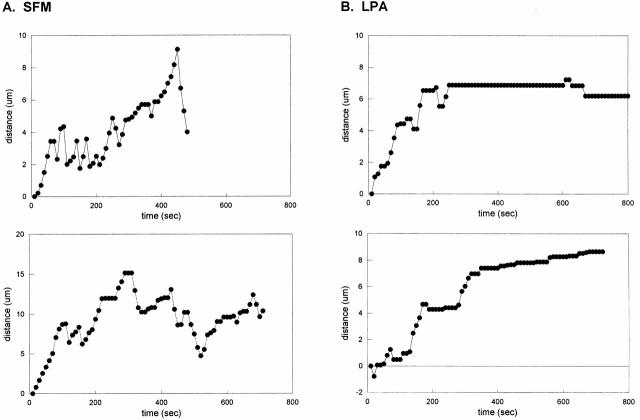
The data above are consistent with our earlier immunofluorescence data suggesting that rho was not dramatically altering the behavior of the bulk MTs, but might affect only a subset of the MTs. To confirm this, we analyzed the data from the time–lapse recordings to see whether the behavior of a subset of MTs was affected. We focused on the length of pauses in individual MTs, since the mean percent time paused showed the greatest difference between serum-free treated and LPA-treated cells. Pauses in individual MTs in serum-free treated cells were very short (usually <1 min) and this can be seen from the plots of the position of individual MT ends over time (“life history” plots; Fig. 8 a shows two typical examples). These MTs undergo typical dynamic instability with periods of growth and shrinkage, punctuated by abrupt transitions between the two states. Pause intervals in these MTs were always <3 min and typically <1 min. Similar results were observed for most of the MTs in LPA-stimulated cells. However, a small percentage of the MTs exhibited strikingly different behaviors with relatively long periods of pause. Life history plots of two of these long-paused MTs from LPA-treated cells are shown in Fig. 8 b. Some of the long-paused MTs did not change position over long periods of time (Fig. 8 b, top; see also Fig. 7), whereas others changed position so slightly that they were below the limit of resolution of our measurements (<0.5 μm/10-s interval) and so were still characterized as pausing (Fig. 8 b, bottom). We do not know whether those in the latter category were actually paused or were just growing very slowly (the growth rates measured over long intervals were in the range of 0.1–0.5 μm/min). It is interesting to note that even the long-paused MTs were not irreversibly stabilized; we observed a number of cases in which long-paused MTs would resume dynamic activity (see Figs. 7 and 8 b).
Figure 8.
Life history plots of MTs in SFM-treated cells and LPA-treated cells. Representative life history plots for typical MTs in control SFM-treated cells (a) and for long-paused MTs in LPA-treated cells (b). Data points indicate the change in position of the end of a single MT at 10-s intervals. MTs in SFM-treated cells undergo frequent transitions between growth and shortening phases, whereas long-paused MTs in LPA-treated cells do not grow or shrink for long periods of time.
To get an idea of how many MTs in the LPA-treated cells exhibited this kind of behavior, we defined long-paused MTs as those exhibiting at least one 3-min pause interval during a recording, since this was longer than any of the pauses seen in serum-free cells. 13% of the MTs in the LPA-treated cells were long-paused according to this criterion. This is consistent with the hypothesis that there is a distinct subset of MTs affected by rho. When we examined the location of the long-paused MTs in cells, we observed an additional feature of these MTs that suggested that they were a separate subset of MTs: virtually all of them were in close proximity to the edge of the cell and presumably near the plasma membrane (see Fig. 7, arrow). This raises the possibility that the factors contributing to the long pauses of these MTs are localized in or near the plasma membrane (see Discussion).
Actin Stress Fibers Are Not Necessary for Glu MT Formation
In our experiments with C3 and val14 rho, the induction or inhibition of Glu MT formation was always accompanied by induction or inhibition of actin stress fiber formation (see Figs. 4 and 5). To determine whether the formation of actin stress fibers was required for the generation of Glu MTs, we treated cells with cytochalasin D before LPA addition to prevent the formation of stress fibers. Cytochalasin D at 0.5 μM was sufficient to completely block the induction of stress fibers, but did not affect the formation of Glu MTs (compare Fig. 9, a and b). Cytochalasin D alone did not induce the formation of stable, Glu MTs. These results show that the formation of actin stress fibers is not a prerequisite for the induction of Glu MTs.
