Abstract
Exposure for 24 h of mucus-secreting HT-29 cells to the sugar analogue GalNAc-α-O-benzyl results in inhibition of Galβ1-3GalNAc:α2,3-sialyltransferase, reduced mucin sialylation, and inhibition of their secretion (Huet, G., I. Kim, C. de Bolos, J.M. Loguidice, O. Moreau, B. Hémon, C. Richet, P. Delannoy, F.X. Real., and P. Degand. 1995. J. Cell Sci. 108:1275–1285). To determine the effects of prolonged inhibition of sialylation, differentiated HT-29 populations were grown under permanent exposure to GalNAc-α-O-benzyl. This results in not only inhibition of mucus secretion, but also in a dramatic swelling of the cells and the accumulation in intracytoplasmic vesicles of brush border–associated glycoproteins like dipeptidylpeptidase-IV, the mucin-like glycoprotein MUC1, and carcinoembryonic antigen which are no longer expressed at the apical membrane. The block occurs beyond the cis-Golgi as substantiated by endoglycosidase treatment and biosynthesis analysis. In contrast, the polarized expression of the basolateral glycoprotein GP 120 is not modified. Underlying these effects we found that (a) like in mucins, NeuAcα2-3Gal-R is expressed in the terminal position of the oligosaccharide species associated with the apical, but not the basolateral glycoproteins of the cells, and (b) treatment with GalNAc-α-O-benzyl results in an impairment of their sialylation. These effects are reversible upon removal of the drug. It is suggested that α2-3 sialylation is involved in apical targeting of brush border membrane glycoproteins and mucus secretion in HT-29 cells.
Enterocytic and mucus-secreting populations isolated from the human colon carcinoma cell line HT-29 have proven extremely useful for the study of intestinal cell differentiation (for review see Zweibaum et al., 1991). The differentiated populations used in this study were isolated from the mainly undifferentiated parental line (Fogh and Trempe, 1975) by selection with increasing concentrations of methotrexate (MTX)1 (Lesuffleur et al., 1990, 1991). Cells selected with 10−6 and 10−5 M MTX form a homogeneous population of mucus-secreting cells (Lesuffleur et al., 1990, 1991), whereas selection with 10−3 M MTX results in a population of enterocytic phenotype (Lesuffleur et al., 1991). Whatever their phenotype, these populations share common differentiation characteristics, namely (a) a postmitotic onset of the differentiation process and (b) a polarized organization of the cell monolayer with the presence of an apical brush border endowed with several glycoproteins such as dipeptidylpeptidase-IV (DPP-IV), the carcinoembryonic antigen (CEA) and the mucin-like glycoprotein MUC1. Mucus-secreting cell populations selected by MTX have been extensively analyzed as to the characteristics of their mucins: at the mRNA level they mainly express MUC5AC (Lesuffleur et al., 1993, 1995); at the protein level they secrete mucus of gastric immunoreactivity (Lesuffleur et al., 1990) with a main oligosaccharide species similar to the clone HT29-16E (Capon et al., 1992), NeuAcα2-3Galβ1-3GalNAc-R (sialyl T antigen) (Lesuffleur et al., 1993; Huet et al., 1995).
Previous results have shown that short-term (24-h) exposure of postconfluent mucus-secreting HT-29 cells to benzyl-2-acetamido-2-deoxy-α-d-galactopyranoside (GalNAc-α-O-benzyl), an inhibitor of mucin O-glycosylation (Kuan et al., 1989; Huang et al., 1992; Byrd et al., 1995), results in a decrease of mucus secretion, a lower sialic acid content of newly synthesized mucins, and an increased content of T antigen (Galβ1-3GalNAc-R) (Huet et al., 1995). Similar effects of GalNAc-α-O-benzyl have been demonstrated in LS174-T colon cancer cells (Kuan et al., 1989) or KATO III gastric cells (Byrd et al., 1995). In the case of HT-29 cells, the change in mucin glycosylation has been shown to be a consequence of the metabolization of GalNAc-α-O-benzyl into Galβ1-3GalNAc-α-O-benzyl which, in turn, is a potent competitive inhibitor of the Galβ1-3GalNAc α2,3-sialyltransferase (Huet et al., 1995; Delannoy et al., 1996), the main sialyltransferase activity expressed in HT-29 cells (Majuri et al., 1995; Delannoy et al., 1996).
Because O-glycosylation may be part of the processing of a wide variety of proteins and the biochemical changes observed after 24 h of treatment may not fully reflect the activity of GalNAc-α-O-benzyl, we set to analyze the effects of a permanent exposure to this drug on the proliferation and differentiation of mucus-secreting HT-29 cells. We found that, in addition to an inhibition of mucus secretion, apical glycoproteins such as the transmembrane glycoprotein MUC1 (Gendler et al., 1987), as well as the N-glycosylated proteins DPP-IV (Semenza, 1989; Misumi et al., 1992) and CEA (Fukushima et al., 1995) considerably accumulate within the cytoplasm and are no longer expressed at the apical membrane, this occurring without modification of the polarization of the cells. Based on this observation we further found that, like in mucins, NeuAcα2-3 is the main sialylated determinant associated with DPP-IV, MUC1, and CEA in differentiated HT-29 cells, whether of mucus-secreting or enterocytic phenotype, and that treatment with GalNAc-α-O-benzyl results in a decreased sialylation of these proteins. These effects of GalNAc-α-O-benzyl are rapidly reversible upon removal of the drug. These results raise the question of whether, in differentiated HT-29 cells, α2-3 sialylation is required for mucus secretion as well as for the correct targeting of apical glycoproteins.
Materials and Methods
Cell Culture
HT-29–differentiated subpopulations were derived from the original cell line (Fogh and Trempe, 1975) that was obtained from the late J. Fogh (Sloan-Kettering Memorial Cancer Center, Rye, NY). The cells adapted to MTX 10−6, 10−5, and 10−3 M (Lesuffleur et al., 1990, 1991) were used after several weekly passages of reversion to drug-free medium (10–40 passages) and are referred to as HT29-RevMTX10−6, RevMTX10−5 (mucus-secreting cells), and RevMTX10−3 (enterocytic cells). Cells were grown in DME (Life Technologies, Inc., Cergy-Pontoise, France) supplemented with 10% inactivated FBS for 30 min at 56°C (Boehringer Mannheim Biochemicals, Mannheim, Germany). GalNAc-α-O-benzyl (Sigma Chemical Co., St. Louis, MO) was solubilized in DME. All experiments and maintenance of the cells were done in 25- or 75-cm2 T flasks (Corning Glass Works, Corning, NY), and in 24-well cell culture clusters (Costar, Cambridge, MA) at 37°C in a 10% CO2 / 90% air atmosphere. Cells were seeded at 2 × 104 cells/cm2. The same conditions were applied to cells grown on tissue culture-treated Transwell polycarbonate membranes with a 24.5-mm diam and a 0.4-μm pore size (Costar). For maintenance purposes, cells were passaged weekly, using 0.025% trypsin in 0.53 mM EDTA in PBS Ca2+Mg2+-free (PBS−). The medium was changed daily in all culture conditions. For growth curves, cells grown in 24-well culture clusters were detached with trypsin and counted with a hemocytometer. Cell volume was determined in a hematocrit. Control Caco-2 cells were cultured as previously reported (Pinto et al., 1983) and analyzed between passages 70 and 80.
Antibodies and Lectins
Mouse mAbs HBB 3/775/42 (Hauri et al., 1985) and G1/136 (Eilers et al., 1989) specific for human DPP-IV and a 120-kD basolateral glycoprotein respectively, were a gift of H.P. Hauri (Biocenter of the University of Basel, Basel, Switzerland). Rat mAb 4H3 against human DPP-IV (Gorvel et al., 1991) was obtained from S. Maroux (CNRS Unité de Recherche Associee 1820, Faculté des Sciences de Saint Jerôme, Marseille, France). Mouse mAb 517 (Le Bivic et al., 1988) against CEA was obtained from A. Le Bivic (Faculté des Sciences de Luminy, Marseille, France). Mouse mAb BC-2 (Xing et al., 1989), which recognizes a sequence in the tandem repeat of the MUC1 gene product was obtained from I. McKenzie (Austin Cancer Research Institute, Heidelberg, Victoria, Australia). Mouse mAb B72.3 against sialyl-Tn (Nuti et al., 1982) was obtained from K.O. Lloyd (Memorial Sloan-Kettering Cancer Center, New York). Mouse mAb TS2/16 against the integrin β1 subunit (Arroyo et al., 1992) was obtained from F. Sanchez-Madrid (Universidad Autónoma de Madrid, Madrid, Spain). Rabbit polyclonal Abs against porcine villin (Robine et al., 1985) and the tight junction protein ZO-1 (Willot et al., 1992) were obtained from D. Louvard (Institut Curie, Paris, France) and J.M. Anderson (Yale University, New Haven, CT), respectively. For the detection of gastric mucins we used the same rabbit polyclonal Ab L56/C as previously used for cloning the L31 mucin cDNA encoding the 3′ end of MUC5AC (Lesuffleur et al., 1995). Fluorescein-conjugated Maackia amurensis agglutinin (MAA) (Wang and Cummings, 1988), Sambucus nigra agglutinin (SNA) (Shibuya et al., 1987), and Peanut (Arachis hypogaea) agglutinin (PNA) (Lotan et al., 1975), which recognize the oligosaccharide species NeuAcα2-3Gal-R, NeuAcα2-6Gal, and Galβ1-3GalNAc-R, respectively, were from Vector Labs Inc. (Burlingame, CA).
Immunofluorescence and Histochemical Staining
Indirect immunofluorescence was performed on cryostat sections of cell layer rolls as reported (Lesuffleur et al., 1990). Briefly, late (day 21) cultures of cells grown in 25-cm2 T flasks were rinsed with Ca2+Mg2+-free PBS, the T flask was cut up with a soldering iron, and then the cell layer gently was scraped with a rubber policeman and poured in a bath of liquid nitrogen. The resulting frozen cell pellet was either stored in liquid nitrogen for further analysis, or immediately processed for cryostat sections. This method has the double advantage of visualizing a large part of the cell layer and allowing the concomitant detection of apical, basolateral, and intracellular proteins on the same section. Double immunofluorescence was performed on sections postfixed with 3.7% paraformaldehyde in PBS− for 10 min at room temperature using secondary antibodies fluorescein-coupled sheep anti–mouse or anti–rabbit Ig (Institut Pasteur Production, Marne-la-Coquette, France) or rhodamine-coupled sheep antiglobulins (Boehringer Mannheim Biochemicals). Desialylation was performed by incubation for 16 h at 37°C of paraformaldehyde-fixed cryostat sections with sialidase from Clostridium perfringens (Sigma Chemical Co.) (50 mU/ml in 50 mM citrate buffer, pH 6.0, 0.9% NaCl, 0.1% CaCl2). For confocal microscopy analysis, cells grown on glass coverslips were fixed with 4% paraformaldehyde for 10 min, incubated with 50 mM NH4Cl for 30 min, and then permeabilized with 0.1% saponin in 1% BSA/ PBS for 30 min. To detect β1 integrin, mAb TS2/16 (0.5 μg/ml in 0.1% saponin in 1% BSA/PBS) was added for 1 h, followed by FITC-conjugated goat anti–mouse; MAA (Vector Labs, Inc.) (20 μg/ml in 0.1% saponin in Tris-HCl, pH 7.5, 15 mM KCl, 5 mM MgCl2) was added for 1 h, followed by streptavidin-rhodamine (Pierce Chemical Co., Rockford, IL). Confocal microscopy analysis was performed using a Leica instrument (model TCS 4D; St. Gallen., Switzerland). Histological staining with alcian blue, pH 2.5, and nuclear red was done on cryostat sections postfixed in absolute ethanol for 10 min at room temperature.
