Abstract
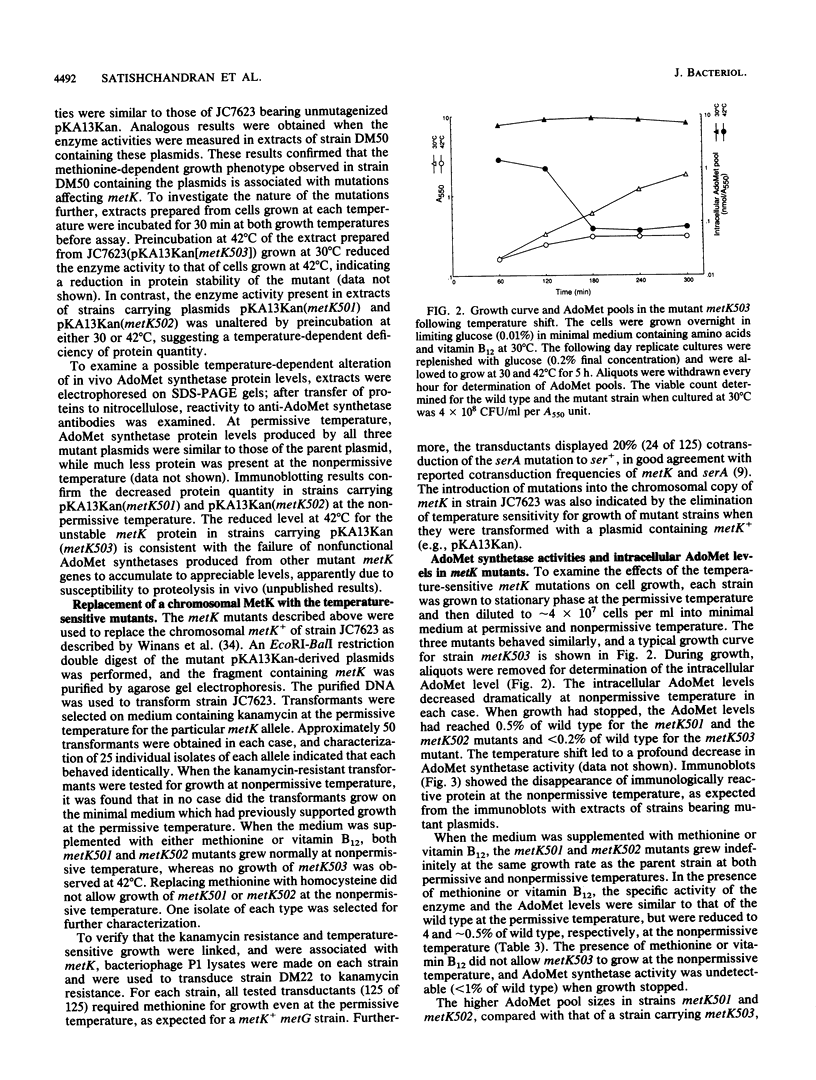
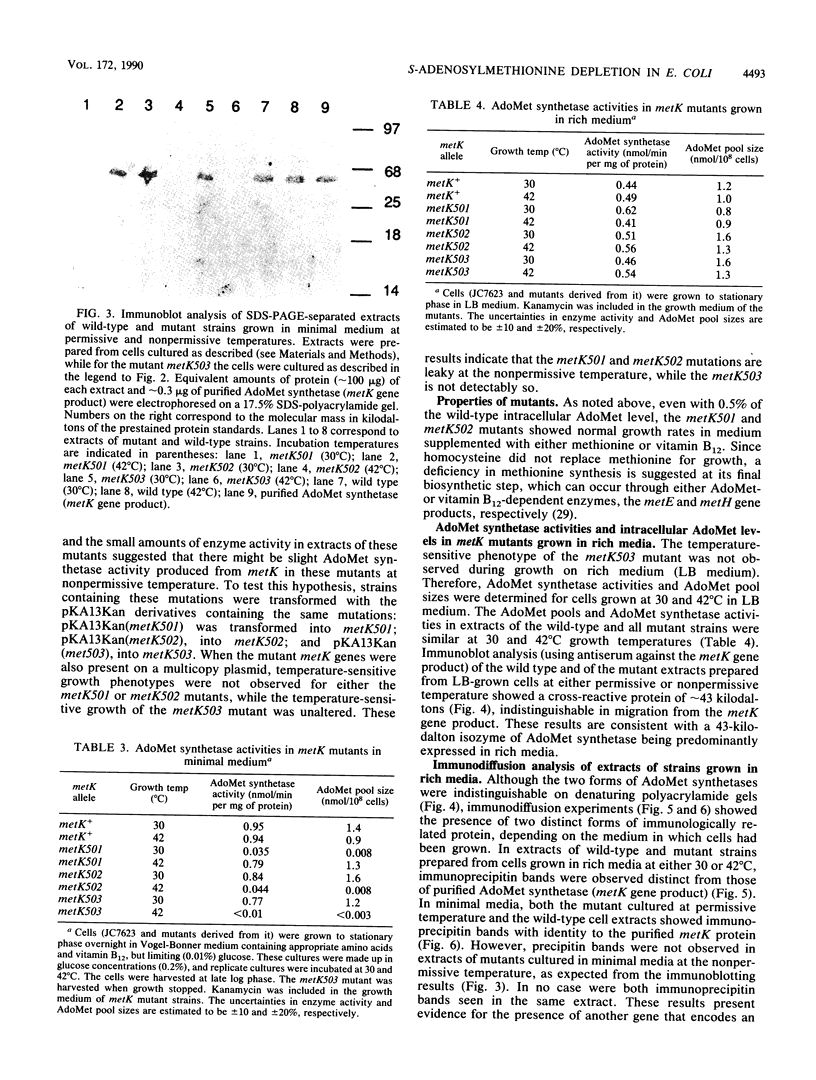
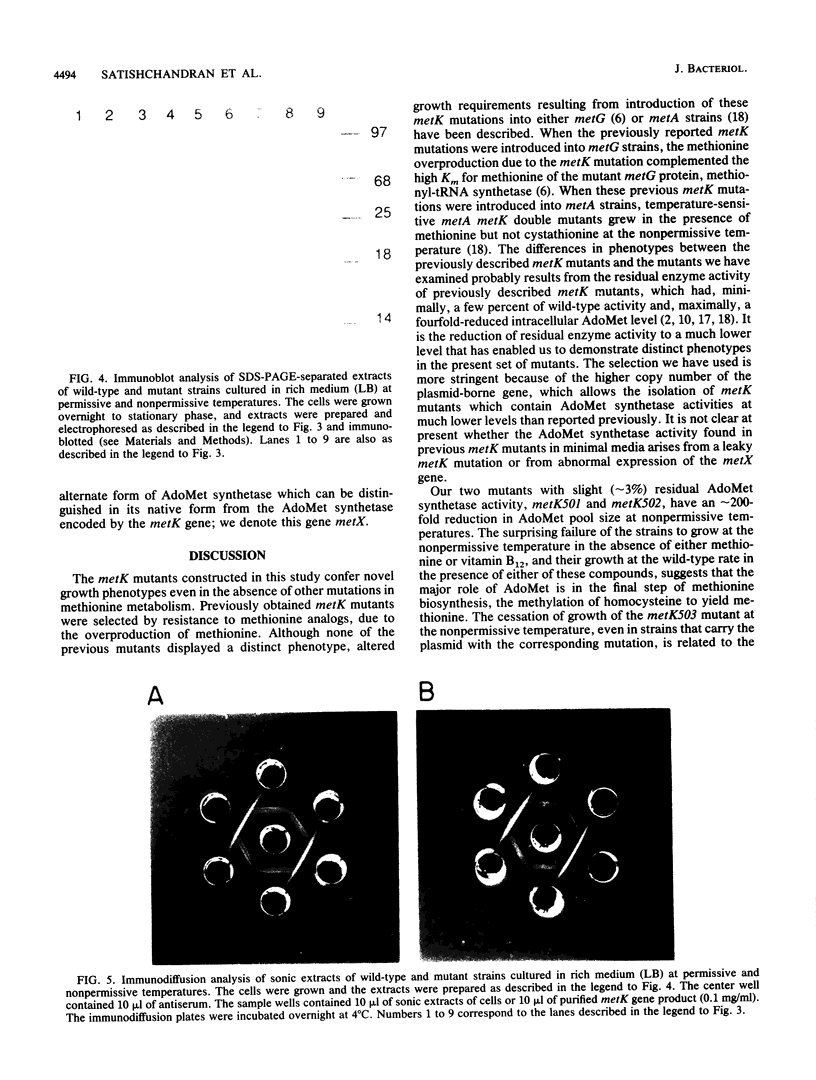
S-Adenosylmethionine (AdoMet) plays a myriad of roles in cellular metabolism. One of the many roles of AdoMet in Escherichia coli and Salmonella typhimurium is as a corepressor of genes encoding enzymes of methionine biosynthesis. To investigate the metabolic effects of large reductions in intracellular AdoMet concentrations in growing cells, we constructed and examined mutants of E. coli which are conditionally defective in AdoMet synthesis. Temperature-sensitive mutants in metK, the structural gene for the S-adenosylmethionine synthetase (AdoMet synthetase) expressed in minimal medium, were constructed by in vitro mutagenesis of a plasmid-borne copy of metK. By homologous recombination, the chromosomal copy was replaced with the mutated metK gene. Both heat- and cold-sensitive mutants were examined. At the nonpermissive temperature, two such mutants had 200-fold-reduced intracellular AdoMet levels and required either methionine or vitamin B12 for growth. In the presence of methionine or vitamin B12, the mutants grew at normal rates even though the AdoMet levels remained 0.5% of wild type. A third mutant when placed at nonpermissive temperature had less than 0.2% of the normal AdoMet level and did not grow on minimal medium even in the presence of methionine or vitamin B12. All of these mutants grew normally on yeast-extract-based medium in which an alternate form of S-adenosylmethionine synthetase was expressed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Borczuk A., Stock A., Stock J. S-adenosylmethionine may not be essential for signal transduction during bacterial chemotaxis. J Bacteriol. 