Abstract
All known proteins that accumulate in the vacuolar space surrounding the obligate intracellular protozoan parasite Toxoplasma gondii are derived from parasite dense granules. To determine if constitutive secretory vesicles could also mediate delivery to the vacuolar space, T. gondii was stably transfected with soluble Escherichia coli alkaline phosphatase and E. coli β-lactamase. Surprisingly, both foreign secretory reporters were delivered quantitatively into parasite dense granules and efficiently secreted into the vacuolar space. Addition of a glycosylphosphatidylinositol membrane anchor rerouted alkaline phosphatase to the parasite surface. Alkaline phosphatase fused to the transmembrane domain and cytoplasmic tail from the endogenous dense granule protein GRA4 localized to dense granules. The protein was secreted into a tuboreticular network in the vacuolar space, in a fashion dependent upon the cytoplasmic tail, but not upon a tyrosine-based motif within the tail. Alkaline phosphatase fused to the vesicular stomatitis virus G protein transmembrane domain and cytoplasmic tail localized primarily to the Golgi, although staining of dense granules and the intravacuolar network was also detected; truncating the cytoplasmic tail decreased Golgi staining and increased delivery to dense granules but blocked delivery to the intravacuolar network. Targeting of secreted proteins to T. gondii dense granules and the plasma membrane uses general mechanisms identified in higher eukaryotic cells but is simplified and exaggerated in scope, while targeting of secreted proteins beyond the boundaries of the parasite involves unusual sorting events.
T oxoplasma gondii is an obligate intracellular protozoan parasite that is the leading cause of focal central nervous system infections in HIV-infected patients. This parasite is capable of invading and replicating within all nucleated mammalian cells. T. gondii resides intracellularly within a vacuole that neither acidifies nor fuses with organelles of the endocytic cascade (for review see Sinai and Joiner, 1997). The intracellular parasite secretes prodigious amounts of proteins into the vacuolar space enclosed by the parasitophorous vacuolar membrane (PVM),1 which surrounds the parasite inside cells (for review see Silverman and Joiner, 1996). These secreted proteins associate with the PVM, with a tubuloreticular network in the vacuolar space (Sibley et al., 1986), or with both.
T. gondii and related Apicomplexan parasites (e.g., Plasmodia, Cryptosporidia, Sarcocystis, Eimeria) contain three morphologically distinct secretory organelles (rhoptries, micronemes, and dense granules), providing a particularly interesting model for studying secretory granule targeting. Dense granules are 200-nm organelles localized throughout the parasite (for review see Cesbron-Delauw, 1994). In contrast to the anterior rhoptries and micronemes, organelles that discharge at the time of invasion (Perkins, 1992), dense granule exocytosis is thought to occur primarily after invasion, and to continue during the intracellular residence of the organism (Dubremetz et al., 1993; Carruthers and Sibley, 1997).
10 dense granule proteins are identified in T. gondii: GRA1–GRA7, two isoforms of the nucleoside triphosphate hydrolase (NTPase), and cyclophilin 18 (for reviews see Cesbron-Delauw, 1994; Silverman and Joiner, 1996). Except for cyclophilin 18, these proteins not only lack significant homology with other proteins in the data base, but they also bear limited amino acid sequence homology with one another. No common sequences are identified that might target these proteins to dense granules. GRA4, GRA5, GRA6, and GRA7 contain putative transmembrane domains, yet all are secreted into the vacuolar space and localize to the vacuolar network (GRA4 and GRA6), to the PVM (GRA5), or to both (GRA7). In contrast, the five major surface proteins of T. gondii, including SAG1, are anchored to the plasma membrane by glycosylphosphatidylinositol (GPI) membrane anchors (Nagel and Boothroyd, 1989; Tomavo et al., 1989) and are not found in the vacuolar space. The vesicles transporting GPI-anchored proteins to the parasite plasma membrane are not identified.
While there is little precedent for understanding protein sorting events to secretory organelles and to the vacuolar space in T. gondii or in any other pathogenic protozoan parasite (Becker and Melkonian, 1996), all secreted proteins in higher eukaryotic cells, whether soluble or membrane associated, follow an identical pathway from the endoplasmic reticulum to the TGN (Burgess and Kelly, 1987). In secretory cells, immature secretory granules (ISG) form in the trans-Golgi and are sorted at that point from constitutive secretory vesicles (CSV) (for review see Urbe et al., 1997). Soluble and membrane granule proteins accumulate in ISG, either by signals mediating entry or by signals mediating retention. Motifs within the ectodomain or cytoplasmic tail of transmembrane proteins may confer information for targeting to or removal from ISG during their formation and subsequent maturation to mature secretory granules (MSG) (Didier et al., 1992; Colomer et al., 1994; Milgram et al., 1996; Dittie et al., 1997; for review see Urbe et al., 1997). GPI-anchored proteins may also be components of secretory granules through signals contained in the ectodomain (Colomer et al., 1996).
To explore the population of vesicles that transport soluble proteins in T. gondii, the parasite was stably transformed with the genes for two soluble foreign secretory reporters, E. coli β-lactamase (BLA) and E. coli alkaline phosphatase (BAP), which should not contain targeting information for delivery to secretory granules. Surprisingly, both reporters localized quantitatively to parasite dense granules, allowing subsequent analysis of targeting signals conferred by addition of membrane anchoring domains to BAP.
Materials and Methods
Buffers
Buffers, including PBS, artificial intracellular salt solution (AISS), electroporation buffer, lysis buffer, and immunoprecipitation wash buffer, were as reported earlier (Ossorio et al., 1994; Roos et al., 1994; Beckers et al., 1995). For secretion assays, AISS buffer with 1× vitamin/amino acid mix (RPMI-1640 Select Amine Kit; GIBCO BRL, Gaithersburg, MD), 5 mM ATP, and protease inhibitors (1 mM PMSF, 5 μM leupeptin, 1 μM aprotinin, 5 μM pepstatin, and 100 μg/ml chymostatin) was used. Biotinylation buffer consisted of 10 mM triethanolamine, pH 9.0, 2 mM CaCl2, 150 mM NaCl.
Reagents
Restriction endonucleases, T4 polymerase, and DNA ligase were from New England Biolabs (Beverly, MA). Taq polymerase was from Perkin-Elmer Cetus (Norwalk, CT). DNA sequenase version 2.0 dideoxy chain termination kit was from U.S. Biochemical (Cleveland, OH). Geneclean II was from Bio 101 (La Jolla, CA). Rabbit antibacterial alkaline phosphatase (BAP) and anti-BLA were from 5 Prime → 3 Prime (Boulder, CO). Methionine-free DME and was from GIBCO BRL. Pro-mix l-35S in vivo cell labeling mix and ECL detection kit were from Amersham Corp. (Arlington Heights, IL). Protein A–Sepharose CL-4B was from Pharmacia Biotech (Piscataway, NJ). Phospholipase C was stored at −20°C in 10 mM Tris, pH 7.5 with 50% glycerol (ICN Pharmaceuticals, Aurora, OH). NHS-SS-biotin, stored at 200 mg/ml in DMSO, and streptavidin beads were from Pierce Chemical Co. (Rockford, IL). All other chemicals were from Sigma Chemical Co. (St. Louis, MO).
Growth of Parasites in Mice and Tissue Culture Cells
T. gondii RH strain tachyzoites lacking hypoxanthine-guanine-xanthine phosphoribosyl transferase (provided by D. Roos, University of Pennsylvania, Philadelphia, PA) were maintained by serial passage in the peritoneum of Swiss-Webster mice or by in vitro culture in Vero cells or human foreskin fibroblasts as previously described (Beckers et al., 1994). Parasites stably expressing BAP or BLA were maintained by serial passage in host cells at 37°C in medium containing 25 μg/ml mycophenolic acid and 50 μg/ml xanthine. RH strain tachyzoites expressing BAP-GPI, BAP-GRA4 constructs, and BAP-G constructs together with the dihydrofolate reductase (DHFR) gene were maintained in medium containing 1 μM pyrimethamine.
Plasmid Constructs
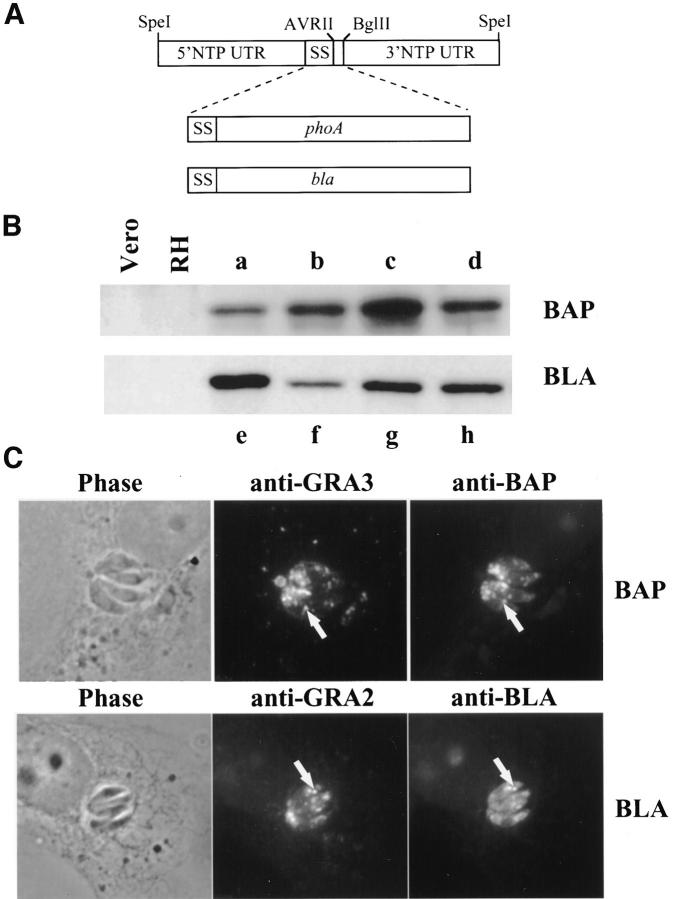
All secretion reporters were initially cloned into the plasmid pNTP/sec (Karsten et al., 1997) for transient expression. This vector contained the 5′ untranslated region (UTR) and 3′ UTR and NH2-terminal signal sequence from the gene for the T. gondii NTPase, an endogenous dense granule protein, as well as an AvrII/BglII cloning site (Fig. 1 A).
