Abstract
The perinucleolar compartment (PNC) is a unique nuclear structure localized at the periphery of the nucleolus. Several small RNAs transcribed by RNA polymerase III and two hnRNP proteins have been localized in the PNC (Ghetti, A., S. Piñol-Roma, W.M. Michael, C. Morandi, and G. Dreyfuss. 1992. Nucleic Acids Res. 20:3671–3678; Matera, A.G., M.R. Frey, K. Margelot, and S.L. Wolin. 1995. J. Cell Biol. 129:1181– 1193; Timchenko, L.T., J.W. Miller, N.A. Timchenko, D.R. DeVore, K.V. Datar, L. Lin, R. Roberts, C.T. Caskey, and M.S. Swanson. 1996. Nucleic Acids Res. 24: 4407–4414; Huang, S., T. Deerinck, M.H. Ellisman, and D.L. Spector. 1997. J. Cell Biol. 137:965–974). In this report, we show that the PNC incorporates Br-UTP and FITC-conjugated CTP within 5 min of pulse labeling. Selective inhibition of RNA polymerase I does not appreciably affect the nucleotide incorporation in the PNC. Inhibition of all RNA polymerases by actinomycin D blocks the incorporation completely, suggesting that Br-UTP incorporation in the PNC is due to transcription by RNA polymerases II and/or III. Treatment of cells with an RNA polymerase II and III inhibitor induces a significant reorganization of the PNC. In addition, double labeling experiments showed that poly(A) RNA and some of the factors required for pre-mRNA processing were localized in the PNC in addition to being distributed in their previously characterized nucleoplasmic domains. Fluorescence recovery after photobleaching (FRAP) analysis revealed a rapid turnover of polypyrimidine tract binding protein within the PNC, demonstrating the dynamic nature of the structure. Together, these findings suggest that the PNC is a functional compartment involved in RNA metabolism in the cell nucleus.
Keywords: perinucleolar compartment; nucleolus, nuclear body; nuclear structure; transcription
The perinucleolar compartment (PNC)1 was first described by the localization of the heterogeneous nuclear ribonucleoprotein I/polypyrimidine tract binding protein (hnRNP I/PTB) (Ghetti et al., 1992). This subnuclear compartment is an irregularly shaped structure with a large variability in size ranging from 0.25 to 1 μm in diameter, and it is localized at the periphery of the nucleolus. The PNC is predominantly found in transformed cells and rarely observed in normal primary cells (Huang et al., 1997). Cell cycle analysis using immunolabeling showed that the PNC dissociates at the beginning of mitosis and reforms at late telophase in the daughter nuclei. Time-lapse observations of living cells transiently expressing green fluorescent protein–tagged PTB (GFP–PTB) revealed that the PNC is a dynamic structure exhibiting discrete movements over time (Huang et al., 1997). Electron microscopic examination of optimally fixed HeLa cells demonstrated that the PNC is composed of multiple thick, electron-dense strands, each measuring ∼80–180 nm in diameter (Huang et al., 1997). Some of these strands are in direct contact with the surface of the nucleolus. Furthermore, deletion mutagenesis studies showed that at least three RNA recognition motifs at either the COOH or NH2 terminus of the PTB protein are required for it to be targeted to the PNC, suggesting that RNA binding is necessary for PTB to be localized in the PNC (Huang et al., 1997). However, the function of the PNC and its relationship to the transformed phenotype remains to be determined.
Thus far, several small RNAs transcribed by RNA polymerase III (RNase MRP RNA, RNase P RNA, and hY RNAs) (Matera et al., 1995; Lee et al., 1996) and two hnRNP proteins, PTB (Ghetti et al., 1992) and CUG-BP/ hNab50 (Timchenko et al., 1996), have been identified in the PNC. One of the hnRNP proteins in the PNC, PTB, is a 57-kD RNA-binding protein that specifically binds pyrimidine-rich sequences (Ghetti et al., 1992). PTB has been shown to be involved in multiple cellular functions, including pre-mRNA splicing (Patton et al., 1993; Singh et al., 1995; Ashiya and Grabowski, 1997), splice site selection in alternative pre-mRNA splicing (Lin and Patton, 1995; Perez et al., 1997), RNA polyadenylation (Lou et al., 1996), and translational regulation of certain viral RNA transcripts (Hellen et al., 1994; Kaminski et al., 1995; Witherell et al., 1995). PTB apparently participates in these functions through the binding of pyrimidine-rich RNA sequences. Therefore, PTB may serve as a bridge between the pyrimidine tract containing RNAs and a variety of cellular factors to fulfill different cellular functions.
CUG-BP/hNab50, a second hnRNP protein localized to the PNC, was initially isolated through a yeast two-hybrid screen because of its interaction with the yeast hnRNP protein Nab2p (Anderson et al., 1993). It was later revealed that CUG-BP/hNab50 binds to the CUG triplet repeats of myotonin protein kinase RNA, which is associated with myotonic dystrophy, an autosomal dominant neuromuscular disease (Timchenko et al., 1996). The CUG-BP/hNab50 protein binds polyadenylated RNA and is distributed predominantly in the nucleoplasm as well as enriched in a locus at the periphery of the nucleolus (Timchenko et al., 1996). Double labeling experiments showed that the perinucleolar localization of the protein coincides with the PNC (Huang, S., and D.L. Spector, unpublished result). More recently, the phosphorylation and intracellular distribution of CUG-BP/hNab50 were shown to be altered in patients with myotonic dystrophy and in a myotonin protein kinase knockout mouse (Robert et al., 1997). However, it was not reported whether the distribution of this protein in the PNC is affected by the change in phosphorylation of the protein. The role of CUG-BP/hNab50 in the PNC is not yet clear.
In addition to the above-described hnRNP proteins, several RNA polymerase III transcripts (hY RNAs, RNase P RNA, and RNase MRP RNA) have also been localized to the PNC (Matera et al., 1995). hY RNAs, which range in size from 69–112 nucleotides, interact with the 60-kD Ro protein to form Ro RNPs. Ro RNPs are present predominantly in the cytoplasm at 1% the level of ribosomes (Wolin and Steitz, 1984), and their function is not known. In spite of the presence of hY1, hY3, and hY5 RNAs, the Ro protein itself was not found in the PNC (Matera et al., 1995). RNase P RNA is involved in tRNA and preribosomal RNA processing (for reviews see Altman, 1990; Clayton, 1994). RNase P RNA is localized in the cytoplasm and diffusely in the nucleoplasm in addition to being present in the PNC (Darr et al., 1992; Matera et al., 1995; Jacobson et al., 1997). When rhodamine-labeled RNase P RNA was injected into the nucleus of NRK cells, the labeled RNA rapidly accumulated in the nucleolus followed by a redistribution into the nucleoplasm (Jacobson et al., 1997). The transient association with the nucleolus suggests that RNase P RNA may be processed and assembled into RNPs or play a role in the nucleolus (Jacobson et al., 1997). RNase MRP, another endoribonuclease, is involved in preribosomal RNA processing (for review see Clayton, 1994). In situ hybridization and microinjection of labeled RNase MRP RNA demonstrated that this RNA is localized in the nucleolus and the PNC (Clayton, 1994; Jacobson et al., 1995; Matera et al., 1995). Not all RNAs transcribed by RNA polymerase III are detected in the PNC. In situ hybridization with specific probes to hY4, 5S rRNA, and U6 snRNA did not reveal hybridization signals in the PNC (Matera et al., 1993, 1995). The significance of the accumulation of several RNA polymerase III transcripts in the PNC is presently unknown.
In this report, we demonstrate that the PNC is structurally distinct from the nucleolus and forms a reticulated mesh on a portion of the nucleolar surface. The PNC incorporates Br-UTP and FITC-CTP after a short pulse using permeabilized cells, suggesting that the PNC is involved in transcription. The structure of the PNC is altered upon the inhibition of RNA synthesis in cultured cells, suggesting that the integrity of the PNC reflects its transcriptional activity. Immunolabeling and in situ hybridization have shown that several pre-mRNA processing factors, including splicing factors (snRNPs and SC35) and a 3′ end processing factor (poly(A) binding protein II [PAB II]), and poly(A) RNA are present in the PNC. In addition, fluorescence recovery after photobleaching (FRAP) analysis has shown that the hnRNP protein, PTB, turns over rapidly in the PNC. Together, these findings suggest that the PNC is a dynamic subnuclear structure that is involved in RNA metabolism in transformed cells.
Materials and Methods
Cell Culture
HeLa cells were grown to subconfluence on 22 × 22-mm glass coverslips in 35-mm Petri dishes in Dulbecco's modified Eagle's minimum essential medium (DMEM) supplemented with 10% fetal calf serum (FCS) (GIBCO-BRL, Life Technologies, Inc., Rockville, MD). The inhibition of RNA polymerase I transcription was achieved by the addition of actinomycin D (0.04 μg/ml for 3 h) (Perry, 1963). The inhibition of RNA polymerase II transcription was achieved by the addition of α-amanitin (50 μg/ ml for 5 h) (Kedinger et al., 1970; Lindell et al., 1970) or 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole (DRB) (25 μg/ml for 3 h) (Sehgal et al., 1976) to the culture medium. Transcription inhibition was reversed when DRB-containing medium was removed from cells and replaced with fresh medium. Cycloheximide (Sigma Chemical Co., St. Louis, MO) was added at 100 μg/ml for 5 h to inhibit protein synthesis and RNA polymerase I activity (Higashi et al., 1968; Willems et al., 1969; O'Keefe et al., 1994).
Three-dimensional Reconstruction of the PNC by Electron Microscopy
Transiently expressed GFP–PTB (Huang et al., 1997) was used as a marker to indicate the localization of the PNC. The corresponding nuclear region identified by fluorescence of the GFP–PTB fusion protein was examined by electron microscopy. Specifically, transfected cells were seeded onto gridded glass coverslips. 12 h after transfection, cells were fixed in 4% paraformaldehyde with 0.05% glutaraldehyde in PBS, and cells that showed the PNC were quickly photographed using an epifluorescence microscope (model FXA; Nikon, Inc., Melville, NY) equipped with a cooled CCD camera (1320 × 1035, 6.7 μm pixel size) (SenSys; Photometrics, Inc., Tuscon, AZ) using Oncor Image software. Subsequently, cells were fixed in 2% glutaraldehyde for 20 min and washed in PBS and 0.1 M cacodylate buffer, pH 7.4. Cells were then postfixed in 1% osmium tetroxide containing 0.15% ferrous cyanide in 0.1 M cacodylate buffer, pH 7.4, for 1 h, dehydrated by incubation in a series of ascending concentrations of ethanol, and embedded in Epon/araldite at 60°C for 48 h. Serial 80-nm-thick sections were poststained with uranyl acetate–lead citrate, mounted on slot grids, and examined at 80 keV with a transmission electron microscope (model 100CX; JEOL U.S.A., Inc., Peabody, MA). The same cells photographed at the fluorescence microscopic level were located, and serial images of the nuclear regions that corresponded to the PNCs were recorded. Outlines of the nucleoli and PNCs were hand traced on 11” × 14” photographic prints and digitized using a digitizing tablet and a software program described by Young et al. (1987). The section planes were aligned, and volumes were surface-rendered using the programs SYNU and SYNU-render as previously described by Hessler et al. (1992).
