Abstract
Cadherin cell–cell adhesion molecules form membrane-spanning molecular complexes that couple homophilic binding by the cadherin ectodomain to the actin cytoskeleton. A fundamental issue in cadherin biology is how this complex converts the weak intrinsic binding activity of the ectodomain into strong adhesion. Recently we demonstrated that cellular cadherins cluster in a ligand-dependent fashion when cells attached to substrata coated with the adhesive ectodomain of Xenopus C-cadherin (CEC1-5). Moreover, forced clustering of the ectodomain alone significantly strengthened adhesiveness (Yap, A.S., W.M. Brieher, M. Pruschy, and B.M. Gumbiner. Curr. Biol. 7:308–315). In this study we sought to identify the determinants of the cadherin cytoplasmic tail responsible for clustering activity. A deletion mutant of C-cadherin (CT669) that retained the juxtamembrane 94–amino acid region of the cytoplasmic tail, but not the β-catenin–binding domain, clustered upon attachment to substrata coated with CEC1-5. Like wild-type C-cadherin, this clustering was ligand dependent. In contrast, mutant molecules lacking either the complete cytoplasmic tail or just the juxtamembrane region did not cluster. The juxtamembrane region was itself sufficient to induce clustering when fused to a heterologous membrane-anchored protein, albeit in a ligand-independent fashion. The CT669 cadherin mutant also displayed significant adhesive activity when tested in laminar flow detachment assays and aggregation assays. Purification of proteins binding to the juxtamembrane region revealed that the major associated protein is p120ctn. These findings identify the juxtamembrane region of the cadherin cytoplasmic tail as a functionally active region supporting cadherin clustering and adhesive strength and raise the possibility that p120ctn is involved in clustering and cell adhesion.
Cadherin cell adhesion molecules are fundamental determinants of tissue organization in developing and adult organisms (Takeichi, 1995; Gumbiner, 1996). Classical cadherins (including E-, N-, and Xenopus C-cadherin) are membrane-spanning glycoproteins that interact with cytoplasmic proteins (catenins) capable of associating with the actin cytoskeleton (Takeichi, 1991, 1995; Yap et al., 1997a ). There is compelling evidence that protein interactions mediated by both the cadherin ectodomain and cytoplasmic tail participate in adhesion. The cadherin ectodomain supports the Ca2+-dependent, homophilic binding that ultimately forms the basis for cadherin adhesiveness. Homophilic binding activity is retained in purified ectodomains of Xenopus C-cadherin and, using a sensitive laminar flow assay, adhesive activity was also detected in CHO cells expressing mutants of C-cadherin lacking the cytoplasmic tail (Brieher et al., 1996). However, tailless mutants supported adhesion that was considerably weaker than that mediated by wild-type C-cadherin (Brieher et al., 1996). Nor did tailless mutants of E-cadherin support adhesion of L cell fibroblasts measured in aggregation assays (Nagafuchi and Takeichi, 1988, 1989; Ozawa et al., 1990). Therefore, additional molecular mechanisms involving the cytoplasmic tail must strengthen the weak intrinsic binding activity of the cadherin ectodomain to support physiological cell adhesion.
Earlier studies identified catenin-binding interactions as one important basis for this adhesive activity. β-catenin binds with high affinity to the COOH-terminal region of the cadherin cytoplasmic tail and serves as an anchor for α-catenin which can, in turn, associate with actin filaments (Aberle et al., 1994; Hulsken et al., 1994; Funayama et al., 1995; Jou et al., 1995; Rimm et al., 1995) and potentially other junctional proteins, such as α-actinin (Knudsen et al., 1995). Cadherin mutants lacking the catenin-binding site were poorly adhesive in aggregation assays when expressed in L cell fibroblasts (Nagafuchi and Takeichi, 1988, 1989; Ozawa et al., 1990). Furthermore, mutant cadherins bearing the cytoplasmic tail or the catenin-binding region alone exerted potent dominant-negative effects upon adhesion when expressed in Xenopus embryos (Kintner, 1992), cultured cells (Fujimori and Takeichi, 1993), and in mouse intestinal epithelia (Hermiston and Gordon, 1995). This inhibitory effect was attributed, at least in part, to titration of β-catenin away from wild-type cadherins by the mutant molecules (Kintner, 1992). Genetic evidence implicating catenins in strong cell adhesion was also obtained in studies of cancer cells (Hirano et al., 1992; Watabe et al., 1994) and embryos (Kofron et al., 1997; Torres et al., 1997) lacking α-catenin and in studies of armadillo (the homologue of β-catenin) in Drosophila (Cox et al., 1996). Taken together, these findings have led to the hypothesis that a protein complex consisting of β- and α-catenin linked to the distal region of the cytoplasmic tail plays a fundamental role in cadherin function, perhaps through association with the actin cytoskeleton (Takeichi, 1991, 1995). The precise molecular mechanism by which this complex determines adhesive strength has yet to be fully elucidated.
Additionally, however, there is evidence that other regions of the cytoplasmic tail, particularly the juxtamembrane region proximal to the catenin-binding site, can influence cadherin function (Kintner, 1992; Riehl et al., 1996; Chen et al., 1997). Cadherin mutants bearing portions of the juxtamembrane region, but not the distal catenin-binding site, also inhibited adhesion when expressed in Xenopus embryos (Kintner, 1992) whereas a mutant of vascular endothelial (VE)1 cadherin possessing the membrane-proximal region but not the distal catenin-binding site supported aggregation of tissue culture cells (Navarro et al., 1995). The juxtamembrane region is also reported to influence the motility of cultured cells (Chen et al., 1997) and neurons (Riehl et al., 1996). Therefore, although there is clear and compelling evidence that catenin-binding by the distal cytoplasmic tail plays an important role in adhesion, other regions of the cytoplasmic tail may also have yet-to-be defined functional contributions.
One mechanism by which cadherin ectodomain and cytoplasmic tail may cooperate in adhesion is through lateral clustering of cadherin molecules. Recently we demonstrated that the distribution of the cadherin ectodomain presented at the cell surface significantly influences adhesive function (Yap et al., 1997b ). We used the FKBP-FK1012 protein oligomerization system (Spencer et al., 1993) to force clustering of a chimeric molecule in which the cytoplasmic tail of Xenopus C-cadherin was replaced by tandem repeats of the FK506-binding protein, FKBP12. Strong adhesion was generated in this system by clustering of the ectodomain alone independent of possible contributions from cytoskeletal interactions or signaling events mediated by the normal cadherin cytoplasmic tail. Therefore, lateral clustering is a potential mechanism to convert the homophilic binding activity of the ectodomain into a state capable of supporting physiological cell adhesion.
We also found that native C-cadherin molecules clustered in a ligand-dependent fashion when cells attached to substrata coated with the adhesive ectodomain of C-cadherin expressed as a recombinant protein (CEC1-5). Cadherin clustering correlated with a significant strengthening of adhesion to CEC-coated substrata (Yap et al., 1997b ). However, those studies suggested that some component of the cytoplasmic tail was necessary for clustering of native cadherins to occur, since the ectodomain-FKBP12 chimera failed to cluster in the absence of FK1012. This suggested that the cytoplasmic tail might contribute to adhesion by driving clustering of the ectodomain presented at the cell surface (Yap et al., 1997b ). Those studies did not identify the region of the cytoplasmic tail responsible for clustering nor was it known whether catenins were necessary for cadherin clustering. In this paper, we therefore sought to identify the cytoplasmic region responsible for cadherin clustering, test its role in adhesion and identify interacting proteins that might be involved.
Materials and Methods
Cadherin Mutants and Transfected Cell Lines
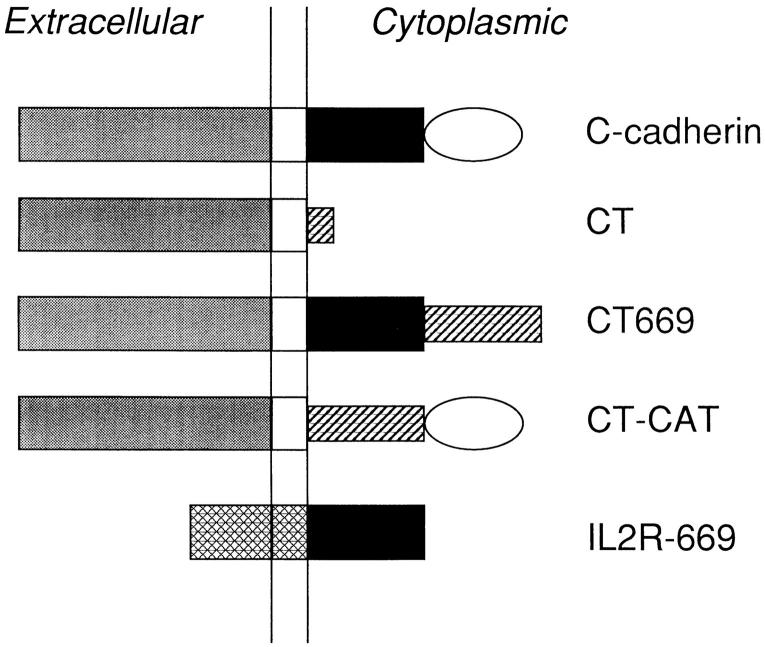
A summary of the C-cadherin mutants is shown in Fig. 1. Construction of the tailless truncation mutant (CT) that lacks the complete predicted cytoplasmic domain and bears a myc-tag at the COOH terminus has been described previously (Brieher et al., 1996). To generate a protein lacking the catenin-binding site (CT669), the cytoplasmic domain was truncated at amino acid position 669 that deletes the terminal 56 amino acids predicted to mediate binding of β-catenin (Nagafuchi and Takeichi, 1988, 1989; Stappert and Kemler, 1994). First, oligonucleotide linkers containing the cloning sites 5′-NdeI-BamHI-NotI-3′ were inserted into the mammalian expression vector pcDNA3, and a BamHI–NotI fragment containing a 6×-myc tag from pCS2 (Fagotto et al., 1996) was ligated into the BamHI– NotI sites. An EcoRV–NdeI fragment from pBS/7B3 containing the signal sequence, ectodomain, transmembrane region and cytoplasmic tail of C-cadherin to amino acid position 669 (Levine et al., 1994) was then inserted to yield the predicted truncation mutant containing a COOH-terminal 6×-myc tag.
