Abstract
In developing Drosophila bristles two species of cross-linker, the forked proteins and fascin, connect adjacent actin filaments into bundles. Bundles form in three phases: (a) tiny bundles appear; (b) these bundles aggregate into larger bundles; and (c) the filaments become maximally cross-linked by fascin. In mutants that completely lack forked, aggregation of the bundles does not occur so that the mature bundles consist of <50 filaments versus ∼700 for wild type. If the forked concentration is genetically reduced to half the wild type, aggregation of the tiny bundles occurs but the filaments are poorly ordered albeit with small patches of fascin cross-linked filaments. In mutants containing an excess of forked, all the bundles tend to aggregate and the filaments are maximally crossbridged by fascin. Alternatively, if fascin is absent, phases 1 and 2 occur normally but the resultant bundles are twisted and the filaments within them are poorly ordered. By extracting fully elongated bristles with potassium iodide which removes fascin but leaves forked, the bundles change from being straight to twisted and the filaments within them become poorly ordered. From these observations we conclude that (a) forked is used early in development to aggregate the tiny bundles into larger bundles; and (b) forked facilitates fascin entry into the bundles to maximally cross-link the actin filaments into straight, compact, rigid bundles. Thus, forked aligns the filaments and then directs fascin binding so that inappropriate cross-linking does not occur.
Keywords: actin, Drosophila, bristles, cross-links, fascin, forked
Bundles of actin filaments are key components in many eukaryotic cells. Obvious examples include microvilli, the acrosomal process of many invertebrate and lower vertebrate sperm, the stereocilia of the inner ear, the bristles and hairs of Drosophila, the sertoli cells of the testes, the ring canals and nurse cell bundles in insect oocytes, the fertilization cone, stress fibers, filopodia, and growth cones. Studies in the 1970s and 1980s focused our attention on the packing of actin filaments into bundles and identified and characterized some of the macromolecular cross-linking components involved. Details on how these cross-linkers were positioned in order to maximize the packing of actin filaments into bundles—no small feat as each actin filament is a helical polymer—followed and are reviewed by DeRosier and Tilney (1982). From the beginning, a number of descriptive studies on how the bundles are generated in vivo were published from which steps in bundle formation could be identified. Experiments followed that aimed to reproduce the in vivo observations by studying the assembly of actin bundles in vitro using purified actin filaments and a single crossbridge. Accordingly, Stokes and DeRosier (1991) investigated factors that control bundle order (the packing of actin filaments in each bundle), bundle size, and bundle number, using fascin, a cross-link isolated from sea urchin eggs. They related these variables to the relative concentrations of actin and fascin.
A careful comparison of the in vivo and in vitro results show that there are serious discrepancies not least of which is the time necessary to form an ordered actin filament bundle in vitro which takes 4–8 d for a small bundle (Stokes and DeRosier, 1991) yet occurs in less than 1 h for the formation of large bundles in vivo (Tilney et al., 1996). Furthermore, there are discrete stages in bundle formation in vivo involving reordering of the filaments in the bundles; these do not occur in vitro. And finally, subsequent studies have made us aware that in most precisely ordered biological bundles formed in vivo there are two and sometimes three macromolecular cross-linkers. Thus, in microvilli, villin and fimbrin are used, in Drosophila bristles fascin and the forked proteins are used (Tilney et al., 1995), in nurse cells in insect oocytes fascin and villin are used (Cant et al., 1994; Mahajan-Miklos and Cooley, 1994), and in stereocilia fimbrin and espin are used (Tilney, L.G., and J.R. Bartles, unpublished observations). That separate cross-links are essential biologically comes from the studies of mutant Drosophila bristles when either fascin or the forked proteins are eliminated (Tilney et al., 1995). The only case known where there is only a single cross-link is in Limulus sperm, but this, judging by the reconstruction of the bundle, is a special case, as the cross-link, scruin, binds to multiple subunits on each actin filament (Schmid et al., 1994; Bullitt et al., 1988).
Why are different cross-links necessary for actin bundle formation in vivo? One idea (Tilney et al., 1996) based upon the time of appearance of the two cross-linkers in bristles during their growth (Wulfkuhle et al., 1998) and on our EM description of stages in bundle formation (Tilney et al., 1995, 1996) is that one cross-link may be used early in bundle formation to tie the filaments together in small bundles so that they can subsequently be zippered together into a precise hexagonally packed unit by fascin. This idea is consistent with earlier observations on the development of microvilli (Chambers and Grey, 1979) and stereocilia (Tilney and DeRosier, 1986).
In this report we have tested the sequential cross-link idea and expanded it using mutants, developmental time points, and extraction methods. From these studies we are beginning to understand why two species of cross-links are essential in vivo and what each contributes during the elongation of a bristle. Our study provides the basis for future in vitro studies using purified cross-links and actin. What we find is that the forked proteins are used early in development to align the filaments into tiny bundles and to aggregate these tiny bundles into the seven to eleven larger bundles found in fully formed bristles. Then, forked proteins somehow orchestrate fascin entry into the bundles which leads to maximally cross-linked, straight, compact and rigid bundles. In essence, the forked proteins hold the bundles in a partially completed state so that fascin can quickly add to generate large bundles with maximal cross-linking. Our study has direct relevance to understanding why other actin bundles (e.g., in microvilli and stereocilia) also contain two or more, albeit different, cross-links for each bundle and why it is essential biologically to have two separate species of cross-links per bundle.
Materials and Methods
Drosophila Stocks
The Oregon-R strain of Drosophila melanogaster was used as the wild type in these studies. The singed3 stock and the forked36a stock were obtained from the Drosophila Stock Center (Indiana University, Bloomington, IN) and maintained as viable homozygotes. The singedX2 and singedS289N stocks were generously supplied by L. Cooley (Yale University, New Haven, CT). The In(1)dl-49, snX2 chromosome was maintained over the In(1)Mud, Mud1 chromosome. Male third instar larvae hemizygous for the In(1)dl-49, snX2 chromosome were selected under a dissecting microscope (where the developing male gonad can be identified) and allowed to pupate. The snS289N chromosome was maintained in males in a stock with C(1)RM-attached X females. Male third instar larvae hemizygous for snS289N were obtained as above. A viable and fertile stock containing two copies of the wild-type forked + gene and four transgenic copies (for a total of six copies per diploid female genome) was generously supplied by N. Petersen (University of Wyoming, Laramie, WY) (Petersen et al., 1994). Female larvae containing 50% of the normal forked protein concentration were f 36a/+ heterozygotes. Flies were maintained on standard cornmeal-molasses-yeast food at 25°C, 60–70% relative humidity, with a 12-h day/night cycle. Complete descriptions of genes and symbols can be found in Lindsley and Zimm (1992) and on FlyBase (FlyBase Consortium, 1998).
Developmental Staging
All animals were staged at the point of puparium formation, an easily recognizable and brief stage lasting 30 min at the beginning of metamorphosis (Bainbridge and Bownes, 1981). White prepupae (0 time) were collected and placed on double-stick scotch tape in a Petri dish that was put back in the incubator at 25°C. Bristle elongation takes place during the interval of 32–48 h after pupariation (Tilney et al., 1996). At the appropriate time the Petri dishes were removed from the incubator and the pupae were dissected.
Dissection of Pupae
We modified our earlier procedure (Tilney et al., 1995). Under ideal moisture conditions, the outer pupal case could be removed with a pair of fine forceps since it is crunchy and tears easily. First, we removed the operculum, and then with one tip of the forceps, tore the outer pupal case down the length of the pupa. The torn sides of the outer pupal case were then stuck down to the double-stick scotch tape. The pupae were lifted free by grabbing the tip of the abdomen, and the pupae were then stuck to another piece of double-stick scotch tape, ventral side down. The pupae were then covered with PBS. With a 5-mm scalpel (Accurate Surgical and Scientific Inc./Kaiser Medical Industrial Co., Westbury, NY), a small incision was made between the developing eyes extending down to the mouth parts. With curved Gills Welsh Vannes micro scissors (Storz Co., St. Louis, MO), a cut was made from the incision down the sides of the head along both sides of the thorax above the wing buds, and then down the sides of the abdomen. A cross-wise cut along the anterior top of the abdomen joined the two side cuts. The cut piece of tissue or dorsal surface of the thorax was removed, placed on is back, and the internal organs and fat bodies were removed with fine forceps. The flight muscles were not removed at this time, since they help the tissue maintain its integrity. Once cleaned, the tissue was fixed for light or electron microscopy.