Transmission EM and Ultrastructural Immunochemistry
Classical transmission EM was performed as previously reported (Lesuffleur et al., 1990, 1991) on cells grown in 25-cm2 plastic flasks. Samples embedded in Epon (Polysciences, Inc., Warington, PA) were reembedded to make sections perpendicular to the bottom of the flask. Thin sections were stained with toluidine blue. Ultrastructural immunochemistry was performed as previously described (Hennebicq-Reig et al., 1996). After rinsing three times in PBS, cells cultured in 25-cm2 flasks were fixed in phosphate buffer containing 4% paraformaldehyde and 0.05% glutaraldehyde. The cell layer was scraped with a rubber policeman, the cell pellet was infiltrated with phosphate buffer containing 2.3 M sucrose and 20% polyvinylpyrolidone, and then frozen in liquid nitrogen. Ultrathin cryosections were successively incubated with PBS containing 10% FBS, mouse mAb HBB 3/775/42 (DPP-IV), rabbit anti–mouse Ig antibody, and 8-nm gold-conjugated protein A. All antibodies and gold-conjugated protein A were diluted in PBS containing 10% FBS. The grids were finally counterstained with methylcellulose uranyl acetate and observed using an electron microscope (model 902; Carl Zeiss, Inc., Thornwood, NY). The same procedure was applied for mAb 517 (CEA) and mAb BC-2 (MUC1).
Northern Blot Analysis
For detection of DPP-IV and villin mRNAs, total RNA was isolated from the cells 16 h after medium change by lysis with guanidium isothiocyanate and centrifugation through a CsCl gradient (Chirgwin et al., 1979). Samples of total RNA, denatured in 1 M glyoxal (Thomas, 1980), were fractionated by electrophoresis through 1% agarose gels and then transferred to nylon (model Hybond N; Amersham Corp., Amersham, UK) in the presence of 20× SSC. Filters were incubated overnight at 42°C in prehybridization buffer containing 50% formamide, 5× SSC, 10× Denhardt's solution, 50 mM sodium phosphate, pH 6.5, and 250 μg/ml sonicated and denatured salmon sperm DNA. Filters were then hybridized with the 32P-labeled probe for 20 h at 42°C in prehybridization buffer containing 10% dextran sulfate (Thomas, 1980). Blots were washed twice with 2× SSC, 0.1% SDS at room temperature, once with 0.1× SSC, 0.1% SDS at 50°C, and once, using the same solution, at 65°C for 15 min. Blots were then processed for autoradiography. To normalize for RNA, filters were dehybridized and stained with methylene blue. Methylene blue staining was preferred to hybridization with actin or glyceraldehyde 3-phosphate dehydrogenase since it was found that the levels of these transcripts differ in dividing and postconfluent cells. DPP-IV was detected with cDNA DPI-101 (Darmoul et al., 1990) and villin with cDNA V19 (Pringault et al., 1986), obtained from D. Louvard. The probe for human ST3Gal I (Kitagawa and Paulson, 1994) was a 537-bp PCR-amplified fragment from HepG2 cells cDNA corresponding to the coding region from nucleotide 361 to 898 (Recchi et al., 1998).
Measurement of Enzyme Activities
DPP-IV activity was measured in the cell homogenates as previously reported (Lesuffleur et al., 1990), according to the method of Nagatsu et al. (1976) using 1.5 mM glycyl-l-proline-4-nitroanilide as substrate. Results are expressed as mU/mg of protein. One unit is defined as the activity that hydrolyzes 1 mmol of substrate/min at 37°C. Proteins were measured with the BCA protein assay reagent (Pierce Chemical Co.). Galβl-3GalNAc α2,3-sialyltransferase activity was measured in cell homogenates prepared by lysing the cells at 0°C with 10 mM sodium cacodylate buffer, pH 6.5, containing 1% Triton X-100 (Sigma Chemical Co.), 20% glycerol, 0.5 mM dithiothreitol, and 5 mM MnCl2 (1 ml per 2.5 × 107 cells). After 10 min of incubation under continuous stirring, cell homogenates were centrifuged at 10,000 g for 15 min and the supernatants were used for enzymatic assay. Protein concentration was determined according to Peterson (1977) using BSA as standard. Cell homogenates (40 μg of protein) were brought to a final volume of 120 μl with 0.1 M sodium cacodylate buffer, pH 6.5, 1% Triton X-100, 0.1 M galactose (as inhibitor of β-galactosidase), 1 mM 2,3-dehydro-2-deoxy-Neu5Ac (as inhibitor of sialidases), 52.9 μM CMP-[14C]- Neu5Ac (0.58 GBq/mmol; 3.68 kBq/120 μl) (Amersham Corp.), containing 1 mM of Galβl-3GalNAcα-O-pNp (Sigma Chemical Co.) and incubated for 1 h at 37°C. The reactions were stopped by adding 1 vol of ethanol. Samples were centrifuged at 3,000 g for 5 min and then supernatants were directly developed by descending paper chromatography with ethyl acetate/pyridine/water (10:4:3 by vol) (Delannoy et al., 1993). Assays were performed in duplicate. The rates of reactions were linear with time, at least for 1 h. The incorporation of [14C]-Neu5Ac was determined by subtraction of the radioactivity measured in the absence of exogenous acceptors and results are expressed as average values in nmol of Neu5Ac transferred per milligram of protein and per hour.
Electrophoresis and Western Blotting
Cells were homogenized by sonication in Tris/Mannitol buffer. Immunoprecipitation of DPP-IV, CEA, and MUC1 was performed as in Hauri et al. (1985), using mAbs 3/775/42, 517, and BC-2 previously coated on protein A–Sepharose beads (Pharmacia Fine Chemicals, Uppsala, Sweden). SDS-PAGE was performed under reducing conditions on 4–20% gradient polyacrylamide gels (Laemmli, 1970) either with 50 μg of total cellular protein per lane or with DPP-IV, CEA, or MUC1 immunoprecipitates. After electrophoresis, proteins were transferred to a nitrocellulose membrane (model BioTrace NT; Gelman Sciences Inc., Ann Arbor, MI) as described in Vaessen et al. (1981). The membranes were then treated for 2 h with polyvinylpyrolidone K-30 (2% in TBS). Immunodetection of DPP-IV, CEA, and MUC-1 was performed with mAbs 4H3, 517, and BC-2, respectively, using secondary antibodies peroxidase-coupled anti-rat or anti-mouse Ig accordingly (Biosys, Compiègne, France). For glycan detection, membranes were incubated with digoxigenin-labeled lectins from Boehringer Mannheim Biochemicals at concentrations of 5 μg/ml in TBS for MAA and SNA, and 2 μg/ml in TBS for PNA-digoxigenin. Then, the nitrocellulose membranes were incubated for 1 h with alkaline phosphatase-labeled antidigoxigenin Fab fragments (1 μg/ml in TBS) (Boehringer Mannheim Biochemicals). After washing, labeled glycoproteins were revealed by 4-nitro blue tetrazolium chloride 5-bromo-4-chloro-3-indolyl-phosphate staining. Desialylation was performed as for cryostat sections before incubation with the digoxigenin-labeled lectins. Digestion with endoglycosidase H (Boehringer Mannheim Biochemicals) was carried out in 0.1 M phosphate buffer, pH 5.5, containing 0.02% SDS, 1% Triton X-100, 1% β-mercaptoethanol, 1 mM phenylmethylsulfonylfluoride, 10 μg/ml leupeptin, 10 μg/ml pepstatin, using 10 mU of endoglycosidase H overnight at 37°C. Digestion with endoglycosidase F/N-glycosidase F (Boehringer Mannheim Biochemicals) was carried out in 0.1 M phosphate buffer, pH 7.0, containing 0.05% SDS, 1% Triton X-100, 1% β-mercaptoethanol, 1 mM phenylmethylsulfonylfluoride, 10 μg/ml leupeptin, and 10 μg/ml pepstatin, using 0.5 U of endoglycosidase F/N-glycosidase F overnight at 37°C. Controls were incubated in the same buffers overnight at 37°C without glycosidase.
Metabolic Labeling and Immunoprecipitation of DPP-IV
Cells were cultured in six-well plates with or without the presence of 2 mM GalNAc-α-O-benzyl until day 11. Subsequently, untreated cells were pulse labeled for 15 min with 200 μCi/well of [35S]-methionine (Amersham Corp.) in 1 ml of methionine-free medium, and then chased for the indicated periods of time with 1 ml 0.01 M methionine in regular medium. The same protocol was applied to treated cells, except for the presence of 2 mM GalNAc-α-O-benzyl throughout the experiment. Cells were rinsed in PBS and lysed in 1 ml of RIPA buffer (0.001 M Tris-HCl, pH 8.0, 0.01 M NaCl, 0.1% SDS, 1% Triton X-100, 0.5% sodium deoxycholate, 1% phenylmethylsulfonylfluoride, 0.001 M sodium ethylene diamine tetraacetate). Aliquots of 50 μg of proteins from cell lysates were incubated with mAb 4H3 overnight at 4°C. Immunocomplexes were collected on protein G–Sepharose 4B (Sigma Chemical Co.), eluted in SDS sample buffer (0.2 M Tris-HCl buffer, pH 6.8, containing 2% SDS and 30% glycerol) at 60°C for 5 min, and then analyzed on 5–30% SDS–polyacrylamide gels. For autoradiography, gels were fixed in 40% ethanol, 10% glycerol, 10% acetic acid (by vol), soaked in Amplify (Amersham Corp.) for 20 min, dried on Whatman paper, and then exposed to Cronex 4 NIF film (Dupont, Les Ulis, France).
Results
GalNAc-α-O-benzyl Treatment Results in a Dose-dependent Decrease of Mucus Secretion and Swelling of Mucus-secreting HT-29 Cells
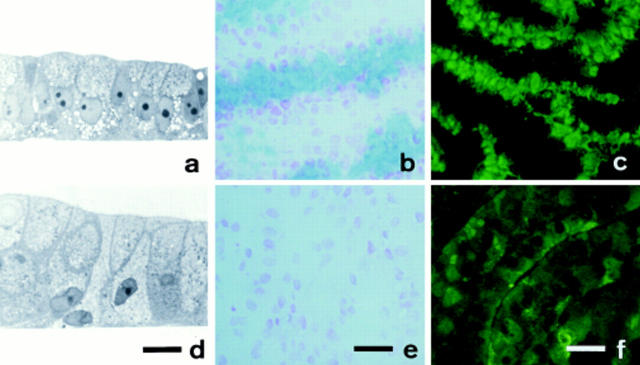
To assay for a dose-dependent effect of GalNAc-α-O-benzyl, mucus-secreting HT-29 cells (RevMTX10−6) were treated, from the day of seeding on, with different concentrations of the drug in the 0.1–2 mM range. Regardless of the concentration used, no effect on cell viability was observed, as assessed by the absence of cells in suspension and trypan blue exclusion. As shown in Fig. 1, it has no effect on the doubling time of the cells in the first days in culture, but results in a dose-dependent lower cell density in the stationary phase. At the highest concentration (2 mM) the cells stop growing before reaching total confluence, with the cell layer occupying 75–80% of the surface of the flask (Fig. 1). This effect is associated with a dramatic swelling of the cells (Figs. 1 and 2), with their cytoplasm appearing filled with a honeycomb-like accumulation of vesicles of various sizes during transmission electron microscopy (see Fig. 4 c). Concomitant with these changes, the mucus gel is totally absent from the cells treated with the highest concentrations, even when the cells are maintained for a longer period (<30 d). The decrease in mucus content was further demonstrated by the analysis of sections of the cell layer (Fig. 2). At the highest concentration (2 mM), there was a total absence of alcian blue–stained material (Fig. 2 e). In addition, the dense immunofluorescent staining of apical mucus droplets observed in control cells with antigastric mucus antibodies was no longer observed in cells treated with 2 mM, having been replaced by a diffuse staining of the cytoplasm (Fig. 2 f).
