Abstract
Cargo is selectively exported from the ER in COPII vesicles. To analyze the role of COPII in selective transport from the ER, we have purified components of the mammalian COPII complex from rat liver cytosol and then analyzed their role in cargo selection and ER export. The purified mammalian Sec23–24 complex is composed of an 85-kD (Sec23) protein and a 120-kD (Sec24) protein. Although the Sec23–24 complex or the monomeric Sec23 subunit were found to be the minimal cytosolic components recruited to membranes after the activation of Sar1, the addition of the mammalian Sec13–31 complex is required to complete budding. To define possible protein interactions between cargo and coat components, we recruited either glutathione-S-transferase (GST)–tagged Sar1 or GST– Sec23 to ER microsomes. Subsequently, we solubilized and reisolated the tagged subunits using glutathione-Sepharose beads to probe for interactions with cargo. We find that activated Sar1 in combination with either Sec23 or the Sec23–24 complex is necessary and sufficient to recover with high efficiency the type 1 transmembrane cargo protein vesicular stomatitis virus glycoprotein in a detergent-soluble prebudding protein complex that excludes ER resident proteins. Supplementing these minimal cargo recruitment conditions with the mammalian Sec13–31 complex leads to export of the selected cargo into COPII vesicles. The ability of cargo to interact with a partial COPII coat demonstrates that these proteins initiate cargo sorting on the ER membrane before budding and establishes the role of GTPase-dependent coat recruitment in cargo selection.
Newly synthesized cargo translocated into the ER is incorporated into small vesicular carriers that mediate transport to Golgi compartments. Although export was previously assumed to occur via a nonselective bulk flow mechanism (Wieland et al., 1987), studies using synchronized in vitro ER export assays have now demonstrated that proteins destined for export are efficiently sorted from resident ER proteins, concentrated, and then packaged into vesicles (Balch et al., 1994; Barlowe et al., 1994; Bannykh et al., 1996; Rowe et al., 1996; for review see Aridor and Balch, 1996a ). The mechanism by which cargo is selected for export remains to be established.
Vesicle budding from the ER is mediated by the COP1II coat complex (Barlowe et al., 1994). COPII is composed of five cytosolic components: the Sar1p GTPase, and the two protein complexes Sec23–24 and Sec13–31. The role of mammalian COPII in ER export was demonstrated by the ability of Sar1 to regulate COPII recruitment and vesicle budding in vivo and in vitro, and through morphological analyses to document the relationship between COPII recruitment and mobilized cargo (Kuge et al., 1993; Aridor et al., 1995; Rowe et al., 1996; Scales et al., 1997; Tang et al., 1997). Under physiological conditions, stable membrane recruitment of soluble COPII components to ER budding sites in mammalian cells requires activation of the Sar1 GTPase (Aridor and Balch, 1996a ; Bannykh et al., 1996; Rowe et al., 1996). Mammalian homologues for yeast Sar1p (Kuge et al., 1994), Sec23p (Orci et al., 1991; Paccaud et al., 1996), and Sec13p (Shaywitz et al., 1995; Tang et al., 1997) have been identified, although functional, intact Sec23–24 or Sec13–31 complexes have not been purified from mammalian cells.
Previous studies have led to the suggestion that the soluble coat complexes found in the cytosol act as sorters for cargo selection. Given this possibility, it has been proposed (Aridor and Balch, 1996a ) that a selective interaction between cargo and COPII components will occur before completion of vesicle budding. To analyze the role of mammalian COPII components in cargo selection and ER export, we purified the functional coat complexes from rat liver cytosol and defined separately the minimal interactions necessary for (a) recruitment of COPII coat components to membranes, (b) cargo selection, and (c) vesicle formation. We found that membrane recruitment of only two COPII components, the small GTPase Sar1 and the Sec23–24 complex, are necessary and sufficient to mobilize the type 1 transmembrane cargo molecule vesicular stomatitis virus glycoprotein (VSV-G) to a detergent-soluble, stable complex that excludes resident ER proteins and is a functional intermediate in COPII vesicle formation. Collectively, our results demonstrate that cargo can interact with cytosolic COPII components while in the ER, and that COPII initiates cargo selection in a Sar1-dependent manner before vesicle budding.
Materials and Methods
Materials
Glutathione-Sepharose (GS) beads were obtained from Pharmacia Biotech. Inc. (Piscataway, NJ); Dyna-beads (M500) were obtained from Dynal (Great Neck, NY). Other antibodies used in this study were gifts from the following laboratories: a polyclonal antibody against Sec23p from R. Schekman (University of California, Berkeley, CA); a monoclonal antibody to VSV-G from T. Kreis (University of Geneva, Geneva, Switzerland; Kreis, 1986); and a polyclonal antibody specific for VSV-G. A clone of mouse Sec23a, provided by J. Rothman (Sloan-Kettering, New York, NY), was sequenced to confirm and identify known mammalian Sec23 proteins (our unpublished data). Polyclonal antibodies to Sec23 were generated against a peptide (DTEHGGSQAR) (residues 707–716) and against GST–Sec23 as described (Dascher et al., 1994). Antibodies to mammalian Sec13 and Sec24 (our unpublished data) were generated against GST chimeras of each of these proteins. Antibodies to immunoglobulin binding protein (BIP) and calnexin were obtained from Stressgen Biotechnologies Corp. (Victoria, British Columbia, Canada). Antibody to ribophorin II was a gift from D. Meyer (University of California, Los Angeles, CA).
Proteins
Mouse Sec23A was expressed as a GST fusion protein in a pGEX/2T vector as described by the manufacturer (Pharmacia Biotech. Inc.). The protein was isolated from the soluble fraction using GS beads as described by the manufacturer (Pharmacia Biotech. Inc.) and then dialyzed against a buffer containing 25 mM Hepes, pH 7.2, 125 mM KOAc, before storage at −80°C. Sar1A mutants were prepared as described (Rowe and Balch, 1995). GST–Sar1–GTP (Sar1[H79G] mutant) was expressed as a GST fusion protein in a pGEX/2T vector as described above.
Purification of the Sec23–24 Complex from Rat Liver Cytosol
Rat livers were homogenized in a blender in 3× vol/wt buffer containing 25 mM Hepes-KOH, pH 7.2, 150 mM KOAc, 250 mM sorbitol, 1 mM DTT, 1 mM EGTA, 1 mM EDTA, 1 mM PMSF, and a complete cocktail of protease inhibitors (buffer A) (Boehringer Mannheim Biochemicals (Indianapolis, IN) at 4°C. The homogenate was centrifuged at 1,000 g for 10 min and then the supernatant was collected and centrifuged at 12,500 g for 20 min. The supernatant was collected and then centrifuged at 186,000 g for 1 h. The supernatant was precipitated with 30% ammonium sulfate and the precipitated material, which contained the Sec23 immunoreactive material, was collected by centrifugation at 16,000 g for 20 min. The ammonium sulfate pellet was resuspended in a buffer B (buffer A supplemented with 1 μg/ml calpain inhibitor 1, 1 μg/ml aprotinin, 0.5 μg/ml leupeptin and 1 μg/ml pepstatin) using a dounce homogenizer, and then centrifuged at 10,000 g for 10 min. The obtained supernatant was loaded on to a gel filtration column (model S-300; Pharmacia Biotech. Inc.) that was preequilibrated with buffer B. The column was eluted at 0.4 ml/min and then fractions were collected. The immunoreactive-containing fractions were pooled and then loaded onto a DEAE-Sepharose column that was eluted in buffer B with a nonlinear salt gradient from 0.15 to 1 M KOAc at a flow rate of 1 ml/min. The immunoreactive peak was pooled and loaded onto an hydroxyapatite column equilibrated with buffer B that was supplemented with 25 mM KH2PO4, pH 6.5 (buffer B). The column was eluted with a gradient of 25 mM to 500 mM KH2PO4, pH 6.5 at a flow rate of 0.8 ml/min. The immunoreactive material was pooled and then dialyzed against a buffer containing 25 mM Hepes-KOH, pH 7.2, and 125 mM KOAc. The Sec23–24 fraction was concentrated to 0.1 mg/ml and then portions were frozen in liquid N2 and then stored at −80°C for subsequent use.
Partial Purification of the Sec13–31 Complex from Rat Liver Cytosol
A 30% ammonium sulfate precipitate was obtained and then loaded onto a S-300 gel filtration column (Pharmacia Biotech. Inc.) as described above. The high molecular weight fraction, which contained the Sec13–31 complex, was pooled and then loaded on to a hydroxyapatite (HAP) column as described above in buffer B and then eluted in buffer B supplemented with 25 mM KH2PO4, pH 6.0. The eluted proteins were pooled and then concentrated to 3 mg/ml, desalted using gel filtration, frozen in a buffer containing 25 mM Hepes-KOH, pH 7.2, and 125 mM KOAc, and then stored at −80°C for subsequent use.
Measurement of Sar1 GTPase Activity
Sar1p GTPase activation (GAP activity) of GST–Sec23 or the Sec23/24 complex was performed as described (Mahajan et al.) (1997) using 0.5 μM Sar1 and 1 μM of recombinant GST–Sec23 or 1 μM of the Sec23/24 purified fraction.
ER Budding Assay
COP II vesicle formation reactions using microsomes prepared from normal rat kidney (NRK) cells were performed and then budding was quantitated using antibodies specific for VSV-G using Western blotting (Rowe et al., 1996). When budding reactions were carried out with purified components, reactions were supplemented with GTP (2 mM).
