Abstract
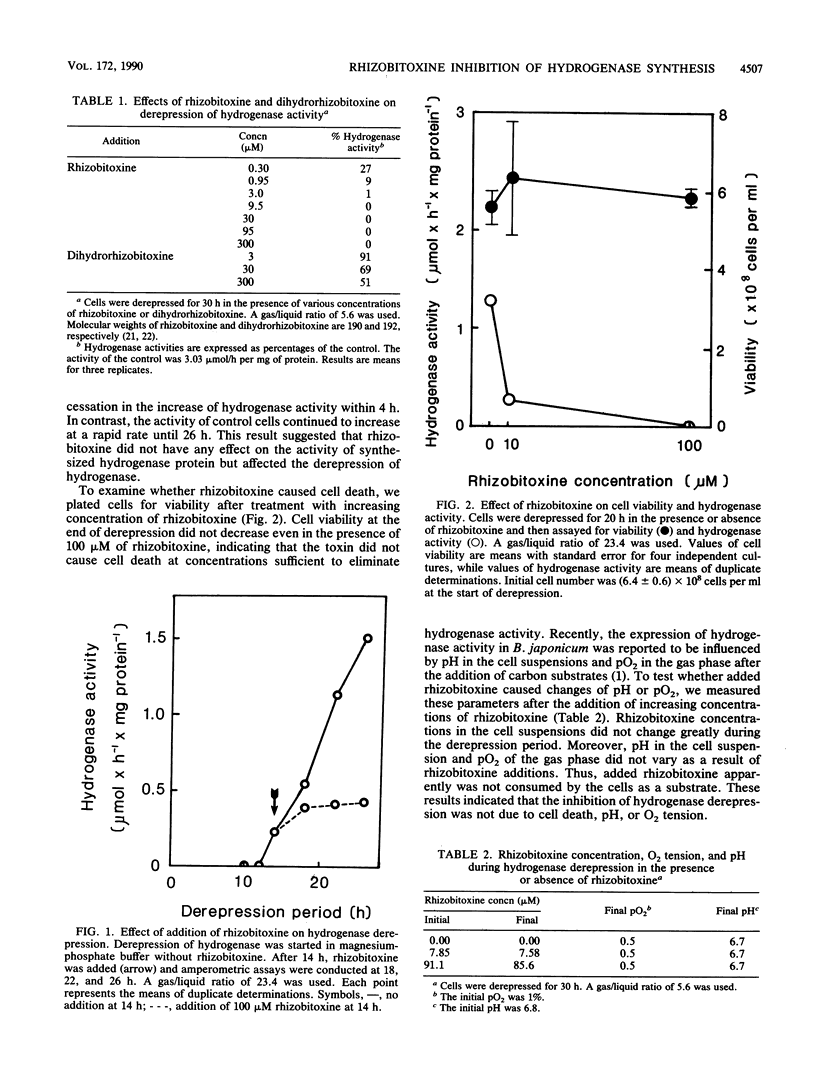
Rhizobitoxine produced by Bradyrhizobium species strongly prevented derepression of hydrogenase expression in free-living Bradyrhizobium japonicum, although the toxin had no effect on the activity of cells which had already synthesized hydrogenase protein. Dihydrorhizobitoxine, a structural analog of rhizobitoxine, proved to be a less potent inhibitor of hydrogenase derepression. Rhizobitoxine did not cause cell death at a concentration sufficient to eliminate hydrogenase expression. The large subunit of hydrogenase was not detectable with antibody after derepression in the presence of rhizobitoxine. The general pattern of proteins synthesized from 14C-labeled amino acids during derepression was not significantly different in the presence or absence of rhizobitoxine. These results indicated that rhizobitoxine inhibited hydrogenase synthesis in free-living B. japonicum. Cystathionine and methionine strongly prevented the inhibition of hydrogenase derepression by rhizobitoxine, suggesting that the inhibition involves the level of sulfur-containing amino acids in the cell.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Emerich D. W., Ruiz-Argüeso T., Ching T. M., Evans H. J. Hydrogen-dependent nitrogenase activity and ATP formation in Rhizobium japonicum bacteroids. J Bacteriol. 1979 Jan;137(1):153–160. doi: 10.1128/jb.137.1.153-160.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans H. J., Harker A. R., Papen H., Russell S. A., Hanus F. J., Zuber M. Physiology, biochemistry, and genetics of the uptake hydrogenase in rhizobia. Annu Rev Microbiol. 1987;41:335–361. doi: 10.1146/annurev.mi.41.100187.002003. [DOI] [PubMed] [Google Scholar]
- Giovanelli J., Owens L. D., Mudd S. H. Mechanism of inhibition of spinach beta-cystathionase by rhizobitoxine. Biochim Biophys Acta. 1971 Mar 10;227(3):671–684. doi: 10.1016/0005-2744(71)90016-7. [DOI] [PubMed] [Google Scholar]
- Hanus F. J., Maier R. J., Evans H. J. Autotrophic growth of H2-uptake-positive strains of Rhizobium japonicum in an atmosphere supplied with hydrogen gas. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1788–1792. doi: 10.1073/pnas.76.4.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lepo J. E., Hanus F. J., Evans H. J. Chemoautotrophic growth of hydrogen-uptake-positive strains of Rhizobium japonicum. J Bacteriol. 1980 Feb;141(2):664–670. doi: 10.1128/jb.141.2.664-670.