Abstract
A 104-kD protein was coimmunoprecipitated with the estrogen receptor from the flowtrough of a phosphocellulose chromatography of MCF-7 cell nuclear extract. mAbs to this protein identified several cDNA clones coding for the human 104-kD major vault protein. Vaults are large ribonucleoprotein particles of unknown function present in all eukaryotic cells. They have a complex morphology, including several small molecules of RNA, but a single protein species, the major vault protein, accounts for >70% of their mass. Their shape is reminiscent of the nucleopore central plug, but no proteins of known function have been described to interact with them. Western blot analysis of vaults purified on sucrose gradient showed the presence of estrogen receptor co-migrating with the vault peak. The AER317 antibody to estrogen receptor coimmunoprecipitated the major vault protein and the vault RNA also in the 20,000 g supernatant fraction. Reconstitution experiments of estrogen receptor fragments with the major vault protein mapped the site of the interaction between amino acids 241 and 280 of human estrogen receptor, where the nuclear localization signal sequences are located. Estradiol treatment of cells increased the amount of major vault protein present in the nuclear extract and coimmunoprecipitated with estrogen receptor, whereas the anti-estrogen ICI182,780 had no effect. The hormone-dependent interaction of vaults with estrogen receptor was reproducible in vitro and was prevented by sodium molybdate. Antibodies to progesterone and glucocorticoid receptors were able to coimmunoprecipitate the major vault protein. The association of nuclear receptors with vaults could be related to their intracellular traffic.
Vault ribonucleoprotein particles are present in all eukaryotic cells and their structure and protein composition are highly conserved (Kedersha et al., 1990). They have a characteristic ovoid shape made by two halves connected on the equatorial plane. Each half contains eight petals and a central ring (Kedersha et al., 1990). The vault particle has a mass of ∼13 MD, corresponding to a sedimentation coefficient of 150 S. One single protein species constitutes >70% of the particle mass and a small molecule of RNA is part of this complex structure. It has been calculated that each petal contains six major vault protein copies and one vRNA molecule, for a total of 96 and 16 molecules, respectively, in each vault particle (Kedersha et al., 1991). Vaults were originally identified in the postmicrosomal supernatant of cell extracts, but it has also been reported that they were present in isolated nuclei (Chugani et al., 1993). Immunocytochemical localization showed perinuclear staining of cultured fibroblasts. Because of their size, shape, and protein and RNA composition, these particles are different from other ribonucleoproteins. The conservation of protein sequence and particle shape between organisms from lower eukaryotes to man is indicative of a central role in cell function. Disruption of major vault proteins in Dictyostelium discoideum results in a phenotype incapable of growing under nutritional stress (Vasu and Rome, 1995). However, the vault function still remains an enigma. Structural similarities and immunocytochemical data suggest that vaults may constitute the central plug of the nucleopore structure, involved in the protein and/or RNA transport between cytoplasm and nucleus (Chugani et al., 1993). More recently it has been demonstrated that the lung resistance–related protein (LRP)1 is the human major vault protein (Scheffer et al., 1995). LRP is a protein overexpressed in many neoplastic tissue and cell lines (Izquierdo et al., 1996). Its expression has been related to a phenotype resistant to chemotherapy (Izquierdo et al., 1995) and it has a high predictive value for a poor response to chemotherapy (List et al., 1996). However, neither the function of this protein in normal cell have been elucidated nor its role in drug resistance mechanism. Several other minor polypeptides are present in the assembled vaults (Kedersha and Rome, 1986), but none of them have been characterized so far. No other proteins with a defined function and interacting with vault particles have been described to date. Data reported in this article indicate that the estrogen receptor (and other steroid receptors) interact with the major vault protein and might, therefore, represent a breakthrough in describing the function of vaults.
Estrogen receptor is the protein responsible for the biological action of estradiol. Binding to specific sequences present in the regulatory region of controlled genes (the hormone responsive element) is a mandatory step in the hormonal mechanism of action (Beato, 1989). Occupation of the hormone binding site modulates the accessibility of the receptor DNA-binding domain to DNA and the transcriptional activation function (Tora et al., 1989b ; Beato et al., 1995). Protein–protein interaction with components of the transcription initiation complex and/or with adapters bridging receptor and transcription initiation complex mediates transcriptional activation (Ing et al., 1992; Cavaillès et al., 1994, 1995; Halachmi et al., 1994; Jacq et al., 1994; Le Douarin et al., 1995; Oñate et al., 1995; Voegel et al., 1996; vom Baur et al., 1996). Other steps involved in the hormone mechanism of action, such as DNA binding (Landel et al., 1994), availability of the C-domain for DNA binding and intracellular partitioning of the receptor may be influenced by protein–protein interaction. Furthermore access to transcriptionally repressed chromatin is probably mediated by the histone acetyltransferase activity of steroid receptor coactivator SRC-1, a protein interacting with steroid receptor in a hormone-dependent manner (Spencer et al., 1997). The first described protein– protein interaction for estrogen receptor has been with a 90-kD heat shock protein (hsp90), forming a quaternary structure known as “native receptor” (Ziemiecki et al., 1986; Redeuihl et al., 1987). The hsp90 does not interfere with the hormone binding, but it shields the DNA-binding domain, preventing the interaction with the hormone- responsive element (Binart et al., 1989). Estradiol binding induces a conformational modification in the receptor molecule, releasing the hsp90 from the hormone binding subunit. hsp90 is a protein present in the cell cytoplasm, but estrogen receptor has been visualized by immunocytochemistry both in the cytoplasm and in the nucleus (King and Greene, 1984; Marchetti et al., 1987). In the hinge region (D domain), three lysine-/arginine-rich motifs have been identified, representing constitutive nuclear localization signals (Picard et al., 1990). A fourth, estrogen-regulated nuclear localization signal has been identified in the hormone binding domain (Ylikomi et al., 1992). Similar nuclear localization signals have also been reported for other steroid receptors. Nuclear localization signals are responsible for nuclear targeting of protein, a process that includes binding to nuclear pore components and energy-dependent transport (Pantè and Aebi, 1996; Nigg, 1997). No interaction of estrogen receptor with known components of the nucleopore or with shuttling carriers has been described so far.
Materials and Methods
Materials
[3H]Estradiol (84–111 Ci/mmol), [32P]α-ATP (3,000 Ci/mmol), peroxidase-conjugated anti–mouse immunoglobulins, and chemiluminescent substrate (ECL) were from Amersham (Aylersbury, Bucks, United Kingdom). Freund's adjuvants, anti–mouse immunoglobulin coupled to agarose, BSA, and rabbit polyclonal antibodies to estrogen receptor (amino acids [aa] 154–171) were from Sigma Chemical Co. (St. Louis, MO). Protein A– and protein G–Sepharose FF-4B, CNBr-activated Sepharose, DEAE Fast Flow column, and pGEX-2TK plasmid were from Pharmacia Biotech Sevrage (Uppsala, Sweden). DTT, tris-HCl, Hepes, acrylamide, bisacrylamide, and β-lactoglobulin were from Serva (Heidelberg, Germany). Tissue culture media, antibiotics, and FCS (Myoclone +) were from Gibco Laboratories (Grand Island, NY). BM-Condimed® H1, tRNA, and protease inhibitors were from Boehringer Mannheim GmbH (Mannheim, Germany). HeLa cell expression library was from CLONTECH (Palo Alto, CA). Anti–mouse IgG isotype were from Caltag Labs (South San Francisco, CA). Restriction enzymes, T7 DNA polymerase, T4 polynucleotide kinase, and other molecular biology reagents were from Promega Corp. (Madison, WI). Dynabeads S-280 were from Dynal AS (Oslo, Norway). Blocking reagent was from Bio-Rad Laboratories (Hercules, CA). Rabbit polyclonal antibodies to progesterone receptor (aa 545–564), to glucocorticoid receptor (aa 5–20), to Nurr77 (aa 4–23), to TFIIB (aa 299–316), and to steroid receptor cofactor-1 (SRC-1) were from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Mouse mAbs to progesterone receptor C-262 was from StressGen Biotechnology Corp. (Victoria BC, Canada). All other reagents were of analytical grade.
Buffers and Solutions
Buffer A: 10 mM Hepes, pH 7.9, 10 mM KCl, 0.5 mM DTT, 1.5 mM MgCl2; buffer C: 20 mM Hepes, pH 7.9, 450 mM NaCl, 0.2 mM DTT, 1.5 mM MgCl2, 0.5 mM EDTA, 0.5 mM PMSF, 25% (vol/vol) glycerol; buffer D: 20 mM Hepes, pH 7.8, 100 mM KCl, 0.5 mM DTT, 0.1 mM EDTA, 15% (vol/vol) glycerol, 10% (wt/vol) sucrose, 0.5 mM PMSF; buffer PC: 20 mM Hepes, pH 7.9, 0.01 mM ZnCl2, 1.5 mM MgCl2, 1 mM DTT, 10% (vol/vol) glycerol; buffer HMEG: 20 mM Hepes, pH 7.9, 2 mM MgCl2, 0.1 mM EDTA, 100 mM KCl, 1 mM DTT, 15% (vol/vol) glycerol, 10% (wt/ vol) sucrose, 0.1% NP-40; buffer H1: 20 mM Hepes, pH 7.9, 2 mM MgCl2, 0.1 mM EDTA, 100 mM KCl, 1 mM DTT, 10% (vol/vol) glycerol, 0.1% NP-40; buffer G0: 20 mM Hepes, pH 7.9, 0.1 mM EDTA, 0.1 M potassium glutamate, 1 mM DTT, 10% (vol/vol) glycerol; buffer G1: buffer G0 containing 1 M guanidium chloride; D'PBS: Dulbecco's PBS without Ca2+ and Mg2+; PBSL: 3 g/liter Na2HPO4, 0.3 g/liter NaH2PO4, 12 g/liter NaCl, 0.05% NP-40, 0.05% Tween 20; WB buffer: PBSL, containing 150 mM NaCl; HAT: 0.1 mM hypoxantine, 0.4 μM aminopterine, 16 μM thymidine; and SSC buffer: 15 mM sodium citrate, pH 7.0, 150 mM NaCl. All buffers were filtered through 0.45-μm pore Millipore filters (Bedford, MA).
