Abstract
The neural isoforms of agrin can stimulate transcription of the acetylcholine receptor (AChR) ε subunit gene in electrically active muscle fibers, as does the motor neuron upon the formation of a neuromuscular junction. It is not clear, however, whether this induction involves neuregulins (NRGs), which stimulate AChR subunit gene transcription in vitro by activating ErbB receptors. In this study, we show that agrin- induced induction of AChR ε subunit gene transcription is inhibited in cultured myotubes overexpressing an inactive mutant of the ErbB2 receptor, demonstrating involvement of the NRG/ErbB pathway in agrin- induced AChR expression. Furthermore, salt extracts from the surface of cultured myotubes induce tyrosine phosphorylation of ErbB2 receptors, indicating that muscle cells express biological NRG-like activity on their surface. We further demonstrate by RT-PCR analysis that muscle NRGs have Ig-like domains required for their immobilization at heparan sulfate proteoglycans (HSPGs) of the extracellular matrix. In extrasynaptic regions of innervated muscle fibers in vivo, ectopically expressed neural agrin induces the colocalized accumulation of AChRs, muscle-derived NRGs, and HSPGs. By using overlay and radioligand-binding assays we show that the Ig domain of NRGs bind to the HSPGs agrin and perlecan. These findings show that neural agrin can induce AChR subunit gene transcription by aggregating muscle HSPGs on the muscle fiber surface that then serve as a local sink for focal binding of muscle-derived NRGs to regulate AChR gene expression at the neuromuscular junction.
The high density accumulation of acetylcholine receptor (AChR)1 channels at the neuromuscular junction (NMJ), required for impulse transmission across the synapse, is the result of transcriptional activation of AChR subunit genes in the subsynaptic muscle nuclei (Brenner et al., 1990; Sanes et al., 1991) and of the insertion of their gene products, the AChR channels, in the synaptic muscle membrane (for review see Sanes, 1997). The AChRs are stabilized in the subsynaptic membrane by anchoring to the cytoskeleton via an elaborate subsynaptic apparatus of highly specialized molecular composition (Fallon and Hall, 1994; Apel and Merlie, 1995; Carbonetto and Lindenbaum, 1995). Both the transcription of AChR genes and the differentiation of the subsynaptic apparatus are under the control of molecules originating from the motor neuron and associated with the synaptic portion of the muscle fiber's basal lamina (BL) (McMahan, 1990; Brenner et al., 1992; Jo and Burden, 1992).
The neural signal proposed to activate AChR gene transcription in muscle is acetylcholine receptor–inducing activity (ARIA; Martinou et al., 1991; Corfas et al., 1993; Chu et al., 1995; Fischbach and Rosen, 1997), a member of the neuregulin (NRG) family of growth and differentiation factors (Falls et al., 1993) arising in several isoforms from a single gene, nrg-1, by alternative mRNA splicing. ARIA/NRG precursors are expressed in motor neurons (Falls et al., 1993) as transmembrane glycoproteins and are cleaved near their transmembrane domains for release (for reviews see Lemke, 1996; Fischbach and Rosen, 1997). The mature form of ARIA/NRG is characterized by an Ig-like domain that binds heparin (Falls et al., 1993) and by a conserved EGF-like domain sufficient to activate receptors of the ErbB family of receptor tyrosine kinases. Neuregulin binding to ErbB receptor heterodimers induces their tyrosine phosphorylation and activates AChR subunit gene transcription in cultured myotubes (Si et al., 1996; Tansey et al., 1996; Altiok et al., 1997).
The neural signal controlling nerve-dependent aggregation of AChR channels in the subsynaptic muscle membrane is agrin (McMahan, 1990), a multidomain heparan sulfate proteoglycan (HSPG) with binding affinities for α-dystroglycan, laminin, and heparin (for review see Denzer et al., 1996). Unlike motor neurons, muscle fibers do not synthesize agrin isoforms active in AChR aggregation (Ferns et al., 1992; Ruegg et al., 1992; Hoch et al., 1993; Gesemann et al., 1995). Agrin is associated with the synaptic BL of the NMJ (Reist et al., 1987) presumably by its binding to laminin (Denzer et al., 1997, 1998). Neuregulins are also bound to synaptic BL (Goodearl et al., 1995; Jo et al., 1995; Sandrock et al., 1995), but the mechanism of this immobilization is not known.
Recent experiments demonstrate, however, that active agrin not only causes the redistribution of cell surface AChRs in cultured myotubes and in vivo but that agrin also induces expression of the AChR ε subunit gene in the absence of nerve-derived NRGs; the expression is resistant to muscle activity as it is at normal synapses (Jones et al., 1996, 1997). In primary myotube cultures, AChR gene transcription induced by agrin depends on its binding to the culture substrate, but conspicuously, does not depend on its AChR aggregating activity (Jones et al., 1996). These findings led us to propose that agrin rather than NRGs may be the key neural factor regulating subsynaptic AChR gene transcription in the muscle fiber. If NRGs are involved in this process they may be derived from the muscle fibers, under the local control of matrix-bound agrin (Jones et al., 1996, 1997). Consistent with this hypothesis, motor neuron–specific agrin isoforms alone, upon expression in extrasynaptic regions of innervated muscle fibers, induce ectopic accumulation of the NRG receptors, ErbB2 and ErbB3 (Rimer, M., I. Cohen, T. Lømo, S.J. Burden, and U.J. McMahan, 1996. Soc. Neurosci. Abstr. 1689; Meier et al., 1997). Furthermore, muscle cells express transcripts encoding ARIA/NRG isoforms (Moscoso et al., 1995; Ng et al., 1997). However, it is not known whether muscle cell–derived NRGs are biologically active.
In this paper, we have tested the hypothesis that muscle cells are a source of functional ARIA/NRG-like biological activity that could be locally concentrated at the muscle surface by agrin to activate AChR subunit gene transcription. We found all elements required for such a process: (a) in cultured myotubes, transcription of AChR ε subunit gene induced by substrate-bound agrin is inhibited when myotubes overexpress an inactive human mutant of ErbB2, HER2KM, as is transcription induced by NRG; (b) NRG transcripts are expressed in innervated muscle and NRG-like biological activity can be extracted from cultured muscle cells; (c) NRG-like immunoreactivity colocalizes with aggregates of newly synthesized AChRs induced in nerve-free segments of innervated muscle fibers by ectopic neural agrin; (d) as proposed by Loeb and Fischbach (1995), the Ig-like domains of muscle derived NRGs mediate direct interaction with glycosaminoglycan (GAG) side chains of HSPGs. Indeed, the HSPGs agrin and perlecan, but not other glycoproteins of the postsynaptic apparatus bind NRGs in overlay and radioligand-binding assays; (e) in addition to agrin, other HSPGs are accumulated in the muscle BL by agrin, thus providing additional binding sites for the localization of NRGs. Taken together, these findings support the hypothesis that agrin regulates synapse-specific AChR gene expression by localizing muscle derived NRGs and activating ErbB receptors.
Materials and Methods
The term neuregulin (NRG) is used in this paper whenever we wish to address transcripts or proteins encoded by the nrg-1 gene (Fischbach and Rosen, 1997) irrespective of species or isoform. Human, rat, and chick NRG isoforms are referred to as heregulin(s) (HRGs), Neu differentiation factor (NDF), or ARIA, respectively (Lemke, 1996; Fischbach and Rosen, 1997). Products of the nrg-2 gene were not considered as they appear not to be expressed in muscle (Carraway et al., 1997; Chang et al., 1997).
AChR ε Subunit Transcription in C2C12 Cells Overexpressing HER2 or HER2KM
Recombinant full-length neural cAgrin7A4B8 was immobilized on 35-mm culture dishes by precoating dishes with 65 μl of 20 μg/ml laminin from EHS tumor (Sigma Chemical Co., St. Louis, MO) followed by spreading 60 μl of 5 μg/ml Agrin7A4B8 diluted in PBS, and then incubation overnight at 4°C. After rinsing, 120,000 C2C12 cells were added to each well and co-transfected with 3 μg of the AChR ε-promoter luciferase reporter construct pLCF216ε (Jones et al., 1996), 250 ng of pCH110 (Pharmacia Biotechnology Inc., Piscataway, NJ) encoding β-galactosidase, and 250 ng of either pHER2/neu or pHER2KM (Wallasch et al., 1995) using the calcium phosphate method (Chen and Okayama, 1987) in serum-free medium (Jones et al., 1996). To exclude unspecific effects on gene regulation after HER2 and HER2KM transfection, we analyzed muscle creatine kinase (MCK) transcription using a 6.5-kbp MCK promoter fragment from pUC118MCK6.5 subcloned into pGL2 (Promega, Heidelberg, Germany). Cells were analyzed 72 h later for luciferase activity using a Luminometer (Turner Designs, Sunnyvale, CA) and relative luciferase units were normalized to β-galactosidase expression. To test for transfection efficiency and protein expression, COS cells were transfected accordingly, proteins extracted in SDS sample buffer and analyzed on Western blots for the expression and tyrosine phosphorylation of HER2 using the antibodies described below.
Reverse Transcriptase–PCR
Extrasynaptic segments of innervated adult rat diaphragm were dissected and polyadenylated mRNA was isolated according to the method of Hengerer (1993). First strand cDNA was synthesized using Superscript reverse transcriptase (Life Technologies, Inc., Gaithersburg, MD) according to the manufacturer's instructions with 50 ng of mRNA primed with 2 pmoles of the 5′-GCAGTAGGCCACCACACACATGATGCC-3′ oligonucleotide (TM-antisense) (Microsynth, Balgach, Switzerland), which corresponds to the transmembrane (TM) region of rat NDF (Wen et al., 1992). The first-strand cDNA was then extracted with phenol/chloroform, precipitated, and then resuspended in 20 μl of water. To amplify an NDF transcript spanning the TM and Ig domains, 10 μl was amplified in a volume of 50 μl with an air thermal cycler (Idaho Technology, Idaho Falls, ID) using a cycle protocol of 94°C for 15 s, 58°C for 15 s, and 72°C for 35 s for 35 cycles and the TM-antisense primer together with an Ig-sense primer 5′-CTCTGGAGAGTATATGTGCAAAGTGATCAGC-3′ that recognizes the 3′ part of the NDF Ig-domain (Wen et al., 1992). To examine whether the resulting Ig/TM product included α or β splice isoforms and to examine the spacer insert between the Ig and EGF domains (for review see Lemke, 1996), the reaction mix was diluted 1:100 and 1 μl was amplified in a 10 μl volume using 35 cycles of 94°C for 1 s, 58°C for 1 s, 72°C for 10 s. Primer combinations were as follows: to examine the spacer insert we used the Ig-sense primer together with the EGF-antisense (5′-CCGTGAAGCACTCGCCCCCATTCACACAG-3′) primer. To detect α- and β-specific isoforms, either a β-specific primer (5′-CCAAAACTACGTAATGGCCAGCTT-3′) or an α-specific primer (5′-TGTACCCATGAAAGTCCAAACCCA-3′) was used in combination with the TM-antisense primer. PCR products were resolved on a 2% agarose gel and transferred to nylon membrane (Boehringer Mannheim GmbH, Mannheim, Germany), which was then hybridized overnight at 42°C with a PCR-generated NDF probe labeled with Digoxygenin-11-dUTP (Boehringer Mannheim GmbH) and spanning the Ig–TM domains, ensuring recognition of all NDF isoforms. Amplified NDF DNA was visualized using alkaline phosphatase–conjugated anti-DIG antibody and the chemiluminescence substrate CDP-Star™ (Boehringer Mannheim GmbH) according to the manufacturer's instructions.