1987 Jul;169(7):3295–3300. doi: 10.1128/jb.169.7.3295-3300.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle S. M., Markham G. D., Hafner E. W., Wright J. M., Tabor H., Tabor C. W. Expression of the cloned genes encoding the putrescine biosynthetic enzymes and methionine adenosyltransferase of Escherichia coli (speA, speB, speC and metK). Gene. 1984 Oct;30(1-3):129–136. doi: 10.1016/0378-1119(84)90113-6. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- CATONI G. L. S-Adenosylmethionine; a new intermediate formed enzymatically from L-methionine and adenosinetriphosphate. J Biol Chem. 1953 Sep;204(1):403–416. [PubMed] [Google Scholar]
- Chater K. F., Lawrence D. A., Rowbury R. J., Gross T. S. Suppression of methionyl transfer RNA synthetase mutants of Salmonella typhimurium by methionine regulatory mutations. J Gen Microbiol. 1970 Sep;63(1):121–131. doi: 10.1099/00221287-63-1-121. [DOI] [PubMed] [Google Scholar]
- Glazer R. I., Peale A. L. Measurement of S-adenosyl-L-methionine levels by SP Sephadex chromatography. Anal Biochem. 1978 Dec;91(2):516–520. doi: 10.1016/0003-2697(78)90538-9. [DOI] [PubMed] [Google Scholar]
- Greene R. C., Hunter J. S., Coch E. H. Properties of metK mutants of Escherichia coli K-12. J Bacteriol. 1973 Jul;115(1):57–67. doi: 10.1128/jb.115.1.57-67.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner E. W., Tabor C. W., Tabor H. Isolation of a metK mutant with a temperature-sensitive S-adenosylmethionine synthetase. J Bacteriol. 1977 Dec;132(3):832–840. doi: 10.1128/jb.132.3.832-840.