Abstract
In epithelial cells, α-, β-, and γ-catenin are involved in linking the peripheral microfilament belt to the transmembrane protein E-cadherin. α-Catenin exhibits sequence homologies over three regions to vinculin, another adherens junction protein. While vinculin is found in cell–matrix and cell–cell contacts, α-catenin is restricted to the latter. To elucidate, whether vinculin is part of the cell–cell junctional complex, we investigated complex formation and intracellular targeting of vinculin and α-catenin. We show that α-catenin colocalizes at cell–cell contacts with endogenous vinculin and also with the transfected vinculin head domain forming immunoprecipitable complexes. In vitro, the vinculin NH2-terminal head binds to α-catenin, as seen by immunoprecipitation, dot overlay, cosedimentation, and surface plasmon resonance measurements. The K d of the complex was determined to 2–4 × 10−7 M. As seen by overlays and affinity mass spectrometry, the COOH-terminal region of α-catenin is involved in this interaction.
Complex formation of vinculin and α-catenin was challenged in transfected cells. In PtK2 cells, intact α-catenin and α-catenin1-670, harboring the β-catenin– binding site, were directed to cell–cell contacts. In contrast, α-catenin697–906 fragments were recruited to cell–cell contacts, focal adhesions, and stress fibers. Our results imply that in vivo α-catenin, like vinculin, is tightly regulated in its ligand binding activity.
Formation of epithelia depends critically on the physical interaction between cells. To this end, both partners develop a series of highly specific, morphologically well defined structures, i.e., tight junctions, cell–cell adherens junctions, and desmosomes (Koch and Franke, 1994). Adherens junctions are specified by transmembrane linker proteins, the cadherins, which mediate the calcium-dependent, homophilic cell–cell adhesion in a wide variety of tissues and species. Truncating the COOH-terminal cytoplasmic domain of E-cadherin is deleterious for epithelial cell–cell adhesion, which emphasizes the importance of linking these transmembrane proteins to the peripheral microfilament belt at the cytoplasmic face of contact sites (Nagafuchi et al., 1994). This linkage is mediated by a complex of three cytosolic proteins, named α-, β-, and γ-catenin (Ozawa et al., 1989). The cadherin– catenin complex, as characterized by immunoprecipitation, contains cadherin/α-catenin and either β- or γ-catenin (Hinck et al., 1994; Näthke et al., 1994). Recent data suggest that α-catenin cannot directly bind to cadherin. Instead, this linkage is mediated through either β- or γ-catenin (Aberle et al., 1994, 1996). More proximally, the link to membrane-apposed actin filaments probably involves direct α-catenin–actin interactions (Rimm et al., 1995), and experimental evidence indicates that this interaction is also indispensable for cell–cell adhesion (Hirano et al., 1992; Torres et al., 1997). Alternatively or in addition, an α-catenin/α-actinin/F-actin complex may be formed, as proposed by Knudsen et al. (1995).
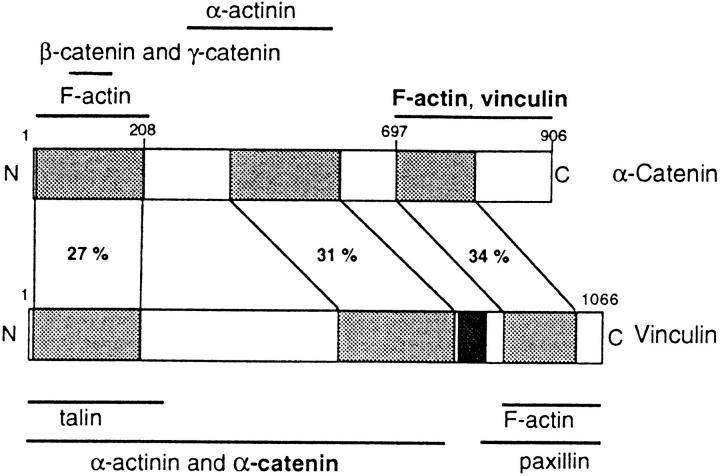
Sequence comparison demonstrated that α-catenin, a protein of 906 amino acids, shares homologies over three extended regions with vinculin (Herrenknecht et al., 1991; Nagafuchi et al., 1991), a structural component of cell–cell as well as of cell–matrix adherens junctions (for references see Burridge et al., 1988; Jockusch et al., 1995; Jockusch and Rüdiger, 1996). This is emphasized in Fig. 1. Vinculin is a multiligand protein of 1,066 amino acids, known to bind to actin filaments (Menkel et al., 1994; Johnson and Craig, 1995) and to microfilament-associated proteins like talin (Otto, 1983; Burridge and Mangeat, 1984) and α-actinin (Belkin and Koteliansky, 1987; Wachsstock et al., 1987). Like vinculin (Isenberg et al., 1982; Johnson and Craig, 1995), α-catenin binds to and bundles actin filaments, but while in vinculin this activity is confined to the COOH-terminal domain (Menkel et al., 1994; Johnson and Craig, 1995; Hüttelmaier et al., 1997), α-catenin contains two actin-binding sites well separated in its sequence (Rimm et al., 1995). Again like vinculin, α-catenin interacts directly with α-actinin (Knudsen et al., 1995; Nieset et al., 1997). Since the rod-like COOH-terminal domain of vinculin can also interact with itself, forming homo-oligomeric aggregates (Otto, 1983; Molony and Burridge, 1985), and since this region shares a high degree of sequence homology with the corresponding region in α-catenin, it was proposed that α-catenin–vinculin heterologous complexes may be formed, involving the COOH-terminal region of both molecules (Herrenknecht et al., 1991; Nagafuchi et al., 1991; Kemler, 1993).
Figure 1.
Comparison of homology domains and ligand binding sites in vinculin and α-catenin. Homology regions are shaded, with the degree of sequence identity given in percent (Herrenknecht et al., 1991). The proline-rich region in vinculin, which has no correspondence in α-catenin, is shown in black. Ligand-binding sites are indicated (for references see Introduction and Discussion), and those sites identified in this study are presented in bold letters. Note that the highest degree of identity is found within the COOH-terminal regions of both molecules.
In this study we tested this hypothesis. By a variety of biochemical methods we demonstrate that vinculin indeed binds to α-catenin, but involves the vinculin head and the α-catenin “tail” domain. Based on colocalization and coimmunoprecipitation studies, we present data showing that both are part of the junctional complex in epithelial cells, thus both contributing to the architecture of cell–cell contacts. Furthermore, transfection studies with α-catenin and deletion fragments thereof are in accordance with the formation of an α-catenin–vinculin complex. Our results suggest that the ligand-binding activities of both α-catenin and vinculin are tightly regulated in vivo.
Materials and Methods
Vectors and Plasmids
A full-length mouse α-catenin cDNA with a COOH-terminal histidine tag cloned in pQE60 (Qiagen, Hilden, Germany) was a kind gift of Dr. Kemler (Max Planck Institue, Freiburg, Germany). The α-catenin COOH-terminal sequence was amplified from this template by PCR. The PCR fragment was cloned into pQE60, to obtain pQE-α-cat697-906 with a COOH-terminal histidine-tag.
For transfection experiments, α-catenin, α-cat697-906, and α-cat1-670 coding sequences were amplified from the prokaryotic expression vector (see above) by PCR. PCR products were ligated into pcDNA-BiP to obtain the eukaryotic expression vectors pcα-catenin-BiP, pcα-cat697-906-BiP, and pcα-cat1-670-BiP. pcDNA-BiP (Rüdiger et al., 1997) is a modification of the pcDNA3 vector (Invitrogene, Leek, Netherlands) carrying a cDNA coding for 10 amino acids of the birch profilin sequence to be used as a COOH-terminal epitope-tag. This tag is specifically recognized by the monoclonal antibody 4A6 (Rothkegel et al., 1996; Wiedemann et al., 1996; Rüdiger et al., 1997). All constructs were sequenced by the dideoxy chain termination technique. For the expression of the vinculin head domain (residues 1–851 of the vinculin sequence; Swissprot P12003) the coding sequence was amplified by PCR from a chicken vinculin cDNA (kind gift of Dr. B. Geiger, Weizmann Institute of Science, Rehovot, Israel) and cloned into pcDNA3 with a COOH-terminal FLAG tag to obtain pcDNA- VH-FLAG.
Expression and Purification of α-Catenin and the Deletion Mutant α-Cat697-906
Escherichia coli M15 (QIAGEN) was transformed with pQE-α-catenin and pQE-α-cat697-906. Expression and purification of the proteins was performed according to Aberle et al. (1994) with a minor modification. The bacteria lysis buffer contained 50 mM Na-phosphate, pH 8.0, 500 mM NaCl, 10 mM 2-mercaptoethanol, 0.5% Triton X-100, 10 μg/ml Pefabloc (Merck, Darmstadt, Germany), 10 μM pepstatin A, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 0.5 g/100 ml CheliteP (Serva, Heidelberg, Germany) as a Ca2+-chelator. CheliteP was removed by centrifugation before applying the sample to the Ni-NTA–Sepharose column (QIAGEN). After affinity purification on Ni-NTA–Sepharose, α-catenin was further purified by anion exchange chromatography on MonoQ HR 5/5 (Pharmacia Biotech Sverige, Uppsala, Sweden), using a 40-ml linear gradient of 0–0.5 M NaCl in 0.1 M Tris-HCl, pH 8.0, supplemented with Pefabloc, pepstatin A, aprotinin, and leupeptin as above. A contaminating, slightly smaller polypeptide present in some preparations was identified by NH2-terminal sequence analysis as a proteolytic fragment, comprising residues 57–906.