Figure 1.
BAP and BLA are stably expressed in T. gondii tachyzoites and localize to parasite dense granules. (A) Organization of the NTPase 5′ UTR containing the promoter (Nakaar et al., 1997), NH2-terminal signal sequence (SS), and 3′ UTR in the plasmid pNTPSec. AvrII and BglII restriction sites were used for inserting the coding regions of E. coli alkaline phosphatase (phoA) and β-lactamase (bla). (B) Expression of the transgenes in single clones from BAP (a–d) or BLA (e–h) transfected parasites was monitored by SDS-PAGE and immunoblot. Also shown are nontransfected parasites (RH) and uninfected Vero cells, indicating no cross-reaction of the antibody with endogenous parasite or cell proteins. (C) Phase contrast and corresponding immunofluorescence images of single clones stained with antibody to the endogenous dense granule protein GRA3 or with antibodies to BAP or to BLA. Precise colocalization of GRA3 with BAP or GRA2 with BLA in dense granules is shown (arrows). (D and E) Ultrathin cryosections of intracellular T. gondii stably expressing BAP or BLA viewed by immunoelectron microscopy. Gold particles were localized in dense granules (DG) (D, BAP; E, BLA) and the intravacuolar network (NW) for BAP (D). Bars, 100 nm.
For the BAP construct, an AvrII/BglII fragment coding for residues 23–449 of the BAP sequence was amplified from plasmid pHI (Inouye et al., 1981). For the BLA gene, an AvrII/BglII fragment coding for BLA was amplified from pBluescript. For the BAP-GPI construct, a PstI/BglII fragment of SAG1 coding for residues 287–319 was amplified from T. gondii genomic DNA (Burg et al., 1988). The digested and purified PCR product together with an AvrII/PstI fragment of BAP were cloned into pNTP/sec cut with AvrII and BglII. BAP-GRA4 was of similar design and contained residues 275–345, encoding the putative transmembrane domain and cytoplasmic tail, fused downstream of BAP. The GRA4 fragment was amplified from genomic DNA (Mevelec et al., 1992). The BAP-GRA4tail− construct was made by introducing a stop codon at residue 298 of the GRA4 domain. BAP-GRA4Y332A was prepared by mutating codon 332 from tyrosine to alanine. BAP-G contained residues 464–511 from the vesicular stomatitis virus G (VSV-G) protein, encoding the transmembrane domain and cytoplasmic tail, introduced downstream of BAP. The VSV-G fragment was amplified from pSVGL1 (Rose and Bergmann, 1983) and cloned with an AvrII/PstI fragment of BAP into pNTP/Sec. To make BAP-Gtail−, a stop codon was introduced at codon 489 of the VSV-G tail to yield a protein with five cytoplasmic residues. BAP-GY501A was constructed by mutating codon 501 (coding for tyrosine) of the VSV-G tail to code for alanine (Thomas et al., 1993).
Plasmids for stable transfection were prepared by digesting the above constructs with SpeI. The SpeI fragments were subcloned into the SpeI site of pminCAT/HXGPRT or pDHFR-TSc3 containing the mutated DHFR gene conferring pyrimethamine resistance (kindly provided by D. Roos) (Donald et al., 1996), as described earlier (Karsten et al., 1997).
Primers
All primers are 5′ to 3′. BAP-AvrII/BglII: CGC CTA GGG ACA CCA GAA ATG CCT GTT C and CGG AGA TCT TTA TTT TCA GCC CCA GGG. BLA: GAA GCC TAG GCC ACC CAG AAA CGC TGG TG and GGA AGA TCT TAC CAA TGC TTA ATG AG. BAP-AvrII/ PstI: CGC CTA GGG ACA CCA GAA ATG CCT GTT C and GGC TGC AGC TTT CAG CCC CAG GGC GGC. SAG1: GGG GCT GCA GGG TCA GCA and GGA GAT CTC ACG CGA CAC AAG CTG CG. GRA4: GGT CGC TGC AGG AAT CCT GAC GGG and CAG AGA TCT CAC TCT TTG CGC TTG CGC ATT C. BAP-GRA4 tail−: GCC AAG ATC TCA CTT GAC AGC CTT TGC. BAP-GRA4 Y332A: GAG AGA TCT CAC TCT TTG CGC ATT CTT TCC AAA TCC TTC AAT AAC TCG GCG GGT GAG GGT CGC GGG. VSV-G: TTC ACT GCA GGC AAA AGC TCT ATT GCC and GCA GAT CTT ACT TTC CAA GTC GGT TC. BAP-G tail−: CGA AAT TAA ATT CTA GAG TTA CCT ATG G. BAP-G Y501A: GCA GAT CTT ACT TTC CAA GTC GGT TCA TCT CTA TGT CTG TAG CAA TCT GTC TTT T.
Transfection and Selection of Stable Lines
Transient transfection was performed as described (Roos et al., 1994). Plasmid DNA for transfection was isolated using Qiagen plasmid kits (Chatsworth, CA). For preparation of stable lines, electroporated parasites were cultured in Vero or HFF cells in selection media and then cloned by growth under soft agar as described earlier (Karsten et al., 1997). A 10-cm Petri dish containing Vero cells was infected with about 200 parasites and incubated at 37°C for 20 h. The monolayer was washed three times with PBS++ (plus Mg2+ and Ca2+) and replaced with a mixture of 2× Vero medium plus either 2 μM pyrimethamine or 50 μg/ml mycophenolic acid and 100 μg/ml xanthine and warm 1.6% agarose (43°C). Dishes were incubated until colonies appeared, and then clones were picked and expanded.
Immunofluorescence and Immunoelectron Microscopy
Immunofluorescence on infected cells or extracellular parasites was done as previously reported (Joiner et al., 1990). Detection was performed with rabbit anti-BAP (1:500) or anti-BLA (1:100) and mouse monoclonal antibodies to GRA3 (T62H11) or GRA2 (T41F51D10) diluted 1: 250 in PBS containing 3% BSA, followed by FITC-conjugated goat anti–rabbit IgG and rhodamine-conjugated goat anti–mouse IgG diluted 1:500 in PBS containing 3% BSA. Coverslips were mounted with Mowiol and observed with an epifluorescence microscope (model Microphot FXA; Nikon, Inc., Melville, NY). Images were captured with a CCD camera (Photometrics, Tuscon, AZ) and processed with Image-Pro Plus (Media Cybernetics, Silver Spring, MD).
For immunoelectron microscopy, monolayers of Vero cells were infected with transgenic T. gondii for 24 h. Infected cells were processed, and sections for immunoelectron microscopy were prepared as previously described (Beckers et al., 1994). Sections were labeled with rabbit anti-BAP (1:250), rabbit anti-BLA (1:100), and mouse anti-SAG1 (T41E5) (1: 2,000) antibody in PBS containing 10% BSA.
SDS-PAGE and Immunoblot
SDS-PAGE and immunoblot were performed according to Laemmli (1970) and Towbin (1979). Immunoblots were developed using rabbit anti-BAP (1:5,000), rabbit anti-BLA (1:1,000), mouse anti-SAG1 (1:1,000), or rabbit anti–cross-reacting determinant (CRD, 1:2,000) (provided by P. Englund, Johns Hopkins University, Baltimore, MD) (Bangs et al., 1985) followed by goat anti–rabbit IgG-HRP or goat anti–mouse IgG-HRP conjugate (1:2,000), and the ECL detection system (Amersham Corp.).