Figure 1.
C-cadherin mutant molecules. A schematic depiction of the full-length C-cadherin molecule consists of the ectodomain (gray), transmembrane region, the 94–amino acid juxtamembrane region of the cytoplasmic tail (black), and catenin-binding site (distal 56 amino acids; open oval). CT consists of the ectodomain and transmembrane regions of C-cadherin alone, the complete cytoplasmic tail being replaced by a myc tag (hatched). The predicted catenin-binding site is deleted in CT669 that retains the 94–amino acid juxtamembrane region of the cytoplasmic tail (filled rectangle) followed by a 6×-myc tag (hatched). In CT-CAT the juxtamembrane region is deleted and replaced by a 5×-myc tag (hatched). IL2R669 consists of the 94–amino acid juxtamembrane region of the C-cadherin cytoplasmic tail fused to the ectodomain and transmembrane regions of the interleukin 2 receptor α-subunit (cross-hatched).
To construct a mutant molecule with an internal deletion of the juxtamembrane region of the cytoplasmic tail, replaced by a 5×-myc sequence to act as an epitope tag and spacer (CT-CAT), an NdeI–XbaI fragment from pBS/7B3 was isolated encoding the catenin-binding region of the C-cadherin cytoplasmic tail (from amino acid 670) and ligated into pBS/ ECFK (Yap et al., 1997b ), which contains an NdeI site at the predicted transmembrane-cytoplasmic junction, to yield a mutant lacking the membrane-proximal region of the cadherin cytoplasmic tail (pBS/CT-CAT). A 5×-myc tag was isolated from pCS2 by polymerase chain reaction using primers that inserted 5′- and 3′-NdeI sites. The PCR fragment was then ligated into pBS/CT-CAT and the complete construct transferred to pCDNA3 by directional cloning using EcoRV and XbaI.
To construct a chimeric protein containing the ectodomain and transmembrane region of the IL2 receptor α-subunit fused to the juxtamembrane region of the C-cadherin cytoplasmic tail (IL2R-669), DNA encoding the cytoplasmic tail from the predicted membrane-cytoplasmic junction to amino acid position 669 was isolated by the polymerase chain reaction using primers that inserted 5′-HindIII and 3′-BamHI sites for cloning purposes. The PCR product was then inserted into pCV-IL2L, which contains the ectodomain and transmembrane region of the interleukin 2 receptor α-subunit (LaFlamme et al., 1992).
All PCR products and ligation sites were sequenced using the Sequenase II kit (following the manufacturer's instructions). Plasmids were expressed in CHO cells using lipofectamine (following the manufacturer's instructions). For stable transfections, cell lines were picked using G418 selection (500–800 μg/ml). Two lines of C-CHO cells were used. Both cell lines spread on CEC1-5–coated substrata, but C-CHO21 cells express cadherin levels ∼4× greater than C-CHO12 cells and were more strongly adhesive in flow assays.
GST Fusion Protein Constructs
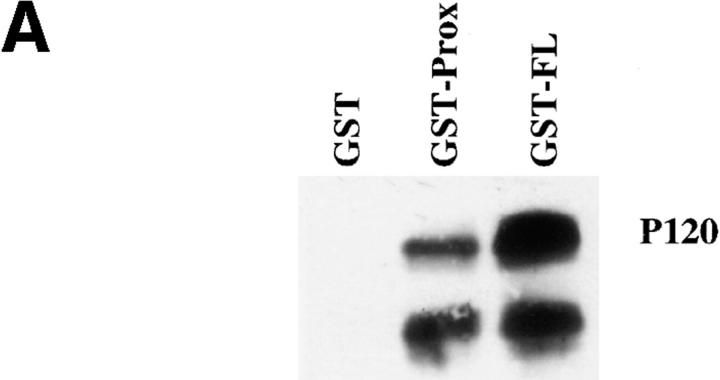
The polymerase chain reaction was performed to obtain fragments encoding either the full-length (nucleotides 2185–2643) or the juxtamembrane region (nucleotides 2185–2415) of the C-cadherin cytoplasmic tail. In both cases a sense primer was used with an EcoRI site at its 5′ site, which allowed subcloning of the fragments into the EcoRI–SmaI sites of pGEX4T-3 (Pharmacia Biotech Sverige, Uppsala, Sweden). All constructs were sequenced.
GST fusion proteins containing the full-length cytoplasmic tail (GST-FL) and the juxtamembrane region of the cytoplasmic tail (GST-Prox) were expressed in BL21 bacteria (Smith and Johnson, 1987), lysed in PBS/ 1% Triton using a French press and purified with glutathione-agarose beads (Sigma Chemical Co., St. Louis, MO). After elution with glutathione and extensive dialysis fusion proteins were bound to fresh glutathione beads to use in the isolation experiments.
Isolation of the 92-kD Protein and Microsequencing
Cells were metabolically labeled overnight using a mixture of [35S]methionine and [35S]cysteine in methionine- and cysteine-free MEM supplemented with 10% regular MEM. Cells were lysed in 1% NP-40 buffer (20 mM Tris, 4 mM EDTA, 150 mM NaCl, pH 7.6) and the soluble fraction was precleared for 3 h at 4°C with glutathione beads. Subsequently, the lysate was incubated with the fusion proteins immobilized on the glutathione beads (5 μg/sample). After extensive washing, the beads and associated proteins were analyzed by 6% SDS-PAGE under reducing conditions.
To obtain sufficient quantities of the 92-kD protein associated with GST-Prox, cells from 260 plates (15-cm diam) were lysed in 1% NP-40 buffer and processed as described above. A total of 100 μg of GST-Prox was used to precipitate proteins from the pooled lysate. After extensive washing the beads were loaded on a 5% SDS-PAGE gel, blotted to nitrocellulose from which the 92-kD protein was excised after staining with Ponceau S. This band was further processed for a combined analysis by mass spectrometry and internal amino acid sequencing essentially as described previously (Erdjument-Bromage et al., 1994). In brief, nitrocellulose-bound protein was trypsin digested in situ and this peptide mixture was subjected to matrix-assisted laserdesorption time-of-flight mass (MALDITOF) spectrometry (Reflex III; Bruker Franzen, Germany). The 32 major peptides were used to search the NRDB protein database (European Bioinformatics Institute, Hinxton, UK) using the Peptide Search algorithm (Mann et al., 1993). Two peptides with mixed mass spectra were subjected to NH2-terminal sequence analysis, using an applied biosynthesis 477A automated sequenator (Perkin-Elmer Corp., Norwalk, CT) as designed by Tempst et al. (1994).
Adhesion Assays
Recombinant C-cadherin ectodomain (CEC1-5) was purified from conditioned media as previously described (Brieher et al., 1996). For adhesion assays glass coverslips and capillaries were coated with CEC1-5 (10 μg/ml in 100 mM NaCl, 20 mM Hepes, and 1 mM CaCl2, pH 7.2) for 8 h and then blocked with 10 mg/ml BSA (overnight at 4°C). Laminar flow adhesion assays were performed as previously described with minor modifications (Brieher et al., 1996; Yap et al., 1997b ). All adhesion assays were performed in the presence of the RGD-containing peptide, GRGDTP (1 mg/ml; Sigma Chemical Co.) to inhibit possible background integrin-mediated adhesiveness (Yap et al., 1997b ). Cells expressing wild-type or mutant cadherins were isolated by incubation with 0.01% crystalline trypsin in Hanks balanced salt solution supplemented with 1 mM CaCl2 (HBSS/ Ca2+; at 37°C for 10 min), conditions that preserve cellular cadherins (Takeichi, 1977). Enzymatic digestion was stopped by addition of soybean trypsin inhibitor (1 mg/ml), cells collected by centrifugation and resuspended in HBSS/Ca2+. Aggregation assays were performed based on previously described methods (Nagafuchi and Takeichi, 1988, 1989). In brief, freshly isolated cells (1-ml aliquots; 2.5 × 105 cells/ml in HBSS/Ca2+) were pipetted into agarose-coated wells of 12-well plates and agitated on an orbital shaker. The numbers of single cells remaining after 45 min were expressed as percentages of the numbers of single cells in the cell suspension immediately before aggregation (Nt/No).
Immunofluorescence Microscopy
Specimens were prepared for immunofluorescent staining by fixation in paraformaldehyde (3% in PBS containing 1 mM CaCl2 and 1 mM MgCl2; 30 min) and permeabilized with Triton X-100 (0.25% in PBS, room T°, 5 min). Reactive aldehyde groups were blocked with glycine (100 mM, 30 min). Nonspecific binding was blocked with 5% nonfat dried milk (in PBS) and all antibody reactions and intermediate washes were performed in blocking buffer. Specimens were incubated with primary antibodies overnight at 4°C, washed, and then incubated with TR- or FITC-conjugated secondary antibodies (room temperature, 1 h). Specimens were examined with a Zeiss Axioskop equipped with ×63 and ×100 plan-APOCHROMAT objectives and photographed using Kodak Elite 400 film. Some samples were examined by confocal laser scanning microscopy using a Biorad MRC 600 confocal microscope mounted on a Zeiss Axioskop equipped with ×40 and ×100 plan-APOCHROMAT objectives and images acquired using the COMOS software supplied with the MRC 600. All images were compiled for publication using Adobe Photoshop.
Western Blotting and Immunoprecipitations
For Western blotting, cells were extracted in 1% NP-40 lysis buffer (1% NP-40, 10 mM Hepes, 150 mM NaCl, 1.5 mM EDTA, pH 7.4) supplemented with protease inhibitors as described previously (Brieher and Gumbiner, 1994). Samples containing equal quantities of total protein were separated by SDS-PAGE and transferred to nitrocellulose. For immunoprecipitation studies cells were lysed with 1% NP-40 extraction buffer, aliquots of NP-40 soluble supernatants containing equal quantities of protein were immunoprecipitated with a polyclonal antibody directed against the C-cadherin ectodomain (Yap et al., 1997b ) or the anti-p120ctn mAb and collected with protein A– or protein G–Sepharose beads. Beads were washed in 1% NP-40 lysis buffer, boiled in SDS-Laemmli buffer containing 50 mM DTT, and separated by SDS-PAGE for immunobloting.
