Abstract
Myosin in adult murine skeletal muscle is composed primarily of three adult fast myosin heavy chain (MyHC) isoforms. These isoforms, MyHC-IIa, -IId, and -IIb, are >93% identical at the amino acid level and are broadly expressed in numerous muscles, and their genes are tightly linked. Mice with a null mutation in the MyHC-IId gene have phenotypes that include growth inhibition, muscle weakness, histological abnormalities, kyphosis (spinal curvature), and aberrant kinetics of muscle contraction and relaxation. Despite the lack of MyHC-IId, IId null mice have normal amounts of myosin in their muscles because of compensation by the MyHC-IIa gene. In each muscle examined from IId null mice, there was an increase in MyHC-IIa– containing fibers. MyHC-IIb content was unaffected in all muscles except the masseter, where its expression was extinguished in the IId null mice. Cross-sectional fiber areas, total muscle cross-sectional area, and total fiber number were affected in ways particular to each muscle. Developmental expression of adult MyHC genes remained unchanged in IId null mice. Despite this universal compensation of MyHC-IIa expression, IId null mice have severe phenotypes. We conclude that despite the similarity in sequence, MyHC-IIa and -IId have unique roles in the development and function of skeletal muscle.
Myosin is the main component of the thick filament of the sarcomere, where it constitutes 40% of the myofibril protein (for a comprehensive review see Warrick and Spudich, 1987). Each hexameric myosin molecule consists of two myosin heavy chains (MyHC,1 220 kD each) and two pairs of nonidentical myosin light chains (∼20 kD each). There are eight known isoforms of sarcomeric MyHC in mammalian skeletal and cardiac muscle. MyHC-α and -β are the major cardiac isoforms (Gulick et al., 1991; Rindt et al., 1993). The β isoform is also referred to as type I MyHC or “slow” MyHC and is expressed in slow skeletal muscle fibers. Two isoforms, embryonic and perinatal, are expressed during development (Periasamy et al., 1984; Bouvagnet et al., 1987). Three adult “fast” isoforms, MyHC-IIa, -IIb, and -IId (also referred to as -IIx), are expressed in fast-twitch fibers (Weydert et al., 1983; Schiaffino et al., 1989; Parker-Thornburg et al., 1992). One MyHC isoform is exclusively expressed in extraocular and pharyngeal muscle (Wieczorak et al., 1985; Lucas et al., 1995).
Striated MyHC genes are located in chromosomal clusters. The cardiac genes, MyHC-α and -β (I, slow), are adjacent to one another on mouse/human chromosomes 14 (Saez et al., 1987; Gulick et al., 1991). The six skeletal MyHCs are encoded by separate genes on mouse and human chromosomes 11 and 17, respectively, and are located within a 350-kb segment (Yoon et al., 1992; Krauter, K., unpublished observations). The MyHC multigene family appears to have arisen from a common ancestral gene based on conservation of genomic structure, clustering of the MyHC genes, and their high sequence conservation, with 78–93% amino acid identity among vertebrate sarcomeric isoforms (Weiss and Leinwand, 1996). The highest degree of conservation occurs between analogous isoforms across species (Weiss and Leinwand, 1996).
Striated MyHC genes are developmentally regulated. MyHC-α and -β (I, slow) genes are expressed in the developing heart of the mouse embryo between 7.5 and 8 days post-coitum (dpc) (Lyons et al., 1990). In skeletal muscle, the embryonic and perinatal MyHC isoforms are expressed at 9.5–10.5 dpc, but only the perinatal isoform persists after birth (Weydert et al., 1987; Lyons et al., 1990). MyHC-I (β, slow) is also expressed early in skeletal muscle development (9.5 dpc) (Lyons et al., 1990). The MyHC-perinatal isoform is eventually replaced by adult MyHC isoforms in skeletal muscle. However, the exact onset of expression of the MyHC-IIa, -IIb, and -IId genes is unknown, but it is thought to occur in mice between late gestation (17.5 dpc) and 5 d after birth (Cox and Buckingham, 1992; DeNardi et al., 1993).
Most vertebrate skeletal muscles express multiple MyHC isoforms, and individual muscle fibers are characterized according to their MyHC content: type IIA (containing MyHC-IIa), type IIB (containing MyHC-IIb), type IID (containing MyHC-IId), and type I (containing MyHC-I [β, slow]) (Pette and Staron, 1990). The maximum velocity of contraction (V max) for fibers composed of a single MyHC increases in the order type I < IIA < IID < IIB (Bottinelli et al., 1991; Galler et al., 1994). Mean cross-sectional areas (CSAs) of fibers also increase in the same order (Hämäläinen and Pette, 1993). MyHC-IIb constitutes 77% of MyHC overall in adult mouse skeletal muscle (Agbulut et al., 1996). Similarly, in rat skeletal muscle, type IIB fibers make up 71% of muscle mass, type IID 18%, type IIA 5%, and type I (β, slow) 6% (Delp and Duan, 1996). Smaller mammals contain a predominance of type IIB fibers, but as the size of the animal increases, the proportion of IIB fibers decreases and the proportion of type IID and IIA fibers increases. In fact, in humans the presence of IIB fibers has not yet been detected despite the identification of a human MyHC-IIb gene and transcript (Schiaffino and Reggiani, 1996). What were previously classified as type IIB fibers in human are now known to contain MyHC-IId (Smerdu et al., 1994; Ennion et al., 1995).
In addition to muscle fibers composed of a single MyHC, hybrid fibers containing two MyHC isoforms can be found in skeletal muscle, but only in certain combinations. Hybrid fibers have been shown to exist in the pairs IIA/I (β, slow), IIA/IID, and IIB/IID, usually with one isoform present in a greater amount (DeNardi et al., 1993; Hämäläinen and Pette, 1995). Hybrid fibers are often found during a transitional phase of muscle, such as a response to a stimulus. Skeletal muscle fibers can alter their MyHC composition in response to “functional demands” of the muscle, including response to electrical stimuli and mechanical factors such as stretch (Termin et al., 1989; Ausoni et al., 1990; Loughna et al., 1990). Innervation and thyroid hormone levels also induce changes in MyHC expression in adult muscle (Pette and Vrbova, 1985; Izumo et al., 1986). Fiber type transitions typically follow a pattern of expression from type I ↔ IIA ↔ IID ↔ IIB, with hybrid fibers present during the transitions (Staron and Johnson, 1993).
The unique kinetic properties of muscle fibers composed of a single MyHC suggest that the MyHC isoforms may be functionally distinct (Hilber et al., 1997). In fact, it has long been known that the contractile velocities of muscles are correlated with their MyHC isoform content (Barany, 1967). There are, however, no biochemical data for the individual adult fast skeletal MyHCs. To investigate the role of the individual adult fast MyHC isoforms in muscle structure and function, we have inactivated the MyHC genes IId and IIb in the mouse (Acakpo-Satchivi et al., 1997). As previously reported, mice null for MyHC-IId and -IIb have reduced body weight, impaired fore- and hindlimb grip strength, and abnormal muscle histology. Additionally, about a third of IId null mice develop kyphosis or severe curvature of the spine. These phenotypes suggest that the myosin genes have unique roles in muscle function and that the absence of one of these gene products causes muscle defects.
In this paper, we demonstrate that the absence of MyHC-IId results in activation of the MyHC-IIa gene. Despite a sufficient quantity of MyHC in muscles of IId null mice, there are significant muscle defects in these mice. In each muscle type analyzed, the relative proportion of type IIA fibers increased. The onset of MyHC-IIa gene activation during development remained unchanged between wild-type and IId null mice and preceded that of the MyHC-IId gene in wild-type animals. The MyHC-IIb content of skeletal muscles in IId null mice remained largely unchanged with the exception of the masseter muscle, which completely lost its normally high content of type IIB fibers in the MyHC-IId null mice. Type IIA cross-sectional fiber areas increased in the tibialis anterior (TA), psoas, and masseter muscles and became as large or larger than type IID fibers in the wild-type mouse. The total muscle CSA and total fiber number were decreased in psoas and masseter, but not in TA muscles of MyHC-IId null mice. We conclude that MyHC-IId has a unique role in the structure and function of the muscle since replacement by MyHC-IIa cannot functionally substitute for its absence.
Materials and Methods
Animals
The generation of MyHC-IId null mice has been previously described (Acakpo-Satchivi et al., 1997). MyHC-IId heterozygous (+/−) mice were used as breeding pairs, and their offspring were genotyped by a PCR technique (Acakpo-Satchivi et al., 1997).
Myofibrils and High-Resolution Gel Electrophoresis
Myofibrils were prepared from muscles of adult male mice according to the procedure by Solaro et al. (1971). Muscles were minced with scissors and homogenized in 1 ml of buffer A (50 mM KCl, 10 mM KPO4, 2 mM MgCl2, 0.5 mM EDTA, 2 mM DTT, pH 7.0). The homogenate was separated by centrifugation at 15,800 g for 15 min at 4°C. The pellet fractions were resuspended in 1 ml of buffer B (buffer A + 1% Triton) and again separated by centrifugation at 15,800 g for 15 min at 4°C. Pellets were resuspended in 1 ml of storage buffer (60 mM KCl, 30 mM imidazole, 2 mM MgCl2, 1 mM DTT, pH 7.0) and kept at −70°C.
For high-resolution gel electrophoresis, MyHC isoforms were separated according to a procedure adapted from Agbulut et al. (1996). 100 ng of total protein from each sample was separated on 8% polyacrylamide gels containing 30% glycerol. The gels were run at 72 V for 30 h at 4°C and then silver stained to visualize the proteins.
For acto:myosin gels, 5 μg of total myofibril protein was separated on 10% polyacrylamide gels. The gels were stained with Coomassie brilliant blue to visualize the proteins. Actin and myosin bands were measured by densitometry.