Quantitation of BLA Released from Extracellular Parasites Treated with Brefeldin A
BLA release from extracellular parasites treated with brefeldin A (BFA) was quantitated by enzymatic assay. Six 10-cm Vero dishes with 2–3 × 107 BLA-expressing parasites, or three 10-cm uninfected Vero dishes as control, were incubated at 37°C for 36–40 h. BFA (50 μg/ml) was added to three infected dishes, and incubation was continued at 37°C for 60 min. A high concentration of a BFA was required to completely disperse the T. gondii Golgi complex, as assessed by immunofluorescence microscopy antiserum (kindly provided by G. Wood, University of Vermont, Burlington, VT) and G. Langsley (Institut Pasteur, Paris, France) to Plasmodium falciparum Rab6. Plates were then put on ice and washed three times with cold secretion buffer. The monolayer was scraped into cold buffer and passed twice through a 27-gauge needle. Then parasites were harvested by centrifugation at 1,450 g for 5 min at 4°C. Parasites were washed 3× at 4°C with secretion buffer. To a total of 2 × 108 extracellular parasites in 1 ml, BFA (20 μg/ml) was added for previously BFA-treated parasites. After incubation at 37°C for 0, 30, 60, and 120 min, 250-μl aliquots were removed and spun at 7,000 rpm for 10 min at 4°C, and the supernatant was transferred to a fresh tube. BLA enzymatic activity in the supernatant was quantitated using described procedures (Laminet and Pluckthun, 1989) with modifications (Karsten et al., 1997).
Surface Delivery of SAG1 in the Presence and Absence of BFA
Delivery of SAG1 to the parasite surface, with or without BFA treatment, was assessed by biotinylation. Infected monolayers (24 h) were suspended in 2 ml/dish methionine and cysteine-free DME with or without 50 μg/ml BFA. After incubation at 37°C for 1 h, 125 μCi pro-mix l-35S in vivo cell labeling mix was added to each dish. Incubation was continued at 37°C for another 60 min, and parasites were liberated as described above.
For surface biotinylation, the parasite pellet was suspended in NHS-SS-biotin (1.5 mg/ml) in biotinylation buffer at 4°C for 30 min with very gentle agitation. Parasites were centrifuged at 1,450 g at 4°C for 5 min and then resuspended in 1 ml PBS2+ with 100 mM glycine and incubated at 4°C for 20 min to quench unreacted biotin.
For immunoprecipitation, parasite pellets were washed, suspended in lysis buffer, and precleared on protein A–Sepharose. To precleared lysates, 5 μl mouse anti-SAG1 (T41E5) and 100 μl protein A–Sepharose beads were added, and incubation continued at 4°C overnight. After washing, the beads were divided into two samples. For surface SAG1 quantification, 100 μl 1% SDS in 50 mM Tris-Cl, pH 7.5, 100 mM NaCl was added, and incubation was continued at 80°C for 10 min to elute bound SAG1. 900 μl lysis buffer was added to the beads, which were centrifuged at 1,500 rpm for 5 min, and the supernatant was transferred to a fresh tube containing 30 μl packed streptavidin beads. After overnight incubation at 4°C, beads were washed. Both total (protein A–Sepharose) and surface SAG1 (streptavidin beads) were quantitated by SDS-PAGE autoradiography and PhosphorImager analysis.
Phospholipase C Treatment
The presence of the GPI anchor was assessed by digestion with phosphatidylinositol-specific phospholipase C (PI-PLC). Parasites were harvested from infected monolayers and washed 3× in cold MEM without FCS. 1.5 × 107 RH strain–, BAP-, or BAP-GPI–expressing parasites were incubated with 0.1 U PI-PLC (ICN Biomedicals, Costa Mesa, CA) in MEM or in MEM without the enzyme for 1 h at 37°C. The reaction volume was 30 μl. Treated parasites were recovered by centrifugation (5 min, 1,450 g, 4°C), and the supernatants were saved. The cells were washed three times in MEM and then lysed in 30 μl SDS loading buffer. All samples were analyzed by SDS-PAGE and immunoblot.
Subcellular Fractionation of Infected Cells
Vero cell monolayers were infected with parasites expressing BAP, BAP-GPI, or BAP-G for 20–40 h. Plates were put on ice and then washed three times with PBS with protease inhibitors. The monolayer was scraped into buffer and passed twice through a 27-gauge needle. The parasites were harvested by centrifugation at 1,450 g for 5 min at 4°C. The supernatant was centrifuged at 10,000 g for 10 min at 4°C to pellet (10K pellet) the bulk of the intravacuolar network and any partially disrupted parasites (Ossorio et al., 1994). The supernatant was centrifuged at 100,000 g for 60 min at 4°C to pellet the remaining network (100K pellet). Proteins within the 100,000 g supernatant (100K supernatant) were precipitated with trichloroacetic acid. All fractions were analyzed by SDS-PAGE and immunoblot.
Results
E. coli Alkaline Phosphatase and β-Lactamase Are Stably Expressed in T. gondii Tachyzoites
Both soluble BAP and BLA were stably expressed in T. gondii. As shown in Fig. 1 B, BAP and BLA were readily detected by immunoblot in extracellular tachyzoites. Both reporters were of the expected size after signal sequence cleavage and comigrated with the endogenous protein expressed in E. coli (not shown), indicating that neither proteolysis, inappropriate processing, nor significant posttranslational modifications occurred in T. gondii.
BAP and BLA Localize to Dense Granules by Immunofluorescence and Immunoelectron Microscopy
Within intracellular parasites, both BAP and BLA localized by immunofluorescence to discrete punctate structures consistent with dense granules (Fig. 1 C). Dense granule localization was confirmed by colocalization of the BAP and BLA signals with the endogenous dense granule proteins GRA2 and GRA3.
Localization of BAP and BLA to dense granules was confirmed by immunoelectron microscopy (Fig. 1, D and E). Both BAP (Fig. 1 D) and BLA (not shown) also associated with the intravacuolar network within the parasitophorous vacuole space, suggesting that localization of soluble proteins to the network morphologically does not depend upon parasite-specific sequences.
Release of BLA from T. gondii Dense Granules Is Not Blocked by Brefeldin A
These results suggest that the pathway for soluble secretory proteins in T. gondii is to dense granules. They do not, however, preclude the simultaneous delivery of soluble secretory proteins into more typical CSV, which also carry soluble cargo to and fuse with the plasma membrane. These vesicles are generally difficult to detect by electron microscopy because of their low abundance, presence in a background of membrane structures, and low concentration of cargo.
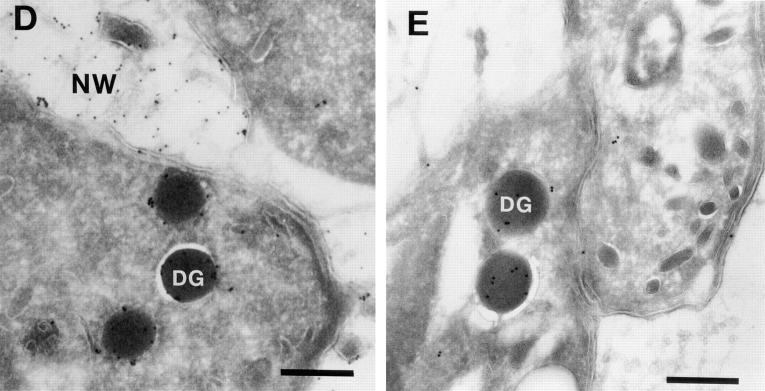
We distinguished between these possibilities using BFA. Secretion from preformed dense core granules is not blocked by BFA (Rosa et al., 1992), whereas CSV, which move rapidly to the plasma membrane, are depleted in the presence of the drug. There was no significant effect on BLA secretion after 1 h of treatment with BFA (Fig. 2 A). Under these same conditions, BFA did block vesicular transport in the parasite, since delivery of the GPI-anchored surface protein SAG1 to the parasite plasma membrane was inhibited by over 85% (Fig. 2 B). Taken together, these results suggest that soluble BAP and BLA are quantitatively delivered to dense granules.
Figure 2.
Short-term BFA treatment does not block BLA secretion but inhibits SAG1 delivery to the plasma membrane. (A) BLA release from extracellular parasites is not blocked by short-term treatment with BFA. Infected monolayers containing BLA-expressing parasites were incubated for 60 min at 37°C with or without BFA, at which point parasites were liberated from the host cells. BLA secreted from the extracellular parasites during continued incubation at 37°C was quantitated by enzymatic assay. There was no difference in the amount of protein released by BFA-treated or untreated parasites. The release over 1 h corresponds to 22–23% of total parasite BLA at time 0. Similar results were observed for release of BAP and the endogenous dense granule protein GRA3 (not shown). (B) Delivery of SAG1 to the parasite surface is inhibited by short-term treatment with BFA. Infected monolayers were treated with or without BFA for 1 h to deplete Golgi-derived constitutive secretory vesicles and then metabolically labeled for an additional hour in the continued presence or absence of BFA. Parasites liberated from the infected cells were surface biotinylated. After surface biotinylation, lysed parasites were immunoprecipitated with SAG1 and protein A beads to quantitate the total (Total lanes) amount of labeled SAG1 present. In half of the sample, biotinylated SAG1 was determined by releasing bound SAG1 from protein A beads and rebinding to Avidin Sepharose beads (Surface lanes). In untreated parasites, 46% of total SAG1 was detected on the parasite surface after 1 h of continuous labeling, whereas less than 7% of total SAG1 was on the surface in BFA-treated parasites.