Agrin and Heregulin
Chick full-length agrin, cAgrin7A4B8, and cAgrin7A0B0 (Denzer et al., 1995), as well as the COOH-terminal agrin fragment c95A0B0 (Gesemann et al., 1995), were purified from conditioned medium of stably transfected HEK 293 cells as described elsewhere (Gesemann et al., 1995; Denzer et al., 1997).
HRGβ1(1–246) cDNA encoding a full-length extracellular domain of the human orthologue of chicken ARIA (Falls et al., 1993; Jeschke et al., 1995) was subcloned into the bacterial expression vector pFLAG-1 (International Biotechnologies Inc., Cambridge, UK), and then used for transformation of Escherichia coli XL-1 blue. Expression of recombinant protein was induced with 1 mM isopropylthio-β-d-galactoside (IPTG), homogenate was enriched for inclusion bodies, and then extracted with 6 M urea followed by extensive dialysis against PBS and purification with anti–FLAG M2 affinity gel (International Biotechnologies Inc., New Haven, CT). Recombinant HRGβ1(177–246) DNA containing a His tag and FLAG epitope (Jeschke et al., 1995) was expressed in bacteria and enriched from periplasmic extract on a His-Trap affinity column (Pharmacia Biotechnology Inc.) according to standard protocols. Cloning of the novel HRG-isoform HRGγ and the isolated Ig domain, HRGΔBbsI, will be described in detail in a separate paper (see Results under Binding of Neuregulin to Agrin Isoforms). Briefly, HRGγ derived from a cDNA library obtained from MDA-MB-231 cells was cloned into the bacterial expression vector pQE30 (Qiagen, Hilden, Germany) resulting in pQE30/HRGγ. The coding sequence of HRGγ was subcloned into pEGFP to provide pEGFP/HRGγ, from which pEGFP/HRGΔBbsI was constructed by linearization with BbsI, fill-in with T4 DNA polymerase, and subsequent digestion with SmaI and religation. Finally, plasmid pQE30/HRGΔBbsI was obtained by replacing the SpeI/HincII fragment of pQE30/HRGγ with the corresponding restriction fragment from plasmid pEGFP/HRGΔBbsI. Bacteria (E. coli XL1 blue) transformed with pQE30/HRGγ or pQE30/ HRGΔBbsI were induced with 0.4 mM IPTG for 5 h, inclusion bodies were solubilized in 6 M urea and purified over a cation exchange column (SP Sepharose Fast Flow; Pharmacia Biotech Sevrage, Uppsala, Sweden) using HPLC equipment. The resulting HRGγ (fraction A) was dialyzed against 50 mM Tris-HCl, 150 mM NaCl, 0.1% Tween 20, and used for most overlay assays and for radiolabeling. For some experiments, this enriched HRGγ/fraction A preparation was further purified by Ni+-NTA (Qiagen) affinity chromatography (HRGγ, fraction C). HRGΔBbsI was purified on His-Trap affinity column in the presence of 6 M urea and stored at 4°C. As a control for nonspecific binding resulting from the presence of a His tag we subcloned the BamHI–HindIII insert of pQE16 (Qiagen) encoding dihydrofolate reductase (DHFR) cDNA ligated to a 6× His tag at its 3′ end into the BamHI–HindIII cut plasmid pQE30. This provided the control protein DHFR flanked with COOH- and NH2-terminal His tags (His-DHFR-His). The NH2-terminal His tag (Arg-Gly-Ser- [His]4) was recognized by the RGS-His antibody (Qiagen). His-DHFR-His was extracted from bacterial inclusion bodies with 6 M urea and used without further purification.
Extraction and purification of recombinant proteins was monitored by SDS-PAGE and Coomassie brilliant blue staining. Protein concentrations of HRGγ and His-DHFR-His were determined by comparing absorptions of Coomassie-stained protein bands with calibration curves obtained with BSA as standard. His- and FLAG-tagged heregulins were analyzed further on immunoblots under standard conditions using anti-His antibodies (Qiagen) and anti–FLAG M1 antibody (International Biotechnologies Inc.) for the detection of HRGβ1(177–246) or anti–FLAG M2 antibody for the detection of HRGβ1(1–246) combined with peroxidase-conjugated anti– mouse antibodies (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA) and chemiluminescence detection using Super Signal™ substrate (Pierce Chemical Co., Rockford, IL). Relative concentrations of HRGβ1(1–246), HRGβ1(177–246), and HRGΔBbsI were adjusted to obtain comparable signals on immunoblots. Fig. 1 shows the heregulin isoforms used in this study and their detection by anti-His or anti-FLAG antibodies.
Figure 1.
Summary diagram of HRG isoforms and their detection. (A) Schematic representation of HRGs indicating the position of the Ig-like domain and EGF-like domain, as well as FLAG and His tags used for purification and detection. HRGβ1(177–246), representing the EGF-like domain, is derived from full-length HRGβ1(1–246), whereas the Ig-like domain is contained in the HRGΔBbsI fragment of HRGγ. (B) Immunoblot of recombinant proteins. HRGγ, HRGΔBbsI, and the control protein His-DHFR-His were detected with anti-His tag primary antibody. HRGβ1(1–246) and HRGβ1(177–246) were detected with anti–FLAG M2 and anti– FLAG M1 primary antibodies, respectively. In this and some of the following figures molecular weights (kD) of standard proteins are indicated.
Extraction of NRG-like Biological Activity from Myotubes and ErbB2 Immunoprecipitation
Extracts from extracellular matrix (ECM) of chick brain and spinal cord cells have been shown previously to contain NRG (Loeb and Fischbach, 1995). Primary rat myotubes cultured according to Brenner et al. (1992) for 6 d and C2C12 myotubes cultured for 5–6 d in differentiation medium were used to test muscle cells for their content of NRG-like biological activity. After washing in ice cold PBS, three to four 30-mm plates were extracted sequentially with 1 ml salt extraction solution (PBS supplemented with 1 mM MgCl2, 2.5 mM CaCl2, and NaCl to 1.0 or 1.5 M) by gentle agitation on ice. After centrifugation at 4°C, salt extracts were dialyzed against 0.5× PBS for several hours and then dialyzed against serum-free DME overnight at 4°C. To monitor NRG-like biological activity, fresh cultures of 6-d-old primary rat myotubes or C2C12 cells differentiated in 30-mm plates were stimulated with 1 ml of this dialyzed, conditioned salt extract for 8 min at 37°C. For comparison, cultures were either incubated with dialysis medium (DME) as unstimulated control or with dialysis medium supplemented with saturating amounts of recombinant HRGβ1(177–246) (stimulated control). After washing in ice-cold PBS, membrane proteins were extracted with 1 ml detergent extraction buffer (50 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM EDTA, 5 mM EGTA, 50 mM NaF, 10 mM sodium-molybdate, 1 mM sodium-orthovanadate, 1% (vol/vol) Triton X-100, 5 μg/ml leupeptin, 5 μg/ml aprotinin, and 1 mM PMSF) and extracts cleared from cell debris by centrifugation at 4°C. ErbB2 was specifically immunoprecipitated with 0.1 μg/ml anti-ErbB2 antibody (C18; Santa Cruz Biotechnology Inc., Santa Cruz, CA) and 10 μl protein A–Sepharose beads for 2–3 h at 4°C. Control experiments showed that this antibody does not cross-react with either ErbB3 or ErbB4 in such detergent- extracts (not shown). After washing three times with 1 ml detergent extraction buffer and once with 1 ml PBS, excess liquid was removed, and then beads were boiled in 20 μl SDS sample buffer. Samples were separated on 6% SDS-PAGE and transferred to nitrocellulose. In our gel system ErbB2 protein migrated as an ∼200-kD band; this was confirmed by immunoblotting with anti-ErbB2 antibodies. For detection of tyrosine phosphorylation, blots were preincubated with 3% BSA in TTBS (25 mM Tris-HCl, pH 7.5, 150 mM NaCl, and 0.1% [vol/vol] Tween-20), incubated with peroxidase-conjugated anti-phosphotyrosine antibody 4G10 (1:3,000; Upstate Biotechnology Inc., Lake Placid, NY) followed by chemiluminescence detection. Densitometric analyses of Western blots were carried out using ImageQuant software and data from different experiments were combined by normalizing the results of each experiment to saturating HRG-stimulated ErbB2 phosphorylation.