Purification of Additional Proteins and Proteolytic Cleavage
Purification of chicken gizzard vinculin and α-actinin was performed according to Feramisco and Burridge (1980). Thermolysin cleavage of α-actinin and purification of the 27- and 53-kD fragments were carried out according to Pavalko and Burridge (1991). Vinculin was digested with V8 protease from Staphylococcus aureus (ICN Biomedicals, Eschwege, Germany), and the 90- and the 29/27-kD fragments were purified as previously described (Kroemker et al., 1994).
Purification of chicken gizzard talin was performed according to standard procedures (O'Halloran et al., 1985).
Ligand Interaction Studies
F-actin binding of proteins was assessed by airfuge sedimentation, according to Menkel et al. (1994). Blot overlays were performed as previously described (Kroemker et al., 1994). To determine dissociation constants (K d) surface plasmon resonance studies were done on a BIACORE 2000 machine (Biacore, Uppsala, Sweden). Roughly 1,000 resonance units (RU)1 of purified α-catenin were coupled to a CM5 sensor chip (corresponding to 1 ng/mm2) according to the manufacturers' protocol. Vinculin and the vinculin head domain were then tested for binding and dissociation kinetics were monitored at the concentrations indicated, using flow rates of 10 μl/min. The k on and k off values were calculated using the BiaEvaluation software. Best fitting of data was obtained by assuming a homogeneous single-site interaction model (A + B = AB).
Affinity Mass Spectrometry and Carboxy-terminal Sequencing
This assay was performed basically as described (Rüdiger et al., 1998). Purified vinculin head fragments (0.7 mg/ml in 10 mM sodium phosphate, pH 7.5) were coupled to MPG Long Chain Alkylamine magnetic beads according to the manufacturer (Controlled Pore Glass, Lincoln Park, NJ). Purified recombinant α-catenin (0.1 mg/ml in 100 mM NH4HCO3, pH 7.8) was incubated overnight with endoproteinase Glu-C (V8, 25 μg/ml; Boehringer Mannheim GmbH, Mannheim, Germany). Proteolysis was stopped by adjusting the pH to 7.0 and addition of diisopropylfluorophosphate (DFP) to a final concentration of 50 μM. A 10 mg/ml suspension of the vinculin head–coated magnetic beads was then incubated for 2 h at room temperature with the V8-treated α-catenin in 100 mM NH4HCO3, 150 mM NaCl, 25 mM potassium phosphate, 240 μM DFP, 4% ethanol, pH 7.5, to allow for the binding of peptides. Beads were then washed thoroughly in PBS and finally in water to remove unspecifically bound α-catenin peptides. For COOH-terminal sequence analysis, vinculin head– coated magnetic beads with bound α-catenin peptides were treated with carboxypeptidase Y (0.2 mg/ml; Sigma Chemical Co., Deisenhofen, Germany) in 10 mM ammoniumacetate, pH 5.5, for 90 s at room temperature. The reaction was stopped by preparing the samples for mass spectrometric analysis according to the sandwich technique (Kussmann et al., 1997). Mass spectrometric analysis was performed using a Bruker REFLEX time-of-flight instrument equipped with a Scout-ion source and pulsed ion extraction. All spectra were recorded at an acceleration voltage of +20 kV in both the linear and the reflector mode with delayed extraction, to achieve high mass resolution.
Cell Extraction, In Situ Cross-linking, and Immunoprecipitation
For immunoprecipitation experiments with the vinculin head domain, transiently transfected PtK2 cells were used. Confluent monolayers of cells, transfected with pcDNA-VH-FLAG (see below), were washed twice with PBS and extracted with lysis buffer (1% Triton X-100, 10% glycerol, 50 mM Tris, pH 7.4, 150 mM NaCl, 2 mM MgCl2, 1 mM DTT, 10 μg/ml aprotinin and leupeptin, 5 μg/ml pepstatin, and 1.2 mM Pefabloc) at 4°C for 10 min. Cells were scraped off the culture dish with a rubber policeman, disrupted by aspiration through a 20-gauge needle, and clarified by centrifugation. The cell lysate was incubated with 10 μg of antibody to either FLAG-tag, α-catenin, or E-cadherin for 2 h at 4°C. Subsequently, 50 μl of a 50% protein A(G)–Sepharose bead slurry (Sigma Chemical Co.) was added. After 1 h, the beads were washed four times with lysis buffer and boiled in SDS-PAGE sample buffer for 10 min to elute proteins bound. Each sample was split into two aliquots, analyzed by SDS-PAGE under reducing conditions, and then electrophoretically transferred to nitrocellulose membranes. The membranes were blocked with 5% skimmed milk powder and incubated for 1 h with primary antibodies (see below). Antibodies were diluted in PBS with 2% BSA. Membranes were sequentially washed in TBS-T, TBS-T with 500 mM NaCl, or TBS-T with 0.5% Triton, incubated with HRP- conjugated secondary antibodies for 1 h, and then extensively washed as above. Chemiluminescence (ECL system; Amersham, Braunschweig, Germany) was used for detection according to the manufacturer's instructions.
In situ cross-linking of proteins was adopted from Hinck et al. (1994), as described below. Monolayers of MDBK cells were rinsed twice with PBS and exposed to 2 ml of PBS containing 200 μg/ml dithiobis(succinimidylproppionate) (DSP; Pierce, Sankt Augustin, Germany) for 20 min at room temperature on a rocking platform. The reaction was stopped by washing the cells in PBS and incubating them for 5 min at room temperature in quenching buffer (PBS/50 mM glycine, pH 7.4). Subsequently, the cells were rinsed twice with PBS and permeabilized for 20 min at 4°C on a rocking platform in CSK buffer (50 mM NaCl, 10 mM Pipes, pH 6.8, 3 mM MgCl2, 0.5% Triton X-100, 300 mM sucrose, 20 mM glycine pH 7.4, 1.2 mM Pefabloc, 10 μg/ml leupeptin, 10 μg/ml aprotinin, and 10 μg/ml pepstatin A). The permeabilized cells were scraped off the culture dish with a rubber policeman, disrupted by repeated aspiration through a 20-gauge needle and sedimented in a microfuge for 10 min. The supernatant (soluble fraction) was removed and the pellet (insoluble fraction) was solubilized in 100 μl SDS immunoprecipitation buffer (15 mM Tris, pH 7.5, 5 mM EDTA, 2.5 mM EGTA, and 1% SDS) at 100°C for 10 min and then diluted to 1 ml with CSK buffer.
For immunoprecipitations, 4–30 μl of the respective antibodies were added to the solubilized insoluble fractions. After 2 h incubation with end-over-end rotation at 4°C, 50 μl of a 50% slurry of protein A(G)–Sepharose (Sigma Chemical Co.) in PBS was added and mixing was continued for 1 h. The samples were washed 4× with TBS-T. To cleave the cross-linker, the immunoprecipitated complexes were finally incubated in sample buffer with 20% mercaptoethanol at 100°C for 10 min. Finally, immunoprecipitates were split into aliquots and analyzed by immunoblotting as above.
Antibodies
A monoclonal antibody directed against the his tag (Dianova, Hamburg, Germany) was employed to monitor the tagged recombinant α-catenin proteins. Proteins carrying the birch profilin tag were recognized by monoclonal antibody 4A6 (Rothkegel et al., 1996; Wiedemann et al., 1996; Rüdiger et al., 1997). The FLAG tag was detected by monoclonal antibody M2 purchased from Eastman Kodak (New Haven, CT). Binding of vinculin, its head, and tail fragments was analyzed with the corresponding monoclonal antibodies against epitopes in both domains (As8 [Kroemker et al., 1994; Menkel et al., 1994] and 4E7 [Kroemker et al., 1994], respectively). A monoclonal antibody against vinculin (hVIN-1) was purchased from Sigma Chemical Co., rabbit antibody 14A (vinculin tail specific) and monoclonal antibody 15E7 (vinculin head specific) were raised in our laboratory. Polyclonal rabbit antibody (L1) against recombinant α-catenin was custom made (Eurogentec, Seraing, Belgium). A polyclonal antibody against α-catenin was purchased from Sigma Chemical Co. A polyclonal rabbit antibody against β-catenin was a kind gift of Dr. R. Kemler (Max Planck Institute, Freiburg, Germany). Monoclonal antibodies specific for β-catenin and E-cadherin were obtained from Dianova. Polyclonal antibodies against α-actinin were purchased from Sigma Chemical Co. Secondary antibodies included FITC-conjugated AffiniPure Fab Fragment Goat anti–mouse IgG (H+L) (Dianova), TRITC-conjugated goat anti– mouse IgG and horseradish peroxidase-coupled rabbit anti–mouse IgG (Sigma Chemical Co.).
Cell Culture, Transfection, and Fluorescence Analysis
PtK2 (Potorous tridactylis, no. CRL 6494; American Type Culture Collection, Rockville, MD) kidney epithelial cells, grown on glass coverslips for 24 h in DME supplemented with 10% FCS, were transfected with pcα-catenin-BiP, pcα-cat1-670, pcα-cat697-906-BiP, or pcDNA-VH-FLAG, respectively, applied as Ca-phosphate precipitates, according to standard protocols. 16 h later, the medium was changed and the cells were further incubated for 24 h before processing them for either immunofluorescence analysis or immunoprecipitation.
Madin–Darby bovine kidney epithelial cells (MDBK; a kind gift of Dr. Kartenbeck, Deutsches Krebsforschungszentrum, Heidelberg, Germany) were grown as described (Kartenbeck et al., 1991; Volberg et al., 1986).