Figure 9.
Cytochalasin D treatment does not block LPA-induced Glu MT formation. A wounded monolayer of serum-starved cells was treated for 15 min with SFM alone (a and c) or SFM containing 0.5 μM cytochalasin D (b and d). LPA was then diluted into the medium to a final concentration of 0.1 μM, and cells were incubated an additional 90 min before fixation and staining. Cytochalasin D blocked LPA-induced stress fibers (d) but not Glu MTs (b). Bar, 20 μm.
Discussion
We have demonstrated that LPA is a major serum factor that can generate rapid changes in the stability of a subset of MTs in serum-starved NIH 3T3 fibroblasts. These effects are mediated by rho GTPase, as inhibition of rho with C3 toxin blocks the LPA effect and constitutively active rho mimics it. The stabilization of MTs induced by rho was selective in that only a subset of the MTs were affected. In many wound-edge cells, the MTs stabilized by active rho were oriented toward the wound edge, showing that a signaling pathway stimulated by active rho is capable of recapitulating the polarized stabilization of MTs in wound edge cells observed with LPA or CS. This is clearly distinct from the global stabilization of MTs induced when MAPs are introduced into cells by microinjection (Drubin and Kirschner, 1986; Dhamodharan and Wadsworth, 1995) or transfection (Kanai et al., 1989; Bramblett et al., 1993). Other reagents that stabilize MTs, like taxol (Gundersen et al., 1987; Mikhailov, A., and G.G. Gundersen, manuscript submitted for publication) and nonhydrolyzable analogues of GTP (Wehland and Sandoval, 1983), appear to affect all MTs in the cell. Rho then is the first molecule identified that participates in the selective stabilization of MTs.
The rapidity of the LPA stabilization of MTs was surprising. In as little as 5 min after application of LPA, we were able to detect MT stability with our dilution assay (Fig. 3). This is a much more rapid change in MT dynamics than has been measured previously, and suggests that the MT system is capable of responding to environmental stimuli as rapidly as the actin system (Condeelis, 1993). Thus MTs, like actin, have the capacity to respond to transient environmental cues. At this point, we have not tried to measure more rapid change in MT dynamics, although it may be possible to detect such changes by directly observing MTs in cells immediately after addition of LPA.
Our direct observations of MT dynamics in LPA-treated cells showed that the primary effect of rho activation on MTs was on whether a MT was involved in dynamic behavior rather than on the parameters of dynamic instability (growth and shortening rates and transition frequencies) that characterize dynamic behavior. The largest difference detected in the average parameters of MTs were a reduction in the time spent pausing and in dynamicity, an overall measure of MT activity. A smaller difference was also detected in the growth rate of MTs, but this was less significant. At the level of individual MTs, we found a subset of MTs that exhibited unusually long pauses, and these account for much of the decreases in the average time spent pausing and dynamicity measured for the entire population. The decreased dynamicity and the small decrease in growth rate could both be a reflection of the increase in pausing. LPA-treated cells undergo fewer transitions, thereby reducing the dynamicity. And because we used a relatively long sampling interval of 10 s, MTs in LPA-treated cells could be undergoing pauses of less than 10 s that would give the appearance of slow growth.
Other groups have reported long-paused MTs in other cells (Cassimeris et al., 1988; Schulze and Kirschner, 1988; Shelden and Wadsworth, 1993) but this marks the first time that the long pauses can be attributed to the activation of a specific signal transduction pathway. We think that the long-paused MTs correspond to Glu MTs because both populations are a subset of the MTs and both are stimulated by LPA and rho. The long pausing events we have observed could clearly contribute to the longevity of the MTs, but several of these 3–7 min long-paused events would be necessary for a MT to become a Glu MT (see above).