Immunoblots and Transfer Blot Overlay Assays
Binding of heregulin was assayed for the chick agrin constructs, cAgrin7A0B0 or cAgrin7A4B8. Proteins were separated by SDS-PAGE on 3–12% gradient gels under reducing conditions and transferred to nitrocellulose (Towbin et al., 1979). Routinely we loaded 100 ng/lane of agrin for immunoblots and up to 250 ng/lane of agrin for transfer blot overlay assays. To test whether agrin proteins were efficiently transferred, blots were incubated with anti-agrin antibodies (No. 3240) raised against the c95 fragment of agrin (Gesemann et al., 1995), followed by peroxidase-conjugated secondary antibodies (Jackson ImmunoResearch Laboratories) and chemiluminescence detection (Pierce Chemical Co.). Transfer blot overlay assays were carried out essentially as described (Yamada et al., 1996). Briefly, unspecific binding sites were blocked with 10 mM triethanolamine, pH 7.6, 140 mM NaCl, 1 mM CaCl2, 1 mM MgCl2, 0.01% Tween 20 (TLBB) supplemented with 5% (wt/vol) nonfat milk powder (MTLBB) for 45 min before they were overlaid with heregulin protein diluted in MTLBB for 1.5 h. In some experiments, MTLBB was supplemented with 10 mM β-mercaptoethanol during incubation with HRG. For detection of His-tagged heregulin proteins, blots were washed three times for 10 min, incubated with anti-His antibody (Qiagen) diluted (1:3,000) in MTLBB for 60 min. After three 10-min washes in TLBB buffer, bound proteins were detected with peroxidase-conjugated goat anti–mouse antibody (1:3,000 in MTLBB; Jackson ImmunoResearch Laboratories) followed by chemiluminescence. For detection of bound FLAG-tagged heregulins, anti–FLAG M1 (detection of HRGβ1[177–246]) and anti–FLAG M2 (detection of HRGβ1[1–246]) primary antibodies were used. For the removal of heparan sulfate glycosaminoglycan side chains, cAgrin7A4B8 and cAgrin7A0B0 at concentrations of 30 μg/ml were diluted threefold with 15 mM Tris-HCl, pH 7.5, 75 mM sodium acetate, pH 7.5, 1 mM CaCl2, and reaction volumes of 30–35 μl were incubated with 0.5 U of heparitinase (Heparinase III, No. H8891; Sigma Chemical Co., St. Louis, MO) for 8 h at 25°C. Reaction was stopped by the addition of SDS sample buffer and deglycosylated proteins were used for overlay assays as described. Untreated controls were incubated in the same buffer but without enzyme added. To test for the influence of Ca2+ on the binding of HRG to agrin, some overlay assays were performed in Ca2+/Mg2+-free TLBB and MTLBB solutions supplemented with 10 mM EDTA.
Solid Phase Radioligand-binding Assay
HRGγ (fraction A; 130 μl of a 1.4 mg/ml protein solution) was iodinated using the chloramine T method (Hunter and Greenwood, 1962) according to Gesemann et al. (1996). Samples of 125I-HRGγ were separated on SDS-PAGE and analyzed by a PhosphorImager (Molecular Dynamics, Inc., Sunnyvale, CA). Microtiter plates (Becton Dickinson Co., Mountain View, CA) were coated with 1 μg/ml Agrin7A0B0 and Agrin7A4B8 or 10 μg/ml of c95A0B0 (Gesemann et al., 1995), laminin-1 prepared from mouse Engelbreth-Holm-Swarm sarcoma (Timpl et al., 1979), tenascin (Chiquet et al., 1991), fibronectin (purified from conditioned fibroblast medium; Chiquet et al., 1991), or perlecan (for review see Timpl, 1993; Timpl and Brown, 1995) diluted in 50 mM sodium bicarbonate, pH 9.6, overnight at 4°C. α-Dystroglycan prepared from chicken lung (Gesemann et al., 1997) and Matrigel™ (Kleinman et al., 1982), were each diluted 1:10 for coating. After coating, wells were blocked with TBS containing 3% BSA, 1.25 mM CaCl2, 1 mM MgCl2 (blocking solution) and incubated for 3 h with 125I-HRGγ diluted 1:200 in blocking solution (final concentration of ∼40 nM). After washing with TBS, 1.25 mM CaCl2, and 1 mM MgCl2 four times, bound radioactivity in each well was counted with a gamma counter.
Heparan sulfate glycosaminoglycan side chains were removed before the incubation with 125I-HRGγ by treating cAgrin7A0B0-coated wells with 0.5 U heparitinase in heparitinase buffer (see above).
Ectopic Expression of Agrin and Immunocytochemistry
Full-length cAgrin7A4B8 or the agrin fragment cN257C95A4B8, a fusion construct of the NH2-terminal laminin-binding domain of agrin (cN257) with the c95A4B8 fragment (Gesemann et al., 1995; Denzer et al., 1997; construct provided by D. Hauser and M.A. Ruegg, Biozentrum, Basel, Switzerland), was expressed at extrasynaptic regions of innervated rat soleus muscle by direct injection of expression plasmid as described previously (Jones et al., 1997). 4–8 wk after injection, muscle was excised, stained with rhodamine-α-bungarotoxin (Molecular Probes, Inc., Eugene, OR), and then processed as whole-mount preparations for anti-HSPG immunostaining or cut into 12-μm frozen sections and stained for the presence of NRG aggregates at sites of ectopic AChR synthesis and accumulation. For staining of HSPG accumulation at sites of ectopic agrin-induced AChRs, dissected muscle was incubated in mAb C17 (Eldridge et al., 1986) diluted 1:100 in 5% horse serum, 1% BSA in PBS for 2 h, washed, and then incubated with BODIPY goat anti–mouse (1:250; Jackson ImmunoResearch Laboratories) for 2 h. Whole-mount preparations were viewed on a laser scanning microscope (Leica, Heerbrugg, Switzerland). For detection of NRG we used the rabbit antiserum sc-348 (Santa Cruz Biotechnology Inc.) specific for membrane-associated NRG precursors with no cross-reactivity to mature forms of NRGs (Moscoso et al., 1995). We also generated a rabbit antiserum anti-HRG No. 76990 raised to bacterially expressed HRGβ1(1–246). For this, rabbits were initially injected with 50 μg of HRGβ1(1–246) antigen expressed in bacteria, extracted from inclusion bodies and dissolved in 2.4 ml Freund's complete adjuvant. Boost injections were carried out with antigen mixed with Freund's incomplete adjuvant, and the IgG fraction from the rabbit serum was obtained by affinity chromatography using the Econo-Pac Serum IgG purification column (Bio-Rad Laboratories, Hercules, CA) according to the manufacturer's instructions. For immunocytochemistry, cryosections were blocked for 5 min with 5% horse serum, 1% BSA in PBS with 0.1% (vol/ vol) Triton X-100 and primary antibodies diluted 1:100 (anti-HRG[622–641]; sc-348) or 1:250 (anti-HRGβ1[1–246]; No. 76990) in incubation buffer (5% horse serum, 1% BSA, in PBS with 0.01% [vol/vol] Triton X-100) for 2 h. After washing, sections were exposed to unconjugated goat anti–rabbit IgG (1:250 in dilution buffer; Cappel, Cochranville, PA) for 2 h, washed, and then incubated with FITC-conjugated rabbit anti–goat IgG (1:250 in dilution buffer; Jackson ImmunoResearch Laboratories) for 2 h.
The concern has been raised (Cohen et al., 1997; Rimer et al., 1997) that an inflammatory response by the use of chicken agrin in rat muscle may be involved in the induction of ectopic AChR clusters by ectopic agrin. However, unlike the injections of chicken agrin plasmid into the muscle bulk, which apparently causes inflammation (Cohen et al., 1997; Rimer et al., 1997), the intracellular injection of much smaller amounts of agrin plasmids into single muscle fibers as carried out in our present and previous experiments (Jones et al., 1997) does not produce any sign of immunological response. Specifically, histological examination of soleus cross-sections in the region of agrin plasmid injection showed no signs of edema, muscle fiber dilatation, or influx of polymorphonuclear granulocytes or of erythrocytes, as can be observed in acute antibody-mediated rejection; nor was there influx of mononuclear cells such as lymphoblasts, lymphocytes, or macrophages, as can occur during a cellular rejection. Perhaps, the immunological response upon plasmid injection to the muscle bulk was elicited by uptake of the cDNA by other cells than muscle and their subsequent presentation of chicken agrin epitopes (Donnelly et al., 1997).
Results
Previous experiments demonstrated that agrin alone is sufficient to initiate transcription of the AChR ε subunit gene (Jones et al., 1996, 1997) and accumulation of adult AChR channels (Jones et al., 1997; Rimer et al., 1997) in muscle. This prompted the hypothesis that NRGs supplied by the nerve terminal are not required to control AChR gene expression. Instead, nerve-derived agrin might initiate AChR subunit gene transcription by recruitment of muscle-derived NRGs and their localization to synaptic BL (Jones et al., 1996). The experiments described below were designed to test this hypothesis.
Blockade of the NRG/ErbB Pathway Blocks Agrin-induced AChR Gene Transcription
If agrin indirectly increases AChR gene expression by recruiting muscle-derived NRG, thus activating the ErbB receptor signaling pathway, inhibition of ErbB receptor activation would be expected to abolish agrin-induced gene transcription. We tested this prediction by overexpressing an inactive HER2 receptor construct (HER2KM) in C2C12 myotubes and monitoring the effect on agrin-induced AChR ε subunit gene induction. HER2KM is a kinase inactive single point mutant of HER2, the human orthologue of rat ErbB2, and HER2KM is thought to form inactive heterodimers with HER3/ErbB3, thereby blocking activation of the ErbB signaling pathway in the myotubes (Wallasch et al., 1995).
An AChR ε subunit promoter luciferase reporter gene construct (Jones et al., 1996) was transiently transfected into C2C12 myoblasts cultured on a laminin substrate impregnated with recombinant full length chick Agrin7A4B8. After differentiation into myotubes, luciferase activity was increased approximately fourfold above that in control cells grown on laminin alone (see Fig. 2). A similar increase in luciferase activity was seen, when parallel cultures were treated with saturating concentrations of a fragment of recombinant heregulinβ1 (HRGβ1[177-246]), which is in the range of induction previously reported by others (Tansey et al., 1996). In contrast, cotransfection of the AChR ε-promoter luciferase reporter construct with a plasmid encoding inactive HER2KM significantly reduced the induction of luciferase activity, both that by substrate-bound agrin and that by HRGβ1, to levels not different from that in control cultures. HER2 and HER2KM acted specifically on the activation of the AChR ε subunit promoter, as no effects were observed on the levels of muscle creatine kinase (MCK) expression.
Figure 2.
Agrin induces AChR gene transcription by an ErbB receptor–mediated pathway. (A) An AChR ε-subunit promoter lucifersase reporter construct was transiently transfected into C2C12 myoblasts grown on either a control laminin substrate (con) or a laminin substrate impregnated with chick neural agrin (Agrin). As a positive control, transfected myoblasts were cultured on laminin and stimulated with a saturating amount of HRGβ1 (HRG). In each case, the functional requirement for the ErbB receptor pathway was tested by cotransfecting an expression plasmid encoding a dominant-negative mutant of the HER2 receptor (HER2KM). For comparison, the increase in luciferase activity directed by the non-mutant HER2 receptor is also shown (HER2). Results represent three independent experiments (mean ± SEM; n = 6–9). Asterisks, different from level of agrin-induced luciferase activity (U < U*; α = 0.01, Mann-Whitney test). (B) Luciferase activity directed by a muscle creatine kinase (MCK) promoter construct remained unchanged after cotransfection of either the HER2KM or HER2 expression plasmids in comparison to cultures transfected with MCK promoter luciferase reporter gene alone (con). Luciferase activities in (A) and (B) were normalized to β-galactosidase and expressed relative to cultures grown on a control laminin substrate.