Immunoisolation of Vesicles
VSV-G–containing vesicles were immunoisolated as described (Rowe et al., 1996).
COPII Recruitment Assay
The membrane recruitment of COPII components with the Sar1-GTP– restricted mutant was performed as described (Aridor et al., 1995). For recruitment of purified Sec23–24 complex or His6–Sec23, 0.5 μg of each were incubated with membranes in the presence of the Sar1–GTP–restricted mutant. When recruitment reactions were carried out with purified components, the reaction mixture was supplemented with 0.1 mM GTP.
GST Complex Isolation
Salt-washed membranes (120–160 μg) were prepared from VSV-infected NRK cells by incubation of microsomes on ice in the presence of 250 mM sorbitol, 35 mM KOAc, 0.5 mM MgOAc, 20 mM Hepes, pH 7.2, and 2.5 M urea for 30 min. Subsequently, the membranes were collected by centrifugation at 12,000 g for 2 min and then resuspended in a buffer containing 20 mM Hepes, pH 7.2, 250 mM sorbitol, 70 mM KOAc, and 1 mM MgOAc. Membranes were then incubated in a budding–transport reaction as described (Rowe et al., 1996) in the presence or absence of GST–Sec23 (11 μg) and the GDP(T39N)- or GTP(H79G)-restricted forms of purified Sar1 proteins (1.5-μM each) and 1 mM GTP for 30 min at the indicated temperature in a final vol of 160 μl as indicated. The reaction was terminated by transfer to ice and then the microsomes were collected by centrifugation at 20,000 g for 10 min. The membranes were then solubilized in a final volume of 1 ml by incubation on ice for 30 min with a buffer containing 20 mM Hepes, pH 7.2, 1 mM MgOAc, and 1% digitonin in the presence of a protease inhibitor cocktail (Rowe et al., 1996) with occasional mixing. The insoluble material was removed by centrifugation at 50,000 g for 15 min at 4°C. GS beads preequilibrated with the solubilization buffer were added to the soluble fraction and then the samples were incubated for an additional 30 min with rocking at 4°C. Subsequently, the GS beads were collected by centrifugation and then washed three times with solubilization buffer. The washed beads were eluted by boiling in SDS sample buffer for 5 min and were then loaded on 7.5% SDS-PAGE gels and analyzed by Western blotting using enhanced chemiluminescence. To determine total protein content, the residual unbound material remaining in the supernatant after pelleting of GS beads was concentrated using CHCl3/MeOH extraction as described (Wessel and Flugge, 1984). The isolation of complexes using GST–Sar1–GTP and Sec23–24 was performed as described for GST-Sec23–isolated complexes and was then analyzed on 10% SDS-PAGE gels.
Immunoelectron Microscopy
Reagents and immunoelectron microscopy were as previously described (Balch et al., 1994; Bannykh et al., 1996).
Results
Purification of Sec23–24 from Rat Liver Cytosol
To analyze the role of COPII in cargo selection and ER export, we first purified the mammalian Sec23–24 complex present in rat liver cytosol to homogeneity. Using a mouse clone of mammalian Sec23 (refer to Materials and Methods), we prepared antipeptide and antiprotein antibodies to Sec23. The mouse clone is 99% homologous to the previously reported human clone of Sec23a (Paccaud et al., 1996). Rabbit polyclonal antibodies recognized an ∼85-kD protein in the cytosol using Western blotting that is also recognized by an antibody generated against yeast Sec23 (Hicke and Schekman, 1989; Hicke et al., 1992), which has been used to morphologically localize Sec23 to the ER in intact pancreatic acinar cells (Orci et al., 1991). Our polyclonal Sec23 antibody immunoprecipitated an ∼85-kD protein complexed with a 120-kD protein from rat liver cytosol (data not shown).
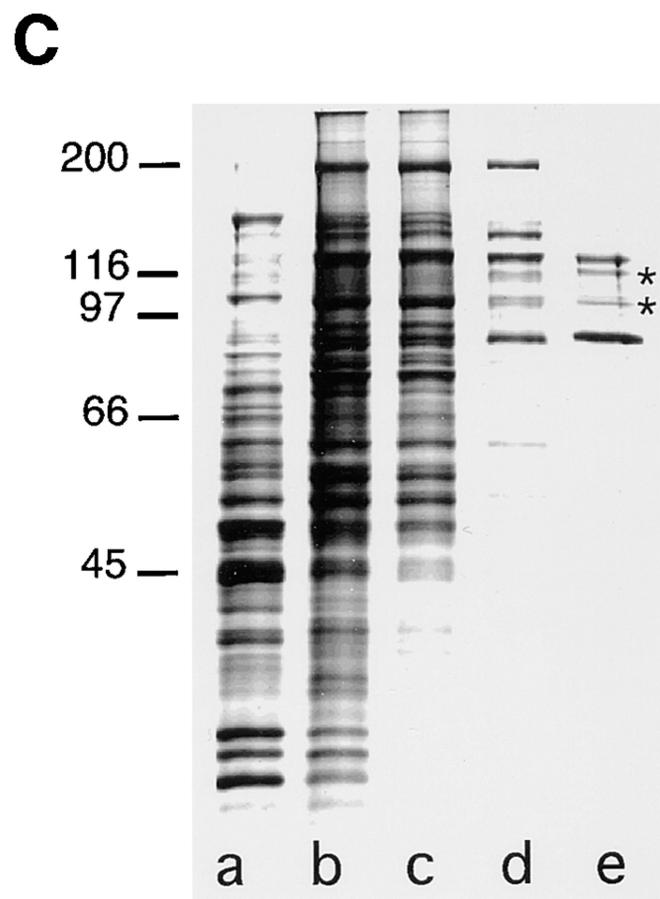
The Sec23–24 complex was purified from rat liver cytosol through sequential steps involving ammonium sulfate precipitation, S-300 gel filtration, DEAE–ion exchange, and HAP chomatography (Fig. 1) (refer to Materials and Methods) based on Western blotting using the Sec23 specific antibody. The purified Sec23–24 complex (Fig. 1 C, lane e) contained two major bands of 85 and 120 kD. The identity of the 85-kD band was established by microsequencing and found to be identical to the human homologue (Paccaud et al., 1996; data not shown). The 120-kD band was identified as the mammalian homologue of yeast Sec24 by microsequencing (data not shown). The Sec24 component was found to particularly labile to partial proteolysis as observed in yeast (Yeung et al., 1995), accounting for faster migrating minor bands in the purified preparation (Fig. 1 C, lane e, arrows) as detected by Western blotting using specific antibody to Sec24 (data not shown).
Figure 1.
Purification of Sec23–24 from rat liver cytosol. The Sec23–24 complex was purified from rat liver cytosol as described in the Materials and Methods. A and B illustrate typical elution profiles from the DEAE and HAP chromatography steps, respectively. (C), A silver-stained gel of representative pooled fractions from crude cytosol (3.3 μg) (lane a), ammonium sulfate precipitate (3 μg) (lane b), S-300–Sepharose (2.8 μg) (lane c), DEAE (0.7 μg) (lane d), and HAP (0.2 μg) (lane e) columns. The asterisks in e, partial proteolytic breakdown products of Sec24 based on Western blotting. Molecular markers are indicated in the left margin of C (kD).
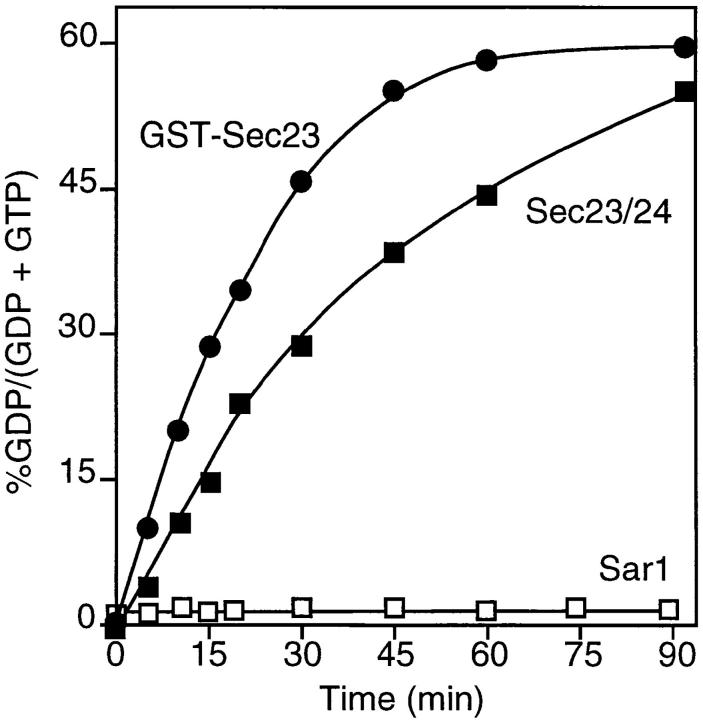
Yeast Sec23 was previously reported to be a Sar1-specific GTPase-activating protein (GAP) (Yoshihisa et al., 1993), accelerating GTP hydrolysis ∼15-fold over the intrinsic rate of hydrolysis. To address the functionality of the purified complex as a Sar1 GAP, we tested the GTP hydrolysis activity in the presence or absence of the purified complex. The purified mammalian Sec23–24 complex accelerated GTP hydrolysis of mammalian Sar1 up to 100-fold the intrinsic rate (Fig. 2), demonstrating its role as a Sar1 GAP. As a control, the complex itself did not show any GTPase activity (data not shown). A similar result was obtained with GST-tagged Sec23, showing that the Sec23 component of the complex contained GAP activity, as reported previously for the yeast complex (Yoshihisa et al., 1993).