How are MTs selectively stabilized by active rho? At this point we cannot draw a detailed molecular mechanism for rho stabilization of MTs. However, by combining our current data with the abundant literature on how small GTPases in the ras superfamily work, we can propose a model with certain defined elements. Rho, like other small GTPases, is isoprenylated in vivo and this is required for its function (Mohr et al., 1990; Kranenburg et al., 1997). This finding and studies showing that rho is translocated to the plasma membrane upon stimulation (Takaishi et al., 1995) suggest that rho is active when associated with the plasma membrane. We suggest that the active, membrane-bound rho locally stimulates a MT stabilizing factor, which in turn stabilizes dynamic MTs whose excursions carry them into the region. This “molecular flypaper” model is attractive since it can account for the selective stabilization of MTs and is consistent with our observations that the long-paused MTs are localized near the plasma membrane.
Rho, like other small GTPases, is thought to be active only when it is in the GTP-bound state. Thus, when rho hydrolyzes its bound GTP, the activation of the MT-stabilizing factor would be turned off and the MT would become dynamic again. Thus, rho may act as a switch between the dynamic and stabilized states of MTs. The longevity of the stabilized state would depend on how long rho remained in the GTP-bound form, and this could be influenced by other factors such as GTPase-activating proteins and guanine nucleotide dissociation inhibitors. In our study we did observe different intervals of pause in the long-paused MTs, but whether this reflects rho GTP hydrolysis is currently unknown.
How is the stabilization of the MT achieved? One possibility is that the stabilizing factor may decrease MT dynamics by sterically capping the end of the MT, since in another study we have found evidence that Glu MTs in TC-7 epithelial cells are physically capped (Infante, A., M. Stein, Y. Zhai, G.G. Borisy, and G.G. Gundersen, manuscript submitted for publication), but other mechanisms are not ruled out by our data. An examination of the known downstream effectors of rho does not reveal an obvious candidate for such a stabilizing factor. Downstream effectors have been identified by two hybrid screens or by affinity chromatography and share the common property of binding specifically to the GTP-bound form of rho. Among this group of proteins are several protein kinases (Leung et al., 1995; Amano et al., 1996; Ishizaki et al., 1996; Matsui et al., 1996; Watanabe et al., 1996), a lipid kinase (Ren et al., 1996), a protein phosphatase-targeting subunit (Kimura et al., 1996), the kinesin-anchoring protein kinectin (Hotta et al., 1996), and a number of proteins of unknown function that may act as adaptor proteins given their protein–protein interaction domains (Madaule et al., 1995; Reid et al., 1996; Watanabe et al., 1996). None of these effectors appear to have a MT-binding domain similar to those described for other MT-interacting proteins. It is possible that rho induces MT stability by activating an intermediate or series of intermediates. Kinectin is an intriguing candidate because it is thought to anchor kinesin to vesicles involved in protein trafficking (Burkhardt, 1996). One of the kinases, rho kinase (ROKα), has been shown recently to be sufficient for triggering stress fiber formation (Leung et al., 1996; Amano et al., 1997). It will be interesting to determine if one or more of these affect MT stability.
From our study and previous work (Ridley and Hall, 1992; Hall, 1994), it is now clear that rho regulates both the actin and MT systems, and in this sense coordinates the activities of these two cytoskeletal systems. Is active rho stimulating each system independently or are the rho stimulated effects on one system dependent on rho effects on the other? We showed here that cytochalasin pretreatment did not prevent LPA from stimulating Glu MT formation, even though it blocked stress fiber formation. This implies that rho-stimulated MT stabilization occurs independently of rho-stimulated effects on actin filaments, although one needs to be careful in interpreting the cytochalasin experiments, since cytochalasin does not completely deplete cells of actin filaments. The converse situation is not as clear. A recent report showed that breaking down MTs with nocodazole in serum-starved fibroblasts induced the formation of stress fibers (Bershadsky et al., 1996) and we have observed similar results in our own experiments (Gundersen, G.G., unpublished observations). Induction of stress fibers by nocodazole may be dependent on active rho, as C3 toxin can block the effect (Enomoto, 1996). This and earlier work (Danowski, 1989) suggest that MTs can affect the actin system. Further studies will be necessary to determine the relationship between the rho-induced changes in MTs and actin filaments.