Fixation and Processing for Light Microscopy
The cut and cleaned thorax was transferred to 4% paraformaldehyde in PBS for 20 min. The tissue was then transferred to 4% paraformaldehyde containing 0.1% Triton X-100 in PBS for an additional 20 min, washed three times in 0.1% Triton X-100 in PBS, and then placed in 0.1% Triton X-100 containing 10−6 M phalloidin conjugated to rhodamine (Sigma Chemical Co., St. Louis, MO) for 30 min. The sample was then placed on a slide and mounted in glycerol Citiflour (Ted Pella, Inc., Redding, CA). A coverslip was applied, and the preparation was sealed with nail polish. Slides were examined either with a universal fluorescent microscope (Carl Zeiss, Inc., Thornwood, NY) or a model TCS 4D confocal microscope (Leica, Heidelberg, Germany).
To establish the presence or absence of fascin or the forked proteins after extraction with potassium iodide (KI),1 we took the detergent or detergent/KI-extracted thoraces and fixed them for 5 min in 4% formaldehyde in PBS and then blocked by PBS-1% BSA and washed three times in PBS. The specimens were incubated in the primary antibody, diluted in PBS containing 1% BSA for 3 h or overnight at 4°C. The thoraces were then washed three times in PBS-1% BSA for 30 min each time and then incubated in secondary antibodies conjugated with fluorescein for 3 h. The specimens were washed and mounted as described above. Confocal images were examined and printed using Adobe Photoshop (Adobe Systems, Inc., Mountain View, CA).
The primary antibodies to the forked proteins were obtained courtesy of N. Petersen and the anti-Drosophila fascin antibodies courtesy of L. Cooley. They were used at concentrations of 1:500 and 1:10, respectively. Secondary antibodies conjugated with fluorescein were obtained from Sigma Chemical Co. (St. Louis, MO).
Fixation and Methods for Transmission Electron Microscopy
Two types of fixatives were used. The most successful was immersion in 2% glutaraldehyde (from an 8% stock purchased form Electron Microscope Sciences, Fort Washington, PA) in 0.05 M phosphate buffer, pH 6.8. Fixation for 1 h was begun at room temperature and the sample was put at 4°C. After 1 h the tissue was then placed in 1% OsO4 in 0.05 phosphate buffer, pH 6.2, at 4°C for 45 min. In the second procedure, fixation by immersion was carried out for 45 min at 4°C. The fixative, which was made just before use, consisted of 1% glutaraldehyde, 1% OsO4 in 0.05 phosphate buffer, pH 6.2. This procedure was designed for fixing actin filaments in situ. A rationale for this fixation is outlined in Tilney and Tilney (1994). After fixation by either method the specimens were washed three times in water at 4°C to remove the phosphate and en bloc stained with 1% uranyl acetate overnight in the dark at 4°C. The specimen was then dehydrated in acetone and flat embedded in Epon. The pupae were oriented before embedding. After polymerization, individual pupae were oriented in the microtome so that transverse sections through the thorax could be obtained. Sections were cut with a diamond knife, stained with uranyl acetate and lead citrate, and then examined with a Philips 200 electron microscope (Philips Scientific, Mahwah, NJ). Because it is essential to have perfect transverse and longitudinal sections through the bristles, an appendage that curves in space, it was necessary to reorient the block, an extremely tedious undertaking. Photographs were taken of sections mounted on uncoated grids.
Scanning Microscopy
Adult Drosophila were fixed for several hours by immersion in 70% alcohol. They were then dehydrated completely, air dried, placed upon stubs, coated with tungsten-platinum, and then examined with a scanning microscope (AMR 1000; Amray Inc., Bedford, MA).
Results
Manipulating Forked Protein Concentration
Background.
We have included this section because to understand the significance of the new data presented in this paper the reader should be cognizant of the data presented in our earlier studies. Unfortunately the significance of some of this background data was not appreciated when the earlier papers were written and thus we have emphasized the important points in this section.
There are three discrete stages in bundle formation that take place as the bristle elongates (Tilney et al., 1996). First, tiny bundles (less than 12 filaments each) appear at the tip of an elongating bristle (phase 1). Most of these tiny bundles are located near the plasma membrane (e.g., Tilney et al., 1996, Fig. 12 a shows 27 cortical bundles and 11 internal bundles). Second, these tiny bundles aggregate into the seven to eleven larger bundles that are attached to the plasma membrane (phase 2) (Tilney et al., 1996). More filaments must appear later as bundles of 180–265 filaments can be seen (e.g., Tilney et al., 1996, Fig. 12 b). Interestingly, the filaments are not hexagonally packed but display liquid order and, in longitudinal section, no 12-nm periodicity indicative of fascin is found. (For further details of why the 12-nm fascin period occurs see Tilney et al., 1995). Not surprisingly, when bristles are stained with antibodies, fascin is not yet present in the bundles, unlike forked which is present from the earliest time point (Wulfkuhle et al., 1998) (our unpublished observations). Third, the filaments in each of the seven to eleven bundles become reordered into hexagonally packed bundles which in longitudinal section show the 12-nm period (phase 3). As expected, antibodies against fascin now stain the bundles. As this reordering occurs more filaments are added to each bundle which now contain between 500 and 650 filaments (Tilney et al., 1996, Fig. 12 c).
Figure 12.
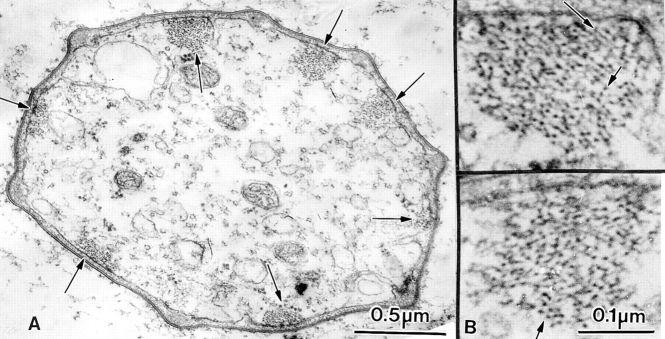
Thin transverse section through a developing bristle of a singedS289N. Although the actin bundles are associated with the limiting plasma membrane they are often on infolded regions of the limiting membrane (arrows). Insets, are two bundles printed at higher magnification. What is striking is that the filaments in these bundles are not hexagonally packed but display liquid order.
Mutants that lack the forked proteins but contain wild-type levels of fascin should form bundles that theoretically correspond to those formed in vitro (Stokes and DeRosier, 1991) where bundles were generated using purified fascin and actin filaments. From our earlier work (Tilney et al., 1995) we know that forked bristles (those that lack the forked proteins) are 55% shorter than wild-type and the fluted nature of the bristle remains. The fluting is linear and the actin bundles by phalloidin staining are straight and not twisted. However, in our thin transverse sections, the actin bundles are tiny relative to the wild type (Fig. 1). The filaments making up the bundle are hexagonally packed and in longitudinal section the 12-nm transverse stripes attributable to the fascin crossbridge are present as predicted.
Figure 1.
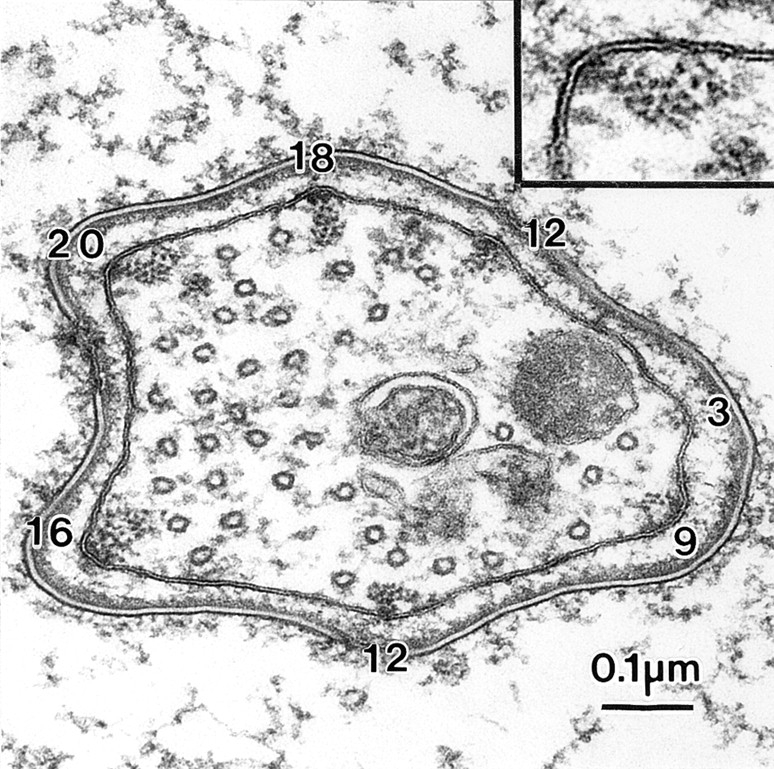
Transverse section through a bristle of the forked36a mutant 48 h after puparium formation. Of interest are the size of the seven actin bundles. The number of filaments per bundle are indicated on the micrograph. One of the bundles is enlarged to show that the filaments are hexagonally packed. Reprinted from Tilney et al. (1995).
