Abstract
The mechanism by which cholera toxin (CT) is internalized from the plasma membrane before its intracellular reduction and subsequent activation of adenylyl cyclase is not well understood. Ganglioside GM1, the receptor for CT, is predominantly clustered in detergent-insoluble glycolipid rafts and in caveolae, noncoated, cholesterol-rich invaginations on the plasma membrane. In this study, we used filipin, a sterol-binding agent that disrupts caveolae and caveolae-like structures, to explore their role in the internalization and activation of CT in CaCo-2 human intestinal epithelial cells. When toxin internalization was quantified, only 33% of surface-bound toxin was internalized by filipin-treated cells within 1 h compared with 79% in untreated cells. However, CT activation as determined by its reduction to form the A1 peptide and CT activity as measured by cyclic AMP accumulation were inhibited in filipin-treated cells. Another sterol-binding agent, 2-hydroxy-β-cyclodextrin, gave comparable results. The cationic amphiphilic drug chlorpromazine, an inhibitor of clathrin-dependent, receptor-mediated endocytosis, however, affected neither CT internalization, activation, nor activity in contrast to its inhibitory effects on diphtheria toxin cytotoxicity. As filipin did not inhibit the latter, the two drugs appeared to distinguish between caveolae- and coated pit–mediated processes. In addition to its effects in CaCo-2 cells that express low levels of caveolin, filipin also inhibited CT activity in human epidermoid carcinoma A431 and Jurkat T lymphoma cells that are, respectively, rich in or lack caveolin. Thus, filipin inhibition correlated more closely with alterations in the biochemical characteristics of CT-bound membranes due to the interactions of filipin with cholesterol rather than with the expressed levels of caveolin and caveolar structure. Our results indicated that the internalization and activation of CT was dependent on and mediated through cholesterol- and glycolipid-rich microdomains at the plasma membrane rather than through a specific morphological structure and that these glycolipid microdomains have the necessary components required to mediate endocytosis.
The mechanism of action of cholera toxin (CT)1 in target cells involves a distinct lag period between toxin binding and adenylyl cyclase activation (reviewed in Fishman et al., 1993). During this time, the toxin must gain entry into the cell and undergo processing to generate small amounts of the A1 peptide (CT-A1), an ADP-ribosyltransferase that catalyzes the transfer of ADP-ribose from NAD+ to the stimulatory G protein, Gs. However, the molecular mechanism of intracellular toxin transport during these intervening sequences is not well understood. Toxin internalization from the plasma membrane has been the focus of many studies. We have previously shown that upon binding through its B subunit (CT-B) to cell surface ganglioside GM1, the intact holotoxin is internalized from the plasma membrane (Orlandi and Fishman, 1993). In the human intestinal epithelial cell line, CaCo-2, the disappearance of both A and B subunits from the cell surface precedes CT-A1 formation and the activation of adenylyl cyclase (Orlandi and Fishman, 1993; Orlandi, 1997).
Using cultured liver cells labeled with CT absorbed to colloidal gold (gold-CT), Montesano et al. (1982) initially identified the plasma membrane structures involved in the binding and subsequent internalization of CT as small, noncoated microinvaginations. Tran et al. (1987) obtained similar results with murine 3T3-L1 fibroblasts. They found that CT internalized through these noncoated regions subsequently enters a tubulovesicular compartment and finally multivesicular bodies. In addition, they observed that in these latter structures, CT colocalizes with ligands known to enter the cell through coated pits. Their results suggested a common intracellular pathway for ligands that enter a cell either through coated or noncoated invaginations on the plasma membrane. The involvement of these smooth membrane invaginations known as caveolae in the internalization of CT was further supported in a study of the ultrastructural distribution of GM1 in human A431 cells (Parton, 1994). GM1 is enriched fourfold in caveolae as identified by the colocalization of gold-CT-B with VIP-21/caveolin, an integral membrane protein frequently associated with caveolar structure (Dupree et al., 1993). Consequently, GM1 and caveolin have become common markers for the identification and purification of caveolae.
Our knowledge of the characteristics and cellular function of caveolae has increased considerably in recent years (reviewed in Parton, 1996; Kurzchalia and Parton, 1996). Originally caveolae were thought to function only in receptor-mediated potocytosis (Anderson et al., 1992); however, speculation of their biological role has since expanded to include such diverse functions as endocytosis independent of the coated pit pathway, sorting and internalization of GPI-anchored proteins, transcytosis, calcium signaling, and signal transduction. Purified endothelial caveolae possess elements essential for intracellular vesicular transport and signal transduction including heterotrimeric G proteins, SNAP, NSF, and GTPases and Src-family kinases (Sargiacomo et al., 1993; Schnitzer et al., 1995a ). Of particular interest is their distinct lipid composition which is enriched in cholesterol, sphingomyelin, and glycosphingolipids but devoid of phospholipids (Brown and Rose, 1992; Fiedler et al., 1993). As such, a fundamental property of these structures is their isolation in low density, detergent-insoluble complexes. The use of sterol binding agents such as filipin, nystatin, and digitonin as well as inhibitors of cholesterol metabolism, has shown that in addition to VIP-21/ caveolin, cholesterol is essential for maintaining caveolar shape and their ability to pinch off to form intracellular vesicles (Rothberg et al., 1992; Smart et al., 1994). Depletion, redistribution, or removal of plasma membrane cholesterol results in the flattening and disassembly of these invaginations, unclustering of receptors, and loss of caveolae-mediated endocytosis (Chang et al., 1992; Schnitzer et al., 1994).
Although it has been inferred from the electron micrographic studies using CT-B to localize GM1 to caveolae that the latter may be the major vehicle for toxin internalization, no evidence has been provided as yet to either directly link caveolae to toxin activation or to rule out the involvement of other subpopulations of GM1 at the cell surface. The latter are randomly distributed in minute amounts in coated pits or in glycolipid microdomains (see Parton, 1994). An earlier study by Sofer and Futerman (1995) suggested that CT is not excluded from clathrin-coated pits and may in fact represent a means by which toxin gains access to the endocytic pathway before its intracellular activation. They reported that inhibitors of the clathrin-dependent pathway, such as cationic amphiphilic drugs (CADs), also acted as partial inhibitors of CT activity. Although only a small percentage of surface-bound CT has been identified in coated pits, only minute quantities of the active CT-A1 have to be generated from the bound CT in order to elicit its cytotoxic effects (Kassis et al., 1982; Orlandi et al., 1993; Orlandi, 1997).
Glycolipid microdomains, though lacking caveolin, bear a striking similarity in composition to caveolae and are similarly enriched in detergent-insoluble extracts (Schnitzer et al., 1995b ). The significance of these domains is best illustrated in cells such as lymphocytes and neuroblastoma cells. Such cells do not express morphologically distinct caveolae and lack any detectable levels of caveolin (Fra et al., 1994; Gorodinsky and Harris, 1995; Parton and Simons, 1995). However, even in the absence of defined caveolae these cells display endocytotic and signal transduction events quite similar to caveolae-mediated functions in nonlymphoid cells (Deckert et al., 1996). As such cells bind and respond to CT (Fishman and Atikkan, 1980; Kassis et al., 1982), the mechanism by which these cells internalize and activate the toxin remains unclear. That these cells contain detergent-insoluble domains rich in cholesterol and glycosphingolipids (Parton and Simons, 1995; Parton, 1996; Simons and Ikonen, 1997), raises the possibility that such caveolae-like glycolipid domains may play a role in toxin action.
In this study, we explored the relationship between CT internalization and intracellular activation in human intestinal CaCo-2 cells as well as human Jurkat T lymphoma cells that lack caveolin and well-defined caveolae (Fra et al., 1994) and human A431 epidermoid carcinoma cells that are rich in both caveolin and caveolae (Parton, 1994). Through the use of several drugs that target their effects on the function and structural characteristics of caveolae, caveolae-like glycolipid rafts, and clathrin-coated pits, we demonstrated that the mechanism of internalization that leads to toxin activation was highly dependent on the clustering of cholesterol within glycolipid domains on the plasma membrane. Our results suggested that both caveolae and caveolae-like glycolipid rafts devoid of caveolar shape or caveolin are indistinguishable with regard to their ability to act as a vehicle for CT entry and activation.