Nuclear Extraction
Cells were extracted following a modification of a protocol described by Fey et al. (1986). Subconfluent cells grown on glass coverslips were rinsed with PBS and incubated at 4°C for 10 min in CSK buffer (100 mM NaCl, 300 mM sucrose, 10 mM Pipes, pH 6.8, 3 mM MgCl2, 1 mM PMSF, and 2 mM vanadyl ribonucleoside complex) containing 0.5% Triton X-100 (vol/ vol). Cells were incubated at 4°C for 5 min in the CSK buffer containing 250 mM ammonium sulfate and 0.5% Triton X-100, treated with DNAse I in CSK buffer with 50 mM NaCl for 30 min at 37°C or room temperature, and finally fixed in 2% paraformaldehyde in PBS.
Transfection
Expression constructs were transiently transfected into HeLa cells by electroporation (Spector et al., 1997). Subconfluent cells in a 100-mm culture dish were trypsinized and collected in DMEM supplemented with 10% FBS. Cells were then mixed with 20 μg of DNA including 7 μg target DNA and 13 μg sheared salmon sperm DNA. 280 μl of cell–DNA mixture was electroporated in a Bio-Rad electroporator (Hercules, CA) at 260 V and 960 μF. Cells were subsequently seeded onto glass coverslips in 35-mm Petri dishes and were grown for either 7 or 24 h.
FRAP
Living cell studies were performed using a 35-mm Petri dish with a coverslip attached to the bottom. Transfected cells were grown on the coverslip for 24 h and observed using an inverted confocal laser-scanning microscope (model 410; Carl Zeiss, Inc., Thornwood, NY). An initial image was acquired, and a FRAP macro was used to photobleach a designated area of the nucleus. The photobleaching was accomplished using the 488-nm argon laser at 50% of its full power (25 mW) for 20 s. Immediately after the bleaching process, an averaged image was obtained. Multiple images were subsequently acquired within 5 min after bleaching, as indicated in Fig. 6.
Figure 6.
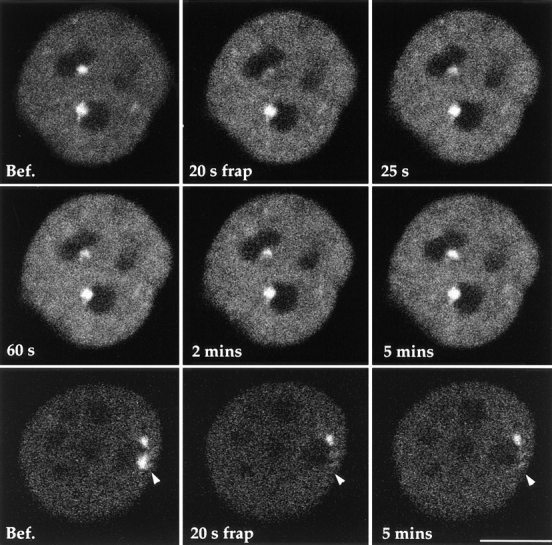
FRAP studies reveal the dynamics of PTB in the PNC. GFP–PTB was transiently expressed in HeLa cells. The fluorescence of the bleached PNC in the living cell (top and middle rows) begins to recover almost immediately after the photobleaching. The fluorescence intensity in the PNC reaches a similar intensity as compared with before photobleaching within 5 min, suggesting that PTB undergoes a rapid turnover in the PNC. However, in fixed cells (bottom row), when the fluorescent PNC is photobleached (arrowheads), the fluorescence in the PNC does not recover even after 5 min. This finding demonstrates that PTB undergoes a rapid turnover in the PNC. Bar, 10 μm.
Nucleotide Incorporation Assay
The transcription assay was modified from published studies (Jackson et al., 1993; Wansink et al., 1993). At 8 or 24 h after transfection, cells were rinsed once with PBS and once with a Tris-glycerol buffer (20 mM Tris-HCl, pH 7.4, 5 mM MgCl2, 25% glycerol, 0.5 mM PMSF, and 0.5 mM EGTA). Cells were then permeabilized in the same buffer containing 5 μg/ml digitonin at room temperature for 3 min. Subsequently, cells were incubated in transcription cocktail (100 mM KCl, 50 mM Tris-HCl, pH 7.4, 5 mM MgCl2, 0.5 mM EGTA, 25% glycerol, 1 mM PMSF, 2 mM ATP, 0.5 mM CTP, 0.5 mM GTP, 0.2 mM Breakup or 0.4 mM FITC-CTP, and 1 U/ml RNasin) for 5 min at 37°C. At the end of the transcription reaction, cells were gently rinsed three times with PBS and then fixed in 2% formaldehyde in PBS. Transcription inhibition was achieved by adding the corresponding drugs to the permeabilization buffer and transcription cocktail.
Immunolabeling
Subconfluent HeLa cells grown on glass coverslips were extracted with CSK buffer containing 0.3% Triton X-100 for 3 min and fixed in freshly made 2% formaldehyde in PBS for 15 min. Cells were washed three times for 10 min each in PBS, and coverslips were incubated with primary antibody for 1 h at room temperature. Antibodies used in this study included anti-PTB primary antibody, SH54 (Huang et al., 1997), at a dilution of 1:300, anti-Sm antibody (Lerner and Steitz, 1979) at a dilution of 1:1,000, anti-B″ at a dilution of 1:10 (Habets et al., 1987, 1989), antifibrillarin (Sigma Chemical Co.) (Ochs et al., 1985) at a dilution of 1:5, anti-SC35 at a dilution of 1:1,000 (Fu and Maniatis, 1990), anti–PAB II at a dilution of 1:10 (Krause et al., 1994), or anticoilin at a dilution of 1:100 (Andrade et al., 1993). Cells were rinsed in PBS, incubated with Texas red–conjugated goat anti–human, FITC- or Texas red–conjugated goat anti–mouse, or Texas red goat anti–rabbit antibody at a dilution of 1:100 for 1 h at room temperature, and then washed three times for 10 min each in PBS. The coverslips were mounted onto glass slides with mounting medium containing 90% glycerol in PBS with 1 mg/ml paraphenylenediamine as an antifade agent. The mounting medium was adjusted to pH 8.0 with 0.2 M bicarbonate buffer (Spector et al., 1997). Cells were examined with a microscope (model FXA; Nikon, Inc.) equipped with epifluorescence and differential interference contrast optics. Images were captured by a cooled CCD camera (1320 × 1035, 6.7 μm pixel size) (SenSys; Photometrics, Inc.) using Oncor Image software.
Results
The PNC Forms a Reticulated Mesh Associated with a Portion of the Nucleolar Surface
Previous examination of thin-sectioned and optimally fixed HeLa cells revealed that the PNC is an electron-dense structure composed of thick strands, some of which are in direct contact with the surface of the nucleolus (Huang et al., 1997). We sought to determine if these thick strands are interconnected and if they form a higher order structure. To resolve the three-dimensional organization of the PNC at high resolution, PNCs in serial thin sections of optimally fixed HeLa cell nuclei were examined by electron microscopy (Materials and Methods). The sections were poststained with uranyl acetate–lead citrate, and the PNC was readily distinguishable from the nucleolus under these conditions of specimen preparation. The PNC was also identified by correlating the cell sections to a fluorescent image of the same cell in which the expression of GFP–PTB (Huang et al., 1997) marked the PNC (Fig. 1). The PNC appeared to be more electron dense than the nucleolus and formed thick strands, some of which intimately associate with the nucleolus. Images of serial sections were aligned, and three-dimensional models were reconstructed using the programs SYNU and SynuRender (Hessler et al., 1992) (Fig. 2). Such analysis has revealed that the PNC is a highly interconnected and reticulated mesh associated with a portion of the nucleolar surface. However, there is considerable variability in the shape, size, and the relationship of the PNC with the nucleolus. In some cells, the PNC is mostly positioned on the surface of the nucleolus (Fig. 2 A), while in others, the PNC extends into the nucleolus like a plug (Fig. 2 B). Occasionally, a portion of the nucleolus extends into the PNC (Fig. 2 C). This variability may represent the dynamic range of PNC:nucleolus interrelationships.
Figure 1.
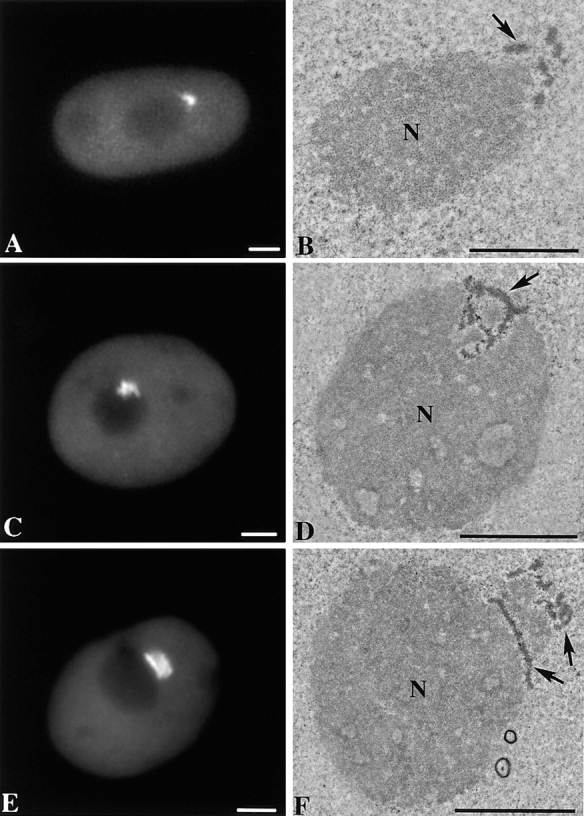
Correlative fluorescent and electron microscopic images of the PNC in the same cells show that the PNC is distinct from and more electron-dense than the nucleolus. HeLa cells transiently expressing GFP–PTB were photographed using a SenSys cooled CCD camera (left column) and subsequently fixed, embedded, thin sectioned, and poststained. The same cell was sought, and the nuclear regions corresponding to the PNC, observed at the light microscopic level, were correlated at the electron microscopic level (right column). Bar, 3 μm.