Antibodies
Cadherins were detected using (a) a pAb directed against the conserved cytoplasmic tail of mouse E-cadherin (Marrs et al., 1993) that recognizes other cadherins, including Xenopus C-cadherin (Yap et al., 1997b ; unpublished data; a generous gift from Dr. J. Marrs, University of Indiana, Indianapolis); and (b) a polyclonal Ab (Yap et al., 1997b ) or mAb 6B6 (Brieher and Gumbiner, 1994) directed against the ectodomain of C-cadherin. β-catenin was detected using a mAb (Transduction Laboratories; Lexington, KY) or with a polyclonal Ab directed against the NH2 terminus (11). p120ctn mAb was purchased from Transduction Labs. mAB 9E10 (Evan et al., 1985) was used to detect myc-tagged proteins. The ectodomain of the human IL2 receptor α-subunit was detected with mAb 3G10 (Boehringer Mannheim Corp., Indianapolis, IN).
Results
Expression and Characterization of C-cadherin Mutants
To examine the contribution of the cadherin cytoplasmic tail to clustering, a series of C-cadherin mutants were designed (Fig. 1). In addition to CT, a mutant lacking the complete cytoplasmic tail, two further mutants were constructed to dissect potential contributions of regions within the tail. (a) A truncation mutant (CT669) lacking the terminal 56 amino acids predicted to mediate catenin binding (Nagafuchi and Takeichi, 1988, 1989; Ozawa et al., 1990; Stappert and Kemler, 1994) was designed in an attempt to detect any activity associated with the 94–amino acid juxtamembrane region of the cytoplasmic tail. (b) Conversely, to assess the potential specific adhesive contribution of the catenin-binding site, the terminal 56 amino acids were retained in the mutant CT-CAT, but the juxtamembrane region was replaced by a 5x-myc tag, acting as a spacer of approximately similar molecular mass to the deleted region of the cytoplasmic tail. Upon stable transfection in CHO cells CT669 and CT-CAT were expressed as polypeptides of molecular mass 125 and 116 kD, respectively (Fig. 2 A), consistent with the predicted contribution of the myc tags to molecular mass.
Figure 2.
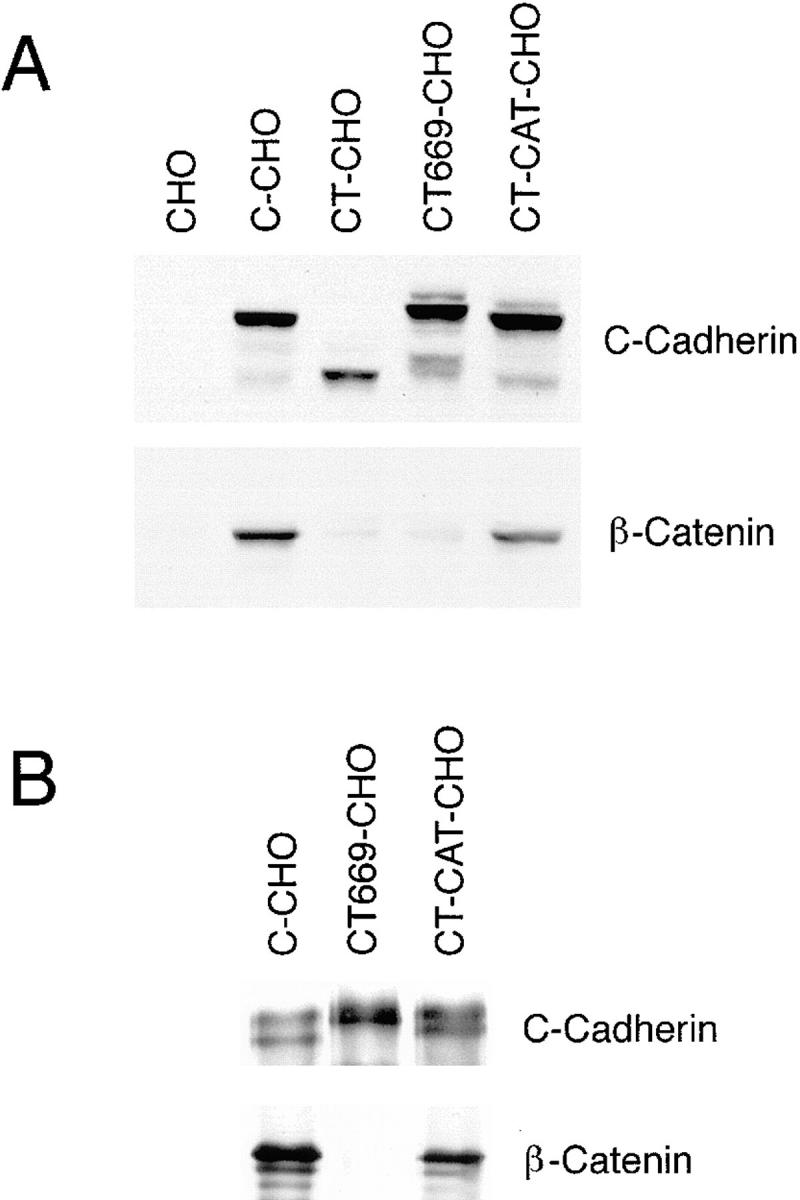
Expression of C-cadherin and mutant cadherin proteins in CHO cells. (A) Comparison between cadherin expression and cellular β-catenin levels. Western blots of lysates from parental CHO cells and CHO cells stably expressing wild-type C-cadherin (C-CHO, clone 12), tailless cadherin mutant (CT-CHO, clone 8), a deletion mutant lacking the catenin-binding region (CT669-CHO, clone A1) and a deletion mutant lacking the juxtamembrane region of the cytoplasmic tail (CT-CAT-CHO, clone 13) were probed for the C-cadherin ectodomain (top), then stripped and reprobed for β-catenin (bottom). Identical amounts of total cellular protein were loaded in each lane. Both the CT669 and CT-CAT mutants display polypeptide bands that run at or slightly higher than wild-type C-cadherin, consistent with the addition of multi-copy myc-epitope tags. Total cellular β-catenin levels were increased in cells expressing wild-type C-cadherin and CT-CAT, but not in cells expressing the CT or CT669 mutants. (B) β-catenin coimmunoprecipitates with wild-type C-cadherin and CT-CAT, but not with CT669. Lysates from C-CHO, CT669-CHO, and CT-CAT-CHO cells were immunoprecipitated with a pAb directed against the C-cadherin ectodomain, transferred to nitrocellulose and probed for C-cadherin (top) or β-catenin (bottom). C-cadherin immunoblots identify mature and precursor forms of the wild-type and mutant cadherins.
To confirm the predicted effects of these deletions on catenin-binding activity, we tested the ability of wild-type and mutant C-cadherin molecules to coimmunoprecipitate β-catenin. As shown in Fig. 2 B, a polyclonal antibody directed against the cadherin ectodomain immunoprecipitated wild-type C-cadherin, CT669, and CT-CAT, and lesser amounts of precursor proteins, from CHO cells stably transfected with these molecules. Immunoblots identified β-catenin in cadherin immunoprecipitates from C-CHO and CT-CAT-CHO cells, but not from CT669-CHO cells. These studies were performed under mild buffer conditions that do not affect the high affinity cadherin–β-catenin interaction (McCrea and Gumbiner, 1991). In addition, expression of cadherins generally increases total cellular β-catenin levels, presumably due to metabolic stabilization of β-catenin bound to cadherins at the cell membrane (Kowalczyk et al., 1994; Finnemann et al., 1997). This phenomenon was observed in C-CHO cells and CT-CAT-CHO cells, but CT669-CHO cells and CT-CHO cells showed total cellular β-catenin levels comparable to parental CHO cells (Fig. 2 A), providing indirect biochemical evidence that CT669 did not retain significant β-catenin–binding activity. These biochemical data are further supported by the observation (described below) that β-catenin failed to colocalize with CT669 in immunofluorescence studies (Fig. 3). Taken together, these findings indicated that β-catenin–binding activity was lost in the CT669 mutant, but retained in the CT-CAT mutant, as predicted from earlier mapping studies performed with other classical cadherin molecules.
Figure 3.
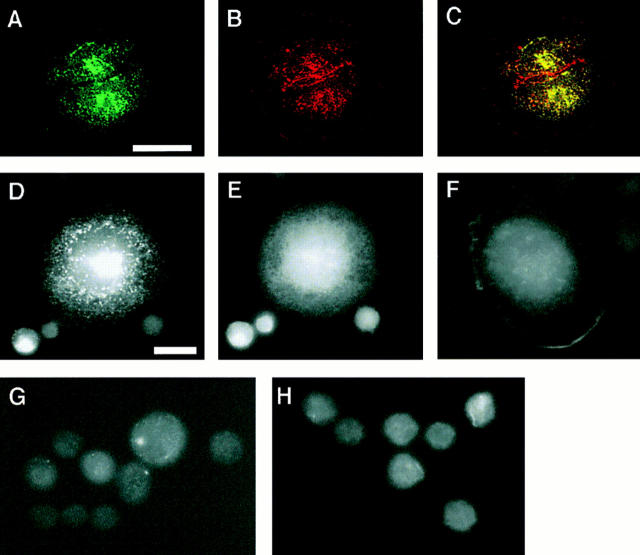
Clustering activity of wild-type and mutant C-cadherin molecules. CHO cells stably expressing C-cadherin or mutant molecules were allowed to attach for 1 h to glass substrata coated with CEC1-5 (A–E, G, and H) or poly-l-lysine (F) and then fixed and stained for the cadherin molecule (A, C, D, F, G, and H) or β-catenin (B, C, and E) by simultaneous dual-label immunofluorescence microscopy. Wild-type C-cadherin was detected using a polyclonal antibody raised against the whole cytoplasmic tail of mouse E-cadherin; mutants CT, CT669, and CT-CAT were detected by staining for the myc epitope tag. Specimens A–C were examined by confocal laser scanning microscopy with the plane of focus at the cell– substrate interface; all other samples were examined by epi-illumination microscopy. (A–C) Wild-type C-cadherin localized in clusters (A) that colocalized with β-catenin (B), as seen by the yellow fluorescence in the overlay image (C). Some β-catenin that did not colocalize with C-cadherin was detected at borders where the cells were close but not directly touching; this is likely to be in a cytoplasmic pool. (D and E) CT669 clustered in cells attached to substrata coated with CEC1-5 (D), but not with poly-l-lysine (F). CT669 clusters did not colocalize with β-catenin that remained diffusely distributed within cells (E). The image in E was overexposed for clarity in reproduction and therefore may give a misleading impression of β-catenin expression in CT669-CHO cells, which was similar to untransfected CHO cells (Fig. 2). Neither CT (G) nor CT-CAT (H) clustered upon attachment to CEC1-5–coated substrata. Note that CT-CHO and CT-CAT-CHO cells spread less on CEC1-5 than either C-CHO or CT669-CHO cells. Bars: (A–C) 25 μm; (D–H) 10 μm.