Materials and Methods
Materials
CT, rhodamine-conjugated CT-B (Rh-CTB), diphtheria toxin (DT) and Pseudomonas exotoxin A were obtained from List Biological Laboratories (Campbell, CA). Other reagents were obtained as follows: filipin complex, chlorpromazine, and 3-isobutyl-1-methylxanthine (IBMX) from Sigma; brefeldin A (BFA) from Epicentre Technologies (Madison, WI); Na125I (carrier-free) and 125I-protein A (9.5 μCi/μg) from Dupont-New England Nuclear (Boston, MA). 2-hydroxypropyl-β-cyclodextrin was graciously provided by Dr. Peter Pentchev (National Institutes of Health, Bethesda, MD) and additionally purchased from Research Plus Inc. (Bayonne, NJ).
Cells and Cell Culture
Cells were obtained from the American Type Culture Collection (Rockville, MD). CaCo-2 cells were grown in MEM supplemented with nonessential amino acids, sodium pyruvate, 2 mM glutamine, and 20% NuSerum IV (Collaborative Biomedical, Bedford, MA; Orlandi and Fishman, 1993). A431 and Jurkat cells were grown in DME and RPMI-1640 media supplemented with 10% FBS, respectively. For assaying cAMP accumulation, CaCo-2 cells were grown in 24 × 16-mm clusters; for assaying the formation of CT-A1, in 6 × 35-mm clusters; for CT internalization and degradation experiments in 12 × 22-mm clusters; for detergent extraction, in 75 cm2 flasks; and for fluorescence microscopy, in 8-well chamber slides (Lab-Tek from Nunc, Naperville, IL).
Indirect Immunofluorescence Microscopy
CaCo-2 cells were labeled with Rh-CTB using a modification of the procedure of Sofer and Futerman (1995). In brief, cells were washed to remove any serum, incubated for 1 h at 37°C in MEM buffered with 25 mM Hepes plus 0.01% BSA with no addition; 1 μg/ml filipin; 10 μg/ml chlorpromazine; or, both together. All subsequent incubations contained the inhibitors. Cells were cooled to 15°C and incubated in the same medium containing 5 nM Rh-CT-B for 30 min. The labeled cells then were washed and either fixed immediately or incubated an additional 30 min at 37°C. The cells were fixed with 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4, for 20 min at 37°C, and washed three times in PBS. The slides were then mounted with a coverslip and the cells observed by fluorescence microscopy using a Zeiss Axiophot microscope equipped with a Plan-APOCHROMAT 63X (1.4 NA) objective and photographed with Kodak TMAX 400 film.
Accumulation of cAMP
Cells (CaCo-2 and A431 in monolayer; Jurkat in suspension) were incubated at 37°C in serum-free medium buffered with 25 mM Hepes and containing 1 mM IBMX and 0.01% BSA with 30 pM CT for 2 h unless otherwise indicated. The cells then were extracted with 0.1 M HCl, and the extracts assayed for cAMP by radioimmune assay (Zaremba and Fishman, 1984). Routinely, filipin or other drugs were added 1 h before the addition of toxin. For treatment with 2-hydroxypropyl-β-cyclo-dextrin, cells were first cultured for 48 h in serum-free MEM containing 0.1% fatty acid-free BSA. Cells were then incubated with 2-hydroxypropyl-β-cyclodextrin in serum-free medium buffered with 25 mM Hepes containing 0.01% for the indicated times.
Triton X-100 Solubility and Analysis of Detergent-insoluble Extracts
CaCo-2 or Jurkat cells (∼1 × 107) were treated with and without 1 μg/ml filipin for 1 h, and then incubated with 125I-CT at 4°C for 1 h. After washing in PBS (± filipin), the cells were pelleted by centrifuging (the CaCo-2 cells were first detached by gentle scraping). The cell pellets were then extracted with a buffer containing with 50 mM Tris-HCl, pH 7.4, 300 mM sucrose, 2 mM phenyl-methylsulfonylfluoride, and 1% Triton X-100 with or without 1 μg/ml filipin for 30 min at 4°C. The samples were then centrifuged at 10,000 g for 10 min, and the supernatants, designated as the soluble fractions, were counted for 125I-CT. Analysis of detergent extracts by floatation on continuous sucrose gradients was adapted from previously described procedures (Smart et al., 1994; Fra et al., 1994). CaCo-2 and Jurkat cell pellets (∼1 × 107 cells) were prepare as described above, and extracted for 30 min at 4°C in 1 ml of 50 mM Tris, pH 7.4, 150 mM NaCl, 1% Triton X-100 ± 1 μg/ml filipin and a mixture of protease inhibitors (5 μg/ml each of leupeptin, soybean trypsin inhibitor, and benzamidine; and 1 mM phenylmethylsulfonylfluoride). The extracts were adjusted to 40% sucrose and 2-ml portions were layered under a 10-ml 10–30% linear sucrose gradient. Samples were centrifuged for 16 h at 38,000 rpm at 4°C using a SW40 rotor. Fractions (∼0.5ml) were collected, counted for 125I-CT, and analyzed for caveolin by immunoblotting using a dot-blot apparatus (Schleicher & Schuell, Inc., Keene, NH), anti-caveolin and anti-rabbit-HRP as described below.
Other Methods
Established methods were used to determine the generation of A1 peptide by intact cells (Kassis et al., 1982) and the degradation of bound 125I-CT (Fishman, 1982). The immunological detection of cell surface CT using anti-CT-A1 antibodies (Orlandi and Fishman, 1993) was performed as previously described (Fishman, 1982). DT cytotoxicity was assayed as before (Orlandi, 1997).
Results
Effects of Filipin and Chlorpromazine on the Internalization of CT
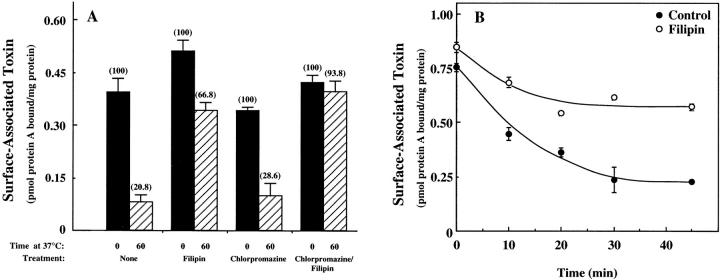
To investigate the pathway(s) of CT internalization and activation in human intestinal epithelial CaCo-2 cells, we first examined the effects of two classes of drugs on the uptake of the toxin from the cell surface. Sterol-binding agents such as filipin bind to cholesterol, a major component of glycolipid microdomains and caveolae, and disrupt caveolar structure and function (Rothberg et al., 1990, 1992). In contrast, cationic amphiphilic drugs (CADs) such as chlorpromazine act on the clathrin-dependent pathway and inhibit receptor-mediated endocytosis by reducing the number of coated pit–associated receptors at the cell surface and causing the accumulation of clathrin and AP-2 in an endosomal compartment (Wang et al., 1993; Sofer and Futerman, 1995). Cells were treated with either filipin or chlorpromazine, exposed to CT at 4°C for 30 min, washed and warmed to 37°C for 60 min. To quantify CT internalization, we used an anti-CT-A1 antiserum (Orlandi and Fishman, 1993) followed by 125I-labeled protein A to detect cell surface CT immunoreactivity. In untreated cells, 79% of the surface-bound CT was internalized after 60 min at 37°C compared with only 33% in cells continuously exposed to filipin (Fig. 1 A). Similar results were obtained when antiserum against the holotoxin was used (data not shown). Cells treated with filipin also exhibited a small increase in total toxin binding. As filipin treatment results in a flattening of the plasma membrane and a substantial loss of caveolar structure in endothelial cells (Schnitzer et al., 1994), these changes may provide better access of the toxin to GM1. In comparison to the effects observed in filipin-treated cells, chlorpromazine-treated cells exhibited a slight reduction in the level of CT binding and only marginally less toxin internalization than untreated cells (Fig. 1 A). The combination of filipin and chlorpromazine, however, resulted in nearly complete inhibition of CT internalization. The disappearance of toxin from the cell surface of both untreated and filipin-treated cells was time-dependent, but in the latter cells, was considerably slower and appeared to plateau after 20 min (Fig. 1 B).
Figure 1.
Effect of filipin and chlorpromazine on the internalization of surface-bound CT in CaCo-2 cells. (A) Cells were incubated for 1 h at 37°C in MEM, Hepes, and 0.01% BSA with no addition; 1 μg/ml filipin; 10 μg/ml chlorpromazine; or both. Then the cells were chilled to 4°C and incubated with 10 nM CT for 1 h in the same medium. Cells were either washed and exposed to anti-CT-A1 antibodies for 1 h at 4°C or incubated in medium with the same additions for 1 h at 37°C before antibody exposure. Immunodetection of surface-bound CT was quantified with 125I-protein A as described in Materials and Methods. (B) Time course of CT internalization in untreated (•) and filipin-treated (○) cells.