Figure 2.
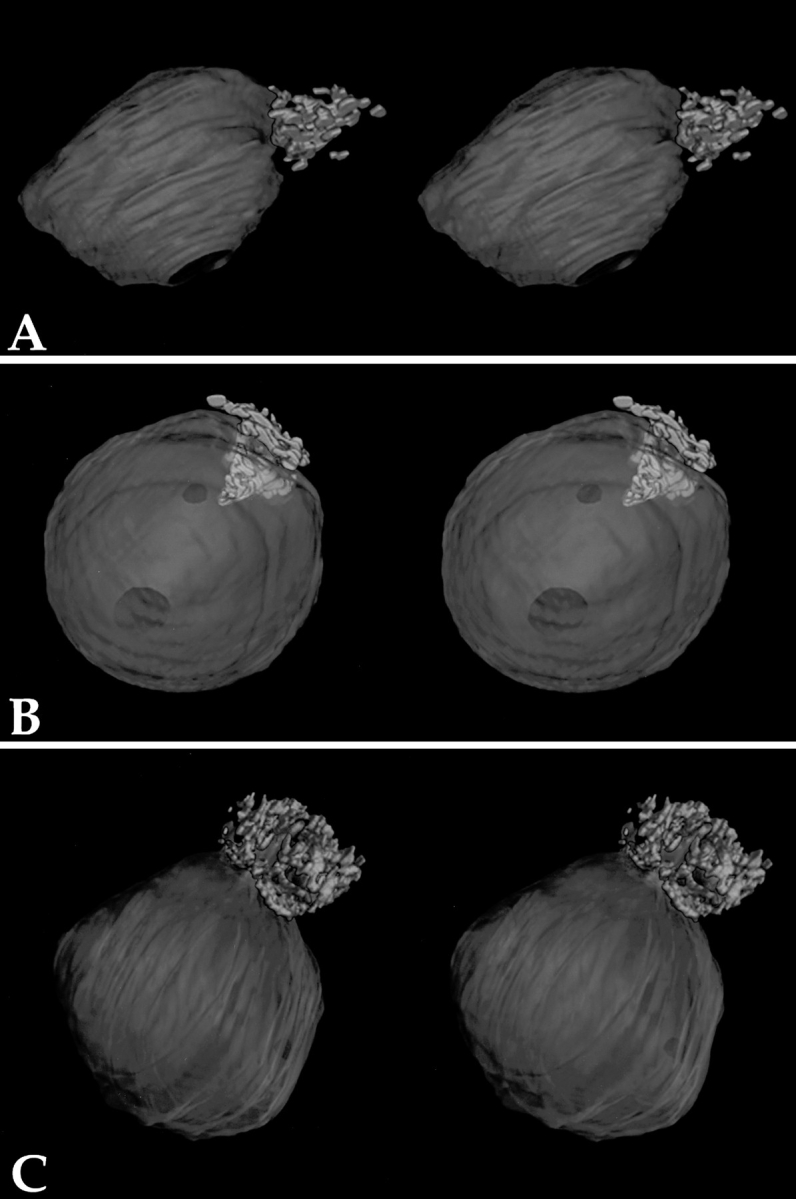
Three-dimensional computer rendering of the PNC from serial section electron microscopy reveals that the PNC forms a highly interconnected reticulated meshwork on a portion of the nucleolar surface (stereo image pairs). Three examples illustrate the variability of the association between the PNC and the nucleolus among different cells. The green represents the PNC, and the purple represents the nucleolus.
The PNC Is Actively Involved in Transcription
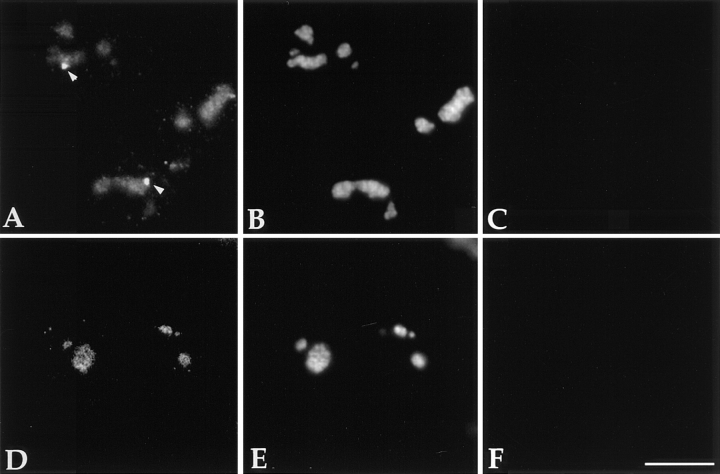
Based on the abundance of RNAs and RNA-binding proteins in the PNC, we were interested in examining if this subnuclear compartment is involved in transcription. In situ Br-UTP incorporation experiments were performed to evaluate the transcriptional activity in the PNC. Cells transiently expressing GFP–PTB, which marks the PNC, were briefly permeabilized and incubated in a transcription cocktail containing Br-UTP using a modified protocol based on the work of Wansink et al. (1993) and Jackson et al. (1993). Cells were then fixed, and the nuclear sites of Br-UTP incorporation were detected by immunolabeling with a monoclonal antibody that specifically recognizes the Br epitope. Simultaneous detection of the incorporation sites (red signal) and the PNC, marked by GFP–PTB (green signal), demonstrates that the PNC coincides with sites of Br-UTP incorporation (Fig. 3, top row). In fact, the Br-UTP incorporation is often more prominent in the PNC than in most of the nucleoplasm. However, the size and shape of the sites of Br-UTP incorporation often do not completely overlap with the PNC. The incorporation was inhibited when it was performed at 4°C and was not detected in the presence of RNase A (data not shown), suggesting that the nucleotides are incorporated into the RNA in an energy-dependent reaction. The nucleotide incorporation could result from either transcription or posttranscriptional modification of nascent RNA, including editing and uridinylation. It has previously been shown that U is posttranscriptionally added to certain small RNA polymerase III transcripts, including RNase MRP RNA (Hashimoto and Steitz, 1983; Yuan et al., 1989), which is found in the PNC. To determine the nature of the Br-UTP incorporation in the PNC, we examined whether the incorporation is DNA dependent and whether the PNC incorporates nucleotides other than Br-UTP. We found that the Br-UTP incorporation in the PNC and the entire nucleus (Fig. 3, second row) was inhibited in the presence of a high concentration of actinomycin D (50 μg/ml) (Perry, 1963), which intercalates into DNA and prevents it from being used as a transcription template. Furthermore, addition of FITC-CTP to the transcription cocktail resulted in an incorporation in the PNC that is similar to the incorporation of Br-UTP (Fig. 3, third row). These findings further demonstrate that the nucleotide incorporation into the PNC is the consequence of transcription.
Figure 3.
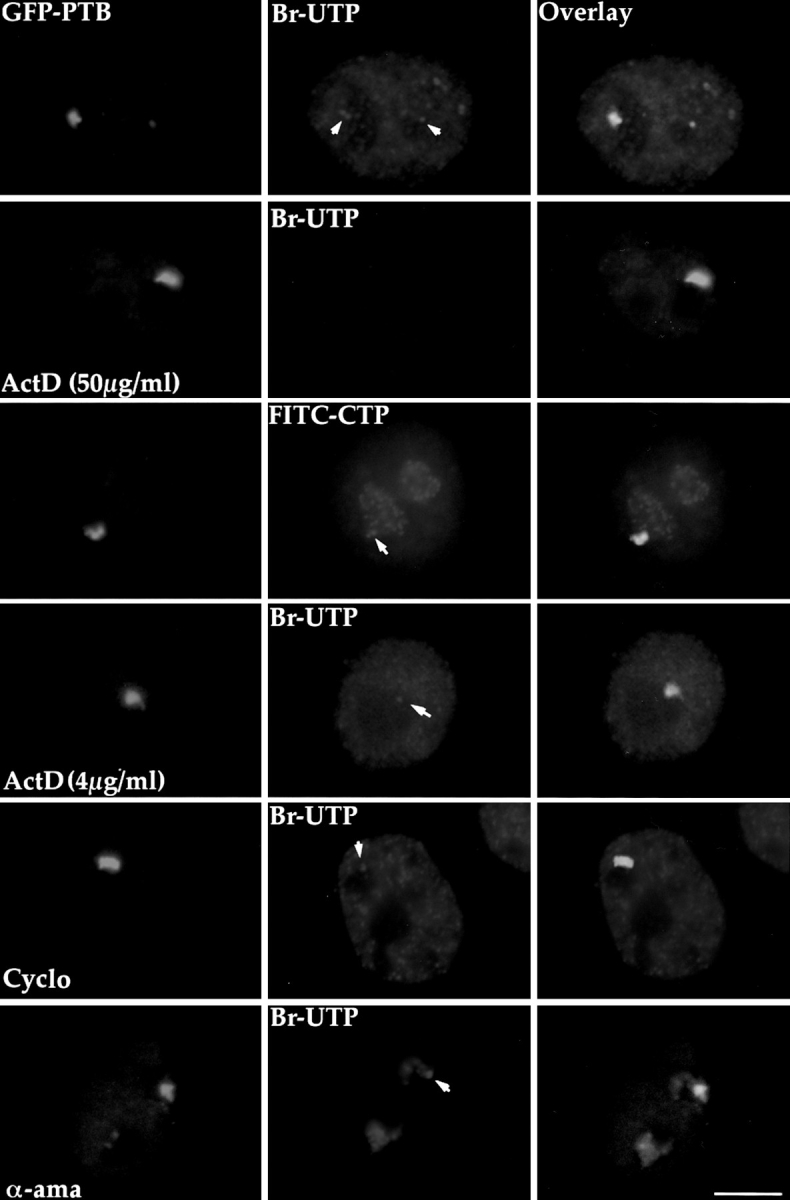
The PNC actively incorporates Br-UTP in a transcription assay using permeabilized cells. The left panels are images of PNCs, marked by expression of GFP–PTB. The middle panels represent the labeled nucleotide incorporation pattern in the same cells. The right panels are overlays from the first two panels. Most PNCs incorporate Br-UTP above the basal level of incorporation in the nucleoplasm (top row, arrows). In some cases, the incorporation of Br-UTP only occurs in part of the PNC. When transcription by all RNA polymerases are inhibited in the presence of a high concentration of actinomycin D (50 μg/ ml), Br-UTP incorporation is not observed in either the PNC or the nucleus (second row). The PNC also incorporates FITC-CTP (third row, arrow). Addition of a low concentration of actinomycin D (fourth row, arrow) or pretreatment using cycloheximide (100 μg/ml) for 3 h before the assay (fifth row, arrow), both of which selectively inhibit RNA polymerase I transcription, do not significantly affect the Br-UTP incorporation in the PNC. Surprisingly, when RNA polymerases II and III are inhibited by the addition of α-amanitin (300 μg/ml), the incorporation of Br-UTP in the PNC is also not obviously changed (bottom row, arrow). Bar, 10 μm.