For fluorescence analysis, cells were fixed in 3.7% paraformaldehyde for 15 min and extracted with 0.2% Triton X-100 for 30 min. For double labeling experiments of endogenous α-catenin (or transfected α-catenin, α-cat1-670, or α-cat697-906) and either β-catenin or vinculin, the cells were first incubated with anti-vinculin (anti–β-catenin) followed by an excess of FITC-conjugated goat anti–mouse Fab-fragments (Dianova). After extensive washing (3× 10 min PBS), the cells were incubated with mAB 4A6 specific for the birch profilin tag sequence, in conjunction with a TRITC-labeled secondary antibody to detect α-catenin, α-cat697-906, or α-cat1-670, respectively. F-actin was stained with FITC-phalloidin. All preparations were examined in a Zeiss Axiophot microscope equipped with epifluorescence. They were photographed on Kodak Tri-X-Pan. Alternatively, images were recorded with a CCD camera (VarioCam; PCO Computer Optics GmbH, Kehlheim, Germany) and processed using Adobe Photoshop.
Results
The Transfected Vinculin Head Domain Is Targeted to Cell–Cell Contacts in PtK2 Cells
Vinculin was identified as a major structural component of cell–matrix and cell–cell adherens junctions (Geiger et al., 1980). The molecule contains a potent talin-binding site in its head domain that was suggested to be responsible for its targeting to cell–matrix contacts (Bendori et al., 1989; Jones et al., 1989). Since talin is not found in cell–cell adherens junctions, we asked for a potential binding partner of vinculin in these structures. We addressed this question by transfecting vinculin head and tail domains, respectively, into PtK2 epithelial cells, to identify the domain responsible for cell–cell contact targeting. We used PtK2 epithelial cells, since we obtained high transfection rates and these cells contain cadherins of the uvomorulin type (Girard and Senecal, 1995), rendering them suitable for the study of catenin targeting. In these cells, like in other epithelial cells, endogenous α-catenin colocalizes with β-catenin, E-cadherin, and vinculin at cell–cell contact sites but is absent from focal contacts, which are rich in vinculin (not shown). The transfected vinculin tail fragment, detected by an antibody specific for the avian vinculin tail sequence, was seen along stress fibers, at focal contacts and in association with the peripheral microfilament belt, in accordance with its actin-binding properties, as described earlier (Hüttelmaier et al., 1997; Menkel et al., 1994). The transfected vinculin head fragment was equipped with a FLAG-tag, to discriminate it from endogenous proteins. It was also found at focal contacts (Fig. 2 A), possibly due to its talin-binding site, as discussed above. In addition, the vinculin head domain was detected at cell–cell contacts (arrowheads in Fig. 2 A). Since this fragment lacks an actin-binding site and talin is absent from cell–cell contacts, this observation suggests the existence of a novel binding partner for the vinculin head present in cell–cell contacts.
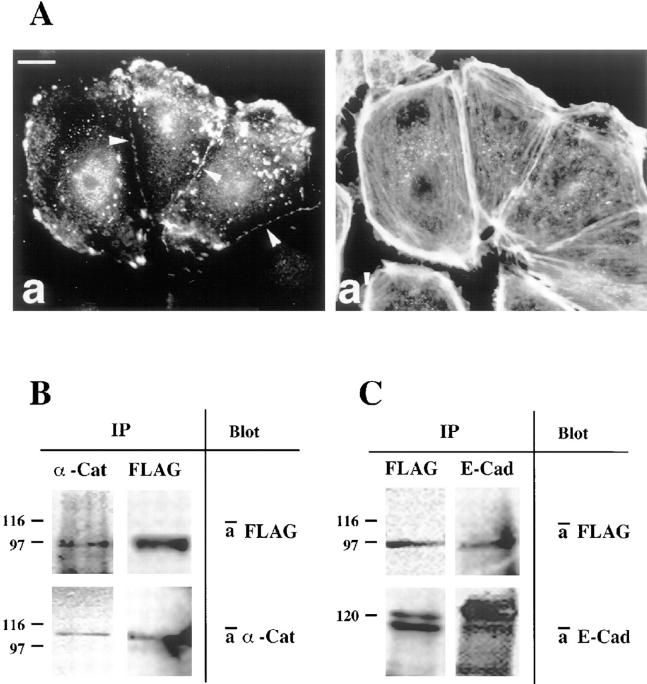
Figure 2.
Association of the vinculin head domain with the E-cadherin–catenin complex. (A) Cellular localization of the transfected vinculin head domain. Transfected PtK2 cells were double stained for the exogenous vinculin head domain (a) using an antibody against the FLAG tag (see text) and for F-actin (a′). Note that the transfected vinculin head domain targets to cell– cell contacts (arrowheads) and to focal contacts. (B and C) Immunoprecipitation analyses. When α-catenin was precipitated from Triton lysates with anti–α-catenin antibodies, the vinculin head fragment coprecipitated (B, left panels). When the transfected vinculin head domain was precipitated with the anti-FLAG antibody, α-catenin coprecipitated (B, right panels) and also E-cadherin coprecipitated (C, left panels). Vice versa, when E-cadherin was precipitated, the vinculin head was coprecipitated (C, right panel). Antibodies used for immunodetection after blotting are indicated on the right for each panel. Bar: (A) 10 μm.
In addition to a detergent-insoluble, cytoskeleton-bound pool of junctional complexes, epithelial cells contain detergent-soluble structures comprising the same components. Both types, however, are membrane associated (Hinck et al., 1994; Näthke et al., 1994). Based on this assumption, we tested whether the transfected vinculin head domain might be present in immunoprecipitates derived from such complexes in detergent extracts (Fig. 2, B and C). When the vinculin head domain was precipitated with the anti-FLAG antibody from 1% Triton X-100 lysates of transiently transfected PtK2 cells, α-catenin (Fig. 2 B), β-catenin (not shown), and also E-cadherin (Fig. 2 C) were coprecipitated. Vice versa, when α-catenin was precipitated with specific antibodies, the transfected vinculin head domain was coprecipitated (Fig. 2 B), and when E-cadherin was precipitated, the transfected vinculin head was found (Fig. 2 C). These data suggest that vinculin is a constituent of the cadherin–catenin complex in PtK2 epithelial cells.
In Situ Cross-linking Reveals Complexes Containing Endogenous α-Catenin and Vinculin in MDBK Cells
Additional evidence for an in vivo interaction between vinculin and α-catenin was obtained with immunoprecipitation using nontransfected cells. Since adherens junction proteins engaged in attaching the F-actin to the plasma membrane are detergent-insoluble as discussed by Hinck et al. (1994) and by Itoh et al. (1997), intact complexes cannot be solubilized. Therefore, we used in situ cross-linking to prevent complex dissociation upon cell extraction. Live MDBK cells were incubated with the membrane-permeable cross-linker DSP in vivo and extracted with 0.5% Triton X-100. The detergent-insoluble cytoskeletal fraction was then solubilized in buffer containing 1% SDS and these samples were used for immunoprecipitation. Results from these studies are shown in Fig. 3. When α-catenin was precipitated from such samples with a polyclonal antibody, vinculin coprecipitated. Vice versa, when vinculin was precipitated with a combination of 14A (vinculin tail specific) and 15E7 (vinculin head specific), α-catenin coprecipitated. In addition to vinculin, a smaller protein of ∼90 kD was precipitated by the vinculin antibodies. This protein may correspond to the vinculin head domain, which is easily generated by proteolysis, as was for example described for aged platelets (Reid et al., 1993) and is also seen in extracts of other cells (our own unpublished observation).
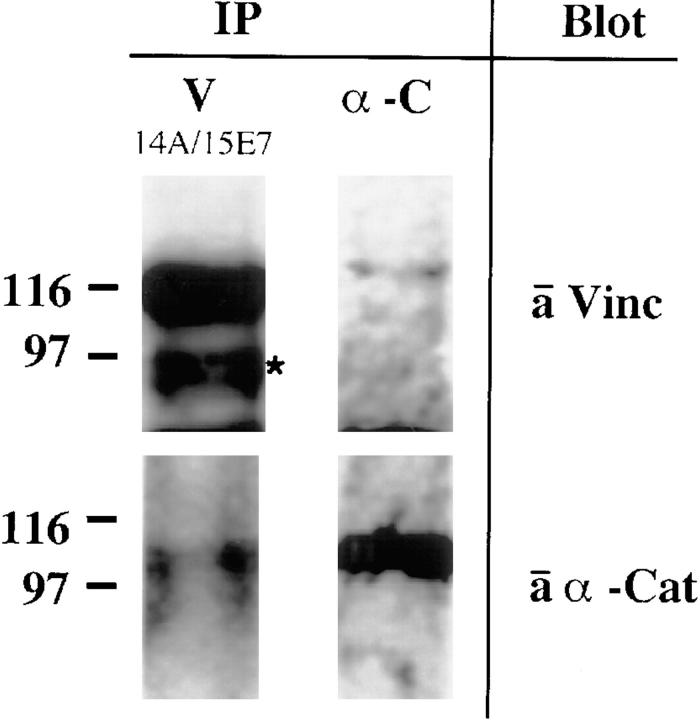
Figure 3.
Immunoprecipitation analysis after in situ cross-linking. After cross-linking proteins in live MDBK cells with DSP, cells were Triton extracted. The Triton-insoluble fraction was solubilized with SDS and these lysates were used for immunoprecipitation analysis. DSP complexes were eluted from the antibodies and simultaneously cleaved by boiling in reducing sample buffer. Immunoprecipitates were probed with specific antibodies against vinculin (hVIN-1) and α-catenin (polyclonal), as indicated on the right. (Left) Vinculin was precipitated with a mixture of 14A (tail specific) and 15E7 (head specific), α-catenin was coprecipitated. The band marked by an asterisk is probably a vinculin degradation product. (Right) α-Catenin was precipitated from identical lysates with α-catenin–specific antibodies, vinculin was coprecipitated.
Thus, we conclude that endogenous vinculin and α-catenin are part of the E-cadherin–catenin complex in MDBK epithelial cells.