New Observations. No Forked Protein, Newly Emerged Bristles.
The bristles first emerge in the 34-h pupae. Transverse sections through emerging bristles of the mutant forked 36a (Fig. 2) reveal that as in the wild-type, tiny actin bundles attached to the plasma membrane appear. The number of filaments in each bundle seldom exceeds 12 (Fig. 2, inset). Since the forked proteins are absent in this null mutant these filaments must be cross-linked by another crossbridge. From our mutant analysis (Tilney et al., 1995) this cross-link should be fascin. Of particular interest is that the number of bundles is more than in the wild type. In Fig. 2 there are 46 membrane-associated bundles versus 27 typically found in the wild type. There is only one internal bundle but throughout the cytoplasm there appear to be individual actin filaments. All filaments are oriented parallel to the elongating axis of the bristle as is the large population of microtubules.
Figure 2.
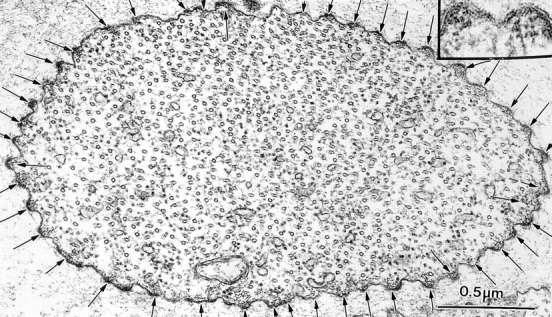
Transverse section through a newly emerged bristle of a 34-h forked36a mutant pupa. Arrows point out the 46 tiny actin bundles. Inset, two of these bundles are enlarged.
No Forked Protein, Fully Elongated Bristles.
From Fig. 1 we see that the diameter of the bundles at the tip of a fully elongated bristle are smaller than the wild type. In this micrograph there are 12, 18, 20, 16, 12, 9, and 3 actin filaments per bundle.
In other transverse sections through bristles the numbers of filaments per cross section are also small in number like that seen at the tip. As mentioned in Tilney et al. (1996) elongation of a bristle occurs at the tip. Therefore, a bundle near the base of a fully elongated bristle has had approximately 14 h to grow, differentiate, and expand by addition of more filaments. We have managed to cut transverse sections through two bristles at their base so we could count actin filament numbers. (We should note that obtaining perfect transverse sections through a bristle is no small feat since each bristle is curved and thus, the block must be continuously readjusted before sectioning; if the angle is off by more than a few degrees one cannot count the number of actin filaments.) In one of these sections we counted the number of filaments per bundle (Fig. 3). The values were 24, 47, 51, and 47 compared with 500–700 filaments per bundle in the wild type. As expected, the filaments are hexagonally packed because fascin is present. The bundles tend to be rectangular in shape.
Figure 3.
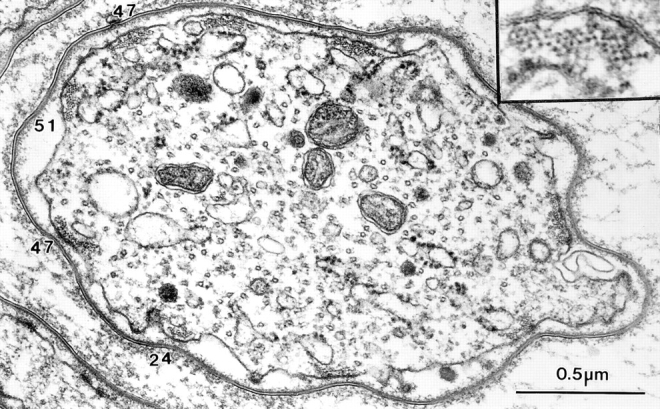
Transverse section through the base of a bristle of a 44-h (nearly mature length) forked36a mutant. The numbers indicate the number of filaments in their respective bundles. Inset, filaments in the bundle are packed hexagonally as one would expect as this mutant contains fascin but no forked proteins.
To summarize, in the absence of the forked proteins, many tiny bundles form at the tip of the bristle but most fail to aggregate and subsequently disappear. As the bristle matures some additional filaments become incorporated and are cross-linked by fascin into the bundles. However, the size of the mature bundles is considerably smaller in forked mutant bristles (≤50 filaments/bundle) when compared with wild type (e.g., 500–700 filaments/bundle).
Reduced Forked Protein Concentration, Fully Elongated Bundles.
By constructing forked36a/+ females we could examine animals with an ∼50% reduction in the amount of forked proteins.
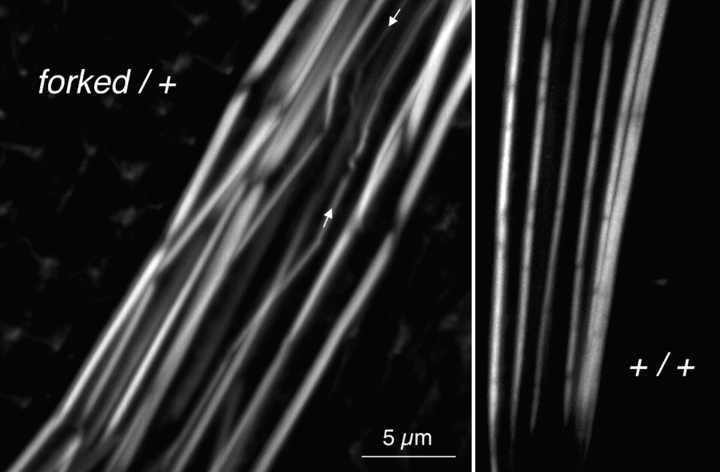
By immunofluorescence of phalloidin-stained bristles this mutant contains the appropriate number of bundles per bristle and the bundles generally run parallel to each other (Fig. 4). Furthermore, the units or modules that by being attached end to end form each bundle (Tilney et al., 1996) are usually found in transverse register. What is different is that the bundles are more variable in thickness with many appearing somewhat swollen and less compact than in the wild type (Fig. 4). Some of the thinner bundles are also wavy in profile (Fig. 4).
Figure 4.
Confocal images of portions of two bristles stained with fluorescent phalloidin to indicate the actin distribution. Left, forked36a/+ expressing less than the normal amount of forked proteins. Of interest is that the bundles are swollen relative to the wild type (right, same magnification). Some of the modules composing the bundles in the heterozygote are present at an acute angle, and some thin bundles are wavy in their trajectory (arrows).
The swollen, less compact nature of the bristles can be understood from thin transverse sections cut through bristles (Fig. 5). The actin filaments making up the bundles display liquid order. There are small patches in these bundles where the filaments are hexagonally packed (e.g., Fig. 5 B) but elsewhere there are spaces in the pattern where the filaments are less ordered. In many cases the diameter of the bundles is greater than that of the wild type due to the more disordered arrangement of the filaments. Nevertheless, the number of filaments per bundle is always less than in bundles in wild-type bristles of the same diameter. In longitudinal section through the bundles often we see the 12-nm period (Fig. 6). A careful comparison of this pattern of crossbridges relative to the wild-type is very instructive because it reveals that the crossbridges only span across a few filaments and not across all the filaments in the bundle. These areas correspond to the few areas in the transverse sections where the filaments are hexagonally packed (Fig. 5 B, arrows). Particularly interesting is that in longitudinal section the 12-nm period extends between the cross-linked pairs of filaments for a long distance. Thus if fascin comes in, it zippers adjacent filaments together all along their length.
Figure 5.
(A) Thin section through a bristle of a mutant that contains only one copy of the forked gene (forked36a/+) but wild-type with respect to the singed gene. Seven actin filament bundles are associated with the limiting plasma membrane (arrows). (B) Two actin bundles present in A are enlarged so that the packing of the actin filaments in the bundles is obvious. The filaments are clearly not hexagonally packed but there are regions indicated by the arrows in which the filaments are closely packed or appear in linear rows.