Preparation of Nuclear Extract and 20,000 g Supernatant
MCF-7 cells were grown in 140-mm-diam Petri dishes or in 175-cm2 flasks in RPMI 1640 Dulbecco's essential medium containing 5% horse serum (heat inactivated), 5% fetal calf serum (heat inactivated), 7 ng/ml (2 × 10−4 USP units/ml) bovine insulin, and 1 nM cortisol. Estradiol deprivation was obtained by growing the cells for a week in medium deprived of phenol red and added with charcoal-treated sera. Charcoal treatment was performed by incubation of sera with 0.25% charcoal for 30 min at 56°C followed by 30 min at 37°C. The procedure was performed twice and the charcoal separated by centrifugation and filtration. Subconfluent cells were harvested and processed as described to obtain the nuclear extract (Dingam et al., 1983). The sample was dialyzed versus buffer D added with 10% (wt/vol) sucrose and 15% (vol/vol) glycerol, and then clarified at 14,000 g for 15 min. Protein concentration was usually between 8 and 9 mg/ml. The extract was stored at −70°C up to 4 wk. The 20,000 g supernatant was obtained by centrifugation at 20,000 g of the homogenate after the removal of nuclei.
Nuclear Extract Fractionation
Phosphocellulose chromatography of cell extract was performed after a described procedure (Reinberg and Roeder, 1987) and its modifications (Tanese et al., 1991; Pugh and Tijan, 1991). The sample (∼200 mg of protein extracted from 2 × 25 ml of packed MCF-7 cell) was diluted with an equal volume of PC buffer containing 0.1 M KCl and applied to a 20-ml phosphocellulose column equilibrated in the same buffer. The flowthrough protein peak was collected and the column eluted three times with 60 ml of PC buffer containing 0.3, 0.5, or 0.75 M KCl. Washes (40 ml) were inserted between elutions and discarded. The collected peak fractions were dialyzed with HMEG buffer, containing 0.1 M KCl and 0.05% NP-40. Protein recovery was >85%, distributed as follows: 40–45% in the flowthrough fraction, 15–28% in the 0.3 M KCl, 11–15% in the 0.5 M KCl, 8–12% in the 0.75 M KCl. The samples were stored frozen in aliquots at −70°C for up to 4 wk.
Preparation of Vault-enriched Fractions
Cells were harvested, sequentially washed in 50 vol of Dulbecco's PBS and 5 vol of buffer A, resuspended in 2 vol of water, and then homogenized using four strokes of a Dounce homogenizer. The homogenate was added with 1/10 of volume of 200 mM Hepes, pH 7.9, 110 mM potassium acetate, 50 mM sodium acetate, 20 mM MgCl2, 20 mM DTT, and centrifuged at 2,000 g for 5 min at 4°C. The supernatant, referred to as postnuclear supernatant, was subsequently centrifuged at 41,000 g for 2 h at 4°C. The pellet, resuspended with buffer H1 containing 20% glycerol, was centrifuged at 20,000 g for 5 min at 4°C, and the supernatant referred to as vault-enriched fraction.
Preparation of mAbs
Production of mAbs AER314 and AER317 to calf uterus estrogen receptor has been previously described (Abbondanza et al., 1993). The mAb 1603 to the human estrogen receptor synthetic fragment was prepared using the protein purified from Escherichia coli cells transfected with pGEX/hER281-595. The recombinant protein was purified using a two- dimensional 16-BAC/SDS-PAGE system as described (Macfarlane, 1989), and then electroeluted. Antibodies production was performed as described elsewhere (Abbondanza et al., 1993). To prepare antibodies to the 104-kD protein, ∼5 μg of protein, eluted from AER314 or AER317 immunoprecipitates of phosphocellulose fractionation of 1 g of nuclear extract proteins, were precipitated with TCA overnight, and resuspended with 0.2 ml of 10 mM NaOH, 0.1% SDS buffer, pH 7.5, with 0.11 vol of 10× PBS. Four Balb-C mice were immunized with that amount of protein, according to the protocol described (Abbondanza et al., 1993). Spleen cells (7.5 × 107) were fused with an equal number of Sp0Ag-14 myeloma cells in the presence of 50% polyethylenglycol 1500, and then seeded in four 96-well microtiter plates in DME/RPMI 1640 medium (1:1), containing 15% Myoclone plus, 10% BM condimed H1 and HAT. After 14 d, supernatants were screened by Western blot analysis of nuclear extract proteins, using a Multiscreen device (Bio-Rad Laboratories). Wells whose supernatant showed a positive signal for a 104-kD band were selected and the cells cloned by limited dilution in the same medium without HAT. Selected positive clones (series 10nn) were expanded and supernatants collected. Supernatants were added with 50 mM Tris-HCl, pH 7.5, and 0.05% sodium azide, and then stored at 4°C up to 1 yr.
Immunoprecipitation
The samples (nuclear extract, phosphocellulose column fractions, or 20,000 g supernatant) were diluted with an equal amount of PC buffer containing 0.1 M KCl and incubated overnight with the mAb (2–5 μg/10 mg of protein) in the presence of the capturing resin. Antibody–antigen complexes were captured either by appropriate preabsorbed sheep anti– mouse IgG isotype coupled to protein G–Sepharose (25 μg/50 μl) for antigen preparation or by anti–mouse IgG coupled to CNBr-activated Sepharose (50 μl) for analytical purposes. At the end of incubation, resins were sequentially washed with 30 vol of buffer H1 (three times for 10 min), 10 vol of buffer G0 (2 min), 10 vol of buffer G1, containing 0.2 M guanidium chloride (2 min). Proteins were eluted with 4 vol of buffer G1, containing 15 μg/ml of β-lactoglobulin, clarified at 3,000 g for 1 min and precipitated with a final concentration of 10% TCA. Precipitated proteins were washed twice with diethyl ether-ethanol (1:1), air dried, and then resuspended for immunization or in sample buffer for SDS-PAGE analysis.
Sucrose Gradients
5- or 40-ml 30–60% sucrose gradients were prepared in HEMG buffer containing 0.1 M KCl. The applied samples were not >1/15 of the gradient volume. The 5-ml gradients were centrifuged in a Beckman Vti65 rotor at 50,000 rpm up to 2.8 × 1011 ω2t; 40-ml gradients were centrifuged in a Beckman Vti55 rotor at 50,000 rpm up to 7.8 × 1011 ω2t. At the end of the run, gradients were collected in 20 fractions.
Preparation of Synthetic Estrogen Receptor Fragments
All the procedures, not described in details, were performed according to standardized protocols (Ausubel et al., 1996). Fusion protein of estrogen receptor fragments and glutathione-S-transferase (GST) were obtained by subcloning the human estrogen receptor cDNA in the EcoRI site of commercial pGEX-2TK plasmid. With such insertion, the human estrogen receptor cDNA sequence was off the GST open reading frame. To obtain the fragments of estrogen receptor starting from aa 65, the plasmid was further digested with BamHI and NotI, and then the ends were filled and ligated. This plasmid was further digested (a) with StuI and ligated to obtain the fragment from aa 65 to 203 (pGEX/hER65–203); (b) with HindIII, filled, and then ligated to obtain the fragment from aa 65 to 339 (pGEX/hER65–339); (c) with BglII, filled, and then ligated to obtain the fragment from aa 65 to 423 (pGEX/hER65–423); (d) with AvaI and NgoMI, filled, and then ligated to obtain the fragment from aa 241 to 595 (pGEX/hER241–595). Another fusion protein, containing the human estrogen receptor fragment from aa 281 to 595 (pGEX/hER281–595) was obtained by subcloning the filled H14 cDNA BamHI fragment in the filled EcoRI site of pGEX-2TK plasmid. With such insertion, the human estrogen receptor cDNA sequence resulted in frame with the GST open reading frame. A 795-bp fragment was excised from this plasmid by digestion with HindIII and AatII and replaced with a similar fragment excised from pGEX/hER65–595, to obtain a fragment with a glycine at position 400 (Tora et al., 1989a ). The fragment was dephosphorylated and purified by electrophoresis before ligation. The plasmids were transfected in the JM109 E. coli strain. Bacterial growth and fusion protein purification were performed according to supplier's instructions with the following modification: after cell lysis by addition of lysozyme and three cycles of freezing-thawing, the lysate was added with 0.5% NP-40, 0.5 mg/ml bacitracin, 10 μg/ml leupeptin, and 1 mM PMSF, and then centrifuged at 18,000 rpm for 15 min. Synthetic proteins were tested by SDS-PAGE followed by Western blot analysis with mAbs to estrogen receptor of the AER3nn series and for estradiol binding activity (Moncharmont et al., 1991).