Addition of a GPI Anchor to BAP Results in Expression on the Plasma Membrane
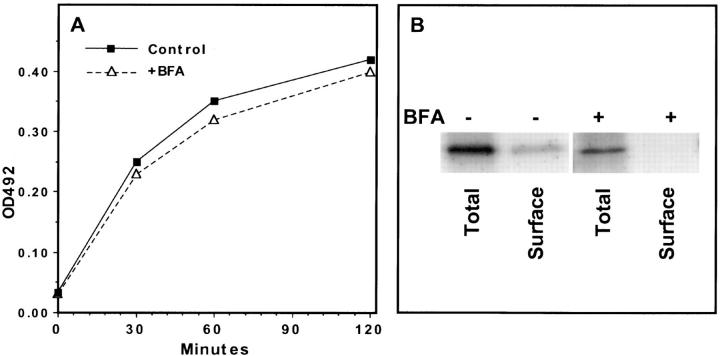
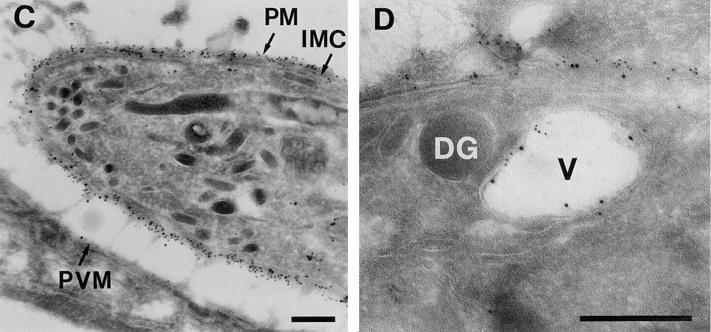
We therefore tested whether a GPI anchor was sufficient to route BAP out of dense granules and into CSV. The 33 residue GPI anchor addition site from SAG1 was added to the COOH terminus of BAP (Fig. 3 A). Alkaline phosphatase with a GPI anchor (BAP-GPI) was clearly expressed on the surface of T. gondii as shown by immunofluorescence microscopy (Fig. 3 B). Immunoelectron microscopy showed colocalization of BAP-GPI with the surface antigen SAG1 (Fig. 3, C and D). No localization to dense granules was found, whereas both BAP-GPI and SAG1 were detected in association with the membrane at the periphery of large electron-lucent vesicles of larger than 500 nm in diameter (Fig. 3 D).
Figure 3.
GPI-anchored alkaline phosphatase localizes to the plasma membrane by immunofluorescence and immunoelectron microscopy. (A) Design of BAP-GPI construct showing the addition of the SAG1-GPI anchor addition site to BAP. (B) Infected cells containing parasites transfected with BAP-GPI were fixed and permeabilized with either methanol (MeOH), which optimizes detection of proteins on the parasite surface or secreted into the vacuolar space, or with paraformaldehyde and Triton X-100 (PFA/ Tx), which optimizes staining of dense granules and other organelles within the parasite. Fixed samples were stained with the anti-GRA3 mAb and the anti-BAP antiserum. Note the presence for BAP-GPI of surface staining (MeOH, anti-BAP), but the absence of dense granule staining (PFA/Tx, anti-BAP), when compared with staining for GRA3. (C and D) Cryosections of BAP-GPI–expressing parasites were labeled with antibodies to SAG1 (5-nm gold) and antibodies to BAP (10-nm gold). 5- and 10-nm gold particles colocalize at the plasma membrane (PM) and inner membrane complex (IMC) of transgenic parasites. In perinuclear areas, membranes of large electron-lucent vesicles (V) were labeled with gold particles of both sizes (C). Bars, 200 nm.
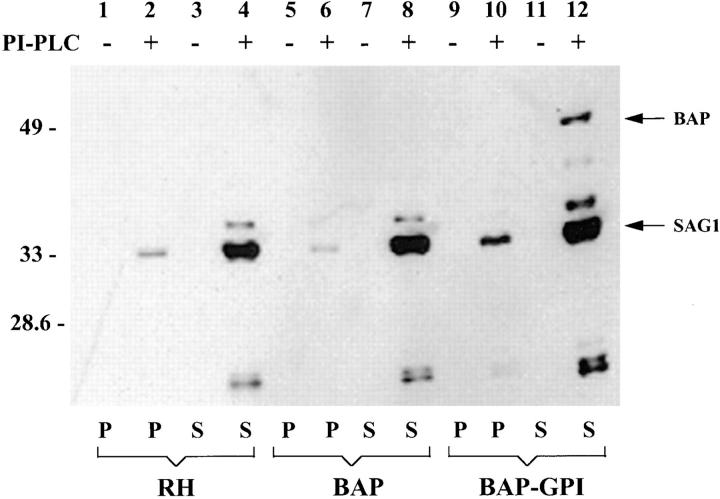
We confirmed that BAP-GPI was linked to the plasma membrane of T. gondii by a GPI anchor (Fig. 4). Parasites expressing BAP-GPI were treated with PI-PLC, and the release of BAP containing the CRD, indicative of cleavage of a GPI-membrane anchor, was demonstrated by immunoblot.
Figure 4.
Phospholipase C treatment exposes a CRD epitope in BAP-GPI. RH strain, BAP-expressing, or BAP-GPI–expressing parasites were treated with PI-PLC (+) or incubated in medium (−). After treatment, the parasites were centrifuged, and the resulting supernatants (S) and pellets (P) were analyzed by SDS-PAGE and immunoblot with antibodies to the CRD (Bangs et al., 1985), an epitope of the GPI anchor that is exposed only after cleavage with PLC. The CRD epitope was exposed on endogenous parasite proteins released into the supernatant, regardless of whether wild-type RH strain parasites or transgenic parasites expressing BAP or BAP-GPI parasites were tested (lanes 4, 8, and 12). These bands correspond to the endogenous surface proteins SAG1 (30 kD), SAG2 (22 kD), SAG3 (43 kD), p23, and p35. A small amount of SAG1 expressing the CRD epitope was present on the parasite surface after PI-PLC treatment (lanes 2, 6, and 10). In contrast, only BAP-GPI–expressing parasites treated with PI-PLC released BAP into the supernatant (lane 12).
Taken together with the results in Figs. 2 and 3, these data indicate that a GPI anchor is sufficient to target proteins to the plasma membrane in T. gondii, and that the predominant pathway for GPI-anchored proteins is to the plasma membrane.
Addition to BAP of the Putative Transmembrane Domain and Cytoplasmic Tail from GRA4 Results in Delivery to Dense Granules and Secretion into the Vacuolar Space
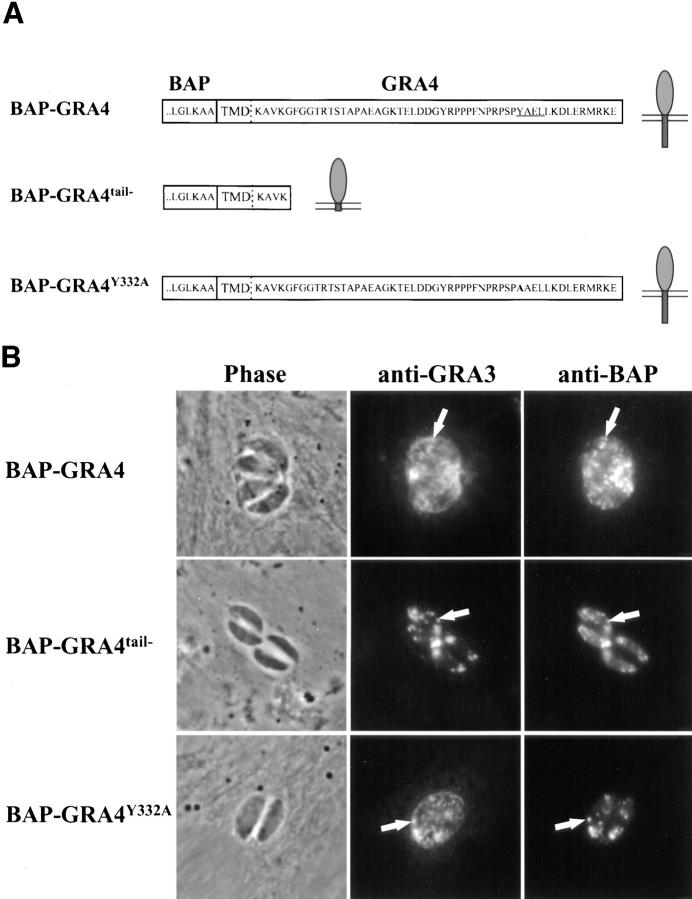
We next fused BAP to the putative transmembrane domain and tail from GRA4 (Mevelec et al., 1992), one of four dense granule proteins in T. gondii that contain a linear stretch of hydrophobic amino acids near the COOH terminus (Fig. 5 A). While predicted to be a type I integral membrane protein (and one of only a handful of such proteins identified to date in the organism), endogenous GRA4 is nonetheless secreted into the vacuolar space in association with the intravacuolar network.
Figure 5.