To identify which RNA polymerase is involved in the Br-UTP incorporation in the PNC, transcription inhibitors that preferentially inhibit different RNA polymerases were added individually to the transcription cocktail. To inhibit RNA polymerase I activity, two alternative approaches were used. Actinomycin D, which inhibits RNA polymerase I at low concentration (Perry, 1963), was added, and the concentration used was titrated such that the nucleolar incorporation of Br-UTP was significantly reduced and the nucleoplasmic incorporation did not exhibit a detectable change (Fig. 3, fourth row). At this concentration of actinomycin D, no significant change was observed in the Br-UTP incorporation in the PNC (Fig. 3, fourth row). Alternatively, cells were pretreated with cycloheximide (100 μg/ml) for up to 5 h before the Br-UTP incorporation assay. Cycloheximide has been shown to inhibit protein synthesis as well as RNA polymerase I transcription by limiting essential labile factors (Higashi et al., 1968; Willems et al., 1969; O'Keefe et al., 1994). The indirect inhibition of RNA polymerase I by inhibiting protein synthesis enables cycloheximide to circumvent the potential accessibility problem that other drugs might have. The treatment of cycloheximide inhibited most of Br-UTP incorporation in the nucleolus but did not appreciably alter the incorporation in the PNC (Fig. 3, fifth row). The similar results obtained by using two different inhibitory mechanisms suggests that RNA polymerase I is unlikely to be responsible for the Br-UTP incorporation in the PNC. To examine whether RNA polymerases II and/or III are involved, we added α-amanitin into the transcription cocktail. α-Amanitin specifically and selectively inhibits RNA polymerase II at a low concentration of 50 μg/ml (Stripe and Fiume, 1967; Weinmann et al., 1975) and inhibits both polymerases II and III at a high concentration of 300 μg/ ml (Weinmann and Roeder, 1974). Surprisingly, the addition of α-amanitin at either the low or high concentration did not significantly change the Br-UTP incorporation in the PNC (Fig. 3, bottom row), despite a significant reduction in nucleoplasmic incorporation. Since the Br-UTP incorporation in the PNC is due to the transcription that occurs within the 5 min of pulse labeling as described above, and since we have ruled out the involvement of RNA polymerase I transcription, it is therefore most likely that RNA polymerase II and/or III are responsible for the synthesis of nascent RNA in the PNC. A reasonable explanation for a lack of transcription inhibition may be the inability of α-amanitin to gain access into the PNC.
The Structural Integrity of the PNC Is Sensitive to Transcriptional Inhibition
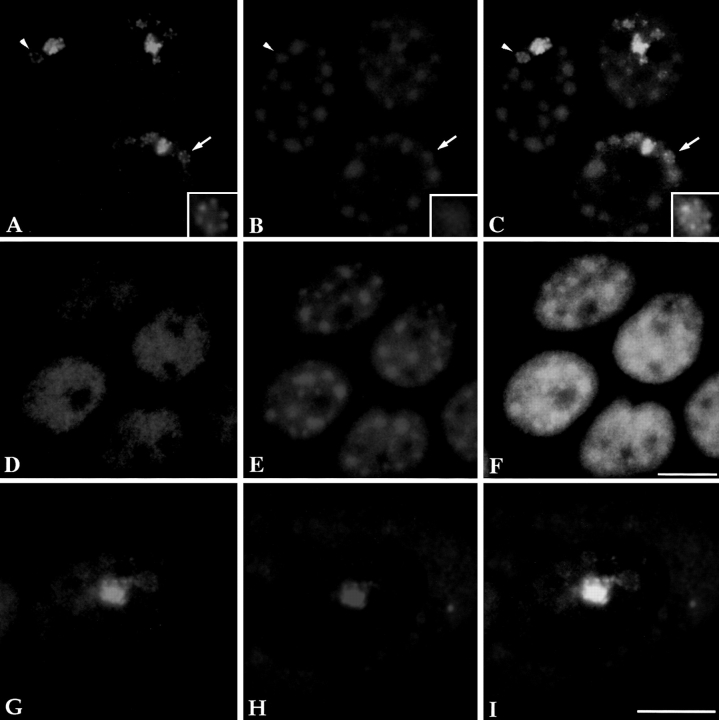
To examine if inhibition of transcription would influence the structure of the PNC in vivo, HeLa cells were treated with α-amanitin at 50 μg/ml for 5 h. The structure of the PNC showed a marked alteration after inhibition of RNA polymerase II (Fig. 4, A and C). The PNC is no longer a compact structure with clear boundaries after α-amanitin treatment. PTB (green) seems to extend from what appears to be the original site of the PNC into the nucleoplasm and, in many cases, forms a series of dots in a rosette with a hollow center (Fig. 4 A, arrow and arrowhead; area at the arrow is enlarged in the inset). When double labeled with the anti-Sm antibody (red), a colocalization was observed between the original sites of the PNC and snRNPs. Interestingly, the extended rosette-shaped dots surround the round snRNP clusters (Fig. 4 C, arrow and arrowhead; area at the arrow is enlarged in the inset). Such alterations appear to form a gradient that decreases with distance from the original locus (the strongly stained region) of the PNC. In contrast, inhibition of RNA polymerase II transcription exerts little to no effect on the diffuse nuclear distribution pattern of PTB in cells that do not have a PNC (Fig. 4 D), demonstrating that the observed alteration is specific to the PNC. Similar changes were also observed when cells were treated with DRB, another RNA polymerase II inhibitor that inhibits a major nuclear Ser/Thr protein kinase, casein kinase II (Sehgal et al., 1976; Zandomeni and Weinmann, 1984). These changes were reversible after cells were grown in drug-free medium for 2 h (data not shown), indicating that the observed alteration in the structure of the PNC is unlikely to be the result of cell death. These findings show that the integrity of the PNC is dependent upon the transcriptional activity of RNA polymerase II.
Figure 4.
The structure of the PNC changes upon transcription inhibition by α-amanitin (an inhibitor of RNA polymerase II and/or III) in cells transiently expressing GFP– PTB (A). When double labeled with the Sm antibody (B), the extended PNC appears to form rosettes surrounding the round Sm clusters (C, inset). This change was not observed in cells that do not contain a PNC (D–F), suggesting that the change is specific to a reorganization of the PNC. When the distribution of GFP–PTB (G) and CUG-BP/hNab50 (H) was compared upon transcription inhibition by α-amanitin, a similar reorganization of the PNC in the nucleus was observed for both hnRNP proteins (G–I) in spite of the fact that the majority of the CUG-BP/hNab50 protein becomes predominantly localized in the cytoplasm (H). Bar, 10 μm.
Examination of another hnRNP protein, CUG-BP/ hNab50, during transcription inhibition using monoclonal antibody 3B1 (Timchenko et al., 1996), which specifically recognizes CUG-BP/hNab50, revealed a similar reorganization of the PNC. However, in contrast to PTB, most of the soluble nucleoplasmic CUG-BP/hNab50 is localized to the cytoplasm when RNA polymerase II transcription is inhibited by α-amanitin. This finding suggests that CUG-BP/hNab50 may belong to the group of hnRNPs that shuttle between the nucleus and cytoplasm in a transcription-dependent manner (for review see Siomi and Dreyfuss, 1997). However, the fraction of CUG-BP/hNab50 that is present in the PNC remains in the nucleus and is colocalized with PTB in the structurally altered PNC (Fig. 4, G–I). In cells that do not contain a PNC, CUG-BP/hNab50 is not detected in the nucleus upon transcription inhibition (data not shown).
When cells in culture were treated for 2–5 h with actinomycin D at a concentration that either selectively inhibits RNA polymerase I (0.04 μg/ml) or all RNA polymerases (10 μg/ml) (Perry, 1963), the PNC was no longer detected by immunolabeling using monoclonal antibodies SH54 or 3B1 (data not shown). However, when cells were treated with cycloheximide (a protein synthesis inhibitor) at 100 μg/ml for up to 5 h, little change was observed in the structure of the PNC (data not shown). Since cycloheximide also inhibits RNA polymerase I activity by inhibiting the synthesis of necessary labile factors (Higashi et al., 1968; Willems et al., 1969; O'Keefe et al., 1994), the resistance to cycloheximide treatment suggests that the structural integrity of the PNC is not dependent on RNA polymerase I transcription or protein synthesis. This finding is consistent with the observation in permeabilized cells that the Br-UTP incorporation in the PNC does not reflect RNA polymerase I transcription. As previous studies showed that treatment with actinomycin D causes nucleolar fragmentation (Reynolds et al., 1964; Ochs et al., 1985), it is not clear whether the disappearance of the PNC after actinomycin D treatment is due to other effects of the drug rather than the inhibition of RNA polymerase I activity.
The PNC Contains Poly(A) RNA and Pre-mRNA Processing Factors
Incorporation of Br-UTP together with the presence of RNA transcripts and hnRNP proteins in the PNC suggest that the PNC is involved in RNA metabolism. To further investigate the function of the PNC, we examined it for the presence of polyadenylated RNA, pre-mRNA splicing factors, and a 3′ end processing factor. In situ hybridization in HeLa cells using a biotinylated oligo (dT)50 probe (Huang et al., 1994) revealed that poly(A) RNA is present in the PNC in addition to being distributed throughout the nucleus in a diffuse and speckled pattern (Fig. 5, top row). The labeling of the poly(A) RNA in some PNCs is at a similar intensity as the larger nuclear speckles. In other PNCs, the labeling intensity of poly(A) RNA is indistinguishable from the diffuse nuclear labeling (data not shown).
Figure 5.