α-Catenin Interacts with Vinculin
For reasons already discussed in Herrenknecht et al. (1991) and Nagafuchi et al. (1991), a candidate binding partner for vinculin within the cadherin–catenin complex might be α-catenin. Based on their assumption, and on our immunoprecipitation data (see above), we tested this hypothesis in a variety of assays. First, we performed dot overlays (Fig. 4).
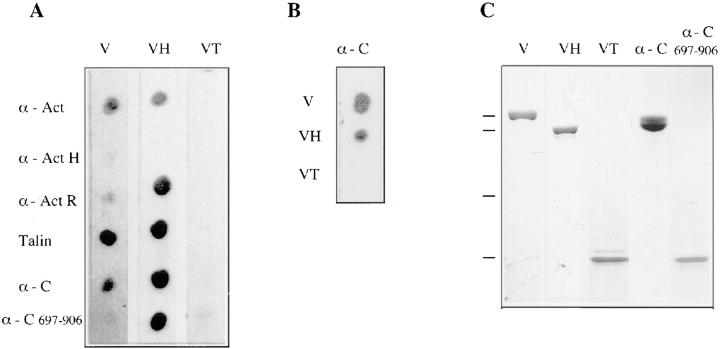
Figure 4.
Analysis of α-catenin–vinculin interactions in dot overlays. (A and B) Proteins dotted onto nitrocellulose are indicated on the left. Proteins added as probes are indicated on the top. The following abbreviations are used: vinculin (V), vinculin head (VH), vinculin tail (VT), α-actinin (α-Act), α-actinin1-265 (α-ActH), α-actinin rod domain (α-ActR), α-catenin (α-C), and α-catenin697-906 (α-C697-906). The following antibodies were employed to monitor protein interactions: As8, directed against an epitope in the vinculin head fragment was used to detect intact vinculin and the NH2-terminal vinculin head domain, 4E7 directed against the vinculin tail domain, and the his-tag antibody to detect recombinant α-catenin, respectively. (C) Coomassie stained gel to show the purity of vinculin, α-catenin, and fragments derived thereof that were used (A and B).
Recombinant α-catenin and its truncated fragment (α-cat697-906) were spotted onto nitrocellulose and incubated with vinculin, its proteolytic 90-kD head or its 27-kD tail domain, respectively. Binding was monitored with specific monoclonal antibodies. Purified talin, α-actinin, and its thermolysin-derived fragments served as controls. As seen in Fig. 4 A, intact vinculin bound strongly to talin, less well to α-catenin and only faintly to α-cat697-906. The isolated vinculin head strongly decorated intact α-catenin and its COOH-terminal fragment. This reaction was even stronger than that seen for the α-actinin COOH-terminal rod domain, a well characterized binding partner of the vinculin head (Kroemker et al., 1994; McGregor et al., 1994). In contrast, the vinculin tail fragment neither bound to α-catenin nor to its COOH-terminal part. As shown in Fig. 4 B, overlays in the reverse order, i.e., with vinculin and its head and tail fragments dotted onto nitrocellulose, probed with α-catenin, and monitored with the his-tag antibody, corroborated these results: α-catenin binds to vinculin and its NH2-terminal head fragment but not to the COOH-terminal vinculin tail. Purity controls of the protein preparations employed show that there were no contaminating proteins present that might have mediated this interaction (Fig. 4 C). Thus, our results imply a direct binding of the COOH-terminal region of α-catenin to the 90-kD head domain of vinculin. An interaction between α-catenin and α-actinin was not detected (not shown) corroborating other data that demonstrate that this complex only forms when α-catenin is associated with the catenin– cadherin complex (Knudsen et al., 1995; Nieset et al., 1997). Talin-α-catenin binding was also not seen, which may be related to the fact that these two proteins do not colocalize in cells.
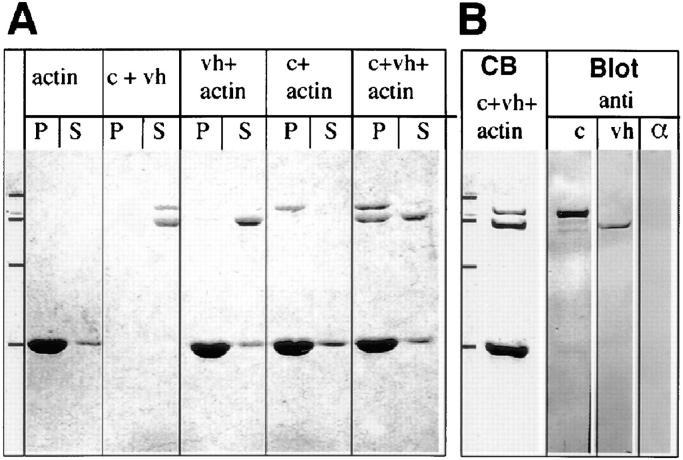
We used cosedimentation to further analyze the interaction of the vinculin head domain with α-catenin. As shown in Fig. 5 A, a mixture of purified α-catenin and the isolated vinculin head did not sediment in the absence of F-actin, whereas α-catenin quantitatively sedimented in the presence of F-actin, as reported by Rimm et al. (1995). The vinculin head fragment did not cosediment with F-actin, in accordance with the localization of the F-actin–binding site within the vinculin tail (Menkel et al., 1994; Hüttelmaier et al., 1997). However, in the presence of α-catenin, the vinculin head cosedimented with F-actin and α-catenin (Fig. 5 A), indicating that α-catenin mediated the sedimentation of the vinculin head when bound to F-actin. Similar amounts of vinculin head and α-catenin cosedimented, arguing for the formation of a 1:1 complex. To exclude that contaminating α-actinin, which contains binding sites for F-actin (Kuhlmann et al., 1992), the vinculin head (Kroemker et al., 1994; McGregor et al., 1994), and α-catenin (Knudsen et al., 1995), might mediate this interaction, an identical mixture of the proteins as used for cosedimentation was analyzed by immunoblotting. Fig. 5 B shows that the α-catenin, vinculin, and F-actin preparations were free of α-actinin. Thus, our data indicate the formation of a ternary complex in which the vinculin head is linked to F-actin via α-catenin.
Figure 5.
Sedimentation assays with F-actin, the vinculin head fragment, and α-catenin. (A) Coomassie blue–stained SDS gels of supernatants (S) and pellets (P). Complex formation of α-catenin (c) and vinculin head (vh) in cosedimentation studies with F-actin (actin). F-actin was 160 pmol in all samples where present. Vinculin head (60 pmol) and α-catenin (20 pmol) were added either alone or in combination, as indicated. (B) Coomassie blue– stained gel (CB) of a mixture of c, vh, and actin and immunoblots of identical mixtures probed for α-catenin (anti-c), vinculin head (anti-vh), and contaminating α-actinin (α). Molecular mass markers, indicated in each panel, are from top to bottom: 116, 96, 66, and 45 kD.
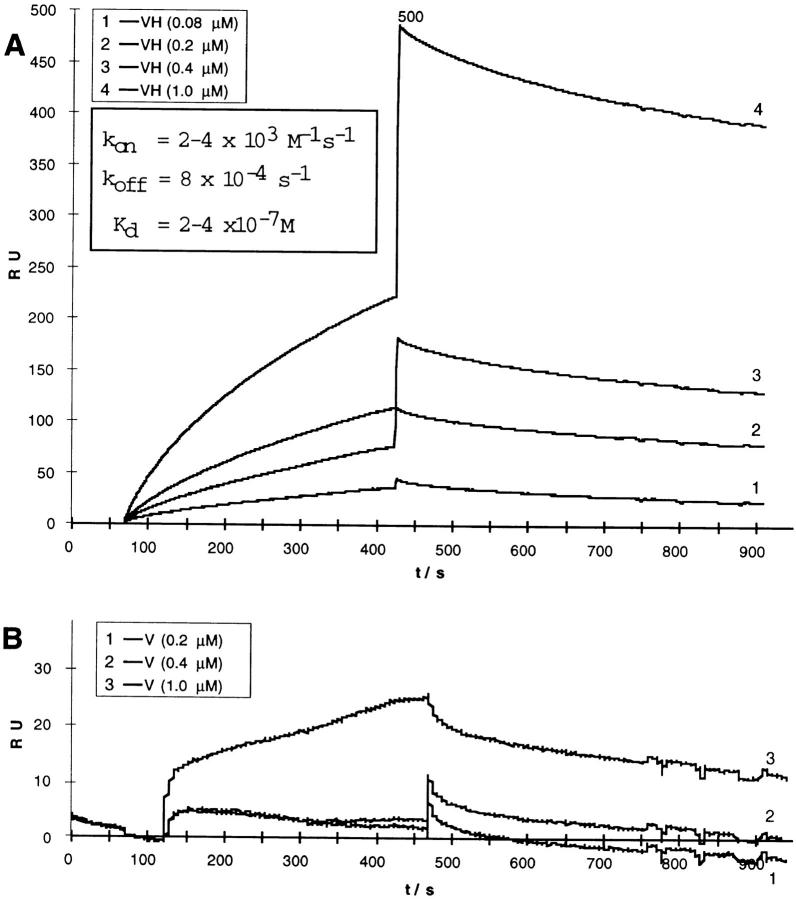
Finally, we analyzed the kinetic properties of the complex formation between α-catenin and the vinculin head fragment by surface plasmon resonance measurements. α-Catenin was immobilized on the sensor chip surface. Binding and dissociation of either vinculin or the vinculin head were monitored in terms of RUs (Fig. 6). When the isolated vinculin head was used, binding and dissociation was easily monitored in the range of 0.08–1 μM of vinculin head. The data obtained (Fig. 6 A) were used to calculate dissociation kinetics, yielding a k off of 8 × 10−4s−1. The k on value was in the range of 2–4 × 103 M−1s−1, depending on the fitting method used. Thus, the dissociation constant of the complex is K d = 2–4 × 10−7 M. In the case of intact vinculin, binding was barely detectable (Fig. 6 B). Binding of intact vinculin accounted for not more than 15 RU, even at high concentrations (10 μM) and reliable data fitting was impossible.