Figure 6.
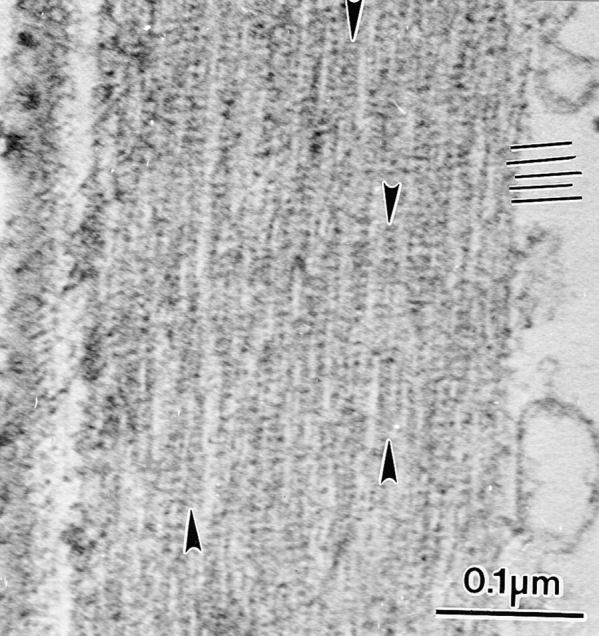
Longitudinal section through an actin bundle in a bristle from the same mutants depicted in Figs. 4 and 5 where a reduced amount of forked protein is present. By turning the micrograph 90° one can see the 12-nm period due to fascin (black lines). A careful examination of this micrograph, however, reveals that the filaments are in small clusters (arrows). Thus, the 12-nm stripe extends across these clusters but does not extend across the space between adjacent clusters. The 12-nm stripe continues down the length of each cluster.
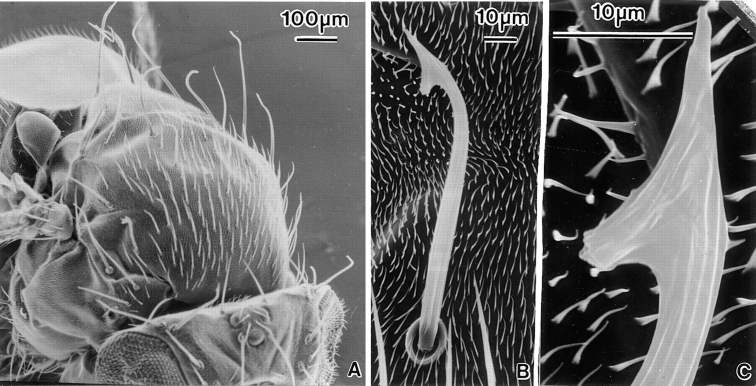
Increased Forked Protein Concentration.
We also examined a mutant containing six copies of the forked gene (Petersen et al., 1994). By scanning microscopy the macrochaetes and some of the microchaetes often had a fish hook appearance (Petersen et al., 1994) (Fig. 7, A and B). At higher resolution, the grooves in the main shaft terminated at the barb of the fish hook and a new set at an acute angle to the first group appeared in the barb (Fig. 7 C). Thin sections cut through the barb portion of bristles from this mutant were especially revealing (Fig. 8 A). The bundles were very large and often irregular in shape. The filaments within these bundles were hexagonally packed (Fig. 8 B) and in longitudinal section showed the 12-nm period indicative of fascin (Fig. 8 C). However, there are gaps in all the bundles where no filaments could be found giving the bundles a moth-eaten appearance (Fig. 8, A and B). We also find in this mutant that in addition to the seven to eleven membrane-associated bundles, there are internal bundles composed of hexagonally packed filaments (Fig. 9 A). These internal bundles tend to aggregate together on one half of the bristle shaft. In longitudinal section these internal bundles display the 12-nm period of fascin. Longitudinal sections also revealed that many membrane-associated bundles appear to be composed of closely applied subbundles each containing ∼50–100 filaments (Fig. 9 B). Thus, many of the gaps seen in transverse section are in reality areas where subbundles do not join perfectly into a single hexagonally packed bundle and/or that additional filaments have not been added to fill in spaces between subbundles as they aggregate during development. Thus, from our thin sections, bristles in this mutant appear to contain more bundles than in the wild type but still have only seven to eleven membrane-associated bundles. These membrane-associated bundles are much larger due to the aggregation of many more smaller bundles than occurs in wild-type bristles. Even so, in all cases the filaments making up the bundles seem to be maximally cross-linked by fascin. There seems to be no liquid ordered filaments.
Figure 7.
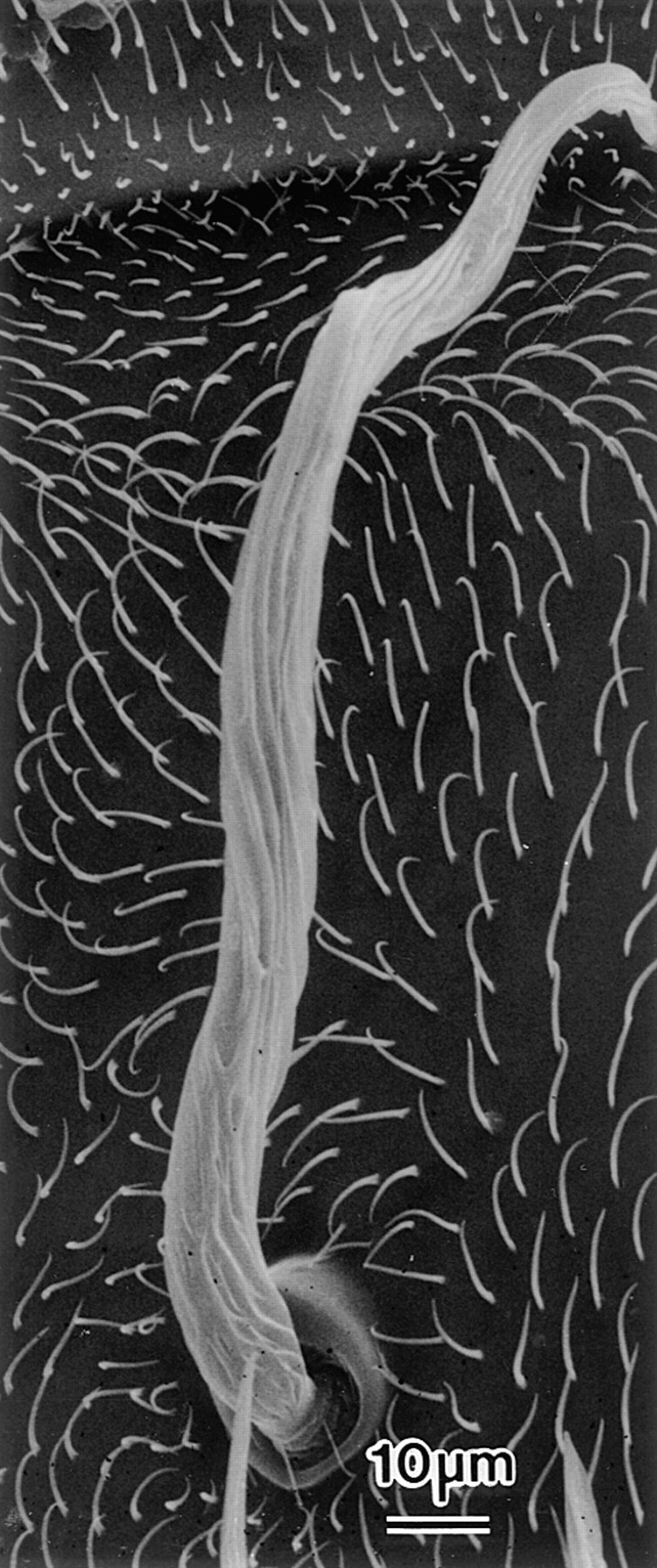
Scanning electron micrographs of a mutant stock containing six copies of the forked gene. (A) View of the thorax. The macrochaete and some of the microchaetes are severely bent and gnarled at their tips. (B) Higher magnification of an individual bristle. Bristles such as these resemble fish hooks. (C) A higher magnification view of the barbed region of the bristle in (B). Of interest is that the longitudinal fluting that runs the length of the bristle is interrupted at the barbed portion where new grooves form at a 45° angle.
Figure 8.