The reduction in agrin- and HRGβ1-induced luciferase activities by HER2KM was not a nonspecific effect arising from overexpression of HER2KM. First, unlike HER2KM, cotransfection of an expression plasmid encoding active, wild-type HER2 did increase luciferase activity even in the absence of agrin. This increase produced by HER2 alone may be due to the formation of autophosphorylated HER2 homodimers, as transfection of HER2 alone into COS cells produced—when compared with transfection with HER2KM—a substantial level of phosphorylated HER2, despite the absence of ligand (data not shown; Wallasch et al., 1995). Second, expression of the two plasmids was similar, as tested in COS cells by Western blotting HER2 and HER2KM protein levels (not shown).
Expression of Neuregulins Containing Ig and EGF Domains in Muscle
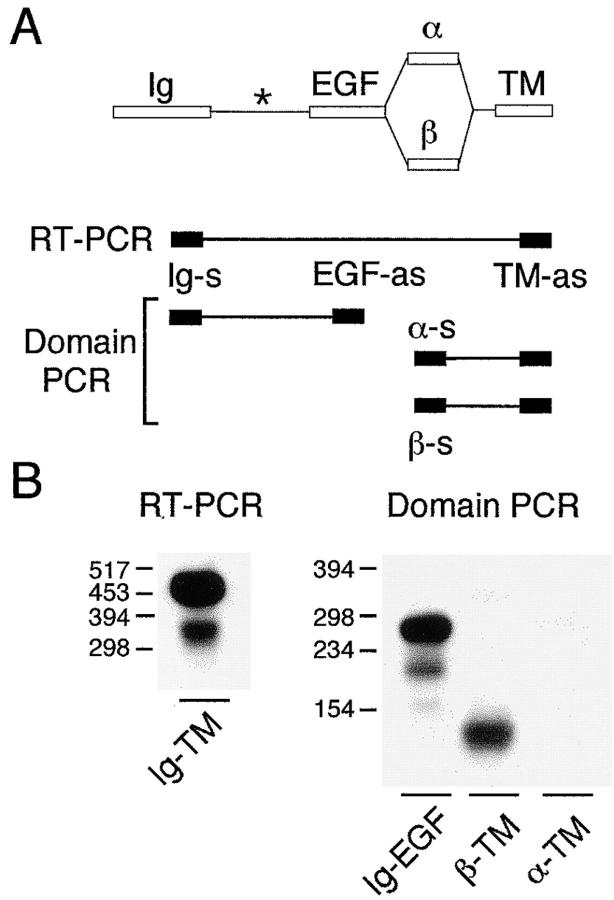
It has been proposed by Loeb and Fischbach (1995) that immobilization of NRGs to the ECM might be via their Ig-like domains binding to HSPGs. For maximal induction of AChR gene transcription by ErbB receptor activation, β isoforms of NRGs are required (Fischbach and Rosen, 1997). We therefore examined whether NRG β isoforms are expressed in innervated adult muscle. To determine whether active NRGs containing the Ig and EGF domains are expressed in adult skeletal muscle, we isolated mRNA from the extrasynaptic region of innervated rat diaphragm. Using reverse transcriptase PCR (Fig. 3 A), NRG transcripts spanning the Ig domain through to the transmembrane domain were amplified. The resulting products (Fig. 3 B, left panel) were then subjected to a second round of PCR using primers which specifically amplified either α- or β-NRG isoforms or amplified the spacer insert between the immunoglobulin and EGF domain. In this way we could amplify β-specific, but not α-specific isoforms that are cotranscribed with the Ig domain (Fig. 3 B, right panel). Further, the calculated molecular weight of the β isoform suggested it to be β1 (148 bp) or β2 (124 bp), but not β4 (205 bp; Wen et al., 1994). This is in agreement with experiments carried out in adult chicken muscle in which the expression of β1-NRG is predominant but very little α-NRG is transcribed (Ng et al., 1997). Three PCR products were amplified using the Ig-EGF primers, which flanked the alternatively spliced spacer domain. Based upon the expected molecular weight, the predominant spacer domain isoform in nonsynaptic region of rat diaphragm is the 34–amino acid (277 bp) variant, although lower amounts of both the 17–amino acid (226 bp) and 0–amino acid (175 bp) isoforms were also amplified (Fig. 3 B, right panel).
Figure 3.
NRG gene transcripts that include Ig and EGF domains are present in the nerve-free region of adult muscle. (A) NRG transcripts were amplified by reverse transcriptase PCR (RT-PCR) using mRNA from the extrasynaptic region of innervated diaphragm muscle and primers specific for the immunoglobulin (Ig) and transmembrane (TM) domains. To examine specific domains within these transcripts, PCR was done using primers spanning Ig-EGF, α-TM, or β-TM as indicated by the connected shaded boxes, whose relative positions within NRG are shown schematically (top). Primer nomenclature is as described in Materials and Methods. s, sense primer; as, antisense primer; asterisk, spacer domain. (B) Products of RT-PCR (left) and PCR of different domains (right) visualized by hybridization with an NDF probe. Amplified domains are indicated below each figure and molecular weight markers (bp) are indicated to the left of each figure.
Taken together, these results suggest that neuregulin transcripts in extrasynaptic regions of innervated adult muscle encode a protein containing functional domains essential for binding to HSPGs and activating the ErbB receptor tyrosine kinases.
Neuregulin-like Biological Activity Can Be Extracted from Cultured Myotubes
We next tested whether NRG-like biological activity is indeed secreted by muscle cells. For this, primary rat myotube cultures were extracted with high salt in physiological buffer. This treatment did not induce obvious cell lysis as determined by visual inspection at the end of the salt buffer incubation, indicating that this mainly removed cell surface– and ECM-immobilized components. Dialyzed extracts were subsequently applied onto fresh myotube cultures for ErbB receptor stimulation. Treatment of cultures with the salt extracts stimulated tyrosine phosphorylation of ErbB2 fourfold, whereas treatment with saturating, recombinant HRGβ1(177–246) produced an ≤20-fold increase (Fig. 4, A and B). Since NRGs activate ErbB2/ErbB3 heterodimers at the NMJ (Altiok et al., 1995; for review see Fischbach and Rosen, 1997), this suggests that the primary cultures contained NRG-like activity. To exclude the possibility that the extracted NRG originated from fibroblasts contaminating our primary cultures, we also examined salt extracts of myotubes formed from the C2C12 cell line. As with the primary cultures, phosphorylation of ErbB2 was observed when such extracts were applied to fresh C2C12 myotubes (Fig. 4 C). From these experiments we conclude that cultured myotubes express NRG-like biological activity as monitored by ErbB2 phosphorylation. However, it is not clear how these NRGs would become localized to the synapse during NMJ formation.
Figure 4.
NRG-like biological activity is extracted from rat and mouse muscle cells. Conditioned salt-extracts (NaCl) were prepared from rat primary cultures (RMT) (A and B) and mouse C2C12 cells (C) and fresh cultures of the same type were used for stimulation. ErbB2 immunoprecipitation from detergent extracts followed by mAb 4G10 Western blotting revealed changes in the level of tyrosine phosphorylation of ErbB2 (arrow) in RMTs (A) and C2C12 myotubes (C). Parallel cultures stimulated with recombinant HRGβ1(177–246) (HRG) at saturating concentrations or unsupplemented serum-free medium (con) were used as positive and negative controls, respectively. (B) Quantitative densitometric analysis from three experiments as shown in A indicating levels of tyrosine phosphorylation of ErbB2 immunoprecipitated from unstimulated control RMT cultures (con; n = 7) or after stimulation with conditioned NaCl extract (NaCl; n = 10) or recombinant HRGβ1(177–246) (HRG; n = 7). Asterisk, significantly different from control (U < U*; α = 0.01, Mann-Whitney test).
Neural Agrin Induces Accumulation of NRG at Ectopic Postsynaptic Specializations
At normal neuromuscular synapses NRGs are accumulated (Jo et al., 1995), and ectopic expression of agrin in innervated muscle fibers induced accumulations of ErbB receptors (Meier et al., 1997). This prompted us to test whether agrin might induce the local accumulation of muscle-derived NRG. For this purpose, we injected innervated rat soleus muscle in vivo with expression plasmids encoding neural cAgrin7A4B8 (Jones et al., 1997). Double labeling of ectopic, agrin-induced AChR aggregates with rhodamine-α-bungarotoxin and with antibodies specific for NRGs revealed the accumulation of muscle-derived neuregulins at sites of ectopic AChR aggregation (Fig. 5). Antisera raised against either the extracellular or intracellular domains of NRG resulted in similar staining patterns. This indicates that membrane bound NRG precursors are accumulated at sites of AChR synthesis and aggregation in the nerve-free region of muscle fibers expressing agrin.
Figure 5.

Ectopic expression of neural cAgrin7A4B8 induces the accumulation of muscle NRG at sites of AChR aggregation. (A and C) Colocalization of AChR stained with Rh-α-BGT (arrows) and anti-HRG antibodies specific for membrane associated HRG precursors (B, HRG(622–641)) or with antibodies raised against the extracellular region of HRGβ1 (D, HRGβ1(1–246)). Bar, 50 μm.
Binding of Neuregulin to Agrin Isoforms
Since NRGs bind to heparin (Falls et al., 1993) and agrin is a HSPG (Denzer et al., 1995; Tsen et al., 1995), we examined whether recombinant NRG isoforms directly bound to recombinant chick agrin. When tested in transfer blot overlay assays, both HRGβ1(1–246) and HRGγ, a novel NRG isoform (Schoumacher, F., H. Mueller, and U. Eppenberger, manuscript in preparation), bound to immobilized full-length neural cAgrin7A4B8, an ∼400–600-kD glycoprotein (Fig. 6, A and B). These results indicate that HRG isoforms bind directly to immobilized full-length neural agrin and that this interaction does not require the presence of the complete EGF-like domain of HRG.
Figure 6.