Differential Effects of Filipin and Chlorpromazine on CT Activation in CaCo-2 Cells
We next examined the effects of filipin on toxin activation and activity. Although the majority of surface-bound toxin entered the cell through a filipin-sensitive mechanism, repeated experiments suggested that even in the presence of filipin, ∼30–40% of the toxin appeared to be internalized (as judged by its loss of immunological reactivity with anti-CT-A1 and -CT antibodies within 60 min at 37°C. Although the bulk of cell surface GM1 is localized in caveolae, some also is distributed among nondescript, more homogeneous regions of the plasma membrane, and to a far lesser degree within coated pits (Parton, 1994). It was necessary, therefore, to determine whether filipin affected the ability of CT to activate adenylyl cyclase as only minute amounts of internalized toxin are required to exert its cytotoxic effects on target cells. Whereas cells treated with 1 μg/ml filipin (1.53 μM) had exhibited a slight increase in toxin binding, they displayed a complete inhibition of CT stimulation of cAMP accumulation (Fig. 2 A). The inhibition of CT activity by filipin was concentration-dependent with an IC50 of 0.5 μM, and was not due to the inhibition of adenylyl cyclase itself as cAMP accumulation stimulated by 100 μM forskolin was similar in untreated and filipin-treated cells (data not shown). Neither chlorpromazine nor imipramine (another CAD) at concentrations as high as 50 μM significantly affected the CT-stimulated cAMP response (Fig. 2 A). Only at concentrations >100 μM did these drugs begin to cause a decrease in toxin activity. These results are in contrast to a study on CT activity in hippocampal neurons, in which the CADs chlorpromazine (25 μM), imipramine (100 μM), and sphingosine (5 μM) are found to partially inhibit CT-stimulated cAMP accumulation by 45, 29, and 31%, respectively (Sofer and Futerman, 1995).
Figure 2.
Inhibition of toxin activity and activation in CaCo-2 cells by filipin but not CADs. (A) CaCo-2 cells were treated with increasing concentrations of filipin (•), chlorpromazine (○), or imipramine (▪) for 1 h at 37°C. The cells were then incubated an additional 2 h at 37°C with 0.03 nM CT and assayed for cAMP accumulation as described in Materials and Methods. (B) CaCo-2 cells were incubated for 1 h at 37°C in the absence (UNTREATED) or presence of the indicated effector (1 μg/ml BFA and filipin; 10 μg/ml chlorpromazine and imipramine). The cells then were incubated with 125I-CT for 1 h at 4°C in media containing the effector, washed and either harvested or incubated an additional 1 h at 37°C in fresh medium with effectors. All cells were then analyzed for the formation of CT-A1 peptide as described in Materials and Methods.
The activation of adenylyl cyclase and the concomitant increase in the intracellular levels of cAMP by CT requires that the internalized toxin first be reduced to form small amounts of the A1 peptide (Kassis et al., 1982). CaCo-2 cells treated with filipin were unable to generate any detectable levels of CT-A1 compared with untreated cells (Fig. 2 B). The ability of filipin to block CT reduction was similar to that of BFA (Fig. 2 B; Orlandi et al., 1993). Neither chlorpromazine nor imipramine had any effect on the formation of CT-A1.
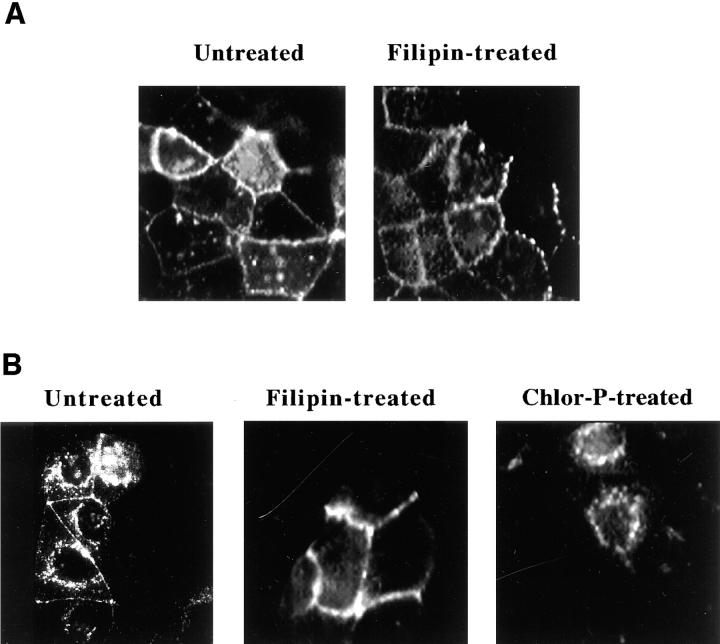
Fluorescence microscopy further illustrated the contrasting effects of filipin and chlorpromazine on toxin internalization and activation. To monitor CT distribution in the presence of these effectors, control and treated cells were labeled with Rh-CT-B at 15°C and incubated for 30 min at 37°C. Control cells exhibited a largely perinuclear fluorescence pattern with a concomitant loss of fluorescence at the plasma membrane (Fig. 3). In contrast, Rh-CT-B was found only at the cell surface of filipin-treated cells indicating that these cells were unable to significantly internalize it. Other fields from filipin-treated cells occasionally showed some internalized fluorescence; however, the patterns were diffuse and remained in the region underlying the plasma membrane. Chlorpromazine-treated cells displayed a perinuclear fluorescent pattern similar to untreated cells. These results were in agreement with CT internalization and activation data shown in Figs. 1 and 2, respectively.
Figure 3.
Effects of filipin and chlorpromazine on the distribution of rhodamine-conjugated CT-B in CaCo-2 cells detected by direct fluorescence microscopy. Cells were incubated for 1 h at 37°C in MEM, Hepes, and 0.01% BSA with no addition; 1 μg/ml filipin; or 25 μg/ml chlorpromazine. The cells were then labeled with 5 nM Rh-CT-B for 30 min at 15°C in fresh medium in the presence or absence of the same effectors. Cells were washed once and incubated an additional 30 min at 4°C (A) or 37°C (B) in fresh medium with effectors and fixed. The distribution of Rh-CT-B was then visualized by direct fluorescence as described in Materials and Methods.
Examination of the Relationship Between Caveolin, Caveolae, and Cholesterol in CT Activation
Thus far, the results with filipin indicated that CT internalization and activation occurred through cholesterol-rich glycolipid microdomains to include the possible involvement of caveolae. To examine this relationship further, we next compared the effects of filipin on CT activation in CaCo-2, A431, and Jurkat T-lymphoma cells. While it is well established that A431 cells contain caveolin and caveolae, Jurkat cells express neither. Studies with CaCo-2 cells, however, have produced conflicting reports on the presence of caveolin and caveolae (Mayor et al., 1994; Mirre et al., 1996). In agreement with Mayor et al. (1994), we confirmed the presence of low levels of caveolin in these cells by RT-PCR, indirect immunofluorescence, and immunoblotting albeit at a considerably lower level than that expressed by A431 cells and in contrast to its known absence in Jurkat cells (data not shown). For detection of caveolin by RT-PCR, synthetic primers were designed from the 5′ and 3′ sequences of human caveolin mRNA that encompassed a portion of the 5′-flanking region and the first 97 amino acids of the protein (Glenney, 1992). The 5′-nucleotide sequence was (sense strand from 5′ to 3′) TTCATCCAGCCACGGGCCAGCATGTCTGGG. The 3′-nucleotide sequence was (antisense strand from 5′ to 3′) CTTCCAAATGCCGTGAAAACTGTGTGTCCC. Indirect immunofluorescence and immunoblotting assays were performed using affinity-purified polyclonal rabbit antisera against the first 97 amino acids of human caveolin obtained from Transduction Laboratories (Lexington, KY).
As one of the markers predominantly used to define the presence of caveolar structure, caveolin expression in CaCo-2 cells suggested the presence of caveolae and hence their possible role in facilitating toxin entry.