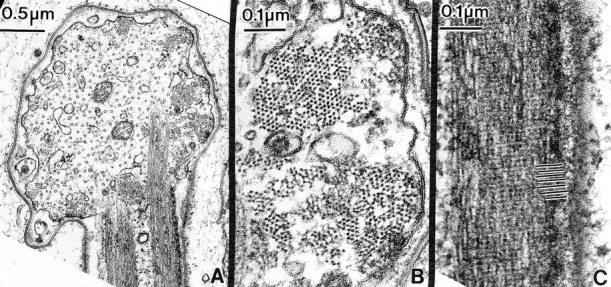
Thin section cut through the barbed portion of a developing fish hook bristle tip similar to the one shown in Fig. 7 C, from a mutant containing six copies of the forked gene. (A) The main shaft of the bristle extends upwards. This section contains two actin filament bundles in longitudinal section (enlarged in C). As in Fig. 7, the barbed portion forms at nearly right angles to the shaft proper and contains eight membrane-associated actin filament bundles (enlarged in B) and numerous microtubules in a parallel array. (B) Higher magnification of two actin bundles cut in transverse section. Most significant is the filaments in the bundles are hexagonally packed albeit with gaps or holes in the bundles. (C) Longitudinal section through an actin bundle in the shaft. If this figure is viewed from the side we can easily discern the 12-nm period (black lines) due to the fascin cross-link.
Figure 9.
Thin section through the fully elongated bristles from a mutant containing six copies of the forked gene. (A) In this transverse section through a bristle we find that the actin bundles are larger than the wild type and tend to aggregate together. Arrows indicate eight of the membrane-associated bundles. These bundles now associate with some internal bundles to form large masses of filaments. Of interest is that most of the bundles are located in the lower half of this cell. The dendrite (D) of the nerve is on the opposite side where there are no bundles. (B) Longitudinal section cut through an actin bundle. Notice that it is composed of a number of subbundles.
Altering the Fascin Cross-link
Background.
Cant et al. (1993) analyzed the singedX2 and singed3 mutations. Although neither appears to be a complete null, immunofluorescence with antibodies directed against the singed product—fascin—showed that the mutant bristles did not show any appreciable fluorescence. Although the length of the mutant microchaete bristles were ∼90% that of the wild type (Tilney et al., 1995) all the bristles appeared twisted, often curled, or singed. The packing of the filaments was no longer hexagonal, but instead showed a liquid order (Tilney et al., 1995). In longitudinal section through the bundles the 12-nm cross striations were no longer present due to the absence of fascin. Nevertheless, actin filaments are present in bundles albeit the number of filaments per bundle is considerably less than the wild type. The number of bundles and the asymmetry in the position of the bundles was unaffected. What is particularly striking is that by immunofluorescence the actin bundles are not straight but are twisted (e.g., Tilney et al., 1996, Fig. 14), which we presume accounts for the twisted appearance of the bristles seen by scanning microscopy (e.g., Tilney et al., 1995, Fig. 7).
New Observations. No Fascin, Early Stages in Bristle Elongation.
As in the wild-type and forked mutants tiny bundles of actin appear near the plasma membrane at the tip of elongating singedX2 bristles. There are also a few bundles and some solitary actin filaments in the center of the extension. By 37 h the tiny bundles begin to aggregate into the seven to eleven larger bundles seen in the mature bristles. This process of aggregation is particularly striking in the singed mutants. What one sees are ribbon-shaped aggregations of filaments (Fig. 10); these aggregations are adjacent to the plasma membrane. Each linear aggregate is composed of individual bundles of approximately a dozen filaments attached side by side. That the packing of the actin filaments in each tiny bundle or in the aggregate lacks hexagonal packing as would be expected. Thus, in the mutant where the forked proteins but little or no fascin are present, aggregation occurs in association with the plasma membrane, but in resulting bundles the filaments are poorly ordered.
Figure 10.
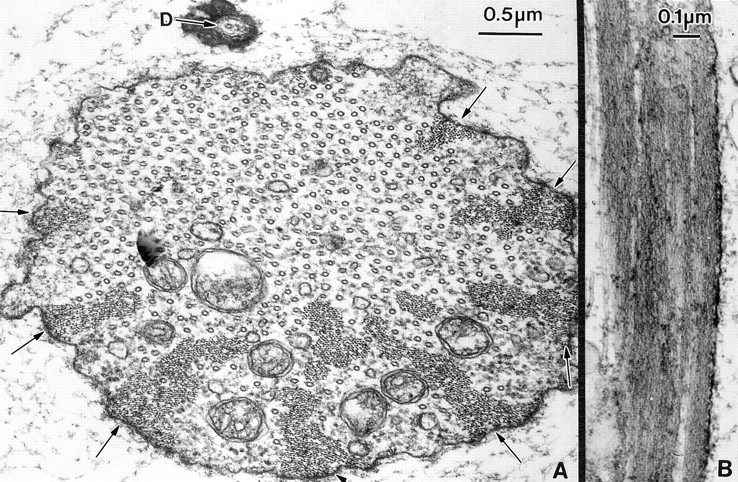
Thin section through a newly emerging bristle from a singedX2 animal 36 h after pupation. Associated with the limiting plasma membrane are nine bundles of actin filaments. Of great interest are that the bundles are rectangular in shape. Arrows, linear series of tiny bundles.
Fully Elongated Bristles in a Mutant Lacking Functional Fascin.
Recently we have examined the singedS289N allele described by Cant and Cooley (1996). Although this mutant produces nearly normal amounts of protein, the mutant protein appears to be completely inactive. When we examined surviving homozygous male pupae we see that both macrochaetes and microchaetes are twisted and the grooves which one sees by scanning microscopy also twist along their length. These grooves are deep and often split or fuse with each other (Fig. 11).
Figure 11.
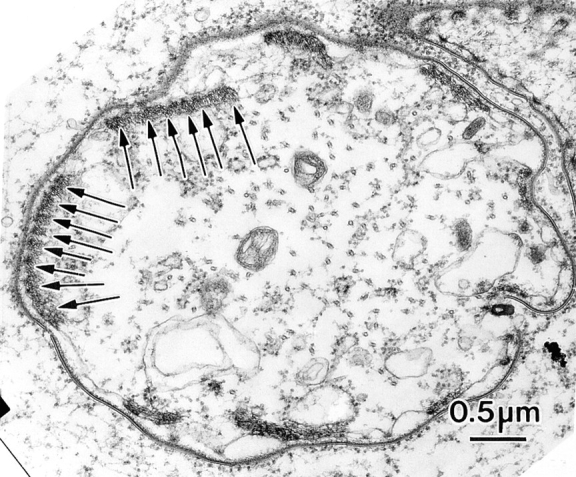
Scanning electron micrograph of a fully elongated bristle from a singedS289N adult. Of particular interest is that the bristle is twisted and the grooves which are indicative of the position of the actin bundles twist around the bristle.
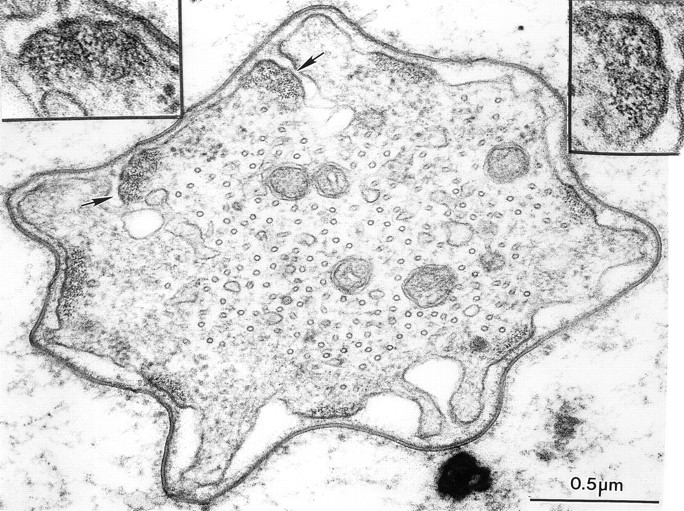
But what is interesting about this mutant are the actin bundles as visualized in thin section. These bundles, albeit small, are rectangular in shape and are usually associated with the membrane. As expected, the filaments within each bundle are not hexagonally packed but display a liquid-like arrangement (Fig. 12, insets). Of particular interest is that in this mutant there are also internal bundles, but in contrast to the forked mutant which overproduces the forked protein, each bundle is generally associated with or connected to a vesicle or within an infolding of the plasma membrane. We found a number of instances in which the peripherally located rectangular bundles are actually positioned along an infolding of the plasma membrane rather than the surface proper (Fig. 12). In longitudinal section part of the bundles are attached to the plasma membrane and half extend inward into the cytoplasm proper. Moreover, in longitudinal section, each bundle seems to be an aggregate of many smaller bundles as if after aggregation of the bundles there is an imperfect filling in of space between the surface of these bundles.