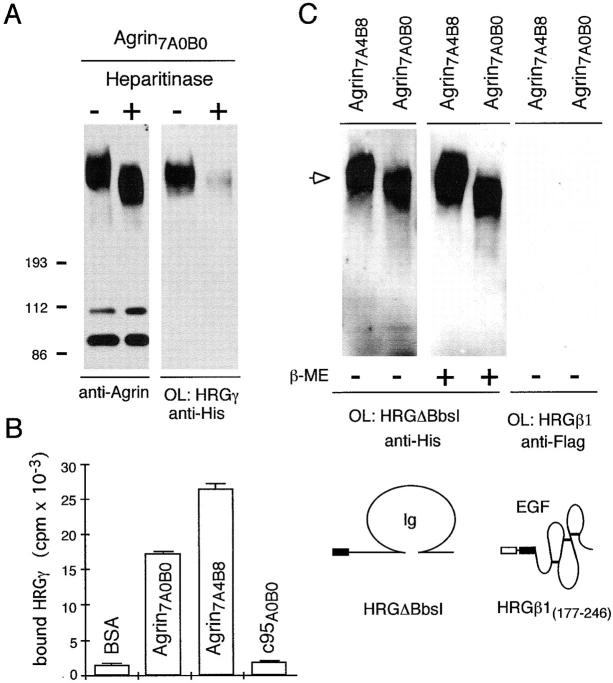
Recombinant heregulins bind to the heparan sulfate proteoglycans agrin and perlecan. (A) Left lane, immunoblot with anti-agrin antibodies for detection of immobilized full-length Agrin7A4B8 (arrow) and smaller degradation products. Center lane, transfer blot overlay assay (OL) with HRGβ1(1–246) followed by anti-FLAG primary antibody and POD-conjugated secondary antibodies for detection. Right lane (negative control), incubation of immobilized agrin with anti-Flag antibody and POD-conjugated secondary antibodies alone. (B) Left lane, immunoblot with anti-agrin antibodies as in A. Center lane, overlay assay with HRGγ, followed by anti-His primary and POD-conjugated secondary antibodies. Right lane (negative control), overlay of immobilized agrin with the control protein His-DHFR-His and anti-His antibody detection to test for nonspecific binding. Schematic representations of HRG isoforms used for overlay assays are shown. (C) Solid phase radioligand-binding assay with 125I-HRGγ revealed strong binding to Agrin7A0B0 and perlecan. Binding to α-dystroglycan, laminin-1, tenascin, fibronectin, and Matrigel™ were comparable to BSA. Mean values ± SEM (n = 3) of bound 125I-HRGγ (in cpm) are shown.
Next, we examined whether HRGγ binds to Agrin7A0B0, a muscle-specific full-length isoform that lacks AChR aggregating activity (Ferns et al., 1992, 1993; Gesemann et al., 1995), but induces AChR subunit gene expression in cultured myotubes (Jones et al., 1996). In solid phase radioligand-binding assays (Fig. 6 C), as well as in transfer blot overlay assays (Fig. 7 A) we detected binding of HRGγ to Agrin7A0B0, but only when Ca2+ was present (data not shown). We then asked whether NRGs bind to other, non-agrin glycoprotein components of muscle synaptic membrane, such as α-dystroglycan, or of the BL, such as perlecan, tenascin, fibronectin, and laminin-1. In overlay assays, HRGγ did not bind to immobilized α-dystroglycan purified from chicken lung, nor to laminin-1, and nidogen separated under reducing conditions on SDS-PAGE (data not shown). This was confirmed by solid phase radioligand-binding assays in which the proteins were coated in non-denatured and non-reduced configuration to microtiter wells (Fig. 6 C). Similar results were obtained for fibronectin, tenascin, and Matrigel™. 125I-HRGγ did, however, exhibit strong affinity for the muscle ECM component perlecan (Timpl, 1993). A possible explanation for the lack of binding to Matrigel™, which contains perlecan, might be the reduced accessibility of perlecan incorporated into the laminin and collagen matrix. Thus, HRGγ exhibits only a weak nonspecific binding to BSA and to non-HSPG proteins, suggesting that this low level of binding most likely reflects nonspecific interactions. Specific binding was only seen for the HSPGs agrin and perlecan. Therefore, we asked whether their binding to NRGs might be mediated by their negatively charged GAG side chains, as proposed by Loeb and Fischbach (1995). This would be consistent with the previous finding that neuregulins bind heparin, a polymer of sulfated glycosaminoglycans (Falls et al., 1993).
Figure 7.
Binding of HRGs to agrin is mediated by GAG chains at the NH2-terminal part of agrin and requires the Ig-like domain of NRGs. (A) Transfer blot overlay assay of native full-length cAgrin7A0B0 (Heparitinase−) or enzyme-treated cAgrin7A0B0 (Heparitinase+) with HRGγ (OL: HRGγ). Treatment with heparitinase causes a shift in the molecular weight of full-length agrin as indicated by immunoblot analysis using anti-agrin antibodies (anti-Agrin) and strongly reduces binding of HRGγ. (B) HRGγ binds to full-length agrin isoforms but not to COOH-terminal agrin fragments. Solid phase radioligand-binding assay of 125I-HRGγ to immobilized full-length agrin isoforms, Agrin-c95A0B0 and BSA. Mean values ± SEM (n = 3) of bound 125I-HRGγ (in cpm). (C) The Ig-like domain of HRGs is sufficient to bind to full-length agrin. Transfer blot overlay assays (OL) of Agrin7A4B8 and Agrin7A0B0 with HRGΔBbsI in the presence (+) or absence (−) of 10 mM β-mercaptoethanol (β-ME) and detection with anti-His primary antibody (arrow). The Ig domain of HRGs binds to immobilized agrin even under reducing conditions, indicating that the disulfide bond of the HRG Ig domain might not be critical for interaction with agrin. In contrast, no binding is detected in overlay assays with HRGβ1(177–246) and anti–FLAG M1 primary antibody. Schematic representations of the HRG fragments used in this overlay experiment are indicated.
The Ig-like Domain of Neuregulins Mediates Binding to the GAG Chains of Agrin
As expected for NRG binding to agrin and perlecan via GAG chains, overlay, and radioligand-binding assays showed that binding of HRGγ to immobilized full-length agrin isoforms cAgrin7A4B8 and cAgrin7A0B0 was completely inhibited by heparin at concentrations as low as 2 μg/ml, the lowest concentration tested (data not shown). Similar inhibition was also seen for perlecan at heparin concentrations 20 μg/ml (data not shown). In addition, enzymatic digestion of Agrin7A0B0 with heparitinase, an enzyme that cleaves GAG side chains from proteoglycans, resulted in a shift to lower apparent molecular weight, as reported earlier (Denzer et al., 1995), and in substantially reduced binding of HRGγ to cAgrin7A0B0 depleted of GAG chains (Fig. 7 A). This result was confirmed by solid phase radioligand-binding assays where treatment of cAgrin7A0B0-coated wells with heparitinase reduced binding of 125I-HRGγ to the wells ∼20-fold (n = 4; data not shown), comparable to binding to immobilized BSA. Finally, if binding of NRGs to agrin were mediated by GAG chains, then NRGs should not bind to shorter COOH-terminal fragments of agrin-lacking GAG chains. Therefore, we tested binding of 125I-HRGγ to immobilized agrin-c95A0B0, a COOH-terminal fragment lacking conserved GAG side chain attachment sites (Denzer et al., 1995; Tsen et al., 1995). HRGγ clearly bound to both full-length agrin isoforms, although with different apparent affinity most likely representing different levels of glycosylation because of clonal selection of agrin-producing HEK 293 cells. Binding of 125I-HRGγ to c95A0B0, however, was comparable with nonspecific binding to BSA (Fig. 7 B).
Taken together, these experiments demonstrate that binding of HRG to agrin is restricted to a region located NH2-terminal to the first laminin G–like domain (Gesemann et al., 1995), and is mediated by GAG side chains. They also demonstrate that the region of agrin required for HRG binding does not overlap with the region of agrin that is sufficient to cause AChR aggregation.
Indirect evidence led to the conclusion that interaction of neuregulins with components of the synaptic BL might be mediated by the Ig-like domain of NRGs (Loeb and Fischbach, 1995). To test this directly, we expressed truncated HRG protein containing either the Ig-like domain, HRGΔBbsI, or the EGF-like domain, HRGβ1(177–246), and tested whether these isolated domains bound immobilized full-length agrin isoforms. Overlay assays (Fig. 7 C) revealed binding of HRGΔBbsI, i.e., the Ig-like domain, but not the HRGβ1(177–246) EGF-like domain to agrin. This experiment demonstrates that the Ig-like domain of neuregulins is sufficient to account for the binding to the synaptic BL proteoglycan agrin.
Agrin Induces the Accumulation of HSPGs at the Muscle Surface
The experiments described so far are consistent with a mechanism whereby agrin induces AChR gene transcription by binding muscle-derived NRGs by their Ig domains to its GAG side chains. The following observations suggest, however, that neural agrin may not be the sole binding partner for muscle NRGs: (a) ectopic NRG accumulations expressed in response to neural agrin only partially overlaps with ectopic agrin deposits (see also Meier et al., 1997); and (b) neural agrin induces the accumulation of muscle-derived HSPGs on the muscle surface. This was seen in experiments in which chick agrin pcN257C95A4B8, a fusion construct consisting of the ∼25-kD laminin-binding NtA domain (Denzer et al., 1997) ligated to c95A4B8 (Gesemann et al., 1995) was injected into myofibers. This agrin construct lacks the region that carries GAG chains but is sufficient to bind to laminin and to induce the synthesis and accumulation of extrasynaptic AChRs when injected as expression plasmids into extrasynaptic regions of innervated rat muscle fibers (Fig. 8). Double labeling of pcN257C95A4B8-injected muscle with Rh-α-BGT and antibodies specific for rat HSPGs (Eldridge et al., 1986) revealed close colocalization of ectopic AChR aggregates and HSPGs. This suggests that nerve-derived agrin isoforms expressed ectopically in innervated muscle induce the accumulation of HSPGs that can serve as local sink for the immobilization of muscle-derived NRGs. The nature of the HSPGs that are accumulated by agrin is presently unknown.
Figure 8.

Ectopic expression of agrin cN257C95A4B8 induces the extrasynaptic accumulation of AChRs and colocalization of muscle-derived HSPGs. Three superimposed laser confocal images taken at 0.5-μm intervals from a muscle fiber to show ectopic AChR aggregates induced by the expression of agrin cN257C95A4B8, colocalized with muscle-derived HSPGs. Staining with Rh-α-BGT to visualize AChRs and anti-HSPG antibody followed by BODIPY-conjugated secondary antibodies. Asterisk, blood vessel stained positive for HSPGs. Bar, 10 μm.