Figure 2.
Sec23 and the Sec23–24 are functional GAP proteins for the mammalian Sar1 protein. Recombinant Sar1 was incubated in the presence or absence of GST– Sec23 or purified Sec23–24 and then GAP activity was monitored as described in the Materials and Methods.
Sec23 Is the Minimal Cytosolic COPII Component That Can Be Recruited to Membranes in Response to Sar1 Activation
To begin to identify the possible protein interactions initiating cargo sorting into COPII vesicles, we examined the recruitment of purified Sec23–24 to ER membranes. Incubation of microsomes with rat liver cytosol led to a temperature- and Sar1-dependent recruitment of the Sec23 component to membranes when measured by pelleting of total membranes (Fig. 3 A, c). This result is consistent with our previous observation that in semi-intact cells, after activation of the Sar1 GTPase, COPII is recruited specifically to the ER and can be colocalized with the mobilized VSV-G based on indirect immunofluorescence (Aridor et al., 1995).
Figure 3.

Sec 23 represents the minimal coat component that is recruited to the membranes by the Sar1 GTPase. (A) Microsomes were incubated with rat liver cytosol for 10 min on ice or at 32°C in the absence or presence of 1 μg of activated Sar1–GTP mutant with or without the ATP regenerating system. In all cases, Sar1–GTP was added together with 100 μM GTP. Membranes were pelleted, washed, and then the amount of Sec23 bound was determined by Western blotting with Sec23-specific antibody as described in the Materials and Methods. (B) Microsomes were incubated with the ATP regenerating system, GTPγS (100 μM), Sar1–GTP, and/or 2 mM GTP as indicated. Membranes were pelleted, washed, and then Western blots were quantitated as described in the Materials and Methods. The amount of Sec23 bound is reported as the percentage of maximal binding observed in incubations containing Sar1–GTP and the ATP regenerating system. (C) Microsomes were incubated with purified Sec23–24 complex or His6-tagged Sec23 with the indicated reagents as described for A. Membranes were pelleted, washed, and then the amount of Sec23 bound was determined by Western blotting. The typical results of three independent experiments are shown.
Stable recruitment of COPII from cytosol to membranes was observed only in the presence of an ATP regenerating system, micromolar concentrations of GTP and the Sar1–GTP–restricted mutant to prevent coat disassembly (Fig. 3 A). The ATP regenerating system could not be replaced by inclusion of the nonhydrolyzable analogue of ATP, ATPγS (data not shown). The requirement for the ATP regenerating system was still observed when recruitment was initiated in the absence of Sar1–GTP, but in the presence of the nonhydrolyzable analogue of GTP, GTPγS, activated to the endogenous cytosolic Sar1 protein (Fig. 3 B, a–c). COPII recruitment required membrane-associated components as limited proteolysis of the membranes prevented binding (data not shown). No recruitment was observed when excess GTP was added together with GTPγS in the absence of ATP (Fig. 3 B, e) and excess GTP significantly reduced GTPγS-induced recruitment in the presence of ATP (Fig. 3 B, d). GTPγS is required, therefore, to stabilize endogenous Sar1 in its active state to detect COPII binding to membranes. In contrast, addition of millimolar concentrations of GTP with the Sar1–GTP mutant in the absence of the ATP-regenerating system efficiently supported recruitment (Fig. 3 B, f). Some of the GTP may be used to generate trace levels of ATP, or added hydrolyzable nucleotide may be used by a protein that does not differentiate between ATP and GTP such as casein kinase II. Interestingly, casein kinase II–like enyzmes have been implicated in coat recruitment (Bonifacino et al., 1996). In any case, these results suggest that Sec23 recruitment involves an additional ATP/GTP-dependent function that is dependent on hydrolysis and is distinct from that of Sar1. A similar requirement has been found for the ARF1-dependent recruitment of AP1 and AP3 adaptor complexes involved in clathrin-coated vesicle formation (Traub et al., 1993; Simpson et al., 1996).
To define the minimal cytosolic components that could be recruited to membranes in the presence of activated Sar1, we examined the recruitment of purified Sec23–24 complex from rat liver cytosol. As observed for recruitment from cytosol, recruitment of purified Sec23–24 was temperature, ATP, and Sar1–GTP dependent (Fig. 3, compare A and C, a–d). Moreover, identical results were also observed when recombinant His6-tagged–Sec23 monomer was used in the absence of the Sec24 protein (Fig. 3 C, lanes e–h). Thus, the ATP/GTP requirement for Sec23 recruitment is due to an activity present on membranes, and Sec23 represents the minimal cytosolic component of the COPII machinery that can be stably recruited to microsomal membranes after activation of the Sar1 GTPase.
VSV-G Interacts with the COPII Machinery in a Sar1-dependent Manner
To address the role of COPII components in cargo selection and vesicle budding, we used an assay that reconstitutes the release of the type 1 transmembrane protein VSV-G into COPII–coated vesicles from mammalian microsomes (Rowe et al., 1996). To follow vesicle budding in vitro, a postnuclear supernatant fraction is prepared from homogenates of cells containing VSV-G in the ER. Membranes are incubated in a transport cocktail containing cytosol from rat liver and an energy source in the form of ATP. The fraction of VSV-G exported from the ER is measured using differential centrifugation to separate more rapidly sedimenting ER and Golgi membranes that are recovered in a medium speed (16,000 g) pellet (MSP) from slowly sedimenting ER-derived vesicles that are released into the medium speed supernatant (MSS). Carrier vesicles present in the MSS are subsequently recovered in a high speed (100,000 g) pellet (HSP), and the amounts of VSV-G in the MSP or HSP fractions are measured by SDS-PAGE and quantitative Western blotting.
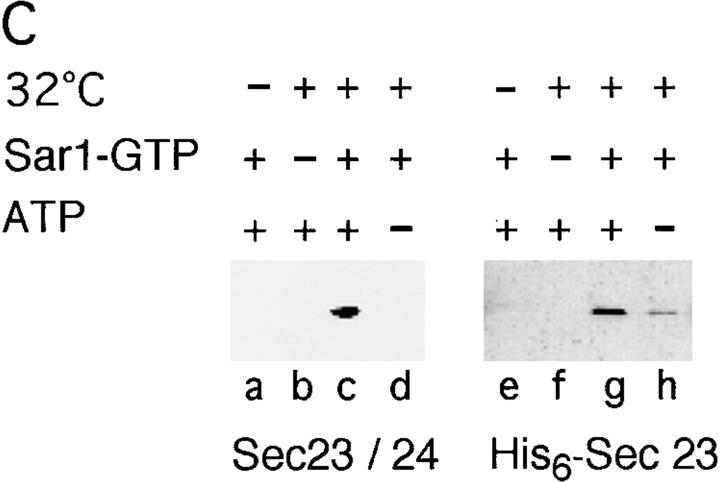
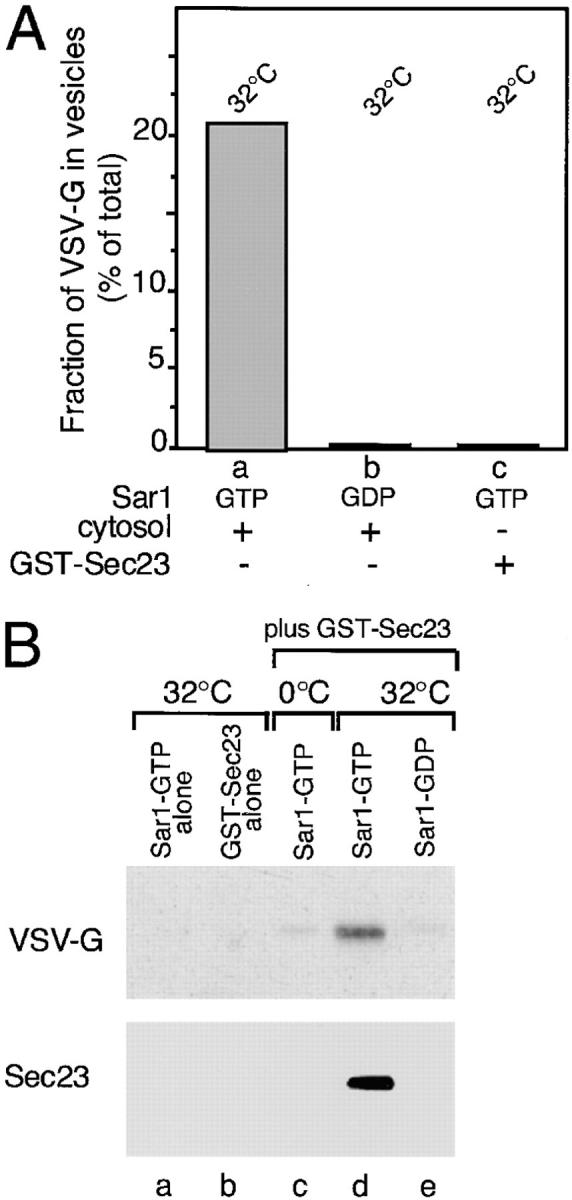
To define the minimal components required for vesicle budding, membranes were washed with high salt to remove any residual bound Sar1, Sec23–24, and Sec13–31 complex (Barlowe et al., 1994; data not shown). These membranes remained export competent, since incubation with cytosol and Sar1–GTP led to the accumulation of VSV-G–containing vesicles (Fig. 4 A, a). Budding was inhibited by incubation with the Sar1A-GDP–restricted mutant (Fig. 4 A, b), a result consistent with our previous demonstration that VSV-G export from the ER is regulated by the Sar1 GTPase (Rowe et al., 1996). High salt-washed membranes retained their ability to recruit a GST-tagged Sec23 (GST–Sec23) monomer in a Sar1A-dependent manner (Fig. 4 B). However, addition of activated Sar1A and GST–Sec23 did not support COPII vesicle formation (Fig. 4 A, c). These results indicate that GST–Sec23, in the absence of Sec24 and Sec13–31, is not sufficient for budding.
