Abstract
There is a growing body of evidence to implicate reversible tyrosine phosphorylation as an important mechanism in the control of the adhesive function of cadherins. We previously demonstrated that the receptor protein tyrosine phosphatase PTPμ associates with the cadherin–catenin complex in various tissues and cells and, therefore, may be a component of such a regulatory mechanism (Brady-Kalnay, S.M., D.L. Rimm, and N.K. Tonks. 1995. J. Cell Biol. 130:977– 986). In this study, we present further characterization of this interaction using a variety of systems. We observed that PTPμ interacted with N-cadherin, E-cadherin, and cadherin-4 (also called R-cadherin) in extracts of rat lung. We observed a direct interaction between PTPμ and E-cadherin after coexpression in Sf9 cells. In WC5 cells, which express a temperature-sensitive mutant form of v-Src, the complex between PTPμ and E-cadherin was dynamic, and conditions that resulted in tyrosine phosphorylation of E-cadherin were associated with dissociation of PTPμ from the complex. Furthermore, we have demonstrated that the COOH-terminal 38 residues of the cytoplasmic segment of E-cadherin was required for association with PTPμ in WC5 cells. Zondag et al. (Zondag, G., W. Moolenaar, and M. Gebbink. 1996. J. Cell Biol. 134: 1513–1517) have asserted that the association we observed between PTPμ and the cadherin–catenin complex in immunoprecipitates of the phosphatase arises from nonspecific cross-reactivity between BK2, our antibody to PTPμ, and cadherins. In this study we have confirmed our initial observation and demonstrated the presence of cadherin in immunoprecipitates of PTPμ obtained with three antibodies that recognize distinct epitopes in the phosphatase. In addition, we have demonstrated directly that the anti-PTPμ antibody BK2 that we used initially did not cross-react with cadherin. Our data reinforce the observation of an interaction between PTPμ and E-cadherin in vitro and in vivo, further emphasizing the potential importance of reversible tyrosine phosphorylation in regulating cadherin function.
The cadherins are a major family of calcium-dependent, homophilic cell adhesion molecules that are concentrated at specialized contact points in the cell termed adherens junctions (for review see Gumbiner, 1996). The cadherins are transmembrane proteins that possess an extracellular segment, characterized by the presence of calcium-binding motifs, and an intracellular segment that is highly conserved between members of the family (for review see Takeichi, 1995). The intracellular segment serves as the site of interaction with proteins termed catenins (α-, β-, and γ-catenin) (for review see Gumbiner, 1995). It appears that β-catenin and γ-catenin/ plakoglobin, which are related to the product of the segment polarity gene armadillo, bind directly to the cytoplasmic segment of cadherin, whereas α-catenin, which is related to the cytoskeleton-associated protein vinculin, binds to β/γ catenin and functions to link the complex to the actin cytoskeleton (for review see Gumbiner, 1995). The intracellular, catenin-binding segment of the cadherins is essential for adhesion; mutations in this segment can disrupt adhesion even in the presence of an intact extracellular segment (Nagafuchi and Takeichi, 1988; Ozawa et al., 1989). Thus, cadherin-mediated adhesion requires the intact cadherin–catenin complex and association with the actin cytoskeleton.
Mutations have been detected in components of the cadherin–catenin complex in several tumors, and destabilization of cadherin-mediated adhesion has been linked with invasion and malignant progression (for reviews see Birchmeier and Behrens, 1994, Birchmeier, 1995). In addition, the junctions in normal cells are dynamic and tyrosine phosphorylated rapidly and reversibly (Volberg et al., 1991). There is now a growing body of evidence to link the loss of adhesive function and the destabilization of adherens junctions with changes in the state of phosphorylation of tyrosyl residues in components of the cadherin– catenin complex (for reviews see Birchmeier and Behrens, 1994; Brady-Kalnay and Tonks, 1995). Expression of the protein tyrosine kinase (PTK)1 v-Src causes aberrant tyrosine phosphorylation that results in disruption of adherens junctions, in the absence of an effect on desmosomes and tight junctions (Warren and Nelson, 1987). Similarly, treatment of MDCK cells with vanadate, a broad specificity inhibitor of members of the protein tyrosine phosphatase (PTP) family of enzymes, results in tyrosine phosphorylation of proteins at adherens junctions and the deterioration of junctional structures (Volberg et al., 1992). Furthermore, β-catenin was observed to be heavily phosphorylated on tyrosyl residues in rat fibroblasts transformed by v-Src, coincident with changes in cell–cell aggregation (Matsuyoshi et al., 1992). In addition, these effects were abrogated by the PTK inhibitor herbimycin A and promoted by the PTP inhibitor vanadate. Interestingly, a temperature-sensitive mutant of v-Src destabilized cadherin-dependent adhesion at the permissive temperature, coincident with tyrosine phosphorylation of E-cadherin, β-catenin, or cytoskeletal components (Behrens et al., 1993; Takeda et al., 1995). The EGF receptor and Met, the receptor for scatter factor, phosphorylate components of the cadherin–catenin complex, and the EGF receptor has been observed to bind directly to β-catenin and to associate with the cadherin–catenin complex in epithelial cells (Hoschuetzky et al., 1994; Ochiai et al., 1994; Shibamoto et al., 1994). These observations suggest that the integrity of adherens junctions is regulated in part at the level of reversible tyrosine phosphorylation that results from the coordinated and competing actions of PTKs and PTPs. Therefore, a prerequisite to understanding fully the significance of tyrosine phosphorylation in the control of cadherin–catenin function will be the identification and characterization of specific PTKs and PTPs that associate with and modify the phosphorylation status of these proteins.
In examining the physiological significance of the tyrosine phosphorylation of the cadherin–catenin complex, we have obtained data that implicate the receptor PTP, PTPμ, as a potential regulator of this complex. PTPμ is characterized by an extracellular segment that contains one MAM (Meprin/A5/PTPμ) domain, one immunoglobulin domain, and four fibronectin type III repeats (Gebbink et al., 1991). This combination of motifs suggested that PTPμ may function in cell–cell adhesion. In fact, we (Brady-Kalnay et al., 1993) and others (Gebbink et al., 1993; Sap et al., 1994) demonstrated that PTPμ, and the structurally related PTPκ, participate in homophilic binding interactions. Ectopic expression of recombinant PTPμ in Sf9 cells induces aggregation of these normally nonadhesive cells (Brady-Kalnay et al., 1993; Gebbink et al., 1993). Subsequently, we determined that the homophilic binding site within the extracellular segment of PTPμ resides in the immunoglobulin domain (Brady-Kalnay and Tonks, 1994). In addition, it has been shown that the MAM domain plays a role in cell–cell aggregation possibly by “sorting” of PTPμ from closely related molecules, such as PTPκ, during cell aggregation (Zondag et al., 1995). More recent data suggest that one aspect of PTPμ function in vivo may be to affect cell adhesion by regulating the adhesive properties of the cadherin–catenin complex. We observed that in the MvLu lung cell line, which expresses PTPμ, catenins, and cadherins endogenously, immunoprecipitates of PTPμ contained cadherins, α-catenin, and β-catenin (Brady-Kalnay et al., 1995). In fact, at least 80% of the total cellular cadherins appeared to be associated with PTPμ in MvLu cells. Similarly, complexes between PTPμ and cadherins were detected in rat heart, lung, and brain tissues, where PTPμ is expressed at high levels (Brady-Kalnay et al., 1995). The results of binding studies in vitro suggest that this association results from a direct interaction between the intracellular segment of PTPμ and the intracellular domain of E-cadherin (Brady-Kalnay et al., 1995). Our results raised the possibility that a component of the cadherin–catenin complex may be an endogenous substrate for PTPμ. Subsequently, several other laboratories have reported the observation of interactions between cadherin–catenin complexes and both receptor and nontransmembrane PTPs in a variety of cell systems (Balsamo et al., 1996; Fuchs et al., 1996; Kypta et al., 1996; Aicher et al., 1997; Cheng et al., 1997).