RNA Analysis
RNA coimmunoprecipitated from nuclear extract or 20,000 g supernatant was extracted with an equal volume of non-buffered phenol/chloroform (1:1) and ethanol precipitated at −20°C for 1 h in the presence of 0.3 μl/ sample of SeeDNA (Amersham). The pellet was washed twice with 75% ethanol, dried, resuspended in denaturing solution, denatured, and then electrophoresed on a 2.2% agarose gel as described (Ausubel et al., 1996). Nucleic acid was transferred onto a nylon membrane (Hybond-N; Amersham) by capillarity, and the membrane further processed according to manufacturer's instructions. The membrane was hybridized with the radiolabeled probe at 42°C overnight, and washed with 2× SSC containing 0.1% SDS at room temperature (Ausubel et al., 1996). Human vault RNA (vRNA) probe was prepared by reverse transcription and PCR amplification of total MCF-7 RNA. Total RNA extraction (guanidine isothiocyanate method), reverse transcription, and PCR amplification were performed according to published protocols (Ausubel et al., 1996). The primers 5′-GGYCAGCWWYAGCTCAGCGGTTACTTC-3′ and 5′-GYCAGRKRGCGCCCGCGGGTYTCGAACC-3′ used for the amplification were derived from rat and bullfrog vRNA sequences (Kickhoefer et al., 1993). The resulting product, purified by agarose electrophoresis, was radiolabeled by Multiprime DNA labeling systems (Amersham) and used as probe for the Northern blot analysis. To confirm that the amplified product was the human vRNA, the fragment was subcloned in pBluescriptSK. 12 clones were analyzed by insert sequencing (Sanger et al., 1977). Two different fragments (hvRNA82 and hvRNA92) were cloned of 82 and 92 bp, respectively, sharing a high percent of identity with rat and bullfrog vRNAs. These sequence data are available from GenBank/EMBL/DDBJ under accession No. AF058296 and AF058297, respectively.
Estrogen Receptor Affinity Chromatography
Estrogen receptor fragments–GST fusion proteins were coupled to glutathione–Sepharose according to manufacturer's instructions. Aliquots (2 mg) of the nuclear extracts were added with 0.5% NP-40, diluted with an equal volume of buffer H1 and incubated overnight at 4°C with 25 μl of resin. The resins were then washed, as indicated above, and eluted with sample buffer (Laemmli, 1970). The samples were subjected to SDS-PAGE followed by Western blot analysis with the mAb 1032 to the major vault protein.
Screening of a HeLa Cell cDNA Library
A commercial λgt-11 expression library from HeLa S3 cell line was plated and screened according to manufacturer's directions. Two mAbs to the 104-kD protein (1032 and 1014) were used for the screening. The antibody presence on the nitrocellulose replicas was revealed by peroxidase-coupled anti–mouse IgG using chemiluminescent substrate (ECL; Amersham) according to manufacturer's instructions. The inserts of two positive clones were amplified using specific λgt-11 primers and subcloned in pGEM. Sequence analysis was performed on both strands of the inserts (700 and 500 bp, respectively), using T7 DNA polymerase (Sanger et al., 1977).
Electrophoresis and Western Blot Analysis
SDS-PAGE and Coomassie blue staining of proteins were performed as described elsewhere (Laemmli, 1970). For Western blot analysis proteins were electrophoretically transferred to a 0.45-μm nitrocellulose sheet in WB buffer (Towbin et al., 1979). Nitrocellulose reactive groups were then blocked for 1 h with a 5% solution of blocking reagent in WB buffer. Primary antibody incubation was performed overnight at 4°C or for 3 h at room temperature using a 1:10 or 1:20 dilution of hybridoma cell supernatant in WB buffer containing 2% solution of blocking reagent (0.25 ml/ cm2). At the end of incubation, blots were washed once for 15 min and three times for 5 min with WB buffer. Antibody reaction was revealed by incubation for 1 h at room temperature with enzyme-coupled anti–mouse IgG (1:10,000 dilution for HRP-coupled antibodies; Amersham or 1:30,000 dilution for alkaline phosphatase–coupled antibodies; Sigma Chemical Co.) in WB buffer containing 0.5% solution of blocking reagent (0.25 ml/cm2), followed by a washing cycle (as above) and using chemiluminescent substrate (ECL; Amersham) (Immunolite; Bio-Rad Laboratories) according to manufacturer's instructions.
Results
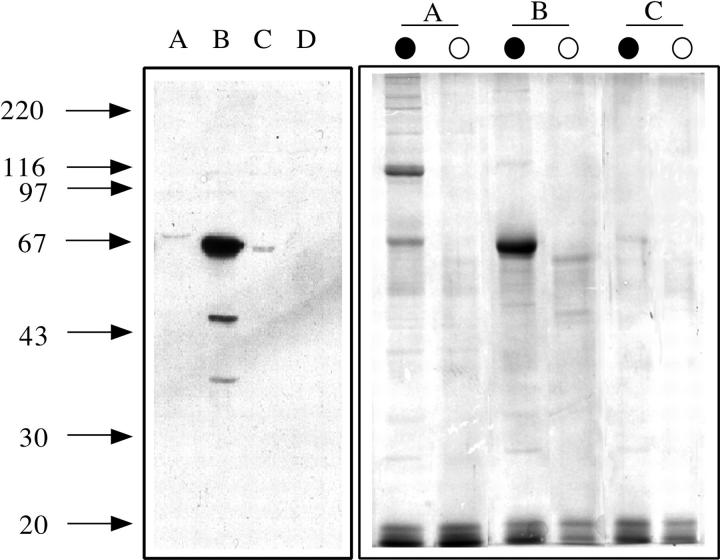
MCF-7 cells are widely used as a model for the molecular analysis of estrogens actions. To identify and purify proteins associated with the estrogen receptor, nuclear extract was prepared from MCF-7 cells and applied to a phosphocellulose column in the presence of 0.1 M KCl. The column was eluted with three different salt concentrations (0.3, 0.5, and 0.75 M KCl). Western blot analysis with an mAb to estrogen receptor of the flowthrough and three elution fractions is presented in Fig. 1 (left). This analysis was performed with the antibody AER317, directed to an epitope localized in the last 70 aas of the estrogen receptor molecule. The antibody revealed an intense 67-kD band in the 0.3 M KCl fraction, indicating that most of the receptor molecules were eluted in it. Fainter bands of the same molecular weight were visible in the flowthrough and in the 0.5 M KCl fractions. Similar results were obtained with other mAbs to estrogen receptor, namely AER314 (epitope mapping between aa 125 and 165) and 1603 (epitope mapping between aa 500 and 595) (data not shown). Immunoprecipitation of the fractions with the mAb AER317, followed by SDS-PAGE analysis, showed that a 104-kD protein was coimmunoprecipitated with the 67-kD receptor protein only in the flowthrough fraction (Fig. 1, right). Another mAb, interacting with the NH2-terminal of the estrogen receptor molecule (AER314), was unable to immunoprecipitate any protein in the flowthrough fraction (data not shown). This 104-kD band was the most prominent, but a few faint high molecular weight bands and bands in the 20–40-kD range were also visible. This result indicated that the estrogen receptor in the flowthrough fraction was presumably complexed with other protein moieties and that in this complex the epitope for the AER314 antibody was masked.
Figure 1.
Western blot analysis of estrogen receptor in the nuclear extract separated by phosphocellulose chromatography and identification of proteins coimmunoprecipitated with the estrogen receptor. Arrows on the left side indicate the migration of standard proteins of the indicated molecular weight (kD). (Left) SDS-PAGE followed by Western blot analysis with the mAb to estrogen receptor AER317 of the flowthrough (lane A), 0.3 M (lane B), 0.5 M (lane C), or 0.75 M KCl (lane D) fractions of the phosphocellulose chromatography of MCF-7 cell nuclear extract. (Right) SDS-PAGE analysis followed by Coomassie blue staining of the gel of proteins immunoprecipitated with the mAb to estrogen receptor AER317 (lanes marked by solid circle) or control IgG (lanes marked by open circle) of the flowthrough (lanes A), 0.3 M (lanes B), or 0.5 M (lanes C) fractions of the phosphocellulose chromatography of nuclear extract.
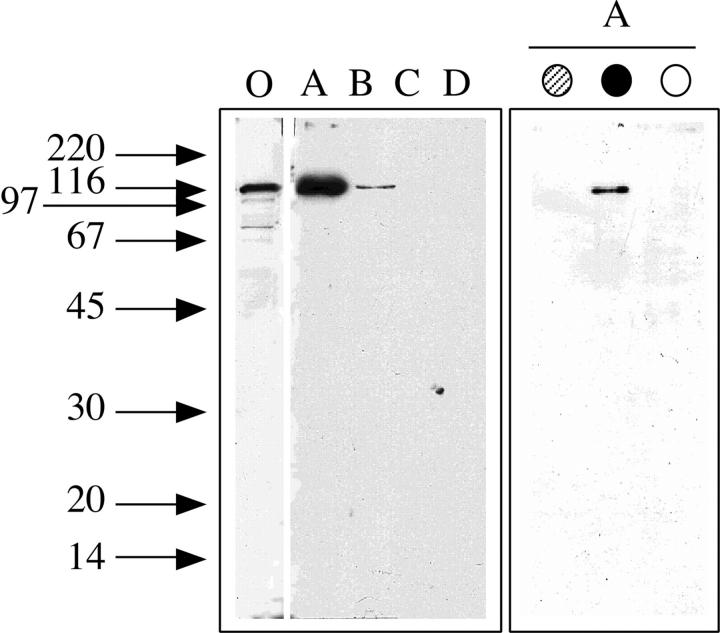
To characterize these proteins, the AER317 immunoprecipitates of the phosphocellulose column flowthrough fraction were used to immunize mice and their spleen cells were used for hybridoma production. Four cloned hybridoma cell lines (1011, 1014, 1027, and 1032) were selected, by Western blot analysis, for the interaction of their antibody with the 104-kD protein coimmunoprecipitated by the antibody to estrogen receptor AER317. The antibody 1032 recognized a 104-kD protein in the nuclear extract by Western blot analysis (Fig. 2, left). The 104-kD protein reacting with the 1032 antibody was most abundantly present only in the flowthrough fraction of the phosphocellulose column and faintly in the 0.3 M KCl fraction (Fig. 2, left). This protein was coimmunoprecipitated from the flowthrough fraction only by the AER317 antibody and not by the AER314 (Fig. 2, right), as demonstrated by Western blot analysis. These results suggested that these antibodies were directed against the same protein forming complexes with the estrogen receptor molecule and preventing its recognition by the antibody AER314. Similar analysis performed with three other selected mAbs (1011, 1014, and 1027) gave comparable results, even though the antibodies 1014 and 1032 recognized different degradation fragments of the 104-kD band, thus suggesting that they were directed to different epitopes (data not shown).