Discussion
We have demonstrated previously that expression of neural agrin locally induces the transcription of AChR subunit genes (Jones et al., 1996, 1997), a function classically attributed to NRGs released from motor neurons; the same sites also contain ErbB receptors (Rimer, M., I. Cohen, T. Lømo, S.J. Burden, and U.J. McMahan. 1996. Soc. Neurosci. Abstr. 1689; Meier et al., 1997). Based on these findings we proposed that synapse-specific AChR subunit gene expression by agrin is induced indirectly via muscle-derived NRGs binding to agrin-induced components of the synaptic BL (Jones et al., 1996, 1997). The present findings further support this hypothesis: the induction of AChR gene expression by agrin can be blocked by inhibiting the ErbB receptor pathway by overexpression of an inactive mutant of HER2/ErbB2. NRG-like immunoreactivity is accumulated at the sites of agrin-induced AChR gene activation and AChR accumulation. We further show that recombinant NRG isoforms of the type expressed in muscle bind directly to the HSPGs agrin and perlecan but not to several other components found at the muscle basal lamina. Binding is to the GAG chains of these HSPGs and is mediated by the Ig-like domain of NRGs. Synaptic accumulation of NRGs may thus be due, at least in part, to binding to agrin itself. Other HSPGs are accumulated in the synaptic BL under the control of agrin, providing further binding sites for NRGs. Thus, agrin induces on the muscle fiber surface all components for regulating AChR gene expression according to the above model.
Expression of NRGs in Muscle
In the absence of NRGs from other potential sources, NRGs mediating agrin-induced AChR gene transcription in nerve-free myotube cultures must be supplied by the muscle cells themselves. Indeed, cultured muscle cells synthesize NRG-like biological activity, which can be extracted from the cell surface and induces phosphorylation of the ErbB2 receptor tyrosine kinase. An indirect action of agrin via the accumulation of NRG derived from muscle could also explain why agrin's action on AChR expression depends on its binding to substrate (Jones et al., 1996): substrate-bound agrin, but not soluble agrin, could focally enrich and present muscle-derived NRGs on the muscle surface, thus activating AChR gene expression.
Previous work has identified NRG transcripts in muscle, but these studies were done using non-innervated clonal cell lines (Moscoso et al., 1995) that, unlike adult muscle, express substantial amounts of AChR ε subunit transcripts and adult-type AChR channels independently of innervation (Pinset et al., 1991; Shepherd and Brehm, 1994). Moreover, it has not been addressed whether active neuregulin β isoforms are colinearly transcribed with the Ig domain (Ng et al., 1997), a prerequisite for binding of muscle NRGs to HSPGs in the BL. Our study now demonstrates that not only cultured myotubes but also adult muscle fibers express NRG mRNA encoding isoforms with an Ig domain. Such transcripts were resolved both in synaptic (data not shown) and extrasynaptic regions of adult innervated muscle, as predicted earlier (Jones et al., 1997). In agreement with results obtained from Sol8 myotubes (Moscoso et al., 1995) or chicken muscle (Ng et al., 1997) muscle-derived NRGs probably represent NRGβ1 and NRGβ2 isoforms arising from alternative mRNA splicing at a conserved site located between the EGF domain and the transmembrane domain (Lemke, 1996; Fischbach and Rosen, 1997). NRGβ isoforms have been shown to be most potent in activation of ErbB receptor heterodimers and in the induction of AChR ε subunit gene transcription (Fischbach and Rosen, 1997). These findings suggest that the protein products of the muscle-derived NRG transcripts are biologically active and that they contain domains required for binding to synaptic HSPGs.
Requirements for NRG Immobilization in the Synaptic Basal Lamina
The expression of NRG-like activity by cultured muscle cells suggests that (a) the transcripts resolved by reverse transcriptase–PCR in muscle are translated into biologically active NRGs, and (b) that muscle-derived NRGs can be associated with the ECM through ionic interactions, as has been proposed for ARIA/NRG released from cerebellar and spinal cord cells (Loeb and Fischbach, 1995). However, their conclusion that ARIA binds to unidentified HSPGs via the Ig domain was derived indirectly, i.e., based on the heparin inhibition of ErbB phosphorylation by recombinant NRGs. In the present work, we demonstrate the importance of the GAG chains directly by showing that binding between NRGs and agrin is inhibited by enzymatic removal of GAGs, and accordingly, that NRGs do not bind to an ∼95-kD COOH-terminal agrin fragment lacking conserved GAG attachment sites. We have identified two potential binding partners at the NMJ, agrin and perlecan, and have specifically excluded other glycoproteins of the NMJ for NRG binding.
Of the potential NRG-binding HSPG partners identified in this paper, i.e., cAgrin7A4B8, cAgrin7A0B0, and perlecan, neither cAgrin7A0B0 nor perlecan were enriched at extrasynaptic AChR clusters induced by ectopic neural agrin in vivo (Meier et al., 1997; Moll, J., M.A. Ruegg, and H.R. Brenner, unpublished observation), and thus, are not involved in NRG accumulation at such sites. However, this does not exclude a role for cAgrin7A0B0 in NRG accumulation during synaptogenesis, as in nerve–muscle cocultures, muscle-derived agrin is focally expressed at sites of neurally induced AChR clusters (Lieth and Fallon, 1993). Perlecan, on the other hand, is expressed at high levels in nonsynaptic BL throughout the muscle, and thus would not appear to contribute to synaptic localization of NRGs.
Whereas neural agrin alone can induce AChR gene expression via NRG accumulation, its GAG chains do not provide the only binding sites for NRGs at the synapse. In addition, agrin induces one other HSPG that in turn may contribute to localize NRGs to the synapse. Specifically, NRGs are also accumulated at AChR aggregates induced by a COOH-terminal fragment of full-length agrin, agrin cN257C95A4B8, a construct that lacks GAG chains. Consistent with this, deposits of full-length agrin extend further along the muscle fiber surface (Meier et al., 1997) than NRGs, which are tightly colocalized with AChR aggregates and agrin-induced HPSGs (see Figs. 5 and 8). Furthermore, CBA-1 agrinA4B19, another COOH-terminal agrin fragment lacking GAG chains, induces AChR ε subunit gene transcription both in vitro and in vivo (Jones et al., 1996, 1997). Accumulation of newly synthesized HSPGs by agrin was previously shown in cultured myotubes (Wallace, 1989). Finally, focal accumulation of NRGs by agrin might be further enhanced if nerve-released agrin not only caused the accumulation of NRG-binding HSPGs but in addition, also induced NRG gene transcription from myonuclei underlying the postsynaptic membrane.
In contrast to neural agrin, muscle cAgrin7A0B0 does not induce ectopic synaptic membrane (Meier et al., 1997), yet in cultured myotubes its potency to induce AChR ε subunit gene transcription is similar to that of neural agrin7A4B8. This apparent discrepancy might reflect the considerably higher levels of expression of MuSK, NRGs, and ErbB receptors in cultured myotubes over that seen in the non-synaptic segments of mature muscle (Moscoso et al., 1995; Jones, G., and H.R. Brenner, unpublished data). As a consequence, the accumulation of constitutively expressed NRGs to muscle agrin's GAG chains and the activation of constitutively expressed ErbB receptors would be sufficient to stimulate AChR gene expression in cultured myotubes. In contrast, in adult muscle the ectopic induction and accumulation of NRG and ErbB receptors may depend on the activation of MuSK, which is phosphorylated by neural but not by muscle agrin (Glass et al., 1996).
In conclusion, our data demonstrate at sites of ectopic agrin deposits, the presence of the major molecular components required for agrin to stimulate AChR gene expression indirectly via the accumulation of muscle-derived NRGs. According to this model, agrin induces local deposits of muscle HSPGs in the muscle BL. Muscle-derived NRGs then bind with their Ig domains to accumulated HSPGs, creating a local source of this differentiation factor. NRGs would then stimulate muscle ErbB receptors that are accumulated in synaptic muscle membrane under the direction of neural agrin.
Alternative Pathways for Agrin-induced AChR Expression?
Whereas this model can explain how agrin induces AChR gene expression involving NRGs in the absence of a nerve terminal, two questions arise. First, could agrin's action to stimulate the expression of AChR genes also be mediated directly via a cognate receptor in the muscle membrane, such as MuSK (Valenzuela et al., 1995), a muscle-specific receptor tyrosine kinase phosphorylated by agrin and mediating AChR aggregation (Glass et al., 1996)? Indeed, deletion of the MuSK gene inhibits NMJ formation including synapse-specific AChR gene transcription (De Chiara et al., 1996), and deletion of the rapsyn gene, which abolishes the synaptic aggregation of ErbB receptors, does not affect synapse-specific expression of AChR genes (Gautam et al., 1995). On the other hand, direct involvement of MuSK in agrin-induced expression of AChRs is not supported, since only neural isoforms of agrin that cluster AChRs also cause phosphorylation and activation of MuSK (Glass et al., 1996). In contrast, the ability of substrate-bound agrin isoforms to activate AChR gene expression is independent of their AChR clustering activity (Jones et al., 1996). Furthermore, AChR gene expression can be induced in cultured myotubes both by COOH- and by NH2-terminal fragments of agrin (Jones et al., 1996; and unpublished results), making binding to a specific, cognate receptor appear unlikely. The second question is whether or not (during the formation of the normal NMJ) NRGs released from motor nerve terminals are important for the expression of AChR subunit genes at the synapse, as originally proposed (Martinou et al., 1991; Falls et al., 1993). The reduction of AChR levels observed in subsynaptic membrane of mice heterozygous for a deletion of Ig(+)-NRG isoforms (Sandrock et al., 1997) is consistent with both a pre- and a postsynaptic origin of BL-bound NRG. Thus, deleting expression of NRGs selectively either in the muscle fibers or in the motor neurons will be required to determine the relative roles of nerve- and muscle-derived NRGs in activating AChR gene transcription during synapse formation.
Acknowledgments
We thank Drs. M. Jeschke and N. Hynes for cDNA encoding recombinant HRGβ1; Dr. R. Timpl for perlecan; Dr. A. Brancaccio for purified α-dystroglycan; T. Schulthess for laminin-1; Dr. M. Chiquet for tenascin and fibronectin; Drs. C. Wallasch and A. Ullrich for the pHER2 and pHER2KM constructs; Dr. R. Zuellig for the plasmid pUC118MCK6.5, which contains the muscle creatine kinase promoter; and Dr. M.A. Ruegg for agrin expression constructs and for comments on the manuscript. We also thank Dr. L. Landmann for preparing the laser scanning confocal images of anti-HSPG–stained preparations, and Dr. H.-J. Schuurman, Novartis Basel, for help with histology. The mAb C17 developed by Dr. J. Sanes was obtained from the Developmental Studies Hybridoma Bank maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA, under contract NO1-HD-7-3263 from the NICHD. We also thank M. Lichtsteiner for excellent technical assistance.