Figure 6.
(A) Surface plasmon resonance measurement of the vinculin head–α-catenin interaction. α-Catenin was immobilized on the sensor chip and probed with vinculin head fragment (A) or intact vinculin (B) at the concentrations indicated to record k on and k off in terms of RU. Values for k on, k off, and K d were calculated from these data and are presented in the graph. Note the different RU scales in A and B.
Hence, a direct and specific interaction between full-length α-catenin and the vinculin head was seen by three independent biochemical methods.
To characterize the vinculin-binding site in α-catenin more precisely, we performed an affinity mass spectrometric assay. We used magnetic beads coated with the purified vinculin head domain, as a bait for vinculin-binding peptides derived from α-catenin by cleavage with endoproteinase Glu-C (V8). Mass spectrometric analysis of these affinity-purified samples detected one peptide of monoisotopic molecular mass 2,490.70 Da. This corresponds within 0.01% to the calculated molecular mass (2,490.40 D) of the peptide TQTKIKRASQKKHVNPVQALSE comprising α-catenin residues 878–899, which is a predicted V8 cleavage product of α-catenin. To verify the identity of this peptide, we performed COOH-terminal sequence analysis by carboxypeptidase treatment and mass spectrometric analysis and obtained the sequence QALSE, which confirms this prediction. Thus, we affinity purified a COOH-terminal α-catenin fragment that strongly and specifically binds to the vinculin head domain.
Transfected α-Cat697-906 Codistributes with Adherens Junctions and Stress Fibers
To further characterize the putative α-catenin–vinculin complex in epithelial cells, we performed transfection studies with truncated α-catenin mutants.
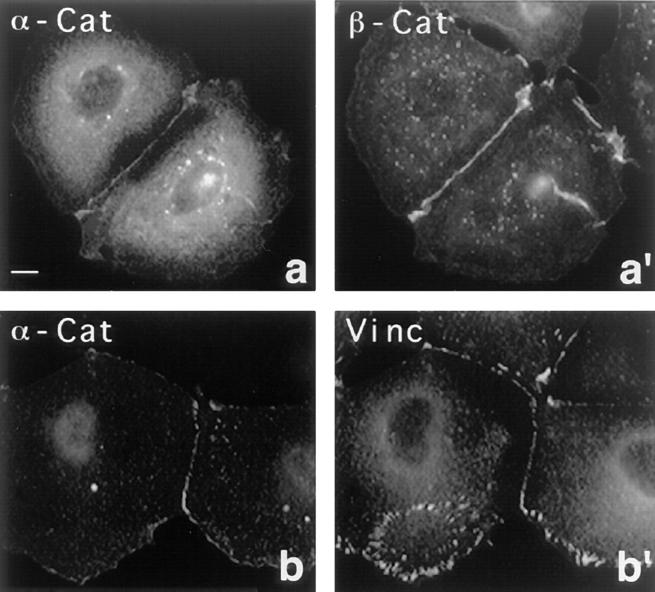
The cellular distribution of transfected α-catenin in PtK2 cells is seen in Fig. 7. The recombinant α-catenin was tagged with the BiP tag to discriminate it from the endogenous protein. As expected, the transfected protein targeted to cell–cell contact sites, identified by immunostaining for β-catenin (Fig. 7, a and a′) and for vinculin (Fig. 7, b and b′). Focal contacts, also detected by staining for vinculin (Fig. 7 b′), were not labeled by intact α-catenin (Fig. 7 b). Hence, the recombinant mouse α-catenin recognized endogenous PtK2 adherens junctions, and the exogenously expressed protein targeted faithfully to its physiological position, the cell–cell contact site.
Figure 7.
Localization of transfected α-catenin in PtK2 cells. The transfected cells were double stained for α-catenin (a and b) using mAB 4A6 against the tag (see text) and for β-catenin (a′) and vinculin (b′). Note that the transfected α-catenin specifically targets to cell–cell contacts, but not to focal contacts. Bar, 10 μm.
Similarly, as shown in Fig. 8, transfected α-cat1-670, comprising the β-catenin–binding site (Huber et al., 1997; Nieset et al., 1997; Obama and Ozawa, 1997; see Fig. 1), was also predominantly recruited to cell–cell contacts (Fig. 8, a and a′, arrowheads) although in this case, an additional nuclear signal was detected (Fig. 8, a and b).
Figure 8.
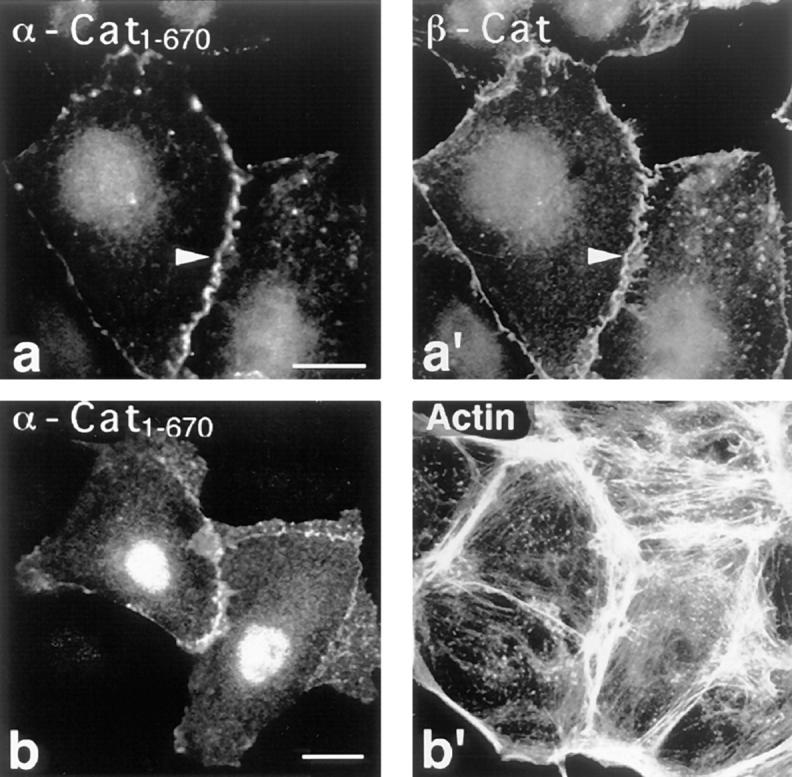
Localization of transfected α-cat1-670 in PtK2 cells. PtK2 cells were double stained for α-cat1-670 (a and b) using mAB 4A6 against the tag and for β-catenin (a′) and F-actin (b′). Note that the transfected α-catenin “head” fragment specifically targets to cell–cell contacts (arrowheads). Bar, 10 μm.
In contrast, when the epitope tagged α-cat697-906 was transiently expressed in PtK2 cells, the fragment was not only found at cell–cell contact sites as identified by colocalization with β-catenin (Fig. 9, a and a′), but also in focal adhesions. This was deduced from images as seen in Fig. 9 (a–c) and proven by double staining for vinculin (Fig. 9, b and b′), and for additional focal contact proteins like paxillin, talin, and α-actinin (not shown). Thus, in transfected cells, the α-catenin COOH-terminal fragment colocalized with vinculin, in accordance with the assumption that it comprises a binding site for vinculin. Furthermore, α-cat697-906 was also detected along stress fibers, as seen in the double stain for F-actin (Fig. 9, c and c′). This may be due to its active F-actin–binding site mapped earlier to the COOH-terminal half of the α-catenin sequence (Rimm et al., 1995). The unexpected localization of α-cat697-906 at focal adhesions and along stress fibers was also observed in transfected NIH/3T3 fibroblastic cells and after microinjection of the purified recombinant fragment into PtK2 and LLC-PK1 cells (not shown).
Figure 9.
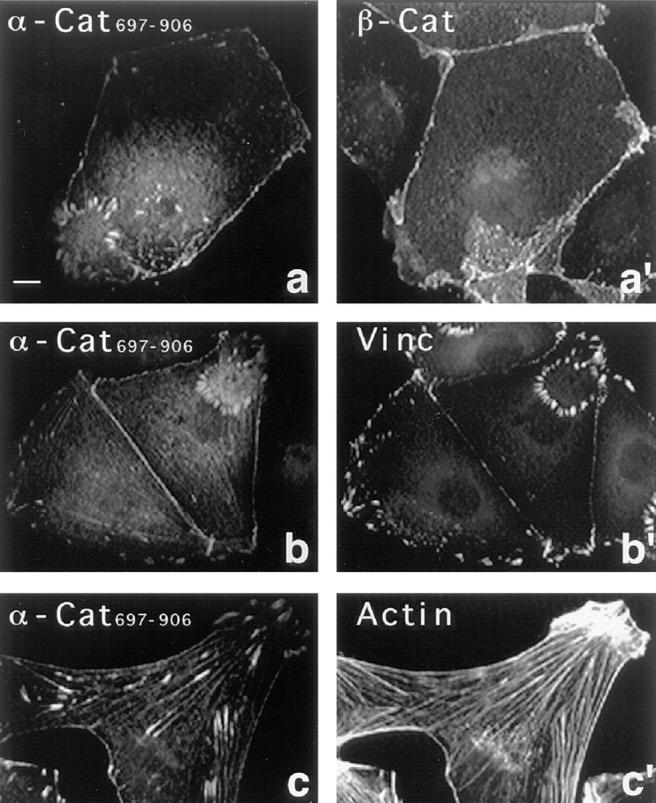
Localization of transfected α-cat697-906 in PtK2 cells. PtK2 cells were double stained for α-cat697-906 (a–c) using mAB 4A6 against the tag and for β-catenin (a′), vinculin (b′), and F-actin (c′). Note that α-cat697-906 is not only seen in cell–cell contacts, but also along stress fibers and in focal adhesions, which sometimes form ring-like arrangements at the ventral membrane of these cells. Bar, 10 μm.