Figure 2.
Western blot analysis of the 104-kD protein in the nuclear extract separated by phosphocellulose chromatography and in the proteins coimmunoprecipitated with the estrogen receptor in the flowthrough fraction. Arrows on the left side indicate the migration of standard proteins of the indicated molecular weight (kD). (Left) SDS-PAGE followed by Western blot analysis with mAb 1032 to the major vault protein of MCF-7 cells nuclear extract (lane O) and of the flowthrough (lane A), 0.3 M (lane B), 0.5 M (lane C), or 0.75 M KCl (lane D) fractions of the phosphocellulose chromatography of nuclear extract. (Right) SDS-PAGE followed by Western blot analysis with mAb 1032 to the major vault protein of proteins immunoprecipitated with the mAbs to estrogen receptor AER314 (gray circle) and AER317 (solid circle) or control IgG (open circle) of the flowthrough fraction of the phosphocellulose chromatography of nuclear extract.
The antibodies 1032 and 1014 recognized a 104-kD band also in HeLa cell nuclear extract (data not shown) and therefore, were used to screen a commercial HeLa cell expression library. Two lambda clones were selected and their inserted DNA sequenced. Sequence comparison with nucleic acid sequence databases indicated a 100% homology of both fragments with the cDNA sequence coding for the LRP/human major vault protein (these sequence data are available from GenBank/EMBL/DDBJ under accession No. X79882), between bases 686–1251 and 301–1001, respectively.
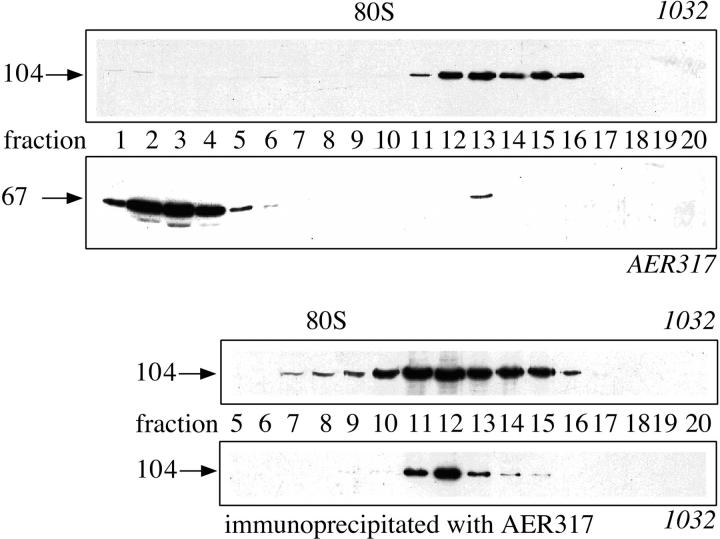
The major vault protein is the major component of vault ribonucleoprotein particles. To investigate whether the estrogen receptor interacted with the assembled particle or with soluble 104-kD protein, vaults were isolated from MCF-7 cell nuclear extract, according to a published procedure (Kedersha and Rome, 1986), by centrifugation on a 30–60% sucrose gradient. Western blot analysis of the gradient fractions with the mAb 1032 to the major vault protein showed an asymmetrical distribution of vaults in the expected region of the gradient, consistent with a sedimentation coefficient of 150 S. Western blot analysis of the same fractions with mAb AER317 indicated the presence of estrogen receptor in the top gradient fractions and in the peak fractions of anti-104-kD protein reactivity (Fig. 3, top). Fractions of a similar preparative gradient were incubated with the AER317 antibody and the immunoprecipitated proteins subjected to SDS-PAGE and Western blot analysis with the 1032 antibody to the major vault protein. The results (Fig. 3, bottom) indicated that the anti-estrogen receptor antibody was able to coprecipitate the 104-kD protein in the vault peak fractions, thus confirming that estrogen receptor was interacting with assembled vaults. In both experiments, the peak of estrogen receptor immunoreactivity corresponded with the left side of the vault peak.
Figure 3.
Western blot analysis and immunoprecipitation of vaults sedimented through a sucrose gradient. Arrows on the left side indicate the molecular weight (kD) of bands calculated from migration of standard proteins; on the top of the frames is reported the sedimentation of 80 S ribosomal subunit (external marker). (Top) SDS-PAGE followed by Western blot analysis with mAbs 1032 to the major vault protein or AER317 to estrogen receptor, as indicated, of fractions of a 5-ml sucrose gradient separation of the flowthrough of phosphocellulose chromatography of nuclear extract. (Bottom) SDS-PAGE followed by Western blot analysis with mAb 1032 to the major vault protein of proteins immunoprecipitated with the mAb to estrogen receptor AER317 (lower frame) from fractions (upper frame) of a 40-ml sucrose gradient separation of the flowthrough of phosphocellulose chromatography of nuclear extract.
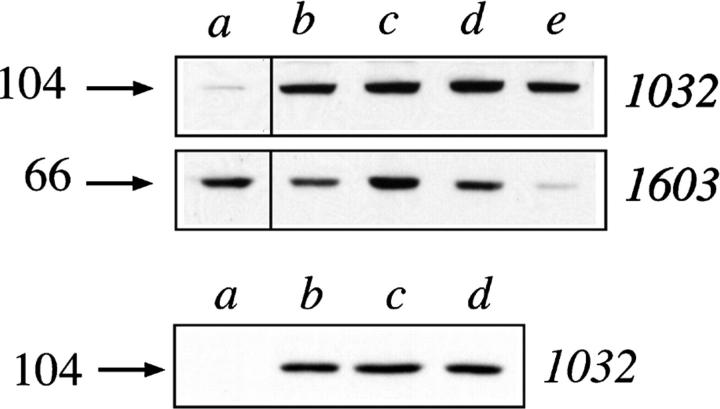
Since vaults were originally described as cytoplasmic particles and estrogen receptor is also present in the 20,000 g supernatant fraction, we performed the immunoprecipitation with the mAb AER317 to estrogen receptor, followed by Western blot analysis with the 1032 antibody to the major vault protein. The result, presented in Fig. 4, showed that the 104-kD major vault protein was coimmunoprecipitated in the 20,000 g supernatant as well as in the nuclear extract from cells grown in undeprived medium. Determination of lactate dehydrogenase activity indicated that the cytoplasmic contamination of nuclear extract was ∼1% (data not shown). To confirm that estrogen receptor was associated with vault particles also in the 20,000 g supernatant fraction, specific vRNA probing was performed in the immunoprecipitates. The RNA extracted from immunoprecipitates was electrophoresed and analyzed by Northern blot using a human vRNA probe. This probe was obtained by reverse transcription followed by PCR amplification of total RNA from MCF-7 cell line, using degenerated consensus primers designed from published rat and bullfrog vRNA sequences (Kickhoefer et al., 1993). Sequence analysis of several cloned PCR products showed two fragments species of 82 and 92 bp, respectively. These two fragments shared ∼86% identity with each other and ∼74–81% identity with rat and bullfrog vRNAs. Agarose electrophoresis followed by Northern blot analysis of the RNA coimmunoprecipitated from either cytosol or nuclear extract showed a band of ∼90 nucleotides (nt). This finding therefore definitely confirmed that estrogen receptor was associated to the assembled vaults.
Figure 4.
Western blot analysis of the 104-kD protein and Northern blot analysis of vRNA coimmunoprecipitated with estrogen receptor in the 20,000 g supernatant of cell extract. (Top) SDS-PAGE followed by Western blot analysis with mAb 1032 to the major vault protein of 20,000 g supernatant (lane a), or nuclear extract (lane b), or of protein immunoprecipitated from 20,000 g supernatant (lanes c and d), or nuclear extract (lane e) with the mAb to estrogen receptor AER317 (lanes d and e), or control IgG (lane c). The arrow on the left side indicates the molecular weight (kD) of the band calculated from migration of standard proteins. (Bottom) Northern blot analysis with a human vRNA probe (see Materials and Methods) of RNA extracted from aliquots of samples indicated in the top panel with the corresponding letters. Arrows on the left side indicate the migration of standard RNA (nt).