Role of Cytoplasmic Domains in Lateral Clustering
To assess clustering activity cells bearing wild-type or mutant cadherins were allowed to attach to glass substrata coated with CEC1-5, then fixed and examined for cadherin clustering by immunofluorescence microscopy. As we previously reported (Yap et al., 1997b ), wild-type C-cadherin localized in prominent clusters at the cell–substrate interface (Fig. 3 A). β-catenin colocalized with many of these C-cadherin clusters (Fig. 3 B; and yellow overlap staining in Fig. 3 C), as well as in a more diffuse pattern, some of which is likely to be in a cytoplasmic pool. In contrast, no clustering of the tailless CT mutant was detectable, even with prolonged periods of attachment (Fig. 3 G). This is consistent with our earlier observation that the cadherin-FKBP12 chimeric protein, which lacks the normal cytoplasmic tail, failed to cluster in the absence of FK1012 (Yap et al., 1997b ).
The CT669 cadherin mutant also localized in prominent clusters, as revealed by staining for the myc epitope tag (Fig. 3 D). However, in these cells the weak β-catenin staining remained diffuse and did not colocalize with CT669 in clusters (Fig. 3 E), a finding consistent with the lack of biochemical association between these proteins. As previously reported for wild-type cadherin (Yap et al., 1997b ), clustering of CT669 was ligand specific. Clustering of CT669 only occurred in cells attached to CEC1-5 (Fig. 3 D), but was not observed when cells were plated onto poly-l-lysine (Fig. 3 F) or plasma fibronectin (not shown). In contrast, no clustering of the CT-CAT mutant was detected at any time (Fig. 3 H). Therefore, the juxtamembrane region of the cytoplasmic tail appeared to support ligand-specific accumulation of C-cadherin in clusters.
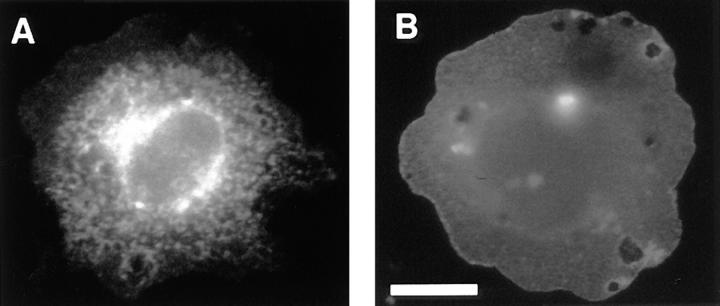
To determine whether the juxtamembrane region of the cytoplasmic tail was sufficient by itself to induce lateral clustering, we constructed a fusion protein consisting of the juxtamembrane 94 amino acids of the C-cadherin cytoplasmic tail fused to the ectodomain and transmembrane region of the IL2-receptor α-subunit (IL2R-669). This, and a control protein consisting of the IL2R elements alone, were transiently expressed in CHO cells. Surface localization of the expressed proteins was determined by immunofluorescent staining using a mAb directed against the IL2R ectodomain in fixed, nonpermeabilized cells attached to poly-l-lysine–coated substrata. Cells expressing the IL2R control protein showed only uniformly diffuse staining (Fig. 4 B). In contrast, cells expressing IL2R-669 showed clustered staining in prominent foci at the cell surface (Fig. 4 A). Clusters of IL2R-669 also stained with a pAb directed against the cadherin cytoplasmic tail but only after permeabilization of the cells (not shown), indicating that the protein was being correctly expressed at the cell surface. Therefore, the juxtamembrane region appeared capable of mediating lateral clustering even in the absence of both the ectodomain or catenin-binding region. Interestingly, IL2R-669 clustered independently of ligand, in contrast to the clustering of C-cadherin and CT669 that depended on cell attachment to CEC1-5.
Figure 4.
Surface localization of a chimeric molecule bearing the juxtamembrane region of the cadherin cytoplasmic tail alone. IL2R-669 and the parental IL2R molecule were transiently expressed in CHO cells and cells attached to poly-l-lysine–coated coverslips. Surface staining of the IL2R ectodomain in fixed, unpermeabilized cells was studied by immunofluorescence microscopy. IL2R-669, bearing the juxtamembrane region of the cadherin cytoplasmic tail stained in prominent clusters (A), whereas staining of the parent IL2R molecule was diffuse (B). Bar, 10 μm.
Functional Activity of Cadherin Cytoplasmic Mutants
Recently. we observed that the strength of adhesion between C-CHO cells and substrata coated with CEC1-5 depended on the duration that cells were initially allowed to attach to substrate under stasis (temporal strengthening) and that this correlated with cadherin clustering (Yap et al., 1997b ). To explore the mechanism underlying this correlation, we tested which of the C-cadherin mutants were capable of supporting temporal strengthening. As positive controls, we used C-CHO cell lines expressing wild-type C-cadherin at levels similar to those of the mutant cadherins.
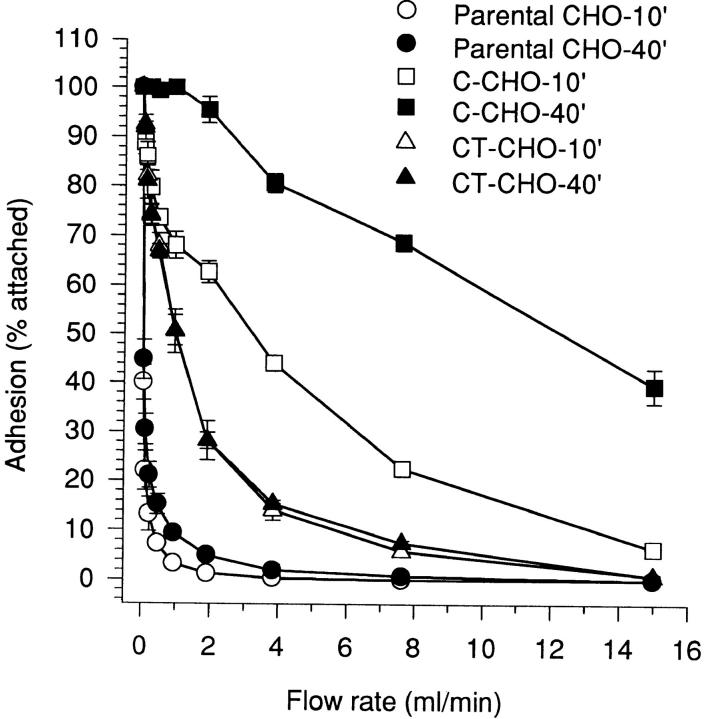
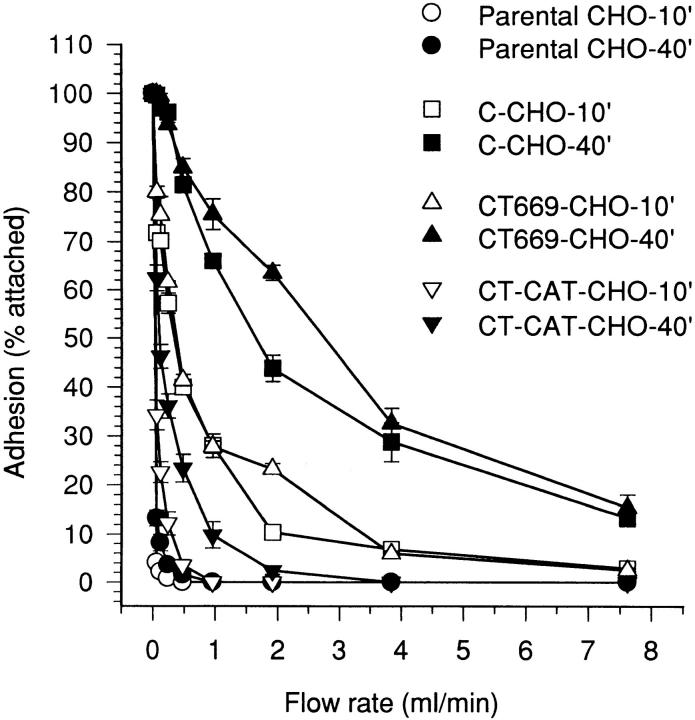
Temporal strengthening of wild-type C-cadherin is demonstrated in Fig. 5. Measured in a sensitive laminar flow adhesion assay, C-CHO cells resisted detachment from substrata coated with CEC1-5 to a significantly greater extent after 40 min static attachment than after an initial 10-min attachment period. As shown previously (Brieher et al., 1996), adhesion of CT-CHO cells (expressing the tailless C-cadherin mutant) was weak but consistently greater than parental CHO cells (Fig. 5). However, in contrast to the significant strengthening displayed by C-CHO cells, CT-CHO cells showed no evidence of temporal strengthening over the range of attachment times studied in these experiments. Thus, although the ectodomain alone can support a measurable level of adhesion when expressed in cells, the cytoplasmic tail is necessary for temporal strengthening to occur.
Figure 5.
Tail-less C-cadherin mutant displays adhesive binding activity but not temporal strengthening of adhesion. CHO cells stably expressing similar levels of C-cadherin (C-CHO clone 21) and tail-less C-cadherin, CT (CT-CHO clone 8) were allowed to attach to glass capillaries coated with CEC1-5 for 10 or 40 min, then adhesive strength measured by resistance to detachment by progressively increasing rates of buffer. Data are means ± SE (n = 3).
