Abstract
Cell-to-cell junction structures play a key role in cell growth rate control and cell polarization. In endothelial cells (EC), these structures are also involved in regulation of vascular permeability and leukocyte extravasation. To identify novel components in EC intercellular junctions, mAbs against these cells were produced and selected using a morphological screening by immunofluorescence microscopy. Two novel mAbs, LIA1/1 and VJ1/16, specifically recognized a 25-kD protein that was selectively localized at cell–cell junctions of EC, both in the primary formation of cell monolayers and when EC reorganized in the process of wound healing. This antigen corresponded to the recently cloned platelet-endothelial tetraspan antigen CD151/PETA-3 (platelet-endothelial tetraspan antigen-3), and was consistently detected at EC cell–cell contact sites. In addition to CD151/PETA-3, two other members of the tetraspan superfamily, CD9 and CD81/ TAPA-1 (target of antiproliferative antibody-1), localized at endothelial cell-to-cell junctions. Biochemical analysis demonstrated molecular associations among tetraspan molecules themselves and those of CD151/ PETA-3 and CD9 with α3β1 integrin. Interestingly, mAbs directed to both CD151/PETA-3 and CD81/ TAPA-1 as well as mAb specific for α3 integrin, were able to inhibit the migration of ECs in the process of wound healing. The engagement of CD151/PETA-3 and CD81/TAPA-1 inhibited the movement of individual ECs, as determined by quantitative time-lapse video microscopy studies. Furthermore, mAbs against the CD151/PETA-3 molecule diminished the rate of EC invasion into collagen gels. In addition, these mAbs were able to increase the adhesion of EC to extracellular matrix proteins. Together these results indicate that CD81/TAPA-1 and CD151/PETA-3 tetraspan molecules are components of the endothelial lateral junctions implicated in the regulation of cell motility, either directly or by modulation of the function of the associated integrin heterodimers.
Intercellular adhesion structures provide, by means of transmembrane proteins selectively localized at the sites of cell–cell contact, the physical strength necessary to build up solid tissues interconnecting the cytoskeleton from the different cells. Junctional structures are also responsible for the polarization of certain cell types, determining different functional subdomains along the plasma membrane, each containing a defined subset of proteins. Tight junctions, composed by the transmembrane protein occludin (Furuse et al., 1993) coupled to cytoplasmic proteins ZO-1, ZO-2, 7H6, cingulin, and symplekin (Keon et al., 1996; for review see Schneeberger et al., 1992; Anderson et al., 1993; Citi 1993), are directly involved in restricting the lateral diffusion of proteins along the plane of the plasma membrane. Adherens junctions, formed by different cadherins (reviewed in Takeichi, 1990; Geiger and Ayalon, 1992; Dejana 1996) linked to the actin cytoskeleton by catenins (Tsukita et al., 1992; Kemler 1993; Cowin and Burke, 1996), initiate cell–cell contacts, nucleate the formation of other junctional structures (Gumbiner et al., 1988), and regulate the expression of the genes involved in the polarized phenotype (McNeill et al., 1990; Marrs et al., 1995). Focal adhesions, in which integrins are the transmembrane adhesion moiety, are mainly responsible for adhesion to the extracellular matrix (Jockusch et al., 1995), which may be sufficient for the establishment of some of the characteristics of a polarized cell phenotype (Drubin and Nelson, 1996). Other junctional complexes like gap junctions, composed by connexin oligomers (for review see Goodenough et al., 1996), do not play a structural role in intercellular adhesion but metabolically couple cells in a determinate tissue.
Intercellular connections are responsible for the main function of endothelial cells as a selective permeable barrier between the bloodstream and the rest of tissues along the body. Endothelial cell-to-cell adhesion also plays the aforementioned general role of cell growth rate control (Caveda et al., 1996) and tissue integrity maintenance. Growth control in endothelium has a great relevance in tumorigenesis, since angiogenesis is one of the main requisites for tumor progression and metastasis (Hanahan and Folkman, 1996). On the other hand, intercellular connections must be modulated by many different stimuli in order to finely regulate the permeability of the endothelial cells (EC)1 monolayer to plasma macromolecules and, in certain tissues and inflammatory conditions, to defined subpopulations of leukocytes present in the bloodstream. Vascular endothelial (VE)-cadherin, an endothelium-specific member of the superfamily of cadherins, seems to be one of the main regulators of permeability in EC monolayers. VE-cadherin is reversibly linked to actin cytoskeleton by catenins and its association with these proteins is rapidly regulated through phosphorylation on catenin tyrosine residues (Lampugnani et al., 1992; Dejana 1996). Other adhesion molecules, such as CD31/PECAM (platelet-endothelial cell adhesion molecule), also localize at intercellular contact sites where it may play a functional role similar to VE-cadherin. CD31 mediates both homophilic as well as heterophilic (CD31-αvβ3) molecular interactions, and is involved in the leukocyte transmigration across the EC monolayer (reviewed in Newman 1997). Certain integrins, such as α2β1 and α5β1, have also been implicated in the maintenance of the EC monolayer integrity (Lampugnani et al., 1991).
The tetraspan superfamily of proteins (TM4) comprises a group of molecules with four membrane-spanning domains, which have been implicated in several cellular functions, as regulation of cell growth and differentiation, cell adhesion, and intracellular signaling (reviewed in Wright and Tomlison, 1994; Hemler et al., 1996; Maecker et al., 1997). In this study, we show the specific intercellular localization of CD9, CD81/TAPA-1 and CD151/PETA-3 tetraspan molecules in EC as well as their specific interaction with α3β1 integrin. These tetraspan molecules appear to have an important role in EC motility, likely by altering cell–matrix adhesion.
Materials and Methods
Cells and Cell Cultures
Human EC from umbilical vein (HUVEC) were obtained and cultured as described previously (Jaffe et al., 1972). In brief, cells were seeded on tissue culture flasks or dishes coated with gelatin 0.5% and grown in 199 medium (Bio Whittaker, Verviers, Belgium) supplemented with 20% FCS (GIBCO BRL, Gaithersburg, MD), 50 IU/ml penicillin, 50 μg/ml streptomycin (ICN Biomedicals, Costa Mesa, CA), 250 μg/ml fungizone (Squibb Industria Farmacéutica, Barcelona, Spain), 50 μg/ml EC growth supplement (ECGS; prepared from bovine brain), and 100 μg/ml heparin (Sigma Chemical Co., St. Louis, MO), and used up to the third passage. Human microvascular endothelial cell line-1 (HMEC-1; Ades et al., 1992) was grown in MCDB-131 medium (GIBCO BRL) supplemented with 20% FCS, 50 IU/ml penicillin, 50 μg/ml streptomycin, 10 ng/ml EGF (Boehringer Mannheim GmbH, Mannheim, Germany), and 1 μg/ml hydrocortisone (Sigma Chemical Co.) on tissue cultured plates precoated with gelatin (0.5%). For immunofluorescence studies, ECs were cultured on glass coverslips (12-mm diam), precoated with gelatin (1%) or human fibronectin (7 μg/ml; Sigma Chemical Co.).
Murine FDC-P1 cells infected with a retrovirus containing CD151 cDNA (FD-CD151) or empty retrovirus (FD-Ruf) were obtained and cultured as described (Ashman et al., 1997).
Antibodies
mAbs TEA1/31 (anti–VE-cadherin; Leach et al., 1993), TP1/15 (anti-CD31; García-Monzón et al., 1995), TS2/16 (anti-β1 integrins; Arroyo et al., 1992), W6/32 (anti–HLA-A,B; Campanero et al., 1991), and 11B1.G4 and 14A2.H1 (anti–CD151/PETA-3; Fitter et al., 1995) have previously been described. mAb GR2110 (anti-CD9) was provided by Dr. F. Garrido (Hospital Virgen de las Nieves, Granada, Spain), 50H.19 (anti-CD9) by Dr. A. Shaw (Cross Cancer Institute, Alberta, Canada), and 5A6 (anti– CD81/TAPA-1) by Dr. S. Levy (Oncology, Stanford University School of Medicine, Stanford, CA). TEA3/18 mAb, clustered as CD63 in the VI International Leukocyte Typing Workshop, and VJ1/14 (anti-β1 integrin) were obtained in our laboratory and their characterization will be described elsewhere. The monoclonal Ig (IgG1,κ) from the P3X63 myeloma cell line was used as negative control. Anti-α2, -α3, -α5, and -β1 integrin chain rabbit polyclonal antibodies directed against the cytoplasmic tail of these molecules were provided by Dr. G. Tarone (Università di Torino, Italy), and anti-α3 rabbit polyclonal antibody as well as P1B5 mAb were purchased from Chemicon International, Inc. (Temecula, CA). Both anti-αv rabbit polyclonal and ABA-6D1 monoclonal Abs were provided by Dr. C. Martinez-A. (Centro Nacional de Biotecnología, Madrid, Spain).
Generation of LIA1/1 and VJ1/16 mAbs
BALB/c mice were injected intraperitoneally with 1.5 × 107 cells (U937 or HMEC-1) on days −45 and −30 and intravenously on day −3. Spleen cells were fused on day 0 with SP2 mouse myeloma cells at a ratio of 4:1 according to standard techniques and distributed on 96-well tissue culture plates (Costar Corp., Cambridge, MA). After 2 wk, hybridoma culture supernatants were harvested and their reactivity was tested against the cell line used in immunization by flow cytometry. Positive supernatants were assayed by immunofluorescence staining of HUVEC, and hybridomas showing a specific cell–cell contact staining were cloned and subcloned by limiting dilution. Both mAbs LIA1/1 and VJ1/16 were IgG1, κ. mAbs were purified from ascitic fluid using an affinity chromatography column of protein A–Sepharose (Pharmacia Biotech Sverige, Uppsala, Sweden) eluted with sodium citrate 0.1 M pH 3.5. Purified antibodies were then either coupled to CNBr-activated CL-4B Sepharose (Pharmacia Biotech Sverige) or biotinylated with N-hydroxysuccinimide-biotin (Pierce Chemical Co., Rockford, IL).
Immunofluorescence Microscopy
Immunofluorescence assays were performed as previously described (Lampugnani et al., 1992), but nonspecific binding sites were blocked by incubation with TNB (0.1 M Tris-HCl, 0.15 M NaCl, 0.5% blocking reagent; Boehringer Mannheim GmbH). Cells were fixed with 4% formaldehyde in PBS supplemented with 1 mM CaCl2 and 1 mM MgCl2 for 15 min at room temperature or with methanol 5 min at −20°C. Secondary FITC-conjugated antibodies to the immunoglobulins of the appropriate species, depending on the primary antibody (anti-mouse Igs from DAKOPATTS, Copenhagen, Denmark or anti-rabbit Igs from Pierce Chemical Co.) were used. When double stained for actin, samples were fixed again with 4% formaldehyde in PBS supplemented with 1 mM CaCl2 and 1 mM MgCl2, permeabilized with 0.1% NP-40 in PBS, washed and incubated with Texas red–phalloidin (Molecular Probes, Inc., Eugene, OR). Samples were examined with a Nikon Labophot-2 photomicroscope with a ×60 oil immersion objective. Images were recorded on TMAX 400 film (Kodak).