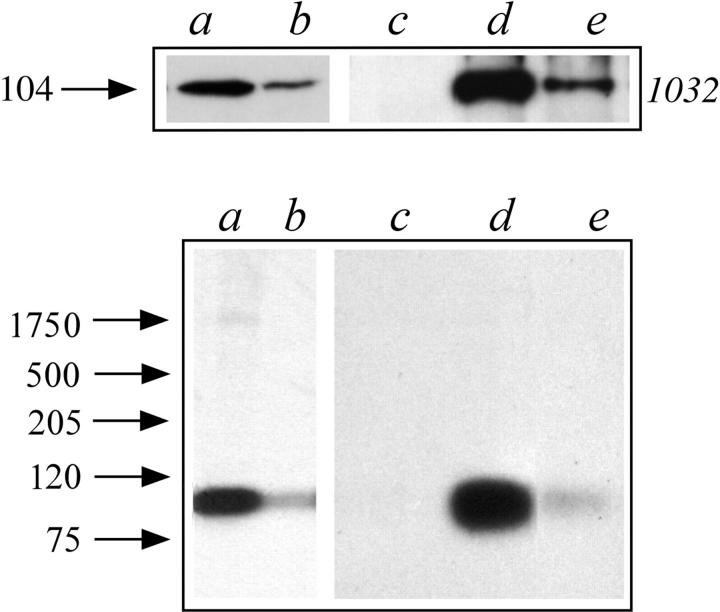
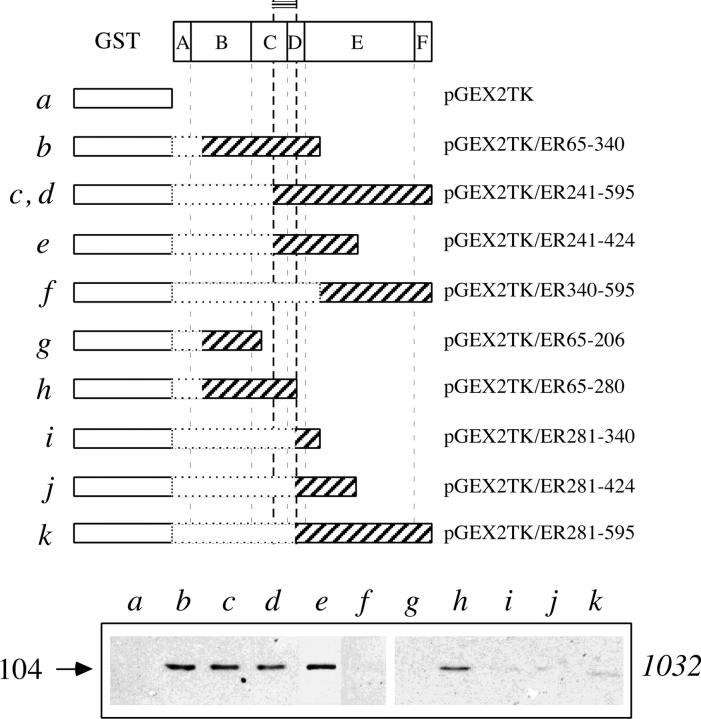
Reconstitution experiments were attempted in order to identify the region of the estrogen receptor molecule involved with the interaction. Fusion protein of estrogen receptor fragments and GST were prepared by expression of deletion mutants generated in the pGEX-2TK vector system. Using an affinity chromatography strategy, the recombinant proteins adsorbed to glutathione-coupled Sepharose (Kaelin et al., 1991) were incubated with aliquots of nuclear extract and the amount of 104-kD vault protein adsorbed was assayed by SDS-PAGE followed by Western blot with antibody 1032. The results are summarized in Fig. 5 and showed that only the fragments containing the sequence between aa 241 and 280 (Fig. 5, b–e and h) were able to retain the 104-kD vault protein. The synthetic receptor fragment interacting with the major vault protein and containing the estradiol binding site (aa 241–595) was incubated with the nuclear extract also in the presence of a physiological concentration of estradiol. The presence of estradiol did not affect the extent of the interaction (Fig. 5, lane d).
Figure 5.
Mapping the estrogen receptor region interacting with the 104-kD protein. SDS-PAGE followed by Western blot analysis with mAb 1032 to the major vault protein of proteins immunopurified on columns containing Sepharose coupled to the indicated fusion proteins. Nuclear extracts were incubated in the absence (lanes a–c and e–k) or in the presence of 10 nM estradiol (lane d). The arrow on the left side indicates the molecular weight (kD) of the band calculated from migration of standard proteins. The domain organization of estrogen receptor is reported in scale. On the top, a double-line segment reports the minimum determined fragment of the estrogen receptor sequence necessary for interaction with the major vault protein.
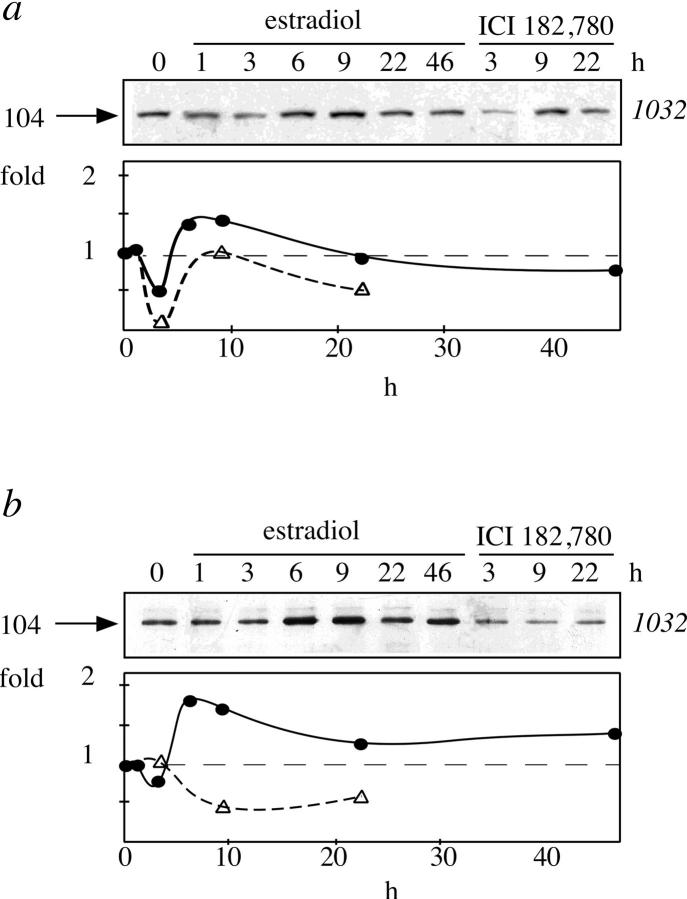
Vault concentration in the nuclear extract was affected by estrogen treatment of MCF-7 cells. The presence of estradiol in the tissue culture medium before cell harvesting produced a biphasic effect. An initial, transient reduction of vault concentration was followed by a moderate and steady increase, peaking at 6–9 h. The pure anti-estrogen ICI182,780 was also responsible for a biphasic effect, but never able to produce an increase of vault concentration at 6–9 h above baseline level. Densitometric analysis of the 104-kD band revealed in the Western blots by the antibody 1032 is presented in Fig. 6 a. Immunoprecipitation of these nuclear extracts with the antibody to estrogen receptor 1603 resulted in a twofold increase of 104-kD vault protein coimmunoprecipitated with estrogen receptor at 6–9 h. Densitometric profile of the 104-kD band, revealed by the antibody 1032 in the Western blots analysis of the immunoprecipitates, is presented in the Fig. 6 b. Treatment with the pure anti-estrogen ICI182,780, on the contrary, produced a reduction of the coimmunoprecipitated 104-kD band.
Figure 6.
Time course estrogen effect on the amount of the 104-kD protein present in the nuclear extract and coimmunoprecipitated with the estrogen receptor. SDS-PAGE followed by Western blot analysis with mAb 1032 to the major vault protein of nuclear extracts (a) or of proteins immunoprecipitated from nuclear extracts with the mAb 1603 to estrogen receptor (b) of cells incubated in the presence of 10 nM estradiol or 100 nM ICI182,780 for the indicated time. The arrows on the left side indicate the molecular weight (kD) of the band calculated from migration of standard proteins; the plots on the bottom are the densitometric analysis of the blots (•, estradiol; ▵, ICI182,780).
The finding presented in Fig. 6 suggested that the association of estrogen receptor with vaults could be hormone dependent. To better document the estradiol effect on the vault-estrogen receptor interaction, we attempted to reproduce it in vitro, using the postnuclear supernatant of hormone-deprived cells, because it contains both vaults and native, uncomplexed receptor (Fig. 7 top, lane a). The postnuclear supernatant was treated with hormones, incubated for 30 min at 30°C, to allow for receptor activation, and then centrifuged to obtain the vault-enriched fraction. These fractions were analyzed by SDS-PAGE, followed by Western blot with the antibodies 1032 to the major vault protein and 1603 to estrogen receptor (Fig. 7 top). The presence of a physiological concentration of estradiol increased the amount of estrogen receptor partitioned with the vault-enriched fraction, whereas the anti-estrogen ICI182,780 was ineffective. Sodium molybdate prevented the association of estrogen receptor with the vault- enriched fraction. Aliquots of the vault-enriched fractions were incubated with the antibody 1603 to estrogen receptor and the immunoprecipitates analyzed by Western blot with the antibody 1032 to the major vault protein (Fig. 7 bottom). The coimmunoprecipitation of the 104-kD vault protein confirmed that estrogen receptor was indeed associated with vault particles. However, the intensity of the 104-kD band from the estrogen-treated aliquot did not show an increase consistent with the increase of estrogen receptor cosedimenting with vaults. This could be due to the use of a limiting concentration of the antibody 1603 used for immunoprecipitation. It could be otherwise explained with the fact that the estradiol effect represents the interaction of more than one receptor molecule with each vault. The results presented in Fig. 7 indicated that also in vitro, in the absence of nuclei, estrogen receptor was able to complex with vaults in an estrogen-dependent manner, whereas the anti-estrogen was ineffective. Furthermore, the absence of estrogen receptor in the vault-enriched fraction in the presence of sodium molybdate indicated that the so-called “native” form of receptor was unable to complex with vaults. Sodium molybdate, in fact, prevents the quaternary structure changes of estrogen receptor induced by temperature and interaction with the ligand, preserving the complex containing the hsp90.