Immunohistochemistry
Muscles were dissected from male mice (age 6–8 wk) and rapidly frozen in liquid nitrogen–cooled isopentane. 10-μm serial sections from the midbelly of each muscle were cut on a microtome at −20°C and stored on coverslips as frozen sections at −20°C. The fixation procedure was as follows: Sections were incubated in acetone solutions (50, 100, 50%) for 5, 2, and 5 min, respectively, at −20°C, washed in 1× PBS, incubated for 10 min in 4% formaldehyde in 1× PBS, and washed in 1× PBS. Sections were then preincubated for 20 min in 0.5% H2O2 in 1× PBS. All subsequent steps were carried out in a humidified chamber. Sections were blocked in 10% normal goat serum in 1× PBS for 1 h at room temperature and then incubated overnight at 4°C with primary antibody solutions containing 1% normal goat serum and 0.5% Tween-20. Sections were washed four times for 5 min each in 1× PBS. Secondary antibodies (fluorescein or HRP conjugated) were applied for 2 h at room temperature. Sections were washed four times for 5 min each in 1× PBS and visualized either by immunofluorescence or by peroxidase staining using a DAB kit (Vector Laboratories, Burlingame, CA) followed by counterstaining with hematoxylin.
Primary antibodies used in these studies were BFF3 (anti–MyHC-IIb), SC71 (anti–MyHC-IIa) (Schiaffino et al., 1986), MHCI (anti–MyHC-I [β, slow]; Novacastra Laboratories, Newcastle, UK), and MHCN (anti-MyHC perinatal, Novacastra Laboratories). Media from BFF3- and SC71-secreting hybridoma cell lines were used undiluted. MHCI ascites was used at a dilution of 1:50 and MHCN ascites at 1:20. Fluorescein- or HRP-conjugated goat anti–mouse secondary antibodies were used at dilutions of 1:200 and were specific for mouse IgG (SC71, MHCI, and MHCN) or mouse IgM (BFF3) (Jackson ImmunoResearch, West Grove, PA). BFF3 and SC71 were gifts of S. Schiaffino (University of Padova, Padova, Italy).
ATPase and NADH-tetrazolium Reductase Histochemistry
Myofibrillar ATPase staining was performed as described by Brooke and Kaiser (1970). In brief, sections were preincubated in alkaline buffer (75 mM glycine, 40 mM CaCl2, 75 mM NaCl, pH 10.3) for 15 min and then incubated in 3 mM ATP in alkaline buffer, pH 9.4, for 45 min. Sections were dehydrated in 50, 70, and 100% ethanol for 2 min each and mounted with Permount (Fisher Scientific, Pittsburgh, PA). For NADH-tetrazolium reductase (NADH-TR) staining, sections were incubated in 0.2 M Tris, pH 7.4, containing 1.5 mM NADH and 1.5 mM nitrotetrazolium blue for 30 min at 37°C. Sections were then placed in acetone solutions (30, 60, 90, 60, and 30%) for 2 min each and mounted with Aquamount (Lerner Labs, Pittsburgh, PA).
Fiber Area Measurements, Muscle CSA Measurements, and Total Fiber Number Calculations
Serial sections from individual muscles of wild-type and MyHC-IId null mice were stained with specific anti-MyHC antibodies as described. A Zidas computerized digitizer (Carl Zeiss, Inc., Thornwood, NY) in combination with a Zeiss microscope fitted with a drawing tube were used for fiber area and muscle area measurements. The scale factor was fixed by measuring a known distance from a micrometer. Measurements were done at 40× magnification for fiber areas and 10× magnification for total muscle CSA. Areas for fiber types were measured from one serial section while a projection of the serial section was used for marking individual fibers. Fibers on the projected section were marked as type IIA, IIB, or I (β, slow) based on antibody staining patterns. Fibers not staining for MyHC-IIa, IIb, or I (β, slow) were scored as type IID fibers. Fibers from two separate regions of each muscle section, containing ∼120–150 fibers each, were counted, and their areas were measured. Results were used to determine the fiber type composition (%) of the entire muscle. In TA and psoas muscles, where fiber type distribution across the muscle was uneven, measurements were taken in each region of the muscle. The total area of each region was measured and used to determine the overall fiber type composition.
Total fiber numbers were calculated for TA, psoas, and masseter by the formula (muscle CSA − interfiber space)/mean fiber CSA. Mean fiber CSA was determined by the formula (percent type IIA/100 × mean IIA fiber CSA) + (percent type IIB/100 × mean IIB fiber CSA) + (percent type IID/100 × mean IID fiber CSA). To determine interfiber space, the areas of all fibers in a 10-cm2 square area on the digitizing field were measured. Relative interfiber space was determined by (1 − total fiber CSA/ total square area). Interfiber space was determined by muscle CSA × (relative interfiber space)/100.
Reverse Transcription PCR of MyHC Genes
RNA was purified from muscles of wild-type and MyHC-IId null mice using the guanidinium-acid phenol method (Chomczynski et al., 1987). Whole embryos were used to prepare RNA at 12.5 and 15.5 dpc. RNA was prepared from the hindlimbs of neonates at 1, 3, and 5 d after birth. First-strand cDNA was synthesized from 2 μg of total RNA using reverse transcriptase (Superscript II RT; GIBCO BRL, Gaithersburg, MD) and 0.5 μg of oligo-dT (17 nucleotides). Oligonucleotides were designed in the 3′ UTRs of five MyHC genes. Specifically, MyHC-IIa (5′ primer, 5′-AAGCGAAGAGTAAGGCTGTC-3′; 3′ primer, 5′-GTGATTGCTTGCAAAGGAAC-3′), MyHC-IIb (5′ primer, 5′-ACAAGCTGCGGGTGAAGAGC-3′; 3′ primer, 5′-CAGGACAGTGACAAAGAACG-3′), MyHC-IId (5′ primer, 5′-CCAAGTGCAGGAAAGTGACC-3′; 3′ primer, 5′-AGGAAGAGACTGACGAGCTC-3′), MyHC-I (β, slow) (5′ primer, 5′-CCAAGGGCCTGAATGAGGAG-3′; 3′ primer, 5′-GCAAAGGCTCCAGGTCTGAG-3′), and MyHC-perinatal (5′ primer, 5′-ACACATCTTGCAGAGGAAGG-3′; 3′ primer, 5′-TAAACCCAGAGAGGCAAGTG-3′).
PCR reactions contained 2 μl of cDNA (1/10 RT reaction), 15 pmol of each primer, and 2 U of taq polymerase (Biolase, Intermountain Scientific, Denver, CO) in a total volume of 50 μl. Reactions were given a hot start at 94°C for 2 min. Amplification conditions were as follows: denaturization at 94°C for 30 s, annealing at 58°C for 30 s, and primer extension at 72°C for 30 s, for a total of 25 cycles (30 cycles for MyHC-IId at developmental time points), followed by one cycle of primer extension at 72°C for 5 min. 10 μl of 6× DNA loading dye (15% ficoll, 0.25% xylene cyanol) was added to each reaction, and 15 μl was separated on a 2% agarose gel containing ethidium bromide.
Results
Skeletal Muscle from MyHC-IId Null Mice Contains Normal Amounts of Myosin
One explanation for muscle defects in MyHC-IId null mice could be an insufficient quantity of myosin in their muscles, which could result in altered stoichiometry of myosin relative to the other sarcomeric proteins. We measured the relative amounts of myosin and actin in masseter and TA muscles from wild-type and IId null mice. Myofibrils prepared from these muscles were separated on 10% polyacrylamide gels and stained with Coomassie blue to visualize the proteins (data not shown). Scanned images of the gels were analyzed by a molecular imaging system. Three samples were run on two separate gels. The myosin to actin ratio in wild-type masseter muscle was 1.80 ± 0.19, while the ratio in IId null masseter muscle was 1.62 ± 0.16. Similarly, in wild-type TA muscle, the myosin to actin ratio was 1.60 ± 0.18, while in IId null TA muscle the ratio was 1.59 ± 0.07. Therefore, no significant difference was found in the myosin to actin ratios between wild-type and MyHC-IId null mice.
MyHC Isoform Composition in Muscles from MyHC-IId Null Mice
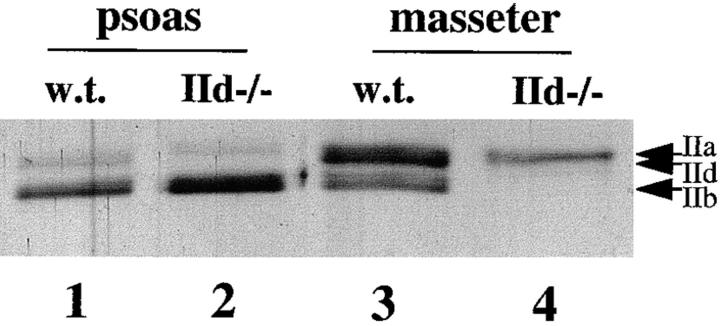
To determine the MyHC composition of muscles from wild-type and MyHC-IId null mice, myofibril preparations from skeletal muscles from these mice were resolved by high-resolution gel electrophoresis (Agbulut et al., 1996). In all muscles examined, MyHC-IId was absent and MyHC-IIa was induced. Fig. 1 shows the MyHC isoform content of the psoas and masseter muscles. Wild-type psoas contained primarily MyHC-IIb, with lesser amounts of IId and IIa (Fig. 1, lane 1). In psoas muscle of IId null mice, MyHC-IIb remained prominent, while the IId band was absent and there was a small increase in MyHC-IIa (Fig. 1, lane 2). Wild-type masseter muscle was composed of MyHC-IIb and -IId (Fig. 1, lane 3). Whereas there was no visible MyHC-IIa in masseter muscle from wild-type mice, masseter from IId null mice contained predominantly MyHC-IIa (Fig. 1, lane 4). Surprisingly, in addition to the increase in MyHC-IIa expression, MyHC-IIb expression was undetectable in the masseter of IId null mice. The proportional increase in MyHC-IIa expression in masseter appears to be much greater than in psoas. Western blots with specific anti-MyHC antibodies confirmed the absence of MyHC-IIb expression in the masseter muscle of IId null mice (data not shown).