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson A. C. The regulation of methionine and s-adenosylmethionine biosynthesis and utilization in mutants of Salmonella typhimurium with defects in s-adenosylmethionine synthetase. Mol Gen Genet. 1974;131(3):263–273. doi: 10.1007/BF00267965. [DOI] [PubMed] [Google Scholar]
- Hobson A. C. The synthesis of S-adenosylmethionine by mutants with defects in S-adenosylmethionine synthetase. Mol Gen Genet. 1976 Feb 27;144(1):87–95. doi: 10.1007/BF00277310. [DOI] [PubMed] [Google Scholar]
- Holloway C. T., Greene R. C., Su C. H. Regulation of S-adenosylmethionine synthetase in Escherichia coli. J Bacteriol. 1970 Nov;104(2):734–747. doi: 10.1128/jb.104.2.734-747.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J. A., Brown L. R., Ferro A. J. Expression of the cloned coliphage T3 S-adenosylmethionine hydrolase gene inhibits DNA methylation and polyamine biosynthesis in Escherichia coli. J Bacteriol. 1987 Aug;169(8):3625–3632. doi: 10.1128/jb.169.8.3625-3632.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys G. O., Willshaw G. A., Smith H. R., Anderson E. S. Mutagenesis of plasmid DNA with hydroxylamine: isolation of mutants of multi-copy plasmids. Mol Gen Genet. 1976 Apr 23;145(1):101–108. doi: 10.1007/BF00331564. [DOI] [PubMed] [Google Scholar]
- Hunter J. S., Greene R. C., Su C. H. Genetic characterization of the metK locus in Escherichia coli K-12. J Bacteriol. 1975 Jun;122(3):1144–1152. doi: 10.1128/jb.122.3.1144-1152.