Figure 7.
Western blot analysis of the estrogen receptor associated with the vault- enriched fraction after cell-free activation of the postnuclear supernatant by hormone and temperature. The arrows on the left side indicate the molecular weight (kD) of bands calculated from migration of standard proteins. (Top) SDS-PAGE followed by Western blot analysis with mAb 1032 to the major vault protein or 1603 to estrogen receptor, as indicated, of postnuclear supernatant (lane a), vault-enriched fractions from aliquots of postnuclear supernatant incubated for 30 min at 30°C (lanes b–e), in the presence of 10 nM estradiol (lane c), 100 nM ICI182,780 (lane d), or 5 mM sodium molybdate (lane e). (Bottom) SDS-PAGE followed by Western blot analysis with mAb 1032 to the major vault protein of proteins immunoprecipitated with control antibodies (lane a) or with the mAb 1603 to estrogen receptor (lanes b–d) from the same samples of lanes b–d of the top panel.
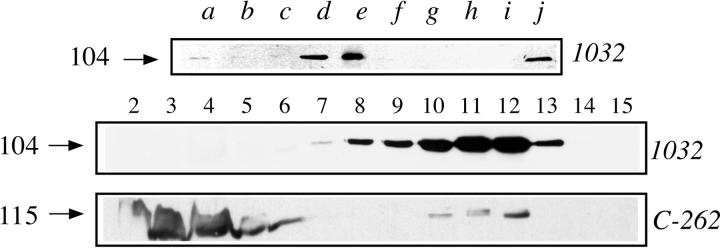
To investigate whether the association with vaults was shared by other proteins involved in the nuclear receptor signaling pathway, we selected three other nuclear receptors and two proteins involved in nuclear receptor transcriptional activation. The nuclear extract of cells grown in complete medium was incubated with antibodies to progesterone and glucocorticoid receptors, to Nurr77, an orphan receptor, to the transcription factor TFIIB or to SRC-1, a coactivator that is required for full transcriptional activity of the steroid receptor superfamily (Oñate et al., 1995), and that recently has been demonstrated to have an intrinsic histone acetyltransferase activity (Spencer et al., 1997). For the last two proteins, association to estrogen receptor had been previously demonstrated. A commercial antiserum to estrogen receptor was also tested. Immunoprecipitated proteins were analyzed by Western blot with the monoclonal antibody 1032 to the major vault protein and the result presented in Fig. 8 (top). The antibody to progesterone receptor was able to coimmunoprecipitate the 104-kD vault protein, even though to a lesser extent than both antibodies to estrogen receptor. The antibodies to glucocorticoid receptor, to Nurr77 and to TFIIB co-immunoprecipitated a very small amount of 104-kD major vault protein, whereas the antibody to SRC-1 did not. To confirm that progesterone receptor was also associated with vault particles, the nuclear extract was applied to a phosphocellulose column and the flowthrough fraction separated on a sucrose gradient. Western blot analysis was performed on the gradient fractions with the antibodies 1032 (to the major vault protein) and C-262 (to progesterone receptor). The result, presented in Fig. 8 (bottom), indicated that a fraction of the total progesterone receptor present in the extract cosedimented with the vault particle peak.
Figure 8.
Western blot analysis of the 104-kD protein and coimmunoprecipitated by antibodies to other nuclear proteins. The arrows on the left side indicate the molecular weight (kD) of the bands calculated from migration of standard proteins. The arrows on the left side indicate the molecular weight (kD) of the bands calculated from migration of standard proteins. (Top) SDS-PAGE followed by Western blot analysis with mAb 1032 to the major vault protein of nuclear extract (lane j) or of proteins immunoprecipitated from the nuclear extract with antibodies to progesterone receptor (lane a), to glucocorticoid receptor (lane b), to Nurr77 (lane c), to estrogen receptor (lanes d and e, commercial polyclonal and AER317, respectively), to TFIIB (lane f), to SRC-1 (lane g), or with mouse (lane h) and rabbit control antibodies (lane i). (Bottom) SDS-PAGE followed by Western blot analysis with mAb 1032 to the major vault protein or C-262 to progesterone receptor, as indicated, of fractions of a 5-ml sucrose gradient separation of the flowthrough of phosphocellulose chromatography of nuclear extract.
Discussion
The interaction of vaults with the estrogen receptor molecule is as puzzling as their involvement with multi-drug resistance mechanisms. No other protein with a defined biological function has been described to be associated with vaults. It is hard, therefore, to predict the biological meaning of the interaction described here within the estradiol mechanism of action. The molecular mechanism of steroid hormone action relays on precise DNA and protein contacts with the receptor molecule (Kumar et al., 1986; Mader et al., 1989; Medici et al., 1991). Specific DNA binding of the receptor to the hormone responsive elements is a necessary but not sufficient step for activating transcription (Mayer et al., 1989). Ligand-activated receptors communicate with the transcriptional apparatus by contact with bridging molecules or directly with basal transcription factors (Ing et al., 1992; Cavaillès et al., 1994, 1995; Halachmi et al., 1994; Jacq et al., 1994; Le Douarin et al., 1995; Oñate et al., 1995; Voegel et al., 1996; vom Baur et al., 1996). It is conceivable that other proteins might interact with the receptor molecule for the correct implementation of other tasks related to signal transduction, such as receptor recycling and/or processing, and intracellular homing. Given the transient nature of these interactions, it was not surprising that only a limited amount of the estrogen receptor molecules present in the nuclear extract was associated with the major vault protein.
Although the selective immunoprecipitation of the 104-kD protein with the AER317 and not with the AER314 antibody suggested that the A/B domain of estrogen receptor was involved in the interaction, a different evidence came from reconstitution experiments with the recombinant receptor fragments. The result presented in Fig. 5 indicated that the region necessary for the interaction was in the COOH-terminal extremity of the DNA-binding domain and in the hinge region (domains C and D), between aa 241 and 340. A fragment containing only part of this sequence showed partial interaction, suggesting the existence of more than one contact involved. These results are consistent with the hypothesis that the interaction with the vault protein mediates the process of nuclear transport of the receptor molecule. In fact, three proto-nuclear localization signals, cooperating for the estradiol-independent nuclear localization of the receptor, have been mapped to the same region (Picard et al., 1990, Ylikomi et al., 1992). This evidence may support the hypothesis that vaults are part of the central plug of the nucleopore (Chugani et al., 1993).
A functional relevance for vaults–estrogen receptor interaction was strongly supported by the evidence that a physiological concentration of estradiol increased the number of estrogen receptor molecules interacting with vaults both in cultured cells and in in vitro preparations. Even though this effect was not dramatic, it was not obtained with the ICI182,780. This molecule is a potent and specific inhibitor of estrogen action and in both animal models and human studies is considered a pure antagonist (Wakeling et al., 1991). The in vitro reconstitution experiments, however, did not show hormone dependency of the interaction between the estrogen receptor fragments and the vault protein. This discrepancy may be explained with the presence of a fourth hormone-regulated nuclear localization signal in the hormone-binding domain of the receptor or by the intervention of other intracellular elements to the assembly of the complex. In the same region of the estrogen receptor, where we mapped the interaction with major vault protein, is localized a sequence necessary for the formation of the 8 S receptor form (Chambraud et al., 1990). This “native” form, found in the cytosol of target cells and unable to bind DNA, is formed by estradiol-binding subunits noncovalently associated with hsp90 (Redeuihl et al., 1987). Ligand binding induces the release of hsp90 from the receptor and its transformation to a DNA-binding form. Although the interaction of hsp90 with the receptor involves several receptor regions, the sequence between aa 251 and 271 is critical for the 8 S formation (Chambraud et al., 1990). Sodium molybdate is able to prevent the ligand- and temperature-induced dissociation of the hsp90 from the hormone-binding subunit, and therefore preserves the 8 S form. In our experiments, addition of sodium molybdate prevented the estrogen-induced interaction of receptor with vaults in the postnuclear supernatant from unstimulated cell. This finding suggested that interaction of vaults and hsp90 with estrogen receptor molecule is mutually exclusive. Hsp90 might sterically hinder the interaction of the receptor with vaults and the estrogen effect might be mediated by the induction of hsp90 dissociation. For the glucocorticoid receptor, stabilization of the complex with hsp90 by sodium molybdate in live cells restricted hormone-dependent nuclear import of receptor (Yang and De Franco, 1996).
A deeper insight into the nature of vault function will certainly explain the function of this interaction in the mechanism of action of estrogenic hormones. Nothing is known about varieties in vault composition. The asymmetrical peak profile of reactivity of sucrose gradient fractions with the 1032 antibody to the major vault protein suggested that the vault population is not homogeneous and that the estrogen receptor interacted only with a sub-population of vaults particles. In this respect, it would be interesting to investigate a possible role of vRNA or other protein moieties in this differential interaction. The RNA coimmunoprecipitated with the major vault protein by the antibody to estrogen receptor contained the human vRNA. The sequence of the fragments cloned from the total MCF-7 cell RNA and used for probing the Northern blot matched the sequences identified as human vRNA genes (Kickhoefer et al., 1998). In particular, the 92-nt fragment had a 95% similarity with HGV1 and the 82-nt fragment had a 94% similarity with HGV2. The differences were limited to the regions covered by degenerated primers. Our electrophoresis separation was unable to separate the two bands and, therefore we were unable to identify which vRNA species was present in the immunoprecipitates. A regulatory rather than structural role for vRNA has been postulated, because the presence of vRNA is not required to maintain the structural integrity of vaults and it has a very distinct tissue-specific expression pattern (Kickhoefer et al., 1993). In addition, comparison of computer-generated secondary structures showed a similarity with other small RNAs (VAI and VAII of adenovirus, Epstein-Barr–encoded RNAs, and ribosomal 5 S) endowed with a regulatory function (Kickhoefer et al., 1993). Coherent to this hypothesis was the finding that vRNA was not necessary for the vaults–estrogen receptor interaction. RNase treatment of nuclear extract did not affect coimmunopurification of vault particles with estrogen receptor (data not shown).