Figure 4.
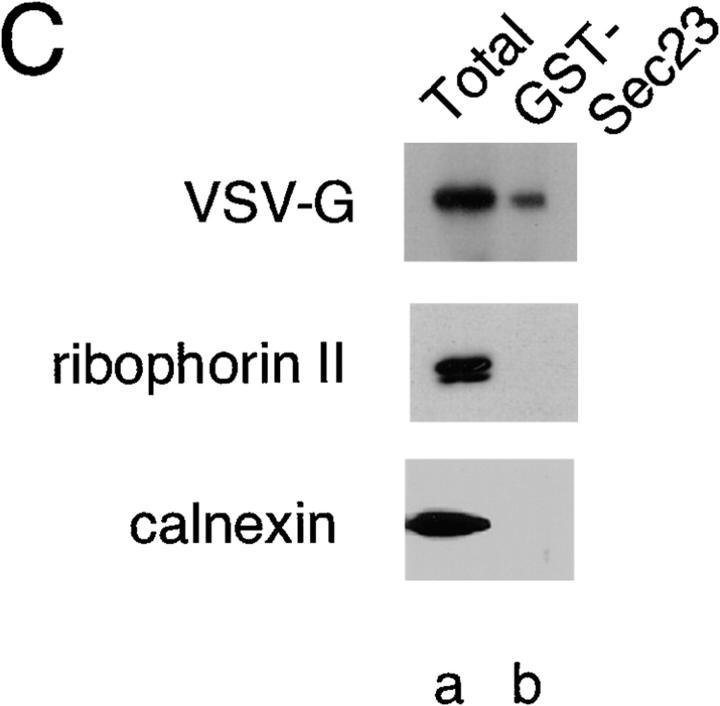
VSV-G can be detected in a complex with Sec23. (A) Salt-washed microsomes prepared as described in Materials and Methods were incubated at 32°C for 30 min in the presence of rat liver cytosol (a and b) or GST–Sec23 (c) in the presence of the inactive Sar1A (GDP- restricted) mutant (b) (1 μg) or the activated Sar1A (GTP-restricted) (a and c) mutant (1 μg). The amount of VSV-G (% of total) released from the ER in COPII vesicles was determined as described (Rowe et al., 1996). (B) Salt-washed microsomes were incubated on ice (c) or at 32°C (a, b, d, and e) for 30 min without (a) or with GST– Sec23 (b–e) in the absence (b) or presence of either the GTP- restricted (a, c, and d) or GDP-restricted (e) Sar1 mutant. Membranes were pelleted, solubilized, and incubated with GS beads and then the amount of GST–Sec23 or VSV-G recovered on the beads was determined using Western blotting as described in Materials and Methods. (C) Salt-washed microsomes were incubated in the presence of the activated Sar1–GTP mutant and then GST–Sec23 was pelleted, solubilized, and then incubated with GS beads as described in Materials and Methods. The amount of VSV-G (top panel), ribophorin II (middle panel) or calnexin (bottom panel) in the total sample (a) or that eluted from beads (b) was determined by Western blotting. The typical results of three independent experiments are shown.
Having defined conditions that do not support vesicle budding, yet support stable recruitment of the GST–Sec23 monomer to membranes, we analyzed whether cargo becomes associated with the recruited Sec23 containing a partial coat. For this purpose, salt-washed microsomes incubated with recombinant GST–Sec23 and/or Sar1A mutants were pelleted, washed, and then solubilized in a detergent containing buffer. After centrifugation to remove insoluble material, the supernatant was incubated with GS beads and then bound GST–Sec23 was quantitated using Western blotting. GST–Sec23 recovery on GS beads was both Sar1A and temperature dependent (Fig. 4 B, b and c). Incubation in the presence of the activated Sar1–GTP– restricted mutant (to prevent coat disassembly) resulted in an ∼100-fold increase in GS bead-bound Sec23 (Fig. 4 B, d). Strikingly, VSV-G was also recovered on GS beads and its recruitment directly mirrored the requirements for GST–Sec23 binding (Fig. 4 B, top). On the average, 15– 20% of the total soluble VSV-G was recovered on GS beads (Fig. 4 C, top, compare a and b), a value comparable to the amount of VSV-G generally released into vesicles in the presence of cytosol and ATP (Fig. 4 A, a). Identical results were observed in a converse set of experiments in which an antibody that recognizes the lumenal domain of VSV-G was used in place of GS beads to immunoprecipitate the VSV-G/GST–Sec23–containing complex (data not shown). Recruitment was selective as ribophorin II and calnexin, abundant ER marker proteins, could not be detected in the protein complexes bound to GS beads (Fig. 4 C, bottom, compare a and b). The soluble chaperone BIP was also excluded from the complex (data not shown). Thus, Sec23 is sufficient to initiate VSV-G recruitment in concert with Sar1A activation, demonstrating a role for Sec23 in cargo sorting that is initiated before vesicle budding.
Sar1 and the Sec23–24 Complex Are Both Required for the Formation of a Cargo Containing Prebudding Intermediate
Although the addition of GST–Sec23 in the absence of the Sec24 component promoted the selective recruitment of cargo, it did not support further vesicle formation. To use the purified Sec23–24 for analysis of cargo recruitment and vesicle formation and to analyze whether Sar1 is a component of the prebudding complex, we modified the activated form of Sar1, Sar1–GTP, with GST to generate a GST/Sar1–GTP chimera (GST–Sar1–GTP). We tested whether the GST–Sar1–GTP chimera would promote the formation of VSV-G–containing vesicles. We took advantage of the fact that after salt wash, Sar1 becomes a limiting component of the budding reaction. This requirement could either be supplemented with excess cytosol (data not shown) or with added exogenous recombinant Sar1 (Fig. 5, c). Washed microsomes were resuspended in a transport reaction mix in the presence of ATP, cytosol, and various Sar1 recombinant proteins for 30 min and then the release of VSV-G to the HSP was measured. Supplementing the assay with either wild-type recombinant Sar1 (Fig. 5 A, compare b to c), Sar1–GTP (Fig. 5 A, compare b to d), or GST–Sar1–GTP (Fig. 5 A, compare b to e) supported efficient vesicle formation.
Figure 5.
GST-tagged Sar1–GTP supports vesicle budding from salt-washed microsomal membranes. (A) Salt-washed microsomes prepared as described in Materials and Methods were incubated in a budding reaction with cytosol on ice (a) or for 30 min at 32°C without (b) or with wild-type Sar1 (1.5 μg) (c), Sar1-GTP (4 μg) (d), or a GST-tagged Sar1–GTP (10 μg) (e) as indicated. The amount of VSV-G (% of total) released from the ER into the HSP was determined as described (Rowe et al., 1996). (B) Salt-washed microsomes were incubated in a budding reaction supplemented with a GST-tagged Sar1–GTP (4 μg) for 30 min at 32°C. The HSP of seven budding reactions was collected and then subjected to immunoisolation with magnetic beads alone (a) or beads coupled with a monoclonal antibody to the VSV-G tail (P5D4) as described (Rowe et al., 1996) (b). GST–Sar1–GTP bound to beads was detected by Western blotting using a polyclonal antibody to Sar1. Molecular weight markers are indicated on the left. The typical results of three independent experiments are shown.
To determine if GST–Sar1–GTP protein was incorporated into VSV-G–containing vesicles, microsomes were incubated in the presence of cytosol and GST–Sar1–GTP for 30 min and then ER-derived vesicles were released into the immunoisolated HSP using magnetic beads coated with anti–VSV-G cytoplasmic tail antibody (P504), an approach we have previously used to characterize both morphologically and biochemically the composition of Sec23-containing COPII vesicles generated in vitro (Rowe et al., 1996). Like Sec23, GST–Sar1–GTP could be readily detected on affinity-purified vesicles using Western blotting with an antibody specific to Sar1 based on its higher molecular weight (47 kD) compared to wild-type Sar1 (25 kD) (Fig. 5 B, arrowhead).