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushner S. R., Nagaishi H., Templin A., Clark A. J. Genetic recombination in Escherichia coli: the role of exonuclease I. Proc Natl Acad Sci U S A. 1971 Apr;68(4):824–827. doi: 10.1073/pnas.68.4.824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham G. D., DeParasis J., Gatmaitan J. The sequence of metK, the structural gene for S-adenosylmethionine synthetase in Escherichia coli. J Biol Chem. 1984 Dec 10;259(23):14505–14507. [PubMed] [Google Scholar]
- Markham G. D., Hafner E. W., Tabor C. W., Tabor H. S-Adenosylmethionine synthetase from Escherichia coli. J Biol Chem. 1980 Oct 10;255(19):9082–9092. [PubMed] [Google Scholar]
- Matthews R. G., Neidhardt F. C. Abnormal induction of heat shock proteins in an Escherichia coli mutant deficient in adenosylmethionine synthetase activity. J Bacteriol. 1988 Apr;170(4):1582–1588. doi: 10.1128/jb.170.4.1582-1588.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raushel F. M., Villafranca J. J. Positional isotope exchange. CRC Crit Rev Biochem. 1988;23(1):1–26. doi: 10.3109/10409238809103118. [DOI] [PubMed] [Google Scholar]
- TABOR H., ROSENTHAL S. M., TABOR C. W. The biosynthesis of spermidine and spermine from putrescine and methionine. J Biol Chem. 1958 Oct;233(4):907–914. [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Methionine adenosyltransferase (S-adenosylmethionine synthetase) and S-adenosylmethionine decarboxylase. Adv Enzymol Relat Areas Mol Biol. 1984;56:251–282. doi: 10.1002/9780470123027.ch4. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Winans S. C., Elledge S. J., Krueger J. H., Walker G. C. Site-directed insertion and deletion mutagenesis with cloned fragments in Escherichia coli. J Bacteriol. 1985 Mar;161(3):1219–1221. doi: 10.1128/jb.161.3.1219-1221.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]