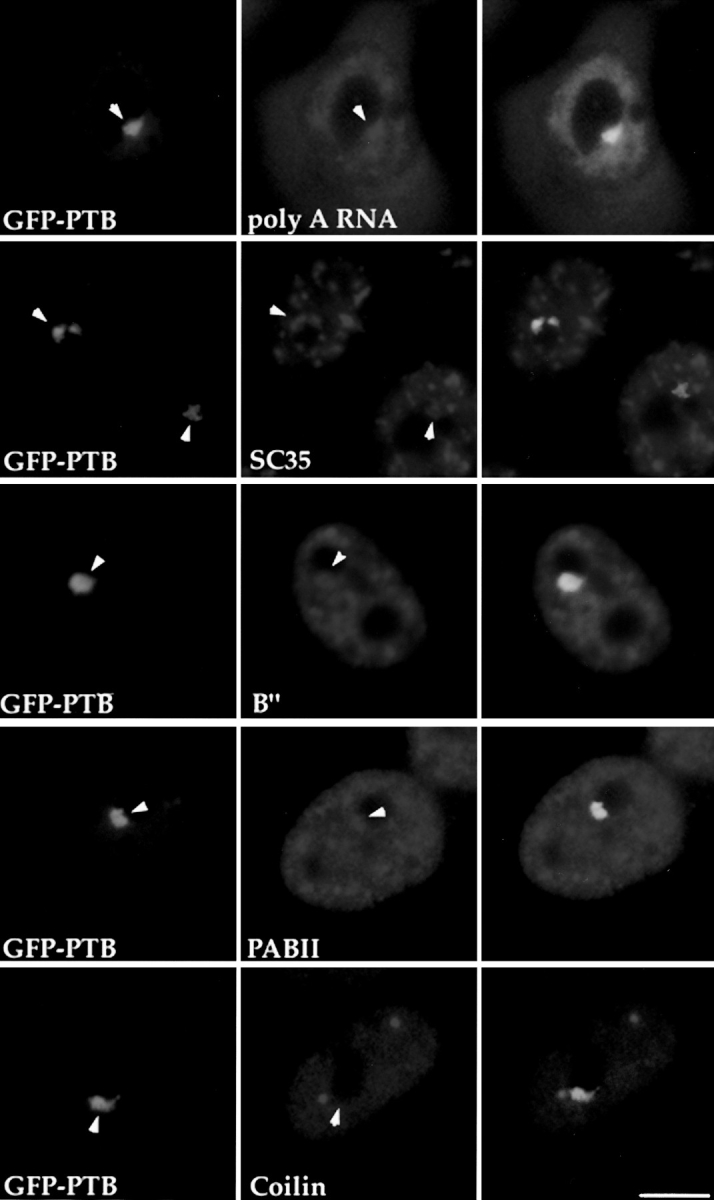
Pre-mRNA processing factors are localized in the PNC. HeLa cells transiently expressing GFP–PTB (left column, arrows indicating the PNC) were immunolabeled with antibodies specifically recognizing several pre-mRNA processing factors as indicated (middle column, arrows showing the same nuclear regions containing these factors). The right column shows the colocalization of the PNC and the RNA processing factors. P80-coilin (bottom row), as a negative control, is not detected in the PNC. Bar, 10 μm.
Immunolabeling of pre-mRNA splicing factors including U2 snRNPs (Habets et al., 1987, 1989), SC35 (Fu and Maniatis, 1990), and a 3′ end processing factor, PAB II (Krause et al., 1994), showed that these factors are present in the PNC in addition to their previously characterized sites of localization (Fig. 5). However, the labeling intensity varies among PNCs. To exclude the possibility that the labeling in the PNC is simply due to the diffuse nuclear staining of these factors, we examined the localization of another diffusely distributed nuclear component, p80 coilin. Coilin, an 80-kD protein, is diffusely distributed in the nucleus in addition to being present in coiled bodies (for reviews see Brasch and Ochs, 1992; Lamond and Carmo-Fonseca, 1993; Gall et al., 1995; Roth, 1995). When coilin was localized with anticoilin antibody (Andrade et al., 1993), no staining of PNCs was observed (Fig. 5, bottom row). The presence of RNA processing factors and poly(A) RNA in the PNC is consistent with the idea that the PNC is a site of transcription and that RNA polymerase II is one of the transcription systems involved. Furthermore, these findings suggest that posttranscriptional processing of certain transcripts may also occur in the PNC.
PTB Turns Over Rapidly in the PNC
To understand the dynamics of the hnRNP proteins in the PNC, we used FRAP to examine the turnover of GFP– PTB in the PNC (see Materials and Methods). Cells transfected with the GFP–PTB construct were examined using a confocal laser-scanning microscope (Carl Zeiss, Inc., Thornwood, NY). Cells with two PNCs were chosen so that one of them served as a marker to monitor the specificity of bleaching and the stability of the focal plane. An initial image was captured before photobleaching. The PNC was photobleached for 20 s by exposure to the 488-nm laser line using the line scanning mode. An averaged image was obtained immediately after photobleaching followed by a series of images at different time points (Fig. 6, top and middle rows). The photobleaching resulted in a nearly complete loss of the GFP–PTB labeling in the targeted PNC. The bleaching was specific since the neighboring PNC, which was separated by a few micrometers, was not affected. 25 s after the bleaching, the GFP–PTB began to reappear in the PNC, and the fluorescent intensity in the PNC recovered to a similar level as before the bleaching process within 5 min. The rapid recovery suggests that the unbleached GFP–PTB in the nucleoplasm quickly enters the PNC. Moreover, the quantitatively large degree of recovery indicates that GFP–PTB is highly mobile in the PNC and that there is not a large immobile fraction of the protein. To exclude the possibility that the recovery of the GFP–PTB labeling in the PNC is due to a spontaneous fluorescence recovery of GFP–PTB, fixed cells expressing GFP–PTB were examined by FRAP in an identical manner (Fig. 6, bottom row). Again, a complete photobleaching of the labeled PNC was achieved without affecting an adjacent PNC. However, recovery of the fluorescence in the bleached PNC was not observed up to 30 min. The result of the control experiment confirmed that the fluorescence recovery observed in living cells is most likely due to the rapid turnover of PTB in the PNC. The rapid turnover of this hnRNP protein in the PNC is consistent with the dynamic nature of a transcription site with regard to RNA synthesis and subsequent transport of processed product away from the transcription site.
The PNC Is Associated with an Insoluble Fraction of the Nucleus
To determine the solubility of the PNC, HeLa cells were first extracted with 0.5% Triton X-100, followed by treatment with 250 mM ammonium sulfate and subsequent DNase I digestion (Fey et al., 1986). Cells were then fixed and immunostained with SH54, a previously characterized monoclonal antibody that specifically recognizes PTB in the PNC (Huang et al., 1997), and human antifibrillarin antibody (Sigma Chemical Co.) (Ochs et al., 1985), which marks the nucleolus. PTB in the PNC remains detectable at the periphery of the nucleolus after the extraction procedure (Fig. 7 A, arrowhead). The PNCs in these preparations appear to be more rounded and smaller in size than in unextracted cells. In contrast to the pool of PTB in the PNC, the diffusely localized nucleoplasmic pool of PTB decreased significantly upon extraction, concomitant with an increase in the staining of the protein in the nucleolus (Fig. 7 A), which was identified by immunolabeling with the antifibrillarin antibody (Fig. 7 B). To examine whether PTB in the nucleolus is derived from the PNC, Detroit 551 cells (normal human fibroblasts), only 2% of which display visible PNCs, were prepared using the same protocol. A similar relocation of nucleoplasmic PTB to the nucleolus was observed in all of the cells in spite of the absence of the PNC in these cells (Fig. 7 D). The simplest explanation is that the extraction leads to the release of PTB from binding to components that are responsible for its diffuse nuclear distribution pattern, and it is then relocated into the nucleoli. These observations demonstrate that the PNC-associated PTB represents a less soluble fraction than the diffuse nuclear population of this protein.
Figure 7.
The PNC is associated with the less soluble fraction of the nucleus. PTB labeling of the PNC is maintained after an extraction using detergent, high salt, and DNAse treatment (A, arrowheads). In contrast, the majority of the diffuse nuclear PTB labeling decreases significantly accompanied by an increased labeling of PTB in the nucleolus (A). Nucleoli labeled with an antibody specifically recognizing fibrillarin do not show a significant change (B). DNA in the extracted cells is no longer detectable when stained with DAPI (C and F). PTB in Detroit 551 cells that do not contain a PNC also show a similar relocalization of the PTB protein (D) into the nucleolus (marked by immunolabeling of fibrillarin in E) upon extraction. Bar, 10 μm.
Discussion
In the present study, we have shown that the PNC actively incorporates Br-UTP and FITC-CTP in a transcription assay using permeabilized cells. Many studies have used this assay to investigate transcription patterns in the cell nucleus (Hozak et al., 1994; Aoki et al., 1997; Fay et al., 1997; Neugebauer and Roth, 1997) since its initial establishment (Jackson et al., 1993; Wansink et al., 1993). Our observation that the PNC actively incorporates labeled nucleotides in a DNA-dependent manner after 5 min of pulse labeling suggests that the PNC is a site of transcription. Inhibition of RNA polymerase I either by addition of actinomycin D in the transcription cocktail or by pretreatment of cells with cycloheximide does not affect the Br-UTP incorporation, indicating that transcription of nascent RNA in the PNC is unlikely to involve RNA polymerase I. This finding is consistent with the report by Matera et al. (1995) that 28S rRNA was not detected in the PNC using in situ hybridization and our previous findings that fibrillarin, which is required for rRNA processing, is not present in the PNC (Huang et al., 1997). RNA polymerases II and/or III may be responsible for the transcription occurring in the PNC, although it is not clear at the present time whether both polymerases are involved. Consistent with this suggestion are the findings that poly(A) RNA and small RNAs transcribed by RNA polymerase III, including hY RNAs, RNase MRP RNA, and RNase P RNA (Matera et al., 1995), are detected in the PNC. Furthermore, immunocytochemical labeling revealed that RNA processing factors, including snRNPs, SC35, and PAB II, are present in the PNC. SnRNPs and SC35 primarily participate in the processing of RNA polymerase II transcripts, and PAB II binds to polyadenylated RNA transcribed by both RNA polymerase II and III. It has become increasingly evident from morphological and biochemical studies that RNA transcription and processing are spatially and temporally linked (Beyer et al., 1980; Beyer and Osheim, 1988; Jiménez-García and Spector, 1993; Xing and Lawrence, 1993; Zhang et al., 1994; Bauren et al., 1996; Huang and Spector, 1996; Du and Warren, 1997; Kim et al., 1997; McCracken et al., 1997; Misteli et al., 1997; Zeng et al., 1997). The presence of RNA processing factors in the PNC supports the notion that the PNC is a site of transcription and suggests that it may also be involved in RNA processing. However, we cannot exclude the possibility that the rapid accumulation of newly synthesized RNA transcripts in the PNC may be the result of transcription elsewhere in the nucleus and the subsequent very rapid translocation of these transcripts to the PNC. As described in a previous study by Matera et al. (1995), the chromosome segment that encodes two of the hY RNAs only showed an occasional association with the PNC. In addition, the La protein known to be at least transiently associated with newly synthesized RNA polymerase III transcripts was not detected in the PNC using immunocytochemical labeling (Matera et al., 1995). Very recently, two other nuclear bodies were found to incorporate tagged nucleotides after a short pulse labeling (LaMorte et al., 1998; Pombo et al., 1998). Promyelocytic leukemia (PML) bodies and coiled bodies rapidly accumulate FITC-UTP upon microinjection into cells, and PML bodies were also shown to contain a transcription factor, cAMP-regulated enhancer binding protein (CREB) binding protein (LaMorte et al., 1998). In addition, the polymorphic interphase karyosomal association (PIKA) domain was shown to be associated with specific chromosomes and to also be active in transcription (Pombo et al., 1998). These observations, together with our finding that the PNC accumulates nascent RNA, suggest that transcription may be spatially regulated in subnuclear compartments. Further studies will identify transcripts that are synthesized and/or processed in the PNC.