Confocal Microscopy
For double staining, cells were incubated with the primary antibody followed by an FITC- or a Cy2-conjugated (Amersham Pharmacia Biotech Inc., Piscataway, NJ) anti-mouse Ig. Then, samples were saturated with mouse serum before the incubation with the second mAb coupled to biotin and revealed with avidin conjugated to Cy-3 (Amersham Pharmacia Biotech Inc.) or to TMRITC (Vector Labs, Inc., Burlingame, CA). Series of optical sections were obtained with a confocal scanning laser microscope (MCR 1024; Bio-Rad Laboratories, Hercules, CA) mounted on a Zeiss Axiovert 135 microscope (Zeiss, Oberkochen, Germany) equipped with a ×63, 1.4 NA planapochromat objective. Fluorescences of Cy2/ FITC and Cy3/TMRITC were obtained with the two major lines at 488 and 514 nm of a 25 mW multilinea Argon laser. The gain and contrast of the photomultiplier were set in order to obtain an optimal detection of the two types of fluorescence while limiting fluorescence overlapping.
Flow Cytometry Analysis
EC either nonactivated or activated 15 h in the presence of 320 U/ml of tumor necrosis factor-α (TNF-α; Wiechen, Vienna, Austria), were trypsinized, washed and resuspended in PBS. The activated state of the ECs was assessed by measuring the expression level of inducible EC markers as E-selectin or vascular cell adhesion molecule (VCAM-1; data not shown). Nonadherent cells were also washed and resuspended in PBS. A total of 5 × 105 cells were incubated with 100 μl of hybridoma culture supernatants for 20 min at 4°C, washed with PBS and then incubated with 100 μl of a 1:50 dilution of an FITC-conjugated anti–mouse Ig. Finally, fluorescence was measured using a FACScan® flow cytometer (Becton Dickinson Labware, Lincoln Park, NJ)
Cell Labeling, Immunoprecipitation, and Western Blot
For metabolic labeling, HUVEC on confluence were washed twice with PBS supplemented with 1 mM CaCl2, 1 mM MgCl2, and incubated at 37°C, 5% CO2 with RPMI-1640 medium free of methionine (ICN Biomedicals, Costa Mesa, CA) supplemented with 20% dialyzed FCS, 50 IU/ ml penicillin, 50 μg/ml streptomycin, ECGS, and heparin. After 45 min, 500 μCi 35S-labeled methionine (Trans 35S-Label™; ICN Pharmaceuticals, Inc., Irvine, CA) were added as well as 10% of the final volume of 199 medium with 20% FCS containing ECGS and heparin. Cells were then cultured overnight at 37°C, 5%CO2, washed twice with PBS 1 mM CaCl2, 1 mM MgCl2, and lysed in PBS, pH 7.5 containing 1% Triton X-100, 1% hemoglobin, 1 mM PMSF (Sigma Chemical Co.) at 4°C. Cell lysates were immediately clarified by centrifugation at 14,000 rpm for 10 min and precleared four times with Gly–Sepharose. For immunoprecipitation, ∼6 × 107 cpm of precleared cell lysates were incubated, for 2 h at 4°C under continuous mixing, with 40 μl of the mAb or Glycine coupled to Sepharose. Immunoprecipitates were then washed twice with lysis buffer 1:10 in PBS 1 mM CaCl2, 1 mM MgCl2, and boiled 5 min at 96°C in Laemmli buffer containing or not 5% 2-mercaptoethanol (reducing or nonreducing conditions, respectively). Samples were then analyzed by SDS-PAGE followed by fluorography and autoradiography.
For Western blot assays, unlabeled cells were lysed under “mild” (TBS 1 mM CaCl2, 1 mM MgCl2, 1% Brij-96, 1% hemoglobin, and 1 mM PMSF) or “stringent” conditions (PBS, 1% Triton X-100, 1% hemoglobin, and 1 mM PMSF). Lysates were immunoprecipitated as before, washed either in 1:10 or undiluted lysis buffer for stringent or mild detergent conditions, separated by SDS-PAGE under nonreducing conditions, and transferred to a nitrocellulose membrane (Protran Nitrocellulose 0.2 μm; Filtration Life-Science, Dassel, Germany). The membrane was then blocked overnight in 10% nonfat milk or 5% BSA in PBS-T (PBS, 0.05% Tween 20), incubated with the primary antibody for 2 h at room temperature under continuous shaking, washed twice with PBS-T before incubation with the secondary antibody to the appropriate Ig species (Pierce Chemical Co.) or streptavidin (DAKOPATTS) conjugated to horseradish peroxidase, and then revealed by enhanced chemiluminescence (ECL; Amersham Pharmacia Biotech Inc.).
Wound Healing Assays
For the measurement of cell migration during wound healing, ECs were seeded on 24-well plates (Costar Corp.) coated with gelatin and grown to confluence. Cells were changed to 199 medium 20% FCS without growth factors 16 h before the experiment and 30 min before the lesion, preincubated with different purified mAbs at 20 μg/ml. Monolayers were then disrupted with a cell scrapper of ∼1.2 mm and photographed at 4, 8, 12, 24, and 28 h in a phase contrast microscope (Nikon ELWD 0.3). Experiments were done in duplicate and two fields of each well were recorded. For immunofluorescence studies during injury repair, cells were maintained in the presence of growth factors and no antibody was added. Cells were scrapped, fixed at the times indicated, and processed as indicated above.
Videomicroscopy Endothelial Cell Motility Assay
Endothelial cells were plated in 199 medium supplemented with 20% FCS on 0.5% gelatin-coated 3.5-cm plastic petri dishes and cultured for 24 h before filming in the absence of growth factors. 30 min before videorecording, different antibodies (final concentration 20 μg/ml) were added to the dishes. Cell videorecords were generated as a sequence of individual digital images (frames) that were obtained every 5 min for a period of 5 h in an Axiovert 135 Zeiss videomicroscope using the IP-LAB-Spectrum software (Signal Analytics Corporation, Vienna, Austria). The tracks of random migration, distances, and average speeds of individual endothelial cells were obtained using the CELL TRACKING software extension developed by Tim Hutton (Confocal Microscopy and Digital Image Unit, Imperial Cancer Research Fund, London, UK).
Collagen Gel Assay
Three-dimensional collagen gels were prepared by diluting type I collagen (ICN Biomedicals Inc.) in serum-free Dulbecco's MEM (Seromed, Heidelberg, Germany) to a final concentration of 500 μg/ml. 500 μl per well of this solution were dispensed onto 24-well plates (Costar Corp.) and allowed to solidify for one h at 37°C. Then, trypsinized HUVECs were plated at confluence in 199 medium (BioWhittaker, Inc., Walkersville, MD) supplemented with 10% FCS (GIBCO BRL) on the surface of the gels. After cell attachment, different mAbs (20 μg/ml) in combination with PMA (20 ng/ml; Sigma Chemical Co.) were added. Collagen gels were photographed after 24 h in a phase contrast microscope (Nikon ELWD 0.3), and migrating cells (dendritic-shaped cells, whose plane of focus was beneath the surface monolayer) were counted in four 20× random fields per well. Experiments were done in duplicate.
Adhesion Assays
For cellular adhesion assays, 96-microwell plates (Nunc-Immuno Plates Maxisorp; Nunc, Inc., Naperville, IL) were coated with different extracellular matrix (ECM) proteins (2 μg/ml fibronectin, 10 μg/ml laminin; Sigma Chemical Co., or Type-I collagen 5 μg/ml; ICN Biomedicals Inc.) for 2 h at 37°C, washed with PBS and blocked with 1% heat denatured (30 min 60°C) BSA for 1 h at 37°C. Then, trypsinized ECs were labeled with the fluorescent probe BCECF-AM (Molecular Probes, Inc.) by incubating the cells in loading buffer (199 medium supplemented with 20 mM Hepes; BioWhittaker, Inc., and 0.1% heat denatured BSA) containing 1 μM BCECF-AM for 15 min at 37°C. Cells were then washed and resuspended in loading buffer containing either 10 μg/ml of different purified mAbs or, anti-α3 P1B5 mAb ascitic fluid diluted 1:100, for 10 min. Then, 3 × 104 cells/well were added to the coated plates, and incubated 15 or 30 min at 37°C. Unbound cells were removed by adding PBS 1 mM CaCl2, 1 mM MgCl2, 20 mM Hepes, 0.1% BSA, sealing with plastic, and inverting the plate for 30 min. The number of cells adhered to the wells was obtained by solubilization with 0.1% SDS in 50 mM Tris, pH 8.5, and fluorescence intensity measurement in a microplate fluorescence reader (Bio-tek FL500). All assays were run by triplicate.
Results
Expression of a Protein of 25-kD (CD151/PETA-3), Specifically Localized at Endothelial Cell-to-Cell Junctions
To search for novel molecules selectively localized at intercellular cell junctions, different mAbs were raised and screened by immunofluorescence techniques on HUVEC monolayers. Two novel mAbs, LIA 1/1 and VJ1/16, specifically recognized a molecule localized at endothelial cell– cell junctions (Fig. 1 a, C and D). When compared to other known EC junctional antigens, their staining pattern was similar to the almost continuous distribution along the cell–cell junctions of VE-cadherin (Fig. 1 a, A) and CD31 (Fig. 1 a, B), but completely different from the punctuated pattern of the gap-junction protein connexin 37 (data not shown). The biochemical analysis of the antigen recognized by LIA 1/1 and VJ 1/16 mAbs showed that both Abs immunoprecipitated a broad band of ∼25 kD from EC lysates metabolically labeled with [35S]methionine, both under reducing and nonreducing conditions (Fig 1 b).
Figure 1.
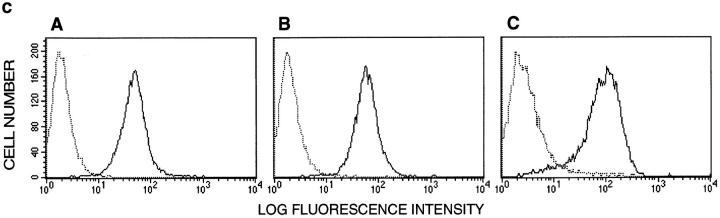
Subcellular localization at cell-to-cell junctions of HUVEC confluent monolayers and cellular distribution of the antigen recognized by LIA1/1 and VJ1/16 mAbs. (a) HUVEC confluent monolayers were stained with mAbs LIA1/1 and VJ1/16 (C and D, respectively), and with mAbs specific to previously characterized markers of EC cell–cell junctions: TEA1/31 anti–VE-cadherin (A) and TP1/15 anti-CD31 (B). The antigen recognized by both mAbs LIA1/1 and VJ1/16 localizes selectively at cell-to-cell junctions. (b) Immunoprecipitation of a 25-kD protein by mAbs LIA1/1 and VJ1/16. HUVECs were metabolically labeled with [35S]methionine and immunoprecipitated with LIA1/1 (lanes D and H),VJ1/16 (lanes C and G), and anti-CD31 TP1/15 (lanes B and F) mAbs coupled to Sepharose, or with Gly-Sepharose (lanes A and E). Immunoprecipitates were subjected to SDS-PAGE under either reducing (lanes A–D) or nonreducing (lanes E–H) conditions. Molecular mass markers are indicated. (c) Expression of LIA1/1 in ECs from different origins and states of activation. Flow cytometry analysis was performed on resting (A) or TNF-α–stimulated HUVECs (B), and HMEC-1 cells (C) stained with the LIA1/1 mAb. Negative control P3X63 mouse myeloma IgG1 is shown in dotted line. Bar, 20 μm.