We then examined the adhesive activity of CT669-CHO and CT-CAT-CHO cells using the laminar-flow assay. As shown in Fig. 6, both CT669-CHO and CT-CAT-CHO cells displayed greater adhesive activity than parental CHO cells. Strikingly, the adhesive activity of CT669-CHO cells was very similar to that displayed by control C-CHO cells. Like C-CHO cells, CT669-CHO cells demonstrated considerable temporal strengthening of adhesion to levels comparable to C-CHO cells. Similar patterns of adhesive behavior were shown by other independent clones of CT669-CHO cells, including lines that expressed lower levels of mutant cadherin protein (not shown). In contrast, adhesion of CT-CAT-CHO cells was weaker than that displayed by C-CHO and CT669-CHO cells (Fig. 6). Comparison of adhesive activity after 10 and 40 min of initial static attachment showed some temporal strengthening in CT-CAT-CHO cells, but to levels of adhesive strength significantly less than either C-CHO or CT669-CHO cells. Therefore, these findings show that the juxtamembrane region of the cytoplasmic tail was capable of strengthening adhesion and supporting strong resistance to detachment.
Figure 6.
Adhesive activity of C-cadherin and mutants assessed by laminar flow assay. Parental CHO cells, C-CHO cells (clone 12), CT669-CHO cells (clone A1), and CT-CAT-CHO cells (clone 13), which were all matched for similar expression levels, were allowed to attach to glass capillaries for 10 or 40 min before adhesive strength was assessed by resistance to detachment by progressively increasing buffer flow rates. Data are means ± SE (n = 3).
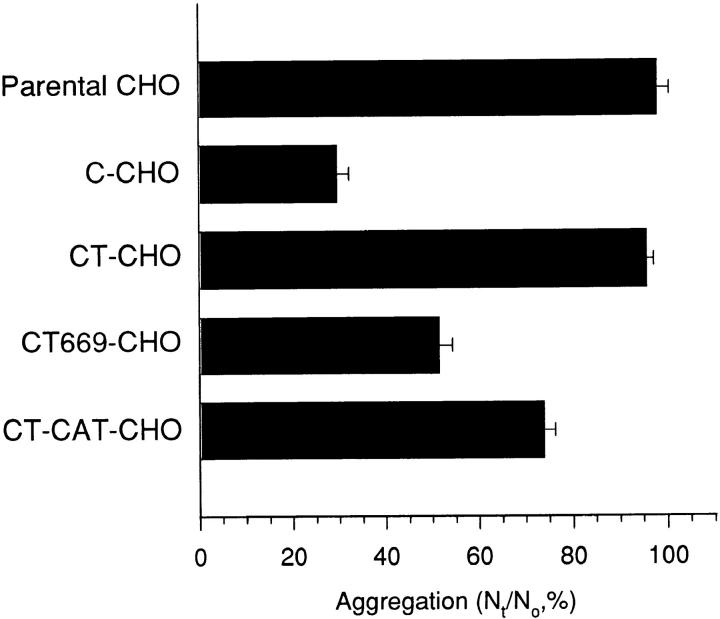
The degree of adhesive activity demonstrated by CT669 was surprising in light of previous studies that demonstrated that mutant E-cadherin molecules lacking the catenin-binding region retained little, if any, adhesive activity when expressed in L cell fibroblasts (Nagafuchi and Takeichi, 1988, 1989; Ozawa et al., 1990). Those studies were performed in aggregation assays that may measure somewhat different parameters of adhesion than the flow assay used in our studies (Kuo et al., 1997). Therefore, we assessed the adhesive activity of the C-cadherin mutants in aggregation assays (Fig. 7). Untransfected parental CHO cells did not aggregate under the conditions used in this study, in contrast to C-CHO cells that showed significant aggregation that was Ca2+-dependent (not shown). CT669-CHO cells also exhibited aggregation activity that was consistently greater than CT-CAT-CHO cells but less pronounced than C-CHO cells. Aggregation of CT669-CHO and CT-CAT-CHO cells was Ca2+-dependent (not shown). Therefore, in our hands significant adhesive activity of CT669 is detectable in aggregation assays as well in the laminar flow detachment assay.
Figure 7.
Adhesive activity of C-cadherin and mutant cadherin molecules assessed by aggregation in suspension. Parental CHO cells, C-CHO cells (clone 12), CT-CHO cells (clone 11), CT669-CHO cells (clone A1) and CT-CAT-CHO cells (clone 13), which were all matched for similar expression levels, were isolated by trypsinization in the presence of Ca2+, then allowed to aggregate in suspension. After 45 min the number of individual cells remaining in suspension (Nt) was counted and expressed as a percentage of the number of cells counted in the freshly isolated suspension (N0). Increased aggregation is reflected in a fall in the Nt/ N0 ratio. Data are means ± SE (n = 3).
Identification of Protein Interactions Associated with the Juxtamembrane Region of the Cadherin Cytoplasmic Tail
The clustering activity associated with the CT669 and IL2R-669 mutants could be mediated either by direct lateral interactions between the juxtamembrane regions of adjacent cadherin molecules or by intermediary proteins that bind to the juxtamembrane region. First, we used bacterially expressed GST- and MBP-fusion proteins bearing the full-length and juxtamembrane cytoplasmic tail regions to test for direct interactions between cytoplasmic domains. Blot overlay assays as well as in vitro reconstitution assays were performed to test if MBP-FL could bind directly to GST-FL and/or GST-Prox. However, we were unable to detect an association despite using a range of different incubation conditions (data not shown). This result is consistent with biochemical studies that have shown that the E-cadherin cytoplasmic domain/β-catenin complex behaves in isolation as a heterodimer (Weis, W., personal communication).
Therefore, we attempted to identify proteins that associate with the membrane proximal region. GST, GST-FL, or GST-Prox bound to glutathione beads were used to precipitate proteins from metabolically labeled HCT116 cells, a human colon carcinoma cell line. Human cells were chosen because the extensive human protein database allows for more reliable identification of any associated proteins after microsequencing.
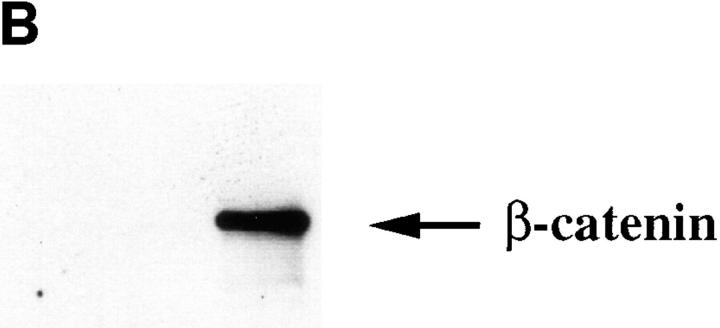
Both GST-FL and GST-Prox, but not GST, precipitated a protein from HCT116 cell lysates that migrated ∼92 kD. Moreover, this protein was the major labeled band associated with GST-Prox (Fig. 8 A). Although the molecular mass of this band was similar to that of β-catenin, GST-prox lacks the predicted β-catenin–binding sequence and, as shown in Fig. 2, β-catenin did not coimmunoprecipitate with CT669 from CHO cells. To confirm that the 92-kD band associated with GST-Prox was not β-catenin, samples from the in vitro binding assay were immunoblotted with a mAb directed against β-catenin. As expected β-catenin was bound by the GST-FL but the similar sized band associated with GST-prox was not recognized by the β-catenin antibody (Fig. 8 B).
Figure 8.
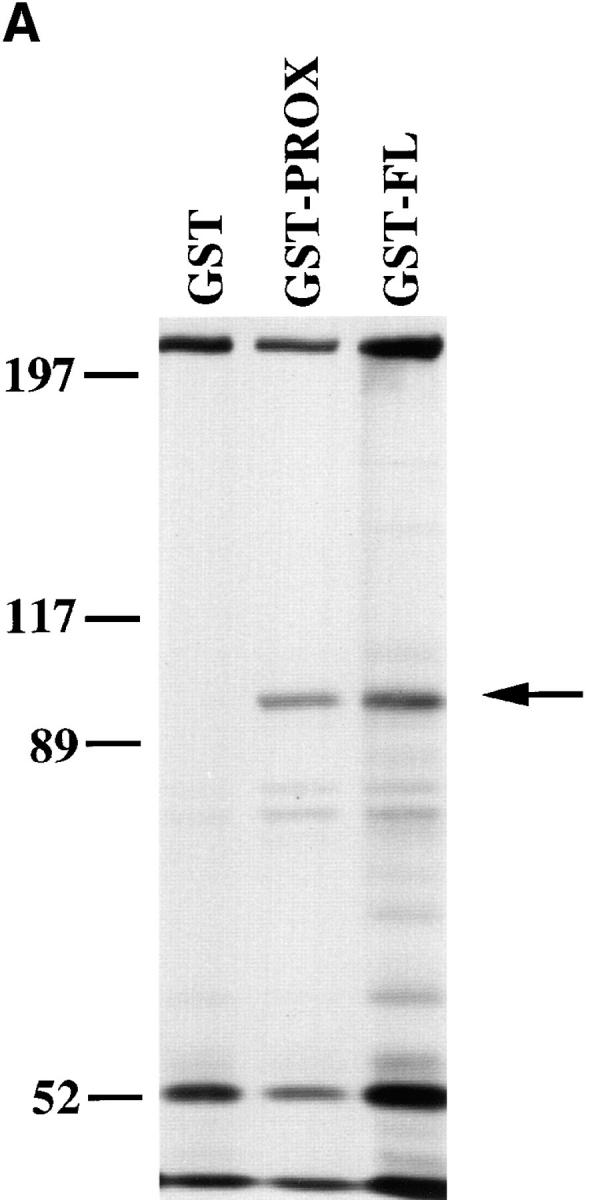
Identification of polypeptides that bind to GST-fusion proteins containing the cadherin cytoplasmic tail. (A) Binding of metabolically labeled proteins to GST fusion proteins. Lysates of 35S-labeled HCT116 cells were incubated with either GST, GST-Prox, or GST-FL. Bound proteins were separated by SDS-PAGE. A 92-kD protein binds to both GST-Prox and GST-FL but not to GST alone. (B) β-catenin bind to GST-FL but not to GST or GST-Prox. A Western blot of proteins bound to the GST fusion proteins was probed with a mAb to β-catenin.