This work was supported by grants from the Swiss National Science Foundation, the Ott-Fonds of the Swiss Academy of Medical Sciences, the Sandoz Stiftung and the Freie Akademische Gesellschaft, Basel (to H.R. Brenner). F. Schoumacher was supported by grants from the Swiss National Science Foundation to Dr. U. Eppenberger and Dr. H. Mueller, and in part by the Stiftung Tumorbank. A.J. Denzer was supported by the Sandoz Stiftung.
Abbreviations used in this paper
- AChR
acetylcholine receptor
- ARIA
acetylcholine receptor–inducing activity
- BL
basal lamina
- DHFR
dihydrofolate reductase
- ECM
extracellular matrix
- GAG
glycosaminoglycan
- HRG
heregulin
- HSPG
heparan sulfate proteoglycan
- MCK
muscle creatine kinase
- NDF
Neu differentiation factor
- NMJ
neuromuscular junction
- NRG
neuregulin
- TM
transmembrane
Footnotes
Address all correspondence to Dr. H.-R. Brenner, Department of Physiology, University of Basel, Vesalgasse 1, CH-4051 Basel, Switzerland. Tel.: (+41) 61 267 35 42. Fax: (+41) 61 267 35 59. E-mail: brenner@ubaclu.unibas.ch
References
- Altiok N, Bessereau JL, Changeux JP. ErbB3 and erbB2/neu mediate the effect of heregulin on acetylcholine receptor gene expression in muscle: differential expression at the endplate. EMBO (Eur Mol Biol Organ) J. 1995;14:4258–4266. doi: 10.1002/j.1460-2075.1995.tb00100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altiok N, Altiok S, Changeux J-P. Heregulin-stimulated acetylcholine receptor gene expression in muscle: requirement for MAP kinase and evidence for a parallel inhibitory pathway independent of electrical activity. EMBO (Eur Mol Biol Organ) J. 1997;16:717–725. doi: 10.1093/emboj/16.4.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apel ED, Merlie JP. Assembly of the postsynaptic apparatus. Curr Opin Neurobiol. 1995;5:62–67. doi: 10.1016/0959-4388(95)80088-3. [DOI] [PubMed] [Google Scholar]
- Brenner HR, Witzemann V, Sakmann B. Imprinting of acetylcholine receptor messenger RNA accumulation in mammalian neuromuscular synapses. Nature. 1990;344:544–547. doi: 10.1038/344544a0. [DOI] [PubMed] [Google Scholar]
- Brenner HR, Herczeg A, Slater CR. Synapse-specific expression of acetylcholine receptor genes and their products at original synaptic sites in rat soleus muscle fibres regenerating in the absence of innervation. Development (Camb) 1992;116:41–53. doi: 10.1242/dev.116.1.41. [DOI] [PubMed] [Google Scholar]
- Carbonetto S, Lindenbaum M. The basement membrane at the neuromuscular junction: a synaptic mediatrix. Curr Opin Neurobiol. 1995;5:596–605. doi: 10.1016/0959-4388(95)80064-6. [DOI] [PubMed] [Google Scholar]
- Carraway KL, Weber JL, Unger MJ, Ledesma J, Naichen Y, Gassmann M, Lai C. Neuregulin-2, a new ligand of ErbB3/ErbB4-receptor tyrosine kinases. Nature. 1997;387:512–516. doi: 10.1038/387512a0. [DOI] [PubMed] [Google Scholar]
- Chang H, Riese DJ, Gilbert W, Stern DF, McMahan UJ. Ligands for ErbB-family receptors encoded by a neuregulin-like gene. Nature. 1997;387:509–512. doi: 10.1038/387509a0. [DOI] [PubMed] [Google Scholar]
- Chen C, Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987;7:2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiquet M, Vrucinic-Fiipi N, Schenk S, Beck K, Chiquet-Ehrismann R. Isolation of chick tenascin variants and fragments. Eur J Biochem. 1991;199:379–388. doi: 10.1111/j.1432-1033.1991.tb16134.x. [DOI] [PubMed] [Google Scholar]
- Chu GC, Moscoso LM, Sliwkowski MX, Merlie JP. Regulation of the acetylcholine receptor ε-subunit gene by recombinant ARIA: an in vitro model for transynaptic gene regulation. Neuron. 1995;14:329–339. doi: 10.1016/0896-6273(95)90289-9. [DOI] [PubMed] [Google Scholar]
- Cohen I, Rimer M, Lomo T, McMahan UJ. Agrin-induced postsynaptic apparatus in skeletal muscle fibers in vivo. Mol Cell Neurosci. 1997;9:237–253. doi: 10.1006/mcne.1997.0623. [DOI] [PubMed] [Google Scholar]
- Corfas G, Falls DL, Fischbach GD. ARIA, a protein that stimulates acetylcholine receptor synthesis, also induces tyrosine phosphorylation of a 185-kDa muscle transmembrane protein. Proc Natl Acad Sci USA. 1993;90:1624–1628. doi: 10.1073/pnas.90.4.1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeChiara TM, Bowen DC, Valenzuela DM, Simmons MV, Poueymirou WT, Thomas S, Kinetz E, Compton DL, Rojas E, Park JS, et al. The receptor tyrosine kinase MuSK is required for neuromuscular junction formation in vivo. Cell. 1996;85:501–512. doi: 10.1016/s0092-8674(00)81251-9. [DOI] [PubMed] [Google Scholar]
- Denzer AJ, Gesemann M, Schumacher B, Ruegg MA. An amino-terminal extension is required for the secretion of chick agrin and its binding to extracellular matrix. J Cell Biol. 1995;131:1547–1560. doi: 10.1083/jcb.131.6.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denzer AJ, Gesemann M, Ruegg MA. Diverse functions of the extracellular matrix molecule agrin. Semin Neurosci. 1996;8:357–366. [Google Scholar]
- Denzer AJ, Brandenberger R, Gesemann M, Chiquet M, Ruegg MA. Agrin binds to the nerve-muscle basal lamina via laminin. J Cell Biol. 1997;137:671–683. doi: 10.1083/jcb.137.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denzer AJ, Schulthess T, Fauser C, Schumacher B, Kammerer RA, Engel J, Ruegg MA. Electron microscopic structure of agrin and mapping of its binding site in laminin-1. EMBO (Eur Mol Biol Organ) J. 1998;17:335–343. doi: 10.1093/emboj/17.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly JJ, Ulmer JB, Shiver JW, Liu MA. DNA vaccines. Annu Rev Immunol. 1997;15:617–648. doi: 10.1146/annurev.immunol.15.1.617. [DOI] [PubMed] [Google Scholar]
- Eldridge CF, Sanes JR, Chiu AY, Bunge RP, Cornbrooks CJ. Basal lamina-associated heparan sulfate proteoglycan in the rat PNS: characterization and localization using monoclonal antibodies. J Neurocytol. 1986;15:37–51. doi: 10.1007/BF02057903. [DOI] [PubMed] [Google Scholar]
- Fallon JR, Hall ZW. Building synapses: agrin and dystroglycan stick together. Trends Neurosci. 1994;17:469–473. doi: 10.1016/0166-2236(94)90135-x. [DOI] [PubMed] [Google Scholar]
- Falls DL, Rosen KM, Corfas G, Lane WS, Fischbach GD. ARIA, a protein that stimulates acetylcholine receptor synthesis, is a member of the Neu ligand family. Cell. 1993;72:801–815. doi: 10.1016/0092-8674(93)90407-h. [DOI] [PubMed] [Google Scholar]
- Ferns MJ, Hoch W, Campanelli JT, Rupp F, Hall ZW, Scheller RH. RNA splicing regulates agrin-mediated acetylcholine receptor clustering activity on cultured myotubes. Neuron. 1992;8:1079–1086. doi: 10.1016/0896-6273(92)90129-2. [DOI] [PubMed] [Google Scholar]
- Ferns MJ, Campanelli JT, Hoch W, Scheller RH, Hall ZW. The ability of agrin to cluster AChRs depends on alternative splicing and on cell surface proteoglycans. Neuron. 1993;11:491–502. doi: 10.1016/0896-6273(93)90153-i. [DOI] [PubMed] [Google Scholar]
- Fischbach GD, Rosen KM. ARIA: a neuromuscular junction neuregulin. Annu Rev Neurosci. 1997;20:429–458. doi: 10.1146/annurev.neuro.20.1.429. [DOI] [PubMed] [Google Scholar]
- Gautam M, Noakes PG, Mudd J, Nichol M, Chu GC, Sanes JR, Merlie JP. Failure of postsynaptic specialization to develop at neuromuscular junctions of rapsyn-deficient mice. Nature. 1995;377:232–236. doi: 10.1038/377232a0. [DOI] [PubMed] [Google Scholar]
- Gesemann M, Denzer AJ, Ruegg MA. Acetylcholine receptor- aggregating activity of agrin isoforms and mapping of the active site. J Cell Biol. 1995;128:625–636. doi: 10.1083/jcb.128.4.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gesemann M, Cavalli V, Denzer AJ, Brancaccio A, Schumacher B, Ruegg MA. Alternative splicing of agrin alters its binding to heparin, dystroglycan, and the putative agrin receptor. Neuron. 1996;16:755–767. doi: 10.1016/s0896-6273(00)80096-3. [DOI] [PubMed] [Google Scholar]
- Gesemann M, Brancaccio A, Schumacher B, Ruegg MA. Agrin is a high-affinity binding protein of dystroglycan in non-muscle tissue. J Biol Chem. 1997;273:600–605. doi: 10.1074/jbc.273.1.600. [DOI] [PubMed] [Google Scholar]
- Glass DJ, Bowen DC, Stitt TN, Radziejewski C, Bruno J, Ryan TE, Gies DR, Shah S, Mattsson K, Burden SJ, et al. Agrin acts via a MuSK receptor complex. Cell. 1996;85:1–20. doi: 10.1016/s0092-8674(00)81252-0. [DOI] [PubMed] [Google Scholar]
- Goodearl ADJ, Yee AG, Sandrock AW, Corfas G, Fishbach GD. ARIA is concentrated in the synaptic basal lamina of the developing chick neuromuscular junction. J Cell Biol. 1995;130:1423–1434. doi: 10.1083/jcb.130.6.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengerer B. A rapid procedure for mRNA extraction from a large number of samples. Biotechniques. 1993;14:522–524. [PubMed] [Google Scholar]
- Hoch W, Ferns M, Campanelli JT, Hall ZW, Scheller RH. Developmental regulation of highly spliced forms of agrin. Neuron. 1993;11:479–490. doi: 10.1016/0896-6273(93)90152-h. [DOI] [PubMed] [Google Scholar]