Figure 1.
MyHC isoform content of muscles from IId null mice determined by high-resolution gel electrophoresis. Myofibrils were prepared from psoas and masseter muscles of wild-type and MyHC-IId null mice (8-wk-old males). Myofibrils were separated by high-resolution gel electrophoresis and silver stained. Lane 1, wild-type psoas (w.t.); lane 2, IId null psoas (IId−/−); lane 3, wild-type masseter (w.t.); lane 4, IId null masseter (IId−/−). The location of MyHC-IIa, -IIb, and -IId bands are indicated.
Immunohistochemical Determination of MyHC Expression in Normal and MyHC-IId Null Skeletal Muscle
Electrophoretic separation of MyHC isoforms gives qualitative and quantitative information about the MyHC composition of specific muscles but does not give information about individual muscle fibers. To determine the histological pattern of MyHC expression in muscles of IId null mice, sections of masseter, psoas, TA, diaphragm, tongue, and extensor digitorum longus (EDL) muscles were stained with anti-MyHC specific antibodies. Fig. 2 shows sections of masseter muscle from wild-type and IId null mice stained with anti–MyHC-IIa and -IIb antibodies. Wild-type masseter muscle contained few fluorescently stained fibers when probed with an anti-MyHC-IIa antibody (Fig. 2 a). The MyHC-IIa–positive fibers depicted in Fig. 2 were only present in a small peripheral area of the muscle. In contrast, all fibers in masseter muscle from MyHC-IId null mice expressed MyHC-IIa (Fig. 2 b). Whereas wild-type masseter contained a significant percentage of MyHC-IIb–containing fibers (Fig. 2 c), staining of masseter muscle from IId null mice with an anti– MyHC-IIb antibody was negative (Fig. 2 d). Staining of masseter muscle from wild-type and IId null mice with antibodies to MyHC-I (β, slow) and MyHC-perinatal was negative (data not shown). These results are consistent with those from high-resolution gels, demonstrating that the MyHC content of masseter from IId null mice is exclusively IIa.
Figure 2.
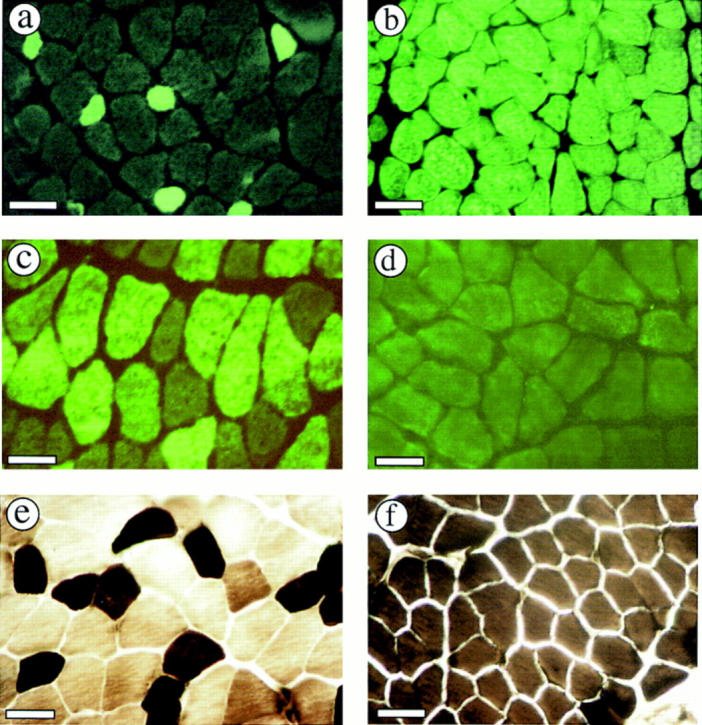
The masseter muscle is uniformly composed of MyHC-IIa in IId null mice. Cross sections of masseter muscle from wild-type (a and c) or MyHC-IId null (b and d) mice (8-wk-old males) were immunohistochemically stained with anti–MyHC-IIa (a and b) or anti–MyHC-IIb (c and d) antibodies. Positive fibers were visualized by fluorescence microscopy. Exposure times were (a) 28 s; (b) 30 s; (c) 35 s; (d) 30 s. Masseter sections from wild-type (e) and MyHC-IId null (f) mice were ATPase stained at pH 10.3 (e and f). Bars: (a and b) 40 μm; (c–f) 30 μm.
An additional classical way to characterize muscle fibers is to stain for ATPase activity (Brooke and Kaiser, 1970). The ATPase stain is a functional assay that complements the antigenic data of the immunostaining. Fig. 2, e and f, shows ATPase staining patterns at pH 10.3 of masseter muscle from wild-type and IId null mice. Traditionally, Type IIB fibers stain lighter at pH 10.3, while type IIA and IID fibers stain dark (Fig. 2 e). In IId null masseter, all fibers stained dark at pH 10.3, reflecting a loss of type IIB fibers (Fig. 2 f). ATPase stains of IId null masseter at pH 4.6 and 4.37 also showed uniform staining of all fibers (medium intensity at pH 4.6, light at pH 4.37), suggesting it is composed of a single fiber type (data not shown).
The MyHC composition of psoas muscle from wild-type and MyHC-IId null mice is illustrated in Fig. 3. Wild-type psoas muscle contains type IIA, IIB, and IID fibers (Jansen et al., 1996). However, psoas muscle contains a spatially uneven distribution of fiber types, with one region of the muscle containing type IIA, IIB, and IID fibers (red portion) and one region composed almost exclusively of type IIB fibers (white portion) (Hämäläinen and Pette, 1995). To determine whether the spatial distribution of fibers was changed in the IId null psoas, serial sections of psoas muscle from wild-type and IId null mice were stained with anti–MyHC-IIa and -IIb antibodies. Photographs in Fig. 3, a–d, are from the red portion of the muscle. The population of MyHC-IIa–containing fibers was low in wild-type psoas (Fig. 3 a). MyHC-IIb–positive fibers from a serial section of the same muscle are shown in Fig. 3 b. Fibers not staining with MyHC-IIa or -IIb antibodies were assumed to be MyHC-IId–containing fibers since psoas muscle from wild-type and IId null mice contained no MyHC-I (β, slow) or MyHC-perinatal–expressing fibers (data not shown). Antibody staining of serial sections of IId null psoas muscle show that it is composed entirely of type IIA and IIB fibers (Fig. 3 c, anti–MyHC-IIa, and Fig. 3 d, anti–MyHC-IIb), with a dramatic increase in the population of type IIA fibers compared with wild type.
Figure 3.
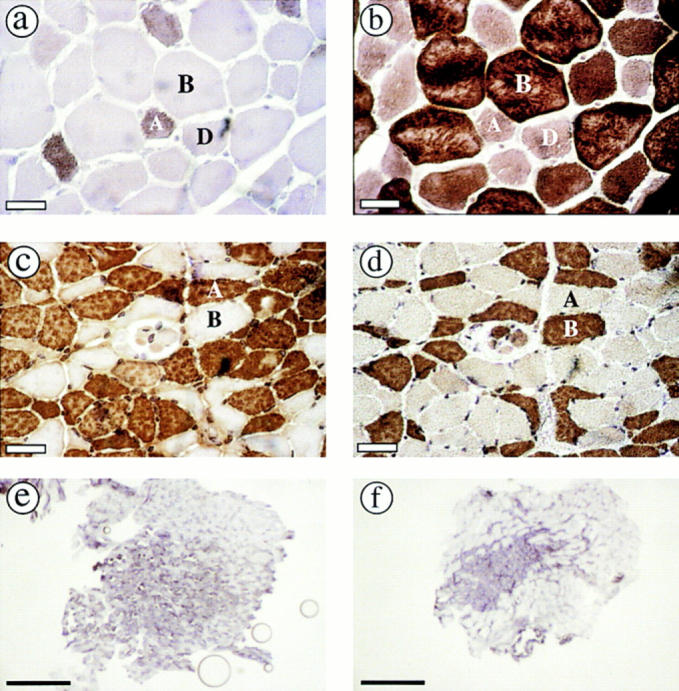
MyHC isoform content of psoas muscles from wild-type and MyHC-IId null mice determined by immunohistochemistry. Serial sections of psoas muscle from wild-type (a and b) and MyHC-IId null (c and d) mice (8-wk-old males) were stained with antibodies to MyHC-IIa (a and c) or MyHC-IIb (b and d). Positive fibers were visualized by peroxidase staining. Sections of psoas muscle from wild-type (e) and MyHC-IId null (f) mice were stained by NADH-TR and photographed at low magnification (4×). Bars: (a–d) 30 μm; (e and f) 0.5 mm.
Type IIA fibers in IId null psoas were localized in a smaller area of the muscle than in wild-type psoas muscle. This is illustrated by low-magnification photographs of psoas muscle stained with NADH-TR in Fig. 3, e and f. The oxidative capacity of muscle fibers can be determined by staining with NADH-TR and follows the pattern type I (dark) > IIA > IID > IIB (light) (Hämäläinen and Pette, 1993). Fiber type distribution in wild-type psoas is shown in Fig. 3 e. Type IIB fibers (light) are present throughout the muscle, whereas type IIA (dark) and type IID (intermediate) fibers are concentrated in the red portion of the muscle. In IId null psoas, the darker staining type IIA fibers were restricted to a smaller area of the muscle (Fig. 3 f).