In this paper, we report the results of a further characterization of the association between PTPμ and cadherin– catenin complexes. We have identified the cadherins that associate with PTPμ in vivo from lysates of rat lung as N-cadherin, E-cadherin, and cadherin-4 (also called R-cadherin). We have used a number of systems to characterize further the association of PTPμ and E-cadherin and have demonstrated that the COOH-terminal 38 residues of the cytoplasmic segment of E-cadherin is necessary for binding of PTPμ. Furthermore, we have shown that conditions that result in tyrosine phosphorylation of E-cadherin also result in dissociation of PTPμ from the complex. A recent article from Zondag et al. (1996) argues that PTPμ does not associate with cadherins and suggests that our observations are the result of nonspecific cross-reactivity between BK2, our antipeptide antibody to PTPμ, and cadherins. We present several lines of data to substantiate the validity of our original observation of the association between PTPμ and the cadherin–catenin complex and to refute the assertion of Zondag et al. that our observation arises from nonspecific antibody cross-reactivity.
Materials and Methods
Expression Vectors and Cell Lines
Expression vectors for wild-type and mutant murine E-cadherins were either generously provided by Masatoshi Takeichi (Kyoto University, Japan) (Nagafuchi and Takeichi, 1988; Nose et al., 1988) or described in Chen et al. (1997). WC5 cells were derived from neonatal rat cerebellar cells by transformation with a mutant of Rous sarcoma virus (LA90) that is temperature-sensitive for transformation (Giotta and Cohn, 1981). At the permissive temperature, 33°C, v-Src is active as a PTK, and the cells are transformed. At 39°C, the v-Src PTK is relatively inactive, and the cells display an epithelial morphology. WC5 cells were maintained at 33°C in DME (GIBCO BRL, Gaithersburg, MD) containing 10% fetal bovine serum and 10 μg/ml gentamicin. The isolation of stable cell lines expressing full-length and deletion constructs of E-cadherin has been described (Chen et al., 1997). At least two clonal cell lines for each E-cadherin deletion mutant were tested in each of the experiments described. MvLu cells were cultured as described previously (Brady-Kalnay et al., 1995). The cell line MCF10AneoN was generously provided by Bonnie Sloan (Wayne State University, Detroit, MI) and cultured as described in Kinch et al. (1997). Sf9 cells (CRL 1711; American Type Culture Collection, Rockville, MD) were maintained at 27°C in Grace's Insect Medium Supplemented (GIBCO BRL) containing 10% fetal bovine serum and 10 μg/ml gentamicin.
Expression in Sf 9 Cells
The following recombinant baculoviruses were used: (a) expressing full-length PTPμ, described previously (Brady-Kalnay et al., 1993); (b) expressing E-cadherin, generated using the BaculoGold Transfection System (Invitrogen Corp., Carlsbad, CA) after ligation of a full-length 2.7-kb cDNA (recovered by restriction digestion of the pBATEM2 plasmid, generously provided by M. Takeichi) into pVL1392 (Invitrogen Corp.); and (c) expressing β-catenin, generously provided by R. Kypta (University of California, San Francisco, CA). Sf9 cells were infected with the recombinant baculoviruses either singly or in pairwise combinations as previously described (Brady-Kalnay et al., 1993). Cells were harvested 48 h after infection by centrifugation at 3,000 g for 5 min and processed for immunoprecipitation and immunoblotting as described below.
Antibodies
Hybridoma cells expressing a rat monoclonal antibody against the extracellular domain of E-cadherin, ECCD-2 (Shiroyashi et al., 1986), were generously provided by Masatoshi Takeichi. Conditioned medium from these cells was used in our experiments. A mouse monoclonal antibody to E-cadherin, antibodies to β-catenin, and antiphosphotyrosine antibody (PY20) were purchased from Transduction Labs (Lexington, KY). In the course of our experiments involving antibody recognition of E-cadherin fusion proteins, we determined that the anti–E-cadherin antibody from Transduction Labs recognized the juxtamembrane half of the intracellular segment. Pan-cadherin antibodies (monoclonal and polyclonal), which react with the conserved COOH-terminal 24 amino acids of the cadherin cytoplasmic segment, were purchased from Sigma Chemical Co. (St. Louis, MO). The cadherin-4 antibody (120A) was generously provided by ICOS Corp. (Seattle, WA). Antibody to N-cadherin (BD7873) was generously provided by Dr. J. Hemperly at Becton Dickinson Labs (Res. Triangle Park, NC) and has been described previously (Payne et al., 1996). Monoclonal antibodies to the intracellular segment of PTPμ (SK series) and monoclonal antibody BK-2, generated against a peptide derived from the extracellular segment of PTPμ, have been described previously (Brady-Kalnay et al., 1993; Brady-Kalnay and Tonks 1994). Polyclonal antibodies to glutathione S transferase (GST) (Brady-Kalnay et al., 1995) and the FG6 monoclonal antibody to PTP1B (Flint et al., 1993) have been described previously.
Binding Assays In Vitro
The GST fusion protein of the extracellular segment of PTPμ (EXTRA-PTPμ) has been described previously (Brady-Kalnay et al., 1993). Two GST/E-cadherin fusion proteins were generated that contain either amino acids 572–631 (the juxtamembrane-half of the cytoplasmic segment, JM E-cad) or amino acids 648–729 (the COOH-terminal, catenin-binding portion of the intracellular segment, CB E-cad). Proteins were expressed in Escherichia coli and purified using glutathione Sepharose (Brady-Kalnay et al., 1995). In slot blot analyses, purified protein samples were adsorbed to nitrocellulose mounted in a slot blot apparatus (Bio-Rad Laboratories, Hercules, CA). The nitrocellulose strip was blocked in 5% nonfat dry milk in TTBS (20 mM Tris-Cl, pH 7.5, 660 mM NaCl, 0.05% Tween-20) and then incubated with primary antibody for 16 h at 4°C. The blot was washed in TTBS and then developed using horseradish peroxidase–conjugated secondary antibodies and Enhanced Chemiluminescence reagents (Amersham Corp., Arlington Heights, IL).
Preparation of Samples for Immunoprecipitation
Triton-soluble lysates of rat lung and MvLu cells were prepared as described (Brady-Kalnay et al., 1995). WC5 cells were lysed in 20 mM Tris, pH 7.5, 2 mM CaCl2, 1% Triton X-100, 5 μg/ml leupeptin, 5 μg/ml aprotinin, 1 mM benzamidine, 200 μM phenyl arsine oxide, 1 mM vanadate, and 0.1 mM molybdate). The buffer was supplemented with 150 mM NaCl for lysis of Sf9 cells. For immunoprecipitation, antibodies were incubated with protein A or protein G beads (Pharmacia Biotech, Piscataway, NJ) for 2 h at room temperature and then washed three times with PBS (9.5 mM phosphate, 137 mM NaCl, pH 7.5) before addition to cell lysates. Purified monoclonal antibodies were used at 0.6 mg of IgG/ml beads, ascites fluid was used at 1 mg of IgG/ml beads, and polyclonal serum was used at 3 mg of IgG/ml beads. Immunoprecipitates were prepared from 200–400 μg of a Triton-soluble lysate of WC5 cells. The immunoprecipitates were washed four times in lysis buffer, and the bound material was eluted by addition of 100 μl of 2× sample buffer and heating for 5 min at 95°C. The proteins were separated by electrophoresis on 6 or 8% SDS polyacrylamide gels and transferred to nitrocellulose or polyvinyl difluoride for immunoblotting.