MT stabilization may be an important component of the rho-dependent response in those systems that have been shown to involve rho. For example, C3 toxin has been shown to inhibit cell motility in sperm, neutrophils, and Swiss 3T3 cells, as well as scatter factor–induced motility in keratinocyes (Stasia et al., 1991; Takaishi et al., 1993, 1995). Rho has also been implicated in endocytosis (Lamaze et al., 1996) and cytokinesis (Takaishi et al., 1995). The possibility that some of the rho effects in these cases are due in part to regulated changes in MT stability will need to be addressed in the future.
Acknowledgments
We thank J. Lessard, J. Kilmartin, K. Aktories, and A. Hall for generously providing reagents.
Abbreviations used in this paper
- Glu
detyrosinated
- LPA
lysophosphatidic acid
- MT
microtubule
- PLB
phospholipase B
- SFM
serum-free medium
- Tyr
tyrosinated
Footnotes
T.A. Cook was supported by an National Institutes of Health training grant through the Integrated Program in Cellular, Molecular, and Biophysical Studies. T. Nagasaki was supported by a Damon Runyon-Walter Winchell Postdoctoral Fellowship. This research was supported by a grant from the American Cancer Society to G.G. Gundersen.
Address all correspondence to Gregg Gundersen, Department of Anatomy and Cell Biology, Columbia University, 630 West 168th Street, New York, NY 10032. Tel.: (212) 305-1899. Fax: (212) 305-3970. E-mail: ggg1@columbia.edu
References
- Amano M, Mukai H, Ono Y, Chihara K, Matsui T, Hamajima Y, Okawa K, Iwamatsu A, Kaibuchi K. Identification of a putative target for rho as the serine-threonine kinase protein kinase N. Science. 1996;271:648–650. doi: 10.1126/science.271.5249.648. [DOI] [PubMed] [Google Scholar]
- Amano M, Chihara K, Kimura K, Fukata Y, Nakamura N, Matsuura Y, Kaibuchi K. Formation of actin stress fibers and focal adhesions enhanced by rho-kinase. Science. 1997;275:1308–1311. doi: 10.1126/science.275.5304.1308. [DOI] [PubMed] [Google Scholar]
- Baas PW, Black MM. Individual microtubules in the axon consist of domains that differ in both composition and stability. J Cell Biol. 1990;111:495–509. doi: 10.1083/jcb.111.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bershadsky A, Chausobsky A, Becker E, Lyubimova A, Geiger B. Involvement of microtubules in the control of adhesion-dependent signal transduction. Curr Biol. 1996;6:1279–1289. doi: 10.1016/s0960-9822(02)70714-8. [DOI] [PubMed] [Google Scholar]
- Bramblett GT, Goedert M, Jakes R, Merrick SE, Trojanowski JQ, Lee VM-Y. Abnormal tau phosphorylation at ser396 in Alzheimer's disease recapitulates development and contributes to reduced microtubule binding. Neuron. 1993;10:1089–1099. doi: 10.1016/0896-6273(93)90057-x. [DOI] [PubMed] [Google Scholar]
- Burridge K, Chrzanowska-Wodnicka M, Zhong C. Focal adhesion assembly. Trends Cell Biol. 1997;7:342–347. doi: 10.1016/S0962-8924(97)01127-6. [DOI] [PubMed] [Google Scholar]
- Bulinski JC, Gundersen GG. Stabilization and posttranslational modification of microtubules during cellular morphogenesis. BioEssays. 1991;13:285–293. doi: 10.1002/bies.950130605. [DOI] [PubMed] [Google Scholar]
- Burkhardt JK. In search of membrane receptors for microtubule-based motors—is kinectin a kinesin receptor? . Trends Cell Biol. 1996;6:127–131. doi: 10.1016/0962-8924(96)20002-9. [DOI] [PubMed] [Google Scholar]