TA, diaphragm, tongue, and EDL muscles from wild-type and MyHC-IId null mice were also immunohistochemically stained with anti-MyHC antibodies to determine the pattern of MyHC expression. In each of these muscles, the number and proportion of type IIA fibers increased in IId null mice compared with wild type without apparent alterations in MyHC-IIb expression (data not shown). Type I (β, slow) fibers were only found in diaphragm, and no MyHC-perinatal–expressing fibers were detected in any muscle. In all muscles examined, there were no fibers that coexpressed MyHC-IIa and -IIb.
Although MyHC-IId comprises <20% of MyHC in mouse, it is widely distributed; almost all muscles contain type IID fibers. A microscope equipped with a computer digitizing system was used to count fiber types as well as measure fiber cross-sectional areas. Four muscles were analyzed in detail in these experiments: TA, diaphragm, psoas, and masseter. These muscles were taken from adult male mice (6–8 wk old). Three mice of each genotype (wild type and IId null) were analyzed. Since no phenotypic or histological differences were previously found between wild-type and MyHC-IId +/− mice, heterozygotes were not included in this study (Acakpo-Satchivi et al., 1997). Serial sections of each muscle were stained with anti–MyHC-IIa, -IIb, and -I (β, slow) antibodies and aligned under two microscopes as described in Materials and Methods. Individual fibers were then counted as type IIA, IIB, or I (β, slow). Since there is currently no antibody that specifically recognizes MyHC-IId, fibers not staining with any of these antibodies were counted as type IID fibers. The results of these analyses are summarized in Table I. Numbers represent averages of three mice of each genotype (wild type and IId null).
Table I.
Fiber Type Composition of Muscles from Wild-Type (w.t.) and MyHC-IId Null Mice
| (a) Overall Fiber Type Composition | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tibialis anterior | Diaphragm | Psoas | Masseter | |||||||||||||
| MyHC | w.t. | IId−/− | w.t. | IId−/− | w.t. | IId−/− | w.t. | IId−/− | ||||||||
| % | % | % | % | |||||||||||||
| IIA | 9 | 30 | 45 | 82 | 15 | 21 | <1 | 100 | ||||||||
| IIB | 73 | 70 | 0 | 0 | 68 | 79 | 43 | 0 | ||||||||
| IID | 18 | 0 | 40 | 0 | 17 | 0 | 56 | 0 | ||||||||
| I | 0 | 0 | 15 | 18 | 0 | 0 | 0 | 0 | ||||||||
| (b) Fiber Type Composition of Red and White Portions of TA and Psoas Muscles | ||||||||||||||||
| MyHC |
Tibialis anterior | Psoas | ||||||||||||||
| w.t. | IId−/− | w.t. | IId−/− | |||||||||||||
| red | white | red | white | red | white | red | white | |||||||||
| IIA | 18 | 0 | 63 | 0 | 31 | 0 | 69 | 0 | ||||||||
| IIB | 46 | 100 | 37 | 10 | 34 | 100 | 31 | 100 | ||||||||
| IID | 36 | 0 | 0 | 0 | 35 | 0 | 0 | 0 | ||||||||
| I | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||
Fiber types were determined by counting each type in serial cross sections of muscles stained with antimyosin antibodies as described. Two areas per section of diaphragm and masseter were counted; two areas of each region in TA and psoas muscle were counted. Measurements in TA and psoas were weighted according to the percent of total area of each region (red and white). Numbers reflect averages of three wild-type and three MyHC-IId null mice. Standard deviations from the mean were 4% for all samples.
Table I shows the overall MyHC content expressed as the percent of positive fibers in TA, psoas, diaphragm, and masseter muscles. In TA, the type IIA fiber content increased from 9 to 30% between wild-type and IId null mice. The type IIB fiber content remained constant at 73 and 77%, in wild-type and IId null mice, respectively. Diaphragm muscle from wild-type mice contained type IIA, IID, and I (β, slow) fibers in the amounts 45, 40, and 15%, respectively. The type IIA fiber content increased to 82% in IId null diaphragm while the type I (β, slow) fiber content remained similar to wild type at 18%. Psoas muscle showed a smaller increase in type IIA fibers overall from 15 to 21% in wild type compared with IId null mice. Type IIB fiber content also increased slightly in IId null psoas from 68 to 79%.
The masseter muscle was unique in its changes between wild-type and MyHC-IId null mice. There are <1% type IIA fibers found in normal mouse masseter, which is composed of 43% type IIB and 56% type IID fibers. As shown in Figs. 1 and 2, masseter from MyHC-IId null mice converted to 100% type IIA fibers. These appear to be pure IIA fibers since staining with anti–MyHC-IIb, –I (β, slow), and –perinatal antibodies was negative. Type IIA fibers were also found to increase in tongue and EDL muscles from IId null mice, which normally contain primarily type IIB and IID fibers (data not shown).
Fiber-type distribution in TA and psoas muscles is spatially uneven and can be divided into two regions based on MyHC fiber-type content. These are classically termed according to the appearance of the muscle as the white portion (or fast-twitch glycolytic), containing all type IIB fibers, and the red portion (or fast-twitch oxidative/glycolytic), containing a mixture of type IIA, IIB, and IID fibers (Barnard et al., 1971; Peter et al., 1972). As reflected in Table I, the type IIA fiber increase in both TA, and psoas was restricted to the red portion of the muscle. The type IIA fibers appear to directly replace type IID fibers in these regions.
Fiber Areas and Muscle CSAs in Skeletal Muscles from IId Null Mice
Fiber areas vary between different fiber types. Type IIB fibers have the fastest rate of contraction and are also the largest in terms of CSA (Schiaffino and Reggiani, 1994). Within an individual muscle, average fiber sizes increase in the order type I < IIA < IID < IIB, with considerable overlap in the size ranges of each fiber type. In addition, for a single fiber type, there is variation in fiber size between different muscles of the same animal (Hämäläinen and Pette, 1995). To determine cross-sectional areas in wild-type and IId null mice, measurements were taken under a microscope as described. Areas were measured for three pairs of mice (males, 6–8 wk). Each pair contained one wild-type and one IId null animal of the same age (littermates). The mean weight of wild-type animals was 26.6 ± 2.8 g, while the mean weight of IId null animals was 18.6 ± 4.5 g. As described previously, TA, psoas, and masseter muscles, but not diaphragm, from IId null mice have smaller masses compared with the same muscles from wild-type mice (Acakpo-Satchivi et al., 1997).
A summary of the mean sizes of the different fiber types in TA, psoas, diaphragm, and masseter is shown in Table II. Sample sizes and statistically significant differences are noted. There were too few type IIA fibers in wild-type masseter to allow measurement and statistical comparison of sizes.
Table II.
Mean Cross-sectional Areas of Muscle Fiber Types in Wild-Type and MyHC-IId Null Mice
| MyHC | Tibialis anterior | Psoas | Diaphragm | Masseter | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| w.t. | IId−/− | w.t. | IId−/− | w.t. | IId−/− | w.t. | IId−/− | |||||||||
| μm2 | μm2 | μm2 | μm2 | |||||||||||||
| IIA | 533 ± 124 | 945 ± 178* | 634 ± 120 | 906 ± 342* ‡ | 781 ± 157 | 788 ± 205 | ND | 728 ± 120‡ | ||||||||
| IIB red | 1447 ± 303 | 2606 ± 707* | 1580 ± 284 | 571 ± 200* | — | — | 1249 ± 267 | — | ||||||||
| IIB white | 1264 ± 240 | 1206 ± 265 | 1329 ± 239 | 1622 ± 370* | — | — | — | — | ||||||||
| IID | 744 ± 143 | — | 926 ± 170 | — | 1166 ± 266 | — | 742 ± 164 | — | ||||||||
| I | — | — | — | — | 730 ± 157 | 750 ± 183 | — | — | ||||||||
Cross-sectional areas were measured in muscles from wild-type (w.t.) and MyHC-IId null (IId−/−) mice (8-wk-old males). Average deviations from the mean are given. Sample sizes were as follows: w.t. TA, IIA = 111, IID = 170, IIB red = 229, IIB white = 112; IId−/− TA, IIA = 138, IIB red = 89, IIB white = 61; w.t. psoas, IIA = 92, IID = 100, IIB red = 102, IIB white = 66; IId−/− psoas, IIA = 227, IIB red = 65, IIB white = 70; w.t. diaphragm, IIA = 156, IID = 117, I = 65; IId−/− diaphragm, IIA = 233; I = 63; w.t. masseter, IIB = 50, IID = 66; IId−/− masseter, IIA = 45. Fiber areas were not determined (ND) for type IIA fibers in wild-type masseter.
P < 0.001 for the same fiber type in IId null mice compared to wild type.
No significant difference found between wild-type IID and IId null-type IIA fiber sizes.