When CaCo-2, A431 and Jurkat cells were treated with various concentrations of filipin before their exposure to CT, the concentration-dependent effects of filipin on the activity of CT were found to differ only slightly (Fig. 4). Thus, the interaction of filipin with plasma membrane cholesterol and its inhibitory effects on CT internalization and activation were found to occur in cells that do not express caveolin and caveolae as well as those that express high levels of caveolin (and well-defined caveolae). Furthermore, we found that in filipin-treated Jurkat cells, CT-A1 formation was totally blocked (data not shown), indicating that the same mechanism of inhibition was occurring in both caveolin-positive and -negative cells.
Figure 4.
Comparison of the concentration-dependent effects of filipin on CT activity in CaCo-2 (•), A431 (○) and Jurkat (□) cells. Cells were treated with increasing concentrations of filipin for 1 h at 37°C, then incubated an additional 2 h at 37°C with 0.03 nM CT and assayed for cAMP accumulation as described in Materials and Methods.
Further Differentiation of CT Internalization Through Coated and Noncoated Pathways
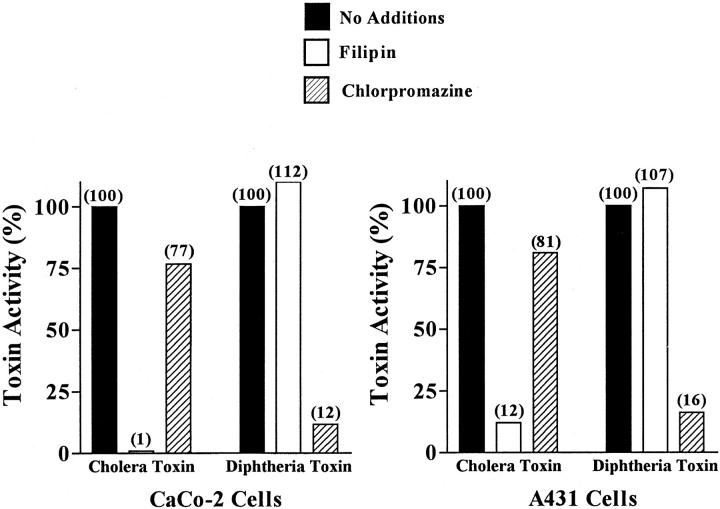
We next used filipin and chlorpromazine to further differentiate the distinct pathways of internalization for CT and DT, another ADP-ribosylating toxin. DT enters the endocytic pathway of target cells through clathrin-coated pits (Moya et al., 1985; Beaumelle et al., 1992). Whereas CT activity was inhibited >95% in filipin-treated CaCo-2 cells, DT activity was slightly enhanced (∼110%; Fig. 5). However, the converse was observed when cells were exposed to 25 μg/ml (70 μM) chlorpromazine. DT cytotoxicity was inhibited by ∼88% whereas CT activity exhibited only a 20% decrease. A similar pattern of results was obtained when A431 cells were treated with either filipin or chlorpromazine and then DT (Fig. 5). Comparable results also were obtained with a third ADP-ribosylating toxin, Pseudomonas exotoxin A, that enters cells through clathrin-coated pits but then follows the same retrograde pathway as CT to the ER, both toxins being blocked by BFA in contrast to DT which is not (see Orlandi et al., 1993). Exotoxin A blocked protein synthesis in control and filipin-treated A431 cells with EC50 values of 90 and 71 ng/ml whereas in chlorpromazine-treated cells, the EC50 was shifted to 402 ng/ml.
Figure 5.
Selective effects of filipin and chlorpromazine on the activity of cholera and diphtheria toxins. CaCo-2 and A431 cells were incubated in the absence or presence of 1 μg/ml filipin or 25 μg/ml chlorpromazine for 1 h at 37°C. Then the cells were exposed to either 0.03 nM CT or 100 ng/ml DT for an additional 2 h at 37°C and assayed for CT stimulation of cAMP accumulation or DT inhibition of protein synthesis as described in Materials and Methods. The results represent the mean ± SEM of two experiments, each done in triplicate. Absolute inhibition of protein synthesis in DT-treated cells was between 30–50%.
The Effects of a Cyclodextrin on CT Internalization and Activation
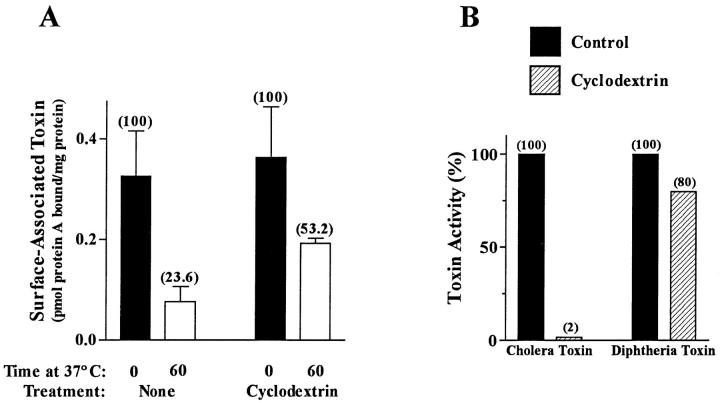
To further explore the role of plasma membrane cholesterol, particularly that associated with detergent-insoluble glycolipid microdomains and caveolae, in mediating CT internalization and activation, we performed similar experiments using another sterol binding agent, 2-hydroxypropyl-β-cyclodextrin. Cyclodextrin has been shown to specifically remove cholesterol from the plasma membrane (Neufeld et al., 1996). Whereas treatment of CaCo-2 cells with cyclodextrin at 100 mg/ml had no significant effect on toxin binding, the internalization of CT was inhibited (Fig. 6 A). As a consequence of this effect, CaCo-2 cells exposed to cyclodextrin no longer responded to CT as measured by cAMP accumulation (Fig. 6 B). As was observed with filipin treatment, the inhibitory effects of cyclodextrin were also selective as the treated CaCo-2 cells retained their sensitivity to DT (Fig. 6 B).
Figure 6.
Effects of cyclodextrin on CT internalization and activation. CaCo-2 cells were cultured for 48 h in serum-free medium containing 0.1% BSA and then incubated for 4 h in serum-free medium containing 0.01% BSA without and with 100 mg/ml cyclodextrin at 37°C. Cells were then assayed for either CT internalization (A) as described in the legend to Fig. 1; or CT and DT cytotoxicities (B) as described in the legend to Fig. 5. Absolute inhibition of protein synthesis in DT-treated cells was 45%.
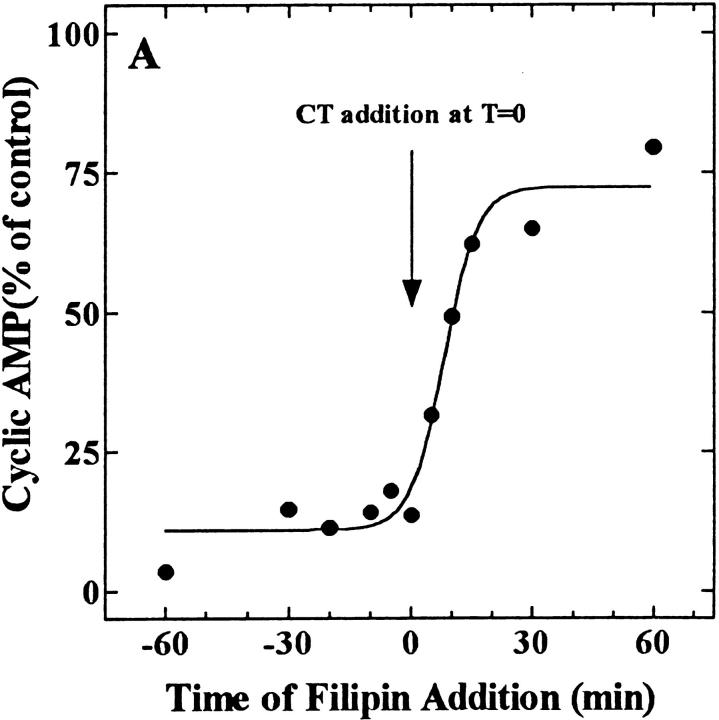
Filipin-induced Inhibition of CT Activity Was Time-dependent and Reversible
Filipin was an effective inhibitor when added to cells either before, or simultaneously with CT (Fig. 7 A). However, the greater the time span between the addition of toxin and the subsequent addition of filipin the less effective filipin was in preventing CT-stimulated cAMP accumulation. In contrast to the nearly complete inhibition of CT action when both were added simultaneously, the addition of filipin as little as 5 min after the toxin resulted in only a 50% inhibition of CT activity. These results suggested that although filipin could rapidly prevent the internalization of CT from the cell surface, once some toxin was internalized (an equally rapid event, see Fig. 1 B), filipin became less effective at preventing the intracellular processing and action of the toxin.