Discussion
In this study, we addressed the following questions. First, why are vinculin and α-catenin, two structural proteins related in sequence, differentially recruited to cell–matrix and cell–cell contacts? Second, is vinculin, a recognized major component of cell–matrix contacts, also part of the cell–cell junctional complex?
The recruitment of intact vinculin to focal adhesions is mainly attributed to its rather high affinity for talin, a protein exclusively seen in cell–matrix contacts (Burridge and Connell, 1983; Drenckhahn et al., 1988). As had been described earlier (Bendori et al., 1989; Menkel et al., 1994) and was confirmed in this study (not shown), the isolated vinculin tail domain, when introduced into host cells by either transfection or microinjection, targets primarily to microfilament bundles and to focal adhesions. In contrast, the transfected head domain displayed no affinity for microfilament bundles but was strongly enriched in focal adhesions, and in addition in cell–cell contacts. Thus, it must be the head domain that is responsible for vinculin recruitment to cell–cell adherens junctions. To analyze whether vinculin is part of the cadherin–catenin complex, which characterizes these junctions, we performed immunoprecipitation studies. Initial attempts to immunoprecipitate this complex failed, probably because the complex is linked to the actin cytoskeleton. This suprastructure cannot be solubilized intact by mild detergent treatment, and higher detergent concentrations destroy the complex. An analogous conclusion was recently drawn by Itoh et al. (1997) for the α-catenin–ZO-1 complex in nonepithelial L-cells. Here, we circumvented this problem by two alternative approaches. First, we transiently transfected PtK2 epithelial cells with the epitope-tagged vinculin head domain. This domain targets to cell–cell contacts, but it cannot bind to actin, as it lacks an F-actin–binding site (Menkel et al., 1994; Johnson and Craig, 1995; Hüttelmaier et al., 1997). Consequently, Triton X-100 soluble cadherin– catenin complexes comprising the vinculin head domain should be immunoprecipitable (Hinck et al., 1994; Itoh et al., 1997). Indeed we detected this fragment in immunoprecipitates comprising E-cadherin, β-catenin, and α-catenin. Second, we used a membrane-permeable chemical cross-linker to form detergent-resistant complexes within MDBK epithelial cells. Under such conditions, we could immunoprecipitate vinculin–α-catenin complexes from the Triton-insoluble, cytoskeletal fraction, using either vinculin- or α-catenin–specific antibodies. Thus, both approaches showed that vinculin is part of the cadherin–catenin junctional complex.
Next, we tested for a direct interaction of α-catenin and vinculin. Hetero-oligomer formation between α-catenin and vinculin had been predicted previously (Herrenknecht et al., 1991; Nagafuchi et al., 1991; Kemler, 1993) but was not demonstrated. Since vinculin homo-oligomers are formed by tail–tail interactions (Molony and Burridge, 1985; Winkler et al., 1996), we initially expected a similar topographical arrangement of the molecules in vinculin–α-catenin hetero-oligomers. However, our dot overlays revealed that the vinculin binding to α-catenin involves the head domain of the former and the putative tail (α-cat697-906) of the latter. Hence, the binding of the vinculin head to the α-catenin “tail” is a heterologous head-to-tail interaction. Furthermore we found that α-catenin mediated cosedimentation of the vinculin head with F-actin and we could affinity purify α-cat878-899 from a V8 digest using immobilized vinculin head fragments. Of course, these data do not preclude putative additional binding sites for vinculin in the NH2-terminal sequence of α-catenin. The kinetic constants for association and dissociation of the α-catenin–vinculin head complex were determined by surface plasmon resonance studies. The calculated K d (2–4 × 10−7 M) is in the range commonly found for the interaction of cytoskeletal components. For example, the affinity of the interaction of vinculin/talin was reported to K d = 2–6 × 10−7 M (Gilmore et al., 1993). On the other hand, the binding of vinculin to α-actinin (Otto, 1983; Wilkins et al., 1983; Kroemker et al., 1994; McGough et al., 1994) is apparently of weaker affinity (K d = 1.3 × 10−5 M; McGregor et al., 1994).
In intramolecular interactions of vinculin, the tail region binds to residues 1–258 of the head (Weekes et al., 1996; our own unpublished observations), resulting in the closed conformation of the vinculin molecule (Jockusch and Rüdiger, 1996). Hence, it seems likely that in the intermolecular interaction described here, the COOH-terminal region of α-catenin that displays the highest degree of homology between vinculin and α-catenin (see Fig. 1) binds to the same vinculin domain, yielding heterologous head-to-tail complexes. In addition, the much lower affinity of intact vinculin as compared with its head domain for α-catenin, as seen in our dot overlays and the surface plasmon resonance measurements, suggests that complex formation is controlled by intramolecular interactions in vinculin, as reported for other vinculin ligands like F-actin, talin, α-actinin, and acidic phospholipids (for references see Jockusch and Rüdiger, 1996).
A direct interaction between vinculin and α-catenin is also consistent with the results obtained by transfection experiments. In transiently transfected PtK2 cells, full-length α-catenin was exclusively found at cell–cell adhesions, whereas endogenous vinculin was seen in both, focal adhesions and cell–cell contacts. Intact α-catenin might be recruited to cell–cell contacts due to its high affinity for β-catenin (K d = 3.8 × 10−8 M), since the β-catenin–binding site had been mapped to residues 48–163 (Obama and Ozawa, 1997). In contrast, transfected α-cat697-906, which lacks the β-catenin binding region, was not only incorporated into cell–cell contacts, but additionally into focal adhesions and along stress fibers. These are the same sites where the vinculin tail fragment is localized (Menkel et al., 1994). Cosedimentation studies of α-cat697-906 with F-actin demonstrated a direct interaction of both proteins (own unpublished results). Hence, the localization of α-cat697-906 along stress fibers is explained by its F-actin–binding site, whereas its recruitment to focal adhesions and cell– cell junctions might be due to an interaction of the transfected protein with endogenous vinculin.
In contrast, both intact vinculin and α-catenin did not bind to stress fibers of cultured cells, even when high levels of the proteins were introduced by either microinjection or overexpression (Rodriguez Fernandez et al., 1992 and this study). This suggests that their actin-binding domains are silent under these conditions and need to be activated. Thus, regulation of ligand binding is apparently also common to both proteins. It remains to be seen whether α-catenin, whose overall structural organization is again similar to that of vinculin (Koslov et al., 1997), is also regulated by a conformational switch and whether PIP2 and Ser/Thr phosphorylation are involved as had been described for vinculin (Gilmore and Burridge, 1996; Schwienbacher et al., 1996; Weekes et al., 1996). Recently, these structural, functional, and regulatory relationships have been reviewed in detail (Rüdiger, 1998).
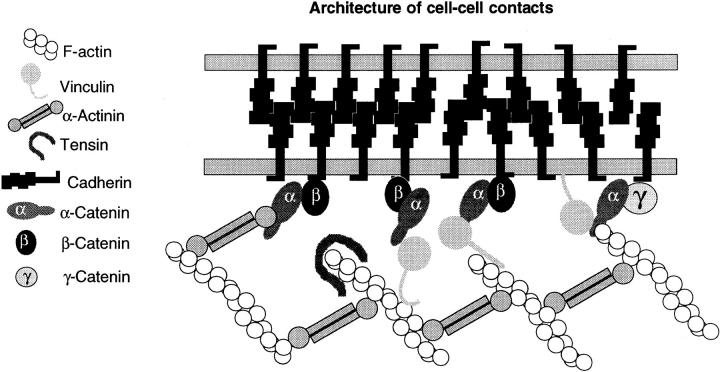
Currently, it remains elusive which of the multiple interactions between junctional proteins are realized in living cells, whether the cells selectively use a specific type, and how such specificity might be regulated in epithelial architecture. The findings reported here add new aspects to our present view on cell–cell adherens junctions as outlined in Fig. 10. The initial model, deduced from immunoprecipitation studies, postulated binding of the cytoplasmic domain of E-cadherin to a heterotrimeric complex of α-, β-, and γ-catenin (Ozawa et al., 1989; Shore and Nelson, 1991; Grunwald, 1993; Kemler, 1992). Based on more recent findings that two distinct complexes, containing E-cadherin and either α- and β-catenin or α- and γ-catenin can be immunoprecipitated, this model was adapted, proposing a coexistence of both complexes in fully polarized epithelial cells (Hinck et al., 1994; Näthke et al., 1994). The most recent observation that α-catenin–cadherin complexes can also directly interact with α-actinin (Knudsen et al., 1995; Nieset et al., 1997), together with the data reported here on complex formation between α-catenin and vinculin, further expand the model, suggesting a highly versatile situation at the plasma membrane of cell–cell contact sites. Actin filaments might associate with α-catenin either directly through its actin-binding site (Rimm et al., 1995), or indirectly through α-actinin or vinculin (Knudsen et al., 1995 and this work). Recent reports of Torres et al. (1997) and Sehgal et al. (1997) are consistent with an essential role for α-catenin in this situation. Torres et al. (1997) showed that a gene trap mutation of mouse α-catenin, affecting the COOH-terminal third of the molecule, leads to a loss-of-function phenotype. Homozygous mice do not develop further than the blastocyst state, due to disruption of the trophoblast epithelium. Similarly, Sehgal et al. (1997) demonstrated that an α-catenin mutant lacking the COOH-terminal 230 amino acids in Xenopus results in impaired blastomere adhesion and loss of the blastocoel. These findings emphasize the significance of our study, since the expression of such mutant α-catenins lacking both, the COOH-terminal F-actin–binding site and the vinculin–binding site, must be deleterious for the establishment of adherens junctions in epithelia.