- Hunter WM, Greenwood FC. Preparation of iodine-131 labeled human growth hormone of high specific activity. Nature. 1962;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Jeschke M, Wels W, Dengler W, Imber R, Stocklin E, Groner B. Target inhibition of tumor-cell growth by recombinant heregulin-toxin fusion proteins. Int J Cancer. 1995;60:730–739. doi: 10.1002/ijc.2910600527. [DOI] [PubMed] [Google Scholar]
- Jo SA, Burden SJ. Synaptic basal lamina contains a signal for synapse-specific transcription. Development (Camb) 1992;115:673–680. doi: 10.1242/dev.115.3.673. [DOI] [PubMed] [Google Scholar]
- Jo SA, Zhu X, Marchionni MA, Burden SJ. Neuregulins are concentrated at nerve-muscle synapses and activate ACh-receptor gene expression. Nature. 1995;373:158–161. doi: 10.1038/373158a0. [DOI] [PubMed] [Google Scholar]
- Jones G, Herczeg A, Ruegg MA, Lichsteiner M, Kröger S, Brenner HR. Substrate-bound agrin induces expression of acetylcholine receptor ε-subunit in cultured mammalian muscle cells. Proc Natl Acad Sci USA. 1996;93:5985–5990. doi: 10.1073/pnas.93.12.5985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G, Meier T, Lichtsteiner M, Witzemann V, Sakmann B, Brenner HR. Induction by agrin of ectopic and functional postsynaptic-like membrane in innervated muscle. Proc Natl Acad Sci USA. 1997;94:2654–2659. doi: 10.1073/pnas.94.6.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman HK, McGarvey ML, Livatta LA, Robey PG, Tryggvason K, Martin GR. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from EHS sarcoma. Biochemistry. 1982;21:6188–6193. doi: 10.1021/bi00267a025. [DOI] [PubMed] [Google Scholar]
- Lemke G. Neuregulins in development. Mol Cell Neurosci. 1996;7:247–262. doi: 10.1006/mcne.1996.0019. [DOI] [PubMed] [Google Scholar]
- Lieth E, Fallon JR. Muscle agrin: neural regulation and localization at nerve-induced acetylcholine receptor clusters. J Neurosci. 1993;13:2509–2514. doi: 10.1523/JNEUROSCI.13-06-02509.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loeb JA, Fishbach GD. ARIA can be released from extracellular matrix through cleavage of a heparin-binding domain. J Cell Biol. 1995;130:127–135. doi: 10.1083/jcb.130.1.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinou JC, Falls DL, Fischbach GD, Merlie JP. Acetylcholine receptor-inducing activity stimulates expression of the ε-subunit gene of the muscle acetylcholine receptor. Proc Natl Acad Sci USA. 1991;88:7669–7673. doi: 10.1073/pnas.88.17.7669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahan UJ. The agrin hypothesis. Cold Spring Harbor Symp Quant Biol. 1990;55:407–418. doi: 10.1101/sqb.1990.055.01.041. [DOI] [PubMed] [Google Scholar]
- Meier T, Hauser DM, Chiquet M, Landmann L, Ruegg MA, Brenner HR. Neural agrin induces ectopic postsynaptic specializations in innervated muscle fibers. J Neurosci. 1997;17:6534–6544. doi: 10.1523/JNEUROSCI.17-17-06534.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscoso LM, Chu GC, Gautam M, Noakes PG, Merlie JP, Sanes JR. Synapse-associated expression of an acetylcholine receptor- inducing protein, ARIA/Heregulin, and its putative receptors, ErbB2 and ErbB3 in developing mammalian muscle. Dev Biol. 1995;172:158–169. doi: 10.1006/dbio.1995.0012. [DOI] [PubMed] [Google Scholar]
- Ng YP, Pun S, Yang JF, Ip NY, Tsim KWK. Chick muscle expresses various ARIA isoforms: regulation during development, denervation and regeneration. Mol Cell Neurosci. 1997;9:132–143. doi: 10.1006/mcne.1997.0613. [DOI] [PubMed] [Google Scholar]
- Pinset C, Mulle C, Benoit P, Changeux J-P, Chelly J, Gros F, Montarras D. Functional adult acetylcholine receptor develops independently of motor innervation in Sol8 mouse skeletal muscle cell line. EMBO (Eur Mol Biol Organ) J. 1991;10:2411–2518. doi: 10.1002/j.1460-2075.1991.tb07780.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reist NE, Magill C, McMahan UJ. Agrin-like molecules at synaptic sites in normal, denervated, and damaged skeletal muscles. J Cell Biol. 1987;105:2457–2469. doi: 10.1083/jcb.105.6.2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimer M, Mathiesen I, Lømo T, McMahan UJ. γ-AChR/ε-AChR switch at agrin-induced postsynaptic-like apparatus in skeletal muscle. Mol Cell Neurosci. 1997;9:254–263. doi: 10.1006/mcne.1997.0622. [DOI] [PubMed] [Google Scholar]
- Ruegg MA, Tsim KWK, Horton SE, Kröger S, Escher G, Gensch EM, McMahan UJ. The agrin gene codes for a family of basal lamina proteins that differ in function and distribution. Neuron. 1992;8:691–699. doi: 10.1016/0896-6273(92)90090-z. [DOI] [PubMed] [Google Scholar]
- Sandrock AW, Goodearl ADJ, Yin Q-W, Chang D, Fischbach GD. ARIA is concentrated in nerve terminals at neuromuscular junctions and at other synapses. J Neurosci. 1995;15:6124–6136. doi: 10.1523/JNEUROSCI.15-09-06124.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandrock AW, Dryer SE, Rosen KM, Gozani SN, Kramer R, Theill LE, Fischbach GD. Maintenance of acetylcholine receptor number by neuregulins at the neuromuscular junction. Science. 1997;276:599–603. doi: 10.1126/science.276.5312.599. [DOI] [PubMed] [Google Scholar]
- Sanes JR. Genetic analysis of postsynaptic differentiation at the vertebrate neuromuscular junction. Curr Opin Neurobiol. 1997;7:93–100. doi: 10.1016/s0959-4388(97)80126-2. [DOI] [PubMed] [Google Scholar]
- Sanes JR, Johnson YR, Kotzbauer PT, Mudd J, Hanley T, Martinou J-C, Merie JP. Selective expression of an acetylcholine receptor-lacZ transgene in synaptic nuclei of adult muscle fibers. Development (Camb) 1991;113:1181–1191. doi: 10.1242/dev.113.4.1181. [DOI] [PubMed] [Google Scholar]
- Shepherd D, Brehm P. Adult forms of nicotinic acetylcholine receptors are expressed in the absence of nerve during differentiation of a mouse skeletal muscle cell line. Dev Biol. 1994;162:549–557. doi: 10.1006/dbio.1994.1108. [DOI] [PubMed] [Google Scholar]
- Si J, Luo Z, Mei L. Induction of acetylcholine receptor gene expression by ARIA requires activation of mitogen-activated protein kinase. J Biol Chem. 1996;271:19752–19759. doi: 10.1074/jbc.271.33.19752. [DOI] [PubMed] [Google Scholar]
- Tansey MG, Chu GC, Merlie JP. ARIA/HRG regulates AChR ε-subunit gene expression at the neuromuscular synapse via activation of phosphatidylinositol 3-kinase and Ras/MAPK pathway. J Cell Biol. 1996;134:465–476. doi: 10.1083/jcb.134.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpl R. Proteoglycans of basement membranes. Experientia (Basel) 1993;49:417–428. doi: 10.1007/BF01923586. [DOI] [PubMed] [Google Scholar]
- Timpl R, Brown JC. Supramolecular assembly of basement membranes. Bioessays. 1995;18:123–132. doi: 10.1002/bies.950180208. [DOI] [PubMed] [Google Scholar]
- Timpl R, Rhode H, Robey PG, Rennard SI, Foidart JM, Martin GR. Laminin-a glycoprotein from basement membranes. J Biol Chem. 1979;254:9933–9937. [PubMed] [Google Scholar]
- Tsen G, Halfter W, Kröger S, Cole GJ. Agrin is a heparan sulfate proteoglycan. J Biol Chem. 1995;270:3392–3399. doi: 10.1074/jbc.270.7.3392. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela DM, Stitt TM, DiStefano PS, Rojas E, Mattsson K, Compton DL, Nunez L, Park JS, Stark JL, Gies DR, et al. Receptor tyrosine kinase specific for the skeletal muscle lineage: expression in embryonic muscle, at the neuromuscular junction, and after injury. Neuron. 1995;15:573–584. doi: 10.1016/0896-6273(95)90146-9. [DOI] [PubMed] [Google Scholar]
- Wallace BG. Agrin-induced specializations contain cytoplasmic, membrane, and extracellular matrix-associated components of the postsynaptic apparatus. J Neurosci. 1989;9:1294–1302. doi: 10.1523/JNEUROSCI.09-04-01294.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallasch C, Weiss FU, Niederfellner G, Jallal B, Issing W, Ullrich A. Heregulin-dependent regulation of HER2/neu oncogenic signaling by heterodimerization with HER3. EMBO (Eur Mol Biol Organ) J. 1995;14:4267–4275. doi: 10.1002/j.1460-2075.1995.tb00101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen D, Peles E, Cupples R, Suggs SV, Bacus SS, Lou Y, Trail G, Hu S, Silbiger SM, Ben R, Levy, et al. Neu differentiation factor: a transmembrane glycoprotein containing an EGF domain and an immunoglobulin homology unit. Cell. 1992;69:559–572. doi: 10.1016/0092-8674(92)90456-m. [DOI] [PubMed] [Google Scholar]
- Wen D, Suggs SV, Karunagaran D, Liu N, Cupples RL, Luo Y, Janssen AM, Ben-Baruch N, Trollinger DB, Jacobsen VL, et al. Structural and functional aspects of the multiplicity of Neu differentiation factors. Mol Cell Biol. 1994;14:1909–1919. doi: 10.1128/mcb.14.3.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada H, Denzer AJ, Hori H, Tanaka T, Anderson LVB, Fujita S, Fukuta-Ohi H, Shimizu T, Ruegg MA, Matsumura K. Dystroglycan is a dual receptor for agrin and laminin-2 in Schwann cell membrane. J Biol Chem. 1996;271:23418–23423. doi: 10.1074/jbc.271.38.23418. [DOI] [PubMed] [Google Scholar]