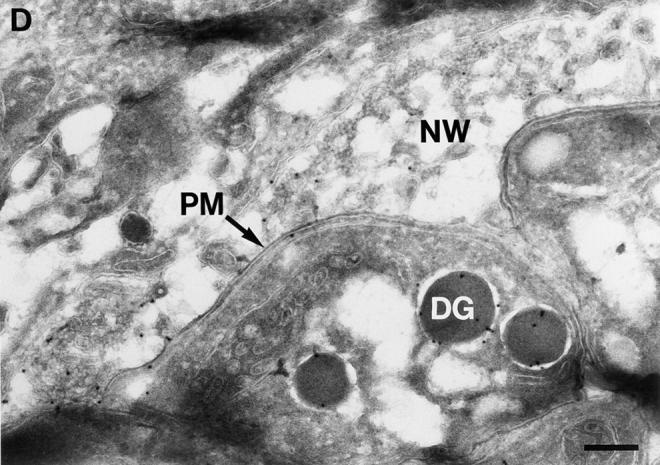
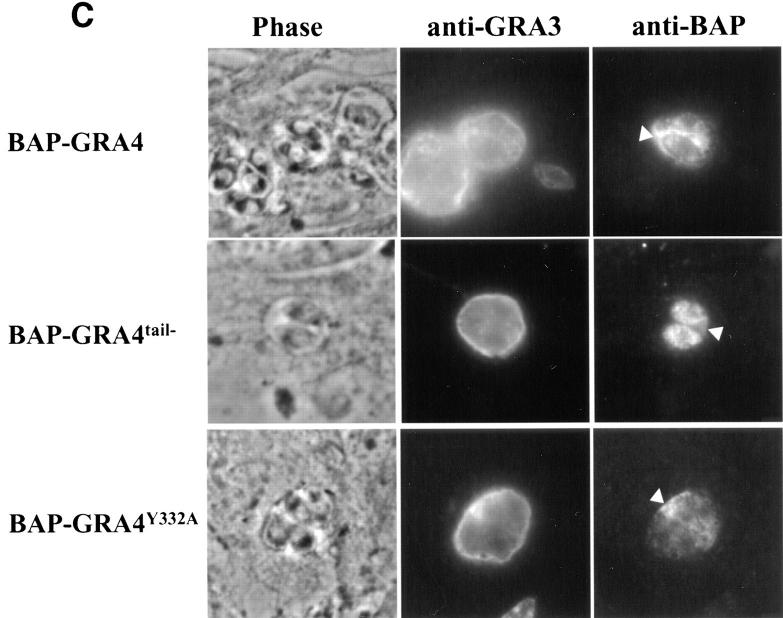
BAP-GRA4 localizes to dense granules and requires the cytoplasmic tail for efficient secretion into the intravacuolar network. (A) Design of BAP-GRA4, BAP-GRA4tail−, and BAP-GRA4Y332A constructs, showing addition of the GRA4 transmembrane and cytoplasmic tail to BAP and the two alterations within the tail. All of the constructs behaved as integral membrane proteins when analyzed in a heterologous in vitro translation/translocation system (not shown). (B) Infected cells containing parasites expressing BAP-GRA4, BAP-GRA4tail−, or BAP-GRA4Y332A were fixed with PFA and permeabilized with Triton X-100 to optimize staining of dense granules and other internal organelles of the parasite. Cells were stained with antibodies to BAP or the dense granule marker GRA3. The BAP signal colocalized with staining for GRA3 in all three chimeras, demonstrating delivery of BAP-GRA4, BAP-GRA4tail−, and BAP-GRA4Y332A to dense granules (arrows). (C) Infected cells containing parasites expressing BAP-GRA4, BAP-GRA4tail−, or BAP-GRA4Y332A were fixed with MeOH to optimize detection of proteins secreted into the vacuolar space. Cells were stained with antibodies to BAP or GRA3. The arrowheads show network staining for the BAP-GRA4 and BAP-GRA4Y332A constructs. Only minimal amounts (arrowhead) of GRA4tail− were detected in the vacuolar space. (D) Cryosections of BAP-GRA4–expressing parasites stained with antibodies to BAP. Gold particles localize primarily to dense granules (DG) and the intravacuolar network (NW), with some staining of the plasma membrane (PM). Bar, 200 nm.
BAP-GRA4 localized to dense granules (Fig. 5 B, arrows; Fig. 5 D) and was secreted in large amounts into the vacuolar space (Fig. 5 C, arrowheads) in association with the intravacuolar network (Fig. 5 D). BAP-GRA4 was also detected by immunoelectron microscopy at the parasite plasma membrane (Fig. 5 D), suggesting that delivery into the vacuolar space followed fusion of dense granules with the plasma membrane.
Truncating the GRA4 Cytoplasmic Tail Blocks Delivery to the Vacuolar Space
Truncating the cytoplasmic tail of GRA4 four residues downstream of the putative transmembrane domain to generate BAP-GRA4tail− did not alter localization to dense granules (Fig. 5 B), but the chimera was not secreted efficiently into the vacuolar space (Fig. 5 C). In contrast, a Y332A substitution within a potential tyrosine-based sorting motif (Fig. 5 A, YAEL) in the GRA4 cytoplasmic tail had no effect on localization to dense granules (Fig. 5 B) or secretion into the vacuolar space (Fig. 5 C).
Addition to BAP of the VSV-G Transmembrane Domain and Cytoplasmic Tail Results in Accumulation within the Golgi
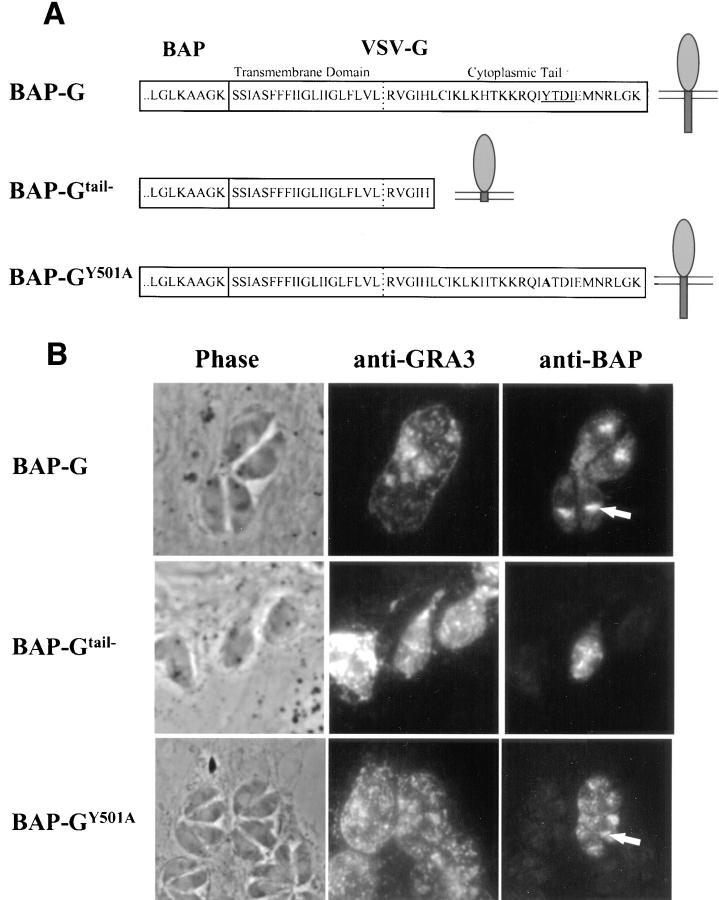
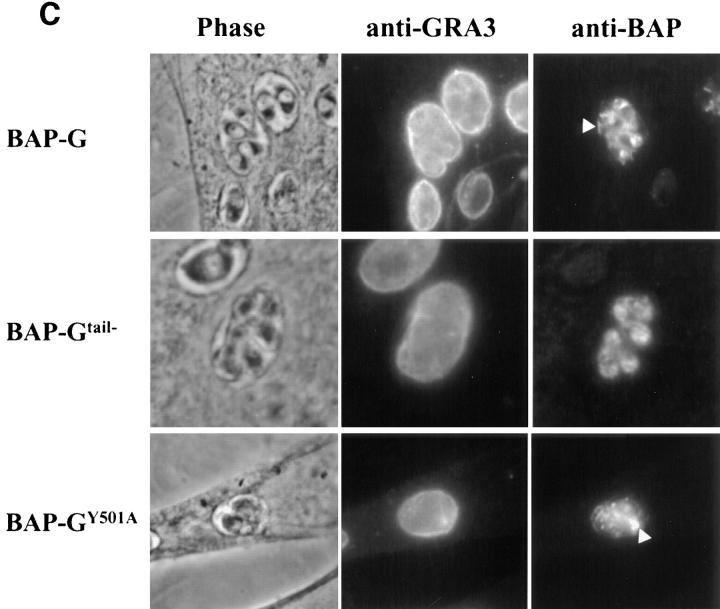
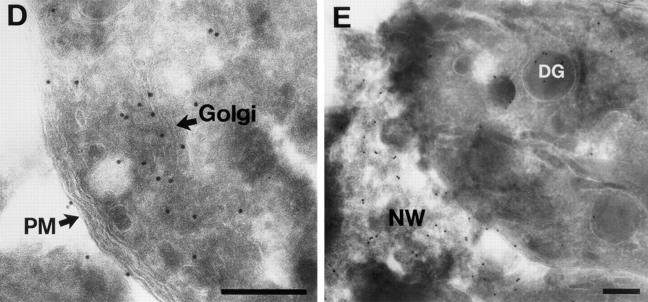
To determine whether the localization observed with BAP-GRA4 and mutants was seen with a foreign transmembrane domain and cytoplasmic tail, we added these regions from VSV-G to BAP to generate BAP-G (Fig. 6 A). BAP-G localized to a crescent-shaped structure anterior to the nucleus (Fig. 6 B, BAP-G, arrows), confirmed by immunoelectron microscopy to be the parasite Golgi (Fig. 6 D). BAP-G was detected to a lesser extent in vesicular structures containing GRA3. Dense granule localization was confirmed by immunoelectron microscopy (Fig. 6 E). BAP-G also localized to the vacuolar space, but not to the vacuolar membrane (Fig. 6 C, BAP-G, arrowhead). The BAP-G signal was detected by electron microscopy at the plasma membrane and in the intravacuolar network (Fig. 6, D and E).