Figure 1.

Dose-dependent effect of GalNAc-α-O-benzyl on cell growth, cell density, and cell volume. HT29-RevMTX10−6 mucus-secreting cells were cultured in 24-well cell culture clusters in the absence (□) or under permanent exposure to 0.5 (♦), 1 (▴), or 2 mM (▪) GalNAc-α-O-benzyl. Values of growth curves are the means of five different passages. SD (data not shown) are less than 5%. (a–d) Phase-contrast microscopy of the cell layer of (a) control cells and cells treated with (b) 0.5, (c) 1, and (d) 2 mM GalNAc-α-O-benzyl after 20 d in culture. Cells treated with 0.1 mM GalNAc-α-O-benzyl were similar to control cells (data not shown). (e and f) Light microscopy in a Malassez cell of cells trypsinized after 20 d in culture: (e) control cells; (f) cells treated with 2 mM GalNAc-α-O-benzyl. The volume of the cells corresponding to e and f, as deduced from hematocrit and cell number, is 1.5 ± 0.1 μm3 per 106 cells in control, versus 8.5 ± 0.3 μm3 per 106 cells in treated cells. Bar, 75 μm.
Figure 2.
Effects of GalNAc-α-O-benzyl on cell morphology and mucus expression in HT29-RevMTX10−6 mucus-secreting cells. Cells were cultured in the absence or under permanent exposure to 2 mM GalNAc-α-O-benzyl and then analyzed after 21 d in culture. Left column, light microscopy of thin sections of the cell layer perpendicular to the surface of the flask. Middle column, alcian blue staining of cryostat sections of cell layers from the same cultures, counterstained with nuclear red. Right column, indirect immunofluorescence staining with pAb L56/C of cryostat sections of the same cell layers. (a–c) Untreated cells; (d–f), cells treated with GalNAc-α-O-benzyl. Bars: left column (17 μm); middle and right columns (40 μm).
Figure 4.
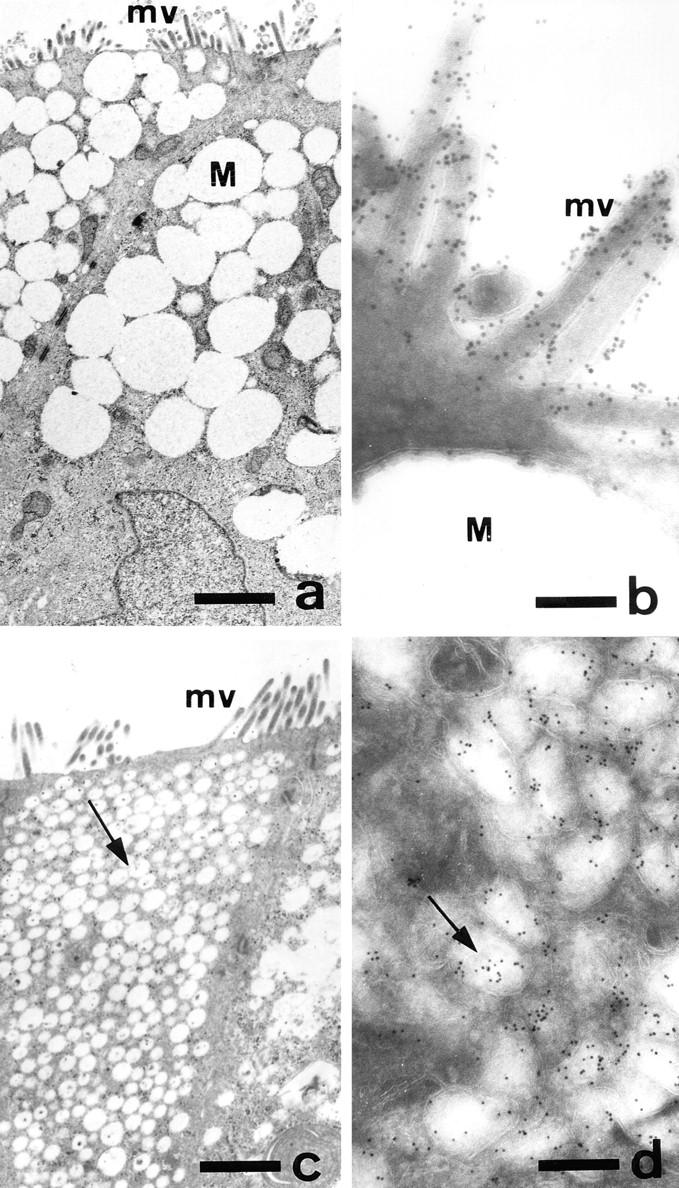
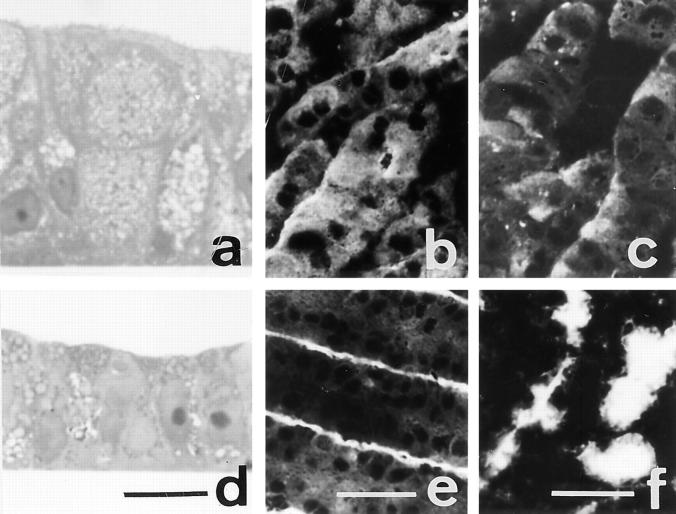
Ultrastructural morphology and localization of DPP-IV in control and GalNAc-α-O-benzyl–treated HT-29 mucus-secreting cells. HT29-RevMTX10−6 cells were analyzed after 21 d of culture in the absence (a and b) or presence (c and d) of 2 mM GalNAc-α-O-benzyl. (a and c) Standard transmission electron microscopy of sections perpendicular to the bottom of the flask showing the apical microvilli in both control and treated cells, the accumulation of mucus droplets (M) in the apical compartment of control cells, and the very numerous vesicles (arrow) in treated cells. (b and d) Immunogold labeling of DPP-IV using mAb HBB 3/775/42: in control cells the gold particles are strictly restricted to the microvilli and absent from the mucus droplets; in treated cells the gold particles are associated with the cytoplasmic vesicles. Similar results were obtained with MUC1 and CEA (data not shown). Bars: (a and c) 1.2 μm; (b) 0.16 μm; (c) 0.28 μm.
Treatment of Mucus-secreting HT-29 Cells with GalNAc-α-O-benzyl Leads to an Intracytoplasmic Accumulation of Brush Border Membrane–associated Glycoproteins
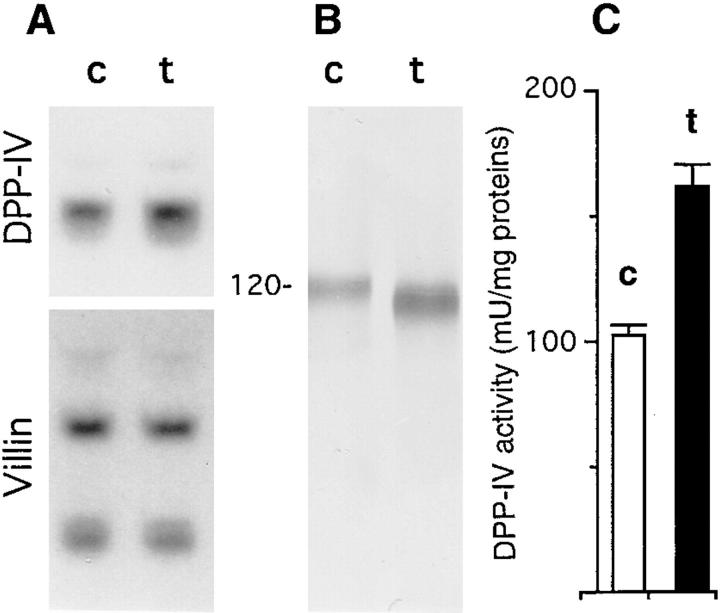
Because the most clear-cut effect of GalNAc-α-O-benzyl was observed at 2 mM, all further experiments were done at this concentration. In control postconfluent HT29-RevMTX10−6 cells, DPP-IV, MUC1, and CEA are exclusively associated with the apical brush border of the cells, as previously reported (Lesuffleur et al., 1993) and shown in Fig. 3, g, i, and k. In contrast, in GalNAc-α-O-benzyl– treated cells, DPP-IV, MUC1, and CEA are present in the totality of the cytoplasm (Fig. 3, h, j, and l); this occurs without modification of the morphological polarity of the cells, substantiated by the apical expression of villin and ZO1 (Fig. 3, a–d). Interestingly, and in contrast to what observed for apical glycoproteins, the treatment had no effect on the basolateral expression of GP120 (Fig. 3, e and f). The cytoplasmic accumulation of apical glycoproteins in treated cells was further confirmed using immunoelectron microscopy: unlike in control cells where they are restricted to the apical brush border, DPP-IV, MUC1, and CEA are localized in the numerous vesicles that fill the cytoplasm (Fig. 4). Using DPP-IV as a marker of the effect, we further found that this altered distribution is associated with an increased level of expression of the enzyme at both mRNA (Fig. 5) and protein level as substantiated by higher enzyme activities and higher protein content, with a lower apparent molecular mass, however, as shown by Western blot (Fig. 5). The same results were obtained with mucus-secreting HT29-RevMTX 10−5 cells and enterocytic HT29-RevMTX10−3 cells (data not shown). The effect of GalNAc-α-O-benzyl on apical glycoproteins is not dependent on the support the cells are cultured on, as exemplified by the swelling of the cells and the intracytoplasmic accumulation of DPP-IV also observed in treated filter-grown cells (data not shown).
Figure 3.