- Cassimeris L, Pryer NK, Salmon ED. Real-time observations of microtubule dynamic instability in living cells. J Cell Biol. 1988;107:2223–2231. doi: 10.1083/jcb.107.6.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Condeelis J. Life at the leading edge: the formation of cell protrusions. Annu Rev Cell Biol. 1993;9:411–444. doi: 10.1146/annurev.cb.09.110193.002211. [DOI] [PubMed] [Google Scholar]
- Danowski BA. Fibroblast contractility and actin organization are stimulated by microtubule inhibitors. J Cell Sci. 1989;93:255–266. doi: 10.1242/jcs.93.2.255. [DOI] [PubMed] [Google Scholar]
- Dhamodharan R, Wadsworth P. Modulation of microtubule dynamic instability in vivo by brain microtubule associated proteins. J Cell Sci. 1995;108:1679–1689. doi: 10.1242/jcs.108.4.1679. [DOI] [PubMed] [Google Scholar]
- Drubin DG, Kirschner MW. Tau protein function in living cells. J Cell Biol. 1986;103:2739–2746. doi: 10.1083/jcb.103.6.2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enomoto T. Microtubule disruption induces the formation of actin stress fibers and focal adhesions in cultured cells: possible involvement of the rho signal cascade. Cell Struct Funct. 1996;21:317–326. doi: 10.1247/csf.21.317. [DOI] [PubMed] [Google Scholar]
- Gundersen GG, Bulinski JC. Microtubule arrays in differentiated cells contain elevated levels of a post-translationally modified form of tubulin. Eur J Cell Biol. 1986;42:288–294. [PubMed] [Google Scholar]
- Gundersen GG, Bulinski JC. Selective stabilization of microtubules oriented toward the direction of cell migration. Proc Natl Acad Sci USA. 1988;85:5946–5950. doi: 10.1073/pnas.85.16.5946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen GG, Kalnoski MH, Bulinski JC. Distinct populations of microtubules: tyrosinated and nontyrosinated alpha-tubulin are distributed differently in vivo. Cell. 1984;38:779–789. doi: 10.1016/0092-8674(84)90273-3. [DOI] [PubMed] [Google Scholar]
- Gundersen GG, Khawaja S, Bulinski JC. Postpolymerization detyrosination of α-tubulin: a mechanism for subcellular differentiation of microtubules. J Cell Biol. 1987;105:251–264. doi: 10.1083/jcb.105.1.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen GG, Khawaja S, Bulinski JC. Generation of a stable, posttranslationally modified microtubule array is an early event in myogenic differentiation. J Cell Biol. 1989;109:2275–2288. doi: 10.1083/jcb.109.5.2275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gundersen GG, Kim I, Chapin CJ. Induction of stable microtubules in 3T3 fibroblasts by TGF-β and serum. J Cell Sci. 1994;107:645–649. doi: 10.1242/jcs.107.3.645. [DOI] [PubMed] [Google Scholar]
- Gurland G, Gundersen GG. Protein phosphatase inhibitors induce the selective breakdown of stable microtubules in fibroblasts and epithelial cells. Proc Nat Acad Sci USA. 1993;90:8827–8831. doi: 10.1073/pnas.90.19.8827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurland G, Gundersen GG. Stable, detyrosinated microtubules function to localize vimentin intermediate filaments in fibroblasts. J Cell Biol. 1995;131:1275–1290. doi: 10.1083/jcb.131.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A. Small GTP-binding proteins and the regulation of the actin cytoskeleton. Annu Rev Cell Biol. 1994;10:31–54. doi: 10.1146/annurev.cb.10.110194.000335. [DOI] [PubMed] [Google Scholar]
- Hotta K, Tanaka K, Mino A, Kohno H, Takai Y. Interaction of the rho family small G proteins with kinectin, an anchoring protein of the kinesin motor. Biochem Biophys Res Commun. 1996;225:69–74. doi: 10.1006/bbrc.1996.1132. [DOI] [PubMed] [Google Scholar]