Figure 6.
BAP-G localizes to the Golgi and requires the cytoplasmic tail for efficient secretion into the intravacuolar network. (A) Design of BAP-G, BAP-Gtail−, and BAP-G Y501A constructs, showing addition of the VSV-G transmembrane and cytoplasmic tail to BAP and the two alterations within the tail. (B) Infected cells containing parasites expressing BAP-G, BAP-Gtail−, or BAP-GY501A were fixed with PFA, permeabilized with Triton X-100, and then stained with antibodies to GRA3 or BAP. The arrows show staining of the parasite Golgi for the BAP-G and BAP-GY501A constructs. Golgi localization was not observed with BAP-Gtail−. Extensive colocalization of BAP-Gtail− and BAP-GY501A with GRA3 was noted, whereas the extent of dense granule staining for BAP-G was variable and less intense. (C) Infected cells containing parasites expressing BAP-G, BAP-Gtail−, or BAP-GY501A were fixed with MeOH. Cells were stained with antibodies to BAP or GRA3. Arrowheads show network staining for BAP-G and BAP-GY501A. Network staining for BAP-Gtail− was minimal. (D and E) Cryosections of BAP-G–expressing parasites stained with antibodies to BAP. Gold particles localize primarily to the Golgi (D), the plasma membrane (PM), and the intravacuolar network (NW), with occasional staining of dense granules (E, DG). Bars, 200 nm.
Partial deletion of the VSV-G cytoplasmic tail alters localization of BAP-G. Motifs in the VSV-G tail dictate either forward transport from the ER to Golgi (Nishimura and Balch, 1997), or basolateral targeting in polarized epithelial cells (Thomas et al., 1993). We therefore truncated the VSV-G tail five residues downstream of the transmembrane domain (Fig. 6 A, BAP-Gtail−). This construct localized to vesicular structures containing GRA3 (Fig. 6 B, BAP-Gtail−). No staining was detected in the region of the parasite Golgi. The BAP-Gtail− chimera was not detected in the vacuolar space (Fig. 6 C, BAP-Gtail−). Substitution of the single tyrosine within the VSV-G tail to alanine (Fig. 6 A, BAP-GY501A) did not eliminate Golgi staining but generally resulted in more extensive delivery of the chimera to vesicular structures containing GRA3 (Fig. 6 B, BAP-GY501A) when compared with BAP-G.
Taken together with the data for BAP-GRA4 and mutants, these results suggest that the cytoplasmic tail and transmembrane domain of an endogenous dense granule protein is either permissive for or mediates targeting to dense granules. The cytoplasmic tail from a nondense granule protein (VSV-G) influences either delivery to or retention within T. gondii dense granules. Finally, the cytoplasmic tail of both an endogenous and a foreign protein are necessary for efficient secretion into the vacuolar space.
Subcellular Fractionation of BAP, BAP-GPI, and BAP-G Confirms Their Microscopic Localization
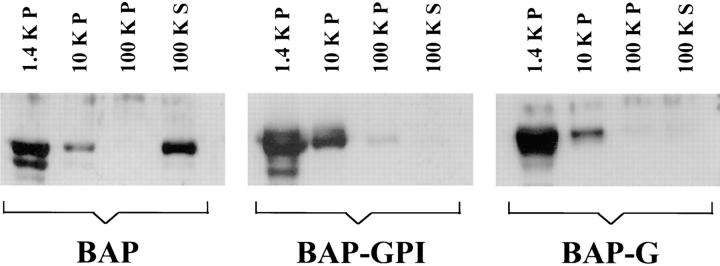
The behavior of BAP, BAP-GPI, and BAP-G was examined after subcellular fractionation of infected host cells (Fig. 7). All reporter proteins were found in large amounts in the 1,450-g parasite pellet, as expected. Of the material not sedimenting with parasites, BAP partitioned predominantly into the 100,000-g supernatant. In contrast, both BAP-GPI and BAP-G partitioned predominantly into the 10,000-g pellet, containing a portion of the intravacuolar network and lysed parasite ghosts. Neither was detected in the 100,000-g supernatant. Thus, all reporters behaved as expected based on their predicted sequence and microscopic localization.
Figure 7.
Subcellular fractionation of infected Vero cells containing transgenic T. gondii Vero cells infected with T. gondii expressing either BAP, BAP-GPI, or BAP-G were disrupted by syringe passage. Intact parasites were pelleted at 1,400 g (1.4K P), and the supernatant, containing material from the vacuolar space and from disrupted parasites, was subjected to further differential centrifugation. Identical parasite equivalents from the 1.4 K P, 10 K P, 100 K P, and 100 K S samples were analyzed by SDS-PAGE immunoblot with antibodies to BAP. Only BAP is a soluble protein in the vacuolar space.
Discussion
We examined sorting of secreted soluble and membrane proteins in the pathogenic protozoan parasite Toxoplasma gondii. While the protein targeting processes within the parasite could be construed as novel, they are also interpretable as simplified and/or exaggerated versions of sorting mechanisms used by mammalian cells. This is the interpretation we favor, as explained below. In contrast, targeting of membrane proteins beyond the confines of the organism and into the surrounding vacuolar space involves unusual sorting events.
Two principle models are suggested to account for delivery of soluble proteins into immature secretory granules budding from the TGN of higher eukaryotic cells: (a) The “sorting for entry”/active sorting model invokes binding of proteins to a secretory granule receptor or acceptor protein that is concentrated in the TGN and mediates delivery to the ISG (Rosa et al., 1989; Cool et al., 1997). (b) The “sorting by retention”/passive sorting model postulates that preferential aggregation of granule proteins, often induced by low pH and high calcium concentrations (Chanat and Huttner, 1991; Colomer et al., 1996), occurs within the ISG, retaining the proteins in the forming granule. In this model, both “constitutive” and “regulated” proteins enter ISG, but the former are removed by a vesicular budding process, generating constitutive secretory vesicles. There is growing evidence to support this model in both endocrine and exocrine cells (Kuliawat and Arvan, 1992, 1994; Castle et al., 1997). Vesicle budding from ISG is thought to be a mechanism to remove excess membrane from the granule during the condensation process leading to formation of MSG. Since neither we nor others have observed structures compatible with ISG in T. gondii, and since budding from ISG is a BFA-sensitive process (De Lisle and Bansal, 1996; Fernandez et al., 1997), it is possible that retention of soluble secreted proteins in T. gondii dense granules reflects the absence of substantial vesicle budding from these structures during their biogenesis. Our results are compatible with a simplified view of the passive sorting model for delivery of soluble proteins to dense granules in T. gondii, in which all soluble proteins are delivered to dense granules and retained by limiting the options for removal.
Could sorting of soluble proteins to dense granules be a consequence of the timing of dense granule gene expression (e.g., sorting by timing [Cabec et al., 1996])? This is unlikely for several reasons: (a) Dense granules are present in T. gondii throughout the cell cycle. (b) Soluble BAP expressed under the control of the SAG1 promoter and using the SAG1 signal sequence is still targeted to dense granules (Karsten, V., and K.A. Joiner, unpublished observations), as is a fusion of the green fluorescent protein to the COOH terminus of SAG1 deleted of the GPI anchor attachment site (Striepen et al., 1998).
Proteins with a GPI anchor are generally transported to the apical cell surface in polarized epithelial cells (Brown et al., 1989; for review see Rodriguez-Boulan and Powell, 1992). It has been suggested that GPI-anchored proteins associate with sphingoglycolipids and form glycolipid rafts in the TGN (for review see Simons and Eikonen, 1997). These microdomains are thought to form spontaneously above a critical concentration of glycolipids and are somehow configured for apical transport. The most likely explanation for our results is that GPI-anchored proteins in T. gondii are transported to the plasma membrane in TGN-derived vesicles, analogous to apical transport vesicles. We cannot, however, exclude the possibility that GPI-anchored proteins are transported initially to dense granules and are subsequently sorted into transport vesicles destined for the plasma membrane. A recent report suggested that SAG1 might form detergent-resistant oligomers when GPI anchored, but to a lesser extent when soluble or when fused to the transmembrane domain of CD46 (Seeber et al., 1998). Of interest, the SAG1-CD46 chimera was delivered to the cell surface, potentially reflecting association of endogenous SAG1 with SAG1-CD46, although transport vesicles were not identified. In fact, vesicles containing the GPI-anchored surface proteins of T. gondii have not been previously identified by either morphologic or biochemical techniques. Given their size (300–500 nm), the large lucent structures in which both SAG1 and BAP-GPI are localized in our study are not likely to be CSV. Of interest, however, is the fact that these structures resemble early endosomes morphologically (Griffiths et al., 1989). Our laboratory has recently identified T. gondii rab5 and rab7 homologues (Stedman, T., and K.A. Joiner, unpublished results), suggesting that structures resembling or functioning as early and late endosomes may ultimately be identified in the parasite.