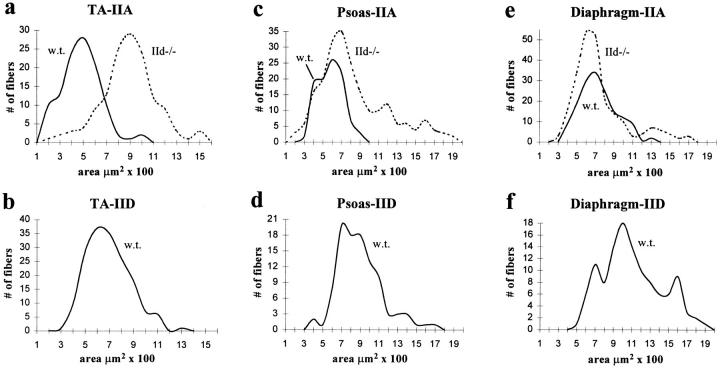
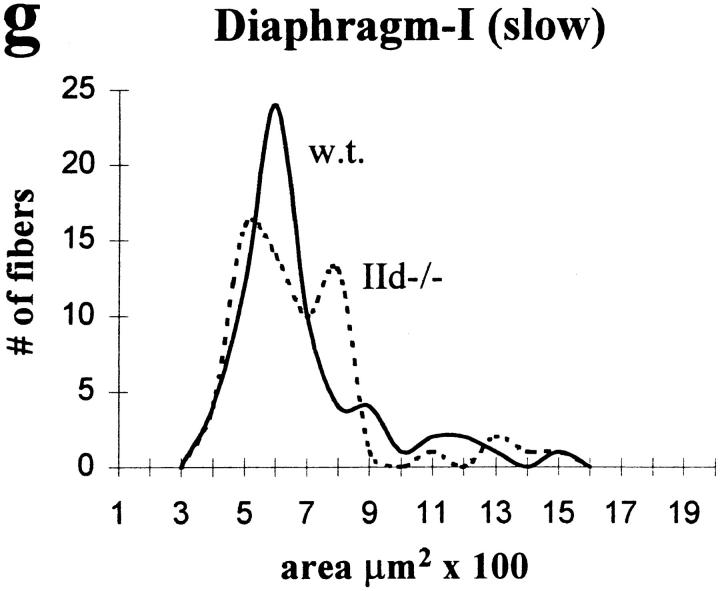
Fiber area measurements from wild-type and IId null mice are shown in Fig. 4 for TA, psoas, and diaphragm. In TA, type IIA fiber size increased in IId null mice (Fig. 4 a). Type IID fiber areas from wild-type TA are shown in Fig. 4 b. Type IIA fibers from IId null TA were significantly larger than wild-type IID fibers from TA (Table II). The mean type IIA fiber size also increased in IId null psoas muscle compared with type IIA fiber size in wild-type psoas (Fig. 4 c). The size range of type IIA fibers (200– 1,900 μm2) in IId null psoas was statistically indistinguishable from type IID fibers from wild-type psoas (300–1,800 μm2) (Fig. 4 d). In contrast to TA and psoas, type IIA fiber areas in wild-type and IId null diaphragm were the same (Fig. 4 e). Type IID fibers in normal diaphragm were larger than type IIA fibers from either wild-type or IId null mice (Fig. 4 f). There was also no difference in the size of type I (β, slow) fibers from wild-type and IId null diaphragm (Fig. 4 g).
Figure 4.
CSAs of type IIA, IID, and I (β, slow) fibers in skeletal muscles from MyHC-IId null mice. CSAs of type IIA fibers from wild-type and MyHC-IId null mice from TA (a), psoas (c), and diaphragm (e) are plotted as the number of fibers counted at each area. Wild type (w.t) are shown as solid lines; IId null (IId−/−) are shown as dotted lines. CSAs of type IID fibers from wild-type TA (b), psoas (d), and diaphragm (f) are also plotted as the number of fibers at each area. CSAs of type I (β, slow) fibers from diaphragms of wild-type (w.t., solid line) and IId null (IId−/−, dotted line) mice are shown in g. Sample numbers (n) are given in Table II.
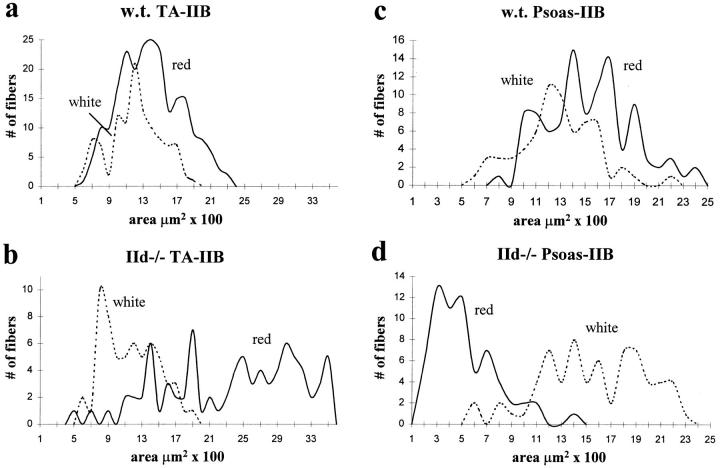
Areas of type IIB fibers from red and white portions of TA and psoas are shown in Fig. 5. Type IIB fiber sizes in normal TA are shown in Fig. 5 a. In IId null TA, type IIB fibers in the red (or mixed fiber) area had a broad range of areas, with a population of very large hypertrophied fibers that were significantly larger than type IIB fibers from the red portion of wild-type TA (Fig. 5 b). On the other hand, type IIB fibers in the white portion of the muscle were the same in wild-type and IId null TA. Type IIB fibers from the red and white portions of wild-type psoas are shown in Fig. 5 c. In contrast to TA, type IIB fibers from the red portion of IId null psoas were much smaller than type IIB fibers from the white portion (Fig. 5 d). These fibers were also significantly smaller than type IIB fibers from both portions of wild-type psoas. Type IIB fibers from the white portion of IId null psoas muscle were slightly larger in size than IIB fibers from white wild-type psoas.
Figure 5.
CSAs of type IIB fibers from MyHC-IId null mice. CSAs measured for type IIB fibers from TA (a and b) and psoas (c and d) are plotted as the number of fibers at each given area. IIB fibers from both wild-type (a and c) and IId null (b and d) TA and psoas are divided into IIB fibers from the red portion (red, solid line) and white portion (white, dotted line) of the muscle. Sample numbers (n) are given in Table II.
Table III presents mean whole muscle CSAs and total fiber numbers determined from muscles of the same mice used in fiber area measurements. Formulas for determining total fiber number are given in Materials and Methods. For TA and psoas, CSAs and total fiber number were determined for both the red and white portions of the muscle. There was no difference in the CSA or the total fiber number between TA muscles from wild-type and IId null mice. In contrast, psoas muscles from IId null mice had smaller CSAs compared with wild-type psoas. Both the red and the white regions of the muscle contributed to the overall smaller size. Psoas of IId null mice also contained fewer total fibers than wild-type psoas. Masseter muscles from IId null mice had smaller CSAs compared with wild-type masseter but contained a similar number of fibers overall.
Table III.
Total Cross-sectional Area and Total Fiber Number of Muscles from Wild-Type and MyHC-IId Null Mice
| Tibialis anterior | Psoas | Masseter | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| w.t. | IId−/− | w.t. | IId−/− | w.t. | IId−/− | |||||||
| Cross-sectional area (μm2 × 106) | ||||||||||||
| red | 1.181 ± 0.113 | 1.774 ± 0.187 | 1.446 ± 0.116 | 0.447 ± 0.041* | — | — | ||||||
| white | 1.125 ± 0.175 | 1.550 ± 0.098 | 1.512 ± 0.015 | 0.982 ± 0.102‡ | — | — | ||||||
| total | 2.306 ± 0.297 | 2.724 ± 0.260 | 2.958± 0.101 | 1.429 ± 0.085* | 2.815 ± 0.200 | 1.912 ± 0.201‡ | ||||||
| Number of fibers | ||||||||||||
| red | 949 ± 95 | 799 ± 115 | 1059 ± 90 | 496 ± 57‡ | — | — | ||||||
| white | 759 ± 87 | 1101 ± 90 | 1067 ± 102 | 571 ± 15‡ | — | — | ||||||
| total | 1708 ± 182 | 1900 ± 27 | 2126 ± 107 | 1067 ± 50* | 2570 ± 183 | 2285 ± 240 | ||||||
Formulas for total fiber number calculations are given in Materials and Methods. For both cross-sectional areas and total fiber numbers, average deviations from the mean are given.
P < 0.001 compared to wild type.
P < 0.005 compared to wild type.
MyHC Gene Expression in Wild-Type and MyHC-IId Null Mice
Since the MyHC-IIa gene compensates for the absence of MyHC-IId, but IId null mice have strong phenotypes, the most likely explanation is that the two genes are not functionally equivalent. However, it is important to determine the timing of MyHC-IIa compensation. If IIa gene expression occurs after a pathologic phenotype has already been established, it would complicate interpretation. We examined the time course of activation of MyHC genes in wild-type and IId null mice using RT-PCR with oligonucleotide primers from the 3′-UTRs that were specific for MyHC-IIa, IIb, IId, I (β, slow), and perinatal.