- Houliston E, Maro B. Posttranslational modification of distinct microtubule subpopulations during cell polarization and differentiation in the mouse preimplantation embryo. J Cell Biol. 1989;108:543–551. doi: 10.1083/jcb.108.2.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishizaki T, Maekawa M, Fujisawa K, Okawa K, Iwamatsu A, Fujita A, Watanabe N, Saito Y, Kakisuka A, Morii N, Narumiya S. The small GTP-binding protein rho binds to and activates a 160 kDa Ser/Thr protein kinase homologous to myotonic dystrophy kinase. EMBO (Eur Mol Biol Organ) J. 1996;15:1885–1893. [PMC free article] [PubMed] [Google Scholar]
- Kanai Y, Takemura R, Oshima T, Mori H, Ihara Y, Yanagisaw M, Masaki Y, Hirokawa N. Expression of multiple tau isoforms and microtubule bundle formation in fibroblasts transfected with a single tau cDNA. J Cell Biol. 1989;109:1173–1184. doi: 10.1083/jcb.109.3.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khawaja S, Gundersen GG, Bulinski JC. Enhanced stability of microtubules enriched in detyrosinated tubulin is not a direct function of detyrosination level. J Cell Biol. 1988;106:141–149. doi: 10.1083/jcb.106.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilmartin JV, Wright B, Milstein C. Rat monoclonal antitubulin antibodies derived by using a nonsecreting rat cell line. J Cell Biol. 1982;93:576–582. doi: 10.1083/jcb.93.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by rho and rho-associated kinase (rho-kinase) Science. 1996;273:245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- Kranenburg O, Poland M, Gebbink M, Oomen L, Moolenar WH. Dissociation of LPA-induced cytoskeletal contraction from stress fiber formation by differential localization of RhoA. J Cell Sci. 1997;110:2417–2427. doi: 10.1242/jcs.110.19.2417. [DOI] [PubMed] [Google Scholar]
- Kreis T. Microtubules containing detyrosinated tubulin are less dynamic. EMBO (Eur Mol Biol Organ) J. 1987;6:2597–2606. doi: 10.1002/j.1460-2075.1987.tb02550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamaze C, Chuang T-H, Terlecky LJ, Bokoch GM, Schmid SL. Regulation of receptor-mediated endocytosis by Rho and Rac. Nature. 1996;382:177–179. doi: 10.1038/382177a0. [DOI] [PubMed] [Google Scholar]
- Lessard JL. Two monoclonal antibodies to actin: one muscle selective and one generally reactive. Cell Motil Cytoskeleton. 1988;10:349–362. doi: 10.1002/cm.970100302. [DOI] [PubMed] [Google Scholar]
- Leung T, Manser E, Tan L, Lim L. A novel serine/threonine kinase binding the ras-related rho A GTPase which translocates the kinase to peripheral membranes. J Biol Chem. 1995;270:29051–29054. doi: 10.1074/jbc.270.49.29051. [DOI] [PubMed] [Google Scholar]
- Leung T, Chen X-Q, Manser E, Lim L. The p160 rhoA-binding kinase ROKa is a member of a kinase family and is involved in the reorganization of the cytoskeleton. Mol Cell Biol. 1996;16:5313–5327. doi: 10.1128/mcb.16.10.5313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRae TH, Langdon CM, Freeman FA. Spatial distribution of posttranslationally modified tubulins in polarized cells of developing Artemia. Cell Motil Cytoskeleton. 1991;18:189–203. doi: 10.1002/cm.970180305. [DOI] [PubMed] [Google Scholar]
- Madaule P, Furuyashiki T, Reid T, Ishizaki T, Watanabe G, Morii N, Narumiya S. A novel partner for the GTP-bound forms of rho and rac. FEBS Lett. 1995;377:243–248. doi: 10.1016/0014-5793(95)01351-2. [DOI] [PubMed] [Google Scholar]
- Matsui T, Amano M, Yamamoto T, Chihara K, Nakafuku M, Ito M, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Rho-associated kinase, a novel serine/threonine kinase, as a putative target for the small GTP-binding protein rho. EMBO (Eur Mol Biol Organ) J. 1996;15:2208–2216. [PMC free article] [PubMed] [Google Scholar]