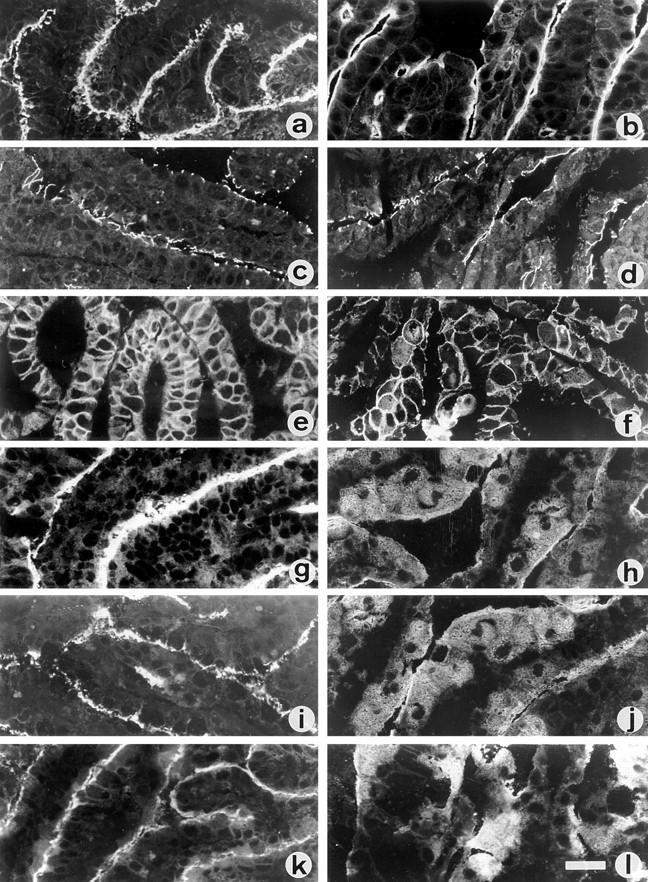
Effect of GalNAc-α-O-benzyl on the cellular distribution of polarized proteins in mucus-secreting HT-29 cells. Indirect immunofluorescence detection of polarized proteins in frozen cryostat sections of the cell layers from control (left column) and 2 mM GalNAc-α-O-benzyl– treated HT29-RevMTX10−6 cells (right column) analyzed after 21 d in culture. Sections of the cell layers were single labeled with antibodies against villin (a and b), ZO1 (c and d), glycoprotein GP120 (e and f), DPP-IV (g and h), MUC1 (i and j), and CEA (k and l). Bar, 40 μm.
Figure 5.
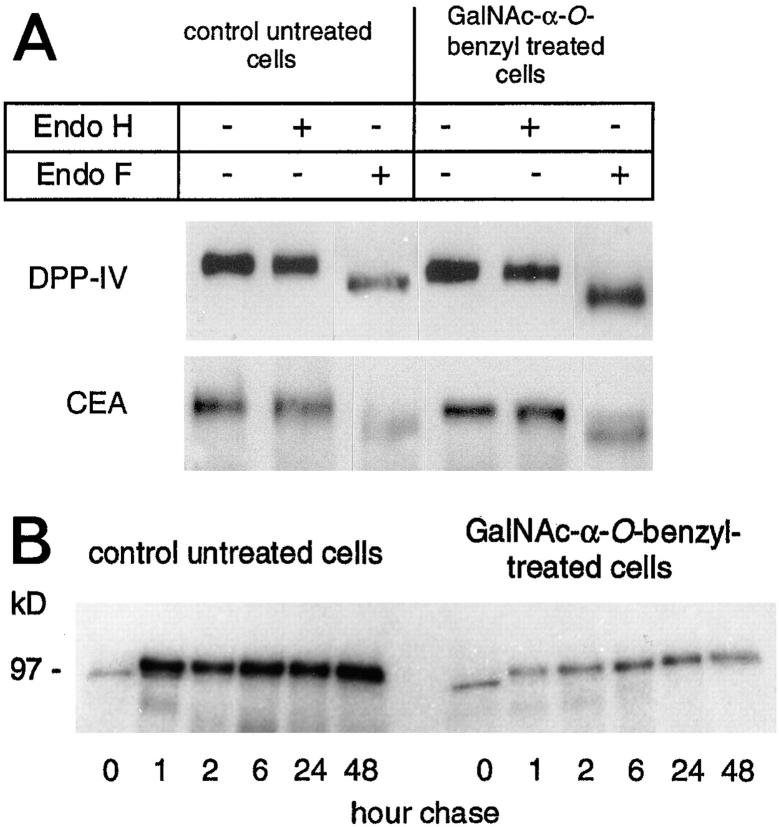
Level of expression of DPP-IV in GalNAc-α-O-benzyl–treated mucus-secreting HT-29 cells. Control (c) and cells treated with 2 mM GalNAc-α-O-benzyl (t) were analyzed after 21 d in culture. (A) Northern blot analysis of DPP-IV, using villin as internal control. (B) Immunoblot analysis, with mAb 4H3 of DPP-IV in cell homogenates. (C) Analysis of DPP-IV enzymatic activity in the cell homogenates from control (open bar) and treated cells (solid bar); results are the means of three different cultures corresponding to three different passages. The apparent discrepancy between the relatively discrete (1.6-fold) increase in DPP-IV activity and the dramatic intracellular accumulation of the enzyme (see Fig. 3, h and Fig. 4, d) can be explained by the fact that the results refer to the specific activity of the enzyme and that other glycoproteins also accumulate. This discrepancy disappears when DPP-IV activity is expressed as mU/106 cells, the values being then 25 ± 1 and 150 ± 6 in control and treated cells, respectively.
The Effects of GalNAc-α-O-benzyl Are Reversible
Switching back the cells to drug-free medium after 20 d of treatment results in a rapid reversal of the phenotype described above: within 24 h the volume of the cells decreases, and in the following days apical glycoproteins redistribute to the apical surface (Fig. 6 e). At the same time, mucus secretion resumes, judged by the occurrence of a visible gel on the surface of the cell layer demonstrated by alcian blue staining (data not shown) and immunofluorescence reactivity to antimucus antibodies of cell layer sections (Fig. 6 f).
Figure 6.
Reversibility of the effects of GalNAc-α-O-benzyl on cell morphology, distribution of DPP-IV, and mucus secretion. HT29-RevMTX10−6 cells were cultured until day 15 in the presence of 2 mM GalNAc-α-O-benzyl and then in drug-free medium. Cultures were analyzed on day 15 (a–c), and 5 d after removal of the drug: (d–f). Left column, thin sections of the cell layer; middle column, indirect immunofluorescence detection of DPP-IV with mAb 3/775/42; right column, indirect immunofluorescence detection of mucus with pAb L56/C in sections of the cell layer. Similar results for DPP-IV were observed as for MUC1 and CEA (data not shown). Bars: left column (16 μm); middle and right columns (80 μm).
NeuAcα2-3Galβ1-3GalNAc Is a Major Oligosaccharide Species Associated with Mucins and Other Glycoproteins from Differentiated HT-29 Cells
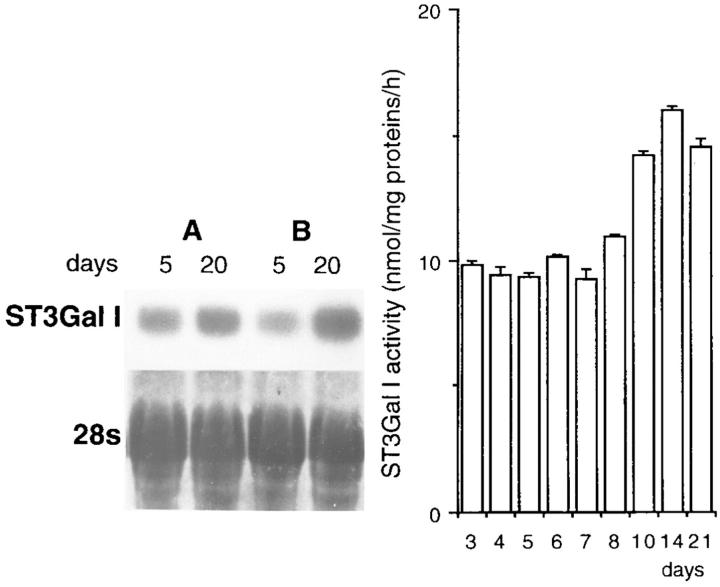
To characterize the oligosaccharide species associated with mucins and other glycoproteins from HT-29–differentiated cells, we used the lectin MAA, which reacts with NeuAcα2-3Gal-R terminal sequence and PNA, which reacts with the O-linked T antigen (Galβ1-3GalNAcα1-O-Ser/Thr). As shown in Fig. 7 via double immunofluorescence, mucus from control HT29-RevMTX10−6 cells is reactive with MAA (Fig. 7, top panel, a–c), whereas only a small proportion of the mucus reacts with PNA (Fig. 7, top panel, d–f). After treatment with sialidase from Clostridium perfringens, all the mucus is reactive with PNA (data not shown), confirming the fact that a large proportion of the mucus expresses the NeuAcα2-3Galβ1-3GalNAc sequence. Because mucus droplets are concentrated in the apical cytoplasm and do not allow to distinguish the reactivity of brush border– associated glycoproteins to lectins in mucus-secreting cells, we used enterocytic HT29-RevMTX10−3 cells to further characterize the reactivity of these glycoproteins to lectins. As shown on cryostat sections from postconfluent cells (Fig. 7, middle panel), MAA shows a strong apical reactivity (Fig. 7, middle panel, a), whereas PNA is unreactive (Fig. 7, middle panel, e). After sialidase treatment, there is no longer any reactivity to MAA (Fig. 7, middle panel, c), whereas PNA shows a strong apical staining (Fig. 7, middle panel, g), thus testifying the presence of a large amount of sialyl-T antigen linked to brush border–associated glycoproteins. In contrast, no basolateral labeling was observed with MAA by confocal microscopy (Fig. 7, bottom panel), thus indicating that sialyl-T antigen is not associated with basolateral glycoproteins in differentiated HT-29 cells. The association of NeuAcα2-3Galβ1-3GalNAcα1-O-Ser/Thr with glycoproteins in differentiated HT-29 cells was confirmed by Western blot analysis of cell homogenates from the different HT-29 cell populations (Fig. 8) which suggest, based on the reactivity to MAA and PNA before and after treatment with sialidase, that NeuAcα2-3Galβ1-3GalNAcα1-O-Ser/ Thr sequence is associated not only to mucins, but also to a number of glycoproteins with a molecular mass in the 80– 400 kD range. Among the glycoproteins that react with MAA are DPP-IV, MUC1, and CEA as shown by immunoblot analysis with MAA of these immunoprecipitated proteins (see Fig. 10). NeuAcα2-3 glycosylation of T antigen is further supported by the observation that ST3Gal I is expressed at all stages of the culture in mucus-secreting as well as in enterocytic HT-29 cells, as substantiated by analysis of ST3Gal I mRNA level and enzyme activity (Fig. 8). Interestingly, no reactivity was observed by immunoblotting cell homogenates with mAb B72.3 against sialyl-Tn (data not shown) or SNA that recognizes the terminal oligosaccharide species NeuAcα2-6Gal (Fig. 9).
Figure 7.