The procedure using GST-Prox was scaled up to purify large amounts of the 92-kD polypeptide (Fig. 9 A), which were transferred to nitrocellulose. After trypsin digestion the peptide mixture was subjected to mass spectrometry analysis resulting in the recovery of 32 major peptide masses, which were used to search the NRDB protein database for possible matches. One protein in the database, human p120ctn, matched to 27 of the 32 peptide masses (Fig. 9 B). p120ctn is a src substrate that has recently been demonstrated to bind to the cadherin complex (Reynolds et al., 1994; Shibamoto et al., 1995; Staddon et al., 1995). Some of the peptides showed mixed mass spectra after separation by HPLC, suggesting the presence of other peptide sequences. Two of these peptides were therefore subjected to NH2-terminal protein sequence analysis to determine the possible presence of another protein. However, both sequences matched perfectly with p120ctn.
Figure 9.


The 92-kD protein bound by GST-Prox is identical to p120ctn. (A) Detection of the 92-kD protein isolated from HCT116 in a large scale experiment. Coomassie brilliant blue staining of a large scale experiment isolating the 92-kD protein from HCT116 lysates using GST-Prox. Arrow marks the 92-kD protein. (B) Peptides identified in the protein sequence of p120ctn. Mass spectrometry analysis identified 27 major fragments that are underlined in the sequence. In bold are the sequences that were found in more than one peptide fragment. The two fragments that were subjected to NH2-terminal sequence analysis are shown in italics.
To confirm that p120ctn binds to GST-FL and GST-Prox, western blot analysis of the proteins in HCT116 lysates that associated with the different GST-fusion proteins was performed. Indeed, both GST-FL and GST-Prox, but not GST alone, bind the 92-kD isoform of p120ctn, the major isoform expressed in HCT116 cells (Mo and Reynolds, 1996), using a mAb to p120ctn that recognizes all major isoforms (results not shown).
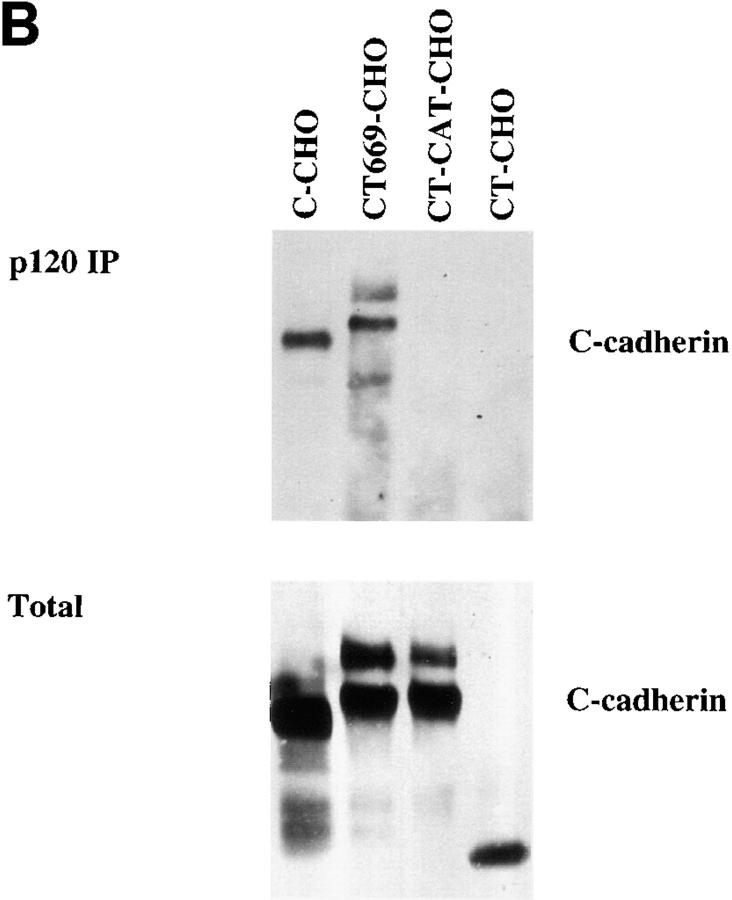
We then sought to confirm that this association also occurred in CHO cells. First, GST fusion proteins bound to glutathione beads were used to isolate proteins from lysates of untransfected CHO cells. As shown in Fig. 10 A both the high molecular mass and lower molecular mass isoforms of p120ctn bound to GST-FL and GST-Prox, but not to GST alone. We then wanted to determine whether p120ctn indeed associated with CT669. Therefore, p120ctn was immunoprecipitated from CHO cells transfected with full-length or mutant C-cadherin. Samples were analyzed by immunoblotting using a pAb that recognized the extracellular domain of C-cadherin. Both full-length C-cadherin and CT669 were found to be associated with p120ctn whereas neither CT-CAT nor CT were detected in the p120ctn immunoprecipitates (Fig. 10 B). Similar amounts of C-cadherin protein were expressed in the different cell lysates (Fig. 10 B), excluding the possibility that the lack of detectable association with CT-CAT was due to lower expression levels in the cells.
Figure 10.
p120ctn associates with the proximal domain of C-cadherin in CHO cells. (A) p120ctn isoforms bind to both GST-Prox and GST-FL. Lysates of CHO cells were incubated with GST, GST-Prox, or GST-FL, transferred to nitrocellulose and probed with a mAb recognizing the major isoforms of p120ctn. (B) Wild-type Cadherin and CT669 but not CT-CAT or CT coimmunoprecipate with p120ctn. Lysates from C-CHO, CT669-CHO, C-CAT-CHO, and CT-CHO cells were immunoprecipitated with a mAb to p120ctn, transferred to nitrocellulose and probed for C-cadherin. In the bottom panel total lysates of these cells were also transferred to nitrocellulose and probed for C-cadherin to compare relative expression levels. C-cadherin immunoblots identify mature and precursor forms of the wild-type and mutant cadherins. Both the CT669 and CT-CAT mutants display polypeptide bands that run at or slightly higher than wild-type C-cadherin, consistent with the addition of muti-copy myc-epitope tags.
Discussion
This study identifies the 94–amino acid juxtamembrane region of the cytoplasmic tail as a functionally important part of a classical cadherin molecule. This region is sufficient to support lateral clustering and exerts a significant influence on adhesive activity. These experiments derive from our previous demonstration that wild-type C-cadherin expressed in CHO cells clusters in a ligand-specific manner upon attachment to CEC1-5–coated substrata (Yap et al., 1997b ). A role for the cytoplasmic tail in cadherin clustering was inferred from the observation that a chimeric molecule in which the cadherin cytoplasmic tail was replaced by tandem repeats of the FK506-binding protein, FKBP12, failed to cluster (Yap et al., 1997b ). Two observations in the current study now identify the juxtamembrane region of the cytoplasmic tail as sufficient to mediate clustering. First, CT669, a mutant that retains the juxtamembrane region of the cytoplasmic tail, clustered in a ligand-specific manner when cells attached to substrata coated with CEC1-5. In contrast, cadherin mutants lacking the complete cytoplasmic tail (CT) or the juxtamembrane region alone (CT-CAT) failed to cluster. Second, the IL2R-669 chimera, but not the parent IL2Rα protein, accumulated in surface clusters when expressed in cells. Therefore, this juxtamembrane region was capable of inducing clustering even without the cadherin ectodomain and transmembrane regions. Taken together these findings indicate that the juxtamembrane region of the cadherin cytoplasmic tail is sufficent for clustering activity.
Our analysis of the cadherin mutants also provides compelling evidence that this juxtamembrane region of the cytoplasmic tail contributes to adhesive function. Thus, the CT669 mutant supported significant levels of adhesion measured in two separate assay systems: a laminar flow detachment assay and cell aggregation assays. Compared with the tailless mutation (CT) that supports measurable adhesion (Brieher et al., 1996), CT669 demonstrated substantially greater levels of adhesive activity in both assays and also showed temporal strengthening in the laminar flow assay. Cells expressing CT669, like C-CHO cells, also spread more extensively on CEC-coated substrata than either CT-CHO- or CT-CAT-CHO cells. How then might significant adhesive strength be supported without the important contribution of the β-catenin–binding region? Recently, we demonstrated that forced clustering of the cadherin ectodomain alone can substantially strengthen adhesion independently of cytoskeletal or signaling events mediated by the cytoplasmic tail (Yap et al., 1997b ). Therefore, we propose that the juxtamembrane region contributes to adhesion by supporting the accumulation of cadherin molecules in clusters after initial ligand binding. In this scheme, changes in adhesive strength would occur through the redistribution of adhesive receptors on the cell surface, a process driven, in turn, by the clustering activity of the juxtamembrane cytoplasmic tail.
A role for the juxtamembrane region in cell adhesion had previously been suggested by reports that a truncation mutant of VE-cadherin retaining this region could support aggregation (Navarro et al., 1995) and by the potent dominant inhibitory activity of this region alone when expressed in early Xenopus embryos (Kintner, 1992). Our current findings, indeed, provide a potential explanation for this inhibitory effect in Xenopus embryos. Overexpression of the juxtamembrane region alone could inhibit adhesion by competing with corresponding regions of wild-type cadherins for protein–protein interactions that mediate clustering.
Many studies have established a clear role for catenins and the β-catenin–binding domain in cadherin-based adhesion (Nagafuchi and Takeichi, 1988, 1989; Ozawa et al., 1990; Hirano et al., 1992; Kintner, 1992; Hermiston and Gordon, 1995; Lee and Gumbiner, 1995; Cox et al., 1996; Kofron et al., 1997; Torres et al., 1997). Earlier studies, based on cell aggregation assays, did not, however, identify a discrete functional role for the juxtamembrane region (Nagafuchi and Takeichi, 1988, 1989; Ozawa et al., 1990). Several factors may account for this disparity between the current and earlier reports. First, our laminar flow detachment assay may measure different biophysical parameters of adhesion than aggregation assays. In particular, the kinetics (on- and off-rates) of the adhesive interaction may influence aggregation assays more than the flow detachment assay (Kuo et al., 1997). Nonetheless, the observation that CT669 mediated aggregation to a significant extent indicates that assay characteristics cannot solely account for the discrepancy with earlier findings. Second, Xenopus C-cadherin may differ from the mammalian E-cadherins studied in earlier reports. There is, indeed, accumulating evidence that cadherins differ in their functional properties (Levine et al., 1994; Marrs et al., 1995; Islam et al., 1996) that may be due both to differences in homophilic binding characteristics (Levine et al., 1994) and also to apparently small divergent regions of the cytoplasmic tails (Marrs et al., 1995). Third, these cadherins were studied in different cellular expression systems. CHO cells seem to support higher levels of cadherin protein expression than mouse L cell fibroblasts (unpublished results) that can influence adhesive activity (Angres et al., 1996; Yap et al., 1997b ). Interestingly, in another study that used CHO cells an adhesive contribution of the juxtamembrane region of VE-cadherin was also reported (Navarro et al., 1995). In all probability a combination of these factors are likely to have facilitated detection of the functional activity of the juxtamembrane region in the current experiments.