Chemical Removal of Cross-links Affects Bundling
To further determine the exact function of individual cross-links we extracted fully elongated wild-type bristles with a variety of reagents to selectively remove one or the other cross-link and then analyzed the morphology of the bristles and their actin bundles. An agent that was particularly revealing was potassium iodide (KI).
Our procedure was as follows. We dissected out the dorsal thorax, then treated the preparation with detergent followed by extraction with KI. The thoraces were then fixed and stained with fluorescently labeled phalloidin and/or antibodies against fascin or the forked proteins labeled with a different fluorochrome. What we noticed is that the actin bundles could still be detected in most bristles even in concentrations of KI as high as 0.6 M, but unlike the controls the bundles are twisted (Fig. 13 A). The bundles were swollen at concentrations of KI above 0.3 M and in some cases the bundles were reduced in number, but 0.65 M was necessary to break down all the filaments.
Figure 13.
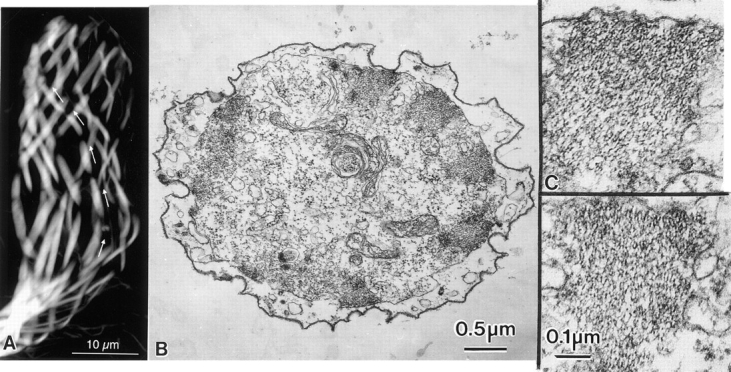
Developing bristles that were extracted with 0.6 M KI. (A) When the thorax is stained after extraction and fixation with fluorescent phalloidin the actin bundles in the bristles are readily visible. Of particular interest is that after extraction of the bristles with KI the bristles change from being straight to being twisted (Fig. 12). The actin bundles are likewise twisted in the bristle (arrows). (B) Thin transverse section of a bristle that had been extracted with 0.6 M KI. Actin bundles are located near the limiting membrane of this bristle. (C) Higher magnification of two bundles illustrated in B. Of interest is that although the actin filaments remain associated with one another in bundles, the filaments are not hexagonally packed and instead show liquid order with respect to each other.
An analysis of the effects of varying concentrations of KI on the presence of fascin or the forked protein is summarized in Table I. At 0.1 M all of the fascin was removed and apparently redistributed within the bristle. With 0.2 M the bundles are fascin free. In contrast, the forked proteins remain at 0.1 M. At higher KI concentrations, forked proteins are extracted from the bundles but concentrate into spots both along and between bundles. These forked protein-positive spots are present even at high KI concentrations, up to 0.6 M.
Table I.
KI Treatment Selectively Removes Actin Cross-links
| % Bristles stained | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Antibody | KI (M) | Uniform | None | Particulate | n | |||||
| Anti-forked | 0 | 100 | 71 | |||||||
| 0.1 | 97 | 3 | 78 | |||||||
| 0.2 | 58 | 42 | 73 | |||||||
| 0.3 | 75 | 25 | 4 | |||||||
| 0.4 | 78 | 23 | 26 | |||||||
| 0.5 | 100 | 4 | ||||||||
| 0.6 | 100 | 6 | ||||||||
| Anti-singed | 0 | 100 | 3 | |||||||
| 0.1 | 100 | 31 | ||||||||
| 0.2 | 92 | 8 | 66 | |||||||
| 0.3 | ||||||||||
| 0.4 | ||||||||||
| 0.5 | ||||||||||
| 0.6 | 100 | 5 | ||||||||
Bristles were detergent extracted, treated with KI, fixed, stained with an antibody directed against one cross-link, and then visualized by confocal microscopy. The actin bundles were either stained evenly (Uniform), not at all (None), or exhibited a very nonuniform speckled appearance (Particulate).
In thin sections through bristles in 0.6 M KI the actin filaments in the bundles show liquid order (Fig. 13, B and C) and in longitudinal section do not display the 12-nm cross striations. These observations are consistent with the antibody studies that show fascin is removed under these circumstances. If the fascin is removed, then the filaments are only held together by the forked protein or aggregates of the forked proteins (the fluorescent spots) or by a yet unidentified cross-link. This in turn leads to poorly ordered filaments whose bundles are twisted.
Discussion
Small Imperfect Bundles Form in the Presence of the Fascin Cross-link
Using one cross-link, fascin, isolated from sea urchin eggs, and purified actin filaments, Stokes and DeRosier (1991) studied bundle formation in vitro. At all concentrations of fascin the bundles in vitro were small and formed very slowly. These observations are relevant here because we study actin bundles in which mutants express fascin but lack the forked proteins appear to be biologically equivalent to bundles formed in vitro. More specifically in the forked36a mutant, a null mutant that fails to express the forked proteins, in newly emerging bristles we find only tiny bundles of ∼10 filaments similar to the situation in the wild-type bristle. However, as the bristles elongate the bundles remain small, being composed of only 10–50 filaments as if the tiny bundles seen in newly emerged bristles fail to aggregate properly and fail to increase in filament number.
By increasing the forked protein concentration to 50% of wild-type levels (e.g., in forked/+ heterozygotes) and in the presence of wild-type amounts of fascin, the bundles are much larger but in most cases the packing of the filaments is in liquid order. Careful examination of these bundles reveals that there are small clusters of well-ordered filaments that display the 12-nm period attributable to fascin. These regions extend for long distances down the bundle as if when filaments pair or small clusters zipper together with fascin, they do so all along their length not just at short intervals. We will come back to this point later in the discussion as it suggests that forked protein is facilitating fascin binding.
What is the Precise Function(s) of the Forked Proteins?
In mutants that lack fascin (singed mutants) rectangular-shaped bundles are present in which the filaments are poorly ordered. Here the filaments are cross-linked together by a different crossbridge, presumably the forked proteins. A similar situation occurs when detergent-treated fully formed bristles are extracted with KI. From antibody staining of KI-extracted bristles it is clear that fascin is completely removed yet the forked proteins remain. Filament order changes from a precise order to a liquid order reminiscent of the singed mutants, which lack fascin at all stages. Thus the forked proteins, directly or indirectly via other components, hold the filaments together but the filaments are poorly ordered.
We also know from the studies of Wulfkuhle et al. (1998) (which we have confirmed) that in wild-type flies, forked proteins appear in the bundles of newly emerged bristles well before fascin. Thus, the tiny filament bundles at the newly emerging bristle tip are first joined together by the forked proteins. The filaments in these bundles are poorly ordered because fascin has not entered the bundles. Subsequently these tiny bundles aggregate into the seven to eleven larger bundles. Only after aggregation is fascin incorporated into the enlarging bundles. This ultimately leads to the filaments becoming maximally cross-linked. When we examine early stages in bundle formation in singed mutants that lack fascin but contain forked protein, in 36-h pupae we see long ribbon-like bundles attached to the limiting plasma membrane in transverse section. Thus in the fascinless mutant there has been a lateral aggregation of the tiny discrete bundles into wider ribbon-like bundles as in the wild type. At later developmental times, shorter, thicker bundles replace the long ribbons as if the ribbons collapse upon themselves. Over the same time scale, in mutants that lack forked proteins but contain wild-type amounts of fascin, aggregation of the tiny bundles does not occur. Accordingly, a second function of the forked proteins must be to aggregate small bundles into larger bundles in phase 2.
The above conclusion is consistent with observations on mutants overexpressing the forked proteins. In this mutant much larger bundles form. They are not flat, and unlike the singed mutant they bulge inward forming large triangular-shaped bundles. Each of these large bundles is composed of numerous subbundles which are aggregated together, presumably by the overexpression of forked protein.