Based upon the extraction behavior and structural alterations during transcription inhibition, two populations of the hnRNP proteins PTB and CUG-BP/hNab50 were identified in the HeLa cell nucleus. One population is diffusely distributed throughout the nucleoplasm, and the other population is enriched in the PNC with a much higher fluorescent intensity, implying a higher protein concentration. The PNC-associated hnRNP proteins are linked with the less soluble fraction of the nucleus and remain associated with the structurally altered PNC during transcription inhibition, whereas the diffusely distributed nuclear populations are removed by the extraction, and CUG-BP/hNab50 is relocated to the cytoplasm during transcription inhibition. These differences suggest that the two populations of hnRNP proteins, the PNC-associated and the diffuse nucleoplasmic, each may interact with a different set of cellular constituents. Since the PNC is predominantly observed in cells with a transformed phenotype, we speculate that the PNC-associated hnRNP proteins may be complexed with cellular components that are altered in terms of their expression or modification in transformed cells. Future studies will focus on the isolation and identification of these constituents.
An increasing number of nuclear bodies or inclusions have been described in recent years from cells or tissues that have altered physiology or are derived from various disease-related samples. For example, coiled bodies, round and electron-dense structures, are often observed in transformed and cancer cell lines (Spector et al., 1992; Ochs et al., 1994). Coiled bodies contain snRNPs, U3 snoRNP, fibrillarin, NOPP140, and p80-coilin (for reviews see Lamond and Carmo-Fonseca, 1993; Gall et al., 1995; Roth, 1995). Several snRNA genes (U1, U2, and U3) are localized adjacent to coiled bodies in a percentage of cases (Frey and Matera, 1995; Smith et al., 1995; Gao et al., 1997). Recently, LaMorte et al. (1998) have localized nascent RNA in coiled bodies, and a transcription factor, TFIIH, was also detected in coiled bodies (Jordan and Carmo-Fonseca, 1997). However, the exact function of coiled bodies remains unknown. A second nuclear structure, “gems” (gemini of coiled body), was shown to be frequently associated with coiled bodies (Liu and Dreyfuss, 1996). Gems contain the survival of motor neurons (SMN) protein that is encoded by a gene responsible for spinal muscular atrophy (Liu et al., 1997). The SMN protein was shown to interact with snRNPs that are involved in pre-mRNA splicing and with a cellular protein called SIP1 through a separate interaction (Liu et al., 1997). The SMN–SIP1 complex was shown to be involved in snRNP biogenesis (Fischer et al., 1997; Liu et al., 1997). PML bodies, also named ND10 (nuclear domain 10) and POD (PML oncogenic domains), change their number and size in acute promyleocytic leukemia (Ascoli and Maul, 1991; Dyck et al., 1994; Koken et al., 1994, 1995; Weis et al., 1994; Terris et al., 1995; for review see Doucas and Evans, 1996). Generally, each cell nucleus contains ∼10–20 PML bodies ranging from 0.3 to 1 μm in diameter. In acute promyleocytic leukemia cells, PML bodies break up into a large number of microparticulates (Dyck et al., 1994). Recently, LaMorte et al. (1998) have localized nascent RNA polymerase II transcripts and CREB binding protein to a subset of PML bodies, suggesting that at least some PML bodies are involved in transcription. Nuclear inclusions, containing Huntington protein, are found in neurons involved in Huntington's Disease (Davies et al., 1997). Patients with Huntington's Disease have an expanded NH2-terminal polyglutamine region in Huntington, and its length influences the extent of Huntington accumulation within the intranuclear inclusions (DiFiglia et al., 1997). The presence of ubiquitin in these structures suggests that the abnormal Huntington is targeted for proteolysis (DiFiglia et al., 1997). Another recent example of a disease-related nuclear inclusion is the change in the subnuclear localization of the ataxin-1 protein, which is associated with the neurodegenerative disorder, Spinocerebellar ataxia type I. The disease-related mutant ataxin-1, with an expanded polyglutamine tract, localizes to a single ∼2-μm structure compared with the normal ataxin-1 protein, which localizes to several 0.5-μm nuclear structures (Skinner et al., 1997). Ataxin-1 interacts with a cerebellar leucine-rich acidic nuclear protein in a manner such that the affinity of the association depends upon the number of glutamines in mutant ataxin-1 (Matilla et al., 1997). All of these examples demonstrate that nuclear structure is altered in response to changes in cellular physiology and/or pathology of cells or tissues. These changes may reflect changes of nuclear function and/or regulation. Although many nuclear bodies and inclusions have been described morphologically, the functional relationship between many of these structural changes and the respective diseases that they are associated with are not clear. Our finding that the PNC is potentially involved in transcription gives us an important clue to further address the molecular basis of the PNC and its relationship with the transformed phenotype.
Acknowledgments
We would like to thank Tamara Howard for her enthusiastic technical assistance. We are very grateful to Dr. Maurice S. Swanson for the 3B1 antibody.
Abbreviations used in this paper
- DRB
5,6-dichloro-1-β-d-ribofuranosylbenzimidazole
- FRAP
fluorescence recovery after photobleaching
- GFP
green fluorescent protein
- hnRNP
heterogeneous ribonucleoprotein
- PAB II
poly(A) binding protein II
- PML
promyleocytic leukemia
- PNC
perinucleolar compartment
- PTB
polypyrimidine binding protein
- SMN
survival of motor neurons
Footnotes
This study is supported by a grant from the National Institute of General Medical Sciences (GM42694) to D.L. Spector and a grant from the National Cancer Institute (K01 CA74988-02) to S. Huang.
Address all correspondence to Sui Huang, Department of Cell and Molecular Biology, Northwestern University Medical School, 303 E. Chicago Ave., Chicago, IL 60611. Tel.: (312) 503-4269. Fax: (312) 503-7912. E-mail: s-huang2@nwu.edu
References
- Altman S. Ribonuclease P. J Biol Chem. 1990;265:20053–20056. [PubMed] [Google Scholar]
- Anderson JT, Wilson SM, Datar KV, Swanson MS. NAB2: a yeast nuclear polyadenylated RNA-binding protein essential for cell viability. Mol Cell Biol. 1993;13:2730–2741. doi: 10.1128/mcb.13.5.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade LEC, Tan EM, Chan EKL. Immunocytochemical analysis of the coiled body in the cell cycle and during cell proliferation. Proc Natl Acad Sci USA. 1993;90:1947–1951. doi: 10.1073/pnas.90.5.1947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki F, Worrad DM, Schultz RM. Regulation of transcriptional activity during the first and second cell cycles in the preimplantation mouse embryo. Dev Biol. 1997;181:296–307. doi: 10.1006/dbio.1996.8466. [DOI] [PubMed] [Google Scholar]
- Ascoli AC, Maul GG. Identification of a novel nuclear domain. J Cell Biol. 1991;112:785–795. doi: 10.1083/jcb.112.5.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashiya M, Grabowski PJ. A neuron-specific splicing switch mediated by an array of pre-mRNA repressor sites: evidence of a regulatory role for the polypyrimidine tract binding protein and a brain-specific PTB counterpart. RNA. 1997;3:996–1015. [PMC free article] [PubMed] [Google Scholar]
- Bauren G, Jiang WQ, Bernholm K, Gu F, Wieslander L. Demonstration of a dynamic, transcription-dependent organization of pre-mRNA splicing factors in polytene nuclei. J Cell Biol. 1996;133:929–941. doi: 10.1083/jcb.133.5.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer AL, Osheim YN. Splice site selection, rate of splicing, and alternative splicing on nascent transcripts. Genes Dev. 1988;2:754–765. doi: 10.1101/gad.2.6.754. [DOI] [PubMed] [Google Scholar]
- Beyer AL, Miller OL, McKnight SL. Ribonucleoprotein structure in nascent hnRNA is nonrandom and sequence-dependent. Cell. 1980;20:75–84. doi: 10.1016/0092-8674(80)90236-6. [DOI] [PubMed] [Google Scholar]
- Brasch K, Ochs RL. Nuclear bodies (NBs): a newly “rediscovered” organelle. Exp Cell Res. 1992;202:211–223. doi: 10.1016/0014-4827(92)90068-j. [DOI] [PubMed] [Google Scholar]
- Clayton DA. A nuclear function for RNase MRP. Proc Natl Acad Sci USA. 1994;91:4615–4617. doi: 10.1073/pnas.91.11.4615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darr SC, Brown JW, Pace NR. The varieties of RNase P. Trends Biol Sci. 1992;17:178–182. doi: 10.1016/0968-0004(92)90262-8. [DOI] [PubMed] [Google Scholar]
- Davies SW, Turmaine M, Cozens BA, DiFiglia M, Sharp AH, Ross CA. Formation of neuronal intranuclear inclusions underlies the neurological dysfunction in mice transgenic for the HD mutation. Cell. 1997;90:537–548. doi: 10.1016/s0092-8674(00)80513-9. [DOI] [PubMed] [Google Scholar]
- DiFiglia M, Sapp E, Chase KO, Davies SW, Bates GP. Aggregation of huntingtin in neuronal intranuclear inclusions and dystrophic neurites in brain. Science. 1997;277:1990–1993. doi: 10.1126/science.277.5334.1990. [DOI] [PubMed] [Google Scholar]
- Doucas V, Evans RM. The PML nuclear compartment and cancer. Biochim Biophys Acta. 1996;1288:M25–M29. doi: 10.1016/s0304-419x(96)00028-5. [DOI] [PubMed] [Google Scholar]