Figure 7.
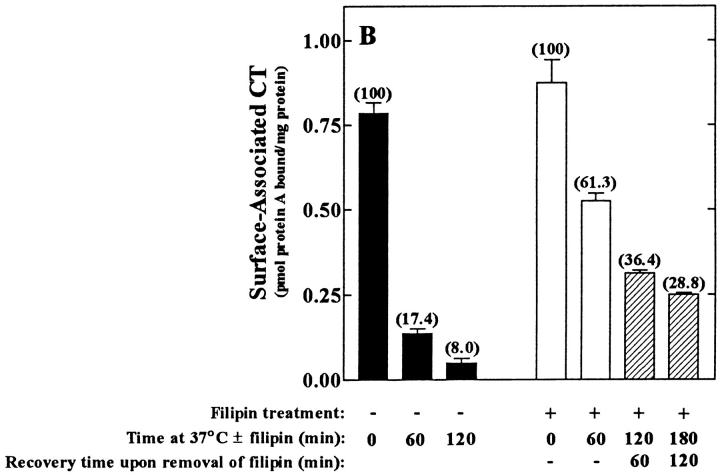
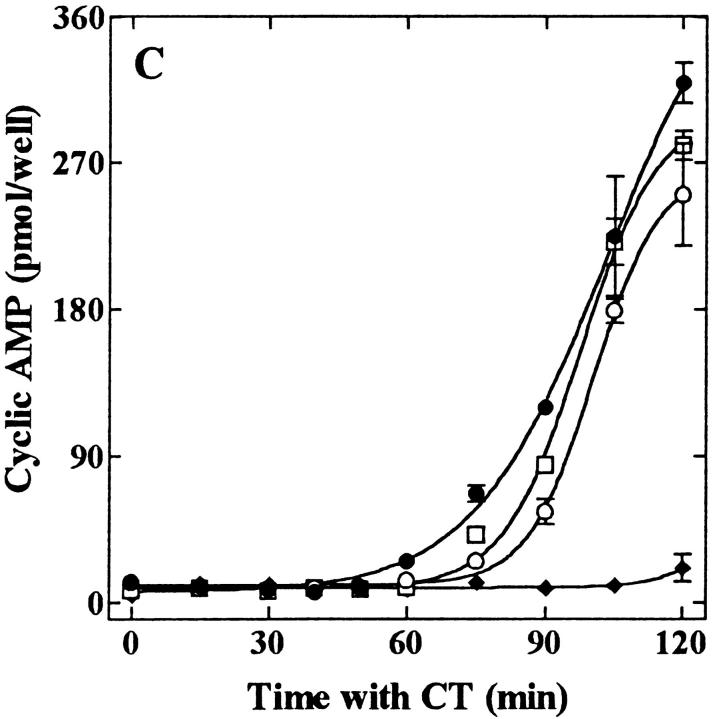
Filipin-mediated inhibition of CT activity in CaCo-2 cells is both rapid (A) and reversible (B and C). (A) Filipin (1 μg/ml) was added to cells either before (− values) or after (+ values) the addition of CT (0.03 nM, final). All the cells were exposed to the toxin for 2 h at 37°C and assayed for the accumulation of cAMP. Results are expressed as a percentage of cAMP formed in the absence of filipin. (B) Cells were treated in the absence (filled bars) or presence (open and cross-hatched bars) of 1 μg/ml filipin for the indicated times at 37°C, chilled to 4°C, and incubated with 10 nM CT for 1 h ± filipin. Cells were either exposed to anti-CT-A1 at 4°C (zero time) or incubated at 37°C with the same additions for the indicated times before antibody exposure. Some of the filipin-treated cells (hatched bars) were washed and incubated in filipin-free medium for an additional 60 and 120 min at 37°C before the addition of antibody at 4°C. Immunodetection of surface-bound CT was quantified with 125I-protein A as described in Materials and Methods. (C) Cells were treated in the presence (♦, ○, □) or absence (•) of 1 μg/ml filipin for 1 h at 37°C and some of the cells were either washed briefly (○); or, for 30 min (□) in filipin-free medium at 37°C. Then all of the cells were incubated with 0.03 nM CT for the indicated times at 37°C and assayed for cAMP accumulation as described in Materials and Methods.
While the results presented above showed that the inhibitory action of filipin was quite rapid, the reversal of its effects was equally rapid and again directly related to the renewed ability of the cells to internalize surface-bound toxin (Fig. 7, B and C). Whereas cells exposed to filipin internalized only 30–40% of the bound toxin, without the formation of A1 peptide, those same cells when placed in fresh medium for 60 and 120 min at 37°C, renewed the uptake of surface-bound toxin to levels approaching those observed in untreated cells (Fig. 7 B) and generated significant levels of A1 peptide (data not shown). Additionally, surface-bound CT did not lose its activity in the presence of filipin. CaCo-2 cells were incubated with CT at 4°C for 1 h, washed to remove unbound toxin and incubated in medium containing filipin at 37°C for 1 h. After the removal of filipin from the culture medium, CT internalization and activation of adenylyl cyclase were restored (Table I). Two hours after the removal of filipin, cAMP levels reached 75–80% of the levels found in cells not exposed to filipin (compare 197 versus 261 pmol cAMP/well). However, the observed lag period between toxin exposure and the onset of cAMP accumulation was increased by ∼15 min. Longer periods of time in filipin-free medium resulted in a shift towards a normal lag period and an even greater recovery (Fig. 7 C).
Table I.
Effects of Filipin on the Activity of Surface-Bound CT in CaCo-2 Cells
| Treatments | CT stimulation of cAMP | |||||
|---|---|---|---|---|---|---|
| CT | Filipin | 2 h | 3 h | |||
| pmol/well | ||||||
| − | − | 9 ± 0.4 | 12 ± 2 | |||
| + | − | 261 ± 7.3 (100) | 485 ± 15 (100) | |||
| + | + | 40 ± 6 (12) | 96 ± 10 (18) | |||
| + | ± | 42 ± 2 (13) | 197 ± 23 (39) | |||
CaCo-2 cells were chilled to 4°C, incubated without (−) and with (+) 0.03 nM CT for 1 h at 4°C, washed and incubated at 37°C for the times indicated in the presence (+) or absence (−) of 1 μg/ml filipin. After 1 h at 37°C, one set of filipin-treated cells (±) were washed and further incubated in filipin-free medium at 37°C for 1 or 2 h. All cells were then assayed for cAMP accumulation as described in Materials and Methods. Values in parentheses denote the net accumulation of cAMP in response to CT as a percent of untreated cells.
Filipin Prevented Toxin Degradation
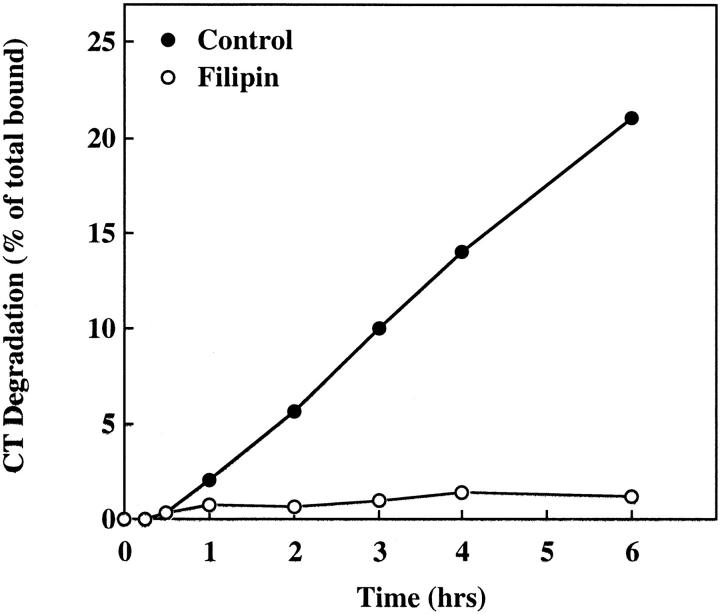
We also compared the rates of CT degradation in untreated and filipin-treated cells. Although only a small percentage of bound CT is required to exert its cytotoxic effects, the majority of internalized toxin is degraded (Fishman, 1982; Orlandi et al., 1993). To measure the rates of CT degradation, cells were treated with or without filipin for 60 min at 37°C, incubated with 125I-CT at 4°C, washed, and warmed to 37°C for the indicated times in fresh medium with or without filipin. The medium was then analyzed for TCA-soluble radioactivity (Fishman, 1982). Consistent with the reduced ability of CT to become internalized from the cell surface in the presence of filipin, toxin degradation was likewise inhibited. Whereas 21% of CT was degraded in untreated cells over a 6 h period at 37°C, no more than 1% was degraded over the same time period in cells continually exposed to filipin (Fig. 8).