Figure 10.
Hypothetical architecture of cell–cell contacts. Different modes of linking F-actin to the plasma membrane are depicted. Actin filaments might be linked to α-catenin and to the adherens junction via α-actinin (Knudsen et al., 1995) or via vinculin (this study). Alternatively, F-actin might directly bind to α-catenin (Rimm et al., 1995). The α/β complex then binds to the transmembrane cadherins, thus tethering neighboring cells together. Note that vinculin can also bind to phospholipids and to α-actinin, providing further possibilities to reinforce the adhesion complex.
Acknowledgments
We thank Drs. R. Kemler and H. Hoschuetzky (Max Planck Institute, Freiburg, Germany) for providing α-catenin cDNA and antibodies, J. Kartenbeck for MDBK cells, C. Schwienbacher for purified talin, K. Schlüter for purified actin, and H. Faulstich (Max Planck Institute, Ladenburg, Germany) for FITC-phalloidin. We are grateful to Dr. B. Haase (BIACORE) for his support with surface plasmon resonance studies and to Dr. M. Kiess (Gesellschaft für Biotechnologische Forschung, Braunschweig, Germany) for NH2-terminal sequence analysis. We are indebted to T. Meßerschmidt for expert technical assistance and photographic artwork.
This study was supported by the Deutsche Forschungsgemeinschaft.
Abbreviations used in this paper
- DFP
diisopropylfluorophosphate
- DSP
dithiobis(succinimidylproppionate)
- MDBK
Madin–Darby bovine kidney epithelial cells
- PIP2
phosphatidylinositol-4,5-bisphosphate
- PtK2
Potourus tridactylus kidney epithelial cells
- RU
resonance units
Footnotes
Address all correspondence to Dr. Manfred Rüdiger, Cell Biology, Zoological Institute, Technical University Braunschweig, 38092 Braunschweig, Germany. Tel.: 0531-391-3191. Fax: 0531-391-8203. E-mail: m.ruediger@tu-bs.de
References
- Aberle H, Butz S, Stappert J, Weissig H, Kemler R, Hoschuetzky H. Assembly of the cadherin catenin complex in vitro with recombinant proteins. J Cell Sci. 1994;107:3655–3663. doi: 10.1242/jcs.107.12.3655. [DOI] [PubMed] [Google Scholar]
- Aberle H, Schwartz H, Kemler R. Cadherin-catenin complex: Protein interactions and their implications for cadherin function. J Cell Biochem. 1996;61:514–523. doi: 10.1002/(SICI)1097-4644(19960616)61:4%3C514::AID-JCB4%3E3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Belkin AM, Koteliansky VE. Interaction of iodinated vinculin, metavinculin and alpha-actinin with cytoskeletal proteins. FEBS Lett. 1987;220:291–294. doi: 10.1016/0014-5793(87)80832-3. [DOI] [PubMed] [Google Scholar]
- Bendori R, Salomon D, Geiger B. Identification of two distinct functional domains on vinculin involved in its association with focal contacts. J Cell Biol. 1989;108:2383–2393. doi: 10.1083/jcb.108.6.2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge K, Connell L. Talin: a cytoskeletal component concentrated in adhesion plaques and other sites of actin-membrane interaction. Cell Motil. 1983;3:405–417. doi: 10.1002/cm.970030509. [DOI] [PubMed] [Google Scholar]
- Burridge K, Mangeat P. An interaction between vinculin and talin. Nature. 1984;308:744–746. doi: 10.1038/308744a0. [DOI] [PubMed] [Google Scholar]
- Burridge K, Fath K, Kelly T, Nuckolls G, Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Drenckhahn D, Beckerle M, Burridge K, Otto J. Identification and subcellular location of talin in various cell types and tissues by means of [125I]vinculin overlay, immunoblotting and immunocytochemistry. Eur J Cell Biol. 1988;46:513–522. [PubMed] [Google Scholar]
- Feramisco JR, Burridge K. A rapid purification of α-actinin, filamin, and a 130,000-dalton protein from smooth muscle. J Biol Chem. 1980;255:1194–1199. [PubMed] [Google Scholar]
- Geiger B, Tokuyasu KT, Dutton AH, Singer SJ. Vinculin, an intracellular protein localized at specialized sites where microfilament bundles terminate at cell membranes. Proc Natl Acad Sci USA. 1980;77:4127–4131. doi: 10.1073/pnas.77.7.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore AP, Burridge K. Regulation of vinculin binding to talin and actin by phosphatidyl-inositol-4-5-bisphosphate. Nature. 1996;381:531–535. doi: 10.1038/381531a0. [DOI] [PubMed] [Google Scholar]
- Gilmore AP, Wood C, Ohanian V, Jackson P, Patel B, Rees DJ, Hynes RO, Critchley DR. The cytoskeletal protein talin contains at least two distinct vinculin binding domains. J Cell Biol. 1993;122:337–347. doi: 10.1083/jcb.122.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girard D, Senecal JL. Characterization of a novel human IgG antibody reactive with Ca(2+)-sensitive cell–cell adhesion epitope of PtK2 epithelial cells. Autoimmunity. 1995;20:237–245. doi: 10.3109/08916939508995701. [DOI] [PubMed] [Google Scholar]
- Grunwald GB. The structural and functional analysis of cadherin calcium-dependent cell adhesion molecules. Curr Opin Cell Biol. 1993;5:797–805. doi: 10.1016/0955-0674(93)90028-o. [DOI] [PubMed] [Google Scholar]
- Herrenknecht K, Ozawa M, Eckerskorn C, Lottspeich F, Lenter M, Kemler R. The uvomorulin-anchorage protein alpha catenin is a vinculin homologue. Proc Natl Acad Sci USA. 1991;88:9156–9160. doi: 10.1073/pnas.88.20.9156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinck L, Nathke IS, Papkoff J, Nelson WJ. Dynamics of cadherin/catenin complex formation: novel protein interactions and pathways of complex assembly. J Cell Biol. 1994;125:1327–1340. doi: 10.1083/jcb.125.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano S, Kimoto N, Shimoyama Y, Hirohashi S, Takeichi M. Identification of a neural alpha-catenin as a key regulator of cadherin function and multicellular organization. Cell. 1992;70:293–301. doi: 10.1016/0092-8674(92)90103-j. [DOI] [PubMed] [Google Scholar]
- Huber O, Krohn M, Kemler R. A specific domain in α-catenin mediates binding to β-catenin and plakoglobin. J Cell Sci. 1997;110:1759–1765. doi: 10.1242/jcs.110.15.1759. [DOI] [PubMed] [Google Scholar]
- Hüttelmaier S, Bubeck P, Rüdiger M, Jockusch BM. Characterization of two F-actin-binding and oligomerization sites in the cell-contact protein vinculin. Eur J Biochem. 1997;247:1136–1142. doi: 10.1111/j.1432-1033.1997.01136.x. [DOI] [PubMed] [Google Scholar]
- Isenberg G, Leonard K, Jockusch BM. Structural aspects of vinculin-actin interactions. J Mol Biol. 1982;158:231–249. doi: 10.1016/0022-2836(82)90431-4. [DOI] [PubMed] [Google Scholar]
- Itoh M, Nagafuchi A, Moroi S, Tsukita S. Involvement of ZO-1 in cadherin-based cell adhesion through its direct binding to or catenin and actin filaments. J Cell Biol. 1997;138:181–192. doi: 10.1083/jcb.138.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jockusch BM, Rüdiger M. Crosstalk between cell adhesion molecules: vinculin as a paradigm for regulation by conformation. Trends Cell Biol. 1996;6:311–315. doi: 10.1016/0962-8924(96)10022-2. [DOI] [PubMed] [Google Scholar]
- Jockusch BM, Bubeck P, Giehl K, Kroemker M, Moschner J, Rothkegel M, Rüdiger M, Schlüter K, Stanke G, Winkler J. The molecular architecture of focal adhesions. Annu Rev Cell Dev Biol. 1995;11:379–416. doi: 10.1146/annurev.cb.11.110195.002115. [DOI] [PubMed] [Google Scholar]
- Johnson RP, Craig SW. F-actin binding site masked by the intramolecular association of vinculin head and tail domains. Nature. 1995;373:261–264. doi: 10.1038/373261a0. [DOI] [PubMed] [Google Scholar]
- Jones P, Jackson P, Price GJ, Patel B, Ohanion V, Lear AL, Critchley DR. Identification of a talin binding site in the cytoskeletal protein vinculin. J Cell Biol. 1989;109:2917–2927. doi: 10.1083/jcb.109.6.2917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kartenbeck J, Schmelz M, Franke WW, Geiger B. Endocytosis of junctional cadherins in bovine kidney epithelial (MDBK) cells cultured in low Ca2+medium. J Cell Biol. 1991;113:881–892. doi: 10.1083/jcb.113.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemler R. Classical cadherins. Semin Cell Biol. 1992;3:149–155. doi: 10.1016/s1043-4682(10)80011-x. [DOI] [PubMed] [Google Scholar]
- Kemler R. From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends Genet. 1993;9:317–321. doi: 10.1016/0168-9525(93)90250-l. [DOI] [PubMed] [Google Scholar]
- Knudsen KA, Soler AP, Johnson KR, Wheelock MJ. Interaction of alpha-actinin with the cadherin/catenin cell–cell adhesion complex via α-catenin. J Cell Biol. 1995;130:67–77. doi: 10.1083/jcb.130.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch PJ, Franke WW. Desmosomal cadherins: another growing multigene family of adhesion molecules. Curr Opin Cell Biol. 1994;6:682–687. doi: 10.1016/0955-0674(94)90094-9. [DOI] [PubMed] [Google Scholar]