The expression of LIA1/1 and VJ1/16 antigen was then analyzed by flow cytometry in EC. We found a high expression in both resting and TNF-α–activated HUVECs as well as in the microvascular endothelial cell line HMEC-1 (Fig. 1 c). The pattern of cellular distribution of LIA1/1 antigen as well as its molecular mass strongly suggested its identity with the platelet and endothelial tetraspan antigen PETA-3 (Sincock et al., 1997) clustered in the Sixth International Leukocyte Typing Workshop as CD151 (Immunol. Today. 1997. 18:100–101).
The identity of the 25-kD antigen recognized by LIA1/1 and VJ1/16 mAbs as CD151/PETA-3, was characterized by Western blot analysis. We found that the anti–CD151/ PETA-3 mAb, 11B1.G4 (Fitter et al., 1995), specifically reacted with the 25-kD band immunoprecipitated by LIA1/1 and VJ1/16 mAbs, (Fig. 2 a, lanes A and B, respectively), with no reactivity towards other tetraspan molecules such as CD9 or CD63 (Fig. 2 a, lanes C and D, respectively). In addition, a flow cytometry analysis was performed on cells transfected with the cDNA coding for CD151/PETA-3 (Fitter et al., 1995), that was independently cloned as SFA-1 (Hasegawa et al., 1996), showing specific recognition by both mAbs LIA1/1 and VJ1/16 of the transfected but not the parental cells (Fig. 2 b). Finally, cross-competitive binding assays with labeled LIA1/1, VJ1/16, and 11B1.G4 anti–CD151/PETA-3 mAbs showed that all three mAbs competed for the binding of labeled LIA1/1 or VJ1/16 mAbs (not shown). These data demonstrated that the LIA1/1 and VJ1/16 mAbs specifically recognize the CD151/PETA-3 tetraspan molecule.
Figure 2.
LIA1/1 and VJ1/16 mAbs specifically recognize the CD151/PETA-3 molecule. (a) The antigen immunoprecipitated by LIA1/1 and VJ1/16 is recognized by anti–CD151/ PETA-3 mAb in Western-blot. EC lysates obtained under “stringent” detergent conditions were immunoprecipitated with LIA1/1 (lane A) and VJ1/ 16 (lane B) as well as with mAbs to other tetraspan antigens present in ECs: GR2110 anti-CD9 (lane C), and TEA3/18 anti-CD63 (lane D). Then, precipitates were subjected to SDS-PAGE, transferred and blotted with the anti–CD151/PETA-3 11B1.G4 mAb. Specific recognition is observed only with the 25 kD antigen immunoprecipitated by LIA1/1 and VJ1/16 (lanes A and B). (b) LIA1/1 and VJ1/16 specifically react with CD151/PETA-3 cDNA expressing cells. LIA1/1, VJ1/16, 11B1.G4, and 14A2.H1 anti–CD151/PETA-3 mAbs were screened on murine FDC-P1 cells infected with a retrovirus containing CD151 cDNA (FD-CD151) or empty retrovirus (FD-Ruf). All mAbs were used as 1/100 dilutions of ascites. Data correspond to the arithmetic mean of mean fluorescence intensities ± 1 SE from triplicate determinations. mAbs 1D4.5 (IgG2a) and 3D3 (IgG1) are, respectively, isotype-matched negative controls for 11B1.G4 and all other mAbs.
Selective Localization of CD151/PETA-3 to Cell–Cell Contacts in EC
We examined in detail the junctional localization of CD151/PETA-3 in HUVEC monolayers by different approaches. Confocal microscopy studies confirmed that the staining with anti-CD151/PETA-3 VJ1/16 and anti–VE-cadherin TEA1/31 mAbs was almost coincident among different optical sections suggesting that CD151 antigen is localized at adherens-junctions (Fig. 3 a). Next we studied the localization of the CD151 antigen in HUVEC monolayers with increasing degrees of confluence. When EC were sparse, the staining was strictly confined to the cell– cell contact areas, being completely absent in isolated cells and the cell borders where no intercellular contact had yet been established. As the cellular density increased and the EC monolayer approached to confluence, the staining pattern was reinforced, always defining the points where adjacent EC interacted (Fig. 3 b, A, and data not shown). In confluent cell monolayers, the pattern of expression of this antigen was that of a typical junctional protein localizing all around the cell margins (Fig. 3 b, C), independently of the substratum where the EC were initially seeded (data not shown). The localization of CD151/PETA-3 in cell– cell contact areas was also evident during the process of cell monolayer repair in wound healing (Fig. 3 c). When an EC confluent monolayer is disrupted, the cells along the margin of the wound progressively invade the damaged area until the continuity of the cell monolayer is reestablished. We found that the CD151/PETA-3 antigen vanished from the margins of the ECs situated on the border of the wound where the cell–cell contact disappeared (Fig. 3 c, A and E). During the invasive movement of the cells into the damaged area, the staining was always confined to those sites where the cell–cell contact was retained.
Figure 3.


Analysis of the junctional character of CD151/PETA-3 in EC monolayers. (a) Comparison by confocal microscopy of the staining in HUVEC confluent monolayers of anti–VE-cadherin and anti–CD151/PETA-3 mAbs. HUVEC confluent monolayers were double-stained with anti–VE-cadherin mAb (TEA1/31) followed by a Cy2-conjugated anti-mouse Ig secondary antibody (A–C), and with biotinylated anti–CD151/PETA-3 (VJ1/16) plus Cy3-streptavidin (D–F) and analyzed by confocal microscopy. Optical sections distanced from each other by 0.4 μm the z axis, starting at the substratum level (A and D). The fluorescence signal in both channels was almost restricted to the same optical section (B and E), indicating the vertical colocalization of both antigens. (b) CD151/PETA-3 staining in HUVEC monolayers at increasing degrees of confluence is restricted to the points of cell–cell contact and is absent from cell margins where no intercellular contact has been established. Immunofluorescence staining was performed in nonconfluent (A), and confluent (C) HUVEC monolayers. B and D show the corresponding staining with phalloidin of the same field. (c) CD151/PETA-3 redistribution during EC monolayer wound healing. Confluent monolayers were disrupted and stained with the LIA1/1 mAb, as stated in Materials and Methods. Wounded edge: (A and E) asterisk shows the position of the wound; proximal to the front (C and G). (A and C) 4 h after the lesion; (E and G) 20 h after the lesion. B, D, F, and H show the corresponding staining with phalloidin of the same field. Note that the staining of LIA1/1 disappears from the margin of the EC cell where cell–cell contact is lost within 4 h, (A) remaining at the intercellular contacts during the process of invasion of the damaged area (C and G). The normal LIA1/1 staining pattern on EC distal from the wounded area did not change during the 20 h of the experiment (not shown). Bars, 20 μm.
Tetraspan Molecules CD9, CD81/TAPA-1 and CD151/PETA-3 Localize at EC Cell-to-Cell Junctions and Are Noncovalently Associated with Each Other and with α3β1 Integrin
Members of the tetraspan superfamily exhibit a complex pattern of cellular distribution and no detailed study of their localization in EC has yet been made, except for CD63 (Vischer and Wagner, 1993). By this reason, we decided to study the expression of other transmembrane 4/tetraspan molecule (TM4) proteins in EC. A high expression of CD9 and CD81/TAPA-1 was detected in the plasma membrane of resting HUVECs, as assessed by flow cytometry (data not shown). The cellular distribution of these antigens studied on HUVEC monolayers by immunofluorescence microscopy, showed that CD9 and CD81/TAPA-1 (Fig. 4 a, A and B) were localized at cell boundaries. In contrast, CD63 staining (Fig. 4 a, C) was, as expected, mainly restricted to lysosomes and intracellular vesicles, that likely corresponded to Weibel-Palade bodies (Vischer and Wagner, 1993). The expression of CD53 was completely absent in this cell type (not shown). Thus, HUVEC express, aside from CD151/PETA-3, considerable levels of CD9 and CD81/ TAPA-1 tetraspan molecules, which all localize at cell–cell junctions.
Figure 4.
Subcellular localization and association of different tetraspan proteins in ECs. (a) Immunofluorescence staining was performed on HUVEC confluent monolayers with GR2110 anti-CD9 (A), 5A6 anti–CD81/TAPA-1 (B), and TEA3/18 anti-CD63 (C), showing the cell–cell junctional pattern of expression of CD9, CD81/TAPA-1 and the staining of lysosomes and Weibel-Palade bodies of CD63. (b) EC lysates obtained under “mild” detergent conditions were immunoprecipitated with 5A6 anti–CD81/ TAPA-1 (lane A), or with LIA1/1 and VJ1/16 anti–CD151/PETA-3 (lanes B and C, respectively), GR2110 anti-CD9 (lane D) and TEA3/18 anti-CD63 (lane E) coupled to Sepharose. Precipitates were subjected to SDS-PAGE and immunoblots revealed with 11B1.G4 anti–CD151/PETA-3, or 50H.19 anti-CD9 mAbs. Coprecipitation of CD81/TAPA-1, CD9 and CD151/PETA-3 was observed using both anti-CD9 and anti–CD151/PETA-3 mAbs. CD63, although expressed by ECs, did neither localize at cell-to-cell junctions nor associate to CD9 or CD151/PETA-3 in these cells. Bar, 20 μm.