- Du L, Warren SL. A functional interaction between the carboxy-terminal domain of RNA polymerase II and pre-mRNA splicing. J Cell Biol. 1997;136:5–18. doi: 10.1083/jcb.136.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyck JA, Maul GG, Miller WH, Jr, Chen JD, Kakizuka A, Evans RM. A novel macromolecular structure is a target of the promyelocyte-retinoic acid receptor oncoprotein. Cell. 1994;76:333–343. doi: 10.1016/0092-8674(94)90340-9. [DOI] [PubMed] [Google Scholar]
- Fay FS, Taneja KL, Shenoy S, Lifshitz L, Singer RH. Quantitative digital analysis of diffuse and concentrated nuclear distributions of nascent transcripts, SC35 and poly(A) Exp Cell Res. 1997;231:27–37. doi: 10.1006/excr.1996.3460. [DOI] [PubMed] [Google Scholar]
- Fey EG, Krochmalnic G, Penman S. The nonchromatin substructures of the nucleus: the ribonucleoprotein (RNP)-containing and RNP-depleted matrices analyzed by sequential fractionation and resinless section electron microscopy. J Cell Biol. 1986;102:1654–1665. doi: 10.1083/jcb.102.5.1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer U, Liu Q, Dreyfuss G. The SMN-SIP1 complex has an essential role in spliceosomal snRNP biogenesis. Cell. 1997;90:1023–1029. doi: 10.1016/s0092-8674(00)80368-2. [DOI] [PubMed] [Google Scholar]
- Frey MR, Matera AG. Coiled bodies contain U7 small nuclear RNA and associate with specific DNA sequences in interphase human cells. Proc Natl Acad Sci USA. 1995;92:5915–5919. doi: 10.1073/pnas.92.13.5915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X-D, Maniatis T. Factor required for mammalian spliceosome assembly is localized to discrete regions in the nucleus. Nature. 1990;343:437–441. doi: 10.1038/343437a0. [DOI] [PubMed] [Google Scholar]
- Gall JG, Tsvetkov A, Wu Z, Murphy C. Is the sphere organelle/ coiled body a universal nuclear component? . Dev Genet. 1995;16:25–35. doi: 10.1002/dvg.1020160107. [DOI] [PubMed] [Google Scholar]
- Gao L, Frey MR, Matera AG. Human genes encoding U3 snRNA associate with coiled bodies in interphase cells and are clustered on chromosome 17p11.2 in a complex inverted repeat structure. Nucleic Acids Res. 1997;25:4740–4747. doi: 10.1093/nar/25.23.4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghetti A, Piñol-Roma S, Michael WM, Morandi C, Dreyfuss G. hnRNP I, the polypyrimidine tract-binding protein: distinct nuclear localization and association with hnRNAs. Nucleic Acids Res. 1992;20:3671–3678. doi: 10.1093/nar/20.14.3671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habets WJ, Hoet MH, De Jong BAW, Van der Kemp A, van Venrooij WJ. Mapping of B cell epitopes on small nuclear ribonucleoproteins that react with human autoantibodies as well as with experimentally induced mouse monoclonal antibodies. J Immunol. 1989;143:2560–2566. [PubMed] [Google Scholar]
- Habets WJ, Sillekens PTG, Hoet MH, Schalken JA, Roebroek AJM, Leunissen JAM, van de Ven WJM. Analysis of a cDNA clone expressing a human autoimmune antigen: full-length sequence of the U2 small nuclear RNA-associated B″ antigen. Proc Natl Acad Sci USA. 1987;84:2421–2425. doi: 10.1073/pnas.84.8.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto C, Steitz JA. Sequential association of nucleolar 7-2 RNA with two different autoantigens. J Biol Chem. 1983;258:1379–1382. [PubMed] [Google Scholar]
- Hellen CU, Pestova TV, Litterst M, Wimmer E. The cellular polypeptide p57 (pyrimidine tract-binding protein) binds to multiple sites in the poliovirus 5′ nontranslated region. J Virol. 1994;68:941–950. doi: 10.1128/jvi.68.2.941-950.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hessler D, Young SJ, Carragher BO, Martone ME, Lamont S, Whittaker M, Milligan RA, Masliah E, Hinshaw JE, Ellisman MH. Programs for visualization in three-dimensional microscopy. Neuroimage. 1992;1:55–67. doi: 10.1016/1053-8119(92)90007-a. [DOI] [PubMed] [Google Scholar]
- Higashi K, Matsuhisa T, Kitao A, Sakamoto Y. Selective suppression of nucleolar RNA metabolism in the absence of protein synthesis. Biochim Biophys Acta. 1968;166:388–393. doi: 10.1016/0005-2787(68)90226-8. [DOI] [PubMed] [Google Scholar]
- Hozak P, Cook PR, Schofer C, Mosgoller W, Wachtler F. Site of transcription of ribosomal RNA and intranucleolar structure in HeLa cells. J Cell Sci. 1994;19:109–113. doi: 10.1242/jcs.107.2.639. [DOI] [PubMed] [Google Scholar]
- Huang S, Spector DL. Intron-dependent recruitment of pre-mRNA splicing factors to sites of transcription. J Cell Biol. 1996;133:719–732. doi: 10.1083/jcb.133.4.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Deerinck T, Ellisman MH, Spector DL. In vivo analysis of the stability and transport of nuclear poly(A)+ RNA. J Cell Biol. 1994;126:877–899. doi: 10.1083/jcb.126.4.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Deerinck T, Ellisman MH, Spector DL. The dynamic organization of the perinucleolar compartment in the mammalian cell nucleus. J Cell Biol. 1997;137:965–974. doi: 10.1083/jcb.137.5.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson DA, Hassan AB, Errington RJ, Cook PR. Visualization of focal sites of transcription within human nuclei. EMBO (Eur Mol Biol Organ) J. 1993;12:1059–1065. doi: 10.1002/j.1460-2075.1993.tb05747.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson MR, Gao LG, Wang YL, Pederson T. Dynamic localization of RNase MRP RNA in the nucleolus observed by fluorescent RNA cytochemistry in living cells. J Cell Biol. 1995;131:1649–1659. doi: 10.1083/jcb.131.6.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson MR, Gao LG, Taneja K, Singer RH, Wang YL, Pederson T. Nuclear domains of the RNA subunit of RNase P. J Cell Sci. 1997;110:829–837. doi: 10.1242/jcs.110.7.829. [DOI] [PubMed] [Google Scholar]
- Jiménez-García LF, Spector DL. In vivo evidence that transcription and splicing are coordinated by a recruiting mechanism. Cell. 1993;73:47–59. doi: 10.1016/0092-8674(93)90159-n. [DOI] [PubMed] [Google Scholar]
- Jordan PC, Carmo-Fonseca M. The cdk7-cyclin H-MAT1 complex associated with TFIIH is localized in coiled bodies. Mol Biol Cell. 1997;8:1207–1217. doi: 10.1091/mbc.8.7.1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski A, Hunt SL, Patton JG, Jackson RJ. Direct evidence that polypyrimidine tract binding protein (PTB) is essential for internal initiation of translation of encephalomyocarditis virus RNA. RNA. 1995;1:924–938. [PMC free article] [PubMed] [Google Scholar]
- Kedinger C, Gniazdowski JL, Mandel JL, Jr, Gissinger F, Chambon P. α-Amanitin: a specific inhibitor of one of two DNA-dependent RNA polymerase activities from calf thymus. Biochem Biophys Res Commun. 1970;38:165–171. doi: 10.1016/0006-291x(70)91099-5. [DOI] [PubMed] [Google Scholar]
- Kim E, Du L, Bregman DB, Warren SL. Splicing factors associate with hyperphosphorylated RNA polymerase II in the absence of pre-mRNA. J Cell Biol. 1997;136:19–28. doi: 10.1083/jcb.136.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koken MH, Puvion-Dutilleul F, Guillemin MC, Viron A, Linares-Cruz G, Stuurman N, de Jong L, Szostecki C, Caivo F, Chomienne C. The t(15:17) translocation alters a nuclear body in a retinoic acid-reversible fashion. EMBO (Eur Mol Biol Organ) J. 1994;13:1073–1083. doi: 10.1002/j.1460-2075.1994.tb06356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koken MH, Linares CG, Quignon F, Viron A, Chelbi AM, Sobczak-Thepot J, Juhlin L, Degos L, Calvo F, de-The H. The PML growth-suppressor has an altered expression in human oncogenesis. Oncogene. 1995;10:1315–1324. [PubMed] [Google Scholar]
- Krause S, Fakan S, Weis K, Wahle E. Immunodetection of poly(A) binding protein II in the cell nucleus. Exp Cell Res. 1994;214:75–82. doi: 10.1006/excr.1994.1235. [DOI] [PubMed] [Google Scholar]
- Lamond AI, Carmo-Fonseca M. The coiled body. Trends Cell Biol. 1993;3:198–204. doi: 10.1016/0962-8924(93)90214-l. [DOI] [PubMed] [Google Scholar]
- LaMorte VJ, Dyck JA, Ochs RL, Evans RM. Localization of nascent RNA and CREB binding protein with the PML-containing nuclear body. Proc Natl Acad Sci USA. 1998;95:4991–4996. doi: 10.1073/pnas.95.9.4991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B, Matera AG, Ward DC, Craft J. Association of RNase mitochondrial RNA processing enzyme with ribonuclease P in higher ordered structures in the nucleolus: a possible coordinate role in ribosome biogenesis. Proc Natl Acad Sci USA. 1996;93:11471–11476. doi: 10.1073/pnas.93.21.11471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner MR, Steitz JA. Antibodies to small nuclear RNAs complexed with proteins are produced by patients with systemic lupus erythematosus. Proc Natl Acad Sci USA. 1979;76:5495–5499. doi: 10.1073/pnas.76.11.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin CH, Patton JG. Regulation of alternative 3′ splice site selection by constitutive splicing factors. RNA. 1995;1:234–245. [PMC free article] [PubMed] [Google Scholar]
- Lindell TJ, Weinberg F, Morries PW, Roeder RG, Rutter WJ. Specific inhibition of nuclear RNA polymerase II by α-amanitin. Science. 1970;170:447–449. doi: 10.1126/science.170.3956.447. [DOI] [PubMed] [Google Scholar]