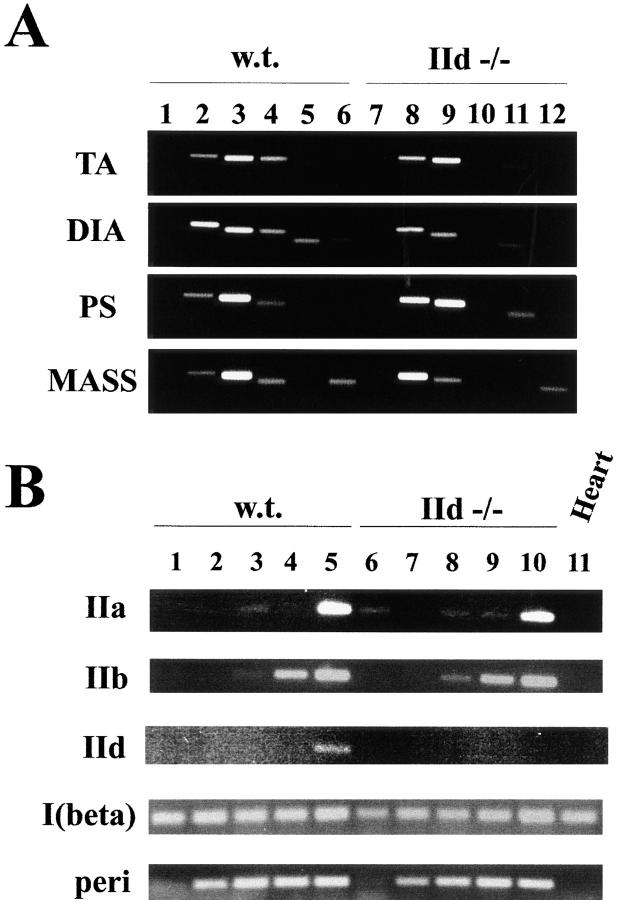
As shown in Fig. 6 A, total RNA was prepared from TA, diaphragm, psoas, and masseter muscles of adult female mice (8 wk). PCR amplification in the presence of all five sets of primers from control reactions in which RT was not added was negative in both wild-type (lane 1) and IId null mice (lane 7). MyHC-IIa mRNA was present in wild-type and IId null TA, diaphragm, and psoas (lanes 2 and 8). By RNase protection, there was a threefold increase in MyHC-IIa mRNA in masseter of IId null mice compared with wild-type masseter (data not shown). MyHC-IIb mRNA was easily detectable in wild-type and IId null TA, diaphragm, and psoas muscles (lanes 3 and 9). MyHC-IIb mRNA was present in diaphragm even though this muscle contains no type IIB fibers. In IId null masseter, MyHC-IIb mRNA levels appeared to have decreased compared with wild type but were not entirely absent (lanes 3 and 9). This was confirmed by RNase protection, which showed there was a sixfold decrease in MyHC-IIb mRNA in IId null mice compared with wild-type animals (data not shown). Whereas MyHC-IId mRNA was present in all four wild-type muscles, it was absent in each of these muscles from IId null mice (lanes 4 and 10). MyHC-I (β, slow) mRNA was found in TA and diaphragm (lanes 5 and 11). MyHC-perinatal mRNA was detected in wild-type diaphragm and masseter (lane 6) and in IId null masseter (lane 12).
Figure 6.
MyHC gene expression in wild-type and IId null mice. RT reactions were performed as described in Materials and Methods. Oligonucleotide primers for PCR were designed in the 3′-UTRs of MyHC genes. PCR amplification of cDNA was performed as described at 25 cycles for all reactions except MyHC-IId at developmental time points (B), which was performed at 30 cycles. One quarter of each reaction was separated on a 2% agarose gel. (A) RNA was prepared from TA, diaphragm, psoas, and masseter muscles of wild-type (lanes 1–6) and IId null (lanes 7–12) female mice (8 wk old). In lanes 1 and 7, PCR was performed in the presence of all sets of MyHC-specific primers with extracts from control reactions in which RT was not added. PCR reactions were specific for MyHC-IIa, lanes 2 and 8; MyHC-IIb, lanes 3 and 9; MyHC-IId, lanes 4 and 10; MyHC-I (β, slow), lanes 5 and 11; and MyHC-perinatal, lanes 6 and 12. (B) RNA was prepared from wild-type (lanes 1–5) and IId null (lanes 6–10) mice at 12.5 dpc (lanes 1 and 6) and 15.5 dpc (lanes 2 and 7) and at 1 (lanes 3 and 8), 3 (lanes 4 and 9), and 5 d (lanes 5 and 10) after birth. At 12.5 and 15.5 dpc, RNA was prepared from whole embryos. At 1, 3, and 5 d after birth, RNA was prepared from hindlimbs. RNA from adult mouse heart (6 wk) was used as a control in lane 11. PCR reactions contained primers specific for MyHC-IIa, -IIb, -IId, -I (β, slow), and perinatal as indicated.
The precise onset of expression of the adult fast MyHC genes in mice is unknown but has been reported to occur between 17.5 dpc and 5 d of postnatal life (Cox and Buckingham, 1992; DeNardi et al., 1993). To determine if the developmental pattern of expression of the adult MyHC genes in IId null mice is altered, MyHC gene expression was examined at five developmental time points in Fig. 6 B in wild-type (lanes 1–5) and IId null (lanes 6–10) mice. RNA was prepared from whole embryos at 12.5 dpc (lanes 1 and 6) and 15.5 dpc (lanes 2 and 7) and from hindlimbs of neonatal mice at days 1 (lanes 3 and 8), 3 (lanes 4 and 9), and 5 (lanes 5 and 10) after birth. Cardiac RNA (lane 11) was included as a negative control for the adult fast and perinatal MyHC genes, which are not expressed in the heart. Some MyHC-IIa mRNA was present at 12.5 dpc in IId null mice (IIa, lane 6). In both wild-type and IId null mice, MyHC-IIa was detectable at days 3, 4, and 5 after birth (IIa, lanes 3–5 and 8–10). MyHC-IIb mRNA was detectable at days 1, 3, and 5 after birth in wild-type and IId null mice (IIb, lanes 3–5 and 8–10). In contrast to MyHC-IIa and -IIb, MyHC-IId mRNA was not detected in wild-type mice until 5 d after birth (IId, lane 5), suggesting that the IId gene is expressed later in development than MyHC-IIa and -IIb. Two different primer pairs in the MyHC-IId 3'UTR gave the same results (data not shown). No MyHC-IId gene expression was detected in developing IId null mice.
In both wild-type and IId null mice, MyHC-I (β, slow) mRNA was easily detectable at each time point (I(beta), lanes 1–10). Only MyHC-I (β, slow) was detected in heart (lane 11). MyHC-perinatal was detectable beginning at 15.5 dpc through 5 d after birth in both wild-type and IId null mice (peri, lanes 2–5 and 7–10).
Discussion
Functional Diversity of Myosin Heavy Chains
Striated MyHC genes are highly conserved, having 78– 93% identity at the amino acid level. One hypothesis is that the multiple MyHC genes are functionally equivalent, and their number exists to provide a safety mechanism. However, experimental evidence suggests that the multiple MyHC genes provide independent functions. Early studies showed that muscle fibers with different MyHC contents had different contractile velocities (Barany, 1967). Likewise, measurements on single fibers from rat, rabbit, and human show differences in ATP hydrolysis and force responses that are correlated with MyHC content (Hilber et al., 1997). As more sequence from skeletal MyHC isoforms becomes available, regions of divergence thought to affect function can be assayed biochemically in vitro (Spudich, 1994). For example, the globular head of myosin contains two flexible loops near the actin and ATP binding sites. Sequences in these loops are not well conserved and may be responsible for differences in the ATPase activities of myosin isoforms.
We have recently shown that inactivation of each of the adult genes, MyHC-IIb or MyHC-IId, in mice leads to muscular defects. Interestingly, the physiological defects exhibited by the two null strains are quite distinct. For example, MyHC-IIb null mice show severe defects in the amount of force generated per CSA, while IId null mice show increased time to contraction and relaxation, but normal amounts of force generation (Acakpo-Satchivi et al., 1997). Additionally, only IId null mice develop kyphosis. Both fore- and hindlimb grip strength are weaker in IId null compared with normal mice, whereas in IIb null mice, only the forelimbs are severely affected (Acakpo-Satchivi et al., 1997). Both lines of null mice have reduced body weight compared with wild-type mice. Despite the fact that MyHC-IId constitutes <20% of the total skeletal MyHC in mice compared with 77% for IIb, the phenotypes of IId null mice are more severe. In the current studies, we have demonstrated that there is a universal increase in MyHC-IIa in MyHC-IId null mice. However, despite the compensational increase in MyHC-IIa, muscles in IId null mice are not normal. One interpretation of this result is that the MyHC-IIa and IId genes are not functionally equivalent. An alternative explanation is that the MyHC-IIa gene is not quantitatively or temporally expressed in a pattern consistent with the MyHC-IId gene. Here, we present data that argue in favor of the former interpretation.
MyHC-IIa Compensation
The genes encoding the skeletal MyHCs are arranged in the order embryonic, IIa, IId, IIb, perinatal, and extraocular within a relatively small region (∼350 kb) on mouse chromosome 11 and human chromosome 17 (Yoon et al., 1992; Weiss et al., 1994; Krauter, K., unpublished observations). The order of MyHC genes is not, however, related to their developmental pattern of expression. This is unusual since the linear order of genes in several other clusters, such as β-globin, has been shown to correlate with their developmental pattern of expression (Dillon and Grosveld, 1993). The upregulation of MyHC-IIa could, however, be a function of the close proximity of the MyHC-IIa and -IId genes on mouse chromosome 11. The two genes are closely linked, with the 3′ region of IIa only 5 kb upstream from the start of the IId coding region (Parker-Thornburg et al., 1992). This distance is similar to that of the two cardiac MyHC genes on chromosome 14, which are separated by 4.5 kb. These genes, MyHC-α and -β (I, slow), are tightly coregulated by factors such as thyroid hormone, which simultaneously decreases β expression (I, slow) and increases α expression (Izumo and Mahdavi, 1988).
The mechanisms determining gene-specific activation of adult fast MyHC genes are not well defined. In mouse, MyHC-IIa, -IIb, and -IId transcripts have been reported to be seen between 17.5 dpc and 5 d postnatal (Cox and Buckingham, 1992; DeNardi et al., 1993), but detailed analyses of the onset of the adult myosin genes has not been studied. Our data suggest that the MyHC-IId gene is expressed later in development (5 d postnatal) than either the MyHC-IIa or -IIb gene in wild-type mice and that there is no temporal alteration in expression of the IIa or IIb genes in IId null mice. Therefore, a change in the developmental timing of expression of the MyHC genes cannot explain the ineffective compensation by IIa. It is also possible that changes in other proteins, such as alternate isoforms of nonmyosin proteins, may contribute to the differences between wild-type and IId null mice. Myofibril preparations separated by gel electrophoresis have not detected any isoform shifts in IId null mice. However, future studies will include more detailed analyses of isoform changes.