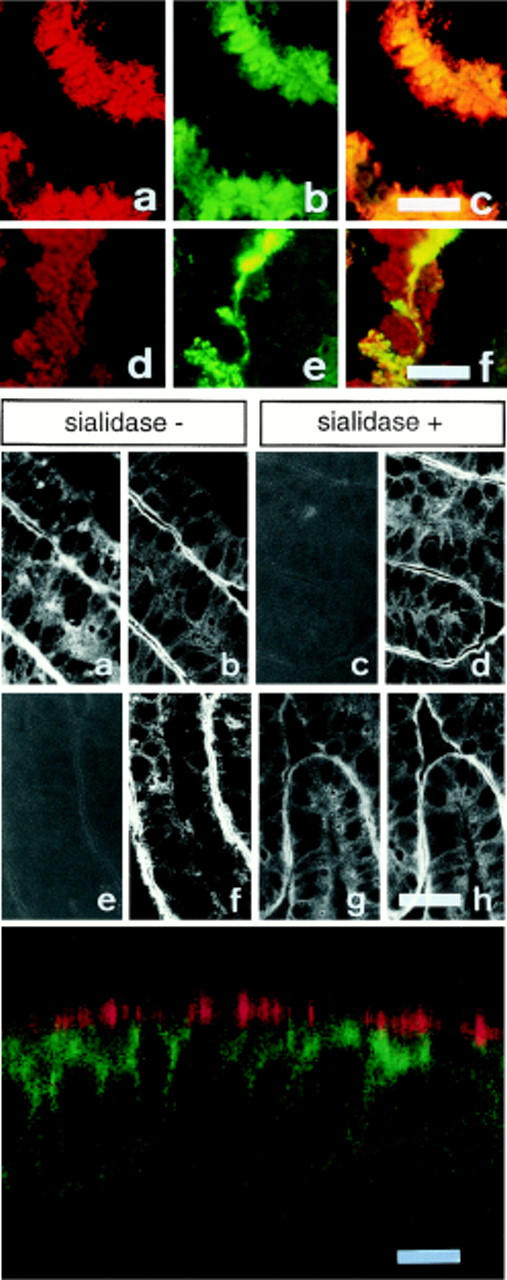
Evidence that NeuAcα2-3Galβ1-3GalNAcα1-O-Ser/Thr is the main oligosaccharide species associated with mucins and apical proteins from differentiated HT-29 cells. Top panel: cryostat sections of cell layers from HT-29 mucus-secreting cells (HT29-RevMTX10−6) were analyzed after 21 d in culture by double immunofluorescence labeling with antigastric mucus Ab L56/C followed by rhodamine-coupled Ig (a and d) and fluorescein-coupled MAA (b) and PNA lectins (e). In c and f, a mixed filter for rhodamine and fluorescein was used. (a–c) Double labeling with Ab L56/C and MAA, showing that the totality of the mucus droplets reacts with MAA; (d–f), double labeling with Ab L56/C and PNA showing that only a small proportion of mucus droplets reacts with PNA. Middle panel (a–h): apical reactivity to MAA and PNA of enterocyte-like HT-29 cells. Frozen cryostat sections from postconfluent (day 21) HT29-RevMTX10−3 cells, treated or not treated with sialidase, were double labeled with fluorescein-conjugated MAA (a and c) or PNA (e and g) and antibodies against villin (b, d, f, and h), used as an apical marker, using rhodamine-coupled Ig as a second antibody. Note in sialidase-treated sections, the disappearance of MAA staining (c) contrasting with the appearance of an apical reactivity to PNA (g). Bottom panel: confocal microscopy analysis of postconfluent HT29-RevMTX10−6 cells (day 21). A section perpendicular to the cell layer was coimmunostained by MAA (red) and mAb TS2/16 against β1 integrin (green). Note the absence of basolateral reactivity to MAA. A similar basolateral pattern as observed with mAb TS2/16 was obtained with mAb G1/136 against GP120 (data not shown). Bars: top and middle panels (40 μm); bottom panel (12 μm).
Figure 8.
Top panel: Western blot analysis of the reactivity to MAA and PNA of cell extracts from differentiated mucus-secreting HT-29 cells. Cell homogenates from postconfluent HT-29-RevMTX10−6 cells were analyzed after (a) 15, (b) 20, and (c) 25 d in culture for their reactivity to MAA and PNA, without or after desialylation of the blot with Clostridium perfringens sialidase. The position of the prestained molecular weight markers is indicated on the left side of the panel. Bottom panel: expression of ST3Gal I. Northern blot analysis of ST3Gal I mRNA in exponentially growing (day 5) and postconfluent (day 20) HT29-RevMTX10−3 (A) and RevMTX10−6 cells (B). The same filter was dehybridized and stained with methylene blue for RNA quantification; only the 28s are shown here. The histogram represents activity (expressed in nmol.mg−1.h−1) of ST3Gal I during the course (from day 3 to 21) in culture of HT29-RevMTX10−6 cells.
Figure 10.
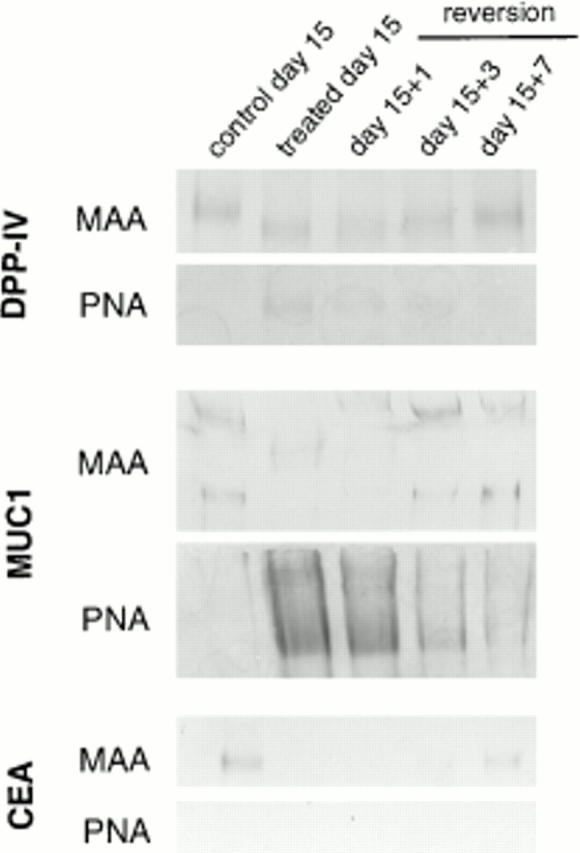
Western blot analysis with MAA and PNA lectins of immunoprecipitated DPP-IV, MUC1, and CEA from control, GalNAc-α-O-benzyl–treated cells and cells reverted to drug-free medium. DPP-IV, MUC1, and CEA were immunoprecipitated from cell homogenates with mAbs 3/775/42, BC-2, and 517, respectively. Note that in control cells DPP-IV, MUC1, and CEA react with MAA, but not with PNA. In treated cells, the reactivity to MAA is decreased for DPP-IV and abolished for MUC1 and CEA, whereas DPP-IV and MUC1, but not CEA, show a reactivity to PNA. The changes in treated cells are reversible upon removal of the drug.
Figure 9.
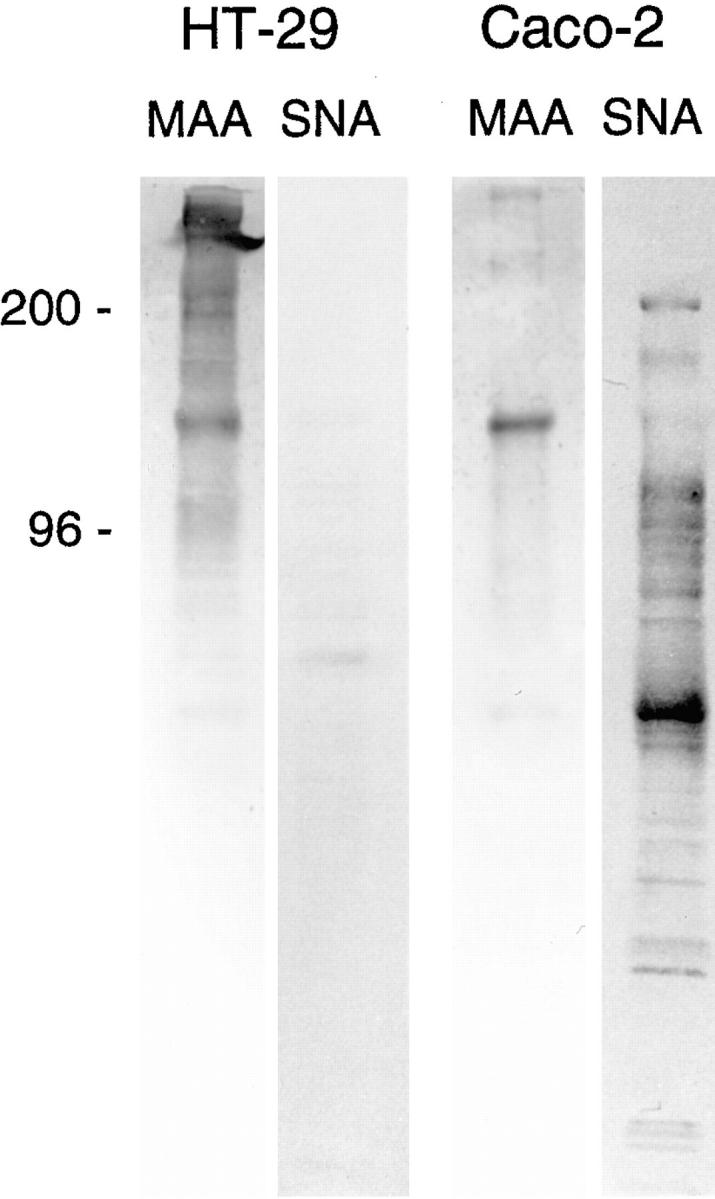
Compared Western blot analysis with MAA and SNA lectins of cell homogenates from postconfluent (day 21) HT29-RevMTX10−5 cells, using as a control postconfluent (day 21) Caco-2 cells. The same quantity of proteins (50 μg) was loaded in each lane. The samples for each lectin were run on the same gel. For clarity they have been rearranged in the figure. Note the almost total absence of SNA-reactive material in HT29 as compared with Caco-2 cells. The absence of SNA-reactivity in HT-29 cells, contrasting with the presence of SNA-reactive apical material in Caco-2 cells, was further confirmed by immunofluorescence of cell layer sections of both populations (data not shown).
Treatment with GalNAc-α-O-benzyl Results in a Decreased α2,3-Sialylation of Glycoproteins
Western blot analysis of the reactivity to lectins of cell homogenates from postconfluent HT29-RevMTX10−6 cells treated with various concentrations of GalNAc-α-O-benzyl shows a dose-dependent decrease of MAA-reacting glycoproteins associated with a dose-dependent increase in PNA-reacting glycoproteins, with a maximum effect observed at a drug concentration of 2 mM (data not shown). This was further confirmed by the observation, using immunofluorescence, that regardless of their phenotype, the cytoplasm of cells treated with 2 mM GalNAc-α-O-benzyl is heavily stained with PNA, in contrast to control cells (data not shown). Western blot analysis of immunoprecipitated DPP-IV, MUC1, and CEA with MAA and PNA shows that MAA reactivity of these glycoproteins is reduced in cells treated with GalNAc-α-O-benzyl. Concomitant to these changes, a reactivity to PNA was observed for MUC1 and DPP-IV, but not for CEA (Fig. 10). The changes in glycosylation are reversible upon removal of the drug; Western blot analysis of the reactivity to lectins of cell homogenates from cells reverted to drug-free medium shows a reappearance of MAA reactivity and the concomitant disappearance of PNA reactivity of a number of glycoproteins in the 80–400 kD range (data not shown). These include MUC1, DPP-IV, and CEA as shown by Western blot analysis with lectins of the immunoprecipitates of these glycoproteins (Fig. 10).
The GalNAc-α-O-benzyl–dependent Secretory Block Occurs beyond the cis-Golgi Compartment of the Cells
To further characterize at which level the block induced by GalNAc-α-O-benzyl occurs, immunoprecipitates of apical glycoproteins from control and treated cells were analyzed for their sensitivity to endoglycosidase H and endoglycosidase F treatment. As exemplified for DPP-IV and CEA (Fig. 11 A), the same results were observed in treated and control cells with both proteins being endoglycosidase H–resistant and endoglycosidase F–sensitive, suggesting that the block occurs after the cis-Golgi. This was further confirmed by analysis of the biosynthesis of DPP-IV that shows the processing of the enzyme is similar in treated and in control cells (Fig. 11 B).