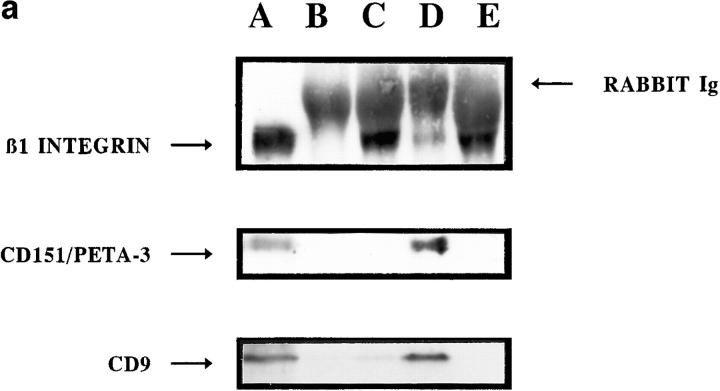
Proteins from the tetraspan superfamily share the capacity to interact noncovalently among themselves and with other integral membrane proteins including β1 integrins (Hemler et al., 1996). Therefore, we studied the possible association of CD151/PETA-3 with CD81/TAPA-1 and CD9 as well as the interaction of these TM4 molecules with β1 integrins in EC. For this purpose, we carried out Western blot analysis on immunoprecipitates of unlabeled EC lysates performed under mild detergent conditions. As shown in Fig. 4 b, coprecipitation of CD81/TAPA-1, CD151/PETA-3, and CD9 was observed in EC immunoprecipitates obtained with either the 5A6 anti–CD81/ TAPA-1 mAb (lane A), the LIA1/1 and VJ1/16 anti– CD151/PETA-3 mAbs (lanes B and C), or the GR2110 anti-CD9 mAb (lane D). In contrast, CD63, that did not localize at lateral junctions, did not coprecipitate CD9 or CD151/PETA-3 (lane E).
On the other hand, CD151/PETA-3 and CD9 molecules were found in anti-β1 (Fig. 5 a, lane A) as well as in anti-α3 (Fig. 5 a, lane D), but not in anti-αv, α5, or α2 immunoprecipitates (Fig. 5 a, lanes B, C, and E, respectively), whereas CD81 was not detected in any of the anti-integrin immunoprecipitates (not shown). Conversely, both the β1 as well as the α3 integrin chains, were easily detected in anti– CD151/PETA-3 and anti-CD9 but not in anti-CD63 or anti–CD81/TAPA-1 immunoprecipitates (Fi.g 5 b, lanes A–H, and data not shown). These associations were specific and independent of the level of integrin expression since both α2β1 and α5β1 heterodimers seemed to be quantitatively far more represented than α3β1 in EC, as shown by the amount of β1 integrin chain coprecipitated with each α chain. No associations of CD9 and CD151/ PETA-3 with other proteins also localized to EC cell–cell junctions, such as VE-cadherin, catenins or CD31 were observed (data not shown).
Figure 5.

Association of TM4 proteins to α3β1 integrin, which is also localized to lateral cell junctions from ECs. (a) EC lysates obtained under “mild” detergent conditions were immunoprecipitated with TS2/16 anti-β1 integrin chain mAb (lane A) and with rabbit polyclonal antibodies against αv, α5, α3, and α2 integrin chains (lanes B–E, respectively). Precipitates were subjected to SDS-PAGE and immunoblots revealed with biotinylated TS2/ 16 anti-β1 integrin, 11B1.G4 anti–CD151/PETA-3, or 50H.19 anti-CD9 mAbs. (b) EC lysates obtained under “mild” detergent conditions were immunoprecipitated with 5A6 anti-CD81 (lane A), LIA1/1 anti–CD151/ PETA-3 (lane B), GR2110 anti-CD9 (lane C) and TEA3/18 anti-CD63 (lane D) mAbs coupled to Sepharose and revealed with anti-α3 integrin polyclonal Ab. EC lysates were also precipitated with VJ1/16 and LIA1/1 anti–CD151/PETA-3 (lanes E and F, respectively), GR2110 anti-CD9 (lane G) and TEA3/18 anti-CD63 (lane H) and blotted with biotinylated TS2/16 anti-β1 integrin mAb. Coprecipitation of α3β1 integrin with CD9 and CD151/PETA-3 is evident in both tetraspan and integrin immunoprecipitates, whereas CD81/TAPA-1 and CD63 do not directly associate to this integrin. (c) Immunofluorescence staining was performed on HUVEC confluent monolayers with rabbit polyclonal antibodies against integrin chains β1 (A), α2 (B), α5 (C), or with the anti-α3 P1B5 (D) and anti-αv ABA-6D1 mAbs (E). F shows the same optical section of a sample doubled stained with P1B5 anti-α3 (green) and LIA1/1 anti–CD151/PETA-3 (red) mAbs. This section showed the maximal staining intensity of both antigens. Bars, 20 μm.
The α2β1 and α5β1 integrins have previously been reported to localize at EC cell-to-cell junctions (Lampugnani et al., 1991), whereas α3β1 has been detected in cell– cell contact sites from other cell types, such as epithelial cells (Larjava et al., 1990). Therefore, we have compared the cellular localization of the different integrins expressed by EC. β1 integrins were detected on EC plasma membrane, showing a reinforced staining at focal adhesions and intercellular boundaries (Fig. 5 c, A). α2 chain was mainly confined at cell–cell contact sites (Fig. 5 c, B), whereas anti-α5 mAbs mainly stained sites of cell–matrix interaction (Fig. 5 c, C), and only in some fields reacted with cell–cell contact sites. The αv integrin chain, which did not coprecipitate with β1 chain in EC (Fig. 5 a, lane B), showed a focal adhesion staining pattern (Fig. 5 c, E). Interestingly, α3 chain was detected in cell-to-cell junctions (Fig 5 c, D), in accordance to its association with TM4 proteins, and clearly colocalized with CD151/PETA-3 (Fig. 5 c, F), as assessed by two-color confocal microscopy analysis.
Antibodies Against CD151/PETA-3 and CD81/TAPA-1 Proteins Inhibit Migration of ECs
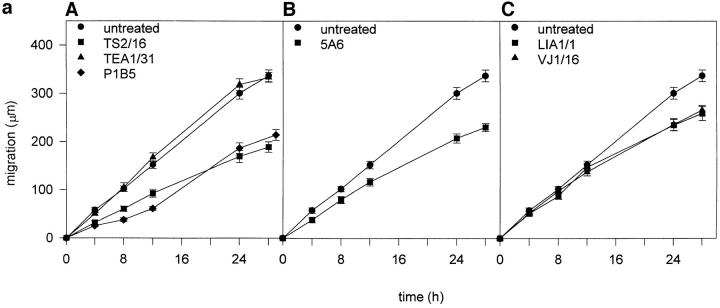
It has previously been reported by different experimental approaches, the involvement of CD9 (Miyake et al., 1991; Jones et al., 1996; reviewed in Maecker et al., 1997), and recently of CD63 (Radford et al., 1997) and CD81/TAPA-1 (Domanico et al., 1997) in tumor cell motility. Therefore, it was of interest to explore the effect of mAbs to different tetraspan molecules on EC motility in a wound healing migration assay. Interestingly, anti–CD81/TAPA-1 5A6 and anti–CD151/PETA-3 LIA1/1 and VJ1/16 mAbs significantly delayed the migration process by which the integrity of the EC monolayer is reestablished (Fig. 6 a, B and C). This effect was also observed with the P1B5 anti-α3 integrin chain mAb (Fig. 6 a, A). In addition, an inhibitory effect was observed with the proactivatory anti-β1 mAb TS2/16, which is able to block leukocyte migration (Weber et al., 1996; Gómez et al., 1997) and increase cell adhesion and spreading (Arroyo et al., 1992). In contrast, the anti– VE-cadherin TEA1/31 mAb, did not significantly affect the rate of migration of EC (Fig. 6 a, A). The anti-CD9 GR2110 showed no effect or a slight inhibitory effect in some migration assays (data not shown).
Figure 6.

Effects of anti-TM4 mAbs on EC migration. (a) Confluent EC monolayers treated with 20 μg/ml of different purified mAbs, or a 1/50 dilution of ascitis fluid of P1B5 anti-α3 integrin mAb, were scrapped and migration of the front of the wound was followed for 28 h. A shows the migration of ECs treated with anti– VE-cadherin TEA1/31, anti-α3 integrin P1B5, and anti-β1 integrin TS2/16 mAb. In panel B, cells were treated with 5A6 anti–CD81/TAPA-1. In panel C cells were preincubated with mAbs anti– CD151/PETA-3 LIA1/1 and VJ1/16. Results correspond to the arithmetic mean ± 1 SE of two experiments performed by duplicate. (b) Antibodies to TM4 molecules reduce EC motility. Videorecords of endothelial cells were generated as described under Materials and Methods in the presence of the following monoclonal antibodies: (A) TEA1/31 anti–VE-cadherin; (B) TS2/16 proactivatory anti-β1 integrin; (C) 5A6 anti–CD81/TAPA-1; and (D) LIA1/1 anti–CD151/PETA-3. The tracks of random migration of six individual cells were followed in each condition. The end point of each cell track are indicated by a filled circle.
The effect of antitetraspan mAbs on the EC motility was further assessed by measuring the rate of random migration of individual ECs by time-lapse videomicroscopy. Representative individual cell migration tracks are shown in Fig. 6 b. In this assay, the anti–CD81/TAPA-1 5A6 as well as the anti–CD151/PETA-3 LIA1/1 significantly decreased cell migration (Fig. 6 b, C and D and Table I), whereas the anti–VE-Cadherin TEA1/31 did not exert any effect (Fig. 6 b, A). The proactivatory anti-β1 integrin TS2/ 16 also diminished migration of endothelial cells (Fig. 6 b, B and Table I).
Table I.
Anti-TM4 mAbs Inhibit EC Random Motility on Gelatin
| mAb | Cell 1 | Cell 2 | Cell 3 | Cell 4 | Cell 5 | Cell 6 | Average speed | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| mean ± SD | ||||||||||||||
| None | 91.81 | 67.54 | 63.01 | 56.42 | 78.84 | 97.12 | 75.79 ± 16.29 | |||||||
| TEA 1/31 (VE-cadherin) | 100.52 | 64.84 | 69.27 | 59.82 | 66.52 | 58.8 | 69.96 ± 15.4 | |||||||
| TS 2/16 (β1) | 35.39 | 36.91 | 50.85 | 27.01 | 27.7 | 25.94 | 33.97 ± 9.4 | |||||||
| 5A6 (CD81) | 27.59 | 23.92 | 25.94 | 35.51 | 41.49 | 19.94 | 29.06 ± 7.96 | |||||||
| LIA 1/1 (CD151/PETA3) | 54.70 | 40.29 | 47.21 | 36.32 | 35.42 | 31.37 | 40.88 ± 8.6 |
Endothelial cells were plated on gelatin (0.5% in DW) coated plastic dishes and cultured for 24 h before filming starts. Films of cells were generated from stacks of individual digital photographs (frames) taken every 5 min for 5 h in an Axiovert 135 Zeiss videomicroscope using the IP-LAB-SPECTRUM software (Signal Analytics Corporation, Vienna, Virginia) and the distances migrated by individual cells were quantitated using the CELL TRACKING software extension developed by Tim Hutton (Confocal Microscopy and Digital Image Unit, Imperial Cancer Research Fund, UK). Numbers represent average speeds of migration of individual cells over a 5-h period in μm/h. mAb TEA 1/31 (anti–VE-cadherin) has no effect on EC motility and was used as a negative control. Anti β1 mAb TS2/16, which activates β1 integrins, has been used as a control with inhibitory effect on EC motility.