- Liu Q, Dreyfuss G. A novel nuclear structure containing the survival of motor neurons protein. EMBO (Eur Mol Biol Organ) J. 1996;15:3555–3565. [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Fischer U, Wang F, Dreyfuss G. The spinal muscular atrophy disease gene product SMN, and its associated protein SIP1 are in a complex with spliceosomal snRNP proteins. Cell. 1997;90:1013–1021. doi: 10.1016/s0092-8674(00)80367-0. [DOI] [PubMed] [Google Scholar]
- Lou H, Gagel RF, Berget SM. An intron enhancer recognized by splicing factors activates polyadenylation. Genes Dev. 1996;10:208–219. doi: 10.1101/gad.10.2.208. [DOI] [PubMed] [Google Scholar]
- Matera AG, Ward DC. Nucleoplasmic organization of small nuclear ribonucleoproteins in cultured human cells. J Cell Biol. 1993;121:715–727. doi: 10.1083/jcb.121.4.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matera AG, Frey MR, Margelot K, Wolin SL. A perinucleolar compartment contains several RNA polymerase III transcripts as well as the polypyrimidine tract-binding protein, hnRNP I. J Cell Biol. 1995;129:1181–1193. doi: 10.1083/jcb.129.5.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matilla A, Koshy BT, Cummings CJ, Isobe T, Orr HT. The cerebellar leucine-rich acidic nuclear protein interacts with ataxin-1. Nature. 1997;389:974–978. doi: 10.1038/40159. [DOI] [PubMed] [Google Scholar]
- McCracken S, Fong N, Yankulov K, Ballantyne S, Pan G, Greenblatt J, Patterson SD, Wickens M, Bentley DL. The C-terminal domain of RNA polymerase II couples mRNA processing to transcription. Nature. 1997;385:357–361. doi: 10.1038/385357a0. [DOI] [PubMed] [Google Scholar]
- Misteli T, Cáceres JF, Spector DL. The dynamics of a pre-mRNA splicing factor in living cells. Nature. 1997;387:523–527. doi: 10.1038/387523a0. [DOI] [PubMed] [Google Scholar]
- Neugebauer KM, Roth MB. Distribution of pre-mRNA splicing factors at sites of RNA polymerase II transcription. Genes Dev. 1997;11:1148–1159. doi: 10.1101/gad.11.9.1148. [DOI] [PubMed] [Google Scholar]
- Ochs RL, Lischwe MA, Spohn WH, Busch H. Fibrillarin: a new protein of the nucleolus identified by autoimmune sera. Biol Cell. 1985;54:123–134. doi: 10.1111/j.1768-322x.1985.tb00387.x. [DOI] [PubMed] [Google Scholar]
- Ochs RL, Stein TWJ, Tan EM. Coiled bodies in the nucleolus of breast cancer cells. J Cell Sci. 1994;107:385–399. doi: 10.1242/jcs.107.2.385. [DOI] [PubMed] [Google Scholar]
- O'Keefe RT, Mayeda A, Sadowski CL, Krainer AR, Spector DL. Disruption of pre-mRNA splicing in vivo results in reorganization of splicing factors. J Cell Biol. 1994;124:249–260. doi: 10.1083/jcb.124.3.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton JG, Porro EB, Galceran J, Tempst P, Nadal-Ginard B. Cloning and characterization of PSF, a novel pre-mRNA splicing factor. Genes Dev. 1993;7:393–406. doi: 10.1101/gad.7.3.393. [DOI] [PubMed] [Google Scholar]
- Perez I, Lin CH, McAfee JG, Patton JG. Mutation of PTB binding sites causes misregulation of alternative 3′ splice site selection in vivo. RNA. 1997;3:764–778. [PMC free article] [PubMed] [Google Scholar]
- Perry RP. Selective effects of actinomycin D on the intracellular distribution of RNA synthesis in tissue culture cells. Exp Cell Res. 1963;29:400–406. [Google Scholar]
- Pombo A, Cuello P, Schul W, Yoon J-B, Roeder RG, Cook PR, Murphy S. Regional and temporal specialization in the nucleus: a transcriptionally-active nuclear domain rich in PTF, Oct1 and PIKA antigens associates with specific chromosomes early in the cell cycle. EMBO (Eur Mol Biol Organ) J. 1998;6:1768–1778. doi: 10.1093/emboj/17.6.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds RC, Montgomery POB, Hughes B. Nucleolar “caps” produced by actinomycin D. Cancer Res. 1964;24:1269–1277. [PubMed] [Google Scholar]
- Robert R, Timchenko NA, Miller JW, Reddy S, Caskey CT, Swanson MS, Timchenko LT. Altered phosphorylation and intracellular distribution of a (CUG)n triplet repeat RNA-binding protein in patients with myotonic dystrophy and in myotonin protein kinase knockout mice. Proc Natl Acad Sci USA. 1997;94:13221–13226. doi: 10.1073/pnas.94.24.13221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth MB. Spheres, coiled bodies and nuclear bodies. Curr Opin Cell Biol. 1995;7:325–328. doi: 10.1016/0955-0674(95)80086-7. [DOI] [PubMed] [Google Scholar]
- Sehgal PB, Darnell JE, Tamm I. The inhibition by DRB (5,6-Dichloro-1-β-d-ribofuranosylbenzimidazole) of hnRNA and mRNA production in HeLa cells. Cell. 1976;9:473–480. doi: 10.1016/0092-8674(76)90092-1. [DOI] [PubMed] [Google Scholar]
- Singh R, Valcarcel J, Green MR. Distinct binding specificities and function of higher eukaryotic polypyrimidine tract-binding proteins. Science. 1995;268:1173–1176. doi: 10.1126/science.7761834. [DOI] [PubMed] [Google Scholar]
- Siomi H, Dreyfuss G. RNA-binding proteins as regulators of gene expression. Curr Opin Genet Dev. 1997;7:345–353. doi: 10.1016/s0959-437x(97)80148-7. [DOI] [PubMed] [Google Scholar]
- Skinner PJ, Koshy BT, Cummings CJ, Klement IA, Helin K. Ataxin-1 with an expanded glutamine tract alters nuclear matrix-associated structures. Nature. 1997;389:971–974. doi: 10.1038/40153. [DOI] [PubMed] [Google Scholar]
- Smith KP, Carter KC, Johnson CV, Lawrence JB. U2 and U1 snRNA gene loci associate with coiled bodies. J Cell Biochem. 1995;59:473–485. doi: 10.1002/jcb.240590408. [DOI] [PubMed] [Google Scholar]
- Spector DL, Lark G, Huang S. Differences in snRNP localization between transformed and nontransformed cells. Mol Biol Cell. 1992;3:555–569. doi: 10.1091/mbc.3.5.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector, D.L., R.D. Goldman, and L.A. Leinwand. 1997. Cells: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 2,100 pp.
- Stripe F, Fiume L. Studies on the pathogenesis of liver necrosis by α-amanitin. Effect of α-amanitin on ribonucleic acid synthesis and on ribonucleic acid polymerase in mouse liver nuclei. Biochem J. 1967;105:779–782. doi: 10.1042/bj1050779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terris B, Baldin V, Dubois S, Degott C, Flejou JF, Henin D, Dejean A. PML nuclear bodies are general targets for inflammation and cell proliferation. Cancer Res. 1995;55:1590–1597. [PubMed] [Google Scholar]
- Timchenko LT, Miller JW, Timchenko NA, DeVore DR, Datar KV, Lin L, Roberts R, Caskey CT, Swanson MS. Identification of a (CUG)n triplet repeat RNA-binding protein and its expression in myotonic dystrophy. Nucleic Acids Res. 1996;24:4407–4414. doi: 10.1093/nar/24.22.4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wansink DG, Schul W, van der Kraan I, van Steensel B, van Driel R, de Jong L. Fluorescent labeling of nascent RNA reveals transcription by RNA polymerase II in domains scattered throughout the nucleus. J Cell Biol. 1993;122:283–293. doi: 10.1083/jcb.122.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinmann R, Roeder RG. Role of DNA-dependent RNA polymerase 3 in the transcription of the tRNA and 5S RNA genes. Proc Natl Acad Sci USA. 1974;71:1790–1794. doi: 10.1073/pnas.71.5.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinmann R, Raskas HJ, Roeder RG. The transcriptional role of host DNA-dependent RNA polymerases in adenovirus-infected KB cells. Cold Spring Harbor Symp Quant Biol. 1975;34:495–500. doi: 10.1101/sqb.1974.039.01.061. [DOI] [PubMed] [Google Scholar]
- Weis K, Rambaud S, Lavau C, Jansen J, Carvalho T, Carmo-Fonseca M, Lamond A, Dejean A. Retinoic acid regulates aberrant nuclear localization of PML-RARα in acute promyelocytic leukemia cells. Cell. 1994;76:345–356. doi: 10.1016/0092-8674(94)90341-7. [DOI] [PubMed] [Google Scholar]
- Willems M, Penman M, Penman S. The regulation of RNA synthesis and processing in the nucleolus during inhibition of protein synthesis. J Cell Biol. 1969;41:177–187. doi: 10.1083/jcb.41.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witherell GW, Schultz-Witherell CS, Wimmer E. Cis-acting elements of the encephalomyocarditis virus internal ribosomal entry site. Virology. 1995;214:660–663. doi: 10.1006/viro.1995.0081. [DOI] [PubMed] [Google Scholar]
- Wolin SL, Steitz JA. The Ro small cytoplasmic ribonucleoproteins: identification of the antigenic protein and its binding site on the Ro RNAs. Proc Natl Acad Sci USA. 1984;81:1996–2000. doi: 10.1073/pnas.81.7.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing Y, Lawrence JB. Nuclear RNA tracks: structural basis for transcription and splicing. Trends Cell Biol. 1993;3:346–353. doi: 10.1016/0962-8924(93)90105-a. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Singh R, Reddy R. Rat nucleolar 7-2 RNA is homologous to mouse mitochondrial RNase mitochondrial RNA-processing RNA. J Biol Chem. 1989;264:14835–14839. [PubMed] [Google Scholar]
- Young SJ, Royer SM, Groves PM, Kinnamon JC. Three-dimensional reconstruction from serial micrographs using an IBM PC. J Electron Microsc Tech. 1987;6:207–215. [Google Scholar]
- Zandomeni R, Weinmann R. Inhibitory effect of 5,6-dichloro-1-β-d-ribofuranosylbenzimidazole on a protein kinase. J Biol Chem. 1984;259:14804–14811. [PubMed] [Google Scholar]
- Zeng C, Kim E, Warren SL, Berget SM. Dynamic relocation of transcription and splicing factors dependent upon transcriptional activity. EMBO (Eur Mol Biol Organ) J. 1997;16:1401–1412. doi: 10.1093/emboj/16.6.1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Taneja KL, Singer RH, Green MR. Localization of pre-mRNA splicing in mammalian nuclei. Nature. 1994;372:809–812. doi: 10.1038/372809a0. [DOI] [PubMed] [Google Scholar]