Besides aggregating the actin filaments together into tiny bundles at the bristle tip, and somehow helping in the aggregation of the tiny disordered bundles into the larger disordered bundles, the forked proteins have a third function. This function (the last) must be to somehow, and we do not yet know precisely how, cooperate in reordering the disordered bundle into a large, ordered, compact, nontwisted, hexagonally packed, maximally cross-linked bundle. In short, the forked proteins must facilitate fascin binding. This conclusion stems from the observation that if only fascin is present in vitro or in vivo (e.g., in forked mutants) or the amount of the forked protein is reduced (e.g., forked/+ heterozygote), large ordered bundles do not form although there are tiny regions of well-ordered fully crossbridged filaments in the heterozygote. Furthermore, if there is an excess of forked protein the filaments are always fully crossbridged by fascin even though there are more bundles produced. Further studies will be necessary to determine how the forked protein facilitates fascin binding.
To maximally cross-link filaments together into a three-dimensional bundle is a much more complex problem then one might expect because what is key is to inhibit inappropriate fascin cross-links so that the resultant bundle has no gaps in it or regions with liquid order between regions of more precise order. If liquid order were to occur the bundles would twist and not maintain strict linearity. Furthermore, the forked protein must somehow manage to increase the efficiency of adding additional filaments in an orderly way to the reordered bundles as they increase dramatically in filament number (Tilney et al., 1996).
We know from the work of Stokes and DeRosier (1991) that actin and fascin form tiny bundles in vitro composed of well-ordered filaments that can increase somewhat in filament number and/or diameter over 4–8 d. In bristles we know that the time it takes to go from a tiny bundle (34-h pupae) to large well-ordered bundles (37-h pupae) occurs in only 3 or 4 h and not the 4–8 d required in vitro. In addition, the ultimate size of the bundles formed in vitro versus in vivo differs by over an order of magnitude. These dramatic differences in time and number must be somehow attributable to the forked proteins as the forked proteins were not present in the in vitro studies.
The Fascin Cross-link Revisited: What Additional Function Exists for This Cross-link?
From earlier studies analyzing the cross-linking of actin filaments with a single crossbridge both in vitro and in vivo (DeRosier and Tilney, 1982) we know that the molar ratio of fascin/actin or fimbrin/actin in stereocilia is 1:4.6. This cross-linking is essential in forming a rigid bundle because if the cross-links are lost, e.g., during noise damage to stereocilia (Tilney et al., 1982) the rigidity of the stereocilia are greatly diminished. Furthermore, in the singed mutants that lack fascin or in bristles treated with 0.2 M KI, characteristically the bristles are/become bent and twisted and lie against the surface of the thorax. Not only does fascin contribute markedly if not almost exclusively to the rigidity of the bristles but it also inhibits the formation of sharp bends and/or twisted profiles. These observations are consistent with diffraction patterns of fascin/actin in in vitro paracrystals (DeRosier and Tilney 1982; DeRosier and Censullo, 1981) or thin longitudinal sections through these crystals or through bristles (Tilney et al., 1995) where the filaments do not twist over each other but instead run perfectly parallel to each other. Thus, not only does fascin maximize the stiffness of the bristles but it also insures that the bristles are not twisted or bent, a situation key for a mechanoreceptor.
Why Are Two Cross-links Essential to Form a Bundle In Vivo?
By comparing our in vivo results on wild-type bristles with those obtained on mutants that lack one or the other cross-link or with the in vitro observations of Stokes and DeRosier (1991), there are a number of reasons why two or more cross-links rather than one make more sense biologically. First, bristles up to 400 μm in length form in less than 14 h. These bristles contain up to 1,000 filaments per bundle. Thus, the time required for one cross-link to form in vitro (8 d for a small bundle of 50–100 filaments) is just not compatible with in vivo formation using two cross-links (14-h) which also results in much larger bundles (∼1,000 filaments). Second, and this point is discussed briefly in Stokes and DeRosier (1991) and in Tilney et al. (1983), the way to form a bundle that is not twisted is to maximally cross-link adjacent actin filaments together. This results in a rigid bundle that will provide a cell extension such as a bristle with its characteristic shape. It is obvious that pairs of filaments maximally cross-linked together can be easily formed but to maximally cross-link filaments in three dimensions as in a bundle requires that the filaments lie in a hexagonal lattice. To develop a hexagonally packed bundle from one that is liquid ordered and/or to form hexagonally from an aggregate of many tiny bundles requires that the cross-links be made and broken until maximum order is achieved. Thus the fascin cross-link, intellectually at least, should have a weak binding constant so the fascin links can come off and go on at more appropriate positions. To hold the filaments together during this ordering phase may require a different species of cross-link that at the same time does not inhibit fascin binding but instead facilitates appropriate fascin cross-linking and/or inhibits inappropriate cross-linking.
We are currently trying to determine how the forked proteins facilitate fascin binding or inhibit inappropriate interactions. One possibility is for the forked proteins to change the twist of the actin filaments and thus make every fascin–actin connection appropriate. Such a model would be consistent with the forked/+ heterozygotes where patches of filaments are maximally cross-linked together by fascin. These cross-links run the full length of the filaments.
Two or More Cross-links per Bundle Is a Common Scenario in Biological Systems
In microvilli of intestinal epithelial cells there are three actin cross-links per bundle, villin, fimbrin, and espin (Bartles et al., 1998), in nurse cell actin bundles there are two cross-links, villin and fascin (Cant et al., 1994; Mahajan-Miklos and Cooley, 1994), in the actin bundle in each stereocilium of the cochlea there is fimbrin and espin (Tilney et al., 1989) (Bartles, J.R., and L.G. Tilney, unpublished observations), and in bristle and hair bundles there is fascin and the forked protein. Significantly in large bundles such as those in stereocilia and in bristles, the formation of a maximally cross-linked bundles albeit by fimbrin or fascin forms in very similar ways. In both cases small bundles of poorly ordered (liquid order) filaments first appear and with time the order increases so that at the end one has maximally cross-linked, hexagonally packed bundles which display the 12-nm period in longitudinal sections. The same developmental sequence also occurs in small bundles. Examples include intestinal microvilli (Chambers and Grey, 1979) and Drosophila nurse cell bundles (Guild et al., 1997). In the latter case a poorly ordered bundle is present in the microvillus and as the bundle moves inward it becomes hexagonally packed. Furthermore, in the intestinal microvilli and in bristles the cross-links appear sequentially. Thus, the forked proteins precede fascin in bristle bundles just as villin precedes fimbrin in intestinal microvillar bundles (Shibayama et al., 1987; Ezzel et al., 1989). There is an additional parallel. Just as bristles that lack the forked protein form but are shorter and have poorly organized actin bundles, microvilli that lack villin also form in villin-deficient mice but they are also shorter and exhibit poorly bundled actin cores (Pinson et al., 1998).
Conclusions
What we proposed to do was to try and understand why two or more cross-links are necessary to form a maximally cross-linked and thus maximally rigid actin bundle. Such bundles could then be used by a cell as cytoskeletal units, the bones of a cell. To answer why questions is difficult, if not pretentious, so what we have done is to try and determine what role each cross-link plays in forming a mature bundle. But what the results from this paper actually do is raise more questions than were initially asked and thus opens new areas for future investigation.
For example, how do the forked proteins facilitate fascin cross-linking and how in the same cell at the same time do cross-links form between actin filaments sequentially (first forked proteins appear on the forming bundles then fascin appears). Furthermore, bristles have mature bundles at the base and less mature or early stage bundles towards the tip. Thus, in the same cytoplasm at the same time all the constituents are present and, yet miraculously, they somehow perform their individual job(s) and interact sequentially to produce straight, rigid, maximally cross-linked bundles.
Although there is abundant evidence that the forked proteins may be a second cross-link, up to now no one has been able to show that purified forked proteins bundle actin in vitro, an experiment that would show conclusively that forked is a cross-linking protein. The reason this information is not available is because recombinant forked protein is insoluble and to purify forked from Drosophila is just not practical. Notwithstanding there is genetic evidence that forked may be a crossbridge as demonstrated by this and earlier papers from our lab and others. Furthermore, antibodies to forked decorate bristle bundles and, if fascin is removed by KI, forked remains on the bundles presumably holding actin filaments together as it does in actin patches early in bundle development (Tilney et al., 1996; Wulfkuhle et al., 1998). Lastly, unpublished results cited in Wulfkuhle et al. (1998), indicate that certain regions of the forked protein can bind to actin filaments in vitro.