Antibodies Against CD151/PETA-3 Decrease the Ability of ECs to Invade Type I Collagen Gels
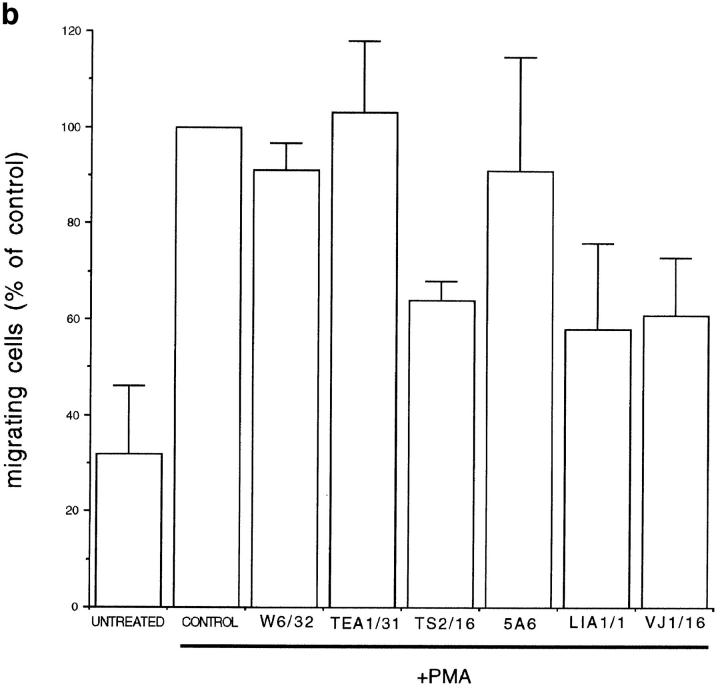
The effect of anti-TM4 mAbs on EC was further assessed in a collagen gel invasion assay. After PMA treatment, these cells are able to migrate into a type I collagen gel and assemble to form tube-like structures (Montesano and Orci, 1985, 1986). This kind of assay has been broadly used as an in vitro approach to study several steps in the angiogenic process (Jain et al., 1997). As shown in Fig. 7 a (D) and b, the anti–CD151/PETA-3 LIA1/1 and VJ1/16 mAbs were able to reduce EC migration and tube formation as compared to control antibodies, whereas the anti–CD81/ TAPA-1 5A6 mAb showed no effect or a slight inhibition on EC migration in this assay (Fig. 7 b). Proactivatory anti-β1 integrin TS2/16 also inhibited both processes (Fig. 7 b). On the other hand, both the anti–VE-Cadherin TEA1/31 (Fig. 7 a, C, and 7 b) and the anti-HLA-A,B W6/ 32 mAbs (Fig. 7 b) neither affected EC invasivity nor their ability to develop cord-like structures.
Figure 7.

Effect of anti-TM4 mAbs on invasion of collagen gels by PMA-treated HUVECs. HUVECs were seeded on the surface of collagen gels, in the presence or not of 20 ng/ml PMA alone (control) or in combination with 20μg/ml of anti–HLA-A,B W6/ 32, anti–VE-Cadherin TEA1/31, anti-β1 integrin TS2/16, anti– CD81/TAPA-1 5A6, or anti–CD151/PETA-3 LIA1/1 and VJ1/16 mAbs for 24 h. (a) Phase-contrast micrographs of migrating cells invading the collagen gels forming tube-like structures. (A) Unstimulated cells; (B) cells treated for 24 h with PMA alone; (C and D) cells treated for 24 h with PMA plus TEA1/31 or LIA1/1, respectively. (b ) Quantitation of migrating cells under the different conditions respect to control cells in the presence of PMA (100%). Experiments were performed by duplicate and represented as the arithmetic mean of the number of migrating cells ± 1 SD of four fields per well from two different experiments.
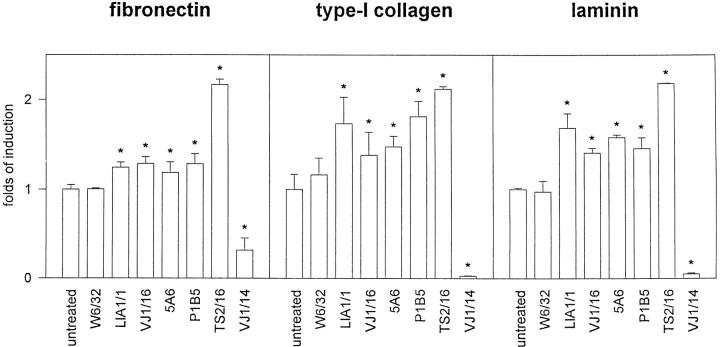
Effect of Anti-TM4 mAbs on EC-ECM Adhesion
To determine the possible mechanism of inhibition of cell migration by anti-TM4 mAbs, we studied the possible role of tetraspan molecules in regulating the activity of their associated α3β1 integrin, that it has been shown to function as a receptor for several ECM proteins, including fibronectin, collagen and laminin (Wayner and Carter, 1987). We found that mAbs against CD81/TAPA-1 and CD151/PETA-3 induced a slight but significant (P < 0.01) increase of EC adhesion to the three substrates studied (Fig. 8). In addition, the proactivatory anti-β1 mAb TS2/ 16 greatly enhanced the cellular adhesion to the different ECM proteins, whereas the blocking anti-β1 mAb VJ1/14 inhibited almost completely EC adhesion (Fig. 8). Lastly, the anti-α3 P1B5 mAb, which has been shown to interfere with fibrosarcoma cell adhesion to different ECM proteins (Wayner and Carter, 1987), did not inhibit EC adhesion, but instead it showed an increment.
Figure 8.
Effect of anti-TM4 mAbs in EC adhesion to different extracellular matrix proteins. Cells were incubated on the coated plates for 15 or 30 min either in the presence or not of 10 μg/ml of the following purified mAbs: anti–HLA-A,B W6/32, anti–CD151/PETA-3 LIA1/1, and VJ1/16, anti–CD81/ TAPA-1 5A6, anti-β1 activatory TS2/16, and anti-β1 blocking VJ1/14. Anti-α3 mAb P1B5 was used as ascitis fluid diluted 1:100. Experiments were performed by triplicate. Data correspond to the arithmetic mean of the percent of cell adhesion ± 1 SD. Levels of basal adhesion were 39.26% ± 1.82 for fibronectin, 33.53% ± 2.85 for collagen, and 25.68% ± 1.55 for laminin. Asterisks indicate significant difference (P < 0.01) compared to basal cell adhesion (Wilcoxon sum rank test).
Discussion
Members of the tetraspan superfamily of proteins have been implicated in diverse cellular functions such as regulation of cell growth and differentiation (Ledbetter et al., 1987; Oren et al., 1990; Kallin et al., 1991; Miyake et al., 1991; Gil et al., 1992; Wice and Gordon, 1995) as well as in cell adhesion (Toothil et al., 1990; Forsyth 1991; Masellis-Smith and Shaw, 1994; Behr and Schriever, 1995). In addition, other reports show their capacity to induce intracellular signals when crosslinked on the cell surface (Olweus et al., 1993; Lebel-Binay et al., 1995; Tai et al., 1996; reviewed in Wright and Tomlison, 1994; Maeker et al., 1997). In this study, we investigated the expression and subcellular localization of different members of this protein superfamily in EC. By using a morphological screening, we selected two monoclonal antibodies that recognize an endothelial cell-to-cell junction molecule, that corresponds to the recently identified tetraspan molecule CD151/PETA-3.
We found that EC express CD151/PETA-3, CD9, CD81/TAPA-1, and CD63. Interestingly, they were all localized at intercellular contact sites with the exception of CD63, which bears a lysosome-targeting signal in its cytoplasmic C-term tail (Metzelaar et al., 1991). The junctional character of these proteins was studied in detail for CD151/PETA-3. The specific localization of this molecule at sites of cell–cell contact is evident not only when the EC monolayer is already confluent, but also during the processes of formation and reestablishment after wounding. In this regard, it has been previously shown that typical junctional molecules, as VE-cadherin, relocalize away from the margins of ECs that are at the wound border, where cell-to-cell contacts are disrupted (Breviario et al., 1995; Lampugnani et al., 1995). Similarly, the expression of CD151/PETA-3 shows a dynamic behavior during the process of migration of ECs into the damaged area, being preserved in the sites where intercellular contact is retained and disappearing from the borders that have lost it. On the other hand, the localization of this antigen at cell– cell junctions is not specific for EC, and CD151/PETA-3 shows a typical junctional staining in all adherent cell types studied that express it (Yáñez-Mó, M., unpublished results). All these data suggest that CD151/PETA-3 is an important element of an intercellular adhesion complex, together with other tetraspan molecules, as CD9 and CD81/TAPA-1.
Tetraspan molecules exhibit a great capacity to interact with other integral proteins in the plasma membrane, such as CD2 (Bell et al., 1992), CD4-CD8 (Imai and Yoshie, 1993), CD19-CD21 (for review Fearon 1993; Bradbury et al., 1993), Fc receptors (Kitani et al., 1991), HLA-DR (Angelisová et al., 1994), tyrosine phosphatases (Carmo and Wright, 1995), heparin-binding EGF-like growth factor (Iwamoto et al., 1994), and PI 4-Kinase (Berditchevski et al., 1997). It has also been reported on different cell lines that members of TM4 associate consistently with integrins β1(Berditchevski et al., 1996; for review see Wright and Tomlison, 1994; Hemler et al., 1996; Maecker et al., 1997), β2 (Skubitz et al., 1996), GpIIb-IIIa (Slupsky et al., 1989), and other TM4 molecules (Imai and Yoshie, 1993; Radford et al., 1996; Rubinstein et al., 1996). It seems that their highly hydrophobic nature greatly favors their interactions with other transmembrane proteins. Our data demonstrate the association of this novel TM4 member CD151/PETA-3 with CD9, CD81/TAPA-1, and α3β1 integrin in ECs, but not with other transmembrane proteins also localized in cell-to-cell junctions, such as VE-cadherin or CD31. In this regard, CD9 has also previously been localized at sites of intercellular contact on monkey kidney epithelial cells where is associated to the same α3β1 integrin heterodimer (Nakamura et al., 1995). In contrast, CD63 does not seem to associate with CD9, CD151/ PETA-3 or β1 integrins in EC, likely because its expression is almost restricted to lysosomes and Weibel-Palade bodies (Vischer and Wagner, 1993). However, when expressed on the cell surface, such as in melanoma cells, CD63 is able to associate with other tetraspan proteins present on this cell type (Radford et al., 1996). Together, these observations further support the specificity of the interactions among CD9, CD81/TAPA-1, and CD151/ PETA-3 on EC, and rule out the possibility of an artefactual association of these molecules during the immunoprecipitation procedure. On the other hand, CD81/TAPA-1, although associated to both CD9 and CD151/PETA-3, does not seem to be directly associated to α3β1 integrin or other β1 integrins in this cell type. However, it still remains to be determined whether the complexes CD9– CD81/TAPA-1 and CD151/PETA-3–CD81/TAPA-1 include α3β1 integrin via its association with CD9 and CD151/PETA-3.