Figure 8.
Filipin inhibits degradation of CT by CaCo-2 cells. Untreated cells (•) and cells treated with 1 μg/ml filipin (○) were incubated with 125I-CT at 4°C, washed to remove any unbound toxin, and incubated with fresh medium with or without filipin at 37°C for the indicated times. Toxin degradation was determined by the levels of TCA-soluble radioactivity that accumulated in the culture medium with time.
The Effects of Filipin on the Detergent Extraction of CT Bound to Cells and on CT in Detergent-resistant Complexes
The interactions between CT and its receptor GM1 at the cell surface were further assessed by examining the effects of filipin on the Triton X-100 solubility of CT bound to cells at 4°C. Hagmann and Fishman (1982) had shown that the majority of CT–ganglioside complexes formed in membranes and intact cells are resistant to extraction with Triton X-100. Likewise, caveolae and glycolipid microdomains, enriched in both ganglioside GM1 and cholesterol have been characterized in terms of their detergent insolubility in the presence of Triton X-100 (Brown and Rose, 1992). Similar to the results of Hagmann and Fishman (1982), when 125I-CT was bound to CaCo-2 cells at 4°C and extracted with 1%Triton X-100 for 30 min at 4°C, 33.7 ± 3.7% of the toxin was found in the soluble fraction. In contrast, 46.6 ± 3.5% of the bound toxin was extracted into the soluble phase in cells treated with 1 μg/ml filipin. The effects of filipin on membrane-bound toxin solubility was even more pronounced in Jurkat cells. In the presence of filipin, >97% of the membrane-bound toxin was found in the soluble fraction compared with 47% in untreated cells.
Triton X-100 detergent extracts of CaCo-2 and Jurkat cells labeled with 125I-CT at 4°C were also analyzed by floatation on continuous sucrose gradients (Fig. 9). Under these conditions, CT–GM1 complexes formed at the cell surface were identified in a peak of radioactivity in the upper regions of the gradient representing detergent-insoluble glycolipid domains (Fig. 9). However, when both cell lines were treated with filipin this detergent-insoluble region displayed a noticeable shift to heavier buoyant densities. The shift in density of the CT-bound peaks indicated that the filipin–sterol complexes formed within cell surface caveolae and glycolipid microdomains may have altered the compositional or biophysical characteristics of these domains. The formation of large peristomal rings of sterols in the presence of filipin as described by Simionescu et al. (1983) may have contributed to this shift in buoyant density. We also analyzed the gradient fractions derived from CaCo-2 cells for caveolin by immunoblotting and found that caveolin colocalized with 125I-CT in extracts from both control and filipin-treated cells (data not shown).
Figure 9.
Analysis of Triton X-100 extracts from CaCo-2 and Jurkat cells on sucrose gradients. Untreated (•) and 1 μg/ml filipin-treated (○) CaCo-2 and Jurkat cells were incubated with 125I-CT for 1 h at 4°C, washed in PBS, scraped, and pelleted. After the cells were extracted for 30 min at 4°C in 1% Triton X-100 solution ± 1 μg/ml filipin, the extracts were centrifuged on a 10–30% linear sucrose gradient as described in Materials and Methods. Fractions (∼0.5 ml) were collected from the top of the gradient and counted for 125I.
Discussion
Based on recent studies of CT activation and intracellular transport (Orlandi et al., 1993, 1997; Lencer et al., 1995; Majoul et al., 1996; Sandvig et al., 1996), a clearer understanding is slowly emerging of the molecular events that occur between toxin binding to ganglioside GM1 on the cell surface and its ultimate activation of adenylate cyclase. Of particular interest is the mechanism by which bound CT is internalized and ultimately targeted to those intracellular sites necessary for its activation. Although most of the bound CT is rapidly internalized from the cell surface, only a small percentage of the internalized toxin is actually responsible for the activation of adenylyl cyclase, the majority of internalized toxin being destined for degradation (Fishman, 1982; Kassis et al., 1982; Orlandi and Fishman, 1993; Orlandi et al., 1993). In this regard, the elegant electron micrographic studies on the internalization and localization of CT and the cell surface distribution of GM1 (Montesano et al., 1982; Tran et al., 1987; Parton, 1994) do not resolve whether more than one pathway exists for toxin entry and subsequent activation.
In this study, we used the polyene antibiotic filipin to show not only that the internalization of CT was mediated by caveolae or caveolae-like domains on the cell surface but that the activation of CT was dependent on its entry through these structures. Filipin interacts with 3-β-hydroxysterols such as cholesterol in the plasma membrane to form planar sterol–filipin complexes and peristomal rings of sterols. Cholesterol is a major component of cell-surface microdomains frequently described in the literature as detergent-insoluble glycolipid-enriched complexes, DIGs, and caveolae (Simons and Ikonen, 1997). The endocytic functions of these entities are influenced by the presence and state of cholesterol (Rothberg et al., 1990; Schnitzer et al., 1994).
We observed that in the presence of filipin, the internalization of surface bound CT in CaCo-2 cells was inhibited using two different assays, a quantitative one using anti-CT-A1 antibodies and 125I-protein A, and direct fluorescence with Rh-CT-B. This inhibition in turn resulted in the blocking of subsequent steps in the intracellular processing of the toxin. These included the inability of the toxin to be reduced to the active A1 peptide, and thereby to activate adenylyl cyclase. In addition, the degradation of CT was blocked in filipin-treated CaCo-2 cells. These effects of filipin are consistent with its primary action being the disruption of the cholesterol-rich microdomains localized to caveolae and caveolae-like structures on the cell surface. In this regard, another sterol binding agent, cyclodextrin, had similar effects on CT internalization and activity.
Further supporting such a mode of action are our observations that the effects of filipin were both rapid and reversible. We found that even when the cells were exposed to filipin and CT at the same time, their response to the toxin was effectively inhibited. When filipin was added after CT, the cells became resistant to its inhibitory effects. Thus, once the toxin has become internalized, it is no longer sensitive to the effects of filipin. The cells also recovered rapidly from the effects of filipin; when they were exposed to CT immediately after being removed from filipin-containing medium, they accumulated almost as much cAMP as control cells. In this regard, CT bound to the surface of filipin-treated cells did not become inactive as once the medium was replaced with filipin-free medium, the cells were able to respond to the toxin by accumulating cAMP. Taken together with the surface labeling experiments, it is clear that filipin treatment has little effect on the ability of CT to bind to its receptor GM1 but prevents the toxin–ganglioside complexes from entering the internalization pathway.
In contrast to the importance of caveolae and caveolae-like microdomains, clathrin-coated pits did not play a significant role in the activation of CT. While the majority of bound CT remained at the cell surface in the presence of filipin, a small percentage still appeared to be internalized (as judged by the loss of cell surface immunoreactivity with CT-A1 antisera). These results may reflect the inability of filipin-treated cells to form fully competent endocytic vesicles capable of transporting toxin from the cell surface. Consequently, a portion of the bound toxin may have become trapped in incompletely-formed vesicles at or near the plasma membrane and were inaccessible to antibody. Another possible explanation may be that a portion of the membrane-bound toxin in these cells was internalized through the coated-pit dependent pathway. We addressed this possibility using CADs that have been shown to reduce the number of cell-surface coated pits and inhibit receptor-mediated endocytosis. When CaCo-2 cells were exposed to CADs such as chlorpromazine, slightly depressed levels of toxin binding and uptake were observed while the combination of chlorpromazine and filipin resulted in the nearly complete inhibition of toxin uptake from the cell surface. These findings suggested some CT was taken up through coated pits, although its entry via this mechanism was not a functional pathway for toxin activation. This was consistent with an early study demonstrating that CT entering cells via transferrin receptors is unable to activate adenylyl cyclase (Pacuszka and Fishman, 1992). Additionally, the contrasting effects of chlorpromazine and filipin (as well as cyclodextrin) on the activity of both CT and DT emphasized the distinct differences between the internalization of DT through clathrin-coated pits and the apparent dependence of CT on caveolae or caveolae-like domains.