- Koslov ER, Maupin P, Pradhan D, Morrow JS, Rimm DL. α-Catenin can form asymmetric homodimeric complexes and/or heterodimeric complexes with beta-catenin. J Biol Chem. 1997;272:27301–27306. doi: 10.1074/jbc.272.43.27301. [DOI] [PubMed] [Google Scholar]
- Kroemker M, Rüdiger AH, Jockusch BM, Rüdiger M. Intramolecular interactions in vinculin control alpha-actinin binding to the vinculin head. FEBS Lett. 1994;355:259–262. doi: 10.1016/0014-5793(94)01216-4. [DOI] [PubMed] [Google Scholar]
- Kuhlmann PA, Hemmings L, Critchley DR. The identification and characterization of an actin-binding site in α-actinin by mutagenesis. FEBS Lett. 1992;304:201–206. doi: 10.1016/0014-5793(92)80619-r. [DOI] [PubMed] [Google Scholar]
- Kussmann M, Nordhoff E, Rahbek-Nielsen H, Haebel S, Rossel-Larsen M, Jakobsen L, Gobom J, Mirgorodskaja E, Kroll-Kristensen A, Palm L, Roepstorff P. Matrix-assited laser desorption/ionization mass spectrometry sample preparation techniques designed for various peptide and protein analytes. J Mass Spectrom. 1997;32:593–601. [Google Scholar]
- McGough A, Way M, De Rosier D. Determination of the alpha- actinin-binding site on actin filaments by cryoelectron microscopy and image analysis. J Cell Biol. 1994;126:433–443. doi: 10.1083/jcb.126.2.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor A, Blanchard AD, Rowe AJ, Critchley DR. Identification of the vinculin-binding site in the cytoskeletal protein alpha-actinin. Biochem J. 1994;301:225–233. doi: 10.1042/bj3010225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menkel AR, Kroemker M, Bubeck P, Ronsiek M, Nikolai G, Jockusch BM. Characterization of an F-actin-binding domain in the cytoskeletal protein vinculin. J Cell Biol. 1994;126:1231–1240. doi: 10.1083/jcb.126.5.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molony L, Burridge K. Molecular shape and self-association of vinculin and metavinculin. J Cell Biochem. 1985;29:31–36. doi: 10.1002/jcb.240290104. [DOI] [PubMed] [Google Scholar]
- Nagafuchi A, Takeichi M, Tsukita S. The 102 kd cadherin-associated protein: similarity to vinculin and posttranscriptional regulation of expression. Cell. 1991;65:849–857. doi: 10.1016/0092-8674(91)90392-c. [DOI] [PubMed] [Google Scholar]
- Nagafuchi A, Ishihara S, Tsukita S. The roles of catenins in the cadherin-mediated cell adhesion: functional analysis of E-cadherin-alpha catenin fusion molecules. J Cell Biol. 1994;127:235–245. doi: 10.1083/jcb.127.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Näthke IS, Hinck L, Swedlow JR, Papkoff J, Nelson WJ. Defining interactions and distributions of cadherin and catenin complexes in polarized epithelial cells. J Cell Biol. 1994;125:1341–1352. doi: 10.1083/jcb.125.6.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieset JE, Redfield AR, Jin F, Knudsen KA, Johnson KR, Wheelock MJ. Characterization of the interactions of alpha-catenin with alpha-actinin and beta-catenin/plakoglobin. J Cell Sci. 1997;110:1013–1022. doi: 10.1242/jcs.110.8.1013. [DOI] [PubMed] [Google Scholar]
- O'Halloran T, Beckerle MC, Burridge K. Identification of talin as a major cytoplasmic protein implicated in platelet activation. Nature. 1985;317:449–451. doi: 10.1038/317449a0. [DOI] [PubMed] [Google Scholar]
- Obama H, Ozawa M. Identification of the domain of alpha-catenin involved in its association with beta-catenin and plakoglobin. J Biol Chem. 1997;272:11017–11020. doi: 10.1074/jbc.272.17.11017. [DOI] [PubMed] [Google Scholar]
- Otto JJ. Detection of vinculin-binding proteins with an 125I-vinculin gel overlay technique. J Cell Biol. 1983;97:1283–1287. doi: 10.1083/jcb.97.4.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa M, Baribault H, Kemler R. The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO (Eur Mol Biol Organ) J. 1989;8:1711–1717. doi: 10.1002/j.1460-2075.1989.tb03563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavalko FM, Burridge K. Disruption of the actin cytoskeleton after microinjection of proteolytic fragments of alpha-actinin. J Cell Biol. 1991;114:481–491. doi: 10.1083/jcb.114.3.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid DM, Jones CE, Luo CY, Shulman NR. Immunoglobulins from normal sera bind platelet vinculin and talin and their proteolytic fragments. Blood. 1993;81:745–751. [PubMed] [Google Scholar]
- Rimm DL, Koslov ER, Kebriaei P, Cianci CD, Morrow JS. α(1)(E)-Catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc Natl Acad Sci USA. 1995;92:8813–8817. doi: 10.1073/pnas.92.19.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez Fernandez, J.L., B. Geiger, D. Salomon, and A. Ben-Ze'ev. Overexpression of vinculin suppresses cell motility in BALB/c 3T3 cells. Cell Motil Cytoskeleton. 1992;22:127–134. doi: 10.1002/cm.970220206. [DOI] [PubMed] [Google Scholar]
- Rothkegel M, Mayboroda O, Rohde M, Wucherpfennig C, Valenta R, Jockusch BM. Plant and animal profilins are functionally equivalent and stabilize microfilaments in living animal cells. J Cell Sci. 1996;109:83–90. doi: 10.1242/jcs.109.1.83. [DOI] [PubMed] [Google Scholar]
- Rüdiger, M. 1998. Vinculin and α-catenin: shared and unique functions in adherens junctions. BioEssays. In press. [DOI] [PubMed]
- Rüdiger M, Jockusch BM, Rothkegel M. Epitope tag-antibody combination useful for the detection of protein expression in prokaryotic and eukaryotic cells. Biotechniques. 1997;23:96–97. doi: 10.2144/97231bm20. [DOI] [PubMed] [Google Scholar]
- Rüdiger, A.-H., U.D. Carl, M. Rüdiger, T. Chakraborty, and J. Wehland. 1998. Interactions and post-translational modifications studied by affinity mass spectrometry. Adv. Mass Spectrom. In press.
- Schwienbacher C, Jockusch BM, Rüdiger M. Intramolecular interactions regulate serine/threonine phosphorylation of vinculin. FEBS Lett. 1996;384:71–74. doi: 10.1016/0014-5793(96)00286-4. [DOI] [PubMed] [Google Scholar]
- Sehgal RNM, Gumbiner BM, Reichardt LF. Antagonism of cell adhesion by an α-catenin mutant, and of the wnt-signaling pathway by α-catenin in Xenopusembryos. J Cell Biol. 1997;139:1033–1046. doi: 10.1083/jcb.139.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shore EM, Nelson WJ. Biosynthesis of the cell adhesion molecule uvomorulin (E-cadherin) in Madin-Darby canine kidney (MDCK) epithelial cells. J Biol Chem. 1991;266:1–9. [PubMed] [Google Scholar]
- Torres M, Stoykova A, Huber O, Chowdhury K, Bonaldo P, Mansouri A, Butz S, Kemler R, Gruss P. An alpha-E-catenin gene trap mutation defines its function in preimplantation development. Proc Natl Acad Sci USA. 1997;94:901–906. doi: 10.1073/pnas.94.3.901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volberg T, Geiger B, Kartenbeck J, Franke WW. Changes in membrane-microfilament interaction in intercellular adherens junctions upon removal of extracellular Ca2+ions. J Cell Biol. 1986;102:1832–1842. doi: 10.1083/jcb.102.5.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wachsstock DH, Wilkins JA, Lin S. Specific interaction of vinculin with alpha-actinin. Biochem Biophys Res Commun. 1987;146:554–560. doi: 10.1016/0006-291x(87)90564-x. [DOI] [PubMed] [Google Scholar]
- Weekes J, Barry ST, Critchley DR. Acidic phospholipids inhibit the intramolecular association between the N- and C-terminal regions of vinculin, exposing actin-binding and protein kinase C phosphorylation sites. Biochem J. 1996;314:827–832. doi: 10.1042/bj3140827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemann P, Giehl K, Almo SC, Fedorov AA, Girvin M, Steinberger P, Rüdiger M, Ortner M, Sippl M, Dolecek C, et al. Molecular and structural analysis of a continuous birch profilin epitope defined by a monoclonal antibody. J Biol Chem. 1996;271:29915–29921. doi: 10.1074/jbc.271.47.29915. [DOI] [PubMed] [Google Scholar]
- Wilkins JA, Chen KY, Lin S. Detection of high molecular weight vinculin binding proteins in muscle and nonmuscle tissues with and electroblot-overlay technique. Biochem Biophys Res Commun. 1983;146:554–560. doi: 10.1016/s0006-291x(83)80245-9. [DOI] [PubMed] [Google Scholar]
- Winkler J, Lünsdorf H, Jockusch BM. The ultrastructure of chicken gizzard vinculin as visualized by high resolution electron microscopy. J Struct Biol. 1996;116:270–277. doi: 10.1006/jsbi.1996.0042. [DOI] [PubMed] [Google Scholar]