Although the interactions among tetraspan molecules and integrins have been widely described (for review Wright and Tomlison, 1994; Hemler et al., 1996; Maecker et al., 1997), there is little evidence regarding a functional role of the tetraspan molecules in these complexes. It is feasible that integrins are responsible for some of the effects seen in cell motility and adhesion triggered by anti-TM4 antibodies. This point is supported by the specific inhibition of these phenomena with anti-integrin blocking antibodies (Masellis-Smith and Shaw, 1994; Behr and Shriever, 1995; Shaw et al., 1995), although some other effects seem to be integrin-independent. Herein, we explored the effect of anti-TM4 mAbs on the motility of EC and found that the anti–CD81/TAPA-1 and -CD151/ PETA-3 mAbs significantly reduced the rate of cellular migration. In addition, both the anti-α3 integrin P1B5 and the proactivatory anti-β1 TS2/16 mAbs had the same inhibitory effect. In this regard, it has been reported that an anti-α3 mAb blocked the motility of melanoma cells without inhibiting cell adhesion (Melchiori et al., 1995). Therefore, we analyzed on EC, the possible correlation between adhesion to ECM proteins and inhibition of cell migration. We found that anti-TM4 mAbs simultaneously induced an inhibitory effect in cell motility and a slight but statistically significant increase in cell adhesion to ECM proteins. A similar dual effect exhibited the anti-α3 mAb P1B5, despite that it has been reported to block cellular adhesion to fibronectin, collagen and laminin (Wayner and Carter, 1987). All these data suggest the existence of TM4-α3β1 functional complexes at EC cell-to-cell contacts in which the tetraspan components of the complex are able to modulate cell motility, likely through regulation of the integrin adhesiveness. However, it remains to be determined whether the TM4-α3β1 complexes that are the targets for motility-regulating antibodies are identical to those at lateral junctions. It also remains to be established whether these effects of TM4 molecules, when engaged by specific mAbs, on cell adhesion and migration are due to direct modulation of the α3β1 function or a consequence of a proactivatory signaling effect on other ECM receptors expressed by ECs. It has been postulated that in ECs α3β1 might act both as an ECM receptor and as an intercellular adhesion moiety (Takeuchi et al., 1994; Weitzman et al., 1994) involved in homophilic (Sriramarao et al., 1993) or heterophilic interactions (Symington et al., 1993), although other reports have proposed a negative role for this integrin in intercellular adhesion (Weitzman et al., 1995).
Cellular migration and invasion are accompanied by remodeling of ECM, which comprises both degradation of preexisting and deposition of new ECM components. Integrins have been shown to be able to regulate these events in several cell types, either by modulating the expression or subcellular localization of different metalloproteases (Werb et al., 1989; Larjava et al., 1993; Langholz et al., 1995; Brooks et al., 1996) or by regulating ECM assembly (Langholz et al., 1995; Wu et al., 1995). In particular, the α3β1 is involved in Type IV collagenase expression and ECM deposition (Larjava et al., 1993; Wu et al., 1995). Large-vessel EC are known to be able to migrate into collagen gels and form tubular structures after treatment with PMA (Montesano and Orci, 1985, 1986; Gamble et al., 1993). This phenomenon involves several events such as degradation of ECM, cell migration, and cellular adhesion in order to form capillary-like cords. Our data showing the inhibitory effect of anti–CD151/PETA-3 mAbs on EC collagen gel invasion indicate that, in addition to their putative role on cell motility, this tetraspan protein could also modulate the possible effect of integrins in endothelial remodeling of ECM. The failure of the anti–CD81/TAPA-1 mAb to significantly inhibit EC migration in this assay, suggests that different tetraspan molecules could participate in either distinct steps of the migration process or other events involved in the EC invasion mechanism. Further research is required to elucidate this issue.
Another possible way of regulation of integrin function by their associated TM4 molecules, could be by determining their cellular localization to a certain subdomain along the plasma membrane. Thus, it is feasible that TM4 proteins, through interactions among the different members of the family, would create a tetraspan web on the cell membrane (Rubinstein et al., 1996; Maecker et al., 1997), that would include the α3β1 integrin, modulating the specificity or function of this adhesion receptor. The existence of this “tetraspan lattice” has been morphologically described in the bladder epithelium, where the TM4 members uroplakins Ia and Ib, interact with the transmembrane proteins uroplakins II and III, and form molecular complexes which stabilize the apical surface of urothelium (Yu et al., 1994).
As pointed out above, cell–cell junctions play crucial roles in cell differentiation and homeostasis. It still remains to be determined whether these TM4-α3β1 complexes play also a role in cell growth control. Integrins, via association with the transmembrane protein caveolin, have recently been involved in the control of cell cycle progression (Wary et al., 1996). Thus, tetraspan molecules, via their interaction with integrins, might be also implicated in these processes. It is also conceivable that the TM4–integrin complexes in EC, due to its localization at the cell–cell contact sites in the endothelial monolayer, could participate in key functions of these cells such as the control of vascular permeability and regulation of leukocyte extravasation. Integrins, which are also localized at cell–cell junctions, have been implicated in the maintenance of EC monolayer integrity (Lampugnani et al., 1991). It remains thus to be further investigated whether their associated TM4 members are also involved in this function.
Acknowledgments
We thank M.A. Olazcarizqueta for technical assistance with confocal microscopy, and Drs. R. González-Amaro and F.W. Luscinskas for critical reading of the manuscript. We would also thank for the support and technical assistance in different aspects to J. Espada, M. Gómez, M. Montoya, M. Nieto, M.A. del Pozo, M.A. Ramirez, P. Sánchez-Mateos, J. Serrador, and M. Vitón. We are grateful to Dr. N. Hogg, T. Hutton, P. Jordan, A. Stokes, and R. Pepperkok (Confocal Microscopy and Digital Image Unit, Imperial Cancer Research Fund, London, UK) for all their help with the videomicroscopy experiments.
This work was supported by grants Fondo Investigaciones Sanitarias 95/0212, SAF 96/0039 from Plan Nacional de Investigación y Desarrollo; grant 07/44/96 from Comunidad Autónoma de Madrid; a grant from Asociación de la Lucha contra el Cáncer to F. Sánchez-Madrid; and grant PM95-0162 to M.O. de Landázuri. C. Cabañas has been partially supported by European Molecular Biology Organization Short Term Fellowship ASTF8757.
Abbreviations used in this paper
- EC
endothelial cells
- ECM
extracellular matrix
- HMEC-1
human microvascular endothelial cell line-1
- HUVEC
human EC from umbilical vein
- PECAM
platelet-endothelial cell adhesion molecule
- TM4
transmembrane 4/tetraspan molecule
- TNF-α
tumor necrosis factor-α
- PETA-3
platelet-endothelial tetraspan antigen-3
- TAPA-1
target of antiproliferative antibody-1;VE, vascular endothelial
Footnotes
Address all correspondence to F. Sánchez-Madrid, Servicio de Inmunología, Hospital de la Princesa, Universidad Autónoma de Madrid, 28006 Madrid, Spain. Tel.: 34-1-4023347. Fax: 34-1-3092496. E-mail: fsmadrid/ princesa@hup.es
References
- Ades EW, Candal FJ, Swerlick RA, George VG, Summers S, Bosse DC, Lawley TJ. HMEC-1: Establishment of an immortalized human microvascular endothelial cell line. J Invest Dermatol. 1992;99:683–690. doi: 10.1111/1523-1747.ep12613748. [DOI] [PubMed] [Google Scholar]
- Anderson JM, Balda MS, Fanning AS. The structure and regulation of tight junctions. Curr Opin Cell Biol. 1993;5:772–778. doi: 10.1016/0955-0674(93)90024-k. [DOI] [PubMed] [Google Scholar]
- Angelisová P, Hilgert I, Horejsi V. Association of four antigens of the tetraspan family (CD37, CD53, TAPA-1, and R2/C33) with MHC class II glycoproteins. Immunogenetics. 1994;39:249–256. doi: 10.1007/BF00188787. [DOI] [PubMed] [Google Scholar]
- Arroyo AG, Sánchez-Mateos P, Campanero MR, Martín-Padura I, Dejana E, Sánchez-Madrid F. Regulation of the VLA integrin-ligand interactions through the β1 subunit. J Cell Biol. 1992;117:659–670. doi: 10.1083/jcb.117.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashman, L.K., S. Fitter, P.M. Sincock, L. Nguyen, and A.C. Cambareri. 1997. Summary report: CD151. In Leucocyte Typing VI. Ed by T. Kishimoto, M. Miyasaka, D. Mason, K. Sugamura, T. Springer, S. Shaw, S.M. Goyert, L. Moretta, H. Zola, A.E.-G.Kr. von dem Borne, K. Okumura, and K. Kikutani. Garland Publishing, New York.