Because GST–Sar1–GTP is active in both coat recruitment and vesicle formation, we examined the ability of this chimera to isolate the VSV-G–containing cargo complex in the presence or absence of Sec23–24 purified from rat liver cytosol. We found that GST–Sar1–GTP promoted a temperature-dependent recruitment of Sec23–24 to microsomal membranes as was observed for GST–Sec23 (refer to Fig. 4 A) (data not shown). Under these conditions, the recruited components did not support vesicle budding in the absence of Sec13–31 (see below and Fig. 7). After incubation, the membranes were solubilized and then the detergent-soluble fraction was incubated with GS beads. Proteins bound to GS beads were subjected to immunoblot and protein analysis. As shown in Fig. 6, incubation of the membranes in the presence of Sec23–24 but in the absence of the GST–Sar1–GTP did not promote cargo binding to GS beads (Fig. 6 A, a). Similarly, addition of GST– Sar1–GTP alone only led to the recovery of a small amount (<1%) of total VSV-G (Fig. 6 A, b), presumably reflecting a residual contamination of salt-washed membranes with COPII components. However, addition of both Sec23–24 and GST–Sar1–GTP led to efficient VSV-G recruitment (Fig. 6 A, c). VSV-G recruitment was temperature dependent (data not shown) and saturable in the presence of increasing Sec23–24 (Fig. 6 D) as further addition of the complex had no effect on the amount of VSV-G recruited to beads or on VSV-G released in the budding assay in a number of experiments (data not shown). The amount of VSV-G recovered under optimal conditions was comparable to that observed by incubation in the presence of GST–Sec23 and Sar1–GTP (refer to Fig. 4). Strikingly, recruitment was selective and excluded ER resident proteins such as ribophorin II (Fig. 6 B) or calnexin (data not shown).
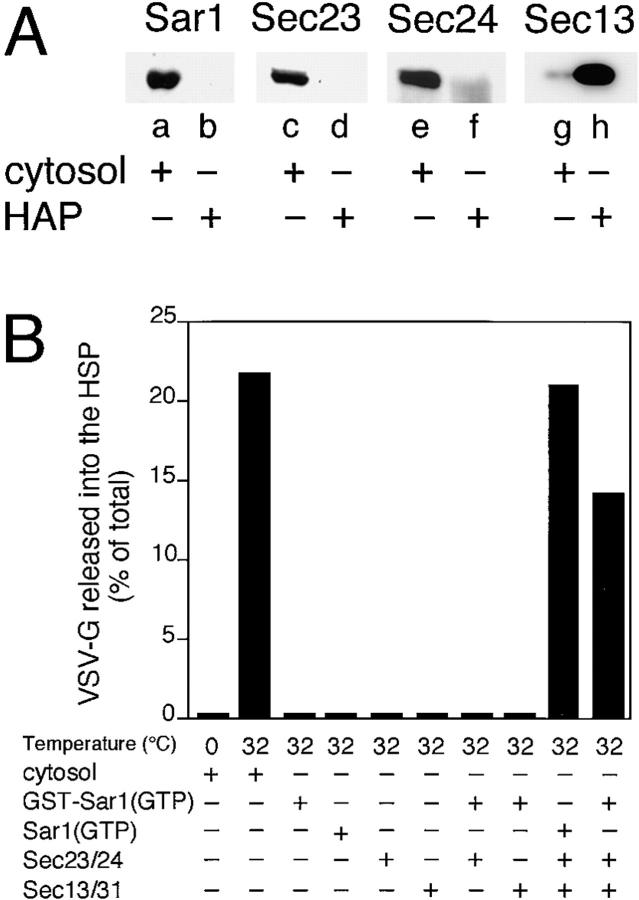
Figure 7.
Vesicle budding from the ER requires Sar1, Sec23–24, and Sec13–31. (A) The mammalian Sec13–31 complex was partially purified from rat liver cytosol as described in the Materials and Methods. The presence of Sar1, Sec23, Sec24, and Sec13 in crude cytosol (a, c, e, and g) and the HAP pool (b, d, f, and h), respectively, was determined by Western blotting with specific antibody (refer to Materials and Methods). Note the absence of Sar1 or Sec23–24 in the HAP fraction highly enriched in the Sec13–31 complex. (B) ER microsomes were incubated in the presence of ATP, GTP, and purified components for 30 min at 32°C and then the amount of VSV-G released into the HSP was determined as described (Rowe et al., 1996). The reactions were carried out in a 40-μl vol supplemented with either 400 μg of rat liver cytosol, 2 μg of GST–Sar1–GTP, 1 μg of His6–Sar1– GTP, 1 μg of Sec23–24 complex, and 42 μg of the Sec13/31–containing fraction as indicated. The typical results of three independent experiments are shown.
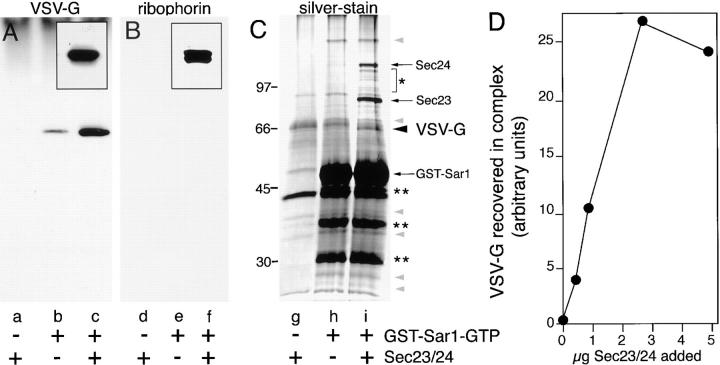
Figure 6.
VSV-G can be isolated in a complex with a GST-tagged Sar1–GTP and the mammalian Sec23–24 complex. (A–C) Salt-washed microsomes were incubated at 32°C for 30 min in the presence of Sec23–24 complex (lanes a, d, and g), GST-tagged Sar1–GTP (lanes b, e, and h), or both (lanes c, f, and i). Membranes were pelleted, solubilized, and then incubated with GS beads as described in Materials and Methods. The amount of VSV-G (A) or ribophorin II (B) recovered on beads was determined using Western blotting as described in Materials and Methods. Insets in A and B show control microsomal membranes on the same blot that were probed for either VSV-G (A) or ribophorin II (B). For immunoblot analysis, the equivalent of four budding reactions (supplemented with a total of 5 μg Sec23–24 (A and B) and 8 μg of GST-tagged Sar1–GTP) were combined. (C) For silver stain analysis, the equivalent of six budding reactions supplemented with 12 μg of GST-tagged Sar1–GTP and 4 μg of Sec23–24 were combined. In C, molecular weight markers are indicated to the left of the gel. *, Sec24 breakdown products; **, GST–Sar1–GTP breakdown products. (D) Salt-washed microsomes were incubated at 32°C for 30 min in the standard budding reaction in the presence of the GST–Sar1–GTP and the indicated amounts of Sec23–24. The relative amounts of VSV-G recovered in the GST–Sar1 complex was determined as described in the Materials and Methods.
Silver staining of the immunoisolated complex demonstrated the efficient recruitment of Sec23–24 (Fig. 6 C, arrows). Although a number of proteins were bound to GS beads in the presence of Sec23–24 alone (Fig. 6 C, g), a control condition that does not support either cargo recruitment or vesicle budding, illustrating nonspecific background binding to GS beads, incubation in the presence of GST–Sar1–GTP alone (Fig. 6 C, h) or in combination with Sec23–24 (Fig. 6 C, i), led to the recruitment of only a limited number of proteins above the background (Fig. 6 C, gray arrowheads). The identity and possible role of these proteins in COPII–mediated cargo selection and vesicle formation are currently under investigation. Importantly, a protein band corresponding to VSV-G based on immunoblotting (Fig. 6, A and C, compare b with i, large arrowhead) was markedly enhanced in the presence of GST– Sar1–GTP and Sec23–24, but not in incubations lacking either of the components. These results directly demonstrate the ability of GST–Sar1 to recruit VSV-G into a cargo-containing complex. Interestingly, the level of recruitment of VSV-G was similar to that of components bound to the complex during initiation of vesicle budding by the addition of Sar1 alone (Fig. 6 C, gray arrowheads), suggesting that a limiting component related to Sar1 recruitment is involved in cargo selection.
The Prebudding Complex Is an Intermediate in ER-to-Golgi Transport
The ability to recruit VSV-G to a detergent-soluble complex containing GST–Sar1 and Sec23–24 led us to examine whether this complex is an intermediate in vesicle budding. For this purpose, a partially purified Sec13–31 complex was prepared from rat liver cytosol. As shown in Fig. 7 A, this fraction lacks Sar1, Sec23, and Sec24, but is substantially enriched (nearly 1,000-fold based on Western blotting) in the Sec13 component. When each of the components was incubated separately with VSV-G containing ER microsomes, vesicle budding was not observed (Fig. 7 B). However, incubation of Sar1–GTP or GST–Sar1–GTP in the presence of both Sec23–24 and Sec13–31 led to efficient recovery of VSV-G in COPII coated vesicles.