Figure 11.
(A) Sensitivity to endoglycosidase H and endoglycosidase F of DPP-IV and CEA from control and GalNAc-α-O-benzyl-treated HT29-RevMTX10−5 cells (day 21). The same quantity of cell homogenate (30 μg of proteins) treated as indicated was loaded in each lane. Western blot analysis of DPP-IV and CEA was performed with mAbs 4H3 and 517, respectively. The samples for each protein were run on the same gel in a different order. For clarity they have been rearranged in the figure. (B) Pulse-chase experiment with DPP-IV. Control and GalNAc-α-O-benzyl–treated cells (day 21) were labeled with [35S]methionine for 15 min followed by a chase in complete medium containing unlabeled methionine. After the indicated periods of chase, DPP-IV was immunoprecipitated with mAb 4H3 and immunoprecipitates were subjected to SDS-PAGE.
Discussion
The present results suggest that in HT-29 cells, α2,3-sialylation plays a crucial role in intracellular transport of brush border membrane–associated glycoproteins and in mucus secretion. They rely on the exceptional conjunction of a number of factors: (a) the availability of polarized cells, isolated from the HT-29 cell line, expressing either an enterocytic or a mucus-secreting differentiated phenotype; (b) the neoplastic nature of these cells which, as such, show a modified pattern of protein glycosylation as compared with their normal counterpart with shorter oligosaccharide side chains (for review see Lesuffleur et al., 1994); (c) the fact that the main sialyltransferase activities expressed in HT-29 cells catalyze the transfer of sialic acid to the 3-position of Gal in the Galβ1-3GalNAc disaccharide sequence (Dall'Olio et al., 1993; Majuri et al., 1995; Delannoy et al., 1996) in contrast to most colon cancers (Sata et al., 1991) or cell lines, including the enterocytic cell line Caco-2, which mainly express the Galβ1-4GlcNAc α2,6-sialyltransferase, ST6Gal I as reported by others (Dall'Olio et al., 1992, 1996), and confirmed here by the reactivity to SNA of Caco-2 glycoproteins; (d) the availability of an O-glycosylation inhibitor, GalNAc-α-O-benzyl (Kuan et al., 1989; Huang et al., 1992; Byrd et al., 1995; DiIulio and Bhavanandan, 1995), which enters the cells and is metabolized into a compound that acts as a competitive inhibitor of Galβ1-3GalNAc α-2,3-sialyltransferases (Huet et al., 1995; Delannoy et al., 1996); and (e) the fact that, in differentiated HT-29 cells, NeuAcα2-3Galβ1-3GalNAc-R is the main oligosaccharide species associated not only with mucins, as previously reported (Capon et al., 1992; Lesuffleur et al., 1993; Huet et al., 1995), but also, as shown here, with a number of glycoproteins of the brush border concomitantly expressed in these cells. The association of NeuAcα2-3Galβ1-3GalNAc-R to apical glycoproteins relies exclusively on their reactivity to MAA, and not to a biochemical characterization that would require a huge amount of cells to be performed. However, the reliability of MAA characterization is validated by a recent structural characterization of carbohydrate chains of the mucus from HT29-RevMTX10−5 mucus-secreting cells (that can be easily performed since large quantities of mucus can be harvested daily) which has confirmed that NeuAcα2-3Galβ1-3GalNAc-R is the main oligosaccharide species associated with the mucus of these cells (Hennebicq-Reig, S., T. Lesuffleur, C. Capon, C. de Bolos, I. Kim, O. Moreau, C. Richet, B. Hémon, M.A. Recchi, E. Maës, J.P. Aubert, F.X. Real, A. Zweibaum, P. Delannoy, P. Degand, and G. Huet, manuscript submitted for publication). Finally, it must be noted, as demonstrated by confocal microscopy, that no MAA reactivity could be detected on the basolateral membrane of the cells.
Based on both the sugar specificity of MAA and PNA lectins and the results of sialidase treatment, the present data show that in addition to mucins (i.e., in HT29-RevMTX10−6 and 10−5), apical O-glycosylproteins such as MUC1 express NeuAcα2-3Galβ1-3GalNAc-R terminal sequences. This being established, four main observations can be drawn from the experiments performed with GalNAc-α-O-benzyl. First, alteration of α2,3-sialylation of mucins and of apical O-glycosylproteins is accompanied by their accumulation into intracytoplasmic vesicles. This accumulation, which is most likely responsible for the dramatic swelling of the cells, is not restricted to O-glycosylproteins, but is also observed for N-glycosylproteins such as DPP-IV and CEA. Regarding CEA, the absence of PNA binding in treated cells may be explained by a weak expression of the Galβ1-3GalNAc sequence, not in contradiction with the fact that the binding of MAA is decreased. Second, the block induced by GalNAc-α-O-benzyl occurs after the cis-Golgi as substantiated by endoglycosidase H resistance of apical glycoproteins and normal processing of DPP-IV. Third, these effects are reversible upon removal of the drug, resulting in a concomitant restoration of α2,3-sialylation and resumption of the secretion of mucins and of the apical delivery of brush border–associated glycoproteins. Fourth, these effects occur without any modification in the morphological polarity of the cells, as shown by the normal polarized expression of villin and ZO1, and without modification of distribution of the basolateral glycoprotein GP120.
How can the fact be explained that GalNAc-α-O-benzyl, an O-glycosylation inhibitor (Kuan et al., 1989; Huang et al., 1992; Byrd et al., 1995) which, in HT-29, is metabolized into a compound, acts as a competitive inhibitor of Galβ1-3GalNAc α2,3-sialyltransferases (Huet et al., 1995; Delannoy et al., 1996) may also inhibit the sialylation of N-glycosylproteins? Recent progress in the molecular cloning of sialyltransferases (for review see Harduin-Lepers et al., 1995; Tsuji, 1996; Tsuji et al., 1996) indicates that three different enzymes (ST3Gal I, ST3Gal II, and ST3Gal IV), encoded by different genes, located on separate chromosomes (Chang et al., 1995) are able to transfer sialic acid residues in the 3-position of Gal onto the disaccharidic Galβ1-3GalNAc-R sequence. The substrate specificity of ST3Gal I and ST3Gal II is strictly restricted to this particular disaccharidic sequence (Kojima et al., 1994) but ST3Gal IV can use both Galβ1-3GalNAc and Galβ1-4GlcNAc as acceptor substrates (Kitagawa and Paulson, 1994). The competitive inhibition of ST3Gal IV by Galβ1-3GalNAc-α-O-benzyl could explain the decrease of α2,3-sialylation of N-glycosylproteins in HT-29 cells. On the other hand, the presence of a high concentration of the competitive substrate (i.e., Galβ1-3GalNAc-α-O-benzyl) may also decrease the concentration of CMP-NeuAc in the Golgi lumen and therefore can compete with the other sialyltransferases expressed in HT-29 cells via the donor substrate.
Whatever the mechanism leading to the decrease of α2,3-sialylation, these results show that lack of terminal NeuAcα2-3Gal-R glycosylation is associated with a blockade of apical targeting of brush border membrane–associated glycoproteins and mucus secretion in HT-29 cells. They further suggest that α2,3-sialylation could play a role in regulating the intracellular traffic of these proteins, and in some way, support the view by Fiedler and Simons (1994) that “it may be that oligosaccharide side chains play a more important role in biosynthetic traffic than hitherto recognized”. Even if the role of glycans in the intracellular targeting of newly synthesized lysosomal enzymes via the mannose-6–phosphate receptor pathways was clearly demonstrated in human colon adenocarcinoma cell lines (Braulke et al., 1992), a role for glycans in the sorting machinery of membrane proteins in polarized cells has long been excluded on the basis of experiments using inhibitors of N-glycosylation such as tunicamycin, which blocks the transfer of Glc3Man9GlcNAc2 from dolichol to Asn, thus resulting in the accumulation of unprocessed proteins in the rough ER (Green et al., 1981), or castanospermine, or 1-deoxymannojirimycin which block processing before trimming of the oligomannose chains (Duronio et al., 1988). Very few recent observations suggest, however, a possible role for terminal glycans in this regulation. Growth hormone, which is nonglycosylated and secreted from both sides of MDCK cell layers, is secreted from the apical side when N-glycosylated (Scheiffele et al., 1995). The sialoglycoprotein gp114 that is expressed on the apical membrane of MDCK cells is misglycosylated and predominantly basolateral in the MDCK mutant MDCKII-RCAr (Le Bivic et al., 1993). The O-glycosylated stalk domain is required for apical sorting of neurotrophin receptors in polarized MDCK cells (Yeaman et al., 1997). In addition to these particularities that concern glycoproteins, it has been shown that among the increasing number of vesicular proteins presumably involved in protein sorting (Rothman, 1994), one of them, VIP36 (Fiedler et al., 1994), presents some homology with leguminous lectins (Fiedler and Simons, 1994) and binds GalNAc (Fiedler and Simons, 1996). Therefore, it is conceivable that terminal NeuAcα2-3Gal glycan sequences as signals, and lectins as receptors for these signals, could be involved in the sorting machinery of glycoproteins in polarized HT-29 cells. Because the glycosylation of both lectin-like resident Golgi proteins and in transit glycoproteins may be affected by GalNAc-α- O-benzyl, the precise level at which the sorting machinery is affected needs to be elucidated.
Whether or not α2,3-sialylation is exclusively involved in the apical sorting of glycoproteins in HT-29 cells cannot be concluded from the present work since it was only focused on brush border membrane glycoproteins and mucins. From the results obtained with Western blot, it is clear that the effects of GalNAc-α-O-benzyl are shared by a number of other proteins that remain to be characterized as to their nature and localization. Nevertheless, the present data show that a shift in the predominant glycosylation pattern of glycoproteins from NeuAcα2-3Galβ1-3GalNAc-R to Galβ1-3GalNAc-R results in what appears as a glycoprotein traffic jam that dramatically alters their normal delivery and also leads to cell hypertrophy.
Analysis of whether the present results uncover a more general involvement of terminal glycans in the intracellular protein transport machinery needs to consider two main evidences. First, there is the high polymorphism of terminal glycans which differ from one individual to another. Second, and with regard to a putative role of animal lectins as receptors for the glycan signal (Fiedler and Simons, 1994), there is the strict oligosaccharide specificity of lectins. This implies that if the results obtained with HT-29 cells rely on a general mechanism, each cellular system, whether from human or animal origin, is unique and only representative of the genetic background of the individual it originates from. With regard to normal or malignant intestinal cells it must be noted that there are only very few indications in which oligosaccharide species are associated with apical glycoproteins. The only available data concern the observation that, in the normal adult intestine, ABH blood group antigens are the main terminal glycans associated with the intestinal brush border hydrolases in humans (Triadou et al., 1983; Green et al., 1988) as well as in rabbits (Gorvel et al., 1982). With regard to differentiated colon cancer cells, the results reported here are the first observation showing that in HT-29 cells, the main terminal oligosaccharide species associated with apical glycoproteins is NeuAcα2-3Gal. Preliminary results obtained in the laboratory indicate that, as in rat developing enterocytes (Roth, 1993), the apical membrane of intestinal enterocytes from 10–20 gestational wk fetuses is strongly reactive with MAA; in contrast, epithelial cells in the adult human small intestine lack MAA reactivity (our unpublished results), differing from the situation found in the adult colon which has been shown to express an apical reactivity to MAA (Sata et al., 1991), but which is devoid of brush border–associated hydrolases. Therefore, the type of glycosylation demonstrated in HT-29–differentiated cells likely represents one more feature of the fetal type of differentiation which is known to be associated with the malignant phenotype (Zweibaum et al., 1991).