Caveolin is both a marker for caveolae and appears to be essential for caveolae formation (Parton, 1996). Not only does caveolin have a high affinity for cholesterol, but appears to interact with GM1 in caveolae (Fra et al., 1995). In this study, we used three different cell lines (CaCo-2, A431, and Jurkat) that express varying levels of caveolin and caveolae to examine the relationship between CT internalization and caveolae and caveolae-like function. While it had previously been reported that CaCo-2 cells do not express caveolin, we have shown here that low levels of the protein are in fact present in this cell line. In all likelihood, the discrepance between our results and those reported by Mirre et al. (1996) may be attributed to the specificity of the reagents and sensitivity of the assays used. Although CaCo-2 cells express low levels of caveolin, our results indicated that it was not required for the activation of CT. We also found that CT was reduced to its A1 peptide and stimulated cAMP accumulation in human Jurkat T lymphoma cells that lack caveolin and caveolae (Fra et al., 1994). The ability of filipin to block the activation and action of CT in Jurkat cells indicates that the complex lipid microdomains of cholesterol, sphingomyelin and glycolipids are the essential plasma membrane entities for a cellular response to the toxin. In this regard, the detergent-resistant properties of cell surface-bound 125I-CT were dramatically altered in both Jurkat and CaCo-2 cells treated with filipin.
The mechanism of CT internalization may be related to the cross-linking of ganglioside GM1 by the pentavalent binding of CT-B. The clustering of GM1–CT complexes in turn may facilitate and enhance GM1-cholesterol interactions and subsequently lead to sequestration within caveolae or caveolae-like domains. In his study on the distribution of GM1 in A431 cells, Parton (1994) suggested that the increased colocalization of GM1 and caveolin within caveolar structures may be related to toxin binding. Thus, 44% of the plasma membrane gold-CT-B is found associated with caveolae in cells labeled at 8°C and then fixed and embedded compared with 22% by post-embedding labeling techniques. This observation is wholly consistent with studies on the distribution of GPI-anchored proteins on the plasma membrane (Mayor et al., 1994; Schnitzer et al., 1995b ). Whereas GPI-anchored proteins were found to reside in microdomains distinct from caveolae, enrichment or partitioning into or near these structures has been observed only upon cross-linking. The increased localization of surface-bound CT into these microdomains initiated by cross-linking of its ganglioside GM1 receptor may trigger toxin internalization. In the presence of filipin, GM1 as well as CT–GM1 complexes may be less able to interact with cholesterol in these microdomains. The increase in detergent extraction of 125I-CT bound to filipin-treated cells and the change in its buoyant density are consistent with such an effect.
Although additional studies are necessary to fully understand the molecular mechanisms that drive the formation and internalization of caveolae and caveolae-like domains, it is quite apparent that they play a significant role in clathrin-independent receptor-mediated endocytosis and signal transduction. The inherent biochemical characteristics of glycolipids and their interactions with other membrane components have long been suspected of aiding in directed intracellular membrane trafficking, particularly in epithelial cells in light of their polar distribution among apical and baso-lateral membrane domains (Simons and van Meer, 1988). It is not surprising then that the glycosphingolipid and cholesterol components of caveolae and caveolae-like domains play a similar role in mediating the endocytic function of these entities. This is evident from the results presented here as well as within the literature that alterations in plasma membrane cholesterol affect clathrin-independent receptor-mediated endocytosis. Likewise, as a core component of caveolae and caveolae-like domains, ganglioside GM1 possesses a similar influence. Although GM1-oligosaccharide provides the recognition site for CT binding, Pacuszka et al. (1991) demonstrated that the nature of the lipid moiety plays an equally essential role in directing CT internalization and activation. Thus, a cholesterol derivative of GM1 is a more effective receptor than native GM1 whereas phospholipid derivatives of GM1 are less effective receptors. The latter observation may be particularly relevant as glycolipid-rich domains are depleted of phospholipids (Fiedler et al., 1993).
This study reinforces these results. Our findings indicated that the internalization and activation of CT was dependent on and mediated through cholesterol- and glycolipid-rich microdomains at the plasma membrane. Whereas this event probably occurs through specific morphological structures such as caveolae in certain cell lines, DIGs also appear to contain all the necessary components to mediate toxin endocytosis. The mechanism by which toxin internalized through these structures is subsequently targeted to sites necessary for activation awaits further study.
Abbreviations used in this paper
- BFA
brefeldin A
- CAD
cationic amphiphilic drug
- CT
cholera toxin
- CT-A,-A1
and -B, A subunit, A1 peptide, and B subunit of CT, respectively
- DIG
detergent-insoluble glycolipid-enriched complexes
- DT
diphtheria toxin
- IBMX
3-isobutyl-1-methylxanthine
- Rh-CTB
rhodamine-conjugated CT-B
Footnotes
Address all correspondence to Palmer A. Orlandi, Food and Drug Administration, CFSAN/OPDFB/DVA/VMB/HFS-327, 200 C. Street, Washington, DC 20204. Tel.: (202) 205-4460. Fax: (202) 205-4939.
References
- Anderson RGW, Kamen BA, Rothberg KG, Lacey SW. Potocytosis: sequestration and transport of small molecules by caveolae. Science. 1992;255:410–411. doi: 10.1126/science.1310359. [DOI] [PubMed] [Google Scholar]
- Beaumelle B, Bensammar L, Bienvenüe A. Selective translocation of the A chain of diphtheria toxin across the membrane of purified endosomes. J Biol Chem. 1992;267:11525–11531. [PubMed] [Google Scholar]
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Chang W-J, Rothberg K, Kamen BA, Anderson RGW. Lowering the cholesterol content of MA104 cells inhibits receptor-mediated transport of folate. J Cell Biol. 1992;118:63–69. doi: 10.1083/jcb.118.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckert M, Tiocchioni M, Bernard A. Endocytosis of GPI- anchored proteins in human lymphocytes: role of glycolipid-based domains, actin cytoskeleton, and protein kinases. J Cell Biol. 1996;133:791–799. doi: 10.1083/jcb.133.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupree P, Parton RG, Raposo G, Kurzchalia TV, Simons K. Caveolae and sorting in the trans-Golgi network of epithelial cells. EMBO (Eur Mol Biol Organ) J. 1993;12:1597–1605. doi: 10.1002/j.1460-2075.1993.tb05804.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler K, Kobayashi T, Kurzchalia TV, Simons K. Glycosphingolipid-enriched, detergent-insoluble complexes in protein sorting in epithelial cells. Biochemistry. 1993;32:6365–6373. doi: 10.1021/bi00076a009. [DOI] [PubMed] [Google Scholar]
- Fishman PH. Internalization and degradation of cholera toxin by cultured cells; relationship to toxin action. J Cell Biol. 1982;93:860–865. doi: 10.1083/jcb.93.3.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman PH, Atikkan EA. Mechanism of action of cholera toxin: effect of receptor density and multivalent binding on activation of adenylate cyclase. J Membr Biol. 1980;54:51–60. doi: 10.1007/BF01875376. [DOI] [PubMed] [Google Scholar]
- Fishman PH, Pacuszka T, Orlandi PA. Gangliosides as receptors for bacterial toxins. Adv Lipid Res. 1993;25:165–187. [PubMed] [Google Scholar]
- Fra AM, Williamson E, Simons K, Parton RG. Detergent-insoluble glycolipid microdomains in lymphocytes in the absence of caveolae. J Biol Chem. 1994;269:30745–30748. [PubMed] [Google Scholar]