- Mikhailov A, Gundersen GG. Centripetal transport of microtubules in motile cells. Cell Motil Cytoskeleton. 1995;32:173–186. doi: 10.1002/cm.970320303. [DOI] [PubMed] [Google Scholar]
- Mohr C, Just I, Hall A, Aktories K. Morphological alterations of Xenopus oocytes induced by valine-14 p21 rho depend on isoprenylation and are inhibited by Clostridium botulinum C3 ADP-ribosyltransferase. FEBS Lett. 1990;275:168–172. doi: 10.1016/0014-5793(90)81464-y. [DOI] [PubMed] [Google Scholar]
- Moolenar WH, Kranenburg O, Postma FR, Zondag GCM. Lysophosphatidic acid: G-protein signaling and cellular responses. Curr Opin Cell Biol. 1997;9:168–173. doi: 10.1016/s0955-0674(97)80059-2. [DOI] [PubMed] [Google Scholar]
- Nagasaki T, Gundersen GG. Depletion of lysophosphatidic acid triggers a loss of oriented detyrosinated microtubules in motile fibroblasts. . J Cell Sci. 1996;109:2461–2469. doi: 10.1242/jcs.109.10.2461. [DOI] [PubMed] [Google Scholar]
- Nagasaki T, Chapin CJ, Gundersen GG. Distribution of detyrosinated microtubules in motile NRK fibroblasts is rapidly altered upon cell-cell contact: implications for contact inhibition of locomotion. Cell Motil Cytoskeleton. 1992;23:45–60. doi: 10.1002/cm.970230106. [DOI] [PubMed] [Google Scholar]
- Nagasaki T, Liao G, Gundersen GG. Isolated plasma membranes induce loss of oriented detyrosinated microtubules and other contact inhibition-like responses in migrating NRK cells. J Cell Sci. 1994;107:3413–3423. doi: 10.1242/jcs.107.12.3413. [DOI] [PubMed] [Google Scholar]
- Pepperkok R, Bre MH, Davoust J, Kreis TE. Microtubules are stabilized in confluent epithelial cells but not in fibroblasts. J Cell Biol. 1990;111:3003–3012. doi: 10.1083/jcb.111.6.3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piperno G, Bre MH, Davoust J, Kreis TE. Microtubules containing acetylated α tubulin in mammalian cells in culture. J Cell Biol. 1987;104:289–302. doi: 10.1083/jcb.104.2.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruss RM, Mirsky R, Raff MC, Thorpe R, Dowding AJ, Anderton BH. All classes of IFs share a common antigenic determinant defines by a monoclonal antibody. Cell. 1981;27:419–428. doi: 10.1016/0092-8674(81)90383-4. [DOI] [PubMed] [Google Scholar]
- Reid T, Furuyashiki T, Ishizaki T, Watanabe G, Fujiwawa K, Morii N, Madaule P, Narumiya S. Rhotekin, a new putative target for rho bearing homology to a serine/threonine kinase, PKN, and rhophilin in the rho-binding domain. J Biol Chem. 1996;271:13556–13560. doi: 10.1074/jbc.271.23.13556. [DOI] [PubMed] [Google Scholar]
- Ren X-D, Bokoch GM, Traynor-Kaplan A, Jenkins GJ, Anderson RA, Schwartz MA. Physical association of the small GTPase rho with a 68-kDa phosphatidylinositol 4-phosphate 5-kinase in Swiss 3T3 cells. Mol Biol Cell. 1996;7:435–442. doi: 10.1091/mbc.7.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- Ridley AJ, Hall A. The small GTP-binding protein rho regulates the assembly of focal adhesions and actin stress fibers in response to growth factors. Cell. 1992;70:389–399. doi: 10.1016/0092-8674(92)90163-7. [DOI] [PubMed] [Google Scholar]
- Rubin EJ, Gill DM, Boquet P, Popoff MR. Functional modification of a 21-kilodalton G protein when ADP-ribosylated by exoenzyme C3 of Clostridium botulinum. Mol Cell Biol. 1988;8:418–426. doi: 10.1128/mcb.8.1.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammak PJ, Borisy GG. Direct observation of microtubule dynamics in living cells. Nature. 1988;332:724–726. doi: 10.1038/332724a0. [DOI] [PubMed] [Google Scholar]