To verify the above requirements for Sar1, the Sec23–24 and the Sec13–31 complexes for the appearance of VSV-G–containing vesicles, we examined the effects of each of these components on vesicle budding in vitro using immunoelectron microscopy. Semi-intact cells, a population of cells in which the plasma membrane is selectively perforated (Beckers et al., 1987; Plutner et al., 1992), faithfully reconstitute cargo selection (Balch et al., 1994) and transport of VSV-G from the ER to Golgi compartments (Davidson and Balch, 1993; Plutner et al., 1991; Nuoffer et al., 1994; Peter et al., 1994; Tisdale et al., 1997). Incubation in the presence of cytosol leads to the sorting and concentration of VSV-G in ER-derived buds and pre-Golgi intermediates (Fig. 8 A, arrowheads) as demonstrated previously using morphometry (Balch et al., 1994; Pind et al., 1994). An identical result was observed in the presence of the COPII components Sar1–GTP, Sec23–24, and Sec13–31 (Fig. 8 B, arrowheads). Typically, VSV-G was concentrated ∼5–15-fold in both ER-associated buds as well as in free vesicles over that observed in the ER membrane. Interestingly, in these incubations containing purified COPII components, we often found VSV-G concentrated on the face of the ER adjacent to regions of high budding activity (Fig. 8 B, top right, arrow), suggesting the budding may be limiting, relative to cargo selection and concentration under these conditions. Since these incubation conditions lack COPI components required for retrograde recycling from pre-Golgi intermediates (Aridor et al., 1995; Rowe et al., 1996) or additional cytosolic factors required for transport to the Golgi (Balch, W.E., unpublished data), the observed concentration of VSV-G in vesicles using only COPII components provides an additional line of evidence that sorting and concentration occurs during cargo selection from the ER (Balch et al., 1994; Bannykh et al., 1996; Rowe et al., 1996).
Figure 8.
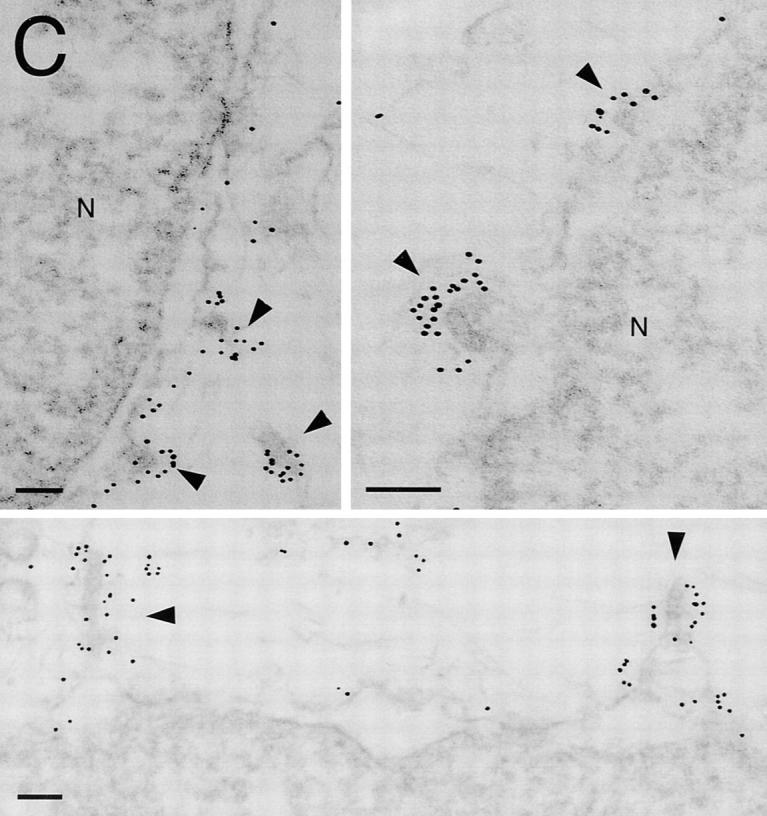
Immunolocalization of VSV-G in semi-intact cells incubated in the presence of cytosol or purified COP II components. Incubation of semiintact cells in the presence of ATP and the indicated components and localization of VSV-G using immunoelectron microscopy (immunodiffusion technique) was carried out as described previously (Balch et al., 1994). Semiintact cells were incubated in the presence of cytosol (A); the Sar1–GTP (9 μg), Sec23–24 (2.5 μg) and Sec13–31 (200 μg) in a 200-μl reaction vol (B); or Sar1–GTP and Sec23–24 in the absence of Sec13–31 or cytosol (C). In A and B, note the increased density of gold particles in ER-associated buds and ER-derived vesicles or pre-Golgi intermediates (arrowheads) when compared to the markedly reduced density of gold particles on total ER membranes. Arrow in top right panel of B highlights regions of localized concentration of VSV-G on ER membranes adjacent to regions of budding activity. Such structures were generally not observed in the presence of cytosol (A). (C) VSV-G can be readily detected in ER- associated buds and regional patches on the ER surface (arrowheads). Bars, 0.1 μM.
In contrast to our ability to readily detect VSV-G in vesicles when semi-intact cells were incubated in the presence of cytosol or the presence of COPII components, VSV-G–containing vesicles were not detected when cells were incubated in the presence of Sar1–GTP alone, consistent with the inability of Sar1–GTP to support budding from ER microsomes in vitro. However, incubation of Sar1 and the Sec23–24 complex together led to a population of structures that appeared as either partial buds or regions of the ER membrane where VSV-G was concentrated in patches relative to its general diffuse distribution before incubation in vitro (Fig. 8 C). These morphological results support our supposition from biochemical studies that Sar1– GTP and the Sec23–24 complex are sufficient to promote an interaction with VSV-G and that all of the components are necessary to direct budding from the ER.
Discussion
In the current study we have demonstrated the role of the mammalian COPII machinery in cargo selection and ER export. We have purified the functional Sec23–24 complex from rat liver cytosol and demonstrated its ability to serve as a Sar1-specific GAP. We have shown that activation of Sar1 leads to the recruitment of Sec23 or the Sec23–24 complex to the ER membrane. After Sar1 activation and recruitment of these components, we have detected a selective interaction of this complex with the cargo molecule VSV-G under conditions that do not support vesicle budding. Subsequent addition of the mammalian Sec13–31 complex results in the packaging of the selected cargo into COPII vesicles. These results now define a role for the COPII Sec23–24 complex in cargo sorting and a role for GTPase-dependent coat recruitment in cargo selection by a cytosolic coat complex during ER export. Each of these points are discussed in further detail below.
Sar1-regulated Sec23–24 Recruitment Selectively Sorts Cargo into a Prebudding Complex
We have found that the Sec23 monomeric subunit or the purified Sec23–24 complex define the minimal cytosolic components of the COPII coat that are recruited to ER membranes after activation of the Sar1 GTPase. These proteins functionally interact with one another, since both the yeast and the mammalian Sec23 components accelerate the intrinsic rate of GTP hydrolysis of Sar1. The activity of the mammalian protein as a Sar1 GAP was particularly notable when compared to the yeast protein (Yoshihisa et al., 1993). Although recruitment of the partial coat does not support vesicle budding, our hypothesis that coat assembly can direct cargo sorting (Aridor and Balch, 1996a ) is now directly supported by the observation that activation of Sar1 GTPase is critically linked to the recruitment of cargo into a prebudding, detergent-soluble protein complex. Related results, showing Sar1–dependent sorting of cargo from yeast ER, have recently been reported (Kuehn et al., 1998). The combined observations establish the evolutionary conservation of the pathway. Using either GST– Sec23 or GST–Sar1, we demonstrated very efficient recovery of VSV-G (∼15–20% of total) into prebudding protein complexes. The formation of the complex was blocked by the Sar1-GDP–restricted mutant that prevents COPII coat assembly indicating the specificity of complex formation for COPII components. The membrane-associated ER resident proteins ribophorin and calnexin, as well as the soluble component BIP, were excluded from the complex demonstrating high selectivity. Exclusion of ER resident proteins during cargo selection has also been observed in immunopurified ER-derived vesicles (Rowe et al., 1996) and semi-intact cells (Balch et al., 1994). Thus, formation of the prebudding complex in vitro reconstitutes the sorting events observed in vivo.
Both Sar1 and the Sec23–24 complex were required for cargo selection as demonstrated from two independent approaches using either the GST–Sec23 protein in the presence of activated Sar1 or activated GST–Sar1 in the presence of purified Sec23–24 complex. The ability to recover the activated Sar1 GTPase, Sec23, or Sec23–24 and cargo in a detergent-soluble complex in the absence of cross-linking reagents suggests a tight association with one another. The interaction between Sar1–GTP and Sec23 is consistent with our observation that mammalian Sec23 is a Sar1 GAP. These results lead us to suggest that Sar1, in its activated state, plays a structural rather than catalytic role in coat recruitment. The interaction of Sar1 with a membrane-associated guanine nucleotide exchange protein (GEP) may promote the formation of a high-affinity contact site on the GTPase for binding to Sec23 to initiate coat recruitment and cargo selection, although the order of these events remains to be determined. The Sec23–24 complex may be required to either interact with and stabilize preselected cargo during budding, or may directly select cargo for delivery to budding sites after Sar1 activation.
It is important to emphasize that the activated Sar1– GTP does not enhance cargo export nor does it affect the observed sorting of cargo into vesicles (Rowe et al., 1996) or prebudding complexes (this study). However, Sar1 activation and inactivation (GTP hydrolysis) are likely to be essential elements of the normal sorting event. Just as Rab proteins may serve as timers for the kinetic proofreading of the assembly of a targeting–fusion complex (Aridor and Balch, 1996b ; Rybin et al., 1996), Sar1 may serve a similar role in which its activation in vivo may function as a kinetic control to transiently stabilize the coat complex for proper cargo selection, packaging, and budding. The fact that we observed an excess of the coat components Sec23 and Sec24 over that of cargo in the presence of activated Sar1–GTP mutant was undoubtedly due to its ability to stabilize this otherwise transient coat assemblages by preventing GTP hydrolysis.