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier R. J., Campbell N. E., Hanus F. J., Simpson F. B., Russell S. A., Evans H. J. Expression of hydrogenase activity in free-living Rhizobium japonicum. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3258–3262. doi: 10.1073/pnas.75.7.3258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier R. J., Hanus F. J., Evans H. J. Regulation of hydrogenase in Rhizobium japonicum. J Bacteriol. 1979 Feb;137(2):825–829. doi: 10.1128/jb.137.2.825-829.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak P. D., Maier R. J. Hydrogenase synthesis in Bradyrhizobium japonicum Hupc mutants is altered in sensitivity to DNA gyrase inhibitors. Appl Environ Microbiol. 1989 May;55(5):1157–1164. doi: 10.1128/aem.55.5.1157-1164.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak P. D., Maier R. J. Inhibition of hydrogenase synthesis by DNA gyrase inhibitors in Bradyrhizobium japonicum. J Bacteriol. 1987 Jun;169(6):2708–2712. doi: 10.1128/jb.169.6.2708-2712.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens L. D., Guggenheim S., Hilton J. L. Rhizobium-synthesized phytototoxin: an inhibitor of beta-cystathionase in Salmonella typhimurium. Biochim Biophys Acta. 1968 May;158(2):219–225. doi: 10.1016/0304-4165(68)90134-7. [DOI] [PubMed] [Google Scholar]
- Owens L. D., Lieberman M., Kunishi A. Inhibition of ethylene production by rhizobitoxine. Plant Physiol. 1971 Jul;48(1):1–4. doi: 10.1104/pp.48.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens L. D., Wright D. A. Production of the Soybean-Chlorosis Toxin by Rhizobium japonicum in Pure Culture. Plant Physiol. 1965 Sep;40(5):931–933. doi: 10.1104/pp.40.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens L. D., Wright D. A. Rhizobial-Induced Chlorosis in Soybeans: Isolation, Production in Nodules, and Varietal Specificity of the Toxin. Plant Physiol. 1965 Sep;40(5):927–930. doi: 10.1104/pp.40.5.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STICKLAND L. H. The determination of small quantities of bacteria by means of the biuret reaction. J Gen Microbiol. 1951 Oct;5(4):698–703. doi: 10.1099/00221287-5-4-698. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Berkum P., Maier R. J. Lack of carbon substrate repression of uptake hydrogenase activity in Bradyrhizobium japonicum SR. J Bacteriol. 1988 Apr;170(4):1962–1964. doi: 10.1128/jb.170.4.1962-1964.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]