The mechanism for biogenesis of the intravacuolar network is unclear. If the network is generated by vesiculation or tubulation from the plasma membrane (Halonen et al., 1996), sorting of BAP-GPI from BAP-G and BAP-GRA4 must occur at that location. Our results suggest that the cytoplasmic tails of membrane proteins could contribute to the budding process. While the mechanism for such an event is not clear, it could be analogous to budding of enveloped viruses, which is dependent upon binding of cytosolic core components of the virus to the cytoplasmic tails of envelope glycoproteins (e.g., VSV-G [Whitt et al., 1989]). Alternatively, the network may be released from a multivesicular structure at a specialized posterior invagination of the parasite (Sibley et al., 1995), in which case sorting mediated by the cytoplasmic tail would occur at an earlier step within the parasite. In either case, there are few precedents for delivery of transmembrane proteins to a membranous network outside cells.
Many transmembrane proteins are sorted out of ISG during maturation to MSG (for review see Urbe et al., 1997) in a process that typically depends upon sequences in the cytoplasmic tail. For example, the peptidylglycine α-amidating monooxygenase is less efficiently retained in granules as a transmembrane protein than after truncation of the cytoplasmic tail (Milgram et al., 1994), potentially analogous to our results with BAP-G. Patches of clathrin on ISG likely represent sites of coated vesicle formation directing removal of selected transmembrane proteins, through interaction with specific motifs in the cytoplasmic tail that mediate recruitment of the AP-1 adaptor (Dittie et al., 1997). The ultimate destination for such vesicles is not known, but could include endosomes, the cell surface, or vesicles recycling back to the TGN. Clathrin-coated vesicles are seen adjacent to the trans-most cisternae of the Golgi in T. gondii (Tilney, L., D. Roos, and M. Shaw, unpublished observations). While their cargo and destination is unknown, it is conceivable that they could deliver BAP-G back to the TGN in a fashion dependent upon the cytoplasmic tail. In such a model, the GRA4 cytoplasmic tail would not be recognized by this sorting machinery, explaining the efficient delivery of BAP-GRA4 to dense granules.
Acknowledgments
This work was supported by Public Health Service Grant RO1 AI30060 from the National Institutes of Health and a Scholar Award in Molecular Parasitology from the Burroughs Wellcome Fund to K.A. Joiner.
Abbreviations used in this paper
- BAP
bacterial alkaline phosphatase
- BFA
brefeldin A
- BLA
bacterial β-lactamase
- CRD
cross-reacting determinant
- CSV
constitutive secretory vesicles
- DHFR
dihydrofolate reductase
- GPI
glycosylphosphatidylinositol
- ISG
immature secretory granules
- MSG
mature secretory granules
- NTPase
nucleoside triphosphate hydrolase
- PI-PLC
phosphatidylinositol-specific phospholipase C
- PVM
parasitophorous vacuolar membrane
- UTR
untranslated region
- VSV-G
vesicular stomatitis virus
Footnotes
Address all correspondence to Keith Joiner, LCI 808, Section of Infectious Diseases, Yale University School of Medicine, 333 Cedar Street, P.O. Box 208022, New Haven, CT 06520-8022. Tel.: (203) 785-4140. Fax: (203) 785-3864. E-mail: Keith.Joiner@yale.edu
Huilin Qi's current address is Department of Cell Biology and Howard Hughes Medical Institute, Yale University School of Medicine.
Con J.M. Beckers' current address is Division of Geographic Medicine, University of Alabama, Birmingham.
References
- Bangs JD, Hereld D, Krakow JL, Hart GW, Englund PT. Rapid processing of the carboxyl terminus of a trypanosome variant surface glycoprotein. Proc Natl Acad Sci USA. 1985;82:3207–3211. doi: 10.1073/pnas.82.10.3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker B, Melkonian M. The secretory pathway of protists: spatial and functional organisation and evolution. Microbiol Rev. 1996;60:697–721. doi: 10.1128/mr.60.4.697-721.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckers CJM, Donald RGK, Roos DS, Luft BJ, Schwab JC, Cao Y, Joiner KA. Inhibition of cytoplasmic and organellar protein synthesis in Toxoplasma gondii: implications for the target of macrolide antibiotics. J Clin Invest. 1995;95:367–376. doi: 10.1172/JCI117665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckers CJM, Dubremetz JF, Mercereau-Puijalon O, Joiner KA. The Toxoplasma gondiirhoptry protein ROP2 is inserted into the parasitophorous vacuole membrane, surrounding the intracellular parasite, and is exposed to the host cell cytoplasm. J Cell Biol. 1994;127:947–961. doi: 10.1083/jcb.127.4.947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown DA, Crise B, Rose JK. Mechanism of membrane anchoring affects polarized expression of two proteins in MDCK cells. Science. 1989;245:1499–1501. doi: 10.1126/science.2571189. [DOI] [PubMed] [Google Scholar]
- Burg JL, Perelman D, Kasper LH, Ware PL, Boothroyd JC. Molecular analysis of the gene encoding the major surface antigen of Toxoplasma gondii. . J Immunol. 1988;141:3584–3591. [PubMed] [Google Scholar]
- Burgess TL, Kelly RB. Constitutive and regulated secretion of proteins. Annu Rev Cell Biol. 1987;3:243–293. doi: 10.1146/annurev.cb.03.110187.001331. [DOI] [PubMed] [Google Scholar]
- Cabec VL, Cowland JB, Calafat J, Borregaard N. Targeting of proteins to granule subsets is determined by timing and not by sorting: the specific granule protein NGAL is localized to azurophil granules when expressed in HL-60 cells. Proc Natl Acad Sci USA. 1996;93:6454–6457. doi: 10.1073/pnas.93.13.6454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carruthers V, Sibley D. Sequential protein secretion from three distinct organelles of Toxoplasma gondiiaccompanies invasion of human fibroblasts. Eur J Cell Biol. 1997;73:114–123. [PubMed] [Google Scholar]
- Castle AM, Huang AY, Castle D. Passive sorting in maturing granules of AtT-20 cells: the entry and exit of salivary amylase and proline rich protein. J Cell Biol. 1997;138:45–54. doi: 10.1083/jcb.138.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cesbron-Delauw MF. Dense-granule organelles of Toxoplasma gondii: their role in the host-parasite relationship. Parasitol Today. 1994;10:293–296. doi: 10.1016/0169-4758(94)90078-7. [DOI] [PubMed] [Google Scholar]
- Chanat E, Huttner W. Milieu-induced, selective aggregation of regulated secretory proteins in the trans-Golgi network. J Cell Biol. 1991;115:1505–1519. doi: 10.1083/jcb.115.6.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colomer V, Lal K, Hoops TC, Rindler MJ. Exocrine granules specific packaging signals are present in the polypeptide moiety of the pancreatic granule membrane protein GP2 and in amylase: implications for protein targeting to secretory granules. EMBO (Eur Mol Biol Organ) J. 1994;13:3711–3719. doi: 10.1002/j.1460-2075.1994.tb06680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colomer V, Kicska GA, Rindler MJ. Secretory granule content proteins and the luminal domains of granule membrane proteins aggregate in vitro at mildly acidic pH. J Biol Chem. 1996;271:48–55. doi: 10.1074/jbc.271.1.48. [DOI] [PubMed] [Google Scholar]
- Cool DR, Normant E, Shen F-S, Chen H-C, Pannell L, Zhang Y, Loh YP. Carboxypeptidase E is a regulated secretory pathway sorting receptor: genetic obliteration leads to endocrine disorders in Cpe fatmice. Cell. 1997;88:73–83. doi: 10.1016/s0092-8674(00)81860-7. [DOI] [PubMed] [Google Scholar]
- De Lisle RC, Bansal R. Brefeldin A inhibits the constitutive-like secretion of a sulfated protein in pancreatic acinar cells. Eur J Cell Biol. 1996;71:62–71. [PubMed] [Google Scholar]
- Didier M, Morrissey JH, Fugate RD, Bainton DF, McEver RP. Cytoplasmic domain of P-selectin (CD62) contains the signal for sorting into the regulated secretory pathway. Mol Biol Cell. 1992;3:309–321. doi: 10.1091/mbc.3.3.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dittie AS, Thomas L, Thomas G, Tooze SA. Interaction of furin in immature secretory granules from neuroendocrine cells with the AP-1 adaptor complex is modulated by casein kinase II phosphorylation. EMBO (Eur Mol Biol Organ) J. 1997;16:4859–4870. doi: 10.1093/emboj/16.16.4859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donald RGK, Carter D, Ullman B, Roos D. Insertional tagging, cloning, and expression of the Toxoplasma gondiihypoxanthine-xanthine-guanine phosphoribosyltransferase gene. J Biol Chem. 1996;271:14010–14019. doi: 10.1074/jbc.271.24.14010. [DOI] [PubMed] [Google Scholar]