Results
PTPμ Interacts with Distinct Members of the Cadherin Superfamily
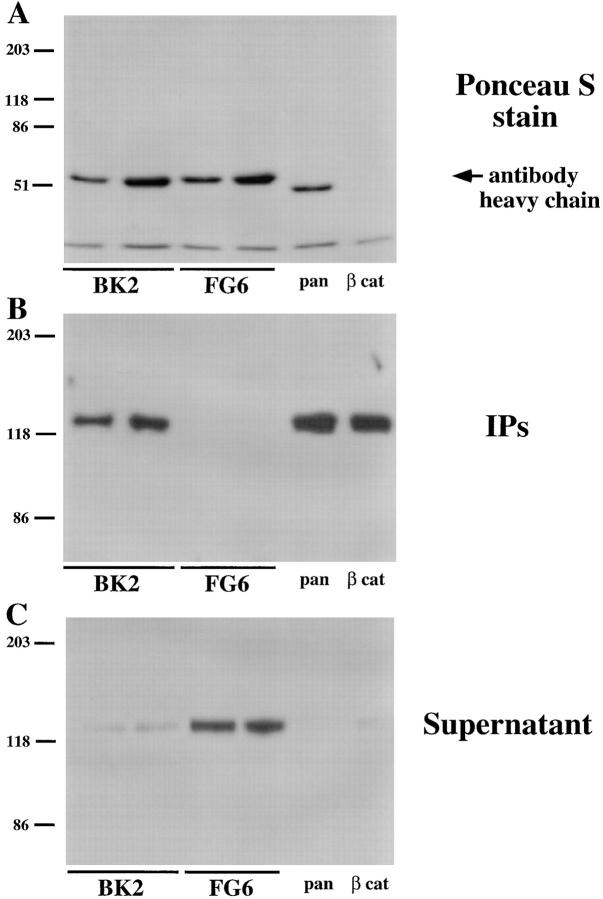
In our initial paper (Brady-Kalnay et al., 1995), we reported that immunoprecipitates of PTPμ from lysates of MvLu cells contained components of the cadherin–catenin complex. Our data indicated that at least 80% of the cadherin in MvLu cell lysates was cleared after immunoprecipitation with antibodies to PTPμ. These results have been called into question (Zondag et al., 1996). To address the reproducibility of this observation, we performed a series of immunoprecipitations from lysates of MvLu cells. We used two different concentrations of anti-PTPμ antibody BK2 or an isotype-matched antibody, FG6, to an unrelated PTP, PTP1B (Flint et al., 1993) as a negative control. In addition, the pan-cadherin antibody and an antibody to β-catenin were included as two positive controls. The relative amounts of antibody heavy chain in each immunoprecipitate are shown in Fig. 1 A. The anti-PTPμ antibody BK2 coimmunoprecipitated cadherin from MvLu cell lysates, to an extent comparable to that seen with the anticadherin and anti–β-catenin antibodies. In contrast, the isotype-matched control antibody to PTP1B did not immunoprecipitate cadherin from MvLu cells at either concentration (Fig. 1 B). In addition, after immunoprecipitation with the various antibodies, we immunoblotted the supernatant that remained to determine the quantity of cadherin that was not precipitated. The results were in agreement with the recovery of cadherin in the immunoprecipitates (Fig. 1 B). Cadherin was cleared from the lysates by immunoprecipitation with anti-PTPμ antibody BK2, to a similar extent as observed in the two positive control immunoprecipitates with pan-cadherin and anti– β-catenin antibodies (Fig. 1 C). These data confirm that the majority of cadherin can be recovered in a complex with PTPμ from MvLu cell lysates. In contrast, control anti-PTP1B antibodies did not clear cadherin from the supernatant (Fig. 1 C).
Figure 1.
Coimmunoprecipitation of PTPμ and cadherin from lysates of MvLu cells. Lysates of MvLu cells were subjected to immunoprecipitation with two different concentrations of anti-PTPμ antibody BK2 or an isotype-matched antibody to PTP1B (FG6), as well as the pan-cadherin and anti–β-catenin antibody. The relative amounts of antibody heavy chain in each immunoprecipitate are shown in a Ponceau S stain of the immunoblot (A). Immunoblots using pan-cadherin antibody were performed on the immunoprecipitates (B). The quantity of cadherin remaining in the supernatant after immunoprecipitation was assessed by immunoblotting (C). The results illustrate that the majority of cadherin in the lysate coimmunoprecipitated with PTPμ.
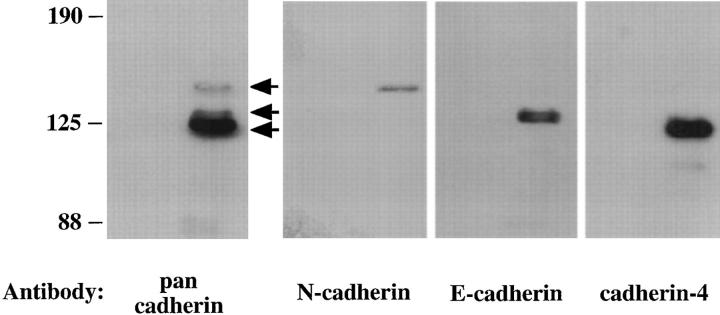
To identify specific cadherins that associate with PTPμ, we performed a series of experiments using extracts of rat lung that express PTPμ endogenously. Immunoprecipitates of PTPμ from rat lung extracts contained three major types of cadherins that were recognized by the pan-cadherin antibody (Fig. 2, arrows). Using antibodies to specific cadherin family members, we determined that PTPμ associated with N-cadherin, E-cadherin, and cadherin-4 (also known as R-cadherin) (Fig. 2).
Figure 2.
Association of PTPμ with N-cadherin, E-cadherin, and cadherin-4 in extracts of rat lung. Extracts of rat lung were immunoprecipitated with mouse IgG (first lane) or BK2 antibody to PTPμ (second lane). These immunoprecipitates were immunoblotted and probed with the pan-cadherin antibody, the N-cadherin antibody, the E-cadherin antibody, or antibody to cadherin-4. Arrows indicate the position of the three bands detected with the pan-cadherin antibody. The data indicate that PTPμ interacts with three distinct members of the cadherin family.
Reconstitution of the Interaction between PTPμ and E-cadherin in Sf 9 Cells
A major contention of the paper by Zondag et al. (1996) was that the association we observed between PTPμ and cadherin was an artifact arising from nonspecific cross- reactivity between the anti-PTPμ antibody, BK2, and cadherins. We have now addressed this issue in a variety of systems.
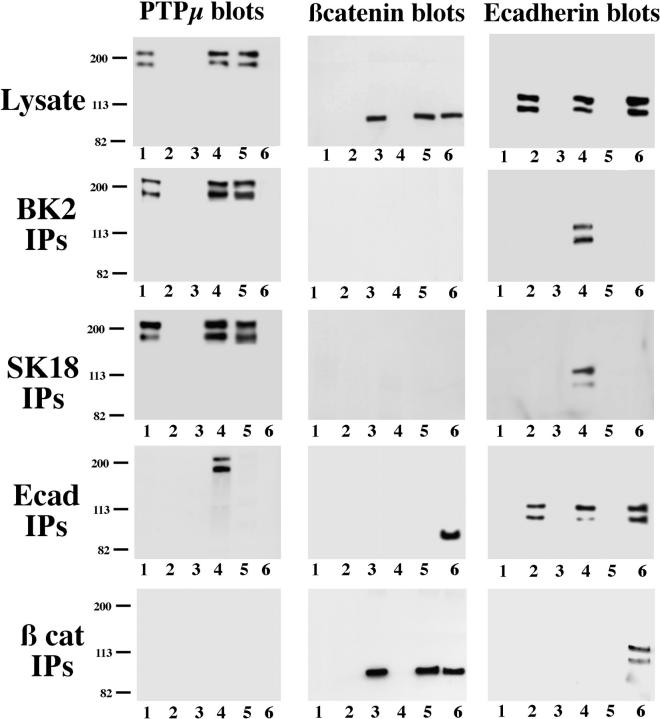
We have reconstituted the complex in Sf9 cells by expression of the individual components using recombinant baculoviruses. PTPμ, E-cadherin, and β-catenin were expressed singly or in pairwise combinations, and expression was verified by immunoblotting cell lysates with the appropriate antibodies (Fig. 3, Lysate). E-cadherin was recovered in anti-PTPμ immunoprecipitates, prepared using the BK2 antibody, only from cells in which both proteins were coexpressed (Fig. 3, BK2 IPs, Ecadherin blots, lane 4). The BK2 antibody did not precipitate E-cadherin from cell lysates in the absence of PTPμ, thus ruling out the possibility that the antibody recognized E-cadherin nonspecifically (Fig. 3, BK2 IPs, Ecadherin blots, lane 2). Similar observations were made when we examined immunoprecipitates with the anti-PTPμ antibody BK2 from lysates of MCF-10A cells, which express large amounts of E-cadherin but do not express detectable levels of PTPμ. Even in the presence of substantial quantities of E-cadherin and the inclusion of large quantities of the antibody, BK2 did not precipitate E-cadherin from lysates of MCF10A cells (data not shown). When the experiment was repeated using a distinct antibody to PTPμ, SK18, which recognizes an epitope in the intracellular portion of the enzyme, the same observation was made. E-cadherin was recovered in anti-PTPμ immunoprecipitates only from lysates of cells coexpressing the phosphatase together with E-cadherin and not cells expressing E-cadherin alone (Fig. 3, SK18 IPs). Furthermore, the formation of a complex was also revealed by the reverse immunoprecipitation/blotting strategy, in that PTPμ was recovered in immunoprecipitates of E-cadherin, but only from lysates of cells in which both proteins were expressed (Fig. 3, Ecad IPs, PTPμ blots, lane 4).