With regard to the oligosaccharidic individual variability it is clear that the results obtained with HT-29 cells cannot be extrapolated as such to other cellular systems. For example, in Caco-2 cells, in which the main expressed sialyltransferase is ST6Gal I (Dall'Olio et al., 1992, 1996) GalNAc-α-O-benzyl has no effect, even at much higher concentrations (10 mM), on the morphology of the cells and the apical polarity of brush border–associated glycoproteins (our unpublished results). This absence of effect is obviously consistent with the fact that in Caco-2 cells, apical glycoproteins such as sucrase-isomaltase or DPP-IV most likely express NeuAcα2-6, as suggested from the SNA reactivity of Caco-2 glycoproteins and that GalNAc-α-O-benzyl has no effect on ST6Gal I.
Finally the present results draw attention to the necessity of characterizing, in each experimental system, which oligosaccharide species is associated with the apical glycoproteins. This is a prerequisite for any further analysis of the role of terminal oligosaccharides in the intracellular traffic of apical glycoproteins. It also implies that new strategies should be developed to specifically block the terminal glycosylation of apical glycoproteins. One challenge is to analyze whether, for example, ABH blood group antigens are involved in the intracellular traffic of intestinal brush border hydrolases in humans.
Acknowledgments
We thank V. van Miegem (CNRS UMR III [Lille]), B. Hémon, and O. Moreau (both from INSERM U377 [Lille]) for excellent technical assistance. We are grateful to the Scientific and Medical Services of the University of Barcelona (Barcelona, Spain) for confocal microscopy analysis.
This work was supported in part by INSERM Consejo Superior de Investigaciones Científicas (CSIC) Cooperation Agreement, and grants from Fondo de Investigacion Sanitaria (94/1128), Generalitat de Catalunya (GRQ 93-01), Comisión Interministerial de Ciencia y Tecnología (CICYT) (SAF 97-0085), and Université Paris XI.
Abbreviations used in this paper
- CEA
carcinoembryonic antigen
- DPP-IV
dipeptidylpeptidase-IV
- GalNAc-α-O-benzyl
benzyl-2-acetamido-2-deoxy-α-d-galactopyranoside
- MAA
Maackia amurensis agglutinin
- MTX
methotrexate
- PNA
Arachis hypogaea agglutinin
- SNA
Sambucus nigra agglutinin. The abbreviations for sialyltransferases are according to the new systematic nomenclature proposed by Tsuji et al. (1996)
Footnotes
Address all correspondence to Alain Zweibaum, INSERM U178, 16 Avenue Paul-Vaillant-Couturier, 94807 Villejuif Cedex, France. Tel.: (33) 1-45-59-50-41. Fax: (33) 1-46-77-02-33. E-mail: zweibaum@infobiogen.fr
References
- Arroyo AG, Sanchez-Mateos P, Campanero MR, Martin-Padura I, Dejana E, Sanchez-Madrid F. Regulation of the VLA integrin–ligand interactions through the β1 subunit. J Cell Biol. 1992;117:659–670. doi: 10.1083/jcb.117.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braulke T, Mach L, Hoflack B, Glossl J. Biosynthesis and endocytosis of lysosomal enzymes in human colon carcinoma SW1116 cells: impaired internalization of plasma membrane-associated cation-independent mannose 6-phosphate receptor. Arch Biochem Biophys. 1992;298:176–181. doi: 10.1016/0003-9861(92)90109-a. [DOI] [PubMed] [Google Scholar]
- Byrd, J.C., R. Dahiya, J. Huang, and Y.S. Kim. 1995. Inhibition of mucin synthesis by benzyl-α-GalNAc in KATO III gastric cancer and Caco-2 colon cancer cells. Eur. J. Cancer. 31A:1498–1505. [DOI] [PubMed]
- Capon C, Laboisse CL, Wieruszeki JM, Maoret JJ, Augeron C, Fournet B. Oligosaccharide structures of mucins secreted by the human colonic cancer cell line CL.16E. J Biol Chem. 1992;267:19248–19257. [PubMed] [Google Scholar]
- Chang ML, Eddy RL, Shows TB, Lau JTY. Three genes that encode human β-galactosidase α-2,3-sialyltransferases. Structural analysis and chromosomal lapping studies. Glycobiology. 1995;5:319–325. doi: 10.1093/glycob/5.3.319. [DOI] [PubMed] [Google Scholar]
- Chirgwin JM, Przybylan AE, McDonald RJ, Rutter WJ. Isolation of biologically active ribonucleic acid from sources enriches in ribonuclease. Biochemistry. 1979;18:5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dall'Olio F, Malagolini N, Serafini-Cessi F. The expression of soluble and cell-bound α-2,6 sialyltransferase in human colonic carcinoma Caco-2 cells correlated with the degree of enterocytic differentiation. Biochem Biophys Res Comm. 1992;184:1405–1410. doi: 10.1016/s0006-291x(05)80039-7. [DOI] [PubMed] [Google Scholar]
- Dall'Olio F, Malagolini N, Guerrini S, Serafini-Cessi F. Resistance to methotrexate is associated with selective changes of α-2,6- and α-2,3-sialyltransferase activities toward N-acetyllactosaminic sequences in human colon cancer cell line HT-29. Biochem Biophys Res Comm. 1993;196:714–720. doi: 10.1006/bbrc.1993.2308. [DOI] [PubMed] [Google Scholar]
- Dall'Olio F, Malagolini N, Guerrini S, Lau JT, Serafini-Cessi F. Differentiation-dependent expression of human β-galactoside α-2,6-sialyltransferase mRNA in colon carcinoma Caco-2 cells. Glycoconj J. 1996;13:115–121. doi: 10.1007/BF01049687. [DOI] [PubMed] [Google Scholar]
- Darmoul D, Lacasa M, Chantret I, Swallow D, Trugnan G. Isolation of cDNA probe for the human intestinal diptidylpeptidase IV and assignment of the gene locus DPPIV to chromosome 2. Annu Hum Genet. 1990;54:191–197. doi: 10.1111/j.1469-1809.1990.tb00377.x. [DOI] [PubMed] [Google Scholar]
- Delannoy P, Pelczar H, Vandamme V, Verbert A. Sialyltransferase activity in FR3T3 cells transformed with ras oncogene: decrease CMP-Neu5Ac:Galβ1-3GalNAc α2,3-sialyltransferase. Glyconj J. 1993;10:91–98. doi: 10.1007/BF00731192. [DOI] [PubMed] [Google Scholar]
- Delannoy P, Kim I, Emery N, De Bolos C, Verbert A, Degand P, Huet G. Benzyl-N-acetyl-α-d-galactosaminide inhibits the sialylation and the secretion of mucins by a mucin secreting HT-29 cell subpopulation. Glycoconj J. 1996;13:717–726. doi: 10.1007/BF00702335. [DOI] [PubMed] [Google Scholar]
- DiIulio NA, Bhavanandan VP. The saccharides of the MUC1 mucin-type glycoprotein, epitectin, produced by H.Ep.2 cells in the presence of aryl-N-acetyl-α-galactosaminides. Glycobiology. 1995;5:195–199. doi: 10.1093/glycob/5.2.195. [DOI] [PubMed] [Google Scholar]
- Duronio V, Jacobs S, Romero PA, Herscovics A. Effect of inhibitors of N-linked oligosaccharide processing on the biosynthesis and function of insulin and insulin-like growth factor-I receptors. J Biol Chem. 1988;263:5436–5445. [PubMed] [Google Scholar]
- Eilers U, Klumperman J, Hauri HP. Nocodazole, a microtubule-active drug, interferes with apical protein delivery in cultured intestinal epithelial cells (Caco-2) J Cell Biol. 1989;108:13–22. doi: 10.1083/jcb.108.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiedler K, Simons K. A putative novel class of animal lectins in the secretory pathway homologous to leguminous lectins. Cell. 1994;77:625–626. doi: 10.1016/0092-8674(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Fiedler K, Simons K. Characterization of VIP 36, an animal lectin homologous to leguminous lectins. J Cell Sci. 1996;109:271–276. doi: 10.1242/jcs.109.1.271. [DOI] [PubMed] [Google Scholar]
- Fiedler K, Parton RG, Kellner R, Etzold T, Simons K. VIP36, a novel component of glycolipid rafts and exocytic carrier vesicles in epithelial cells. EMBO (Eur Mol Biol Organ) J. 1994;13:1729–1740. doi: 10.1002/j.1460-2075.1994.tb06437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogh, J., and G. Trempe. 1975. New Human Tumor Cell Lines. In Human Tumor Cells “In Vitro”. J. Fogh, editor. Plenum Press, New York, 115–141.