The molecular mechanisms by which the MyHC-IIa gene is activated are unknown. The DNA sequence elements that regulate responses of developmental cues and innervation are also not known. Of the three adult MyHC isoforms, only the mouse MyHC-IIb promoter has been partially characterized. The mouse IIb promoter contains elements necessary for muscle-specific gene regulation, including E-box domains, although regulation by myogenic regulators is indirect (Takeda et al., 1995). Recently, the TATA box was shown to be critical for MyHC-IIb transcription (Diagana et al., 1997). When the IIb TATA box was replaced with functional TATA boxes of several other genes, the promoter was no longer functional in muscle cells. We have found differences in both the sequence and the distance from the transcriptional start site of both the TATA and CCAAAT boxes between the mouse MyHC-IIa, -IIb, and -IId genes (unpublished data). These promoters are currently being characterized.
Phenotypic Effects of MyHC-IIa Increase
Type IID fibers lie between type IIA and IIB fibers in both size and speed of contraction. Since transitions in adult skeletal muscle follow the trend I ↔ IIA ↔ IID ↔ IIB, one might expect that MyHC-IId null mice would contain either more type IIB fibers, more type IIA fibers, or both. The observed increase in type IIA fibers represents a transition from “faster” to “slower” fibers in IId null mice. Given that stimulation of muscle generally results in a transition from type IIB → IID → IIA → I fibers, exercising wild-type and IId null mice should provide interesting results on their exercise capacity and endurance.
Since the individual MyHC isoforms have different contractile properties, the increase in the percent of type IIA fibers in skeletal muscles of IId null mice is likely to cause physiological effects on muscle function. Whereas the increase in MyHC-IIa in psoas and TA muscles was not as great as in diaphragm and masseter, these muscles showed the most striking histological defects. In both TA and psoas muscles, the average size of type IIA fibers increased in IId null mice. In TA, type IIA fibers in IId null mice were larger than their type IID counterparts in wild-type mice. In psoas muscle, type IIA fibers from IId null psoas were statistically indistinguishable in size from wild-type IID fibers. Where there were few type IIA fibers to compare in wild-type masseter muscle, the average IIA fiber area of 728 μm2 in IId null mice was larger than type IIA fibers from most wild-type tissues. The diaphragm seems to be the exception, where type IIA as well as I (β, slow) fibers remained similar in size between IId null and wild-type mice. Therefore, type IIA fiber size changes between wild-type and IId null mice varied between different muscles but increased overall. Since type IID fibers are on average larger than type IIA fibers in wild-type mice, the presence of larger IIA fibers in IId null mice raises the question of whether fibers predestined to become type IID have become IIA.
Although MyHC-IIb levels did not change drastically overall, type IIB fiber sizes were dramatically changed between wild-type and IId null mice. For example, in the red portion of both TA and psoas, there was drastic change in IIB fiber size between wild-type and IId null mice (Table II). Interestingly, it did not effect the tissues in the same manner. Type IIB fibers from the red portion of IId null TA were abnormally large compared with IIB fibers from wild-type TA. The opposite was true in IId null psoas, where type IIB fibers in the red portion were much smaller than IIB fibers from wild-type psoas and also type IIA fibers in the same region.
The masseter is unique among IId null muscles in that it expresses 100% MyHC-IIa. There are no other muscles in mice known to contain all type IIA fibers. This is particularly striking since, of the adult MyHCs, IIa composes the lowest amount in muscles of wild-type mice (∼5%). Mandibular muscles are normally composed of fast fiber types, and masseter from both cat and rabbit have been found to contain some fast-contracting MyHC-α (Rowlerson et al., 1981; D'albis et al., 1993). The conversion of mouse masseter from MyHC-IIb and -IId in wild-type to MyHC-IIa, the slowest of the adult fast skeletal isomyosins, may explain the apparent decreased food and water intake of IId null mice compared with wild type (Acakpo-Satchivi et al., 1997). Since there are very few muscles in the mouse composed uniformly of one fiber type, the masseter from IId null mice may be useful for physiological and biochemical studies of MyHC-IIa.
Another interesting property of IId null masseter was the absence of MyHC-IIb protein. In contrast to wild-type masseter, there were clearly no type IIB fibers present in IId null masseter. MyHC-IIb mRNA levels in masseter from IId null mice, although not completely absent, were sixfold lower compared with wild type by RNase protection, suggesting the IIb gene may be downregulated at the transcription level.
Diaphragm muscles from IId null mice generate normal levels of force (Acakpo-Satchivi et al., 1997). However, kinetic properties such as time to peak tension and time to relaxation are longer in IId null diaphragm. This can be explained by conversion of wild-type diaphragm, which contains 45% type IIA fibers, to 82% type IIA fibers in IId null diaphragm. Since the type I (β, slow) fiber content of diaphragm remained the same between wild-type and IId null mice, the slower kinetic properties may reflect the prevalence of MyHC-IIa.
We have found that skeletal muscles in MyHC-IId null mice have an increase in MyHC-IIa. The inability of MyHC-IIa to completely substitute for MyHC-IId function, reflected in the multiple phenotypes of MyHC-IId null mice, suggests that these two very similar genes each have a unique role in muscle structure and function.
Acknowledgments
We thank S. Schiaffino for his gifts of SC71 and BFF3 antibodies.
This work was supported by grants from the National Institutes of Health: NIH R01GM29090 to L.A. Leinwand, NIH F32AR08443 to C.A. Sartorius, and NIH T32GM07288 to B.D. Lu.
Abbreviations used in this paper
- CSA
cross-sectional area
- dpc
days post-coitum
- EDL
extensor digitorum longus
- MyHC
myosin heavy chain
- NADH-TR
NADH-tetrazolium reductase
- RT
reverse transcription
- TA
tibialis anterior
References
- Acakpo-Satchivi LJR, Edelmann W, Sartorius CA, Lu BD, Wahr PA, Watkins SC, Metzger JM, Leinwand LA, Kucherlapati R. Growth and muscle defects in mice lacking an adult myosin heavy chain gene. J Cell Biol. 1997;139:1–11. doi: 10.1083/jcb.139.5.1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agbulut O, Li Z, Mouly V, Butler-Browne GS. Analysis of skeletal and cardiac muscle from desmin knock-out and normal mice by high resolution separation of myosin heavy-chain isoforms. Biol Cell. 1996;88:131–135. [PubMed] [Google Scholar]
- Ausoni S, Gorza L, Schiaffino S, Gundersen K, Lomo T. Expression of myosin heavy chain isoforms in stimulated fast and slow rat muscles. J Neurosci. 1990;10:153–160. doi: 10.1523/JNEUROSCI.10-01-00153.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barany M. ATPase activity of myosin correlated with speed of muscle shortening. J Gen Physiol. 1967;50:197–218. doi: 10.1085/jgp.50.6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard RJ, Edgerton VR, Furukawa T, Peter JB. Histochemical, biochemical and contractile properties of red, white, and intermediate fibers. Am J Physiol. 1971;220:410–414. doi: 10.1152/ajplegacy.1971.220.2.410. [DOI] [PubMed] [Google Scholar]
- Bottinelli R, Schiaffino S, Reggiani C. Force-velocity relationship and myosin heavy chain isoform compositions of skinned fibres from rat skeletal muscle. J Physiol (Lond) 1991;437:655–672. doi: 10.1113/jphysiol.1991.sp018617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvagnet PF, Strehler EE, White GE, Strehler-Page MA, Nadal B - Ginard, and V. Mahdavi. Multiple positive and negative 5′ regulatory elements control the cell-type-specific expression of the embryonic skeletal myosin heavy-chain gene. Mol Cell Biol. 1987;7:4377–4389. doi: 10.1128/mcb.7.12.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke MH, Kaiser KK. Three ‘myosin ATPase' systems: the nature of their pH lability and sulfhydryl dependence. J Histochem Cytochem. 1970;18:670–672. doi: 10.1177/18.9.670. [DOI] [PubMed] [Google Scholar]
- Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–169. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cox RD, Buckingham ME. Actin and myosin genes are transcriptionally regulated during mouse skeletal muscle development. Dev Biol. 1992;149:228–234. doi: 10.1016/0012-1606(92)90279-p. [DOI] [PubMed] [Google Scholar]
- D'albis A, Anger M, Lompre A-M. Rabbit masseter expresses the cardiac α myosin heavy chain gene. FEBS Lett. 1993;324:178–180. doi: 10.1016/0014-5793(93)81388-g. [DOI] [PubMed] [Google Scholar]
- Delp MD, Duan C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Phys. 1996;80:261–270. doi: 10.1152/jappl.1996.80.1.261. [DOI] [PubMed] [Google Scholar]