Since the CT669 and IL2R-669 mutants do not bind β-catenin, this further suggests that β- and α-catenin are not required for cadherin clustering. In this regard, we also found that a construct lacking the juxtamembrane region but retaining the β-catenin–binding site of the cytoplasmic tail (CT-CAT) failed to cluster, implying that the juxtamembrane region of the cytoplasmic tail might be solely responsible for cadherin clustering. However, our CT-CAT mutant, containing a 5x-myc tag spacer, was relatively weakly active in functional assays, compared with earlier findings that the β-catenin–binding region was sufficient to support significant cell aggregation activity (Ozawa et al., 1990). Consequently, we cannot be certain that our CT-CAT mutant retained all the functional activity of the catenin-binding region. Nonetheless, CT-CAT possessed strong catenin-binding activity, demonstrating that β-catenin–binding alone is not sufficient to mediate cadherin clustering. Therefore, although we cannot exclude an additional role of the β-catenin–binding region, our data provide strong evidence that the juxtamembrane region of the cytoplasmic tail mediates distinct interactions that are experimentally sufficient to induce cadherin clustering.
The juxtamembrane region of the cadherin cytoplasmic tail could support clustering either through direct lateral interactions or through the participation of intermediary binding proteins. To date, we have been unable to identify any biochemical evidence for direct lateral interactions between the juxtamembrane regions of cadherin molecules using purified proteins that have been studied under a variety of binding conditions. Although this does not definitively exclude a role for direct lateral interactions, it seems more likely that other, intermediary binding proteins are necessary for clustering. Our current experiments suggest that p120ctn is a possible candidate to perform this role. It was isolated as the major protein that bound to the juxtamembrane region of the cadherin cytoplasmic tail, using a biochemical approach designed to identify as many associating proteins as possible. Further experiments will be needed to address whether p120ctn is required for cadherin clustering. p120ctn has been shown previously to interact with the cadherin complex independently of the β-catenin–binding site (Reynolds et al., 1994; Shibamoto et al., 1995; Staddon et al., 1995; Lampugnani et al., 1997). However, the role of p120ctn in cadherin function has not been established. Although this protein is a substrate for several kinases and the amount associated with the cadherin complex may vary in different cell types, it is unclear whether this variation is regulated nor what role it plays in adhesion (Daniel and Reynolds, 1997).
Interestingly, the clustering activity of the juxtamembrane region also appears to be regulated by the extracellular domain. Whereas clustering of the IL2R-669 chimera occurred in a ligand-independent manner, clustering of wild-type C-cadherin and of the CT669 cadherin mutant was strictly ligand-dependent. In one scenario, the unligated ectodomain might inhibit the clustering activity of the cytoplasmic tail, which is then disinhibited upon ligand binding to the ectodomain. A similar sequence of events, where ligand-binding releases an inhibitory influence of the ectodomain, has been implicated in the ligand-dependent targeting of β1-integrins into focal adhesions (LaFlamme et al., 1992) and activation of signaling by the FGF receptor (Webster and Donoghue, 1997).
These and other recent findings (Brieher et al., 1996; Yap et al., 1997b ) point to a model of cadherin function in which the homophilic binding activity of the ectodomain is strengthened by the clustering activity of the cytoplasmic tail (Yap et al., 1997a ). This clustering activity may be essential to convert the apparently weak intrinsic binding activity of the ectodomain into a state capable of supporting physiological adhesion. Furthermore, alterations in the degree of clustering (the distribution of adhesive receptors presented at the cell surface) supported by the cadherin cytoplasmic tail provide a potential mechanism by which surface adhesiveness can be regulated in response to cellular signals.
Finally, our experiments raise questions about the mechanistic contributions to adhesion served by the juxtamembrane and catenin-binding regions of the cytoplasmic tail. It is possible that both regions participate in the same or similar molecular mechanisms for cell adhesion. Alternatively, it is interesting to consider the possibility that these regions might mediate mechanistically distinct contributions to adhesion. For example, the juxtamembrane region may be principally responsible for clustering, whereas the catenin-binding region may be the major link to the actin cytoskeleton. Actin association might stabilize clusters or be more important for supporting morphogenetic responses to adhesion, such as compaction and epithelialization (Watabe et al., 1994). In addition, the catenin complex is likely to participate in signal transduction mechanisms that regulate cadherin-based adhesion in response to growth factors and other physiological cues (reviewed in Yap et al., 1997a ). With the identification of the juxtamembrane region as a distinct contributor to cadherin function, it should be possible to dissect more thoroughly the specific mechanistic contributions of cadherins and catenins and determine how they are integrated to form fully functional adhesive complexes at the cell surface.
Acknowledgments
We thank Dr. James Marrs for a generous gift of antibodies and Dr. Susan LaFlamme for the IL2α receptor plasmid. This work would not have been possible without the support, imagination, and intellectual energy of our colleagues, Bill Brieher, Francois Fagotto, Cara Gottardi, Kathleen Guger, and all the members of the Gumbiner lab who showed us the way out of the harbor.
We thank the Sloan-Kettering Structural Chemistry Laboratory (supported by grant 5P309CA05746 from the NCI), especially Drs. Hediye Erdjument-Bromage and Paul Tempst, for carrying out the combined analysis of mass spectrometry and amino acid sequencing. Confocal microscopy was performed at the Confocal Microscopy Facility of the University of Queensland, established by grants from the Australian Research Council, and maintained by Mr. Colin Macqueen. This work was supported by National Institutes of Health grant GM52717 awarded to B.M. Gumbiner, the Cancer Center Support grant NCI-P30-CA-08748, and in part by funds from the Dana Fund. A.S. Yap was also supported by the National Health and Medical Research Council of Australia. C.M. Niessen was supported by The Dutch Cancer Society.
Abbreviations used in this paper
- CT
cytoplasmic tail
- VE
vascular endothelial
Footnotes
A.S. Yap and C.M. Niessen contributed equally to the work described in this paper.
References
- Aberle H, Butz S, Stappert J, Weissig H, Kemler R, Hoschuetzky H. Assembly of the cadherin-catenin complex in vitro with recombinant proteins. J Cell Sci. 1994;107:3655–3663. doi: 10.1242/jcs.107.12.3655. [DOI] [PubMed] [Google Scholar]
- Angres B, Barth A, Nelson WJ. Mechanism for transition from initial to stable cell-cell adhesion: kinetic analysis of E-cadherin–mediated adhesion using a quantitative adhesion assay. J Cell Biol. 1996;134:549–557. doi: 10.1083/jcb.134.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brieher WM, Gumbiner BM. Regulation of cadherin function during activin induced morphogenesis of Xenopusanimal caps. J Cell Biol. 1994;126:519–527. doi: 10.1083/jcb.126.2.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brieher WM, Yap AS, Gumbiner BM. Lateral dimerization is required for the homophilic binding activity of C-cadherin. J Cell Biol. 1996;135:487–489. doi: 10.1083/jcb.135.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Paradies NE, Fedor-Chaiken M, Brackenbury R. E-cadherin mediates adhesion and suppresses cell motility via distinct mechanisms. J Cell Sci. 1997;110:345–356. doi: 10.1242/jcs.110.3.345. [DOI] [PubMed] [Google Scholar]
- Cox RT, Kirkpatrick C, Peifer M. Armadillo is required for adherens junction assembly, cell polarity, and morphogenesis during Drosophilaembryogenesis. J Cell Biol. 1996;134:133–148. doi: 10.1083/jcb.134.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM, Reynolds AB. Tyrosine phosphorylation and cadherin/ catenin function. Bioessays. 1997;19:883–891. doi: 10.1002/bies.950191008. [DOI] [PubMed] [Google Scholar]
- Erdjument-Bromage H, Lui MS, Sabatini DM, Snyder SH, Tempst P. High sensitivity sequencing of large proteins: partial structure of the rapamycin-FKBP12 target. Protein Sci. 1994;3:2435–2446. doi: 10.1002/pro.5560031227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evan GI, Lewis GK, Ramsey G, Bishop JM. Isolation of monoclonal antibodies specific for the human c-myc proto-oncogene product. Mol Cell Biol. 1985;5:3610–3616. doi: 10.1128/mcb.5.12.3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagotto F, Funayama N, Gluck U, Gumbiner BM. Binding to cadherins antagonizes the signaling activity of β-catenin during axis formation in Xenopus. . J Cell Biol. 1996;132:1105–1114. doi: 10.1083/jcb.132.6.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnemann S, Mitrik I, Hess M, Otto G, Wedlich D. Uncoupling of XB/U-cadherin-catenin complex formation from its function in cell-cell adhesion. J Biol Chem. 1997;272:11856–11862. doi: 10.1074/jbc.272.18.11856. [DOI] [PubMed] [Google Scholar]