The role of cytosolic coats in cargo sorting has been suggested in previous studies that have detected cargo–coat interactions in vitro using approaches that probe for the binding of coat components to immobilized, signal-containing peptides on ice using detergent-solubilized cell homogenates. For example, interactions between AP1 complexes with the clathrin coat and tyrosine-based sorting motifs (Ohno et al., 1995, 1996; Bonifacino et al., 1996) or interactions between dilysine containing sorting determinants and COPI components (Cosson and Letourner, 1994; Fiedler et al., 1996; Sohn et al., 1997) have been demonstrated. However, it is clear that such interactions take place neither under physiological conditions nor are either of these coats recruited to their target membranes without activation of the small GTPase ARF1. Therefore, the dependency of COPII–mediated cargo selection on Sar1 activation and the role of Sar1 as an integral component of the prebudding complex may be also shared by other cytosolic coat complexes. Indeed, direct interaction between the small GTPase ADP-ribosylation factor ARF1 and the β subunit of COPI during COPI recruitment was recently demonstrated (Zhao et al., 1997), and the activation of ARF1 is required for the generation of the high- affinity recruitment of AP1 to the TGN membrane (Traub et al., 1993). Therefore, under physiological conditions, coat complexes will not bind sorting determinants until as yet unknown events directing GTPase activation occur.
Although our studies largely focused on analysis of recruitment of cargo in the presence of Sar1 and Sec23–24 into prebudding complexes, we found that the subsequent addition of cytosol or just the Sec13–31 complex alone was sufficient to promote vesicle formation. Thus, Sar1 and the Sec23–24 complex are necessary but not sufficient for budding. However, their recruitment represents a bonafide intermediate in the stepwise assembly of a vesicle on the ER membrane. The specific role of Sec13–31 in steps subsequent to cargo selection remains to be determined.
The Molecular Basis for Cargo Selection
What are the determinants that dictate the recognition between cargo and COP II components? We have recently identified a diacidic motif on the VSV-G cytoplasmic tail and a number of other transmembrane proteins that accelerate export from the ER (Nishimura and Balch, 1997). In the absence of the diacidic code, VSV-G is still selectively exported from the ER, but at a markedly (∼10-fold) lower rate, suggesting that the code directs increased affinity for the export machinery. However, at this point we cannot detect significant interaction between the VSV-G cytosolic tail and the purified Sec23/24 complex (Aridor, M., and W.E. Balch, unpublished observations). Therefore, additional membrane proteins may function as adaptors to link cargo to the COPII budding machinery. A number of membrane-associated proteins have been reported to affect selective cargo incorporation and general vesicle formation. Sec23 can interact directly with Sec16, a peripheral membrane component of COPII coats, which may play a role in cargo selection (Campbell and Schekman, 1997; Espenshade et al., 1995; Shaywitz et al., 1997). A prominent protein of similar molecular weight was detected in the cargo-containing complex (Fig. 6 C; top, gray arrowhead). Moreover, members of the p24 family of vesicle proteins (Schimmoller et al., 1995; Stamnes et al., 1995) and the gene products BST1 and BST3 (Elrod-Erickson and Kaiser, 1996) are genetically linked to the regulation of COPII vesicle formation. Mutation of these proteins can cause selective defects in cargo export (Schimmoller et al., 1995) and partially compensate for the loss of the COPII subunit Sec13 while compromising cargo sorting at the ER (Elrod-Erickson and Kaiser, 1996). Interestingly, a double phenylalanine motif in the cytoplasmic tail of p24 family members has been shown to be required for intra-Golgi transport and possibly ER-to-Golgi transport (Fiedler et al., 1996). Residues located in the transmembrane domain of this family of proteins have been found to affect the activity of the phenylalanine motif (Fiedler and Rothman, 1997). These results support the possibility that additional membrane-associated proteins may be required for the sorting of cargo from ER resident proteins. As only a limited number of unidentified proteins (approximately six) were detected in the cargo containing prebudding complex over background, their potential role in promoting COPII interactions and cargo sorting will undoubtedly shed important insight into the cargo selection process.
Acknowledgments
This work was supported by grants from the National Institutes of Health to W.E. Balch (GM 42336, CA 58689, and DK 51870). The electron microscopy made extensive use of Core B in CA 586689. M. Aridor is a recipient of a fellowship grant from the European Molecular Biology Organization (EMBO) and the Human Frontier Sciences Program; S. Bannykh is a recipient of a Cystic Fibrosis Foundation postdoctoral fellowship; C. Nuoffer was a recipient of a fellowship from EMBO and the Swiss National Science Foundation.
Abbreviations used in this paper
- COP
coat protein
- GAP
GTPase-activating protein
- GS
glutathione-Sepharose
- GST
glutathione-S-transferase
- HAP
hydroxylapitate
- HSP
high speed pellet
- VSV-G
vesicular stomatitis virus glycoprotein
Footnotes
Address all correspondence to William E. Balch, Department of Cell Biology, 10550 North Torrey Pines Road, La Jolla, CA 92037. Tel.: (619) 784-2310. Fax: (619) 784-9126. E-mail: webalch@scripps.edu
References
- Aridor M, Balch WE. Principles of selective transport: coat complexes hold the key. Trends Cell Biol. 1996a;6:315–320. doi: 10.1016/0962-8924(96)10027-1. [DOI] [PubMed] [Google Scholar]
- Aridor M, Balch WE. Timing is everything. Nature. 1996b;383:220–221. doi: 10.1038/383220a0. [DOI] [PubMed] [Google Scholar]
- Aridor M, Bannykh SI, Rowe T, Balch WE. Sequential coupling between COPII and COPI vesicle coats in endoplasmic reticulum-to-Golgi transport. J Cell Biol. 1995;131:875–893. doi: 10.1083/jcb.131.4.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balch WE, McCaffery JM, Plutner H, Farquhar MG. Vesicular stomatitis virus glycoprotein is sorted and concentrated during export from the endoplasmic reticulum. Cell. 1994;76:841–852. doi: 10.1016/0092-8674(94)90359-x. [DOI] [PubMed] [Google Scholar]
- Bannykh SI, Rowe T, Balch WE. Organization of endoplasmic reticulum export complexes. J Cell Biol. 1996;135:19–35. doi: 10.1083/jcb.135.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlowe C, Orci L, Yeung T, Hosobuchi M, Hamamoto S, Salama N, Rexach MF, Ravazzola M, Amherdt M, Schekman R. COPII: a membrane coat formed by sec proteins that drive vesicle budding from the endoplasmic reticulum. Cell. 1994;77:895–907. doi: 10.1016/0092-8674(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Beckers CJM, Keller DS, Balch WE. Semi-intact cells permeable to macromolecules: use in reconstitution of protein transport from the endoplasmic reticulum to the Golgi complex. Cell. 1987;50:523–534. doi: 10.1016/0092-8674(87)90025-0. [DOI] [PubMed] [Google Scholar]
- Bonifacino JS, Marks MS, Ohno H, Kirchhausen T. Mechanisms of signal-mediated protein sorting in the endocytic and secretory pathways. Proc Assoc Am Physic. 1996;4:285–295. [PubMed] [Google Scholar]
- Campbell JL, Schekman R. Selective packaging of cargo molecules into endoplasmic reticulum-derived COPII vesicles. Proc Natl Acad Sci USA. 1997;94:837–842. doi: 10.1073/pnas.94.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosson P, Letourner F. Coatomer interaction with di-lysine endoplasmic reticulum retention motifs. Science. 1994;263:1629–1631. doi: 10.1126/science.8128252. [DOI] [PubMed] [Google Scholar]
- Davidson HW, Balch WE. Differential inhibition of multiple vesicular transport steps between the endoplasmic reticulum and trans-Golgi network. J Biol Chem. 1993;268:4216–4226. [PubMed] [Google Scholar]
- Elrod-Erickson MJ, Kaiser CA. Genes that control the fidelity of endoplasmic reticulum to Golgi transport identified as suppressors of vesicle budding mutations. Mol Biol Cell. 1996;7:1043–1058. doi: 10.1091/mbc.7.7.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espenshade P, Gimeno RE, Holzmacher E, Teung P, Kaiser CA. Yeast SEC16 gene encodes a multidomain vesicle coat protein that interacts with Sec23p. J Cell Biol. 1995;131:311–324. doi: 10.1083/jcb.131.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler K, Rothman JE. Sorting determinants in the transmembrane domain of p24 proteins. J Biol Chem. 1997;272:24739–24742. doi: 10.1074/jbc.272.40.24739. [DOI] [PubMed] [Google Scholar]
- Fiedler K, Veit M, Stamnes MA, Rothman JE. Bimodal interaction of coatomer with a family of putative cargo and vesicle coat protein receptors, the p24 proteins. Science. 1996;273:1396–1399. doi: 10.1126/science.273.5280.1396. [DOI] [PubMed] [Google Scholar]