- Saxton WM, Stemple DL, Leslis RJ, Salmon ED, Zavortink M, McIntosh JR. Tubulin dynamics in cultured mammalian cells. J Cell Biol. 1984;99:2175–2186. doi: 10.1083/jcb.99.6.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze E, Kirschner M. Microtubule dynamics in interphase cells. J Cell Biol. 1986;102:1020–1031. doi: 10.1083/jcb.102.3.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze E, Kirschner M. New features of microtubule behaviour observed in vivo. Nature. 1988;334:356–359. doi: 10.1038/334356a0. [DOI] [PubMed] [Google Scholar]
- Schulze E, Asai DJ, Bulinski JC, Kirschner M. Posttranslational modifications and microtubule stability. J Cell Biol. 1987;105:2167–2177. doi: 10.1083/jcb.105.5.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelden E, Wadsworth P. Observation and quantification of individual microtubule behavior in vivo: microtubule dynamics are cell-type specific. J Cell Biol. 1993;120:935–945. doi: 10.1083/jcb.120.4.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DB, Johnson KS. Single-step purification of polypeptides expressed in Escherichia coli as fusion proteins with glutathione-S-transferase. Gene. 1988;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Stasia M-J, Jouan A, Bourmeyster N, Boquet P, Vignais PV. ADP-ribosylation of a small size GTP-binding protein in bovine neutrophils by the C3 exoenzyme of Clostridium botulinum and effect on the cell motility. Biochem Biophys Res Commun. 1991;180:615–622. doi: 10.1016/s0006-291x(05)81110-6. [DOI] [PubMed] [Google Scholar]
- Takaishi K, Kikuchi A, Kuroda S, Kotani K, Sasaki T, Takai Y. Involvement of rho p21 and its inhibitory GDP/GTP exchange protein (rho GDI) in cell motility. Mol Cell Biol. 1993;13:72–79. doi: 10.1128/mcb.13.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaishi K, Sasaki T, Kato M, Yamochi W, Kuroda S, Nakamura T, Takeichi M, Takai Y. Translocation of activated Rho from the cytoplasm to membrane ruffling area, cell-cell adhesion sites and cleavage furrows. Oncogene. 1995;11:39–48. [PubMed] [Google Scholar]
- Warn RM, Harrison A, Planques T, Robert-Nicoud N, Wheland J. Distribution of microtubules containing post-translationally modified alpha-tubulin during Drosophilaembryogenesis. Cell Motil Cytoskeleton. 1990;17:34–45. doi: 10.1002/cm.970170106. [DOI] [PubMed] [Google Scholar]
- Watanabe G, Saito Y, Madaule P, Ishizaki T, Fujisawa K, Morii N, Mukai H, Ono Y, Kakizuka A, Narumiya S. Protein kinase N (PKN) and PKN-related protein rhophilin as targets of small GTPase rho. Science. 1996;271:645–648. doi: 10.1126/science.271.5249.645. [DOI] [PubMed] [Google Scholar]
- Webster DR, Gundersen GG, Bulinski JC, Borisy GG. Differential turnover of tyrosinated and detyrosinated microtubules. Proc Natl Acad Sci USA. 1987;84:9040–9044. doi: 10.1073/pnas.84.24.9040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster DR, Wehland J, Weber K, Borisy GG. Detyrosination of α tubulin does not stabilize microtubules in vivo. J Cell Biol. 1990;111:113–122. doi: 10.1083/jcb.111.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehland J, Sandoval IV. Cells injected with guanosine 5′-[α,β- methylene]triphosphate, an α,β-nonhydrolyzable analog of GTP, show anomalous patterns of tubulin polymerization affecting cell translocation, intracellular movement, and the organization of Golgi elements. Proc Natl Acad Sci USA. 1983;80:1938–1941. doi: 10.1073/pnas.80.7.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]