Figure 3.
Reconstitution of the interaction between PTPμ and E-cadherin in Sf9 cells. Using recombinant baculoviruses, PTPμ, E-cadherin, and β-catenin were expressed singly (lane 1–3, respectively) or in pairwise combinations, PTPμ + E-cadherin (lane 4), PTPμ + β-catenin (lane 5), and E-cadherin + β-catenin (lane 6) in Sf9 cells. Cells were lysed, and 1 μg of lysate protein was blotted directly to confirm expression of the individual proteins. PTPμ was expressed primarily in the full-length form that was not proteolytically processed. Immunoprecipitates were prepared from 40 μg of cell lysate using the antibodies indicated on the left of the figure and blotted with either anti-PTPμ antibody SK15 anti–E-cadherin antibody ECCD-2, or anti–β-catenin antibody (Transduction Labs), as indicated across the top of the figure.
The data also illustrate that PTPμ did not interact with β-catenin in this system (see Fig. 3, anti–β-catenin blots of BK2 and SK18 immunoprecipitates and anti-PTPμ blots of β-catenin immunoprecipitates), whereas β-catenin was recovered in immunoprecipitates of E-cadherin (Fig. 3, Ecad IPs and β cat IPs), indicating that the protein was produced in a conformation appropriate for complex formation. This observation is consistent with our previous results from blot-overlay assays, which revealed a direct interaction between PTPμ and E-cadherin in vitro (Brady-Kalnay et al., 1995) and indicates that the binding of PTPμ to E-cadherin is not mediated by β-catenin.
These data reveal that PTPμ/E-cadherin complexes are recovered by immunoprecipitation with two distinct antibodies to the phosphatase or with antibody to E-cadherin. In addition, anti-PTPμ antibodies did not precipitate E-cadherin in the absence of the phosphatase. Therefore, it is highly unlikely that this result could be explained by nonspecific antibody cross-reactivity.
The PTPμ/E-cadherin Complex Is Dynamic In Vivo
WC5 is a Rous sarcoma virus–transformed, rat cerebellar cell line that expresses a temperature-sensitive mutant of the v-Src PTK. When grown at the nonpermissive temperature (39°C), the Src PTK displays little activity, and the cells manifest properties of astrocytes (Giotta and Cohn, 1981). In contrast, when grown at the permissive temperature (33°C), Src is active and the cells are transformed. The expression of endogenous PTPμ, both the unprocessed (200 kD) and proteolytically processed (100 kD) forms of the enzyme, and ectopically expressed E-cadherin was not affected by switching between permissive and nonpermissive temperatures (Fig. 4 A).
Figure 4.
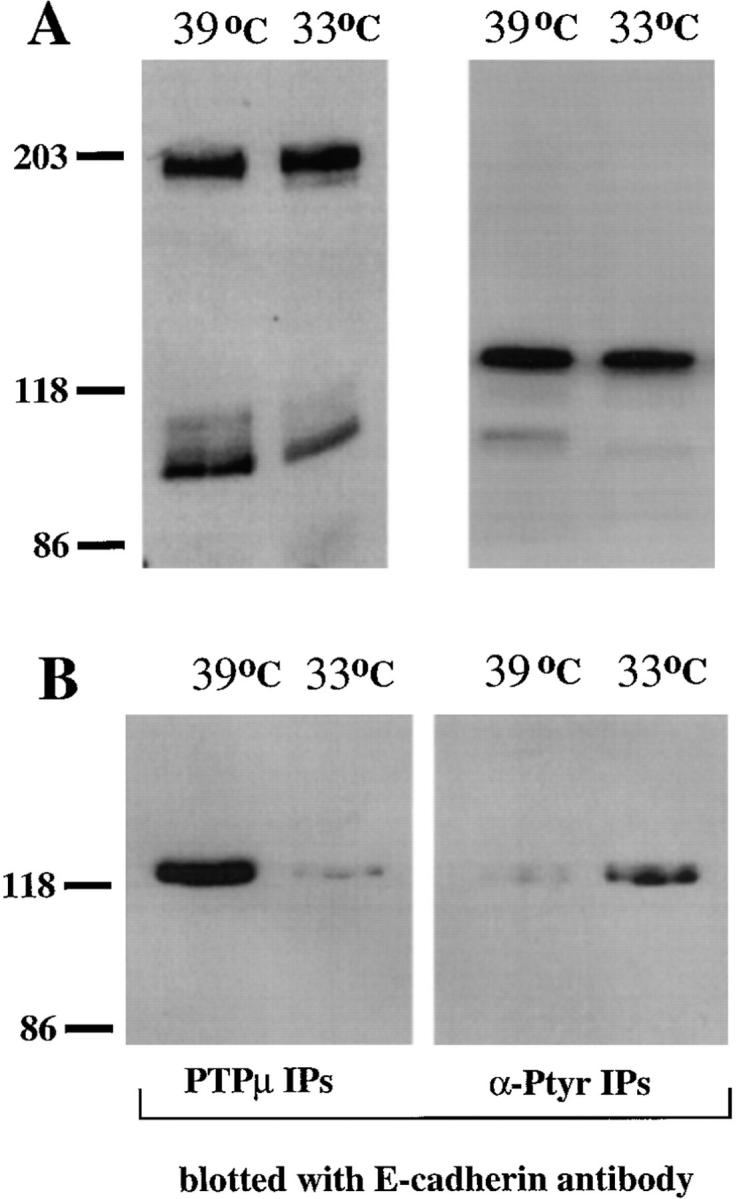
Inverse correlation between tyrosine phosphorylation of E-cadherin and association with PTPμ. (A) Immunoblots of lysates of WC5 cells ectopically expressing full-length E-cadherin grown at either 39 or 33°C. The left panel shows a blot with anti-PTPμ antibody SK15, and the right panel shows a blot with ECCD-2 antibody to E-cadherin. The data illustrate that the levels of PTPμ and E-cadherin are unaltered by the shift in temperature. (B) Immunoprecipitates of WC5 cell lysates prepared in Triton-containing buffer, using either anti-PTPμ antibody BK2 or antiphosphotyrosine antibody PY20. The immunoprecipitates were blotted with the ECCD-2 antibody to E-cadherin.
We used the WC5 cell line that expresses full-length E-cadherin to assess the effect of tyrosine phosphorylation on the PTPμ/cadherin complex by comparing the extent to which E-cadherin coimmunoprecipitated with PTPμ at the permissive and nonpermissive temperatures. As shown in Fig. 4, PTPμ antibodies coimmunoprecipitated E-cadherin well at the nonpermissive temperature (39°C) but poorly at the permissive temperature (33°C) for the Src PTK (Fig. 4 B), despite the fact that expression of E-cadherin was apparently unaltered at 39°C compared with 33°C (Fig. 4 A). Interestingly, E-cadherin was immunoprecipitated with antiphosphotyrosine antibodies at 33°C but to a lesser extent at 39°C. Under harsh detergent conditions (RIPA buffer), we observed that E-cadherin was tyrosine phosphorylated directly at the permissive temperature (data not shown). Therefore, our data indicate an inverse correlation between the presence of PTPμ in the cadherin–catenin complex and the phosphorylation of tyrosyl residues in E-cadherin. The fact that a complex between PTPμ and E-cadherin was detected at 39°C but not at 33°C, using identical conditions for cell lysis and immunoprecipitation at each temperature, indicates that the complex we observe is dynamic. Furthermore, although the potential existence of a phosphorylation-sensitive, cross-reacting epitope is not formally excluded, these data also indicate that it is unlikely that the complex we detect can be explained by nonspecific antibody cross-reactivity.