- Hicke L, Schekman R. Yeast Sec23p acts in the cytoplasm to promote protein transport from the endoplasmic reticulum to the Golgi complex in vivo and in vitro. EMBO (Eur Mol Biol Organ) J. 1989;8:1677–1684. doi: 10.1002/j.1460-2075.1989.tb03559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicke L, Yoshihisa T, Schekman R. Sec23p and a novel 105 kD protein function as a multimeric complex to promote vesicle budding and protein transport from the ER. Mol Biol Cell. 1992;3:667–676. doi: 10.1091/mbc.3.6.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis TE. Microinjected antibodies against the cytoplasmic domain of vesicular stomatitis virus glycoprotein block its transport to the cell surface. EMBO (Eur Mol Biol Organ) J. 1986;5:931–941. doi: 10.1002/j.1460-2075.1986.tb04306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn MJ, Herrmann M, Schekman R. COPII-cargo interactions direct protein sorting into ER-derived transport vesicles. Nature. 1998;391:187–190. doi: 10.1038/34438. [DOI] [PubMed] [Google Scholar]
- Kuge O, Dascher C, Orci L, Rowe T, Amherdt M, Plutner H, Ravazzola M, Tanigawa G, Rothman JE, Balch WE. Sar1 promotes vesicle budding from the endoplasmic reticulum but not Golgi compartments. J Cell Biol. 1994;125:51–65. doi: 10.1083/jcb.125.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuge W, Hara-Kuge S, Orci L, Ravazzola M, Amherdt M, Tanigawa G, Wieland FT, Rothman JE. ζ-COP, a subunit of coatomer, is required for COP-coated vesicle assembly. J Cell Biol. 1993;123:1727–1734. doi: 10.1083/jcb.123.6.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan R, Delphin C, Guan T, Gerace L, Melchior F. A small ubiquitin-related polypeptide involved in targeting RanGAP1 to nuclear pore complex RanBP2. Cell. 1997;88:97–107. doi: 10.1016/s0092-8674(00)81862-0. [DOI] [PubMed] [Google Scholar]
- Nishimura N, Balch WE. A di-acidic signal required for selective export from the endoplasmic reticulum. Science. 1997;277:556–558. doi: 10.1126/science.277.5325.556. [DOI] [PubMed] [Google Scholar]
- Nuoffer CN, Davidson HW, Matteson J, Meinkoth J, Balch WE. A GDP-bound form of Rab1 inhibits protein export from the endoplasmic reticulum and transport between Golgi compartments. J Cell Biol. 1994;125:225–237. doi: 10.1083/jcb.125.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno H, Stewart J, Fournier MC, Bosshart H, Rhee I, Miyatake S, Saito T, Gallusser A, Kirchhausen T, Bonifacino JS. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science. 1995;269:1872–1875. doi: 10.1126/science.7569928. [DOI] [PubMed] [Google Scholar]
- Ohno H, Fournier M-C, Poy G, Bonifacino JS. Structural determinants of interaction of tyrosine-based sorting signals with the adaptor medium chains. J Biol Chem. 1996;271:29009–29015. doi: 10.1074/jbc.271.46.29009. [DOI] [PubMed] [Google Scholar]
- Orci L, Ravazzola M, Meda P, Holcomb C, Moore H-P, Hicke L, Schekman R. Mammalian Sec23p homologue is restricted to the endoplasmic reticulum transitional cytoplasm. Proc Natl Acad Sci USA. 1991;88:8611–8615. doi: 10.1073/pnas.88.19.8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paccaud J-P, Reith W, Carpentier J-L, Ravazzola M, Amherdt M, Schekman R, Orci L. Cloning and functional characterization of mammalian homologues of the COPII component Sec23. Mol Biol Cell. 1996;7:1535–1546. doi: 10.1091/mbc.7.10.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peter F, Nuoffer C, Pind SN, Balch WE. Guanine nucleotide dissociation inhibitor (GDI) is essential for rab1 function in budding from the ER and transport through the Golgi stack. J Cell Biol. 1994;126:1393–1406. doi: 10.1083/jcb.126.6.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pind S, Nuoffer C, McCaffery JM, Plutner H, Davidson HW, Farquhar MG, Balch WE. Rab1 and Ca2+are required for the fusion of carrier vesicles mediating endoplasmic reticulum-to-Golgi transport. J Cell Biol. 1994;125:239–252. doi: 10.1083/jcb.125.2.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plutner H, Cox AD, Pind S, Khosravi-Far R, Bourne JR, Schwaninger R, Der CJ, Balch WE. Rab1b regulates vesicular transport between the endoplasmic reticulum and successive Golgi compartments. J Cell Biol. 1991;115:31–43. doi: 10.1083/jcb.115.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plutner H, Davidson HW, Saraste J, Balch WE. Morphological analysis of protein transport from the endoplasmic reticulum to Golgi membranes in digitonin permeabilized cells: role of the p58 containing compartment. J Cell Biol. 1992;119:1097–1116. doi: 10.1083/jcb.119.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe, T., and W.E. Balch. 1995. Expression and purification of mammalian Sar1. In Methods in Enzymology. W.E. Balch, C.J. Der, and A. Hall, editors. Small GTPases and Their Regulators: Proteins Involved in Transport. Academic Press, San Diego, CA. [DOI] [PubMed]
- Rowe T, Aridor M, McCaffery JM, Plutner H, Balch WE. COPII vesicles derived from mammalian endoplasmic reticulum (ER) microsomes recruit COPI. J Cell Biol. 1996;135:895–911. doi: 10.1083/jcb.135.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybin V, Ullrich O, Rubino M, Alexandrov K, Simon I, Seabra MC, Goody R, Zerial M. GTPase activity of rab5 acts as a timer for endocytic membrane fusion. Nature. 1996;383:266–269. doi: 10.1038/383266a0. [DOI] [PubMed] [Google Scholar]
- Scales SJ, Pepperkok R, Kreis TE. Visualization of ER-to-Golgi transport in living cells reveals a sequential mode of action for COPII and COPI. Cell. 1997;90:1137–1148. doi: 10.1016/s0092-8674(00)80379-7. [DOI] [PubMed] [Google Scholar]
- Schimmoller F, Singer-Kruger B, Schroder S, Kruger U, Barlowe C, Riezman H. The absence of Emp24p, a component of ER-derived COPII-coated vesicles, causes a defect in transport of selected proteins to the Golgi. EMBO (Eur Mol Biol Organ) J. 1995;14:1329–1339. doi: 10.1002/j.1460-2075.1995.tb07119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz DA, Orci L, Ravazzola M, Swaroop A, Kaiser CA. Human SEC13Rp functions in yeast and is located on transport vesicles budding from the endoplasmic reticulum. J Cell Biol. 1995;128:769–777. doi: 10.1083/jcb.128.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaywitz DA, Espenshade PJ, Gimeno RE, Kaiser CA. COPII subunit interactions in the assembly of the vesicle coat. J Biol Chem. 1997;272:25413–25416. doi: 10.1074/jbc.272.41.25413. [DOI] [PubMed] [Google Scholar]
- Simpson F, Bright NA, West MA, Newman LS, Darnell RB, Robinson MS. A novel adaptor-related protein complex. J Cell Biol. 1996;133:749–760. doi: 10.1083/jcb.133.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn K, Orci L, Ravazzola M, Amherdt M, Bremser M, Lottspecich F, Fiedler K, Helms JB, Wieland FT. A major transmembrane protein of Golgi-derived COPI-coated vesicles involved in coatomer binding. J Cell Biol. 1997;135:1239–1248. doi: 10.1083/jcb.135.5.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamnes MA, Craighead MW, Hoe MH, Lampen N, Geromanos S, Tempst P, Rothman JE. An integral membrane component of COPI-coated transport vesicles defines a new family of proteins involved in budding. Proc Natl Acad Sci USA. 1995;92:8011–8015. doi: 10.1073/pnas.92.17.8011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang BL, Peter F, Krijnse-Locker J, Low SH, Griffiths G, Hong W. The mammalian homolog of yeast Sec13p is enriched in the intermediate compartment and is essential for protein transport from the endoplasmic reticulum to the Golgi apparatus. Mol Cell Biol. 1997;1:256–266. doi: 10.1128/mcb.17.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisdale EJ, Plutner H, Matteson J, Balch WE. P53/58 binds COPI and is required for selective transport through the early secretory pathway. J Cell Biol. 1997;137:581–593. doi: 10.1083/jcb.137.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub LM, Ostrom JA, Kornfeld S. Biochemical dissection of AP-1 recruitment onto Golgi membranes. J Cell Biol. 1993;123:561–573. doi: 10.1083/jcb.123.3.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessel D, Flugge UI. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal Biochem. 1984;138:141–143. doi: 10.1016/0003-2697(84)90782-6. [DOI] [PubMed] [Google Scholar]
- Wieland FT, Gleason ML, Serafini TA, Rothman JE. The rate of bulk flow from the endoplasmic reticulum to the cell surface. Cell. 1987;50:289–300. doi: 10.1016/0092-8674(87)90224-8. [DOI] [PubMed] [Google Scholar]
- Yeung T, Yoshihisa T, Schekman R. Purification of the Sec23p-Sec24p complex. Methods Enzymol. 1995;257:145–151. doi: 10.1016/s0076-6879(95)57020-9. [DOI] [PubMed] [Google Scholar]
- Yoshihisa T, Barlowe C, Schekman R. Requirement for a GTPase-activating protein in vesicle budding from the endoplasmic reticulum. Nature. 1993;259:1466–1468. doi: 10.1126/science.8451644. [DOI] [PubMed] [Google Scholar]
- Zhao L, Helms JB, Brugger B, Harter C, Martoglio B, Graf R, Brunner J, Wieland FT. Direct and GTP-dependent interaction of ADP ribosylation factor 1 with coatomer subunit beta. Proc Natl Acad Sci USA. 1997;94:4418–4423. doi: 10.1073/pnas.94.9.4418. [DOI] [PMC free article] [PubMed] [Google Scholar]