- Fukushima K, Ohkura T, Kanai M, Kuroki M, Matsuoka Y, Kobata A, Yamashita K. Carbohydrate structures of a normal counterpart of the carcinoembryonic antigen produced by colon epithelial cells of normal adults. Glycobiology. 1995;5:105–115. doi: 10.1093/glycob/5.1.105. [DOI] [PubMed] [Google Scholar]
- Gendler SJ, Burchell JM, Duhig T, Lamport D, White R, Parker M, Taylor-Papadimitriou J. Cloning of partial cDNA encoding differentiation and tumor-associated mucin glycoproteins expressed by human mammary epithelium. Proc Natl Acad Sci USA. 1987;84:6060–6064. doi: 10.1073/pnas.84.17.6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorvel JP, Wisner-Provost A, Maroux S. Identification of glycoproteins bearing human blood group A determinants in rabbit enterocyte plasma membranes. FEBS (Fed Eur Biochem Soc) Lett. 1982;143:17–20. doi: 10.1016/0014-5793(82)80263-9. [DOI] [PubMed] [Google Scholar]
- Gorvel JP, Ferrero A, Chambraud L, Rigal A, Bonicel J, Maroux S. Expression of sucrase-isomaltase and dipeptidylpeptidase IV in human small intestine and colon. Gastroenterology. 1991;101:618–625. doi: 10.1016/0016-5085(91)90517-o. [DOI] [PubMed] [Google Scholar]
- Green RF, Meiss HK, Rodriguez-Boulan E. Glycosylation does not determine segregation of viral envelope proteins in the plasma membrane of epithelial cells. J Cell Biol. 1981;89:230–239. doi: 10.1083/jcb.89.2.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green, F.R., P. Greenwell, L. Dickson, B. Griffiths, J. Noades, and D.M. Swallow. 1988. Expression of the ABH, Lewis, and Related Antigens of the Glycoproteins of the Human Jejunal Brush Border. In Subcellular Biochemistry. J.R. Harris, editor. Plenum Press, New York. 119–153. [DOI] [PubMed]
- Harduin-Lepers A, Recchi MA, Delannoy P. 1994, the year of sialyltransferases. Glycobiology. 1995;5:741–758. doi: 10.1093/glycob/5.8.741. [DOI] [PubMed] [Google Scholar]
- Hauri HP, Sterchi EE, Bienz D, Fransen J, Marxer A. Expression of intracellular transport of microvillus membrane hydrolases in human intestinal epithelial cells. J Cell Biol. 1985;101:838–851. doi: 10.1083/jcb.101.3.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennebicq-Reig S, Kim I, Janin A, Grard G, Hémon B, Moreau O, Porchet N, Aubert JP, Degand P, Huet G. Regulation of cathepsin D dependent on the phenotype of colon carcinoma cells. Int J Cancer. 1996;68:479–484. doi: 10.1002/(SICI)1097-0215(19961115)68:4<479::AID-IJC13>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Huang J, Byrd JC, Yoor WH, Kim YS. Effect of benzyl-α-GalNac, an inhibitor of mucin glycosylation, on cancer-associated antigens, in human colon cancer cells. Oncol Res. 1992;4:507–515. [PubMed] [Google Scholar]
- Huet G, Kim I, De Bolos C, Loguidice JM, Moreau O, Hemon B, Richet C, Delannoy P, Real FX, Degand P. Characterization of mucins and proteoglycans synthesized by a mucin-secreting HT-29 cell subpopulation. J Cell Sci. 1995;108:1275–1285. doi: 10.1242/jcs.108.3.1275. [DOI] [PubMed] [Google Scholar]
- Kitagawa H, Paulson JC. Cloning of a novel α2,3-sialyltransferase that sialylates glycoprotein and glycolipid carbohydrate groups. J Biol Chem. 1994;269:1394–1401. [PubMed] [Google Scholar]
- Kojima N, Lee YC, Hamamoto T, Kurosawa N, Tsuji S. Kinetic properties and acceptor substrate preferences of two kinds of Galβ1-3GalNac α2,3-sialyltransferase from mouse brain. Biochemistry. 1994;33:5772–5776. doi: 10.1021/bi00185a014. [DOI] [PubMed] [Google Scholar]
- Kuan SF, Byrd JC, Basbaum C, Kim YS. Inhibition of mucin glycosylation by aryl-N-acetyl-alpha-galactosaminides in human colon cancer cells. J Biol Chem. 1989;264:19271–19277. [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Le Bivic A, Hirn M, Reggio H. HT-29 cells are an “in vitro” model for the generation of cell polarity in epithelia during embryonic differentiation. Proc Natl Acad Sci USA. 1988;85:136–140. doi: 10.1073/pnas.85.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bivic A, Garcia M, Rodriguez-Boulan E. Ricin-resistant Madin-Darby canine kidney cells missort a major endogenous apical sialoglycoprotein. J Biol Chem. 1993;268:6909–6916. [PubMed] [Google Scholar]
- Lesuffleur T, Barbat A, Dussaulx E, Zweibaum A. Growth adaptation to methotrexate of HT-29 human colon carcinoma cells is associated with their ability to differentiate into columnar absorptive and mucus-secreting cells. Cancer Res. 1990;50:6334–6343. [PubMed] [Google Scholar]
- Lesuffleur T, Barbat A, Luccioni C, Beaumatin J, Claire M, Kornovski A, Dussaulx E, Zweibaum A. Dihydrofolate reductase gene amplification-associated shift of differentiation in methotrexate-adapted HT-29 cells. J Cell Biol. 1991;115:1409–1418. doi: 10.1083/jcb.115.5.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesuffleur T, Porchet N, Aubert JP, Swallow D, Gum JR, Kim YS, Real FX, Zweibaum A. Differential expression of the human mucin genes MUC1 to MUC5 in relation to growth and differentiation of different mucus-secreting HT-29 cell subpopulations. J Cell Sci. 1993;106:771–783. doi: 10.1242/jcs.106.3.771. [DOI] [PubMed] [Google Scholar]
- Lesuffleur T, Zweibaum A, Real FX. Mucins in normal and neoplastic human gastrointestinal tissues. Crit Rev Oncol-Hematol. 1994;17:153–180. doi: 10.1016/1040-8428(94)90053-1. [DOI] [PubMed] [Google Scholar]
- Lesuffleur T, Roche F, Hill AS, Lacasa M, Fox M, Swallow DM, Zweibaum A, Real FX. Characterization of a mucin cDNA clone isolated from HT-29 mucus-secreting cells: the 3' end of MUC5AC? . J Biol Chem. 1995;270:13665–13673. doi: 10.1074/jbc.270.23.13665. [DOI] [PubMed] [Google Scholar]
- Lotan R, Skutelski E, Danon D, Sharon N. The purification, composition, and specificity of the anti-T lectin from peanut (arachis hypogae) . J Biol Chem. 1975;250:8518–8523. [PubMed] [Google Scholar]
- Majuri ML, Niemela R, Tiisala S, Renkonen O, Renkonen R. Expression and function of α-2,3-sialyl- and α-1,3/1,4-fucosyltransferases in colon adenocarcinoma cell lines: role in synthesis of E-selectin counter-receptors. Int J Cancer. 1995;63:551–559. doi: 10.1002/ijc.2910630416. [DOI] [PubMed] [Google Scholar]
- Misumi Y, Hayashi Y, Arakawa F, Ikehara Y. Molecular cloning and sequence analysis of human dipeptidyl-peptidase IV, a serine proteinase on the cell surface. Biochim Biophys Acta. 1992;1131:333–336. doi: 10.1016/0167-4781(92)90036-y. [DOI] [PubMed] [Google Scholar]
- Nagatsu T, Hino M, Fuyamada H, Hayakawa T, Sakakibara S, Nakagawa Y, Takemoto T. New chromogenic substrates for X-prolyl dipeptidyl aminopeptidase. Anal Biochem. 1976;74:466–476. doi: 10.1016/0003-2697(76)90227-x. [DOI] [PubMed] [Google Scholar]
- Nuti M, Teramoto YA, Mariani-Constantini R, Hand PH, Colcher D, Schlom JA. A monoclonal antibody (B72.3) define patterns of distribution of a novel tumor-associated antigen in human mammary carcinoma cell populations. Int J Cancer. 1982;29:539–545. doi: 10.1002/ijc.2910290509. [DOI] [PubMed] [Google Scholar]
- Peterson GL. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977;83:346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Pinto M, Robine-Léon S, Appay MD, Kedinger M, Triadou N, Dussaulx E, Lacroix B, Simon-Assmann P, Haffen K, Fogh J, Zweibaum A. Enterocyte-like differentiation and polarization of the human colon carcinoma cell line Caco-2 in culture. Biol Cell. 1983;47:323–330. [Google Scholar]
- Pringault E, Arpin M, Garcia A, Finidori J, Louvard D. A human villin cDNA clone to investigate the differentiation of intestinal and kidney cells in vivo and in culture. EMBO (Eur Mol Biol Organ) J. 1986;5:3119–3124. doi: 10.1002/j.1460-2075.1986.tb04618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recchi MA, Harduin-Lepers A, Boilly-Marer Y, Verbert A, Delannoy P. Multiplex RT-PCR method for the analysis of the expression of human sialyltransferases: application to breast cancer cells. Glycoconj J. 1998;15:19–27. doi: 10.1023/a:1006983214918. [DOI] [PubMed] [Google Scholar]
- Robine S, Huet C, Moll R, Sahuquillo-Merino C, Coudrier E, Zweibaum A, Louvard D. Can villin be used to identify malignant and undifferentiated normal digestive epithelial cells? . Proc Natl Acad Sci USA. 1985;82:8488–8492. doi: 10.1073/pnas.82.24.8488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J. Cellular sialoglycoconjugates: a histochemical perspective. Histochem J. 1993;25:687–710. doi: 10.1007/BF00211765. [DOI] [PubMed] [Google Scholar]
- Rothman JE. Mechanisms of intracellular protein transport. Nature. 1994;372:55–63. doi: 10.1038/372055a0. [DOI] [PubMed] [Google Scholar]
- Sata T, Roth J, Zuber C, Stamm B, Heitz PU. Expression of α2,6-linked sialic acid residues in neoplastic but not in normal human colonic mucosa. Am J Pathol. 1991;139:1435–1448. [PMC free article] [PubMed] [Google Scholar]
- Scheiffele P, Peranen J, Simons K. N-glycans as apical sorting signals in epithelial cells. Nature. 1995;378:96–98. doi: 10.1038/378096a0. [DOI] [PubMed] [Google Scholar]
- Semenza G. The insertion of stalked proteins of the brush border membranes: the state of the art in 1988. Biochem Int. 1989;18:15–33. [PubMed] [Google Scholar]
- Shibuya N, Goldstein IJ, Broekaert WF, Nsimba-Lubaki M, Peeter B, Peumans WJ. The elderberry (Sambucus nigra L.)bark lectin recognizes the Neu5Ac(α2,6)Gal/GalNAc sequence. J Biol Chem. 1987;262:1596–1601. [PubMed] [Google Scholar]
- Thomas PS. Hybridization of denatured mRNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci USA. 1980;77:5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triadou N, Audran E, Rousset M, Zweibaum A, Oriol R. Relationship between the secretor status and the expression of ABH blood group antigenic determinants in human intestinal brush border membrane hydrolases. Biochim Biophys Acta. 1983;761:231–236. doi: 10.1016/0304-4165(83)90070-3. [DOI] [PubMed] [Google Scholar]
- Tsuji S. Molecular cloning and functional analysis of sialyltransferases. J Biochem. 1996;120:1–23. doi: 10.1093/oxfordjournals.jbchem.a021369. [DOI] [PubMed] [Google Scholar]
- Tsuji, S., A.K. Datta, and J.C. Paulson. 1996. Systematic nomenclature for sialyltransferases. Glycobiology. 6:V–VII. [DOI] [PubMed]
- Vaessen RTMJ, Kreike J, Groot GSP. Protein transfer to nitrocellulose filters. FEBS (Fed Eur Biochem Soc) Lett. 1981;124:193–196. doi: 10.1016/0014-5793(81)80134-2. [DOI] [PubMed] [Google Scholar]
- Wang WC, Cummings RD. The immobilized leukoagglutinin from the seeds of Maacki amurensisbinds with high affinity to complex-type Asn-linked oligosaccharides containing terminal sialic acid-linked α-2,3 to penultimate galactose residues. J Biol Chem. 1988;263:4576–4585. [PubMed] [Google Scholar]
- Willot E, Balda SM, Heintzelman M, Jameson B, Anderson JM. Localization and differential expression of two isoforms of the tight junction protein ZO-1. Am J Physiol. 1992;262:C1119–C1124. doi: 10.1152/ajpcell.1992.262.5.C1119. [DOI] [PubMed] [Google Scholar]
- Xing P-X, Tjandra JJ, Stacker SA, Teh JG, Thompson CH, McLaughlin PJ, McKenzie IFC. Monoclonal antibodies reactive with mucin expressed in breast cancer. Immunol Cell Biol. 1989;67:183–195. doi: 10.1038/icb.1989.29. [DOI] [PubMed] [Google Scholar]
- Yeaman C, Le Gall AH, Baldwin AN, Monlauzeur L, Le Bivic A, Rodriguez-Boulan E. The O-glycosylated stalk domain is required for apical sorting of neurotrophin receptors in polarized MDCK cells. J Cell Biol. 1997;139:929–940. doi: 10.1083/jcb.139.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweibaum, A., M. Laburthe, E. Grasset, and D. Louvard. 1991. Use of Cultured Cell Lines in Studies of Intestinal Cell Differentiation and Function. In Intestinal Absorption and Secretion. Handbook of Physiology. Volume 4. Section 6. The Gastrointestinal System. M. Field and R.A. Frizzell, editors. American Physiological Society, Bethesda, MD. 223–255.