- Fujimori T, Takeichi M. Disruption of epithelial cell-cell adhesion by exogenous expression of a mutated nonfunctional N-cadherin. Mol BiolCell. 1993;4:37–47. doi: 10.1091/mbc.4.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funayama N, Fagotto F, McCrea P, Gumbiner BM. Embryonic axis induction by the armadillo repeat domain of β-catenin: evidence for intracellular signaling. J Cell Biol. 1995;128:959–968. doi: 10.1083/jcb.128.5.959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner BM. Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- Hermiston ML, Gordon JI. In vivo analysis of cadherin function in the mouse intestinal epithelium: essential roles in adhesion, maintenance of differentiation, and regulation of programmed cell death. J Cell Biol. 1995;129:489–506. doi: 10.1083/jcb.129.2.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano S, Kimoto N, Shimoyama Y, Hirohashi S, Takeichi M. Identification of a neural α-catenin as a key regulator of cadherin function and multicellular organization. Cell. 1992;70:293–301. doi: 10.1016/0092-8674(92)90103-j. [DOI] [PubMed] [Google Scholar]
- Hulsken J, Birchmeier W, Behrens J. E-cadherin and APC compete for the interaction with β-catenin and the cytoskeleton. J Cell Biol. 1994;127:1375–1380. doi: 10.1083/jcb.127.6.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam S, Carey TE, Wolf GT, Wheelock MJ, Johnson KR. Expression of N-cadherin by human squamous carcinoma cells induces a scattered fibroblastic phenotype with disrupted cell-cell adhesion. J Cell Biol. 1996;135:1643–1654. doi: 10.1083/jcb.135.6.1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou T-S, Stewart DB, Stappert J, Nelson WJ, Marrs JA. Genetic and biochemical dissection of protein linkages in the cadherin-catenin complex. Proc Natl Acad Sci USA. 1995;92:5067–5071. doi: 10.1073/pnas.92.11.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kintner C. Regulation of embryonic cell adhesion by the cadherin cytoplasmic domain. Cell. 1992;69:225–236. doi: 10.1016/0092-8674(92)90404-z. [DOI] [PubMed] [Google Scholar]
- Knudsen KA, Soler AP, Johnson KR, Wheelock MJ. Interaction of α-actinin with the cadherin/catenin cell-cell adhesion complex via α-catenin. J Cell Biol. 1995;130:67–77. doi: 10.1083/jcb.130.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kofron M, Spagnuolo A, Klymkowsky M, Wylie C, Heasman J. The roles of maternal α-catenin and plakoglobin in the early Xenopusembryo. Development. 1997;124:1553–1560. doi: 10.1242/dev.124.8.1553. [DOI] [PubMed] [Google Scholar]
- Kowalczyk AP, Palka HL, Luu HH, Nilles LA, Anderson JE, Wheelock MJ, Green KJ. Posttranslational regulation of plakoglobin expression. Influence of the desmosomal cadherins on plakoglobin metabolic stability. J Biol Chem. 1994;269:31214–31223. [PubMed] [Google Scholar]
- Kuo SC, Hammer DA, Lauffenburger DA. Simulation of detachment of specifically bound particles from surfaces by shear flow. Biophys J. 1997;73:517–531. doi: 10.1016/S0006-3495(97)78090-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaFlamme SE, Akiyama SK, Yamada KM. Regulation of fibronectin receptor distribution. J Cell Biol. 1992;117:437–447. doi: 10.1083/jcb.117.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampugnani MG, Corada M, Andriopoulou P, Esser S, Risau W, Dejana E. Cell confluence regulates tyrosine phosphorylation of adherens junction components in endothelial cells. J Cell Sci. 1997;110:2065–2077. doi: 10.1242/jcs.110.17.2065. [DOI] [PubMed] [Google Scholar]
- Lee C-H, Gumbiner BM. Disruption of gastrulation movements in Xenopusby a dominant-negative mutant for C-cadherin. Dev Biol. 1995;171:363–373. doi: 10.1006/dbio.1995.1288. [DOI] [PubMed] [Google Scholar]
- Levine E, Lee CH, Kintner C, Gumbiner BM. Selective disruption of E-cadherin function in early Xenopusembryos by a dominant negative mutant. Development. 1994;120:901–909. doi: 10.1242/dev.120.4.901. [DOI] [PubMed] [Google Scholar]
- Mann M, Hojrup P, Roepstorff P. Use of mass-spectrometric molecular weight information to identify proteins in sequence databases. Biol Mass Spectrom. 1993;22:338–345s. doi: 10.1002/bms.1200220605. [DOI] [PubMed] [Google Scholar]
- Marrs JA, Napolitano EW, Murphy-Erdosh C, Mays RW, Reichardt LF, Nelson WJ. Distinguishing roles of the membrane-cytoskeleton and cadherin mediated cell-cell adhesion in generating different Na+,K+-ATPase distributions in polarized epithelia. J Cell Biol. 1993;123:149–164. doi: 10.1083/jcb.123.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs JA, Andersson-Fisone C, Jeong MC, Cohen-Gould L, Zurzolo C, Nabi IR, Rodriguez-Boulan E, Nelson WJ. Plasticity in epithelial cell phenotype: modulation by expression of different cadherin cell adhesion molecules. J Cell Biol. 1995;129:507–519. doi: 10.1083/jcb.129.2.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrea P, Gumbiner B. Purification of a 92-kD cytoplasmic protein tightly associated with the cell-cell adhesion molecule E-cadherin (uvomorulin): characterization and extractability of the protein complex from the cell cytostructure. J Biol Chem. 1991;266:4514–4520. [PubMed] [Google Scholar]
- Mo YY, Reynolds AB. Identification of p120cas isoforms and heterogenous expression of p120casisoforms in human tumor cell lines. Cancer Res. 1996;56:2633–2640. [PubMed] [Google Scholar]
- Nagafuchi A, Takeichi M. Cell binding function of E-cadherin is regulated by the cytoplasmic domain. EMBO (Eur Mol Biol Organ) J. 1988;7:3679–3684. doi: 10.1002/j.1460-2075.1988.tb03249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagafuchi A, Takeichi M. Transmembrane control of cadherin- mediated cell adhesion: a 94 kD protein functionally associated with a specific region of the cytoplasmic domain of E-cadherin. Cell Regul. 1989;1:37–44. doi: 10.1091/mbc.1.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro P, Caveda L, Breviario F, Mandoteanu I, Lampugnani M-G, Dejana E. Catenin-dependent and -independent functions of vascular endothelial cadherin. J Biol Chem. 1995;270:30965–30972. doi: 10.1074/jbc.270.52.30965. [DOI] [PubMed] [Google Scholar]
- Ozawa M, Ringwald M, Kemler R. Uvomorulin-catenin complex formation is regulated by a specific domain in the cytoplasmic region of the cell adhesion molecule. Proc Natl Acad Sci USA. 1990;87:4246–4250. doi: 10.1073/pnas.87.11.4246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds AB, Daniel J, McCrea PD, Wheelock MJ, Wu J, Zhang Z. Identification of a new catenin: the tyrosine kinase substrate p120casassociates with E-cadherin complexes. Mol Cell Biol. 1994;14:8333–8342. doi: 10.1128/mcb.14.12.8333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riehl R, Johnson K, Bradley R, Grunwald GB, Cornel E, Liliebaum A, Holt CE. Cadherin function is required for axon outgrowth in retinal ganglion cells in vivo. Neuron. 1996;17:837–848. doi: 10.1016/s0896-6273(00)80216-0. [DOI] [PubMed] [Google Scholar]
- Rimm DL, Koslov ER, Kebriaei P, Cianci CD, Morrow JS. α1(E)-Catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc Natl Acad Sci USA. 1995;92:8813–8817. doi: 10.1073/pnas.92.19.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibamoto S, Hayakawa K, Hori T, Miyazawa K, Kitamura N, Johnson K, Wheelock MJ, Matsuyoshi N, Takeichi M, Ito F. Association of p120, a tyrosine kinase substrate, with E-cadherin/catenin complexes. J Cell Biol. 1995;128:949–957. doi: 10.1083/jcb.128.5.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DB, Johnson KS. Single step purification of polypeptides expressed in Escherichia colias fusions with glutathione S-transferase. Gene. 1987;67:31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Spencer DM, Wandless TJ, Schreiber SL, Crabtree GR. Controlling signal transduction with synthetic ligands. Science. 1993;262:1019–1024. doi: 10.1126/science.7694365. [DOI] [PubMed] [Google Scholar]
- Staddon JM, Smales C, Schulze C, Esch FS, Rubin LL. p120, a p120-related protein (p100) and the cadherin/catenin complex. J Cell Biol. 1995;130:369–381. doi: 10.1083/jcb.130.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stappert J, Kemler R. A short core region of E-cadherin is essential for catenin binding and is highly phosphorylated. Cell Adhes Commun. 1994;2:319–327. doi: 10.3109/15419069409014207. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Functional correlation between cell adhesive properties and some cell surface proteins. J Cell Biol. 1977;75:464–474. doi: 10.1083/jcb.75.2.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. Cadherin cell adhesion receptors as a morphogenetic regulator. Science. 1991;251:1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- Takeichi M. Morphogenetic roles of classic cadherins. Curr Opin Cell Biol. 1995;7:619–627. doi: 10.1016/0955-0674(95)80102-2. [DOI] [PubMed] [Google Scholar]
- Tempst P, Geromanos S, Elicione C, Erdjument-Bromage H. Improvements in microsequencer performance for low picomole sequence analysis. Methods Enzymol. 1994;6:248–261. [Google Scholar]
- Torres M, Stoykova A, Huber O, Chowdhury K, Bonaldo P, Mansouri A, Butz S, Kemler R, Gruss P. An α-E-cateningene trap mutation defines its function in preimplantation development. Proc Natl Acad Sci USA. 1997;94:901–906. doi: 10.1073/pnas.94.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe M, Nagafuchi A, Tsukita S, Takeichi M. Induction of polarized cell-cell association and retardation of growth by activation of the E-cadherin-catenin adhesion system in a dispersed carcinoma line. J Cell Biol. 1994;127:247–256. doi: 10.1083/jcb.127.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster MK, Donoghue DJ. Enhanced signaling and morphological transformation by a membrane-localized derivative of the fibroblast growth factor receptor 3 kinase domain. Mol Cell Biol. 1997;17:5739–5747. doi: 10.1128/mcb.17.10.5739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap AS, Brieher WM, Gumbiner BM. Molecular and functional analysis of cadherin-based adherens junctions. Annu Rev Cell Dev Biol. 1997a;13:119–146. doi: 10.1146/annurev.cellbio.13.1.119. [DOI] [PubMed] [Google Scholar]
- Yap AS, Brieher WM, Pruschy M, Gumbiner BM. Lateral clustering of the adhesive ectodomain: a fundamental determinant of cadherin function. Curr Biol. 1997b;7:308–315. doi: 10.1016/s0960-9822(06)00154-0. [DOI] [PubMed] [Google Scholar]