- Behr S, Schriever F. Engaging CD19 or target of an antiproliferative antibody-1 on human B lymphocytes induces binding of B cells to the interfollicular stroma of human tonsils via integrin α4/β1 and fibronectin. J Exp Med. 1995;182:1191–1199. doi: 10.1084/jem.182.5.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell GM, Seaman WE, Niemi EC, Imboden JB. The OX-44 molecule couples to signaling pathways and is associated with CD2 on rat T lymphocytes and in a Natural Killer cell line. J Exp Med. 1992;175:527–536. doi: 10.1084/jem.175.2.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berditchevski F, Zutter MM, Hemler ME. Characterization of novel complexes on the cell surface between integrins and proteins with 4 transmembrane domains (TM4 proteins) Mol Biol Cell. 1996;7:193–207. doi: 10.1091/mbc.7.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berditchevski F, Tolias KF, Wong K, Carpenter CL, Hemler ME. A novel link between integrins, transmembrane-4 superfamily proteins (CD63 and CD81) and phosphatidylinositol 4-kinase. J Biol Chem. 1997;272:2585–2598. doi: 10.1074/jbc.272.5.2595. [DOI] [PubMed] [Google Scholar]
- Bradbury LE, Goldmacher VS, Tedder TF. The CD19 signal transduction complex of B lymphocytes. J Immunol. 1993;151:2915–2927. [PubMed] [Google Scholar]
- Breviario F, Caveda L, Corada M, Martín-Padura I, Navarro P, Golay J, Introna M, Gulino D, Lampugnani MG, Dejana E. Functional properties of human vascular endothelial cadherin (7B4/Cadherin-5), an endothelium-specific cadherin. Arterioscler Thromb Vasc Biol. 1995;15:1229–1239. doi: 10.1161/01.atv.15.8.1229. [DOI] [PubMed] [Google Scholar]
- Brooks PC, Stromblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, Quigley JP, Cheresh DA. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin αvβ3. Cell. 1996;85:683–693. doi: 10.1016/s0092-8674(00)81235-0. [DOI] [PubMed] [Google Scholar]
- Campanero MR, Arroyo AG, Pulido R, Ursa A, de Matías MS, Sánchez-Mateos P, Kassner PD, Chan BMC, Hemler ME, Corbí AL, de Landázuri MO, Sánchez-Madrid F. Functional role of α2/β1 and α4/β1 integrins in leukocyte intercellular adhesion induced through the common β1 subunit. Eur J Immunol. 1992;22:3111–3119. doi: 10.1002/eji.1830221213. [DOI] [PubMed] [Google Scholar]
- Carmo AM, Wright MD. Association of the transmembrane 4 superfamily molecule CD53 with a tyrosine phosphatase activity. Eur J Immunol. 1995;25:2090–2095. doi: 10.1002/eji.1830250743. [DOI] [PubMed] [Google Scholar]
- Caveda L, Martín-Padura I, Navarro P, Breviario F, Corada M, Gulino D, Lampugnani MG, Dejana E. Inhibition of cultured cell growth by vascular endothelial cadherin (Cadherin-5/VE-Cadherin) J Clin Invest. 1996;98:886–893. doi: 10.1172/JCI118870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Citi S. The molecular organization of tight junctions. J Cell Biol. 1993;121:485–489. doi: 10.1083/jcb.121.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowin P, Burke B. Cytoskeleton-membrane interactions. Curr Opin Cell Biol. 1996;8:56–65. doi: 10.1016/s0955-0674(96)80049-4. [DOI] [PubMed] [Google Scholar]
- Dejana E. Endothelial adherens junctions: implications in the control of vascular permeability and angiogenesis. J Clin Invest. 1996;98:1949–1953. doi: 10.1172/JCI118997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domanico SZ, Pelletier AJ, Havran WL, Quaranta V. Integrin α6Aβ1 induces CD81-dependent cell motility without engaging the extracellular matrix migration substrate. Mol Biol Cell. 1997;8:2253–2265. doi: 10.1091/mbc.8.11.2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drubin DG, Nelson WJ. Origins of cell polarity. Cell. 1996;84:335–344. doi: 10.1016/s0092-8674(00)81278-7. [DOI] [PubMed] [Google Scholar]
- Fearon DT. The CD19-CR2-TAPA-1 complex, CD45 and signaling by the antigen receptor of B lymphocytes. Curr Opin Immunol. 1993;5:341–348. doi: 10.1016/0952-7915(93)90051-s. [DOI] [PubMed] [Google Scholar]
- Fitter S, Tetaz TJ, Berndt MC, Ashman LK. Molecular cloning of a cDNA encoding a novel platelet-endothelial cell tetra-span antigen, PETA-3. Blood. 1995;4:1348–1355. [PubMed] [Google Scholar]
- Forsyth KD. Anti-CD9 antibodies augment neutrophil adherence to endothelium. Immunology. 1991;72:292–296. [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S, Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble JR, Matthias LJ, Meyer G, Kaur P, Russ G, Faull R, Berndt MC, Vadas MA. Regulation of in vitro capillary tube formation by anti-integrin antibodies. J Cell Biol. 1993;121:931–943. doi: 10.1083/jcb.121.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Monzón C, Sánchez-Madrid F, García-Buey L, Arroyo AG, García-Sánchez A, Moreno-Otero R. Vascular adhesion molecule expression in viral chronic hepatitis: evidence of neoangiogenesis in portal tracts. Gastroenterology. 1995;108:231–241. doi: 10.1016/0016-5085(95)90029-2. [DOI] [PubMed] [Google Scholar]
- Geiger B, Ayalon O. Cadherins. Annu Rev Cell Biol. 1992;8:307–332. doi: 10.1146/annurev.cb.08.110192.001515. [DOI] [PubMed] [Google Scholar]
- Gil ML, Vita N, Lebel-Binay S, Miloux B, Chalon P, Kaghad M, Marchiol-Fournigault C, Conjeaud H, Caput D, Ferrara P, et al. A member of the tetra spans transmembrane protein superfamily is recognized by a monoclonal antibody raised against an HLA class I-deficient, lymphokine-activated killer-susceptible, B lymphocyte line. J Immunol. 1992;148:2826–2833. [PubMed] [Google Scholar]
- Gómez M, Luque A, del Pozo MA, Hogg N, Sánchez-Madrid F, Cabañas C. Functional relevance during lymphocyte migration and cellular localization of activated β1 integrins. Eur J Immunol. 1997;27:8–16. doi: 10.1002/eji.1830270103. [DOI] [PubMed] [Google Scholar]
- Goodenough DA, Goliger JA, Paul DL. Connexins, connexons, and intercellular communication. Annu Rev Biochem. 1996;65:475–502. doi: 10.1146/annurev.bi.65.070196.002355. [DOI] [PubMed] [Google Scholar]
- Gumbiner B, Stevenson B, Grimaldi A. The role of the cell adhesion molecule uvomorulin in the formation and maintenance of the epithelial junctional complex. J Cell Biol. 1988;107:1575–1587. doi: 10.1083/jcb.107.4.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- Hasegawa H, Utsunomiya Y, Kishimoto K, Yanagisawa K. SFA-1, a novel cellular gene induced by human T-cell leukemia virus type 1, is a member of the transmembrane 4 superfamily. J Virol. 1996;70:3258–3263. doi: 10.1128/jvi.70.5.3258-3263.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemler ME, Mannion BA, Berditchevski F. Association of TM4SF proteins with integrins: relevance to cancer. Biochim Biophys Acta. 1996;1287:67–71. doi: 10.1016/0304-419x(96)00007-8. [DOI] [PubMed] [Google Scholar]
- Imai T, Yoshie O. C33 antigen and M38 antigen recognized by monoclonal antibodies inhibitory to syncitium formation in human T cell leukemia virus type 1 are both members of the transmembrane 4 superfamily and associate with each other and with CD4 or CD8 in T cells. J Immunol. 1993;151:6470–6481. [PubMed] [Google Scholar]
- Iwamoto R, Higashiyama S, Mitamura T, Taniguchi N, Klagsbrun M, Mekada E. Heparin-binding EGF-like growth factor, which acts as the diphteria toxin receptor, forms a complex with membrane protein DRAP27/ CD9, which up-regulates functional receptors and diphteria toxin sensitivity. EMBO (Eur Mol Biol Organ) J. 1994;13:2322–2330. doi: 10.1002/j.1460-2075.1994.tb06516.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe EA, Nachman RI, Becker CG, Minick RC. Culture of human endothelial cells derived from umbilical veins. Circulation. 1972;46:211–253. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RK, Schlenger K, Höckel M, Yuan F. Quantitative angiogenesis assays: progress and problems. Nat Med. 1997;3:1203–1208. doi: 10.1038/nm1197-1203. [DOI] [PubMed] [Google Scholar]
- Jockusch BM, Bubeck P, Giehl K, Kroemer M, Moschner J, Rothkegel M, Rüdiger M, Schlüter K, Stanke G, Winkler J. The molecular architecture of focal adhesions. Annu Rev Cell Dev Biol. 1995;11:379–416. doi: 10.1146/annurev.cb.11.110195.002115. [DOI] [PubMed] [Google Scholar]
- Jones PH, Bishop LA, Watt FM. Functional significance of CD9 association with beta-1 integrins in human epidermal keratinocytes. Cell Adhes Commun. 1996;4:297–305. doi: 10.3109/15419069609010773. [DOI] [PubMed] [Google Scholar]
- Kallin B, de Martin R, Etzold T, Sorrentino V, Philipson L. Cloning of a growth arrest-specific and transforming growth factor β-regulated gene, TI 1, from an epithelial cell line. Mol Cell Biol. 1991;11:5338–5345. doi: 10.1128/mcb.11.10.5338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemler R. From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends Genet. 1993;9:317–321. doi: 10.1016/0168-9525(93)90250-l. [DOI] [PubMed] [Google Scholar]
- Keon BH, Shäfer S, Kuhn C, Grund C, Franke WW. Symplekin, a novel type of tight junction plaque protein. J Cell Biol. 1996;134:1003–1018. doi: 10.1083/jcb.134.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitani S, Berenstein E, Mergenhagen S, Tempst P, Siraganian RP. A cell surface glycoprotein of rat basophilic leukemia cells close to the high affinity IgE receptor (FcεRI) J Biol Chem. 1991;266:1903–1909. [PubMed] [Google Scholar]
- Lampugnani MG, Resnati M, Dejana E, Marchisio PC. The role of integrins in the maintenance of endothelial monolayer integrity. J Cell Biol. 1991;112:479–490. doi: 10.1083/jcb.112.3.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampugnani MG, Resnati M, Raiteri M, Pigott R, Pisacane A, Houen G, Ruco LP, Dejana E. A novel endothelial-specific membrane protein is a marker of cell–cell contacts. J Cell Biol. 1992;118:1511–1522. doi: 10.1083/jcb.118.6.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampugnani MG, Corada M, Caveda L, Breviario F, Ayalon O, Geiger B, Dejana E. The molecular organization of endothelial cell to cell junctions: differential association of plakoglobin, β-catenin, and α-catenin with vascular endothelial cadherin (VE-cadherin) J Cell Biol. 1995;129:203–217. doi: 10.1083/jcb.129.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langholz O, Röckel D, Mauch C, Kozlowska E, Bank I, Krieg T, Eckes B. Collagen and collagenase gene expression in three-dimensional collagen lattices are differentially regulated by α1β1 and α2β1 integrins. J Cell Biol. 1995;131:1903–1915. doi: 10.1083/jcb.131.6.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larjava H, Peltonen J, Akiyama SK, Yamada SS, Gralnick HR, Uitto J, Yamada KM. Novel function for β1 integrins in keratinocyte cell–cell interactions. J Cell Biol. 1990;110:803–815. doi: 10.1083/jcb.110.3.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larjava H, Lyons JG, Salo T, Mäkelä M, Koivisto L, Birkedal-Hansen H, Akiyama SK, Yamada KM, Heino J. Anti-integrin antibodies induce type IV collagenase expression in keratinocytes. J Cell Physiol. 1993;157:190–200. doi: 10.1002/jcp.1041570125. [DOI] [PubMed] [Google Scholar]
- Leach L, Clark P, Lampugnani MG, Arroyo AG, Dejana E, Firth JA. Immunoelectron characterization of the inter-endothelial junctions in human term placenta. J Cell Sci. 1993;104:1073–1081. doi: 10.1242/jcs.104.4.1073. [DOI] [PubMed] [Google Scholar]
- Lebel-Binay S, Lagaudrière C, Fradelizi D, Conjeaud H. CD82, member of the tetra-span-transmembrane protein family, is a costimulatory protein for T cell activation. J Immunol. 1995;155:101–110. [PubMed] [Google Scholar]
- Ledbetter, J.A., G. Shu, and E.A. Clark. 1987. Monoclonal antibodies to a new gp40-45 (CD37) B-cell–associated cluster growth modulate B-cell proliferation. In Leukocyte Typing III. A.J. McMichael, P.C.L. Beverley, S. Cobbold, M.J. Crumpton, W. Gilks, F.M. Gotch, N. Hogg, M. Horton, N. Ling, J.C.M. MacLennan, D.Y. Mason, C. Milstein, D. Spiegelhalter, and H. Waldmann, editors. Oxford University Press, Oxford. 339 pp.