There are two additional puzzles that should be aired. First, we know that besides fascin there must be at least one additional cross-link holding the filaments together into bundles; presumably it is the forked protein or proteins associated with forked, but there may be other cross-links as well. Evidence that more than two may be present comes from observations that in the forked-singed double mutant (a mutant lacking both fascin and forked) tiny membrane-associated monolayers—we refer to them as rafts—appear (Tilney et al., 1995) and stain with phalloidin (Wulfkuhle et al., 1998). Second, before we can speculate or better determine exactly how a second and/or third cross-link interacts with fascin to form a bundle we need to know the stoichiometries of each relative to actin. This must be a topic for further study because at present we cannot determine this value in vivo and only by in vitro studies using the two or three cross-links can we determine this value. Nevertheless to better understand how these cross-links work together to facilitate fascin binding will require this knowledge.
Acknowledgments
This work was supported by a grant from the National Institute of Health (GM-52857) to L.G. Tilney and a grant from The American Cancer Society (RPG-92-014-05) to G.M. Guild.
Footnotes
Many thanks to N. Petersen and L. Cooley for their gifts of fly strains and antibodies against the forked protein and fascin, respectively. We are especially grateful to M. Mooseker who for two summers gave advice, encouragement, space, and equipment while L.G. Tilney was in Woods Hole. We would also like to thank the reviewers of this manuscript for fair and just criticism, and our editor D. DeRosier (Brandeis University) for helping to improve the manuscript.
Address all correspondence to G. Guild, Department of Biology, University of Pennsylvania, Philadelphia, Pennsylvania 19104. Tel.: (215) 898-6388. Fax: (215) 898-8780. E-mail: gguild@sas.upenn.edu
1. Abbreviation used in this paper: KI, potassium iodide.
References
- Bainbridge SP, Bownes M. Staging the metamorphosis of Drosophila melanogaster. . J Embryol Exp Morphol. 1981;66:57–80. [PubMed] [Google Scholar]
- Bartles JR, Wierda A, Zheng L. Identification and characterization of espin, an actin-binding protein localized to the F-actin-rich junctional plaques of Sertoli cell ectoplasmic specializations. J Cell Sci. 1996;109:1229–1239. doi: 10.1242/jcs.109.6.1229. [DOI] [PubMed] [Google Scholar]
- Bartles JR, Zheng L, Li A, Wierda A, Chen B. Small espin: a third actin-bundling protein and potential forked protein ortholog in brush border microvilli. J Cell Biol. 1998;143:107–119. doi: 10.1083/jcb.143.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullitt ESA, DeRosier DJ, Coluccio LM, Tilney LG. Three-dimensional reconstruction of an actin bundle. J Cell Biol. 1988;107:597–611. doi: 10.1083/jcb.107.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cant K, Knowles A, Mooseker MS, Cooley L. Drosophila singed, a fascin homologue, is required for actin bundle formation during oogenesis and bristle extension. J Cell Biol. 1994;125:369–380. doi: 10.1083/jcb.125.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cant K, Cooley L. Single amino acid mutations in Drosophila fascin disrupt actin bundling function in vivo. . Genetics. 1996;143:249–258. doi: 10.1093/genetics/143.1.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers C, Grey RD. Development of the structural components of the brush border in absorptive cells of the chick intestine. Cell Tiss Res. 1979;204:387–405. doi: 10.1007/BF00233651. [DOI] [PubMed] [Google Scholar]
- DeRosier DJ, Tilney LG. How actin filaments pack into bundles. Cold Spr Harb Symp Quant Biol. 1982;81:525–540. doi: 10.1101/sqb.1982.046.01.049. [DOI] [PubMed] [Google Scholar]
- DeRosier DJ, Censullo R. Structure of F-actin needles from extracts of sea urchin oocytes. J Mol Biol. 1981;146:77–99. doi: 10.1016/0022-2836(81)90367-3. [DOI] [PubMed] [Google Scholar]
- Ezzell RM, Chafel MM, Matsudaira PT. Differential localization of villin and fimbrin during development of the mouse visceral endoderm and intestinal epithelium. Development (Camb) 1989;106:407–419. doi: 10.1242/dev.106.2.407. [DOI] [PubMed] [Google Scholar]
- FlyBase Consortium. FlyBase: A Drosophiladatabase. Nucleic Acids Res. 1998;26:85–88. doi: 10.1093/nar/26.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guild GM, Connelly PS, Shaw MK, Tilney LG. Actin filament cables in Drosophilanurse cells are composed of modules that slide passively past one another during dumping. J Cell Biol. 1997;138:783–797. doi: 10.1083/jcb.138.4.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsley, D.L., and G.G. Zimm. 1992. The Genome of Drosophila melanogaster. Academic Press, San Diego, CA. 1,133 pp.
- Mahajan-Mikios S, Cooley L. The villin-like protein encoded by the Drosophila quailgene is required for actin bundle assembly during oogenesis. Cell. 1994;78:291–301. doi: 10.1016/0092-8674(94)90298-4. [DOI] [PubMed] [Google Scholar]
- Petersen NS, Lankenau D-H, Mitchell HK, Young P, Corces VG. Forked proteins are components of fiber bundles present in developing bristles of Drosophila melanogaster. . Genetics. 1994;136:173–182. doi: 10.1093/genetics/136.1.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinson KI, Dunbar L, Samuelson L, Gumucio DL. Targeted disruption of the mouse villin gene does not impair the morphogenesis of microvilli. Dev Dynam. 1998;211:109–121. doi: 10.1002/(SICI)1097-0177(199801)211:1<109::AID-AJA10>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Schmid MF, Agris JM, Jakana J, Matsudaira P, Chiu W. Three-dimensional structure of a single filament in the Limulusacrosomal bundle: Scruin binds homologous helix-loop-beta motifs in actin. J Cell Biol. 1994;124:341–350. doi: 10.1083/jcb.124.3.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibayama T, Carboni JM, Mooseker MS. Assembly of the intestinal brush border: Appearance and redistribution of microvillar core proteins in developing chick enterocytes. J Cell Biol. 1987;105:335–344. doi: 10.1083/jcb.105.1.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes DL, DeRosier DJ. Growth conditions control the size and order of actin bundles in vitro. Biophys J. 1991;59:456–465. doi: 10.1016/S0006-3495(91)82239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney LG, Connelly P, Smith S, Guild GM. F-actin bundles in Drosophilabristles are assembled from modules composed of short filaments. J Cell Biol. 1996;135:1291–1308. doi: 10.1083/jcb.135.5.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney LG, DeRosier DJ. Actin filaments, stereocilia, and hair cells of the bird cochlea IV. How the actin filaments become organized in developing stereocilia and in the cuticular plate. Dev Biol. 1986;116:119–129. doi: 10.1016/0012-1606(86)90048-5. [DOI] [PubMed] [Google Scholar]
- Tilney LG, Saunders JC, Egelman EH, DeRosier DJ. Changes in the organization of actin filaments in the stereocilia of noise-damaged lizard cochleae. Hearing Res. 1982;7:181–197. doi: 10.1016/0378-5955(82)90013-2. [DOI] [PubMed] [Google Scholar]
- Tilney LG, Egelman EH, DeRosier DJ, Saunders JC. Actin filaments, stereocilia, and hair cells of the bird cochlea. II. Packing of actin filaments in the stereocilia and the cuticular plate and what happens to the organization when the stereocilia are bent. J Cell Biol. 1983;96:822–834. doi: 10.1083/jcb.96.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney LG, Tilney MS. Methods to visualize actin polymerization associated with bacterial invasion. Methods Enzymol. 1994;236:476–481. doi: 10.1016/0076-6879(94)36036-7. [DOI] [PubMed] [Google Scholar]
- Tilney LG, Tilney MS, Guild GM. F-actin bundles in Drosophilabristles I. Two filament cross-links are involved in bundling. J Cell Biol. 1995;130:629–638. doi: 10.1083/jcb.130.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney MS, Tilney LG, Stephens RE, Merte C, Drenckhahn D, Cotanche DA, Bretcher A. Preliminary biochemical characterization of the stereocilia and cuticular plate of hair cells of the chick cochlea. J Cell Biol. 1989;109:1711–1723. doi: 10.1083/jcb.109.4.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulfkuhle JD, Petersen NS, Otto JJ. Changes in the F-actin cytoskeleton during neurosensory bristle development in Drosophila: the role of singed and forked proteins. Cell Motil Cytoskel. 1998;40:119–132. doi: 10.1002/(SICI)1097-0169(1998)40:2<119::AID-CM2>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]