- Denardi C, Ausoni S, Moretti P, Gorza L, Velleca M, Buckingham M, Schiaffino S. Type-2X-myosin heavy chain is coded by a muscle fiber type–specific and developmentally regulated gene. J Cell Biol. 1993;123:823–835. doi: 10.1083/jcb.123.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diagana TT, North DL, Jabet C, Fiszman MY, Takeda S, Whalen RG. The transcriptional activity of a muscle-specific promoter depends critically on the structure of the TATA element and its binding protein. J Mol Biol. 1997;265:480–493. doi: 10.1006/jmbi.1996.0752. [DOI] [PubMed] [Google Scholar]
- Dillon N, Grosveld F. Transcriptional regulation of multigene loci: multilevel control. Trends Genet. 1993;9:134–137. doi: 10.1016/0168-9525(93)90208-y. [DOI] [PubMed] [Google Scholar]
- Ennion S, Sant'ana J, Pereira, Sargeant AJ, Young A, Goldspink G. Characterization of human skeletal muscle fibres according to the myosin heavy chains they express. J Muscle Res Cell Motil. 1995;16:35–43. doi: 10.1007/BF00125308. [DOI] [PubMed] [Google Scholar]
- Galler S, Schmitt T, Pette D. Stretch activation, unloaded shortening velocity, and myosin heavy chain isoforms of rat skeletal muscle fibres. J Physiol (Lond) 1994;478:513–521. doi: 10.1113/jphysiol.1994.sp020270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulick J, Subramaniam A, Neumann J, Robbins J. Isolation and characterization of the mouse cardiac myosin heavy chain genes. J Biol Chem. 1991;266:9180–9185. [PubMed] [Google Scholar]
- Hämäläinen N, Pette D. The histochemical profiles of fast fiber types IIB, IID, and IIA in skeletal muscles of mouse, rat and rabbit. J Histochem Cytochem. 1993;41:733–743. doi: 10.1177/41.5.8468455. [DOI] [PubMed] [Google Scholar]
- Hämäläinen N, Pette D. Patterns of myosin isoforms in mammalian skeletal muscle fibres. Microsc Res Tech. 1995;30:381–389. doi: 10.1002/jemt.1070300505. [DOI] [PubMed] [Google Scholar]
- Hilber K, Galler S, Pette D. Functional differences of myosin heavy-chain isoforms in skeletal muscle. Naturwiss. 1997;84:201–204. doi: 10.1007/s001140050378. [DOI] [PubMed] [Google Scholar]
- Izumo S, Mahdavi V. Thyroid hormone receptor isoforms generated by alternative spicing differentially activate myosin HC transcription. Nature. 1988;334:539–542. doi: 10.1038/334539a0. [DOI] [PubMed] [Google Scholar]
- Izumo S, Nadal-Ginard B, Mahdavi V. All members of the MHC multigene family respond to thyroid hormone in a highly tissue-specific manner. Science. 1986;231:597–600. doi: 10.1126/science.3945800. [DOI] [PubMed] [Google Scholar]
- Jansen G, Groenen PJTA, Bächner D, Jap PHK, Coerwinkel M, Oerlemans F, van den Broek W, Gohlsch B, Pette D, Plomp JJ, et al. Abnormal myotonic dystrophy protein kinase levels produce only mild myopathy in mice. Nat Genet. 1996;13:316–324. doi: 10.1038/ng0796-316. [DOI] [PubMed] [Google Scholar]
- Loughna PT, Izumo S, Goldspink G, Nadal-Ginard B. Disuse and passive stretch cause rapid alterations in expression of developmental and adult contractile protein genes in skeletal muscle. Development (Camb) 1990;109:217–223. doi: 10.1242/dev.109.1.217. [DOI] [PubMed] [Google Scholar]
- Lucas C, Rughani A, Hoh JFY. Expression of extraocular myosin heavy chain in rabbit laryngeal muscle. J Muscle Res Cell Motil. 1995;16:368–378. doi: 10.1007/BF00114502. [DOI] [PubMed] [Google Scholar]
- Lyons GE, Ontell M, Cox R, Sassoon D, Buckingham M. The expression of myosin genes in developing skeletal muscle in the mouse embryo. J Cell Biol. 1990;111:1465–1476. doi: 10.1083/jcb.111.4.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker-Thornburg JB, Bauer B, Palerma J, Robbins J. Structural and developmental analysis of two linked myosin heavy chain genes. Dev Biol. 1992;150:99–107. doi: 10.1016/0012-1606(92)90010-e. [DOI] [PubMed] [Google Scholar]
- Periasamy M, Wieczorek DF, Nadal-Ginard B. Characterization of a developmentally regulated perinatal myosin heavy chain gene expressed in skeletal muscle. J Biol Chem. 1984;259:13573–13578. [PubMed] [Google Scholar]
- Peter JB, Barnard RJ, Edgerton VR, Gillespie CA, Stempel KE. Metabolic profiles of three fiber types of skeletal muscles in guinea pigs and rabbits. Biochemistry. 1972;11:2627–2633. doi: 10.1021/bi00764a013. [DOI] [PubMed] [Google Scholar]
- Pette D, Staron RS. Cellular and molecular diversities of mammalian skeletal muscle fibers. Rev Physiol Biochem Pharmacol. 1990;116:1–76. doi: 10.1007/3540528806_3. [DOI] [PubMed] [Google Scholar]
- Pette D, Vrbova G. Neural control of phenotypic expression in mammalian muscle fibers. Muscle Nerve. 1985;8:676–689. doi: 10.1002/mus.880080810. [DOI] [PubMed] [Google Scholar]
- Rindt HJ, Gulick J, Knotts S, Neumann J, Robbins J. In vivo analysis of the murine β-myosin heavy chain gene promoter. J Biol Chem. 1993;268:5332–5338. [PubMed] [Google Scholar]
- Rowlerson A, Pope B, Murray J, Whalen RB, Weeds AG. A novel myosin present in cat jaw-closing muscles. J Muscle Res Cell Motil. 1981;2:5332–5338. [Google Scholar]
- Saez LJ, Gianola KM, McNally EM, Feghali R, Eddy R, Shows TB, Leinwand LA. Human cardiac myosin heavy chain genes and their linkage in the genome. Nucleic Acids Res. 1987;15:5443–5459. doi: 10.1093/nar/15.13.5443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiaffino S, Reggiani C. Myosin isoforms in mammalian skeletal muscle. J Appl Physiol. 1994;77:493–501. doi: 10.1152/jappl.1994.77.2.493. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: gene regulation and functional significance. Physiol Rev. 1996;76:371–423. doi: 10.1152/physrev.1996.76.2.371. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Gorza L, Sartore S, Saggin L, Carli M. Embryonic myosin heavy chain as a differentiation marker of human developing muscle and rhabdomyosarcoma. A monoclonal antibody study. Exp Cell Res. 1986;163:211–220. doi: 10.1016/0014-4827(86)90574-4. [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Gorza L, Sartore S, Saggin L, Ausoni S, Vianello M, Gundersen K, Lomo T. Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. J Muscle Res Cell Motil. 1989;10:197–205. doi: 10.1007/BF01739810. [DOI] [PubMed] [Google Scholar]
- Smerdu V, Karch-Mizrachi I, Campione M, Leinwand L, Schiaffino S. Type IIx myosin heavy chain transcripts are expressed in type IIb fibers of human skeletal muscle. Am J Physiol. 1994;267:C1723–C1728. doi: 10.1152/ajpcell.1994.267.6.C1723. [DOI] [PubMed] [Google Scholar]
- Solaro RJ, Pang DC, Briggs N. The purification of cardiac myofibrils with Triton X-100. Biochim Biophys Acta. 1971;245:259–262. doi: 10.1016/0005-2728(71)90033-8. [DOI] [PubMed] [Google Scholar]
- Spudich JA. How molecular motors work. Nature. 1994;372:515–518. doi: 10.1038/372515a0. [DOI] [PubMed] [Google Scholar]
- Staron RS, Johnson P. Myosin polymorphism and differential expression in adult human skeletal muscle. Comp Biochem Physiol. 1993;106:463–475. doi: 10.1016/0305-0491(93)90120-t. [DOI] [PubMed] [Google Scholar]
- Takeda S, North DL, Diagana T, Miyagoe Y, Lakich MM, Whalen RG. Myogenic regulatory factors can activate TATA-containing promoter elements via an E-box independent mechanism. J Biol Chem. 1995;270:15664–15670. doi: 10.1074/jbc.270.26.15664. [DOI] [PubMed] [Google Scholar]
- Termin A, Staron RS, Pette D. Changes in myosin heavy chain isoforms during chronic low-frequency stimulation of rat fast hindlimb muscles. A single fiber study. Eur J Biochem. 1989;186:749–754. doi: 10.1111/j.1432-1033.1989.tb15269.x. [DOI] [PubMed] [Google Scholar]
- Warrick HM, Spudich JA. Myosin structure and function in cell motility. Annu Rev Cell Biol. 1987;3:379–421. doi: 10.1146/annurev.cb.03.110187.002115. [DOI] [PubMed] [Google Scholar]
- Weiss A, Mayer DCG, Leinwand LA. Diversity of myosin-based motility: multiple genes and functions. Soc Gen Physiol Ser. 1994;49:159–171. [PubMed] [Google Scholar]
- Weiss A, Leinwand LA. The mammalian myosin heavy chain gene family. Annu Rev Cell Dev Biol. 1996;12:417–439. doi: 10.1146/annurev.cellbio.12.1.417. [DOI] [PubMed] [Google Scholar]
- Weydert A, Daubas P, Caravetti M, Minty A, Bugaisky G, Cohen A, Robert B, Buckingham M. Sequential accumulation of mRNAs encoding different myosin heavy chain isoforms during skeletal muscle development in vivodetected with a recombinant plasmid identified as coding for an adult fast myosin heavy chain from mouse skeletal muscle. J Biol Chem. 1983;258:13867–13874. [PubMed] [Google Scholar]
- Weydert A, Barton P, Harris JA, Pinset C, Buckingham M. Developmental pattern of mouse skeletal myosin heavy chain gene transcripts in vivo and in vitro. Cell. 1987;49:121–129. doi: 10.1016/0092-8674(87)90762-8. [DOI] [PubMed] [Google Scholar]
- Wieczorek DF, Periasamy M, Butler-Browne GS, Whalen RG, Nadal-Ginard B. Coexpression of multiple myosin heavy chain genes, in addition to a tissue-specific one, in extraocular musculature. J Cell Biol. 1985;101:618–629. doi: 10.1083/jcb.101.2.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon SJ, Seller SH, Kucherlapati R, Leinwand LA. Organization of the human myosin heavy chain gene cluster. Proc Natl Acad Sci USA. 1992;89:12078–12082. doi: 10.1073/pnas.89.24.12078. [DOI] [PMC free article] [PubMed] [Google Scholar]