- Maecker HT, Todd SC, Levy S. The tetraspanin superfamily: molecular facilitators. FASEB J. 1997;11:428–442. [PubMed] [Google Scholar]
- Marrs JA, Andersson-Fisone C, Jeong MC, Cohen-Gould L, Zurzolo C, Nabi IR, Rodriguez-Boulan E, Nelson WJ. Plasticity in epithelial cell phenotype: modulation by expression of different cadherin cell adhesion molecules. J Cell Biol. 1995;129:507–519. doi: 10.1083/jcb.129.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masellis-Smith A, Shaw ARE. CD9-regulated adhesion. Anti-CD9 monoclonal antibody induce pre-B cell adhesion to bone marrow fibroblasts through de novo recognition of fibronectin. J Immunol. 1994;152:2768–2777. [PubMed] [Google Scholar]
- McNeill H, Ozawa M, Kemler R, Nelson WJ. Novel function of the cell adhesion molecule uvomorulin as an inducer of cell surface polarity. Cell. 1990;62:309–316. doi: 10.1016/0092-8674(90)90368-o. [DOI] [PubMed] [Google Scholar]
- Melchiori A, Mortarini R, Carlone S, Marchisio PC, Anichini A, Noonan DM, Albini A. The α3β1 integrin is involved in melanoma cell migration and invasion. Exp Cell Res. 1995;219:233–242. doi: 10.1006/excr.1995.1223. [DOI] [PubMed] [Google Scholar]
- Metzelaar MJ, Wijngaard PLJ, Peters PJ, Sixma JJ, Nieuwenhuis HK, Clevers HC. CD63 antigen. A novel lysosomal membrane glycoprotein, cloned by a screening procedure for intracellular antigens in eukaryotic cells. J Biol Chem. 1991;266:3239–3245. [PubMed] [Google Scholar]
- Miyake M, Koyama M, Seno M, Ikeyama S. Identification of the motility-related protein (MRP-1), recognized by monoclonal antibody M31-15, which inhibits cell motility. J Exp Med. 1991;174:1347–1354. doi: 10.1084/jem.174.6.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesano R, Orci L. Tumor-promoting phorbol esters induce angiogenesis in vitro. Cell. 1985;42:469–477. doi: 10.1016/0092-8674(85)90104-7. [DOI] [PubMed] [Google Scholar]
- Montesano R, Orci L. Phorbol esters induce angiogenesis in vitro from large-vessel endothelial cells. J Cell Physiol. 1986;130:284–291. doi: 10.1002/jcp.1041300215. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Iwamoto R, Mekada E. Membrane-anchored heparin-binding EGF-like growth factor (HB-EGF) and diphteria toxin receptor-associated protein (DRAP27)/CD9 form a complex with integrin α3β1 at cell–cell contact sites. J Cell Biol. 1995;129:1691–1705. doi: 10.1083/jcb.129.6.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman PJ. The biology of PECAM-1. J Clin Invest. 1997;99:3–8. doi: 10.1172/JCI119129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olweus J, Lund-Johansen F, Horejsi V. CD53, a protein with four membrane-spanning domains, mediates signal transduction in human monocytes and B cells. J Immunol. 1993;151:707–716. [PubMed] [Google Scholar]
- Oren R, Takahashi S, Doss C, Levy R, Levy S. TAPA-1, the target of an antiproliferative antibody, defines a new family of transmembrane proteins. Mol Cell Biol. 1990;10:4007–4015. doi: 10.1128/mcb.10.8.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radford KJ, Thorne RF, Hersey P. CD63 associates with transmembrane 4 superfamily members, CD9 and CD81, and with β1 integrins in human melanoma. Biochem Biophys Res Commun. 1996;222:13–18. doi: 10.1006/bbrc.1996.0690. [DOI] [PubMed] [Google Scholar]
- Radford KJ, Thorne RF, Hersey P. Regulation of tumor cell motility and migration by CD63 in a human melanoma cell line. J Immunol. 1997;158:3353–3358. [PubMed] [Google Scholar]
- Rubinstein E, Naour FL, Lagaudrière-Gesbert C, Billard M, Conjeaud H, Boucheix C. CD9, CD63, CD81, and CD82 are components of a surface tetraspan network connected to HLA-DR and VLA integrins. Eur J Immunol. 1996;26:2657–2665. doi: 10.1002/eji.1830261117. [DOI] [PubMed] [Google Scholar]
- Schneeberger EE, Lynch RD. Structure, function and regulation of cellular tight junctions. Am J Physiol. 1992;262:L647–L661. doi: 10.1152/ajplung.1992.262.6.L647. [DOI] [PubMed] [Google Scholar]
- Shaw AR, Domanska A, Mak A, Gilchrist A, Dobler K, Visser L, Poppema S, Fliegel L, Letarte M, Willet BJ. Ectopic expression of human and feline CD9 in a human B cell line confers beta 1 integrin-dependent motility on fibronectin and laminin substrates and enhanced tyrosine phosphorylation. J Biol Chem. 1995;270:24092–24099. doi: 10.1074/jbc.270.41.24092. [DOI] [PubMed] [Google Scholar]
- Sincock PM, Mayrhofer G, Ashman LK. Localization of the transmembrane 4 superfamily (TM4SF) member PETA-3 (CD151) in normal human tissues: comparison with CD9, CD63 and α5β1 integrin. J Histochem Cytochem. 1997;45:515–525. doi: 10.1177/002215549704500404. [DOI] [PubMed] [Google Scholar]
- Skubitz KM, Campbell KD, Iida J, Skubitz APN. CD63 associates with tyrosine kinase activity and CD11/CD18, and transmits an activation signal in neutrophils. J Immunol. 1996;157:3617–3626. [PubMed] [Google Scholar]
- Slupsky JR, Seehafer JG, Tang S, Masellis-Smith A, Shaw ARE. Evidence that monoclonal antibodies against CD9 antigen induce specific association between CD9 and the platelet glycoprotein IIb/IIIa complex. J Biol Chem. 1989;264:12289–12293. [PubMed] [Google Scholar]
- Sriramarao P, Steffner P, Gehlsen KR. Biochemical evidence for a homophilic interaction of the α3β1 integrin. J Biol Chem. 1993;268:22036–22041. [PubMed] [Google Scholar]
- Symington BE, Takada Y, Carter WG. Interaction of integrins α3β1 and α2β1: potential role in keratinocyte intercellular adhesion. 1993. J Cell Biol. 1993;120:523–535. doi: 10.1083/jcb.120.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai XG, Yashiro Y, Abe R, Toyooka K, Wood CR, Morris J, Long A, Ono S, Kobayashi M, Hamaoka T, et al. A role for CD9 molecules in T cell activation. J Exp Med. 1996;184:753–758. doi: 10.1084/jem.184.2.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. Cadherins: a molecular family important in selective cell– cell adhesion. Annu Rev Biochem. 1990;59:237–252. doi: 10.1146/annurev.bi.59.070190.001321. [DOI] [PubMed] [Google Scholar]
- Takeuchi K, Tsuji T, Hakomori S, Irimura T. Intercellular adhesion induced by anti-α3 integrin (VLA-3) antibodies. Exp Cell Res. 1994;211:133–141. doi: 10.1006/excr.1994.1069. [DOI] [PubMed] [Google Scholar]
- Toothil VJ, van Mourik JA, Niewenhuis HR, Metzelaar MJ, Pearson JD. Characterization of the enhanced adhesion of neutrophil leukocytes to thrombin-stimulated endothelial cells. J Immunol. 1990;145:283–291. [PubMed] [Google Scholar]
- Tsukita S, Tsukita S, Nagafuchi A, Yonemura S. Molecular linkage between cadherins and actin filaments in cell–cell adherens junctions. Curr Opin Cell Biol. 1992;4:834–839. doi: 10.1016/0955-0674(92)90108-o. [DOI] [PubMed] [Google Scholar]
- Vischer UM, Wagner DD. CD63 is a component of Weibel-Palade bodies of human endothelial cells. Blood. 1993;82:1184–1191. [PubMed] [Google Scholar]
- Wary KK, Miniero F, Isakoff SJ, Marcantonio EE, Giancotti FG. The adaptor protein Shc couples a class of integrins to the control of cell cycle progression. Cell. 1996;87:733–743. doi: 10.1016/s0092-8674(00)81392-6. [DOI] [PubMed] [Google Scholar]
- Wayner E, Carter WG. Identification of multiple cell adhesion receptors for collagen and fibronectin in human fibrosarcoma cells possessing unique α and common β subunits. J Cell Biol. 1987;105:1873–1884. doi: 10.1083/jcb.105.4.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber C, Alon R, Moser B, Springer TA. Sequential regulation of α4β1 and α5β1 integrin avidity by CC chemokines in monocytes: implications for transendothelial chemotaxis. J Cell Biol. 1996;134:1063–1073. doi: 10.1083/jcb.134.4.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitzman JB, Pasqualini R, Takada Y, Hemler ME. The function and distinctive regulation of the integrin VLA-3 in cell adhesion, spreading and homotypic cell aggregation. J Biol Chem. 1994;12:8651–8657. [PubMed] [Google Scholar]
- Weitzman JB, Chen A, Hemler ME. Investigation of the role of β1 integrins in cell–cell adhesion. J Cell Sci. 1995;108:3635–3644. doi: 10.1242/jcs.108.11.3635. [DOI] [PubMed] [Google Scholar]
- Werb Z, Tremble PM, Behrendtsen O, Crowly E, Damsky CH. Signal transduction through the fibronectin receptor induces collagenase and stromelysin gene expression. J Cell Biol. 1989;109:877–889. doi: 10.1083/jcb.109.2.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wice BM, Gordon JI. A tetraspan membrane glycoprotein produced in the human intestinal epithelium and liver that can regulate cell density-dependent proliferation. J Biol Chem. 1995;270:21907–21918. doi: 10.1074/jbc.270.37.21907. [DOI] [PubMed] [Google Scholar]
- Wright MD, Tomlinson MG. The ins and outs of the transmembrane 4 superfamily. Immunol Today. 1994;15:588–594. doi: 10.1016/0167-5699(94)90222-4. [DOI] [PubMed] [Google Scholar]
- Wu C, Chung AE, Mc JA, Donald A novel role for α3β1 integrins in extracellular matrix assembly. J Cell Sci. 1995;108:2511–2523. doi: 10.1242/jcs.108.6.2511. [DOI] [PubMed] [Google Scholar]
- Yu J, Lin JH, Wu XR, Sun TT. Uroplakins Ia and Ib, two major differentiation products of bladder epithelium, belong to a family of four transmembrane domain (4TM) proteins. J Cell Biol. 1994;125:171–182. doi: 10.1083/jcb.125.1.171. [DOI] [PMC free article] [PubMed] [Google Scholar]