We have no specific data about the stoichiometry of the vault-receptor interaction. The intensity of the major vault protein bands coimmunoprecipitated with estrogen receptor suggested that estradiol treatment may induce an increase in both the number of vaults involved and receptor molecules interacting with each particle.
Although vaults were originally identified in the postmicrosomal supernatant of cell extracts, it has also been reported that they were present in isolated nuclei and that they can be extracted with 1% Triton X-100 or 150 mM NaCl washes of nuclei (Chugani et al., 1993). In our experiments, vaults were originally identified in the nuclear extract from cultured MCF-7 cells. The nuclear extract was prepared according to a protocol optimized for the recovery of cell-free transcription activity by RNA polymerase rather than for integrity of the nuclear preparation. The amount of 104-kD major vault protein present in our nuclear extract was ∼1/10th of that present in the cytosol fraction. Coimmunoprecipitation of vaults with the estrogen receptor also in the postnuclear supernatant or in the vault-enriched fraction indicated that the interaction of estrogen receptor was not selective for vaults present in the nuclear extract. However, a precise intracellular localization of vaults interacting with estrogen receptor would require a double-labeling immunocytochemistry study and it is out of the aim of this report.
Taken together, these evidences allowed us to speculate that the interaction between estrogen receptor and vaults is probably related to the intracellular transport. The existence of an energy-dependent nucleo–cytoplasmic shuttle mechanism has been demonstrated for progesterone and estrogen receptors (Guiochon-Mantel et al., 1991). An active, regulated transport of estrogen receptor between nucleus and cytoplasm might establish a further control step in the mechanism of hormone action. Differential partitioning of receptor molecules between intracellular compartments (nucleus/cytoplasm) might modulate the sensitivity of a target cell to hormone, adjusting the intranuclear receptor concentration. It is reasonable to assume that such control mechanism could be modified by estradiol itself—thus participating to the receptor downregulation effect—or by interfering signal transduction pathways or by other integrated intracellular functions. The evidence that progesterone receptor cosedimented with vaults and that antibodies to glucocorticoid receptor and to Nurr77 were able to immunoprecipitate the 104-kD major vault protein suggested that interaction with this particle is a property common to more than one member of the nuclear receptor superfamily. If this is the case, vaults might be involved in a general mechanism of nucleo–cytoplasmic shuttle for modulation of signal transduction of steroid hormones.
Abbreviations used in this paper
- aa
amino acids
- GST
glutathione- S-transferase
- LRP
lung resistance–related protein
- hsp90
90-kD heat shock protein
- vRNA
vault RNA
Footnotes
Plasmids pHEG0, pHE14, and pHE15 were a kind gift of Prof. P. Chambon (Institut de Génetique et de Biologie Moléculaire du CNRS, Strasbourg, France).
This investigation was supported by the Italian Ministry for University and Scientific and Technological Research and the Italian Association for Cancer Research (AIRC).
Address all correspondence to Bruno Moncharmont, Istituto di Patologia generale ed Oncologia, Facoltà di Medicina e Chirurgia, Seconda Università degli studi di Napoli, Larghetto Sant' Aniello a Caponapoli, 2 I-80138 Naples, Italy. Tel.: +39 81 5665686. Fax: +39 81 5665695. E-mail: mobruno @unina.it
References
- Abbondanza C, De Falco A, Nigro V, Medici N, Armetta I, Molinari AM, Moncharmont B, Puca GA. Characterization and epitope mapping of a new panel of monoclonal antibodies to estradiol receptor. Steroids. 1993;58:4–12. doi: 10.1016/0039-128x(93)90011-b. [DOI] [PubMed] [Google Scholar]
- Ausubel, F.M., R. Breint, R.E. Kingston, D.D. Moore, J.G. Seidman, J.G. Smith, and K. Struhl. 1996. Current Protocols in Molecolar Biology. Vol. 3. Wiley & Sons Inc., New York.
- Beato M. Gene regulation by steroid hormones. Cell. 1989;56:335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Beato M, Herrlich P, Schütz G. Steroid hormone receptors: many actors in search for a plot. Cell. 1995;83:851–857. doi: 10.1016/0092-8674(95)90201-5. [DOI] [PubMed] [Google Scholar]
- Binart N, Chambraud B, Dumas B, Rowlands DA, Bigogne C, Levin JM, Garnier J, Baulieu EE, Catelli MG. The cDNA-derived amino acid sequence of chick heat shock protein Mr90,000 (HSP 90) reveals a “DNA like” structure: potential site of interaction with steroid receptors. Biochem Biophys Res Commun. 1989;159:140–147. doi: 10.1016/0006-291x(89)92415-7. [DOI] [PubMed] [Google Scholar]
- Cavaillès V, Dauvois S, Danielian PS, Parker MG. Interaction of proteins with transcriptionally active estrogen receptors. Proc Natl Acad Sci USA. 1994;91:10009–10013. doi: 10.1073/pnas.91.21.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavaillès V, Dauvois S, L'Horset F, Lopez G, Hoare S, Kushner PJ, Parker GP. Nuclear factor RIP140 modulates transcriptional activation by the estrogen receptor. EMBO (Eur Mol Biol Organ) J. 1995;14:3741–3751. doi: 10.1002/j.1460-2075.1995.tb00044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambraud B, Berry M, Redeuilh G, Chambon P, Baulieu E-E. Several regions of human estrogen receptor are involved in the formation of receptor-heat shock protein 90 complexes. J Biol Chem. 1990;265:20686–20691. [PubMed] [Google Scholar]
- Chugani DC, Rome LH, Kedersha NL. Evidence that vault ribonucleoprotein particles localize to the nuclear pore complex. J Cell Sci. 1993;106:23–29. doi: 10.1242/jcs.106.1.23. [DOI] [PubMed] [Google Scholar]
- Dingam JD, Lebivitz RM, Roeder RG. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiochon-Mantel A, Lescop P, Christin-Maitre S, Loosfelt H, Perrot-Applanat M, Milgrom E. Nucleocytoplasmic shuttling of the progesterone receptor. EMBO (Eur Mol Biol Organ) J. 1991;10:3851–3859. doi: 10.1002/j.1460-2075.1991.tb04954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halachmi S, Marden E, Martin G, MacKay H, Abbondanza C, Brown M. Estrogen receptor-associated proteins: possible mediators of hormone-induced transcription. Science. 1994;264:1455–1458. doi: 10.1126/science.8197458. [DOI] [PubMed] [Google Scholar]
- Ing NH, Beekman JM, Tsai SY, Tsai MJ, O'Malley BW. Members of the steroid hormone receptor superfamily interact with TFIIB (S300-II) J Biol Chem. 1992;267:17617–17623. [PubMed] [Google Scholar]
- Izquierdo MA, van der Zee AG, Vermorken JB, van der Valk P, Belien JA, Giaccone G, Scheffer GL, Flens MJ, Pinedo HM, Kenemans P, et al. Drug resistance-associated marker Lrp for prediction of response to chemotherapy and prognoses in advanced ovarian carcinoma. J Natl Cancer Inst. 1995;87:1230–1237. doi: 10.1093/jnci/87.16.1230. [DOI] [PubMed] [Google Scholar]
- Izquierdo MA, Shoemaker RH, Flens MJ, Scheffer GL, Wu L, Prather TR, Scheper RJ. Overlapping phenotypes of multidrug resistance among panels of human cancer-cell lines. Int J Cancer. 1996;65:230–238. doi: 10.1002/(SICI)1097-0215(19960117)65:2<230::AID-IJC17>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Jacq X, Brou C, Lutz J, Davidson I, Chambon P, Tora L. Human TAFII30 is present in a distinct TFIID complex and is required for transcriptional activation by the estrogen receptor. Cell. 1994;79:107–117. doi: 10.1016/0092-8674(94)90404-9. [DOI] [PubMed] [Google Scholar]
- Kaelin WG, Palas DC, De Capio JA, Kaye FJ, Livingston DM. Identification of cellular proteins that can interact specifically with the T/E1A-binding region of the retinoblastoma gene product. Cell. 1991;64:521–532. doi: 10.1016/0092-8674(91)90236-r. [DOI] [PubMed] [Google Scholar]
- Kedersha NL, Rome LH. Isolation and characterization of a novel ribonucleoprotein particle: Large structures contain a single species of small RNA. J Cell Biol. 1986;103:699–709. doi: 10.1083/jcb.103.3.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha NL, Miquel M-C, Bittner D, Rome LH. Vaults II. Ribonucleoprotein structures are highly conserved among higher and lower eukaryotes. J Cell Biol. 1990;110:895–901. doi: 10.1083/jcb.110.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kedersha NL, Heuser JE, Chugani DC, Rome LH. Vaults III. Vault ribonucleoprotein particles open into flower-like structures with octagonal symmetry. J Cell Biol. 1991;112:225–235. doi: 10.1083/jcb.112.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kickhoefer VA, Searles RP, Kedersha NL, Garber ME, Johnson DL, Rome LH. Vault ribonucleoprotein particles from rat and bullfrog contain a related small RNA that is transcribed by RNA polymerase III. J Biol Chem. 1993;268:7868–7873. [PubMed] [Google Scholar]