Identification of the Catenin-binding Domain within the Intracellular Segment of E-cadherin as the Site of Interaction with PTPμ
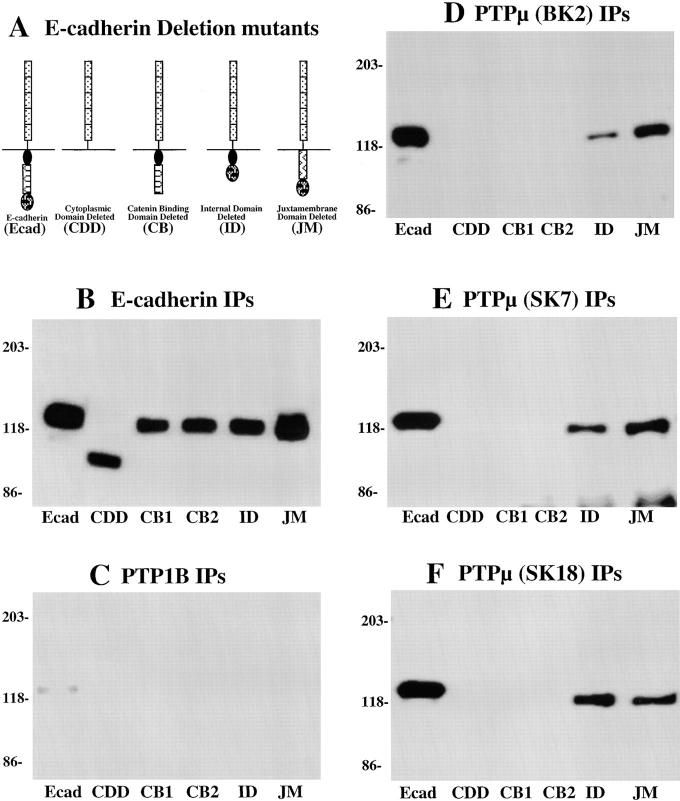
In our previous study, we demonstrated that E-cadherin interacts with PTPμ both in vitro and in vivo. Therefore, we set out to identify the binding site for PTPμ in E-cadherin. In our initial report, we demonstrated that the intracellular segment of PTPμ interacted directly with the intracellular segment of E-cadherin (Brady-Kalnay et al., 1995). To determine more precisely the location of the PTPμ-binding site within the cytoplasmic segment of E-cadherin, we used the WC5 cells, which express PTPμ endogenously but do not express endogenous E-cadherin. We used a series of WC5 cell lines that express ectopically various forms of E-cadherin containing deletions in the cytoplasmic segment. We tested for the effects of the deletions in E-cadherin on its ability to associate with PTPμ in a cellular context by immunoprecipitating PTPμ from the various WC5 cell lysates and determining whether E-cadherin coimmunoprecipitated with the phosphatase. As shown in Fig. 5 A, WC5 cell lines expressing five deletion mutants of E-cadherin (Chen et al., 1997) were used. Specifically, these included (a) the full-length E-cadherin molecule as a control (Ecad), (b) a control from which the entire cytoplasmic segment of E-cadherin was deleted (CDD), (c) a mutant in which the COOH-terminal 38 residues were deleted (CB), (d) a mutant in which the internal domain of the intracellular segment was deleted (ID), and (e) a mutant in which the juxtamembrane domain of E-cadherin was deleted (JM).
Figure 5.
Identification of the segment of E-cadherin that is required for interaction with PTPμ. (A) Schematic diagram of the E-cadherin mutants that were expressed ectopically in the WC5 cell line. In addition to wild-type E-cadherin, there were mutants containing the following deletions: the entire cytoplasmic segment (CDD), the catenin binding domain (CB), the internal domain (ID), or the juxtamembrane domain (JM). B–F illustrate a series of immunoprecipitates that have been blotted with the ECCD-2 antibody to the extracellular segment of E-cadherin. (B) E-cadherin (ECCD-2). All the E-cadherin deletion mutants were expressed to similar levels and migrated at the appropriate molecular weight. (C) PTP1B (FG6). (D) PTPμ (BK2). (E) PTPμ (SK7). (F) PTPμ (SK18). These results illustrate that only forms of E-cadherin that contain the COOH-terminal, catenin-binding domain associated with PTPμ.
The WC5 cell lines expressed each of the E-cadherin deletion mutants to similar levels. Each of the mutants was precipitated with antibodies to the extracellular segment of E-cadherin and migrated at the expected molecular weight (Fig. 5 B). A monoclonal antibody to the cytosolic PTP, PTP1B (Flint et al., 1993), was used as a negative control, and although capable of immunoprecipitating PTP1B (data not shown), it did not immunoprecipitate E-cadherin (Fig. 5 C). Using the BK2 antibody to PTPμ, which recognizes a peptide sequence in the MAM domain within the extracellular segment of PTPμ (Brady-Kalnay and Tonks, 1994), we observed that E-cadherin mutants bearing a deletion of the COOH-terminal 38 amino acids did not associate with PTPμ (Fig. 5 D). Thus, PTPμ failed to coimmunoprecipitate E-cadherin from two distinct WC5 lines expressing such E-cadherin deletion mutants (CB1 and CB2) and from a line expressing E-cadherin from which the entire cytoplasmic segment was deleted (CDD). These results indicate that the COOH-terminal 38 residues of E-cadherin is required for the interaction with PTPμ.
Originally, our observation of association between PTPμ and cadherin was founded primarily upon experiments performed with one antibody to the phosphatase, designated BK2, which recognized an epitope in the extracellular segment of the enzyme. We generated previously the SK series of monoclonal antibodies to the intracellular domain of PTPμ (Brady-Kalnay et al., 1993). By using histidine-tagged fusion proteins comprising various portions of the intracellular segment of PTPμ, we identified two classes of SK antibodies. One class recognized epitopes in the juxtamembrane segment of the phosphatase whereas the other recognized epitopes in the first phosphatase domain (data not shown). To extend the scope of our analysis of the association of PTPμ and cadherins, we tested whether the SK series monoclonal antibodies were able to immunoprecipitate the PTPμ/E-cadherin complex from the various WC5 cell lines, concentrating on one with an epitope in the juxtamembrane segment of PTPμ (SK7) and another that recognized the first PTP domain (SK18). As shown in Fig. 5, E and F, these antibodies, like BK2, immunoprecipitated the PTPμ/E-cadherin complex, but only from cells expressing forms of E-cadherin in which the COOH-terminal 38 residues were present. Similar data were obtained with all SK series antibodies tested (data not shown).
The Anti-PTPμ Antibody, BK2, Does Not Cross-react with Cadherins
The BK2 antibody was generated against a peptide derived from the NH2 terminus of human PTPμ (Brady-Kalnay et al., 1994) and did not show cross-reactivity with the PTPμ-like enzymes PTPκ and PTPλ/PCP-2. The peptide sequence displayed no obvious similarity to the intracellular segment of cadherin, which contains the site of interaction with PTPμ (Brady-Kalnay et al., 1995 and Fig. 5). Despite the lack of obvious sequence similarity between this peptide and the cadherins, we addressed the issue of cross-reactivity further in the following experiment.
We examined whether the anti-PTPμ antibody BK2 recognized a GST–E-cadherin fusion protein, purified after expression in E. coli, in a direct binding assay under nondenaturing conditions in vitro. Our original study demonstrated an interaction between the intracellular segment of PTPμ and the intracellular segment of E-cadherin (Brady-Kalnay et al., 1995). Furthermore, the data presented here in Fig. 5 show that the COOH-terminal 38 residues of E-cadherin were required for association with PTPμ. Therefore, any potential cross-reacting epitopes would be in this segment. We tested whether the BK2 antibody binds to the intracellular segment of E-cadherin under nondenaturing conditions. We used the following four GST fusion proteins: (a) GST alone, (b) the juxtamembrane half of the intracellular segment of E-cadherin (JM E-cad), (c) the COOH-terminal, catenin-binding portion of the intracellular segment of E-cadherin (CB E-cad), and (d) the extracellular domain of PTPμ (EXTRA PTPμ). An antibody to GST recognized all of the fusion proteins (Fig. 6 A). One of the commercially available antibodies reacted with the juxtamembrane half of E-cadherin (Fig. 6 B), whereas the polyclonal, pan-cadherin antibody reacted with the catenin-binding half of the E-cadherin intracellular segment (Fig. 6 C). The SK15 antibody, to the intracellular segment of PTPμ, did not recognize any of the fusion proteins (Fig. 6 D), whereas antibody BK2 interacted only with the extracellular segment of PTPμ (Fig. 6 E). Thus, under these conditions, the BK2 antibody did not recognize the intracellular segment of E-cadherin, which contains the binding site for PTPμ (Fig. 5), even when 10 μg of the purified protein was applied to the nitrocellulose filter to ensure maximal binding of the protein to the nitrocellulose, and the blot was overexposed. These data provide further indication that the BK2 antibody does not recognize E-cadherin nonspecifically.