- Dubremetz JF, Achbarou A, Bermudes D, Joiner KA. Kinetics of apical organelle exocytosis during Toxoplasma gondiihost cell interaction. Parasitol Res. 1993;79:402–408. doi: 10.1007/BF00931830. [DOI] [PubMed] [Google Scholar]
- Fernandez CJ, Haugwitz M, Eaton B, Moore HP. Distinct molecular events during secretory granule biogenesis revealed by sensitivities to brefeldin A. Mol Biol Cell. 1997;8:2171–2185. doi: 10.1091/mbc.8.11.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths G, Back R, Marsh M. A quantitative analysis of the endocytic pathway in baby hamster kidney cells. J Cell Biol. 1989;109:2703–2720. doi: 10.1083/jcb.109.6.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halonen SK, Weidner E, Siebenaller JF. Evidence for heterotrimeric GTP-binding proteins in Toxoplasma gondii. . J Eukaryot Microbiol. 1996;43:187–192. doi: 10.1111/j.1550-7408.1996.tb01389.x. [DOI] [PubMed] [Google Scholar]
- Inouye H, Michaelis S, Wright A, Beckwith J. Cloning and restriction mapping of the alkaline phosphatase structural gene (phoA) of Escherichia coliand generation of deletion mutants in vitro. J Bacteriol. 1981;146:668–675. doi: 10.1128/jb.146.2.668-675.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner KA, Fuhrman SA, Mietinnen H, Kasper LL, Mellman I. Toxoplasma gondii: fusion competence of parasitophorous vacuoles in Fc receptor transfected fibroblasts. Science. 1990;249:641–646. doi: 10.1126/science.2200126. [DOI] [PubMed] [Google Scholar]
- Karsten V, Qi H, Beckers CJM, Joiner KA. Targeting the secretory pathway of Toxoplasma gondii. . Methods (Orlando) 1997;13:103–111. doi: 10.1006/meth.1997.0503. [DOI] [PubMed] [Google Scholar]
- Kuliawat R, Arvan P. Protein targeting via the “constitutive-like” secretory pathway in isolated pancreatic islets: passive sorting in the immature granule compartment. J Cell Biol. 1992;118:521–529. doi: 10.1083/jcb.118.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuliawat R, Arvan P. Distinct molecular mechanisms for protein sorting within immature secretory granules of pancreatic B-cells. J Cell Biol. 1994;126:77–86. doi: 10.1083/jcb.126.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laminet AA, Pluckthun A. The precursor of β-lactamase: purification, properties, and folding kinetics. EMBO (Eur Mol Biol Organ) J. 1989;8:1469–1477. doi: 10.1002/j.1460-2075.1989.tb03530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mevelec MN, Chardes T, Mercereau-Puijalon O, Bourguin I, Archbarou A, Dubremetz JF, Bout D. Molecular cloning of GRA4, a dense Toxoplasma gondiidense granule protein, recognized by mucosal IgA antibodies. Mol Biochem Parasitol. 1992;56:227–238. doi: 10.1016/0166-6851(92)90172-g. [DOI] [PubMed] [Google Scholar]
- Milgram SL, Eipper BA, Mains RE. Differential trafficking of soluble and integral membrane secretory granule-associated proteins. J Cell Biol. 1994;124:33–41. doi: 10.1083/jcb.124.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milgram SL, Mains RE, Eipper BA. Identification of routing determinants in the cytosolic domain of a secretory granule-associated integral membrane protein. J Biol Chem. 1996;271:17526–17535. doi: 10.1074/jbc.271.29.17526. [DOI] [PubMed] [Google Scholar]
- Nagel SD, Boothroyd JC. The major surface antigen, P30, of Toxoplasma gondiiis anchored by a glycolipid. J Biol Chem. 1989;264:5569–5574. [PubMed] [Google Scholar]
- Nakaar, V., D. Bermudes, K.R. Peck, and K.J. Joiner. 1997. Upstream elements are required for expression of nucleoside triphosphate hydrolase genes of Toxoplasma gondii. Mol. Biochem. Parasitol. In press. [DOI] [PubMed]
- Nishimura N, Balch WE. A di-acidic signal required for selective export from the endoplasmatic reticulum. Science. 1997;277:556–558. doi: 10.1126/science.277.5325.556. [DOI] [PubMed] [Google Scholar]
- Ossorio PN, Dubremetz JF, Joiner KA. A soluble secretory protein of the intracellular parasite Toxoplasma gondiiassociates with the parasitophorous vacuole membrane through hydrophobic interactions. J Biol Chem. 1994;269:15350–15357. [PubMed] [Google Scholar]
- Perkins M. Rhoptry organelles of apicomplexan parasites. Parasitol Today. 1992;8:28–32. doi: 10.1016/0169-4758(92)90308-o. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E, Powell SK. Polarity of epithelial and neuronal cells. Annu Rev Cell Biol. 1992;8:395–427. doi: 10.1146/annurev.cb.08.110192.002143. [DOI] [PubMed] [Google Scholar]
- Roos DS, Donald RGK, Morrissette NS, Moulton ALC. Molecular tools for genetic dissection of the protozoan parasite Toxoplasma gondii. . Methods Cell Biol. 1994;45:27–63. doi: 10.1016/s0091-679x(08)61845-2. [DOI] [PubMed] [Google Scholar]
- Rosa P, Weiss U, Pepperkok R, Ansorge W, Niehrs C, Stelzer EH, Huttner WB. An antibody against secretogranin I (chromogranin B) is packaged into secretory granules. J Cell Biol. 1989;109:17–34. doi: 10.1083/jcb.109.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa P, Barr FA, Stinchcombe JC, Binacchi C, Huttner W. Brefeldin A inhibits the formation of constitutive secretory vesicles and immature secretory granules from the trans-Golgi network. Eur J Cell Biol. 1992;59:265–274. [PubMed] [Google Scholar]
- Rose J, Bergmann J. Altered cytoplasmic domains affect intracellular transport of the vesicular stomatitis virus glycoprotein. Cell. 1983;34:513–524. doi: 10.1016/0092-8674(83)90384-7. [DOI] [PubMed] [Google Scholar]
- Seeber F, Dubremetz JF, Boothroyd JC. Analysis of Toxoplasma gondiistably transfected with a transmembrane variant of its major surface protein SAG1. J Cell Sci. 1998;111:23–29. doi: 10.1242/jcs.111.1.23. [DOI] [PubMed] [Google Scholar]
- Sibley LD, Krahenbuhl JL, Adams GMW, Weidner E. Toxoplasma modifies macrophage phagosomes by secretion of a vesicular network rich in surface proteins. J Cell Biol. 1986;103:867–874. doi: 10.1083/jcb.103.3.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibley LD, Niesman IR, Parmley SF, Cesbron-Delauw M-F. Regulated secretion of multi-lamellar vesicles leads to formation of a tubulo-vesicular network in host-cell vacuoles occupied by Toxoplasma gondii. . J Cell Sci. 1995;108:1669–1677. doi: 10.1242/jcs.108.4.1669. [DOI] [PubMed] [Google Scholar]
- Silverman, J.A., and K.A. Joiner. 1996. Toxoplasma/host cell interaction. In Host Response to Intracellular Pathogens. S.H.E. Kaufman, editor. R.G. Landers, Austin, TX. 313–338.
- Simons K, Eikonen E. Functional rafts in cell membranes. Nature. 1997;387:569–572. doi: 10.1038/42408. [DOI] [PubMed] [Google Scholar]
- Sinai A, Joiner KA. Safe haven: the cell biology of non-fusogenic pathogen vacuoles. Annu Rev Microbiol. 1997;27:415–462. doi: 10.1146/annurev.micro.51.1.415. [DOI] [PubMed] [Google Scholar]
- Striepen B, He CY, Matrajt M, Soldati D, Roos DS. Expression, selection and organellar targeting of the green fluorescent protein in Toxoplasma gondii. . Mol Biochem Parasitol. 1998;92:328–338. doi: 10.1016/s0166-6851(98)00011-5. [DOI] [PubMed] [Google Scholar]
- Thomas DC, Brewer CB, Roth MB. Vesicular stomatitis virus glycoprotein contains a dominant cytoplasmic basolateral sorting signal critically dependent upon tyrosine. J Biol Chem. 1993;268:3313–3320. [PubMed] [Google Scholar]
- Tomavo S, Schwarz R, Dubremetz JF. Evidence for glycosyl-phosphatidyl inositol anchor of Toxoplasma gondiisurface antigens. Mol Cell Biol. 1989;9:4576–4580. doi: 10.1128/mcb.9.10.4576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbe S, Tooze SA, Barr FA. Formation of secretory vesicles in the biosynthetic pathway. Biochim Biophys Acta. 1997;1358:6–22. doi: 10.1016/s0167-4889(97)00050-5. [DOI] [PubMed] [Google Scholar]
- Whitt MA, Chong L, Rose JK. Glycoprotein cytoplasmic domain sequences required for rescue of a vesicular stomatitis virus glycoprotein mutant. J Virol. 1989;63:3569–3578. doi: 10.1128/jvi.63.9.3569-3578.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]