- Kickhoefer VA, Rajavel KS, Sceffer GL, Dalton WS, Scheper RJ, Rome LH. Vaults are up-regulated in multidrug-resistant cancer cell lines. J Biol Chem. 1998;273:8971–8974. doi: 10.1074/jbc.273.15.8971. [DOI] [PubMed] [Google Scholar]
- King WJ, Greene GL. Monoclonal antibodies localize oestrogen receptor in the nuclei of target celis. Nature. 1984;307:745–747. doi: 10.1038/307745a0. [DOI] [PubMed] [Google Scholar]
- Kumar V, Green S, Staub A, Chambon P. Localisation of the oestradiol-binding and putative DNA-binding domains of the human oestrogen receptor. EMBO (Eur Mol Biol Organ) J. 1986;5:2231–2236. doi: 10.1002/j.1460-2075.1986.tb04489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Landel CC, Kushner PJ, Greene GL. The interaction of human estrogen receptor with DNA is modulated by receptor-associated proteins. Mol Endocrinol. 1994;8:1407–1419. doi: 10.1210/mend.8.10.7854357. [DOI] [PubMed] [Google Scholar]
- Le Douarin B, Zechel C, Garnier JM, Lutz Y, Tora L, Pierrat P, Heery D, Gronemeyer H, Chambon P, Losson R. The N-terminal part of TIF1, a putative mediator of the ligand-dependent activation function (AF-2) of nuclear receptors, is fused to B-raf in the oncogenic protein T18. EMBO (Eur Mol Biol Organ) J. 1995;14:2020–2033. doi: 10.1002/j.1460-2075.1995.tb07194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- List AF, Spier CS, Grogan TM, Johnson C, Roe D, Greer JP, Wolff SN, Broxterman HJ, Scheffer GL, Scheper RJ, Dalton WS. Overexpression of the major vault transporter protein lung-resistance protein predicts treatment outcome in acute myeloid leukemia. Blood. 1996;87:2464–2469. [PubMed] [Google Scholar]
- Macfarlane DE. Two dimensional benzyldimethyl-n-hexadecylammonium chloride → sodium dodecylsulfate preparative polyacrylamide gel electrophoresis: a high capacity high resolution technique for the purification of proteins from complex mixtures. Anal Biochem. 1989;176:457–463. doi: 10.1016/0003-2697(89)90342-4. [DOI] [PubMed] [Google Scholar]
- Mader S, Kumar V, de Verneuil H, Chambon P. Three amino acids of the oestrogen receptor are essential to its ability to distinguish an oestrogen from a glucocorticoid-responsive element. Nature. 1989;338:271–274. doi: 10.1038/338271a0. [DOI] [PubMed] [Google Scholar]
- Marchetti E, Querzoli P, Moncharmont B, Parikh I, Bagni A, Marzola A, Fabris G, Nenci I. Immunocytochemical demonstration of estrogen receptors by monoclonal antibodies in human breast cancer: correlation with estrogen receptor assay by dextran-coated charcoal method. Cancer Res. 1987;47:2508–2513. [PubMed] [Google Scholar]
- Mayer M-E, Gronemeyer H, Turcotte B, Bocquel M-T, Tasset D, Chambon P. Steroid hormone receptors compete for factors that mediate their enhancer function. Cell. 1989;57:433–442. doi: 10.1016/0092-8674(89)90918-5. [DOI] [PubMed] [Google Scholar]
- Medici N, Nigro V, Abbondanza C, Moncharmont B, Molinari AM, Puca GA. In vitro binding of the purified hormone-binding subunit of the estrogen receptor to oligonucleotides containing natural or modified sequences of an estrogen responsive element. Mol Endocrinol. 1991;5:555–563. doi: 10.1210/mend-5-4-555. [DOI] [PubMed] [Google Scholar]
- Moncharmont B, Ramp G, De Goeij CCJ, Sluyser M. Comparison of the estrogen receptor in hormone-dependent and hormone-independent Grunder strain mouse mammary tumors. Cancer Res. 1991;51:3843–3848. [PubMed] [Google Scholar]
- Nigg EA. Nucleocytoplasmic transport: signals, mechanism and regulation. Nature. 1997;386:779–787. doi: 10.1038/386779a0. [DOI] [PubMed] [Google Scholar]
- Oñate SA, Tsai SY, Tsai MJ, O'Malley BW. Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science. 1995;270:1354–1357. doi: 10.1126/science.270.5240.1354. [DOI] [PubMed] [Google Scholar]
- Pantè N, Aebi U. Toward the molecular dissection of protein import into nuclei. Curr Opin Cell Biol. 1996;8:397–406. doi: 10.1016/s0955-0674(96)80016-0. [DOI] [PubMed] [Google Scholar]
- Picard D, Kumar V, Chambon P, Yamamoto KR. Signal transduction by steroid hormones: nuclear localization is differentially regulated in estrogen and glucocorticoid receptors. Cell Regul. 1990;1:291–299. doi: 10.1091/mbc.1.3.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugh BF, Tijan R. Transcription from a TATA-less promoter requires a multisubunit TFIID complex. Genes Dev. 1991;5:1935–1945. doi: 10.1101/gad.5.11.1935. [DOI] [PubMed] [Google Scholar]
- Redeuihl GJ, Moncharmont B, Secco CM, Baulieu E-E. Subunit composition of the molybdate-stabilized “8–9 S” nontransformed estradiol receptor purified from calf uterus. J Biol Chem. 1987;262:6969–6975. [PubMed] [Google Scholar]
- Reinberg D, Roeder RG. Factors involved in specific transcription by mammalian RNA polymerase II. Purification and functional analysis of initiation factors IIB and IIE. J Biol Chem. 1987;262:3310–3321. [PubMed] [Google Scholar]
- Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffer GL, Wijngaard PL, Flens MJ, Izquierdo MA, Slovak ML, Pinedo HM, Meijer CJ, Clevers HC, Scheper RJ. The drug resistance-related protein LRP is the human major vault protein. Nat Med. 1995;1:578–582. doi: 10.1038/nm0695-578. [DOI] [PubMed] [Google Scholar]
- Spencer TE, Jenster G, Burcin MM, Allis CD, Zhou J, Mizzen CA, McKenna NJ, Oñate SA, Tsai SY, Tsai MJ, O'Malley BW. Steroid receptor coactivator-1 is a histone acetyltransferase. Nature. 1997;389:194–198. doi: 10.1038/38304. [DOI] [PubMed] [Google Scholar]
- Tanese N, Pugh BF, Tijan R. Coactivators for a proline-rich activator purified from the multisubunit human TFIID complex. Genes Dev. 1991;5:2212–2224. doi: 10.1101/gad.5.12a.2212. [DOI] [PubMed] [Google Scholar]
- Tora L, Mullick A, Metzger D, Ponglikitmongkol M, Park I, Chambon P. The cloned human oestrogen receptor contains a mutation which alters its hormone binding properties. EMBO (Eur Mol Biol Organ) J. 1989a;8:1981–1986. doi: 10.1002/j.1460-2075.1989.tb03604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tora L, White J, Brou C, Tasset D, Webster N, Scheer E, Chambon P. The human estrogen receptor has two independent nonacidic transcriptional activation functions. Cell. 1989b;54:481–487. doi: 10.1016/0092-8674(89)90031-7. [DOI] [PubMed] [Google Scholar]
- Towbin H, Staehelin D, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedures and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasu KS, Rome LH. Dictyosteliumvaults: disruption of the major proteins reveals growth and morphological defects and uncovers a new associated protein. J Biol Chem. 1995;279:16588–16594. doi: 10.1074/jbc.270.28.16588. [DOI] [PubMed] [Google Scholar]
- Voegel JJ, Heine MJS, Zechel C, Gronemeyer H, Chambon P. TIF2, a 160 kDa transcriptional mediator for the ligand-dependent activation function AF–2 of nuclear receptors. EMBO (Eur Mol Biol Organ) J. 1996;15:3667–3675. [PMC free article] [PubMed] [Google Scholar]
- vom Baur E, Zechel C, Heery DM, Heine MJS, Garnier JM, Vivat V, Le Douarin B, Gronemeyer H, Chambon P, Losson R. Differential ligand-dependent interactions between the AF-2 activating domain of nuclear receptors and the putative transcriptional intermediary factors mSUG1 and TIF1. EMBO (Eur Mol Biol Organ) J. 1996;15:110–124. [PMC free article] [PubMed] [Google Scholar]
- Wakeling AE, Dukes M, Bowler J. A potent specific pure antiestrogen with clinical potential. Cancer Res. 1991;51:3867–3873. [PubMed] [Google Scholar]
- Yang J, De Franco DB. Assessment of glucocorticoid receptor-heat shock protein 90 interactions in vivo during nucleocytoplasmic trafficking. Mol Endocrinol. 1996;10:3–13. doi: 10.1210/mend.10.1.8838140. [DOI] [PubMed] [Google Scholar]
- Ylikomi T, Bocquel MT, Berry M, Gronemeyer H, Chambon P. Cooperation of proto-signals for nuclear accumulation of estrogen and progesterone receptors. EMBO (Eur Mol Biol Organ) J. 1992;11:3681–3694. doi: 10.1002/j.1460-2075.1992.tb05453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemiecki A, Catelli M-G, Joab I, Moncharmont B. Association of the heat shock protein hsp90 with steroid hormone receptors and tyrosine kinase oncogene products. Biochem Biophys Res Commun. 1986;138:1298–1307. doi: 10.1016/s0006-291x(86)80424-7. [DOI] [PubMed] [Google Scholar]