Figure 6.
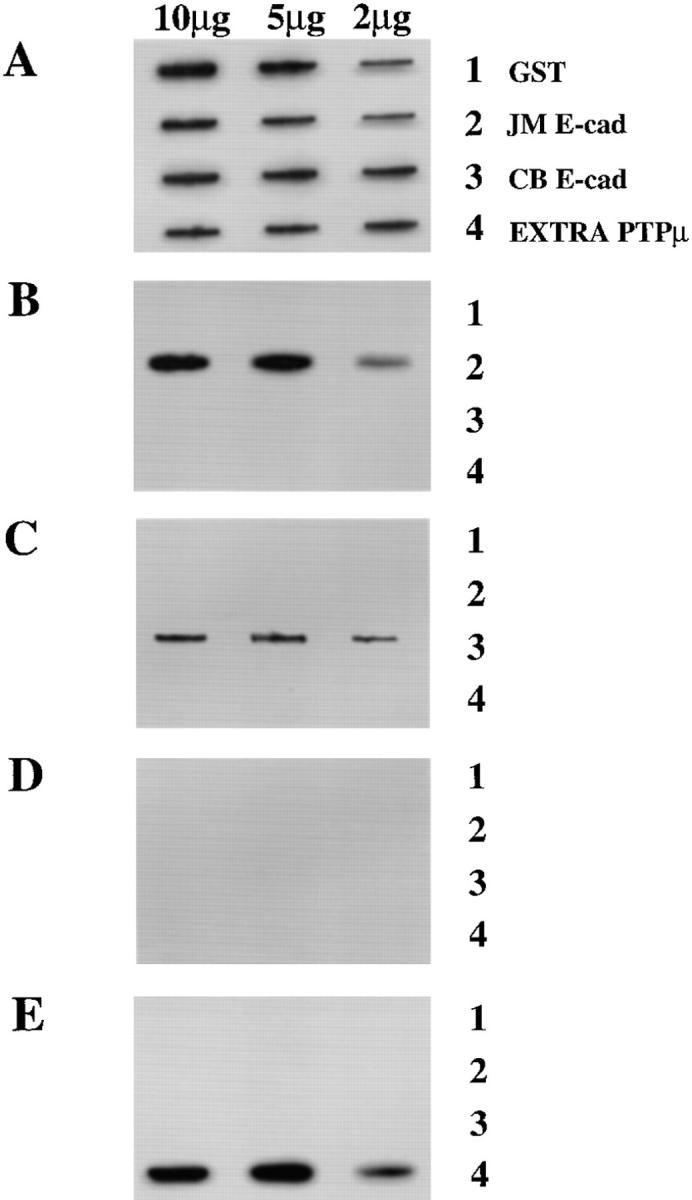
The BK2 antibody does not cross-react with the intracellular segment of E-cadherin. Purified GST fusion proteins (2, 5, or 10 μg) were applied to the wells of a slot blot apparatus and adsorbed to nitrocellulose. Identical nitrocellulose strips were incubated with antibodies to (A) GST, (B) the juxtamembrane half of the intracellular segment of E-cadherin (Transduction Labs), (C) the catenin-binding half of the intracellular segment of E-cadherin (Sigma pan-cadherin), (D) antibody to the intracellular segment of PTPμ (SK15), or (E) the BK2 antibody the extracellular segment of PTPμ. The data illustrate that the BK2 antibody does not cross-react with the intracellular segment of E-cadherin, which is the portion of E-cadherin required for association with PTPμ.
Discussion
Components of adherens junctions are subjected to rapid, reversible tyrosine phosphorylation in a cellular context (Volberg et al., 1991). Tyrosine phosphorylation of the cadherin–catenin complex has been observed under a variety of conditions, including in response to oncoprotein PTKs, such as Src (Matsuyoshi et al., 1992; Behrens et al., 1993), or to oncogenic forms of Ras (Kinch et al., 1995) and following stimulation of receptor PTKs, such as EGF receptor and Met (Shibamoto et al., 1994). In addition, PTKs such as EGF receptor and c-erbB2 have been observed to associate with the cadherin–catenin complex in vivo (Hoschuetzky et al., 1994; Ochiai et al., 1994). The reversibility of tyrosine phosphorylation in vivo depends upon the coordinated action of both PTKs and PTPs. Therefore, to understand fully the regulation of cadherin function by reversible tyrosine phosphorylation, it will be necessary to identify and characterize the phosphatases that act upon adhesion complexes in vivo. Our observation of association between a receptor PTP, PTPμ, and cadherins in various tissues and cells is consistent with a role for this phosphatase in regulating cadherin function and lends further support to the regulatory importance of tyrosine phosphorylation in cell adhesion.
In this study, we have demonstrated that PTPμ interacted with N-cadherin, E-cadherin, and cadherin-4 (also called R-cadherin) in extracts of rat lung. Although PTPμ can interact with several cadherins, it displays a restricted tissue distribution. Therefore, one would anticipate that, if regulation of cadherin function by reversible tyrosine phosphorylation was a general phenomenon, there would be additional PTPs that function in a manner analogous to PTPμ in other cell types. Subsequent to our original demonstration of association between PTPμ and the cadherin– catenin complex (Brady-Kalnay et al., 1995), several reports have appeared that substantiate the general principle that members of the PTP family may be important regulators of cadherin-mediated adhesion. Association of a number of receptor and nontransmembrane PTPs with different members of the cadherin family in a variety of cell systems has now been reported. PTPκ, a receptor PTP that is closely related in structure to PTPμ (∼75% sequence identity with the same overall arrangement of structural motifs), has been shown to associate directly with β-catenin and plakoglobin (Fuchs et al., 1996). Interestingly, PTPκ displays a much broader expression pattern than PTPμ (Jiang et al., 1993) and therefore may interact with cadherin–catenin complexes in many tissues. To date, four other PTPs have been shown to interact with cadherin– catenin complexes. Most recently, LAR (Aicher et al., 1997) and a novel receptor PTP, termed PTPλ (a close relative of PTPs μ and κ; Cheng et al., 1997), were also shown to associate with β-catenin. These authors observed that the association with β-catenin, like that involving PTPκ (Fuchs et al., 1996), required the intracellular segment of the phosphatase (Aicher et al., 1997; Cheng et al., 1997). A LAR-like receptor PTP was found to associate with the cadherin–catenin complex in PC12 cells, and this association appears to be regulated by nerve growth factor–induced tyrosine phosphorylation of the PTP itself (Kypta et al., 1996). In addition, a PTP1B-like cytoplasmic phosphatase has been shown to interact with N-cadherin (Balsamo et al., 1996). The authors suggest that the association of the PTP with N-cadherin facilitates dephosphorylation of β-catenin, which is required for N-cadherin–mediated adhesion and its association with the actin cytoskeleton.
In contrast to this consensus view of the potential importance of PTPs in regulating the tyrosine phosphorylation of cadherin–catenin complexes in vivo, one report (Zondag et al., 1996) has questioned the validity of our original observation. Therefore, we will respond in detail to the various issues raised in the paper by Zondag et al., in an attempt both to resolve this controversy and clarify the various issues. Zondag et al. report the generation of antibodies to an undefined epitope(s) in the ectodomain of PTPμ that fail to coimmunoprecipitate cadherin–catenin complexes. After successive rounds of immunoprecipitation with one of these antibodies, 3D7, to “clear” PTPμ from the cell lysate, the authors subjected the cleared lysate to immunoprecipitation with our antibody BK2. Even though they were unable to detect PTPμ in the cleared lysate by immunoblotting with their antibodies, they still observed cadherin in BK2 immunprecipitates. From this, the authors concluded that the interaction we observed was due to nonspecific cross-reactivity between BK2 and cadherin. There are two problems with this experiment and the conclusion drawn from it. Firstly, the authors did not blot the cleared lysate with BK2 to check whether there was a pool of PTPμ that was not recognized by their antibodies but was detected by BK2. Secondly, although the authors made the strong assertion of nonspecific cross- reactivity between BK2 and cadherin, they failed to demonstrate such cross-reactivity in a direct binding assay.