- Fra AM, Masserini M, Palestini P, Sonnino S, Simons K. A photo-reactive derivative of ganglioside GM1 specifically cross-links VIP21-caveolin on the cell surface. FEBS Lett. 1995;375:11–14. doi: 10.1016/0014-5793(95)95228-o. [DOI] [PubMed] [Google Scholar]
- Glenney JR. The sequence of human caveolin reveals identity with VIP21, a component of transport vesicles. FEBS Lett. 1992;314:45–48. doi: 10.1016/0014-5793(92)81458-x. [DOI] [PubMed] [Google Scholar]
- Gorodinsky A, Harris DA. Glycolipid-anchored proteins in neuroblastoma cells form detergent-resistant complexes without caveolin. J Cell Biol. 1995;129:619–627. doi: 10.1083/jcb.129.3.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagmann J, Fishman PH. Detergent extraction of cholera toxin and gangliosides from cultured cells and isolated membranes. Biochem Biophys Acta. 1982;720:181–187. doi: 10.1016/0167-4889(82)90010-6. [DOI] [PubMed] [Google Scholar]
- Kassis S, Hagmann J, Fishman PH, Chang PP, Moss J. Mechanism of action of cholera toxin on intact cells. J Biol Chem. 1982;257:12148–12152. [PubMed] [Google Scholar]
- Kurzchalia TV, Parton RG. And still they are moving....dynamic properties of caveolae. FEBS Lett. 1996;389:52–54. doi: 10.1016/0014-5793(96)00585-6. [DOI] [PubMed] [Google Scholar]
- Lencer WI, Constable C, Moe S, Jobling MG, Webb HM, Ruston S, Madara JL, Hirst TR, Holmes RK. Targeting of cholera toxin and Escherichia coliheat labile toxin in polarized epithelia: role of COOH-terminal KDEL. J Cell Biol. 1995;131:951–962. doi: 10.1083/jcb.131.4.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majoul IV, Bastiaens PIH, Soling H-D. Transport of external lys-asp-glu-leu (KDEL) protein from the plasma membrane to the endoplasmic reticulum: studies with cholera toxin. J Cell Biol. 1996;133:777–789. doi: 10.1083/jcb.133.4.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor S, Rothberg K, Maxfield FR. Sequestration of GPI- anchored proteins in caveolae triggered by cross-linking. Science. 1994;264:1948–1951. doi: 10.1126/science.7516582. [DOI] [PubMed] [Google Scholar]
- Mirre C, Monlauzeur L, Garcia M, Delgrossi M-H, Le Bivic A. Detergent-resistant membrane microdomains from CaCo-2 cells do not contain caveolin. Am J Physiol. 1996;271:C887–C894. doi: 10.1152/ajpcell.1996.271.3.C887. [DOI] [PubMed] [Google Scholar]
- Montesano R, Roth J, Robert A, Orci L. Non-coated membrane invaginations are involved in binding and internalization of cholera and tetanus toxins. Nature. 1982;296:651–653. doi: 10.1038/296651a0. [DOI] [PubMed] [Google Scholar]
- Moya M, Dautry-Varsat A, Goud B, Louvard D, Boquet P. Inhibition of coated pit formation in hep2cells blocks the cytotoxicity of diphtheria toxin but not ricin toxin. J Cell Biol. 1985;101:548–559. doi: 10.1083/jcb.101.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld EB, Cooney AD, Pitha J, Dawidowicz EA, Dwyer NK, Pentchev PG, Blanchette-Mackie EJ. Intracellular trafficking of cholesterol monitored with a cyclodextrin. J Biol Chem. 1996;271:21604–21613. doi: 10.1074/jbc.271.35.21604. [DOI] [PubMed] [Google Scholar]
- Orlandi PA. Protein disulfide isomerase-mediated reduction of the A-subunit of cholera toxin in a human intestinal cell line. J Biol Chem. 1997;272:4591–4599. [PubMed] [Google Scholar]
- Orlandi PA, Curran PK, Fishman PH. Brefeldin A blocks the response of cultured cells to cholera toxin: implications for intracellular trafficking in toxin action. J Biol Chem. 1993;268:12010–12016. [PubMed] [Google Scholar]
- Orlandi PA, Fishman PH. Orientation of cholera toxin bound to target cells. J Biol Chem. 1993;268:17038–17044. [PubMed] [Google Scholar]
- Pacuszka T, Fishman PH. Intoxication of cultured cells by cholera toxin: evidence for different pathways when bound to ganglioside GM1or neoganglioproteins. Biochemistry. 1992;31:4773–4778. doi: 10.1021/bi00135a005. [DOI] [PubMed] [Google Scholar]
- Pacuszka T, Bradley RM, Fishman PH. Neoglycolipid analogues of ganglioside GM1 as functional receptors of cholera toxin. Biochemistry. 1991;30:2563–2570. doi: 10.1021/bi00224a001. [DOI] [PubMed] [Google Scholar]
- Parton RG. Ultrastructural localization of gangliosides; GM1is concentrated in caveolae. J Histol Cytol. 1994;42:155–166. doi: 10.1177/42.2.8288861. [DOI] [PubMed] [Google Scholar]
- Parton RG. Caveolae and caveolins. Curr Opin Cell Biol. 1996;8:542–548. doi: 10.1016/s0955-0674(96)80033-0. [DOI] [PubMed] [Google Scholar]
- Parton RG, Simons K. Digging into caveolae. Science. 1995;269:1398–1399. doi: 10.1126/science.7660120. [DOI] [PubMed] [Google Scholar]
- Rothberg KG, Ying Y-S, Kamen BA, Anderson RGW. Cholesterol controls the clustering of the glycosphingolipid-anchored membrane receptor for 5-methyltetrahydrofolate. J Cell Biol. 1990;111:2931–2938. doi: 10.1083/jcb.111.6.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg KG, Heuser JE, Donzell WC, Ying Y-S, Glenney JR, Anderson RGW. Caveolin, a protein component of caveolae membrane coats. Cell. 1992;68:673–682. doi: 10.1016/0092-8674(92)90143-z. [DOI] [PubMed] [Google Scholar]
- Sandvig K, Oystein G, van Deurs B. Thapsigargin-induced transport of cholera toxin to the endoplasmic reticulum. Proc Natl Acad Sci USA. 1996;93:12339–12343. doi: 10.1073/pnas.93.22.12339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargiacomo M, Sudol M, Tang Z, Lisanti MP. Signal transducing molecules and glycosyl-phosphatidylinositol-linked proteins form a caveolin-rich insoluble complex in MDCK cells. J Cell Biol. 1993;122:789–807. doi: 10.1083/jcb.122.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer JE, Liu J, Oh P. Endothelial caveolae have the molecular transport machinery for vesicle budding, docking and fusion including VAMP, NSF, SNAP, annexins and GTPases. J Biol Chem. 1995a;270:14399–14404. doi: 10.1074/jbc.270.24.14399. [DOI] [PubMed] [Google Scholar]
- Schnitzer JE, McIntosh DP, Dvorak AM, Liu J, Oh P. Separation of caveolae from associated microdomains of GPI-anchored proteins. Science. 1995b;269:1435–1439. doi: 10.1126/science.7660128. [DOI] [PubMed] [Google Scholar]
- Schnitzer JE, Oh P, Pinney E, Allard J. Filipin-sensitive caveolae mediated transport in endothelium: reduced transcytosis, scavenger endocytosis and capillary permeability of select macromolecules. J Cell Biol. 1994;127:1217–1232. doi: 10.1083/jcb.127.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionescu N, Lupu F, Simionescu M. Rings of membrane sterols surround the openings of vesicles and fenestrae, in capillary endothelium. J Cell Biol. 1983;97:1592–1600. doi: 10.1083/jcb.97.5.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons K, Ikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Simons K, van Meer G. Lipid sorting in epithelial cells. Biochemistry. 1988;27:6197–6202. doi: 10.1021/bi00417a001. [DOI] [PubMed] [Google Scholar]
- Smart EJ, Ying Y-S, Conrad PA, Anderson RGW. Caveolin moves from caveolae to the Golgi apparatus in response to cholesterol oxidation. J Cell Biol. 1994;127:1185–1197. doi: 10.1083/jcb.127.5.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofer A, Futerman AH. Cationic amphiphilic drugs inhibit the internalization of cholera toxin to the golgi apparatus and the subsequent elevation of cyclic amp. J Biol Chem. 1995;270:12117–12122. doi: 10.1074/jbc.270.20.12117. [DOI] [PubMed] [Google Scholar]
- Tran D, Carpentier J-L, Sawano F, Gorden P, Orci L. Ligands internalized through coated or noncoated invaginations follow a common intracellular pathway. Proc Natl Acad Sci USA. 1987;84:7957–7961. doi: 10.1073/pnas.84.22.7957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L-H, Rothberg KG, Anderson RGW. Mis-assembly of clathrin lattices on endosomes reveals a regulatory switch for coated pit formation. J Cell Biol. 1993;123:1107–1117. doi: 10.1083/jcb.123.5.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaremba TG, Fishman PH. Desensitization of catecholamine-stimulated adenylate cyclase and down-regulation of beta-adrenergic receptors in rat glioma C6 cells: role of cyclic AMP and protein synthesis. Mol Pharmacol. 1984;26:206–213. [PubMed] [Google Scholar]