The following observations refute their argument. First, we used Sf9 cells, which do not contain detectable levels of endogenous PTPμ or E-cadherin, to express these proteins and reconstitute the complex. Through this approach we demonstrated that E-cadherin was only recovered in immunoprecipitates of PTPμ from lysates of cells in which both proteins had been coexpressed. Furthermore, the complex was detected in the reciprocal experiment, in which PTPμ was recovered in immunoprecipitates of E-cadherin, but again only from lysates of cells in which both proteins had been coexpressed. Second, and importantly, BK2 did not recognize E-cadherin in a direct binding assay in vitro, using purified components under nondenaturing conditions. Third, BK2 did not immunoprecipitate E-cadherin from lysates of MCF10A cells, which do not express PTPμ to a level that can be detected by antibody BK2 but express substantial levels of E-cadherin. The authors present data from a similar experiment in COS cells, which they state lacks endogenous PTPμ. They report that cadherin is detected in BK2 immunoprecipitates whether or not PTPμ was expressed ectopically. However, using a number of antibodies to PTPμ, including BK2, we detected expression of this protein in COS cells (data not shown), and thus coprecipitation of cadherin would not be unexpected. The reason for this discrepancy is unclear, although the authors did not test for the presence of PTPμ in COS cell lysates by blotting with BK2. Fourth, in WC5 cells transformed by temperature-sensitive v-Src and expressing E-cadherin ectopically, immunoprecipitates of PTPμ from lysates of cells cultured at the nonpermissive temperature contained coprecipitating cadherin, whereas at the permissive temperature the levels of associated cadherin were reduced substantially (Fig. 4). It is unlikely that BK2 would display cross-reactivity only in lysates from one temperature condition. Finally, we have demonstrated interaction between PTPμ and various members of the cadherin family using different antibodies that recognize at least three distinct epitopes in the phosphatase.
Zondag et al. (1996) presented several additional arguments to question the validity of the association we observed between PTPμ and cadherin. For example, they cited their failure to detect the phosphatase in anticadherin immunoprecipitates as further evidence that PTPμ and cadherin do not interact. However, it is important to note that in these experiments the authors used a pan-cadherin antibody that is directed against the COOH-terminal sequence that contains the segment of E-cadherin that is required for interaction with PTPμ. Therefore, the absence of PTPμ from these immunoprecipitates could easily be explained by steric hindrance introduced by antibody binding to cadherin. In addition, the authors dismiss our demonstration of direct interaction in blot-overlay binding studies as the result of production of the cadherin and PTPμ fusion proteins in bacteria, which, they suggest, is likely to yield misfolded or denatured protein and result in a high risk of nonspecific protein–protein interactions. However, the PTPμ used as probe was produced in insect Sf9 cells, not bacteria, and was catalytically functional and therefore was not denatured or misfolded. Furthermore, we included controls in the experiment to show that, as would be expected, under identical conditions E-cadherin bound β-catenin (specifically the NH2-terminal segment and not the COOH-terminal segment of β-catenin) but not α-catenin. Therefore, it is unlikely that our results can be explained by nonspecific association or improper protein folding.
The observation that PTPμ could interact with several cadherins prompted us to investigate the binding site for PTPμ on the cadherins. For these studies, we used a series of WC5 rat astrocyte-like cell lines, which express PTPμ endogenously and express ectopically mutant forms of E-cadherin that lacked various portions of the cytoplasmic segment. The results indicated that the COOH-terminal 38 residues, which overlap with the catenin-binding domain, were required for the interaction with PTPμ. A number of factors suggest that this is likely to be a direct binding site: (a) We have demonstrated previously that the intracellular segment of PTPμ interacts directly with the intracellular segment of E-cadherin in vitro; (b) we have shown here (Fig. 3) that PTPμ and E-cadherin interact after coexpression in Sf9 cells and, considering the extent of overexpression achieved in this system, it is unlikely that the interaction is mediated by an endogenous Sf9 cell protein; and (c) deletions of other portions of the E-cadherin cytoplasmic segment had little effect on the association with PTPμ in WC5 cells. Although we did not detect a direct interaction between PTPμ and β-catenin in vitro or in Sf9 cells, we have detected both cadherin and β-catenin in immunoprecipitates of PTPμ (Brady-Kalnay et al., 1995). In light of data suggesting that E-cadherin functions as a dimer (Brieher et al., 1996; Nagar et al., 1996), it is possible that one E-cadherin molecule of the dimer may bind PTPμ while the other interacts with β-catenin. The observation that the COOH-terminal 38 residues of E-cadherin are required for interaction with PTPμ raises the possibility that the association may be regulated by β-catenin in vivo.
In summary, we believe that our data have established convincingly the existence of a complex between PTPμ and various members of the family of cadherins in a number of different cell systems. In addition, we believe that we have presented data to refute convincingly the assertions of Zondag and colleagues (1996) that the association we observe between PTPμ and cadherins is artifactual. Our observations highlight further the potential importance of reversible tyrosine phosphorylation in regulating the adhesive properties of the cadherin family of cell adhesion molecules.
Acknowledgments
We thank Ferdose Sartawi, Jullia Rosdahl, Leif Stordal, and Martha Daddario for technical assistance.
Abbreviations used in this paper
- GST
glutathione S transferase
- PTK
protein tyrosine kinase
- PTP
protein tyrosine phosphatase
Footnotes
S. Brady-Kalnay is funded by a Junior Faculty Investigator Award from the Case Western Reserve University/University Hospitals Ireland Cancer Center from an ACS Institutional Research Grant (IRG 186), a Case Western Reserve University Research Initiation Grant, and a grant from the American Cancer Society, Ohio Division Inc., Cuyahoga Unit. N.K. Tonks is funded by a grant from the National Institutes of Health (GM55989).
Address all correspondence to N.K. Tonks, Cold Spring Harbor Laboratory, One Bungtown Road, Cold Spring Harbor, NY 11724-2208. Tel.: (516) 367-8846. Fax: (516) 367-6812. E-mail: tonks@cshl.org
References
- Aicher B, Lerch MM, Müller T, Schilling J, Ullrich A. Cellular redistribution of protein tyrosine phosphatases LAR and PTPσ by inducible proteolytic processing. J Cell Biol. 1997;138:681–696. doi: 10.1083/jcb.138.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balsamo J, Leung TC, Ernst H, Zanin MKB, Hoffman S, Lilien J. Regulated binding of a PTP1B-like phosphatase to N-cadherin: control of cadherin-mediated adhesion by dephosphorylation of β catenin. J Cell Biol. 1996;134:801–813. doi: 10.1083/jcb.134.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens J, Vakaet L, Friis R, Winterhager E, Van Roy F, Mareel M, Birchmeier W. Loss of epithelial differentiation and gain of invasiveness correlates with tyrosine phosphorylation of the E-cadherin/β catenin complex in cells transformed with a temperature-sensitive v-src gene. J Cell Biol. 1993;120:757–766. doi: 10.1083/jcb.120.3.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birchmeier W. E-cadherin as a tumor (invasion) suppressor gene. BioEssays. 1995;17:97–99. doi: 10.1002/bies.950170203. [DOI] [PubMed] [Google Scholar]
- Birchmeier W, Behrens J. Cadherin expression in carcinomas: role in the formation of cell junctions and the prevention of invasiveness. Biochim Biophys Acta. 1994;1198:11–26. doi: 10.1016/0304-419x(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Brady-Kalnay S, Flint AJ, Tonks NK. Homophilic binding of the receptor-type protein tyrosine phosphatase PTPμ mediates cell–cell aggregation. J Cell Biol. 1993;122:961–972. doi: 10.1083/jcb.122.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady-Kalnay S, Tonks NK. Identification of the homophilic binding site of the receptor protein tyrosine phosphatase PTPμ. J Biol Chem. 1994;269:28472–28477. [PubMed] [Google Scholar]
- Brady-Kalnay SM, Tonks NK. Protein tyrosine phosphatases as adhesion receptors. Curr Opin Cell Biol. 1995;7:650–657. doi: 10.1016/0955-0674(95)80106-5. [DOI] [PubMed] [Google Scholar]
- Brady-Kalnay SM, Rimm DL, Tonks NK. The receptor protein tyrosine phosphatase PTPμ associates with cadherins and catenins in vivo. J Cell Biol. 1995;130:977–986. doi: 10.1083/jcb.130.4.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brieher WM, Yap AS, Gumbiner BM. Lateral dimerization is required for the homophilic binding activity of C-cadherin. J Cell Biol. 1996;135:487–496. doi: 10.1083/jcb.135.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Paradies N, Fedor-Chaiken M, Brackenbury R. E-cadherin mediates adhesion and suppresses cell motility via distinct mechanisms. J Cell Sci. 1997;110:345–356. doi: 10.1242/jcs.110.3.345. [DOI] [PubMed] [Google Scholar]
- Cheng J, Wu K, Armanini M, O'Rourke N, Dowbenko D, Lasky LA. A novel protein-tyrosine phosphatase related to the homotypically adhering κ and μ receptors. J Biol Chem. 1997;272:7264–7277. doi: 10.1074/jbc.272.11.7264. [DOI] [PubMed] [Google Scholar]
- Flint A, Gebbink M, Franza B, Hill D, Tonks NK. Multi-site phosphorylation of the protein tyrosine phosphatase, PTP1B: identification of cell cycle regulated and phorbol ester stimulated sites of phosphorylation. EMBO (Eur Mol Biol Organ) J. 1993;12:1937–1946. doi: 10.1002/j.1460-2075.1993.tb05843.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs M, Muller T, Lerch MM, Ulrich A. Association of human protein-tyrosine phosphatase κ with members of the armadillo family. J Biol Chem. 1996;271:16712–16719. doi: 10.1074/jbc.271.28.16712. [DOI] [PubMed] [Google Scholar]
- Gebbink M, van Etten I, Hateboer G, Suijkerbuijk R, Beijersbergen R, van Kessel A, Moolenaar W. Cloning, expression and chromosomal localization of a new putative receptor-like protein tyrosine phosphatase. FEBS Lett. 1991;290:123–130. doi: 10.1016/0014-5793(91)81241-y. [DOI] [PubMed] [Google Scholar]
- Gebbink MFBG, Zondag GCM, Wubbolts RW, Beijersbergen RL, van Etten I, Moolenaar WH. Cell-cell adhesion mediated by a receptor-like protein tyrosine phosphatase. J Biol Chem. 1993;268:16101–16104. [PubMed] [Google Scholar]
- Giotta GJ, Cohn M. The expression of glial fibrillary acidic protein in a rat cerebellar cell line. J Cell Physiol. 1981;107:219–230. doi: 10.1002/jcp.1041070207. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Signal transduction by β-catenin. Curr Opin Cell Biol. 1995;7:634–640. doi: 10.1016/0955-0674(95)80104-9. [DOI] [PubMed] [Google Scholar]
- Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- Hoschuetzky H, Aberle H, Kemler R. β catenin mediates the interaction of the cadherin catenin complex with epidermal growth factor receptor. J Cell Biol. 1994;127:1375–1380. doi: 10.1083/jcb.127.5.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Wang H, D'Eustachio P, Musacchio J, Schlessinger J, Sap J. Cloning and characterization of R-PTP-κ, a new member of the receptor protein tyrosine phosphatase family with a proteolytically cleaved cellular adhesion molecule-like extracellular region. Mol Cell Biol. 1993;13:2942–2951. doi: 10.1128/mcb.13.5.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinch MS, Clark GJ, Der CJ, Burridge K. Tyrosine phosphorylation regulates the adhesions of Ras-transformed breast epithelia. J Cell Biol. 1995;130:461–471. doi: 10.1083/jcb.130.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinch MS, Petch L, Zhong C, Burridge K. E-cadherin engagement stimulates tyrosine phosphorylation. Cell Adhes Commun. 1997;4:425–437. doi: 10.3109/15419069709004459. [DOI] [PubMed] [Google Scholar]
- Kypta R, Su H, Reichardt L. Association between a transmembrane protein tyrosine phosphatase and the cadherin–catenin complex. J Cell Biol. 1996;134:1519–1529. doi: 10.1083/jcb.134.6.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyoshi N, Hamaguchi M, Taniguchi S, Nagafuchi A, Tsukita S, Takeichi M. Cadherin-mediated cell–cell adhesion is perturbed by v-src tyrosine phosphorylation in metastatic fibroblasts. J Cell Biol. 1992;118:703–714. doi: 10.1083/jcb.118.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuchi A, Takeichi M. Cell binding function of E-cadherin is regulated by the cytoplasmic domain. EMBO (Eur Mol Biol Organ) J. 1988;7:3679–3684. doi: 10.1002/j.1460-2075.1988.tb03249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagar B, Overduin M, Ikura M, Rini JM. Structural basis of calcium-induced E-cadherin rigidification and dimerization. Nature. 1996;380:360–364. doi: 10.1038/380360a0. [DOI] [PubMed] [Google Scholar]
- Nose A, Nagafuchi A, Takeichi M. Expressed recombinant cadherins mediate cell sorting in model systems. Cell. 1988;54:993–1001. doi: 10.1016/0092-8674(88)90114-6. [DOI] [PubMed] [Google Scholar]
- Ochiai A, Akimoto S, Kanai Y, Shibata T, Oyama T, Hirohashi S. c-erbB-2 gene product associates with catenin in human cancer cells. Biochem Biophys Res Commun. 1994;205:73–78. doi: 10.1006/bbrc.1994.2631. [DOI] [PubMed] [Google Scholar]
- Ozawa M, Baribault H, Kemler R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO (Eur Mol Biol Organ) J. 1989;8:1711–1717. doi: 10.1002/j.1460-2075.1989.tb03563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne HR, Hemperly JJ, Lemmon V. N-cadherin expression and function in cultured oligodendrocytes. Dev Br Res. 1996;97:9–15. doi: 10.1016/s0165-3806(96)00124-1. [DOI] [PubMed] [Google Scholar]
- Sap J, Jiang YP, Friedlander D, Grumet M, Schlessinger J. Receptor tyrosine phosphatase R-PTP-κ mediates homophilic binding. Mol Cell Biol. 1994;14:1–9. doi: 10.1128/mcb.14.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibamoto S, Hayakawa M, Takeuchi K, Hori T, Oku N, Miyazawa K, Kitamura N, Takeichi M, Ito F. Tyrosine phosphorylation of β catenin and plakoglobin enhanced by hepatocyte growth factor and epidermal growth factor in human carcinoma cells. Cell Adhes Commun. 1994;1:295–305. doi: 10.3109/15419069409097261. [DOI] [PubMed] [Google Scholar]
- Shiroyashi Y, Nose A, Iwasaki K, Takeichi M. N-linked oligosaccharides are not involved in the function of a cell-cell binding glycoprotein E-cadherin. Cell Struct Funct. 1986;11:245–252. doi: 10.1247/csf.11.245. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Morphogenetic roles of classic cadherins. Curr Biol. 1995;7:619–627. doi: 10.1016/0955-0674(95)80102-2. [DOI] [PubMed] [Google Scholar]
- Takeda H, Nagafuchi A, Yonemura S, Tsukita S, Behrens J, Birchmeier W, Tsukita S. V-src kinase shifts the cadherin-based cell adhesion from the strong to the weak state and β catenin is not required for the shift. J Cell Biol. 1995;131:1839–1847. doi: 10.1083/jcb.131.6.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volberg T, Geiger B, Dror R, Zick Y. Modulation of intercellular adherens-type junctions and tyrosine phosphorylation of their components in RSV-transformed cultured chick lens cells. Cell Regul. 1991;2:105–120. doi: 10.1091/mbc.2.2.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volberg T, Zick Y, Dror R, Sabanay I, Gilon C, Levitzki A, Geiger B. The effect of tyrosine-specific protein phosphorylation on the assembly of adherens-type junctions. EMBO (Eur Mol Biol Organ) J. 1992;11:1733–1742. doi: 10.1002/j.1460-2075.1992.tb05225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren SL, Nelson WJ. Nonmitogenic morphoregulatory action of pp60v-src on multicellular epithelial structures. Mol Cell Biol. 1987;7:1326–1337. doi: 10.1128/mcb.7.4.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zondag G, Koningstein G, Jiang YP, Sap J, Moolenaar WH, Gebbink M. Homophilic interactions mediated by receptor tyrosine phosphatases μ and κ. J Biol Chem. 1995;270:14247–14250. doi: 10.1074/jbc.270.24.14247. [DOI] [PubMed] [Google Scholar]
- Zondag G, Moolenaar W, Gebbink M. Lack of association between receptor protein tyrosine phosphatase RPTPμ and cadherins. J Cell Biol. 1996;134:1513–1517. doi: 10.1083/jcb.134.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]